Abstract
Stem or progenitor cells are an essential component for the development, homeostasis, expansion, and regeneration of many tissues. Within white adipose tissue (WAT) reside vascular-resident adipose progenitor cells (APCs) that can proliferate and differentiate into either white or beige/brite adipocytes, which may control adiposity. Recent studies have begun to show that APCs can be manipulated to control adiposity and counteract ‘diabesity’. However, much remains unknown about the identity of APCs and how they may control adiposity in response to homeostatic and external cues. Here, we discuss recent advances in our understanding of adipose progenitors and cover a range of topics, including the stem cell/progenitor lineage, their niche, their developmental and adult roles, and their role in cold-induced beige/brite adipocyte formation.
Evidence for Adipose Progenitor Cells
Adipose tissues are widely distributed in stereotypic positions throughout the body [1]. This distribution can specify function, spanning diverse roles such as protection against trauma, cold, and starvation [2]. Yet, the ability of adipose tissue to expand in response to caloric excess can lead to obesity and its associated metabolic disorders (diabetes, hypertension, cardiovascular disease, atherosclerosis, cancer, etc.), which can have profound physiological, psychological, sociological, and economical ramifications [3,4]. While controlled caloric intake and increased fitness can address the obesity pandemic, it may also be addressed by identifying therapies that can manipulate adipose tissue formation, mass, and function. However, such a metabolic ‘silver bullet’ remains elusive. Targeting adipocytes themselves has proved to be only modestly or temporarily effective. For example, although liposuction and abdominoplasty remove unwanted adipose tissue, the adipose tissue compensates by regenerating its mass [5]. This reconstitution suggests that APCs are involved in the responses to injury or trauma and, conceivably, that stem/progenitor cells may also regulate tissue homeostasis and expansion. The possibility of a stem compartment is also supported by other findings. For instance, high fat diet (HFD) and exercise appear to regulate the adipose stem compartment to produce the number of cells (stem and adipocytes) necessary to meet metabolic demand [6–9]. The adipose stem compartment also seems subject to pharmacological manipulation; for example, the antidiabetes drug thiazolidinedione (TZD) has been shown to drive APC commitment to adipocytes [10]. Thus, the adipose stem compartment may be a modulatory nexus to counteract adiposity and metabolic dysfunction. Although our understanding of adipose stem biology is in its infancy, recent efforts to characterize APCs, their niche, and how they control adiposity and metabolic dysfunctions have begun to bear fruit. In this review, we delineate these findings and discuss unresolved questions.
WAT Development, Homeostasis and Expansion
Three phases of WAT (see Glossary) biology exist: (i) the development of adipose tissue (organogenesis); (ii) the homeostasis or maintenance of adipose tissue; and (iii) the expansion of adipose tissue to external stimuli, such as caloric excess and cold exposure. Recent studies into these three facets have begun to make inroads into this relatively poorly understood area of adipose tissue biology, and the findings indicate that progenitor/stem cells contribute to each phase. In this review, we discuss the role of APCs in adipose tissue development, homeostasis, and expansion, and in thermogenic responses.
WAT Development
Developmental Timing
Many organ systems require a specialized developmental cell, which coordinates the development, pattern, and formation of the tissue [11–13]. Recent studies suggest that adipose tissues require a specialized developmental cell type that patterns and forms the depots. Studies directed at murine adipose tissue organogenesis have indicated that subcutaneous and visceral adipose depots (SAT and VAT, respectively; Box 1) form in an ordered and timed manner throughout embryogenesis and within the first few weeks of birth [13–15]. SAT depots begin to develop during embryogenesis and the progenitor compartment is established for all SAT depots before the first few days of life. For example, the SAT depots, inguinal WAT (IGW) and periscapular WAT (PSCW), are specified between embryonic days E13.5 and E18.5 [13–15]. VAT depots principally form postnatally: the perigonadal (PGW) lineage forms approximately between postnatal day 3 (P3) and the second week of life. The mesenteric (MSW) VAT adipose compartment completes its organogenesis lineage establishment between the second and third weeks of life [13,14]. The retroperitoneal (RPW) VAT depot is formed in-between these pre- and postnatal stages, and has a morphogenesis, texture, and histology that also seems intermediate [13] (Figure 1). Early-to-mid embryonic establishment of SAT progenitors and tissues also occurs in humans. Human APCs begin to accumulate lipid during the second trimester of embryogenesis [16]. As embryogenesis continues, adipose depots and progenitors elaborate and the process is completed within the following weeks, just before birth [16,17]. However, little is known about the in utero or postnatal timing of human VAT [18,19]. Collectively, studies from both rodents and humans indicate that WAT organogenesis unfolds in a systematic and developmentally timed manner and that embryonic specification may denote the function and requirement of adipose tissue during development.
Box 1. Adipose Tissue: Back to Basics.
Lessons from Histology
Histological studies from the first half of the 20th century suggested that WAT comprises specialized cells termed ‘adipocytes’ that store lipid, rather than comprising connective tissue intercalated with lipid droplets [77]. WAT is not only specialized in lipid storage, but also acts as an endocrine organ by maintaining systemic metabolism, such as insulin sensitivity and lipid homeostasis. Decades of additional research has uncovered key roles for adipose tissue in physiology and metabolism, such as appetite, sexual reproduction, and thermogenic regulation [78]. The classic histological efforts delineated two types of adipose tissue: WAT [2] and brown adipose tissue (BAT). Fatty energy stores are liberated from WAT into the bloodstream upon demand and are brought to the appropriate cells, organs, and tissues for utilization [2]. BAT has a unique interscapular location, distinguished histology, and markedly different function due to the expression of mitochondrial uncoupling protein 1 (UCP1) [53], which functions to uncouple the electron transport chain to produce heat [53]. This thermogenic capability of BAT is essential for hibernating animals, which require heat to increase their core body temperature after a long bout of torpor [53]
Anatomy and Types
WAT is anatomically separated into two broad adipose compartments: subcutaneous (SAT), just below the dermis, and visceral (VAT), within the body cavity [1]. The SAT and VAT compartments themselves contain several distinct adipose depots. For example, murine SAT includes periscapular and inguinal depots; VAT includes perirenal, perigonadal, and mesenteric depots [1,18]. SAT and VAT have defined anatomical locations and distinctive developmental timing, texture, vascularity, adipocyte size, and gene expression [18,19]. These traits may confer body-fat distribution, body mass index, and insulin and glucose sensitivity, and have important functional attributes. For example, several studies indicate that humans with increased VAT have a higher prevalence of metabolic dysfunction than those with increased SAT [20,21].
Figure 1. Adipose Tissue Organogenesis.
