Abstract
Translational bioinformatics and data science play a crucial role in biomarker discovery as it enables translational research and helps to bridge the gap between the bench research and the bedside clinical applications. Thanks to newer and faster molecular profiling technologies and reducing costs, there are many opportunities for researchers to explore the molecular and physiological mechanisms of diseases. Biomarker discovery enables researchers to better characterize patients, enables early detection and intervention/prevention and predicts treatment responses. Due to increasing prevalence and rising treatment costs, mental health (MH) disorders have become an important venue for biomarker discovery with the goal of improved patient diagnostics, treatment and care. Exploration of underlying biological mechanisms is the key to the understanding of pathogenesis and pathophysiology of MH disorders. In an effort to better understand the underlying mechanisms of MH disorders, we reviewed the major accomplishments in the MH space from a bioinformatics and data science perspective, summarized existing knowledge derived from molecular and cellular data and described challenges and areas of opportunities in this space.
Keywords: translational bioinformatics, neuroscience, biomarker discovery, data science, mental health informatics
INTRODUCTION
How bioinformatics and data science contribute to biomarker discovery in MH
Thanks to the digitization of healthcare data, massive amounts of data are being generated and collected from electronic health record (EHR) systems, medical imaging, laboratory and genomics tests, mobile health and wearable technology. This surge in Big Data, projected to reach the zettabytes range annually [1, 2]. With advances in artificial intelligence (AI) methodologies and cloud computing technologies, scientists are able to apply machine learning (ML) and AI-based deep learning techniques to structured and unstructured data on a scale that was previously unimaginable.
In this Big Data revolution, bioinformatics and data science play a crucial role as it enables scientists to extract and integrate biological information from the DNA, mRNA, microRNA, genes, proteins and metabolites, environmental and lifestyle factors. The scalable computational power of cloud computing empowers researchers to delve into complex disease mechanisms, enabling a systems-level understanding [3, 4].
The US Food and Drug Administration (FDA) has defined various categories of biomarkers and their various areas of applications. They include diagnostic, prognostic and theranostic biomarkers and can enable identification of various disease subtypes, better prediction of disease progression and better monitoring of treatment response [5, 6]. A good biomarker must be reliable, reproducible and independently confirmed by more than one study [7].
In recent years, mental health (MH) disorders have become a promising venue for biomarker discovery and for improved patient outcomes due to increasing prevalence and rising treatment costs [6]. Exploration of underlying biological mechanisms is the key to the pathogenesis and pathophysiology of mental disorders [8]. This is also keeping with National Institute of Mental Health (NIMH)’s Research Domain Criteria (RDoC) which is a framework that enables the study of the mechanisms of mental illness [6]. At present, very few biomarker tests have been approved for use in the clinic for MH, making this research even more important [9]. The progressive identification of new biomarkers in the MH space could enable researchers to build advanced clinical description support systems (CDSS) empowered by sophisticated AI models to advance personalized medicine [10].
Translational bioinformatics plays a vital role in biomarker discovery as it bridges the gap from the bench to the bedside. In order to better understand the various mechanisms of mental illnesses, we reviewed the major accomplishments in MH translational research from a bioinformatics and data science perspective, summarized computationally enabled discoveries of potential molecular and cellular biomarkers and described challenges and areas of opportunities for further exploration in this space.
This review is based on extensive search of relevant publications. A multiterm query for the following terms was performed in the NCBI Pubmed repository (bioinformatics OR transcriptome OR Proteomics OR genomics OR sequencing OR infection OR microbiome OR microRNA OR gene expression OR multiomics OR NGS OR RNA-seq OR RNAseq) AND (brain OR mental illness OR psychiatric OR psychiatry OR depression OR schizophrenia OR bipolar OR bi-polar OR autism OR anxiety OR PTSD OR Addiction OR Neurodegenerative diseases OR dementia OR memory loss. This resulted in 195,104 results from publications in 2009–2019, and 200 of the most relevant publications were downloaded for detailed review. The publications were then tagged based on two categories—category of MH disorder and molecular technology.
The scope of this review article was limited to the most common MH disease categories including major depressive disorders (MDD), Alzheimer’s disease (AD) and common disorders including schizophrenia (SCZ), bipolar disorder (BD), autism spectrum disorder (ASD), attention-deficit/hyperactivity disorder (ADHD) and posttraumatic stress disorder (PTSD). The word ‘biomarker’ was not used in the query as that may have resulted in publications unrelated to molecular and cellular mechanisms. As a result, the final list of publications was 173 (Tables 1 and 2).
Table 1.
Summary of articles categorized by the type of omics analysis
| Category of omics study | Number of published articles | Percentage of published articles |
|---|---|---|
| Proteomics | 46 | 27 |
| NGS; genomics | 36 | 21 |
| Cellular | 21 | 12 |
| Microbiome | 17 | 10 |
| Multiomics | 16 | 9 |
| microRNA | 13 | 8 |
| Gene expression | 11 | 6 |
| Epigenomics | 11 | 6 |
| Imaging | 2 | 1 |
| Total | 173 | 100 |
Table 2.
Summary of articles categorized by MH disorder
| Category of MH disorder | Number of published articles | Percentage of published articles |
|---|---|---|
| Alzheimers | 35 | 20 |
| Major depressive disorders | 33 | 19 |
| Neurodegenerative disorders (in general) | 26 | 15 |
| Neuropsychiatric disorders (in general) | 19 | 11 |
| PTSD, general anxiety | 18 | 10 |
| ASD and ADHD | 15 | 9 |
| Other (neurological disorders, psychiatry) | 15 | 9 |
| SCZ and BD | 12 | 7 |
| Total | 173 | 100 |
Summary of diseases reviewed
AD is a progressive neurodegenerative disorder that is estimated to affect one in nine senior adults. Its risk factors include age, family history and surrounding environment [11]. Many studies have been conducted to understand the underlying molecular mechanisms but no cure has been found so far [12]. MDDs is one of the most common MH disorders in the USA [13] and affects about 4.7% of the people in the world [14]. MDD which is associated with high mortality includes clinical depression, BD, suicide and other mood disorders. MDD is known to be heterogeneous and caused by a combination of genetic, environmental and psychological factors, and not many biomarkers are known to be effective in this domain [13].
According to the World Health Organization (WHO), SCZ is a psychiatric disorder that affects 1 in 300 people worldwide. SCZ is not as well studied as other psychiatric disorders like MDD. BD is a mental illness associated with extreme changes in mood from high to low and vice versa [15]. ADHD is one of the most common neurodevelopmental disorders that begin in early childhood. ASD is another neurological disorder that also begins in early childhood and impairs the ability to communicate and interact [16]. Anxiety disorders, such as PTSD among others, are one of the most common classes of psychiatric disorders and are known to be familial and heritable to a moderate degree [17].
Potential biomarkers from proteomics studies in various MH-related disorders
One of the most studied molecular datatypes in AD is proteomics-derived protein-based biomarkers. Advances in proteomics have allowed development of new biomarker discovery methods for early detection and diagnosis [18, 19]. The articles reviewed highlight the pivotal role of proteomics in unraveling intricate molecular mechanisms associated with AD, including the identification of protein-based biomarkers for early detection and diagnosis. Noteworthy findings encompass the characterization of over 400 proteins linked to amyloid plaques [20], the influence of hyperphosphorylated tau protein on neuronal health [21] and the discovery of key proteins such as glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in AD progression [22, 25]. The mitochondrial dimension of AD is explored through proteomic analysis of mitochondrial proteomes, revealing distinct patterns between early-onset and late-onset AD [23, 24]. Additionally, mitochondrial dysfunction, oxidative stress and alterations in protein homeostasis and energy production emerged as crucial contributors to AD pathophysiology [25–27]. Exploration of the proteomic landscape of the hippocampal tissues pinpointed to the changes in protein expression and potential implications for calcium signaling and extracellular matrix dynamics [28]. In the clinic, reduced glucose utilization is used as a biomarker for AD detection [29].
We found proteomics to be one of the most common molecular profiling platforms applied in MDD as well. One hundred and seventy-one serum proteins were identified, and serum analytes were linked to diverse cell communication in MDD patients [30]. Changes in protein abundance that were associated with several biological functions, including inflammation, transcription, cell metabolism and cytoskeleton organization [31] and those related to energy metabolism-related were also identified [7, 32].
In MDD, the protein HINT1 displayed increased brain levels, while SCZ exhibited lowered HINT1 levels [32]. Other aberrations associated with SCZ included glutamate receptor N-methyl-d-aspartate receptor (NMDA-R) and gamma-aminobutyric acid (GABA) [33]. Clinicians needed to identify and differentiate BD from MDD at the first depressive episode as the treatment course is different. Ren et al. [34] studied the differences between the two disorders by using a proteomics technology that applied isobaric tags for relative and absolute quantification (iTRAQ) technology combined with liquid chromatography–tandem mass spectrometry (LC–MS/MS). The authors found nine proteins significantly changed between MDD and BD and shortlisted B2RAN2 and ENG as potential biomarkers to distinguish BP from MDD.
The p140Cap protein interactome network associated with the SRCIN1 gene has been found associated with SCZ, ASD and BD [35]. Da Silva et al’s [36] proteomic profiling elucidated molecular mechanisms underlying the effects of methylphenidate in ADHD, highlighting potential links between pathways related to neurotransmitter release and GABA transmission, with drug response. The findings are summarized in Table 3.
Table 3.
Potential biomarkers from proteomics studies in various MH-related disorders
| Author name or study ID | Disorder | Protein(s)/biological functions affected | Implications | Source |
|---|---|---|---|---|
| [22] | AD | GAPDH | Interaction with β-amyloid precursor protein [22] | Various |
| [24] | AD | Respiratory mitochondrial complex subunits including those from the NDUFA and NDUFB subunits of the enzyme NADH dehydrogenase | Mitochondrial proteomic differences in early-onset and late-onset AD [24] | Brain tissue |
| Multiple studies: Moya-Alvarado et al. [25–27] | AD | Proteins associated with mitochondria, phosphorylation and oxidation | Impaired glucose metabolism and energy production [25–27] | Multiple studies from blood and brain tissue |
| Hondius et al. [28] | AD | Calcium-dependent signaling proteins, extracellular matrix components | Protein expression changes in hippocampus [28] | Brain |
| [138] | AD | Novel peptide sequences identified (SpotLight) | Antibody variable region associations with potential to provide disease origin insights [138] | Blood |
| [29] | AD | Reduced glucose utilization | Biomarker for AD detection [29] | Brain |
| Bot et al./Netherlands Study of Depression and Anxiety | MDD | 171 serum proteins and serum analytes | Linked to diverse cell communication, signal transduction processes, immune response and protein metabolism [30] | Serum |
| Gellen et al. | MDD | Changes in protein abundance | Linked to several biological functions, including inflammation, transcription, cell metabolism and cytoskeleton organization [31] | Animal model |
| Comes et al. [139] | MDD | 141 peptides and analytics analytes with combined m/z 1017, m/z 1042 and m/z 1479 | Potential biomarkers | Blood |
| Multiple studies: [32, 140]; [7, 32] | MDD | DPYSL2 also known as CRMP2; CA2 and ALDOC | Regulating axonal guidance, neuronal growth cone collapse and cell migration [32, 140]; energy metabolism [7, 32] | Studies from blood and brain tissue |
| [32] | MDD versus SCZ | HINT1 increased in MDD and lower in SCZ. | Differential protein levels in brain of MDD and SCZ patients | Blood and urine |
| [139] | BD | Alpha-2-macroglobulin, Apolipoprotein A-I and C4b-binding protein alpha chain, Complement C3, Glutathione-S-transferase A3, hemopexin, Immunoglobulin M, Kit ligand, Macrophage migration inhibitory factor, MMP7 and sex hormone-binding globulin | Proteins belonging to the following pathways associated with BD: FXR/RXR activation, LXR/RXR activation, acute phase response signaling, clathrin-mediated endocytosis signaling and atherosclerosis signaling | Blood |
| Ren et al. [34] | BD versus MDD | Proteins upregulated: B2RAN2, B4E1B2, APOA1, ENG, SBSN and QSOX2. Proteins downregulated: ORM1, MRC2 and SLPI downregulated | B2RAN2 and ENG as potential biomarkers to differentiate BD and MDD | Blood plasma |
| Ristori et al. [10] | ASD | (APOE and APOA1) and (FN1) | Large presence of apolipoproteins proteins and fibronectin | Studies from blood and brain tissue |
| Junaid et al. [141] | ASD | Glyoxalase I (Glo1) [141] | Increase in polarity | Brain |
| [35] | SCZ, ASD and BD | p140Cap protein interactome network associated with the SRCIN1 gene | Common interactome network | Various |
Potential biomarkers from genomics/NGS studies in various MH-related disorders
Affordable high throughput genome sequencing has spurred a wave of new studies utilizing next-generation sequencing (NGS) to uncover biomarkers and untangle the intricate pathology of MH disorders like AD [37]. Bertram et al’s [38] NGS investigations unveiled mutations in genes APP, TREM2 and PLD3. Verheijen et al. studied distinct subtypes of AD including early onset Alzheimer’s disease (EOAD) and late onset Alzheimer’s disease (LOAD). EOAD affects people before the age of 65 and often hereditary; was associated with genes like APP, PSEN1 and PSEN2 and characterized by amyloid accumulation. On the contrary, LOAD, affecting those over 65, with about 80% genetic contribution, is notably linked to APOE ε4 allele, which is a major risk factor [39].
Increased hypothalamic–pituitary–adrenal (HPA) axis activity has been known to occur in MDD leading to reduced mood and cognitive dysfunction [40], Nashed et al’s study on cancer-induced depression via RNA-seq revealed pathways tied to neuronal development, intracellular signaling, memory and learning [41]. NR3C2 and NR3C1 genes encoding mineralocorticoid and glucocorticoid receptors emerged as MDD risk factors, affecting HPA axis and cognitive functions [42]. Other candidate genes that have been linked with MDD include SNPs of CRHR1 that function through the HPA axis. [17, 43]. RGS2 gene has been found associated with multiple MH disorders including PTSD, generalized anxiety disorder (GAD) and PD [17].
Pies et al. studied biomarkers in SCZ and identified four main potential biomarkers that included mutations in neuregulin-1 NRGI, a cell adhesion molecule which acts on the EGFR family of receptors. Mutations in this gene have been linked with increased risk of SCZ [7, 44]. Mutations in DISC1 have been found in multiple MH disorders including BD, SCZ, MDD and ASD [35, 45–47]. Li et al. studied 36 studies on 4 neuropsychiatric disorders including ASD, epileptic encephalopathy, intellectual disability, SCZ profiled by WES/WGS and found 764 candidate genes in these disorders. Of these, 53 genes were found in more than one disorder and indicated a shared etiology of those disorders, with de novo mutations in SCN2A mutations common to all. [48]. Common pathways found between SCZ and ASD were synaptogenesis and synapse function and epigenetic process [49]. RGS2 gene has been found associated with PTSD, GAD and PD genes [17].
