Abstract
Background:
Maternal exposure to weather-related extreme heat events (EHEs) has been associated with congenital heart defects (CHDs) in offspring. Certain medications may affect an individual’s physiologic responses to EHEs. We evaluated whether thermoregulation-related medications modified associations between maternal EHE exposure and CHDs.
Methods:
We linked geocoded residence data from the U.S. National Birth Defects Prevention Study, a population-based case-control study, to summertime EHE exposures. An EHE was defined using the 90th percentile of daily maximum temperature (EHE90) for each of six climate regions during postconceptional weeks 3–8. Adjusted odds ratios (aORs) and 95% confidence intervals (CIs) for associations between EHE90 and the risk of CHDs were estimated by strata of maternal thermoregulation-related medication use and climate region. Interaction effects were evaluated on multiplicative and additive scales.
Results:
Over 45% of participants reported thermoregulation-related medication use during the critical period of cardiogenesis. Overall, these medications did not significantly modify the association between EHEs and CHDs. Still, medications that alter central thermoregulation increased aORs (95% CI) of EHE90 from 0.73 (0.41, 1.30) among non-users to 5.09 (1.20, 21.67) among users in the Southwest region, U.S. This effect modification was statistically significant on the multiplicative (P = 0.03) and additive scales, with an interaction contrast ratio (95% CI) of 1.64 (0.26, 3.02).
Conclusion:
No significant interaction was found for the maternal use of thermoregulation-related medications with EHEs on CHDs in general, while medications altering central thermoregulation significantly modified the association between EHEs and CHDs in Southwest U.S. This finding deserves further research.
Keywords: Extreme heat events, Heart defects, Medication use during pregnancy, Thermoregulation, NBDPS
1. Introduction
Congenital heart defects (CHDs) are common birth defects worldwide. In the United States, CHD prevalence ranges from 0.4 to 1 per 100 liveborn infants, and 24% of all infant deaths due to birth defects are affected by CHDs (Benjamin et al., 2018). These defects greatly impair the quality of life among affected individuals and involve substantial medical expenditures for the family and society (Benjamin et al., 2018). For example, it was reported that hospitalization costs for individuals of all ages with CHDs exceeded $6.1 billion in 2013 (Benjamin et al., 2018; Arth et al., 2017). Previous literature has shown that non-inherited risk factors may contribute to the occurrence of CHDs, including certain maternal illnesses, medication use (Donofrio et al., 2014), and environmental exposures, such as ambient heat exposure during pregnancy (Lin et al., 2018).
As climate change has become an emergent issue in recent decades, extreme weather events are expected to become more frequent and longer in duration (Watts et al., 2021), which has the potential to increase CHDs (Zhang et al., 2019). Our previous research from the National Birth Defects Prevention Study (NBDPS) observed that, while the generally null results were found, 3–15 days of an extreme heat event (EHE) in some climate regions during the critical period for heart development was significantly associated with a higher occurrence of ventricular septal defects (Lin et al., 2018), consistent with previous studies by Agay-Shay et al. (2013) in Israel and Auger et al. (2017) in Quebec.
However, as individuals with different physiological characteristics may be predisposed to different risks of heat-related morbidity and mortality (Ebi et al., 2021), pregnant women are not affected by EHEs equally. Some risk factors that may increase women’s susceptibility to exposure to EHEs are outdoor activity; indoor air conditioning; older maternal age; pregnancy-related conditions; and use of certain medications (Laurent et al., 2018, Konkel, 2019, Macintyre et al., 2018; Hansen et al., 2008). These medications, notably diuretic, psychotropic, and anticholinergic medications, have been implicated in affecting physiologic thermoregulation and increasing the risk of heat-related illness or death (Dang et al., 2022) in the general population. For example, increased mortality has been observed among tranquilizer users during an EHE in the United States and France (Kilbourne et al., 1982; Ledrans et al., 2004). It is postulated that some prescription and over-the-counter (OTC) medications may affect hydration status, electrolyte balance, hemodynamics, thermoregulatory set point, or alertness, and also interfere with sweat production. This could further increase the effects of maternal exposure to EHEs on the risk of CHDs in offspring (Hajat et al., 2010; Westaway et al., 2015).
Few studies have examined whether and how maternal medication use may modify the association between EHE and CHDs. Further, no prior studies have assessed the health effects of specific medication classes in conjunction with temperature extremes and climate region differences. To fill these knowledge gaps, we used data from the NBDPS to evaluate whether maternal use of medications that may affect thermoregulation during pregnancy modified the association between summertime EHEs and the occurrence of CHDs in offspring. Because NBDPS study sites were located in several different climate regions (National Centers For Environmental Information, 2017), we also assessed whether there were regional differences in the modification effects of medication on the EHE-CHD relationship.
2. Methods
2.1. Study design and population
The NBDPS was a population-based case-control study in the United States that aimed to determine risk factors for major structural birth defects. An overview of the design and methodology of the NBDPS has previously been published (Reefhuis et al., 2015). We studied women whose pregnancies resulted in CHD cases and non-malformed livebirth controls during the period October 1, 1997, through December 31, 2007 (except for Utah and North Carolina with EDDs from 2003 to 2007) and whose addresses were geocoded (>97%) in eight NBDPS sites (not all catchment areas are statewide): Arkansas (AR, statewide), Texas (TX, selected counties), North Carolina (NC, selected counties), Georgia (GA, selected counties), New York (NY, selected counties), Utah (UT, statewide), California (CA, selected counties) and Iowa (IA, statewide) (Reefhuis et al., 2015). Case children were defined as those born alive, those born still, and those electively terminated from each site’s birth defects surveillance system. The clinical geneticists reviewed the abstracted medical information, including standardized definitions of defects and diagnostic procedures that should be performed to confirm the findings (Rasmussen et al., 2003). Control children were non-malformed liveborn children, randomly selected from either birth certificates or birth hospitals in the same catchment areas as the case children. These children, as well as those whose mothers did not speak English and/or Spanish and/or were incarcerated, were not eligible for the NBDPS.
The study participants were required to provide written informed consent before the study commenced. The study was conducted according to the principles of the Helsinki Declaration. Institutional Review Boards at the Centers for Disease Control and Prevention (CDC) and each study site approved the NBDPS.
2.2. Data collection
Data on demographics and meteorology have already been described (Lin et al., 2018). Participants were interviewed between six weeks and two years after their EDDs using computer-assisted telephone technology. Interview questions covered demographics, health conditions, medication use, pregnancy history, complications, and exposures at home and work.
