Abstract
We reviewed the literature on the importance of selected anti‐high‐risk human papillomavirus (HR‐HPV) antibodies (namely, 16/18 and early oncoproteins E6 and E7) as potential serological markers for early detection of individuals at high risk of cervical cancer. We searched for studies in PubMed and Embase databases published from 2010 to 2020 on antibodies against HR‐HPV E6 and E7 early proteins and cervical cancer. Pooled sensitivity and specificity for HPV16 and HPV18 antibodies were calculated using a bivariate hierarchical random‐effects model. A total of 69 articles were identified; we included three studies with 1550 participants. For the three HPV16/18 E6 and E7 antibody tests, enzyme‐linked immunosorbent assay‐based assays had a sensitivity of 18% for detecting CIN2+ (95% confidence interval [CI]: 15–21) and a specificity of 96% (95% CI: 92–98), for slot‐blot, sensitivity was 28.9% (95% CI: 23.3–35.1) and specificity was 72% (95% CI: 66.6–77.0) for detecting CIN2+, and for multiplex HPV serology assay based on a glutathione S‐transferase, sensitivity was 16% (95% CI: 8.45–28.6) and specificity was 98% (95% CI: 97–99) for detecting invasive cervical cancer. HR‐HPV16/18 E6 and E7 serological markers showed high specificity, but sensitivity was suboptimal for the detection of cervical cancer in either population screening settings or as point‐of‐care screening tests.
Keywords: cervical cancer, E6 and E7 proteins antibody, human papillomavirus, sensitivity, specificity
1. INTRODUCTION
In high‐income countries, organized and effective screening of cervical cancer using Papanicolaou (pap) smears and more recently human papillomavirus (HPV) DNA testing (self‐collected vaginal sample) have contributed to a decline in incident cervical cancer cases. 1 However, in low‐ and middle‐income countries (LMICs), despite efforts to implement organized cervical cancer screening with cytology or HPV testing, coverage and uptake rates are very low, with only about 30% of women having undergone opportunistic cervical cancer screening. 2 , 3 , 4 , 5 The great benefits and small harm of pap screening have been well documented. 6 , 7 However, some sociocultural barriers (i.e., health‐seeking behaviors, an embarrassment for pelvic examination by male providers, a perception that the pap smear screening process is painful and can include bleeding with a long turnaround time) exist around the genital examination, as shown in a recent review. 8 Therefore, conventional methods for screening cervical cancer that involve genital examination could be considered less desirable or acceptable to women. These include the more current HPV DNA or messenger ribonucleic acid (mRNA) tests that are favored over ThinPrep cytological test and cytology (Pap tests). 9
In all the aforementioned screening test scenarios, large proportions of women who screen positive for being at high risk of developing cervical cancer require recall and confirmatory diagnostic testing, and then an additional recall to receive treatment. Visual inspection of the cervix with acetic acid (VIA) is an alternative cervical cancer “screen and treat” screening method that is deemed more viable in LMIC, thus avoiding one recall step. 10 However, studies in LMIC have shown mixed results on the performance of VIA in these settings. 7 , 10 , 11 Besides, VIA has a problem of high false‐positive and false‐negative results and depends on the operator's experience. 12 , 13 As a result, the incidence rates of cervical cancer remain high in LMIC, 5 and reducing incidence rates of cervical cancers in these settings remains a challenge. Hence, a novel efficient screening tool that is less invasive and more acceptable to women is needed.
In May 2018, the General Director of the World Health Organization (WHO) made a global call for action to eliminate cervical cancer using novel technologies for the identification of premalignant tumors at the earliest stage. 14 , 15 The 90–70–90 global cervical cancer elimination strategies call for 90% of girls to be fully vaccinated by the HPV vaccine by the age of 15 years, 70% of women should have been screened with high‐throughput tests by the age of 35 years and again by the age of 45 years, and 90% of women diagnosed with cervical cancer disease to be on treatment by the year 2030. 14
One of the promising strategies is the use of serum profiling antibodies to HPV16 and HPV18 early oncoproteins E6 and E7 as biomarkers for early detection of invasive cervical cancer (ICC). 16 The E6 protein binds to the E3 ubiquitin ligase E6‐associated protein and to the tumor suppressor protein p53, which is involved in the control of the cell growth process and apoptosis. 17 The presence of E7 in human cells prevents phosphorylation of the retinoblastoma protein leading to uncontrolled cell proliferation. 18 Out of the seven early proteins, both E6/E7 oncoproteins are involved in the transformation of cells, mitosis, and immortalization of cervical cancer cells, 19 and have shown the strongest associations with cervical cancer. 20 , 21 , 22 , 23 Since several antibody‐based finger prick “point‐of‐care” tests are now readily available for screening other infectious agents such as HIV, 24 which have a short turnaround time (<1 h), a yet‐to‐be‐developed rapid antibody‐based HPV finger prick screening test for detecting cervical cancer may be more acceptable than conventional cervical cancer screening methodologies requiring a genital examination. If such a serological test can be carried out cheaply and has a rapid turnaround time, then it could save time and avoid repeat visits. 25
However, the value of high‐risk HPV (HR‐HPV) E6 and E7 serology as an alternative, less‐invasive point‐of‐care screening tool for detecting at‐risk individuals has not been fully investigated. In addition, globally there are no currently developed and USA Food and Drug Administration (FDA)‐approved serological markers for screening cervical cancer in the general population. Studies from the early 1990s 26 , 27 showed some potential with sensitivities around 27%–41% and specificities around 99%–100%. We, therefore, performed a systematic review aimed at assessing the utility of HPV antibodies, especially of HR‐HPV16/18 E6 and E7 serological markers, in the detection of cervical cancer and cervical intraepithelial neoplasia 2/3 (CIN2/3).
