Abstract
The nitric oxide (NO)–soluble guanylate cyclase (sGC)–cyclic guanosine monophosphate (cGMP) pathway plays a key role in the pathogenesis of pulmonary hypertension (PH). Targeted treatments include phosphodiesterase type 5 inhibitors (PDE5i) and sGC stimulators. The sGC stimulator riociguat is approved for the treatment of pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH). sGC stimulators have a dual mechanism of action, enhancing the sGC response to endogenous NO and directly stimulating sGC, independent of NO. This increase in cGMP production via a dual mechanism differs from PDE5i, which protects cGMP from degradation by PDE5, rather than increasing its production. sGC stimulators may therefore have the potential to increase cGMP levels under conditions of NO depletion that could limit the effectiveness of PDE5i. Such differences in mode of action between sGC stimulators and PDE5i could lead to differences in treatment efficacy between the classes. In addition to vascular effects, sGC stimulators have the potential to reduce inflammation, angiogenesis, fibrosis and right ventricular hypertrophy and remodelling. In this review we describe the evolution of treatments targeting the NO–sGC–cGMP pathway, with a focus on PH.
Shareable abstract
Soluble guanylate cyclase (sGC) stimulators may have advantages over phosphodiesterase-5 inhibitors for treating pulmonary arterial hypertension, arising from their different mode of action on the nitric oxide−sGC−cyclic guanosine monophosphate pathway. https://bit.ly/3vLODUW
Introduction
Pulmonary hypertension (PH) encompasses a group of conditions in which loss and obstruction of the pulmonary vascular bed leads to rises in pulmonary arterial pressure (PAP) and pulmonary vascular resistance (PVR), which can induce progressive right ventricular (RV) dysfunction, RV failure and death [1, 2]. PH is defined as a mean PAP (mPAP) >20 mmHg at rest, with precapillary PH defined as mPAP >20 mmHg, PVR >2 Wood units and pulmonary artery wedge pressure ≤15 mmHg [2]. PH can complicate most cardiovascular and respiratory diseases [2]. The clinical classification of PH defines five groups, as follows: pulmonary arterial hypertension (PAH; group 1), PH associated with left heart disease (PH-LHD; group 2), PH associated with lung diseases and/or hypoxia (PH-CLD; group 3), PH associated with pulmonary artery obstructions (group 4, which includes chronic thromboembolic PH (CTEPH)), and PH with unclear and/or multifactorial mechanisms (group 5) [2]. PH is estimated to affect 1% of the population worldwide and up to 10% of persons aged >65 years [3]. Groups 2 and 3 are the most common forms [3].
PAH and CTEPH are rare [3], but they have received intense attention in recent decades due to clinical trials resulting in effective therapies. Several classes of drug that target the pathogenesis of PAH have been developed and approved. These include drugs acting via the nitric oxide (NO)–soluble guanylate cyclase (sGC)–cyclic guanosine monophosphate (cGMP) pathway, namely phosphodiesterase type 5 inhibitors (PDE5i) and the sGC stimulator riociguat [2, 4–6], as well as prostanoids and endothelin receptor antagonists (ERAs). In CTEPH, attention has concentrated on surgical treatment by pulmonary endarterectomy (PEA), which is potentially curative, with low operative mortality, in eligible patients [7]. A more recent interventional treatment for inoperable CTEPH is balloon pulmonary angioplasty [7]. To date, only two targeted drugs have been approved for the treatment of CTEPH. Riociguat was the first and is indicated for patients who are inoperable or have persistent or recurrent CTEPH after PEA [2]. The prostanoid treprostinil has been granted a similar indication in Europe [8].
Several clinical risk scores have been developed to predict the prognosis of patients with PAH. Examples include scores derived from the French [9, 10], Swedish [11], Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA) [12, 13] and Registry to Evaluate Early and Long-term PAH Disease Management (REVEAL) registries [14–17]. The objective of treatment is to move patients to a lower risk score or category. Approximately 60% of patients remain at intermediate risk at 3 months to 2 years after initiation of monotherapy or combination therapy [18]. Using an expanded four-strata model, the intermediate risk category can be subdivided into intermediate–low and intermediate–high risk categories [12]. Notably, data from a post hoc analysis of the phase IV Riociguat rEplacing PDE5i therapy evaLuated Against Continued PDE5i thErapy (REPLACE) study showed that the overall benefit of switching from PDE5i to riociguat in patients with PAH at intermediate risk (see below) was maintained when patients were further subdivided into intermediate–low and intermediate–high risk groups [19]. This review describes the evolution of treatments targeting the NO–sGC–cGMP pathway from PDE5i to sGC stimulators and discusses the potential clinical advantages of sGC stimulators for some patients with PAH based on their distinct modes of action.
Clinical data for sGC stimulators in PH
PAH
Studies of switching from PDE5i to sGC stimulators
As the majority of patients receiving PAH-approved treatments, including PDE5i, do not achieve a low-risk profile [9, 11, 18], the most recent phase of the riociguat clinical development programme investigated the effects of switching from PDE5i to riociguat in patients with PAH with an insufficient treatment response [20, 21].
The REPLACE study, which used a prospective, randomised, open-label, blinded end-point design, investigated patients with PAH at intermediate risk, defined as a World Health Organization functional class (WHO FC) of III and a 6-min walk distance (6MWD) of 165–440 m despite stable treatment with tadalafil or sildenafil with or without background ERA. Patients switched to riociguat after a wash-out period (n=111) or continued PDE5i therapy (n=115) [21]. The composite primary end-point of REPLACE was clinical improvement at week 24, defined as absence of clinical worsening and at least two of the following three variables: 6MWD increase by ≥10% or ≥30 m from baseline; WHO FC I/II; or N-terminal prohormone of brain natriuretic peptide (NT-proBNP) level reduction of ≥30% from baseline. This composite clinical improvement end-point signifies a shift in assessed primary end-points and reflects patients' and physicians' objectives and expectations. Clinical improvement was achieved by 41% and 20% of patients in the riociguat and PDE5i groups, respectively (OR 2.78, 95% CI 1.53–5.06; p=0.0007) [21]. Improvements were also observed for secondary end-points at week 24, as follows: the mean treatment difference for 6MWD was 23 m (95% CI 5–40; p=0.054); a higher proportion of patients in the riociguat group showed an improved WHO FC compared with the PDE5i group (mean difference: −0.26, 95% CI −0.42–−0.11; p=0.0007); and the mean treatment difference for NT-proBNP levels was −170 pg·mL−1 (95% CI −426–87; p=0.11). Clinical worsening events occurred in one patient (1%) in the riociguat group and 10 patients (9%) in the PDE5i group (OR 0.10, 95% CI 0.01–0.73; p=0.0047). Safety results for riociguat were consistent with those previously observed in patients with PAH [22, 23] and CTEPH [24, 25]. In the riociguat group, adverse events (AEs) leading to study drug discontinuation were reported in 5% of patients (RV failure, upper abdominal pain, diarrhoea, fatigue, dizziness, headache, dyspnoea and hypotension (each in one patient), and exertional dyspnoea (in two patients)). In the PDE5i group, one patient (1%) discontinued due to an AE of drug therapy. AEs of special interest (all symptomatic hypotension) were reported in 5% and 2% of patients, respectively [21]. Data from REPLACE suggest a way for physicians and patients to optimise the NO–sGC–cGMP pathway by switching to riociguat, rather than moving immediately to triple combination therapy.
REPLACE built upon the exploratory, open-label, uncontrolled phase IIIb Riociguat clinical Effects Studied in Patients with Insufficient Treatment response to PDE5 inhibitors (RESPITE) study, in which patients with PAH and an insufficient response to tadalafil or sildenafil, with or without background ERA, were switched to riociguat after a wash-out period [20]. At week 24, 6MWD increased from baseline by +31±63 m (95% CI 13–49 m; p=0.0010) and there were improvements in other end-points, including NT-proBNP levels, WHO FC and haemodynamic parameters [20]. Riociguat was well tolerated after switching from PDE5i.
Other key studies
The randomised, double-blind, phase III Pulmonary Arterial Hypertension Soluble Guanylate Cyclase–Stimulator Trial 1 (PATENT-1) compared riociguat at a maximum dose of 2.5 mg three times daily with placebo in 443 patients with PAH [22]. Riociguat significantly improved 6MWD (the primary end-point) versus placebo. Improvements were also seen versus placebo in various secondary end-points, haemodynamic parameters [26, 27] and health-related quality of life (HRQoL) measures [22]. Riociguat was well tolerated, with 3% and 7% of patients in the riociguat 2.5 mg and placebo groups, respectively, discontinuing study drug due to AEs [22]. The improvements seen in 6MWD and WHO FC persisted for 2 years in the PATENT-2 long-term extension study and riociguat continued to show a favourable safety profile [23]. The uncontrolled, noninterventional EXPosurE Registry RiociguaT in patients with PH (EXPERT) registry of riociguat in clinical practice also reported that riociguat was well tolerated in patients with PAH [28].
The randomised, double-blind, placebo-controlled PATENT PLUS study investigated the effects of combined riociguat and sildenafil in 18 patients with PAH [29]. The primary end-point—maximum change in supine systolic blood pressure from baseline—was not met and there were high rates of discontinuation due to hypotension, serious AEs and deaths in the long-term extension study [29]. The concomitant use of riociguat and PDE5i is contraindicated [30, 31].
Recent reviews have described the clinical trials of riociguat in PAH and other indications [6, 32].
CTEPH
The randomised, double-blind, placebo-controlled, phase III Chronic Thromboembolic Pulmonary Hypertension Soluble Guanylate Cyclase–Stimulator Trial 1 (CHEST-1) assessed riociguat at a maximum dose of 2.5 mg three times daily versus placebo in 261 patients with inoperable CTEPH or persistent/recurrent PH after PEA [24]. Riociguat significantly improved 6MWD (the primary end-point), secondary end-points including haemodynamic parameters and HRQoL measures [24, 27, 33]. Riociguat was well tolerated with 3% of patients discontinuing study drug due to AEs, compared with 2% of patients in the placebo group [24]. Echocardiographic, haemodynamic and clinical data from CHEST-1 and the open-label, uncontrolled early access study (n=262), which enrolled patients with inoperable or persistent/recurrent CTEPH receiving riociguat, reported beneficial effects on RV structure and function [34–37]. Improvements in 6MWD and WHO FC in CHEST-1 persisted for 2 years in the long-term extension study, CHEST-2 (n=237), and treatment continued to be well tolerated [25]. Data from patients with CTEPH in EXPERT [38] and the early access study [37] also indicated good tolerability of riociguat in this setting.
