Abstract
Aims
To evaluate associations of metabolic profiles and biomarkers with brain atrophy, lesions, and iron deposition to understand the early risk factors associated with dementia.
Materials and methods
Using data from 26 239 UK Biobank participants free from dementia and stroke, we assessed the associations of metabolic subgroups, derived using an artificial neural network approach (self‐organizing map), and 39 individual biomarkers with brain MRI measures: total brain volume (TBV), grey matter volume (GMV), white matter volume (WMV), hippocampal volume (HV), white matter hyperintensity (WMH) volume, and caudate iron deposition.
Results
In metabolic subgroup analyses, participants characterized by high triglycerides and liver enzymes showed the most adverse brain outcomes compared to the healthy reference subgroup with high‐density lipoprotein cholesterol and low body mass index (BMI) including associations with GMV (β standardized −0.20, 95% confidence interval [CI] −0.24 to −0.16), HV (β standardized −0.09, 95% CI −0.13 to −0.04), WMH volume (β standardized 0.22, 95% CI 0.18 to 0.26), and caudate iron deposition (β standardized 0.30, 95% CI 0.25 to 0.34), with similar adverse associations for the subgroup with high BMI, C‐reactive protein and cystatin C, and the subgroup with high blood pressure (BP) and apolipoprotein B. Among the biomarkers, striking associations were seen between basal metabolic rate (BMR) and caudate iron deposition (β standardized 0.23, 95% CI 0.22 to 0.24 per 1 SD increase), GMV (β standardized −0.15, 95% CI −0.16 to −0.14) and HV (β standardized −0.11, 95% CI −0.12 to −0.10), and between BP and WMH volume (β standardized 0.13, 95% CI 0.12 to 0.14 for diastolic BP).
Conclusions
Metabolic profiles were associated differentially with brain neuroimaging characteristics. Associations of BMR, BP and other individual biomarkers may provide insights into actionable mechanisms driving these brain associations.
Keywords: brain iron, brain volume, metabolic profiling, self‐organizing map, white matter hyperintensities
1. INTRODUCTION
Alterations in brain structure and function can be detected long before the symptoms of cognitive impairment are diagnosed. 1 , 2 Understanding the metabolic factors and profiles associated with dementia‐related brain changes could help determine early risk factors for dementia. Metabolic risk factors in an individual rarely present on their own and may be accompanied and/or complicated by other protective and adverse risk factors. Population subgrouping can be a useful method to assess risks associated with profiles of factors that aggregate together in the population, and to provide information on the preventative approach best suited to individuals with a particular profile.
Using a data‐driven, artificial neural network approach, a self‐organizing map [SOM] that detects multivariable patterns in complex datasets, we recently stratified the White British contingent of the UK Biobank population into six subgroups based on metabolic profiling using the extensive data for biomarkers relating to cardiovascular health, diabetes, kidney and liver function, bone and joints, cancer, and obesity (Table 1). 3 , 4 These markers clustered into subgroups in a way that was strongly characteristic of later disease incidence. In comparison to Subgroup IV, selected as a reference based on its favourable status with regards to lipids and body fat and low risks of cardiometabolic diseases and morbidity, the other five subgroups showed differential risks of several age‐related diseases including all‐cause dementia, cancer, diabetes, rheumatoid arthritis, and ischaemic heart disease. 3
TABLE 1.
Summary of characteristics of six self‐organizing map‐derived metabolic subgroup biomarker profiles
| I. High ApoB and BP without hyperglycaemia | II. High triglycerides and liver enzymes | III. High BMI and CRP and cystatin C | IV. High HDLC and low BMI | V. High sex hormones and low calcium | VI. High urinary excretion without kidney stress |
|---|---|---|---|---|---|
|
High BP (systolic and diastolic) High LDLC and ApoB High albumin, total protein, and calcium |
High body fat, BMI High basal metabolic rate a High triglycerides High liver enzymes (ALT, AST, GGT) High blood sugar (glucose, HbA1c) High alkaline phosphatase High diastolic BP Low HDLC Low IGF‐1 Low 25‐hydroxyvitamin D |
High body fat, BMI High basal metabolic rate a High CRP High kidney markers (urate, cystatin C, microalbumin, urea, creatinine, urinary creatinine) Low HDLC and ApoA1 Low albumin |
Low BMI Low basal metabolic rate a High HDLC and ApoA1 Low urinary creatinine, K+, Na+ High albumin |
Low BMI Low BP (systolic and diastolic) Low albumin, total protein, and calcium Low urate High oestradiol High SHBG High testosterone (in males) |
High urea, urinary creatinine, K+ |
Note: Summary of subgroup characteristics described previously in more detail. 3
Abbreviations: ALT, alanine aminotransferase; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; AST, aspartate aminotransferase; BMI, body mass index; BP, blood pressure; CRP, C‐reactive protein; GGT, gamma glutamyltransferase; HbA1c, glycated haemoglobin; HDLC, high‐density lipoprotein cholesterol; IGF‐1, insulin‐like growth factor 1; K+, potassium; LDLC, low‐density lipoprotein cholesterol; Na+, sodium; SHBG, sex hormone‐binding globulin.
Standardized deviations from the mean (zero) for basal metabolic rate (not reported in original study) are, for Subgroups I to VI, respectively; 0.1, 0.4, 0.5, −0.4, −0.2, 0.1.
In this study, we investigated the relationships between the SOM‐defined metabolic subgroups (and their biomarker components) and neuroimaging markers of brain morphology and iron deposition. We used MRI brain data from the largest investigation of its kind 5 (N > 26 000) allowing us to focus on dementia‐related measures at the preclinical stage.
2. MATERIALS AND METHODS
2.1. Participants
The UK Biobank is a prospective cohort study of 503 000 participants who were aged 37 to 73 years at recruitment between 2006 and 2010. 4 Baseline information was collected at recruitment using questionnaires, verbal interviews, physical examination, and blood and urine sampling, for an extensive range of demographic, lifestyle, physical and biochemical variables. Our analyses were restricted to unrelated White participants of British ancestry, with baseline information recorded for metabolic biomarkers used for subgroup profiling (Supplementary Figure 1). We identified and excluded individuals with a history of dementia (n = 284) or stroke (n = 5169) at baseline based on “first occurrence” disease information. 6 Our target sample comprised participants with brain MRI imaging information available through participation in an ongoing UK Biobank substudy initiative that began in 2014 and aims to collect imaging data for 100 000 participants. 5 After exclusion of participants with outlier values for brain MRI data (±3 standard deviations [SD] from the mean), 26 239 participants remained for analyses involving total brain volume (TBV), grey matter volume (GMV) and white matter volume (WMV), 26 228 for hippocampal volume (HV), 25 354 for white matter hyperintensity (WMH) volume, and 23 603 for caudate iron deposition (Supplementary Figure 1). Ethical approval for the UK Biobank was granted by the National Information Governance Board for Health and Social Care and North West Multicentre Research Ethics Committee (11/NW/0382), and our research study was conducted under application 10 171. All participants provided electronic consent for their anonymized data and samples to be used for health‐related research, and for being re‐contacted for further substudies.