2. METHODS
This systematic review and meta‐analysis were registered and published with PROSPERO (CRD42020206036). In the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA), 28 guidelines were followed to evaluate the usefulness of HPV16/18 E6 and E7 serological markers in the detection of CIN2/3 and cervical cancer.
2.1. Search strategies
The search for studies was done in the following electronic databases from the period 2010 to 2020: PubMed and Embase, using the following search words “HPV,” “E6 and E7 protein,” “CIN2,” CIN3,” and “cervical cancer”. We did not include high‐grade squamous intraepithelial lesion (HSIL) in the search terms because HSIL is based on cytology, while CIN2/3 are based on histology, which incorporates HSIL. After title and abstract review, where possible full copies of the text of appropriate published articles were obtained and assessed for inclusion. Additional searches were done on the references of the initial studies. Our review focused on HPV16 and HPV18 because they are the most common serotypes and are found in 72% of cervical cancer. 29
2.2. Criteria for study selection
The study selection involved exporting all identified studies (69) combined in Mendeley into Covidence (https://www.covidence.org/). Covidence is a useful web tool for screening and selecting studies for conducting systematic reviews. For accuracy and consistency, the two independent reviewers (M. G. S. and T. R.) screened the full texts. Disagreements were resolved through discussion; any unresolved disagreements were resolved by the third reviewer (M. M.).
2.3. Inclusion
Studies were selected if they were in English, cross‐sectional, retrospective case–control, or prospective cohort (or nested case–control). Cases were defined as ICC or CIN 2/3. We used author‐defined definitions of controls, which broadly used similarly aged women without HPV‐related conditions. Diagnostic accuracy‐test studies in which the HPV16/18 E6 and E7 serological markers were compared to a cytopathology or histopathology reference standard were included.
2.4. Exclusion
Studies with a sample size of less than 200 that could overestimate the effect size 30 (of the small studies excluded some used an HPV E6/E7 mRNA marker and the others used a urine Trovagene HPV test), conference papers, abstracts only, and reviews were excluded. Also, studies that assessed the presence of HPV types in ICC or CIN2/3 were excluded. Given the regular improvements in serological techniques, studies were excluded if they were published before the year 2010. We excluded only laboratory‐based studies (which did not include controls) and those studies that focused on CIN1 (wrong outcome). Any studies presenting HPV16/18 E6 and E7 DNA within samples of the cervical biopsy were not included because we aimed to assess the utility of anti‐HPV antibodies, especially of HR‐HPV16/18 E6 and E7 in the serum of patients with ICC or CIN2/3. We further excluded papers that were not on serological testing, that is, the Onco E6 test because it detects the protein itself, whereas the HPV E6/E7 mRNA tests detect the RNA encoding for the oncoproteins. Our study focused on the test accuracy of HPVE6 and E7 oncoprotein antibodies.
2.5. Participants
The population of interest were women with cervical cancer and CIN2/3. Studies were included if they reported women with cytologically/histologically confirmed cervical cancer or high‐risk lesions (e.g., CIN2 and CIN3) in comparison to women with the normal cervical tissue.
2.6. Index test
The index test was HPV16/18 E6 and E7 and E4 protein serological markers from the serum of the participants. Determination of the threshold for positive or negative results was based on test cut‐off values proposed by the manufacturer or the study authors based on validated cut‐off points.
2.7. Reference standard
The reference test was histopathologic confirmation of cervical cancer or CIN2+ in paraffin‐embedded tissue sections or cytological confirmation of CIN2/3 or cervical cancer. Methods of diagnosis were noted.
2.8. Study outcome
The outcome of the study was sensitivity, specificity, and diagnostic odds ratios (DORs) for CIN2/3 and cervical cancer.
2.9. Data extraction
The two reviewers independently extracted data (M. G. S. and T. R.) for all the papers meeting the inclusion and exclusion criteria. The following information was extracted from the eligible studies: author; year of publication; study location; sample size; the number of cases and controls, cut‐offs, and test manufacturer. We extracted percentages of HPV16/18 E6 and E7 (antibodies seropositive) from precancers and cervical cancer cases and controls according to predefined cut‐off values. We then extracted frequencies for true positives (TP), false positives (FP), true negatives (TN), and false negatives (FN) for HPV16 (E6 and E7) or HPV18 (E6 and E7) antibodies. In some of the studies, more than one HPV‐type antigen was assessed on the same individuals participating in the study. In such cases, each serological biomarker was considered an independent study when extracting the data.
2.10. Study quality assessment
Assessment of the quality of the studies was done using the Quality Assessment of Diagnostic Accuracy Studies‐2 (QUADAS‐2) tool. 31 Quality assessments of the studies were based on: a selection of participants' characteristics; the number of included participants with cervical cancer or CIN2+; description of the index test (HPV16/18 E6 and E7) serological markers and the reference test (histology or cytology). The studies were grouped into high quality, low quality, and unclear. Any disagreements were resolved through discussion with the third reviewer (M. M.).