Other types of PH
The randomised, double-blind, placebo-controlled, phase IIb DYNAMIC study compared riociguat with placebo in 114 patients with PH associated with heart failure with preserved ejection fraction (HFpEF). Riociguat was generally well tolerated and led to significant improvements in cardiac output at rest and in some haemodynamic parameters, although clinical symptoms were not significantly changed and dropout rates were higher with riociguat than with placebo [39].
The phase IIb LEPHT study of riociguat in patients with PH-LHD failed to meet its primary end-point of change in mPAP after 16 weeks, although PVR, cardiac index and stroke volume index were significantly improved [40]. The single-dose DILATE-1 study in PH-LHD showed no significant reduction in mPAP with riociguat versus placebo [41]. The safety of riociguat in these studies was similar to that observed in PAH or CTEPH [40, 41].
For the treatment of PH-CLD, inhaled therapies have potential advantages over oral drugs, including improved tolerability, reduction of systemic AEs and avoidance of ventilation/perfusion mismatch [42]. Inhaled NO has been shown to improve mPAP and PVR index in PH associated with COPD [43] and pulmonary fibrosis [44, 45]. However, NO donors are subject to tachyphylaxis and production of peroxynitrite [46]. Pilot studies of riociguat in PH associated with COPD [47] and PH associated with interstitial lung disease (PH-ILD) [48] suggested haemodynamic benefits and good tolerability. In a randomised, placebo-controlled, phase IIb trial in patients with idiopathic interstitial pneumonia (IIP)-associated PH (RISE-IIP); however, riociguat was associated with an increased risk of serious AEs and mortality versus placebo, leading to an unfavourable risk–benefit profile [49, 50].
Effect on RV function
As RV afterload is a key determinant of right heart failure (HF) in PAH and CTEPH [51], effective treatment would be expected to improve or preserve RV function. Echocardiographic measurements that can be used to evaluate RV function and size include RV ejection fraction, tricuspid annular plane systolic excursion (TAPSE), fractional area change and global longitudinal strain.
A post hoc analysis from PATENT-1 and CHEST-1 showed that riociguat improved measurements of RV function including stroke volume, cardiac efficiency, RV work and RV power [34]. Retrospective echocardiographic studies of PATENT-1, CHEST-1, their long-term extensions, the early access study and PATENT PLUS reported that riociguat was associated with reductions in RV area and right atrial area, reductions in RV wall thickness and improvements in TAPSE and tricuspid regurgitation velocity [35, 36]. A retrospective study using speckle-tracking echocardiography in 45 patients with PAH or CTEPH reported that riociguat induced reverse RV remodelling, with significant decreases in RV diameter, RV end-diastolic and -systolic area indices, RV global longitudinal strain, and increased fractional area change [52]. These observations suggest improvement of RV contractile function by riociguat regardless of RV loading [52]. The effects of riociguat on RV structure and function have recently been reviewed [53].
Mechanism of action of sGC stimulators and activators
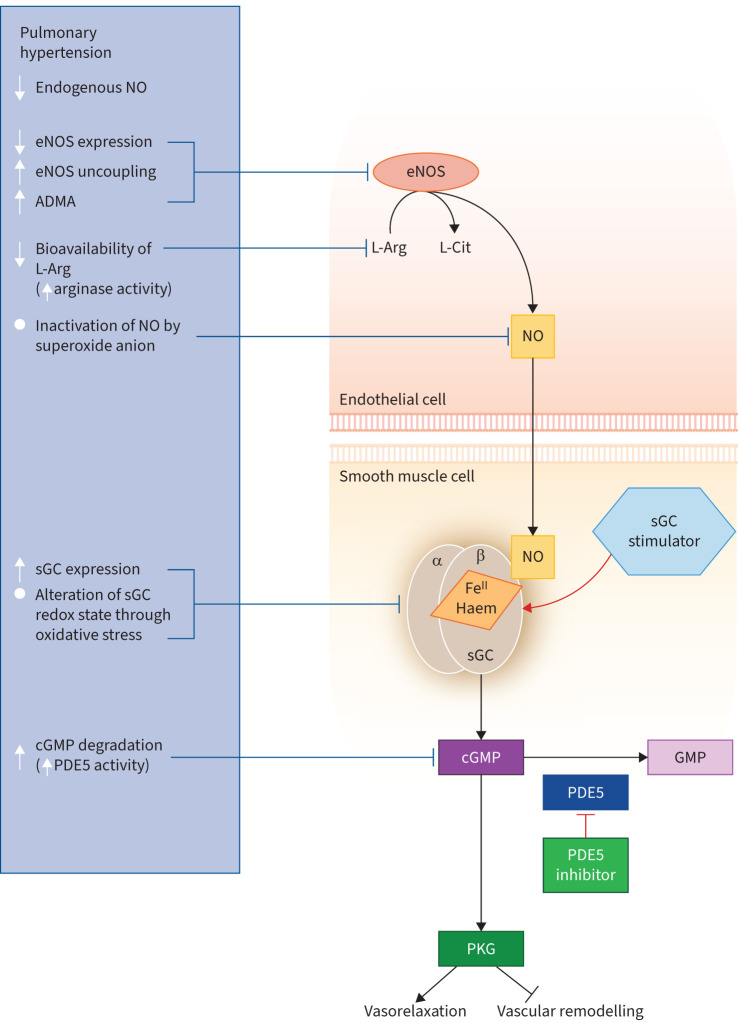
The NO–sGC–cGMP pathway is a key signalling pathway in cardiovascular, cardiopulmonary and cardiorenal regulation [46, 54, 55]. This pathway requires L-arginine, synthesised from L-citrulline by arginosuccinate synthase and arginosuccinate lyase [56]. L-Arginine is a substrate for NO synthases (NOS), which generate NO with the involvement of various cofactors including tetrahydrobiopterin and nicotinamide adenine dinucleotide phosphate [54]. sGC consists of an α- and a β-subunit with a NO-binding haem structure [46, 54]. NO diffuses through cell membranes and binds to cytosolic sGC, producing a conformational change in the enzyme and stimulating its catalytic site, which converts guanosine triphosphate to cGMP [46, 54, 55]. cGMP binds to and activates cGMP-activated protein kinases, cGMP-regulated ion channels and cGMP-regulated phosphodiesterases (PDEs) [46, 57, 58]. cGMP plays an important role in the regulation of vascular tone and maintains tissue homeostasis by various mechanisms including antifibrotic and anti-inflammatory effects [46, 55, 59]. The action of cGMP is terminated by two mechanisms: cleavage by PDEs, of which the most important is phosphodiesterase type 5 (PDE5); and extrusion by multidrug resistance proteins [60]. Dimethylarginine dimethylaminohydrolase metabolises asymmetric dimethylarginine (ADMA) and can increase bioavailable NO [61].
NO−sGC−cGMP signalling can be dysregulated in several ways in cardiovascular, cardiopulmonary and cardiorenal diseases including PAH and other forms of PH (figure 1) [46, 55, 62]. Oxidative stress alters the redox state of sGC, leading to formation of the oxidised and finally haem-free sGC (also called apo-sGC), which is nonesponsive to NO [46, 55, 62, 63]. Endothelial dysfunction impairs NO production, resulting in decreased NO bioavailability and reduced tissue cGMP levels [46, 55, 63]. Other factors that can impair the NO–sGC–cGMP pathway include reduction of L-arginine levels by arginase, reduced availability of L-arginine, downregulation of NOS in the vascular endothelium, inactivation of NO by superoxide anion, increased plasma concentrations of the endogenous NOS inhibitor ADMA, impaired sGC transcription and reduced stability of sGC mRNA [46, 55, 62].
FIGURE 1.
Steps in the nitric oxide (NO)–soluble guanylate cyclase (sGC)–cyclic guanosine monophosphate (cGMP) pathway that may be disrupted in pulmonary hypertension. ADMA: asymmetric dimethylarginine; eNOS: endothelial nitric oxide synthase; FeII: ferrous iron; GMP: guanosine monophosphate; L-Arg: L-arginine; L-Cit: L-citrulline; PDE5: phosphodiesterase type 5; PKG: protein kinase G. Reproduced from [63] with permission.
RV remodelling in patients with PH in response to increased PVR can become maladaptive because of increased long-term RV afterload resulting in RV eccentric hypertrophy, uncoupling and systolic and diastolic dysfunction [51, 64, 65]. Disruption of the NO–sGC–cGMP pathway is associated with numerous disorders that can contribute to the development of HF, including ventricular fibrosis, stiffening and hypertrophy [66]. sGC stimulators can act to increase cGMP levels and potentially improve these mechanisms [66].
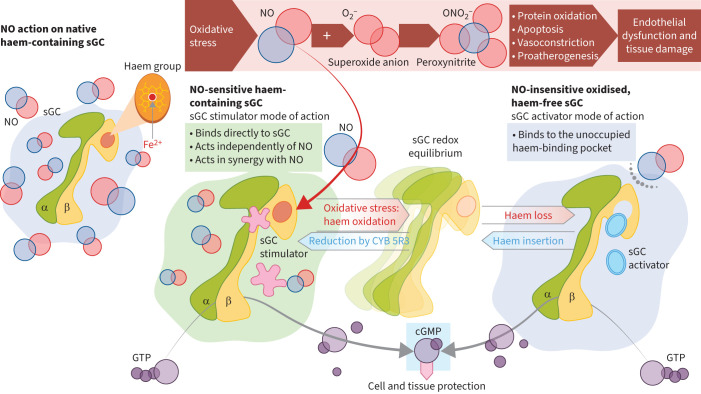
sGC stimulators have a dual mode of action (figure 2) [46, 54, 55, 67–69]. They bind to the haem-containing form of sGC, stabilising the enzyme in its active structural conformation, independent of NO binding [46, 54, 55]. In addition, they render sGC more responsive to endogenous NO by stabilising the NO–haem complex [46, 54, 55]. sGC stimulators can therefore increase cGMP production even when NO production is impaired or absent, in contrast to PDE5i (discussed in detail below), which inhibit cGMP degradation and therefore require sufficient endogenous cGMP production, which may be impaired in PH and other disorders [46, 54, 55].
FIGURE 2.
Mechanisms of action of soluble guanylate cyclase (sGC) stimulators and activators. α: α-subunit of sGC; β: β-subunit of sGC; cGMP: cyclic guanosine monophosphate; CYB 5R3: cytochrome B5 reductase 3; Fe2+: iron ion oxidised in +2 state; GTP: guanosine triphosphate; NO: nitric oxide; O2–: superoxide anion; ONO2–: peroxynitrite. Reproduced and modified from [46].
