Summary
Identification and differentiation of appropriate indications on hip preserving with bone grafting therapy remains a crucial challenge in the treatment of osteonecrosis of the femoral head (ONFH). A prospective cohort study on bone grafting therapy for ONFH aimed to evaluate hip survival rates, and to establish a risk scoring derived from potential risk factors (multivariable model) for hip preservation. Eight variables were identified to be strongly correlated with a decreased rate of hip survival post-therapy, and a comprehensive risk scoring was developed for predicting hip-preservation outcomes. The C-index stood at 0.72, and the areas under the receiver operating characteristics for the risk score’s 5- and 10-year hip failure event predictions were 0.74 and 0.72, respectively. This risk score outperforms conventional methods in forecasting hip preservation. Bone grafting shows sustained benefits in treating ONFH when applied under the right indications. Furthermore, the risk scoring proves valuable as a decision-making tool, facilitating risk stratification for ONFH treatments in future.
Subject areas: Predicting model
Graphical abstract
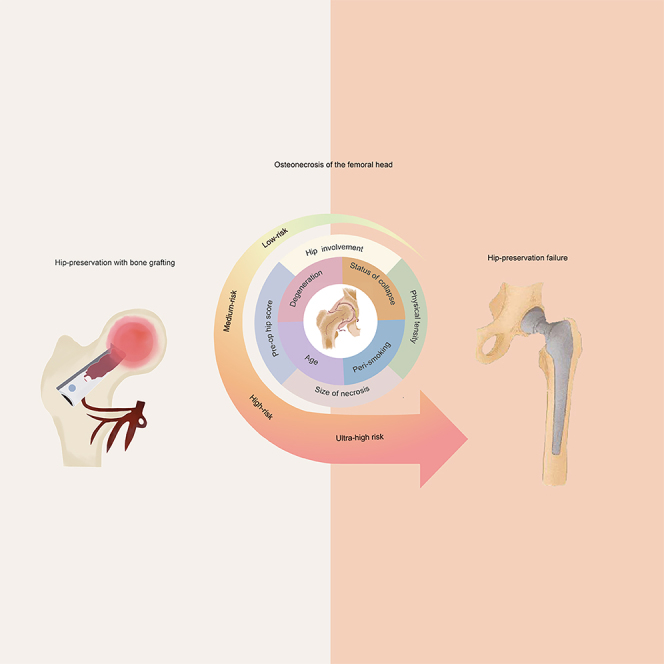
Highlights
-
•
It is vital to stratify the risks of osteonecrosis for hip salvaging with bone grafting
-
•
Eight key predictors are identified as strongly correlated with decreased hip survival
-
•
A new score which related to predictive hip survival is clarified with bone grafting
-
•
The score can be a useful tool for decision-making of hip preservation for the necrosis
Predicting model
Introduction
Osteonecrosis of the femoral head (ONFH) is one of the most common limb-related disability globally. Most untreated ONFHs are associated with progressively increasing pain, leading to collapse of articular cartilage and development of osteoarthritis within a few years.1,2 ONFH mainly affects young and middle-aged adults.2,3 For younger ONFH patients who have high activity demands, surgical procedures of hip preservation should be the main curative treatment for consideration.4
Hip-preserving procedures of various bone grafting play an important role in the treatment of ONFH, with pain relief, halting or delaying progression.5 Appropriate surgical indication is the key factor for evaluating its clinical value on one hip-preserving procedure in ONFH. However, the current evidence of surgical indications (such as according to imaging stages of ONFH, patient age, etc.) for hip preservation is still insufficient and has rendered much debate.6,7,8 For example, some experts deemed that hip preservation with bone grafting is not appropriate in situations where the collapse of the femoral head exceeds 2 mm or when any level of collapse is present5,8; maybe this represents a predominate opinion, which is related to the trend of excessive use of hip replacement methods for management of ONFH globally.9,10 However, some evidence has favored that bone grafting still benefits severely collapsed femoral head in young adults or adolescents.11 Therefore, robust evidence on research on indications for hip preserving with bone grafting in ONFH is imperative to break through the non-uniform treatment algorithm. To our knowledge, to date, there are just only imaging variables or imaging scores derived from smaller samples for evaluating disease progression (mainly focused on predicting collapse) of ONFH.3,12,13,14,15,16,17 As a complex disease, a comprehensive risk score derived from high-quality evidence on long-term hip preserving with bone grafting in ONFH is currently lacking and imperative, which may play an important role in facilitating clinical strategic decision and prognosis prediction for hip preservation.
As an important methed of bone grafting, free vascularized fibular grafting (FVFG) is considered one of the most promising procedures to surgically salvage the hip in ONFH due to its advantages of biological reconstruction.18,19 In this study, FVFG therapy was used as a representative sample of bone grafting for ONFH patients, to investigate the long-term effect on hip preservation, and to develop a comprehensive risk score for surgical decision-making and predicting prognosis. We examined a large-scale longitudinal cohort of patients from a real-world clinical practice with FVFG to treat ONFH, with long and full follow-up period adjusting the results for confounding by the known potential prognostic factors.19,20,21,22,23
Results
Baseline characteristic of patients
The final study population included 854 patients (1,206 hips) (Figure 1). Participants had a mean age ± standard deviation, 35.5 ± 10.4 years (ranged: 13–59 years); 72.4% (618/854 Patients) were male. Association Research Circulation Osseous (ARCO) staging of ONFH with 1,206 hips was carried out as seen in preoperative images: 299 hips (24.8%) without collapse were classified as stage II, and 907 hips (75.2%) with collapsed as stage IIIA to stage IV. The detailed baseline variables are presented in Table 1.
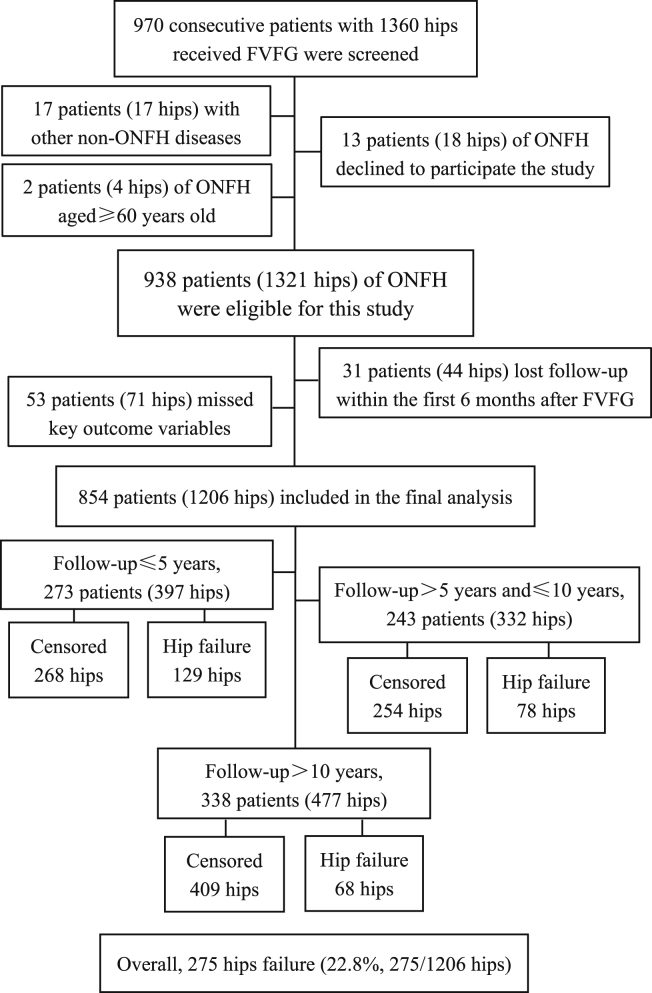
Figure 1.
