Abstract
Objectives:
Postprandial hyperglycemia is common in type 2 diabetes even in those with acceptable glycemic control and conveys an increased risk of cardiovascular morbidity and mortality. Although obstructive sleep apnea (OSA) has been associated with altered glucose metabolism, data regarding its association with postprandial hyperglycemia in type 2 diabetes are limited. Thus, the current study sought to characterize the association between OSA and postprandial hyperglycemia in adults with type 2 diabetes.
Methods:
A cross-sectional study of adults with type 2 diabetes was conducted. Home sleep testing was used to assess OSA severity as determined by the oxygen desaturation index (ODI). Self-monitoring of blood glucose (SMBG) was performed before and 2-hours after breakfast, lunch and dinner for three days. The association between OSA and glucose levels before and after each meal was examined using multivariable logistic regression.
Results:
The study sample consisted of 195 adults of which 52% were men. OSA severity, as assessed by by ODI quartiles, was associated with higher postprandial glucose values after dinner but not after breakfast or lunch. The adjusted odds ratios (95% confidence intervals) for a higher post-dinner glucose level for four ODI quartiles were 1.00 (Reference), 2.16 (0.96, 4.87), 2.23 (1.03, 4.83), and 2.58 (1.18, 5.94). Stratified analyses showed that this association was present in men but not women.
Conclusions:
Increasing OSA severity is associated with postprandial hyperglycemia in type 2 diabetes and may contribute to impaired glycemic control. Future studies examining the impact of OSA treatment on glucose metabolism should consider meal-related glycemic excursions as a potentially important outcome.
Keywords: diabetes, obstructive sleep apnea, postprandial glucose, hyperglycemia
1. INTRODUCTION
Obstructive sleep apnea is a treatable sleep disorder that is common in patients with type 2 diabetes mellitus1–3. Research over the last decade suggests that in type 2 diabetes, increasing OSA severity is associated with worsening glycemic control as determined by hemoglobin A1c (HbA1c)4–7. Randomized control trials, however, have not shown improvements in HbA1c with positive airway pressure (PAP) treatment in adults with type 2 diabetes and OSA8,9. While several limitations could explain the discordance between observational and interventional studies (e.g., poor adherence to PAP therapy), an important consideration in the available randomized trials is that glycemic status was characterized with measures such as HbA1c, fasting glucose, or fasting insulin. While these metrics have been important in establishing the potential association between OSA and altered glucose metabolism, they do not provide information on whether OSA influences the degree of meal-related excursions in glucose levels in type 2 diabetes.
There is substantial evidence demonstrating that a higher HbA1c is associated with increased risk of diabetes-associated complications10–12 and lowering HbA1c results in a significant risk reduction in both adverse microvascular and macrovascular outcomes. While HbA1c is valuable in assessing long-term glycemic control, assessment of meal-related excursions in glycemic patterns through self-monitoring blood glucose (SMBG) is of clinical relevance. Postprandial hyperglycemia hinders optimal glycemic control in type 2 diabetes and increases the risk for death from macrovascular disease, independent of HbA1c levels13–17. Thus, in adults with type 2 diabetes, characterizing the effects of OSA on postprandial hyperglycemia, may help define the value of assessing meal-related glucose excursions particularly for future studies investigating the impact of OSA treatment on glucose metabolism. Given that both type 2 diabetes and OSA are pervasive and independently associated with adverse cardiovascular outcomes, attaining a more comprehensive understanding of intraday meal-related excursions in glucose values in OSA and type 2 diabetes is of value. Thus, the objective of the current study were to characterize the association between OSA and postprandial hyperglycemia and identify factors that modify the association.
2. MATERIALS AND METHODS
2.1. Study Sample
Adults with type 2 diabetes, not requiring insulin therapy, were recruited from the general community through letters and advertisements. Eligibility criteria included a HbA1c value ≥ 6.5% and presence of OSA. Exclusion criteria included any ongoing therapy for OSA (e.g., PAP therapy, oral appliance), insulin therapy for type 2 diabetes, inability to complete the required assessments including the home sleep apnea test, participation in an ongoing weight loss program, change in glycemic medications in the previous six weeks, current oral steroid use, or any unstable medical condition, such as unstable angina or uncontrolled hypertension. Demographic information such as age, sex, race, body mass index (BMI), and total fat mass as determined by the DEXA scan was collected. The research protocol was approved by an Institutional Review Board on human research (IRB Approval Number: NA_00036672).
