Abstract
Background
Posttraumatic stress disorder (PTSD) has been associated with ischemic heart disease in women veterans, but evidence for associations with other cardiovascular disorders remains limited in this population. This retrospective longitudinal cohort study evaluated the association of PTSD with incident stroke/transient ischemic attack (TIA) in women veterans.
Methods and Results
Veterans Health Administration electronic health records were used to identify women veterans aged ≥18 years engaged with Veterans Health Administration health care from January 1, 2000 to December 31, 2019. We identified women veterans with and without PTSD without a history of stroke or TIA at start of follow‐up. Propensity score matching was used to match groups on age, race or ethnicity, traditional cardiovascular risk factors, female‐specific risk factors, a range of mental and physical health conditions, and number of prior health care visits. PTSD, stroke, TIA, and risk factors used in propensity score matching were based on diagnostic codes. Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% CIs for associations of PTSD with an incident stroke/TIA composite. Subanalyses considered stroke and TIA separately, plus age‐ and race‐ or ethnicity‐stratified analyses were carried out.
The analytic sample included 208 092 women veterans (104 046 with and 104 046 without PTSD). PTSD was associated with a greater rate of developing stroke/TIA (HR, 1.33 [95% CI, 1.25–1.42], P<0.001). This elevated risk was especially pronounced in women <50 years old and in Hispanic/Latina women.
Conclusions
Findings indicate a strong association of PTSD with incident stroke/TIA in women veterans. Research is needed to determine whether addressing PTSD and its downstream consequences can offset this risk.
Keywords: PTSD, stroke, transient ischemic attack, women veterans
Subject Categories: Cerebrovascular Disease/Stroke
Nonstandard Abbreviations and Acronyms
- PSM
propensity score matching
- VHA
Veterans Health Administration
- XGB
Extreme gradient boosting
Clinical Perspective.
What Is New?
Using longitudinal electronic health record data, we demonstrated that posttraumatic stress disorder was associated with a greater rate of developing stroke (particularly ischemic and undefined stroke) and transient ischemic attack in a large sample of women veterans
Posttraumatic stress disorder emerged as an especially potent risk factor for stroke/transient ischemic attack for women veterans <50 years of age and for Hispanic/Latina women veterans
What Are the Clinical Implications?
Findings highlight the potential benefit of screening for stroke/transient ischemic attack risk in women veterans with posttraumatic stress disorder and emphasize the importance of integrated mental and cardiovascular health care after trauma
Stroke is the fifth leading cause of death in the United States, with 1 individual estimated to die from stroke every 3.5 minutes. 1 , 2 Stroke prevalence is estimated at 3.2% of the adult US population, with ≈795 000 individuals experiencing a new or recurrent stroke event each year in the United States. 3 The effects of stroke can be long lasting, potentially resulting in physical, functional, mental, or cognitive impairment. 3 , 4 , 5 , 6 Additionally, transient ischemic attacks (TIAs) are increasingly recognized as clinical phenomena that result from ischemia and can produce lasting brain injury. 7
Multiple disparities have been observed in rates of stroke/TIA and related outcomes. For example, lifetime risk of stroke is higher in women than in men, 3 and each year, more women die of stroke compared with men. 1 , 2 Women also have worse functional outcomes after stroke than men. 8 Additionally, compared with non‐Hispanic White individuals, higher rates of stroke have been observed in non‐Hispanic Black and Hispanic/Latino individuals. 9 , 10 , 11 Differences in the burden of stroke risk factors such as hypertension, dyslipidemia, diabetes, smoking, physical inactivity, and atrial fibrillation may explain, in part, excess stroke vulnerability in women and individuals of minoritized racial and ethnic groups. 8 , 11 Female‐specific risk factors for stroke have been identified as well, including gestational hypertension and diabetes, earlier age at menarche and menopause, and use of hormone therapy. 12
Growing research has also linked psychosocial factors to stroke, and these factors may be particularly relevant for cardiovascular health in women. 13 , 14 Contingent on experiencing a traumatic event, posttraumatic stress disorder (PTSD) has been linked to risk of cardiovascular disease (CVD), including stroke. 15 The majority of individuals (50%–89%) will experience trauma over their lifespan, 16 , 17 and PTSD is twice as common in women than in men in the general population, with ≈1 in 10 women and 1 in 20 men developing PTSD in their lifetime. 16 Moreover, PTSD is more prevalent in veterans of wars, especially those involved in combat. 18 , 19 , 20 , 21
Across several longitudinal studies, PTSD has been found to precede and predict incident stroke or TIA. 22 , 23 , 24 , 25 , 26 This research has been conducted in large population‐based health registry samples of men and women, 22 , 24 disaster first responders, 26 community‐dwelling women, 25 and predominantly (≈88%) male samples of younger veterans. 23 , 27 However, despite rapidly growing numbers of women veterans, research on PTSD and stroke/TIA in this population is relatively lacking. This empirical gap is notable, as women veterans have been identified as an important, yet underrepresented, population in cardiovascular research and care. Indeed, there have been calls to action to focus research specifically on the unique set of cardiovascular risks faced by women veterans. 28 Notably, women veterans have rates of traditional and psychosocial cardiovascular risk factors, including trauma exposure and PTSD, that are greater compared with either civilian women or men veterans. 28 Women veterans are also younger and more diverse with respect to race and ethnicity than their male counterparts 29 and thus may carry distinct stroke risks compared with men veterans.
In the current study, we addressed this gap by examining the extent to which PTSD was associated with incident stroke, including ischemic and hemorrhagic subtypes, and TIA specifically in women veterans, the fastest growing group within users of the Veterans Health Administration (VHA) health care system. 29 Furthermore, we performed stratified analyses based on age and race or ethnicity to evaluate potential subgroup differences given the more diverse nature of the women veteran population with respect to these demographic factors.
Methods
Data Source
Because of the sensitive nature of the data presented in this article, on request to the corresponding author, data will be available to approved individuals cleared by the National Data Service Research and Development, and the privacy officer at the Department of Veterans Affairs. In this retrospective longitudinal study, national VHA electronic health records (EHRs), including outpatient, inpatient, and purchased care (ie, services received from external clinicians but paid for by the VHA), were used to identify the study cohort of women veterans and obtain relevant variables (as described in a later section). VHA Health Factor data were also used to define smoking status. This study was approved by the University of California, Los Angeles Institutional Review Board. As a retrospective longitudinal analysis of the medical records, consent was not required. Further, we followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines in preparing this article.
Study Cohort
The VHA Corporate Data Warehouse 30 was used to identify a cohort of women veterans aged 18 years or older who engaged with the VHA health care system from January 1, 2000 to December 31, 2019 (Figure 1). Women who did not pass data quality control (eg, invalid death date or age), and those without any encounters after their first health care encounter during the study period, were excluded. An index visit date marking start of follow‐up was selected as either the first visit with a PTSD diagnosis for women with PTSD or the first visit occurring at least 1 year after the initial health care encounter for women without PTSD. The 12‐month period preceding the index visit date served as a baseline, whereby data on clinical risk factors for stroke and TIA were assessed. Women with <12 months of baseline data were excluded, as were women who diagnosed with PTSD, stroke, or TIA before or during this baseline period. The remaining 630 710 women comprised the prematch sample.
