Abstract
Two experiments were conducted using 120 pigs to test the hypothesis that supplementation of β-mannanase could reduce digesta viscosity, enhance nutrient digestion, and improve intestinal health and growth of nursery pigs. In experiment 1, 48 crossbred barrows were randomly allotted to four treatments with increasing levels of β-mannanase at 0, 200, 400, and 600 U/kg in feeds. All pigs were euthanized on day 12 to collect jejunal digesta to measure digesta viscosity and ileal digesta to measure apparent ileal digestibility (AID) of dry matter (DM), gross energy (GE), neutral detergent fiber (NDF), and acid detergent fiber (ADF). In experiment 2, 72 nursery pigs were randomly allotted to three treatments with increasing levels of β-mannanase at 0, 400, and 600 U/kg in feeds. Plasma collected on day 9 was used to measure tumor necrosis factor-α (TNF-α), immunoglobulin G (IgG), malondialdehyde (MDA), and protein carbonyl (PC). All pigs were euthanized on day 10 to collect duodenal and jejunal tissues to evaluate the production of TNF-α, IL-6, and MDA, morphology, crypt cell proliferation, and expression of tight junction proteins in the jejunum. Data were analyzed using the MIXED procedure for polynomial contrasts and the NLMIXED procedure for broken-line analysis of SAS. In experiment 1, β-mannanase supplementation tended to have quadratic effects on digesta viscosity (P = 0.085) and AID of GE (P = 0.093) in the pigs. In experiment 2, jejunal digesta viscosity of the pigs was reduced (P < 0.05) when β-mannanase was supplemented at 360 U/kg of feed. β-Mannanase supplementation linearly reduced (P < 0.05) TNF-α, IgG, MDA, and PC in the duodenum, and TNF-α, IgG, and MDA in the jejunum of the pigs. β-Mannanase supplementation linearly increased (P < 0.05) villus height to crypt depth ratio and crypt cell proliferation in the jejunum. β-Mannanase supplementation tended to linearly improve (P = 0.083) expression of zonula occludens-1 in the jejunum. In conclusion, supplementation of β-mannanase at 360 U/kg reduced the digesta viscosity and up to 600 U/kg positively affected intestinal health and growth of pigs by reducing inflammation and oxidative stress whilst enhancing structure and barrier function in the jejunum.
Keywords: inflammation, jejunum, β-mannanase, nursery pigs, oxidative stress, viscosity
β-Mannanase effectively reduced digesta viscosity through hydrolysis of β-mannan in feeds and thus enhanced intestinal health of nursery pigs by reducing inflammation and oxidative stress whilst improving intestinal barrier function in the jejunum.
Introduction
Nursery pigs are commonly susceptible to the challenges that can negatively affect their immature digestive systems and potentially impair growth and health (Kim and Duarte, 2021). The small intestine is a crucial part of the gastrointestinal tract responsible for feed digestion, absorption, and protection from toxins and external invasions (Lallès et al., 2007; Zheng et al., 2021). One of the significant limitations for newly weaned pigs is their inability to digest complex carbohydrates, including non-starch polysaccharides (NSP) found in feeds. β-Mannans and β-galactomannans are NSP abundant in protein supplements such as copra meal, palm kernel meal, and soybean meal (Kiarie et al., 2013; Jang et al., 2023a). Once ingested, β-mannans exhibit hygroscopic properties, increasing digesta viscosity in the small intestine, which can hinder the movement of digesta through the intestine, impede the access of digestive enzymes to digesta, reduce the surface area available for nutrient digestion, and impair nutrient absorption and growth of pigs (Jha and Mishra, 2021; Kiarie et al., 2021).
