Abstract
Human immunodeficiency virus type 1 (HIV-1) uses a variety of chemokine receptors as coreceptors for virus entry, and the ability of the virus to be neutralized by antibody may depend on which coreceptors are used. In particular, laboratory-adapted variants of the virus that use CXCR4 as a coreceptor are highly sensitive to neutralization by sera from HIV-1-infected individuals, whereas primary isolates that use CCR5 instead of, or in addition to, CXCR4 are neutralized poorly. To determine whether this dichotomy in neutralization sensitivity could be explained by differential coreceptor usage, virus neutralization by serum samples from HIV-1-infected individuals was assessed in MT-2 cells, which express CXCR4 but not CCR5, and in mitogen-stimulated human peripheral blood mononuclear cells (PBMC), where multiple coreceptors including CXCR4 and CCR5 are available for use. Our results showed that three of four primary isolates with a syncytium-inducing (SI) phenotype and that use CXCR4 and CCR5 were neutralized poorly in both MT-2 cells and PBMC. The fourth isolate, designated 89.6, was more sensitive to neutralization in MT-2 cells than in PBMC. We showed that the neutralization of 89.6 in PBMC was not improved when CCR5 was blocked by having RANTES, MIP-1α, and MIP-1β in the culture medium, indicating that CCR5 usage was not responsible for the decreased sensitivity to neutralization in PBMC. Consistent with this finding, a laboratory-adapted strain of virus (IIIB) was significantly more sensitive to neutralization in CCR5-deficient PBMC (homozygous Δ32-CCR5 allele) than were two of two SI primary isolates tested. The results indicate that the ability of HIV-1 to be neutralized by sera from infected individuals depends on factors other than coreceptor usage.
Human immunodeficiency virus type 1 (HIV-1), the etiologic agent of AIDS, utilizes the HLA class II receptor, CD4, as its primary receptor to gain entry into cells (17, 30). Entry is initiated by a high-affinity interaction between CD4 and the surface gp120 of the virus (32). Subsequent to this interaction, conformational changes that permit fusion of the viral membrane with cellular membranes occur within the viral transmembrane gp41 (9, 58, 59). In addition to CD4, one or more recently described viral coreceptors are needed for fusion to take place. These coreceptors belong to a family of seven-transmembrane G-protein-coupled proteins and include the CXC chemokine receptor CXCR4 (3, 4, 24, 44), the CC chemokine receptors CCR5 (1, 12, 13, 18, 21, 23, 45) and, less commonly, CCR3 and CCR2b (12, 21), and two related orphan receptors termed BONZO/STRL33 and BOB (19, 34). Coreceptor usage by HIV-1 can be blocked by naturally occurring ligands, including SDF-1 for CXCR4 (4, 44), RANTES, MIP-1α, and MIP-1β in the case of CCR5 (13, 45), and eotaxin for CCR3 (12).
The selective cellular tropisms of different strains of HIV-1 may be determined in part by coreceptor usage. For example, all culturable HIV-1 variants replicate initially in mitogen-stimulated human peripheral blood mononuclear cells (PBMC), but only a minor fraction are able to infect established CD4+ T-cell lines (43). This differential tropism is explained by the expression of CXCR4 together with CCR5 and other CC chemokine coreceptors on PBMC and the lack of expression of CCR5 on most T-cell lines (5, 10, 19, 35, 39, 50, 53). Indeed, low-passage field strains (i.e., primary isolates) of HIV-1 that fail to replicate in T-cell lines use CCR5 as their major coreceptor and are unable to use CXCR4 (1, 12, 18, 21, 23, 28). Because these isolates rarely produce syncytia in PBMC and fail to infect MT-2 cells, they are often classified as having a non-syncytium-inducing (NSI) phenotype. Primary isolates with a syncytium-inducing (SI) phenotype are able to use CXCR4 alone or, more usually, in addition to CCR5 (16, 20, 51). HIV-1 variants that have been passaged multiple times in CD4+ T-cell lines, and therefore considered to be laboratory adapted, exhibit a pattern of coreceptor usage that resembles that of SI primary isolates. Most studies have shown that the laboratory-adapted strain IIIB uses CXCR4 alone (3, 13, 20, 24, 51) and that MN and SF-2 use CXCR4 primarily and CCR5 to a lesser degree (11, 13). Sequences within the V3 loop of gp120 have been shown to be important, either directly or indirectly, for the interaction of HIV-1 with both CXCR4 (52) and CCR5 (12, 14, 54, 60). This region of gp120 contains multiple determinants of cellular tropism (43) and is a major target for neutralizing antibodies to laboratory-adapted HIV-1 but not to primary isolates (29, 46, 57).
It has been known for some time that the ability of sera from HIV-1-infected individuals to neutralize laboratory-adapted strains of HIV-1 does not predict their ability to neutralize primary isolates in vitro (7). In general, the former viruses are highly sensitive to neutralization whereas the latter viruses are neutralized poorly by antibodies induced in response to HIV-1 infection (7, 43). Importantly, neutralizing antibodies generated by candidate HIV-1 subunit vaccines have been highly specific for laboratory-adapted viruses (26, 37, 38). In principle, the dichotomy in neutralization sensitivity between these two categories of virus could be related to coreceptor usage. To test this, we investigated whether the use of CXCR4 in the absence of CCR5 would render SI primary isolates highly sensitive to neutralization in vitro by sera from HIV-1-infected individuals. Two similar studies using human monoclonal antibodies and soluble CD4 have been reported (31a, 55).
MATERIALS AND METHODS
Viruses.
SI primary isolates V89872, V67970, and H69172 and NSI primary isolates P59423, W25798, and W79290 have all been described previously (40). HIV-1 89.6 is a primary isolate obtained from the PBMC of a 47-year-old patient with AIDS who received no antiretroviral therapy (15). This isolate is dualtropic for lymphocytes and macrophages (15) and can utilize CXCR4, CCR5, and to a lesser extent CCR3 and CCR2b for entry (12, 21). All primary isolates were obtained by PBMC coculture and were of low passage number (one or two passages of the original coculture supernatant) in human PBMC exclusively. HIV-1 IIIB, MN, and SF-2 were obtained from either Robert C. Gallo (IIIB and MN) or the NIH AIDS Research and Reference Reagent Program (SF-2) and have been described elsewhere (25, 33); these viruses were grown in H9 cells.
Cells.
