Abstract
Objective
Low back pain is one of the main causes of disability in the world. Although regenerative medicine may represent breakthroughs in the management of low back pain, its use remains controversial. Therefore, we conducted a meta-analysis to evaluate the clinical efficacy of platelet-rich plasma (PRP) injection therapy versus different control groups for chronic low back pain during 4 weeks, 3 months, and 6 months.
Methods
Different electronic databases were searched for randomized controlled trials up to August 2023. Mean changes from baseline in pain and Oswestry Disability Index (ODI) scores at 4 weeks, 3 months, and 6 months and standard deviations of outcome were recorded.
Results
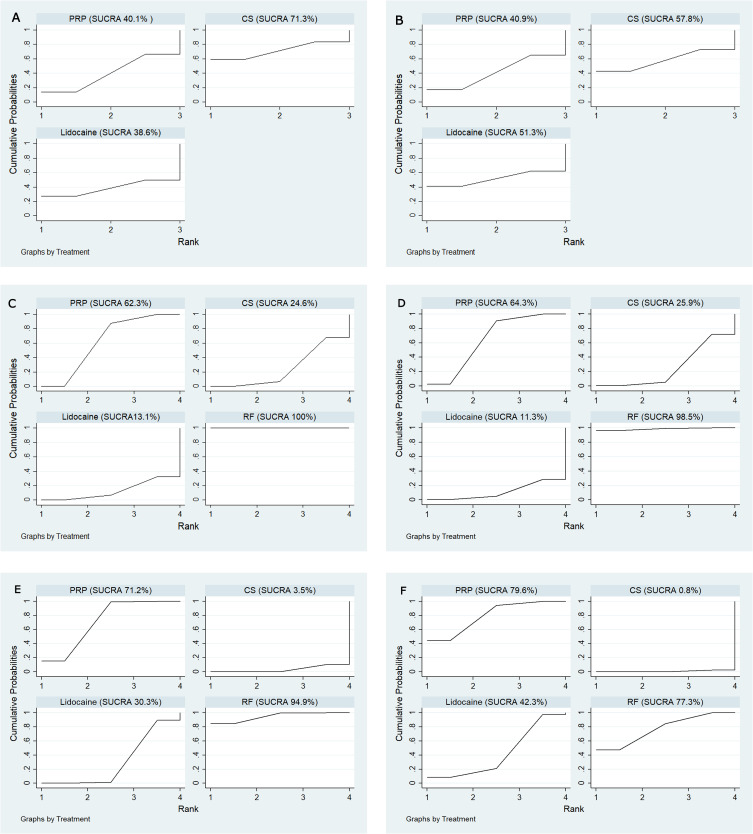
Four articles with 154 cases were finally included in this meta-analysis. After 4 weeks, corticosteroid (CS) was the optimal treatment option for chronic low back pain in terms of improvement in pain and disability index (surface under the cumulative ranking curve [SUCRA]=71.3%, SUCRA=57.8%, respectively). After 3 months, radiofrequency (RF) emerged as the best therapy in pain (SUCRA=100%) and disability index (SUCRA=98.5%), followed by PRP (SUCRA=62.3%, SUCRA=64.3%, respectively), CS (SUCRA=24.6%, SUCRA=25.9%, respectively) and lidocaine (SUCRA=13.1%, SUCRA=11.3%, respectively). At 6 months, RF was most likely to be the best treatment in pain (SUCRA=94.9%) and disability index (SUCRA=77.3%), followed by PRP (SUCRA=71.2%, SUCRA=79.6%, respectively). However, compared with the last follow-up, there was a slight downward trend in improvement pain and disability index with RF, while PRP was still an upward trend.
Conclusion
This study demonstrated better short-term improvement of chronic low back pain with CS after 4 weeks. PRP and RF improvement effects matched, but follow-up of at least 6 months showed that PRP seemed to be more advantageous in improvement in disability indices. Considering the limitations of this study, these conclusions still need to be verified by more comparative RCTs and a longer follow-up period.
Keywords: platelet-rich plasma, chronic low back pain, meta-analysis, randomized controlled trial, clinical efficacy
Introduction
Low back pain (LBP) is a highly prevalent symptom experienced by nearly everyone and is a leading cause of disability globally.1,2 LBP is defined as pain (with or without pain in one or both legs) lasting at least one day, located posteriorly, ranging from the lower border of the 12th rib to the lower gluteal crease.3–5 LBP symptoms can arise from various anatomical issues, including bones, nerve roots, fascial structures, joints, muscles, intervertebral discs, and abdominal organs.6,7 Precisely defining the source of LBP is impossible for most patients. Nonspecific LBP accounts for approximately 90–95% of all cases. Improvement in approximately 75% of LBP patients is measured by increased pain and disability within 1 month, but in approximately 25% of cases, LBP is a chronic condition.8 Chronic LBP is a prevalent issue on a global scale for which there is currently a lack of effective interventions to address this problem, leading to decreased physical activity and heightened disability. The increasing incidence of LBP imposes a heavy socioeconomic burden on patients and the healthcare sector, and worldwide, LBP is a leading cause of lost productivity and disability globally, with enormous socioeconomic and health impacts,9,10 is now acknowledged as a critical public health concern. Therefore, patients with LBP require extensive multidisciplinary and multimodal care.11,12
Platelet-rich plasma (PRP) is defined as autologous blood with a platelet concentration higher than the physiological baseline. It is prepared by centrifugation of the blood to obtain a concentrated solution of platelet.13,14 Since its first reported clinical application, it has gained popularity for its potential to repair damaged tissue and treat various degenerative and traumatic musculoskeletal diseases,15 such as tendinopathy, osteoarthritis, and ligamentous injuries.16–18 Given that the human intervertebral disc is an avascular tissue with an extremely low regenerative capacity and that the disc in healthy adults has little direct blood supply,19 there has been growing interest in the use of PRP injections for the treatment of chronic LBP. Many researchers have looked at the connection between PRP and disc degeneration, including in vitro, in vivo, preclinical animal studies, and human clinical trials.20–23 However, most studies on PRP injections for persistent LBP were limited to small case reports and studies with small sample sizes. Therefore, we conducted a meta-analysis of all known pertinent clinical trials of PRP injection therapy versus different control groups for chronic low back pain to evaluate the efficacy of different injections for persistent LBP.
