Abstract
Cancers of epithelial origin such as breast, prostate, cervical, gastric, colon and lung cancer account for a large proportion of deaths worldwide. Better treatment of metastasis, the main cause of cancer deaths, is therefore urgently required. Several of these tumours have been shown to have an abnormally high concentration of Na+ ([Na+]) and emerging evidence points to this accumulation being due to elevated intracellular [Na+]. This poses intriguing questions about the cellular mechanisms underlying Na+ dysregulation in cancer, and its pathophysiological significance. Elevated intracellular [Na+] may be due to alterations in activity of the Na+/K+‐ATPase, and/or increased influx via Na+ channels and Na+‐linked transporters. Maintenance of the electrochemical Na+ gradient across the plasma membrane is vital to power many cellular processes that are highly active in cancer cells, including glucose and glutamine import. Na+ channels are also upregulated in cancer cells, which in turn promotes tumour growth and metastasis. For example, ENaC and ASICs are overexpressed in cancers, increasing invasion and proliferation. In addition, voltage‐gated Na+ channels are also upregulated in a range of tumour types, where they promote metastatic cell behaviours via various mechanisms, including membrane potential depolarisation and altered pH regulation. Together, recent findings relating to elevated Na+ in the tumour microenvironment and how this may be regulated by several classes of Na+ channels provide a link between altered Na+ handling and poor clinical outcome. There are new opportunities to leverage this altered Na+ microenvironment for therapeutic benefit, as exemplified by several ongoing clinical trials.
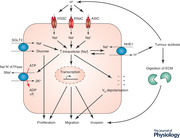
Keywords: breast cancer, metastasis, migration, proliferation, sodium, ion channels
Abstract figure legend Mechanisms of Na+ channel and transporter‐dependent proliferation, migration and invasion. Na+ enters through VGSC, ENaC and ASIC channels and through the SGLT2 cotransporter and the NHE1 exchanger. These mechanisms may be responsible for elevating intracellular [Na+]. Na+ is removed from the cell via the Na+/K+‐ATPase. VGSCs depolarise the cell membrane potential (V m), which leads to increased migration. VGSCs also regulate transcription of genes involved in proliferation, migration and invasion. VGSCs increase the activity of NHE1, further elevating intracellular [Na+] and extracellular [H+]. This acidifies the extracellular environment and aids cellular invasion through extracellular matrix. The low extracellular pH will then affect the Na+ channels, increasing the inward Na+ current through these in a positive feedback mechanism which increases intracellular [Na+] and extracellular [H+].

Introduction
Cancers of epithelial origin such as breast, prostate, cervical, gastric, colon and lung cancer, account for a large proportion of deaths worldwide. Breast cancer is the most commonly diagnosed cancer in the UK, making up 15% of total cancer cases and 7% of cancer deaths (Cancer Research UK, 2022a, 2022b). Although treatments targeting hormone receptors and human epidermal growth factor receptor 2 (HER2) have improved outcomes in oestrogen receptor (ER), progesterone receptor (PR) or HER2 positive cancers, 15−20% of breast cancers cannot be targeted with such therapies because they lack these receptors and are classed as triple negative (Brouckaert et al., 2012). Metastatic breast cancer may be managed by chemotherapy but cannot currently be cured and triple negative metastatic breast cancer has the poorest median survival of 8.8 months (Lobbezoo et al., 2013). Better prevention and treatment of metastatic breast cancer is therefore urgently required.
[Na+] is elevated in tumours
Several types of tumours have been shown to have an abnormally high concentration of Na+ (Leslie et al., 2019). This was demonstrated in the early 1980s with flame photometry and X‐ray microanalysis of freeze‐dried brain sections, showing elevated Na+ concentration ([Na+]) in glioma allograft tumours compared to contralateral brain regions (Hürter et al., 1982). The intracellular [Na+] was also discovered to be higher in cancerous cells than in non‐cancerous cells, and higher in rapidly proliferating cells than slowly proliferating cells (Cameron et al., 1980). More recently elevated tumour [Na+] has been detected using 23Na‐magnetic resonance imaging (MRI) in brain, breast, uterine and prostate cancer (Barrett et al., 2018; Jacobs et al., 2009; James et al., 2022 Ouwerkerk et al., 2003, 2007) (Fig. 1 A). It is unclear whether this elevation is due to an increased [Na+] in the intracellular or extracellular compartments. Another possibility is that it is due to an increased relative volume of extracellular fluid, since [Na+] is an order of magnitude higher in extracellular fluid (140–150 mm) than in intracellular fluid (10–15 mm) (Hille, 2001; Madelin et al., 2014) (Fig. 1 B).
Figure 1. Potential interplay between elevated tissue [Na+] and cellularity in breast cancer.
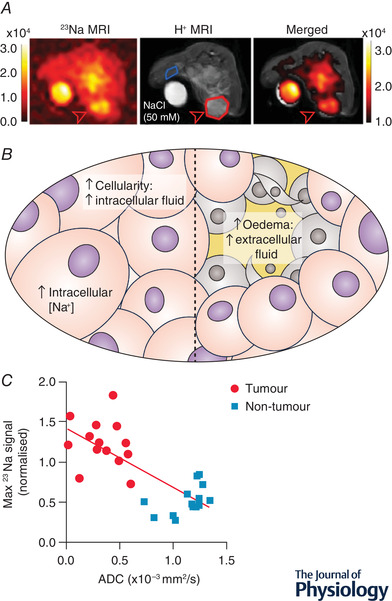
A, 23Na‐MRI images of a mouse mammary tumour (open red arrow), showing elevated [Na+] in the tumour compared to healthy contralateral tissue (adapted from James et al., 2022). B, possible causes of increased tumour [Na+]. Left: increased intracellular [Na+] must explain the change if the cellularity is increased, and the extracellular fluid volume is decreased. Right: oedema in glioma or necrotic tumours causes an increase in extracellular fluid volume which would increase the total tissue [Na+]. C, inverse correlation between tumour [Na+] and apparent diffusion coefficient (ADC) which estimates extracellular fluid volume (adapted from James et al., 2022).
It is possible that the relative importance of each of these factors depends on the site of a tumour. Gliomas, for example, cause brain oedema: an increase in extracellular fluid volume via increased capillary permeability, and reduced absorption of tissue fluid into parenchymal cells (Papadopoulos et al., 2004). Such an increase in the extracellular fluid volume fraction can be estimated by measuring the apparent diffusion coefficient (ADC) of water using diffusion‐weighted 1H‐MRI (Le Bihan et al., 1986), since it is assumed that diffusion will be greater in the extracellular environment than inside cells. In glioma, successful chemotherapy treatment inducing tumour necrosis caused an increase in ADC as well as [Na+], suggesting that the increase in [Na+] was due to an increased extracellular volume fraction. In many tumours, however, there appears to be a reduction in extracellular volume compared to healthy tissue. When benign breast lesions were compared to breast cancer tumours, there was an inverse correlation between [Na+] and ADC (Zaric et al., 2016), with higher [Na+] and lower ADC in the cancerous tumours. This would suggest that the elevation in total tissue [Na+] in breast cancer is due to an increase in intracellular [Na+]. Interestingly, chemotherapy treatment of breast cancer reduced tissue [Na+] (Jacobs et al., 2011, 2010). This reduction of [Na+] was also seen with chemotherapy treatment in a mouse model of breast cancer, with no change in ADC (James et al., 2022) (Fig. 1 C). Despite these treatments leading to cell death, which would be expected to increase the extracellular fluid volume fraction, in each study the total tissue [Na+] decreased. Considered together, these studies in breast cancer suggest that intracellular [Na+] is elevated and may be reduced by chemotherapy treatment. This poses an intriguing question about the cellular mechanisms underlying Na+ dysregulation in cancer.