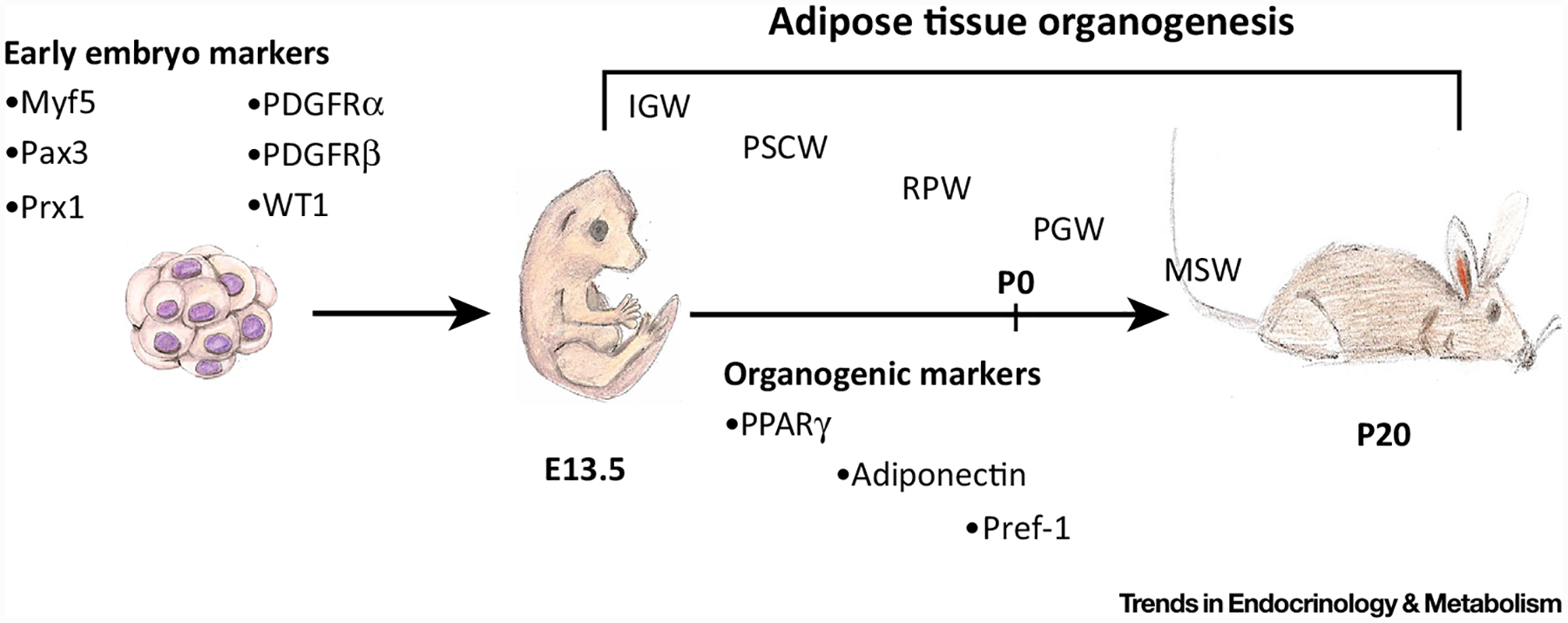
White adipose tissues are formed embryonically and postnatally. A variety of tools have been used to broadly identify early embryonic markers of adipose tissue, such as Myf5, Pax3, and Prx1. Around embryonic day (E)13.5, subcutaneous inguinal and periscapular adipose tissues are specified, patterned, and formed by more restrictive adipose lineage markers, such as PPARγ, adiponectin, and Pref-1. As embryogenesis continues (E18.5), the retroperitoneal adipose depot becomes specified, patterned, and formed. Both the perigonadal and mesenteric white adipose tissues are specified postnatally, beginning at postnatal day 2 (P)2 and ending near P20. Within the first month of life, all adipose depots are specified, formed, lipid filled, and functional. Designation of white adipose depots: IGW, Inguinal; MSW, mesenteric; PGW, perigonadal; PSCW, periscapular; RPW, retroperitoneal.
Developmental Origin
The subcutaneous embryonic and visceral postnatal organogenesis may reflect different developmental origins. Cell marking studies performed in mice indicate that SAT depots emerge from a field of cells marked by the homeobox gene Prx1. However, some VAT depots seem to originate from a field of cells marked by Wilms Tumor 1 (WT1), but the contribution seems to vary depending on the VAT depots [20,21]. Guertin and colleagues performed several elegant studies using Myf5-Cre-driven indelible reporters. They found that Myf5-Cre marked a subset of progenitor cells within PSCW SAT but not IGW SAT or PGW VAT, potentially uncovering key aspects of adipose organogenic mechanisms [22–24]. All SAT and VAT depots originate from cells that express platelet-derived growth factor receptor alpha (PDGFRα), consistent with the broad expression of PDGFRα throughout early and mid-embryogenesis [25,26]. However, it seems that PDGFRα has less of a role in adult adipose tissue homeostasis based on the lack of adipocyte labeling, except under HFD conditions. In general, it appears that a variety of mesodermal genetic tools mark WAT and BAT depots, but it will be beneficial to identify more specific markers to track and manipulate adipose tissue development, if such adipose-specific markers exist (Table 1).
Table 1.
Marking the Adipose Lineage
| Lineage Marker | WAT Marked | Phase of Adipose Tissue Biology | Refs |
|---|---|---|---|
| aP2-Cre | All SAT and VAT | Development and homeostasis | [32,79] |
| Lysm-Cre | Bone marrow WAT | Undetermineda | [80] |
| Myf5-Cre | SAT, periscapular; VAT, retroperitoneal | Undetermineda | [24] |
| Pax3-Cre | SAT, periscapular; VAT, retroperitoneal | Undetermineda | [24] |
| PDGFRα/-Cre | All SAT and WAT | Undetermineda | [25] |
| PDGFRβ-Cre | SAT, inguinal; VAT, retroperitoneal | Undetermineda | [32] |
| Prx-1-Cre | SAT only, inguinal and periscapular | Undetermineda | [21] |
| SM22-Cre | SAT, inguinal; VAT, perigonadal | Thermogenicb; not developmental | [32,76] |
| Sox10-Cre | Head-neck WAT | Undetermineda | [81] |
| Ve-Cadherin-Cre | All SAT and VAT | Developmental and homeostasisb | [82] |
| Adiponectin-rtTA; AdipoChaser | All SAT and VAT | All phases | [14] |
| Myh11-CreERT2 | Undetermined | Thermogenicb | [75] |
| NG2-CreERT2 | SAT | Thermogenicb | [76] |
| PPARγ-tTA AdipoTrak | All SAT and VAT | All phases | [13,32,76] |
| PDGFRα/-CreERT2 | VAT | Expansion and thermogenic | [26] |
| PDGFRβ-rtTA MuralChaser | SAT, inguinal; VAT, perigonadal | Expansion and thermogenic | [42] |
| Pref-1-rtTA | All SAT and VAT | Developmental and homeostasisb | [43] |
| SMA-CreERT2 | All SAT and VAT | Homeostasis and thermogenicb; not developmental | [13,76] |
| WT1-CreERT2 | VAT | Developmental and homeostasisb | [20] |
These genetic models cannot decipher when (timing) and where (adipocyte or progenitor) the Cre driver is actively marking the adipose lineage.
Other phases of adipose tissue biology have not been assessed.
