Abstract
Background
Clostridium piliforme (causative agent of Tyzzer disease) infects various animals, including primates, and hence a threat to animal and human health worldwide. At present, it is detected using traditional methods, such as path morphology, polymerase chain reaction and enzyme‐linked immunosorbent assay. Therefore, it is necessary to develop convenient, efficient visual molecular biological methods for detecting C. piliforme.
Objectives
To establish a method with good specificity, high sensitivity and simple operation for the detection of C. piliforme.
Methods
In this study, we designed internal and external primers based on the conserved 23S rRNA region of C. piliforme to develop a biotin‐labelled diarrhoea‐suffered loop‐mediated isothermal amplification (LAMP) system for detecting of C. piliforme and assessed the specificity, sensitivity and repeatability of the LAMP system.
Results
The LAMP system did not exhibit cross‐reactivity with 24 other common pathogenic species, indicating that it had good specificity. The minimum concentration of sensitivity was 1 × 10−7 ng/μL. Mouse models (Meriones unguiculatus) of Tyzzer disease were established and a LAMP−lateral flow dipstick (LAMP–LFD) was developed for detecting C. piliforme. The detection rate of C. piliforme was 5.08% in clean‐grade animals and 9.96% in specific‐pathogen‐free‐grade animals from Jiangsu, Zhejiang and Shanghai. In addition, the detection rates of C. piliforme were 10.1%, 8.6% and 20%, in animals from Hangzhou, Wenzhou and Shaoxing, respectively. The detection rate of C. piliforme was higher in experimental animals used in schools than in those used in companies and research institutes.
Conclusions
The LAMP–LFD method established in this study can be used to detect C. piliforme in animals handled in laboratory facilities of universities, pharmaceutical enterprises and research and development institutions.
Keywords: Clostridium piliforme, diagnosis, LAMP–LFD, rapid detection, Tyzzer
In this study, we developed a method for the rapid detection of Clostridium piliforme. This method has good specificity, high sensitivity, simple operation and potential for basic promotion application.
1. INTRODUCTION
Clostridium piliforme (the causative agent of Tyzzer disease) was discovered by Ernest Tyzzer in 1917 (Tyzzer, 1917). It infects various animals and causes liver and intestinal necrosis, diarrhoea and death (Tyzzer disease). C. piliforme infection primarily causes asymptomatic or subclinical diseases (Fries, 1979). Animals infected with C. piliforme may suddenly develop illness owing to immunosuppression, environmental changes and other factors, resulting in the interruption of experiments such as tumour transplantation. Therefore, it is one of the pathogens that should be eliminated in clean‐grade laboratory animals. C. piliforme has been detected in patients with human immunodeficiency virus infection, suggesting that patients with a compromised immune system are susceptible to C. piliforme infection (Smith et al., 1996). A serological study involving keepers, related personnel and unrelated personnel who had close contact with laboratory animals reported that the positive rate of C. piliforme detection was higher in personnel who had close contact with laboratory animals (85.7%) than in related personnel (40.5%) and unrelated personnel (22.0%) (Jufang & Jiaming, 1998). Therefore, C. piliforme poses a threat to both laboratory animals and related personnel.
Ganoe et al. (2020) suggested that Tyzzer disease might have contributed to the significant decline in the population of North American muskrats over the last 50 years. A high detection rate of C. piliforme has been observed in rodent laboratory animals. Jie et al. (2015), Jinchun et al. (2017) and Jie et al. (2021) examined pathogen infections in rats and mice in Beijing, Shanghai and Guangdong Province during 2009−2013, 2010−2013 and 2013−2015, respectively. C. piliforme was detected at all time points in the three studies. These findings suggest that C. piliforme infection is a serious public health problem affecting the quality of laboratory animals and the health of the researchers involved.
C. piliforme can be cultured only under in vitro conditions using chicken embryos and specific cell lines (Navarro & Uzal, 2020). Because the embryos and cells used are highly inactive under conventional conditions, the preservation and transmission of the strain are difficult. The biological characteristics, pathogenesis and genetics of C. piliforme remain elusive; moreover, no recent advancements have been made in strategies for detecting the pathogen or diagnosing and preventing Tyzzer disease. At present, C. piliforme is detected via pathological examination, immunofluorescence assay (IFA), polymerase chain reaction (PCR) and enzyme‐linked immunosorbent assay (ELISA) based on a 196‐bp fragment of the 16S rRNA of C. piliforme (Goto & Itoh, 1994).
The Chinese standard GB/T14926.10‐2008 for the detection of C. piliforme in laboratory animals excludes the cortisone excitation test proposed by the original standard and uses only serological antibody detection. Histopathological tests are time‐consuming and require experienced personnel, whereas IFA and ELISA frequently yield false‐negative results in window‐phase or latently infected animals. Moreover, it is difficult to purify antigens for ELISA (Yanbo & Junxia, 2017). These limitations impede the early detection of C. piliforme. Therefore, the above‐mentioned methods are not suitable for diagnosing Tyzzer disease in primary laboratory animal testing and inspection facilities.
Loop‐mediated isothermal amplification (LAMP) is a molecular technique with high specificity in which a set of four specific primers binds to six regions of DNA (F3c, F1c, F2c, B2c, B1c and B3c). The high tolerance of LAMP to enzyme inhibitors allows for onsite assessment of clinical and biological samples without sample enrichment (Das et al., 2022). LAMP assays have been successfully used to detect the pathogens of many infectious diseases, including SARS‐CoV‐2 (Nuchnoi et al., 2023; Ooi et al., 2022), porcine epidemic diarrhoea virus and porcine circovirus type 2 (Areekit et al., 2022), West Nile virus (Tomar et al., 2022), herpesvirus of turkeys (Mescolini et al., 2022), Treponema pallidum (Becherer et al., 2020), Haemophilus ducreyi (Becherer et al., 2020), Ascaridia galli (Panich et al., 2023), Toxoplasma gondii (Xue et al., 2021), Ebola virus (Bonney et al., 2020) and Helicobacter pylori (Horiuchi et al., 2019; Panich et al., 2023). However, LAMP may result in non‐specific binding owing to the formation of primer dimers (Garg et al., 2022). The use of multiple primers increases the risk of primer dimer formation, which can lead to template‐free amplification, producing false‐positive results (Rolando et al., 2020). Therefore, LAMP product amplificatio should be validated using specific DNA probes or restriction endonucleases (Ghaith & Abu Ghazaleh, 2021; Ku et al., 2022).
Although colourimetric indicators, such as SYBR dye, are highly sensitive and time‐efficient and require simple visual testing of the product, they can bind to any double‐stranded DNA with low levels of specificity and produce false‐positive results (Mamba et al., 2018). In a study, a novel technique based on LAMP and a lateral flow dipstick (LFD) was developed for early detection of C. piliforme. This technique reduced the risk of false‐positive results caused by primer dimer formation (Tomar et al., 2022). Labelling the primers enables the visualization of LAMP products in combination with LFD, and the results can be judged by observing the control and the test lines, reducing the likelihood of false‐positive results caused by the non‐specific binding of amplification products. LAMP products combined with test strips can achieve rapid, low‐cost, miniaturized and easy‐to‐operate on‐field detection. This combination strategy can overcome the lack of testing equipment and technical strength and is, therefore, of great significance for controlling the infection and transmission of C. piliforme.
