Abstract
Recent studies have demonstrated that the β-chemokines RANTES, MIP-1α, and MIP-1β suppress human immunodeficiency virus type 1 (HIV-1) replication in vitro and may play an important role in protecting exposed but uninfected individuals from HIV-1 infection. However, levels of β-chemokines in AIDS patients are comparable to and can exceed levels in nonprogressing individuals, indicating that global β-chemokine production may have little effect on HIV-1 disease progression. We sought to clarify the role of β-chemokines in nonprogressors and AIDS patients by examination of β-chemokine production and HIV-1 infection in patient T-lymphocyte clones established by herpesvirus saimiri immortalization. Both CD4+ and CD8+ clones were established, and they resembled primary T cells in their phenotypes and expression of activated T-cell markers. CD4+ T-cell clones from all patients had normal levels of mRNA-encoding CCR5, a coreceptor for non-syncytium-inducing (NSI) HIV-1. CD4+ clones from nonprogressors and CD8+ clones from AIDS patients secreted high levels of RANTES, MIP1α, and MIP-1β. In contrast, CD4+ clones from AIDS patients produced no RANTES and little or no MIP-1α or MIP-1β. The infection of CD4+ clones with the NSI HIV-1 strain ADA revealed an inverse correlation to β-chemokine production; clones from nonprogressors were poorly susceptible to ADA replication, but clones from AIDS patients were highly infectable. The resistance to ADA infection in CD4+ clones from nonprogressors could be partially reversed by treatment with anti-β-chemokine antibodies. These results indicate that CD4+ cells can be protected against NSI-HIV-1 infection in culture through endogenously produced factors, including β-chemokines, and that β-chemokine production by CD4+, but not CD8+, T cells may constitute one mechanism of disease-free survival for HIV-1-infected individuals.
The development of AIDS involves complex mechanisms (10). While most individuals progress to AIDS within 10 years of human immunodeficiency virus type 1 (HIV-1) infection, about 5% stay disease free, with stable CD4+ cell counts 7 or more years after infection (10, 13). These categories of patients are termed progressors and long-term nonprogressors, respectively (10, 13). The reasons for a lack of disease progression are still unclear but likely involve several factors, including effective HIV-1-specific immune responses or infection with less-virulent HIV-1 strains (6, 10, 13, 21). Another potential mechanism to mitigate HIV-1 infection and cytopathicity involves the production of ligands which compete for the cellular coreceptors for HIV-1. Recent studies have demonstrated that certain members of the β-chemokine family, such as RANTES, MIP-1α, and MIP-1β, can play a critical role in vitro in the suppression of non-syncytium-inducing (NSI) strains of HIV-1 (1, 7–9, 29). These β-chemokines inhibit HIV-1 by blocking the C-C chemokine receptor-5 (CCR5), which has been identified as a necessary coreceptor for NSI strains of HIV-1 (8, 9). However, the role of the β-chemokines in HIV-1 disease progression in vivo remains unclear. Although one study has shown that bulk CD4+ and CD8+ T cells from HIV-1-infected asymptomatic subjects produce moderately elevated levels of β-chemokines that might affect HIV-1 replication (11), other studies indicate no correlation between β-chemokine levels and nonprogression (5, 28, 32). Indeed, in one of these studies, increased levels of RANTES and MIP-1α were reported in sera from AIDS patients but not in sera from nonprogressors (32). In the present study, we explored the apparently conflicting roles of HIV-1 infection on β-chemokine production and disease progression by using immortalized T-cell clones derived from peripheral blood lymphocytes (PBL) of progressors and long-term nonprogressors.
In recent years, selected strains of herpesvirus saimiri (HVS) have been employed to immortalize human CD4+ and CD8+ T cells (reviewed in reference 16). We (25) and others (2, 17, 31) have shown that HVS-immortalized T cells retain the phenotype of conventionally cultured T cells, including the T-cell receptor and antigen-specific responses, and maintain important functional pathways. HVS-transformed CD4+ cells from normal donors also retain susceptibility to infection with HIV-1 (including strains with limited host cell range), suggesting that no incompatibility exists between HVS immortalization and HIV-1 replication (19, 23). We recently described the generation in culture of long-term CD4+ and CD8+ T-cell clones with HVS from PBL of HIV-1-infected subjects (24). Similar to HVS-immortalized clones from normal donors, T-cell clones from HIV-1-infected subjects maintained a normal, functional phenotype (24). In this report, we investigate β-chemokine production and the role of β-chemokines in HIV-1 infection in HVS-immortalized CD4+ and CD8+ T-cell clones developed from HIV-1-positive nonprogressors or AIDS patients.