Oxidative stress associated with cardiopulmonary diseases could shift intracellular levels of native sGC toward oxidised and haem-free apo-sGC forms, which are unresponsive to NO, impairing the efficacy of NO and limiting the efficacy of PDE5i. sGC stimulators also require haem-containing sGC and have effects on apo-sGC only at very high concentrations not reached in clinical practice [46, 70]. Therefore, the efficacy of sGC stimulators could also be reduced by persisting oxidative stress. These observations triggered the search for and the development of sGC activators that, unlike sGC stimulators, are haem-independent and bind to the unoccupied haem-binding pocket of apo-sGC, enhancing cGMP production under conditions of oxidative stress (figure 2) [46, 55, 56, 71, 72]. sGC activators currently in clinical development include runcaciguat and mosliciguat, discussed below.
Clinical development of sGC stimulators involved the synthesis and testing of many compounds to increase potency and specificity, eliminate off-target effects and produce pharmacokinetic properties suitable for therapy [46, 55]. In addition to vascular effects in models of PH and other disorders, pre-clinical studies in various animal models (some with early compounds that did not reach clinical application), including cGMP measurements by radioimmunoassay, have shown that sGC stimulators reduce inflammation, angiogenesis, fibrosis and RV hypertrophy and remodelling [46, 54, 55, 59, 73–75]. In vitro, cGMP inhibits extracellular matrix formation, tissue growth factor β-induced collagen and fibronectin production and fibroblast-to-myoblast differentiation [59]. The experimental sGC stimulator BAY 41-2272 was reported to reduce myofibroblasts and perivascular collagen accumulation in a rat model of hypertensive cardiac disease [76] and to inhibit fibroblast-to-myofibroblast differentiation [77]. Riociguat also decreased skin fibrosis in various preclinical models for systemic sclerosis [78]. Besides antifibrotic efficacy, cGMP signalling is also involved in the control of vascular smooth muscle cell (VSMC) growth [79], and in vivo and in vitro studies have shown that BAY 41-2272 inhibits neointimal growth by reducing VSMC proliferation and migration [79]. In various animal models of RV hypertrophy, riociguat has been reported to reduce RV hypertrophy, collagen accumulation and pulmonary vascular remodelling, to improve or preserve RV function, and to reduce the expression of transforming growth factor β (TGF-β) and its effects on fibroblasts [80–82]. In a mouse model of RV hypertrophy induced by pulmonary artery banding, treatment with riociguat was associated with significantly increased RV ejection fraction, reduced RV end-systolic and -diastolic volumes, improvements in RV stroke volume and prevention of RV fibrosis compared with placebo [82]. In a mouse model of PH-LHD, riociguat was associated with reduced left ventricular (LV) collagen content and pulmonary vascular remodelling, although there was no significant effect on LV hypertrophy [83]. In some rodent models of pulmonary fibrosis and PH, riociguat was reported to have greater effects than sildenafil on pulmonary haemodynamics, RV structure and RV function, possibly because of their different mechanisms of action [81, 84, 85].
The mode of action of sGC stimulators is illustrated in the supplementary video.
Clinical data for PDE5i in PH
PAH
PDE5i are well established in the treatment of PAH, following clear evidence of efficacy and tolerability in clinical trials and extensive experience in routine practice.
The first PDE5i approved for the treatment of PAH was sildenafil, taken three times daily. This was followed by tadalafil, taken once daily. Vardenafil was given twice daily in the EVALUATION trial (see below) but is not currently licensed in PAH. Key trials of PDE5i in PAH include SERAPH [86], SUPER [87] and SUPER-2 [88] with sildenafil in adults, STARTS [89] and STARTS-2 [90] with sildenafil in paediatric PAH, PHIRST [91] and PHIRST-2 [92] with tadalafil in adults, EVALUATION [93] with vardenafil in adults, and AMBITION [94], which compared tadalafil with ambrisentan and the combination of both drugs in adults. These trials and some other studies of sildenafil are summarised in table 1. They have shown that PDE5i improve exercise capacity, haemodynamic parameters and functional capacity in diverse populations of patients with PAH. These benefits are maintained at long-term follow-up and treatment is generally well tolerated. AMBITION showed that initial combination therapy resulted in a significantly lower risk of clinical failure than either monotherapy [94]. Improvements in various end-points have been reported with the addition of sildenafil to epoprostenol [98], but the addition of sildenafil to bosentan has given inconsistent results [99, 100].
TABLE 1.
Key trials of phosphodiesterase type 5 inhibitors (PDE5i) in pulmonary arterial hypertension (PAH)
| Trial | Active treatment; comparator | Population | Duration | Primary end-point | Statistically significant secondary end-points |
| Sildenafil | |||||
| Randomised, double-blind trial [95] | 25−100 mg three times daily Placebo (crossover) |
Primary PH (n=22) | 6 weeks | Exercise time ↑ 44% (p<0.0001) at end of sildenafil phase versus end of placebo phase | CI ↑ (p<0.0001) Dyspnoea ↓ (p=0.009) Fatigue ↓ (p=0.04) on heart failure QoL score |
| SUPER [87] | 20 mg three times daily (n=69) 40 mg three times daily (n=68 randomised, 67 treated) 80 mg three times daily (n=71) Placebo (n=70) |
IPAH (n=175) PAH-CTD (n=84) PAH-CHD (n=18) |
12 weeks | 6MWD ↑ 13%, 13%, and 15% versus baseline with 20 mg, 40 mg and 80 mg, respectively (all p<0.001) | Compared with placebo: mPAP ↓ (p<0.05) with each dose PVR ↓ (p≤0.01) with each dose CI ↑ (p≤0.05) with 40 mg and 80 mg WHO FC (p≤0.003 for each dose) |
| SUPER-2 Open-label extension of SUPER [88] |
At 3 years (n=183) 20 mg three times daily (5%) 40 mg three times daily (8%) 80 mg three times daily (87%) |
IPAH/HPAH (63%) APAH (37%) |
Median 1242 days | 6MWD ↑ in 46% of patients, ↓ in 18% | WHO FC improved in 29% of patients, maintained in 31% |
| Low-dose study [96] | 1 mg three times daily (n=41) 5 mg three times daily (n=43) 20 mg three times daily (n=45) |
IPAH (74%) Other (26%) |
12 weeks | 6MWD ↑ 14 m, 41 m, and 38 m versus baseline with 1 mg, 5 mg and 20 mg, respectively (p=0.011 for 20 mg versus 1 mg) | PVR ↓ versus baseline with 20 mg BNP ↓ (p=0.005 for 20 mg versus 1 mg) Pro-BNP ↓ (p=0.009 for 20 mg versus 1 mg) |
| Severe PAH study [97] | 25−100 mg three times daily Placebo (crossover) |
IPAH (n=10) Eisenmenger syndrome (n=10) |
6 weeks | 6MWD ↑ versus baseline (p<0.0001) | PAP ↓ versus baseline NYHA class improved versus baseline Exercise duration ↑ versus baseline Mets ↑ versus baseline (All p=0.0001) |
| STARTS-1# [89] | Low dose (n=42) Medium dose (n=55) High dose (n=77) Placebo (n=60) |
Paediatric PAH (n=235) | 16 weeks | Peak oxygen consumption for three doses combined ↑8% versus placebo (p=0.056) | PVRI ↓ versus placebo with high dose (p<0.001) mPAP ↓ versus placebo with high dose (p=0.006) CI ↑ versus placebo with high dose (p=0.017) |
| STARTS-2: open-label extension of STARTS-1 [90] | Received therapy for >3 years (n=166) | Paediatric PAH | Median 4.1 years | 3-year survival: Low dose 93% Medium dose 91% High dose 87% Placebo 96% |
NA |
| SERAPH [86] | 50 mg twice daily then 50 mg three times daily (n=14) or bosentan 62.5 mg twice daily then 125 mg twice daily (n=12) | IPAH (n=23) PAH-CTD (n=3) |
16 weeks | Sildenafil group: RV mass ↓ versus baseline (p=0.015) | Sildenafil group versus baseline: 6MWD ↑ (p<0.01) CI ↑ (p<0.01) QoL improved (p<0.01) BNP ↓ (p<0.05) |
| Tadalafil | |||||
| PHIRST [91] | 2.5 mg·day−1 (n=82) 10 mg·day−1 (n=80) 20 mg·day−1 (n=82) 40 mg·day−1 (n=79) Placebo (n=82) |
IPAH/HPAH (n=247) Anorexigen use (n=16) PAH-CTD (n=95) ASD (n=32) Repair of VSD or PDA (n=15) |
16 weeks | Placebo-corrected 6MWD ↑ versus baseline with 40 mg (p<0.01) | Tadalafil 40 mg versus baseline: Time to clinical worsening improved (p=0.041) Incidence of clinical worsening ↓ (p=0.038) QoL improved (p<0.02) mPAP ↓ (p=0.01) PVR ↓ (p=0.039) CI ↑ (p=0.028) |
| PHIRST-2: open-label extension of PHIRST [92] | 20 mg·day−1 (n=63) 40 mg·day−1 (n=294) |
IPAH/HPAH (n=223) Anorexigen use (n=15) PAH-CTD (n=78) ASD (n=29) Repair of VSD or PDA (n=12) |
52 weeks | Improvements in 6MWD with 20 mg or 40 mg in PHIRST maintained | Overall survival 97% at week 68 Clinical worsening 27% in 20 mg group, 22% in 40 mg group |
| AMBITION [94] | Tadalafil 40 mg·day−1 plus placebo (n=121) Ambrisentan 10 mg·day−1 plus placebo (n=126) Tadalafil 40 mg·day−1 plus ambrisentan 10 mg·day−1 (n=253) |
IPAH (n=265) HPAH (n=14) PAH-CTD (n=187) PAH-CHD (n=9) PAH-HIV (n=9) Drug/toxin exposure (n=16) |
Event-driven: mean duration of study drug use 517 days | First event of clinical failure: 18% of patients with combination; 34% with ambrisentan; 28% with tadalafil (p=0.005 for combination versus tadalafil) |
6MWD ↑ versus baseline greater with combination than tadalafil (p=0.003) or ambrisentan (p<0.001) NT-proBNP ↓ versus baseline greater with combination than tadalafil (p<0.001) or ambrisentan (p<0.01) Satisfactory clinical response rate greater with combination with tadalafil (p=0.03) |
| Vardenafil | |||||
| EVALUATION [93] | Vardenafil 5 mg·day−1 then 5 mg twice daily (n=44) Placebo (n=22) |
IPAH (n=39) PAH-CTD (n=19) Repaired left-to-right shunt (n=6) |
12 weeks | Placebo-corrected 6MWD ↑ versus baseline (p<0.001) | mPAP ↓ (p=0.047) PVR ↓ (p=0.003) CI ↑ (p=0.005) WHO FC improved (p=0.032) Borg dyspnoea index improved (p=0.046) Clinical worsening ↓ (p=0.044) |
6MWD: 6-min walk distance; AMBITION: Ambrisentan and Tadalafil in Patients with Pulmonary Arterial Hypertension; APAH: associated PAH; ASD: atrial septal defect; BNP: brain natriuretic peptide; CI: cardiac index; EVALUATION: Efficacy and Safety of Vardenafil in the Treatment of Pulmonary Arterial Hypertension; FC: functional class; HPAH: heritable PAH; IPAH: idiopathic PAH; LV: left ventricular; Mets: metabolic equivalent unit; mPAP: mean pulmonary arterial pressure; NA: not applicable; NT-proBNP: N-terminal prohormone of brain natriuretic peptide; NYHA: New York Heart Association; PAH-CHD: PAH associated with congenital heart disease; PAH-CTD: PAH associated with connective tissue disease; PAH-HIV: PAH associated with HIV; PAP: pulmonary arterial pressure; PDA: patent ductus arteriosus; PH: pulmonary hypertension; PHIRST: Pulmonary Arterial Hypertension and Response to Tadalafil; pro-BNP: prohormone of brain natriuretic peptide; PVR: pulmonary vascular resistance; PVRI: pulmonary vascular resistance index; QoL: quality of life; RV: right ventricular; SERAPH: Sildenafil versus Endothelin Receptor Antagonist for Pulmonary Hypertension; STARTS: Sildenafil in Treatment-Naïve Children, Aged 1−17 Years, With Pulmonary Arterial Hypertension; SUPER: Sildenafil Use in Pulmonary Arterial Hypertension; VSD: ventricular septal defect; WHO: World Health Organization. #: Dose selected according to body weight.
