Abstract
Antibody disulfide bond (DSB) reduction during manufacturing processes is a widely observed phenomenon attributed to host cell reductases present in harvest cell culture fluid. Enzyme-induced antibody reduction leads to product fragments and aggregates that increase the impurity burden on the purification process. The impact of reduction on bivalent bispecific antibodies (BisAbs), which are increasingly entering the clinic, has yet to be investigated. We focused on the reduction and reoxidation properties of a homologous library of bivalent BisAb formats that possess additional single-chain Fv (scFv) fragments with engineered DSBs. Despite all BisAbs having similar susceptibilities to enzymatic reduction, fragmentation pathways were dependent on the scFv fusion site. Reduced molecules were allowed to reoxidize with and without low pH viral inactivation treatment. Both reoxidation studies demonstrated that multiple, complex BisAb species formed as a result of DSB mis-pairing. Furthermore, aggregate levels increased for all molecules when no low pH treatment was applied. Combined, our results show that complex DSB mis-pairing occurs during downstream processes while aggregate formation is dependent on sample treatment. These results are applicable to other novel mAb-like formats containing engineered DSBs, thus highlighting the need to prevent reduction of novel protein therapeutics to avoid diminished product quality during manufacturing.
Keywords: bispecific antibody, reduction, aggregation, disulfide bond mis-pair, appended scFv-IgG bispecific antibody
Introduction
Bispecific antibodies (BisAbs) are an emerging class of biotherapeutics that are designed to bind two distinct epitopes. Multivalent antigen-binding allows BisAbs to have a wide range of therapeutic applications, including treatments for lupus, rheumatoid arthritis, osteoporosis, lymphoma, and leukemia (Sedykh, Prinz, Buneva, & Nevinsky, 2018). There are currently around 100 different engineered BisAb formats reported in the literature with unique combinations of antigen-binding domains (Brinkmann & Kontermann, 2017). One class of BisAb molecules focuses on appending single-chain Fv (scFv) fragments to full-length IgG heavy chains. The additional scFv fragments contain antigen-binding VH and VL domains that are commonly linked together with a Gly/Ser repeat peptide, with an engineered disulfide bond (DSB) for improved biochemical stability (Figure 1A) (Benschop et al., 2019; Mack, Riethmüller, & Kufer, 1995; McCall et al., 2001).
Figure 1.
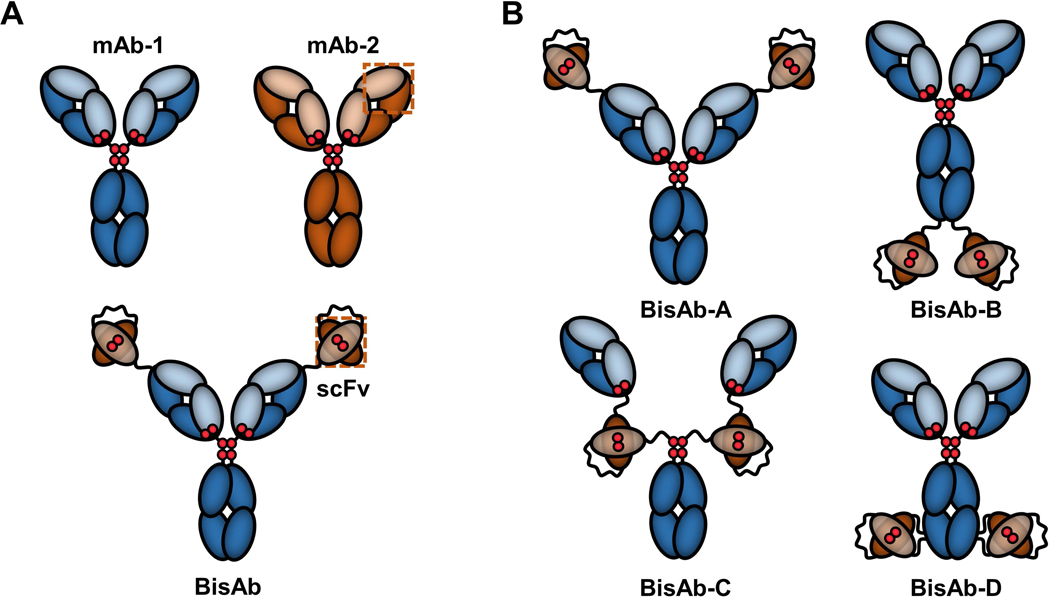
Cartoon representations of IgG-single-chain Fv (scFv) fusion antibodies. (a) Example of a bivalent bispecific antibody (BisAb) formed with the full construct of one parental monoclonal antibody (mAb) linked to scFv fragments derived from the VH and VL antigen-binding domains of a separate parental mAb. Gly/Ser linker peptides are shown as curvy lines. Interchain IgG and interdomain scFv disulfides are represented as red diploids. (b) Fusion of scFv fragments to different parental mAb heavy chain sites yield four unique, symmetrical BisAb formats
While BisAbs have shown promising biological activity in early-stage research programs, the additional scFv fragments have posed unexpected challenges to BisAb manufacturing. It was recently reported that the engineered scFv cysteines of a scFv-fusion BisAb produced monomer size variants and stable dimers with reduced bioactivity as a result of DSB mis-pairing (Cao et al., 2018). Unlike BisAbs, monoclonal antibody (mAb) fragmentation and aggregate formation during manufacturing processes has been previously studied. Reduction of mAbs has been shown to be a result of cell lysis from shear forces during harvest operations, thereby releasing intracellular reductases that can act on the mAb (Handlogten, Zhu, & Ahuja, 2017; Hutchinson, Bingham, Murrell, Farid, & Hoare, 2006; Koterba, Borgschulte, & Laird, 2012; Trexler-Schmidt et al., 2010). Once a mAb has been reduced, fragments may reform the intact antibody with no significant change in bioactivity (Wang, Liu, Cai, Huang, & Flynn, 2015). However, the presence of reduced mAbs in harvested cell culture fluid can also lead to increased aggregation during the low pH viral inactivation step of the antibody purification process (Chung et al., 2017). Removal of antibody aggregates is thus required to ensure that they do not elicit patient immune responses or impact drug efficacy (Fan et al., 2012; Rosenberg, 2006). Controlling and understanding factors impacting product quality are crucial components of process development. There is thus a demonstrated need to examine BisAb susceptibility to CHO cell reductases and resulting impurity formation that may impact product quality and process robustness.