2.2. Home Sleep Apnea Test
The ApneaLink Plus®, a type III portable monitoring device, was given to all participants by trained research assistants for home sleep apnea testing18. Nasal airflow was recorded with a nasal cannula connected to a pressure transducer. Pulse oximetry was used to assess oxyhemoglobin saturation, and respiratory effort was measured with a pneumatic sensor attached to an effort belt. At least four hours of interpretable recording time was required for inclusion in the study. For the current analyses, the oxygen desaturation index (ODI) was chosen as a metric of OSA severity given the higher fidelity of the pulse oximetry signal compared to other respiratory signals such as the nasal cannula or the effort belt. Initially, the overnight recordings were subjected to automated scoring of the desaturation events using the 4% threshold. Thereafter, each recording was manually reviewed by a board certified sleep physician. The ODI was calculated as the number of oxygen desaturations of 4% or more over the total recording time in hours.
2.3. Self-Monitoring of Blood Glucose
SMBG was performed with finger sticks before and after breakfast, lunch, and dinner for three consecutive days in a majority of participants. A commercially available glucometer (Abbott FreeStyle®) was provided along with the glucose measuring strips. Glucose values were assessed before and approximately two hours after breakfast, lunch, and dinner, and at bedtime. Study participants were instructed not to consume snacks between meals. The associated Abbott FreeStyle® software was used to record the values from the 7-point SMBG and participants were asked to also record the values in a diary.
2.4. Statistical Analyses
The primary independent variable was the pre- and post-meal glucose value averaged over the three days and then categorized using quartiles to be modeled with ordinal logistic regression. Using quartiles to generate categories eases interpretation given that glucose values are typically interpreted based on specific thresholds. Thus, the regression coefficients from the ordinal logistic model represent the odds of being in a higher category of glucose values for a unit increase in the independent variable (e.g., ODI). To further ease exposition and enhance interpretability, ODI was also categorized using quartiles to quantify OSA severity. The ODI was categorized by the following quartile cut-points: <10.5 events/hr (reference), 10.5–14.8 events/hr, 14.9–23.3 events/hr, and ≥ 23.3 events/hr. Covariates such as age, sex, race, BMI, total body fat, and HbA1c were included in the multivariable models to adjust for potential confounding. Sensitivity analyses were also conducted using conventional cut-points for the ODI including < 5.0, 5.0–14.9, 15.0–29.9, and ≥ 30.0 events/hr. Results from these models were similar to that based on ODI quartiles and thus to avoid potentially biased results, ODI quartiles were used to asses a dose-response association. Models were stratified by sex given that the known sex-based differences in severity of OSA and type 2 diabetes. In these latter models, quartiles of OSA severity were determined separately for men and women. Sensitivity analyses with models with and without the number and type of hypoglycemic measures showed no material change in the regression coefficients and thus the most parsimonious models without the medications are shown. All analyses were done using STATA 15.0 (College Station, Texas).
3. RESULTS
The sample size was 195 adults with type 2 diabetes. Table 1 shows baseline data on demographics, anthropometrics, HbA1c, and OSA severity stratified by sex. Given that the target sample was adults with type 2 diabetes, it consisted of older and obese participants with over half of them being men. The mean HbA1c was 7.5% (SD: 0.92%), suggesting that glycemic control, on average, was fairly acceptable. HbA1c did not differ significantly between men and women. The average BMI and total fat were, however, higher in women compared to men. Additionally, while the mean ODI was statistically different between men and women (21.5 versus 17.6 events/hour; p < 0.01), the proportion of men and women in each quartile of ODI was not statistically not different.
Table 1.
Characteristics of the Sample
| Variable | All Subjects (N=195) | Men (N=103) | Women (N=92) | p-value |
|---|---|---|---|---|
| Age, years, mean (SD) | 60.0 (9.2) | 59.5 (9.6) | 60.5 (8.7) | 0.48 |
| BMI, kg/m2, mean (SD) | 33.7 (5.6) | 32.5 (5.0) | 35.1 (5.8) | < 0.001 |
| Total fat, grams, mean (SD) | 38,254 (11,535) | 34,848 (10,905) | 42,067 (11,070) | < 0.001 |
| Race,% | 0.12 | |||
| White | 57 | 63 | 49 | |
| African-American | 33 | 27 | 40 | |
| Other | 10 | 10 | 11 | |
| HbAlc%,mean(SD) | 7.50 (0.92) | 7.55 (0.95) | 7.44 (0.89) | 0.42 |
| ODI, mean (SD) | 19.7 (14.0) | 21.5 (15.0) | 17.6 (12.6) | 0.05 |
| ODI (events/hr) quartile, % | 0.07 | |||
| I (< 10.5 events/hr) | 25 | 23 | 27 | |
| II (10.5 – 14.8 events/hr) | 25 | 24 | 26 | |
| III (14.9 – 23.3 events/hr) | 25 | 21 | 31 | |
| IV (≥ 23.3 event/hr) | 25 | 32 | 16 | |
| Percent time with O2 < 90% | 18.4 (21.3) | 17.0 (18.8) | 19.9 (23.9) | 0.34 |
BMI: body mass index; HbA1c: hemoglobin A1c; ODI: oxygen desaturation index (events/hr); p-value comparing men and women.
