Abstract
Background
Chronic obstructive pulmonary disease (COPD) and hyperuricaemia are both characterised by systemic inflammation. Preventing chronic diseases among the population with common metabolic abnormality is an effective strategy. However, the association of hyperuricaemia with the higher incidence and risk of COPD remains controversial. Therefore, replicated researches in populations with distinct characteristics or demographics are compellingly warranted.
Methods
This cohort study adopted a design of ambispective hospital-based cohort. We used propensity score matching (PSM) and inverse probability of treatment weighting (IPTW) to minimise the effects of potential confounding factors. A Cox regression model and restricted cubic spline (RCS) model were applied further to assess the effect of serum urate on the risk of developing COPD. Finally, we conducted a two-sample Mendelian randomisation (MR) analysis to explore evidence of causal association.
Results
There is a higher incidence in the population with hyperuricaemia compared with the population with normal serum urate (22.29/1000 person-years vs 8.89/1000 person-years, p=0.009). This result is robust after performing PSM (p=0.013) and IPTW (p<0.001). The Cox model confirms that hyperuricaemia is associated with higher risk of developing COPD (adjusted HR=3.35 and 95% CI=1.61 to 6.96). Moreover, RCS shows that the risk of developing COPD rapidly increases with the concentration of serum urate when it is higher than the reference (420 µmol/L). Finally, in MR analysis, the inverse variance weighted method evidences that a significant causal effect of serum urate on COPD (OR=1.153, 95% CI=1.034 to 1.289) is likely to be true. The finding of MR is robust in the repeated analysis using different methods and sensitivity analysis.
Conclusions
Our study provides convincing evidence suggesting a robust positive association between serum urate and the risk of developing COPD, and indicates that the population with hyperuricaemia is at high risk of COPD in the Chinese population who seek medical advice or treatment in the hospital.
Keywords: COPD epidemiology, Clinical Epidemiology
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Robust positive association between high serum urate and higher risk of developing chronic obstructive pulmonary disease (COPD) remains controversial because there are conflicting interpretations of whether serum urate has a protective or detrimental effect with respect to lung function.
A limited number of epidemiological researches have explored whether high serum urate can lead to worsened lung function or higher risk of developing COPD, but results have been rather inconsistent.
WHAT THIS STUDY ADDS
This study is the first hospital-based cohort study in Chinese population to explore the effect of serum urate on the risk of developing COPD.
We found that there are higher incidence and risk of COPD in the population with hyperuricaemia compared with the population with normal serum urate.
The results of Mendelian randomisation analysis suggested that a significant causal effect of serum urate on the risk of developing COPD is likely to be true.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our study provides compelling evidence suggesting that the population with hyperuricaemia is at high risk of COPD in the Chinese population who seek medical advice or treatment in the hospital.
Our findings have guiding implications for clinical practice and indicate that intervening high levels of serum urate may be an effective strategy for the prevention of COPD.
Further researches, such as field trials or community intervention trials, are warranted to be carried out for investigating whether the management of the levels of serum urate could potentially lower the risk of developing COPD.
Introduction
The Global Burden of Disease Study described that the global prevalence of chronic obstructive pulmonary disease (COPD) in the population aged 40 years or older was 9–10%.1 2 In China, COPD was accounted for a quarter of the global population with COPD,3 and the prevalence was 13.6% in a nationally representative population aged 40 years or older.4 COPD is still an under-recognised and underdiagnosed disease characterised by chronic systemic inflammation.5 In addition, the disease burden of COPD is projected to continuously increase in the coming decades due to ageing of the population, smoking and air pollution.6 We strive to identify the high-risk population of COPD, in the hope that the intervention for population at high risk of COPD can successfully reduce the risk of developing COPD.
Urate is found at high concentrations in respiratory tissues and epithelial lining fluid of airways.7 Hyperuricaemia is also associated with the presence of systemic inflammation8 9 and is thought to inflict poor clinical consequences of respiratory inflammatory diseases, such as the acute exacerbation and mortality of patients with COPD.10 11 It seems biologically plausible that serum urate plays a role in the onset of COPD. However, causal association of high serum urate with higher risk of developing COPD remains controversial because there are conflicting interpretations of whether serum urate has a protective or detrimental effect with respect to lung function.
From a biomolecular mechanism perspective, serum urate could have opposing properties.12–14 While urate is an endogenous antioxidant that may protect the lung from oxidative damage caused by cigarette smoke and air pollution,7 15 it also, paradoxically, has been considered to possess pro-oxidant and inflammation-stimulatory effects, as serum urate can induce the expression of inflammatory markers16 17 and activate NLRP3 inflammasome.18 19 In addition, a limited number of epidemiological researches have explored whether high serum urate can lead to worsened lung function or higher risk of developing COPD, but results have been rather inconsistent.20–23 Actually, having inconsistent results is a frequent phenomenon in epidemiological researches because of potential confounders and heterogeneity of different populations.
Thus, replicated researches in populations with distinct characteristics or demographics are compellingly warranted. To the best of our knowledge, this is the first hospital-based cohort study in the Chinese population to explore the effect of serum urate on the incidence and risk of COPD. In the present study, we primarily focused on the population who seeks medical advice or treatment in the hospital. Thus, it was worth noting that, different from other epidemiological studies, this cohort study was carried out among the characteristic population rather than the community population. Other than that, our study also performed a two-sample Mendelian randomisation (MR) analysis using public summary statistics of Genome Wide Association Study (GWAS) to explore evidence of causal association between serum urate and the risk of developing COPD. MR analysis is regarded as a natural randomised controlled trial; therefore, it is an armoury of epidemiological analysis methods for strengthening causal inference.
Materials and methods
Study design and population
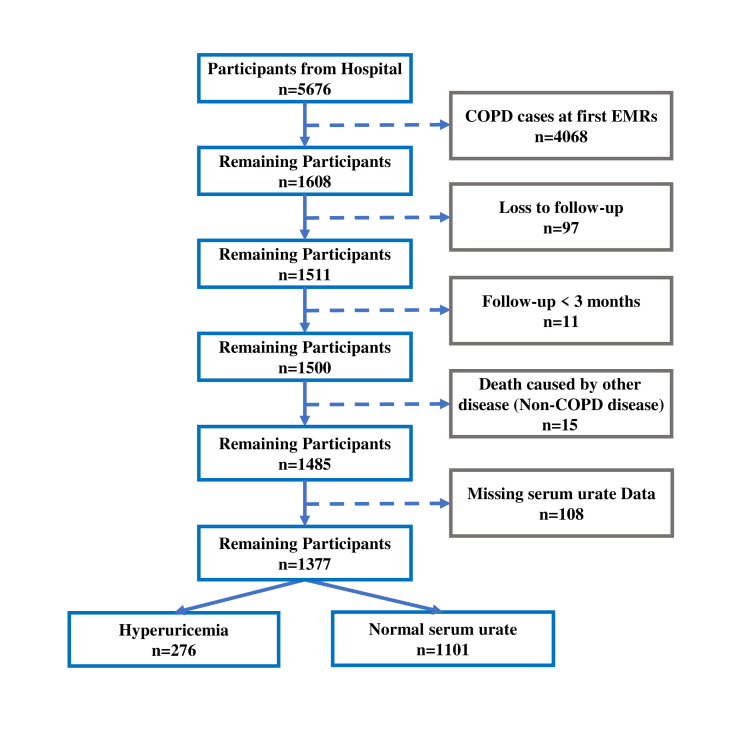
This cohort study adopted a design of ambispective hospital-based cohort. First, we conducted a retrospective cohort study using electronic medical records (EMRs) of the Department of Respiratory and Critical Care Medicine from four public hospitals (Shenzhen Longhua District Central Hospital, Dongguan Binhaiwan Central Hospital, Songshan Lake Central Hospital of Dongguan, Guangzhou Panyu District Central Hospital). EMRs were collected from 1 January 2016 to 30 June 2021. A total of 12 962 EMRs were obtained and included a total of 5676 individuals. As shown in a flow diagram (figure 1), we excluded individuals who were diagnosed as COPD at first EMR, loss to follow-up, had follow-up time less than 3 months, died because of non-COPD and had missing serum urate date; 1377 individuals were eventually eligible for inclusion in the analysis.