Extracellular pH is reduced in tumours
A more well‐known ionic dysregulation in the tumour microenvironment is the reduction of extracellular pH seen in many tumours (Reshkin et al., 2014; White et al., 2017). This is likely to be linked to the Warburg effect; where cancer cells use glycolysis for ATP production even when oxygen is not limiting (Vander Heiden et al., 2009; Warburg, 1956). Despite the excessive production of acidic metabolites, there appears to be a strong drive for cancer cells to maintain a neutral or slightly alkaline intracellular pH, perhaps to remove inhibition of further glycolysis. In fact, intracellular alkalinisation is an early event in oncogenesis caused by induction of the viral oncogene HPV16 E7 (Reshkin et al., 2000). pH regulation is tightly linked to Na+ homeostasis since many of the cellular pH regulatory mechanisms depend on the inward Na+ gradient (Fig. 2). Of particular note are the Na+/H+ exchanger, NHE1, and the Na+–bicarbonate cotransporter, NBCn1, both of which are upregulated and highly active in breast cancer (Amith et al., 2015; Boedtkjer et al., 2013; Cardone et al., 2005; McIntyre et al., 2016; Toft et al., 2021). These studies indicate that inhibition of Na+‐dependent pH regulatory mechanisms shows promise in treating chemotherapy‐resistant hypoxic tumours.
Figure 2. Na+ channels and transporters in cancer cells as discussed in this article.
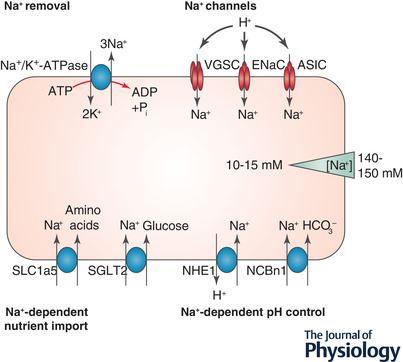
Na+/K+‐ATPase generates the Na+ gradient across the plasma membrane. Na+ channels such as VGSC, ENaC and ASIC lead to Na+ entry, particularly when the extracellular pH is low. Amino acids and glucose are imported via Na+‐dependent transporters SLC1a5 and SGLT2. The intracellular pH is maintained at a neutral or slightly alkaline pH by Na+‐dependent pH regulatory mechanisms NHE1 and NCBn1 (adapted from Leslie et al. 2019).
Na+/K+‐ATPase
The plasma membrane potential and Na+ gradient is generated by the Na+/K+‐ATPase, which consumes a large proportion of the total cellular ATP supply. This proportion can be 40% in kidney cells and more than this in the brain (Attwell & Laughlin, 2001; Whittam & Willis, 1963), but it is not yet known in cancer cells. Na+/K+‐ATPase activity responds to changes in intracellular [Na+] by direct binding of cytoplasmic Na+ (Therien & Blostein, 2000) and via increasing activity of the salt‐inducible kinase SIK1 (Sjöström et al., 2007). Reduced sensitivity of Na+/K+‐ATPase to intracellular [Na+] via modulation of SIK1 signalling could be responsible for the increase in intracellular [Na+] seen in tumours. Indeed, SIK1 is downregulated in many cancers including breast cancer (Ponnusamy & Manoharan, 2021), supporting this possibility. Alternatively, Na+/K+‐ATPase activity might be limited by ATP supply in cancer cells, leading to accumulation of intracellular [Na+].
Na+‐dependent nutrient import into cancer cells
Maintenance of the electrochemical Na+ gradient across the plasma membrane is vital to power many cellular processes that are highly active in cancer. For example, glucose is imported via the Na+–glucose co‐transporter SGLT2 (Fig. 2), which is functionally active in prostate and pancreatic cancer (Scafoglio et al., 2015). This transporter aids tumour growth and metastasis, and is upregulated in lung cancer metastases compared to primary tumours (Ishikawa et al., 2001). Cancer cells have a higher glucose intake than normal cells to fuel their highly glycolytic metabolism and this is the basis of 2‐deoxy‐2‐[18F]fluoro‐d‐glucose positron emission tomography scans for detection of metastases (Czernin, 2002).
Just as cancer cells rely heavily on glucose, many are also ‘addicted’ to glutamine (Bhutia et al., 2015), although in healthy tissue this is a non‐essential amino acid. Glutamine is important for regulation of protein synthesis via control of mammalian target of rapamycin complex 1 (mTORC1) and is vital for cell growth (Wise & Thompson, 2010). The Na+ gradient is used to import glutamine and other amino acids into cancer cells via SLC1a5/ASCT2 (Fig. 2), which is upregulated and highly active in many cancer types (Dolinska et al., 2003; Hassanein et al., 2013; van Geldermalsen et al., 2016; Witte et al., 2002).
Na+ channels in cancer cells
Despite the need to maintain a Na+ gradient across the plasma membrane, several Na+ channels are upregulated in cancers, allowing further Na+ entry into cancer cells and depleting the Na+ gradient (Fig. 2). The physiological reason for this increase in Na+ channels is still unclear although they promote tumour growth and metastasis via several mechanisms. One such channel is the epithelial Na+ channel ENaC, which is primarily responsible for reabsorbing Na+ (and therefore water) in the kidney collecting duct. ENaC protein is increased in hepatic cellular carcinoma tissue compared to matched healthy tissue and it increases proliferation, migration and invasion of hepatic cancer cells (Bondarava et al., 2009; Jin et al., 2015). ENaC is also upregulated at the mRNA level in melanoma, breast, hepatic and brain cancer cell lines (Amara et al., 2016; Bondarava et al., 2009; Kapoor et al., 2009; Yamamura et al., 2008). ENaC is closely related to the acid‐sensing ion channel (ASIC) which is also overexpressed in cancers including colorectal carcinoma where it increases invasion and proliferation via the NFAT1 axis, particularly in acidic conditions such as those found in the tumour microenvironment (Zhou et al., 2017). ASICs are also involved in invasion and epithelial to mesenchymal transition, a step which is required for cancer cells to metastasize, in pancreatic cancer and hepatic cancer (Jin et al., 2015; Zhu et al., 2017). Both ENaC and ASIC increase permeability to Na+ in low extracellular pH (Boscardin et al., 2016; Collier & Snyder, 2009), and could be responsible for increasing intracellular [Na+], particularly in acidic tumours.
Another group of Na+ channels of importance to oncogenesis are the voltage‐gated Na+ channels (VGSCs). These are classically responsible for depolarising the cell membrane potential, which initiates action potentials in electrically excitable cells such as neurons and myocytes. VGSCs have been identified in breast, colon, prostate, ovarian and lung cancers amongst others (Diaz et al., 2007; House et al., 2010; Laniado et al., 1997; Roger et al., 2003, 2007). VGSC activity increases directional migration and invasion of breast, prostate and lung cancer cells through extracellular matrix (Brackenbury et al., 2007; Fraser et al., 2005; Grimes et al., 1995; Laniado et al., 1997; Roger et al., 2003, 2007; Smith et al., 1998). In vivo, VGSC activity increases xenograft breast tumour growth and metastasis (Nelson et al., 2015b). Expression of VGSC α‐subunit mRNA is upregulated in prostate, breast, cervical and ovarian cancer (Diss et al., 2005; Gao et al., 2010; Hernandez‐Plata et al., 2012; Yang et al., 2012) and protein expression of the cardiac VGSC α‐subunit Nav1.5 is associated with increased lymph node invasion in breast cancer (Fraser et al., 2005; Nelson et al., 2015b; Yamaci et al., 2017).