The generation with HVS of long-term T-cell clones from HIV-1-infected subjects has been described previously (24, 26). In brief, PBL were separated from heparinized blood by Ficoll-Hypaque gradient (Sigma Chemical Co., St. Louis, Mo.) and resuspended in RPMI 1640 medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, penicillin (100 U/ml), streptomycin (100 μg/ml) (all from Life Technologies, Grand Island, N.Y.), and 20 U of human interleukin-2 (IL-2) (Boehringer Mannheim, Indianapolis, Ind.) per ml. Ro 31-8959, an HIV-1 protease inhibitor (a generous gift from I. Duncan) that inhibits the spread of HIV-1, was added into the medium for the initial 21 days of culture at a final concentration of 10−5 M (22). Cells were infected with HVS, group C, strain 488-77 at a multiplicity of infection of 0.1 as previously described (24). Three to five days after infection, infected PBL were cloned by seeding at 0.5 to 1 cells/well in 96-well plates containing X-irradiated allogeneic PBL (105 cells/well). Growing clones were expanded without any further addition of feeder cells. The T-cell clones from the nonprogressors used in this study and the respective blood donors have been previously described (24). AIDS patients’ PBL were obtained from subjects enrolled at the Clinical Trial Unit of St. Luke’s-Roosevelt Hospital Center; all donors had a circulating CD4+ T-cell count of <400 per mm3. To increase the proportion of HVS-immortalized CD4+ cells from AIDS patients, CD4+ cells were purified from PBL by anti-CD4 antibody (Ab)-coated magnetic beads prior to infection with HVS (26). MHCD4 and CHCD4, HVS-immortalized CD4+ clones from normal donors used in this study, have also been described previously (25). Stable T-cell clones obtained 3 to 4 months after HVS infection were characterized for surface antigen display by fluorescence-activated cell sorter analysis as previously described (27). The monoclonal fluorescein isothiocyanate- or phosphatidylethanolamine-labeled Abs included OKT4 (anti-CD4), OKT8 (anti-CD8), OKT11 (anti-CD2), OKT3 (anti-CD3) (all from Biosource International, Camarillo, Calif.), anti-CD14, anti-CD20, and BMA-031 (anti-TCRα/β) (all from Becton Dickinson, San Jose, Calif.). For analysis of TCR-Vβ expression of the T-cell clones, we used a panel of anti-TCR-Vβ Abs, including anti-Vβ2 and -Vβ3 Abs (Acac Co., Westbrook, Maine), and Vβ5a, Vβ5, Vβ5c, Vβ6a, Vβ8a, Vβ12a, Vβ13, and Vβ17 Abs (all from T Cell Sciences, Cambridge, Mass.).
A total of 25 HVS-immortalized CD4+ T-cell clones and 6 CD8+ T-cell clones were selected for the present studies. Like HVS-immortalized T-cell clones from normal donors (16, 25), clones from HIV-1-positive subjects expressed T-cell markers CD2, CD3, and TCRα/β but not CD14 or CD20 (Table 1). Most of these clones were in an activated state as indicated by the expression of activation markers HLA-DR and CD25 (data not shown). As reported previously (24), each T-cell clone we obtained expressed either CD4 or CD8, and no double-positive clones were obtained. All T-cell clones from HIV-1-positive patients tested thus far carried an α/β T-cell receptor, similar to the majority of T-cell clones from normal donors (25). Although the CD4+ clones from the AIDS patients used in this study were generated with purified CD4+ cells (26), compared to total PBL in nonprogressors or other AIDS patients (24), no phenotypic or functional difference was observed among these CD4+ clones, indicating that this slight modification in the protocol had not preferentially selected any specific subclass of CD4+ cells. Most of the HVS-immortalized T-cell clones from normal donors are of the Th1 type, i.e., they produce gamma interferon (IFN-γ) but no IL-4 (16, 25). We tested the Th1 or Th2 phenotypes of these clones by measuring IFN-γ and IL-4 production. IL-4 and IFN-γ were detected in the culture supernatants of individual T-cell clones with commercial enzyme-linked immunosorbent assay (ELISA) kits (Biosource International) as previously described (25). Similar to the T-cell clones from the normal donors (16, 25), all CD4+ and CD8+ clones established from the PBL of either nonprogressors or AIDS patients constitutively produced variable levels of IFN-γ but not IL-4 (Table 1 and data not shown), indicating that these clones had a Th1 phenotype.
TABLE 1.