Figure 1.
Flow diagram for selecting research subjects. Of the 5676 participants obtained from electronic medical records (EMRs), we excluded individuals who were diagnosed as COPD at first EMR, loss to follow-up, had follow-up time less than 3 months, died because of non-COPD and had missing serum urate date; 1377 participants were eventually eligible for inclusion in the analysis after data cleaning in accordance with the flow diagram. COPD, chronic obstructive pulmonary disease.
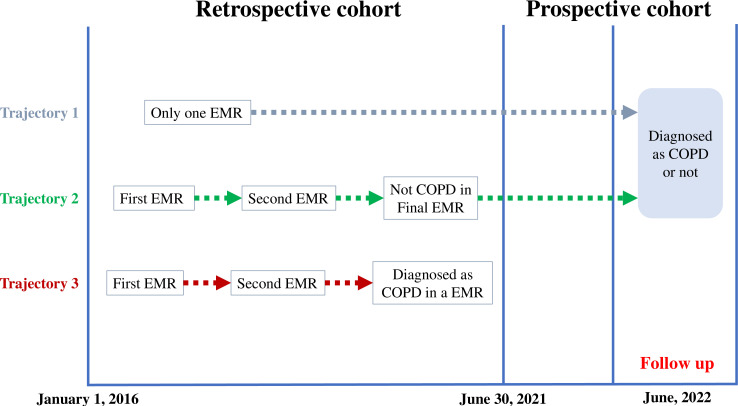
The specific procedure of this cohort was illustrated in a schematic diagram (figure 2). First, we conducted a retrospective cohort study based on EMRs of 5 years. Each EMR is regarded as a follow-up. We treated first EMR of every participant as baseline. Second, when the EMRs first showed that the participant was diagnosed with COPD, we considered this EMR as the occurrence of outcome. Finally, we conducted a follow-up work in the month of June 2022 for participants who had not been diagnosed as COPD in the final EMR. Specifically, in the month of June 2022, we provided a free lung function test for participants who were requested to follow up, and professional respiratory physicians diagnosed whether these participants had COPD. Additionally, we provided a free test of serum urate for participants who only had one EMR.
Figure 2.
Schematic diagram for the execution of the hospital-based cohort study. This hospital-based cohort study adopted a design of ambispective cohort. Each EMR is regarded as a follow-up. As shown in trajectory 1, for some participants who only have one EMR, we conducted follow-up spirometry and serum urate test in the month of June 2022 for them. Similarly, as shown in trajectory 2, we conducted a follow-up work in the month of June 2022 for participants who had not been diagnosed as COPD in the final EMR. As shown in trajectory 3, when the EMRs first showed that the participant was diagnosed with COPD, we considered this EMR as the occurrence of outcome. COPD, chronic obstructive pulmonary disease; EMR, electronic medical record.
It is important to note that all four hospitals have concealed the identifying labels of each participant and replaced them with analytical labels that do not disclose any personal information about the participants. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.24
Patient and public involvement
Participants and their families did not take part in the design, conduct, reporting or dissemination of this study.
Data collection and definition of variables
Information on participants was obtained from EMRs. The population with hyperuricaemia was considered to be the exposed group. The saturation of urate is 420 µmol/L (regardless of gender) in blood so that higher concentrations of serum urate can result in deposition of urate in tissues. China multidisciplinary expert consensus on diagnosis and treatment of hyperuricaemia and related diseases described that hyperuricaemia was defined as concentrations of serum urate higher than 420 µmol/L (regardless of gender).25 According to this criterion, individuals with hyperuricaemia in our study were defined as those with concentrations of serum urate higher than 420 µmol/L (regardless of gender) on two separate EMRs. The outcome is the diagnosis of COPD made by professional respiratory physicians, and the diagnostic standard of COPD is the ratio of forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC) less than 0.70 on post-bronchodilator spirometry. High creatinine level was defined as concentrations of blood creatinine higher than 106 µmol/L in male or 97 µmol/L in female. Demographic characteristics of participants included age (middle-age: 40–59 or old age: ≥60 years), gender (male and female), body mass index (BMI) (Chinese standards: underweight: <18.5, normal: ≥18.5 and <24, overweight: ≥24 and <28, obesity: ≥28), and smoking (yes: current and ever or no: never) and drinking status (yes: current and ever or no: never). Additionally, medical history (yes or no) included chronic bronchitis, bronchiectasis, tuberculosis, pneumonia and diabetes.
Propensity score matching and inverse probability of treatment weighting
To minimise the effects of potential confounding factors, we employed propensity score matching (PSM) and inverse probability of treatment weighting (IPTW) strategies. Specifically, we treated the above-mentioned variables (age, gender, BMI, smoking status, drinking status, chronic bronchitis, bronchiectasis, tuberculosis, pneumonia and diabetes) as potential confounding factors, and estimated the propensity score by using a multivariable logistic regression analysis that incorporated these as covariates. PSM analysis adopted a design with 2:1 control–exposure ratio and performed nearest neighbour matching. In IPTW analysis, individuals with hyperuricaemia were weighted by the inverse of the propensity score and individuals with normal serum urate were weighted by the inverse of 1 minus the propensity score. We examined covariate balance after PSM or IPTW by using standardised mean difference (SMD) of 0.1 or more as the cut-off for imbalance.
Cox regression model analysis
Four sets of Cox regression models were built for verifying the association between hyperuricaemia and the risk of developing COPD, among which model 1 was unadjusted; model 2 was adjusted for personal basic information that included age, gender, BMI and creatinine level; model 3 was additionally adjusted for information of environmental exposure that included smoking and drinking status; and model 4 was further adjusted for medical history that included chronic bronchitis, bronchiectasis, tuberculosis, pneumonia and diabetes.