2.2. Biomarker traits and stratification into metabolic subtypes
Study participants were subdivided into six metabolic subgroups based on biochemical and anthropometric traits using a SOM approach, as previously described, 3 an artificial neural network technique designed to detect multivariable patterns in complex datasets. 7 Traits used to sort individuals into subgroups included markers relating to cardiovascular health (apolipoprotein A1 [ApoA1], apolipoprotein B [ApoB], high‐density lipoprotein cholesterol [HDLC], direct low‐density lipoprotein cholesterol [LDLC], lipoprotein[a], total cholesterol, triglycerides), diabetes (glucose, glycated haemoglobin), inflammation (C‐reactive protein [CRP]), kidney function (creatinine, creatinine in urine, cystatin C, microalbumin, phosphate, potassium, sodium, total protein, urate and urea), liver function (alanine aminotransferase [ALT], aspartate aminotransferase [AST], gamma glutamyltransferase [GGT], albumin, direct bilirubin, total bilirubin), bone and joint (alkaline phosphatase, calcium, 25‐hydroxyvitamin D [25(OH)D], rheumatoid factor), cancer (insulin‐like growth factor 1 [IGF‐1], oestradiol, sex hormone‐binding globulin [SHBG], testosterone), and physiology (body fat percentage, body mass index [BMI], diastolic blood pressure [BP], systolic BP). We assessed the relationships between the metabolic subgroups and the individual biomarkers listed above, with MRI brain outcomes. We also included basal metabolic rate (BMR) as one of the physiological biomarkers, which is a UK Biobank measure derived from body impedance, a component of the SOM training variables. Further information regarding biomarker assay methodology is described in Supplementary Methods. All components of the SOM were standardized by age and sex for defining the six subgroups with diverse multivariable metabolic profiles that were not confounded by statins, antihypertensives or diabetic medications. 3
2.3. Brain MRI outcomes
The UK Biobank‐processed brain volume data 8 collected between 2014 and 2019 were used for our analyses. Details on brain MRI data acquisition are provided in the Supplementary Methods. Volumetric data included GMV, WMV, TBV (derived as the sum of GMV and WMV), HV (sum of left and right HVs) and volume of WMHs. The most common dementia type, Alzheimer's disease, is associated with greater iron deposition in the basal ganglia (including the caudate, putamen and globus pallidus). 9 , 10 For estimation of iron deposition, we selected the caudate as a representative region since iron deposition in this region has shown some correlation with measures of cognitive deficit. 10 For caudate iron deposition analyses, median T2* values (in milliseconds) for the left and right caudate were converted to R2* (R2* = 1/T2*) such that a higher R2* value indicated greater iron deposition, and a single caudate R2* value was calculated as the mean of the left and right caudate R2* values. All brain volumes and R2* data were normalized to total head size, and WMH volume data were inverse normal transformed to approximate normal distribution.
2.4. Covariates
As covariates we included information on age, sex, assessment centre, socioeconomic factors (education, Townsend deprivation index, employment), and lifestyle factors (smoking, alcohol consumption, physical activity, stress, diet) to account for potential confounding. A dietary iron covariate was included as a lifestyle factor for caudate iron deposition analyses only. Covariates were based on self‐reported data from the baseline assessment, except for the Townsend deprivation index which was derived from participants' post codes as recorded in the National Health Service primary care trust registries. 11 Categories for education, employment, physical activity, stressful events in the past 2 years, and smoking status are outlined in the Supplementary Methods, as is the derivation of the healthy diet score, and dietary iron covariate.
2.5. Statistical analysis
For metabolic subgroup and biomarker analyses, linear regression was used to evaluate differences in standardized brain MRI outcomes. For subgroup analyses, differences are in comparison to Subgroup IV (high HDLC and low BMI), selected based on having low adiposity and low burden of a number of common age‐related diseases. 3 This subgroup also had most preserved overall brain volumes. A binary variable was generated for each of the six metabolic subgroups, creating five indicator variables and the reference group. For the main biomarker analyses, and for restricted biomarker analyses in individuals aged 60 years and above, differences in standardized brain MRI outcomes are presented per 1‐SD change in biomarker level. Biomarker measures were first sex‐standardized, and log‐transformed except for oestradiol, microalbumin and rheumatoid factor, which were analysed without log transformation due to many zero values (below detection). To account for multiple testing across 39 biomarkers included in the analyses, we applied Bonferroni correction, with a threshold of P < 0.0013. Sex interactions with the biomarker‐brain MRI associations were assessed using an interaction term, and a Bonferroni‐adjusted threshold of P < 0.0013. Where an interaction was detected, the population was then stratified by sex and associations were evaluated in males and females separately.
For analyses of metabolic subgroup associations with brain MRI outcomes, we made adjustments for covariates in four models. Model 1 adjusted for basic covariates (age, sex and assessment centre), Model 2 for basic plus socioeconomic‐related covariates (education, Townsend deprivation index, and employment), Model 3 for basic plus lifestyle factors (smoking, alcohol consumption, level of physical activity, recent stress, and healthy diet, and, for caudate iron deposition analyses only, a dietary iron variable), and Model 4 for all covariates. For biomarker analyses, we adjusted for basic covariates, socio‐economic‐related covariates, and lifestyle factors (including the dietary iron covariate for analyses of caudate iron deposition). Adjustment for sex was omitted in the sex‐stratified association analyses. In all analyses, we applied a complete case strategy given only approximately 2% of covariate information was missing. Analyses were carried out using Stata/SE 16.1 (StataCorp) and R 3.5.0 software (R Foundation for Statistical Computing).
3. RESULTS
3.1. Population characteristics
The study sample consisted of up to 26 239 participants of whom 52% were female. Brain MRI variables were associated with a range of health and lifestyle covariates (Table 2, Supplementary Table 1). Younger age, higher education level, having never been a smoker, greater number of working hours, and higher level of physical exercise showed expected associations with greater HV, and lower WMH volume. Caudate iron deposition levels tended to increase with age, as expected, and were higher in current and ex‐smokers, but also in those with higher education level, greater number of working hours, and more strenuous exercise levels. Those who reported experiencing recent stress due to serious illness, injury or assault tended to have lower GMV and higher WMH volume. The average (± SD) time from baseline to neuroimaging data collection was 8.7 (± 1.7) years.
TABLE 2.
