Summary
Algae are diverse organisms with significant biotechnological potential for resource circularity. Taking inspiration from fermentative microbes, engineering algal genomes holds promise to broadly expand their application ranges. Advances in genome sequencing with improvements in DNA synthesis and delivery techniques are enabling customized molecular tool development to confer advanced traits to algae. Efforts to redesign and rebuild entire genomes to create fit-for-purpose organisms currently being explored in heterotrophic prokaryotes and eukaryotic microbes could also be applied to photosynthetic algae. Future algal genome engineering will enhance yields of native products and permit the expression of complex biochemical pathways to produce novel metabolites from sustainable inputs. We present a historical perspective on advances in engineering algae, discuss the requisite genetic traits to enable algal genome optimization, take inspiration from whole-genome engineering efforts in other microbes for algal systems, and present candidate algal species in the context of these engineering goals.
Keywords: synthetic biology, synthetic genomics, algal biotechnology, metabolic engineering, engineering biology, light-driven biotechnology
Graphical abstract

Goold et al. review the history of algal genetic engineering in the context of the advent of whole-genome engineering in bacteria and yeasts mediated by DNA synthesis and its application to complete recoding of chromosomes/genomes. The authors discuss innate genetic traits that can practically enable genome optimization in algae, candidate algae that may be suited to these engineering goals, and new avenues for engineering advanced traits into algal cells to enhance their use in sustainable light-driven bioprocesses.
Introduction
Algae: A disparate grouping of unrelated protists
Algae represent a broadly distributed and diverse group of organisms, ranging from large multicellular species resembling plants, such as kelp, to simple unicellular protists, such as picoeukaryotes. Despite their shared ancestral origins, algae exhibit ecological, genetic, and physiological diversity that exceeds that of most other taxa.1 In general, algae have plastids that contain remnant genomes originating from a singular primary endosymbiotic event involving uptake of a cyanobacterium by a eukaryotic protist. Most, but not all, algae retain the capacity for photosynthesis. After the initial endosymbiotic event, three main groups of algae arose and served as the foundation for what are now the dominant photosynthetic eukaryotes, including land plants.2 Although the diversity of algae extends to secondary, tertiary, and even quaternary endosymbiotic groups of organisms,3 we will discuss only primary endosymbiotic algae belonging to the red, green, and Glaucophyceae groupings that exist within the plant kingdom.
Red, green, and Glaucophyta algae
Rhodophyta (red), Chlorophyta (green), and Glaucophyta are the three primary lineages of algae.4 Rhodophyta and Glaucophyta retain phycobilisomes, which are ancestral cyanobacterial light-harvesting structures, with Rhodophyta using both red phycoerythrobilin and blue phycocyanobilin pigments for light capture, while Glaucophyta only employ blue phycobilins.5,6 The Chlorophyta perform light-harvesting using chlorophyll a and b within plant-like chloroplasts. The blue-green-hued Glaucophyta plastids, called cyanelles, retain bacterial-like peptidoglycan walls,7 which are found also in some chlorophyta, byrophytes, and tracheophytes.8 Photosynthetic eukaryotes evolved a single-step biosynthesis pathway for the most prevalent plastid lipid, monogalactosyldiacylglycerol, compared to a two-step process in the cyanobacterial progenitors.9 Starch storage is another shared trait that evolved in eukaryotic algae.6
Algae can be used in biotechnology for bioconversion of low-value inputs, such as carbon dioxide, nitrogen/phosphorus streams, and trace elements often found in wastewaters, into biomass and higher-value bioproducts (Figure 1). Photosynthetic algal cultures require only minimal chemical inputs compared to fermentative organisms that rely on reduced carbon sources for growth, and many algae also thrive under mixotrophic conditions, making them ideal candidates for upcycling complex wastewaters.10 Examples of algae-derived products include nutraceuticals made from whole-cell biomass or carotenoid-enriched extracts of unmodified Dunaliella salina and Haematococcus lacustris (formerly pluvialis). The Trebouxiophyte alga Chlorella vulgaris has GRAS (generally recognized as safe) status and has been cultivated as an alternative food for decades.11 Several companies are looking at classically mutated variants of green algae with reduced pigments and fermentative growth to use their biomass as single-cell protein for human food (Triton Algae Innovations [https://tritonai.com] and Algenuity [https://www.algenuity.com]). Rhodophyta have long been used for food and agar, a natural hydrocolloid that is a fundamental product for microbiology.12
Figure 1.
The algal cell is a platform for harnessing energy from photosynthesis to convert basic inputs into more complex chemicals
A simplified cellular architecture, illustrated here, shows a primary endosymbiotic alga containing a plastid that was derived from uptake of a cyanobacterium by an ancestral protist. Genomes are present in the nucleus (purple), mitochondria (light gray and white), and plastid (green). Various other subcellular compartments characteristic of eukaryotes are illustrated, including the ER (gray cisternae), Golgi apparatus (light-gray cisternae), lipid droplets (orange), and vacuoles or other microbodies (dark gray). Eukaryotic algae accumulate starch for carbon storage (white). In addition to photosynthetic production of oxygen (O2), some species can generate molecular hydrogen (H2) under specific conditions. Algae transform water, CO2, and inorganic nutrients into biomass containing valuable molecules with diverse applications. Efficient uptake of dissolved nutrients can mitigate eutrophication and yield clean water. Genetic engineering of algal genomes opens possibilities beyond natural products to generate valuable biochemicals and recombinant proteins from minimal inorganic inputs.
Algal biotechnology mainly focuses on unicellular or multicellular microbial species cultivated in photobioreactors of various dimensions or open pond systems.11 Enclosed systems such as photobioreactors and fermentors offer superior control over culture conditions, invasive species, and pests but are more expensive than open (pond) systems.13 Algae, in open or closed photobioreactors, can be cultivated on non-arable land using brackish or salt water and can have higher theoretical productivity in biomass per unit time than traditional land crops.14,15,16
Opportunities for engineering algae
Beyond fundamental research, genetic engineering of algal genomes to yield alternative traits and novel products now provides opportunities to add additional layers of value to their cultivation. The promise of algal biotechnology theoretically lies in the use of photosynthetic microbes that can be grown in wastewater using solar energy to capture nutrients that would otherwise be discarded and convert them into specific chemicals of interest.
The organellar compartmentalization of algal cells provides a platform for the production of either non-glycosylated recombinant proteins in the chloroplast17 or glycosylation of proteins in the endoplasmic reticulum (ER) and subsequent secretion.18 Algal cells may also be favorable eukaryotic environments for metabolic engineering approaches to produce specialty metabolites by expressing vascular plant enzymes.19,20 However, several barriers hinder the broad implementation of algal species as host cell systems for molecular engineering. Some species may be easy to cultivate but are not genetically tractable, thereby necessitating substantial effort to achieve genetic manipulation.
Photosynthetic growth, while advantageous from a sustainability perspective, also necessitates infrastructure considerations to ensure adequate light penetration and carbon dioxide supply into cultivation vessels, factors that generally yield lower volumetric cell densities than fermentation. Nevertheless, these challenges present opportunities, and several research groups have modified diverse species of algae to make various products, covered in multiple reviews: hydrogen and diesel biofuels,21 fragrances, flavors, cosmetics,19,22 and pharmaceuticals.23,24 The key advantage algae bring to biotechnology is their capacity for photosynthesis and propensity for inorganic nutrient uptake, which enables them to use the energy from sunlight for the synthesis of complex chemicals from repurposed waste streams (illustrated in Figure 2). Native natural products can be sourced from algal biomass, and genetic engineering can be used to enhance yields of these products or generate non-native biochemicals in the algae.
Figure 2.
Native and engineered algae for biotechnology
(A) Algae cultures are scaled up from small volumes in flasks (upper left) to several thousand liters in raceway ponds (upper right) or tubular photobioreactors (lower middle). Photos of outdoor algal cultivation are of the Phase I facilities of Development of Algal Biotechnology in Kingdom of Saudi Arabia (DAB-KSA) – Beacon Development project funded by the Ministry of Environment Water and Agriculture and run by Dr. Claudio Fuentes Grünewald at King Abdullah University of Science & Technology (KAUST) Campus from 2023 (photo credit author K.J.L.). The product of algal cultivation is biomass with species-specific composition of pigments and other compounds; from left to right are dried green algae biomass from Tetraselmis, Chlorella, Haematococcus, and Dunaliella sourced from Algikey, Portugal, in 2022 (photo credit Sergio Gutiérrez).
(B) Engineering of algae involves the transformation of designer DNA, followed by the selection of transformant colonies and their subsequent isolation and characterization. The workflow for C. reinhardtii transformant recovery and high-throughput screening on agar plates is shown (top). Scale-up of engineered algae is the same as for wild-type strains, with increasing culture sizes providing the inoculum until production volumes are achieved (bottom) (pictures and images provided by Dr. Mark Seger of Arizona Center for Algae Technology & Innovation).
(C) Left: among other compounds, C. reinhardtii has been genetically modified to produce heterologous isoprenoids, including patchoulol,25,26,27 sclareol,28 bisabolene,29 casbene,30,31 and volatile isoprene.32 Middle: P. moriformis was engineered to make tailored triacylglycerol oils. The images show a high-stability oil (left panel) that remains unfouled after 10 days of continuous deep frying, compared to high-oleic Canola oil (right panel). The relative percentages of major fatty acids in the oils are indicated above the pictures (reproduced from INFORM magazine,33 with permission). Right: C. reinhardtii (top34,35), C. merolae (middle36), and A. protothecoides (bottom37) have all been engineered to produce orange-red ketocarotenoids such as canthaxanthin and astaxanthin (structures shown above) using the C. reinhardtii β-carotene ketolase 1 (BKT1). Wild-type strains are on the left, and modified strains are on the right. The C. reinhardtii pictures were provided by Dr. Thomas Baier and Jacop Kneip, Universität Bielefeld. The C. merolae pictures are from Seger et al.36A. protothecoides (photo by author J.L.M.) was grown heterotrophically on organic carbon and is non-photosynthetic in this state. The yellow pigmentation in wild-type Auxenochlorella is primarily from lutein.
Algal biomass can be separated into hydrophobic and hydrophilic fractions and may also include secreted products such as extracellular polysaccharides. Sourcing multiple products of value from a single cultivated organism is promoted as a “biorefinery” concept, akin to the fractionation of crude oil in a petroleum refinery. In this context, engineered algae could yield multiple native and engineered target products simultaneously. For example, our recent report showcased the potential of engineered algae grown in post-anaerobic-treatment wastewater to simultaneously generate biomass, purify the water of ammonium and phosphorus, remove CO2, and produce a heterologous terpenoid.10 Another report demonstrated genetic engineering for the concomitant production of ketocarotenoids in algal biomass and volatile isoprene hydrocarbons in the culture headspace.32 These examples illustrate the potential of engineered algae as light-driven, multi-product-generating cellular platforms for waste-stream revalorization.
Reliable and advanced genetic modification has been confined to a limited number of model algae (examples in Figure 2). However, improving knowledge of newly characterized species with favorable genetic and growth characteristics is expanding the repertoire of algae that are suitable for engineering approaches. This has been facilitated by the advent of cost-effective whole-genome sequencing and synthesis of custom DNA molecular tools for genetic modifications in emerging species. As markets and product demands constantly evolve, this review refrains from endorsing specific biochemical targets for algal engineering. Rather, we will emphasize features of importance and the potential value of using algae as platforms for synthetic biology and metabolic and genome engineering, as well as for basic discovery. Advances in genome manipulation, both for small-scale targeted engineering and more comprehensive whole-genome redesign, will play pivotal roles in defining future applications for these diverse organisms.
Key developments in engineering algal genomes
Algae that are genetically tractable model organisms
Classical genetics in algae has been instrumental in generating auxotrophic strains and dissecting essential pathways since the 1960s.38 These approaches were primarily focused on the unicellular Chlorophyta, Chlamydomonas reinhardtii, which continues to be the premier model organism for genetic, biochemical, and biophysical studies of photosynthesis and eukaryotic cilia/flagella.39 The nuclear and organellar genomes have all been transformed,40,41,42 and the development of improved markers and gene expression toolkits is ongoing.43,44 However, the availability of genomic data for other algae is rapidly increasing, facilitating the design of gene expression cassettes for genetic interrogation of emerging species.1,45
These datasets have enabled the development of molecular tools for nuclear transformation of a number of red and green algae, although successful transformation of Glaucophyta has not yet been reported.16 Examples of species in which new molecular tools have enabled transformation include Ostreococcus tauri,46 Auxenochlorella protothecoides,37 and Galdieria partida.47 Transformation of Volvox carteri has also been demonstrated for fundamental analyses.48,49 Molecular genetic tools have been developed for Ulva mutabilis, the first multicellular green alga besides V. carteri to be amenable to biotechnological exploitation.50 However, transformation of some algae is inconsistent or difficult to reproduce or has not gained traction with a broad community of researchers, e.g., Chromochloris zofingensis51 and numerous Chlorella spp.52
In addition to nuclear genome engineering, plastid genome engineering in algae is possible, and even genetic transplantation of whole plastid genomes between species has been reported.53 In addition to C. reinhardtii, the chloroplast genome of the red alga Cyanidioschyzon merolae 10D has been transformed,36,54 as has the fast-growing green Picochlorum spp.55 (discussed later). Much remains to be understood regarding transgene expression in algae, and there are numerous species-specific features that require consideration for each organism in which engineering is a goal.
Chlamydomonas reinhardtii as a long-standing model alga
C. reinhardtii has served as the prototypical green alga for laboratory investigation since its first isolation in Amherst, USA, in 1945 and remains the most extensively studied algal species to date. It has a division time of 6–8 h; can grow photoautotrophically, photoheterotrophically, or heterotrophically on media containing acetate as a fixed carbon source; and is easy to isolate into axenic cultures. With chloroplasts similar to those of plants, C. reinhardtii has been adopted as a model for chlorophyceae photosynthesis. Beyond that it has been used to study starch biosynthesis, circadian rhythms, microtubule formation and flagellar movement, and sexual reproduction, and the light-gated ion channels discovered in Chlamydomonas have opened up the field of optogenetics.56
Transformation of DNA into C. reinhardtii can be achieved by agitation in the presence of glass beads, biolistic bombardment with DNA-coated nanoparticles, or electroporation.57 As is typical of primary endosymbiotic algae and plants, C. reinhardtii cells maintain separate nuclear and plastid genomes, each harboring unique features and potentials for genetic engineering.