In this study, we established a sensitive and rapid method for detecting C. piliforme. A set of highly specific primers was designed to effectively amplify the 23S rRNA gene of C. piliforme (National Center for Biotechnology Information [NCBI] GeneBank: DQ352811.1, NIH National Library of Medicine). To the best of our knowledge, this study is the first to develop LAMP–LFD for detecting C. piliforme.
2. MATERIALS AND METHODS
2.1. Bacterial and viral strains
C. piliforme and 24 other pathogenic species were used to evaluate the specificity of LAMP and LAMP–LFD (Table 1). All strains were cultured in lysogeny broth (LB)/agar, blood agar plate or specific culture broth/agar (except for C. piliforme) at 37°C.
TABLE 1.
Bacterial strains used in this study.
| Bacterial strain | Origin |
|---|---|
| Salmonella enterica | ATCC15611 |
| Popoff serovar Choleraesuis | ATCC10708 |
| Salmonella enterica | ATCC13314 |
| Salmonella enterica subsp. Enterica | ATCC13076 |
| Escherichia coli | ATCC25922 |
| Shigella boydii | ATCC 9207 |
| Pasteurella pneumotropica | ATCC35149 |
| Salmonella enterica subsp. enterica serovar Pullorum | ATCC13036 |
| Shigella dysenteriae | CGMCC1.1869 |
| Salmonella typhimurium | CGMCC1.1194 |
| Shigella flexneri | CGMCC1.1868 |
| Bordetella bronchiseptica | CMCC58401 |
| Corynebacterium kutscheri | CMCC65013 |
| Klebsiella pneumonia | CMCC46108 |
| Staphylococcus aureus | CMCC26112 |
| Pseudomonas aeruginosa | Isolate (HMC, Mouse) |
| Listeria monocytogenes | Isolate (ZJU) |
| Muribacter muris | Isolate (ZJU, Mouse) |
| Edwardsiella tarda | Isolate (NIFDC, Fish) |
| Streptococcus suis II | Isolate (ZJU) |
| Klebsiella oxytoca | Isolate (Zhejiang Vital River Laboratory Animal Technology Co., Ltd, Cavia porcellus) |
| Aeromonas hydrophila | Isolate (NIFDC, Fish) |
| Salmonella paratyphi A | Isolate (Hangzhou Red Cross Hospital, Human) |
| Klebsiella sp. | Isolate (HMC) |
| Clostridium piliforme | Isolate (HMC, Meriones unguiculatus) |
Abbreviations: HMC, Hangzhou Medical College; NIFDC, National Institutes for Food and Drug Control; ZJU, Zhejiang University.
2.2. Collection of bacterial samples and extraction of DNA
C. piliforme DNA was extracted from Meriones unguiculatus. Total bacterial DNA was extracted from microorganisms using the TIANamp Bacteria DNA Kit (TIANGEN, #DP302‐02) according to the manufacturer's instructions. The extracted DNA was quantified on a NanoDrop 2000 spectrometer (Thermo Fisher Scientific) and stored at −20°C.
2.3. Design of LAMP primers specific for C. piliforme
The 23S rRNA gene sequence of C. piliforme was downloaded from NCBI. The Primer Explorer V5 software was used to design primers and probes specific for 23S rRNA. These primer sequences were tested using against the basic local alignment search tools, and sequences with high specificity were selected from each group. Each set of primers and probes was composed of forward inner primers (FIPs), reverse inner primers (BIPs), forward outer primers (F3), reverse outer primers (B3), loop primers (LF) and a probe. And the 5′‐end of FIPs was labelled with biotin, whereas the probe was labelled with carboxyfluorescein (FAM).
2.4. LAMP reaction system
LAMP reaction was performed according to the method developed by Notomi et al. (2000). The reaction mixture had a final volume of 25 μL, comprising ThermoPol Reaction Buffer (NEB, #B9004S), MgSO4 (NEB, #B1003S), dNTPs (Sangon Biotech, #B500055‐0500), external primers (F3 and B3), internal primers (biotin‐FIP and BIP), Bst 2.0 DNA polymerase (NEB, #M0537S), LAMP fluorescent dye (NEB, #B1700S), LF, template and diethyl pyrocarbonate (DEPC)‐treated water (Sangon Biotech, #B501005‐0500). To prepare the optimal reaction system for LAMP, the final concentration of dNTPs was set to 1.0, 1.2, 1.4, 1.6, 1.8 and 2.0 mM; the final concentration of Mg2+ was set to 0–6 mM; and the internal‐to‐external primer ratios were set to 2:1, 4:1, 6:1, 8:1, 10:1, 12:1, 14:1 and 16:1. The system was optimized at 58, 60, 62, 64 and 66°C, and loop primers were added to accelerate the reaction. The reaction mixture was amplified on the CFX96 Touch Real‐Time PCR Detection System (Bio‐Rad) at 58–66°C for 1 h and terminated at 85°C for 10 min. The amplified product was analysed via fluorescence signal acquisition.
2.5. Assessment of the specificity and repeatability of LAMP and LAMP–LFD methods
The specificity of LAMP and LAMP–LFD was verified according to standard protocols. Genomic DNA extracted from the 24 pathogenic strains was used as the control, the DNA template of C. piliforme was used as the positive control and DEPC‐treated water was used as the negative control. The samples were analysed on the CFX96 Touch Real‐Time PCR Detection System and LFD. The template was used at concentrations of 2.5, 2.5 × 10−1, 2.5 × 10−2 and 2.5 × 10−3 ng/μL for intra‐ and intergroup repetitive experiments. For intragroup repetitive experiments, four replicates were set for each concentration. For intergroup repetitive experiments, three replicates were set for each concentration gradient. The coefficients of variation and mean values were calculated.
2.6. Assessment of the sensitivity of LAMP and LAMP–LFD methods
The positive control template (genomic DNA of C. piliforme) was serially diluted 10‐folded to assess the sensitivity of LAMP and LAMP–LFD. These dilutions ranged from 1 to 1 × 10−9 ng/μL, with DEPC‐treated water being used as the negative control. Each concentration was tested three times. PCR was performed using external primers (F3 and B3) as conventional primers. The reaction mixture had a final volume of 25 μL and consisted of 12.5‐μL Premix Taq (Takara, RR902A), 5‐pmol F3 and B3 and 1‐μL template. The PCR conditions were set as follows: initial denaturation at 94°C for 5 min; denaturation at 94°C for 1 min, annealing at 55°C for 30 s, extension at 72°C for 30 s for 35 cycles and final extension at 72°C for 10 min. The PCR products were separated on 1.5% agarose gels, stained with Yea Red (Yeasen, #10202ES76) and visualized on the ChemiDoc System (Bio‐Rad).
