Abstract
Studies have shown a relationship between circadian rhythm disruptions and type-2 diabetes. This investigation examined the effects of circadian disruption (6-h phase advances) on the progression of diabetes in a type-2 diabetic mouse model –TALLYHO/JngJ – and whether wheel-running can alleviate the effects of the phase advances. 6-h advances alter fasting glucose, glucose tolerance and insulin production. Wheel-running reduced body mass, improved glucose tolerance and reduced insulin in TALLYHO/JngJ and alleviated some of the changes in diabetic symptoms due to 6-h advances. These results indicate that individuals with type-2 diabetes can benefit from physical activity and exercise can be a countermeasure to offset the effects of an acute phase advance.
Keywords: Advance, jet-lag, mouse, shift-work, TALLYHO/JngJ, type-2 diabetes
Introduction
Type-2 diabetes mellitus (T2DM) is the most common form of diabetes, accounting for ~90% of diabetes cases and affecting over 27 million Americans (American Diabetes Association, 2014). T2DM is often accompanied by obesity and dyslipidemia, as well as long-term physiological complications, such as kidney and nerve damage (Leahy, 2005). Hyperglycemia and poor glucose tolerance for T2DM can be caused either by reduced levels of insulin release due to impaired pancreatic islet β-cell function or destruction (Cnop et al., 2005), or through insulin resistance, where insulin is produced, but glucose uptake by tissues is severely impaired (Weyer et al., 2001). In humans, the etiology of T2DM seems to be caused by a combination of genetic predisposition and risk factors, such as high-calorie diets and reduced activity.
The pancreas, like most peripheral organs, has its own intrinsic circadian oscillator, with rhythmic expression of circadian genes, such as period and bmal1 (Muhlbauer et al., 2004), which in turn regulates the secretion of insulin in a circadian manner (Peschke & Peschke, 1998). Lesions or ablations of the suprachiasmatic nucleus (SCN), which controls the circadian phase of peripheral oscillators, disrupted insulin release, impaired glucose homeostasis and increased body mass (la Fleur et al., 2001). Circadian mutant mice also illustrate the importance of circadian rhythms in glucose and insulin homeostasis. For example, Clock mutant mice have altered gluconeogenesis (Rudic et al., 2004) and reduced insulin release due to smaller pancreatic islets and poorer glucose tolerance (Marcheva et al., 2010), which likely contributes to their obesity and metabolic syndrome (Turek et al., 2005). Complete circadian arrhythmia caused by Bmal1-KO reduced insulin levels and obesity, which were almost completely reversed when “rhythms” were rescued (Sadacca et al., 2010; Shi et al., 2013). Thus, circadian rhythms and diabetes are intimately connected, as disruptions to both systemic circadian regulation of metabolic tissues or the molecular clock can lead to and promote the progression of diabetes.
Shift-work, night-work, rotating work schedules and jet-lag due to frequent travel are more and more prevalent in our society. Shift-work and poor sleep is associated with poor health outcomes, including the progression of T2DM (Morikawa et al., 2005). One contributing factor to this relationship may be the increased consumption of carbohydrate and/or fat-laden foods, along with altered meal patterns, in those experiencing chronic shift-work schedules (Lowden et al., 2010). Individuals with sleep disturbances also have a higher risk of developing T2DM (Kawakami et al., 2004). Moreover, individuals with a genetic predisposition to diabetes have altered daily insulin secretion rhythms (Boden et al., 1999), which might make them more susceptible to external disruptions of the circadian cycle.
We investigated the effects of circadian disruption on the progression of T2DM using a mouse model with polygenic etiology and obese phenotypes. Male TALLYHO/JngJ (TH) mice have been proposed as a translational animal model of the human disease, as they develop T2DM at approximately 10 weeks of age, experiencing hyperglycemia, hyperinsulinemia, insulin resistance and obesity (Kim et al., 2001, 2006; Stewart et al., 2010). Our studies investigated the effects of an acute circadian disruptor on the progression of diabetes phenotypes using 6-h phase advances. We also examined the effects of running-wheel access to determine if they can alleviate the effects of circadian disruption on the progression of diabetes. Recent studies investigating the effects of circadian disruption in the form of shifting light/dark cycles (Gale et al., 2011; Iwamoto et al., 2014) have used multiple 6–8-h shifts per week to produce complete circadian desynchrony. Our study investigated how diabetic mice would respond behaviorally and physiologically the day following a single 6-h shift every 4–6 weeks (mimicking a single west–east airline travel event or a single change in a shift-schedule).