2.11. Statistical analyses
To achieve our objective, we performed a descriptive analysis of different serological markers by calculating the sensitivity and specificity, using the TP, FP, TN, and FN results and their 95% confidence intervals (CIs). To quantify pooled effects (Supporting Information: Figure S2), random‐effects models for meta‐analysis were performed. Cochrane's Q‐statistic test and the I 2 tests were used to examine heterogeneity.
Second, we assessed the DOR for HPV16/18 E6 and E7 and other seropositive E oncoprotein (E4) antibodies and for CIN2/3 and cervical cancer from the different studies. Other studies have suggested that E4 plays an important role in the detection of cervical cancer when used in combination with other markers. 32 We pooled the sensitivity and specificity for HPV16/18 E6 and E7 serological markers to detect CIN2/3 and cervical cancer. Analysis by different serological screening tests (enzyme‐linked immunosorbent assay [ELISA], multiplex HPV serology assay based on a glutathione S‐transferase capture immunoassay in combination with fluorescent beads [multiplex HPV serology immunofluorescent assay] and immunoenzymatic assay [slot‐blot]) was performed for comparison of pooled effects and heterogeneity. In the primary studies, cases and controls were matched by age.
To estimate sensitivity and specificity, a bivariate random‐effects model 33 was applied using “Metandi” and “Midas” in STATA. Metandi fits a two‐level mixed logistic regression model, with independent binomial distributions for the TP and TN conditional on the sensitivity and specificity in an individual study, and a bivariate hierarchical normal model for the logit transformation of sensitivity and specificity between studies. Midas calculates summary receiver‐operating characteristics (SROC) (Supporting Information: Figure S3), summary likelihood, and DOR. Hierarchical SROC (HSROC) was used to account for the correlation between sensitivity and specificity (Supporting Information: Figure S4). The HSROC accounts for threshold effects and between‐ and within‐study variability.
Statistical significance was considered at a p value of 0.05, and all analyses were performed using STATA version 16 and the Review Manager (Version 5.3; Cochrane Collaboration). All statistical tests were two‐sided.
3. RESULTS
We first identified 69 records (papers) through PubMed library searches and references (Figure 1). We retrieved 44 potential papers after a screening of titles and abstracts. Twenty‐three papers were eligible for inclusion in the systematic review. Out of 23 papers, only 3 papers were included with 1550 women. A total of 11 HPV16/18 oncoprotein antibodies (studies) were obtained from different serological test methods. Two‐thirds of the studies were a case–control design and one study was a prospective diagnostic accuracy study (Table 1). Assessment of the methodological quality of the individual studies was done using QUADAS‐2 (Supporting Information: Figure S1 and Table 2).
Figure 1.
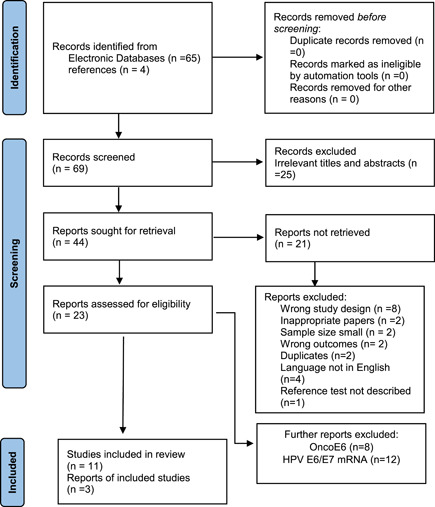
The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses diagram of the studies in the systematic review of HPV16 and HPV18 E6/E7 serological markers. HPV, human papillomavirus.
Table 1.
Summary of the three study characteristics included in the review: CIN2, CIN3, and ICC
| No. | Study ID (author and year) | Study reference number | Country | Study design | Index test | Reference method | Sample size | Median age | CIN2+, n (%) | ICC, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Salazar‐Piña (2016) 20 | d1 | Mexico | Diagnostic test accuracy study | HPV16 E4 and E7 immunoenzymatic assay (slot‐blot) | Histopathology | 485 | 40.0 | 106 (29.9) | 15 (3.1) |
| 2 | Jin (2018) 34 | d2 | South Korea | Case–control study | HPV E6 and E7 (ELISA) | Cytology | 249 | 44.3 | 144 (57.8) | ‐ |
| 3 | Combes (2014) 35 | d3 | Algeria and India | Case–control study | HPV16 L1, E1,E2, E4, E6, and E7 (multiplex HPV serology immunofluorescent assay) | Cytology | 816 | 50.9 | ‐ | 307 (37.6) |
Abbreviations: CIN, cervical intraepithelial neoplasia; ELISA, enzyme‐linked immunosorbent assay; HPV, human papillomavirus; ICC, invasive cervical cancer.
Table 2.