Phenotypes of CD4+ clones from nonprogressors and AIDS patientsa
| Patient no. (HIV-1 disease status) | Clone | TCR α/β | CD2 | CD3 | CD14 | CD20 | Cytokine (U/ml)
|
|
|---|---|---|---|---|---|---|---|---|
| IFN-γ | IL-4 | |||||||
| 1 (NPb) | NP1-1 | + | + | + | − | − | 246 | 0 |
| NP1-2 | + | + | + | − | − | 194 | 0 | |
| NP1-3 | + | + | + | − | − | 258 | 0 | |
| NP1-4 | + | + | + | − | − | 208 | 0 | |
| NP1-5 | + | + | + | − | − | 188 | 0 | |
| NP1-6 | + | + | + | − | − | 144 | 0 | |
| NP1-7 | + | + | + | − | − | 267 | 0 | |
| 2 (NP) | NP2-1 | + | + | + | − | − | 75 | 0 |
| NP2-4 | + | + | + | − | − | 108 | 0 | |
| NP2-5 | + | + | + | − | − | 88 | 0 | |
| 3 (AIDS) | AD1-1 | + | + | + | − | − | 44 | 0 |
| AD1-3 | + | + | + | − | − | 66 | 0 | |
| AD1-6 | + | + | + | − | − | 248 | 0 | |
| AD1-8 | + | + | + | − | − | 113 | 0 | |
| AD1-12 | + | + | + | − | − | 23 | 0 | |
| AD1-13 | + | + | + | − | − | 131 | 0 | |
| AD1-15 | + | + | + | − | − | 70 | 0 | |
| AD1-18 | + | + | + | − | − | 42 | 0 | |
| AD1-19 | + | + | + | − | − | 41 | 0 | |
| AD1-23 | + | + | + | − | − | 70 | 0 | |
| AD1-30 | + | + | + | − | − | 218 | 0 | |
| 4 (AIDS) | AD2-1 | + | + | + | − | − | 196 | 0 |
| AD2-2 | + | + | + | − | − | 210 | 0 | |
| AD2-4 | + | + | + | − | − | 76 | 0 | |
| AD2-8 | + | + | + | − | − | 121 | 0 | |
| None | MHCD4 | + | + | + | − | − | 131 | 0 |
| None | CHCD4 | + | + | + | − | − | 117 | 0 |
Phenotypes were obtained by fluorescence-activated cell sorter analyses with appropriate negative and positive controls and by ELISA (for cytokines) as described previously (25).
NP, nonprogressor.
Recent studies have indicated that the β-chemokines RANTES, MIP-1α, and MIP-1β can block NSI HIV-1 replication in culture and may play a role in HIV-1 pathogenesis (1, 7–9, 29). We tested the production of RANTES, MIP-1α, and MIP-1β by HVS-immortalized CD4+ and CD8+ clones from HIV-1-positive subjects, using a sandwich ELISA (R&D Systems, Minneapolis, Minn.). As summarized in Table 2, none of the CD4+ clones from AIDS patients tested produced RANTES, only one clone produced a minimal level of MIP-1α, and several clones produced low levels of MIP-1β. In contrast to CD4+ clones from AIDS patients, all CD4+ clones from both nonprogressors tested produced high levels of RANTES, MIP-1α, and MIP-1β (Table 2). The levels of β-chemokine production by these clones did not change significantly after stimulation with phytohemagglutinin (data not shown). Although most of the HVS-immortalized CD4+ clones from normal donors did not produce RANTES, MIP-1α, or MIP-1β, many other clones from these donors did spontaneously produce these β-chemokines (Table 2 and data not shown), indicating that the constitutive production of these factors by CD4+ clones from nonprogressors may not be due to the selective immortalization of cells at a specific stage of cellular differentiation. In contrast to the deficient β-chemokine production by CD4+ clones from AIDS patients, a high level of β-chemokine secretion was observed in a majority of CD8+ clones from patients in both categories (Table 2) as well as in HVS-immortalized CD8+ clones from normal donors (Table 2 and unpublished data). Thus, both CD4+ and CD8+ clones from nonprogressors produce high levels of RANTES, MIP-1α, and MIP-1β, but CD4+ clones from AIDS patients are deficient in the production of RANTES and secrete little or no MIP-1α or MIP-1β. Since the CD4+ clones from nonprogressors expressed β-chemokines, while the clones from AIDS patients did not (Table 2), we wondered whether any difference among these clones existed in the expression of CCR5, the receptor for these factors as well as the coreceptor for NSI HIV-1 strains (1, 8, 9, 14). To detect mRNA encoding CCR5, reverse transcription-PCR was performed as previously described (24) with various T-cell clones from HIV-1-infected subjects and normal donors and SupT1 cells as the control. PCR was performed with primers for CCR5 that encompass most of the CCR5 gene open reading frame as described previously (14). All CD4+ clones tested, whether from nonprogressors, AIDS patients, or normal donors, expressed comparable levels of CCR5 mRNA. HIV-1-susceptible SupT1 cells also expressed equivalent levels of CCR5 (data not shown).
TABLE 2.
