Abstract
An adenovirus vector encoding murine Fas ligand (mFasL) under an inducible control was derived. In vivo ectopic expression of mFasL in murine livers induced an inflammatory cellular infiltration. Furthermore, ectopic expression of mFasL by myocytes did not allow prolonged vector-mediated transgene expression. Thus, ectopic expression of functional mFasL in vector-transduced cells does not appear to confer, by itself, an immunoprivileged site sufficient to mitigate adenovirus vector immunogenicity.
Recombinant adenovirus vectors have found broad utility for a variety of gene therapy applications (9, 57). This fact derives principally from their ability to accomplish efficient in vivo gene transfer in a variety of organ contexts. Despite this property, successful use of these agents for gene therapy purposes has been significantly limited to date, largely because an invariable consequence of in situ cellular transduction by adenovirus vectors at distinct parenchymal sites has been shown to be a significant host immunological response against transduced cells (52, 56, 60). A number of specific immune effector mechanisms, together with nonspecific defense mechanisms, are called into play to eliminate an infecting virus (57). This process has been associated with attenuation of expression of the transferred therapeutic gene based, at least in part, on loss of the vector-transduced cells (12, 61, 63). Since the cells infected with recombinant adenoviruses are usually rapidly eliminated, it is likely that the host immune system plays a major role in preventing sustained expression of the foreign genes. Innate and adaptive immune response-related clearance of adenovirus vectors in vivo has been described (59). The importance of the immune response against adenovirus vectors was first suggested by reports of long-term recombinant gene expression and less inflammatory reaction after a single adenovirus administration to neonatal animals, which have an immature immune system (64). Similar results were obtained in mice with severe immunodeficiency and in nude mice (6). Subsequently, several groups have demonstrated that infection of an immunocompetent host with recombinant adenoviruses elicits a CD8+ cytotoxic T-cell (CTL) response that eliminates virus-infected cells within 28 days of infection (12, 61, 62). Thus, strategies for prolonging the expression of therapeutic genes delivered by adenovirus, even in the context of diseases in which transient effects may be sought, such as cancer, are essential requirements for achieving clinical utility.
Based on a growing understanding of the immunological phenomena underlying this process, a variety of distinct strategies have been proposed to attenuate vector immunogenicity (31). To maximize the therapeutic potential of adenovirus vectors, various treatments administered at the time of vector delivery, aimed at modifying the host immune response, are being developed. In this regard, it has been postulated that the major stimulus for host immune responses is the expression of endogenous viral genes by transduced cells (63). Consequently, strategies to reduce endogenous viral gene expression through additional deletions in several gene regions of recombinant adenovirus vectors have been developed (16, 55). Direct strategies to abrogate presentation of viral antigens by antigen-presenting cells to the immune system have also been studied. Methods used have included cytotoxic drugs, antilymphocyte agents, cyclosporine, FK506, and deoxyspergualin (21, 28, 53, 58). In addition, interruption of the specific interaction between major histocompatibility complex class I molecules in antigen-primed cells and helper T lymphocytes has been proposed. Interventions explored in this context have included the use of agents that block costimulatory signals, such as CTLA4lg and anti-CD40 ligand (19, 25). Thus, considerable efforts have been directed at mitigating host response to the vector-transduced cells as a means to prolong transgene expression for gene therapy purposes.
From a conceptual standpoint, these strategies seek to render vector-transduced cells into immunologically privileged sites to avoid their recognition by the host immune system. In this regard, host mechanisms for establishing such immune privilege have been recognized to occur in selected endogenous physiological contexts. Immune privilege in sites like the eye, testis, and brain allows foreign agents and tissues to persist in those locations, and this phenomenon has long held the promise of solving the problems of autoimmunity, graft rejection, and potentially vector immunogenicity (14, 18, 29, 39, 45, 54). One clinical example of the function of an immunologically privileged site is the success of human corneal transplants, where a very high percentage of transplants engraft without tissue matching or immunosuppressive therapy. Stuart et al. recently demonstrated that Fas ligand (FasL) expression on the cornea is a major factor in corneal allograft survival; thus, they provide an explanation for one of the most successful tissue transplants performed in humans (50). FasL is a member of the tumor necrosis factor family and induces apoptosis in cells that express Fas (7, 20, 43, 51, 54). FasL is expressed by activated CTLs as well as NK cells and works as a death induction factor (33, 35). It has been proposed that when activated inflammatory cells enter the eye or testis, they are immediately killed through the Fas-FasL pathway (17, 34). In this regard, Lau et al. showed that syngeneic myoblasts expressing murine FasL (mFasL) protected allogenic pancreatic islets concomitantly transplanted under the kidney capsule (27). Furthermore, the protective effect of FasL was also observed when testis-derived Sertoli cells survived and provided local immunosuppression for xenografts in rat brains (47). Recently, Xu et al. have shown an evasion of the immune CTL response by induction of FasL expression on simian immunodeficiency virus-infected cells (60). Malignant melanoma and hepatocellular carcinomas have been found to express FasL, suggesting that these tumor cells can evade the immune attack through their expression of FasL (20, 49). These remarkable observations have led to the hypothesis that ectopic FasL expression may have the potential to render a site impervious to the consequences of immune recognition. It thus seemed reasonable to use such an approach to achieve immune protection of vector-transduced cells in a gene therapy context. We hypothesized that such immune protection of cells transduced by adenovirus might be induced via methods directed at local augmentation of expression of the FasL molecule. Furthermore, this attenuation of the host immune response might thus allow prolongation of the transgene expression that derives from transduction with recombinant adenovirus vectors.
