Key Points
Question
What is the HIV incidence among cisgender women with different adherence trajectories to preexposure prophylaxis (PrEP) with emtricitabine and tenofovir disoproxil fumarate?
Findings
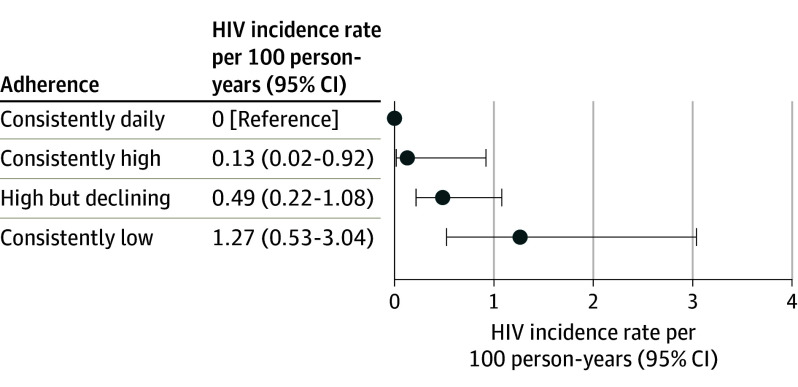
In this pooled analysis of 6296 participants from 11 postapproval studies of PrEP in cisgender women, the overall HIV incidence was 0.72 per 100 person-years. The HIV incidence rates per 100 person-years for different PrEP adherence trajectories were 0 for consistently daily (7 doses/week), 0.13 for consistently high (4-6 doses/week), 0.49 for high but declining (from a mean of 4-6 doses/week and then declining), and 1.27 for consistently low (less than 2 doses/week) adherence.
Meaning
In a pooled analysis of postapproval studies, cisgender women with daily or consistently high adherence to emtricitabine and tenofovir disoproxil fumarate for PrEP experienced very low HIV incidence.
Abstract
Importance
Emtricitabine and tenofovir disoproxil fumarate (F/TDF) for HIV preexposure prophylaxis (PrEP) is highly effective in cisgender men who have sex with men (MSM) when adherence is high (>4 doses/week). Real-world effectiveness and adherence with F/TDF for PrEP in cisgender women is less well characterized.
Objective
To characterize the effectiveness of F/TDF for PrEP and its relationship with adherence in cisgender women.
Design, Setting, and Participants
Data were pooled from 11 F/TDF PrEP postapproval studies conducted in 6 countries that included 6296 cisgender women aged 15 to 69 years conducted from 2012 to 2020. HIV incidence was evaluated according to adherence level measured objectively (tenofovir diphosphate concentration in dried blood spots or tenofovir concentration in plasma; n = 288) and subjectively (electronic pill cap monitoring, pill counts, self-report, and study-reported adherence scale; n = 2954) using group-based trajectory modeling.
Exposures
F/TDF prescribed orally once a day. HIV incidence was analyzed in subgroups based on adherence trajectory.
Main Outcomes and Measures
HIV incidence.
Results
Of the 6296 participants, 46% were from Kenya, 28% were from South Africa, 21% were from India, 2.9% were from Uganda, 1.6% were from Botswana, and 0.8% were from the US. The mean (SD) age at PrEP initiation across all studies was 25 (7) years, with 61% of participants being younger than 25 years. The overall HIV incidence was 0.72 per 100 person-years (95% CI, 0.51-1.01; 32 incident HIV diagnoses among 6296 participants). Four distinct groups of adherence trajectories were identified: consistently daily (7 doses/week), consistently high (4-6 doses/week), high but declining (from a mean of 4-6 doses/week and then declining), and consistently low (less than 2 doses/week). None of the 498 women with consistently daily adherence acquired HIV. Only 1 of the 658 women with consistently high adherence acquired HIV (incidence rate, 0.13/100 person-years [95% CI, 0.02-0.92]). The incidence rate was 0.49 per 100 person-years (95% CI, 0.22-1.08) in the high but declining adherence group (n = 1166) and 1.27 per 100 person-years (95% CI, 0.53-3.04) in the consistently low adherence group (n = 632).
Conclusions and Relevance
In a pooled analysis of 11 postapproval studies of F/TDF for PrEP among cisgender women, overall HIV incidence was 0.72 per 100 person-years; individuals with consistently daily or consistently high adherence (4-6 doses/week) to PrEP experienced very low HIV incidence.
This pooled analysis of postapproval studies examines the association between baseline demographics and other characteristics and PrEP adherence trajectories with HIV incidence in women.
Introduction
The World Health Organization recommends the inclusion of oral preexposure prophylaxis (PrEP) containing tenofovir disoproxil fumarate (TDF) as an option for HIV prevention in individuals at substantial risk of acquiring HIV as part of combination HIV prevention approaches.1 The combination of emtricitabine and TDF (F/TDF) was approved for HIV PrEP to prevent sexual acquisition of HIV infection in adults by the US Food and Drug Administration (FDA) in 20122 and was extended to include adolescents in 2018.3 However, the relative efficacy of F/TDF for PrEP in cisgender women compared with men who have sex with men (MSM) has been questioned due to negative results in some randomized clinical trials in women4,5,6 and because of reports of differential concentrations of tenofovir in vaginal vs rectal mucosa.7,8 Mucosal concentration data, in particular, have informed distinct approaches to the counseling of women compared with MSM, with women being advised that near-perfect daily adherence is required for optimal efficacy of F/TDF for PrEP.7,9,10
Among cisgender MSM, the relationship between adherence (the number of F/TDF doses taken per week estimated from tenofovir diphosphate concentrations in dried blood spots [DBS]) and prevention efficacy has been described.11 Analysis of the iPrEx Open Label Extension study found no new HIV infections in participants with tenofovir diphosphate concentrations indicative of taking at least 4 doses per week.12 These findings were consistent with modeling data that estimated an HIV risk reduction of 76% for 2 doses per week, 96% for 4 doses per week, and 99% for 7 doses per week among cisgender MSM.13
Since the approval of F/TDF for PrEP, several global postapproval studies have been conducted to better define the effectiveness of F/TDF for PrEP in real-world settings and other contexts outside of randomized clinical trials, including in cisgender women. In the present analysis, data were pooled from 11 such studies in 6 countries (Botswana, India, Kenya, South Africa, Uganda, and the US) including 6296 women to evaluate F/TDF PrEP adherence and HIV incidence. Group-based trajectory modeling was employed,14,15,16,17 allowing leverage of a diverse range of quantitative and qualitative adherence data to examine the adherence-effectiveness relationship of F/TDF for PrEP in cisgender women. Using this approach, this study sought to determine the association between baseline demographics and other characteristics and adherence trajectories with HIV incidence in women in postapproval settings.