Figure 1. Consolidated Standards of Reporting Trials diagram for creation of the study cohort.
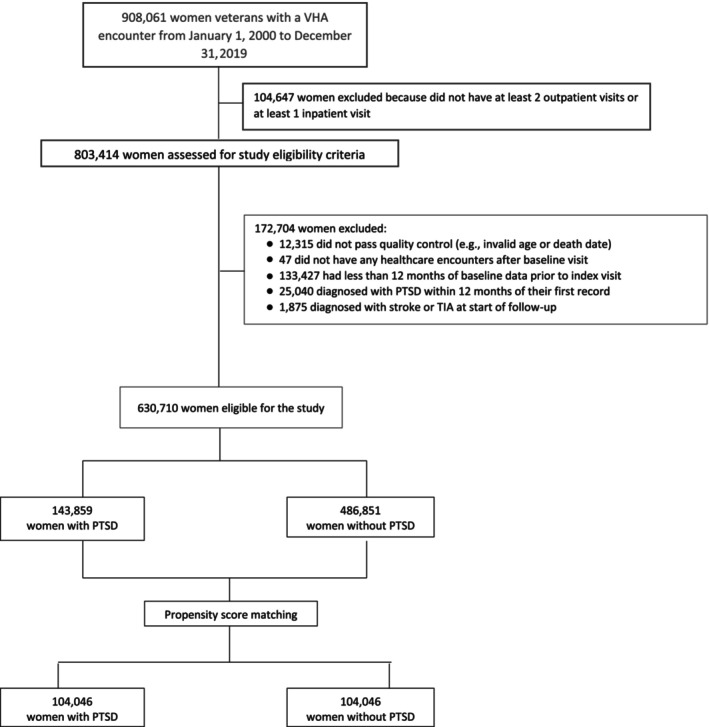
PTSD indicates posttraumatic stress disorder; and TIA, transient ischemic attack.
Variable Definitions
Exposure
Exposure was defined as having a PTSD diagnosis (ever versus never). Consistent with prior VHA EHR research, 31 , 32 , 33 , 34 , 35 , 36 , 37 PTSD was defined using International Classification of Diseases, Ninth Revision (ICD‐9) or Tenth Revision (ICD‐10) diagnosis codes. We required PTSD documentation at 1 or more inpatient or 2 or more outpatient visits. 31 , 32 , 33 , 35 This approach to defining PTSD based on these EHR specifications has been found to have high sensitivity (0.99) and specificity (0.96). 33
Outcomes
Our primary outcome was a composite of incident stroke (ischemic or hemorrhagic) or TIA. Similar to the approach described for PTSD, stroke and TIA were defined by at least 1 inpatient or at least 2 outpatient documentations of ICD‐9 or ICD‐10 diagnosis codes. This approach increases the precision of identifying events, especially in outpatient records, and has been used to define stroke and TIA in prior VHA EHR research. 23 In addition to the composite stroke/TIA outcome, we examined incident stroke, including ischemic, hemorrhagic, and otherwise undefined stroke subtypes, and incident TIA separately as secondary outcomes.
Demographic and Cardiovascular Risk Factors
Demographic factors (age, race or ethnicity), VHA visit characteristics (year of index visit, number of health care encounters during the 12‐month baseline), traditional CVD risk factors (hyperlipidemia, hypertension, diabetes, smoking), female‐specific risk factors (gestational hypertension and diabetes, placental disorders, preeclampsia), and other mental and physical health conditions (anxiety, depression, schizophrenia, alcohol dependence, nonalcohol drug dependence, obesity, chronic kidney disease, hypothyroidism, hyperthyroidism, adrenal and pituitary disorders) were extracted from the EHR and used in propensity score matching (PSM). Age (continuous, plus grouped as 18–29, 30–39, 40–49, 50–59, 60+ years for stratified analyses) and race or ethnicity (categorized as American Indian or Alaskan Native, Asian or Pacific Islander, Hispanic/Latina, non‐Hispanic Black, non‐Hispanic White, unknown) were self‐reported. Year of index visit, along with the number of health care encounters during the baseline 12‐month period, was also extracted from the EHR. As detailed in our paper on PTSD and ischemic heart disease in women veterans, 31 ICD codes were used to define cardiovascular risk factors in the 12 months before and on the index visit date, using the same inpatient and outpatient validation criteria described previously, with 2 exceptions. Obesity was defined by ICD codes or body mass index ≥30, calculated as the most recent weight (in kilograms) at or before the index visit divided by the mean height (in square meters). 32 Smoking status was categorized as current or not current/unknown at index visit based on Health Factors data. 38 , 39 , 40
Statistical Analysis
To derive estimates of the marginal association of PTSD with incident stroke and TIA among women with PTSD diagnosis, we used PSM. Propensity scores were estimated for all 630 710 women meeting study criteria outlined in Figure 1 using extreme gradient boosting (XGB), 41 a tree‐based machine learning algorithm that iteratively models residuals to improve fit. 42 Such machine learning techniques have been demonstrated to produce less biased estimates than standard parametric and semiparametric techniques because they are less prone to misspecification (eg, overfitting, assumptions of linearity). 43 To deter overfitting of the propensity model, we randomly partitioned the sample into training and test sets along a 70/30 split. The training set was used to develop and tune the model, which included as predictors the aforementioned demographic and clinical variables, and the test set was used to evaluate the models' performance on heretofore unseen data. K‐fold, specifically 10‐fold, cross‐validation was applied to the training set to derive optimal values of the XGB hyperparameters corresponding to eta, maximum tree depth, ratio of predictors subsampled, percentage of observations subsampled, and boosting iterations. Hyperparameters were optimized via grid search on accuracy of predictions. Once the optimal model hyperparameters were determined through the cross‐validation process, the model was applied to the full training and test sets, and Cohen's kappa agreement between observed and predicted values was calculated for each. According to Cohen, 44 kappa values near 0 indicate no agreement, 0.41–0.60 moderate agreement, and 0.81–1.00 almost perfect agreement. As a final step, the XGB model was used to generate propensity scores representing predicted probabilities of PTSD status for the entire matched sample.
Nearest neighbor matching was performed on the XGB propensity scores, using a caliper of 0.25 and restricting matches to women with no more than a 2‐year age difference. Standardized mean differences were used to confirm covariate balance among women veterans with and without PTSD. To assess the marginal association of PTSD with the stroke/TIA composite among women veterans, we conducted Cox survival models that accounted for the shared variance among matched pairs using the grouped jackknife method. 45 The Fine–Gray method 46 was used to account for the competing risk of death. The proportional hazards assumption was assessed via examination of Schoenfeld residual plots and tests of proportionality, where statistical significance indicates potential violation. We first modeled time until all stroke/TIA events as a function of PTSD status, with censoring applied to patients who died or were not diagnosed with stroke or TIA before end of study. We then modeled stroke and TIA events separately, followed by separate models for stroke subtypes (ischemic, hemorrhagic, undefined). As a final step, we conducted age‐ and race‐ or ethnicity‐stratified analyses of the association of PTSD with the composite stroke/TIA outcome.