Restricted cubic spline analysis
We used a restricted cubic spline (RCS) model to quantify the dose–response relationship between the concentration of serum urate and the risk of developing COPD. Three knots at 10th, 50th and 90th percentiles of serum urate concentration were used in plotted smooth curves, and covariates for adjusting in the RCS model were the same as above-mentioned potential confounding factors.
Two-sample MR study
We retrieve public GWAS datasets on the MR-Base platform developed by the Medical Research Council Integrative Epidemiology Unit.26 Serum urate-related single nucleotide polymorphisms (SNPs) were obtained from GWAS (GWAS ID: ieu-a-1055) of the Global Urate Genetics Consortium. The summary statistics of COPD GWAS (GWAS ID: finn-b-J10_COPD) were obtained from FinnGen database.
Furthermore, we conducted a two-sample MR analysis complying with the STROBE-MR guidelines27 and the guidelines for performing MR.28 Instrumental variables (IVs) were selected as exposure of MR study by adopting a series of approaches. First, we selected SNPs that were highly associated with levels of serum urate in terms of genome-wide significance (p<5×10−8). Second, we performed clumping process to ensure the independence of SNPs based on a low linkage disequilibrium with other SNPs (r2<0.001) within a clumping distance of 10 000 kb. Third, we excluded SNPs that were not present in the summary statistics of COPD GWAS. We calculated the individual and overall F-statistics for IVs, and F-statistic >10 was used as evidence to identify whether those SNPs were strong IVs.
The inverse variance weighted (IVW) was the main method implemented for MR analysis to figure out the causal effect, while additional methods, such as maximum likelihood, weighted median, weighted mode and MR-Egger, were used for sensitivity analysis to examine the robustness of the results. We further used IVW and MR-Egger methods to perform Cochran’s Q test to assess heterogeneity, and used the Egger regression intercept and MR-PRESSO to estimate the magnitude of horizontal pleiotropy. Leave-one-out analysis was conducted by removing one SNP at a time to determine whether the causal association was driven by a single SNP. We also performed reverse-direction MR analysis to estimate the effect of COPD on levels of serum urate. This MR study used only publicly available GWAS statistics from previously published works, making it exempt from institutional review board review.
Multivariable MR is an MR analysis that adjusts for potential confounding phenotypes, and can be used to assess whether potential confounding phenotypes introduce bias due to pleiotropy, resulting in yielding an independent effect of serum urate on COPD after adjusting the effect of potential confounding phenotypes. In this multivariable MR, we considered four variables as covariates, including estimated glomerular filtration rate based on creatinine (ebi-a-GCST90103634), alcohol consumption (ieu-a-1283), PM2.5 (ukb-b-10817) and pack years of smoking (ukb-b-10831). The multivariable IVW is the common method for multivariable MR.
Statistical analysis
We used PASS V.15 to calculate the sample size. The calculation parameters of sample size are as follows: reference incidence of COPD=7.8% (10-year cumulative incidence in community population),28 alpha=0.05, power=0.8, sample size of exposed group=276. We hypothesised that the population with hyperuricaemia has more than twofold higher risk of developing COPD than those with normal serum urate (rate ratio, RR ≥2). The results of sample size estimation show that the sample size of our control group is sufficient for statistical analysis (online supplemental figure 1). On the contrary, we calculated the statistical power of our results according to sample size of this study and RR obtained from the analysis. The calculation parameters of statistical power are as follows: sample size of exposed group=276, sample size of control group=1101, reference incidence of COPD=7.8%, alpha=0.05.
bmjresp-2023-002203supp001.pdf (1,023.2KB, pdf)
Participants who had missing data or who were loss to follow-up were excluded from the analysis. Statistical significance was defined as p<0.05 for two-sided tests. The incidence of COPD was described by person-year incidence, and incidence comparison was carried out by ‘iri’ function in Stata V.17.0. All other statistical analyses were performed using R software (V.4.0.1).
Result
The demographic data of hospital-based cohort study
Of the 5676 participants obtained from EMRs, 1377 participants are eventually eligible for inclusion in the analysis after data cleaning, as shown in the flow diagram (figure 1). There are 276 participants with hyperuricaemia and 1101 participants with normal serum urate. Original demographic data of 1377 participants are summarised in table 1, and this table simultaneously summarised matched demographic data processed by PSM and IPTW. Balanced diagnostics demonstrate that the groups were well matched in terms of age, gender, BMI, smoking status, drinking status, chronic bronchitis, bronchiectasis, tuberculosis, pneumonia and diabetes, indicated by SMD <0.1 for all variables (online supplemental figure 2).
Table 1.
Demographic data of participants in the hospital-based cohort study
| Original data | Propensity score matching | Inverse probability of treatment weighting | |||||||
| Characteristic | Normal | Hyperuricaemia | P value | Normal | Hyperuricaemia | P value | Normal | Hyperuricaemia | P value |
| N=1101 | N=276 | N=527 | N=276 | N=1377.8 | N=1369.5 | ||||
| Age | 0.501 | 0.519 | 0.567 | ||||||
| 40–59 | 554 (50.3%) | 132 (47.8%) | 238 (45.2%) | 132 (47.8%) | 683.0 (49.6%) | 649.4 (47.4%) | |||
| ≥60 | 547 (49.7%) | 144 (52.2%) | 289 (54.8%) | 144 (52.2%) | 694.7 (50.4%) | 720.1 (52.6%) | |||
| Gender | <0.001 | 0.786 | 0.809 | ||||||