Methods
Study Selection
Data were analyzed from prospective studies involving oral F/TDF for PrEP in cisgender women and adolescents conducted between 2012, the year F/TDF for PrEP was approved for use in the US, and 2020. These studies were performed as part of the postmarketing commitment to the FDA and were either demonstration projects (n = 7) or open-label studies (n = 4), with 7 conducted in sub-Saharan Africa,18,19,20,21,22,23,24,25 2 in India,26,27 and 2 in the US28,29 (Table). Study populations varied, but all included individuals who could benefit from PrEP and persons in serodiscordant (1 partner living with HIV and 1 partner without HIV) relationships, adolescent females and young women, and female sex workers.
Table. Postapproval Studies of Emtricitabine and Tenofovir Disoproxil Fumarate for Preexposure Prophylaxis (PrEP) Among Cisgender Women in 6 Countries.
| Source | Country | Study population | No. of participants | Measures used for adherence trajectory | No. of participants with adherence data (No. with objective measuresa) | Age range, y | New HIV diagnosis, No. | Start date | End date |
|---|---|---|---|---|---|---|---|---|---|
| Partners Demo21 | Kenya, Uganda | Serodiscordant couples | 334 | Tenofovir diphosphate concentration in DBS, plasma tenofovir concentration, and electronic monitoring | 329 (88) | 18-54 | 8 | 11/30/12 | 5/2/16 |
| TDF2 OLE22 | Botswana | Young heterosexual adults | 102 | Tenofovir diphosphate concentration in DBS | 45 (45) | 23-44 | 0 | 3/6/13 | 7/17/14 |
| PrEPception28 | US | HIV-negative women and male partners living with HIV | 24 | Tenofovir diphosphate concentration in DBS | 16 (16) | 22-47 | 0 | 2/25/14 | 4/7/18 |
| Kolkata PrEP Demo27 | India | Female sex workers | 678 | 5-Level physician-reported summary | 678 | 19-50 | 0 | 1/18/15 | 9/21/19 |
| Kenya PrEP18 | Kenya | General population | 1347 | Electronic monitoring, self-report, and pill count | 507 | 15-59 | 3 | 6/4/15 | 12/6/17 |
| Ashodaya PrEP26 | India | Female sex workers | 647 | Self-report | 646 | 19-51 | 0 | 3/3/16 | 12/15/18 |
| Safer Conception25 | Kenya | Serodiscordant couples | 40 | Electronic monitoring | 40 | 20-47 | 0 | 3/23/16 | 2/21/18 |
| CRUSH29 | US | Women in an urban community clinic | 25 | Tenofovir diphosphate concentration in DBS | 7 (7) | 22-69 | 0 | 5/13/16 | 8/30/17 |
| MPYA19,20 | Kenya | Young women (aged 15-24 y) | 348 | Tenofovir diphosphate concentration in DBS and electronic monitoring | 348 (14) | 18-24 | 3 | 12/21/16 | 2/23/20 |
| 3Ps for Prevention24 | South Africa | Young women (aged 16-25 y) | 200 | 2-Level physician-reported summary | 186 | 16-25 | 1 | 5/23/17 | 5/21/18 |
| POWER23 | Kenya, South Africa | Young women (aged 16-25 y) | 2551 | Tenofovir diphosphate concentration in DBS and 5-level physician-reported summary | 152 (67) | 16-25 | 17 | 6/14/17 | 12/4/20 |
| Total | 6296 | 2954 (237) | 32 |
Abbreviations: CRUSH, Connecting Resources for Urban Sexual Health; MPYA, Monitoring PrEP Among Young Adult Women; POWER, Prevention Options for Women Evaluation Research.
The number of participants with objective adherence measures (at least 1 measurement of tenofovir diphosphate concentration in dried blood spots [DBS] or tenofovir concentration in plasma) in the trajectory modeling (ie, 237 individuals had objective adherence measure data and 2717 participants were assessed using exclusively subjective measurements in the trajectories). Adherence was categorized as consistently daily (7 doses/wk), high (4-6 doses/wk), moderate (2-3 doses/wk), and low (<2 doses/wk) as described in the Methods. The optimal (most stable) model from group-based trajectory modeling resulted in 4 groups with distinct patterns of adherence. Three groups had stable adherence over time, regardless of the model (3 or 4 groups) used: consistently daily (7 doses/wk), consistently high (4-6 doses/wk), and consistently low (<2 doses/wk). The fourth group had dynamic adherence over time, which went from initially high to declining (from 4-6 to 2-3 doses/wk).
