Abstract
Exogenous electrical fields have been explored in regenerative medicine to increase cellular expression of pro-regenerative growth factors. Adipose-derived stem cells (ASCs) are attractive for regenerative applications, specifically for neural repair. Little is known about the relationship between low-level electrical stimulation (ES) and ASC regenerative potentiation. In this work, patterns of ASC expression and secretion of growth factors (i.e., secretome) were explored across a range of ES parameters. ASCs were stimulated with low-level stimulation (20 mV/mm) at varied pulse frequencies, durations, and with alternating versus direct current. Frequency and duration had the most significant effects on growth factor expression. While a range of stimulation frequencies (1, 20, 1000 Hz) applied intermittently (1 h × 3 days) induced upregulation of general wound healing factors, neural-specific factors were only increased at 1 Hz. Moreover, the most optimal expression of neural growth factors was achieved when ASCs were exposed to 1 Hz pulses continuously for 24 h. In evaluation of secretome, apparent inconsistencies were observed across biological replications. Nonetheless, ASC secretome (from 1 Hz, 24 h ES) caused significant increase in neurite extension compared to non-stimulated control. Overall, ASCs are sensitive to ES parameters at low field strengths, notably pulse frequency and stimulation duration.
Keywords: Frequency, Secretome, Growth factor, Neural regeneration, Cell-based therapies, SH-SY5Y, Neurite outgrowth, Schwann cell
INTRODUCTION
Endogenous electric fields present at damaged tissue sites play a significant role in controlling tissue repair and regeneration.20 These phenomena have been recapitulated for therapeutic purposes using externally applied electrical stimulation (ES) to direct cell migration, cell proliferation, and nerve sprouting at wounds.7,20 It has been documented that ES has beneficial effects on neurite outgrowth, axonal regeneration, and muscle regrowth after injury.4,7,8 ES provides guidance cues to direct cell behavior, complementing other signals such as those for chemotaxis,9,20,32 but also indirectly promotes regeneration and growth by increasing growth factor production in stimulated cells.
An advantage of ES is that it is a non-drug approach for promoting beneficial cell behavior, particularly in difficult regenerative contexts such as central nervous system (CNS) repair.11 ES has been used in other neural applications as well to promote axonal outgrowth and to activate or facilitate activation of neuronal networks around lesions.13 Although promising results have been found with direct ES of neural tissue, performing such procedures in vivo is quite invasive, as most ES methods require electrode implantation. In studies regarding peripheral nerve injury, it has been suggested that ES effects on regeneration can be attributed to secondary responses of stimulated cells. For example, ES has been found to not only induce upregulation of neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) in neurons,4 but also to stimulate release of these factors from nearby Schwann cells.34 Further, Koppes et al. have discovered that ES of neuron and Schwann cell co-cultures significantly increases neurite outgrowth compared to ES of neurons alone,16 suggesting that stimulation of trophic factor release from support cells could be critical.
Hence, a promising therapeutic option for neural injuries has emerged with the use of secreted products (i.e., secretome) of electrically stimulated cells, rather than ES on the damaged tissue itself. ES of various native cell types, such as cardiac cells and Schwann cells, has enabled regulation of secretome factors, cell alignment, and contractility.15,26 Rackauskas et al. have shown that the frequency of pulsed ES can control the amount of vascular endothelial growth factor (VEGF) secreted by cardiomyocytes.26 In addition to tissue resident cells, various sources of stem cells, including mesenchymal stem cells (MSCs) and neural stem cells (NSCs), have also been explored to determine if ES can elicit production of a broad range of pro-regenerative molecules that may be applicable in multiple regenerative contexts.2,22,40 Studies on MSCs have also demonstrated a synergistic effect in clinical applications of stem cells with exogenous ES.18
In this study, a particular subtype of MSCs, adipose-derived stem cells (ASCs), were investigated following ES. ASCs are attractive given their ability to secrete a cocktail of pro-regenerative and neurotrophic factors and their abundant sourcing methods by liposuction.29 Although still not well characterized, studies have shown that ASCs have potential for enhanced therapeutic responses to exogenous ES.2,22,30 ASCs have been reported to respond to intermittent ES (1–4 h/day) by directional migration towards the cathode and by upregulating specific mRNA such as VEGF.22,38 Additionally, Beugels et al. have demonstrated increased ASC secretion of VEGF after continuous ES (72 h, 400 mV/mm, 2 Hz).2 Since it is documented that ASCs secrete VEGF in response to ES, it is possible that this response may extend to neurotrophic factors as well. One study demonstrated that biomolecular stimulation of ASCs could simultaneously increase secreted levels of VEGF, BDNF, and GDNF in ASCs to make their secretome effective for neural repair.14 However, the effects of ES (and specifically low-level ES) on ASC expression and secretion of neurotrophic factors are largely unknown and require further exploration.
Overall, exogenous ES, ASC-based therapies, and stem cell secretome have each shown beneficial effects on neural regeneration and have merited consideration in combination for this work. In this study, we investigated the effect of low-level ES on ASC expression and production of growth factors. Despite existing studies on ASC response to ES, it is not currently known how ASCs respond to low-level fields that are physiologically similar to neural cell signals and exogenous signals that are known to elicit neural cell response and axonal growth (i.e., stimulation with frequency ≤ 20 Hz and field magnitude < 100 mV/mm).15,25,31,39 This study sought to investigate several ES parameters relevant to other neural-based studies regarding ASC response and production of pro-regenerative factors. Given that the study focused on low-level electrical fields, results could be relevant to transplanted ASCs, but the primary focus was to understand ASC secretome production to advance cell-free delivery options for neural injuries. The ES parameters studied were frequency of alternating current (AC) pulses, AC versus direct current (DC), and time of stimulation, as contributors to ASC behavior. The goal was to induce pro-regenerative production of multiple growth factors as a result of ES and to determine which of the ES parameters had the greatest effect on cell behavior. Additionally, we present two custom ES bioreactors that are adaptable to a range of ES modalities. Our results provide insights/considerations for modeling applied versus true electrical field strengths as a function of bioreactor geometry and material design.