| Female | 564 (51.2%) | 81 (29.3%) | 161 (30.6%) | 81 (29.3%) | 644.7 (46.8%) | 628.1 (45.9%) | |||
| Male | 537 (48.8%) | 195 (70.7%) | 366 (69.4%) | 159 (70.7%) | 733.1 (53.2%) | 741.4 (54.1%) | |||
| BMI | 0.007 | 0.912 | 0.954 | ||||||
| Underweight | 117 (10.6%) | 20 (7.2%) | 42 (8.0%) | 20 (7.2%) | 137.1 (10.0%) | 121.4 (8.9%) | |||
| Normal | 545 (49.5%) | 119 (43.1%) | 232 (44.0%) | 119 (43.1%) | 663.0 (48.1%) | 662.0 (48.3%) | |||
| Overweight | 330 (30.0%) | 94 (34.1%) | 180 (34.2%) | 94 (34.1%) | 424.1 (30.8%) | 423.8 (30.9%) | |||
| Obese | 109 (9.9%) | 43 (15.6%) | 73 (13.9%) | 43 (15.6%) | 153.6 (11.1%) | 162.3 (11.8%) | |||
| Creatinine level | <0.001 | <0.001 | <0.001 | ||||||
| Normal | 1096 (99.5%) | 265 (96.0%) | 525 (99.6%) | 265 (96.0%) | 1371.2 (99.5%) | 1306.9 (95.4%) | |||
| High | 5 (0.5%) | 11 (4.0%) | 2 (0.4%) | 11 (4.0%) | 6.6 (0.5%) | 62.6 (4.6%) | |||
| Smoking | 0.074 | 0.69 | 0.904 | ||||||
| No | 893 (81.1%) | 210 (76.1%) | 409 (77.6%) | 210 (76.1%) | 1104.1 (80.1%) | 1101.9 (80.5%) | |||
| Yes | 208 (18.9%) | 66 (23.9%) | 118 (22.4%) | 66 (23.9%) | 273.7 (19.9%) | 267.6 (19.5%) | |||
| Drinking | 0.111 | 0.965 | 0.931 | ||||||
| No | 1040 (94.5%) | 253 (91.7%) | 485 (92.0%) | 253 (91.7%) | 1292.6 (93.8%) | 1283.0 (93.7%) | |||
| Yes | 61 (5.5%) | 23 (8.3%) | 42 (8.0%) | 23 (8.3%) | 85.1 (6.2%) | 86.4 (6.3%) | |||
| Chronic bronchitis | 0.037 | 0.661 | 0.821 | ||||||
| No | 1012 (91.9%) | 242 (87.7%) | 469 (89.0%) | 242 (87.7%) | 1254.0 (91.0%) | 1240.3 (90.6%) | |||
| Yes | 89 (8.1%) | 34 (12.3%) | 58 (11.0%) | 34 (12.3%) | 123.8 (9.0%) | 129.2 (9.4%) | |||
| Bronchiectasis | <0.001 | 0.81 | 0.765 | ||||||
| No | 891 (80.9%) | 252 (91.3%) | 477 (90.5%) | 252 (91.3%) | 1143.2 (83.0%) | 1121.9 (81.9%) | |||
| Yes | 210 (19.1%) | 24 (8.7%) | 50 (9.5%) | 24 (8.7%) | 234.5 (17.0%) | 247.6 (18.1%) | |||
| Tuberculosis | 0.26 | 0.548 | 0.677 | ||||||
| No | 1043 (94.7%) | 256 (92.8%) | 496 (94.1%) | 256 (92.8%) | 1300.2 (94.4%) | 1300.4 (95.0%) | |||
| Yes | 58 (5.3%) | 20 (7.2%) | 31 (5.9%) | 20 (7.2%) | 77.5 (5.6%) | 69.0 (5.0%) | |||
| Pneumonia | 0.74 | 0.39 | 0.821 | ||||||
| No | 468 (42.5%) | 121 (43.8%) | 213 (40.4%) | 121 (43.8%) | 588.1 (42.7%) | 573.0 (41.8%) | |||
| Yes | 633 (57.5%) | 155 (56.2%) | 341 (59.6%) | 155 (56.2%) | 789.6 (57.3%) | 796.8 (58.2%) | |||
| Diabetes | 1.000 | 0.867 | 0.616 | ||||||
| No | 954 (86.6%) | 239 (86.6%) | 460 (87.3%) | 239 (86.6%) | 1192.7 (86.6%) | 1164.6 (85.3%) | |||
| Yes | 147 (13.4%) | 37 (13.4%) | 67 (12.7%) | 37 (13.4%) | 185.0 (13.4%) | 201.9 (14.7%) | |||
BMI, body mass index.
Higher incidence and risk of COPD in the population with hyperuricaemia
Among the 1377 participants who did not have COPD at baseline in our hospital-based cohort study, 37 new cases of COPD developed during the follow-up period, resulting in a crude COPD incidence of 11.76/1000 person-years. As shown in table 2, there is higher incidence of COPD in the population with hyperuricaemia (22.29/1000 person-years) compared with the population with normal serum urate (8.89/1000 person-years). It is demonstrated that the population with hyperuricaemia has a higher risk of developing COPD (p=0.009; RR=2.51 and 95% CI=1.21 to 5.06). More importantly, it is still suggested that hyperuricaemia is associated with a higher risk of developing COPD after performing PSM (p=0.013, RR=2.85 and 95% CI=1.17 to 7.38) or IPTW (p<0.001, RR=2.71 and 95% CI=1.73 to 4.37). The Kaplan-Meier curves of the probabilities of developing COPD were shown in online supplemental figure 3. The statistical power of our results is higher than 90% (online supplemental figure 4).
Table 2.
The incidence and risk of COPD in the hospital-based cohort
| Normal serum urate | Hyperuricaemia | ||||||||
| New COPD cases | Person-year | Incidence (/1000 person-years) |
RR | New COPD cases | Person-year | Incidence (/1000 person-years) |
RR | P value | |
| Original data | 22 | 2474.04 | 8.89 | Ref | 15 | 672.80 | 22.29 | 2.51 (1.21 to 5.06) | 0.009 |
| PSM | 9 | 1149.39 | 7.83 | Ref | 15 | 672.80 | 22.29 | 2.85 (1.17 to 7.38) | 0.013 |
| IPTW | 27 | 3087.93 | 8.74 | Ref | 78 | 3287.36 | 23.73 | 2.71 (1.73 to 4.37) | <0.001 |
COPD, chronic obstructive pulmonary disease; IPTW, inverse probability of treatment weighting; PSM, propensity score matching; RR, rate ratio.
Moreover, as shown in table 3, the population with hyperuricaemia has a 2.42-fold (model 1: HR=2.42 and 95% CI=1.26 to 4.77, p=0.008) higher risk of developing COPD than those with normal serum urate in univariate Cox regression analysis. Furthermore, multivariate Cox regression analysis also confirms that hyperuricaemia remains independently associated with higher risk of COPD (model 2 was adjusted for personal basic information including age, gender, BMI and creatinine level: adjusted HR=2.32 and 95% CI=1.15 to 4.70, p=0.019; model 3 was additionally adjusted for information of environmental exposure including smoking and drinking: adjusted HR=2.32 and 95% CI=1.14 to 4.71, p=0.020; model 4 was further adjusted for medical history including chronic bronchitis, bronchiectasis, tuberculosis, pneumonia and diabetes: adjusted HR=3.35 and 95% CI=1.61 to 6.96, p=0.001).
Table 3.
Cox regression model
| Model | HR | 95% CI | P value |
| Model 1* | 2.42 | 1.26 to 4.67 | 0.008 |
| Model 2† | 2.32 | 1.15 to 4.70 | 0.019 |
| Model 3‡ | 2.32 | 1.14 to 4.71 | 0.020 |
| Model 4§ | 3.35 | 1.61 to 6.96 | 0.001 |
*Univariate Cox regression model.
†Multivariable Cox regression models adjusted for personal basic information: age (40–59 or ≥60 years), gender (male or female), BMI (underweight, normal, overweight or obese) and creatinine level (normal or high).
‡Additionally adjusted for environmental exposure: smoking (yes or no) and drinking (yes or no).
§Further adjusted for medical history: chronic bronchitis (yes or no), bronchiectasis (yes or no), tuberculosis (yes or no), pneumonia (yes or no) and diabetes (yes or no).