Incidence
Between November 2012 and December 2020, a total of 6296 cisgender women aged 15 to 69 years initiated F/TDF for PrEP as participants in 11 prospective studies (Table). Participants were followed up regularly (follow-up visit frequencies across studies ranged from a mean of every 1.1-4.5 months) and underwent HIV testing using protocols in place at each study site. The “on-PrEP” HIV incidence rate was calculated by dividing the number of new HIV infection diagnoses by cumulative person-years of follow-up, determined as the time between PrEP initiation and the earliest date of HIV diagnosis, PrEP discontinuation, study end, or 96 weeks. The HIV incidence rate was calculated overall and in groups defined by adherence trajectories, as detailed below, to estimate effectiveness of F/TDF in preventing HIV acquisition using Poisson regression.
Adherence Measures
Adherence data were available for a subset (n = 2955 [47%]) of participants. Adherence was assessed by a variety of methods, which were dictated by the protocol for each independent study (Table). The majority were subjective measures (92%), including electronic pill cap monitoring, pill counts, self-report, or a study-reported adherence scales. Objective adherence measures included quantification of tenofovir diphosphate in DBS in 209 participants, reflecting adherence over the past 8 to 12 weeks,30 or of tenofovir in plasma in 46 participants, reflecting adherence over the past 2 to 7 days.31 Of the 46 participants with plasma tenofovir data, 18 also had tenofovir diphosphate in DBS measured. Adherence was categorized as daily (7 doses/week), high (4-6 doses/week), moderate (2-3 doses/week), or low (less than 2 doses/week) according to the following established cutoffs for tenofovir diphosphate in DBS: greater than or equal to 1249 fmol/punch, 699 to 1250 fmol/punch, 350 to 700 fmol/punch, and less than 350 fmol/punch, respectively.11 For the 46 participants who had tenofovir plasma data that were used in the group-based trajectory modeling (ie, not including those with concurrent tenofovir diphosphate measurements in DBS), participants with plasma concentrations greater than or equal to 40 ng/mL were assigned to the high adherence group. This threshold was selected based on prior studies suggesting that 40 ng/mL reflects recent adherence32,33 and that participants with pill coverage of at least 80% had plasma tenofovir concentration greater than 40 ng/mL.31 Participants with concentrations less than 40 ng/mL were included in the low adherence group. If more than 1 type of adherence monitoring variable was available, a single measure was used based on the following priority: (1) tenofovir diphosphate concentration in DBS, (2) TFV concentration in plasma, (3) physician- or study-reported 5-level summary (very good, good, fair, poor, very poor), (4) physician- or study-reported 2-level summary (yes, no), (5) electronic adherence monitoring device, (6) self-report, and (7) pill count.
Group-Based Trajectory Modeling
Group-based trajectory modeling14 was used to identify patterns of PrEP adherence over 96 weeks. This approach has been applied to PrEP adherence studies previously.15,16,17 Data were available to support trajectory modeling in 2954 women. Linear, quadratic, or cubic models were used to allow the data to cluster into 2 to 6 groups of adherence trajectories based on ordinal adherence metrics. An ordinal number was assigned indicating overall adherence to denote the cutoffs in doses per week across all adherence measurements. For example, if adherence (regardless of method) was categorized as 7 doses per week, the assignment for that measurement was 4 on a scale of 1 to 4. Using a specified number of trajectories and various shapes (eg, linear, quadratic), the model estimated the posterior probability of an individual belonging to each group and the individual was classified based on the highest posterior probability. The final models were selected and groups were defined based on bayesian or Akaike information criterion. Individual trajectories were further examined using spaghetti plots.
Multinomial ordinal logistic regression models were used to calculate odds ratios (ORs) and associated 95% CIs in evaluating the association between baseline participant characteristics and group-based adherence trajectory categories. Mixed models with random effect by each individual study were used to account for clustering within the study. Incidence rate ratios (IRRs) were calculated to compare HIV incidence rates across different adherence trajectories, using the low adherence group as the reference.
All statistical analyses were performed using SAS software version 9.3 (SAS Institute) except for the trajectory group modeling, which was conducted using the R version 3.00 with the gbmt package (R Foundation; https://cran.r-project.org/web/packages/gbmt/index.html).
All studies were reviewed and approved by appropriate institutional review boards or similar authorities prior to implementation. Data used in this analysis were previously deidentified and members of the study team had no access to any identifiable participant information.
Results
Participant Characteristics
Of the 6296 participants, 2886 (46%) were from Kenya, 1751 (28%) were from South Africa, 1325 (21%) were from India, 183 (2.9%) were from Uganda, 102 (1.6%) were from Botswana, and 49 (0.8%) were from the US. The mean (SD) age at PrEP initiation across all 11 studies was 25 (7) years, with 61% of participants being younger than 25 years. The majority of participants were married (52%) and nulliparous (54%), and 21% reported engaging in transactional sex (eTable 1 in Supplement 1).
HIV Incidence
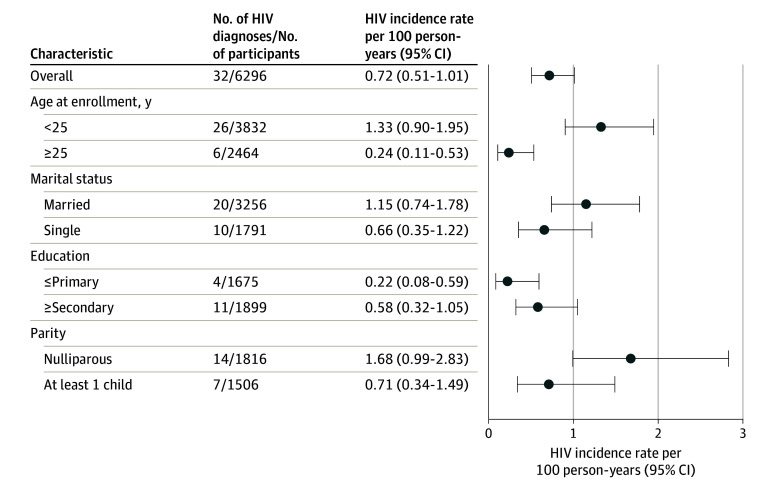
There were 32 incident HIV diagnoses among the 6296 participants, which is an overall HIV incidence rate of 0.72 per 100 person-years (95% CI, 0.51-1.01). HIV incidence was higher in younger (<25 years), married, and nulliparous participants (Figure 1). HIV incidence in other subgroups is shown in eTable 2 in Supplement 1.
