Abstract
Тhe poor prognosis of patients initially diagnosed at an advanced stage of colorectal cancer (CRC) and the heterogeneity within the same tumor stage define the need for additional predictive biomarkers. Tumor buds are proposed as a poor prognostic factor for CRC, however, they are still not implemented into routine pathology reporting. In turn, the chitinase-3-like protein 1 (CHI3L1) also known as YKL-40, is regarded as a candidate circulating biomarker and therapeutic target in CRC. The aim of our study was to investigate tissue YKL-40 localization and tumor budding in CRC. Thirty-one CRC patients and normal colonic tissues were examined. The correlation between YKL-40 levels, tumor budding and clinocopathological parameters was evaluated by polychoric correlation analysis. The immunohistochemical assessment revealed high YKL-40 expression in CRC in contrast to normal mucosa. Specifically, intense YKL-40 staining was detected in the front of tumor invasion compared with tumor parenchyma and noncancerous tissue. We present novel data for increased YKL-40 expression in tumor buds within the front of tumor invasion. We assume that the combination of this morphological parameter with the tissue level of the pleotropic YKL-40 glycoprotein could serve as a future prognostic biomarker for CRC stratification and treatment.
Keywords: YKL-40, Tumor budding, Colorectal cancer
1. Introduction
Colorectal cancer (CRC) is the third most common neoplasm worldwide and is one of the leading causes of death among oncological patients [1]. Progress is being made regarding early detection, risk stratification, and treatment principles. However, there are still unexplained aspects of how genetic alterations in CRC modulate cancer cell biology and shape the heterotypic interactions across cells in the tumor microenvironment (TME) [2].
Histomorphologic analysis remains an important tool to stratify malignant lesions but heterogeneity within the same tumor stages defines the need for additional prognostic biomarkers. Tumor budding was reported for the first time as “sprouting” at the invasive edge of carcinomas [3]. Since then, tumor budding is object of intensive investigations. It is considered as a histopathologic marker of an aggressive tumor process. Tumor budding is characterized by isolated or small clusters of tumor cells that are removed from the neoplastic epithelium and migrate a short distance into the neoplastic stroma. Tumors which display this feature have a more aggressive behaviour [4,5]. Some authors reported that the evaluation of budding could be a useful prognostic marker in CRC patients [6], but still the detailed molecular mechanisms of this formation remain unknown [7].
In the past two decades, tumor buds were proposed as a poor prognostic factor for CRC but they are still not implemented into routine pathology reporting. The American Joint Committee on Cancer and the College of American Pathologists guidelines for CRC include tumor budding as an optional field with a recommendation to report it in all stage I and stage II cases [7]. Single cells or clusters of up to four cells at the invasive margin of CRC could be classified as peritumoral budding (tumor buds at the tumor front) [8].
There are a number of predictive and prognostic genetic, molecular and proteomic markers that entered the clinical practice in CRC diagnosis and therapy [9]. However, there is not a single reliable biomarker for prediction of clinical outcome and response to treatment of CRC patients, which imposes the usage of a constellation of different laboratory and clinical parameters. Many efforts are now addressed to identify new molecules that can be also used as drug targets. Potential diagnostic, prognostic, and predictive CRC biomarkers such as proteins [1], epigenetic changes [10], non-coding RNAs [11] and fecal metabolites [12] are investigated. Most of them are tightly linked to proliferation, metastasis, and drug resistance [13].
In this varied field, the Chitinase-3-like protein 1 (CHI3L1) also known as YKL-40, rises a lot of interest as a candidate circulating biomarker and therapeutic target in CRC [14,15]. YKL-40 is a 40 kDa heparin- and chitin-binding glycoprotein, which belongs to the family of glycosyl hydrolases, but lacks enzymatic properties [16]. It is secreted by a multitude of cells including neutrophils, macrophages, stem cells, fibroblast-like synovial cells, chondrocytes, endothelial cells, vascular smooth muscle cells and hepatic stellate cells, as well as by tumor cells [17,18]. The gene for YKL-40 (CHI3L1) is located on chromosome 1q32.1 [19] and is overexpressed in normal cells with high proliferation rates [20]. YKL-40 plays a major role in different physiological processes involved in tissue remodelling and cellular adaptation to modifications of the TME along with macrophage differentiation and dendritic cells recruitment [21].
YKL-40 dysregulation has been linked to chronic inflammatory diseases and cancer, although its function has not yet been fully clarified in these pathological conditions. Recent evidence in different human cancers and animal tumor models links YKL-40 gene overexpression to higher cell proliferation, angiogenesis and vasculogenic mimicry, migration, and invasion [22,23]. For example, YKL-40 promotes cancer angiogenesis in glioblastoma and in breast and colon cancer [24]. Overexpression of YKL-40 is associated with extracellular tissue remodelling in glioblastoma as well as in non-small cell lung cancer (NSCLC) and prostate cancer, where it directly regulates the expression of Epithelial Mesenchymal Transition (EMT) genes [25,26]. Moreover, since YKL-40 is a secreted inflammatory protein, elevated patient serum concentrations have been reported to correlate with advanced tumor stage, poor outcome and limited response to therapy in various malignant diseases, including CRC [27,28]. Furthermore, in another survey, HCT116 and CaCo2 cells overexpressing YKL-40 were shown to exhibit increased motility, invasion and proliferation, and YKL-40 up-regulation was associated with EMT signalling activation. Of note, tissue YKL-40 overexpression has been proposed to increase the metastatic potential of CRC [29].
All these studies provide important evidence in support of the possible use of YKL-40 as a novel therapeutic target, as recently suggested by preclinical studies based on the application of an anti-YKL-40 antibody to treat brain tumors [30].
An increasing number of studies focused on CRC highlight serum YKL-40 level as a risk predictor and as an independent prognostic biomarker [20,31]. Also, the mechanism behind the short survival of CRC patients with elevated serum YKL-40 remains still to be fully elucidated. Moreover, the prognostic value of YKL-40 in patients with mCRC has not been examined yet.
In the current pilot study, we investigate tissue YKL-40 expression and tumor budding in CRC searching a relationship between protein level, tumor budding and clínico-pathological parameters. We present novel data on association of YKL-40 immunoreactivity with tumor front (budding) in adenocarcinomatous cells.