Flowchart illustrating study population
Abbreviations: FVFG, free vascularized fibular grafting; ONFH, osteonecrosis of the femoral head; Hip failure, Converted to THA or need to THA.
Table 1.
Baseline characteristics stratified by the outcome of hip failure events in ONFH patients treated with FVFG
| Variables | Case (%) | Hips (%) | No failure events | Failure events | p | |
|---|---|---|---|---|---|---|
| Etiology (n = 854)a | trauma-induced | 217 (25.4%) | 217 (18.0%) | 170 (78.3%) | 47 (21.7%) | 0.005 |
| steroid-induced | 354 (41.5%) | 583 (48.3%) | 449 (77.0%) | 134 (23.0%) | ||
| alcohol-induced | 140 (16.4%) | 216 (17.9%) | 151 (69.9%) | 65 (30.1%) | ||
| idiopathic | 143 (16.7%) | 190 (15.8%) | 161 (84.7%) | 29 (15.3%) | ||
| Gender (n = 854) | male | 618 (72.4%) | 882 (73.1%) | 675 (76.5%) | 207 (23.5%) | 0.363 |
| female | 236 (27.6%) | 324 (26.9%) | 256 (79.0%) | 68 (21.0%) | ||
| Ethnicity (n = 854) | Han Chinese | 836 (97.9%) | 1178 (97.7%) | 913 (77.5%) | 265 (22.5%) | 0.099 |
| ethnic minorities | 18 (2.1%) | 28 (2.3%) | 18 (64.3%) | 10 (35.7%) | ||
| Surgical age, years | mean ± SD | 854 (100%) | 1206 (100%) | 34.3 ± 10.2 | 39.5 ± 10.0 | <0.001 |
| (n = 854) | ≤ 35 | 416 (48.7%) | 593 (49.2%) | 494 (83.3%) | 99 (16.7%) | <0.001 |
| 36 – 50 | 355 (41.6%) | 504 (41.8%) | 374 (74.2%) | 130 (25.8%) | ||
| > 50 | 83 (9.7%) | 109 (9.0%) | 63 (57.8%) | 46 (42.2%) | ||
| Preoperative smoking | no/ceased | 654 (77.1%) | 914 (76.2%) | 724 (79.2%) | 190 (20.8%) | 0.005 |
| (n = 848) | smoking | 194 (22.9%) | 286 (23.8%) | 204 (71.3%) | 82 (28.7%) | |
| Heavy alcohol | no/ceased | 662 (78.1%) | 926 (77.2%) | 734 (79.3%) | 192 (20.7%) | 0.003 |
| Consumption (n = 848) | heavy drinking | 186 (21.9%) | 274 (22.8%) | 194 (70.8%) | 80 (29.2%) | |
| Perioperative smoking | no | 818 (96.5%) | 1156 (96.3%) | 906 (78.4%) | 250 (21.6%) | <0.001 |
| (n = 848) | yes | 30 (3.5%) | 44 (3.7%) | 22 (50.0%) | 22 (50.0%) | |
| Involved hip (n = 854) | unilateral | 414 (48.5%) | 414 (34.3%) | 335 (80.9%) | 79 (19.1%) | 0.026 |
| bilateral | 440 (51.5%) | 792 (65.7%) | 596 (75.3%) | 196 (24.7%) | ||
| Occupational intensity | low | 456 (54.7%) | 640 (54.1%) | 518 (80.9%) | 122 (19.1%) | 0.001 |
| (n = 833) | moderate-to-high | 377 (45.3%) | 542 (45.9%) | 396 (73.1%) | 146 (26.9%) | |
| ARCO imaging stage | stage II | 299 (24.8%) | 268 (89.6%) | 31 (10.4%) | <0.001 | |
| stage IIIA | 321 (26.6%) | 249 (77.6%) | 72 (22.4%) | |||
| stage IIIB | 242 (20.1%) | 177 (73.1%) | 65 (26.9%) | |||
| stage IIIC | 303 (25.1%) | 215 (71.0%) | 88 (29.0%) | |||
| stage IV | 41 (3.4%) | 22 (53.7%) | 19 (46.3%) | |||
| Location | inner or middle | 154 (12.8%) | 132 (85.7%) | 22 (14.3%) | 0.007 | |
| lateral | 1052 (87.2%) | 799 (76.0%) | 253 (24.0%) | |||
| Size of necrosis lesion | ≤ 30% | 381 (31.6%) | 335 (87.9%) | 46 (12.1%) | <0.001 | |
| > 30% | 825 (68.4%) | 596 (72.2%) | 229 (27.8%) | |||
| Status of collapse | no depression | 299 (24.7%) | 268 (89.6%) | 31 (10.4%) | <0.001 | |
| < 2 mm | 489 (40.6%) | 378 (77.3%) | 111 (22.7%) | |||
| ≥ 2 mm | 418 (34.7%) | 285 (68.2%) | 133 (31.8%) | |||
| Articular surface | none or <15% | 615 (51.0%) | 511 (83.1%) | 104 (16.9%) | <0.001 | |
| involvement | 15% – 30% | 259 (21.5%) | 193 (74.5%) | 66 (25.5%) | ||
| > 30% | 332 (27.5%) | 227 (68.4%) | 105 (31.6%) | |||
| Acetabular | none or mild | 1165 (96.6%) | 909 (78.0%) | 256 (22.0%) | <0.001 | |
| Degeneration | moderate-to-severe | 41 (3.4%) | 22 (53.7%) | 19 (46.3%) | ||
| Preoperative hip function | fair to excellent | 662 (54.9%) | 565 (85.3%) | 97 (14.7%) | <0.001 | |
| poor (< 70 points) | 544 (45.1%) | 366 (67.3%) | 178 (32.7%) |
Study population consisted of 854 patients (total data of 1,206 hips was analyzed).
Follow-up outcomes
At a median of 110.3 months (interquartile range [IQR]: 29.3 – 137.8 months, range: 3 – 214 months) of follow-up, 11 patients (16 hips) died from diseases unrelated to the surgery treatment; of these 11 patients, a hip failure event had occurred in eight hips and another eight hips were censored. A total of 275 hips (22.8%) had experienced hip failure; of these, 143 hips received total hip arthroplasty (THA) and 132 hips needed THA. The median time of occurring hip failures was 68.2 months (IQR: 14.9 – 120.4 months) after FVFG.
Overall, hip function improved significantly after bone grafting. Harris hip score (HHS) scores improved from a pre-FVFG mean score of 70.1 ± 16.4 points to 80.9 ± 14.0 points at the final follow-up for 1,161 hips of complete records (p < 0.001). In this study, thirty-nine patients were involved, of which 66 hips received the surgery, and 45 hips of them converted to receive THA without a detailed HHS score in the final follow-up were given a “poor” score by default. Of all 1,206 hips, preoperatively the percentage of recorded “poor” HHS scores was 45.1% (544/1,206 hips), but postoperatively the percentage of “poor” HHS scores decreased significantly to 19.5% (235/1,206 hips) at the final follow-up (p < 0.001).