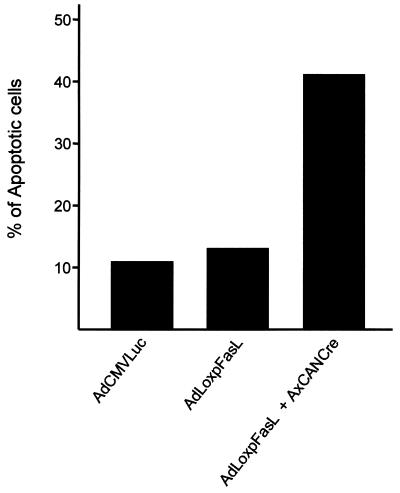
The initial step in this endeavor was the construction of a replication-incompetent, recombinant adenovirus vector expressing mFasL. A first consideration was the fact that coexpression of Fas and FasL in the same cellular context results in an autocrine loop that induces apoptosis (26). Thus, a Fas-positive phenotype in available packaging cells (293 cells) would potentially undermine efforts to derive a FasL-expressing vector. In this regard, at 10 h after infection with 5 PFU per cell of AdCMVLuc (irrelevant virus), AdLoxpFasL alone, or AdLoxpFasL plus AdCANCre, both of which express the inducible mFasL, we analyzed apoptosis in 293 cells. Cells were washed with phosphate-buffered saline and resuspended at 106 cells/ml. Early detection of apoptosis was performed with an ApoAlert Annexin V Fluos staining kit as instructed by the manufacturer (Boehringer Mannheim, Indianapolis, Ind.). Detection of apoptosis is based in changes occurring on the cell surface during early stages of apoptosis; specifically, translocation of phosphatidylserine from the interior side of the plasma membrane to the outer leaflet is detected. This is the basis for the high-affinity binding of annexin V to phosphatidylserine. For this analysis, 293 cells were incubated with annexin V-biotin in a HEPES buffer for 15 min at room temperature. Fluorescence-activated cell sorting analysis was performed in 104 events in a pool of cells from quadruplicate experiments, and data were expressed as a percentage of apoptotic cells (Fig. 1). The percentage of cells within each region was calculated by using CellFIT version 1.0 (Becton Dickinson).
FIG. 1.
Apoptosis analysis of 293 cells expressing mFasL, performed as described in the text.
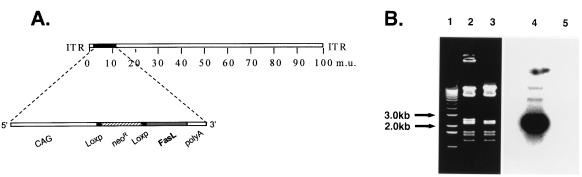
We thus conceptualized a strategy to express FasL in an inducible context, based on an application of the Cre/Loxp system to the recombinant adenovirus context (5). Figure 2A depicts the structure of our designed vector, whereby FasL is functionally separated from the modified chicken β-actin promoter with the cytomegalovirus immediate-early enhancer (CAG) (37) by a Loxp-flanked stuffer segment. The encoded Cre recombinase protein would be predicted to excise the stuffer and allow functional reconstitution of the expression cassette at the deleted E1 site of the recombinant adenovirus vector. Of note, Kanegae et al. (22) and Anton and Graham (5) have both described the utility of such a Loxp-based inducible system in the context of the adenovirus genome. Thus, this strategy based on inducible gene expression offered the means to construct such a vector expressing FasL, regardless of the expression of Fas by the viral vector packaging cell line.
FIG. 2.
Construction of a recombinant adenovirus vector encoding mFasL. (A) Map of the recombinant adenovirus vector. An expression cassette is inserted into the deleted E1A region. This cassette allows inducible expression of mFasL from the CAG promoter after excising of the stuffer Neor gene flanked by the Loxp sites. ITR, adenovirus inverted terminal repeats. Map units (m.u.) 0 to 100 are indicated. (B) Confirmation of identity of the adenovirus vector encoding mFasL, AdLoxpFasL. Adenovirus genome DNA was subjected to restriction endonuclease digestion with XhoI and analyzed by gel electrophoresis and Southern blotting. For Southern blotting, a 32P-labeled mFasL probe was used. Lane 1, 1-kb DNA ladder; lane 2, XhoI digestion of AdLoxpFasL; lane 3, XhoI digestion of E1-deleted adenovirus vector lacking the mFasL gene; lane 4, Southern blot analysis as for lane 2; lane 5, Southern blot analysis as for lane 3.
The recombinant adenovirus AdLoxpFasL was constructed by the two-plasmid homologous recombination method of Parks et al. (40). To generate a plasmid expressing mFasL incorporating the Cre/Loxp system, a β-actin promoter-driven neomycin resistance (Neor) gene flanked by two Loxp sites was subcloned as a HindIII-SalII fragment from plasmid pCANLNLW (provided by I. Saito, Tokyo, Japan) into the multiple cloning site of the adenovirus shuttle plasmid pΔESP1B (Microbix, Inc., Ontario, Canada). The resultant plasmid, pΔEloxP, sequentially contains 0.5 map units of sequence from the left end of the adenovirus type 5 genome, the β-actin promoter, the first Loxp site, the Neor gene, a second directional repeat Loxp site, and a unique SwaI site, followed by simian virus 40 poly(A) signal sequences and finally map units 9 through 16 of the adenovirus genome. Full-length mFasL was excised from the plasmid pcDNA3-mFasL with BamHI and XhoI, blunt ended with Klenow fragment, and then subcloned into the SwaI site of pΔEloxP. Restriction endonuclease digestion and direct sequence analysis confirmed the orientation and sequence of the inserted mFasL. The resultant plasmid, pΔE1loxPF, was then cotransfected into the adenovirus packaging cell line 293 together with the adenovirus packaging plasmid pJM17 (Microbix), by using Lipofectin (BRL, Gaithersburg, Md.) as previously described (44). After cotransfection, cells were overlaid with Dulbecco’s modified Eagle’s medium-F12 (Mediatech/Cellgro) supplemented with 2.5% heat-inactivated fetal bovine serum (FBS) (HyClone, Logan, Utah) and 0.65% Noble agar (Difco, Detroit, Mich.). Plaques were picked approximately 10 days posttransfection and carried through three additional isolation steps. The identity of the resultant adenovirus vector, AdLoxpFasL, was confirmed by restriction endonuclease digestion with XhoI and Southern blot analysis using standard procedures (Fig. 2B).
