Abstract
The effect of ivermectin (IVM) in treating coronavirus disease 2019 (COVID-19) is still controversial, yet the drug has been widely used in the world. The aim of this review was to systematically evaluate the clinical outcomes of IVM in patients with COVID-19. From inception to June 22, 2023, the PubMed, EMBASE, Web of Science (WOS), and scopus databases were searched for relevant observational studies on the risk of RA in migraineurs. We searched PubMed/Medline, EMBASE, the Cochrane Library, Web of Science, medRxiv, and bioRxiv to collect all relevant publications from inception to June 22, 2023. Primary outcomes were all-cause mortality rate, mechanical ventilation (MV) requirement, PCR negative conversion, and adverse events (AEs). Revman 5.4 was used to assess the risk of bias (RoB) and quality of evidence. Thirty-three RCTs (n = 10,489) were included. No significant difference in all-cause mortality rates or PCR negative conversion between IVM and controls. There were significant differences in MV requirement (RR 0.67, 95% CI 0.47–0.96) and AEs (RR 0.87, 95% CI 0.80–0.95) between the two groups. Ivermectin could reduce the risk of MV requirement and AEs in patients with COVID-19, without increasing other risks. In the absence of a better alternative, clinicians could use it with caution.
Keywords: Ivermectin, Treatment, COVID-19, SARS-CoV-2, Meta-analysis
Graphical abstract
1. Introduction
Despite the concerted efforts and the relatively successful vaccination against the coronavirus disease 2019 (COVID-19) around the world [1,2], the pandemic is likely to last for a long time due to the emergence of multiple variants and anti-vaccine movements worldwide [[3], [4], [5]]. In this context, the potential of several drugs to alleviate the symptoms of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or the symptoms of COVID-19 has been evaluated [6]. However, to date, few pharmacotherapies have shown efficacy in reducing the rate of hospitalizations, mortality, or mechanical ventilation (MV) [7,8].
Drug repositioning, also known as drug recycling or drug repurposing, is an effective approach to find new indications for approved drug. A repositioned drug has all reliable data of safety and pharmacokinetic profiling [9,10]. Therefore, drug repositioning is highly efficient, low-cost and riskless. Which can significantly reduce the time required to produce an effective drug to treat COVID-19. Ivermectin (IVM), a semisynthetic, anti-parasite agent [11], has attracted much attention as a potential drug for COVID-19 [12], and has been widely used off label to control COVID-19 [[13], [14], [15]]. In cells infected by SARS-CoV-2, IVM has been shown to inhibit both viral adherence and replication, and can reduce the concentration of viral RNA by nearly 5000-fold [[16], [17], [18]]. Which raised hopes of clinical benefit to the treatment of COVID-19. However, the concentration in cell culture was equivalent to >50-fold the normal maximum safe dosage allowed for patients daily, which raised concerns about the efficacious dose and tolerability of ivermectin for the treatment of SARS-CoV-2 infection in humans [18].
Multiple clinical trials have been conducted to evaluate clinical outcomes [[19], [20], [21], [22], [23], [24], [25], [26]], with contradictory outcomes, and some of these studies have been withdrawn or retracted for fear of serious data inconsistencies or research fraud [[27], [28], [29]]. It is imperative to synthesize evidence for clinicians and communities. Systematic reviews have been performed on this topic, but in the majority of them a retracted trial accounted for more than 10% of the overall effect [27,[30], [31], [32]], which overestimated the benefits. However, we additionally evaluated hospital admission, mortality, arrhythmia, and compliance. In addition, we enrolled more studies, and the sample size was larger.
Because the available evidence on the benefits of IVM in the treatment of people with COVID-19 remains controversial and there is a risk of serious adverse events (SAEs), the WHO living guideline recommends IVM for COVID-19 only within clinical trials, and the Infectious Diseases Society of America's guideline suggests against IVM for treating patients with COVID-19 [6,33]. Therefore, there is still a lack of up-to-date and reliable evidence synthesis of the effect of IVM in patients with COVID-19. The aim of this study was to synthesize the evidence to critically appraise the therapeutic effects and adverse events (AEs) of IVM for COVID-19.
2. Method
This systematic review was carried out based on recommendations from the Cochrane Handbook [34] and the latest Preferred Reporting Items for Systematic Reviews of Interventions (PRISMA 2020) statements [35], and the protocol of our study was prospectively registered in PROSPERO (CRD42022364559).
2.1. Search strategy
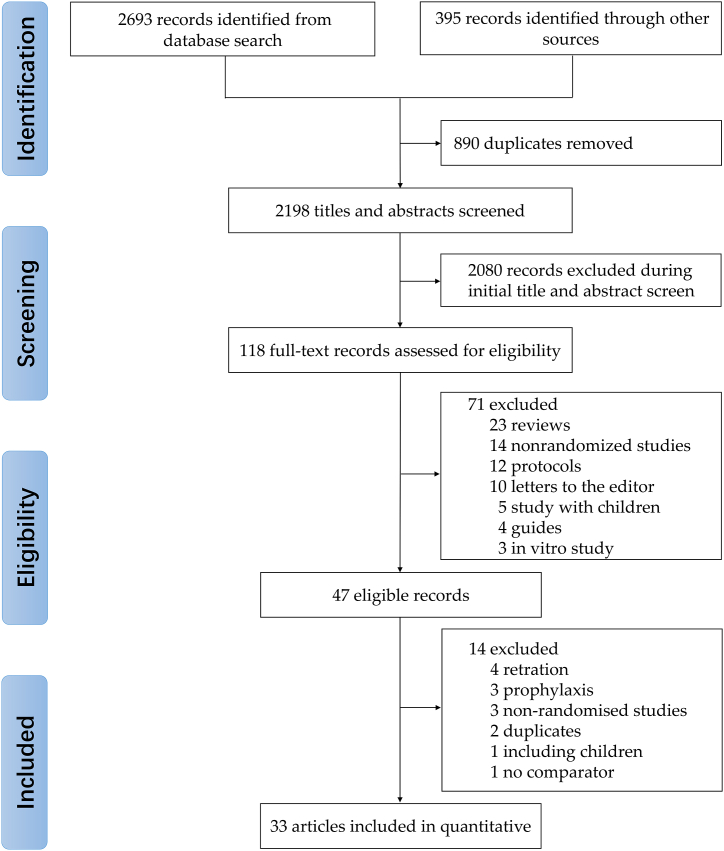
We searched PubMed/Medline, EMBASE, the Cochrane Library, Web of Science, medRxiv, and bioRxiv to collect all relevant publications from inception to June 22, 2023. There were no restrictions on language, publication status, date, region, or participant demographics. Descriptors were identified in Medical Subject Headings (MeSH), Embase Subject Headings (Emtree) and Descritores em Ciências da Saúde (Decs). The Cochrane-validated filter for randomized controlled trials was used [36]. The search strategy was adjusted based on descriptors in each specific database and the complete search strategy is shown in Fig. 1. In addition, references of all included studies were also searched manually to identify any potential qualified studies.
Fig. 1.
PRISMA flow diagram.
2.2. Eligibility criteria
We included randomized controlled trials (RCTs) reporting benefit or harm outcomes of IVM for treating adults with COVID-19, irrespective of COVID-19 severity. Controls were placebo or the standard of care (SOC). Case reports, case series, policy reports, conference abstracts, commentaries, and editorials were excluded. Studies evaluated the use of IVM as adjuvant or combination therapy were excluded. Studies assessing IVM as prophylaxis against COVID-19 infection were also excluded.
2.3. Outcomes
Primary outcomes were all-cause mortality rate, length of hospital stay (LOS), PCR negative conversion, and AEs; and secondary outcomes included symptoms resolved, viral clearance, admission to intensive care unit (ICU), MV requirement, discharged from hospital, hospitalization due to progression, and SAEs. AEs were defined based on Common Terminology Criteria for Adverse Events (CTCAE), and SAEs were defined based on Food and Drug Administration (FDA) and National Cancer Institute (NCI).
2.4. Data extraction
Two investigators (ZS and SS) independently screened titles and abstracts, and then evaluated full texts of selected abstracts. Disagreements were resolved through discussion or by a third investigator (YZ).
We developed a Microsoft Excel template to extract data systematically, including study design (methods, location, eligible criteria, follow-up duration and sample size), participant characteristics (disease severity, sex and age), intervention and comparator characteristics (dosage and frequency of IVM/comparator, comparator, outcome measures at baseline, and measures of outcome from baseline to the end of follow-up). In studies with more than two study arms, only data from arms relevant to our review were extracted.
We planned to contact authors of (potentially) eligible studies to provide relevant information. If a study reported interquartile range and median, methods recommended by Wan et al. [37] and Luo et al. [38] were used to estimate the standard deviation (SD) and mean deviation (MD) for data pooling if there were no significant bias on the ground of the test by Shi et al. [39].
2.5. Quality assessment
Two authors (ZS and LZ) independently evaluated trials for risk of bias using the revised Cochrane risk-of-bias tool for randomized trials (RoB 2.0) [40]. The overall certainty of the body of evidence was rated according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, allowing for overall risk of bias, consistency of effect, imprecision, indirectness and publication bias to assess the certainty of the body of evidence [41,42]. In case of serious concerns in any of these domains, the quality of evidence will be rated down. The overall RoB 2.0 judgment was incorporated into the GRADE assessment.
2.6. Statistical synthesis
LOS was expressed as mean difference; for other outcomes, we pooled RR and its 95% confidence interval (CI) using a fixed-effects or random-effects model depending on the presence of heterogeneity [43,44]. Heterogeneity between studies was assessed using Cochran's Q test with a significance level of P < 0.10 and further quantified using I2 statistics. When values were less than 25%, 25–75%, and more than 75%, respectively, I2 values were rated as low, moderate, and high degrees of heterogeneity.
Subgroup analysis was conducted for all-cause mortality rate by the controls, and sensitivity analysis was conducted for all-cause mortality rate by severity of COVID-19.
Funnel plot was drawn to investigate the possibility of publication bias, and the symmetry of the funnel plot was visually evaluated [45].
Analyses were performed using the RevMan 5.4 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020).
3. Results
3.1. Literature selection and study characteristics
Through a systematic database search, we identified 2693 records and 395 additional records from the gray literature. 118 full-text articles or unpublished datasets were evaluated for eligibility after the removal of 890 duplicates and 2198 at title and abstract review stages. Full-text articles for the remaining 47 records were retrieved, of which three were excluded for evaluating IVM for COVID-19 prophylaxis [[46], [47], [48]], four due to retraction [28,29,49,50], three for the study design (not RCT) [[51], [52], [53]], two for duplicates [54,55], one due to including children [56], and one for no control group [57]. No additional studies were retrieved from reference lists of the enrolled studies. Therefore, 33 studies were qualified and included in our systematic review (Fig. 1).