Figure 1. HIV Incidence in Postapproval Studies of Emtricitabine and Tenofovir Disoproxil Fumarate for Preexposure Prophylaxis in Cisgender Women in 6 Countries, 2012-2020.
Adherence Measures
Measures of adherence were available for 2955 participants and are reported by visit. The number of participants with objective adherence data declined from 147 at week 16 to 22 at week 96 (Figure 2A). Among those with objective adherence data, the percentage with high or daily adherence varied between 20% and 33%. Of the 2887 participants with subjective adherence data, a similar decline in the number of participants with reported adherence data was observed, with the percentage reporting high or daily adherence varying between 67% and 82% until week 80, then dropping to 13% at week 96 (Figure 2B). Subjectively reported adherence was substantially higher compared with objective measures at all points throughout participation, with the exception of the final visit.
Figure 2. Adherence by Visit to Emtricitabine and Tenofovir Disoproxil Fumarate for Preexposure Prophylaxis in Cisgender Women (n = 2955) .
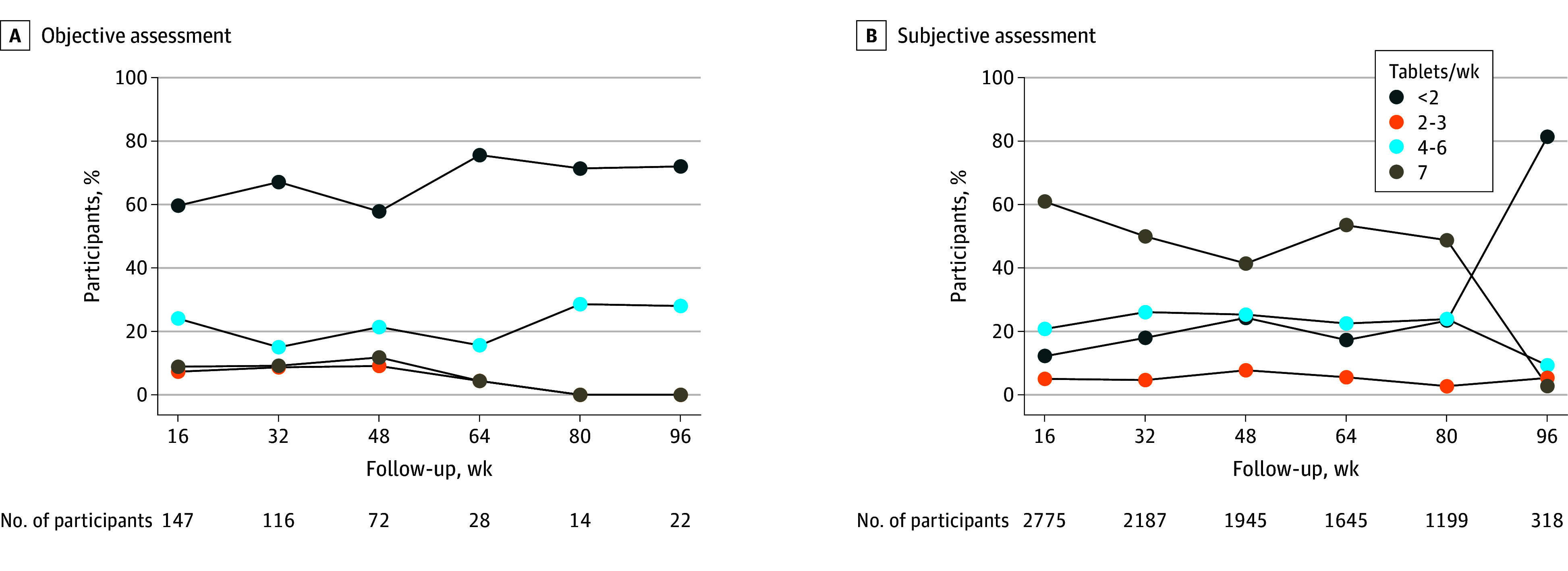
Totals do not sum to 2955 because adherence data were collected at different time points in different studies. Across all time points a total of 288 participants had objective adherence data available and 2717 had only subjective adherence data available. In cases in which multiple measures were available within a single 16-week period, the highest level of adherence was used. A, Objective assessments of adherence included tenofovir concentration measurements in dried blood spots or plasma. B, Subjective assessment of adherence included electronic pill cap monitoring, pill counts, self-report, or a study-reported adherence scale.
Group-Based Adherence Trajectories
The various adherence measures used in modeling the adherence trajectory groups are summarized in eTable 3 in Supplement 1. The optimal (most stable) model from group-based trajectory modeling resulted in 4 groups with distinct patterns of adherence. Three groups had stable adherence over time, regardless of the model (3 or 4 groups) used: consistently daily (7 doses/week), consistently high (4-6 doses/week), and consistently low (less than 2 doses/week). The fourth group had dynamic adherence over time: initially high then declining (from a mean of 4-6 to 2-3 doses/week; Figure 3). Linear regression was applied to the adherence scores (including subjective and objective measurements) for the 1166 individuals who were clustered into the high then declining group. The linear regression trend is shown in Figure 3A. The regression trend reveals a linear decline, with “high” representing the Y-intercept of the regression line, signifying a group-based starting point with a mean of 4 to 6 doses per week. The negative slope of the regression line indicates the downward trend after this initial starting point. Therefore, the mean of 4 to 6 doses per week is not based on individual data or a specific period, rather it reflects a group-level overall starting point. Not all individuals initiated from the intercept and the declines did not occur within a specific or consistent timeframe.
