Abstract
Recently, efficient and long-term in vivo gene transfer by recombinant adeno-associated virus type 2 (rAAV) vectors has been demonstrated in a variety of tissues. Further improvement in vector titer and purity will expedite this in vivo exploration and provide preclinical information required for use in human gene therapy. In an effort to obtain higher titers, we constructed a novel AAV helper plasmid which utilizes translational control of AAV Rep genes (J. Li et al., J. Virol. 71:5236–5243, 1997). To address the issue of purity, in this study we report the first rAAV production method which is completely free of adenovirus (Ad) helper virus. The new production system uses a plasmid construct which contains a mini-Ad genome capable of propagating rAAV in the presence of AAV Rep and Cap genes. This construct is missing some of the early and most of the late Ad genes and is incapable of producing infectious Ad. Transfection of 293 cells with the new mini-Ad helper and AAV packaging plasmids results in high-titer rAAV vectors with yields greater than 1,000 transducing units, or 105 viral particles per cell. When rAAV vectors were produced by using this production scheme and compared to traditional heat-inactivated rAAV preparations in vitro and in vivo, we observed transduction equivalent to or better than normal levels. The complete removal of infectious Ad from AAV production should facilitate a better understanding of immune response to AAV vectors in vivo, eliminate the need for developing replication-competent Ad assays, and provide a more defined reagent for clinical use.
Recombinant adeno-associated virus (rAAV) vectors are promising alternative gene delivery systems, based on the defective and nonpathogenic parvovirus adeno-associated virus type 2 (AAV-2) (2, 18, 26, 33, 41, 45). All vectors are derived from a plasmid substrate which retains only the AAV 145-bp inverted terminal repeats (ITRs) flanking the transgene cassette of choice. The deleted viral coding sequences are present on a separate template, referred to as an AAV helper or packaging plasmid (20, 39). Generation of rAAV requires transfection of the vector and packaging constructs into adenovirus (Ad)-infected cells (33). Due to the lack of homology between vector and helper sequences, rAAV produced in this system is essentially free of wild-type (wt) AAV (39). The ability to generate rAAV free of wt virus minimizes the possibility of undesirable viral gene expression that has caused host immune reactions seen with other vectors (49, 50). Coupled with the ability to transduce both dividing and nondividing cells, recent in vivo studies with rAAV have resulted in efficient and long-term gene transfer in a variety of tissues, including lung (1, 15, 16), muscle (6, 12, 21, 24, 32, 46), central nervous system (23, 29, 34, 47), liver (25, 38, 40), and retina (14).
Interestingly, these advances in rAAV transduction in vivo have been directly facilitated by the ability to produce and purify vector particles. It is not surprising, given the cryptic nature of this virus, that new insights into its biology have affected vector production schemes. For example, the AAV cis-acting ITRs, which function as the origin of DNA replication, packaging, and integration signals for vector DNA, also serve as regulatory elements for wt AAV gene expression (28). Although involved in wt AAV gene expression, the ITRs are excluded from the AAV helper plasmids in order to avoid generation of wt recombinants (20, 31, 39). The AAV p5 promoter sequence also exhibits an enhancer-like function which appears redundant to the ITR for regulating AAV p19 and p40 for Rep52/40 and capsid (Cap) gene expression (28, 35–37). The identification of this fact necessitates that AAV gene expression from the packaging plasmid be optimized for Rep and Cap expression in order to achieve efficient vector replication and packaging (27, 43). Previously we have shown that overexpression of AAV Rep78/68 proteins by substituting the p5 promoter with strong heterologous promoters resulted in considerably lower rAAV yield. In contrast, reduction of Rep78/68 expression by attenuated translation initiation has resulted in much higher rAAV yields (27). These findings suggest that proper regulation of AAV gene expression plays a crucial role in rAAV production and that manipulation of the packaging plasmids to optimize the AAV gene expression can lead to improved vector yields. Moreover, it is equally critical that proper helper functions are provided from essential Ad genes, which not only regulate AAV gene expression but also alter the cellular environment to suit AAV propagation.
A hallmark feature of AAV is its requirement for coinfection with an unrelated virus, such as Ad, to provide essential helper functions for the productive life cycle. A number of Ad genes, including the E1a, E1b, E2a, E4, and VA RNA genes, possessing these helper functions have been identified. E1a serves as a transactivator, up-regulating the transcriptional activity of numerous Ad genes as well as the AAV Rep and Cap genes. By interacting with E4, the E1b gene can facilitate the timely transportation of viral mRNAs. The E4 gene, particularly open reading frame 6, is also involved in facilitating AAV DNA replication. E2a and VA RNA act to enhance the viral mRNA stability and efficiency of translation, especially for AAV Cap transcripts (for a review, see reference 33 and references therein). To produce rAAV, the Ad helper functions are usually provided by Ad particle infection after cotransfection of both the rAAV vector and the AAV packaging plasmids.
Although this procedure for introducing Ad helper genes is most efficient, a number of problems are generated as a result of this infection. The primary concern is the need to remove the contaminating Ad particles. In addition, the inherent competition between AAV and Ad for critical viral gene functions affects the final yield of vectors generated. Complete removal of Ad has relied on physical techniques such as CsCl2 gradients, column chromatography, and a heat-denaturing step to inactivate any residual Ad particles that may still be present. While most of these procedures have succeeded to various degrees, the potential for Ad contamination is an unwanted risk, and the presence of Ad denatured proteins is unacceptable for clinical use. Recent efforts have focused on improving both the rAAV packaging plasmids and the vector titers (5, 7, 13, 17, 27, 43). Although adequately high titers of rAAV can be achieved after purification and concentration, the vector yields on a per-cell basis (102 to 104 particles/cell) still have potential for improvement compared with wild-type AAV yields (greater than 105 particles/cell [44]). In addition, the presence of Ad or Ad proteins will always be a potential source of contamination that will induce unwanted immune response to rAAV transduced cells in vivo (11, 32).
To address these concerns, we made a novel AAV packaging construct, pXX2, that increases the rAAV yield by 15-fold compared to the conventional packaging plasmid pAAV/Ad (39). In addition, we also describe a new vector production method that is completely free of Ad. The Ad helper functions are delivered from a plasmid, pXX6, which contains the essential helper genes but lacks the Ad structural and replication genes. Furthermore, combination of these two new plasmids increases rAAV vector yields 40-fold. Finally, both in vitro and in vivo examination of such Ad-free rAAV vectors revealed identical or better infectivity and transduction compared to conventional procedures that use Ad particles as the helper. These advances should significantly impact the study of rAAV vectors in their role as a viral delivery system for human gene therapy.
MATERIALS AND METHODS
Construction of AAV packaging and Ad helper plasmids.