An interesting discovery was serotonin transporter SLC6A4 gene was found associated with both ASD [50] and tandem repeats in the promoter region of this gene was associated with PTSD [17]. Mutations in dopamine transporter and D4 receptor have shown to have potential as biomarkers in ADHD [51, 52]. Overall, we saw the genes commonly found in biomarker studies to be linked with monoaminergic neurotransmitter systems, neuropeptides and HPA axis function and an increased activity of transporter genes in the SLC family. Table 4 summarizes these findings in more detail.
Table 4.
Potential biomarkers from genomics/NGS studies in various MH-related disorders
| Author name or study ID | Disorder | Genes/biological functions affected | Implications/key findings | Source |
|---|---|---|---|---|
| Bertram et al.’s [38] | AD | APP, TREM2 and PLD3 | Gene mutations associated with AD | Various |
| Iacono et al. [142] | AD | A study of mouse models in AD using single-cell RNA sequencing (scRNA-seq) and functional analysis identified genes associated with gene expression or metabolic processes | Genes linked with multiple mouse organs were found to be associated | Brain |
| Verheijen [39] | EOAD | Increased accumulation of the amyloid-β (Aβ)1–42 peptide. Genes associated included amyloid precursor protein (APP), presenilin 1 (PSEN1) and presenilin 2 (PSEN2) | Hundreds of pathogenic mutations were found in this inherited disorder | brain |
| Verheijen [39] | LOAD | APOE ε4 allele | Well-known risk factor | Brain |
| Pantazatos et al. [143] | MDD | Humanin-like-8 (MTRNRL8), interleukin-8 (IL8) and serpin peptidase inhibitor, clade H (SERPINH1) and chemokine ligand 4 (CCL4) | Altered gene expression identified using RNA-seq | Brain |
| Nashed et al. [41] | MDD | Neuronal development, intracellular signaling, learning and memory | Pathways implicated in depression using RNA-seq | Brain |
| Pantazatos et al. [144] | MDD | SSAT and SATX isoforms, SAT1 | Low gene expressions in MDD | Brain |
| Pirooznia et al. [145] | MDD | Calcium signaling and dendrite regulation | Exons of synaptic genes potentially involved in the etiology of MDD | Brain |
| Howard et al. [146] | MDD | 102 genomic variants and 269 genes including SORCS3 and NEGR1 | Meta-analysis study of three large genome-wide association studies (GAWAS) studies | Brain |
| Keller et al. [42] | MDD | Variants in the NR3C1 gene including rs33388, rs10052957, rs10482633, rs41423247. variants in the NR3C2 gene included rs1879829, rs3910052, rs4835488, rs6535578, rs7658048 and rs5522 | NR3C2, NR3C1 variants affecting HPA axis and cognition | Brain |
| Belzeaux et al. [147] | MDD | RORA, GCET2 and SMARCC2 | Three potential biomarkers for treatment response | Various |
| Feng et al. [148] | MDD | EEF2, RPL26L1, RPLP0, PRPF8, LSM3, DHX9, RSRC1 and AP2B1 | potential pathogenic genes associated with MDD and potential therapeutic targets | Various |
| Multiple studies [17, 43] | MDD | SNPs of CRHR1 | Candidate genes reported | various; blood |
| Multiple studies [149–151] | MDD | Whole-genome sequencing was used to identify SNPs: one near gene SIRT1, an enzyme that deacetylates proteins that contribute to cellular regulation and the other SNP in an intron of LHPP gene [149–151] | Single nucleotide polymorphisms (SNPs) associated with MDD | Various; blood; saliva |
| Multiple studies [35, 152, 153] | SCZ and BD | Mutations and DNA methylation in BRD1 protein | Genetic associations | Various; blood; blood |
| Pies et al. [7, 44] | SCZ | Mutations in neuregulin-1 NRGI | Potential biomarkers for SCZ; increased risk of SCZ | Various; various |
| Multiple studies [35, 154, 155] | SCZ | ZNF804A [35, 155] and CRMP2 mutations [35, 154] | Increased risk of SCZ | Various; blood; mouse models, cell lines and DNA constructs |
| [49] | SCZ | Voltage-gated calcium channels, ARC-associated scaffold and FMRP interactors | The affected functional gene sets were identified using whole exome sequencing (WES) | Induced pluripotent stem cells (iPSC) |
| Demkow et al. [133] | ASD, ADHD | NGS testing justification in various clinical scenarios | Enables search for inherited conditions and new de novo mutations | Various |
| Goes et al. [156] | ASD | RPGRIP1L, FRAS1, AHNAK, KDM5B and SLC12A4 | Shortlisted genes implicated in ASD using WES | DNA from lymphoblastoid cell lines |
| Multiple studies [51, 52] | ADHD | Mutations in dopamine transporter and D4 receptor | Potential biomarkers | Various |
| Li et al. [48] | ASD, epileptic encephalopathy (EE), intellectual disability (ID), SCZ | 53 shared genes among four disorders, including SCN2A | Indicates a shared etiology of these disorders | Various |
| [49] | SCZ and ASD | Synaptogenesis and synapse function and epigenetic process | Common pathways found | Induced pluripotent stem cells (iPSC) |
| Wen et al. [157] | ASD | Mutations in MECP2 | Used WES to identify several loss-of-function mutations that could lead to ASD | Peripheral blood |
| Multiple studies: Sjaarda et al. [17, 50] | ASD and PTSD | Serotonin transporter SLC6A4 | Mutations linked to ASD and prenatal stress; GWAS-identified polymorphisms associated with PTSD | Mouse model; various |
| [17] | PTSD, generalized anxiety disorder (GAD) and Parkinson (PD) | RGS2 | Only a few findings have been confirmed by multiple studies | Various |
Potential biomarkers from gene expression studies in various MH-related disorders
A wide array of gene expression studies was examined across various MH disorders, offering interesting insights into the underlying molecular mechanisms. Wang et al. [53] suggest a potential link between abnormal AMP expression and AD onset in flies. Forero et al. [54] conducted a substantial meta-analysis of gene expression studies in MDD, revealing differentially expressed genes across various brain regions including blood, amygdala, cerebellum, anterior cingulate cortex (ACC) and prefrontal cortex (PFC) regions and highlighting 23 confirmed genes from their findings (Table 4). Dysregulated genes associated with MDD include SLC1A2 (glutamate transporter), GABRD (GABA receptor [54, 55]), genes in the HTR serotonergic family [56] and PXMP2 (ROS metabolism) [54]. Xiao et al’s [57] study on SCZ and BD revealed altered mRNA levels of RELN, while Kuan et al’s [58] research from World Trade Center responders who had PTSD identified 99 differentially expressed genes, including the upregulation of FKBP5 in PTSD responders. Overall, we can see diverse gene expression patterns associated with different MH disorders, providing valuable insights into potential biomarkers and therapeutic targets. Table 5 summarizes these differentially expressed genes and findings in more detail.
Table 5.
Potential biomarkers from gene expression studies in various MH-related disorders
| Author name or study ID | Disorder | DEGs/biological functions affected | Implications/key findings | Source |
|---|---|---|---|---|
| Wu et al. [158] | AD | ITGB5, RPH3A, GNAS, THY1 and SEPT6 | Associated with AD disease progression | Brain tissue |
| Wang et al. [53] | AD | Abnormal AMP expression | Onset and development of AD in flies | Brain tissue |
| Forero et al. [60] | MDD | A list of 23 genes including ABCG4, ACTA2, AGAP1, AP2B1, ATP1A3, ATP2B1, ATP5A1, BMI1, C10orf10, C3orf70, CAMK2A, CD24, CDC37, CDH13, CDKN1B, CDO1, CLDND1, CPLX1, CSRNP3, GLDN, GRM8, IL17RD, TUSC3 | Short list of 23 genes confirmed in other studies as well | Meta-analyses for GWES of MDD for four brain regions and for blood |
| Multiple studies: [54–56] | MDD | Glutamate transporter gene SLC1A2 [54]; GABAergic gene GABRD that codes for gamma-aminobutyric acid type A receptor delta subunit [54, 55]; genes in the serotonergic family including HTR1A, HTR1B, HTR2A, HTR2C [56] and the PXMP2 gene which is involved in reactive oxygen species (ROS) metabolism [54] | Dysregulated in various regions of the brain in MDD | Brain and peripheral tissues; blood |
| Multiple studies: [49, 159, 160, 161] | SCZ | GFAP [159], GLUL [160] and S100B [49, 161] | Genes implicated in SCZ | Brain tissue; brain tissue; blood |
| Xiao et al. [57] | SCZ and BD | mRNA levels of RELN were affected in patients | The study of the methylome and transcriptome | Brain tissue |
| Ansel et al. [74] | ASD | DIO2, Cirbp, DNMT3A, DNMT3B, TET1, TET3 | Dysregulated genes identified | Multiple sources |
| McCaffrey et al. [162] | ADHD | ABCB5, RGS2, GAK, GIT1 | This study of RNA markers could be studied further towards design of targets for diagnostics and therapeutics in ADHD | Blood |
Potential biomarkers from microRNA studies in various MH-related disorders
Forero et al. performed one of the largest meta-analysis of gene expression studies in MDD that covered 24 datasets that included a total of 753 samples. The authors identified 35, 793, 231, 668 and 252 genes differentially expressed from studies analyzed in the blood, amygdala, cerebellum, ACC and PFC regions, respectively [59, 60]. One particular microRNA reported in most of the studies was miR-132 which is one of the microRNAs regulating expression of BDNF, one of the key players in brain plasticity. This microRNA also targets the gens MAOA and SLC6A3 that are implicated in neuropsychiatric disorders [61, 62]. Kohen et al. applied RNA-seq to patients with MH disorders including SCZ, MDD and BD and found that the level of expression of another microRNA: miR-182 was changed in these disorders. miR-182 was also found activated in patients with BD and healthy controls, while it was found downregulated in MDD and SCZ [63]. Nakata et al. [64] studied microRNA expression in peripheral blood from adults with high functioning ASD and compared with healthy controls and discovered miR-6126 as downregulated in ASD. Gupta et al. studied PTSD data from military veterans and found circulatory microRNAs to play an important role. Specifically, microRNAs associated with HPA axis regulation through FKBP5 were found to play a key role in PTSD [65]. Detailed findings are summarized in Table 6. Overall, these diverse miRNAs implicated in MH disorders offers valuable insights into potential mechanisms and therapeutic avenues.
Table 6.
Potential biomarkers from microRNA studies in various MH-related disorders
| Author name or study ID | Disorder | Molecular features/biological functions affected | Implications/key findings | Source |
|---|---|---|---|---|
| Pang et al. [163] | AD | hsa-let-7d, hsa-miR-144, hsa-miR-374a, and hsa-miR-106b targeting genes in pathways PI3K-AKT signaling pathway, MAPK signaling pathway, oxidative phosphorylation, synaptic vesicle cycle, cell–cell adhesion, cytokine-mediated signaling pathway, proteasome, arginine, proline metabolism and pentose phosphate pathway [163] | Analysis of two important regions of the brain—entorhinal cortex (EC) and hippocampus (HIP) of AD patients revealed microRNAs that targeted genes in specific pathways | Blood |
| Multiple studies: Forero et al. [55, 59, 60, 164] | MDD | DEGs in various brain regions. MicroRNAs including hsa-miR-32, hsa-miR-33, hsa-miR-122, hsa-miR-429 associated with MDD. These microRNAs also known to regulate other MDD genes including GABA receptors, NOTCH2 and HNRNPU [60, 164]. Other miRNAs implicated include hsa-miR-370, hsa-miR-411, hsa-miR-433, hsa-miR-487b and hsa-miR-539 [165] | Studies analyzed in the blood, amygdala, cerebellum, ACC and PFC regions revealed microRNAs linked to chronic stress and fear and GABA receptors linked to chronic stress and fear [55, 164] | Various brain regions |
| Wang et al. [166–168] | SCZ | hsa-miR409-3p [166–168] which targets genes associated with SCZ including FAM117B, GABRA1, GAD1, and NUMBL. hsa-miR-370 targets several SCZ associated genes including BDNF, NRG1 and SYN2 [166, 169]. Other microRNAs affected include miR-30e, miR-7, miR-195, miR-34a and miR-346 | microRN As that targets genes associated with SCZ | Tissue; blood; blood |
| Multiple studies [61, 170] | MDD and BD | miR-652 [61, 170] | microRNA affected in both MDD and BD | Various sources (microRNA affected in both MDD and BD); blood |
| Kohen et al. [63] | SCZ, MDD and BD | miR-182 | RNA-seq revealed this microRNA was found activated in patients with BD and healthy controls while downregulated in MDD and SCZ [63] | Brain tissue |
| Multiple studies [61, 62] | Multiple neuropsychiatric disorders | miR-132, one of the microRNAs regulating expression of BDNF, and targets the genes MAOA and SLC6A3 | One of the key players in brain plasticity and implicated in neuropsychiatric disorders [61, 62] | Various sources (microRNA affected in both MDD and BD); various sources |
| Srivastav et al. [171] | ADHD | microRNAs regulated the gene expression of BDNF, DAT1, HTR2C, HTR1B and SNAP-25 | These microRNAs were also linked to ADHD etiology [171] | Various sources |
| Nakata et al. [64] | ASD | miR-6126 downregulated in ASD | Study of microRNA expression in peripheral blood from adults with high functioning ASD compared with healthy controls | Blood |
| Gupta et al. [65] | ADHD | microRNAs associated with HPA axis regulation through FKBP5 were found to play a key role in PTSD | Circulatory microRNAs to play an important role in PTSD | Blood |
| Martin et al. [172] | PTSD | Four upregulated microRNAs (miR-19a-3p, miR-101–3p, miR-20a-5p and miR-20b-5p) and four downregulated microRNAs (miR-15b-3p, miR-125b-5p, miR-128-3p and miR-486-3p) in PTSD samples | Implications of microRNA dysregulation in PTSD patients | Blood |
Potential biomarkers from epigenomics studies in various MH-related disorders
Zhang et al. employed whole genome bisulfite sequencing to identify novel differentially methylated sites in genes DLGPAP1, TMEM51 and EIF2AK2 that could serve as potential biomarkers for AD. [66]. Li et al’s review of 67 studies highlighted hypermethylation in BDNF and SLC6A4 as associated with depression [67, 68]. Kuan et al. [69] studied epigenome-wide association studies (EWASs) of MDD of 473 World Trade Center responders and found phosphatidylinositol signaling and cell cycle pathways affected. DNA methylation changes in genes CAMK2A, SLC1A2, HTR1A and HTR1B have also been implicated in MDD [68, 70, 71]. Epigenetic changes in gene BDNF or receptor TRKB were found in multiple psychiatric disorders including MDD, BD, SCZ and borderline personality disorder [72].
Epigenetic changes in serotonin transporter SLC6A4 have implications in MDD, BD, PTSD, SCZ, and ADHD.