We present the in vitro reduction and reoxidation properties of a set of BisAb formats (IgG-scFv fusions) using the thioredoxin enzyme system to induce DSB reduction. The thioredoxin pathway was specifically selected because it is a well-characterized enzymatic system that has been demonstrated to reduce mAbs during manufacturing operations (Handlogten et al., 2017; Koterba et al., 2012; Magnusson, Björnstedt, & Holmgren, 1997). Due to the reported link between mAb reduction and aggregation during the low pH viral inactivation step of the purification process, we additionally assessed the reoxidation behavior of enzyme-reduced BisAbs with and without low pH treatment followed by room temperature storage, both of which occur during typical BisAb production. Our results demonstrate that BisAb reduction behavior is format-dependent, and subsequent reoxidation leads to aggregation and impurity formation that is distinct from the parental mAb. Importantly, the majority of BisAb impurities are the result of DSB mis-pairing, which indicates that the impact of reduction on downstream processing is much more severe for BisAbs than mAbs.
Materials and Methods
BisAb Structure Preparation
Homology modeling in the Molecular Operating Environment (MOE) (Chemical Computing Group) was performed to prepare separate structures for the parental mAb and the scFv fragment using the Amber10:EHT force field. Crystal structures with high sequence identity to each BisAb component from the Protein Data Bank (PDB) were used as templates. The full-length parental mAb model was constructed based on its heavy chain (PDB template: 1HZH) and light chain (PDB template: 6CYF) sequences. The scFv fragment included the cysteine-engineered mAb-2 VH and VL sequences separated by a (Gly/Ser4)4 peptide (PDB template: 5GRU). Three unique scFv models were then generated by building the (Gly/Ser4)2 linker peptides onto the C-terminus, N-terminus, or both termini for BisAb-A, BisAb-B, and BisAbs-C,D, respectively. To prepare the final BisAb structures, scFv-linker models for each format were grafted onto the parental mAb heavy chains at the designated format position. Further energy minimization was performed using the same force field as initial homology models.
Cell Culture and Production of Antibodies
CHO cell culture fluid for all molecules was generated in 3 L stirred glass bioreactors using proprietary media and nutrient feeds with initial working volumes of 1.5 L as previously described (Handlogten et al., 2017). BisAbs and the parental mAb were purified by protein A capture using MabSelectSuRe resin (GE Healthcare) and an ÄKTA avant 150 system (GE Healthcare). Low-pH viral inactivation was performed by titrating the protein A capture product to pH 3.5 with 1 M acetic acid, followed by neutralization to pH 7.4 with 1 M tris(hydroxymethyl)aminomethane (Tris) base. The product was then concentrated to approximately 30 mg/mL via centrifugation at 4000g using Amicon Ultra-15 centrifugal filter units with an Ultracel-30 membrane (Millipore). Preparative size-exclusion chromatography (SEC) was performed to isolate monomeric BisAbs and the parental mAb using an ÄKTA Explorer 100 system and HiLoad Superdex 200 column (GE Healthcare).
In vitro Reduction of Antibodies by Thioredoxin System
Antibody reduction in vitro utilized purified antibodies and components of the thioredoxin enzymatic pathway (thioredoxin reductase, thioredoxin, and NADPH). Solutions containing thioredoxin system enzymes were made with purified rat recombinant TrxR1 (Cayman Chemical) and human recombinant Trx1 (Abcam) in 50 mM Tris buffer, pH 7.4 (reaction buffer) at concentrations of 0.1 μM and 2.0 μM, respectively. Antibodies were spiked into the enzyme solutions at 1.2 mg/mL, and reactions started upon addition of NADPH (Sigma-Aldrich) to a final concentration of 0.24 mM. Samples were flash-frozen at −80˚C at 0, 5, 15, 30, 45, 60, and 120 minutes after addition of NADPH.
Quantification of Antibody Components by Non-Reduced Capillary Electrophoresis
Quantification of intact antibody species and fragments were quantified using non-reduced capillary electrophoresis (NR-CE). Briefly, samples were analyzed using a LabChip GXII (Perkin-Elmer) and were prepared following the manufacturer’s protocol using non-reducing conditions unless otherwise indicated. Quantification of antibody species was performed using LabChip GXII software. Reduction rates were calculated by fitting percent intact protein a first-order exponential decay using non-linear regression and error analysis with Origin Pro.
Reoxidation of Thioredoxin-Reduced Antibodies
Antibodies were reduced by thioredoxin using the method described for in vitro reduction and held for 30 days at room temperature in cryovials (ThermoFisher, Catalog #368632) with ~2.0 mL of headspace containing air. Non-reduced antibodies were additionally held at room temperature for 30 days to serve as controls. Thioredoxin/Thioredoxin Reductase were not removed because the required co-factor for activity, NADPH, degrades within 24 hours at room temperature in aqueous solutions (Wu, Wu, & Knight, 1986). HP-SEC was used to quantify aggregate percentage. Samples were then analyzed with NR-CE to quantify reformation of intact monomer and the presence of reduced species remaining after the 30-day hold.
Reoxidation of Thioredoxin-Reduced Antibodies Treated with Low pH and Neutralization
Antibodies were reduced by thioredoxin using the method described for kinetics experiments, with the modification of 4 mg/mL antibody and 1 mM NADPH to account for dilution after low pH treatment. The same TrxR1 and Trx1 concentrations used in the kinetics and hold studies were sufficient with higher antibody concentrations since excess NADPH was provided. After 120 minutes, samples were titrated with 1 M acetic acid to pH 3.5, incubated at room temperature for 1 hour, and neutralized to pH 7.4 with 1 M Tris base. Low pH-treated samples were then held for 30 days at room temperature in cryovials. Non-reduced antibodies were additionally treated with low pH and neutralized, then held at room temperature for 30 days to serve as controls. HP-SEC was used to quantify aggregate percentage. Samples were then analyzed as described above using NR-CE to quantify reformation of intact monomer and the presence of reduced species remaining after the 30-day hold.
Antibody Aggregate Quantification
Percentage of antibody aggregates was determined by HP-SEC. Briefly, an Agilent HPLC system (Agilent 1200) was used with a 7.8 × 300 mm TSKgel G3000SW XL column (ToSoh Bioscience) at a flow rate of 1 mL/min and 0.1 M sodium phosphate, 0.1 M sodium sulfate, pH 6.8 for the mobile phase buffer. Protein detection and percent aggregate were then determined by absorbance at 280 nm.
LC-MS Analysis of Reoxidized BisAb Fragments
LC-MS analysis of reoxidized BisAb samples was performed as previously described (Cao et al., 2018). Briefly, samples were deglycosylated with PNGase F prior to reversed-phase chromatography separation and MS using a Synapt G2 mass spectrometer in conjunction with an ultra-performance LC (UPLC) system (Waters).
Results and Discussion
BisAb Homology Models
To evaluate reduction and reoxidation behavior due to differences in BisAb format/structure, we selected a homologous library of symmetric BisAbs with formats A, B, C, and D (Figure 1B). Homology models of BisAbs were constructed to estimate location of the scFv domains and interchain DSBs in three-dimensional space (Figure 2). Each model contains a full-length IgG1 mAb (parental mAb) and appended scFv fragments containing the amino sequences from the VH and VL domains of a separate IgG1 mAb.
Figure 2.