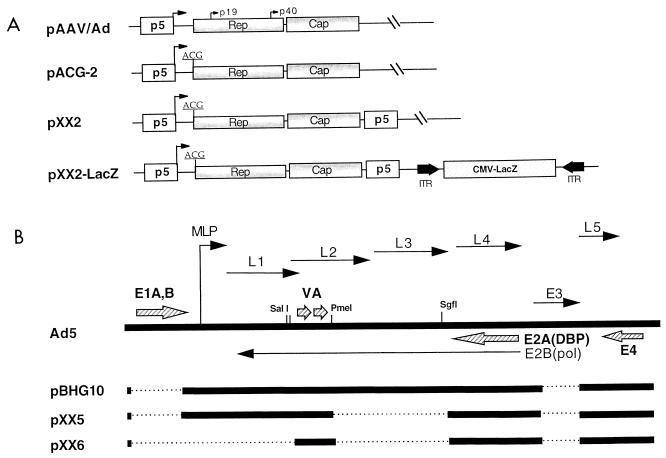
The AAV packaging plasmids were constructed based on plasmid pAAV/Ad (39). Construction of packaging plasmid pACG2 was reported previously (27). Packaging plasmid pXX2 was constructed from pACG2 by inserting a promoter p5 element downstream of the capsid gene. In detail, pACG2 was partially digested with XbaI following complete digestion by NsiI. The large fragment containing the plasmid backbone and the AAV gene cassette was purified. This fragment was ligated to an XbaI-PstI fragment isolated from pACG2 containing the p5 promoter. The new construct, pXX2, has two copies of p5 flanking the AAV coding regions (Fig. 1A).
FIG. 1.
Construction of AAV packaging and Ad helper plasmids. (A) AAV packaging plasmids AAV/Ad, ACG-2, XX2, and XX2-LacZ all contain the endogenous promoter p5 (open box) and Rep and Cap genes (shaded box). Plasmids ACG-2, XX2, and XX2-LacZ utilize an artificial ACG start codon for Rep protein synthesis (27). In addition, plasmids XX2 and XX2-LacZ contain an extra copy of the p5 promoter element downstream of the Cap gene. Also included in pXX2-LacZ is an AAV-LacZ vector containing a cytomegalovirus (CMV)-LacZ gene cassette (hatched box) flanked by the AAV ITRs (solid arrow). (B) Construction of Ad helper plasmids. The thick solid lines represent Ad DNA sequences. The dotted lines are the Ad DNA sequences deleted in constructs pBHG10, pXX5, and pXX6. The arrowhead lines are the Ad early and late genes or RNA transcripts. L1 to L5 are the Ad late gene transcripts made by the Ad major late promoter (MLP). Ad E1A, E1B, E2A, E4, and VA RNA genes (large hatched arrows) are the essential genes supplying the helper functions for AAV production. DBP, single-stranded DNA binding protein.
Plasmid pXX2-LacZ, containing both the AAV-LacZ vector cassette and the packaging genes, was constructed from pdx31-LacZ (29) and pXX2. In detail, an Sse8387I linker (5′ CGCCTGCAGG 3′) was first cloned into the ClaI site of pXX2, generating pXX2-Sse. The AAV-LacZ vector cassette was then excised from plasmid pdx31-LacZ by PstI digestion and cloned into the Sse8387I site of pXX2-Sse, generating pXX2-LacZ (Fig. 1A).
The Ad helper plasmid pXX5 was constructed by deleting an 8-kb PmeI-SgfI fragment from plasmid pBHG10 (3), which already had deletions in E1 and E3 genes as well as the Ad packaging signal sequence. Plasmid pXX5 has further lost the hexon, penton, core protein, and DNA polymerase genes. The Ad helper plasmid pXX6 was constructed by cloning the large ClaI-SalI fragment of pXX5 into ClaI-SalI sites of the high-copy-number plasmid pBluescript KS(+)II (Stratagene). pXX6 has further lost an 8.5-kb fragment containing the Ad terminal protein gene, as well as the Ad major late promoter (Fig. 1B).
Western analyses of Ad proteins.
Western blots of Ad structural proteins were carried out by a previously published method (22), with modifications. Briefly, a cell pellet from one half of a 10-cm-diameter dish was lysed in 250 μl of radioimmunoprecipitation assay buffer (10 mM Tris-Cl [pH 8.2], 1% Triton X-100, 1% sodium dodecyl sulfate [SDS], 150 mM NaCl). The samples were separated on SDS–10% polyacrylamide gels and transferred to a nitrocellulose membrane. After blocked in 10% nonfat dry milk in Tris-buffered saline (TBS; 50 mM Tris-Cl [pH 7.5], 200 mM NaCl) for 1 h, the membranes were incubated at room temperature for 30 min with a rabbit anti-Ad type 5 (Ad5) fiber protein antibody (19) (1:2,000 dilution in TBS containing 1% nonfat milk and 0.5% Tween 20). Following primary antibody incubation and rinses, the membranes were incubated at room temperature for 1 h with goat anti-rabbit antibody conjugated with horseradish peroxidase (1:5,000 dilution in TBS containing 2% dry milk; Sigma). After three washes with TBS containing 0.5% Tween 20 and one with TBS alone, the specific protein bands were visualized with chemiluminescence reagent (DuPont) and exposed to X-ray film.
Production and measurement of titers of rAAV vectors.
The rAAV-LacZ vector was made by cotransfection methods as previously described (27, 46). Briefly, 1 to 2 h before transfection, each 10-cm-diameter plate of human 293 cells (70 to 80% confluent) was fed with 10 ml of fresh Iscove modified Dulbecco medium (Gibco) containing 10% fetal bovine serum (HyClone) without antibiotics. A total of 25 μg of plasmid DNA was dissolved in 1 ml of 0.25 M CaCl2 and then quickly mixed with 1 ml of HEPES-buffered saline (50 mM HEPES, 280 mM NaCl, 1.5 mM Na2HPO4 [pH 7.12]) and added to the cells. At 8 to 12 h after transfection, the medium was replaced with fresh Dulbecco modified Eagle medium (Gibco) containing 10% fetal bovine serum and antibiotics. When Ad5 (dl309) was used as the helper virus at a multiplicity of infection (MOI) of 2, the cells were harvested at 48 h postinfection. When the Ad plasmids were used to supply the helper functions, the cytopathic effect was less intense and delayed for more than 12 h longer than that of Ad infection. However, the cells were still harvested at 48 h posttransfection unless specified otherwise. After low-speed centrifugation on a tabletop centrifuge, the cell pellets were resuspended in 1 ml of 100 mM NaCl–10 mM Tris-HCl (pH 8.5) and subjected to four cycles of freeze-thaw and removal of cell debris. The rAAV virus lysate was heated at 56°C for 30 min to inactivate the Ad and stored at −20°C before use. Following coinfection of 293 cells with various dilutions of the rAAV stocks and Ad5 dl309 (MOI of 1) for 24 h, the titers of AAV-LacZ viruses were determined by counting the blue cells after 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining. Large-scale rAAV preparation and CsCl density gradient purification were carried out by a previously published method (46).