Identification of a multivariable risk factors model for hip survival
When comparing the outcomes of participants who had an event of hip failure and those who did not, we observed fifteen baseline variables in the univariate analysis that were significantly different in the two groups (Table 1). Perioperative smoking was also significantly different between the two groups (Table 1).
Results of further multivariable analyses for 1,179 hips that had complete data are shown in Table 2. Eight significant variables from this analysis showed that older surgical age, more severe depression with collapse, moderate-to-severe degree of acetabular degeneration, larger size of necrosis, moderate-to-high physical intensity, bilateral hip involvement, poor preoperative HHS, and perioperative smoking were associated with increased hip failure after FVFG treatment (p < 0.05). The top four risk factors for predicting hip failure included the following: surgical age (>50 years; hazard ratio [HR] 2.53, 95% confidence interval [CI], 1.55 – 4.13), peri-smoking (HR 2.38, 1.26 – 4.50), acetabular degeneration (moderate to severe; HR 2.23, 1.16 – 4.29), and collapse status (depression ≥ 2 mm; HR 2.16, 1.29 – 3.61) (Table 2).
Table 2.
Multivariable model analysis for hip survival after treated with bone grafting (FVFG) on ONFH
| Variables | Fragile Cox modela |
||
|---|---|---|---|
| β | Adjusted HR (95% CI) | Risk score | |
| Surgical age | |||
| ≤ 35 years | 1.0 (Ref) | 0 | |
| 36 – 50 years | 0.436b | 1.546 (1.105 – 2.163) | 1 |
| > 50 years | 0.927 | 2.526 (1.547 – 4.125) | 2 |
| Status of collapse | |||
| No collapsed | 1.0 (Ref) | 0 | |
| Collapsed < 2 mm | 0.459 | 1.583 (1.000 – 2.531) | 1 |
| Collapsed ≥ 2 mm | 0.769 | 2.158 (1.288 – 3.614) | 2 |
| Degeneration (moderate-to-severe) | 0.802 | 2.229 (1.160 – 4.285) | 2 |
| Size (> 30%) | 0.592 | 1.808 (1.201 – 2.723) | 1.5 |
| Hip involvement (bilateral ONFH) | 0.671 | 1.956 (1.400 – 2.733) | 1.5 |
| Physical tensity (moderate-to-high) | 0.601 | 1.823 (1.338 – 2.484) | 1.5 |
| Pre-HHS (< 70 points) | 0.589 | 1.802 (1.318 – 2.464) | 1.5 |
| Peri-smoking | 0.865 | 2.375 (1.255 – 4.496) | 2 |
From completed datasets of 1,179 hips.
Using the minimum β value as the scoring reference, and other values divided by the minimum β value to form a new risk score (based on the rounding principle).
Development of a comprehensive risk scoring for hip preservation
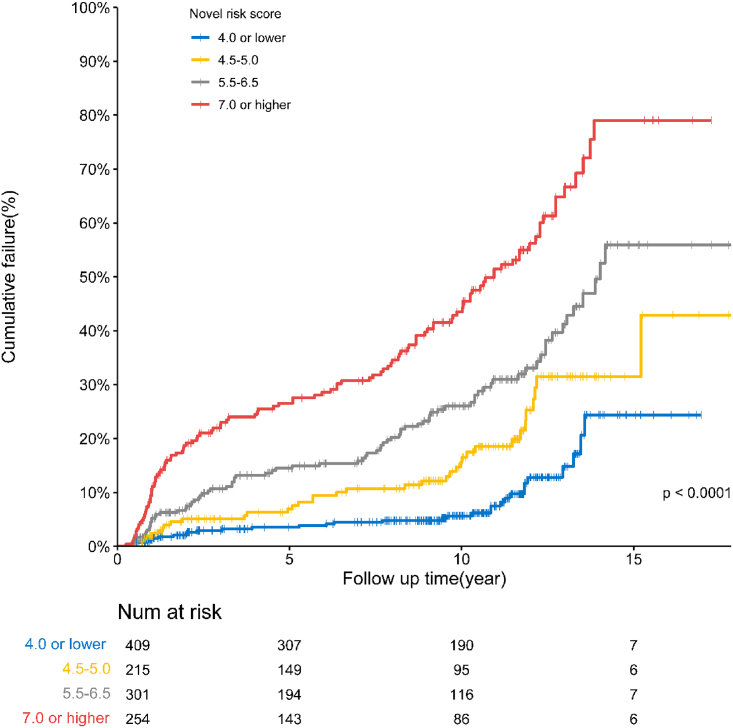
A comprehensive risk score was developed to predict the effect of hip survival using the eight variables in the final fragile Cox model. The median score was 5.0 (IQR: 4.0 to 6.5); scores ranged from 0 to 14.0 in this cohort. We stratified the risk scores into four levels (by the 25th, median, and 75th): low-risk (score ≤ 4.0), medium-risk (score between 4.5 and 5.0), high-risk (score between 5.5 and 6.5), and ultra-high-risk (score ≥ 7.0) (Table 3). The estimated annual hazard of a hip failure was 0.5% (95% CI: 0.3% – 0.6%) in patients with a low-risk level, 1.0% (0.7% – 1.3%) with a medium-risk level, 1.7% (1.4% – 2.0%) with a high-risk level, and 3.9% (3.3% – 4.5%) with an ultra-high-risk level. And the estimated cumulative survival or failure rates between these four risk score levels were calculated as well (p < 0.001, see Table 3, and Figure 2). The relationship between risk score and predicting the probability of hip failure at 5-, 10-, and 15-year after FVFG was shown directly in Figure 3A.
Table 3.
Relationship between risk-score levels and estimated hip survival/failure event with bone grafting for ONFHs
| Risk-score level | Hips (n) | Failure event |
Estimate survival time |
Estimated cumulative survival rate |
Estimated annual hazard of failure rate |
|---|---|---|---|---|---|
| (n, %) | (means, M) | mean (95% CI) | mean (95% CI) | ||
| Low risk (≤4.0) | 409 | 32 (7.8%) | 184.9 | 93.4% (91.4% – 95.5%) | 0.5% (0.3% – 0.6%) |
| Medium risk (4.5 – 5.5) | 215 | 36 (16.7%) | 178.5 | 87.3% (84.1% – 90.6%) | 1.0% (0.7% – 1.3%) |
| High risk (6.0 – 6.5) | 301 | 82 (27.2%) | 154.8 | 81.4% (78.5% – 84.3%) | 1.7% (1.4% – 2.0%) |
| Ultra-high risk (≥ 7.0) | 254 | 116 (46.1%) | 117.4 | 71.3% (67.8% – 74.8%) | 3.9% (3.3% – 4.5%) |
| Overall | 1179 | 267 (22.6%) | 165.5 | 79.2% (77.1% – 81.3%) | 1.9% (1.7% – 2.1%) |
Results of Kaplan-Meier survival analysis; there were significant differences between each score level (p < 0.01).
Figure 2.
Cumulative failure rate of 1,179 hips with four risk score levels determined by Kaplan-Meier survival curves
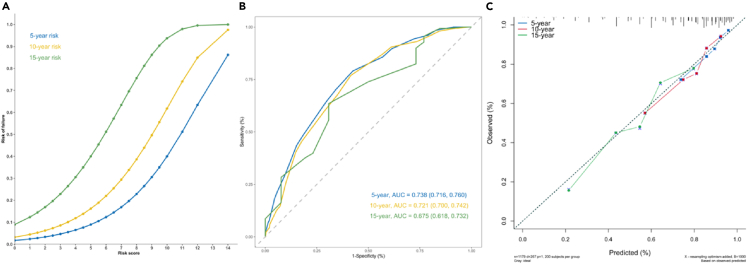
Figure 3.