Methods
All animal studies were carried out with the approval from Bridgewater State University’s Institutional Animal Care and Use Committee (IACUC).
Characterizing the circadian locomotor activity phenotype of TH mice
In order to assess the effects of 6-h phase shifts on the diabetes in TH mice, we first characterized the behavioral circadian free-running rhythm of TH mice to determine if they would be a good model for circadian rhythm studies. Eight, 5-week-old, male TH mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA) and were fed standard chow (Lab Diet 5001) and water ad libitum. They were individually housed in running-wheel cages (StarrLife Sciences, Oakmont, PA, USA, wheel diameter: 23 cm) and initially maintained in a 12:12 light–dark cycle (LD; ~50 lux) for 2 weeks. After the entrainment period, all animals were placed into constant darkness (DD). Locomotor activity was monitored by the Vital View Data Acquisition System (StarrLife Sciences) in 10-min bins. Running-wheel activity data were used to determine the free-running locomotor activity rhythm, using the automatic onset detection calculation and Chi-square periodogram in ClockLab (Actimetrics, Wilmette, IL, USA) for both pre-onset diabetes (Weeks 7–9) and post-onset diabetes (Weeks 10–12), as well as overall activity as measured by number of wheel-turns per day. The onset of diabetes was approximated by observing an increase in non-fasting blood glucose levels to above 250 mg/dL at Week 10 (method described below), as previously characterized by Kim et al. (2006). Paired t-tests were used to determine if the behavioral free-running period changed after the approximate onset of diabetes at Week 10 in TH mice.
The effects of wheel-running and phase shifts on the symptoms of T2DM in TH mice
As the T2DM in TH mice is polygenic in nature, a regular genetic control does not exist, as is the case for other polygenic mouse models, and in fact, many other studies have used C57BL/6 (B6) mice as their controls (Kim & Saxton, 2012; Kim et al., 2001, 2006; Leiter, 2009; Mao et al., 2014). Due to this fact, we decided to use TH mice maintained in LD in non-wheel housing as the control group to determine how diabetic individuals respond to access to physical activity and/or phase shifts. Male TH mice (5 weeks) were obtained from Jackson Laboratories, were placed into 12:12 LD for 3 weeks, and given standard chow and water ad libitum. Approximately half of each genotype were placed individually into either a running-wheel cage (W) or a non-running-wheel cage (NW), which uses IR-beams to measure home-cage locomotor activity (StarrLife Sciences). Then, half of each housing condition were either kept in the 12:12 LD cycle or exposed to six 6-h phase advances, one advance every 4–6 weeks, which led to the following four groups: NW LD (control group, n = 7), W LD (exercise group, n = 6),, NW shift (shifted group, n = 7) and W shift (exercise-shifted group, n = 7). An activity bout analysis (using ClockLab) was conducted to examine bout length, counts per bout, and bouts per day under each treatment to ascertain activity differences among the genotypes, housing and lighting conditions, as described in Ahmad et al. (2013) from the week after the arrival until the insulin assay was performed (see below). In addition, the average daily levels of wheel-running or home-cage activity during the light and dark phases, as well as the ratio of light-to-dark activity (e.g. a larger LD ratio would indicate a higher proportion of light activity to total activity), were calculated for the entirety for the experiment in LD mice, and for each epoch for the shifted mice, using Actiview (StarrLife Sciences). Total amount of weight gained throughout the experiment was calculated by subtracting the body mass at Week 30 and Week 6. Additionally, weekly measurements of water and food intake were also measured, taking into account possible food hoarding and spillage by manually searching the bedding for leftover pieces of chow.
Five intraperitoneal glucose tolerance tests (GTTs) (2 g/kg) were conducted at Zeitgeber time (ZT) 4 (i.e. 4 h after the lights turn on), once every 4 weeks starting at Week 8 until Week 24 for all animals. The time ZT 4 was chosen as it is the time of the most impaired glucose tolerance and most likely would be affected by any circadian disruptions. For the shifted animals, the 6-h advance occurred 1 day prior to the advance, so that those mice had one full cycle in the new LD phase. Food was removed, running-wheels locked to prevent wheel-turning by the mice and new bedding was given 12 h prior to the test. Blood glucose was measured using a One-Touch Ultra-2 Glucose Monitor, first prior to the glucose injection (time 0) and then 30, 60 and 120 min post-injection.