Developmental APCs
Current evidence suggests that WAT development relies on one or several developmental or organogenic progenitors to pattern and form the SAT and VAT depots. To identify such a developmental cell, one group used direct in vivo examination, cell marking, and in vitro testing to identify that developmental APCs express perilipin and adiponectin. Importantly, these cells were capable of replication. Moreover, the authors noted that these proliferating perilipin+ cells appeared in clusters located along the growing vasculature within WAT [27]. In agreement, Jiang et al. showed that developmental APCs also reside juxtaposed to blood vessels within developing WAT [13]. To more restrictively probe the WAT developmental stem/progenitor compartment, Rodeheffer and colleagues used a leptin-luciferase reporter mouse to track adipose tissue development [15]. Exploiting this model, they found that leptin-luciferase activity could be detected as early as E16.5 and its activity progressively increased until birth. Genetic analysis of leptin-luciferase+ cells revealed a unique genetic signature as WAT developed. This genetic signature also appeared to demonstrate some differences to the classical NIH3T3-L1 adipocyte cell culture model used to study adipogenesis [15] (Box 3). These genes provide potential clues about the WAT developmental program; however, how this genetic network controls or initiates WAT development remains unanswered. Along the same lines, researchers have begun to identify genes that regulate WAT development versus homeostasis [6,28]. For example, both C/EBPα and AKT2 are involved in maintaining WAT homeostasis and expansion, but not WAT development [6,28].
Box 3. Adipogenesis: How to Make a Fat Cell.
Decades of elegant adipocyte research has focused on in vitro cell culture modeling, studying what has been coined ‘adipogenesis’ [88]. Adipogenesis was first described to demonstrate the transition of cultured and confluent NIH-3T3-L1 fibroblast cells to individual lipid-laden mature adipocytes. Fibroblast-like cells were incubated with a powerful recipe comprising thought-to-be adipogenic ‘inducers’: cAMP inducers, glucocorticoid agonists, and insulin or insulin-like growth factor (IGF) [89]. These pioneering studies paved the way for understanding adipocyte differentiation. For decades, researchers have teased apart various transcriptional components that regulate this complex differentiation process [88]. Advances derived from the NIH-3T3-L1 cellular system have been the identification of relevant molecules expressed during fat accumulation, particularly the transcriptional regulatory program. Among these, PPARγ, a nuclear hormone receptor, is necessary and sufficient for adipocyte formation. Several regulators of the differentiation program converge upon PPARγ to influence the conversion of progenitor cells to mature adipocytes [90]. In addition to transcriptional regulators, multiple signaling molecules, such as insulin, thyroid hormone, and TGFβ superfamily members, have been elucidated, and appear to initiate the adipogenic cascade (reviewed in [91]). Once adipogenic signals have been induced, a transcriptional cascade occurs, leading to the activation of multiple factors, such as Kruppel-like factor 4 (KLF4), SMAD1/5/8, CREB, glucocorticoid receptor, C/EBPβ, and C/EBPδ. Other transcription factors, such as thyroid receptor, C/EBPα, and LXR, are involved in adipocyte maturation and fat storage [91]. The appearance of a unilocular lipid droplet and the expression of lipid storage proteins characterize terminal differentiation [91]. Many signaling molecules, hormones, vitamins, and transcriptional factors positively regulate adipocyte differentiation, but just as many block or inhibit adipocyte maturation, such as WNT signaling, hedgehog, and retinoic acid [92,93]. In vitro adipogenesis has led the way for the basic biological understanding of adipogenesis; however, many aspects of in vitro adipocyte formation may not be conserved in vivo; thus, a potential challenge for adipose tissue biologists is to determine whether these molecules and their signaling control adipose tissue formation in vivo.
Collectively, these seminal findings spanning WAT organogenesis to the embryonic cellular origins of WAT to identifying primitive developmental adipose stem cells have set the foundation for the next step in WAT developmental studies. For example, studies that try to identify the molecular underpinnings, such as the transcriptional and signaling networks that control developmental APCs, could unearth basic developmental questions. Additionally, identifying key genes that control adipose tissue development may allow for the engineering of unique genetic tools to more restrictively mark and track developmental APCs.
WAT Homeostasis
The Adult APCs
Adipocyte turnover and ultrastructural studies suggest that APCs continually supply the tissue with new adipocytes throughout the lifespan of the organism [29,30]. Indeed, through the use of flow cytometric studies and genetic modeling, scientists have begun to uncover adult APCs in both rodents and humans [31–33]. However, multiple origins have been suggested for APCs (reviewed in [34]). A commonality from several independent research groups is the notion that adult APCs reside in the stromal vascular fraction (SVF) of WAT [31,32] (Box 2). Adipose tissue can be separated into two compartments based upon buoyant density: a floated fraction that contains lipid-laden white adipocytes and the SVF [35]. Within the SVF are cells that, when cultured in adipogenic medium, can form cells that contain lipid droplets, have some resemblance to adipocytes, and express adipocyte markers [36]. Using flow cytometry-based techniques, a population of cells from the SVF has been identified that express several cell surface markers and stem cell genes, such as Sca1+, CD24+, CD29+, CD34+ and PDFGRα+, and that can adipogenesis ectopically [31,32]. Additional studies have begun to generate a lineage progression framework from more stem-like CD24+ cells that can give rise to CD24– cells, which seem to be more committed to adipocyte differentiation [25]. PDGFRα also marks both populations of APCs [25].Overall, it appears that APCs may exist in various stages of adipocyte lineage commitment and that not all APCs are equal.
Box 2. The APC Niche: A Vascular Zip Code.
The niche is a major regulator of stem cell biology [83]. It controls stem cell properties, such as proliferation, migration, paracrine and endocrine signaling, and quiescence and differentiation [83]. Elegant in vivo studies from the 1940s and electron microscope studies from the 1960s demonstrated that broblast-like cells reside on WAT blood vessels [81,84]. These electron microscope images showed vascular-residing cells peeling away from the blood vessel as they transitioned into lipid-filled cells, or adipocytes [84]. Recently, genetic fate-mapping studies have positioned APC to the vascular niche [13,42]. These progenitor cells reside in perivascular positions along blood vessels within the adipose tissue. These vascular-residing progenitor cells appear to resemble mural cells in that they express a multitude of not only mural cell markers, such as SMA, but also adipocyte lineage markers, such as PPARg, ZFP423, and Pref-1 [13,42]. Although the niche is a critical regulatory component of stem cell biology, it has been difficult to examine the adipose tissue niche. One such difficulty has been the inability to isolate and examine the intact niche. This could be addressed by using an organotypic culture procedure termed ‘stromal vascular particulates’ (SVPs), which maintain the native structure of the microenvironment. Isolation of SVPs showed APCs wrapped around the blood vessel. Upon adipogenic signals, these cells peel away from the SVP, becoming lipid-filled adipocytes loosely associated with the vascular unit [13,32]. Several other studies have implicated the blood vessel niche as the microenvironment that controls APC number and adipose tissue mass [85]. The relation between blood vessels and APCs appears to be reciprocal [86]. That is, APCs stimulate blood vessel formation and blood vessels stimulate APCs expansion and differentiation [1,87]. Taken together, these studies support the notion that the vasculature is the niche for APCs. Future studies aimed at examine the APC niche may highlight important regulators of adipose tissue biology
Notably, a similar population of murine SVF cells has been identified that have adipogenic potential but, surprisingly, these APCs express PPARγ, a nuclear hormone receptor and master regulator of adipocyte differentiation [32,37,38] (Box 3). Using genetic-tracing methodologies, it was found that PPARg-expressing APCs are critical for adipocyte formation in vitro and in vivo [13,32]. In vivo tracking of PPARγ+ cells indicated that these cells reside within the blood vessel walls of WAT [13,32] (Figure 2). In line with a vascular residency, these APCs resemble mural cells (aka: pericytes and vascular smooth muscle cells) because they express several mural cell markers, such as PDGFRβ and alpha smooth muscle actin (SMA) [13]. Importantly, these cells fate mapped into mature adipocytes under normal chow homeostatic conditions with an estimate of 10–20% of new adipocytes forming per month depending on the WAT depots [13]. Likewise, human studies based upon radioactive isotopic decay suggested that adipocytes are also continually turned over and replenished, with estimates of approximately 9% per year [30]. However, does this appreciable rate of adipocyte formation in murine models have an effect on adipose tissue homeostasis and systemic metabolism? Indeed, deletion of PPARγ in SMA+ cells demonstrated that these cells are required for new adipocyte formation. The inability to form new mature white adipocytes under normal homeostatic conditions resulted in blocked adipose tissue expansion and decreased glucose sensitivity [13]. These studies support the notion that APCs are used to maintain WAT and that, if WAT homeostasis is disrupted, metabolic dysfunction ensues.