2. Materials and methods
2.1. Patient samples
A retrospective record review was performed on 31 patients who had undergone surgical resection for CRC at Service d’Anatomie et de Cytologie Pathologiques, Grand Hôpital de l’Est Francilien, Jossigny, France and at the Department of General and Clinical Pathology, Medical University of Plovdiv, Bulgaria.
During the primary review of tissue samples and clinical records of the basic group of patientsр the following selection criteria were set: optimal tissue preservation with few or no surgical autolysis according to light microscopic evaluation; histologically present CRC; budding reported only in nonmucinous and nonsignet ring cell adenocarcinoma areas of the tumor; neither prior radiation treatment nor neoadjuvant therapy. Clinical and follow-up data were obtained from medical records and surgical pathology files. For each patient the following demographic and clinicopathological characteristics were considered: age, sex, tumor localization, tumor differentiation, pathological tumor stage (pTNM), presence of microsatellite instability or genetic somatic mutation, and budding.
Informed consent was obtained from all patients and the survey was approved by the Ethics Committee at the Medical University of Plovdiv (Protocol No 4/08.06. 2022). CRC tissues were collected at the time of surgical resection, fixed in 10% neutral buffered formaldehyde. Small pieces of tissues at the border with a non-tumorous colon (around 2 × 2 × 2 to 3 × 3 × 3 cm depending on the size) were embedded in paraffin. Normal samples from distal tumoral colonic tissue from the main group of patients and 5 non-neoplastic colon tissue samples (surgical margins of operative material from sigmoid diverticulosis; age-matched patients) were used as internal and external normal controls, respectively.
Standard 4-μm-thick paraffin sections were stained with haematoxylin-eosin (HE) and haematoxylin-eosin-safran (HES) or were used for immunohistochemical analyses.
2.2. Immunohistochemistry
Briefly, tissue sections were deparaffinized and rehydrated. Antigen retrieval was achieved with 0.01 M citrate buffer (pH 6.0) for 20 min at 95 °C water bath. Endogenous peroxide was quenched by 3% H2O2, followed by protein block according to manufacturer's instructions (Leica, NovocastraTM Peroxidase Detection System, Cat No RE7110-K). All samples were incubated with primary rabbit polyclonal anti-YKL-40 antibody (Abcam, ab180569; working dilution, 1:100) at 4 °C, overnight. For each case a negative control was prepared by replacing the primary antibody by 1% BSA/TBS. Samples were then incubated with biotinylated secondary antibody for 1 h and with streptavidin-HRP for 30 min, at room temperature (RT). DAB was used as a chromogen. Following counterstaining with Novocastra haematoxylin, the slides were mounted with mounting medium (Biognost, Cat № BM-500). Digital slides were visualized using the CaseViewer (3DHistech) software.
The intensity of YKL-40 expression was calculated according to a semi quantitative scale [20,32,33]. The findings were stratified using a scale of 0–3: a score of 0 denoted no detectable expression, 1 denoted “weak” expression (in <10% of the total cells in one visual field at 400× magnification), 2 denoted “moderate” expression (>10%–50% of the total cells in one visual field at 400× magnification) and 3 denoted “strong” expression (in >50% of the total cells in one visual field at 400× magnification).
2.3. Tumor budding assessment in CRC
The quantitative morphologic analysis of tumor budding was performed by a senior pathologist (DD) as recommended from the International Tumor Budding Consensus Conference (ITBCC) of 2016. Tumor budding was scored using a 3-tier system (Bd1-Bd3) according to the number of buds evident in the highest count after scanning 10 separate fields (at 20× objective lens) along the invasive front of the tumor. The number of tumor buds was based on HE assessment [34]. A correction for microscope eyepiece field diameter as well as bud count normalization to a field area of 0.785 mm2 was performed [32].
2.4. Statistical analysis
The data were explored for missing values, where none were detected, and distributional assumption tests were then performed. Fisher's exact test was used to assess the association among the studied ordinal variables (tumor budding, YKL-40 expression, histological differentiation of the tumor). Exact confidence intervals of the proportions were calculated using the binominal distribution. Statistical data processing was conducted using Fisher-Freeman-Halton test and GraphPad Prism10.
Given that most of the variables of interest were binary or ordered categorical, we employed polychoric correlation analysis (or polyserial correlations for the tests with Age) to examine the patterns of associations in our dataset. Polychoric correlations were generated as maximum likelihood estimates under the assumption that the observed ordinal variables reflected continuous latent constructs [35]. For these tests, we used the package “polychoric” in Stata v. 18 [36]. The correlations of interest were presented in a tabular form. Results were considered statistically significant at the p < 0.05 level.
To control the proportion of type I errors (false discovery rate) due to multiple testing within a given statistical family of tests (e.g., in a correlation matrix with multiple bivariate tests), we applied the Benjamini–Hochberg correction method [37].
3. Results
3.1. Demographic and clinicopathological characteristics of CRC patients
The sample comprised a total of 31 CRC patients with a mean age of 71 ± 10 (years). Fourteen (45,2%) were females and 17 males (54,8%). The clinicopathological characteristics of the patients examined are summarized in Table 1. Patient survival is strictly correlated with the pT stage in the TNM classification and ranges from 3 to 12 years after initial diagnosis, to date (data not shown).
Table 1.
Demographic and clinicopathological characteristics of CRC patients.
| Characteristics | Patients |
|---|---|
|
Number Age (years), mean |
n = 31 70, 9 ± 10, 2 |
|
Sex Female Male |
14 (45,2 %) 17 (54, 8 %) |
|
Primary site Right colon Left colon Other (rectum, colon transversum) |
14 (45,2 %) 14 (45,2 %) 3 (9,7 %) |
|
Histology Conventional Not determined |
29 2 |
|
Histological grade (G) G1-G2 (low grade) G3 (high grade) |
27 (87,1%) 4 (3 %) |
|
Tumor stage pT1 pT2 pT3 pT4 |
1 (3,2%) 3 (9,7%) 15 (48,4%) 12 (38,7%) |
|
Lymphatic and vascular invasion Present Absent |
17 (54,8%) 14 (45,2%) |
|
Microsatellite instability Present Absent |
12 (38,7%) 19 (61,3%) |
|
Genetic somatic mutations K-Ras N-Ras Others (PIK3CA/p.E545K) |
25 (80,6%) 2 (6,5%) 4 (12,9%) |
Legend: G1-well-differentiated tumor; G2-moderately differentiated tumor; G3-poorly differentiated tumor.