Analyses were conducted using R, version 4.1.2. XGB and k‐fold cross‐validation were conducted using the caret package for R, version 6.0–94. Nearest neighbor PSM was conducted using the MatchIt package, version 4.5.3, and Cox models were conducted using the survival package, version 3.5–5. A 2‐sided P value<0.05 indicated statistical significance.
Results
Participant Characteristics
Baseline characteristics and standardized mean differences for the prematch and matched samples of women veterans with and without PTSD are displayed in Table 1. In contrast to the prematch sample, the matched sample achieved good balance across all covariates, with corresponding standardized mean differences <0.10. The final XGB model used to produce propensity scores for matching demonstrated moderate fit on both the training and test sets (both Cohen's kappas=0.51), thus indicating good generalizability of the model. Number of visits, year of index visit, depression, and anxiety conveyed the most importance in the propensity model (Figure 2).
Table 1.
Baseline Demographics and Clinical Features of the Prematch and Matched Samples of Women Veterans With and Without PTSD
| Prematch sample | Matched sample | |||||
|---|---|---|---|---|---|---|
| No PTSD | PTSD | No PTSD | PTSD | |||
| (n=486 851) | (n=143 859) | SMD | (n=104 046) | (n=104 046) | SMD | |
| Age, y | 43.24±15.76 | 40.29±11.61 | −0.213 | 39.13±11.67 | 39.16±11.66 | 0.003 |
| Age group, y | ||||||
| 18–29 | 123 376 (25.3%) | 34 287 (23.8%) | −0.035 | 29 806 (28.7%) | 29 623 (28.5%) | −0.004 |
| 30–39 | 100 803 (20.7%) | 40 939 (28.5%) | 0.181 | 28 239 (27.1%) | 28 355 (27.3%) | 0.003 |
| 40–49 | 113 560 (23.3%) | 35 664 (24.8%) | 0.034 | 24 880 (23.9%) | 25 009 (24.0%) | 0.003 |
| 50–59 | 82 261 (16.9%) | 25 517 (17.7%) | 0.022 | 16 410 (15.8%) | 16 298 (15.7%) | −0.003 |
| 60+ | 66 851 (13.7%) | 7452 (5.2%) | −0.295 | 4711 (4.5%) | 4761 (4.6%) | 0.002 |
| Race or ethnicity | ||||||
| Asian or Pacific Islander | 11 310 (2.3%) | 3274 (2.3%) | −0.003 | 2691 (2.59%) | 2542 (2.4%) | −0.009 |
| Non‐Hispanic Black | 115 746 (23.8%) | 45 981 (32.0%) | 0.183 | 31 143 (29.9%) | 31 458 (30.2%) | 0.007 |
| Hispanic/Latina | 31 648 (6.5%) | 13 041 (9.1%) | 0.096 | 9692 (9.3%) | 9772 (9.4%) | 0.003 |
| Unknown | 77 394 (15.9%) | 7802 (5.4%) | −0.344 | 5601 (5.4%) | 6348 (6.1%) | 0.031 |
| American Indian or Alaska Native | 3612 (0.7%) | 1793 (1.3%) | 0.051 | 1203 (1.2%) | 1273 (1.2%) | 0.006 |
| Non‐Hispanic White | 247 141 (50.8%) | 71 968 (50.0%) | −0.015 | 53 716 (51.6%) | 52 653 (50.6%) | −0.020 |
| Baseline conditions | ||||||
| Hyperlipidemia | 24 117 (5.0%) | 24 234 (16.9%) | 0.389 | 10 023 (9.6%) | 11 496 (11.1%) | 0.047 |
| Hypertension | 40 683 (8.4%) | 26 296 (18.3%) | 0.295 | 12 606 (12.1%) | 13 780 (13.2%) | 0.034 |
| Diabetes | 10 049 (2.1%) | 8484 (5.9%) | 0.197 | 3199 (3.1%) | 3809 (3.7%) | 0.033 |
| Smoking | 33 221 (6.8%) | 42 208 (29.3%) | 0.612 | 20 753 (20.0%) | 23 067 (22.2%) | 0.055 |
| Obesity | 63 803 (13.1%) | 56 119 (39.0%) | 0.618 | 29 999 (28.8%) | 31 962 (30.7%) | 0.041 |
| Chronic kidney disease | 801 (0.2%) | 938 (0.7%) | 0.077 | 305 (0.3%) | 372 (0.4%) | 0.011 |
| Neuroendocrine disorders | 13 475 (2.8%) | 10 318 (7.2%) | 0.204 | 4618 (4.4%) | 5240 (5.0%) | 0.028 |
| Female‐specific risk factors | 473 (0.1%) | 1434 (1.0%) | 0.122 | 327 (0.3%) | 570 (0.6%) | 0.036 |
| Depression | 8382 (1.7%) | 34 980 (24.3%) | 0.713 | 7457 (7.2%) | 10 278 (9.9%) | 0.097 |
| Bipolar disorder | 3271 (0.7%) | 8958 (6.2%) | 0.308 | 2239 (2.2%) | 3309 (3.2%) | 0.064 |
| Anxiety | 7434 (1.5%) | 27 068 (18.8%) | 0.597 | 6413 (6.2%) | 8680 (8.3%) | 0.084 |
| Schizophrenia | 1266 (0.3%) | 1271 (0.9%) | 0.083 | 434 (0.4%) | 563 (0.5%) | 0.018 |
| Alcohol use disorder | 1348 (0.3%) | 5591 (3.9%) | 0.255 | 1139 (1.1%) | 1706 (1.6%) | 0.047 |
| Drug use disorder | 900 (0.2%) | 4726 (3.3%) | 0.239 | 796 (0.8%) | 1325 (1.3%) | 0.051 |
| Number of baseline health care visits | 4.98 (9.64) | 17.78 (22.21) | 0.747 | 11.33 (14.43) | 12.46 (16.45) | 0.073 |
| Year of index visit date | ||||||
| 2000 | 8 (0.0%) | 0 (0.0%) | −0.006 | 1 (0.0%) | 0 (0.0%) | −0.004 |
| 2001 | 48 441 (10.0%) | 988 (0.7%) | −0.422 | 848 (0.8%) | 986 (1.0%) | 0.014 |
| 2002 | 29 389 (6.0%) | 1532 (1.1%) | −0.271 | 1390 (1.3%) | 1503 (1.4%) | 0.009 |