Methods
Literature Search Strategy
This meta-analysis was conducted and reported per the guidelines of Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA). Randomized controlled trials (RCTs) up to August 2023 were searched in the databases of PubMed, Embase, and the Cochrane Library. The search terms were as follows: “platelet-rich plasma” or “PRP” and “low back pain”.
Inclusion and Exclusion Criteria
According to the PICOS (Population, Intervention, Comparison, Outcomes, Study) strategy, the eligibility criteria for included studies were listed below by population, intervention, comparator, outcomes, and study characteristics. (1) participants: the subjects were adult patients (aged >18 years) diagnosed with chronic LBP; (2) intervention: studies were performed PRP injection for chronic LBP; (3) comparator: comparative group receiving intervention or placebo, or usual care; (4) outcomes: the study reported the following outcome measurements: numeric rating scale (NRS) score, visual analog scale (VAS) score and Oswestry Disability Index (ODI), and mean changes from baseline in pain and ODI scores at 4 weeks, 3 months and 6 months and standard deviations of outcome were recorded; (5) study type: all studies were RCTs; (6) written in or translated into English. The exclusion criteria included the following: (1) The language was not English; (2) the data were incomplete, and (3) Review articles, case reports, or letters.
Data Extraction
Two reviewers independently screened retrieved article titles and abstracts, removed duplicates, and made study selections based on inclusion and exclusion criteria. If a decision could not be made based on the abstract, the full text was retrieved. The following data were extracted from potentially relevant studies: first author, year of publication, study design, age, sex, body mass index, method of administration, follow-up, and outcome data. We categorized the outcome data according to the following periods: assessments at 4 weeks, 3 months, and 6 months follow-up were considered. We excluded outcome data if we were unable to extract outcome data from the study, such as if the data was transformed into a graph without precise values, or if the data was reported in an unusable manner. Any disagreements about study selection or data extraction were resolved through discussion.
Quality Assessment and Bias Risk
The “bias risk” assessment of the potential of each RCT was evaluated based on The Cochrane Collaboration’s tool24 and the software Review Manager software 5.3 (RevMan5.3, Oxford, UK). The following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases were assessed. The risk of bias for each study was independently assessed by two investigators. Any disagreements were resolved through consultation with a third researcher. The publication bias of studies was evaluated by symmetry of funnel plots.
Statistical Analysis
We developed a frequentist network meta-analysis model to compare the effect of PRP injection therapy and different control groups on patient-reported VAS and ODI outcomes for at least 6 months after treatment for chronic LBP. The Q statistic was used to evaluate the homogeneity and consistency assumptions under the random effects model.25 The global I2 was used to assess statistical heterogeneity.26 Inconsistency was evaluated using node splitting and their reported P values if there were direct or indirect comparisons between treatments in several trials.27 The distinctions between mixed (both direct and indirect) and direct treatment effects within the network were used to infer indirect evidence. To calculate mixed effects for each treatment comparison, network meta-analysis models were employed. The mixed effect size was defined as potentially clinically significant when its value was between 0.5 and 1.0, and as clinically significant when its value was >1.0.28,29 Since both VAS and ODI scores are continuous variables, we assessed the standardized mean difference (SMD) of the change from baseline and 95% confidence interval (CI) of continuous results from the same assessment instrument and the same unit of measurement. For studies reporting pain using the NRS, the values were converted to VAS scale equivalents, and outcome measures reported by included RCTs were uniformly converted to VAS pain scales from 0 to 10. Surface Under the Cumulative Ranking Curve (SUCRA) was used to rate the various therapies,30 with an SUCRA value of 100 denoting the most successful intervention and an SUCRA value of 0 denoting the least effective intervention. All analyses were run in STATA software v.14.0 (Stata, College Station, TX, USA), and P<0.05 was considered statistically significant.
Results
Literature Search
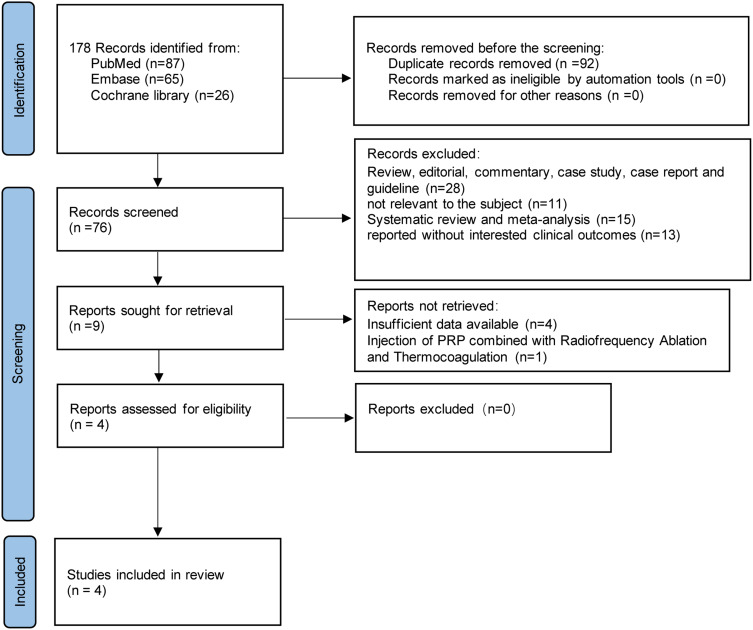
Through a literature search, a total of 178 articles were identified from electronic databases. A total of 92 duplicate articles were eliminated, and 76 articles were eliminated after a review of titles and abstracts. The remaining nine potentially eligible articles were evaluated by full-text reading and screening. Ultimately, four articles21,23,31,32 with appropriate outcome measures that met the eligibility criteria were included. The PRISMA flowchart of the studies in this review was presented in Figure 1. A total of 154 cases were enrolled in the study. The follow-up period ranged from 4 weeks to 52 weeks. However, Goyal’s study did not have relevant results of 4-week follow-up. Table 1 provides details information on the characteristics of the studies in the included articles.
