Abstract
There is substantial variability in percent total weight loss (%TWL) following bariatric surgery. Functional brain imaging may explain more variance in post-surgical weight loss than psychological or metabolic information. Here we examined the neuronal responses during anticipatory cues and receipt of drops of milkshake in 52 prebariatric surgery men and women with severe obesity (OW, BMI = 35–60 kg/m2) (23 sleeve gastrectomy (SG), 24 Roux-en-Y gastric bypass (RYGB), 3 laparoscopic adjustable gastric banding (LAGB), 2 did not undergo surgery) and 21 healthy-weight (HW) controls (BMI = 19–27 kg/m2). One-year post-surgery weight loss ranged from 3.1 to 44.0 TWL%. Compared to HW, OW had a stronger response to milkshake cues (compared to water) in frontal and motor, somatosensory, occipital, and cerebellar regions. Responses to milkshake taste receipt (compared to water) differed from HW in frontal, motor, and supramarginal regions where OW showed more similar response to water. One year post-surgery, responses to high-fat milkshake cues normalized in frontal, motor, and somatosensory regions. This change in brain response was related to scores on a composite health index. We found no correlation between baseline response to milkshake cues or tastes and%TWL at 1-yr post-surgery. In RYGB participants only, a stronger response to low-fat milkshake and water cues (compared to high-fat) in supramarginal and cuneal regions respectively was associated with more weight loss. A stronger cerebellar response to high-fat vs low-fat milkshake receipt was also associated with more weight loss. We confirm differential responses to anticipatory milkshake cues in participants with severe obesity and HW in the largest adult cohort to date. Our brain wide results emphasizes the need to look beyond reward and cognitive control regions. Despite the lack of a correlation with post-surgical weight loss in the entire surgical group, participants who underwent RYGB showed predictive power in several regions and contrasts. Our findings may help in understanding the neuronal mechanisms associated with obesity.
Keywords: Obesity, Bariatric surgery, Reward, fMRI, Weight loss
1. Introduction
Bariatric surgery is an effective intervention for severe obesity, however, there is substantial variability in the percentage of total weight loss (%TWL) after bariatric surgery. Patients typically lose 8–38% of their body weight post-surgery, depending on their pre-surgical BMI and type of surgery they underwent (Courcoulas et al., 2013; Higa et al., 2011; Hsieh et al., 2014; Lauti et al., 2016). However, within the first three years after surgery, 5–20% of patients regain a significant portion of weight lost post-surgery (Courcoulas et al., 2013; Lauti et al., 2016), which can increase to > 30% in the following years (Courcoulas et al., 2018; Lauti et al., 2016). In addition, 25–35% of patients have inadequate initial weight loss (Higa et al., 2011). Psychological or metabolic indicators have failed to explain this variance in post-surgery weight loss (Courcoulas et al., 2015). Brain activity during rest or a task as measured with functional brain imaging, however, has been shown by several studies to be associated with post-surgical weight loss (Cerit et al., 2019; Holsen et al., 2018; Ness et al., 2014). In addition, neuroimaging may provide a better understanding of the neuronal mechanisms underlying obesity and weight loss. In this paper we focus on the bariatric surgery perspective of anticipatory cues and taste receipt; for more mechanistic and physiological information on neural control of responses to food cues in murine models, see (Andermann and Lowell, 2017; Livneh et al., 2020; Livneh et al., 2017).
The above studies have looked at different tasks or resting state, and different regions of interest. Higher activity in frontal cognitive control regions while participants looked at pictures of food (compared to non-food) was correlated with 3 and 6 months post-surgery weight loss (Ness et al., 2014) whereas higher activity in reward regions was associated with unsuccessful weight loss after psychosocial intervention (Murdaugh et al., 2012). Participants with stronger activity in the nucleus accumbens (NAcc) and hypothalamus when trying to enhance their amount of craving while looking at a picture of palatable food had less 1-year post-surgery weight loss (Holsen et al., 2018). Last, during resting state, stronger functional connectivity between the NAcc and insula, and hypothalamus and precentral gyrus was correlated with more 1-yr post-surgery weight loss (Cerit et al., 2019).
Several studies have reported that individuals with obesity (OW) have a different neuronal response to the anticipatory cue and receipt of milkshake drops compared to healthy-weight (HW) adults and adolescents (Babbs et al., 2013; Ng et al., 2011; Stice et al., 2008b, 2008a) and children (Bohon, 2017). Moreover, the neuronal response may be indicative of future weight gain in HW and OW adults (Geha et al., 2013; Sun et al., 2015) and HW adolescents (Stice et al., 2015; Stice and Yokum, 2018). However, despite these differences and associations with future weight change, we found no prior studies that had examined post-surgical changes in brain response during a milkshake task, or associations of pre-surgery brain response with post-surgical weight loss.
Currently, literature on the milkshake task and obesity in adults is characterized by small sample sizes (generally less than 20 participants per group) and predefined regions of interest, limiting generalizability and leading to inconsistent conclusions. The same holds true for studies on associations between pre-surgery imaging and post-surgery weight loss. In addition, the use of arbitrary weight loss criteria is not a perfect indicator of “success” following bariatric surgery: co-morbidities such as fasting blood sugar, plasma lipids, exercise tolerance, and mood can all improve post-surgically. Indeed, although 25–33% of the patients who underwent bariatric surgery had inadequate weight loss, only 14% did not benefit health-wise (Higa et al., 2011).
To help overcome these limitations, we used an unbiased whole brain approach in 52 pre-surgery patients with severe obesity and 21 HW controls. To the best of our knowledge, this is the first study investigating the milkshake task in adults with severe obesity, and the largest cohort of adults with overweight or obesity to date. Outcome measures were 1-yr post-surgery %TWL and a health-improvement score. We expected to replicate findings from existing literature on the differential response to milkshake taste and cue between OW and HW (a stronger response in somatosensory regions to taste and cue; the literature is not in agreement on the direction of effect in reward regions). In addition, we investigated post-surgery changes (compared to pre-surgery) and whether such changes correlated with outcome at 1-yr post-surgery. Last, we sought for associations between pre-surgery response and 1-yr post-surgery outcome.
2. Materials and methods
2.1. Participants
Eighty-two men and women (age 18–65 years) were recruited from April 2013 to January 2016 from Hartford Hospital’s Metabolic and Bariatric Surgical Center. Participants qualified for RYGB, SG, or LAGB surgery if they had a BMI > 40 or > 35 kg/m2 and one or more obesity-related medical comorbidities, and had to receive surgical clearance from a multidisciplinary team. Participants were scanned before beginning their glycogen-depleting diet in preparation for bariatric surgery. Thirty-one healthy-weight participants were recruited from the community and hospital employees through flyers. To allow for normal weight fluctuations, BMI during scanning was > 25 kg/m2 for 5 participants (ranging from 25.1 to 26.7). Exclusion criteria for the study were: history of prior bariatric surgery; current tobacco use; pregnancy; current or lifetime Axis-I psychotic or substance use disorder as assessed by the Structured Clinical Interview Diagnosis for DSM-IV (SCID-IV); history of traumatic brain injury; IQ < 70 (based on Wechsler Abbreviated Scale of Intelligence); claustrophobia; positive urine for marijuana, cocaine, or opiates; positive alcohol breath test; or any MRI-contraindications (e.g., presence of metal implants). The study was approved by Hartford Hospital’s Institutional Review Board and all participants gave their written informed consent.
Of the 82 included patients, 12 participants dropped out (scheduling problems (N = 4) or MRI-related claustrophobia (N = 8)), 18 participants were excluded from analyses for the following reasons: lactose intolerance (N = 3); did not complete the task (N = 4); only scanned on day 1 and could not be scheduled for the second scan day (N = 4); incorrect scan sequence (N = 3); technical scanner problems (N = 2); had already started their diet (N = 2). Four control participants failed to meet inclusion criteria (head injury, low IQ, left handed, significant health problems) and two dropped out (scheduling problems and MRI-related claustrophobia). Four control participants did not have data for this task: incorrect scan sequence (N = 2); skipped the task due to time restraints (N = 1), claustrophobia (N = 1);. Final included number of participants was 52 with obesity (OW) and 21 with healthy weight (HW). See Table 1 for participant demographics.
Table 1.