| 2003 | 25 634 (5.3%) | 1947 (1.4%) | −0.220 | 1751 (1.7%) | 1838 (1.8%) | 0.006 |
| 2004 | 23 720 (4.9%) | 2803 (2.0%) | −0.162 | 2662 (2.6%) | 2612 (2.5%) | −0.003 |
| 2005 | 21 687 (4.5%) | 3469 (2.4%) | −0.112 | 3060 (2.9%) | 3060 (2.9%) | 0.000 |
| 2006 | 20 678 (4.3%) | 3716 (2.6%) | −0.092 | 3194 (3.1%) | 3224 (3.1%) | 0.002 |
| 2007 | 20 838 (4.3%) | 4823 (3.4%) | −0.048 | 4338 (4.2%) | 4073 (3.9%) | −0.013 |
| 2008 | 21 425 (4.4%) | 5621 (3.9%) | −0.025 | 4891 (4.7%) | 4689 (4.5%) | −0.009 |
| 2009 | 22 905 (4.7%) | 6450 (4.5%) | −0.011 | 5231 (5.0%) | 5206 (5.0%) | −0.001 |
| 2010 | 24 030 (4.9%) | 6964 (4.8%) | −0.004 | 5657 (5.4%) | 5606 (5.4%) | −0.002 |
| 2011 | 24 132 (5.0%) | 7708 (5.4%) | 0.018 | 6410 (6.2%) | 6150 (5.9%) | −0.010 |
| 2012 | 25 415 (5.2%) | 8704 (6.1%) | 0.036 | 6797 (6.5%) | 6830 (6.6%) | 0.001 |
| 2013 | 25 177 (5.2%) | 10 214 (7.1%) | 0.080 | 7970 (7.7%) | 7820 (7.5%) | −0.005 |
| 2014 | 25 764 (5.3%) | 11 469 (8.0%) | 0.108 | 8513 (8.2%) | 8514 (8.2%) | 0.000 |
| 2015 | 25 821 (5.3%) | 13 100 (9.1%) | 0.147 | 8950 (8.6%) | 9108 (8.8%) | 0.005 |
| 2016 | 28 631 (5.9%) | 13 479 (9.4%) | 0.132 | 8417 (8.1%) | 8336 (8.0%) | −0.003 |
| 2017 | 27 639 (5.7%) | 13 412 (9.3%) | 0.139 | 7746 (7.4%) | 8113 (7.8%) | 0.013 |
| 2018 | 25 288 (5.2%) | 14 403 (10.0%) | 0.183 | 8326 (8.0%) | 8568 (8.2%) | 0.009 |
| 2019 | 20 229 (4.2%) | 13 057 (9.1%) | 0.199 | 7894 (7.6%) | 7810 (7.5%) | −0.003 |
Data are reported as mean±SD or /requency (%). PTSD indicates posttraumatic stress disorder; and SMD, standardized mean difference.
Figure 2. Variable importance from extreme gradient boosting propensity model.
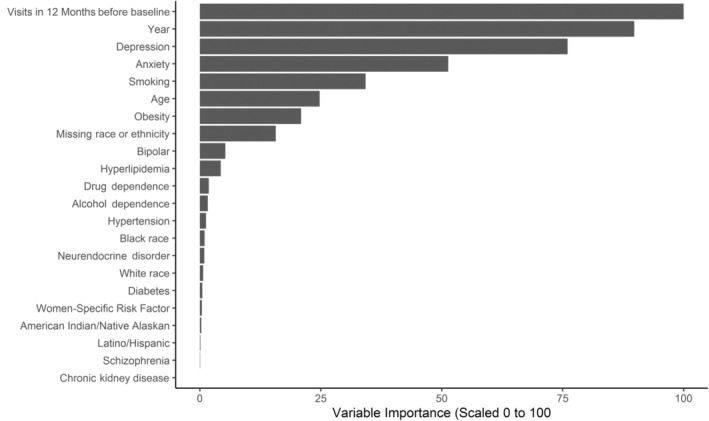
Women veterans in the analytic sample (N=208 092) had a mean age of 39.15 years, with <5% of the study population aged 60 years or older. The population was diverse with respect to race or ethnicity as well (51% non‐Hispanic White, 30% non‐Hispanic Black, 9.3% Hispanic/Latina, 2.5% Asian or Pacific Islander, 1.2% American Indian or Alaskan Native).
PTSD and Incident Stroke/TIA
The Cox models all demonstrated appropriate assumptions of proportional hazards according to Schoenfeld residual plots and tests of statistical significance (P≥0.16). The mean length of follow‐up for women veterans with PTSD was 6.42 years (SD=4.49) and 5.82 years (SD=4.58) for women veterans without PTSD. During that time, 1544 (1.5%) matched controls died before end of follow‐up, as did 1555 (1.5%) women with PTSD. Over follow‐up, there were 3883 stroke or TIA events; 1573 (1.5%) were among women veterans without PTSD, and 2310 (2.2%) were among women veterans with PTSD (Table 2). For both women veterans with and without PTSD, the majority of these events were strokes (64.6% and 71.9%, respectively). Illustration of the survival analysis for patients with PTSD versus those without PTSD (Figure 3) presents Kaplan–Meier survival curves for the stroke/TIA composite (A), stroke (B), and TIA (C) for women veterans with and without PTSD. PTSD was significantly associated with an elevated rate of developing stroke or TIA (hazard ratio [HR], 1.33 [95% CI, 1.25–1.42]; Table 2). In separate analyses, PTSD was also significantly related to incident stroke (HR, 1.19 [95% CI, 1.10–1.29]) and incident TIA (HR, 1.66 [95% CI, 1.48–1.87]). Analyses of stroke subtypes revealed that PTSD was significantly associated with a heightened rate of developing ischemic and undefined stroke but not hemorrhagic, stroke (Table 2).
Table 2.
Associations of PTSD With Incident Stroke and TIA in Women Veterans
| No PTSD | PTSD | Cox regression results | ||
|---|---|---|---|---|
| Outcome | No. (%) events | No. (%) events | HR (95% CI) | P value |
| Stroke/TIA composite | 1573 (1.5%) | 2310 (2.2%) | 1.33 (1.25–1.42) | <0.001 |
| Stroke | 1131 (1.1%) | 1493 (1.4%) | 1.19 (1.10–1.29) | <0.001 |
| Hemorrhagic | 117 (0.1%) | 125 (0.1%) | 0.96 (0.75–1.24) | 0.75 |
| Ischemic | 806 (0.8%) | 1068 (1.0%) | 1.19 (1.09–1.30) | <0.001 |
| Undefined | 208 (0.2%) | 300 (0.3%) | 1.31 (1.10–1.57) | 0.003 |
| TIA | 442 (0.4%) | 817 (0.8%) | 1.66 (1.48–1.87) | <0.001 |
HR indicates hazard ratio; PTSD, posttraumatic stress disorder; and TIA, transient ischemic attack.
Figure 3. Survival analysis for patients with PTSD versus those without PTSD.