Mechanisms of VGSC‐induced migration and invasion
Several mechanisms have been identified to explain VGSC‐induced invasion of cancer cells. These describe actions of both pore‐forming α‐subunits and auxiliary β‐subunits. VGSC α‐subunits are large (∼270 kDa) proteins containing four homologous domains, each with six transmembrane α‐helices (Catterall, 2014). The Na+ pore opens transiently in response to depolarisation of the cell membrane potential and this event is quickly followed by channel inactivation due to movement of an intracellular inactivation loop. Sometimes these channels fail to inactivate, allowing a long‐lasting Na+ current into the cell. This ‘persistent’ current is usually 1−3% the size of the transient current but can be responsible for a significant Na+ influx (Alzheimer et al., 1993; Eijkelkamp et al., 2012). The α‐subunits each have specific tissue distribution and they are classified as tetrodotoxin‐sensitive or ‐resistant (Savio‐Galimberti et al., 2012).
VGSC β‐subunits are small transmembrane proteins with an extracellular immunoglobulin domain, through which they act as cell adhesion molecules. β1 is the most highly expressed isoform in breast cancer, where it is upregulated compared to healthy tissue (Chioni et al., 2009; Nelson et al., 2014). β1 increases tumour growth and metastasis in a xenograft model of breast cancer (Nelson et al., 2014). This may be due to its ability to induce functional expression of a tetrodotoxin‐sensitive α‐subunit (Haworth et al., 2021), or due to cell adhesion‐mediated signalling via the src family fyn kinase, an action which depends on a Na+ current through the α‐subunit (Brackenbury et al., 2010, 2008). In contrast, β4 acts as a tumour suppressor, preventing migration and invasion independent of α‐subunit activity (Bon et al., 2016).
Nav1.5 was shown to act as a master regulator of a network of invasion genes in colon cancer (House et al., 2010, 2015). In these studies, pharmacological elevation of the persistent Na+ current upregulated the protein kinase A/extracellular signal‐regulated kinase/c‐JUN/ETS‐like gene 1/ETS‐1 transcriptional pathway, possibly by interacting with the small GTPase Rap1, which is activated by depolarisation of the cell membrane potential. A similar mechanism was identified to control migration, whereby VGSC‐induced cell membrane depolarisation activates another small GTPase, Rac1 (Yang et al., 2020). This GTPase initiates branching of the actin cytoskeleton necessary for formation of lamellipodia at the leading edge of migrating cells (Pullar et al., 2006; Wu et al., 2009). The paradigm linking depolarisation and small GTPase activation was first described for K‐Ras: depolarisation was shown to alter interactions between charged phospholipids at the plasma membrane, specifically phosphatidylserine in the inner leaflet, leading to nanoclustering of anchored small GTPases and their subsequent activation (Zhou et al., 2015).
A separate mechanism for VGSC‐mediated invasion is promotion of H+ extrusion from breast cancer cells via increasing NHE1 activity (Brisson et al., 2013, 2011; Gillet et al., 2009). Reducing the extracellular pH via this system activates enzymes which degrade the extracellular matrix, particularly the cysteine cathepsins. These studies showed that extracellular pH is particularly low in caveolae (lipid‐raft‐associated invaginations of the plasma membrane), which leads to effective breakdown of extracellular matrix around the invadopodia. The mechanism underlying this promotion of NHE1 activity is not obvious since Na+ entry would be expected to reduce the driving force for NHE1 H+ extrusion. Interestingly, Nav1.5 colocalised with NHE1 in these lipid rafts, which prompted the authors to propose that Nav1.5 might increase NHE1 activity via an allosteric interaction. They also showed that low pH caused an increase in NHE1‐mediated Li+ uptake into cells, and the pH sensitivity was stronger in the presence of Nav1.5 (Brisson et al., 2013). The authors concluded that Nav1.5 increases the pH sensitivity of NHE1. An additional possibility is that low pH increases Li+ uptake via Nav1.5, since this channel has also been shown to be pH sensitive (Onkal et al., 2019). Indeed, the H+ efflux was dependent on Na+ conductance through Nav1.5 (Brisson et al., 2011), which raises the question of whether the Na+ influx itself might be involved in increasing H+ extrusion through NHE1, rather than a direct interaction between the Nav1.5 and NHE1 proteins.
SIK1 acts as a tumour suppressor and is downregulated in many cancers including breast cancer. Knock‐down of SIK1 increases VGSC‐induced invasiveness in breast cancer cells (Gradek et al., 2019). Given that high intracellular [Na+] normally activates SIK1, which then inhibits glycolysis (Ponnusamy & Manoharan, 2021), knock‐down of SIK1 reduces its inhibition of glycolysis and may therefore increase H+ production, thus promoting low pH‐dependent invasion. Na+ entry through VGSCs may also upregulate glycolysis in cancer cells by promoting Na+/K+‐ATPase activity to maintain the Na+ gradient across the plasma membrane (Soltoff & Mandel, 1984; Therien & Blostein, 2000). Na+/K+‐ATPase preferentially uses ATP derived from glycolysis in skeletal muscle, vascular smooth muscle and breast cancer cells (Dutka & Lamb, 2007; Epstein et al., 2014; James et al., 1996; Paul et al., 1979), so upregulation of Na+/K+‐ATPase activity might be expected to increase the rate of glycolysis. Intriguingly, the non‐voltage‐gated isoform of the VGSC α‐subunit, Nax, engages in lactate signalling in glial cells (Berret et al., 2013; Shimizu et al., 2007). This channel increases glucose uptake alongside H+ and lactate production in response to elevated extracellular [Na+] (Nomura et al., 2019). Nax channels, which have substantial homology to voltage‐gated isoforms of VGSCs, interact directly with the α‐subunit of the Na+/K+‐ATPase to mediate this Na+‐dependent increase in glycolysis (Shimizu et al., 2007). Interestingly, the gene encoding Nax, SCN7A, recently emerged as a key gene associated with tumour mutational burden in gastric cancer (Li et al., 2022). Thus, the importance of Na+ channels in regulating progression across different cancer types may be greater than previously anticipated.
Interplay between elevated intracellular [Na+] and extracellular [H+]
As well as promoting extracellular acidification, VGSCs are in turn regulated by low extracellular pH. An acidic extracellular pH reduces the transient Na+ current but greatly increases the persistent Na+ current through VGSCs, particularly in the most commonly found isoform in breast and colon cancer cells, Nav1.5 (Ghovanloo et al., 2018; Khan et al., 2006, 2002). VGSCs are not the only Na+ channels which open in response to low extracellular pH, since ENaC and ASIC channels also increase Na+ entry in acidic extracellular pH (Boscardin et al., 2016; Collier & Snyder, 2009). Together, VGSC‐induced extracellular acidification and acid‐induced Na+ entry through Na+ channels may produce a positive feedback mechanism leading to elevated intracellular [Na+] and extracellular [H+]. This mechanism may help to explain why [Na+] is elevated in many tumours, and it may provide clues about new ways to target cancer therapeutically.
Clinical implications
The utility of 23Na MRI in monitoring response to chemotherapy is starting to become apparent, because this imaging modality can detect physiological changes in tumours before there has been a change in tumour size (Jacobs et al., 2011, 2010; James et al., 2022). Fast assessment of response to chemotherapy reduces the side effects associated with ineffective treatments and hastens the introduction of effective ones, so this imaging modality has the potential to improve treatment of cancer patients. In addition, VGSC expression correlates with poor prognosis in breast cancer and could therefore be used as a biomarker (Fraser et al., 2005).