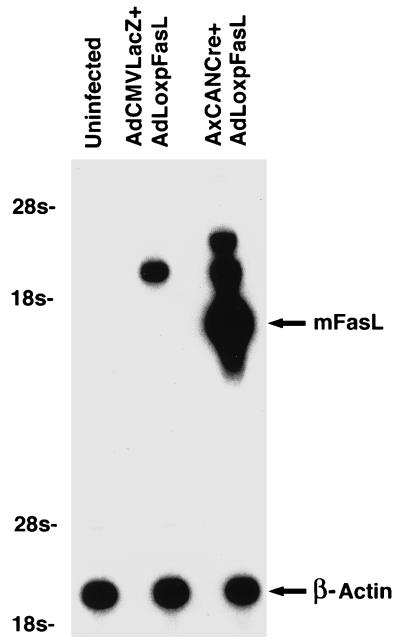
We next sought to confirm the expression of mFasL from the derived adenovirus vector AdLoxpFasL. Such analysis was required to confirm the relative absence of expression from the noninduced vector configuration, as well as the mFasL expression after induction with Cre recombinase in cells transduced with the inducible vector. For this analysis, murine B6 lpr/lpr (lpr stands for lymphoproliferation) macrophages were used. These cells derive from a transgenic mouse deficient in Fas expression and thus permit FasL expression without the potential consequences of induction of apoptosis by the interaction between Fas and FasL (3, 36). Cells were grown in RPMI 1640 medium supplemented with 10% FBS in a humidified 5% CO2 atmosphere and seeded at 105 cells in 60-mm2 plates. After overnight culture, cells were coinfected with AdLoxpFasL and AxCANCre, a Cre-expressing recombinant adenovirus vector, for induction of mFasL expression in effector cells (22). As a control, cells were also coinfected with AdLoxpFasL plus a non-Cre-expressing recombinant adenovirus, AdCMVLacZ. A multiplicity of infection of 5 PFU/cell was used for each vector. Infections were allowed to proceed for 1 h in culture medium containing 2% FBS, followed by incubation for 24 h in RPMI 1640 supplemented with 10% FBS. Uninfected controls, not exposed to viral vectors, were maintained and processed in the same manner. Total RNA extraction and Northern blotting were then performed by techniques described elsewhere (65). Probes for this analysis included a 960-bp fragment of the mFasL cDNA amplified by PCR or a murine β-actin cDNA. In this analysis, the addition of the adenovirus expressing Cre recombinase resulted in a marked induction of mFasL expression in the target cells. Specifically, a readily detectable band corresponding to the full-length mFasL cDNA could be noted when RNA from the experimental group including both AxCANCre and AdLoxpFasL was analyzed. A band corresponding to the induced expression of the mFasL cDNA was not noted in other groups. Interestingly, a band of higher molecular weight was detected in the group including the AdCMVLacZ and AdLoxpFasL vectors, in relatively reduced amounts. The size of the band, as well as its context, suggests that a low level of spontaneous expression of mFasL occurred with these vectors. Levels of mFasL, however, were dramatically less than those noted when AxCANCre and AdLoxpFasL were administered. Analysis of the β-actin transcripts showed comparable levels in each group. Thus, the AdLoxpFasL vector is capable of expressing high levels of mFasL in target cells under the control of Cre recombinase (Fig. 3).
FIG. 3.
Analysis of gene expression characteristic of AdLoxpFasL by Northern blot analysis. Murine B6 lpr/lpr mouse macrophages were infected with either AdLoxpFasL plus AdCMVLacZ (lane 2) or AdLoxpFasL plus AxCANCre (lane 3). Lane 1 is an uninfected control. Twenty-four hours postinfection, total RNA was isolated and probed with mFasL and β-actin cDNAs.
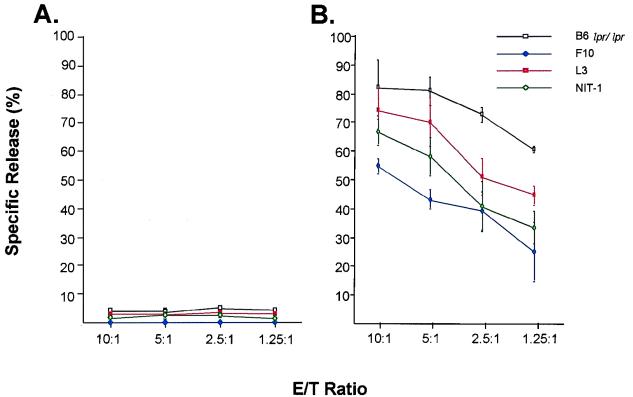
We next sought to confirm the functional activity of the mFasL encoded in the vector AdLoxpFasL. For this analysis, we employed a 51Cr release assay in which a FasL-sensitive cell line (A20) was used as the target cell. The A20 cells were labeled with [51Cr]sodium chromate (50 μCi/105 cells; Amersham, Arlington Heights, Ill.) for 1 h at 37°C. After extensive washing, the labeled A20 cells were then added to various murine cell lines, including B6 lpr/lpr macrophages, NIT-1 insulinoma cells, L3 microglioma cells, and F10 astrocytoma cells, at various effector-to-target (E/T) ratios. After 6 h of incubation, the specific release of the radioactive marker was determined by gamma scintigraphy as previously described (66). These cells had been preinfected with AdCMVLacZ and AdLoxpFasL or with AxCANCre and AdLoxpFasL at 5 PFU/cell. The spontaneous release of 51Cr was determined by incubating the 51Cr-labeled A20 with medium alone, whereas the maximum release was determined by adding sodium dodecyl sulfate solution (SDS) to a final concentration of 0.05%. The percentage of specific release was calculated as follows: % specific lysis = [(experimental 51Cr release) − (spontaneous 51Cr release)]/[(maximum 51Cr release) − (spontaneous 51Cr release)]. Infection with AdCMVLacZ and AdLoxpFasL did not induce or enhance killing in any of the tested cell lines (Fig. 4A). In marked contrast, an increase in cell killing, as manifested by 51Cr release, was noted for all infected cell lines with an increasing E/T ratio (Fig. 4B) when AxCANCre and AdLoxpFasL were used. This finding is consistent with the concept that the adenovirus vector effectively mediated ectopic mFasL expression. Furthermore, it was demonstrated that the induced mFasL expression rendered target cells sensitive to killing mediated by Fas. Thus, the AdLoxpFasL vector is capable of expressing physiologically relevant amounts of functional mFasL after induction by adenovirus-mediated expression of Cre recombinase.
FIG. 4.
Characterization of the function of ectopic expression of mFasL in macrophages. The lpr/lpr macrophages were infected with either AdLoxpFasL plus AdCMVLacZ (A) or AdLoxpFasL plus AxCANCre (B) and mixed with 51Cr-labeled A20 cells at the indicated E/T ratios; after 6 h of incubation, the specific release of radioactive marker was determinated.