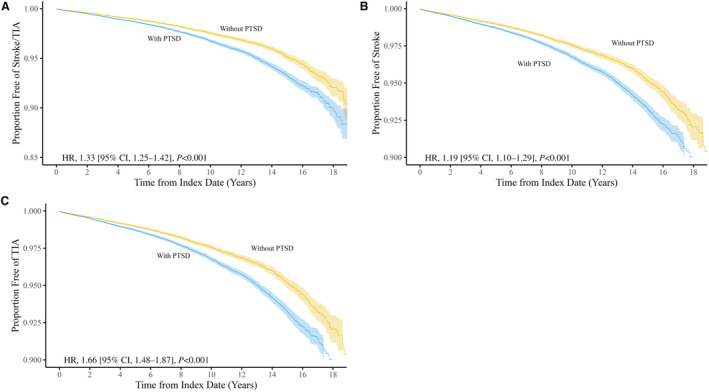
A, Comparison of stroke/TIA between the PTSD and no‐PTSD cohorts. B, Comparison of stroke between the PTSD and no‐PTSD cohorts. C, Comparison of TIA between the PTSD and no‐PTSD cohorts. HR indicates hazard ratio; PTSD, posttraumatic stress disorder; and TIA, transient ischemic attack.
Stratified Analyses of PTSD and Incident Stroke/TIA: Age and Race or Ethnicity
The results of analyses stratified by age and race or ethnicity are reported in Table 3. PTSD was associated with elevated rates of the incident stroke/TIA composite for all age groups except women veterans aged 60 years and older. However, the latter population comprised <5% of the total study population, and the PTSD effect estimate still conveyed elevated risk. In analyses stratified by race or ethnicity, PTSD was associated with significantly elevated rates of developing stroke/TIA for Hispanic/Latina (HR, 2.27 [95% CI, 1.66–3.12]), non‐Hispanic White (HR,1.40 [95% CI, 1.28–1.52]), non‐Hispanic Black women (HR, 1.12 [95% CI, 1.00–1.25]), and women of other/unknown race or ethnicity (HR, 1.40 [95% CI: 1.04–1.89]). In contrast, no significant associations were observed for American Indian or Alaskan Native, or Asian or Pacific Islander women.
Table 3.
Age‐ and Race‐ or Ethnicity‐Stratified Analyses of Association of PTSD With Incident Stroke/TIA in Women Veterans
| No PTSD | PTSD | Cox regression results | |||
|---|---|---|---|---|---|
| No. | No. (%) events | No. (%) events | HR (95% CI) | P value | |
| Age, y | |||||
| 18–29 | 59 429 | 76 (0.3%) | 133 (0.4%) | 1.54 (1.16–2.04) | 0.003 |
| 30–39 | 56 594 | 190 (0.7%) | 333 (1.2%) | 1.56 (1.31–1.87) | <0.001 |
| 40–49 | 49 889 | 532 (2.1%) | 812 (3.2%) | 1.41 (1.27–1.58) | <0.001 |
| 50–59 | 32 708 | 556 (3.4%) | 761 (4.7%) | 1.29 (1.15–1.43) | <0.001 |
| 60+ | 9472 | 219 (4.6%) | 271 (5.7%) | 1.10 (0.92–1.32) | 0.28 |
| Race or ethnicity | |||||
| Asian or Pacific Islander | 5233 | 21 (0.8%) | 23 (0.9%) | 0.99 (0.55–1.79) | 0.98 |
| Non‐Hispanic Black | 62 601 | 547 (1.8%) | 664 (2.1%) | 1.12 (1.00–1.25) | 0.049 |
| Hispanic/Latina | 19 464 | 53 (0.5%) | 135 (1.4%) | 2.27 (1.66–3.12) | <0.001 |
| Other/Unknown | 11 949 | 65 (1.2%) | 129 (2.0%) | 1.40 (1.04–1.89) | 0.026 |
| American Indian or Alaskan Native | 2476 | 13 (1.1%) | 30 (2.4%) | 1.89 (0.99–3.60) | 0.054 |
| Non‐Hispanic White | 106 369 | 874 (1.6%) | 1329 (2.5%) | 1.40 (1.28–1.52) | <0.001 |
HR indicates hazard ratio; PTSD, posttraumatic stress disorder; and TIA, transient ischemic attack.
Discussion
This study represents the largest and most comprehensive investigation of the association of PTSD with incident stroke/TIA in women veterans, a growing population with high level of trauma exposure and cardiovascular risk that nevertheless has been underrepresented in research on the link between PTSD and CVD. 28 Our investigation has several important findings. First, PTSD was a risk factor for incident stroke/TIA based on a composite outcome after matching for a comprehensive set of cardiovascular risk factors. Second, in separate subanalyses, PTSD was also significantly associated with heightened rates of developing stroke, TIA, ischemic stroke, and undefined stroke. Third, PTSD was a significant risk factor for developing stroke/TIA across all age groups, except for women veterans aged 60 and older. Fourth, differences in the PTSD‐stroke/TIA relation emerged for different racial and ethnic groups, with significantly elevated HRs observed for Hispanic/Latina, non‐Hispanic Black, and non‐Hispanic White women and women of other race or ethnicity. These findings are consistent with our previous study demonstrating a significant association between PTSD and incident ischemic heart disease in women veterans. Together, these data suggest that women veterans with PTSD may be at elevated risk of developing various cardiovascular disorders and that this risk may be pronounced for certain subgroups.
Our results extend growing work demonstrating a link between PTSD and stroke/TIA in various trauma‐exposed samples, including population‐based registry members, community‐based samples, and veterans. 22 , 23 , 25 , 26 , 27 Despite differences in covariates and analytic approaches across investigations, the effect sizes in our sample of women veterans were generally similar to those detected in women in these other samples. 22 , 25 Further, we identified links between PTSD and incident ischemic and undefined (but not hemorrhagic) stroke, as in some, 23 , 24 but not all, 22 , 27 prior work. In general, hemorrhagic stroke is less prevalent than ischemic stroke, 47 and hemorrhagic stroke comprised the smallest proportion of stroke events in the current study. Thus, we may have been underpowered for this subtype analysis in particular.
Understanding the mechanisms linking PTSD with incident stroke/TIA can inform potential risk screening and intervention efforts. PTSD has been associated with a number of behavioral risk factors for stroke/TIA, including poor diet, physical inactivity, smoking, insomnia, and medication nonadherence. 34 , 48 , 49 , 50 PTSD is also highly comorbid with psychiatric disorders that have also been linked to stroke (eg, depression), 51 , 52 and pharmacotherapy commonly prescribed for individuals with PTSD or depression has been associated with elevated stroke risk. 27 PTSD is also characterized by numerous physiological processes that can contribute to stroke/TIA, including dysregulation of the hypothalamic–pituitary–adrenal axis, sympathetic‐adrenal‐medullary system, and immune system. 53 , 54 To date, research in community‐dwelling women found that a variety of behavioral (eg, physical activity, diet quality, smoking) and medical (eg, obesity, hypertension, type 2 diabetes) risk factors may explain only ≈50% of the PTSD–CVD relationship (based on a composite of myocardial infarction and stroke), 25 suggesting that comprehensive approaches are needed to fully delineate relevant pathway variables. Furthermore, although our groups of women veterans with and without PTSD were balanced with respect to a variety of psychiatric disorders (anxiety, depression, schizophrenia, alcohol dependence, and nonalcohol drug dependence) through PSM, more research is needed to better understand the manifestations of posttraumatic psychopathology that may be most relevant to subsequent risk of CVD, including stroke/TIA. For example, PTSD and depression have a number of overlapping symptoms (eg, anhedonia, sleep and concentration difficulties), and research that considers cross‐cutting dimensions of psychopathology may help to identify particular symptoms to address in more targeted screening and intervention efforts.