3.2. Evaluation of tumor budding
Tumor budding was classified as high (Bd2 and Bd3) in 19 tumors (61,3%) of which 16 were moderate and low differentiated (Fig. 1A). Absence or Bd1 was found in 12 (38,7%) patients.
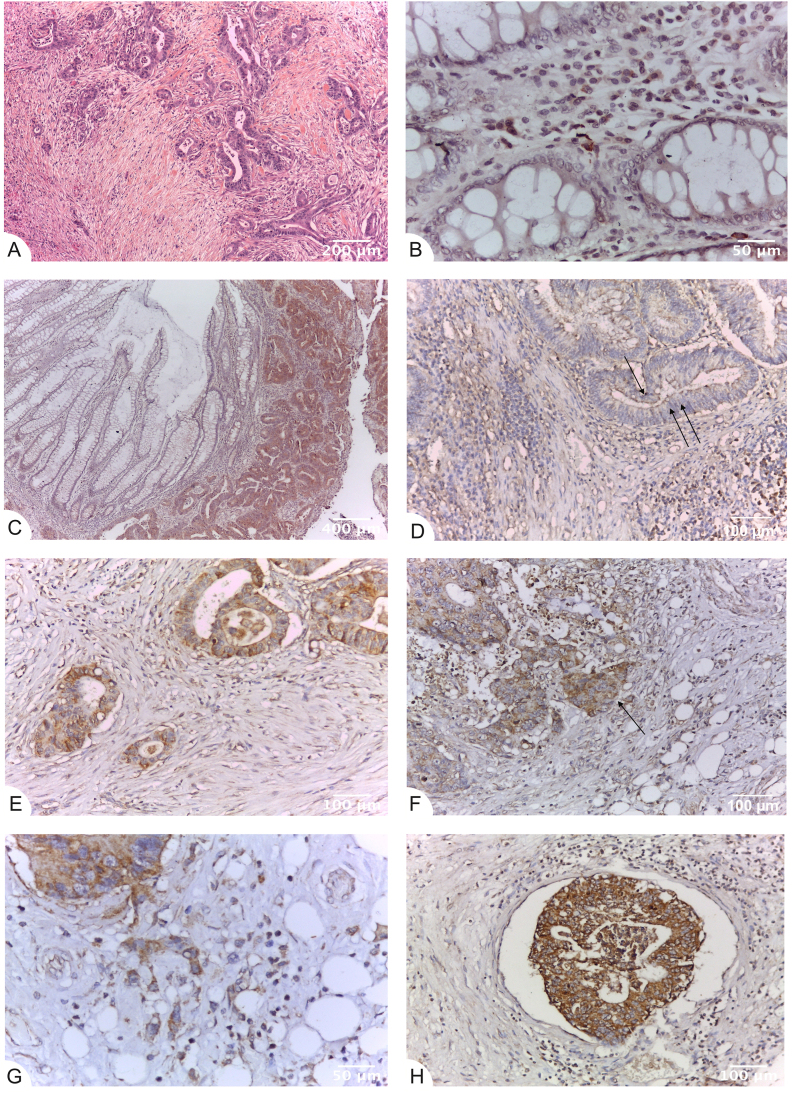
Fig. 1.
Strong YKL-40 expression in tumor buds within the front of tumor invasion in CRC. Tissue sections (A) Tumor budding (Bd3) (left) in the front of tumor invasion of colorectal carcinoma in standard stain. HES, x100; (B) Lack of YKL-40 immunoreactivity in the glands (0) and weak glycoprotein expression (1+) in macrophages within the mucous chorion of normal control colon. Immunohistochemistry (IHC), anti-YKL-40, ×400 (original magnification); (C), (D) Higher immunohistochemical expression of YKL-40 in colorectal carcinoma (right) compared to the normal mucosa. Apical membranous and vacuolar YKL-40 expression (arrow) of low (D) to moderate (C) intensity in the glandular tumor parenchyma. IHC, anti-YKL-40, x50(C), and ×200(D)(original magnification); (E) Intensive immunostaining of YKL-40 (2+ and 3+) in the front of tumor invasion. IHC, anti-YKL-40, ×200 (original magnification); (F) Higher immunohistochemical expression (arrow, centre) compared to the tumor parenchyma (left and top). IHC, anti-YKL-40, ×200 (original magnification); (G) Intensive immunostaining (3+) of YKL-40 in the tumor budding (Bd3) in the front of tumor invasion-tumor and stromal cells. IHC, anti-YKL-40, ×400 (original magnification); (H) Strong expression of YKL-40 (3+) in a peritumoral venous tumor embolus. IHC, anti-YKL-40, ×200 (original magnification). YKL-40 expression levels in tumor parenchyma, stroma and front are assessed on adenocarcinomatous cells (cytokeratin 20+/cytokeratin 7-/CDX2+ markers).
3.3. Evaluation of YKL-40 expression in CRC and normal colon tissues
The expression of YKL-40 in 31 CRC specimens and distal normal peritumoral tissue mucosa and external controls was investigated by immunohistochemistry. Positive expression (brown) staining of YKL-40 was noticed in the cell cytoplasm. Lack of YKL-40 reactivity (0) in normal colon glandular tissue and weak glycoprotein expression (1+) in macrophages within tumor stroma were observed (Fig. 1B). The immunohistochemical expression of YKL-40 was higher in CRC compared to the normal mucosa (Fig. 1C). Low to moderate (1+ to 2+) YKL-40 expression was noted in the glandular tumor parenchyma of CRC patients (Fig. 1D). Intensive immunostaining of YKL-40 (2+ and 3+) in the front of tumor invasion was found (Fig. 1E) in comparison with tumor parenchyma (Fig. 1F) and normal controls (Fig. 1B and D).
Strong expression of YKL-40 (3+) in the tumor budding (Bd3) in the front of tumor invasion within tumor and stromal cells (Fig. 1G) and in peritumoral venous tumor emboli was detected (Fig. 1H).
A significant difference in the localization of YKL-40 expression in the tumor parenchyma and at the front of tumor invasion was also found. In tumor parenchymal cells, the expression was membranous and in the apical part of vacuoles (Fig. 1D). In tumor cells with budding at the invasion front, YKL-40 showed a diffusely cytoplasmic expression (Fig. 1B, C, D, F).