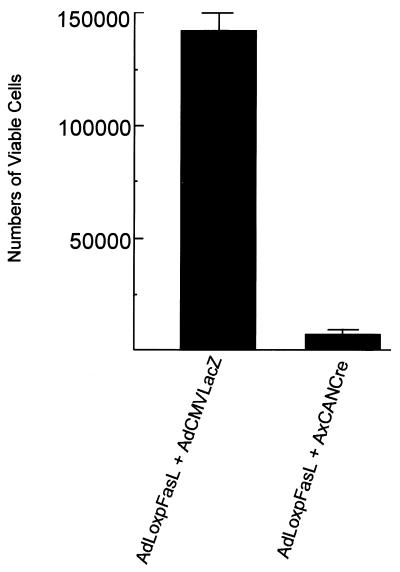
Whereas this experiment confirmed that mFasL augmentation could enhance cell killing via physiologic pathways of cellular interaction, we also sought to demonstrate the consequences of Fas and FasL coexpression within the same target cell. Such a finding of an autocrine pathway of induction of apoptosis would validate our strategy for construction of the adenovirus vector expressing FasL in an inducible manner. Furthermore, such a finding would have consequences for the manner whereby ectopic FasL expression would be achieved for applications to attenuate vector immunogenicity. To validate this concept, we induced expression of mFasL in HeLa and 293 cells, which already express Fas (30). For this analysis, 105 HeLa cells were plated in six-well tissue culture plates and cultured in Dulbecco’s modified Eagle’s medium-F12 supplemented with 10% FBS at 37°C and 5% CO2 atmosphere for 24 h. The cells were then infected with the various combinations of adenovirus vectors, as described above, at 10 PFU/cell and washed extensively 1 h postinfection. At 24 h postinfection, the cells were stained with trypan blue, and triplicates were counted to determine the number of viable cells. In this analysis, induced expression of mFasL in HeLa cells resulted in a dramatic decrement in viable cell numbers (Fig. 5). This result was not seen with noninduced AdLoxpFasL. Thus, the expression of mFasL, in the context of a target cell expressing Fas, can induce an autocrine suicide event. This finding thus rationalizes the use of adenovirus-mediated ectopic expression of mFasL exclusively in tissue contexts in which Fas is not expressed.
FIG. 5.
Induction of autocrine suicide event by Fas-expressing target cells by AdLoxpFasL. HeLa cells were infected with AdLoxpFasL plus AdCMVLacZ or with AdLoxpFasL plus AxCANCre. After 24 h, cells were stained with trypan blue and analyzed in triplicate to determine the number of viable cells.
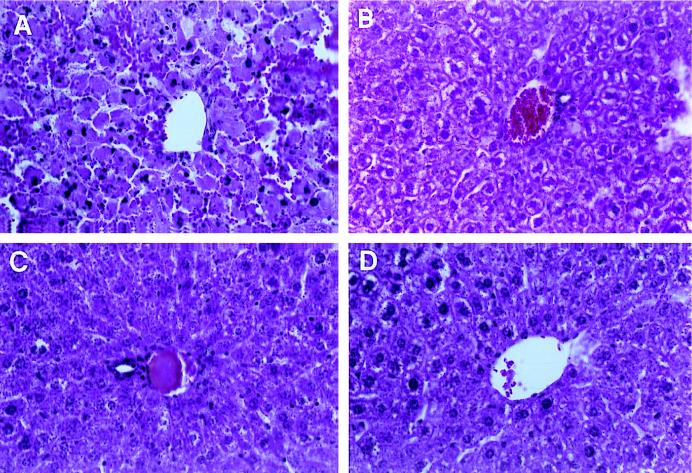
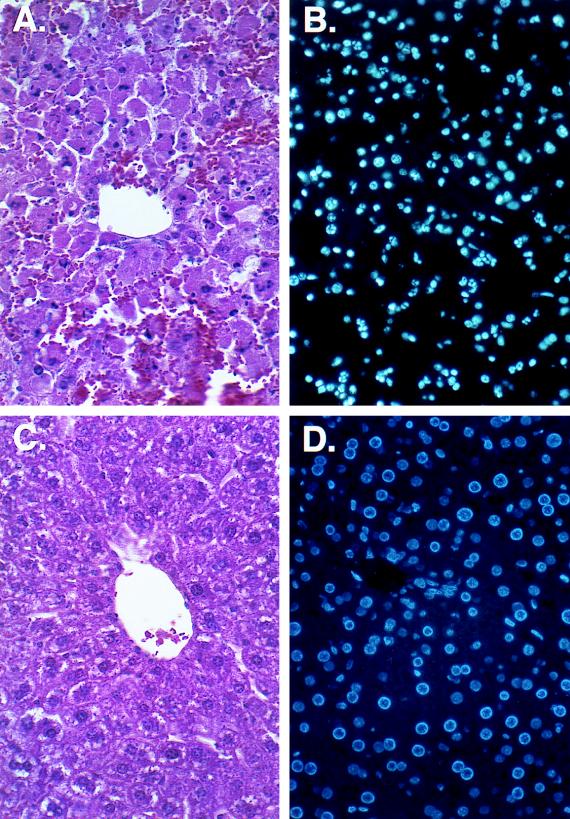
As an additional test of the biologic effect of expression of mFasL at ectopic sites, we used AdLoxpFasL to achieve in situ expression of FasL in the liver. For this experiment, adult female C57BL/6 and B6 lpr/lpr mice were injected via the tail vein with adenovirus vector constructs. This means of delivery is known to achieve principally hepatocyte transduction (60). Animals were challenged with both AdCMVLacZ and AdLoxpFasL, or with AxCANCre and AdLoxpFasL, at 5 × 109 PFU per animal. Twenty-four hours after injection, livers were harvested and analyzed by immunohistochemistry to study the hepatic parenchyma. For the B6 lpr/lpr mice, both combinations of delivered viruses did not elicit any changes in histopathology compared to livers from control uninfected mice (data not shown). In contrast, in the group of C57BL/6 mice, which received the AxCANCre and AdLoxpFasL vectors, hepatocytes demonstrated pyknotic nuclei, as well as a significant influx of inflammatory cells (granulocytes and lymphocytes) which were distributed throughout the hepatic parenchyma. This infiltrate was not seen either in the group that received AdCMVLacZ and AdLoxpFasL or in the control group that received no virus (Fig. 6). In further analysis, detection of apoptosis in hepatic parenchyma was performed. To this end, in situ detection of apoptotic cells with Hoechst 33258 (1 μg/ml) showed that a proportion of cells underwent apoptotic changes (10, 15). In this regard, nuclei showed condensed chromatin under the fluorescence microscope (UV filter) in the treated animals with the AxCANCre and AdLoxpFasL vectors but not in the control groups (Fig. 7). The fact that this inflammatory phenomenon and apoptosis were noted only in the group in which expression of mFasL was activated by Cre recombinase suggests that it was the expression of mFasL per se which induced these alterations.