Figure 1.
PRISMA flow diagram of the systematic review and meta-analysis.
Table 1.
Characteristics of the Included Studies
| First Author (Year) | Sample Size | Diagnostic | BMI (kg/m2) | M/F | Mean Age, y | Intervention | Injection Site | Follow-Ups | Outcome Measurements |
|---|---|---|---|---|---|---|---|---|---|
| Akeda K et al 202223 | 16 | Discogenic Low Back Pain | NR | 6/3 | 35.1 | PRP | Intradiscal injection | 4, 8, 12, 26, and 52 weeks | VAS, ODI |
| NR | 5/2 | 27.9 | CS | ||||||
| Won SJ et al 202221 | 30 | Chronic Low Back Pain | 25.1±4.1 | 6/10 | 50.5±17.0 | PRP | Tenderness points in the lumbosacral spine and lumbopelvic region | 4 weeks, 3 months, and 6 months | VAS, ODI |
| 22.9±2.7 | 6/8 | 51.0±18.1 | Lidocaine | ||||||
| Saraf A et al 202331 | 60 | Discogenic Low Back Pain | 23.21±4.68 | 15/14 | 42.03±11.31 | PRP | Transforaminal injections | 1, 3, and 6 months | VAS, ODI |
| 22.05±3.03 | 16/15 | 45.83±12.35 | CS | ||||||
| Goyal T et al 202232 | 48 | Discogenic Low Back Pain | 24.5±1.65 | 13/11 | 35.12±9.36 | PRP | Intradiscal injection | 3, 6 months | NRS, ODI |
| 24.11±1.56 | 13/11 | 33.25±9.16 | RF |
Abbreviations: BMI, body mass index; M, male; F, female; PRP, platelet-rich plasma; CS, Corticosteroid; RF, radiofrequency; VAS, visual analog scale; NRS, numeric rating scale; ODI, Oswestry Disability Index.
Risk of Bias Assessment
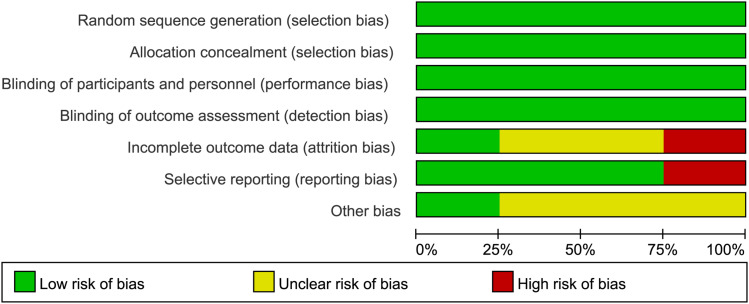
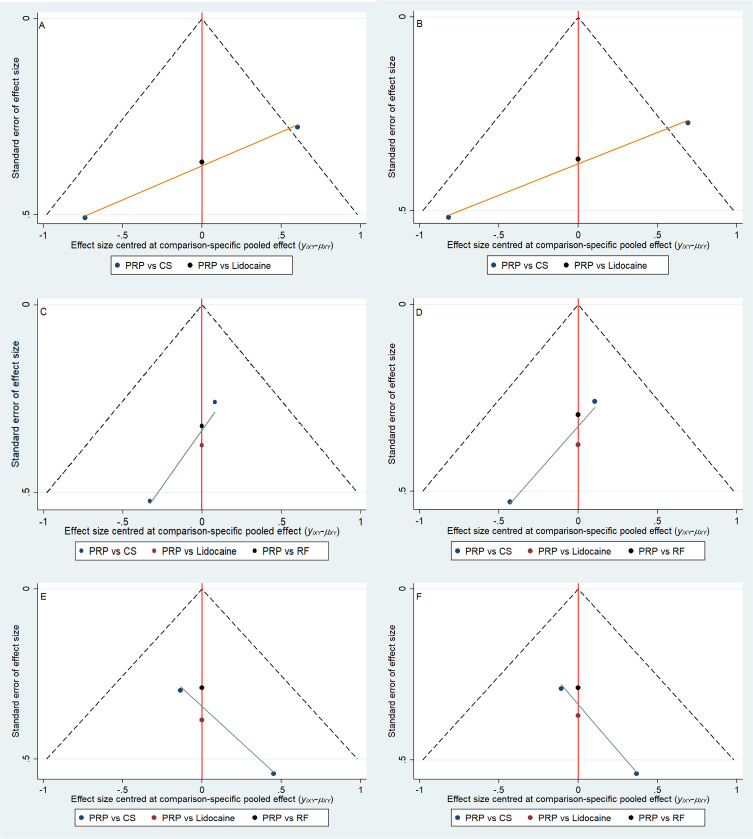
The overall risk of bias in each study was considered “low” when more than four Cochrane Collaboration Tools items related to “low risk” were considered applicable; the overall risk of bias in each study was considered applicable when two to three items were applicable Considered “moderate”; “high” when less than two “low risk” projects or more than one “high risk” project were considered applicable. Except for Goyal’s study,32 which had a moderate risk of bias, the other trials were well-designed and had a low risk of bias (Figure 2). The publication bias of the studies was assessed by the symmetry of the funnel plot. As shown in Figure 3, the shape of the funnel plot was roughly symmetrical.
Figure 2.
Risk bias assessment for randomized controlled trials.
Figure 3.