In vivo vector delivery into muscle tissue.
Swiss Webster mice were purchased from Taconic (Germantown, N.Y.) and handled in accordance with the institutional guidelines of the University of North Carolina. Before virus injection, 5-week-old mice were anesthetized with 2.5% Avertin intraperitoneally. Ten microliters of AAV-LacZ (107 infectious units) was injected into the hindleg tibialis anterior muscles percutaneously. At various time points, the mice were euthanized and the muscle tissue was harvested and rapidly frozen in liquid nitrogen. Cryostat sectioning of the tissue was performed at 20-μm thickness with a Leica microtome. The sections were then fixed and X-Gal stained as previously described (46).
RESULTS
Generation of Ad helper plasmids for rAAV production.
We have recently reported that unregulated overexpression of AAV Rep78/68 inhibits rAAV production, while attenuated Rep78/68 synthesis increases the rAAV yield. The attenuation was achieved by mutating the original ATG translation start codon into an inefficient ACG codon (27). Because deletion of promoter p5 as a cis-acting element can cause down-regulation of AAV promoters p19 and p40 (28, 35–37), we reasoned that the addition of an extra copy of the p5 promoter element may up-regulate the expression of p19 and p40. Consequently, the expression of Rep52/40 and the capsid proteins would increase. Interestingly, Rep52/40 is involved in AAV single-stranded DNA formation, which is part of the packaging process (4), while the capsid proteins are required for assembling viral particles. Therefore, the collective effects of these helper plasmid changes may result in higher rAAV particle yield. Because p5 can function as an enhancer for p19 and p40, we inserted the element downstream of the Cap coding sequences in plasmid pACG2. This packaging plasmid, pXX2, contains two copies of p5 sequences flanking the AAV coding regions (Fig. 1A).
rAAV particles are typically produced by cotransfection of the vector and packaging plasmids (39), a procedure that may limit the equal uptake of these constructs by the cells. To test whether combining the vector and the packaging cassettes into one plasmid would alleviate this putative limiting factor, an AAV-LacZ vector cassette was cloned into the packaging plasmid pXX2, generating plasmid pXX2-LacZ, containing both the vector and the packaging cassettes (Fig. 1A).
To eliminate the potential problem of Ad contamination during rAAV production, we initially used a defective Ad plasmid, pBHG10 (3), to supply the Ad helper functions (Fig. 1B). This plasmid lacks Ad E1a and E1b genes as well as the viral packaging signal. The missing E1a and E1b genes in plasmid pBHG10 can be complemented when transfected into human 293 cells. Since all of the Ad genes can be expressed, plasmid pBHG10 is expected to supply the full helper functions for rAAV production. However, this plasmid still presents two problems. First, although it is unable to produce Ad particles due to the lack of packaging signal (3), it still can potentially recombine with the left-hand Ad genome integrated in 293 cells to generate wild-type Ad. Second, since all Ad structural and replication genes are functional in pBHG10, they not only compete with rAAV for the cellular resources of DNA and protein synthesis but also produce some cytotoxic structural proteins (30). Cytotoxic proteins such as the fiber can still be a contaminant that affects the purity of rAAV stocks. Based on these concerns, we have deleted an 8-kb DNA fragment in the central region of the Ad coding sequences from pBHG10, resulting in the destruction of hexon, penton, core protein, and Ad DNA polymerase genes (Fig. 1B). Therefore, the new plasmid, pXX5, is unable to generate Ad particles or most of the structural proteins (data not shown). This construct was assayed for AAV helper function and tested positive (data not shown). To further remove the unnecessary Ad genes and increase the plasmid DNA yield, a third Ad helper plasmid was constructed by cloning a large fragment of pXX5 containing E2a, E4, and VA RNA genes into the high-copy-number plasmid pBluescript KS(+)II to generate pXX6 (Fig. 1B). This plasmid has an additional 8.5-kb deletion at the left-hand end of the Ad genome, including the major late promoter which regulates all Ad late genes. This plasmid, pXX6, by design should be defective for Ad virion production and the ability to produce Ad structural proteins.
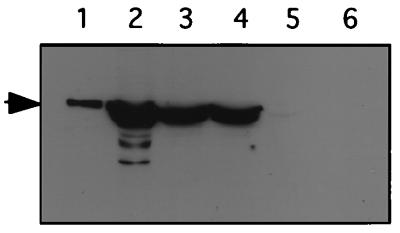
Despite the deletion of the major late promoter, the coding sequence of the Ad fiber protein gene is still present in pXX6. To examine if the gene is still expressed, we carried out Western analysis using an antibody against the Ad5 fiber protein (19). As shown in Fig. 2, only trace amounts of fiber proteins were detected in cells transfected with pXX6 (Fig. 2, lane 5). This extremely low level of fiber gene expression may result from a cryptic promoter, since the Ad major late promoter is deleted. In contrast, cells infected with Ad particles produced the highest level of fiber protein (Fig. 2, lane 2). As expected, cells transfected with plasmid pBHG10 or pXX5 (Fig. 2, lanes 3 and 4) also produced abundant fiber protein. Even though the Ad major late promoter on pBHG10 and pXX5 is fully functional, the fiber protein produced is less than that seen with Ad infection (Fig. 2; compare lanes 3 and 4 to lane 2). These results indicate that plasmid pXX6 is a better choice for generating rAAV devoid of both Ad and the majority of structural proteins.
FIG. 2.
Western analysis of the Ad5 fiber protein with an antibody against the C-terminal knob domain (19). Lanes: 1, CsCl gradient purified Ad particles as the positive control; 2, lysate of Ad5-infected 293 cells; 3 to 5, lysates of 293 cells transfected with plasmids pBHG10, pXX5, and pXX6 respectively; 6, lysate of mock-transfected 293 cells.
Optimizing AAV packaging plasmids that increase vector yield.
To investigate the utility of the new packaging plasmids pACG2 and pXX2 for rAAV production, we used an AAV-LacZ construct (pdx31-AAV) as a reporter vector plasmid and compared vector yields to those for the established AAV helper pAAV/Ad (29). The transducing rAAV titers can be readily assessed by X-Gal staining of infected cells. To examine the packaging efficiency, the AAV-LacZ vector plasmid was cotransfected with various packaging plasmids into 293 cells. At 48 h after Ad infection, the cells were harvested and subjected to four cycles of freeze-thaw. After removal of cell debris and heat inactivation, the rAAV vector titers in the crude lysate were determined by infection of HeLa cells and X-Gal staining. Serial dilutions were performed so that positive cells would represent 1 transducing unit (t.u.) of rAAV-LacZ (data not shown). As shown in Table 1, packaging plasmid pACG2 results in approximately an eightfold increase in rAAV vector yield over its parental plasmid pAAV/Ad. This increase is attributed to the attenuated Rep78/68 synthesis as previously described (27). Furthermore, plasmid pXX2, which contains an additional p5 promoter downstream of the AAV Cap region, resulted in an additional 1.8-fold increase over its parental plasmid pACG2. This additional increase in overall vector yield is consistent with an enhancer function associated with p5 as previously reported (28, 35–37). It is noteworthy that for pXX2 helper construct, the best viral yields were obtained at a vector/packaging plasmid ratio of 3:1. Together, the ATG-to-ACG mutation of Rep78/68 start codon coupled with the addition of p5 sequences downstream of the Cap gene generated an overall 15-fold increase in rAAV yields compared to the parental packaging plasmid pAAV/Ad (39).