FIG. 6.
Effect of ectopic expression of mFasL expression in the liver. Adult female C57BL/6 mice were injected intravenously with AdLoxpFasL plus AxCANCre (A), AdCMVLacZ (B), AdLoxpFasL plus AdCMVLacZ (C), or phosphate-buffered saline alone (D). Livers were harvested at 24 h postinjection, and sections were prepared for histological analysis staining with hematoxylin and eosin. Magnification, ×320.
FIG. 7.
In situ detection of apoptotic cells in the liver. Adult female C57BL/6 mice were injected intravenously with AdLoxpFasL plus AxCANCre (A and B) or AdCMVLacZ (C and D). Livers were harvested at 24 h postinjection, and sections were prepared for histological analysis staining with hematoxylin and eosin (A and C) or with Hoechst 33258 reagent to detect apoptosis (B and D). Magnification, ×320.
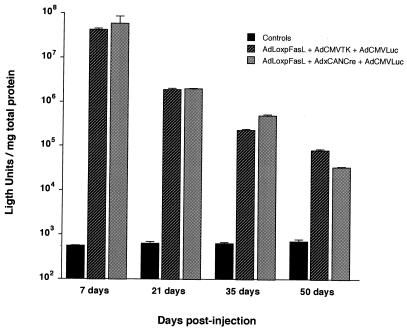
To explore the potential of mFasL to mitigate vector immunogenicity, it was necessary to use a target tissue not characterized by the expression of Fas. In this regard, myocytes are known to lack expression of Fas (46). Furthermore, there is a significant amount of data characterizing the temporal pattern of adenovirus vector-mediated transgene expression in the muscle (2, 41). Specifically, it has been shown that direct administration of adenovirus vectors by intramuscular (i.m.) injection can achieve infection of a significant number of mature myofibers (1, 42). Of note, a well-characterized host immune response is induced after i.m. delivery of adenovirus vectors, including a CTL-mediated eradication of transduced cells (62). We thus sought to mitigate this process by ectopic expression of mFasL in vector-modified myocytes. For this experiment, adult female BALB/c mice were injected i.m. via the intraglossal route to achieve transduction of mature myofibers. Groups of animals received no vector, AdLoxpFasL plus AdCMVTK plus AdCMVLuc, or AdLoxpFasL plus AxCANCre plus AdCMVLuc (109 PFU of each virus per animal). The combination of viruses in the second group included an adenovirus encoding the luciferase reporter gene, AdCMVLuc, to allow measurement of transgene expression, plus AdLoxpFasL with an irrelevant control adenovirus, AdCMVTK, which would not be predicted to induce mFasL expression. The combination of viruses in the third group contained the virus expressing the reporter gene, and also contained AdLoxpFasL plus AxCANCre, to achieve induction of mFasL expression. Thus, this experiment would allow direct comparison of the pattern of transgene expression mediated by the adenovirus vector in the presence or absence of mFasL coexpression.
In this experiment, all uninfected control groups demonstrated an absence of luciferase expression in harvested myofibers, as expected. For the group without induced mFasL expression, an initial high level of gene expression which was readily detectable by day 7 postinfection was achieved. These levels of gene transfer underwent attenuation in a time-dependent manner such that by day 50 postinfection, the magnitudes of luciferase gene expression were nearly 4 orders of magnitude less than those observed at day 7 (Fig. 8). This pattern of nonpersistence of transgene expression is analogous to that described by other authors and reflects the consequences of the host immune response to the vector-transduced cells (2, 41). When this experiment was repeated except with in situ induction of mFasL, the pattern of transgene expression did not differ from that noted in the noninduced group (Fig. 8); a rapid reduction in transgene expression was noted such that by day 50 postinjection, a decrement of more than 3 orders of magnitude was noted. Of note, analysis of the infected muscle sites by reverse transcription-PCR confirmed expression of mFasL in the induced group. In addition, in the noninduced group, lower levels of mFasL could be detected (data not shown). Thus, autocrine suicide of infected muscle cells (negative Fas receptor) expressing mFasL was not likely the basis of diminishing transgene expression in the group with induction of mFasL. It thus appeared that in this organ context, simple ectopic expression of mFasL did not achieve the desired end of establishing the vector-infected cell as an immunologically privileged site.
FIG. 8.
Effect of ectopic expression of mFasL expression on longevity of transgene expression in murine myocytes. Adult female BALB/c mice were injected intraglossally with the indicated vectors; control animals were not injected. At various times postinjection, luciferase activity was determined in harvested tissue. Each histogram represents the mean ± standard deviation for seven animals.
A recent report by Muruve et al. has also described the construction and characterization of a recombinant adenovirus vector expressing mFasL (32). Many of their findings are of interest in the context of the results of this study. These investigators used a cytomegalovirus-driven mFasL cassette in their adenovirus vector. For this construction, they experienced difficulty deriving the virion and have noted consistently low viral titer yields. This was attributed, in part, to the fact that the adenovirus packaging cell line 293 is known to express Fas (32). Thus, the resulting autocrine loop has confounded their vector derivation and propagation efforts. Anticipation of this issue led us to develop an adenovirus vector with an inducible system. In this manner, we have readily obtained the desired recombinant vectors and obtained viral titers commensurate with those of standard recombinant adenovirus vectors. Thus, from a strictly practical standpoint, we have derived a benefit from maintaining the mFasL in an inducible state in the context of the adenovirus vector. An additional aspect of the report of Muruve et al. was the finding that systemic injection of their vector induced widespread death of hepatocytes, a phenomenon consistent with the effects of anti-Fas antibody (38). Furthermore, transduced pancreatic allografts underwent apoptotic cell death, resulting in nonfunctional grafts when transplanted into syngeneic or allogenic recipients. The latter phenomenon is significant, as Fas is widely expressed. Whereas Muruve et al. did not explicitly use FasL to prolong transgene expression in cells infected by adenovirus vectors, their results did provide insight into the complexity of the Fas-FasL pathway. In this context, Allison et al. (4) reported that expression of functional FasL in the pancreatic islets of transgenic mice failed to protect these islets from allogenic transplant rejection. In addition, these genetically modified cells induced a granulocytic infiltrate that damaged the islets (4, 8, 23). Further of note, Seino et al. reported that FasL expression in tumor cells could induce a granulocyte-mediated rejection (48). A further new study challenges the immunoprotective effect of FasL; Kang et al. have shown that adenovirus-mediated expression of FasL in pancreatic islet allografts induces neutrophilic infiltration and islet destruction (23). One more example of the complexity of the Fas-FasL system is the discovery that various disease states result from dysregulation of the system, including lymphoproliferative autoimmune syndromes, hepatitis, Hashimoto’s thyroiditis, and glomerular cell apoptosis (11, 13). Finally, Kayagaki et al. observed that naturally occurring alleles of FasL have different abilities to trigger apoptosis through Fas, suggesting that polymorphism of FasL affects the biological activity (24).
