Abstract
Background
Preoperative kidney dysfunction is a risk factor for right heart failure (RHF) after implantation of a left ventricular assist device (LVAD). However, characteristic kidney function trajectories before and after post‐LVAD RHF are uncertain, so we investigated this.
Methods and Results
We identified individuals who received primary continuous‐flow LVAD implantation from July 1, 2014 to December 31, 2017 in the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) data set. Incident RHF was ascertained using the INTERMACS definition at 1 and 3 months and classified as transient or persistent. Kidney function trajectories before and after RHF onset, and relationships of baseline kidney function with RHF risk at the different time points, were assessed. We identified 8076 LVAD recipients who met inclusion criteria. Incident RHF was present at 1 month in 26.4%. There were 4850 individuals with follow‐up at 3 months, with incident RHF in 4.2%. Kidney function trajectories differed from pre‐LVAD implantation to 1‐month follow‐up by RHF category, with those developing persistent RHF having no improvement in baseline kidney function. For trajectories before the 3‐month RHF ascertainment time, the shape was similar for those with and without RHF, with lower estimated glomerular filtration rate levels among those who developed RHF. Baseline estimated glomerular filtration rate levels below the normal range were associated with higher risk of RHF at 1 and 3 months.
Conclusions
In LVAD recipients, preimplantation kidney function and subsequent kidney function trajectories differed substantially by RHF at 1 and 3 months postimplantation, even after adjustment for several confounders. This may demonstrate bidirectional associations between kidney function and right ventricular function in LVAD recipients.
Keywords: kidney function, left ventricular assist device, right heart failure, trajectory
Subject Categories: Cardiorenal Syndrome, Heart Failure
Clinical Perspective.
What Is New?
Kidney function trajectories differed markedly before incident right heart failure (RHF) after left ventricular assist device (LVAD) implantation, and these trajectories differed by whether RHF was transient or persistent.
Those with RHF at 1‐month post‐LVAD that persisted had a trajectory that showed no improvement in kidney function following LVAD implantation.
Pre‐LVAD estimated glomerular filtration rate (eGFR) demonstrated strong, nonlinear associations with RHF at 1 or 3 months. eGFR levels below normal were associated with increasing risk of RHF at these time points, while changes in eGFR levels within the normal range were not associated with substantial change in risk.
What Are the Clinical Implications?
The study provides insight into the relationship between kidney dysfunction and RHF in LVAD recipients.
Notable was the absence of increased kidney function from before LVAD implantation to 1 month in those who manifested persistent RHF onset at this time, suggesting lack of kidney function improvement after LVAD implantation as an additional clinical risk factor for RHF.
Additionally, we clarified the shape of the relationship between baseline kidney function and risk of RHF, showing that lower eGFR within the abnormal range is associated with progressive increase in RHF risk at both time points, while higher eGFR within the normal range did not change the risk.
Right heart failure (RHF) following durable left ventricular assist device (LVAD) implantation remains a frequent and harmful complication, affecting an estimated 20% to 40% of recipients. 1 Kidney dysfunction before LVAD implantation is associated with higher risk of subsequent RHF, and measures of baseline kidney function are used in several RHF prediction models. 2 , 3 Additionally, impaired kidney function at baseline is associated with higher mortality risk after LVAD placement. 4 , 5 Kidney function trajectories demonstrating worsening function or lack of improvement following LVAD implantation portend worse outcomes. 6
Right heart dysfunction and kidney dysfunction are pathophysiologically intertwined. Much remains unknown about these relationships in the setting of durable mechanical circulatory support, but extrapolation from other disease processes provides insight. Elevated right‐sided cardiac filling pressures can cause kidney dysfunction through direct hemodynamic and nonhemodynamic mechanisms. 7 Transmission of high venous pressures to the kidney experimentally can cause rapid interstitial pressure increase resulting in decreased filtration. 7 , 8 Over time, elevated venous pressures can cause irreversible kidney damage (termed congestive nephropathy) through neurohormonal activation, endothelial damage, inflammation, and oxidative damage. 9
Kidney dysfunction may contribute directly to development and worsening of right heart dysfunction as well. However, most research has focused on the effects of right heart dysfunction on kidney function, 10 on the cross‐sectional relationship between right heart dysfunction and kidney dysfunction, 11 or on the effects of kidney dysfunction on left ventricular function. 12 , 13 The most apparent mechanism for kidney dysfunction to affect right heart function is through intravascular volume expansion resulting from sodium retention, increasing ventricular preload. Dysregulation of the neuro‐hormonal axis, with sympathetic nervous and renin‐angiotensin‐aldosterone system activation, may directly mediate the deleterious effects of kidney dysfunction on the right heart. 14 Additionally, kidney dysfunction can contribute to pulmonary vascular dysfunction and remodeling, 15 , 16 increasing right ventricular (RV) afterload and in the long run decreasing function. This pulmonary vascular dysfunction may occur through decreased nitric oxide signaling and increased endothelin‐1 levels due to kidney dysfunction. 17
RHF can manifest early or late following LVAD implantation, with the prevalence and incidence of RHF appearing to decline with increasing time following LVAD implantation. 18 , 19 Early RHF is reported to be less persistent than RHF that occurs later, and it has been suggested that early and later RHF can be considered distinct entities. 18 Persistence of RHF has been shown to be associated with adverse outcomes. 18
We sought to study the relationship of kidney function trajectories after LVAD implantation and development of RHF. Specifically, we studied the following: (1) how kidney function trajectories following LVAD implantation differ between those who subsequently develop RHF and those who do not, (2) How kidney function trajectories differ following development of RHF, and (3) how baseline estimated kidney function relates to risk of development of RHF.
METHODS
The data are available from the National Heart, Lung, and Blood Institute. 20 Analysis code is available from the corresponding author upon reasonable request.