Demographics of included participants, and participant group with follow-up information.
| HW | OW, baseline | OW, with %TWL | OW, with FUP scan | HW vs OW P (t or χ2) | |
|---|---|---|---|---|---|
| N | 21 | 52 | 48 | 28 | – |
| Age (years) | 36.8 (13.1, 18.2-60.0) | 40.1 (11.5, 18.2-58.5) | 40.1 (11.5, 18.2-58.5) | 41.8 (10.8, 19.4-58.5) | 0.326 |
| Sex, fem/male % | 52/ 48% | 75/ 25% | 73/ 27% | 79/ 21% | 0.109 |
| Baseline BMI (kg/m2) | 23.1 (2.1, 19.0-26.7) | 44.5 (6.1, 35.1-60.2) | 44.4 (6.0, 35.1-60.2) | 44.1 (6.6, 35.1-60.2) | < 0.0001 |
| RYGB/SG/LAGB - N | – | 24/ 23/ 3 | 24/ 21/ 3 | 13/ 13/ 2 | – |
| RYGB/SG/LAGB - % | – | 46/ 44/ 6% a | 50/ 44/ 6% | 46/ 46/ 7% | – |
| 12 month % TWL | – | 26.5% (9.7, 3.1–44.0) | 26.5% (9.7, 3.1–44.0) | 25.3% (7.8, 9.6–38.8) | – |
| Race, white/AA% | 67/ 29% b | 75/ 25% | 77/ 23% | 79/ 21% | 0.276 |
| Non-Hisp / Hisp% | 90/ 5% c | 85/ 15% | 83/ 17% | 86/ 14% | 0.437 |
Data are presented as mean (sd, range)
HW = healthy weight; OW = participants with severe obesity; FUP = follow up; AA = African American; Hisp = Hispanic
Two participants (4%) did not undergo surgery.
One participant identified themselves as a Pacific Islander.
Missing for one participant.
2.2. Composite health outcome measure
In addition to weight loss, bariatric surgery has an important effect on health; even when weight loss is inadequate (Higa et al., 2011). Therefore, we also investigated the correlation between baseline brain response and a more comprehensive composite health measure. Principal component analysis was used to summarize multiple dimensions of health outcomes, including changes in weight, mental health, quality of life, and metabolic measures, accounting for their inter-correlations. To construct this score, difference scores were derived from weight, total cholesterol, fasting plasma glucose, fasting plasma insulin, hemoglobin A1c, HOMA-IR (Homeostatic Model Assessment for Insulin Resistance), total score on Depression Anxiety Stress Scales (Lovibond and Lovibond, 1995), and quality of life measures pre-surgically and 1-year post-surgery. A covariance matrix of these outcome variables was created, eigenvectors computed and the first principal component extracted. Loadings of this component represent the relative contributions of each variable to the overall composite measure. The composite score was defined as a linear transformation of clinical measures weighted by their component loadings as below (IWQOL = impact of weight on quality of life; DASS = depression and anxiety scale):
2.3. Experimental design
Participants were scheduled for fMRI scanning on two days, on average 3 weeks before surgery. All participants completed fMRI tasks around the same time in the morning following a participant-confirmed 8 h overnight fast. Scanning sessions were divided into two different days due to large number of fMRI tasks.
The milkshake task was the first MRI paradigm (of several) to be completed on the second scan day. Participants were shown pictures of high-fat milkshake, low-fat milkshake, or water, accompanied by their respective written words. In the explanation of the task, participants were told that they would sometimes receive a drop of the pictured liquid in their mouth via a pump and oral manifold. Despite the instruction, only high-fat milkshake was used for all milkshake trials, and a tasteless solution for the water trials. Thus, the difference between the high-fat and low-fat taste-phase is only different by expectation, as the somatosensory input is the same for both milkshake conditions.
This event-related paradigm was adapted from a prior study that shows that an isocaloric milkshake with a low-fat label can lead to a different activation response in OW compared HW (Ng et al., 2011). Pre-stimulus cues consisted of colored pictures of glasses of water, regular high-fat milkshake, or low-fat milkshake with corresponding labels. The pictures signaled the delivery of 0.75 ml regular milkshake for both the high-fat and low-fat milkshake conditions, and a tasteless solution for water (Gearhardt et al., 2011). A tasteless solution (25 mM KCl and 2.5 mM NaHCO3) was used because water has a taste that activates the taste cortex (Zald and Pardo, 2000). The gustatory stimuli (milkshake/tasteless) were delivered over 3 s, 3–7 s after the cue onset using a custom 3D printed plastic manifold. The manifold was placed above participant’s mouth prior to the scan to precisely deliver measured drops of the liquids. After the delivery of the taste stimuli, a fixation cross appeared for 6–11 s to allow the subject to swallow. There was no rinse; total task duration was ~13.5 min per run. The task was divided in two runs with 60 cues presented in each run. Over the two runs, 63% (76 out of 120) of the cues were associated with delivery of a taste. Pictures were presented using MATLAB with a LCD projector and the taste-solutions were delivered using programmable syringe pumps (Braintree Scientific BS-8000) controlled by Cogent Graphics (a graphics toolbox for MATLAB, developed by John Romaya at the Laboratory of Neurobiology at the Wellcome Department of Imaging Neuroscience) to ensure consistent volume, rate and timing of taste delivery.
2.4. Scan parameters and preprocessing
Neuroimaging data were acquired at the Olin Neuropsychiatry Research Center on a wide-bore Siemens Skyra 3T scanner with software VD13A-SP04 (Siemens, Erlangen, Germany) using a 64 channel head coil. Five high-resolution T1-weighted images (Axial MPRAGE, TR = 2200 ms, TE = 2.88 ms, TI = 794 ms, Flip angle = 13 °, voxel size = 0.8 mm isotropic, FOV = 176 × 256 × 167 mm, iPAT = 3) were collected to achieve better signal to noise ratio. The T1 images were aligned and averaged, producing a single high quality T1. Functional data were acquired using a multiband gradient-echo sequence (Axial, TR = 475 ms, TE = 30 ms, FOV = 240 mm, flip angle = 60°, acquisition matrix = 80 × 80, voxel size = 3 mm isotropic, number of slices = 48, multi band factor = 8, iPAT = 1).
Data were processed using a standard FSL (version 5.0.8) FEAT pipeline (Smith et al., 2004; Woolrich et al., 2004, 2001) including high-pass filter at 0.01 Hz, realignment with MCFLIRT, fieldmap correction, boundary based registration to T1 via a single band reference image, nonlinear registration to standard MNI space (resampling to 2 mm isotropic), FILM prewhitening (Woolrich et al., 2001), Gaussian smoothing at 6 mm full-width half maximum.
ACompCor (Behzadi et al., 2007; Muschelli et al., 2014) as implemented in nipype (version 1.6.0; in python version 3.8.5) (Gorgolewski et al., 2011) was used for motion correction. Structural T1 scans were segmented with FAST (Zhang et al., 2001); cerebrospinal fluid (CSF) and white matter (WM) masks were warped to the functional scans and thresholded at 99% tissue probability. For WM masks, voxels were included with a intensity of > 0.9 after 3D 3-voxel kernel mean filtering. For CSF masks, this was > 0.75. More aggressive erosion lead to empty masks. CSF and WM masks were combined to one mask. Components that explained 50% of the variance were saved and used as confound regressors.
2.5. Statistical analysis
Each task was modeled using FSL FEAT including all task-related events. Gamma function (Phase = 0; Stdev = 3; Mean lag = 6) was used for convolution with event regressors. Twelve standard motion parameters (x, y, z, roll, yaw, pitch, and their derivatives) were added as regressors of no interest. In addition, components from aCompCor were added as confounds. Three participants (2 RYGB, 1 SG) had framewise displacement of > 0.5 mm for > 25% of the volumes. Analyses were repeated excluding these participants, and analyses were repeated added mean FD as a covariate of no interest.
The following second level analyses were tested: HW vs OW; change from baseline to 1-yr post-surgery in OW; correlation between change and post-surgery outcome; correlation between baseline and 1-yr post-surgery outcome. Cross-sectional second level analyses were done with PALM (Winkler et al., 2014) in Matlab 2018b with 5000 permutations and the recommended tail acceleration (Winkler et al., 2016). Longitudinal second level analyses were done with the Sandwich Estimator (Guillaume et al., 2014), as implemented in FSL. The model included all baseline OW participants (N = 52), and those with a follow-up scan (N = 28). Threshold-free cluster enhancement (TFCE) (Smith and Nichols, 2009) settings were set the same for PALM and SWE, using the default settings height = 2, extent = 0.5, cluster = 6. Age, age2, sex, and their interactions, and baseline BMI were included as regressors of no interest. The following six contrasts were examined: High-fat Cue > Low-fat Cue (HiLo-Cue); Low-fat Cue > Water Cue (LoWa-Cue); High-fat Cue > Water Cue (HiWa-Cue); High-fat Taste > Low-fat Taste (HiLo-Taste); Low-fat Taste > Water Taste (LoWa-Taste); High-fat Taste > Water Taste (HiWa-Taste). Whole brain gray matter mask was created by thresholding FSL’s tissue template at 50%. Differences between each of the contrasts were examined for the following comparisons: baseline OW pre-surgery vs HW; OW pre-surgery vs post-surgery; correlations of OW pre-surgery BOLD response with one year post-surgery %TWL and health index; correlations of OW pre- to post-surgery change with%TWL and health index. Significance was determined through TFCE, with whole-brain FWE-correction at p < 0.05. Only clusters with k ≥ 10 voxels are reported. Unthresholded statistical maps have been uploaded to NeuroVault.org (Gorgolewski et al., 2015) and are available at https://neurovault.org/collections/LHFDZIWH/. Due to the large T values (as is common with TFCE), we uploaded both the TFCE maps and the voxel-wise z-maps.