Summary of grey matter, hippocampal and white matter hyperintensity volumes and caudate iron deposition levels, stratified by baseline characteristics of the UK Biobank participants in this study
| n (%) | GMV, cm3 Median (IQR) | HV, cm3 Median (IQR) | WMH volume, cm3 Median (IQR) | C‐Fe, s−1 Median (IQR) | |
|---|---|---|---|---|---|
| Total | 26 239 (100) | 792.6 (760.6‐825.2) | 7.69 (7.14‐8.24) | 3.64 (1.96‐7.34) | 18.3 (16.7‐20.2) |
| Sex | |||||
| Male | 12 492 (47.6) | 776.8 (746.9‐806.5) | 7.94 (7.35‐8.51) | 3.79 (2.02‐7.70) | 19.8 (18.3‐21.4) |
| Female | 13 747 (52.4) | 806.9 (776.2‐839.2) | 7.50 (7.00‐7.99) | 3.50 (1.90‐7.04) | 17.1 (16.0‐18.5) |
| P | <1.0 × 10−300 | <1.0 × 10−300 | 0.003 | <1.0× 10−300 | |
| Age | |||||
| 40‐49 years | 6902 (26.3) | 829.5 (802.1‐855.7) | 7.92 (7.41‐8.49) | 2.00 (1.20‐3.41) | 18.0 (16.6‐19.7) |
| 50‐59 years | 10 889 (41.5) | 795.0 (768.8‐821.5) | 7.75 (7.23‐8.26) | 3.56 (2.07‐6.46) | 18.2 (16.7‐20.1) |
| 60‐70 years | 8448 (32.2) | 760.8 (734.4‐787.1) | 7.39 (6.86‐7.92) | 6.53 (3.54‐12.39) | 18.7 (17.0‐20.6) |
| P | <1.0 × 10−300 | <1.0 × 10−300 | <1.0 × 10−300 | 1.7 × 10−31 | |
| Education | |||||
| None | 1644 (6.3) | 777.8 (749.4‐807.8) | 7.39 (6.89‐7.93) | 5.47 (2.89‐10.4) | 18.0 (16.4‐19.9) |
| NVQ/CSE/A‐levels | 8183 (31.2) | 796.3 (762.1‐829.9) | 7.65 (7.09‐8.19) | 3.59 (1.95‐7.10) | 18.2 (16.6‐20.1) |
| Degree/professional | 16 352 (62.3) | 792.4 (760.9‐824.3) | 7.73 (7.20‐8.29) | 3.52 (1.89‐7.12) | 18.4 (16.8‐20.2) |
| Missing | 60 (0.2) | 782.1 (754.4‐809.4) | 7.45 (6.76‐8.01) | 5.99 (3.35‐10.91) | 18.7 (16.9‐21.0) |
| P | 0.01 | 3.7 × 10−31 | 8.7 × 10−4 | 3.2 × 10−13 | |
| Employment | |||||
| No | 1530 (5.8) | 803.5 (772.7‐835.7) | 7.57 (7.07‐8.13) | 3.28 (1.84‐6.27) | 17.6 (16.3‐19.4) |
| Retired | 6606 (25.2) | 764.2 (736.9‐792.4) | 7.41 (6.88‐7.97) | 6.19 (3.34‐11.9) | 18.6 (16.9‐20.6) |
| 1st quartile (lowest work hours) | 4096 (15.6) | 801.3 (769.5‐835.3) | 7.62 (7.11‐8.13) | 3.40 (1.81‐6.99) | 17.6 (16.3‐19.3) |
| 2nd quartile | 2983 (11.4) | 807.5 (775.3‐839.7) | 7.74 (7.23‐8.26) | 3.01 (1.67‐5.73) | 17.9 (16.4‐19.7) |
| 3rd quartile | 5742 (21.9) | 804.6 (773.6‐835.0) | 7.82 (7.28‐8.37) | 2.95 (1.65‐5.40) | 18.3 (16.8‐20.2) |
| 4th quartile (highest work hours) | 5034 (19.2) | 797.1 (768.3‐826.2) | 7.96 (7.41‐8.51) | 2.96 (1.68‐5.56) | 19.0 (17.4‐20.7) |
| Missing | 248 (0.9) | 797.4 (764.2‐831.5) | 7.70 (7.14‐8.24) | 3.80 (2.12‐8.42) | 18.0 (16.5‐20.0) |
| P | 1.0 × 10−24 | 3.2 × 10−26 | 3.8 × 10−22 | 0.002 | |
| Smoking | |||||
| Never | 16 146 (61.5) | 797.5 (766.5‐829.8) | 7.70 (7.23‐8.31) | 3.36 (1.82‐6.73) | 18.1 (16.6‐19.9) |
| Ex‐smoker | 8490 (32.4) | 782.5 (750.4‐815.9) | 7.64 (7.16‐8.21) | 4.16 (2.21‐8.34) | 18.6 (16.9‐20.5) |
| Current | 1557 (5.9) | 790.6 (756.2‐825.2) | 7.73 (7.19‐8.26) | 3.80 (2.11‐8.37) | 18.9 (17.2‐20.9) |
| Missing | 46 (0.2) | 780.7 (748.1‐800.1) | 7.44 (7.08‐8.01) | 4.31 (2.14‐8.41) | 18.3 (16.5‐20.2) |
| P | 1.1 × 10−47 | 1.8 × 10−9 | 4.7 × 10−18 | 3.2 × 10−24 | |
| Physical exercise | |||||
| None | 823 (3.1) | 799.7 (767.0‐831.5) | 7.52 (7.02‐8.04) | 4.02 (2.07‐7.32) | 17.6 (1.3‐19.6) |
| Light/moderate | 21 277 (81.1) | 791.2 (758.8‐823.7) | 7.66 (7.12‐8.21) | 3.79 (2.03‐7.70) | 18.3 (16.7‐20.1) |
| Strenuous activity | 4010 (15.3) | 798.9 (768.6‐829.8) | 7.92 (7.35‐8.48) | 2.91 (1.63‐5.54) | 18.7 (17.1‐20.3) |
| Missing | 129 (0.5) | 782.4 (745.2‐821.6) | 7.52 (6.98‐8.17) | 3.50 (1.66‐7.43) | 18.4 (16.9‐20.2) |
| P | 8.6 × 10−5 | 3.6 × 10−15 | 0.0002 | 0.003 | |
| Stressful events in last 2 years | |||||
| None | 15 462 (58.9) | 789.7 (756.4‐823.0) | 7.69 (7.14‐8.24) | 3.72 (1.99‐7.54) | 18.4 (16.8‐20.2) |
| Serious illness, injury, or assault | 1581 (6.0) | 789.3 (764.2‐823.0) | 7.67 (7.14‐8.24) | 4.07 (2.07‐8.39) | 18.6 (17.0‐20.4) |
| Family death or illness | 7402 (28.2) | 796.9 (777.6‐837.9) | 7.67 (7.14‐8.23) | 3.54 (1.92‐7.12) | 18.1 (16.6‐20.0) |
| Marital separation or divorce | 519 (2.0) | 804.5 (770.6‐836.0) | 7.79 (7.26‐8.35) | 2.99 (1.65‐5.82) | 18.1 (16.6‐20.1) |
| Financial difficulties | 1235 (4.7) | 802.2 (757.5‐834.4) | 7.75 (7.23‐8.35) | 3.13 (1.76‐6.36) | 18.4 (16.8‐20.2) |
| Missing | 30 (0.1) | 789.5 (767.1‐818.2) | 7.67 (7.04‐8.01) | 4.41 (2.38‐6.26) | 17.5 (16.1‐19.7) |
| P | 0.02 | 0.17 | 0.001 | 0.15 | |
Note: P values were from likelihood ratio comparisons of linear regression models and included adjustments for sex, age and assessment centre. Standard deviations for brain MRI markers are 46.95 cm3 for GMV, 1.06 cm3 for HV, 8.21 cm3 for WMH volume, and 2.45 s−1 for caudate iron (R2*).