In addition to demonstrating that PTSD was associated with incident stroke/TIA in the overall sample of women veterans, we identified age‐ and race‐ or ethnicity‐related subgroups for whom this risk was particularly prominent. Similar to other research on PTSD and CVD in women, 13 , 14 , 25 , 31 younger women with PTSD had the greatest risks for incident stroke/TIA. Indeed, the PTSD‐stroke/TIA link was most pronounced for women <50 years old. These findings may have multiple explanations. For example, CVD risks accumulate with advancing age, and the relative impact of PTSD as a CVD risk factor may be attenuated at older ages when other risk factors are also prevalent. At younger ages, when there are fewer other CVD risk factors, the PTSD‐stroke/TIA relation may thus appear more pronounced. Furthermore, with advancing age, it is possible that PTSD may resolve naturally or due to treatment, thereby reducing its impact on stroke/TIA. There is also initial evidence that PTSD symptom improvement or treatment may be associated with reductions in a CVD risk factor (hypertension), 55 , 56 and PTSD remission (versus ongoing symptoms) has been associated with reduced risk of CVD onset. 57 Future research is needed to directly test these hypotheses.
Differences in the PTSD‐stroke/TIA relation also emerged for women veterans of different race or ethnicity. PTSD was associated with significantly elevated rates of developing stroke/TIA for Hispanic/Latina, non‐Hispanic Black, and non‐Hispanic White women and women of other/unknown race or ethnicity, with Hispanic/Latina women with PTSD having >2‐fold greater risk of stroke/TIA. No significant associations were observed for American Indian or Alaskan Native or Asian or Pacific Islander women. However, caution should be used in interpreting the results for these latter populations due to the relatively small subgroup size and low absolute number of events. Nevertheless, our findings parallel some of the larger literature on racial and ethnic disparities in stroke/TIA, with particularly heightened risk observed in Hispanic/Latina women. 11 Research on the factors underlying these differences is needed. However, current research points to differences in the prevalence of CVD risk factors, access to care, treatment adherence, and systemic or provider‐based bias as potential explanations. 28 , 58 , 59
Limitations
The present results are subject to limitations characterizing retrospective analyses of administrative diagnostic data, including misclassification, misestimation of onset time, ascertainment bias, and issues with representativeness. Although PSM, particularly when implemented with machine learning, minimizes biases due to measured confounds, estimates are subject to bias from unmeasured confounders. For instance, due to limitations of the EHRs, not all potential risk factors, such as menopausal status, diet, or physical activity could be assessed. Furthermore, we did not account for pharmacotherapy as a factor relevant to stroke/TIA. Such analysis requires incorporating various information (eg, medication class, dosage, length of therapy, adherence) and was beyond the scope of this project. We also did not have information about trauma exposure, PTSD severity, or receipt of interventions. Moreover, women with PTSD who were not matched to controls, numbering 39 813 (27.7%), were not represented in our sample, thus limiting generalizability. Relatedly, our results are based on a large cohort of women veterans and may not be generalizable to other women and men. However, our study also had many strengths. This is the largest investigation of the association of PTSD with stroke/TIA in women veterans, analyzing over 1 million years of health data. In addition, we matched the cohorts with respect to a comprehensive list of potential risk factors in addition to traditional risk factors. Finally, because of the large sample size, we were able to perform stratified analysis based on age and race or ethnicity, thus uncovering subgroups with particularly elevated risk.
Conclusions
Our study suggests that PTSD is an important risk factor for stroke/TIA in women veterans, especially at younger ages and in the Hispanic/Latina population. These results reinforce our call to action to improve the cardiovascular care of women veterans. 28 Furthermore, our findings highlight the potential benefit of screening for stroke/TIA risk in women veterans with PTSD and emphasize the importance of integrated mental and cardiovascular health care after trauma. Additional research is required to assess the extent to which PTSD predicts a broader range of cardiovascular disorders and whether interventions targeting PTSD can offset the elevated cardiovascular risk.
Sources of Funding
This research was funded through a grant from the Department of Defense Office of Congressionally Directed Medical Research Programs Proposal Number PR171210, Award Number W81XWH‐18‐1‐0725).
Disclosures
None.
Supporting information
Data S1.
This article was sent to Jose R. Romero, MD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.033032
For Sources of Funding and Disclosures, see page 10.
References
- 1. Multiple cause of death, CDC Wonder Online Database. Centers for Disease Control and Prevention, National Center for Health Statistics. 2021. Accessed April 1, 2021. https://wonder.cdc.gov/mcd‐icd10.html [Google Scholar]
- 2. National Vital Statistics System: public use data file documentation: mortality multiple cause‐of‐death micro‐data files. Centers for Disease Control and Prevention, National Center for Health Statistics. 2021.. Accessed April 8, 2021. https://www.cdc.gov/nchs/nvss/mortality_public_use_data.htm
- 3. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore‐Mensah Y, et al. Heart disease and stroke statistics‐2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention . Prevalence and most common causes of disability among adults–United States, 2005. MMWR Morb Mortal Wkly Rep. 2009;58:421–426. [PubMed] [Google Scholar]
- 5. Towfighi A, Ovbiagele B, El Husseini N, Hackett ML, Jorge RE, Kissela BM, Mitchell PH, Skolarus LE, Whooley MA, Williams LS, et al. Poststroke depression: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48:e30–e43. doi: 10.1161/STR.0000000000000113 [DOI] [PubMed] [Google Scholar]