Post hoc tests for sex effects were done in Matlab (2018b) with two sample t-tests on the mean value of the significant voxels in the contrast map after regressing out nuisance variables (age, age2, sex, and their interactions, baseline BMI). Sex was self-reported; no patient had a history of gender reassignment surgery or identified their gender differently than their birth-assigned sex.
2.6. Additional region of interest approach
As mentioned above, we used a whole-brain approach to allow for generalizability and thorough statistical testing. We repeated the analyses for the follow up correlation with a mask including regions reported to be correlated with weight loss after surgery or non-surgical weight loss intervention: superior, middle, and medial frontal cortex, inferior temporal gyrus, posterior cingulate gyrus (PCC) (Ness et al., 2014); NAcc (Holsen et al., 2018); insula (Cerit et al., 2019); anterior cingulate cortex (ACC), frontal operculum, insula (Murdaugh et al., 2012). Bilateral anatomical regions were taken from the Harvard-Oxford atlas (Desikan et al., 2006).
3. Results
3.1. Response to milkshake in HW
In our small sample of N = 21 HW, we found that response in the right inferior frontal gyrus (peak at MNI 56, 12, 14) was lower during receipt of a high-fat compared to low-fat milkshake (HiLo-Taste; p = 0.032, z = 1083, k = 248, see Fig. 1, Table S1). This finding was still present after correcting for mean FD.
Fig. 1.
Healthy weight participants had a stronger response to low-fat compared to high-fat milkshake. Maps are thresholded at p < 0.05, whole-brain corrected with TFCE and FWE. Boxplots show the mean BOLD response over all significant voxels for the significant contrast.
3.2. Response to milkshake in HW and OW at baseline
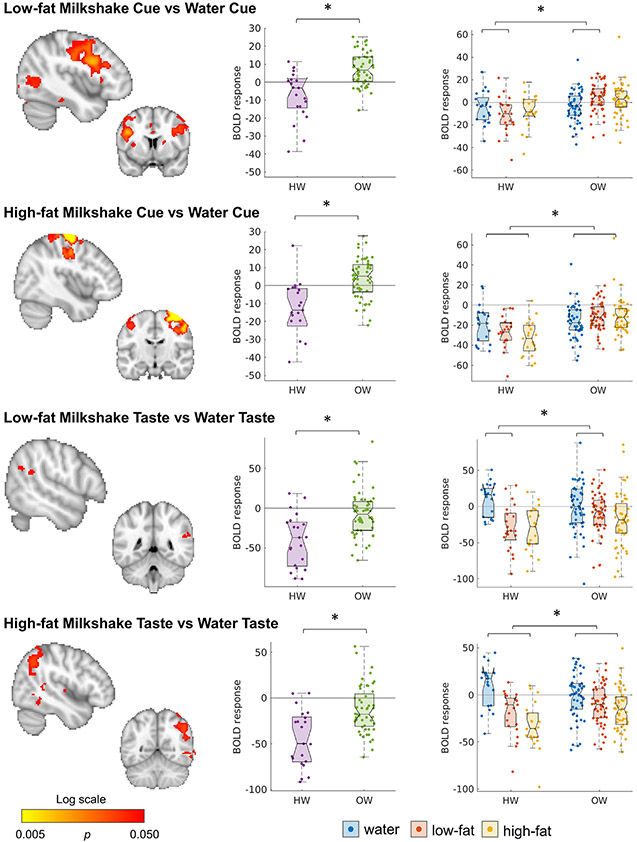
Where HW had a negative response to low-fat milkshakes cues compared to water cue (LoWa-Cue), OW (before surgery) showed a positive response. This was seen in areas throughout the brain including frontal, pre- and postcentral, and visual regions (Fig. 2, Tables S2, S3). The effect was similar over all regions in the contrast (thalamus, caudate, insula, frontal regions, pre- and postcentral gyrus, cerebellum, occipital regions, and brainstem, see Fig. S1a,b). For the HiWa-Cue the blunted response was limited to a cluster around the pre- and postcentral gyrus, predominantly in the right hemisphere (Fig. 1B, Table S2). Brain responses to receipt of low-fat or high-fat milkshake (compared to receipt of a tasteless solution) also differed from HW: where HW showed a positive response to water and a negative response to milkshake, OW had a more similar response to both stimuli. This difference was located more in the parietal lobule (Fig. 2, Table S2)
Fig. 2.
Different activation in HW and OW in response to a low-fat or high-fat cue preceding the possible receipt of a milkshake drop (compared to a cue indicating receipt water), and receipt of low-fat and high-fat milkshake taste (compared to a tasteless solution). Maps are thresholded at p < 0.05, whole-brain corrected with TFCE and FWE. Boxplots show the mean BOLD response over all significant voxels of the significant contrasts. Boxplots are shown for all conditions using the mask of the significant contrast. See also Tables S2-3; Fig. S1a,b.
Because of the significantly higher motion in OW (mean (SD) of mean FD = 0.26 (0.09) for OW, and 0.19 (0.06) for HW, p = 0.0010), analyses were repeated with mean FD added as a nuisance variable. This diminished the differences reported above for LoWa-Cue (a small cluster in the left inferior frontal gyrus remained significant (k = 20)) and HiWa-Cue (a small cluster in the precentral gyrus remained significant (k = 49)). For the HiWa-Taste the group differences were no longer significant. The contrast of LoWa-Taste on the other hand became stronger and the cluster expanded to the right temporal cortex.
When repeating the analyses excluding three participants (OW) with excessive motion (> 25% frames with FD > 0.5), significance was lost for LoWa-Cue, remained the same for HiWa-Cue and HiLo-Taste. For the HiWa-Taste, only a small cluster in the posterior supramarginal gyrus remained significant (k = 39).
NEO personality traits (Costa and McCrae, 1992) differed between HW and OW in the neuroticism (OW > HW, Hedges’ g = −0.80, p = 0.004) and openness dimension (HW > OW, g = 0.58, p = 0.036). Other NEO personality traits and mood based on the Depression Anxiety Stress Scales (DASS, Lovibond and Lovibond, 1995) and the Beck Depression Inventory (BDI, Beck et al., 1996) did not differ between HW and OW at baseline (ps ≥ 0.08). See for more detail Table S4. Since the milkshake task is a passive task that is not related to impulsivity, it is unlikely that these differences are related to our results.
