Abstract
Background
Numerous surgical approaches exist for the treatment of pilonidal disease. Current literature on treatment is of poor quality, limiting the ability to define optimal intervention. The aim of this study was to provide real-world data on current surgical practice and report patient and risk-adjusted outcomes, informing future trial design.
Methods
This UK-wide multicentre prospective cohort study, including patients (aged over 16 years) who had definitive treatment for symptomatic pilonidal disease, was conducted between May 2019 and March 2022. Patient and disease characteristics, and intervention details were analysed. Data on patient-reported outcomes, including pain, complications, treatment failure, wound issues, and quality of life, were gathered at various time points up to 6 months after surgery. Strategies were implemented to adjust for risk influencing different treatment choices and outcomes.
Results
Of the 667 participants consenting, 574 (86.1%) were followed up to the study end. Twelve interventions were observed. Broadly, 59.5% underwent major excisional surgery and 40.5% minimally invasive surgery. Complications occurred in 45.1% of the cohort. Those who had minimally invasive procedures had better quality of life and, after risk adjustment, less pain (score on day 1: mean difference 1.58, 95% c.i. 1.14 to 2.01), fewer complications (difference 17.5 (95% c.i. 9.1 to 25.9)%), more rapid return to normal activities (mean difference 25.9 (18.4 to 33.4) days) but a rate of higher treatment failure (difference 9.6 (95% c.i. 17.3 to 1.9)%). At study end, 25% reported an unhealed wound and 10% had not returned to normal activities.
Conclusion
The burden after surgery for pilonidal disease is high and treatment failure is common. Minimally invasive techniques may improve outcomes at the expense of a 10% higher risk of treatment failure.
This article describes one of the largest real-world experiences of surgery for pilonidal disease. The results are different from those reported in the literature. Many patients experience protracted recovery, and failure is common. Use of minimally invasive techniques is likely to reduce the burden of postoperative recovery substantially, for both patient well-being, direct healthcare costs, and costs to the wider economy, but with a higher risk of recurrence.
Introduction
Pilonidal disease (PD) is an acquired disease driven by the embedding of loose hair within hair follicles usually located in the natal cleft. This triggers an inflammatory response leading to the formation of midline pits, sinuses, or abscesses. Individuals present with a painful abscess or develop a chronic cycle of pain and discharge from openings of interconnected subcutaneous tracts. Symptoms cause significant disruption to education, employment, relationships, and quality of life. The condition is common worldwide, and affects at least 26 per 100 000 of the population—predominantly young people in education or employment1.
Management of PD is usually surgical; many interventions have been described, the majority involving excision of varying amounts of skin and subcutaneous tissue around the pits, with or without primary skin closure with sutures or flaps. Ideal outcomes include early return to normal activity and minimal complications related to infection, bleeding, recurrence, or healing. There is no consensus on what interventions offer the best outcomes, with the available literature reporting results from mostly low-quality, single-centre, retrospective cohorts2. Studies are limited by inconsistent reporting of outcomes, lack of disease stratification, and absence of patient-reported outcomes3. The limited data make it difficult to progress patient care and impair ability to design high-quality randomized studies.
This lack of consensus and the poor quality of current research was recognized by the National Institute for Health Research, which commissioned the PITSTOP study with the aim of improving understanding of disease management and guiding future research. PITSTOP comprised a multimethod assessment of current practice and outcomes in the treatment of PD, with a multicentre prospective cohort study at its centre. The cohort study aimed to provide real-world data on surgical interventions used in current UK practice, and report patient and risk-adjusted outcomes of these interventions, providing data to inform the design of pragmatic randomized trials.
Methods
Study design
This was a multicentre prospective cohort study conducted in the UK between May 2019 and March 2022. The study was registered in the ISRCTN registry (95551898), and ethical approval was secured from a National Health Service (NHS) Research Ethics Committee before commencement (18/EE/0370). The study is reported with reference to the STROBE and GRIPP2SF guidelines4,5.
Settings and participants
Data were collected on patients aged over16 years who had definitive non-emergency treatment for symptomatic PD at UK NHS hospitals. Pregnant patients were excluded. Participants identified from primary- and secondary-care referrals and surgery waiting lists were given an approved participant information sheet detailing the study, with follow-up by a dedicated research nurse. For those eligible and willing to participate, written informed consent was obtained before patient data collection. Consistent with standard practice, the surgeon discussed the condition, possible interventions, and their advantages and disadvantages. Patients were given a minimum of 24 h between receiving the participant information sheet and consent, and completed forms were uploaded to a REDCap database.