MT-2 (27) and CEMx174 (49) are human CD4+ lymphoblastoid cell lines that are highly permissive to infection by laboratory-adapted variants and SI primary isolates of HIV-1, and neither cell line expresses transcripts for CCR5 (10). Normal PBMC were prepared from buffy coats from healthy, HIV-1-negative individuals obtained through the Durham Regional Red Cross. These PBMC were presumed to be CCR5 positive because of their ability to support the replication of NSI isolates of HIV-1. Additional PBMC were obtained from a healthy, HIV-1-negative individual who is homozygous for a 32-bp deletion in the CCR5 allele (homozygous Δ32-CCR5). The homozygous Δ32-CCR5 allele in these PBMC was confirmed by electrophoretic mobility analysis of PCR products as described previously (35). PBMC were isolated by centrifugation over lymphocyte separation medium (Organon-Teknika/Akzo, Durham, N.C.). Cells at the interface were washed twice in growth medium (RPMI 1640 supplemented with 20% heat-inactivated fetal bovine serum and 50 μg of gentamicin/ml) and suspended at a density of 2 × 106 cells/ml in growth medium containing phytohemagglutinin-P (5 μg/ml). The cells were incubated for 3 days at 37°C in 5% CO2–95% humidified air, washed twice with growth medium, and resuspended in growth medium containing 4% human interleukin-2 (IL-2) for use in neutralization assays.
Serum samples and chemokines.
Serum samples were obtained from HIV-1-infected individuals residing in the Durham, N.C., area. An exception was individual 10, who belonged to a cohort of HIV-1-infected long-term nonprogressors (40). Each individual had been infected for at least 2 years, was asymptomatic, and had >500 CD4+ lymphocytes per mm3. Informed consent was obtained from each individual before their blood was drawn. All serum samples were heat inactivated at 56°C for 1 h prior to use. Recombinant human RANTES, MIP-1α, and MIP-1β were purchased from R&D Systems, Minneapolis, Minn.
Assessment of coreceptor usage.
Viruses were assessed for coreceptor usage by using U87-CD4-CXCR4 and U87-CD4-CCR5 cells as described previously (10, 18). These cells were generously provided by Dan Littman (Skirball Institute, New York, N.Y.). Usage was considered positive when viral p24 was detected after 5 to 7 days of incubation with virus.
Virus neutralization assays.
Antibody-mediated virus neutralization was measured by a reduction in either virus-induced cell killing or viral p24 synthesis in infection-susceptible cells. The cell-killing assay was performed with either MT-2 cells (used with all viruses except SF-2) or CEMx174 cells (used with SF-2) as described previously (41). CEMx174 cells were chosen over MT-2 cells for assays with SF-2 because of the greater cytopathic effects of this virus in the former cell line. Briefly, 50 μl of cell-free virus containing 1,000 50% tissue culture infective doses (TCID50) was added to multiple dilutions of test sera in 100 μl of growth medium in triplicate wells of 96-well microtiter plates. Virus-serum mixtures were incubated at 37°C for 1 h, and then MT-2 cells (5 × 104 cells in 100 μl) or CEMx174 cells (105 cells in 100 μl) were added to each well. Infection led to extensive syncytium formation and virus-induced cell killing in approximately 3 to 5 days in the absence of antibodies. Neutralization was measured by staining viable cells with Finter’s neutral red in poly-l-lysine-coated plates as described previously (41). Percent protection was determined by calculating the difference in absorption (A540) between test wells (cells, serum sample, and virus) and virus control wells (cells and virus) and dividing this result by the difference in absorption between cell control wells (cells only) and virus control wells. Neutralization was measured at a time when virus-induced cell killing in virus control wells was greater than 70% but less than 100%. Neutralizing antibody titers are given as the reciprocals of the serum dilutions required to protect 50% of cells from virus-induced killing.
Virus neutralization in PBMC was measured by a reduction in p24 synthesis essentially as described previously (40). Diluted serum samples were incubated with virus (1,000 TCID50) in a total of 50 μl in triplicate for 1 h at 37°C in 96-well U-bottom culture plates. Six wells containing only virus (no test sample) were included as controls. Following a 1-h incubation at 37°C, phytohemagglutinin-stimulated PBMC (4 × 105 cells in 150 μl of IL-2 growth medium) were added to each well and incubated for 3 h at 37°C. Cells were then washed three times with 200 μl of growth medium to remove the virus inoculum and antibodies. Washed cells were resuspended in 200 μl of IL-2 growth medium and incubated in fresh 96-well U-bottom plates until p24 production reached a peak. Immediately after resuspension, 25 μl was removed and mixed with 225 μl of 0.5% Triton X-100 spiked with a known amount of p24 to test for interference from anti-p24 antibody in the antigen detection enzyme-linked immunosorbent assay. Culture supernatants (25 μl) were collected on a daily basis thereafter and mixed with 225 μl of 0.5% Triton X-100 for the quantification of p24 produced by infection. Viral p24 was quantified with an antigen enzyme-linked immunosorbent assay as described by the supplier (DuPont, Wilmington, Del.). The 25-μl volume of culture fluid removed each day was replaced with 25 μl of fresh growth medium with or without IL-2, as before. Unless stated otherwise, neutralization was measured at a time prior to when p24 production in virus control wells had reached its peak, which is when optimum sensitivity is achieved in this assay (61). A similar set of assays measured neutralization by a reduction in p24 synthesis in MT-2 cells. These latter assays were performed in growth medium without IL-2 and used 5 × 104 MT-2 cells per well. Neutralization in both cases was considered positive when p24 synthesis was reduced by >80% relative to the virus control. Culture fluids harvested after the last washing showed no interference with p24 detection.
Antibody-mediated neutralization of virus in the presence and absence of CC chemokines was measured in human PBMC as described above except that the PBMC were preincubated for 1 h at 37°C in IL-2 growth medium with and without the CC chemokines RANTES, MIP-1α, and MIP-1β (each at 500 ng/ml) prior to assay. This concentration of RANTES alone has been shown to exceed the 90% inhibitory dose for a majority of NSI isolates in PBMC (28, 56). The chemokines were maintained at this concentration in the growth medium at all steps throughout the entire neutralization assay. This same mixture of CC chemokines caused a complete block in the replication of two of two NSI primary isolates in PBMC in our laboratory (data not shown).
RESULTS
Coreceptor usage.