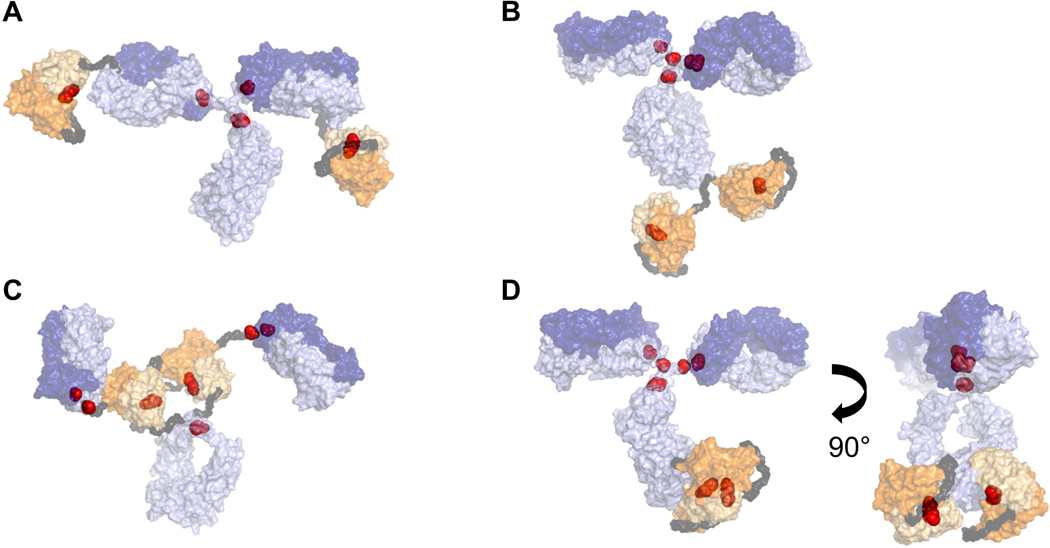
Putative structural homology models for (a) BisAb-A, (b) BisAb-B, (c) BisAb-C, and (d) BisAb-D, which includes two orientations to observe the scFv fragments. Surface representations are shown for the full-length parental mAb (purple), scFv fragments (orange), and Gly/Ser linker peptides (dark gray). Interchain IgG and interdomain scFv disulfide molecules are represented as red spheres. Heavy chain and light chain sequences are depicted in light and dark shades, respectively. BisAb, bispecific antibody; mAb, monoclonal antibody; scFv, single-chain Fv
BisAb-A contains the scFv fragment fused to the N-terminus of the IgG VH domain (Coloma & Morrison, 1997), while BisAb-B includes the scFv fragment attached to the C-terminus of the IgG CH3 domain (Dimasi et al., 2009). The BisAb-C format’s scFv fragment is located between the IgG Fab and Fc regions, directly above the hinge (DiGiandomenico et al., 2014), while the BisAb-D format includes the scFv fragment inserted in the middle of the IgG CH3 domain (Cao et al., 2018). Both BisAb-C and BisAb-D used Gly/Ser peptide linkers at the N-and C-termini of the scFv fragments to connect each end to the IgG sequence.
BisAb Reduction Kinetics and Fragmentation Pathways
As mAb reduction has been linked to CHO cell reductases, we opted to study in vitro BisAb reduction behavior using enzymes as opposed to a reagent such as dithiothreitol (DTT) to better mimic reduction that can occur during manufacturing operations. The susceptibility of BisAbs and the parental mAb to reduction by thioredoxin was determined by fitting the percent of intact molecule (LHHL) to a first-order exponential decay for each molecule (Figure 3A, Supplementary Figure S1). All molecules displayed similar reduction rates, with BisAb-C reducing slightly slower than other BisAbs and the parental mAb. This indicates that reduction occurs rapidly, and the presence of an additional interdomain DSB on the scFv does not present steric competition for reduction by thioredoxin. It is important to note that NR-CE cannot detect reduction of BisAb interdomain scFv DSBs. All scFv DSBs are located within BisAb heavy chains, so there is no appreciable change in BisAb heavy chain molecular weight that can be detected upon scFv DSB reduction by NR-CE.
Figure 3.
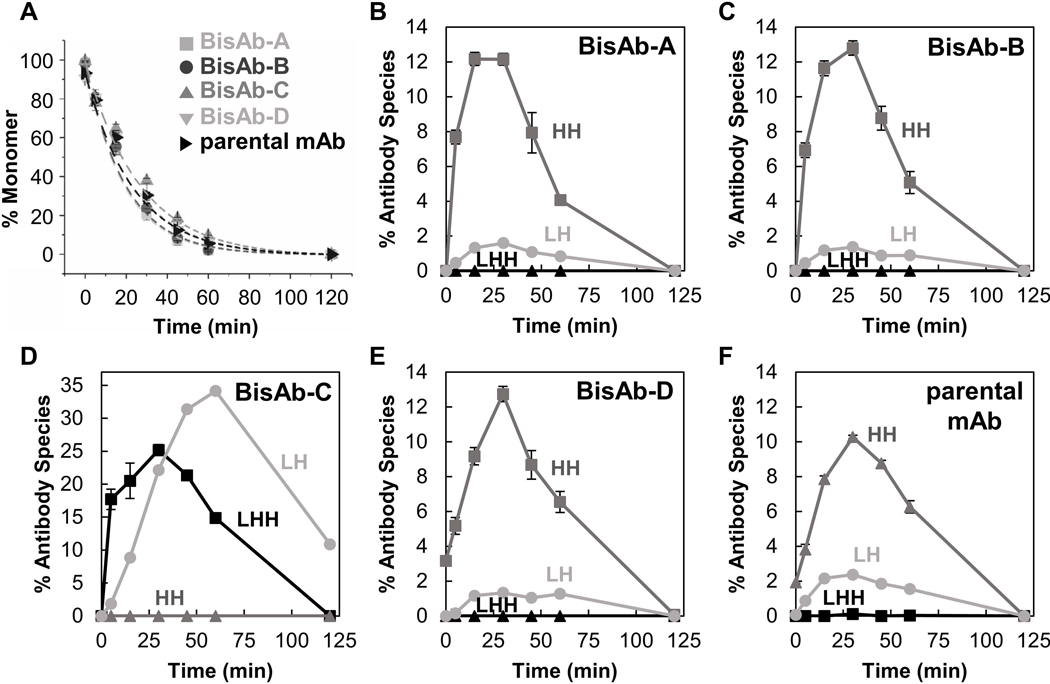
Antibody reduction kinetics (a) and quantification of intermediate species (b–f) generated during reaction with the thioredoxin enzyme system. Kinetic curves were fit to a first-order exponential decay. Intermediate fragments include those missing one light chain (LHH, black squares), heavy chain dimers (HH, dark gray triangles), and half-antibodies (LH, light gray circles). Error bars represent the standard deviation of triplicates
As each molecule went from intact to reduced, several intermediates formed including antibodies missing a light chain (LHH), heavy chain dimers (HH), and half-antibodies (LH) (Figure 3B-F, Supplementary Figure S2). Corresponding LHHL, light chain, and heavy chain percentages are provided in Supplementary Figure S3. Heavy chain dimers were the dominant intermediate species observed during reduction of the parental mAb and BisAb formats A, B, and D. This reduction behavior has been previously observed for IgG1 (κ and λ) mAbs (Liu, Chumsae, Gaza-Bulseco, Hurkmans, & Radziejewski, 2010; Montaño & Morrison, 2002), consistent with the parental mAb class and light chain isotype (IgG1-κ). As BisAb-A, BisAb-B, and BisAb-D show high levels of HH, the hinge region’s relative stability against reduction appears to be retained in these formats and is likely an inherited property of the parental mAb. However, relative L-H and H-H DSB stability may be different in other BisAb molecules regardless of format because L-H stability is dependent on light chain isotype (Liu et al., 2010; Montaño & Morrison, 2002; Wang et al., 2015).
