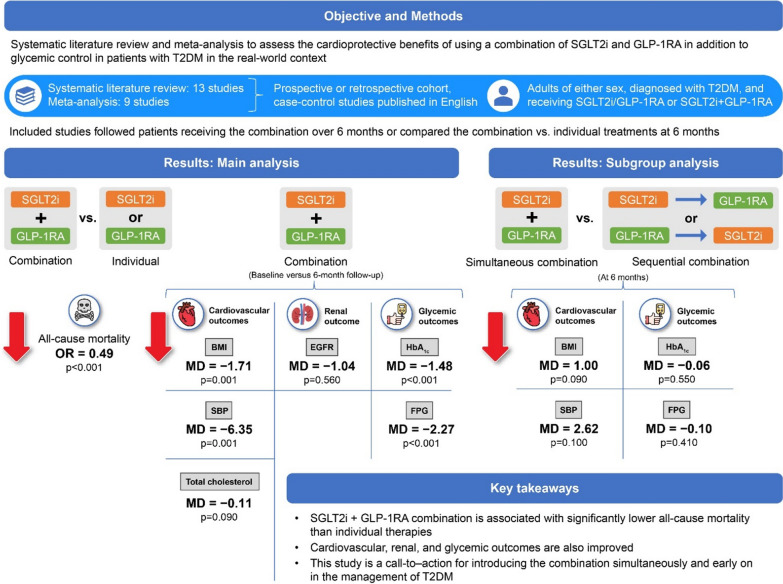
Abstract
Background
Randomized controlled trials and real-world studies suggest that combination therapy with sodium–glucose transport protein 2 inhibitors (SGLT2is) and glucagon-like peptide-1 receptor agonists (GLP-1RAs) is associated with improvement in fasting plasma glucose (FPG), glycated hemoglobin (HbA1c), systolic blood pressure (SBP), body mass index (BMI), and total cholesterol levels. However, a systematic review of available real-world evidence may facilitate clinical decision-making in the real-world scenario. This meta-analysis assessed the safety and effectiveness of combinations of SGLT2is + GLP-1RAs with a focus on their cardioprotective effects along with glucose-lowering ability in patients with type 2 diabetes mellitus (T2DM) in a real-world setting.
Methods
Electronic searches were performed in the PubMed/MEDLINE, PROQuest, Scopus, CINAHL, and Google Scholar databases. Qualitative analyses and meta-analyses were performed using the Joanna Briggs Institute SUMARI software package and Review Manager v5.4, respectively.
Results
The initial database search yielded 1445 articles; of these, 13 were included in this study. The analyses indicated that SGLT2is + GLP-1RAs combinations were associated with significantly lower all-cause mortality when compared with individual therapies (odds ratio [95% confidence interval [CI] 0.49 [0.41, 0.60]; p < 0.00001). Significant reductions in BMI (− 1.71 [− 2.74, − 0.67]; p = 0.001), SBP (− 6.35 [− 10.17, − 2.53]; p = 0.001), HbA1c levels (− 1.48 [− 1.75, − 1.21]; p < 0.00001), and FPG (− 2.27 [− 2.78, − 1.76]; p < 0.00001) were associated with the simultaneous administration of the combination. Changes in total cholesterol levels and differences between simultaneous and sequential combination therapies for this outcome were not significant.
Conclusion
This systematic review and meta-analysis based on real-world data suggests that the combination of SGLT2is + GLP-1RAs is associated with lower all-cause mortality and favorable improvements in cardiovascular, renal, and glycemic measurements. The findings drive a call-to–action to incorporate this combination early and simultaneously in managing T2DM patients and achieve potential cardiovascular benefits and renal protection.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-024-02192-4.
Keywords: Diabetes mellitus, Sodium–glucose transport protein 2 inhibitor, Glucagon-like peptide-1 receptor agonist, Cardiovascular, Meta-analysis, Observational studies
Introduction
Diabetes is a significant predisposing factor for microvascular and macrovascular complications with cardiovascular events 2–3 times more likely to occur in patients with diabetes than in those without diabetes [1]. Conventionally, the management of type 2 diabetes mellitus (T2DM) has been glucocentric rather than focusing on reducing cardiovascular events [2]. However, over the last few years, sodium–glucose transport protein 2 inhibitors (SGLT2is) and glucagon-like peptide-1 receptor agonists (GLP-1RAs) have been shown to reduce the risk of all-cause mortality, cardiovascular mortality, and kidney failure [3]. Recent meta-analyses of the major SGLT2i cardiovascular outcome trials (CVOTs) reported a reduced risk of all-cause mortality and major adverse cardiovascular events (MACE) in T2DM patients using SGLT2i [4, 5]. Similarly, recent meta-analyses of GLP-1 CVOTs reported a reduction in MACE, relative risk of CV deaths, and all-cause mortality [6, 7]. Like SGLT2is [8, 9], the CV effects of GLP-1RAs are reported to be independent of glucose reduction as shown in the multicenter, double-blind, placebo-controlled SELECT study (N = 17,604), in which the GLP-1RA semaglutide significantly reduced the incidence of cardiovascular mortality and that of non-fatal myocardial infarction as well as non-fatal stroke when compared to the placebo (6.5% vs. 8.0%, p < 0.001) in non-diabetes patients with preexisting cardiovascular disease and obesity [10]. While the exact mechanism of action of these two classes of drugs on reducing mortality is still being investigated, several meta-analyses have shown significantly reduced glycated hemoglobin (HbA1c), body weight, body mass index (BMI), systolic blood pressure (SBP), and low-density lipoprotein cholesterol (LDL-C) when either of these drugs was used individually. However, the reduction in these parameters was more significant when the two classes were used in combination with minimal safety concerns resulting in clinical guidelines recommending their use in T2DM patients with established atherosclerotic cardiovascular disease (ASCVD) or with multiple CV risk factors without ASCVD irrespective of the HbA1c level or use of other glucose-lowering medications [7, 11–16]. There has been no direct evidence regarding the effects of the combination of SGLT2is and GLP-1RAs on mortality and other cardiovascular outcomes because of a lack of randomized controlled trials (RCTs) comparing combination versus individual therapies. However, a retrospective study and a subsequent meta-analysis reported significantly reduced risks of MACE, cardiovascular mortality, hypertensive heart failure, and all-cause mortality compared with SGLT2i/GLP1RA monotherapy [17, 18].
To date, there has been no meta-analysis conducted to assess the effect of SGLT2i and GLP-1 combination therapy in the real-world context where results can differ significantly from results from randomized controlled trials (RCTs). Our meta-analysis of real-world data is focused on the impact of such a combination on all-cause mortality and the management of T2DM patients.
Review question
What is the effectiveness and safety of combinations of SGLT2is + GLP-1RAs in the management of T2DM among adults?
Methods
This systematic literature review (SLR) was conducted as per the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) reporting guidelines. The protocol was registered with the International Prospective Register of Systematic Reviews PROSPERO (registration number: CRD42023434707).
Inclusion criteria
This SLR considered observational real-world studies (prospective cohort, retrospective cohort, and case–control studies published in English) that evaluated the effectiveness and safety of combination therapies of SGLT2is and GLP-1RAs in the management of T2DM in adults (≥ 18 years), irrespective of sex, race, ethnicity, or nationality. Systematic reviews, clinical trials, conference abstracts, case series, and case reports were excluded from the analysis. The classification of patients as having T2DM and selection for treatment were determined by the authors of each study included in this SLR. All included studies either followed patients receiving the combination treatment and compared baseline values with those at the end of the follow-up (at least 6 months) or compared the combination treatment against individual treatments at the 6-month follow-up.
Search strategy
A broad search of MEDLINE (PubMed) was initially undertaken to identify related articles. The index terms derived were then used to develop the search strategy for the PubMed, PROQuest, Scopus, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Google Scholar (first 100 articles) databases (Additional file 1: Table S1). The search was performed from inception until May 2023. Citation screening using backward and forward citations of included studies was additionally performed. Only those studies published in English were included; no restrictions on publication dates were set on any search.
Selection of studies
All the citations identified after the search were collated on SR-Accelerator [19], and duplicates were removed. The screening of titles along with abstracts was carried out by two independent authors (AA and HS) as per the review inclusion criteria. Subsequently, when the full texts were screened, articles were closely analyzed for compliance with the inclusion and exclusion criteria in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram. Additionally, a literature mapping was performed using Litmaps® to indicate the relationship among included articles using their citations.
The included studies were assessed for quality using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Cohort Studies [20]. Each study was critically appraised by two independent reviewers (AA and HS) based on criteria such as the similarity of study groups, reliability and validity of exposure measurement, identification and handling of confounding factors, freedom from the outcome (at the start of the study), outcome measurement, follow-up time, completeness of follow-up, and appropriateness of statistical analyses. All disagreements regarding appraisals were resolved through discussions. Details are provided in Additional file 2.
All the studies included in this SLR underwent data extraction by two independent authors (AA and HS) with piloted data extraction sheets. Details of the data extraction process are presented in Additional file 2.
Study outcomes
The outcomes of interest were all-cause mortality, cardiovascular risk factors (BMI, SBP, and total cholesterol), renal outcomes (eGFR and albuminuria), and glycemic outcomes (HbA1c and FPG). Adverse events were also analyzed qualitatively. The term ‘baseline” represented the time when the patients initiated treatment with either the combination or individual treatments, and this definition was consistent across the included studies.
Subgroup analysis
A subgroup analysis was performed based on whether the patients received the combination simultaneously and had not received either SGLT2is or GLP-1RAs prior to baseline and whether the patients received the combination sequentially, i.e., they were already receiving either SGLT2i or GLP-1RA and the other drug was introduced. The two sequential combination therapy subgroups included patients who were receiving SGLT2i with GLP-1RA added on and patients who were receiving GLP-1RA with SGLT2i added on.
Synthesis of data
All the extracted data were pooled with the help of a statistical meta-analysis model in the Review Manager v5.4 software (RevMan, Cochrane Collaboration software). For continuous data, effect sizes were presented as weighted (or standardized) final mean differences and their 95% confidence intervals (CIs); for dichotomous data, these were presented as odds ratios and 95% CIs. The I2 statistic for heterogeneity among the included studies was calculated, wherein I2 values of < 30%, 30%–59%, 60%–90%, and > 90% corresponded to low, moderate, substantial, and high heterogeneity, respectively. All analyses were carried out using a random-effects model.
If statistical pooling was not possible, the findings were presented as a narrative, considering the population characteristics, study design, data source, and assessment of the outcome measure.
Assessing certainty in the findings
Certainties in the quality of the evidence and the estimated effects of the results in this SLR were assessed as per the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) [21]. The findings were summarized using the GRADEPro GDT v.4 software (McMaster University, ON, Canada), and include all the results on all-cause mortality and changes in HbA1c, FPG, BMI, SBP, GFR, and total cholesterol levels.