TABLE 1.
Comparison of rAAV titers produced by different packaging plasmids
| Packaging plasmida | Vector/packaging plasmid ratio | rAAV-LacZ yield (t.u./10-cm-diam plate)b | t.u./cellc |
|---|---|---|---|
| 3:1 | 8.5 × 107 | 17 | |
| pAAV/Ad | 1:1 | 1.4 × 108 | 28 |
| 1:3 | 1.0 × 108 | 20 | |
| 3:1 | 5.0 × 108 | 100 | |
| pACG-2 | 1:1 | 1.1 × 109 | 220 |
| 1:3 | 8.0 × 108 | 160 | |
| 3:1 | 2.0 × 109 | 400 | |
| pXX2 | 1:1 | 1.4 × 109 | 280 |
| 1:3 | 1.0 × 108 | 200 | |
| pXX2-LacZ | 1:1 | 1.3 × 109 | 260 |
For the purpose of comparison, the LacZ-AAV titers from pAAV/Ad and pACG-2 are adapted from previously published results (27).
Average of three experiments obtained from a 10-cm plate of human 293 cells; determined by infecting 293 cells with Ad at an MOI of 1 and various dilution of heat-inactivated rAAV virus stocks. After X-Gal staining, each blue cell was translated into 1 t.u.
Obtained by dividing the titers (total t.u.) from each 10-cm-diameter plate by the total number of 293 cells (approximately 5 × 106).
rAAV yields from single packaging/vector combination plasmids.
Typically, the rAAV vector cassette and the packaging gene cassette are introduced on two individual plasmids. Cotransfection experiments rely on efficient delivery of both plasmids into the same cell. We combined the AAV-LacZ vector cassette into the packaging plasmid pXX2, generating a single plasmid, pXX2-LacZ, to directly address this concern. This manipulation ensures the presence of the vector and packaging genes at equal molar ratios in every transfected cell. We compared the single vector/helper plasmid pXX2-LacZ transfection and the cotransfection of the separate vector AAV-LacZ and packaging plasmid pXX2. The two methods generated very similar rAAV vector yields (Table 1), indicating that combining the vector and packaging cassette into a single plasmid offers no noticeable benefit.
Production of high-titer rAAV completely free of Ad.
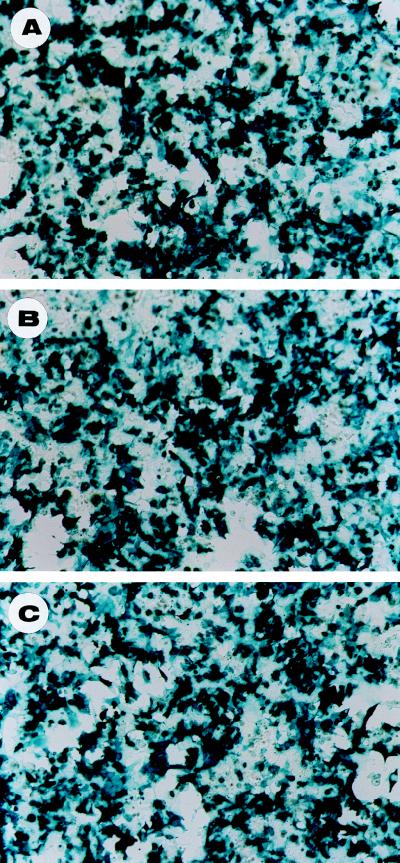
Since cotransfection of single rather than double AAV vector/helper constructs did not appear to have a major impact on vector yield, we next addressed the role of providing the Ad helper function from a plasmid backbone. To examine the helper functions of Ad plasmids on the production of rAAV vectors, we carried out cotransfection of three plasmids (AAV vector and packaging construct, with Ad helper plasmids). Even though the experiment described above suggested that transfection of two rather than one plasmid had little effect on vector yield, we wanted to rule out any concern of efficiently introducing three essential constructs into the same target cell. Since the optimized transfection condition for calcium phosphate method is 25 μg of total DNA per 10-cm-diameter plate of 293 cells (27), we measured transfection efficiency by using AAV-LacZ plasmid as the reporter and pBluescript KS(−)II as the carrier DNA. The ratios of vector AAV-LacZ to carrier plasmid were 1:0, 1:1, and 1:10, respectively, while the total DNA remained constant at 25 μg for each 10-cm-diameter plate. X-Gal staining revealed similar overall percentages of blue cells (above 70%) transfected at various vector/carrier DNA ratios. No obvious difference in staining intensity among various ratios was observed (Fig. 3). These observations suggest that multiple plasmid transfection is not rate limiting, supporting the work by others (51), and also provide necessary information required to evaluate our three plasmid approach.
FIG. 3.
Comparison of transfection efficiencies with different vector/carrier DNA ratios. Samples of 293 cells were stained with X-Gal at 24 h after transfection with 25 μg of vector plasmid pdx31-LacZ (1:0) (A), 4 μg of pdx-31-LacZ and 21 μg of carrier plasmid pBluescript KS (1:5.25) (B), or 2.5 μg of pdx31-LacZ and 22.5 μg of pBluescript KS (1:9 ratio) (C). No obvious difference in percentage and intensity of the blue cells can be noticed at different vector/carrier DNA ratios.
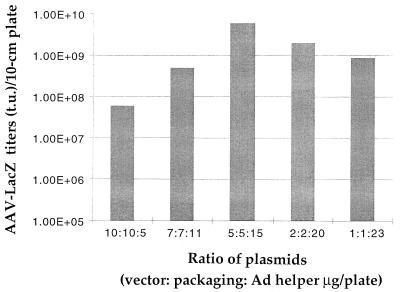
After defining the transfection conditions, we examined rAAV yields at different ratios of AAV vector, AAV packaging, and Ad helper plasmids. The plasmids used were pdx31-LacZ (vector), pXX2 (packaging), and pXX6 (Ad helper), respectively. As shown in Fig. 4, the best rAAV-LacZ yield was obtained at a 5:5:15 ratio of these constructs (vector/packaging/helper in terms of micrograms of DNA for each 10-cm-diameter plate), which is roughly in a 1:1:1 molar ratio. The rAAV yield (6 × 109 t.u./10-cm-diameter plate) achieved under this condition is noticeably (threefold) higher than those obtained by Ad infection (2 × 109 t.u./10-cm-diameter plate [Table 1]). When less than 15 μg of Ad plasmid pXX6 was used, the rAAV yields were significantly reduced. This information suggests that a rate-limiting gene product is supplied from this helper. However, increased amount of Ad plasmid had no significant effect on rAAV yield, suggesting that the Ad helper functions were optimal when introduced at a molar ratio equal to that of the AAV vector/packaging plasmids.
