Abstract
Colorectal cancer (CRC) is one of the most common causes of cancer morbidity in both sexes but shows sex differences. First, sex-specific differences in tumor recurrence and survival rates have been reported. For example, the development of CRC is found about 1.5 times higher and 4–8 years earlier in males compared to females, suggesting the protective role of estrogen in the disease. Furthermore, female patients have a higher risk of developing right-sided (proximal) colon cancer than male patients, which is known to have more aggressive clinical character compared to left-sided (distal) colon cancer. That is, left and right CRCs show differences in carcinogenic mechanism, that the chromosomal instability pathway is more common in left colon cancer while the microsatellite instability and serrated pathways are more common in right colon cancer. It is thought that there are sex-based differences on the background of carcinogenesis of CRC. Sex differences of CRC have two aspects, sexual dimorphism (biological differences in hormones and genes) and gender differences (non-biological differences in societal attitudes and behavior). Recently, sex difference of colon adenoma pathway and sexual dimorphism in the biology of gene and protein expression, and in endocrine cellular signaling in the CRC carcinogenesis have been accumulated. In addition, behavioral patterns can lead to differences in exposure to risk factors such as drinking or smoking, diet and physical activity. Therefore, understanding sex/gender-related biological and sociocultural differences in CRC risk will help in providing strategies for screening, treatment and prevention protocols to reduce the mortality and improve the quality of life. In this review, sex/gender differences in colon adenoma pathway and various aspects such as clinicopathological, biological, molecular, and socio-cultural aspects of CRC were described.
Keywords: Colorectal neoplasms, Early detection of cancer, Primary prevention, Sex, Therapeutics
INTRODUCTION
Colorectal cancer (CRC) is a significant cause of morbidity and mortality in the world, ranked third most common cancer globally in 2012 and expected to reach 2.2 million by 2030 [1,2]. CRC shows sex differences, attributable to sexual dimorphism (biological differences in hormones and genes) and gender differences (non-biological differences in societal attitudes and behavior) [3]. CRC occurs more frequently in males than in females [4] and the incidence and mortality of CRC in populations over 65 years old are higher in females than those in males, implying that CRC is a major health threat among older females [5,6]. Considering the longer life expectancy of females compared to that of males, sex/gender-targeted strategies to prevent and treat CRC should be properly delivered to improve the quality of life, especially in older females [5].
The biological aspects of these differences are explained by sex differences in sex hormones, gut microbiota and molecular properties [7]. For instance, CRC can be classified as right-sided (proximal) and left-(distal) colon cancer clinically, and right colon cancer which is known to be more aggressive compared to left colon cancer [5] is reported to be more prevalent in females than in males [8]. In carcinogenic sequence of CRC, The chromosomal instability (CIN) pathway begins with mutations in the tumor suppressor genes is more common mechanism in left colon cancer, while the microsatellite instability (MSI) pathway begins with initial alteration of the Wnt signaling followed by B-Raf proto-oncogene serine/threonine-protein kinase (BRAF) mutation is more frequent in right colon cancer [9]. In addition to the adenoma to carcinoma sequence, colorectal carcinogenesis can occur via the serrated pathway characterized by mutations in RAS and RAF, which is also more common in right colon cancer [10]. This difference between sexes in carcinogenesis may be one explanation for the relationship between sex and tumor location.
In term of gender, the diet, smoking, alcohol, and physical activities affect the development of CRC. In this review, sex and gender differences of CRC have been briefly reviewed to provide holistic approach for the sex/gender-targeted CRC screening, treatment, and prevention.
SEX DIFFERENCES IN CLINICAL CHARACTERISTICS
The Global Cancer Observatory 2019 shows that the incidence and survival patterns for CRC vary across the world [11], and the incidence of CRC shows sex difference that is consistently higher in males than in females [11]. Mortality rates from CRC also shows sex differences, which are higher in males compared to females consistently across different regions of the world [3]. This would be the result of a combination of sexual dimorphism and gender differences, and it is difficult to determine how much each factor is attributable to the sex/gender differences in CRC incidence and mortality rates [3]. Maybe it can be different depending on age, race and region.
1. Epidemiology
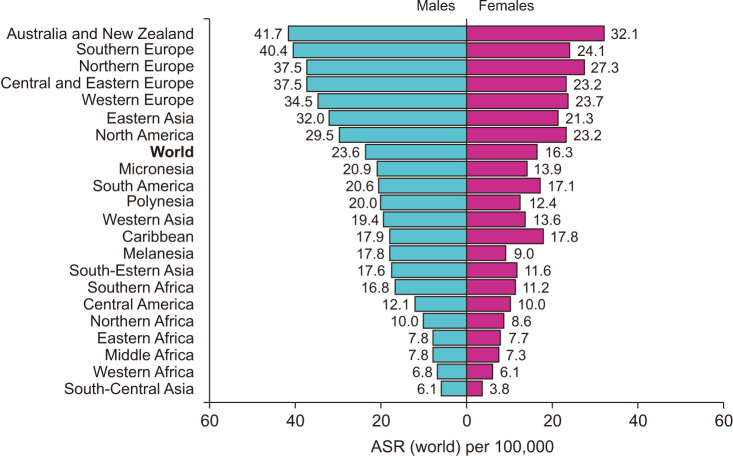
According to the Global Cancer Observatory, the age-standardized incidence rates (ASIR) per 100,000 in males is 45% higher (23.6 per 100,000 person-year) compared to females (16.3 per 100,000 person-year) (Fig. 1) [11]. Also, males have a 50% higher cumulative risk (CR) to develop CRC than females (CR 2.75 vs. 1.83) [3]. In addition, cancer statistics in Korea showed that ASIR per 100,000 for incidence in 2017 was 30.8 per 100,000 persons, 39.9 in males and 23.0 in females [4]. According to age-frames, sex disparity in younger patients is not clear since the incidence of CRC under 50 years old females are very low. That is, the tendency of CRC to occur more in males than in females becomes apparent from 45–50 years of age in USA, UK and China, and females have lower incidence rates than age-matched males in every age-group above 50 years old (Fig. 2) [12,13]. This results in 4–8 years delay in females compared to males, so for example females aged 65 have similar CRC incidence to males aged 60 [14]. This difference in CRC incidence between sexes has been observed steadily in USA [15] and Korea [16].
Fig. 1. Age-standardized (ASR) incidence rates of colorectal cancer (CRC) depending on sex. The incidence rate for CRC vary markedly across the world. Data from International Agency for Research on Cancer (IARC) (Global Cancer Observatory. Colorectal cancer [Internet]. International Agency for Research on Cancer; c2020 [cited 2023 Mar 5]) [11].
Fig. 2. Incidence and mortality of colorectal cancer (CRC) by age and sex worldwide. Incidence rates and mortality rates of CRC increase with age. The rates are higher in males than in females, with the difference becoming evident after age 50 years. Rates are per 100,000 persons per year. F: female, M: male. Data from the article of Keum and Giovannucci (Nat Rev Gastroenterol Hepatol 2019;16:713-32) [13] with original copyright holder’s permission.
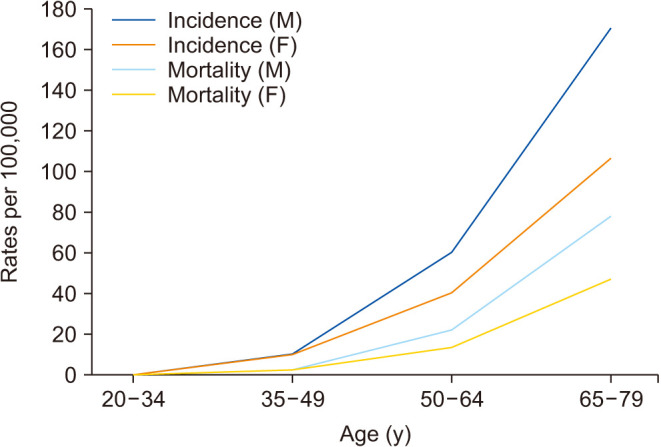
For the reason that sex differences are observed in the incidence of CRC, factors such as obesity and smoking have been proposed [17,18]. Population-based data collected in the 1970s reported that the ASIR for CRC were similar between sexes [19]. However, within the past few decades, the incidence of CRC in males has increased and exceeded especially not only in high incidence populations such as New Zealand, the USA, Canada, Australia, and the UK [1] but also in the low-risk populations such as Hong Kong, Japan, and Singapore [1]. The importance of the environment and lifestyle has been observed in migrant studies, confirming that the incidence of CRC in males rises more rapidly than that in females when they immigrate to high incidence areas from low incidence areas [19].
In addition, a protective role for estrogen in the prevention of CRC has been proposed. For example, the incidence of CRC in the postmenopausal females steadily increases which is very different from that of males [4]. This can be interpreted as sex hormones have a direct impact on the incidence of CRC or may be because hormone abundance affects the contribution of other established risk factors [1]. In animal studies, the CRC incidence was definitely higher in male mice than females in azoxymethane/dextran sodium sulfate (AOM/DSS)-induced colon cancer mouse model, and the treatment of 17β-estradiol (E2) during the DSS inflammation period prevented the development of CRC [18]. In addition, endogenous and exogenous testosterone presented a stimulating effect on AOM/DSS-induced colitis and carcinogenicity [20]. Taken together, sex hormones may contribute to CRC risk, to be discussed in detail in the following sections.
2. Prognosis
Mortality rates from CRC are higher in males compared to females and this is consistently seen across different regions of the world [3]. According to the Global Cancer Observatory, the age-standardized mortality rate for males is 50% higher (10.8 per 100,000 person-year) than for females (7.2 per 100,000 person-year) [11]. In specific, a German population-based cohort study including 185,967 patients showed that females had significantly better overall (hazard ratio [HR], 0.853) and recurrence-free survival (HR, 0.857) than males [21]. Similar findings were observed in the USA [15] and Korea [16]. A meta-analysis from 2017 including 37 clinical trials also showed that females had better overall (HR, 0.87) and cancer-specific survival (HR, 0.92) than males [22]. In the same way, the EUROCARE-4 study that analyzed data of patients diagnosed between 1995 and 1999 from 23 European countries showed females had a 2.2% advantage in 5-year average and region-adjusted survival for CRC [23]. However, the most recent EUROCARE-5 study, which evaluated patients diagnosed between 2000 and 2007 from 29 European countries did not show significant female advantage in CRC survival [24]. In addition, a cross-sectional study from the UK including 164,980 CRC patients showed no significant age-standardized survival benefit for females compared to males [25]. Generally, the benefit in CRC survival, which has been attributable to sexual dimorphism, has been associated with the premenopausal stage in females [3]. That is, premenopausal patients have better 5-year survival rates than age-matched male patients, and younger females (18–44 years) show lower mortality compared to older females (over 50 years) [26,27]. In contrast, female CRC patients over 65 years old showed worse survival rates than age-matched male patients [3].
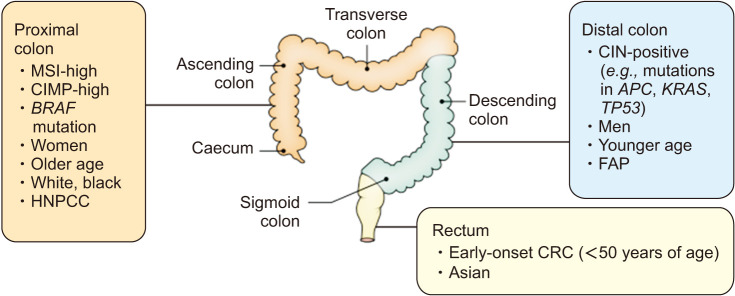
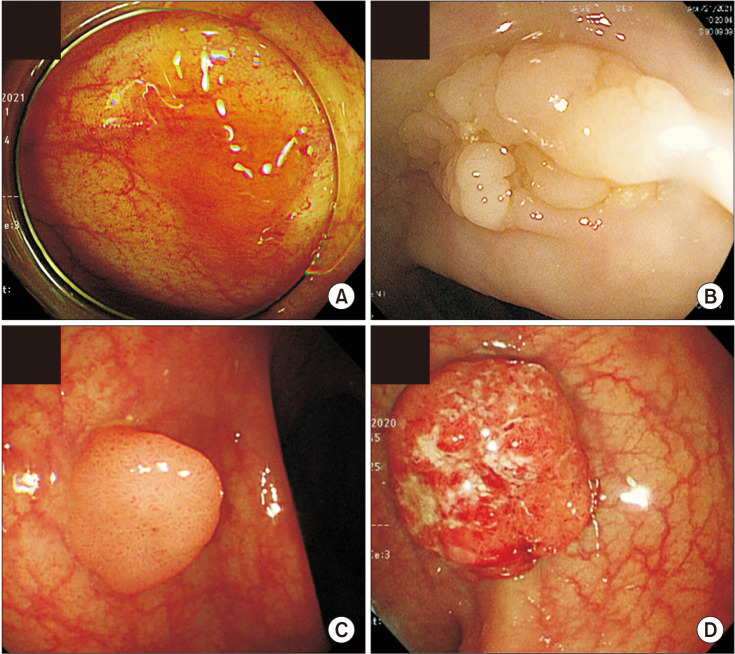
This could be because that female patients over 65 years old tend to be diagnosed in a more advanced stage and have a more aggressive cancer type than male patients [5,28,29,30,31]. Right-sided (proximal) tumors occur predominantly in females and older patients [5], associated with vague symptoms and is less differentiated compared to left-sided colon cancers (Fig. 3) [13,22]. In a Japanese study analyzed 62,350 colon cancer patients, five-year net survivals for subjects with left- and right-sided colon cancer were 74.0% (95% confidence interval [CI], 73.4–74.7) and 70.4% (95% CI, 69.7–71.0), respectively [32]. Compared to left-sided colon cancers, the excess hazard ratio (EHR) for right-sided colon cancers was 1.20 (95% CI, 1.16–1.25) after adjustment for age, sex, and stage [32]. Furthermore, a meta-analysis reported that patients with right colon cancers had an 18% increase in mortality risk, independent of stage [33]. Metastatic CRC patients with right-sided tumors also had worse survival rates compared to left-sided tumors [34]. It’s probably due to that right-sided tumors are more difficult to be diagnosed by colonoscopy due to their flat shape compared to left-sided tumors in the form of polyp (Fig. 4) [5,35]. That is, the lack of gender-specific screening tools could be an explanation for higher mortality and shorter 5-year survival rate of females around the world. Besides, right- and left-sided colon cancers show different molecular features such as immune infiltration, differentiation or MSI. For example, high-MSI tumors, comprising close to 15% of all CRC cases, are known to locate predominantly in the right-sided colon [5,36], to be discussed separately.
Fig. 3. Anatomical subtypes of colorectal cancer (CRC) and their associations with tumor molecular features and other factors. MSI: microsatellite instability, CIMP: CpG island methylator phenotype, HNPCC: hereditary non-polyposis colorectal cancer, CIN: chromosomal instability, FAP: familial adenomatous polyposis. Adapted from the article of Keum and Giovannucci (Nat Rev Gastroenterol Hepatol 2019;16:713-32) [13] with original copyright holder’s permission.
Fig. 4. Morphological differences between right-sided tumors (A, B) and left-sided tumors (C, D). Right-sided tumors are flat-shaped compared to polypoid left-sided tumors, therefore difficult to be detected early.
In overall, females have a lower risk to develop CRC and generally show better prognosis than male patients. However, right-sided colon cancers are more common in females that tend to be diagnosed in advanced stage and have worse prognosis than males, especially in older females. That is, the prognosis of CRC according to sex appears to be affected by a combination of multiple factors and is difficult to interpret. There are several explanations for this paradox, including the protective effect of female hormone estrogen including hormone replacement treatment [21] that female sex hormones might exert a protective immunologic effect on the inflammatory response [37], and heterogenous nature of right-sided tumors to have not only aggressive feature but also MSI, which is known to be associated with improved outcomes [37]. However, the results are not consistent across studies and the exact mechanism still remains unclear and needs further investigation on the survival advantage in feamels and role of sex hormone in CRC.
SEX DIFFERENCES IN PATHOGENESIS
As mentioned above, CRC seems to develop by the combined effect of sexual dimorphism and gender differences [3]. In biological aspects, 17β-E2 plays a key role in the prevention of CRC in females, while social and behavioral factors such as diet is very important in the increase of CRC in males. The amount each factor contributes still has not fully discovered yet.
1. Lifestyles and risk factors
Diet, exercise and other lifestyle factors have been associated with CRC risk [38]. A recent study observed that lifestyle factors such as diet and smoking are linked with specific molecular CRC subtypes [39]. In addition, the impact of lifestyle factors on CRC risk seems to have sex difference, that a high inflammatory profile was related to higher risk for CRC in males, while not in females [40].