Loke et al. [73] studied epigenetic changes associated with Autism and identified five candidate genes (OXTR, GAD1, EN2, RELN, MECP2) whose methylation was affected in the brains of ASD patients. One of the very commonly studied methylation changes is the addition of a methyl group on the fifth carbon of cytosine (e.g. 5-methylcytosine 5mC). This epigenetic marker in involved in important functions including X-chromosome inactivation, chromatin structure, gene silencing and genomic imprinting. Disruptions in 5mc has recently been linked to ASD with promising relevant in the clinic [74–76]. Kuan et al. [69] conducted EWASs of PTSD responders and found genes enriched in the following pathways including stress response, inflammation and physical health. Detailed findings are summarized in Table 7.
Table 7.
Potential biomarkers from epigenomics studies in various MH-related disorders
| Author name or study ID | Disorder | Molecular features/biological functions affected | Study summary/key findings | Source |
|---|---|---|---|---|
| Zhang et al. [66] | AD | Novel differentially methylated sites in genes DLGPAP1, TMEM51 and EIF2AK2 | Potential biomarkers for AD identified through whole-genome bisulfite sequencing in mouse brains | Brain |
| Li et al. [67, 68] | MDD | Hypermethylation in BDNF and SLC6A4 associated with MDD. DNA methylation changes in genes linked to MDD. [67, 68] | Review of 67 studies to summarize the relationship between DNA and depression | Blood; various |
| Multiple studies: Kuan et al. [68–71] | MDD | Phosphatidylinositol signaling and cell cycle pathways affected in MDD. Genes CAMK2A, SLC1A2, HTR1A and HTR1B also implicated | EWASs | Various; blood; various; various |
| [72] | MDD, BD, SCZ | Epigenetic changes in gene BDNF or receptor TRKB | Could be a potential biomarker as it was found in multiple psychiatric disorders | Various |
| Multiple studies [35, 57, 152, 153] | SCZ and BD | Mutations and DNA methylation in BRD1 protein. Methylation changes in RELN, PPP3CC, DNMT1, DTNBP1, NOS1, HTR1E, GRM5, PRIMA1, HTR2A and HTR2A [57] | The study of the methylome and transcriptome in SCZ and BD found changes in the methylation of many genes | Various; brain; various; brain; |
| Neumann et al [173] | ADHD | DNA methylation in CREB5 which is known to be important for neurite outgrowth was associated with ADHD [173] | DNA methylation at birth was associated with ADHD by performing an epigenome-wide association study (EWAS) | Blood |
| [72, 174] | MDD, BD, PTSD, SCZ and ADHD | Epigenetic changes in serotonin transporter SLC6A4 | Could be a potential biomarker as it was found in multiple MH disorders | Blood; various |
| Loke et al. [73] | ASD | (OXTR, GAD1, EN2, RELN, MECP2) | Identified five candidate genes whose methylation was affected in the brains of ASD patients [73] | Various |
| Multiple studies [74–76] | ASD | 5mC is a methylation marker involved in important functions including X-chromosome inactivation, chromatin structure, gene silencing and genomic imprinting | 5mc methylation could be a potential marker in the clinic [74–76] | Various; brain; brain |
| [175] | PTSD | Methylation levels of FKBP5 and SLC6A4 genes studied for associations with PTSD | Epigenetic insights into genes associated with PTSD | Blood |
| Kual et al. health [69] | PTSD | Genes enriched in the following pathways including stress response, inflammation and physical health [69]. Epigenetic changes in HDAC4 | Epigenetic changes were found in a gene in the blood of patients with PTSD | Blood |
Potential biomarkers from imaging studies in various MH-related disorders
Imaging biomarkers play a crucial role in not only understanding MH disorders but also help with early detection in many MH disorders. In AD, functional and structural MRI, along with amyloid imaging using PET tracers, aid in detecting changes and amyloid plaques in the brain [77–79]. For MDDs, MRIs reveal structural abnormalities in regions like the PFC, cingulate cortex, thalamus and hippocampus, offering insights into potential pathogenesis [80]. In ASD, white matter microstructure and amygdala growth abnormalities impacted brain networks in early life [81]. MRIs were also found to differentiate ADHD patients from controls based on alterations in the cortical shape in areas of the brain [82]. Such an approach could be explored further for clinical use to identify clinical symptoms and treatment response [83]. Detailed findings are summarized in Table 8.
Table 8.
Potential biomarkers from imaging studies in various MH-related disorders
| Author name or study ID | Disorder | Molecular features/biological functions affected | Study summary/key findings | Source |
|---|---|---|---|---|
| [77] | Alzheimer’s disease (AD) | Functional and structural MRI | Functional and structural magnetic resonance imaging (MRI) can be used to indicate the changes in the cerebrospinal fluid (CSF) | Resting-state functional MRI (rfMRI) |
| [78, 79] | AD | Many AD patients have amyloid-β (Aβ) plaques present in their brains long before they develop the disease. Amyloid imaging i.e., using PET tracers for detecting changes and amyloid plaques | This amyloid imaging can help with early detection purposes, but are expensive and have a hazard of radiation [78, 79] | PET; PET |
| [80] | MDD | MRI reveals structural abnormalities in PFC, cingulate cortex, thalamus and hippocampus | These abnormal brain functions may also be associated with the pathogenesis of MDD and could be studied further for early diagnosis and intervention [80] | MRI |
| [176] | SCZ and BD | Genetic variants of the genes GFAP [159], GLUL [160] and S100B [49, 161] associated with cytoskeletal effects manifested in brain imaging | Potential for use in early detection | Various |
| Lainhart et al [81] | ASD | White matter microstructure and amygdala growth abnormalities impact brain networks in early life | Potential for use in early detection in ASD | MRI |
| Sun et al [82] | ADHD | MRIs differentiate ADHD patients from controls based on cortical shape alterations | Potential biomarkers for ADHD | Anatomic and diffusion-tensor magnetic resonance (MR) imaging |
| Zilcha-Mano et al. [83] | PTSD | Resting state MRIs and ML identify unique brain abnormalities for clinical differentiation and treatment response | Such an approach could be explored further for clinical use to identify clinical symptoms and treatment response [83] | Resting-state magnetic resonance images |
Potential biomarkers from copy number studies in various MH-related disorders
Copy number variations (CNVs) in the genomic regions are linked to various MH disorders. From this summary, it is clear that there are common genomic regions of copy number instability across various MH disorders including 1p, 1q, 15q, 16p and 22q.
In AD, variations in regions of chromosome 1 and 2 were found including 1p36, 1q21,1q32, 2p23 and 2q14 [84]. People who inherit one copy of the APOE isoform APOE ε4 have an increased chance of AD and those with two copies have an even greater risk [85]. In another study, MDD patients had a higher mitochondrial DNA copy number and could be relevant to the pathophysiology of MDD [86]. For SCZ and BD, Xiao et al. pinpointed CNV ‘hot spots’, i.e. regions of large CNV as 1q32 and 22q11.22 [87, 88]. Krgović et al. predicted that the rate of CNVs in patients with ADHD was 1.33 times higher when compared to healthy controls [89]. Duplications of 15q13.3 and 16p13.11 regions were found in ADHD patients [89, 90], while deletions in the 22q11.2 and deletions/duplications in 16p11.2 were commonly observed in ASD patients [91]. The mitochondria has been found to play a crucial role in neurodegenerative diseases including AD, MDD, BD and others [23] [86]. Mitochondrial DNA copy number was found significantly lower in PTSD patients, which may reflect impaired energy metabolism [92]. Detailed findings are summarized in Table 9.
Table 9.
Potential biomarkers from copy number studies in various MH-related disorders
| Author name or study ID | Disorder | Molecular features/biological functions affected | Study summary/key findings | Source |
|---|---|---|---|---|
| Cuccaro et al [84] | AD | CNV identified in genomic regions1p36, 1q21,1q32, 2p23, and 2q14 [84] | Identification of CNVs in specific regions associated with AD (1p, 1q, 2p, 2q) | DNA |
| Multiple studies [85, 177] | AD | People who inherit one copy of the APOE isoform APOE ε4 have an increased chance of AD and those with two copies have an even greater risk [85] | APOE ε4 allele is known to affect normal brain function and early onset of memory loss. Potential biomarker | Blood |
| Chung et al [86] | MDD | Higher mitochondrial DNA copy number in MDD patients | Potential relevance of mitochondrial DNA copy number to MDD pathophysiology | Peripheral blood |
| Xiao et al. [87, 88] | MDD | Genomic regions 1q32 and 22q11.22 identified as ‘hot spots’ i.e. regions of large copy number variation for SC and BD [87, 88] | Identification of genomic regions with large CNVs in SCZ and BD | Genotyping and DNA pooling; genotyping |
| Krgović et al. [89] | ADHD | Patients with ADHD show higher CNV rate compared to healthy control | Association of higher CNV rate with ADHD | Genome-wide study |
| [89, 90] | ADHD | Duplications in 15q13.3, 16p13.11 regions found in ADHD patients | CNV in chromosomal regions 15p and 16p associated with ADHD | Genome-wide study; blood |
| [91] | ASD | Duplications in the 16p11.2 regions, and deletions in the 22q11.2 region | CNV in chromosomal regions 16p and 22q associated with ADHD | Genetic study |
| [23, 86] | AD, MDD and BD | Energy production impaired and higher levels of oxidative stress | The crucial role of mitochondria in neurodegenerative diseases | Mitochrondrial DNA |
| Bersani et al. studied [92] | PTSD | Lower mitochondrial DNA copy number (mtDNAcn) in PTSD patients | Impaired energy metabolism potentially reflected by mtDNAcn in PTSD patients | Mitochrondrial DNA |
Potential biomarkers from metabolomics and glycomics studies in various MH-related disorders
Metabolites are the substrates and products of metabolism and include sugars, lipids, amino acids, fatty acids, phenolic compounds and alkaloids among others [93]. Glycans are long chain essential carbohydrate molecules that serve structure, energy storage and regulatory purposes [94]. The advantage of using metabolites as biomarkers is that they are found in blood and serum. They can be extracted and analyzed using noninvasive and inexpensive analysis techniques. Changes in glycosylation typically occur during disease progression and have been increasingly studied for biomarker development [95]. Table 10 shows key takeaways on research in this area.
Table 10.
Potential biomarkers from metabolomics and glycomics studies in various MH-related disorders
| Author name or study ID | Disorder | Molecular features/biological functions affected | Study summary/key findings | Source |
|---|---|---|---|---|
| Mapstone et al. [96] | AD | Identification of a lipid panel in peripheral blood predicting AD development with 90% accuracy | Promising noninvasive biomarkers for early AD detection | Blood |
| Frenkel-Pinter et al. [97] | AD | Altered levels of glycans involved in protein O-GlcNAcylation and N-/O-glycosylation | Potential glyco-based AD biomarkers | Brain regions and serum |
| Hashimoto et al. [98] | MDD | Downregulation of prune metabolism and involvement of amino acid metabolism in MDD pathogenesis [98] | Identification of affected metabolic pathway in MDD | Various |
| Okamoto et al. [99] | SCZ | Lower peak values of metabolites in SCZ patients, affecting pathways like glutamate metabolism | Metabolic differences in SCZ patients, contributing to a better understanding of the disorder | Serum |
| Ren et al. [100] | BD | Identified potential BD biomarkers: lactate, trimethylamine oxide, N-acetyl glycoprotein and α-glucose | Discovery of metabolites with potential biomarker utility for BD | Serum |
| Orozco et al. [101] | ASD | Linked 11 plasma metabolites to ASD outcomes, revealing disturbances in one-carbon metabolism and the tricarboxylic acid cycle | Insights into metabolic changes in ASD and their potential roles | Plasma |
| Tian et al. [102] | ADHD | Identified differentially changed metabolites including FAPy-adenine and dopamine 4-sulfate | Metabolomic profiling in ADHD, highlighting potential metabolic contributors to the disorder | Urine |
| Karabatsiakis et al. [103] | PTSD metabolite | 13 significant metabolite changes including glycerophospholipids and an endocannabinoid signaling | Insights into metabolic alterations in PTSD patients using peripheral blood, and suggesting candidate markers and pathways of interest | Blood |
Mapstone et al. [96] identified a set of 10 lipids from the peripheral blood of people who went on to develop AD 2–3 years later with 90% accuracy. Frenkel-Pinter et al. [97] studied the changes in glycosylation pathways associated with AD, found changed levels of glycans involved in protein O-GlcNAcylation and N-/O-glycosylation and proposed for the use as novel glyco-based biomarkers for AD [97]. Hashimoto et al. [98] found purine metabolism downregulation in MDD patients and amino acid metabolism involvement in MDD pathogenesis. Okamoto et al. [99] noted reduced metabolite peak values in SCZ, affecting pathways like glutamate metabolism. Ren et al. [100] identified potential BD biomarkers: lactate, trimethylamine oxide, N-acetyl glycoprotein and α-glucose. Orozco et al. [101] linked 11 plasma metabolites to ASD outcomes, revealing disturbances in one-carbon metabolism and the tricarboxylic acid cycle. Tian et al’s [102] ADHD study identified differentially changed metabolites including FAPy-adenine and dopamine 4-sulfate. Karabatsiakis et al’s [103] PTSD research found 13 significant metabolite changes including glycerophospholipids and an endocannabinoid signaling metabolite.
Potential biomarkers from multiomics studies in various MH-related disorders
Utilizing multiple omics data types enhances our understanding of brain-related disorders [39, 104]. In AD, multiomics approaches have been applied, integrating genomics, epigenomics, transcriptomics and proteomics data to gain insights into AD pathogenesis and identify potential biomarkers.
In MDD, integration of metabolomics and proteomics unveiled intricate molecular alterations that could contribute to the pathophysiology, offering insights for potential therapeutic strategies [105]. A multiomics analysis (RNA-seq, microRNA, ChiPseq) discovered dysregulation of nuclear FGFR1 signaling in SCZ, indicating a potential therapeutic target [106]. Another multiomics analysis comparing ASD and SCZ revealed affected biological processes including neural development, synaptic dysfunction neural networks, and enriched chromatin modification in ASD [107]. Another multiomics investigation unveiled the molecular interplay between genetic variation, gene expression and methylation, providing insights into ADHD-related mechanisms [108]. This multiomics approach is instrumental in understanding the complex biology of neuropsychiatric disorders, offering potential avenues for treatment and biomarker discovery. Table 11 summarizing key findings from these studies.
Table 11.