Variables
Basic demographic data were collected, including age, sex, employment status, BMI, time spent seated, and smoking status. Disease characteristics included site and extent of sinus, whether disease was primary or recurrent, and presence of pus. Type of disease was classified according to the International Pilonidal Society classification6 into four categories: type 1—midline pits/cavity only; type 2—lateral disease extension; type 3—disease below the coccyx; and type 4—recurrent disease after surgery with intent to cure.
Procedure details were recorded at the time of surgery, including excision type, wound closure, and dressing use. Previous work7 suggested that approximately 10 different interventions are used to treat PD in practice, although individual surgeons tend to use a narrower range depending on experience. For this study, procedures were classified into minimally invasive (for example, pit picking, glue, endoscopic resection, laser, seton, Bascoms I), and major excisional (excision and leave open or closure in midline/asymmetric, or using rotational flaps) procedures. Outcomes included treatment failure, wound healing, return to usual activities, pain, and quality of life.
Health status was measured using the EQ-5D-5L™ tool8 (EuroQol Group, Rotterdam, the Netherlands) at baseline, on day 7 after surgery, at the first postoperative clinic visit, and 6 months after surgery. Pain was assessed on a scale from 0 to 10 (10 being worst pain imaginable) at the same time points and on the day after surgery. Return to normal activities was reported by patients, and recorded on day 7, at the first postoperative clinic visit, and 6 months after surgery. Time to healing was assessed at the clinic visit and at 6 months. Wound impact was measured using the Cardiff Wound Impact Score9 at the same time points.
Complications were assessed on days 1 and 7, at the first postoperative clinic visit, and 6 months after surgery. These included bleeding, seroma, infection, flap necrosis, haematoma, maceration, dehiscence, discharge, and other procedure-related complications. Definitions are available in Table S1. Treatment failure was defined by the need for a further surgical procedure for PD, patient-reported ‘recurrence’, or an adverse event or complication reported that was consistent with unresolved PD.
Data collection and follow-up
Data were collected by trained research personnel. Participants completed baseline questionnaires after eligibility and consent had been confirmed. Other outcome data were collected on days 1 and 7, at the first postoperative clinic visit, and 6 months after the procedure. Participant data were also collected opportunistically at the end of the study.
Public and patient involvement
Two public and patient involvement representatives contributed to the study. The aim was to ensure that the study design addressed key patient concerns and priorities. A patient focus group was used to provide input into the study design, especially key outcomes, measurement approaches, and time points.
Statistical analysis
Demographic and outcome data are reported using descriptive statistics. Analysis was prespecified in a statistical analysis plan. All analyses were performed in Stata® version 1 (StataCorp, College Station, TX, USA), and, where available, the characteristics of patients with and without data at 6 months were compared descriptively.
Several strategies were implemented to adjust for risk potentially influencing treatment choices and outcomes. The following features were considered as potential risk factors: sex, BMI, depth of natal cleft, presence and type of gluteal hair, smoking status, pit density, presence of unilateral or bilateral disease, distance from furthest lateral opening to nearest pit, presence of pus, and type of disease. A regression model was developed exploring factors associated with the outcome of interest. Factors were included based on Akaike’s information criteria and the size of the c-statistic of the model. A model including all factors, and one adjusting for type of disease alone, were also fitted. All models were discussed and agreed with a core study clinical team before comparative data were revealed. Continuous outcomes were modelled using linear regression, and differences between treatment groups with 95% confidence intervals were estimated from the regression coefficient for the procedure group. Binary outcomes were modelled using logistic regression and absolute differences in proportions were assessed using the difference in marginal probabilities. Time to wound healing and time to return to normal activities were modelled using Cox regression.
Propensity score approaches were undertaken using inverse probability weighting (IPW) and propensity score matching. Features associated with treatment choice were assessed using logistic regression in which treatment choice was the outcome. Co-variables were identified analogously to the regression model. The same propensity score adjustments were used for each outcome. The propensity score-adjusted models were then used to calculate predicted outcomes in both arms, following which their difference and 95% confidence interval were estimated.
The final approach of augmented IPW incorporates regression modelling and IPW, and simultaneously models both treatment selection and outcome using the co-variates employed in the previous models. The differences in predicted outcomes and their 95% confidence intervals were estimated for each outcome.
Sample size
The aim was to recruit approximately 800 patients, with at least 100 within each of the most common operative strategies. Doing so allowed proportions to be estimated for each management strategy to a standard error of 5% or less, and pain numerical rating scale to within a standard error of 0.2 points assuming that a standard deviation of a 10-point scale would not exceed 2 units.