The distribution of pre- and post-meal glucose levels across breakfast, lunch, and dinner are shown in Figure 1. Across the four ODI categories, pre or post-glucose values were comparable for three meals. However, the difference (Δ), defined as the post-pre meal glucose values, did vary as a function of ODI for dinner but not for breakfast or lunch (Table 2). The median difference (Δ) in glucose values with dinner was 26.0, 32.3, 44.3, and 44.6 mg/dl for the four ODI quartiles (p=0.038 for linear trend), respectively. Thus, compared to the first quartile (ODI < 10.5 events/hr), the median post-pre differences (Δ) with dinner were 5.4, 19.0, and 16.7 mg/dl higher in the 2nd, 3rd, and 4th ODI quartiles after adjusting for age, sex, race, and BMI indicating a greater postprandial glucose excursion with dinner with higher ODI levels.
Figure 1.
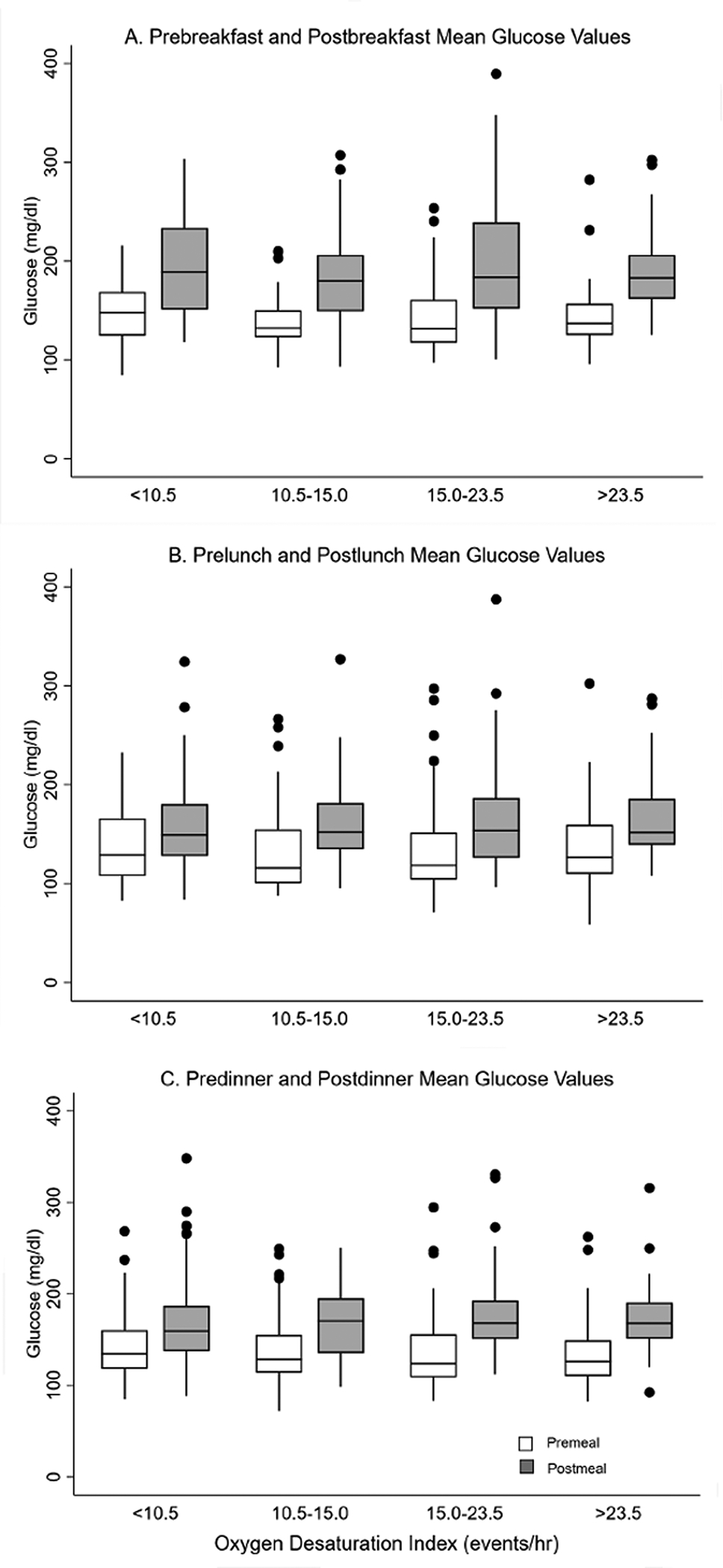
Unadjusted pre-meal (white) and post-meal glucose (grey) values by OSA severity
Table 2.