Several retrospective studies indicate that treatment with local anaesthetics around the time of surgical tumour removal lengthens the disease‐free interval (Djamgoz et al., 2019; Forget et al., 2019; Lopez‐Charcas et al., 2021). Local anaesthetics act by inhibiting VGSCs, although their cancer‐protective effects may be due to their ability to reduce the required doses of inhalational and opioid anaesthetics, which can be immunosuppressive. Another therapeutic use for VGSC inhibitors is prevention of seizures. Anti‐epileptic drugs prescribed to breast cancer patients substantially improved survival after radiotherapy for brain metastases (Reddy et al., 2015). The anti‐epileptic drug phenytoin also delayed tumour growth and metastasis in an in vivo model of breast cancer (Nelson et al., 2015a). A third type of VGSC inhibitors used clinically is the class I antiarrhythmic drugs. Ranolazine, a class 1b antiarrhythmic which inhibits the persistent Na+ current, reduced metastasis in in vivo models of breast and prostate cancer (Bugan et al., 2019; Driffort et al., 2014). Clinical trials of drugs targeting Na+ channels and transporters in cancer were reviewed in Leslie et al. (2019) and there are currently two prospective trials examining the systemic or intratumoral delivery of the local anaesthetic lidocaine in the perioperative period (NCT01916317, R. A. Badwe, 2013; NCT02786329, D. Ionescu, 2016).
Conclusion
In conclusion, we have summarised the recent findings relating to elevated Na+ in the tumour microenvironment and how this may be regulated by several classes of Na+ channels and transporters. In particular, our understanding of how Na+ channels, particularly VGSCs, contribute to cancer cell invasion and metastasis has grown based on recent studies, such that a picture is now emerging which may start to explain a key mechanistic link between altered Na+ handling in solid tumours and poor clinical outcome. Given the accessibility and pharmacological tractability of these channels and transporters, there are opportunities to leverage the changes in Na+ transport seen in cancer for therapeutic benefit, as exemplified by several ongoing clinical studies.
Additional information
Competing interests
None.
Author contributions
T.K.L. and W.B. both contributed to the conception and design of the work and drafting the work and revising it critically for important intellectual content. Both authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by Cancer Research UK (A25922) and Breast Cancer Now (2015NovPhD572).
Supporting information
Peer Review History
Biographies
Theresa Leslie completed a VetMB at the University of Cambridge in 2007 and practiced as a veterinary surgeon for several years before embarking on a PhD in Will Brackenbury's group in 2016, funded by the charity Breast Cancer Now. She recently graduated from her PhD. Her project focused on the role of voltage‐gated sodium channels in regulating breast cancer invasion and metastasis.

Will Brackenbury is a Senior Lecturer in Biomedical Sciences at the University of York. Following a PhD in Cell Physiology at Imperial College London, and a postdoc in Pharmacology at the University of Michigan, he started his ion channel research laboratory at York in 2011 as an MRC Fellow. His research centres on sodium channel signalling in solid tumours.

Handling Editors: Peying Fong & Helle Praetorius
The peer review history is available in the Supporting information section of this article (https://doi.org/10.1113/JP282306#support‐information‐section).
References
- Alzheimer, C. , Schwindt, P. C. , & Crill, W. E. (1993). Modal gating of Na+ channels as a mechanism of persistent Na+ current in pyramidal neurons from rat and cat sensorimotor cortex. Journal of Neuroscience, 13(2), 660–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara, S. , Ivy, M. T. , Myles, E. L. , & Tiriveedhi, V. (2016). Sodium channel γENaC mediates IL‐17 synergized high salt induced inflammatory stress in breast cancer cells. Cellular Immunology, 302, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amith, S. R. , Fong, S. , Baksh, S. , & Fliegel, L. (2015). Na+/H+ exchange in the tumour microenvironment: Does NHE1 drive breast cancer carcinogenesis? International Journal of Developmental Biology, 59(7–9), 367–377. [DOI] [PubMed] [Google Scholar]
- Attwell, D. , & Laughlin, S. B. (2001). An energy budget for signaling in the grey matter of the brain. Journal of Cerebral Blood Flow and Metabolism, 21(10), 1133–1145. [DOI] [PubMed] [Google Scholar]
- Barrett, T. , Riemer, F. , McLean, M. A. , Kaggie, J. , Robb, F. , Tropp, J. S. , Warren, A. , Bratt, O. , Shah, N. , Gnanapragasam, V. J. , Gilbert, F. J. , Graves, M. J. , & Gallagher, F. A. (2018). Quantification of total and intracellular sodium concentration in primary prostate cancer and adjacent normal prostate tissue with magnetic resonance imaging. Investigative Radiology, 53(8), 450–456. [DOI] [PubMed] [Google Scholar]
- Berret, E. , Nehmé, B. , Henry, M. , Toth, K. , Drolet, G. , & Mouginot, D. (2013). Regulation of central Na+ detection requires the cooperative action of the NaX channel and α1 isoform of Na+/K+‐ATPase in the Na+‐sensor neuronal population. Journal of Neuroscience, 33(7), 3067–3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutia, Y. D. , Babu, E. , Ramachandran, S. , & Ganapathy, V. (2015). Amino acid transporters in cancer and their relevance to “glutamine addiction”: Novel targets for the design of a new class of anticancer drugs. Cancer Research, 75(9), 1782–1788. [DOI] [PubMed] [Google Scholar]
- Boedtkjer, E. , Moreira, J. M. A. , Mele, M. , Vahl, P. , Wielenga, V. T. , Christiansen, P. M. , Jensen, V. E. D. , Pedersen, S. F. , & Aalkjaer, C. (2013). Contribution of Na+,HCO3 –‐cotransport to cellular pH control in human breast cancer: A role for the breast cancer susceptibility locus NBCn1 (SLC4A7). International Journal of Cancer, 132(6), 1288–1299. [DOI] [PubMed] [Google Scholar]
- Bon, E. , Driffort, V. , Gradek, F. , Martinez‐Caceres, C. , Anchelin, M. , Pelegrin, P. , Cayuela, M. L. , Marionneau‐Lambot, S. , Oullier, T. , Guibon, R. , Fromont, G. , Gutierrez‐Pajares, J. L. , Domingo, I. , Piver, E. , Moreau, A. , Burlaud‐Gaillard, J. , Frank, P. G. , Chevalier, S. , Besson, P. , & Roger S. (2016). SCN4B acts as a metastasis‐suppressor gene preventing hyperactivation of cell migration in breast cancer. Nature Communications, 7(1), 13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondarava, M. , Li, T. , Endl, E. , & Wehner, F. (2009). alpha‐ENaC is a functional element of the hypertonicity‐induced cation channel in HepG2 cells and it mediates proliferation. Pflugers Archiv: European Journal of Physiology, 458(4), 675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscardin, E. , Alijevic, O. , Hummler, E. , Frateschi, S. , & Kellenberger, S. (2016). The function and regulation of acid‐sensing ion channels (ASICs) and the epithelial Na channel (ENaC): IUPHAR Review 19. British Journal of Pharmacology, 173(18), 2671–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury, W. J. , Calhoun, J. D. , Chen, C. , Miyazaki, H. , Nukina, N. , Oyama, F. , Ranscht, B. , & Isom, L. L. (2010). Functional reciprocity between Na+ channel Nav1.6 and beta1 subunits in the coordinated regulation of excitability and neurite outgrowth. Proceedings of the National Academy of Sciences, USA, 107(5), 2283–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury, W. J. , Chioni, A. M. , Diss, J. K. , & Djamgoz, M. B. (2007). The neonatal splice variant of Nav1.5 potentiates in vitro invasive behaviour of MDA‐MB‐231 human breast cancer cells. Breast Cancer Research and Treatment, 101(2), 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury, W. J. , Davis, T. H. , Chen, C. , Slat, E. A. , Detrow, M. J. , Dickendesher, T. L. , Ranscht, B. , & Isom, L. L. (2008). Voltage‐gated Na+ channel beta1 subunit‐mediated neurite outgrowth requires Fyn kinase and contributes to postnatal CNS development in vivo. Journal of Neuroscience, 28(12), 3246–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson, L. , Driffort, V. , Benoist, L. , Poet, M. , Counillon, L. , Antelmi, E. , Rubino, R. , Besson, P. , Labbal, F. , Chevalier, S. , Reshkin, S. J. , Gore, J. , & Roger, S. (2013). NaV1.5 Na+ channels allosterically regulate the NHE‐1 exchanger and promote the activity of breast cancer cell invadopodia. Journal of Cell Science, 126, 4835–4842. [DOI] [PubMed] [Google Scholar]
- Brisson, L. , Gillet, L. , Calaghan, S. , Besson, P. , Le Guennec, J. Y. , Roger, S. , & Gore, J. (2011). Na(V)1.5 enhances breast cancer cell invasiveness by increasing NHE1‐dependent H+ efflux in caveolae. Oncogene, 30(17), 2070–2076. [DOI] [PubMed] [Google Scholar]
- Brouckaert, O. , Wildiers, H. , Floris, G. , & Neven, P. (2012). Update on triple‐negative breast cancer: Prognosis and management strategies. International Journal of Women's Health, 4, 511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugan, I. , Kucuk, S. , Karagoz, Z. , Fraser, S. P. , Kaya, H. , Dodson, A. , Foster, C. S. , Altun, S. , & Djamgoz, M. B. A. (2019). Anti‐metastatic effect of ranolazine in an in vivo rat model of prostate cancer, and expression of voltage‐gated sodium channel protein in human prostate. Prostate Cancer and Prostatic Diseases, 22(4), 569–579. [DOI] [PubMed] [Google Scholar]
- Cameron, I. L. , Smith, N. K. , Pool, T. B. , & Sparks, R. L. (1980). Intracellular concentration of sodium and other elements as related to mitogenesis and oncogenesis in vivo. Cancer Research, 40, 1493–1500. [PubMed] [Google Scholar]
- Cancer_Research_UK (2022a). Breast cancer mortality. https://www.cancerresearchuk.org/health‐professional/cancer‐statistics/statistics‐by‐cancer‐type/breast‐cancer#heading‐Two Accessed May 2022.