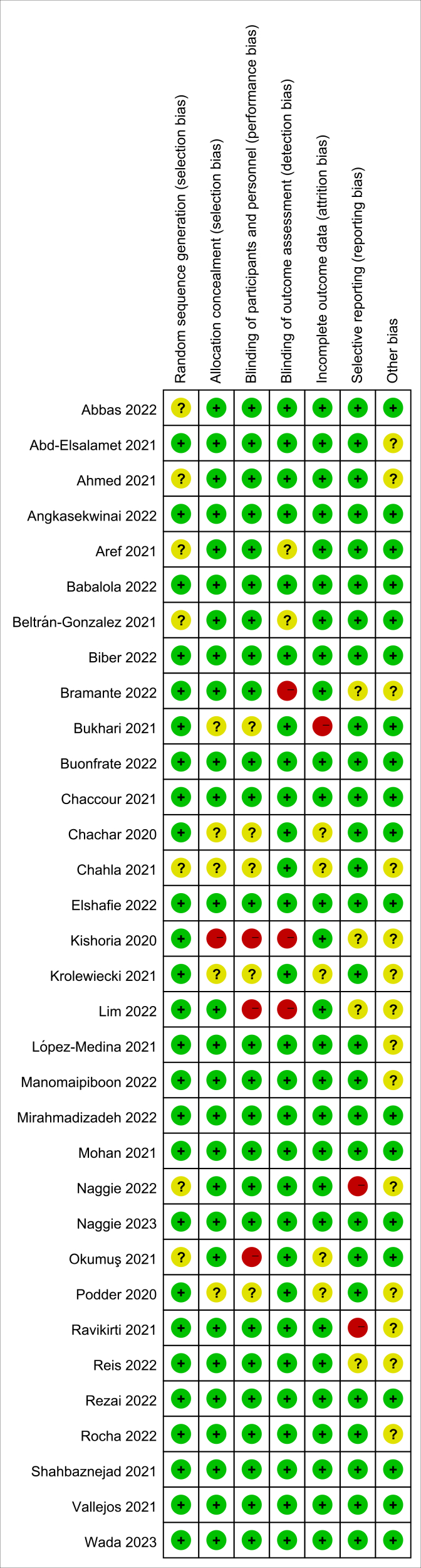
Table 1 summarized the main characteristics of the included studies. COVID-19 disease severity was asymptomatic/mild in 12 randomized controlled trials, moderate in 1, mild and moderate in 17, severe in 2, and moderate and severe in 1. Studies were done in 19 countries: Argentina (n = 3 studies) [[58], [59], [60]], Bangladesh (n = 2) [61,62], Brazil (n = 1) [10], China (n = 1) [63], Colombia (n = 1) [64], Egypt (n = 3) [23,65,66], India (n = 3) [[67], [68], [69]], Iran (n = 3) [26,70,71], Israel (n = 1) [72], Italy (n = 1) [22], Japan, (n = 1) [73], Malaysia (n = 1) [24], Mexico (n = 2) [74,75], Nigeria (n = 1) [76], Pakistan (n = 2) [77,78], Spain (n = 1) [79], Thailand (n = 2) [25,80], Turkey (n = 1) [81], and USA (n = 3) [21,82,83]. Of all 33 studies, 7 had an overall high risk of bias, 13 had some concerns of bias, and 13 had a low risk (Supplementary Fig. 1).
Table 1.
Characteristics of studies entered into meta-analysis.
| Study Authors (Year) | Name of publication | Country (Sample Size) | IVM Dose and Duration | ControlGroup | COVID-19 Severity by WHO Classification | Patient Age, Mean (SD) or Median (IQR), y | Patients, % |
Evaluated outcomes | Duration of Follow-up, d | Funding | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Laboratory-confirmed COVID-19 | Hospitalized | Female Sex | CVD or CHD | DM | HTN | ||||||||||
| Abbas et al. (2022) [63] | Indian J. Pharm. Sci. | China (n = 202) | 300 μg/kg per day for 5 days | Placebo | Mild in 100% | IVM: 38.33 (6.84) Control: 37.33 (5.84) | 100 | 100 | 55.4 | 0 | 0 | 0 | All-cause mortality rate, Symptoms resolved, SAEs, Hospitalization due to progression | 21 | ND |
| Abd-Elsalam et al. (2021) [65] | J. Med. Virol. | Egypt (n = 164) | 12 mg (single dose) for 3 days + SOC | SOC | Mild to moderate | IVM: 42.38 (16.02) Control: 39.38 (16.92) | 100 | 100 | 50 | ND | 16.5 | 19.5 | MV requirement, All-cause mortality rate, LOS | 30 | ND |
| Ahmed et al. (2021) [61] | Int. J. Infect. Dis. | Bangladesh (n = 48) | 12 mg once daily for 5 d | Placebo | Mild in 100% | 42 (NR) | 100 | 100 | 54 | 0 | 0 | 0 | Remission of symptoms, LOS, SAEs, Oxygen requirement, Time to viral clearance | 14 | Industry |
| Angkasekwinai et al. (2022) [80] | Antibiotics | Thailand (n = 447) | 400–600 μg/kg, once daily for 2 days | Placebo | Mild in 11.6%, moderate in 88.4% | 39.5 (12.1) | 100 | 7.4 | 56.8 | 1.8 | 6.9 | 11.2 | All-cause mortality rate, AEs, Symptoms resolved, SAEs, | 28 | Government |
| Aref et al. (2021) [66] | Int. J. Nanomed. | Egypt (n = 114) | spray twice daily + SOC | SOC | Mild in 100% | 45.1 (18.9) | 100 | 0 | 28.1 | 3.5 | 12.3 | 17.5 | PCR negative conversion, Progress to more severe disease, Duration taken for negative, AEs | 18 | Government |
| Babalola et al. (2022) [76] | QJM | Nigeria (n = 62) | given every 84 h, twice a week for 2 weeks + SOC: A1: 6 mg; A2: 12 mg | Placebo + SOC | Asymptomatic or mild/moderate symptoms | 44.1 (14.7) | 100 | ND | 30.6 | ND | 3.2 | 14.5 | All-cause mortality rate, Duration taken for negative, AEs, SAEs | 42 | ND |
| Beltrán-Gonzalez et al. (2022) [74] | Infect. Dis. Rep. | Mexico (n = 106) | 12 mg or 18 mg, according to patient weight | Placebo | Severe in 100% | 53 (16.9) | 100 | 100 | 37.8 | ND | 33.9 | 32.1 | All-cause mortality rate, clinical recovery, LOS, AEs, Respiratory deterioration | 28 | Government |
| Biber et al. (2022) [72] | Int. J. Infect. Dis. | Israel (=89) | Ivermectin 0.2 mg/kg for 3 days | Placebo | Mild to moderate, not requiring O2 and asymptomatic cases | 35 (28–47) (IQR) | 100 | 0 | 21.3 | ND | ND | ND | PCR negative conversion, AEs, Hospitalization due to progression, SAEs | 21 | ND |
| Bramante et al. (2022) [21] | New Engl. J. Med. | USA (n = 1323) | 390–470 μg/kg per day, for 3 days | Placebo | Mild in 100% | 46 ([IQR]37–55) | 100 | 0 | 56 | 22.8 | 1.6 | ND | Hypoxemia, emergency department visit, Hospitalization, mortality | 14 | ND |
| Bukhari et al. (2021) [77] | Medrxiv | Pakistan (n = 86) | Single dose: 12 mg | SOC | Mild in most patients (percentage unclear) | 39 (42) | 100 | 100 | 15 | 5.8 | 12 | 14 | Time to viral clearance, AEs | 28 | ND |
| Buonfrate et al. (2022) [22] | Int. J. AG. | Italy (n = 93) | Single dose A1: 600 μg/kg; A2: 1200 μg/kg | Placebo | Mild in 83.9%, moderate in 16.1% | 47 (31–58) | 100 | 100 | 41.9 | 23.4 | 4.7 | ND | Viral clearance, Hospitalization due to progression, Mean durations of symptoms, Mean reduction in viral load, SAEs | 14 | Government |
| Chaccour et al. (2021) [79] | EClinicalMedicine | Spain (n = 24) | Single dose 400 μg/kg | Placebo | Mild in 100% | 26 (19–36) | 100 | 0 | 50 | 0 | 0 | 0 | All-cause mortality rate, AEs, PCR at d 7 | 28 | Government |
| Chachar et al. (2020) [78] | Int. J. Sci. | Pakistan (n = 50) | 12 mg, 12 mg at 12 h, and 12 mg at 24 h | SOC | Mild in 100% | 42 (16) | 100 | 0 | 38 | 8 | 40 | 26 | Asymptomatic at d 7 | 7 | ND |
| Chahla et al. (2021) [58] | medRxiv | Argentina (n = 172) | 24 mg every 7 days for 4 weeks + SOC | SOC | Mild in 100% | IVM: 40 (19–53) Placebo: 37.5 (31,49) IQR | 100 | 0 | 52.3 | ND | 6.4 | 11 | Symptoms resolved, Discharged from hospital | 28 | Government |
| Elshafie et al. (2022) [23] | Expert Rev. Anti. Infect. Ther. | Egypt (n = 206) | 36 mg on day 1, 3, 6 | Placebo | moderate to severe | 59 (16) | 35.4 | 100 | 46.6 | 9.7 | 27.7 | 38.3 | All-cause mortality rate, AEs, Time or number of recovery | 90 | ND |
| Kishoria et al. (2020) [67] | Indian J. Res. | India (n = 32) | 12 mg + SOC | SOC | Asymptomatic/Mild patients in 100% | 38 | 100 | 100 | 28.1 | ND | ND | ND | PCR negative conversion, Discharged from hospital | 6 | ND |
| Krolewiecki et al. (2021) [59] | EClinicalMedicine | Argentina (n = 45) | 0.6 mg/kg once daily for 5 d | SOC | Mild in 87%, moderate in 13% | 41 (12) | 100 | 100 | 44 | ND | 16 | 13 | Viral load at d 5, IVM plasma level | 30 | Government |
| Lim et al. (2022) [24] | JAMA Intern. Med. | Malaysia (n = 490) | 0.4 mg/kg body weight daily for 5 days + SOC | SOC | Mild in 34.1%, moderate in 65.9% | 62.5 (8.7) | 100 | 0 | 54.5 | 11.6 | 53.5 | 75.3 | All-cause mortality rate, LOS, Symptoms resolved, Admission to ICU, MV requirement, Progress to more severe disease | 28 | ND |
| López-Medina et al. (2021) [64] | JAMA | Colombia (n = 398) | 300 μg/kg once daily for 5 d | Placebo | Mild in 100% | 37 (29–48) | 100 | 1 | 78 | 1.7 | 6 | 13 | All-cause mortality rate, Time to complete resolution, AEs, SAEs, Escalation of care | 21 | Government |