Although coreceptor usage by the 89.6 primary isolate had been defined previously (12, 21), coreceptors used by the other primary isolates studied here remained unknown. We assessed the coreceptor usage of these isolates, in addition to the stocks of IIIB, MN, and SF-2 used in our experiments, by measuring their ability to replicate in two U87-CD4 indicator cell lines that stably expressed either CXCR4 or CCR5 after transfection. The results in Table 1 show that our particular stocks of IIIB, MN, and SF-2 can use CXCR4 but not CCR5, while the NSI isolates can use CCR5 but not CXCR4. The SI isolates were capable of using both CXCR4 and CCR5, although it is possible that these latter viruses consist of a mixture of quasispecies that use either or both coreceptors.
TABLE 1.
Coreceptor usage by laboratory-adapted variants and primary isolates of HIV-1
| Virus | Categorya | Phenotype | p24
production (ng/ml) inb:
|
|
|---|---|---|---|---|
| U87-CD4-CXCR4 cells | U87-CD4-CCR5 cells | |||
| IIIB | LA | SI | 2,335 | <1 |
| MN | LA | SI | 116 | <1 |
| SF-2 | LA | SI | 9 | <1 |
| V89872 | PI | SI | 714 | 430 |
| V67970 | PI | SI | 404 | 514 |
| H69172 | PI | SI | 765 | 965 |
| P59423 | PI | NSI | <1 | 33 |
| W25798 | PI | NSI | <1 | 78 |
| W79290 | PI | NSI | <1 | 397 |
LA, laboratory adapted; PI, primary isolate.
U87-CD4 cell lines transfected to express either CXCR4 or CCR5 were inoculated with cell-free viruses, incubated overnight, and then washed to remove the virus. Infection was assessed by p24 production 5 to 7 days later.
Neutralization sensitivity of SI primary isolates in MT-2 cells compared with that in PBMC.
Our first goal was to determine whether SI primary isolates differ from laboratory-passaged viruses in their sensitivity to neutralization in MT-2 cells with sera from HIV-1-infected individuals. The results in Table 2 show that laboratory-passaged stocks of IIIB and MN were much more sensitive to neutralization in MT-2 cells than were three of three SI primary isolates. The most potent neutralization of an SI primary isolate was seen for serum W05477 when tested against isolate V67970, which had a titer of 79. Isolate V67970 was neutralized weakly by three additional serum samples in MT-2 cells. Isolate H69172 was neutralized weakly by only one serum sample, whereas isolate V89872 was insensitive to neutralization with all five serum samples. Similar results were obtained when the SI primary isolates were assayed in human PBMC (Table 2). The few outcomes in PBMC that were discordant with outcomes in MT-2 cells were marginal in magnitude. In contrast to SI primary isolates, all five serum samples neutralized IIIB and MN, with titers that were often at least 10- to 100-fold higher than those obtained with the primary isolates.
TABLE 2.
Neutralization of primary isolates of HIV-1 in MT-2 cells and human PBMC by sera from HIV-1-infected individuals
| Seruma | Neutralizing antibody
titer inb:
|
||||
|---|---|---|---|---|---|
| MT-2
cells
|
MT-2 cells/PBMC
|
||||
| IIIB | MN | V89872 | V67970 | H69172 | |
| T84468 | 248 | 2,241 | <5/<5 | <5/<5 | <5/<5 |
| Y44003 | 21 | 3,149 | <5/<5 | 6/<5 | <5/<5 |
| W05477 | 191 | 670 | <5/<5 | 79/25 | <5/<5 |
| V97490 | 97 | 336 | <5/5 | 12/<5 | 6/<5 |
| AA4875 | 165 | 763 | <5/<5 | 14/5 | <5/<5 |
Sera are from HIV-1-infected individuals.
Titers measured in MT-2 cells are the reciprocal serum dilutions at which 50% of cells were protected from virus-induced cell killing; titers measured in PBMC are the reciprocal serum dilutions that reduced p24 synthesis by >80%.
Since virus neutralization in both assays was measured by different endpoints (i.e., a reduction in cell killing in MT-2 cells versus a reduction in p24 synthesis in PBMC), we next investigated the outcome when a reduction in p24 synthesis was used as the endpoint in the MT-2 assay. Serum samples from four HIV-1-infected individuals that contained high-titer neutralizing antibodies against IIIB, MN, and SF-2 in the MT-2 cell-killing assay had very poor neutralizing activity against each of two primary isolates when assayed in MT-2 cells by a reduction in p24 synthesis (Table 3). Specifically, only one virus-serum combination was positive for neutralization (serum T00953 with virus V67970 reduced p24 by >80%). Mild infection enhancement was seen with some serum samples (i.e., negative values), but the enhancement never exceeded a doubling of p24 synthesis. These results are further evidence that SI primary isolates are neutralized poorly in MT-2 cells with serum samples from HIV-1-infected individuals.
TABLE 3.
Neutralization of laboratory-passaged viruses and primary isolates of HIV-1 in MT-2 cells by sera from HIV-1-infected individuals
| Seruma | Neutralizing antibody
titer forb:
|
||||
|---|---|---|---|---|---|
| IIIB | MN | SF-2 | V89872 | V67970 | |
| T00953 | 127 | 178 | 245 | <5 | ≥5 |
| V91008 | 8,434 | 495 | 2,285 | <5 | <5 |
| W97464 | 516 | 156 | 1,850 | <5 | <5 |
| P46471 | 1,688 | 682 | 3,442 | <5 | <5 |
Sera were tested at a 1:5 dilution against primary isolates in MT-2 cells.
Titers for IIIB, MN, and SF-2 are the reciprocal dilutions at which 50% of cells were protected from virus-induce cell killing. Neutralization of primary isolates was considered positive when p24 synthesis was reduced >80% relative to the virus control (no test serum). All viruses were assayed in MT-2 cells except SF-2, which was assayed in CEMx174 cells.
Neutralization of 89.6 in PBMC when CCR5 usage is blocked by CC chemokines.
Primary isolate 89.6 differed from the other primary isolates by being highly sensitive to neutralization in MT-2 cells. As shown in Table 4, sera from three infected individuals (10, W97464, and T00953) had neutralization titers ranging from 96 to 758 when measured against 89.6 in MT-2 cells, although the titers were much lower when measured in PBMC (Table 4). Since this virus is able to use CCR5 in addition to CXCR4 (12, 21), and since MT-2 cells express only CXCR4 (10, 39), we next examined whether the decreased sensitivity to neutralization in PBMC was related to CCR5 usage.
TABLE 4.