At Week 30, whole blood was collected at ZT 4 to perform a lipid panel and insulin ELISA assays (the setup for these assays was performed as described previously for the GTT, except that the food removal and wheel-locking occurred 4 h prior to the test, not 12 h prior). Levels of total cholesterol, HDL cholesterol and triglycerides were measured, using the CardioChek system (Polymer Technology Systems Diagnostics, Indianapolis, IN, USA), by applying 15 μL of whole blood onto the test strip. Serum for measuring insulin concentrations was obtained by centrifuging whole blood at 4°C for 20 min at 2000g. Insulin levels were calculated using the Ultra-Sensitive Mouse Insulin ELISA Kit (Crystal Chem, Downers Grove, IL, USA).
Statistical analyses
The time to re-entrainment to the new photoperiod after the 6-h phase shift was calculated by manually counting the number of days until the animal’s activity onset was at the start of the new lights-on time and was analyzed using an independent t-test. Area under the curve (AUC) was calculated for each mouse for the GTT. Two-way ANOVAs were conducted to determine differences among the different groups, with Tukey HSD post-hoc pairwise comparisons, for food and water intake, weight gain, AUC for GTTs, activity patterns and insulin and lipid levels.
Results
Evaluation of the behavioral circadian rhythm of TH mice in DD
There were no significant changes in the average free-running period pre (23.72 ± 0.07 h) and post (23.75 ± 0.05 h) the onset of diabetes or the average wheel-turns per day (Pre = 27.46 ± 5.10 and Post = 22.76 ± 6.61) (both p > 0.10) (Supplemental Figure 1). TH mice are able to successfully entrain to an LD cycle and show a robust free-running rhythm in DD and have circadian periods comparable to other mouse strains.
Locomotor activity under LD and 6 h phase-advancing conditions
TH mice exhibited stable entrainment under an LD cycle and were able to resynchronize to a shifted LD cycle within a few days, in either wheel or non-wheel housing conditions (Supplemental Figure 2). The means and SEM of all of the activity parameters analyzed are summarized in Table 1. While no differences were found for the bout analysis between mice in LD or shifted conditions, TH mice supplied with a wheel had overall increased locomotor activity (F1,23 = 29.66, p < 0.001). The bout analysis revealed that the increased activity was due to increased counts per bout (F1,23 = 26.45, p < 0.001), not due to increased length of time per bout (F1,23 = 3.19, p < 0.087; all p > 0.10). Additionally, TH without a wheel exhibited increased number of bouts per day (F1,23 = 11.95, p = 0.002). In summary, mice without running-wheels had similar amounts of time being active (bout length), but exhibited reduced levels of locomotor activity (counts per bout) compared to animals provided with a wheel.
Table 1.
Activity profile and bout analysis (mean ± SEM) for all housing and lighting conditions for each genotype.
| Wheel access | Lighting condition | N | Activity per 10 min bin | Light activity | Dark activity | Light to dark activity ratio | Days to re-entrainment | Activity bout length (min) | Counts per bout | Bouts per day |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Y | LD | 6 | 40.73 ± 4.82a | 9.53 ± 0.92a | 69.41 ± 10.06a | 0.15 ± 0.01a | N/A | 49.05 ± 4.02a | 703.60 ± 107.25a | 8.25 ± 0.56a |
| N | LD | 7 | 19.65 ± 3.60b | 10.74 ± 1.57a | 28.64 ± 6.00b | 0.41 ± 0.04b | N/A | 40.18 ± 4.63a | 246.30 ± 53.99b | 10.37 ± 0.51b |
| Y | Shift | 7 | 38.81 ± 5.08a | 2.54 ± 0.38b | 65.78 ± 7.81a | 0.05 ± 0.01c | 1.97 ± 0.14a | 36.91 ± 3.60a | 566.25 ± 102.65a | 8.99 ± 0.41a |
| N | Shift | 7 | 16.97 ± 1.36b | 4.65 ± 1.03b | 28.77 ± 2.80b | 0.17 ± 0.04a | 2.13 ± 0.31a | 43.91 ± 4.13a | 218.12 ± 29.91b | 11.02 ± 0.81b |
TH mice given running-wheels exhibited increased locomotor activity and better light-to-dark activity ratios. Mice without a running-wheel showed increased number of activity bouts per day, but reduced activity per bout compared to mice with a running-wheel. Running-wheel access did not affect time to re-entrainment in mice experiencing the 6-h phase advances. Different letters indicate a significant difference from each other at p < 0.05.