The non-nuclear genomes of C. reinhardtii
The plastid genome of C. reinhardtii is evolutionarily linked to those of modern-day plants.2 Plastid compartments are a distinct microenvironment from the cytoplasm, and most of the proteins that function in the plastid are nuclear-encoded, translated on cytoplasmic ribosomes, and imported into the organelle.58 The chloroplast is more suitable for biotechnological applications such as recombinant protein overexpression than the mitochondrial genome.42,59 The chloroplast genome copy number ranges from 40 to 100,60 requiring selection pressure to achieve homoplasmy, a state in which all genome copies are the same, following a transformation event. However, this copy number can facilitate high levels of recombinant protein expression. The chloroplast genome has a strong A/T codon bias compared to the nuclear genome, and transformed DNA is integrated by homologous recombination (HR), a feature that enables precise targeting of transgene insertion.58 The C. reinhardtii plastid can function as a compartment for recombinant protein accumulation, and it has been shown that disulfide bond formation as well as proper folding of complex non-native proteins can occur within the organelle.61,62
The C. reinhardtii plastid genome has been proposed as a miniature test bed for synthetic genomics.63,64 Codon reassignment was demonstrated here, taking advantage of the absence of the TGA stop codon in any coding sequence. A tryptophan tRNA was recoded to recognize UGA, enabling the plastid expression of recombinant proteins that cannot be translated in other hosts.65 The use of antibiotic markers in plastid transformation can be avoided by using native genes to complement mutants with disrupted plastid-encoded photosynthetic genes.66,67 Plastid expression of the Pseudomonas stutzeri WM88 phosphite oxidoreductase (ptxd) gene was shown to enable use of inorganic phosphite as a phosphorus source. This metabolic enhancement has dual functions as a transformation marker and in reducing contamination by other organisms that cannot metabolize phosphite.68,69 Numerous recombinant proteins have been produced in this organelle, and iterative transformations have been made possible by the development of chloroplast selection marker recycling methods.70
The plastid genome of C. reinhardtii will likely be the first to be synthetically redesigned, as efforts have commenced to completely recode this in several research groups. At the time of writing, C. reinhardtii plastid genome engineering projects are under way in the groups of Saul Purton (University College London), Alison Smith and Jason Chin (Cambridge),71 Tobias Erb (Max-Planck-Institute for Terrestrial Microbiology, Marburg), Duanmu Deqiang (Huazhong Agricultural University, Wuhan), and Zhangli Hu, Chaogang Wang, and Bin Jia (Shenzhen University).72
Major milestones in the development of C. reinhardtii nuclear genome transformation
Chlamydomonas was one of the first algae to have a high-quality genome sequence publicly available.73 Initially, forward genetics screens were the focus of C. reinhardtii research. Early chemical and radiation mutagenesis studies generated cell-wall-deficient strains,74 starchless mutants,75 strains used in uncovering the xanthophyll cycle and its role in photoprotection,76 and lipid biosynthesis mutants,77,78 among many others. A key development in C. reinhardtii genetic engineering was the glass-bead-mediated transformation protocol, which enabled random integration of DNA into the nuclear genome of cell-wall-deficient or -removed strains,40 thereby enabling some expression of transgenes. Transformation and insertional mutagenesis were initially achieved using endogenous genomic or cDNA sequences to complement auxotrophs, strains lacking a native metabolic capacity.79 However, achieving reliable transgene expression was previously a challenge.
Some antibiotic resistance markers that could be employed in early C. reinhardtii transformation experiments included a Streptoalloteichus hindustanus (Shble)80,81 gene for bleomycin family antibiotic resistance and the high-GC-content Streptomyces hygroscopicus aphVII82 and S. rimosus aphVIII83 genes for hygromycin and paromomycin resistance, respectively.81,83 These studies revealed the importance of codon optimization and selecting appropriate endogenous promoters and terminators in C. reinhardtii gene expression cassette design.84 It was demonstrated that inclusion of the first intron of the Ribulose-1,5 Bisphosphate Carboxylase/Oxygenase Small Subunit 2 (RBCS2) gene improved the expression of antibiotic resistance markers,80,85 as well as luciferase and fluorescent reporters,85,86,87 and substantial improvements in nuclear transgene overexpression were achieved through the repetitive spreading of this intron throughout synthetically designed transgenes.25,88 The strong tendency of Chlamydomonas to inactivate transgenic DNA sequences during integration into the nuclear genome has also been mitigated by the development of domesticated strains with mutations in an Sir2-type histone deacetylase involved in epigenetic gene silencing.89,90
The Chlamydomonas synthetic biology toolkit now includes an almost gapless 114-Mb nuclear genome assembly,91 multiple transformation markers,92,93 defined parameters for transgene expression,94 localization signals that can be used to direct proteins to specific subcellular compartments and fluorescent proteins tags for visualization,95,96 and ribonucleoprotein (RNP)-mediated gene editing.97,98,99 Libraries of knockout mutants have also been generated for use by the research community,100 and these developments combine to now enable sophisticated metabolic and genome engineering in Chlamydomonas. The community-developed modular cloning “MoClo” kit for C. reinhardtii continues to be a major collaborative effort toward standardizing Chlamydomonas engineering.44 Key biotechnology applications using this alga include demonstrating synthesis of valuable commodity biochemicals, including terpenoids such as isoprene32 and polyamines such as cadaverine.101 These efforts illustrate the utility of engineered algae for producing tailored chemicals, particularly in the context of wastewater valorization.10 Biotherapeutic applications include engineered biocontrol of pathogens in aquaculture102 and expression of anti-cancer immunotoxins.62
Future crucial genome engineering developments to enable use of C. reinhardtii as a chassis organism
The extensive expertise that has been accumulated in the field of Chlamydomonas biology makes this alga an attractive system for engineering purposes. However, there are significant challenges that must be overcome if it is to reach its potential as a platform for genome-level engineering. An important consideration is its relatively large genome size (114 Mbp,91 Figure 3), which is currently beyond the scope for complete synthetic redesign. Additionally, transgene integration into the nuclear genome occurs primarily by random non-homologous end joining (NHEJ) rather than HR,103 so making large-scale targeted DNA replacements is challenging. Deletion or downregulation of components of the NHEJ pathway for DNA repair such as Ku80 increased the frequency of HR-mediated integration by up to 70% in Kluyveromcyes marxianus and other yeasts.104 RNP-mediated gene editing to make knockouts and targeted insertions of small transgene cassettes has been demonstrated in Chlamydomonas,98,105,106 and the frequency of homology-directed repair (HDR) in gene editing was improved by mutagenesis of Ku80.107 However, targeted integration of large, complex, multi-gene expression constructs for synthetic biology has not yet been demonstrated.
Figure 3.
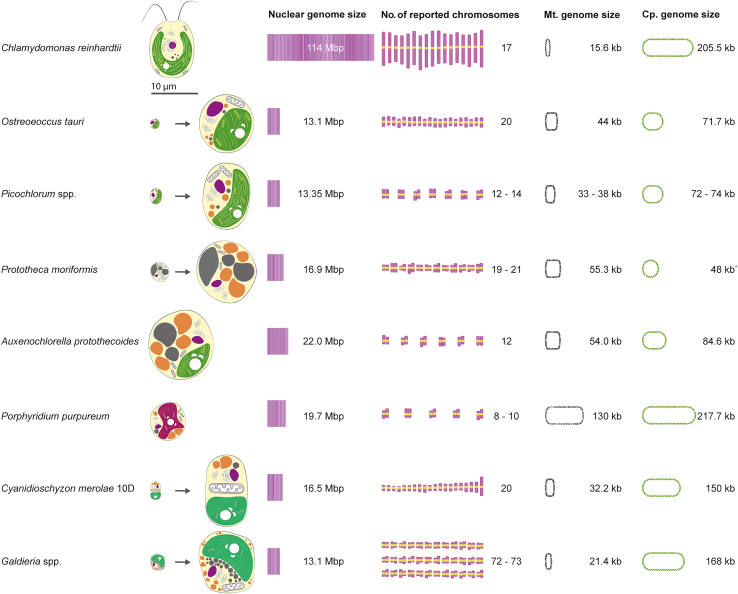
Algal genomes that are candidates for engineering and resynthesis
The nuclear genomes of the algae in this list are substantially smaller than the model Chlorophyta C. reinhardtii, making them more suitable for complete genome redesign. Cartoons representing each genus or species illustrate the relative cell sizes and pigmentation differences. The ploidy of the vegetative state is indicated by paired chromosomes for diploid and single chromosomes for haploid. The total haploid numbers of chromosomes in each nuclear genome, and the sizes of the mitochondrial and plastid genomes, are indicated. Genome data are from the following references: C. reinhardtii,91O. tauri,108Picochlorum spp.,109Prototheca spp.,110A. protothecoides (unpublished data), P. purpureum,111C. merolae 10D,112 and Galdieria spp.113 Plastids (green, except phycoerythrin-containing P. purpureum and heterotrophic Prototheca spp.), nuclei (purple), starch deposits (white), vacuoles and microbodies (gray), lipid bodies (orange), and mitochondria, ER, and Golgi (illustrated cartoons).
Improvements in gene-editing protocols have shown promise for targeting integration of gene expression cassettes through HDR.99 However, recycling of marker genes in the nuclear genome has not yet been reported, so the number of successive engineering events that can be performed is limited by the availability of selection markers. Automation of genetic engineering, facilitated by robotic handling114 in conjunction with high-throughput analytics and phenomic screens,115 will play a role in increasing the speed of algal strain development.116 Older techniques, such as protoplast fusion, whereby two cells can be physically merged and aspects of their genomes combined, may be useful in engineered strains when mating or marker recycling (discussed in the next section) is not feasible.117,118
While C. reinhardtii is likely to maintain its position as the premier model green alga, its complex nuclear genome may be too large for total redesign engineering to generate a completely controllable cell chassis. Other algae, with simpler genome architectures and the ability to perform HR, may be better suited for such investigations and are discussed in the next section (Figure 3).
The possibilities of synthetic genomics in algae
Desirable traits to enable synthetic genome design in algae
Beyond the targeted disruption of genes and the insertion of cassettes for gene expression is the concept of whole-genome redesign; this strategy involves expanding functionality by systematically re-engineering and replacing entire chromosomes to eventually build a synthetic tailored genome. Essential aspects of redesign include removing redundancies in codon usage and thereby liberating tRNAs for recoding alternative amino acids, removing repetitive genome sequences, disentangling overlapping coding sequences, adding PCR tags to genes, incorporating specific recombination sites to enable whole-genome plasticity, or adding synthetic supernumerary neochromosomes—de novo chromosomes that are not found in nature—for specific purposes.119,120 The Synthetic Yeast Genome Project (Sc2.0) (collected articles of which can be found at https://www.cell.com/consortium/synthetic-yeast-genome) is a global consortium effort to redesign the genome of this model organism, aiming to rebuild the 11.3-Mb Saccharomyces cerevisiae genome with many of these features. This project is replacing wild-type chromosomes with synthetic sequences to remove non-essential introns and tandem repeats in coding sequences, along with long terminal repeats from viral DNA. Consolidated tRNAs are also being relocated to a neochromosome.
To leverage these technologies in algae and truly customize algal genomes, it will likely be only practical to focus on those with naturally small, non-redundant genomes that contain close-to-minimal gene sets. For organisms with larger genomes, such as C. reinhardtii, engineered rearrangements in native chromosomes may be facilitated if improvements in HDR can be realized.121 However, genomes with lower total nucleotide footprints will be simpler to build de novo. Smaller genomes may have fewer hidden genetic elements, such as non-coding RNA within introns, or other uncharacterized genome structure features that may hinder engineering efforts.
The generation of redesigned genomes is currently realized in yeast by stepwise replacement of sections of native chromosomes with redesigned variants. For this purpose, HR of transformed DNA into the genome is essential, along with efficient counter-selectable transformation markers. Chromosome section replacement can be enacted by alternating between two selectable markers and stepwise walking across chromosome sections. A technique for this is called switching auxotrophies progressively for integration, as used in S. cerevisiae.122 Marker genes such as URA3, encoding orotidine 5-phosphate decarboxylase, or MAA7, encoding tryptophan synthase β subunit, can be positively selected for restoration of uracil or tryptophan prototrophy, respectively. Excision of the markers (negative selection) restores auxotrophy and enables survival on 5-fluororotic acid or 5-fluoroanthranilate, which are otherwise converted into toxic products, leading to cell death.123,124 Targeted modifications from CRISPR-Cas techniques can also assist in fine-tuning sequences within newly designed chromosome sections.120,122,125
Rapid growth is another key trait required to enable future genome engineering in algal platform strains. All genetic modification goals necessitate multiple rounds of transformation; therefore, a rapid doubling time is crucial to achieve genome-scale alterations within a reasonable time frame. The ability to grow heterotrophically can also be useful for the host cell, improving the likelihood of recovery after transformation events replacing large chromosomal sections, which can interrupt core cellular functions such as photosynthesis.126 C. reinhardtii has been an advantageous model for teasing apart photosynthetic mechanisms, since non-photosynthetic mutants can be recovered on acetic acid.57
Haploidy can be another advantage, since genomic modifications can be more complex when there are two potential targets, but diploidy can also offer robustness and the ability to remove one copy of essential genes. Sexual reproduction can expedite the construction of partially synthetic chromosomes in tandem, and meiotic crossover can be exploited to assemble multiple parts into a full-length functional synthetic chromosome in a daughter cell.127 Small genomes with high numbers of chromosomes can be advantageous for minimizing the sizes of synthetic chromosomes that have to be replaced and for consolidating genes for particular biochemical pathways onto specific chromosomes. Lower inherent intron density in the starting strain reduces the effort required to identify hidden regulatory elements that are contained in those features.