Results
Participants
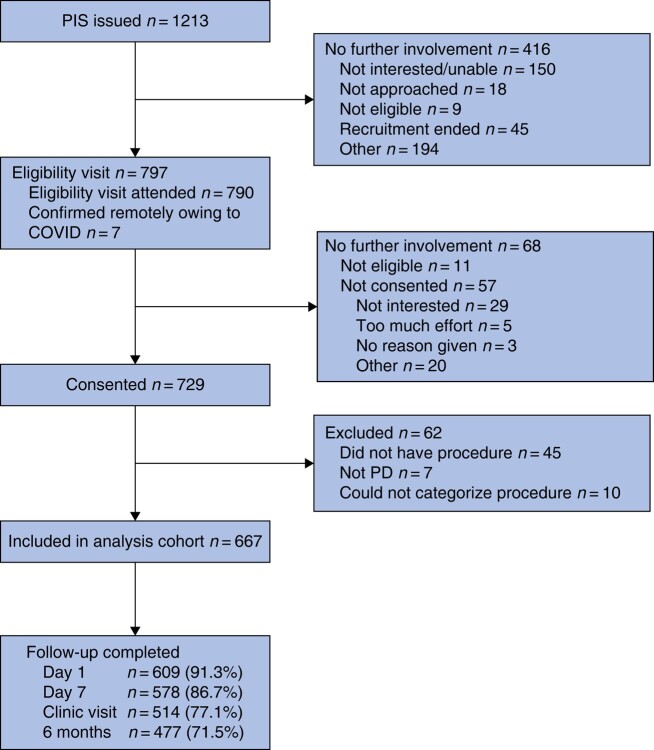
Thirty-one UK sites recruited participants over the 34-month interval. Figure 1 shows the flow chart for 729 consented participants. Sixty-two participants were excluded from analyses for reasons shown in Fig. 1, leaving 667 for analysis, of whom 574 participants (86.1%) were followed up at the end of the study, and 477 (71.5%) provided follow-up data at 6 months. Patient characteristics are presented in Table S2.
Fig. 1.
Flow chart for PITSTOP study
PIS, patient information sheet; PD, pilonidal disease.
Procedures
Of the 667 patients, 397 (59.5%) had a major excisional procedure; the most frequently performed was the Karydakis procedure (164 of 397), followed by Bascoms cleft lift (86). Among the 270 patients (40.5%) who had minimally invasive procedures, sinus curettage with glue was the most frequently performed procedure (106), followed by pit picking (60), and endoscopic pilonidal sinus treatment (44) (Table 1). Median duration of surgery was 15 (i.q.r. 9–24) min for minimally invasive procedures and 45 (30–60) min for major excisional procedures, with 95.0% performed as a day case. Factors associated with treatment selection are summarized in Fig. S1.
Table 1.
Procedures undertaken throughout study
| Procedure type | n | Procedure category | n | Procedure | n |
|---|---|---|---|---|---|
| Skin excisional procedures | 397 (60) | Asymmetric closure and rotational flap | 272 (41) | Bascoms cleft lift | 86 (13) |
| Rotational flap | 22 (3) | ||||
| Karydakis | 164 (25) | ||||
| Leave open | 49 (7) | Leave open | 43 (6) | ||
| Leave open (marsupialization) | 6 (1) | ||||
| Midline closure | 76 (11) | Midline closure | 76 (11) | ||
| Skin-preserving procedures | 270 (40) | Minimally invasive surgery | 270 (40) | Bascoms I | 39 (6) |
| EPSIT | 44 (7) | ||||
| Glue | 106 (16) | ||||
| Laser | 11 (2) | ||||
| Pit picking | 60 (9) | ||||
| Seton | 10 (2) |
Values are n (%). EPSIT, endoscopic pilonidal sinus treatment.
Over half (56.0%) of participants with type 1 disease (only midline pit or sinuses) had minimally invasive procedures, whereas 53.0% of those with recurrent disease had major excision and asymmetric closure. For all disease categories, there were several participants who received each treatment type, suggesting variety in the types of procedure considered appropriate for patients with different disease characteristics.
Patient-reported outcomes
Health utilities calculated using the EQ-5D-5L™ showed divergence in health utility, with better scores reported by the minimally invasive procedure group beginning 1 week after surgery and continuing at clinic follow-up (Table S3). The Cardiff Wound Impact Score was similar across treatments at clinic review and at 6 months.