1) Diet
Females have generally healthier dietary habits than males, with higher fiber and lower meat consumption, and less alcohol intake [3]. Interestingly, a number of studies have reported that dietary factors are associated differently with CRC depending on sex [40] and the location of tumors [5]. The European prospective investigation into cancer and nutrition study has reported that a high inflammatory profile (proinflammatory diet+sedentarism+obesity) showed a strong association with higher risk for CRC in males (HR, 2.11; 95% CI, 1.50–2.97) while not in females [40]. Interestingly, high carbohydrate intake increased right-sided colon cancer in females, but increased rectal cancer in males [41], while high fat and protein intakes increased risks of right- and left-sided colon cancers, respectively [42,43]. Recent evidence from a large Canadian population-based case-control study suggested that high intake of polyunsaturated fat, trans-fat, cholesterol, sucrose, and lactose was associated with the increased risk of right-sided colon cancer [44]. Meat consumption increased the risk of left-sided colon cancer [45,46,47], whereas total iron and iron from supplements [47], high calcium intake [48,49,50] and higher serum/plasma 25-hydroxyvitamin D level [51] were inversely associated with distal colon cancer. Consumption of soy products containing phytoestrogens also has been reported to be inversely associated with the risk of CRC [52,53,54,55], that a recent meta-analysis found that soy consumption was associated with an approximately 21% reduction in CRC risk in females. This is presumably due to the structural and metabolic similarities of soy isoflavones to estrogen [52], supported by the evidence found in an experimental study that higher intake of phytoestrogens increases estrogen receptor α (ERα) expression, decreases apoptosis, and induces inflammation markers in colonic mucosa of female mice [56]. Additional large population-based studies would be needed for estimating the sex-specific dietary risk and providing guidelines for cancer-preventive diet.
2) Physical activity levels, obesity, metabolic syndrome
A number of prospective and retrospective studies support an inverse association between physical activity and risk of colon, but not rectal cancer, in spite of the wide variation in physical assessment methodology among studies [57]. In a prospective study of nurses, females who were in the upper quintile of activity showed almost half the risk of developing colon cancer compared to non-active females (relative risk [RR], 0.54; 95% CI, 0.33–0.90) [58]. Similar finding was observed in the Health Professionals Follow-up Study, a large prospective study in males [59]. Several biological mechanisms have been proposed for this inverse association between physical activity and colon cancer. First, a potential mechanism acting through prostaglandin E2 (PGE2) synthesis was suggested, that high physical activity seemed to have an inverse correlation with PGE2 concentration in the rectal mucosa [60]. Second, hyperinsulinemia related to physical inactivity, high body mass, and central deposition of adipose tissue may also be an important mechanism of CRC occurrence [57]. That is, association between CRC and diabetes mellitus [61] and insulin [62] had been found in recent studies, and insulin is known to be a mitogen for normal and neoplastic colonic epithelial cells [59]. Estrogen might play a role in the metabolic CRC development process, that hyperinsulinemia was associated with CRC progression only in postmenopausal females in previous study [63]. In addition, there was a report that the association between body mass index (BMI) and colon cancer in females was found in only premenopausal females but not in postmenopausal females [64]. This would be because that higher BMI and lower physical activity levels have been positively associated with higher levels of circulating estrogens in postmenopausal females [65], since adipose tissue is an important source of estrogens. To sum up, the effect of obesity on colorectal neoplasia in premenopausal females acts via the insulin/insulin growth factor pathway, while the opposite effect of insulin and estrogen in obesity offset in postmenopausal females [57].
Meanwhile, sex difference is observed in terms of the risk of obesity and metabolic syndrome (MetS) on the incidence of CRC. In a follow-up study of 408,931 Korean adults, being underweight (<18.5 kg/m2) reduced the risk for CRC among females (adjusted HR, 0.646; 95% CI, 0.484–0.863) whereas higher BMI significantly increased the risk in males and in the elderly [66]. In addition, obesity (≥25 kg/m2), diabetes mellitus, and hypertension were identified as risk factors for CRC in males but not in females [66]. MetS and its components has been thought to be involved in the development of CRC, but the effect seemed to be different depending on gender and location of CRC. In a study analyzed the data of 22,809,722 Korean individuals who underwent regular health check-ups between 2009 and 2012, the HR for CRC development in patients with MetS was 1.22 (95% CI, 1.20–1.24) and this association was more prominent in males than in females (HR, 1.41; 95% CI, 1.37–1.44 vs. HR, 1.23; 95% CI, 1.20–1.27, p for interaction <0.001) [67]. Additionally, left-sided colon cancers were more associated with MetS among males compared to females (HR, 1.70; 95% CI, 1.61–1.80 vs. HR, 1.43; 95% CI, 1.33–1.54) while right colon cancers showed a stronger association with MetS among females than males (HR, 1.63; 95% CI, 1.49–1.78 vs. HR, 1.34; 95% CI, 1.24–1.44) (all p for interaction <0.001, respectively) [67].
3) Alcohol
Alcohol consumption is one of the most important known risk factors for cancers in human [68], and there are clear evidences that alcohol consumption increases cancer risks in many organs including colon and rectum [69]. Ethanol itself can cause local irritation of the upper gastrointestinal (GI) tract [70] and could stimulate carcinogenesis by inhibiting DNA methylation [71], and furthermore acetaldehyde, a metabolic product of ethanol, is classified as a human carcinogen by the IARC [72]. For instance, the risk of CRC by alcohol was 1.42 times higher in a Japanese cohort study [73], and light drinking (less than 10 g per day) as well as moderate to heavy alcohol consumption significantly increased the risks of the CRC (HR, 1.12; 95% CI, 1.11–1.14) compared to non-drinkers after adjusting for age, sex, smoking, exercise, income, BMI, and diabetes in a population-based prospective cohort of 23,323,730 adults who had undergone a biennial health check-ups between the years 2009 and 2012 in Korea [71].
Sociological and cultural aspects of alcohol drinking vary, and the toxic threshold of ethanol may differ by individual and by sex [71]. It might be related to the variant allele of ALDH2, which breaks down acetaldehyde to acetate in the metabolism of alcohol, that genetic variation is much higher in Asians (28%–45%) than in other ethnic groups [74]. Interestingly, the increase in CRC risk with the amount of alcohol consumption was not significant in females in previous study [71], similar with previous community-based study observed 6,291 residents (aged 55 and older) of Ganghwa-do for 20.8 years from 1985 to 2005 in Korea, which reported the risk of CRC as 1.87 times higher in heavy drinkers than in non-drinkers [75]. In this study, the mortality associated with soju (Korean traditional alcohol with higher amount of ethanol) was significantly higher than that associated with makgeolli (Korean traditional alcohol with lower amount of ethanol) in males, but this difference was not significant in females [75]. This could be due to the amount of consumption was larger and the duration of consumption was longer in males than in females.
4) Smoking
A higher risk of adenomatous polyps has been consistently observed among smokers with RRs ranging from 1.4 to 3.6 [57,76] and an induction period of 30–40 years between smoking and risk of CRC has been proposed based on results from two large cohort studies [77,78]. Most of studies have reported positive associations between cigarette smoking and CRC, and overall evidence supports the hypothesis that tobacco smoke is an initiator of colorectal carcinogenesis [57]. There are several studies did not support an association though [79,80,81,82], mainly conducted in Sweden, suggestive of the influence of other factors such as heredity [79,81,82]. In the latest and largest published study to date, based on data from the Cancer Prevention Study II, 312,332 males and 469,019 females were followed prospectively and the RR for CRC mortality in current smokers was 1.32 (95% CI, 1.16–1.49) for females and 1.41 (95% CI, 1.26–1.58) for males [83,84], and increased risk was evident after 20 or more years of smoking for males and females combined as compared with never smokers [84]. A follow-up review continued to support the adverse effect of tobacco on risk of CRC [85]. However, there is no data regarding the sex/gender difference of smoking effect on the CRC, so further studies are needed.
2. Sex hormones
Sex hormones derived from cholesterol and are classified into estrogens (17β-estradiol, estriol, and estrone), androgens (testosterone), and progestogens (progesterone) [3]. They are produced in the testis (males) and ovaries (females), but also in the adipose tissue, adrenal glands, brain, skin, and bone [3]. Among these sex hormones, the mechanisms of estrogen with its receptors and testosterone on the development of CRC are being actively discovered. Above all, adipose tissue can be an important source of estrogens in postmenopausal females and obese males [3], that higher BMI and lower physical activity levels have been positively associated with higher levels of circulating estrogens in postmenopausal females and males [85,86].
1) Estrogen
In general, estrogen acts as a cancer-protective hormone through its anti-inflammatory activities, but the roles of individual estrogen receptors are often controversial in carcinogenesis of CRC [87]. Estrogen prevents the development of CRC via suppressing inflammation that provides the tumor microenvironment. For example, in previous study investigated the effect of E2 treatment in CCD841CoN, a female human colonic epithelial cell line, ERα expression has no significant change after E2 treatment but E2 treatment consistently increased ERβ expression [88]. In addition, estrogen was found to inhibit inflammation through down-regulation of nuclear factor-κB (NF-κB) and cyclooxygenase-2 (COX-2) expression and induction of anti-oxidant enzymes such as heme oxygenase-1 and NAD(P)H-quinone oxidoreductase-1, which is downstream of nuclear factor erythroid 2-related factor 2 (Nrf2) [88]. E2 was found to increase Nrf2 activity in breast cancer cell line [89] and colonic epithelial cells (CCD841CoN) [88], and Nrf2 downregulates pro-inflammatory signaling by suppressing NF-κB [90], regulates the expression of anti-oxidant enzymes [91], and activates NLRP3 inflammasome [92] that leads to immune modulation through caspase-1 related activities such as pyroptosis [93]. In another previous study in AOM/DSS-induced colon cancer mouse model, E2 inhibited the initiation of CRC by regulating Nrf2-related pathways, and a dual role of Nrf2 in modulating inflammation and carcinogenesis was observed [94]. To sum up, it might induce pyroptosis in the DSS-induced inflammation stage, but promote cancer progression once tumor formation is initiated via immune modulation of NLRP3 inflammasome-induced interleukin (IL)-1β and IL-18 in the tumor microenvironment [95]. E2 prevents carcinogenesis, whereas in the absence of E2, a cancer inducing microenvironment is created through NF-κB activation [94].
In addition to protection of endogenous estrogen in females against the development of proximal colon cancer, exogenous E2 replacement in ovariectomy (OVX) female mice showed protective effects against AOM/DSS-induced colitis and carcinogenesis in a female AOM/DSS mouse model [96]. OVX significantly increased tumor number and incidence rate in only the proximal colon after AOM/DSS treatment and these increases were significantly reduced by E2 supplementation, while OVX did not affect CRC development in the distal colon. In this study, the increase in Nrf2 and antioxidant enzyme gene expression by E2 supplementation in the OVX_AOM/DSS group blocked the expression of NF-κB-regulated proinflammatory cytokines (i.e., TNF-α and IL-6) and COX-2 [96].
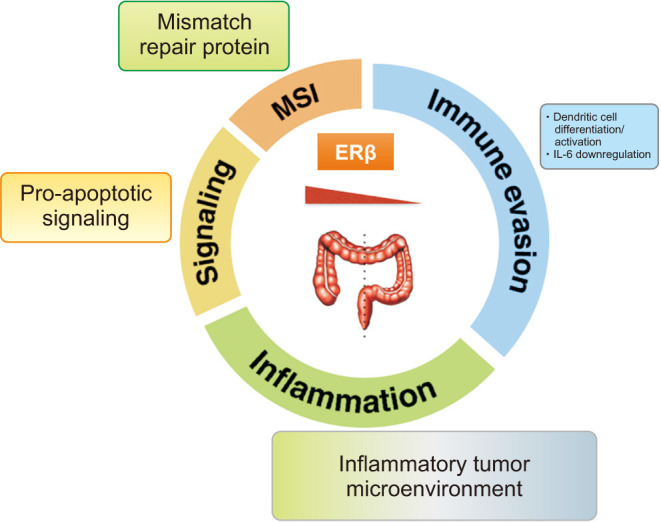
The protective role of estrogen in the female against CRC has been mainly associated with expression levels of the ERβ in intestinal epithelial cells [97,98]. That is, ERβ is the prevalent estrogen receptor in normal colon mucosa and shows a significantly reduced expression in CRC [99]. ERβ showed tumor-suppressive function in CRC through activation of pro-apoptotic signaling, regulation of mismatch repair proteins, and modulation of the inflammatory tumor microenvironment (Fig. 5) [94,97]. Thus, selective loss of ERβ within the large intestine promotes tumorigenesis [97,99], that ERβ1, ERβ2, and ERβ5 isoforms are expressed in the colon with variable levels along the crypt axis [100] and are reduced in tumor cells compared to normal colon [101,102]. ERβ inhibits proliferation by blocking of the cell cycle in G1-S phase [103], stimulating apoptosis [104] and induces anti-inflammatory signaling in CRC cells [105]. Other protective mechanisms include upregulation of tight junction proteins occludin (OCLN) and the F11 receptor/junctional adhesion molecule-A to preserve homeostasis of paracellular permeability [106], that the mRNA expressions of tight junction proteins Zonula occludens-1, OCLN, and Claudin4, were decreased by AOM/DSS-treatment while were recovered by supplementation of 17β-E2 [107]. Estrogen has been shown to increase ERβ levels, and this could be a mechanism by which estrogen confers protection against CRC [108].
Fig. 5. Estrogens regulate the cellular effects through their intracellular receptors, estrogen receptors (ER)α and ERβ. ERβ showed tumor-suppressive function in colorectal cancer through activation of pro-apoptotic signaling, regulation of mismatch repair proteins, and modulation of the inflammatory tumor microenvironment. MSI: microsatellite instability, IL: interleukin. Adapted from the article of Caiazza et al (Front Oncol 2015;5:19) [97].
In addition to the protective activity of estrogen in CRC via ER, the role of membrane-bound G protein coupled estrogen receptor (GPER) is suggested [109,110]. Diverse functions of GPER in the colon include the regulation of visceral hypersensitivity and gut motility, immune responses in inflammatory bowel diseases (IBD) [111,112], and the modulation of cell migration and proliferation in CRC cell lines [113]. GPER is known to stimulate gut motility [114], and a sex-dependent regulation of GPER expression and signal transduction for mucosal inflammation has been proposed in IBD such as Crohn’s disease and ulcerative colitis [115] which can provoke CRC, especially in male. Thus, estrogen and GPER modulate physiological intestinal functions [3], and that estrogen effects in advanced CRC tumors may be transduced via GPER given the relative absence of expression of ERα or ERβ in CRC patients and cell lines studied [3]. GPER has been described as a tumor promoter in certain cancers such as breast cancer via activation of epidermal growth factor receptor, STAT5 and MAPK/extracellular regulated kinase (ERK) pathways [116]. However, in CRC, the expression of GPER is reported to act variously as a tumor suppressor or promoter depending on the stage of the disease and expression levels of ER and GPER [117], and the role of GPER in CRC looks like to be both sexually dimorphic and dependent on the stage of the disease [3]. For example, the expression of GPER was significantly decreased with increasing stage and lymph node metastasis of CRC patients [118]. That is, as colon cancer progresses to advanced stage disease, GPER expression was found to be greatly reduced in cancerous tissue compared to adjacent healthy colon and low GPER expression was associated with reduced survival [119]. In addition, high GPER expression was associated with poor relapse free survival in females with stage 3/4 but not in stage 1/2 CRC while there was no correlation of GPER expression in males with disease of any stage [21].
2) Progesterone
Progesterone has also been considered as a potential contributor to CRC protection. Recent evidence implicated that the effect of progesterone reducing CRC would be additive to estrogen signaling via the estrogen receptor ERβ [120], so the protective role of progesterone looks like to be specific to postmenopausal incidence of CRC [121]. However, the effect of progesterone on the CRC is seems to be minor so far.