Median (25th–75th percentile) values of differences in (post–pre) glucose values.
| Oxygen desaturation index (ODI) quartile (events/hr) | |||||
|---|---|---|---|---|---|
| Meal | 1st (< 10.5) | 2nd (10.5–14.8) | 3rd (14.9–23.3) | 4th (> 23.3) | p-value* |
| Breakfast | 41.7 (19.0–70.0) | 41.0 (14.7–70.3) | 46.3 (21.7–85.7) | 41.8 (15.7–66.8) | 0.74 |
| Lunch | 30.0 (8.3–53.3) | 26.7 (−1.0–7.0) | 31.7 (9.0–62.3) | 25.2 (4.0–47.2) | 0.94 |
| Dinner | 26.0 (−2.0–53.3) | 32.3 (49.5–52.0) | 44.3 (16.3–52.7) | 44.6 (16.3–62.3) | 0.038 |
p-value for linear trend across ODI quartiles for each meal
Glucose values for each meal were subsequently categorized into quartiles for ease of interpretation and modeled with multivariable ordinal logistic regression. As before, the distribution of pre-meal glucose values for the three meals were comparable across ODI quartiles (Figure 1). Thus, all subsequent analyses focused on associations between OSA severity and post-meal glucose values. Table 3 shows the fully-adjusted odds ratios of being in higher category of glucose value for each of the three meals. No systematic trend was observed in post-meal values for breakfast or lunch as a function of ODI quartile. However, a higher ODI value was associated with higher post-dinner glucose value even with adjustments for pre-dinner glucose value. To account for potential confounders, other covariates including age, race, sex, BMI, percent of total body fat, and degree of glycemic control (HbA1c) were included in the multivariable model and resulted in minimal attenuation of the odds ratios relating ODI category to post-prandial hyperglycemia. Further adjustments for the number and types of diabetes medications showed no attenuation in the odds ratios and thus the most parsimonious model without medications is presented in Table 3. Sensitivity analyses including sex-based stratified analyses showed that the association between increasing OSA severity and higher postprandial glucose values with the dinner meal was notable in men but not women. For these analyses, quartiles of ODI were determined based on sex given the observed difference in ODI between men and women (Table 4). Finally, to examine whether degree of hypoxemia during sleep, as assessed by the absolute and percent time spent with an oxygen saturation less than 90% was also included in all multivariable models. These analyses showed that the frequency of oxygen desaturation (ODI) but not the percent or time below an oxygen saturation below 90% was independently associated post-dinner hyperglycemia.
Table 3.
Adjusted odds ratios (ORs) of being in a higher quartile of post-meal glucose by OSA severity
| Breakfast | Lunch | Dinner | |
|---|---|---|---|
| Model 1 | |||
| ODI quartiles (events/hr) | |||
| 1: < 10.5 | 1.00 | 1.00 | 1.00 |
| 2: 10.5–14.8 | 0.87 (0.41, 1.82) | 1.19 (0.58, 2.45) | 1.85 (0.86, 3.98) |
| 3: 14.9–23.3 | 1.06 (0.49, 2.30) | 1.07 (0.51, 2.27) | 1.89 (0.89, 3.99) |
| 4: > 23.3 | 1.03 (0.50, 2.16) | 1.07 (0.52, 2.24) | 2.15 (1.00, 4.60) |
| p-value for linear trend | 0.79 | 0..92 | 0.06 |
| Model 2 | |||
| ODI quartiles (events/hr) | |||
| 1: < 10.5 | 1.00 | 1.00 | 1.00 |
| 2: 10.5–14.8 | 0.93 (0.43, 2.03) | 1.39 (0.65, 2.98) | 2.16 (0.96, 4.87) |
| 3: 14.9–23.3 | 1.17 (0.53, 2.57) | 1.25 (0.58, 2.69) | 2.23 (1.03, 4.83) |
| 4: > 23.3 | 1.26 (0.57, 2.79) | 1.55 (0.69, 3.46) | 2.58 (1.18, 5.94) |
| p-value for linear trend | 0.45 | 0.36 | 0.03 |
Adjusted ORs (95% CI) of being in a higher quartile of post-meal glucose. Model 1 includes pre-meal glucose values as the only covariate. Model 2 includes pre-meal glucose values, age, sex, race, body mass index (BMI), total fat, and hemoglobin A1c (HbA1c) as covariates. Oxygen desaturation index: ODI (events/hr)
Table 4.