Results
Search results
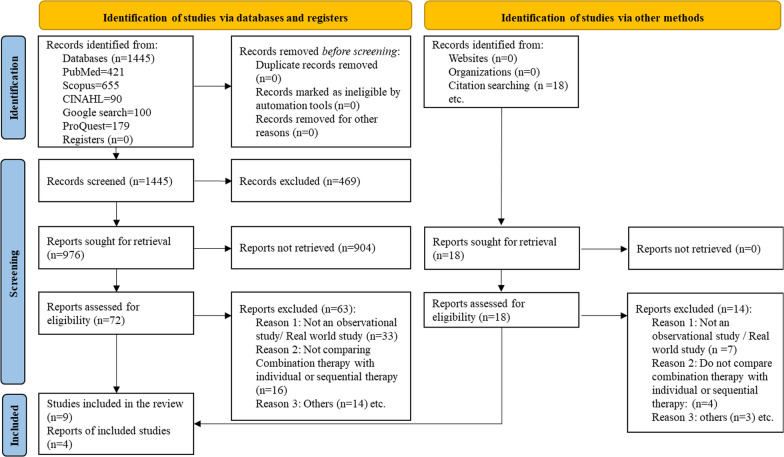
Of the 1445 articles obtained in the initial database search, 976 were identified based on their titles and abstracts. Manual screening of grey literature resulted in an addition of 18 articles. The full texts of 90 articles were retrieved, 77 studies were excluded (Additional file 1: Table S2), and 13 were included in the study [22–34]. Figure 1 shows the PRISMA flowchart.
Fig. 1.
PRISMA flowchart
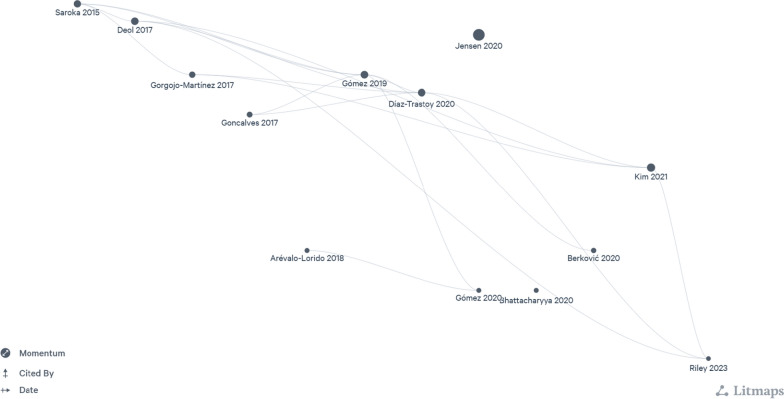
Thirteen studies were critically appraised; following this, data were extracted from these studies and analyzed. The literature mapping of the studies included in this SLR is shown in Fig. 2. Quantitative meta-analysis was performed using 8 studies [23, 26–31, 33]. The remaining 5 studies were considered to be ineligible for inclusion in this meta-analysis as mean values were not available (n = 1), follow-up duration was less than 6 months (n = 2), and only differences in outcomes were provided instead of absolute values (n = 2) (Additional file 1: Table S3).
Fig. 2.
Literature mapping of the included studies (Litmaps®)
Each point on the map represents one included study in the systematic literature review. The size of each point is a function of the “momentum” of the study, which is calculated based on the “cited by” count and weighted by recency. The most recent articles appear on the right-hand side of the map and the most cited articles appear at the top, so recent popular studies appear in the top right-hand corner.
Ten studies were assessed to be of moderate-to–low quality (score 6–8), and three studies were of low quality (score ≤5) (Table 1). Low-quality studies typically had issues in their methodology, such as unclear or inadequate management of confounding factors, unreliable or invalid outcome measurements, incomplete follow-up without adequate exploration of reasons, and inappropriate or unclear use of statistical analysis. These deficiencies suggest potential biases in the studies, which could affect the trustworthiness of their findings and limit their contributions to evidence-based practice.
Table 1.
Critical appraisal for methodological quality
| Author name and year | Were the two groups similar and recruited from the same population? | Were the exposures measured similarly to assign people to both exposed and unexposed groups? | Was the exposure measured in a valid and reliable way? | Were confounding factors identified? | Were strategies to deal with confounding factors stated? | Were the groups/participants free of the outcome at the start of the study (or at the moment of exposure)? | Were the outcomes measured in a valid and reliable way? | Was the follow-up time reported and sufficient to be long enough for outcomes to occur? | Was follow-up complete, and if not, were the reasons for loss to follow-up described and explored? | Were strategies to address incomplete follow-up utilized? | Was appropriate statistical analysis used? | Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Berkovic et al. 2020 | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✓ | ✓ | N/A | ✓ | 9 |
| Gorgojo-Martínez et al. 2017 | ✓ | ✓ | ✓ | ✗ | ? | N/A | ✓ | ✓ | ✓ | N/A | ✓ | 7 |
| Kim et al. 2022 | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✓ | ✓ | N/A | ✓ | 9 |
| Deol et al. 2017 | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✓ | ✓ | N/A | ✓ | 9 |
| Carretero-Gómez et al. 2019 | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ✓ | ✓ | N/A | ? | 8 |
| Arevalo et al. 2018 | ? | ✓ | ✓ | ✗ | ✗ | N/A | ✓ | ✓ | ✓ | N/A | ? | 5 |
| Diaz et al. 2020 | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | ? | ? | N/A | ? | 6 |
| Carretero-Gómez et al. 2020 | ✓ | ✓ | ✓ | ? | ? | N/A | ✓ | ✓ | ✓ | N/A | ✓ | 7 |
| Riley et al. 2023 | ✓ | ✓ | ✓ | ✓ | ? | N/A | ✓ | ✗ | ✗ | N/A | ? | 5 |
| Goncalves et al. 2017 | ✓ | ✓ | ? | ✓ | ✗ | N/A | ? | ✓ | ✓ | N/A | ✗ | 5 |
| Bhattacharyya et al. 2020 | ✓ | ✓ | ✓ | ✓ | ✗ | N/A | ✓ | ✓ | ✓ | N/A | ✗ | 7 |
| Saroka et al. 2015 | ✓ | ✓ | ? | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | 8 |
| Jensen et al. 2020 | ? | ✗ | ✓ | ✓ | ✓ | N/A | ✓ | ✓ | ✓ | N/A | ✓ | 7 |
✓: Yes; ✗: No; ?: Unclear; N/A: Not applicable
Given that meta-analysis and subgroup analyses included a relatively small number of studies, inadequate data were available to provide a statistical estimate of publication bias.
Qualitative analysis (systematic review)
All the studies included in this SLR are real-world observational studies published during 2015–2023, had sample sizes ranging from 15 to 2.2 million, and a follow-up ranging from 3 months to 20 years (Table 2). Patients in the included studies were 49.5–70.4 years old, and 43.6%–65.5% were males (Table 3). Prior comorbidities included cardiovascular disease, hypertension, hyperlipidemia, and obesity. Patients in the included studies reported the concomitant use of other antidiabetic drugs such as metformin, sulfonylureas, and insulin, among others (Table 4).
Table 2.
Characteristics of the included studies
| Study | Country | Participant count | Cohorts, n | Follow-up time point (s) | Study design | Included in the meta-analysis (Y/N) |
|---|---|---|---|---|---|---|
| Arévalo et al. [22] | Spain | 17 |
Simultaneous SGLT2i + GLP-1RA (n = 2) Sequential GLP-1RA + SGLT2i add-on (n = 10) Sequential SGLT2i + GLP-1RA add-on (n = 5) |
6 months | Multicenter, prospective cohort study | N |
| Berkovic et al. [23] | Croatia | 200 |
Simultaneous SGLT2i + GLP-1RA (n = 76) Sequential GLP-1RA + SGLT2i add-on (n = 76) Sequential SGLT2i + GLP-1RA add-on (n = 48) |
6 months 12 months |
Retrospective and prospective, multicenter, observational cohort study | Y |
| Bhattacharyya et al. [24] | India | 15 | GLP-1RA (dulaglutide) + SGLT2i + metformin with or without insulin | 3 months | Retrospective, single-center, observational study | N |
| Carretero Gómez et al. [27] | Brazil | 113 |
Simultaneous SGLT2i + GLP-1RA (n = 30) Sequential GLP-1RA + SGLT2i add-on (n = 59) Sequential SGLT2i + GLP-1RA add-on (n = 24) |
3 months 6 months |
Prospective, observational, multicenter cohort study | Y |
| Carretero Gómez et al. [28] | Brazil | 178 |
Simultaneous SGLT2i + GLP-1RA (n = 52) Sequential GLP-1RA + SGLT2i add-on (n = 76) Sequential SGLT2i + GLP-1RA add-on (n = 50) |
6 months | Prospective, observational, multicenter cohort study | Y |
| Deol et al. [25] | UK | 79 | Sequential GLP-1RA + SGLT2i add-on (n = 79) | 3–6 months | Retrospective, single-center, observational cohort study | N |
| Díaz-Trastoy et al. [26] | Spain | 212 |
Simultaneous SGLT2i + GLP-1RA (n = 38) Sequential GLP-1RA + SGLT2i add-on (n = 85) Sequential SGLT2i + GLP-1RA add-on (n = 89) |
5.5 months 16.4 months |
Retrospective, single-center, observational cohort study | Y |
| Goncalves et al. [29] | USA | 79 |
Simultaneous SGLT2i + GLP-1RA (n = 33) Sequential GLP-1RA + SGLT2i add-on (n = 46) |
Simultaneous SGLT2i + GLP-1RA: 14 months Sequential GLP-1RA + SGLT2i add-on: 18 months |
Retrospective, single-center, observational cohort study | Y |
| Gorgojo-Martínez et al. [32] | Spain | 213 |
Sequential GLP-1RA + SGLT2i (dapagliflozin) add-on (n = 109) SGLT2i (dapagliflozin) (n = 103) |
6 months 12 months |
Retrospective, single-center, observational cohort study | N |
| Jensen et al. [30] | Denmark | 66,807 |
SGLT2i (n = 3405) GLP-1RA (n = 11,436) SGLT2i + GLP-1RA (n = 1823) |
20 years | Time-to–event cohort study based on the Danish National Registry | Y |
| Kim et al. [31] | Korea | 104 | SGLT2i + GLP-1RA (n = 104) |
6 months 12 months |
Retrospective, single-center, observational cohort study | Y |
| Riley et al. [33] | Global | 2.2 million |
SGLT2i (n = 1,43,600) GLP-1RA (n = 1,86,841) SGLT2i + GLP-1RA (n = 1,08,504) |
5 years | Retrospective, observational cohort study based on inpatient and outpatient electronic medical records from TriNetX | Y |
| Saroka et al. [34] | USA | 75 | Sequential GLP-1RA + SGLT2i (canagliflozin) add-on (n = 75) | 40 months | Retrospective, pre-post, single-center, observational cohort study | N |
GLP-1RA Glucagon-like peptide-1 receptor agonist, SGLT2i Sodium–glucose transport protein 2 inhibitor, UK United Kingdom, USA United States of America
Table 3.