Abbreviations: C‐Fe, caudate iron (R2*); GMV, grey matter volume; HV, hippocampal volume; IQR, interquartile range; WMH, white matter hyperintensity; WMV, white matter volume.
3.2. Associations between metabolic subgroup and brain MRI parameters
Metabolic subgroup associations with neuroimaging markers after full covariate adjustment are shown in Figure 1, and data for all adjustment models are presented in Supplementary Table 2. Adiposity‐related subgroups II (high triglycerides and liver enzymes) and III (high BMI, CRP and cystatin C) showed the most adverse brain outcomes compared to the reference Subgroup IV (high HDLC and low BMI). Subgroup II was associated with greater caudate iron deposition (β standardized 0.30, 95% confidence interval [CI] 0.25 to 0.34) and WMH volume (β standardized 0.22, 95% CI 0.18 to 0.26), and lower GMV (β standardized −0.20, 95% CI −0.24 to −0.16) and HV (β standardized −0.09, 95% CI −0.13 to −0.04), with similar associations observed for Subgroup III. Subgroup I (high ApoB and BP without hyperglycaemia) was associated with lower GMV, WMV and TBV, and higher WMH volume and caudate iron deposition, while Subgroup VI (high urinary excretion without kidney stress) was associated with lower GMV and higher caudate iron deposition. Subgroup V (high sex hormones and low calcium) was associated with lower HV, lower WMH volume, and slightly more caudate iron deposition than the reference Subgroup IV (ie, second to lowest caudate iron deposition overall).
FIGURE 1.
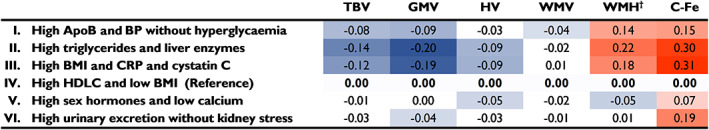
Metabolic subgroup associations with brain MRI parameters. Linear regression was used to evaluate subgroup differences in brain volumes and caudate iron deposition in comparison to Subgroup IV. Analyses are adjusted for basic covariates (age, sex and assessment centre), socioeconomic‐related covariates (education, Townsend deprivation index and employment) and lifestyle factors (smoking, alcohol consumption, level of physical activity, recent stress and healthy diet). For caudate iron deposition analyses only, an additional covariate for dietary iron was included in lifestyle factors. All brain MRI data were normalized to total head size, and *white matter hyperintensity (WMH) volume data were inverse normal transformed to approximate normal distribution. Estimate values with P values <0.05 are indicated in colour, with orange indicating higher values, and blue indicating lower values, and more intense colour representing greater deviation of the point estimate from zero. Confidence interval and P‐value information can be found in Supplementary Table 2. Abbreviations: ApoB, apolipoprotein B; BMI, body mass index; BP, blood pressure; C‐Fe, caudate iron (R2*); CRP, C‐reactive protein; GMV, grey matter volume; HDLC, high density lipoprotein cholesterol; HV, hippocampal volume; TBV, total brain volume; WMV, white matter volume
3.3. Associations between serum and physiological biomarkers and brain MRI parameters
We assessed associations between biomarkers and brain MRI variables across the entire study population, to assess which biomarkers might be driving the subgroup associations. Results from fully adjusted analyses of biomarker‐brain outcome associations are presented in Figure 2 (heat map) and Supplementary Table 3 (extended data).
FIGURE 2.
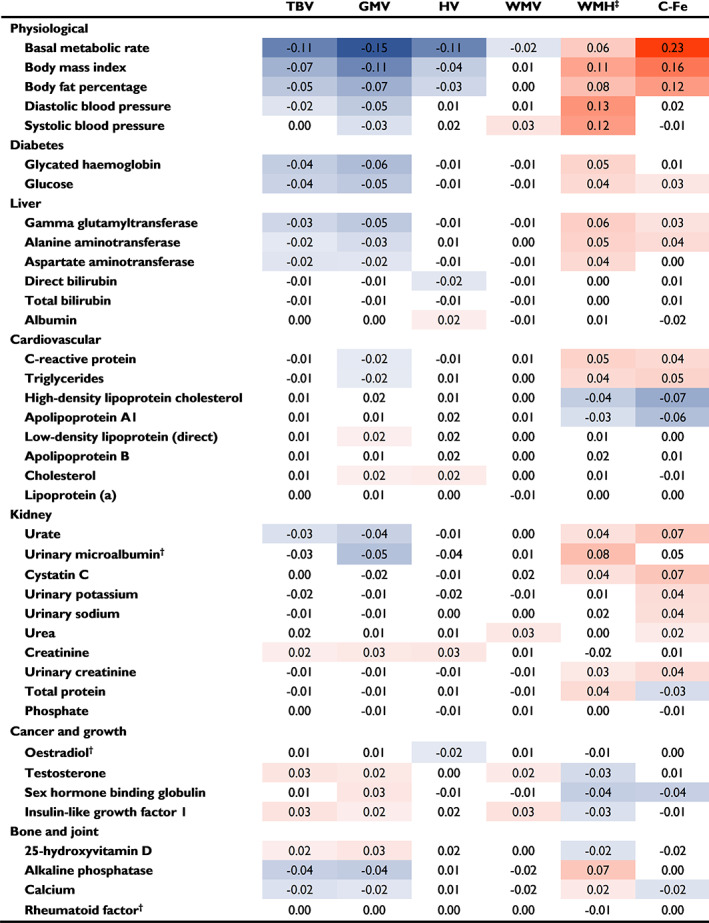
Metabolic biomarker associations with brain MRI parameters. Standardized beta estimates are shown for each neuroimaging marker, per one‐standard deviation higher metabolic biomarker level. Biomarker data were sex‐standardized, and log‐transformed †with the exceptions of oestradiol, microalbumin and rheumatoid factor, which were analysed without log transformation due to many values being zero. All analyses were adjusted for age, sex, assessment centre, socioeconomic (education, Townsend deprivation index, employment) and lifestyle factors (smoking, alcohol consumption, physical activity, stress, diet). Caudate iron deposition analyses were also adjusted for dietary iron. All brain MRI data were normalized to total head size, and *white matter hyperintensity (WMH) volume data were inverse normal transformed to approximate normal distribution. Estimate values with P values below the Bonferroni‐adjusted threshold (P < 0.0013) are indicated in colour, with orange indicating higher values, and blue indicating lower values, and more intense colour representing greater deviation of the point estimate from zero. Confidence interval and P‐value information can be found in Supplementary Table 3. Abbreviations: C‐Fe, caudate iron (R2*); GMV, grey matter volume; HV, hippocampal volume; TBV, total brain volume; WMV, white matter volume
Higher BMR, a trait of Subgroups II and III, was associated with striking brain outcomes including greater caudate iron deposition (β standardized 0.23, 95% CI 0.22 to 0.24, per 1‐SD increase in biomarker), lower TBV mainly reflective of lower GMV (β standardized −0.15, 95% CI −0.16 to −0.14), lower HV (β standardized −0.11, 95% CI −0.12 to −0.10), and greater WMH volume (β standardized 0.06, 95% CI 0.05 to 0.07). Higher BMI and body fat percentage showed similar associations. Additional metabolic features that also cluster in liver‐stressed Subgroup II such as higher diabetes markers (glycated haemoglobin and glucose), liver enzymes (GGT, ALT and AST) and triglycerides, and lower 25(OH)D and IGF‐1, were also associated with lower GMV and TBV, higher WMH volume, and for some, higher caudate iron deposition. Lower levels of IGF‐1 were also associated with lower WMV.