2.7. Construction of mouse models of C. piliforme infection
A total of 29 3‐week‐old male mice (M. unguiculatus) were divided into experimental and control groups. The experimental group containing 24 mice was divided into 4 groups, whereas the control group had 5 mice. After 3 days of stabilization, liver tissues infected with C. piliforme frozen at −80°C were quickly homogenized with sterile PBS without leaving any evident granules. Mice in the experimental group were administered 350 μL of the liver homogenate via gavage, whereas those in the control group were administered 350 μL of normal saline via gavage. The health status of all mice was monitored daily, and their droppings were collected. On day 8, the mice were sacrificed under pentobarbital anaesthesia, and their tissues and organs were collected. Liver tablets were prepared for Giemsa staining, and the collected samples were tested and compared with the set using the nested PCR method established by Niepceron and Licois (2010). The primer sequences used for nested PCR are mentioned in Table 2. The reaction mixture had a final volume of 25 μL and consisted of 2.5 μL of 10× PCR buffer, 2 μL of 2.5‐mM dNTPs, 0.5 μL of 10‐mM primers (OP1 and OP2), 0.15 μL of 5‐U/mL rTaq (Takara, R001A) and 1 μL of template. The PCR conditions were set as follows: initial denaturation at 94°C for 1 min; denaturation at 94°C for 30 s, annealing at 50°C for 30 s and extension at 72°C for 1 min for 25 cycles. The first‐stage amplification product was diluted 10‐fold, and 1 μL was added to enter the second‐stage reaction system, and the primers were used with PiliF and PiliR. The PCR conditions were set as follows: initial denaturation at 94°C for 1 min; denaturation at 94°C for 30 s, annealing at 55°C for 30 s and extension at 72°C for 1 min for 30 cycles. The amplified products were separated on a 1% agarose gel, and the results were visualized on a gel‐imaging system.
TABLE 2.
Sequence and length of loop‐mediated isothermal amplification (LAMP) primers.
| Name | Primer sequence (5′ → 3′) | Length |
|---|---|---|
| LAMP | ||
| F3 | GCTCTGCTACTGTATACTGAA | 21 |
| B3 | ACAATTCGACTATCTCTCATCA | 22 |
| FIP | CCGCTACTTAGGAAATCGATTTTTCGGGGAACGTTGTGAACTG | 43 |
| BIP | CGAGCGAAAGGGAAAGAGGCATGGATTTTGCAGTCCTCAA | 40 |
| LF | CTCTTCCTGTTGCTACTTAGATGTT | 25 |
| Probe | GCCAAACCATAAAGCGTGC | 19 |
| Nested PCR | ||
| OP1 | CCTAACACATGCAAGTC | 17 |
| OP2 | GGCATGATGATTTGACG | 17 |
| PiliF | TGGGATAACATCGAGAAATC | 20 |
| PiliR | TACGTAGYCTGTCAATGGT | 19 |
Abbreviations: B3, reverse outer primer; BIP, reverse inner primer; F3, forward outer primer; FIP, forward inner primer; LF, loop primer; PCR, polymerase chain reaction.
2.8. Application of established LAMP–LFD for detecting C. piliforme
We established a LAMP–LFD method for detecting C. piliforme in mouse liver tissues. DNA was extracted from 399 clean‐grade and in specific‐pathogen‐free (SPF)‐grade mouse liver tissue samples collected from Jiangsu province, Zhejiang province and Shanghai, China, and amplified using LAMP–LFD to detect C. piliforme.
3. RESULTS
3.1. Establishment of the LAMP reaction system
Between the two pairs of primers designed for LAMP, primer 1 had no amplification curve, whereas primer 2 had an amplification curve (Figure 1a,b). Therefore, primer 2 was used for subsequent experiments (Table 2). The specific position of the primer and probe is mentioned in Figure S1a. Located between B1c and B2 primers, the probe was used for molecular hybridization to detect the biotinylated LAMP products of FAM. The final product was a 196‐bp target fragment. All primers were obtained from Sangon Biotech Co., Ltd.
FIGURE 1.
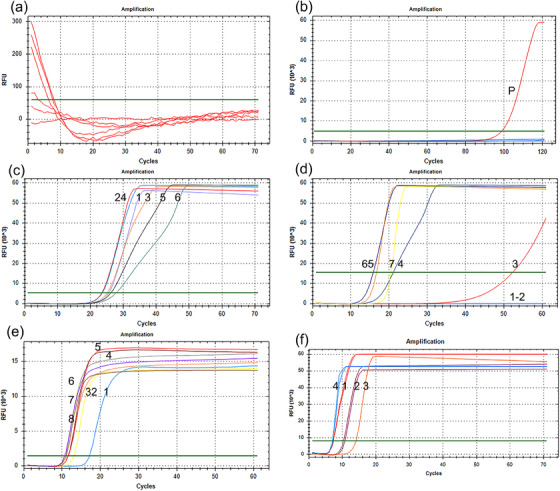
Establishment of loop‐mediated isothermal amplification (LAMP) reaction system. The green line represents the threshold. (a) Amplification curve of primer 1; (b) amplification curve of primer 2; (c) the final concentrations of dNTPs were 1.0, 1.2, 1.4, 1.6, 1.8 and 2.0 mM; (d) the final concentrations of Mg2+ were 0–6 mM; (e) the inner‐to‐outer primer ratios were 2:1, 4:1, 6:1, 8:1, 10:1, 12:1, 14:1 and 16:1; (f) numbers 1−4 represent lysogeny broth (LB), LB + loop primer (LF), no LF and LF, respectively.
Furthermore, each parameter of the reaction system was optimized; the results showed that the amplification curve initially appeared at the final dNTP concentration of 1.6 mM (Figure 1c) and the final Mg2+ concentration of 5 mM (Figure 1d). When the inner‐to‐outer primer ratio was 8:1, 10:1, 12:1, 14:1 or 16:1, the amplification curves appeared almost simultaneously (Figure 1e). Therefore, the inner‐to‐outer primer ratio was determined to be 8:1 according to the literature. Given that the addition of a loop primer can reduce the reaction time, two loop primers (LF and LB) were designed. The amplification curve was initially observed when LF was added (Figure 1f) and when the reaction temperature was set to 62°C (Figure S2a–e). Therefore, the final reaction mixture was prepared using 2.5‐μL ThermoPol reaction buffer, 5‐mM MgSO4, 1.6‐mM dNTPs, 5‐pmol external primers (F3 and B3), 40‐pmol internal primers (biotin‐FIP and BIP), 8‐U Bst 2.0 DNA polymerase (8 U/μL), 0.5 μL LAMP fluorescent dye, 20‐pmol loop primer (LF), 1‐μL template and DEPC‐treated water. F3 and B3 were used to construct C. piliforme plasmids. The results of PCR and sequence comparison are shown in Figure S1c,d. As shown in Figure S1b. A C. piliforme–positive plasmid was successfully constructed.