Characteristics of participants in the included studies
| Study | Participant count | Males, % | Participant age, years, mean ± SD | Duration of diabetes, years | Baseline HbA1c, mean ± SD, % | Baseline SBP, mean ± SD, mmHg | Baseline BMI, mean ± SD, kg/m2 | Comorbidities |
|---|---|---|---|---|---|---|---|---|
| Arévalo et al. [22] | 17 | 52.9 | 66.8 ± 8.3 (median) | N/A | 8.1 ± 0.8 | 124 ± 11 | 30.8 ± 3.8 | N/A |
| Berkovic et al. [23] | 200 | N/A | 62.1 ± 9.5 | 11.7 ± 6.2 | 8.32 ± 1.26 | N/A | 39.41 ± 5.49 | N/A |
| Bhattacharyya et al. [24] | 15 | 60.0 | 49.5 ± 9.3 | 7.6 ± 1.2 | 8.61 ± 1.41 | 129.87 ± 4.81 | 32.27 ± 4.67 | N/A |
| Carretero Gómez et al. [27] | 113 | 65.5 | 70.4 ± 8.8 | N/A | 8.04 ± 1.2 | 136.1 ± 17 | 36.5 ± 6.6 | Hypertension (69.9%), dyslipidemia (82.3%), coronary artery disease (19.5%), heart failure (9.73%) |
| Carretero Gómez et al. [28] | 178 | 58.6 | 61.9 ± 10.0 | 10.0 ± 6.7 | 8.2 ± 0.9 | 138.3 ± 16.9 | 36.2 ± 10 | Hypertension (80.9%), dyslipidemia (81.4%), coronary artery disease (7.9%) |
| Deol et al. [25] | 79 | 51.1 | 57.4 ± 7.8 | 13.1 ± 7.2 | 8.8 ± 1.47 | 134 ± 16 | 38.4 ± 6.3 | N/A |
| Díaz-Trastoy et al. [26] | 212 | 47.6 | 61.5 ± 9.6 | 12.3 ± 7.0 | 8.4 ± 1.2 | 137.4 ± 17.9 | 37.7 ± 8.1 | Hypertension (51.1%), retinopathy (14.6%), nephropathy (10.4%), peripheral neuropathy (3.3%), autonomic neuropathy (1.9%), cardiovascular disease (11.8%), cerebrovascular disease (1.4%), heart failure (0.5%), pulmonary edema (0.9%) |
| Goncalves et al. [29] | 79 |
Sequential GLP-1RA + SGLT2i add-on: 50.0 Simultaneous SGLT2i + GLP-1RA: 48.0 |
Sequential GLP-1RA + SGLT2i add-on: Median 60.5 ± 7.1 Simultaneous SGLT2i + GLP-1RA: Median 51.0 ± 10.0 |
Simultaneous SGLT2i + GLP-1RA: 9.3 ± 6.0 Sequential GLP-1RA + SGLT2i add-on: 11.0 ± 6.0 |
Sequential GLP-1RA + SGLT2i add-on: 8.9 ± 1.3 Simultaneous SGLT2i + GLP-1RA: 9.1 ± 1.4 |
Sequential GLP-1RA + SGLT2i add-on: 133 ± 17 Simultaneous SGLT2i + GLP-1RA: 135 ± 16 |
Sequential GLP-1RA + SGLT2i add-on: 37.2 ± 5.2 Simultaneous SGLT2i + GLP-1RA: 39.2 ± 8.2 |
N/A |
| Gorgojo-Martínez et al. [32] | 213 |
Sequential GLP-1RA + SGLT2i (dapagliflozin) add-on: 59.6 SGLT2i (dapagliflozin): 47.1 |
Sequential GLP-1RA + SGLT2i (dapagliflozin) add-on: 59.1 ± 10.7 SGLT2i (dapagliflozin): 59.7 ± 10.8 |
Sequential GLP-1RA + SGLT2i (dapagliflozin) add-on: 12.4 SGLT2i (dapagliflozin): 9.1 |
Sequential GLP-1RA + SGLT2i (dapagliflozin) add-on: 7.4 ± 1.3 SGLT2i (dapagliflozin): 7.3 ± 1.3 |
Sequential GLP-1RA + SGLT2i (dapagliflozin) add-on: 139.5 ± 15.5 SGLT2i (dapagliflozin): 139.3 ± 13.5 |
Sequential GLP-1RA + SGLT2i (dapagliflozin) add-on: 35.0 ± 4.2 SGLT2i (dapagliflozin): 35.9 ± 8.2 |
Sequential GLP-1RA + SGLT2i (dapagliflozin) add-on: Hypertension (82.6%), hypercholesterolemia (93.6%), hypertriglyceridemia (63.3%), current smoker (17.4%), diabetic retinopathy (15.6%), diabetic renal disease (29.4%), diabetic neuropathy (11.1%), coronary artery disease (13.8%), stroke (5.5%), peripheral artery disease (12.8%) |
| Jensen et al. [30] | 66,807 |
SGLT2i: 63.8 GLP-1RA: 52.1 SGLT2i + GLP-1RA: 64.8 |
SGLT2i: 59.0 ± 12.0 GLP-1RA: 58.0 ± 12.0 SGLT2i + GLP-1RA: 57.0 ± 11.0 |
SGLT2i: 6.0 ± 5.0 GLP-1RA: 7.0 ± 5.0 SGLT2i + GLP-1RA: 9.0 ± 5.0 |
N/A | |||
| Kim et al. [31] | 104 | 48.7 | 51.1 ± 10.6 | N/A | 9.02 ± 1.39 | 132.78 ± 16.49 | 28.78 ± 4.28 | N/A |
| Riley et al. [33] | 2.2 million |
SGLT2i: 59.6 GLP-1RA: 43.6 SGLT2i + GLP-1RA: 50.9 |
SGLT2i: 62.8 ± 12.2 GLP-1RA: 58.7 ± 13.0 SGLT2i + GLP-1RA: 58.7 ± 11.5 |
N/A | N/A | |||
| Saroka et al. [34] | 75 | 56.0 | 58.0 ± 8.6 | 13.8 ± 6.4 | 7.94 ± 0.69 | 121 ± 11 | 39.4 ± 9.4 | Hypertension (90.7%), dyslipidemia (94.7%), microvascular disease (32.0%), macrovascular disease (22.7%) |
BMI Body mass index, GLP-1RA glucagon-like peptide-1 receptor agonist, HbA1c glycated hemoglobin, N/A not available, SBP systolic blood pressure, SD standard deviation, SGLT2i sodium–glucose transport protein 2 inhibitor
Table 4.
Antidiabetic drugs used by patients in the included studies
| Study | Number of patients (N) | SGLT2i, % | GLP-1RA, % | Other concurrent antidiabetic medications, % |
|---|---|---|---|---|
| Arévalo et al. [22] | 17 |
Dapagliflozin: 47.1% Canagliflozin: 29.4% Empagliflozin: 23.5% |
Liraglutide: 82.3% Dulaglutide: 11.8% Albiglutide: 5.9% |
N/A |
| Berkovic et al. [23] | 200 |
SGLT2i Insulin: 32.4% Metformin: 94.1% SU: 11.8% Other: 8.8% GLP-1RA Insulin: 45.2% Metformin: 85.5% SU: 19.4% Other: 16.1% SGLT2i + GLP-1A Insulin: 26.2% Metformin: 78.6% SU: 14.3% Other: 19.0% |
||
| Bhattacharyya et al. [24] | 15 | Canagliflozin, empagliflozin, or dapagliflozin: 100% | Dulaglutide: 100% |
Sitagliptin/vildagliptin/linagliptin + glimepiride: 100% Insulin (8.35 ± 0.45 U): 26.7% |
| Carretero Gómez et al. [27] | 113 |
Canagliflozin: 58.4% Dapagliflozin: 26.5% Empagliflozin: 15.04% |
Liraglutide: 52.2% Dulaglutide: 29.2% Exenatide LAR: 8.84% Lixisenatide: 5.31% Albiglutide: 4.42% |
Insulin (39.4 ± 19.5 U): 46% |
| Carretero Gómez et al. [28] | 178 |
Canagliflozin: 46.6% Dapagliflozin: 29.8% Empagliflozin: 23.6% |
Liraglutide: 52.2% Dulaglutide: 33.1% Exenatide LAR: 7.3% Lixisenatide: 4.5% Albiglutide: 2.8% |
N/A |
| Deol et al. [25] | 79 |
Dapagliflozin: 97.3% Canagliflozin: 2.7% |
Insulin (89.0 ± 51.0 U): 62.2% Metformin: 78.4% |
|
| Díaz-Trastoy et al. [26] | 212 |
Dapagliflozin: 45.8% Empagliflozin: 35.8% Canagliflozin: 18.4% |
Dulaglutide: 52.4% Liraglutide: 26.9% Exenatide LAR: 17% Lixisenatide: 3.8% |
Metformin: 86.8% Sulfonylureas: 10.8% DPP-4 inhibitors: 1.9% Pioglitazone: 0.9% Repaglinide: 0.9% Insulin (59.3 ± 38.7 U): 41% |
| Goncalves et al. [29] | 79 |
Canagliflozin: 75.0% Empagliflozin: 35.0% |
Liraglutide: 66% | N/A |
| Gorgojo-Martínez et al. [32] | 213 | Dapagliflozin: 100% |
Liraglutide: 72.5% Exenatide (once weekly): 20.2% Exenatide (twice daily): 2.8% Lixisenatide: 4.6% |
Sequential GLP-1RA + SGLT2i add-on Metformin: 96.3% SU: 13.8% Glitazones: 7.3% Insulin (56.6 ± 41.6 U): 48.6% SGLT2i Metformin: 83.7% SU: 19.2% DPP-4 inhibitors: 41.3% Insulin (45.3 ± 30.5 U): 35.6% |
| Jensen et al. [30] | 66,807 |
Dapagliflozin: 49.7% Canagliflozin: 3.4% Empagliflozin: 46.9% |
Liraglutide: 96.2% Exenatide: 2.2% Lixisenatide: 0.1% Dulaglutide: 1.5% |
Metformin: 100% |
| Kim et al. [31] | 104 | Dapagliflozin or empagliflozin: 100% | Dulaglutide: 100% |
Metformin: 98.1% SU: 81.7% Insulin (53.38 ± 24.57 U): 10.6% |
| Riley et al. [33] | 2.2 million | N/A | N/A | N/A |
| Saroka et al. [34] | 75 | Canagliflozin: 100% |
Liraglutide: 62.7% Exenatide (once weekly): 25.3% Exenatide (twice daily): 12.0% |
Metformin: 78.7% Insulin: 60% Thiazolidinediones: 25.3% SU: 16% Colesevelam: 4% DPP-4 inhibitor: 2.7% Meglitinide: 1.3% |
DPP Dipeptidyl peptidase, GLP-1RA glucagon-like peptide-1 receptor agonist, N/A not available, SGLT2i sodium–glucose transport protein 2 inhibitor, SU Sulfonylurea
Genital infections, urinary tract infections, abdominal pain, nausea, bloated abdomen, diarrhea, polyuria, asthenia, yeast infections, dry mouth, and hypotension were among the commonly reported adverse events in the included studies. In the study by Carretero-Gomez et al. [27], symptomatic hypoglycemia was reported in < 10% of the patients and one death due to subarachnoid hemorrhage was reported. Treatment with SGLT2i was discontinued due to genital mycotic infection, worsening of renal function, leg amputation, and bariatric surgery. Treatment with GLP-1RA was discontinued due to gastrointestinal effects, insulin intensification, and worsening of renal function. In the study by Kim et al. [31], gastrointestinal adverse effects were commonly reported after 3 months, but their incidence was reduced at later time points. Mild hypoglycemia was also reported in < 5% of the patients. Major adverse effects such as ketoacidosis, pancreatitis, fractures, or acute renal failure were not reported.