β-Mannanase specifically targets β-mannans and β-galactomannans, hydrolyzing β-1,4-mannosidic bonds (Ximenes et al., 2005; Tiwari et al., 2018) resulting in reduced digesta viscosity and improved nutrient digestibility (Jeon et al., 2019; Genova et al., 2023). Furthermore, the hydrolysis of β-mannans into mannan oligosaccharides can provide prebiotic effects (Halas and Nochta, 2012; Agazzi et al., 2020) and inactivate toxic compounds such as aflatoxin (Kim et al., 2016) benefiting the intestinal environment in nursery pigs. Beneficial effects of β-mannanase supplementation have been shown to be related to reduced digesta viscosity, enhanced NSP digestion, and improved growth performance (Balasubramanian et al., 2018), whereas its potential mechanisms related to mucosal health warrant investigation. Therefore, it was hypothesized that supplementation of β-mannanase could reduce digesta viscosity and increase nutrient digestion by enhancing intestinal health thereby improving the growth performance of nursery pigs. To test the hypothesis, the objective of this study was to evaluate the effects of increasing supplementation levels of β-mannanase on inflammatory status, oxidative stress status, humoral immune status, structural damages and repairing, and barrier function in the jejunum as well as nutrient digestibility and growth performance of nursery pigs.
Materials and Methods
The protocol of this experiment was reviewed and approved by the Institutional Animal Care and Use Committee at North Carolina State University (Raleigh, NC, USA). Two experiments were conducted under the guidelines in the care and management of animals (Federation of Animal Science Societies, 2020) at research facilities at North Carolina State University (Raleigh, NC, USA).
Animals and experimental feeds
In experiment 1, 40 barrows were weaned at 21 d of age and fed a common diet for 21 d prior to the initiation of the study. After 21 d feeding of a common feed, barrows with 15.7 ± 1.1 kg of body weight (BW) were allotted to four treatments based on a randomized complete block design with initial BW (heavy and light) as a block. Each treatment had 12 replicates and the pigs were individually placed in metabolism pens (0.6 m × 1.8 m) equipped with a stainless-steel feeder attached to the front of the cage, nipple water next to the feeder, and slatted flooring (Passos et al., 2015a). β-Mannanase was supplemented at 0, 250, 500, and 750 mg/kg into the basal diet to provide 0, 200, 400, and 600 U/kg, respectively. The basal feeds contained 58.2% corn, 27.0% soybean meal, 10.0% corn distiller’s dried grains with solubles (DDGS), and 4.5% others (Table 1). Pigs were provided with the experimental feeds at a fixed amount based on the BW of pigs (daily feed allowance = 0.09 × BW0.75 kg) for 12 d. Feeds were provided two times per day (0700 and 1700 hours). The BW of individual pigs and daily feed disappearance were recorded to determine average daily gain (ADG), average daily feed intake (ADFI), and feed efficiency (gain to feed ratio [G:F]) until day 11 and all pigs were euthanized on day 12.
Table 1.
Composition of experimental feeds1 used in experiment 1 (as-fed basis)
| Item | Basal feed |
|---|---|
| Feedstuff, % | |
| Corn, yellow | 58.22 |
| Soybean meal, 48% crude protein | 27.00 |
| Corn DDGS2 | 10.00 |
| l-Lys HCl | 0.20 |
| Salt | 0.30 |
| Vitamin and mineral premix3 | 0.18 |
| Monocalcium phosphate | 0.50 |
| Limestone | 1.30 |
| Poultry fat | 2.00 |
| Titanium dioxide | 0.30 |
| Total | 100.00 |
| Calculated composition | |
| ME, Mcal/kg | 3.34 |
| Crude protein, % | 20.63 |
| SID4 Lys, % | 1.02 |
| SID Thr, % | 0.62 |
| SID Trp, % | 0.21 |
| Total mannan5, % | 0.31 |
| Analyzed composition | |
| Dry matter, % | 90.42 |
| Crude protein, % | 21.41 |
| Ca, % | 0.55 |
| P, % | 0.63 |
| NDF6, % | 12.71 |
| ADF7, % | 3.54 |
1β-Mannanase was added replacing the corn at 0, 250, 500, or 750 mg/kg of the basal feed to adjust as 0, 200, 400, or 600 U/kg. Analyzed β-mannanase activities in the experimental diet were 0, 265 ± 3, 438 ± 3, or 623 ± 5 U/kg of feeds.
2DDGS: distiller’s dried grains with solubles.