True positive, false positive, false negative, and true negative of different tests for the detection of CIN2/3 and ICC
| No. | Study ID (1st author and year) | Study reference number | CIN2, CIN3, or cervical cancer) | Cut‐off (threshold) | Test (manufacturer) | Marker name (index test) | Full name | TP | FP | FN | TN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Salazar‐Piña (2016) 20 | d1a | CIN2+ | 2 AU/ml | Qiagen | HPV16 E7 (slot‐blot) | HPV 16 E7 antibodies immunoenzymatic assay (slot‐blot) | 48 | 58 | 73 | 92 |
| Salazar‐Piña (2016) 20 | d1b | CIN2+ | 7 AU/ml | Qiagen | HPV16 E4 (slot‐blot) | HPV16 E4 antibodies immunoenzymatic assay (slot‐blot) | 22 | 26 | 99 | 124 | |
| 2 | Jin (2018) 34 | d2a | CIN2+ | N/A | Greiner Bio‐One | HPV16 E6 (ELISA) | Enzyme‐linked immunosorbent assay | 41 | 2 | 118 | 47 |
| Jin (2018) 34 | d2b | CIN2+ | N/A | Greiner Bio‐One | HPV16 E7 (ELISA) | Enzyme‐linked immunosorbent assay | 28 | 2 | 131 | 47 | |
| Jin (2018) 34 | d2c | CIN2+ | N/A | Greiner Bio‐One | HPV18 E6 (ELISA) | Enzyme‐linked immunosorbent assay | 29 | 2 | 130 | 47 | |
| Jin (2018) 34 | d2d | CIN2+ | N/A | Greiner Bio‐One | HPV18 E7 (ELISA) | Enzyme‐linked immunosorbent assay | 26 | 2 | 133 | 47 | |
| 3 | Combes (2014) 35 | d3a | ICC | 394–714 MFI | Luminex Corp. | HPV16E6 (multiplex) | Multiplex HPV serology immunofluorescent assay | 99 | 4 | 208 | 323 |
| Combes (2014) 35 | d3b | ICC | 395–714 MFI | Luminex Corp. | HPV16E7 (multiplex) | Multiplex HPV serology immunofluorescent assay | 86 | 10 | 221 | 317 | |
| Combes (2014) 35 | d3c | ICC | 396–714 MFI | Luminex Corp. | HPV18E6 (multiplex) | Multiplex HPV serology immunofluorescent assay | 13 | 9 | 294 | 318 | |
| Combes (2014) 35 | d3d | ICC | 397–714 MFI | Luminex Corp. | HPV18E7 (Multiplex) | Multiplex HPV serology immunofluorescent assay | 39 | 5 | 268 | 322 | |
| Combes (2014) 35 | d3e | ICC | 394–714 MFI | Luminex Corp. | HPV16 E4 (multiplex) | Multiplex HPV serology immunofluorescent assay | 53 | 4 | 254 | 323 |
Abbreviations: CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; ICC, invasive cervical cancer; N/A, not available.
There was a considerable degree of heterogeneity for sensitivity between the studies for combined CIN2/3 and ICC, which fluctuated between 0 and 0.40, while specificity was very good for most of the biomarkers. Thus, we did not pool the studies (Figure 2A–C). For the stratified analysis by tumor stage, sensitivity was very low for CIN2/3, which ranged between 0 and 0.20 for the biomarkers, while specificity was above 0.90 for most of the biomarkers. For ICC, the sensitivity ranged between 0.20 and 0.40 and specificity ranged between 0.96 and 0.97.
Figure 2.
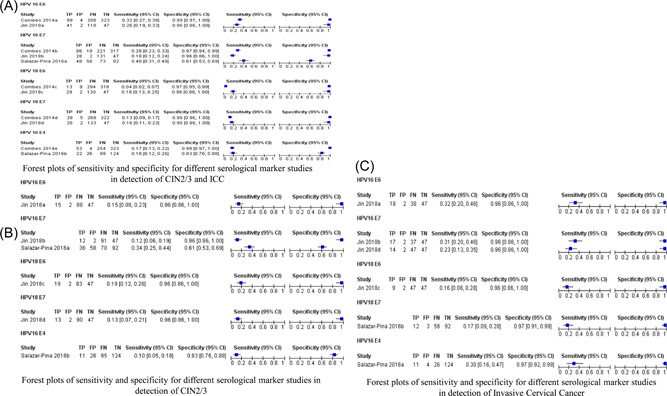
Forest plots of sensitivity and specificity for different serological marker studies in the detection of CIN2/3 and ICC. (A) Forest plots of sensitivity and specificity for different serological markers studies in the detection of combined CIN2/3 and ICC. (B) Forest plots of sensitivity and specificity for different serological marker studies in the detection of CIN2/3. (C) Forest plots of sensitivity and specificity for different serological marker studies in the detection of ICC. CI, confidence interval; CIN, cervical intraepithelial neoplasia; FN, false negative; FP, false positive; HPV, human papillomavirus; ICC, invasive cervical cancer; TN, true negative; TP, true positive.
Different assays were used to characterize the association between HPV E6 and E7 antibodies and cervical cancer. In the studies that used ELISA, the pooled sensitivity was 18% (95% CI: 15–21) and specificity was 96% (95% CI: 92–98). For the studies that used multiplex HPV serology assay, the pooled sensitivity was 16% (95% CI: 8.45–28.6) and specificity was 98% (95% CI: 97–99) (Table 3). For the studies that used immunoenzymatic assay (slot‐blot), the pooled sensitivity was 28.9% (95% CI: 23.3–35.1) and specificity was 72% (95% CI: 66.6–77.0) (Table 3). The multiplex immunofluorescent assay had a DOR of 9.72 (95% CI: 3.95–23.93), the ELISA had a DOR of 5.00 (95% CI: 2–11), and immunoenzymatic assay had a DOR of 1.05 (95% CI: 0.72–1.52) (Table 3).