The most pronounced difference between BisAb formats is the reduction pathway for BisAb-C, which produced significant levels of LH and LHH fragments. Half antibody formation is also characteristic of IgG4 mAbs due to hinge flexibility (Bloom, Madanat, Marriott, Wong, & Chan, 1997; Moritz & Stracke, 2017; Silva, Vetterlein, Jose, Peters, & Kirby, 2015). The additional scFv fragments and (Gly4/Ser)2 peptide linkers above the hinge region for the BisAb-C format likely result in highly dynamic Fab arms, which would make the hinge disulfides more accessible to thioredoxin as illustrated in Figure 4. Although LH formation was not unique to BisAb-C, percentages for other antibodies were typically in the range of 1–2%. Appearance of LHH fragments was observed solely during BisAb-C reduction, supporting the hypothesis that readily accessible hinge disulfides may slow interchain L-H DSB cleavage compared to homologous BisAbs and the parental mAb in this study. Competition of reduction in H-H and L-H cannot be generalized for all BisAb formats due to contributions from light chain isotype.
Figure 4.
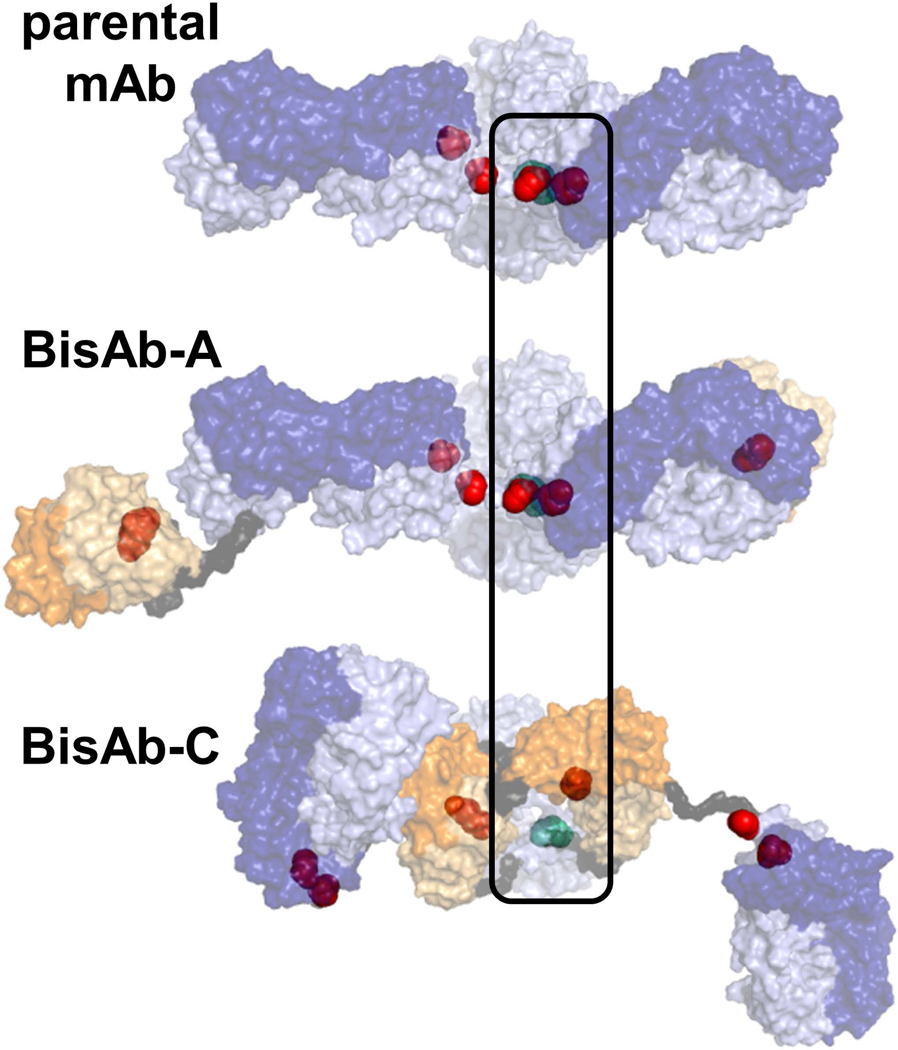
Estimated hinge interchain disulfide bond accessibility based on top-down orientation of homology models. Interchain disulfides are represented as spheres, with the hinge disulfides colored teal
BisAb Reoxidation Profile Following Reduction by Thioredoxin
In addition to characterizing the reduction behavior of BisAbs, the impurity profile upon reoxidation was examined. While an in vitro reoxidation study does not perfectly replicate conditions of stored harvested cell culture, a controlled environment allows for meaningful interpretations of reduction-induced BisAb impurity formation. We reduced BisAbs and the parental mAb with the thioredoxin system and allowed reoxidation for 30 days at room temperature in vials containing air headspace. All BisAbs and the parental mAb were completely reduced at the start of the 30 day hold. Throughout the hold study, changes in aggregate were assessed by high-performance size-exclusion chromatography (HP-SEC) (Table 1, Supplementary Table S1). The percentage of aggregate increased for all antibodies following the 30-day incubation at room temperature. With the exception of BisAb-C, all other BisAb formats showed a larger increase in aggregate levels than the parental mAb. The greatest changes in aggregate were observed for BisAb-D (7.0%), followed by BisAb-B (6.3%) and BisAb-A (3.0%). BisAb-C aggregate levels were comparable to those observed for parental mAb reoxidation (1–2% increase). All control samples that were not subjected to reduction treatment did not show any change in aggregate content during the hold duration.
Table 1.