Figure 3. Longitudinal Patterns of Adherence to Emtricitabine and Tenofovir Disoproxil Fumarate for Preexposure Prophylaxis in Cisgender Women (n = 2954).
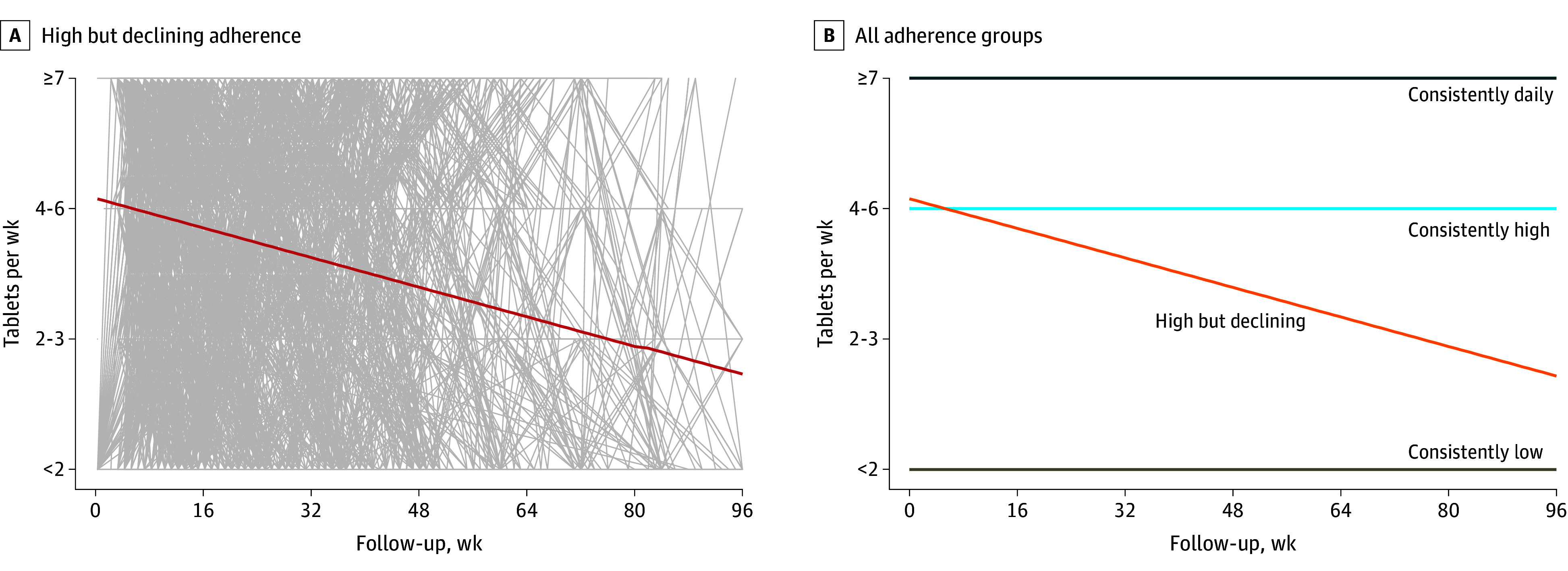
A. Individual lines for each participant in the high but declining group are shown in gray, with the linear regression trendline for the group shown as a red line. B. Trendlines for all 4 groups, showing the 3 levels of stable adherence and contrast with the high but declining group.
Baseline participant characteristics associated with adherence trajectory categories are summarized in eTable 4 in Supplement 1. Participants younger than 25 years had lower odds of having consistently daily (OR, 0.42 [95% CI, 0.25-0.71], consistently high (OR, 0.82 [95% CI, 0.53-1.27]), or high but declining (OR, 0.51 [95% CI, 0.37-0.69]) (vs consistently low) adherence trajectory compared with women 25 years or older. Nulliparous participants had lower odds of having high but declining (OR, 0.57 [95% CI, 0.34-0.97]) (vs consistently low), consistently daily (OR 0.55 [95% CI, 0.24-1.26]), or consistently high (OR, 0.79 [95% CI, 0.44-1.40]) adherence compared with participants with at least 2 children, but the latter estimates were not statistically significant.
HIV Incidence Rates Among Women by Group-Based Adherence Trajectory
A total of 12 incident HIV infections were observed among the 2955 participants with adherence data, compared with 20 diagnoses among 3341 participants without adherence data. None of the 498 participants with consistently daily adherence by group-based trajectory acquired HIV (Figure 4); because there were no cases of infection, an IRR could not be calculated for this group. Only 1 of the 658 participants with consistently high adherence (4-6 doses/week) acquired HIV, resulting in an HIV incidence rate estimate of 0.13 per 100 person-years (95% CI, 0.02-0.92) for this group. The incidence rate was 0.49 per 100 person-years (95% CI, 0.22-1.08) in the high but declining adherence group (n = 1166) and 1.27 per 100 person-years (95% CI, 0.53-3.04) in the consistently low adherence group (n = 632). Individuals with consistently high adherence (IRR, 0.10 [95% CI, 0.01-0.88]) and individuals with high but declining adherence (IRR, 0.38 [95% CI, 0.12-1.26]) had lower HIV incidence compared with individuals with consistently low adherence.
Figure 4. HIV Incidence Rates Among Cisgender Women by Adherence Trajectory (n = 2954).
Adherence was categorized as consistently daily (7 doses/week), high (4-6 doses/week), moderate (2-3 doses/week), and low (less than 2 doses/week), as described in the Methods. The optimal (most stable) model from group-based trajectory modeling resulted in 4 groups with distinct patterns of adherence. Three groups had stable adherence over time, regardless of the model (3 or 4 groups) used: consistently daily (7 doses/week), consistently high (4-6 doses/week), and consistently low (less than 2 doses/week). The fourth group had dynamic adherence over time: initially high then declining (from 4-6 to 2-3 doses/week).
