Abstract
Across the globe, approximately one in 10 babies are born preterm, that is, before 37 weeks of a typical 40 weeks of gestation. Up to 50% of preterm born infants develop brain injury, encephalopathy of prematurity (EoP), that substantially increases their risk for developing lifelong defects in motor skills and domains of learning, memory, emotional regulation, and cognition. We are still severely limited in our abilities to prevent or predict preterm birth. No longer just the “support cells,” we now clearly understand that during development glia are key for building a healthy brain. Glial dysfunction is a hallmark of EoP, notably, microgliosis, astrogliosis, and oligodendrocyte injury. Our knowledge of glial biology during development is exponentially expanding but hasn't developed sufficiently for development of effective neuroregenerative therapies. This review summarizes the current state of knowledge for the roles of glia in infants with EoP and its animal models, and a description of known glial‐cell interactions in the context of EoP, such as the roles for border‐associated macrophages. The field of perinatal medicine is relatively small but has worked passionately to improve our understanding of the etiology of EoP coupled with detailed mechanistic studies of pre‐clinical and human cohorts. A primary finding from this review is that expanding our collaborations with computational biologists, working together to understand the complexity of glial subtypes, glial maturation, and the impacts of EoP in the short and long term will be key to the design of therapies that improve outcomes.
Keywords: astrocytes, cytokine and chemokine receptors, development, growth factor, mechanisms of glia cell injury, microglial cells, oligodendrocytes
Main Points
Encephalopathy of prematurity (EoP) leads to cognitive disorders in millions annually.
There are multiple reciprocal molecular interactions between microglia, border‐associated macrophages, astrocytes, and oligodendrocyte precursors–oligodendrocytes in the genesis of EoP.
1. INTRODUCTION
Glia are a diverse group of non‐neuronal cells in the central nervous system (CNS) that support and modulate neuronal function. Whilst traditionally overlooked as “glue” or “support cells”, an explosion of research has revealed the immense significance of glia in health and disease. Glia include astrocytes, polydendrocytes, oligodendrocytes, and microglia which are all highly interactive with one another and with neurons. These interactions, including providing metabolic support, regulating ion and neurotransmitter balance, participating in synaptic pruning and myelination, and contributing to immune responses and inflammation in the CNS. Altogether these functions underpin complex network formation, neurotransmission, and brain homeostasis—all the functions that make the human brain a vessel for human thought and consciousness. By unraveling the complexities of glial cells, we hope to gain insights into the underlying mechanisms of brain disorders, leading to the development of novel therapeutic strategies and interventions targeting glial dysfunction to improve outcomes for infants with encephalopathy of prematurity (EoP).
1.1. Encephalopathy of prematurity: epidemiology and impact
Preterm infants constitute a large patient group, as 7%–15% of all babies globally are born preterm, at less than 37 of 40 typical weeks of gestation. In the European Union alone, 400,000 babies are born prematurely each year. In 2010, the Global Burden of Disease Study estimated that preterm birth was the most common cause of death and disability in children under the age of 5 years. Preterm birth causes more deaths than malaria or pneumonia, resulting in the loss of 77 million (95% CI of 66–88 million) disability adjusted life years (DALYs) (DALYs & Collaborators, 2016). The emotional cost to affected individuals and their families is immeasurable, but the lifetime cost of care for one child with cerebral palsy is approximately 1.1 million Euros (Kruse et al., 2009).
In well‐equipped healthcare settings, more than 50% of babies born at less than 28 weeks of gestation will survive. However, 30% of the babies born between 28‐ and 32‐weeks of gestation will develop a lifelong disability, including cerebral palsy, impaired cognitive function, and psychiatric disorders, such as attention deficit and autism spectrum disorders (ADHD and ASD) (Pierrat et al., 2017). Even babies born between 32 and 37 weeks (i.e., “late preterm”) are at increased risk of neonatal mortality and morbidity, including increased rates of cerebral palsy and lower cognitive performance (Kajantie et al., 2019).
As seen in many diseases affecting the developing brain, boys are more likely to be affected than girls (Peelen et al., 2016), and have higher rates of preterm birth and poorer outcomes (O'Driscoll et al., 2018). Many genetic, biochemical and structural differences between male and female fetuses convey this altered risk (Rosenkrantz et al., 2019; Varner et al., 2020).
1.2. Encephalopathy of prematurity: neuropathology and imaging in humans
The constellation of brain injuries sustained by preterm‐born infants is called EoP. This term was first coined in 1993 (Lin et al., 1993), to replace the previously used Little's disease to describe disturbances in tone in children born preterm or small for gestational age without an overt acute clinical neurological illness in early life. Based on post‐mortem and magnetic resonance imaging (MRI) studies, we define the key hallmarks of EoP as gliosis (increased cell number and altered morphology), white matter injury (WMI) linked to oligodendrocyte maturation arrest and delayed myelination, dysmaturation of some interneuron subsets, abnormal cortical microstructure, and reduced gyrification index. Over time, the proportion of infants developing necrotic foci (focal oligodendrocyte death) and axonopathy (swollen axon terminals, engorged varicosities with neurotransmitter granules, and enlarged axons full of highly phosphorylated neurofilaments) has substantially reduced but these more severe outcomes are still observed in some infants (Buser et al., 2012).
Our understanding of the neuropathology in EoP has been driven by research of human post‐mortem cohorts including from France, the United States, and the United Kingdom. OF note, studies were undertaken in France in very preterm born infants (25–29 postconceptional weeks, pcw) (Verney et al., 2012) that compared neuropathology in preterm infants with and without WMI. In infants with diffuse WMI, microglia had reduced morphological complexity (assumed to reflect immune activation) and were increased in number (IBA1), there was increased phagocytosis (CD68 positive cell number), and decreased astrogliosis (GFAP and MCT1 staining). However, in the few small focal necrotic lesions present there were no astrocyte changes. The authors reported no change in the numbers of oligodendrocyte lineage cells (Olig2) between preterm infants with or without WMI (diffuse or focal necrotic) (Verney et al., 2012). These findings supported a study from a cohort of late preterm‐born US infants in which, similarly, white matter was injured without the loss of oligodendrocytes (Billiards et al., 2008). However, oligodendrocyte cell death has been reported in cases of late preterm birth from a separate US cohort (Back, Luo, et al., 2005). A series of neuropathological studies in the United States also found significant white matter necrosis around the ventricles in infants who were diagnosed with periventricular leukomalacia (PVL, a severe, cystic form of WMI) (25 pcw‐term) (Haynes et al., 2008; Haynes & van Leyen, 2013; Kinney et al., 2012; Ligam et al., 2009). Of note, the incidence of cystic WMI (PVL) and cerebral palsy has dramatically decreased over the last decades in most high resource health care settings (Hamrick et al., 2004; Smithers‐Sheedy et al., 2022), thanks to the improved perinatal care including transfer of women about to deliver preterm to tertiary (high) level care hospitals, optimization of the nature of prenatal administration of steroids to promote lung maturation in a way that doesn't negatively impact brain development, less invasive ventilation methods, approaches to limit painful procedure exposure and parental nutrition.
Microgliosis (increased number and more amoeboid morphology) is a hallmark of EoP across post‐mortem studies (Billiards et al., 2008; Haynes et al., 2008; Haynes & van Leyen, 2013; Kinney et al., 2012; Ligam et al., 2009; Verney et al., 2012; Verney, Monier, et al., 2010). Microglia in preterm infants have been shown to increase expression of the pro‐inflammatory associated markers inducible nitric oxide synthase (iNOS) and the NMDA receptor (Verney, Monier, et al., 2010). Astrogliosis (increased number and area coverage) is reported in infants of later gestational ages (Buser et al., 2012; Haynes et al., 2003) in agreement with the peak of astrocyte development post 26 gestational weeks. However, it is also suggested that astrogliosis is more prominent in cases with severe injury or where injury has been present for some time (Back, Luo, et al., 2005). An ongoing confounder in studies of preterm‐born infants is that the timing of brain change onset is unknown, although evidence suggests there are structural and biochemical changes well before birth (Cook et al., 2019; Denney et al., 2021; Hornaday et al., 2022; Jelliffe‐Pawlowski et al., 2018; Story et al., 2021). In preterm‐born infants with cystic WMI from US cohorts, astrocytes have been reported to increase expression of the pro‐inflammatory marker nitrotyrosine (Haynes et al., 2003) and expression of the glutamate transporter EAAT (Desilva et al., 2008). Astrocyte cell body and the end feet coverage of blood vessels have also been investigated in a cohort of infants from the United States (El‐Khoury et al., 2006). This study demonstrated that with increasing gestational age (from 16 to 40 weeks), astrocyte cell body and end feet coverage increased across the brain. However, in infants born preterm GFAP‐positive end feet coverage was lower in the germinal matrix compared to the cortex and white matter, perhaps contributing to vulnerability of the blood vessels of the preterm born infant germinal matrix to hemorrhage.
Post‐mortem studies of infants (24–29 postconceptional weeks, pcw) diagnosed with cystic WMI who were born in the UK report fewer neurons in the thalamus (Vontell et al., 2013). Similarly, in studies of neuropathology on infants diagnosed with cystic WMI who were late preterm (32 pcw‐term) who died in the United States, there were fewer neurons in the thalamus and cerebral cortex together with astrogliosis and microgliosis (Andiman et al., 2010; Ligam et al., 2009; Pierson et al., 2007). Obvious neuronal injury has not been reported in cases of diffuse WMI (Back, Luo, et al., 2005; Pierson et al., 2007). However, axonopathy is reported in diffuse WMI cases (Back, Tuohy, et al., 2005; Buser et al., 2012; Verney et al., 2012) and in cases with cystic WMI (Ligam et al., 2009). However, changes in the distribution of interneuron subtypes are reported in post‐mortem studies, including in the cohort of English infants born very premature with diffuse WMI (Stolp et al., 2019), and in two cohorts of US‐born infants (Lacaille et al., 2021; Panda et al., 2018). Gene studies strongly support these cell number and distribution changes in a large cohort of infants, wherein preterm birth altered the maturation index of the GABAergic system, based on expression of 14 genes (Lacaille et al., 2021). Of particular interest, the maturation index was affected in male, but not female preterm infants.
Human studies are immensely informative but must be interpreted through several lenses: some countries/cultures/specific hospitals redirect care towards palliative support for infants sooner or at a less severe stage of injury (note above, France versus the United States). These practices impact the severity and evolution of brain injury. In all cases there are significant delays in post‐mortem tissue collection, therefore impacting markers (for instance) of cell death and autophagy. Also, the delay between preterm birth and death can be variable, potentially influencing neuropathological findings. However, most of the reported cases have a relatively short duration of post‐natal life as most deaths of very preterm neonates are linked to withdrawal of care that is most often decided in the first days. The limited number of reported cases with a longer survival does not allow us to draw any valid conclusion about the impact on neuropathological findings. It is also essential to consider that it is the most injured infants who die, impacting our understanding of the mechanism of injury for less severely affected infants.
Imaging studies, whilst arguably less informative about the mechanisms of injury, overcome the substantial limitations on data from post‐mortem studies. Imaging studies provide access to the brain in less severely injured infants at multiple time points and in a high proportion of infants. Magnetic resonance imaging (MRI), magnetic resonance spectroscopy (MRS), and ultrasound (US) are routinely applied to understand injury and predict outcomes, and these are thoroughly reviewed elsewhere (Dudink et al., 2020; Inder et al., 2021). MRI, although more expensive and technically challenging than ultrasound, is better at identifying patterns of grey and WMI (Kwon et al., 2014) and allows the quantitative assessment of the integrity, growth, and connectivity of the brain (Doria et al., 2014). However, it is worth noting the development of in‐unit MRI technology will increase the accessibility and frequency of use of this technology (Cawley et al., 2022). Neonatal MRI has uncovered critical impacts of preterm birth, such as reduced cortical development (surface area and less complex structure) (Ajayi‐Obe et al., 2000) and functional consequences such as disrupted network integration (Gilchrist et al., 2022), changes that last well into adulthood (Nosarti et al., 2014). The severity of injury at term equivalent, as identified by MRI, is predictive of adverse outcomes at two years of age (Woodward et al., 2006).
Magnetic resonance spectroscopy measures biochemical changes that can be interpreted as indicators of brain injury or changes in brain maturation. For example, proton (1H) MRS can detect increases in N‐acetyl aspartate (NAA) synthesized in neurons or axonal mitochondria over time during development reflecting increased oligodendrocyte proliferation and differentiation. Conversely, on 1H MRS choline (Cho) and lactate decrease with development, reflecting membrane turnover and maturation of enzyme systems in the brain, respectively (Robertson & Cox, 2001). MRS indices have been shown to correlate with poor outcomes in preterm‐born infants (Gire et al., 2022; Hyodo et al., 2018), especially N‐NAA/Cho ratios, reviewed in (Cebeci et al., 2022). However, the voxel size of MRS is approximately three times larger compared to MRI, thus reducing the specificity of the analysis. As such the specific biological function of these metabolites may be less critical than their predictive utility, as when we work such large voxels the metabolites will have been produced by multiple different cells, so it is difficult to interpret the data. MRS changes, like MRI, persist in infants born preterm, with changes in MRS at 3 years old in prematurely born children associated with executive function deficits (Schnider et al., 2020).
An imaging modality with the potential to shine a light on the glial response to injury is positron emission tomography (PET), especially via the use of the astrocyte and microglia‐associated ligand translocator protein (TSPO) and the microglial ionotropic purinoceptor P2X7. The development of third‐generation TSPO tracers and first‐generation P2X7 tracers as potential diagnostic tools are reviewed here (Di Virgilio et al., 2023; Singh et al., 2023). However, in the context of EoP and despite PET ligands as a valuable technique in animal models, concerns about radiation exposure are still significant inhibitors in the development of neonatal‐focused PET imaging. We expect that as the value of PET tracer studies is demonstrated in adults in the coming years, they will find a place in guiding the delivery of long‐lasting immune‐modulatory “tertiary phase treatments” to improve outcomes after EoP (Fleiss et al., 2012).
1.3. Encephalopathy of prematurity: etiology
Histological chorioamnionitis, defined as acute inflammation (including neutrophil infiltration) of the amnion and chorion (Kim et al., 2015), is the primary factor associated with preterm delivery outside the context of medically indicated preterm deliveries (Maisonneuve et al., 2020; Palmsten et al., 2018). Over 40% of infants born spontaneously before 32 weeks of gestation are exposed to histological chorioamnionitis (Bierstone et al., 2018; Maisonneuve et al., 2020). This relationship is even more pronounced in extremely preterm infants with up to 70% of infants born at 22–25 pcw, the threshold of viability, exposed to histological chorioamnionitis (Maisonneuve et al., 2020). Multiple cohort studies have indicated a robust link between chorioamnionitis and an increased risk of cystic WMI and cerebral palsy (Kaukola et al., 2006; Tsamantioti et al., 2022; Venkatesh et al., 2020), supported further by similar findings from multiple systematic reviews of the field over time and location (Maisonneuve et al., 2020; Shi et al., 2017; Tsamantioti et al., 2022; Venkatesh et al., 2020). However, it is worth noting that this association between chorioamnionitis and poor outcomes hasn't always been reported (Shi et al., 2017), possibly due to differences in definitions and neurological follow‐up. Interestingly, preterm birth itself is a risk factor for the development of autism spectrum disorder, and the presence of chorioamnionitis further increases the risk of autism spectrum disorder by up to a factor of 17 (Moster et al., 2008).
Evidence for the presence of hypoxic‐ischemic (HI) injury in preterm‐born infants is more difficult to characterize, as the main diagnostic tool, the Sarnat score (a combined electroencephalogram [EEG] and neurologic exam) is agnostic to the cause of encephalopathy. For instance, EEG changes (including burst suppression) are reported in term infants diagnosed with HIE (Iyer et al., 2014), and preterm born infants exposed to perinatal inflammation (Helderman et al., 2010). In addition, a recent study of the Sarnat score found poor agreement even between well‐trained operators in infants below 32 weeks of gestation and stated that “further research into the development of a standardized, gestational age‐specific, assessment tool for classification of HIE in (preterm born) infants is needed” (Pavageau et al., 2020). Further complicating the issue of characterizing HI in preterm‐born infants is the poor predictive ability of blood gases. Specifically, historical observations of blood gasses and outcome, discussed in (Bobrow & Soothill, 1999), suggest that fetal acidosis is a poor predictor of outcome and that possibly the infant is remarkably resistant to acidosis compared to the adult. Using the Sarnat score, moderate‐to‐severe HI was diagnosed in 3.7% of preterm infants in a US cohort collected from 2008 to 2011 (Galinsky et al., 2018; Manuck et al., 2016). Other studies using approaches, including the Sarnat and blood gasses, have reported lower rates, between 0.6% and 0.8%, of preterm born infants (Chalak et al., 2012; Pavageau et al., 2020).
Experimental and epidemiological evidence suggests that the factor linking chorioamnionitis and poor neurological outcomes is neuroinflammation driven by systemic inflammation (Favrais et al., 2011; Hagberg et al., 2015; Kelly et al., 2021; Krishnan et al., 2017; Paton et al., 2019; Schmidt et al., 2016; Shiow et al., 2017; Van Steenwinckel et al., 2019; Verney et al., 2012). The fetal and infant immune responses are complex (Menon et al., 2009), and many studies link elevated levels of immune markers to poor outcomes (Hornaday et al., 2022). It is also possible now to take complex multi‐marker approaches to screening for predictive or risk biomarkers (Aung et al., 2019; Cordeiro et al., 2016; Leviton et al., 2015) that we hope will provide more understanding of systemic inflammation and outcomes (O'Shea et al., 2012). It is clear though, that although no one inflammatory marker is the “key” and that intermittent or sustained systemic inflammation is more detrimental to the brain than inflammation of shorter (Kuban et al., 2017), reviewed in (Humberg et al., 2020). Other conditions before or after delivery of the preterm infant may induce or worsen systemic inflammation. These conditions include hypoxic‐ischemic events, mechanical ventilation (which induces pulmonary and systemic inflammation) and leads to brain injury (Allison et al., 2019; Bose et al., 2013), neonatal sepsis, or necrotizing enterocolitis (NEC) (Pierrat et al., 2017), which typically occurs a few days after birth but causes significant systemic inflammation.
1.4. Animal models of EoP
An unavoidable and ongoing friction in the field of EoP is the debate about the factors (environmental, genetic, and social) that precipitate the onset of EoP, including what is necessary or sufficient. This knowledge is central to designing models with the greatest translational value. In high‐resource settings, substantial improvements in antenatal and perinatal care have reduced the overall severity of brain injury (Jahan et al., 2021; Smithers‐Sheedy et al., 2022). We have also improved our ability to monitor and interpret the biochemical events occurring in utero and perinatally (near‐infrared spectroscopy, fetal heart rate, MRS, ultrafast Doppler). These monitoring approaches and information from animal models have shifted the etiological paradigm from “HI only” towards the concept of “HI + inflammation” and now towards an increasingly “inflammation‐focused” etiology for most infants with EoP (Gilles et al., 2017). Thus, it could be argued that all parties now agree that neuroinflammation is necessary to damage the brain in a way that directly leads to EoP. However, whether those complex neuroinflammatory events were initiated by HI and in what infants is still a matter of ongoing debate. Another key consideration is the contribution from genes and environmental factors that may act as drivers of risk alone or that may sensitize to a precipitating factor but not cause injury alone. The potential for a more complex model of causal attribution is illustrated by an increasing number of studies linking genetic factors to more severe injury profiles in infants diagnosed with neonatal encephalopathy (NE) or cerebral palsy (Calkavur et al., 2011; Varner et al., 2020), and reviewed in (McIntyre et al., 2021). Historically, injury in these infants was described as being caused by birth asphyxia alone, with significant medico‐legal implications.
Outlined in Table 1 are a collection of approaches for modeling EoP, to highlight the diversity in species and etiological modeling undertaken leading to similar phenotypes. Preterm‐born infants are also at increased risk of other forms of brain injury including germinal matrix or intraventricular hemorrhage (Twilhaar et al., 2018). Although these are separate clinical entities from EoP, studies of preclinical models and infants post‐mortem also show that microgliosis, astrogliosis and oligodendrocyte injury are also common in these insults (Chamnanvanakij et al., 2002; Jinnai et al., 2020; Romantsik et al., 2022; Truttmann et al., 2020). It is worth noting that the gestational length in animals used to model EoP and the proportion of brain development occurring in utero varies greatly. For example, sheep gestation is approximately 144 days, and arguably the brain at term in the sheep is equivalent to a 2‐week‐old term‐born human infant (Back et al., 2006). In contrast, the mouse has a gestation of approximately 20 days and gives birth to offspring with brain development roughly equivalent to a 23‐week gestation human (Craig et al., 2003). These comparisons are primarily made based on neuronal maturation and migration patterns, and oligodendrocyte differentiation and myelination. A benefit of working with altricial rodents is that it is possible to injure the brain where there is a functional gut and lung, removing the variables of oxygen or other nutrient supply in understanding brain injury.
TABLE 1.
A selected list of models of EoP to demonstrate the breadth of species and insult being explored and finding changes in glia.
| Insult | Human cohort Eq. | Approx. human age Eq. | Species | Age at injury | Microgliosis | Astrogliosis | WMI | Oligo. dysmat‐uration | Oligo death or loss | Axonapathy | Interneuronopathy | Altered neuronal microstructure | Reduced gyrification | Behavioural defects | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Excitotoxicity | Hypoxia, ischemia, excessive inflammation | 32wGA | Mouse | P5 | Yes | Yes | Yes (focal) | ? | Yes | Yes | Yes | ? | NA | No | (Dommergues et al., 2000; Marret et al., 1995) |
| Hyperoxia | Suboptimal ventilation | 28‐38wGA | Mouse | P3‐5, P6‐8 | Yes | Yes | Yes | Yes | Yes | ? | ? | Yes | NA | Yes | (Brehmer et al., 2012; Ritter et al., 2013; Schmitz et al., 2011) |
| Hypoxia ischemia (prenatal) | Placental abruption, HIE | 28wGA | Sheep | 105dGA (150d = term) | Yes | Yes | Yes (diffuse and focal | Yes | Yes | Yes | Yes | Yes | Yes | Yes | (Davidson et al., 2021; Gussenhoven et al., 2021; Jisa et al., 2018; Li et al., 2016) |
| Hypoxia Ischemia (postnatal) | Placental abruption, HIE | 24‐37wGA |
Mouse Rat |
P2‐5 P2‐7 |
Yes | Yes | Yes (focal) | No | Yes | Yes | Yes | Yes | NA | Yes | (Albertsson et al., 2014; McQuillen et al., 2003) |
| Chronic hypoxia (postnatal) | Poor ventilation, poor lung function (BPD) | 32‐36wGA |
Mouse Ferret |
P6‐8 P10‐20 |
Yes | Yes | Yes (decreased WM volumes) | Yes | No | Yes | ? | Yes | Yes | ? | (Jablonska et al., 2012; Scafidi et al., 2014; Tao et al., 2012) |
| Intermittent hypoxia | Apnea of prematurity | 24‐40wGA |
Mouse Rat |
P2‐10 P2‐P14 |
Yes | Yes | Yes (diffuse) | Yes | No | Yes | ? ( inflamm‐ation) | Yes | NA | Yes | (Cai et al., 2012; Darnall et al., 2017; Juliano et al., 2015) |
| Prematurity itself and ventilation | Preterm ventilated | 28wGA | Baboon | 125dGA (180d = term) | NA | Yes | Yes (decreased WM volume) | NA | NA | NA | Yes | NA | Yes | NA | (Loeliger et al., 2006; Verney, Rees, et al., 2010) |
| Reactive astrogliosis (organotypic brain slice) | Chorio‐amnionitis, sepsis | 23wGA onwards |
Mouse Rat |
P1 | Yes | Yes | Yes (diffuse) | Yes | No | ? | ? | ? | NA | NA | (Dean et al., 2011; Miron et al., 2010) |
| Systemic inflammatory exposure (intraamniotic) | Chorio‐amnionitis | 30‐35wGA | Sheep |
110‐125dGA (150d = term) |
Yes | Yes | Yes (diffuse + small foci) | Yes | No (except longest exp.) | ? | ? | Yes | Yes | Yes | (Gussenhoven et al., 2018; Kuypers et al., 2013; Ophelders et al., 2016) |
| Systemic inflammatory exposure (prenatal intravenous) | Chorio‐amnionitis, sepsis | 28wGA | Sheep |
105d GA (150d = term) |
Yes | Yes | Yes (diffuse + range of focal lesions) | Yes | Not consistently | Yes | Yes | Yes | ? | Yes | (Galinsky, Dhillon, et al., 2020; Yawno et al., 2013) |
| Systemic inflammatory exposure (postnatal) | Chorio‐amnionitis |
23‐32wGA 28‐40wGA |
Mouse |
P1‐P5 P3‐P11 |
Yes | Yes | Yes (diffuse) |
Yes |
No | Yes | Yes | Yes | NA | Yes | (Du et al., 2011; Favrais et al., 2011; Schang et al., 2014) |
| Systemic inflammatory exposure (intrauterine) plus postnatal hypoxia | Chorio‐amnionitis and perinatal hypoxia | 20wGA + 30wGA | Rat |
E18 (LPS) P4 (Hypoxia) |
Yes | Yes | Yes (diffuse) | Yes | No | No | Yes | Yes | Yes | Yes | (Vaes et al., 2020; Vaes et al., 2021) |
Note: Shaded areas correspoind to “yes”. Non shaded areas correspond to “no” or “NA”.