Plots of the new risk score associated with predicting 5-, 10-, and 15-year hip failure after FVFG therapy
(A) Plot of the risk score associated with predicting 5-, 10-, and 15-year probability of hip failure after FVFG.
(B) The AUROCs of the risk score for a hip failure event prediction at 5, 10, and 15 years.
(C) Calibration plots for the risk-score model for predicting 5-, 10-, and 15-year hip failure risk.
Evaluation of risk scores with a nomogram plot showed an acceptable performance in predicting 5- and 10-year incidence of hip failure event. The C-index was 0.72 (95% CI: 0.68 – 0.75), and the area under the receiver operating characteristics (AUROC) of the risk score for a hip failure event prediction at 5, 10, and 15 years was, respectively, 0.74 (95% CI: 0.72 – 0.76), 0.72 (0.70 – 0.74), and 0.68 (0.62 – 0.73) (Figure 3B). Calibration curves of the model for 5-, 10-, and 15-year hip failure risk showed that these predicted probabilities were close to the observed incidences (Figure 3C).
Internal validation for predicting score model
The original data were randomized into two parts (validation split), and then they were evaluated by discrimination and calibration, again using 1,000 bootstrap samples. The validation of C-index was 0.74 (0.70 – 0.78); the AUROCs for predicting accuracy of hip failure risk at 5, 10, and 15 years in internal validation set are shown in Figure S1. All of them showed an acceptable prediction effect. The calibration plots of the scores for 5-, 10-, and 15- year hip failure risk in the validation set are shown in Figure S1. Calibration was also done by testing for agreement between the observed and predicted hip failure in the validation/entire cohort sets using the Nam-D’Agostino test. The Nam-D’Agostino test of the risk-score model indicated that there was appropriate agreement between the observed and predicted hip failure in the validation and entire cohort sets (see Figure S2). All the aforementioned results on internal validation indicated that the risk-score model is reliable and feasible.
Outperforms conventional methods in forecasting hip preservation
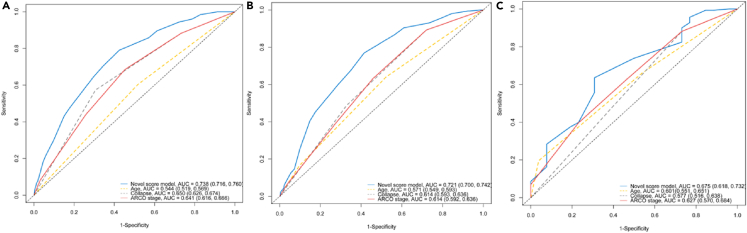
Due to scarcity of other comparable risk-score models in predicting risk for hip failure, we compared the performance of the risk model against previously identified methods or key risk variables. Preoperative ONFH stage (i.e., ARCO imaging stage), collapse status of the femoral head, and surgical age were compared with our risk-score model using AUROCs. The AUROC plots indicated that the area under the curve (AUC) value for predicting 5-, 10-, and 15-year hip failure risk with the score model was superior to any of the other three methods during the same follow-up periods (Figure 4).
Figure 4.
Comparison of AUROC plots of integrated risk-score model and three methods conventionally believed to be dominant risk-prediction variables for 5-, 10-, and 15-year hip failure
Plots of (A), (B), and (C) show AUC values of the score model for 5-, 10-, and 15-year predicting of hip failure risk compared with three methods (age at surgery, degree of collapse, and ARCO stage), respectively. Integrated risk-score model shown in blue.
Sensitivity analysis
The sensitivity analysis was performed using the subgroup samples of patients (age ≤ 50 years, collapse of the femoral head < 2 mm) from the risk-score model. Of the cases analyzed, 15.6% (108/691 surgical hips) presented at their last follow-up with hip failure events with a median of 112.5 months follow-up. The AUC values for predicting accuracy of hip failure risk at 5-, 10- year, and 15- year were 0.71, 0.73, 0.63, respectively (see Figure S3).
Discussion
To our knowledge, the present study assembled one of the largest cohorts of patients in which bone grafting (FVFG) was used to treat ONFH with the aim of preserving hip(s). Most of ONFHs (75.2% hips) were post-collapsed, having an ARCO stage of IIIA to IV (Table 1). HHS at long-term follow-up showed that bone grafting significantly improved disabling symptoms of ONFH. Only 22.8% hips converted to or needed THA (hip failure) after a median follow-up of 110.3 months. The results of the present study reconfirmed that FVFG has significant positive effects in improving hip function, relieving pain, maintaining femoral head stability, or delaying disease progression under appropriate indications.19,37,38
According to those potential factors reported with the ability to affect the prognosis of ONFH,4,14,19,20,23,39 we performed a multivariable analysis to control the influence of potential confounding factors and identified eight key variables that are closely associated with hip survival after the surgery. Besides the status of collapse, surgical age, and extent of hip joint degeneration, those aforementioned variables are key risk factors commonly emphasized; in this study, five other risk factors are also identified that influenced hip-preservation prognosis: larger size (more than 30%) of necrosis lesion, (degree of moderate-to-high) intensity of occupational or daily activity, presence of bilateral ONFH, low preoperative hip function (i.e., poor HHS), and perioperative smoking. Our result confirmed that the larger size of necrosis lesion remains one of important risk factors for hip-preservation prognosis, which is similar to the previous reports.14,40 Bilateral involvement and occupations requiring moderate-to-high intensity physical labor may increase the burden on the affected hip due to long-term and repeated weight bearing;19 perioperative smoking is identified to be a key risk factor for hip survival with FVFG therapy. As we all know, in terms of microvascular surgery, perioperative smoking could cause circulatory crisis, arterial spasm, and thrombosis, which will increase the failure rate in microvascular tissue transfer.41,42 The possible mechanisms of the harmful substance nicotine in tobacco on vascular microcirculation have been studied.43,44 So, the importance of educating patients to avoid smoking perioperatively for this surgery should be re-emphasized. And low HHS is also confirmed to be associated with poor prognosis on hip preservation for ONFH, which is consistent with previous literature.45
Compared to previous studies,4,20,37,38,39 this study included a good representation of bone grafting for ONFH cases with non-collapsed and collapsed femoral heads. Firstly, comprehensive variables between baseline (demographic, characteristic of clinical and imaging, etc.) and perioperative period were collected for screening the potential risk factors. Secondly, the participants were followed up for a sufficiently long time; we clearly defined the criteria for recruiting and enrolling patients into the large-scale real-world follow-up cohort and outlined a reliable endpoint event. Thirdly, confounding factors were well controlled using a new Cox model (considering the random effect of individual hip(s) in cases), and a reliable internal verification and subgroup analysis was conducted. The aforementioned advantages demonstrate that this research has avoided or reduced selection bias and confounding bias as much as possible, and it also enlightens that the constructive risk model is more robust and reliable for risk predicting of long-term hip preservation in ONFH when compared to previous models.46,47
The most important advance of this study was that, we proposed a comprehensive quantitative risk scoring for predicting hip-preservation prognosis and preliminarily verified its performance in comparison with conventional methods used for evaluation of ONFH. In our knowledge, maybe it is the first time that, based on large-scale sample of population, an integrated predictive risk scoring has been reported to be closely related to long-term hip-preservation effect for ONFH. The risk-score model added value for predicting prognosis of hip survival and facilitated the management of decision-making for future ONFHs. We had a stratified risk score that indicated different treatment options. For example, an ONFH with a score of ≥7 should be considered a contraindication for bone grafting treatment in future, as this score level of patient will likely not benefit. Since ONFH is a complex refractory disease, multi-factor comprehensive evaluation has become an increasingly useful trend.14 Therefore, the quantitative risk scoring developed from this study may bring positive value in stratification for ONFH management, due to its more precise surgical indications for hip preservation than those using conventional methods (such as imaging stages or degree of collapse, patient age) in previous studies.2,3,5,14,23
In conclusion, the study confirmed that bone grafting shows sustained benefits in treating ONFH when applied under the right indications. The developed risk-scoring system proves valuable as a decision-making tool, facilitating risk stratification for ONFH treatments. Future external validation is warranted.