Potential biomarkers from multiomics studies in various MH-related disorders
| Author name or study ID | Disorder | Molecular features/biological functions affected | Study summary/key findings | Source |
|---|---|---|---|---|
| Pang et al. [163] | Alzheimer’s disease (AD) | Measured genes and microRNAs expression, systems biology analysis | Identification of potential AD biomarkers, better understand AD pathogenesis | Entorhinal cortex, hippocampus and blood |
| Song et al. [178] | AD | Summarized studies and results based on the genome, transcriptome and epigenome, curated data into a database called AlzBase | Advancements towards candidate biomarkers and new hypotheses | Various |
| De Yager et al. [179] | AD | Multiomics analysis of the frontal cortex regions. The data had come from over 3000 patients that included 1179 samples from whole genome sequencing (WGS), 740 samples from DNA methylation, 712 samples from chromatin immunoprecipitation with sequencing (Chip-seq), 638 samples from RNA sequencing (RNA-seq) and 702 samples from microRNA expression profiling. The patients profiled were part of the Religious Orders Study (ROS) or the Rush Memory and Aging Project (MAP) [179, 180] | This dataset includes controls well and hence allows users to repurpose and offers opportunities for new findings | Various |
| Wang et al. [181] | AD | Generated WGS, whole exome sequencing (WES), RNA-seq and proteomic data from 258 AD brains along with clinical and pathophysiological data called the Mount Sinai cohort [181, 182] | This large-scale study of matched multiomics data in AD and control brains servers as an important resource for further analyses. The raw and processed data are publicly available. | Brain |
| Zhang et al. [105] | MDD | Studied the brains of chronic unpredictable mild stressed rat models by application of both metabolomics and proteomics. Significant changes were found in 30 metabolites and 170 proteins, related to these biological processes including impairment in amino acid metabolism and protein synthesis/degradation; dysregulation of glutamate and glycine metabolism; disturbances in fatty acid and glycerophospholipid metabolism; abnormal expression of synapse-associated proteins | Such multiomics studies could improve our understanding of the biology behind MDD and enable better treatments | Gas chromatography/mass spectrometry (GC–MS) |
| Narla et al. [106] | SCZ | Applied multiomics analysis including RNA-seq, microRNA and ChiPseq to find dysregulation of nuclear FGFR1 signaling in SCZ patients | Potential as a therapeutic target for SCZ | Plasmids expressing FGFR1 constructs and Human induced pluripotent stem cell lines, neuron committed cells |
| Goes et al. [156] | BD | Performed a large-scale meta-analysis using whole-exome sequencing (WES)and found three genes affected: MLK4, APPL2 and HSP90AA1 | Identification of BD-affected genes | Various |
| Pineda-Cirera et al. [108] | ADHD | Studied genetic variation that influences brain methylation. They found that genetic variants for ADHD were correlated with higher gene expression and lower methylation of ARTN and PIDD1. On the other hand, Genetic variants for ADHD were correlated with a lower gene expression and higher methylation of C2orf82 [108] | Interplay of gene expression and methylation changes in ADHD | Brain |
| Hubers et al. [183] | ADHD | Performed an integrative analysis of genomics, epigenomics and metabolomics data from in 596 twins (cases and controls) from the Netherlands Twin Register (NTR) and looked for associations with ADHD. The top differentially changed features included TMEM, STAP2 and DNA methylation in MAD1L1 [183] | Identification of differentially changed features related to ADHD | Urine, buccal cell swabs |
| Nomura et al. [107] | ASD versus SCZ | Performed a multiomics analysis to compare ASD and SCZ and found the several biological processed affected in both disorders including neural development, synaptic dysfunctions and neural network. The authors also found chromatin modification process to be enriched only in ASD samples | Shared and distinct biological processes in ASD and SCZ | Various |
| Dean et al. [184] | PTSD | Studied warzone-related PTSD using multiomics technologies including genetics, DNA methylation, proteomics, metabolomics, immune cell counts, cell aging, endocrine markers, microRNAs and cytokines. They applied multistep ML models to identify candidate biomarkers for PTSD. At the end of their multi step analysis, 10 top performing candidate biomarkers were identified as most relevant to PTSD, including methylation markers cg01208318, cg20578780, cg15687973 (PDE9A) and 75,938,326 C2orf3; microRNA markers hsa-mir-133a-1-3p, hsa-mir-192-5p, hsa-miR-9-1-5p, metabolite marker gamma glutamyltyrosine; clinical labs insulin and mean platelet volume and physiological marker heart rate |
Identification of top-performing candidate biomarkers for PTSD | Blood |
Potential biomarkers from cellular data in various MH-related disorders
Cellular data in the form biological functions and pathways offer another dimension to better understand the underlying mechanism in various MH disorders. While some biological functions may have come up along with the molecular markers in previous sections, the current section focuses solely on features at the cellular level. These could be pathways, biological functions and cellular processes associated with various MH disorders. Table 12 summarizes these key features. These could be used as potential targets for further research and potential therapeutic interventions.
Table 12.
Potential biomarkers from cellular data in various MH-related disorders
| Author name or study ID | Disorder | Molecular features/biological functions affected | Study summary/key findings | Source |
|---|---|---|---|---|
| Sancesario et al. [185] | AD | A meta-analysis of 96 articles related to Alzheimer’s disease that included 12 meta-analyses, 21 re-analyses of existing data and 63 original studies. Studies of brain tissues identified the following affected pathways including dopamine metabolism, mitochondrial function, oxidative stress, protein degradation, neuroinflammation, vesicular transport and synaptic transmission. Studies of the blood identified the following affected pathways including pathways involved in immune function, inflammation, RNA processing, protein chaperones, mitochondrial function and programmed cell death. | Pathways identified in both blood and brain tissue were mitochondrial function, protein degradation and inflammation indicated that AD was a systemic disease and not localized to any one region [185] | Both blood and brain tissue |
| Reitz et al [186] | AD | These included amyloid pathway, immune and inflammation system, lipid transport and metabolism, synaptic cell functioning, Tau pathology, cell migration, hippocampal synaptic function, cytoskeletal function and axonal transport and microglial and myeloid cell function | Identification of major pathways associated with AD, including immune system involvement and synaptic function | Various |
| Mirza et al. [187] | AD | The study of the electrophysiological changes indicated the following pathways contributed to the pathophysiology of AD including Glutamate receptor signaling, CREB signaling, dopamine- DARPP32 feedback in cAMP signaling and fMLP signaling in neutrophils | Implicates various neurotransmitter systems | Various |
| Li et al. [188] | AD | Identified nitric oxide, reactive oxygen species in macrophages (NOROS), NFkB and mitochondrial dysfunction and the major pathways associated with late onset AD (LOAD) [188] | Major pathways associated with late-onset AD, highlighting oxidative stress and immune response | Various |
| Mengsi et al. [158] | AD | Used gene expression data from 76 AD patients and discovered that the GABAergic (related to neurotransmitter GABA) system, neurons and synaptic function were affected in AD | AD affects neurotransmitter systems and synaptic function | Brain |
| Di Resta et al. [12] | AD | Reviewed the AD disease from an omics perspective. The molecular analyses shed light on AD pathogenesis, the cellular level analysis provided a systems biology perspective that could enable more effective treatment options | Concluded that an integration of molecular and cellular level analyses could better help with understanding of this complex disease | Various |
| Pang et al. [163] | AD | Several functional genes expressed together were affected in AD patients, including ERBB2, ERBB4, OCT3, MIF, CDK13 and GPI. Several pathways were found to be significantly dysregulated in EC and HIP brain regions, including PI3K-AKT signaling pathway, MAPK signaling pathway, oxidative phosphorylation, synaptic vesicle cycle, cell–cell adhesion, cytokine-mediated signaling pathway, proteasome, arginine and proline metabolism and pentose phosphate pathway | Analysis of two important brain regions in AD patients the entorhinal cortex (EC) and hippocampus (HIP) compared to normal controls | EC and HIP |
| [189, 190] | AD | In AD patients, the protein Tau is no longer able to help with forming structures that transport nutrients within nerve cells, which eventually leads to cell death. Hyperphosphorylation, i.e. signaling mechanisms used by the cell to regulate mitosis of this tau protein is known to be associated with AD | Importance of tau protein dysfunction and hyperphosphorylation | Clones of human brain τ isoforms |
| [191, 192] | AD | Researchers found S100A9 increased in the brains of AD patients. S100A9 is a calcium binding protein that plays an important role in the regulation of inflammatory processes and immune response [191, 192] | Increased S100A9 | Various; ultracentrifugation-electrostatic repulsion hydrophilic interaction chromatography (UC-ERLIC) coupled mass spectrometry-based proteomics profiling of soluble and aggregated amyloidal plaque |
| Howard et al. [146] | MDD | Performed gene set enrichment analysis (GSEA) on the 269 genes that they found to be associated with MDD and found the following pathways as significantly enriched including post synapse, synapse, neuron spine, excitatory synapse, behavior, cognition, neuron projection, modulation of synaptic transmission and regulation of synapse structure or activity [146, 193, 194] | Pathways associated with MDD, revealing potential therapeutic targets | Various |
| Pantazatos et al. [143] | MDD | Used RNA-seq and found the following pathways affected in MDD including lower expression of immune-related pathways like chemokine receptor activity, chemotaxis and cytokine biosynthesis and angiogenesis and vascular development | Insights into immune system involvement in MDD. | Brain |
| Forero et al. [60]. | MDD | Performed one of the largest meta-analysis of gene expression studies in MDD that covered 24 datasets that included a total of 753 samples. A functional analysis of the DEGs in MDD identified the following biological processes and KEGG pathways enriched with DEGs including synaptic transmission, neuron projection, Alzheimer’s disease pathway and proteasome pathways | Identification of biological processes and pathways in MDD | Various |
| [139] | MDD | Studies have also identified a change in the abundance of pro-inflammatory and oxidative stress response proteins in MDD. Other pathways implicated include LXR/RXR activation, acute phase response signaling, FXR/RXR activation, agranulocyte adhesion diapedesis and granulocyte adhesion diapedesis | Changes in the abundance of pro-inflammatory and oxidative stress response proteins in MDD | Blood |
| Multiple studies [7, 32, 137, 140, 193, 195] | MDD | Dysfunctional metabolic pathways for ATP production have been observed in MDD [193, 195]. This includes mitochondrial dysfunction and issues with glucose transporter proteins [32, 140]. Changes in energy metabolism-related proteins have also been identified [7, 32]. Studies have explored dysregulation in the glutamate system in MDD, particularly in the context of ketamine studies [137] | Dysregulation in metabolic and glutamate systems | Various; various; various, various, brain; various |
| Silva-Costa et al. [196] | The study of proteomic-based biomarker studies associated with MDD found gene biomarkers as part of several biological processes including inflammatory system, immune and inflammatory systems, lipid metabolism, carboxypeptidase activity, retinoid metabolic process, artery morphogenesis, coagulatory systems, cell communication, protein metabolism, regulation of the nervous system, energy metabolism, oxidative stress, and cell communication and oligodendrogenesis [196]. | Identifies potential gene biomarkers associated with MDD. | various | |
| Multiple studies [197]. [198]. | MDD | Mehta et al. reviewed gene expression and RNA-seq studies from postmortem brain and peripheral blood for potential links to MDD and found biological processes that include inflammatory response, cell survival, apoptosis and oxidative stress [197]. Lin and Tsai [198] also reviewed gene expression-based studies in MDD to identify biomarkers related to peripheral immune response and growth factors, endocrine factors and metabolic markers | Several systems implicated including immune and inflammatory, oxidative stress and more | Postmortem brain and peripheral blood; peripheral blood cells |
| [139]. [199]. | SCZ | Multiple proteins associated with SCZ belonged to the following pathways including LXR/RXR activation, FXR/RXR activation, hepatic fibrosis and hepatic stellate cell activation and atherosclerosis signaling [139]. A total of 99 peptides were found associated with BD and 202 peptides [139]. The pathways altered in SCZ included oxidative phosphorylation, mitochondrial dysfunction, EIF2 signaling, protein ubiquitination Pathway, mTOR signaling, CDK5 signaling, among others [199] | Pathways associated with SCZ, suggesting targets for further research and potential therapeutic interventions. | Blood; prefrontal pyramidal cells |
| Multiple studies [49, 159–161, 176, 200–202] | SCZ | Genetic variants of the genes GFAP [159], GLUL [160] and S100B [161] [49] were implicated including astrocyte function, including signal transduction, tyrosine kinase signaling, G protein–coupled receptor signaling, small GTPase-mediated signaling, cell adhesion and gene transcription [49, 200]. Other cell processes found altered in SCZ include reduced migration in neural precursor cells [201], Cytoskeletal effects [176], aberrations in mitochondrial function [202] | Cell processes found altered in SCZ | Various; brain; brain; various; various; brain; brain; fibroblasts |
| Depino et al. [203] | SCZ | A review of animal models of SCZ found changes in dopaminergic function and reduction in neurogenesis (the process that produces the cells of the nervous system) | Altered dopaminergic function and neurogenesis in SCZ | Animal models |
| Multiple studies: Wang et al. [204]. | SCZ | A review of RNA-seq-based studies in SCZ found GABA function, glutamate function, myelin and oligodendrocyte related processes affected. Other biological processes related to immune and inflammatory pathways (including genes IL6 and SERPINA3) and response to virus or bacteria were also affected [204] | Biological processes affected in SCZ | Various |
| [7, 44] | SCZ | Patients with SCZ were found to have abnormal smooth-pursuit eye movement and reduced anterior cingulate volumes; enlarged lateral and third ventricular volumes and white matters abnormalities [7, 44] | Potential biomarkers and structural anomalies associated with SCZ | Various; various |
| [7, 205, 206] | SCZ | Changes in oligodendrocytes, energy metabolism (NADPH) [7, 206], glutamatergic neurotransmission and cannabinoid metabolism [7, 205] | Dysregulations in SCZ | Various; cerebrospinal fluid (CSF); postmortem mediodorsal thalamus (MDT) |
| Arion et al. [199] | SCZ | Transcriptome alterations in pyramidal cells of prefrontal cortex (SCZ patients) | Transcriptome changes specific to SCZ, less prominent in BD and MDD | Prefrontal pyramidal cells |
| [61] | SCZ and MDD | Oxidative phosphorylation was the most affected pathway in MDD whereas glycolysis pathway was the most affected in SCZ brains. Pathways found commonly affected between SCZ and MDD include WNT pathway, MAPK, PTEN signaling pathways, glutamate signaling that includes SLC38A2, GRM7, GRIA2; neurodevelopment-related genes including RUNX3, ITGB1, FMR1, STAT3 and SCZ susceptibility genes PDGFRA and PPARG [61] | Shared and distinct pathways in SCZ and MDD; potential targets for understanding and treating these disorders | Blood, serum and plasma |
| [207] | BD | Mitochondrial dysfunction may be associated with decreased in mitochondrial respiration, downregulation of proteins involved in mitochondrial respiration. It could also cause changes in mitochondrial morphology, increased mitochondrial DNA polymorphisms [207] | Mitochondrial dysfunction’s potential contribution to BD progression | Various |
| [7, 208, 209] | ASD | Fragile X syndrome is the most commonly studied genetic cause for Autism [7, 208]. Hormozdiari et al. studied WES data from 1116 patients with Autism to identify two sets of gene networks. One set was found associated with Wnt, Notch, SWI/SNF and NCOR complexes. The second set was associated with synaptic function, including long-term potentiation and calcium signaling [209] | Gene network analysis reveals two sets of genes associated with ASD, shedding light on molecular pathways involved in synaptic function and complex biological processes | Various; various; various |
| [7, 141, 210, 211] | ASD | Research has linked ASD with several biological processes including oxidative stress and mitochondrial dysfunction [210], increased polarity of glyoxalase 1 (GLO1) [141] and protein phosphorylation [7, 211] | Biological processes associated with ASD | Various; brain; various; saliva |
| Multiple studies [7, 162, 212–214] | ADHD | Biological processes affected in ADHD include prefrontal dopamine deficiency, central dopaminergic dysfunction, changes in oxidative metabolism and immunity [7, 212, 213]. In recent years, dopaminergic and noradrenergic systems have risen as potential genetic and biochemical markers in ADHD diagnosis [214]. McCaffrey et al. [162] studied RNA markers in case-controlled subjects and a study of twins which revealed the genes in the galactose metabolism pathway as affected | Biological processes associated with ADHD that could be studied further towards design of targets for diagnostics and therapeutics | Various; blood; various; blood; various |
| [58, 65] | PTSD | RNA-seq analysis in individuals with PTSD revealed glucocorticoid receptor signaling and immunity-related pathways. The authors found the key biological processes associated with PTSD to be immune dysregulation and HPA axis [58]. Gupta et al. studied PTSD data from military veterans, and the micoRNAs associated with immune response inflammation were found to play a pivotal role in PTSD in veterans [65] | The key biological processes associated with PTSD are immune dysregulation and HPA axis | Blood; various |
Noteworthy themes of interest
Throughout this comprehensive review, we have encountered several noteworthy discoveries.