Abbreviations: “?” unknown; BPD, bronchopulmonary dysplasia; Chorio, chorioamnionitis; Eq., equivalent; HI, hypoxia ischemia; HIE, hypoxic ischemic encephalopathy; LPS, lipopolysaccharide; NA, not applicable; P, postnatal day; SI, systemic inflammation; wGA, weeks gestational age.
Although many studies into EoP are undertaken in vivo, another valuable approach in modeling EoP is ex vivo slice culture. This approach, which can be applied across diverse species, such as ferret, rabbit and mouse (Miron et al., 2010; Vinukonda et al., 2012; Wood et al., 2022) has the benefits of maintaining tissue architecture and cell‐cell interactions and progression of oligodendrocyte maturation and myelination, but in a situation where these outcome measures can be assessed longitudinally. In addition, these approaches allow for the study of drugs and other tools that are too high risk to test in vivo safety but are of interest for understanding the fundamental biology of injury mechanisms. Work with this approach shows that the creation of the slice itself leads to oligodendrocyte maturation arrest and hypomyelination linked to astrogliosis and microgliosis (Holloway et al., 2021). The authors used the complex cell‐cell interactions of the model to characterize the damaging impact of astrocyte‐derived hyaluronan on oligodendrocyte maturation (Dean et al., 2011). Slice cultures have also revealed the specific impacts of the immune response to blood, critical for understanding the pathophysiology of intraventricular hemorrhage (Vinukonda et al., 2012), including that gliosis is driven by plasma factors more than the presence of red blood cells.
Another approach, that has yet to gain significant traction in perinatal medicine, is the use of patient‐specific induced pluripotent stem cell (iPSC) derived brain organoids to study the impacts of genetics and insults. We predict that using patient‐derived iPSCs to grow brain organoids could uncover key links between genetic variants and environmental factors in altering brain structure from the start of development. The approach has already proven its value by providing deep insights into the mechanisms of basic brain development (Qian et al., 2020; Sabate‐Soler et al., 2022) and disorders such as Parkinson's and Alzheimer's disease (Huang et al., 2022; Jarazo et al., 2022).
It is impossible (although attractive) to advocate for the best model for EoP—as the model needs to match the specific patient group and we know that many variables impact outcomes. For example, if you wish to understand how to improve outcomes for infants with asphyxia, such as linked to placental abruption, then an in‐utero asphyxia (or an ex‐utero HI) model would be ideal. However, if you want to understand injury occurring in the large proportion of infants exposed to inflammation, then a model applying an immune activator is optimal. However, the timing and severity of this also must be carefully tuned to the population of interest. For example, to understand processes occurring in the context of very preterm birth, with “moderate/subclinical” chorioamnionitis but a relatively uncomplicated post‐natal course then perhaps a model of exposure to systemic inflammation alone (Du et al., 2011; Favrais et al., 2011) would be ideal. In contrast, to study the impacts of fulminant chorioamnionitis and birth with severe lung injury, a combination model of inflammation and hypoxia may be the perfect option (Vaes et al., 2020). We also know that the “best model” can be one with less complexity, allowing for the specific contributions of specific variables to be explored and understood. With these “reductionist approaches” a great deal of essential knowledge has been gained especially at the start of the translational pipeline for screening time and dose responses, reviewed in (Ramanantsoa et al., 2013). An example is the induction of a focal cortical injury with an excitotoxic injection. This model, which is easy to perform, has high throughput and low complexity has been valuable for screening drugs, especially dose and timing studies (Haldipur et al., 2014) that are difficult in more complex models and although reductionist allows for the specific impacts of between focal CNS injury without interaction with the systemic immune response (Blaise et al., 2017). Finally, some models are reducing in relevance in high‐resource settings, such as the hyperoxia model. However, hyperoxia remains an ongoing problem in low‐resource settings, especially with the development of relatively affordable oxygen concentrator technology (Ng et al., 2022).
2. GLIAL CELLS AND MECHANISMS
2.1. Microglial origins in rodents and humans
Microglia are the macrophages of the brain parenchyma and the predominant cells driving the immune response in the CNS. Unlike most tissue‐resident macrophages, microglia are yolk sac (YS)‐derived without a monocyte intermediate originating from classic hematopoietic stem cells (Sheng et al., 2015). Instead, microglia originate from c‐Kitlo CD41lo progenitors emerging from the YS around embryonic day 7.25 (E7.25) in the mouse (Ginhoux et al., 2010). Specifically, these progenitors are named early erythroid myeloid progenitors (eEMPs). Microglia derived from eEMP are detectable in brain parenchyma in mice at E8.5 (Ginhoux et al., 2010; Kierdorf et al., 2013) and in humans between the 4 and 5th postconceptional week; before the onset of substantial neurogenesis (Billiards et al., 2006; Menassa et al., 2022; Verney, Monier, et al., 2010). In the human fetal brain, microglia penetrate the brain parenchyma at 4.5–5.5 WG (Verney, Monier, et al., 2010) via the choroid plexus, meninges, and ventricles. The microglia then form clusters during development (i) between the subplate and cortical plate (10–12 WG) where the first synapses are detected (Monier et al., 2007), (ii) in the corpus callosum (16 WG), and (iii) around the anterior horn of the lateral ventricle, the site of major axonal crossroads (19–30 WG) (Ashwell, 1991; Verney et al., 2012; Verney, Monier, et al., 2010). These specific brain routes of entries and locations are similar in mice. Microglia arise from the bloodstream, the ventricular space or the meninges and have demonstrated the same stepwise colonization and proliferation pattern across species (Ginhoux et al., 2013).
Brain colonization by YS‐derived microglia continues until the blood‐brain barrier is substantially formed at E15.5 in mice (Ben‐Zvi et al., 2014). Microglia penetrate the brain parenchyma in the human fetus at 4.5–5.5 WG (Verney, Monier, et al., 2010) via the choroid plexus, meninges, and ventricles. In human, the BBB becomes functional as early as 12 weeks' gestation (Grontoft, 1954) and the relationship between microglial invasion and the BBB is less clear than in the mouse. During early stages of development, microglia are identifiable by their amoeboid shape, remarkable capacity to proliferate, and specific patterns of gene expression, reflective of their roles in brain building. This contrasts with adult microglia with their roles in homeostasis and complex morphology (Ginhoux et al., 2010; Kierdorf et al., 2013). In the human infant, proliferation peaks in the subplate and surrounding areas by 15–20 pcw, with cells observed to begin migrating into the brain (Menassa et al., 2022). There is an accumulation of amoeboid proliferating microglia in and around the developing white matter in premature neonates, which can largely explain the vulnerability of the white matter in these neonates (Monier et al., 2007; Monier et al., 2006). Marked microglial reactivity in white matter is one of the characteristics classically observed in human EoP (Buser et al., 2012; Haynes et al., 2003; Verney et al., 2012). Figure 1 is a summary of the roles of glia in development and EoP.
FIGURE 1.
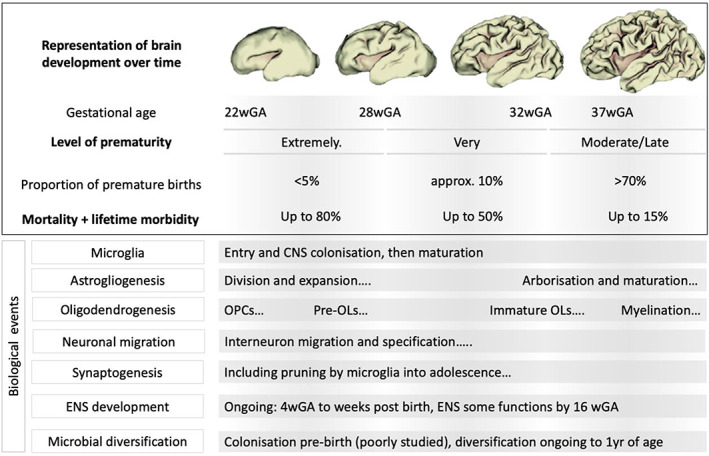
A representation of brain development across stages of gestation explaining the severity of prematurity, outcomes and then the events occurring across development that may be impacted by the events leading to encephalopathy of prematurity (EoP) and EoP itself.
Whilst overall proliferation appears to peak at 6 months of age in the human, total microglia cell number continues to increase and peaks at 1.5 years of age—possibly reflecting the important roles not only in early brain building, but also in sculping the developing connectome into early childhood (Askew et al., 2017). In the mouse, microglia number increases steadily during the first two postnatal weeks (Alliot et al., 1999) to reach a density similar to that of the adult brain. The first 2 weeks in the mouse is comparable to 23 pcw to 3 months of age in the human (Chen et al., 2017). Across species microglia eventually tile the entire brain via ramified processes, surveying the parenchyma to maintain homeostasis (neuromodulation, synaptic homeostasis and plasticity, phagocytosis of apoptotic neurons and debris, etc.) (Li & Barres, 2018).
2.2. Sex differences in microglial organization and phenotype
Sex differences in microglia begin with dimorphisms in the colonization of the developing brain with more microglia in the male brain early in development in the mouse (Schwarz et al., 2012) and microglia then act to sculpt the brain in sex dependent ways (Bordt et al., 2020; Lenz & McCarthy, 2015), impacting behavior (Smith & Bilbo, 2019). Sex differences in the adult are postulated to underpin differences in many neurological and neurodegenerative disorders (Chen et al., 2021; Kodama & Gan, 2019; Ugidos et al., 2022). Not only does the transcriptome vary by sex, but there are increased microglial densities and soma sizes in the adult mouse male hippocampus, cortex and amygdala, compared to the female brain (Acaz‐Fonseca et al., 2015). However, transcriptomics analysis suggests that in the embryonic mouse there are fewer differences between male and female microglia, but that dimorphism increases over time (Hammond et al., 2019; Hanamsagar et al., 2018; Villa et al., 2018). In the pig, sexual dimorphism is also reported in late gestation by Antonson et al. (2019). A microglia developmental index (Tay et al., 2019; Tay et al., 2017; Tremblay et al., 2010) suggests that from E18 male mouse microglia are developmentally delayed compared to their female counterparts and further work indicates this difference might include that female microglia are skewed toward an anti‐inflammatory response (Hammond et al., 2019). Microglia express receptors for both estrogen and testosterone (Acaz‐Fonseca et al., 2015) and estrogen is reported to induce a male microglial phenotype in P2, P5, and P8 female mice, an effect which persisted into adulthood (Villa et al., 2018). Interestingly the transcriptomic differences between male and female adult microglia are maintained following transplantation into the brain of mice of the opposite sex (Villa et al., 2018). There have been two studies of prenatal human microglia that assessed sexual dimorphism and found none: Kracht et al. (2020) analyzing microglia from 9 to 18 weeks of gestation (23 samples, scRNAseq) and Thion et al. (2018) from 14 to 23 weeks of gestation (10 samples, bulk RNAseq). Based on the findings from rodents, where microglia are less sexually dimorphic at younger ages, whether there is sexual dimorphism in the brain of preterm born infants (22–37 weeks gestation) will require analysis of additional samples.
2.3. Microglial reactivity and states
From the earliest stages of colonization, microglia sense the micro‐environment of the brain. Their sensing functions are primarily driven by a diverse battery of receptors to detect cytokines/chemokines, damage‐associated molecular patterns, and pathogen‐associated molecular patterns (Smolders et al., 2019). Activation of the pathways downstream of these receptors may trigger the conversion of “homeostatic” microglia into “reactive” states. There are arguably as many types of reactive microglia as there are types of brain injuries, diseases, and types of people, with their unique genetic end environmentally driven characteristics. The M1 versus M2 nomenclature emerged in the early 2000s to refer to the inflammatory versus pro‐resolution/alternatively reactive microglial phenotypes and was based on the terminology used by immunologists to describe lymphocyte reactivity states. The validity of this binary for microglial has been clearly and rightly debunked as microglial reactivity is a complex dynamic process regulated both temporally and spatially and is dependent on the specific nature of the brain injury (Chhor et al., 2017; Hammond et al., 2019; Hellstrom Erkenstam et al., 2016). Nevertheless, a nomenclature to simply and clearly define and describe the reactive states of microglia is a requirement if we are to communicate our findings linking microglia development, disease, and function. The current recommendation for how to describe microglia includes using multiple markers including protein, gene and morphological/ultrastructural markers to classify the microglial state. Then, to clearly define the state relative to the nature of the insult, species, age, sex, and spatial location (Paolicelli et al., 2022). Subtypes of microglia that are well established in the literature include dark microglia, linked to their color on electron microscopy imaging (Bisht et al., 2016), disease‐associated microglia (DAM), and senescent microglia which are less “functionally active” but the markers characterizing this are still being debated (Ng et al., 2023). All of these different states add to the kaleidoscope of roles and (dys)function of microglia that need to be considered to understand the development of EoP and its long‐term impacts (Fleiss & Gressens, 2012). If we think finding a consensus to describe microglia in our relatively well‐controlled pre‐clinical models is complicated, then defining microglia in our post‐mortem studies is even more problematic. Even studies with access to the largest preterm human cohorts contain samples across gestational age, survival time, genetics, and prenatal and postnatal course and thus are unique. As such, circumspection about the generalizability of any findings will be best practice, allowing us to find commonalities between studies over time.
2.4. Microglial reactivity in models of EoP
Studies have established that exposure to inflammatory challenges such as those associated with chorioamnionitis, sepsis and lung injury activates microglia to an “immune‐responsive” state and that this microglial response is necessary to lead to oligodendrocyte injury and hypomyelination (Dommergues et al., 2003; Van Steenwinckel et al., 2019). “Immune responsive” here is used to describe changes in morphology to a more amoeboid shape, increased number of cells and up‐regulation of the expression of markers of a pro‐inflammatory function, such as inducible nitric oxide synthase (iNOS), cyclo‐oxygenase‐2 (COX2), interleukin (IL)‐6, and tumor necrosis factor‐alpha (TNF‐α) (Chhor et al., 2013; Dean et al., 2010) and down‐regulation of markers associated with a repair or regeneration state, such as insulin growth factor IGF‐1 (Van Steenwinckel et al., 2019). Similar to findings in immune activation models of EoP, in a model of hypoxic‐ischemic EoP microglial reactivity and recruitment in the white matter correlated with a decrease in the number of oligodendrocyte progenitors (Falahati et al., 2013). Across models of the different facets of EoP not only is there an immediate and robust immune‐reactivity, but reactivity can persist into young adulthood (Galinsky, van de Looij, et al., 2020; Gussenhoven et al., 2018; Jinnai et al., 2020; Morin et al., 2022; Romantsik et al., 2022; Snyder et al., 2018; Vaes et al., 2021). Transcriptomics analyses have shown that microglia involved in immune‐reactive or inflammatory processes disengage from their developmental functions (Krishnan et al., 2017; Matcovitch‐Natan et al., 2016). The specific impact of the loss of developmental functions is only just beginning to be explored but is likely to be responsible for some of the negative impacts of microglia immune activation on brain development. Male sex is associated with poorer outcomes for infants born preterm, and sex alters the responses to injury in models of EoP (Barkhuizen et al., 2019; Favrais et al., 2011; Le Dieu‐Lugon et al., 2020; Mayoral et al., 2009) and across models of perinatal brain injuries in diverse species (Charriaut‐Marlangue et al., 2017; Fleiss et al., 2011; Fleiss et al., 2012). Underpowered studies have made it challenging to uncover sexual dimorphism, however (Ankeny et al., 2023).
Exposure of animals to lipopolysaccharide (LPS), a component of the cell wall of gram‐negative bacteria, is one way to model EoP. Depending on the dose and timing of LPS in these models injury includes white matter lesions driven by oligodendrocyte dysmaturation (Dean et al., 2009; van de Looij et al., 2012) or cell death including the loss of O4+ immature and O1+ mature oligodendrocyte immunoreactivity and hypomyelination (Galinsky, Dhillon, et al., 2020; Paton et al., 2019; Rousset et al., 2006; Snyder et al., 2018). This WMI is consistently accompanied by substantial microglial reactivity, with cells becoming less ramified in morphology and expressing high levels of the pro‐inflammatory markers IL‐1β, iNOS, and TNF‐α (Fan et al., 2005; Galinsky et al., 2018). LPS activates microglial TLR4 and CD14 receptors and induces the release of reactive oxygen species which at a high enough dose of LPS is sufficient to cause pre‐oligodendrocyte death (Lehnardt et al., 2002) leading to diffuse and cystic WMI (Garnier et al., 2006; Pang et al., 2003). The pro‐inflammatory cytokines IL‐1β and TNF‐α, released by immune reactive microglia, can also directly inhibit the proliferation and maturation of oligodendrocyte precursors (OPCs) (Taylor et al., 2010; Xie et al., 2016). Interestingly, in “two‐hit models” of perinatal brain injury, including EoP, a relationship exists between timing and dose of exposure to LPS and its impact on the brain. For instance, exposure to LPS before a “second hit” (further inflammation or HI) is protective in some paradigms but in contrast is LPS sensitizing to the severity of a second hit in other paradigms (Brehmer et al., 2012; Holloway et al., 2021; Lu et al., 2023; Mathai et al., 2013). This complex time and LPS dose relationship is reviewed by Galinsky et al. (2018).
2.5. Microglia identity, heterogeneity, and developmental functions
The population‐wide identifiers of microglia, stratification into states and understanding of the functions of microglia have been considerably refined in the past decade thanks to the emergence of tools and technologies, including conditional knockout models, microglia depletion approaches, flow cytometry analysis and cell sorting, quantitative mass spectrometry and genome‐wide expression profiling of microglia at bulk, spatial and at the single‐cell levels. As a tissue‐resident macrophage of the CNS, murine microglia express multiple macrophage markers including, integrin CD11B, the leukocyte common antigen (LCA; also called CD45), the surface glycoproteins F4/80, the fractalkine receptor CX3CR1, the calcium‐binding protein IBA1, and the colony‐stimulating factor receptor CSF‐1R, which drives a critical signaling pathway for microglial development and maintenance (Prinz & Mildner, 2011). Microglia also have a unique proteomic and transcriptomic signature. Microglia (specifically, depending on brain region, species and insult) express a combination of markers that allows them to be identified specifically including P2Y purinoergic receptor 12 (P2ry12), transmembrane protein 119 (Tmem119), sialic acid binding Ig‐like lectin H (Siglech), G protein coupled receptor 34 (Gpr34), suppressor of cytokine signaling 3 (Socs3), β‐hexosaminidase subunit β (Hexb), olfactomedin‐like protein 3 (Olfml3) and Fc receptor‐like S, scavenger receptor (Fcrls). Some of these markers are also observed in human microglia, including P2RY12 and TMEM119 (Butovsky et al., 2014; Butovsky & Weiner, 2018; Satoh et al., 2016; Zhu et al., 2017).
Microglia show significant heterogeneity across development. The variety of microglial states across time is directly linked to changes in the functions of these cells over time including supporting the proliferation and migration of neurons and supporting myelin maturation and maintenance, making microglia a central element in brain development (Hammond et al., 2019; Li et al., 2019; Matcovitch‐Natan et al., 2016; McNamara et al., 2023). In human development, the end of the second and third trimesters of gestation can be considered to occur from E15 to approximately P10 in mice (Chen et al., 2017). The developing brain‐associated microglia between E14 and a few weeks after birth in the mouse has been given the classification of “pre‐adult” microglia, based on high expression of genes related to synaptic pruning and neural maturation (Matcovitch‐Natan et al., 2016). Previous studies support the specific role of microglia in neuronal development at this time, including that they modulate laminar positioning of cortical interneuron at E16.5 and E18.5 and axonal outgrowth of dopaminergic axons (Squarzoni et al., 2014). Microglia play a major role in synaptic refinement via pruning and actively engulfing synaptic material which influences the building of functional brain connectivity during early stages of post‐natal brain development (Kim et al., 2017; Lehrman et al., 2018; Paolicelli et al., 2011). In addition, microglia are critical modulators of neuronal survival and apoptotic neuronal clearance. Specifically, it has been demonstrated that cortical neurons of layer V require microglia‐derived IGF1 for survival during postnatal development (Ueno et al., 2013). Amoeboid and phagocytic microglia clusters are observed in regions where programmed cell death of neurons occurs. The phagocytic clearance of these apoptotic neurons by microglia requires specific recognition via cell surface signals, leading to the binding and engulfment of neurons by microglia (Witting et al., 2000).
Considering the pre‐adult microglia state, studies of sc‐RNA sequencing data have further highlighted that during the window of development from early pre and post‐natal development it is possible to identify at least four types of pre‐adult microglia (i) Ms4a7‐expressing microglia at E14.5, that are transcriptionally similar to border‐associated macrophages but defined as embryonic microglia in which a microglia‐specific identity had not yet been achieved (Hammond et al., 2019), (ii) proliferative microglia, expressing cell cycle genes and representing 35%–40% of microglia at E14.5 and P5, and in P7 post‐natal brain (Hammond et al., 2019) (iii) immature microglia expressing higher levels of microglia‐specific homeostatic genes (Q. Li et al., 2019), and (iv) primitive/embryonic‐like microglia in the postnatal brain with an over‐representation of ribosomal components and lower levels of MG‐specific homeostatic genes (Q. Li et al., 2019). The heterogeneity of microglial populations identified as “pre‐adult microglia” calls for in‐depth studies to understand the developmental functions of microglial states in this window and relevance to preterm born infants. In addition, more studies are needed on how damage such as that resulting from neonatal brain lesions could disrupt specific microglial states and their associated brain‐building functions to be able to establish specific protective strategies. Matcovitch‐Natan et al. (2016) successfully characterized the effects of maternal immune activation on microglial development at E12.5 and E14.5 in the mouse. Their finding detailed that microglial “development” was accelerated following exposure to polyinosinic:polycytidylic acid (Poly I:C) at the pre‐adult microglial stage, causing premature expression of genes associated with and adult microglial profile. These interruptions to normal microglial development may result in chronic and acute developmental abnormalities and diseases. Furthermore, a similar study in piglets concluded that there were interruptions to microglial development following maternal immune activation, and that these are highly sex‐dependent (Antonson et al., 2019).
Recent work demonstrated that microglia associated with mouse models of Alzheimer's disease comprise two ontogenetically and functionally distinct cell lineages (Silvin et al., 2022). One is an embryonically derived DAM sharing a common gene expression signature with developmental (fetal and early post‐natal) microglia, including expression of integrin Itgax (CD11C) that has protective functions. The second is a monocyte expressing disease inflammatory macrophage population that accumulates in the brain during aging (Silvin et al., 2022). This study highlights that in the context of neurodegenerative pathology, there may be a reactivation of a developmentally associated microglia profile, which may be an attempt to protect the brain and limit the progression of pathology.