- Cancer_Research_UK (2022b). Breast cancer statistics. https://www.cancerresearchuk.org/health‐professional/cancer‐statistics/statistics‐by‐cancer‐type/breast‐cancer#heading‐Zero Accessed May 2022.
- Cardone, R. A. , Casavola, V. , & Reshkin, S. J. (2005). The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nature Reviews. Cancer, 5(10), 786–795. [DOI] [PubMed] [Google Scholar]
- Catterall, W. A. (2014). Structure and function of voltage‐gated sodium channels at atomic resolution. Experimental Physiology, 99(1), 35–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chioni, A. M. , Brackenbury, W. J. , Calhoun, J. D. , Isom, L. L. , & Djamgoz, M. B. (2009). A novel adhesion molecule in human breast cancer cells: Voltage‐gated Na+ channel beta1 subunit. International Journal of Biochemistry & Cell Biology, 41, 1216–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier, D. M. , & Snyder, P. M. (2009). Extracellular protons regulate human ENaC by modulating Na+ self‐inhibition. Journal of Biological Chemistry, 284(2), 792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernin, J. (2002). Clinical applications of FDG‐PET in oncology. Acta Medica Austriaca, 29(5), 162–170. [DOI] [PubMed] [Google Scholar]
- Diaz, D. , Delgadillo, D. M. , Hernandez‐Gallegos, E. , Ramirez‐Dominguez, M. E. , Hinojosa, L. M. , Ortiz, C. S. , Berumen, J. , Camacho, J. , & Gomora, J. C. (2007). Functional expression of voltage‐gated sodium channels in primary cultures of human cervical cancer. Journal of Cellular Physiology, 210(2), 469–478. [DOI] [PubMed] [Google Scholar]
- Diss, J. K. , Stewart, D. , Pani, F. , Foster, C. S. , Walker, M. M. , Patel, A. , & Djamgoz, M. B. (2005). A potential novel marker for human prostate cancer: Voltage‐gated sodium channel expression in vivo. Prostate Cancer and Prostatic Diseases, 8(3), 266–273. [DOI] [PubMed] [Google Scholar]
- Djamgoz, M. B. A. , Fraser, S. P. , & Brackenbury, W. J. (2019). In vivo evidence for voltage‐gated sodium channel expression in carcinomas and potentiation of metastasis. Cancers, 11(11), 1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinska, M. , Dybel, A. , Zablocka, B. , & Albrecht, J. (2003). Glutamine transport in C6 glioma cells shows ASCT2 system characteristics. Neurochemistry International, 43(4–5), 501–507. [DOI] [PubMed] [Google Scholar]
- Driffort, V. , Gillet, L. , Bon, E. , Marionneau‐Lambot, S. , Oullier, T. , Joulin, V. , Collin, C. , Pages, J. C. , Jourdan, M. L. , Chevalier, S. , Bougnoux, P. , Le Guennec, J. Y. , Besson, P. , & Roger, S. (2014). Ranolazine inhibits NaV1.5‐mediated breast cancer cell invasiveness and lung colonization. Molecular Cancer, 13(1), 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutka, T. L. , & Lamb, G. D. (2007). Na+‐K+ pumps in the transverse tubular system of skeletal muscle fibers preferentially use ATP from glycolysis. American Journal of Physiology. Cell Physiology, 293(3), C967–C977. [DOI] [PubMed] [Google Scholar]
- Eijkelkamp, N. , Linley, J. E. , Baker, M. D. , Minett, M. S. , Cregg, R. , Werdehausen, R. , Rugiero, F. , & Wood, J. N. (2012). Neurological perspectives on voltage‐gated sodium channels. Brain, 135(9), 2585–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein, T. , Xu, L. , Gillies, R. J. , & Gatenby, R. A. (2014). Separation of metabolic supply and demand: Aerobic glycolysis as a normal physiological response to fluctuating energetic demands in the membrane. Cancer & Metabolism, 2, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget, P. , Aguirre, J. A. , Bencic, I. , Borgeat, A. , Cama, A. , Condron, C. , Eintrei, C. , Eroles, P. , Gupta, A. , Hales, T. G. , Ionescu, D. , Johnson, M. , Kabata, P. , Kirac, I. , Ma, D. , Mokini, Z. , Guerrero Orriach, J. L. , Retsky, M. , Sandrucci, S. , Siekmann, W. , Štefančić, L. , Votta‐Vellis, G. , Connolly, C. , & Buggy, D. (2019). How anesthetic, analgesic and other non‐surgical techniques during cancer surgery might affect postoperative oncologic outcomes: A summary of current state of evidence. Cancers, 11(5), 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser, S. P. , Diss, J. K. J. , Chioni, A. M. , Mycielska, M. E. , Pan, H. Y. , Yamaci, R. F. , Pani, F. , Siwy, Z. , Krasowska, M. , Grzywna, Z. , Brackenbury, W. J. , Theodorou, D. , Koyuturk, M. , Kaya, H. , Battaloglu, E. , De Bella, M. T. , Slade, M. J. , Tolhurst, R. , Palmieri, C. , Jiang, J. , Latchman, D. S. , Coombes, R. C. , & Djamgoz, M. B. A. (2005). Voltage‐gated sodium channel expression and potentiation of human breast cancer metastasis. Clinical Cancer Research, 11(15), 5381–5389. [DOI] [PubMed] [Google Scholar]
- Gao, R. , Shen, Y. , Cai, J. , Lei, M. , & Wang, Z. (2010). Expression of voltage‐gated sodium channel alpha subunit in human ovarian cancer. Oncology Reports, 23, 1293–1299. [DOI] [PubMed] [Google Scholar]
- Ghovanloo, M. R. , Peters, C. H. , & Ruben, P. C. (2018). Effects of acidosis on neuronal voltage‐gated sodium channels: Nav1.1 and Nav1.3. Channels, 12(1), 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillet, L. , Roger, S. , Besson, P. , Lecaille, F. , Gore, J. , Bougnoux, P. , Le Lalmanach, G. , & Guennec, J. Y. (2009). Voltage‐gated sodium channel activity promotes cysteine cathepsin‐dependent invasiveness and colony growth of human cancer cells. Journal of Biological Chemistry, 284(13), 8680–8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradek, F. , Lopez‐Charcas, O. , Chadet, S. , Poisson, L. , Ouldamer, L. , Goupille, C. , Jourdan, M. L. , Chevalier, S. , Moussata, D. , Besson, P. , & Roger, S. (2019). Sodium channel Na v 1.5 controls epithelial‐to‐mesenchymal transition and invasiveness in breast cancer cells through its regulation by the salt‐inducible kinase‐1. Scientific Reports, 9(1), 18652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes, J. A. , Fraser, S. P. , Stephens, G. J. , Downing, J. E. G. , Laniado, M. E. , Foster, C. S. , Abel, P. D. , & Djamgoz, M. B. A. (1995). Differential expression of voltage‐activated Na+ currents in 2 prostatic tumor cell lines – Contribution to invasiveness in‐vitro. FEBS Letters, 369(2–3), 290–294. [DOI] [PubMed] [Google Scholar]