Higher diastolic BP was associated with greater WMH volume (0.13, 0.12 to 0.14), as was higher systolic BP, in accordance with subgroups with high BP (Subgroups I and II) having greater volume, and low BP Subgroup V having lower volume of WMHs.
Higher testosterone level was associated with higher GMV (0.02, 0.01 to 0.03), WMV (0.02, 0.01 to 0.04) and TBV (0.03, 0.02 to 0.04) and lower WMH volume (−0.03, −0.04 to −0.02), while higher oestradiol level was associated with lower HV (−0.02, −0.03 to −0.01). Higher SHBG level was associated with higher GMV, lower WMH volume and lower caudate iron deposition. The associations of these individual sex hormone‐related factors concur with those observed for ‘high hormones’ Subgroup V, in which these factors cluster; although low BP may be the main driver of the association with low WMH volume for this subgroup. Additionally, lower albumin was associated with lower HV and may be related to the lower HV in Subgroup V.
Several kidney biomarkers, as well as the inflammation marker CRP, showed brain MRI associations consistent with the lower GMV, higher WMH volume and higher caudate iron deposition observed in Subgroup III, the subgroup with characteristic inflammation and kidney stress. Most notable were associations of urinary microalbumin with WMH volume (0.08, 0.05 to 0.10), and urate and cystatin C with caudate iron deposition (0.07, 0.05 to 0.08 for each). Kidney marker associations with higher caudate iron deposition and lower GMV were also consistent with the associations of Subgroup VI (which has high kidney excretion but lacks signs of inflammation or urinary microalbumin). Not all kidney markers were associated with adverse brain outcomes; higher serum creatinine level was associated with greater HV, GMV and TBV, while higher urea level was associated with greater WMV.
High‐density lipoprotein cholesterol was inversely associated with caudate iron deposition (−0.07, −0.09 to −0.06) and WMH volume (−0.04, −0.05 to −0.03), as was ApoA1. High HDLC is a trait of reference Subgroup IV and may contribute to the relatively low caudate iron deposition and WMH volume of this group. Conversely, low levels of these biomarkers in adiposity‐related Subgroups II and III may be related to their higher caudate iron deposition levels and WMH volumes. Weaker or no associations were observed for other cholesterol‐related markers (direct LDLC, ApoB, total cholesterol, lipoprotein[a]).
3.4. Biomarker‐brain MRI sensitivity analyses
Because brain atrophy, iron deposition and WMH volume are measures that become more pronounced with age, we evaluated whether biomarker‐brain MRI associations were maintained if the population was restricted to older participants (aged 60 years and above; Supplementary Figure 2, and Supplementary Table 4). For these analyses, we had 8281 participants for TBV, GMV and WMV, 8276 for HV, 7979 for WMH volume, and 7419 for caudate iron deposition. The findings in the older age group were generally similar to the analysis conducted in the full sample. Furthermore, an additional three associations were exposed in the 60+ age group; higher BMI and lower SHBG were associated with higher WMV (β standardized 0.05, 95% CI 0.02 to 0.07, and −0.05, −0.07 to −0.03, respectively, per 1‐SD increase in biomarker), and higher IGF‐1 was associated with higher HV (β standardized 0.03, 95% CI 0.01 to 0.05).
We also assessed differences in biomarker‐brain MRI associations by sex in the main population, using full covariate adjustment and a Bonferroni‐adjusted threshold of P < 0.0013 (Supplementary Figure 3). In general, adverse associations of higher adiposity, BMR, liver stress, urate, sodium in urine, and lower 25(OH)D tended to be more pronounced or only observed in males. However, in females, higher BMI and lower SHBG were linked to greater WMV, and higher cholesterol was associated with greater HV.
4. DISCUSSION
In this study we investigated the relationships between physiological and biochemical biomarkers of metabolism (clustered in SOM‐derived metabolic subgroups, and individually), and dementia‐related brain MRI parameters. Compared to the metabolically favourable Subgroup IV, representing a subgroup with preserved brain volumes and low caudate iron deposition levels, the five other metabolic subgroups displayed differential associations with brain MRI outcomes. Profiles with high BMR and adiposity measures, and traits of liver or kidney stress (Subgroups II and III) had the most pronounced adverse brain outcomes, echoed to a lesser extent in subgroups with high ApoB and BP (Subgroup I), and high urinary excretion (Subgroup VI), while Subgroup V with high hormone levels had lower HV, despite preserved overall brain volume and lower burden of WMHs. Several important associations with brain morphology and caudate iron deposition were identified for biomarkers related to BMR, obesity, BP and HDLC.