Consistent with a key role of microglia in the onset of WMI in preterm developing brains, a specific CD11C+ microglial subset expressing genes for neuronal and glial survival, migration, and differentiation was identified in primary myelinating areas (mainly in the corpus callosum and cerebellar white matter) during the first post‐natal week in the mouse. These neonatal‐specific CD11C+ cells constitute a significant source of insulin growth factor 1 (IGF1) which regulates myelin thickness. Using CD11cCre‐GFP Igf1fl/fl mice, it was established that an IGF‐1 deficit in a CD11C+ cells induced a significant decrease of mRNA encoding myelin proteins (including Plp, Mag, and Mbp), and hypomyelination of axons in corpus callosum. These findings demonstrate that IGF1‐producing CD11c+ cells play a critical role in primary myelination (Wlodarczyk et al., 2017). Using large‐scale transcriptional profiling, a unique molecular signature of a subset of microglia located in the white matter was then later established. The distinct transcriptomic signature of these microglia included expression of the Clec7a, Spp1, Igf1, Anxa5, Itgax (encoding for CD11C), and Gpnmb genes (Hagemeyer et al., 2017). In a model of EoP induced by IL‐1β exposure, reactive microglia were characterized by a long‐lasting decrease of Igf1 mRNA. The disturbance of this specific state of microglia could be explained at least in part by the hypomyelination of corpus callosum seen in these animals (Van Steenwinckel et al., 2019).
In addition to differences in distributions of the microglia described above, there are regional differences in the basal state of the microglia transcriptional profile, most well established in the grey matter versus the white matter, within regions and in adult studies of disease (Amor et al., 2022; Spencer et al., 2019; van der Poel et al., 2019). However, transcriptomic profiling of microglia in the cerebrum and the cerebellum in an inflammation‐induced model of EoP demonstrated a common reactive signature characterized by an over‐representation of Gene Ontology terms related to inflammation and cell proliferation (Klein et al., 2022). Further analysis revealed a specific cerebellar signature with significant enrichment of interferon (IFN) signaling, particularly type II IFN (IFN‐γ) signaling following systemic inflammatory exposure, which was not significantly observed in the microglia of the cerebrum. A type II interferon signature in microglia/macrophages was also identified via spatial transcriptomics of human infant cerebellum after adverse birth events (Holloway et al., 2023). As such, the specific response of cerebellar microglia may potentially contribute to the shaping of cerebellar lesions and associated deficits which are relatively common in preterm‐born infants (Haldipur et al., 2011; Spoto et al., 2021).
2.6. Specifically targeting microglia to improve deficits associated with EoP
In neonatal rodents, in some models the selective ablation of microglia increases the levels of proinflammatory cytokines and chemokines and exacerbates brain injury (Faustino et al., 2011; Lalancette‐Hebert et al., 2007); a finding that was initially surprising. However, as we understood the importance of microglia during neonatal development, it became clear that total microglial depletion was not the best approach for treating EoP especially if the depletion was prolonged. In contrast, treating perinatal brain inflammation by specifically targeting microglia has been demonstrated as an efficient approach to protecting the developing brain. These targeted approaches include nanoparticle drug delivery systems, which are an effective solution for specifically targeting microglia in the CNS. It is now well established that microglia take up poly(lactic‐co‐glycolic) acid (PLGA)‐ and l‐tyrosine poly‐ phosphate (LTP)‐based nanoparticles and hydroxyl poly(amido‐amine) (PAMAM) dendrimers including 3DNA nanostructures without inducing the reactive functional aspects of these cells (Cahalane et al., 2020; Lloyd et al., 2019; Van Steenwinckel et al., 2019; Zhang et al., 2016).
In vitro studies show that microglia can take up drugs delivered using nanoparticles in a cell‐autonomous manner. For instance, in LPS‐stimulated SIMA9 microglial cells, treatment with the anti‐inflammatory drug Rolipam conjugated to PLGA nanoparticles decreased LPS‐induced release of TNF‐α. Interestingly, this is nanoparticle specific as LPS‐induced IL‐6 release is suppressed by LTP‐Rolipram nanoparticles but not by PLGA‐Rolipam nanoparticles. In addition, Celastrol an NR4A1 agonist that alleviates inflammation and induces autophagy incorporated into PAMAM dendrimers decreased the release of NO and IL‐6 induced by LPS, such as NO and IL‐6 (Boridy et al., 2012). In addition, treatment with PAMAM dendrimers conjugated to minocycline, a highly lipophilic second‐generation semisynthetic derivative of tetracycline inhibited the release of NO from LPS‐stimulation of a murine BV‐2 microglial cell line (Sharma et al., 2017).
Nanoparticles also have a demonstrated ability to cross the blood‐brain barrier and be taken up specifically by microglia in both mice and rabbits (Kannan et al., 2012; Sharma et al., 2017; Van Steenwinckel et al., 2019). In a rabbit model of cerebral palsy induced by prenatal exposure to LPS, a dendrimer‐minocycline conjugate administered by an intravenous route was specifically taken up by reactive microglia in periventricular white matter areas, including the corpus callosum and the lateral ventricle (Sharma et al., 2017). In the rabbit model of cerebral palsy induced by exposure to LPS during gestation, a single injection of PAMAM dendrimer conjugated to N‐acetylcysteine (NAC) an amino acid stimulating the antioxidant glutathione production) leads to colocalization in reactive microglia and astrocytes in the periventricular region and attenuates neuroinflammatory processes, greatly improving motor function (Kannan et al., 2012). This NAC dendrimer is also protective in a mouse model of HI‐induced neonatal white matter injury, at subacute or delayed time points after injury. The D‐NAC dendrimer could attenuate the pro‐inflammatory response up to 9 days after injury, while not impacting the anti‐inflammatory response. The D‐NAC therapy also improves myelination, resulting in a reduction of white matter injury (Nance et al., 2015). Oral pediatric formulation containing D‐NAC is now available and could be an effective option for treating neuroinflammation (Yellepeddi et al., 2018).
In a mouse model of systemic inflammation induced EoP, 3DNA nanoparticles targeting microglia have been shown to reduce brain injury. Unbiased transcriptomic analyses of microglia in a mouse model of EoP induced by systemic inflammatory activation (early postnatal IL‐1β exposure in the mouse) have shown a significant decrease of canonical WNT signaling in reactive microglia. Multiple pharmacological and genetic approaches in vitro and in vivo demonstrated that the level of WNT pathway activity in microglia is inversely correlated with proinflammatory marker expression level. Targeting microglia specifically with 3DNA nanoparticles conjugated to a WNT activator reduced IL‐1β exposure‐induced white matter injury by modulating microglial reactivity and improving myelination and persistent cognitive deficit in adults (Van Steenwinckel et al., 2019). In the same model of systemic inflammation‐driven EoP, analysis of the microglial “micro”‐transcriptome and an in vitro study of primary microglia have demonstrated a major role of miR‐146b‐5p on the regulation of pro‐inflammatory processes in pro‐inflammatory immune reactive microglia. The immunomodulatory miR‐146 family acts via a negative feedback mechanism in a wide variety of immune cells to prevent overstimulation of the inflammatory response. Targeting microglia specifically with 3DNA nanoparticles conjugated to synthetic miR‐146b‐5p demonstrated the ability of miR‐146b‐5p to shut down this immune reactivity, improving myelination and cognitive deficits (Bokobza et al., 2022).
2.7. Astrocytes in the developing brain
Astrocytes are the most numerous glial in the CNS, and together with oligodendrocytes and neurons, they are derived from neuroepithelium lining the developing neural tube. Astrocyte proliferation begins around 26 wGA in humans, peaking around 28–30 wGA. Astrogenesis in the mouse brain is initiated at E16‐18 (Nagao et al., 2016). The timing of the peak in astrocyte production could be particularly relevant for preterm neonates given that astrocytes play a major role in brain development and homeostasis. In particular, astrocytes support neuronal growth and axonal guidance (Doherty et al., 1990; Kanemaru et al., 2007; Tomaselli et al., 1988) and as astrocytes surround and contact most neuronal synapses, releasing soluble molecules necessary for synapse formation and proper brain connectivity (Allen & Eroglu, 2017; Elmariah et al., 2005; Hughes et al., 2010; Ullian et al., 2001). Astrocytes also play a major role in myelination; for example, white matter astrocytes express higher levels of GFAP (often used as a marker of immune reactivity, astrogliosis) which is essential for normal white matter architecture and blood‐brain barrier integrity (Cai et al., 2007), illustrated by the fact that GFAP deficits lead to late‐onset CNS demyelination (Liedtke et al., 1996) and in mice carrying a null mutation in GFAP, abnormal myelination, poor vascularization of the white matter and impairment of the blood‐brain‐barrier has been described (Liedtke et al., 1996). Astrocytes support myelination through their communication with oligodendrocytes via gap junctions, transfer of lipids and secretion of pro‐myelination factors (Dupree et al., 1998; Magnotti et al., 2011; Menichella et al., 2003; Odermatt et al., 2003; Stankoff et al., 2002; Sutor et al., 2000).
2.8. Astrogliosis in EoP
Compared to microglia, relatively less is known about the role of astrocytes in EoP, so we also include a discussion of their roles in other forms of neonatal white matter injury. Reactivity of astrocytes to damage or inflammation induces the release of cytokines, chemokines and growth factors which actively participate in the neuroinflammatory response (Liddelow et al., 2017; Shiow et al., 2017). Astrocytes, like microglia, respond to multidimensional parameters to establish distinct astrocyte phenotypes and play a detrimental or beneficial role depending on developmental stage, injury or disease (Escartin et al., 2021; Liddelow et al., 2017). Using in vitro techniques, it has been demonstrated that blocking differentiation of OPCs into oligodendrocytes by chemically‐induced hypoxic stress was restored by brain‐derived neurotrophic factor released from astrocytes (Miyamoto et al., 2015). This important finding suggests that appropriate modulation of astrocytes could represent a powerful strategy to support oligodendrogenesis.
Astrogliosis describes a complex morpho‐functional remodeling in response to immune activation (injury, pathogen). In human preterm infants, diffuse WM lesions are accompanied by diffuse astrogliosis and increased hyaluronic acid (HA), suggesting the occurrence of extracellular matrix remodeling within the lesion (Buser et al., 2012). Extracellular high molecular weight HA can be broken down by hyaluronidase activity or reactions with reactive oxygen species (Litwiniuk et al., 2016). In addition, high molecular weight HA and fragments of different sizes can influence various biological processes through interactions with receptors such as CD44. It has also been demonstrated that a high molecular weight form of HA synthesized by astrocytes in chronic demyelinated lesions inhibits the maturation of OPCs into myelin‐producing cells (Back, Tuohy, et al., 2005). The hyaluronan (HA) receptor CD44, an independent marker of astrogliosis, is also upregulated in HA‐rich WMI lesions (Buser et al., 2012; Srivastava et al., 2020). In cases of human term neonatal HI encephalopathy with subcortical WMI, the production of the COX2‐inducing E2 pro‐inflammatory mediator was strongly induced in reactive astrocytes (Shiow et al., 2017). In the same study, looking at a model of inflammation‐associated EoP, prostaglandin E2 (PGE2) derived from astrocytes directly inhibited OPC maturation (Shiow et al., 2017). In a mouse model of neonatal HI, a lineage tracing approach revealed a preferential generation of subventricular zone (SVZ)‐derived white matter astrocytes, rather than oligodendrocytes, suggesting impairments in the specification of glial precursors within the neonatal SVZ during recovery from neonatal HI (Bain et al., 2010) that might also impact preterm infants. A detailed study of the gene expression of GABAergic targets in the prefrontal cortex of post‐mortem preterm born infants revealed an astrocyte specific dysfunction, predominately in males (Lacaille et al., 2021). Transcriptome analyses have also clearly outlined that mouse astrocytes are sexually dimorphic, male astrocytes mature faster and dimorphism peaks at P7, declining with age (Rurak et al., 2022). Astrocyte dimorphism impacts their responses in utero in a manner relevant to preterm‐born infants. Specifically, administration of synthetic glucocorticoids to rats during pregnancy (on E18), to mimic treatment to mature the fetal lung in women with threatened preterm labor), causes changes in adulthood in the number, density and distribution of astrocytes, predominantly in males (McArthur et al., 2016).
2.9. Targeting astrocytes in EoP
Specific modulation of reactive astrocytes in models of HI encephalopathy demonstrates some protective effects on the developing brain. For example, in a model of term infant HI, GFAP and vimentin knockout increases the number of surviving newborn neurons (Jarlestedt et al., 2010). Hypoxia‐inducible factor‐1α (HIF‐1α), a sensitive regulator of oxygen homeostasis, is known to play an extensive role in the pathophysiology of stroke, including neuronal survival, neuroinflammation, angiogenesis, glucose metabolism, and blood‐brain barrier regulation (He et al., 2021). A delayed expression of HIF‐1α was observed in astrocytes at 7 days after induction of HI in rat pups to mimic term infant NE. Inhibiting this delayed HIF‐1α expression decreased astrogliosis, reduced damage to myelination and memory performance (Wang et al., 2022). Also, in a term infant model of HI injury in the rat, overexpression of receptors for complement peptide C3a (C3a) under the control of the GFAP promoter, reduced hippocampal neurodegeneration and reactive gliosis (Pozo‐Rodrigalvarez et al., 2021). In a very limi study of a neonatal model of sepsis induced by LPS a deficit of astrocytic Cx43 was established using Cx43flox/+: hGFAP‐Cre mice. This resulted in weakened inflammatory responses characterized by reduced upregulation of pro‐inflammatory cytokines and reduced microglial reactivity (Zhou et al., 2015), but these finding needs to be validated. Finally, in a model of preterm HI‐induced EoP in sheep, the blockade of astrocytic connexin 43 hemichannels was able to significantly reduce the severity of EEG dysfunction and partially recover deficits in the neuropathology (Davidson et al., 2014). As such, astrogliosis and microgliosis seem to represent a major process that requires careful analysis at multiple levels to characterize potential opportunities to develop targeted therapeutic strategies.
2.10. Oligodendrocytes in EoP
Historically, oligodendrocytes were considered just a “victim” in EoP. In more severe models of injury and cases of EoP within the focal white matter lesions oligodendrocyte death is observed (Du et al., 2011; Haynes et al., 2003; Rousset et al., 2006; Scafidi et al., 2014; Serdar et al., 2018). In cohorts with less severe outcomes (dWMI) and comparable models, dysmaturation/hypomyelination without cell death is more common (Billiards et al., 2008; Favrais et al., 2011; Li et al., 2018; Verney et al., 2012). Interestingly, recent work shows that oligodendrocytes themselves produce inflammatory and trophic factors which are hypothesized to participate in the inflammatory response, reviewed in (Kirby & Castelo‐Branco, 2021; Madeira et al., 2022) and even play a role in circuit formation (Buchanan et al., 2023). For instance, in a model of systemic‐inflammation driven EoP with diffuse WMI (Favrais et al., 2011), TLR3, IL‐1β, IFN‐β, chemokine ligand (Ccl) 2, and Cxcl10 are elevated in the immune‐activated oligodendrocytes, and this effect was greater in the pre‐OLs compared to the OPCs (Boccazzi et al., 2021). This is linked to impacts of inflammation to open the chromatin conformation around transcription factors in the oligodendrocytes (Schang et al., 2022).
A greater understanding of oligodendrocyte biology also highlights roles for OPCs, also known as NG2 glia or polydendrocytes as more than a progenitor pool. Not all OPC mature into mature oligodendrocytes, and during development and into adulthood OPC are being shown to play active roles in synaptic pruning, playing a role in experience‐dependent mechanisms of synaptic refinement in the thalamocortical relays in mice (Auguste et al., 2022) and capable of phagocytosing whole cells in the context of models of multiple sclerosis (Nguyen & Pender, 1998). The phagocytotic abilities of the oligodendrocytes enable them to function as antigen‐presenting cells, and they express MHC I and MHC II which are increased in the context of IFNγ exposure (Kirby et al., 2019), a cytokine associated with WMI in preterm born infants (Hansen‐Pupp et al., 2005) and in models of EoP (Jellema et al., 2015; Van Steenwinckel et al., 2019).
Oligodendrocyte development and function, like that of microglia and astrocytes, is sexually dimorphic, manifesting as differences in total myelin in adulthood in humans and rodents (Cerghet et al., 2006; Seeker & Williams, 2022). In a study including transcriptomics, proliferation, migration, myelination and cytotoxicity assays in mouse OPCs (Yasuda et al., 2020), female OPCs have a higher capacity for proliferation and migration, male OPCs have a higher capacity for differentiation and myelination, and female OPCs are more resistant to oxygen‐glucose deprivation.
This is a relatively new field, and no studies specifically target oligodendrocyte immune function as a therapy for EoP. However, this approach has been used successfully in a model of multiple sclerosis, with nanoparticle‐mediated delivery of the immunomodulatory and pro‐myelinogenic factor LIF to increase in vivo myelination (Rittchen et al., 2015). However, it is an obvious extension of the idea of oligodendrocytes being immunomodulatory targets that all our current therapies (melatonin, EPO, hypothermia, etc.) may partially reduce inflammatory reactions by acting directly on oligodendrocytes. This requires further study to identify which agents are most effective, to see if combination therapies can be better designed for modulating glia (R. Pang et al., 2021) and to understand cross‐talk between cell types as oligodendrocytes have been shown to mediate anti‐inflammatory processes (Madsen et al., 2020).
2.11. The role of non‐CNS cell types
Typically, CNS macrophages are classified into two groups based on their location: microglia, which are found within the brain tissue itself, and border‐associated macrophages (BAMs), which inhabit various border tissues of the CNS such as the meningeal membranes, the choroid plexus, and perivascular spaces (Munro et al., 2022).
2.11.1. Diversity of BAMs
Meningeal macrophages
The three anatomical layers that surround the CNS, known as the meninges, consist of the dura mater, which is located beneath the skull and contains lymphatic vessels and fenestrated blood vessels, followed by the arachnoid mater, and finally the pia mater, a thinner tissue that adheres to the CNS (Rua & McGavern, 2018). The arachnoid and pia mater are called the “leptomeninges.” While it was previously believed that the CNS was immune‐privileged, recent findings indicate that the meninges contain a dense network of immune cells, such as resident meningeal macrophages, dendritic cells, B cells, and T cells (Goldmann et al., 2016; Louveau et al., 2015; Mrdjen et al., 2018; Rua & McGavern, 2018).
Interestingly, dural BAMs can be divided into two distinct subsets based on their gene expression patterns: MHCIIlowCD206high cells present initially, and MHCIIhigh CD206low cells which appear later (Mrdjen et al., 2018; Rua & McGavern, 2018; Van Hove et al., 2019). Leptomeningeal BAMs are more homogeneous, consisting primarily of MHCIIlowCD206high cells, and like microglia they self‐maintain independently of monocyte input (Goldmann et al., 2016).
Choroid plexus macrophages
During normal conditions, the choroid plexus plays a role in both the secretion and regulation of cerebrospinal fluid (CSF), while also participating in the elimination of waste products and metabolites (Bedussi et al., 2015). BAMs can be found both in the stroma and at the apical site (Kolmer cells) of the choroid plexus (Munro et al., 2022). Stromal choroid plexus macrophages are enriched in MHC‐II upon aging, similar to dural macrophages (Goldmann et al., 2016). On the apical side, Kolmer's epiplexus cells have a transcriptomic signature closer to microglia than BAMs, and lineage tracing studies indicate that they self‐maintain, like microglia and leptomeningeal macrophages (Van Hove et al., 2019).
Perivascular macrophages
The perivascular spaces surrounding blood vessels within the brain tissue also contain macrophages (Munro et al., 2022). Under normal circumstances, these compartments are predominantly inaccessible to molecules originating from the bloodstream, unless there is a selective transportation mechanism in place (Banks 2016). Perivascular macrophages (PVMs) are essential in the immune response in the brain as they communicate with surrounding endothelial cells and permit the transmigration of other immune cells to elicit a reaction to insult or injury (Faraco et al., 2017).
2.11.2. BAM origins
In mice, the CSF system forms at embryonic day E9.5 when the neural tube undergoes closure, trapping amniotic fluid inside (Munro et al., 2022). Macrophages begin arriving in the embryo proper from E9.5, following the stepwise establishment of the embryonic circulation (Q. Li & Barres, 2018; Perdiguero & Geissmann, 2016).
BAMs initially derive from YS–generated progenitors during embryogenesis, and in the dura and choroid plexus, these cells are partially replaced by monocyte‐derived macrophages in adulthood, that preferentially differentiate in MHC‐IIhigh BAMs (Goldmann et al., 2016; Rua et al., 2019). Recent findings suggest that some of the myeloid cells in the dura arise from myeloid cell reservoirs in the adjacent skull bone marrow rather than from circulatory routes (Cugurra et al., 2021).
On the basis of their transcriptomes, BAMs have been linked with many biological processes, such as lipid metabolism, stimulus detection, antigen presentation, and phagocytosis (Van Hove et al., 2019). Using drug and transgenic approaches to manipulate BAMs, several groups have studied their role in cognition, CSF flow and inflammation.
2.11.3. Roles for BAM
Cognition
Kipnis and colleagues demonstrated the crucial involvement of meningeal IL‐4 derived in exerting beneficial effects on learning and memory by modulating the phenotype of meningeal myeloid cells (e.g., BAMs). In the absence of IL‐4, these meningeal myeloid cells adopt a pro‐inflammatory bias, which correlates with impaired cognitive performance (Derecki et al., 2010). A recent study showed that deletion of the transcription factor SMAD4 in microglia and embryonic‐derived BAMs using Crybb1‐Cre caused a developmental arrest of microglia. Interestingly, instead of developing into typical microglia, cells acquired a specification signature resembling that of BAMs and this was associated with impairment of mouse memory skills (Brioschi et al., 2023). Additionally, PVMs are implicated in cognitive impairment and blood‐brain barrier dysfunction related to hypertension, as well as neurovascular alterations observed in Alzheimer's disease (Mildenberger et al., 2022). On the other hand, PVMs have been found to play a beneficial role in clearing amyloid‐β in cerebral amyloid angiopathy, illustrating their diverse involvement in various disease processes (Mildenberger et al., 2022).
CSF flow
Mice lacking macrophages (such as Csf1r ‐/‐), display enlarged ventricles and hydrocephaly, suggesting that CNS macrophages regulate CSF homeostasis (Mildenberger et al., 2022). In addition, PVM play a role in regulating the dynamics of CSF flow. Specifically, PVMs are found near the brain arterial tree and their depletion through pharmacological or genetic means resulted in the accumulation of extracellular matrix proteins, obstructing the access of CSF to perivascular spaces. As a consequence, this impairs CNS perfusion and clearance (Drieu et al., 2022).
Inflammation
Several studies have investigated the fate and protective function of BAMs following infection by pathogens introduced either locally or from the periphery. One study focused on the intracranial injection of lymphocytic choriomeningitis virus (LCMV), which caused infection and death of resident dural meningeal macrophages. It also resulted in the long‐term engraftment of monocytes derived from the blood in the dura mater, but these monocytes displayed impaired immune functions (Rua et al., 2019). Furthermore, dural BAMs responded to systemic inflammation induced by peripheral injection of LPS, SARS‐CoV‐2, or LCMV (Rebejac et al., 2022). In contrast to intracranial infection, peripheral LCMV infection does not produce symptoms in immunocompetent mice. However, depleting dural meningeal macrophages increased brain viral load and led to fatal meningitis, mimicking the effects of an intracranial infection (Rebejac et al., 2022). In another infection model, peripheral infection of mice with Trypanosoma brucei, the causative agent of sleeping sickness, resulted in parasite invasion of the brain borders and parenchyma. This triggered a temporary recruitment of monocytes, and long‐term transcriptional changes were more noticeable in BAMs, particularly those in the choroid plexus, compared to microglia (De Vlaminck et al., 2022). Depletion of resident macrophages, which affects microglia as well as leptomeningeal and dural BAMs, led to an increased parasite load. Likewise, depletion of BAMs (Cui et al., 2020; Pinho‐Ribeiro et al., 2023) resulted in an elevated bacterial load following infection with Streptococcus agalactiae or Streptococcus pneumoniae.
These studies were performed in adult mice, but little is known about the behavior of BAMs upon inflammation in early life. Injection of a viral genome mimetic, polyinosinic‐polycytidylic acid to pregnant dams at E12.5 (a model of maternal immune activation) caused accumulation of epiplexus macrophages, associated with increase in CCL2 (Cui et al., 2020), however the causal link with long‐term neurodevelopmental deficits was not assessed.