Neutralization of HIV-1 89.6 in MT-2 cells and human PBMC with sera from HIV-1-infected individuals
Serum samples are from HIV-1-infected individuals.
Titers in MT-2 cells are the reciprocal serum dilutions at which 50% of cells were protected from virus-induced cell killing; titers for primary isolates are the last serum dilutions at which p24 synthesis was reduced >80% relative to the virus control (no test serum).
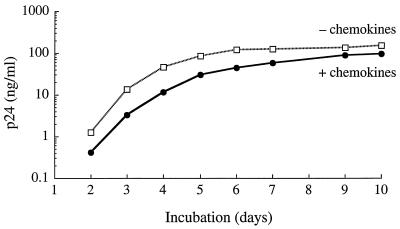
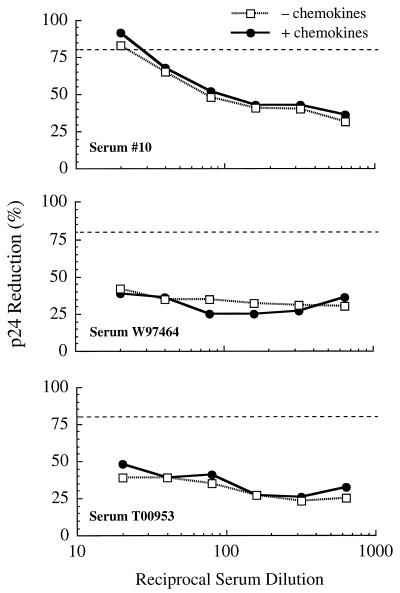
For this assessment, each of the three serum samples was tested for its ability to neutralize 89.6 in human PBMC in the presence and absence of a mixture of RANTES, MIP-1α, and MIP-1β to block CCR5. Figure 1 shows that the mixture of CC chemokines had minimal effects on the replication of 89.6, which is in agreement with the ability of this virus to utilize coreceptors other than CCR5 (e.g., CXCR4) and suggests that CCR5 is used poorly by this isolate, at least when other coreceptors are available. The serum neutralization curves showed that the virus was no more or less sensitive to neutralization in the presence of the CC chemokines (Fig. 2). Positive neutralization (>80% reduction in p24) was detected with serum 10 only. Neutralization curves with this serum sample were nearly identical under both conditions, corresponding to neutralization titers of 22 and 30 in the absence and presence of CC chemokines, respectively. These titers were much lower than the titer of 758 detected in MT-2 cells and were consistent with our previous results for PBMC (Table 4). No neutralization was detected with the remaining two serum samples in either the presence or the absence of CC chemokines. Although one of these samples (T00953) neutralized 89.6 in PBMC previously (Table 4), the titer (1:5) was lower than the initial 1:20 serum dilution used for the experiment depicted in Fig. 2. These results indicate that CCR5 usage does not explain the differential neutralization of 89.6 in MT-2 cells compared with PBMC.
FIG. 1.
Replication of HIV-1 89.6 in human PBMC in the presence and absence of CC chemokines. PBMC were incubated for 1 h at 37°C in the presence and absence of a mixture of RANTES, MIP-1α, and MIP-1β (each at 500 ng/ml) in 96-well culture plates. Cells were then transferred to each of 6 wells of a 96-well culture plate containing cell-free virus, and the incubation continued for another 3 h. The cells were washed three times with growth medium, resuspended in IL-2 growth medium, and incubated for 14 days. Viral p24 in culture fluids was quantified on the days indicated.
FIG. 2.
Antibody-mediated neutralization of HIV-1 89.6 in human PBMC in the presence and absence of CC chemokines. Neutralization of HIV-1 89.6 was assessed in human PBMC with serum samples from three HIV-1-infected individuals in the presence and absence of RANTES, MIP-1α, and MIP-1β (each at 500 ng/ml) as described in Materials and Methods. Viral p24 in culture fluids was quantified on day 5 of incubation. Dotted lines correspond to 80% reduction in p24 synthesis relative to the virus control (no test serum). Average concentrations of p24 in virus control wells on days 2 to 10 are shown in Fig. 1.
Antibody-mediated neutralization of SI primary isolates in homozygous Δ32-CCR5 PBMC.
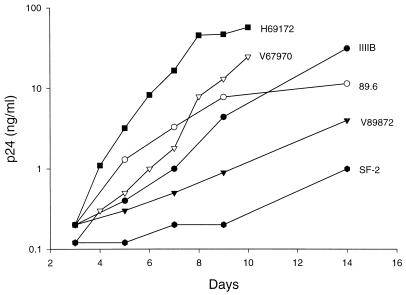
As another approach to test whether the poor neutralization of primary isolates in vitro is associated with CCR5 usage, neutralization assays were performed with PBMC that were genetically deficient in CCR5 (homozygous for the Δ32-CCR5 allele). The homozygous Δ32-CCR5 PBMC chosen for this evaluation were first characterized for the ability to support the replication of SI and NSI viruses as a way of confirming their functional coreceptor status. As expected, SI primary isolates H69172, V67970, and, to a lesser extent, 89.6 and V89872 were able to replicate (Fig. 3). Two laboratory-adapted variants, IIIB and SF-2, also replicated well (Fig. 3). Not shown in Fig. 3 are results for the laboratory-adapted strain MN and the NSI primary isolates P59423, W25798, and W79290, which did not grow; cultures were negative for p24 on days 3, 5, 7, 9, and 14. Since each of the NSI isolates has been shown to replicate to high levels in normal PBMC within 6 days, using the same inoculum size (47), their inability to replicate in the homozygous Δ32-CCR5 PBMC agrees with the lack of expression of CCR5. The inability of MN to replicate in these cells was unexpected, since this virus can use CXCR4, and could reflect a greater complexity of coreceptor usage by MN than is currently recognized. We should also note that this stock of MN failed to replicate in normal human PBMC that were capable of supporting the replication of NSI isolates (data not shown).
FIG. 3.
Virus replication in homozygous Δ32-CCR5 PBMC. PBMC (Δ32-CCR5) were inoculated with virus in triplicate wells of 96-well culture plates and incubated for 1 day. The virus inoculum was removed by a series of washes, and the cells were incubated in fresh IL-2 growth medium for an additional 13 days (14 days in total). Viral p24 in culture fluids was quantified on the days indicated.