Limitations of the study
This study has some limitations. First, we failed to analyze some variables (e.g., BMI, laboratory indicators, date of weight bearing after surgery) because of incomplete or missing data. These variables might have influenced the multivariable model if included. Second, the receiver operating characteristic (ROC) and calibration plot for the risk-score model for predicting 15-year THA risk is relatively unsatisfied or unstable due to the limited number of patients with long-term follow-ups of > 15 years. However, we obtained satisfactory results for internal validation of the risk-score model. Third, related few cohorts have had long-term follow-ups with bone grafting for treating ONFH globally; this requires large and qualified sample sizes to develop a stable model with external data verification. We recommend conducting similar studies or applying this method to test its rationality, feasibility and reliability. Therefore, caution is needed when extending the conclusion of the risk score associated with hip preservation to other bone grafting methods for treating ONFH.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and algorithms | ||
| SAS 9.4 | SAS Institute | https://www.sas.com |
| R language (Version 4.2.0) | The R Project for statistical computing | http://www.r-project.org |
Resource availability
Lead contact
-
•
Further information and requests should be directed to the lead contact, Changqing Zhang (zhangcq@sjtu.edu.cn).
Materials availability
-
•
This study did not generate any unique reagents.
Data and code availability
-
•
This paper does not report the original code.
-
•
The data sources of this study are presented in the “STAR Methods” sections.
-
•
Any additional information required to the data reported in this paper is available from the lead contact upon request.
Experimental model and study participant details
Study design and subjects
The prospective cohort study was conducted at a single medical center in China, and the aforementioned data was sourced from our offline registration system specifically designed for hip preservation with bone grafting (FVFG) for treating ONFH. From April 2001 to December 2010, sequentially screened and enrolled in-patients who met the criteria for receiving FVFG treatment from the data registration system. This study is part of a real-world cohort study on bone transplantation for hip preservation in the treatment of ONFH. The study complied with the ethical principles regarding human experimentation in the Declaration of Helsinki and was approved by the ethics committee of Shanghai Sixth People’s Hospital (no. 2013-45-(1)). Each participant provided written informed consent.
All participants included in the study met the following criteria: (1) had a preoperative diagnosis of ONFH rated at stage II - IV according to Association Research Circulation Osseous (ARCO) international classification of osteonecrosis;24 and (2) were younger than 60 years old on admission for FVFG surgery. Patients were excluded when any of the following criteria were met: (1) declined to sign the informed consent form or explicitly refused to participate; (2) participants who were censored for less than six months after discharge and (or) those who lacked key outcome variables, such as hip failure or the latest hip function scores, were also excluded. A total of 970 patients (1360 hips) who had received FVFG treatment were screened for inclusion/exclusion 938 patients (1321 hips) of ONFH were eligible for this study. The final study population included 854 patients (Female / Male= 236/618) with 1206 hips (Figure 1); 97.9% of the participants (836/854) in this study are Han Chinese, and the other 2.1% are ethnic minorities in China.
Method details
Collection of variables
Baseline demographic characteristics (gender, surgical age, ethnicity), health history (etiology), lifestyle (smoking,25 heavy alcohol consumption,26,27 physical activity28,29), ONFH laterality, ONFH imaging data, preoperative hip-function status, and perioperative smoking were collected.
Preoperative imaging characteristics of the ONFH, including the size of lesions; location; degree of collapse; extent of a crescent sign or surface collapse; degree of acetabular degeneration; and imaging stage. For the femoral head was collapsed apparently, only X-ray images (anteroposterior and frog-leg lateral view) were needed for assessment. If suspected collapse or no collapse, joint images were assessed by using X-ray, CT, and (or) MRI. The detailed method of imaging measurement was referred to previously described,24 which gave an overview how to evaluate or measure the necrosis location (medial, central, lateral), necrosis extent (minimal or < 15%, moderate or 15% - 30%, extensive or > 30%), percent of surface collapse (< 15%, 15% – 30%, > 30%) and dome depression (< 2 mm, 2 - 4 mm, > 4mm), and classified the ARCO imaging (stage I to IV). Participants’ hip function was assessed using the Harris hip score (HHS) scale.30
Definition of variables
Preoperative smoking statuses were classified as no smoking, ceased smoking (cessation of smoking for more than 6 months) and currently smoking (at least one cigarette per day) before two weeks admission in this study.25
Perioperative smoking was defined as smoking during the time from 2 - 3 weeks prior to admission for surgery to four weeks after operation in this study.
Heavy alcohol consumption was defined as alcohol intake of ≥ 15 grams per day (female) and ≥ 30 grams per day (male),26 (early) cessation of heavy drinking was defined as reporting no heavy drinking days in the past three months and drinking with weekly limits, or abstinence at one year.27
Our two daily occupational exertion intensity categories were derived using a two-step reclassification according to the nine International Standard Classification of Occupations of 1988 (ISCO - 88). First, using the nine ISCO - 88 categories, we assigned the participants’ daily exertion intensity into one of three groups: (1) low intensity, which included managers, scientists, and office workers; (2) moderate intensity, which included technicians, service workers, and machine operators; or (3) high intensity, which included farmers or agricultural workers, craftsmen, and laborers.28,29 Second, participants were assigned to one of two intensity categories based on their individual answers on a questionnaire. Thus, the two categories used for analysis were low intensity and moderate-to-high intensity.
The degree of acetabular degeneration was defined as follows: Mild degeneration was characterized by sclerosis of the joint surface; moderate-to-severe degeneration was characterized by the presence of osteophytes and/or joint stenosis.
Harris hip score (HHS) scale. HHS is the main tool for evaluating individual hip disabilities and outcomes of treatment, especially in hip-joint preservation studies.30 HHS has domains that measure pain; walking function (gait, assistance when walking, walking distance); daily functional activities; deformity; and range of motion. It categorizes hip function into four grades: excellent (90 - 100 points); good (80 - 89 points); fair (70 - 79 points); and poor (< 70 points).31
Surgical technique
The surgical technique we used for bone grafting (FVFG) has been described in detail previously.32 All operations were performed by the same group of senior surgeons (CZ, JS, DJ). The surgical technique, which in overview, comprises the vascularized fibular harvest (a lateral approach at the midportion of the leg was made to harvest the fibular graft, and the length of the fibular graft was 6-8 cm on average), removal of the necrotic tissue from the femoral head, hip debridement, fibular implantation and fixation, and vascular anastomosis (anastomosis of the peroneal vessels to the ascending branch of the lateral femoral circumflex vessels was performed).