Algae with natural features that hold potential for whole-genome engineering
Ostreococcus spp.
Ostreococcus has emerged as a model picoeukaryote, particularly for investigations into the ecological niche-specific adaptations exhibited by four ecotypes or species—O. tauri, O. lucimarinus, O. mediterraneus, and O. sp RCC809, which were isolated from different marine environments128—and in studies of circadian rhythms.129 O. tauri possesses a compact haploid genome and one of the smallest known chloroplast genomes (Figure 3), making it a good candidate for exploring minimal gene sets. Its nuclear genome is 12.56 Mb spanning 20 chromosomes, its mitochondrial genome is 44.3 kb, and its chloroplast genome is 71.7 kb.108 Doubling times for O. tauri have been reported between 13.3 and 6 h.130,131 Approximately 39% of genes are predicted to contain introns, which is considerably lower than the 92% in C. reinhardtii but significantly higher than the 5% of S. cerevisiae. O. tauri has been transformed by electroporation132 and polyethylene glycol (PEG) treatment.46 Efficient HR, requiring short homology arms for gene targeting, has been reported only once133 and may require further characterization. Chromosomes 2 and 19 are particularly noteworthy for synthetic genomics applications, as they harbor the majority of its transposable elements and many genes encoding transporters, resembling prokaryotic genome islands that are enriched in genes acquired through horizontal gene transfer (HGT).134
Picochlorum spp.
Picochlorum spp. represent a genus of high-light and thermotolerant, euryhaline picoeukaryotic Trebouxiophytes that have been isolated from a range of aquatic environments.135 Picochlorum spp. have high photoautotrophic biomass productivity, with doubling times as short as 2 h reported for P. celeri.136 Genome sequences are available for several Picochlorum spp.,109 at least some of which are diploid, and the haploid sizes of their nuclear genomes range from 13 to 14 Mb (Figure 1).137 P. celeri has a diploid genome of 27.43 Mb across 15 chromosomal contigs. Other species such as P. soloecismus and P. sp. SENEW3 have genomes as small as 13.5 Mb, with chloroplast and mitochondrial genomes of 72.7 kb and 38.8 kb, respectively. Improved thermotolerance in P. sp. BPE23 was achieved through adaptive laboratory evolution and was associated with chromosome duplications.138
Electroporation and biolistics were used to transform the P. renovo nucleus and chloroplast, respectively,55,139 and RNP-mediated gene editing enabled the targeted disruption of nitrate reductase and carotenoid isomerase genes in P. celeri.140 Efficient secretion was driven by N-terminal fusions of native secretory peptides to fluorescent proteins in P. renovo, and this strain has been suggested as a platform for photoproduction of industrially relevant enzymes.139 Development of molecular genetic tools is in an early stage for Picochlorum, but substantial progress has been made despite the relatively short duration of research.137
Prototheca spp.
Solazyme/TerraVia Holdings produced “tailored oils”' for fuels, nutrition, and industrial applications by modifying fatty acid and lipid biosynthesis in the non-photosynthetic Trebouxiophyte, Prototheca moriformis UTEX 1435. These activities represent probably the most advanced industrial use and genetic engineering of algae to date, yet key advances in manipulating Prototheca fatty acid/lipid composition are only reported in patent filings. Prototheca was selected as the industrial engineering platform on the basis of its robust fermentation performance, high lipid yield on glucose, and genetically tractable nature.141 As is common for many oleaginous organisms, triacylglyceride (TAG) oil production in Prototheca was induced by nitrogen depletion in the presence of excess sugar.141 Heterotrophic doubling time on glucose was reported to be 4 h.142,143 A public genome sequence for P. moriformis, which is diploid,141 is not yet available, but the haploid sizes of the nuclear genomes of related Prototheca spp. range between 16.7 and 20 Mb.110,144 Although Prototheca spp. are obligate heterotrophs, they retain residual plastids, which are the site of bulk fatty acid biosynthesis.110
Genetic engineering in Prototheca was achieved by biolistic bombardment, and efficient nuclear gene targeting was found to occur by HR.141 Selections for transformation included neomycin antibiotic resistance, rescue of thiamine auxotrophy, and glycosyl hydrolases that expanded the range of disaccharide sugar feedstocks suitable for fermentation.141 Prototheca was also modified to express fungal pathways facilitating xylose uptake and conversion to xylulose, along with an Arabidopsis plastid transporter to enable incorporation of xylulose-5-phosphate into the pentose phosphate pathway. These modifications enabled xylose assimilation and enhanced utilization of cellulosic sugars in industrial fermentation.145 Several additional metabolic engineering examples are highlighted in detail in the next paragraph to provide a cross-section of the approaches made possible by the genetic tractability of this alga. Currently, Prototheca stands as a prime example of the potential applications for genome engineering in algae; however, these species cannot be used in light-driven production systems.
Prototheca lipid is predominantly TAG, resembling olive oil, with palmitate (C16:0), oleate (C18:1n-9), and linoleate (C18:2n-6) as the predominant fatty acids. Strains accumulating high levels of C8:0, C10:0, C12:0, and C14:0 were engineered by overexpression of acyl-acyl carrier protein (ACP) thioesterases, β-ketoacyl-ACP synthases, and acyltransferases with substrate specificities toward medium-chain fatty acids (MCFAs).146,147,148 High-oleic oils (>90%), with very low levels of saturated and polyunsaturated fatty acids, were generated by increasing elongation and stearoyl-ACP desaturase (SAD) activities in the chloroplast to enhance production of monounsaturated fatty acids, while downregulating the microsomal fatty acid desaturase FAD2, which converts oleate to linoleate.149,150,151 Strains enriched with up to 70% stearate-oleate-stearate “structured fat” were created by expressing a C18:0-specific Garcinia mangostana FATA1 thioesterase, downregulating endogenous SAD2 and FAD2, and co-expressing cocoa acyltransferases exhibiting a high degree of discrimination against esterification of saturated fatty acids at the sn-2 position.152 Prototheca “algae butter” was compatible with cocoa butter and comparable in performance to shea stearin in confectionary applications.153
Fatty acid elongases (FAEs) from Crambe abyssinica and other species were used to modify the cytoplasmic elongation pathway, converting C18:1n-9 to C22:1n-9 (erucic acid), a fatty acid used in the manufacture of film plastics.150 The efficiency of elongation was enhanced by Lands cycle acyltransferases, which increased the exchange of acyl groups between diacylglycerol, membrane phospholipids, and the acyl-coenzyme A (CoA) pool.150 Up to 20% erucic acid content was achieved by expressing FAEs, upregulating homomeric acetyl-CoA carboxylase, and co-expressing β-ketoacyl-CoA reductase, 3-hydroxyacyl-CoA dehydratase, and enoyl-CoA reductase transgenes.150
Prototheca was also employed as a platform for cannabinoid biosynthesis by Purissima.154 Enzymes from Cannabis sativa to make olivetolic acid were co-expressed with prenyl transferases and cannabinoid synthase genes, and accumulation of olivetolic acid, cannibigerolic acid, cannabidiolic acid, and tetrahydrocannabinolic acid in Prototheca oil was confirmed by chromatography and mass spectrometry.154 These examples of Prototheca metabolic engineering for commercial applications illustrate the possibilities of novel products achievable when an alga exhibits transformability, gene targeting by HR in the nuclear genome, and stable transgene expression. These features are requisite for future genome-scale manipulations.
Auxenochlorella protothecoides
Auxenochlorella protothecoides is the closest photosynthetic relative to Prototheca spp.,110 and thus far all of the engineering capabilities developed for Prototheca can be applied to Auxenochlorella.37 Doubling times from 6 h heterotrophically on glucose to 16 h photoautotrophically have been reported.155,156 Photoautotrophic lipid accumulation of up to 40% biomass has been reported,157 and mixotrophic degradation and use of cellulosic material as a substrate for growth has been observed.158 In contrast to Prototheca, which are obligate heterotrophs, Auxenochlorella retains the capacity for photosynthesis but suppresses photosynthetic complex accumulation and switches to heterotrophic growth in the presence of organic carbon sources.159
Transformation of A. protothecoides UTEX 250 using a lithium acetate/PEG procedure and efficient gene targeting by HR were reported by Phycoil Biotechnology International.37 Successful transformation and nuclear gene targeting are possible in two additional genetically distinct A. protothecoides strains, UTEX 25 and 2341, and high-quality, gapless nuclear, and organellar genome assemblies of A. protothecoides UTEX 250 have been generated (Figure 3) (unpublished data). The transformation markers developed for use in Prototheca were found to also function in A. protothecoides, and both heterologous and endogenous promoters have proven effective in driving transgene expression.37
Engineering approaches to improve the value of Auxenochlorella biomass include disrupting one or both alleles of lycopene cyclase epsilon (LCYE) to change lutein/zeaxanthin ratios with concomitant expression of the C. reinhardtii β-carotene ketolase (BKT1), leading to the accumulation of oils containing varying mixtures of orange-red ketocarotenoids (Figure 2).37 These engineering efforts were inspired by the demonstration of modified ketocarotenoid profiles in C. reinhardtii with BKT1 recoding and overexpression.34,35,160,161 Strains with increased polyunsaturated fatty acids and reduced ω-6/ω-3 ratios were generated by knockin of strong promoters at the FAD3 locus, accompanied by the expression of Arabidopsis PDCT and Linum usitatissimum (flax) FAD3 transgenes.37 Disruption of both alleles of squalene epoxidase (SQE) yielded strains accumulating over 1,000 ppm of squalene, comparable to the content of olive oil.37 Co-expression of the FATB2 thioesterase and KASA1 β-ketoacyl-ACP synthase from Cuphea wrightii in Auxenochlorella led to accumulation of up to 35% MCFA (unpublished data). These results suggest that A. protothecoides is equivalent to P. moriformis as a platform for lipid and isoprenoid pathway engineering, with the added advantage of phototrophic or mixotrophic growth.162
Prototheca and Auxenochlorella have significant potential as platforms for applied genetic and metabolic engineering, but their suitability for complete genome redesign remains to be demonstrated. Diploidy may facilitate complex chromosome rearrangements by providing redundancy, but it may also pose challenges in achieving complete synthetic replacements. These species originally found traction in industrial sectors with limited knowledge sharing and are only now emerging as model organisms for academic research with publicly available genome and systems data.158,163,164 Facile HR, robust heterotrophic growth, and their oleaginous natures make them attractive hosts for future genome engineering approaches.
Porphyridium purpureum
The red alga Porphyridium purpureum is noteworthy for its genetic flexibility, having acquired up to 9% of its genome by HGT111; an implication of this could be that P. purpureum may have a natural propensity for production of foreign proteins. Indeed, bacterial origins of replication enabled plasmid vectors to be maintained in the P. purpureum nucleus as stable episomes, and expressed transgenes can accumulate up to 5% of total soluble protein.165 Circular plasmids were transformed by biolistic bombardment, and the efficiency of co-transformation with 1:1 mixtures of two plasmids was 100%.165
HR in the Porphyridium nuclear genome has not been observed, but gene editing with CRISPR-Cas9 RNP was used to knock out the chlorophyll a synthase gene, resulting in strains with enhanced phycoerythrin production.166 Transformation and RNP-mediated HDR are fundamental tools that may enable genome engineering approaches in P. purpureum. As illustrated in Figure 3, the P. purpureum nuclear genome is relatively compact at 19.7 Mb, with 8,355 predicted genes.111 There are no reports describing a Porphyridium sexual cycle, but genes that are essential for meiosis are present in the genome.111 Porphyridium growth can be enhanced by supplementation with some organic carbon sources, but even under mixotrophy, doubling times are still over 2 days long compared to rapid growers such as Picochlorum.167,168
Porphyridium is an example of an organism that could be cultivated for biorefinery processing, since these species can produce large amounts of sulfated extracellular polysaccharides for cosmetic and pharmaceutical applications, phycobilins, which are of interest as biodegradable dyes, and the polyunsaturated fatty acids arachidonic acid and eicosapentaenoic acid.169 Genome engineering could be employed to generate strains with improved capacities to synthesize these, and other, value-added natural products.
Cyanidioschyzon merolae 10D
C. merolae 10D is a polyextremophilic red alga that is a model for investigating the unique mechanisms of photosynthesis at low pH and high temperatures. It is an obligate phototroph, originating from acidic hot springs and lacking a cell wall.170 In contrast with other red algae, the members of the Cyanidiophyceae have lost phycoerythrin biosynthesis, resulting in a cyan-green coloration.170,171 C. merolae exhibits a 9-h doubling time and can be cultivated at 42°C and pH 2 or lower.172 These conditions can confer significant advantages for scale-up, as the energy requirements are lower for heating than cooling, and low pH is useful for mitigating contamination.172
Its 16.5-Mb haploid nuclear genome spans 20 chromosomes, with approximately 55% GC content, and it possesses 32-kb mitochondrial and 150-kb plastid genomes.112 Only 0.5% of C. merolae nuclear genes contain introns.170 Transformation into the nuclear genome is achieved using a PEG-mediated protocol, and 500-bp homology arms are sufficient for gene targeting by HR.173 Transformation and maintenance of circular episomal plasmids has been observed by our group.
C. merolae strains have been engineered to enable heterotrophic growth by expressing sugar transporters,174 for increased lipid yields with a cyanobacterial acyl-ACP reductase,175 and for production of valuable ketocarotenoids.36 The compact and intron-poor nuclear genome, along with its genetic tractability, metabolic flexibility, and adaptations to extreme growth conditions, positions C. merolae as a promising candidate for transgene overexpression and genome redesign.
Galdieria spp.