Subgroups II and III, representing individuals with high BMI, adverse metabolic profiles, and increased dementia risk, 3 were associated with lower HV, GMV and TBV, and the greatest burdens of WMHs and caudate iron deposition. Biomarker analyses highlighted high BMR and adiposity markers and low HDLC as potentially key drivers of subgroup‐brain MRI associations in these subgroups. Higher BP, diabetes markers, liver enzymes, triglycerides, and low IGF‐1 and 25(OH)D may also be related to Subgroup II brain outcomes, while inflammation and kidney stress may be related to adverse brain outcomes in Subgroup III. Some associations were expected based on previous findings, including associations of obesity 12 , 13 , 14 and diabetes 15 , 16 with lower HV, GMV and TBV, and higher WMH volume. Obesity and insulin resistance have been linked to greater caudate iron deposition, 17 while higher BP has been linked to greater severity of WMHs. 18 We saw expected associations of low 25(OH)D with lower GMV and TBV, and greater WMH volume as previously described. 19 , 20 , 21 , 22 , 23 , 24 Evidence from Mendelian randomization studies suggests that higher levels of genetically predicted circulating 25(OH)D decrease the risks of Alzheimer's disease 25 , 26 , 27 , 28 and overall dementia. 24 Our subgrouping approach revealed that particular traits such as higher liver enzymes, blood sugar indicators, alkaline phosphatase levels and diastolic BP were factors that cluster with lower 25(OH)D levels in the population and also share a common brain MRI association profile, raising the possibility that beneficial effects of higher 25(OH)D level may be linked to counteracting these cardiometabolic disturbances.
Our findings of associations of liver enzyme GGT with lower GMV, TBV and higher WMH volume and caudate iron deposition are consistent with previous observational studies reporting its associations with adverse affects for cognition and dementia‐related outcomes. 29 , 30 , 31 , 32 GGT plays an important role in protecting cells against oxidative damage by mediating the uptake of the antioxidant glutathione, 33 and higher levels may be reflective of greater oxidative stress.
The group with a high sex hormone profile, Subgroup V, had lower HV, lower burden of WMHs, and higher caudate iron deposition than reference Subgroup IV (although it had the second lowest caudate iron deposition level overall). Subgroup V features, such as being lean with low BP, having high SHBG levels, and (for males) having higher testosterone levels may be linked to the preserved overall brain volume and lower burden of WMHs in this subgroup. However, higher oestradiol and lower albumin levels are features that may be related to the lower HV of this subgroup. Consistent with the current findings, we have previously observed lower risk of hypertension and higher risk of all‐cause dementia for Subgroup V compared to Subgroup IV. 3 Our findings are consistent with previous studies linking higher oestradiol levels to lower HV, 34 , 35 and are compatible with reports linking low albumin levels to greater dementia‐related risks. 36 , 37
The most striking biomarker‐brain MRI associations seen in this study were for BMR. Although strongly linked to adiposity, BMR showed markedly larger estimates for brain MRI outcomes than did obesity measures, especially higher caudate iron deposition, lower GMV and lower HV. The UK Biobank BMR measure is derived from body impedance, accurately reflecting resting energy expenditure determined by respiratory breath gas analysis, according to the recording device manufacturers. 38 Patients with dementia have been reported to have higher resting energy expenditure. 39 , 40 Furthermore, in healthy White subjects, variance in brain volumes (especially grey matter) has been shown to explain much of the residual variance in resting energy expenditure not accounted for by fat‐free mass. 41 BMR is related to the function of mitochondria (the cellular organelles where respiration occurs), and many links have been made between perturbation of mitochondrial function and dementia. 42 Although it is an essential energy‐producing process vital for neuronal function, mitochondrial respiration generates oxidants, and higher BMR could promote harmful effects of oxidative stress if not adequately counterbalanced by the body's antioxidant capacity. Further studies are warranted to explore the biological underpinnings of the striking brain MRI associations observed with this biomarker, which is infrequently measured in relation to brain imaging outcomes.
Some strengths of this study include the utilization of a large population, representing the largest ever MRI neuroimaging cohort, 5 utilization of our SOM‐derived metabolic profiling approach, and the inclusion of caudate iron deposition as an outcome in our analyses, facilitated by the collection of T2* data by the UK Biobank. Brain iron levels increase with age, 43 and brain iron accumulation has long been associated with neurodegeneration and dementia. 44 In Alzheimer's disease, higher levels of iron have been observed in deep grey matter, including the caudate region of the basal ganglia, 9 , 10 where it has been shown to correlate with measures of Alzheimer's disease severity. 10 Our study has revealed several metabolic factors that are associated with this phenotype, most notably, higher BMR and obesity markers. The inverse relationship observed between HDLC and caudate iron deposition is also worth further investigation; indeed, food groups linked to healthy lipid profiles such as nuts, healthy oils and fish, have recently been shown to moderate the effects of age on brain iron concentration, and working memory performance. 45
Data‐driven metabolic profiling using the SOM approach allows hypothesis‐free subgrouping of a population, based on the way different traits cluster together in that population. Some limitations of the approach used in this study must also be acknowledged. For example, while SOM subgroups are based on aggregation of metabolic factors in the population, variation will still be evident within each subgroup, and not every individual will perfectly fit a particular subgroup profile. While our study has a prospective design, causality cannot be conclusively inferred by the observed associations, necessitating further investigations to validate and determine the causality of these relationships. We cannot exclude the possibilities of reverse causation (such as where early neurodegenerative processes are affecting metabolism), or residual confounding by unknown factors. The UK Biobank cohort is subject to healthy volunteer bias and, as such, may not be reflective of the general population, 46 and because our cohort was limited to participants of White British ancestry, our findings need to be evaluated in other ethnic groups.
Metabolic profiling provides important insight into population diversity and multimorbidity, and the SOM‐defined metabolic subgroups have previously shown differential associations with the prevalence and incidence of all‐cause dementia. 3 In the current study, which evaluated metabolic subgroup and individual biomarker relationships with dementia‐related brain MRI outcomes among a cohort that was free of dementia and stroke at baseline, we have observed differential patterns of neuroimaging outcomes across metabolic subgroups and identified metabolic biomarkers that may be driving these brain associations. We have demonstrated notable differences in brain neuroimaging characteristics by metabolic profile, with the associations of BMR, BP and other individual biomarkers providing insights into actionable mechanisms that may drive these subgroup associations.
AUTHOR CONTRIBUTIONS
Elina Hyppönen conceptualized and supervised the study and advised on analyses. Amanda Lumsden was involved in conceptualization, investigation, and preparation of tables and figures and wrote the original draft. Anwar Mulugeta conducted the formal analyses, curated the data and prepared figures and tables. Ville‐Petteri Mäkinen contributed to conceptualization and advised on analyses. All authors interpreted results, reviewed and edited the manuscript and approved the final version for submission.
CONFLICT OF INTEREST
The authors report no competing interests.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14853.