The increase in activity on the wheel came from increased dark phase activity (F1,23 = 33.27, p < 0.001), not from differences in light activity (p = 0.13). Additionally, animals in LD had increased activity in the light phase (F1,23 = 38.13, p < 0.001), but not the dark phase (p = 0.80), compared to animals undergoing the advance. A wheel-by-light interaction was uncovered for the LD ratio (F1,23 = 6.09, p = 0.022), and pairwise comparisons uncovered that TH no-wheel LD mice exhibited significantly higher light–dark ratios compared to animals given a wheel in LD and mice under shifted conditions (all p < 0.001). However, no difference was found between wheel LD mice and wheel-shifted mice (p = 0.10). Last, a t-test revealed no difference between no-wheel and wheel groups for time to re-entrainment (p = 0.30).
Food, fluid and body weight measurements
Wheel-running (F1,24 = 17.55, p < 0.001) and 6-h phase advances (F1,24 = 16.97, p < 0.001) produced a significant increase in weekly food consumption per body weight. No differences were found in water consumption (F1,24 = 0.02, p = 0.91) among any of the groups. Mice in LD and no-wheel housing gained significantly more weight by the end of the experiment compared to animals in LD with a wheel (F1,23 = 6.71, p = 0.016; p = 0.002) and under 6-h advances (p < 0.001). Wheel-running negated the difference in weight gain between LD and phase-advanced mice (p = 0.15) (Supplemental Figure 3).
Glucose tolerance, blood insulin and lipid levels under LD and shifting conditions
At all weeks, except Week 12, TH mice kept in an LD cycle (regardless of wheel access) experienced reduced glucose tolerance, as indicated by increased AUC for the GTT, compared to phase-advanced animals (all p < 0.001) (Figure 1). Wheel-running produced no improvement to glucose tolerance (all p > 0.10), until at Week 24, where glucose tolerance was markedly improved in wheel LD compared to no-wheel LD animals (F1,21 = 8.43; p = 0.009). Still, animals with wheel access in LD had significantly poorer glucose tolerance compared to shifted animals with a wheel (p = 0.003) and without one (p = 0.009).
Figure 1.
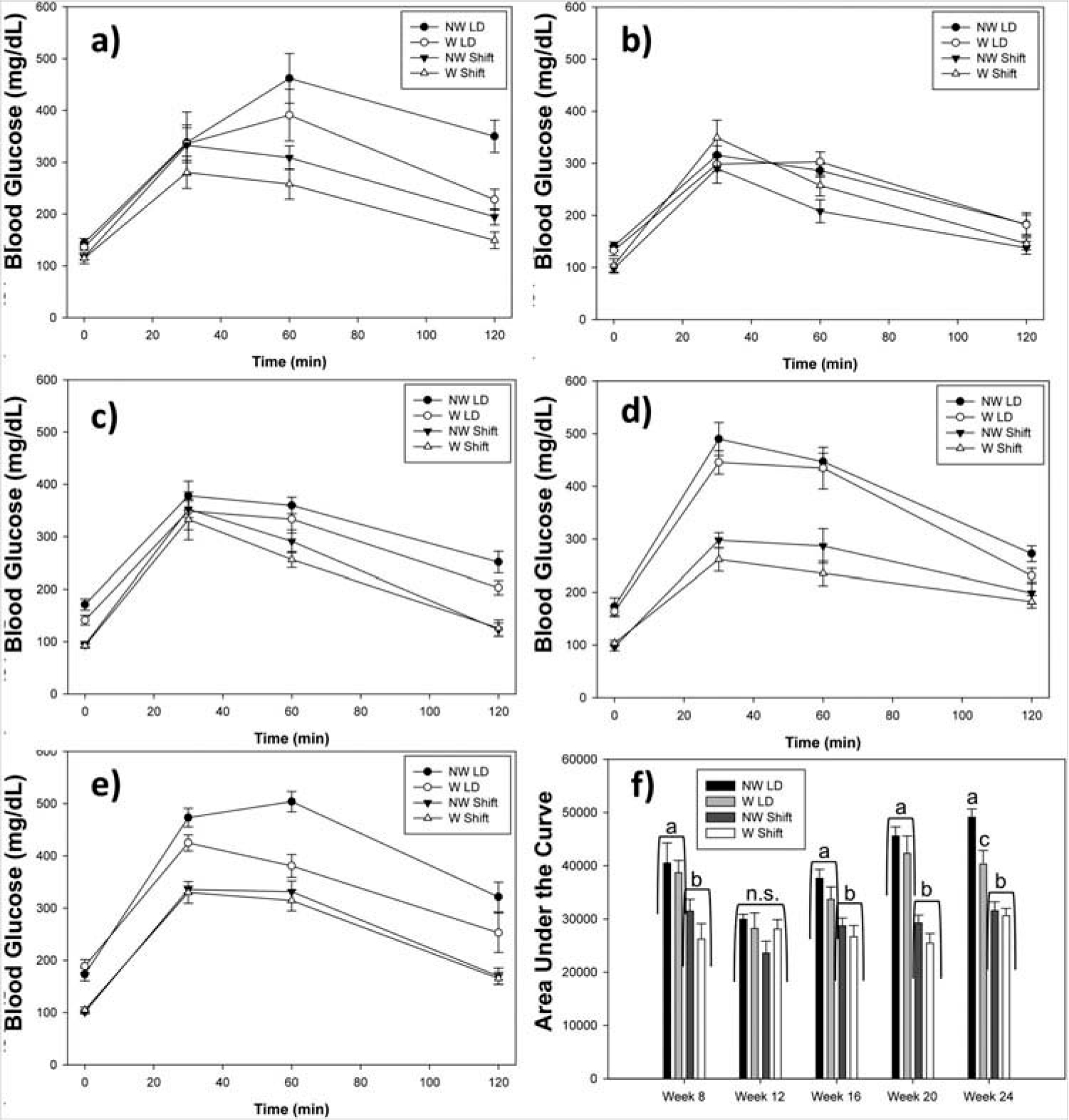
The effects of running-wheel access on glucose tolerance. (a) Week 8, (b) Week 12, (c) Week 16, (d) Week 20, (e) Week 24, (f) area under the curve (AUC). Shifted mice exhibited reduced AUC compared to animals held in an LD cycle, at all weeks except for Week 12 (all p < 0.05). Means ± SEM. Filled-in shapes refer to animals without running-wheels and open shapes refer to animals with wheels. Circles refer to animals in LD and triangles refer to animals receiving the shift. Different letters indicate a significant difference from each other at p < 0.05; n.s. indicates no significant differences during that week.