β-chemokine production and HIV-1 status of T-cell clones from HIV-1-infected individuals
| Clonea | CD4/CD8 | HIV statusb
|
β-Chemokine (pg/ml)
|
|||
|---|---|---|---|---|---|---|
| DNA | p24 | RANTES | MIP-1α | MIP-1β | ||
| NP1-1 | CD4 | + | − | 5,510 | 7,940 | 15,984 |
| NP1-2 | CD4 | − | − | 13,794 | 21,747 | 18,984 |
| NP1-3 | CD4 | − | − | 38,053 | 21,915 | 18,912 |
| NP1-4 | CD4 | − | − | 27,484 | 19,866 | 20,634 |
| NP1-5 | CD4 | − | − | 12,520 | 20,403 | 20,280 |
| NP1-6 | CD4 | − | − | 13,666 | 20,319 | 19,356 |
| NP1-7 | CD4 | − | − | 6,599 | 19,983 | 19,695 |
| NP2-1 | CD4 | − | − | 3,733 | 5,080 | 20,616 |
| NP2-4 | CD4 | − | − | 21,435 | 6,715 | 17,865 |
| NP2-5 | CD4 | − | − | 18,187 | 9,755 | 13,731 |
| NP2-2 | CD8 | NAc | NA | 0 | 207 | 3,532 |
| NP2-3 | CD8 | NA | NA | 10,220 | 21,560 | 39,888 |
| AD1-1d | + | + | 0 | 0 | 0 | |
| AD1-3d | + | + | 0 | 0 | 0 | |
| AD1-6 | CD4 | − | − | 0 | 0 | 4,143 |
| AD1-8d | + | + | 0 | 276 | 2,256 | |
| AD1-12 | CD4 | − | − | 0 | 0 | 0 |
| AD1-13 | CD4 | − | − | 0 | 0 | 1,985 |
| AD1-15 | CD4 | − | − | 0 | 0 | 0 |
| AD1-18d | + | + | 0 | 0 | 3,534 | |
| AD1-19 | CD4 | − | − | 0 | 0 | 0 |
| AD1-23 | CD4 | − | − | 0 | 0 | 0 |
| AD1-30 | CD4 | − | − | 0 | 0 | 0 |
| AD1-27 | CD8 | NA | NA | 20,780 | 0 | 41,400 |
| AD1-28 | CD8 | NA | NA | 15,860 | 34,328 | 44,184 |
| AD2-1 | CD4 | − | − | 0 | 0 | 618 |
| AD2-2 | CD4 | − | − | 0 | 0 | 0 |
| AD2-4 | CD4 | − | − | 0 | 0 | 0 |
| AD2-8 | CD4 | − | − | 0 | 0 | 0 |
| AD2-3 | CD8 | NA | NA | 10,700 | 39,200 | 38,624 |
| AD2-7 | CD8 | NA | NA | 12,325 | 41,480 | 44,184 |
| MHCD4 | CD4 | NA | NA | 0 | 0 | 0 |
| CHCD4 | CD4 | NA | NA | 8,770 | 36,768 | 34,576 |
| KRCD8 | CD8 | NA | NA | 15,285 | 36,616 | 40,392 |
T-cell clones are as described in Table 1. MHCD4 and CHCD4 are CD4+ T-cell clones, and KRCD8 is a CD8+ HVS-immortalized T-cell clone from normal donors (25).
HIV-1 DNA is by PCR, and p24 is by ELISA.
NA, not applicable.
This HIV-1-producing clone was surface-CD4 negative but expressed CD4 mRNA (data not shown).
Occasional HVS-immortalized CD4+ clones from nonprogressors as well as AIDS patients have been found to carry HIV-1 DNA (24). Indeed, we have recently reported that selected CD4+ clones from one AIDS patient spontaneously produced infectious HIV-1 (26). We examined the HIV-1 status of CD4+ clones by PCR to detect HIV-1 DNA and by p24 core antigen assay for productive HIV-1 infection as described previously (4, 30). None of the CD4+ clones developed from nonprogressors produced HIV-1 as tested by p24 measurement (Table 2) and by virus transmission assays by coculture with SupT1 or HeLa-CD4 cells (data not shown), but one clone was found to carry latent HIV-1 DNA (Table 2). In contrast, about one-third of T-cell clones from one AIDS patient, AD1, carried HIV-1 DNA and constitutively produced HIV-1 (Table 2). The HIV-1 produced by these cells was infectious, and some clones produced T-cell-tropic virus, while others produced dual-tropic HIV-1 (26a). The four CD4+ clones established from the PBL of the second AIDS patient, AD2, were negative for HIV-1 DNA and p24 production. It is presently unclear whether the productive HIV-1 infection observed in some patients’ T-cell clones (Table 2) occurred during immortalization in vitro or whether HVS was able to immortalize cells which carried virus in vivo. As expected, CD4 was downmodulated in HIV-1-producing T-cell clones (Table 2). The significance of nonproductive HIV-1 infection in the CD4+ clone NP1-1 (Table 2) is unclear at present.