Supporting information
Appendix S1 Supporting Information
ACKNOWLEDGMENTS
The authors wish to thank all the participants of the UK Biobank without whom this work could not be undertaken. Open access publishing facilitated by University of South Australia, as part of the Wiley ‐ University of South Australia agreement via the Council of Australian University Librarians.
Lumsden AL, Mulugeta A, Mäkinen V‐P, Hyppönen E. Metabolic profile‐based subgroups can identify differences in brain volumes and brain iron deposition. Diabetes Obes Metab. 2023;25(1):121‐131. doi: 10.1111/dom.14853
Funding information E.H. received grant funding from the National Health and Medical Research Council Australia (GNT1157281) which supported this research.
DATA AVAILABILITY STATEMENT
Data availability statement;All data and code from this study will be made available to approved users of the UK Biobank, upon application.
REFERENCES
- 1. Apostolova LG, Mosconi L, Thompson PM, et al. Subregional hippocampal atrophy predicts Alzheimer's dementia in the cognitively normal. Neurobiol Aging. 2010;31(7):1077‐1088. doi: 10.1016/j.neurobiolaging.2008.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beason‐Held LL, Goh JO, An Y, et al. Changes in brain function occur years before the onset of cognitive impairment. J Neurosci. 2013;33(46):18008‐18014. doi: 10.1523/JNEUROSCI.1402-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mulugeta A, Hyppönen E, Ala‐Korpela M, Mäkinen VP. Cross‐sectional metabolic subgroups and 10‐year follow‐up of cardiometabolic multimorbidity in the UK Biobank. Sci Rep. 2022;12(1):8590. doi: 10.1038/s41598-022-12198-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sudlow C, Gallacher J, Allen N, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miller KL, Alfaro‐Almagro F, Bangerter NK, et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci. 2016;19(11):1523‐1536. doi: 10.1038/nn.4393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. UK Biobank . First occurrence of health outcomes defined by 3‐character ICD10 code. UK Biobank Showcase; 2019. https://biobank.ndph.ox.ac.uk/showcase/ukb/docs/first_occurrences_outcomes.pdf
- 7. Gao S, Mutter S, Casey A, Makinen VP. Numero: a statistical framework to define multivariable subgroups in complex population‐based datasets. Int J Epidemiol. 2019;48(2):369‐374. doi: 10.1093/ije/dyy113 [DOI] [PubMed] [Google Scholar]
- 8. Alfaro‐Almagro F, Jenkinson M, Bangerter NK, et al. Image processing and quality control for the first 10,000 brain imaging datasets from UK Biobank. Neuroimage. 2018;166:400‐424. doi: 10.1016/j.neuroimage.2017.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Damulina A, Pirpamer L, Soellradl M, et al. Cross‐sectional and longitudinal assessment of brain iron level in Alzheimer disease using 3‐T MRI. Radiology. 2020;296:192541. doi: 10.1148/radiol.2020192541 [DOI] [PubMed] [Google Scholar]
- 10. Du L, Zhao Z, Cui A, et al. Increased iron deposition on brain quantitative susceptibility mapping correlates with decreased cognitive function in Alzheimer's disease. ACS Chem Nerosci. 2018;9(7):1849‐1857. doi: 10.1021/acschemneuro.8b00194 [DOI] [PubMed] [Google Scholar]
- 11. Phillimore P, Beattie A, Townsend P. Widening inequality of health in northern England, 1981‐91. BMJ. 1994;308(6937):1125‐1128. doi: 10.1136/bmj.308.6937.1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fernández‐Andújar M, Morales‐García E, García‐Casares N. Obesity and gray matter volume assessed by neuroimaging: a systematic review. Brain Sci. 2021;11(8):999. doi: 10.3390/brainsci11080999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Han YP, Tang X, Han M, et al. Relationship between obesity and structural brain abnormality: accumulated evidence from observational studies. Ageing Res Rev. 2021;71:101445. doi: 10.1016/j.arr.2021.101445 [DOI] [PubMed] [Google Scholar]
- 14. Mulugeta A, Lumsden A, Hyppönen E. Unlocking the causal link of metabolically different adiposity subtypes with brain volumes and the risks of dementia and stroke: a Mendelian randomization study. Neurobiol Aging. 2021;102:161‐169. doi: 10.1016/j.neurobiolaging.2021.02.010 [DOI] [PubMed] [Google Scholar]
- 15. Wang DQ, Wang L, Wei MM, et al. Relationship between type 2 diabetes and white matter hyperintensity: a systematic review. Front Endocrinol (Lausanne). 2020;11:595962. doi: 10.3389/fendo.2020.595962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schneider ALC, Selvin E, Sharrett AR, et al. Diabetes, prediabetes, and brain volumes and subclinical cerebrovascular disease on MRI: the atherosclerosis risk in communities neurocognitive study (ARIC‐NCS). Diabetes Care. 2017;40(11):1514‐1521. doi: 10.2337/dc17-1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blasco G, Puig J, Daunis IEJ, et al. Brain iron overload, insulin resistance, and cognitive performance in obese subjects: a preliminary MRI case‐control study. Diabetes Care. 2014;37(11):3076‐3083. doi: 10.2337/dc14-0664 [DOI] [PubMed] [Google Scholar]
- 18. Wartolowska KA, Webb AJS. Midlife blood pressure is associated with the severity of white matter hyperintensities: analysis of the UK Biobank cohort study. Eur Heart J. 2021;42(7):750‐757. doi: 10.1093/eurheartj/ehaa756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brouwer‐Brolsma EM, van der Zwaluw NL, van Wijngaarden JP, et al. Higher serum 25‐hydroxyvitamin D and lower plasma glucose are associated with larger gray matter volume but not with white matter or Total brain volume in Dutch community‐dwelling older adults. J Nutr. 2015;145(8):1817‐1823. doi: 10.3945/jn.115.214197 [DOI] [PubMed] [Google Scholar]
- 20. Croll PH, Boelens M, Vernooij MW, et al. Associations of vitamin D deficiency with MRI markers of brain health in a community sample. Clin Nutr. 2021;40(1):72‐78. doi: 10.1016/j.clnu.2020.04.027 [DOI] [PubMed] [Google Scholar]
- 21. Chung PW, Park KY, Kim JM, et al. 25‐Hydroxyvitamin D status is associated with chronic cerebral small vessel disease. Stroke. 2015;46(1):248‐251. doi: 10.1161/STROKEAHA.114.007706 [DOI] [PubMed] [Google Scholar]
- 22. Annweiler C, Bartha R, Karras SN, Gautier J, Roche F, Beauchet O. Vitamin D and white matter abnormalities in older adults: a quantitative volumetric analysis of brain MRI. Exp Gerontol. 2015;63:41‐47. doi: 10.1016/j.exger.2015.01.049 [DOI] [PubMed] [Google Scholar]