Quantitative analysis (meta-analysis)
All-cause mortality
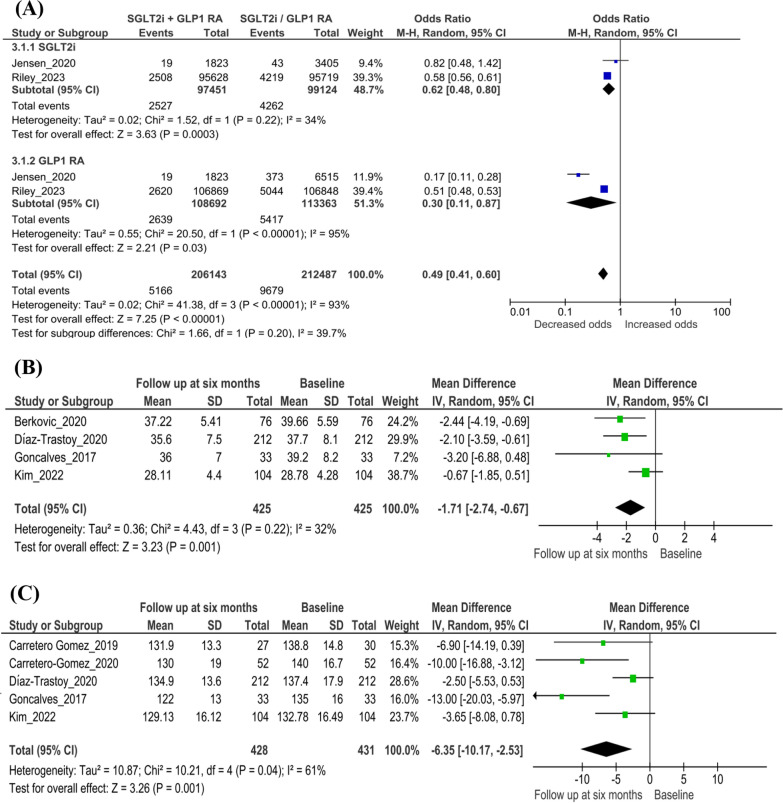
The number of all-cause mortality events was significantly lower with the combination therapy than with SGLT2i (p = 0.0003) or GLP-1RA (p = 0.03), with an overall decrease in the odds for all-cause mortality with the combination therapy (n = 2 studies; odds ratio [95% CI] 0.49 [0.41, 0.60]; I2 = 93.0%; p < 0.00001) (Fig. 3A).
Fig. 3.
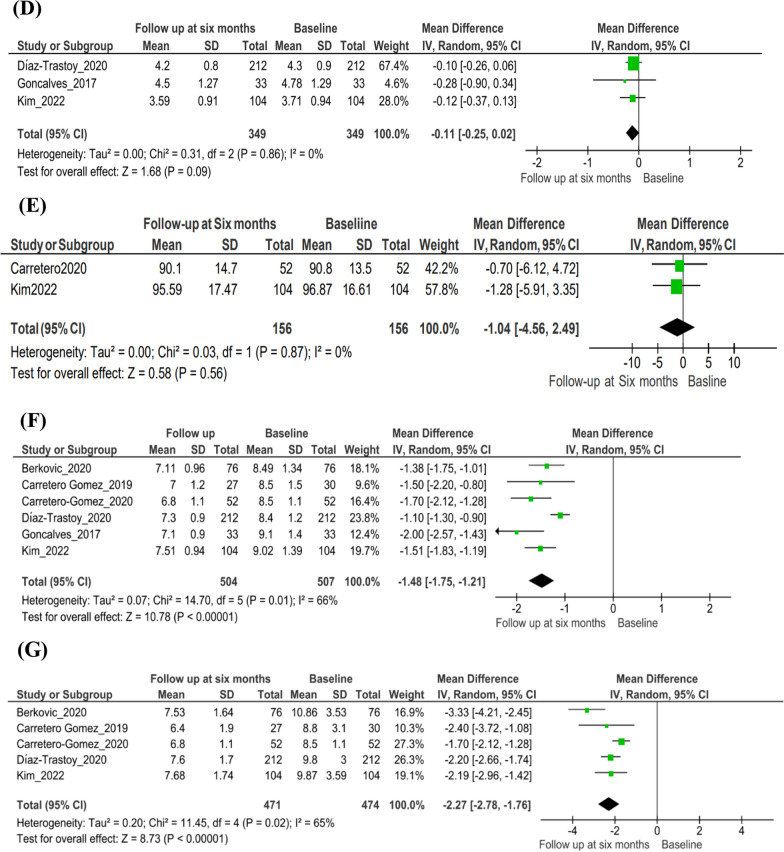
Findings from the main analysis. A Odds of all-cause mortality with SGLT2i + GLP-1RA combination therapy versus SGLT2i or GLP-1RA therapy. B Changes in BMI with combination therapy at the baseline versus the 6-month follow-up. C Changes in SBP with combination therapy at the baseline versus the 6-month follow-up. D Changes in total cholesterol with combination therapy at the baseline versus the 6-month follow-up. E Changes in eGFR with simultaneous combination therapy at the baseline versus the 6-month follow-up. F Changes in HbA1c with combination therapy at the baseline versus the 6-month follow-up. G Changes in FPG with combination therapy at the baseline versus the 6-month follow-up. BMI Body mass index, CI confidence interval, eGFR estimated glomerular filtration rate, FPG fasting plasma glucose, M–H Mantel–Haenszel, GLP-1RA glucagon-like peptide-1 receptor agonist, HbA1c glycated hemoglobin, IV importance value, SBP systolic blood pressure, SD standard deviation, SGLT2i Sodium–glucose transport protein 2 inhibitor
Cardiovascular risk factors
The combination significantly reduced BMI and SBP at follow-up (BMI: n = 4 studies; mean difference [95% CI] − 1.71 [− 2.74, − 0.67]; I2 = 32%; p = 0.001, Fig. 3B; SBP: n = 5 studies; mean difference [95% CI] − 6.35 [− 10.17, − 2.53]; I2 = 61%; p = 0.001, Fig. 3C). Although there was a decrease in the mean total cholesterol levels at follow-up from baseline with the combination therapy, the difference was not significant (n = 3 studies; mean difference [95% CI] − 0.11 [− 0.25, 0.02]; I2 = 0%; p = 0.09) (Fig. 3D).
Renal outcomes
The combination therapy was not associated with significant decreases in eGFR levels at follow-up from baseline (n = 2 studies; mean difference [95% CI] − 1.04 [− 4.56, 2.49]; I2 = 0%; p = 0.56) (Fig. 3E). Two studies (Carretero-Gomez et al. [28] and Diaz-Trastoy et al. [26]) reported results related to albuminuria. In the study by Carretero-Gomez et al. [28], there was a significant reduction in total urinary albumin-to–creatinine ratio (UACR; − 15.14 mg/g; p < 0.0001) and macroalbuminuria (UACR > 30 mg/g; − 63.18 mg/g; p < 0.0001) at 26 weeks. In the study by Diaz-Trastoy et al. [26], albuminuria data were available for 127 of 212 patients, 10 patients progressed from normoalbuminuria to micro- and macroalbuminuria, and 13 patients showed regression.
Glycemic outcomes
Treatment with the SGLT2i + GLP-1RA combination was also associated with improved glycemic control, expressed as a significant decrease in HbA1c and FPG levels at the 6-month follow-up from baseline (HbA1c: n = 7 studies; mean difference [95% CI] − 1.48 [− 1.75, − 1.21]; I2 = 66.0%; p < 0.00001, Fig. 3F; FPG: n = 5 studies; mean difference [95% CI] − 2.27 [− 2.78, − 1.76]; I2 = 65.0%; p < 0.00001, Fig. 3G).