Galdieria spp. are red algae that have been isolated from similarly extreme environments as C. merolae. Unique among the Cyanidiophyceae, they are facultative heterotrophs capable of using as many as 30 carbon sources while retaining the ability to grow photoautotrophically or mixotrophically. Galdieria spp. possess compact nuclear genomes ranging in size from 11 to 16 Mb, with 72–73 chromosomes depending on the species, and about 45% GC content, despite occupying an environmental niche similar to that of C. merolae.113,176 Approximately 1% of the genome was acquired by HGT.176
Galdieria display many features that would be beneficial for genome engineering; G. partita was discovered to have a sexual cycle, with meiotic conversion of cell-walled, vegetative diploid cells into motile cell-wall-less haploid gametes under pH 1, which can mate to produce diploid progeny.47 Meiotic crossovers can facilitate the stepwise assembly of synthetic chromosomes or transfer of transgenes.
Haploid cells of G. partita have been transformed successfully by adapting the C. merolae PEG-mediated protocol; gene targeting by HR was reported to occur efficiently, as was marker recycling.47 The only drawback to engineering Galdieria is its relatively slow 16-h doubling time,177,178 but this may be mitigated by faster growth under mixotrophic conditions.179 To date, only one study has demonstrated genetic manipulation in this genus, so continuing efforts are warranted to explore its potential as a synthetic biology platform.
Novel genera
Bioprospecting for novel species is another avenue for discovering algae with the appropriate traits for genome engineering. New algae species worthy of further investigation for biotechnological applications should grow rapidly, have relatively small, genetically tractable genomes, preferably be capable of efficient HR, and have high flux to valuable natural products.180
Medakamo hakoo, an ultrasmall (1 μm diameter) Trebouxiophyte discovered in an aquarium in 2015, is an example of a promising new algae species, possessing a compact 15-Mb nuclear genome and unusual cell division involving generation of four daughter cells per cell cycle. M. hakoo is in a sister clade to Botryococcus and despite its small size may have the potential to produce starch and high titers of TAGs. These natural features warrant further investigation, and successful transformation could open avenues for the use of this species for synthetic biology and biotechnology.181
Broad perspectives of synthetic genomics and the paradigm of whole-genome approaches to engineering
From pathways to genomes
Chromosome- and genome-scale engineering approaches in other organisms have been made possible by advances in DNA sequencing, DNA synthesis, and transformation of large customized DNA fragments.182 Algal genome engineering will for some time likely remain limited to the introduction of short biochemical pathways or to protein overexpression. The plastid presents a logical starting point for synthetic genome redesign, and partial replacement of nuclear chromosome sections may be possible before whole synthetic chromosome replacements become a reality.121 Neochromosomes containing multi-gene pathways in addition to RNP-mediated editing of the genome could enhance algae with valuable new traits or products before complete genomic redesigns are realized. In yeast, 56 genetic changes in total were employed to yield strains producing monoterpene indole alkaloids, combining 30 heterologous genes and multiple genome edits but without a synthetic genomics approach incorporating partial or complete chromosome redesign.183
To facilitate neochromosome construction for algal applications, techniques such as the “telomerator” could be employed. This is a seed sequence used to add telomeric caps to small DNA fragments to enable the creation of synthetic small chromosomes.184 Neochromosomes were employed in yeast to encode a humanized version of the adenine biosynthesis pathway185 and express an array of pan-genomic elements from industrial or environmental strains for evaluation of phenotypic diversity.186 A 190-kb neochromosome relocated all 275 yeast tRNA genes to reduce the genomic instability associated with tRNA elements.119
The potential for completely re-engineering algal genomes is of interest to develop future cell lines that function more like programmable biological machines. Engineered algae of the future with entirely recoded genomes will be a chassis for light-driven bioproduction and waste circularization concepts, with a range of features that make them controllable and elegant to modify. Some of these potential features are illustrated in Figure 4.
Figure 4.
The wild-type alga compared to the genome-engineered alga of the future
Left: the wild-type alga maintains three separate genomes in the plastid, mitochondria, and nucleus. The nuclear genome may be haploid or diploid in vegetative cells, and genes for all cellular processes are distributed throughout the chromosomes. Genetic loci on any of the genomes may be modified by gene targeting and editing, and transgenes can be integrated and expressed. Simple heterologous pathways can be introduced, but broad genomic alterations, multi-locus targeting, and reliable expression of multiple transgenes of complex biochemical pathways is still challenging. The wild-type or domesticated alga is susceptible to pathogens, represented by blue hexagons, and accumulates both native and engineered products through synthetic biology. Right: the engineered alga of the future will feature completely recoded and redesigned genomes. The nuclear genome, either haploid or diploid, will be dispersed across numerous small autonomously replicating chromosomes, neochromosomes, or episomes. Plastid genomes may be rearranged, with additional gene transfer to the nucleus when advantageous. Redundancies and repetitions in the genome will be eliminated except where necessary to increase gene copy numbers for improved protein expression. Genes will be grouped into neighborhoods according to function to facilitate modular engineering, and recombination elements will be inserted to enable broad-scale genome shuffling on demand. The recoded algal genomes will be intrinsically resistant to pathogen attack and will encode defenses against competitors and grazers, along with biocontainment strategies such as engineered auxotrophy or mating incompatibility. Genome landing pads will streamline the integration of multi-transgene pathways and accelerate the biosynthesis of novel products, symbolized by colored hexagons in the plastid.
Lessons from synthetic genomics in relation to algae
Biocontainment, genome architecture engineering, and synthetic speciation
JCVI-Syn1.0 was the first example of an entirely synthetic genome and its successful transplantation into a restriction-deficient strain of Mycoplasma mycoides.187 This demonstrated that a chemically synthesized copy of a genome, with significant modifications, could be used to replace a native genome. Among other features, watermarks and defined sequence tags were included, features that influenced latter genetic designs in more complex organisms. The current stage of the synthetic mycoplasma genome, JCVI-Syn3a, has shown that minimization of genomes can reduce redundancy and complexity and increase the predictability of a biological system. The genetic dissection of highly redundant gene families can unveil otherwise cryptic functions in genome stability that might be otherwise overlooked in the native genome context; analysis in a minimal genome is a useful way to fully characterize individual gene function of highly redundant genes.188 This minimization approach, applied to algal genomes, could elucidate the roles of many genes that have only been assigned functions based on predictive annotation or models.
Within the Sc2.0 project, one included redesign feature is the placement of numerous loxP sites in terminator regions of coding sequences throughout the engineered genome.189 Through inducible expression of the Cre recombinase, recombination between intergenic symmetrical lox sites (lox-p-sym) stimulates genome-wide terminator swapping, deletions, inversions, and duplications as well as translocations. However, unlike mutagenesis methods, this inducible randomization generally does not affect gene-coding sequences.189 This technique is called SCRaMbLE (synthetic chromosome rearrangement and modification by lox-p-mediated evolution) and can be used to investigate rapid evolution of yeast strains with improved features such as tolerance to high pH190 and heterologous metabolite production.191 Similar features could be incorporated into future synthetic algal genomes to facilitate directed evolution, metabolic engineering, and reverse engineering or to improve strain growth performance.
Biocontainment becomes increasingly important as engineered organisms are deployed. Synthetic transgenes could be unwittingly introduced into the environment through outbreeding and HGT, risking adverse ecological outcomes. A number of mechanisms have been proposed to mitigate these risks, including codon reassignment and synthetic auxotrophy through dependence on non-natural amino acids.192 Conditional suicide systems and tox/antitox gene pairs can also create boundaries for genetic escape.193,194 Salmonella typhimurium and Escherichia coli strains expressing modified tRNAs to recode codon usage were resistant to viral infection and incompatible with bacterial HGT.192 Similar approaches could be applied for crop protection and biocontainment in algae with engineered genomes.
Another approach to minimize genetic escape involves karyotype engineering to limit mating compatibility with wild-type strains. Circularization of a synthetic S. cerevisiae chromosome V demonstrated the flexibility of eukaryotic cells to function normally with uncommon chromosomal arrangements and the feasibility of more complex genome architectural design approaches.195 Reproductively isolated S. cerevisiae strains where the whole genome is merged into one chromosome196 were also created by RNP-mediated gene editing, but it should be noted that the inability to mate with wild type does not necessarily prevent genetic transfer into the environment. Algal species, such as those featured in Figure 3, may be interesting for such approaches, with those containing small genomes and performing HR being specifically promising candidates.
Final notes
The future of algal synthetic biology offers hope for the efficient photosynthetic conversion of nutrients from waste streams into value-added biochemicals. Drawing inspiration from engineered genomes of bacteria and yeasts, we now have the foundational tools of high-quality algal genome assemblies, efficient transformation, some species with efficient HR, and maturing RNP-mediated gene editing. These tools, applied in hosts with favorable molecular genetic features, will enable genome-scale modification and redesign in several algae species. The seven algal genera detailed in “algae with natural features that hold potential for whole-genome engineering” are candidates for additional development, but bioprospecting efforts should continue to seek fast-growing, genetically tractable species with superior traits for genome engineering. Genome minimization, chromosome architecture restructuring and refactoring, and the transplantation of metabolic pathways onto neochromosomes are all becoming feasible approaches in algae systems and can be applied to genome-scale engineering projects that were once purely hypothetical.
Acknowledgments
K.J.L. acknowledges baseline research funding from King Abdullah University of Science and Technology (KAUST). J.L.M. acknowledges DOE Office of Science, Office of Biological and Environmental Research. External support for Macquarie University’s Synthetic Biology initiative is acknowledged from Bioplatforms Australia, the New South Wales (NSW) Chief Scientist and Engineer, and the NSW Government’s Department of Primary Industries. Australian Government funding through its investment agency, the Australian Research Council, toward the Macquarie University-led ARC Center of Excellence for Synthetic Biology is gratefully acknowledged by H.D.G. Figures 1, 3, and 4 were created by Heno Hwang, scientific illustrator at KAUST.
Declaration of interests
J.L.M. is a paid consultant for Phycoil Biotechnology International, Inc., and a shareholder in Phycoil Biotechnology Korea, Inc. J.L.M. is an inventor on three patents related to Prototheca moriformis and Auxenochlorella protothecoides engineering that are listed in the references.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xgen.2024.100505.