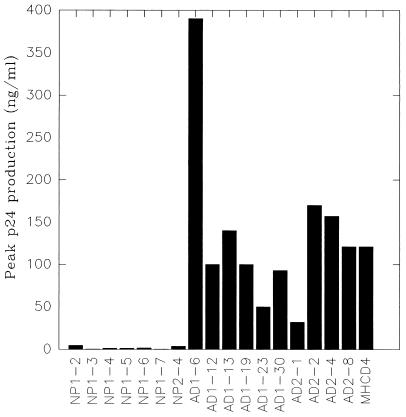
Several studies have demonstrated that the β-chemokines RANTES, MIP-1α, and MIP-1β are able to suppress the replication of HIV-1 of the NSI phenotype by blocking the C-C chemokine coreceptor CCR5 (1, 8, 9). Since the CD4+ clones from nonprogressors produced β-chemokines, while the CD4+ clones from AIDS patients did not (Table 2), even though all of these clones expressed comparable levels of CCR5 (data not shown), we tested the susceptibility of selected CD4+ clones from nonprogressors and AIDS patients to infection with NSI HIV-1. CD4+ clones were infected with NSI strain HIV-1ADA with 0.5 pg of viral p24 antigen per cell as previously described (30). Infection was monitored by the measurement of p24 levels in culture supernatants by ELISA with the HIV-1 antigen detection kit (Coulter, Hialeah, Fla.). Cell viability was monitored by trypan blue exclusion at regular intervals (23). All HIV-1 DNA–negative CD4+ clones from AIDS patients tested were readily infectable by HIV-1ADA, producing peak p24 levels of up to 390 ng/ml at 2 to 3 weeks after infection (Fig. 1). In contrast, all seven CD4+ clones from nonprogressors examined in this assay were poorly susceptible to infection with the same virus, and peak p24 levels were only between 0.2 and 4.5 ng/ml (Fig. 1). We did not observe a significant reduction of virus production in CD4+ clones from AIDS patients which produced low levels of MIP-1α or MIP-1β compared to the level in CD4+ clones which did not produce chemokines, indicating that a low level of chemokine production alone may not be sufficient to prevent infection by HIV-1ADA (Table 2 and Fig. 1). MHCD4, an HVS-immortalized CD4+ T-cell clone from a normal donor, which does not secrete β-chemokines (Table 2), was also highly susceptible to HIV-1ADA (Fig. 1). These results suggest that the high-level production of β-chemokines by CD4+ clones from nonprogressors may be responsible for the observed resistance of these clones to HIV-1 infection in culture. Interestingly, two HIV-1-producing clones from AIDS patients also secreted low levels of MIP1-α and/or MIP-1β, indicating that the spontaneous production of these chemokines at low levels does not inhibit HIV-1 replication (Table 2).
FIG. 1.
Infection of different CD4+ clones from nonprogressors and AIDS patients by HIV-1ADA. An equal number of cells from each clone was infected with HIV-1ADA (0.5 pg of p24 per cell) as described in the text. Culture supernatants were collected at regular intervals and assayed for p24 production. Peak virus production for the clones shown was between 2 and 3 weeks after infection.
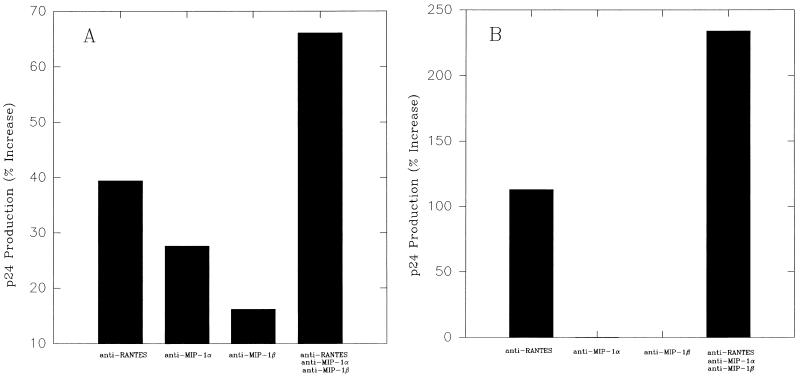
The role of β-chemokines endogenously produced by the CD4+ clones from nonprogressors in protection against HIV-1ADA was further tested by chemokine neutralization and the monitoring of virus replication. To test the inhibitory role of β-chemokines in infection with HIV-1ADA viruses, β-chemokine-producing clones from nonprogressors were cultured in the presence of anti-RANTES, -MIP-1α, and -MIP-1β neutralizing Abs (10 μg/ml) (all from R&D Systems) either separately or in combination for 24 h and then infected with HIV-1ADA as described above. Infected cultures were maintained in their respective Ab-containing medium. Culture supernatants were collected at regular intervals and assayed for HIV-1 production by p24 assay as described above. As shown in two representative experiments in Fig. 2 with HIV-1ADA-resistant clones NP1-3 and NP1-6, HIV-1 production was enhanced up to more than 60% when NP1-3 cells were infected in the presence of neutralizing Abs against RANTES, MIP-1α, and MIP-1β either alone or in combination, with maximum enhancement observed when all three Abs were added together (Fig. 2A). Similarly, the addition of anti-RANTES Ab enhanced HIV-1ADA replication in NP1-6 cells, and although neither anti-MIP-1α nor -MIP-1β alone significantly enhanced virus production, the combination of all three anti-β-chemokine Abs produced the most enhancing effect, indicating that these β-chemokines probably acted synergistically toward inducing HIV-1 inhibition (Fig. 2B). It should be noted that the observed enhancement was from the very low level of HIV-1 infection in these clones and that the infection of NP1-3 and NP1-6 cells in the presence of antichemokine Abs did not restore virus production to levels comparable to those seen in AIDS patients’ clones or in clones from normal donors (MHCD4) (Fig. 1). This result indicates that other antiviral factors, in addition to β-chemokines, produced by the nonprogressors’ CD4+ cells may be involved in resistance against HIV-1ADA.