While no functional study is currently available regarding the role of BAMs in EoP, it is tempting to speculate that BAMs might play a role through loss of trophic and/or gain of toxic functions. Indeed, in adult mice, BAMs protect and nurture the brain, and these functions are impaired upon infection (Rebejac et al., 2022). It is thus likely that in early‐life, BAMs downregulate their homeostatic program upon inflammation, which could impair brain development. In addition, BAMs could also promote and relay inflammation to the underlying parenchyma, thus creating changes in the developmental trajectory, and long‐term cognitive issues
2.11.4. Modulators of glial function: the role of gut‐brain‐axis communication
The gut‐brain axis refers to the physiological pathways enabling communication between the central and enteric nervous systems (ENSs), via neural, endocrine, immune, and humoral signals. The ENS houses 200–600 million neurons (Furness et al., 2014) and is commonly referred to as the “second brain”. Like the brain, the ENS encompasses a vast network of interconnected neurons which are involved in regulating autonomic processes within the gastrointestinal tract. Enteric neurons are organized into ganglia within the ENS, adjacent to resident glial cells, referred to as enteric glial cells (EGCs). In addition to the gut microbiota being important in regulating gastrointestinal function, it is vital in regulating brain function via the gut‐brain axis. An absence of gut microbiota, as studied in germ‐free mice, affects behavior and cognitive function, resulting in decreased spatial and working memory function (Gareau et al., 2011), altered hippocampal neurogenesis and functional connectivity (Scott et al., 2020), as well as reduced anxiety‐like (Neufeld et al., 2011) and social behaviors (Desbonnet et al., 2014). Germ‐free mice also exhibit defects in microglia, resulting in impaired immune function (Erny et al., 2015). This body of work demonstrates the critical ability of the microbiome to shape neurodevelopment, including cognitive function and immune cell development.
In addition to altering microglia located in the brain, depletion of the gut microbiome also affects development of enteric glia. Like brain astrocytes, EGCs support enteric neuronal function, and regulate neurotransmitter balance, and maintain epithelial barrier integrity (Neunlist et al., 2014). They can also adopt a pro‐inflammatory reactive phenotype in inflammatory gastrointestinal disorders and bacterial and viral infections (Ochoa‐Cortes et al., 2016). The ENS houses the largest population of glia outside of the brain (Rosenberg & Rao, 2021). Population of the lamina propria by enteric glial cells occurs during early postnatal stages and the abundance of EGCs remain consistent after weaning as they are continuously renewed during adult life (Kabouridis et al., 2015). This renewal of EGCs in the gut is regulated by the host microbiota. This has been demonstrated by decreased numbers of EGCs as a result of antibiotic‐induced depletion of the microbiota (Kabouridis et al., 2015; Poon et al., 2022; Vicentini et al., 2021), which appears to occur in a sex‐dependent manner (Poon et al., 2022). In addition, a loss of enteric neurons was also observed following antibiotic treatment in mice which was accompanied by slower transit time and increased intestinal permeability (Vicentini et al., 2021). Of interest, recovery of the gastrointestinal microbiota after antibiotic withdrawal also promoted enteric neurogenesis and restored both gut function as well as enteric neuronal and glial population numbers (Vicentini et al., 2021). These findings highlight the importance of the microbiome in regulating neuroglial networks and gastrointestinal function. Thus, it is evident that the gut microbiome is fundamental in shaping the development and function of the nervous system, which is highly relevant in neurodevelopmental disorders, including those resulting from premature birth.
The developmental stage at which an infant is born can affect their microbiome composition. The microbiome of infants at term is temporarily dynamic after birth. Initially, the microbial population possesses low diversity and abundant gut bacteria, hosting aerobes and facultative anaerobes (reviewed in (Healy et al., 2022)). These bacteria consume oxygen available in the lumen of the gut creating an anoxic environment, which enables proliferation by obligate anaerobic bacteria, increasing in diversity and abundance to resemble growth toward an adult human gut microbiome population (Foong et al., 2020). Premature birth results in impaired maturation of the gut microbiome, as well as the gastrointestinal tract and immune system. The initial colonizers of the gut are similar in term and preterm infants, however variables including mode of delivery (vaginal vs. caesarean), feeding method and duration (breastfeeding vs. formula), and antibiotic exposure results in in differences of the abundance of different populations of microbiota (Healy et al., 2022).
Recent evidence suggests that the gut microbiota of preterm infants is associated with early cognitive and behavioral neurodevelopment (Chen et al., 2023; Sarkar et al., 2022) and has been identified as a potential biomarker of neurodevelopmental delay in preterm infants (Roze et al., 2020). Disruption of the maturation and development of the microbiome has also been linked to a number of fatal diseases in preterm infants, including NEC and late‐onset sepsis (Masi & Stewart, 2019; Thanert et al., 2021). NEC is a disease involving severe intestinal inflammation, cellular damage, and necrosis of gastrointestinal tissue. If left untreated, this damage can lead to perforation of the intestine, peritonitis, sepsis and death (Neu & Walker, 2011). A significant role of microbial dysbiosis is thought to contribute to NEC, in addition to intestinal immaturity including impaired permeability and motility, and an underdeveloped and highly reactive immune system (Tanner et al., 2015). Alterations in the diversity of viral components of the gastrointestinal microbiome have also been reported prior to the onset of NEC (Kaelin et al., 2022). Experimentally, the hypothesis that immature microbiome development is involved in the pathogenesis of NEC is strengthened by animal research demonstrating that administration of trinitrobenzene sulfonate, an immune stimulant used to model NEC in rodents, is ineffective in germ‐free mice due to the absence of gut bacteria (MohanKumar et al., 2017).
While enteric glial cells have yet to be well‐studied in preterm infants per se, some research has been conducted in preterm infants with NEC. For example, Fagbemi and coworkers identified that the chemokine CCL20 was upregulated in 9 of 20 preterm infants with acute NEC and strongly expressed by glial cells, and 11 cases showed reduced expression of glial cells (Fagbemi et al., 2013). Similarly, a preclinical model of NEC exhibits depletion of enteric glia which is mediated via the toll‐like receptor 4 (TLR4), and this is accompanied by reduced intestinal motility and inflammation (Kovler et al., 2021). Given our growing understanding of the role of the ENS and, more specifically, the microbiome in modulating neurodevelopment and immune function, the bacterial composition of preterm infants and potential interventions must be studied in greater depth.
Probiotics have been proposed as a potential therapeutic in mitigating the adverse effects of microbial dysbiosis in preterm infants. For example, probiotic administration has previously been reported to reduce the incidence of NEC (Sun et al., 2017; Underwood, 2019; Zhu et al., 2019), sepsis, mortality, as well as reducing the duration of hospitalization post birth compared with controls (Sun et al., 2017). Beneficial impacts of probiotics have also been observed in preclinical animal models of NEC including decreased inflammation, improved intestinal barrier function, and decreased incidence and severity of NEC (Underwood, 2019).
Another potential treatment for complications arising from preterm birth is the application of bacteriophage therapy. Bacteriophages (phage) constitute most of the viral population within the gut microbiome. These phages are unique from other viruses in their specificity in targeting and infecting bacteria. While dysbiosis of gut bacterial populations are increasingly profiled in relation to disease states, perturbations to the balance of phage communities within the gut are less well characterized (Shamash & Maurice, 2022). The absence of viral particles in the meconium, the earliest infant stool sample, suggests that the gut does not have an established virome at birth and becomes colonized with phage within the first week of life (Breitbart et al., 2008). Potential therapeutic benefits proposed by manipulating phage are additionally of interest due to an increasing prevalence of antibiotic resistance in healthcare. Phage are highly specific in targeting their bacterial host, a trait that makes them suitable for targeting specific bacterial species (Furfaro et al., 2017) within gut microbial communities, unlike broad‐spectrum antibiotics which inhibit a wider range of bacteria and can commonly disrupt the human microbiome to result in detrimental effects. Indeed, antibiotic treatment to address bacterial infection in preterm infants is associated with widespread perturbation of the microbiome (Gibson et al., 2016) and a higher incidence of NEC, late‐onset sepsis, and death (Kuppala et al., 2011). While applications of bacteriophage‐mediated therapies are still emerging, promising results in mediating multi‐drug resistant and antibiotic‐resistant bacterial infections in preclinical animal models and in human trials have been reported (Lin et al., 2017). In summary, the use of bacteriophage therapy in the context of premature complications and NEC may enable specific targeting of a source of infection without disturbing the balance of beneficial bacteria within the gut microbiome.
An enhanced understanding of the complex neuroglial networks within the gut and their relationship with host microbial communities may prove vital in treating disorders affecting neurodevelopment and cognition. Additional research is required to clarify the roles of bacteria, viruses and bacteriophage in modulating enteric neuronal and glial function in the pathophysiology of preterm infants. Addressing microbial dysbiosis via probiotics and the potential application of bacteriophage therapy to treat early‐life infections are emerging therapeutic options to reduce the potentially fatal complications of preterm birth.
In conclusion, glia are critical for brain development, and together the derailment of these normal processes and their “immune activation” causes brain injury in the preterm born infant (summarized in Figure 2). There has been an explosion in data describing glial function and responses in some contexts, like normal mouse development and disease, and in human post‐mortem studies of disorders such as Autism, Parkinson's and Alzheimer's disease, using cutting‐edge omics approaches (Velmeshev et al., 2019). However, in perinatal brain injury, most studies are arguably lower in technology and depth of analysis. The main reason for this is that the field of perinatal brain injury is relatively small and modestly funded compared to adult neurological disease subspecialties. Also, human studies are stymied by less tissue donation than for adult disorders and far more unknowns about patient history (when did injury start, how severe was the onset, what was the specific nature of the injury, how well did the infant adapt), plus the complex treatments offered to the infants (steroids, ventilation, antibiotics, etc.) that all alter the outputs of later analyses. However, as the technologies and approaches move through the fields of neurological disorders including reducing costs and technological requirements, more studies using combined neuropathology and genetics across human and periclinal models are creating increasing waves of valuable knowledge. With these knowledge gains and biomedical engineering advances (CRISPR, nanoparticles, etc.) therapies targeting glia phenotypes to steer these cells to repair and regenerate the brain can be developed in future.
FIGURE 2.
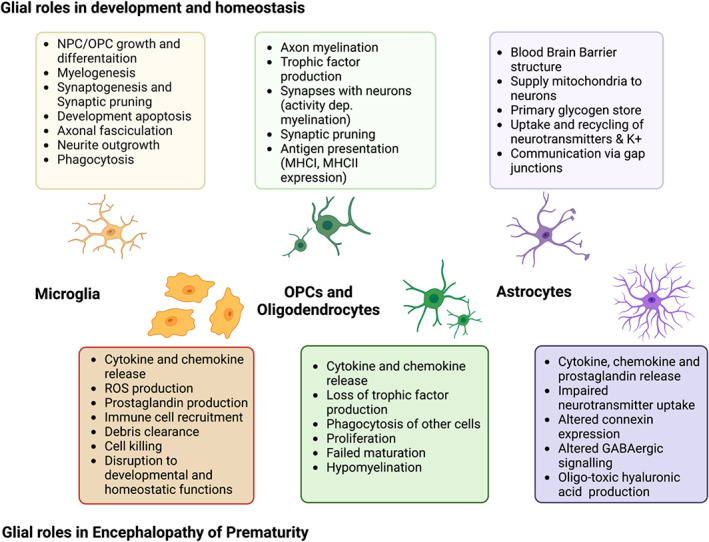
A summary of the role of microglia, oligodendrocyte precursor cells (OPCs), oligodendrocytes, and astrocytes in brain development and homeostasis and in encephalopathy of prematurity.
AUTHOR CONTRIBUTIONS
All authors contributed to the planning, writing, and revision of the manuscript. BF designed the Figures and put together the Table.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
Pierre Gressens is supported by grants from INSERM, Université Paris Cité, ANR, ERANET‐NEURON (VasOX), Fondation pour la Recherche sur le Cerveau, Fondation Princesse Grace de Monaco, Fondation des Gueules Cassées, and “Investissement d'Avenir ANR‐11‐INBS‐0011” NeurATRIS. Veronique E. Miron is supported by the John David Eaton Chair in Multiple Sclerosis Research from St. Michael's Hospital Foundation, and a Medical Research Council Senior Non‐Clinical Fellowship. Bobbi Fleiss and Isabelle K. Shearer are supported by the Cerebral Palsy Alliance and the Kinghorn Foundation. Pierre Gressens, Juliette Van Steenwinckel, Cindy Bokobza, Mireille Laforge, and Bobbi Fleiss are supported by Horizon 2020 Framework Program of the European Union (grant agreement no. 874721/PREMSTEM). Elisa L. Hill‐Yardin, Samantha M. Matta, Isabelle K. Shearer, and Bobbi Fleiss are supported by the National Health and Medical Research Foundation of Australia. Elisa L. Hill‐Yardin is also supported by Axial Therapeutics and Fondation Jérôme Lejeune. Rejane Rua is supported by the European Research Council, as well as by institutional funding from the Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique and Aix‐Marseille‐Université.
Van Steenwinckel, J. , Bokobza, C. , Laforge, M. , Shearer, I. K. , Miron, V. E. , Rua, R. , Matta, S. M. , Hill‐Yardin, E. L. , Fleiss, B. , & Gressens, P. (2024). Key roles of glial cells in the encephalopathy of prematurity. Glia, 72(3), 475–503. 10.1002/glia.24474
Juliette Van Steenwinckel, Cindy Bokobza, Bobbi Fleiss, and Pierre Gressens contributed equally to this study.
Contributor Information
Bobbi Fleiss, Email: bobbi.fleiss@rmit.edu.au.
Pierre Gressens, Email: pierre.gressens@inserm.fr.
DATA AVAILABILITY STATEMENT
As this is a Review paper, data deszcribed in this paper belong to Authors of the original papers. Requests for data should be addressed to these Authors.
REFERENCES
- Acaz‐Fonseca, E. , Duran, J. C. , Carrero, P. , Garcia‐Segura, L. M. , & Arevalo, M. A. (2015). Sex differences in glia reactivity after cortical brain injury. Glia, 63(11), 1966–1981. 10.1002/glia.22867 [DOI] [PubMed] [Google Scholar]
- Ajayi‐Obe, M. , Saeed, N. , Cowan, F. M. , Rutherford, M. A. , & Edwards, A. D. (2000). Reduced development of cerebral cortex in extremely preterm infants. Lancet, 356(9236), 1162–1163. 10.1016/s0140-6736(00)02761-6 [DOI] [PubMed] [Google Scholar]
- Albertsson, A. M. , Bi, D. , Duan, L. , Zhang, X. , Leavenworth, J. W. , Qiao, L. , … Wang, X. (2014). The immune response after hypoxia‐ischemia in a mouse model of preterm brain injury. Journal of Neuroinflammation, 11(1), 153. 10.1186/s12974-014-0153-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, N. J. , & Eroglu, C. (2017). Cell biology of astrocyte‐synapse interactions. Neuron, 96(3), 697–708. 10.1016/j.neuron.2017.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alliot, F. , Godin, I. , & Pessac, B. (1999). Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Research. Developmental Brain Research, 117(2), 145–152. 10.1016/s0165-3806(99)00113-3 [DOI] [PubMed] [Google Scholar]
- Allison, B. J. , LaRosa, D. A. , Barton, S. K. , Hooper, S. , Zahra, V. , Tolcos, M. , … Polglase, G. R. (2019). Dose‐dependent exacerbation of ventilation‐induced lung injury by erythropoietin in preterm newborn lambs. Journal of Applied Physiology, 126(1), 44–50. 10.1152/japplphysiol.00800.2018 [DOI] [PubMed] [Google Scholar]
- Amor, S. , McNamara, N. B. , Gerrits, E. , Marzin, M. C. , Kooistra, S. M. , Miron, V. E. , & Nutma, E. (2022). White matter microglia heterogeneity in the CNS. Acta Neuropathologica, 143(2), 125–141. 10.1007/s00401-021-02389-x [DOI] [PubMed] [Google Scholar]
- Andiman, S. E. , Haynes, R. L. , Trachtenberg, F. L. , Billiards, S. S. , Folkerth, R. D. , Volpe, J. J. , & Kinney, H. C. (2010). The cerebral cortex overlying periventricular leukomalacia: analysis of pyramidal neurons. Brain Pathology, 20(4), 803–814. 10.1111/j.1750-3639.2010.00380.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankeny, R. A. , Whittaker, A. L. , Ryan, M. , Boer, J. , Plebanski, M. , Tuke, J. , & Spencer, S. J. (2023). The power of effective study design in animal experimentation: Exploring the statistical and ethical implications of asking multiple questions of a data set. Brain, Behavior, and Immunity, 112, 163–172. 10.1016/j.bbi.2023.06.012 [DOI] [PubMed] [Google Scholar]
- Antonson, A. M. , Lawson, M. A. , Caputo, M. P. , Matt, S. M. , Leyshon, B. J. , & Johnson, R. W. (2019). Maternal viral infection causes global alterations in porcine fetal microglia. Proceedings of the National Academy of Sciences of the United States of America, 116(40), 20190–20200. 10.1073/pnas.1817014116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwell, K. (1991). The distribution of microglia and cell death in the fetal rat forebrain. Brain Research. Developmental Brain Research, 58(1), 1–12. 10.1016/0165-3806(91)90231-7 [DOI] [PubMed] [Google Scholar]
- Askew, K. , Li, K. , Olmos‐Alonso, A. , Garcia‐Moreno, F. , Liang, Y. , Richardson, P. , … Gomez‐Nicola, D. (2017). Coupled proliferation and apoptosis maintain the rapid turnover of microglia in the adult brain. Cell Reports, 18(2), 391–405. 10.1016/j.celrep.2016.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auguste, Y. S. S. , Ferro, A. , Kahng, J. A. , Xavier, A. M. , Dixon, J. R. , Vrudhula, U. , … Cheadle, L. (2022). Oligodendrocyte precursor cells engulf synapses during circuit remodeling in mice. Nature Neuroscience, 25(10), 1273–1278. 10.1038/s41593-022-01170-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung, M. T. , Yu, Y. , Ferguson, K. K. , Cantonwine, D. E. , Zeng, L. , McElrath, T. F. , … Meeker, J. D. (2019). Prediction and associations of preterm birth and its subtypes with eicosanoid enzymatic pathways and inflammatory markers. Scientific Reports, 9(1), 17049. 10.1038/s41598-019-53448-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back, S. A. , Luo, N. L. , Mallinson, R. A. , O'Malley, J. P. , Wallen, L. D. , Frei, B. , … Montine, T. J. (2005). Selective vulnerability of preterm white matter to oxidative damage defined by F2‐isoprostanes. Annals of Neurology, 58(1), 108–120. 10.1002/ana.20530 [DOI] [PubMed] [Google Scholar]
- Back, S. A. , Riddle, A. , & Hohimer, A. R. (2006). Role of instrumented fetal sheep preparations in defining the pathogenesis of human periventricular white‐matter injury. Journal of Child Neurology, 21(7), 582–589. [DOI] [PubMed] [Google Scholar]
- Back, S. A. , Tuohy, T. M. , Chen, H. , Wallingford, N. , Craig, A. , Struve, J. , … Sherman, L. S. (2005). Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nature Medicine, 11(9), 966–972. 10.1038/nm1279 [DOI] [PubMed] [Google Scholar]
- Bain, J. M. , Ziegler, A. , Yang, Z. , Levison, S. W. , & Sen, E. (2010). TGFbeta1 stimulates the over‐production of white matter astrocytes from precursors of the “brain marrow” in a rodent model of neonatal encephalopathy. PLoS One, 5(3), e9567. 10.1371/journal.pone.0009567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkhuizen, M. , van Mechelen, R. , Vermeer, M. , Chedraui, P. , Paes, D. , van den Hove, D. L. A. , … Gavilanes, A. W. D. (2019). Systemic multipotent adult progenitor cells improve long‐term neurodevelopmental outcomes after preterm hypoxic‐ischemic encephalopathy. Behavioural Brain Research, 362, 77–81. 10.1016/j.bbr.2019.01.016 [DOI] [PubMed] [Google Scholar]
- Bedussi, B. , van Lier, M. G. , Bartstra, J. W. , de Vos, J. , Siebes, M. , VanBavel, E. , & Bakker, E. N. (2015). Clearance from the mouse brain by convection of interstitial fluid towards the ventricular system. Fluids and Barriers of the CNS, 12, 23. 10.1186/s12987-015-0019-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐Zvi, A. , Lacoste, B. , Kur, E. , Andreone, B. J. , Mayshar, Y. , Yan, H. , & Gu, C. (2014). Mfsd2a is critical for the formation and function of the blood‐brain barrier. Nature, 509(7501), 507–511. 10.1038/nature13324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierstone, D. , Wagenaar, N. , Gano, D. L. , Guo, T. , Georgio, G. , Groenendaal, F. , … Miller, S. P. (2018). Association of histologic chorioamnionitis with perinatal brain injury and early childhood neurodevelopmental outcomes among preterm neonates. JAMA Pediatrics, 172(6), 534–541. 10.1001/jamapediatrics.2018.0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiards, S. S. , Haynes, R. L. , Folkerth, R. D. , Borenstein, N. S. , Trachtenberg, F. L. , Rowitch, D. H. , … Kinney, H. C. (2008). Myelin abnormalities without oligodendrocyte loss in periventricular leukomalacia. Brain Pathology, 18(2), 153–163. 10.1111/j.1750-3639.2007.00107.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiards, S. S. , Haynes, R. L. , Folkerth, R. D. , Trachtenberg, F. L. , Liu, L. G. , Volpe, J. J. , & Kinney, H. C. (2006). Development of microglia in the cerebral white matter of the human fetus and infant. Journal of Comparative Neurology, 497(2), 199–208. 10.1002/cne.20991 [DOI] [PubMed] [Google Scholar]
- Bisht, K. , Sharma, K. P. , Lecours, C. , Gabriela Sanchez, M. , El Hajj, H. , Milior, G. , … Tremblay, M. E. (2016). Dark microglia: A new phenotype predominantly associated with pathological states. Glia, 64(5), 826–839. 10.1002/glia.22966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaise, B. J. , Schwendimann, L. , Chhor, V. , Degos, V. , Hodson, M. P. , Dallmann, G. , … Fleiss, B. (2017). Persistently altered metabolic phenotype following perinatal excitotoxic brain injury. Developmental Neuroscience, 39(1‐4), 182–191. 10.1159/000464131 [DOI] [PubMed] [Google Scholar]