3The vitamin premix provided per kg of complete diet: 8,433 IU of vitamin A, 1,202 IU of vitamin D3 as activated animal sterol, 48 IU of vitamin E, 4.0 mg of vitamin K as menadione dimethylpyrimidinol bisulfate, 6.0 mg of riboflavin, 36.2 mg of niacin, 24.1 mg of d-pantothenic acid as calcium pantothenate, 1.8 mg of folic acid, 0.24 mg of d-biotin, 0.031 mg of vitamin B12. The trace mineral premix provided per kg of complete diet: 16.5 mg of Cu as CuSO4, 0.3 mg I as ethylenediamine dihydriodide, 165 mg of Fe as FeSO4, 40 mg of Mn as MnSO4, 0.3 mg of Se as Na2SeO3, and 165 mg of Zn as ZnO.
4SID: standardized ileal digestible.
5Calculated based on Kiarie et al. (2021).
6NDF: neutral detergent fiber.
7ADF: acid detergent fiber.
In experiment 2, 72 pigs were weaned at 21 d of age and fed a common diet for 24 d prior to the initiation of the study. The common diet contained the nutrients meeting the requirement for the pigs suggested by NRC (2012). After 24 d feeding of a common diet, pigs (36 barrows and 36 gilts; initial BW 15.5 ± 2.3 kg) were allotted to three treatments based on a randomized complete block design with initial BW (heavy, middle, and light) and sex (barrows and gilts) as blocks. Each treatment had 24 replicates and the pigs were individually placed in pens (1.4 m × 3.9 m) equipped with a stainless-steel feeder attached to the front of the cage, nipple water next to the feeder, and slatted flooring (Choi et al., 2023). β-Mannanase was supplemented at 0, 500, and 750 mg/kg into the basal diet to provide 0, 400, and 600 U/kg, respectively. The basal feeds contained 51.9% corn, 33.0% soybean meal, 10.0% corn DDGS, and 5.1% others (Table 2). Pigs were provided with the experimental feeds and water ad libitum for 10 d. The BW of individual pigs and feed disappearance were recorded to determine ADG, ADFI, and G:F. All pigs were euthanized on day 10 immediately after recording the BW and feed disappearance.
Table 2.
Composition of experimental feeds1 used in experiment 2 (as-fed basis)
| Item | Basal feed |
|---|---|
| Feedstuff, % | |
| Corn, yellow | 51.87 |
| Soybean meal, 48% crude protein | 33.00 |
| Corn DDGS2 | 10.00 |
| l-Lys HCl | 0.30 |
| dl-Met | 0.05 |
| l-Thr | 0.05 |
| Salt | 0.30 |
| Vitamin and mineral premix3 | 0.18 |
| Dicalcium phosphate | 0.55 |
| Limestone | 1.20 |
| Poultry fat | 2.00 |
| Total | 100.00 |
| Calculated composition | |
| ME, Mcal/kg | 3.39 |
| Crude protein, % | 23.16 |
| SID4 Lys, % | 1.25 |
| SID Thr, % | 0.75 |
| SID Trp, % | 0.24 |
| Total mannan5, % | 0.62 |
| Analyzed composition | |
| Dry matter, % | 91.28 |
| Crude protein, % | 21.41 |
| Ca, % | 0.65 |
| P, % | 0.60 |
| NDF6, % | 8.60 |
| ADF7, % | 4.01 |
1β-Mannanase was added at 0, 500, or 750 mg/kg of basal feed to adjust as 0, 400, or 600 unit/kg. Analyzed β-mannanase activities in the experimental diet were 0, 477 ± 32, or 697 ± 21 U/kg of feeds.
2DDGS, distiller’s dried grains with solubles.
3The vitamin premix provided per kg of complete diet: 8,433 IU of vitamin A, 1,202 IU of vitamin D3 as activated animal sterol, 48 IU of vitamin E, 4.0 mg of vitamin K as menadione dimethylpyrimidinol bisulfate, 6.0 mg of riboflavin, 36.2 mg of niacin, 24.1 mg of d-pantothenic acid as calcium pantothenate, 1.8 mg of folic acid, 0.24 mg of d-biotin, 0.031 mg of vitamin B12. The trace mineral premix provided per kg of complete diet: 16.5 mg of Cu as CuSO4, 0.3 mg I as ethylenediamine dihydriodide, 165 mg of Fe as FeSO4, 40 mg of Mn as MnSO4, 0.3 mg of Se as Na2SeO3, and 165 mg of Zn as ZnO.
4SID, standardized ileal digestible.
5Calculated based on Kiarie et al. (2021).
6NDF, neutral detergent fiber.