Discussion
This pooled analysis of more than 6000 cisgender women using F/TDF for PrEP showed higher HIV incidence among younger, married, and nulliparous women, which is consistent with previous reports.34 Among the nearly 3000 individuals with adherence data, the effectiveness of F/TDF in preventing HIV infection was very high in women who demonstrated consistently high (4-6 doses/week) or consistently daily (7 doses/week) adherence. This interpretation is comparable to the established relationship between effectiveness and adherence observed in cisgender MSM. Even with the relatively low number of incident HIV diagnoses, a clear relationship was observed between evidence (both objective and subjective) of higher adherence with lower risk of HIV infection.
Current guidance for PrEP use among cisgender women highlights the more rigid recommendations relative to those for MSM. In its 2021 PrEP Clinical Guideline Update,35 the US Centers for Disease Control and Prevention advised clinicians to counsel patients on the importance of daily dosing, noting that there is less “forgiveness” for missed PrEP doses for cisgender women than for MSM. These recommendations are largely based on pharmacokinetic modeling that suggests that cervicovaginal tissue concentrations of tenofovir diphosphate are lower than those observed in rectal tissue with similar dosing, although a correlation between tissue tenofovir diphosphate concentration and PrEP efficacy has not been definitively demonstrated.7,9 However, even with low overall adherence to oral F/TDF in some randomized clinical trials among cisgender women, a direct relationship between objective measures of adherence and efficacy was observed.31,36
A 2023 analysis evaluating the F/TDF adherence-efficacy relationship in the HPTN 083 and 084 trials demonstrated that, although there are differences in the pharmacologic forgiveness profile between cisgender men and women, the protective efficacy of F/TDF against HIV remained high (>80%) in cisgender women, despite less than daily dosing.10 Indeed, participants with tenofovir diphosphate concentrations indicative of taking 4 to 6 doses per week had a nearly 90% HIV risk reduction in the HPTN 084 trial, consistent with the results observed in the present study. Modeling studies leveraging F/TDF clinical trial data in cisgender women have demonstrated similar findings, underscoring how differences in efficacy between cisgender men and women that were previously observed were likely driven by adherence rather than biological differences.37,38
Women may be motivated to use oral F/TDF for reasons that relate directly to the timing of HIV risk or to perceived benefit. For example, high uptake of F/TDF use was recently reported among HIV-exposed women with plans for pregnancy in rural Uganda.32 Sexual behaviors that expose women to HIV acquisition also change over time, as demonstrated in the HPTN 064 study, in which social factors were found to influence sustained high-risk behavior, and should be integrated into decisions about approach to PrEP use.39 These findings highlight how the broader context surrounding individuals’ HIV prevention needs should be integrated into decisions about PrEP use and that cisgender women need not be restricted to a rigid daily regimen, similar to the adherence forgiveness that has been reported in cisgender MSM.
Limitations
This analysis has several limitations. First, data were pooled from heterogeneous postapproval studies that differed in important ways, including (1) approaches to collecting the objective and subjective measures of adherence, (2) varied geographies with different HIV incidence, (3) distinct inclusion and exclusion criteria reflecting diverse behaviors associated with HIV acquisition among the participants, (4) approaches to mode of PrEP delivery (eg, home PrEP delivery, use of mobile clinic), and (5) differences in sociodemographics related to country setting. Second, adherence data, especially from objective measures, were only available for a subset of participants, limiting generalizability. The use of plasma tenofovir concentrations to categorize participants into adherence categories may be susceptible to misclassification because plasma tenofovir has a short window of detection. However, no changes were observed in sensitivity analyses excluding the 46 participants for whom plasma tenofovir measures were used in group-based trajectory modeling (data not shown). Importantly, objective adherence data were available only for a minority of participants and were largely representative of the total population. As previously reported, it was also observed that participants’ self-reported adherence was substantially overestimated compared with objective measures.4,5,40 This reinforces the notion that considerable caution should be applied when interpreting self-reported adherence to PrEP. Third, the accuracy of the group-based trajectory methodology used could be improved with a greater sample size and longer duration of follow-up.
Conclusion
This analysis of the effectiveness of F/TDF for PrEP in cisgender women in real-world settings supports that HIV prevention effectiveness is high with adherence to greater than 4 doses per week, which is comparable to the adherence-efficacy relationship for PrEP use in cisgender MSM. This finding may inform provision of F/TDF for PrEP to, and counseling for, cisgender women, emphasizing that although daily adherence is optimal, a minimum of 4 doses per week of F/TDF is expected to provide effective protection for most females.
Educational Objective: To identify the key insights or developments described in this article.
-
Why did the authors undertake a pooled analysis of the efficacy of HIV preexposure prophylaxis (PrEP) in cisgender women?
Negative results in some trials have led to doubts regarding efficacy of HIV PrEP in cisgender women compared with men who have sex with men.
Poor compliance has undermined most prior efforts to demonstrate efficacy of PrEP in cisgender women.
Risk reduction efforts have largely focused on barrier methods, such as condoms, leading to uncertain results in prior trials of PrEP.
-
What did the authors find regarding adherence and incident HIV infections?
All adherence levels were associated with new infections at incidence rates greater than or equal to 0.49 per 100 person-years.
All women enrolled in the study were sufficiently adherent, so no difference in HIV incidence was found.
No women with consistently daily adherence acquired HIV and declining levels of adherence were associated with higher incidence rates.
-
What do the authors indicate that their findings suggest?
Adherence reporting, although often subjective, proved to be highly reliable and consistent with objective measures.