Figure 2. Adult Adipose Tissue Homeostasis and Beiging.
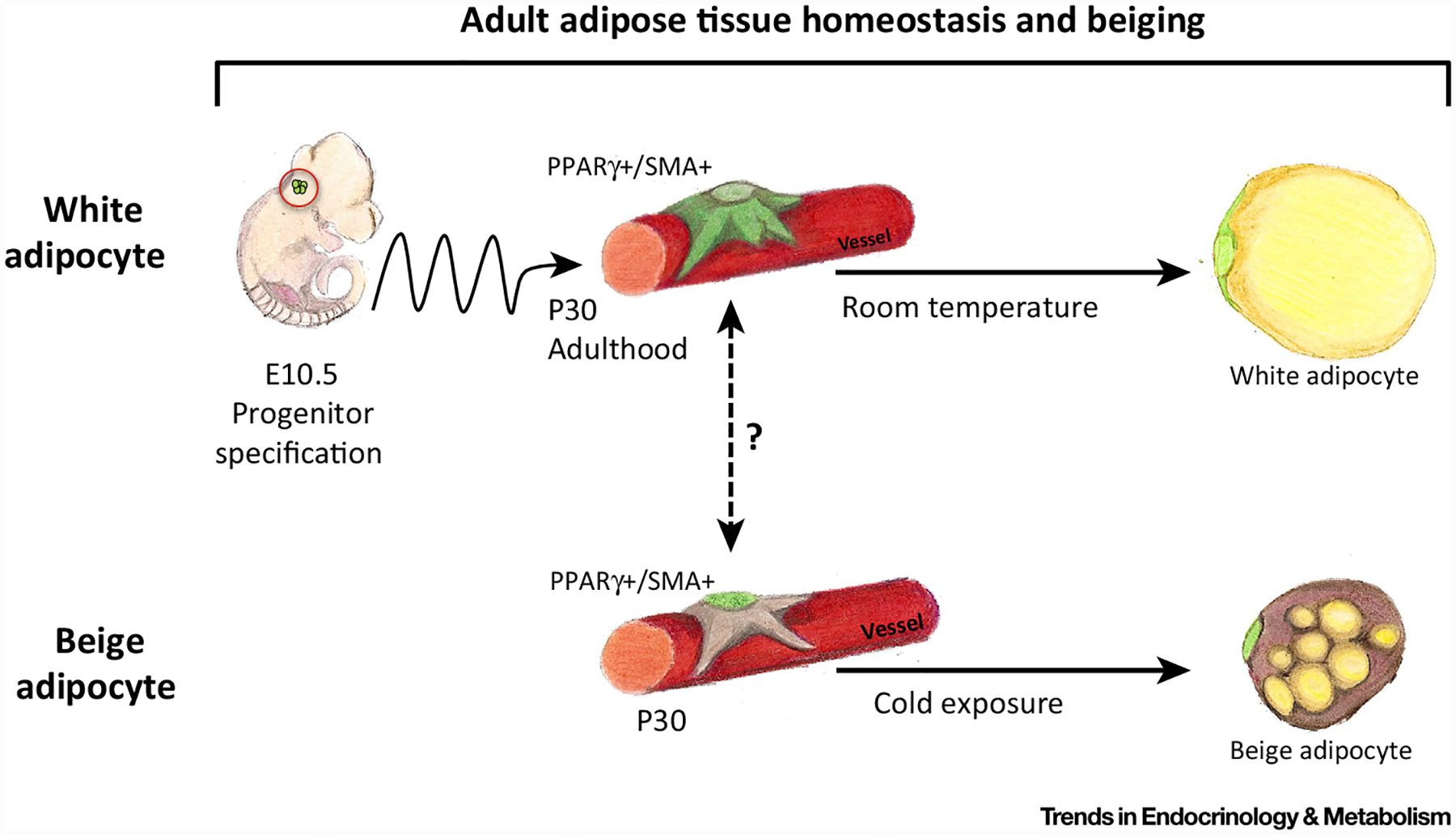
White adipocytes formation: adult white adipocyte progenitor cells (APCs) emanate from different lineages than do developmental APCs. However, adult APCs are specified at or near embryonic day (E)10.5; these cells then undergo a rostral–caudal migration throughout embryogenesis, eventually arriving at the adipose depots at approximately postnatal day (P)30. At P30, adult E10.5-specified APC then occupy vascular niche positioning and express mural cell and APC markers. Under homeostatic and adipogenic cues, these cells then transition from the blood vessel niche as they differentiate into mature unilocular white adipocytes. Beige/brite adipocyte formation: in cold-stimulated adult mice, vascular residing alpha smooth muscle actin (SMA)+ beige/brite progenitor cells leave their perivascular niche and differentiate into beige/brite adipocytes. The relation between adult vascular-residing white APC and vascular-residing beige/brite adipocyte progenitors is currently unknown, as is the embryonic specification of beige/brite progenitors.
Similarly, Gupta and colleagues identified a perivascular cell that expresses zinc finger protein 423 (ZFP423) and also PPARγ and other mural markers, such as PDGFRβ and SMA [39–41]. However, PDGFRβ/ZFP423+ cells only fate mapped into mature adipocytes under HFD conditions [42]. The difference in fate-mapping potential between PDGFRβ/ZFP423+ and SMA PPARγ+ cells needs further investigation. Correspondingly, PDGFRα+ cells have been identified as a progenitor source for white adipocytes, but only appear to contribute to VAT expansion [26]. These PDGFRα+ cells reside in the blood vessel adventitial region and resemble fibroblast-like cells [26]. These cells express neither mural cell markers nor PPARγ and fate mapped into perigonadal adipocytes under HFD conditions [26]. What might account for the discrepancies in adipocyte generation in different rodent models? Several reasons may exist: (i) specificity of genetic tools used to mark the APC and adipocyte compartment; (ii) the mouse: age, gender, and strain; (iii) the type of WAT depot under investigation; (iv) the location analyzed within the WAT depot; and (v) the reliance on the notion that APCs must divide (BrdU fate mapping: see ‘WAT Expansion’). What is clear from these studies is that some APCs do reside in adipose tissues. A future goal would be to tease apart the lineage and cellular differences and similarities among these various cell types. That is, does one cell type beget the other, or are they independent? In addition, are different cell types utilized under different dietary conditions? Answers to these lineage relation questions may be able to provide clues into when, why, and how APCs are engaged to differentiate into mature adipocytes under different cues.