3.2. Specificity and repeatability of the established LAMP and LAMP–LFD methods
We tested the specificity of the established LAMP and LAMP–LFD methods using genomic DNA extracted from the 24 pathogenic strains and C. piliforme. Only the genomic DNA extracted from C. piliforme was amplified in both LAMP and LAMP–LFD, whereas DNA templates from other strains showed no signal (Figure 2a,c). These results indicated that the LAMP and LAMP–LFD methods had good specificity. Intragroup repetitive experiments were performed using the template at four concentrations (2.5, 2.5 × 10−1, 2.5 × 10−2 and 2.5 × 10−3 ng/μL) (Figure 2b). The coefficients of variation and mean values estimated in both intra‐ and intergroup repetitive experiments are mentioned in Table 3.
FIGURE 2.
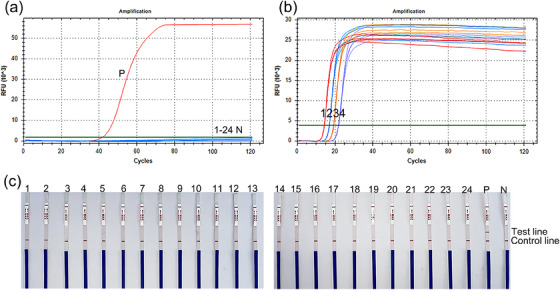
Specificity and repeatability of the established loop‐mediated isothermal amplification (LAMP) and loop‐mediated isothermal amplification−lateral flow dipstick (LAMP–LFD) methods. (a) Real‐time fluorescence curves of LAMP; the black curve is the amplification curve of Clostridium piliforme; (b) repeatability of LAMP; numbers 1−4 represent 2.5, 2.5 × 10−1, 2.5 × 10−2 and 2.5 × 10−3 ng/μL, respectively; (c) detection via LAMP–LFD: Numbers 1−24 are Listeria monocytogenes, Salmonella enterica, Shigella dysenteriae, Salmonella typhimurium, Popoff serovar Choleraesuis, S. enterica, Muribacter muris, Salmonella enterica subsp. enterica, Bordetella bronchiseptica, Edwardsiella tarda, Escherichia coli. Corynebacterium kutscheri, Streptococcus suis, Klebsiella oxytoca, Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus, Aeromonas hydrophila, Salmonella paratyphi A, Pasteurella pneumotropica, Shigella boydii, Shigella flexneri, Klebsiella sp., Salmonella enterica subsp. enterica serovar Pullorum. P: C piliforme, N: negative control.
TABLE 3.
Coefficients of variation and mean values in intra‐ and intergroup repetitive experiments.
| Template concentration (ng/μL) | Intragroup repetitive experiment | Intergroup repetitive experiments | ||
|---|---|---|---|---|
| Mean values/Cq | CV/% | Mean values/Cq | CV/% | |
| 2.5 | 14.28 | 0.41 | 20.62 | 7.3 |
| 2.5 × 10−1 | 16.98 | 0.37 | 24.83 | 3.0 |
| 2.5×10−2 | 19.21 | 0.24 | 28.12 | 3.5 |
| 2.5×10−3 | 21.82 | 0.61 | 33.68 | 1.5 |
3.3. Sensitivity of LAMP–LFD and PCR in detecting C. piliforme
The sensitivity of LAMP–LFD was assessed using the genomic DNA of C. piliforme at different concentrations. The genomic DNA of C. piliforme was serially diluted 10‐fold from 1 to 1 × 10−9 ng/μL for real‐time LAMP and LAMP–LFD assays. The real‐time LAMP signal curve showed that the detection limit for C. piliforme was 1 × 10−7 ng/μL at 70 min (Figure 3a,b). Similar results were observed in LAMP–LFD (Figure 3c). In addition, the results of PCR results showed that the lowest concentration detected was 1 × 10−5 ng/μL (Figure 3d).
FIGURE 3.
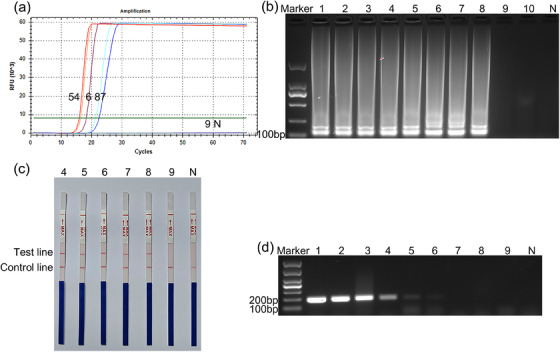
Sensitivity of loop‐mediated isothermal amplification−lateral flow dipstick (LAMP–LFD) and polymerase chain reaction (PCR). (a) Real‐time fluorescence curve of sensitivity of loop‐mediated isothermal amplification (LAMP) for detecting Clostridium piliforme; numbers 4−9 represent 1 × 10−3, 1 × 10−4, 1 × 10−5, 1 × 10−6, 1 × 10−7 and 1 × 10−8 ng/μL of C. piliforme DNA, respectively; lane N represents negative control; (b) agarose gel electrophoresis of LAMP products. Lane marker: 2000‐bp DNA marker, numbers 1−10 represent 1, 1 × 10−1, 1 × 10−2, 1 × 10−3, 1 × 10−4, 1 × 10−5, 1 × 10−6, 1 × 10−7, 1 × 10−8 and 1×10−9 ng/μL of C. piliforme DNA, respectively, Lane N: negative control; (c) detection of C. piliforme via LAMP–LFD. Numbers 4−9 represent 1 × 10−3, 1 × 10−4, 1 × 10−5, 1 × 10−6, 1 × 10−7 and 1 × 10−8 ng/μL of C. piliforme DNA, respectively, N: negative control; (d) agarose gel electrophoresis of PCR products. Lane marker: 1000‐bp DNA marker, numbers 1−9: represent 1, 1 × 10−1, 1 × 10−2, 1 × 10−3, 1 × 10−4 ng/μL, 1 × 10−5, 1 × 10−6, 1 × 10−7 and 1 × 10−8 ng/μL of C. piliforme DNA, respectively. Lane N: negative control.
3.4. Testing of infected samples
After the mice in the experimental group were dissected, evident white lesions were observed in the liver. In addition, a few mice had a black and congested cecum and exhibited hardening of the cecum (Figure 4a,b). On the contrary, no evident abnormality was observed in the livers of mice in the control group (Figure 4c,d). Nested PCR was also positive for infection samples (Figure 4e). A total of 29 3‐week‐old male mice (M. unguiculatus) were divided into experimental and control groups. The above material and method mention it. Giemsa staining oil microscopy can find that most filamentous bacteria are highly suspected of C. piliforme, whereas the control group has no obvious change (Figure 4f−i). In addition, C. piliforme was detected in mouse faeces daily. After nucleic acid extraction of infected samples, C. piliforme was detected in the experimental group but not in the control group (Figure 4j).