Two of the above SI primary isolates were assessed for the ability to be neutralized in the homozygous Δ32-CCR5 PBMC with serum samples from the four HIV-1-infected individuals listed in Table 3, plus one additional serum sample, D75899, that neutralizes a broad spectrum of primary isolates (61). Serum from a healthy, noninfected individual (D01) was used as a negative control. All serum samples were tested at a 1:5 dilution. Table 5 shows that neither virus was neutralized by the negative control serum. Of the five HIV-1-positive sera, potent neutralization of V67970 and H69172 was seen with three and two sera, respectively. The remaining virus-serum combinations produced negligible neutralization (<80%). Similar patterns of neutralization of V67970 by these serum samples have been observed previously in normal PBMC (47, 61).
TABLE 5.
Antibody-mediated neutralization of SI primary isolates in homozygous Δ32-CCR5 PBMC
| Seruma | Neutralizing antibody
titer for primary isolateb:
|
|
|---|---|---|
| V67970 | H69172 | |
| T00953 | ≥5 | ≥5 |
| V91008 | ≥5 | <5 |
| W97464 | <5 | <5 |
| P46471 | <5 | <5 |
| D75899 | ≥5 | ≥5 |
| D01 | <5 | <5 |
All serum samples except D01 were from HIV-1-infected individuals; sample D01 came from a healthy, noninfected individual and was used as a negative control.
Primary isolates V67970 and H69172 were grown in normal PBMC and assayed in homozygous Δ32-CCR5 PBMC. Virus neutralization was assessed at a 1:5 dilution of serum samples. Neutralization was measured on day 8 for V67970 (11.6 ng of p24/ml in absence of test serum) and on day 7 for H69172 (18.0 ng of p24/ml in absence of test serum). Neutralization was considered positive when p24 synthesis was reduced >80% relative to the virus control (no test serum).
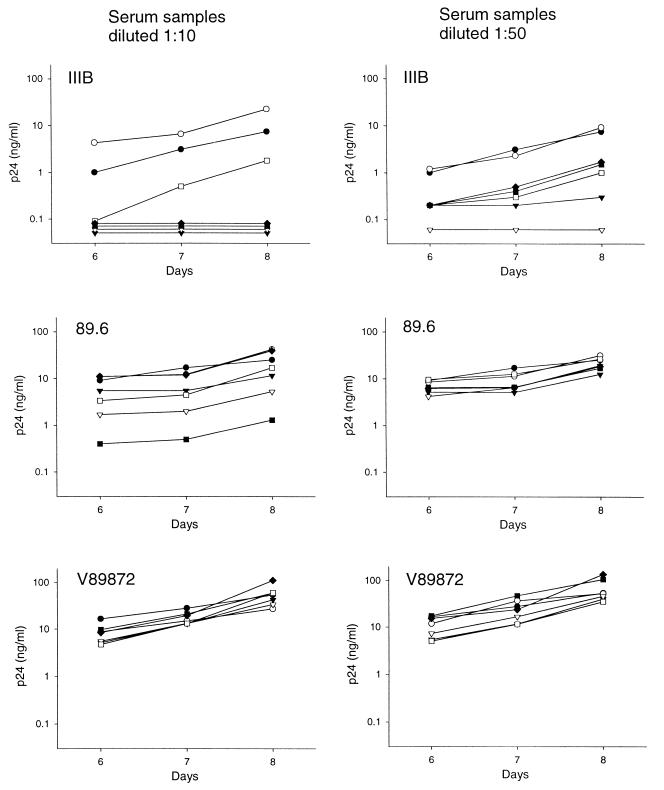
A second experiment was performed with an additional preparation of the homozygous Δ32-CCR5 PBMC (i.e., a later blood sample from the same donor as before), but this time the serum samples were tested for the ability to neutralize IIIB and the primary isolates 89.6 and V89872. Sera were evaluated at 1:10 and 1:50 dilutions, and p24 was quantified on days 6, 7, and 8, when virus production in the absence of test serum was just peaking. Three time points were used here as a way of distinguishing different potencies of virus neutralization, where the detection of neutralization may decrease with increasing incubation time as nonneutralized virions multiply (61). The results showed that IIIB was significantly more sensitive to neutralization than were the primary isolates (Fig. 4). Specifically, each of the five serum samples from infected individuals caused >80% reduction in IIIB p24 synthesis relative to the virus control (no test serum) at both serum dilutions tested, regardless of the day of assay. In fact, p24 was undetectable at all time points with four of the serum samples tested at a 1:10 dilution. The one sample that was not 100% neutralizing at a 1:10 dilution (V91008) was the most potent serum in MT-2 cells (Table 3), indicating that IIIB may sometimes be less sensitive to neutralization in PBMC than it is in MT-2 cells. By comparison, isolate 89.6 was neutralized by two of five serum samples at a 1:10 dilution only (samples P46471 and T00953). Our results showed also that isolate V89872 was relatively insensitive to neutralization by both dilutions of all serum samples. Except for some mild infection enhancement seen for IIIB at a 1:10 serum dilution, the negative control sample (D01) had little effect on all three viruses.
FIG. 4.
Antibody-mediated virus neutralization in homozygous Δ32-CCR5 PBMC. Serum samples from five HIV-1-infected individuals and one HIV-1-negative individual were assessed for the ability to neutralize the laboratory-adapted strain, IIIB, and the primary isolates, 89.6 and V89872, in homozygous Δ32-CCR5 PBMC. Suspensions of cell-free virus were incubated for 1 h at 37°C in triplicate with 1:10-diluted and 1:50-diluted serum samples in 96-well culture plates. PBMC were added, incubated for 3 h at 37°C, and then washed three times with growth medium. PBMC were resuspended in IL-2 growth medium and incubated for 8 days. Viral p24 production in culture supernatants was quantified on days 6, 7, and 8 of incubation. Values less than 0.1 ng of p24/ml were below the limit of detection in these assays. Serum samples: •, no test serum; ○, D01; ▾, D75899; ▿, P46471; ▪, T00953; □, V91008; ⧫, W97464.
DISCUSSION
This study aimed to determine whether the dichotomy in neutralization sensitivity between laboratory-adapted variants and primary isolates of HIV-1 could be explained by differential coreceptor usage. Specifically, we sought to determine whether either the exclusive use of CXCR4 or the inability to use CCR5 was associated with a high level of sensitivity to neutralizing antibodies. The premise for these studies is found in the fact that antibody-mediated neutralization of laboratory-adapted viruses is most commonly measured in CD4+ T-cell lines that express CXCR4 but not CCR5, such as MT-2 cells, whereas the neutralization of primary isolates is measured in PBMC that express multiple coreceptors, including CCR5 and CXCR4.