7ADF, acid detergent fiber.
The experimental feeds used in experiments 1 and 2 were formulated to provide nutrients to meet or exceed requirements suggested by NRC (2012) and formulated at the North Carolina Feed Educational Unit (Raleigh, NC, USA). All diets were in mash form. The supplemental levels of β-mannanase were set up based on the significant biological responses in previous studies (Yoon et al. 2010; Lv et al., 2013; Kim et al., 2017). Beta-mannanase (800,000 U/kg; CTCBIO Inc., Seoul, South Korea) was produced through the fermentation by Bacillus subtilis isolate WL-7 (GenBank AAT27435.1) on Luria broth. One unit of β-mannanase activity was defined as the amount of enzyme required to release 1 µmole of mannose reducing sugars equivalents per minute from 1.0% locust bean gum in 200 mM sodium phosphate buffer, pH 6.0 at 50 °C.
Sample collection
In experiment 1, at the end of the studies, all pigs were euthanized by exsanguination after the penetration of a captive bolt to the head to collect digesta samples. Immediately after the euthanasia, the ileal portion (a portion of 20 cm prior to the ileocecal connection) of the small intestine was used to obtain digesta in the ileum. The distal portion of the jejunum (20 cm) was also used to collect jejunal digesta to measure viscosity using a viscometer (Brookfield Digital Viscometer, Model DV-II Version 2.0, Brookfield Engineering Laboratories, Inc., Stoughton, MA). Digesta from the ileum were stored in a sterile container and kept frozen at −20 °C.
In experiment 2, blood samples were collected (10 mL) using vacutainer tubes containing dipotassium EDTA (BD, Franklin Lakes, NJ) from the jugular vein of pigs in all pens on day 9. Blood sampling was done without prior fasting at 0900 hours when pigs were active in eating behavior (Hyun et al., 1997; Nyachoti et al., 2004). The tubes were centrifuged at 3,000 × g for 15 min and then the plasma was aliquoted into 1.5 mL tubes and stored at −80 °C until analysis. On the last day of the experiment, all pigs were euthanized by the penetration of a captive bolt followed by exsanguination. After euthanasia, tissues from the duodenum (5 cm after the pyloric-duodenal junction) and distal jejunum (3 m distal to the pyloric-duodenal junction) were collected and flushed with sterile saline solution. The first 10 cm was used to collect jejunal mucosa by scraping the mucosal layer in the jejunum using a glass microscope slide and the remaining 5 cm was stored in 10% formalin buffer for histological evaluation. Duodenal and jejunal mucosa and plasma samples were used to measure tumor necrosis factor-α (TNF-α) and immunoglobulin G (IgG) as indicators of immune status and malondialdehyde (MDA), and protein carbonyl (PC) as indicators of oxidative stress status.
Viscosity of jejunal digesta
Viscosity was immediately measured in the same day after obtaining jejunal digesta based on the methods described by Passos et al. (2015b). The jejunal digesta were homogenized and then transferred to 15 mL tubes (each sample repeated four times). The tubes were centrifuged at 1,500 × g for 5 min at 4 °C and then the supernatant was again centrifuged at 10,000 × g for 10 min at 4 °C. The second supernatant was placed in ice to measure viscosity immediately. A viscometer (Brookfield Digital Viscometer, Model DV-II Version 2.0, Brookfield Engineering Laboratories, Inc.) was set at 25 °C, and 1.0 mL of the supernatant was placed in the viscometer.
Apparent ileal digestibility
Titanium dioxide (TiO2) was added to the feeds at 0.3% as an indigestible external marker for the last 5 days of the first experiment. Titanium dioxide (TiO2) concentrations of the diets and ileal digesta were determined by the procedure described in Chen et al. (2017). The apparent ileal digestibility (AID) of nutrients was calculated according to the following equation:
where TiO2diet and TiO2digesta are the TiO2 contents in the diet and ileal digesta, respectively (g/kg; dry matter [DM] basis); and NUTRdigesta and NUTRdiet are the nutrient contents in the ileal digesta and diet, respectively (DM basis). Ileal digesta were stored at −20 °C and freeze-dried (24D 48, Virtis, Gardiner, NY). The feeds and freeze-dried digesta samples were ground and analyzed for TiO2 concentration following the methodology described by Williams et al. (1962), neutral detergent fiber (NDF) following Van Soest et al. (1991), and acid detergent fiber (ADF) based on (Method 973.18, AOAC, 2006). AID of NDF and ADF were calculated using titanium concentration in the feeds and digesta.