These results parallel the limitations of our study in that ectopic FasL expression per se was not sufficient to prolong expression of a vector-encoded transgene by attempting to diminish immunological eradication of vector-transduced cells. Thus, several issues with respect to the use of FasL have arisen in these studies. First, while intended to allow an immunologically privileged site, the ectopic expression of FasL can actually elicit an inflammatory influx. Second, the coexpression of Fas and an ectopic FasL can induce an autocrine loop with induction of target cell apoptosis. In addition, the magnitude and temporal pattern of FasL expression may be key determinants of its efficacy in this context. These issues were not addressed in our study. The complex aspects of FasL biology can confound direct attempts to explain this axis in many tissue contexts and can be frankly deleterious at some organ sites through elicitation of parenchymal apoptosis. Thus, a more complete understanding of the Fas-FasL pathway will be required before strategies to exploit the system for mitigating vector immunogenicity may be contemplated.
Acknowledgments
We thank Christi Stuart for technical support.
This work was supported in part by grants NIH RO1-HL 50255, NIH RO1-CA 74242, and U.S. Army DAMD-17-94-J4398 and by a grant from the American Lung Association.
REFERENCES
- 1.Acsadi G, Lochmuller H, Jani A, Huard J, Massie B, Prescott S, Simoneau M P B, Karpati G. Dystrophin expression in muscles of mdx mice after adenovirus-mediated in vivo gene transfer. Hum Gene Ther. 1996;7:129–140. doi: 10.1089/hum.1996.7.2-129. [DOI] [PubMed] [Google Scholar]
- 2.Acsadi G, Massie B, Jani A. Adenovirus-mediated gene transfer into striated muscles. J Mol Med. 1995;73:165–180. doi: 10.1007/BF00188137. [DOI] [PubMed] [Google Scholar]
- 3.Adachi M, Watanabe-Fukanaga R, Nagata S. Aberrant transcription caused by the insertion of an early transposable element in an intron of the Fas antigen gene of lpr mice. Proc Natl Acad Sci USA. 1993;90:1756–1760. doi: 10.1073/pnas.90.5.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allison J, Georgiou H M, Strasser A, Vaux D L. Transgenic expression of CD95 ligand on islet b cells induces a granulocytic infiltration but does not confer immune privilege upon islet allografts. Proc Natl Acad Sci USA. 1997;94:3943–3947. doi: 10.1073/pnas.94.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anton M, Graham F L. Site-specific recombination mediated by an adenovirus vector expressing the Cre recombinase protein: a molecular switch for control of gene expression. J Virol. 1995;69:4600–4606. doi: 10.1128/jvi.69.8.4600-4606.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barr D, Tubb J, Ferguson D, Scaria A, Lieber A, Wilson C, Perkins J, Kay M A. Strain related variations in adenovirally mediated transgene expression from mouse hepatocytes in vivo: comparisons between immunocompetent and immunodeficient inbred strains. Gene Ther. 1995;2:151–155. [PubMed] [Google Scholar]
- 7.Berke G. Killing mechanisms of cytotoxic lymphocytes. Curr Opin Hematol. 1997;4:32–40. doi: 10.1097/00062752-199704010-00006. [DOI] [PubMed] [Google Scholar]
- 8.Chervonsky A V, Wang Y, Wong F S, Visintin I, Flavell R A, Janeway C A, Jr, Matis L A. The role of Fas in autoimmune diabetes. Cell. 1997;89:17–24. doi: 10.1016/s0092-8674(00)80178-6. [DOI] [PubMed] [Google Scholar]
- 9.Douglas, J. T., and D. T. Curiel. 1997. Adenoviruses as vectors for gene therapy. Sci. Med. 44–53.
- 10.Elstein K H, Zucker R M. Comparison of cellular and nuclear flow cytometric techniques for discriminating apoptotic subpopulations. Exp Cell Res. 1994;211:322–331. doi: 10.1006/excr.1994.1094. [DOI] [PubMed] [Google Scholar]
- 11.Fisher G, Rosenberg R J, Straus S E, Dale J K, Middelton L A, Lin A Y, Strober W, Lenardo M J, Puck J M. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81:935–946. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 12.Fisher K J, Choi H, Burda J, Chen S J, Wilson J M. Recombinant adenovirus deleted of all viral genes for gene therapy of cystic fibrosis. Virology. 1996;217:11–22. doi: 10.1006/viro.1996.0088. [DOI] [PubMed] [Google Scholar]
- 13.French L, Tschopp J. Thyroiditis and hepatitis: Fas on the road to disease. Nat Med. 1997;3:387–388. doi: 10.1038/nm0497-387. [DOI] [PubMed] [Google Scholar]
- 14.French L E, Tschopp J. Constitutive Fas ligand expression in several non-lymphoid mouse tissues: implications for immune-protection and cell turnover. Behring Inst Mitt. 1996;97:156–160. [PubMed] [Google Scholar]
- 15.Fukuo, K., T. Nakahashi, S. Nomura, S. Hata, T. Suhara, M. Shimizu, M. Tamatani, S. Morimoto, Y. Kitamura, and T. Ogihara. 1997. Possible participation of Fas-mediated apoptosis in the mechanism of atherosclerosis. Gerontology 43(Suppl. 1):35–42. [DOI] [PubMed]
- 16.Gao G P, Yang Y, Wilson J M. Biology of adenovirus vectors with E1 and E4 deletions for liver-directed gene therapy. J Virol. 1996;70:8934–8943. doi: 10.1128/jvi.70.12.8934-8943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green D R, Ware C F. Fas-ligand: privilege and peril. Proc Natl Acad Sci USA. 1997;94:5986–5990. doi: 10.1073/pnas.94.12.5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffith T S, Brunner T, Fletcher S M, Green D R, Ferguson T A. Fas ligand-induced apoptosis as a mechanism of immune. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 19.Guerette B, Vilquin J T, Gingras M, Gravel C, Wood K J, Tremblay J P. Prevention of immune reactions triggered by first-generation adenoviral vectors by monoclonal antibodies and CTLA4lg. Hum Gene Ther. 1996;7:1455–1463. doi: 10.1089/hum.1996.7.12-1455. [DOI] [PubMed] [Google Scholar]