FIGURE 4.
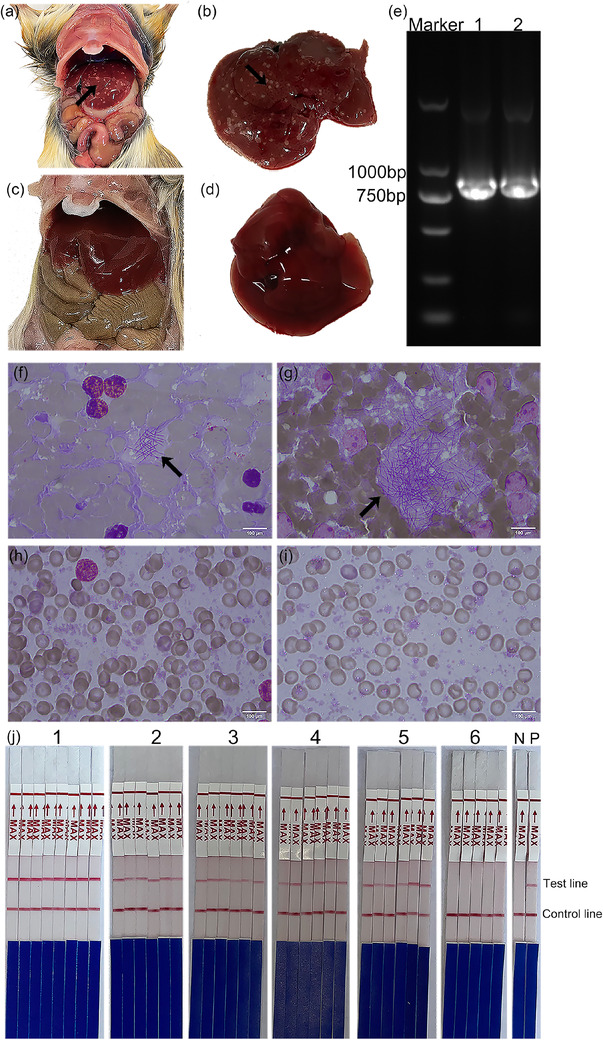
Infected samples were detected via loop‐mediated isothermal amplification−lateral flow dipstick (LAMP–LFD). (a and b) Scattered white lesions were observed on the livers of mice in the experimental group; the lesions are indicated by black arrows; (c and d) the livers of mice in the control group were normal; (e) results of nested polymerase chain reaction (PCR) of infected samples; (f and g) results of Giemsa staining of mouse liver compression tablets in the experimental group. The black arrows indicate bacteria that are highly suspected of being C. piliforme; (h and i) results of Giemsa staining of mouse liver compression tablets in the control group; (j) number 1, results of pathongen detection in stool samples via LAMP–LFD on days 1−8 in the experimental group; numbers 2−5, results of LAMP–LFD in the experimental group; number 6, results of LAMP–LFD in the control group, N: negative control; P: C. piliforme.
3.5. Detection of C. piliforme in clinical samples
We collected 399 clinical samples from 20 centres in Hangzhou, Ningbo, Jiaxing, Shaoxing, Wenzhou, Jinhua, Nanjing and Shanghai, including schools, companies and research institutes. Overall, 8.52% (34/399) of the DNA of C. piliforme was detected in mouse liver tissue via LAMP–LFD. The positive rates of C. piliforme detection in clean‐ and SPF‐grade mice are shown in Table 4. The positive rate of C. piliforme detection in LAMP–LFD was 8.52%, which was higher than that observed in ELISA (1.75%) (Table 5). The positive rates of C. piliforme detection were 8.6%, 10.1% and 20.0% in Wenzhou, Hangzhou and Shaoxing, respectively (Figure 5). The detection rate of C. piliforme was higher in schools than in companies (19/210, 9.0%). However, C. piliforme was not detected in samples collected from research institutes.
TABLE 4.
The mouse samples detected by loop‐mediated isothermal amplification−lateral flow dipstick (LAMP–LFD).
| Mouse | ||
|---|---|---|
| Grade | Positive sample | Total |
| Clean | 6 (5.08%) | 118 |
| SPF | 28 (9.96%) | 281 |
| Total | 34 (8.52%) | 399 |
Abbreviation: SPF, specific‐pathogen‐free.
TABLE 5.
Results of enzyme‐linked immunosorbent assay (ELISA) and loop‐mediated isothermal amplification−lateral flow dipstick (LAMP–LFD) for detection of Clostridium piliforme in mouse liver and serum.
| Sample (n = 399) | ||
|---|---|---|
| Test methods | Positive | Negative |
| LAMP–LFD | 34 (8.52%) | 365 (91.48%) |
| ELISA | 7 (1.75%) | 392 (98.25%) |
FIGURE 5.
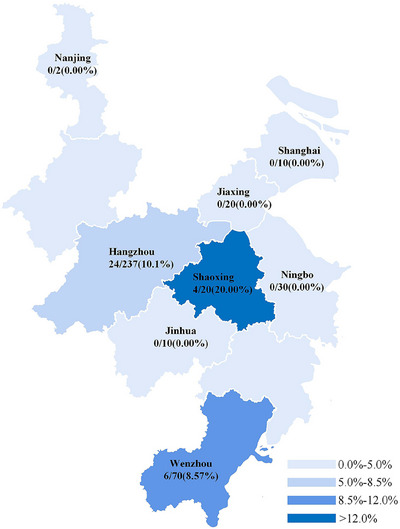
Detection of C. piliforme in clinical samples. Rate of C. piliforme detection via loop‐mediated isothermal amplification−lateral flow dipstick (LAMP–LFD) in eight cities.
4. DISCUSSION
Only a few cases of C. piliforme infection have been reported recently (Ellero et al., 2021; Ganoe et al., 2020; Jacobson et al., 2022; Rho et al., 2022), and no advancements have been made in C. piliforme detection methods. Tyzzer disease caused by the C. piliforme infection has a high mortality rate because the symptoms usually appear shortly before animal death and effective drugs are lacking. Consequently, huge economic losses are incurred by commercial and experimental animal breeding sites infected with C. piliforme. Therefore, developing rapid, economic and efficient detection methods and field tests is important for the early diagnosis, control and treatment of Tyzzer disease. The diagnosis of C. piliforme infection relies on the general and histological manifestations of the liver, bowel or myocardial lesions. In addition, special staining methods can be used; for example, the combination of silver staining and PCR can improve pathogen detection rates. Niepceron and Licois (2010) established a nested PCR method with high sensitivity for the detection of C. piliforme; Tosa et al. (2019) established a multiplex immunochromatographic method for rapid and simple detection of multiple pathogens, including C. piliforme. Rapid, instant and accurate nucleic acid detection is necessary in certain industries; however, some centres are not equipped with complex instruments, such as a PCR system (Wang et al., 2023; Yan et al., 2023).