3.4. Quantitative analysis of YKL-40 expression
In the next step, we compared quantitatively YKL-40 expression in different tumor regions – tumor parenchyma, stroma and tumor front in the adenocarcinomatous cells (cytokeratin 20+/cytokeratin 7-/CDX2+). Summarized data of YKL-40 expression in different tumor regions are presented in Table 2, while Fig. 2 compares YKL-40 in tumor front and tumor parenchyma.
Table 2.
Immunohistochemical expression of YKL-40 of different tumor areas in CRC colon.
| Expression of YKL-40 |
CRC, tumor parenchyme |
CRC, tumor stroma |
CRC, tumor front |
CRC tumor normal parenchyme |
CRC tumor normal stroma |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % (95% CI) |
N | % (95% CI) |
N | % (95% CI) |
N | % (95% CI) |
N | % (95% CI) |
|
| No expression detected | 0 | 0 (0,0–11,2) |
0 | 0 (0,0–11,2) |
0 | 0 (0,0–11,2) |
5 | 16,1 [5,5–7,7–33] |
4 | 12,9 (3,6–29,83) |
| Weak expression (+) |
7 | 22,6 [1,6–9,9–41] |
5 | 16,1 [5,5–7,7–33] |
1 | 3,2 (0,1–16,7) |
24 | 77,7 [4,9–58,58–90] |
19 | 61,3 [2–15,15–42,42–78] |
| Moderate expression (++) |
18 | 58,1 [1–5,5–39,39–75] |
21 | 67,7 [3,6–48,48–83] |
0 | 0 (0,0–11,2) |
2 | 6,5 (0,8–21,4) |
8 | 25,8 (11,9–44,61) |
| Strong expression (+++) | 6 | 19,4 [5,5–7,7–37] |
5 | 16,1 [5,5–7,7–33] |
30 | 96,8 [3–9,9–83,83–99] |
0 | 0 (0,0–11,2) |
0 | 0 (0,0–11,2) |
Legend: 95% CI – 95% Confidence interval.
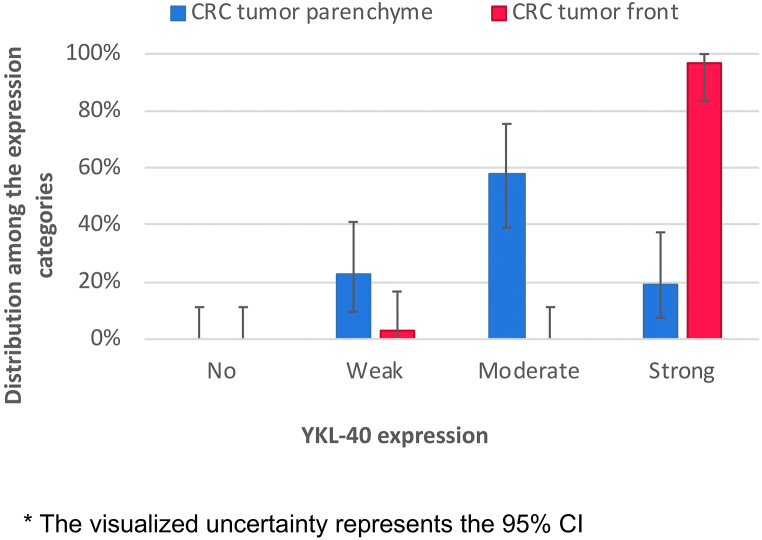
Fig. 2.
Definitive difference in YKL-40 expression between tumor parenchyma and tumor front. * The visualized uncertainty represents the 95% CI.
Strong YKL-40 immunoreactivity was determined in the tumor front of 30 patient samples (96,8%), while positivity in the tumor parenchyma was detected only in 6 patients (19,6%). These observations suggest a definitive difference between tumor parenchyma and tumor front in cases of strong YKL-40 expression (Fig. 2).
As expected, there was no intensive staining for YKL-40 in normal colon. Table 2 summarizes the level of immunohistochemical expression and its localization in CRC colonic tissues. The frequency modulation and the statistically significative differences in the intensity of YKL-40 expression in the tumor parenchyma and in the tumor stroma relative to the tumor front and to the negative controls are presented in Fig. 3.
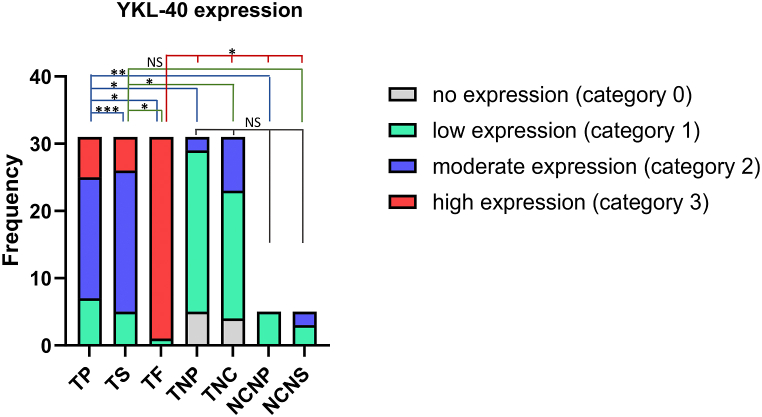
Fig. 3.
Difference in the intensity of YKL-40 expression in tumor parenchyma, stroma and front in CRC and in normal colon. Statistical significative differences of YKL-40 expression in the tumor parenchyma and in the tumor stroma relative to the tumor front and negative controls, respectively (tissue samples: CRC n = 31, normal colon n = 5). YKL-40 expression levels were categorized into four groups (category 0- no expression, 1- low expression, 2- moderate expression, 3- high expression) in the tissue regions analyzed: (TP- tumor parenchyma; TS- tumor stroma; TF- tumor front; TNP- tumor normal parenchyma; TNS- tumor normal stroma; PNC- parenchyma normal colon; SNC- stroma normal colon). Fisher-Freeman-Halton test. Differences are statistically significant with a Benjamini-Hochberg corrected p value (*p < 0,001; **p = 0,0022; ***p = 0,0023); not significant (NS), p > 0.05). For exact uncorrected and corrected p-values, see Supplementary Table S2.