3) Testosterone
Androgen receptors (ARs) have been reported to be expressed in CRC [122]. ARs are the binding site for dihydrotestosterone (DHT) and there are two isoforms of the AR, AR-A and AR-B [123]. Both of these were detected in healthy colonic mucosa whereas only AR-A was detected in neoplastic colonic mucosa [124], that suggests that neoplastic colonic mucosa is characterized by loss of expression of the AR-B isoform [123]. However, the role of testosterone in CRC is somewhat unclear so far. Firstly, there was no significant clinical correlation between testosterone concentrations and outcomes in CRC patients [125,126]. A recent study in Japan including 185 CRC patients and 361 controls who were postmenopausal showed that higher testosterone levels were significantly associated with CRC risk (odds ratio [OR], 2.1; 95% CI, 1.11–3.99) [127]. However, some other studies reported that lower androgenicity in males, as a result of reduced AR activity by hypermethylation or lower circulating dehydroepiandrosterone sulfate, has been associated increased CRC risk [128,129]. A recent USA study including 732 CRC patients and 1,156 controls from 4 prospective cohorts also reported that high testosterone levels in males were significantly associated with lower RR for CRC (highest vs. lowest quartile 0.65) while no association in postmenopausal females was observed [130]. In addition, a prospective study analyzed 107,859 males diagnosed with prostate cancer from the American SEER-Medicare database showed that orchiectomy and long-term androgen-deprivation 3 (more than 25 months) were significantly associated with higher CRC risk (HR, 1.37; 95% CI, 1.14–1.66 and HR, 1.31; 95% CI, 1.12–1.53; respectively) [131]. There may be confounding factors leading to the different CRC risk associations in different populations as these are epidemiological observational studies, so there are limitations to the interpretation of the results [3].
Expression of the AR has also been studied. For example, in a case-control study including 550 CRC patients and 540 controls, longer cytosine-adenine-guanine (CAG) repeats in the AR gene that reduce the transcription rates, have been associated with higher CRC risk and lower 5-year median overall survival (HR, 1.4; 95% CI, 1.04–1.79) in CRC patients [132]. In contrast, no clear association between CAG repeats in AR and CRC prognosis was observed in a German population-based study of 1,798 CRC patients and 1,810 controls [133]. Some smaller prospective population-based studies failed to detect any association of testosterone with CRC risk, adding complexity [126,134]. In summary, the role of testosterone in CRC is not yet clear, but there seems to be some age-dependent differential effects of the hormone, tumor stage and tissue environment factors [3].
3. Molecular characteristics and immunity
CRC shows stage-independent variability in patient outcomes supposedly due to molecular heterogeneity. CIN, which is associated with 60%–70% of CRC, is more often observed in left-sided colon cancer, and defective genes include adenomatous polyposis coli (APC), Kirstenras, deleted in CRC, and p53 [135,136,137]. On the other hand, MSI-high, CpG island methylator phenotype (CIMP)-high, and BRAF mutation are often observed in right-sided colon cancer (Table 1) [31,136,137]. Hereditary non-polyposis CRC is more likely to develop tumors on the right side of the colon, whereas familial adenomatous polyposis is associated with left-sided colon cancer [138,139]. This MSI status has been shown to impact sensitivity to CRC treatments and prognosis [3]. For example, immunotherapy has been shown to improve prognosis, particularly of MSI-high tumor carrying patients, whereas anti-EGFR and 5-FU adjuvant therapies exert little and no benefit to high frequency MSI (MSI-H) tumors in contrast to microsatellite stable tumors [140].
Table 1. Molecular features of preneoplastic lesions and CRC by site.
| CIMP-high | MSI-high | MLH1 methylation | BRAF mutation | CIN | ||
|---|---|---|---|---|---|---|
| Preneoplastic lesions | ||||||
| Sessile serrated adenoma (right-sided) | + | +/- | +/- | + | - | |
| Conventional adenoma (right and left-sided) | - | - | - | - | + | |
| CRCs | ||||||
| Right-sided CRC | High prevalence | High prevalence | High prevalence | High prevalence | Low prevalence | |
| Left-sided CRC | Low prevalence | Low prevalence | Low prevalence | Low prevalence | High prevalence | |
CRC: colorectal cancer, CIMP: CpG island methylator phenotype, MSI: microsatellite instability, CIN: chromosomal instability.
Adapted from the article of Lee et al. (J Natl Compr Canc Netw 2017;15:411-9) [31] with original copyright holder’s permission.
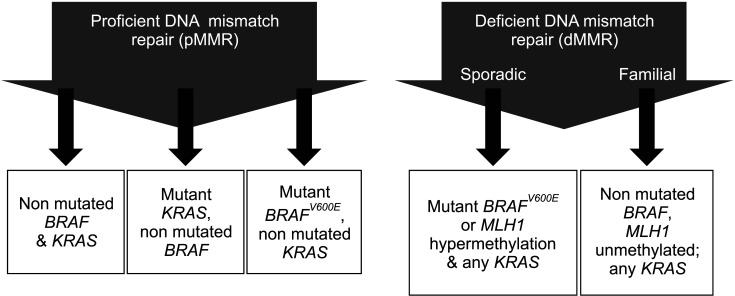
Commonly observed alterations are broadly classified into: (1) hypermutated tumors (up to 16%) of which three-quarters show MSI-H and one-quarter have somatic MMR gene and polymerase ε (POLE) mutations [141], (2) non-hypermutated tumors (up to 84%) with multiple somatic copy number alterations and aneuploidy that contain activating mutations in KRAS and phosphoinositide 3-kinase catalytic subunit alpha (PIK3CA) and loss of heterozygosity of APC and TP53 tumor suppressor genes, and (3) CIMP (up to 20%) that is commonly observed in MSI-H tumors and in some non-hypermutated CRCs [142]. CIMP is characterized by a high frequency of genome-wide DNA methylation of CpG islands [143,144], promotes tumorigenesis by methylation-mediated transcriptional repression. CIMP-positive tumors are typically proximal, higher grade, have deficient DNA mismatch repair (dMMR) resulting in MSI-H [143,145,146], and known to be more frequent in females and older individuals [142]. CRCs with dMMR/MSI-H include both familial and sporadic types, that about two-thirds dMMR CRCs are sporadic [143,144] and the other third are due to germline mutations in MMR genes (MLH1, MSH2, MSH6, PMS2) that cause Lynch syndrome, and BRAFV600E mutations are strongly enriched in sporadic CRCs with dMMR and CIMP (Fig. 6) [141,147,148,149].
Fig. 6. Classification and survival of colorectal cancer according to the proficiency of DNA mismatch repair. Adapted from the article of Sinicrope et al (Gastroenterology 2015;148:88-99) [148].
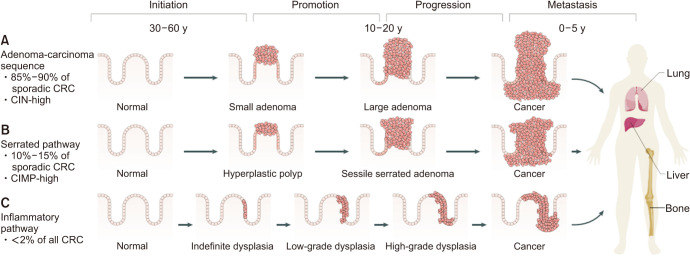
1) Adenoma-carcinoma sequence and serrated pathways
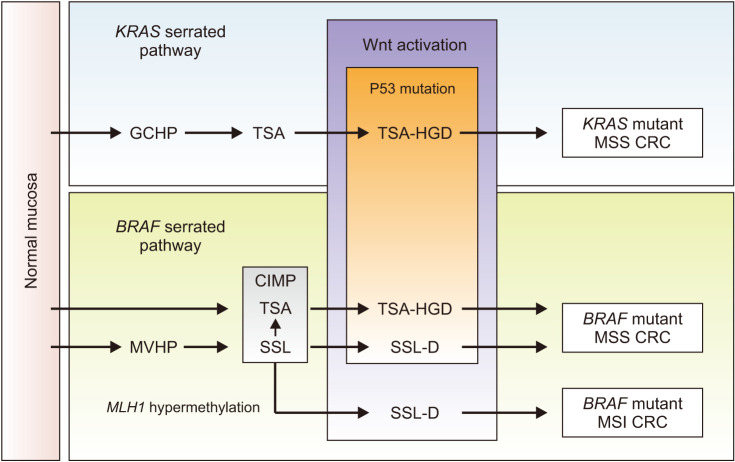
Conventional adenoma-to-carcinoma sequence is based on two mechanisms of tumorigenesis: CIN or MSI (Fig. 7) [9,13]. The CIN pathway begins with mutations in the tumor suppressor gene APC and progresses into adenocarcinoma upon acquisition of additional mutations in the genes KRAS, SMAD4, and TP53, with dysregulation of the Wnt/β-catenin, MAPK, PI3K and TGF-β signaling pathways [9]. Alternatively, the MSI pathway begins with initial alteration of the Wnt signaling followed by BRAF mutation and alterations of the genes TGFBR2, IGF2R, and BAX [9]. In addition to the adenoma to carcinoma sequence, colorectal carcinogenesis can occur via the serrated pathway, that approximately 25% of sporadic CRCs are known to arise via serrated precursor lesions [10]. Serrated polyps include hyperplastic polyps, sessile serrated lesions, and traditional serrated adenomas. The serrated pathway is characterized by mutations in RAS and RAF, disruptions to the Wnt signaling pathway, and widespread methylation of CpG islands (Fig. 8) [10]. First, mutation in a gene that regulates mitogen-activated protein kinase pathway (such as in KRAS or in most cases BRAF) occurs, and mutations in BRAF result in methylation of CpG islands, called the CIMP [144,150]. CIMP results in silencing of many genes, including some tumor suppressor genes. Progression of serrated polyps is also associated with activation of the Wnt signaling pathway as in the case of conventional adenomas that is usually an early step in carcinogenesis [10].
Fig. 7. Pathways of colorectal carcinogenesis. (A) adenoma-carcinoma sequence, (B) serrated pathway, and (C) inflammatory pathway. CRC: colorectal cancer, CIN: chromosomal instability, CIMP: CpG island methylator phenotype. Adapted from the article of Keum and Giovannucci (Nat Rev Gastroenterol Hepatol 2019;16:713-32) [13] with original copyright holder’s permission.
Fig. 8. KRAS and BRAF serrated pathways. GCHP: goblet cell-rich hyperplastic polyp, TSA: traditional serrated adenoma, HGD: high grade dysplasia, MSS: microsatellite stable, CRC: colorectal cancer, MVHP: microvesicular hyperplastic polyp, CIMP: CpG island methylator phenotype, SSL-D: sessile serrated lesion with dysplasia, MSI: microsatellite instability. Adapted from the article of Crockett and Nagtegaal (Gastroenterology 2019;157:949-66.e4) [10] with original copyright holder’s permission.
Proximal colon cancer is more prevalent in females than in males (34% vs. 25%, respectively, in the European Prospective Investigation into Cancer cohort) [151], and the difference between sexes in the serrated pathway may be the underlying cause. For example, in recent study in Japan reported that serrated adenoma/polyp (SSA/P) lesions were mostly located in the proximal colon, and SSA/P with dysplasia or invasive carcinoma was more associated with female sex compared with SSA/P without dysplasia [152]. Furthermore, a recent study in Korea also exhibited the molecular differences such as programmed cell death-ligand 1 (PD-L1), MMR/MSI status and EGFR expression in CRC according to sex and tumor location that females with proximal CRC showed a markedly higher incidence of dMMR/MSI-high status, suggesting a possible underlying mechanism of sex-specific colorectal carcinogenesis [153]. From these results, the association with SSA/P with dysplasia or invasive carcinoma in proximal colon and female sex is suggested, and additional research focusing on sex differences in molecular pathways is needed.
2) KRAS and BRAF oncogenes
KRAS and NRAS mutation status predict the efficacy and clinical benefit of anti-EGFR antibodies [154,155,156]. More than one-third of CRCs carry mutations in exon 2 of KRAS, and an additional 15% carry mutations in exons 3 and 4 of KRAS and in exons 2, 3, and 4 of NRAS, that predict resistance to anti-EGFR antibody therapy [154,155,157,158]. In addition, mutations in the PIK3CA (exon 20 vs. exon 9), which is part of the EGFR signaling pathway, may confer resistance to anti-EGFR therapies [158]. KRAS exon 2 mutations are reported to be associated with poor clinical outcome, as shown in recent data from adjuvant studies in node-positive CRCs [159,160] and the adverse impact of KRAS mutations on prognosis seems to be stronger in the distal colon cancers compared to the proximal cancers [161], although the data are somewhat conflicting [149,162,163].
A point mutation (V600E) in the BRAF oncogene is known to be mutually exclusive with mutation in KRAS [164] and associated with a poor prognosis in metastatic [165] and node-positive CRCs [153,159,162], shows sex difference. First, sessile SSA/Ps, early precursor lesions in the serrated neoplasia pathway and results in BRAF-mutated CRCs, are known to be associated with advanced age, female sex, and proximal colon [152]. Additionally, BRAF-associated CRC was reported to be associated with older age, female sex, proximal tumor location and poor differentiation [166,167]. In addition, patients with tumors containing mutations in BRAF had markedly worsened prognosis than patient without these mutations [168,169]. The adverse prognostic impact of BRAFV600E mutant could be explained by feedback activation of EGFR when BRAF is inhibited, that is related to resistance to vemurafenib, the BRAF/MEK/ERK signaling pathway inhibitor [170].
3) DNA mismatch repair/microsatellite instability
CRCs with dMMR/MSI-H are reported to have a favorable prognosis compared to pMMR/MSS cancers consistently [158,171,172,173], and the difference in survival appears to be stronger in earlier stage cancers compared to later stage diseases [174]. Recent National Comprehensive Cancer Network (NCCN) guidelines recommend MMR/MSI testing for all newly diagnosed CRC cases, since these tests provides prognostic information. Meanwhile, the comprehensive effect of MMR/MSI and CIMP on prognosis is conflicting, that a meta-analysis concluded that CIMP was associated with a poor prognosis in both MSI-H and MSS tumors [175], while poorer survival was observed in those lacking MLH1 methylation compared to those with MLH1 hypermethylation in another study using CIMP-positive tumors [176]. CIMP has been shown to occur in sporadic cancers and has been associated with older age, female sex, proximal location, mucinous histology, and MSI, presence of BRAF mutation and K-ras mutations and wild type p53 in several cohort and population-based studies [177].
Tumors with dMMR/MSI-H are hypermutated and express abundant frameshift peptides, and these peptides are reported to serve as neoantigens eliciting active immune responses [178], and dMMR/MSI-H CRCs show increased expression of immune checkpoint (IC) proteins (PD-1, PD-L1, CTLA-4, LAG-3, IDO) [179]. As an extension of this, metastatic CRCs with MSI-H showed better treatment response to pembrolizumab, an anti-PD-1 antibody compared to pMMR/MSS CRCs (response rate 62% vs. 0%) in a recent phase II study [180]. There is also sex difference in dMMR, that tumors with dMMR are reported to be more common in female sex, younger age, right colon, greater tumor burden, and poorer differentiation [181], and females with proximal CRC showed a significant association with dMMR/MSI-high and high EGFR expression in recent study [153]. There is differential prognosis by stage between patients with right- and left-sided CRC, that right-sided tumors have a slightly better prognosis in stage II colon cancer, but worse prognosis in stage III and IV colon cancers [31]. This would be likely associated with the higher prevalence of good-prognosis MSI-high tumors in right-sided stage II cancers, in spite of aggressive characteristic of right-sided cancer [31], a significant difference in male and female colon cancer.
4) Programmed cell death-ligand 1 and immune pathway
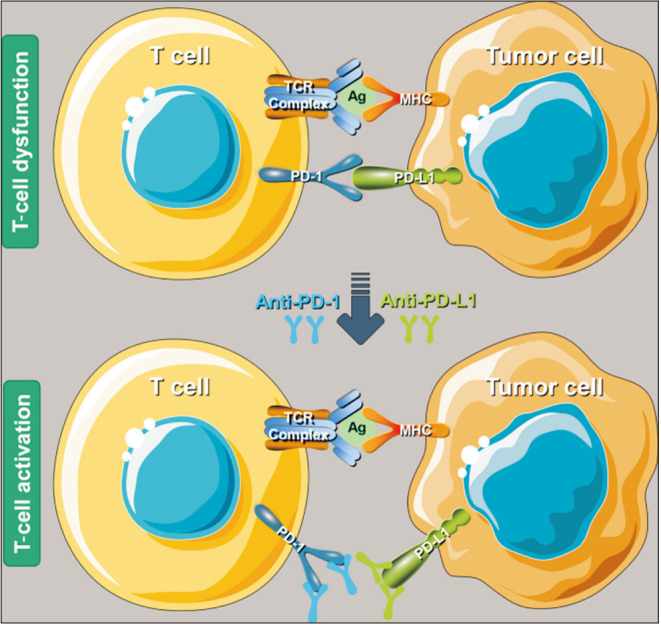
PD-1 and PD-L1 are a pair of ICs that work as the brake on the immune system and play a crucial role in the tumor immune escaping process [182]. PD-1 is the most important receptor for activating T-cell expression and mediating immunosuppression, while the PD-L1 facilitates apoptosis of activated T-cells via causing T-cells dysfunction and anergy [183,184]. The overexpression of PD-L1 weakens the cytolytic activity of T cells and promotes the occurrence and invasion of tumors [185]. In opposite, PD-1/PD-L1 inhibitors could stop T-cell apoptosis and dysfunction, which further enhances the activation of T cells (Fig. 9) [186]. PD-L1 is highly expressed on the surface of many tumor cells, which can also induce immune cells to secrete immunosuppressive factors and further inhibit the killing effect of the antitumor immunity [187]. Anti-PD-1/PD-L1 therapy can bind to PD-1 and PD-L1 correspondingly, further preventing the combination of PD-1 on the surface of T cells and PD-L1 on the surface of tumor cells [188]. Current NCCN guidelines version 2.2021 suggested that anti-PD-1/PD-L1 therapy not only can be applicable to stage IV dMMR/MSI-H CRC patients but also be used as part of neoadjuvant therapy. Currently, nivolumab and pembrolizumab received the accelerated approval of the U.S. Food and Drug Administration as the second-line treatment for patients with dMMR/MSI-H mCRC in 2017 [189], and pembrolizumab or nivolumab, alone or in combination with ipilimumab was recommended as a first-line treatment option for patients with dMMR/MSI-H mCRC in consideration of the above outcomes, whether it is eligible for intensive therapy in NCCN guidelines version 2.2021 [190].