Funnel plot. (A) VAS at 4 weeks; (B) ODI at 4 weeks; (C) VAS at 3 months; (D) ODI at 3 months;(E) VAS at 6 months; (F) ODI at 6 months.
Network Meta-Analysis
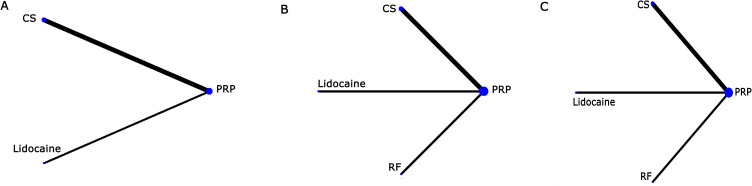
A network diagram of available comparisons and studies were shown in Figure 4. The assumption of between-design consistency assumed that a design-by-treatment interaction random effects model was satisfied for both VAS at 4 weeks (Q statistic=0.44, P=0.51), 3 months (Q statistic=4.94, P=0.052) and 6 months (Q statistic=1.09, P=0.30) follow-up and ODI at 4 weeks (Q statistic=0.99, P=0.32), 3 months (Q statistic=4.89, P=0.058) and 6 months (Q statistic=0.00, P=1.00) follow-up, suggesting that there was no significant difference within the network between direct and indirect evidence.
Figure 4.
Network of comparative interventions. The size of each treatment node corresponds to the patient’s number of randomly assigned treatments. (A) VAS and ODI at 4 weeks; (B) VAS and ODI at 3 months; (C) VAS and ODI at 6 months.
VAS
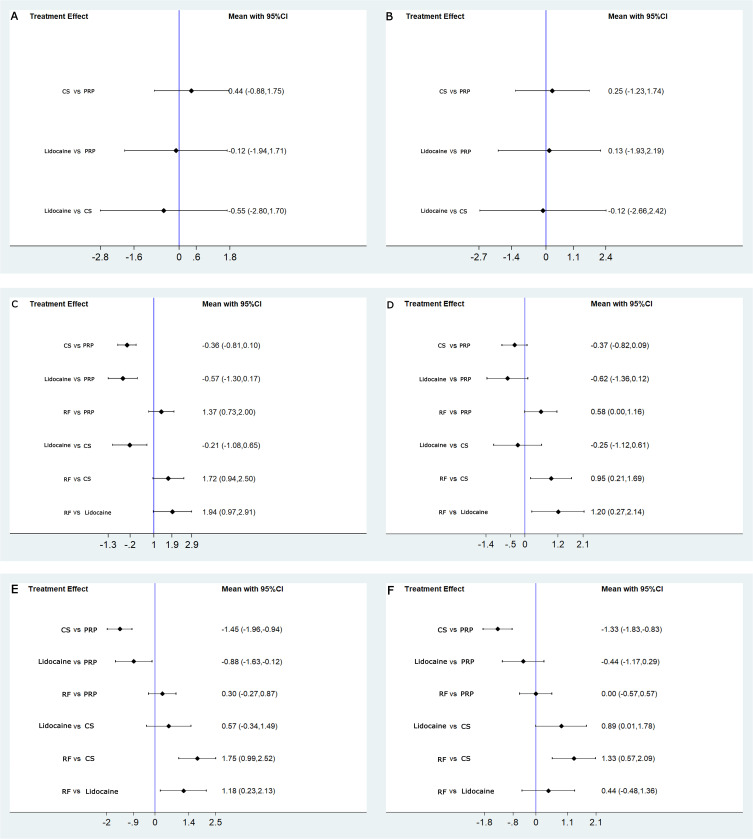
Comprehensive observation showed that the pain scores of all patients were significantly reduced compared to before injection, and in line with the minimal clinical important difference (MCID). For LBP patients, an MCID value of 1.5 for the effect in VAS is recommended, with a 30% change from baseline.33 Pain intensity decreased at 4 weeks were not significantly different between patients who received PRP injections compared with those who received CS (0.44, 95% CI-0.88 to 1.75, p=0.52) and lidocaine (−0.12, 95% CI-1.94 to 1.71, p=0.90). Pain intensity decreased at 3 months, there was no statistical difference between patients injected with PRP and patients injected with CS (−0.36, 95% CI-0.81 to 0.10, p=0.13) and lidocaine (−0.57, 95% CI-1.30 to 0.17, p=0.13), and there was a significant difference between patients injected with PRP and RF (1.37, 95% CI 0.73 to 2.00, p=0.00). At treatment follow-up, pain intensity decreased at 6 months and was also significantly different in patients who received PRP injections compared with those who received CS and lidocaine; the between-group differences were −1.45 (95% CI −1.96 to −0.94, p=0.00) and −0.88 (95% CI −1.63 to −0.12, p=0.023), respectively. However, there was no significant difference in pain intensity between the PRP injection and RF (0.30, 95% CI −0.27 to 0.87, p=0.296) at 6 months. The findings between-group differences were shown in Table 2.
Table 2.
The Effect Sizes of PRP Injection Therapy versus Different Control Group for Chronic Low Back Pain (Direct Evidence)
| Comparison at 4 Weeks | Comparison at 3 Months | Comparison at 6 Months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Effect Size (95% CI) | Z | P | Effect Size (95% CI) | Z | P | Effect Size (95% CI) | Z | P | |
| Pain | |||||||||
| PRP vs CS | 0.44 (−0.88, 1.75) | 0.65 | 0.52 | −0.36 (−0.81, 0.10) | −1.53 | 0.13 | −1.45 (−1.96, −0.94) | −5.54 | 0.00 |
| PRP vs Lidocaine | −0.12 (−1.94, 1.71) | −0.12 | 0.90 | −0.57 (−1.30, 0.17) | −1.52 | 0.13 | −0.88 (−1.63, −0.12) | −2.27 | 0.02 |
| PRP vs RF | — | — | — | 1.37 (0.73, 2.00) | 4.23 | 0.00 | 0.30 (−0.27, 0.87) | 1.05 | 0.30 |
| Disability Index | |||||||||
| PRP vs CS | 0.25 (−1.23, 1.74) | 0.33 | 0.74 | −0.37 (−0.82, 0.089) | −1.58 | 0.12 | −1.33 (−1.83, −0.83) | −5.18 | 0.00 |
| PRP vs Lidocaine | 0.13 (−1.93, 2.19) | 0.12 | 0.90 | −0.62 (−1.36, 0.12) | −1.65 | 0.099 | −0.44 (−1.17, 0.29) | −1.18 | 0.24 |
| PRP vs RF | — | — | — | 0.58 (0.0040, 1.16) | 1.97 | 0.048 | 2.18e−12 (−0.57, 0.57) | 0.00 | 1.000 |
Abbreviations: PRP, platelet-rich plasma; CS, Corticosteroid; RF, radiofrequency; CI, Confidence intervals.