Cohort
We identified individuals who received primary isolated continuous flow LVAD implantation from July 1, 2014 to December 31, 2017 in the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) data set from the National Heart, Lung, and Blood Institute. We excluded those who received dialysis before LVAD implantation and those with <1 month of follow‐up after LVAD implantation. The INTERMACS protocol specifies follow‐up data collection at the post‐LVAD time points of 1 week, and 1, 3, 6, and 12 months, although the actual times of follow‐up vary. Analysis of the INTERMACS database was determined not to be human subjects research by the Baylor College of Medicine Institutional Review Board; the INTERMACS protocol was approved by Institutional Review Boards at each participating center and informed consent was not required because it was deemed a quality‐improvement project. 21
RHF Assessment
To enable focus on kidney function trajectory over the first 1 month following LVAD implantation (during which there is normally substantial increase in estimated glomerular filtration rate [eGFR]), 22 we focused on RHF at the 1‐ and 3‐month time points. Because there is substantial spread in actual time points of post‐LVAD data collection in INTERMACS (Figure 1), we ascertained 1‐month RHF from data collected 0.75 to 1.5 months postimplantation, and 3‐month RHF from data collected 2.5 to 3.5 months following implantation. RHF was ascertained according to the INTERMACS 2014 definition, which requires both elevated central venous pressure (measurement of right atrial pressure >16 mm Hg, significantly dilated inferior vena cava with absence of respiratory variation by echocardiography, or jugular venous distension visible halfway up the neck of an upright patient) and clinical abnormalities attributable to elevated central venous pressure (peripheral edema graded >2+, ascites or hepatomegaly on physical examination or imaging, or evidence of worsening hepatic congestion or liver dysfunction [total bilirubin >2 mg/dL or creatinine >2 mg/dL]). 18 , 23 We further divided RHF into transient (no RHF at the subsequent time point) or persistent (RHF present at the subsequent time point).
Figure 1. Cohort and timeline summary for RHF determination following LVAD implantation.
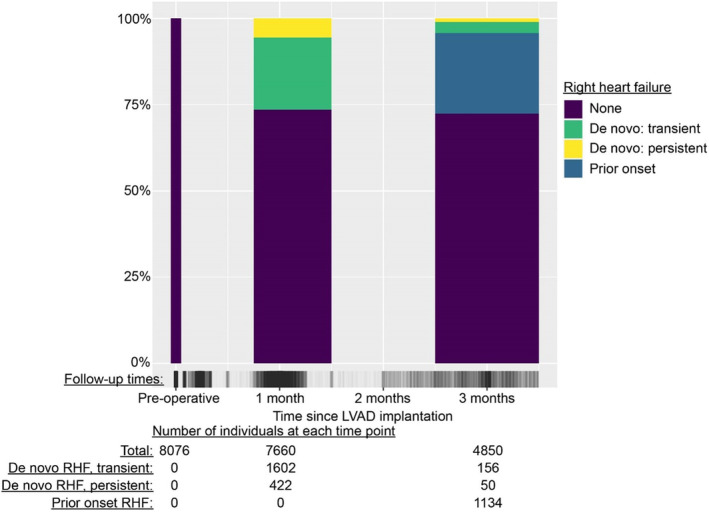
Individuals were included in the overall cohort if they had at least 1 follow‐up at the time of the 1‐month follow‐up assessment or later. The INTERMACS protocol targeted follow‐up visits at 1 week, and 1, 3, 6, and 12 months, although actual follow‐up times necessarily varied. For assessing RHF at the 1‐month and 3‐month follow‐ups, the time periods of 0.75 to 1.5 months postimplantation, and 2.5 to 3.5 months postimplantation were used, respectively (represented by widths of the columns). The times with patient data available are represented by the vertical gray dashes on the x axis. INTERMACS indicates Interagency Registry for Mechanically Assisted Circulatory Support; LVAD, left ventricular assist device; and RHF, right heart failure.
Kidney Function Estimation and Covariates
Kidney function was estimated using serum creatinine concentrations from the individual centers provided to INTERMACS and the 2021 Chronic Kidney Disease Epidemiology Collaboration equation. 24 To reflect low kidney function in those receiving dialysis during follow‐up visits without imposing identical values, for these visits eGFR was imputed randomly from the lowest centile of eGFR values from patients not receiving dialysis at that visit. Other covariates of interest assessed from the INTERMACS data at baseline were use of IV inotropes, left ventricular ejection fraction, right ventricular dysfunction, previous tricuspid valve repair or replacement, diabetes, preoperative mechanical ventilation, baseline blood urea nitrogen to creatinine ratio, device type (centrifugal or axial pump), and primary cardiac diagnosis. These covariates were treated as confounders and included in regression models because they have been demonstrated to be important predictors of RHF (with potential causal implications) 18 and also identify potential causes of kidney dysfunction.
Statistical Analysis
Baseline variables were compared among different RHF groups. Continuous variables are presented as median with interquartile range, while categorical variables are presented as number and percentage (n, %). Group comparisons were performed using nonparametric 1‐way ANOVA for continuous variables and χ2 test for categorical variables. P<0.05 was considered statistically significant. For modeling eGFR trajectories, separate linear mixed models were created for the pre‐RHF time periods (from pre‐LVAD to 1 or 3 months, depending on the RHF ascertainment time), and the post‐RHF time periods (from RHF ascertainment time to 12 months). For these models, eGFR was regressed on RHF status, baseline covariates and actual follow‐up times, and all variables were interacted with follow‐up time. Random intercepts were included in the eGFR trajectory models. Given the relatively small number of individuals with RHF onset at 3 months, we did not separate transient and persistent RHF at this time point for trajectory modeling.
Based on published shapes of average eGFR changes following LVAD implantation, 6 , 22 the regression models for eGFR change before and after the 1 month RHF ascertainment window were chosen to be linear, as was the model for eGFR change following the 3‐month RHF ascertainment window. For the trajectory from pre‐LVAD to the 3‐month RHF ascertainment window, we used piecewise linear models that allowed inflection at 1 month, to enable fit of the generally observed early increase up to 1 month followed by later declines. Pearson residuals were plotted against fitted values, and normality of residuals were assessed for each model, and the results were acceptable. Results are presented graphically and numerically using estimated marginal means of eGFR, predictions that are averaged over all values of categorical variables and use the means of continuous variables.
Relationships of baseline eGFR with incident RHF at 1 month and 3 months following LVAD implantation were investigated among those who had RHF ascertainment data available during these 2 time points using logistic regression, adjusting for baseline covariates. The eGFR term was modeled using restricted cubic splines with 5 knots (placed at the 5th, 25th, 50th, 75th, and 95th eGFR percentile).
The cumulative incidence of receiving dialysis during follow‐up was estimated with competing risks of dialysis, heart transplantation, device explantation, and death. Gray's test was used to compare cumulative incidence of dialysis by RHF group. 25 Analyses were completed using R version 4.2.1 (www.R‐project.org).