- Bobrow, C. S. , & Soothill, P. W. (1999). Causes and consequences of fetal acidosis. Archives of Disease in Childhood. Fetal and Neonatal Edition, 80(3), F246–F249. 10.1136/fn.80.3.f246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccazzi, M. , van Steenwinckel, J. , Schang, A. L. , Faivre, V. , le Charpentier, T. , Bokobza, C. , … Gressens, P. (2021). The immune‐inflammatory response of oligodendrocytes in a murine model of preterm white matter injury: the role of TLR3 activation. Cell Death & Disease, 12(2), 166. 10.1038/s41419-021-03446-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokobza, C. , Joshi, P. , Schang, A. L. , Csaba, Z. , Faivre, V. , Montane, A. , … van Steenwinckel, J. (2022). miR‐146b protects the perinatal brain against microglia‐induced hypomyelination. Annals of Neurology, 91(1), 48–65. 10.1002/ana.26263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordt, E. A. , Ceasrine, A. M. , & Bilbo, S. D. (2020). Microglia and sexual differentiation of the developing brain: A focus on ontogeny and intrinsic factors. Glia, 68(6), 1085–1099. 10.1002/glia.23753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boridy, S. , Soliman, G. M. , & Maysinger, D. (2012). Modulation of inflammatory signaling and cytokine release from microglia by celastrol incorporated into dendrimer nanocarriers. Nanomedicine (London, England), 7(8), 1149–1165. 10.2217/nnm.12.16 [DOI] [PubMed] [Google Scholar]
- Bose, C. L. , Laughon, M. M. , Allred, E. N. , O'Shea, T. M. , van Marter, L. J. , Ehrenkranz, R. A. , … Investigators, E. S. (2013). Systemic inflammation associated with mechanical ventilation among extremely preterm infants. Cytokine, 61(1), 315–322. 10.1016/j.cyto.2012.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehmer, F. , Bendix, I. , Prager, S. , van de Looij, Y. , Reinboth, B. S. , Zimmermanns, J. , … Gerstner, B. (2012). Interaction of inflammation and hyperoxia in a rat model of neonatal white matter damage. PLoS One, 7(11), e49023. 10.1371/journal.pone.0049023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbart, M. , Haynes, M. , Kelley, S. , Angly, F. , Edwards, R. A. , Felts, B. , … Rohwer, F. (2008). Viral diversity and dynamics in an infant gut. Research in Microbiology, 159(5), 367–373. 10.1016/j.resmic.2008.04.006 [DOI] [PubMed] [Google Scholar]
- Brioschi, S. , Belk, J. A. , Peng, V. , Molgora, M. , Rodrigues, P. F. , Nguyen, K. M. , … Colonna, M. (2023). A cre‐deleter specific for embryo‐derived brain macrophages reveals distinct features of microglia and border macrophages. Immunity, 56(5), 1027–1045.e1028. 10.1016/j.immuni.2023.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan, J. , da Costa, N. M. , & Cheadle, L. (2023). Emerging roles of oligodendrocyte precursor cells in neural circuit development and remodeling. Trends in Neurosciences, 46(8), 628–639. 10.1016/j.tins.2023.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser, J. R. , Maire, J. , Riddle, A. , Gong, X. , Nguyen, T. , Nelson, K. , … Back, S. A. (2012). Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Annals of Neurology, 71(1), 93–109. 10.1002/ana.22627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky, O. , Jedrychowski, M. P. , Moore, C. S. , Cialic, R. , Lanser, A. J. , Gabriely, G. , … Weiner, H. L. (2014). Identification of a unique TGF‐beta‐dependent molecular and functional signature in microglia. Nature Neuroscience, 17(1), 131–143. 10.1038/nn.3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky, O. , & Weiner, H. L. (2018). Microglial signatures and their role in health and disease. Nature Reviews. Neuroscience, 19(10), 622–635. 10.1038/s41583-018-0057-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalane, C. , Bonezzi, J. , Shelestak, J. , Clements, R. , Boika, A. , Yun, Y. H. , & Shriver, L. P. (2020). Targeted delivery of anti‐inflammatory and imaging agents to microglial cells with polymeric nanoparticles. Molecular Pharmaceutics, 17(6), 1816–1826. 10.1021/acs.molpharmaceut.9b00489 [DOI] [PubMed] [Google Scholar]
- Cai, J. , Chen, Y. , Cai, W. H. , Hurlock, E. C. , Wu, H. , Kernie, S. G. , … Lu, Q. R. (2007). A crucial role for Olig2 in white matter astrocyte development. Development, 134(10), 1887–1899. 10.1242/dev.02847 [DOI] [PubMed] [Google Scholar]
- Cai, J. , Tuong, C. M. , Zhang, Y. , Shields, C. B. , Guo, G. , Fu, H. , & Gozal, D. (2012). Mouse intermittent hypoxia mimicking apnoea of prematurity: effects on myelinogenesis and axonal maturation. Journal of Pathology, 226(3), 495–508. 10.1002/path.2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkavur, S. , Akisu, M. , Olukman, O. , Balim, Z. , Berdeli, A. , Cakmak, B. , … Kultursay, N. (2011). Genetic factors that influence short‐term neurodevelopmental outcome in term hypoxic‐ischaemic encephalopathic neonates. Journal of International Medical Research, 39(5), 1744–1756. 10.1177/147323001103900517 [DOI] [PubMed] [Google Scholar]
- Cawley, P. A. , Nosarti, C. , & Edwards, A. D. (2022). In‐unit neonatal magnetic resonance imaging‐new possibilities offered by low‐field technology. Journal of Perinatology, 42(7), 843–844. 10.1038/s41372-022-01401-w [DOI] [PubMed] [Google Scholar]
- Cebeci, B. , Alderliesten, T. , Wijnen, J. P. , van der Aa, N. E. , Benders, M. , de Vries, L. S. , … Groenendaal, F. (2022). Brain proton magnetic resonance spectroscopy and neurodevelopment after preterm birth: a systematic review. Pediatric Research, 91(6), 1322–1333. 10.1038/s41390-021-01539-x [DOI] [PubMed] [Google Scholar]
- Cerghet, M. , Skoff, R. P. , Bessert, D. , Zhang, Z. , Mullins, C. , & Ghandour, M. S. (2006). Proliferation and death of oligodendrocytes and myelin proteins are differentially regulated in male and female rodents. Journal of Neuroscience, 26(5), 1439–1447. 10.1523/JNEUROSCI.2219-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalak, L. F. , Rollins, N. , Morriss, M. C. , Brion, L. P. , Heyne, R. , & Sanchez, P. J. (2012). Perinatal acidosis and hypoxic‐ischemic encephalopathy in preterm infants of 33 to 35 weeks' gestation. Journal of Pediatrics, 160(3), 388–394. 10.1016/j.jpeds.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamnanvanakij, S. , Margraf, L. R. , Burns, D. , & Perlman, J. M. (2002). Apoptosis and white matter injury in preterm infants. Pediatric and Developmental Pathology, 5(2), 184–189. 10.1007/s10024001-0205-0 [DOI] [PubMed] [Google Scholar]
- Charriaut‐Marlangue, C. , Besson, V. C. , & Baud, O. (2017). Sexually dimorphic outcomes after neonatal stroke and hypoxia‐ischemia. International Journal of Molecular Sciences, 19(1), 61. 10.3390/ijms19010061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Li, H. , Zhao, T. , Chen, K. , Chen, M. H. , Sun, Z. , … Cong, X. S. (2023). The Impact of early life experiences and gut microbiota on neurobehavioral development in preterm infants: A longitudinal cohort study. Microorganisms, 11(3), 814. 10.3390/microorganisms11030814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, V. S. , Morrison, J. P. , Southwell, M. F. , Foley, J. F. , Bolon, B. , & Elmore, S. A. (2017). Histology atlas of the developing prenatal and postnatal mouse central nervous system, with emphasis on prenatal days E7.5 to E18.5. Toxicologic Pathology, 45(6), 705–744. 10.1177/0192623317728134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Hong, T. , Chen, F. , Sun, Y. , Wang, Y. , & Cui, L. (2021). Interplay between microglia and Alzheimer's disease‐focus on the most relevant risks: APOE genotype, sex and age. Frontiers in Aging Neuroscience, 13, 631827. 10.3389/fnagi.2021.631827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhor, V. , Le Charpentier, T. , Lebon, S. , Ore, M. V. , Celador, I. L. , Josserand, J. , … Fleiss, B. (2013). Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain, Behavior, and Immunity, 32, 70–85. 10.1016/j.bbi.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhor, V. , Moretti, R. , Le Charpentier, T. , Sigaut, S. , Lebon, S. , Schwendimann, L. , … Fleiss, B. (2017). Role of microglia in a mouse model of paediatric traumatic brain injury. Brain, Behavior, and Immunity, 63, 197–209. 10.1016/j.bbi.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, J. , Bennett, P. R. , Kim, S. H. , Teoh, T. G. , Sykes, L. , Kindinger, L. M. , … Terzidou, V. (2019). First trimester circulating microRNA biomarkers predictive of subsequent preterm delivery and cervical shortening. Scientific Reports, 9(1), 5861. 10.1038/s41598-019-42166-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro, C. N. , Savva, Y. , Vaidya, D. , Argani, C. H. , Hong, X. , Wang, X. , & Burd, I. (2016). Mathematical modeling of the biomarker milieu to characterize preterm birth and predict adverse neonatal outcomes. American Journal of Reproductive Immunology, 75(5), 594–601. 10.1111/aji.12502 [DOI] [PubMed] [Google Scholar]
- Craig, A. , Ling Luo, N. , Beardsley, D. J. , Wingate‐Pearse, N. , Walker, D. W. , Hohimer, A. R. , & Back, S. A. (2003). Quantitative analysis of perinatal rodent oligodendrocyte lineage progression and its correlation with human. Experimental Neurology, 181(2), 231–240. [DOI] [PubMed] [Google Scholar]
- Cugurra, A. , Mamuladze, T. , Rustenhoven, J. , Dykstra, T. , Beroshvili, G. , Greenberg, Z. J. , … Kipnis, J. (2021). Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science, 373(6553), eabf7844. 10.1126/science.abf7844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, J. , Shipley, F. B. , Shannon, M. L. , Alturkistani, O. , Dani, N. , Webb, M. D. , … Lehtinen, M. K. (2020). Inflammation of the embryonic choroid plexus barrier following maternal immune activation. Developmental Cell, 55(5), 617–628.e616. 10.1016/j.devcel.2020.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalys, G. B. D. , & Collaborators, H. (2016). Global, regional, and national disability‐adjusted life‐years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990‐2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet, 388(10053), 1603–1658. 10.1016/S0140-6736(16)31460-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnall, R. A. , Chen, X. , Nemani, K. V. , Sirieix, C. M. , Gimi, B. , Knoblach, S. , … Hunt, C. E. (2017). Early postnatal exposure to intermittent hypoxia in rodents is proinflammatory, impairs white matter integrity, and alters brain metabolism. Pediatric Research, 82(1), 164–172. 10.1038/pr.2017.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, J. O. , Drury, P. P. , Green, C. R. , Nicholson, L. F. , Bennet, L. , & Gunn, A. J. (2014). Connexin hemichannel blockade is neuroprotective after asphyxia in preterm fetal sheep. PLoS One, 9(5), e96558. 10.1371/journal.pone.0096558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, J. O. , van den Heuij, L. G. , Fraser, M. , Wassink, G. , Miller, S. L. , Lim, R. , … Bennet, L. (2021). Window of opportunity for human amnion epithelial stem cells to attenuate astrogliosis after umbilical cord occlusion in preterm fetal sheep. Stem Cells Translational Medicine, 10(3), 427–440. 10.1002/sctm.20-0314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vlaminck, K. , Van Hove, H. , Kancheva, D. , Scheyltjens, I. , Pombo Antunes, A. R. , Bastos, J. , … Movahedi, K. (2022). Differential plasticity and fate of brain‐resident and recruited macrophages during the onset and resolution of neuroinflammation. Immunity, 55(11), 2085–2102 e2089. 10.1016/j.immuni.2022.09.005 [DOI] [PubMed] [Google Scholar]
- Dean, J. M. , Farrag, D. , Zahkouk, S. A. , El Zawahry, E. Y. , Hagberg, H. , Kjellmer, I. , & Mallard, C. (2009). Cerebellar white matter injury following systemic endotoxemia in preterm fetal sheep. Neuroscience, 160(3), 606–615. 10.1016/j.neuroscience.2009.02.071 [DOI] [PubMed] [Google Scholar]
- Dean, J. M. , Riddle, A. , Maire, J. , Hansen, K. D. , Preston, M. , Barnes, A. P. , … Back, S. A. (2011). An organotypic slice culture model of chronic white matter injury with maturation arrest of oligodendrocyte progenitors. Molecular Neurodegeneration, 6, 46. 10.1186/1750-1326-6-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean, J. M. , Wang, X. , Kaindl, A. M. , Gressens, P. , Fleiss, B. , Hagberg, H. , & Mallard, C. (2010). Microglial MyD88 signaling regulates acute neuronal toxicity of LPS‐stimulated microglia in vitro. Brain, Behavior, and Immunity, 24(5), 776–783. 10.1016/j.bbi.2009.10.018 [DOI] [PubMed] [Google Scholar]
- Denney, J. M. , Nelson, E. , Wadhwa, P. , Waters, T. , Mathew, L. , Goldenberg, R. L. , & Culhane, J. F. (2021). Cytokine profiling: variation in immune modulation with preterm birth vs. uncomplicated term birth identifies pivotal signals in pathogenesis of preterm birth. Journal of Perinatal Medicine, 49(3), 299–309. 10.1515/jpm-2020-0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki, N. C. , Cardani, A. N. , Yang, C. H. , Quinnies, K. M. , Crihfield, A. , Lynch, K. R. , & Kipnis, J. (2010). Regulation of learning and memory by meningeal immunity: a key role for IL‐4. Journal of Experimental Medicine, 207(5), 1067–1080. 10.1084/jem.20091419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet, L. , Clarke, G. , Shanahan, F. , Dinan, T. G. , & Cryan, J. F. (2014). Microbiota is essential for social development in the mouse. Molecular Psychiatry, 19(2), 146–148. 10.1038/mp.2013.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desilva, T. M. , Billiards, S. S. , Borenstein, N. S. , Trachtenberg, F. L. , Volpe, J. J. , Kinney, H. C. , & Rosenberg, P. A. (2008). Glutamate transporter EAAT2 expression is up‐regulated in reactive astrocytes in human periventricular leukomalacia. Journal of Comparative Neurology, 508(2), 238–248. 10.1002/cne.21667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio, F. , Vultaggio‐Poma, V. , Falzoni, S. , & Giuliani, A. L. (2023). The coming of age of the P2X7 receptor in diagnostic medicine. International Journal of Molecular Sciences, 24(11), 9465. 10.3390/ijms24119465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty, P. , Fruns, M. , Seaton, P. , Dickson, G. , Barton, C. H. , Sears, T. A. , & Walsh, F. S. (1990). A threshold effect of the major isoforms of NCAM on neurite outgrowth. Nature, 343(6257), 464–466. 10.1038/343464a0 [DOI] [PubMed] [Google Scholar]
- Dommergues, M. A. , Patkai, J. , Renauld, J. C. , Evrard, P. , & Gressens, P. (2000). Proinflammatory cytokines and interleukin‐9 exacerbate excitotoxic lesions of the newborn murine neopallium. Annals of Neurology, 47(1), 54–63. [PubMed] [Google Scholar]
- Dommergues, M. A. , Plaisant, F. , Verney, C. , & Gressens, P. (2003). Early microglial activation following neonatal excitotoxic brain damage in mice: A potential target for neuroprotection. Neuroscience, 121(3), 619–628. [DOI] [PubMed] [Google Scholar]
- Doria, V. , Arichi, T. , & Edwards, D. A. (2014). Magnetic resonance imaging of the preterm infant brain. Current Pediatric Reviews, 10(1), 48–55. 10.2174/157339631001140408120821 [DOI] [PubMed] [Google Scholar]
- Drieu, A. , Du, S. , Storck, S. E. , Rustenhoven, J. , Papadopoulos, Z. , Dykstra, T. , … Kipnis, J. (2022). Parenchymal border macrophages regulate the flow dynamics of the cerebrospinal fluid. Nature, 611(7936), 585–593. 10.1038/s41586-022-05397-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, X. , Fleiss, B. , Li, H. , D'Angelo, B. , Sun, Y. , Zhu, C. , … Wang, X. (2011). Systemic stimulation of TLR2 impairs neonatal mouse brain development. PLoS One, 6(5), e19583. 10.1371/journal.pone.0019583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudink, J. , Jeanne Steggerda, S. , Horsch, S. , & Eur, U. G. (2020). State‐of‐the‐art neonatal cerebral ultrasound: technique and reporting. Pediatric Research, 87(1), 3–12. 10.1038/s41390-020-0776-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupree, J. L. , Coetzee, T. , Suzuki, K. , & Popko, B. (1998). Myelin abnormalities in mice deficient in galactocerebroside and sulfatide. Journal of Neurocytology, 27(9), 649–659. 10.1023/a:1006908013972 [DOI] [PubMed] [Google Scholar]
- El‐Khoury, N. , Braun, A. , Hu, F. , Pandey, M. , Nedergaard, M. , Lagamma, E. F. , & Ballabh, P. (2006). Astrocyte end‐feet in germinal matrix, cerebral cortex, and white matter in developing infants. Pediatric Research, 59(5), 673–679. 10.1203/01.pdr.0000214975.85311.9c [DOI] [PubMed] [Google Scholar]
- Elmariah, S. B. , Oh, E. J. , Hughes, E. G. , & Balice‐Gordon, R. J. (2005). Astrocytes regulate inhibitory synapse formation via Trk‐mediated modulation of postsynaptic GABAA receptors. The Journal of Neuroscience, 25(14), 3638–3650. 10.1523/JNEUROSCI.3980-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erny, D. , Hrabe de Angelis, A. L. , Jaitin, D. , Wieghofer, P. , Staszewski, O. , David, E. , … Prinz, M. (2015). Host microbiota constantly control maturation and function of microglia in the CNS. Nature Neuroscience, 18(7), 965–977. 10.1038/nn.4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin, C. , Galea, E. , Lakatos, A. , O'Callaghan, J. P. , Petzold, G. C. , Serrano‐Pozo, A. , … Verkhratsky, A. (2021). Reactive astrocyte nomenclature, definitions, and future directions. Nature Neuroscience, 24(3), 312–325. 10.1038/s41593-020-00783-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagbemi, A. O. , Torrente, F. , Puleston, J. , Lakhoo, K. , James, S. , & Murch, S. H. (2013). Enteric neural disruption in necrotizing enterocolitis occurs in association with myenteric glial cell CCL20 expression. Journal of Pediatric Gastroenterology and Nutrition, 57(6), 788–793. 10.1097/MPG.0b013e3182a86fd4 [DOI] [PubMed] [Google Scholar]
- Falahati, S. , Breu, M. , Waickman, A. T. , Phillips, A. W. , Arauz, E. J. , Snyder, S. , … Fatemi, A. (2013). Ischemia‐induced neuroinflammation is associated with disrupted development of oligodendrocyte progenitors in a model of periventricular leukomalacia. Developmental Neuroscience, 35(2‐3), 182–196. 10.1159/000346682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, L. W. , Pang, Y. , Lin, S. , Rhodes, P. G. , & Cai, Z. (2005). Minocycline attenuates lipopolysaccharide‐induced white matter injury in the neonatal rat brain. Neuroscience, 133(1), 159–168. 10.1016/j.neuroscience.2005.02.016 [DOI] [PubMed] [Google Scholar]
- Faraco, G. , Park, L. , Anrather, J. , & Iadecola, C. (2017). Brain perivascular macrophages: characterization and functional roles in health and disease. Journal of Molecular Medicine (Berlin, Germany), 95(11), 1143–1152. 10.1007/s00109-017-1573-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustino, J. V. , Wang, X. , Johnson, C. E. , Klibanov, A. , Derugin, N. , Wendland, M. F. , & Vexler, Z. S. (2011). Microglial cells contribute to endogenous brain defenses after acute neonatal focal stroke. Journal of Neuroscience, 31(36), 12992–13001. 10.1523/JNEUROSCI.2102-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favrais, G. , van de Looij, Y. , Fleiss, B. , Ramanantsoa, N. , Bonnin, P. , Stoltenburg‐Didinger, G. , … Gressens, P. (2011). Systemic inflammation disrupts the developmental program of white matter. Annals of Neurology, 70(4), 550–565. 10.1002/ana.22489 [DOI] [PubMed] [Google Scholar]
- Fleiss, B. , Coleman, H. A. , Castillo‐Melendez, M. , Ireland, Z. , Walker, D. W. , & Parkington, H. C. (2011). Effects of birth asphyxia on neonatal hippocampal structure and function in the spiny mouse. International Journal of Developmental Neuroscience, 29(7), 757–766. 10.1016/j.ijdevneu.2011.05.006 [DOI] [PubMed] [Google Scholar]
- Fleiss, B. , & Gressens, P. (2012). Tertiary mechanisms of brain damage: a new hope for treatment of cerebral palsy? Lancet Neurology, 11(6), 556–566. 10.1016/S1474-4422(12)70058-3 [DOI] [PubMed] [Google Scholar]
- Fleiss, B. , Nilsson, M. K. , Blomgren, K. , & Mallard, C. (2012). Neuroprotection by the histone deacetylase inhibitor trichostatin A in a model of lipopolysaccharide‐sensitised neonatal hypoxic‐ischaemic brain injury. Journal of Neuroinflammation, 9(1), 70. 10.1186/1742-2094-9-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foong, J. P. P. , Hung, L. Y. , Poon, S. , Savidge, T. C. , & Bornstein, J. C. (2020). Early life interaction between the microbiota and the enteric nervous system. American Journal of Physiology. Gastrointestinal and Liver Physiology, 319(5), G541–G548. 10.1152/ajpgi.00288.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furfaro, L. L. , Chang, B. J. , & Payne, M. S. (2017). Applications for bacteriophage therapy during pregnancy and the perinatal period. Frontiers in Microbiology, 8, 2660. 10.3389/fmicb.2017.02660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness, J. B. , Callaghan, B. P. , Rivera, L. R. , & Cho, H. J. (2014). The enteric nervous system and gastrointestinal innervation: integrated local and central control. Advances in Experimental Medicine and Biology, 817, 39–71. 10.1007/978-1-4939-0897-4_3 [DOI] [PubMed] [Google Scholar]
- Galinsky, R. , Dhillon, S. K. , Dean, J. M. , Davidson, J. O. , Lear, C. A. , Wassink, G. , … Gunn, A. J. (2020). Tumor necrosis factor inhibition attenuates white matter gliosis after systemic inflammation in preterm fetal sheep. Journal of Neuroinflammation, 17(1), 92. 10.1186/s12974-020-01769-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinsky, R. , Lear, C. A. , Dean, J. M. , Wassink, G. , Dhillon, S. K. , Fraser, M. , … Gunn, A. J. (2018). Complex interactions between hypoxia‐ischemia and inflammation in preterm brain injury. Developmental Medicine and Child Neurology, 60(2), 126–133. 10.1111/dmcn.13629 [DOI] [PubMed] [Google Scholar]
- Galinsky, R. , van de Looij, Y. , Mitchell, N. , Dean, J. M. , Dhillon, S. K. , Yamaguchi, K. , … Gunn, A. J. (2020). Magnetic resonance imaging correlates of white matter gliosis and injury in preterm fetal sheep exposed to progressive systemic inflammation. International Journal of Molecular Sciences, 21(23), 8891. 10.3390/ijms21238891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau, M. G. , Wine, E. , Rodrigues, D. M. , Cho, J. H. , Whary, M. T. , Philpott, D. J. , … Sherman, P. M. (2011). Bacterial infection causes stress‐induced memory dysfunction in mice. Gut, 60(3), 307–317. 10.1136/gut.2009.202515 [DOI] [PubMed] [Google Scholar]
- Garnier, Y. , Berger, R. , Alm, S. , von Duering, M. U. , Coumans, A. B. , Michetti, F. , … Gazzolo, D. (2006). Systemic endotoxin administration results in increased S100B protein blood levels and periventricular brain white matter injury in the preterm fetal sheep. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 124(1), 15–22. 10.1016/j.ejogrb.2005.05.014 [DOI] [PubMed] [Google Scholar]