Home addresses were collected from participants for at least one month between 3 months before and the end of pregnancy. National Centers for Atmospheric Research conducted summertime daily temperature measurements for each participating site. The CDC geocoded all maternal self-reported residences, linked them to the nearest weather monitoring station, and assigned temperature measurements from those stations to each participant. We computed data imputation using the average length of stay in one residence of participants reporting complete residential histories if they reported multiple residences but had missing dates during which they moved between residences (16.4% of the study population). Climate regions included in this analysis are as follows (National Centers for Environmental Information, 2017): South (Arkansas, Texas), Southeast (North Carolina, Georgia), Northeast (New York), Southwest (Utah), West (California), and Midwest (Iowa). Every state and region generally define summer as June, July, and August.
2.3. Exposure definition
The critical period for heart development was defined as post-conceptional weeks 3–8 in the summer season (Lin et al., 2018). The date of conception was defined with a hierarchical approach relying primarily on self-reported EDD, supplemented with clinical data, as necessary. Daily maximum temperature (Tmax) was used to define EHE indicators as at least three consecutive days with daily Tmax above the 90th percentile of the Tmax distribution for summer that year by station (EHE90). We created exposure indices as any EHE90 exposure during post-conceptional weeks 3–8 or none in summer.
2.4. Effect modification assessment
The NBDPS interview included questions regarding maternal medication use during pregnancy, including start and stop dates and duration and frequency of use. We defined thermoregulation-related medications as those that can affect the thermoregulatory response based on the mechanism by which medications may affect the thermoregulatory response (Hajat et al., 2010). These were examined as potential effect modifiers for the association between EHE and CHDs (Table 1). Specific medication classes were listed and classified into three grouping levels based on Hajat’s review of the potential ways certain medications can affect the risk of heat-associated illness (Hajat et al., 2010). The most specific category of medications was defined as Level 1 medications and included 21 individual medication classes. These individual medication classes were grouped into eight Level 2 categories based on the mechanism by which medications may affect the thermoregulatory response: changing heat perception; impacting renal function; causing vasoconstriction; shifting fluid balance; increasing metabolic heat production; decreasing heart rate or contractility; increasing dehydration; or altering central thermoregulation/decreasing cognitive alertness (subsequently referred to as “altering central thermoregulation”). Level 3 was a single category that comprised all thermoregulation-related medications. We considered women exposed to these medications if they reported use at least once at any point during the month before conception through the end of the first trimester (defined as the 90 days after the estimated date of conception), since women may be confused with the timing of pregnancy recognition compared to the timing of conception. Also, some medications taken the month before conception may have lingering effects extending into the 1st trimester of pregnancy.
Table 1.
Classification of thermoregulation-related medications used in the study participants.
| Medication class (Level 1) | Mode of action (Level 2) | Any thermoregulation-related medication (Level 3) |
| Antimigraine, Anticonvulsant, Non-steroidal anti-inflammatory drugs, Sedative | Change heat perception | |
| Angiotensin-converting enzyme inhibitors, Angiotensin receptor blockers, Non-steroidal anti-inflammatory drugs | Impact renal function | |
| Sympathomimetics, Antihistamines | Cause vasoconstriction | |
| Laxatives | Shift the fluid balance | |
| Sympathomimetics | Increase metabolic heat production | |
| Anticholinergics, Anticonvulsants, Antidepressants, Antipsychotics (excluding antidepressants), Sedatives | Alter central thermoregulation | |
| β blockers, Calcium channel blockers | Decrease heart rate or contractility | |
| Angiotensin-converting enzyme inhibitors, Diuretics | Cause dehydration |
In the sensitivity analysis to address the issue of taking medication as needed (PRN), we coded mothers who reported non-steroidal anti-inflammatory drugs (NSAIDs) PRN as no medication use since NSAIDs are more likely to be taken this way compared to other studied medications.
Furthermore, to better connect the window of exposure for medications to the window of exposure to EHE (gestational 3–8 weeks), we coded mothers taking medication if they reported use at least once at any point during the month before conception through the end of the 2nd month of pregnancy (8 weeks after conception).
2.5. Confounders and statistical analysis
We selected the following confounders based on existing literature and a directed acyclic graph (DAG): maternal age at delivery (continuous), race/ethnicity (dichotomized as non-Hispanic white, other due to small numbers in some regions); and education at delivery (<12, ≥12 years). We estimated adjusted odds ratios (aORs) and 95% confidence intervals (CIs) for the association between EHE90 and CHDs by strata of maternal thermoregulation-related medication use (yes/no) with unconditional logistic regression, adjusting for the confounders described above. All estimates were further stratified by climate region. We evaluated the modification effects of medication use, by level, on the association between EHE90 and all CHDs examined or CHD subtype grouping (septal defects, conotruncal heart defects, atrioventricular septal defects, anomalous pulmonary venous return, and left or right outflow tract obstruction defects) on both multiplicative and additive scales. The multiplicative scale was calculated by evaluating the product term for EHE90 and medications included in each category. The statistical significance of the product term was determined by using the Wald statistic. We assessed the interaction contrast ratios (ICR) for the additive scale. The computation for ICR was: ICR = OR11-OR10-OR01 + 1, where OR11 denotes co-exposure (EHE90 and indicated medication), OR10 or OR01 denotes the presence of one exposure (EHE90 or indicated medication), and the absence of the other (Knol et al., 2011). An ICR above 0 indicates a super-additive effect, whereas an ICR below 0 indicates a sub-additive effect. Confidence intervals (95%) for ICRs were computed by the multivariate delta method: ICR ± 1.96√ var(ICR) (Nie et al., 2010).
We analyzed each CHD subtype separately, as permitted by sample size, to reduce etiologic heterogeneity among CHD cases. We only calculated the modification effect of the Level 2 and Level 3 medication groups since stratified analysis by the Level 1 medication made the sample sizes very small, and many ORs could not be calculated. Similarly, analyses for more specific CHD subtypes, such as ventricular septal and atrial septal defects, were not performed due to very small sample sizes. Statistical tests were two-sided with a significance level of P < 0.05. Data were analyzed using SAS (v.9.4; SAS Institute, Inc., Cary, NC).
Reruns of the final models were performed to examine the impact of residual confounding or uncontrolled confounders on our findings: 1) including EDD year as a variable in regression models, allowing the potential impact of year to be taken into account; 2) exclusion of mothers with pre-gestational diabetes (n = 84), families with a history of CHDs (n = 110), and multiple births (n = 190); 3) using the Bayesian analysis approach (Greenland 2006; Greenland, 2007) to address the multiple comparison concerns; 4) addressing the issue of taking PRN, by coding mothers who reported NSAIDs PRN as no medication use (n = 110); 5) conducting two sub-analyses, one restricted to participants with at least half of the critical period for heart development during the summer season (21 days, n = 1473) and a second restricted to participants with the whole critical period for heart development during the summer season (42 days, n = 780).