Table 3.
Bivariate meta‐analysis of different HPV16 and HPV18 serological markers: pooled sensitivity and specificity by assay type
| Marker name | Study reference number | Number of studies | Endpoint | Sensitivity (95% CI) | Specificity (95% CI) | DOR (95% CI) | |
|---|---|---|---|---|---|---|---|
| HPV serology (ELISA) | HPV16 and HPV18 (E6 and E7) | d2a, d2b, d2c, d2d | 4 | CIN2+ | 18.0 (15–21) | 96.0 (92.0–98.0) | 5.00 (2.00–11.00) |
|
HPV serology (multiplex HPV serology immunofluorescent assay) |
HPV16 and HPV18 E6, E7, and E4 | d3a, d3b, d3c, d3d, d3e | 5 | ICC | 16.0 (8.45–28.6) | 98.0 (97.0–99.0) | 9.72 (3.95–23.93) |
|
HPV serology (immunoenzymatic assay (slot‐blot)) |
HPV16 E7 and E4 | d1a, d1b | 2 | CIN2+ | 28.9 (23.3–35.1) | 72.0 (66.6–77.0) | 1.05 (0.72–1.52) |
Abbreviations: CI, confidence interval; DOR, diagnostic odds ratio; ELISA, enzyme‐linked immunosorbent assay; HPV, human papillomavirus; ICC, invasive cervical cancer.
Across the studies, HPV antibody sensitivity was low, ranging from 4% to 40%, while the specificity was high, ranging from 61% to 99%. There was a considerable degree of heterogeneity between the studies; thus, we did not pool the studies (Figure 2). From the HSROC curve, HPV16 E6 antibody presence appears to be a better marker compared to the other markers (Figure 3).
Figure 3.
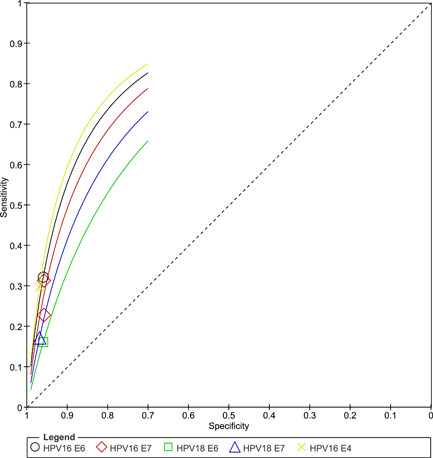
Summary of the hierarchial summary receiver‐operating characteristic plots of sensitivity and specificity for HPV16 and HPV18 protein serological markers, namely, E6, E7, and E4. HPV, human papillomavirus.
4. DISCUSSION
Antibodies to HPV oncoproteins have been suggested as important serological markers in the detection of cervical cancer. Our systematic review and meta‐analysis focused on the sensitivity and specificity of HPV16/18 early oncoprotein antibodies. Our main findings suggest that HPV16 and HPV18 E6 and E7 antibodies have high specificity but low sensitivity to detect cases with cervical cancer or CIN2/3 precancerous lesions. Another key finding is that there is scant literature on E6/E7 as a biomarker for ICC and CIN2/3.
We found that HPV16 and HPV18 E6 and E7 antibodies have higher specificity but lower sensitivity. Our estimates are similar to the estimates pre‐2010 of Sun et al. 36 in Brazil where they reported specificity of 99.5% and sensitivity of 40.7% for HPV16 E6 or E7 (higher antibody titers). Similarly, in Japan, the findings of Sasagawa et al., 27 using ELISA test with E6 or E7 proteins as antigens, had first shown in 1992 that 27% of cervical cancer and 19% of CIN3 were positive for anti‐HPV16 E6 antibody, while 33% in cervical cancer and 8% in CIN3 were positive for anti‐HPV16 E7. Therefore, low sensitivity in these serological tests may represent a lower immune response against HPV16/18 E6 or E7 protein in many patients with these malignant lesions. Another explanation for the low sensitivity of these assays might be due to low immune responses against HPV16/18. The serological responses to HPV16/18 E6 and E7 are likely to be surrogate markers for cytotoxic immune responses by cytotoxic T lymphocyte (CTL) targeting these proteins. Such CTLs function to kill CIN or cancer cells positive with HPV16 or HPV18. 37 In addition, our findings showed that the HPV16 E6 antibody is a relatively better serological marker compared to the other biomarkers. Similarly, a recent systematic review and meta‐analysis on blood‐based biomarkers of HPV‐associated cancer found HPV16 E6 antibodies to be a better serology marker. 38 Although HPV16 E6 was shown to be a better biomarker, its sensitivity is still low for it to be used as a screening tool. Thus, additional studies are needed for its validation with other biomarkers. 39
HPV16 E6 and E7 antibodies have been associated with the tumor stage of cervical cancer. 37 , 39 , 40 , 41 , 42 In our study, we found that the sensitivity for CIN2/3 was lower compared to the sensitivity for ICC. Our findings are in agreement with the findings of Park et al., 40 who reported that seropositive HPV16 E6 and E7 antibodies were associated with the stage of the tumor. Their findings showed that seropositivity for HPV16 E6 was 51% and 38% for HPV16 E7 in Stage II and HPV16 E6 (69%) and HPV16 E7 (56%) for Stage III/IV, respectively. In addition, in squamous carcinoma seropositivity was 54% for HPV16 E6% and 32% for HPV16 E7. However, a radioimmunoprecipitation assay (RIPA) was used making comparison difficult between assays. Similarly, in Korea, Chee et al., 37 using RIPA, found that HPV16 E6 and E7 antibodies were associated with the clinical stage of the tumor for HPV16 E6 (CIN [4.2%], Stage I [12.5%], Stage IIa [35.7%], Stage IIb [100%], Stage III [100%]) and HPV16 E7 (CIN [4.2%], Stage I [43.8%], Stage IIa [57.1%], Stage IIb [60%], Stage III [100%]).