FIG. 2.
Enhancement of HIV-1ADA replication in nonprogressor clone NP1-3 (A) and NP1-6 (B) by treatment with anti-RANTES, MIP-1α, and MIP-1β Abs. Cells were cultured for 24 h in the presence of 10 μg of each Ab or isotype control Ab (control) per ml either separately or in combination as shown. Cells were then infected with HIV-1ADA as in the experiment shown in Fig. 1 and cultured in the respective Ab-containing medium. Virus production (p24) was measured after 2 weeks and is presented as the increase (percentage) over that of the control. Results of two independent experiments are shown.
Taken together, our results demonstrate that elevated levels of endogenous β-chemokine production by CD4+, but not CD8+, T-cell clones correlate with a nonprogressor status of HIV-1-infected individuals and may contribute to the resistance of these clones to NSI HIV-1 infection in vitro. Although these conclusions were drawn from the study of four patients, a large number of T-cell clones, all of which showed a similar phenomenon, were studied in these experiments. While most of the CD8+ clones from either AIDS patients or nonprogressors produced high levels of β-chemokines, only CD4+ clones from nonprogressors produced β-chemokines and were largely resistant to HIV-1ADA infection in vitro. These results suggest that the overall levels of β-chemokines in plasma from HIV-1-infected individuals are potentially less important for protection from NSI HIV-1 infection than the source of the cells producing the chemokines. Our results are consistent with the recent report of Scala et al. (28) and provide an explanation for paradoxical findings by other investigators who reported either elevated (33) or comparable (5, 28) levels of β-chemokines in AIDS patients compared to those in nonprogressors. Given the demonstrated ability of β-chemokines to inhibit NSI HIV-1 infection in vitro (1, 8, 9), it is surprising that AIDS patients have high levels of both β-chemokines and replicating HIV-1. Our results suggest that CD8+ cells alone may be responsible for the production of β-chemokines in AIDS patients and that the failure of CD4+ cells to secrete the chemokines may contribute to the progression of disease in these subjects. Conversely, the ability of CD4+ T cells of nonprogressors to produce high levels of chemokines may play an important role in the inhibition of NSI HIV-1 replication and the control of disease progression. Other groups (28) have also reported high levels of β-chemokine production by CD4+ cells from nonprogressors. Further studies with additional T-cell clones will be required to determine whether β-chemokine production by CD4+ cells is a critical feature of nonprogression.
An interesting question is why HIV-1 disease progressed in AIDS patients in spite of the elevated production of β-chemokines by the patients’ CD8+ T cells (5, 28, 33 and Table 2). Three explanations may be proposed for this apparent paradox. First, it has been shown that β-chemokines are effective only against NSI strains of HIV-1 (7, 8), while HIV-1 disease progression often correlates with the emergence of SI strains (10, 13). Thus, control of NSI HIV-1 infection by β-chemokines may not influence disease progression. Second, the spread of HIV-1 occurs more effectively through cell-cell contact (13). Since our data indicate that β-chemokines found in AIDS patients may be produced primarily by CD8+ T cells (Table 2), these β-chemokines may not be able to block CCR5 coreceptors on CD4+ cells that are not always close to CD8+ T cells. In contrast, the endogenous production of β-chemokines by CD4+ cells in nonprogressors could provide a readily available source of CCR5 coreceptor ligands to block receptor utilization on CD4+ cells and prevent viral infection. Third, several recent studies have suggested that soluble factors other than β-chemokines, produced by CD8+ cells, can play an important role in suppressing HIV-1 replication (3, 12, 15, 18, 20). It is possible that, although they produce high levels of β-chemokines, CD8+ cells from AIDS patients do not secrete other HIV-1-suppressing factors and thus fail to prevent HIV-1 replication and disease progression.