A systematic review of sildenafil, tadalafil or vardenafil monotherapy studies showed that patients with PAH were more likely to improve their WHO FC versus placebo, to have an improvement in 6MWD of ≥48 m and to have improved survival [101]. AEs of PDE5i include headache, gastrointestinal disorders, flushing, muscle aches, joint pains, bronchitis, anaemia, abnormal hepatic function and upper respiratory tract infection [101, 102].
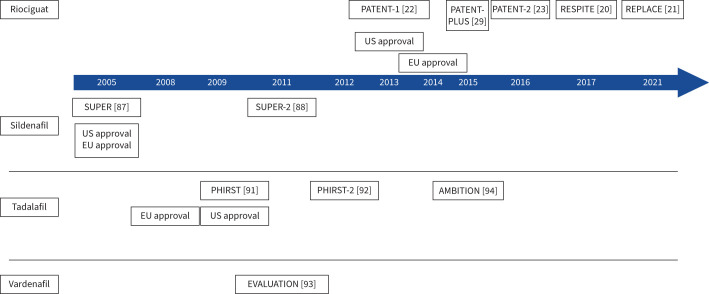
Figure 3 summarises the major clinical trials and approvals of riociguat and PDE5i in the treatment of PAH [20–23, 29, 87, 88, 91–94].
FIGURE 3.
Major clinical trials of riociguat and phosphodiesterase type 5 inhibitors in the treatment of pulmonary arterial hypertension. Dates of approval in the United States of America (US) and European Union (EU) are also shown. AMBITION: Ambrisentan and Tadalafil in Patients with Pulmonary Arterial Hypertension; EVALUATION: Efficacy and Safety of Vardenafil in the Treatment of Pulmonary Arterial Hypertension; PATENT-1: Pulmonary Arterial Hypertension Soluble Guanylate Cyclase–Stimulator Trial 1; PATENT-2: Pulmonary Arterial Hypertension Soluble Guanylate Cyclase–Stimulator Trial 2; PHIRST: Pulmonary Arterial Hypertension and Response to Tadalafil; PHIRST-2: Pulmonary Arterial Hypertension and Response to Tadalafil-2; REPLACE: Riociguat rEplacing PDE5i therapy evaLuated Against Continued PDE5i thErapy; RESPITE: Riociguat clinical Effects Studied in Patients with Insufficient Treatment response to PDE5 inhibitors; SUPER: Sildenafil Use in Pulmonary Arterial Hypertension; SUPER-2: Sildenafil Use in Pulmonary Arterial Hypertension-2.
CTEPH
Open-label studies reported improvements in haemodynamics and functional capacity with sildenafil in inoperable CTEPH [103, 104]. In a randomised, double-blind, placebo-controlled pilot study of sildenafil, significant improvements in WHO FC and PVR were reported after 12 weeks; however, there was no significant improvement in the primary end-point of 6MWD at 12 weeks [105]. No larger trials of sildenafil in CTEPH have since been carried out and no trials of tadalafil or vardenafil in this indication have been reported. No PDE5i are approved for use in patients with CTEPH [106], although registry data indicate that they are commonly prescribed [107].
Other types of PH
In the randomised, placebo-controlled SIOVAC trial in patients with residual PH due to LHD (valvular heart disease), patients who received sildenafil were less likely to improve and more likely to experience clinical worsening than those receiving placebo [108]. Small studies of sildenafil in patients with PH associated with HFpEF showed improvements in haemodynamics or RV function [109–111], but another trial did not confirm these observations [112].
Trials of PDE5i in PH associated with COPD have shown inconsistent effects on haemodynamics and exercise capacity [113–115].
Mechanism of action of PDE5i
PDE5i, including sildenafil, tadalafil, vardenafil, udenafil, mirodenafil and lodenafil, are competitive blockers of substrate binding at the catalytic site of PDE5; they increase cGMP levels by inhibiting the hydrolysis of cGMP (figure 1) [46, 54, 55, 60, 116]. Their action therefore depends on the production of endogenous cGMP and their effectiveness may be compromised in patients with a defective NO–sGC–cGMP pathway [117]. There are 11 major classes of PDE [116]. PDE5, PDE6 and PDE9 are selective for cGMP, PDE4, PDE7 and PDE8 are selective for cyclic adenosine monophosphate, and PDE1, PDE2, PDE3, PDE10 and PDE11 have mixed specificity [116]. PDE5 is widely distributed in tissues and is therefore a major target for pharmacological intervention [116]. PDE5i efficacy may be compromised by other cGMP-metabolising PDEs compensating when PDE5i are used and in tissues in which PDE5 is not the major route of cGMP metabolism [55]. These mechanisms may explain why a substantial proportion of patients with erectile dysfunction (ED) do not sufficiently respond to PDE5i. Tachyphylaxis has also been suggested as a reason for the failure of PDE5i in some patients with ED [118], but the relevance of this observation to PH is unclear. PDE5 exists in multiple isoforms, but no currently marketed PDE5i is selective for a specific isoform [60].
In bleomycin-induced PH in rats, sildenafil improved RV systolic pressure and pulmonary acceleration time/ejection time ratio [84]. In rats with PH induced by the vascular endothelial growth factor receptor antagonist SU5416 and hypoxia, or by transverse aortic constriction [81, 83], and in mice with pressure overload RV hypertrophy induced by pulmonary artery banding [82], sildenafil preserved RV structure and function. The antifibrotic effects of sildenafil appear to be mediated through reduction of DNA synthesis, increases in cGMP levels and inhibition of TGF-β, similar to those of riociguat [59, 81, 82, 119].
Current status of sGC stimulators and PDE5i in PH
Guidelines for the management of PH, published in 2022, recommend initial oral combination therapy with an ERA and a PDE5i for the treatment of patients with PAH at low or intermediate risk, and initial therapy with an ERA, a PDE5i and a prostanoid for patients at high risk [2]. Initial monotherapy is recommended only for patients in specific subgroups, such as elderly patients and those with cardiopulmonary comorbidities [2]. Subsequent management depends on the risk status attained with initial therapy [2]. In patients who achieve a low-risk status with their initial PAH therapy, continuation of treatment is recommended. In patients who are at intermediate–low risk despite ERA/PDE5i therapy, adding selexipag should be considered to reduce the risk of clinical worsening. Switching from PDE5i to riociguat may also be considered in these patients. In patients who are at intermediate–high or high risk while receiving oral therapies, the addition of intravenous (i.v.) epoprostenol or i.v./subcutaneous (s.c.) treprostinil and referral for lung transplantation evaluation should be considered [2, 120, 121]. If adding i.v./s.c. prostacyclin analogues is unfeasible, adding selexipag or switching from PDE5i to riociguat may be considered.
Riociguat is also recommended for the treatment of inoperable CTEPH, as evaluated by a CTEPH team including at least one experienced PEA surgeon, and in persistent/recurrent CTEPH after surgical treatment, whereas PDE5i are not recommended for the treatment of CTEPH [2, 7]. Neither riociguat nor PDE5i are indicated for the treatment of PH groups 2, 3 or 5 [2, 30, 31, 122–125]. Riociguat is contraindicated in PH-IIP, following the unfavourable results mentioned above [49, 50].
No specific dose adjustments are needed for riociguat when combined with ERAs or prostanoids [30, 31]. Bosentan is a moderate inducer of CYP3A4, CYP2C9 and possibly of CYP2C19, and has been reported to reduce exposure to sildenafil [122, 123, 126] and tadalafil [124, 125], which are cleared predominantly by CYP3A4. PDE5i are generally effective and well tolerated in patients with PAH, the once-daily dosing of tadalafil is convenient and generic formulations of PDE5i are widely available.
In recent studies, 47−58% of patients with PAH were reported to be receiving PDE5i, whereas only 6% were receiving riociguat [127, 128].
sGC activators
The sGC activator cinaciguat demonstrated improvements in haemodynamics and dyspnoea scores in acute decompensated HF after continuous infusion [129], but further clinical development was terminated early because of an unfavourable risk–benefit profile with an excess of hypotensive events [72]. Cinaciguat has been shown to have antihypertrophic effects in vitro, with significant reductions in neonatal rat cardiomyocyte size and protein synthesis [130]. Preclinical studies demonstrated that the sGC activator runcaciguat reduced proteinuria and markers of kidney damage in rat models of chronic kidney disease. Runcaciguat is in clinical development for the oral treatment of patients with chronic kidney disease and nonproliferative diabetic retinopathy [131, 132].