- Gibson, M. K. , Wang, B. , Ahmadi, S. , Burnham, C. A. , Tarr, P. I. , Warner, B. B. , & Dantas, G. (2016). Developmental dynamics of the preterm infant gut microbiota and antibiotic resistome. Nature Microbiology, 1, 16024. 10.1038/nmicrobiol.2016.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist, C. P. , Thompson, D. K. , Kelly, C. E. , Beare, R. , Adamson, C. , Dhollander, T. , … Anderson, P. J. (2022). The structural connectome and internalizing and externalizing symptoms at 7 and 13 years in individuals born very preterm and full term. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 7(4), 424–434. 10.1016/j.bpsc.2021.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles, F. , Gressens, P. , Dammann, O. , & Leviton, A. (2017). Hypoxia‐ischemia is not an antecedent of most preterm brain damage: the illusion of validity. Developmental Medicine and Child Neurology, 60(2), 120–125. 10.1111/dmcn.13483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux, F. , Greter, M. , Leboeuf, M. , Nandi, S. , See, P. , Gokhan, S. , … Merad, M. (2010). Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science, 330(6005), 841–845. 10.1126/science.1194637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux, F. , Lim, S. , Hoeffel, G. , Low, D. , & Huber, T. (2013). Origin and differentiation of microglia. Frontiers in Cellular Neuroscience, 7, 45. 10.3389/fncel.2013.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gire, C. , Berbis, J. , Dequin, M. , Marret, S. , Muller, J. B. , Saliba, E. , & Tosello, B. (2022). A correlation between magnetic resonance spectroscopy (1‐H MRS) and the neurodevelopment of two‐year‐olds born preterm in an EPIRMEX cohort study. Frontiers in Pediatrics, 10, 936130. 10.3389/fped.2022.936130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann, T. , Wieghofer, P. , Jordao, M. J. , Prutek, F. , Hagemeyer, N. , Frenzel, K. , … Prinz, M. (2016). Origin, fate and dynamics of macrophages at central nervous system interfaces. Nature Immunology, 17(7), 797–805. 10.1038/ni.3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grontoft, O. (1954). Intracranial haemorrhage and blood‐brain barrier problems in the new‐born: A pathologico‐anatomical and experimental investigation. Acta Pathologica et Microbiologica Scandinavica, 100, 8–109. [PubMed] [Google Scholar]
- Gussenhoven, R. , Ophelders, D. , Dudink, J. , Pieterman, K. , Lammens, M. , Mays, R. W. , … Jellema, R. K. (2021). Systemic multipotent adult progenitor cells protect the cerebellum after asphyxia in fetal sheep. Stem Cells Translational Medicine, 10(1), 57–67. 10.1002/sctm.19-0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussenhoven, R. , Westerlaken, R. J. J. , Ophelders, D. , Jobe, A. H. , Kemp, M. W. , Kallapur, S. G. , … Wolfs, T. (2018). Chorioamnionitis, neuroinflammation, and injury: timing is key in the preterm ovine fetus. Journal of Neuroinflammation, 15(1), 113. 10.1186/s12974-018-1149-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg, H. , Mallard, C. , Ferriero, D. M. , Vannucci, S. J. , Levison, S. W. , Vexler, Z. S. , & Gressens, P. (2015). The role of inflammation in perinatal brain injury. Nature Reviews. Neurology, 11(4), 192–208. 10.1038/nrneurol.2015.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemeyer, N. , Hanft, K. M. , Akriditou, M. A. , Unger, N. , Park, E. S. , Stanley, E. R. , … Prinz, M. (2017). Microglia contribute to normal myelinogenesis and to oligodendrocyte progenitor maintenance during adulthood. Acta Neuropathologica, 134(3), 441–458. 10.1007/s00401-017-1747-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldipur, P. , Bharti, U. , Alberti, C. , Sarkar, C. , Gulati, G. , Iyengar, S. , … Mani, S. (2011). Preterm delivery disrupts the developmental program of the cerebellum. PLoS One, 6(8), e23449. 10.1371/journal.pone.0023449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldipur, P. , Dupuis, N. , Degos, V. , Moniaux, N. , Chhor, V. , Rasika, S. , … Gressens, P. (2014). HIP/PAP prevents excitotoxic neuronal death and promotes plasticity. Annals of Clinical Translational Neurology, 1(10), 739–754. 10.1002/acn3.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond, T. R. , Dufort, C. , Dissing‐Olesen, L. , Giera, S. , Young, A. , Wysoker, A. , … Stevens, B. (2019). Single‐cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell‐state changes. Immunity, 50(1), 253–271.e256. 10.1016/j.immuni.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrick, S. E. , Miller, S. P. , Leonard, C. , Glidden, D. V. , Goldstein, R. , Ramaswamy, V. , … Ferriero, D. M. (2004). Trends in severe brain injury and neurodevelopmental outcome in premature newborn infants: the role of cystic periventricular leukomalacia. Journal of Pediatrics, 145(5), 593–599. 10.1016/j.jpeds.2004.05.042 [DOI] [PubMed] [Google Scholar]
- Hanamsagar, R. , Alter, M. D. , Block, C. S. , Sullivan, H. , Bolton, J. L. , & Bilbo, S. D. (2018). Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia, 66(2), 460. 10.1002/glia.23277 [DOI] [PubMed] [Google Scholar]
- Hansen‐Pupp, I. , Harling, S. , Berg, A. C. , Cilio, C. , Hellstrom‐Westas, L. , & Ley, D. (2005). Circulating interferon‐gamma and white matter brain damage in preterm infants. Pediatric Research, 58(5), 946–952. 10.1203/01.PDR.0000182592.76702.E8 [DOI] [PubMed] [Google Scholar]
- Haynes, R. L. , Billiards, S. S. , Borenstein, N. S. , Volpe, J. J. , & Kinney, H. C. (2008). Diffuse axonal injury in periventricular leukomalacia as determined by apoptotic marker fractin. Pediatric Research, 63(6), 656–661. 10.1203/PDR.0b013e31816c825c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes, R. L. , Folkerth, R. D. , Keefe, R. J. , Sung, I. , Swzeda, L. I. , Rosenberg, P. A. , … Kinney, H. C. (2003). Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. Journal of Neuropathology and Experimental Neurology, 62(5), 441–450. [DOI] [PubMed] [Google Scholar]
- Haynes, R. L. , & van Leyen, K. (2013). 12/15‐lipoxygenase expression is increased in oligodendrocytes and microglia of periventricular leukomalacia. Developmental Neuroscience, 35(2‐3), 140–154. 10.1159/000350230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Q. , Ma, Y. , Liu, J. , Zhang, D. , Ren, J. , Zhao, R. , … Yang, Y. (2021). Biological functions and regulatory mechanisms of hypoxia‐inducible factor‐1alpha in ischemic stroke. Frontiers in Immunology, 12, 801985. 10.3389/fimmu.2021.801985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy, D. B. , Ryan, C. A. , Ross, R. P. , Stanton, C. , & Dempsey, E. M. (2022). Clinical implications of preterm infant gut microbiome development. Nature Microbiology, 7(1), 22–33. 10.1038/s41564-021-01025-4 [DOI] [PubMed] [Google Scholar]
- Helderman, J. B. , Welch, C. D. , Leng, X. , & O'Shea, T. M. (2010). Sepsis‐associated electroencephalographic changes in extremely low gestational age neonates. Early Human Development, 86(8), 509–513. 10.1016/j.earlhumdev.2010.06.006 [DOI] [PubMed] [Google Scholar]
- Hellstrom Erkenstam, N. , Smith, P. L. , Fleiss, B. , Nair, S. , Svedin, P. , Wang, W. , … Mallard, C. (2016). Temporal characterization of microglia/macrophage phenotypes in a mouse model of neonatal hypoxic‐ischemic brain injury. Frontiers in Cellular Neuroscience, 10, 286. 10.3389/fncel.2016.00286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway, R. K. , Ireland, G. , Sullivan, G. , Becher, J. C. , Smith, C. , Boardman, J. P. , … Miron, V. E. (2021). Microglial inflammasome activation drives developmental white matter injury. Glia, 69(5), 1268–1280. 10.1002/glia.23963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway, R. K. , Zhang, L. , Molina‐Gonzalez, I. , Ton, K. , Nicoll, J. A. R. , Boardman, J. P. , … Miron, V. E. (2023). Localized microglia dysregulation impairs central nervous system myelination in development. Acta Neuropathologica Communications, 11(1), 49. 10.1186/s40478-023-01543-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornaday, K. K. , Wood, E. M. , & Slater, D. M. (2022). Is there a maternal blood biomarker that can predict spontaneous preterm birth prior to labour onset? A systematic review. PLoS One, 17(4), e0265853. 10.1371/journal.pone.0265853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, S. , Zhang, Z. , Cao, J. , Yu, Y. , & Pei, G. (2022). Chimeric cerebral organoids reveal the essentials of neuronal and astrocytic APOE4 for Alzheimer's tau pathology. Signal Transduction and Targeted Therapy, 7(1), 176. 10.1038/s41392-022-01006-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, E. G. , Elmariah, S. B. , & Balice‐Gordon, R. J. (2010). Astrocyte secreted proteins selectively increase hippocampal GABAergic axon length, branching, and synaptogenesis. Molecular and Cellular Neurosciences, 43(1), 136–145. 10.1016/j.mcn.2009.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humberg, A. , Fortmann, I. , Siller, B. , Kopp, M. V. , Herting, E. , Gopel, W. , … Priming Immunity at the beginning of life, C . (2020). Preterm birth and sustained inflammation: consequences for the neonate. Seminars in Immunopathology, 42(4), 451–468. 10.1007/s00281-020-00803-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyodo, R. , Sato, Y. , Ito, M. , Sugiyama, Y. , Ogawa, C. , Kawai, H. , … Hayakawa, M. (2018). Magnetic resonance spectroscopy in preterm infants: association with neurodevelopmental outcomes. Archives of Disease in Childhood. Fetal and Neonatal Edition, 103(3), F238–F244. 10.1136/archdischild-2016-311403 [DOI] [PubMed] [Google Scholar]
- Inder, T. E. , de Vries, L. S. , Ferriero, D. M. , Grant, P. E. , Ment, L. R. , Miller, S. P. , & Volpe, J. J. (2021). Neuroimaging of the preterm brain: Review and recommendations. The Journal of Pediatrics, 237(276‐287), e274. 10.1016/j.jpeds.2021.06.014 [DOI] [PubMed] [Google Scholar]
- Iyer, K. K. , Roberts, J. A. , Metsaranta, M. , Finnigan, S. , Breakspear, M. , & Vanhatalo, S. (2014). Novel features of early burst suppression predict outcome after birth asphyxia. Annals of Clinical Translational Neurology, 1(3), 209–214. 10.1002/acn3.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonska, B. , Scafidi, J. , Aguirre, A. , Vaccarino, F. , Nguyen, V. , Borok, E. , … Gallo, V. (2012). Oligodendrocyte regeneration after neonatal hypoxia requires FoxO1‐mediated p27Kip1 expression. Journal of Neuroscience, 32(42), 14775–14793. 10.1523/JNEUROSCI.2060-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan, I. , Muhit, M. , Hardianto, D. , Laryea, F. , Chhetri, A. B. , Smithers‐Sheedy, H. , … Khandaker, G. (2021). Epidemiology of cerebral palsy in low‐ and middle‐income countries: preliminary findings from an international multi‐centre cerebral palsy register. Developmental Medicine and Child Neurology, 63(11), 1327–1336. 10.1111/dmcn.14926 [DOI] [PubMed] [Google Scholar]
- Jarazo, J. , Barmpa, K. , Modamio, J. , Saraiva, C. , Sabate‐Soler, S. , Rosety, I. , … Schwamborn, J. C. (2022). Parkinson's disease phenotypes in patient neuronal cultures and brain organoids improved by 2‐hydroxypropyl‐beta‐cyclodextrin treatment. Movement Disorders, 37(1), 80–94. 10.1002/mds.28810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarlestedt, K. , Rousset, C. I. , Faiz, M. , Wilhelmsson, U. , Stahlberg, A. , Sourkova, H. , … Pekny, M. (2010). Attenuation of reactive gliosis does not affect infarct volume in neonatal hypoxic‐ischemic brain injury in mice. PLoS One, 5(4), e10397. 10.1371/journal.pone.0010397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellema, R. K. , Ophelders, D. R. , Zwanenburg, A. , Nikiforou, M. , Delhaas, T. , Andriessen, P. , … Kramer, B. W. (2015). Multipotent adult progenitor cells for hypoxic‐ischemic injury in the preterm brain. Journal of Neuroinflammation, 12, 241. 10.1186/s12974-015-0459-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelliffe‐Pawlowski, L. L. , Rand, L. , Bedell, B. , Baer, R. J. , Oltman, S. P. , Norton, M. E. , … Ryckman, K. K. (2018). Prediction of preterm birth with and without preeclampsia using mid‐pregnancy immune and growth‐related molecular factors and maternal characteristics. Journal of Perinatology, 38(8), 963–972. 10.1038/s41372-018-0112-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinnai, M. , Koning, G. , Singh‐Mallah, G. , Jonsdotter, A. , Leverin, A.‐L. , Svedin, P. , … Hagberg, H. (2020). A model of germinal matrix hemorrhage in preterm rat pups. Frontiers in Cellular Neuroscience, 14, 535320. 10.3389/fncel.2020.535320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jisa, K. A. , Clarey, D. D. , & Peeples, E. S. (2018). Magnetic resonance imaging findings of term and preterm hypoxic‐ischemic encephalopathy: A review of relevant animal models and correlation to human imaging. Open Neuroimaging Journal, 12, 55–65. 10.2174/1874440001812010055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano, C. , Sosunov, S. , Niatsetskaya, Z. , Isler, J. A. , Utkina‐Sosunova, I. , Jang, I. , … Ten, V. (2015). Mild intermittent hypoxemia in neonatal mice causes permanent neurofunctional deficit and white matter hypomyelination. Experimental Neurology, 264, 33–42. 10.1016/j.expneurol.2014.11.010 [DOI] [PubMed] [Google Scholar]
- Kabouridis, P. S. , Lasrado, R. , McCallum, S. , Chng, S. H. , Snippert, H. J. , Clevers, H. , … Pachnis, V. (2015). Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron, 85(2), 289–295. 10.1016/j.neuron.2014.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin, E. A. , Rodriguez, C. , Hall‐Moore, C. , Hoffmann, J. A. , Linneman, L. A. , Ndao, I. M. , … Lim, E. S. (2022). Longitudinal gut virome analysis identifies specific viral signatures that precede necrotizing enterocolitis onset in preterm infants. Nature Microbiology, 7(5), 653–662. 10.1038/s41564-022-01096-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajantie, E. , Strang‐Karlsson, S. , Evensen, K. A. I. , & Haaramo, P. (2019). Adult outcomes of being born late preterm or early term – What do we know? Seminars in Fetal & Neonatal Medicine, 24(1), 66–83. 10.1016/j.siny.2018.11.001 [DOI] [PubMed] [Google Scholar]
- Kanemaru, K. , Okubo, Y. , Hirose, K. , & Iino, M. (2007). Regulation of neurite growth by spontaneous Ca2+ oscillations in astrocytes. The Journal of Neuroscience, 27(33), 8957–8966. 10.1523/JNEUROSCI.2276-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan, S. , Dai, H. , Navath, R. S. , Balakrishnan, B. , Jyoti, A. , Janisse, J. , … Kannan, R. M. (2012). Dendrimer‐based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Science Translational Medicine, 4(130), 130ra146. 10.1126/scitranslmed.3003162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaukola, T. , Herva, R. , Perhomaa, M. , Paakko, E. , Kingsmore, S. , Vainionpaa, L. , & Hallman, M. (2006). Population cohort associating chorioamnionitis, cord inflammatory cytokines and neurologic outcome in very preterm, extremely low birth weight infants. Pediatric Research, 59(3), 478–483. 10.1203/01.pdr.0000182596.66175.ee [DOI] [PubMed] [Google Scholar]
- Kelly, S. B. , Stojanovska, V. , Zahra, V. A. , Moxham, A. , Miller, S. L. , Moss, T. J. M. , … Galinsky, R. (2021). Interleukin‐1 blockade attenuates white matter inflammation and oligodendrocyte loss after progressive systemic lipopolysaccharide exposure in near‐term fetal sheep. Journal of Neuroinflammation, 18(1), 189. 10.1186/s12974-021-02238-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierdorf, K. , Erny, D. , Goldmann, T. , Sander, V. , Schulz, C. , Perdiguero, E. G. , … Prinz, M. (2013). Microglia emerge from erythromyeloid precursors via Pu.1‐ and Irf8‐dependent pathways. Nature Neuroscience, 16(3), 273–280. 10.1038/nn.3318 [DOI] [PubMed] [Google Scholar]
- Kim, C. J. , Romero, R. , Chaemsaithong, P. , Chaiyasit, N. , Yoon, B. H. , & Kim, Y. M. (2015). Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. American Journal of Obstetrics and Gynecology, 213(4), S29–S52. 10.1016/j.ajog.2015.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. J. , Cho, M. H. , Shim, W. H. , Kim, J. K. , Jeon, E. Y. , Kim, D. H. , & Yoon, S. Y. (2017). Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Molecular Psychiatry, 22(11), 1576–1584. 10.1038/mp.2016.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney, H. C. , Haynes, R. L. , Xu, G. , Andiman, S. E. , Folkerth, R. D. , Sleeper, L. A. , & Volpe, J. J. (2012). Neuron deficit in the white matter and subplate in periventricular leukomalacia. Annals of Neurology, 71(3), 397–406. 10.1002/ana.22612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby, L. , & Castelo‐Branco, G. (2021). Crossing boundaries: Interplay between the immune system and oligodendrocyte lineage cells. Seminars in Cell & Developmental Biology, 116, 45–52. 10.1016/j.semcdb.2020.10.013 [DOI] [PubMed] [Google Scholar]
- Kirby, L. , Jin, J. , Cardona, J. G. , Smith, M. D. , Martin, K. A. , Wang, J. , … Calabresi, P. A. (2019). Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination. Nature Communications, 10(1), 3887. 10.1038/s41467-019-11638-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, L. , Van Steenwinckel, J. , Fleiss, B. , Scheuer, T. , Buhrer, C. , Faivre, V. , … Schmitz, T. (2022). A unique cerebellar pattern of microglia activation in a mouse model of encephalopathy of prematurity. Glia, 70(9), 1699–1719. 10.1002/glia.24190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama, L. , & Gan, L. (2019). Do microglial sex differences contribute to sex differences in neurodegenerative diseases? Trends in Molecular Medicine, 25(9), 741–749. 10.1016/j.molmed.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovler, M. L. , Gonzalez Salazar, A. J. , Fulton, W. B. , Lu, P. , Yamaguchi, Y. , Zhou, Q. , … Hackam, D. J. (2021). Toll‐like receptor 4‐mediated enteric glia loss is critical for the development of necrotizing enterocolitis. Science Translational Medicine, 13(612), eabg3459. 10.1126/scitranslmed.abg3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kracht, L. , Borggrewe, M. , Eskandar, S. , Brouwer, N. , de Sousa, C. , Lopes, S. M. , Laman, J. D. , … Eggen, B. J. L. (2020). Human fetal microglia acquire homeostatic immune‐sensing properties early in development. Science, 369(6503), 530–537. 10.1126/science.aba5906 [DOI] [PubMed] [Google Scholar]
- Krishnan, M. L. , van Steenwinckel, J. , Schang, A. L. , Yan, J. , Arnadottir, J. , Le Charpentier, T. , … Gressens, P. (2017). Integrative genomics of microglia implicates DLG4 (PSD95) in the white matter development of preterm infants. Nature Communications, 8(1), 428. 10.1038/s41467-017-00422-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse, M. , Michelsen, S. I. , Flachs, E. M. , Bronnum‐Hansen, H. , Madsen, M. , & Uldall, P. (2009). Lifetime costs of cerebral palsy. Developmental Medicine and Child Neurology, 51(8), 622–628. 10.1111/j.1469-8749.2008.03190.x [DOI] [PubMed] [Google Scholar]
- Kuban, K. C. , Joseph, R. M. , O'Shea, T. M. , Heeren, T. , Fichorova, R. N. , Douglass, L. , … Extremely Low Gestational Age Newborn Study, I . (2017). Circulating inflammatory‐associated proteins in the first month of life and cognitive impairment at age 10 years in children born extremely preterm. Journal of Pediatrics, 180(116‐123), e111. 10.1016/j.jpeds.2016.09.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppala, V. S. , Meinzen‐Derr, J. , Morrow, A. L. , & Schibler, K. R. (2011). Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. The Journal of Pediatrics, 159(5), 720–725. 10.1016/j.jpeds.2011.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers, E. , Jellema, R. K. , Ophelders, D. R. , Dudink, J. , Nikiforou, M. , Wolfs, T. G. , … Kramer, B. W. (2013). Effects of intra‐amniotic lipopolysaccharide and maternal betamethasone on brain inflammation in fetal sheep. PLoS One, 8(12), e81644. 10.1371/journal.pone.0081644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon, S. H. , Vasung, L. , Ment, L. R. , & Huppi, P. S. (2014). The role of neuroimaging in predicting neurodevelopmental outcomes of preterm neonates. Clinics in Perinatology, 41(1), 257–283. 10.1016/j.clp.2013.10.003 [DOI] [PubMed] [Google Scholar]
- Lacaille, H. , Vacher, C. M. , & Penn, A. A. (2021). Preterm birth alters the maturation of the GABAergic system in the human prefrontal cortex. Frontiers in Molecular Neuroscience, 14, 827370. 10.3389/fnmol.2021.827370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalancette‐Hebert, M. , Gowing, G. , Simard, A. , Weng, Y. C. , & Kriz, J. (2007). Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. The Journal of Neuroscience, 27(10), 2596–2605. 10.1523/JNEUROSCI.5360-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Dieu‐Lugon, B. , Dupre, N. , Legouez, L. , Leroux, P. , Gonzalez, B. J. , Marret, S. , … Cleren, C. (2020). Why considering sexual differences is necessary when studying encephalopathy of prematurity through rodent models. European Journal of Neuroscience, 52(1), 2560–2574. 10.1111/ejn.14664 [DOI] [PubMed] [Google Scholar]
- Lehnardt, S. , Lachance, C. , Patrizi, S. , Lefebvre, S. , Follett, P. L. , Jensen, F. E. , … Vartanian, T. (2002). The toll‐like receptor TLR4 is necessary for lipopolysaccharide‐induced oligodendrocyte injury in the CNS. The Journal of Neuroscience, 22(7), 2478–2486. 10.1523/JNEUROSCI.22-07-02478.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrman, E. K. , Wilton, D. K. , Litvina, E. Y. , Welsh, C. A. , Chang, S. T. , Frouin, A. , … Stevens, B. (2018). CD47 protects synapses from excess microglia‐mediated pruning during development. Neuron, 100(1), 120–134.e126. 10.1016/j.neuron.2018.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz, K. M. , & McCarthy, M. M. (2015). A starring role for microglia in brain sex differences. The Neuroscientist, 21(3), 306–321. 10.1177/1073858414536468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leviton, A. , Gressens, P. , Wolkenhauer, O. , & Dammann, O. (2015). Systems approach to the study of brain damage in the very preterm newborn. Frontiers in Systems Neuroscience, 9, 58. 10.3389/fnsys.2015.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Yawno, T. , Sutherland, A. , Loose, J. , Nitsos, I. , Bischof, R. , … Miller, S. L. (2016). Preterm white matter brain injury is prevented by early administration of umbilical cord blood cells. Experimental Neurology, 283, 179–187. 10.1016/j.expneurol.2016.06.017 [DOI] [PubMed] [Google Scholar]
- Li, J. , Yawno, T. , Sutherland, A. E. , Gurung, S. , Paton, M. , McDonald, C. , … Miller, S. L. (2018). Preterm umbilical cord blood derived mesenchymal stem/stromal cells protect preterm white matter brain development against hypoxia‐ischemia. Experimental Neurology, 308, 120–131. 10.1016/j.expneurol.2018.07.006 [DOI] [PubMed] [Google Scholar]
- Li, Q. , & Barres, B. A. (2018). Microglia and macrophages in brain homeostasis and disease. Nature Reviews. Immunology, 18, 225–242. 10.1038/nri.2017.125 [DOI] [PubMed] [Google Scholar]
- Li, Q. , Cheng, Z. , Zhou, L. , Darmanis, S. , Neff, N. F. , Okamoto, J. , … Barres, B. A. (2019). Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single‐cell RNA sequencing. Neuron, 101(2), 207–223.e210. 10.1016/j.neuron.2018.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow, S. A. , Guttenplan, K. A. , Clarke, L. E. , Bennett, F. C. , Bohlen, C. J. , Schirmer, L. , … Barres, B. A. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature, 541(7638), 481–487. 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke, W. , Edelmann, W. , Bieri, P. L. , Chiu, F. C. , Cowan, N. J. , Kucherlapati, R. , & Raine, C. S. (1996). GFAP is necessary for the integrity of CNS white matter architecture and long‐term maintenance of myelination. Neuron, 17(4), 607–615. 10.1016/s0896-6273(00)80194-4 [DOI] [PubMed] [Google Scholar]
- Ligam, P. , Haynes, R. L. , Folkerth, R. D. , Liu, L. , Yang, M. , Volpe, J. J. , & Kinney, H. C. (2009). Thalamic damage in periventricular leukomalacia: novel pathologic observations relevant to cognitive deficits in survivors of prematurity. Pediatric Research, 65(5), 524–529. 10.1203/PDR.0b013e3181998baf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, D. M. , Koskella, B. , & Lin, H. C. (2017). Phage therapy: An alternative to antibiotics in the age of multi‐drug resistance. World Journal of Gastrointestinal Pharmacology and Therapeutics, 8(3), 162–173. 10.4292/wjgpt.v8.i3.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, J. P. , Goh, W. , Brown, J. K. , & Steers, A. J. (1993). Heterogeneity of neurological syndromes in survivors of grade 3 and 4 periventricular haemorrhage. Childs Nervous System, 9(4), 205–214. 10.1007/BF00303571 [DOI] [PubMed] [Google Scholar]