Findings from the subgroup analyses
Cardiovascular risk factors
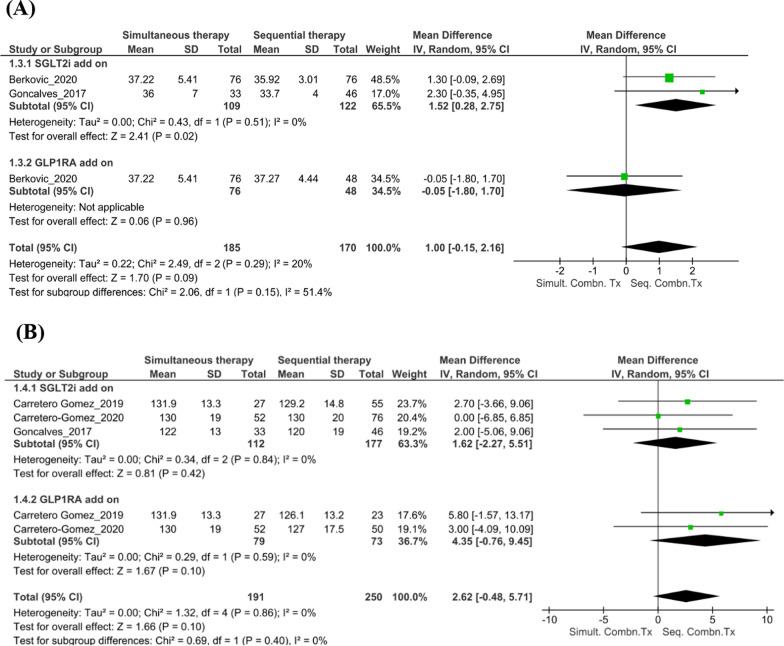
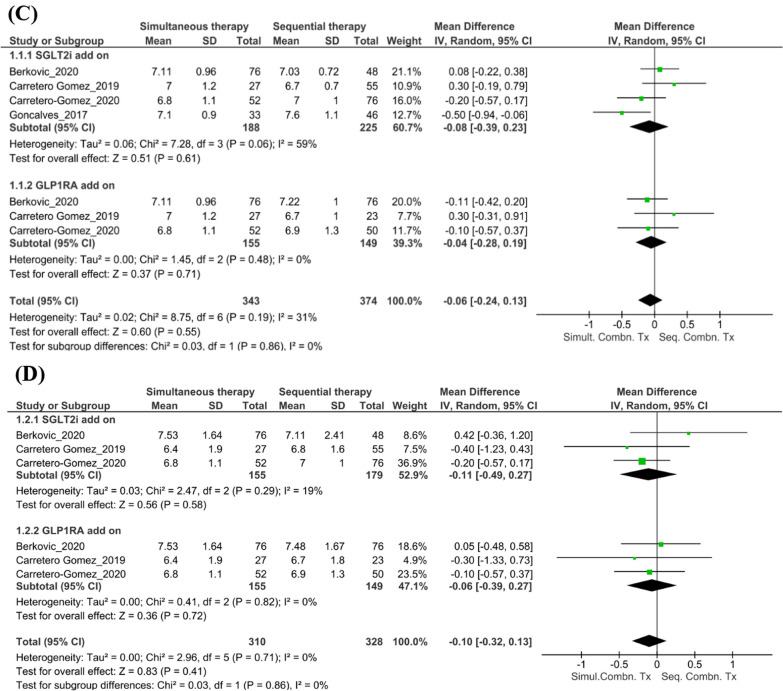
The pattern of administration of the combination therapy did not significantly affect BMI at follow-up (n = 2 studies; mean [95% CI] 1.00 [− 0.15, 2.16]; I2 = 20%; p = 0.09, Fig. 4A). However, the reduction in BMI was significant when patients were already on GLP-1RA and received add-on SGLT2i (n = 2 studies; mean [95% CI] 1.52 [0.28, 2.75]; I2 = 0%; p = 0.02). Although there was a reduction in the SBP levels in those on sequential combination therapy compared with those on simultaneous combination therapy at 6 months follow-up, this change was not significant (n = 5 studies; mean [95% CI] 2.62 [− 0.48, 5.71]; I2 = 0%; p = 0.10, Fig. 4B).
Fig. 4.
Findings from the subgroup analyses. A Changes in BMI with simultaneous versus sequential combination therapy at the 6-month follow-up. B Changes in SBP with simultaneous versus sequential combination therapy at the 6-month follow-up. C Changes in HbA1c levels with simultaneous versus sequential combination therapy at the 6-month follow-up. D Changes in FPG levels with simultaneous versus sequential combination therapy at the 6-month follow-up. BMI body mass index, CI confidence interval, FPG fasting plasma glucose, GLP-1RA glucagon-like peptide-1 receptor agonist, HbA1c glycated hemoglobin, IV importance value, SBP systolic blood pressure, SD standard deviation, SGLT2i sodium–glucose transport protein 2 inhibitor, Seq. Combn. Tx sequential combination therapy, Simult. Combn. Tx simultaneous combination therapy
Renal outcomes
In the study by Carretero-Gomez et al. [28], the extent of reduction in the total UACR was significant and similar when the combination was started simultaneously or when a GLP-1RA was added to ongoing SGLT2i therapy (− 17.19 mg/g and − 16.4 mg/g, respectively; p < 0.0001). A greater reduction in macroalbuminuria was observed when an SGLT2i was added to a GLP-1RA than when a GLP-1RA was added to an SGLT2i (− 116.7 mg/g [n = 24] and − 55.5 mg/g [n = 21]; p < 0.005).
Glycemic outcomes
For the subgroup analyses, outcomes at the 6-month follow-up were compared between patients treated with the combination of SGLT2i + GLP-1RA administered simultaneously versus sequentially. Pooled estimates for changes in HbA1c levels and FPG levels showed no significant differences between the simultaneous combination therapy and sequential combination therapy at follow-up (HbA1c: n = 4 studies; mean [95% CI] − 0.06 [− 0.24, 0.13]; I2 = 31.0%; p = 0.55, Fig. 4C; FPG: n = 2 studies; mean [95% CI] − 0.10 [− 0.32, 0.13]; I2 = 0%; p = 0.41, Fig. 4D).
Due to the lack of studies comparing simultaneous combination and sequential combination therapies at follow-up, meta-analysis could not be performed for all-cause mortality outcomes, and changes in eGFR and total cholesterol levels.
GRADE analysis
According to the GRADE analysis (Table 5), the certainty of evidence for the outcomes of all-cause mortality and changes in BMI, SBP, HbA1c, eGFR, and total cholesterol levels was “low.” The certainty of evidence for the outcome of changes in FPG was “very low.”
Table 5.
Findings of the GRADE analysis
| Combination of SGLT2i and GLP-1RA therapy compared to SGLT2i or GLP-1RA therapy for type 2 diabetes |
|
Patient or population: T2DM Interventiona: Combination of SGLT2i and GLP-1RA therapy Comparisona: SGLT2i or GLP-1RA therapy |
| Outcome | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments |
|---|---|---|---|---|---|
| Risk with combination of SGLT2i and GLP-1RA therapy | |||||
| All-cause mortality | 22 per 1000 (19 to 27) | RR 0.49 (0.41 to 0.60) | 4,18,630 (2 observational studies) |
⨁⨁◯◯ Low |
Combination of SGLT2i and GLP-1RA therapy may result in a large reduction in all-cause mortality due to T2DM |
|
Change in HbA1c Follow-up: 6 months |
MD 1.48 lower (1.75 lower to 1.21 lower) | – | 1011 (7 observational studies) |
⨁⨁◯◯ Low |
Combination of SGLT2i and GLP-1RA therapy may result in a slight (but important) reduction in HbA1C |
|
Change in FPG Follow-up: 6 months |
MD 2.27 lower (2.78 lower to 1.76 lower) | – | 945 (5 observational studies) |
⨁◯◯◯ Very low |
Combination of SGLT2i and GLP-1RA therapy may reduce FPG but the evidence is very uncertain |
|
Change in BMI Follow-up: mean 6 months |
MD 1.71 lower (2.74 lower to 0.67 lower) | – | 850 (4 observational studies) |
⨁⨁◯◯ Low |
The combination of SGLT2i and GLP-1RA therapy may reduce BMI |
|
Change in SBP Follow-up: 6 months |
MD 6.35 lower (10.17 lower to 2.53 lower) | – | 859 (5 observational studies) |
⨁⨁◯◯ Low |
Evidence suggests that the combination of SGLT2i and GLP-1RA therapy results in a large reduction in SBP |
|
Change in eGFR Follow-up: 6 months |
MD 1.04 lower (4.56 lower to 2.49 higher) | – | 312 (2 observational studies) |
⨁⨁◯◯ Low |
The combination of SGLT2i and GLP-1RA therapy may reduce eGFR |
|
Change in total cholesterol Follow-up: 6 months |
MD 0.11 lower (0.25 lower to 0.02 higher) | – | 698 (3 observational studies) |
⨁⨁◯◯ Low |
The combination of SGLT2i and GLP-1RA therapy may reduce total cholesterol |
BMI Body mass index, CI confidence interval, eGFR estimated glomerular filtration rate, FPG fasting plasma glucose, GLP-1RA glucagon-like peptide-1 receptor agonist, GRADE Grading of Recommendations, Assessment, Development and Evaluation, HbA1c glycated hemoglobin, MD mean difference, RR relative risk, SBP systolic blood pressure, SD standard deviation, SGLT2i sodium–glucose transport protein 2 inhibitor, T2DM type 2 diabetes mellitus
aIntervention and comparator for changes in HbA1c, FPG, BMI, SBP, eGFR, and total cholesterol was the combination of SGLT2i + GLP-1RA administered simultaneously, but MD was calculated based on the difference in the parameters between baseline and follow-up at 6 months
Discussion
Our meta-analysis of real world data showed a significant reduction in all-cause mortality as well as significant reductions in HbA1c, SBP, and body weight in T2DM patients treated with SGLT2i + GLP-1RA combination compared to either 2 drug class used alone.
Individual use of SGLT2is and GLP-1RAs can significantly improve cardiovascular outcomes and reduce mortality. This association has been reported in several landmark RCTs and meta-analyses [7, 11, 12, 35–41]. Empagliflozin was associated with a reduction in CV mortality, nonfatal MI, or nonfatal stroke, as well as a reduction in all-cause mortality [35–37], whereas DECLARE showed dapagliflozin reduced all-cause mortality in patients with heart failure with reduced ejection fraction (HFrEF) but not in those without HFrEF [39] The CANVAS and CANVAS-R studies on canagliflozin have reported similar benefits [38]. Death from CV causes and all-cause mortality were reduced in participants receiving liraglutide in the LEADER study [42, 43], while the SUSTAIN-6 study demonstrated lower CV deaths in patients receiving subcutaneous semaglutide [44]. Oral semaglutide significantly reduced CV risk factors such as HbA1c, body weight, and SBP with nearly 50% reduction in CV and all-cause mortality in the PIONEER-6 trial [45]. The SOUL study demonstrated the non-inferiority of semaglutide to placebo in terms of CV mortality outcomes [46]. The REWIND study reported a lower incidence of MACE-3 and other CV outcomes [41], whereas, albiglutide reduced CV events driven by a reduction in myocardial infarction with similar CV deaths [40].
In the absence of studies directly comparing SGLT2i/GLP-1RA combination versus either of these being used alone, meta-analyses of CVOTs and observational studies have assessed whether the concurrent use of SGLT2is + GLP-1RAs provides an additional advantage in reducing the incidence of MACE, cardiorenal endpoints, and cardiovascular mortality. A retrospective cohort study evaluating the added benefits of administering GLP-1RAs + SGLT2is in patients with T2DM (N = 5576) showed that the addition of GLP-1RA reduced the risk of composite all-cause mortality, myocardial infarction, and stroke by 67% [17]. However, an analysis of three US claims datasets from 2013 to 2018 for all-cause mortality among patients (N = 12,584) who received add-on SGLT2i while already receiving GLP-1RA therapy showed that although the SGLT2i addition reduced the risk of MACE and hospitalizations related to heart failure, it did not reduce all-cause mortality [47]. In a study by Jensen et al. [30], treatment with metformin + SGLT2is and metformin + GLP-1RAs was associated with a reduced risk of MACE, all-cause mortality, and severe hypoglycemia; however, patients who received triple therapy with SGLT2i + GLP-1RAs + metformin had the lowest risk of all three outcomes.
Although initial metanalyses on SGLT21/GLP-1 combination reporting greater reductions in HbA1c, SBP, and body weight were not powered or did not assess MACE or all-cause mortality, a later meta-analysis of CVOTs using SGLT2i or GLP-1 reported significant reductions in MACE (30%), cardiovascular mortality/hospitalization due to heart failure (31%), and all-cause mortality (57%) compared with monotherapy with either SGLT2i or GLP-1RA [18, 48]. Our real-world meta-analysis supports further the reduction in all-cause mortality, suggesting better cardiovascular protection when these two drug classes with different mechanisms of action are used together.