A particular stock of HIV-1 was considered to be a primary isolate if it had been grown only in PBMC and was of low passage number. All of our primary isolates were passaged no more than twice in PBMC exclusively. We partially mimicked the conditions under which laboratory-adapted variants are highly sensitive to neutralization by assessing the ability of SI primary isolates to be neutralized in MT-2 cells by sera from HIV-1-infected individuals. The fact that three of four SI primary isolates were neutralized poorly in both MT-2 cells and PBMC suggests that preferential use of CXCR4 does not render SI primary isolates highly sensitive to neutralization. We chose SI primary isolates that were capable of using both CXCR4 and CCR5, and although these viruses could have contained a mixture of quasispecies that use either or both coreceptors, infection in MT-2 cells would have been selective for those that use CXCR4.
A fourth primary isolate, designated 89.6, was highly sensitive to neutralization in MT-2 cells but not in PBMC. Because this virus is capable of using both CCR5 and CXCR4, it was possible that in this case, the exclusive use of CXCR4 in MT-2 cells improved its sensitivity to neutralization. This proved not to be true, however, since 89.6 remained relatively insensitive to neutralization in PBMC when RANTES, MIP-1α, and MIP-1β were added to block the use of CCR5. IIIB also showed some evidence of being less sensitive to neutralization in PBMC, and this virus is known to use only CXCR4 as a coreceptor. Along these same lines, we observed a dichotomy in neutralization sensitivity between the IIIB laboratory-adapted strain of virus and SI primary isolates in homozygous Δ32-CCR5 PBMC, where IIIB was significantly more sensitive to neutralization than were primary isolates. These results, taken together, indicate that CCR5 usage does not explain why primary isolates are less sensitive to neutralization than laboratory-adapted strains.
Since our results identified no obvious effects of CXCR4 and CCR5 on the detection of virus neutralization by sera from infected individuals, coreceptor usage probably does not determine the ability of HIV-1 to be neutralized by antibody. A similar conclusion has been reached in two independent studies that used human monoclonal antibodies and soluble CD4 (31a, 55). Other evidence in support of this conclusion may be found in the fact that SI and NSI primary isolates are equally difficult to neutralize in PBMC despite their differential use of CXCR4 and CCR5 (8, 31, 40, 42, 47). Other factors are more likely to explain why laboratory-adapted HIV-1 is highly sensitive to neutralization compared to primary isolates. One possibility is that envelope glycoprotein folding, subunit-subunit interactions, or positioning of glycosylation sites affects the exposure of neutralization epitopes on laboratory-adapted variants and primary isolates differently (7, 43). Antibodies to these epitopes may be able to neutralize the virus regardless of which coreceptors are used, as long as the epitope is exposed for antibody to bind. An example is the V3 loop of gp120, which contains multiple neutralization epitopes that are readily exposed on laboratory-adapted viruses (29, 46, 57) but are hidden in the native structure of gp120 on primary isolates (6). These epitopes could be the same as, or different from, those in the V3 loop that are suspected to play a role in coreceptor interactions (12, 14, 52, 54, 60).
The manner in which the envelope glycoproteins interact with their coreceptors might be another factor that affects virus neutralization by antibody. For example, different strains of HIV-1 seem to use different binding motifs on both CXCR4 (33, 52) and CCR5 (2, 22, 36, 48), making it possible that virus neutralization depends on which motifs are used. Interestingly, a recent study with monoclonal antibody 12G5 provided evidence that IIIB and 89.6 use different motifs on CXCR4 (52), although we showed that both viruses are highly neutralizable in MT-2 cells, where CXCR4 is presumably the sole coreceptor. Finally, our results do not exclude the possibility that coreceptors other than CXCR4 and CCR5 can influence the neutralization of HIV-1 by antibody.
We emphasize that our findings do not imply that antibodies to the coreceptor binding site(s) on viral envelope glycoproteins would be nonneutralizing. In fact, several lines of evidence support the notion that such antibodies could be highly effective at neutralizing a broad spectrum of HIV-1 variants (12, 14, 54, 60). Neutralization epitopes that reside in coreceptor binding sites on the viral envelope glycoproteins would be expected to be highly conserved so as to maintain coreceptor usage. However, these epitopes might become exposed transiently only after gp120-CD4 binding (54, 60), thereby reducing their immunogenicity and restricting their accessibility to antibodies. Our results agree that if these antibodies exist, they are probably not present in significant quantities in sera from HIV-1-infected individuals.
ACKNOWLEDGMENTS
We thank Hua-Xin Liao, Duke University Medical Center, for performing genetic assessments of CCR5.
This work was supported by grants AI-15106, AI-28662, AI-35502, AI-36082, and AI-41420 from the National Institutes of Health. J.P.M. is an Elizabeth Glaser Scientist of the Pediatric AIDS Foundation.