LAMP is a highly specific and sensitive detection technique developed by Notomi et al. (2000). It enables rapid DNA amplification and involves the use of Bst 2.0 DNA polymerase with chain replacement activity and a set of four specially designed primers, which can recognize six different sequences on the target DNA. To date, no study has reported the use of LAMP for detecting C. piliforme. In this study, we designed a set of primers specific for the 23S rRNA sequence of C. piliforme to establish a LAMP–LFD method for detecting the pathogen.
LAMP products are usually identified via gel electrophoresis, magnesium pyrophosphate turbidity determination and fluorescence‐based assays. Because LAMP involves the use of four or six primers, the likelihood of primer dimer formation is high, resulting in false‐positive results owing to template‐free amplification (Rolando et al., 2020; Schneider et al., 2019). LFD is used to detect LAMP products (Mescolini et al., 2022; Tomar et al., 2022). LAMP–LFD is more specific to the biotin and fluorescein labelling of LAMP products obtained by molecular probe hybridization and combined with a double sandwich.
In this study, when LAMP products were tested via agarose gel electrophoresis, the positive samples showed typical amplification bands with evident boundaries, whereas the negative samples showed amplification bands without evident boundaries. Although positive amplification could be determined, the risk of obtaining false‐positive results was unavoidable. No amplification bands appeared in negative samples when real‐time PCR and LFD were used for detection. These results indicate that agarose gel electrophoresis may not be an appropriate technique for detecting LAMP products.
The kinetics of LAMP reaction remain elusive. To date, no study has quantified the rate of strand displacement synthesis in LAMP reaction (Dangerfield et al., 2023). Real‐time kinetic data of LAMP were used here to predict the concentration curves of different amplicon subtypes. This method may provide information for designing downstream detection strategies (Kaur et al., 2020).
Although loop primers are not essential for LAMP, they can be used to accelerate amplification (Dangerfield et al., 2023). In this study, when two loop primers (LF and LB) were used simultaneously, the reaction time was shortened by approximately 15 min. Moreover, when only one loop primer (LF or LB) was added, the reaction time was further shortened. In particular, the amplicons were detected within 10 min. Therefore, we speculate that only one loop primer is required to accelerate amplification.
Effective drugs and vaccines against C. piliforme infection are lacking. Therefore, it is necessary to eliminate the source of infection and control its spread. The LAMP–LFD method established in this study does not require complex instruments and can be used in the most basic settings. The method did not exhibit cross‐reactivity with 24 pathogenic bacteria commonly detected in experimental animals, indicating that it had good specificity. The detection limit of LAMP–LFD reached 1 × 10−7 ng/μL, whereas that of PCR was 1 × 10−5 ng/μL, which was substantially higher than that of traditional PCR. After establishing mouse models of C. piliforme infection, we collected and tested the faeces of mice daily. The results showed the presence of C. piliforme in faeces every day. These findings indicate that we can reduce the harm caused to laboratory animals while collecting whole blood by using their faeces for detecting C. piliforme. This measure may improve animal welfare and contribute to the healthy development of laboratory animals.
For the 399 clinical samples tested in this study, the detection rate of C. piliforme was substantially higher in LAMP–LFD than in ELISA. The detection rates of C. piliforme were 10.1%, 8.57% and 20% in Hangzhou, Wenzhou, and Shaoxing respectively. In addition, the detection rate was higher in school experimental animals than in companies and research institutes. These results emphasize that experimental animals should be cautiously fed and their health status should be regularly monitored. Efforts should be made to avoid the losses caused by the outbreak of Tyzzer disease and reduce the risk of drawing erroneous conclusions owing to the presence of pathogens in laboratory animals.
5. CONCLUSION
In conclusion, the LAMP–LFD method established in this study has good specificity and high sensitivity for the detection of C. piliforme. The detection limit of LAMP–LFD is higher than that of PCR. LAMP–LFD is a rapid method that can be used for detecting C. piliforme in regions with insufficient equipment.
AUTHOR CONTRIBUTIONS
Data curation; investigation; writing – original draft: Junhao Tao. Data curation; formal analysis; investigation; methodology; software; writing – original draft: Huiqiong Yan. Data curation; formal analysis; software: Sisi Chen. Resources; software: Jiangtao Du. Data curation; methodology; resources; software: Shasang Zhou. Data curation; formal analysis; resources; software; validation: Honggang Guo. Funding acquisition; resources: Lingqun Lu. Resources; validation: Jie Fang. Data curation; resources; software: Xiaoyin Jin. Data curation; resources; visualization: Zhiyuan Wang. Funding acquisition; supervision; visualization: Huazhong Ying. Funding acquisition; investigation; project administration; writing – review and editing: Wei Han and Fangwei Dai.
CONFLICT OF INTEREST STATEMENT
The authors have declared no conflicts of interest.
ETHICS STATEMENT
The animal study was reviewed and approved by Zhejiang Experimental Animal Quality Supervision and Testing Station (reference no. ZJCLA‐IACUC‐20040018). All work using animals was approved by the Ethics Committee for Experimental Animal Welfare of Zhejiang Academy of Medical Sciences and conformed with the Helsinki Declaration of 1975 revised in 2008.
PEER REVIEW
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer‐review/10.1002/vms3.1318.
Supporting information
Figure S1 (a) Position of each primer in the sequence; (b) C. piliforme plasmid sequencing map; (c) plasmid construction colony PCR; (d) alignment map of plasmid sequencing.
Figure S2 Optimization of temperature in the LAMP reaction system. (a–e) 58, 60, 62, 64, 66°C, respectively.
ACKNOWLEDGEMENTS
The authors would like to thank Zhejiang Experimental Animal Quality Supervision and Testing Station for providing samples and complying with laboratory animal welfare guidelines. We would like to thank KetengEdit (www.ketengedit.com) for its linguistic assistance during the preparation of this manuscript.
Tao, J. , Yan, H. , Chen, S. , Du, J. , Zhou, S. , Guo, H. , Lu, L. , Fang, J. , Jin, X. , Wang, Z. , Ying, H. , Han, W. , & Dai, F. (2024). Establishment and application of a loop‐mediated isothermal amplification−lateral flow dipstick (LAMP–LFD) method for detecting Clostridium piliforme . Veterinary Medicine and Science, 10, e1318. 10.1002/vms3.1318
Contributor Information
Wei Han, Email: hanwei3612@163.com.
Fangwei Dai, Email: fangweidai@163.com.
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available on request from the corresponding author.