3.5. Correlation analysis between YKL-40 expression and clinicopathological parameters
To further elucidate the relationship between YKL- 40 expression levels, tumor budding and clinicopathological parameters we performed polychoric correlation analysis. This matrix has been compiled by reporting the most significant correlation coefficients, where the p-values followed additional Benjamini-Hochberg correction. Statistically significant correlations and associations were obtained between tumor budding and lymph node involvement, invasion in lymphatics and blood vessels, respectively (r = 0.818, p = 0.0032; r = 0.800, p = 0.0036; r = 0.997, p = 0.0036). A moderate correlation between YKL-40 protein levels in tumor stroma and the number of somatic mutations detected (r = 0.593) was registered. Also a link between YKL-40 protein levels in the tumor front and the vascular invasion (r = 0.946) was found. However when the Benjamini-Hochberg correction was applied these correlations turned to be not significan (p = 0.16, p = 0.877) (Table 3). The exact uncorrected and corrected p-values are presented in Supplementary Table S1.
Table 3.
Polychoric correlation matrix between YKL-40 expression and budding and other clinical variables.
| G | pT | pN | L | V | Mutations | MSS/ MSI |
Age | Female vs Male | |
|---|---|---|---|---|---|---|---|---|---|
| Tumor parenchyma | 0.199 | −0.183 | −0.120 | −0.347 | −0.143 | 0.175 | −0.225 | −0.040 | 0.066 |
| Tumor stroma | 0.003 | −0.307 | −0.113 | −0.156 | −0.239 | 0.593 | −0.341 | −0.100 | −0.668 |
| Tumor front | – | −0.996 | −0.251 | −0.951 | 0.946 | −0.069 | – | −0.004 | – |
| Tumor normal parenchyma | −0.065 | −0.455 | 0.159 | 0.140 | 0.196 | 0.233 | −0.376 | −0.091 | −0.991a |
| Tumor normal stroma | −0.165 | −0.085 | 0.212 | 0.123 | 0.373 | 0.044 | −0.239 | 0.034 | −0.419 |
| Budd | 0.021 | 0.106 | 0.818a | 0.800a | 0.997a | 0.013 | −0.160 | 0.006 | −0.329 |
Abbreviations: G-histological stage; pT-tumor stage; pN- invasion in lymph nodes; L-lymphatics; V-blood vessels; MSS/MSI- microsatellite stability/microsatellite instability.
Correlation is statistically significant with a Benjamini-Hochberg corrected p value < 0.05. For exact uncorrected and corrected p-values, see Supplementary Table S1.
Collectively, our results on YKL-40 levels and tumor budding in CRC suggest a possible role for this glycoprotein in tumor invasion. These data also address the prognostic significance of YKL-40 as a potential marker for CRC diagnosis, stratification, and treatment.
4. Discussion
Despite the increasing recommendations for early detection screening and the continuous advancements in treatment protocols, approximately 25% of patients are initially diagnosed at an advanced stage with metastases. About 20% of these cases may develop metastatic CRC (mCRC), which is the main cause of CRC mortality [38,39].
Currently, the clinical stage determined in accordance with the TNM (tumor, lymph nodes, metastasis) classification provides information for risk stratification and for the choice of appropriate therapy. Nevertheless, the heterogeneous nature of tumors and the varying responses of primary and metastatic tumors to different treatment options often cause diverse patient outcome also within the same prognostic group or in patients with the same tumor stage [40]. In addition, drug resistance is still a major barrier to effective cancer treatment [41].
The prognostic importance of CRC tissue levels of YKL-40 is not fully clear yet. To our knowledge, the present study is the first to assess the significance of the tumor budding in CRC and the clinicopathological value of YKL-40 expression in different tumor regions. In addition, the impact of YKL-40 according to the immune score is discussed. Compared to normal cells, YKL-40 expression is shown to be higher in various cancer cells [15,42,43]. Here, in the CRC study group examined, we have also clearly detected elevated YKL-40 in the tumor parenchyma, stroma and front of adenocarcinomatous cells. Of note, in our survey, we are showing that YKL-40 is not only expressed in tumor cells, but it is intensively present in the tumor front (both in tumor and stromal cells), indicating a role in the invasive potential of cancer cells. In support of this data is the observation by Xu CJ et al. who reported a proliferation of stromal myofibroblasts in colon carcinoma that resulted in fibrosis in the area of tumor budding [44]. These findings are in accordance with other investigations focused on the prognostic impact of YKL-40 immunohistochemical expression in CRC patients [42,45]. YKL- 40 has also been shown to be involved in promoting CRC cell migration and invasion by activating the EMT, probably through the PI3K/AKT signalling pathway [29].
Tumor budding is thought to be an emerging prognostic biomarker in a variety of solid cancers, including CRC [45,46]. It represents a dynamic process involving tumor cell dissociation from the main tumor tissue [47]. In CRC, tumor budding is correlated with the prediction of lymph node metastasis in pT1 cases [48] and a poor relapse in stage II colon cancer [49]. Additionally, tumor budding is also discussed as a marker associated with EMT transition where tumor buds interact with diverse components of the tumor stroma [50].
Yamada et al. used tissue microarray to show that a set of five EMT-related proteins have a minor role in tumor bud formation. This finding provoked the search for new protein molecules associated with tumor budding and progression [51]. Furthermore, YKL-40 promotes invasion and metastasis of bladder cancer by regulating EMT [52]. Thus, YKL-40 may be a good candidate and supported by our data, together with tumor budding, they may serve as prognostic biomarkers in colon cancer. In the future, it would be of great interest to investigate and follow up YKL-40 expression in metastases within the CRC group examined, with respect to tumor budding with the aim of unraveling its predictive significance in cancer recurrence.
Recent research focusing on YKL-40 tissue expression in CRC and Inflammatory Bowel Disease (IBD) revealed a significant upregulation in colonic epithelial cells under inflammatory conditions and a particular ability of YKL-40 to exacerbate intestinal inflammation by enhancing bacterial adhesion and invasion in colonic epithelial cells [31,53]. We are unaware of earlier research on both YKL-40 and tumor budding in CRC, therefore, our present results are the first to reveal moderate to strong expression of YKL-40 in tumor stroma and tumor front in 67,7% and 96, 8% of CRC cases, respectively. The relationships between YKL-40 protein levels in tumor stroma and tumor front with the presence of somatic mutations and vascular invasion, respectively, are other interesting findings of our study (data not shown). They support the fact that genetic mutations can cause important stromal changes and activation, and indicate a possible role for YKL-40 in cancer progression and invasion. In turn, the association found between MSS/MSI and aging in our analysis is consistent with previously published data, as microsatellite instability is a hallmark of age-related defects in DNA mismatch repair activity [54,55].