Supplemental information
References
- 1.Goodstein D.M., Shu S., Howson R., Neupane R., Hayes R.D., Fazo J., Mitros T., Dirks W., Hellsten U., Putnam N., Rokhsar D.S. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 2012;40:D1178–D1186. doi: 10.1093/nar/gkr944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keeling P.J. Diversity and evolutionary history of plastids and their hosts. Am. J. Bot. 2004;91:1481–1493. doi: 10.3732/ajb.91.10.1481. [DOI] [PubMed] [Google Scholar]
- 3.Keeling P.J. The endosymbiotic origin, diversification and fate of plastids. Phil. Trans. R. Soc. B. 2010;365:729–748. doi: 10.1098/rstb.2009.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodríguez-Ezpeleta N., Brinkmann H., Burey S.C., Roure B., Burger G., Löffelhardt W., Bohnert H.J., Philippe H., Lang B.F. Monophyly of Primary Photosynthetic Eukaryotes: Green Plants, Red Algae, and Glaucophytes. Curr. Biol. 2005;15:1325–1330. doi: 10.1016/j.cub.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 5.Green B.R. What Happened to the Phycobilisome? Biomolecules. 2019;9:748. doi: 10.3390/biom9110748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ball S., Colleoni C., Cenci U., Raj J.N., Tirtiaux C. The evolution of glycogen and starch metabolism in eukaryotes gives molecular clues to understand the establishment of plastid endosymbiosis. J. Exp. Bot. 2011;62:1775–1801. doi: 10.1093/jxb/erq411. [DOI] [PubMed] [Google Scholar]
- 7.Reyes-Prieto A., Bhattacharya D. Phylogeny of Nuclear-Encoded Plastid-Targeted Proteins Supports an Early Divergence of Glaucophytes within Plantae. Mol. Biol. Evol. 2007;24:2358–2361. doi: 10.1093/molbev/msm186. [DOI] [PubMed] [Google Scholar]
- 8.MacLeod A.I., Knopp M.R., Gould S.B. A mysterious cloak: the peptidoglycan layer of algal and plant plastids. Protoplasma. 2024;261:173–178. doi: 10.1007/s00709-023-01886-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petroutsos D., Amiar S., Abida H., Dolch L.-J., Bastien O., Rébeillé F., Jouhet J., Falconet D., Block M.A., McFadden G.I., et al. Evolution of galactoglycerolipid biosynthetic pathways – From cyanobacteria to primary plastids and from primary to secondary plastids. Prog. Lipid Res. 2014;54:68–85. doi: 10.1016/j.plipres.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 10.De Freitas B.B., Overmans S., Medina J.S., Hong P.-Y., Lauersen K.J. Biomass generation and heterologous isoprenoid milking from engineered microalgae grown in anaerobic membrane bioreactor effluent. Water Res. 2023;229 doi: 10.1016/j.watres.2022.119486. [DOI] [PubMed] [Google Scholar]
- 11.Hallmann A. Algal transgenics and biotechnology. Transgenic Plant J. 2007;1:81–98. [Google Scholar]
- 12.Kim J.K., Yarish C., Hwang E.K., Park M., Kim Y. Seaweed aquaculture: Cultivation technologies, challenges and its ecosystem services. ALGAE. 2017;32:1–13. doi: 10.4490/algae.2017.32.3.3. [DOI] [Google Scholar]
- 13.Janssen M., Tramper J., Mur L.R., Wijffels R.H. Enclosed outdoor photobioreactors: Light regime, photosynthetic efficiency, scale-up, and future prospects. Biotechnol. Bioeng. 2003;81:193–210. doi: 10.1002/bit.10468. [DOI] [PubMed] [Google Scholar]
- 14.Sheehan J., Dunahay T., Benemann J., Roessler P. National Renewable Energy Laboratory (NREL); 1998. A Look Back at the U.S. Department of Energy’s Aquatic Species Program: Biodiesel from Algae; Close-Out Report. [DOI] [Google Scholar]
- 15.Barbosa M.J., Janssen M., Südfeld C., D’Adamo S., Wijffels R.H. Hypes, hopes, and the way forward for microalgal biotechnology. Trends Biotechnol. 2023;41:452–471. doi: 10.1016/j.tibtech.2022.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Walker T.L., Collet C., Purton S. Algal transgenics in the genomic era 1: genetic engineering of algae. J. Phycol. 2005;41:1077–1093. doi: 10.1111/j.1529-8817.2005.00133.x. [DOI] [Google Scholar]
- 17.Almaraz-Delgado A.L., Flores-Uribe J., Pérez-España V.H., Salgado-Manjarrez E., Badillo-Corona J.A. Production of therapeutic proteins in the chloroplast of Chlamydomonas reinhardtii. AMB Expr. 2014;4:57. doi: 10.1186/s13568-014-0057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasala B.A., Mayfield S.P. The microalga Chlamydomonas reinhardtii as a platform for the production of human protein therapeutics. Bioeng. Bugs. 2011;2:50–54. doi: 10.4161/bbug.2.1.13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lauersen K.J. Eukaryotic microalgae as hosts for light-driven heterologous isoprenoid production. Planta. 2019;249:155–180. doi: 10.1007/s00425-018-3048-x. [DOI] [PubMed] [Google Scholar]
- 20.Wichmann J., Lauersen K.J., Kruse O. Green algal hydrocarbon metabolism is an exceptional source of sustainable chemicals. Curr. Opin. Biotechnol. 2020;61:28–37. doi: 10.1016/j.copbio.2019.09.019. [DOI] [PubMed] [Google Scholar]
- 21.Kruse O., Hankamer B. Microalgal hydrogen production. Curr. Opin. Biotechnol. 2010;21:238–243. doi: 10.1016/j.copbio.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Einhaus A., Baier T., Kruse O. Molecular design of microalgae as sustainable cell factories. Trends Biotechnol. 2023 doi: 10.1016/j.tibtech.2023.11.010. Published online December 12, 2023. [DOI] [PubMed] [Google Scholar]
- 23.Taunt H.N., Stoffels L., Purton S. Green biologics: The algal chloroplast as a platform for making biopharmaceuticals. Bioengineered. 2018;9:48–54. doi: 10.1080/21655979.2017.1377867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Specht E., Miyake-Stoner S., Mayfield S. Micro-algae come of age as a platform for recombinant protein production. Biotechnol. Lett. 2010;32:1373–1383. doi: 10.1007/s10529-010-0326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lauersen K.J., Baier T., Wichmann J., Wördenweber R., Mussgnug J.H., Hübner W., Huser T., Kruse O. Efficient phototrophic production of a high-value sesquiterpenoid from the eukaryotic microalga Chlamydomonas reinhardtii. Metab. Eng. 2016;38:331–343. doi: 10.1016/j.ymben.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Gutiérrez S., Overmans S., Wellman G.B., Samaras V.G., Oviedo C., Gede M., Szekely G., Lauersen K.J. A synthetic biology and green bioprocess approach to recreate agarwood sesquiterpenoid mixtures. Green Chem. 2024 doi: 10.1039/D3GC03708H. Published online December 14, 2023. [DOI] [Google Scholar]
- 27.Abdallah M.N., Wellman G.B., Overmans S., Lauersen K.J. Combinatorial Engineering Enables Photoautotrophic Growth in High Cell Density Phosphite-Buffered Media to Support Engineered Chlamydomonas reinhardtii Bio-Production Concepts. Front. Microbiol. 2022;13:885840–885912. doi: 10.3389/fmicb.2022.885840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Einhaus A., Steube J., Freudenberg R.A., Barczyk J., Baier T., Kruse O. Engineering a powerful green cell factory for robust photoautotrophic diterpenoid production. Metab. Eng. 2022;73:82–90. doi: 10.1016/j.ymben.2022.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Wichmann J., Baier T., Wentnagel E., Lauersen K.J., Kruse O. Tailored carbon partitioning for phototrophic production of (E)-α-bisabolene from the green microalga Chlamydomonas reinhardtii. Metab. Eng. 2018;45:211–222. doi: 10.1016/j.ymben.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Lauersen K.J., Wichmann J., Baier T., Kampranis S.C., Pateraki I., Møller B.L., Kruse O. Phototrophic production of heterologous diterpenoids and a hydroxy-functionalized derivative from Chlamydomonas reinhardtii. Metab. Eng. 2018;49:116–127. doi: 10.1016/j.ymben.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Mehrshahi P., Nguyen G.T.D.T., Gorchs Rovira A., Sayer A., Llavero-Pasquina M., Lim Huei Sin M., Medcalf E.J., Mendoza-Ochoa G.I., Scaife M.A., Smith A.G. Development of Novel Riboswitches for Synthetic Biology in the Green Alga Chlamydomonas. ACS Synth. Biol. 2020;9:1406–1417. doi: 10.1021/acssynbio.0c00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yahya R.Z., Wellman G.B., Overmans S., Lauersen K.J. Engineered production of isoprene from the model green microalga Chlamydomonas reinhardtii. Metab. Eng. Commun. 2023;16 doi: 10.1016/j.mec.2023.e00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peitz M., Bond R. A Customized Approach to Frying Oil. INFORM magazine. 2014;2 [Google Scholar]
- 34.Perozeni F., Cazzaniga S., Baier T., Zanoni F., Zoccatelli G., Lauersen K.J., Wobbe L., Ballottari M. Turning a green alga red: engineering astaxanthin biosynthesis by intragenic pseudogene revival in Chlamydomonas reinhardtii. Plant Biotechnol. J. 2020;18:2053–2067. doi: 10.1111/pbi.13364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amendola S., Kneip J.S., Meyer F., Perozeni F., Cazzaniga S., Lauersen K.J., Ballottari M., Baier T. Metabolic Engineering for Efficient Ketocarotenoid Accumulation in the Green Microalga Chlamydomonas reinhardtii. ACS Synth. Biol. 2023;12:820–831. doi: 10.1021/acssynbio.2c00616. [DOI] [PubMed] [Google Scholar]
- 36.Seger M., Mammadova F., Villegas-Valencia M., Bastos de Freitas B., Chang C., Isachsen I., Hemstreet H., Abualsaud F., Boring M., Lammers P.J., Lauersen K.J. Engineered ketocarotenoid biosynthesis in the polyextremophilic red microalga Cyanidioschyzon merolae 10D. Metab. Eng. Commun. 2023;17 doi: 10.1101/2023.02.27.530181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moseley J., Lee B., IM C.S., Kim J., Kim D., Bhat R. Production of lipids and terpenoids in auxenochlorella protothecoides. US patent application publication US20220154229 A1. 2022 filed November 5, 2021, and published May 19, 2022. [Google Scholar]
- 38.Loppes R. A new class of arginine-requiring mutants in Chlamydomonas reinhardtii. Mol. Gen. Genet. 1969;104:172–177. doi: 10.1007/BF00272799. [DOI] [PubMed] [Google Scholar]
- 39.Salomé P.A., Merchant S.S. A Series of Fortunate Events: Introducing Chlamydomonas as a Reference Organism. Plant Cell. 2019;31:1682–1707. doi: 10.1105/tpc.18.00952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kindle K.L. High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA. 1990;87:1228–1232. doi: 10.1016/S0076-6879(98)97005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kindle K.L., Richards K.L., Stern D.B. Engineering the chloroplast genome: Techniques and capabilities for chloroplast transformation in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA. 1991;88:1721–1725. doi: 10.1073/pnas.88.5.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Remacle C., Cardol P., Coosemans N., Gaisne M., Bonnefoy N. High-efficiency biolistic transformation of Chlamydomonas mitochondria can be used to insert mutations in complex I genes. Proc. Natl. Acad. Sci. USA. 2006;103:4771–4776. doi: 10.1073/pnas.0509501103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gutiérrez S., Lauersen K.J. Gene Delivery Technologies with Applications in Microalgal Genetic Engineering. Biology. 2021;10:265. doi: 10.3390/biology10040265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crozet P., Navarro F.J., Willmund F., Mehrshahi P., Bakowski K., Lauersen K.J., Pérez-Pérez M.E., Auroy P., Gorchs Rovira A., Sauret-Gueto S., et al. Birth of a Photosynthetic Chassis: A MoClo Toolkit Enabling Synthetic Biology in the Microalga Chlamydomonas reinhardtii. ACS Synth. Biol. 2018;7:2074–2086. doi: 10.1021/acssynbio.8b00251. [DOI] [PubMed] [Google Scholar]
- 45.Nelson D.R., Hazzouri K.M., Lauersen K.J., Jaiswal A., Chaiboonchoe A., Mystikou A., Fu W., Daakour S., Dohai B., Alzahmi A., et al. Large-scale genome sequencing reveals the driving forces of viruses in microalgal evolution. Cell Host Microbe. 2021;29:250–266.e8. doi: 10.1016/j.chom.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Sanchez F., Geffroy S., Norest M., Yau S., Moreau H., Grimsley N. Simplified Transformation of Ostreococcus tauri Using Polyethylene Glycol. Genes. 2019;10:399. doi: 10.3390/genes10050399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirooka S., Itabashi T., Ichinose T.M., Onuma R., Fujiwara T., Yamashita S., Jong L.W., Tomita R., Iwane A.H., Miyagishima S.Y. Life cycle and functional genomics of the unicellular red alga Galdieria for elucidating algal and plant evolution and industrial use. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2210665119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hallmann A., Rappel A. Genetic engineering of the multicellular green alga Volvox: A modified and multiplied bacterial antibiotic resistance gene as a dominant selectable marker. Plant J. 1999;17:99–109. doi: 10.1046/j.1365-313X.1999.00342.x. [DOI] [PubMed] [Google Scholar]
- 49.Hallmann A., Wodniok S. Swapped green algal promoters: aphVIII-based gene constructs with Chlamydomonas flanking sequences work as dominant selectable markers in Volvox and vice versa. Plant Cell Rep. 2006;25:582–591. doi: 10.1007/s00299-006-0121-x. [DOI] [PubMed] [Google Scholar]
- 50.Blomme J., Liu X., Jacobs T.B., De Clerck O. A molecular toolkit for the green seaweed Ulva mutabilis. Plant Physiol. 2021;186:1442–1454. doi: 10.1093/plphys/kiab185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu J., Sun Z., Gerken H., Huang J., Jiang Y., Chen F. Genetic engineering of the green alga Chlorella zofingiensis: a modified norflurazon-resistant phytoene desaturase gene as a dominant selectable marker. Appl. Microbiol. Biotechnol. 2014;98:5069–5079. doi: 10.1007/s00253-014-5593-y. [DOI] [PubMed] [Google Scholar]