MATERIALS AND METHODS
Bioreactor Fabrication and Design
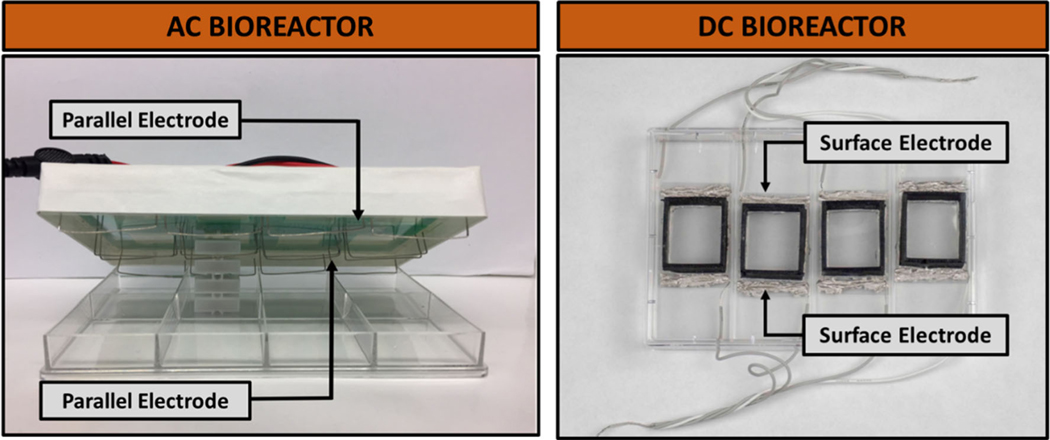
Two different bioreactors were used in this work, which are referred to as either “AC bioreactor” or “DC bioreactor.” The AC bioreactor provides AC stimulation through culture media using submerged electrodes, whereas the DC bioreactor passes DC through a conductive substrate with electrodes attached outside of the culture area. Images of both bioreactors are found in Fig. 1. The AC bioreactor is composed of platinum electrodes secured to the top lid of an 8-well tissue culture plate (Thermo Fisher Scientific 12–565-497 or 167064) using a printed circuit board to connect the positive and negative electrodes appropriately. The platinum wires (Fisher Scientific AA10286BY) are bent into an “L” shape and are submerged in media when placed on a respective well plate. The AC bioreactor was manufactured under similar principle to previous publications by Mobini et al.21,22 The lid is interchangeable between any similar 8-well plate, which allows for easy handling and sterilization. This bioreactor applies an electrical field up to ~ 30 mV/mm (maximized when applied voltage is 1 V) before surpassing estimated electrolysis limits based on the double-layer capacitance theory.12,28 Other versions of this bioreactor have been used previously to study the effect of ES on ASCs22 and MSCs.23 The culture area is 10.5 cm2 for the AC bioreactor and 5.25 cm2 for the DC bioreactor.
FIGURE 1.
Bioreactors used for exogenous ES application. The alternating current (AC) bioreactor contains parallel platinum electrodes spaced about 3.4 cm apart in a standard 8-well plate. The direct current (DC) bioreactor is made up of a conductive indium tin oxide (ITO) substrate with surface electrodes epoxied about 3.4 cm apart, on the outside of a rectangular nylon well.
The DC bioreactor consists of a conductive indium tin oxide (ITO) substrate upon which cells are cultured. The bioreactor wells were manufactured using 3D-printed nylon structures attached to the ITO substrate using silicone glue. Individual wells were 3D-printed using nylon material (Ultimaker 2.85 mm NFC, Dynamism #1609), soaked in 70% ethanol, followed by ddH2O to clean. For complete sterilization, the bioreactors were exposed to ethylene oxide. Prior to seeding cells, wells were rinsed with sterile PBS. Supplemental Fig. S1 shows that the materials for this reactor are not toxic and allow for high viability of ASCs over a multiday period required for testing. This DC bioreactor applies the electric field to the cells through the ITO substrate by passing current between two electrodes epoxied on the substrate outside of the 28 × 24.5 mm wells, the same distance apart as the electrodes in the AC bioreactor (approximately 3.4 cm). The design of this bioreactor is highly customizable and can be adapted for various well sizes and field strengths depending on user need.
The DC bioreactor was designed such that the current will move between the two electrodes through the ITO surface, meaning that there are no capacitive effects associated with the electrical field calculation (voltage/distance). However, for the AC bioreactor, there are capacitive effects associated with current moving through culture media. Characterization of these effects were first carried out using electrochemical impedance and circuit modeling (described in Supplemental section b and Fig. S2).
ASC Culture
Human ASCs were purchased from Lonza (PT-5006) and expanded in complete growth medium consisting of DMEM-F12 (Caisson DF:15), 10% fetal bovine serum (FBS, Atlanta Biologicals, S11150), and 1% penicillin/streptomycin (P/S, Fisher Scientific, 091674049). Passages 3–6 were used in experiments. For non-tissue culture-treated plates (i.e., those used in studies with the AC bioreactor), a poly-D-lysine coating (PDL, Fisher Scientific ICN15017510) was first applied by adding 20 μg/mL PDL in water to wells (3 mL/well). For passaging, cells were incubated at 37 °C for 4.5 min in 0.25% trypsin/EDTA (Thermo Fisher 25200072). For the AC bioreactor, cells were seeded at 1 × 105/well, and for the DC bioreactor, ASCs were seeded at 7.5 × 104/well.
Electrical Stimulation
After seeding, cells were incubated overnight, after which the growth medium was switched to StemPro MSC SFM XenoFree complete medium (StemPro basal medium with 1% StemPro supplement and 1% P/S). Secretome media choice is described in more detail in Supplemental Fig. S3. ES was applied by attaching bioreactors to a either pulse generator (LXI TG2512A) for AC, or an ELC AL 924A DC voltage supply for DC. In all cases, a voltage amplitude (Vamp) of 674–700 mV was applied to achieve an electric field of approximately 20 mV/mm in either bioreactor. AC stimulation regimes consisted of pulse waves applied at 0.5 ms pulse widths with 0 offset and frequencies of 1, 20, of 1000 Hz. Two stimulation regimes were assessed: intermittent (1 h × 3 days) or continuous (24 h). Non-electrically stimulated control plates were included in each experiment, and three biological replicates were conducted per regime tested.
Secretome and Cell Sample Collection
Cell processing was performed 24 h after ES ended. First, secretome was pooled for cells from the same biological replicates and stimulation runs. Secretome was centrifuged at 1000 rpm for 4 min to remove debris and then was frozen at − 80 °C. Next, cell lysis buffer was prepared with 10 μL of β-mercaptoethanol (Sigma-Aldrich M3148) per 1 mL of RLT Buffer Plus (Qiagen). 350 μL of this solution was added to each bioreactor well. Cells were immediately lysed for RNA extraction after secretome removal.