3. Results
3.1. Characteristics of participants and climate region
We identified 5848 CHD cases and 5742 controls with geocoded residence data from the NBDPS. Among them, 2070 cases and 2109 controls had at least one day of the critical period for heart development during the summer season. The maternal characteristics of the 2109 controls were similar to all NBDPS controls (Supplemental Table 1). The characteristics of the participants by case and control child status were described previously (Lin et al., 2018). Briefly, compared to control children, case children had a lower proportion with maternal age at delivery <19 years old (9.39% vs. 10.90% in controls), a higher proportion with maternal education at delivery <12 years (44.89% vs. 42.81%), more with maternal pre-pregnancy body mass index (BMI) >30 kg/m2 (22.45% vs. 18.20%), reported greater maternal caffeine use pre-pregnancy during the critical period for heart development (45.50% vs. 43.33%) and had a higher proportion with a family history of CHDs (3.73% vs. 1.18%). In addition, a higher proportion of case children were male (53.88% vs. 51.16%). The temperature mean and range varied widely across different states/regions (Supplemental Table 2). The South region (Arkansas, Texas) had the highest mean EHE90 (36.2–37.2 °C) thresholds, followed by the Southwest region (Utah, 36.7 °C) and the Southeast region (North Carolina, 34.1 °C; Georgia, 35.5 °C), whereas the Northeast region (New York) had the lowest mean EHE90 (30.6 °C) threshold.
3.2. Maternal medication use
As shown in Table 2, among the study participants, 48.8% of case and 45.3% of control mothers reported taking any thermoregulation-related medication (medication Level 3) from the month before pregnancy to the end of the first trimester. Level 1 medications with more than 50 mothers reporting use included NSAIDs, laxatives, antihistamines, sympathomimetics, and antidepressants. Among NSAID users, 19.5% took PRN. Use by the level of medication and region is reported in Supplemental Tables 3-5. In summary, case participants in the Midwest had the highest proportion of medication exposure (55.1%), while the West had the lowest (38.2%).
Table 2.
Frequency of thermoregulation-related medication usea during the period from the month before pregnancy through the end of the 1st trimester among participants who had at least one gestational day during 3–8 weeks in summer in eight states, NBDPS, 1997–2007.
| Medicationb | Case (n = 2070) n (%) |
Control (n = 2109) n (%) | |
|---|---|---|---|
| Level 1 Medication Categories | Level 2 Categories c |
||
| 1. Non-steroidal anti-inflammatory | 563 (27.7) | 520 (25.0) | 1, 2 |
| 2. Laxatives | 256 (12.4) | 276 (13.1) | 4 |
| 3. Antihistamines | 241 (11.7) | 227 (10.8) | 3 |
| 4. Sympathomimetics | 189 (9.1) | 199 (9.4) | 3, 5 |
| 5. Antidepressants | 115 (5.6) | 81 (3.8) | 6 |
| 6. Sedatives | 31 (1.5) | 23 (1.1) | 1, 6 |
| 7. Anticonvulsants | 27 (1.3) | 16 (0.8) | 1, 6 |
| 8. β blockers | 16 (0.8) | 5 (0.2) | 7 |
| 9. Centrally-acting antiadrenergics | 16 (0.8) | 9 (0.4) | NA |
| 10. Diuretics | 12 (0.6) | 3 (0.1) | 8 |
| 11. Antipsychotics (excluding antidepressants) | 9 (0.4) | 6 (0.3) | 6 |
| 12. Anticholinergics | 8 (0.4) | 6 (0.3) | 6 |
| 13. Angiotensin-converting enzyme inhibitors | 7 (0.3) | 4 (0.2) | 2, 8 |
| 14. Calcium channel blockers | 6 (0.3) | 0 | 7 |
| 15. Antimigraine | 6 (0.3) | 9 (0.4) | 1 |
| 16. Antihypertensives, not otherwise specified | 4 (0.2) | & | NA |
| 17. Angiotensin receptor blocker | & | & | 2 |
| Level 2 Medication Categories | |||
| 1.Medications that may change heat perception | 589 (28.5) | 539 (25.6) | |
| 2. Medications that may impact renal function | 568 (27.4) | 524 (24.9) | |
| 3. Medications that may cause vasoconstriction | 352 (17.0) | 354 (16.8) | |
| 4.Medications that may shift fluid balance | 256 (12.4) | 276 (13.1) | |
| 5.Medications that may increase metabolic heat production | 189 (9.1) | 199 (9.4) | |
| 6.Medications that may alter central thermoregulation | 158 (7.6) | 115 (5.5) | |
| 7.Medications that may decrease heart rate or contractility | 22 (1.1) | 5 (0.2) | |
| 8.Medications that may lead to dehydration | 19 (0.9) | 6 (0.3) | |
| Level 3 Medication Category | |||
| Any thermoregulation-related medication (all Level 1 medications) | 1010 (48.8) | 956 (45.3) | |
Abbreviation: NBDPS, National Birth Defects Prevention Study. &, number less than 3 was suppressed to prevent inadvertent disclosure of participant identities.
Exposure to medication was defined as self-reported use at least once at any point during the month before conception through the end of the first trimester.
Not mutually exclusive categories.
The Arabic number in this column indicated the level 2 Category of medication as follows: 1, medications that may change heat perception; 2, medications that may impact renal function; 3, medications that may cause vasoconstriction; 4, medications that may shift fluid balance; 5, medications that may increase metabolic heat production; 6, medications that may alter central thermoregulation; 7, medications that may decrease heart rate or contractility; and 8, medications that may lead to dehydration.