Adjusted odds ratios of being in a higher quartile of post-meal glucose by OSA severity
| Breakfast | Lunch | Dinner | |
|---|---|---|---|
| Men | |||
| ODI quartiles (events/hr) | |||
| 1: < 10.9 | 1.00 | 1.00 | 1.00 |
| 2: 10.9–16.1 | 0.97 (0.32, 2.93) | 1.27 (0.43, 3.73) | 5.69 (1.57, 20.56) |
| 3: 16.6–27.0 | 1.60 (0.53, 4.83) | 1.43 (0.48, 4.26) | 7.00 (2.00, 24.51) |
| 4: > 27.5 | 0.62 (0.19, 2.02) | 1.60 (0.51, 5.03) | 6.44 (1.73, 24.04) |
| p-value for linear trend | 0.68 | 0.40 | <0.01 |
| Women | |||
| ODI quartiles (events/hr) | |||
| I: < 10.4 | 1.00 | 1.00 | 1.00 |
| II: 10.4–14.2 | 0.83 (0.24, 2.86) | 1.79 (0.53, 6.04) | 0.77 (0.23, 2.59) |
| III: 14.4–18.4 | 1.85 (0.52, 6.61) | 0.91 (0.28, 2.98) | 0.94 (0.31, 2.89) |
| IV: > 18.5 | 1.11 (0.38, 3.90) | 1.92 (0.55, 6.69) | 1.62 (0.46, 5.70) |
| p-value for linear trend | 0.55 | 0.59 | 0.45 |
Adjusted ORs (95% CI) of being in a higher quartile of post-meal glucose. Model 1 includes pre-meal glucose values as the only covariate. Model 2 includes pre-meal glucose values, age, sex, race, BMI, total fat, and HbA1c as covariates.
4. CONCLUSIONS
The results of the current study demonstrate several findings that offer insight into the association between OSA and glucose metabolism in type 2 diabetes. First, despite adjustment for multiple covariates, increasing OSA severity was associated with higher differences in the post-pre glucose values with dinner but not with breakfast or lunch. Second, while the post-pre differences with dinner were higher, the increase was driven by a higher post-dinner glucose level. Third, the noted association between dinner-related hyperglycemia and OSA was present only in men. Finally, the frequency of oxygen desaturation on the home sleep apnea test rather than absolute or percent time below an oxygen saturation of 90% was associated with higher post-dinner glucose values.
The current study adds to the relatively limited body of literature suggesting that meal-related changes in glucose values are relevant in characterizing the association between OSA and glucose metabolism. Even in the absence of overt type 2 diabetes, OSA been noted to be associated with measures of glycemic variability19,20, including postprandial glucose values20. In those with type 2 diabetes, the data are scant and conflicting. Similar to the results of the current study, Khaire et al. found OSA to be associated with glycemic variability, as assessed by the mean amplitude of glycemic excursions, in adults with non-insulin requiring type 2 diabetes21. This finding, however, has not been consistent across other studies, as patients with type 2 diabetes and comorbid heart failure did not demonstrate an association between OSA severity and glycemic variability. In one of the earliest studies examining the effects of PAP therapy on glycemic control in obese patients with type 2 diabetes, postprandial glucose values on continuous glucose monitoring pre- and post-CPAP use in small sample of 24 subjects varied with PAP therapy22. Adherence with PAP (i.e., average use of > 4 hours per night over a period of three months), was associated with a reduction in postprandial glucose values after breakfast, lunch, and dinner. In particular, PAP treatment reduced the total number of glucose values above 200 mg/dl. In the current investigation with both overweight and obese adults, breakfast demonstrated the largest difference in pre- and post-breakfast glucose values in unadjusted analyses. However, in the adjusted model postprandial dinner values demonstrated the most robust association with increasing OSA severity. In the United States, dinner is typically the largest meal of the day23,24 and thus imposes the greatest glycemic load. Parallel to the notion that an exercise challenge during a cardiac stress test can unmask underlying ischemic heart disease that is not evident at rest, the dinner meal specifically represents a significant challenge to glucose homeostasis that allowed uncovering of an association between OSA and post-dinner hyperglycemia that was not apparent in the fasting state or with smaller meals. Moreover, it is important to recognize that because medications for glucose control were taken predominantly in the morning with or right after breakfast, the ensuing glucose-lowering effect would be most apparent with the lunch meal25,26. Thus, it is not surprising that post-pre meal differences (Table 2) were largest with breakfast (prior to any medication effect) and smallest with lunch. By dinner time, medication effect is likely to decrease, and the largest meal of the day is consumed. With the large metabolic challenge from dinner, the resulting impact of OSA on postprandial hyperglycemia became most apparent.