The strengths of our systematic review and meta‐analysis include the application of the standard systematic review protocol, using Covidence software to screen the studies, involvement of second and third reviewers at all stages of screening, and using QUADAS‐2 to assess the quality of the studies' methodology. Our study had several limitations: After all the exclusions, there were only a few studies included that assessed the antibodies against HPV E6 and E7 oncoprotein for detection of CIN2/3 and cervical cancer. There was a high level of heterogeneity for the included studies; therefore, it was impossible to have pooled estimates of all the studies. Due to the different assays used, we were unable to pool all the studies together. In addition, due to the selection bias that comes with case–control design, we were unable to pool the studies together. 43 Our systematic review and meta‐analysis only covered studies published in the last 10 years and might have missed studies published in other languages, or that were not appearing during the literature search.
This systematic review and meta‐analysis focused on the utility (e.g., sensitivity and specificity) of HPV antibodies, especially of HPV16/18 E6 and E7 serological markers in the detection of ICC and CIN2/3. Recently, the WHO in their new cervical cancer guidelines suggested HPV antibodies and oncoproteins as possible future screening tests. 44 If such a test could be incorporated into population screening using point‐of‐care testing of appropriately aged nonvaccinated women (e.g., 30–65 years as recommended by cervical screening guidelines), 44 it would improve screening rates, reduce losses to follow‐up (people, samples, and results) and ultimately lead to early detection of the disease in limited‐resource settings. Individuals who have been seropositive to both HPV E6/E7 antibodies are at higher risk for cervical cancer, 45 which makes them potentially useful biomarker candidates for early detection of cervical cancer. 46 However, we found that evidence is still limited for HPV antibodies to be translated into an effective detection tool for cervical cancer detection. Further laboratory studies are required on the development of more accurate HPV serological biomarkers for them to be successfully translated into an effective screening or point‐of‐care detection tool for cervical cancer.
Our systematic review and meta‐analysis further add to the debate that HPV16 and 18 E6 and E7 antibodies could be useful biomarkers for the screening of cervical cancer. There seems very little improvement over time in the utility of serological markers in detecting cervical cancer or CIN3. Although the specificity of HPV serological markers is high, their sensitivity is suboptimal for the detection of cervical cancer.
AUTHOR CONTRIBUTIONS
Mwiza Gideon Singini conceptualized the study, performed data analysis, and drafted the manuscript. Freddy Sitas, Tim Waterboer, Chantal Babb de Villiers, and Elvira Singh edited the manuscript. Melitah Motlhale and Thendo Ramaliba assisted with data extraction. Mazvita Muchengeti, Abram Bunya Kamiza, and Robert Newton assisted in proofreading the manuscript. Wenlong Carl Chen helped with the formatting of figures. Debbie Bradshaw, Tim Waterboer, Christopher G. Mathew, Wenlong Carl Chen, Freddy Sitas, Elvira Singh, Mazvita Muchengeti, Abram Bunya Kamiza, and Robert Newton are the members of the ERICA‐SA collaborative study. All authors read and provided feedback to improve the final version of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supplementary information.
ACKNOWLEDGMENTS
We thank all those who provided feedback to improve the manuscript. This study was supported by the South African Medical Research Council (with funds received from the South African National Department of Health) and the UK Medical Research Council (with funds from the UK Government's Newton Fund) (MRC‐RFA‐SHIP 01‐2015). The funders were not involved in the conceptualization, review, or approval of the manuscript. This project forms part of an international research program aimed at identifying evolving risk factors for cancer in African populations (ERICA‐SA) (https://www.samrc.ac.za/intramural-research-units/evolving-risk-factors-cancers-african-populations-erica-sa).