RESULTS
We identified 8076 LVAD recipients who met the inclusion criteria (Table). Age was median (interquartile range, 59 [49–66]) years and 21.3% were female. The treatment goal was destination therapy in 48.4%. A follow‐up visit occurred in 7660 LVAD recipients during the 1‐month ascertainment window (Figure 1). Incident RHF was present at 1 month in 2024 (26.4%), with 1602 (20.9%) having transient RHF (ie, RHF was present only at this follow‐up visit) and 422 (5.5%) having RHF that persisted to the next follow‐up time. Baseline characteristics differed by RHF category at 1 month (Table). Those who ultimately manifested RHF at the 1‐month point had a higher prevalence of baseline cardiogenic shock, higher body mass index, and higher prevalence of INTERMACS‐defined chronic kidney disease. They also had a higher prevalence of baseline severe RV dysfunction and pre‐LVAD temporary mechanical circulatory support, in addition to lower serum albumin, lower hemoglobin, and higher white blood cell count.
Table 1.
Baseline Characteristics Overall and by Presence of Incident Right Heart Failure at 1 Month
| Characteristic | Overall | Missing or unknown, n (%) | Right heart failure at 1 mo | P value† | ||
|---|---|---|---|---|---|---|
| None | Transient | Persistent | ||||
| Number* | 8076 | 5636 | 1602 | 422 | ||
| Demographics | ||||||
| Age, y | 59 [49–66] | 0 (0%) | 58 [48–66] | 60 [51–67] | 59 [49–65] | <0.001 |
| Female sex | 1724 (21.3%) | 0 (0%) | 1194 (21.2%) | 344 (21.5%) | 98 (23.2%) | 0.61 |
| Race | 252 (3.1%) | 0.004 | ||||
| White | 5173 (66.1%) | 3639 (66.9%) | 973 (62.2%) | 265 (63.9%) | ||
| Black | 2069 (26.4%) | 1383 (25.4%) | 478 (30.6%) | 124 (29.9%) | ||
| Asian | 137 (1.8%) | 97 (1.8%) | 27 (1.7%) | 6 (1.4%) | ||
| Other | 448 (5.7%) | 322 (5.9%) | 86 (5.5%) | 20 (4.8%) | ||
| Hispanic ethnicity | 539 (6.7%) | 204 (2.5%) | 361 (6.6%) | 126 (8.0%) | 32 (7.8%) | 0.106 |
| Device strategy | 0 (0.0%) | 0.005 | ||||
| Destination therapy | 3911 (48.4%) | 2709 (48.1%) | 828 (51.7%) | 179 (42.4%) | ||
| Bridge to transplant | 2142 (26.5%) | 1522 (27.0%) | 391 (24.4%) | 131 (31.0%) | ||
| Bridge to candidacy | 1987 (24.6%) | 1380 (24.5%) | 372 (23.2%) | 112 (26.5%) | ||
| Other | 36 (0.4%) | 25 (0.4%) | 11 (0.7%) | 0 (0.0%) | ||
| INTERMACS profile | 0 (0.0%) | <0.001 | ||||
| Critical cardiogenic shock (1) | 1189 (14.7%) | 723 (12.8%) | 325 (20.3%) | 82 (19.4%) | ||
| Progressive decline (2) | 2778 (34.4%) | 1809 (32.1%) | 638 (39.8%) | 167 (39.6%) | ||
| Stable, inotrope‐dependent (3) | 3033 (37.6%) | 2241 (39.8%) | 517 (32.3%) | 135 (32.0%) | ||
| Resting symptoms or lesser severity (4–7) | 1076 (13.3%) | 863 (15.3%) | 122 (7.6%) | 38 (9.0%) | ||
| Device type | 0 (0.0%) | <0.001 | ||||
| Axial flow | 5404 (66.9%) | 3839 (68.1%) | 1017 (63.5%) | 243 (57.6%) | ||
| Centrifugal flow | 2672 (33.1%) | 1797 (31.9%) | 585 (36.5%) | 179 (42.4%) | ||
| Patient characteristics | ||||||
| Body mass index, kg/m2 | 27.68 [23.84–32.58] | 26 (0.3%) | 27.52 [23.72–32.32] | 27.86 [23.80–33.07] | 28.30 [24.74–33.68] | 0.004 |
| Diabetes, severe by INTERMACS | 353 (4.4%) | 0 (0.0%) | 248 (4.4%) | 68 (4.2%) | 12 (2.8%) | 0.31 |
| CKD, INTERMACS classification | 565 (7.0%) | 0 (0.0%) | 344 (6.1%) | 153 (9.6%) | 33 (7.8%) | <0.001 |
| Primary cardiac disease cause | 67 (0.8%) | 0.581 | ||||
| Ischemic cardiomyopathy | 3264 (42.5%) | 2266 (42.3%) | 672 (44.2%) | 164 (40.7%) | ||
| Idiopathic | 2602 (33.9%) | 1797 (33.5%) | 516 (33.9%) | 139 (34.5%) | ||
| Infectious/inflammatory | 225 (2.9%) | 164 (3.1%) | 42 (2.8%) | 9 (2.2%) | ||
| Toxic cardiomyopathy | 139 (1.8%) | 97 (1.8%) | 27 (1.8%) | 10 (2.5%) | ||
| Other specified cause | 1448 (18.9%) | 1039 (19.4%) | 263 (17.3%) | 81 (20.1%) | ||
| Cardiovascular parameters | ||||||
| LV ejection fraction <20% | 5410 (69.9%) | 335 (4.1%) | 3787 (70.1%) | 1059 (69.0%) | 275 (67.6%) | 0.426 |
| Severe RV dysfunction | 901 (13.6%) | 1439 (17.8%) | 592 (12.9%) | 188 (14.0%) | 66 (18.9%) | 0.005 |
| Inotrope use | 6766 (84.1%) | 27 (0.3%) | 4597 (81.9%) | 1426 (89.3%) | 381 (90.3%) | <0.001 |
| Systolic blood pressure, mm Hg | 105 [95–116] | 128 (1.5%) | 105 [95–116] | 105 [95–116] | 106 [97–118] | 0.11 |
| Diastolic blood pressure, mm Hg | 65 [58–73] | 148 (1.8%) | 65 [58–73] | 65 [58–73] | 65 [58–73] | 0.915 |
| Prior tricuspid valve repair/replacement | 52 (0.6%) | 0 (0.0%) | 30 (0.5%) | 13 (0.8%) | 6 (1.4%) | 0.054 |
| Temporary mechanical circulatory support | 2144 (31.4%) | 1238 (15.3%) | 1312 (28.2%) | 583 (40.2%) | 144 (38.2%) | <0.001 |