Postoperative management, follow-ups and endpoints
At hospital discharge following FVFG surgery, we orally instructed participants about active and passive functional exercises for their lower extremities and about the schedule we wanted them to follow for progressively increasing weight-bearing; this information was also given to them in writing. In general, our approach to postoperative weight-bearing is tailored to the individual needs of each patient, guided by their specific clinical presentation and surgical findings. patients are told to avoid bearing weight on the extremity for six weeks to three months according to individual status. For patients diagnosed with early-stage avascular necrosis of the femoral head (ARCO II or earlier), who demonstrate intraoperative stability, we advocate a cautious progression to weight-bearing. Typically, this begins with light weight-bearing exercises at about 6 weeks post-surgery, with a gradual increase to full weight-bearing by 3 - 4 months, contingent on the patient's response and recovery. In contrast, patients with more advanced necrosis (ARCO III or later), or those with intraoperative instability, are advised to delay the start of weight-bearing exercises until approximately 3 months post-surgery. The goal for these patients is to advance to full weight-bearing by the 5 - 6 months mark, again depending on individual progress and stability.
Participants were followed up with radiographic and clinical examinations every three months for the first postoperative year and semiannually for the second year. Afterwards, annual follow-up was required, but the actual frequency of follow-up visits was relatively intermittent due to patients’ causes. In this study, the most recent follow-up took place through outpatient evaluation or through remote video interview. During the follow-up period, changes in HHS, baseline characteristic of ONFH imaging and radiographic progression, hip survival status, death, and the time of hip failure or death was recorded. A team (YF, DJ, JS) was responsible for reviewing the radiographic images and radiographic progression of hip joint (osteoarthritis and its severity), when disputes arose regarding the judgment and interpretation of certain radiographic images, the team members persevered in their discussions until they reached a consensus. The evaluation of HHS scores at baseline and follow-up was guided and assisted by the investigators, the patient filled out or reported verbally; and the verification of HHS information was completed by a team (SC, ZZ, HH).
Follow-up endpoints or cut-off was set as follows: censored any time after 6 months post-FVFG surgery, date of diagnosis or occurrence of a hip failure, death, or the phased study end date (i.e., December 31, 2019), whichever came first.
Outcomes and definitions
The primary outcome was the cumulative rate of hip failure. Hip failure was defined as target hip(s) needing hip replacement or converted to total hip arthroplasty (THA). At follow-up, needing hip replacement was judged if the femoral head (hip joint) had progressively occurred severe osteoarthritis (Kallgren-Lawrence grade III - IV), with a concomitant of poor (< 70 points) in HHS score (including increasing pain despite medication, unacceptably severe limitations in hip joint function).
Quantification and statistical analysis
Categorical variables were summarized using frequency distribution and percentage, while continuous variables were summarized as mean ± standard deviation (SD) or median and interquartile range (IQR), as appropriate. Categorical variables between groups were compared using the Chi-square test. Continuous variables were compared using an independent-samples t test or pair-samples t test, or nonparametric test, where appropriate according to distribution pattern. The cumulative incidence of hip failure was calculated using the Kaplan-Meier survival method, and differences between groups were evaluated using the log-rank test. For consideration of unequal number between subjects of patient and hip (s) and modifying the potential baseline hazard function, a Cox proportional hazards regression model with a random effect (frailty Cox model)33,34 was used to identify independent risk factors and calculate their hazard ratios (HRs) and 95% confidence intervals (CIs). The forward stepwise method of partial likelihood ratio test was used. Covariates selection for entering multivariable model were based on the results of univariate analyses, except for the imaging stage (as a composite imaging variable); A criterion of probability was then set for the forward stepwise method, with αentry = 0.05 and αremoval = 0.1. Independent risk factors were selected that had effects on development of hip failure(s). Missing data was not subjected to multiple imputation when conducting multivariable analysis.
Following described strategies,35,36 the risk-prediction score (model) was calculated. First, we calculated a ratio for each variable by dividing each beta coefficient by the smallest coefficient from the final frailty Cox model, and then rounded that number of ratios to the nearest integer or half integer and assigned this number as the variable’s score.35,36 Second, we generated a score for each individual based on the sum of the variable scores; this score was incorporated into the final model. As with previous study,36 the model scores in this cohort were stratified into four risk levels based on the distribution of the median and IQR. Harrell’s C-index and area under the receiver operating characteristic (ROC) curves (AUROCs) were used to evaluate the prediction model’s discrimination at 5-, 10-, and 15-year. The prediction model was calibrated using calibration curves and Nam-D’Agostino test. Internal validation was performed according to a 1:1 holdout (validation split) cross validation using 1000 bootstrap samples. The C-index, AUROCs, calibration plots, and Nam-D’Agostino test were used for internal validation.
The ROC curve was also used to evaluate the difference in prediction accuracy between the comprehensive index (risk score) and some conventional variables (such as imaging stage, collapse status of preoperative femoral head, and surgical age). Additionally, we performed a sensitivity analysis (or subgroup analysis) to assess the predicting effect of a given AUC value when adjusting the samples for surgical age (≤ 50 years) and limited collapse < 2 mm. The level of significance was set at P < 0.05 (two-sided test). All statistical analyses were conducted using SAS version 9.4 (SAS Institute) and the R statistical package (version 4.2.0, http://www.r-project.org).
Additional resources
-
•
This study did not generate or contributed to a new website/forum.
Acknowledgments
We want to acknowledge and thank all the participants involved in this study and investigators for their efforts in creating this ONFH dataset.
We thank fundings for supporting this study: Shanghai Municipal Health Commission key priority discipline project, Shanghai Spinal Disease and Trauma Orthopedics Research Center Project, Three-year action project to promote clinical skills and clinical innovation in municipal hospitals of Shanghai Hospital Development Center (no. SHDC2023CRS049), and National Health Commission of the People's Republic of China (no. 201402016).
Author contributions
S.C. and C.Z. contributed to the conception and/or design of the work. All authors contributed to the acquisition, analysis, or interpretation of data for the work. S.C., K.F., Q.C., and C.Z. worked on the initial draft of the manuscript. S.C. and Q.C. did the statistical analysis. All authors had the opportunity to revise the work critically for important intellectual content. S.C. and C.Z. have directly accessed and verified the underlying data reported in the manuscript. All authors agreed the final version to be published. C.Z. is the guarantor.
Declaration of interests
The authors declare no competing interests.
Published: February 27, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.109332.