Adding further to the progenitor fray is the observation that adult perivascular APCs have embryonic origins [13,43]. Current evidence suggests that adult APCs emanate from a small group of cells present in the E10.5 embryo [13,43]. These E10.5 cells express PPARγ and Pref-1, two genes that are also expressed postnatally in bone fide APCs [13,43]. PPARγ E10.5 fate-mapping studies showed that these cells lineage traced into adult, but not developing, WAT (SAT and VAT) [13]. Notably, E10.5 adult APCs do not reside in WAT before or during WAT development [13]. After WAT development, PPARγ+ E10.5 adult progenitor cells in ltrate the WAT depots and occupy vascular residency (Figure 2). Moreover, deleting PPARγ in these E10.5 cells resulted in abnormal WAT homeostasis (decreased adiposity and glucose sensitivity) but did not alter adipose tissue development [13]. Another study using Pref-1 labeling found similar results: Pref-1 marked a field of cells at E10.5 located in the dorsal mesenteric region that produced adipocytes [43]. However, the Pref-1 studies did not distinguish the temporal relation of specification between the developmental and adult pools. Recent studies suggest that retinoic acid upregulates Pref-1, but does this important embryonic hormone regulate Pref-1 under this biological setting [44]? Other questions relating to downstream pathways and genes that regulate and/or are regulated by Pref-1 and PPARγ could provide key insights into lineage specification and tissue development and homeostasis. Such insights might highlight how adult adipose tissues are developed and subsequently maintained throughout the lifespan of the organism.
Other Adipocyte Progenitor Cell Sources
Even though several studies have suggested WAT-resident APCs, others have suggested auxiliary sources, such as multipotent hematopoietic progenitors [45]. Through the use of imaging and bone marrow transplantation studies, researchers have identified multipotent hematopoietic cells that are able to populate and contribute to adipocyte formation [46]. However, several transplant and genetic mouse studies are at odds, which has led to a heated debate as to whether bone marrow progenitors can differentiate into white adipocytes [47,48]. Recently, an elegant and sophisticated study was performed to address whether human bone marrow cells could produce SAT adipocytes. These researchers utilized genomic differences between donor and recipient cells to find that human bone marrow progenitors contributed to approximately 10% of SAT adipocyte number over the entire lifespan of the recipient [49]. This study corroborates that bone marrow progenitors can give rise to adipocytes, but raises the interesting notion that other lineages can serve as a reservoir for adipocytes under various conditions. Additionally, what is the relation between the identified perivascular sources and the multipotent hematopoietic stem cells? It will be exciting to distinguish the temporal relation(s) or difference(s) between these alternative sources and lineages of WAT homeostasis and expansion.
WAT Expansion: Plumped Up
Both WAT hypertrophy and hyperplasia are key responses to a HFD or Western diet [14]. However, it has been difficult to assess the relative contribution of hypertrophy and hyperplasia. To measure hyperplasia, scientists have relied on methods to measure DNA synthesis (BrdU) of the SVF compartment and to monitor the ability of SV cells to transition from the progenitor to adipocyte compartment. Studies examining the rodent APC compartment have proposed that 4.8% of progenitors are dividing at a given time and that the APC pool is continually replenished [50]. However, these DNA synthesis studies may not accurately reflect the overall contribution of the adipocyte progenitor compartment to adipocyte differentiation. Furthermore, this model also relies on the notion that APCs must proliferate before adipocyte differentiation. To overcome this, new genetic tools have been developed to access the overall contribution of hypertrophy and hyperplasia to WAT expansion. These recent efforts have shown that new adipocytes form in response to HFD but primarily in VAT depots [14]. In rodents, the generation of new adipocytes in response to a HFD appears to depend on the age of the animal. For example, juvenile mice fed HFD showed robust adipogenesis, whereas adult mice showed lower adipogenic potential [51]. Aging, in general, also appears to decrease the progenitor compartment activity (proliferation and differentiation) and this may be a component of metabolic failure that is triggered by dysfunctional adipocytes as we age [52].
Not only are new adipocytes formed under caloric excess, but the APC compartment also appears to be active. To begin to tease apart when and where APCs might proliferate under HFD conditions, Rodeheffer and colleagues found that, soon after HFD administration, APCs are prompted to enter the cell cycle [6]. The propagatory phase is relatively short lived. Yet, the burst of proliferation leads to a marked increase in the number of progenitors, potentially held as a reserve. Indeed, the newly formed progenitor cells are not immediately utilized to make adipocytes; instead, they are used after several weeks of HFD to generate new white fat cells [6]. However, the identity of signals and factors that govern this response remains unknown. Collectively, although it appears that new adipocytes are generated under HFD conditions, other factors, such as age, duration, and gender, should be taken into consideration when studying these events.
Beige/Brite Adipocyte Lineage: Beige/Brite Is the New Black
Brown adipose tissue (BAT) generates heat rather than energy (ATP) [53]. It does so, in part, by expressing the mitochondrial protein uncoupling protein 1 (UCP1), which uncouples the electron transport chain [53]. In addition to UCP1, there may be other unique energy expenditure features of BAT, such as arginine/creatine metabolism, that assist in thermogenic activity [54]. This key thermogenesis feature of BAT has been shown to be essential for hibernating animals, which require heat to increase their core body temperature after a long bout of torpor [55]. During the 1980s, it was realized that rodents exposed to cold induced the formation of brown-like adipocytes within WAT [56]. Successive studies showed that β-adrenergic agonists stimulated a related phenomenon, identifying the sympathetic nervous system as a regulator of endothermia [57]. In rodents, it was thought that these brown-like adipocytes induced by cold or β-adrenergic stimuli within WAT depots, were related to classical interscapular brown adipocytes. However, recent efforts have begun to distinguish them into a third type of adipocyte, termed beige or brite (brown-in white adipose tissues) [58,59]. Beige/brite adipocytes are inducible, multilocular, can express UCP1, and are thermogenic [58]. Even though both brown and beige/brite adipocytes express a core of common thermogenic genes, they differentially express other genes, such as CD137 and TMEM26, which are highly enriched in beige/brite adipocytes [58]. However, further characterization is needed to fully identify whether beige/brite adipocytes are analogs to, or distinct from, brown adipocytes [60].