| IABP | 1399 (17.3%) | 0 (0.0%) | 935 (16.6%) | 309 (19.3%) | 90 (21.3%) | 0.004 |
| ECMO | 200 (2.5%) | 0 (0.0%) | 114 (2.0%) | 64 (4.0%) | 16 (3.8%) | <0.001 |
| Mechanical ventilation | 386 (4.8%) | 0 (0.0%) | 256 (4.5%) | 89 (5.6%) | 29 (6.9%) | 0.038 |
| Laboratory values | ||||||
| Hemoglobin, g/dL | 11.3 [9.7–12.7] | 23 (0.3%) | 11.5 [9.9–12.8] | 10.6 [9.2–12.2] | 10.5 [9.3–12.0] | <0.001 |
| WBC, 103/μL | 7.8 [6.3–10.0] | 22 (0.3%) | 7.7 [6.2–9.8] | 8.2 [6.6–10.7] | 8.2 [6.4–10.6] | <0.001 |
| Platelets, 103/μL | 190 [146–243] | 28 (0.3%) | 191 [148–246] | 186 [138–239] | 179 [132–228] | <0.001 |
| Total bilirubin, mg/dL | 0.9 [0.6–1.5] | 359 (4.4%) | 0.9 [0.6–1.4] | 1.1 [0.7–1.8] | 1.0 [0.7–1.7] | <0.001 |
| BUN, mg/dL | 24 [17–35] | 27 (0.3%) | 23 [17–33] | 27 [19–39] | 28 [19–44] | <0.001 |
| Serum creatinine, mg/dL | 1.2 [1.0–1.6] | 7 (0.1%) | 1.2 [1.0–1.6] | 1.3 [1.1–1.7] | 1.4 [1.1–1.9] | <0.001 |
| eGFR, mL/min per 1.73 m2 | 64 [47–85] | 7 (0.1%) | 66 [48–87] | 58 [43–78] | 54 [38–74] | <0.001 |
| Albumin, g/dL | 3.5 [3.1–3.8] | 443 (5.5%) | 3.5 [3.1–3.9] | 3.3 [2.9–3.8] | 3.4 [3.0–3.8] | <0.001 |
Median [interquartile range] is given for continuous variables, and n (%) for categorical variables. BUN indicates blood urea nitrogen; CKD, chronic kidney disease; ECMO, extracorporeal membrane oxygenation; eGFR, estimated glomerular filtration rate; IABP, intraaortic balloon pump; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; LV, left ventricular; RV, right ventricular; and WBC, white blood cells.
Overall column includes individuals who did not have a follow‐up visit within the 1‐mo ascertainment window but did have later follow‐up visit; thus, the number overall is greater than the sum of the 3 other groups.
Statistical comparisons were performed using the nonparametric 1‐way ANOVA for continuous variables, and χ2 test for categorical variables.
There were 4850 individuals with follow‐up data available from the 3‐month ascertainment window (the reasons for missing 3‐month data and comparisons of baseline variables between the groups with available and missing data are given in Tables S1 and S2, along with comparison of survival estimates for the 2 groups in Figure S1; the groups were similar). Incident RHF was present in 206 (4.2%), with 156 (3.2%) having transient RHF and 50 (1.0%) having persistent RHF. Prior RHF diagnosis was present in 1134 (23.4%) at 3 months.
The median number of eGFR values in cohort follow‐up was 5. Median time of final eGFR value was 6.6 months after LVAD implantation, and values at 12‐month follow‐up were available for 2895 (38.5% of the cohort). Trajectories of eGFR from pre‐LVAD to the 1‐month follow‐up (adjusted for potential confounders) differed substantially by presence and type of RHF at 1 month (Figure 2A). Baseline eGFR (adjusted least‐squares mean) ranged from a high of 71.9 (95% CI, 71.2–72.5) mL/min per 1.73 m2 with no RHF at 1 month to a low of 58.2 (95% CI, 55.7–60.7) mL/min per 1.73 m2 with development of persistent RHF at 1 month (a difference of 13.2 [95% CI, 10.8–15.7] mL/min per 1.73 m2). One‐month eGFR change was a maximum of 8.0 (95% CI, 7.4–8.7) mL/min per 1.73 m2 with no RHF at 1 month, compared with a slight decrease with the development of persistent RHF at 1 month (−0.2 [95% CI, −2.5 to 2.1]).
Figure 2. New‐onset RHF at 1 month post LVAD implantation: kidney function trajectories (and 95% CIs) before and after RHF ascertainment at 1‐month follow‐up visit.
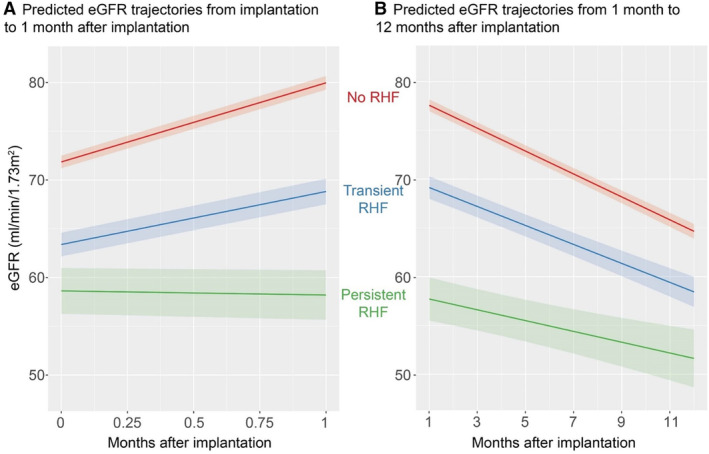
A, Predicted eGFR trajectories from the time of implantation to 1 month following implantation, by RHF status at 1 month. B, Predicted eGFR trajectories from 1 month to 12 months following LVAD implantation, by RHF status at 1 month. Predictions are estimated marginal means of eGFR. These predictions are based on the means of continuous variables and averaging over predictions using all values of categorical variables. Covariates were age, sex, INTERMACS profile, diabetes, use of IV inotropes at implantation, LVEF<20% at implantation, severe RV dysfunction at implantation, previous tricuspid valve repair or replacement, mechanical ventilation at baseline, blood urea nitrogen to creatinine ratio at baseline, device type (centrifugal or axial flow), and primary cardiac disease. eGFR indicates estimated glomerular filtration rate; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; RHF, right heart failure; and RV, right ventricle.