A potential angiogenic role of YKL-40 in CRC in an animal model was reported by Kawada et al. [56]. These results were supported by the fact that YKL-40 expression was regulated by a variety of pro-inflammatory cytokines [28]. Tissue overexpression of YKL-40 plays an important role in stromal cells and in the TME, by promoting chemotaxis of macrophages and increasing the density of microvessels [57].
Additionally, in a previous multi-cohort study of public human CRC expression profiles and our own clinical data (n = 1533) we detected elevated YKL-40 expression which correlated with shorter survival in patients with advanced CRC. We investigated the prognostic impact of YKL-40 by analyzing data of CRC patients with different stages. In four out five datasets, Kaplan–Meier curves showed shorter survival in patients with high YKL-40 expression levels than in patients with low YKL-40. Indicating that YKL-40 upregulation is related to a poor prognosis of CRC [29].
We found tumor tissue-specific modulation in YKL-40 expression in CRC. In particular, much higher levels were detected at the front of the tumor, relative to the tumor parenchyma and stroma. The restriction of YKL-40 expression at the tumor front, together with tumor budding at the tumor front and the apparent association between budding and clinicopathological features such as invasion in lymphatics and blood vessels, indicate a possible role for YKL-40 in cancer progression. There are several key data published in the literature, supporting our findings. The group of Graves et al. found increased secretion of a cell surface mucin-glycoprotein in breast cancer, podocalyxin, which facilitated tumor spread and regulated tumor budding and invasion [58]. In this regard, Shino et al. reported the presence of cytoplasmic pseudo-fragments around budding foci in CRC, which resulted from the activation of lysosomal and cytosolic enzymes and glycoproteins in the cytoplasm of tumor cells within the tumor front [59].
However, although there is an obviously enhanced expression of YKL-40 in tumor buds, we did not find significant correlation between expression level, tumor grade or budding score. A limitation of our study is the small number of examined cases, which could partially explain the lack of correlation observed. Also, it should be considered that in normal colonic tissue there are no buds nor strong YKL-40 expression. In addition, since there is almost no variation in YKL-40 expression at the tumor front in our group of CRC patients, the analysis of this parameter is not that informative. Another drawback of the report is the lack of data on the clinical outcome and survival as this is a prospective pilot study. Taking into account the modest statistical power of our analyses, the borderline-significant associations we observed could still be considered clinically important with a relative prognostic impact. We believe that more translational studies including experiments on CRC cell lines, as well as examining a larger CRC cohort could have a prognostic value and might implicate new reliable biomarkers.
In conclusion, we present novel data on YKL-40 expression and tumor budding in CRC suggesting that this glycoprotein with diverse functions might be associated with tumor aggressiveness. A new insight into the clinical relevance of the identified signatures, the spatial immunochemical expression of YKL-40 and tumor budding is provided.
Ethics statement
-
•
This study was reviewed and approved by the Ethics Committee at the Medical University of Plovdiv, with the protocol number: (4/08.06. 2022).
-
•
All participants/patients (or their proxies/legal guardians) provided informed consent to participate in the study.
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request. Contact Tsvetomira Ivanova, Victoria Sarafian.
CRediT authorship contribution statement
Maria Kazakova: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Funding acquisition, Formal analysis, Conceptualization. Tsvetomira Ivanova: Writing – review & editing, Supervision, Project administration, Methodology, Funding acquisition, Formal analysis, Data curation, Conceptualization. Dorian Dikov: Writing – review & editing, Writing – original draft, Formal analysis. Diana Molander: Writing – review & editing, Formal analysis, Data curation. Kiril Simitchiev: Writing – review & editing, Formal analysis. Yordan Sbirkov: Writing – review & editing, Project administration, Formal analysis. Angel M. Dzhambov: Writing – review & editing, Formal analysis. Victoria Sarafian: Writing – review & editing, Supervision, Project administration, Methodology, Funding acquisition, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study is financed by the European Union-NextGenerationEU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project № BG-RRP-2.004-0007-C01 and by the Bulgarian National Science Fund - grant project № КП-06 ПН63/7 from 2022/BG-175467353-2022-04-0056.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e27570.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Rawla P., Sunkara T., Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Przegląd Gastroenterol. 2019;14:89–103. doi: 10.5114/pg.2018.81072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J., Ma X., Chakravarti D., et al. Genetic and biological hallmarks of colorectal cancer. Genes Dev. 2021;35:787–820. doi: 10.1101/gad.348226.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morodomi T., Isomoto H., Shirouzu K., et al. An index for estimating the probability of lymph node metastasis in rectal cancers: lymph node metastasis and the histopathology of actively invasive regions of cancer. Cancer. 1989;63:539–543. doi: 10.1002/1097-0142(19890201)63:3<539::aid-cncr2820630323>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Mitrovic B., Handley K., Assarzadegan N., et al. Prognostic and predictive value of tumor budding in colorectal cancer. Clin. Colorectal Cancer. 2021;20:256–264. doi: 10.1016/j.clcc.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Basile D., Broudin C., Emile J.F., et al. Tumor budding is an independent prognostic factor in stage III colon cancer patients: a post-hoc analysis of the IDEA-France phase III trial (PRODIGE-GERCOR) Ann. Oncol. 2023;33:628–637. doi: 10.1016/j.annonc.2022.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Betge J., Kornprat P., Pollheimer M., et al. Tumor budding is an independent predictor of outcome in AJCC/UICC stage II colorectal cancer. Ann. Surg Oncol. 2012;19:3706–3712. doi: 10.1245/s10434-012-2426-z. [DOI] [PubMed] [Google Scholar]
- 7.Ueno H., Mochizuki H., Hashiguchi Y., et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004;127:385–394. doi: 10.1053/j.gastro.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 8.Lugli A., Vlajnic T., Giger O., et al. Intratumoral budding as a potential parameter of tumor progression in mismatch repair-proficient and mismatch repair deficient colorectal cancer patients. Hum. Pathol. 2011;42:1833–1840. doi: 10.1016/j.humpath.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Erstad D., Tumusiime G., Cusack J., et al. Prognostic and predictive biomarkers in colorectal cancer: implications for the clinical surgeon. Ann. Surg Oncol. 2015;22:3433–3450. doi: 10.1245/s10434-015-4706-x. [DOI] [PubMed] [Google Scholar]