Intestinal morphology and Ki-67 in crypt cells
Two parts of mid-jejunum were fixed in 10% buffered formalin for 48 h after tissue sampling, and then transferred to a 70% ethanol solution and sent to North Carolina State University Histology Laboratory (College of Veterinary Medicine, Raleigh, NC) for dehydration, embedment, and hematoxylin and eosin staining as well as immunohistochemistry for staining Ki67+ antibody to measure the proliferation of enterocytes according to Deng et al. (2022). Villus height and crypt depth (VH:CD) were measured using a camera (Infinity 2-2 digital CCD) attached to a microscope (Olympus CX31, Lumenera Corporation, Ottawa, Canada) as described in previous studies (Deng et al., 2022; Moita and Kim, 2023). Lengths of 15 well-oriented intact villi and their associated crypts were measured in each slide. The villi length was measured from the top of the villi to the villi-crypt junction, and the CD was measured from the villi-crypt junction to the bottom of the crypt. Then, the VH:CD ratio was calculated. Images of 15 intact crypts from each slide were cropped and the Image JS software was used for calculating the ratio of Ki-67 positive cells to total cells in the crypt (%) as described in previous studies (Duarte et al., 2019; Kim et al., 2019). Morphological evaluation and crypt cell proliferation were executed by the same person.
Inflammatory cytokine, immunoglobulin, and oxidative damage products
Duodenal and jejunal mucosa samples (1 g) were taken and added with 2 mL of phosphate-buffered saline solution into 5 mL polypropylene tubes. The mucosa samples were homogenized using a tissue homogenizer (Tissuemiser, Thermo Fisher Scientific Inc., Rockford, IL) for 30 s on ice and transferred to a new 2 mL microcentrifuge tube for centrifugation for 15 min at 14,000 × g at 4 °C as described in previous studies (Jang et al., 2020; Duarte et al., 2023). The supernatant was aliquot into four sets of 0.25 mL into polypropylene tubes and stored at −80 °C for further analysis. The concentration of total protein was analyzed by using Pierce BCA Protein Assay Kit (#23225, Thermo Fisher Scientific, Waltham, MA) following the manufacturer protocol. Mucosa samples were diluted (1:60) to reach the working range of 20 to 2,000 μg/mL. The absorbance was measured at 562 nm. The total protein concentration was calculated by the standard curve and used to normalize the concentrations of protein measures. Concentrations of TNF-α and IgG in the mucosa and plasma samples were determined using ELISA kits for TNF-α (#PTA00, R&D Systems, Minneapolis, MN) and IgG (#E101-104, Bethyl Laboratories Inc., Montgomery, TX) following the manufacturer’s protocols. The concentrations of MDA and PC were measured by commercial assay kits (#STA-330 and STA-310, respectively, Cell Biolabs, Inc., San Diego, CA) following the manufacture protocols. The working range of TNF-α standards was 0 to 1,500 pg/mL. The minimum detectable TNF-α was 2.8 pg/mL, and the intra- and interassay coefficients of variation (CV) were 4.1% and 8.3%, respectively. The working range of IgG standards was 0 to 500 ng/mL. The minimum detectable IgG was 0.69 ng/mL, and the intra- and interassay CV were 4.4% and 5.7%, respectively. The working range of MDA standards was 0 to 125 μM. The minimum detectable MDA was 0.98 μM and the intra-assay and interassay CV were 7.8% and 7.3%, respectively. The working range of PC standards was 0 to 7.5 nmol/mg. The minimum detectable PC was 0.38 nmol/mg and the intra-assay and interassay CV were 5.0% and 3.0%, respectively.