Trajectories of eGFR from RHF ascertainment at 1‐month postimplantation to 12 months postimplantation showed declining eGFR trajectories with all RHF categories (Figure 2B). The largest decline was without RHF (−12.7 [95% CI, −13.4 to −12.1] mL/min per 1.73 m2 over 11 months); the smallest decline was with RHF (−6.6 [95% CI, −9.3 to −3.9] mL/min per 1.73 m2).
A total of 710 individuals received dialysis during follow‐up, for a cumulative incidence of 8.7% over 12 months of follow‐up: 4.2% (95% CI, 3.7%–4.7%) in those without RHF at 1 month, 20.3% (95% CI, 18.3%–22.3%) in those with transient RHF at 1 month, and 27.9% (95% CI, 23.7%–32.3%) for those with persistent RHF at 1 month (P<0.001).
For outcomes of incident RHF at the 3‐month follow‐up, eGFR patterns from the pre‐RHF period (0–3 months) were similar in shape regardless of ultimate RHF development, with early increase followed by a decline from months 1 to 3 (Figure 3A). While the shape was similar, the eGFR values differed markedly by the development of RHF, with lower eGFR throughout with RHF. Marginal eGFR values for the post‐3‐month period showed declines in eGFR values in both groups (Figure 3B).
Figure 3. New‐onset RHF at 3 months post LVAD implantation: kidney function trajectories (and 95% CIs) before and after RHF ascertainment at 3‐month follow‐up visit.
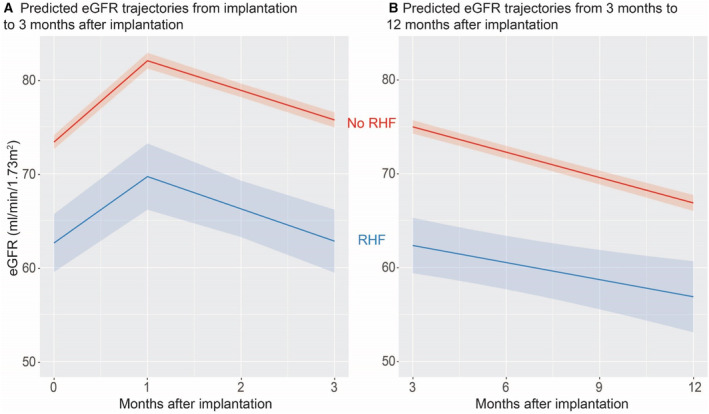
A, Predicted eGFR trajectories from the time of implantation to 3 months following implantation, by RHF status at 3 months. B, Predicted eGFR trajectories from 3 months to 12 months following LVAD implantation, by RHF status at 3 months. Predictions are estimated marginal means of eGFR. These predictions are based on the means of continuous variables and averaging over predictions using all values of categorical variables. Covariates were age, sex, INTERMACS profile, diabetes, use of IV inotropes at implantation, LVEF<20% at implantation, severe RV dysfunction at implantation, previous tricuspid valve repair or replacement, mechanical ventilation at baseline, blood urea nitrogen to creatinine ratio at baseline, device type (centrifugal or axial flow), and primary cardiac disease. eGFR indicates estimated glomerular filtration rate; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; RHF, right heart failure; and RV, right ventricle.
The relationship of pre‐LVAD eGFR with the RHF outcome at 1 month, after adjustment for potential confounders, is shown in Figure 4A. There is a monotonic relationship between eGFR and odds of RHF at 1 month, with lower eGFR associated with higher odds of having RHF at 1 month. Compared with baseline eGFR of 60 mL/min per 1.73 m2, an eGFR of 30 mL/min per 1.73 m2 is associated with 1.6 (95% CI, 1.3–1.8) times higher odds of RHF at 1 month, and eGFR of 90 mL/min per 1.73 m2 is associated with 0.76 (95% CI, 0.63–0.90) times the odds. The relationship becomes nearly flat above an eGFR of 90 mL/min per 1.73 m2. A similar relationship is observed between baseline eGFR and RHF at 3 months, although point estimates of risk slightly increase at higher baseline eGFR, and confidence intervals are wider (Figure 4B).
Figure 4. Adjusted odds ratios (and 95% CIs) for incident RHF following LVAD implantation by preimplantation kidney function estimates.