Risk-adjusted outcomes
The primary comparison between treatments was made between 397 major excisional procedures and 270 minimally invasive procedures. No factors were found to be collinear. Non-linearity of continuous features (BMI, natal cleft depth, pit spread, and pit distance) was investigated and all were deemed to be sufficiently modelled using linear terms. For the propensity score modelling, sufficient overlap in risk score was observed for all outcomes, and so risk-adjusted analysis was deemed appropriate for major excisional versus minimally invasive procedures.
The propensity score model identified sex, presence of pus, and type of disease as the most important features in treatment choice. Patients were more likely to have a major excisional procedure if they were women, had pus, or had recurrent disease, and least likely to have a major excisional procedure if they had type 1 disease. These factors were used in the propensity-adjusted models for all outcome comparisons.
Complications
Nearly half of participants experienced a complication during follow up (301, 45.1%), most commonly infection (26.0%) and discharge (18.0%). Complication rates were broadly similar across the three excisional groups, but lower for patients who received minimally invasive surgery, particularly with respect to bleeding, dehiscence, and infection. Major excisional procedures were associated with an increased risk of complications after adjustment for factors affecting treatment choice and outcome (adjusted risk difference 17.5 (95% c.i. 9.1 to 25.9)%) (Table 2).
Table 2.
Outcome comparison for major excisional versus minimally invasive procedures
| Outcome | Major excisional procedure | Minimally invasive procedure | Unadjusted analysis | Adjusted analysis |
||||
|---|---|---|---|---|---|---|---|---|
| n | Value | n | Value | n | Difference* | n | Difference* | |
| Pain score, mean (s.d.) | ||||||||
| Day 1 | 364 | 4.22(2.53) | 242 | 2.60(2.24) | 606 | MD 1.62 (1.23, 2.02) | 536 | MD 1.58 (1.14, 2.01)‡ |
| Day 7 | 353 | 3.44(2.50) | 221 | 1.86(2.18) | 574 | MD 1.58 (1.18, 1.98) | 512 | MD 1.53 (1.12, 1.95)‡ |
| Complications | 207 of 385 (54) | 94 of 258 (36) | 643 | RD 17.3 (9.6, 25.0)% | 579 | RD 17.5 (9.1, 25.9)%§ | ||
| Recurrence within 6 months | 51 of 337 (15) | 61 of 229 (27) | 566 | RD −11.5 (−18.4, −4.6)% | 514 | RD −9.6 (−17.3, −1.9)%§ | ||
| Time to return to normal activity (days), median (i.q.r.)† | 366 | 32 (14–62) | 241 | 7 (4–21) | 607 | 21.0 (16.3, 25.7) | 502 | 25.9 (18.4, 33.4)¶ |
| Time to healing (days), median (i.q.r.)† | 336 | 70 (31–152) | 217 | 30 (14–154) | 553 | MD 39.7 (27.0, 52.4) | 452 | MD 53.5 (28.8, 78.2)# |
Values are n (%) unless otherwise indicated; *values in parentheses are 95% confidence intervals. Reference group: skin-preserving procedure. †Analysed using augmented inverse probability weighting. Adjusted for ‡sex, type of disease, lateral distance, and presence of pus; §sex, Wysocki classification, BMI, and presence of pus; ¶sex, Wysocki classification, natal cleft depth, lateral distribution, and presence of pus; #sex, Wysocki classification, and presence of pus. MD, mean difference; RD, risk difference.
Pain
Minimally invasive procedures were associated with lower levels of pain. The observed difference in pain between procedures was of a similar magnitude at 1 day and 1 week after surgery (pain score on day 1: adjusted mean difference 1.58, 95% c.i. 1.14 to 2.01) (Table 2).
