Abstract
Objective
The C reactive protein polymyalgia rheumatica activity score (CRP-PMR-AS) is a composite index that includes CRP levels and was developed specifically for PMR. As treatments such as interleukin-6 antagonists can normalise CRP levels, the erythrocyte sedimentation rate (ESR) of PMR-AS, the clinical (clin)-PMR-AS and the imputed-CRP (imp-CRP)-PMR-AS have been developed to avoid such bias. Our primary objective was to measure the correlation of these activity scores. Our secondary objective was to evaluate the concordance between different cutoffs of the PMR-ASs.
Method
Data from the Safety and Efficacy of tocilizumab versus Placebo in Polymyalgia rHeumatica With glucocORticoid dEpendence (SEMAPHORE) trial, a superiority randomised double-blind placebo-controlled trial, were subjected to post hoc analysis to compare the efficacy of tocilizumab versus placebo in patients with active PMR. The CRP-PMR-AS, ESR-PMR-AS, clin-PMR-AS and imp-CRP-PMR-AS were measured at every visit. The concordance and correlation between these scores were evaluated using kappa correlation coefficients, Bland-Altman correlations, intraclass correlation coefficients (ICCs) and scatter plots.
Results
A total of 101 patients were included in the SEMAPHORE trial, and 100 were analysed in this study. The correlation between the PMR-ASs was excellent, as the ICC and kappa were >0.85 from week 4 until week 24 (CRP-PMR-AS ≤10 or >10). Bland-Altman plots revealed that the differences between the CRP-PMR-AS and the other three
scores were low. The cut-off values for the clin-PMR-AS were similar to those for the CRP-PMR-AS 86% of the time.
Conclusion
The correlation between all the PMR-ASs was excellent, reflecting the low weight of CRP. In clinical trials using drugs that have an impact on CRP, the derived activity scores can be used.
Trial registration number
NTC02908217.
Keywords: biological therapy, inflammation, polymyalgia rheumatica
WHAT IS ALREADY KNOWN ON THIS TOPIC.
The disease activity score developed for polymyalgia rheumatica (PMR) is the algebraic sum of C reactive protein (CRP) and clinical items.
Among patients with active PMR receiving glucocorticoid therapy, the addition of tocilizumab, compared with placebo, resulted in improved disease activity scores; however, tocilizumab decreases in the CRP level.
WHAT THIS STUDY ADDS
We studied the concordance and correlation of the disease activity score using the CRP level (CRP-PMR-AS) with the derived activity scores which do not use CRP level.
The clinical parameters, erythrocyte sedimentation rate and linear regression were used.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
When patients with PMR are treated with interleukin-6 receptor inhibitors, even if the CRP level is affected by the treatment, the disease activity score combined with the CRP level can be used in follow-up and help the therapeutic decisions.
Introduction
Polymyalgia rheumatica (PMR) is a chronic inflammatory disease that occurs in people >50 years of age and is characterised by an increase in acute inflammatory reactants, inflammatory pain in the girdles, inflammatory lumbar or cervical pain, morning stiffness and nocturnal awakenings.1 2 The reference treatment is glucocorticoids for up to 18 months.3 Treatment is managed by practitioners during the follow-up based on clinical and inflammatory parameters and the absence of giant cell arteritis.
Disease activity scores are recommended for chronic diseases to target treatment efficacy homogeneously, to define remission and to stop treatment to limit the duration of exposure. A disease activity score must be simple and practical to use. This score must discriminate between clinical situations and identify differences between the treatment group and control group in trials. It must be truthful, unbiased and relevant.4 The score must also be based on characteristic symptoms of the disease. It should not be influenced by confounding factors such as a treatment that can normalise one of the parameters regardless of its effectiveness. In addition, then, the score must be validated.
In PMR, the tools used in therapeutic trials to evaluate disease activity have been heterogeneous, using (alone or in combination) the absence of inflammation on biological parameters or the clinical improvement of the patients (subjective parameters) assessed by the investigator and/or the ability to decrease the dosage of glucocorticoids. In 2004, an EULAR activity score, the polymyalgia rheumatica activity score (PMR-AS), was devised by a group of experts5 and published by Leeb and Bird6 for monitoring treatment in clinical practice and for use in therapeutic trials. The C reactive protein polymyalgia rheumatica activity score (CRP-PMR-AS) was used in this study to distinguish this score from the other ones proposed. After a literature review, the identification of several biological and clinical items by experts was validated in a cohort of 76 patients with PMR and in a replication cohort, and a core set of response criteria was defined. This final core set included five parameters, with one biological item (CRP), one patient-reported outcome (pain intensity), one morning stiffness duration, one physician’s evaluation of global activity and one clinical item (elevation of the upper limbs) examination. The CRP-PMR-AS has thresholds defining remission (<1.5) and high disease activity (>17) with good sensitivity for improvement or relapse and a high internal validation.6 Since its development, it has been used in several therapeutic trials.7–9
In PMR, the inability to stop using glucocorticoids is referred to as glucocorticoid dependence and occurs in 50% of patients during the first year of treatment10; additionally, glucocorticoid dependence may lead to long-term exposure (4–8 years) in 25% of patients11 12 and the occurrence of glucocorticoid-related adverse events (ie, osteoporotic fracture, diabetes mellitus, hypertension, infections, etc).13 Strategies for lowering exposure to glucocorticoids were developed based on the use of interleukin (IL)-6 receptor antagonists14–16 in patients with active PMR despite treatment with prednisone, which resulted in a greater proportion of patients with low disease activity based on the CRP-PMR-AS (<10) and greater glucocorticoid discontinuation. Other treatments, such as rituximab,17 abatacept,18 tofacitinib19 and baricitinib,20 are under evaluation using the CRP-PMR-AS as the primary end point. In patients treated with an IL-6R antagonist or Janus kinase inhibitor, neither the CRP level21 nor the erythrocyte sedimentation rate (ESR) level is reliable.22 Indeed, the inhibition of the IL-6 pathway quickly induces a decrease in CRP blood levels before the first therapeutic effect. Flares with normal acute phase reactant levels are often observed.23 A dissociation between the CRP concentration/ESR and disease activity is possible, as the ESR is dependent on other parameters (age, anaemia, hyperglobulinaemia, etc) and has a long half-life.