Twelve-hour fasting glucose (Time 0) was also significantly reduced in phase-advanced mice at all weeks (all p < 0.01). In order to rule out possible differences in fasting glucose between the TH mice selected for each condition, a “no-shift control” blood glucose measurement was performed at the same ZT at Weeks 22 and 26 (2 weeks after the prior GTT) after a 12-h fast in the same animals. t-Tests with the Bonferroni correction showed that TH mice when exposed to the shift had reduced levels of fasting blood glucose at both Week 20 (100 ± 5) and Week 24 (101 ± 3) compared to when the animals were not shifted at Weeks 22 (169 ± 8) and 26 (114 ± 6) (p = 0.001 and p = 0.084, respectively), indicating that it was the 6-h phase advance that led to the reduced glucose.
Long-term wheel-running (F1,21 = 7.77; p = 0.011; p < 0.001) and 6-h advances (p = 0.004) reduced the hyperinsulemia normally exhibited by TH mice (without wheel access) at Week 30; however, animals with wheel access in LD exhibited similar insulin levels to shifted animals on a wheel (p = 0.98) (Table 2). Six-hour advances produced reductions in total cholesterol (F1,21 = 19.97; p < 0.001). Additionally, wheel-running also negated the significant reductions in total cholesterol levels seen in LD vs. shifted animals (p = 0.17). Last, wheel-running produced a marginal reduction on whole-blood triglyceride levels (F1,21 = 3.56; p = 0.073), while 6-h advances produced significant reductions in triglycerides (F1,21 = 10.08; p = 0.005).
Table 2.
Insulin levels and lipid panel profile (means ± SEM) for 30-week-old TH mice under different housing and lighting conditions.
| Wheel access | Lighting condition | Insulin (ng/mL) | Total cholesterol (mg/dL) | HDL cholesterol (mg/dL) | Triglyceride (mg/dL) |
|---|---|---|---|---|---|
|
| |||||
| N | LD | 5.56 ± 0.70a | 175 ± 6a | >85a | 469 ± 19a |
| Y | LD | 1.45 ± 0.30b | 135 ± 7b | >85a | 381 ± 21bA |
| N | Shift | 3.03 ± 0.79c | 107 ± 5b | >85a | 347 ± 39b |
| Y | Shift | 1.39 ± 0.19b | 116 ± 4b | >85a | 293 ± 53b |
Running-wheel access and 6-h advances reduced insulin, cholesterol, and triglyceride levels. Additionally, insulin levels were reduced in animals experiencing the 6-h advance in animals without a running wheel, but this effect was not found in animals with a running-wheel. Different letters indicate a significant difference from each other at p < 0.05; ^: p = 0.073.