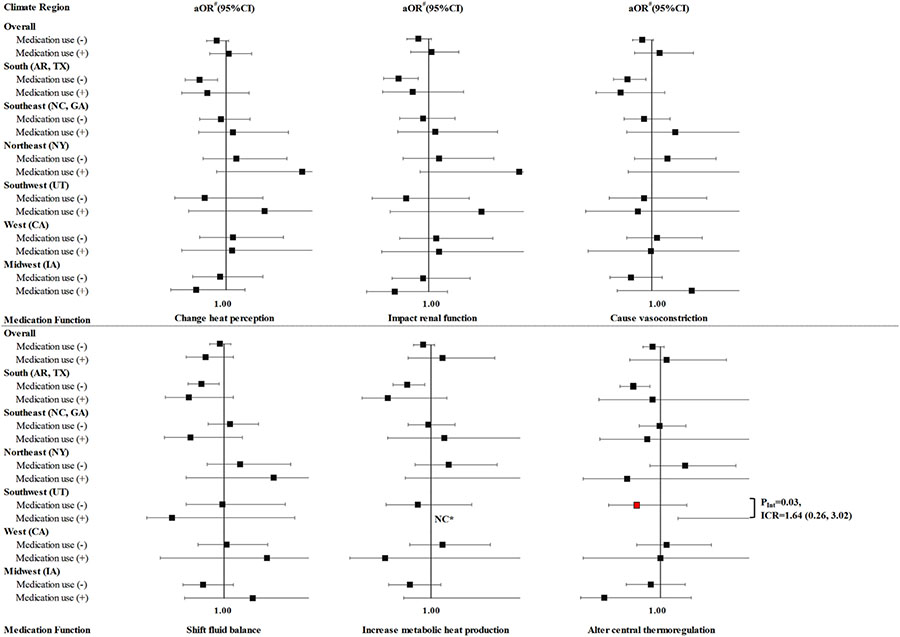
3.3. Modification of maternal medication use on the effect of EHE
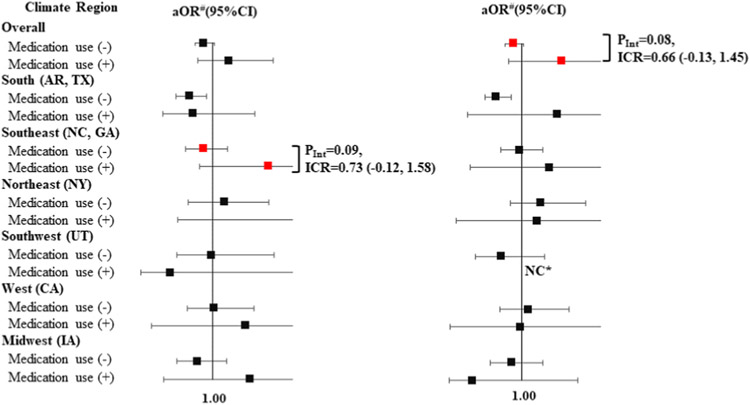
Overall, thermoregulation-related medication use did not modify the association between EHE90 and total CHDs (Table 3). For Level 2 medications (Fig. 1 & Supplemental Table 6), the use of medications altering central thermoregulation increased the aOR (95% CI) of EHE90 from 0.73 (0.41, 1.30) among non-users to 5.09 (1.20, 21.67) among the users in the Southwest. This effect modification was statistically significant on the multiplicative (P = 0.03) and additive scales [ICR (95% CI):1.64 (0.26, 3.02)]. Medications altering central thermoregulation also showed a non-significant super-additivity of the aOR of EHE90 for total CHDs in the South region (Arkansas, Texas) [ICR = 0.38] (Supplemental Table 6). A similar pattern was not observed in the other climate regions. For Level 1 medications (Fig. 2 & Supplemental Table 7), there was some evidence of super-additivity of antihistamines in the Southeast regions with an ICR of 0.73, but the result was borderline significant. We also observed that the association between EHEs and total CHDs changed by strata of antidepressant use overall, changing from aOR (95% CI) 0.90 (0.79,1.02) among non-users to 1.50 (0.84, 2.68) among users [multiplicative scale (P = 0.08), ICR (95%): 0.66 (−0.13, 1.45)].
Table 3.
Effect modification of maternal use of any thermoregulation-related (Level 3) medicationsa during the period from the month before pregnancy through the end of the 1st trimester on the association between a summertime EHE90 in the residential area during the maternal critical period and CHDs among offspring in eight states, NBDPS, 1997–2007.
| Climate Region | No medication use |
Medication use reported |
Multiplicative scale |
Additive scale |
||
|---|---|---|---|---|---|---|
| N of Cases exp/unexp | aOR# (95% CI) | N of Cases exp/unexp | aORb (95% CI) | P value | ICR | |
| Overall | 604/456 | 0.92 (0.77, 1.09) | 586/424 | 0.92 (0.77, 1.11) | 0.97 | −0.02 (−0.28, 0.25) |
| South (AR, TX) | 160/176 | 0.70 (0.51, 0.97) | 148/151 | 0.69 (0.49, 0.98) | 0.98 | −0.02 (−0.45, 0.41) |
| Southeast (NC, GA) | 104/116 | 0.92 (0.63, 1.33) | 126/122 | 1.03 (0.72, 1.48) | 0.63 | 0.13 (−0.40, 0.66) |
| Northeast (NY) | 46/64 | 1.09 (0.66, 1.80) | 59/50 | 1.39 (0.81, 2.41) | 0.66 | 0.27 (−0.64, 1.18) |
| Southwest (UT) | 90/17 | 0.58 (0.25, 1.33) | 98/20 | 1.26 (0.63, 2.55) | 0.18 | 0.59 (−0.05, 1.23) |
| West (CA) | 153/38 | 1.17 (0.72, 1.90) | 91/27 | 0.88 (0.47, 1.67) | 0.45 | −0.37 (−1.50, 0.76) |
| Midwest (IA) | 51/45 | 0.95 (0.56, 1.62) | 64/54 | 0.73 (0.46, 1.18) | 0.35 | −0.35 (−1.17, 0.46) |
Abbreviation: aOR, adjusted odds ratio; AR, Arkansas; CA, California; CHD, congenital heart defects; CI, confidence interval; EHE, extreme heat event; EHE90, EHE was defined using the 90th percentile of daily maximum temperature for each study region during postconceptional weeks 3–8; GA, Georgia; IA, Iowa; ICR, interaction contrast ratio; NBDPS, National Birth Defects Prevention Study; NC, North Carolina; NY, New York; TX, Texas; UT, Utah.
Exposure to medication was defined as self-reported use at least once at any point during the month before conception through the end of the first trimester.
Reference group was participants unexposed to both EHE90 and medication use; adjusted for maternal age (continuous), maternal race/ethnicity, and maternal education level.
Fig. 1. Stratified aORs of the association between a summertime EHE90 in the residential area during the maternal critical period and CHDs among offspring, by maternal use of thermoregulation-related medication, by mechanism (Level 2) in eight states during the period from the month before pregnancy through the end of the 1st trimester, NBDPS, 1997–2007.
Exposure to medication was defined as self-reported use at least once during the month before conception through the end of the first trimester. # Referred to the participants unexposed to EHE90 and adjusted for maternal age (continuous), maternal race/ethnicity, and maternal education level. NC* indicated estimates for case groups with <3 exposed cases were not calculated. PInt indicated P value for EHE90*medication use. Abbreviationsa OR, adjusted odds ratio; AR, Arkansas; CA, California; CHD, congenital heart defects; CI, confidence interval; EHE, extreme heat event; EHE90, EHE was defined using the 90th percentile of daily maximum temperature for each study region during postconceptional weeks 3–8; GA, Georgia; IA, Iowa; ICR, interaction contrast ratio; NBDPS, National Birth Defects Prevention Study; NC, North Carolina; NY, New York; TX, Texas; UT, Utah.