3.3. Post-surgery changes
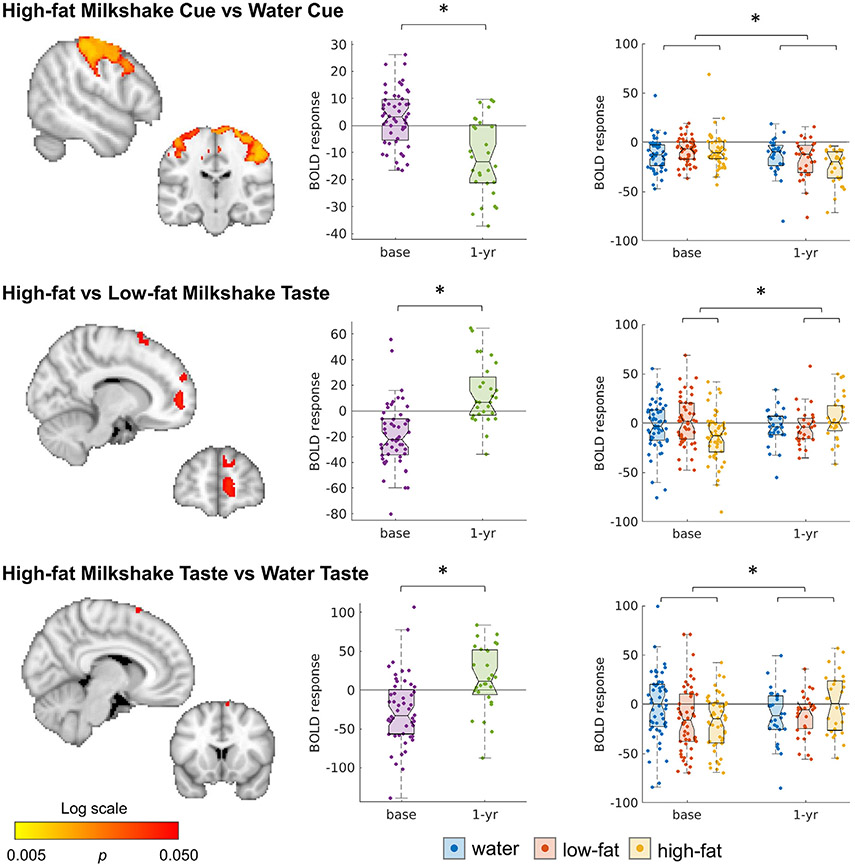
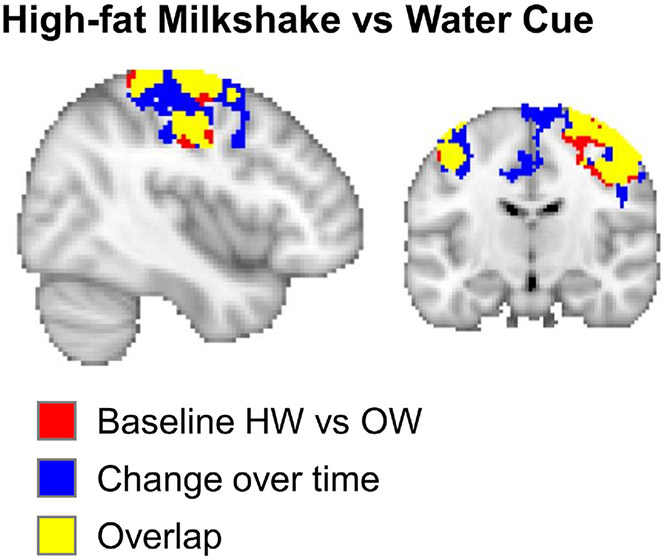
OW participants showed pronounced changes in their response to HiWa-Cue in a large cluster including among others the superior frontal gyrus, pre- and post-central gyrus and the supplementary motor cortex (Fig. 3, Table S5). This region overlapped with the differences between OW and HW at baseline (see Fig. 4), indicating that the OW normalized their brain responses (compare Figs. 2 and 3). Reponses to milkshake receipt (HiLo-Taste and HiWa-Taste) also changed after surgery in several clusters in the frontal cortex (Fig. 4, Table S5).
Fig. 3.
Significant change between baseline and 1-yr post-surgery in participants with obesity. Maps are thresholded at p < 0.05, whole-brain corrected with TFCE and FWE. Boxplots show the mean BOLD response over all significant voxels for the specific contrast. Boxplots are shown for all conditions using the mask of the significant contrast. See also Table S5.
Fig. 4.
Overlap (yellow) between the group differences between OW and HW (red), and the change over time (blue).
When including mean FD as a covariate in the model, results remained the same for HiWa-Cue, and became stronger for the HiLo-Taste and HiWa-Taste. After excluding motion outliers (N = 3 at baseline, 2 at follow-up), results were similar. Motion did not change over time as measured by a paired t-test (mean (sd) FD = 0.23 (0.06), 0.21 (0.12) for pre- and post-surgery respectively; t(27) = 1.03, p = 0.31). To increase power by adding all baseline participants with obesity, we also checked the change over time in a linear model (lmer(meanFD ~ time + (1 ∣ Subj)). This also showed no significant change (χ2 (1) = 3.63, p = 0.057.
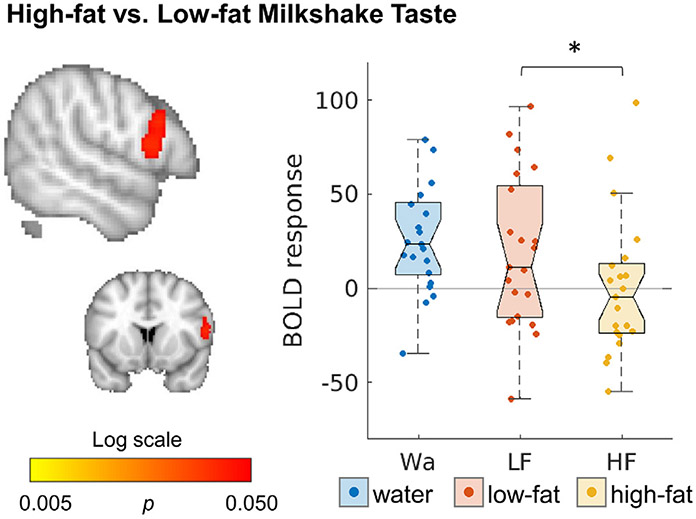
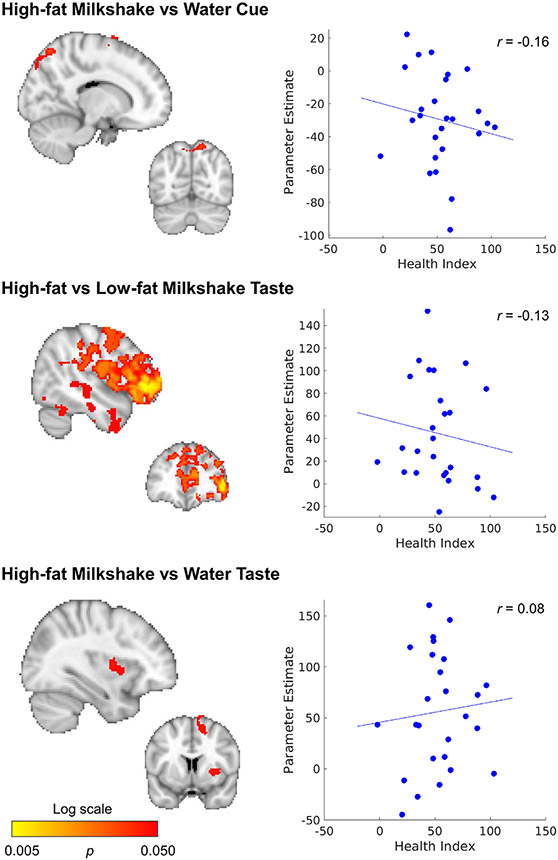
Changes after surgery were correlated with composite health index scores, but not with weight loss. A correlation between the composite health index and change in response to HiLo-Taste, was predominantly seen in the right hemisphere, and was strongest in the frontal pole (Fig. 5, Tables S6-7). Smaller clusters were found for HiWa-Cue (frontal, pre-, and post central gyrus, superior occipital cortex) and HiWa-Taste (insula, superior frontal, supplementary motor cortex, right putamen).
Fig. 5.
Correlation between change over time (1-yr post-surgery minus baseline) and composite health index. Maps are thresholded at p < 0.05, whole-brain corrected with TFCE and FWE. See also Tables S6,7.
When including mean FD as a covariate in the model, results remained the same. Only one person qualified as a motion outlier and they had a composite score of 49, which was close to the mean (53, with sd = 24). Therefore, this person is unlikely to have had a large influence on the results. Change in motion was not correlated with the composite health index (r = −0.11, p = 0.59).