Fig. 9. Schematic mechanism of programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) inhibitors to restore T-cell functions. TCR: T cell receptor, MHC: major histocompatibility complex, Ag: antigen. Adapted from the article of Bie et al (Front Oncol 2022;12:769124) [188].
It is expected that there will be a sex difference in expression of ICs, but there are only few studies so far. In recent study in mice CRC model, co-treatment with E2 and anti-PD-L1 antibodies significantly inhibited mouse colon 38 (MC38) tumor growth and reduced PD-L1-expressing cells in male mice compared to treatment with either E2 or anti-PD-L1 antibodies alone [191]. Furthermore, combination treatment with E2 and anti-PD-L1 decreased tumor-associated macrophages (TAM) population (CD11b+F4/80+) in the tumor mass while increasing M1 TAMs (CD11b+F4/80+CD86+), suggesting that estrogen inhibits MC38 tumor growth by downregulating PD-L1 expression and regulating tumor-associated cell populations and boosts the effect of anti-PD-L1 antibody in the MC38 tumor model [191]. In another human study, PD-L1 expression was higher in serrated polyps than in conventional adenomas, higher in cancer cells than in normal control or adenoma cells and seemed to be inversely correlated with male proximal CRC patients (OR, 0.28; p=0.034) (Fig. 10) [153]. Sex differences in expression of ICs are important because they can lead to differences in responses to ICI treatment. Studies have reported differences in immunogenic missense mutation load, treatment response (durable clinical benefit), and survival between males and females in lung cancer [192] and melanoma [193,194]. In the case of CRC, there are lack of data on sex difference so far, and further studies on sex differences will be needed to predict treatment response as ICI treatment is expected to be activated in the future.
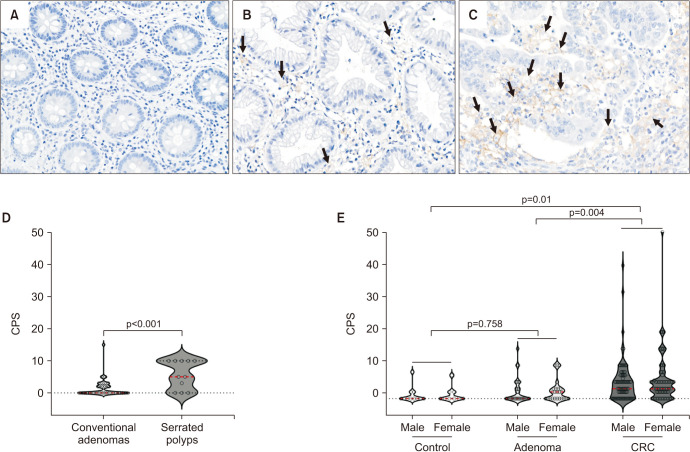
Fig. 10. Expression of programmed cell death ligand 1 (PD-L1) in colorectal tissues depending on histology. Representative immunohistochemical results of PD-L1 expression (arrows): (A) negative normal control, (B) sessile serrated lesion (adenoma), and (C) colorectal cancer (CRC) (magnification, ×20). (D) A statistically significant increase in the average PD-L1 combined positive score (CPS) value was observed in serrated polyps within colorectal adenomas. (E) CRC showed a significantly higher average PD-L1 CPS score than control and colorectal adenomas. The CPS was defined and calculated as follows: total number of PD-L1 positive cells including tumor and mononuclear inflammatory cells, divided by the number of all viable tumor cells in colorectal adenomas and CRCs, and multiplication with 100. Adapted from the article of Choi et al (PLoS One 2023;18:e0282017) [153].
4. Gut microbiome and its metabolite
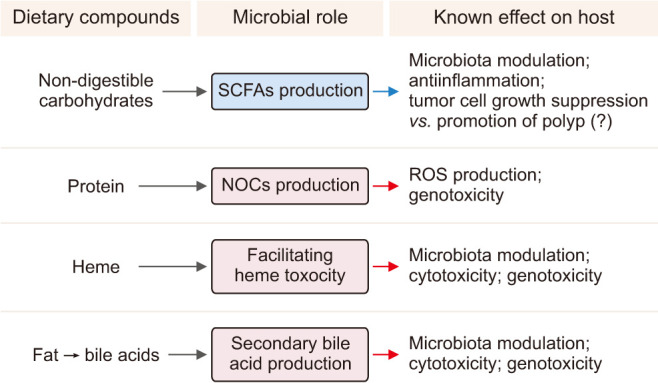
The gut microbiome is located in close proximity to the colorectal epithelium and there is cross interaction. Dysbiosis, a state of pathological imbalance in the gut microbiome contribute to CRC [195]. The development of CRC has been associated with an overall reduction in microbial diversity [196] and specific enrichment of individual bacteria such as Fusobacterium nucleatum [197] and loss of potentially protective bacteria such as Roseburia [198]. In addition, the metabolic factors of microbiota such as bile acid and butyrate affect colon carcinogenesis (Fig. 11) [199].
Fig. 11. Key microbiota metabolites which are related with colon cancer. SCFAs: short-chain fatty acids, NOCs: nitroso compounds, ROS: reactive oxygen species. Adapted from the article of Yoon and Kim (J Cancer Prev 2018;23:117-25) [199].
1) Gut microbiome
As mentioned before, dysbiosis contribute to CRC and there are hypotheses suggested. First, several studies in animal models have reported that estrogen signaling can help to maintain microbiome diversity [3] and OVX has led to microbial dysbiosis although this was affected by strain and diet [200]. Furthermore, dietary supplementation with the hormone E2 has been shown to increase microbial diversity in healthy male mice and to impact the ratio of bacteria in the microbiome of a CRC-induced mouse model [201].
The potential protective effect of estrogen was examined in a recent study using the AOM/DSS mouse model of intestinal specific ERβ deletion in the colitis-induced CRC model, suggesting that ERβ expression influenced gut microbiome diversity and attenuated these diseases [202]. Similarly, another study showed a reduction in gut microbiota diversity with the development of CRC, which was exacerbated in the absence of ERβ [203]. In addition, significant increases in the microbial diversity (Chao1 index) in females, males supplemented with E2, and males treated with AOM/DSS/E2 compared with normal males were observed [201]. In AOM/DSS-treated male mice, E2 supplementation showed significantly lower level of the Firmicutes/Bacteroidetes (F/B) ratio, and the ratio of commensal bacteria to opportunistic pathogens was higher in females and E2-treated males compared to normal males and females subjected to OVX [201]. These findings suggest that estrogen alters the gut microbiota in ICR (CrljOri:CD1) mice, particularly AOM/DSS-treated males, by decreasing the F/B ratio and changing Shannon and Simpson index, and highlights a possibility that estrogen could cause changes in the gut microbiota, thereby reducing the risk of developing CRC [201].
A recent study showed interesting data, that the sex and age-dependent gut microbial differences seen in the healthy controls disappeared as CRC progressed [204]. That is, Lactate-producing bacteria (Bifidobacterium adolescentis, Bifidobacterium catenulatum group and Lactobacillus ruminis) was more abundant in female than in males, and Actinobactetia and butyrate-producing bacteria (Agathobaculum butyriciproducens and Blautia faecis) were more abundant in the younger patients than in older patients in healthy controls. However these sex and age-dependent differences were not observed in the adenoma and CRC groups, suggesting the potential carcinogenic role of gut microbial change in CRC [204]. Summarizing, it is thought that changes in gut microbiota under the influence of sex hormones such as estrogen will contribute to the sex difference in the occurrence of CRC.
2) Gut microbiome metabolites
Metabolic property of gut bacterial population has been investigated in relation to tumorigenesis. The well-known factor is short-chain fatty acids (SCFAs), nitroso compounds (NOCs), heme and secondary bile acids [199]. The SCFAs, namely acetate, butyrate, and propionate are quantitatively and metabolically the most important microbial end-products of the human colon fermentation process [205,206]. Among them, butyrate produced by fermentation of dietary fiber is considered to be the main reason for the health benefit from the indigestible carbohydrate [207]. Most butyrate producers in the human colon belong to the Firmicutes phylum and in particular Clostridium clusters IV and XIVa [205]. Butyrate is the preferred energy source for the colonic mucosa, and it suppresses the growth of tumor cells [208]. Butyrate induces cell differentiation, promotes cell apoptosis and reduces tumor cell invasiveness [209]. The most investigated mechanism is that butyrate inhibits histone deacetylases and thus results in inactivation of many oncogenic signaling pathways [208]. The metabolic rearrangement in cancerous colonocytes is an appropriate means for providing biomaterials as well as energy that are essential for growth (Warburg effect). Human study revealed that high red meat consumption increased the levels of pro-oncogenic microRNA including miR17–92 cluster in rectal biopsies, and increased butyrate supply through consumption of a butyrylated-resistant starch restored the miR17–92 miRNAs to baseline levels [210]. Butyrate insufficiency may contribute to the development of inflammatory conditions because the acid has been shown to induce the differentiation of colonic T regulatory lymphocytes, which suppress inflammatory and allergic responses [211,212]. Actually, butyrate was shown to suppress development of colon carcinogenesis in ApcMin/+ mice due to its Gpr109a agonist property through T regulatory cell differentiation [213]. There was a age difference in this butyrate producing gut microbiota, that major bacterial taxa changed in 31-week-old rats when gut microbiota in cecal contents of 6-, 31-, and 74-week-old and 2-year-old male Fischer-344 rats (corresponding to 5, 30, 60, and 80-year-old humans in terms of age) were analyzed using 16S ribosomal RNA metagenome sequencing [214]. Especially, Lachnospiraceae, a SCFAs-producing family, increased at this age and unknown species EU622775_s and EU622773_s showed strong relationship with cecal butyrate level at 31-weeks of age, suggesting that butyrate production is different during lifespan with a peak in the middle age in rat [214]. However, there is no research regarding the sex difference of microbiota in the patients with CRC so far.
3) Nitroso compounds/heme
NOCs are known to exert highly carcinogenic effects following the formation of potent DNA alkylating agents during metabolism [215]. Although most dietary nitrate and nitrite are absorbed in upper GI tract and excreted in the urine, people consuming large amount of red meat can have nitrosating agents through colon [216]. Heme iron is more abundant in red meat than white meat and fish, and they mediate transportation of nitrosating agents [216]. Dietary heme was also reported to alter the microflora by decreasing the number of gram-positive bacteria, leading to expansion of Gram-negative community [217,218]. In addition, a recent study reported that dietary heme induces gut dysbiosis such as a decrease in α-diversity, a reduction of Firmicutes and an increase of Proteobacteria, particularly Enterobacteriaceae [219], similar to DSS-induced colitis model. A reduction in fecal butyrate levels and an increase of large adenomatous polyps were also found in mice fed the heme supplemented diet compared to control mice [219]. However, there is no research regarding the sex difference of NOCs or heme in the patients with CRC, so far.
4) Bile acids
As bile acids are involved in the absorption of dietary fat in the intestine [220], high-fat diets induce an increase in bile secretion. Secondary bile acids are the metabolites produced by intestinal bacteria from primary bile acids (cholic and chenodeoxycholic acids). Large bowel anaerobic bacteria deconjugates and dehydroxylates, cleaving glycine and taurine residues to form the secondary bile acids such as deoxycholic acid and lithocholic acid [206,221]. Metagenomic analysis showed that the microbial bile salt hydrolase activity is identified in all major bacterial divisions in the gut [222]. Bile salt hydrolase confer bile tolerance and hence improvement in survival of bacteria in murine intestine [222]. However, to the host, continuous exposure to the certain hydrophobic bile acids may induce oxidative DNA damage that might lead to tumorigenesis [220]. In serum and bile of patients with colonic adenomas, more deoxycholic acid was detected than in healthy controls [223]. Secondary bile acids are toxic to several cell systems at physiological concentrations [223]. Direct installation of secondary bile acids in the large bowel can be tumor promoting. Infusion of deoxycholic acid led to damage of the mucosa, provoking increased cell proliferation [224]. Deoxycholic acid has been reported to cause resistance to apoptosis, as suggested from tissue specimens [225] and cell-line studies [226]. However, there is no research regarding the sex difference of secondary bile acids in the patients with CRC, so far.
TREATMENT AND CANCER SURVEILLANCE
1. Treatment
First of all, it is necessary to consider fertility in the treatment of female CRC patients. A retrospective study of females receiving adjuvant 5-fluorouracil, leucovorin, oxaliplatin (FOLFOX) chemotherapy reported that 41% experienced amenorrhea during chemotherapy and 16% exhibited persistent amenorrhea 1 year after the completion of treatment, that chemotherapy may affect early menopause and fertility [227]. Second, it is necessary to consider gender-specific recurrence and survival differences. For example, the genotype of the TP53 tumor suppressor gene was predictive of survival following adjuvant chemotherapy in females with stage III colon cancer [228]. In cases of stage II and III CRC patients, polymorphisms in PLS3 and LCP1 were associated with tumor recurrence in females with proximal CRC [229]. In addition, the effect of sex on drug efficacy and toxicity is also should be considered. Females were less likely to receive 5-fluorouracil treatment and showed a shorter duration of treatment compared to males in a study of 1,785 CRC patients aged more than 65 years old, which were possibly associated with the observation that females were more prone to dehydration than males [230]. Indeed, females experienced more severe toxicity including stomatitis, leukopenia, alopecia, and diarrhea compared to male when receiving 5-fluorouracil-based treatment [231]. Recent studies reported that stage III female colon cancer patients tend to omit adjunctive chemotherapy sessions compared to male counterparts [232,233,234]. Also, a greater percentage of elderly female patients and female patients with a prolonged hospital stay exhibited a higher rate of discontinuation [232]. Taken together, research efforts towards the development of anti-cancer drugs displaying less toxicity to the reproductive system are required [5].
2. Cancer screening
Gender differences in CRC screening have been noted with females tending to consider CRC as a male disease and taking time to choose females gastroenterologists [235], whereas males tend to undergo colonoscopy right away more than females [236]. In addition, as mentioned above, right-sided tumors occur predominantly in females than in males [5], and right- and left-sided tumors are different in morphology that right-sided tumors are more often flat while left-sided tumors are more often polypoid [35]. Therefore, appropriate bowel preparation before colonoscopy is important for early detection of flat-shaped right colon cancer as inadequate bowel preparation affects the effectiveness and accuracy of examination [237]. Several studies have reported that age, sex/gender, physical activity, and disease are associated with bowel preparation [238], and there might be several reasons. First, the length of colon is known to be longer in females than in males [239]. In previous studies, male sex [240] and men gender [241] were associated with inadequate bowel preparation, and males were associated with prolonged cecal intubation time [242]. In contrast, males showed higher success rate of cecal intubation within 20 minutes than females [243]. Females showed better bowel preparation than males, but significantly longer cecal intubation time was needed in females in study of 12,561 patients (6,148 females and 6,413 males) [237]. In contrast, withdrawal time was significantly longer in men, which was caused by the higher rate of colonoscopy biopsy in males and higher polyp detection rate in males [237]. In summary, sex-based strategy in indication, follow-up interval, bowel preparation method and withdrawal of colonoscopy would be needed.