FIG. 4.
Comparison of different vector/packaging/Ad helper plasmid ratios on the yields of rAAV vectors.
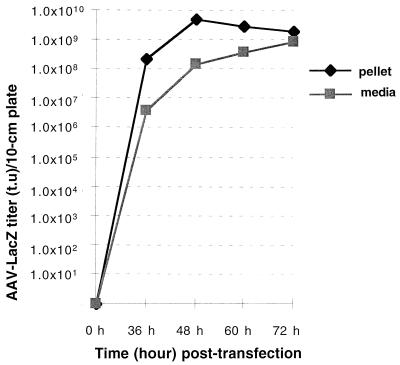
Previously, we found that rAAV yields peaked at 48 h after Ad infection. For Ad helper plasmid pXX6, time course experiments were carried out to examine the effect on rAAV yields between 36 and 72 h posttransfection. As shown in Fig. 5, peak viral yield was obtained at 48 h after introduction of Ad plasmid DNA, similar to our previous published results (27). Extending the time points resulted in more rAAV particles being released into the cell culture media (Fig. 5). We have applied the Ad-free method in large-scale rAAV production using various vector plasmids. We observed similar results with various AAV vectors (in the range of about 10-fold), suggesting that this procedure was not unique to the original constructs tested and could be applied to potentially all rAAV vectors currently being used (Table 2). In summary, this three-plasmid transfection strategy has increased rAAV yields up to 40-fold over transfection with the traditional pAAV/Ad packaging plasmid in Ad-infected cells. More importantly, this genetic approach has ensured the removal of unwanted Ad particles and the majority of Ad structural proteins.
FIG. 5.
Time course of rAAV yields, using Ad plasmid pXX6 as the helper.
TABLE 2.
Ad-free rAAV titers obtained from large-scale preparations of different vector constructs
| rAAV vector | Packaging plasmid | rAAV yield (t.u. [109]/10-cm-diam plate)a | t.u./cellb | Particles (105)/cellc |
|---|---|---|---|---|
| pdx31-LacZ | pXX2 | 6.1 | 1,100 | 9.4 |
| pdx31-LacZ | pXX10d | 3.5 | 640 | NDf |
| pAB-11 | pXX2 | 5.0 | 900 | 8.0 |
| pDD-GFP-Neoe | pXX2 | 1.1 | 200 | ND |
| pTRhFIXm1 | pXX2 | ND | ND | 1.2 |
Average of two experiments using 20 plates (15-cm diameter) of human 293 cells. The rAAVs were purified by double CsCl density gradient purification before measurement of the titers. The titers given are the equivalent titers from a 10-cm-diameter plate of 293 cells (t.u./10-cm-diameter plate = total rAAV titer yielded from 20 15-cm-diameter plates divided by 20 and then divided by 2.25, since the surface area of a 15-cm-diameter plate equals 2.25 of a 10-cm-diameter plate). The t.u. were measured by infecting HeLa cells with Ad at an MOI of 1 and various dilutions of rAAV virus stocks. After X-Gal staining, each blue cell was translated into 1 t.u.
Obtained by dividing the total vector titers (t.u.) from 20 15-cm-diameter plates by the total number of 293 cells (approximately 20 × 1.25 × 107 = 2.5 × 108).
Obtained by dividing the total particle yields (from DNA dot blot) by the total numbers of cells from which the viruses were made.
An rAAV packaging plasmid derived from pACG2 by replacing the capsid gene of AAV-2 with that of another parvovirus (27a).
The yield was determined after three rounds of CsCl density gradient purification. The t.u. of this vector is measured similarly to the t.u. of LacZ vectors except it is based on the number of green fluorescent cells.
ND, not determined.
Efficient in vivo transduction by rAAV vectors generated by using Ad helper constructs.
Our preliminary analysis in vitro of rAAV vectors generated using Ad helper plasmids suggested that both infectivity and transduction were equivalent to or better than those for vector particles generated by using Ad. However, recent studies have determined that the presence of Ad can facilitate AAV transduction in primary cells or after in vivo delivery (9, 11). Therefore, we assayed the Ad-free rAAV vectors for efficient transduction in primary cultured cells and nondividing cells, such as myotubes and neurons, in vivo.
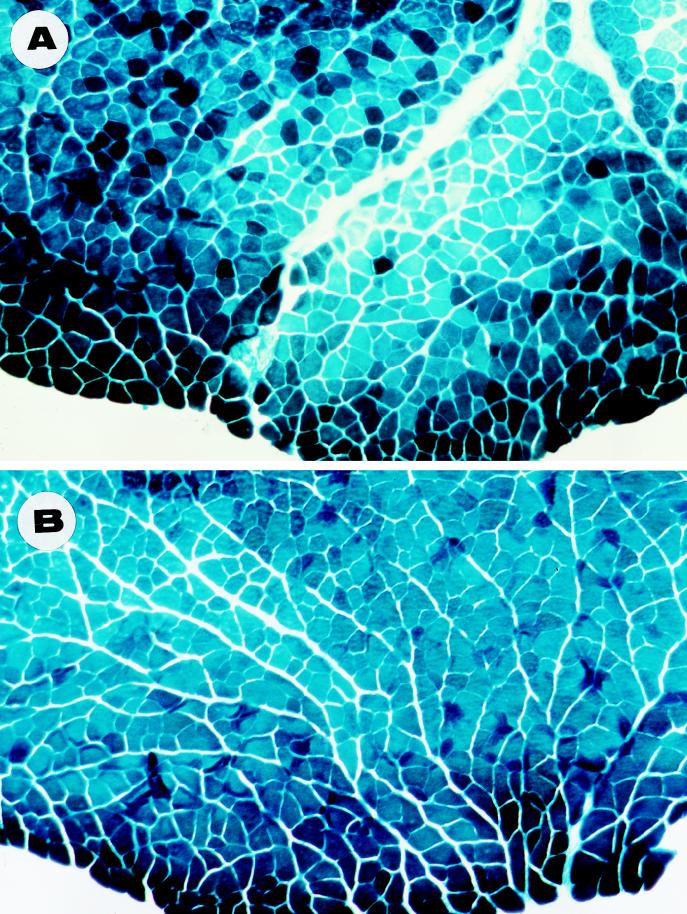
To test if the AAV-LacZ vector produced by the Ad-free method could still achieve equivalent results in vivo, we chose skeletal muscle of immunocompetent mice as the target, since the same tissue has been successfully transduced with AAV-LacZ vectors produced by using Ad infection production methods (12, 24, 46). For each animal, a total of 107 t.u. of AAV-LacZ was injected into the hindleg muscles of 5-week-old Swiss Webster mice (Taconic), which are outbred and immunocompetent. Two weeks and 4 months after vector delivery, the animals were sacrificed and the muscle tissues were analyzed by cryosection and X-Gal staining (46). As shown in Fig. 6, transduction in the muscle tissues was efficient at both short-term (3 weeks) and long-term (4 months) time points. No sign of cellular immune response in vector-transduced cells was observed. These results unequivocally demonstrate that rAAV can efficiently transduce nondividing muscle cells in vivo in the absence of possible Ad contamination. We have also achieved efficient in vivo transduction in rat brain by using rAAV vectors made in the same Ad-free manner, suggesting that these highly defined vector preparations should be extremely valuable in deriving AAV vector transduction data in vivo (data not shown).