- Litwiniuk, M. , Krejner, A. , Speyrer, M. S. , Gauto, A. R. , & Grzela, T. (2016). Hyaluronic acid in inflammation and tissue regeneration. Wounds, 28(3), 78–88. [PubMed] [Google Scholar]
- Lloyd, A. F. , Davies, C. L. , Holloway, R. K. , Labrak, Y. , Ireland, G. , Carradori, D. , … Miron, V. E. (2019). Central nervous system regeneration is driven by microglia necroptosis and repopulation. Nature Neuroscience, 22(7), 1046–1052. 10.1038/s41593-019-0418-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeliger, M. , Inder, T. , Cain, S. , Ramesh, R. C. , Camm, E. , Thomson, M. A. , … Rees, S. M. (2006). Cerebral outcomes in a preterm baboon model of early versus delayed nasal continuous positive airway pressure. Pediatrics, 118(4), 1640–1653. 10.1542/peds.2006-0653 [DOI] [PubMed] [Google Scholar]
- Louveau, A. , Harris, T. H. , & Kipnis, J. (2015). Revisiting the mechanisms of CNS immune privilege. Trends in Immunology, 36(10), 569–577. 10.1016/j.it.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, D. , Evangelou, A. V. , Shankar, K. , Dewji, F. I. , Lin, J. , & Levison, S. W. (2023). Neuroprotective effect of lipopolysaccharides in a dual‐hit rat pup model of preterm hypoxia‐ischemia. Neuroscience Letters, 795, 137033. 10.1016/j.neulet.2022.137033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira, M. M. , Hage, Z. , & Tsirka, S. E. (2022). Beyond myelination: Possible roles of the immune proteasome in oligodendroglial homeostasis and dysfunction. Frontiers in Neuroscience, 16, 867357. 10.3389/fnins.2022.867357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen, P. M. , Desu, H. L. , de Rivero Vaccari, J. P. , Florimon, Y. , Ellman, D. G. , Keane, R. W. , … Brambilla, R. (2020). Oligodendrocytes modulate the immune‐inflammatory response in EAE via TNFR2 signaling. Brain, Behavior, and Immunity, 84, 132–146. 10.1016/j.bbi.2019.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnotti, L. M. , Goodenough, D. A. , & Paul, D. L. (2011). Deletion of oligodendrocyte Cx32 and astrocyte Cx43 causes white matter vacuolation, astrocyte loss and early mortality. Glia, 59(7), 1064–1074. 10.1002/glia.21179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonneuve, E. , Lorthe, E. , Torchin, H. , Delorme, P. , Devisme, L. , L'Helias, L. F. , … Group, E.‐O. W . (2020). Association of chorioamnionitis with cerebral palsy at two years after spontaneous very preterm birth: The EPIPAGE‐2 cohort study. Journal of Pediatrics, 222(71‐78), e76. 10.1016/j.jpeds.2020.03.021 [DOI] [PubMed] [Google Scholar]
- Manuck, T. A. , Rice, M. M. , Bailit, J. L. , Grobman, W. A. , Reddy, U. M. , Wapner, R. J. , … Human Development Maternal‐Fetal Medicine Units . (2016). Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. American Journal of Obstetrics and Gynecology, 215(1), e101–e114. 10.1016/j.ajog.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marret, S. , Mukendi, R. , Gadisseux, J. F. , Gressens, P. , & Evrard, P. (1995). Effect of ibotenate on brain development: an excitotoxic mouse model of microgyria and posthypoxic‐like lesions. Journal of Neuropathology and Experimental Neurology, 54(3), 358–370. [DOI] [PubMed] [Google Scholar]
- Masi, A. C. , & Stewart, C. J. (2019). The role of the preterm intestinal microbiome in sepsis and necrotising enterocolitis. Early Human Development, 138, 104854. 10.1016/j.earlhumdev.2019.104854 [DOI] [PubMed] [Google Scholar]
- Matcovitch‐Natan, O. , Winter, D. R. , Giladi, A. , Vargas Aguilar, S. , Spinrad, A. , Sarrazin, S. , … Amit, I. (2016). Microglia development follows a stepwise program to regulate brain homeostasis. Science, 353(6301), aad8670. 10.1126/science.aad8670 [DOI] [PubMed] [Google Scholar]
- Mathai, S. , Booth, L. C. , Davidson, J. O. , Drury, P. P. , Fraser, M. , Jensen, E. C. , … Bennet, L. (2013). Acute on chronic exposure to endotoxin in preterm fetal sheep. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 304(3), 189–197. 10.1152/ajpregu.00388.2012 [DOI] [PubMed] [Google Scholar]
- Mayoral, S. R. , Omar, G. , & Penn, A. A. (2009). Sex differences in a hypoxia model of preterm brain damage. Pediatric Research, 66(3), 248–253. 10.1203/PDR.0b013e3181b1bc34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur, S. , Pienaar, I. S. , Siddiqi, S. M. , & Gillies, G. E. (2016). Sex‐specific disruption of murine midbrain astrocytic and dopaminergic developmental trajectories following antenatal GC treatment. Brain Structure & Function, 221(5), 2459–2475. 10.1007/s00429-015-1049-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre, S. , Nelson, K. B. , Mulkey, S. B. , Lechpammer, M. , Molloy, E. , Badawi, N. , … Publications, C. (2021). Neonatal encephalopathy: Focus on epidemiology and underexplored aspects of etiology. Seminars in Fetal & Neonatal Medicine, 26(4), 101265. 10.1016/j.siny.2021.101265 [DOI] [PubMed] [Google Scholar]
- McNamara, N. B. , Munro, D. A. D. , Bestard‐Cuche, N. , Uyeda, A. , Bogie, J. F. J. , Hoffmann, A. , … Miron, V. E. (2023). Microglia regulate central nervous system myelin growth and integrity. Nature, 613(7942), 120–129. 10.1038/s41586-022-05534-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillen, P. S. , Sheldon, R. A. , Shatz, C. J. , & Ferriero, D. M. (2003). Selective vulnerability of subplate neurons after early neonatal hypoxia‐ischemia. Journal of Neuroscience, 23(8), 3308–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menassa, D. A. , Muntslag, T. A. O. , Martin‐Estebane, M. , Barry‐Carroll, L. , Chapman, M. A. , Adorjan, I. , … Gomez‐Nicola, D. (2022). The spatiotemporal dynamics of microglia across the human lifespan. Developmental Cell, 57(17), 2127–2139.e2126. 10.1016/j.devcel.2022.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menichella, D. M. , Goodenough, D. A. , Sirkowski, E. , Scherer, S. S. , & Paul, D. L. (2003). Connexins are critical for normal myelination in the CNS. The Journal of Neuroscience, 23(13), 5963–5973. 10.1523/JNEUROSCI.23-13-05963.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon, R. , Peltier, M. R. , Eckardt, J. , & Fortunato, S. J. (2009). Diversity in cytokine response to bacteria associated with preterm birth by fetal membranes. American Journal of Obstetrics and Gynecology, 201(3), 301–306. 10.1016/j.ajog.2009.06.027 [DOI] [PubMed] [Google Scholar]
- Mildenberger, W. , Stifter, S. A. , & Greter, M. (2022). Diversity and function of brain‐associated macrophages. Current Opinion in Immunology, 76, 102181. 10.1016/j.coi.2022.102181 [DOI] [PubMed] [Google Scholar]
- Miron, V. E. , Ludwin, S. K. , Darlington, P. J. , Jarjour, A. A. , Soliven, B. , Kennedy, T. E. , & Antel, J. P. (2010). Fingolimod (FTY720) enhances remyelination following demyelination of organotypic cerebellar slices. American Journal of Pathology, 176(6), 2682–2694. 10.2353/ajpath.2010.091234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto, N. , Maki, T. , Shindo, A. , Liang, A. C. , Maeda, M. , Egawa, N. , … Arai, K. (2015). Astrocytes promote oligodendrogenesis after white matter damage via brain‐derived neurotrophic factor. The Journal of Neuroscience, 35(41), 14002–14008. 10.1523/JNEUROSCI.1592-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MohanKumar, K. , Namachivayam, K. , Cheng, F. , Jiang, R. H. , Flores‐Torres, J. , Torres, B. A. , & Maheshwari, A. (2017). Trinitrobenzene sulfonic acid‐induced intestinal injury in neonatal mice activates transcriptional networks similar to those seen in human necrotizing enterocolitis. Pediatric Research, 81(1‐1), 99–112. 10.1038/pr.2016.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier, A. , Adle‐Biassette, H. , Delezoide, A. L. , Evrard, P. , Gressens, P. , & Verney, C. (2007). Entry and distribution of microglial cells in human embryonic and fetal cerebral cortex. Journal of Neuropathology and Experimental Neurology, 66(5), 372–382. 10.1097/nen.0b013e3180517b46 [DOI] [PubMed] [Google Scholar]
- Monier, A. , Evrard, P. , Gressens, P. , & Verney, C. (2006). Distribution and differentiation of microglia in the human encephalon during the first two trimesters of gestation. The Journal of Comparative Neurology, 499(4), 565–582. 10.1002/cne.21123 [DOI] [PubMed] [Google Scholar]
- Morin, C. , Guenoun, D. , Sautet, I. , Faivre, V. , Csaba, Z. , Schwendimann, L. , … Bokobza, C. (2022). The impact of mouse preterm birth induction by RU‐486 on microglial activation and subsequent hypomyelination. International Journal of Molecular Sciences, 23(9), 4867. 10.3390/ijms23094867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moster, D. , Lie, R. T. , & Markestad, T. (2008). Long‐term medical and social consequences of preterm birth. New England Journal of Medicine, 359(3), 262–273. 10.1056/NEJMoa0706475 [DOI] [PubMed] [Google Scholar]
- Mrdjen, D. , Pavlovic, A. , Hartmann, F. J. , Schreiner, B. , Utz, S. G. , Leung, B. P. , … Becher, B. (2018). High‐dimensional single‐cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity, 48(3), 599. 10.1016/j.immuni.2018.02.014 [DOI] [PubMed] [Google Scholar]
- Munro, D. A. D. , Movahedi, K. , & Priller, J. (2022). Macrophage compartmentalization in the brain and cerebrospinal fluid system. Science Immunology, 7(69), eabk0391. 10.1126/sciimmunol.abk0391 [DOI] [PubMed] [Google Scholar]
- Nagao, M. , Ogata, T. , Sawada, Y. , & Gotoh, Y. (2016). Zbtb20 promotes astrocytogenesis during neocortical development. Nature Communications, 7, 11102. 10.1038/ncomms11102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance, E. , Porambo, M. , Zhang, F. , Mishra, M. K. , Buelow, M. , Getzenberg, R. , … Kannan, S. (2015). Systemic dendrimer‐drug treatment of ischemia‐induced neonatal white matter injury. Journal of Controlled Release, 214, 112–120. 10.1016/j.jconrel.2015.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu, J. , & Walker, W. A. (2011). Necrotizing enterocolitis. The New England Journal of Medicine, 364(3), 255–264. 10.1056/NEJMra1005408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld, K. A. , Kang, N. , Bienenstock, J. , & Foster, J. A. (2011). Effects of intestinal microbiota on anxiety‐like behavior. Communicative & Integrative Biology, 4(4), 492–494. 10.4161/cib.4.4.15702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunlist, M. , Rolli‐Derkinderen, M. , Latorre, R. , Van Landeghem, L. , Coron, E. , Derkinderen, P. , & De Giorgio, R. (2014). Enteric glial cells: recent developments and future directions. Gastroenterology, 147(6), 1230–1237. 10.1053/j.gastro.2014.09.040 [DOI] [PubMed] [Google Scholar]
- Ng, E. , Dundek, M. , & Burke, T. F. (2022). Evaluation of an innovative low flow oxygen blender system for global access. Frontiers in Pediatrics, 10, 981821. 10.3389/fped.2022.981821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, P. Y. , McNeely, T. L. , & Baker, D. J. (2023). Untangling senescent and damage‐associated microglia in the aging and diseased brain. The FEBS Journal, 290(5), 1326–1339. 10.1111/febs.16315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, K. B. , & Pender, M. P. (1998). Phagocytosis of apoptotic lymphocytes by oligodendrocytes in experimental autoimmune encephalomyelitis. Acta Neuropathologica, 95(1), 40–46. 10.1007/s004010050763 [DOI] [PubMed] [Google Scholar]
- Nosarti, C. , Nam, K. W. , Walshe, M. , Murray, R. M. , Cuddy, M. , Rifkin, L. , & Allin, M. P. (2014). Preterm birth and structural brain alterations in early adulthood. NeuroImage Clinical, 6, 180–191. 10.1016/j.nicl.2014.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa‐Cortes, F. , Turco, F. , Linan‐Rico, A. , Soghomonyan, S. , Whitaker, E. , Wehner, S. , … Christofi, F. L. (2016). Enteric glial cells: A new frontier in neurogastroenterology and clinical target for inflammatory bowel diseases. Inflammatory Bowel Diseases, 22(2), 433–449. 10.1097/MIB.0000000000000667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt, B. , Wellershaus, K. , Wallraff, A. , Seifert, G. , Degen, J. , Euwens, C. , … Willecke, K. (2003). Connexin 47 (Cx47)‐deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS. The Journal of Neuroscience, 23(11), 4549–4559. 10.1523/JNEUROSCI.23-11-04549.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Driscoll, D. N. , McGovern, M. , Greene, C. M. , & Molloy, E. J. (2018). Gender disparities in preterm neonatal outcomes. Acta Paediatrica, 107(9), 1494–1499. 10.1111/apa.14390 [DOI] [PubMed] [Google Scholar]
- Ophelders, D. R. , Gussenhoven, R. , Lammens, M. , Kusters, B. , Kemp, M. W. , Newnham, J. P. , … Wolfs, T. G. (2016). Neuroinflammation and structural injury of the fetal ovine brain following intra‐amniotic Candida albicans exposure. Journal of Neuroinflammation, 13, 29. 10.1186/s12974-016-0492-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea, T. M. , Allred, E. N. , Kuban, K. C. , Dammann, O. , Paneth, N. , Fichorova, R. , … Extremely Low Gestational Age Newborn Study, I . (2012). Elevated concentrations of inflammation‐related proteins in postnatal blood predict severe developmental delay at 2 years of age in extremely preterm infants. Journal of Pediatrics, 160(3), 395–401 e394. 10.1016/j.jpeds.2011.08.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmsten, K. , Nelson, K. K. , Laurent, L. C. , Park, S. , Chambers, C. D. , & Parast, M. M. (2018). Subclinical and clinical chorioamnionitis, fetal vasculitis, and risk for preterm birth: A cohort study. Placenta, 67, 54–60. 10.1016/j.placenta.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda, S. , Dohare, P. , Jain, S. , Parikh, N. , Singla, P. , Mehdizadeh, R. , … Ballabh, P. (2018). Estrogen treatment reverses prematurity‐induced disruption in cortical interneuron population. Journal of Neuroscience, 38(34), 7378–7391. 10.1523/JNEUROSCI.0478-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang, R. , Avdic‐Belltheus, A. , Meehan, C. , Martinello, K. , Mutshiya, T. , Yang, Q. , … Robertson, N. J. (2021). Melatonin and/or erythropoietin combined with hypothermia in a piglet model of perinatal asphyxia. Brain Communications, 3(1), fcaa211. 10.1093/braincomms/fcaa211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang, Y. , Cai, Z. , & Rhodes, P. G. (2003). Disturbance of oligodendrocyte development, hypomyelination and white matter injury in the neonatal rat brain after intracerebral injection of lipopolysaccharide. Brain Research. Developmental Brain Research, 140(2), 205–214. 10.1016/s0165-3806(02)00606-5 [DOI] [PubMed] [Google Scholar]
- Paolicelli, R. C. , Bolasco, G. , Pagani, F. , Maggi, L. , Scianni, M. , Panzanelli, P. , … Gross, C. T. (2011). Synaptic pruning by microglia is necessary for normal brain development. Science, 333(6048), 1456–1458. 10.1126/science.1202529 [DOI] [PubMed] [Google Scholar]
- Paolicelli, R. C. , Sierra, A. , Stevens, B. , Tremblay, M. E. , Aguzzi, A. , Ajami, B. , … Wyss‐Coray, T. (2022). Microglia states and nomenclature: A field at its crossroads. Neuron, 110(21), 3458–3483. 10.1016/j.neuron.2022.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton, M. C. B. , Allison, B. J. , Fahey, M. C. , Li, J. , Sutherland, A. E. , Pham, Y. , … McDonald, C. A. (2019). Umbilical cord blood versus mesenchymal stem cells for inflammation‐induced preterm brain injury in fetal sheep. Pediatric Research, 86(2), 165–173. 10.1038/s41390-019-0366-z [DOI] [PubMed] [Google Scholar]
- Pavageau, L. , Sanchez, P. J. , Steven Brown, L. , & Chalak, L. F. (2020). Inter‐rater reliability of the modified Sarnat examination in preterm infants at 32‐36 weeks' gestation. Pediatric Research, 87(4), 697–702. 10.1038/s41390-019-0562-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelen, M. J. , Kazemier, B. M. , Ravelli, A. C. , De Groot, C. J. , Van Der Post, J. A. , Mol, B. W. , … Kok, M. (2016). Impact of fetal gender on the risk of preterm birth, a national cohort study. Acta Obstetricia et Gynecologica Scandinavica, 95(9), 1034–1041. 10.1111/aogs.12929 [DOI] [PubMed] [Google Scholar]
- Perdiguero, E. G. , & Geissmann, F. (2016). The development and maintenance of resident macrophages. Nature Immunology, 17(1), 2–8. 10.1038/ni.3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrat, V. , Marchand‐Martin, L. , Arnaud, C. , Kaminski, M. , Resche‐Rigon, M. , Lebeaux, C. , … Group, E.‐W . (2017). Neurodevelopmental outcome at 2 years for preterm children born at 22 to 34 weeks' gestation in France in 2011: EPIPAGE‐2 cohort study. BMJ, 358, j3448. 10.1136/bmj.j3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson, C. R. , Folkerth, R. D. , Billiards, S. S. , Trachtenberg, F. L. , Drinkwater, M. E. , Volpe, J. J. , & Kinney, H. C. (2007). Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathologica, 114(6), 619–631. 10.1007/s00401-007-0295-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho‐Ribeiro, F. A. , Deng, L. , Neel, D. V. , Erdogan, O. , Basu, H. , Yang, D. , … Chiu, I. M. (2023). Bacteria hijack a meningeal neuroimmune axis to facilitate brain invasion. Nature, 615(7952), 472–481. 10.1038/s41586-023-05753-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon, S. S. B. , Hung, L. Y. , Wu, Q. , Parathan, P. , Yalcinkaya, N. , Haag, A. , … Foong, J. P. P. (2022). Neonatal antibiotics have long term sex‐dependent effects on the enteric nervous system. The Journal of Physiology, 600(19), 4303–4323. 10.1113/JP282939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo‐Rodrigalvarez, A. , Li, Y. , Stokowska, A. , Wu, J. , Dehm, V. , Sourkova, H. , … Pekna, M. (2021). C3a receptor signaling inhibits neurodegeneration induced by neonatal hypoxic‐ischemic brain injury. Frontiers in Immunology, 12, 768198. 10.3389/fimmu.2021.768198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz, M. , & Mildner, A. (2011). Microglia in the CNS: immigrants from another world. Glia, 59(2), 177–187. 10.1002/glia.21104 [DOI] [PubMed] [Google Scholar]
- Qian, X. , Su, Y. , Adam, C. D. , Deutschmann, A. U. , Pather, S. R. , Goldberg, E. M. , … Ming, G. L. (2020). Sliced human cortical organoids for modeling distinct cortical layer formation. Cell Stem Cell, 26(5), 766–781.e769. 10.1016/j.stem.2020.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanantsoa, N. , Fleiss, B. , Bouslama, M. , Matrot, B. , Schwendimann, L. , Cohen‐Salmon, C. , … Gallego, J. (2013). Bench to cribside: the path for developing a neuroprotectant. Translational Stroke Research, 4(2), 258–277. 10.1007/s12975-012-0233-2 [DOI] [PubMed] [Google Scholar]
- Rebejac, J. , Eme‐Scolan, E. , Arnaud Paroutaud, L. , Kharbouche, S. , Teleman, M. , Spinelli, L. , … Rua, R. (2022). Meningeal macrophages protect against viral neuroinfection. Immunity, 55(11), 2103–2117.e2110. 10.1016/j.immuni.2022.10.005 [DOI] [PubMed] [Google Scholar]
- Rittchen, S. , Boyd, A. , Burns, A. , Park, J. , Fahmy, T. M. , Metcalfe, S. , & Williams, A. (2015). Myelin repair in vivo is increased by targeting oligodendrocyte precursor cells with nanoparticles encapsulating leukaemia inhibitory factor (LIF). Biomaterials, 56, 78–85. 10.1016/j.biomaterials.2015.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter, J. , Schmitz, T. , Chew, L. J. , Buhrer, C. , Mobius, W. , Zonouzi, M. , & Gallo, V. (2013). Neonatal hyperoxia exposure disrupts axon‐oligodendrocyte integrity in the subcortical white matter. Journal of Neuroscience, 33(21), 8990–9002. 10.1523/JNEUROSCI.5528-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson, N. L. , & Cox, I. J. (2001). Magnetic resonance spectroscopy of the neonatal brain. In Rutherford M. A. (Ed.), MRI of the neonatal brain. W B Saunders Co Ltd. [Google Scholar]
- Romantsik, O. , Ross‐Munro, E. , Gronlund, S. , Holmqvist, B. , Brinte, A. , Gerdtsson, E. , … Ley, D. (2022). Severe intraventricular hemorrhage causes long‐lasting structural damage in a preterm rabbit pup model. Pediatric Research, 92(2), 403–414. 10.1038/s41390-022-02075-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg, H. J. , & Rao, M. (2021). Enteric glia in homeostasis and disease: From fundamental biology to human pathology. iScience, 24(8), 102863. 10.1016/j.isci.2021.102863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkrantz, T. S. , Hussain, Z. , & Fitch, R. H. (2019). Sex differences in brain injury and repair in newborn infants: Clinical evidence and biological mechanisms. Frontiers in Pediatrics, 7, 211. 10.3389/fped.2019.00211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset, C. I. , Chalon, S. , Cantagrel, S. , Bodard, S. , Andres, C. , Gressens, P. , & Saliba, E. (2006). Maternal exposure to LPS induces hypomyelination in the internal capsule and programmed cell death in the deep gray matter in newborn rats. Pediatric Research, 59(3), 428–433. 10.1203/01.pdr.0000199905.08848.55 [DOI] [PubMed] [Google Scholar]
- Roze, J. C. , Ancel, P. Y. , Marchand‐Martin, L. , Rousseau, C. , Montassier, E. , Monot, C. , … Group, E. S . (2020). Assessment of neonatal intensive care unit practices and preterm newborn gut microbiota and 2‐year neurodevelopmental outcomes. JAMA Network Open, 3(9), e2018119. 10.1001/jamanetworkopen.2020.18119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rua, R. , Lee, J. Y. , Silva, A. B. , Swafford, I. S. , Maric, D. , Johnson, K. R. , & McGavern, D. B. (2019). Infection drives meningeal engraftment by inflammatory monocytes that impairs CNS immunity. Nature Immunology, 20(4), 407–419. 10.1038/s41590-019-0344-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rua, R. , & McGavern, D. B. (2018). Advances in meningeal immunity. Trends in Molecular Medicine, 24(6), 542–559. 10.1016/j.molmed.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rurak, G. M. , Simard, S. , Freitas‐Andrade, M. , Lacoste, B. , Charih, F. , Van Geel, A. , … Salmaso, N. (2022). Sex differences in developmental patterns of neocortical astroglia: A mouse translatome database. Cell Reports, 38(5), 110310. 10.1016/j.celrep.2022.110310 [DOI] [PubMed] [Google Scholar]
- Sabate‐Soler, S. , Nickels, S. L. , Saraiva, C. , Berger, E. , Dubonyte, U. , Barmpa, K. , … Schwamborn, J. C. (2022). Microglia integration into human midbrain organoids leads to increased neuronal maturation and functionality. Glia, 70(7), 1267–1288. 10.1002/glia.24167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar, A. , Prescott, S. M. , Dutra, S. , Yoo, J. Y. , Gordon, J. , Shaffer, E. , … Groer, M. E. (2022). Relationships of the very low birth weight infant microbiome with neurodevelopment at 2 and 4 years of age. Developmental Psychobiology, 64(7), e22317. 10.1002/dev.22317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh, J. , Kino, Y. , Asahina, N. , Takitani, M. , Miyoshi, J. , Ishida, T. , & Saito, Y. (2016). TMEM119 marks a subset of microglia in the human brain. Neuropathology, 36(1), 39–49. 10.1111/neup.12235 [DOI] [PubMed] [Google Scholar]