- 10.Jia Y., Guo M. Epigenetic changes in colorectal cancer. Chin. J. Cancer. 2013;32:21–30. doi: 10.5732/cjc.011.10245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomes S., Simões A., Pereira D., et al. miR-143 or miR-145 overexpression increases cetuximab-mediated antibody-dependent cellular cytotoxicity in human colon cancer cells. Oncotarget. 2016;7:9368–9387. doi: 10.18632/oncotarget.7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yagin F., Alkhateeb A., Colak C., et al. A fecal-microbial-extracellular-vesicles-based metabolomics machine learning framework and biomarker discovery for predicting colorectal cancer patients. Metabolites. 2023;13:589. doi: 10.3390/metabo13050589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strippoli A., Cocomazzi A., Basso M., et al. c-MYC expression is a possible keystone in the colorectal cancer resistance to EGFR inhibitors. Cancers. 2020;12:638. doi: 10.3390/cancers12030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansen J., Jensen V., Roslind A., et al. Serum YKL-40, a new prognostic biomarker in cancer patients? Cancer Epidemiol. Biomarkers Prev. 2006;15:194–202. doi: 10.1158/1055-9965.EPI-05-0011. [DOI] [PubMed] [Google Scholar]
- 15.Schultz N., Johansen J. YKL-40-A protein in the field of translational medicine: a role as a biomarker in cancer patients? Cancers. 2010;2:1453–1491. doi: 10.3390/cancers2031453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu B., Trinh K., Figueira W., et al. Isolation and sequence of a novel human chondrocyte protein related to mammalian members of the chitinase protein family. J. Biol. Chem. 1996;271:19415–19420. doi: 10.1074/jbc.271.32.19415. [DOI] [PubMed] [Google Scholar]
- 17.Recklies A., White C., Ling H. The chitinase 3-like protein human cartilage glycoprotein 39 (HC-gp39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal regulated kinase- and protein kinase B-mediated signaling pathways. Biochem. J. 2002;365:119–126. doi: 10.1042/BJ20020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Ceuninck F., Gaufillier S., Bonnaud A., et al. YKL-40 (cartilage gp-39) induces proliferative events in cultured chondrocytes and synoviocytes and increases glycosaminoglycan synthesis in chondrocytes. Biochem. Biophys. Res. Commun. 2001;285:926–931. doi: 10.1006/bbrc.2001.5253. [DOI] [PubMed] [Google Scholar]
- 19.Rehli M., Krause S., Andreesen R. Molecular characterization of the gene for human cartilage gp-39(CHI3L1), a member of the chitinase protein family and marker for late stages of macrophage differentiation. Genomics. 1997;43:221–225. doi: 10.1006/geno.1997.4778. [DOI] [PubMed] [Google Scholar]
- 20.Kim S., Das K., Noreen S., et al. Prognostic implications of immunohistochemically detected YKL-40 expression in breast cancer. World J. Surg. Oncol. 2007;5:17. doi: 10.1186/1477-7819-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roslind A., Johansen J. YKL-40: a novel marker shared by chronic inflammation and oncogenic transformation. Methods Mol. Biol. 2009;511:159–184. doi: 10.1007/978-1-59745-447-6_7. [DOI] [PubMed] [Google Scholar]
- 22.Shao R., Francescone R., Ngernyuang N., et al. Anti-YKL-40 antibody and ionizing irradiation synergistically inhibit tumor vascularization and malignancy in glioblastoma. Carcinogenesis. 2014;35:373–382. doi: 10.1093/carcin/bgt380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansen J. Changes of biochemical markers of bone turnover and YKL-40 following hormonal treatment for metastatic prostate cancer are related to survival. Clin. Cancer Res. 2007;13:3244–3249. doi: 10.1158/1078-0432.CCR-06-2616. [DOI] [PubMed] [Google Scholar]
- 24.Jensen V., Johansen J., Price A. High levels of serum HER-2/neu and YKL-40 independently reflect aggressiveness of metastatic breast cancer. Clin. Cancer Res. 2003;9:4423–4434. [PubMed] [Google Scholar]
- 25.Geng B., Pan J., Zhao T., et al. Chitinase 3-like 1-CD44 interaction promotes metastasis and epithelial-to-mesenchymal transition through beta-catenin/Erk/Akt signaling in gastric cancer. J. Exp. Clin. Cancer Res. 2018;37:208. doi: 10.1186/s13046-018-0876-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao T., Su Z., Li Y., et al. Chitinase-3 like-protein-1 function and its role in diseases. Signal Transduct. Targeted Ther. 2020;5:201. doi: 10.1038/s41392-020-00303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roslind A., Knoop S., Jensen B., et al. YKL-40 protein expression is not a prognostic marker in patients with primary breast cancer. Breast Cancer Res. Treat. 2020;112:275–285. doi: 10.1007/s10549-007-9870-7. [DOI] [PubMed] [Google Scholar]
- 28.Bhat K., Pelloski C., Zhang Y., et al. Selective repression of YKL-40 by NF kappa B in glioma cell lines involves recruitment of histone deacetylase-1 and -2. FEBS Lett. 2008;582:3193–3200. doi: 10.1016/j.febslet.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 29.De Robertis M., Greco M., Cardone R., et al. Upregulation of YKL-40 promotes metastatic phenotype and correlates with poor prognosis and therapy response in patients with colorectal cancer. Cells. 2022;11:3568. doi: 10.3390/cells11223568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao R., Hamel K., Petersen L., et al. YKL-40, a secreted glycoprotein, promotes tumor angiogenesis. Oncogene. 2009;28:4456–4468. doi: 10.1038/onc.2009.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johansen J., Christensen I., Jørgensen L., et al. Serum YKL-40 in risk assessment for colorectal cancer: a prospective study of 4,496 subjects at risk of colorectal cancer. Cancer Epidemiol. Biomarkers Prev. 2015;24:621–626. doi: 10.1158/1055-9965.EPI-13-1281. [DOI] [PubMed] [Google Scholar]
- 32.Francescone R., Scully S., Faibish M., et al. Role of YKL-40 in the angiogenesis, radioresistance, and progression of glioblastoma. J. Biol. Chem. 2011;286:15332–15343. doi: 10.1074/jbc.M110.212514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang D., Zhai B., Hu F., et al. High YKL-40 Serum concentration is correlated with prognosis of Chinese patients with breast cancer. PLoS One. 2012;7 doi: 10.1371/journal.pone.0051127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lugli A., Kirsch R., Ajioka Y., et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) Mod. Pathol. 2017;30:1299–1311. doi: 10.1038/modpathol.2017.46. [DOI] [PubMed] [Google Scholar]
- 35.Drasgow F. Copyright john Wiley & Sons. Polychoric and polyserial correlations. Encyclopaedia of Statistical Sciences. 1988;7:68–74. [Google Scholar]
- 36.Kolenikov S. Polychoric, by any other ‘namelist.’ Retrieved from Stata website: https://www.stata.com/meeting/chicago16/slides/chicago16_kolenikov.pdf.2016. .