CONCLUSIONS
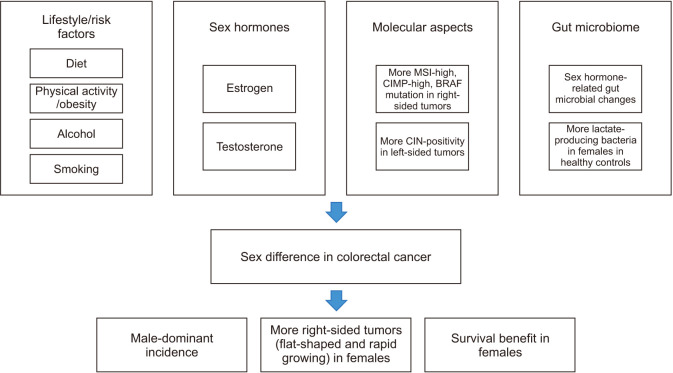
Clinical and preclinical studies have indicated that there are sex- and gender-associated differences in CRC development. Both genetic and environmental factors play roles in sex/gender differences in CRC, that sex hormones including estrogen and androgen contribute to CRC risk, but this is modulated by lifestyle and environmental factors such as diet, alcohol drinking, tobacco smoking and exercise (Fig. 12). Males are at a higher risk of CRC than females, but females have a higher risk of developing right-sided colon cancer than males, which seems to be associated with differences in carcinogenic sequence of CRC, that the CIN pathway is more common in left colon cancer while the MSI and serrated pathways are more common in right colon cancer. In addition, molecular heterogeneity including CIN, oncogene mutation and dMMR is a factor that determines the difference between left- and right-sided tumors in males and females. It is necessary to consider sex/gender differences in terms of effectiveness and toxicities of drugs in treatment of CRC, and in terms of indication, follow-up interval, bowel preparation method and withdrawal of colonoscopy in screening of CRC. Summarizing, biological and pathophysiological differences in CRC between sexes need to be clarified, and gender-specific analyses should be conducted to provide optimal cancer treatment and prevention strategies to reduce CRC both in males and females.
Fig. 12. Sex difference in colorectal cancer. MSI: microsatellite instability, CIMP: CpG island methylator phenotype, BRAF: B-Raf proto-oncogene serine/threonine-protein kinase, CIN: chromosomal instability.
Acknowledgements
None.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: This work was supported by a grant from the National Research Foundation of Korea (NRF) funded by the government of the Republic of Korea (2019R1A2C2085149).
- Conceptualization: NK.
- Funding acquisition: NK.
- Writing – original draft: YC.
- Writing – review & editing & final approval: All authors.
References
- 1.Haziman AA, Ravinderan S, Thangavelu T, Thomas W. A novel role for estrogen-induced signaling in the colorectal cancer gender bias. Ir J Med Sci. 2019;188:389–395. doi: 10.1007/s11845-018-1867-1. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 3.Abancens M, Bustos V, Harvey H, McBryan J, Harvey BJ. Sexual dimorphism in colon cancer. Front Oncol. 2020;10:607909. doi: 10.3389/fonc.2020.607909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong S, Won YJ, Park YR, Jung KW, Kong HJ, Lee ES Community of Population-Based Regional Cancer Registries. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2017. Cancer Res Treat. 2020;52:335–350. doi: 10.4143/crt.2020.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SE, Paik HY, Yoon H, Lee JE, Kim N, Sung MK. Sex- and gender-specific disparities in colorectal cancer risk. World J Gastroenterol. 2015;21:5167–5175. doi: 10.3748/wjg.v21.i17.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012 v1.0. IARC CancerBase No. 11. IARC Press; 2012. [Google Scholar]
- 7.Ahlquist T, Lind GE, Costa VL, Meling GI, Vatn M, Hoff GS, et al. Gene methylation profiles of normal mucosa, and benign and malignant colorectal tumors identify early onset markers. Mol Cancer. 2008;7:94. doi: 10.1186/1476-4598-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pal SK, Hurria A. Impact of age, sex, and comorbidity on cancer therapy and disease progression. J Clin Oncol. 2010;28:4086–4093. doi: 10.1200/JCO.2009.27.0579. [DOI] [PubMed] [Google Scholar]
- 9.De Palma FDE, D’Argenio V, Pol J, Kroemer G, Maiuri MC, Salvatore F. The molecular hallmarks of the serrated pathway in colorectal cancer. Cancers (Basel) 2019;11:1017. doi: 10.3390/cancers11071017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crockett SD, Nagtegaal ID. Terminology, molecular features, epidemiology, and management of serrated colorectal neoplasia. Gastroenterology. 2019;157:949–966.e4. doi: 10.1053/j.gastro.2019.06.041. [DOI] [PubMed] [Google Scholar]
- 11.Global Cancer Observatory. Colorectal cancer [Internet] International Agency for Research on Cancer; c2020. [cited 2023 Mar 5]. Available from: https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf. [Google Scholar]
- 12.International Agency for Research on Cancer. CI5plus: cancer incidence in five continents time trends [Internet] World Health Organization; [cited 2023 Mar 5]. Available from: https://ci5.iarc.fr/ci5plus/default.aspx. [Google Scholar]
- 13.Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713–732. doi: 10.1038/s41575-019-0189-8. [DOI] [PubMed] [Google Scholar]
- 14.Brenner H, Hoffmeister M, Arndt V, Haug U. Gender differences in colorectal cancer: implications for age at initiation of screening. Br J Cancer. 2007;96:828–831. doi: 10.1038/sj.bjc.6603628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Cancer Institute. SEER*Explorer: An interactive website for SEER cancer statistics [Internet] National Cancer Institute; [cited 2023 Mar 5]. Available from: https://seer.cancer.gov/explorer/application.html?site=20&data_type=1&graph_type=1&compareBy=sex&chk_sex_3=3&chk_sex_2=2&race=1&age_range=1&hdn_stage=101&rate_type=2&advopt_precision=1&advopt_display=2. [Google Scholar]
- 16.National Cancer Center. Annual report of cancer statistics in Korea in 2017. Ministry of Health and Welfare; 2019. Dec, Report No.: 117044. [Google Scholar]
- 17.O’Mahony F, Thomas W, Harvey BJ. Novel female sex-dependent actions of oestrogen in the intestine. J Physiol. 2009;587(Pt 21):5039–5044. doi: 10.1113/jphysiol.2009.177972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Son HJ, Sohn SH, Kim N, Lee HN, Lee SM, Nam RH, et al. Effect of estradiol in an azoxymethane/dextran sulfate sodium-treated mouse model of colorectal cancer: implication for sex difference in colorectal cancer development. Cancer Res Treat. 2019;51:632–648. doi: 10.4143/crt.2018.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rennert G, Rennert HS, Pinchev M, Lavie O, Gruber SB. Use of hormone replacement therapy and the risk of colorectal cancer. J Clin Oncol. 2009;27:4542–4547. doi: 10.1200/JCO.2009.22.0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song CH, Kim N, Nam RH, Choi SI, Yu JE, Nho H, et al. Testosterone strongly enhances azoxymethane/dextran sulfate sodium-induced colorectal cancer development in C57BL/6 mice. Am J Cancer Res. 2021;11:3145–3162. [PMC free article] [PubMed] [Google Scholar]
- 21.Schmuck R, Gerken M, Teegen EM, Krebs I, Klinkhammer-Schalke M, Aigner F, et al. Gender comparison of clinical, histopathological, therapeutic and outcome factors in 185,967 colon cancer patients. Langenbecks Arch Surg. 2020;405:71–80. doi: 10.1007/s00423-019-01850-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Wang G, He J, Ren S, Wu F, Zhang J, et al. Gender differences in colorectal cancer survival: a meta-analysis. Int J Cancer. 2017;141:1942–1949. doi: 10.1002/ijc.30827. [DOI] [PubMed] [Google Scholar]
- 23.Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R EUROCARE Working Group. EUROCARE-4. Survival of cancer patients diagnosed in 1995-1999. Results and commentary. Eur J Cancer. 2009;45:931–991. doi: 10.1016/j.ejca.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 24.De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, et al. EUROCARE-5 Working Group. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE--5-a population-based study. Lancet Oncol. 2014;15:23–34. doi: 10.1016/S1470-2045(13)70546-1. [DOI] [PubMed] [Google Scholar]
- 25.White A, Ironmonger L, Steele RJC, Ormiston-Smith N, Crawford C, Seims A. A review of sex-related differences in colorectal cancer incidence, screening uptake, routes to diagnosis, cancer stage and survival in the UK. BMC Cancer. 2018;18:906. doi: 10.1186/s12885-018-4786-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.International Agency for Research on Cancer. Cancer Tomorrow: a tool that predicts the future cancer incidence and mortality burden worldwide from the current estimates in 2020 up until 2040 [Internet] World Health Organization; [cited 2023 Mar 5]. Available from: https://gco.iarc.fr/tomorrow/graphicisotype?type=0&type_sex=0&mode=population&sex=0&populations=900&cancers=8&age_group=value&apc_male=0&apc_female=0&single_unit=100000&print=0. [Google Scholar]
- 27.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 28.Koo JH, Jalaludin B, Wong SK, Kneebone A, Connor SJ, Leong RW. Improved survival in young women with colorectal cancer. Am J Gastroenterol. 2008;103:1488–1495. doi: 10.1111/j.1572-0241.2007.01779.x. [DOI] [PubMed] [Google Scholar]
- 29.Majek O, Gondos A, Jansen L, Emrich K, Holleczek B, Katalinic A, et al. GEKID Cancer Survival Working Group. Sex differences in colorectal cancer survival: population-based analysis of 164,996 colorectal cancer patients in Germany. PLoS One. 2013;8:e68077. doi: 10.1371/journal.pone.0068077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hendifar A, Yang D, Lenz F, Lurje G, Pohl A, Lenz C, et al. Gender disparities in metastatic colorectal cancer survival. Clin Cancer Res. 2009;15:6391–6397. doi: 10.1158/1078-0432.CCR-09-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee MS, Menter DG, Kopetz S. Right versus left colon cancer biology: integrating the consensus molecular subtypes. J Natl Compr Canc Netw. 2017;15:411–419. doi: 10.6004/jnccn.2017.0038. [DOI] [PubMed] [Google Scholar]
- 32.Nakagawa-Senda H, Hori M, Matsuda T, Ito H. Prognostic impact of tumor location in colon cancer: the Monitoring of Cancer Incidence in Japan (MCIJ) project. BMC Cancer. 2019;19:431. doi: 10.1186/s12885-019-5644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrelli F, Tomasello G, Borgonovo K, Ghidini M, Turati L, Dallera P, et al. Prognostic survival associated with left-sided vs right-sided colon cancer: a systematic review and meta-analysis. JAMA Oncol. 2017;3:211–219. doi: 10.1001/jamaoncol.2016.4227. [DOI] [PubMed] [Google Scholar]
- 34.Gasser E, Braunwarth E, Riedmann M, Cardini B, Fadinger N, Presl J, et al. Primary tumour location affects survival after resection of colorectal liver metastases: a two-institutional cohort study with international validation, systematic meta-analysis and a clinical risk score. PLoS One. 2019;14:e0217411. doi: 10.1371/journal.pone.0217411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaku E, Oda Y, Murakami Y, Goto H, Tanaka T, Hasuda K, et al. Proportion of flat- and depressed-type and laterally spreading tumor among advanced colorectal neoplasia. Clin Gastroenterol Hepatol. 2011;9:503–508. doi: 10.1016/j.cgh.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 36.Ward R, Meagher A, Tomlinson I, O'Connor T, Norrie M, Wu R, et al. Microsatellite instability and the clinicopathological features of sporadic colorectal cancer. Gut. 2001;48:821–829. doi: 10.1136/gut.48.6.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quirt JS, Nanji S, Wei X, Flemming JA, Booth CM. Is there a sex effect in colon cancer? Disease characteristics, management, and outcomes in routine clinical practice. Curr Oncol. 2017;24:e15–e23. doi: 10.3747/co.24.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conti L, Del Cornò M, Gessani S. Revisiting the impact of lifestyle on colorectal cancer risk in a gender perspective. Crit Rev Oncol Hematol. 2020;145:102834. doi: 10.1016/j.critrevonc.2019.102834. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, He X, Ugai T, Haruki K, Lo CH, Hang D, et al. Risk factors and incidence of colorectal cancer according to major molecular subtypes. JNCI Cancer Spectr. 2020;5:pkaa089. doi: 10.1093/jncics/pkaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jakszyn P, Cayssials V, Buckland G, Perez-Cornago A, Weiderpass E, Boeing H, et al. Inflammatory potential of the diet and risk of colorectal cancer in the European prospective investigation into cancer and nutrition study. Int J Cancer. 2020;147:1027–1039. doi: 10.1002/ijc.32870. [DOI] [PubMed] [Google Scholar]
- 41.Keshava C, Frye BL, Wolff MS, McCanlies EC, Weston A. Waf-1 (p21) and p53 polymorphisms in breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:127–130. Erratum in: Cancer Epidemiol Biomarkers Prev 2004;13:1682. [PubMed] [Google Scholar]
- 42.McMichael AJ, Potter JD. Diet and colon cancer: integration of the descriptive, analytic, and metabolic epidemiology. Natl Cancer Inst Monogr. 1985;69:223–228. [PubMed] [Google Scholar]
- 43.West DW, Slattery ML, Robison LM, Schuman KL, Ford MH, Mahoney AW, et al. Dietary intake and colon cancer: sex- and anatomic site-specific associations. Am J Epidemiol. 1989;130:883–894. doi: 10.1093/oxfordjournals.aje.a115421. [DOI] [PubMed] [Google Scholar]
- 44.Hu J, La Vecchia C, Negri E, Mery L. Nutrients and risk of colon cancer. Cancers (Basel) 2010;2:51–67. doi: 10.3390/cancers2010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hjartåker A, Aagnes B, Robsahm TE, Langseth H, Bray F, Larsen IK. Subsite-specific dietary risk factors for colorectal cancer: a review of cohort studies. J Oncol. 2013;2013:703854. doi: 10.1155/2013/703854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larsson SC, Rafter J, Holmberg L, Bergkvist L, Wolk A. Red meat consumption and risk of cancers of the proximal colon, distal colon and rectum: the Swedish mammography cohort. Int J Cancer. 2005;113:829–834. doi: 10.1002/ijc.20658. [DOI] [PubMed] [Google Scholar]
- 47.Ferrucci LM, Sinha R, Huang WY, Berndt SI, Katki HA, Schoen RE, et al. Meat consumption and the risk of incident distal colon and rectal adenoma. Br J Cancer. 2012;106:608–616. doi: 10.1038/bjc.2011.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stemmermann GN, Nomura A, Chyou PH. The influence of dairy and nondairy calcium on subsite large-bowel cancer risk. Dis Colon Rectum. 1990;33:190–194. doi: 10.1007/BF02134177. [DOI] [PubMed] [Google Scholar]
- 49.Wu K, Willett WC, Fuchs CS, Colditz GA, Giovannucci EL. Calcium intake and risk of colon cancer in women and men. J Natl Cancer Inst. 2002;94:437–446. doi: 10.1093/jnci/94.6.437. [DOI] [PubMed] [Google Scholar]
- 50.Oh K, Willett WC, Wu K, Fuchs CS, Giovannucci EL. Calcium and vitamin D intakes in relation to risk of distal colorectal adenoma in women. Am J Epidemiol. 2007;165:1178–1186. doi: 10.1093/aje/kwm026. [DOI] [PubMed] [Google Scholar]
- 51.Touvier M, Chan DS, Lau R, Aune D, Vieira R, Greenwood DC, et al. Meta-analyses of vitamin D intake, 25-hydroxyvitamin D status, vitamin D receptor polymorphisms, and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2011;20:1003–1016. doi: 10.1158/1055-9965.EPI-10-1141. [DOI] [PubMed] [Google Scholar]
- 52.Yan L, Spitznagel EL, Bosland MC. Soy consumption and colorectal cancer risk in humans: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19:148–158. doi: 10.1158/1055-9965.EPI-09-0856. [DOI] [PubMed] [Google Scholar]
- 53.Yang G, Shu XO, Li H, Chow WH, Cai H, Zhang X, et al. Prospective cohort study of soy food intake and colorectal cancer risk in women. Am J Clin Nutr. 2009;89:577–583. doi: 10.3945/ajcn.2008.26742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cotterchio M, Boucher BA, Manno M, Gallinger S, Okey A, Harper P. Dietary phytoestrogen intake is associated with reduced colorectal cancer risk. J Nutr. 2006;136:3046–3053. doi: 10.1093/jn/136.12.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Budhathoki S, Joshi AM, Ohnaka K, Yin G, Toyomura K, Kono S, et al. Soy food and isoflavone intake and colorectal cancer risk: the Fukuoka colorectal cancer study. Scand J Gastroenterol. 2011;46:165–172. doi: 10.3109/00365521.2010.522720. [DOI] [PubMed] [Google Scholar]
- 56.Bises G, Bajna E, Manhardt T, Gerdenitsch W, Kallay E, Cross HS. Gender-specific modulation of markers for premalignancy by nutritional soy and calcium in the mouse colon. J Nutr. 2007;137(1 Suppl):211S–215S. doi: 10.1093/jn/137.1.211S. [DOI] [PubMed] [Google Scholar]
- 57.Martínez ME. Primary prevention of colorectal cancer: lifestyle, nutrition, exercise. Recent Results Cancer Res. 2005;166:177–211. doi: 10.1007/3-540-26980-0_13. [DOI] [PubMed] [Google Scholar]
- 58.Martínez ME, Giovannucci E, Spiegelman D, Hunter DJ, Willett WC, Colditz GA. Leisure-time physical activity, body size, and colon cancer in women. Nurses' Health Study Research Group. J Natl Cancer Inst. 1997;89:948–955. doi: 10.1093/jnci/89.13.948. [DOI] [PubMed] [Google Scholar]
- 59.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122:327–334. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 60.Martínez ME, Heddens D, Earnest DL, Bogert CL, Roe D, Einspahr J, et al. Physical activity, body mass index, and prostaglandin E2 levels in rectal mucosa. J Natl Cancer Inst. 1999;91:950–953. doi: 10.1093/jnci/91.11.950. [DOI] [PubMed] [Google Scholar]
- 61.Hu FB, Manson JE, Liu S, Hunter D, Colditz GA, Michels KB, et al. Prospective study of adult onset diabetes mellitus (type 2) and risk of colorectal cancer in women. J Natl Cancer Inst. 1999;91:542–547. doi: 10.1093/jnci/91.6.542. [DOI] [PubMed] [Google Scholar]
- 62.Schoen RE, Tangen CM, Kuller LH, Burke GL, Cushman M, Tracy RP, et al. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst. 1999;91:1147–1154. doi: 10.1093/jnci/91.13.1147. [DOI] [PubMed] [Google Scholar]
- 63.Ho GY, Wang T, Gunter MJ, Strickler HD, Cushman M, Kaplan RC, et al. Adipokines linking obesity with colorectal cancer risk in postmenopausal women. Cancer Res. 2012;72:3029–3037. doi: 10.1158/0008-5472.CAN-11-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hampe J, Lynch NJ, Daniels S, Bridger S, Macpherson AJ, Stokkers P, et al. Fine mapping of the chromosome 3p susceptibility locus in inflammatory bowel disease. Gut. 2001;48:191–197. doi: 10.1136/gut.48.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McTiernan A, Wu L, Chen C, Chlebowski R, Mossavar-Rahmani Y, Modugno F, et al. Women's Health Initiative Investigators. Relation of BMI and physical activity to sex hormones in postmenopausal women. Obesity (Silver Spring) 2006;14:1662–1677. doi: 10.1038/oby.2006.191. [DOI] [PubMed] [Google Scholar]
- 66.Shin CM, Han K, Lee DH, Choi YJ, Kim N, Park YS, et al. Association among obesity, metabolic health, and the risk for colorectal cancer in the general population in Korea using the National Health Insurance Service-national sample cohort. Dis Colon Rectum. 2017;60:1192–1200. doi: 10.1097/DCR.0000000000000876. [DOI] [PubMed] [Google Scholar]
- 67.Choi YJ, Lee DH, Han KD, Shin CM, Kim N. Abdominal obesity, glucose intolerance and decreased high-density lipoprotein cholesterol as components of the metabolic syndrome are associated with the development of colorectal cancer. Eur J Epidemiol. 2018;33:1077–1085. doi: 10.1007/s10654-018-0440-6. [DOI] [PubMed] [Google Scholar]
- 68.World Cancer Research Fund, American Institute for Cancer Research (AICR) Food, nutrition, physical activity, and the prevention of cancer: a global perspective. AICR; 2007. [Google Scholar]
- 69.International Agency for Research on Cancer (IARC) IARC monographs on the evaluation of carcinogenic risks to humans. Vol. 96, Alcohol consumption and ethyl carbamate. IARC Press; 2010. [PMC free article] [PubMed] [Google Scholar]
- 70.World Health Organization. IARC monographs on the evaluation of the carcinogenic risks to humans. Vol. 44, Alcohol drinking. IARC Press; 1988. [Google Scholar]
- 71.Choi YJ, Lee DH, Han KD, Kim HS, Yoon H, Shin CM, et al. The relationship between drinking alcohol and esophageal, gastric or colorectal cancer: a nationwide population-based cohort study of South Korea. PLoS One. 2017;12:e01857782018. doi: 10.1371/journal.pone.0185778. Erratum for: PLoS One 2018;13:e0197765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.International Agency for Research on Cancer (IARC) Reevaluation of some organic chemicals, hydrazine and hydrogen peroxide. IARC; 1999. [Google Scholar]
- 73.Mizoue T, Inoue M, Wakai K, Nagata C, Shimazu T, Tsuji I, et al. Research Group for Development and Evaluation of Cancer Prevention Strategies in Japan. Alcohol drinking and colorectal cancer in Japanese: a pooled analysis of results from five cohort studies. Am J Epidemiol. 2008;167:1397–1406. doi: 10.1093/aje/kwn073. [DOI] [PubMed] [Google Scholar]
- 74.Goedde HW, Agarwal DP, Fritze G, Meier-Tackmann D, Singh S, Beckmann G, et al. Distribution of ADH2 and ALDH2 genotypes in different populations. Hum Genet. 1992;88:344–346. doi: 10.1007/BF00197271. [DOI] [PubMed] [Google Scholar]
- 75.Yi SW, Sull JW, Linton JA, Nam CM, Ohrr H. Alcohol consumption and digestive cancer mortality in Koreans: the Kangwha cohort study. J Epidemiol. 2010;20:204–211. doi: 10.2188/jea.JE20090077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Giovannucci E, Martínez ME. Tobacco, colorectal cancer, and adenomas: a review of the evidence. J Natl Cancer Inst. 1996;88:1717–1730. doi: 10.1093/jnci/88.23.1717. [DOI] [PubMed] [Google Scholar]
- 77.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Kearney J, et al. A prospective study of cigarette smoking and risk of colorectal adenoma and colorectal cancer in U.S. men. J Natl Cancer Inst. 1994;86:183–191. doi: 10.1093/jnci/86.3.183. [DOI] [PubMed] [Google Scholar]
- 78.Giovannucci E, Colditz GA, Stampfer MJ, Hunter D, Rosner BA, Willett WC, et al. A prospective study of cigarette smoking and risk of colorectal adenoma and colorectal cancer in U.S. women. J Natl Cancer Inst. 1994;86:192–199. doi: 10.1093/jnci/86.3.192. [DOI] [PubMed] [Google Scholar]
- 79.Nordlund LA, Carstensen JM, Pershagen G. Cancer incidence in female smokers: a 26-year follow-up. Int J Cancer. 1997;73:625–628. doi: 10.1002/(sici)1097-0215(19971127)73:5<625::aid-ijc2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 80.Tavani A, Pregnolato A, La Vecchia C, Negri E, Talamini R, Franceschi S. Coffee and tea intake and risk of cancers of the colon and rectum: a study of 3,530 cases and 7,057 controls. Int J Cancer. 1997;73:193–197. doi: 10.1002/(sici)1097-0215(19971009)73:2<193::aid-ijc5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 81.Baron JA, Gerhardsson de Verdier M, Ekbom A. Coffee, tea, tobacco, and cancer of the large bowel. Cancer Epidemiol Biomarkers Prev. 1994;3:565–570. [PubMed] [Google Scholar]
- 82.Nyrén O, Bergström R, Nyström L, Engholm G, Ekbom A, Adami HO, et al. Smoking and colorectal cancer: a 20-year follow-up study of Swedish construction workers. J Natl Cancer Inst. 1996;88:1302–1307. doi: 10.1093/jnci/88.18.1302. [DOI] [PubMed] [Google Scholar]
- 83.Xu X, Duncan AM, Wangen KE, Kurzer MS. Soy consumption alters endogenous estrogen metabolism in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2000;9:781–786. [PubMed] [Google Scholar]
- 84.Chao A, Thun MJ, Jacobs EJ, Henley SJ, Rodriguez C, Calle EE. Cigarette smoking and colorectal cancer mortality in the cancer prevention study II. J Natl Cancer Inst. 2000;92:1888–1896. doi: 10.1093/jnci/92.23.1888. [DOI] [PubMed] [Google Scholar]
- 85.Liedtke S, Schmidt ME, Becker S, Kaaks R, Zaineddin AK, Buck K, et al. Physical activity and endogenous sex hormones in postmenopausal women: to what extent are observed associations confounded or modified by BMI? Cancer Causes Control. 2011;22:81–89. doi: 10.1007/s10552-010-9677-4. [DOI] [PubMed] [Google Scholar]
- 86.Lukanova A, Lundin E, Zeleniuch-Jacquotte A, Muti P, Mure A, Rinaldi S, et al. Body mass index, circulating levels of sex-steroid hormones, IGF-I and IGF-binding protein-3: a cross-sectional study in healthy women. Eur J Endocrinol. 2004;150:161–171. doi: 10.1530/eje.0.1500161. [DOI] [PubMed] [Google Scholar]
- 87.Stevanato Filho PR, Aguiar Júnior S, Begnami MD, Ferreira FO, Nakagawa WT, Spencer RMSB, et al. Estrogen receptorβ as a prognostic marker of tumor progression in colorectal cancer with familial adenomatous polyposis and sporadic polyps. Pathol Oncol Res. 2018;24:533–540. doi: 10.1007/s12253-017-0268-5. [DOI] [PubMed] [Google Scholar]
- 88.Son HJ, Kim N, Song CH, Lee SM, Lee HN, Surh YJ. 17β-Estradiol reduces inflammation and modulates antioxidant enzymes in colonic epithelial cells. Korean J Intern Med. 2020;35:310–319. doi: 10.3904/kjim.2018.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu J, Williams D, Walter GA, Thompson WE, Sidell N. Estrogen increases Nrf2 activity through activation of the PI3K pathway in MCF-7 breast cancer cells. Exp Cell Res. 2014;328:351–360. doi: 10.1016/j.yexcr.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 90.Li W, Khor TO, Xu C, Shen G, Jeong WS, Yu S, et al. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem Pharmacol. 2008;76:1485–1489. doi: 10.1016/j.bcp.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Niture SK, Khatri R, Jaiswal AK. Regulation of Nrf2-an update. Free Radic Biol Med. 2014;66:36–44. doi: 10.1016/j.freeradbiomed.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao C, Gillette DD, Li X, Zhang Z, Wen H. Nuclear factor E2-related factor-2 (Nrf2) is required for NLRP3 and AIM2 inflammasome activation. J Biol Chem. 2014;289:17020–17029. doi: 10.1074/jbc.M114.563114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Suzuki R, Kohno H, Sugie S, Tanaka T. Sequential observations on the occurrence of preneoplastic and neoplastic lesions in mouse colon treated with azoxymethane and dextran sodium sulfate. Cancer Sci. 2004;95:721–727. doi: 10.1111/j.1349-7006.2004.tb03252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fabbi M, Carbotti G, Ferrini S. Context-dependent role of IL-18 in cancer biology and counter-regulation by IL-18BP. J Leukoc Biol. 2015;97:665–675. doi: 10.1189/jlb.5RU0714-360RR. [DOI] [PubMed] [Google Scholar]
- 96.Song CH, Kim N, Lee SM, Nam RH, Choi SI, Kang SR, et al. Effects of 17β-estradiol on colorectal cancer development after azoxymethane/dextran sulfate sodium treatment of ovariectomized mice. Biochem Pharmacol. 2019;164:139–151. doi: 10.1016/j.bcp.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 97.Caiazza F, Ryan EJ, Doherty G, Winter DC, Sheahan K. Estrogen receptors and their implications in colorectal carcinogenesis. Front Oncol. 2015;5:19. doi: 10.3389/fonc.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maingi JW, Tang S, Liu S, Ngenya W, Bao E. Targeting estrogen receptors in colorectal cancer. Mol Biol Rep. 2020;47:4087–4091. doi: 10.1007/s11033-020-05414-6. [DOI] [PubMed] [Google Scholar]
- 99.Principi M, Barone M, Pricci M, De Tullio N, Losurdo G, Ierardi E, et al. Ulcerative colitis: from inflammation to cancer. Do estrogen receptors have a role? World J Gastroenterol. 2014;20:11496–11504. doi: 10.3748/wjg.v20.i33.11496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fiorelli G, Picariello L, Martineti V, Tonelli F, Brandi ML. Functional estrogen receptor beta in colon cancer cells. Biochem Biophys Res Commun. 1999;261:521–527. doi: 10.1006/bbrc.1999.1062. [DOI] [PubMed] [Google Scholar]
- 101.Wong NA, Malcomson RD, Jodrell DI, Groome NP, Harrison DJ, Saunders PT. ERbeta isoform expression in colorectal carcinoma: an in vivo and in vitro study of clinicopathological and molecular correlates. J Pathol. 2005;207:53–60. doi: 10.1002/path.1807. [DOI] [PubMed] [Google Scholar]
- 102.Wada-Hiraike O, Imamov O, Hiraike H, Hultenby K, Schwend T, Omoto Y, et al. Role of estrogen receptor beta in colonic epithelium. Proc Natl Acad Sci U S A. 2006;103:2959–2964. doi: 10.1073/pnas.0511271103. Erratum in: Proc Natl Acad Sci U S A 2006;103:8299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martineti V, Picariello L, Tognarini I, Carbonell Sala S, Gozzini A, Azzari C, et al. ERbeta is a potent inhibitor of cell proliferation in the HCT8 human colon cancer cell line through regulation of cell cycle components. Endocr Relat Cancer. 2005;12:455–469. doi: 10.1677/erc.1.00861. Erratum in: Endocr Relat Cancer 2005;12:682. [DOI] [PubMed] [Google Scholar]
- 104.Qiu Y, Waters CE, Lewis AE, Langman MJ, Eggo MC. Oestrogen-induced apoptosis in colonocytes expressing oestrogen receptor beta. J Endocrinol. 2002;174:369–377. doi: 10.1677/joe.0.1740369. [DOI] [PubMed] [Google Scholar]
- 105.Hases L, Indukuri R, Birgersson M, Nguyen-Vu T, Lozano R, Saxena A, et al. Intestinal estrogen receptor beta suppresses colon inflammation and tumorigenesis in both sexes. Cancer Lett. 2020;492:54–62. doi: 10.1016/j.canlet.2020.06.021. [DOI] [PubMed] [Google Scholar]
- 106.Braniste V, Leveque M, Buisson-Brenac C, Bueno L, Fioramonti J, Houdeau E. Oestradiol decreases colonic permeability through oestrogen receptor beta-mediated up-regulation of occludin and junctional adhesion molecule-a in epithelial cells. J Physiol. 2009;587(Pt 13):3317–3328. doi: 10.1113/jphysiol.2009.169300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Song CH, Kim N, Sohn SH, Lee SM, Nam RH, Na HY, et al. Effects of 17β-estradiol on colonic permeability and inflammation in an azoxymethane/dextran sulfate sodium-induced colitis mouse model. Gut Liver. 2018;12:682–693. doi: 10.5009/gnl18221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barzi A, Lenz AM, Labonte MJ, Lenz HJ. Molecular pathways: estrogen pathway in colorectal cancer. Clin Cancer Res. 2013;19:5842–5848. doi: 10.1158/1078-0432.CCR-13-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jacenik D, Beswick EJ, Krajewska WM, Prossnitz ER. G protein-coupled estrogen receptor in colon function, immune regulation and carcinogenesis. World J Gastroenterol. 2019;25:4092–4104. doi: 10.3748/wjg.v25.i30.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jacenik D, Krajewska WM. Significance of G protein-coupled estrogen receptor in the pathophysiology of irritable bowel syndrome, inflammatory bowel diseases and colorectal cancer. Front Endocrinol (Lausanne) 2020;11:390. doi: 10.3389/fendo.2020.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jacenik D, Zielińska M, Mokrowiecka A, Michlewska S, Małecka-Panas E, Kordek R, et al. G protein-coupled estrogen receptor mediates anti-inflammatory action in Crohn's disease. Sci Rep. 2019;9:6749. doi: 10.1038/s41598-019-43233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xu S, Wang X, Zhao J, Yang S, Dong L, Qin B. GPER-mediated, oestrogen-dependent visceral hypersensitivity in stressed rats is associated with mast cell tryptase and histamine expression. Fundam Clin Pharmacol. 2020;34:433–443. doi: 10.1111/fcp.12537. [DOI] [PubMed] [Google Scholar]
- 113.Manjegowda MC, Limaye AM. DNA methylation dependent suppression of GPER1 in colorectal cancer. Med Res Arch. 2019;6:1–7. [Google Scholar]
- 114.Guérin A, Mody R, Fok B, Lasch KL, Zhou Z, Wu EQ, et al. Risk of developing colorectal cancer and benign colorectal neoplasm in patients with chronic constipation. Aliment Pharmacol Ther. 2014;40:83–92. doi: 10.1111/apt.12789. [DOI] [PubMed] [Google Scholar]