- 52.Yang B., Liu J., Jiang Y., Chen F. Chlorella species as hosts for genetic engineering and expression of heterologous proteins: Progress, challenge and perspective. Biotechnol. J. 2016;11:1244–1261. doi: 10.1002/biot.201500617. [DOI] [PubMed] [Google Scholar]
- 53.O’Neill B.M., Mikkelson K.L., Gutierrez N.M., Cunningham J.L., Wolff K.L., Szyjka S.J., Yohn C.B., Redding K.E., Mendez M.J. An exogenous chloroplast genome for complex sequence manipulation in algae. Nucleic Acids Res. 2012;40:2782–2792. doi: 10.1093/nar/gkr1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zienkiewicz M., Krupnik T., Drożak A., Golke A., Romanowska E. Transformation of the Cyanidioschyzon merolae chloroplast genome: prospects for understanding chloroplast function in extreme environments. Plant Mol. Biol. 2017;93:171–183. doi: 10.1007/s11103-016-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dahlin L.R., Guarnieri M.T. Heterologous expression of phosphite dehydrogenase in the chloroplast or nucleus enables phosphite utilization and genetic selection in Picochlorum spp. Algal Res. 2022;62 doi: 10.1016/j.algal.2021.102604. [DOI] [Google Scholar]
- 56.Grossman A., Wollman F.A. 3rd Edition. Elsevier; 2023. The Chlamydomonas Sourcebook. [DOI] [Google Scholar]
- 57.Rochaix J.D. Chlamydomonas reinhardtii as the photosynthetic yeast. Ann Rev Gen. 1995;29:209–230. doi: 10.1146/annurev.ge.29.120195.001233. [DOI] [PubMed] [Google Scholar]
- 58.Surzycki R., Greenham K., Kitayama K., Dibal F., Wagner R., Rochaix J.-D., Ajam T., Surzycki S. Factors effecting expression of vaccines in microalgae. Biologicals. 2009;37:133–138. doi: 10.1016/j.biologicals.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 59.Purton S., Szaub J.B., Wannathong T., Young R., Economou C.K. Genetic engineering of algal chloroplasts: Progress and prospects. Russ. J. Plant Physiol. 2013;60:491–499. doi: 10.1134/S1021443713040146. [DOI] [Google Scholar]
- 60.Lau K.W., Ren J., Wu M. Redox Modulation of Chloroplast DNA Replication in Chlamydomonas reinhardtii. Antioxid. Redox Signal. 2000;2:529–535. doi: 10.1089/15230860050192305. [DOI] [PubMed] [Google Scholar]
- 61.Mayfield S.P., Franklin S.E., Lerner R.A. Expression and assembly of a fully active antibody in algae. Proc. Natl. Acad. Sci. USA. 2003;100:438–442. doi: 10.1073/pnas.0237108100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tran M., Van C., Barrera D.J., Pettersson P.L., Peinado C.D., Bui J., Mayfield S.P. Production of unique immunotoxin cancer therapeutics in algal chloroplasts. Proc. Natl. Acad. Sci. USA. 2013;110:E15–E22. doi: 10.1073/pnas.1214638110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dyo Y.M., Purton S. The algal chloroplast as a synthetic biology platform for production of therapeutic proteins. Microbiology. 2018;164:113–121. doi: 10.1099/mic.0.000599. [DOI] [PubMed] [Google Scholar]
- 64.Jackson H.O., Taunt H.N., Mordaka P.M., Smith A.G., Purton S. The Algal Chloroplast as a Testbed for Synthetic Biology Designs Aimed at Radically Rewiring Plant Metabolism. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.708370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Young R.E.B., Purton S. Codon reassignment to facilitate genetic engineering and biocontainment in the chloroplast of Chlamydomonas reinhardtii. Plant Biotechnol. J. 2016;14:1251–1260. doi: 10.1111/pbi.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen H.-C., Melis A. Marker-free genetic engineering of the chloroplast in the green microalga Chlamydomonas reinhardtii. Plant Biotechnol. J. 2013;11:818–828. doi: 10.1111/pbi.12073. [DOI] [PubMed] [Google Scholar]
- 67.Wannathong T., Waterhouse J.C., Young R.E.B., Economou C.K., Purton S. New tools for chloroplast genetic engineering allow the synthesis of human growth hormone in the green alga Chlamydomonas reinhardtii. Appl. Microbiol. Biotechnol. 2016;100:5467–5477. doi: 10.1007/s00253-016-7354-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cutolo E., Tosoni M., Barera S., Herrera-Estrella L., Dall'Osto L., Bassi R. A chimeric hydrolase-PTXD transgene enables chloroplast-based heterologous protein expression and non-sterile cultivation of Chlamydomonas reinhardtii. Algal Res. 2021;59 [Google Scholar]
- 69.Changko S., Rajakumar P.D., Young R.E.B., Purton S. The phosphite oxidoreductase gene, ptxD as a bio-contained chloroplast marker and crop-protection tool for algal biotechnology using Chlamydomonas. Appl. Microbiol. Biotechnol. 2020;104:675–686. doi: 10.1007/s00253-019-10258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jackson H.O., Taunt H.N., Mordaka P.M., Kumari S., Smith A.G., Purton S. CpPosNeg: A positive-negative selection strategy allowing multiple cycles of marker-free engineering of the Chlamydomonas plastome. Biotechnol. J. 2022;17 doi: 10.1002/biot.202200088. [DOI] [PubMed] [Google Scholar]
- 71.Jackson H.O., Taunt H.N., Mordaka P.M., Smith A.G., Purton S. The Algal Chloroplast as a Testbed for Synthetic Biology Designs Aimed at Radically Rewiring Plant Metabolism. Front. Plant Sci. 2021;12:708370–708415. doi: 10.3389/fpls.2021.708370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guo C., Zhang G., Wang H., Mei R., Li X., Li H., Jia B., Wang C., Hu Z. The “replacing surgery” of cpDNA: de novo chemical synthesis and in vivo functional testing of Chlamydomonas chloroplast genome. bioRxiv. 2023 doi: 10.1101/2023.01.05.522807. Preprint at. [DOI] [Google Scholar]
- 73.Merchant S.S., Prochnik S.E., Vallon O., Harris E.H., Karpowicz S.J., Witman G.B., Terry A., Salamov A., Fritz-Laylin L.K., Maréchal-Drouard L., et al. The Chlamydomonas Genome Reveals the Evolution of Key Animal and Plant Functions. Science. 2007;318:245–250. doi: 10.1126/science.1143609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fuentes C., Vanwinkle-swift K. Isolation and characterization of a cell wall-defective mutant of Chlamydomonas monoica (Chlorophyta)1. J. Phycol. 2003;39:1261–1267. doi: 10.1111/j.0022-3646.2003.03-087.x. [DOI] [Google Scholar]
- 75.Zabawinski C., Van Den Koornhuyse N., D’Hulst C., Schlichting R., Giersch C., Delrue B., Lacroix J.-M., Preiss J., Ball S. Starchless Mutants of Chlamydomonas reinhardtii Lack the Small Subunit of a Heterotetrameric ADP-Glucose Pyrophosphorylase. J. Bacteriol. 2001;183:1069–1077. doi: 10.1128/JB.183.3.1069-1077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Niyogi K.K., Bjorkman O., Grossman A.R. Chlamydomonas Xanthophyll Cycle Mutants Identified by Video Imaging of Chlorophyll Fluorescence Quenching. Plant Cell. 1997;9:1369–1380. doi: 10.1105/tpc.9.8.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li X., Moellering E.R., Liu B., Johnny C., Fedewa M., Sears B.B., Kuo M.-H., Benning C. A Galactoglycerolipid Lipase Is Required for Triacylglycerol Accumulation and Survival Following Nitrogen Deprivation in Chlamydomonas reinhardtii. Plant Cell. 2012;24:4670–4686. doi: 10.1105/tpc.112.105106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moellering E.R., Miller R., Benning C. In: Lipids in Photosynthesis Advances in Photosynthesis and Respiration. Wada H., Murata N., editors. Springer Netherlands; 2009. Molecular Genetics of Lipid Metabolism in the Model Green Alga Chlamydomonas reinhardtii; pp. 139–155. [DOI] [Google Scholar]
- 79.Kindle K.L., Schnell R.A., Fernández E., Lefebvre P.A. Stable nuclear transformation of Chlamydomonas using the Chlamydomonas gene for nitrate reductase. J. Cell Biol. 1989;109:2589–2601. doi: 10.1083/jcb.109.6.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lumbreras V., Stevens D.R., Purton S. Efficient foreign gene expression in Chlamydomonas reinhardtii mediated by an endogenous intron: Foreign gene expression in Chlamydomonas. Plant J. 1998;14:441–447. doi: 10.1046/j.1365-313X.1998.00145.x. [DOI] [Google Scholar]
- 81.Stevens D.R., Rochaix J.D., Purton S. The bacterial phleomycin resistance gene ble as a dominant selectable marker in Chlamydomonas. Mol. Gen. Genet. 1996;251:23–30. doi: 10.1007/BF02174340. [DOI] [PubMed] [Google Scholar]
- 82.Berthold P., Schmitt R., Mages W. An Engineered Streptomyces hygroscopicus aph 7″ Gene Mediates Dominant Resistance against Hygromycin B in Chlamydomonas reinhardtii. Protist. 2002;153:401–412. doi: 10.1078/14344610260450136. [DOI] [PubMed] [Google Scholar]
- 83.Sizova I., Fuhrmann M., Hegemann P. A Streptomyces rimosus aphVIII gene coding for a new type phosphotransferase provides stable antibiotic resistance to Chlamydomonas reinhardtii. Gene. 2001;277:221–229. doi: 10.1016/S0378-1119(01)00616-3. [DOI] [PubMed] [Google Scholar]
- 84.Labadorf A., Link A., Rogers M.F., Thomas J., Reddy A.S., Ben-Hur A. Genome-wide analysis of alternative splicing in Chlamydomonas reinhardtii. BMC Genom. 2010;11:114. doi: 10.1186/1471-2164-11-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eichler-Stahlberg A., Weisheit W., Ruecker O., Heitzer M. Strategies to facilitate transgene expression in Chlamydomonas reinhardtii. Planta. 2009;229:873–883. doi: 10.1007/s00425-008-0879-x. [DOI] [PubMed] [Google Scholar]
- 86.Fuhrmann M., Oertel W., Hegemann P. A synthetic gene coding for the green fluorescent protein (GFP) is a versatile reporter in Chlamydomonas reinhardtii. Plant J. 1999;19:353–361. doi: 10.1046/j.1365-313X.1999.00526.x. [DOI] [PubMed] [Google Scholar]
- 87.Lauersen K.J., Kruse O., Mussgnug J.H. Targeted expression of nuclear transgenes in Chlamydomonas reinhardtii with a versatile, modular vector toolkit. Appl. Microbiol. Biotechnol. 2015;99:3491–3503. doi: 10.1007/s00253-014-6354-7. [DOI] [PubMed] [Google Scholar]
- 88.Baier T., Wichmann J., Kruse O., Lauersen K.J. Intron-containing algal transgenes mediate efficient recombinant gene expression in the green microalga Chlamydomonas reinhardtii. Nucleic Acids Res. 2018;46:6909–6919. doi: 10.1093/nar/gky532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Neupert J., Gallaher S.D., Lu Y., Strenkert D., Segal N., Barahimipour R., Fitz-Gibbon S.T., Schroda M., Merchant S.S., Bock R. An epigenetic gene silencing pathway selectively acting on transgenic DNA in the green alga Chlamydomonas. Nat. Commun. 2020;11:6269. doi: 10.1038/s41467-020-19983-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Neupert J., Karcher D., Bock R. Generation of Chlamydomonas strains that efficiently express nuclear transgenes. Plant J. 2009;57:1140–1150. doi: 10.1111/j.1365-313X.2008.03746.x. [DOI] [PubMed] [Google Scholar]
- 91.Craig R.J., Gallaher S.D., Shu S., Salomé P.A., Jenkins J.W., Blaby-Haas C.E., Purvine S.O., O’Donnell S., Barry K., Grimwood J., et al. The Chlamydomonas Genome Project, version 6: Reference assemblies for mating-type plus and minus strains reveal extensive structural mutation in the laboratory. Plant Cell. 2023;35:644–672. doi: 10.1093/plcell/koac347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mussgnug J.H. Genetic tools and techniques for Chlamydomonas reinhardtii. Appl. Microbiol. Biotechnol. 2015;99:5407–5418. doi: 10.1007/s00253-015-6698-7. [DOI] [PubMed] [Google Scholar]
- 93.Perozeni F., Baier T. Current Nuclear Engineering Strategies in the Green Microalga Chlamydomonas reinhardtii. life. 2023;13 doi: 10.3390/life13071566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jaeger D., Baier T., Lauersen K.J. Intronserter, an advanced online tool for design of intron containing transgenes. Algal Res. 2019;42 doi: 10.1016/j.algal.2019.101588. [DOI] [Google Scholar]
- 95.Gutiérrez S., Wellman G.B., Lauersen K.J. Teaching an old ‘doc’ new tricks for algal biotechnology: Strategic filter use enables multi-scale fluorescent protein signal detection. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.979607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rasala B.A., Barrera D.J., Ng J., Plucinak T.M., Rosenberg J.N., Weeks D.P., Oyler G.A., Peterson T.C., Haerizadeh F., Mayfield S.P. Expanding the spectral palette of fluorescent proteins for the green microalga Chlamydomonas reinhardtii. Plant J. 2013;74:545–556. doi: 10.1111/tpj.12165. [DOI] [PubMed] [Google Scholar]
- 97.Shin S.-E., Lim J.-M., Koh H.G., Kim E.K., Kang N.K., Jeon S., Kwon S., Shin W.-S., Lee B., Hwangbo K., et al. CRISPR/Cas9-induced knockout and knock-in mutations in Chlamydomonas reinhardtii. Sci. Rep. 2016;6 doi: 10.1038/srep27810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nievergelt A.P., Diener D.R., Bogdanova A., Brown T., Pigino G. Efficient precision editing of endogenous Chlamydomonas reinhardtii genes with CRISPR-Cas. Cell Rep. Methods. 2023;3 doi: 10.1016/j.crmeth.2023.100562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nievergelt A.P., Diener D.R., Bogdanova A., Brown T., Pigino G. Protocol for precision editing of endogenous Chlamydomonas reinhardtii genes with CRISPR-Cas. STAR Protoc. 2023;5 doi: 10.1016/j.xpro.2023.102774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li X., Patena W., Fauser F., Jinkerson R.E., Saroussi S., Meyer M.T., Ivanova N., Robertson J.M., Yue R., Zhang R., et al. A genome-wide algal mutant library and functional screen identifies genes required for eukaryotic photosynthesis. Nat. Genet. 2019;51:627–635. doi: 10.1038/s41588-019-0370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dementyeva P., Freudenberg R.A., Baier T., Rojek K., Wobbe L., Weisshaar B., Kruse O. A novel, robust and mating-competent Chlamydomonas reinhardtii strain with an enhanced transgene expression capacity for algal biotechnology. Biotechnol. Rep. 2021;31 doi: 10.1016/j.btre.2021.e00644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Charoonnart P., Worakajit N., Zedler J.A.Z., Meetam M., Robinson C., Saksmerprome V. Generation of microalga Chlamydomonas reinhardtii expressing shrimp antiviral dsRNA without supplementation of antibiotics. Sci. Rep. 2019;9:3164. doi: 10.1038/s41598-019-39539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gumpel N.J., Rochaix J.D., Purton S. Studies on homologous recombination in the green alga Chlamydomonas reinhardtii. Curr. Genet. 1994;26:438–442. doi: 10.1007/BF00309931. [DOI] [PubMed] [Google Scholar]