Although the exact mechanisms by which SGLT2is and GLP-1RAs reduce CV and all-cause mortality are not yet fully understood, endothelial dysfunction is a common pathogenetic feature underlying HF, especially with preserved ejection fraction, T2DM, and frailty, and empagliflozin has recently been demonstrated to act via regulation of microRNAs and reduction of mitochondrial oxidative stress [49, 50]. The cardiovascular benefits of empagliflozin were evident through the significant improvement in the 5-m gait speed test within 3 months of treatment in older and frail individuals with T2DM and hypertension [50]. The combination of empagliflozin and liraglutide reduced central systolic blood pressure, perfused boundary region, and arterial stiffness in patients with T2DM to a greater extent than insulin in addition to similar glycemic effects, suggesting that the combination should be preferred over traditional insulin plus metformin in patients with T2DM and high cardiovascular risk [51]. Vascular remodeling is a pathological process in cardiovascular diseases, and GLP-1RAs, including semaglutide, have been shown to reduce vessel remodeling through their anti-inflammatory and anti-proliferative effects independent of their interaction with GLP-1R [52].
The present analysis indicates that simultaneous treatment with the combination of SGLT2is + GLP-1RAs is effective in significantly reducing baseline HbA1c and FPG levels in patients with T2DM at follow-up. Accumulated data underscore the advantages of combining SGLT2is and GLP-1RAs for cardiovascular, renal, and metabolic health in T2DM patients at a low risk of developing hypoglycemia [53]. Individuals newly diagnosed with T2DM frequently present with multiple comorbid conditions that increase their susceptibility to CVD, such as hypertension, dyslipidemia, and obesity [54]. The severity of these comorbidities is especially high in patients after 1 year of being diagnosed with T2DM and presenting with HbA1c levels of > 6.5% [55]. Hence, it is crucial to attain early and sustained glycemic control to prevent diabetes-related complications.
GLP1-RAs promote pancreatic insulin secretion to regulate the levels of glucose in the blood. Apart from their role in insulin secretion, GLP-1RAs delay gastric emptying, stimulate the appetite centers in the brain to induce early satiety, and consequently reduce food consumption [13, 26, 56]. SGLT2is also contribute to weight loss as a result of caloric loss through glycosuria; however, the weight loss may be less than expected due to increased food intake, especially if adequately intensive lifestyle changes are not implemented [57]. A retrospective search of the electronic prescriptions of patients with T2DM (N = 446,798) for SGLT2i + GLP-1RA treatment in Spain (2018) showed that the combination resulted in faster weight loss and greater HbA1c reduction when administered simultaneously than when the drugs were administered sequentially [26]. This real-world data analysis also shows a significant decrease in BMI with the combination therapy. Notably, there was a higher reduction in BMI when an SGLT2i was added to the GLP-1RA therapy rather than vice versa.
A significant decrease in SBP was seen at 6 months follow-up compared to baseline among patients who received simultaneous SGLT2i + GLP-1RA therapy. While no significant difference in the SBP between patients who received the simultaneous versus sequential combination therapy was apparent, the reduction in SBP was greater in patients who first received SGLT2is and then received GLP-1RAs. This is consistent with a previous review that focused on data from placebo-controlled trials regarding the antihypertensive effects of GLP-1RAs, SGLT2is, and DPP-4 inhibitors; this review reported that SGLT2is are more potent than the other two drug classes in reducing SBP and diastolic blood pressure among patients with T2DM [58]. The decrease in SBP can be attributed to the natriuresis induced by SGLT2is [59]. GLP-1RAs exhibit a nephroprotective effect by directly interacting with renal cells; they prevent glomerular hyperfiltration by promoting diuresis and natriuresis [60].
In the present analysis, the difference between the baseline and the follow-up eGFR was statistically non-significant in patients with T2DM who received the combination; however, the duration of follow-up in these real-world studies was not long enough to observe any improvement in kidney function that was demonstrated in larger SGLT2i trials, e.g., the DECLARE TIMI58 trial (median follow-up: 4.2 years [39]) and CANVAS trial (mean follow-up: 3.6 years [38]). More recently, the FLOW study, which focused on evaluating the nephroprotective and cardioprotective efficacy of semaglutide, a GLP-1RA, in patients with T2DM and CKD (N = 3534), was stopped prematurely as it met certain pre-specified efficacy criteria, with a very high likelihood of study success on reducing the progression of renal disease [61]. Microalbuminuria is an independent risk factor for progressive CKD and cardiovascular events, especially in patients with T2DM [62]. Post hoc analyses of the double-blind, placebo-controlled Semaglutide Treatment Effect in People with obesity (STEP) 2 trial, which involved overweight/obese patients with T2DM (N = 1210), showed that those on semaglutide had an improved UACR status. Additionally, treatment with semaglutide led to a reduction in the proportion of patients with microalbuminuria (UACR 30–300 mg/g) compared to treatment with placebo at the end of week 68 (11.5% vs. 22.4%) [63]. SGLT2is are recommended for the treatment of patients with T2DM and CKD (eGFR ≥ 30 mL/min/1.73 m2 and UACR > 30 mg/g), given their nephroprotective effects via the renin–angiotensin–aldosterone system [64]. However, for those with impaired renal function (eGFR < 30 mL/min/1.73 m2), the lowering of HbA1c levels by SGLT2is is negligible [65]. This again supports the rationale for combining SGLT2is with GLP-1RAs, which exert their glucose-lowering effect independent of kidney function. GLP-1RAs are reported to inhibit the development and/or progression of kidney disease in patients with T2DM while reducing the risk of kidney damage [66].
Estimates for CV mortality could not be derived in this meta-analysis, given the lack of real-world studies reporting CV mortality outcomes and meeting the study eligibility criteria. The Journal of the American College of Cardiology 2020 expert consensus report on the combined use of these drugs suggested the need for more evidence-based studies supporting the use of this combination for its CV benefits but concluded that the concurrent use of both SGLT2is and GLP-1RAs is permissible when indicated, considering the patient benefits established in numerous trials [67]. Guidelines and recommendations from various medical bodies, including the American Diabetes Association (ADA), European Society of Cardiology, and Diabetes Canada, have advocated for adding an SGLT2i following the use of a GLP-1RA, or vice versa, for T2DM patients with a high risk of developing ASCVD and those with CKD [68–70].
Despite the substantial amount of literature proposing the advantages of using SGLT2is + GLP-1RAs, a retrospective cross-sectional two-center study conducted in Riyadh, Saudi Arabia (January–December 2020), showed that physicians were under-prescribing these drugs; the study showed that endocrinologists most frequently prescribed SGLT2is or GLP-1RAs (60.6%), followed by internal medicine physicians (11.4%), cardiologists (9.8%), and nephrologists (2.0%) [71]. While RCT meta-analyses and findings from the present real-world analysis demonstrate the added benefit of combination therapy among patients with T2DM [13, 14, 16, 51, 72–74], very little has truly been translated into practice. Despite recommendations to include the combination in patients with T2DM, cardiologists view diabetes care independently of cardiovascular care, and consequently, are reluctant to prescribe SGLT2is + GLP-1RAs for their cardiovascular benefits in the management of patients with T2DM [75, 76]. Recognizing this reluctance, it is important to analyze the barriers associated with integrating these treatments into routine clinical practice to enhance cardiovascular outcomes [71]. Combination therapy with SGLT2is + GLP-1RAs can address multiple components of the ominous octet (insulin and glucagon secretion, hepatic glucose production, gastrointestinal incretin defect, appetite and weight loss, and muscle and hepatic insulin sensitivity), improve cardiovascular risk, and prevent diabetic nephropathy and should therefore be considered as an option during the early treatment stages of patients with T2DM. Combination therapy should perhaps be considered for first-line treatment in patients with T2DM who are at high risk of cardiovascular and renal disease as it is not associated with any notable safety issues or adverse event outcomes [77]. Treatment goals for patients with T2DM should be focused on the timely control of HbA1c levels along with the prevention of microvascular and macrovascular complications [78].
This systematic review has some limitations. First, publication bias assessment was not possible as relatively few studies were identified for each of the outcomes presented in this SLR. Second, there was considerable heterogeneity in patient demographics (e.g., age), duration of diabetes, medical history (e.g., baseline HbA1c), treatment history, and concomitant medications for T2DM (e.g., insulin, metformin, or other glucose-lowering agents) among the included studies. Most of the studies did not report the duration for which the patients received treatment with SGLT2i or GLP-1RA until add-on was initiated, which would have provided insights into the extent of heterogeneity in the timing of therapy titration. Accordingly, for all the outcomes, the certainty of evidence was deemed low-to–very low in the GRADE analysis. Third, unadjusted mortality values have been presented in the current meta-analysis. Given that both SGLT2is and GLP-1RAs strongly influence BMI and microalbuminuria, it will be interesting to note how mortality varies as a function of these parameters. Such adjustment analyses could not be conducted due to lack of number of studies reporting mortality outcomes. Fourth, a meta-analysis for cardiovascular mortality could not be performed due to the lack of sufficient data. This calls for more and larger-sized real-world studies to strengthen the evidence that supports the early use of these combinations to improve cardiovascular outcomes and glycemic control in patients with T2DM.
Conclusion
This SLR and meta-analysis of real-world studies suggests that the combination of SGLT2is + GLP-1RAs is associated with significantly lower all-cause mortality than individual therapies, with an improvement in cardiovascular, renal, and glycemic measurements. Providing evidence that supports the advantages of introducing the combination early can significantly strengthen the foundation for making confident clinical decisions. Moreover, the simultaneous use of these drugs could prove more beneficial than sequential combination therapy in patients with T2DM, and if similar results are reported with the use of oral GLP-1RAs, it may be easier to initiate the combination earlier in the disease course.
Supplementary Information
Additional file 1: Table S1. Search strategy for the systematic literature review. Table S2. List of studies excluded at the full-text screening stage with reasons for exclusion. Table S3. List of studies excluded from the meta-analysis and reasons for exclusion.
Additional file 2. Assessment of study quality and data extraction.
Acknowledgements
We would like to thank BioQuest Solutions Pvt. Ltd. for providing medical writing support and editorial assistance.
Abbreviations
- ADA
American Diabetes Association
- BMI
Body Mass Index
- CKD
Chronic kidney disease
- eGFR
Estimated glomerular filtration rate
- FPG
Fasting plasma glucose
- GLP-1RAs
Glucagon-like peptide-1 receptor agonists
- GRADE
Grading of Recommendations, Assessment, Development and Evaluation
- JBI
Joanna Briggs Institute
- LDL-C
Lipoprotein cholesterol
- MACE
Major Adverse Cardiovascular Events
- RCTs
Randomized controlled trials
- SGLT2is
Sodium–glucose transport protein 2 inhibitors
- SBP
Systolic blood pressure
- T2DM
Type 2 diabetes mellitus
- MD
Mean difference
- OR
Odds ratio
Author contributions
All authors have contributed equally and significantly to the conceptualization and investigation of the study. All authors have read and reviewed the final draft of this manuscript, take responsibility for the integrity and accuracy of this manuscript, and have given their approval for this version to be published.