- Scafidi, J. , Hammond, T. R. , Scafidi, S. , Ritter, J. , Jablonska, B. , Roncal, M. , … Gallo, V. (2014). Intranasal epidermal growth factor treatment rescues neonatal brain injury. Nature, 506(7487), 230–234. 10.1038/nature12880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schang, A. L. , Van Steenwinckel, J. , Chevenne, D. , Alkmark, M. , Hagberg, H. , Gressens, P. , & Fleiss, B. (2014). Failure of thyroid hormone treatment to prevent inflammation‐induced white matter injury in the immature brain. Brain, Behavior, and Immunity, 37, 95–102. 10.1016/j.bbi.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schang, A. L. , Van Steenwinckel, J. , Ioannidou, Z. S. , Lipecki, J. , Rich‐Griffin, C. , Woolley‐Allen, K. , … Gressens, P. (2022). Epigenetic priming of immune/inflammatory pathways activation and abnormal activity of cell cycle pathway in a perinatal model of white matter injury. Cell Death & Disease, 13(12), 1038. 10.1038/s41419-022-05483-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, A. F. , Kannan, P. S. , Chougnet, C. A. , Danzer, S. C. , Miller, L. A. , Jobe, A. H. , & Kallapur, S. G. (2016). Intra‐amniotic LPS causes acute neuroinflammation in preterm rhesus macaques. Journal of Neuroinflammation, 13(1), 238. 10.1186/s12974-016-0706-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz, T. , Ritter, J. , Mueller, S. , Felderhoff‐Mueser, U. , Chew, L. J. , & Gallo, V. (2011). Cellular changes underlying hyperoxia‐induced delay of white matter development. Journal of Neuroscience, 31(11), 4327–4344. 10.1523/JNEUROSCI.3942-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnider, B. , Tuura, R. , Disselhoff, V. , Latal, B. , Wehrle, F. M. , Hagmann, C. F. , & EpoKids Research, G . (2020). Altered brain metabolism contributes to executive function deficits in school‐aged children born very preterm. Pediatric Research, 88(5), 739–748. 10.1038/s41390-020-1024-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, J. M. , Sholar, P. W. , & Bilbo, S. D. (2012). Sex differences in microglial colonization of the developing rat brain. Journal of Neurochemistry, 120(6), 948–963. 10.1111/j.1471-4159.2011.07630.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, G. A. , Terstege, D. J. , Vu, A. P. , Law, S. , Evans, A. , & Epp, J. R. (2020). Disrupted neurogenesis in germ‐free mice: effects of age and sex. Frontiers in Cell and Development Biology, 8, 407. 10.3389/fcell.2020.00407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeker, L. A. , & Williams, A. (2022). Oligodendroglia heterogeneity in the human central nervous system. Acta Neuropathologica, 143(2), 143–157. 10.1007/s00401-021-02390-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serdar, M. , Herz, J. , Kempe, K. , Winterhager, E. , Jastrow, H. , Heumann, R. , … Bendix, I. (2018). Protection of oligodendrocytes through neuronal overexpression of the small GTPase Ras in hyperoxia‐induced neonatal brain injury. Frontiers in Neurology, 9, 175. 10.3389/fneur.2018.00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamash, M. , & Maurice, C. F. (2022). Phages in the infant gut: a framework for virome development during early life. The ISME Journal, 16(2), 323–330. 10.1038/s41396-021-01090-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, R. , Kim, S. Y. , Sharma, A. , Zhang, Z. , Kambhampati, S. P. , Kannan, S. , & Kannan, R. M. (2017). Activated microglia targeting dendrimer‐minocycline conjugate as therapeutics for neuroinflammation. Bioconjugate Chemistry, 28(11), 2874–2886. 10.1021/acs.bioconjchem.7b00569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng, J. , Ruedl, C. , & Karjalainen, K. (2015). Most tissue‐resident macrophages except microglia are derived from fetal hematopoietic stem cells. Immunity, 43(2), 382–393. 10.1016/j.immuni.2015.07.016 [DOI] [PubMed] [Google Scholar]
- Shi, Z. , Ma, L. , Luo, K. , Bajaj, M. , Chawla, S. , Natarajan, G. , … Tan, S. (2017). Chorioamnionitis in the development of cerebral palsy: A meta‐analysis and systematic review. Pediatrics, 139(6), e20163781. 10.1542/peds.2016-3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiow, L. R. , Favrais, G. , Schirmer, L. , Schang, A. L. , Cipriani, S. , Andres, C. , … Rowitch, D. H. (2017). Reactive astrocyte COX2‐PGE2 production inhibits oligodendrocyte maturation in neonatal white matter injury. Glia, 65(12), 2024–2037. 10.1002/glia.23212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvin, A. , Uderhardt, S. , Piot, C. , Da Mesquita, S. , Yang, K. , Geirsdottir, L. , … Ginhoux, F. (2022). Dual ontogeny of disease‐associated microglia and disease inflammatory macrophages in aging and neurodegeneration. Immunity, 55(8), 1448–1465.e1446. 10.1016/j.immuni.2022.07.004 [DOI] [PubMed] [Google Scholar]
- Singh, P. , Singh, D. , Srivastava, P. , Mishra, G. , & Tiwari, A. K. (2023). Evaluation of advanced, pathophysiologic new targets for imaging of CNS. Drug Development Research, 84(3), 484–513. 10.1002/ddr.22040 [DOI] [PubMed] [Google Scholar]
- Smith, C. J. , & Bilbo, S. D. (2019). Microglia sculpt sex differences in social behavior. Neuron, 102(2), 275–277. 10.1016/j.neuron.2019.03.039 [DOI] [PubMed] [Google Scholar]
- Smithers‐Sheedy, H. , Waight, E. , Goldsmith, S. , Reid, S. , Gibson, C. , Watson, L. , … Australian Cerebral Palsy Register . (2022). Declining trends in birth prevalence and severity of singletons with cerebral palsy of prenatal or perinatal origin in Australia: A population‐based observational study. Developmental Medicine and Child Neurology, 64(9), 1114–1122. 10.1111/dmcn.15195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders, S. M. , Kessels, S. , Vangansewinkel, T. , Rigo, J. M. , Legendre, P. , & Brone, B. (2019). Microglia: Brain cells on the move. Progress in Neurobiology, 178, 101612. 10.1016/j.pneurobio.2019.04.001 [DOI] [PubMed] [Google Scholar]
- Snyder, J. M. , Wood, T. R. , Corry, K. , Moralejo, D. H. , Parikh, P. , & Juul, S. E. (2018). Ontogeny of white matter, toll‐like receptor expression, and motor skills in the neonatal ferret. International Journal of Developmental Neuroscience, 70, 25–33. 10.1016/j.ijdevneu.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer, S. J. , Basri, B. , Sominsky, L. , Soch, A. , Ayala, M. T. , Reineck, P. , … Barrientos, R. M. (2019). High‐fat diet worsens the impact of aging on microglial function and morphology in a region‐specific manner. Neurobiology of Aging, 74, 121–134. 10.1016/j.neurobiolaging.2018.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoto, G. , Amore, G. , Vetri, L. , Quatrosi, G. , Cafeo, A. , Gitto, E. , … Di Rosa, G. (2021). Cerebellum and prematurity: A complex interplay between disruptive and dysmaturational events. Frontiers in Systems Neuroscience, 15, 655164. 10.3389/fnsys.2021.655164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squarzoni, P. , Oller, G. , Hoeffel, G. , Pont‐Lezica, L. , Rostaing, P. , Low, D. , … Garel, S. (2014). Microglia modulate wiring of the embryonic forebrain. Cell Reports, 8(5), 1271–1279. 10.1016/j.celrep.2014.07.042 [DOI] [PubMed] [Google Scholar]
- Srivastava, T. , Sherman, L. S. , & Back, S. A. (2020). Dysregulation of hyaluronan homeostasis during white matter injury. Neurochemical Research, 45(3), 672–683. 10.1007/s11064-019-02879-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankoff, B. , Aigrot, M. S. , Noel, F. , Wattilliaux, A. , Zalc, B. , & Lubetzki, C. (2002). Ciliary neurotrophic factor (CNTF) enhances myelin formation: a novel role for CNTF and CNTF‐related molecules. The Journal of Neuroscience, 22(21), 9221–9227. 10.1523/JNEUROSCI.22-21-09221.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolp, H. B. , Fleiss, B. , Arai, Y. , Supramaniam, V. , Vontell, R. , Birtles, S. , … Gressens, P. (2019). Interneuron development is disrupted in preterm brains with diffuse white matter injury: Observations in mouse and human. Frontiers in Physiology, 10, 955. 10.3389/fphys.2019.00955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story, L. , Davidson, A. , Patkee, P. , Fleiss, B. , Kyriakopoulou, V. , Colford, K. , … Rutherford, M. (2021). Brain volumetry in fetuses that deliver very preterm: An MRI pilot study. NeuroImage Clinical, 30, 102650. 10.1016/j.nicl.2021.102650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, J. , Marwah, G. , Westgarth, M. , Buys, N. , Ellwood, D. , & Gray, P. H. (2017). Effects of probiotics on necrotizing enterocolitis, sepsis, intraventricular hemorrhage, mortality, length of hospital stay, and weight gain in very preterm infants: A meta‐analysis. Advances in Nutrition, 8(5), 749–763. 10.3945/an.116.014605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutor, B. , Schmolke, C. , Teubner, B. , Schirmer, C. , & Willecke, K. (2000). Myelination defects and neuronal hyperexcitability in the neocortex of connexin 32‐deficient mice. Cerebral Cortex, 10(7), 684–697. 10.1093/cercor/10.7.684 [DOI] [PubMed] [Google Scholar]
- Tanner, S. M. , Berryhill, T. F. , Ellenburg, J. L. , Jilling, T. , Cleveland, D. S. , Lorenz, R. G. , & Martin, C. A. (2015). Pathogenesis of necrotizing enterocolitis: modeling the innate immune response. The American Journal of Pathology, 185(1), 4–16. 10.1016/j.ajpath.2014.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao, J. D. , Barnette, A. R. , Griffith, J. L. , Neil, J. J. , & Inder, T. E. (2012). Histopathologic correlation with diffusion tensor imaging after chronic hypoxia in the immature ferret. Pediatric Research, 71(2), 192–198. 10.1038/pr.2011.32 [DOI] [PubMed] [Google Scholar]
- Tay, T. L. , Carrier, M. , & Tremblay, M. E. (2019). Physiology of microglia. Advances in Experimental Medicine and Biology, 1175, 129–148. 10.1007/978-981-13-9913-8_6 [DOI] [PubMed] [Google Scholar]
- Tay, T. L. , Savage, J. C. , Hui, C. W. , Bisht, K. , & Tremblay, M. E. (2017). Microglia across the lifespan: from origin to function in brain development, plasticity and cognition. The Journal of Physiology, 595(6), 1929–1945. 10.1113/JP272134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, D. L. , Pirianov, G. , Holland, S. , McGinnity, C. J. , Norman, A. L. , Reali, C. , … Mehmet, H. (2010). Attenuation of proliferation in oligodendrocyte precursor cells by activated microglia. Journal of Neuroscience Research, 88(8), 1632–1644. 10.1002/jnr.22335 [DOI] [PubMed] [Google Scholar]
- Thanert, R. , Keen, E. C. , Dantas, G. , Warner, B. B. , & Tarr, P. I. (2021). Necrotizing enterocolitis and the microbiome: Current status and future directions. The Journal of Infectious Diseases, 223(12), S257–S263. 10.1093/infdis/jiaa604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thion, M. S. , Low, D. , Silvin, A. , Chen, J. , Grisel, P. , Schulte‐Schrepping, J. , … Garel, S. (2018). Microbiome influences prenatal and adult microglia in a sex‐specific manner. Cell, 172(3), 500–516 e516. 10.1016/j.cell.2017.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli, K. J. , Neugebauer, K. M. , Bixby, J. L. , Lilien, J. , & Reichardt, L. F. (1988). N‐cadherin and integrins: two receptor systems that mediate neuronal process outgrowth on astrocyte surfaces. Neuron, 1(1), 33–43. 10.1016/0896-6273(88)90207-3 [DOI] [PubMed] [Google Scholar]
- Tremblay, M. E. , Lowery, R. L. , & Majewska, A. K. (2010). Microglial interactions with synapses are modulated by visual experience. PLoS Biology, 8(11), e1000527. 10.1371/journal.pbio.1000527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truttmann, A. C. , Ginet, V. , & Puyal, J. (2020). Current evidence on cell death in preterm brain injury in human and preclinical models. Frontiers in Cell and Development Biology, 8, 27. 10.3389/fcell.2020.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsamantioti, E. , Lisonkova, S. , Muraca, G. , Ortqvist, A. K. , & Razaz, N. (2022). Chorioamnionitis and risk of long‐term neurodevelopmental disorders in offspring: a population‐based cohort study. American Journal of Obstetrics and Gynecology, 227(2), 217–287. 10.1016/j.ajog.2022.03.028 [DOI] [PubMed] [Google Scholar]
- Twilhaar, E. S. , Wade, R. M. , de Kieviet, J. F. , van Goudoever, J. B. , van Elburg, R. M. , & Oosterlaan, J. (2018). Cognitive outcomes of children born extremely or very preterm since the 1990s and associated risk factors: A meta‐analysis and meta‐regression. JAMA Pediatrics, 172(4), 361–367. 10.1001/jamapediatrics.2017.5323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno, M. , Fujita, Y. , Tanaka, T. , Nakamura, Y. , Kikuta, J. , Ishii, M. , & Yamashita, T. (2013). Layer V cortical neurons require microglial support for survival during postnatal development. Nature Neuroscience, 16(5), 543–551. 10.1038/nn.3358 [DOI] [PubMed] [Google Scholar]
- Ugidos, I. F. , Pistono, C. , Korhonen, P. , Gomez‐Budia, M. , Sitnikova, V. , Klecki, P. , … Malm, T. (2022). Sex differences in poststroke inflammation: a focus on microglia across the lifespan. Stroke, 53(5), 1500–1509. 10.1161/STROKEAHA.122.039138 [DOI] [PubMed] [Google Scholar]
- Ullian, E. M. , Sapperstein, S. K. , Christopherson, K. S. , & Barres, B. A. (2001). Control of synapse number by glia. Science, 291(5504), 657–661. 10.1126/science.291.5504.657 [DOI] [PubMed] [Google Scholar]
- Underwood, M. A. (2019). Probiotics and the prevention of necrotizing enterocolitis. Journal of Pediatric Surgery, 54(3), 405–412. 10.1016/j.jpedsurg.2018.08.055 [DOI] [PubMed] [Google Scholar]
- Vaes, J. E. G. , Kosmeijer, C. M. , Kaal, M. , van Vliet, R. , Brandt, M. J. V. , Benders, M. , & Nijboer, C. H. (2020). Regenerative therapies to restore interneuron disturbances in experimental models of encephalopathy of prematurity. International Journal of Molecular Sciences, 22(1), 211. 10.3390/ijms22010211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaes, J. E. G. , van Kammen, C. M. , Trayford, C. , van der Toorn, A. , Ruhwedel, T. , Benders, M. , … Nijboer, C. H. (2021). Intranasal mesenchymal stem cell therapy to boost myelination after encephalopathy of prematurity. Glia, 69(3), 655–680. 10.1002/glia.23919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Looij, Y. , Lodygensky, G. A. , Dean, J. , Lazeyras, F. , Hagberg, H. , Kjellmer, I. , … Sizonenko, S. V. (2012). High‐field diffusion tensor imaging characterization of cerebral white matter injury in lipopolysaccharide‐exposed fetal sheep. Pediatric Research, 72(3), 285–292. 10.1038/pr.2012.72 [DOI] [PubMed] [Google Scholar]
- van der Poel, M. , Ulas, T. , Mizee, M. R. , Hsiao, C. C. , Miedema, S. S. M. , … Huitinga, I. (2019). Transcriptional profiling of human microglia reveals grey‐white matter heterogeneity and multiple sclerosis‐associated changes. Nature Communications, 10(1), 1139. 10.1038/s41467-019-08976-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hove, H. , Martens, L. , Scheyltjens, I. , De Vlaminck, K. , Pombo Antunes, A. R. , De Prijck, S. , … Movahedi, K. (2019). A single‐cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nature Neuroscience, 22(6), 1021–1035. 10.1038/s41593-019-0393-4 [DOI] [PubMed] [Google Scholar]
- Van Steenwinckel, J. , Schang, A. L. , Krishnan, M. L. , Degos, V. , Delahaye‐Duriez, A. , Bokobza, C. , … Gressens, P. (2019). Decreased microglial Wnt/beta‐catenin signalling drives microglial pro‐inflammatory activation in the developing brain. Brain, 142(12), 3806–3833. 10.1093/brain/awz319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varner, M. W. , Costantine, M. M. , Jablonski, K. A. , Rouse, D. J. , Mercer, B. M. , Leveno, K. J. , … Human Development Maternal‐Fetal Medicine Units, N . (2020). Sex‐specific genetic susceptibility to adverse neurodevelopmental outcome in offspring of pregnancies at risk of early preterm delivery. American Journal of Perinatology, 37(3), 281–290. 10.1055/s-0039-1678535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velmeshev, D. , Schirmer, L. , Jung, D. , Haeussler, M. , Perez, Y. , Mayer, S. , … Kriegstein, A. R. (2019). Single‐cell genomics identifies cell type‐specific molecular changes in autism. Science, 364(6441), 685–689. 10.1126/science.aav8130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh, K. K. , Leviton, A. , Hecht, J. L. , Joseph, R. M. , Douglass, L. M. , Frazier, J. A. , … Kuban, K. C. K. (2020). Histologic chorioamnionitis and risk of neurodevelopmental impairment at age 10 years among extremely preterm infants born before 28 weeks of gestation. American Journal of Obstetrics and Gynecology, 223(5), e710–e741. 10.1016/j.ajog.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verney, C. , Monier, A. , Fallet‐Bianco, C. , & Gressens, P. (2010). Early microglial colonization of the human forebrain and possible involvement in periventricular white‐matter injury of preterm infants. Journal of Anatomy, 217(4), 436–448. 10.1111/j.1469-7580.2010.01245.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verney, C. , Pogledic, I. , Biran, V. , Adle‐Biassette, H. , Fallet‐Bianco, C. , & Gressens, P. (2012). Microglial reaction in axonal crossroads is a hallmark of noncystic periventricular white matter injury in very preterm infants. Journal of Neuropathology and Experimental Neurology, 71(3), 251–264. 10.1097/NEN.0b013e3182496429 [DOI] [PubMed] [Google Scholar]
- Verney, C. , Rees, S. , Biran, V. , Thompson, M. , Inder, T. , & Gressens, P. (2010). Neuronal damage in the preterm baboon: impact of the mode of ventilatory support. Journal of Neuropathology and Experimental Neurology, 69(5), 473–482. 10.1097/NEN.0b013e3181dac07b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicentini, F. A. , Keenan, C. M. , Wallace, L. E. , Woods, C. , Cavin, J. B. , Flockton, A. R. , … Sharkey, K. A. (2021). Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome, 9(1), 210. 10.1186/s40168-021-01165-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa, A. , Gelosa, P. , Castiglioni, L. , Cimino, M. , Rizzi, N. , Pepe, G. , … Maggi, A. (2018). Sex‐specific features of microglia from adult mice. Cell Reports, 23(12), 3501–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinukonda, G. , Hu, F. , Upreti, C. , Ungvari, Z. , Zia, M. T. , Stanton, P. K. , & Ballabh, P. (2012). Novel organotypic in vitro slice culture model for intraventricular hemorrhage of premature infants. Journal of Neuroscience Research, 90(11), 2173–2182. 10.1002/jnr.23102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vontell, R. , Supramaniam, V. , Thornton, C. , Wyatt‐Ashmead, J. , Mallard, C. , Gressens, P. , … Hagberg, H. (2013). Toll‐like receptor 3 expression in glia and neurons alters in response to white matter injury in preterm infants. Developmental Neuroscience, 35(2‐3), 130–139. 10.1159/000346158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. J. , Li, Z. H. , Gao, R. W. , Chen, Q. F. , Lin, J. , Xiao, M. L. , … Chen, C. (2022). Effects of delayed HIF‐1alpha expression in astrocytes on myelination following hypoxia‐ischaemia white matter injury in immature rats. Translational Pediatrics, 11(1), 20–32. 10.21037/tp-21-407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witting, A. , Muller, P. , Herrmann, A. , Kettenmann, H. , & Nolte, C. (2000). Phagocytic clearance of apoptotic neurons by Microglia/Brain macrophages in vitro: involvement of lectin‐, integrin‐, and phosphatidylserine‐mediated recognition. Journal of Neurochemistry, 75(3), 1060–1070. [DOI] [PubMed] [Google Scholar]
- Wlodarczyk, A. , Holtman, I. R. , Krueger, M. , Yogev, N. , Bruttger, J. , Khorooshi, R. , … Owens, T. (2017). A novel microglial subset plays a key role in myelinogenesis in developing brain. The EMBO Journal, 36(22), 3292–3308. 10.15252/embj.201696056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, T. R. , Hildahl, K. , Helmbrecht, H. , Corry, K. A. , Moralejo, D. H. , Kolnik, S. E. , … Nance, E. (2022). A ferret brain slice model of oxygen‐glucose deprivation captures regional responses to perinatal injury and treatment associated with specific microglial phenotypes. Bioengineering & Translational Medicine, 7(2), e10265. 10.1002/btm2.10265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward, L. J. , Anderson, P. J. , Austin, N. C. , Howard, K. , & Inder, T. E. (2006). Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. New England Journal of Medicine, 355(7), 685–694. 10.1056/NEJMoa053792 [DOI] [PubMed] [Google Scholar]
- Xie, D. , Shen, F. , He, S. , Chen, M. , Han, Q. , Fang, M. , … Deng, Y. (2016). IL‐1beta induces hypomyelination in the periventricular white matter through inhibition of oligodendrocyte progenitor cell maturation via FYN/MEK/ERK signaling pathway in septic neonatal rats. Glia, 64(4), 583–602. 10.1002/glia.22950 [DOI] [PubMed] [Google Scholar]
- Yasuda, K. , Maki, T. , Kinoshita, H. , Kaji, S. , Toyokawa, M. , Nishigori, R. , … Takahashi, R. (2020). Sex‐specific differences in transcriptomic profiles and cellular characteristics of oligodendrocyte precursor cells. Stem Cell Research, 46, 101866. 10.1016/j.scr.2020.101866 [DOI] [PubMed] [Google Scholar]
- Yawno, T. , Schuilwerve, J. , Moss, T. J. , Vosdoganes, P. , Westover, A. J. , Afandi, E. , … Miller, S. L. (2013). Human amnion epithelial cells reduce fetal brain injury in response to intrauterine inflammation. Developmental Neuroscience, 35(2‐3), 272–282. 10.1159/000346683 [DOI] [PubMed] [Google Scholar]
- Yellepeddi, V. K. , Mohammadpour, R. , Kambhampati, S. P. , Sayre, C. , Mishra, M. K. , Kannan, R. M. , & Ghandehari, H. (2018). Pediatric oral formulation of dendrimer‐N‐acetyl‐l‐cysteine conjugates for the treatment of neuroinflammation. International Journal of Pharmaceutics, 545(1‐2), 113–116. 10.1016/j.ijpharm.2018.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, F. , Lin, Y. A. , Kannan, S. , & Kannan, R. M. (2016). Targeting specific cells in the brain with nanomedicines for CNS therapies. Journal of Controlled Release, 240, 212–226. 10.1016/j.jconrel.2015.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J. J. , Cheng, C. , Qiu, Z. , Zhou, W. H. , & Cheng, G. Q. (2015). Decreased connexin 43 in astrocytes inhibits the neuroinflammatory reaction in an acute mouse model of neonatal sepsis. Neuroscience Bulletin, 31(6), 763–768. 10.1007/s12264-015-1561-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, C. , Kros, J. M. , van der Weiden, M. , Zheng, P. , Cheng, C. , & Mustafa, D. A. (2017). Expression site of P2RY12 in residential microglial cells in astrocytomas correlates with M1 and M2 marker expression and tumor grade. Acta Neuropathologica Communications, 5(1), 4. 10.1186/s40478-016-0405-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, X. L. , Tang, X. G. , Qu, F. , Zheng, Y. , Zhang, W. H. , & Diao, Y. Q. (2019). Bifidobacterium may benefit the prevention of necrotizing enterocolitis in preterm infants: A systematic review and meta‐analysis. International Journal of Surgery, 61, 17–25. 10.1016/j.ijsu.2018.11.026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
As this is a Review paper, data deszcribed in this paper belong to Authors of the original papers. Requests for data should be addressed to these Authors.