Antibody aggregate levels from reoxidized samples assessed by HP-SEC.
| Antibody | T=0 % Agg | T=30 days % Agg | Δ % Agg |
|---|---|---|---|
| BisAb-A | 2.0 | 5.0 | 3.0 |
| BisAb-B | 0.7 | 7.0 | 6.3 |
| BisAb-C | 2.0 | 3.0 | 1.0 |
| BisAb-D | 3.0 | 10.0 | 7.0 |
| parental mAb | 0.7 | 3.0 | 2.3 |
To further characterize antibody reoxidation patterns, we assessed antibody species present at the end of the hold study. Ideal reoxidation of mAb interchain disulfides would produce LHHL and potentially partially reoxidized species LHH, HH, and LH. These fragments are expected for typical antibodies and can be identified using NR-CE (Figure 5). Atypical fragments are classified as “new species.” Comparison of reoxidized and partially-reduced samples showed that reduced parental mAb stored for 30 days formed a pronounced new species migrating at the size of light chain dimer (LL). Other new parental mAb species are present in addition to LL, albeit at much lower levels. It is difficult to distinguish LL from H in the parental mAb by NR-CE due to the similar molecular weights of both species. As LL is the result of DSB mis-pairing, analysis with CE under reducing conditions (R-CE) allowed the identification of LL and H. Reoxidized parental mAb showed only L and H species with R-CE, which demonstrates that new species are the result of DSB mis-pairs (Figure 5A). Light chain dimer formation is less problematic because light chain dimer is removed during conventional protein A capture. However, formation of light chain dimer results in loss of product yield.
Figure 5.
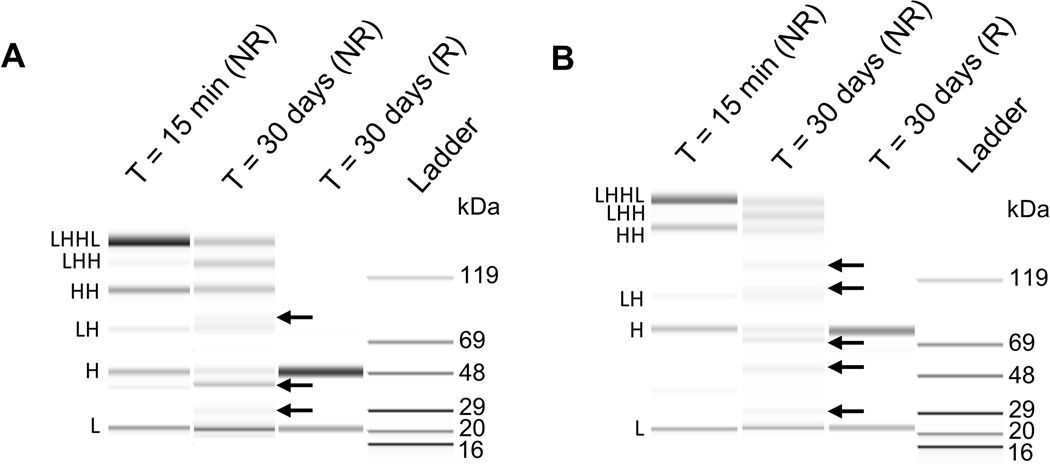
Identification method for new species formed during the reoxidation period of thioredoxin-reduced antibodies. Representative gel-like images are shown for (a) parental mAb and (b) BisAb-A. Nonreduced capillary electrophoresis was performed for partially reduced and reoxidized antibody samples. Partially reduced samples were collected 15 min after incubation with the thioredoxin enzyme system. Samples treated with dithiothreitol (R) are shown for comparison. Arrows indicate new species. BisAb, bispecific antibody; mAb, monoclonal antibody
In contrast, comparison of reoxidized and partially-reduced BisAb samples showed five new species that did not correlate with the expected molecular weights of antibody fragments (LHH, HH, HL) or intact BisAb (LHHL), including one species that migrates at an apparent molecular weight between LH and HH (Figure 5B). Light chain dimer, ~50 kDa, is easily distinguished from BisAb heavy chains, which migrate at approximately 75–80 kDa due to the additional scFv fragments. R-CE analysis further shows that new BisAb species formed after reoxidation are due to DSB mis-pairing since all species reduce completely to L and H. The extent of BisAb mis-pairing suggests that this behavior is due to the appended scFv fragments, as indicated by additional mis-paired BisAbs formed compared to the parental mAb. It is possible that structural rearrangement of mis-paired BisAbs (Figure 6) could induce migration shifts by gel-based methods, leading to the appearance of multiple bands. Therefore, intact mass spectrometry was used to establish that the components present in reoxidized BisAbs have the exact masses corresponding to configurations LHH, HH, LH, L, and H fragments. Analysis by mass spectrometry demonstrated that only masses for the expected BisAb fragments were observed (Supplemental Figures S5-S8) thereby confirming that new species observed by NR-CE are all DSB mis-pairs. These results additionally confirmed that there are no other fragments formed in reoxidized samples such as scFv cleavage from the BisAb. It is expected that BisAb scFv interdomain disulfides would be targeted by thioredoxin in addition to sites on the IgG1-like portion of the molecule based on previous mAb reduction studies (Chung et al., 2017). Cao et al. have demonstrated that BisAb scFv DSBs can be reduced, leading to dimer formation in a different molecule possessing the BisAb-D format. These results support the hypothesis that CHO cell reductases such as thioredoxin can produce BisAbs with free scFv thiols that may yield complex fragments and aggregates upon reoxidation.
Figure 6.