FIG. 6.
In vivo transduction of muscle tissues by an rAAV-LacZ vector generated with the Ad-free method. The muscle tissues were cryosectioned and X-Gal stained at 3 weeks (A) and 4 months (B) following intramuscular delivery of 107 infectious units of rAAV vector, which was purified by double CsCl gradient centrifugation.
In addition, an rAAV plasmid construct harboring human factor IX cDNA was used to generate viral stocks by either the Ad-free method or the conventional Ad infection method. When the same amount of viral particles (determined by DNA dot blot) from either viral stock was used to infect primary cultures of fibroblasts from a hemophilic dog, the Ad-free rAAV produced threefold more human factor IX than with the conventional vector approach (32a). These results indicate that Ad-free rAAV vectors have no dependence on Ad or Ad proteins for efficient transduction of nondividing cells in vivo. In addition, these reagents should now provide the first opportunity to study the effect of host immune response to rAAV transduction without influences of Ad contamination.
DISCUSSION
To further improve the yield of rAAV, we have constructed a novel AAV packaging plasmid, pXX2, which increases vector yields approximately 15-fold over the conventional packaging plasmid pAAV/Ad (27, 39). In addition, by supplying the Ad helper functions from a defective Ad miniplasmid, we have completely prevented the generation of Ad particles. Surprisingly, this modification to the vector production scheme did not result in a decrease in rAAV production. In vivo examination of the Ad-free rAAV vector in muscle tissues demonstrated efficient and long-term transduction without signs of cellular immune reaction. Production of high-titer Ad-free rAAV will expedite the applications of this vector system in the research of gene therapy in vivo. More importantly, this genetic approach has eliminated the need for Ad removal by chromatography, heat denaturation, or other physical steps routinely used when following the previous production procedures. Eliminating the need for replication-competent Ad assays should provide a more defined reagent for preclinical and clinical phase I studies.
Because AAV is a defective parvovirus, its propagation is dependent on the essential helper functions provided by a helper virus, usually Ad. Although helper-independent replication of AAV can be accomplished when the host cells are stressed with genotoxic treatments, such as irradiation or certain chemicals, only 1% of the cells demonstrate limited replication (48). Ad infection is still the most effective way to supply the helper functions for AAV propagation. Complete removal of Ad particles from the crude rAAV stocks has been attempted by various approaches. For example, temperature-sensitive replication mutants of Ad have been tried. These genetic approaches reduce but fail to prevent Ad contamination completely (33a). The first demonstration that purified Ad5 DNA could supply adequate helper functions to rAAV production was provided by Ferrari et al. (9). With this approach, the residual 10% of the viral genome, including the origin and packaging signal, was removed (9). The missing E1 gene products were provided by using 293 cells. While successful in principle, this method required purification of Ad DNA as a starting material, a laborious and inefficient process. In addition, the risk of generating wt Ad still existed due to overlap between the truncated Ad genome and viral sequences presence in 293 cells. The ability to generate Ad-free AAV vectors was significant but required further modifications to become practical (10).
To overcome these obstacles, we have constructed various Ad miniplasmids to substitute for the Ad virion DNA component (9). Substituting Ad helper functions from virus to plasmid DNA for rAAV production has several advantages. First, plasmids such as pXX6 give rise to much higher DNA yield (1 to 3 mg of DNA/liter of bacterial culture) compared to DNA isolated from Ad virions (30 μg/10-cm-diameter plate). Second, there is no risk of generating Ad particles. Third, because of extensive deletions in Ad late genes which are unnecessary for AAV replication, not only are the rAAV preps Ad free, but unwanted Ad structural proteins have been eliminated. This single modification should have the biggest impact in vivo analysis of rAAV transduction. In addition, the rAAV yields produced with Ad helper plasmid transfections are up to threefold higher than those obtained with the Ad infection (Tables 1 and 2). In this study the rAAV LacZ yield was greater than 5.5 × 109 t.u./10-cm-diameter plate, or more than 200 to 1,000 t.u./cell, about a 40-fold increase over the titers obtained from the conventional method, using pAAV/Ad as the packaging plasmid and Ad particles as helper (1.4 × 108 t.u./plate, or 28 t.u./cell [Table 1]). We attribute this increase to the fact that after Ad transfection, competition for Ad gene products is removed, since these Ad miniconstructs do not replicate or package. In addition, since heat treatment of AAV stocks becomes unnecessary, the overall stability of the vectors may be improved. While these benefits appear obvious, the removal of Ad infection from the production scheme should also improve the reproducibility of generating rAAV vectors. For example as we previously reported that Ad infection can often impact final yields, depending on time of addition (27). A one-step transfection method should be more reproducible and can also be applied to AAV packaging cell lines (unpublished observations). One important criterion for an advancement to a production procedure is applicability to various vector constructs. In numerous small- and large-scale productions, we have consistently obtained high-titer rAAV vectors (Table 2). In addition, equivalent results have been obtained with the same method by a different group: a greater than 60-fold increase in vector yields was achieved over the conventional method (52). A plausible explanation for the reproducibility is that once a reliable transfection method is established, the plasmid concentration and ratio can be accurately controlled. In contrast, helper Ad infection is sensitive to cell density and timing of infection. Unlike Ad plasmid DNA, the titers of helper Ad stocks and MOI are more prone to variations from batch to batch.
Contrary to retroviral and Ad packaging cell lines, use of AAV has centered around the transient transfection procedure. Recent development of rAAV packaging cell lines by incorporating both vector and packaging elements into the same cell has eliminated the requirement for plasmid transfection. This important improvement can potentially lead to more reproducible large-scale vector production. However, the rAAV titers generated from the current packaging cell lines are still significantly lower than for the conventional transfection methods. For example, packaging cell lines used by Tamayose et al. generated rAAV vector with titers of 106 t.u. per 10-cm-diameter plate, or less than 1 t.u./cell (42). The packaging cell lines of Clark et al. generated vector titers of up to 20 t.u./cell (7). However, it is noteworthy that the titers obtained by Clark et al. were measured on a special cell line (C12), where the rAAV vector DNA can be amplified by the integrated AAV Rep gene in the cells when coinfected with Ad (7, 8). Amplification of vector DNA would lead to higher transgene expression and enhanced reporter detection. In fact, the authors showed that the apparent titers measured on cell line C12 were about 5 to 10 times higher than the titers measured on normal 293 or HeLa cells, which cannot amplify the vector DNA (7, 8). Based on these observations, the rAAV yields generated from current packaging cells lines are at least 2 orders of magnitude lower than those of current transfection methods (27, 43). Until more efficient packaging cell lines are made, the transient transfection method remains a viable and the most productive way to produce rAAV vectors. Our preliminary results using Ad miniplasmids and AAV packaging cells lines suggest that it may be possible to derive a helper cell line that will carry all of the critical components required for efficient rAAV production (rAAV vector, packaging plasmid, and AAV helper functions now provided by Ad miniplasmids.