As in rheumatoid arthritis with the Clinical Disease Activity index (CDAI),24 the derived score could be used in PMR if the CRP level is modified by the treatment or unavailable. The clinical PMR-AS (clin-PMR-AS)25 was described in 2018 to avoid bias through this normalisation. Otherwise, it is possible to use a statistical method to impute the CRP level in the imputed CRP activity score (imp-CRP-PMR-AS).25 These scores are used in the current study, although they are limited because they are less often used in the literature and are less validated than the primary score, CRP-PMR-AS.
In this study, the main objective was to measure the correlation of the CRP-PMR-AS with the ESR-PMR-AS, clin-PMR-AS and imp-CRP-PMR-AS among patients treated either with tocilizumab or with corticosteroid therapy using data from the Safety and Efficacy of tocilizumab versus Placebo in Polymyalgia rHeumatica With glucocORticoid dEpendence (SEMAPHORE) (Clinicaltrials.gov identifier: NTC02908217), a superiority randomised double-blind parallel placebo-controlled trial.15
Patients and methods
Patient data
The data were collected from the SEMAPHORE trial, where patients with PMR with an inability to decrease their glucocorticoid dosage <10 mg/day were randomised to receive an infusion of tocilizumab (8 mg/kg) or placebo.15 The primary outcome was CRP-PMR-AS <10 and either a prednisone dosage <5 mg/day or a decrease of ≥10 mg from baseline at week 24. The primary end point was evaluated at week 24. Secondary outcomes included the ESR-PMR-AS, clin-PMR-AS and imp-CRP-PMR-AS at each visit at weeks 0, 4, 8, 12, 16, 20 and 24.
Evaluation of disease activity scores
The CRP-PMR-AS is the algebraic sum of morning stiffness in minutes×0.1; degree of elevation of the upper limbs (EUL) (range 0–3; 3 indicates worse clinical abnormality: 3=none, 2=below the shoulder girdle, 1=up to the shoulder girdle, 0=above the shoulder girdle); a 10-point visual analogue scale (VAS) for pain intensity from the patient (pVAS; 10 indicates worse pain); a 10-point VAS global assessment from the physician (phVAS; 10 indicates worse health) and CRP level in mg/dL.6 Initially, remission was defined as a score <1.5, low disease activity was defined as a score between 1.5 and 7, medium disease activity was defined as a score between 7 and 17 and high disease activity was defined as a score >17.26 It has been shown that a flare can be considered when CRP-PMR-AS is >1027 28 because it is used in practice to guide treatment adjustments.29
The ESR-PMR-AS6 (range 0–100, higher scores indicate worse disease activity, no minimal clinically important difference (MCID)), was calculated with the ESR (in millimetres per hour×0.1) is used instead of CRP. No cut-off has been defined for flare or remission.
The clin-PMR-AS25 (range 0–70; higher score indicates worse disease activity; no MCID established) was calculated by summing the clinical parameters: morning stiffness (in minutes×0.1)+EUL (0–3 scale)+phVAS (0–10 scale)+pVAS (0–10 scale).
The imp-CRP-PMR-AS25 (range 0–100; higher scores indicate worse disease activity, no MCID established) was created using linear regression between the CRP-PMR-AS and clin-PMR-AS. The formula (imp-CRP-PMR-AS=1.12 (clin-PMR-AS)+0.26) was obtained in a previous study.25
Statistical analysis
The correlation between the different activity scores was evaluated as follows. Scatter plots were created based on the whole population with inclusion data and week 24. Additional scatter plots were generated with data from the tocilizumab group and placebo group. Bland-Altman plots were used to assess the agreement between the scores,30 and the CRP-PMR-AS was chosen as the gold standard. The mean differences must be close to zero unless there is a measurement error between the gold standard and the tested score. Indeed, if the variability of the differences was only linked to analytical imprecision of each of the two methods, the average of these differences should be zero. The reliability of the difference in activity scores was evaluated by the intraclass correlation coefficient (ICC). We compared the CRP-PMR-AS and each other score at every visit. An ICC of >0.8 was considered reliable. Cohen’s kappa was used to evaluate the agreement between the CRP-PMR-AS and the different methods used to determine patient status given by the clin-PMR-AS, imp-CRP-PMR-AS and ESR-PMR-AS using different cutoffs (1.5, 7, 10, 17) to dichotomise the CRP-PMR-AS. The result of the agreement varies between −1 and 1 and represents no agreement when <0, none to slight agreement when <0.20, weak agreement when <0.40, moderate agreement when <0.60, substantial agreement when <0.80 and almost perfect agreement when >0.81.31
The contingency table illustrates the features of the disease (remission, low activity, high activity) in terms of the absolute frequency of the clin-PMR-AS and CRP-PMR-AS (with and without CRP) at inclusion and week 24, at the end of protocol treatment, using the same cutoffs as CRP-PMR-AS.