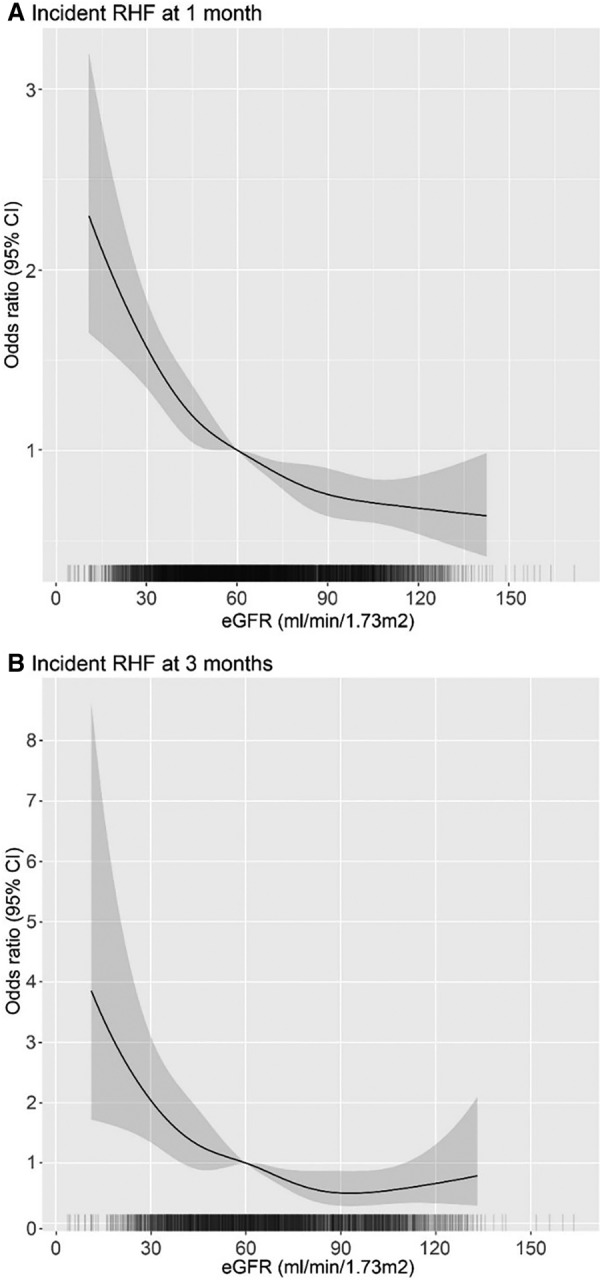
A, Odds ratios for incident RHF at 1 month following LVAD implantation across preimplantion kidney function level. The cohort included all individuals with follow‐up at the 1‐month point. B, Odds ratio for incident RHF at 3 months following LVAD implantation across preimplantation kidney function level. The cohort included all individuals with follow‐up at the 3‐month point. Models were adjusted for age, sex, INTERMACS profile, diabetes, and use of IV inotropes at implantation, LVEF<20% at implantation, severe RV dysfunction at implantation, previous tricuspid valve repair or replacement, mechanical ventilation at baseline, blood urea nitrogen to creatinine ratio at baseline, device type (centrifugal or axial flow), and primary cardiac disease. eGFR indicates estimated glomerular filtration rate; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; RHF, right heart failure; and RV, right ventricle.
DISCUSSION
Using a large national cohort of LVAD recipients, we identified novel differences in kidney function trajectories before and after the development of RHF following LVAD implantation, after adjustment for several potential confounders. Additionally, we found that lower baseline kidney function estimates were associated with substantially increased risk of RHF at 1‐ and 3‐month time points after adjustment for several potential confounders.
Kidney function trajectories leading to 1‐month follow‐up showed marked differences among the groups with no RHF, transient RHF, or persistent RHF onset at 1 month. Both the pre‐LVAD kidney function level and the 1‐month change differed markedly among the groups. Most notable was that persistent RHF development at 1 month was associated with an essentially flat eGFR trajectory from the time of implantation. Kidney‐centric and RV‐centric explanations for this may both be relevant, at a cohort and an individual level. A kidney‐centric explanation is that the presence of irreversible, nonhemodynamic parenchymal kidney disease at baseline (due to, for example, diabetes, ischemic nephropathy, or congestive nephropathy), or significant cardiac surgery–associated acute kidney injury, could have made individuals more susceptible to the added stresses to the RV by the LVAD, ultimately setting the stage for RV decompensation. Severe kidney disease (chronic or acute) could contribute to this decompensation susceptibility by impairing intravascular volume autoregulation, increasing neurohormonal activation, inhibiting nitric‐oxide‐mediated pulmonary vasodilation, or through kidney disease–induced pulmonary vascular remodeling. A potential RV‐centric explanation could be made that residual confounding from pre‐existing RV dysfunction at baseline, despite adjustment for several related baseline variables, was the cause of the observed baseline kidney dysfunction. In this scenario, baseline RV dysfunction leads to persistently elevated central venous pressures following LVAD implantation and lack of improvement in hemodynamically mediated, potentially reversible kidney dysfunction, resulting in no improvement in eGFR while simultaneously putting the individual at higher risk of RV decompensation.
With the assessment of incident RHF at the 3‐month time point, the pre‐RHF development kidney function trajectories differed from those seen with RHF at the 1‐month time point. With or without RHF at 3 months, an early increase in eGFR in the first month was followed by substantial decline. The major difference, however, was in the levels at which these trajectories took place, with lower eGFR throughout the trajectory in the setting of eventual RHF. Similar kidney‐centric and RV‐centric considerations as discussed above apply here, with the modification that any chronic or acute kidney disease may have tended to be milder in the group with RHF development at 3 months, and all had some degree of reversibility, at least temporarily. Alternatively, the trajectory to RHF at 3 months could reflect a slower, more drawn‐out RV decompensation process manifesting with delayed development of RHF.
The post‐RHF ascertainment window of up to 12 months showed similarities among all groups. First, eGFR trended down in all groups, even in those who never developed RHF. The eGFR downward trend seemed to be slightly faster without RHF; the reason for this is unclear, and considerations include body composition changes (with no RHF being associated with more gain of muscle mass and thus decreased eGFR without affecting kidney function), or potentially to overall decreasing kidney function related to lack of pulsatility and other possible mechanisms of kidney damage from long‐term LVAD support. 26
Finally, when evaluating how baseline kidney function relates to risk of subsequent RHF at 1‐ and 3‐month time points, it is notable how consistent the relationship is, with higher risk estimates for lower kidney function, even after adjusting for other factors. At the higher end of eGFR, where the eGFR equations become very inaccurate and where concerns about low muscle mass causing low serum creatinine level are a concern, there was a flattening of risk estimate, but no substantial increasing risk; this suggested that concerns about baseline eGFR estimates being highly confounded by body composition may be limited. 26 The considerations underlying these relationships are similar to those discussed above in examining the pre‐RHF kidney function trajectories. Despite the limitations of eGFR for assessing kidney function in this setting (lack of validation in this setting, high inaccuracy previously demonstrated in heart failure), baseline eGFR showed a strong relationship with risk for developing RHF.