RNA Extraction and Polymerase Chain Reaction (PCR)
RNA was extracted from cell lysates using the RNeasy Plus kit (Qiagen 74134) according to the manufacturer instructions. Briefly, lysates were spun through gDNA Eliminator spin columns (included in kit), and then 350 μL of 70% ethanol was added per sample. Samples were transferred to RNeasy spin columns before washing with recommended buffers. RNA was eluted in 25 μL water prior to determining RNA concentration and purity with a NanoDrop spectrophotometer. Appropriate sample purity was considered a 260/280 ratio of between 1.8 and 2.1. RNA was reverse transcribed using High-capacity cDNA Reverse Transcription kit (Thermo Fisher 4368814) per the manufacturer’s instructions.
PCR primers were designed using Primer Express software (version 3.0.1) for the following genes: VEGF, tissue inhibitor of metalloproteinases 1 (TIMP1), BDNF, and GDNF (Table 1). VEGF and TIMP1 were chosen as factors involved in generalized wound healing that were previously documented as altered in ASCs under other ES conditions.2,30 BDNF and GDNF were studied given their expression by ASCs and their ubiquitous actions in neural regeneration.14,19 Hypoxanthine-guanine phosphoribosyl-transferase (HPRT1) was used as the housekeeping gene. Quantitative reverse transcription PCR (RT-qPCR) was performed using 30 μL reaction volumes with PowerUp SYBR Green master mix (Thermo Fisher A25742) in a QuantiStudio 6 Flex system. All reactions were optimized for appropriate amplification at a Tm of 58 °C. Gene expression was quantified using the ΔΔCt method.
TABLE 1.
Primer sequences for target genes.
| Gene | Species | Forward (5′ to 3′) | Reverse | Tm |
|---|---|---|---|---|
| VEGF-A | Human | CAC CCA CCC ACA TAC ATA CAT | AGT CTC TCA TCT CCT CCT CTT C | 58 °C |
| TIMP-1 | ACT GTT GGC TGT GAG GAA TG | GAA GAA AGA TGG GAG TGG GAA C | ||
| BDNF | AGA GGC TTG ACA TCA TTG GCT G | CAA AGG CAC TTG ACT ACT GAG CAT C | ||
| GDNF | CAC CAG ATA AAC AAA TGG CAG TGC | CGA CAG GTC ATC ATC AAA GGC G | ||
| HPRT1 | TTG CTG ACC TGC TGG ATT AC | CTT GCG ACC TTG ACC ATC TT |
Enzyme-Linked Immunosorbent Assay (ELISA)
Several ELISAs were used in this study to evaluate secretome composition. In all ELISAs, secretome was concentrated using centrifugal filter units (Fisher Scientific UFC800324). Secretome was centrifuged in the filters at 4000×g for 20 min at 4 °C, resulting in volume reduction from 4 mL to approximately 500–700 μL (total protein was measured with Pierce 660 assay to ensure consistencies in concentrating the samples). First, a human VEGF ELISA (Thermo Fisher Scientific KHG10111 and BMS277–2) was performed on secretome collected from frequency-testing regimes in the AC bioreactor (20 mV/mm field strength at 1, 20, and 1000 Hz). For the continuous stimulation (i.e., 24 h, 1 Hz regime), a human BDNF ELISA (RayBiotech, ELH-BDNF-2) and a human GDNF ELISA (RayBiotech ELH-GDNF-1) were performed to assess neural-specific factors. ELISAs were prepared and performed according to manufacturer instructions.
Scratch Assay
A fibroblast scratch assay is a commonly employed 2D in vitro model for simplistic wound healing in which a scratch is introduced to a nearly confluent layer of cells and allowed to close over time. Other groups have utilized this assay to assess the effects of MSC secretome on wound healing and angiogenesis in vitro.3,24,27 We performed a scratch assay to test fibroblast proliferation and migration in response to secretome from ES applied at various frequencies, given that general wound healing genes (either VEGF or TIMP-1) were upregulated at all frequencies assessed. Human dermal fibroblasts (Sciencell 2320) were expanded in DMEM-F12, 10% FBS, and 1% P/S. Fibroblasts were passaged and resuspended in a low-serum medium consisting of DMEM-F12, 2% FBS, and 1% P/S. Cells were then seeded into 48-well plates at a density of 25,000 cells per well and grown to confluence for 1 day, after which a scratch was made down the center of each well using a P1000 pipette tip. Media was aspirated from each well after scratching to remove any cell debris and replaced with appropriate secretome medium. Cells were imaged immediately after scratching (0 h), as well as 6, 12, 24, and 30 h afterwards, or until the scratch was closed. Scratch width was measured using ImageJ at 10+ locations along the length to obtain an average value for each well at each time point. Then, a percent closure rate was obtained by first normalizing the scratch widths to each sample’s individual width at time 0, and then graphing “percent closed” versus time and recording the slope of each trend line.
Neurite Outgrowth Assay
SH-SY5Y neuroblastoma cells were purchased from ATCC (CRL-2266). Cells were seeded in 48-well plates at 1.6 × 104/well and cultured for 2 days prior to initiating differentiation with medium containing 3% FBS and 10 μM retinoic acid (i.e., to induce neurite extension). Following 2 days in differentiation media, cells were switched to one of the following groups: secretome from non-stimulated (NoES) ASCs, secretome from ASCs stimulated for 24 h at 1 Hz (20 mV/mm), StemPro media (no cell conditioning), or differentiation media (positive control). Brightfield images were taken on a Zeiss Axio Observer inverted microscope at days 0 and 5. For analysis, 10 cells were randomly assigned in each frame using a random ROI function in ImageJ and neurite length was quantified. Average neurite length per well was used in the final analysis.
Statistics
In all studies, three biological replicates (i.e., a unique passage and plating of cells for stimulation) were performed with 3–4 technical replicates (i.e., individual wells) each. For more than two groups, ANOVA followed by post-hoc Tukey’s HSD was performed using JMP (University of Florida license). If assumptions for normality (Anderson-Darling test) or equal variance (Levene’s test) were not verified, Wilcoxon or Welch’s tests were used, respectively. For analyses with two groups, appropriate t-tests were performed. A statistically significant result was considered to have a p value < 0.05.