- 23. Feng C, Tang N, Huang H, Zhang G, Qi X, Shi F. 25‐Hydroxy vitamin D level is associated with total MRI burden of cerebral small vessel disease in ischemic stroke patients. Int J Neurosci. 2019;129(1):49‐54. doi: 10.1080/00207454.2018.1503182 [DOI] [PubMed] [Google Scholar]
- 24. Navale SS, Mulugeta A, Zhou A, Llewellyn DJ, Hypponen E. Vitamin D and brain health: an observational and Mendelian randomization study. Am J Clin Nutr. 2022;116:531‐540. doi: 10.1093/ajcn/nqac107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mokry LE, Ross S, Morris JA, Manousaki D, Forgetta V, Richards JB. Genetically decreased vitamin D and risk of Alzheimer disease. Neurology. 2016;87(24):2567‐2574. doi: 10.1212/WNL.0000000000003430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Larsson SC, Traylor M, Markus HS, Michaelsson K. Serum parathyroid hormone, 25‐hydroxyvitamin D, and risk of Alzheimer's disease: a Mendelian randomization study. Nutrients. 2018;10(9):1243. doi: 10.3390/nu10091243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang L, Qiao Y, Zhang H, et al. Circulating vitamin D levels and Alzheimer's disease: a Mendelian randomization study in the IGAP and UK biobank. J Alzheimers Dis. 2020;73(2):609‐618. doi: 10.3233/JAD-190713 [DOI] [PubMed] [Google Scholar]
- 28. Meng L, Wang Z, Ming YC, Shen L, Ji HF. Are micronutrient levels and supplements causally associated with the risk of Alzheimer's disease? A two‐sample Mendelian randomization analysis. Food Funct. 2022;13(12):6665‐6673. doi: 10.1039/d1fo03574f [DOI] [PubMed] [Google Scholar]
- 29. Tang Z, Chen X, Zhang W, et al. Association between gamma‐glutamyl transferase and mild cognitive impairment in Chinese women. Front Aging Neurosci. 2021;13:630409. doi: 10.3389/fnagi.2021.630409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee YB, Han K, Park S, et al. Gamma‐glutamyl transferase variability and risk of dementia: a nationwide study. Int J Geriatr Psychiatry. 2020;35(10):1105‐1114. doi: 10.1002/gps.5332 [DOI] [PubMed] [Google Scholar]
- 31. Kunutsor SK, Laukkanen JA. Gamma glutamyltransferase and risk of future dementia in middle‐aged to older Finnish men: a new prospective cohort study. Alzheimers Dement. 2016;12(9):931‐941. doi: 10.1016/j.jalz.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 32. Praetorius Björk M, Johansson B. Gamma‐Glutamyltransferase (GGT) as a biomarker of cognitive decline at the end of life: contrasting age and time to death trajectories. Int Psychogeriatr. 2018;30(7):981‐990. doi: 10.1017/s1041610217002393 [DOI] [PubMed] [Google Scholar]
- 33. Yavuz BB, Yavuz B, Halil M, et al. Serum elevated gamma glutamyltransferase levels may be a marker for oxidative stress in Alzheimer's disease. Int Psychogeriatr. 2008;20(4):815‐823. doi: 10.1017/s1041610208006790 [DOI] [PubMed] [Google Scholar]
- 34. den Heijer T, Geerlings MI, Hofman A, et al. Higher estrogen levels are not associated with larger hippocampi and better memory performance. Arch Neurol. 2003;60(2):213‐220. doi: 10.1001/archneur.60.2.213 [DOI] [PubMed] [Google Scholar]
- 35. Smeeth DM, Dima D, Jones L, et al. Polygenic risk for circulating reproductive hormone levels and their influence on hippocampal volume and depression susceptibility. Psychoneuroendocrinology. 2019;106:284‐292. doi: 10.1016/j.psyneuen.2019.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu JJ, Weng SC, Liang CK, et al. Effects of kidney function, serum albumin and hemoglobin on dementia severity in the oldest old people with newly diagnosed Alzheimer's disease in a residential aged care facility: a cross‐sectional study. BMC Geriatr. 2020;20(1):391. doi: 10.1186/s12877-020-01789-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Llewellyn DJ, Langa KM, Friedland RP, Lang IA. Serum albumin concentration and cognitive impairment. Curr Alzheimer Res. 2010;7(1):91‐96. doi: 10.2174/156720510790274392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tanita . Body composition analyzer BC‐418MA: instruction manual; 2005. https://tanita.eu/media/wysiwyg/manuals/medical-approved-body-composition-monitors/mc-418-instruction-manual.pdf
- 39. Doorduijn AS, de van der Schueren MAE, van de Rest O, et al. Energy intake and expenditure in patients with Alzheimer's disease and mild cognitive impairment: the NUDAD project. Alzheimers Res Ther. 2020;12(1):116. doi: 10.1186/s13195-020-00687-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sergi G, De Rui M, Coin A, Inelmen EM, Manzato E. Weight loss and Alzheimer's disease: temporal and aetiologic connections. Proc Nutr Soc. 2013;72(1):160‐165. doi: 10.1017/S0029665112002753 [DOI] [PubMed] [Google Scholar]
- 41. Geisler C, Hubers M, Granert O, Muller MJ. Contribution of structural brain phenotypes to the variance in resting energy expenditure in healthy Caucasian subjects. J Appl Physiol (1985). 2018;125(2):320‐327. doi: 10.1152/japplphysiol.00690.2017 [DOI] [PubMed] [Google Scholar]
- 42. Wang W, Zhao F, Ma X, Perry G, Zhu X. Mitochondria dysfunction in the pathogenesis of Alzheimer's disease: recent advances. Mol Neurodegener. 2020;15(1):30. doi: 10.1186/s13024-020-00376-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bartzokis G, Mintz J, Sultzer D, et al. In vivo MR evaluation of age‐related increases in brain iron. AJNR Am J Neuroradiol. 1994;15(6):1129‐1138. [PMC free article] [PubMed] [Google Scholar]
- 44. Benarroch EE. Brain iron homeostasis and neurodegenerative disease. Neurology. 2009;72(16):1436‐1440. doi: 10.1212/WNL.0b013e3181a26b30 [DOI] [PubMed] [Google Scholar]
- 45. Zachariou V, Bauer CE, Seago ER, et al. Healthy dietary intake moderates the effects of age on brain iron concentration and working memory performance. Neurobiol Aging. 2021;106:183‐196. doi: 10.1016/j.neurobiolaging.2021.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of sociodemographic and health‐related characteristics of UK biobank participants with those of the general population. Am J Epidemiol. 2017;186(9):1026‐1034. doi: 10.1093/aje/kwx246 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Data Availability Statement
Data availability statement;All data and code from this study will be made available to approved users of the UK Biobank, upon application.