- 6. Levine DA, Galecki AT, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, Wadley VG. Trajectory of cognitive decline after incident stroke. JAMA. 2015;314:41–51. doi: 10.1001/jama.2015.6968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Easton JD, Johnston SC. Time to retire the concept of transient ischemic attack. JAMA. 2022;327:813–814. doi: 10.1001/jama.2022.0300 [DOI] [PubMed] [Google Scholar]
- 8. Madsen TE, Howard VJ, Jiménez M, Rexrode KM, Acelajado MC, Kleindorfer D, Chaturvedi S. Impact of conventional stroke risk factors on stroke in women: an update. Stroke. 2018;49:536–542. doi: 10.1161/STROKEAHA.117.018418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morgenstern LB, Smith MA, Sánchez BN, Brown DL, Zahuranec DB, Garcia N, Kerber KA, Skolarus LE, Meurer WJ, Burke JF, et al. Persistent ischemic stroke disparities despite declining incidence in Mexican Americans. Ann Neurol. 2013;74:778–785. doi: 10.1002/ana.23972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiménez MC, Manson JE, Cook NR, Kawachi I, Wassertheil‐Smoller S, Haring B, Nassir R, Rhee JJ, Sealy‐Jefferson S, Rexrode KM. Racial variation in stroke risk among women by stroke risk factors. Stroke. 2019;50:797–804. doi: 10.1161/STROKEAHA.117.017759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gardener H, Sacco RL, Rundek T, Battistella V, Cheung YK, Elkind MS. Race and ethnic disparities in stroke incidence in the Northern Manhattan Study. Stroke. 2020;51:1064–1069. doi: 10.1161/STROKEAHA.119.028806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Demel SL, Kittner S, Ley SH, McDermott M, Rexrode KM. Stroke risk factors unique to women. Stroke. 2018;49:518–523. doi: 10.1161/STROKEAHA.117.018415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shah AJ, Ghasemzadeh N, Zaragoza‐Macias E, Patel R, Eapen DJ, Neeland IJ, Pimple PM, Zafari AM, Quyyumi AA, Vaccarino V. Sex and age differences in the association of depression with obstructive coronary artery disease and adverse cardiovascular events. J Am Heart Assoc. 2014;3:e000741. doi: 10.1161/JAHA.113.000741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vaccarino V, Sullivan S, Hammadah M, Wilmot K, Al Mheid I, Ramadan R, Elon L, Pimple PM, Garcia EV, Nye J, et al. Mental stress‐induced‐myocardial ischemia in young patients with recent myocardial infarction: sex differences and mechanisms. Circulation. 2018;137:794–805. doi: 10.1161/CIRCULATIONAHA.117.030849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Edmondson D, von Känel R. Post‐traumatic stress disorder and cardiovascular disease. Lancet Psychiatry. 2017;4:320–329. doi: 10.1016/S2215-0366(16)30377-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012 [DOI] [PubMed] [Google Scholar]
- 17. Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM‐IV and DSM‐5 criteria. J Trauma Stress. 2013;26:537–547. doi: 10.1002/jts.21848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351:13–22. doi: 10.1056/NEJMoa040603 [DOI] [PubMed] [Google Scholar]
- 19. Dohrenwend BP, Turner JB, Turse NA, Adams BG, Koenen KC, Marshall R. The psychological risks of Vietnam for U.S. veterans: a revisit with new data and methods. Science. 2006;313:979–982. doi: 10.1126/science.1128944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fulton JJ, Calhoun PS, Wagner HR, Schry AR, Hair LP, Feeling N, Elbogen E, Beckham JC. The prevalence of posttraumatic stress disorder in Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans: a meta‐analysis. J Anxiety Disord. 2015;31:98–107. doi: 10.1016/j.janxdis.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 21. Kulka RA, Schlenger WE, Fairbank JA, Hough RL, Jordan BK, Marmar CR. Trauma and the Vietnam War Generation: Report of Findings from the National Vietnam Veterans Readjustment Study. Brunner/Mazel; 1990. [Google Scholar]
- 22. Gradus JL, Farkas DK, Svensson E, Ehrenstein V, Lash TL, Milstein A, Adler N, Sørensen HT. Associations between stress disorders and cardiovascular disease events in the Danish population. BMJ Open. 2015;5:e009334. doi: 10.1136/bmjopen-2015-009334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosman L, Sico JJ, Lampert R, Gaffey AE, Ramsey CM, Dziura J, Chui PW, Cavanagh CE, Brandt C, Haskell S, et al. Posttraumatic stress disorder and risk for stroke in young and middle‐aged adults: a 13‐year cohort study. Stroke. 2019;50:2996–3003. doi: 10.1161/STROKEAHA.119.026854 [DOI] [PubMed] [Google Scholar]
- 24. Song H, Fang F, Arnberg FK, Mataix‐Cols D, Fernández de la Cruz L, Almqvist C, Fall K, Lichtenstein P, Thorgeirsson G, Valdimarsdóttir UA. Stress related disorders and risk of cardiovascular disease: population based, sibling controlled cohort study. BMJ. 2019;365:l1255. doi: 10.1136/bmj.l1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sumner JA, Kubzansky LD, Elkind MS, Roberts AL, Agnew‐Blais J, Chen Q, Cerdá M, Rexrode KM, Rich‐Edwards JW, Spiegelman D, et al. Trauma exposure and posttraumatic stress disorder symptoms predict onset of cardiovascular events in women. Circulation. 2015;132:251–259. doi: 10.1161/CIRCULATIONAHA.114.014492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Remch M, Laskaris Z, Flory J, Mora‐McLaughlin C, Morabia A. Post‐traumatic stress disorder and cardiovascular diseases: a cohort study of men and women involved in cleaning the debris of the world trade center complex. Circ Cardiovasc Qual Outcomes. 2018;1:e004572. doi: 10.1161/CIRCOUTCOMES.117.004572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gaffey AE, Rosman L, Burg MM, Haskell SG, Brandt CA, Skanderson M, Dziura J, Sico JJ. Posttraumatic stress disorder, antidepressant use, and hemorrhagic stroke in young men and women: a 13‐year cohort study. Stroke. 2021;52:121–129. doi: 10.1161/STROKEAHA.120.030379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Han JK, Yano EM, Watson KE, Ebrahimi R. Cardiovascular care in women veterans: a call to action. Circulation. 2019;139:1102–1109. doi: 10.1161/CIRCULATIONAHA.118.037748 [DOI] [PubMed] [Google Scholar]
- 29. Frayne S, Phibbs C, Saechao F, Friedman S, Shaw J, Romodan Y. Sourcebook: women veterans in the Veterans Health Administration. Volume 4: longitudinal trends in sociodemographics, utilization, health profile, and geographic distribution. Women's Health Services, Veterans Health Administration, Department of Veterans Affairs. 2018.