RESULTS
Bioreactor Characterization
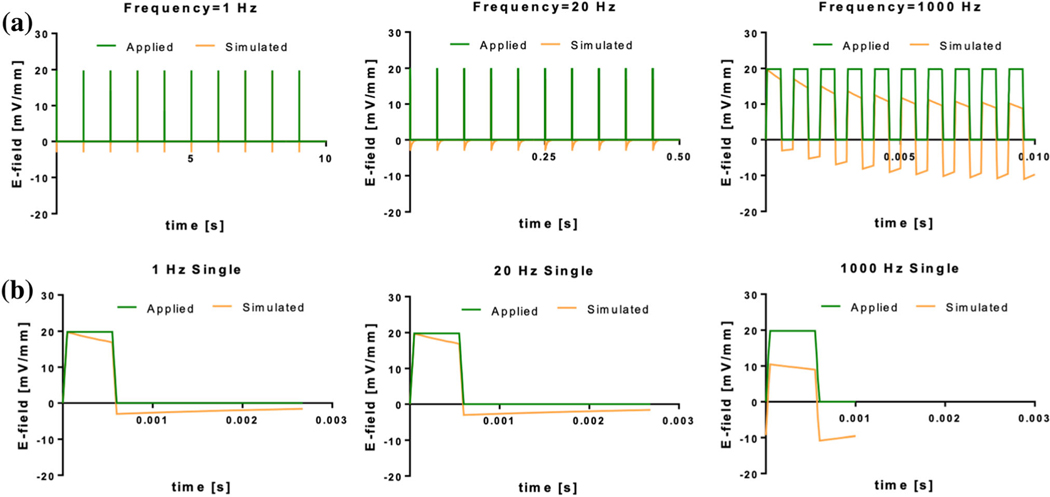
In the AC bioreactor, the applied voltage drops across the capacitive double layer and resistive media. Voltage across the resistive media as a function of time was computed using transient circuit analysis, and electric field was computed by dividing voltage by the electrode separation distance. The electric field values were compared to an applied source for different pulse waveform frequencies in Fig. 2. While the 1 and 20 Hz groups had field values with minimal discrepancy from the source, there was a notable shift in the 1000 Hz electrical field due to incomplete dissipation of the double layer capacitor in one pulse period. Thus, the system equilibrated to bipolar application of the field at one half the applied magnitude.
FIGURE 2.
Applied and simulated electrical field strengths for 1 Hz, 20 Hz, and 1000 Hz frequencies in the AC bioreactor. A 500 ls, 674 mV (20 mV/mm) square pulse was applied for each frequency. Graphs for (a) the first 10 pulses and (b) a single pulse (after equilibration) for each frequency are shown. Note that single profiles are meant only to capture differences in magnitude of the applied versus actual profiles. Time scales for frequencies in experiments should consider only profiles shown in (a).
ASCs Exhibit Frequency-Dependent Responses to ES via AC Pulses
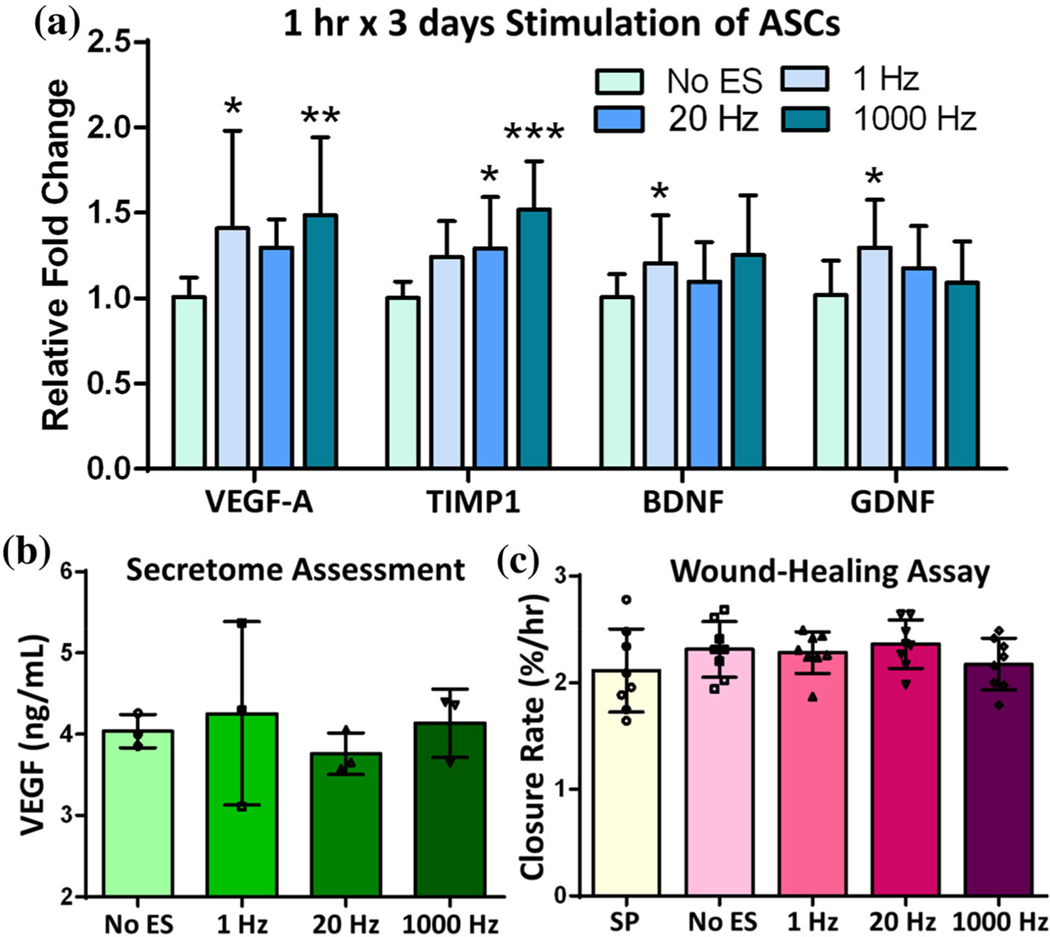
To evaluate effects of stimulation frequency, ASCs were exposed to intermittent stimulation (1 h × 3 days) at either 1 Hz, 20 Hz or 1000 Hz in the AC bioreactor. Fig. 3a depicts frequency-dependent alterations in gene expression for VEGF-A, TIMP1, BDNF and GDNF. The 1 Hz frequency elicited a significant upregulation in all but TIMP1, whereas 20 Hz only caused upregulation of TIMP1. At 1000 Hz, the general wound healing factors (TIMP1 and VEGF-A) were significantly upregulated while the neural-specific factors (BDNF and GDNF) were not. Next, secretome was assessed to determine if the gene expression profiles were sufficient to cause protein levels to be altered. VEGF was selected for this analysis given its abundant wound healing functions and that it had the highest average fold change across the frequencies. ELISA results reported in Fig. 3b suggest that there were no significant differences in VEGF-A protein levels within the secretome derived from any stimulation frequency. Functionally, the secretome from stimulated ASCs also did not cause changes in closure rate of fibroblasts following a scratch injury (Fig. 3c).