Dysregulation in the immune and inflammatory systems
Researchers have found that the immune system and inflammatory responses undergo a systemic change in patients affected with neurodegenerative diseases including dementia and neurodegeneration [109, 110]. Increased levels of circulating cytokines and other pro-inflammatory markers have been found and its role in these diseases is being studied in more detail [111, 112].
Heightened activity within the HPA axis
The heightened activity within the HPA axis has been consistently observed across various MH disorders and in this review. Furthermore, multiple NGS studies have implicated the role of serotonin transporter SLC6A4 in MH disorders. An interesting parallel was found in the involvement of the dopamine transporter gene SLC6A3 in neuropsychiatric conditions [113]. While dopamine predominantly resides in the brain and serotonin predominantly in the gut, both neurotransmitters play pivotal roles not only in MH but also in gut health [114]. Presently, the interactions between these neurotransmitters remain currently unclear. However, a detailed exploration of complex interconnections in the body (i.e. ‘axis’) may provide valuable insights interactions [115]. There are various connections in the body such as gut–brain, gut–lung and gut–skin axes. Interestingly, these axes are also linked with the immune system. Below, we discuss in more detail the gut–brain axis and potential for the gut microbiome in MH disorders as a future direction.
The gut–brain axis and the microbiome: an emerging biomarker in MH
The gut microbiome, comprising trillions of microorganisms in the gastrointestinal tract, is a novel biomarker with far-reaching implications for MH. Linked through the gut–brain axis, it exerts bidirectional influence over human behavior and brain function via the immune, nervous and endocrine systems [116]. Dysregulations in gut microbiota have been associated with neurodegenerative diseases, mood disorders and depression, often driven by chronic inflammation converting into mood symptoms [116, 117]. Stress influences neuroendocrine hormones affecting bacterial growth, and consequently, behavior, metabolism, appetite and immunity. Microbiome research is particularly extensive in MDD and neuropsychiatric conditions like SCZ and BD. MDD shows microbiota alterations, while SCZ and BD exhibit systemic immune changes [116–118]. Furthermore, ASD and ADHD have their unique microbiota profiles. Preclinical AD is associated with gut microbiome shifts correlating with pathological markers. [119–121, 214]. Complementary therapies like ‘psychobiotics’ and fecal transplants are being explored alongside traditional treatments, promising new avenues for MH understanding and intervention [122].
DISCUSSION
A systems biology approach
We used the potential NGS biomarkers indicated in this article to demonstrate how the results could give us insights to the underlying mechanisms of Alzheimer’s. We performed a systems biology analysis using an online interaction network tool StringDB (https://string-db.org/) [123] and subsequently an enrichment analysis using online tool EnrichR (https://maayanlab.cloud/Enrichr/) [124]. Figure 1 shows a gene interaction network obtained from the potential NGS biomarkers. The lines that connect the genes are the edges of this network and indicate gene associations obtained from various types of evidences including curated databases, experimentally determined connections or predicted interactions by published literature. From our enrichment analyses, we identified many of the genes to be associated with regulation of amyloid fibril formation and positive regulation of amyloid-beta clearance validating the gene results and its disease association (Figure 2).
Figure 1.
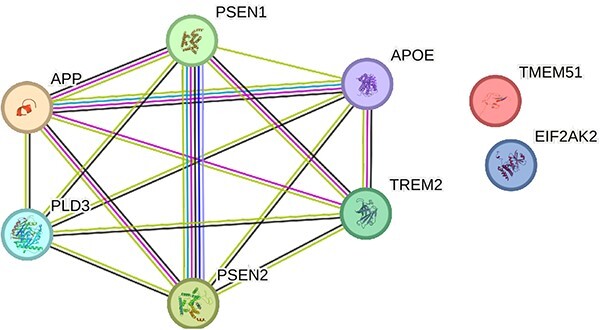
Alzheimer’s disease gene interaction network input to this network: TREM2, PLD3, DLGPAP1, TMEM51, EIF2AK2, APP, PSEN1, PSEN2, APOE. Genes that are known to interact with each other are connected by cyan lines (information obtained from curated databases) or magenta lines (experimentally determined connections). The genes that could be in the same neighborhood are connected by green lines, those that could have gene fusions are linked by red lines and those genes that could co-occur are linked by blue lines.
Figure 2.
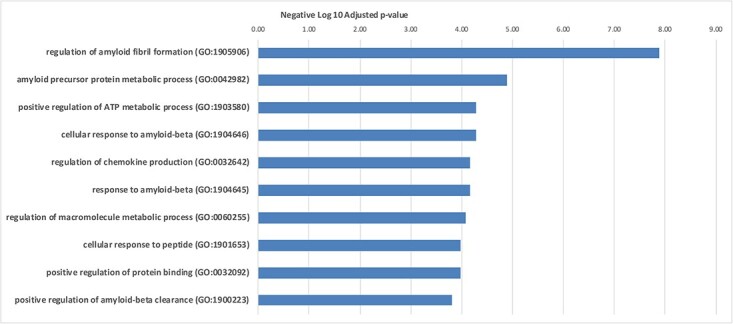
Top enriched gene ontology biological processes in AD. The x axis shows the negative log base 10 of the adjusted P value. The y axis indicates the various GO terms enriched.
A similar analysis was performed using the potential biomarker genes associated with MDD. We used StringDB which generated two gene interaction networks (Figure 3). Enrichment analysis was performed on the gene network on the left side using EnrichR and was found to be involved in serotonin signaling receptor pathway and serotonin metabolism. Serotonin binding deficits have been well documented in MDD literature [125]. Enrichment analysis performed on the genes network on the right side revealed RNA editing mechanisms which have been documented in association with MDD in a publication [126].
Figure 3.
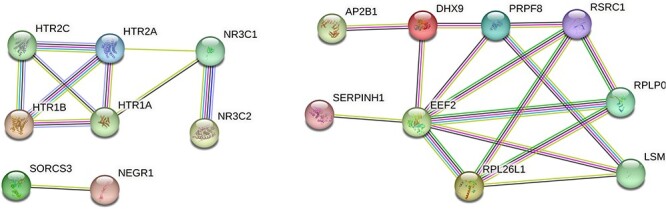
Gene interaction networks in MDD Input gene list: SORCS3, NEGR1, NR3C2, NR3C1, MTRNRL8, SERPINH1, CCL4, SLC1A2, GABRD, HTR1A, HTR1B, HTR2A, HTR2C, PXMP2, EEF2, RPL26L1, RPLP0, PRPF8, LSM3, DHX9, RSRC1, AP2B1 Genes that are known to interact with each other are connected by cyan lines (information obtained from curated databases) or magenta lines (experimentally determined connections). The genes that could be in the same neighborhood are connected by green lines, those that could have gene fusions are linked by red lines and those genes that could co-occur are linked by blue lines.
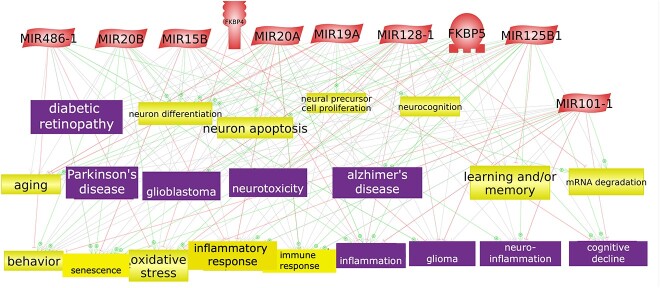
A third analysis was performed using the potential biomarker microRNAs and genes associated with PTSD (Figure 4). These molecular features were enriched by the annotation of common cell processes and diseases relevant to MH. This analysis was performed using Elsevier Pathway Studio software (www.pathwaystudio.com).
Figure 4.
microRNAs and genes associated with PTSD enriched with common cell processes and diseases. The figure shows an interaction network between the microRNAs and genes associated with PTSD. First connections between the microRNAs and genes were found and then the common cell processes and diseases were added to the network. The microRNAs are represented as red parallelograms, the cell processes are the yellow colored boxes and the diseases are the purple colored boxes.
Such an evidence-based analysis demonstrates the power of connecting the candidate biomarkers to biology using a systems biology approach. It not only pinpoints the biological processes affected but also creates ideas and opportunities for new hypotheses generation and experiments for therapeutic intervention.
Knowledge extracted and cataloged from research in the molecular and cellular domains could enable us to identify specialized pathways relevant to the MH domain. This would enable very sophisticated downstream system biology analysis and that could offer new insights into mechanisms of MH disorders. Such a cellular level analysis in conjunction with a multiomics analysis could help understand the functioning of the disorder at a systems level and improve our understanding of the disorders.
Taking advantage of publicly available MH datasets and resources
Throughout this review process for this article, we encountered many important large datasets and/or resources relevant to this topic. Many of these resources follow the FAIR principles of Findable, Accessible, Interoperable and Reproducible [127] and could empower researchers to kick-start their analyses without the need to apply for a federal grant to gather sample data. Data from such studies could be used in meta-analyses or case control association studies using powerful modern ML tools or AI-based models. Reuse of such publicly available resources enable researchers to perform an in-depth data analysis for their discovery or validation experiments. These resources have been summarized in Supplementary File 1.
Integration of molecular technologies in the clinic for MH disorders
The integration of molecular technologies into the clinical realm for MH disorders is a promising avenue for enhancing diagnosis and treatment. In the context of MDD, traditional diagnostic methods involving questionnaires and clinical assessments are being complemented by molecular omics technologies [128]. Researchers are exploring the potential clinical application of pharmacogenomic testing in MDD [129]. Antidepressant drugs have been found to influence the epigenome through multiple mechanisms. Drug Genipin has been found to reduce activity of enzyme DNMT1 which preserves the DNA methylation patterns during replication [130]. Another drug Paroxetine was found to change phosphorylation of DNMT1 which again affects enzyme activity. Other drugs known as histone deacetylase (HDAC) inhibitors have been found to have antidepressant effects through regulation of gene transcription [131]. HDAC inhibitors and cyclooxygenase-2 (COX-2) inhibitors also show promise in treating MDD in animal models [7, 132]. Other examples include the use of pharmacogenomic markers of CYP450 to predict drug response or adverse effects in psychiatric drugs. Another example includes confirming drug treatment in certain genetic conditions including Down syndrome, Fragile X syndrome, phenylketonuria and 22q11 deletion syndrome [133]. Understanding the functions and biological processes of the potential biomarkers would allow scientists to explore how they could be integrated into the clinic.
Towards clinical decision support systems
In the realm of clinical cancer care, sequencing-based results and reports, known as Molecular Diagnostic (MolDx) panels, are gradually making their way into practice. The FDA has approved an increasing number of NGS-based biomarkers that are covered by insurance for both diagnostic and treatment purposes. Genetic counseling often accompanies these tests, ensuring informed decision-making. A notable example in the field of MH is the GeneSight test (genesight.com) [134], which assesses genetic variants to guide drug selection for psychiatric disorders. These advancements open the door to creating tailored molecular diagnostic panels for each MH disorder. These panels could be integrated into CDSSs, along with patient medical and drug history, to suggest suitable medications. Clinicians could then use this information to devise more effective treatment plans, reducing the likelihood of adverse reactions or poor responses, ultimately lowering hospital readmissions and costs. This progress holds the potential to drive personalized medicine forward through sophisticated ML models within CDSS.
Challenges
Challenges and future directions in MH research are multifaceted. Patients with mental illness are at an elevated risk for other health issues like cardiovascular disease [135] and type 2 diabetes [136], emphasizing the need to bridge the gap between psychiatric disorders and their physiological manifestations. While progress has been made in identifying biomarkers, actionable ones are still limited [49], requiring more validation and research. There is a growing demand for increased funding in MH research, as well as the development of noninvasive diagnostic procedures, expanded insurance coverage and cost-effective diagnostic and treatment options [7].
Future directions
In the realm of future directions for MH research, several promising avenues emerge. Firstly, there is a growing interest in creating diagnostic panels using the potential biomarkers discussed in this article. This approach, already successful in cancer diagnostics and often covered by insurance, holds the potential to streamline and enhance MH disorder diagnoses, making them more accessible and cost-effective.
Moreover, the application of these potential biomarkers could lead to more sophisticated interaction analyses, a few examples of which were showcased in this article. These can provide deeper insights into the underlying mechanisms of these disorders. This includes exploring intricate relationships between various biological markers, shedding light on the complex nature of MH conditions.
To gain a comprehensive understanding, researchers could employ advanced statistical analyses, such as correlation or ML approaches to examine the multiomics biomarkers across various MH disorders listed in this article. This approach could uncover shared or distinct patterns, aiding in tailored diagnostic and treatment strategies.
Additionally, the gut–brain axis and its connections to the immune system represent an intriguing area for further exploration. Investigating the role of the gut microbiome in MH disorders could yield critical insights into the connection between the gastrointestinal system and brain function, potentially opening new avenues for intervention.
Lastly, it is essential to expand this research beyond the disorders already discussed. Applying similar methodologies to other MH conditions could broaden our understanding of the molecular underpinnings of these disorders, ultimately paving the way for more effective diagnostics and treatments across the MH spectrum.