| Manomaipiboon et al. (2022) [25] | Trials | Thailand (n = 72) | 12 mg per day, for 5 days | SOC | Mild to moderate | 48.57 (14.8) | 100 | 100 | 62.5 | 2.8 | 23.6 | 40.3 | PCR negative conversion, Symptoms resolved, Mean durations of symptoms | 28 | Government |
| Mirahmadizadeh et al. (2022) [26] | Respirology | Iran (n = 391) | 3 mg for 2 days, cumulative dose of 24 mg | Placebo | Mild in 100% | 39 (17) | 100 | 0 | 49 | 1.1 | 5 | 6.9 | All-cause mortality rate, Symptoms resolved, MV requirement, Hospitalization due to progression, AEs, SAEs, Mean durations of symptoms | Government | |
| Mohan et al. (2021) [68] | J. Infect. Themother. | India (n = 157) | A1: Ivermectin 12 mg (single dose); A2: Ivermectin 24 mg (single dose) | Placebo | Mild in 64%, moderate in 36% | 35.3 (10.4) | 100 | 100 | 11.2 | 0.8 | 8.8 | 11.2 | PCR negative conversion, Progress to more severe disease, Discharged from hospital, AEs, SAEs | 14 | Government |
| Naggie et al. (2022) [82] | JAMA | USA (n = 1591) | Ivermectin 400 μg/kg for 3 days | Placebo | Mild-to-moderate | 48 (12) | 100 | 0 | 59 | ND | 11.5 | 26 | All-cause mortality rate, Admission to ICU, MV requirement, Hospitalization due to progression, Mean durations of symptoms, AEs, SAEs | 28 | Government |
| Naggie et al. (2023) [83] | JAMA | USA (n = 1206) | Ivermectin 600 μg/kg for 6 days | Placebo | Mild-to-moderate | IVM: 47 (38–58) Control:48 (39–58) | 100 | 0 | 59.1 | 3.9 | 9 | 26.3 | Time to sustained recovery, All-cause hospitalization rate, All-cause mortality rate | 28 | Government |
| Okumuş et al. (2021) [81] | BMC Infect. Dis. | Turkey (n = 60) | 0.2 mg/kg for 5 days + SOC | SOC | Severe pneumonia | IVM: 58.17 (11.52) Control: 66.23 (13.31) | 100 | 100 | 33.3 | 23.3 | 31.6 | 45 | All-cause mortality rate, PCR negative conversion, Clinical improvement, AEs, SAEs | 5 | Government |
| Podder et al. (2020) [62] | IMC J. Med. Sci. | Bangladesh (n = 62) | Single dose: 200 μg/kg | SOC | Mild in 81%, moderate in 19% | 39 (12) | 100 | ND | 29 | ND | ND | ND | Time to full recovery, Viral clearance | 10 | Government |
| Ravikirti et al. (2021) [69] | J. Pharm. Sci. | India (n = 115) | 12 mg/d for 2 days | Placebo | Mild in 79%, moderate in 21% | 53 (15) | 100 | 100 | 28 | 11 | 36 | 35 | All-cause mortality rate, Admission to ICU, MV requirement, Viral clearance at d 6 | 10 | ND |
| Reis et al. (2022) [10] | New Engl. J. Med. | Brazil (n = 679) | Ivermectin: 400 μg per kilogram of body weight once daily for 3 days + SOC | Placebo + SOC | Mild-to-moderate | 49 (38–57) | 100 | 0 | 58.2 | 1.8 | 12.9 | 8.4 | MV requirement, AEs, Hospitalization due to progression, Viral clearance | 21 | Government |
| Rezai et al. (2022) [70] | Front. Med. | Iran (n = 1158) | 0.4 mg/kg per day for 3 days + SOC | Placebo + SOC | Moderate in 53.9%, severe in 46.1% | 44.9 (5–96) | 100 | 52.6 | 50 | 7.7 | 20.1 | 18.7 | Clinical improvement, Recovery, LOS, ICU admission, MV requirement, AEs, Mortality | 7 | ND |
| Rocha et al. (2022) [75] | BMC Infect. Dis. | Mexico (n = 56) | 12 mg per day for 3 days + SOC | Placebo + SOC | asymptomatic and mild | IVM: 40.4 (15.2) Control:36.4 (13) | 100 | 100 | 67.8 | 3/30: 1/26 | 2/30: 1/26 | 4/30 1/26 | PCR negative conversion, Symptoms resolved, Progress to more severe disease, AEs | 14 | Industry |
| Shahbaznejad et al. (2021) [71] | Clin. Ther. | Iran (n = 69) | 0.2 mg/kg + SOC | SOC | Moderate in 55%, severe in 45% | 46.4 (22.5) | 64 | 100 | 47.8 | ND | ND | ND | Duration of hospital stay, Overall clinical improvement | 10 | ND |
| Vallejos et al. (2021) [60] | BMC Infect. Dis. | Argentina (n = 501) | IVM: BW < 80 kg: 12 mg at inclusion and another 12 mg after 24 h; 80 kg < BW < 110 kg:18 mg at inclusion and another 18 mg after 24 h; 110 kg < BW: 24 mg at inclusion and another 24 mg after 24 h + SOC | Placebo + SOC | Mild in 100% | 42.5 (15.5) | 100 | 0 | 47.3 | 2.6 | 9.6 | 23.6 | All-cause mortality rate, PCR negative conversion, MV requirement, Hospitalization due to progression, AEs, SAEs | 30 | ND |
| Wada et al. (2023) [73] | Front. Med. | Japan (n = 221) | 200 μg/kg | Placebo | Asymptomatic or mild/moderate symptoms | 47.7 (15.0) | 100 | 0 | 35.4 | ND | 13.7 | ND | Time to a negative, All-cause mortality rate, MV requirement, Hospitalization due to progression, AEs | 45 | Government |
AEs: adverse events, CHD: coronary heart disease, CVD: cerebrovascular disease, DM: diabetes mellitus, HTN: hypertension, ICU: Intensive Care Unit, IVM: ivermectin, LOS: length of hospital stay, MV: mechanical ventilation, ND: No data available, PCR: polymerase chain reaction, SAEs: severe adverse events, SD: standard deviation, SOC: standards of care, IQR: interquartile range.
3.2. Meta-analysis
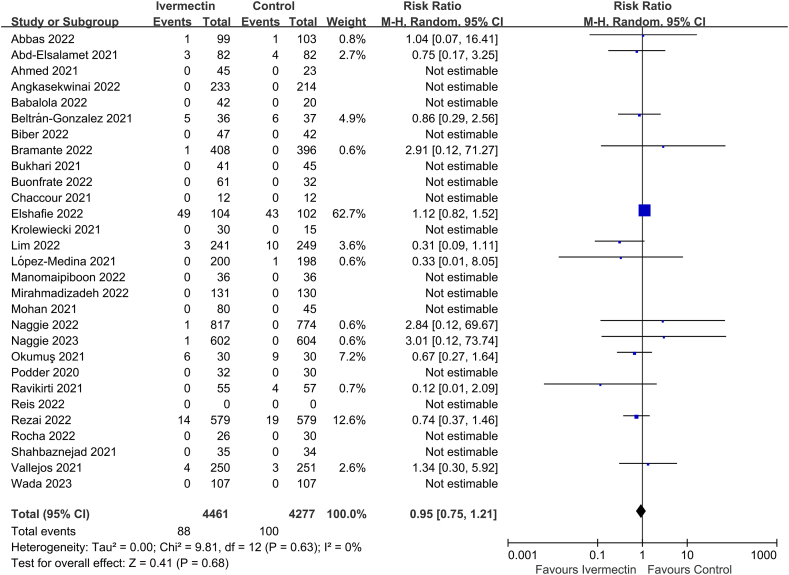
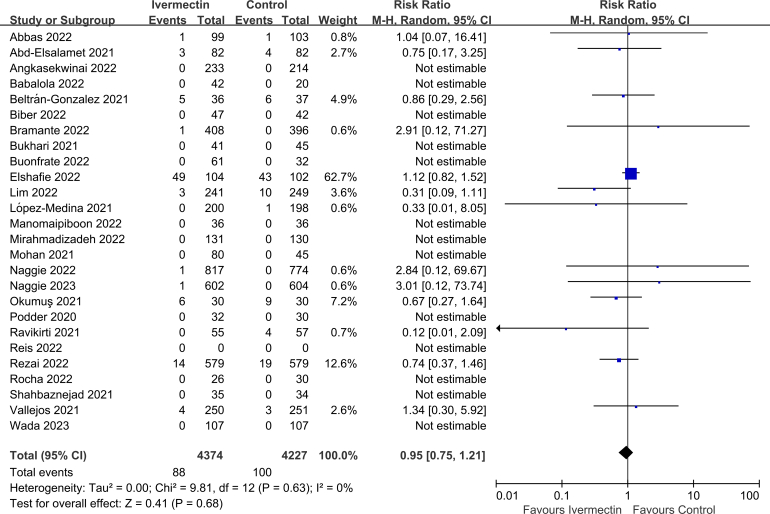
Twenty-nine of the thirty-three studies reported on all-cause mortality rate (n = 8738 participants) [10,[21], [22], [23], [24], [25], [26],[59], [60], [61], [62], [63], [64], [65],[68], [69], [70], [71], [72], [73], [74], [75], [76], [77],[79], [80], [81], [82], [83]]. There was no significant difference in all-cause mortality rate between IVM and controls (RR 0.95, 95% CI 0.75–1.21, I2 = 0%; Fig. 2).
Fig. 2.
Effect of ivermectin on all-cause mortality rates in patients with COVID-19.
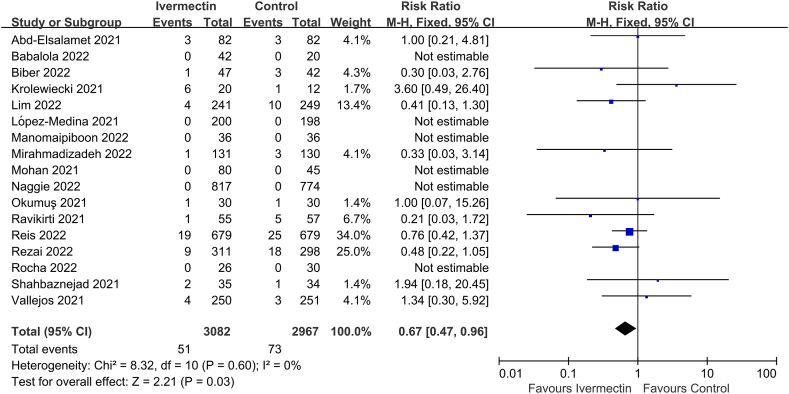
Seventeen of the thirty-three studies reported on MV requirement (n = 6049 participants) [10,[24], [25], [26],59,60,64,65,[68], [69], [70], [71], [72],75,76,81,82], and significant difference was observed between the two groups (RR 0.67, 95% CI 0.47–0.96, I2 = 0%; Fig. 3).
Fig. 3.
Effect of ivermectin on mechanical ventilation requirement in patients with COVID-19.
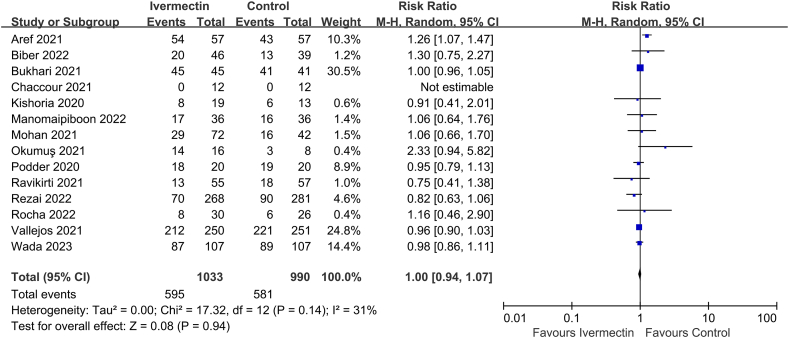
13 of the 33 studies reported on PCR negative conversion (n = 2023 participants) [25,60,62,[66], [67], [68], [69], [70],72,73,75,77,79,81], but no significant difference was observed between the two groups (RR 1.00, 95% CI 0.94–1.07, I2 = 31%; Fig. 4).
Fig. 4.
Effect of ivermectin on PCR negative conversion in patients with COVID-19.
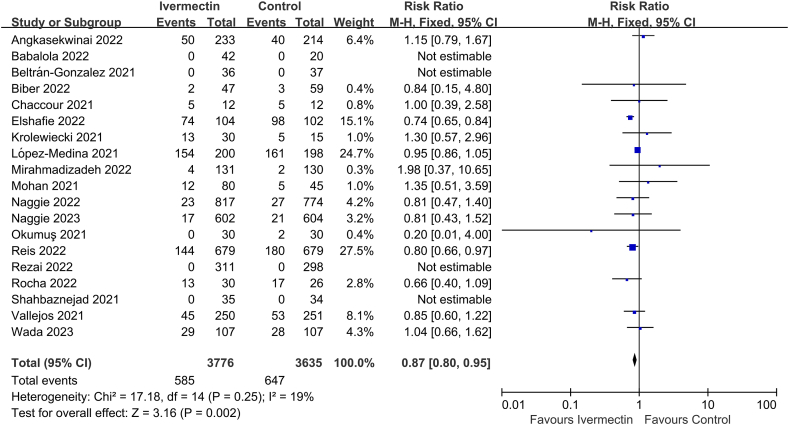
19 of the 33 studies reported on AEs (n = 7411 participants) [10,23,26,59,60,64,68,[70], [71], [72], [73], [74], [75], [76],[79], [80], [81], [82], [83]], and significant difference was observed between IVM and controls (RR 0.87, 95% CI 0.80–0.95, I2 = 19%; Fig. 5).