Results
Patient data
The SEMAPHORE trial was conducted in 17 centres in France. A total of 101 patients were included in the SEMAPHORE trial, and 100 were analysed for this study and received tocilizumab (49 patients) or placebo (51 patients) from inclusion to week 24. One patient on tocilizumab was excluded before receiving the first infusion because he developed giant cell arteritis. At baseline, the median (with IQR) CRP level was 0.9 mg/dL (0.4–1.7) in both groups, the median ESR was 28.3 mm/hour in the tocilizumab group and 24.3 mm/hour in the placebo group. The patient VAS was 5.4 in the tocilizumab group and 6.0 in the placebo group, and the physician VAS was 5.5 in the tocilizumab group and 5.3 in the placebo group. The distribution of the upper limb elevation did not differ between the two groups (data not shown).
Correlation between the CRP-PMR-AS and the other activity scores
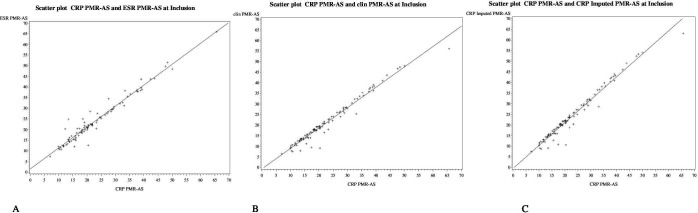
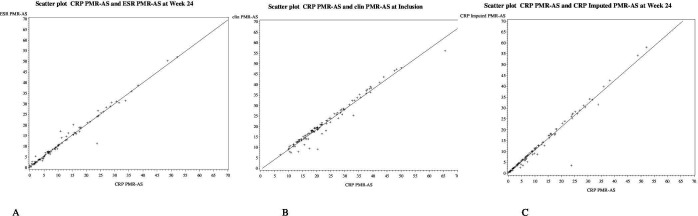
In the whole population of the trial, as shown in the scatter plots (figure 1), the relationship was linear between the CRP-PMR-AS and the other scores at inclusion before the first infusion and at week 24 (figure 2). The results were similar in each treatment group (placebo or tocilizumab) at inclusion and at week 24 (online supplemental figures 1 and 2). Therefore, although tocilizumab induces a dramatic decrease in the CRP level, the correlation between the different PMR-ASs was excellent. The scatter plots showed little dispersion and good correlation.
Figure 1.
Scatter plots between the CRP-PMR-AS* and the ESR-PMR-AS† (A), clin-PMR-AS‡ (B) and imp-CRP PMR-AS§ (C) at inclusion for all the patients (n= 100). Trendlines are represented by a continuous line. The CPR-PMR-AS is the algebraic sum of morning stiffness in minutes multiplied by 0.1, ability to elevate the upper limbs on a scale of 0 to 3, physicians global assessment on a 10-point visual analog scale, patient-reported pain intensity on a 10-point VAS, and CRP level in mg/dL. Higher values indicate greater disease activity. Values below 7 define low disease activity; between 7 and 17, moderate disease activity; and greater than 17, high activity. † The ESR-PMR-AS uses the ESR in minutes multiplied by 0.1 instead of the CRP level. ‡ The clin-PMR-AS is the sum of the clinical items. § The imp-CRP PMR-AS is calculated with the formula 1.12(clin-PMR-AS)+0.26. PMR: polymyalgia rheumatica; AS: activity score; ESR: erythrocyte sedimentation rate; CRP: C reactive protein; clin: clinical; imp-CRP: imputed CRP.
Figure 2.
Scatter plots between the C reactive protein polymyalgia rheumatica activity score (CRP-PMR-AS)* and the erythrocyte sedimentation rate (ESR)-PMR-AS (A), clinical (clin)-PMR-AS (B) and imputed (imp)-CRP-PMR-AS (C) at week 24 for all the patients (n=100). Trending lines are represented by continuous lines. PMR: polymyalgia rheumatica; AS: activity score; ESR: erythrocyte sedimentation rate; CRP: C reactive protein; clin: clinical; imp-CRP: imputed CRP
rmdopen-2023-003741supp001.pdf (260.5KB, pdf)
Discrepancies between the different activity scores, with or without the CRP
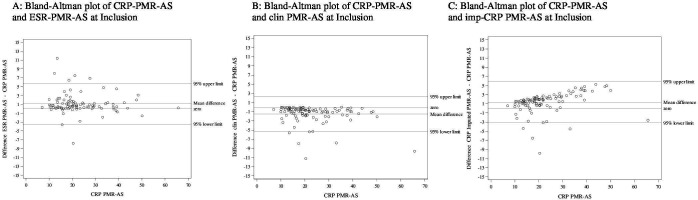
To describe the dispersion of the results between the different activity scores, each score was analysed using Bland-Altman plots at inclusion (figure 3A-C) and after the end of the treatment (week 24) (figure 4) using the CRP-PMR-AS as a reference. Differences were low irrespective of the CRP-PMR-AS value at inclusion. As expected, the clin-PMR-AS (figure 3B) yielded a systematic lower value with a mean difference and 95% CI upper limit of approximately 3 to 5 at inclusion. The spread was more visible when the CRP-PMR-AS was compared with the imp-CRP-PMR-AS and when the CRP-PMR-AS increased (figure 3C). An increase in the imp-CRP-PMR-AS resulted in a greater score.
Figure 3.
Bland-Altman plots using C reactive protein polymyalgia rheumatica activity score (CRP-PMR-AS)* as the gold standard at inclusion for all patients (n=100). ESR, erythrocyte sedimentation rate. The y-axis represents the difference between the CRP-PMR-AS and ESR-PMR-AS (A), the difference between the CRP-PMR-AS and clin-PMR-AS (B) and the difference between the CRP-PMR-AS and imp-CRP PMR-AS (C). The 95% upper and lower limits are presented, and the mean differences and zero line are presented. PMR: polymyalgia rheumatica; AS: activity score; ESR: erythrocyte sedimentation rate; CRP: C reactive protein; clin: clinical; imp-CRP: imputed CRP
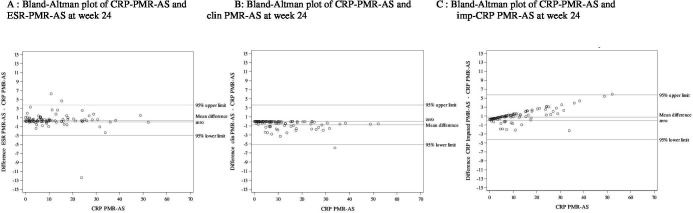
Figure 4.