REFERENCES
- Areekit, S. , Tangjitrungrot, P. , Khuchareontaworn, S. , Rattanathanawan, K. , Jaratsing, P. , Yasawong, M. , Chansiri, G. , Viseshakul, N. , & Chansiri, K. (2022). Development of duplex LAMP technique for detection of porcine epidemic diarrhea virus (PEDV) and porcine circovirus type 2 (PCV 2). Current Issues in Molecular Biology, 44(11), 5427–5439. 10.3390/cimb44110368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becherer, L. , Knauf, S. , Marks, M. , Lueert, S. , Frischmann, S. , Borst, N. , von Stetten , F. , Bieb, S. , Adu‐Sarkodie, Y. , Asiedu, K. , Mitjà, O. , & Bakheit, M. (2020). Multiplex mediator displacement loop‐mediated isothermal amplification for detection of Treponema pallidum and Haemophilus ducreyi . Emerging Infectious Diseases, 26(2), 282–288. 10.3201/eid2602.190505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonney, L. C. , Watson, R. J. , Slack, G. S. , Bosworth, A. , Wand, N. I. V. , & Hewson, R. (2020). A flexible format LAMP assay for rapid detection of Ebola virus. PLoS Neglected Tropical Diseases, 14(7), e0008496. 10.1371/journal.pntd.0008496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangerfield, T. L. , Paik, I. , Bhadra, S. , Johnson, K. A. , & Ellington, A. D. (2023). Kinetics of elementary steps in loop‐mediated isothermal amplification (LAMP) show that strand invasion during initiation is rate‐limiting. Nucleic Acids Research, 51(1), 488–499. 10.1093/nar/gkac1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, D. , Lin, C. W. , & Chuang, H. S. (2022). LAMP‐based point‐of‐care biosensors for rapid pathogen detection. Biosensors (Basel), 12(12), 1068. 10.3390/bios12121068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellero, N. , Lanci, A. , Avallone, G. , Mariella, J. , Castagnetti, C. , Muscatello, L. V. , Di Maio, C. , & Freccero, F. (2021). The first case of Tyzzer's disease in a young foal in Italy: A case report. Veterinaria Italiana, 57(3), 239–246. 10.12834/VetIt.1983.12227.1 [DOI] [PubMed] [Google Scholar]
- Fries, A. S. (1979). Studies on Tyzzer's disease: A long‐term study of the humoral antibody response in mice, rats and rabbits. Laboratory Animals, 13(1), 37–41. 10.1258/002367779781071267 [DOI] [PubMed] [Google Scholar]
- Ganoe, L. S. , Brown, J. D. , Yabsley, M. J. , Lovallo, M. J. , & Walter, W. D. (2020). A review of pathogens, diseases, and contaminants of muskrats (Ondatra zibethicus) in North America. Frontiers in Veterinary Science, 7, 233. 10.3389/fvets.2020.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg, N. , Ahmad, F. J. , & Kar, S. (2022). Recent advances in loop‐mediated isothermal amplification (LAMP) for rapid and efficient detection of pathogens. Current Research in Microbial Sciences, 3, 100120. 10.1016/j.crmicr.2022.100120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaith, D. M. , & Abu Ghazaleh, R. (2021). Carboxamide and N‐alkylcarboxamide additives can greatly reduce non specific amplification in loop‐mediated isothermal amplification for foot‐and‐mouth disease virus (FMDV) using Bst 3.0 polymerase. Journal of Virological Methods, 298, 114284. 10.1016/j.jviromet.2021.114284 [DOI] [PubMed] [Google Scholar]
- Goto, K. , & Itoh, T. (1994). Detection of Bacillus piliformis by specific amplification of ribosomal sequences. Jikken Dobutsu. Experimental Animals, 43(3), 389–394. [PubMed] [Google Scholar]
- Horiuchi, S. , Nakano, R. , Nakano, A. , Hishiya, N. , Uno, K. , Suzuki, Y. , Tanouchi, A. , Kakuta, N. , Masui, T. , Jojima, N. , & Yano, H. (2019). Development of a loop‐mediated isothermal amplification assay for rapid Helicobacter pylori detection. Journal of Microbiological Methods, 163, 105653. 10.1016/j.mimet.2019.105653 [DOI] [PubMed] [Google Scholar]
- Jacobson, S. A. , Ferro, P. J. , Navarro, M. A. , Uzal, F. A. , & Edwards, E. E. (2022). Clostridium piliforme and canine distemper virus coinfection in 2 domestic dog littermates and a gray fox kit. Journal of Veterinary Diagnostic Investigation, 34(5), 894–897. 10.1177/10406387221109899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jie, F. , Cheng, G. , Jin‐Xing, L. , Sheng‐Chang, W. , Jie, Z. , Xiao‐Feng, W. , Cheng, G. , & Zheng‐Hong, X. (2015). Analysis on pathogen infection of mice and rats in Shanghai from 2010 to 2013. Laboratory Animal and Comparative Medicine, 35(5), 398–402. 10.3969/j.issn.1674-5817.2015.05.011 [DOI] [Google Scholar]
- Jie, W. , Jian, H. , & Wenju, L. (2021). Analysis of random testing results of laboratory animals in Beijing area from 2017 to 2019. Laboratory Animal Science, 38(5), 19‐. 10.3969/j.issn.1006-6179.2021.05.005 [DOI] [Google Scholar]
- Jinchun, P. , Weibo, Z. , Meiling, C. , Ruike, W. , Fangui, Z. , Shuwu, H. , Huiwen, Z. , & Zuo, Z. (2017). Microbiological and parasitological investigation in laboratory mice and rats in Guangdong Province from 2013 to 2015. Chinese Journal of Comparative Medicine, 27(02), 64–69. +85 10.3969/j.issn.1671-7856.2017.02.012 [DOI] [Google Scholar]
- Jufang, Y. , & Jiaming, T. (1998). Serological investigation on the infection of Tyzzer in the population. Shanghai Laboratory Animal Science, Z1, 210. [Google Scholar]
- Kaur, N. , Thota, N. , & Toley, B. J. (2020). A stoichiometric and pseudo kinetic model of loop mediated isothermal amplification. Computational and Structural Biotechnology Journal, 18, 2336–2346. 10.1016/j.csbj.2020.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku, J. , Chauhan, K. , Hwang, S. H. , Jeong, Y.‐J. , & Kim, D.‐E. (2022). Enhanced specificity in loop‐mediated isothermal amplification with poly(ethylene glycol)‐engrafted graphene oxide for detection of viral genes. Biosensors (Basel), 12(8), 661. 10.3390/bios12080661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamba, T. S. , Mbae, C. K. , Kinyua, J. , Mulinge, E. , Mburugu, G. N. , & Njiru, Z. K. (2018). Lateral flow loop‐mediated isothermal amplification test with stem primers: Detection of Cryptosporidium species in Kenyan children presenting with diarrhea. Journal of Tropical Medicine, 2018, 7659730. 10.1155/2018/7659730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescolini, G. , Baigent, S. J. , Catelli, E. , & Nair, V. K. (2022). Rapid, sensitive, and species‐specific detection of conventional and recombinant herpesvirus of turkeys vaccines using loop‐mediated isothermal amplification coupled with a lateral flow device readout. Frontiers in Veterinary Science, 9, 873163. 10.3389/fvets.2022.873163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro, M. A. , & Uzal, F. A. (2020). Pathobiology and diagnosis of clostridial hepatitis in animals. Journal of Veterinary Diagnostic Investigation, 32(2), 192–202. 10.1177/1040638719886567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niepceron, A. , & Licois, D. (2010). Development of a high‐sensitivity nested PCR assay for the detection of Clostridium piliforme in clinical samples. Veterinary Journal, 185(2), 222–224. 10.1016/j.tvjl.2009.05.002 [DOI] [PubMed] [Google Scholar]