- 20.Hahne M, Rimoldi D, Schroter M, Romero P, Schreier M, French L E, Schneider P, Bornard T, Fontana A, Lienard D, Cerottine J C, Tschopp J. Melanoma cell expression of Fas (Apo-1/CD95) ligand: implications for tumor immune escape. Science. 1996;274:1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 21.Jooss K, Yang Y, Wilson J M. Cyclophosphamide diminishes inflammation and prolongs transgene expression following delivery of adenoviral vectors to mouse liver and lung. Hum Gene Ther. 1996;7:1555–1566. doi: 10.1089/hum.1996.7.13-1555. [DOI] [PubMed] [Google Scholar]
- 22.Kanegae Y, Lee G, Sato Y, Tanaka M, Nakai M, Sakaki T, Sugano S, Saito I. Efficient gene activation in mammalian cells by using recombinant adenovirus expressing site-specific Cre recombinase. Nucleic Acids Res. 1995;23:3816–3821. doi: 10.1093/nar/23.19.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang S M, Schneider D B, Lin Z, Hanahan D, Dichek D A, Stock P G, Baekkeskov S. Fas ligand expression in islets of Langerhans does not confer immune privilege and instead targets them for rapid destruction. Nat Med. 1997;3:738–743. doi: 10.1038/nm0797-738. [DOI] [PubMed] [Google Scholar]
- 24.Kayagaki N, Yamaguchi N, Nagao F, Matsuo S, Maeda H, Okumura K, Yagita H. Polymorphism of murine Fas ligand that affects the biological activity. Proc Natl Acad Sci USA. 1997;94:3914–3919. doi: 10.1073/pnas.94.8.3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolls J K, Lei D, Odom G, Nelson S, Summer W R, Gerber M A, Shellito J E. Use of transient CD4 lymphocyte depletion to prolong transgene expression of E1-deleted adenoviral vectors. Hum Gene Ther. 1996;7:489–497. doi: 10.1089/hum.1996.7.4-489. [DOI] [PubMed] [Google Scholar]
- 26.Larsen C P, Alexander D Z, Hendrix R, Ritchie S C, Pearson T C. Fas-mediated cytotoxicity. An immunoeffector or immunoregulatory pathway in T cell-mediated immune responses? Transplantation. 1995;60:221–224. doi: 10.1097/00007890-199508000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Lau H T, Yu M, Fontana A, Stoeckert C J., Jr Prevention of islet allograft rejection with engineered myoblasts expressing FasL in mice. Science. 1996;273:109–112. doi: 10.1126/science.273.5271.109. [DOI] [PubMed] [Google Scholar]
- 28.Lochmuller H, Petrof B J, Allen C, Prescott S, Massie B, Karpati G. Immunosuppression by FK506 markedly prolongs expression of adenovirus-delivered transgene in skeletal muscles of adult dystrophic [mdx] mice. Biochem Biophys Res Commun. 1995;213:569–574. doi: 10.1006/bbrc.1995.2169. [DOI] [PubMed] [Google Scholar]
- 29.Lynch D H, Ramsdell F, Alderson M R. Fas and FasL in the homeostatic regulation of immune responses. Immunol Today. 1995;16:569–574. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 30.Mandal M, Maggirwar S B, Sharma N, Kaufmann S H, Sun S C, Kumar R. Bcl-2 prevents CD95 (Fas/APO-1)-induced degradation of lamin B and poly(ADP-ribose) polymerase and restores the NF-kappaB signaling pathway. J Biol Chem. 1996;271:30354–30359. doi: 10.1074/jbc.271.48.30354. [DOI] [PubMed] [Google Scholar]
- 31.McFadden G, Graham K, Barry M. New strategies of immune modulation by DNA viruses. Transplant Proc. 1996;28:2085–2088. [PubMed] [Google Scholar]
- 32.Muruve D A, Nicolson A G, Manfro R C, Strom T B, Sukhatme V P, Libermann T A. Adenovirus-mediated expression of Fas ligand induces hepatic apoptosis after systemic administration and apoptosis of ex vivo-infected pancreatic islet allografts and isografts. Hum Gene Ther. 1997;8:955–963. doi: 10.1089/hum.1997.8.8-955. [DOI] [PubMed] [Google Scholar]
- 33.Nagata S. A death factor—the other side of the coin. Behring Inst Mitt. 1996;97:1–11. [PubMed] [Google Scholar]
- 34.Nagata S. Fas ligand and immune evasion. Nat Med. 1996;2:1306–1307. doi: 10.1038/nm1296-1306. [DOI] [PubMed] [Google Scholar]
- 35.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 36.Nagata S, Suda T. Fas and Fas ligand: lpr and gld mutations. Immunol Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 37.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–200. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 38.Ogasawara J, Watanabe-Fukanaga R, Adachi M, Matsuzawa A, Kasugal T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 39.Ozdemirli M, El-Khatib M, Foote L C, Wang J K, Marshak-Rothstein A, Rothstein T L, Ju S T. Fas (CD95)/Fas ligand interactions regulate antigen-specific, major histocompatibility complex-restricted T/B cell proliferative responses. Eur J Immunol. 1996;26:415–419. doi: 10.1002/eji.1830260222. [DOI] [PubMed] [Google Scholar]
- 40.Parks R J, Chen L, Anton M, Sankar U, Rudnicki M A, Graham F L. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc Natl Acad Sci USA. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petrof B J, Acsadi G, Jani A, Massie B, Bourdon J, Matusiewicz N, Yang L, Lochmuller H, Karpati G. Efficiency and functional consequences of adenovirus-mediated in vivo gene transfer to normal and dystrophic (mdx) mouse diaphragm. Am J Respir Cell Mol Biol. 1995;13:508–517. doi: 10.1165/ajrcmb.13.5.7576685. [DOI] [PubMed] [Google Scholar]