Fig. 5.
Effect of ivermectin on adverse events in patients with COVID-19.
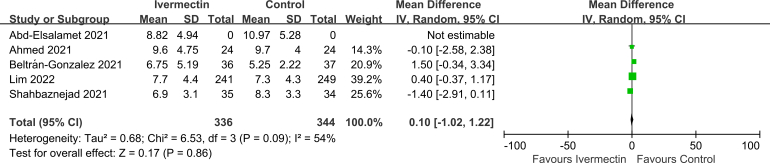
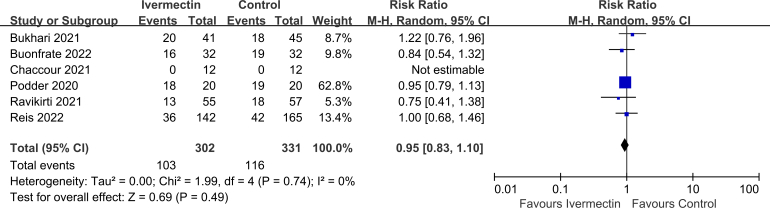
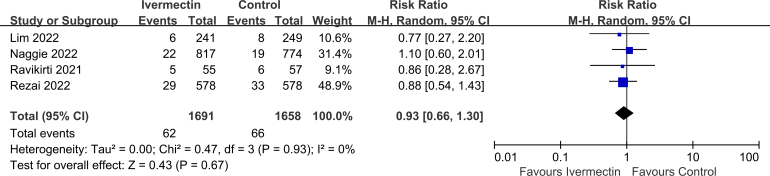
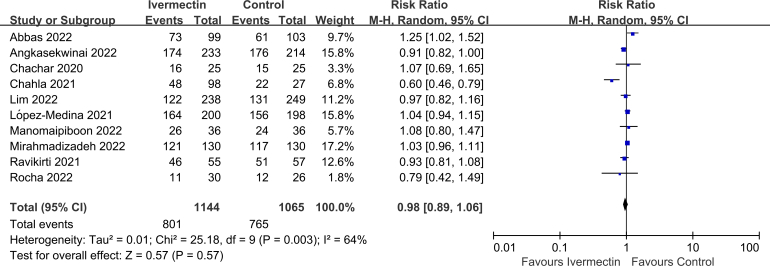
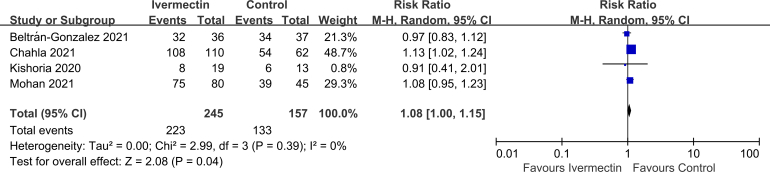
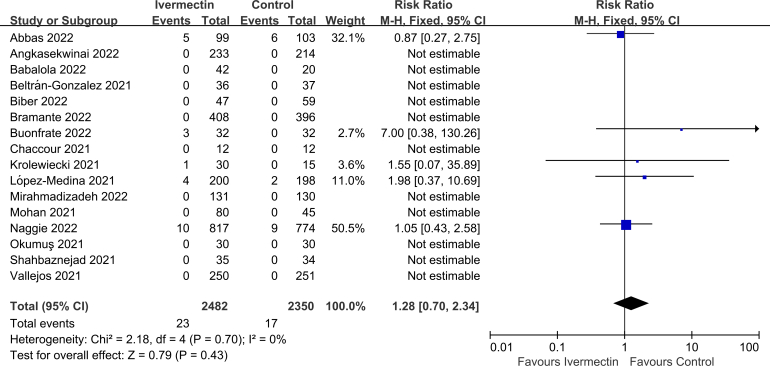
No significant difference was observed in LOS (MD 0.10, 95% CI -1.02 to 1.22, I2 = 54%; Supplementary Fig. 2), viral clearance (RR 0.95, 95% CI 0.83–1.10, I2 = 0%; Supplementary Fig. 3), admission to ICU (RR 0.93, 95% CI 0.66–1.30, I2 = 0%; Supplementary Fig. 4), symptoms resolved (RR 0.98, 95% CI 0.89–1.06, I2 = 64%; Supplementary Fig. 5), discharged from hospital (RR 1.08, 95% CI 1.00–1.15, I2 = 0%; Supplementary Fig. 6), or SAEs (RR 1.28, 95% CI 0.70–2.34, I2 = 0%; Supplementary Fig. 7) between the two groups.
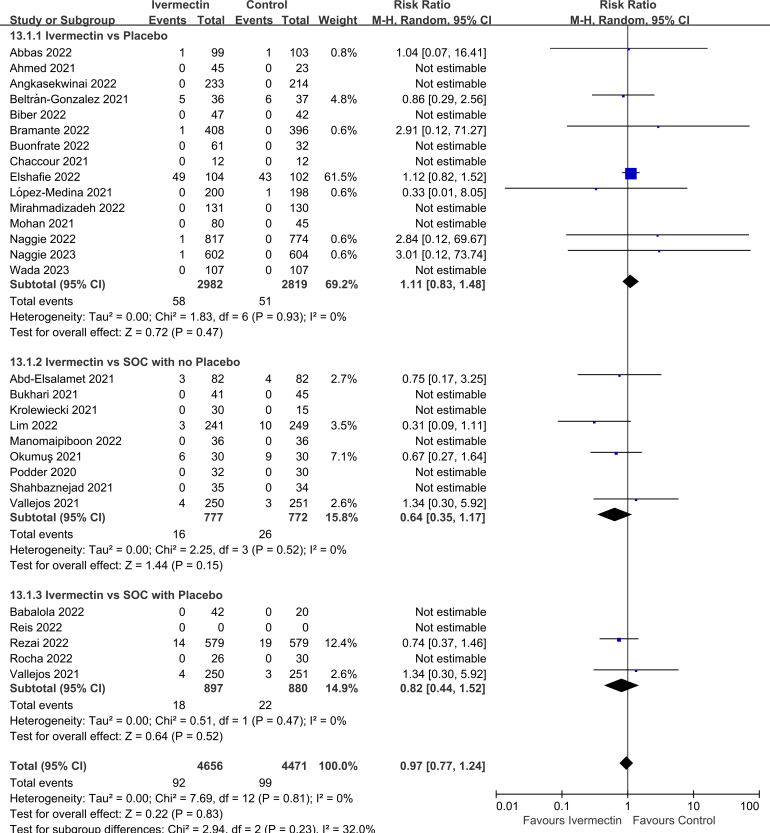
3.3. Subgroup analyses
Subgroup analysis was performed by the controls. There was no significant difference in all-cause mortality rate between IVM and placebo (RR 1.11, 95% CI 0.83–1.48, I2 = 0%), between IVM and SOC with no placebo (RR 0.64, 95% CI 0.35–1.17, I2 = 0%), or between IVM and SOC with placebo (RR 0.82, 95% CI 0.44–1.51, I2 = 0%; Supplementary Fig. 8).
3.4. Sensitivity analysis and publication bias
Sensitivity analysis excluding small sample size studies (n < 60) showed that there was no significant difference in all-cause mortality rate (RR 0.95, 95% CI 0.75–1.21, I2 = 0%; Supplementary Fig. 9), which remained consistent with the overall analysis.
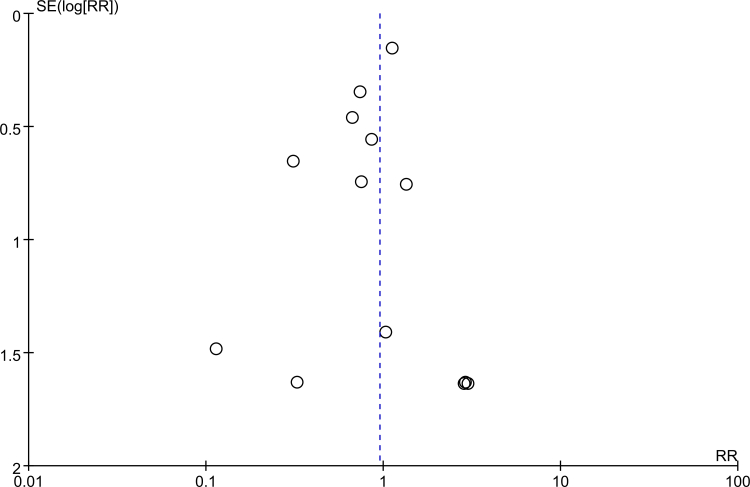
The funnel plot for all-cause mortality rate was symmetrical by visual inspection, indicating no publication bias in this study (Supplementary Fig. 10).
4. Discussion
Our meta-analysis included thirty-three RCTs (n = 10,489), which comprehensively reviewed the evidence on IVM treatment of patients with COVID-19 up to June 22, 2023 and showed that IVM did not have an effect in reducing the risk of mortality in patients with COVID-19. However, IVM had an effect on reducing the risk of AEs and MV requirement. Additionally, IVM did not increase the risk of PCR negative conversion, LOS, viral clearance, admission to ICU, symptoms resolved, discharged from hospital, hospitalization due to progression, or SAEs. Subgroup analysis based on different controls showed that IVM did not have an effect on reducing all-cause mortality rates in patients with COVID-19. In addition, subgroup analysis by severity of COVID-19 patients, IVM showed no effect on all-cause mortality rate between IVM and SOC, either. Which was consistent with the overall analysis when excluded small sample size studies (n < 60) or studies with follow-up < 21 days.
Despite not being recommended in the current guidelines by WHO or IDSA [33,84], IVM was one of the drugs and substances frequently used in self-medication in patients with COVID-19 [[85], [86], [87]]. Self-medication was related to the massive dissemination of misleading information, individual fear of contracting the virus, and limited access to healthcare services [85]. With the publication of overwhelming number of studies on COVID-19, it is an insurmountable challenge to keep the living reviews updated. IVM has been the subject of two living reviews. One living review was updated in August 2022, and pooled the evidence from forty-nine studies. Which suggested a reduction in mortality with IVM (RR 0.69, 95% CI 0.50–0.95), however, the effect was no longer apparent when included only the studies of low risk of bias (RR 1.00, 95% CI 0.80–1.24) [88]. The other suggested that IVM might reduce the mortality in patients with COVID-19 compared with control (RR 0.63, 95% CI 0.37–1.05), and highlighted the fact that the certainty of the evidence for IVM is low [89]. The two living reviews were not updated after the studies of Elgazzar et al., Pott-Junior et al., and Samaha et al. were retracted. Evidence shows that the use of hydroxychloroquine (HCQ) is harmful [90]. Therefore, future updates should not keep in the pooled analysis the studies that compared IVM with HCQ.
Different from our results, another review [91] included some studies in which IVM was compared with HCQ [19,57,92]; and another recent review [93] included the Pott-Junior et al. retracted trial [28]. We strongly believe that such studies should not be included in the best available body of evidence now. In additional, several studies have been published after these two reviews [21,23,26,63,70,80].