- Hassanein, M. , Hoeksema, M. D. , Shiota, M. , Qian, J. , Harris, B. K. , Chen, H. , Clark, J. E. , Alborn, W. E. , Eisenberg, R. , & Massion, P. P. (2013). SLC1A5 mediates glutamine transport required for lung cancer cell growth and survival. Clinical Cancer Research, 19(3), 560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth, A. , Hodges, S. , Isom, L. , Baumann, C. , & Brackenbury, W. (2021). Subcellular dynamics and functional activity of the cleaved Na+ channel β1 subunit intracellular domain. bioRxiv, 10.1101/2021.12.29.474414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez‐Plata, E. , Ortiz, C. S. , Marquina‐Castillo, B. , Medina‐Martinez, I. , Alfaro, A. , Berumen, J. , Rivera, M. , & Gomora, J. C. (2012). Overexpression of NaV 1.6 channels is associated with the invasion capacity of human cervical cancer. International Journal of Cancer, 130(9), 2013–2023. [DOI] [PubMed] [Google Scholar]
- Hille, B. (2001). Ion channels of excitable membranes. Sinauer Associates Inc. [Google Scholar]
- House, C. D. , Vaske, C. J. , Schwartz, A. M. , Obias, V. , Frank, B. , Luu, T. , Sarvazyan, N. , Irby, R. , Strausberg, R. L. , Hales, T. G. , Stuart, J. M. , & Lee, N. H. (2010). Voltage‐gated Na+ channel SCN5A is a key regulator of a gene transcriptional network that controls colon cancer invasion. Cancer Research, 70(17), 6957–6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House, C. D. , Wang, B. D. , Ceniccola, K. , Williams, R. , Simaan, M. , Olender, J. , Patel, V. , Baptista‐Hon, D. T. , Annunziata, C. M. , Gutkind, J. S. , Hales, T. G. , & Lee, N. H. (2015). Voltage‐gated Na+ channel activity increases colon cancer transcriptional activity and invasion via persistent MAPK signaling. Scientific Reports, 5(1), 11541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hürter, T. , Bröcker, W. , & Bosma, H. J. (1982). Investigations on vasogenic and cytotoxic brain edema, comparing results from X‐ray microanalysis and flame photometry. Microscopica Acta, 85, 285–293. [PubMed] [Google Scholar]
- Ishikawa, N. , Oguri, T. , Isobe, T. , Fujitaka, K. , & Kohno, N. (2001). SGLT gene expression in primary lung cancers and their metastatic lesions. Japanese Journal of Cancer Research, 92(8), 874–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs, M. A. , Ouwerkerk, R. , Kamel, I. , Bottomley, P. A. , Bluemke, D. A. , & Kim, H. S. (2009). Proton, diffusion‐weighted imaging, and sodium (23Na) MRI of uterine leiomyomata after MR‐guided high‐intensity focused ultrasound: A preliminary study. Journal of Magnetic Resonance Imaging, 29(3), 649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs, M. A. , Ouwerkerk, R. , Wolff, A. C. , Gabrielson, E. , Warzecha, H. , Jeter, S. , Bluemke, D. A. , Wahl, R. , & Stearns, V. (2011). Monitoring of neoadjuvant chemotherapy using multiparametric, 2 3Na sodium MR, and multimodality (PET/CT/MRI) imaging in locally advanced breast cancer. Breast Cancer Research and Treatment, 128(1), 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs, M. A. , Stearns, V. , Wolff, A. C. , Macura, K. , Argani, P. , Khouri, N. , Tsangris, T. , Barker, P. B. , Davidson, N. E. , Bhujwalla, Z. M. , Bluemke, D. A. , & Ouwerkerk, R. (2010). Multiparametric magnetic resonance imaging, spectroscopy and multinuclear (23Na) imaging monitoring of preoperative chemotherapy for locally advanced breast cancer. Academic Radiology, 17(12), 1477–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, A. D. , Leslie, T. K. , Kaggie, J. D. , Wiggins, L. , Patten, L. , Murphy, O. , 'Duinn, J. , Langer, S. , Labarthe, M. C. , Riemer, F. , Baxter, G. , McLean, M. A. , Gilbert, F. J. , Kennerley, A. J. , & Brackenbury, W. J. (2022). Sodium accumulation in breast cancer predicts malignancy and treatment response. British Journal of Cancer, 127(2), 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, J. H. , Fang, C. H. , Schrantz, S. J. , Hasselgren, P. O. , Paul, R. J. , & Fischer, J. E. (1996). Linkage of aerobic glycolysis to sodium‐potassium transport in rat skeletal muscle. Implications for increased muscle lactate production in sepsis. Journal of Clinical Investigation, 98(10), 2388–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, C. , Ye, Q.‐H. , Yuan, F.‐L. , Gu, Y.‐L. , Li, J.‐P. , Shi, Y.‐H. , Shen, X.‐M. , Bo, L. , & Lin, Z.‐H. (2015). Involvement of acid‐sensing ion channel 1α in hepatic carcinoma cell migration and invasion. Tumour Biology, 36(6), 4309–4317. [DOI] [PubMed] [Google Scholar]
- Kapoor, N. , Bartoszewski, R. , Qadri, Y. J. , Bebok, Z. , Bubien, J. K. , Fuller, C. M. , & Benos, D. J. (2009). Knockdown of ASIC1 and epithelial sodium channel subunits inhibits glioblastoma whole cell current and cell migration. Journal of Biological Chemistry, 284(36), 24526–24541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, A. , Kyle, J. W. , Hanck, D. A. , Lipkind, G. M. , & Fozzard, H. A. (2006). Isoform‐dependent interaction of voltage‐gated sodium channels with protons. Journal of Physiology, 576(2), 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, A. , Romantseva, L. , Lam, A. , Lipkind, G. , & Fozzard, H. A. (2002). Role of outer ring carboxylates of the rat skeletal muscle sodium channel pore in proton block. Journal of Physiology, 543(1), 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laniado, M. E. , Lalani, E. N. , Fraser, S. P. , Grimes, J. A. , Bhangal, G. , Djamgoz, M. B. , & Abel, P. D. (1997). Expression and functional analysis of voltage‐activated Na+ channels in human prostate cancer cell lines and their contribution to invasion in vitro. American Journal of Pathology, 150, 1213–1221. [PMC free article] [PubMed] [Google Scholar]
- Le Bihan, D. , Breton, E. , Lallemand, D. , Grenier, P. , Cabanis, E. , & Laval‐Jeantet, M. (1986). MR imaging of intravoxel incoherent motions: Application to diffusion and perfusion in neurologic disorders. Radiology, 161(2), 401–407. [DOI] [PubMed] [Google Scholar]
- Leslie, T. K. , James, A. D. , Zaccagna, F. , Grist, J. T. , Deen, S. , Kennerley, A. , Riemer, F. , Kaggie, J. D. , Gallagher, F. A. , Gilbert, F. J. , & Brackenbury, W. J. (2019). Sodium homeostasis in the tumour microenvironment. Biochimica et Biophysica Acta – Reviews on Cancer, 1872(2), 188304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. , Zhou, K. , Li, M. , Hu, Q. , Wei, W. , Liu, L. , & Zhao, Q. (2022). Identification of SCN7A as the key gene associated with tumor mutation burden in gastric cancer. BMC Gastroenterology, 22(1), 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobbezoo, D. J. , van Kampen, R. J. , Voogd, A. C. , Dercksen, M. W. , van den Berkmortel, F. , Smilde, T. J. , van de Wouw, A. J. , Peters, F. P. , van Riel, J. M. , Peters, N. A. , de Boer, M. , Borm, G. F. , & Tjan‐Heijnen, V. C. (2013). Prognosis of metastatic breast cancer subtypes: The hormone receptor/HER2‐positive subtype is associated with the most favorable outcome. Breast Cancer Research and Treatment, 141(3), 507–514. [DOI] [PubMed] [Google Scholar]