HR, Hazard Ratio.
Non-linear dose–response relationship between the concentration of serum urate and the risk of COPD
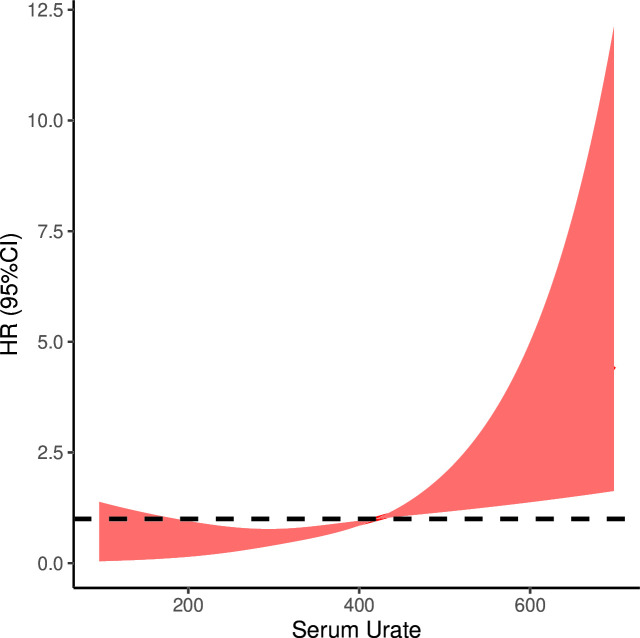
As shown in figure 3, there is a non-linear dose–response relationship between the concentration of serum urate and the risk of COPD after adjusting potential confounding factors that include age, gender, BMI, smoking status, drinking status, chronic bronchitis, bronchiectasis, tuberculosis, pneumonia and diabetes. Setting serum urate of 420 µmol/L as the reference value (OR=1), the RCS analysis demonstrated a rapid increase in the risk of developing COPD with increasing concentration of serum urate when it was higher than the reference value (p=0.0004).
Figure 3.
The result of restricted cubic spline analysis. There was a significant non-linear dose–response relationship between the concentration of serum urate and the risk of COPD after adjusting potential confounding factors. Setting serum urate of 420 µmol/L as the reference (OR=1), the risk of COPD rapidly increased with the concentration of serum urate when it was higher than the reference. COPD, chronic obstructive pulmonary disease. HR, hazard ratio.
Causal association of serum urate levels with the risk of COPD is likely to be true based on the MR analysis
A brief introduction of GWAS included in MR analysis is shown in online supplemental table 1. There are 27 SNPs significantly associated with serum urate, among which one SNP (rs1471633) that was not found in summary statistics of outcome GWAS and two palindromic SNPs (rs17632159 and rs6830367) were removed, leaving independent 24 SNPs identified as IVs for serum urate. F-statistic of each IV is greater than 10, which indicates that every IV used in MR analysis is not considered as a weak instrument. Basic information regarding IVs is shown in online supplemental table 2. Horizontal pleiotropy test shows that there is no horizontal pleiotropy between IVs and outcome (online supplemental table 3).
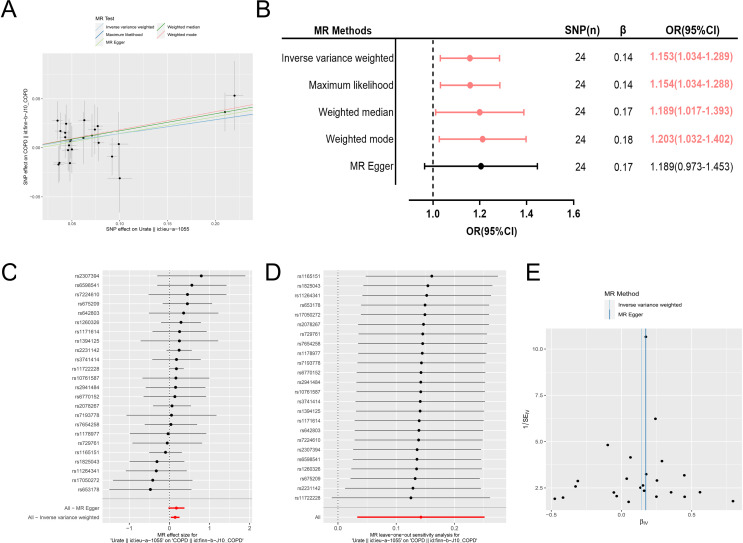
Univariable MR results are described in figure 4. Specifically, as shown in figure 4A, all five MR methods show a positive effect of serum urate on COPD. As shown in figure 4B, the IVW method yields a significant positive effect of serum urate on the risk of COPD (OR=1.153, 95% CI=1.034 to 1.289, p=0.010), and the statistical results of three other methods also yield the same significant positive effect. It is worth noting that MR-Egger may have lower statistical power to detect a significant positive effect compared with other methods. Thus, the consistency of the results across four methods suggests that our finding is robust. The positive effects of each IV on COPD are exhibited in a forest plot (figure 4C). Leave-one-out analyses indicate that the results are stable and not driven by any single SNP (figure 4D). As shown in figure 4E, a funnel plot shows that there is a symmetrical distribution of the point estimate of the positive association effect, and this result indicates that the underlying bias is unlikely to affect the positive association. Moreover, the homogeneity test shows no potential homogeneity in this MR analysis, which further indicates that our results are not biased (online supplemental table 4). Finally, reverse MR analysis does not provide any reliable evidence to suggest that COPD may be the cause of elevated serum urate levels (online supplemental table 5).
Figure 4.
(A–E) The results of univariable two-sample Mendelian randomisation (MR) analysis. (A) All five MR methods showed a positive effect of serum urate on COPD. (B) The IVW method yielded a significant positive effect of serum urate on the risk of COPD, and the statistical results of three other methods also yielded the same significant positive effect. (C) The forest plot showed the positive effects of each IV on COPD. (D) Leave-one-out analyses indicated that the results were stable and not driven by any single SNP. (E) The funnel plot showed a symmetrical distribution of the point estimate of the positive association effect, and this result indicated that the underlying bias was unlikely to affect the positive association. COPD, chronic obstructive pulmonary disease; IV, instrumental variable; IVW, inverse variance weighted; SNP, single nucleotide polymorphism.
Multivariable two-sample MR to adjust the effect of serum urate on the risk of COPD
As shown in table 4, compared with univariate MR, multivariable MR identified independent 16 SNPs as IVs for serum urate. After adjusting four major confounding factors, the multivariable IVW method provided further suggestive evidence supporting the notion that serum urate remains independently positively associated with the risk of COPD (OR=1.137, 95% CI=1.014 to 1.274, p=0.027).
Table 4.