- Notomi, T. , Okayama, H. , Masubuchi, H. , Yonekawa, T. , Watanabe, K. , Amino, N. , & Hase, T. (2000). Loop‐mediated isothermal amplification of DNA. Nucleic Acids Research, 28(12), E63. 10.1093/nar/28.12.e63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuchnoi, P. , Piromtong, P. , Siribal, S. , Anansilp, K. , Thichanpiang, P. , & Okada, P. A. (2023). Applicability of a colorimetric reverse transcription loop‐mediated isothermal amplification (RT‐LAMP) assay for SARS‐CoV‐2 detection in high exposure risk setting. International Journal of Infectious Diseases: IJID, 128, 285–289. 10.1016/j.ijid.2023.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi, K. H. , Liu, M. M. , Moo, J. R. , Nimsamer, P. , Payungporn, S. , Kaewsapsak, P. , & Tan, M. H. (2022). A sensitive and specific fluorescent RT‐LAMP assay for SARS‐CoV‐2 detection in clinical samples. ACS Synthetic Biology, 11(1), 448–463. 10.1021/acssynbio.1c00538 [DOI] [PubMed] [Google Scholar]
- Panich, W. , Tejangkura, T. , & Chontananarth, T. (2023). Feasibility of a DNA biosensor assay based on loop‐mediated isothermal amplification combined with a lateral flow dipstick assay for the visual detection of Ascaridia galli eggs in faecal samples, Avian Pathology, 52(3), 209–218. 10.1080/03079457.2023.2196251 [DOI] [PubMed] [Google Scholar]
- Rho, J. , Park, H.‐S. , Won, Y.‐S. , Kwun, H.‐J. , & Son, H.‐Y. (2022). Fatal systemic infection of Clostridium tarantellae in a wild Korean Raccoon dog (Nyctereutes procyonoides koreensis). Journal of Wildlife Diseases, 58(2), 421–424. 10.7589/JWD-D-21-00007 [DOI] [PubMed] [Google Scholar]
- Rolando, J. C. , Jue, E. , Barlow, J. T. , & Ismagilov, R. F. (2020). Real‐time kinetics and high‐resolution melt curves in single‐molecule digital LAMP to differentiate and study specific and non‐specific amplification. Nucleic Acids Research, 48(7), e42. 10.1093/nar/gkaa099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, L. , Blakely, H. , & Tripathi, A. (2019). Mathematical model to reduce loop mediated isothermal amplification (LAMP) false‐positive diagnosis. Electrophoresis, 40(20), 2706–2717. 10.1002/elps.201900167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, K. J. , Skelton, H. G. , Hilyard, E. J. , Hadfield, C. T. , Moeller, R. S. , Tuur, S. , Decker, C. , Wagner, K. F. , & Angritt, P. (1996). Bacillus piliformis infection (Tyzzer's disease) in a patient infected with HIV‐1: Confirmation with 16S ribosomal RNA sequence analysis. Journal of the American Academy of Dermatology, 34(2 Pt 2), 343–348. 10.1016/s0190-9622(07)80005-3 [DOI] [PubMed] [Google Scholar]
- Tomar, P. S. , Patel, S. , Dash, P. K. , & Kumar, J. S. (2022). Simple and field amenable loop‐mediated isothermal amplification‐lateral flow dipstick assay for detection of west Nile virus in human clinical samples. Journal of Applied Microbiology, 133(6), 3512–3522. 10.1111/jam.15783 [DOI] [PubMed] [Google Scholar]
- Tosa, N. , Ishida, T. , Yoshimatsu, K. , Hayashimoto, N. , Shiokawa, K. , Takakura, A. , & Arikawa, J. (2019). Multiplex immunochromatographic assay for serologic diagnosis of major infectious diseases in laboratory mice. Journal of the American Association for Laboratory Animal Science, 58(6), 790–795. 10.30802/aalas-jaalas-19-000008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyzzer, E. E. (1917). A fatal disease of the Japanese waltzing mouse caused by a spore‐bearing bacillus (Bacillus piliformis, N. SP.). The Journal of Medical Research, 37(2), 307–338.5. [PMC free article] [PubMed] [Google Scholar]
- Wang, B. , Gan, Q. , Tong, Y. , Qiao, Y. , Han, M. , Zhang, R. , Han, Q. , Li, C. , Bai, S. , Xu, L. , Yin, Y. , Zhang, C. , Munkhtsetseg, B. , Zhao, X. , Meng, M. , & Xi, R. (2023). A visual diagnostic detection of Helicobacter pylori and the gastric carcinoma‐related virulence genes (cagA and vacA) by a fluorescent loop‐mediated isothermal amplification (LAMP). Talanta, 256, 124260. 10.1016/j.talanta.2023.124260 [DOI] [PubMed] [Google Scholar]
- Xue, Y. , Kong, Q. , Ding, H. , Xie, C. , Zheng, B. , Zhuo, X. , Ding, J. , Tong, Q. , Lou, D. , Lu, S. , & Lv, H. (2021). A novel loop‐mediated isothermal amplification‐lateral‐flow‐dipstick (LAMP‐LFD) device for rapid detection of Toxoplasma gondii in the blood of stray cats and dogs. Parasite, 28, 41. 10.1051/parasite/2021039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, Y. , Yang, T. , Luo, Z. , Li, D. , Li, L. , & Lin, X. (2023). Ultrasensitive quantification of pathogens in milliliters of beverage by filtration‐based digital LAMP. Food Chemistry, 408, 135226. 10.1016/j.foodchem.2022.135226 [DOI] [PubMed] [Google Scholar]
- Yanbo, Z. , & Junxia, W. (2017). Evaluation of testing methods in laboratory animal Clostridium piliforme, Mycoplasma pulmonis and rabbit hemorrhagic disease virus. Laboratory Animal Science, 34(02), 71–74. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 (a) Position of each primer in the sequence; (b) C. piliforme plasmid sequencing map; (c) plasmid construction colony PCR; (d) alignment map of plasmid sequencing.
Figure S2 Optimization of temperature in the LAMP reaction system. (a–e) 58, 60, 62, 64, 66°C, respectively.
Data Availability Statement
The data supporting the findings of this study are available on request from the corresponding author.