Bland-Altman plots using the C reactive protein polymyalgia rheumatica activity score (CRP-PMR-AS) as the gold standard at week 24 for all patients (n=100). ESR, erythrocyte sedimentation rate. Abreviations: PMR : polymyalgia rheumatica ; AS : activity score ; ESR : erythrocyte sedimentation rate ; CRP : C reactive protein ; clin : clinical ; imp-CRP : imputed CRP. Footnote:The y-axis represents the difference between the CRP-PMR-AS and ESR-PMR-AS (A), the difference between the CRP-PMR-AS and clin-PMR-AS (B) and the difference between the CRP-PMR-AS and imp-CRP PMR-AS (C). 95% upper and lower limits, the zero line and mean differences are presented. The discordant point represents a patient with a high CRP level (26 mg/L) with no sign of a flare of PMR.
At week 24, the differences became narrower (figure 4), regardless of the treatment group (online supplemental figures 3 and 4).
Agreement between the different PMR-AS
The ICCs between the CRP-PMR-AS and ESR-PMR-AS, between the CRP-PMR-AS and clin-PMR-AS and between the CRP-PMR-AS and imp-CRP-PMR calculated for the global sample were all 0.99 (95% CI 0.99 to 0.99). The ICCs were all >0.98 at inclusion and week 24 (online supplemental table 1). The ICCs remained high when calculated for each treatment group (online supplemental tables 2 and 3), with the highest being 1 between the CRP-PMR-AS and clin-PMR-AS and between the CRP-PMR-AS and ESR-PMR-AS at week 24 in the tocilizumab group. Kappa coefficients were high at each visit calculated for the whole population (table 1), regardless of the chosen cut-off and for each treatment group (data not shown). Indeed, the highest kappa coefficient considering all visits was 0.94 between the CRP-PMR-AS and the clin-PMR-AS with the cut-off value of 17 and between the CRP-PMR-AS and the ESR-PMR-AS with the cut-off value of 10. The lowest kappa coefficient (0.81) was found between the CRP-PMR-AS and the ESR-PMR-AS with the cut-off value of 1.5 (table 1).
Table 1.
Kappa coefficient between the different PMR-ASs* for the whole population, according to the different cut-off values of the CRP-PMR-AS
| Cut-off 0 to 1.5 vs >1.5 Kappa (95% CI) |
Cut-off 0 to 7 vs >7 Kappa (95% CI) |
Cut-off 0 to 10 vs >10 Kappa (95% CI) |
Cut-off 0 to 17 vs >17 Kappa (95% CI) |
|
| CRP-PMR-AS versus ESR-PMR-AS | 0.75 (0.70 to 0.80) | 0.87 (0.84 to 0.90) | 0.90 (0.87 to 0.93) | 0.89 (0.86 to 0.93) |
| CRP-PMR-AS versus clin-PMR-AS | 0.91 (0.88 to 0.94) | 0.93 (0.90 to 0.95) | 0.93 (0.90 to 0.95) | 0.94 (0.92 to 0.97) |
| CRP-PMR-AS versus imp-CRP-PMR-AS | 0.92 (.89 to 0.95) | 0.92 (0.89 to 0.94) | 0.91 (0.89 to 0.94) | 0.92 (0.89 to 0.95) |
Global kappa coefficient was calculated for the whole population (n=100) of the SEMAPHORE trial at all visits between inclusion and week 24.
The results are presented as kappa coefficients with 95% CIs.
*Details on the activity scores are provided in the caption of figure 1.
AS, activity score; clin, clinical; CRP, C reactive protein; ESR, erythrocyte sedimentation rate; imp, imputed; PMR, polymyalgia rheumatica; SEMAPHORE, Safety and Efficacy of tocilizumab versus Placebo in Polymyalgia rHeumatica With glucocORticoid dEpendence.
Evaluation of the classification of disease activity according to the CRP-PMR-AS and clin-PMR-AS
At week 24, the clin-PMR-AS was able to categorise 94% of patients in the placebo group in the same disease activity group as the CRP-PMR-AS; three patients were reclassified. The clin-PMR-AS classified 38/50 patients, and the CRP-PMR-AS classified 35/50 patients under the therapeutic threshold (under 10). In the tocilizumab group, 100% of the patients were categorised into the same activity group as the clin-PMR-AS and the CRP-PMR-AS; the clin-PMR-AS classified 37/47 patients under the 10 mark, as did the CRP-PMR-AS in the tocilizumab group (table 2).
Table 2.
Contingency table at the primary end point (week 24) in the placebo group (n=51) and tocilizumab group (n=49) according to the CRP-PMR-AS* represented in the columns and the clin-PMR-AS in the rows
| Placebo group Clin-PMR-AS |
CRP-PMR-AS | 0 to ≤1.5 | >1.5 to ≤7 | >7 to ≤10 | >10 to ≤17 | >17 | Total |
| 0 to ≤1.5 | 6 | 2 | 0 | 0 | 0 | 8 | |
| >1.5 to ≤7 | 0 | 18 | 3 | 0 | 0 | 21 | |
| >7 to ≤10 | 0 | 0 | 6 | 3 | 0 | 9 | |
| >10 to ≤17 | 0 | 0 | 0 | 6 | 0 | 6 | |
| >17 | 0 | 0 | 0 | 0 | 6 | 6 | |
| Total | 6 | 20 | 9 | 9 | 6 | 50 |
| Tocilizumab group Clin-PMR-AS |
CRP-PMR-AS | 0 to ≤1.5 | >1.5 to ≤7 | >7 to ≤10 | >10 to ≤17 | >17 | Total |
| 0 to ≤1.5 | 12 | 0 | 0 | 0 | 0 | 12 | |
| >1.5 to ≤7 | 0 | 19 | 1 | 0 | 0 | 20 | |
| >7 to ≤10 | 0 | 0 | 5 | 0 | 0 | 5 | |
| >10 to ≤17 | 0 | 0 | 0 | 5 | 0 | 5 | |
| >17 | 0 | 0 | 0 | 0 | 5 | 5 | |
| Total | 12 | 19 | 6 | 5 | 5 | 47 |
Every cut-off of the CRP-PMR-AS is presented and compared with the clin-PMR-AS results. The number of patients with the same results for both scores are presented in grey.
There was one missing data point for the placebo group and two missing data points for the tocilizumab group.
*Details of the activity scores are provided in the caption of figure 1.
PMR, polymyalgia rheumatica; AS, activity score; CRP, C reactive protein; clin, clinical.
At inclusion, as shown in the contingency tables in online supplemental table 5, 96% of the patients were classified in the same activity group as the clin-PMR-AS and CRP-PMR-AS, in the placebo group and 92% in the tocilizumab group (under the 10 mark). The dichotomised clin-PMR-AS and CRP-PMR-AS were concordant regardless of the cut-off considered.
Discussion
In our study, we evaluated the correlation between different PMR-AS scores and the concordance between the different cut-off values. Indeed, despite the low impact of CRP on the global CRP-PMR-AS score, the presence of CRP induced some bias in therapeutic trials. We found strong agreement between the CRP-PMR-AS and ESR-PMR-AS and between the clin-PMR-AS and imp-CRP-PMR-AS at inclusion and at any time during the study. The concordance between the CRP-PMR-AS and the ESR-PMR-AS, clin-PMR-AS and imp-CRP-PMR-AS were excellent for the whole population of patients with glucocorticoid dependence and for each treatment group (tocilizumab vs placebo), as shown in the online supplemental figures 3, 4 and table 4. After a few infusions of tocilizumab, the CRP levels decreased drastically in the treatment group, but the correlation and agreement remained strong at week 4 and week 8. The kappa coefficient in our study between the CRP-PMR-AS and the imp-CRP-PMR-AS in our study was approximately 0.91, regardless of the chosen cut-off. Similar results for the kappa coefficient between the CRP-PMR-AS and imp-CRP-PMR-AS, 0.93 in the Tocilizumab Effect iN pOlymyalgia Rheumatica (TENOR) cohort,25 were published previously. The clin-PMR-AS was concordant with the CRP-PMR-AS in the literature as well,25 explained by the normalisation of CRP levels when patients are treated.
In our study, the imp-CRP-PMR-AS yielded higher activity scores than the CRP-PMR-AS as indicated by Bland-Altman plots (the higher the CRP-PMR-AS was, the greater the difference between the imp-CRP-PMR-AS and the CRP-PMR-AS was). This finding reflects the weight of the increase in CRP level in the CRP-PMR-AS, which is poorly accounted for in the imp-CRP-PMR-AS, probably because the imp-CRP-PMR-AS was created based on a cohort of patients with recent-onset PMR and higher levels of CRP.
One patient in the placebo group at week 24 had an elevated CRP level (23.5 mg/L). This patient had undergone cystoscopy a few days before for bladder polyps, but the subject had no sign of a PMR flare. Another patient had a difference in the mean over the 95% upper limit (mean difference 5.87). That patient had a CRP level of 58 mg/L, a neutrophil count of 9 G/L and no data on possible fever. The glucocorticoid dosage was 4 mg/day. These data suggest an infection, but the patient also had clinical and ultrasonographic signs of a flare-up of PMR.
Activity scores with reliable, credible evaluation criteria that reflect the patient’s clinical situation are important in clinical practice and in therapeutic trials. These criteria, when used in trials, should not be influenced by the evaluated treatments because they create bias. This issue is increasingly prevalent in PMR since treatments such as IL-6R inhibitors are used and modify inflammatory parameters after the first intake. Substitution criteria that are not influenced by the intrinsic effect of the treatment are therefore needed. Trials in rheumatoid arthritis, Still’s disease or giant cell arteritis treated with tocilizumab are also a relevant issue because they used usual activity scores with CRP levels included (eg, DAS28, ACR 50).32–35
A new set of outcome measures has already been explored: muscle tenderness on pressure was too subjective, myalgia was too dependent on pain (VAS) and alpha globulin was not specific enough.5 Other outcomes, such as an ultrasonography score or PET scan, were studied in clinical trials for diagnosis but seem to have a low responsiveness to change after the treatment started36–38; additionally, these outcomes are less accessible, quite expensive and influenced by mechanical damage. With actual knowledge, the glucocorticoid toxicity index39 could be included in the new set for trials with corticosteroid-sparing treatment. Patient-reported outcomes are becoming increasingly important40–42 and are included in the PMR-Impact scale43; however, these outcomes have not been used in trial or in practice, and patient responsiveness and interpretability are not well known. More specific scores are developed in parallel as quality of life parameters (HAQ, Medical Otcome Study Short Form 36 (MOSSF-36 or SF-36)) and could be used as secondary outcomes because they have high variability in patients and are always related to objective improvement.44 But the function in PMR and the quality of life are improved by the glucocorticoid treatment, which is increased in case of signs of relapse. In studies that evaluate a new treatment in addition of the standard treatment by glucocorticoid, a score like the HAQ can be biased by the glucocorticoid dosage increasing or lowering, independently of efficacy of the studied new treatment.
The CRP-PMR-AS described in 2004 is very simple to use, representative of the disease and defined by a group of experts, and can be used in clinical trials in the PMR45 46 instead of in general subjective data (practitioner opinion concerning remission or flare).40 This could allow a better understanding of the therapeutic efficacy of different treatments for PMR. We have shown here that the CRP-PMR-AS has a high internal consistency and provides an option in which it can be used without inflammatory parameters in clinical trials and in practice, thus providing guidance regarding treatment duration or intensification.
This study has several limitations. First, the CRP-PMR-AS has been recently studied and some limitations have been highlighted. There are no data on reliability or measurement error and few data on construct validity and responsiveness.47–49 However, there is no better available activity measure for PMR.
Second, there was no evaluation of the correlation of these scores when patients presented higher levels of CRP. In fact, the median CRP concentration at inclusion was 0.9 mg/dL, and recent PMR was not evaluated in this trial. However, this was done in the TENOR study, which showed a good concordance between the PMR-AS activity scores.7
Third, the ESR is also partially affected by an IL-6 antagonist.22 In trials, the correlation between the ESR-PMR-AS and CRP-PMR-AS has been determined to be very good.6 However, in the acute phase, the ESR-PMR-AS yields a higher score. In our study, the ESR-PMR-AS did not yield a higher score, and the correlation was even stronger.
In conclusion, the concordance between the CRP-PMR-AS and the ESR-PMR-AS, clin-PMR-AS or imp-CRP-PMR-AS is excellent; the clin-PMR-AS is strongly correlated with the CRP-PMR-AS, and the same cutoffs can be used to make therapeutic decisions. The clin-PMR-AS is simple and reliable and is an excellent option for evaluating activity in PMR and for comparing the intervention results of the therapeutic trials, subject to the comparability of the samples included, as well as in clinical practice.
Acknowledgments
The abstract of this work has been presented at the ACR Congress 2022, EULAR Congress 2023 and SFR (French Rheumatology Society) Congress 2022.
Footnotes
Twitter: @crichez33, @alain.saraux
Contributors: JD'A, AS and VD-P contributed to all part of the manuscript, literature search, conceptualisation, design, data collection, methodology, data analysis, data interpretation, validation, writing, recruitment of the patients and supervision. EN and AS contributed to literature search, design, methodology, data collection, data analysis and supervision of data analysis, data interpretation, validation and writing. AL, GCA, ED, CR, M-ET, DW, ET, AP, J-EG, RF, BF, LC, PH, CLH, BD, GD, IC-V, DC, DG, TM contributed to literature search, data collection, resources, data interpretation, writing and recruitment of the patients. VD-P is responsible for the overall content as the guarantor.
Funding: The funders of the SEMAPHORE study were the French National Programme for Clinical Research and the study sponsor. Roche-Chugai provided a grant for the SEMAPHORE study and donated the tocilizumab.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study was approved by Comité de Protection des Personnes Ouest VI, Brest, France.
References
- 1. Buttgereit F, Dejaco C, Matteson EL, et al. Polymyalgia Rheumatica and giant cell arteritis: A systematic review. JAMA 2016;315:2442–58. 10.1001/jama.2016.5444 [DOI] [PubMed] [Google Scholar]
- 2. Dasgupta B, Cimmino MA, Maradit-Kremers H, et al. Provisional classification criteria for Polymyalgia Rheumatica: a European League against rheumatism/American college of rheumatology collaborative initiative. Ann Rheum Dis 2012;71:484–92. 10.1136/annrheumdis-2011-200329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dejaco C, Kerschbaumer A, Aletaha D, et al. Treat-to-target recommendations in giant cell arteritis and Polymyalgia Rheumatica. Ann Rheum Dis 2024;83:48–57. 10.1136/ard-2022-223429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boers M, Kirwan JR, Wells G, et al. Developing core outcome measurement sets for clinical trials: OMERACT filter 2.0. Journal of Clinical Epidemiology 2014;67:745–53. 10.1016/j.jclinepi.2013.11.013 [DOI] [PubMed] [Google Scholar]
- 5. Leeb BF, Bird HA, Nesher G, et al. EULAR response criteria for Polymyalgia Rheumatica: results of an initiative of the European collaborating Polymyalgia Rheumatica group (subcommittee of ESCISIT). Ann Rheum Dis 2003;62:1189–94. 10.1136/ard.2002.002618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leeb BF, Bird HA. A disease activity score for Polymyalgia Rheumatica. Ann Rheum Dis 2004;63:1279–83. 10.1136/ard.2003.011379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Devauchelle-Pensec V, Berthelot JM, Cornec D, et al. Efficacy of first-line Tocilizumab therapy in early Polymyalgia Rheumatica: a prospective longitudinal study. Ann Rheum Dis 2016;75:1506–10. 10.1136/annrheumdis-2015-208742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marsman DE, Bolhuis TE, den Broeder N, et al. Polymyalgia Rheumatica treatment with methotrexate in optimal dose in an early disease phase (PMR MODE): study protocol for a multicenter double-blind placebo controlled trial. Trials 2022;23:318. 10.1186/s13063-022-06263-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bolhuis TE, Marsman DE, den Broeder AA, et al. 1-year results of treatment with Rituximab in Polymyalgia Rheumatica: an extension study of a randomised double-blind placebo-controlled trial. Lancet Rheumatol 2023;5:e208–14. 10.1016/S2665-9913(23)00032-2 [DOI] [PubMed] [Google Scholar]
- 10. González-Gay MA, Matteson EL, Castañeda S. Polymyalgia Rheumatica. Lancet 2017;390:1700–12. 10.1016/S0140-6736(17)31825-1 [DOI] [PubMed] [Google Scholar]
- 11. Kremers HM, Reinalda MS, Crowson CS, et al. Relapse in a population based cohort of patients with Polymyalgia Rheumatica. J Rheumatol 2005;32:65–73. [PubMed] [Google Scholar]
- 12. Salvarani C, Cantini F, Niccoli L, et al. Acute-phase reactants and the risk of relapse/recurrence in Polymyalgia Rheumatica: a prospective followup study. Arthritis Rheum 2005;53:33–8. 10.1002/art.20901 [DOI] [PubMed] [Google Scholar]
- 13. Rossini M, Viapiana O, Vitiello M, et al. Prevalence and incidence of Osteoporotic fractures in patients on long-term glucocorticoid treatment for rheumatic diseases: the glucocorticoid induced osteoporosis tool (GIOTTO) study. Reumatismo 2017;69:30–9. 10.4081/reumatismo.2017.922 [DOI] [PubMed] [Google Scholar]
- 14. Lally L, Forbess L, Hatzis C, et al. Brief report: A prospective open-label phase IIa trial of Tocilizumab in the treatment of Polymyalgia Rheumatica: TCZ IN THE TREATMENT OF PMR. Arthritis & Rheumatology 2016;68:2550–4. 10.1002/art.39740 Available: https://acrjournals.onlinelibrary.wiley.com/toc/23265205/68/10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Devauchelle-Pensec V, Carvajal-Alegria G, Dernis E, et al. Effect of Tocilizumab on disease activity in patients with active Polymyalgia Rheumatica receiving glucocorticoid therapy: A randomized clinical trial. JAMA 2022;328:1053–62. 10.1001/jama.2022.15459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bonelli M, Radner H, Kerschbaumer A, et al. Tocilizumab in patients with new onset Polymyalgia Rheumatica (PMR-SPARE): a phase 2/3 randomised controlled trial. Ann Rheum Dis 2022;81:838–44. 10.1136/annrheumdis-2021-221126 [DOI] [PubMed] [Google Scholar]
- 17. Marsman DE, den Broeder N, van den Hoogen FHJ, et al. Efficacy of Rituximab in patients with Polymyalgia Rheumatica: a double-blind, randomised, placebo-controlled, proof-of-concept trial. The Lancet Rheumatology 2021;3:e758–66. 10.1016/S2665-9913(21)00245-9 [DOI] [Google Scholar]
- 18. Toussirot E, Michaud M, Wendling D, et al. Abatacept as Adjunctive therapy in refractory Polymyalgia Rheumatica. J Rheumatol 2021;48:1888–9. 10.3899/jrheum.210455 [DOI] [PubMed] [Google Scholar]
- 19. Zhang L, Li J, Yin H, et al. Efficacy and safety of tofacitinib in patients with Polymyalgia Rheumatica: a phase 2 study. Ann Rheum Dis 2023;82:722–4. 10.1136/ard-2022-223562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. University Hospital, Brest . Baricitinib healing effect in earLy pOlymyalgia Rheumatica (BACHELOR study). 2022. Available: https://clinicaltrials.gov/study/NCT04027101
- 21. Berman M, Berliner S, Bashouti N, et al. Reduced C-reactive protein level at hospital admission in patients treated with Tocilizumab – an attention may be required. Heliyon 2023;9:e16665. 10.1016/j.heliyon.2023.e16665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schmidt-Arras D, Rose-John S. IL-6 pathway in the liver: from Physiopathology to therapy. J Hepatol 2016;64:1403–15. 10.1016/j.jhep.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 23. Stone JH, Tuckwell K, Dimonaco S, et al. Glucocorticoid dosages and acute-phase Reactant levels at giant cell arteritis flare in a randomized trial of Tocilizumab. Arthritis Rheumatol 2019;71:1329–38. 10.1002/art.40876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takanashi S, Kaneko Y, Takeuchi T. CDAI and Das28 in the management of rheumatoid arthritis in clinical practice. Ann Rheum Dis 2020;79:671–4. 10.1136/annrheumdis-2019-216607 [DOI] [PubMed] [Google Scholar]
- 25. Devauchelle-Pensec V, Saraux L, Berthelot JM, et al. Assessing Polymyalgia Rheumatica activity when C-reactive protein is unavailable or Uninterpretable. Rheumatology (Oxford) 2018;57:666–70. 10.1093/rheumatology/kex477 [DOI] [PubMed] [Google Scholar]
- 26. Leeb BF, Rintelen B, Sautner J, et al. The Polymyalgia Rheumatica activity score in daily use: proposal for a definition of remission. Arthritis Rheum 2007;57:810–5. 10.1002/art.22771 [DOI] [PubMed] [Google Scholar]
- 27. Binard A, de Bandt M, Berthelot J-M, et al. Performance of the Polymyalgia Rheumatica activity score for diagnosing disease flares. Arthritis Rheum 2008;59:263–9. 10.1002/art.23338 [DOI] [PubMed] [Google Scholar]
- 28. Binard A, Lefebvre B, De Bandt M, et al. Validity of the Polymyalgia Rheumatica activity score in primary care practice. Ann Rheum Dis 2009;68:541–5. 10.1136/ard.2008.088526 [DOI] [PubMed] [Google Scholar]
- 29. Cleuziou C, Binard A, De Bandt M, et al. Contribution of the Polymyalgia Rheumatica activity score to glucocorticoid dosage adjustment in everyday practice. J Rheumatol 2012;39:310–3. 10.3899/jrheum.110866 [DOI] [PubMed] [Google Scholar]