A novel sGC activator, mosliciguat (BAY 1237592), has been specifically designed for local inhaled application in the lung [46]. Studies of inhaled mosliciguat in animal models of PH demonstrated reduced pulmonary artery pressure without reduced systemic artery pressure or ventilation/perfusion mismatch over a broad dose range with a long duration of action. Mosliciguat also showed bronchodilatory effects in an acetylcholine-induced rat model of PH [133].
Future research/remaining questions
As our understanding of the NO–sGC–cGMP pathway has evolved, different drugs targeting this pathway, from PDE5i to sGC-modulating drugs, have been developed. These led to treatment options that may provide improved outcomes for some patients as suggested by the REPLACE trial (see above) [21]. Extensive additional research is required to provide a clearer understanding of the differences between PDE5i and sGC stimulators, how they may relate to clinical outcomes, and for which patients each class of agent is most appropriate [46]. Further investigations of the multiple effects observed in various organ systems and tissues from pre-clinical studies with sGC stimulators or activators is needed, as not all preclinical observations may translate into clinical benefits.
New modes of delivery for PAH-targeted drugs, such as inhaled or nanoparticle-based formulations, are under investigation. An inhaled formulation of vardenafil (RT-234) is in clinical development. A first-in-human study in healthy volunteers demonstrated RT-234 to be rapidly absorbed after inhalation, with dose-proportional systemic exposure, and is generally safe and well tolerated [134]. A phase IIb trial (Vardenafil Inhaled for Pulmonary Arterial Hypertension PRN Phase 2B (VIPAH-PRN 2B); NCT04266197) is investigating the effects of inhaled RT-234 on exercise parameters in patients with PAH [135]. RT-234 uses the AOPTM dry powder inhaler, which patients with PAH are able to use [136]. An inhaled sGC stimulator, MK-5475, reduced PVR without effects on systemic blood pressure or heart rate in a single-dose, phase I study in patients with PAH [137]. A phase II/III study (Phase 2/3 Study of an Inhaled sGC Stimulator in PAH (INSIGNIA-PAH); NCT04732221) of inhaled MK-5475 in patients with PAH is in progress [138].
The impact of mosliciguat on PVR is being evaluated in patients with PAH or CTEPH in a phase I study (ATMOS; NCT03754660) [139]. Whole-genome sequencing and genome-wide association studies have revealed that NO–sGC–cGMP signalling is impaired in various cardiovascular diseases. Reversing this impairment using sGC stimulators or sGC activators may provide a targeted treatment [140–142]. With the identification of genetic variants that modulate NO signalling and cGMP production, we are encouraged to make rational choices about optimal therapeutic strategies.
Questions for future research
Further understanding of the multiple effects observed in various organ systems and tissues from preclinical studies with sGC stimulators or activators.
Potential for new modes of delivery such as inhaled or nanoparticle-based formulations.
Clarification of the therapeutic potential of sGC activators in different diseases.
Role of personalised therapy in cardiovascular diseases based on genetic variants that modulate NO signalling and cGMP production.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary video ERR-0183-2023.SUPPLEMENT (54.6MB, mp4)
Acknowledgements
Medical writing services were provided by Richard Murphy of Adelphi Communications Ltd (Macclesfield, UK) funded by Bayer AG (Berlin, Germany) in accordance with Good Publications Practice (GPP3).
Provenance: Submitted article, peer reviewed.
Conflict of interest: R.L. Benza reports receiving grants from Actelion, Bayer AG, Bellerophon Therapeutics and Eiger Biopharmaceuticals. E. Grünig reports fees for lectures and/or consultations from Actelion, Bayer AG, GlaxoSmithKline, Merck Sharp & Dohme Corp., Pfizer and United Therapeutics outside the submitted work. P. Sandner and J.-P. Stasch are employees of Bayer AG, Wuppertal, Germany. G. Simonneau reports personal fees and nonfinancial support from Actelion, Bayer AG and Merck Sharp & Dohme outside the submitted work.
Support statement: Medical writing services provided by Adelphi Communications Ltd were funded by Bayer AG. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Humbert M, Guignabert C, Bonnet S, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J 2019; 53: 1801887. doi: 10.1183/13993003.01887-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2022; 30: 2200879. doi: 10.1183/13993003.00879-2022 [DOI] [PubMed] [Google Scholar]
- 3.Hoeper MM, Humbert M, Souza R, et al. A global view of pulmonary hypertension. Lancet Respir Med 2016; 4: 306–322. doi: 10.1016/S2213-2600(15)00543-3 [DOI] [PubMed] [Google Scholar]
- 4.Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019; 53: 1801889. doi: 10.1183/13993003.01889-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Humbert M, Ghofrani HA. The molecular targets of approved treatments for pulmonary arterial hypertension. Thorax 2016; 71: 73–83. doi: 10.1136/thoraxjnl-2015-207170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sommer N, Ghofrani HA, Pak O, et al. Current and future treatments of pulmonary arterial hypertension. Br J Pharmacol 2021; 178: 6–30. doi: 10.1111/bph.15016 [DOI] [PubMed] [Google Scholar]
- 7.Kim NH, Delcroix M, Jais X, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J 2019; 53: 1801915. doi: 10.1183/13993003.01915-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.SciPharm Sàrl . Trepulmix: EPAR – Product Information. Date last accessed: 20 April 2022. Date last updated: 23 January 2022. www.ema.europa.eu/en/documents/product-information/trepulmix-epar-product-information_en.pdf
- 9.Boucly A, Weatherald J, Savale L, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J 2017; 50: 1700889. doi: 10.1183/13993003.00889-2017 [DOI] [PubMed] [Google Scholar]
- 10.Boucly A, Weatherald J, Savale L, et al. External validation of a refined four-stratum risk assessment score from the French pulmonary hypertension registry. Eur Respir J 2022; 59: 2102419. doi: 10.1183/13993003.02419-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kylhammar D, Kjellstrom B, Hjalmarsson C, et al. A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur Heart J 2018; 39: 4175–4181. doi: 10.1093/eurheartj/ehx257 [DOI] [PubMed] [Google Scholar]
- 12.Hoeper MM, Pausch C, Olsson KM, et al. COMPERA 2.0: a refined 4-strata risk assessment model for pulmonary arterial hypertension. Eur Respir J 2021; 60: 2102311. doi: 10.1183/13993003.02311-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoeper MM, Pittrow D, Opitz C, et al. Risk assessment in pulmonary arterial hypertension. Eur Respir J 2018; 51: 1702606. doi: 10.1183/13993003.02606-2017 [DOI] [PubMed] [Google Scholar]
- 14.Benza RL, Gomberg-Maitland M, Elliott CG, et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest 2019; 156: 323–337. doi: 10.1016/j.chest.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 15.Benza RL, Gomberg-Maitland M, Miller DP, et al. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest 2012; 141: 354–362. doi: 10.1378/chest.11-0676 [DOI] [PubMed] [Google Scholar]
- 16.Benza RL, Kanwar MK, Raina A, et al. Development and validation of an abridged version of the REVEAL 2.0 risk score calculator, REVEAL Lite 2, for use in patients with pulmonary arterial hypertension. Chest 2021; 159: 337–346. doi: 10.1016/j.chest.2020.08.2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010; 122: 164–172. doi: 10.1161/CIRCULATIONAHA.109.898122 [DOI] [PubMed] [Google Scholar]
- 18.Hoeper MM, Kramer T, Pan Z, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J 2017; 50: 1700740. doi: 10.1183/13993003.00740-2017 [DOI] [PubMed] [Google Scholar]
- 19.Benza RL, Simonneau G, Ghofrani H, et al. Analysis of the COMPERA 2.0 risk assessment tool in the REPLACE study. Pulm Circ 2022; 12: e12153. doi: 10.1002/pul2.12153 [DOI] [Google Scholar]
- 20.Hoeper MM, Simonneau G, Corris P, et al. RESPITE: switching to riociguat in PAH patients with inadequate response to PDE5i. Eur Respir J 2017; 50: 1602425. doi: 10.1183/13993003.02425-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoeper MM, Al-Hiti H, Benza RL, et al. Switching to riociguat versus maintenance therapy with phosphodiesterase-5 inhibitors in patients with pulmonary arterial hypertension (REPLACE): a multicentre, open-label, randomised controlled trial. Lancet Respir Med 2021; 9: 573–584. doi: 10.1016/S2213-2600(20)30532-4 [DOI] [PubMed] [Google Scholar]
- 22.Ghofrani HA, Galiè N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 2013; 369: 330–340. doi: 10.1056/NEJMoa1209655 [DOI] [PubMed] [Google Scholar]
- 23.Ghofrani HA, Grimminger F, Grünig E, et al. Predictors of long-term outcomes in patients treated with riociguat for pulmonary arterial hypertension: data from the PATENT-2 open-label, randomised, long-term extension trial. Lancet Respir Med 2016; 4: 361–371. doi: 10.1016/S2213-2600(16)30019-4 [DOI] [PubMed] [Google Scholar]
- 24.Ghofrani HA, D'Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013; 369: 319–329. doi: 10.1056/NEJMoa1209657 [DOI] [PubMed] [Google Scholar]
- 25.Simonneau G, D'Armini AM, Ghofrani HA, et al. Predictors of long-term outcomes in patients treated with riociguat for chronic thromboembolic pulmonary hypertension: data from the CHEST-2 open-label, randomised, long-term extension trial. Lancet Respir Med 2016; 4: 372–380. doi: 10.1016/S2213-2600(16)30022-4 [DOI] [PubMed] [Google Scholar]