Fig. 2. Stratified aORs of the association between a summertime EHE90 in the residential area during the maternal critical period and CHDs among offspring, by maternal use of antihistamines and antidepressants (Level 1 medications) in eight states, during the period from the month before pregnancy through the end of the 1st trimester, NBDPS, 1997–2007.
Exposure to medication was defined as self-reported use at least once during the month before conception through the end of the first trimester. # Referred to the participants unexposed to EHE90 and adjusted for maternal age (continuous), maternal race/ethnicity, and maternal education level. NC* indicated estimates for case groups with <3 exposed cases were not calculated. PInt indicated P value for EHE90*medication use.
Most of the results were null concerning the modification effect of Level 2 and Level 3 medications on CHD subtypes (data not shown). However, as shown in Table 4, the use of any thermoregulation-related medication increased the aOR (95% CI) of EHE90 from 0.21 (0.07, 0.59) among non-users to 3.12 (0.81, 11.97) among users for septal defects in the Southwest, and from 0.33 (0.09, 1.21) among non-users to 2.92 (1.03, 8.28) among users for right outflow tract defects in the Northeast. These effect modifications were statistically significant on the multiplicative (P = 0.003, and P = 0.01, respectively) and additive scales [ICR (95%CI):1.12 (0.26, 3.02), and 1.89 (0.54, 3.25), respectively].
Table 4.
aEffect modification of maternal use of any thermoregulation-related (Level 3) medicationsa during the period from the month before pregnancy through the end of the 1st trimester on the association between a summertime EHE90 in the residential area during the maternal critical period and septal defects or RVOTO among offspring in Southwest and Northeast, respectively, NBDPS, 1997–2007.
| CHD Subtype & Climate Region | No medication use |
Medication use reported |
Multiplicative scale |
Additive Scale |
||
|---|---|---|---|---|---|---|
| N of Cases exp/unexp | aORb (95% CI) | N of Cases exp/unexp | aORb (95% CI) | P value | ICR | |
| Septal defects in Southwest (UT) | 23/10 | 0.21 (0.07, 0.59) | 34/3 | 3.12 (0.81, 11.97) | 0.003 | 1.12 (0.76, 1.49) |
| RVOTO in Northeast (NY) | 3/15 | 0.33 (0.09, 1.21) | 15/6 | 2.92 (1.03, 8.28) | 0.011 | 1.89 (0.54, 3.25) |
Abbreviation: aOR, adjusted odds ratio; CI, confidence interval; EHE, extreme heat event; EHE90, EHE was defined using the 90th percentile of daily maximum temperature for each study regi1on during postconceptional weeks 3–8; ICR, interaction contrast ratio; NBDPS, National Birth Defects Prevention Study; NY, New York; RVOTO, right ventricular outflow tract obstruction; UT, Utah.
Exposure to medication was defined as self-reported use at least once at any point during the month before conception through the end of the first trimester.
Reference group was participants unexposed to EHE90; adjusted for maternal age (continuous), maternal race/ethnicity, and maternal education level.
3.4. Sensitivity analyses
To further investigate the positive results in the Southwest region, we ran multiple sensitivity analyses to evaluate the modification by medications that alter central thermoregulation for the association between EHE90 and CHDs. This was limited to total CHDs to have sufficient case numbers. To estimate the potential impact of year, we added the variable of EDD year into the models and found that the results’ magnitudes and statistical significances, both overall and in the Southwest region, remained similar to the original ones (data not shown). In the multiple sensitivity analyses (data not shown), we first excluded mothers with pre-gestational diabetes, family history of CHD, or multiple births, and reran the models; we also found similar results. Moreover, to estimate the potential impact of multiple comparisons, we used Bayesian analysis and observed similar findings. The ranges of ORs by medication use before and after the multiple comparisons correction were basically the same.
To address the issue of reporting medication use as PRN, interaction on multiplicative and additive scales were calculated for the medication groups that contained NSAIDs, such as any heat-related medication, medications that affect heat perception, medications that impact renal function, and NSAIDs by themselves, after coding mothers who reported NSAIDs PRN as no medication use. These results were similar to the original (data not shown).
Finally, when we restricted the analysis to participants having at least 21 days of the critical period for heart development during the summer season, we observed the interaction of medications that alter central thermoregulation, and EHE90 remained statistically significant on the multiplicative scale (P = 0.05), and with borderline significance on the additive scale [ICR (95%CI):1.51 (−0.01, 3.03)] in Southwest. However, due to the small sample size, most results were null or could not be calculated when using the definition of having the entire critical period for heart development during the summer season.
To address the issue of the exposure window for medications and EHE, we reran all analyses by excluding the medication use during the 3rd month of pregnancy from the medication exposure. All results were shown to be similar to the original. Comparing the medication exposed number in Table 2 and Supplemental Table 8, we found that 5 (among 115) case participants and 3 (among 81) control participants took antidepressants, and 7 (among 158) case participants and 9 (among 115) control participants took medications that may alter central thermoregulation, only in the third month of pregnancy. The modification effect of medications altering central thermoregulation remained statistically significant with the same estimates as the main analysis. As in the previous analysis, the use of any thermoregulation-related medication modified the EHE90 effect on septal defects in the Southwest, and right outflow tract defects in the Northeast, with statistical significance on the multiplicative (P = 0.01, and P = 0.03, respectively), and additive scales [ICR (95%CI): 1.05 (0.70, 1.40), and 1.58 (0.24, 2.92), respectively].
4. Discussion
4.1. Main finding
We observed that using thermoregulation-related medications during the critical period for heart development is common (almost 50% of study participants) among pregnant women in the NBDPS. In addition, we found no significant interaction for the maternal use of thermoregulation-related medications with EHEs on CHDs overall, while medications that alter central thermoregulation may increase the strength of the effect of EHEs on CHDs in the Southwest with statistical significance on the multiplicative and additive scale.
4.2. Thermoregulation-related medication use among pregnant women
In our study population, thermoregulation-related medications were commonly used during the period from the month before pregnancy through the end of the 1st trimester. Among the medications surveyed in this study, NSAIDs were the most prevalent (>25%), which is consistent with the prevalence rate of 21.9% for ibuprofen within the first trimester of the enrolled mothers reported by prior studies (Thorpe et al., 2013). However, 19.5% of NSAID users took it PRN. According to the literature, antihistamines are also commonly reported in pregnancy, and their use has increased in recent years (Thorpe et al., 2013), which was evident in the prevalence of antihistamine use of 11.2% in the current study. Meanwhile, the prevalence of antidepressant use was 5.6% in case and 3.8% in control participants in the current study. Consistently, the prevalence of selective serotonin reuptake inhibitors (SSRIs) in the NBDPS has also increased over time, as reported previously (from 2.3% in 2009 to 5.8% in 2011 among pregnant controls) (Dawson et al., 2015).