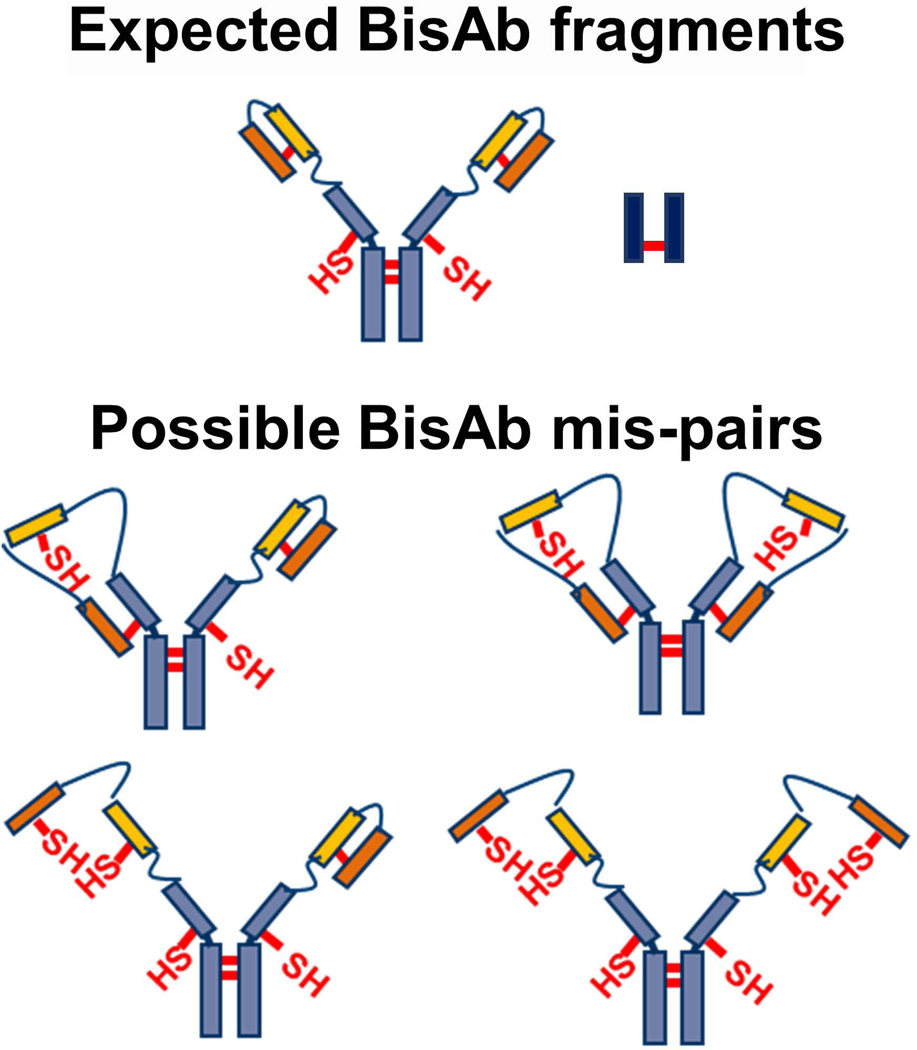
Potential new species formed during reduced BisAb reoxidation. (Top) BisAb heavy and light chain dimers are expected fragments. (Bottom) Free thiols on BisAb heavy chain dimers, for example, may react with free thiols present in reduced scFv fragments to produce mispaired disulfide bonding. BisAb, bispecific antibody; scFv, single-chain Fv
Table 2 and Supplementary Table S2 summarize the percentage of intact monomer, expected antibody fragments, and new species that were determined by NR-CE. Reduced BisAb-C reformed 56% intact monomer, which was greater than intact monomer that reformed for all other tested antibodies. It is possible that the ability of BisAb-C to reform monomers to a greater extent than other BisAb formats contributed to its minimal change in aggregate levels detected during the hold study (Table 1). Overall, the percentage of new species formed for BisAb-A, BisAb-B, and BisAb-D was ~30%, while BisAb-C produced ~15% new species. High levels of new species observed for BisAbs were reflected in the band migrating between LH and HH, light chain dimer, and for BisAb-C, an additional band migrating above LHHL (Supplemental Figure S4). Though new species and fragment levels were comparable between BisAb-A, BisAb-B, and BisAb-D, it is possible that improved BisAb-A monomer formation contributed to decreased aggregation (Table 1). Parent mAb new species formed were ~10% and was mainly light chain dimer. Additionally, parental mAb reoxidation behavior differs from previous reoxidation studies with IgG1 mAbs in which intact monomer reformed rapidly, and light chain dimer was not observed (Wang et al., 2015). Differences are likely due to specific properties of the antibodies and/or sample storage, oxygen level, or mixing.
Table 2.
Antibody species from reoxidized samples quantified by fragment analysis.
| Antibody | Control (Not reduced), % Monomer | Reduced, % Monomer | Reduced, % New Species | Reduced, % Fragment |
|---|---|---|---|---|
| BisAb-A | 95 | 12 | 29 | 59 |
| BisAb-B | 96 | 6 | 34 | 60 |
| BisAb-C | 98 | 56 | 15 | 29 |
| BisAb-D | 94 | 6 | 26 | 68 |
| parental mAb | 90 | 13 | 10† | 77 |
Light chain dimer
BisAb Reoxidation Profile with Low pH Treatment
Low pH viral inactivation is a common step in the antibody purification process and has been shown to affect mAb reoxidation and aggregation behavior (Vázquez-Rey & Lang, 2011). To determine the effect of low pH viral inactivation on aggregate formation in reduced BisAb samples, a separate study was performed in which antibodies reduced by the thioredoxin system underwent low pH treatment and neutralization. After complete reduction by thioredoxin, antibodies were titrated to pH 3.5 with acetic acid and neutralized to pH 7.4 with Tris base. Low-pH treated, reduced antibodies were then allowed to reoxidize for 30 days at room temperature.
Table 3 includes aggregate analysis for reoxidized antibodies that were initially reduced and treated with low pH/neutralization. Unlike the reoxidation study with room temperature storage alone, low-pH treated samples did not show noticeably increased aggregate levels after reoxidation. The highest aggregate percentage observed was for the BisAb-A sample at 1.6% aggregate. Interestingly, BisAb-D did not show any increase in aggregate levels after the reoxidation period even though BisAb-D aggregate formation was most severe with room temperature incubation alone. Aggregate formation during the reoxidation period does not appear to be influenced by initial aggregate present since BisAb-A and the parental mAb started with comparable levels of aggregate in both studies yet produced much lower aggregate formation when exposed to low pH. Overall, the reoxidation behavior for thioredoxin-reduced antibodies that were subjected to low pH/neutralization did not result in increased aggregate levels as expected based on previous studies with an IgG2 mAb (Chung et al., 2017). Several possibilities may explain this discrepancy including differences in IgG subclass. For example, it has been previously reported that the IgG2 subclass has a higher propensity to aggregate compared to IgG1 (Franey, Brych, Kolvenbach, & Rajan, 2010). IgG2 mAbs have two additional DSBs in the hinge region compared to IgG1 mAbs, suggesting that interchain DSBs may influence aggregation behavior.
Table 3.
Antibody aggregate levels from reoxidized samples treated with low pH and neutralization.
| Antibody | T=0 % Agg | T=30 days % Agg | Δ % Agg |
|---|---|---|---|
| BisAb-A | 1.5 | 1.6 | 0.1 |
| BisAb-B | 1.2 | 1.3 | 0.1 |
| BisAb-C | 0.1 | 0.8 | 0.7 |
| BisAb-D | 1.5 | 1.5 | 0.0 |
| parental mAb | 0.6 | 0.9 | 0.3 |
We additionally compared intact monomer, new species, and fragment levels for low-pH treated, reoxidized antibodies (Table 4) with the previous reoxidation study using the same species identification methods. Similar to the decreased aggregate formation observed for low pH-treated BisAbs, there was a decrease in percentage of new species present after reoxidation for low pH/neutralization-treated antibodies. Light chain dimer, for example, was not observed for any samples treated with low pH, which was an unexpected result. This led to no new species present for the parental mAb. The new species persisting for low pH-treated BisAb-A, BisAb-B, and BisAb-D belonged to the same band migrating between LH and HH that was observed for reoxidized BisAb samples without low pH/neutralization (Figure 5B). The BisAb-C band migrating above LHHL also remained with low pH treatment.
Table 4.