Cisgender women need not be restricted to a rigid daily regimen of PrEP.
Discussion of sexual behavior has no role in PrEP.
eTable 1. Baseline Participant Characteristics, Post-approval Studies of Emtricitabine/Tenofovir Disoproxil Fumarate for Pre-Exposure Prophylaxis in Cisgender Women, Six Countries, 2012–2020 (N=6296)
eTable 2. HIV incidence in Subgroups, Post-approval Studies of Emtricitabine/Tenofovir Disoproxil Fumarate for Pre-Exposure Prophylaxis in Cisgender Women, Six Countries, 2012–2020 (N=6296)
eTable 3. Adherence Measurements Utilized in Assigning Adherence Trajectory Groups
eTable 4. Association between Baseline Participant Characteristics and Adherence Trajectory Categories in Cisgender Women who had Adherence Trajectories, 2012-2020
Data sharing statement
References
- 1.World Health Organization . Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach: 2021. update. Updated July 16, 2021. Accessed October 10, 2022. https://apps.who.int/iris/handle/10665/342899 [PubMed]
- 2.US Food and Drug Administration . FDA approves first drug for reducing the risk of sexually acquired HIV infection. Published July 16, 2012. Accessed April 3, 2023. https://www.hiv.gov/blog/fda-approves-first-drug-for-reducing-the-risk-of-sexually-acquired-hiv-infection/
- 3.National Institute of Child Health and Human Development . Item of interest: FDA approves PrEP therapy for adolescents at risk of HIV. Published May 16, 2018. Accessed April 11, 2023. https://www.nichd.nih.gov/newsroom/news/051618-PrEP
- 4.Marrazzo JM, Ramjee G, Richardson BA, et al. ; VOICE Study Team . Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6):509-518. doi: 10.1056/NEJMoa1402269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Damme L, Corneli A, Ahmed K, et al. ; FEM-PrEP Study Group . Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411-422. doi: 10.1056/NEJMoa1202614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baeten JM, Donnell D, Ndase P, et al. ; Partners PrEP Study Team . Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399-410. doi: 10.1056/NEJMoa1108524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cottrell ML, Yang KH, Prince HM, et al. A translational pharmacology approach to predicting outcomes of preexposure prophylaxis against HIV in men and women using tenofovir disoproxil fumarate with or without emtricitabine. J Infect Dis. 2016;214(1):55-64. doi: 10.1093/infdis/jiw077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patterson KB, Prince HA, Kraft E, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3(112):112re4. doi: 10.1126/scitranslmed.3003174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Cremades M, Vučićević K, Hendrix CW, et al. Characterizing HIV-preventive, plasma tenofovir concentrations-a pooled participant-level data analysis from human immunodeficiency virus preexposure prophylaxis clinical trials. Clin Infect Dis. 2022;75(11):1873-1882. doi: 10.1093/cid/ciac313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson PL, Marzinke MA, Glidden DV. Updating the adherence-response for oral F-TDF for PrEP among cisgender women. Clin Infect Dis. 2023;76(10):1850-1853. doi: 10.1093/cid/ciad021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson PL, Liu AY, Castillo-Mancilla JR, et al. Intracellular tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following directly observed therapy. Antimicrob Agents Chemother. 2017;62(1):e01710-17. doi: 10.1128/AAC.01710-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grant RM, Anderson PL, McMahan V, et al. ; iPrEx study team . Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14(9):820-829. doi: 10.1016/S1473-3099(14)70847-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson PL, Glidden DV, Liu A, et al. ; iPrEx Study Team . Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4(151):151ra125. doi: 10.1126/scitranslmed.3004006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagin DS. Group-based trajectory modeling: an overview. Ann Nutr Metab. 2014;65(2-3):205-210. doi: 10.1159/000360229 [DOI] [PubMed] [Google Scholar]
- 15.Musinguzi N, Pyra M, Bukusi EA, Mugo NR, Baeten JM, Haberer JE; MPYA Study Team . Trajectories of oral PrEP adherence among young Kenyan women: implications for promoting effective PrEP use. AIDS Behav. 2023;27(1):171-181. doi: 10.1007/s10461-022-03753-y [DOI] [PubMed] [Google Scholar]
- 16.Cooney EE, Reisner SL, Saleem HT, et al. ; American Cohort To Study HIV Acquisition Among Transgender Women (LITE) Study Group . Prevention-effective adherence trajectories among transgender women indicated for PrEP in the United States: a prospective cohort study. Ann Epidemiol. 2022;70:23-31. doi: 10.1016/j.annepidem.2022.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stoner MCD, Rucinski KB, Giovenco D, et al. Trajectories of PrEP adherence among young women aged 16 to 25 in Cape Town, South Africa. AIDS Behav. 2021;25(7):2046-2053. doi: 10.1007/s10461-020-03134-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masyuko S, Mukui I, Njathi O, et al. Pre-exposure prophylaxis rollout in a national public sector program: the Kenyan case study. Sex Health. 2018;15(6):578-586. doi: 10.1071/SH18090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haberer JE, Bukusi EA, Mugo NR, et al. ; MPYA Study Team . Effect of SMS reminders on PrEP adherence in young Kenyan women (MPYA study): a randomised controlled trial. Lancet HIV. 2021;8(3):e130-e137. doi: 10.1016/S2352-3018(20)30307-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haberer JE, Mugo N, Bukusi EA, et al. Understanding pre-exposure prophylaxis adherence in young women in Kenya. J Acquir Immune Defic Syndr. 2022;89(3):251-260. doi: 10.1097/QAI.0000000000002876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baeten JM, Heffron R, Kidoguchi L, et al. ; Partners Demonstration Project Team . Integrated delivery of antiretroviral treatment and pre-exposure prophylaxis to HIV-1-serodiscordant couples: a prospective implementation study in Kenya and Uganda. PLoS Med. 2016;13(8):e1002099. doi: 10.1371/journal.pmed.1002099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henderson F, Taylor A, Chirwa L, et al. Characteristics and oral PrEP adherence in the TDF2 open- label extension in Botswana. Presented at: 8th IAS Conference on HIV Pathogenesis, Treatment & Prevention; 19-22 July 2015; Vancouver, Canada. Accessed February 16, 2024. https://onlinelibrary.wiley.com/doi/epdf/10.7448/IAS.18.5.20479