Multivariable MR results for adjusting the effect of serum urate on the risk of COPD
| Exposure | SNP (n) | β | SE | OR (95% CI) | P value |
| Serum urate | 16 | 0.13 | 0.06 | 1.137 (1.014 to 1.274) | 0.027 |
| eGFR (creatinine) | 182 | 0.23 | 0.54 | 1.258 (0.434 to 3.647) | 0.672 |
| Alcohol consumption | 3 | 0.11 | 0.32 | 1.119 (0.592 to 2.114) | 0.729 |
| PM2.5 | 3 | 0.70 | 0.51 | 2.016 (0.745 to 5.456) | 0.168 |
| Pack years of smoking | 5 | 1.73 | 0.20 | 5.643 (3.845 to 8.282) | <0.001 |
COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; MR, Mendelian randomisation; PM2.5, particulate matter air pollution; SNP, single nucleotide polymorphism.
Discussion
To the best of our knowledge, this study is the first hospital-based cohort study conducted on the Chinese population to explore the effect of serum urate on the risk of developing COPD. We find that there are higher incidence and risk of developing COPD in the population with hyperuricaemia compared with the population with normal serum urate. On the one hand, we are more confident in this finding after performing PSM and IPTW to minimise the effects of some confounding factors. However, we did not balance the difference in creatinine levels because it seems not biologically plausible that the creatinine levels in the hyperuricaemia group would be similar to the creatinine levels in the normal serum urate group. We also consider that high serum urate due to the diseases reflected by creatinine levels may also be able to exacerbate inflammatory damage of lung tissue and promote chronic respiratory inflammation eventually progressing to COPD. Moreover, insufficient number of individuals with high creatinine levels can affect the effectiveness of matching. On the other hand, we used other statistical methods for sensitivity analysis. Specifically, the Cox regression model confirms that hyperuricaemia was independently associated with higher risk of COPD. RCS analysis shows that the risk of developing COPD rapidly increases with the concentration of serum urate when it was higher than the reference value (420 µmol/L).
Furthermore, we performed MR analysis to acquire additional evidence to support our results. The univariable and multivariable MR analyses both suggest that a significant causal effect of serum urate on the risk of developing COPD is likely to be true if one exists. In this MR analysis, all IVs were significantly associated with serum urate levels in terms of genome-wide significance (p<5×10–8), which indicates that this MR analysis satisfies the relevant assumption. The exclusion-restriction assumption might be violated due to horizontal pleiotropy. On the other hand, the horizontal pleiotropy test shows that there is no horizontal pleiotropy between IVs and outcome, which indicates that this MR analysis satisfies the exclusion-restriction assumption. It is worth noting that there is currently no method to test whether the independence assumption is violated. Considering that the independence assumption might be violated mainly due to confounding phenotypes, we performed multivariable MR to adjust for the effects of major confounding phenotypes, whereby adjusting for the effects of IVs associated with confounding phenotypes is able to reduce the likelihood that IVs of serum urate violate the independence assumption. Overall, the finding of MR is robust in the repeated analysis using different methods and sensitivity analysis.
Urate is a biomarker of xanthine oxidase activity, which is known to be an important source of reactive oxygen species.29 In vitro and in vivo studies support the hypothesis that serum urate has a positive association with inflammatory markers and contributes to systemic sterile inflammation.12 Mechanically, urate can upregulate the expression of proinflammatory cytokine, such as interleukin (IL)-6, IL-18, C reactive protein and tumour necrosis factor-alpha.12 In addition, hyperuricaemia can lead to serum urate level exceeding the solubility threshold; it precipitates into urate crystals, and urate can act as a danger‐associated molecular pattern to induce enhanced and maladaptive immune responses.9 Martinon et al indicated that urate-driven inflammation in vitro is dependent on the assembly of the NLRP3 inflammasome.30 Chen et al indicated that urate can stimulate macrophages to activation of nuclear factor kappa B and IL‐1β expression.31 Moreover, urate can induce epigenetic regulation, such as histone methylation and microRNA upregulation, to affect the expression of inflammatory cytokine.9
Hyperuricaemia and the urate released from persistent hypoxia damage in tissue may destroy the balance between the antioxidant and inflammation-stimulatory properties of urate, which makes the latter predominate.32 Serum urate has been linked to the pathogenesis of several diseases characterised by systemic inflammation,33 especially gout and cardiovascular diseases. COPD is also a disease characterised by systemic inflammation that gradually develops from chronic lung inflammation. In addition, urate is found at high concentrations in respiratory tissues and epithelial lining fluid; thus, the role of serum urate in the onset of respiratory inflammatory diseases cannot be ignored. Thus, the activation of pro-oxidant and inflammation-stimulatory effects induced by elevated levels of serum urate is a potential mechanism underlying the causal effect of serum urate on the onset of COPD. For example, urate can activate NLRP3 inflammasome to induce the activation of leucocytes that cause damage to endothelial cells,30 while as demonstrated by some studies, pulmonary endothelial dysfunction is involved in the pathogenesis of COPD.34 35 In addition, Liu et al found that extracellular vesicles isolated from patients with hyperuricaemia might exacerbate both systemic inflammation and airway inflammatory response via the senescence-related pathway.36
From a public health perspective, we would like to identify whether the population with hyperuricaemia is at high risk of COPD. Some studies have reported findings that are consistent with our own.20 22 37 38 For example, Kobylecki et al measured lung function and serum urate in 114 979 individuals who were of Danish descent.20 Their cohort study suggested that high plasma urate was associated with worsened lung function and higher risk of COPD. It was a pity that, when they carried out MR analysis, the result did not support a causality between high plasma urate and FEV1% predicted, FVC% predicted, symptoms of airway disease or COPD in a large general population. However, the positive effect yielded from our MR analysis was concluded based on the summary statistics of GWAS in a European population. MR study is a form of IV analysis, whereby one or more genetic variants can be used as a proxy for a specific exposure to test a causal effect of exposure on outcome, which can be regarded as a natural randomised controlled trial.27 It can be seen that even with such a powerful analytical method as MR, the results of a population in one country may not be applicable to populations in other countries, which may be related to the differences in IVs of different ethnic groups.
Conversely, Horsfall et al similarly conducted a large sample size of a cohort study, but found that low serum urate was associated with higher risk of COPD.23 According to what we know, there are currently only these two cohort studies in the world to explore whether high levels of serum urate can lead to the higher incidence and risk of COPD. Obviously, the interpretation of previous studies calls for discretion because of potential confounders and heterogeneity of different populations. Thus, there is a compelling need for replicated or further researches in populations with distinct characteristic or demographics.
Different from other studies, we primarily focused on the population who seek medical advice or treatment in a hospital rather than the community population. Such population has a higher risk of COPD, especially those who seek medical advice in the department of respiratory diseases or receive lung function test. It is biologically plausible that high urate levels would increase the risk of COPD in such a population, as urate possesses pro-oxidant and inflammation-stimulatory effects. In addition, they have better medical compliance and can positively respond to follow-up work so that the rate of loss to follow-up in our cohort was only about 6%. It was worth noting that, when medical history which included chronic bronchitis, bronchiectasis, tuberculosis, pneumonia and diabetes was incorporated into the Cox model (model 4), the HR of model 4 significantly increased compared with the other three models. Thus, our results emphasised the importance of intervening in serum urate level in the populations with some medical history. Although it remains uncertain whether the conclusion of our study can be applied to a larger Chinese community population, our study is unique and specific in that it was restricted to individuals who seek medical advice in hospital or receive lung function test, reducing the potential bias from some population stratification. The elucidation of the relationship between hyperuricaemia and COPD has guiding implications for clinical practice.