Return to normal activity and wound healing
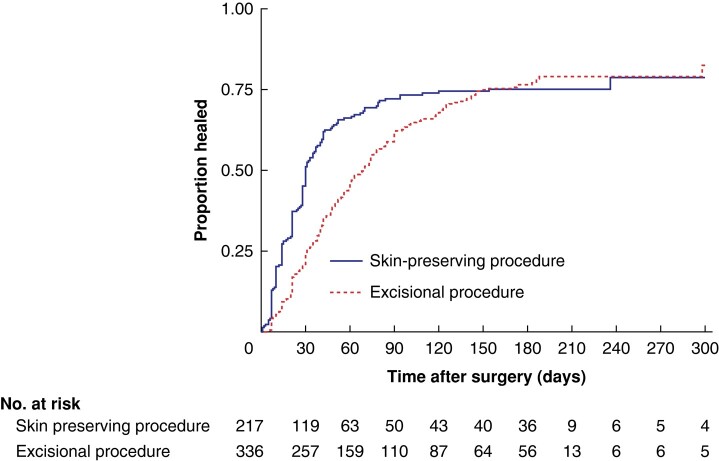
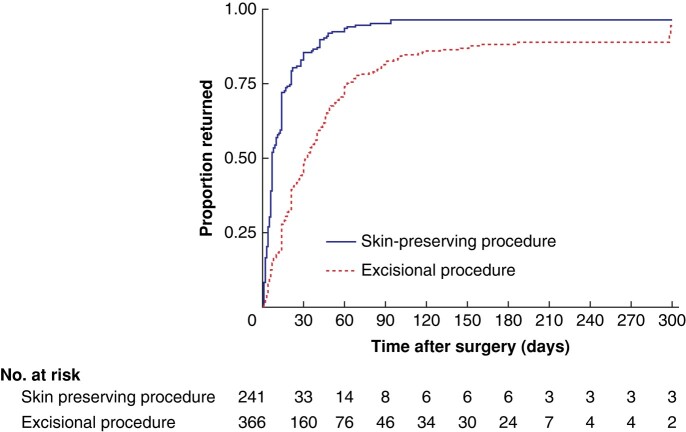
Nearly all participants had returned to normal activity by the end of follow-up, but only 75% reported complete wound healing (Fig. 2). The median time to return to normal activity was shorter among those who had minimally invasive procedures compared with patients who had major excisional procedures (7 versus 32 days) (Table 2). At 6 months, 12% of participants who had major excisional procedures and 4% of those who had minimally invasive procedures were yet to return to normal activity (assuming that patients lost to follow-up before 6 months had the same rate of return to normal activity as the observed group) (Fig. 3). If a best-case scenario was used, in which patients lost to follow-up were assumed to have recovered completely, the proportion of participants who had not returned to normal activity at 6 months would have been 4% for major excisional procedures and 2% for minimally invasive procedures.
Fig. 2.
Time-to-event analysis for wound healing by procedure type
Fig. 3.
Time-to-event analysis for return to normal activities by procedure type
Treatment failure
Major excisional procedures were associated with a significant reduction in treatment failure of around 10–12% (adjusted risk difference −9.6 (95% c.i. −17.3 to −1.9)%) (Table 2). Other risk-adjusted models are presented in Table S4. Pairwise comparisons of those undergoing asymmetric versus minimally invasive procedures produced results similar to those of the analysis that included all major excisional procedures (Table S5).
Discussion
The PITSTOP cohort reflects one of the largest prospectively collected real-world data sets on the outcomes of treatment for PD. The study has broadly demonstrated that surgery results in a substantial burden for many patients who have a high rate of complications, protracted recovery, and a significant risk of recurrent disease. Of the interventions, major excisional procedures were associated with more pain and prolonged recovery; however, the treatment failure rate was lower than that for minimally invasive techniques. These differences remained consistent even after risk adjustment for patient demographics and disease severity.
Characteristics of patients included in this cohort are largely concordant with those previously published, showing a tendency towards male sex and raised BMI1. This is a challenging demographic to recruit to research studies, as rates of loss to follow-up can be high10. The retention rate of 86% observed in this study is at odds with the 100% long-term follow-up reported in many case series11–13.
Surveys reported over the past decade have demonstrated surgeon preferences in treatment of PD7,14, with asymmetric closure techniques favoured. The results of these surveys triangulate with the present finding that 41% of patients underwent such procedures. Although the same surveys reported the increasing use of minimally invasive techniques, these were used in just 40% of the present cohort. Although no one factor determines selection of surgical treatment, a range of procedures offered across different PD disease types was noted. Taken in context with risk-adjusted outcomes, this raises questions about whether major excisional procedures reflect overtreatment of a group of patients with limited disease. Even for those with more severe disease, although the risk of treatment failure may be higher with minimally invasive techniques, previous discrete-choice experiments indicated some patient groups are prepared to trade this higher risk for more rapid recovery15.
A significant proportion of patients took a long time to heal. One-quarter of patients had not healed or had persistent disease at 6 months. Furthermore, around 10% of patients in this study had not resumed normal activities by 6 months, and around 20% reported treatment failure. This is in contrast to many other, mainly single-centre, studies16–18, which reported extremely low treatment failure rates.