Discussion
It is the first time, to our knowledge, that the TH mouse was utilized in a circadian study. Their free-running rhythms were assessed to make certain they had normal circadian periods and to see if they could entrain to a LD cycle. In both wheel and non-running-wheel cages, TH mice are able to synchronize and re-entrain to a LD cycle and have circadian activity behavior similar to other mouse strains. The circadian locomotor activity pattern and free-running rhythm did not change after the normal onset of diabetes in this mouse strain around Week 10, despite seeing a progression of diabetic phenotype through impaired glucose tolerance. During the 6-h phase advances, however, that impairment of glucose tolerance was much less pronounced, even at Week 8, before the onset of full-blown diabetes. Still, while glucose levels and tolerance have not been completely altered yet, it is worth noting that young TH mice (Week 8 and earlier) exhibit some diabetic phenotypes including increased insulin, cholesterol and triglyceride levels, obesity and larger pancreatic islets (Kim et al., 2006; Stewart et al., 2010). So while the younger TH mice were not fully diabetic yet, the circadian disruption still produced a decrease in glucose tolerance and fasting glucose similar to TH mice at Weeks 16, 20 and 24, indicating the effects of the circadian disruptions might be similar among pre-diabetic and diabetic individuals. Still, male TH mice exhibit the traits associated with obese, type-2 diabetics, and since they have a clear and stable behavioral circadian rhythm, this mouse may be a good model for testing the effects of circadian disruption on T2DM.
Although disturbances to the circadian rhythm can come in many forms (including jet-lag and shift-work), they have all been connected to health problems as alterations have been shown to interrupt metabolic and endocrine functions, affect body weight regulation and affect glucose/lipid homeostasis in a wide variety of ways (Scheer et al., 2009). In the current study, animals experiencing the monthly 6-h advance had alterations to their insulin levels and glucose tolerance compared to the mice held in a LD cycle. While normally exhibiting hyperinsulinemia, TH mice exposed to the shifted cycle had lower insulin levels compared to LD controls. A recent study found a similar result, where HIP rats when exposed to repeated 6-h advances exhibited reduced insulin secretion in response to glucose administration (Gale et al., 2011). These results indicate that no matter how T2DM manifests itself (hyperinsulinemia in TH versus loss of β-cells for HIP), severe alterations to the phase of a lighting cycle seem to produce decreases in insulin secretion. Regardless of lighting condition, mice with access to running-wheels had similar levels of insulin to each other and reduced relative to mice without access in LD. Additionally, the effects of running-wheel access on other measures were also independent of lighting condition, as body mass, cholesterol, and triglyceride levels (at Week 30) were similar across groups all experimental groups. Each of these measures were actually reduced compared to mice without running-wheels, further suggesting wheel-running, or chronic exercise, promotes healthy outcomes.
A surprising result was the reduced glucose levels found in shifted TH mice, as chronobiological disruptions normally elevate blood glucose levels (Gale et al., 2011; Varcoe et al., 2011). A possibility might be that TH mice are more susceptible to circadian disruptions compared to other mouse strains and that we are measuring these responses during a different phase of their glucose rhythm. While TH mice can entrain to a LD cycle, they seem to have more activity in the light or inactive phase of their day, as evidenced by their increased light-to-dark ratio in LD found in this study. This result is corroborated by a recent study showing that TH mice feed and drink more during the inactive phase (i.e. the light phase) of their daily cycle than non-diabetic mice (Mao et al., 2014). These results might suggest that there may be desynchrony between their master clock in the SCN and peripheral oscillators, such as the pancreas and liver, which control metabolic functions, even under a stable LD cycle. As T2DM alter insulin secretion rhythms (Boden et al., 1999) and other rhythms including melatonin (Mantele et al., 2012), the peripheral oscillator might be at a different phase compared to their behavioral rhythm, which means we measured the glucose tolerance in TH mice at a different “physiological” circadian phase compared to their “behavioral” phase. The fact that TH mice seemingly immediately shift to the new LD cycle (about two days) might support the notion that these mice might behaviorally entraining at a quicker pace than physiologically, which might exacerbate the phase difference between central and peripheral oscillators.