- 104.Choo J.H., Han C., Kim J.-Y., Kang H.A. Deletion of a KU80 homolog enhances homologous recombination in the thermotolerant yeast Kluyveromyces marxianus. Biotechnol. Lett. 2014;36:2059–2067. doi: 10.1007/s10529-014-1576-4. [DOI] [PubMed] [Google Scholar]
- 105.Chen H., Yang Q.-L., Xu J.-X., Deng X., Zhang Y.-J., Liu T., Rots M.G., Xu G.-L., Huang K.-Y. Efficient methods for multiple types of precise gene-editing in Chlamydomonas. Plant J. 2023;115:846–865. doi: 10.1111/tpj.16265. [DOI] [PubMed] [Google Scholar]
- 106.Sizova I., Kelterborn S., Verbenko V., Kateriya S., Hegemann P. Chlamydomonas POLQ is necessary for CRISPR/Cas9-mediated gene targeting. G3 (Bethesda). 2021;11:jkab114. doi: 10.1093/g3journal/jkab114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ferenczi A., Chew Y.P., Kroll E., von Koppenfels C., Hudson A., Molnar A. Mechanistic and genetic basis of single-strand templated repair at Cas12a-induced DNA breaks in Chlamydomonas reinhardtii. Nat. Commun. 2021;12 doi: 10.1038/s41467-021-27004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Blanc-Mathieu R., Verhelst B., Derelle E., Rombauts S., Bouget F.-Y., Carré I., Château A., Eyre-Walker A., Grimsley N., Moreau H., et al. An improved genome of the model marine alga Ostreococcus tauri unfolds by assessing Illumina de novo assemblies. BMC Genom. 2014;15:1103. doi: 10.1186/1471-2164-15-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Foflonker F., Mollegard D., Ong M., Yoon H.S., Bhattacharya D. Genomic Analysis of Picochlorum Species Reveals How Microalgae May Adapt to Variable Environments. Mol. Biol. Evol. 2018;35:2702–2711. doi: 10.1093/molbev/msy167. [DOI] [PubMed] [Google Scholar]
- 110.Suzuki S., Endoh R., Manabe R.I., Ohkuma M., Hirakawa Y. Multiple losses of photosynthesis and convergent reductive genome evolution in the colourless green algae Prototheca. Sci. Rep. 2018;8:940. doi: 10.1038/s41598-017-18378-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bhattacharya D., Price D.C., Chan C.X., Qiu H., Rose N., Ball S., Weber A.P.M., Arias M.C., Henrissat B., Coutinho P.M., et al. Genome of the red alga Porphyridium purpureum. Nat. Commun. 2013;4:1941. doi: 10.1038/ncomms2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Matsuzaki M., Misumi O., Shin-i T., Maruyama S., Takahara M., Miyagishima S.Y., Mori T., Nishida K., Yagisawa F., Nishida K., et al. Genome sequence of the ultrasmall unicellular red alga Cyanidioschyzon merolae 10D. Nature. 2004;428:653–657. doi: 10.1038/nature02398. [DOI] [PubMed] [Google Scholar]
- 113.Downing J.M., Lock S.C.L., Iovinella M., Davey J., Mackinder L.C.M., Chong J.P.J., Ashton P.D., Feichtinger G.A., James S., Jeffares D., et al. Comparisons between Complete Genomes of the Eukaryotic Extremophile Galdieria sulphuraria Reveals Complex Nuclear Chromosomal Structures. bioRxiv. 2022 doi: 10.1101/2022.10.04.510839. Preprint at. [DOI] [Google Scholar]
- 114.Hillson N., Caddick M., Cai Y., Carrasco J.A., Chang M.W., Curach N.C., Bell D.J., Le Feuvre R., Friedman D.C., Fu X., et al. Building a global alliance of biofoundries. Nat. Commun. 2019;10:2040. doi: 10.1038/s41467-019-10079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Herdean A., Sutherland D.L., Ralph P.J. Phenoplate: An innovative method for assessing interacting effects of temperature and light on non-photochemical quenching in microalgae under chemical stress. N. Biotechnol. 2022;66:89–96. doi: 10.1016/j.nbt.2021.10.004. [DOI] [PubMed] [Google Scholar]
- 116.Fauser F., Vilarrasa-Blasi J., Onishi M., Ramundo S., Patena W., Millican M., Osaki J., Philp C., Nemeth M., Salomé P.A., et al. Systematic characterization of gene function in the photosynthetic alga Chlamydomonas reinhardtii. Nat. Genet. 2022;54:705–714. doi: 10.1038/s41588-022-01052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kong F., Li M., Liu K., Ge Y., Yamasaki T., Beyly-Adriano A., Ohama T., Li-Beisson Y. Efficient approaches for nuclear transgene stacking in the unicellular green microalga Chlamydomonas reinhardtii. Algal Res. 2023;71 doi: 10.1016/j.algal.2023.103048. [DOI] [Google Scholar]
- 118.Matagne R.F., Deltour R., Ledoux L. Somatic fusion between cell wall mutants of Chlamydomonas reinhardtii. Nature. 1979;278:344–346. doi: 10.1038/278344a0. [DOI] [Google Scholar]
- 119.Schindler D., Walker R.S.K., Jiang S., Brooks A.N., Wang Y., Müller C.A., Cockram C., Luo Y., García A., Schraivogel D., et al. Design, Construction, and Functional Characterization of a tRNA Neochromosome in Yeast. Cell. 2023;186:5237–5253.e22. doi: 10.1016/j.cell.2023.10.015. [DOI] [PubMed] [Google Scholar]
- 120.Shen Y., Stracquadanio G., Wang Y., Yang K., Mitchell L.A., Xue Y., Cai Y., Chen T., Dymond J.S., Kang K., et al. SCRaMbLE generates designed combinatorial stochastic diversity in synthetic chromosomes. Genome Res. 2016;26:36–49. doi: 10.1101/gr.193433.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Coradini A.L.V., Ville C.N., Krieger Z.A., Roemer J., Hull C., Yang S., Lusk D.T., Ehrenreich I.M. Building synthetic chromosomes from natural DNA. Nat. Commun. 2023;14:8337. doi: 10.1038/s41467-023-44112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Richardson S.M., Mitchell L.A., Stracquadanio G., Yang K., Dymond J.S., DiCarlo J.E., Lee D., Huang C.L.V., Chandrasegaran S., Cai Y., et al. Design of a synthetic yeast genome. Science. 2017;355:1040–1044. doi: 10.1126/science.aaf4557. [DOI] [PubMed] [Google Scholar]
- 123.Boeke J.D., La Croute F., Fink G.R. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol. Gen. Genet. 1984;197:345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- 124.Toyn J.H., Gunyuzlu P.L., Hunter White W., Thompson L.A., Hollis G.F. A counterselection for the tryptophan pathway in yeast: 5-fluoroanthranilic acid resistance. Yeast. 2000;16:553–560. doi: 10.1002/(SICI)1097-0061(200004)16:6<553::AID-YEA554>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 125.DiCarlo J.E., Norville J.E., Mali P., Rios X., Aach J., Church G.M. Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Res. 2013;41:4336–4343. doi: 10.1093/nar/gkt135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mitchell L.A., Wang A., Stracquadanio G., Kuang Z., Wang X., Yang K., Richardson S., Martin J.A., Zhao Y., Walker R., et al. Synthesis, debugging, and effects of synthetic chromosome consolidation: synVI and beyond. Science. 2017;355 doi: 10.1126/science.aaf4831. [DOI] [PubMed] [Google Scholar]
- 127.Zhang W., Zhao G., Luo Z., Lin Y., Wang L., Guo Y., Wang A., Jiang S., Jiang Q., Gong J., et al. Engineering the ribosomal DNA in a megabase synthetic chromosome. Science. 2017;355 doi: 10.1126/science.aaf3981. [DOI] [PubMed] [Google Scholar]
- 128.Clayton S., Lin Y.-C., Follows M.J., Worden A.Z. Co-existence of distinct Ostreococcus ecotypes at an oceanic front: Ocean fronts promote biodiversity. Limnol. Oceanogr. 2017;62:75–88. doi: 10.1002/lno.10373. [DOI] [Google Scholar]
- 129.Morant P.-E., Thommen Q., Pfeuty B., Vandermoere C., Corellou F., Bouget F.-Y., Lefranc M. A robust two-gene oscillator at the core of Ostreococcus tauri circadian clock. Chaos. 2010;20 doi: 10.1063/1.3530118. [DOI] [PubMed] [Google Scholar]
- 130.Smallwood C.R., Hill E.A., Chrisler W., Brookreson J., Evans J.E. Optimizing bioreactor growth of the smallest eukaryote. bioRxiv. 2018 doi: 10.1101/291211. Preprint at. [DOI] [Google Scholar]
- 131.Farinas B., Mary C., De O Manes C.-L., Bhaud Y., Peaucellier G., Moreau H. Natural Synchronisation for the Study of Cell Division in the Green Unicellular Alga Ostreococcus tauri. Plant Mol. Biol. 2006;60:277–292. doi: 10.1007/s11103-005-4066-1. [DOI] [PubMed] [Google Scholar]
- 132.van Ooijen G., Knox K., Kis K., Bouget F.-Y., Millar A.J. Genomic Transformation of the Picoeukaryote Ostreococcus tauri. JoVE. 2012;65 doi: 10.3791/4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lozano J.-C., Schatt P., Botebol H., Vergé V., Lesuisse E., Blain S., Carré I.A., Bouget F.-Y. Efficient gene targeting and removal of foreign DNA by homologous recombination in the picoeukaryote Ostreococcus. Plant J. 2014;78:1073–1083. doi: 10.1111/tpj.12530. [DOI] [PubMed] [Google Scholar]
- 134.Derelle E., Ferraz C., Rombauts S., Rouzé P., Worden A.Z., Robbens S., Partensky F., Degroeve S., Echeynié S., Cooke R., et al. Genome analysis of the smallest free-living eukaryote Ostreococcus tauri unveils many unique features. Proc. Natl. Acad. Sci. USA. 2006;103:11647–11652. doi: 10.1073/pnas.0604795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.da Roza P.A., Goold H.D., Paulsen I.T. Picochlorum sp. SENEW3. Trends Genet. 2022;38:209–210. doi: 10.1016/j.tig.2021.09.012. [DOI] [PubMed] [Google Scholar]
- 136.Weissman J.C., Likhogrud M., Thomas D.C., Fang W., Karns D.A., Chung J.W., Nielsen R., Posewitz M.C. High-light selection produces a fast-growing Picochlorum celeri. Algal Res. 2018;36:17–28. doi: 10.1016/j.algal.2018.09.024. [DOI] [Google Scholar]
- 137.Becker S.A., Spreafico R., Kit J.L., Brown R., Likhogrud M., Fang W., Posewitz M.C., Weissman J.C., Radakovits R. Phased Diploid Genome Sequence for the Fast-Growing Microalga Picochlorum celeri. Microbiol. Resour. Announc. 2020;9 doi: 10.1128/MRA.00087-20. 00087-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Barten R., van Workum D.-J.M., de Bakker E., Risse J., Kleisman M., Navalho S., Smit S., Wijffels R.H., Nijveen H., Barbosa M.J. Genetic mechanisms underlying increased microalgal thermotolerance, maximal growth rate, and yield on light following adaptive laboratory evolution. BMC Biol. 2022;20:242. doi: 10.1186/s12915-022-01431-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dahlin L.R., Guarnieri M.T. Development of the high-productivity marine microalga, Picochlorum renovo, as a photosynthetic protein secretion platform. Algal Res. 2021;54 doi: 10.1016/j.algal.2021.102197. [DOI] [Google Scholar]
- 140.Krishnan A., Cano M., Burch T.A., Weissman J.C., Posewitz M.C. Genome editing using Cas9-RNA ribonucleoprotein complexes in the high-productivity marine alga Picochlorum celeri. Algal Res. 2020;49 doi: 10.1016/j.algal.2020.101944. [DOI] [Google Scholar]
- 141.Franklin S., Somanchi A., Wee J., Rudenko G., Moseley J.L., Rakitsky W., Zhao X., Bhat R. Tailored oils produced from recombinant oleaginous microorganisms. US patent US20190100780 A1. 2019 filed September 7, 2018, and granted April 4, 2019. [Google Scholar]
- 142.Bumbak F., Cook S., Zachleder V., Hauser S., Kovar K. Best practices in heterotrophic high-cell-density microalgal processes: achievements, potential and possible limitations. Appl. Microbiol. Biotechnol. 2011;91:31–46. doi: 10.1007/s00253-011-3311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Running J.A., Severson D.K., Schneider K.J. Extracellular production of L -ascorbic acid by Chlorella protothecoides, Prototheca species, and mutants of P. moriformis during aerobic culturing at low pH. J. Ind. Microbiol. Biotechnol. 2002;29:93–98. doi: 10.1038/sj.jim.7000275. [DOI] [PubMed] [Google Scholar]
- 144.Bakuła Z., Siedlecki P., Gromadka R., Gawor J., Gromadka A., Pomorski J.J., Panagiotopoulou H., Jagielski T. A first insight into the genome of Prototheca wickerhamii, a major causative agent of human protothecosis. BMC Genom. 2021;22:168. doi: 10.1186/s12864-021-07491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chua P., Somanchi A. Genetically engineered microorganisms that metabolize xylose. US patent US9499845 B2. 2016 filed September 1, 2014, and granted November 22, 2016. [Google Scholar]
- 146.Franklin S., Somanchi A., Wee J., Rudenko G., Moseley J.L., Rakitsky W. Tailored oils produced from recombinant heterotrophic microorganisms. US patent US9657299 B2. 2017 filed December 18, 2015, and granted May 23, 2017. [Google Scholar]
- 147.Davis D., Rudenko G.N., Somanchi A., Casolari J., Franklin S., Ewing A. Ketoacyl ACP synthase genes and uses thereof. US patent US9969990 B2. 2019 filed April 10, 2018, and granted June 11, 2019. [Google Scholar]
- 148.Ferreira J., Wee J.L., KO N.-H. β-ketoacyl-ACP synthase IV variants. WIPO patent application publication WO2021146520 A1. 2021 filed January 15, 2021, and published July 22, 2021. [Google Scholar]
- 149.Franklin S., Rakitsky W., Rudenko G., Zhao X., Rodriguez F.A., Lu W., Wee J. Microbial oils with lowered pour points, dielectric fluids produced therefrom, and related methods. US patent US10167489 B2. 2019 filed June 10, 2016, and granted January 1, 2019. [Google Scholar]