FIGURE 3.
AC Stimulation: Low-level, intermittent (1 h × 3 days) ES of ASCs. (a) Gene expression results for general wound healing factors VEGF-A and TIMP1 and neural growth factors BDNF and GDNF. (b) VEGF protein levels in secretome media from ASCs stimulated with different pulse frequencies. (c) Secretome from stimulated ASCs was incubated on dermal fibroblasts to determine scratch closure rate (%/h). Data are mean ± std, n = 11–12/group across 3 biological replicates for PCR, n = 3/group biological replicates for ELISA and n = 8 wells/group across 3 biological replicates for wound healing assay. *p value < 0.05, **p value < 0.01, ***p value < 0.0001 compared to NoES.
Intermittent DC Stimulation Induces Minimal ASC Responses
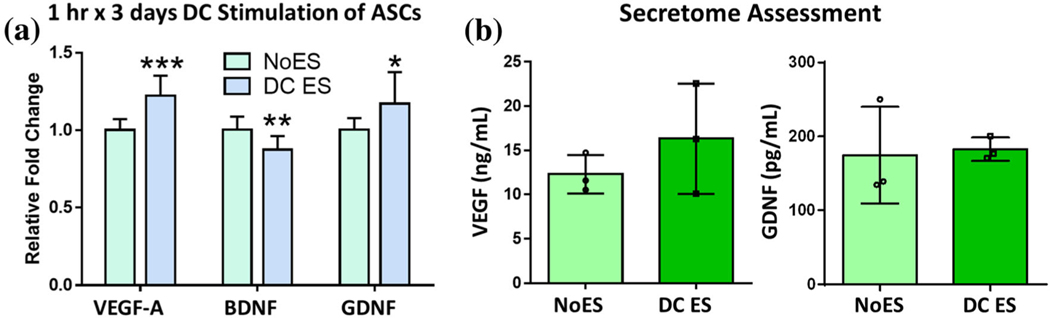
Because most existing ES studies use DC application, we manufactured a DC bioreactor so that comparisons between AC and DC could be made directly. Figure 4 provides both gene and protein expression results for the growth factors previously studied under AC stimulation. Figure 4a indicates a significant increase in VEGF and GDNF gene expression (p value < 0.0001, 0.022, respectively) and a decrease in BDNF expression (p value = 0.0017) compared to the NoES control. These changes in gene expression did not result in significant changes in the secretome protein levels of VEGF or GDNF, as measured by ELISA (Fig. 4b).
FIGURE 4.
DC Stimulation: ASCs underwent intermittent stimulation (1 h × 3 days) at low field strength. (a) Growth factor gene expression results for ASCs exposed to DC stimulation. (b) ELISA results for VEGF and GDNF in ASC secretome following ES. Data are mean ± std, n = 11–12/group across 3 biological replicates for PCR and n = 3/group biological replicates for ELISA. *p value < 0.05, **p value < 0.01, ***p value < 0.0001 compared to NoES.
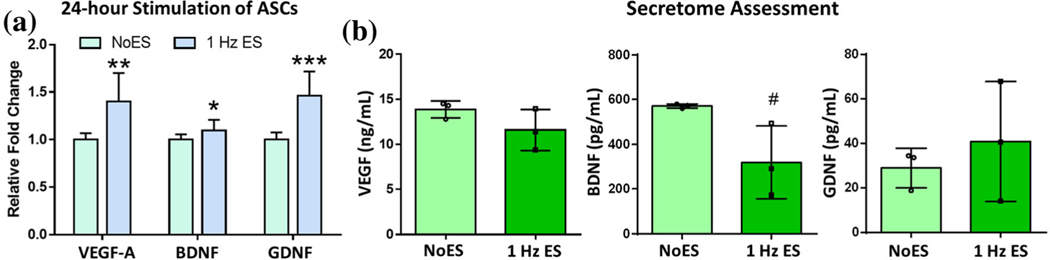
Continuous ES Induces Higher, More Varied Expression of ASC-Derived Growth Factors
By increasing the time of low-level stimulation to 24 h continuous (1 Hz pulses), VEGF, BDNF and GDNF gene expressions were significantly elevated compared to the NoES control (Fig. 5a). These results were similar in magnitude of the fold change to the 1 h × 3 days results when compared to the control for all genes (p value = 0.0058 for VEGF, 0.022 for BDNF and 0.0001 for GDNF). ELISAs were performed on the secretome and revealed that there were no significant differences in VEGF, BDNF, or GDNF protein levels; however, there was higher variation in the ES samples compared to the NoES group (Fig. 5b). VEGF and BDNF trended towards lower protein levels in the secretome. For GDNF, two biological replicates had higher GDNF protein levels, whereas one was lower than NoES, indicating potential differences in run-to-run ES or secretome processing.
FIGURE 5.
ASCs were exposed to continuous stimulation (24 h) at 1 Hz pulse frequency. (a) Growth factor gene expression results for ASCs exposed to continuous stimulation. (b) ELISA results for VEGF, BDNF and GDNF in ASC secretome following 24 h of ES. Data are mean ± std, n = 11/group across 3 biological replicates and n = 3/group biological replicates for ELISA. *p value < 0.05, **p value < 0.01, ***p value < 0.0001, #p value = 0.055 compared to NoES.