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Atchison R E, Goslin J, Monteclaro F S, Franci C, Diglio L, Charo I F, Goldsmith M A. Multiple extracellular elements of CCR5 and HIV-1 entry: dissociation from response to chemokines. Science. 1996;274:1924–1926. doi: 10.1126/science.274.5294.1924. [DOI] [PubMed] [Google Scholar]
- 3.Berson J F, Long D, Doranz B J, Rucker J, Jirik F R, Doms R W. A seven transmembrane domain receptor involved in fusion and entry of T-cell-tropic human immunodeficiency virus type 1 strains. J Virol. 1996;70:6288–6295. doi: 10.1128/jvi.70.9.6288-6295.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–832. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 5.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bou-Habib D C, Roderiquez G, Oravecz T, Berman P W, Lusso P, Norcross M A. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from antibody neutralization. J Virol. 1994;68:6006–6013. doi: 10.1128/jvi.68.9.6006-6013.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton, D. R., and D. C. Montefiori. 1997. The antibody response in HIV-1 infection. AIDS 11(Suppl. A):S87–S98. [PubMed]
- 8.Cao Y, Qin L, Zhang L, Safrit J, Ho D D. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med. 1995;332:201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 9.Chan D C, Fass D, Berger J M, Kim P S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, Zhou P, Ho D D, Landau N R, Marx P A. Genetically divergent strains of simian immunodeficiency virus use CCR5 as a coreceptor for entry. J Virol. 1997;71:2705–2714. doi: 10.1128/jvi.71.4.2705-2714.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng-Mayer C, Liu R, Landau N R, Stamatatos L. Macrophage tropism of human immunodeficiency virus type 1 and utilization of the CC-CKR5 coreceptor. J Virol. 1997;71:1657–1661. doi: 10.1128/jvi.71.2.1657-1661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 13.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 14.Cocchi F, DeVico A L, Garzino-Demo A, Cara A, Gallo R C, Lusso P. The V3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection. Nat Med. 1996;2:1244–1247. doi: 10.1038/nm1196-1244. [DOI] [PubMed] [Google Scholar]
- 15.Collman R, Balliet J W, Gregory A A, Friedman H, Kolson D L, Nathanson N, Srinivasan A. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J Virol. 1992;66:7517–7521. doi: 10.1128/jvi.66.12.7517-7521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Changes in coreceptor use correlates with disease progression in HIV-1 infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalgleish A G, Beverly P C L, Clapham P R, Crawford D H, Greaves M F, Weiss R A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 18.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 19.Deng H, Unutmaz D, KewalRamani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 20.Dittmar M T, McKnight A, Simmons G, Clapham P R, Weiss R A, Simmonds P. HIV-1 tropism and co-receptor use. Nature. 1997;385:495–496. doi: 10.1038/385495a0. [DOI] [PubMed] [Google Scholar]
- 21.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 22.Doranz B J, Lu Z-H, Rucker J, Zhang T-Y, Sharron M, Cen Y-H, Wang Z-X, Guo H-H, Du J-G, Accavitti M A, Doms R W, Peiper S C. Two distinct CCR5 domains can mediate coreceptor usage by human immunodeficiency virus type 1. J Virol. 1997;71:6305–6314. doi: 10.1128/jvi.71.9.6305-6314.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 24.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 25.Gallo R C, Salahuddin S Z, Popovic M, Shearer G M, Kaplan M, Haynes B F, Palker T J, Redfield R, Oleske J, Safai B, White G, Foster P, Markham P D. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1994;224:500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- 26.Hanson C V. Measuring vaccine-induced HIV neutralization: report of a workshop. AIDS Res Hum Retroviruses. 1994;10:645–648. doi: 10.1089/aid.1994.10.645. [DOI] [PubMed] [Google Scholar]
- 27.Harada S, Koyanagi Y, Yamamoto N. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science. 1985;229:563–566. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- 28.Jansson M, Popovic M, Karlsson A, Cocchi F, Rossi P, Albert J, Wigzell H. Sensitivity to inhibition by β-chemokines correlates with biological phenotypes of primary HIV-1 isolates. Proc Natl Acad Sci USA. 1996;93:15382–15387. doi: 10.1073/pnas.93.26.15382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Javaherian K, Langlois A J, McDanal C, Ross K L, Eckler L I, Jellis C L, Profy A T, Rusche J R, Bolognesi D P, Putney S D, Matthews T J. Principal neutralizing domain of the human immunodeficiency virus type 1 envelope protein. Proc Natl Acad Sci USA. 1989;86:6768–6772. doi: 10.1073/pnas.86.17.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman J-C, Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984;312:767–770. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 31.Kostrikis L G, Cao Y, Ngai H, Moore J P, Ho D D. Quantitative analysis of serum neutralization of human immunodeficiency virus type 1 from subtypes A, B, C, D, E, F, and I: lack of direct correlation between neutralization serotypes and genetic subtypes and evidence for prevalent serum-dependent infectivity enhancement. J Virol. 1996;70:445–458. doi: 10.1128/jvi.70.1.445-458.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31a.LaCasse R A, Follis K E, Moudgil T, Trahey M, Binley J M, Planelles V, Zolla-Pazner S, Nunberg J H. Coreceptor utilization by human immunodeficiency virus type 1 is not a primary determinant of neutralization sensitivity. J Virol. 1998;72:2491–2495. doi: 10.1128/jvi.72.3.2491-2495.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lasky L A, Nakamura G, Smith D H, Fennie C, Shimasaki C, Patzer E, Berman P, Gregory T, Capon D J. Delineation of a region of the human immunodeficiency virus gp120 glycoprotein critical for interaction with the CD4 receptor. Cell. 1987;50:975–985. doi: 10.1016/0092-8674(87)90524-1. [DOI] [PubMed] [Google Scholar]
- 33.Levy J A, Hoffman A D, Kramer S M, Landis J A, Shimabukuro J M, Oshiro L S. Isolation of lymphocytopathic retrovirus from San Francisco patients with AIDS. Science. 1984;225:840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- 34.Liao F, Alkhatib G, Peden K C, Sharma G, Berger E A, Farber J M. STRL33, a novel chemokine receptor-like protein functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med. 1997;185:2015–2023. doi: 10.1084/jem.185.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stuhlmann H, Koup R A, Landau N R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–378. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 36.Lu Z-H, Berson J F, Chen Y-H, Turner J D, Zhang T-Y, Sharron M, Jenks M H, Wang Z-X, Kim J, Rucker J, Hoxie J A, Peiper S C, Doms R W. Evolution of HIV-1 coreceptor usage through interactions with distinct CCR5 and CXCR4 domains. Proc Natl Acad Sci USA. 1997;94:6426–6431. doi: 10.1073/pnas.94.12.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mascola J R, Snyder S W, Weislow O S, Belay S M, Belshe R B, Schwartz D H, Clements M L, Dolin R, Graham B S, Gorse G J, Keefer M C, McElrath M J, Walker M C, Wagner K F, McNeil J G, McCutchan F E, Burke D S. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. J Infect Dis. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- 38.Matthews T J. Dilemma of neutralization resistance of HIV-1 field isolates and vaccine development. AIDS Res Hum Retroviruses. 1994;10:631–632. doi: 10.1089/aid.1994.10.631. [DOI] [PubMed] [Google Scholar]