Analyses examining the association between OSA and post-prandial hyperglycemia were stratified by sex given the well-established differences in the clinical presentation and outcomes for both OSA27–29 and type 2 diabetes30–32 in men and women. Moreover, sex has been recognized as an important biological modifier of health-related outcomes related to metabolism33. In the current study, stratified analyses by sex demonstrated that the association between OSA and post-prandial glucose values was present in men, but not women, independent of OSA severity or glycemic control. The available literature describing sex-specific differences in the association of OSA and glucose metabolism is limited to study samples without type 2 diabetes and has yielded mixed results. In a community-based sample of 400 women without type 2 diabetes, a dose-dependent association was seen between AHI and both plasma glucose and serum insulin concentrations. Furthermore, insulin sensitivity was markedly lower in women with severe OSA compared to those without OSA (i.e., AHI ≥ 30 versus < 5 events/hour).34 A sex-based difference was also noted in the association of OSA and incident type 2 diabetes in a sleep clinic-based cohort, with OSA predicting the development of type 2 diabetes in women but not men35. In contrast, in a study of 145 young persons without type 2 diabetes, insulin secretion was higher in men than women36. The results herein demonstrate that there are sex-specific differences in glycemic measures in those with type 2 diabetes which vary as a function of OSA severity, with men demonstrating an independent association between OSA and higher post-dinner glucose values. The current investigation also showed that the frequency of oxygen desaturation rather than cumulative time below 90% oxygen saturation was associated with higher post-dinner hyperglycemia. A few previous studies examining the association between metrics of OSA and glycemic outcomes in persons with37,38 and without39,40 type 2 diabetes have demonstrated that time below an oxygen saturation of 90% is independently associated with greater impairments in glycemic measures. In contrast, there is evidence that across the various OSA metrics, the frequency of desaturation events is most robustly associated with worse glycemic outcomes6,41. Interestingly, findings from a study that adjusted for total body fat in addition to BMI and assessed incident type 2 diabetes in men with OSA suggested that total fat may possibly mediate the association between cumulative oxygen desaturation time and glycemic outcomes41. In the current study, total body fat was also considered and likewise showed that an association between oxygen desaturation frequency may more accurately capture the association between OSA-associated hypoxemia-reoxygenation cycling and glycemic measures in type 2 diabetes.
There are several strengths and limitations of the current study that merit discussion. Strengths include the almost equal distribution of men and women in the sample, the robust sample size, and adjustment for potential confounders such as total fat, BMI, degree of glycemic control, and pre-meal glucose levels. Limitations include the cross sectional analyses that precludes making inferences about causal effects between OSA and post-prandial hyperglycemia, the use of a type 3 portable sleep monitoring device, and the lack of adjustment for variation in meal patterns across weekdays versus the weekend. Nonetheless, the high correlation between AHI and ODI42 would suggest that any bias introduced by using a home sleep apnea test is likely nondifferential across ODI categories. Other limitations to consider are the lack of nutrition information that was not available and may account for some variability in post-prandial glucose values and the fact that subjects had relatively well controlled type 2 diabetes as evidenced by a mean HbA1c of 7.5%.
5. Conclusion
In summary, the results herein demonstrate an independent association between OSA and glucose metabolism in persons with type 2 diabetes that is more fully captured with the inclusion of post-meal glucose measurements. Future studies are needed to examine whether such associations also exist in those with type 1 diabetes and whether OSA therapy with CPAP can alter meal-related dynamic changes in glucose levels given the easy of which they can acquired.