In summary, this study demonstrates that CD4+ T-cell clones from nonprogressors, but not from AIDS patients, produce high levels of endogenous β-chemokines that can protect these cells against infection with HIV-1ADA in vitro. However, CD8+ clones from AIDS patients as well as nonprogressors also produced increased levels of these β-chemokines. These findings suggest one explanation for the apparent discrepancy between the high levels of β-chemokines in plasma from AIDS patients and the absence of protection against HIV-1 infection in these subjects. Finally, these results underscore the importance of endogenous β-chemokine production by CD4+ T cells in protecting against HIV-1 infection.
ACKNOWLEDGMENTS
We thank G. McKinley, P. Gupta, and J. Sonnabend for providing the PBL samples, R. C. Desrosiers for the HVS, and I. Duncan (Roche Products, Ltd., London, England) for Ro 31-8959. We thank G. Li for technical assistance and L. Peters for preparation of the manuscript. We are grateful to M. J. Potash for critically reviewing the manuscript.
K.S. is an Aaron Diamond Fellow, and this work was supported by a fellowship from the Aaron Diamond Foundation to K.S. and by PHS grants to D.J.V. and K.S.
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, and MIP-1β receptor as a fusin cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Broker B M, Tsygankov A Y, Muller-Fleckenstein I, Guse A H, Chitaev N A, Biesinger B, Fleckenstein B, Emmrich F. Immortalization of human T cell clones by herpesvirus saimiri: signal transduction analysis reveals functional CD3, CD4, and IL-2 receptors. J Immunol. 1993;151:1184–1192. [PubMed] [Google Scholar]
- 3.Chen Y, Dampf D, Kulka K, Saha K, Chen M, Gupta P. 9th annual meeting of the National Cooperative Vaccine Development and AIDS. 1997. Differential suppression of HIV-1 by CD8+ T cells; p. 159. [Google Scholar]
- 4.Chowdhury I H, Chao W, Potash M J, Sova P, Gendelman H E, Volsky D J. vif-negative human immunodeficiency virus type 1 persistently replicates in primary macrophages, producing attenuated progeny virus. J Virol. 1996;70:5336–5345. doi: 10.1128/jvi.70.8.5336-5345.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clerici M, Balotta C, Trabattoni D, Papagno L, Ruzzante S, Rusconi S, Fusi M L, Colombo M C, Galli M. Chemokine production in HIV-1-seropositive long-term asymptomatic individuals. AIDS. 1996;10:1432–1433. doi: 10.1097/00002030-199610000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Clerici M, Shearer G M. The TH1-TH2 hypothesis of HIV-1 infection: new insights. Immunol Today. 1995;15:575–581. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 7.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-1-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 8.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Marzio P D, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 9.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by chemokine receptor CC-CKR5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 10.Haynes B F, Pantaleo G, Fauci A S. Toward an understanding of the correlates of protective immunity to HIV-1 infection. Science. 1996;271:324–328. doi: 10.1126/science.271.5247.324. [DOI] [PubMed] [Google Scholar]
- 11.Kinter A L, Ostrowski M, Goletti D, Oliva A, Weissman D, Gantt K, Hardy E, Jackson R, Ehler L, Fauci A S. HIV-1 replication in CD4+ T cells of HIV-1-infected individuals is regulated by a balance between the viral suppressive effects of endogenous β-chemokines and the viral inductive effects of other endogenous cytokines. Proc Natl Acad Sci USA. 1996;93:14076–14081. doi: 10.1073/pnas.93.24.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leith J G, Copeland K F T, McKay P J, Richards C D, Rosenthal K L. CD8+ T cell-mediated suppression of HIV-1 long terminal repeat-driven gene expression is not modulated by the CC chemokines RANTES, macrophage-inflammatory protein (MIP)-1α and MIP-1β. AIDS. 1997;11:575–580. doi: 10.1097/00002030-199705000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Levy J A. HIV-1 pathogenesis and long-term survival. AIDS. 1993;7:1401–1410. doi: 10.1097/00002030-199311000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stullmann H, Koup R A, Landau N R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 15.Mackewicz C E, Barker E, Levy J A. Role of β-chemokines in suppressing HIV-1 replication. Science. 1996;274:1393–1394. doi: 10.1126/science.274.5291.1393. [DOI] [PubMed] [Google Scholar]