Funding
None.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/1/2024
A Correction to this paper has been published: 10.1186/s12933-024-02283-2
References
- 1.Ma C-X, Ma X-N, Guan C-H, Li Y-D, Mauricio D, Fu S-B. Cardiovascular disease in type 2 diabetes mellitus: progress toward personalized management. Cardiovasc Diabetol. 2022;21:74. doi: 10.1186/s12933-022-01516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Padhi S, Nayak AK, Behera A. Type II diabetes mellitus: a review on recent drug based therapeutics. Biomed Pharmacother. 2020;131:110708. doi: 10.1016/j.biopha.2020.110708. [DOI] [PubMed] [Google Scholar]
- 3.Palmer SC, Tendal B, Mustafa RA, Vandvik PO, Li S, Hao Q, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2021 doi: 10.1136/bmj.m4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 5.Marilly E, Cottin J, Cabrera N, Cornu C, Boussageon R, Moulin P, et al. SGLT2 inhibitors in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials balancing their risks and benefits. Diabetologia. 2022;65:2000–2010. doi: 10.1007/s00125-022-05773-8. [DOI] [PubMed] [Google Scholar]
- 6.Lee MMY, Kristensen SL, Gerstein HC, McMurray JJV, Sattar N. Cardiovascular and mortality outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A meta-analysis with the FREEDOM cardiovascular outcomes trial. Diabetes Metab Syndr. 2022;16:102382. doi: 10.1016/j.dsx.2021.102382. [DOI] [PubMed] [Google Scholar]
- 7.Sattar N, Lee MMY, Kristensen SL, Branch KRH, Del Prato S, Khurmi NS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9:653–662. doi: 10.1016/S2213-8587(21)00203-5. [DOI] [PubMed] [Google Scholar]
- 8.Zou C-Y, Liu X-K, Sang Y-Q, Wang B, Liang J. Effects of SGLT2 inhibitors on cardiovascular outcomes and mortality in type 2 diabetes. Medicine (Baltimore) 2019;98:e18245. doi: 10.1097/MD.0000000000018245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teo YH, Teo YN, Syn NL, Kow CS, Yoong CSY, Tan BYQ, et al. Effects of sodium/glucose cotransporter 2 (SGLT2) inhibitors on cardiovascular and metabolic outcomes in patients without diabetes mellitus: a systematic review and meta-analysis of randomized-controlled trials. J Am Heart Assoc. 2021;10:e019463. doi: 10.1161/JAHA.120.019463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lincoff AM, Brown-Frandsen K, Colhoun HM, Deanfield J, Emerson SS, Esbjerg S, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023 doi: 10.1056/NEJMoa2307563. [DOI] [PubMed] [Google Scholar]
- 11.Ali MU, Mancini GBJ, Fitzpatrick-Lewis D, Lewis R, Jovkovic M, Zieroth S, et al. The Effectiveness of sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide-1 receptor agonists on cardiorenal outcomes: systematic review and meta-analysis. Can J Cardiol. 2022;38:1201–1210. doi: 10.1016/j.cjca.2022.05.011. [DOI] [PubMed] [Google Scholar]
- 12.McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021;6:148. doi: 10.1001/jamacardio.2020.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo M, Gu J, Teng F, Chen J, Ma X, Chen Q, et al. The efficacy and safety of combinations of SGLT2 inhibitors and GLP-1 receptor agonists in the treatment of type 2 diabetes or obese adults: a systematic review and meta-analysis. Endocrine. 2020;67:294–304. doi: 10.1007/s12020-019-02175-6. [DOI] [PubMed] [Google Scholar]
- 14.Mantsiou C, Karagiannis T, Kakotrichi P, Malandris K, Avgerinos I, Liakos A, et al. Glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter-2 inhibitors as combination therapy for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obesity Metabolism. 2020;22:1857–1868. doi: 10.1111/dom.14108. [DOI] [PubMed] [Google Scholar]
- 15.Li C, Luo J, Jiang M, Wang K. The efficacy and safety of the combination therapy with GLP-1 receptor agonists and SGLT-2 inhibitors in type 2 diabetes mellitus: a systematic review and meta-analysis. Front Pharmacol. 2022;13:838277. doi: 10.3389/fphar.2022.838277/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patoulias D, Stavropoulos K, Imprialos K, Katsimardou A, Kalogirou M-S, Koutsampasopoulos K, et al. Glycemic efficacy and safety of glucagon-like peptide-1 receptor agonist on top of sodium-glucose co-transporter-2 inhibitor treatment compared to sodium-glucose co-transporter-2 inhibitor alone: A systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2019;158:107927. doi: 10.1016/j.diabres.2019.107927. [DOI] [PubMed] [Google Scholar]
- 17.Lopez PD, Bhatia K, Bohra C, Mahmood K, Baruch L, Eng C. Benefits of adding glucagon-like peptide 1 receptor agonists to sodium-glucose co-transporter 2 inhibitors in diabetic patients with atherosclerotic disease and heart failure. Am J Cardiol. 2022;181:87–93. doi: 10.1016/j.amjcard.2022.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Du L, Qin J, Wang D, Zhao Y, Xu N, Wu C, et al. Meta-analysis assessing the effectiveness of SGLT2i+GLP1RA combination therapy versus monotherapy on cardiovascular and cerebrovascular outcomes in diabetic patients. Front Physiol. 2022 doi: 10.3389/fphys.2022.1028486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark J, Glasziou P, Del Mar C, Bannach-Brown A, Stehlik P, Scott AM. A full systematic review was completed in 2 weeks using automation tools: a case study. J Clin Epidemiol. 2020;121:81–90. doi: 10.1016/j.jclinepi.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Chapter 7: Systematic reviews of etiology and risk. JBI manual for evidence synthesis; 2020. https://synthesismanual.jbi.global
- 21.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arévalo-Lorido JC, Gómez JC, Huelgas RG, De Lucas DG, Polo LM, Aguilar JMV, et al. Lowering blood pressure with the combination of a sodium-glucose cotransporter 2 inhibitor and a glucagon-like peptide-1 receptor agonist in type 2 diabetic patients: a clinical evidence. High Blood Press Cardiovasc Prev. 2018;25:417–420. doi: 10.1007/s40292-018-0280-1. [DOI] [PubMed] [Google Scholar]
- 23.Berkovic MC, Bilic-Curcic I, Bozek T, Mahecic DH, Majanovic SK, Canecki-Varzic S, et al. Glucagon-like-1 receptor agonists and sodium/glucose cotransporter-2 inhibitors combination—are we exploiting their full potential in a real life setting? WJD. 2020;11:540–552. doi: 10.4239/wjd.v11.i11.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhattacharyya S. Clinical effectiveness of combination therapy with dulaglutide, SGLT2 inhibitor and metformin with or without insulin in Indian adults with type 2 diabetes: a real-world retrospective study. Clinical Diabetology. 2020;9:233–238. doi: 10.5603/DK.2020.0026. [DOI] [Google Scholar]
- 25.Deol H, Lekkakou L, Viswanath AK, Pappachan JM. Combination therapy with GLP-1 analogues and SGLT-2 inhibitors in the management of diabesity: the real world experience. Endocrine. 2017;55:173–178. doi: 10.1007/s12020-016-1125-0. [DOI] [PubMed] [Google Scholar]
- 26.Díaz-Trastoy O, Villar-Taibo R, Sifontes-Dubón M, Mozo-Peñalver H, Bernabeu-Morón I, Cabezas-Agrícola JM, et al. GLP1 receptor agonist and SGLT2 inhibitor combination: an effective approach in real-world clinical practice. Clin Ther. 2020;42:e1–12. doi: 10.1016/j.clinthera.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Carretero Gómez J, Arévalo Lorido JC, Gómez Huelgas R, García De Lucas D, Mateos Polo L, Varela Aguilar JM, et al. Combination therapy with glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors in older patients with type 2 diabetes: a real-world evidence study. Can J Diabetes. 2019;43:186–192. doi: 10.1016/j.jcjd.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Carretero Gómez J, Ena J, Seguí Ripoll JM, Carrasco-Sanchez FJ, Gómez Huelgas R, Mateos Polo L, et al. Early biomarkers of diabetic kidney disease. A focus on albuminuria and a new combination of antidiabetic agents. Int J Clin Pract. 2020 doi: 10.1111/ijcp.13586. [DOI] [PubMed] [Google Scholar]
- 29.Goncalves E, Bell DSH. Glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter-2 inhibitors: sequential or simultaneous start? Diabetes Obes Metab. 2017;19:909–911. doi: 10.1111/dom.12897. [DOI] [PubMed] [Google Scholar]
- 30.Jensen MH, Kjolby M, Hejlesen O, Jakobsen PE, Vestergaard P. Risk of major adverse cardiovascular events, severe hypoglycemia, and all-cause mortality for widely used antihyperglycemic dual and triple therapies for type 2 diabetes management: a cohort study of all danish users. Diabetes Care. 2020;43:1209–1218. doi: 10.2337/dc19-2535. [DOI] [PubMed] [Google Scholar]
- 31.Kim HS, Yoon T, Jung CH, Park J-Y, Lee WJ. Clinical efficacy of sodium-glucose cotransporter 2 inhibitor and glucagon-like peptide-1 receptor agonist combination therapy in type 2 diabetes mellitus: real-world study. Diabetes Metab J. 2022;46:658–662. doi: 10.4093/dmj.2021.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorgojo-Martínez JJ, Serrano-Moreno C, Sanz-Velasco A, Feo-Ortega G, Almodóvar-Ruiz F. Real-world effectiveness and safety of dapagliflozin therapy added to a GLP1 receptor agonist in patients with type 2 diabetes. Nutr Metab Cardiovasc Dis. 2017;27:129–137. doi: 10.1016/j.numecd.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Riley DR, Essa H, Austin P, Preston F, Kargbo I, Ibarburu GH, et al. All-cause mortality and cardiovascular outcomes with sodium-glucose Co-transporter 2 inhibitors, glucagon-like peptide-1 receptor agonists and with combination therapy in people with type 2 diabetes. Diabetes Obesity Metabolism. 2023;25:2897–2909. doi: 10.1111/dom.15185. [DOI] [PubMed] [Google Scholar]
- 34.Saroka RM, Kane MP, Busch RS, Watsky J, Hamilton RA. SGLT-2 inhibitor therapy added to GLP-1 agonist therapy in the management of T2DM. Endocr Pract. 2015;21:1315–1322. doi: 10.4158/EP15877.OR. [DOI] [PubMed] [Google Scholar]
- 35.Wagdy K. The EMPEROR-reduced trial: SGLT2 inhibitors for heart failure get more support. Glob Cardiol Sci Pract. 2020;2020:e202031. doi: 10.21542/gcsp.2020.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagdy K, Nagy S. EMPEROR-preserved: SGLT2 inhibitors breakthrough in the management of heart failure with preserved ejection fraction. Glob Cardiol Sci Pract. 2021;2021:e202117. doi: 10.21542/gcsp.2021.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 38.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 39.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 40.Hernandez AF, Green JB, Janmohamed S, D'Agostino RB, Granger CB, Jones NP, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet (London, England) 2018;392:1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 41.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. The Lancet. 2019;394:121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 42.Verma S, Bhatt DL, Bain SC, Buse JB, Mann JFE, Marso SP, et al. Effect of liraglutide on cardiovascular events in patients with type 2 diabetes mellitus and polyvascular disease: results of the LEADER trial. Circulation. 2018;137:2179–2183. doi: 10.1161/CIRCULATIONAHA.118.033898. [DOI] [PubMed] [Google Scholar]