- 115.Jacenik D, Cygankiewicz AI, Mokrowiecka A, Małecka-Panas E, Fichna J, Krajewska WM. Sex- and age-related estrogen signaling alteration in inflammatory bowel diseases: modulatory role of estrogen receptors. Int J Mol Sci. 2019;20:3175. doi: 10.3390/ijms20133175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Meng R, Qin Q, Xiong Y, Wang Y, Zheng J, Zhao Y, et al. NHERF1, a novel GPER associated protein, increases stability and activation of GPER in ER-positive breast cancer. Oncotarget. 2016;7:54983–54997. doi: 10.18632/oncotarget.10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jung J. Role of G protein-coupled estrogen receptor in cancer progression. Toxicol Res. 2019;35:209–214. doi: 10.5487/TR.2019.35.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Skrzypczak M, Goryca K, Rubel T, Paziewska A, Mikula M, Jarosz D, et al. Modeling oncogenic signaling in colon tumors by multidirectional analyses of microarray data directed for maximization of analytical reliability. PLoS One. 2010;5:e13091. doi: 10.1371/journal.pone.0013091. Erratum in: PLoS One 2010;5:10.1371/annotation/8c585739-a354-4fc9-a7d0-d5ae26fa06ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sasso CV, Santiano FE, Campo Verde, Zyla LE, Semino SN, Guerrero-Gimenez ME, et al. Estradiol and progesterone regulate proliferation and apoptosis in colon cancer. Endocr Connect. 2019;8:217–229. doi: 10.1530/EC-18-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Meijer BJ, Wielenga MCB, Hoyer PB, Amos-Landgraf JM, Hakvoort TBM, Muncan V, et al. Colorectal tumor prevention by the progestin medroxyprogesterone acetate is critically dependent on postmenopausal status. Oncotarget. 2018;9:30561–30567. doi: 10.18632/oncotarget.25703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Slattery ML, Sweeney C, Murtaugh M, Ma KN, Wolff RK, Potter JD, et al. Associations between ERalpha, ERbeta, and AR genotypes and colon and rectal cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:2936–2942. doi: 10.1158/1055-9965.EPI-05-0514. [DOI] [PubMed] [Google Scholar]
- 123.Catalano MG, Pfeffer U, Raineri M, Ferro P, Curto A, Capuzzi P, et al. Altered expression of androgen-receptor isoforms in human colon-cancer tissues. Int J Cancer. 2000;86:325–330. doi: 10.1002/(sici)1097-0215(20000501)86:3<325::aid-ijc4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 124.Roshan MH, Tambo A, Pace NP. The role of testosterone in colorectal carcinoma: pathomechanisms and open questions. EPMA J. 2016;7:22. doi: 10.1186/s13167-016-0071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) prospective population study. Circulation. 2007;116:2694–2701. doi: 10.1161/CIRCULATIONAHA.107.719005. [DOI] [PubMed] [Google Scholar]
- 126.Hyde Z, Flicker L, McCaul KA, Almeida OP, Hankey GJ, Chubb SA, et al. Associations between testosterone levels and incident prostate, lung, and colorectal cancer. A population-based study. Cancer Epidemiol Biomarkers Prev. 2012;21:1319–1329. doi: 10.1158/1055-9965.EPI-12-0129. [DOI] [PubMed] [Google Scholar]
- 127.Mori N, Sawada N, Iwasaki M, Yamaji T, Goto A, Shimazu T, et al. Circulating sex hormone levels and colorectal cancer risk in Japanese postmenopausal women: the JPHC nested case-control study. Int J Cancer. 2019;145:1238–1244. doi: 10.1002/ijc.32431. [DOI] [PubMed] [Google Scholar]
- 128.Xia T, Sun H, Huang H, Bi H, Pu R, Zhang L, et al. Androgen receptor gene methylation related to colorectal cancer risk. Endocr Connect. 2019;8:979–987. doi: 10.1530/EC-19-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Alberg AJ, Gordon GB, Hoffman SC, Comstock GW, Helzlsouer KJ. Serum dehydroepiandrosterone and dehydroepiandrosterone sulfate and the subsequent risk of developing colon cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:517–521. [PubMed] [Google Scholar]
- 130.Yang W, Giovannucci EL, Hankinson SE, Chan AT, Ma Y, Wu K, et al. Endogenous sex hormones and colorectal cancer survival among men and women. Int J Cancer. 2020;147:920–930. doi: 10.1002/ijc.32844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gillessen S, Templeton A, Marra G, Kuo YF, Valtorta E, Shahinian VB. Risk of colorectal cancer in men on long-term androgen deprivation therapy for prostate cancer. J Natl Cancer Inst. 2010;102:1760–1770. doi: 10.1093/jnci/djq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Huang R, Wang G, Song Y, Wang F, Zhu B, Tang Q, et al. Polymorphic CAG repeat and protein expression of androgen receptor gene in colorectal cancer. Mol Cancer Ther. 2015;14:1066–1074. doi: 10.1158/1535-7163.MCT-14-0620. [DOI] [PubMed] [Google Scholar]
- 133.Rudolph A, Shi H, Försti A, Hoffmeister M, Sainz J, Jansen L, et al. Repeat polymorphisms in ESR2 and AR and colorectal cancer risk and prognosis: results from a German population-based case-control study. BMC Cancer. 2014;14:817. doi: 10.1186/1471-2407-14-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ørsted DD, Nordestgaard BG, Bojesen SE. Plasma testosterone in the general population, cancer prognosis and cancer risk: a prospective cohort study. Ann Oncol. 2014;25:712–718. doi: 10.1093/annonc/mdt590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jacobs ET, Thompson PA, Martínez ME. Diet, gender, and colorectal neoplasia. J Clin Gastroenterol. 2007;41:731–746. doi: 10.1097/MCG.0b013e3180338e56. [DOI] [PubMed] [Google Scholar]
- 137.Missiaglia E, Jacobs B, D'Ario G, Di Narzo AF, Soneson C, Budinska E, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014;25:1995–2001. doi: 10.1093/annonc/mdu275. Erratum in: Ann Oncol 2015;26:445. [DOI] [PubMed] [Google Scholar]
- 138.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101:403–408. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 139.Lynch HT, Watson P, Lanspa SJ, Marcus J, Smyrk T, Fitzgibbons RJ, Jr, et al. Natural history of colorectal cancer in hereditary nonpolyposis colorectal cancer (Lynch syndromes I and II) Dis Colon Rectum. 1988;31:439–444. doi: 10.1007/BF02552613. [DOI] [PubMed] [Google Scholar]
- 140.Shen H, Yang J, Huang Q, Jiang MJ, Tan YN, Fu JF, et al. Different treatment strategies and molecular features between right-sided and left-sided colon cancers. World J Gastroenterol. 2015;21:6470–6478. doi: 10.3748/wjg.v21.i21.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sinicrope FA, Okamoto K, Kasi PM, Kawakami H. Molecular biomarkers in the personalized treatment of colorectal cancer. Clin Gastroenterol Hepatol. 2016;14:651–658. doi: 10.1016/j.cgh.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci U S A. 1999;96:8681–8686. doi: 10.1073/pnas.96.15.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, Faasse MA, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787–793. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 145.Hawkins N, Norrie M, Cheong K, Mokany E, Ku SL, Meagher A, et al. CpG island methylation in sporadic colorectal cancers and its relationship to microsatellite instability. Gastroenterology. 2002;122:1376–1387. doi: 10.1053/gast.2002.32997. [DOI] [PubMed] [Google Scholar]
- 146.Ogino S, Kawasaki T, Kirkner GJ, Kraft P, Loda M, Fuchs CS. Evaluation of markers for CpG island methylator phenotype (CIMP) in colorectal cancer by a large population-based sample. J Mol Diagn. 2007;9:305–314. doi: 10.2353/jmoldx.2007.060170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Domingo E, Espín E, Armengol M, Oliveira C, Pinto M, Duval A, et al. Activated BRAF targets proximal colon tumors with mismatch repair deficiency and MLH1 inactivation. Genes Chromosomes Cancer. 2004;39:138–142. doi: 10.1002/gcc.10310. [DOI] [PubMed] [Google Scholar]
- 148.Sinicrope FA, Shi Q, Smyrk TC, Thibodeau SN, Dienstmann R, Guinney J, et al. Molecular markers identify subtypes of stage III colon cancer associated with patient outcomes. Gastroenterology. 2015;148:88–99. doi: 10.1053/j.gastro.2014.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sinicrope FA, Mahoney MR, Yoon HH, Smyrk TC, Thibodeau SN, Goldberg RM, et al. Alliance for Clinical Trials in Oncology. Analysis of molecular markers by anatomic tumor site in stage III colon carcinomas from adjuvant chemotherapy trial NCCTG N0147 (alliance) Clin Cancer Res. 2015;21:5294–5304. doi: 10.1158/1078-0432.CCR-15-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bond CE, Liu C, Kawamata F, McKeone DM, Fernando W, Jamieson S, et al. Oncogenic BRAF mutation induces DNA methylation changes in a murine model for human serrated colorectal neoplasia. Epigenetics. 2018;13:40–48. doi: 10.1080/15592294.2017.1411446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Murphy N, Ward HA, Jenab M, Rothwell JA, Boutron-Ruault MC, Carbonnel F, et al. Heterogeneity of colorectal cancer risk factors by anatomical subsite in 10 European countries: a multinational cohort study. Clin Gastroenterol Hepatol. 2019;17:1323–1331.e6. doi: 10.1016/j.cgh.2018.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Murakami T, Sakamoto N, Nagahara A. Clinicopathological features, diagnosis, and treatment of sessile serrated adenoma/polyp with dysplasia/carcinoma. J Gastroenterol Hepatol. 2019;34:1685–1695. doi: 10.1111/jgh.14752. [DOI] [PubMed] [Google Scholar]
- 153.Choi J, Kim N, Nam RH, Kim JW, Song CH, Na HY, et al. Influence of location-dependent sex difference on PD-L1, MMR/MSI, and EGFR in colorectal carcinogenesis. PLoS One. 2023;18:e0282017. doi: 10.1371/journal.pone.0282017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 155.Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–1075. doi: 10.1016/S1470-2045(14)70330-4. [DOI] [PubMed] [Google Scholar]
- 156.Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 157.Bokemeyer C, Bondarenko I, Hartmann JT, de Braud F, Schuch G, Zubel A, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22:1535–1546. doi: 10.1093/annonc/mdq632. [DOI] [PubMed] [Google Scholar]
- 158.De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753–762. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 159.Blons H, Emile JF, Le Malicot K, Julié C, Zaanan A, Tabernero J, et al. Prognostic value of KRAS mutations in stage III colon cancer: post hoc analysis of the PETACC8 phase III trial dataset. Ann Oncol. 2014;25:2378–2385. doi: 10.1093/annonc/mdu464. Erratum in: Ann Oncol 2015;26:822-5. [DOI] [PubMed] [Google Scholar]
- 160.Yoon HH, Tougeron D, Shi Q, Alberts SR, Mahoney MR, Nelson GD, et al. Alliance for Clinical Trials in Oncology. KRAS codon 12 and 13 mutations in relation to disease-free survival in BRAF-wild-type stage III colon cancers from an adjuvant chemotherapy trial (N0147 alliance) Clin Cancer Res. 2014;20:3033–3043. doi: 10.1158/1078-0432.CCR-13-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28:466–474. doi: 10.1200/JCO.2009.23.3452. [DOI] [PubMed] [Google Scholar]
- 162.Hutchins G, Southward K, Handley K, Magill L, Beaumont C, Stahlschmidt J, et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol. 2011;29:1261–1270. doi: 10.1200/JCO.2010.30.1366. Erratum in: J Clin Oncol 2011;29:2949. [DOI] [PubMed] [Google Scholar]
- 163.Mouradov D, Domingo E, Gibbs P, Jorissen RN, Li S, Soo PY, et al. Survival in stage II/III colorectal cancer is independently predicted by chromosomal and microsatellite instability, but not by specific driver mutations. Am J Gastroenterol. 2013;108:1785–1793. doi: 10.1038/ajg.2013.292. [DOI] [PubMed] [Google Scholar]
- 164.Rajagopalan H, Bardelli A, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature. 2002;418:934. doi: 10.1038/418934a. [DOI] [PubMed] [Google Scholar]
- 165.Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, et al. MRC COIN Trial Investigators. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103–2114. doi: 10.1016/S0140-6736(11)60613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Clancy C, Burke JP, Kalady MF, Coffey JC. BRAF mutation is associated with distinct clinicopathological characteristics in colorectal cancer: a systematic review and meta-analysis. Colorectal Dis. 2013;15:e711–e718. doi: 10.1111/codi.12427. [DOI] [PubMed] [Google Scholar]
- 167.Sasaki Y, Hamaguchi T, Yamada Y, Takahashi N, Shoji H, Honma Y, et al. Value of KRAS, BRAF, and PIK3CA mutations and survival benefit from systemic chemotherapy in colorectal peritoneal carcinomatosis. Asian Pac J Cancer Prev. 2016;17:539–543. doi: 10.7314/apjcp.2016.17.2.539. [DOI] [PubMed] [Google Scholar]
- 168.Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 169.Wu M, Kim YS, Ryu HS, Choi SC, Kim KY, Park WC, et al. MSI status is associated with distinct clinicopathological features in BRAF mutation colorectal cancer: a systematic review and meta-analysis. Pathol Res Pract. 2020;216:152791. doi: 10.1016/j.prp.2019.152791. [DOI] [PubMed] [Google Scholar]
- 170.Lito P, Pratilas CA, Joseph EW, Tadi M, Halilovic E, Zubrowski M, et al. Relief of profound feedback inhibition of mitogenic signaling by RAF inhibitors attenuates their activity in BRAFV600E melanomas. Cancer Cell. 2012;22:668–682. doi: 10.1016/j.ccr.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gavin PG, Colangelo LH, Fumagalli D, Tanaka N, Remillard MY, Yothers G, et al. Mutation profiling and microsatellite instability in stage II and III colon cancer: an assessment of their prognostic and oxaliplatin predictive value. Clin Cancer Res. 2012;18:6531–6541. doi: 10.1158/1078-0432.CCR-12-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Platz EA, De Marzo AM, Giovannucci E. Failure to detect prostate cancer in the PSA era: comments on N Engl J Med 2003; 349: 215-224 and N Engl J Med 2003; 349: 335-342. Cancer Causes Control. 2004;15:91–94. doi: 10.1023/B:CACO.0000016580.49577.09. [DOI] [PubMed] [Google Scholar]
- 173.Sinicrope FA, Foster NR, Thibodeau SN, Marsoni S, Monges G, Labianca R, et al. DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J Natl Cancer Inst. 2011;103:863–875. doi: 10.1093/jnci/djr153. Erratum in: J Natl Cancer Inst 2011;103:1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Klingbiel D, Saridaki Z, Roth AD, Bosman FT, Delorenzi M, Tejpar S. Prognosis of stage II and III colon cancer treated with adjuvant 5-fluorouracil or FOLFIRI in relation to microsatellite status: results of the PETACC-3 trial. Ann Oncol. 2015;26:126–132. doi: 10.1093/annonc/mdu499. [DOI] [PubMed] [Google Scholar]
- 175.Phipps AI, Limburg PJ, Baron JA, Burnett-Hartman AN, Weisenberger DJ, Laird PW, et al. Association between molecular subtypes of colorectal cancer and patient survival. Gastroenterology. 2015;148:77–87.e2. doi: 10.1053/j.gastro.2014.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Levine AJ, Phipps AI, Baron JA, Buchanan DD, Ahnen DJ, Cohen SA, et al. Clinicopathologic risk factor distributions for MLH1 promoter region methylation in CIMP-positive tumors. Cancer Epidemiol Biomarkers Prev. 2016;25:68–75. doi: 10.1158/1055-9965.EPI-15-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Arain MA, Sawhney M, Sheikh S, Anway R, Thyagarajan B, Bond JH, et al. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol. 2010;105:1189–1195. doi: 10.1038/ajg.2009.699. [DOI] [PubMed] [Google Scholar]