The disease burden caused by COPD in China has risen to third place in 2017.39 A national cross-sectional study shows that the prevalence of spirometry-defined COPD was 8.6% in China, which indicates that the number of patients with COPD in China exceeds to 100 million.40 In addition, hyperuricaemia has become the second most common metabolic abnormality after diabetes in China.40 Preventing chronic diseases among the population with common metabolic abnormality is an effective strategy. In other words, the intervention for a common metabolic abnormality can help in preventing the onset of other chronic diseases. Serum urate is a widely available, low-cost and rapidly measured biochemical parameter, and serum urate measurement may still provide valuable information to the clinician on the risk of developing COPD in addition to information from systemic inflammatory state and other relevant inflammatory factors, such as smoking and food intake. Such understanding indicates that controlling the levels of serum urate may become an important measure for preventing the onset of COPD. Therefore, further researches, such as field trials or community intervention trials, are warranted to be carried out for investigating whether the management of the levels of serum urate through lifestyle modifications or pharmacological intervention could potentially lower the risk of developing COPD.
There are some limitations in our study that should be considered. First, our MR analysis was performed based on summary statistics of GWAS in a European population, and there is still a lack of publicly available summary statistics of GWAS for serum urate and COPD in the Chinese population. Second, the concentrations of serum urate may not be a good proxy for the concentrations of urate in the epithelial lining fluid. It is possible that urate in the epithelial lining fluid might play a different role than serum urate. Third, PSM methods may not be appropriate to control some potential risk factors highly associated with hyperuricaemia, such as food intake, therapy or medication, renal function and more, as it is not biologically plausible that these factors were balanced between the hyperuricaemia and normal groups. These factors may contribute to the development of chronic lung inflammation into COPD by inducing other non-urate substances. Finally, several possible factors that may influence the risk of developing COPD were not adjusted in our analysis, such as passive smoking and atmospheric air pollution. However, after adjustment for major confounders, a robust positive association has been yielded with a high degree of confidence.
Conclusions
Our study provides preliminary and compelling evidence suggesting a robust positive association between serum urate and the risk of developing COPD, and indicates that the population with hyperuricaemia is at high risk of developing COPD in the Chinese population who seek medical advice or treatment in the hospital. These findings have guiding implications for clinical practice, and indicate that the intervention of high levels of serum urate may be an effective strategy for the prevention of COPD.
bmjresp-2023-002203supp002.pdf (51.2KB, pdf)
Acknowledgments
Thank you to Zeqin Huang, Wenhui Lun, Yongsun Dong, Zhi Li, Chen Xie, Lu Chen, Yonghui Yu, Shizhen Chen, Liming Lu, Hongmei Huang, Ling Lu, Jiamin Liang, Yuepeng Sui, Ziqi Yu, Linyuan Liu and Amei Zhuo (Institute of Public Health, Guangzhou Medical University) for their contribution to the data entry.
Footnotes
BR, DX and YD contributed equally.
Contributors: JL and BR are the guarantors responsible for the overall content, had full responsibility for the work and the conduct of the study, had access to the data, controlled the decision to publish, and had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design—JL. Data curation—BR and JL. Acquisition of data—JY and XZ. Analysis or interpretation of data—BR, DX and YD. Methodology—JC and YD. Administrative, technical or material support—DH, CX, CC and YL. Funding acquisition—JL and CX. Writing (drafting of the manuscript)—BR. Writing (review and editing of the manuscript)—XL and AL. Critical revision of the manuscript for important intellectual content—XW and YD. Validation—BR and DX. Visualisation—BR and DX. Supervision—JL.
Funding: This study was supported by the National Natural Science Foundation of China (82373678, 82173609 (JL)), Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (2017BT01S155 (JL)), Natural Science Foundation of Guangdong Province (2023A1515011627 (CX)), and Dongguan Science and Technology of Social Development Program (20221800905212 (CX)).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The summary statistics of GWAS were public. They can be retrieved on the MR-Base platform (https://www.mrbase.org/), and the data can be directly invoked for analysis using R packages of TwoSampleMR. The data of the cohort are not publicly available. If there is a need or interest, the corresponding author can be contacted for discussion.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was reviewed and approved by the Ethics Committee of Guangzhou Medical University (2021020012), Shenzhen Longhua District Central Hospital (2021-027-01), Dongguan Binhaiwan Central Hospital (2022090), Songshan Lake Central Hospital of Dongguan (201911220626920) and Guangzhou Panyu District Central Hospital (2020-018-07). Informed consent has been obtained from all participants.