Previous studies in several cohorts have demonstrated that RHF developing months after LVAD implantation is common and associated with adverse outcomes. 18 , 27 Various kidney function measures have also been studied for association with risk of RHF. A recently published analysis using the Society of Thoracic Surgeons INTERMACS database found that for every 5 mg/dL increase in baseline blood urea nitrogen, the odds of incident RHF at 3 months increased by 6% on adjusted analysis. 18 That analysis also demonstrated the favorable overall survival course for resolved RHF following LVAD implantation compared with persistent RHF. A systematic review of prediction models for RHF following LVAD implantation identified 7 externally validated prediction models, most focused on early (<30 day) RHF onset. 2 Of these, 3 included measures of baseline kidney dysfunction as prediction variables. 2 Previous analysis of INTERMACS from our group has demonstrated substantial heterogeneity in kidney function trajectory following LVAD implantation. 6 A prior INTERMACS analysis has evaluated unadjusted long‐term eGFR averages following RHF assessment at 3 months up to 2 years of follow‐up, and found early eGFR decline up to ≈12 months followed by stabilization, with lower average eGFR in those with RHF. 27
While the presence of RHF is treated as a dichotomous condition in this and other analyses, right ventricular dysfunction exists on a continuum in advanced HF and with LVAD support, and can be present to varying degrees before LVAD implantation and throughout the post‐LVAD implantation course. 27 This RV dysfunction can be driven by intrinsic myocardial disease, elevated RV afterload, and elevated RV preload. 27 Goals of developing and testing clinical strategies to maintain optimal RV filling pressures, to improve or at least preserve RV function, to improve elevated pulmonary vascular resistance, and to optimally manage LVAD pump parameters to reduce the risk of late RHF have been advocated. 27 Prior analysis has demonstrated that the onset of RHF after LVAD implantation is associated with both lower preimplantation eGFR and with lower subsequent eGFR. 27 Our analysis has extended these results by adjusting for potential confounding factors, including baseline RV and LV function.
One limitation of our study is that kidney dysfunction is one of the criteria for INTERMACS‐defined RHF, resulting in some interlinking of kidney dysfunction and RHF at the definitional level. In addition, survival bias may affect the post‐RHF kidney trajectories, because eGFRs were assessed from surviving patients, and it is known that RHF is associated with a higher risk of death. 18 Another limitation is that exact time of RHF onset is unknown; rather, it was assessed at different prespecified approximate time points set out in INTERMACS. Additionally, given the low numbers, we were unable to separate kidney function trajectories for those with transient versus persistent RHF onset at 3 months. There is a risk of informative censoring from missing values and missing follow‐up. The INTERMACS data set available from the National Heart, Lung, and Blood Institute ends at December 31, 2017, and thus does not include individuals receiving the currently used centrifugal flow LVAD device; however, the risks of RHF and kidney dysfunction were similar in the trial comparing the currently used centrifugal pump device to the previous generation axial flow device. 28
In conclusion, kidney function trajectories differed by development of incident RHF at 1 month and 3 months postimplantation, even after adjustment for confounders. This may provide additional support for the bidirectional relationships between kidney function and RV function in LVAD recipients. In addition, these findings support additional research into strategies to reduce risk of RHF in LVAD recipients with baseline kidney dysfunction and to better understand the mechanisms underlying the observed relationships.
Sources of Funding
Dr Walther is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (grant/award number: K23DK122131). Dr Navaneethan is supported by National Heart, Lung, and Blood Institute (NHLBI) grant (K24 HL161414) and institutional Garabed Eknoyan MD Endowed Professorship. This manuscript was prepared using INTERMACS research materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of INTERMACS or the NIH.
Disclosures
Outside the submitted work, Dr Navaneethan reported receiving personal fees from ACI Clinical, AstraZeneca (data safety monitoring board), Bayer, Boehringer Ingelheim, Eli Lilly and Co, GSK, Intercept Pharma, Vertex, and Vifor. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
Figure S1
Reference 29
This manuscript was sent to Sula Mazimba, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.031305
For Sources of Funding and Disclosures, see page 10.
See Editorial by Roehm and Agdamag.
References
- 1. Pagani FD. Right heart failure after left ventricular assist device placement: medical and surgical management considerations. Cardiol Clin. 2020;38:227–238. doi: 10.1016/j.ccl.2020.01.005 [DOI] [PubMed] [Google Scholar]
- 2. Frankfurter C, Molinero M, Vishram‐Nielsen JKK, Foroutan F, Mak S, Rao V, Billia F, Orchanian‐Cheff A, Alba AC. Predicting the risk of right ventricular failure in patients undergoing left ventricular assist device implantation: a systematic review. Circ Heart Fail. 2020;13:e006994. doi: 10.1161/CIRCHEARTFAILURE.120.006994 [DOI] [PubMed] [Google Scholar]
- 3. Bellavia D, Iacovoni A, Scardulla C, Moja L, Pilato M, Kushwaha SS, Senni M, Clemenza F, Agnese V, Falletta C, et al. Prediction of right ventricular failure after ventricular assist device implant: systematic review and meta‐analysis of observational studies. Eur J Heart Fail. 2017;19:926–946. doi: 10.1002/ejhf.733 [DOI] [PubMed] [Google Scholar]
- 4. Kirklin JK, Naftel DC, Kormos RL, Pagani FD, Myers SL, Stevenson LW, Givertz MM, Young JB. Quantifying the effect of cardiorenal syndrome on mortality after left ventricular assist device implant. J Heart Lung Transplant. 2013;32:1205–1213. doi: 10.1016/j.healun.2013.09.001 [DOI] [PubMed] [Google Scholar]
- 5. Cowger J, Sundareswaran K, Rogers JG, Park SJ, Pagani FD, Bhat G, Jaski B, Farrar DJ, Slaughter MS. Predicting survival in patients receiving continuous flow left ventricular assist devices: the HeartMate II risk score. J Am Coll Cardiol. 2013;61:313–321. doi: 10.1016/j.jacc.2012.09.055 [DOI] [PubMed] [Google Scholar]