3.4. Baseline correlations with outcome
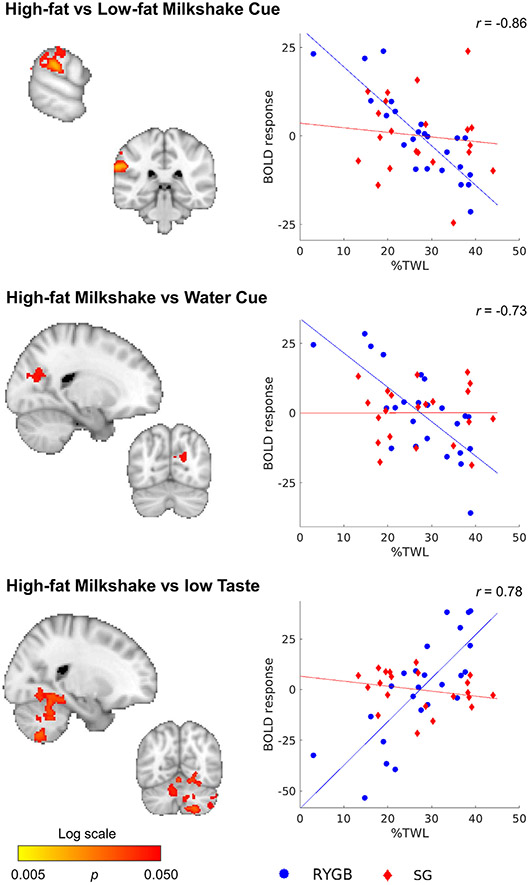
Baseline scans were not correlated with%TWL nor composite health index one year post-surgery, also not when looking at predefined regions of interest based on the literature (ps > 0.121). When running the analyses separately for SG and RYGB, baseline scans of the participants who underwent RYGB (N = 24) were correlated with%TWL for three contrasts (HiLo-Cue, HiWa-Cue, HiLo-Taste; Fig. 6, Table S8). For HiLo-Cue, responses in the supramarginal gyrus, occipital cortex, and cerebellum were negatively correlated with%TWL 1 year after surgery (r = −0.86). See Figure S2 for region-wise visualization. The response to HiWa-Cue around the cuneal cortex was also negatively correlated to%TWL (r = −0.73). Cerebellar response to HiLo-Taste was positively correlated with%TWL. Note that despite the instructions given to participants, both high-fat and low-fat milkshake receipts were isocaloric high-fat. The correlation between%TWL and the contrast in the significant voxels was r = 0.78. Note that the correlations inflated due to selection bias of the voxels in the significant cluster (Kriegeskorte et al., 2009). Responses to HiWa-Taste or LoWa-Taste were not correlated with%TWL. There were no correlations between baseline scan and composite health index (p > 0.213). No associations between baseline scans and outcomes were found for the SG group (N = 21, p > 0.115). No associations were found in the whole group (N = 48) when adding surgery type as a nuisance variable (p > 0.112).
Fig. 6.
Response to high-fat vs low-fat milkshake cues and taste correlated with%TWL in RYGB (blue circles), but not SG (red diamonds) participants. Note that participants received high-fat taste in both conditions, but were told that it was low-fat in the low-fat condition. Map is thresholded at p < 0.05, whole-brain corrected with TFCE and FWE. Note that the correlation is inflated due to selection bias of the voxels in the significant cluster (Kriegeskorte et al., 2009). See also Table S8; Fig. S2.
After including FD as a covariate to the model, as well as after removing 2 motion outliers, results were similar but with smaller clusters for HiLo-Cue and HiLo-Taste. Results were no longer significant for HiWa-Cue. Baseline motion was not correlated with%TWL in the whole group (r = 0.16, p = 0.27) nor in RYGB participants only (r = 0.16, p = 0.45).
3.5. Supplementary analyze
To check whether significant results were due to a sex effect, two-sample t-tests were performed for the significant clusters. This was done for the whole group, as well as HW and OW separately, and baseline and follow-up for the change over time. For contrasts with a brain wide significant cluster (LoWa-Cue in HW vs OW; HiLo-Taste in change over time correlation with composite health index; HiLo-Cue in baseline correlation with%TWL), effects were also tested in large regions of interest. For all such tests, only one reached significance. In the comparison of HW vs OW, in HiWa-Taste women HW (N = 11) had a lower response to the high-fat taste than did HW men (N = 10) (t(19) = −3.3, p = 0.004). Note the small sample sizes.
4. Discussion
In a cohort of participants with morbid obesity we confirmed previous reports of a differential response to anticipatory cues and milkshake receipt in participants with obesity compared to healthy-weight controls. This effect was attributable to OW having a more similar response to water and milkshake, whereas HW have a more distinct differences in their responses to water and milkshake cues and receipts. Brain responses to high-fat milkshake cues (compared to water) in somatosensory regions were normalized (i.e. more resembles those of HW participants) 1-yr post-surgery compared to pre-surgery. Changes in predominantly frontal responses were correlated to the composite health index. Last, correlations between weight loss and pre-surgery brain responses during the milkshake task was only seen in RYGB participants in areas surrounding the supramarginal gyrus, cuneus, and cerebellum.
To our knowledge, this is the first study to report on the milkshake task in participants with obesity before and after bariatric surgery. Despite promising correlations between brain responses during this task and weight gain in healthy-weight adolescents (Stice et al., 2015; Stice and Yokum, 2018) and healthy to obese weight adults (Geha et al., 2013; Sun et al., 2015), our study found no correlations with %TWL one year post-surgery in our group of 52 participants. However, when examining only participants who underwent RYGB, responses to high-fat vs low-fat milkshake cues located in the supramarginal gyrus, superior lateral occipital cortex and cerebellum were negatively correlated to %TWL, and cerebellar responses to high-fat vs (indicated) low-fat taste receipt were positively correlated with %TWL. The taste-phase is only different by expectation, as the somatosensory input is the same between both milkshake conditions. Indeed, healthy-weight participants (HW) had a differential response to high-fat milkshake taste compared to the low-fat taste (HiLo-Taste), despite that the somatosensory input is the same for both milkshakes. This suggests that in HW, the right inferior frontal cortex is involved in top-down processing of the perceived taste. However, this contrast (HiLo-Taste) did not differ between OW and HW as reported by Ng et al. (2011). The cerebellum is involved in reward expectation and prediction (Hull, 2020), and is associated with addiction and drug craving (Cservenka et al., 2015; Miquel et al., 2016; Moreno-Rius and Miquel, 2017; Moulton et al., 2014; Wagner and Luo, 2020) and is acutely activated by drugs of abuse (Moulton et al., 2014). Interestingly, a recent study showed its involvement in taste discrimination (Avery et al., 2020). Our results suggest that a stronger reward and/or expectation-driven cerebellar response to the high-fat milkshake taste (compared to the low-fat indicated milkshake) may be related to more weight loss. Together, this shows the importance of including the cerebellum in whole brain analyses.
Despite these plausible relations between cerebellar response to reward receipt and post-surgical %TWL, this effect was only seen in RYGB participants. First and foremost, the small sample size could explain different findings between surgery types. Although this is a large sample in the literature of neuroimaging and bariatric surgery, small samples sizes run the risk of false positives. This was nicely demonstrated by Stice and Yokum (2018) in the context of this specific task, but see for more in depth overviews (Bossier et al., 2020; Turner et al., 2018; Varoquaux, 2018). Second, several studies reported that post-intervention change in brain response differs across surgery types (Baboumian et al., 2019; Faulconbridge et al., 2016) and non-surgical weight loss programs compared to LAGB (Bruce et al., 2014). The fact that brain changes may depend on surgery type allows the possibility that a specific intervention “targets” a specific brain circuit, which could explain how activation of specific regions can correlate with %TWL in RYGB but not SG. Third, patients who opt for RYGB may be drawn from a different population than those opting for SG. Indeed, on average RYGB patients have a higher BMI and more comorbidities (Nasser et al., 2020). This difference was also seen in our sample (summed over presence of diabetes, hypertension, hyperlipidemia, GERD, sleep apnea, χ2 (1, N = 47) = 9.6, p = 0.002), although baseline BMI did not differ between SG and RYGB participants. Different patient characteristics and comorbidities may be associated with different brain characteristics and brain responses. Fourth, on average RYGB leads to more weight loss than SG (Courcoulas et al., 2015; Li et al., 2016; McTigue et al., 2020), possibly also leading to a difference in variation of %TWL between the two groups. However, there was no weight loss difference between surgery types in our sample and from Fig. 2B we can infer that the correlation in RYGB participants was not driven by the presence of more participants with more %TWL. In summary, it is plausible that cerebellar neuronal responses to the receipt of a drop of milkshake are associated with weight loss one year post-surgery in RYGB but not SG participants. Nevertheless, as noted, the results should be interpreted with caution as the sample size is small (N = 24).
Regions previously associated with post-surgery weight loss were not correlated with %TWL in our study. The most readily explanation for that is that none of the previous studies employed the gustatory food cue task. However, this task (when whole brain corrected) has been reported to correlate with future weight gain in HW adolescents (Stice et al., 2015; Stice and Yokum, 2018) and HW and OW adults (Geha et al., 2013). However, adolescents may have different brain responses during this task as they undergo a period of intense brain development (Durston and Casey, 2006) and have a higher reward-sensitivity and reward system activation (van Duijvenvoorde et al., 2016). In addition, as both the current study and others have found, OW brains, compared to HW brains, respond differently to anticipatory cues and milkshake receipts (Babbs et al., 2013; Ng et al., 2011; Stice et al., 2008b, 2008a). Thus, networks and processes associated with weight gain in HW can differ from those in OW. Last, two studies reported that the associations between change in BMI and neuronal responses of the milkshake task depended on the presence of the Taq polymorphism (Stice et al., 2008a; Sun et al., 2015), although this finding was not replicated (Stice and Yokum, 2018).