Confusion arises around the definition of recurrence. Although this could be considered as wound healing with disease then re-arising, studies often include disease that never heals after surgery. True recurrence is probably uncommon and requires a protracted length of time to detect precisely19. It was not possible to evaluate this outcome within the limitations of the present study design and funding envelope. The definition used here included a composite of patient-reported recurrence and/or evidence of further intervention at least 6 months after surgery.
This work has limitations. Data were available for analysis for just 71.5% of patients at 6 months despite dedicated central research follow-up support. Recruitment and follow-up intervals crossed multiple COVID-19 waves, which doubtless had an impact, enhancing difficulties in retaining the demographic of the study as discussed above. Other limitations include heterogeneity related to the range of treatments: 12 in total. The small numbers of some procedures made analysis of individual interventions statistically unsound. To address this issue, interventions were categorized into two groups with similar underlying surgical principles; minimally invasive procedures focus on destruction/removal of the pit/underlying cavity, whereas major excisional procedures remove the disease and surrounding skin with or without closure of the remaining wound. This broad categorization may be criticized, particularly for the major excisional group, where excision and leave open or closure in the midline techniques may result in inferior outcomes compared with asymmetric closure techniques20–22. However, meaningful statistical analysis was possible for the asymmetric group, and similar differences were observed.
This paper represents a large, prospective, real-world data set, capturing the broad variation in interventions and outcomes. This includes patient-reported outcomes, allowing quality of life to be profiled after different interventions. Outcomes can be considered robust given the comprehensive risk adjustment approaches used. This study also included engagement with patients in the design and interpretation, ensuring relevance to the target population.
Patients with PD are typically of working age, and economically active1. This is reflected in present cohort, with the majority of patients in employment, providing caring roles, or in full-time education. The impact of delayed healing or impaired return to normal activities has repercussions beyond the individual. Inability to work results in lost productivity to the economy, potential reliance on sick pay, and reduced spending and tax return. This is in addition to ongoing costs of dressings and healthcare worker time in treating those with open wounds or wound breakdown. PD is therefore an economically important disease, particularly if a group of widely used treatments can remove a significant proportion of patients from full activity for several months. Given this, it is surprising that more prominence and priority has not been given to this condition.
Policymakers and clinicians should consider outcomes from their local systems and explore whether there is scope to increase the use of minimally invasive procedures, reducing wound morbidity and encouraging early return to work. It is important not to make the treatment worse than the disease. Researchers will find this study useful in several ways. It provides real-world data relevant to the design of a pragmatic RCT. It also suggests that an approach comparing major excisional with minimally invasive surgery is appropriate, and has identified variables to inform stratification of randomization. Furthermore, it provides a range of outcomes that might be relevant to future work. Although a core outcome set is required, it is likely that recovery, pain, return to normal activities, and quality of life will be key outcomes.
The real-world experience of surgery for PD is different from that reported in the literature. Many patients experience protracted recovery, and failure is common. Using minimally invasive techniques is likely to reduce the burden of postoperative recovery substantially, in terms of both patient well-being and economic cost, but with a higher risk of recurrence.
Collaborators
K. Ali (Hinchingbrooke Hospital, UK), R. Brady (Newcastle upon Tyne Hospitals NHS Foundation Trust, UK), G. Branagan (Salisury NHS Foundation Trust, UK), S. Chaudri (Leicester General Infirmary, UK), F. Di Fabio (Basingstoke Hospital, UK), G. Dennison (Yeovil District Hospital NHS Trust, UK), D. Donnelly (Manchester Royal Infirmary, UK), M. Evans (Swansea Bay University Hospital, UK), F. Gerald, S. Gonzalez (University of Sheffield, UK), J. Grainger (Countess of Chester Hospital, UK), A. Hardy (Peterborough City Hospital, UK), N. Husain (Royal Derby Hospital, UK), S. Kapur, K. Keogh, M. Lim, P. Mackey (Royal Devon and Exeter NHS Foundation Trust, UK), Y. Maeda (Queen Elizabeth Hospital, Glasgow, UK), S. Mangam (Queen Elizabeth The Queen Mother Hospital, UK), F. Mazarelo (Manchester University NHS Foundation Trust, UK), K. Muhammad (Tameside and Glossop Integrated Care NHS Foundation Trust, UK), N. Pawa (Chelsea and Westminster Hospitals NHS Trust, UK), L. Pearce (Salford Royal NHS Foundation Trust, UK), J. Pitt (East Suffolk and North Essex NHS Foundation Trust, UK), R. Rajaganeshan (St Helens and Knowsley Teaching Hospitals NHS Trust, UK), P. Shackley (University of Sheffield, UK), R. Simmonds (University of Sheffield, UK), R. Stevenson (Glasgow Royal Infirmary, UK), J. Torkington (University Hospitals of Wales, UK), P. Vaughan-Shaw (Western General Hospital, UK), Vimalachandran Dale (Countess of Chester Hospital, UK), J. Wilson (Wirral University Teaching Hospital, UK).