- 178.Schwitalle Y, Kloor M, Eiermann S, Linnebacher M, Kienle P, Knaebel HP, et al. Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology. 2008;134:988–997. doi: 10.1053/j.gastro.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 179.Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43–51. doi: 10.1158/2159-8290.CD-14-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Liang Y, Cai X, Zheng X, Yin H. Analysis of the clinicopathological characteristics of stage I-III colorectal cancer patients deficient in mismatch repair proteins. Onco Targets Ther. 2021;14:2203–2212. doi: 10.2147/OTT.S278029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bailey SR, Maus MV. Gene editing for immune cell therapies. Nat Biotechnol. 2019;37:1425–1434. doi: 10.1038/s41587-019-0137-8. [DOI] [PubMed] [Google Scholar]
- 183.Sugiura D, Okazaki IM, Maeda TK, Maruhashi T, Shimizu K, Arakaki R, et al. PD-1 agonism by anti-CD80 inhibits T cell activation and alleviates autoimmunity. Nat Immunol. 2022;23:399–410. doi: 10.1038/s41590-021-01125-7. [DOI] [PubMed] [Google Scholar]
- 184.Kamada T, Togashi Y, Tay C, Ha D, Sasaki A, Nakamura Y, et al. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci U S A. 2019;116:9999–10008. doi: 10.1073/pnas.1822001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhang X, Yang Z, An Y, Liu Y, Wei Q, Xu F, et al. Clinical benefits of PD-1/PD-L1 inhibitors in patients with metastatic colorectal cancer: a systematic review and meta-analysis. World J Surg Oncol. 2022;20:93. doi: 10.1186/s12957-022-02549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Huang MY, Jiang XM, Wang BL, Sun Y, Lu JJ. Combination therapy with PD-1/PD-L1 blockade in non-small cell lung cancer: strategies and mechanisms. Pharmacol Ther. 2021;219:107694. doi: 10.1016/j.pharmthera.2020.107694. [DOI] [PubMed] [Google Scholar]
- 188.Bie F, Tian H, Sun N, Zang R, Zhang M, Song P, et al. Research progress of anti-PD-1/PD-L1 immunotherapy related mechanisms and predictive biomarkers in NSCLC. Front Oncol. 2022;12:769124. doi: 10.3389/fonc.2022.769124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fan A, Wang B, Wang X, Nie Y, Fan D, Zhao X, et al. Immunotherapy in colorectal cancer: current achievements and future perspective. Int J Biol Sci. 2021;17:3837–3849. doi: 10.7150/ijbs.64077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:329–359. doi: 10.6004/jnccn.2021.0012. [DOI] [PubMed] [Google Scholar]
- 191.Song CH, Kim N, Nam RH, Choi SI, Jang JY, Kim JW, et al. Combination treatment with 17β-estradiol and anti-PD-L1 suppresses MC38 tumor growth by reducing PD-L1 expression and enhancing M1 macrophage population in MC38 colon tumor model. Cancer Lett. 2022;543:215780. doi: 10.1016/j.canlet.2022.215780. [DOI] [PubMed] [Google Scholar]
- 192.Scott SC, Shao XM, Niknafs N, Balan A, Pereira G, Marrone KA, et al. Sex-specific differences in immunogenomic features of response to immune checkpoint blockade. Front Oncol. 2022;12:945798. doi: 10.3389/fonc.2022.945798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Shi F, Zhang W, Yang Y, Yang Y, Zhao J, Xie M, et al. Sex disparities of genomic determinants in response to immune checkpoint inhibitors in melanoma. Front Immunol. 2021;12:721409. doi: 10.3389/fimmu.2021.721409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.van der Kooij MK, Dekkers OM, Aarts MJB, van den Berkmortel FWPJ, Boers-Sonderen MJ, de Groot JWB, et al. Sex-based differences in treatment with immune checkpoint inhibition and targeted therapy for advanced melanoma: a nationwide cohort study. Cancers (Basel) 2021;13:4639. doi: 10.3390/cancers13184639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dulal S, Keku TO. Gut microbiome and colorectal adenomas. Cancer J. 2014;20:225–231. doi: 10.1097/PPO.0000000000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Brennan CA, Garrett WS. Gut microbiota, inflammation, and colorectal cancer. Annu Rev Microbiol. 2016;70:395–411. doi: 10.1146/annurev-micro-102215-095513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22:299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Geng J, Fan H, Tang X, Zhai H, Zhang Z. Diversified pattern of the human colorectal cancer microbiome. Gut Pathog. 2013;5:2. doi: 10.1186/1757-4749-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yoon K, Kim N. The effect of microbiota on colon carcinogenesis. J Cancer Prev. 2018;23:117–125. doi: 10.15430/JCP.2018.23.3.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Org E, Mehrabian M, Parks BW, Shipkova P, Liu X, Drake TA, et al. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes. 2016;7:313–322. doi: 10.1080/19490976.2016.1203502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Song CH, Kim N, Nam RH, Choi SI, Lee HN, Surh YJ. 17β-Estradiol supplementation changes gut microbiota diversity in intact and colorectal cancer-induced ICR male mice. Sci Rep. 2020;10:12283. doi: 10.1038/s41598-020-69112-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Pedram A, Razandi M, Lewis M, Hammes S, Levin ER. Membrane-localized estrogen receptor α is required for normal organ development and function. Dev Cell. 2014;29:482–490. doi: 10.1016/j.devcel.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ibrahim A, Hugerth LW, Hases L, Saxena A, Seifert M, Thomas Q, et al. Colitis-induced colorectal cancer and intestinal epithelial estrogen receptor beta impact gut microbiota diversity. Int J Cancer. 2019;144:3086–3098. doi: 10.1002/ijc.32037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Song CH, Kim N, Nam RH, Choi SI, Jang JY, Kim EH, et al. Changes in gut microbiome composition according to disease progression, sex, and age in patients with colorectal adenoma and adenocarcinoma. J Korean Cancer Assoc [Google Scholar]
- 205.Ou J, Carbonero F, Zoetendal EG, DeLany JP, Wang M, Newton K, et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr. 2013;98:111–120. doi: 10.3945/ajcn.112.056689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Brownawell AM, Caers W, Gibson GR, Kendall CW, Lewis KD, Ringel Y, et al. Prebiotics and the health benefits of fiber: current regulatory status, future research, and goals. J Nutr. 2012;142:962–974. doi: 10.3945/jn.112.158147. [DOI] [PubMed] [Google Scholar]
- 207.Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol. 2016;7:979. doi: 10.3389/fmicb.2016.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Encarnação JC, Abrantes AM, Pires AS, Botelho MF. Revisit dietary fiber on colorectal cancer: butyrate and its role on prevention and treatment. Cancer Metastasis Rev. 2015;34:465–478. doi: 10.1007/s10555-015-9578-9. [DOI] [PubMed] [Google Scholar]
- 209.Donohoe DR, Collins LB, Wali A, Bigler R, Sun W, Bultman SJ. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol Cell. 2012;48:612–626. doi: 10.1016/j.molcel.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wu X, Wu Y, He L, Wu L, Wang X, Liu Z. Effects of the intestinal microbial metabolite butyrate on the development of colorectal cancer. J Cancer. 2018;9:2510–2517. doi: 10.7150/jca.25324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Humphreys KJ, Conlon MA, Young GP, Topping DL, Hu Y, Winter JM, et al. Dietary manipulation of oncogenic microRNA expression in human rectal mucosa: a randomized trial. Cancer Prev Res (Phila) 2014;7:786–795. doi: 10.1158/1940-6207.CAPR-14-0053. [DOI] [PubMed] [Google Scholar]
- 212.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. Erratum in: Nature 2014;506:254. [DOI] [PubMed] [Google Scholar]
- 213.Conlon MA, Bird AR, Clarke JM, Le Leu RK, Christophersen CT, Lockett TJ, et al. Lowering of large bowel butyrate levels in healthy populations is unlikely to be beneficial. J Nutr. 2015;145:1030–1031. doi: 10.3945/jn.114.209460. [DOI] [PubMed] [Google Scholar]
- 214.Choi SI, Son JH, Kim N, Kim YS, Nam RH, Park JH, et al. Changes in cecal microbiota and short-chain fatty acid during lifespan of the rat. J Neurogastroenterol Motil. 2021;27:134–146. doi: 10.5056/jnm20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zeng H, Lazarova DL, Bordonaro M. Mechanisms linking dietary fiber, gut microbiota and colon cancer prevention. World J Gastrointest Oncol. 2014;6:41–51. doi: 10.4251/wjgo.v6.i2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kim DH, Jin YH. Intestinal bacterial beta-glucuronidase activity of patients with colon cancer. Arch Pharm Res. 2001;24:564–567. doi: 10.1007/BF02975166. [DOI] [PubMed] [Google Scholar]
- 217.Kulkarni N, Reddy BS. Inhibitory effect of Bifidobacterium longum cultures on the azoxymethane-induced aberrant crypt foci formation and fecal bacterial beta-glucuronidase. Proc Soc Exp Biol Med. 1994;207:278–283. doi: 10.3181/00379727-207-43817. [DOI] [PubMed] [Google Scholar]
- 218.Rowland IR, Granli T, Bøckman OC, Key PE, Massey RC. Endogenous N-nitrosation in man assessed by measurement of apparent total N-nitroso compounds in faeces. Carcinogenesis. 1991;12:1395–1401. doi: 10.1093/carcin/12.8.1395. [DOI] [PubMed] [Google Scholar]
- 219.Kobayashi J. Effect of diet and gut environment on the gastrointestinal formation of N-nitroso compounds: a review. Nitric Oxide. 2018;73:66–73. doi: 10.1016/j.niox.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 220.IJssennagger N, Derrien M, van Doorn GM, Rijnierse A, van den Bogert B, Müller M, et al. Dietary heme alters microbiota and mucosa of mouse colon without functional changes in host-microbe cross-talk. PLoS One. 2012;7:e49868. doi: 10.1371/journal.pone.0049868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ferrario C, Taverniti V, Milani C, Fiore W, Laureati M, De Noni I, et al. Modulation of fecal Clostridiales bacteria and butyrate by probiotic intervention with Lactobacillus paracasei DG varies among healthy adults. J Nutr. 2014;144:1787–1796. doi: 10.3945/jn.114.197723. Erratum in: J Nutr 2015;145:839. [DOI] [PubMed] [Google Scholar]
- 222.Gamage SMK, Dissabandara L, Lam AK, Gopalan V. The role of heme iron molecules derived from red and processed meat in the pathogenesis of colorectal carcinoma. Crit Rev Oncol Hematol. 2018;126:121–128. doi: 10.1016/j.critrevonc.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 223.Ijssennagger N, Belzer C, Hooiveld GJ, Dekker J, van Mil SW, Müller M, et al. Gut microbiota facilitates dietary heme-induced epithelial hyperproliferation by opening the mucus barrier in colon. Proc Natl Acad Sci U S A. 2015;112:10038–10043. doi: 10.1073/pnas.1507645112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Constante M, Fragoso G, Calvé A, Samba-Mondonga M, Santos MM. Dietary heme induces gut dysbiosis, aggravates colitis, and potentiates the development of adenomas in mice. Front Microbiol. 2017;8:1809. doi: 10.3389/fmicb.2017.01809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Barrasa JI, Olmo N, Lizarbe MA, Turnay J. Bile acids in the colon, from healthy to cytotoxic molecules. Toxicol In Vitro. 2013;27:964–977. doi: 10.1016/j.tiv.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 227.Cercek A, Siegel CL, Capanu M, Reidy-Lagunes D, Saltz LB. Incidence of chemotherapy-induced amenorrhea in premenopausal women treated with adjuvant FOLFOX for colorectal cancer. Clin Colorectal Cancer. 2013;12:163–167. doi: 10.1016/j.clcc.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 228.Warren RS, Atreya CE, Niedzwiecki D, Weinberg VK, Donner DB, Mayer RJ, et al. Association of TP53 mutational status and gender with survival after adjuvant treatment for stage III colon cancer: results of CALGB 89803. Clin Cancer Res. 2013;19:5777–5787. doi: 10.1158/1078-0432.CCR-13-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ning Y, Gerger A, Zhang W, Hanna DL, Yang D, Winder T, et al. Plastin polymorphisms predict gender- and stage-specific colon cancer recurrence after adjuvant chemotherapy. Mol Cancer Ther. 2014;13:528–539. doi: 10.1158/1535-7163.MCT-13-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Oliver JS, Martin MY, Richardson L, Kim Y, Pisu M. Gender differences in colon cancer treatment. J Womens Health (Larchmt) 2013;22:344–351. doi: 10.1089/jwh.2012.3988. [DOI] [PubMed] [Google Scholar]
- 231.Sloan JA, Goldberg RM, Sargent DJ, Vargas-Chanes D, Nair S, Cha SS, et al. Women experience greater toxicity with fluorouracil-based chemotherapy for colorectal cancer. J Clin Oncol. 2002;20:1491–1498. doi: 10.1200/JCO.2002.20.6.1491. [DOI] [PubMed] [Google Scholar]
- 232.van der Geest LG, Portielje JE, Wouters MW, Weijl NI, Tanis BC, Tollenaar RA, et al. All Nine Hospitals in the Leiden Region of the Comprehensive Cancer Centre The Netherlands. Complicated postoperative recovery increases omission, delay and discontinuation of adjuvant chemotherapy in patients with Stage III colon cancer. Colorectal Dis. 2013;15:e582–e591. doi: 10.1111/codi.12288. [DOI] [PubMed] [Google Scholar]
- 233.Paulson EC, Wirtalla C, Armstrong K, Mahmoud NN. Gender influences treatment and survival in colorectal cancer surgery. Dis Colon Rectum. 2009;52:1982–1991. doi: 10.1007/DCR.0b013e3181beb42a. [DOI] [PubMed] [Google Scholar]
- 234.Dobie SA, Baldwin LM, Dominitz JA, Matthews B, Billingsley K, Barlow W. Completion of therapy by Medicare patients with stage III colon cancer. J Natl Cancer Inst. 2006;98:610–619. doi: 10.1093/jnci/djj159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Friedemann-Sánchez G, Griffin JM, Partin MR. Gender differences in colorectal cancer screening barriers and information needs. Health Expect. 2007;10:148–160. doi: 10.1111/j.1369-7625.2006.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ritvo P, Myers RE, Paszat L, Serenity M, Perez DF, Rabeneck L. Gender differences in attitudes impeding colorectal cancer screening. BMC Public Health. 2013;13:500. doi: 10.1186/1471-2458-13-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hwang YJ, Shin DW, Kim N, Yoon H, Shin CM, Park YS, et al. Sex difference in bowel preparation quality and colonoscopy time. Korean J Intern Med. 2021;36:322–331. doi: 10.3904/kjim.2019.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Gandhi K, Tofani C, Sokach C, Patel D, Kastenberg D, Daskalakis C. Patient characteristics associated with quality of colonoscopy preparation: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16:357–369.e10. doi: 10.1016/j.cgh.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 239.Khashab MA, Pickhardt PJ, Kim DH, Rex DK. Colorectal anatomy in adults at computed tomography colonography: normal distribution and the effect of age, sex, and body mass index. Endoscopy. 2009;41:674–678. doi: 10.1055/s-0029-1214899. [DOI] [PubMed] [Google Scholar]
- 240.Ness RM, Manam R, Hoen H, Chalasani N. Predictors of inadequate bowel preparation for colonoscopy. Am J Gastroenterol. 2001;96:1797–1802. doi: 10.1111/j.1572-0241.2001.03874.x. [DOI] [PubMed] [Google Scholar]
- 241.Rotondano G, Rispo A, Bottiglieri ME, De Luca L, Lamanda R, Orsini L, et al. Quality of bowel cleansing in hospitalized patients undergoing colonoscopy: a multicentre prospective regional study. Dig Liver Dis. 2015;47:669–674. doi: 10.1016/j.dld.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 242.Akere A, Otegbayo JA. Complete colonoscopy: impact of patients' demographics and anthropometry on caecal intubation time. BMJ Open Gastroenterol. 2016;3:e000076. doi: 10.1136/bmjgast-2016-000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Chung JI, Kim N, Um MS, Kang KP, Lee D, Na JC, et al. Learning curves for colonoscopy: a prospective evaluation of gastroenterology fellows at a single center. Gut Liver. 2010;4:31–35. doi: 10.5009/gnl.2010.4.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]