Singini MG, Singh E, Bradshaw D, et al. Usefulness of high‐risk HPV early oncoprotein (E6 and E7) serological markers in the detection of cervical cancer: a systematic review and meta‐analysis. J Med Virol. 2022;95:e27900. 10.1002/jmv.27900
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Peto PJ, Gilham PC, Fletcher O, Matthews FE. The cervical cancer epidemic that screening has prevented in the UK. Lancet. 2004;17 364(9430):249‐256. [DOI] [PubMed] [Google Scholar]
- 2. Wanyenze RK, Bwanika JB, Beyeza‐Kashesya J, et al. Uptake and correlates of cervical cancer screening among HIV‐infected women attending HIV care in Uganda. Glob Health Action. 2017;10(1):1380361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Botha M, Dreyer G. Guidelines for cervical cancer screening in South Africa. South African J Gynaecol Oncol. 2017;9:1. [Google Scholar]
- 4. Erku DA, Netere AK, Mersha AG, Abebe SA, Mekuria AB, Belachew SA. Comprehensive knowledge and uptake of cervical cancer screening is low among women living with HIV/AIDS in Northwest Ethiopia. Gynecol Oncol Res Pract. 2017;4(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Finocchario‐Kessler S, Wexler C, Maloba M, Mabachi N, Ndikum‐Moffor F, Bukusi E. Cervical cancer prevention and treatment research in Africa: a systematic review from a public health perspective. BMC Women's Health. 2016;16(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Petry KU, Wörmann B, Schneider A. Benefits and risks of cervical cancer screening. Oncol Res Treat. 2014;37(suppl 3):37‐57. [DOI] [PubMed] [Google Scholar]
- 7. Sankaranarayanan R. Screening for cancer in low‐ and middle‐income countries. Ann Glob Health. 2014;80:412‐417. [DOI] [PubMed] [Google Scholar]
- 8. Chidyaonga‐Maseko F, Chirwa ML, Muula AS. Underutilization of cervical cancer prevention services in low and middle income countries: a review of contributing factors. Pan Afr Med J. 2015;21:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shi WJ, Liu H, Wu D, Tang ZH, Shen YC, Guo L. E6/E7 proteins are potential markers for the screening and diagnosis of cervical pre‐cancerous lesions and cervical cancer in a Chinese population. Oncol Lett. 2017;14(5):6251‐6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mabeya H, Khozaim K, Liu T, et al. Comparison of conventional cervical cytology versus visual inspection with acetic acid among human immunodeficiency virus‐infected women in Western Kenya. J Low Genit Tract Dis. 2012;16(2):92‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson LG, Armstrong A, Joyce CM, Teitelman AM, Buttenheim AM. Implementation strategies to improve cervical cancer prevention in sub‐Saharan Africa: a systematic review. Implement Sci. 2018;13(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hegde D, Shetty H, Shetty PK, Rai S. Diagnostic value of acetic acid comparing with conventional Pap smear in the detection of colposcopic biopsy‐proved CIN. J Cancer Res Ther. 2011;7(4):454‐458. [DOI] [PubMed] [Google Scholar]
- 13. Kremer WW, Van Zummeren M, Breytenbach E, et al. The use of molecular markers for cervical screening of women living with HIV in South Africa. AIDS. 2019;33(13):2035‐2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. WHO . Global strategy to accelerate the elimination of cervical cancer as a public health problem and its associated goals and targets for the period 2020–2030. United Nations Gen Assem. 2020;2(1):1‐3. [Google Scholar]
- 15. To eliminate cervical cancer in the next 100 years , implementing an effective strategy is critical. WHO. 2020‐02‐04. Accessed February 4, 2021. https://www.who.int/news/item/04-02-2020-to-eliminate-cervical-cancer-in-the-next-100-years
- 16. Ewaisha R, Panicker G, Maranian P, Unger ER, Anderson KS. Serum immune profiling for early detection of cervical disease. Theranostics. 2017;7(16):3814‐3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu L‐L, Kang L‐N, Zhao F‐H, et al. Elevated expression of human papillomavirus‐16/18 E6 oncoprotein associates with persistence of viral infection: a 3‐year prospective study in China. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1167‐1174. [DOI] [PubMed] [Google Scholar]
- 18. Cuzick J, Bergeron C, von Knebel Doeberitz M, et al. New technologies and procedures for cervical cancer screening. Vaccine. 2012;30(suppl 5):F107‐F116. [DOI] [PubMed] [Google Scholar]
- 19. Yim E‐K, Park J‐S. Biomarkers in cervical cancer. Biomark Insights. 2007;1:215‐225. [PMC free article] [PubMed] [Google Scholar]
- 20. Salazar‐Piña DA, Pedroza‐Saavedra A, Cruz‐Valdez A, et al. Validation of serological antibody profiles against human papillomavirus type 16 antigens as markers for early detection of cervical. Cancer Med. 2016;95(6):e2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Müller M, Viscidi RP, Ulken V, et al. Antibodies to the E4, E6, and E7 proteins of human papillomavirus (HPV) type 16 in patients with HPV‐associated diseases and in the normal population. J Invest Dermatol. 1995;104(1):138‐141. [DOI] [PubMed] [Google Scholar]
- 22. Muller M, Gausepohl H, De Martynoff G, Frank R, Brasseur R, Gissmann L. Identification of seroreactive regions of the human papillomavirus type 16 protein E4, E6, E7 and L1. J Gen Virol. 1990;71(pt 11) 11):2709‐2717. [DOI] [PubMed] [Google Scholar]