ODI
The ODI evaluated findings regarding disability indices. All four groups showed significant improvements in disability indices compared to before injection. These improved results met the requirements of MCID. For patients with LBP, the recommended MCID value for disability indices is 10, a change of 30% from baseline.33 In this study, all participants had a decrease in disability indices at 4 weeks, 3 months, and 6 months follow-up, but none of the disability indices were statistically significant, except the PRP injections compared with RF were statistically significant at 3 months (0.58, 95% CI 0.0040 to 1.16, p=0.048) and PRP injections compared with CS injections that were statistically significant at 6 months (−1.33, 95% CI −1.83 to −0.83, p=0.00). The findings between-group differences were shown in Table 2.
Mixed Effects
In terms of pain improvement and changes in disability indices after 4 weeks, no statistically significant differences were found between pairwise comparisons of PRP, CS, and lidocaine. Concerning pain improvement after 3 months, the effect of RF treatment was found to be sufficiently large to be potentially clinically meaningful compared with PRP (1.37, 95% CI 0.73 to 2.00), CS (1.72, 95% CI 0.94 to 2.50), and lidocaine (1.94, 95% CI 0.97 to 2.91), whereas there may be potential clinical differences between PRP and lidocaine (−0.57, 95% CI −1.30 to 0.17). On changes in disability indices after 3 months, only RF treatments compared with lidocaine (1.20, 95% CI 0.27 to 2.14) were found to have a sufficiently large effect to be of potential clinical significance. After 6 months of follow-up, both mixed treatment comparisons were considered clinically meaningful in terms of pain improvement, except that no statistically significant differences were found for RF comparison with PRP (0.30, 95% CI −0.27 to 0.87). Whereas the changes in disability indices after 6 months showed that both PRP and RF treatments had statistically significant compared with CS treatments, and with large and equal effect sizes, the between-group differences were −1.33 (95% CI −1.83 to −0.83) and 1.33 (95% CI 0.57 to 2.09), respectively. In other words, PRP and RF treatments achieved the same improvement in disability indices after 6 months for participants. The results were illustrated in Figure 5 and Table 3.
Figure 5.
Forest plot for mixed comparisons. (A) VAS at 4 weeks; (B) ODI at 4 weeks; (C) VAS at 3 months; (D) ODI at 3 months; (E) VAS at 6 months; (F) ODI at 6 months.
Table 3.
Mixed Effects for Pain and Disability Index
| PRP | CS | Lidocaine | RF | ||
|---|---|---|---|---|---|
| Pain | |||||
| 4 weeks | PRP | 0.44 (−0.88, 1.75) | −0.12 (−1.94, 1.71) | ||
| CS | −0.55 (−2.80, 1.70)a | ||||
| Lidocaine | |||||
| RF | |||||
| 3 months | PRP | −0.36 (−0.81, 0.10) | −0.57 (−1.30, 0.17)a | 1.37 (0.73, 2.00)b | |
| CS | −0.21 (−1.08, 0.65) | 1.72 (0.94, 2.50)b | |||
| Lidocaine | 1.94 (0.97, 2.91)b | ||||
| RF | |||||
| 6 months | PRP | −1.45 (−1.96, −0.94)b | −0.88 (−1.63, −0.12)a | 0.30 (−0.27, 0.87) | |
| CS | 0.57 (−0.34, 1.49)a | 1.75 (0.99, 2.52)b | |||
| Lidocaine | 1.18 (0.23, 2.13)b | ||||
| RF | |||||
| Disability Index | |||||
| 4 weeks | PRP | 0.25 (−1.23, 1.74) | 0.13 (−1.93, 2.19) | ||
| CS | −0.12 (−2.66, 2.42) | ||||
| Lidocaine | |||||
| RF | |||||
| 3 months | PRP | −0.37 (−0.82, 0.09) | −0.62 (−1.36, 0.12)a | 0.58 (0.00, 1.16)a | |
| CS | −0.25 (−1.12, 0.61) | 0.95 (0.21, 1.69)a | |||
| Lidocaine | 1.20 (0.27, 2.14)b | ||||
| RF | |||||
| 6 months | PRP | −1.33 (−1.83, −0.83)b | −0.44 (−1.17, 0.29) | 0.00 (−0.57, 0.57) | |
| CS | 0.89 (0.01, 1.78)a | 1.33 (0.57, 2.09)b | |||
| Lidocaine | 0.44 (−0.48, 1.36) | ||||
| RF | |||||
Notes: Bolded values indicate statistically significant difference between the respective treatments. aValues≥0.5 indicate a statistically significant difference between treatments that also represented a potential clinically important difference. bValues≥1.0 indicate a statistically significant difference between treatments that was clinically meaningful.
Abbreviations: PRP, platelet-rich plasma; CS, Corticosteroid; RF, radiofrequency.