Recent technological (imaging) and methodological (monitoring in vivo BAT activity) advances have led to the identification and revitalization of brown and cold-inducible BAT as a potential therapy for metabolic dysfunction for humans [61–65]. This renewal has been spearheaded due to human research that demonstrates the presence of brown and/or beige/brite adipocytes [62,63,66–69]. Biopsies of human supraclavicular WAT after cold exposure demonstrated the presence of inducible thermogenic brown-like adipocytes [70]. Molecular profiling studies indicate that the thermogenic adipocytes that appear in humans are more like rodent beige/brite adipocytes rather than the classical rodent brown adipocytes [58,59]. Thus, the ability to manipulate the formation or function of each adipocyte lineage could be a component in the diabesity pharmacopeia. However, whether beige/brite adipocytes are therapeutically relevant in the fight against human metabolic dysfunction remains controversial, although we favor a therapeutic role.
Since the discovery of beige/brite adipocytes, the cellular lineage that generates these cellular furnaces has troubled scientists and has remained murky [53]. An early popular notion was that beige/brite adipocytes derive from the trans-differentiation of mature white adipocytes [71]. This notion has been rekindled by genetic studies examining adipocyte cell marking, which have suggested that white adipocytes could convert from white to inducible beige/brite adipocytes [72]. However, another adipocyte fate-mapping study (AdipoChaser) is at odds with the white to beige/brite conversion possibility [14]. This may be related, in part, to the type of genetic tools and drugs used to induce labeling recombination [73]. In agreement with the interconversion hypothesis, another study identified that, after cold exposure in mice, beige/brite adipocytes are not eliminated, but convert to white adipocytes [74]. However, upon additional cold exposure, these phenotypic white, once beige/brite, adipocytes revert to being beige/brite adipocytes. This study suggests that these ‘interconverting adipocytes’ have retained a beige/brite genetic memory that allows them to become reactivated when stimulated. Of note, after re-exposure to cold, new unlabeled beige/brite adipocytes were also generated that were not from the previously marked beige/brite adipocytes [74]. Thus, other cellular sources might exist. In agreement with this notion, several other studies have proposed a perivascular source for cold-induced beige/brite adipocytes, a resemblance to WAT progenitors. For example, smooth muscle genetic fate-mapping studies have suggested cells marked by Myh11, PDGFRβ, and SMA can generate beige/brite adipocytes in response to cold exposure [42,75,76]. In a recent fate-mapping study, SMA+ perivascular cells generated 50–70% of new beige/brite adipocytes after 1 week of cold exposure [76]. Conversely, other smooth muscle genetic fate-mapping tools, such as Myh11 and PDGFRβ, only label beige/brite adipocytes after 2 weeks of cold exposure [42,75]. What accounts for the differences between 1-week versus 2-weeks of cold exposure is not clear. Flow cytometric studies indicate that different populations of smooth muscle cells may exist that express one or multiple smooth muscle markers, which suggests that different cellular populations are used after different durations of cold exposure. Regardless of the contribution, two methodologies, blockade of adipocyte differentiation or a cell killing strategy, tested the ability of SMA+ cells to generate beige/brite adipocytes. Remarkably, blocking adipogenesis within SMA+ cells or ablating SMA+ cells led to the failure of cold-induced beige/brite adipocytes. The absence of beige/brite adipocytes also appeared to impair metabolism; mice were unable to either defend their temperature or lower plasma glucose [76]. This was not the case when adipogenesis was blocked in Myh11+ cells: mice formed beige/brite adipocytes, defended their body temperature, and reduced their plasma glucose after 2 weeks of cold exposure [76]. Further studies aimed at delineating the relation and/or differences between cellular sources may help unravel the molecular underpinnings and signals that govern beige/brite adipocyte formation during prolonged cold exposure.
Concluding Remarks and Future Perspectives
Here, we have pieced together the current understanding of how APCs regulate adipose tissue development, homeostasis, expansion, and thermogenesis. This is an emerging eld that is evolving rapidly (see Outstanding Questions). However, the adipose stem/progenitor field has lagged behind and is only now beginning to catch up with other advanced lineages, such as the hematopoietic and neuronal fields, to define and characterize the cells that generate adipocytes. Recent efforts have provided hints of several aspects of APCs, such as their lineage and/or anlagen, niche, and role in tissue development and homeostasis. In addition to these fundamental aspects of stem cells, recent in vivo studies have made inroads towards defining molecular attributes and signatures, such as transcriptional networks and signaling pathways that regulate adipocyte formation. However, reconciling current knowledge into an outline of how the APC lineage is established and how these progenitors control WAT biology is difficult. Many discrepancies exist within the published data, which could be attributed to transgenic mouse models, mouse gender, mouse age, mouse strain, WAT depot under investigation, position of analysis within the WAT depot, and specificity of cell sorting antibodies. Nevertheless, this is an exciting time for the field with many new discoveries ahead that will begin to address these fundamental WAT questions. Such insights may begin to paint a picture of the APC lineage and its role in WAT development, homeostasis, expansion, and thermogenesis.
Outstanding Questions.
How do APCs pattern, organize, and form both the developmental and adult adipose tissues? What are the transcriptional and signaling networks involved in both of these phases? Are these same phases and networks observed in human adipose organogenesis and homeostasis?
What is the role of PPARγ in the adipose lineage? Does PPARγ regulate stem cell attributes, niche interaction, or only adipocyte differentiation?
How do other proposed cellular sources of WAT relate to the identified perivascular adipose progenitors? Are these other sources required for adipose tissue development or homeostasis?
What function do dermal and bone marrow adipocytes perform: functional, metabolic, niche, or other?
How does a HFD alter adipose progenitor cell dynamics (proliferation, migration, niche positioning, and differentiation)? Are different progenitor cells used in response to a HFD compared with a normal diet or homeostatic conditions?
What factors regulate adult APC niche interactions? What are the consequences when these signals are disrupted genetically, pharmacologically, or via diet? What is the niche for developmental APCs?
Are beige and white adipocytes generated from the same SMA+ progenitors or from a different SMA pool? What is the lineage relation between SMA, Myh11, and PDGFRb with regards to generating cold-induced beige adipocytes after 1 week versus 2 weeks of cold exposure? What are the relative contributions from other cellular sources to beiging?
Are there differences between cold-induced and b-adrenergic-induced beige adipocytes?
Trends.
Subcutaneous and visceral white adipose depots have different embryonic and postnatal development from different adipose progenitor sources.
APCs contribute to adipocyte formation under both homeostatic and environmental cues.
White APCs reside in a perivascular niche resembling a subset of mural cells.
Beige APCs reside in a perivascular niche and, upon cold exposure, form beige adipocytes, a potential therapy to combat excess fat.
Acknowledgments
We thank all current and former members of the Graff lab for their helpful comments and discussion points leading to refinement of our concepts. We also thank the NIH and NIDDK (R01-DK066556, R01-DK064261 and R01-DK088220 to J.M.G) for support. D.C.B was supported by NIDDK F32-DK101153 and is currently supported by K01-DK109027. J.M.G is a cofounder and shareholder of Reata Pharmaceuticals. We apologize for not citing important work that has challenged and pioneered the way for the above studies, due to space limitations.
Glossary
- Beige/brite adipocytes
a cold and b3 adrenergic-inducible multilocular adipocyte that can express UCP1 and has thermogenic capacity.
- Brown adipose tissue (BAT)
multilocular, mitochondria-rich adipocytes with thermogenic function. They express uncoupling protein 1 (UCP1), which uncouples the electron transport chain to generate heat rather than chemical energy (ATP).