- Lopez‐Charcas, O. , Pukkanasut, P. , Velu, S. E. , Brackenbury, W. J. , Hales, T. G. , Besson, P. , Gomora, J. C. , & Roger, S. (2021). Pharmacological and nutritional targeting of voltage‐gated sodium channels in the treatment of cancers. iScience, 24(4), 102270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madelin, G. , Kline, R. , Walvick, R. , & Regatte, R. R. (2014). A method for estimating intracellular sodium concentration and extracellular volume fraction in brain in vivo using sodium magnetic resonance imaging. Scientific Reports, 4(1), 4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre, A. , Hulikova, A. , Ledaki, I. , Snell, C. , Singleton, D. , Steers, G. , Seden, P. , Jones, D. , Bridges, E. , Wigfield, S. , Li, J.‐L. , Russell, A. , Swietach, P. , & Harris, A. L. (2016). Disrupting hypoxia‐induced bicarbonate transport acidifies tumor cells and suppresses tumor growth. Cancer Research, 76(13), 3744–3755. [DOI] [PubMed] [Google Scholar]
- Nelson, M. , Millican‐Slater, R. , Forrest, L. C. , & Brackenbury, W. J. (2014). The sodium channel β1 subunit mediates outgrowth of neurite‐like processes on breast cancer cells and promotes tumour growth and metastasis. International Journal of Cancer, 135(10), 2338–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, M. , Yang, M. , Dowle, A. A. , Thomas, J. R. , & Brackenbury, W. J. (2015a). The sodium channel‐blocking antiepileptic drug phenytoin inhibits breast tumour growth and metastasis. Molecular cancer, 14(1), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, M. , Yang, M. , Millican‐Slater, R. , & Brackenbury, W. J. (2015b). Nav1.5 regulates breast tumor growth and metastatic dissemination in vivo. Oncotarget, 6(32), 32914–32929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura, K. , Hiyama, T. Y. , Sakuta, H. , Matsuda, T. , Lin, C. H. , Kobayashi, K. , Kuwaki, T. , Takahashi, K. , Matsui, S. , & Noda, M. (2019). [Na+] increases in body fluids sensed by central Na x induce sympathetically mediated blood pressure elevations via H+‐dependent activation of ASIC1a. Neuron, 101(1), 60–75.e66. [DOI] [PubMed] [Google Scholar]
- Onkal, R. , Fraser, S. P. , & Djamgoz, M. B. A. (2019). Cationic modulation of voltage‐gated sodium channel (Nav1.5): Neonatal versus adult splice variants‐1. Monovalent (H+) ions. Bioelectricity, 1(3), 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouwerkerk, R. , Bleich, K. B. , Gillen, J. S. , Pomper, M. G. , & Bottomley, P. A. (2003). Tissue sodium concentration in human brain tumors as measured with 23Na MR imaging. Radiology, 227(2), 529–537. [DOI] [PubMed] [Google Scholar]
- Ouwerkerk, R. , Jacobs, M. A. , Macura, K. J. , Wolff, A. C. , Stearns, V. , Mezban, S. D. , Khouri, N. F. , Bluemke, D. A. , & Bottomley, P. A. (2007). Elevated tissue sodium concentration in malignant breast lesions detected with non‐invasive 23Na MRI. Breast Cancer Research and Treatment, 106(2), 151–160. [DOI] [PubMed] [Google Scholar]
- Papadopoulos, M. C. , Saadoun, S. , Binder, D. K. , Manley, G. T. , Krishna, S. , & Verkman, A. S. (2004). Molecular mechanisms of brain tumor edema. Neuroscience, 129(4), 1009–1018. [DOI] [PubMed] [Google Scholar]
- Paul, R. J. , Bauer, M. , & Pease, W. (1979). Vascular smooth muscle: aerobic glycolysis linked to sodium and potassium transport processes. Science, 206(4425), 1414–1416. [DOI] [PubMed] [Google Scholar]
- Ponnusamy, L. , & Manoharan, R. (2021). Distinctive role of SIK1 and SIK3 isoforms in aerobic glycolysis and cell growth of breast cancer through the regulation of p53 and mTOR signaling pathways. Biochimica et Biophysica Acta – Molecular Cell Research, 1868(5), 118975. [DOI] [PubMed] [Google Scholar]
- Pullar, C. , Baier, B. , Kariya, Y. , Russell, A. , Horst, B. , Marinkovich, M. , & Isseroff, R. (2006). beta4 integrin and epidermal growth factor coordinately regulate electric field‐mediated directional migration via Rac1. Molecular Biology of the Cell, 17(11), 4925–4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, J. P. , Dawood, S. , Mitchell, M. , Debeb, B. G. , Bloom, E. , Gonzalez‐Angulo, A. M. , Sulman, E. P. , Buchholz, T. A. , & Woodward, W. A. (2015). Antiepileptic drug use improves overall survival in breast cancer patients with brain metastases in the setting of whole brain radiotherapy. Radiotherapy and Oncology, 117(2), 308–314. [DOI] [PubMed] [Google Scholar]
- Reshkin, S. J. , Bellizzi, A. , Caldeira, S. , Albarani, V. , Malanchi, I. , Poignee, M. , Alunni‐Fabbroni, M. , Casavola, V. , & Tommasino, M. (2000). Na+/H+ exchanger‐dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation‐associated phenotypes. FASEB Journal, 14(14), 2185–2197. [DOI] [PubMed] [Google Scholar]
- Reshkin, S. J. , Greco, M. R. , & Cardone, R. A. (2014). Role of pHi, and proton transporters in oncogene‐driven neoplastic transformation. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences, 369(1638), 20130100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger, S. , Besson, P. , & Le Guennec, J. Y. (2003). Involvement of a novel fast inward sodium current in the invasion capacity of a breast cancer cell line. Biochimica et Biophysica Acta, 1616(2), 107–111. [DOI] [PubMed] [Google Scholar]
- Roger, S. , Rollin, J. , Barascu, A. , Besson, P. , Raynal, P. I. , Iochmann, S. , Lei, M. , Bougnoux, P. , Gruel, Y. , & Le Guennec, J. Y. (2007). Voltage‐gated sodium channels potentiate the invasive capacities of human non‐small‐cell lung cancer cell lines. International Journal of Biochemistry & Cell Biology, 39, 774–786. [DOI] [PubMed] [Google Scholar]