SUCRA
The four competing therapies were then ranked according to pain and disability indices results. In this study, changes in pain scores after 4 weeks for participants, the SUCRA-based intervention ranking indicated that CS was at the top of the ranking (SUCRA =74.5%), followed by PRP (SUCRA =51.5%), lidocaine (SUCRA=48.1%) and R (SUCRA=25.9%), respectively. But changes in pain scores after 3 months showed that RF had the highest likelihood of being the best treatment (SUCRA=100%), followed by PRP (SUCRA=62.3%), CS (SUCRA=24.6%) and lidocaine (SUCRA =13.1%). Changes in pain scores after 6 months for participants showed that RF had the highest likelihood of being the best treatment (SUCRA=94.9%), followed by PRP (SUCRA=71.2%), lidocaine (SUCRA =30.3%) and CS (SUCRA=3.5%).
Concerning changes in disability indices after 4 weeks for participants, the SUCRA-based intervention ranking indicated that CS was at the top of the ranking (SUCRA=67.0%), followed by lidocaine (SUCRA=59.7%), PRP (SUCRA=55.0%) and RF (SUCRA=18.3%), respectively. The changes in disability indices after 3 months for participants showed that RF had the highest likelihood of being the best treatment (SUCRA=98.5%), followed by PRP (SUCRA=64.3%), CS (SUCRA=25.9%) and lidocaine (SUCRA=11.3%). But the changes in disability indices after 6 months for participants showed that PRP had the highest likelihood of being the best treatment (SUCRA=79.6%), followed by RF (SUCRA=77.3%), lidocaine (SUCRA=42.3%) and CS (SUCRA=0.8%). The SUCRA results were illustrated in Figure 6.
Figure 6.
Treatments were ranked according to surface under the cumulative ranking curve. (A) VAS at 4 weeks; (B) ODI at 4 weeks; (C) VAS at 3 months; (D) ODI at 3 months; (E) VAS at 6 months; (F) ODI at 6 months.
No relevant adverse events were reported in all four included studies.
Discussion
In this study, we evaluated the effectiveness of PRP injections compared with different control group injections in patients with chronic LBP. We evaluated all data from each included study and observed that PRP injections, CS, lidocaine, and RF were associated with a reduction in pain and disability indices after 4 weeks, 3 months, and 6 months. To our knowledge, this represents the first comprehensive analysis that compared the therapeutic efficacy of PRP to CS, lidocaine, and RF in terms of easing pain and enhancing functional disability in patients with chronic LBP.
The main finding of this network meta-analysis was that CS at 4 weeks had a superior short-term improvement in chronic LBP symptoms compared to the pretreatment baseline post-injection patient follow-up. Other improvements were ranked by PRP, lidocaine, although neither of them showed statistically significant improvement in pairwise comparisons. In other words, CS had relatively excellent short-term improvement effects on pain and disability indices after 4 weeks of treatment, while PRP and lidocaine injections had similar short-term effects. Since Goyal’s study did not have results of 4 weeks follow-up, there were no relevant comparative results for RF. During further follow-up of the patient after 3 months of treatment, this study showed that patients with chronic LBP symptoms had significantly improved after receiving PRP and RF treatment, and the patients who received RF treatment had the best improvement in pain and disability index, followed by PRP. However, the improvement of the pain and disability index by CS showed a downward trend and lidocaine improved the least. Patients were followed for a longer period time 6 months after injection. It was found that the pain and disability index of LBP patients also improved significantly after receiving PRP and RF treatment, and the improvement of PRP showed an upward trend, while the improvement of RF showed a slight downward trend, but the degree of improvement between PRP and RF was equivalent and there was no significant difference. On the contrary, CS had the worst improvement in the pain and disability index of LBP patients after 6 months, indicating that with the extension of follow-up time, the long-term effect of PRP and RF treatment was better than that of CS. No adverse reactions were reported during the follow-up period. These results preliminarily confirmed the safety, feasibility, and effectiveness of PRP in the treatment of chronic LBP.
Although the mechanism remains unclear, it is generally accepted that there are several proposed potential mechanisms behind the pain-relieving and enhanced function disability of intradiscal PRP injection. PRP has been reported to have three to eight times the concentration of platelets compared to whole blood. It contains higher levels of cytokines and growth factors that can promote angiogenesis, accelerate endothelial regeneration, stimulate tissue repair and healing processes, and increase collagen content in different types of tissues.34–38 When platelets are activated in PRP, they can produce many growth factors, including platelet-derived growth factor, basic fibroblast growth factor, transforming growth factor-β, insulin-like growth factor, vascular endothelial growth factor, etc. They can promote the production of collagen II and chondrocytes, prevent apoptosis of mesenchymal stem cells and chondrocytes, and avert the catabolic effects of inflammatory cytokines like interleukin-1β and matrix metalloproteinase. Additionally, PRP also contains plasma proteins that are thought to play a critical role in the healing process of connective tissues such as Sox9, aggrecan, collagen type I, and collagen type II. Due to its effect on increasing osmotic pressure, aggregated proteins play a role in absorbing water as well as providing tensile strength and anchoring tissue to the bone via increasing collagen. By surpassing the nuclear factor-kB signaling pathway, PRP injection transforms this pathological state into one that is anabolic and anti-inflammatory.39–44 Moreover, PRP is derived from the patient’s own body, and there are antimicrobial proteins in platelets that can migrate to the injury site, which makes autologous PRP injection potentially safer.
Overall, this study showed that the use of PRP injections in the treatment of chronic LBP works well. The main significant advantage of this therapy is the safety of autologous PRP itself. The injection sites for PRP in the four included studies were intradiscal injections and tender points in the lumbosacral and lumbopelvic regions. Except for a few transient adverse effects (injection site pain), none of the studies reported any serious adverse events or complications from the injections. Since autologous PRP is obtained from the patient’s blood, PRP therapy has a lower risk of disease infection and allergic reaction. A strength of this study was the availability of similar quantitative scores for the four treatments across three periods of time. Furthermore, different database searches improved the search quality of our study. However, the limited number and sample size of controlled studies were currently our limitations. Due to individual differences, differences in PRP concentration and quality will also affect the efficacy, and the optimal time of PRP injection and the optimal concentration of platelets in PRP have yet to be standardized. In the future, the effectiveness of PRP treatment needs to be further verified through RCTs with multi-center, larger sample size, longer follow-up time, and more objective indicators, and a unified diagnosis and treatment plan and clinical application standards should be formulated. As an emerging treatment method for chronic LBP, PRP has broad application prospects and is worthy of further research and exploration.