Antibody species from reoxidized samples treated with low pH and neutralization.
| Antibody | Control (Not reduced), % Monomer | Reduced, % Monomer | Reduced, % New Species | Reduced, % Fragment |
|---|---|---|---|---|
| BisAb-A | 89 | 59 | 11 | 30 |
| BisAb-B | 90 | 51 | 13 | 36 |
| BisAb-C | 94 | 42 | 11 | 47 |
| BisAb-D | 81 | 34 | 20 | 46 |
| parental mAb | 85 | 19 | 0 | 81 |
Differences in levels of aggregate and antibody species between the two reoxidation studies are due to the low pH treatment since antibodies were stored under the same conditions, neutral pH and room temperature. Tris and acetate buffers were considered appropriate for titration since the reduced samples were in Tris buffer initially, and the use of different acids for low pH treatment has not had an impact on aggregation for multiple in-house molecules with reduction. We propose that antibody electrostatic surface properties at low pH drive differences in aggregation and reoxidation behavior. At pH 3.5, the BisAb and parent mAb light and heavy chain fragments possess a high number of positively charged surface residues from protonated carboxylate side chains (Supplemental Figure S9). Electrostatic repulsion between fragments during the low pH incubation period may be crucial for preventing long-term new species and aggregate formation. The pronounced decrease in levels of light chain dimer, BisAb-specific new species, and aggregates with the mimicked viral inactivation step could be explained by this electrostatic repulsion effect. Exposure of IgG mAbs to low pH or increasing ionic strength, which is expected to occur upon titration with acetic acid and Tris buffers (Trexler-Schmidt et al., 2009), has been implicated in promoting aggregate formation through partial unfolding (Buchner et al., 1991; Ejima et al., 2007) and/or colloidal instability (Jin et al., 2019; Saito et al., 2013). Interestingly, for the BisAbs and parental mAb in this study, increased conductivity and low pH exposure improve long-term molecule stability. Since the improved stability at reduced pH and increased conductivity was observed for both the parental mAb and the BisAbs, this trait in the BisAbs may be an inherited property from the parental mAb. However, future studies will be required to fully elucidate the phenomena leading to decreased aggregation propensity during low pH treatment, as the incubation period is relatively short (one hour) relative to the total storage time (30 days) at neutral pH.
Conclusions
In summary, we have demonstrated that the BisAb formats in this study and the parental mAb exhibit similar susceptibilities to reduction by thioredoxin and that the antibody format influences intermediate species pathways during enzymatic reduction. Additionally, the reoxidation behavior of BisAbs has more severe consequences than mAbs and is dependent on sample treatment after reduction. For downstream processing, our results suggest that stored harvested cell culture fluid containing reduced BisAb product may lead to both aggregate formation and DSB mis-pairing if purification does not immediately occur. However, immediate purification of reduced BisAb cell culture fluid would likely not prevent formation of disulfide-scrambled BisAb fragments under oxidizing conditions. The appearance of multiple BisAb mis-paired fragments in reoxidized samples poses a much greater challenge to downstream purification, as these may not be easily removed during polishing steps. Precautions should therefore be taken to avoid reduction of molecules with engineered DSBs during manufacturing. Beyond the BisAb formats presented in this study, there are many new mAb-like molecules in the clinic that rely on engineered DSBs for their structures (Dahlén, Veitonmäki, & Norlén, 2018; Kontermann & Brinkmann, 2015). Our results demonstrating DSB-dependent mis-pairing and aggregation are likely applicable to these novel constructs and may aid future considerations for molecule design and screening.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Krista Evans, Judith Klover, and Thomas Albanetti for their work developing the cell lines expressing the BisAbs used in this work.
Funding
This work was supported in part by the National Institutes of Health (NIGMS, 5T32 GM008715).
Footnotes
Disclosure of Potential Conflicts of Interest
The authors have no conflict of interest to declare.
Supplementary Material
Supplemental data for this article can be accessed online.
References
- Benschop RJ, Chow C-K, Tian Y, Nelson J, Barmettler B, Atwell S, … Allan BW (2019). Development of tibulizumab, a tetravalent bispecific antibody targeting BAFF and IL-17A for the treatment of autoimmune disease. mAbs, 1–16. doi: 10.1080/19420862.2019.1624463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom JW, Madanat MS, Marriott D, Wong T, & Chan SY (1997). Intrachain disulfide bond in the core hinge region of human IgG4. Protein Science, 6(2), 407–415. doi:doi: 10.1002/pro.5560060217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann U, & Kontermann RE (2017). The making of bispecific antibodies. mAbs, 9(2), 182–212. doi: 10.1080/19420862.2016.1268307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner J, Renner M, Lilie H, Hinz HJ, Jaenicke R, Kiefhaber T, & Rudolph R. (1991). Alternatively folded states of an immunoglobulin. Biochemistry, 30(28), 6922–6929. doi: 10.1021/bi00242a016 [DOI] [PubMed] [Google Scholar]
- Cao M, Wang C, Chung WK, Motabar D, Wang J, Christian E, … Liu. (2018). Characterization and analysis of scFv-IgG bispecific antibody size variants. mAbs, 10(8), 1236–1247. doi: 10.1080/19420862.2018.1505398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WK, Russell B, Yang Y, Handlogten M, Hudak S, Cao M, … Zhu M. (2017). Effects of antibody disulfide bond reduction on purification process performance and final drug substance stability. Biotechnology and Bioengineering, 114(6), 1264–1274. doi:doi: 10.1002/bit.26265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coloma MJ, & Morrison SL (1997). Design and production of novel tetravalent bispecific antibodies. Nature Biotechnology, 15, 159. doi: 10.1038/nbt0297-159 [DOI] [PubMed] [Google Scholar]
- Dahlén E, Veitonmäki N, & Norlén P. (2018). Bispecific antibodies in cancer immunotherapy. Therapeutic advances in vaccines and immunotherapy, 6(1), 3–17. doi: 10.1177/2515135518763280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiandomenico A, Keller AE, Gao C, Rainey GJ, Warrener P, Camara MM, … Stover CK. (2014). A multifunctional bispecific antibody protects against Pseudomonas aeruginosa. Science Translational Medicine, 6(262), 262ra155. Retrieved from http://stm.sciencemag.org/content/6/262/262ra155.abstract [DOI] [PubMed] [Google Scholar]