- Savio‐Galimberti, E. , Gollob, M. H. , & Darbar, D. (2012). Voltage‐gated sodium channels: Biophysics, pharmacology, and related channelopathies. Frontiers in Pharmacology, 3, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scafoglio, C. , Hirayama, B. A. , Kepe, V. , Liu, J. , Ghezzi, C. , Satyamurthy, N. , Moatamed, N. A. , Huang, J. , Koepsell, H. , Barrio, J. R. , & Wright, E. M. (2015). Functional expression of sodium‐glucose transporters in cancer. Proceedings of the National Academy of Sciences, USA, 112(30), E4111‐4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu, H. , Watanabe, E. , Hiyama, T. Y. , Nagakura, A. , Fujikawa, A. , Okado, H. , Yanagawa, Y. , Obata, K. , & Noda, M. (2007). Glial Nax channels control lactate signaling to neurons for brain [Na+] sensing. Neuron, 54(1), 59–72. [DOI] [PubMed] [Google Scholar]
- Sjöström, M. , Stenström, K. , Eneling, K. , Zwiller, J. , Katz, A. I. , Takemori, H. , & Bertorello, A. M. (2007). SIK1 is part of a cell sodium‐sensing network that regulates active sodium transport through a calcium‐dependent process. Proceedings of the National Academy of Sciences, USA, 104(43), 16922–16927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, P. , Rhodes, N. P. , Shortland, A. P. , Fraser, S. P. , Djamgoz, M. B. , Ke, Y. , & Foster, C. S. (1998). Sodium channel protein expression enhances the invasiveness of rat and human prostate cancer cells. FEBS Letters, 423(1), 19–24. [DOI] [PubMed] [Google Scholar]
- Soltoff, S. P. , & Mandel, L. J. (1984). Active ion transport in the renal proximal tubule. II. Ionic dependence of the Na pump. Journal of General Physiology, 84(4), 623–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therien, A. G. , & Blostein, R. (2000). Mechanisms of sodium pump regulation. American Journal of Physiology. Cell Physiology, 279(3), C541–C566. [DOI] [PubMed] [Google Scholar]
- Toft, N. J. , Axelsen, T. V. , Pedersen, H. L. , Mele, M. , Burton, M. , Balling, E. , Johansen, T. , Thomassen, M. , Christiansen, P. M. , & Boedtkjer, E. (2021). Acid‐base transporters and pH dynamics in human breast carcinomas predict proliferative activity, metastasis, and survival. eLife, 10, e68447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Geldermalsen, M. , Wang, Q. , Nagarajah, R. , Marshall, A. D. , Thoeng, A. , Gao, D. , Ritchie, W. , Feng, Y. , Bailey, C. G. , Deng, N. , Harvey, K. , Beith, J. M. , Selinger, C. I. , O'Toole, S. A. , Rasko, J. E. J. , & Holst, J. (2016). ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple‐negative basal‐like breast cancer. Oncogene, 35(24), 3201–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden, M. G. , Cantley, L. C. , & Thompson, C. B. (2009). Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science, 324(5930), 1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg, O. (1956). On the origin of cancer cells. Science, 123(3191), 309–314. [DOI] [PubMed] [Google Scholar]
- White, K. A. , Grillo‐Hill, B. K. , & Barber, D. L. (2017). Cancer cell behaviors mediated by dysregulated pH dynamics at a glance. Journal of Cell Science, 130(4), 663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittam, R. , & Willis, J. S. (1963). Ion movements and oxygen consumption in kidney cortex slices. Journal of Physiology, 168(1), 158–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise, D. R. , & Thompson, C. B. (2010). Glutamine addiction: A new therapeutic target in cancer. Trends in Biochemical Sciences, 35(8), 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte, D. , Ali, N. , Carlson, N. , & Younes, M. (2002). Overexpression of the neutral amino acid transporter ASCT2 in human colorectal adenocarcinoma. Anticancer Research, 22, 2555–2557. [PubMed] [Google Scholar]
- Wu, Y. I. , Frey, D. , Lungu, O. I. , Jaehrig, A. , Schlichting, I. , Kuhlman, B. , & Hahn, K. M. (2009). A genetically encoded photoactivatable Rac controls the motility of living cells. Nature, 461(7260), 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaci, R. F. , Fraser, S. P. , Battaloglu, E. , Kaya, H. , Erguler, K. , Foster, C. S. , & Djamgoz, M. B. A. (2017). Neonatal Nav1.5 protein expression in normal adult human tissues and breast cancer. Pathology, Research and Practice, 213(8), 900–907. [DOI] [PubMed] [Google Scholar]
- Yamamura, H. , Ugawa, S. , Ueda, T. , & Shimada, S. (2008). Expression analysis of the epithelial Na+ channel delta subunit in human melanoma G‐361 cells. Biochemical and Biophysical Research Communications, 366(2), 489–492. [DOI] [PubMed] [Google Scholar]
- Yang, M. , James, A. D. , Suman, R. , Kasprowicz, R. , Nelson, M. , O'Toole, P. J. , & Brackenbury, W. J. (2020). Voltage‐dependent activation of Rac1 by Nav1.5 channels promotes migration. Journal of Cellular Physiology, 235(4), 3950–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, M. , Kozminski, D. J. , Wold, L. A. , Modak, R. , Calhoun, J. D. , Isom, L. L. , & Brackenbury, W. J. (2012). Therapeutic potential for phenytoin: Targeting Na(v)1.5 sodium channels to reduce migration and invasion in metastatic breast cancer. Breast Cancer Research and Treatment, 134(2), 603–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaric, O. , Pinker, K. , Zbyn, S. , Strasser, B. , Robinson, S. , Minarikova, L. , Gruber, S. , Farr, A. , Singer, C. , Helbich, T. H. , Trattnig, S. , & Bogner, W. (2016). Quantitative sodium MR imaging at 7 T: Initial results and comparison with diffusion‐weighted imaging in patients with breast tumors. Radiology, 280(1), 39–48. [DOI] [PubMed] [Google Scholar]
- Zhou, Y. , Wong, C. O. , Cho, K. J. , van der Hoeven, D. , Liang, H. , Thakur, D. P. , Luo, J. , Babic, M. , Zinsmaier, K. E. , Zhu, M. X. , Hu, H. , Venkatachalam, K. , & Hancock, J. F. (2015). Membrane potential modulates plasma membrane phospholipid dynamics and K‐Ras signaling. Science, 349(6250), 873–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Z. , Song, J. , Li, W. , Liu, X. , Cao, L. , Wan, L. , Tan, Y. , Ji, S. , Liang, Y. , & Gong, F. (2017). The acid‐sensing ion channel, ASIC2, promotes invasion and metastasis of colorectal cancer under acidosis by activating the calcineurin/NFAT1 axis. Journal of Experimental & Clinical Cancer Research, 36, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, S. , Zhou, H.‐Y. , Deng, S.‐C. , Deng, S.‐J. , He, C. , Li, X. , Chen, J.‐Y. , Jin, Y. , Hu, Z.‐L. , Wang, F. , Wang, C.‐Y. , & Zhao, G. (2017). ASIC1 and ASIC3 contribute to acidity‐induced EMT of pancreatic cancer through activating Ca/RhoA pathway. Cell Death & Disease, 8, e2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Peer Review History