CONCLUSION
Neuroscience research has made tremendous progress in understanding the processes that govern the system. But not much progress has been made in translating this research into the clinic to treat psychiatric disorders. Biomarkers can bridge this gap which is why it is crucial to extract knowledge from molecular and cellular research using a broad array of bioinformatics and data analytics methods, tools and resources [121, 137]. The progressive identification of new biomarkers in the MH space could enable researchers to build advanced CDSS empowered by sophisticated ML models to advance personalized medicine.
Our findings suggest that MH disorders we reviewed in this paper involve complex molecular and cellular changes, affecting various pathways and processes, including protein function, phosphorylation, inflammation and immune responses, many of which could lead to new diagnostic or prognostic biomarkers. It also highlights the tremendous scope and opportunity for application of molecular and cellular data to further MH research. As we strive to integrate MH disorders into mainstream EHR systems, the power of translational bioinformatics and systems medicine will enable us to overcome the stigma associated with these disorders and accelerate new funding for research studies, in silico and lab analyses and findings.
Key Points
Biomarkers in mental health disorders: The article discusses various potential biomarkers associated with different mental health disorders, shedding light on the molecular and cellular aspects of conditions like AD, MDD, SCZ, BD, ASD and ADHD.
Omics technologies: The work emphasizes the importance of omics technologies, including genomics, proteomics, epigenetics, DNA copy number, microRNA and multi-omics analysis, in identifying and understanding these potential biomarkers. These technologies provide valuable insights into the biological processes underlying mental health disorders.
Gut–brain axis and the immune system: The article highlights the emerging role of the gut–brain axis in mental health. It discusses how the gut microbiome and its interactions with the brain through the immune and endocrine systems can influence mental health conditions, paving the way for potential diagnostic and therapeutic interventions.
Example evidence-based analysis: We used the published results in mental health disorders and performed evidence-based analysis to demonstrate the power of connecting the candidate biomarkers to biology using a systems biology approach. It not only pinpoints the biological processes affected but also creates ideas and opportunities for new hypotheses generation and experiments for therapeutic intervention.
Clinical integration: The work explores the integration of molecular technologies, such as pharmacogenomic testing, into clinical practice for mental health disorders. It discusses how these technologies can aid in personalized treatment plans and improve treatment outcomes.
Supplementary Material
Author Biographies
Krithika Bhuvaneshwar is a senior bioinformatician and research instructor at the Georgetown University Innovation Center for Biomedical Informatics (Georgetown-ICBI). She has expertise in bioinformatics analysis and systems biology research and combines her interdisciplinary skills in bioinformatics and applied biostatistics for numerous projects.
Yuriy Gusev is an associate professor of bioinformatics and the bioinformatics lead at the Georgetown University Innovation Center for Biomedical Informatics (Georgetown-ICBI). He has over 20 years of experience in academic and industry research in the fields of bioinformatics, systems biology, biomarker discovery and computational modeling of biological systems.
Contributor Information
Krithika Bhuvaneshwar, Innovation Center for Biomedical Informatics (ICBI), Georgetown University, Washington DC, 20007, USA.
Yuriy Gusev, Innovation Center for Biomedical Informatics (ICBI), Georgetown University, Washington DC, 20007, USA.
AUTHOR CONTRIBUTIONS
K.B. conceptualized and wrote the paper. Y.G. reviewed and edited the paper.
FUNDING
The work was partly funded and in part supported by the National Center For Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) under Award Number UL1TR001409. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. Luo J, Wu M, Gopukumar D, Zhao Y. Big data application in biomedical research and health care: a literature review. Biomed Inform Insights 2016;8:BII.S31559–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stephens ZD, Lee SY, Faghri F, et al. Big data: astronomical or genomical? PLoS Biol 2015;13(7):e1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lin Y, Qian F, Shen L, et al. Computer-aided biomarker discovery for precision medicine: data resources, models and applications. Brief Bioinform 2019;20(3):952–75. [DOI] [PubMed] [Google Scholar]
- 4. Toga AW, Foster I, Kesselman C, et al. Big biomedical data as the key resource for discovery science. J Am Med Inform Assoc 2015;22(6):1126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhuvaneshwar K, Gusev Y. Bioinformatics in mental health: deriving knowledge from molecular and cellular data (Chapter 11). In: Tenenbaum JD, Ranallo PA (eds). Mental Health Informatics Enabling a Learning Mental Healthcare System. 1st edition. Springer, 2021, 265–94. [Google Scholar]
- 6. Tenenbaum JD, Bhuvaneshwar K, Gagliardi JP, et al. Translational bioinformatics in mental health: open access data sources and computational biomarker discovery. Brief Bioinform 2019;20(3):842–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sokolowska I, Ngounou Wetie AG, Wormwood K, et al. The potential of biomarkers in psychiatry: focus on proteomics. J Neural Transm (Vienna) 2015;122(Suppl 1):S9–18. [DOI] [PubMed] [Google Scholar]
- 8. Herron JW, Nerurkar L, Cavanagh J. Neuroimmune biomarkers in mental illness. Curr Top Behav Neurosci 2018;40:45–78. [DOI] [PubMed] [Google Scholar]
- 9. Biologically-inspired biomarkers for mental disorders. EBioMedicine 2017;17:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ristori MV, Mortera SL, Marzano V, et al. Proteomics and metabolomics approaches towards a functional insight onto autism spectrum disorders: phenotype stratification and biomarker discovery. Int J Mol Sci 2020;21(17):6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Breijyeh Z, Karaman R. Comprehensive review on Alzheimer's disease: causes and treatment. Molecules 2020;25(24):5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Di Resta C, Ferrari M. New molecular approaches to Alzheimer's disease. Clin Biochem 2019;72:81–6. [DOI] [PubMed] [Google Scholar]
- 13. Otte C, Gold SM, Penninx BW, et al. Major depressive disorder. Nat Rev Dis Primers 2016;2:16065. [DOI] [PubMed] [Google Scholar]
- 14. Perez-Caballero L, Torres-Sanchez S, Romero-Lopez-Alberca C, et al. Monoaminergic system and depression. Cell Tissue Res 2019;377(1):107–13. [DOI] [PubMed] [Google Scholar]
- 15. Institute of Medicine (US) Committee on Nervous System Disorders in Developing Countries . Neurological, Psychiatric, and Developmental Disorders: Meeting the Challenge in the Developing World. National Academies Press (US), 2001. 10.17226/10111. [DOI] [PubMed] [Google Scholar]
- 16. Morris-Rosendahl DJ, Crocq MA. Neurodevelopmental disorders-the history and future of a diagnostic concept. Dialog Clin Neurosci 2020;22(1):65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smoller JW. The genetics of stress-related disorders: PTSD, depression, and anxiety disorders. Neuropsychopharmacology 2016;41(1):297–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lista S, Zetterberg H, O'Bryant SE, et al. Evolving relevance of neuroproteomics in Alzheimer's disease. Methods Mol Biol 2017;1598:101–15. [DOI] [PubMed] [Google Scholar]
- 19. Brinkmalm A, Portelius E, Ohrfelt A, et al. Explorative and targeted neuroproteomics in Alzheimer's disease. Biochim Biophys Acta 2015;1854(7):769–78. [DOI] [PubMed] [Google Scholar]
- 20. Liao L, Cheng D, Wang J, et al. Proteomic characterization of postmortem amyloid plaques isolated by laser capture microdissection. J Biol Chem 2004;279(35):37061–8. [DOI] [PubMed] [Google Scholar]
- 21. Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 2011;1(1):a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Butterfield DA, Hardas SS, Lange ML. Oxidatively modified glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and Alzheimer's disease: many pathways to neurodegeneration. J Alzheimers Dis 2010;20(2):369–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Srivastava S. The mitochondrial basis of aging and age-related disorders. Genes (Basel) 2017;8(12):398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adav SS, Park JE, Sze SK. Quantitative profiling brain proteomes revealed mitochondrial dysfunction in Alzheimer's disease. Mol Brain 2019;12(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moya-Alvarado G, Gershoni-Emek N, Perlson E, Bronfman FC. Neurodegeneration and Alzheimer's disease (AD). What can proteomics tell us about the Alzheimer's brain? Mol Cell Proteomics 2016;15(2):409–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Butterfield DA, Di Domenico F, Swomley AM, et al. Redox proteomics analysis to decipher the neurobiology of Alzheimer-like neurodegeneration: overlaps in Down's syndrome and Alzheimer's disease brain. Biochem J 2014;463(2):177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sultana R, Boyd-Kimball D, Poon HF, et al. Redox proteomics identification of oxidized proteins in Alzheimer's disease hippocampus and cerebellum: an approach to understand pathological and biochemical alterations in AD. Neurobiol Aging 2006;27(11):1564–76. [DOI] [PubMed] [Google Scholar]
- 28. Hondius DC, van Nierop P, Li KW, et al. Profiling the human hippocampal proteome at all pathologic stages of Alzheimer's disease. Alzheimers Dement 2016;12(6):654–68. [DOI] [PubMed] [Google Scholar]
- 29. Mosconi L, Pupi A, De Leon MJ. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer's disease. Ann N Y Acad Sci 2008;1147:180–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bot M, Chan MK, Jansen R, et al. Serum proteomic profiling of major depressive disorder. Transl Psychiatry 2015;5(7):e599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gellen B, Volgyi K, Gyorffy BA, et al. Proteomic investigation of the prefrontal cortex in the rat clomipramine model of depression. J Proteomics 2017;153:53–64. [DOI] [PubMed] [Google Scholar]
- 32. Martins-de-Souza D. Proteomics, metabolomics, and protein interactomics in the characterization of the molecular features of major depressive disorder. Dialog Clin Neurosci 2014;16(1):63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Adams RA, Huys QJ, Roiser JP. Computational psychiatry: towards a mathematically informed understanding of mental illness. J Neurol Neurosurg Psychiatry 2016;87(1):53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ren J, Zhao G, Sun X, et al. Identification of plasma biomarkers for distinguishing bipolar depression from major depressive disorder by iTRAQ-coupled LC-MS/MS and bioinformatics analysis. Psychoneuroendocrinology 2017;86:17–24. [DOI] [PubMed] [Google Scholar]
- 35. Park DI, Turck CW. Interactome studies of psychiatric disorders. Adv Exp Med Biol 2019;1118:163–73. [DOI] [PubMed] [Google Scholar]
- 36. da Silva BS, Leffa DT, Beys-da-Silva WO, et al. Integrative proteomics and pharmacogenomics analysis of methylphenidate treatment response. Transl Psychiatry 2019;9(1):308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kikuchi M, Nakaya A. Bioinformatics analysis of Alzheimer's disease. Brain Nerve 2017;69(7):835–42. [DOI] [PubMed] [Google Scholar]
- 38. Bertram L. Next generation sequencing in Alzheimer's disease. Methods Mol Biol 2016;1303:281–97. [DOI] [PubMed] [Google Scholar]
- 39. Verheijen J, Sleegers K. Understanding Alzheimer disease at the Interface between genetics and Transcriptomics. Trends Genet 2018;34(6):434–47. [DOI] [PubMed] [Google Scholar]
- 40. Mikulska J, Juszczyk G, Gawronska-Grzywacz M, Herbet M. HPA Axis in the Pathomechanism of depression and schizophrenia: new therapeutic strategies based on its participation. Brain Sci 2021;11(10):1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nashed MG, Linher-Melville K, Frey BN, Singh G. RNA-sequencing profiles hippocampal gene expression in a validated model of cancer-induced depression. Genes Brain Behav 2016;15(8):711–21. [DOI] [PubMed] [Google Scholar]
- 42. Keller J, Gomez R, Williams G, et al. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry 2017;22(4):527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heim C, Bradley B, Mletzko TC, et al. Effect of childhood trauma on adult depression and neuroendocrine function: sex-specific moderation by CRH receptor 1 gene. Front Behav Neurosci 2009;3:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pies R. Psychiatric diagnosis and the Pathologist's view of schizophrenia. Psychiatry (Edgmont) 2008;5(7):62–5. [PMC free article] [PubMed] [Google Scholar]
- 45. Hashimoto R, Numakawa T, Ohnishi T, et al. Impact of the DISC1 Ser704Cys polymorphism on risk for major depression, brain morphology and ERK signaling. Hum Mol Genet 2006;15(20):3024–33. [DOI] [PubMed] [Google Scholar]
- 46. Kilpinen H, Ylisaukko-Oja T, Hennah W, et al. Association of DISC1 with autism and Asperger syndrome. Mol Psychiatry 2008;13(2):187–96. [DOI] [PubMed] [Google Scholar]
- 47. Thomson PA, Wray NR, Millar JK, et al. Association between the TRAX/DISC locus and both bipolar disorder and schizophrenia in the Scottish population. Mol Psychiatry 2005;10(7):657–68 616. [DOI] [PubMed] [Google Scholar]
- 48. Li J, Cai T, Jiang Y, et al. Genes with de novo mutations are shared by four neuropsychiatric disorders discovered from NPdenovo database. Mol Psychiatry 2016;21(2):290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Falk A, Heine VM, Harwood AJ, et al. Modeling psychiatric disorders: from genomic findings to cellular phenotypes. Mol Psychiatry 2016;21(9):1167–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sjaarda CP, Hecht P, McNaughton AJM, et al. Interplay between maternal Slc6a4 mutation and prenatal stress: a possible mechanism for autistic behavior development. Sci Rep 2017;7(1):8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Froehlich TE, McGough JJ, Stein MA. Progress and promise of attention-deficit hyperactivity disorder pharmacogenetics. CNS Drugs 2010;24(2):99–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McGough JJ. Attention deficit hyperactivity disorder pharmacogenetics: the dopamine transporter and D4 receptor. Pharmacogenomics 2012;13(4):365–8. [DOI] [PubMed] [Google Scholar]
- 53. Wang M, Peng IF, Li S, Hu X. Dysregulation of antimicrobial peptide expression distinguishes Alzheimer's disease from normal aging. Aging (Albany NY) 2020;12:690–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ciobanu LG, Sachdev PS, Trollor JN, et al. Differential gene expression in brain and peripheral tissues in depression across the life span: a review of replicated findings. Neurosci Biobehav Rev 2016;71:281–93. [DOI] [PubMed] [Google Scholar]
- 55. Feng Y, Kapornai K, Kiss E, et al. Association of the GABRD gene and childhood-onset mood disorders. Genes Brain Behav 2010;9(6):668–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Serotonergic . https://www.sciencedirect.com/topics/neuroscience/serotonergic (5 January 2021, date last accessed).