- 42.Quantin B, Perricaudet L D, Tajbakhsh S, Mandel J L. Adenovirus as an expression vector in muscle cells in vivo. Proc Natl Acad Sci USA. 1992;89:2581–2584. doi: 10.1073/pnas.89.7.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rathmell J C, Cooke M P, Ho W Y, Grein J, Townsend S E, Davis M M, Goodnow C C. CD95 (Fas)-dependent elimination of self-reactive B cells upon interaction with CD4+ T cells. Nature. 1995;376:181–184. doi: 10.1038/376181a0. [DOI] [PubMed] [Google Scholar]
- 44.Rosenfeld M E, Wang M, Siegal G P, Alvarez R D, Mikheeva G, Krasnykh V, Curiel D T. Adenoviral-mediated delivery of herpes simplex virus thymidine kinase results in tumor reduction and prolonged survival in SCID mouse model of human ovarian carcinoma. J Mol Med. 1996;74:455–462. doi: 10.1007/BF00217521. [DOI] [PubMed] [Google Scholar]
- 45.Saas P, Walker P R, Hahne M, Quiquerez A L, Schnuriger V, Perrin G, French L, Van Meir E G, de Tribolet N, Tschopp J, Dietrich P Y. Fas ligand expression by astrocytoma in vivo: maintaining immune privilege in the brain? J Clin Invest. 1997;99:1173–1178. doi: 10.1172/JCI119273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sahashi K, Ibi T, Ling J. Immunostaining of anti-Fas IgG1 antibody in diseased human muscle. Rinsho Shinkeigaku. 1995;35:764–769. [PubMed] [Google Scholar]
- 47.Sanberg P R, Borlongan C V, Saporta S, Cameron F. Testis-derived Sertoli cells survive and provide localized immunoprotection for xenografts in rat brain. Nat Biotechnol. 1996;14:1692–1695. doi: 10.1038/nbt1296-1692. [DOI] [PubMed] [Google Scholar]
- 48.Seino K, Kayagaki N, Okumura K, Yagita H. Antitumor effect of locally produced CD95 ligand. Nat Med. 1997;3:165–170. doi: 10.1038/nm0297-165. [DOI] [PubMed] [Google Scholar]
- 49.Strand S, Hofmann W J, Hug H, Muller M, Otto G, Strand D, Mariani S M, Stremmel W, Krammer P H, Galle P R. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells—a mechanism of immune evasion? Nat Med. 1996;2:1361–1366. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- 50.Stuart P M, Griffith T S, Usui N, Pepose J, Yu X, Ferguson T A. CD95 ligand (FasL)-induced apoptosis is necessary for corneal allograft survival. J Clin Invest. 1997;99:396–402. doi: 10.1172/JCI119173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 52.Tripathy S K, Black H B, Goldwasser E, Leiden J M. Immune response to transgene-encoded proteins limit the stability to gene expression after injection of replication-defective adenovirus vectors. Nat Med. 1996;2:545–555. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 53.Vilquin J T, Guerette B, Kinoshita I, Roy B, Goulet M, Gravel C, Roy R, Tremblay J P. FK506 immunosuppression to control the immune reactions triggered by first-generation adenovirus-mediated gene transfer. Hum Gene Ther. 1995;6:1391–1401. doi: 10.1089/hum.1995.6.11-1391. [DOI] [PubMed] [Google Scholar]
- 54.Walker P R, Saas P, Dietrich P Y. Role of Fas ligand (CD95L) in immune escape: the tumor cell strikes back. J Immunol. 1997;158:4521–4524. [PubMed] [Google Scholar]
- 55.Wang Q, Finer M H. Second-generation adenovirus vector. Nat Med. 1996;2:714–716. doi: 10.1038/nm0696-714. [DOI] [PubMed] [Google Scholar]
- 56.Wilson J M. Gene therapy for cystic fibrosis: challenges and future directions. J Clin Invest. 1995;96:2547–2554. doi: 10.1172/JCI118318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson J M. Adenoviruses as gene-delivery vehicles. N Engl J Med. 1996;334:1185–1187. doi: 10.1056/NEJM199605023341809. [DOI] [PubMed] [Google Scholar]
- 58.Wolff G, Worgall S, van Rooijen N, Song W R, Harvey B G, Crystal R G. Enhancement of in vivo adenovirus-mediated gene transfer and expression by prior depletion of tissue macrophages in the target organ. J Virol. 1997;71:624–629. doi: 10.1128/jvi.71.1.624-629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Worgall S, Wolff G, Falck-Pedersen E, Crystal R G. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum Gene Ther. 1997;8:437–444. doi: 10.1089/hum.1997.8.1-37. [DOI] [PubMed] [Google Scholar]
- 60.Xu X N, Screaton G R, Gotch F M, Dong T, Tan R, Almond N, Walker B, Stebbings R, Kent K, Nagata S, Stott J E, McMichael A J. Evasion of cytotoxic T lymphocyte (CTL) responses by Nef-dependent induction of Fas ligand (CD95L) expression on simian immunodeficiency virus-infected cells. J Exp Med. 1997;186:7–16. doi: 10.1084/jem.186.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang Y, Ertl H C, Wilson J M. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 62.Yang Y, Haecker S E, Su Q, Wilson J M. Immunology of gene therapy with adenoviral vectors in mouse skeletal muscle. Hum Mol Genet. 1996;5:1703–1712. doi: 10.1093/hmg/5.11.1703. [DOI] [PubMed] [Google Scholar]
- 63.Yang Y, Su Q, Wilson J M. Role of viral antigens in destructive cellular immune responses to adenovirus vector-transduced cells in mouse lungs. J Virol. 1996;70:7209–7212. doi: 10.1128/jvi.70.10.7209-7212.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zepeda M L, Wilson J M. Neonatal cotton rats do not exhibit destructive immune response to adenoviral vectors. Gene Ther. 1997;3:973–979. [PubMed] [Google Scholar]
- 65.Zhang H G, Blackburn W D, Jr, Minghetti P P. Characterization of a SV40-transformed rheumatoid synovial fibroblast cell line which retains genotypic expression patterns: a model for evaluation of anti-arthritic agents. In Vitro Cell Dev Biol Anim. 1997;33:37–41. doi: 10.1007/s11626-997-0020-7. [DOI] [PubMed] [Google Scholar]
- 66.Zhou T, Cheng J, Yang P, Wang Z, Liu C, Su X, Bluethmann H, Mountz J D. Inhibition of Nur77/Nurr1 leads to inefficient clonal deletion of self-reactive T cells. J Exp Med. 1996;183:1879–1892. doi: 10.1084/jem.183.4.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]