Supplementary Material
Acknowledgements
This work is attributed to the University of Sheffield.
Contributor Information
Steven R Brown, Sheffield Clinical Trials Research Unit, School of Health and Related Research (ScHARR), University of Sheffield, Sheffield, UK.
Daniel Hind, Sheffield Clinical Trials Research Unit, School of Health and Related Research (ScHARR), University of Sheffield, Sheffield, UK.
Emily Strong, Sheffield Clinical Trials Research Unit, School of Health and Related Research (ScHARR), University of Sheffield, Sheffield, UK.
Mike Bradburn, Sheffield Clinical Trials Research Unit, School of Health and Related Research (ScHARR), University of Sheffield, Sheffield, UK.
Farhat Din, Academic Coloproctology, Institute of Genetics and Cancer, University of Edinburgh, Western General Hospital, Edinburgh, UK.
Ellen Lee, Sheffield Clinical Trials Research Unit, School of Health and Related Research (ScHARR), University of Sheffield, Sheffield, UK.
Jon Lund, Department of Surgery, Derby Royal Infirmary, University Hospitals of Derby and Burton, Derby, UK.
Christine Moffatt, Institute of Care Excellence, Nottingham University Hospitals NHS Trust, Nottingham, UK.
Jonathan Morton, Department of Surgery, Addenbrookes Hospital, Cambridge University Hospitals, Cambridge, UK.
Asha Senapati, Department of Surgery, St Mark’s Hospital, London, UK; Department of Surgery, Queen Alexandra Hospital, Portsmouth, UK.
Helen Jones, Department of Surgery, Oxford University Hospitals NHS Foundation Trust, Oxford, UK.
Matthew J Lee, Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK.
PITSTOP Management Group:
K Ali, R Brady, G Branagan, S Chaudri, F Di Fabio, G Dennison, D Donnelly, M Evans, F Gerald, S Gonzalez, J Grainger, A Hardy, N Husain, S Kapur, K Keogh, M Lim, P Mackey, Y Maeda, S Mangam, F Mazarelo, K Muhammad, N Pawa, L Pearce, J Pitt, R Rajaganeshan, P Shackley, R Simmonds, R Stevenson, J Torkington, P Vaughan-Shaw, Vimalachandran Dale, and J Wilson
Funding
This work was funded by the Health technology Assessment Programme grant HTA 17/17/02.
Author contributions
Steven Brown (Conceptualization, Funding acquisition, Investigation, Visualization, Writing—original draft, Writing—review & editing), Daniel Hind (Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Software, Writing—original draft, Writing—review & editing), Emily Strong (Data curation, Project administration, Writing—original draft, Writing—review & editing), Mike Bradburn (Data curation, Formal analysis, Methodology, Project administration, Validation, Visualization, Writing—original draft, Writing—review & editing), Farhat Din (Conceptualization, Funding acquisition, Investigation, Writing—review & editing), Ellen Lee (Data curation, Formal analysis, Methodology, Validation, Visualization, Writing—review & editing), Jon Lund (Conceptualization, Funding acquisition, Investigation, Writing—review & editing), Christine Moffatt (Conceptualization, Funding acquisition, Writing—review & editing), Jonathan Morton (Conceptualization, Funding acquisition, Investigation, Writing—review & editing), Asha Senapati (Conceptualization, Funding acquisition, Investigation, Writing—review & editing), Helen Jones (Conceptualization, Funding acquisition, Investigation, Writing—review & editing), and Matthew Lee (Conceptualization, Funding acquisition, Methodology, Visualization, Writing—original draft, Writing—review & editing)
Disclosure
M.B. is a current member of the Health Technology Assessment (HTA) Commissioning Committee. S.R.B. was a member of HTA Commissioning Committee from October 2017 to September 2019. D.H. was a member of the HTA Clinical Evaluation and Trials Committee and HTA Fast Track Committee—June 2021. The authors declare no other conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
Data availability
Data are available on request from the Sheffield Clinical Trials Research Unit, School of Health and Related Research (ScHARR), University of Sheffield, Sheffield, UK.