4.3. Effect modification of medication on the association between EHEs and CHDs
We observed that medications that alter central thermoregulation may increase the effect of EHEs on CHDs in the Southwest region (Utah), but a similar modification effect was not found in general. Physiologic effects of medications on thermoregulation and reports that thermoregulation-related medications may increase the risk of heat-related mortality or adverse health outcomes justify concern that the combined effects of EHEs and thermoregulation-related medications may adversely affect pregnant women and their fetuses (Gessel and Lin, 2020; Sagy et al., 2016; Zhang et al., 2016). However, current research on the interaction between heat and medication is limited. No existing studies evaluate this interaction concerning its effect on birth outcomes or CHDs.
4.4. Geographic differences
The interaction between medications that alter central thermoregulation, primarily antidepressants, and EHE90 on CHDs was only observed in the Southwest (Utah) but not in other regions included in this study. While this finding may be due to chance, the differences in local micro-environments, climate and weather patterns, and socio-demographic profiles may modify the EHE-CHDs relationship for this region. One notable observation is that the women in the Southwest region of our study reported the highest prevalence of using medications that alter central thermoregulation during the period from the month before pregnancy through the end of the 1st trimester (15.6% in the cases, Supplemental Table 4), twice the proportion of women reporting these medications in other NBDPS regions. The low proportion of women using medications that alter central thermoregulation in the other regions may have limited our ability to detect interaction effects. Furthermore, the comparison of the maternal characteristics by region (Supplemental Table 9) showed that the participants in the Southwest region had the lowest proportion of black non-Hispanics, the highest proportion of family history of CHDs, and high parity (>2) compared to mothers in other regions. While we cannot interpret our findings based on the limited data, we postulated that these differences in maternal characteristics might also be related to physical activity patterns, dietary habits, and unknown confounding factors which may interact with or affect maternal heat exposure or medication metabolism and accumulation in the body.
4.5. Biological mechanism
Biological mechanisms for the modification of maternal medication use on the association between EHEs and CHDs are unknown. Pharmacokinetics and heat intolerance are possible biological pathways. The effects of certain medications on physiological or behavioral thermoregulation can lead to increased heat strain. For instance, there is a well-established association between antipsychotics and heat intolerance and heat stroke (Bhanushali and Tuite, 2004; Reilly and Kirk, 2007). Such medications as serotonin, dopamine, and noradrenaline could suppress the anterior hypothalamus’s thermoregulatory center, resulting in sweating capacity loss. Meanwhile, these medications increase cutaneous vasoconstriction, reducing convection and radiation-mediated heat dissipation (Lee et al., 2015; Reilly and Kirk, 2007; Stadnyk and Glezos, 1983; and Cusack et al., 2011). Specifically, for antidepressants, the mechanism for effect modification is complicated. Antidepressants with anticholinergic effects, like tricyclic antidepressants (TCAs), can alter central thermoregulation. TCAs, serotonin, noradrenaline reuptake inhibitors (SNRIs), and SSRIs impair sweating. Additionally, these medications cause sedation and cognitive impairment, which could reduce alertness, judgment, and perception of hot weather. TCAs could also cause hypotension and reduced cardiac output, whereas SSRIs and venlafaxine are commonly associated with hyponatremia (Westaway et al., 2015).
4.6. Strengths and limitations
To our knowledge, this is the first study assessing the interaction between maternal medication use and high ambient temperatures on the occurrence of CHDs. The study used data from the NBDPS that included diverse populations from eight U.S. states, with substantial climate variation over a 10-year period. These nationwide-representative data also enabled us to evaluate the effects of thermoregulation-related medications used by pregnant women in the United States. Centralized geocoding increased the consistency of the data across NBDPS sites and improved the quality control of the geocoded data. Furthermore, a clinical case review of abstracted medical record information was done systematically by trained clinical geneticists using standardized diagnostic criteria (Soim et al., 2018), and detailed information was collected through the questionnaire for medication use and potential confounders.
A major concern in this case-control study is recall bias which may cause inaccurate measurement of medication use and potential confounding variables since data were collected up to 2 years after EDDs. To minimize recall bias, several strategies were used, such as asking for specific diseases or medication names, timing, and duration of use, and using a pregnancy calendar. In addition, a list of specific responses was provided for most interview questions. Meanwhile, data from existing and objective sources were used to determine the primary exposure (extreme heat) and the outcome (CHDs). Due to this, it appears that differential recall between the cases and controls is null.
Another concern is the different time windows used for medications (monthly) versus EHE (daily), i.e., monthly medication use could not be verified on the same day as the EHE, which makes interpreting the interaction effects difficult. However, most medications studied, except NSAIDs, laxatives, and antihistamines, are used to treat chronic conditions and would likely have been used continuously throughout the first trimester. Although up to 19.5% of NSAID users reported PRN use, reanalysis of the medication groups that included NSAIDs, counting PRN use as nonexposed, remained similar to the original. Moreover, sensitivity analysis, including only participants who had at least half of the critical period for heart development during the summer season, found similar results. In addition, as we considered women may be confused with the timing of pregnancy recognition, the actual time of medication use, and some medications having lingering effects; exposure to medications was defined using the time window during the month before conception through the end of the first trimester. We also conducted a sensitivity analysis to exclude the third month of pregnancy from the time window. The results using different time windows for medication exposure remained very consistent.
Furthermore, we cannot separate the effects of medication from the risks associated with the underlying disease as these two factors are highly correlated. Additionally, the effects of other potential confounders such as occupational exposures, activity patterns and hydration were not evaluated in this study.
Another limitation is our inability to assess the modifying effect of medications that alter central thermoregulation by CHD subtype or for certain medications due to inadequate sample sizes, although it is the largest population-based study for birth defects or CHDs in the U.S. However, any thermoregulation-related medication significantly modifies the association between EHEs and septal defects in Southwest (UT) and right outflow tract defects in the Northeast (NY). It is worth noting that we observed borderline statistical significance with synergic effects for medications altering central thermoregulation in the South region (Arkansas, Texas), antihistamines in Southeast (NC, GA), and antidepressants in overall regions. Future larger studies or pooled analyses by merging different study population may help increase the study power, and detect the interaction effects of these medications with heat exposure on CHD more easily.