- Hypertrophy
the enlargement of pre-existing adipocytes, which can expand to accommodate excess dietary nutrients, storing them as triglycerides
- Hyperplasia
the proliferation and expansion of the APC and stromal vascular compartment within adipose depots in response to caloric excess.
- Subcutaneous adipose tissue (SAT)
white adipose tissues that form just below the dermis. Inguinal (posterior) and periscapular (anterior) are distinct depots of SAT.
- Stromal vascular fraction (SVF)
the numerous cell types that comprise the nonadipocyte compartment of adipose depots. Cell types include: endothelial cells, mural/smooth muscle cells, fibroblasts, neuronal cells, inflammatory cells, and APCs
- Visceral adipose tissue (VAT)
white adipose tissues that form within the body cavity. Perigonadal, retroperitoneal, and mesenteric are distinct depots of VAT.
- White adipose tissue (WAT)
lipid-storing unilocular adipocytes that store energy in the form of triglycerides
References
- 1.Berry DC et al. (2013) The developmental origins of adipose tissue. Development 140, 3939–3949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosen ED and Spiegelman BM (2006) Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444, 847–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spiegelman BM and Flier JS (2001) Obesity and the regulation of energy balance. Cell 104, 531–543 [DOI] [PubMed] [Google Scholar]
- 4.WHO (2013) Obesity and Overweight, WHO [Google Scholar]
- 5.Sterodimas A et al. (2012) Thirty-four years of liposuction: past, present and future. Eur. Rev. Med. Pharmacol. Sci 16, 393–406 [PubMed] [Google Scholar]
- 6.Jeffery E et al. (2015) RAPCid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat. Cell Biol 17, 376–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gollisch KS et al. (2009) Effects of exercise training on subcutaneous and visceral adipose tissue in normal- and high-fat diet-fed rats. Am. J. Physiol. Endocrinol. Metabo 297, E495–E504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakurai T et al. (2010) Effects of exercise training on adipogenesis of stromal-vascular fraction cells in rat epididymal white adipose tissue. Acta Physiol. 200, 325–338 [DOI] [PubMed] [Google Scholar]
- 9.Zeve D et al. (2016) Exercise-induced skeletal muscle adaptations alter the activity of adipose progenitor cells. PLoS ONE 11, e0152129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang W et al. (2011) Thiazolidinediones regulate adipose lineage dynamics. Cell Metab. 14, 116–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang Y et al. (2012) SnapShot: adipocyte life cycle. Cell 150, 234–234 e232 [DOI] [PubMed] [Google Scholar]
- 12.Gesta S et al. (2007) Developmental origin of fat: tracking obesity to its source. Cell 131, 242–256 [DOI] [PubMed] [Google Scholar]
- 13.Jiang Y et al. (2014) Independent stem cell lineages regulate adipose organogenesis and adipose homeostasis. Cell Rep. 9, 1007–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang QA et al. (2013) Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med 19, 1338–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birsoy K et al. (2011) Analysis of gene networks in white adipose tissue development reveals a role for ETS2 in adipogenesis. Development 138, 4709–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poissonnet CM et al. (1983) Growth and development of human adipose tissue during early gestation. Early Hum. Dev 8, 1–11 [DOI] [PubMed] [Google Scholar]
- 17.Poissonnet CM et al. (1984) The chronology of adipose tissue appearance and distribution in the human fetus. Early Hum. Dev 10, 1–11 [DOI] [PubMed] [Google Scholar]
- 18.Fox K et al. (1993) Abdominal fat deposition in 11-year-old children. Int. J. Obes. Relat. Metab. Disord 17, 11–16 [PubMed] [Google Scholar]
- 19.Siegel MJ et al. (2007) Total and intraabdominal fat distribution in preadolescents and adolescents: measurement with MR imaging. Radiology 242, 846–856 [DOI] [PubMed] [Google Scholar]
- 20.Chau YY et al. (2014) Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat. Cell Biol 16, 367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez-Gurmaches J et al. (2015) Highly selective in vivo labeling of subcutaneous white adipocyte precursors with Prx1-Cre. Stem Cell Reports 4, 541–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seale P et al. (2008) PRDM16 controls a brown fat/skeletal muscle switch. Nature 454, 961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seale P et al. (2009) Transcriptional control of brown adipocyte development and physiological function–of mice and men. Genes Dev. 23, 788–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez-Gurmaches J and Guertin DA (2014) Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat. Commun 5, 4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berry R and Rodeheffer MS (2013) Characterization of the adipocyte cellular lineage in vivo. Nat. Cell Biol 15, 302–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee YH et al. (2012) In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell Metab. 15, 480–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong KY et al. (2015) Perilipin+ embryonic preadipocytes actively proliferate along growing vasculatures for adipose expansion. Development 142, 2623–2632 [DOI] [PubMed] [Google Scholar]
- 28.Wang QA et al. (2015) Distinct regulatory mechanisms governing embryonic versus adult adipocyte maturation. Nat. Cell Biol 17, 1099–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cinti S et al. (1984) A morphological study of the adipocyte precursor. J. Submicrosc. Cytol 16, 243–251 [PubMed] [Google Scholar]
- 30.Spalding KL et al. (2008) Dynamics of fat cell turnover in humans. Nature 453, 783–787 [DOI] [PubMed] [Google Scholar]
- 31.Rodeheffer MS et al. (2008) Identification of white adipocyte progenitor cells in vivo. Cell 135, 240–249 [DOI] [PubMed] [Google Scholar]
- 32.Tang W et al. (2008) White fat progenitor cells reside in the adipose vasculature. Science 322, 583–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimmerlin L et al. (2010) Stromal vascular progenitors in adult human adipose tissue. Cytometry. Part A 77, 22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Billon N and Dani C (2012) Developmental origins of the adipocyte lineage: new insights from genetics and genomics studies. Stem Cell Rev. 8, 55–66 [DOI] [PubMed] [Google Scholar]
- 35.Church CD et al. (2014) Isolation and study of adipocyte precursors. Methods Enzymol. 537, 31–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bjorntorp P et al. (1978) Isolation and characterization of cells from rat adipose tissue developing into adipocytes. J. Lipid Res 19, 316–324 [PubMed] [Google Scholar]
- 37.Tontonoz P et al. (1994) mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 8, 1224–1234 [DOI] [PubMed] [Google Scholar]
- 38.Chawla A and Lazar MA (1994) Peroxisome proliferator and retinoid signaling pathways co-regulate preadipocyte phenotype and survival. Proc. Natl. Acad Sci. U.S.A 91, 1786–1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shankar K et al. (2015) Cucumis melo ssp. agrestis var. agrestis ameliorates HFD-induced dyslipidemia in Syrian golden hamsters and inhibits adipogenesis in 3T3-L1 adipocytes. Pharmacogn. Mag 11, S501–S510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta RK et al. (2010) Transcriptional control of preadipocyte determination by Zfp423. Nature 464, 619–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta RK et al. (2012) Zfp423 expression identi es committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab. 15, 230–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vishvanath L et al. (2016) Pdgfrbeta(+) mural preadipocytes contribute to adipocyte hypErplasia induced by high-fat-diet feeding and prolonged cold exposure in adult mice. Cell Metab. 23, 350–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hudak CS et al. (2014) Pref-1 marks very early mesenchymal precursors required for adipose tissue development and expansion. Cell Rep. 8, 678–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berry DC et al. (2012) Retinoic acid upregulates preadipocyte genes to block adipogenesis and suppress diet-induced obesity. Diabetes 61, 1112–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crossno JT Jr et al. (2006) Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J. Clin. Invest 116, 3220–3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sera Y et al. (2009) Hematopoietic stem cell origin of adipocytes. Exp. Hemato 37, 1108–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomiyama K et al. (2008) Characterization of transplanted green fluorescent protein+ bone marrow cells into adipose tissue. Stem Cells 26, 330–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koh YJ et al. (2007) Bone marrow-derived circulating progenitor cells fail to transdifferentiate into adipocytes in adult adipose tissues in mice. J. Clin. Invest 117, 3684–3695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryden M et al. (2015) Transplanted bone marrow-derived cells contribute to human adipogenesis. Cell Metab. 22, 408–417 [DOI] [PubMed] [Google Scholar]