One strength of this review is that, in addition to the traditional search, the Living Overview of Evidence database (L.OVE, issued by Epistemonikus) was also used to conduct a comprehensive search. L. OVE is a digital tool that can compile studies from several databases (including preprint databases), and is updated through computational algorithms [94]. Which may be more convinient to update the search regularly and more efficient than the traditional search [95]. Misleading information may be obtained when inappropriate methods are used. Therefore, another strength of this review is the application of strict methodological criteria. Our review analyzed not only studies comparing IVM with placebo, but also those comparing IVM with SOC, in a stratified analysis. Additionally, stratified analysis based on the severity of COVID-19 was performed.
This study has several limitations. First, all-cause mortality rate, MV requirement, PCR negative conversion, and AEs are primary outcomes, and the certainty of the evidence for these outcomes were ranked as moderate owing to some concerns about imprecision and risk of bias. Methodological limitations were mainly associated with data integrity, design of included studies, and potential conflicts of interests. Additional concerns were that some studies did not pre-register prior to recruiting participants, while others had revised the protocol. However, as LOS was negative, these potential sources of bias may not have had a significant impact on these outcomes. Another limitation lies in the low incidence of event. Among the 29 studies examining for all-cause mortality, 15 did not have any events. Therefore, the overall all-cause mortality rate should be very low in this context. The majority of the included studies enrolled participants with mild-to-moderate COVID-19, but the severity of disease varied among them. To overcome this limitation, a subgroup analysis was conducted and no significant effect modification was found in each subgroup. Which also applies for MV requirement. Last, our meta-analysis included studies with small sample size, which may overstate the benefits of this intervention. To address this limitation, we performed a sensitivity analysis by excluding studies with small sample size, which found significant effect modification of MV requirement.
The urgent need for COVID-19 treatment options has spurred a surge in RCTs, which led to the perform and publication of studies with uneven quality and notable methodological shortcomings. This provided fertile ground for even poorly evidence to be exaggerated not only on social media but also in the scientific literature [27]. The consequence is very serious because the outcomes from studies with high risk of bias would spread rapidly in clinical practice, and these drugs would also be incorporated into public policies or as SOC among different countries and regions hastily. To make matters worse, some researchers and institutions were reluctant to change the protocols after the evidences against the use of certain drugs [15]. Consequently, studies have enrolled uneven and clinically inappropriate options due to defining their comparators as standard of care. For example, a research protocol was registered in Brazil [96] following evidence that HCQ can increase the risk of all-cause mortality. In May 2020, the WHO recommended against the use of antibiotics as standard of care in COVID-19 patients without evidence of bacterial pneumonia; however, two later studies kept antibiotics in their definition of standard of care [22,81].
Furthermore, we sought to minimize potential biases during the review process based on the methods recommended by the Cochrane Collaboration [96] and prescribed in our PROSPERO protocol.
In a recent publication, the authors reported that in addition to the retracted trials, several others claiming benefits for IVM may be equally of concern. Which emphasized that trial registry updates could not explain the incompatibility between published participant demographics and timelines that are inconsistent with the authenticity of the data collection [27]. Therefore, it is critical for reviewers to keep updated with potential new retractions in time before following strict methodological standards. A number of supporters, many of whom are anti-vaccination activists, have continued to vigorously promote the use of IVM, claiming that real evidence has been ignored. Some websites have released systematic reviews on the effectiveness of IVM for COVID-19 (covid19criticalcare.com) and (https://ivmmeta.com). Most of which are not peer-reviewed, do not present the eligible criteria used in the selection process, and do not display statistical criteria for assessing the effectiveness and heterogeneity among included studies. These websites, according to Roman et al., provide misinformation to health professionals, patients, and the general population who are unable to critically analyze scientific studies. Our thorough and transparent review may contribute to disseminate authentic evidence. Although the incidences of AEs and PCR negative conversion were lower in IVM group, the incidence of SAEs was comparable between the two groups. As associated with a certain clinical benefit, these should be taken into account in the management of patients with COVID-19.
5. Conclusion
In summary, ivermectin could reduce the risk of mechanical ventilation requirement and adverse events in patients with COVID-19, without increasing other risks. Despite no conclusive evidence or guidelines recommending ivermectin as a therapeutic drug for COVID-19, clinicians could use it with caution in the absence of better alternatives, and self-medication of ivermectin is not recommended for patients with COVID-19.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of interest's statement
The authors declare no competing interests.
Data availability statement
Data included in article/supp. Material/referenced in article. Data will be made available on request.
CRediT authorship contribution statement
Zhilong Song: Writing – original draft, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Senyuan Shi: Validation, Software, Resources, Project administration, Methodology, Investigation. Yongli Zhang: Writing – review & editing, Visualization, Supervision, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e27647.
Contributor Information
Zhilong Song, Email: song011009@163.com.
Senyuan Shi, Email: 13569514405@163.com.
Yongli Zhang, Email: zhangyongli0378@sina.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
figs4.
figs5.
figs6.
figs7.
figs8.
figs9.
figs10.
References
- 1.Yin J., Chen Y., Li Y., Wang C., Zhang X. Immunogenicity and efficacy of COVID-19 vaccines in people living with HIV: a systematic review and meta-analysis. Int. J. Infect. Dis. 2022;124:212–223. doi: 10.1016/j.ijid.2022.10.005. https://doi:10.1016/j.ijid.2022.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin J., Chen Y., Li Y., Zhang X., Wang C. Seroconversion rate after COVID-19 vaccination in patients with solid cancer: a systematic review and meta-analysis. Hum. Vaccines Immunother. 2022;18(6) doi: 10.1080/21645515.2022.2119763. https://doi:10.1080/21645515.2022.2119763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carpiano R.M., Callaghan T., DiResta R., Brewer N.T., Clinton C., Galvani A.P., Lakshmanan R., Parmet W.E., Omer S.B., Buttenheim A.M., Benjamin R.M., Caplan A., Elharake J.A., Flowers L.C., Maldonado Y.A., Mello M.M., Opel D.J., Salmon D.A., Schwartz J.L., Sharfstein J.M., Hotez P.J. Confronting the evolution and expansion of anti-vaccine activism in the USA in the COVID-19 era. Lancet. 2023;401(10380):967–970. doi: 10.1016/S0140-6736(23)00136-8. https://doi:10.1016/s0140-6736(23)00136-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falcon M., Rodríguez-Blázquez C., Romay-Barja M., Ayala A., Burgos A., De Tena-Dávila M.J., Forjaz M.J. COVID-19 vaccine hesitancy in Spain and associated factors. Front. Public Health. 2023;11 doi: 10.3389/fpubh.2023.1129079. https://doi:10.3389/fpubh.2023.1129079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abu El Kheir-Mataria W., Saleh B.M., El-Fawal H., Chun S. COVID-19 vaccine hesitancy among parents in Low- and Middle-Income Countries: a meta-analysis. Front. Public Health. 2023;11 doi: 10.3389/fpubh.2023.1078009. https://doi:10.3389/fpubh.2023.1078009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Update to living WHO guideline on drugs for covid-19. BMJ. 2022;378:o2224. doi: 10.1136/bmj.o2224. https://doi:10.1136/BMJ.o2224 [DOI] [PubMed] [Google Scholar]
- 7.Ader F., Bouscambert-Duchamp M., Hites M., Peiffer-Smadja N., Poissy J., Belhadi D., Diallo A., Lê M.P., Peytavin G., Staub T., Greil R., Guedj J., Paiva J.A., Costagliola D., Yazdanpanah Y., Burdet C., Mentré F. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial. Lancet Infect. Dis. 2022;22(2):209–221. doi: 10.1016/S1473-3099(21)00485-0. https://doi:10.1016/s1473-3099(21)00485-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses. Lancet. 2022;399(10339):1941–1953. doi: 10.1016/S0140-6736(22)00519-0. https://doi:10.1016/s0140-6736(22)00519-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Low Z.Y., Farouk I.A., Lal S.K. Drug repositioning: new Approaches and future Prospects for Life-debilitating diseases and the COVID-19 pandemic outbreak. Viruses. 2020;12(9) doi: 10.3390/v12091058. https://doi:10.3390/v12091058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reis G., Silva E., Silva D.C.M., Thabane L., Milagres A.C., Ferreira T.S., Dos Santos C.V.Q., Campos V.H.S., Nogueira A.M.R., de Almeida A., et al. Effect of early treatment with ivermectin among patients with covid-19. N. Engl. J. Med. 2022;386(18):1721‐1731. doi: 10.1056/NEJMoa2115869. https://doi:10.1056/NEJMoa2115869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chikezie F.M., Opara K.N., Ubulom P.M.E., Yaro C.A., Al-Akeel R.K., Osei-Atweneboana M.Y., Alexiou A., Papadakis M., Batiha G.E. Onchocerciasis transmission status in some endemic communities of Cross River State, Nigeria after two decades of mass drug administration with ivermectin. Sci. Rep. 2023;13(1):5413. doi: 10.1038/s41598-023-31446-6. https://doi:10.1038/s41598-023-31446-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arévalo A.P., Pagotto R., Pórfido J.L., Daghero H., Segovia M., Yamasaki K., Varela B., Hill M., Verdes J.M., Duhalde Vega M., Bollati-Fogolín M., Crispo M. Ivermectin reduces in vivo coronavirus infection in a mouse experimental model. Sci. Rep. 2021;11(1):7132. doi: 10.1038/s41598-021-86679-0. https://doi:10.1038/s41598-021-86679-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mega E.R. Latin America's embrace of an unproven COVID treatment is hindering drug trials. Nature. 2020;586(7830):481–482. doi: 10.1038/d41586-020-02958-2. https://doi:10.1038/d41586-020-02958-2 [DOI] [PubMed] [Google Scholar]