Supplemental information
References
- 1.Mont M.A., Zywiel M.G., Marker D.R., McGrath M.S., Delanois R.E. The natural history of untreated asymptomatic osteonecrosis of the femoral head: a systematic literature review. J. Bone Joint Surg. Am. 2010;92:2165–2170. doi: 10.2106/JBJS.I.00575. [DOI] [PubMed] [Google Scholar]
- 2.Ligh C.A., Nelson J.A., Fischer J.P., Kovach S.J., Levin L.S. The Effectiveness of Free Vascularized Fibular Flaps in Osteonecrosis of the Femoral Head and Neck: A Systematic Review. J. Reconstr. Microsurg. 2017;33:163–172. doi: 10.1055/s-0036-1594294. [DOI] [PubMed] [Google Scholar]
- 3.Yoon B.H., Mont M.A., Koo K.H., Chen C.H., Cheng E.Y., Cui Q., Drescher W., Gangji V., Goodman S.B., Ha Y.C., et al. The 2019 Revised Version of Association Research Circulation Osseous Staging System of Osteonecrosis of the Femoral Head. J. Arthroplasty. 2020;35:933–940. doi: 10.1016/j.arth.2019.11.029. [DOI] [PubMed] [Google Scholar]
- 4.Eward W.C., Rineer C.A., Urbaniak J.R., Richard M.J., Ruch D.S. The vascularized fibular graft in precollapse osteonecrosis: is long-term hip preservation possible? Clin. Orthop. Relat. Res. 2012;470:2819–2826. doi: 10.1007/s11999-012-2429-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mont M.A., Salem H.S., Piuzzi N.S., Goodman S.B., Jones L.C. Nontraumatic Osteonecrosis of the Femoral Head: Where Do We Stand Today?: A 5-Year Update. J. Bone Joint Surg. Am. 2020;102:1084–1099. doi: 10.2106/JBJS.19.01271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moya-Angeler J., Gianakos A.L., Villa J.C., Ni A., Lane J.M. Current concepts on osteonecrosis of the femoral head. World J. Orthop. 2015;6:590–601. doi: 10.5312/wjo.v6.i8.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao D., Zhang F., Wang B., Liu B., Li L., Kim S.Y., Goodman S.B., Hernigou P., Cui Q., Lineaweaver W.C., et al. Guidelines for clinical diagnosis and treatment of osteonecrosis of the femoral head in adults (2019 version) J. Orthop. Translat. 2020;21:100–110. doi: 10.1016/j.jot.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGrory B.J., York S.C., Iorio R., Macaulay W., Pelker R.R., Parsley B.S., Teeny S.M. Current practices of AAHKS members in the treatment of adult osteonecrosis of the femoral head. J. Bone Joint Surg. Am. 2007;89:1194–1204. doi: 10.2106/JBJS.F.00302. [DOI] [PubMed] [Google Scholar]
- 9.Ng M.K., Gordon A.M., Piuzzi N.S., Wong C.H.J., Jones L.C., Mont M.A. Trends in Surgical Management of Osteonecrosis of the Femoral Head: A 2010 to 2020 Nationwide Study. J. Arthroplasty. 2023;38:S51–S57. doi: 10.1016/j.arth.2023.03.071. [DOI] [PubMed] [Google Scholar]
- 10.Sodhi N., Acuna A., Etcheson J., Mohamed N., Davila I., Ehiorobo J.O., Jones L.C., Delanois R.E., Mont M.A. Management of osteonecrosis of the femoral head an up-to-date analysis of operative trends. Bone Joint Lett. J. 2020;102-B:122–128. doi: 10.1302/0301-620x.102b7.Bjj-2019-1611.R1. [DOI] [PubMed] [Google Scholar]
- 11.Ding H., Gao Y.S., Chen S.B., Jin D.X., Zhang C.Q. Free vascularized fibular grafting benefits severely collapsed femoral head in concomitant with osteoarthritis in very young adults: a prospective study. J. Reconstr. Microsurg. 2013;29:387–392. doi: 10.1055/s-0033-1343836. [DOI] [PubMed] [Google Scholar]
- 12.Huang Z., Li T., Lin N., Cui Q., Chen W. Evaluation of Radiographic Outcomes after Core Decompression for Osteonecrosis of the Femoral Head: The Beijing University of Chinese Medicine X-ray Evaluation Method. J. Bone Joint Surg. Am. 2022;104:25–32. doi: 10.2106/JBJS.20.00478. [DOI] [PubMed] [Google Scholar]
- 13.Takashima K., Sakai T., Hamada H., Takao M., Sugano N. Which Classification System Is Most Useful for Classifying Osteonecrosis of the Femoral Head? Clin. Orthop. Relat. Res. 2018;476:1240–1249. doi: 10.1007/s11999.0000000000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee G.C., Steinberg M.E. Are we evaluating osteonecrosis adequately? Int. Orthop. 2012;36:2433–2439. doi: 10.1007/s00264-012-1658-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ha Y.C., Jung W.H., Kim J.R., Seong N.H., Kim S.Y., Koo K.H. Prediction of collapse in femoral head osteonecrosis: a modified Kerboul method with use of magnetic resonance images. J. Bone Joint Surg. Am. 2006;88(Suppl 3):35–40. doi: 10.2106/JBJS.F.00535. [DOI] [PubMed] [Google Scholar]
- 16.Wei Q.S., Li Z.Q., Hong Z.N., Hong G.J., Pang F.X., Yang P., Yang F., Yuan Y.J., Zhuang Z.K., He W. Predicting Collapse in Osteonecrosis of the Femoral Head Using a New Method: Preserved Angles of Anterior and Lateral Femoral Head. J. Bone Joint Surg. Am. 2022;104:47–53. doi: 10.2106/JBJS.20.00507. [DOI] [PubMed] [Google Scholar]
- 17.Lin T., Cai K., Yang P., WuRi S., Chen W., Deng P., Li Z., Chen Z., He W., Zhang Q., Wei Q. Composite indices of femoral neck strength predicts the collapse of steroid-associated osteonecrosis of the femoral head: a retrospective study. BMC Musculoskelet. Disord. 2022;23:722. doi: 10.1186/s12891-022-05622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mankin H.J. Nontraumatic necrosis of bone (osteonecrosis) N. Engl. J. Med. 1992;326:1473–1479. doi: 10.1056/NEJM199205283262206. [DOI] [PubMed] [Google Scholar]
- 19.Korompilias A.V., Lykissas M.G., Beris A.E., Urbaniak J.R., Soucacos P.N. Vascularised fibular graft in the management of femoral head osteonecrosis: twenty years later. J. Bone Joint Surg. Br. 2009;91:287–293. doi: 10.1302/0301-620X.91B3.21846. [DOI] [PubMed] [Google Scholar]
- 20.Nakasone S., Takao M., Sakai T., Nishii T., Sugano N. Does the extent of osteonecrosis affect the survival of hip resurfacing? Clin. Orthop. Relat. Res. 2013;471:1926–1934. doi: 10.1007/s11999-013-2833-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomaru Y., Yoshioka T., Sugaya H., Kumagai H., Hyodo K., Aoto K., Wada H., Akaogi H., Yamazaki M., Mishima H. Ten-year results of concentrated autologous bone marrow aspirate transplantation for osteonecrosis of the femoral head: a retrospective study. BMC Musculoskelet. Disord. 2019;20:410. doi: 10.1186/s12891-019-2797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morita D., Hasegawa Y., Okura T., Osawa Y., Ishiguro N. Long-term outcomes of transtrochanteric rotational osteotomy for non-traumatic osteonecrosis of the femoral head. Bone Joint Lett. J. 2017;99-B:175–183. doi: 10.1302/0301-620X.99B2.BJJ-2016-0417.R2. [DOI] [PubMed] [Google Scholar]
- 23.Mont M.A., Cherian J.J., Sierra R.J., Jones L.C., Lieberman J.R. Nontraumatic Osteonecrosis of the Femoral Head: Where Do We Stand Today? A Ten-Year Update. J. Bone Joint Surg. Am. 2015;97:1604–1627. doi: 10.2106/JBJS.O.00071. [DOI] [PubMed] [Google Scholar]