Lacking information on maternal gene variation was also a limitation because participants may metabolize medications differently due to gene variation that may impact the effect of these medications. Additional analyses of these potential effect modifications in large study populations and assessing gene variation related to medication metabolism are needed to address these limitations in the future. The fact that our main positive results arose from just one site and no other areas in the study brings into question the generalizability. Finally, we are concerned that our positive results in Utah may be a chance finding. However, we rechecked all results using Bayesian analysis, and found the previously positive estimates remained significant, which lessens but does not totally eliminate such concern.
5. Conclusions
We observed that use of thermoregulation-related medications is common among pregnant women. No significant interaction between the maternal use of these medications and EHEs on CHDs was found overall, while an increased risk of EHE-associated risk of CHD was observed for medications altering central thermoregulation in the Southwest region of the United States (Utah). Additional large studies would be needed to further validate the effect of medication use on the association between EHE and CHDs in different geographic regions and CHD subtypes.
Supplementary Material
Acknowledgments
This project was supported through Centers for Disease Control and Prevention (CDC) cooperative agreements under PA #96043, PA #02081, FOA #DD09-001, FOA #DD13-003, and NOFO #DD18-001 to the Centers for Birth Defects Research and Prevention participating in the National Birth Defects Prevention Study (NBDPS) and/or the Birth Defects Study To Evaluate Pregnancy exposures (BD-STEPS). Coding of drug information in the National Birth Defects Prevention Study used the Slone Drug Dictionary under license from the Slone Epidemiology Center of Boston University.
The authors thank the support from the NBDPS and all participants of the NBDPS. In addition, we thank Dr. David Savitz for his advice on study design, Dr. Wan-Hsiang’s support on data management and imputation, Dr. Syni-An Hwang, Dr. Edward Fitzgerald, Dr. Tom Luben, and Dr. Gary M. Shaw’s guidance and support on project implementation, Dr. Alison Woomert’s help on data access, and the California Department of Public Health Maternal Child and Adolescent Health Division for providing data for these analyses.
Funding
This study was supported by ES021359 from the NIH, United States and U38EH000184-05 from the Centers for Disease Control and Prevention, United States;81903287 from the National Natural Science Foundation, China and 2020B1111170011 from the Department of Science and Technology of Guangdong Province, China. The funders of this study had no role in the study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the manuscript for publication.
Footnotes
CRediT author statement
Yanqiu Ou: Conceptualization, Writing-Original draft preparation. Eleni A. Papadopoulos: Methodology, Software, Reviewing and Editing. Sarah C. Fisher, Marilyn L. Browne: Data curation, Supervision, Reviewing and Editing. Ziqiang Lin, Aida Soim, Yi Lu, Scott Sheridan, Peter H. Langlois, Paul A. Romitti, Erin M. Bell, Marcia L. Feldkamp, and Sadia Malik: Reviewing and Editing. Jennita Reefhuis: Validation, Reviewing and Editing. Shao Lin: Conceptualization, Supervision, Reviewing and Editing.
Disclaimer
The views expressed in this manuscript are those of the authors and do not necessarily represent the views or policies or the official position of the Centers for Disease Control and Prevention, the U.S. Environmental Protection Agency, nor the California Department of Public Health.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2022.114217.
Data availability
Data will be made available on request.
References
- Agay-Shay K, Friger M, Linn S, Peled A, Amitai Y, Peretz C, 2013. Ambient temperature and congenital heart defects. Hum. Reprod 28 (8), 2289–2297. 10.1093/humrep/det244. [DOI] [PubMed] [Google Scholar]
- Auger N, Fraser WD, Sauve R, Bilodeau-Bertrand M, Kosatsky T, 2017. Risk of congenital heart defects after ambient heat exposure early in pregnancy. Environ. Health Perspect 125 (1), 8–14. 10.1289/EHP171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arth AC, Tinker SC, Simeone RM, Ailes EC, Cragan JD, Grosse SD, 2017. Inpatient hospitalization costs associated with birth defects among persons of all ages - United States, 2013. MMWR Morb. Mortal. Wkly. Rep 66 (2), 41–46. 10.15585/mmwr.mm6602a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanushali MJ, Tuite PJ, 2004. The evaluation and management of patients with neuroleptic malignant syndrome. Neurol. Clin 22 (2), 389–411. 10.1016/j.ncl.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, et al. , 2018. Heart disease and stroke statistics-2018 update: a report from the American heart association. Circulation 137 (12), e67–e492. 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- Cusack L, Crespigny CD, Athanasos P, 2011. Heatwaves and their impact on people with alcohol, drug and mental health conditions: a discussion paper on clinical practice considerations. J. Adv. Nurs 67 (4), 915–922. 10.1111/j.1365-2648.2010.05551.x. [DOI] [PubMed] [Google Scholar]
- Dang TN, Vy NTT, Thuong DTH, Phung D, Van Dung D, Le An P, 2022. Main and added effects of heatwaves on hospitalizations for mental and behavioral disorders in a tropical megacity of Vietnam. Environ. Sci. Pollut. Res. Int 10.1007/s11356-022-19898-1. [DOI] [PubMed] [Google Scholar]
- Donofrio MT, Moon-Grady AJ, Hornberger LK, Copel JA, Sklansky MS, Abuhamad A, Cuneo BF, Huhta JC, Jonas RA, Krishnan A, et al. , 2014. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation 129 (21), 2183–2242. 10.1161/01.cir.0000437597.44550.5d. [DOI] [PubMed] [Google Scholar]
- Dawson AL, Razzaghi H, Arth A, Canfield MA, Parker SE, Parker J, 2015. National birth defects prevention study. Maternal exposures in the national birth defects prevention study: time trends of selected exposures. Birth Defects Res. A Clin. Mol. Teratol 103 (8), 703–712. 10.1002/bdra.23377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebi KL, Capon A, Berry P, Broderick C, de Dear R, Havenith G, Honda Y, Kovats RS, Ma W, Malik A, et al. , 2021. Hot Weather and Heat Extremes: Health Risks. The Lancet, vol. 398, pp. 698–708. 10.1016/s0140-6736(21)01208-3, 10301. [DOI] [PubMed] [Google Scholar]