- 14.Chua K.P., Conti R.M., Becker N.V. US insurer spending on ivermectin prescriptions for COVID-19. JAMA. 2022;327(6):584–587. doi: 10.1001/jama.2021.24352. https://doi:10.1001/JAMA.2021.24352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furlan L., Caramelli B. The regrettable story of the "covid kit" and the "early treatment of covid-19" in Brazil, lancet reg. Health Am. 2021;4 doi: 10.1016/j.lana.2021.100089. https://doi:10.1016/j.lana.2021.100089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Low Z.Y., Yip A.J.W., Lal S.K. Repositioning Ivermectin for Covid-19 treatment: molecular mechanisms of action against SARS-CoV-2 replication. BBA-Mol. Basis Dis. 2022;1868(2) doi: 10.1016/j.bbadis.2021.166294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozer M., Goksu S.Y., Conception R., Ulker E., Balderas R.M., Mahdi M., Manning Z., To K., Effendi M., Anandakrishnan R., Whitman M., Gugnani M. Effectiveness and safety of Ivermectin in COVID-19 patients: a prospective study at a safety-net hospital. J. Med. Virol. 2022;94(4):1473–1480. doi: 10.1002/jmv.27469. https://doi:10.1002/jmv.27469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020;178 doi: 10.1016/j.antiviral.2020.104787. https://doi:10.1016/j.antiviral.2020.104787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niaee M., Namdar P., Allami A., Zolghadr L., Javadi A., Karampour A., Varnaseri M., Bijani B., Cheraghi F., Naderi Y., et al. Ivermectin as an adjunct treatment for hospitalized adult COVID-19 patients: a randomized multi-center clinical trial, Asian Pac. J. Trop. Med. 2021;14(6):266‐273. https://doi:10.4103/1995-7645.318304 [Google Scholar]
- 20.Lazar Neto F., Salzstein G.A., Cortez A.L., Bastos T.L., Baptista F.V.D., Moreira J.A., Lauterbach G.P., de Oliveira J.C., de Assis F.C., Aguiar M.R.A., de Deus A.A., Dias M.F.D.S., Sousa F.C.B., Duailibi D.F., Kondo R.H., de Moraes A.C.F., Martins M.A. Comparative assessment of mortality risk factors between admission and follow-up models among patients hospitalized with COVID-19. Int. J. Infect. Dis. 2021;105:723–729. doi: 10.1016/j.ijid.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bramante C.T., Huling J.D., Tignanelli C.J., Buse J.B., Liebovitz D.M., Nicklas J.M., Cohen K., Puskarich M.A., Belani H.K., Proper J.L., Siegel L.K., Klatt N.R., Odde D.J., Luke D.G., Anderson B., Karger A.B., Ingraham N.E., Hartman K.M., Rao V., Hagen A.A., Patel B., Fenno S.L., Avula N., Reddy N.V., Erickson S.M., Lindberg S., Fricton R., Lee S., Zaman A., Saveraid H.G., Tordsen W.J., Pullen M.F., Biros M., Sherwood N.E., Thompson J.L., Boulware D.R., Murray T.A. Randomized trial of metformin, ivermectin, and fluvoxamine for covid-19. N. Engl. J. Med. 2022;387(7):599–610. doi: 10.1056/NEJMoa2201662. https://doi:10.1056/NEJMoa2201662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buonfrate D., Chesini F., Martini D., Roncaglioni M.C., Ojeda Fernandez M.L., Alvisi M.F., De Simone I., Rulli E., Nobili A., Casalini G., et al. High-dose ivermectin for early treatment of COVID-19 (COVER study): a randomised, double-blind, multicentre, phase II, dose-finding, proof-of-concept clinical trial. Int. J. AG. 2022;59(2) doi: 10.1016/j.ijantimicag.2021.106516. https://doi:10.1016/j.ijantimicag.2021.106516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elshafie A.H., Elsawah H.K., Hammad M., Sweed E.M., Seif A.S., Abdel Ghaffar M.M., Goda F.M., Mosalam E.M., Abdallah M.S. Ivermectin role in COVID-19 treatment (IRICT): single-center, adaptive, randomized, double-blind, placebo-controlled, clinical trial. Expert Rev. Anti Infect. Ther. 2022;20(10):1341–1350. doi: 10.1080/14787210.2022.2098113. https://doi:10.1080/14787210.2022.2098113 [DOI] [PubMed] [Google Scholar]
- 24.Lim S.C.L., Hor C.P., Tay K.H., Mat Jelani A., Tan W.H., Ker H.B., Chow T.S., Zaid M., Cheah W.K., Lim H.H., et al. Efficacy of ivermectin treatment on disease progression among adults with mild to moderate COVID-19 and comorbidities: the I-tech randomized clinical trial. JAMA Intern. Med. 2022;182(4):426‐435. doi: 10.1001/jamainternmed.2022.0189. https://doi:10.1001/JAMAinternmed.2022.0189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manomaipiboon A., Pholtawornkulchai K., Poopipatpab S., Suraamornkul S., Maneerit J., Ruksakul W., Phumisantiphong U., Trakarnvanich T. Efficacy and safety of ivermectin in the treatment of mild to moderate COVID-19 infection: a randomized, double-blind, placebo-controlled trial. Trials. 2022;23(1):714. doi: 10.1186/s13063-022-06649-3. https://doi:10.1186/s13063-022-06649-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mirahmadizadeh A., Semati A., Heiran A., Ebrahimi M., Hemmati A., Karimi M., Basir S., Zare M., Charlys da Costa A., Zeinali M., Sargolzaee M., Eilami O. Efficacy of single-dose and double-dose ivermectin early treatment in preventing progression to hospitalization in mild COVID-19: a multi-arm, parallel-group randomized, double-blind, placebo-controlled trial. Respirology. 2022;27(9):758–766. doi: 10.1111/resp.14318. https://doi:10.1111/resp.14318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrence J.M., Meyerowitz-Katz G., Heathers J.A.J., Brown N.J.L., Sheldrick K.A. The lesson of ivermectin: meta-analyses based on summary data alone are inherently unreliable. Nat. Med. 2021;27(11):1853–1854. doi: 10.1038/s41591-021-01535-y. https://doi:10.1038/s41591-021-01535-y [DOI] [PubMed] [Google Scholar]
- 28.Pott-Junior H., Paoliello M.M.B., Miguel A.Q.C., da Cunha A.F., de Melo Freire C.C., Neves F.F., da Silva de Avó L.R., Roscani M.G., Dos Santos S.S., Chachá S.G.F. Use of ivermectin in the treatment of Covid-19: a pilot trial. Toxicol Rep. 2021;8:505–510. https://doi:10.1016/j.toxrep.2021.03.003 [Google Scholar]
- 29.Samaha A.A., Mouawia H., Fawaz M., Hassan H., Salami A., Bazzal A.A., Saab H.B., Al-Wakeel M., Alsaabi A., Chouman M., Moussawi M.A., Ayoub H., Raad A., Hajjeh O., Eid A.H., Raad H. Effects of a single dose of ivermectin on viral and clinical outcomes in asymptomatic SARS-CoV-2 infected subjects: a pilot clinical trial in Lebanon. Viruses. 2021;13(6) doi: 10.3390/v13060989. https://doi:10.3390/v13060989 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Bryant A., Lawrie T.A., Dowswell T., Fordham E.J., Mitchell S., Hill S.R., Tham T.C. Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines. Am. J. Ther. 2021;28(4):e434–e460. doi: 10.1097/MJT.0000000000001402. https://doi:10.1097/mjt.0000000000001402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siemieniuk R.A., Bartoszko J.J., Zeraatkar D., Kum E., Qasim A., Martinez J.P.D., Izcovich A., Lamontagne F., Han M.A., Agarwal A., Agoritsas T., Azab M., Bravo G., Chu D.K., Couban R., Devji T., Escamilla Z., Foroutan F., Gao Y., Ge L., Ghadimi M., Heels-Ansdell D., Honarmand K., Hou L., Ibrahim Q., Khamis A., Lam B., Mansilla C., Loeb M., Miroshnychenko A., Marcucci M., McLeod S.L., Motaghi S., Murthy S., Mustafa R.A., Pardo-Hernandez H., Rada G., Rizwan Y., Saadat P., Switzer C., Thabane L., Tomlinson G., Vandvik P.O., Vernooij R.W., Viteri-García A., Wang Y., Yao L., Zhao Y., Guyatt G.H., Brignardello-Petersen R. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. doi: 10.1136/bmj.m2980. https://doi:10.1136/BMJ.m2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill A., Garratt A., Levi J., Falconer J., Ellis L., McCann K., Pilkington V., Qavi A., Wang J., Wentzel H. Meta-analysis of randomized trials of ivermectin to treat SARS-CoV-2 infection. Open Forum Infect. Dis. 2021;8(11) doi: 10.1093/ofid/ofab358. https://doi:10.1093/ofid/ofab358 ofab358. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Bhimraj A., Morgan R.L., Shumaker A.H., Baden L., Cheng V.C.C., Edwards K.M., Gallagher J.C., Gandhi R.T., Muller W.J., Nakamura M.M., O'Horo J.C., Shafer R.W., Shoham S., Murad M.H., Mustafa R.A., Sultan S., Falck-Ytter Y. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19. Clin. Infect. Dis. 2022;ciac724 doi: 10.1093/cid/ciaa478. https://doi:10.1093/cid/ciac724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higgins J.P.T., Sally G. Wiley; New Jersey: 2008. Cochrane Handbook for Systematic Reviews of Interventions. [Google Scholar]
- 35.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R., Glanville J., Grimshaw J.M., Hróbjartsson A., Lalu M.M., Li T., Loder E.W., Mayo-Wilson E., McDonald S., McGuinness L.A., Stewart L.A., Thomas J., Tricco A.C., Welch V.A., Whiting P., Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. https://doi:10.1136/BMJ.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.H. Julian, LassersonToby, C. Jackie, T. David, T. James, F. Ella, C. Rachel. Standards for the conduct and reporting of new Cochrane Intervention Reviews, reporting of protocols and the planning, conduct and reporting of updates. https://community.cochrane.org/mecir-manual. Accessed August 13 2019.
- 37.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. https://doi:10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo D., Wan X., Liu J., Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018;27(6):1785–1805. doi: 10.1177/0962280216669183. https://doi:10.1177/0962280216669183 [DOI] [PubMed] [Google Scholar]
- 39.Shi J., Luo D., Wan X., Liu Y., JimingLiu, Bian Z., Tong T. Detecting the skewness of data from the sample size and the five-number summary. Stat. Methods Med. Res. 2023;32(7):1338–1360. doi: 10.1177/09622802231172043. https://arxiv.org/abs/2010.05749 [DOI] [PubMed] [Google Scholar]
- 40.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.Y., Corbett M.S., Eldridge S.M., Emberson J.R., Hernán M.A., Hopewell S., Hróbjartsson A., Junqueira D.R., Jüni P., Kirkham J.J., Lasserson T., Li T., McAleenan A., Reeves B.C., Shepperd S., Shrier I., Stewart L.A., Tilling K., White I.R., Whiting P.F., Higgins J.P.T. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. https://doi:10.1136/BMJ.l4898 [DOI] [PubMed] [Google Scholar]
- 41.Guyatt G.H., Oxman A.D., Vist G.E., Kunz R., Falck-Ytter Y., Alonso-Coello P., Schünemann H.J. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. https://doi:10.1136/BMJ.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schünemann H.J., Vist G.E., Higgins J.P., Santesso N., Deeks J.J., Glasziou P., Akl E.A., Guyatt G.H., Group o.b.o.t.C.G.M. Cochrane Handbook for Systematic Reviews of Interventions. Wiley; 2019. Interpreting results and drawing conclusions. [Google Scholar]
- 43.Hartung J., Knapp G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat. Med. 2001;20(24):3875–3889. doi: 10.1002/sim.1009. https://doi:10.1002/sim.1009 [DOI] [PubMed] [Google Scholar]
- 44.DerSimonian R., Laird N. Meta-analysis in clinical trials, Control. Clin. Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. https://doi:10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 45.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. https://doi:10.1136/BMJ.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seet R.C.S., Quek A.M.L., Ooi D.S.Q., Sengupta S., Lakshminarasappa S.R., Koo C.Y., So J.B.Y., Goh B.C., Loh K.S., Fisher D., et al. Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: an open-label randomized trial. Int. J. Infect. Dis. 2021;106:314‐322. doi: 10.1016/j.ijid.2021.04.035. https://doi:10.1016/j.ijid.2021.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shoumann W.M., Hegazy A.A., Nafae R.M., Ragab M.I., Samra S.R., AnasIbrahim D., Hal-Mahrouky T., Sileem A.E. Use of ivermectin as a potential chemoprophylaxis for covid-19 in Egypt: a randomised clinical trial. J. Clin. Diagn. Res. 2021;15(2):OC27‐OC32. https://doi:10.7860/JCDR/2021/46795.14529 [Google Scholar]
- 48.Behera P., Patro B.K., Padhy B.M., Mohapatra P.R., Bal S.K., Chandanshive P.D., Mohanty R.R., Ravikumar S.R., Singh A., Singh S.R., Pentapati S.S.K., Nair J., Batmanbane G. Prophylactic role of ivermectin in severe acute respiratory syndrome coronavirus 2 infection among healthcare workers. Cureus. 2021;13(8) doi: 10.7759/cureus.16897. https://doi:10.7759/cureus.16897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reardon S. Flawed ivermectin preprint highlights challenges of COVID drug studies. Nature. 2021;596(7871):173–174. doi: 10.1038/d41586-021-02081-w. https://doi:10.1038/d41586-021-02081-w [DOI] [PubMed] [Google Scholar]
- 50.Elgazzar A., Eltaweel A., Youssef S.A., Hany B., Hafez M., Moussa H. Research Square; 2021. Efficacy and Safety of Ivermectin for Treatment and Prophylaxis of COVID-19 Pandemic.https://doi:10.21203/rs.3.rs-100956/v4 [Google Scholar]
- 51.Hazan S., Dave S., Gunaratne A.W., Dolai S., Clancy R.L., McCullough P.A., Borody T.J. Effectiveness of ivermectin-based multidrug therapy in severely hypoxic, ambulatory COVID-19 patients. Future Microbiol. 2022;17:339–350. doi: 10.2217/fmb-2022-0014. https://doi:10.2217/fmb-2022-0014 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Gorial F.I., Mashhadani S., Sayaly H.M., Dakhil B.D., AlMashhadani M.M., Aljabory A.M., Abbas H.M., Ghanim M., Rasheed J.I. 2020. Effectiveness of Ivermectin as Add-On Therapy in COVID-19 Management (Pilot Trial), medRxiv. [Google Scholar]
- 53.Zubair S.M., Zahid A., Shahzad T., Zubairi A.B.S., Khan J.A., Irfan M. Role of ivermectin in hospitalized patients with mild to moderate COVID-19. Eur. Respir. J. 2021;58(SUPPL 65) https://doi:10.1183/13993003.congress-2021.PA3666 [Google Scholar]
- 54.Naggie S., Boulware D.R., Lindsell C.J., Stewart T.G., Lim S.C., Cohen J., Kavtaradze D., Amon A.P., Gabriel A., Gentile N., Felker G.M., Rothman R.L., Jayaweera D., McCarthy M.W., Sulkowski M., Wilson S., DeLong A., Remaly A., Wilder R., Collins S., Dunsmore S.E., Adam S.J., Thicklin F., Hanna G.J., Ginde A.A., Castro M., McTigue K., Shenkman E., Hernandez A.F., Accelerating Covid-19 Therapeutic Interventions and Vaccines (ACTIV)-6 Study Group and Investigators Effect of higher-dose ivermectin for 6 days vs placebo on time to sustained recovery in outpatients with COVID-19: A randomized clinical trial. JAMA. 2023;329(11):888–897. doi: 10.1001/jama.2023.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naggie S., Boulware D.R., Lindsell C.J., Stewart T.G., Gentile N., Collins S., McCarthy M.W., Jayaweera D., Castro M., Sulkowski M., McTigue K., Thicklin F., Felker G.M., Ginde A.A., Bramante C.T., Slandzicki A.J., Gabriel A., Shah N.S., Lenert L.A., Dunsmore S.E., Adam S.J., DeLong A., Hanna G., Remaly A., Wilder R., Wilson S., Shenkman E., Hernandez A.F., Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-6) Study Group and Investigators Effect of ivermectin vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: A randomized clinical trial. JAMA. 2022;328(16):1595–1603. doi: 10.1001/jama.2022.18590. Erratum in: JAMA 329 (2) (2023) 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.George B., Moorthy M., Kulkarni U., Selvarajan S., Rupali P., Christopher D.J., Balamugesh T., Rose W., Lakshmi K.M., Devasia A.J., Fouzia N.A., Korula A., Lionel S., Abraham A., Mathews V. Single dose of ivermectin is not useful in patients with hematological disorders and COVID-19 illness: a phase II B open labelled randomized controlled trial. Indian J. Hematol. Blood Transfus. 2022;38(4):615–622. doi: 10.1007/s12288-022-01546-w. https://doi:10.1007/s12288-022-01546-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Galan L.E.B., Santos N.M.D., Asato M.S., Araújo J.V., de Lima Moreira A., Araújo A.M.M., Paiva A.D.P., Portella D.G.S., Marques F.S.S., Silva G.M.A., et al. Phase 2 randomized study on chloroquine, hydroxychloroquine or ivermectin in hospitalized patients with severe manifestations of SARS-CoV-2 infection. Pathog. Glob. Health. 2021;115(4):235‐242. doi: 10.1080/20477724.2021.1890887. https://doi:10.1080/20477724.2021.1890887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chahla R.E., Medina Ruiz L., Mena T., Brepe Y., Terranova P., Ortega E.S., Barrenechea G.G., Goroso D.G., Peral de Bruno M.d.l.A. medRxiv; 2021. IVERMECTIN REPROPOSING FOR COVID-19 TREATMENT OUTPATIENTS IN MILD STAGE IN PRIMARY HEALTH CARE CENTERS. [Google Scholar]
- 59.Krolewiecki A., Lifschitz A., Moragas M., Travacio M., Valentini R., Alonso D.F., Solari R., Tinelli M.A., Cimino R.O., Álvarez L., Fleitas P.E., Ceballos L., Golemba M., Fernández F., Fernández de Oliveira D., Astudillo G., Baeck I., Farina J., Cardama G.A., Mangano A., Spitzer E., Gold S., Lanusse C. Antiviral effect of high-dose ivermectin in adults with COVID-19: a proof-of-concept randomized trial. EClinicalMedicine. 2021;37 doi: 10.1016/j.eclinm.2021.100959. https://doi:10.1016/j.eclinm.2021.100959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vallejos J., Zoni R., Bangher M., Villamandos S., Bobadilla A., Plano F., Campias C., Chaparro Campias E., Medina M.F., Achinelli F., et al. Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial. BMC Infect. Dis. 2021;21(1):635. doi: 10.1186/s12879-021-06348-5. https://doi:10.1186/s12879-021-06348-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahmed S., Karim M.M., Ross A.G., Hossain M.S., Clemens J.D., Sumiya M.K., Phru C.S., Rahman M., Zaman K., Somani J., et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int. J. Infect. Dis. 2021;103:214‐216. doi: 10.1016/j.ijid.2020.11.191. https://doi:10.1016/j.ijid.2020.11.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Podder C.S., Chowdhury N., Sina M.I., Haque W.M.M.U. Outcome of ivermectin treated mild to moderate COVID-19 cases: a single-centre, open-label, randomised controlled study. IMC J. Med. Sci. 2020;14(2):11–18. [Google Scholar]
- 63.Abbas K.U., Muhammad S., Ding S.F. The effect of ivermectin on reducing viral symptoms in patients with mild COVID-19. Indian J. Pharmaceut. Sci. 2022;84:87‐91. https://doi:10.36468/pharmaceutical-sciences.spl.416 [Google Scholar]
- 64.López-Medina E., López P., Hurtado I.C., Dávalos D.M., Ramirez O., Martínez E., Díazgranados J.A., Oñate J.M., Chavarriaga H., Herrera S., Parra B., Libreros G., Jaramillo R., Avendaño A.C., Toro D.F., Torres M., Lesmes M.C., Rios C.A., Caicedo I. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. JAMA. 2021;325(14):1426–1435. doi: 10.1001/jama.2021.3071. https://doi:10.1001/JAMA.2021.3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abd-Elsalam S., Noor R.A., Badawi R., Khalaf M., Esmail E.S., Soliman S., Abd El Ghafar M.S., Elbahnasawy M., Moustafa E.F., Hassany S.M., et al. Clinical study evaluating the efficacy of ivermectin in COVID-19 treatment: a randomized controlled study. J. Med. Virol. 2021;93(10):5833‐5838. doi: 10.1002/jmv.27122. https://doi:10.1002/jmv.27122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aref Z.F., Bazeed S., Hassan M.H., Hassan A.S., Rashad A., Hassan R.G., Abdelmaksoud A.A. Clinical, biochemical and molecular evaluations of ivermectin mucoadhesive nanosuspension nasal spray in reducing upper respiratory symptoms of mild COVID-19. Int. J. Nanomed. 2021;16:4063–4072. doi: 10.2147/IJN.S313093. https://doi:10.2147/ijn.S313093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kishoria N., Mathur S., Parmar V., Kaur R., Agarwal H., Parihar B., Verma S. Ivermectin as adjuvant to hydroxycholoroquine in patients resistant to standard treatment for Sars-Cov-2: results of an open-label randomized clinical study. Indian J. Res. 2020;9(8):1–4. [Google Scholar]
- 68.Mohan A., Tiwari P., Suri T.M., Mittal S., Patel A., Jain A., Velpandian T., Das U.S., Boppana T.K., Pandey R.M., et al. Single-dose oral ivermectin in mild and moderate COVID-19 (RIVET-COV): a single-centre randomized, placebo-controlled trial. J. Infect. Themother. 2021;27(12):1743‐1749. doi: 10.1016/j.jiac.2021.08.021. https://doi:10.1016/j.jiac.2021.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ravikirti n., Roy R., Pattadar C., Raj R., Agarwal N., Biswas B., Manjhi P.K., Rai D.K., Shyama n., Kumar A., et al. Evaluation of ivermectin as a potential treatment for mild to moderate COVID-19: a double-blind randomized placebo controlled trial in eastern India. J. Pharm. Pharmaceut. Sci. 2021;24:343‐350. doi: 10.18433/jpps32105. https://doi:10.18433/jpps32105 [DOI] [PubMed] [Google Scholar]
- 70.Rezai M.S., Ahangarkani F., Hill A., Ellis L., Mirchandani M., Davoudi A., Eslami G., Roozbeh F., Babamahmoodi F., Rouhani N., Alikhani A., Najafi N., Ghasemian R., Mehravaran H., Hajialibeig A., Navaeifar M.R., Shahbaznejad L., Rahimzadeh G., Saeedi M., Alizadeh-Navai R., Moosazadeh M., Saeedi S., Razavi-Amoli S.K., Rezai S., Rostami-Maskopaee F., Hosseinzadeh F., Movahedi F.S., Markowitz J.S., Valadan R. Non-effectiveness of ivermectin on inpatients and outpatients with COVID-19; results of two randomized, double-blinded, placebo-controlled clinical trials. Front. Med. 2022;9 doi: 10.3389/fmed.2022.919708. https://doi:10.3389/fmed.2022.919708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shahbaznejad L., Davoudi A., Eslami G., Markowitz J.S., Navaeifar M.R., Hosseinzadeh F., Movahedi F.S., Rezai M.S. Effects of ivermectin in patients with COVID-19: a multicenter, double-blind, randomized, controlled clinical trial. Clin. Therapeut. 2021;43(6):1007‐1019. doi: 10.1016/j.clinthera.2021.04.007. https://doi:10.1016/j.clinthera.2021.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Biber A., Harmelin G., Lev D., Ram L., Shaham A., Nemet I., Kliker L., Erster O., Mandelboim M., Schwartz E. The effect of ivermectin on the viral load and culture viability in early treatment of nonhospitalized patients with mild COVID-19–a double-blind, randomized placebo-controlled trial. Int. J. Infect. Dis. 2022;122:733–740. doi: 10.1016/j.ijid.2022.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wada T., Hibino M., Aono H., Kyoda S., Iwadate Y., Shishido E., Ikeda K., Kinoshita N., Matsuda Y., Otani S., Kameda R., Matoba K., Nonaka M., Maeda M., Kumagai Y., Ako J., Shichiri M., Naoki K., Katagiri M., Takaso M., Iwamura M., Katayama K., Miyatsuka T., Orihashi Y., Yamaoka K. Efficacy and safety of single-dose ivermectin in mild-to-moderate COVID-19: the double-blind, randomized, placebo-controlled CORVETTE-01 trial. Front. Med. 2023;10 doi: 10.3389/fmed.2023.1139046. https://doi:10.3389/fmed.2023.1139046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beltran Gonzalez J.L., González Gámez M., Mendoza Enciso E.A., Esparza Maldonado R.J., Hernández Palacios D., Dueñas Campos S., Robles I.O., Macías Guzmán M.J., García Díaz A.L., Gutiérrez Peña C.M., Martinez Medina L., Monroy Colin V.A., Arreola Guerra J.M. Efficacy and safety of ivermectin and hydroxychloroquine in patients with severe COVID-19: a randomized controlled trial. Infect. Dis. Rep. 2022;14(2):160–168. doi: 10.3390/idr14020020. https://doi:10.3390/idr14020020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rocha C.d.l., Cid-Lopez M.A., Venegas-Lopez B.I., Gómez-Mendez S.C., Sánchez-Ortiz A., Pérez-Ríos A.M., Llamas-Velazquez R.A., Meza-Acuña A.I., Vargas-Íñiguez B., Rosales-Galván D. Ivermectin compared with placebo in the clinical course in Mexican patients with asymptomatic and mild COVID-19: a randomized clinical trial. BMC Infect. Dis. 2022;22(1):917. doi: 10.1186/s12879-022-07890-6. https://doi:10.1186/s12879-022-07890-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Babalola O.E., Bode C.O., Ajayi A.A., Alakaloko F.M., Akase I.E., Otrofanowei E., Salu O.B., Adeyemo W.L., Ademuyiwa A.O., Omilabu S. Ivermectin shows clinical benefits in mild to moderate COVID19: a randomized controlled double-blind, dose-response study in Lagos. QJM. 2022;114(11):780–788. doi: 10.1093/qjmed/hcab035. https://doi:10.1093/qjmed/hcab035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shah Bukhari K.H., Asghar A., Perveen N., Hayat A., Mangat S.A., Butt K.R., Abdullah M., Fatima T., Mustafa A., Iqbal T. Efficacy of ivermectin in COVID-19 patients with mild to moderate disease. MedRxiv. 2021 2021-02. [Google Scholar]
- 78.Chachar A.Z.K., Khan K.A., Asif M., Tanveer K., Khaqan A., Basri R. Effectiveness of ivermectin in SARS-CoV-2/COVID-19 patients. Int. J. Sci. 2020;9(9):31–35. [Google Scholar]
- 79.Chaccour C., Casellas A., Blanco-Di Matteo A., Pineda I., Fernandez-Montero A., Ruiz-Castillo P., Richardson M.A., Rodríguez-Mateos M., Jordán-Iborra C., Brew J., Carmona-Torre F., Giráldez M., Laso E., Gabaldón-Figueira J.C., Dobaño C., Moncunill G., Yuste J.R., Del Pozo J.L., Rabinovich N.R., Schöning V., Hammann F., Reina G., Sadaba B., Fernández-Alonso M. The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial. EClinicalMedicine. 2021;32 doi: 10.1016/j.eclinm.2020.100720. https://doi:10.1016/j.eclinm.2020.100720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Angkasekwinai N., Rattanaumpawan P., Chayakulkeeree M., Phoompoung P., Koomanachai P., Chantarasut S., Wangchinda W., Srinonprasert V., Thamlikitkul V. Safety and efficacy of ivermectin for the prevention and treatment of COVID-19: a double-blinded randomized placebo-controlled study. Antibiotics (Basel) 2022;11(6) doi: 10.3390/antibiotics11060796. https://doi:10.3390/antibiotics11060796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Okumuş N., Demirtürk N., Çetinkaya R.A., Güner R., Avcı İ Y., Orhan S., Konya P., Şaylan B., Karalezli A., Yamanel L., Kayaaslan B., Yılmaz G., Savaşçı Ü., Eser F., Taşkın G. Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients. BMC Infect. Dis. 2021;21(1):411. doi: 10.1186/s12879-021-06104-9. https://doi:10.1186/s12879-021-06104-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Naggie S. Ivermectin for treatment of mild-to-moderate COVID-19 in the outpatient setting: a decentralized, placebo-controlled, randomized, platform clinical trial. JAMA. 2022;328(16):1595–1603. doi: 10.1001/jama.2022.18590. https://doi:10.1001/JAMA.2022.18590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Naggie S., Boulware D.R., Lindsell C.J., Stewart T.G., Slandzicki A.J., Lim S.C., Cohen J., Kavtaradze D., Amon A.P., Gabriel A., Gentile N., Felker G.M., Jayaweera D., McCarthy M.W., Sulkowski M., Rothman R.L., Wilson S., DeLong A., Remaly A., Wilder R., Collins S., Dunsmore S.E., Adam S.J., Thicklin F., Hanna G.J., Ginde A.A., Castro M., McTigue K., Shenkman E., Hernandez A.F. Effect of higher-dose ivermectin for 6 Days vs placebo on time to sustained recovery in outpatients with COVID-19: a randomized clinical trial. JAMA. 2023;329(11):888–897. doi: 10.1001/jama.2023.1650. https://doi:10.1001/JAMA.2023.1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Agarwal A., Rochwerg B., Lamontagne F., Siemieniuk R.A., Agoritsas T., Askie L., Lytvyn L., Leo Y.S., Macdonald H., Zeng L., Amin W., Barragan F.A.J., Bausch F.J., Burhan E., Calfee C.S., Cecconi M., Chanda D., Dat V.Q., De Sutter A., Du B., Freedman S., Geduld H., Gee P., Gotte M., Harley N., Hashimi M., Hunt B., Jehan F., Kabra S.K., Kanda S., Kim Y.J., Kissoon N., Krishna S., Kuppalli K., Kwizera A., Lado Castro-Rial M., Lisboa T., Lodha R., Mahaka I., Manai H., Mino G., Nsutebu E., Preller J., Pshenichnaya N., Qadir N., Relan P., Sabzwari S., Sarin R., Shankar-Hari M., Sharland M., Shen Y., Ranganathan S.S., Souza J.P., Stegemann M., Swanstrom R., Ugarte S., Uyeki T., Venkatapuram S., Vuyiseka D., Wijewickrama A., Tran L., Zeraatkar D., Bartoszko J.J., Ge L., Brignardello-Petersen R., Owen A., Guyatt G., Diaz J., Kawano-Dourado L., Jacobs M., Vandvik P.O. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379. doi: 10.1136/bmj.m3379. https://doi:10.1136/BMJ.m3379 [DOI] [PubMed] [Google Scholar]
- 85.Baracaldo-Santamaría D., Pabón-Londoño S., Rojas-Rodriguez L.C. Drug safety of frequently used drugs and substances for self-medication in COVID-19, Ther. Adv. Drug Saf. 2022;13 doi: 10.1177/20420986221094141. https://doi:10.1177/20420986221094141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vasquez-Elera L.E., Failoc-Rojas V.E., Martinez-Rivera R.N., Morocho-Alburqueque N., Temoche-Rivas M.S., Valladares-Garrido M.J. Self-medication in hospitalized patients with COVID-19: a cross-sectional study in northern Peru. Germs. 2022;12(1):46–53. doi: 10.18683/germs.2022.1305. https://doi:10.18683/germs.2022.1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang S., Shen S., Hou N. Is ivermectin effective in treating COVID-19? Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.858693. https://doi:10.3389/fphar.2022.858693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pan American Health Organization, Ongoing Living Update of Potential COVID-19 Therapeutics Options: Summary of Evidence. In Traducciones 2019-2024. https://iris.paho.org/handle/10665.2/52719. Accessed 30 Nov 2023.
- 89.Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2021;373:n967. doi: 10.1136/bmj.n967. https://doi:10.1136/BMJ.n967 [DOI] [PubMed] [Google Scholar]
- 90.Axfors C., Schmitt A.M., Janiaud P., Van't Hooft J., Abd-Elsalam S., Abdo E.F., Abella B.S., Akram J., Amaravadi R.K., Angus D.C., Arabi Y.M., Azhar S., Baden L.R., Baker A.W., Belkhir L., Benfield T., Berrevoets M.A.H., Chen C.P., Chen T.C., Cheng S.H., Cheng C.Y., Chung W.S., Cohen Y.Z., Cowan L.N., Dalgard O., de Almeida E.V.F.F., de Lacerda M.V.G., de Melo G.C., Derde L., Dubee V., Elfakir A., Gordon A.C., Hernandez-Cardenas C.M., Hills T., Hoepelman A.I.M., Huang Y.W., Igau B., Jin R., Jurado-Camacho F., Khan K.S., Kremsner P.G., Kreuels B., Kuo C.Y., Le T., Lin Y.C., Lin W.P., Lin T.H., Lyngbakken M.N., McArthur C., McVerry B.J., Meza-Meneses P., Monteiro W.M., Morpeth S.C., Mourad A., Mulligan M.J., Murthy S., Naggie S., Narayanasamy S., Nichol A., Novack L.A., O'Brien S.M., Okeke N.L., Perez L., Perez-Padilla R., Perrin L., Remigio-Luna A., Rivera-Martinez N.E., Rockhold F.W., Rodriguez-Llamazares S., Rolfe R., Rosa R., Røsjø H., Sampaio V.S., Seto T.B., Shahzad M., Soliman S., Stout J.E., Thirion-Romero I., Troxel A.B., Tseng T.Y., Turner N.A., Ulrich R.J., Walsh S.R., Webb S.A., Weehuizen J.M., Velinova M., Wong H.L., Wrenn R., Zampieri F.G., Zhong W., Moher D., Goodman S.N., Ioannidis J.P.A., Hemkens L.G. Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials. Nat. Commun. 2021;12(1):2349. doi: 10.1038/s41467-021-22446-z. https://doi:10.1038/s41467-021-22446-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marcolino M.S., Meira K.C., Guimarães N.S., Motta P.P., Chagas V.S., Kelles S.M.B., de Sá L.C., Valacio R.A., Ziegelmann P.K. Systematic review and meta-analysis of ivermectin for treatment of COVID-19: evidence beyond the hype. BMC Infect. Dis. 2022;22(1):639. doi: 10.1186/s12879-022-07589-8. https://doi:10.1186/s12879-022-07589-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chowdhury A., Shahbaz M., Karim R., Islam J., Dan G., He S.X. A comparative study on ivermectin-doxycycline and hydroxychloroquine-azithromycin therapy on COVID-19 patients. Eurasian J Med Oncol. 2021;5(1):63–70. https://doi:10.1016/j.biopha.2021.111956 [Google Scholar]
- 93.Popp M., Reis S., Schießer S., Hausinger R.I., Stegemann M., Metzendorf M.I., Kranke P., Meybohm P., Skoetz N., Weibel S. Ivermectin for preventing and treating COVID-19. Cochrane Database Syst. Rev. 2022;6(6):Cd015017. doi: 10.1002/14651858.CD015017.pub3. https://doi:10.1002/14651858.CD015017.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pan American Health O. Ongoing living update of COVID-19 therapeutic options: summary of evidence. Rapid Review. 2022:462. 4 May 2022. [Google Scholar]
- 95.Araya V., Kraemer P., Burdiles P., Herrera P., Castillo C., Sepulveda D., Quiñelen E., Pimentel L., Contreras M., Bravo R., Bravo G.B., Morel M., Ortiz L., Vergudo F., Zavala C., Lobos D., Llovet V., Moll C., Rada G., N I. The Living Overview of Evidence database (LOVE) may be more efficient than a traditional search of systematic reviews and randomized trials. 2020. https://abstracts.cochrane.org/2019-santiago/living-overview-evidence-database-love-may-be-more-efficient-traditional-search
- 96.Higgins J., T J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A. 2022. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2.https://community.cochrane.org/mecir-manual.2022 (updated February 2021) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. Material/referenced in article. Data will be made available on request.