- 23.Celum CL, Bukusi EA, Bekker LG, et al. ; POWER Study Team . PrEP use and HIV seroconversion rates in adolescent girls and young women from Kenya and South Africa: the POWER demonstration project. J Int AIDS Soc. 2022;25(7):e25962. doi: 10.1002/jia2.25962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Celum CL, Gill K, Morton JF, et al. Incentives conditioned on tenofovir levels to support PrEP adherence among young South African women: a randomized trial. J Int AIDS Soc. 2020;23(11):e25636. doi: 10.1002/jia2.25636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heffron R, Ngure K, Velloza J, et al. Implementation of a comprehensive safer conception intervention for HIV-serodiscordant couples in Kenya: uptake, use and effectiveness. J Int AIDS Soc. 2019;22(4):e25261. doi: 10.1002/jia2.25261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reza-Paul S, Lazarus L, Maiya R, et al. The Ashodaya PrEP project: lessons and implications for scaling up PrEP from a community-led demonstration project among female sex workers in Mysore, India. Glob Public Health. 2020;15(6):889-904. doi: 10.1080/17441692.2020.1724316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jana S, Ray P, Roy S, et al. Successful integration of HIV pre-exposure prophylaxis into a community-based HIV prevention program for female sex workers in Kolkata, India. Int J STD AIDS. 2021;32(7):638-647. doi: 10.1177/0956462420983992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leech AA, Biancarelli D, Aaron E, et al. HIV pre-exposure prophylaxis for conception among HIV serodiscordant couples in the United States: a cohort study. AIDS Patient Care STDS. 2020;34(7):295-302. doi: 10.1089/apc.2020.0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koester KA, Dufour MSK, Bourdeau B, Packard R, Herb F, Myers JJ. HIV pre-exposure prophylaxis (PrEP) Adherence and reasons for discontinuation among women in the CRUSH-PrEP for Women Demonstration Project. Presented at: 14th International Conference on HIV Treatment and Prevention Adherence; June 17-19 2019; Miami, Florida. [Google Scholar]
- 30.Brooks KM, Anderson PL. Pharmacologic-based methods of adherence assessment in HIV prevention. Clin Pharmacol Ther. 2018;104(6):1056-1059. doi: 10.1002/cpt.1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donnell D, Baeten JM, Bumpus NN, et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr. 2014;66(3):340-348. doi: 10.1097/QAI.0000000000000172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthews LT, Atukunda EC, Owembabazi M, et al. High PrEP uptake and objective longitudinal adherence among HIV-exposed women with personal or partner plans for pregnancy in rural Uganda: a cohort study. PLoS Med. 2023;20(2):e1004088. doi: 10.1371/journal.pmed.1004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mallayasamy S, Chaturvedula A, Fossler MJ, et al. ; Partners Demonstration Project Team . Tenofovir plasma concentration from preexposure prophylaxis at the time of potential HIV exposure: a population pharmacokinetic modeling and simulation study involving serodiscordant couples in East Africa. Antimicrob Agents Chemother. 2019;63(8):e00446-19. doi: 10.1128/AAC.00446-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balkus JE, Brown E, Palanee T, et al. An empiric HIV risk scoring tool to predict HIV-1 acquisition in African women. J Acquir Immune Defic Syndr. 2016;72(3):333-343. doi: 10.1097/QAI.0000000000000974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.US Centers for Disease Control . Preexposure prophylaxis for the prevention of HIV infection in the United States: 2021 Update: a clinical practice guideline. Accessed January 9, 2022. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
- 36.Delany-Moretlwe S, Hughes JP, Bock P, et al. ; HPTN 084 study group . Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial. Lancet. 2022;399(10337):1779-1789. doi: 10.1016/S0140-6736(22)00538-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L, Iannuzzi S, Chaturvedula A, et al. Model-based predictions of protective HIV pre-exposure prophylaxis adherence levels in cisgender women. Nat Med. 2023;29(11):2753-2762. doi: 10.1038/s41591-023-02615-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore M, Stansfield S, Donnell DJ, et al. Efficacy estimates of oral pre-exposure prophylaxis for HIV prevention in cisgender women with partial adherence. Nat Med. 2023;29(11):2748-2752. doi: 10.1038/s41591-023-02564-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Justman J, Befus M, Hughes J, et al. Sexual behaviors of US women at risk of HIV acquisition: a longitudinal analysis of findings from HPTN 064. AIDS Behav. 2015;19(7):1327-1337. doi: 10.1007/s10461-014-0992-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015;5(4):470-482. doi: 10.1007/s13142-015-0315-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline Participant Characteristics, Post-approval Studies of Emtricitabine/Tenofovir Disoproxil Fumarate for Pre-Exposure Prophylaxis in Cisgender Women, Six Countries, 2012–2020 (N=6296)
eTable 2. HIV incidence in Subgroups, Post-approval Studies of Emtricitabine/Tenofovir Disoproxil Fumarate for Pre-Exposure Prophylaxis in Cisgender Women, Six Countries, 2012–2020 (N=6296)
eTable 3. Adherence Measurements Utilized in Assigning Adherence Trajectory Groups
eTable 4. Association between Baseline Participant Characteristics and Adherence Trajectory Categories in Cisgender Women who had Adherence Trajectories, 2012-2020
Data sharing statement