- 6. Walther CP, Benoit JS, Lamba HK, Civitello AB, Erickson KF, Mondal NK, Liao KK, Navaneethan SD. Distinctive kidney function trajectories following left ventricular assist device implantation. J Heart Lung Transplant. 2022;41:1798–1807. doi: 10.1016/j.healun.2022.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Verbrugge FH, Guazzi M, Testani JM, Borlaug BA. Altered hemodynamics and end‐organ damage in heart failure: impact on the lung and kidney. Circulation. 2020;142:998–1012. doi: 10.1161/CIRCULATIONAHA.119.045409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gottschalk CW, Mylle M. Micropuncture study of pressures in proximal tubules and peritubular capillaries of the rat kidney and their relation to ureteral and renal venous pressures. Am J Physiol. 1956;185:430–439. doi: 10.1152/ajplegacy.1956.185.2.430 [DOI] [PubMed] [Google Scholar]
- 9. Husain‐Syed F, Grone HJ, Assmus B, Bauer P, Gall H, Seeger W, Ghofrani A, Ronco C, Birk HW. Congestive nephropathy: a neglected entity? Proposal for diagnostic criteria and future perspectives. ESC Heart Fail. 2021;8:183–203. doi: 10.1002/ehf2.13118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bansal S, Prasad A, Linas S. Right heart failure‐unrecognized cause of cardiorenal syndrome. J Am Soc Nephrol. 2018;29:1795–1798. doi: 10.1681/ASN.2018020224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dini FL, Demmer RT, Simioniuc A, Morrone D, Donati F, Guarini G, Orsini E, Caravelli P, Marzilli M, Colombo PC. Right ventricular dysfunction is associated with chronic kidney disease and predicts survival in patients with chronic systolic heart failure. Eur J Heart Fail. 2012;14:287–294. doi: 10.1093/eurjhf/hfr176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wali RK, Wang GS, Gottlieb SS, Bellumkonda L, Hansalia R, Ramos E, Drachenberg C, Papadimitriou J, Brisco MA, Blahut S, et al. Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end‐stage renal disease. J Am Coll Cardiol. 2005;45:1051–1060. doi: 10.1016/j.jacc.2004.11.061 [DOI] [PubMed] [Google Scholar]
- 13. Hawwa N, Shrestha K, Hammadah M, Yeo PSD, Fatica R, Tang WHW. Reverse remodeling and prognosis following kidney transplantation in contemporary patients with cardiac dysfunction. J Am Coll Cardiol. 2015;66:1779–1787. doi: 10.1016/j.jacc.2015.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schefold JC, Filippatos G, Hasenfuss G, Anker SD, von Haehling S. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol. 2016;12:610–623. doi: 10.1038/nrneph.2016.113 [DOI] [PubMed] [Google Scholar]
- 15. Bollenbecker S, Czaya B, Gutierrez OM, Krick S. Lung‐kidney interactions and their role in chronic kidney disease‐associated pulmonary diseases. Am J Physiol Lung Cell Mol Physiol. 2022;322:L625–L640. doi: 10.1152/ajplung.00152.2021 [DOI] [PubMed] [Google Scholar]
- 16. Edmonston DL, Parikh KS, Rajagopal S, Shaw LK, Abraham D, Grabner A, Sparks MA, Wolf M. Pulmonary hypertension subtypes and mortality in CKD. Am J Kidney Dis. 2019;75:713–724. doi: 10.1053/j.ajkd.2019.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Husain‐Syed F, McCullough PA, Birk HW, Renker M, Brocca A, Seeger W, Ronco C. Cardio‐pulmonary‐renal interactions: a multidisciplinary approach. J Am Coll Cardiol. 2015;65:2433–2448. doi: 10.1016/j.jacc.2015.04.024 [DOI] [PubMed] [Google Scholar]
- 18. Kapelios CJ, Lund LH, Wever‐Pinzon O, Selzman CH, Myers SL, Cantor RS, Stehlik J, Chamogeorgakis T, McKellar SH, Koliopoulou A, et al. Right heart failure following left ventricular device implantation: natural history, risk factors, and outcomes: an analysis of the STS INTERMACS database. Circ Heart Fail. 2022;15:e008706. doi: 10.1161/CIRCHEARTFAILURE.121.008706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kapelios CJ, Charitos C, Kaldara E, Malliaras K, Nana E, Pantsios C, Repasos E, Tsamatsoulis M, Toumanidis S, Nanas JN. Late‐onset right ventricular dysfunction after mechanical support by a continuous‐flow left ventricular assist device. J Heart Lung Transplant. 2015;34:1604–1610. doi: 10.1016/j.healun.2015.05.024 [DOI] [PubMed] [Google Scholar]
- 20. Interagency Registry for Mechanically Assisted Circulatory Support (Intermacs). National Heart Lung and Blood Institute. Biologic Specimen and Data Repository Information Coordinating Center. National Heart, Lung, and Blood Institute. 2020. Accessed January 1, 2024. https://biolincc.nhlbi.nih.gov/studies/intermacs/ [Google Scholar]
- 21. Interagency registry for mechanically assisted circulatory support. Manual of Operations and Procedures, Version 5.0. 2016.
- 22. Brisco MA, Kimmel SE, Coca SG, Putt ME, Jessup M, Tang WW, Parikh CR, Testani JM. Prevalence and prognostic importance of changes in renal function after mechanical circulatory support. Circ Heart Fail. 2014;7:68–75. doi: 10.1161/CIRCHEARTFAILURE.113.000507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hall SA, Copeland H, Alam A, Joseph SM. The "right" definition for post‐left ventricular assist device right heart failure: the more we learn, the less we know. Front Cardiovasc Med. 2022;9:893327. doi: 10.3389/fcvm.2022.893327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, Crews DC, Doria A, Estrella MM, Froissart M, et al. New creatinine‐and cystatin C–based equations to estimate GFR without race. N Engl J Med. 2021;385:1737–1749. doi: 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gray RJ. A class of K‐sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. doi: 10.1214/aos/1176350951 [DOI] [Google Scholar]
- 26. Walther CP, Civitello AB, Liao KK, Navaneethan SD. Nephrology considerations in the management of durable and temporary mechanical circulatory support. Kidney360. 2022;3:569–579. doi: 10.34067/KID.0003382021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rame JE, Pagani FD, Kiernan MS, Oliveira GH, Birati EY, Atluri P, Gaffey A, Grandin EW, Myers SL, Collum C, et al. Evolution of late right heart failure with left ventricular assist devices and association with outcomes. J Am Coll Cardiol. 2021;78:2294–2308. doi: 10.1016/j.jacc.2021.09.1362 [DOI] [PubMed] [Google Scholar]
- 28. Mehra MR, Uriel N, Naka Y, Cleveland JC Jr, Yuzefpolskaya M, Salerno CT, Walsh MN, Milano CA, Patel CB, Hutchins SW, et al. A fully magnetically levitated left ventricular assist device—final report. N Engl J Med. 2019;380:1618–1627. doi: 10.1056/NEJMoa1900486 [DOI] [PubMed] [Google Scholar]
- 29. Yang D, Dalton JE. A Unified Approach to Measuring the Effect Size Between Two Groups Using SAS. Paper presented at: SAS Global Forum 2012; April 22–25, 2012; Orlando, FL. Accessed January 5, 2024. https://support.sas.com/resources/papers/proceedings12/335‐2012.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figure S1
Reference 29