To the best of our knowledge, our milkshake study includes the largest cohort of adults with severe obesity to date. We found a strong, widespread stronger response in OW compared to HW during the anticipatory cue for the delivery of a drop of milkshake, and a much smaller blunted response to the receipt of high-fat or (indicated) low-fat milkshake. Most milkshake studies reported a difference between OW and HW for the taste-phase but not during the anticipatory cue (Babbs et al., 2013; Bohon, 2017; Stice et al., 2011). The two studies that found a different response to the anticipatory cue also reported a stronger response in OW (Ng et al., 2011; Stice et al., 2008b). Findings on responses to taste-receipt are inconsistent; both diminished (Babbs et al., 2013; Stice et al., 2008a) and stronger response to taste (Ng et al., 2011; Stice et al., 2011) in reward regions have been reported. These studies included younger participants (14–25 years), and looked at predefined regions with a liberal statistical threshold. One study with a larger sample size (N = 55 + 53) and whole brain approach reported a stronger response in somatosensory processing areas in adolescents at high compared to low risk for obesity (Shaerrer et al., 2018). In summary, different results can be explained by small sample sizes; a predefined ROI approach which reduces generalizability and lowers the statistical threshold; liberal statistics; and different group characteristics (e.g. BMI, age).
Another source of variance is amount of motion in the scanner, and the method of motion correction. OW had higher within-scanner motion than HW, and correction for motion reduced the group differences. Despite the reduction in significance, we emphasize that scans were motion corrected (aCompCor), which increased power over all analyses (our initial analyses were done without aCompCor). In addition, correcting for mean FD may be throwing the baby out with the bathwater because of the inherent genetic relation between motion and BMI (Hodgson et al., 2017). Although a recent study did show a decrease in motion after surgery (Beyer et al., 2020), we did not find such changes in our sample. Nevertheless, motion remains an important source of bias, and its relation to variables of interest complicates interpretation.
Interestingly, in our data, the difference between HW and OW response to the anticipatory cue was more widespread for the low-fat than for the high-fat milkshake cue. Several studies support a differential response to visual images of high- and low-energy-dense foods (Siep et al., 2009; Zoon et al., 2018a), possibly because unhealthy foods elicit ambivalent responses (craving but avoiding) (Deluchi et al., 2017; Nijs and Franken, 2012). A more variable response to high-fat milkshake cue could explain the more focused motor related response to the anticipatory cue, whereas the low-fat cue led in addition to the motor related response, also to responses in regions related to reward expectation and anticipation (cerebellum, caudate, thalamus), evaluation (cingulate), visual processing (cuneus, precuneus, calcarine gyrus), and cognitive control (frontal cortex).
One year after bariatric surgery, brain responses to milkshake cues were decreased in primary motor and somatosensory cortices, whereas brain responses to milkshake taste receipt were increased in frontal regions. These results are in agreement with the literature on visual food cues which also report decreases in primary motor cortex after weight loss intervention (Murdaugh et al., 2012), and increases in frontal regions after bariatric surgery (Baboumian et al., 2019; Bruce et al., 2012; Zoon et al., 2018b). The decrease in motor response overlapped with the difference between HW and OW, and suggests that surgery normalized the brain responses to anticipatory high-fat milkshake cues (HiWa-Cues). Moreover, OW who had a more normalized response to HiWa-Cues in motor regions after surgery, had a better health outcome after surgery. The composite health index includes weight loss, improvement in depression, anxiety, impact of weight on quality of life, and cholesterol. Since weight loss was not correlated with changes in brain responses, this correlation may reflect improvements in psychological state. However, because of the complexity of the change over time (because it includes four variables) and the composite health index, in combination with a small sample size (N = 28 with baseline and follow-up scan) and small correlations, the results need to be interpreted with caution.
Several limitations to this study should be taken into account. First, although our sample size is relatively large in this field, several studies have shown that sample sizes need to be much larger to allow for reliable replication (Bossier et al., 2020; Turner et al., 2018; Varoquaux, 2018). The lack of a HW-follow-up group also limits statistical interpretation. We did do our best to limit the false positive rate by using whole brain non-parametric statistical methods (cf, Eklund et al., 2016). Second, although BMI is an easy and often used metric of obesity, it is not a good proxy for the underlying metabolic alterations. It is possible that brain differences between OW and HW are less driven by BMI and more by other metrics of obesity such as waist circumference and fasting insulin levels (Farruggia et al., 2020). This could increase variability in obese samples and add to the lack of convergence in the literature. A recent meta-analysis has shown that for the visual food cue, there are no differences between brain responses of OW and HW, but that age may be an important factor (Morys et al., 2020). Third, the sample included participants who underwent different surgery types, and post-surgery brain changes differ may across surgery types (Baboumian et al., 2019; Faulconbridge et al., 2016). This may lead to increased variation in pre- to post-surgery brain changes, which could explain the absence of longitudinal changes in our study. Fourth, the balance between men and women was unequal. Previous studies have demonstrated that women respond differently to visual food images (Chao et al., 2017). However, sex was included as a nuisance variable in all analyses and there were no sex differences in our findings. In addition, men and women did not differ in terms of surgery type, baseline BMI, or %TWL; although men did have more comorbidities (higher prevalence of sleep apnea, higher HDL values). Last, we did not have information on subjective ratings of milkshake or artificial saliva pleasantness, or the frequency of personal milkshake consumption. Experienced pleasantness may depend on BMI (Gobbi et al., 2020) and correlate with local brain responses (Small et al., 2003).
In conclusion, our results of brain wide blunted response of individuals with severe obesity (compared to healthy weight individuals) during anticipatory cues emphasizes the need to look beyond reward and cognitive control regions. In addition, cerebellar activation during the milkshake task might hold predictive information on post-surgery weight loss, however, since we only found that in a sub-sample, this finding needs to replicated in a larger sample.
Supplementary Material
Funding
This work was funded by Hartford Hospital internal funding.
Footnotes
Credit authorship contribution statement
Marinka M.G. Koenis: Methodology, Formal analysis, Writing – original draft, Visualization, Writing – review & editing. Pavlos K. Papasavas: Conceptualization, Resources, Investigation, Writing – review & editing, Supervision, Funding acquisition. Ronald J. Janssen: Methodology, Writing – review & editing. Darren S. Tishler: Resources, Investigation, Writing – review & editing, Supervision. Godfrey D. Pearlson: Conceptualization, Writing – review & editing, Supervision, Funding acquisition.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neuroimage.2021.118623.
Declaration of Competing Interest
Outside the submitted work: DST: Received consulting fees from Medtronic, Olympus Corporation, ConMed, and Ethicon. PKP: Received consulting fees from Olympus Corporation. MMGK and GDP report no conflicts of interest.
Data availability statement
The data that support the findings of this study are not publicly available due to restrictions imposed by the administering institution and privacy of the participants. The authors will share them by request from any qualified investigator after completion of a data sharing agreement.