SY5Y Neurite Outgrowth Assay
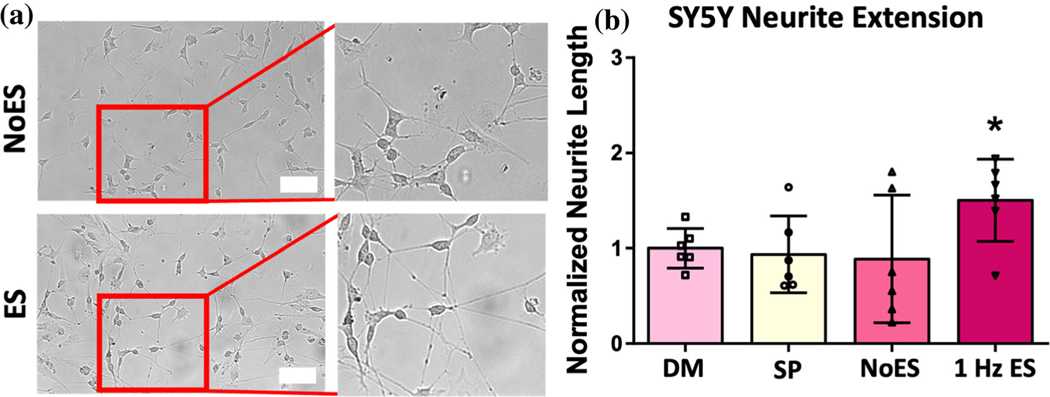
Given the upregulated gene expression in the 24-h stimulation group, secretome from these samples was used to assess effects on neurite outgrowth. Partially differentiated SY5Y cells were incubated with secretome for 5 days, and results of neurite extension over this time are provided in Fig. 6. Secretome from the ES group caused significantly higher neurite outgrowth over the timeframe compared to the NoES control (p value = 0.0215). Differentiation media (DM) and StemPro (SP) with no ASC conditioning were run as additional controls. The DM group was considered a positive control as these cells were cultured in media with retinoic acid to induce neurite extension.17 ES was not significantly different from DM.
FIGURE 6.
SY5Y neurite extension model. Secretome from ASCs exposed to continuous (24 h) ES at 1 Hz was incubated on partially differentiated SY5Y cells. (a) Representative images of SY5Y cells after 5 days of incubation in secretome media. Scale bar is 100 μm. (b) Neurite length as measured by average extension from cell bodies after 5 days in secretome media. ‘DM’ denotes differentiation media and ‘SP’ denotes StemPro media (with no cell conditioning). Data are mean ± std, n = 6/group, normalized to differentiation media (DM) group at 1. *p value < 0.05 compared to NoES control.
DISCUSSION
In this work, we report the effects of both AC and DC stimulation, of various durations and frequencies, on human ASCs. Using an established parallel-electrode (i.e., AC) bioreactor, as well as a novel ITO DC bioreactor, we explore how both intermittent and continuous ES regimes affect ASC expression and secretion of pro-regenerative and neurotrophic factors. Although the AC bioreactor employed in these studies has been previously published,21 it has not yet been characterized using electrochemical impedance spectroscopy and transient circuit analysis to model the voltage across the resistive media. Results of this study indicated that considerations need to be made when working with higher frequencies (i.e., 1000 Hz) as these frequencies may cause shifts in the magnitude of the electrical field.
Initially, ASCs were intermittently stimulated at an applied 20 mV/mm, making this study novel in the report of ASC frequency-dependent responses at low electrical field strengths. Results of intermittent stimulation suggested that 1 Hz pulses were most effective at increasing gene expression of neural-specific growth factors in particular. Interestingly, other studies have indicated the effectiveness of low-frequency (i.e., 20 Hz or less) AC ES on a variety of neural cell types for eliciting specific cellular responses,25,31,39 such as increased neurite extension from cortical neurons (2 Hz, 80 mV/mm) or increased Schwann cell growth factor secretion (1 Hz, 500 mV/mm).10,31 For this reason, we preliminarily compared ASC response to ES to that of human Schwann cells in Supplemental Data (Fig. S4).
As numerous researchers have reported effects of DC stimulation on cells,1,22,30,36 we performed stimulation experiments using the same intermittent time pattern (1 h × 3 days) in the DC bioreactor. We found similar trends in ASC gene expression as in the AC stimulation experiments, with significantly increased relative fold changes of VEGF-A and GDNF, however there was a decrease in expression of BDNF between ES and NoES. Additionally, no differences in protein secretion of VEGF or GDNF were detected between stimulated and non-stimulated groups. Hence, low-level intermittent ES (20 mV/mm) elicited similar effects on ASCs, whether delivered through AC or DC stimulation, with AC inducing upregulation of more genes overall. This may be because AC affords the exploration of frequency-dependent mechanisms of cell response to low-level stimulation that are important in neurophysiological contexts.6,33
From these results and other studies in the literature, it was hypothesized that perhaps higher expression of growth factors could be achieved by using continuous stimulation rather than intermittent stimulation. So, in the next assessment, continuous stimulation was applied to cells for 24 h at 1 Hz in the AC bioreactor. This frequency was chosen because VEGF-A, BDNF, and GDNF were all significantly upregulated (via gene expression) after 1 Hz intermittent stimulation. With longer stimulation time (24 h), all three growth factors were significantly upregulated at the gene level compared to non-stimulated (NoES) controls, with GDNF achieving the greatest significant difference. The result that GDNF may be more electrically inducible than BDNF is consistent with other studies.35 Further, ELISA results revealed a trend towards decreased VEGF and BDNF secretion and increased GDNF secretion. It should be noted that in at least one of three replicates, there is a clear difference in growth factor levels between NoES and ES. The results may indicate variations in ASC responses to ES as a factor of cell source, passage, proliferation, or other influence that is different run-to-run. These differences may be interesting to explore in future studies, especially given that in the SY5Y assay, cells cultured in secretome from stimulated ASCs experienced significantly greater neurite extension than those cultured in secretome from non-stimulated ASCs. This result was from a batch of cells that did have increased growth factor secretion. Although this result suggests a difference in the pro-regenerative nature of ASCs under continuous low-level stimulation as compared to no stimulation, the effect may be convoluted by variations in biological replications of this phenomenon. Such variations may be due to methods used to assess secretome or to the stability of the specific factors themselves. Currently, few studies have investigated biological replication or batch-to-batch variation of ASCs under ES.