- Dimasi N, Gao C, Fleming R, Woods RM, Yao X-T, Shirinian L, … Wu H. (2009). The Design and Characterization of Oligospecific Antibodies for Simultaneous Targeting of Multiple Disease Mediators. Journal of Molecular Biology, 393(3), 672–692. doi: 10.1016/j.jmb.2009.08.032 [DOI] [PubMed] [Google Scholar]
- Ejima D, Tsumoto K, Fukada H, Yumioka R, Nagase K, Arakawa T, & Philo JS (2007). Effects of acid exposure on the conformation, stability, and aggregation of monoclonal antibodies. Proteins: Structure, Function, and Bioinformatics, 66(4), 954–962. doi: 10.1002/prot.21243 [DOI] [PubMed] [Google Scholar]
- Fan X, Brezski RJ, Fa M, Deng H, Oberholtzer A, Gonzalez A, … An Z. (2012). A single proteolytic cleavage within the lower hinge of trastuzumab reduces immune effector function and in vivo efficacy. Breast Cancer Research : BCR, 14(4), R116–R116. doi: 10.1186/bcr3240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franey H, Brych SR, Kolvenbach CG, & Rajan RS (2010). Increased aggregation propensity of IgG2 subclass over IgG1: Role of conformational changes and covalent character in isolated aggregates. Protein Science, 19(9), 1601–1615. doi:doi: 10.1002/pro.434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handlogten MW, Zhu M, & Ahuja S. (2017). Glutathione and thioredoxin systems contribute to recombinant monoclonal antibody interchain disulfide bond reduction during bioprocessing. Biotechnology and Bioengineering, 114(7), 1469–1477. doi:doi: 10.1002/bit.26278 [DOI] [PubMed] [Google Scholar]
- Hutchinson N, Bingham N, Murrell N, Farid S, & Hoare M. (2006). Shear stress analysis of mammalian cell suspensions for prediction of industrial centrifugation and its verification. Biotechnology and Bioengineering, 95(3), 483–491. doi: 10.1002/bit.21029 [DOI] [PubMed] [Google Scholar]
- Jin W, Xing Z, Song Y, Huang C, Xu X, Ghose S, & Li ZJ (2019). Protein aggregation and mitigation strategy in low pH viral inactivation for monoclonal antibody purification. mAbs, null-null. doi: 10.1080/19420862.2019.1658493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontermann RE, & Brinkmann U. (2015). Bispecific antibodies. Drug Discovery Today, 20(7), 838–847. doi: 10.1016/j.drudis.2015.02.008 [DOI] [PubMed] [Google Scholar]
- Koterba KL, Borgschulte T, & Laird MW (2012). Thioredoxin 1 is responsible for antibody disulfide reduction in CHO cell culture. Journal of Biotechnology, 157(1), 261–267. doi: 10.1016/j.jbiotec.2011.11.009 [DOI] [PubMed] [Google Scholar]
- Liu H, Chumsae C, Gaza-Bulseco G, Hurkmans K, & Radziejewski CH (2010). Ranking the Susceptibility of Disulfide Bonds in Human IgG1 Antibodies by Reduction, Differential Alkylation, and LC−MS Analysis. Analytical Chemistry, 82(12), 5219–5226. doi: 10.1021/ac100575n [DOI] [PubMed] [Google Scholar]
- Mack M, Riethmüller G, & Kufer P. (1995). A small bispecific antibody construct expressed as a functional single-chain molecule with high tumor cell cytotoxicity. Proceedings of the National Academy of Sciences, 92(15), 7021. Retrieved from http://www.pnas.org/content/92/15/7021.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson CGM, Björnstedt M, & Holmgren A. (1997). Human IgG is substrate for the thioredoxin system: Differential cleavage pattern of interchain disulfide bridges in IgG subclasses. Molecular Immunology, 34(10), 709–717. doi: 10.1016/S0161-5890(97)00092-8 [DOI] [PubMed] [Google Scholar]
- McCall AM, Shahied L, Amoroso AR, Horak EM, Simmons HH, Nielson U, … Weiner LM. (2001). Increasing the Affinity for Tumor Antigen Enhances Bispecific Antibody Cytotoxicity. The Journal of Immunology, 166(10), 6112. Retrieved from http://www.jimmunol.org/content/166/10/6112.abstract [DOI] [PubMed] [Google Scholar]
- Montaño RF, & Morrison SL (2002). Influence of the Isotype of the Light Chain on the Properties of IgG. The Journal of Immunology, 168(1), 224. doi: 10.4049/jimmunol.168.1.224 [DOI] [PubMed] [Google Scholar]
- Moritz B, & Stracke JO (2017). Assessment of disulfide and hinge modifications in monoclonal antibodies. Electrophoresis, 38(6), 769–785. doi: 10.1002/elps.201600425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg AS (2006). Effects of protein aggregates: An immunologic perspective. The AAPS Journal, 8(3), E501–E507. doi: 10.1208/aapsj080359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Hasegawa J, Kobayashi N, Tomitsuka T, Uchiyama S, & Fukui K. (2013). Effects of Ionic Strength and Sugars on the Aggregation Propensity of Monoclonal Antibodies: Influence of Colloidal and Conformational Stabilities. Pharmaceutical Research, 30(5), 1263–1280. doi: 10.1007/s11095-012-0965-4 [DOI] [PubMed] [Google Scholar]
- Sedykh SE, Prinz VV, Buneva VN, & Nevinsky GA (2018). Bispecific antibodies: design, therapy, perspectives. Drug Design, Development and Therapy, 12, 195–208. doi: 10.2147/DDDT.S151282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J-P, Vetterlein O, Jose J, Peters S, & Kirby H. (2015). The S228P Mutation Prevents in Vivo and in Vitro IgG4 Fab-arm Exchange as Demonstrated using a Combination of Novel Quantitative Immunoassays and Physiological Matrix Preparation. The Journal of Biological Chemistry, 290(9), 5462–5469. doi: 10.1074/jbc.M114.600973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trexler-Schmidt M, Sze-Khoo S, R. Cothran A, Q. Thai B, Sargis S, Lebreton B, … S. Blank G. (2009). Purification Strategies to Process 5 g/L Titers of Monoclonal Antibodies. Biopharm International, 8–15. [Google Scholar]
- Trexler-Schmidt M, Sargis S, Chiu J, Sze-Khoo S, Mun M, Kao YH, & Laird MW (2010). Identification and prevention of antibody disulfide bond reduction during cell culture manufacturing. Biotechnology and Bioengineering, 106(3), 452–461. doi:doi: 10.1002/bit.22699 [DOI] [PubMed] [Google Scholar]
- Vázquez-Rey M, & Lang DA (2011). Aggregates in monoclonal antibody manufacturing processes. Biotechnology and Bioengineering, 108(7), 1494–1508. doi:doi: 10.1002/bit.23155 [DOI] [PubMed] [Google Scholar]
- Wang T, Liu YD, Cai B, Huang G, & Flynn GC (2015). Investigation of antibody disulfide reduction and re-oxidation and impact to biological activities. Journal of Pharmaceutical and Biomedical Analysis, 102, 519–528. doi: 10.1016/j.jpba.2014.10.023 [DOI] [PubMed] [Google Scholar]
- Wu JT, Wu LH, & Knight JA (1986). Stability of NADPH: effect of various factors on the kinetics of degradation. Clinical Chemistry, 32(2), 314. Retrieved from http://clinchem.aaccjnls.org/content/32/2/314.abstract [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