References
- Andermann ML, Lowell BB, 2017. Toward a wiring diagram understanding of appetite control. Neuron 95 (4), 757–778. doi: 10.1016/j.neuron.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery JA, Liu AG, Ingeholm JE, Riddell CD, Gotts SJ, Martin A, 2020. Taste quality representation in the human brain. J. Neurosci 40, 1042–1052. doi: 10.1523/JNEUROSCI.1751-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbs RK, Sun X, Felsted J, Chouinard-Decorte F, Veldhuizen MG, Small DM, 2013. Decreased caudate response to milkshake is associated with higher body mass index and greater impulsivity. Physiol. Behav 121, 103–111. doi: 10.1016/j.physbeh.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baboumian S, Pantazatos SP, Kothari S, McGinty J, Holst J, Geliebter A, 2019. Functional magnetic resonance imaging (fMRI) of neural responses to visual and auditory food stimuli pre and post roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG). Neuroscience 409, 290–298. doi: 10.1016/j.neuroscience.2019.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK, 1996. Manual For the Beck Depression Inventory-II. Psychological Corporation, San Antonio, TX. [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT, 2007. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37, 90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer F, Prehn K, Wüsten KA, Villringer A, Ordemann J, Flöel A, Witte AV, 2020. Weight loss reduces head motion: revisiting a major confound in neuroimaging. Hum. Brain Mapp 41, 2490–2494. doi: 10.1002/hbm.24959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohon C., 2017. Brain response to taste in overweight children: a pilot feasibility study. PLoS ONE 12. doi: 10.1371/journal.pone.0172604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossier H, Roels SP, Seurinck R, Banaschewski T, Barker GJ, Bokde ALW, Quinlan EB, Desrivières S, Flor H, Grigis A, Garavan H, Gowland P, Heinz A, Ittermann B, Martinot JL, Artiges E, Nees F, Orfanos DP, Poustka L, Fröhner Dipl-Psych JH, Smolka MN, Walter H, Whelan R, Schumann G, Moerkerke B, 2020. The empirical replicability of task-based fMRI as a function of sample size. Neuroimage 212, 116601. doi: 10.1016/j.neuroimage.2020.116601. [DOI] [PubMed] [Google Scholar]
- Bruce AS, Bruce JM, Ness AR, Lepping RJ, Malley S, Hancock L, Powell J, Patrician TM, Breslin FJ, Martin LE, Donnelly JE, Brooks WM, Savage CR, 2014. A comparison of functional brain changes associated with surgical versus behavioral weight loss. Obesity 22, 337–343. doi: 10.1002/oby.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce JM, Hancock L, Bruce A, Lepping RJ, Martin L, Lundgren JD, Malley S, Holsen LM, Savage CR, 2012. Changes in brain activation to food pictures after adjustable gastric banding. Surg. Obes. Relat. Dis 8, 602–608. doi: 10.1016/j.soard.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Cerit H, Davidson P, Hye T, Moondra P, Haimovici F, Sogg S, Shikora S, Goldstein JM, Evins AE, Whitfield-Gabrieli S, Stoeckel LE, Holsen LM, 2019. Resting-state brain connectivity predicts weight loss and cognitive control of eating behavior after vertical sleeve gastrectomy. Obesity 27, 1846–1855. doi: 10.1002/oby.22607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao AM, Loughead J, Bakizada ZM, Hopkins CM, Geliebter A, Gur RC, Wadden TA, 2017. Sex/gender differences in neural correlates of food stimuli: a systematic review of functional neuroimaging studies. Obes. Rev 18, 687–699. doi: 10.1111/obr.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, McCrae RR, 1992. NEO Personality Inventory-Revised (NEO PI-R). Psychological Assessment Resources, Odessa, FL. [Google Scholar]
- Courcoulas AP, Christian NJ, Belle SH, Berk PD, Flum DR, Garcia L, Horlick M, Kalarchian MA, King WC, Mitchell JE, Patterson EJ, Pender JR, Pomp A, Pories WJ, Thirlby RC, Yanovski SZ, Wolfe BM, 2013. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA J. Am. Med. Assoc 310, 2416–2425. doi: 10.1001/jama.2013.280928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courcoulas AP, Christian NJ, O’Rourke RW, Dakin G, Patchen Dellinger E, Flum DR, Melissa Kalarchian PD, Mitchell JE, Patterson E, Pomp A, Pories WJ, Spaniolas K, Steffen K, Wolfe BM, Belle SH, 2015. Pre-operative factors and 3-year weight change in the longitudinal assessment of bariatric surgery (LABS) consortium. Surg. Obes. Relat. Dis 11, 1109–1118. doi: 10.1016/j.soard.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courcoulas AP, King WC, Belle SH, Berk P, Flum DR, Garcia L, Gourash W, Horlick M, Mitchell JE, Pomp A, Pories WJ, Purnell JQ, Singh A, Spaniolas K, Thirlby R, Wolfe BM, Yanovski SZ, 2018. Seven-year weight trajectories and health outcomes in the longitudinal assessment of bariatric surgery (LABS) study. JAMA Surg. 153, 427–434. doi: 10.1001/jamasurg.2017.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Jones SA, Nagel BJ, 2015. Reduced cerebellar brain activity during reward processing in adolescent binge drinkers. Dev. Cogn. Neurosci 16, 110–120. doi: 10.1016/j.dcn.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluchi M, Costa FS, Friedman R, Gonçalves R, Bizarro L, 2017. Attentional bias to unhealthy food in individuals with severe obesity and binge eating. Appetite 108, 471–476. doi: 10.1016/J.APPET.2016.11.012. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ, 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Durston S, Casey BJ, 2006. What have we learned about cognitive development from neuroimaging? Neuropsychologia 44, 2149–2157. doi: 10.1016/j.neuropsychologia.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H, 2016. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. U. S. A 113, 7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farruggia MC, van Kooten MJ, Perszyk EE, Burke MV, Scheinost D, Constable RT, Small DM, 2020. Identification of a brain fingerprint for overweight and obesity. Physiol. Behav 222. doi: 10.1016/j.physbeh.2020.112940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulconbridge LF, Ruparel K, Loughead J, Allison KC, Hesson LA, Fabricatore AN, Rochette A, Ritter S, Hopson RD, Sarwer DB, Williams NN, Geliebter A, Gur RC, Wadden TA, 2016. Changes in neural responsivity to highly palatable foods following roux-en-Y gastric bypass, sleeve gastrectomy, or weight stability: an fMRI study. Obesity 24, 1054–1060. doi: 10.1002/oby.21464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhardt AN, Yokum S, Orr PT, Stice E, Corbin WR, Brownell KD, 2011. Neural correlates of food addiction. Arch. Gen. Psychiatry 68, 808–816. doi: 10.1001/archgenpsychiatry.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha PY, Aschenbrenner K, Felsted J, O’Malley SS, Small DM, 2013. Altered hypothalamic response to food in smokers. Am. J. Clin. Nutr 97, 15–22. doi: 10.3945/ajcn.112.043307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi S, Weber SC, Graf G, Hinz D, Asarian L, Geary N, Leeners B, Hare TA, Tobler PN, 2020. Reduced neural satiety responses in women affected by obesity. Neuroscience 447. doi: 10.1016/j.neuroscience.2020.07.022. [DOI] [PubMed] [Google Scholar]
- Gorgolewski K, Burns CD, Madison C, Clark D, Halchenko YO, Waskom ML, Ghosh SS, 2011. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in Python. Front. Neuroinform 5. doi: 10.3389/fninf.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski KJ, Varoquaux G, Rivera G, Schwarz Y, Ghosh SS, Maumet C, Sochat VV, Nichols TE, Poldrack RA, Poline J-B, Yarkoni T, Margulies DS, 2015. NeuroVault.org: a web-based repository for collecting and sharing unthresholded statistical maps of the human brain. Front. Neuroinform 9, 8. doi: 10.3389/fninf.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaume B, Hua X, Thompson PM, Waldorp L, Nichols TE, 2014. Fast and accurate modeling of longitudinal and repeated measures neuroimaging data. Neuroimage 94, 287–302. doi: 10.1016/j.neuroimage.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa K, Ho T, Tercero F, Yunus T, Boone KB, 2011. Laparoscopic rouxen-Y gastric bypass: 10-year follow-up. Surg. Obes. Relat. Dis 7, 516–525. doi: 10.1016/j.soard.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Hodgson K, Poldrack RA, Curran JE, Knowles EE, Mathias S, Göring HHH, Yao N, Olvera RL, Fox PT, Almasy L, Duggirala R, Barch DM, Blangero J, Glahn DC, 2017. Shared genetic factors influence head motion during MRI and body mass index. Cereb. Cortex 27, 5539–5546. doi: 10.1093/cercor/bhw321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsen LM, Davidson P, Cerit H, Hye T, Moondra P, Haimovici F, Sogg S, Shikora S, Goldstein JM, Evins AE, Stoeckel LE, 2018. Neural predictors of 12-month weight loss outcomes following bariatric surgery. Int. J. Obes 42, 785–793. doi: 10.1038/ijo.2017.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh T, Zurita L, Grover H, Bennett A, Farrokhyar F, Gmora S, Anvari M, Hong D, 2014. 10-year outcomes of the vertical transected gastric bypass for obesity: a systematic review. Obes. Surg doi: 10.1007/s11695-013-1161-2. [DOI] [PubMed] [Google Scholar]
- Hull C., 2020. Prediction signals in the cerebellum: beyond supervised motor learning. Elife doi: 10.7554/eLife.54073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI, 2009. Circular analysis in systems neuroscience: the dangers of double dipping. Nat. Neurosci 12, 535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauti M, Kularatna M, Hill AG, MacCormick AD, 2016. Weight regain following sleeve gastrectomy-a systematic review. Obes. Surg doi: 10.1007/s11695-016-2152-x. [DOI] [PubMed] [Google Scholar]