In our assessments, we have seen trends of significant increases in gene expression detected after ES that are similar in magnitude (< 2-fold) to another ES study on ASCs.30 Changes in gene expression on this level did not reliably result in increased protein secretion, which is also consistent with other studies and could be due to time differences in gene expression versus protein secretion.2,15 In one study, however, Beugels et al. observed increased VEGF levels in secretome from ASCs electrically stimulated at 400 mV/mm with 3, 6, and 12 ms pulse duration at 2 Hz frequency.2 The authors reported that these stimulation levels may have yielded intracellular artifacts resulting from cell detachment during stimulation. Differences across these studies may merit investigation of higher electrical fields, but also methods to capture endogenous growth factors for cell-free therapies might prove valuable.
Limitations of this work include the maximum field strength able to be achieved by the AC bioreactor. Water electrolysis threshold limits in the AC bioreactor prevented exploration of higher field strengths with the current design. It could be beneficial to develop another bioreactor to explore AC stimulation at higher field strengths, which are suggested to elicit greater protein secretion.2 Further, although this work has mainly focused on general cell response and the composition and effect of cell secretions on other cells, it is important to consider the molecular mechanisms at work that could lead to these observed trends. For example, one mechanistic theory is that ES activates voltage-gated ion channels (VGICs) in ASCs, causing ion fluxes that subsequently influence cell behavior.5,37 Although some researchers have begun looking into such mechanisms,37 there is still much unknown regarding how ES affects ion influx and subsequent cellular pathways. More knowledge in this space could help inform ES parameters to optimize secretome production for various regenerative applications.
This study investigated the effects of low-level stimulation regimes on ASC gene expression and secretome production. The purpose of investigating low-level fields that are consistent with physiological levels was to understand ASC responses that could be relevant to either direct cell transplantation strategies or to exogenous applications to produce secretome for therapeutic delivery. The challenge that remains is a lack of knowledge as to what ES parameters govern cellular responses to endogenous electrical fields produced in wounds and what the parameters in a wound environment are exactly. Results of this study indicated that ASCs do not have consistent patterns of response to 20 mV/mm ES regimes, and therefore, future work should focus on understanding the biological mechanisms of electrically induced cellular responses within physiological contexts. There are many electrical parameters and differences across experimental designs (i.e., bioreactors) to consider, leading to difficulties in iterative approaches to maximize effective stimulation given current knowledge in the field.
To our knowledge, this work contributes unique findings regarding the effects of both AC and DC low-level stimulation on ASCs, specifically for production of neurotrophic factors. Further, we have introduced a novel, customizable bioreactor (with a conductive substrate) that is relatively simple and inexpensive to manufacture. This bioreactor could serve as an accessible and economical option for researchers to perform various electrical stimulation parameters on the cell types of their choice.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to acknowledge Dr. Keith March (UF Medicine) for discussions on this work. We also thank the Thermo Fisher Aspire program for providing complimentary ELISAs. Funding for this work was provided by NIH R21 NS111398.
Footnotes
SUPPLEMENTARY INFORMATION
The online version contains supplementary material available at https://doi.org/10.1007/s10439-021-02875-z.
REFERENCES
- 1.Bertucci C, Koppes R, Dumont C, and Koppes A. Neural responses to electrical stimulation in 2D and 3D in vitro environments. Brain Res. Bull 152:265–284, 2019. [DOI] [PubMed] [Google Scholar]
- 2.Beugels J, Molin DGM, Ophelders DRMG, Rutten T, Kessels L, Kloosterboer N, de Grzymala AAP, Kramer BWW, van der Hulst RRWJ, and Wolfs TGAM. Electrical stimulation promotes the angiogenic potential of adipose-derived stem cells. Sci. Rep 9:12076, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter K, Lee HJ, Na KS, Fernandes-Cunha GM, Blanco IJ, Djalilian A, and Myung D. Characterizing the impact of 2D and 3D culture conditions on the therapeutic effects of human mesenchymal stem cell secretome on corneal wound healing in vitro and ex vivo. Acta Biomaterialia. 99:247–257, 2019. 10.1016/j.actbio.2019.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elzinga K, Tyreman N, Ladak A, Savaryn B, Olson J, and Gordon T. Brief electrical stimulation improves nerve regeneration after delayed repair in Sprague Dawley rats. Exp. Neurol 269:142–153, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Forostyak O, Butenko O, Anderova M, Forostyak S, Sykova E, Verkhratsky A, and Dayanithi G. Specific profiles of ion channels and ionotropic receptors define adipose- and bone marrow derived stromal cells. Stem Cell Res. 16:622–634, 2016. [DOI] [PubMed] [Google Scholar]
- 6.Gellner A-K, Reis J, and Fritsch B. Glia: a neglected player in non-invasive direct current brain stimulation. Front. Cell. Neurosci 10:188, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon T. Electrical stimulation to enhance axon regeneration after peripheral nerve injuries in animal models and humans. Neurotherapeutics. 13:295–310, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon T, Brushart TM, and Chan KM. Augmenting nerve regeneration with electrical stimulation. Neurol. Res 30:1012–1022, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Gordon T, Sulaiman OAR, and Ladak A. Chapter 24 electrical stimulation for improving nerve regeneration: where do we stand? Int. Rev. Neurobiol 87:433–444, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Huang J, Ye Z, Hu X, Lu L, and Luo Z. Electrical stimulation induces calcium-dependent release of NGF from cultured Schwann cells. Glia. 58:622–631, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Huang Y, Li Y, Chen J, Zhou H, and Tan S. Electrical stimulation elicits neural stem cells activation: new perspectives in CNS repair. Front. Hum. Neurosci 9:586, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ike IS, Sigalas I, and Iyuke S. Understanding performance limitation and suppression of leakage current or self-discharge in electrochemical capacitors: a review. Phys. Chem. Chem. Phys 18:661–680, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Jack AS, Hurd C, Martin J, and Fouad K. Electrical stimulation as a tool to promote plasticity of the injured spinal cord. J. Neurotrauma 37:1933–1953, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kingham PJ, Kolar MK, Novikova LN, Novikov LN, and Wiberg M. Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells Dev. 23:741–754, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Koppes AN, Nordberg AL, Paolillo GM, Goodsell NM, Darwish HA, Zhang L, and Thompson DM. Electrical stimulation of schwann cells promotes sustained increases in neurite outgrowth. Tissue Eng. Part A 20:494–506, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koppes AN, Seggio AM, and Thompson DM. Neurite outgrowth is significantly increased by the simultaneous presentation of Schwann cells and moderate exogenous electric fields. J. Neural Eng.8(4):046023, 2011. 10.1088/1741-2560/8/4/046023. [DOI] [PubMed] [Google Scholar]