- 30. Altman DG, Bland JM. Measurement in medicine: the analysis of method comparison studies. The Statistician 1983;32:307. 10.2307/2987937 [DOI] [Google Scholar]
- 31. McHugh ML. Interrater reliability: the Kappa Statistic. Biochem Med 2012;276–82:276–82. 10.11613/BM.2012.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stone JH, Tuckwell K, Dimonaco S, et al. Trial of Tocilizumab in giant-cell arteritis. N Engl J Med 2017;377:317–28. 10.1056/NEJMoa1613849 [DOI] [PubMed] [Google Scholar]
- 33. Kaneko Y, Kameda H, Ikeda K, et al. Tocilizumab in patients with adult-onset still’s disease refractory to glucocorticoid treatment: a randomised, double-blind, placebo-controlled phase III trial. Ann Rheum Dis 2018;77:1720–9. 10.1136/annrheumdis-2018-213920 [DOI] [PubMed] [Google Scholar]
- 34. Ogata A, Tanimura K, Sugimoto T, et al. Phase III study of the efficacy and safety of subcutaneous versus intravenous Tocilizumab monotherapy in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2014;66:344–54. 10.1002/acr.22110 Available: https://acrjournals.onlinelibrary.wiley.com/toc/21514658/66/3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. De Benedetti F, Brunner HI, Ruperto N, et al. Randomized trial of Tocilizumab in systemic juvenile idiopathic arthritis. N Engl J Med 2012;367:2385–95. 10.1056/NEJMoa1112802 [DOI] [PubMed] [Google Scholar]
- 36. Sondag M, Guillot X, Verhoeven F, et al. Utility of 18F-Fluoro-Dexoxyglucose positron emission tomography for the diagnosis of Polymyalgia Rheumatica: a controlled study. Rheumatology (Oxford) 2016;55:1452–7. 10.1093/rheumatology/kew202 [DOI] [PubMed] [Google Scholar]
- 37. Huwart A, Garrigues F, Jousse-Joulin S, et al. Ultrasonography and magnetic resonance imaging changes in patients with Polymyalgia Rheumatica treated by Tocilizumab. Arthritis Res Ther 2018;20:11. 10.1186/s13075-017-1499-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brinth LS, Hansen A, Jensen DV, et al. Diagnostic value of composite and simplified FDG-PET/CT scores in Polymyalgia Rheumatica and the influence of recent glucocorticoid treatment—A retrospective diagnostic cohort study. Diagnostics (Basel) 2023;13:514. 10.3390/diagnostics13030514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stone JH, McDowell PJ, Jayne DRW, et al. The glucocorticoid toxicity index: measuring change in glucocorticoid toxicity over time. Semin Arthritis Rheum 2022;55:S0049-0172(22)00061-0. 10.1016/j.semarthrit.2022.152010 [DOI] [PubMed] [Google Scholar]
- 40. Matteson EL, Maradit-Kremers H, Cimmino MA, et al. Patient-reported outcomes in Polymyalgia Rheumatica. J Rheumatol 2012;39:795–803. 10.3899/jrheum.110977 [DOI] [PubMed] [Google Scholar]
- 41. Strand V, Tundia N, Bergman M, et al. Upadacitinib improves patient-reported outcomes vs placebo or Adalimumab in patients with rheumatoid arthritis: results from SELECT-COMPARE. Rheumatology (Oxford) 2021;60:5583–94. 10.1093/rheumatology/keab158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hiligsmann M, Rademacher S, Kaal KJ, et al. The use of routinely collected patient-reported outcome measures in rheumatoid arthritis. Semin Arthritis Rheum 2018;48:357–66. 10.1016/j.semarthrit.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 43. Twohig H, Mitchell C, Mallen CD, et al. Development and Psychometric evaluation of the PMR-impact scale: a new patient reported outcome measure for Polymyalgia Rheumatica. Rheumatology (Oxford) 2023;62:758–65. 10.1093/rheumatology/keac317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kalke S, Mukerjee D, Dasgupta B. A study of the health assessment questionnaire to evaluate functional status in Polymyalgia Rheumatica. Rheumatology (Oxford) 2000;39:883–5. 10.1093/rheumatology/39.8.883 [DOI] [PubMed] [Google Scholar]
- 45. Camellino D, Matteson EL, Buttgereit F, et al. Monitoring and long-term management of giant cell arteritis and Polymyalgia Rheumatica. Nat Rev Rheumatol 2020;16:481–95. 10.1038/s41584-020-0458-5 [DOI] [PubMed] [Google Scholar]
- 46. Moreel L, Doumen M, Betrains A, et al. The future of Polymyalgia Rheumatica research: what can we learn from rheumatoid arthritis. Joint Bone Spine 2023;90:S1297-319X(23)00008-8. 10.1016/j.jbspin.2023.105529 [DOI] [PubMed] [Google Scholar]
- 47. Bolhuis TE, Nizet LEA, Owen C, et al. Measurement properties of the Polymyalgia Rheumatica activity score: A systematic literature review. J Rheumatol 2022;49:627–34. 10.3899/jrheum.211292 [DOI] [PubMed] [Google Scholar]
- 48. Yates M, Owen CE, Muller S, et al. Feasibility and face validity of outcome measures for use in future studies of Polymyalgia Rheumatica: an OMERACT study. J Rheumatol 2020;47:1379–84. 10.3899/jrheum.190575 [DOI] [PubMed] [Google Scholar]
- 49. Twohig H, Owen C, Muller S, et al. Outcomes measured in Polymyalgia Rheumatica and measurement properties of instruments considered for the OMERACT core outcome set: A systematic review. J Rheumatol 2021;48:883–93. 10.3899/jrheum.200248 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003741supp001.pdf (260.5KB, pdf)
Data Availability Statement
Data are available on reasonable request.