- 16.Meinl E, Hohlfeld R, Wekerle H, Fleckenstein B. Immortalization of human T cells by herpesvirus saimiri. Immunol Today. 1995;16:55–58. doi: 10.1016/0167-5699(95)80087-5. [DOI] [PubMed] [Google Scholar]
- 17.Mittrucker H W, Muller-Fleckenstein I, Fleckenstein B, Fleischer B. Herpesvirus saimiri-transformed human T lymphocytes: normal functional phenotype and preserved T cell receptor signaling. Int Immunol. 1993;5:985–990. doi: 10.1093/intimm/5.8.985. [DOI] [PubMed] [Google Scholar]
- 18.Moriuchi H, Moriuchi M, Combadiere C, Murphy P M, Fauci A S. CD8+ T cell-derived soluble factor(s), but not β-chemokines RANTES, MIP-1α, and MIP-1β, suppress HIV-1 replication in monocyte/macrophage. Proc Natl Acad Sci USA. 1996;93:15341–15345. doi: 10.1073/pnas.93.26.15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nick S, Fickenscher H, Biesinger B, Born G, Jahn G, Fleckenstein B. Herpesvirus saimiri transformed human T cell lines: a permissive system for human immunodeficiency viruses. Virology. 1993;194:875–877. doi: 10.1006/viro.1993.1334. [DOI] [PubMed] [Google Scholar]
- 20.Paliard X, Lee A Y, Walker C M. RANTES, MIP-1α, and MIP-1β are not involved in the inhibition of HIV-1SF33 replication mediated by CD8+ T cell clones. AIDS. 1996;10:1317–1321. doi: 10.1097/00002030-199610000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Pantaleo G, Menzo S, Vaccarezza M. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N Engl J Med. 1995;332:209–216. doi: 10.1056/NEJM199501263320402. [DOI] [PubMed] [Google Scholar]
- 22.Roberts N, et al. Rational design of peptide-based HIV-1 proteinase inhibitors. Science. 1990;248:358–362. doi: 10.1126/science.2183354. [DOI] [PubMed] [Google Scholar]
- 23.Saha K, Caruso M, Volsky D J. Human immunodeficiency virus type 1 (HIV-1) infection of herpesvirus saimiri-immortalized human CD4-positive T lymphoblastoid cells: evidence of enhanced HIV-1 replication and cytopathic effects caused by endogenous IFN-γ. Virology. 1997;231:1–9. doi: 10.1006/viro.1997.8485. [DOI] [PubMed] [Google Scholar]
- 24.Saha K, Sova P, Chao W, Chess L, Volsky D J. Generation of CD4+ and CD8+ T cell clones from PBLs of HIV-1 infected subjects using herpesvirus saimiri. Nat Med. 1996;2:1272–1275. doi: 10.1038/nm1196-1272. [DOI] [PubMed] [Google Scholar]
- 25.Saha K, Ware R, Yellin M J, Chess L, Lowy I. Herpesvirus saimiri-transformed human CD4+ T cells can provide polyclonal B cell help via the CD40 ligand as well as the TNF-α pathway and through release of lymphokines. J Immunol. 1996;157:3876–3885. [PubMed] [Google Scholar]
- 26.Saha K, McKinley G, Volsky D J. Improvement of herpesvirus saimiri (HVS) T cell immortalization procedure to generate multiple CD4+ T cell clones from peripheral blood lymphocytes of AIDS patients. J Immunol Methods. 1997;206:21–23. doi: 10.1016/s0022-1759(97)00080-x. [DOI] [PubMed] [Google Scholar]
- 26a.Saha, K., and D. J. Volsky. Unpublished data.
- 27.Saha K, Wong P K Y. ts1, a temperature-sensitive mutant of Moloney murine leukemia virus TB, can infect both CD4+ and CD8+ T cells but requires CD4+ T cells in order to cause paralysis and immunodeficiency. J Virol. 1992;66:2639–2646. doi: 10.1128/jvi.66.5.2639-2646.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scala E, D’Offizi G, Rooso R, Turriziani O, Ferrara R, Mazzone A M, Antonelli G, Aiuti F, Paganelli R. C-C chemokines, IL-16, and soluble antiviral factor activity are increased in cloned T cells from subjects with long-term nonprogressive HIV-1 infection. J Immunol. 1997;158:4485–4492. [PubMed] [Google Scholar]
- 29.Schmidtmayerova H, Sherry B, Bukrinsky M. Chemokines and HIV-1 replication. Nature. 1996;382:767. doi: 10.1038/382767a0. [DOI] [PubMed] [Google Scholar]
- 30.Volsky D J, Simm M, Shahabuddin M, Li G, Chao W, Potash M J. Interference to human immunodeficiency virus type 1 infection in the absence of downmodulation of the principal virus receptor, CD4. J Virol. 1996;70:3823–3833. doi: 10.1128/jvi.70.6.3823-3833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber F, Meinl E, Drexler K, Czlonkowska A, Huber S, Fickenscher H, Muller-Fleckenstein I, Wekerle H, Hohlfeld R. Transformation of human T cell clones by herpesvirus saimiri: intact antigen recognition by autonomously growing myelin basic protein-specific T cells. Proc Natl Acad Sci USA. 1993;90:11049–11053. doi: 10.1073/pnas.90.23.11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zanussi S, Andrea M D, Simonelli C, Tirelli V, DePaoli P. Serum levels of RANTES and MIP-1α in HIV-1-positive long-term survivors and progressor patients. AIDS. 1996;10:1431–1432. doi: 10.1097/00002030-199610000-00018. [DOI] [PubMed] [Google Scholar]