- 17.Kovalevich J, and Langford D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol. Biol 1078:9–21, 2013. 10.1007/978-1-62703-640-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leppik L, Zhihua H, Mobini S, Thottakkattumana Parameswaran V, Eischen-Loges M, Slavici A, Helbing J, Pindur L, Oliveira KMC, Bhavsar MB, Hudak L, Henrich D, and Barker JH. Combining electrical stimulation and tissue engineering to treat large bone defects in a rat model. Sci. Rep 8:6307, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopatina T, Kalinina N, Karagyaur M, Stambolsky D, Rubina K, Revischin A, Pavlova G, Parfyonova Y, and Tkachuk V. Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth De Novo. PLoS ONE. 6:e17899, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Messerli MA, and Graham DM. Extracellular electrical fields direct wound healing and regeneration. Biol. Bull 221(1):79–92, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Mobini S, Leppik L, and Barker JH. Direct current electrical stimulation chamber for treating cells in vitro. BioTechniques. 60:95–98, 2016. [DOI] [PubMed] [Google Scholar]
- 22.Mobini S, Leppik L, ThottakkattumanaParameswaran V, and Barker JH. In vitro effect of direct current electrical stimulation on rat mesenchymal stem cells. PeerJ. 5:e2821, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mobini S, Talts Ü-L, Xue R, Cassidy NJ, and Cartmell SH Electrical stimulation changes human mesenchymal stem cells orientation and cytoskeleton organization. J. Biomater. Tissue Eng 7(9):839–933, 2017. [Google Scholar]
- 24.Park S-R, Kim J-W, Jun H-S, Roh JY, Lee H-Y, and Hong I-S. Stem cell secretome and its effect on cellular mechanisms relevant to wound healing. Mol. Ther 26:606–617, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quan X, Huang L, Yang Y, Ma T, Liu Z, Ge J, Huang J, and Luo Z. Potential mechanism of neurite outgrowth enhanced by electrical stimulation: involvement of MicroRNA-363–5p targeting DCLK1 expression in rat. Neurochem. Res 42:513–525, 2017. [DOI] [PubMed] [Google Scholar]
- 26.Rackauskas G, Saygili E, Rana OR, Saygili E, Gemein C, Laucevicius A, Aidietis A, Marinskis G, Serpytis P, Plisiene J, Pauza DH, and Schauerte P. Subthreshold high-frequency electrical field stimulation induces VEGF expression in cardiomyocytes. Cell Transpl. 24:1653–1659, 2015. [DOI] [PubMed] [Google Scholar]
- 27.Ribeiro TO, Silveira BM, Meira MC, Carreira ACO, Sogayar MC, Meyer R, and Fortuna V. Investigating the potential of the secretome of mesenchymal stem cells derived from sickle cell disease patients. PLoS ONE. 14:e0222093, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross Macdonald J, and Barlow CA. Theory of double-layer differential capacitance in electrolytes. J. Chem. Phys 36:3062, 1962. [Google Scholar]
- 29.Salgado JA, Reis LR, Sousa N, and Gimble MJ. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther. 5:103–110, 2010. [DOI] [PubMed] [Google Scholar]
- 30.Tandon N, Goh B, Marsano A, Chao PHG, Montouri-Sorrentino C, Gimble J, and Vunjak-Novakovic G. Alignment and elongation of human adipose-derived stem cells in response to direct-current electrical stimulation. Conference Proceedings of Annual International Conference of the IEEE Engineering in Medicine and Biology Society 1:6517–6521, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang-Schomer MD 3D axon growth by exogenous electrical stimulus and soluble factors. Brain Res. 1678:288–296, 2018. [DOI] [PubMed] [Google Scholar]
- 32.Thakral G, LaFontaine J, Najafi B, Talal TK, Kim P, and Lavery LA. Electrical stimulation to accelerate wound healing. Diabet. Foot Ankle 4:22081, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vöröslakos M, Takeuchi Y, Brinyiczki K, Zombori T, Oliva A, Fernández-Ruiz A, Kozák G, Kincses ZT, Iványi B, Buzsáki G, and Berényi A. Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nat. Commun 9:483, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilhelm JC, Xu M, Cucoranu D, Chmielewski S, Holmes T, Lau KS, Bassell GJ, and English AW. Cooperative roles of BDNF expression in neurons and schwann cells are modulated by exercise to facilitate nerve regeneration. J. Neurosci 32:5002–5009, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willand MP, Rosa E, Michalski B, Zhang JJ, Gordon T, Fahnestock M, and Borschel GH. Electrical muscle stimulation elevates intramuscular BDNF and GDNF mRNA following peripheral nerve injury and repair in rats. Neuroscience. 334:93–104, 2016. [DOI] [PubMed] [Google Scholar]
- 36.Yang G, Long H, Ren X, Ma K, Xiao Z, Wang Y, and Guo Y. Regulation of adipose-tissue-derived stromal cell orientation and motility in 2D- and 3D-cultures by direct-current electrical field. Dev. Growth Differ 59:70–82, 2017. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Li M, Kang ET, and Neoh KG. Electrical stimulation of adipose-derived mesenchymal stem cells in conductive scaffolds and the roles of voltage-gated ion channels. Acta Biomaterialia. 32:46–56, 2016. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Neoh KG, and Kang ET. Electrical stimulation of adipose-derived mesenchymal stem cells and endothelial cells co-cultured in a conductive scaffold for potential orthopaedic applications. J. Tissue Eng. Regen. Med 12:878–889, 2017. [DOI] [PubMed] [Google Scholar]
- 39.Zhou WT, Qin Ni Y, Bing Jin Z, Zhang M, Hong Wu J, Zhu Y, Zhi Xu G, and Kang Gan D. Electrical stimulation ameliorates light-induced photoreceptor degeneration in vitro via suppressing the proinflammatory effect of microglia and enhancing the neurotrophic potential of Müller cells. Exp. Neurol 238:192–208, 2012. [DOI] [PubMed] [Google Scholar]
- 40.Zhu R, Sun Z, Li C, Ramakrishna S, Chiu K, and He L. Electrical stimulation affects neural stem cell fate and function in vitro. Exp Neurol. 319:112963, 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.