In conclusion, high-titer rAAV vectors generated by transient transfection using Ad minichromosome described here is an excellent method for generating sufficient rAAV for preclinical and phase I clinical studies. The ability to provide a more refined (Ad-free) AAV vector should have a major impact on analyzing this delivery system in vivo and should provide a method for generating safer rAAV vectors for clinical studies.
ACKNOWLEDGMENTS
We thank R. Pickles for the gift of anti-Ad5 fiber antibody.
This work was supported by Public Health Service grants HL48347 and HL51818 from the National Institutes of Health. J.L. and X.X. received salary support from Somatix Therapy Corporation.
REFERENCES
- 1.Afione S A, Conrad C K, Kearns W G, Chunduru S, Adams R, Reynolds T C, Guggino W B, Cutting G R, Carter B J, Flotte T R. In vivo model of adeno-associated virus vector persistence and rescue. J Virol. 1996;70:3235–3241. doi: 10.1128/jvi.70.5.3235-3241.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berns K I, Giraud C. Adenovirus and adeno-associated virus as vectors for gene therapy. Ann NY Acad Sci. 1995;772:95–104. doi: 10.1111/j.1749-6632.1995.tb44735.x. [DOI] [PubMed] [Google Scholar]
- 3.Bett A J, Haddara W, Prevec L, Graham F L. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci USA. 1994;91:8802–8806. doi: 10.1073/pnas.91.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chejanovsky N, Carter B J. Mutagenesis of an AUG codon in the adeno-associated virus rep gene: effects on viral DNA replication. Virology. 1989;173:120–128. doi: 10.1016/0042-6822(89)90227-4. [DOI] [PubMed] [Google Scholar]
- 5.Chiorini J A, Wendtner C M, Urcelay E, Safer B, Hallek M, Kotin R M. High-efficiency transfer of the T cell co-stimulatory molecule B7-2 to lymphoid cells using high-titer recombinant adeno-associated virus vectors. Hum Gene Ther. 1995;6:1531–1541. doi: 10.1089/hum.1995.6.12-1531. [DOI] [PubMed] [Google Scholar]
- 6.Clark K R, Sferra T J, Johnson P R. Recombinant adeno-associated viral vectors mediate long-term transgene expression in muscle. Hum Gene Ther. 1997;8:659–669. doi: 10.1089/hum.1997.8.6-659. [DOI] [PubMed] [Google Scholar]
- 7.Clark K R, Voulgaropoulou F, Fraley D M, Johnson P R. Cell lines for the production of recombinant adeno-associated virus. Hum Gene Ther. 1995;6:1329–1341. doi: 10.1089/hum.1995.6.10-1329. [DOI] [PubMed] [Google Scholar]
- 8.Clark K R, Voulgaropoulou F, Johnson P R. A stable cell line carrying adenovirus-inducible rep and cap genes allows for infectivity titration of adeno-associated virus vectors. Gene Ther. 1996;3:1124–1132. [PubMed] [Google Scholar]
- 9.Ferrari F K, Samulski T, Shenk T, Samulski R J. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrari F K, Xiao X, McCarty D, Samulski R J. New developments in the generation of Ad-free, high-titer rAAV gene therapy vectors. Nat Med. 1997;3:1295–1297. doi: 10.1038/nm1197-1295. [DOI] [PubMed] [Google Scholar]
- 11.Fisher K J, Gao G P, Weitzman M D, DeMatteo R, Burda J F, Wilson J M. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher K J, Jooss K, Alston J, Yang Y, Haecker S E, High K, Pathak R, Raper S E, Wilson J M. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 13.Fisher K J, Kelley W M, Burda J F, Wilson J M. A novel adenovirus-adeno-associated virus hybrid vector that displays efficient rescue and delivery of the AAV genome. Hum Gene Ther. 1996;7:2079–2087. doi: 10.1089/hum.1996.7.17-2079. [DOI] [PubMed] [Google Scholar]
- 14.Flannery J G, Zolotukhin S, Vaquero M I, LaVail M M, Muzyczka N, Hauswirth W W. Efficient photoreceptor-targeted gene expression in vivo by recombinant adeno-associated virus. Proc Natl Acad Sci USA. 1997;94:6916–6921. doi: 10.1073/pnas.94.13.6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flotte T, Carter B, Conrad C, Guggino W, Reynolds T, Rosenstein B, Taylor G, Walden S, Wetzel R. A phase I study of an adeno-associated virus-CFTR gene vector in adult CF patients with mild lung disease. Hum Gene Ther. 1996;7:1145–1159. doi: 10.1089/hum.1996.7.9-1145. [DOI] [PubMed] [Google Scholar]
- 16.Flotte T R, Afione S A, Conrad C, McGrath S A, Solow R, Oka H, Zeitlin P L, Guggino W B, Carter B J. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc Natl Acad Sci USA. 1993;90:10613–10617. doi: 10.1073/pnas.90.22.10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flotte T R, Barraza-Ortiz X, Solow R, Afione S A, Carter B J, Guggino W B. An improved system for packaging recombinant adeno-associated virus vectors capable of in vivo transduction. Gene Ther. 1995;2:29–37. [PubMed] [Google Scholar]
- 18.Flotte T R, Carter B J. Adeno-associated virus vectors for gene therapy. Gene Ther. 1995;2:357–362. [PubMed] [Google Scholar]
- 19.Henry L J, Xia D, Wilke M E, Deisenhofer J, Gerard R D. Characterization of the knob domain of the adenovirus type 5 fiber protein expressed in Escherichia coli. J Virol. 1994;68:5239–5246. doi: 10.1128/jvi.68.8.5239-5246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermonat P L, Muzyczka N. Use of adeno-associated virus as a mammalian DNA cloning vector: transduction of neomycin resistance into mammalian tissue culture cells. Proc Natl Acad Sci USA. 1984;81:6466–6470. doi: 10.1073/pnas.81.20.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herzog R W, Hagstrom J N, Kung S H, Tai S J, Wilson J M, Fisher K J, High K A. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc Natl Acad Sci USA. 1997;94:5804–5809. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunter L A, Samulski R J. Colocalization of adeno-associated virus Rep and capsid proteins in the nuclei of infected cells. J Virol. 1992;66:317–324. doi: 10.1128/jvi.66.1.317-324.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplitt M G, Leone P, Samulski R J, Xiao X, Pfaff D W, O’Malley K L, During M J. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994;8:148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- 24.Kessler P D, Podsakoff G M, Chen X, McQuiston S A, Colosi P C, Matelis L A, Kurtzman G J, Byrne B J. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc Natl Acad Sci USA. 1996;93:14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koeberl D D, Alexander I E, Halbert C L, Russell D W, Miller A D. Persistent expression of human clotting factor IX from mouse liver after intravenous injection of adeno-associated virus vectors. Proc Natl Acad Sci USA. 1997;94:1426–1431. doi: 10.1073/pnas.94.4.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kotin R M. Prospects for the use of adeno-associated virus as a vector for human gene therapy. Hum Gene Ther. 1994;5:793–801. doi: 10.1089/hum.1994.5.7-793. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Samulski R J, Xiao X. Role of highly regulated rep gene expression in adeno-associated virus vector production. J Virol. 1997;71:5236–5243. doi: 10.1128/jvi.71.7.5236-5243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a.Li, J., et al. Unpublished data.