- 150.Franklin S., Bhat R., Zhao X. Oleaginous microalgae having an LPAAT ablation. US patent application publication US20160348119 A1. 2016 filed April 6, 2016, and published December 1, 2016. [Google Scholar]
- 151.Richardson C.E., Hennebelle M., Otoki Y., Zamora D., Yang J., Hammock B.D., Taha A.Y. Lipidomic Analysis of Oxidized Fatty Acids in Plant and Algae Oils. J. Agric. Food Chem. 2017;65:1941–1951. doi: 10.1021/acs.jafc.6b05559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Moseley J.L., Casolari J., Zhao X., Ewing A., Somanchi A., Franklin S., Davis D. Novel acyltransferases, variant thioesterases, and uses thereof. US patent application publication US20180142218 A1. 2018 filed October 4, 2017, and published May 24, 2018. [Google Scholar]
- 153.Ghazani S.M., Zou L., Rakitsky W.G., Marangoni A.G. Algal Butter, a Novel Cocoa Butter Equivalent: Chemical Composition, Physical Properties, and Functionality in Chocolate. J. Americ. Oil Chem. Soc. 2018;95:1239–1251. doi: 10.1002/aocs.12127. [DOI] [Google Scholar]
- 154.Rudenko G., Davis D., Cho C., Kong I., Pandey A., Frykman S. Neurotransmitters and methods of making the same. WIPO patent application publication WO2023064731 A1. 2023 filed October 10, 2022, and published April 2023. [Google Scholar]
- 155.Ahmad I., Hellebust J.A. Regulation of Chloroplast Development by Nitrogen Source and Growth Conditions in a Chlorella protothecoides Strain. Plant Physiol. 1990;94:944–949. doi: 10.1104/pp.94.3.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Krzemińska I., Piasecka A., Nosalewicz A., Simionato D., Wawrzykowski J. Alterations of the lipid content and fatty acid profile of Chlorella protothecoides under different light intensities. Bioresour. Technol. 2015;196:72–77. doi: 10.1016/j.biortech.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 157.Gris B., Sforza E., Vecchiato L., Bertucco A. Development of a Process for an Efficient Exploitation of CO2 Captured from Flue Gases as Liquid Carbonates for Chlorella protothecoides Cultivation. Ind. Eng. Chem. Res. 2014;53:16678–16688. doi: 10.1021/ie502336d. [DOI] [Google Scholar]
- 158.Vogler B.W., Starkenburg S.R., Sudasinghe N., Schambach J.Y., Rollin J.A., Pattathil S., Barry A.N. Characterization of plant carbon substrate utilization by Auxenochlorella protothecoides. Algal Res. 2018;34:37–48. doi: 10.1016/j.algal.2018.07.001. [DOI] [Google Scholar]
- 159.Matsuka M., Otsuka H., Hase E. Changes in contents of carbohydrate and fatty acid in the cells of Chlorella protothecoides during the processes of de- and re-generation of chloroplasts. Plant Cell Physiol. 1966;7:651–662. doi: 10.1093/oxfordjournals.pcp.a079217. [DOI] [Google Scholar]
- 160.Cazzaniga S., Perozeni F., Baier T., Ballottari M. Engineering astaxanthin accumulation reduces photoinhibition and increases biomass productivity under high light in Chlamydomonas reinhardtii. Biotechnol. Biofuels. 2022;15:77. doi: 10.1186/s13068-022-02173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tran N.T., Kaldenhoff R. Metabolic engineering of ketocarotenoids biosynthetic pathway in Chlamydomonas reinhardtii strain CC-4102. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-67756-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xiong W., Gao C., Yan D., Wu C., Wu Q. Double CO2 fixation in photosynthesis–fermentation model enhances algal lipid synthesis for biodiesel production. Bioresour. Technol. 2010;101:2287–2293. doi: 10.1016/j.biortech.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 163.Gao C., Wang Y., Shen Y., Yan D., He X., Dai J., Wu Q. Oil accumulation mechanisms of the oleaginous microalga Chlorella protothecoides revealed through its genome, transcriptomes, and proteomes. BMC Genom. 2014;15:582. doi: 10.1186/1471-2164-15-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xing G., Yuan H., Yang J., Li J., Gao Q., Li W., Wang E. Integrated analyses of transcriptome, proteome and fatty acid profilings of the oleaginous microalga Auxenochlorella protothecoides UTEX 2341 reveal differential reprogramming of fatty acid metabolism in response to low and high temperatures. Algal Res. 2018;33:16–27. doi: 10.1016/j.algal.2018.04.028. [DOI] [Google Scholar]
- 165.Li Z., Bock R. Replication of bacterial plasmids in the nucleus of the red alga Porphyridium purpureum. Nat. Commun. 2018;9:3451. doi: 10.1038/s41467-018-05651-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jeon M.S., Han S.-I., Jeon M., Choi Y.-E. Enhancement of phycoerythrin productivity in Porphyridium purpureum using the clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 ribonucleoprotein system. Bioresour. Technol. 2021;330 doi: 10.1016/j.biortech.2021.124974. [DOI] [PubMed] [Google Scholar]
- 167.Coward T., Fuentes-Grünewald C., Silkina A., Oatley-Radcliffe D.L., Llewellyn G., Lovitt R.W. Utilising light-emitting diodes of specific narrow wavelengths for the optimization and co-production of multiple high-value compounds in Porphyridium purpureum. Bioresour. Technol. 2016;221:607–615. doi: 10.1016/j.biortech.2016.09.093. [DOI] [PubMed] [Google Scholar]
- 168.Jiao K., Xiao W., Xu Y., Zeng X., Ho S.H., Laws E.A., Lu Y., Ling X., Shi T., Sun Y., et al. Using a trait-based approach to optimize mixotrophic growth of the red microalga Porphyridium purpureum towards fatty acid production. Biotechnol. Biofuels. 2018;11:273–311. doi: 10.1186/s13068-018-1277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li S., Ji L., Shi Q., Wu H., Fan J. Advances in the production of bioactive substances from marine unicellular microalgae Porphyridium spp. Bioresour. Technol. 2019;292 doi: 10.1016/j.biortech.2019.122048. [DOI] [PubMed] [Google Scholar]
- 170.Miyagishima S.-Y., Tanaka K. The Unicellular Red Alga Cyanidioschyzon merolae— The Simplest Model of a Photosynthetic Eukaryote. Plant Cell Physiol. 2021;62:926–941. doi: 10.1093/pcp/pcab052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Misumi O., Matsuzaki M., Nozaki H., Miyagishima S.y., Mori T., Nishida K., Yagisawa F., Yoshida Y., Kuroiwa H., Kuroiwa T. Cyanidioschyzon merolae Genome. A Tool for Facilitating Comparable Studies on Organelle Biogenesis in Photosynthetic Eukaryotes. Plant Physiol. 2005;137:567–585. doi: 10.1104/pp.104.053991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Minoda A., Sakagami R., Yagisawa F., Kuroiwa T., Tanaka K. Improvement of culture conditions and evidence for nuclear transformation by homologous recombination in a red alga, Cyanidioschyzon merolae 10D. Plant Cell Physiol. 2004;45:667–671. doi: 10.1093/pcp/pch087. [DOI] [PubMed] [Google Scholar]
- 173.Fujiwara T., Ohnuma M., Kuroiwa T., Ohbayashi R., Hirooka S., Miyagishima S.-Y. Development of a Double Nuclear Gene-Targeting Method by Two-Step Transformation Based on a Newly Established Chloramphenicol-Selection System in the Red Alga Cyanidioschyzon merolae. Front. Plant Sci. 2017;8 doi: 10.3389/fpls.2017.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fujiwara T., Hirooka S., Mukai M., Ohbayashi R., Kanesaki Y., Watanabe S., Miyagishima S.Y. Integration of a Galdieria plasma membrane sugar transporter enables heterotrophic growth of the obligate photoautotrophic red alga Cynanidioschyzon merolae. Plant Direct. 2019;3 doi: 10.1002/pld3.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sumiya N., Kawase Y., Hayakawa J., Matsuda M., Nakamura M., Era A., Tanaka K., Kondo A., Hasunuma T., Imamura S., Miyagishima S.y. Expression of Cyanobacterial Acyl-ACP Reductase Elevates the Triacylglycerol Level in the Red Alga Cyanidioschyzon merolae. Plant Cell Physiol. 2015;56:1962–1980. doi: 10.1093/pcp/pcv120. [DOI] [PubMed] [Google Scholar]
- 176.Rossoni A.W., Price D.C., Seger M., Lyska D., Lammers P., Bhattacharya D., Weber A.P. The genomes of polyextremophilic cyanidiales contain 1% horizontally transferred genes with diverse adaptive functions. eLife. 2019;8 doi: 10.7554/eLife.45017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Abiusi F., Trompetter E., Hoenink H., Wijffels R.H., Janssen M. Autotrophic and mixotrophic biomass production of the acidophilic Galdieria sulphuraria ACUF 64. Algal Res. 2021;60 doi: 10.1016/j.algal.2021.102513. [DOI] [Google Scholar]
- 178.Graziani G., Schiavo S., Nicolai M.A., Buono S., Fogliano V., Pinto G., Pollio A. Microalgae as human food: chemical and nutritional characteristics of the thermo-acidophilic microalga Galdieria sulphuraria. Food Funct. 2013;4:144–152. doi: 10.1039/C2FO30198A. [DOI] [PubMed] [Google Scholar]
- 179.Lammers P., Seger M., Park W., Csakan N. Methods of increasing biomass productivity in algae cultures. US patent US11814616 B2. 2023 filed December 14, 2018, and granted November 14, 2023. [Google Scholar]
- 180.Magoni C., Bertacchi S., Giustra C.M., Guzzetti L., Cozza R., Ferrari M., Torelli A., Marieschi M., Porro D., Branduardi P., Labra M. Could microalgae be a strategic choice for responding to the demand for omega-3 fatty acids? A European perspective. Trends Food Sci. Technol. 2022;121:142–155. doi: 10.1016/j.tifs.2022.01.030. [DOI] [Google Scholar]
- 181.Kato S., Misumi O., Maruyama S., Nozaki H., Tsujimoto-Inui Y., Takusagawa M., Suzuki S., Kuwata K., Noda S., Ito N., et al. Genomic analysis of an ultrasmall freshwater green alga, Medakamo hakoo. Commun. Biol. 2023;6:89. doi: 10.1038/s42003-022-04367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hughes R.A., Ellington A.D. Synthetic DNA Synthesis and Assembly: Putting the Synthetic in Synthetic Biology. Cold Spring Harb. Perspect. Biol. 2017;9:a023812. doi: 10.1101/cshperspect.a023812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhang J., Hansen L.G., Gudich O., Viehrig K., Lassen L.M.M., Schrübbers L., Adhikari K.B., Rubaszka P., Carrasquer-Alvarez E., Chen L., et al. A microbial supply chain for production of the anti-cancer drug vinblastine. Nature. 2022;609:341–347. doi: 10.1038/s41586-022-05157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mitchell L.A., Boeke J.D. Circular permutation of a synthetic eukaryotic chromosome with the telomerator. Proc. Natl. Acad. Sci. USA. 2014;111:17003–17010. doi: 10.1073/pnas.1414399111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Agmon N., Temple J., Tang Z., Schraink T., Baron M., Chen J., Mita P., Martin J.A., Tu B.P., Yanai I., et al. Phylogenetic debugging of a complete human biosynthetic pathway transplanted into yeast. Nucleic Acids Res. 2020;48:486–499. doi: 10.1093/nar/gkz1098. gkz1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kutyna D.R., Onetto C.A., Williams T.C., Goold H.D., Paulsen I.T., Pretorius I.S., Johnson D.L., Borneman A.R. Construction of a synthetic Saccharomyces cerevisiae pan-genome neo-chromosome. Nat. Commun. 2022;13:3628. doi: 10.1038/s41467-022-31305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lartigue C., Glass J.I., Alperovich N., Pieper R., Parmar P.P., Hutchison C.A., Smith H.O., Venter J.C. Genome Transplantation in Bacteria: Changing One Species to Another. Science. 2007;317:632–638. doi: 10.1126/science.1144622. [DOI] [PubMed] [Google Scholar]
- 188.Breuer M., Earnest T.M., Merryman C., Wise K.S., Sun L., Lynott M.R., Hutchison C.A., Smith H.O., Lapek J.D., Gonzalez D.J., et al. Essential metabolism for a minimal cell. eLife. 2019;8 doi: 10.7554/eLife.36842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wu Y., Zhu R.-Y., Mitchell L.A., Ma L., Liu R., Zhao M., Jia B., Xu H., Li Y.-X., Yang Z.-M., et al. In vitro DNA SCRaMbLE. Nat. Commun. 2018;9:1935. doi: 10.1038/s41467-018-03743-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ma L., Li Y., Chen X., Ding M., Wu Y., Yuan Y.-J. SCRaMbLE generates evolved yeasts with increased alkali tolerance. Microb. Cell Fact. 2019;18:52. doi: 10.1186/s12934-019-1102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jia B., Wu Y., Li B.-Z., Mitchell L.A., Liu H., Pan S., Wang J., Zhang H.-R., Jia N., Li B., et al. Precise control of SCRaMbLE in synthetic haploid and diploid yeast. Nat. Commun. 2018;9:1933. doi: 10.1038/s41467-018-03084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nyerges A., Vinke S., Flynn R., Owen S.V., Rand E.A., Budnik B., Keen E., Narasimhan K., Marchand J.A., Baas-Thomas M., et al. A swapped genetic code prevents viral infections and gene transfer. Nature. 2023;615:720–727. doi: 10.1038/s41586-023-05824-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Balan A., Schenberg A.C.G. A conditional suicide system for Saccharomyces cerevisiae relying on the intracellular production of the Serratia marcescens nuclease. Yeast. 2005;22:203–212. doi: 10.1002/yea.1203. [DOI] [PubMed] [Google Scholar]
- 194.Kristoffersen P., Jensen G.B., Gerdes K., Piškur J. Bacterial Toxin-Antitoxin Gene System as Containment Control in Yeast Cells. Appl. Environ. Microbiol. 2000;66:5524–5526. doi: 10.1128/AEM.66.12.5524-5526.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Xie Z.-X., Li B.-Z., Mitchell L.A., Wu Y., Qi X., Jin Z., Jia B., Wang X., Zeng B.-X., Liu H.-M., et al. “Perfect” designer chromosome V and behavior of a ring derivative. Science. 2017;355 doi: 10.1126/science.aaf4704. [DOI] [PubMed] [Google Scholar]
- 196.Shao Y., Lu N., Wu Z., Cai C., Wang S., Zhang L.-L., Zhou F., Xiao S., Liu L., Zeng X., et al. Creating a functional single-chromosome yeast. Nature. 2018;560:331–335. doi: 10.1038/s41586-018-0382-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.