- 37.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B. 1995;57:289–300. [Google Scholar]
- 38.Zhai Z., Yu X., Yang B., et al. Colorectal cancer heterogeneity and targeted therapy: clinical implications, challenges and solutions for treatment resistance. Semin. Cell Dev. Biol. 2017;64:107–115. doi: 10.1016/j.semcdb.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 39.Xie Y., Chen X., Fang J. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Targeted Ther. 2020;5:22. doi: 10.1038/s41392-020-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vetter L., Merkel S., Bénard A., et al. Colorectal cancer in Crohn's colitis is associated with advanced tumor invasion and a poorer survival compared with ulcerative colitis: a retrospective dual-center study. Int. J. Colorectal Dis. 2021;36:141–150. doi: 10.1007/s00384-020-03726-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Misale S., Di Nicolantonio F., Sartore-Bianchi A., et al. Resistance to anti-EGFR therapy in colorectal cancer: from heterogeneity to convergent evolution. Cancer Discov. 2014;4:1269–1280. doi: 10.1158/2159-8290.CD-14-0462. [DOI] [PubMed] [Google Scholar]
- 42.Hacking S.M., Chakraborty B., Nasim R., et al. A holistic appraisal of stromal differentiation in colorectal cancer: biology, histopathology, computation, and genomics. Pathol. Res. Pract. 2021;220 doi: 10.1016/j.prp.2021.153378. [DOI] [PubMed] [Google Scholar]
- 43.Steponaitis G., Skiriutė D., Kazlauskas A., et al. Diagn. Pathol. 2016;11:42. doi: 10.1186/s13000-016-0492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu C.J., Mikami T., Nakamura T., et al. Tumor budding, myofibroblast proliferation, and fibrosis in obstructing colon carcinoma: the roles of Hsp47 and basic fibroblast growth factor. Pathol. Res. Pract. 2013;209:69–74. doi: 10.1016/j.prp.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Kai K., Kohya N., Kitahara K., et al. Tumor budding and dedifferentiation in gallbladder carcinoma: potential for the prognostic factors in T2 lesions. Virchows Arch. 2011;459:449–456. doi: 10.1007/s00428-011-1131-9. [DOI] [PubMed] [Google Scholar]
- 46.Ohike N., Coban I., Kim G.E., et al. Tumor budding as a strong prognostic indicator in invasive ampullary adenocarcinomas. Am. J. Surg. Pathol. 2010;34:1417–1424. doi: 10.1097/PAS.0b013e3181f0b05a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lugli A., Zlobec I., Berger M.D., et al. Tumour budding in solid cancers. Nat. Rev. Clin. Oncol. 2021;18:101–115. doi: 10.1038/s41571-020-0422-y. [DOI] [PubMed] [Google Scholar]
- 48.Bosch S., Teerenstra S., de Wilt J., et al. Predicting lymph node metastasis in pT1 colorectal cancer: a systematic review of risk factors providing rationale for therapy decisions. Endoscopy. 2013;45:827–841. doi: 10.1055/s-0033-1344238. [DOI] [PubMed] [Google Scholar]
- 49.Ueno H., Ishiguro M., Nakatani E., et al. Prospective multicenter study on the prognostic and predictive impact of tumor budding in stage II colon cancer: results from the SACURA Trial J Clin Oncol. 2019;37:1886–1894. doi: 10.1200/JCO.18.02059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lugli A., Zlobec I., Berger M.D., et al. Tumour budding in solid cancers. Nat. Rev. Clin. Oncol. 2020;123:700–708. doi: 10.1038/s41571-020-0422-y. [DOI] [PubMed] [Google Scholar]
- 51.Yamada N., Sugai T., Eizuka M., et al. Tumor budding at the invasive front of colorectal cancer may not be associated with the epithelial-mesenchymal transition. Hum. Pathol. 2017;60:151–159. doi: 10.1016/j.humpath.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 52.Hao H., Wang L., Chen H., et al. YKL-40 promotes the migration and invasion of prostate cancer cells by regulating epithelial mesenchymal transition. Am. J Transl Res. 2017;15:3749–3757. [PMC free article] [PubMed] [Google Scholar]
- 53.Eurich K., Segawa M., Toei-Shimizu S., et al. Potential role of chitinase 3-like-1 in inflammation-associated carcinogenic changes of epithelial cells. World J. Gastroenterol. 2009;15:5249–5259. doi: 10.3748/wjg.15.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boland C., Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073–2087. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smwdt L., Lemahieu J., Palmans S., et al. Microsatellite instable vs stable colon carcinomas: analysis of tumour heterogeneity, inflammation and angiogenesis. BJC. 2015;113:500–509. doi: 10.1038/bjc.2015.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kawada M., Seno H., Kanda K., et al. Chitinase 3-like 1 promotes macrophage recruitment and angiogenesis in colorectal cancer. Oncogene. 2012;31:3111–3123. doi: 10.1038/onc.2011.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shao R., Francescone R., Ngernyuang N., et al. Anti-YKL-40 antibody and ionizing irradiation synergistically inhibit tumor vascularization and malignancy in glioblastoma. Carcinogenesis. 2014;35:373. doi: 10.1093/carcin/bgt380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Graves M.L., Cipollone J.A., Austin P., et al. The cell surface mucin podocalyxin regulates collective breast tumor budding. Breast Cancer Res. 2016;18:11. doi: 10.1186/s13058-015-0670-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shinto E., Mochizuki H., Ueno H., et al. A novel classification of tumour budding in colorectal cancer based on the presence of cytoplasmic pseudo-fragments around budding foci. Histopathology. 2005;47:25–31. doi: 10.1111/j.1365-2559.2005.02162.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request. Contact Tsvetomira Ivanova, Victoria Sarafian.