- 43.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 45.Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–851. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 46.McGuire DK, Busui RP, Deanfield J, Inzucchi SE, Mann JFE, Marx N, et al. Effects of oral semaglutide on cardiovascular outcomes in individuals with type 2 diabetes and established atherosclerotic cardiovascular disease and/or chronic kidney disease: design and baseline characteristics of SOUL, a randomized trial. Diabetes Obes Metab. 2023;25:1932–1941. doi: 10.1111/dom.15058. [DOI] [PubMed] [Google Scholar]
- 47.Dave CV, Kim SC, Goldfine AB, Glynn RJ, Tong A, Patorno E. Risk of cardiovascular outcomes in patients with type 2 diabetes after addition of sglt2 inhibitors versus sulfonylureas to baseline GLP-1RA therapy. Circulation. 2021;143:770–779. doi: 10.1161/CIRCULATIONAHA.120.047965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh AK, Singh R. Metabolic and cardiovascular benefits with combination therapy of SGLT-2 inhibitors and GLP-1 receptor agonists in type 2 diabetes. WJC. 2022;14:329–342. doi: 10.4330/wjc.v14.i6.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mone P, Lombardi A, Kansakar U, Varzideh F, Jankauskas SS, Pansini A, et al. Empagliflozin improves the MicroRNA signature of endothelial dysfunction in patients with heart failure with preserved ejection fraction and diabetes. J Pharmacol Exp Ther. 2023;384:116–122. doi: 10.1124/jpet.121.001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mone P, Varzideh F, Jankauskas SS, Pansini A, Lombardi A, Frullone S, et al. SGLT2 inhibition via empagliflozin improves endothelial function and reduces mitochondrial oxidative stress: insights from frail hypertensive and diabetic patients. Hypertension. 2022;79:1633–1643. doi: 10.1161/HYPERTENSIONAHA.122.19586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ikonomidis I, Pavlidis G, Thymis J, Birba D, Kalogeris A, Kousathana F, et al. Effects of glucagon-like peptide-1 receptor agonists, sodium-glucose cotransporter-2 inhibitors, and their combination on endothelial glycocalyx, arterial function, and myocardial work index in patients with type 2 diabetes mellitus after 12-month treatment. JAHA. 2020;9:e015716. doi: 10.1161/JAHA.119.015716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jensen DM, Skovsted GF, Bonde MFB, Bentzon JF, Rolin B, Franck G, et al. Semaglutide treatment attenuates vessel remodelling in ApoE-/- mice following vascular injury and blood flow perturbation. Atheroscler Plus. 2022;49:32–41. doi: 10.1016/j.athplu.2022.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gourdy P, Darmon P, Dievart F, Halimi J-M, Guerci B. Combining glucagon-like peptide-1 receptor agonists (GLP-1RAs) and sodium-glucose cotransporter-2 inhibitors (SGLT2is) in patients with type 2 diabetes mellitus (T2DM) Cardiovasc Diabetol. 2023;22:79. doi: 10.1186/s12933-023-01798-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Busch RS, Kane MP. Combination SGLT2 inhibitor and GLP-1 receptor agonist therapy: a complementary approach to the treatment of type 2 diabetes. Postgrad Med. 2017;129:686–697. doi: 10.1080/00325481.2017.1342509. [DOI] [PubMed] [Google Scholar]
- 55.Laiteerapong N, Ham SA, Gao Y, Moffet HH, Liu JY, Huang ES, et al. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (the diabetes & aging study) Diabetes Care. 2019;42:416–426. doi: 10.2337/dc17-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koliaki C, Doupis J. Incretin-based therapy: a powerful and promising weapon in the treatment of type 2 diabetes mellitus. Diab Ther. 2011;2:101–121. doi: 10.1007/s13300-011-0002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Janež A, Fioretto P. SGLT2 inhibitors and the clinical implications of associated weight loss in type 2 diabetes: a narrative review. Diabetes Ther. 2021;12:2249–2261. doi: 10.1007/s13300-021-01104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liakos CI, Papadopoulos DP, Sanidas EA, Markou MI, Hatziagelaki EE, Grassos CA, et al. Blood pressure-lowering effect of newer antihyperglycemic agents (SGLT-2 inhibitors, GLP-1 receptor agonists, and DPP-4 inhibitors) Am J Cardiovasc Drugs. 2021;21:123–137. doi: 10.1007/s40256-020-00423-z. [DOI] [PubMed] [Google Scholar]
- 59.Dutka M, Bobiński R, Ulman-Włodarz I, Hajduga M, Bujok J, Pająk C, et al. Sodium glucose cotransporter 2 inhibitors: mechanisms of action in heart failure. Heart Fail Rev. 2021;26:603–622. doi: 10.1007/s10741-020-10041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Granata A, Maccarrone R, Anzaldi M, Leonardi G, Pesce F, Amico F, et al. GLP-1 receptor agonists and renal outcomes in patients with diabetes mellitus type 2 and diabetic kidney disease: state of the art. Clin Kidney J. 2022;15:1657–1665. doi: 10.1093/ckj/sfac069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rossing P, Baeres FMM, Bakris G, Bosch-Traberg H, Gislum M, Gough SCL, et al. The rationale, design and baseline data of FLOW, a kidney outcomes trial with once-weekly semaglutide in people with type 2 diabetes and chronic kidney disease. Nephrol Dial Transplant. 2023;38:2041–2051. doi: 10.1093/ndt/gfad009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Márquez DF, Ruiz-Hurtado G, Segura J, Ruilope L. Microalbuminuria and cardiorenal risk: old and new evidence in different populations. F1000Res. 2019 doi: 10.12688/f1000research.17212.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heerspink HJL, Apperloo E, Davies M, Dicker D, Kandler K, Rosenstock J, et al. Effects of semaglutide on albuminuria and kidney function in people with overweight or obesity with or without type 2 diabetes: exploratory analysis from the STEP 1, 2, and 3 trials. Diabetes Care. 2023;46:801–810. doi: 10.2337/dc22-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jeong SJ, Lee SE, Shin DH, Park IB, Lee HS, Kim K-A. Barriers to initiating SGLT2 inhibitors in diabetic kidney disease: a real-world study. BMC Nephrol. 2021;22:177. doi: 10.1186/s12882-021-02381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mancini GBJ, O’Meara E, Zieroth S, Bernier M, Cheng AYY, Cherney DZI, et al. Canadian cardiovascular society guideline for use of GLP-1 receptor agonists and SGLT2 inhibitors for cardiorenal risk reduction in adults. Can J Cardiol. 2022;38:1153–1167. doi: 10.1016/j.cjca.2022.04.029. [DOI] [PubMed] [Google Scholar]
- 66.Rowlands J, Heng J, Newsholme P, Carlessi R. Pleiotropic effects of GLP-1 and analogs on cell signaling, metabolism, and function. Front Endocrinol. 2018;9:672. doi: 10.3389/fendo.2018.00672/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Das SR, Everett BM, Birtcher KK, Brown JM, Januzzi JL, Kalyani RR, et al. Expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes. J Am Coll Cardiol. 2020;76:1117–1145. doi: 10.1016/j.jacc.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.ADA American diabetes association standards of medical care in diabetes–2017. Diabetes Care. 2022;44:S1–232. [Google Scholar]
- 69.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 70.Houlden RL. Diabetes Canada 2018 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2018;42:S1–5. doi: 10.1016/j.jcjd.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 71.Korayem GB, Alshaya OA, Alghamdi AA, Alanazi SS, Almutib RT, Alsaileek M, et al. The prescribing pattern of sodium-glucose cotransporter-2 inhibitors and glucagon-like peptide-1 receptor agonists in patient with type two diabetes mellitus: A two-center retrospective cross-sectional study. Front Public Health. 2022 doi: 10.3389/fpubh.2022.1031306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ludvik B, Frías JP, Tinahones FJ, Wainstein J, Jiang H, Robertson KE, et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6:370–381. doi: 10.1016/S2213-8587(18)30023-8. [DOI] [PubMed] [Google Scholar]
- 73.Zinman B, Bhosekar V, Busch R, Holst I, Ludvik B, Thielke D, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7:356–367. doi: 10.1016/S2213-8587(19)30066-X. [DOI] [PubMed] [Google Scholar]
- 74.Jabbour SA, Frías JP, Guja C, Hardy E, Ahmed A, Öhman P. Effects of exenatide once weekly plus dapagliflozin, exenatide once weekly, or dapagliflozin, added to metformin monotherapy, on body weight, systolic blood pressure, and triglycerides in patients with type 2 diabetes in the DURATION-8 study. Diabetes Obes Metab. 2018;20:1515–1519. doi: 10.1111/dom.13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vaduganathan M, Patel RB, Singh A, McCarthy CP, Qamar A, Januzzi JL, et al. Prescription of glucagon-like peptide-1 receptor agonists by cardiologists. J Am Coll Cardiol. 2019;73:1596–1598. doi: 10.1016/j.jacc.2019.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vaduganathan M, Sathiyakumar V, Singh A, McCarthy CP, Qamar A, Januzzi JL, et al. Prescriber patterns of SGLT2i after expansions of U.S. food and drug administration labeling. J Am College Cardiol. 2018;72:3370–3372. doi: 10.1016/j.jacc.2018.08.2202. [DOI] [PubMed] [Google Scholar]
- 77.DeFronzo RA. Combination therapy with GLP -1 receptor agonist and SGLT2 inhibitor. Diabetes Obes Metab. 2017;19:1353–1362. doi: 10.1111/dom.12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, et al. Management of hyperglycemia in type 2 diabetes, 2022. a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2022;2022(45):2753–2786. doi: 10.2337/dci22-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Search strategy for the systematic literature review. Table S2. List of studies excluded at the full-text screening stage with reasons for exclusion. Table S3. List of studies excluded from the meta-analysis and reasons for exclusion.
Additional file 2. Assessment of study quality and data extraction.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed.