Abstract
Background & Aims
The investigational first-generation core inhibitor vebicorvir (VBR) demonstrated safety and antiviral activity over 24 weeks in two phase IIa studies in patients with chronic HBV infection. In this long-term extension study, patients received open-label VBR with nucleos(t)ide reverse transcriptase inhibitors (NrtIs).
Methods
Patients in this study (NCT03780543) previously received VBR + NrtI or placebo + NrtI in parent studies 201 (NCT03576066) or 202 (NCT03577171). After receiving VBR + NrtI for ≥52 weeks, stopping criteria (based on the treatment history and hepatitis B e antigen status in the parent studies) were applied, and patients either discontinued both VBR + NrtI, discontinued VBR only, or continued both VBR + NrtI. The primary efficacy endpoint was the proportion of patients with HBV DNA <20 IU/ml at 24 weeks off treatment.
Results
Ninety-two patients entered the extension study and received VBR + NrtI. Long-term VBR + NrtI treatment led to continued suppression of HBV nucleic acids and, to a lesser extent, HBV antigens. Forty-three patients met criteria to discontinue VBR + NrtI, with no patients achieving the primary endpoint; the majority of virologic rebound occurred ≥4 weeks off treatment. Treatment was generally well tolerated, with few discontinuations due to adverse events (AEs). There were no deaths. Most AEs and laboratory abnormalities were related to elevations in alanine aminotransferase and occurred during the off-treatment or NrtI-restart phases. No drug–drug interactions between VBR + NrtI and no cases of treatment-emergent resistance among patients who adhered to treatment were observed.
Conclusions
Long-term VBR + NrtI was safe and resulted in continued reductions in HBV nucleic acids following completion of the 24-week parent studies. Following treatment discontinuation, virologic relapse was observed in all patients. This first-generation core inhibitor administered with NrtI for at least 52 weeks was not sufficient for HBV cure.
Clinical trial number
Impact and implications
Approved treatments for chronic hepatitis B virus infection (cHBV) suppress viral replication, but viral rebound is almost always observed after treatment discontinuation, highlighting an unmet need for improved therapies with finite treatment duration producing greater therapeutic responses that can be sustained off treatment. First-generation core inhibitors, such as vebicorvir, have mechanisms of action orthogonal to standard-of-care therapies that deeply suppress HBV viral replication during treatment; however, to date, durable virologic responses have not been observed after treatment discontinuation. The results reported here will help researchers with the design and interpretation of future studies investigating core inhibitors as possible components of finite treatment regimens for patients with cHBV. It is possible that next-generation core inhibitors with enhanced potency may produce deeper and more durable antiviral activity than first-generation agents, including vebicorvir.
Keywords: Hepatitis, Core inhibitor, Antiviral, Off-treatment, Open-label, Nucleos(t)ide reverse transcriptase inhibitor, Viral relapse, Hepatitis B virus
Graphical abstract
Highlights
-
•
The HBV core inhibitor vebicorvir (VBR) + NrtI was evaluated in phase II studies.
-
•
Long-term VBR + NrtI is generally well tolerated in patients with chronic HBV infection.
-
•
Reductions in HBV DNA and pgRNA were observed with long-term VBR + NrtI treatment.
-
•
In patients who stopped treatment, a sustained virologic response was not observed.
-
•
Drug–drug interactions and viral resistance were not observed in this study.
Introduction
Chronic HBV infection (cHBV) represents a significant public health burden. Worldwide, ∼296 million people have cHBV, and ∼900,000 die annually from HBV-related causes, primarily from complications of cirrhosis and/or hepatocellular carcinoma.[1], [2], [3] Effective cHBV treatment is essential to reduce these risks. Current treatment includes finite injectable IFNα and chronic oral nucleos(t)ide reverse transcriptase inhibitors (NrtIs). IFNα4,5 and NrtIs6,7 both demonstrate on-treatment antiviral activity, but durable off-treatment virologic responses are rare. For therapeutic regimens to achieve ‘functional cure’ (defined as sustained suppression of HBV DNA <lower limit of quantification [LLOQ] for ≥6 months post-treatment and undetectable HBsAg with/without HBsAg seroconversion),8 novel combination approaches incorporating mechanisms complementary to existing treatments are needed.
Vebicorvir (VBR) is an investigational, novel, pangenotypic, first-generation core inhibitor that inhibits HBV replication via mechanisms distinct from NrtIs: inhibition of pregenomic RNA (pgRNA) encapsidation, which prevents assembly and release of viral particles, and disruption of viral capsids, which prevents the formation of covalently closed circular (ccc)DNA. In phase IIa studies, when combined with NrtIs, VBR led to deeper reductions in HBV DNA and pgRNA vs. NrtI monotherapy over 24 weeks of treatment in both virologically-suppressed (VS; study 201)9 and treatment-naive (TN; study 202)10 patients with cHBV. However, mean reductions in HBsAg in patients who received VBR + NrtI in studies 201 and 202 were not significantly different from patients who received placebo (PBO) + NrtI. This report presents the results of an open-label, long-term extension in which patients who previously participated in study 201 or 202 received VBR + NrtI for up to 148 weeks.
Patients and methods
Study population and design
Study 211 was a phase II, open-label, multicentre extension study (NCT03780543) evaluating the safety and efficacy of VBR + NrtI in patients with cHBV who had previously completed 24 weeks of treatment in either study 201 (NCT03576066)9 or 202 (NCT03577171).10 Patients were enrolled from 24 sites in the USA, Canada, Hong Kong, New Zealand, and the UK. Complete inclusion and exclusion criteria and details on treatment compliance are provided in the Supplementary materials.
All patients in study 211 received open-label 300 mg VBR (Assembly Biosciences, Inc., South San Francisco, CA, USA), administered as three 100-mg tablets once daily along with standard-of-care NrtI per the manufacturer’s instructions. The VBR dose regimen was the same as that used in the parent studies, which was determined from the phase Ib study.11 Most patients from study 201 took tenofovir disoproxil fumarate or tenofovir alafenamide as their NrtI at baseline, whereas all patients from study 202 received entecavir (ETV) along with VBR or PBO.
Patients could be treated for up to 148 weeks. The actual duration of treatment for each patient in study 211 was based on their respective HBV treatment history (i.e. VS or TN) and HBeAg status (positive or negative) at baseline in their parent study, along with their individual virologic response in study 211 at Week (W) 52. Based on these factors, each patient was assigned to one of three protocol-specified treatment actions (TAs): discontinue both VBR + NrtI, discontinue VBR only and continue NrtI alone, or continue both VBR + NrtI (Table S1).
Individual safety and virologic responses in study 211 were influenced by patient characteristics at baseline in the parent studies and the respective treatments received—that is, VBR + NrtI or PBO + NrtI. Therefore, data from study 211 are reported according to HBeAg status and treatment assignment in the parent study. Additionally, study 211 data are reported from three treatment phases: ‘on-treatment’, during VBR + NrtI therapy; ‘off-treatment’, for patients who discontinued VBR + NrtI; and ‘after NrtI restart’, for patients requiring reintroduction of antivirals after stopping both VBR + NrtI (Table S2). The study design is shown in Fig. S1.
This study was conducted in accordance with the principles of the Declaration of Helsinki, Council for International Organizations of Medical Sciences International Ethical Guidelines, and applicable Good Clinical Practice guidelines. Investigative sites obtained written informed consent before patients were enrolled.
Endpoints
The primary endpoint was the proportion of patients with HBV DNA <20 IU/ml (LLOQ) at 24 weeks off treatment. Secondary endpoints included incidence of adverse events (AEs), premature discontinuations as a result of AEs, abnormal safety laboratory results, proportion of patients with abnormal alanine aminotransferase (ALT) at study 211 baseline who achieved normal ALT at end of treatment (EOT) and end of study (EOS), and incidence of viral rebound off treatment. A complete list of exploratory endpoints is included in the Supplementary material.
Safety assessments
Primary safety assessments included the number of AEs, defined as any untoward medical occurrence in a treated patient regardless of the causal relationship with treatment. The severity of each AE and laboratory abnormality was assessed by the investigator according to the Division of AIDS Toxicity Grading of Laboratory Abnormalities and Clinical AEs. Additional definitions of AEs are described in the Supplementary material. Full schedules of efficacy, safety, and pharmacokinetic (PK) assessments are described in Tables S3–S6 and the Supplementary material.
Assays
Methodological details for measuring HBV DNA, HBV pgRNA, total nucleic acids (TNA; HBV DNA + pgRNA), and antigens are described in the Supplementary material.
To assess for potential HBV sequence changes associated with viral resistance or blunted treatment response, serum samples from patients with on-treatment viral rebound (≥1 log10 HBV DNA increase from the on-treatment nadir) were selected for sequencing of HBV core and polymerase/reverse transcriptase genes. Plasma samples quantifying VBR and NrtI concentrations were collected predose at W48 and analysed at the bioanalytical laboratory (Agilex, Thebarton, Australia) using validated methodologies.
Statistical analysis
Baseline characteristics and demographics were summarised using the all-enrolled analysis set, which included all patients enrolled in study 211. Efficacy-related endpoints were assessed using the full analysis set, which included all patients who received any dose of study drug and had at least one postdose assessment for the endpoint of interest.
The safety population included all patients who received at least one dose of study drug. PK evaluation included all patients in the safety population who had available VBR or NrtI PK data. Sample size was not based on statistical considerations and consequently descriptive statistics are used throughout. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patient disposition
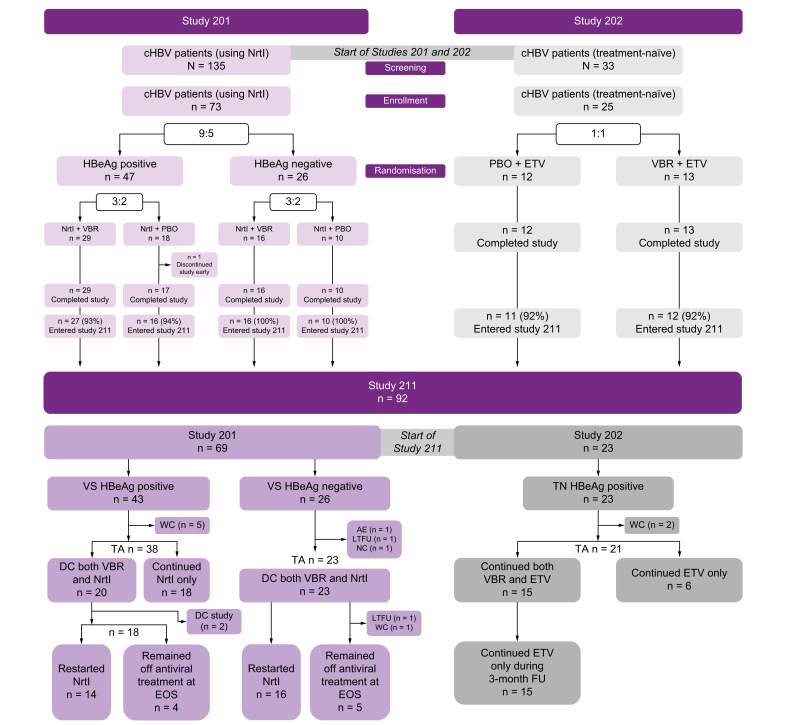
Overall, 92/98 (94%) patients (69/73 [95%] from study 201; 23/25 [92%] from study 202) were enrolled between December 2018 and June 2019. Patient disposition is shown in Fig. 1. All enrolled patients received VBR + NrtI during the on-treatment phase. Forty-three of 69 (62%) patients from study 201 discontinued VBR + NrtI during study 211, with 18/69 (26%) discontinuing VBR and continuing NrtI. Of the 43 patients from study 201 who discontinued VBR + NrtI, 30/43 (70%) restarted NrtI after discontinuation, with nine of 43 (21%) remaining off treatment at EOS. No patients from study 202 discontinued both VBR + ETV, with six of 23 (26%) discontinuing VBR and continuing ETV. The duration of time patients spent in the on- and off-treatment phase is described in the Supplementary material.
Fig. 1.
Study disposition in patients from studies 201 and 202 taking VBR in study 211.
Study 211 outcomes are based on the treatment history of the patients in parent studies 201 and 202. AE, adverse event; cHBV, chronic hepatitis B virus infection; DC, discontinued; EOS, end of study; ETV, entecavir; FU, follow-up; LTFU, lost to follow-up; NC, non-compliance; NrtI, nucleos(t)ide reverse transcriptase inhibitor; PBO, placebo; TA, treatment action; TN, treatment-naive; VBR, vebicorvir; VS, virologically-suppressed; WC, withdrew consent.
Baseline demographics and disease characteristics
At study 211 baseline, overall mean age was 44 years, with 62/92 (67%) patients aged <50 years. Most patients were male (52/92; 57%) and Asian (80/92; 87%). Some baseline demographics varied according to the respective parent study, with greater proportions of patients originating from study 201 being older (mean age 46 vs. 36 years) and male (64% vs. 35%) vs. those from study 202 (Table S7). Additional baseline disease characteristics for patients in study 211 are shown in Table S8 and described in further detail in the Supplementary material.
Primary efficacy endpoint
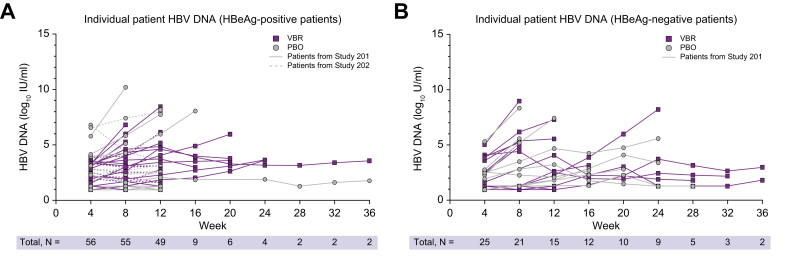
Forty-three patients (20 HBeAg-positive, 23 HBeAg-negative) from study 201 met the protocol-specified TA criteria to discontinue VBR + NrtI. Following discontinuation, all patients experienced virologic relapse with no patients achieving HBV DNA <LLOQ at 24 weeks off treatment (Fig. 2). Of the patients who were HBeAg-negative, 16/23 (70%) relapsed by the 4W follow-up visit, whereas seven of 23 (30%) relapsed by the 12W or 16W follow-up visits. Of the patients who were HBeAg-positive, 17/18 (94%) relapsed by the 4W follow-up visit (one patient had relapsed by the 12W follow-up visit). No patients from study 202 met the TA criteria to discontinue VBR + ETV.
Fig. 2.
Individual patient HBV DNA during the off-treatment phase in patients from study 211.
HBV DNA levels in (A) patients who were HBeAg-positive and (B) patients who were HBeAg-negative during the off-treatment phase in study 211. Parent study designation as well as treatment received during the parent studies are shown. No patients remained <LLOQ of 20 IU/ml (1.3 log10 IU/ml) off treatment. LLOQ, lower limit of quantification; PBO, placebo, VBR, vebicorvir.
Changes in HBV nucleic acids and antigens during the on-treatment phase
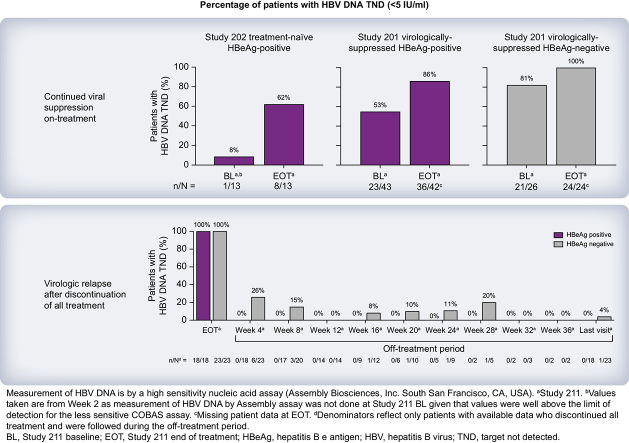
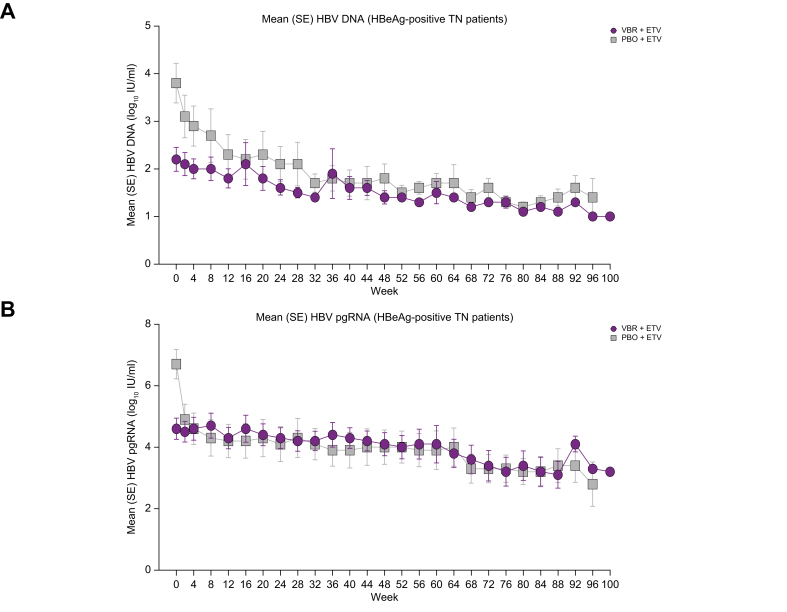
Among patients who were HBeAg-negative who received PBO + NrtI and VBR + NrtI in study 201, most had HBV DNA target not detected (TND) at on-treatment baseline and at EOT using the COBAS TaqMan assay (Table 1). With the Assembly assay, a greater percentage of patients with available samples had HBV DNA TND at on-treatment baseline, with all patients who were HBeAg-negative having HBV DNA TND at EOT. Approximately half of patients who were HBeAg-positive from study 201 who received PBO + NrtI and VBR + NrtI had HBV DNA TND at on-treatment baseline and 30–50% had HBV DNA TND at EOT by the COBAS TaqMan assay. When assessed by the Assembly assay, five of 16 (31%) and 18/27 (67%) patients who were HBeAg-positive who received PBO + NrtI and VBR + NrtI, respectively, during study 201 had HBV DNA TND at on-treatment baseline, with numerically more patients having HBV DNA TND at EOT in study 211. When assessed by the Assembly assay, no patients from study 202 had HBV DNA TND at on-treatment baseline, with none of five and one of eight (13%) patients who received PBO + NrtI and VBR + NrtI, respectively, during study 202 having HBV DNA TND at W2 in study 211. When assessed using the COBAS TaqMan assay, no patients from study 202 had HBV DNA TND at on-treatment baseline and at W2 in study 211. Per the COBAS TaqMan assay, six of 23 (26%) patients from study 202 had HBV DNA TND at EOT (the percentage of patients was greater when assessed by the Assembly assay [eight of 13 (62%)]; Table 1). Because mean baseline levels of HBV DNA were greater in patients who received PBO + NrtI from study 202 vs. patients who received VBR + NrtI (Table S8), there were greater mean reductions in HBV DNA at EOT among patients who received PBO + ETV in study 202 vs. patients who received VBR + ETV (Fig. 3A).
Table 1.
Observed changes in HBV DNA and pgRNA during the on-treatment phase in study 211 (FAS).
| Patients originating from study 201 (on-treatment phase) | ||||||
|---|---|---|---|---|---|---|
| VS HBeAg (-) |
VS HBeAg (+) |
|||||
| PBO + NrtI n = 10 |
VBR + NrtI n = 16 |
Total n = 26 |
PBO + NrtI n = 16 |
VBR + NrtI n = 27 |
Total n = 43 |
|
| HBV DNA TND | ||||||
| Baseline∗ | 6/10 (60) | 13/16 (81) | 19/26 (73) | 8/15 (53) | 16/27 (59) | 24/43 (57) |
| HBV DNA TND | ||||||
| EOT∗ | 8/10 (80) | 13/16 (81) | 21/26 (81) | 8/16 (50) | 8/27 (30) | 16/43 (37) |
| HBV DNA TND | ||||||
| Baseline† | 9/10 (90) | 12/16 (75) | 21/26 (81) | 5/16 (31) | 18/27 (67) | 23/43 (53) |
| HBV DNA TND | ||||||
| EOT† | 10/10 (100) | 14/14 (100) | 24/24 (100) | 13/15 (87) | 23/27 (85) | 36/42 (86) |
| HBV DNA change from baseline, log10 IU/ml, mean (SD) | ||||||
| EOT | -0.1 (0.14) | 0.2 (0.81) | 0.1 (0.64) | 0.0 (0.23) | 0.2 (0.61) | 0.1 (0.51) |
| HBV pgRNA change from baseline, log10 U/ml, mean (SD) | ||||||
| EOT | 0 (0.00) | 0.1 (0.38) | 0.1 (0.29) | -1.4 (1.02) | 0 (0.47) | -0.5 (0.98) |
| HBV TNA change from baseline, log10 U/ml, mean (SD)‡ | ||||||
| EOT | 0 (0.00) | 0 (0.00) | 0 (0.00) | -1.2 (1.10) | 0.0 (0.14) | -0.5 (0.91) |
| Patients originating from study 202 (TN HBeAg +; on-treatment phase) | |||
|---|---|---|---|
| PBO + ETV n = 11 |
VBR + ETV n = 12 |
Total N = 23 |
|
| HBV DNA TND | |||
| Baseline∗ | 0 | 0 | 0 |
| Week 2∗ | 0 | 0 | 0 |
| HBV DNA TND | |||
| EOT∗ | 3/11 (27) | 3/12 (25) | 6/23 (26) |
| HBV DNA TND | |||
| Baseline† | ND§ | ND§ | ND§ |
| Week 2† | 0¶ | 1/8 (13) | 1/13 (8) |
| HBV DNA TND | |||
| EOT† | 5/5 (100) | 3/8 (38) | 8/13 (62) |
| HBV DNA change from baseline, log10 IU/ml, mean (SD) | |||
| EOT | -2.1 (1.08) | -0.8 (0.70) | -1.4 (1.09) |
| HBV pgRNA change from baseline, log10 U/ml, mean (SD) | |||
| EOT | -3.0 (1.38) | -0.6 (0.75) | -1.7 (1.60) |
Data shown are n/N (%) unless otherwise stated.
EOT, end of treatment; ETV, entecavir; FAS, full analysis set; LOD, limit of detection; ND, not determined; NrtI, nucleos(t)ide reverse transcriptase inhibitor; PBO, placebo; pgRNA, pregenomic RNA; TN, treatment-naive; TNA, total nucleic acids; TND, target not detected; VBR, vebicorvir; VS, virologically-suppressed.
Assessed by COBAS TaqMan; LOD = 10 IU/ml.
Assessed by Assembly Biosciences, Inc. HBV DNA assay; LOD = 5 IU/ml.
TNA = HBV DNA + HBV pgRNA.
Patient HBV DNA TND levels were ND given that values were well above the LOD for the less sensitive COBAS assay.
The denominator for patients originating from study 202 is 5 for PBO + ETV.
Fig. 3.
HBV DNA and pgRNA change from baseline in patients from study 202 (FAS; on-treatment phase).
Changes from baseline in (A) HBV DNA and (B) HBV pgRNA in study 211 from patients previously enrolled in study 202. ETV, entecavir; FAS, full analysis set; PBO, placebo; pgRNA, pregenomic RNA; TN, treatment-naive; VBR, vebicorvir.
Changes in HBV pgRNA and TNA during the on-treatment phase are shown in Table 1. Because mean baseline levels of pgRNA were greater in patients who received PBO + NrtI from study 201 vs. patients who received VBR + NrtI, there were greater mean reductions in pgRNA levels among patients who received PBO + NrtI in study 201 at EOT vs. patients who received VBR + NrtI in study 201. These findings were also observed for TNA, as only patients who were HBeAg-positive showed a mean change from on-treatment baseline in TNA at EOT. Greater reductions in HBV pgRNA in patients who received VBR + ETV vs. patients who received PBO + ETV from study 202 led to different levels of HBV pgRNA between treatment groups at on-treatment baseline. Given this, greater mean reductions from on-treatment baseline in mean HBV pgRNA were observed among study 202 patients who had received PBO + ETV vs. VBR + ETV at EOT (Fig. 3B).
Minimal mean changes from on-treatment baseline at EOT in HBV antigens were observed (Table S9). At EOT, one patient (PBO + ETV) from study 202 achieved HBeAg seroconversion (defined as antigen loss with the appearance of antibodies), which was maintained through EOS. At EOS, two additional patients achieved HBeAg seroconversion (both received VBR + NrtI in study 201). No patients achieved HBsAg seroconversion.
Changes in HBV nucleic acids and antigens in patients who discontinued both VBR + NrtI during the off-treatment phase
Mean HBV DNA and TNA increases from off-treatment baseline at the end of the off-treatment period were greater among patients who were HBeAg-positive vs. HBeAg-negative from study 201 (Table 2). The one patient who was HBeAg-positive from study 201 with pgRNA results available, who discontinued both VBR + NrtI, had a change from baseline to end of off-treatment phase of 4.7 log10 U/ml. Individual patient HBV DNA levels by HBeAg status in the parent studies throughout the off-treatment phase are shown in Fig. 2. During the off-treatment phase, 17/20 (85%) patients who were HBeAg-positive and 16/23 (70%) patients who were HBeAg-negative relapsed with HBV DNA levels >2,000 IU/ml.
Table 2.
Observed changes in HBV DNA and pgRNA during the off-treatment phase in patients from study 211 (FAS).
| Patients originating from study 201 (off-treatment phase) | ||
|---|---|---|
| VS HBeAg (-) discontinue both n = 23 |
VS HBeAg (+) discontinue both n = 18 |
|
| HBV DNA baseline, log10 IU/ml | 1.0 (0.11) | 1.2 (0.16) |
| Change from baseline at end of off-treatment | 3.6 (2.41) | 4.8 (2.55) |
| HBV pgRNA baseline, log10 U/ml | NA | 1.5 (ND)∗ |
| Change from baseline at end of off-treatment | NA | 4.7 (ND)∗ |
| HBV TNA baseline, log10 U/ml | 1.3 (0.00) | 1.3 (0.06) |
| Change from baseline at end of off-treatment | 2.6 (2.29) | 4.0 (2.51) |
Data shown are mean (SD).
FAS, full analysis set; NA, not applicable; ND, not determined; pgRNA, pregenomic RNA; TNA, total nucleic acids; VS, virologically-suppressed.
n = 1 with available pgRNA results.
Mean antigen levels tended to be slightly greater at the end of the off-treatment period vs. off-treatment baseline in patients who discontinued both VBR + NrtI during the off-treatment phase (Table S10). Mean HBV DNA declined ∼4 log10 from baseline among patients who discontinued VBR + NrtI when they restarted NrtI (Table S11). Mean decreases in HBV antigens among patients who restarted NrtI after discontinuing VBR + NrtI were ≤1 log10 for all antigens (Table S12). Among patients who discontinued VBR and continued NrtI/ETV during the off-treatment phase, the mean increase from baseline in HBV DNA was ∼1 log10 IU/ml for patients from study 202, with no notable changes observed among patients from study 201. Mean increases from baseline in HBV pgRNA and TNA were numerically greater for patients from study 201 vs. 202. Mean HBV pgRNA and TNA increased from baseline during the off-treatment phase among patients who continued NrtI/ETV (Table S13; Fig. S2). When VBR was discontinued but NrtI/ETV was continued, there was a rebound in mean pgRNA of ∼2 log10 U/ml, as early as the 4W follow-up visit, that persisted throughout EOS. Mean HBV DNA rebounded ∼1 log10 IU/ml in patients from study 202 but did not rebound in patients from study 201. No notable changes were observed in mean antigen levels from off-treatment baseline to last visit among patients who discontinued VBR and continued NrtI/ETV only (Table S14; Fig. S3).
Safety
Mean (SD) treatment duration during study 211 was 63.5 (17.5) weeks, with most patients (66/92; 72%) receiving study drugs between 48 and 72 weeks. Overall exposure ranged from 1.1 to 103.9 weeks.
A summary of treatment-emergent adverse events (TEAEs) and AEs by treatment phase is reported in Table 3. During the on-treatment phase, 54/92 (59%) patients reported one or more TEAEs. One patient who was HBeAg-positive who received PBO + ETV in study 202 developed on-treatment serious AEs (SAEs) not related to study drug, and another patient (HBeAg-positive who received VBR + NrtI in study 202) developed TEAEs possibly related to study drug—see Supplementary material for more information. No grade 4 TEAEs or deaths occurred during the on-treatment phase. The most common TEAEs were upper respiratory tract infection (10/92; 11%), nasopharyngitis (six of 92; 7%), and fatigue (five of 92; 5%), most of which were grade 1. No trends in the incidence of TEAEs related to treatment received or HBeAg status in the parent studies were noted. On-treatment rash occurred in nine of 69 (13%) patients from study 201 (all grade 1 with variable onset) and none in study 202. All but one case resolved before EOS, and none led to study drug discontinuation. No patients met the ALT flare criteria. Treatment-emergent laboratory abnormalities are summarised in Table S15.
Table 3.
Summary of safety by treatment phase in patients from study 211 (SAS).
| On-treatment phase | |||||
|---|---|---|---|---|---|
| Patients originating from study 201 |
Patients originating from study 202 |
||||
| Patients reporting: | VS HBeAg (-) n = 26 | VS HBeAg (+) n = 43 | Total n = 69 | TN HBeAg (+) n = 23 | Overall total n = 92 |
| TEAE | 16 (62) | 26 (60) | 42 (61) | 12 (52) | 54 (59) |
| Grade 1 | 9 (35) | 11 (26) | 20 (29) | 6 (26) | 26 (28) |
| Grade 2 | 7 (27) | 14 (33) | 21 (30) | 3 (13) | 24 (26) |
| Grade 3 | 0 | 1 (2) | 1 (1) | 3 (13) | 4 (4) |
| TEAE related to study drug | 3 (12) | 5 (12) | 8 (12) | 2 (9) | 10 (11) |
| TE SAE | 0 | 0 | 0 | 1 (4) | 1 (1) |
| TEAE leading to study drug discontinuation | 0 | 0 | 0 | 2 (9) | 2 (2) |
| TEAEs found in ≥5% of the total patient population | |||||
| Upper respiratory tract infection | 3 (12) | 6 (14) | 9 (13) | 1 (4) | 10 (11) |
| Nasopharyngitis | 1 (4) | 3 (7) | 4 (6) | 2 (9) | 6 (7) |
| Fatigue | 1 (4) | 3 (7) | 4 (6) | 1 (4) | 5 (5) |
| Discontinued both VBR + NrtI (off-treatment phase, patients originating from study 201) | |||
|---|---|---|---|
| Patients reporting: | VS HBeAg (-)n = 23 | VS HBeAg (+)n = 18 |
Total N = 41 |
| AE | 10 (43) | 7 (39) | 17 (41) |
| Grade 1 | 2 (9) | 3 (17) | 5 (12) |
| Grade 2 | 5 (22) | 3 (17) | 8 (20) |
| Grade 3 | 3 (13) | 1 (6) | 4 (10) |
| AE related to study drug | 0 | 0 | 0 |
| SAE | 2 (9) | 0 | 2 (5) |
| AEs found in ≥5% of the total patient population | |||
| ALT increased | 6 (26) | 5 (28) | 11 (27) |
| AST increased | 2 (9) | 0 | 2 (5) |
| Headache | 1 (4) | 1 (6) | 2 (5) |
| Nausea | 2 (9) | 0 | 2 (5) |
| Back pain |
2 (9) |
0 |
2 (5) |
|
Discontinued both VBR + NrtI, then restarted NrtI (NrtI-restart phase, patients originating from study 201) | |||
| Patients reporting: |
VS HBeAg (-) n = 16 |
VS HBeAg (+) n = 14 |
Total N = 30 |
| AE | 3 (19) | 6 (43) | 9 (30) |
| Grade 1 | 1 (6) | 2 (14) | 3 (10) |
| Grade 2 | 0 | 3 (21) | 3 (10) |
| Grade 4 | 2 (13) | 1 (7) | 3 (10) |
| AE related to study drug | 0 | 0 | 0 |
| SAE | 0 | 0 | 0 |
| AEs found in ≥5% of the total patient population | |||
| ALT increased |
2 (13) |
3 (21) |
5 (17) |
|
Continued NrtI/ETV (off-treatment phase) | |||
|
Patients originating from study 201 |
Patients originating from study 202 |
||
|
Patients reporting: |
VS HBeAg (+) continue NrtI only n = 18 |
TN HBeAg (-) continue ETV only n = 6 |
|
| AE | 0 | 1 (17) | |
| Grade 1 | 0 | 1 (17) | |
Data shown are n (%).
AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ETV, entecavir; NrtI, nucleos(t)ide reverse transcriptase inhibitor; SAE, serious adverse event; SAS, safety analysis set; TE, treatment-emergent; TEAE, treatment-emergent adverse event; TN, treatment-naive; VBR, vebicorvir; VS, virologically-suppressed.
During the off-treatment phase, there were no notable differences in safety between patients who were HBeAg-positive or -negative from study 201 who discontinued both VBR + NrtI; 17/41 (41%) patients reported an AE (see Supplementary material for information surrounding two patients [both HBeAg-negative] who reported SAEs). No grade 4 AEs or deaths occurred in patients who discontinued both VBR + NrtI. The most common AEs were increases in ALT, increases in aspartate aminotransferase (AST), back pain, headache, and nausea. Two patients had a grade 3 laboratory abnormality (ALT and AST increase), and one had a grade 4 laboratory abnormality (ALT increase). During the NrtI-restart phase, most AEs and laboratory abnormalities were related to ALT and AST increases with no SAEs or deaths. Following NrtI restart, ALT returned to prediscontinuation levels with no events of hepatic decompensation. After discontinuing both VBR + NrtI, 22 patients had increases in ALT: one grade 4, two grade 3, and 12 grade 2. Further detail on ALT elevations and normalisations is provided in the Supplementary material.
Emergence of resistance-associated variants
Nine patients experienced on-treatment viral rebound, and Sanger sequencing showed no core inhibitor binding pocket substitutions or NrtI resistance mutations for eight out of nine patients tested at all time points. One patient had a T109I resistance-associated substitution in the core gene. This patient (who received VBR + NrtI in study 201) had persistently low HBV DNA through on-treatment W32 and had the T109I mutation detected as a mixture as early as W4 during study 201. Five of the nine patients had virologic rebound in a setting of noncompliance with study drug, including the patient with T109I detected in the core gene.
Pharmacokinetics
Summary statistics for predose concentrations of VBR, ETV, and tenofovir are described in Table S16. In general, W48 predose VBR and NrtI concentrations were comparable with those observed in the parent studies, supporting the lack of a drug–drug interaction.12
Discussion
This study was designed to assess the safety and antiviral activity of long-term, open-label VBR + NrtI in patients with cHBV. In patients originating from studies 201 and 202, long-term VBR + NrtI led to further reductions in HBV DNA and pgRNA at EOT in study 211 and was generally well tolerated, with few discontinuations and no deaths. No resistance-associated substitutions were observed in patients who were adherent to study drug. PK data from this study are consistent with the parent studies and support a lack of drug–drug interactions between VBR and NrtIs following longer-term administration.
Mean HBV DNA and pgRNA declined during the on-treatment period among patients receiving open-label VBR. Compared with study 211 baseline, there was an increase in the percentage of patients achieving HBV DNA TND at EOT. The observed on-treatment change in viral parameters varied and was influenced by the previous status of patients in the parent studies. Administration of VBR + NrtI resulted in slight numeric, but not clinically significant, decreases in HBV antigens. Additionally, neither HBsAg loss nor HBsAg seroconversion was observed in any patient, which likely correlates with intrahepatic viral persistence.13 Therefore, we conclude that long-term treatment with VBR + NrtI does not result in functional cure.
Long-term administration of VBR + NrtI was well tolerated, with most TEAEs being grade 1/2 and no grade 4 TEAEs or deaths reported. The nature and frequency of the observed TEAEs were similar between patients who were HBeAg-positive and -negative and those who were TN or VS at the start of the parent studies. Most grade 3/4 AEs and laboratory abnormalities occurred in the off-treatment or NrtI-restart phases and were related to elevations in ALT following cessation of antiviral treatment. Among patients meeting predefined criteria for stopping antiviral therapy, discontinuation of VBR + NrtI was well tolerated, with no hepatic decompensation events and limited AEs and ALT elevations.
Although long-term treatment with VBR + NrtI provided deep reductions in HBV DNA and pgRNA at EOT, failure to maintain HBV DNA <LLOQ after cessation of all antiviral therapy points to the need for more potent and/or additional therapies and novel combinations to work toward finite treatment and functional cure. The failure thus far to achieve functional cure off treatment is likely because of the inability of current treatment regimens to interfere with cccDNA formation and maintenance. Although combination therapy with clinically approved NrtIs and IFNα results in greater HBsAg loss than either monotherapy, the fact that neither of these agents eliminates cccDNA means that off-treatment viral rebound is likely.14 Therefore, it will be of great importance for future agents to have greater efficacy against the cccDNA reservoir. Although first-generation core inhibitors, such as VBR, demonstrate trough plasma concentrations above the protein-adjusted EC50 (paEC50) values for HBV DNA and cccDNA formation, next-generation core inhibitors may have enhanced potency with paEC50s multiple-fold (up to 900-fold higher vs. VBR) above that required for inhibition of capsid disassembly and prevention of cccDNA formation, often called ‘secondary mechanisms’ of core inhibitors (beyond their effects on capsid assembly).[15], [16], [17] Future studies incorporating next-generation core inhibitors may show higher levels of antiviral activity than what was observed in this study.
An important caveat in this study is the treatment assignment and HBeAg status of patients in the parent studies. In general, patients who were HBeAg-negative and received VBR + NrtI in study 201 entered study 211 with low levels of viral parameters, which made assessing changes in these parameters in study 211 difficult. Conversely, patients who were HBeAg-positive from study 202 and received PBO + NrtI had high levels of viral parameters at study 211 baseline, resulting in viral changes that were more apparent with long-term VBR + NrtI. Furthermore, this study followed patients who discontinued all antiviral treatment (i.e. VBR + NrtI) for approximately 4–36 weeks. Given that levels of certain viral parameters, such as HBsAg, may take years to diminish under antiviral treatment,13 it is likely that this short period of follow-up did not capture potential longer-term declines in HBsAg in these patients. As no patients met the primary study endpoint, NrtIs were generally restarted, and consequently the period of follow-up when patients were not receiving any antiviral treatment was insufficient to determine if there were any continued declines in viral parameters. In summary, open-label, long-term, once-daily VBR + NrtI was safe and well tolerated, with few discontinuations and no deaths. Although deeper levels of viral suppression were observed with the VBR + NrtI combination, durable virologic outcomes were not observed in patients who met the criteria to discontinue antiviral treatment. VBR has been investigated further in two triple-combination, open-label, phase II studies – with both NrtI and IFNα, and with NrtI and the investigational RNA inhibitor AB-729. VBR did not show superior efficacy in key viral parameters in these triple-combination studies vs. dual combinations without VBR – hence clinical development of VBR was discontinued.
Financial support
This study was sponsored and funded by Assembly Biosciences, Inc., South San Francisco, CA, USA.
Authors’ contributions
All authors meet authorship criteria set forth by the International Committee for Medical Journal Editors, have significantly contributed to and approved the final submitted version of the manuscript, and take responsibility for the integrity of the work. Study oversight: M-FY, SF, XM, TTN, TH, H-WH, ME, RGN, JSP, IMJ, WSA, S-HH, EJG, SJK, LMS, MBo, FW, AR, MBe, NR, SC, DTD, PYK, ERS, HSB, JL, KA, MSS. Experiments and procedures: M-FY, SF, XM, TTN, TH, H-WH, ME, RGN, JSP, IMJ, WSA, S-HH, EJG, RY, MBo, FW, AR, MBe, NR, SC, DTD, PYK, ERS, HSB, JL, KA, MSS. Data acquisition: M-FY, SF, XM, TTN, TH, H-WH, ME, RGN, JSP, IMJ, WSA, S-HH, EJG, RY, JM, MBo, FW, AR, MBe, NR, SC, DTD, PYK, ERS, HSB, JL, KA, MSS. Data analysis: M-FY, SF, IMJ, JM, SJK, LMS, JL, KA, MSS. Data interpretation: All authors. Critical revision of the manuscript: All authors.
Data availability statement
Data can be made available to researchers upon reasonable request.
Conflicts of interest
M-FY reports being an advisor/consultant for AbbVie, AiCuris, Aligos Therapeutics, Antios Therapeutics, Arbutus Biopharma, Arrowhead Pharmaceuticals, Assembly Biosciences, Clear B Therapeutics, Dicerna Pharmaceuticals, Finch Therapeutics, Fujirebio Incorporation, GlaxoSmithKline, Gilead Sciences, Immunocore, Janssen, Roche, Sysmex Corporation, Tune Therapeutics, Vir Biotechnology, and Visirna Therapeutics and receiving grant/research support from AbbVie, Arrowhead Pharmaceuticals, Assembly Biosciences, Fujirebio Incorporation, Gilead Sciences, Immunocore, Roche, and Sysmex Corporation. SF reports receiving fees for speaking and teaching and/or serving on advisory committees for AbbVie, Assembly Biosciences, Gilead Sciences, Janssen, Lupin, Novo Nordisk, Pfizer, and Springbank Pharma. XM reports being a consultant and being on the speakers' bureau for Gilead Sciences. TTN reports receiving research grant support from Assembly Biosciences and Gilead Sciences. TH reports being on the advisory committee, review panel, or consulting for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Mallinckrodt Pharmaceuticals, Merck, and Organovo and receiving research support from AbbVie, Allergan, Assembly Biosciences, Astra Zeneca, Boehringer Ingelheim, Bristol-Myers Squibb, CARA, Cytodyn, DURECT Corporation, Enanta Pharmaceuticals, Galectin Therapeutics, Gilead Sciences, Grifols, Intercept Pharmaceuticals, Janssen, Merck, Mirum, Novartis, Novo Nordisk, Nucorion Pharmaceuticals, Pfizer, Salix Pharmaceuticals, Sonic Incytes, Terns Pharmaceuticals, and Valeant. H-WH reports serving on the National Advisory Board and receives research grant support from Gilead Sciences. ME reports receiving grants from AbbVie, Bristol-Myers Squibb, Eisai, Gilead Sciences, and Roche and serving on advisory boards for AbbVie, Bristol-Myers Squibb, Gilead Sciences, and Merck. RGN reports having served on advisory boards and as a speaker for Gilead Sciences, Janssen, and Merck and having conducted research for AbbVie, Gilead Sciences, Janssen, and Merck. JSP reports receiving research grants from Assembly Biosciences and GlaxoSmithKline and consulting fees from Gilead Sciences. IMJ reports being a consultant or on advisory boards for AbbVie, Aligos Therapeutics, Arbutus Biopharma, Gilead Sciences, Intercept Pharmaceuticals, Janssen, and Roche; having conducted research (all payments to institution) for Assembly Biosciences, Bristol-Myers Squibb, Cymabay, Eli Lilly, Enanta Pharmaceuticals, Genfit, Gilead Sciences, Intercept, Janssen, Merck, and Novo Nordisk; receiving payment from the Chronic Liver Disease Foundation for manuscript preparation; and participating on a Data Monitoring Committee for Altimmune, Arrowhead Pharmaceuticals, Galmed, GlaxoSmithKline, and Takeda. WSA reports being a member of the speaking bureau for Gilead Sciences and Intercept Pharmaceuticals and has received research grants from Genfit, GlaxoSmithKline, Intercept Pharmaceuticals, Ipsen, Madrigal, Mirum, Pfizer, and Zydus. S-HH reports being a consultant and being on the speakers' bureau for Gilead Sciences. EJG reports serving on advisory boards for AbbVie, Aligos Therapeutics, Assembly Biosciences, Gilead Sciences, GlaxoSmithKline, Intellia, Janssen, Roche, Vir Biotechnology, and Virion and having served as a speaker for Abbott Diagnostics, AbbVie, and Gilead Sciences. KZ, RY, and SJK report being employees of and holding stock interest in Assembly Biosciences. JM and LMS report being former employees of and holding stock interest in Assembly Biosciences. MBo reports being a member of the speaking bureau for AbbVie, Gilead Sciences, and Intercept Pharmaceuticals and has received research support from Assembly Biosciences, Boehringer Ingelheim, Gilead Sciences, Intercept Pharmaceuticals, Inventiva, and Viking Therapeutics. FW reports being a study investigator for AbbVie. AR reports receiving grant support, lecture fees, and advisory board fees from AbbVie, Celgene, Gilead Sciences, Intercept Pharmaceuticals, Merck, and Novartis. MBe reports having no conflicts of interest. NR reports advising, being on the speakers’ bureau for, and receiving grants from AbbVie and Gilead Sciences; being on the speakers’ bureau for Onyx and Salix; and having received grants from Bristol-Myers Squibb and Merck. SC reports receiving clinical trial–related payments from Assembly Biosciences. DTD reports being a consultant for Gilead Sciences and Intercept Pharmaceuticals. PYK reports being an advisor/consultant for AbbVie, Aligos Therapeutics, Antios Therapeutics, Enanta Pharmaceuticals, Gilead Sciences, and Janssen and receives grant/research supports from Altimmune, Arrowhead Pharmaceuticals, Assembly Biosciences, Bristol-Myers Squibb, Eiger Biopharmaceuticals, and Target Registries. ERS reports receiving research and grant support from Assembly Biosciences, Celgene, the University of Florida (TARGET), and Vir Biotechnology and receives royalties from the Schiff Diseases of the Liver, 12th edition. HSB reports having consultancy agreements with and receiving research support from Bristol-Myers Squibb and Gilead Sciences. JL reports having no conflicts of interest. KA reports being on the advisory board, a consultant, and a speaker for AbbVie, Aligos, Arbutus Biopharma, Assembly Biosciences, Bristol-Myers Squibb, Gilead Sciences, Immunocore, Janssen, Merck, Novartis, Roche, Shinogi, Sobi, and Vir Biotechnology and receiving grants from Bristol-Myers Squibb, Gilead Sciences, and Roche. MSS reports receiving grants from AbbVie, Assembly Biosciences, GlaxoSmithKline, Janssen, the National Institutes of Health, and Vir Biotechnology and receiving personal fees from AbbVie, Antios Therapeutics, Arbutus Biopharma, Atea Pharmaceuticals, Gilead Sciences, GlaxoSmithKline, F2G, Immunocore, Precision Biosciences, and Virion Therapeutics.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We thank all the patients, investigators, and site staff who participated in this study. Writing and editorial support were provided by Gregory Suess, PhD, CMPP of AlphaBioCom, a Red Nucleus company, and were funded by Assembly Biosciences, Inc., South San Francisco, CA, USA.
Footnotes
Author names in bold designate shared co-first authorship.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100999.
Supplementary data
The following are the supplementary data to this article:
References
- 1.EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . 2020. Hepatitis B: key facts.https://www.who.int/news-room/fact-sheets/detail/hepatitis-b Available from: [Google Scholar]
- 3.World Health Organization . World Health Organization; Geneva: 2021. Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021: accountability for the global health sector strategies 2016–2021: actions for impact. [Google Scholar]
- 4.Lau G.K., Piratvisuth T., Luo K.X., et al. Peginterferon alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682–2695. doi: 10.1056/NEJMoa043470. [DOI] [PubMed] [Google Scholar]
- 5.Janssen H.L., van Zonneveld M., Senturk H., et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet. 2005;365:123–129. doi: 10.1016/S0140-6736(05)17701-0. [DOI] [PubMed] [Google Scholar]
- 6.Buti M., Gane E., Seto W.K., et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1:196–206. doi: 10.1016/S2468-1253(16)30107-8. [DOI] [PubMed] [Google Scholar]
- 7.Chan H.L., Fung S., Seto W.K., et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1:185–195. doi: 10.1016/S2468-1253(16)30024-3. [DOI] [PubMed] [Google Scholar]
- 8.Cornberg M., Lok A.S., Terrault N.A., et al. Guidance for design and endpoints of clinical trials in chronic hepatitis B – report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference. Hepatology. 2020;71(3):1070–1092. doi: 10.1002/hep.31030. [DOI] [PubMed] [Google Scholar]
- 9.Yuen M.F., Agarwal K., Ma X., et al. Safety and efficacy of vebicorvir in virologically suppressed patients with chronic hepatitis B virus infection. J Hepatol. 2022;77:642–652. doi: 10.1016/j.jhep.2022.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Sulkowski M.S., Agarwal K., Ma X., et al. Safety and efficacy of vebicorvir administered with entecavir in treatment-naïve patients with chronic hepatitis B virus infection. J Hepatol. 2022;77:1265–1275. doi: 10.1016/j.jhep.2022.05.027. [DOI] [PubMed] [Google Scholar]
- 11.Yuen M.F., Agarwal K., Gane E.J., et al. Safety, pharmacokinetics, and antiviral effects of ABI-H0731, a hepatitis B virus core inhibitor: a randomised, placebo-controlled phase 1 trial. Lancet Gastroenterol Hepatol. 2020;5:152–166. doi: 10.1016/S2468-1253(19)30346-2. [DOI] [PubMed] [Google Scholar]
- 12.Zomorodi K., Wang G., Knox S.J., et al. Evaluation of the drug-drug interaction profile of vebicorvir, a first-generation hepatitis B core inhibitor: findings from phase 1 and phase 2a studies. J Hepatol. 2022;77 SAT-388. [Google Scholar]
- 13.Moini M., Fung S. HBsAg loss as a treatment endpoint for chronic HBV infection: HBV cure. Viruses. 2022;14:657. doi: 10.3390/v14040657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcellin P., Ahn S.H., Ma X., et al. Combination of tenofovir disoproxil fumarate and peginterferon α-2a increases loss of hepatitis B surface antigen in patients with chronic hepatitis B. Gastroenterology. 2016;150:134–144.e110. doi: 10.1053/j.gastro.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 15.Cai D., Evanchik M., Yan R., et al. Second generation hepatitis B virus core inhibitors ABI-H2158 and ABI-H3733 have enhanced potency and target coverage for both antiviral inhibition and covalently closed circular DNA establishment activities. J Hepatol. 2021;75:S290. [Google Scholar]
- 16.Unchwaniwala N., Delaney W., Kitrinos K. ABI-4334, a novel inhibitor of hepatitis B virus core protein, promotes formation of empty capsids and prevents cccDNA formation by disruption of incoming capsids. J Hepatol. 2022;77 SAT-383. [Google Scholar]
- 17.Shen M., Zong Z., Mohammed N., et al. Improving the pharmacokinetic profile of the hepatitis B virus core inhibitor ABI-H3733 following oral administration: results from new formulation activities. J Hepatol. 2022;77 SAT-389. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be made available to researchers upon reasonable request.