- 39.McKnight A, Wilkinson D, Simmons G, Talbot S, Picard L, Ahuja M, Marsh M, Hoxie J A, Clapham P R. Inhibition of human immunodeficiency virus fusion by a monoclonal antibody to a coreceptor (CXCR4) is both cell type and virus strain dependent. J Virol. 1997;71:1692–1696. doi: 10.1128/jvi.71.2.1692-1696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montefiori D C, Pantaleo G, Fink L M, Zhou J T, Zhou J Y, Bilska M, Miralles G D, Fauci A S. Neutralizing and infection-enhancing antibody responses to human immunodeficiency virus type 1 in long-term nonprogressors. J Infect Dis. 1996;173:60–67. doi: 10.1093/infdis/173.1.60. [DOI] [PubMed] [Google Scholar]
- 41.Montefiori D C, Robinson W E, Jr, Schuffman S S, Mitchell W M. Evaluation of antiviral drugs and neutralizing antibodies to human immunodeficiency virus by a rapid and sensitive microtiter infection assay. J Clin Microbiol. 1988;26:231–235. doi: 10.1128/jcm.26.2.231-235.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore J P, Cao Y, Leu J, Qin L, Korber B, Ho D D. Inter- and intraclade neutralization of human immunodeficiency virus type 1: genetic clades do not correspond to neutralization serotypes but partially correspond to gp120 antigenic serotypes. J Virol. 1996;70:427–444. doi: 10.1128/jvi.70.1.427-444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore, J. P., and D. D. Ho. 1995. HIV-1 neutralization: the consequences of viral adaptation to growth on transformed T cells. AIDS 9(Suppl. A):S117–S136. [PubMed]
- 44.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J-L, Arenzana-Seisdedos F, Schwartz O, Heard J-M, Clark-Lewis I, Legler D F, Loetcher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 45.Oravecz T, Marina P, Norcross M A. β-chemokine inhibition of monocytotropic HIV-1 infection. J Immunol. 1996;157:1329–1332. [PubMed] [Google Scholar]
- 46.Palker T J, Clark M E, Langlois A J, Matthews T J, Weinhold K J, Randall R R, Bolognesi D P, Haynes B F. Type-specific neutralization of the human immunodeficiency virus with antibodies to env-encoded synthetic peptides. Proc Natl Acad Sci (USA) 1988;85:1932–1936. doi: 10.1073/pnas.85.6.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pilgrim A K, Pantaleo G, Cohen O J, Fink L M, Zhou J Y, Zhou J T, Bolognesi D P, Fauci A S, Montefiori D C. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term nonprogressive infection. J Infect Dis. 1997;176:924–932. doi: 10.1086/516508. [DOI] [PubMed] [Google Scholar]
- 48.Rucker J, Samson M, Doranz B J, Libert F, Berson J F, Yi Y, Smyth R J, Collman R G, Broder C C, Vassart G, Doms R W, Parmentier M. Regions in β-chemokine receptors CCR5 and CCR2b that determine HIV-1 cofactor specificity. Cell. 1996;87:437–446. doi: 10.1016/s0092-8674(00)81364-1. [DOI] [PubMed] [Google Scholar]
- 49.Salter R D, Howell D N, Cresswell P. Gene regulating HLA class I antigen expression in T-B lymphoblast hybrids. Immunogenetics. 1985;21:235–246. doi: 10.1007/BF00375376. [DOI] [PubMed] [Google Scholar]
- 50.Samson M, Libert F, Doranz B J, Rucker J, Liesnard C, Farber C-M, Saragosti S, Lapoumeroulie C, Cogniaux J, Forceille C, Muyldermans G, Verhofstede C, Burtonboy G, Georges M, Imai T, Rana S, Yi Y, Smyth R J, Collman R G, Doms R W, Vassart G, Parmentier M. Resistance to HIV-1 infection of Caucasian individuals bearing mutant alleles of the CKR5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 51.Simmons G, Wilkinson D, Reeves J D, Dittmar M T, Beddows S, Weber J, Carnegie G, Desselberger U, Gray P W, Weiss R A, Clapham P R. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J Virol. 1996;70:8355–8360. doi: 10.1128/jvi.70.12.8355-8360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strizki J M, Turner J D, Collman R G, Hoxie J, Gonzalez-Scarano F. A monoclonal antibody (12G5) directed against CXCR-4 inhibits infection with dual-tropic human immunodeficiency virus type 1 isolate HIV-189.6 but not the T-tropic isolate HIV-1HxB. J Virol. 1997;71:5678–5683. doi: 10.1128/jvi.71.7.5678-5683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taub D D, Conlin K, Lloyd A R, Oppenheim J J, Kelvin D J. Preferential migration of activated CD4+ and CD8+T cells in response to MIP-1α and MIP-1β. Science. 1993;260:355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 54.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 55.Trkola A, Ketas T, KewalRamani V N, Endorf F, Binley J M, Katinger H, Robinson J, Littman D R, Moore J P. Neutralization sensitivity of human immunodeficiency virus type 1 primary isolates to antibodies and CD4-based reagents is independent of coreceptor usage. J Virol. 1998;72:1876–1885. doi: 10.1128/jvi.72.3.1876-1885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trkola A, Paxton W A, Monard S P, Hoxie J A, Siani M A, Thompson D A, Wu L, Mackay C R, Horuk R, Moore J P. Genetic subtype-independent inhibition of human immunodeficiency virus type 1 replication by CC- and CXC-chemokines. J Virol. 1998;72:396–404. doi: 10.1128/jvi.72.1.396-404.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vancott T C, Polonis V R, Loomis L D, Michael N L, Nara P L, Birx D L. Differential role of V3-specific antibodies in neutralization assays involving primary and laboratory-adapted isolates of HIV type 1. AIDS Res Hum Retroviruses. 1995;11:1379–1390. doi: 10.1089/aid.1995.11.1379. [DOI] [PubMed] [Google Scholar]
- 58.Weissenhorn W, Wharton S A, Calder L J, Earl P L, Moss B, Aliprandis E, Skehel J J, Wiley D C. The ectodomain of HIV-1 env subunit gp41 forms a soluble, alpha-helical, rod-like oligomer in the absence of gp120 and the N-terminal fusion peptide. EMBO J. 1996;15:1507–1514. [PMC free article] [PubMed] [Google Scholar]
- 59.Wild C T, Dubay J W, Greenwell T, Baird T, Jr, Oas T G, McDanal C, Hunter E, Matthews T. Propensity for a leucine zipper-like domain of human immunodeficiency virus type 1 gp41 to form oligomers correlates with a role in virus-induced fusion rather than assembly of the glycoprotein complex. Proc Natl Acad Sci USA. 1994;91:12676–12680. doi: 10.1073/pnas.91.26.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 61.Zhou J Y, Montefiori D C. Antibody-mediated neutralization of primary isolates of human immunodeficiency virus type 1 in peripheral blood mononuclear cells is not affected by the initial activation state of the cells. J Virol. 1997;71:2512–2517. doi: 10.1128/jvi.71.3.2512-2517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]