- 26.Galiè N, Grimminger F, Grünig E, et al. Comparison of hemodynamic parameters in treatment-naïve and pre-treated patients with pulmonary arterial hypertension in the randomized phase III PATENT-1 study. J Heart Lung Transplant 2017; 36: 509–519. doi: 10.1016/j.healun.2016.12.012 [DOI] [PubMed] [Google Scholar]
- 27.Thenappan T, Al-Naamani N, Ghio S, et al. Effect of riociguat on pulmonary arterial compliance in the PATENT and CHEST studies. Pulm Circ 2020; 10: 2045894020963836. doi: 10.1177/2045894020963836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoeper MM, Gomez Sanchez MA, Humbert M, et al. Riociguat treatment in patients with pulmonary arterial hypertension: final safety data from the EXPERT registry. Respir Med 2021; 177: 106241. doi: 10.1016/j.rmed.2020.106241 [DOI] [PubMed] [Google Scholar]
- 29.Galiè N, Muller K, Scalise AV, et al. PATENT PLUS: a blinded, randomised and extension study of riociguat plus sildenafil in PAH. Eur Respir J 2015; 45: 1314–1322. doi: 10.1183/09031936.00105914 [DOI] [PubMed] [Google Scholar]
- 30.Bayer AG . Adempas: EPAR – Product Information. Date last accessed: 27 January 2022. Date last updated: 31 August 2023. www.ema.europa.eu/en/documents/product-information/adempas-epar-product-information_en.pdf
- 31.Bayer AG . Adempas US prescribing information. Date last accessed: 27 January 2022. Date last updated: September 2021. https://labeling.bayerhealthcare.com/html/products/pi/Adempas_PI.pdf
- 32.Klinger JR, Chakinala MM, Langleben D, et al. Riociguat: clinical research and evolving role in therapy. Br J Clin Pharmacol 2021; 87: 2645–2662. doi: 10.1111/bcp.14676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim N, D'Armini A, Grimminger F, et al. Haemodynamic effects of riociguat in inoperable/recurrent chronic thromboembolic pulmonary hypertension. Heart 2017; 103: 599–606. doi: 10.1136/heartjnl-2016-309621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benza RL, Ghofrani HA, Grünig E, et al. Effect of riociguat on right ventricular function in patients with pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2021; 40: 1172–1180. doi: 10.1016/j.healun.2021.06.020 [DOI] [PubMed] [Google Scholar]
- 35.Marra AM, Egenlauf B, Ehlken N, et al. Change of right heart size and function by long-term therapy with riociguat in patients with pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Int J Cardiol 2015; 195: 19–26. doi: 10.1016/j.ijcard.2015.05.105 [DOI] [PubMed] [Google Scholar]
- 36.Marra AM, Halank M, Benjamin N, et al. Right ventricular size and function under riociguat in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension (the RIVER study). Respir Res 2018; 19: 258. doi: 10.1186/s12931-018-0957-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McLaughlin V, Jansa P, Nielsen-Kudsk JE, et al. Riociguat in patients with chronic thromboembolic pulmonary hypertension: results from an early access study. BMC Pulm Med 2017; 17: 216–224. doi: 10.1186/s12890-017-0563-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghofrani HA, Gomez Sanchez MA, Humbert M, et al. Riociguat treatment in patients with chronic thromboembolic pulmonary hypertension: final safety data from the EXPERT registry. Respir Med 2020; 178: 106220. doi: 10.1016/j.rmed.2020.106220 [DOI] [PubMed] [Google Scholar]
- 39.Dachs TM, Duca F, Rettl R, et al. Riociguat in pulmonary hypertension and heart failure with preserved ejection fraction: the haemoDYNAMIC trial. Eur Heart J 2022; 43: 3402–3413. doi: 10.1093/eurheartj/ehac389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonderman D, Ghio S, Felix SB, et al. Riociguat for patients with pulmonary hypertension due to systolic left ventricular dysfunction: a phase IIb double-blind, randomized, placebo-controlled, dose-ranging hemodynamic study. Circulation 2013; 128: 502–511. doi: 10.1161/CIRCULATIONAHA.113.001458 [DOI] [PubMed] [Google Scholar]
- 41.Bonderman D, Pretsch I, Steringer-Mascherbauer R, et al. Acute hemodynamic effects of riociguat in patients with pulmonary hypertension associated with diastolic heart failure (DILATE-1): a randomized, double-blind, placebo-controlled, single-dose study. Chest 2014; 146: 1274–1285. doi: 10.1378/chest.14-0106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hill NS, Preston IR, Roberts KE. Inhaled therapies for pulmonary hypertension. Respir Care 2015; 60: 794–802. doi: 10.4187/respcare.03927 [DOI] [PubMed] [Google Scholar]
- 43.Vonbank K, Ziesche R, Higenbottam TW, et al. Controlled prospective randomised trial on the effects on pulmonary haemodynamics of the ambulatory long term use of nitric oxide and oxygen in patients with severe COPD. Thorax 2003; 58: 289–293. doi: 10.1136/thorax.58.4.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nathan SD, Flaherty KR, Glassberg MK, et al. A randomized, double-blind, placebo-controlled study of pulsed, inhaled nitric oxide in subjects at risk of pulmonary hypertension associated with pulmonary fibrosis. Chest 2020; 158: 637–645. doi: 10.1016/j.chest.2020.02.016 [DOI] [PubMed] [Google Scholar]
- 45.King CS, Flaherty KR, Glassberg MK, et al. A phase-2 exploratory randomized controlled trial of INOpulse in patients with fibrotic interstitial lung disease requiring oxygen. Ann Am Thorac Soc 2022; 19: 594–602. doi: 10.1513/AnnalsATS.202107-864OC [DOI] [PubMed] [Google Scholar]
- 46.Sandner P, Follmann M, Becker-Pelster E, et al. Soluble GC stimulators and activators: past, present and future. Br J Pharmacol 2021; in press [ 10.1111/bph.15698] [DOI] [PubMed] [Google Scholar]
- 47.Ghofrani HA, Staehler G, Grünig E, et al. Acute effects of riociguat in borderline or manifest pulmonary hypertension associated with chronic obstructive pulmonary disease. Pulm Circ 2015; 5: 296–304. doi: 10.1086/680214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoeper MM, Halank M, Wilkens H, et al. Riociguat for interstitial lung disease and pulmonary hypertension: a pilot trial. Eur Respir J 2013; 41: 853–860. doi: 10.1183/09031936.00213911 [DOI] [PubMed] [Google Scholar]
- 49.Nathan SD, Behr J, Collard HR, et al. Riociguat for idiopathic interstitial pneumonia-associated pulmonary hypertension (RISE-IIP): a randomised, placebo-controlled phase 2b study. Lancet Respir Med 2019; 7: 780–790. doi: 10.1016/S2213-2600(19)30250-4 [DOI] [PubMed] [Google Scholar]
- 50.Nathan SD, Cottin V, Behr J, et al. Impact of lung morphology on clinical outcomes with riociguat in patients with pulmonary hypertension and idiopathic interstitial pneumonia: a post hoc subgroup analysis of the RISE-IIP study. J Heart Lung Transplant 2021; 40: 494–503. doi: 10.1016/j.healun.2021.02.006 [DOI] [PubMed] [Google Scholar]
- 51.Vonk Noordegraaf A, Chin KM, Haddad F, et al. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update. Eur Respir J 2019; 53: 1801900. doi: 10.1183/13993003.01900-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murata M, Kawakami T, Kataoka M, et al. Clinical significance of guanylate cyclase stimulator, riociguat, on right ventricular functional improvement in patients with pulmonary hypertension. Cardiology 2021; 146: 130–136. doi: 10.1159/000510860 [DOI] [PubMed] [Google Scholar]
- 53.Benza RL, Langleben D, Hemnes A, et al. Riociguat and the right ventricle in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2022; 31: 220061. doi: 10.1183/16000617.0061-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sandner P, Becker-Pelster EM, Stasch JP. Discovery and development of sGC stimulators for the treatment of pulmonary hypertension and rare diseases. Nitric Oxide 2018; 77: 88–95. doi: 10.1016/j.niox.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 55.Sandner P, Zimmer DP, Milne GT, et al. Soluble guanylate cyclase stimulators and activators. Handb Exp Pharmacol 2021; 264: 355–394. doi: 10.1007/164_2018_197 [DOI] [PubMed] [Google Scholar]
- 56.Haines RJ, Pendleton LC, Eichler DC. Argininosuccinate synthase: at the center of arginine metabolism. Int J Biochem Mol Biol 2011; 2: 8–23. [PMC free article] [PubMed] [Google Scholar]
- 57.Hofmann F. The cGMP system: components and function. Biol Chem 2020; 401: 447–469. doi: 10.1515/hsz-2019-0386 [DOI] [PubMed] [Google Scholar]
- 58.Adler J, Kuret A, Langst N, et al. Targets of cGMP/cGKI in cardiac myocytes. J Cardiovasc Pharmacol 2020; 75: 494–507. doi: 10.1097/FJC.0000000000000817 [DOI] [PubMed] [Google Scholar]
- 59.Sandner P, Stasch JP. Anti-fibrotic effects of soluble guanylate cyclase stimulators and activators: a review of the preclinical evidence. Respir Med 2017; 122: Suppl. 1, S1–S9. doi: 10.1016/j.rmed.2016.08.022 [DOI] [PubMed] [Google Scholar]
- 60.Baillie GS, Tejeda GS, Kelly MP. Therapeutic targeting of 3ʹ,5ʹ-cyclic nucleotide phosphodiesterases: inhibition and beyond. Nat Rev Drug Discov 2019; 18: 770–796. doi: 10.1038/s41573-019-0033-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palm F, Onozato ML, Luo Z, et al. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. Am J Physiol Heart Circ Physiol 2007; 293: H3227–H3245. doi: 10.1152/ajpheart.00998.2007 [DOI] [PubMed] [Google Scholar]
- 62.Ghofrani HA, Humbert M, Langleben D, et al. Riociguat: mode of action and clinical development in pulmonary hypertension. Chest 2017; 151: 468–480. doi: 10.1016/j.chest.2016.05.024 [DOI] [PubMed] [Google Scholar]
- 63.Stasch JP, Evgenov OV. Soluble guanylate cyclase stimulators in pulmonary hypertension. Handb Exp Pharmacol 2013; 218: 279–313. doi: 10.1007/978-3-642-38664-0_12 [DOI] [PubMed] [Google Scholar]
- 64.Simonneau G, Torbicki A, Dorfmuller. P, et al. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160112. doi: 10.1183/16000617.0112-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Naeije R, Badagliacca R. The overloaded right heart and ventricular interdependence. Cardiovasc Res 2017; 113: 1474–1485. doi: 10.1093/cvr/cvx160 [DOI] [PubMed] [Google Scholar]