Conclusion
In this meta-analysis of the effectiveness of PRP injections versus different control groups in patients with chronic LBP, CS injections showed better short-term improvement after 4 weeks. PRP injections and RF improvement effects matched, but at least 6 months of follow-up showed that PRP injections seemed to be more advantageous in terms of improvement of disability indices, because the disability indices effects of PRP injections can be sustained and significant, and the long-term effects were more predictive excellent. Considering the limitations of this study, these conclusions still need to be verified by a large number of comparative RCTs and longer follow-up times.
Acknowledgments
We thank the reviewers for their thorough review of our manuscript.
Funding Statement
This research received no grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and confirm that this report complies with those standards.
Disclosure
The authors declare no potential conflicts of interest in this work.
References
- 1.Kahere M, Hlongwa M, Ginindza TG. A scoping review on the epidemiology of chronic low back pain among adults in Sub-Saharan Africa. Int J Environ Res Public Health. 2022;19(5):2964. doi: 10.3390/ijerph19052964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Onuora S. Low back pain is a growing concern. Nat Rev Rheumatol. 2023;19(8):462. doi: 10.1038/s41584-023-00999-1 [DOI] [PubMed] [Google Scholar]
- 3.Mosabbir A. Mechanisms behind the development of chronic low back pain and its neurodegenerative features. Life. 2022;13(1). doi: 10.3390/life13010084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kreiner DS, Matz P, Bono CM, et al. Guideline summary review: an evidence-based clinical guideline for the diagnosis and treatment of low back pain. Spine J. 2020;20(7):998–1024. doi: 10.1016/j.spinee.2020.04.006 [DOI] [PubMed] [Google Scholar]
- 5.Wang F, Cheung CW, Wong SSC. Regenerative medicine for the treatment of chronic low back pain: a narrative review. J Int Med Res. 2023;51(2):3000605231155777. doi: 10.1177/03000605231155777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anders C, Hübner A. Influence of elastic lumbar support belts on trunk muscle function in patients with non-specific acute lumbar back pain. PLoS One. 2019;14(1):e0211042. doi: 10.1371/journal.pone.0211042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wieczorek A, Campau E, Pionk E, et al. A closer look into the association between the sacroiliac joint and low back pain. Spartan Med Res J. 2021;6(1):21971. doi: 10.51894/001c.21971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carregaro RL, Tottoli CR, Rodrigues DDS, et al. Low back pain should be considered a health and research priority in Brazil: lost productivity and healthcare costs between 2012 to 2016. PLoS One. 2020;15(4):e0230902. doi: 10.1371/journal.pone.0230902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knezevic NN, Candido KD, Vlaeyen JWS, et al. Low back pain. Lancet. 2021;398(10294):78–92. doi: 10.1016/S0140-6736(21)00733-9 [DOI] [PubMed] [Google Scholar]
- 10.Mattiuzzi C, Lippi G, Bovo C. Current epidemiology of low back pain. J Hosp Manag Health Policy. 2020;4:15. doi: 10.21037/jhmhp-20-17 [DOI] [Google Scholar]
- 11.Wu A, March L, Zheng X, et al. Global low back pain prevalence and years lived with disability from 1990 to 2017: estimates from the Global Burden of Disease Study 2017. Ann Transl Med. 2020;8(6):299. doi: 10.21037/atm.2020.02.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nees TA, Riewe E, Waschke D, et al. Multidisciplinary pain management of chronic back pain: helpful treatments from the patients’ perspective. J Clin Med. 2020;9(1):145. doi: 10.3390/jcm9010145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Hamed FS, Abu-Nada L, Rodan R, et al. Differences in platelet-rich plasma composition influence bone healing. J Clin Periodontol. 2021;48(12):1613–1623. doi: 10.1111/jcpe.13546 [DOI] [PubMed] [Google Scholar]
- 14.Saqlain N, Mazher N, Fateen T, et al. Comparison of single and double centrifugation methods for preparation of Platelet-Rich Plasma (PRP). Pak J Med Sci. 2023;39(3):634–637. doi: 10.12669/pjms.39.3.7264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen RT, Borg-Stein J, McInnis K. Applications of platelet-rich plasma in musculoskeletal and sports medicine: an evidence-based approach. Pm&r. 2011;3(3):226–250. doi: 10.1016/j.pmrj.2010.11.007 [DOI] [PubMed] [Google Scholar]
- 16.Paget LDA, Reurink G, de Vos RJ, et al. Platelet-rich plasma injections for the treatment of ankle osteoarthritis. AM J SPORT MED. 2023;51(10):2625–2634. doi: 10.1177/03635465231182438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Liu Z, Tang J, et al. Fibroblast growth factor 2-induced human amniotic mesenchymal stem cells combined with autologous platelet rich plasma augmented tendon-to-bone healing. J Orthop Transl. 2020;24:155–165. doi: 10.1016/j.jot.2020.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossi LA, Piuzzi N, Tanoira I, et al. Subacromial platelet-rich plasma injections produce significantly worse improvement in functional outcomes in patients with partial supraspinatus tears than in patients with isolated tendinopathy. ARTHROSCOPY. 2023;39(9):2000–2008. doi: 10.1016/j.arthro.2023.03.019 [DOI] [PubMed] [Google Scholar]
- 19.Roberts S, Evans H, Trivedi J, et al. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88(Suppl 2):10–14. doi: 10.2106/JBJS.F.00019 [DOI] [PubMed] [Google Scholar]