References
- 1. Søndenaa K, Andersen E, Nesvik I, Søreide JA. Patient characteristics and symptoms in chronic pilonidal sinus disease. Int J Colorectal Dis 1995;10:39–42 [DOI] [PubMed] [Google Scholar]
- 2. Kumar M, Clay WH, Lee MJ, Brown SR, Hind D. A mapping review of sacrococcygeal pilonidal sinus disease. Tech Coloproctol 2021;25:675–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown SR, Lund JN. The evidence base for pilonidal sinus surgery is the pits. Tech Coloproctol 2019;23:1173–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med 2007;4:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ 2017;358:j3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee MJ, Strong EB, Lund J, Hind D, Brown SR; the PITSTOP Management Group . A survey of treatment preferences of UK surgeons in the treatment of pilonidal sinus disease. Colorectal Dis 2023;25:2010–2016 [DOI] [PubMed] [Google Scholar]
- 7. Wysocki AP. Towards a classification for sacrococcygeal pilonidal disease-Berlin 2017. Pilonidal Sinus J 2018;4:5–12 [Google Scholar]
- 8. Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Price P, Harding K. Cardiff wound impact schedule: the development of a condition-specific questionnaire to assess health-related quality of life in patients with chronic wounds of the lower limb. Int Wound J 2004;1:10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care 2004;42:200–209 [DOI] [PubMed] [Google Scholar]
- 11. Milone M, Velotti N, Manigrasso M, Anoldo P, Milone F, De Palma GD. Long-term follow-up for pilonidal sinus surgery: a review of literature with metanalysis. Surgeon 2018;16:315–320 [DOI] [PubMed] [Google Scholar]
- 12. Elbanna HG, Emile SH, Youssef M, Thabet W, El-Hamed TM, Ghnnam WM. Novel approach of treatment of pilonidal Sinus disease with thrombin gelatin matrix as a sealant. Dis Colon Rectum 2016;59:775–780 [DOI] [PubMed] [Google Scholar]
- 13. Dag A, Colak T, Turkmenoglu O, Sozutek A, Gundogdu R. Phenol procedure for pilonidal sinus disease and risk factors for treatment failure. Surgery 2012;151:113–117 [DOI] [PubMed] [Google Scholar]
- 14. Shabbir J, Chaudhary BN, Britton DC. Management of sacrococcygeal pilonidal sinus disease: a snapshot of current practice. Int J Colorectal Dis 2011;26:1619–1620 [DOI] [PubMed] [Google Scholar]
- 15. Wickramasekera N, Strong E, Shackley P, Callaghan T, Lee M, Hind D et al. Patient preferences for pilonidal sinus treatments: a discrete choice experiment survey. Colorectal Dis 2023;25:984–994 [DOI] [PubMed] [Google Scholar]
- 16. Stauffer VK, Luedi MM, Kauf P, Schmid M, Diekmann M, Wieferich K et al. Common surgical procedures in pilonidal sinus disease: a meta-analysis, merged data analysis, and comprehensive study on recurrence. Sci Rep 2018;8:3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Steele SR, Perry WB, Mills S, Buie WD; Standards Practice Task Force of the American Society of Colon and Rectal Surgeons . Practice parameters for the management of pilonidal disease. Dis Colon Rectum 2013;56:1021–1027 [DOI] [PubMed] [Google Scholar]
- 18. Baur T, Stauffer VK, Vogt AP, Kauf P, Schmid M, Luedi MM et al. Recurrence rates after uncommon surgical procedures for pilonidal sinus disease. Coloproctology 2019;41:96–100 [Google Scholar]
- 19. Bi S, Sun K, Chen S, Gu J. Surgical procedures in the pilonidal sinus disease: a systematic review and network meta-analysis. Sci Rep 2020;10:13720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Al-Khamis A, McCallum I, King PM, Bruce J. Healing by primary versus secondary intention after surgical treatment for pilonidal sinus. Cochrane Database Syst Rev 2010;CD006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McCallum IJD, King PM, Bruce J. Healing by primary closure versus open healing after surgery for pilonidal sinus: systematic review and meta-analysis. BMJ 2008;336:868–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Karydakis GE. Easy and successful treatment of pilonidal sinus after explanation of its causative process. Aust N Z J Surg 1992;62:385–389 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on request from the Sheffield Clinical Trials Research Unit, School of Health and Related Research (ScHARR), University of Sheffield, Sheffield, UK.