- 24.Steinberg M.E., Steinberg D.R. Classification systems for osteonecrosis: an overview. Orthop. Clin. North Am. 2004;35:273–283. doi: 10.1016/j.ocl.2004.02.005. vii-viii. [DOI] [PubMed] [Google Scholar]
- 25.Santos A.C., Ebrahim S., Barros H. Alcohol intake, smoking, sleeping hours, physical activity and the metabolic syndrome. Prev. Med. 2007;44:328–334. doi: 10.1016/j.ypmed.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Cao Y., Willett W.C., Rimm E.B., Stampfer M.J., Giovannucci E.L. Light to moderate intake of alcohol, drinking patterns, and risk of cancer: results from two prospective US cohort studies. BMJ. 2015;351:h4238. doi: 10.1136/bmj.h4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palzes V.A., Kline-Simon A.H., Satre D.D., Sterling S., Weisner C., Chi F.W. Predictors of early and sustained cessation of heavy drinking over 5 years among adult primary care patients. Addiction. 2022;117:82–95. doi: 10.1111/add.15612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brighenti-Zogg S., Mundwiler J., Schüpbach U., Dieterle T., Wolfer D.P., Leuppi J.D., Miedinger D. Physical Workload and Work Capacity across Occupational Groups. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duncan M.J., Badland H.M., Mummery W.K. Physical activity levels by occupational category in non-metropolitan Australian adults. J. Phys. Act. Health. 2010;7:718–723. doi: 10.1123/jpah.7.6.718. [DOI] [PubMed] [Google Scholar]
- 30.Smith M.V., Klein S.E., Clohisy J.C., Baca G.R., Brophy R.H., Wright R.W. Lower extremity-specific measures of disability and outcomes in orthopaedic surgery. J. Bone Joint Surg. Am. 2012;94:468–477. doi: 10.2106/JBJS.J.01822. [DOI] [PubMed] [Google Scholar]
- 31.Nilsdotter A., Bremander A. Measures of hip function and symptoms: Harris Hip Score (HHS), Hip Disability and Osteoarthritis Outcome Score (HOOS), Oxford Hip Score (OHS), Lequesne Index of Severity for Osteoarthritis of the Hip (LISOH), and American Academy of Orthopedic Surgeons (AAOS) Hip and Knee Questionnaire. Arthritis Care Res. 2011;63(Suppl 11):S200–S207. doi: 10.1002/acr.20549. [DOI] [PubMed] [Google Scholar]
- 32.Gao Y.S., Chen S.B., Jin D.X., Sheng J.G., Cheng X.G., Zhang C.Q. Modified surgical techniques of free vascularized fibular grafting for treatment of the osteonecrosis of femoral head: results from a series of 407 cases. Microsurgery. 2013;33:646–651. doi: 10.1002/micr.22149. [DOI] [PubMed] [Google Scholar]
- 33.Austin P.C. A Tutorial on Multilevel Survival Analysis: Methods, Models and Applications. Int. Stat. Rev. 2017;85:185–203. doi: 10.1111/insr.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang H.K., Liu P.P.S., Hsu J.Y., Lin S.M., Peng C.C.H., Wang J.H., Loh C.H. Fracture risks among patients with atrial fibrillation receiving different oral anticoagulants: a real-world nationwide cohort study. Eur. Heart J. 2020;41:1100–1108. doi: 10.1093/eurheartj/ehz952. [DOI] [PubMed] [Google Scholar]
- 35.McMahon G.M., Zeng X., Waikar S.S. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern. Med. 2013;173:1821–1828. doi: 10.1001/jamainternmed.2013.9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang H., Bae S.H., Nam H., Lee H.L., Lee S.W., Yoo S.H., Song M.J., Kwon J.H., Nam S.W., Choi J.Y., et al. A risk prediction model for hepatocellular carcinoma after hepatitis B surface antigen seroclearance. J. Hepatol. 2022;77:632–641. doi: 10.1016/j.jhep.2022.03.032. [DOI] [PubMed] [Google Scholar]
- 37.Richard M.J., DiPrinzio E.V., Lorenzana D.J., Whitlock K.G., Hein R.E., Urbaniak J.R. Outcomes of free vascularized fibular graft for post-traumatic osteonecrosis of the femoral head. Injury. 2021;52:3653–3659. doi: 10.1016/j.injury.2021.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Yoo M.C., Kim K.I., Hahn C.S., Parvizi J. Long-term followup of vascularized fibular grafting for femoral head necrosis. Clin. Orthop. Relat. Res. 2008;466:1133–1140. doi: 10.1007/s11999-008-0204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berend K.R., Gunneson E.E., Urbaniak J.R. Free vascularized fibular grafting for the treatment of postcollapse osteonecrosis of the femoral head. J. Bone Joint Surg. Am. 2003;85:987–993. doi: 10.2106/00004623-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Boontanapibul K., Huddleston J.I., 3rd, Amanatullah D.F., Maloney W.J., Goodman S.B. Modified Kerboul Angle Predicts Outcome of Core Decompression With or Without Additional Cell Therapy. J. Arthroplasty. 2021;36:1879–1886. doi: 10.1016/j.arth.2021.01.075. [DOI] [PubMed] [Google Scholar]
- 41.Gu Y.D., Zhang G.M., Zhang L.Y., Li F.G., Jiang J.F. Clinical and experimental studies of cigarette smoking in microvascular tissue transfers. Microsurgery. 1993;14:391–397. doi: 10.1002/micr.1920140608. [DOI] [PubMed] [Google Scholar]
- 42.van Adrichem L.N., Hoegen R., Hovius S.E., Kort W.J., van Strik R., Vuzevski V.D., van der Meulen J.C. The effect of cigarette smoking on the survival of free vascularized and pedicled epigastric flaps in the rat. Plast. Reconstr. Surg. 1996;97:86–96. doi: 10.1097/00006534-199601000-00015. [DOI] [PubMed] [Google Scholar]
- 43.Messner B., Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2014;34:509–515. doi: 10.1161/ATVBAHA.113.300156. [DOI] [PubMed] [Google Scholar]
- 44.Leone A., Landini L. Vascular pathology from smoking: look at the microcirculation! Curr. Vasc. Pharmacol. 2013;11:524–530. doi: 10.2174/1570161111311040016. [DOI] [PubMed] [Google Scholar]
- 45.Migliorini F., Maffulli N., Baroncini A., Eschweiler J., Tingart M., Betsch M. Prognostic factors in the management of osteonecrosis of the femoral head: A systematic review. Surgeon. 2023;21:85–98. doi: 10.1016/j.surge.2021.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Pan J., Ding Q., Lv S., Xia B., Jin H., Chen D., Xiao L., Tong P. Prognosis after autologous peripheral blood stem cell transplantation for osteonecrosis of the femoral head in the pre-collapse stage: a retrospective cohort study. Stem Cell Res. Ther. 2020;11:83. doi: 10.1186/s13287-020-01595-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao E.Z., Liu Z.H., Zeng W.N., Ding Z.C., Luo Z.Y., Zhou Z.K. Nomogram to predict collapse-free survival after core decompression of nontraumatic osteonecrosis of the femoral head. J. Orthop. Surg. Res. 2021;16:519. doi: 10.1186/s13018-021-02664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
This paper does not report the original code.
-
•
The data sources of this study are presented in the “STAR Methods” sections.
-
•
Any additional information required to the data reported in this paper is available from the lead contact upon request.