- 20.Wang SZ, Rui YF, Tan Q, et al. Enhancing intervertebral disc repair and regeneration through biology: platelet-rich plasma as an alternative strategy. Arthritis Res Ther. 2013;15(5):220. doi: 10.1186/ar4353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Won SJ, Kim DY, Kim JM. Effect of platelet-rich plasma injections for chronic nonspecific low back pain: a randomized controlled study. Medicine. 2022;101(8):e28935. doi: 10.1097/MD.0000000000028935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xuan Z, Yu W, Dou Y, et al. Efficacy of platelet-rich plasma for low back pain: a systematic review and meta-analysis. J Neurol Surg Part A. 2020;81(6):529–534. doi: 10.1055/s-0040-1709170 [DOI] [PubMed] [Google Scholar]
- 23.Akeda K, Ohishi K, Takegami N, et al. Platelet-rich plasma releasate versus corticosteroid for the treatment of discogenic low back pain: a double-blind randomized controlled trial. J Clin Med. 2022;11(2). doi: 10.3390/jcm11020304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011:343 d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White IR, Barrett JK, Jackson D, et al. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods. 2012;3(2):111–125. doi: 10.1002/jrsm.1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neupane B, Richer D, Bonner AJ, et al. Network meta-analysis using R: a review of currently available automated packages. PLoS One. 2014;9(12):e115065. doi: 10.1371/journal.pone.0115065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29(7–8):932–944. doi: 10.1002/sim.3767 [DOI] [PubMed] [Google Scholar]
- 28.Jevsevar DS, Shores PB, Mullen K, et al. Mixed treatment comparisons for nonsurgical treatment of knee osteoarthritis: a network meta-analysis. J Am Acad Orthop Sur. 2018;26(9):325–336. doi: 10.5435/JAAOS-D-17-00318 [DOI] [PubMed] [Google Scholar]
- 29.Johnston BC, Thorlund K, Schünemann HJ, et al. Improving the interpretation of quality of life evidence in meta-analyses: the application of minimal important difference units. Health Qual Life Outcomes. 2010;8:116. doi: 10.1186/1477-7525-8-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2010;64(2):163–171. doi: 10.1016/j.jclinepi.2010.03.016 [DOI] [PubMed] [Google Scholar]
- 31.Saraf A, Hussain A, Sandhu AS, et al. Transforaminal injections of platelet-rich plasma compared with steroid in lumbar radiculopathy: a prospective, double-blind randomized study. Indian J Orthop. 2023;57(7):1126–1133. doi: 10.1007/s43465-023-00898-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goyal T, Paswan A, Jain D, et al. Comparative evaluation of efficacy of percutaneous intradiscal radiofrequency ablation and platelet rich plasma injection for discogenic low back pain: a prospective randomized trial. J Musculoskelet Res. 2022. doi: 10.1142/s0218957722500099 [DOI] [Google Scholar]
- 33.Singjie LC, Kusuma SA, Saleh I, et al. The potency of platelet-rich plasma for chronic low back pain: a systematic review and meta-analysis of randomized controlled trial. Asian Spine J. 2023;17(4):782–789. doi: 10.31616/asj.2022.0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L, Zhang C, Song D, et al. Combination of percutaneous endoscopic lumbar discectomy and platelet-rich plasma hydrogel injection for the treatment of lumbar disc herniation. J Orthop Surg Res. 2023;18(1):609. doi: 10.1186/s13018-023-04093-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawabata S, Akeda K, Yamada J, et al. Advances in platelet-rich plasma treatment for spinal diseases: a systematic review. Int J Mol Sci. 2023;24(8). doi: 10.3390/ijms24087677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akeda K, Fujiwara T, Takegami N, et al. Retrospective analysis of factors associated with the treatment outcomes of intradiscal platelet-rich plasma-releasate injection therapy for patients with discogenic low back pain. Medicina Lithuania. 2023;59(4). doi: 10.3390/medicina59040640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaye AD, Edinoff AN, Rosen YE, et al. Regenerative medicine: pharmacological considerations and clinical role in pain management. Curr Pain Headache R. 2022;26(10):751–765. doi: 10.1007/s11916-022-01078-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grossen AA, Lee BJ, Shi HH, et al. Platelet-rich plasma injections: pharmacological and clinical considerations in pain management. Curr Pain Headache R. 2022;26(10):741–749. doi: 10.1007/s11916-022-01082-2 [DOI] [PubMed] [Google Scholar]
- 39.Knezevic NN, Mandalia S, Raasch J, et al. Treatment of chronic low back pain-new approaches on the horizon. J Pain Res. 2017;10:1111–1123. doi: 10.2147/JPR.S132769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang SZ, Jin JY, Guo YD, et al. Intervertebral disc regeneration using platelet‑rich plasma‑containing bone marrow‑derived mesenchymal stem cells: a preliminary investigation. Mol Med Rep. 2016;13(4):3475–3481. doi: 10.3892/mmr.2016.4983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins T, Alexander D, Barkatali B. Platelet-rich plasma: a narrative review. Efort Open Rev. 2021;6(4):225–235. doi: 10.1302/2058-5241.6.200017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akeda K, Yamada J, Linn ET, et al. Platelet-rich plasma in the management of chronic low back pain: a critical review. J Pain Res. 2019;12:753–767. doi: 10.2147/JPR.S153085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohammed S, Yu J. Platelet-rich plasma injections: an emerging therapy for chronic discogenic low back pain. J Spine Surg. 2018;4(1):115–122. doi: 10.21037/jss.2018.03.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang Y, Yang M, Ke S, et al. Effect of platelet-rich plasma on intervertebral disc degeneration in vivo and in vitro: a critical review. Oxid Med Cell Longev. 2020;2020:8893819. doi: 10.1155/2020/8893819 [DOI] [PMC free article] [PubMed] [Google Scholar]