- Gessel T, Lin CY, 2020. Exertional heatstroke in a marathon runner complicated by concurrent use of an antipsychotic medication affecting thermoregulation. Clin. J. Sport Med 30 (5), e166–e168. 10.1097/JSM.0000000000000750. [DOI] [PubMed] [Google Scholar]
- Greenland S., 2006. Bayesian perspectives for epidemiological research: I. Foundations and basic methods. Int. J. Epidemiol 35, 765–775. 10.1093/ije/dyi312. [DOI] [PubMed] [Google Scholar]
- Greenland S., 2007. Bayesian perspectives for epidemiological research. II.Regression analysis. Int. J. Epidemiol 36, 195–202. 10.1093/ije/dyl289. [DOI] [PubMed] [Google Scholar]
- Hansen A, Bi P, Nitschke M, Ryan P, Pisaniello D, Tucker G, 2008. The effect of heat waves on mental health in a temperate Australian city. Environ. Health Perspect 116 (10), 1369–1375. 10.1289/ehp.11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajat S, O’Connor M, Kosatsky T, 2010. Health effects of hot weather: from awareness of risk factors to effective health protection. Lancet 375 (9717), 856–863. 10.1016/S0140-6736(09)61711-6. [DOI] [PubMed] [Google Scholar]
- Kilbourne EM, Choi K, Jones TS, Thacker SB, 1982. Risk factors for heatstroke. A case-control study. JAMA 247 (24), 3332–3336. [PubMed] [Google Scholar]
- Knol MJ, VanderWeele TJ, Groenwold RHH, Klungel OH, Rovers MM, Grobbee DE, 2011. Estimating measures of interaction on an additive scale for preventive exposures. Eur. J. Epidemiol 26 (6), 433–438. 10.1007/s10654-011-9554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel L., 2019. Taking the Heat: potential fetal health effects of hot temperatures. Environ. Health Perspect 127 (10) 10.1289/ehp6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Lin Z, Ou Y, Soim A, Shrestha S, Lu Y, Sheridan S, Luben TJ, Fitzgerald E, Bell E, et al. , 2018. Maternal ambient heat exposure during early pregnancy in summer and spring and congenital heart defects - a large US population-based, case-control study. Environ. Int 118, 211–221. 10.1016/j.envint.2018.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CP, Chen PJ, Chang CM, 2015. Heat stroke during treatment with olanzapine, trihexyphenidyl, and trazodone in a patient with schizophrenia. Acta Neuropsychiatr. 27 (6), 380–385. 10.1017/neu.2015.29. [DOI] [PubMed] [Google Scholar]
- Ledrans M, Pirard P, Tillaut H, Pascal M, Vandentorren S, Suzan F, Salines G, Tertre AL, Medina S, Maulpoix A, et al. , 2004. The heat wave of August 2003: what happened? Rev. Prat 54 (12), 1289–1297. [PubMed] [Google Scholar]
- Laurent JGC, Williams A, Oulhote Y, Zanobetti A, Allen JG, Spengler JD, 2018. Reduced cognitive function during a heat wave among residents of non-air-conditioned buildings: an observational study of young adults in the summer of 2016. PLoS Med. 15 (7), e1002605 10.1371/journal.pmed.1002605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre HL, et al. , 2018. Assessing urban population vulnerability and environmental risks across an urban area during heatwaves - implications for health protection. Sci. Total Environ 610–611, 678–690. 10.1016/j.scitotenv.2017.08.062. [DOI] [PubMed] [Google Scholar]
- National Centers For Environmental Information, 2017. U.S. Climate Regions.
- Nie L, Chu H, Li F, Cole SR, 2010. Relative excess risk due to interaction: resampling-based confidence intervals. Epidemiology 21 (4), 552–556. 10.1097/EDE.0b013e3181e09b0b. [DOI] [PubMed] [Google Scholar]
- Reefhuis J, Gilboa SM, Anderka M, Browne ML, Feldkamp ML, Hobbs CA, Jenkins MM, Langlois PH, Newsome KB, Olshan AF, et al. , 2015. The national birth defects prevention study: a review of the methods. Birth Defects Res. A Clin. Mol. Teratol 103 (8), 656–669. 10.1002/bdra.23384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly TH, Kirk MA, 2007. Atypical antipsychotics and newer antidepressants. Emerg. Med. Clin 25 (2), 477–497. 10.1016/j.emc.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Olney RS, Holmes LB, Lin AE, Keppler-Noreuil KM, Moore CA, 2003. National Birth Defects Prevention Study. Guidelines for case classification for the national birth defects prevention study. Birth Defects Res. A Clin. Mol. Teratol 67, 193–201. 10.1002/bdra.10012. [DOI] [PubMed] [Google Scholar]
- Stadnyk AN, Glezos CP, 1983. Drug-induced heat stroke. Can. Med. Assoc. J 128 (8), 957–959. 10.1097/00000542-197309000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soim A, Sheridan SC, Hwang SA, Hsu WH, Fisher SC, Shaw GM, Feldkamp ML, Romitti PA, Reefhuis J, Langlois PH, et al. , 2018. A population-based case-control study of the association between weather-related extreme heat events and orofacial clefts. Birth Defects Res. 110 (19), 1468–1477. 10.1002/bdr2.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagy I, Vodonos A, Novack V, Rogachev B, Haviv YS, Barski L, 2016. The combined effect of high ambient temperature and antihypertensive treatment on renal function in hospitalized elderly patients. PLoS One 11 (12), e0168504. 10.1371/journal.pone.0168504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe PG, Gilboa SM, Hernandez-Diaz S, Lind J, Cragan JD, Briggs G, Kweder S, Friedman JM, Mitchell AA, Honein MA, et al. , 2013. Medications in the first trimester of pregnancy: most common exposures and critical gaps in understanding fetal risk. Pharmacoepidemiol. Drug Saf 22 (9), 1013–1018. 10.1002/pds.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts N, et al. , 2021. The 2020 report of the Lancet Countdown on health and climate change: responding to converging crises. Lancet 397 (10269), 129–170. 10.1016/s0140-6736(20)32290-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westaway K, Frank O, Husband A, McClure A, Shute R, Edwards S, Curtis J, Rowett D, 2015. Medicines can affect thermoregulation and accentuate the risk of dehydration and heat-related illness during hot weather. J. Clin. Pharm. Therapeut 40, 363–367. 10.1111/jcpt.12294. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Nitschke M, Krackowizer A, Dear K, Pisaniello D, Weinstein P, Tucker G, Shakib S, Bi P, 2016. Risk factors of direct heat-related hospital admissions during the 2009 heatwave in Adelaide, Australia: a matched case-control study. BMJ Open 6 (6), e010666. 10.1136/bmjopen-2015-010666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Spero TL, Nolte CG, Garcia VC, Lin Z, Romitti PA, Shaw GM, Sheridan SC, Feldkamp ML, Woomert A, et al. , 2019. Projected Changes in Maternal Heat Exposure during Early Pregnancy and the Associated Congenital Heart Defect Burden in the United States, vol. 8, e010995. 10.1161/JAHA.118.010995, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.