- 28.McCarty D M, Christensen M, Muzyczka N. Sequences required for coordinate induction of adeno-associated virus p19 and p40 promoters by Rep protein. J Virol. 1991;65:2936–2945. doi: 10.1128/jvi.65.6.2936-2945.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCown T J, Xiao X, Li J, Breese G R, Samulski R J. Differential and persistent expression patterns of CNS gene transfer by an adeno-associated virus (AAV) vector. Brain Res. 1996;713:99–107. doi: 10.1016/0006-8993(95)01488-8. [DOI] [PubMed] [Google Scholar]
- 30.McCoy R D, Davidson B L, Roessler B J, Huffnagle G B, Janich S L, Laing T J, Simon R H. Pulmonary inflammation induced by incomplete or inactivated adenoviral particles. Hum Gene Ther. 1995;6:1553–1560. doi: 10.1089/hum.1995.6.12-1553. [DOI] [PubMed] [Google Scholar]
- 31.McLaughlin S K, Collis P, Hermonat P L, Muzyczka N. Adeno-associated virus general transduction vectors: analysis of proviral structures. J Virol. 1988;62:1963–1973. doi: 10.1128/jvi.62.6.1963-1973.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monahan, P. E., J. Tazelaar, X. Xiao, T. C. Nicols, D. A. Bellinger, M. S. Read, C. E. Walsh, and R. J. Samulski. Direct intramuscular injection with recombinant AAV vectors results in sustained expression in a dog model of hemophilia. Gene Ther., in press. [DOI] [PubMed]
- 32a.Monahan, P. E., et al. Unpublished data.
- 33.Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 33a.Muzyczka, N. Personal communication.
- 34.Peel A L, Zolotukhin S, Schrimsher G W, Muzyczka N, Reier P J. Efficient transduction of green fluorescent protein in spinal cord neurons using adeno-associated virus vectors containing cell type-specific promoters. Gene Ther. 1997;4:16–24. doi: 10.1038/sj.gt.3300358. [DOI] [PubMed] [Google Scholar]
- 35.Pereira D J, McCarty D M, Muzyczka N. The adeno-associated virus (AAV) Rep protein acts as both a repressor and an activator to regulate AAV transcription during a productive infection. J Virol. 1997;71:1079–1088. doi: 10.1128/jvi.71.2.1079-1088.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pereira D J, Muzyczka N. The adeno-associated virus type 2 p40 promoter requires a proximal Sp1 interaction and a p19 CArG-like element to facilitate Rep transactivation. J Virol. 1997;71:4300–4309. doi: 10.1128/jvi.71.6.4300-4309.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pereira D J, Muzyczka N. The cellular transcription factor SP1 and an unknown cellular protein are required to mediate Rep protein activation of the adeno-associated virus p19 promoter. J Virol. 1997;71:1747–1756. doi: 10.1128/jvi.71.3.1747-1756.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ponnazhagan S, Mukherjee P, Yoder M C, Wang X S, Zhou S Z, Kaplan J, Wadsworth S, Srivastava A. Adeno-associated virus 2-mediated gene transfer in vivo: organ-tropism and expression of transduced sequences in mice. Gene. 1997;190:203–210. doi: 10.1016/s0378-1119(96)00576-8. [DOI] [PubMed] [Google Scholar]
- 39.Samulski R J, Chang L S, Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J Virol. 1989;63:3822–3822. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snyder R O, Miao C H, Patijn G A, Spratt S K, Danos O, Nagy D, Gown A M, Winther B, Meuse L, Cohen L K, Thompson A R, Kay M A. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet. 1997;16:270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]
- 41.Srivastava A. Parvovirus-based vectors for human gene therapy. Blood Cells. 1994;20:531–536. [PubMed] [Google Scholar]
- 42.Tamayose K, Hirai Y, Shimada T. A new strategy for large-scale preparation of high-titer recombinant adeno-associated virus vectors by using packaging cell lines and sulfonated cellulose column chromatography. Hum Gene Ther. 1996;7:507–513. doi: 10.1089/hum.1996.7.4-507. [DOI] [PubMed] [Google Scholar]
- 43.Vincent K A, Piraino S T, Wadsworth S C. Analysis of recombinant adeno-associated virus packaging and requirements for rep and cap gene products. J Virol. 1997;71:1897–1905. doi: 10.1128/jvi.71.3.1897-1905.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao, X. Unpublished observations.
- 45.Xiao X, Li J, McCown T J, Samulski R J. Gene transfer by adeno-associated virus vectors into the central nervous system. Exp Neurol. 1997;144:113–124. doi: 10.1006/exnr.1996.6396. [DOI] [PubMed] [Google Scholar]
- 46.Xiao X, Li J, Samulski R J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao X, McCown T J, Li J, Breese G R, Morrow A L, Samulski R J. Adeno-associated virus (AAV) vector antisense gene transfer in vivo decreases GABA (A) alpha1 containing receptors and increases inferior collicular seizure sensitivity. Brain Res. 1997;756:76–83. doi: 10.1016/s0006-8993(97)00120-0. [DOI] [PubMed] [Google Scholar]
- 48.Yakobson B, Koch T, Winocour E. Replication of adeno-associated virus in synchronized cells without the addition of a helper virus. J Virol. 1987;61:972–981. doi: 10.1128/jvi.61.4.972-981.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Y, Li Q, Ertl H C, Wilson J M. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Y, Nunes F A, Berencsi K, Furth E E, Gonczol E, Wilson J M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao J, Schmieg F I, Simmons D T, Molloy G R. Mouse p53 represses the rat brain creatine kinase gene but activates the rat muscle creatine kinase gene. Mol Cell Biol. 1994;14:8483–8492. doi: 10.1128/mcb.14.12.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zolotukhm, S. Personal communication.