- 57. Xiao Y, Camarillo C, Ping Y, et al. The DNA methylome and transcriptome of different brain regions in schizophrenia and bipolar disorder. PloS One 2014;9(4):e95875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kuan PF, Waszczuk MA, Kotov R, et al. Gene expression associated with PTSD in world trade Center responders: an RNA sequencing study. Transl Psychiatry 2017;7(12):1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mouillet-Richard S, Baudry A, Launay JM, Kellermann O. MicroRNAs and depression. Neurobiol Dis 2012;46(2):272–8. [DOI] [PubMed] [Google Scholar]
- 60. Forero DA, Guio-Vega GP, Gonzalez-Giraldo Y. A comprehensive regional analysis of genome-wide expression profiles for major depressive disorder. J Affect Disord 2017;218:86–92. [DOI] [PubMed] [Google Scholar]
- 61. Gruzdev SK, Yakovlev AA, Gruzdev SK, et al. The missing link: how exosomes and miRNAs can help in bridging psychiatry and molecular biology in the context of depression, bipolar disorder and schizophrenia. Cell Mol Neurobiol 2019;39(6):729–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Qian Y, Song J, Ouyang Y, et al. Advances in roles of miR-132 in the nervous system. Front Pharmacol 2017;8:770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kohen R, Dobra A, Tracy JH, Haugen E. Transcriptome profiling of human hippocampus dentate gyrus granule cells in mental illness. Transl Psychiatry 2014;4(3):e366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nakata M, Kimura R, Funabiki Y, et al. MicroRNA profiling in adults with high-functioning autism spectrum disorder. Mol Brain 2019;12(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gupta S, Guleria RS, Szabo YZ. MicroRNAs as biomarker and novel therapeutic target for posttraumatic stress disorder in veterans. Psychiatry Res 2021;305:114252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang S, Qin C, Cao G, et al. Genome-wide analysis of DNA methylation profiles in a senescence-accelerated mouse prone 8 brain using whole-genome bisulfite sequencing. Bioinformatics 2017;33(11):1591–5. [DOI] [PubMed] [Google Scholar]
- 67. Li M, D'Arcy C, Li X, et al. What do DNA methylation studies tell us about depression? A systematic review. Transl Psychiatry 2019;9(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hack LM, Fries GR, Eyre HA, et al. Moving pharmacoepigenetics tools for depression toward clinical use. J Affect Disord 2019;249:336–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kuan PF, Waszczuk MA, Kotov R, et al. An epigenome-wide DNA methylation study of PTSD and depression in world trade Center responders. Transl Psychiatry 2017;7(6):e1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Albert PR, Le Francois B, Vahid-Ansari F. Genetic, epigenetic and posttranscriptional mechanisms for treatment of major depression: the 5-HT1A receptor gene as a paradigm. J Psychiatry Neurosci 2019;44(3):164–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vialou V, Feng J, Robison AJ, Nestler EJ. Epigenetic mechanisms of depression and antidepressant action. Annu Rev Pharmacol Toxicol 2013;53:59–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kular L, Kular S. Epigenetics applied to psychiatry: clinical opportunities and future challenges. Psychiatry Clin Neurosci 2018;72(4):195–211. [DOI] [PubMed] [Google Scholar]
- 73. Loke YJ, Hannan AJ, Craig JM. The role of epigenetic change in autism spectrum disorders. Front Neurol 2015;6:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ansel A, Rosenzweig JP, Zisman PD, et al. Variation in gene expression in autism Spectrum disorders: an extensive review of transcriptomic studies. Front Neurosci 2016;10:601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cheng Y, Li Z, Manupipatpong S, et al. 5-Hydroxymethylcytosine alterations in the human postmortem brains of autism spectrum disorder. Hum Mol Genet 2018;27(17):2955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Madrid A, Papale LA, Alisch RS. New hope: the emerging role of 5-hydroxymethylcytosine in mental health and disease. Epigenomics 2016;8(7):981–91. [DOI] [PubMed] [Google Scholar]
- 77. Huang CC, Huang WM, Chen CH, et al. The Alzheimer's disease neuroimaging I, Lin CP: the combination of functional and structural MRI is a potential screening tool in Alzheimer's disease. Front Aging Neurosci 2018;10:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rowe CC, Villemagne VL. Brain amyloid imaging. J Nucl Med 2011;52(11):1733–40. [DOI] [PubMed] [Google Scholar]
- 79. Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh compound-B. Ann Neurol 2004;55(3):306–19. [DOI] [PubMed] [Google Scholar]
- 80. Qiu H, Li J. Major depressive disorder and magnetic resonance imaging: a mini-review of recent progress. Curr Pharm Des 2018;24(22):2524–9. [DOI] [PubMed] [Google Scholar]
- 81. Lainhart JE. Brain imaging research in autism spectrum disorders: in search of neuropathology and health across the lifespan. Curr Opin Psychiatry 2015;28(2):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sun H, Chen Y, Huang Q, et al. Psychoradiologic utility of MR imaging for diagnosis of attention deficit hyperactivity disorder: a Radiomics analysis. Radiology 2018;287(2):620–30. [DOI] [PubMed] [Google Scholar]
- 83. Zilcha-Mano S, Zhu X, Suarez-Jimenez B, et al. Diagnostic and predictive neuroimaging biomarkers for posttraumatic stress disorder. Biol Psychiatry Cogn Neurosci Neuroimag 2020;5(7):688–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cuccaro D, De Marco EV, Cittadella R, Cavallaro S. Copy number variants in Alzheimer's disease. J Alzheimers Dis 2017;55(1):37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Alzheimer's Disease Genetics Fact Sheet. https://www.nia.nih.gov/health/alzheimers-disease-genetics-fact-sheet (5 January 2021, date last accessed).
- 86. Chung JK, Lee SY, Park M, et al. Investigation of mitochondrial DNA copy number in patients with major depressive disorder. Psychiatry Res 2019;282:112616. [DOI] [PubMed] [Google Scholar]
- 87. Nothen MM, Nieratschker V, Cichon S, Rietschel M. New findings in the genetics of major psychoses. Dialog Clin Neurosci 2010;12(1):85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Malhotra D, Sebat J. CNVs: harbingers of a rare variant revolution in psychiatric genetics. Cell 2012;148(6):1223–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Krgović D. Role of copy number variations in ADHD. In: Kumperščak HG (ed). ADHD - From Etiology to Comorbidity. Intechopen, 2021. [Google Scholar]
- 90. Williams NM, Franke B, Mick E, et al. Genome-wide analysis of copy number variants in attention deficit hyperactivity disorder: the role of rare variants and duplications at 15q13.3. Am J Psychiatry 2012;169(2):195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Velinov M. Genomic copy number variations in the autism clinic-work in progress. Front Cell Neurosci 2019;13:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bersani FS, Morley C, Lindqvist D, et al. Mitochondrial DNA copy number is reduced in male combat veterans with PTSD. Prog Neuropsychopharmacol Biol Psychiatry 2016;64:10–7. [DOI] [PubMed] [Google Scholar]
- 93. Soo EC, Hui JP. Metabolomics in glycomics. Methods Mol Biol 2010;600:175–86. [DOI] [PubMed] [Google Scholar]
- 94. Glycan . https://www.sciencedirect.com/topics/neuroscience/glycan (5 January 2021, date last accessed).
- 95. Kam RKT, Poon TCW. The potentials of glycomics in biomarker discovery. Clin Proteomics 2008;4(3–4):67–79. [Google Scholar]
- 96. Mapstone M, Cheema AK, Fiandaca MS, et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med 2014;20(4):415–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Frenkel-Pinter M, Shmueli MD, Raz C, et al. Interplay between protein glycosylation pathways in Alzheimer's disease. Sci Adv 2017;3(9):e1601576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hashimoto K. Metabolomics of major depressive disorder and bipolar disorder: overview and future perspective. Adv Clin Chem 2018;84:81–99. [DOI] [PubMed] [Google Scholar]
- 99. Okamoto N, Ikenouchi A, Watanabe K, et al. A metabolomics study of serum in hospitalized patients with chronic schizophrenia. Front Psych 2021;12:763547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ren Y, Bao S, Jia Y, et al. Metabolic profiling in bipolar disorder patients during depressive episodes. Front Psych 2020;11:569612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Orozco JS, Hertz-Picciotto I, Abbeduto L, Slupsky CM. Metabolomics analysis of children with autism, idiopathic-developmental delays, and down syndrome. Transl Psychiatry 2019;9(1):243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tian X, Liu X, Wang Y, et al. Urinary metabolomic study in a healthy children population and metabolic biomarker discovery of attention-deficit/hyperactivity disorder (ADHD). Front Psych 2022;13:819498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Karabatsiakis A, Hamuni G, Wilker S, et al. Metabolite profiling in posttraumatic stress disorder. J Mol Psychiatry 2015;3(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Avramouli A, Vlamos PM. Integrating omic technologies in Alzheimer's disease. Adv Exp Med Biol 2017;987:177–84. [DOI] [PubMed] [Google Scholar]
- 105. Zhang Y, Yuan S, Pu J, et al. Integrated metabolomics and proteomics analysis of hippocampus in a rat model of depression. Neuroscience 2018;371:207–20. [DOI] [PubMed] [Google Scholar]
- 106. Narla ST, Lee YW, Benson CA, et al. Common developmental genome deprogramming in schizophrenia - role of integrative nuclear FGFR1 signaling (INFS). Schizophr Res 2017;185:17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Nomura J, Mardo M, Takumi T. Molecular signatures from multi-omics of autism spectrum disorders and schizophrenia. J Neurochem 2021;159(4):647–59. [DOI] [PubMed] [Google Scholar]
- 108. Pineda-Cirera L, Shivalikanjli A, Cabana-Dominguez J, et al. Exploring genetic variation that influences brain methylation in attention-deficit/hyperactivity disorder. Transl Psychiatry 2019;9(1):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Franceschi C, Bonafe M, Valensin S, et al. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 2000;908:244–54. [DOI] [PubMed] [Google Scholar]
- 110. Costantini E, D'Angelo C, Reale M. The role of immunosenescence in neurodegenerative diseases. Mediators Inflamm 2018;2018:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Weiskopf D, Weinberger B, Grubeck-Loebenstein B. The aging of the immune system. Transpl Int 2009;22(11):1041–50. [DOI] [PubMed] [Google Scholar]
- 112. Qin L, Jing X, Qiu Z, et al. Aging of immune system: immune signature from peripheral blood lymphocyte subsets in 1068 healthy adults. Aging (Albany NY) 2016;8(5):848–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Reith MEA, Kortagere S, Wiers CE, et al. The dopamine transporter gene SLC6A3: multidisease risks. Mol Psychiatry 2022;27(2):1031–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chen Y, Xu J, Chen Y. Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients 2021;13(6):2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Fischer AG, Ullsperger M. An update on the role of serotonin and its interplay with dopamine for reward. Front Hum Neurosci 2017;11:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Clapp M, Aurora N, Herrera L, et al. Gut microbiota's effect on mental health: the gut-brain axis. Clin Pract 2017;7(4):987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Skonieczna-Zydecka K, Marlicz W, Misera A, et al. Microbiome-the missing link in the gut-brain axis: focus on its role in gastrointestinal and mental health. J Clin Med 2018;7(12):521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wong ML, Inserra A, Lewis MD, et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol Psychiatry 2016;21(6):797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Mangiola F, Ianiro G, Franceschi F, et al. Gut microbiota in autism and mood disorders. World J Gastroenterol 2016;22(1):361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sherwin E, Sandhu KV, Dinan TG, Cryan JF. May the force be with you: the light and dark sides of the microbiota-gut-brain axis in neuropsychiatry. CNS Drugs 2016;30(11):1019–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bull-Larsen S, Mohajeri MH. The potential influence of the bacterial microbiome on the development and progression of ADHD. Nutrients 2019;11(11):2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Bastiaanssen TFS, Cussotto S, Claesson MJ, et al. Gutted! Unraveling the role of the microbiome in major depressive disorder. Harv Rev Psychiatry 2020;28(1):26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Szklarczyk D, Gable AL, Nastou KC, et al. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res 2021;49(D1):D605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kuleshov MV, Jones MR, Rouillard AD, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res 2016;44(W1):W90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Bartlett EA, Zanderigo F, Shieh D, et al. Serotonin transporter binding in major depressive disorder: impact of serotonin system anatomy. Mol Psychiatry 2022;27:3417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Salvetat N, Checa-Robles FJ, Patel V, et al. A game changer for bipolar disorder diagnosis using RNA editing-based biomarkers. Transl Psychiatry 2022;12(1):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Wilkinson MD, Dumontier M, Aalbersberg IJ, et al. The FAIR guiding principles for scientific data management and stewardship. Sci Data 2016;3:160018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bilello JA. Seeking an objective diagnosis of depression. Biomark Med 2016;10(8):861–75. [DOI] [PubMed] [Google Scholar]
- 129. Bousman CA, Arandjelovic K, Mancuso SG, et al. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenomics 2019;20(1):37–47. [DOI] [PubMed] [Google Scholar]
- 130. Ye D, Zhang L, Fan W, et al. Genipin normalizes depression-like behavior induced by prenatal stress through inhibiting DNMT1. Epigenetics 2018;13(3):310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Fuchikami M, Yamamoto S, Morinobu S, et al. The potential use of histone deacetylase inhibitors in the treatment of depression. Prog Neuropsychopharmacol Biol Psychiatry 2016;64:320–4. [DOI] [PubMed] [Google Scholar]
- 132. Muller N, Myint AM, Schwarz MJ. Inflammatory biomarkers and depression. Neurotox Res 2011;19(2):308–18. [DOI] [PubMed] [Google Scholar]
- 133. Demkow U, Wolanczyk T. Genetic tests in major psychiatric disorders-integrating molecular medicine with clinical psychiatry-why is it so difficult? Transl Psychiatry 2017;7(6):e1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Genesight test. https://genesight.com/ (31 March 2021, date last accessed).