- 66.Breitenstein S, Roessig L, Sandner. P, et al. Novel sGC stimulators and sGC activators for the treatment of heart failure. Handb Exp Pharmacol 2017; 243: 225–247. [DOI] [PubMed] [Google Scholar]
- 67.Liu R, Kang Y, Chen L. Activation mechanism of human soluble guanylate cyclase by stimulators and activators. Nat Commun 2021; 12: 5492. doi: 10.1038/s41467-021-25617-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beuve A. Wedging open a catalytic site. Elife 2019; 8: e52418. doi: 10.7554/eLife.52418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Horst BG, Yokom AL, Rosenberg. DJ, et al. Allosteric activation of the nitric oxide receptor soluble guanylate cyclase mapped by cryo-electron microscopy. eLife 2019; 8: e50634. doi: 10.7554/eLife.50634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stasch JP, Becker EM, Alonso-Alija. C, et al. NO-independent regulatory site on soluble guanylate cyclase. Nature 2001; 410: 212–215. doi: 10.1038/35065611 [DOI] [PubMed] [Google Scholar]
- 71.Cordwin DJ, Berei TJ, Pogue KT. The role of sGC stimulators and activators in heart failure with reduced ejection fraction. J Cardiovasc Pharmacol Ther 2021; 26: 593–600. doi: 10.1177/10742484211042706 [DOI] [PubMed] [Google Scholar]
- 72.Gheorghiade M, Greene SJ, Filippatos. G, et al. Cinaciguat, a soluble guanylate cyclase activator: results from the randomized, controlled, phase IIb COMPOSE programme in acute heart failure syndromes. Eur J Heart Fail 2012; 14: 1056–1066. doi: 10.1093/eurjhf/hfs093 [DOI] [PubMed] [Google Scholar]
- 73.Sravani S, Saifi MA, Godugu C. Riociguat ameliorates kidney injury and fibrosis in an animal model. Biochem Biophys Res Commun 2020; 530: 706–712. doi: 10.1016/j.bbrc.2020.07.128 [DOI] [PubMed] [Google Scholar]
- 74.Li L, Yin M, Hu. L, et al. Novel pyrazolo[3,4-b] pyridine derivative (HLQ2g) attenuates hypoxic pulmonary hypertension via restoring cGKI expression and BMP signaling pathway. Front Pharmacol 2021; 12: 691405. doi: 10.3389/fphar.2021.691405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zagorski J, Neto-Neves E, Alves NJ, et al. Modulation of soluble guanylate cyclase ameliorates pulmonary hypertension in a rat model of chronic thromboembolic pulmonary hypertension by stimulating angiogenesis. Physiol Rep 2022; 10: e15156. doi: 10.14814/phy2.15156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Masuyama H, Tsuruda T, Sekita. Y, et al. Pressure-independent effects of pharmacological stimulation of soluble guanylate cyclase on fibrosis in pressure-overloaded rat heart. Hypertens Res 2009; 32: 597–603. doi: 10.1038/hr.2009.64 [DOI] [PubMed] [Google Scholar]
- 77.Beyer C, Zenzmaier C, Palumbo-Zerr. K, et al. Stimulation of the soluble guanylate cyclase (sGC) inhibits fibrosis by blocking non-canonical TGFβ signalling. Ann Rheum Dis 2015; 74: 1408–1416. doi: 10.1136/annrheumdis-2013-20450 [DOI] [PubMed] [Google Scholar]
- 78.Dees C, Beyer C, Distler. A, et al. Stimulators of soluble guanylate cyclase (sGC) inhibit experimental skin fibrosis of different aetiologies. Ann Rheum Dis 2015; 74: 1621–1625. doi: 10.1136/annrheumdis-2014-206809 [DOI] [PubMed] [Google Scholar]
- 79.Joshi CN, Martin DN, Fox. JC, et al. The soluble guanylate cyclase stimulator BAY 41-2272 inhibits vascular smooth muscle growth through the cAMP-dependent protein kinase and cGMP-dependent protein kinase pathways. J Pharmacol Exp Ther 2011; 339: 394–402. doi: 10.1124/jpet.111.183400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schermuly RT, Stasch JP, Pullamsetti. SS, et al. Expression and function of soluble guanylate cyclase in pulmonary arterial hypertension. Eur Respir J 2008; 32: 881–891. doi: 10.1183/09031936.00114407 [DOI] [PubMed] [Google Scholar]
- 81.Lang M, Kojonazarov B, Tian. X, et al. The soluble guanylate cyclase stimulator riociguat ameliorates pulmonary hypertension induced by hypoxia and SU5416 in rats. PLoS One 2012; 7: e43433. doi: 10.1371/journal.pone.0043433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rai N, Veeroju S, Schymura Y, et al. Effect of riociguat and sildenafil on right heart remodeling and function in pressure overload induced model of pulmonary arterial banding. Biomed Res Int 2018; 2018: 3293584. doi: 10.1155/2018/3293584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pradhan K, Sydykov A, Tian X, et al. Soluble guanylate cyclase stimulator riociguat and phosphodiesterase 5 inhibitor sildenafil ameliorate pulmonary hypertension due to left heart disease in mice. Int J Cardiol 2016; 216: 85–91. doi: 10.1016/j.ijcard.2016.04.098 [DOI] [PubMed] [Google Scholar]
- 84.Evgenov OV, Zou L, Zhang M, et al. Nitric oxide-independent stimulation of soluble guanylate cyclase attenuates pulmonary fibrosis. BMC Pharmacol 2011; 11: Suppl. 1, 9. doi: 10.1186/1471-2210-11-S1-O921878090 [DOI] [Google Scholar]
- 85.Chamorro V, Morales-Cano D, Milara J, et al. Riociguat versus sildenafil on hypoxic pulmonary vasoconstriction and ventilation/perfusion matching. PLoS One 2018; 13: e0191239. doi: 10.1371/journal.pone.0191239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilkins MR, Paul GA, Strange JW, et al. Sildenafil versus endothelin receptor antagonist for pulmonary hypertension (SERAPH) study. Am J Respir Crit Care Med 2005; 171: 1292–1297. doi: 10.1164/rccm.200410-1411OC [DOI] [PubMed] [Google Scholar]
- 87.Galiè N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005; 353: 2148–2157. doi: 10.1056/NEJMoa050010 [DOI] [PubMed] [Google Scholar]
- 88.Rubin LJ, Badesch DB, Fleming TR, et al. Long-term treatment with sildenafil citrate in pulmonary arterial hypertension: SUPER-2. Chest 2011; 150: 1274–1283. doi: 10.1378/chest.10-0969 [DOI] [PubMed] [Google Scholar]
- 89.Barst RJ, Ivy DD, Gaitan G, et al. A randomized, double-blind, placebo-controlled, dose-ranging study of oral sildenafil citrate in treatment-naive children with pulmonary arterial hypertension. Circulation 2012; 125: 324–334. doi: 10.1161/CIRCULATIONAHA.110.016667 [DOI] [PubMed] [Google Scholar]
- 90.Barst RJ, Beghetti M, Pulido T, et al. STARTS-2: long-term survival with oral sildenafil monotherapy in treatment-naive pediatric pulmonary arterial hypertension. Circulation 2014; 129: 1914–1923. doi: 10.1161/CIRCULATIONAHA.113.005698 [DOI] [PubMed] [Google Scholar]
- 91.Galiè N, Brundage BH, Ghofrani HA, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation 2009; 119: 2894–2903. doi: 10.1161/CIRCULATIONAHA.108.839274 [DOI] [PubMed] [Google Scholar]
- 92.Oudiz RJ, Brundage BH, Galiè N, et al. Tadalafil for the treatment of pulmonary arterial hypertension: a double-blind 52-week uncontrolled extension study. J Am Coll Cardiol 2012; 60: 768–774. doi: 10.1016/j.jacc.2012.05.004 [DOI] [PubMed] [Google Scholar]
- 93.Jing ZC, Yu ZX, Shen JY, et al. Vardenafil in pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled study. Am J Respir Crit Care Med 2011; 183: 1723–1729. doi: 10.1164/rccm.201101-0093OC [DOI] [PubMed] [Google Scholar]
- 94.Galiè N, Barbera JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015; 373: 834–844. doi: 10.1056/NEJMoa1413687 [DOI] [PubMed] [Google Scholar]
- 95.Sastry BK, Narasimhan C, Reddy NK, et al. Clinical efficacy of sildenafil in primary pulmonary hypertension: a randomized, placebo-controlled, double-blind, crossover study. J Am Coll Cardiol 2004; 43: 1149–1153. doi: 10.1016/j.jacc.2003.10.056 [DOI] [PubMed] [Google Scholar]
- 96.Vizza CD, Sastry BK, Safdar Z, et al. Efficacy of 1, 5, and 20 mg oral sildenafil in the treatment of adults with pulmonary arterial hypertension: a randomized, double-blind study with open-label extension. BMC Pulm Med 2017; 17: 44. doi: 10.1186/s12890-017-0374-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Singh TP, Rohit M, Grover A, et al. A randomized, placebo-controlled, double-blind, crossover study to evaluate the efficacy of oral sildenafil therapy in severe pulmonary artery hypertension. Am Heart J 2006; 151: 851.e1–851.e5. doi: 10.1016/j.ahj.2005.09.006 [DOI] [PubMed] [Google Scholar]
- 98.Simonneau G, Rubin LJ, Galiè N, et al. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Ann Intern Med 2008; 149: 521–530. doi: 10.7326/0003-4819-149-8-200810210-00004 [DOI] [PubMed] [Google Scholar]
- 99.Iversen K, Jensen AS, Jensen TV, et al. Combination therapy with bosentan and sildenafil in Eisenmenger syndrome: a randomized, placebo-controlled, double-blinded trial. Eur Heart J 2010; 31: 1124–1131. doi: 10.1093/eurheartj/ehq011 [DOI] [PubMed] [Google Scholar]
- 100.Vizza CD, Jansa P, Teal S, et al. Sildenafil dosed concomitantly with bosentan for adult pulmonary arterial hypertension in a randomized controlled trial. BMC Cardiovasc Disord 2017; 17: 239. doi: 10.1186/s12872-017-0674-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barnes H, Brown Z, Burns A, et al. Phosphodiesterase 5 inhibitors for pulmonary hypertension. Cochrane Database Syst Rev 2019; 1: CD012621. doi: 10.1002/14651858.CD012621.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.ClinicalTrials.gov . Special investigation for long-term use of sildenafil (regulatory post marketing commitment plan). Date last accessed 10 October 2022. Date last updated: 1 February 2021. www.clinicaltrials.gov/ct2/show/results/NCT00666198
- 103.Ghofrani HA, Schermuly RT, Rose F, et al. Sildenafil for long-term treatment of nonoperable chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med 2003; 167: 1139–1141. doi: 10.1164/rccm.200210-1157BC [DOI] [PubMed] [Google Scholar]
- 104.Reichenberger F, Voswinckel R, Enke B, et al. Long-term treatment with sildenafil in chronic thromboembolic pulmonary hypertension. Eur Respir J 2007; 30: 922–927. doi: 10.1183/09031936.00039007 [DOI] [PubMed] [Google Scholar]