- Li J, Lai D, Wu D, 2016. Laparoscopic roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy to treat morbid obesity-related comorbidities: a systematic review and meta-analysis. Obes. Surg doi: 10.1007/s11695-015-1996-9. [DOI] [PubMed] [Google Scholar]
- Livneh Y, Ramesh RN, Burgess CR, Levandowski KM, Madara JC, Fenselau H, Goldey GJ, Diaz VE, Jikomes N, Resch JM, Lowell BB, Andermann ML, 2017. Homeostatic circuits selectively gate food cue responses in insular cortex. Nature 546 (7660), 611–616. doi: 10.1038/nature22375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livneh Y, Sugden AU, Madara JC, Essner RA, Flores VI, Sugden LA, Resch JM, Lowell BB, Andermann ML, 2020. Estimation of current and future physiological states in insular cortex. Neuron 105 (6), 1094–1111. doi: 10.1016/j.neuron.2019.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond SH, Lovibond PF, 1995. Manual For the Depression Anxiety Stress Scales, 2nd ed. Psychology Foundation, Sydney. [Google Scholar]
- McTigue KM, Wellman R, Nauman E, Anau J, Coley RY, Odor A, Tice J, Coleman KJ, Courcoulas A, Pardee RE, Toh S, Janning CD, Williams N, Cook A, Sturtevant JL, Horgan C, Arterburn D, McBride CL, McClay J, Clark JM, Inge TH, Lent MR, Schlundt DG, Duke M, Smith SR, Odegaard AO, Desai NK, Tavakkoli A, Cirelli E, Xanthakos SA, Rasmussen-Torvik LJ, Michalsky MP, Daley MF, Purcell G, Murali S, Emiliano A, Kost RG, Apovian CM, Hess D, Blalock CA, Malanga E, Desai JR, Nadglowski J, Holmes JH, Vitello J, Horberg MA, Greenlee RT, Fitzpatrick SL, Zeiger R, Conroy MB, Bell DS, Ard J, Bian J, Chan B, Edwards MA, Wee C, Jones DB, Kraschnewski JL, Reichard K, Gordon HS, Meltzer DO, Roe ED, Richardson W, Malhotra S, Cowell LG, Bazzano LA, Brown JS, Cook AJ, 2020. Comparing the 5-year diabetes outcomes of sleeve gastrectomy and gastric bypass the national patient-centered clinical research network (PCORNet) bariatric study. JAMA Surg. 155. doi: 10.1001/jamasurg.2020.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel M, Vazquez-Sanroman D, Carbo-Gas M, Gil-Miravet I, Sanchis-Segura C, Carulli D, Manzo J, Coria-Avila GA, 2016. Have we been ignoring the elephant in the room? Seven arguments for considering the cerebellum as part of addiction circuitry. Neurosci. Biobehav. Rev doi: 10.1016/j.neubiorev.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Moreno-Rius J, Miquel M, 2017. The cerebellum in drug craving. Drug Alcohol. Depend doi: 10.1016/j.drugalcdep.2016.12.028. [DOI] [PubMed] [Google Scholar]
- Morys F, García-García I, Dagher A, 2020. Is obesity related to enhanced neural reactivity to visual food cues? A review and meta-analysis. Soc. Cogn. Affect. Neurosci doi: 10.1093/scan/nsaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton EA, Elman I, Becerra LR, Goldstein RZ, Borsook D, 2014. The cerebellum and addiction: insights gained from neuroimaging research. Addict. Biol 19, 317–331. doi: 10.1111/adb.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdaugh DL, Cox JE, Cook EW, Weller RE, 2012. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage 59, 2709–2721. doi: 10.1016/j.neuroimage.2011.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschelli J, Nebel MB, Caffo BS, Barber AD, Pekar JJ, Mostofsky SH, 2014. Reduction of motion-related artifacts in resting state fMRI using aCompCor. Neuroimage 96, 22–35. doi: 10.1016/j.neuroimage.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser H, Ivanics T, Carlin AM, 2020. Factors influencing the choice between laparoscopic sleeve gastrectomy and roux-en-Y gastric bypass. Surg. Endosc doi: 10.1007/s00464-020-07933-6. [DOI] [PubMed] [Google Scholar]
- Ness A, Bruce J, Bruce A, Aupperle R, Lepping R, Martin L, Hancock L, Patrician T, Malley S, Selim N, Savage CR, 2014. Pre-surgical cortical activation to food pictures is associated with weight loss following bariatric surgery. Surg. Obes. Relat. Dis 10, 1188–1195. doi: 10.1016/j.soard.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Ng J, Stice E, Yokum S, Bohon C, 2011. An fMRI study of obesity, food reward, and perceived caloric density does a low-fat label make food less appealing? Appetite 57, 65–72. doi: 10.1016/j.appet.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijs IMT, Franken IHA, 2012. Attentional processing of food cues in overweight and obese individuals. Curr. Obes. Rep 1, 106–113. doi: 10.1007/s13679-012-0011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaerrer GE, Stice E, Burger KS, 2018. Adolescents at high risk of obesity show greater striatal response to increased sugar content in milkshakes. Am. J. Clin. Nutr 107, 859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A, 2009. Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav. Brain Res 198, 149–158. doi: 10.1016/j.bbr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Small DM, Jones-Gotman M, Dagher A, 2003. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage 19, 1709–1715. doi: 10.1016/S1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- Smith S, Nichols T, 2009. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44, 83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM, 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 (Suppl 1), S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stice E, Burger KS, Yokum S, 2015. Reward region responsivity predicts future weight gain and moderating effects of the TaqIA allele. J. Neurosci 35, 10316–10324. doi: 10.1523/JNEUROSCI.3607-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Small DM, 2008a. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 322, 449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM, 2008b. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J. Abnorm. Psychol 117, 924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, 2018. Relation of neural response to palatable food tastes and images to future weight gain: using bootstrap sampling to examine replicability of neuroimaging findings. Neuroimage 183, 522–531. doi: 10.1016/j.neuroimage.2018.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger KS, Epstein LH, Small DM, 2011. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J. Neurosci 31, 4360–4366. doi: 10.1523/JNEUROSCI.6604-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Kroemer NB, Veldhuizen MG, Babbs AE, De Araujo IE, Gitelman DR, Sherwin RS, Sinha R, Small DM, 2015. Basolateral amygdala response to food cues in the absence of hunger is associated with weight gain susceptibility. J. Neurosci 35, 7964–7976. doi: 10.1523/JNEUROSCI.3884-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BO, Paul EJ, Miller MB, Barbey AK, 2018. Small sample sizes reduce the replicability of task-based fMRI studies. Commun. Biol 1, 1–10. doi: 10.1038/s42003-018-0073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijvenvoorde ACK, Peters S, Braams BR, Crone EA, 2016. What motivates adolescents? Neural responses to rewards and their influence on adolescents’ risk taking, learning, and cognitive control. Neurosci. Biobehav. Rev doi: 10.1016/j.neubiorev.2016.06.037. [DOI] [PubMed] [Google Scholar]
- Varoquaux G., 2018. Cross-validation failure: small sample sizes lead to large error bars. Neuroimage doi: 10.1016/j.neuroimage.2017.06.061. [DOI] [PubMed] [Google Scholar]
- Wagner MJ, Luo L, 2020. Neocortex-cerebellum circuits for cognitive processing. Trends Neurosci. doi: 10.1016/j.tins.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Douaud G, Nichols TE, Smith SM, 2016. Faster permutation inference in brain imaging. Neuroimage 141, 502–516. doi: 10.1016/j.neuroimage.2016.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE, 2014. Permutation inference for the general linear model. Neuroimage 92, 381–397. doi: 10.1016/J.NEUROIMAGE.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM, 2004. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage 21, 1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM, 2001. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage 14, 1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV, 2000. Cortical activation induced by intraoral stimulation with water in humans. Chem. Senses 25, 267–275. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Brady M, Smith S, 2001. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging 20, 45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Zoon HFA, de Bruijn SEM, Jager G, Smeets PAM, de Graaf C, Janssen IMC, Schijns W, Deden L, Boesveldt S, 2018a. Altered neural inhibition responses to food cues after Roux-en-Y Gastric Bypass. Biol. Psychol 137, 34–41. doi: 10.1016/J.BIOPSYCHO.2018.06.005. [DOI] [PubMed] [Google Scholar]
- Zoon HFA, de Bruijn SEM, Smeets PAM, de Graaf C, Janssen IMC, Schijns W, Aarts EO, Jager G, Boesveldt S, 2018b. Altered neural responsivity to food cues in relation to food preferences, but not appetite-related hormone concentrations after RYGB-surgery. Behav. Brain Res 353, 194–202. doi: 10.1016/j.bbr.2018.07.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not publicly available due to restrictions imposed by the administering institution and privacy of the participants. The authors will share them by request from any qualified investigator after completion of a data sharing agreement.