References
- 1.Gangji W, Xiaofang C, Xiaofang C, et al. Relationship between smoking and the risk of morbidity of chronic obstructive pulmonary diseases among residents aged 30 years and above in Sichuan province: a prospective study. Zhonghua Liu Xing Bing Xue Za Zhi 2023;44:778–85. [DOI] [PubMed] [Google Scholar]
- 2.Christenson SA, Smith BM, Bafadhel M, et al. Chronic obstructive pulmonary disease. Lancet 2022;399:2227–42. 10.1016/S0140-6736(22)00470-6 [DOI] [PubMed] [Google Scholar]
- 3.Yin P, Wu J, Wang L, et al. The burden of COPD in China and its provinces: findings from the global burden of disease study 2019. Front Public Health 2022;10:859499. 10.3389/fpubh.2022.859499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang L, Gao P, Bao H, et al. Chronic obstructive pulmonary disease in China: a nationwide prevalence study. Lancet Respir Med 2018;6:421–30. 10.1016/S2213-2600(18)30103-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fabbri LM, Rabe KF. From COPD to chronic systemic inflammatory syndrome? Lancet 2007;370:797–9. 10.1016/S0140-6736(07)61383-X [DOI] [PubMed] [Google Scholar]
- 6.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006;3:e442. 10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Vliet A, O’Neill CA, Cross CE, et al. Determination of low-molecular-mass antioxidant concentrations in human respiratory tract lining fluids. Am J Physiol 1999;276:L289–96. 10.1152/ajplung.1999.276.2.L289 [DOI] [PubMed] [Google Scholar]
- 8.Ruggiero C, Cherubini A, Ble A, et al. Uric acid and inflammatory markers. Eur Heart J 2006;27:1174–81. 10.1093/eurheartj/ehi879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabău G, Crișan TO, Klück V, et al. Urate-induced immune programming: consequences for gouty arthritis and hyperuricemia. Immunol Rev 2020;294:92–105. 10.1111/imr.12833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartziokas K, Papaioannou AI, Loukides S, et al. Serum uric acid as a Predictor of mortality and future exacerbations of COPD. Eur Respir J 2014;43:43–53. 10.1183/09031936.00209212 [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Liu L, Liang R, et al. Hyperuricemia is a biomarker of early mortality in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2015;10:2519–23. 10.2147/COPD.S87202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyngdoh T, Marques-Vidal P, Paccaud F, et al. Elevated serum uric acid is associated with high circulating inflammatory Cytokines in the population-based Colaus study. PLoS One 2011;6:e19901. 10.1371/journal.pone.0019901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortese F, Giordano P, Scicchitano P, et al. Uric acid: from a biological advantage to a potential danger. A focus on cardiovascular effects. Vascul Pharmacol 2019;120:106565. 10.1016/j.vph.2019.106565 [DOI] [PubMed] [Google Scholar]
- 14.Sautin YY, Johnson RJ. Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 2008;27:608–19. 10.1080/15257770802138558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eiserich JP, van der Vliet A, Handelman GJ, et al. Dietary antioxidants and cigarette smoke-induced Biomolecular damage: a complex interaction. Am J Clin Nutr 1995;62(6 Suppl):1490S–1500S. 10.1093/ajcn/62.6.1490S [DOI] [PubMed] [Google Scholar]
- 16.Kang D-H, Park S-K, Lee I-K, et al. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol 2005;16:3553–62. 10.1681/ASN.2005050572 [DOI] [PubMed] [Google Scholar]
- 17.Ruggiero C, Cherubini A, Miller E, et al. Usefulness of uric acid to predict changes in C-reactive protein and Interleukin-6 in 3-year period in Italians aged 21 to 98 years. Am J Cardiol 2007;100:115–21. 10.1016/j.amjcard.2007.02.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braga TT, Forni MF, Correa-Costa M, et al. Soluble uric acid activates the Nlrp3 Inflammasome. Sci Rep 2017;7:39884. 10.1038/srep39884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H, Ma Y, Cao R, et al. Soluble uric acid induces myocardial damage through activating the Nlrp3 Inflammasome. J Cell Mol Med 2020;24:8849–61. 10.1111/jcmm.15523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobylecki CJ, Vedel-Krogh S, Afzal S, et al. Plasma Urate, lung function and chronic obstructive pulmonary disease: a Mendelian Randomisation study in 114 979 individuals from the general population. Thorax 2018;73:748–57. 10.1136/thoraxjnl-2017-210273 [DOI] [PubMed] [Google Scholar]
- 21.Song JU, Hwang J, Ahn JK. Serum uric acid is positively associated with pulmonary function in Korean health screening Examinees. Mod Rheumatol 2017;27:1057–65. 10.1080/14397595.2017.1285981 [DOI] [PubMed] [Google Scholar]
- 22.Jeong H, Baek S-Y, Kim S-W, et al. Gender-specific Association of serum uric acid and pulmonary function: data from the Korea national health and nutrition examination survey. Medicina 2021;57:953. 10.3390/medicina57090953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horsfall LJ, Nazareth I, Petersen I. Serum uric acid and the risk of respiratory disease: a population-based cohort study. Thorax 2014;69:1021–6. 10.1136/thoraxjnl-2014-205271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elm E von, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–8. 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chinese Multidisciplinary expert consensus on the diagnosis and treatment of hyperuricemia and related diseases. Chinese Medical Journal 2017;130:2473–88. 10.4103/0366-6999.216416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hemani G, Zheng J, Elsworth B, et al. The MR-base platform supports systematic causal inference across the human Phenome. Elife 2018;7:e34408. 10.7554/eLife.34408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skrivankova VW, Richmond RC, Woolf BAR, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA 2021;326:1614–21. 10.1001/jama.2021.18236 [DOI] [PubMed] [Google Scholar]
- 28.Burgess S, Davey Smith G, Davies NM, et al. Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open Res 2019;4:186. 10.12688/wellcomeopenres.15555.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sjödin B, Hellsten Westing Y, Apple FS. Biochemical mechanisms for oxygen free radical formation during exercise. Sports Med 1990;10:236–54. 10.2165/00007256-199010040-00003 [DOI] [PubMed] [Google Scholar]
- 30.Martinon F, Pétrilli V, Mayor A, et al. Gout-associated uric acid crystals activate the Nalp3 Inflammasome. Nature 2006;440:237–41. 10.1038/nature04516 [DOI] [PubMed] [Google Scholar]
- 31.Chen CJ, Shi Y, Hearn A. Myd88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by Monosodium Urate crystals. Journal of Clinical Investigation 2006;116:2262–71. 10.1172/JCI28075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarangi R, Varadhan N, Bahinipati J. Serum uric acid in chronic obstructive pulmonary disease: A hospital based case control study. JCDR 2017;11:BC09–13. 10.7860/JCDR/2017/29300.10605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alvarez-Lario B, MacArron-Vicente J. Is there anything good in uric acid QJM 2011;104:1015–24. 10.1093/qjmed/hcr159 [DOI] [PubMed] [Google Scholar]
- 34.Arao T, Takabatake N, Sata M, et al. In vivo evidence of endothelial injury in chronic obstructive pulmonary disease by lung Scintigraphic assessment of (123)I-metaiodobenzylguanidine. J Nucl Med 2003;44:1747–54. [PubMed] [Google Scholar]
- 35.Tuder RM, Zhen L, Cho CY, et al. Oxidative stress and apoptosis interact and cause emphysema due to vascular endothelial growth factor receptor blockade. Am J Respir Cell Mol Biol 2003;29:88–97. 10.1165/rcmb.2002-0228OC [DOI] [PubMed] [Google Scholar]
- 36.Liu X, Li Z, Zheng Y, et al. Extracellular Vesicles isolated from hyperuricemia patients might aggravate airway inflammation of COPD via Senescence-associated pathway. J Inflamm (Lond) 2022;19:18. 10.1186/s12950-022-00315-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aida Y, Shibata Y, Osaka D, et al. The relationship between serum uric acid and Spirometric values in participants in a health check: the Takahata study. Int J Med Sci 2011;8:470–8. 10.7150/ijms.8.470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang H, Wang Z, Xiao S, et al. Association between serum uric acid and lung function in people with and without chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2022;17:1069–80. 10.2147/COPD.S356797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the global burden of disease study 2017. The Lancet 2019;394:1145–58. 10.1016/S0140-6736(19)30427-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China pulmonary health [CPH] study): a national cross-sectional study. Lancet 2018;391:1706–17. 10.1016/S0140-6736(18)30841-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2023-002203supp001.pdf (1,023.2KB, pdf)
bmjresp-2023-002203supp002.pdf (51.2KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The summary statistics of GWAS were public. They can be retrieved on the MR-Base platform (https://www.mrbase.org/), and the data can be directly invoked for analysis using R packages of TwoSampleMR. The data of the cohort are not publicly available. If there is a need or interest, the corresponding author can be contacted for discussion.