In this study, body mass and glucose tolerance did not change significantly between running-wheel and non-running-wheel mice in LD until Week 24, where TH mice seem to benefit from long-term running-wheel access. Additionally, at Week 30, TH mice were displaying lower blood insulin, cholesterol and triglycerides, despite having increased food intake with running-wheel access similar to other strains of mice (Swallow et al., 2001). So while a reduction in caloric intake cannot explain the improved diabetic symptoms in TH mice with a wheel, these improvements may be due to improved glucose uptake through upregulated glucose transporters. Normally, impaired insulin signaling due to T2DM can lead to the downregulation of the insulin-responsive glucose transporter, GLUT4 (Ostenson, 2001). Wheel-running has been shown to upregulate expression of GLUT4 in skeletal muscle (Gulve et al., 1993). These results indicate that a possible upregulation of GLUT4 transporters might be a mechanism of how wheel-running might help TH mice. Obesity and insulin resistance can also lead to reductions in GLUT4 by itself (Favaretto et al., 2014), and as obesity is often accompanied by low physical activity, there appears to be an onus on type-2 diabetics to maintain an exercise regimen in order to stave off the development of T2DM, regardless if they lose weight or not. Even with a running-wheel, TH mice are still considered obese, with a body weight significantly higher than other inbred mouse strains, but they seem to benefit from wheel access.
In conclusion, we report that insulin and glucose tolerance are significantly altered the day after a 6-h phase advance compared to a stable LD cycle in a type-2 diabetic mouse – TALLYHO/JngJ. Additionally, total body mass gain at the end of the experiment was also reduced in mice experiencing the 6-h phase advances. While ineffective in altering glucose tolerance early on, wheel-running did produce improved glucose tolerance, insulin levels and reduced obesity in TH mice. These results indicate that physical activity can improve the symptoms of T2DM and might be a method used to prevent some (but not all) of the negative health consequences associated with jet-lag and shift-work.
Supplementary Material
Acknowledgements
The authors would like to thank Nicole Arruda, Isabella De Pina Monteiro, Rachel Gelineau, Gina Nash, Dylon Pyne and Josh West for their help with animal maintenance and weekly measurements.
Funding
This work was supported by BSU ATP, CARS and Daniel Smith Awards.
Footnotes
Declaration of interest
The authors declare no competing financial interests.
References
- Ahmad ST, Steinmetz SB, Bussey HM, et al. (2013). Larval ethanol exposure alters free-running circadian rhythm and per Locus transcription in adult D. melanogaster period mutants. Behav Brain Res. 241:50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. (2014). Diagnosis and classification of diabetes mellitus. Diabetes Care. 37:S81–S90. [DOI] [PubMed] [Google Scholar]
- Boden G, Chen X, Polansky M. (1999). Disruption of circadian insulin secretion is associated with reduced glucose uptake in first-degree relatives of patients with type 2 diabetes. Diabetes. 48:2182–8. [DOI] [PubMed] [Google Scholar]
- Cnop M, Welsh N, Jonas JC, et al. (2005). Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: Many differences, few similarities. Diabetes. 54:S97–107. [DOI] [PubMed] [Google Scholar]
- Favaretto F, Milan G, Collin GB, et al. (2014). GLUT4 defects in adipose tissue are early signs of metabolic alterations in Alms1GT/GT, a mouse model for obesity and insulin resistance. PLoS One. 9:e109540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale JE, Cox HI, Qian J, et al. (2011). Disruption of circadian rhythms accelerates development of diabetes through pancreatic beta-cell loss and dysfunction. J Biol Rhythms. 26:423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulve EA, Rodnick KJ, Henriksen EJ, Holloszy JO. (1993). Effects of wheel running on glucose transporter (GLUT4) concentration in skeletal muscle of young adult and old rats. Mech Ageing Dev. 67:187–200. [DOI] [PubMed] [Google Scholar]
- Iwamoto A, Kawai M, Furuse M, Yasuo S. (2014). Effects of chronic jet lag on the central and peripheral circadian clocks in CBA/N mice. Chronobiol Int. 31:189–98. [DOI] [PubMed] [Google Scholar]