- 50.Rigamonti A et al. (2011) Rapid cellular turnover in adipose tissue. PLoS ONE 6, e17637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim SM et al. (2014) Loss of white adipose hyperplastic potential is associated with enhanced susceptibility to insulin resistance. Cell Metab. 20, 1049–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tchkonia T et al. (2010) Fat tissue, aging, and cellular senescence. Aging Cell. 9, 667–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cannon B and Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol. Rev 84, 277–359 [DOI] [PubMed] [Google Scholar]
- 54.Kazak L et al. (2015) A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige/brite fat. Cell 163, 643–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith RE and Hock RJ (1963) Brown fat: thermogenic effector of arousal in hibernators. Science 140, 199–200 [DOI] [PubMed] [Google Scholar]
- 56.Young P et al. (1984) Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett. 167, 10–14 [DOI] [PubMed] [Google Scholar]
- 57.Cousin B et al. (1992) Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J. Cell Sci 103, 931–942 [DOI] [PubMed] [Google Scholar]
- 58.Wu J et al. (2012) Beige/brite adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lidell ME et al. (2014) Two types of brown adipose tissue in humans. Adipocyte 3, 63–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harms M and Seale P (2013) Brown and beige/brite fat: development, function and therapeutic potential. Nat. Med 19, 1252–1263 [DOI] [PubMed] [Google Scholar]
- 61.Cypess AM et al. (2009) Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med 360, 1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van der Lans AA et al. (2013) Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J. Clin. Invest 123, 3395–3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Marken Lichtenbelt WD et al. (2009) Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med 360, 1500–1508 [DOI] [PubMed] [Google Scholar]
- 64.Blondin DP et al. (2014) Increased brown adipose tissue oxidative capacity in cold-acclimated humans. J. Clin. Endocrinol. Metab 99, E438–E446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sidossis L and Kajimura S (2015) Brown and beige/brite fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J. Clin. Invest 125, 478–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Borga M et al. (2014) Brown adipose tissue in humans: detection and functional analysis using PET (positron emission tomography), MRI (magnetic resonance imaging), and DECT (dual energy computed tomography). Methods Enzymol. 537, 141–159 [DOI] [PubMed] [Google Scholar]
- 67.Virtanen KA et al. (2009) Functional brown adipose tissue in healthy adults. N. Engl. J. Med 360, 1518–1525 [DOI] [PubMed] [Google Scholar]
- 68.Yoneshiro T et al. (2011) Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity 19, 13–16 [DOI] [PubMed] [Google Scholar]
- 69.Yoneshiro T et al. (2013) Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Invest 123, 3404–3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shinoda K et al. (2015) Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat. Med 21, 389–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barbatelli G et al. (2010) The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Ame. J. Physiol. Endocrinol. Metab 298, E1244–E1253 [DOI] [PubMed] [Google Scholar]
- 72.Lee YH et al. (2015) Cellular origins of cold-induced brown adipocytes in adult mice. FASEB J. 29, 286–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ye R et al. (2015) Impact of tamoxifen on adipocyte lineage tracing: Inducer of adipogenesis and prolonged nuclear translocation of Cre recombinase. Mol. Metab 4, 771–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rosenwald M et al. (2013) Bi-directional interconversion of brite and white adipocytes. Nat. Cell Biol 15, 659–667 [DOI] [PubMed] [Google Scholar]
- 75.Long JZ et al. (2014) A smooth muscle-like origin for beige/brite adipocytes. Cell Metab. 19, 810–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berry DC et al. (2016) Mouse strains to study cold-inducible beige/brite progenitors and beige/brite adipocyte formation and function. Nat. Commun 7, 10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Clark ER (1940) Microscopic studies of the new formation of fat in living adult rabbits. Am. J. Anat 67, 255–285 [Google Scholar]
- 78.Rosen ED and Spiegelman BM (2014) What we talk about when we talk about fat. Cell 156, 20–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abel ED et al. (2001) Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 409, 729–733 [DOI] [PubMed] [Google Scholar]
- 80.Majka SM et al. (2010) De novo generation of white adipocytes from the myeloid lineage via mesenchymal intermediates is age, adipose depot, and gender specific. Proc. Natl. Acad Sci. U.S.A 107, 14781–14786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Billon N et al. (2007) The generation of adipocytes by the neural crest. Development 134, 2283–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tran KV et al. (2012)The vascular endothelium of the adiposetissue gives rise to both white and brown fat cells. Cell Metab. 15, 222–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morrison SJ and Scadden DT (2014) The bone marrow niche for haematopoietic stem cells. Nature 505, 327–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Napolitano L (1963) The differentiation of white adipose cells. An electron microscope study. J. Cell Biol 18, 663–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Crandall DL et al. (1997) A review of the microcirculation of adipose tissue: anatomic, metabolic, and angiogenic perspectives. Microcirculation 4, 211–232 [DOI] [PubMed] [Google Scholar]
- 86.Cao Y (2007) Angiogenesis modulates adipogenesis and obesity. J. Clin. Invest 117, 2362–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Traktuev DO et al. (2009) Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ. Res 104, 1410–1420 [DOI] [PubMed] [Google Scholar]
- 88.Rosen ED and Spiegelman BM (2000) Molecular regulation of adipogenesis. Annu. Rev. Cell Dev. Biol 16, 145–171 [DOI] [PubMed] [Google Scholar]
- 89.Green H and Kehinde O (1975) An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell 5, 19–27 [DOI] [PubMed] [Google Scholar]
- 90.Tontonoz P and Spiegelman BM (2008) Fat and beyond: the diverse biology of PPARgamma. Annu. Rev. Biochem 77, 289–312 [DOI] [PubMed] [Google Scholar]
- 91.Cristancho AG and Lazar MA (2011) Forming functional fat: a growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol 12, 722–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Berry DC and Noy N (2009) All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor beta/delta and retinoic acid receptor. Mol. Cell. Biol 29, 3286–3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smas CM et al. (1997) Cleavage of membrane-associated pref-1 generates a soluble inhibitor of adipocyte differentiation. Mol. Cell. Biol 17, 977–988 [DOI] [PMC free article] [PubMed] [Google Scholar]