- 105.Suntharalingam J, Treacy CM, Doughty NJ, et al. Long-term use of sildenafil in inoperable chronic thromboembolic pulmonary hypertension. Chest 2008; 134: 229–236. doi: 10.1378/chest.07-2681 [DOI] [PubMed] [Google Scholar]
- 106.Delcroix M, Torbicki A, Gopalan D, et al. ERS statement on chronic thromboembolic pulmonary hypertension. Eur Respir J 2021; 57: 2002828. doi: 10.1183/13993003.02828-2020 [DOI] [PubMed] [Google Scholar]
- 107.Guth S, D'Armini AM, Delcroix M, et al. Current strategies for managing chronic thromboembolic pulmonary hypertension: results of the worldwide prospective CTEPH Registry. ERJ Open Res 2021; 7: 00850-2020. doi: 10.1183/23120541.00850-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bermejo J, Yotti R, Garcia-Orta R, et al. Sildenafil for improving outcomes in patients with corrected valvular heart disease and persistent pulmonary hypertension: a multicenter, double-blind, randomized clinical trial. Eur Heart J 2018; 39: 1255–1264. doi: 10.1093/eurheartj/ehx700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guazzi M, Vicenzi M, Arena R, et al. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation 2011; 124: 164–174. doi: 10.1161/CIRCULATIONAHA.110.983866 [DOI] [PubMed] [Google Scholar]
- 110.Reichenbach A, Al-Hiti H, Malek I, et al. The effects of phosphodiesterase 5 inhibition on hemodynamics, functional status and survival in advanced heart failure and pulmonary hypertension: a case-control study. Int J Cardiol 2013; 168: 60–65. doi: 10.1016/j.ijcard.2012.09.074 [DOI] [PubMed] [Google Scholar]
- 111.Belyavskiy E, Ovchinnikov A, Potekhina A, et al. Phosphodiesterase 5 inhibitor sildenafil in patients with heart failure with preserved ejection fraction and combined pre- and postcapillary pulmonary hypertension: a randomized open-label pilot study. BMC Cardiovasc Disord 2020; 20: 408. doi: 10.1186/s12872-020-01671-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hoendermis ES, Liu LC, Hummel YM, et al. Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: a randomized controlled trial. Eur Heart J 2015; 36: 2565–2573. doi: 10.1093/eurheartj/ehv336 [DOI] [PubMed] [Google Scholar]
- 113.Vitulo P, Stanziola A, Confalonieri M, et al. Sildenafil in severe pulmonary hypertension associated with chronic obstructive pulmonary disease: a randomized controlled multicenter clinical trial. J Heart Lung Transplant 2017; 36: 166–174. doi: 10.1016/j.healun.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 114.Rao RS, Singh S, Sharma BB, et al. Sildenafil improves six-minute walk distance in chronic obstructive pulmonary disease: a randomised, double-blind, placebo-controlled trial. Indian J Chest Dis Allied Sci 2011; 53: 81–85. [PubMed] [Google Scholar]
- 115.Blanco I, Santos S, Gea J, et al. Sildenafil to improve respiratory rehabilitation outcomes in COPD: a controlled trial. Eur Respir J 2013; 42: 982–992. doi: 10.1183/09031936.00176312 [DOI] [PubMed] [Google Scholar]
- 116.Tzoumas N, Farrah TE, Dhaun N, et al. Established and emerging therapeutic uses of PDE type 5 inhibitors in cardiovascular disease. Br J Pharmacol 2020; 177: 5467–5488. doi: 10.1111/bph.14920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Andersson KE. PDE5 inhibitors – pharmacology and clinical applications 20 years after sildenafil discovery. Br J Pharmacol 2018; 175: 2554–2565. doi: 10.1111/bph.14205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McMahon CN, Smith CJ, Shabsigh R. Treating erectile dysfunction when PDE5 inhibitors fail. BMJ 2006; 332: 589–592. doi: 10.1136/bmj.332.7541.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wharton J, Strange JW, Moller GM, et al. Antiproliferative effects of phosphodiesterase type 5 inhibition in human pulmonary artery cells. Am J Respir Crit Care Med 2005; 172: 105–113. doi: 10.1164/rccm.200411-1587OC [DOI] [PubMed] [Google Scholar]
- 120.Olsson KM, Richter MJ, Kamp JC, et al. Intravenous treprostinil as an add-on therapy in patients with pulmonary arterial hypertension. J Heart Lung Transplant 2019; 38: 748–756. doi: 10.1016/j.healun.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 121.Bartolome SD, Sood N, Shah TG, et al. Mortality in patients with pulmonary arterial hypertension treated with continuous prostanoids. Chest 2018; 154: 532–540. doi: 10.1016/j.chest.2018.03.050 [DOI] [PubMed] [Google Scholar]
- 122.Pfizer . Revatio: EPAR – Product Information. Date last accessed: 9 May 2019. Date last updated: 15 January 2019. www.ema.europa.eu/en/documents/product-information/revatio-epar-product-information_en.pdf
- 123.Pfizer . Revatio. US prescribing information. Date last accessed: 10 October 2022. Date last updated: January 2014. www.accessdata.fda.gov/drugsatfda_docs/label/2014/021845s011%2C022473s004%2C0203109s002lbl.pdf
- 124.Eli Lilly . Adcirca: EPAR – Product Information. Date last accessed: 9 May 2019. Date last updated: 20 September 2023. www.ema.europa.eu/en/documents/product-information/adcirca-epar-product-information_en.pdf
- 125.Eli Lilly . Adcirca. Prescribing information. Date last accessed: 9 May 2019. Date last updated: September 2020. https://pi.lilly.com/us/adcirca-pi.pdf
- 126.Grünig E, Ohnesorge J, Benjamin N, et al. Plasma drug concentrations in patients with pulmonary arterial hypertension on combination treatment. Respiration 2017; 94: 26–37. doi: 10.1159/000470916 [DOI] [PubMed] [Google Scholar]
- 127.Gall H, Felix JF, Schneck FK, et al. The Giessen Pulmonary Hypertension Registry: survival in pulmonary hypertension subgroups. J Heart Lung Transplant 2017; 36: 957-–967. . doi: 10.1016/j.healun.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 128.Ogbomo A, Tsang Y, Kariburyo F, et al. Real-world analysis of treatment patterns among hospitalized patients with pulmonary arterial hypertension. Pulm Ther 2021; 7: 575–590. doi: 10.1007/s41030-021-00173-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lapp H, Mitrovic V, Franz N, et al. Cinaciguat (BAY 58-2667) improves cardiopulmonary hemodynamics in patients with acute decompensated heart failure. Circulation 2009; 119: 2781–2788. doi: 10.1161/CIRCULATIONAHA.108.800292 [DOI] [PubMed] [Google Scholar]
- 130.Irvine JC, Ganthavee V, Love JE, et al. The soluble guanylyl cyclase activator BAY 58-2667 selectively limits cardiomyocyte hypertrophy. PLoS One 2012; 7: e44481. doi: 10.1371/journal.pone.0044481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Benardeau A, Kahnert A, Schomber T, et al. Runcaciguat, a novel soluble guanylate cyclase activator, shows renoprotection in hypertensive, diabetic, and metabolic preclinical models of chronic kidney disease. Naunyn Schmiedebergs Arch Pharmacol 2021; 394: 2363–2379. doi: 10.1007/s00210-021-02149-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hahn MG, Lampe T, El Sheikh S, et al. Discovery of the soluble guanylate cyclase activator runcaciguat (BAY 1101042). J Med Chem 2021; 64: 5323–5344. doi: 10.1021/acs.jmedchem.0c02154 [DOI] [PubMed] [Google Scholar]
- 133.Becker-Pelster EM, Hahn MG, Delbeck M, et al. Inhaled mosliciguat (BAY 1237592): targeting pulmonary vasculature via activating apo-sGC. Respir Res 2022; 23: 272. doi: 10.1186/s12931-022-02189-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eldon MA, Parsley EL, Maurer M, et al. Safety, tolerability, and pharmacokinetics of RT234 (vardenafil inhalation powder): a first-in-human, ascending single- and multiple-dose study in healthy subjects. J Aerosol Med Pulm Drug Deliv 2021; 34: 251–261. doi: 10.1089/jamp.2020.1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.ClinicalTrials.gov . Vardenafil inhaled for pulmonary arterial hypertension PRN phase 2B study (VIPAH-PRN 2B). Date last accessed: 10 October 2022. Date last updated: 10 February 2023. https://clinicaltrials.gov/ct2/show/NCT04266197
- 136.Sahay S, Holy R, Lyons S, et al. Impact of human behavior on inspiratory flow profiles in patients with pulmonary arterial hypertension using AOS dry powder inhaler device. Pulm Circ 2021; 11: 2045894020985345. doi: 10.1177/2045894020985345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bajwa EK, Cislak D, Palcza J, et al. Effects of an inhaled soluble guanylate cyclase (sGC) stimulator MK-5475 in pulmonary arterial hypertension (PAH). Respir Med 2023; 206: 107065. doi: 10.1016/j.rmed.2022.107065 [DOI] [PubMed] [Google Scholar]
- 138.ClinicalTrials.gov. A study of the efficacy and safety of MK-5475 in participants with pulmonary arterial hypertension (INSIGNIA-PAH: phase 2/3 study of an inhaled sGC stimulator in PAH) (MK-5475-007). Date last accessed: 10 October 2022. Date last updated: 2 February 2024. https://clinicaltrials.gov/ct2/show/NCT04732221
- 139.ClinicalTrials.gov . This study tests the safety of inhaled BAY1237592, how the drug is tolerated and how it effects patients with high blood pressure in the arteries of the lungs in the two different disease groups pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH) (ATMOS). Date last accessed: 10 October 2022. Date last updated: 28 September 2023. https://clinicaltrials.gov/ct2/show/NCT03754660
- 140.Emdin CA, Khera AV, Klarin D, et al. Phenotypic consequences of a genetic predisposition to enhanced nitric oxide signaling. Circulation 2018; 137: 222–232. doi: 10.1161/CIRCULATIONAHA.117.028021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kessler T, Wobst J, Wolf B, et al. Functional characterization of the GUCY1A3 coronary artery disease risk locus. Circulation 2017; 136: 476–489. doi: 10.1161/CIRCULATIONAHA.116.024152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Leineweber K, Moosmang S, Paulson D. Genetics of NO deficiency. Am J Cardiol 2017; 120: S80–S88. doi: 10.1016/j.amjcard.2017.06.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary video ERR-0183-2023.SUPPLEMENT (54.6MB, mp4)