- 30. Fihn SD, Francis J, Clancy C, Nielson C, Nelson K, Rumsfeld J, Cullen T, Bates J, Graham GL. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33:1203–1211. doi: 10.1377/hlthaff.2014.0054 [DOI] [PubMed] [Google Scholar]
- 31. Ebrahimi R, Lynch KE, Beckham JC, Dennis PA, Viernes B, Tseng CH, Shroyer ALW, Sumner JA. Association of posttraumatic stress disorder and incident ischemic heart disease in women veterans. JAMA Cardiol. 2021;6:642–651. doi: 10.1001/jamacardio.2021.0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scherrer JF, Salas J, Cohen BE, Schnurr PP, Schneider FD, Chard KM, Tuerk P, Friedman MJ, Norman SB, van den Berk‐Clark C, et al. Comorbid conditions explain the association between posttraumatic stress disorder and incident cardiovascular disease. J Am Heart Assoc. 2019;8:e011133. doi: 10.1161/JAHA.118.011133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harrington KM, Quaden R, Stein MB, Honerlaw JP, Cissell S, Pietrzak RH, Zhao H, Radhakrishnan K, Aslan M, Gaziano JM, et al. Validation of an electronic medical record‐based algorithm for identifying posttraumatic stress disorder in U.S. veterans. J Trauma Stress. 2019;32:226–237. doi: 10.1002/jts.22399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cohen BE, Marmar C, Ren L, Bertenthal D, Seal KH. Association of cardiovascular risk factors with mental health diagnoses in Iraq and Afghanistan war veterans using VA health care. JAMA. 2009;302:489–492. doi: 10.1001/jama.2009.1084 [DOI] [PubMed] [Google Scholar]
- 35. Gravely AA, Cutting A, Nugent S, Grill J, Carlson K, Spoont M. Validity of PTSD diagnoses in VA administrative data: comparison of VA administrative PTSD diagnoses to self‐reported PTSD checklist scores. J Rehabil Res Dev. 2011;48:21–30. doi: 10.1682/JRRD.2009.08.0116 [DOI] [PubMed] [Google Scholar]
- 36. Frayne SM, Miller DR, Sharkansky EJ, Jackson VW, Wang F, Halanych JH, Berlowitz DR, Kader B, Rosen CS, Keane TM. Using administrative data to identify mental illness: what approach is best? Am J Med Qual. 2010;25:42–50. doi: 10.1177/1062860609346347 [DOI] [PubMed] [Google Scholar]
- 37. Abrams TE, Vaughan‐Sarrazin M, Keane TM, Richardson K. Validating administrative records in post‐traumatic stress disorder. Int J Methods Psychiatr Res. 2016;25:22–32. doi: 10.1002/mpr.1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Golden SE, Hooker ER, Shull S, Howard M, Crothers K, Thompson RF, Slatore CG. Validity of Veterans Health Administration structured data to determine accurate smoking status. Health Informatics J. 2020;26:1507–1515. doi: 10.1177/1460458219882259 [DOI] [PubMed] [Google Scholar]
- 39. McGinnis KA, Brandt CA, Skanderson M, Justice AC, Shahrir S, Butt AA, Brown ST, Freiberg MS, Gibert CL, Goetz MB, et al. Validating smoking data from the Veteran's Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res. 2011;13:1233–1239. doi: 10.1093/ntr/ntr206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Melzer AC, Pinsker EA, Clothier B, Noorbaloochi S, Burgess DJ, Danan ER, Fu SS. Validating the use of Veterans Affairs tobacco health factors for assessing change in smoking status: accuracy, availability, and approach. BMC Med Res Methodol. 2018;18:39. doi: 10.1186/s12874-018-0501-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen T, He T, Benesty M, Khotilovich V, Tang Y, Cho H. Xgboost: Extreme Gradient Boosting. R package version 0.4‐2 1, no.4 2015; 1–4.
- 42. Lee BK, Lessler J, Stuart EA. Improving propensity score weighting using machine learning. Stat Med. 2010;29:337–346. doi: 10.1002/sim.3782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Westreich D, Lessler J, Funk MJ. Propensity score estimation: neural networks, support vector machines, decision trees (CART), and meta‐classifiers as alternatives to logistic regression. J Clin Epidemiol. 2010;63:826–833. doi: 10.1016/j.jclinepi.2009.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. doi: 10.1177/001316446002000104 [DOI] [Google Scholar]
- 45. Lipsitz SR, Dear KB, Zhao L. Jackknife estimators of variance for parameter estimates from estimating equations with applications to clustered survival data. Biometrics. 1994;50:842–846. doi: 10.2307/2532797 [DOI] [PubMed] [Google Scholar]
- 46. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 47. Fang MC, Coca Perraillon M, Ghosh K, Cutler DM, Rosen AB. Trends in stroke rates, risk, and outcomes in the United States, 1988 to 2008. Am J Med. 2014;127:608–615. doi: 10.1016/j.amjmed.2014.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. van den Berk‐Clark C, Secrest S, Walls J, Hallberg E, Lustman PJ, Schneider FD, Scherrer JF. Association between posttraumatic stress disorder and lack of exercise, poor diet, obesity, and co‐occuring smoking: a systematic review and meta‐analysis. Health Psychol. 2018;37:407–416. doi: 10.1037/hea0000593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Meinhausen C, Prather AA, Sumner JA. Posttraumatic stress disorder (PTSD), sleep, and cardiovascular disease risk: a mechanism‐focused narrative review. Health Psychol. 2022;41:663–673. doi: 10.1037/hea0001143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Taggart Wasson L, Shaffer JA, Edmondson D, Bring R, Brondolo E, Falzon L, Konrad B, Kronish IM. Posttraumatic stress disorder and nonadherence to medications prescribed for chronic medical conditions: a meta‐analysis. J Psychiatr Res. 2018;102:102–109. doi: 10.1016/j.jpsychires.2018.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB. Depression and risk of stroke morbidity and mortality: a meta‐analysis and systematic review. JAMA. 2011;306:1241–1249. doi: 10.1001/jama.2011.1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shalev AY, Freedman S, Peri T, Brandes D, Sahar T, Orr SP, Pitman RK. Prospective study of posttraumatic stress disorder and depression following trauma. Am J Psychiatry. 1998;155:630–637. doi: 10.1176/ajp.155.5.630 [DOI] [PubMed] [Google Scholar]
- 53. Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I. Biological studies of post‐traumatic stress disorder. Nat Rev Neurosci. 2012;13:769–787. doi: 10.1038/nrn3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Katrinli S, Oliveira NCS, Felger JC, Michopoulos V, Smith AK. The role of the immune system in posttraumatic stress disorder. Transl Psychiatry. 2022;12:313. doi: 10.1038/s41398-022-02094-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Burg MM, Brandt C, Buta E, Schwartz J, Bathulapalli H, Dziura J, Edmondson DE, Haskell S. Risk for incident hypertension associated with posttraumatic stress disorder in military veterans and the effect of posttraumatic stress disorder treatment. Psychosom Med. 2017;79:181–188. doi: 10.1097/PSY.0000000000000376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Scherrer JF, Salas J, Friedman MJ, Cohen BE, Schneider FD, Lustman PJ, van den Berk‐Clark C, Chard KM, Tuerk P, Norman SB, et al. Clinically meaningful posttraumatic stress disorder (PTSD) improvement and incident hypertension, hyperlipidemia, and weight loss. Health Psychol. 2020;39:403–412. doi: 10.1037/hea0000855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gilsanz P, Winning A, Koenen KC, Roberts AL, Sumner JA, Chen Q, Glymour MM, Rimm EB, Kubzansky LD. Post‐traumatic stress disorder symptom duration and remission in relation to cardiovascular disease risk among a large cohort of women. Psychol Med. 2017;47:1370–1378. doi: 10.1017/S0033291716003378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Feagin J, Bennefield Z. Systemic racism and U.S. health care. Soc Sci Med. 2014;103:7–14. doi: 10.1016/j.socscimed.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 59. Rose DE, Farmer MM, Yano EM, Washington DL. Racial/ethnic differences in cardiovascular risk factors among women veterans. J Gen Intern Med. 2013;28:S524–S528. doi: 10.1007/s11606-012-2309-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.