Supplementary Material
HIGHLIGHTS:
Increasing obstructive sleep apnea (OSA) in type 2 diabetes is associated with postprandial hyperglycemia, a risk factor for adverse cardiac outcomes.
OSA severity is associated with higher postprandial glucose levels after dinner independent of age, total body fat, pre-meal glucose levels, and degree of glycemic control.
There are sex-specific differences in the association between OSA and postprandial hyperglycemia.
Funding:
RNA was funded by National Institutes of Health Grant HL118414. NMP was funded by National Institutes of Health Grants HL146709 and HL117167. The funding source had no role in the study design, collection of data, analysis or interpretation of the data, writing of the report, or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests: none
References
- 1.Einhorn D, Stewart DA, Erman MK, Gordon N, Philis-Tsimikas A, Casal E. Prevalence of sleep apnea in a population of adults with type 2 diabetes mellitus. Endocr Pract. 2007;13(4):355–362. [DOI] [PubMed] [Google Scholar]
- 2.Foster GD, Sanders MH, Millman R, et al. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009;32(6):1017–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reutrakul S, Mokhlesi B. Obstructive Sleep Apnea and Diabetes: A State of the Art Review. Chest. 2017;152(5):1070–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med. 2010;181(5):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kent BD, Grote L, Ryan S, et al. Diabetes mellitus prevalence and control in sleep-disordered breathing: the European Sleep Apnea Cohort (ESADA) study. Chest. 2014;146(4):982–990. [DOI] [PubMed] [Google Scholar]
- 6.Pillai A, Warren G, Gunathilake W, Idris I. Effects of sleep apnea severity on glycemic control in patients with type 2 diabetes prior to continuous positive airway pressure treatment. Diabetes Technol Ther. 2011;13(9):945–949. [DOI] [PubMed] [Google Scholar]
- 7.Priou P, Le Vaillant M, Meslier N, et al. Association between obstructive sleep apnea severity and glucose control in patients with untreated versus treated diabetes. J Sleep Res. 2015;24(4):425–431. [DOI] [PubMed] [Google Scholar]
- 8.Shaw JE, Punjabi NM, Naughton MT, et al. The Effect of Treatment of Obstructive Sleep Apnea on Glycemic Control in Type 2 Diabetes. Am J Respir Crit Care Med. 2016;194(4):486–492. [DOI] [PubMed] [Google Scholar]
- 9.West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax. 2007;62(11):969–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diabetes Control Complications Trial Research Group, Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. [DOI] [PubMed] [Google Scholar]
- 11.King P, Peacock I, Donnelly R. The UK prospective diabetes study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br J Clin Pharmacol. 1999;48(5):643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavalot F, Petrelli A, Traversa M, et al. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab. 2006;91(3):813–819. [DOI] [PubMed] [Google Scholar]
- 14.Gerich JE. Clinical significance, pathogenesis, and management of postprandial hyperglycemia. Arch Intern Med. 2003;163(11):1306–1316. [DOI] [PubMed] [Google Scholar]
- 15.Levitan EB, Song Y, Ford ES, Liu S. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med. 2004;164(19):2147–2155. [DOI] [PubMed] [Google Scholar]
- 16.Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003;26(3):881–885. [DOI] [PubMed] [Google Scholar]
- 17.Sorkin JD, Muller DC, Fleg JL, Andres R. The relation of fasting and 2-h postchallenge plasma glucose concentrations to mortality: data from the Baltimore Longitudinal Study of Aging with a critical review of the literature. Diabetes Care. 2005;28(11):2626–2632. [DOI] [PubMed] [Google Scholar]
- 18.Ng SS, Chan TO, To KW, et al. Validation of a portable recording device (ApneaLink) for identifying patients with suspected obstructive sleep apnoea syndrome. Intern Med J. 2009;39(11):757–762. [DOI] [PubMed] [Google Scholar]
- 19.Nakata K, Miki T, Tanno M, et al. Distinct impacts of sleep-disordered breathing on glycemic variability in patients with and without diabetes mellitus. PLoS One. 2017;12(12):e0188689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng CS, Cao YA, Tian YH, Zhang WL, Xia J, Yang L. Features of continuous glycemic profile and glycemic variability in patients with obstructive sleep apnea syndrome. Diabetes Res Clin Pract. 2017;134:106–112. [DOI] [PubMed] [Google Scholar]