- Kawakami N, Takatsuka N, Shimizu H. (2004). Sleep disturbance and onset of type 2 diabetes. Diabetes Care. 27:282–3. [DOI] [PubMed] [Google Scholar]
- Kim JH, Saxton AM. (2012). The TALLYHO mouse as a model of human type 2 diabetes. Methods Mol Biol. 933:75–87. [DOI] [PubMed] [Google Scholar]
- Kim JH, Sen S, Avery CS, et al. (2001). Genetic analysis of a new mouse model for non-insulin-dependent diabetes. Genomics. 74:273–86. [DOI] [PubMed] [Google Scholar]
- Kim JH, Stewart TP, Soltani-Bejnood M, et al. (2006). Phenotypic characterization of polygenic type 2 diabetes in TALLYHO/JngJ mice. J Endocrinol. 191:437–46. [DOI] [PubMed] [Google Scholar]
- la Fleur SE, Kalsbeek A, Wortel J, et al. (2001). A daily rhythm in glucose tolerance: A role for the suprachiasmatic nucleus. Diabetes. 50:1237–43. [DOI] [PubMed] [Google Scholar]
- Leahy JL. (2005). Pathogenesis of type 2 diabetes mellitus. Arch Med Res. 36:197–209. [DOI] [PubMed] [Google Scholar]
- Leiter EH. (2009). Selecting the “right” mouse model for metabolic syndrome and type 2 diabetes research. Methods Mol Biol. 560:1–17. [DOI] [PubMed] [Google Scholar]
- Lowden A, Moreno C, Holmback U, et al. (2010). Eating and shift work – effects on habits, metabolism and performance. Scand J Work Environ Health. 36:150–62. [DOI] [PubMed] [Google Scholar]
- Mantele S, Otway DT, Middleton B, et al. (2012). Daily rhythms of plasma melatonin, but not plasma leptin or leptin mRNA, vary between lean, obese and type 2 diabetic men. PLoS One. 7:e37123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Dillon KD, McEntee MF, et al. (2014). Islet insulin secretion, β-cell mass, and energy balance in a polygenic mouse model of type 2 diabetes with obesity. J Inborn Errors Metabol Screen. 2:1–6. [Google Scholar]
- Marcheva B, Ramsey KM, Buhr ED, et al. (2010). Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 466:627–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa Y, Nakagawa H, Miura K, et al. (2005). Shift work and the risk of diabetes mellitus among Japanese male factory workers. Scand J Work Environ Health. 31:179–83. [DOI] [PubMed] [Google Scholar]
- Muhlbauer E, Wolgast S, Finckh U, et al. (2004). Indication of circadian oscillations in the rat pancreas. FEBS Lett. 564:91–6. [DOI] [PubMed] [Google Scholar]
- Ostenson CG. (2001). The pathophysiology of type 2 diabetes mellitus: An overview. Acta Physiol Scand. 171:241–7. [DOI] [PubMed] [Google Scholar]
- Peschke E, Peschke D. (1998). Evidence for a circadian rhythm of insulin release from perifused rat pancreatic islets. Diabetologia. 41:1085–92. [DOI] [PubMed] [Google Scholar]
- Rudic RD, McNamara P, Curtis AM, et al. (2004). BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2:1893–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadacca LA, Lamia KA, deLemos AS, et al. (2010). An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia. 54:120–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS, Shea SA. (2009). Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 106:4453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi SQ, Ansari TS, McGuinness OP, et al. (2013). Circadian disruption leads to insulin resistance and obesity. Curr Biol. 23:372–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart TP, Kim HY, Saxton AM, Kim JH. (2010). Genetic and genomic analysis of hyperlipidemia, obesity and diabetes using (C57BL/6J × TALLYHO/JngJ) F2 mice. BMC Genom. 11:713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow JG, Koteja P, Carter PA, Garland T Jr. (2001). Food consumption and body composition in mice selected for high wheel-running activity. J Comp Physiol B. 171:651–9. [DOI] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, et al. (2005). Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 308:1043–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varcoe TJ, Wight N, Voultsios A, et al. (2011). Chronic phase shifts of the photoperiod throughout pregnancy programs glucose intolerance and insulin resistance in the rat. PLoS One. 6:e18504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyer C, Tataranni PA, Bogardus C, Pratley RE. (2001). Insulin resistance and insulin secretory dysfunction are independent predictors of worsening of glucose tolerance during each stage of type 2 diabetes development. Diabetes Care. 24:89–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