- 23. Estêvão D, Costa NR, Gil da Costa RM, Medeiros R. Hallmarks of HPV carcinogenesis: the role of E6, E7 and E5 oncoproteins in cellular malignancy. Biochim Biophys Acta. 2019;1862(2):153‐162. [DOI] [PubMed] [Google Scholar]
- 24. Jerene D, Abebe W. HIV Testing Services. HIV Self‐Testing and Partner; 2017:7. [Google Scholar]
- 25. Kozel TR, Burnham‐Marusich AR. Point‐of‐care testing for infectious diseases: past, present, and future. J Clin Microbiol. 2017;55(8):2313‐2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sun Y, Eluf‐Neto J, Bosch FX, et al. Human papillomavirus‐related serological markers of invasive cervical carcinoma in Brazil. Cancer Epidemiol Biomarkers Prev. 1994;3:341‐347. [PubMed] [Google Scholar]
- 27. Sasagawa T, Inoue M, Tanizawa O, Yutsudo M, Hakura A. Identification of antibodies against human papillomavirus type 16 E6 and E7 proteins in sera of patients with cervical neoplasias. Japanese. J Cancer Res. 1992;83(7):705‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151:264‐269. [DOI] [PubMed] [Google Scholar]
- 29. de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;2(8):e180‐e190. [DOI] [PubMed] [Google Scholar]
- 30. Zhang Z, Xu X, Ni H. Small studies may overestimate the effect sizes in critical care meta‐analyses: a meta‐epidemiological study. Crit Care. 2013;17(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Whiting PF, Rutjes AWS, Westwood ME, et al. Quadas‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529‐536. [DOI] [PubMed] [Google Scholar]
- 32. Griffin H, Wu Z, Marnane R, et al. E4 antibodies facilitate detection and type‐assignment of active HPV infection in cervical disease. PLoS One. 2012;7(12):e49974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reitsma JB, Glas AS, Rutjes AWS, Scholten RJPM, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58(10):982‐990. [DOI] [PubMed] [Google Scholar]
- 34. Jin Y, Choi JW, Kim HJ, et al. Profiling of serum antibodies against human papillomavirus antigens in Korean women with cervical intraepithelial neoplasia and cervical cancer. Cancer Med. 2018;7(11):5655–5664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Combes JD, Pawlita M, Waterboer T, et al. Antibodies against high‐risk human papillomavirus proteins as markers for invasive cervical cancer. Int J Cancer. 2014;135(10):2453–2461. [DOI] [PubMed] [Google Scholar]
- 36. Sun Y, Eluf‐Neto J, Bosch FX, et al. Serum antibodies to human papillomavirus 16 proteins in women from Brazil with invasive cervical carcinoma. Cancer Epidemiol Prev Biomarkers. 1999;8(10):935‐940. [PubMed] [Google Scholar]
- 37. Chee YH, Namkoong SE, Kim DH, Kim SJ, Park JS. Immunologic diagnosis and monitoring of cervical cancers using in vitro translated HPV proteins. Gynecol Oncol. 1995;57(2):226‐231. [DOI] [PubMed] [Google Scholar]
- 38. Balachandra S, Kusin SB, Lee R, et al. Blood‐based biomarkers of human papillomavirus‐associated cancers: a systematic review and meta‐analysis. Cancer. 2021;127(6):850‐864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fisher SG, Benitez‐Bribiesca L, Nindl I, et al. The association of human papillomavirus type 16 E6 and E7 antibodies with stage of cervical cancer. Gynecol Oncol. 1996;61(1):73‐78. [DOI] [PubMed] [Google Scholar]
- 40. Park DS, Selvey LA, Kelsall SR, Frazer IH. Human papillomavirus type 16 E6, E7 and L1 and type 18 E7 proteins produced by recombinant baculoviruses. J Virol Methods. 1993;45:303‐318. [DOI] [PubMed] [Google Scholar]
- 41. Park JS, Park DC, Kim CJ, et al. HPV‐16‐related proteins as the serologic markers in cervical neoplasia. Gynecol Oncol. 1998;69(1):47‐55. [DOI] [PubMed] [Google Scholar]
- 42. Baay MF, Duk JM, Groenier KH, et al. Relation between HPV‐16 serology and clinico‐pathological data in cervical carcinoma patients: prognostic value of anti‐E6 and/or anti‐E7 antibodies. Cancer Immunol Immunother. 1997;44(4):211‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Beynon R, Hawkins J, Laing R, et al. The diagnostic utility and cost‐effectiveness of selective nerve root blocks in patients considered for lumbar decompression surgery: a systematic review and economic model. Health Technol Assess. 2013;17(19):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. WHO .WHO guideline for screening and treatment of cervical pre‐cancer lesions for cervical cancer prevention. 2nd ed. 2021. https://www.who.int/publications/i/item/9789240030824 [PubMed] [Google Scholar]
- 45. Meschede W, Zumbach K, Braspenning J, et al. Antibodies against early proteins of human papillomaviruses as diagnostic markers for invasive cervical cancer. J Clin Microbiol. 1998;36(2):475‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gutierrez‐Xicotencatl L, Salazar‐Piña A, Chihu‐Amparan L, Pedroza‐Saavedra A. Serological biomarkers for the prediction and detection of human papillomavirus associated cancers. In: Athari SS, ed. Immunoregulatory Aspects of Immunotherapy. InTech; 2018. http://www.intechopen.com/books/immunoregulatory-aspects-of-immunotherapy/serological-biomarkers-for-the-prediction-and-detection-of-human-papillomavirus-associated-cancers [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