- 21.Khaire SS, Gada JV, Utpat KV, Shah N, Varthakavi PK, Bhagwat NM. A study of glycemic variability in patients with type 2 diabetes mellitus with obstructive sleep apnea syndrome using a continuous glucose monitoring system. Clin Diabetes Endocrinol. 2020;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med. 2005;165(4):447–452. [DOI] [PubMed] [Google Scholar]
- 23.Kant AK, Graubard BI. 40-year trends in meal and snack eating behaviors of American adults. J Acad Nutr Diet. 2015;115(1):50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheer FA, Morris CJ, Shea SA. The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity (Silver Spring). 2013;21(3):421–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20(6):953–966. [DOI] [PubMed] [Google Scholar]
- 26.Sola D, Rossi L, Schianca GP, et al. Sulfonylureas and their use in clinical practice. Arch Med Sci. 2015;11(4):840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin CM, Davidson TM, Ancoli-Israel S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev. 2008;12(6):481–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161(5):1465–1472. [DOI] [PubMed] [Google Scholar]
- 29.Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164(4):406–418. [DOI] [PubMed] [Google Scholar]
- 30.Arnetz L, Ekberg NR, Alvarsson M. Sex differences in type 2 diabetes: focus on disease course and outcomes. Diabetes Metab Syndr Obes. 2014;7:409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kautzky-Willer A, Harreiter J, Pacini G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr Rev. 2016;37(3):278–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Legato MJ, Gelzer A, Goland R, et al. Gender-specific care of the patient with diabetes: review and recommendations. Gend Med. 2006;3(2):131–158. [DOI] [PubMed] [Google Scholar]
- 33.Reusch JEB, Kumar TR, Regensteiner JG, Zeitler PS, Conference P. Identifying the Critical Gaps in Research on Sex Differences in Metabolism Across the Life Span. Endocrinology. 2018;159(1):9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Theorell-Haglow J, Berne C, Janson C, Lindberg E. Obstructive sleep apnoea is associated with decreased insulin sensitivity in females. Eur Respir J. 2008;31(5):1054–1060. [DOI] [PubMed] [Google Scholar]
- 35.Celen YT, Hedner J, Carlson J, Peker Y. Impact of gender on incident diabetes mellitus in obstructive sleep apnea: a 16-year follow-up. J Clin Sleep Med. 2010;6(3):244–250. [PMC free article] [PubMed] [Google Scholar]
- 36.Temple KA, Leproult R, Morselli L, Ehrmann DA, Van Cauter E, Mokhlesi B. Sex Differences in the Impact of Obstructive Sleep Apnea on Glucose Metabolism. Front Endocrinol (Lausanne). 2018;9:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leong WB, Banerjee D, Nolen M, Adab P, Thomas GN, Taheri S. Hypoxemia and glycemic control in type 2 diabetes mellitus with extreme obesity. J Clin Endocrinol Metab. 2014;99(9):E1650–1654. [DOI] [PubMed] [Google Scholar]
- 38.Torrella M, Castells I, Gimenez-Perez G, et al. Intermittent hypoxia is an independent marker of poorer glycaemic control in patients with uncontrolled type 2 diabetes. Diabetes Metab. 2015;41(4):312–318. [DOI] [PubMed] [Google Scholar]
- 39.Appleton SL, Vakulin A, Wittert GA, et al. The association of obstructive sleep apnea (OSA) and nocturnal hypoxemia with the development of abnormal HbA1c in a population cohort of men without diabetes. Diabetes Res Clin Pract. 2016;114:151–159. [DOI] [PubMed] [Google Scholar]
- 40.Kendzerska T, Gershon AS, Hawker G, Tomlinson G, Leung RS. Obstructive sleep apnea and incident diabetes. A historical cohort study. Am J Respir Crit Care Med. 2014;190(2):218–225. [DOI] [PubMed] [Google Scholar]
- 41.Appleton SL, Vakulin A, McEvoy RD, et al. Nocturnal Hypoxemia and Severe Obstructive Sleep Apnea are Associated with Incident Type 2 Diabetes in a Population Cohort of Men. J Clin Sleep Med. 2015;11(6):609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dawson A, Loving RT, Gordon RM, et al. Type III home sleep testing versus pulse oximetry: is the respiratory disturbance index better than the oxygen desaturation index to predict the apnoea-hypopnoea index measured during laboratory polysomnography? BMJ Open. 2015;5(6):e007956. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