- 135. Correll CU, Solmi M, Veronese N, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry 2017;16(2):163–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Annamalai A, Kosir U, Tek C. Prevalence of obesity and diabetes in patients with schizophrenia. World J Diabetes 2017;8(8):390–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Jones KA, Menniti FS, Sivarao DV. Translational psychiatry–light at the end of the tunnel. Ann N Y Acad Sci 2015;1344:1–11. [DOI] [PubMed] [Google Scholar]
- 138. Lundstrom SL, Zhang B, Rutishauser D, et al. SpotLight proteomics: uncovering the hidden blood proteome improves diagnostic power of proteomics. Sci Rep 2017;7:41929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Comes AL, Papiol S, Mueller T, et al. Proteomics for blood biomarker exploration of severe mental illness: pitfalls of the past and potential for the future. Transl Psychiatry 2018;8(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Hensley K, Venkova K, Christov A, et al. Collapsin response mediator protein-2: an emerging pathologic feature and therapeutic target for neurodisease indications. Mol Neurobiol 2011;43(3):180–91. [DOI] [PubMed] [Google Scholar]
- 141. Junaid MA, Kowal D, Barua M, et al. Proteomic studies identified a single nucleotide polymorphism in glyoxalase I as autism susceptibility factor. Am J Med Genet A 2004;131(1):11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Iacono G, Massoni-Badosa R, Heyn H. Single-cell transcriptomics unveils gene regulatory network plasticity. Genome Biol 2019;20(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Pantazatos SP, Huang YY, Rosoklija GB, et al. Whole-transcriptome brain expression and exon-usage profiling in major depression and suicide: evidence for altered glial, endothelial and ATPase activity. Mol Psychiatry 2017;22(5):760–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Pantazatos SP, Andrews SJ, Dunning-Broadbent J, et al. Isoform-level brain expression profiling of the spermidine/spermine N1-acetyltransferase1 (SAT1) gene in major depression and suicide. Neurobiol Dis 2015;79:123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Pirooznia M, Wang T, Avramopoulos D, et al. High-throughput sequencing of the synaptome in major depressive disorder. Mol Psychiatry 2016;21(5):650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Howard DM, Adams MJ, Clarke TK, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci 2019;22(3):343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Belzeaux R, Lin R, Ju C, et al. Transcriptomic and epigenomic biomarkers of antidepressant response. J Affect Disord 2018;233:36–44. [DOI] [PubMed] [Google Scholar]
- 148. Feng J, Zhou Q, Gao W, et al. Seeking for potential pathogenic genes of major depressive disorder in the gene expression omnibus database. Asia Pac Psychiatry 2019;12:e12379. [DOI] [PubMed] [Google Scholar]
- 149. Sharma A. Systems genomics support for immune and inflammation hypothesis of depression. Curr Neuropharmacol 2016;14(7):749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Cui L, Gong X, Tang Y, et al. Relationship between the LHPP gene polymorphism and resting-state brain activity in major depressive disorder. Neural Plast 2016;2016:9162590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. consortium C . Sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature 2015;523(7562):588–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Nyegaard M, Severinsen JE, Als TD, et al. Support of association between BRD1 and both schizophrenia and bipolar affective disorder. Am J Med Genet B Neuropsychiatr Genet 2010;153B(2):582–91. [DOI] [PubMed] [Google Scholar]
- 153. Severinsen JE, Bjarkam CR, Kiaer-Larsen S, et al. Evidence implicating BRD1 with brain development and susceptibility to both schizophrenia and bipolar affective disorder. Mol Psychiatry 2006;11(12):1126–38. [DOI] [PubMed] [Google Scholar]
- 154. Liu Y, Pham X, Zhang L, et al. Functional variants in DPYSL2 sequence increase risk of schizophrenia and suggest a link to mTOR signaling. G3 (Bethesda) 2014;5(1):61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Zhou Y, Dong F, Lanz TA, et al. Interactome analysis reveals ZNF804A, a schizophrenia risk gene, as a novel component of protein translational machinery critical for embryonic neurodevelopment. Mol Psychiatry 2018;23(4):952–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Goes FS, Pirooznia M, Parla JS, et al. Exome sequencing of familial bipolar disorder. JAMA Psychiatry 2016;73(6):590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Wen Z, Cheng TL, Li GZ, et al. Identification of autism-related MECP2 mutations by whole-exome sequencing and functional validation. Mol Autism 2017;8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Wu M, Fang K, Wang W, et al. Identification of key genes and pathways for Alzheimer’s disease via combined analysis of genome-wide expression profiling in the hippocampus. Biophys Rep 2019;5(2):98–109. [Google Scholar]
- 159. Webster MJ, O'Grady J, Kleinman JE, Weickert CS. Glial fibrillary acidic protein mRNA levels in the cingulate cortex of individuals with depression, bipolar disorder and schizophrenia. Neuroscience 2005;133(2):453–61. [DOI] [PubMed] [Google Scholar]
- 160. Steffek AE, McCullumsmith RE, Haroutunian V, Meador-Woodruff JH. Cortical expression of glial fibrillary acidic protein and glutamine synthetase is decreased in schizophrenia. Schizophr Res 2008;103(1–3):71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Zhai J, Zhang Q, Cheng L, et al. Risk variants in the S100B gene, associated with elevated S100B levels, are also associated with visuospatial disability of schizophrenia. Behav Brain Res 2011;217(2):363–8. [DOI] [PubMed] [Google Scholar]
- 162. McCaffrey TA, 3rd St Laurent G, Shtokalo D, et al. Biomarker discovery in attention deficit hyperactivity disorder: RNA sequencing of whole blood in discordant twin and case-controlled cohorts. BMC Med Genomics 2020;13(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Pang X, Zhao Y, Wang J, et al. The bioinformatic analysis of the dysregulated genes and MicroRNAs in entorhinal cortex, hippocampus, and blood for Alzheimer's disease. Biomed Res Int 2017;2017:9084507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Jovasevic V, Corcoran KA, Leaderbrand K, et al. GABAergic mechanisms regulated by miR-33 encode state-dependent fear. Nat Neurosci 2015;18(9):1265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Bobinska K, Szemraj J, Mossakowska-Wojcik J, et al. The significance of microRNAs in the course of rDD. Pharmacol Rep 2017;69(2):206–12. [DOI] [PubMed] [Google Scholar]
- 166. Wang X, Gardiner EJ, Cairns MJ. Optimal consistency in microRNA expression analysis using reference-gene-based normalization. Mol Biosyst 2015;11(5):1235–40. [DOI] [PubMed] [Google Scholar]
- 167. Beveridge NJ, Cairns MJ. MicroRNA dysregulation in schizophrenia. Neurobiol Dis 2012;46(2):263–71. [DOI] [PubMed] [Google Scholar]
- 168. Wang J, Wang Y, Yang J, Huang Y. microRNAs as novel biomarkers of schizophrenia (review). Exp Ther Med 2014;8(6):1671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Liu C, Zhang F, Li T, et al. MirSNP, a database of polymorphisms altering miRNA target sites, identifies miRNA-related SNPs in GWAS SNPs and eQTLs. BMC Genomics 2012;13:661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Walker RM, Rybka J, Anderson SM, et al. Preliminary investigation of miRNA expression in individuals at high familial risk of bipolar disorder. J Psychiatr Res 2015;62:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Srivastav S, Walitza S, Grunblatt E. Emerging role of miRNA in attention deficit hyperactivity disorder: a systematic review. Atten Defic Hyperact Disord 2018;10(1):49–63. [DOI] [PubMed] [Google Scholar]
- 172. Martin CG, Kim H, Yun S, et al. Circulating miRNA associated with posttraumatic stress disorder in a cohort of military combat veterans. Psychiatry Res 2017;251:261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Neumann A, Walton E, Alemany S, et al. Association between DNA methylation and ADHD symptoms from birth to school age: a prospective meta-analysis. Transl Psychiatry 2020;10(1):398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Palma-Gudiel H, Fananas L. An integrative review of methylation at the serotonin transporter gene and its dialogue with environmental risk factors, psychopathology and 5-HTTLPR. Neurosci Biobehav Rev 2017;72:190–209. [DOI] [PubMed] [Google Scholar]
- 175. Bishop JR, Lee AM, Mills LJ, et al. Methylation of FKBP5 and SLC6A4 in relation to treatment response to mindfulness based stress reduction for posttraumatic stress disorder. Front Psych 2018;9:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Lim KC, Crino PB. Focal malformations of cortical development: new vistas for molecular pathogenesis. Neuroscience 2013;252:262–76. [DOI] [PubMed] [Google Scholar]
- 177. Di Battista AM, Heinsinger NM, Rebeck GW. Alzheimer's disease genetic risk factor APOE-epsilon4 also affects normal brain function. Curr Alzheimer Res 2016;13(11):1200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Song F, Han G, Bai Z, et al. Alzheimer's disease: genomics and beyond. Int Rev Neurobiol 2015;121:1–24. [DOI] [PubMed] [Google Scholar]
- 179. De Jager PL, Ma Y, McCabe C, et al. A multi-omic atlas of the human frontal cortex for aging and Alzheimer's disease research. Sci Data 2018;5:180142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Allen GI, Amoroso N, Anghel C, et al. Crowdsourced estimation of cognitive decline and resilience in Alzheimer's disease. Alzheimers Dement 2016;12(6):645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Wang M, Beckmann ND, Roussos P, et al. The Mount Sinai cohort of large-scale genomic, transcriptomic and proteomic data in Alzheimer's disease. Sci Data 2018;5:180185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. AMP-AD Knowledge Portal – The Mount Sinai Brain Bank (MSBB) study. https://adknowledgeportal.synapse.org (4 March 2024, date last accessed)
- 183. Hubers N, Hagenbeek FA, Pool R, et al. Integrative multi-omics analysis of genomic, epigenomic, and metabolomics data leads to new insights for attention-deficit/hyperactivity disorder. medRxiv 2022;195(2):521, 521. [DOI] [PubMed] [Google Scholar]
- 184. Dean KR, Hammamieh R, Mellon SH, et al. Multi-omic biomarker identification and validation for diagnosing warzone-related post-traumatic stress disorder. Mol Psychiatry 2020;25(12):3337–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Sancesario GM, Bernardini S. Alzheimer's disease in the omics era. Clin Biochem 2018;59:9–16. [DOI] [PubMed] [Google Scholar]
- 186. Reitz C. Genetic diagnosis and prognosis of Alzheimer's disease: challenges and opportunities. Expert Rev Mol Diagn 2015;15(3):339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Mirza Z, Rajeh N. Identification of electrophysiological changes in Alzheimer's disease: a microarray based transcriptomics and molecular pathway analysis study. CNS Neurol Disord Drug Targets 2017;16(9):1027–38. [DOI] [PubMed] [Google Scholar]
- 188. Li X, Long J, He T, et al. Integrated genomic approaches identify major pathways and upstream regulators in late onset Alzheimer's disease. Sci Rep 2015;5:12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Alonso A, Zaidi T, Novak M, et al. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci USA 2001;98(12):6923–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Gong CX, Iqbal K. Hyperphosphorylation of microtubule-associated protein tau: a promising therapeutic target for Alzheimer disease. Curr Med Chem 2008;15(23):2321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Cristovao JS, Gomes CM. S100 proteins in Alzheimer's disease. Front Neurosci 2019;13:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Adav SS, Gallart-Palau X, Tan KH, et al. Dementia-linked amyloidosis is associated with brain protein deamidation as revealed by proteomic profiling of human brain tissues. Mol Brain 2016;9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Martins-de-Souza D, Guest PC, Harris LW, et al. Identification of proteomic signatures associated with depression and psychotic depression in post-mortem brains from major depression patients. Transl Psychiatry 2012;2(3):e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Martins-de-Souza D, Guest PC, Vanattou-Saifoudine N, et al. Phosphoproteomic differences in major depressive disorder postmortem brains indicate effects on synaptic function. Eur Arch Psychiatry Clin Neurosci 2012;262(8):657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Kahl KG, Stapel B, Frieling H. Link between depression and cardiovascular diseases due to epigenomics and proteomics: focus on energy metabolism. Prog Neuropsychopharmacol Biol Psychiatry 2019;89:146–57. [DOI] [PubMed] [Google Scholar]
- 196. Silva-Costa LC, Carlson PT, Guest PC, et al. Proteomic markers for depression. Adv Exp Med Biol 2019;1118:191–206. [DOI] [PubMed] [Google Scholar]
- 197. Mehta D, Menke A, Binder EB. Gene expression studies in major depression. Curr Psychiatry Rep 2010;12(2):135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Lin E, Tsai SJ. Genome-wide microarray analysis of gene expression profiling in major depression and antidepressant therapy. Prog Neuropsychopharmacol Biol Psychiatry 2016;64:334–40. [DOI] [PubMed] [Google Scholar]
- 199. Arion D, Huo Z, Enwright JF, et al. Transcriptome alterations in prefrontal pyramidal cells distinguish schizophrenia from bipolar and major depressive disorders. Biol Psychiatry 2017;82(8):594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Goudriaan A, de Leeuw C, Ripke S, et al. Specific glial functions contribute to schizophrenia susceptibility. Schizophr Bull 2014;40(4):925–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Brennand K, Savas JN, Kim Y, et al. Phenotypic differences in hiPSC NPCs derived from patients with schizophrenia. Mol Psychiatry 2015;20(3):361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Mertens J, Wang QW, Kim Y, et al. Differential responses to lithium in hyperexcitable neurons from patients with bipolar disorder. Nature 2015;527(7576):95–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Depino AM. Perinatal inflammation and adult psychopathology: from preclinical models to humans. Semin Cell Dev Biol 2018;77:104–14. [DOI] [PubMed] [Google Scholar]
- 204. Wang X, Cairns MJ. Understanding complex transcriptome dynamics in schizophrenia and other neurological diseases using RNA sequencing. Int Rev Neurobiol 2014;116:127–52. [DOI] [PubMed] [Google Scholar]
- 205. Vasic N, Connemann BJ, Wolf RC, et al. Cerebrospinal fluid biomarker candidates of schizophrenia: where do we stand? Eur Arch Psychiatry Clin Neurosci 2012;262(5):375–91. [DOI] [PubMed] [Google Scholar]
- 206. Martins-de-Souza D, Maccarrone G, Wobrock T, et al. Proteome analysis of the thalamus and cerebrospinal fluid reveals glycolysis dysfunction and potential biomarkers candidates for schizophrenia. J Psychiatr Res 2010;44(16):1176–89. [DOI] [PubMed] [Google Scholar]
- 207. Scaini G, Andrews T, Benevenuto D, et al. Chapter 5 – Mitochondrial pathways in bipolar disorder: Mechanisms and implications. In: Quevedo J, Carvalho AF, Vieta E (eds). Neurobiology of Bipolar Disorder. Academic Press, 2021, pp. 61–9.
- 208. Hagerman R, Lauterborn J, Au J, Berry-Kravis E. Fragile X syndrome and targeted treatment trials. Results Probl Cell Differ 2012;54:297–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Hormozdiari F, Penn O, Borenstein E, Eichler EE. The discovery of integrated gene networks for autism and related disorders. Genome Res 2015;25(1):142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Junaid MA, Pullarkat RK. Proteomic approach for the elucidation of biological defects in autism. J Autism Dev Disord 2001;31(6):557–60. [DOI] [PubMed] [Google Scholar]
- 211. Castagnola M, Messana I, Inzitari R, et al. Hypo-phosphorylation of salivary peptidome as a clue to the molecular pathogenesis of autism spectrum disorders. J Proteome Res 2008;7(12):5327–32. [DOI] [PubMed] [Google Scholar]
- 212. Swanson JM, Kinsbourne M, Nigg J, et al. Etiologic subtypes of attention-deficit/hyperactivity disorder: brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychol Rev 2007;17(1):39–59. [DOI] [PubMed] [Google Scholar]
- 213. Ceylan MF, Sener S, Bayraktar AC, Kavutcu M. Changes in oxidative stress and cellular immunity serum markers in attention-deficit/hyperactivity disorder. Psychiatry Clin Neurosci 2012;66(3):220–6. [DOI] [PubMed] [Google Scholar]
- 214. Scassellati C, Bonvicini C. Chapter 4: Role of Dopaminergic and Noradrenergic Systems as Potential Biomarkers in ADHD Diagnosis and Treatment. In: Norvilitis JM (ed). ADHD – New Directions in Diagnosis and Treatment. IntechOpen, 2015;66(3):220–6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.