Tight junction proteins
Four samples of jejunal tissue in each treatment were used to measure tight junction protein as described by Kim et al. (2019). Tissue samples (50 mg) from the jejunum were weighed and suspended into 0.5 mL RIPA lysis and extraction buffer containing 5 µL protease inhibitor cocktail. Tissue samples were homogenized (Tissuemiser; Thermo Fisher Scientific Inc) on ice. The homogenate was centrifuged at 10,000 × g at 4 °C for 10 min to collect supernatant. The protein concentration of the supernatant was adjusted to 2 µg/µL by using a BCA protein assay as mentioned above. The adjusted supernatant was denatured at 100 °C for 5 min in the water bath and was loaded in each well for SDS-PAGE. After SDS-PAGE, the gel was moved to a polyvinylidene difluoride membrane for transferring a target protein to the membrane. Protein was electrophoretically transferred at 90 mV for 1 h. These were then blocked in 5% skim milk, and incubated overnight at 4 °C with primary antibodies against claudin (Sigma, St. Louis, MO) diluted 1:400, occludin (Abcam, Cambridge, United Kingdom) diluted 1:100, zonula occludens-1 (ZO-1; Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:150, and β-actin (Cell Signaling Technology, Danvers, MA). The membrane was subsequently washed and incubated for 1 h at room temperature with horseradish-conjugated secondary antibodies. Immuno-positive bands were visualized by a chemiluminescent method (ECL, Amersham). The band density of tight junction proteins and housekeeping protein were measured with an image analyzer software (LI-COR Biosciences, Lincoln, NE). Each experiment sample was run in triplicate. Exposure times were adjusted so that the darkest bands did not saturate the film. The band densities were determined by summing all the gray level pixel values, after background subtraction, between the boundaries of the bands denoted by the lines. To avoid errors due to differences in signal intensity between the three blots, the average value of triplicate band density was used to calculate the relative band density of tight junction proteins. Relative band density was determined by dividing the band density of the tight junction protein by the band density of β-actin.
Statistical analysis
A randomized complete block design was used in this study with blocking criteria (in experiment 1, initial BW; in experiment 2, sex and initial BW). In the model, a fixed effect was the β-mannanase supplementation and random effects were sex and initial BW. The data were analyzed using Mixed procedure of SAS (version 9.4, SAS Inst., Inc., Cary, NC). In both studies, a pig was the experimental unit. Polynomial contrasts were pre-planned to evaluate the dose–response to β-mannanase supplementation. The contrasts were determined using the Interactive Matrix Language (IML) procedure of SAS to generate coefficients for the unevenly spaced orthogonal contrasts. These coefficients generated by the IML procedure were then used in the Mixed procedure for contrasts. The viscosity data was further analyzed using the NLMIXED procedure to determine the breakpoint to obtain the optimal β-mannanase supplemental level, as previously described (Jang et al., 2021). The predictor was set by multiplying the β-mannanase inclusion (U/kg of feed) with the overall ADFI to account for the feed consumption of the animals through the experimental period (U/d). The P values less than 0.05 were considered statistically significant and between 0.05 and 0.10 were considered tendency.
Results
Experiment 1
The initial BW of pigs at the beginning of the experiment was 15.5 kg and there was no difference among treatments (Table 3). Increasing supplemental levels of β-mannanase from 0 to 600 U/kg in the feeds tended to linearly reduce (P = 0.076) digesta viscosity (5.23 to 4.38 centipoise [cP]) in the jejunum of pigs (Table 4). Increasing supplemental levels of β-mannanase from 0 to 600 U/kg in the feeds tended to have quadratic effects (P = 0.085) on digesta viscosity (minimum 3.87 cP at 419 U/kg of β-mannanase) in the jejunum of pigs. Increasing supplemental levels of β-mannanase from 0 to 600 U/kg in the feeds also tended to have quadratic effects (P = 0.093) on AID of gross energy (minimum 58.2% at 254 U/kg of β-mannanase) in the pigs. However, there was no difference in AID of DM, NDF, and ADF in nursery pigs by increasing supplemental levels of β-mannanase from 0 to 600 U/kg.
Table 3.
Growth performance of nursery pigs in experiment 1
| Item | β-mannanase, U1/kg | SEM | P value | ||||
|---|---|---|---|---|---|---|---|
| 0 | 200 | 400 | 600 | Linear | Quadratic | ||
| Body weight, kg | |||||||
| day 0 | 15.7 | 15.9 | 15.6 | 15.7 | 0.8 | 0.765 | 0.771 |
| day 11 | 19.5 | 19.6 | 19.3 | 19.4 | 0.9 | 0.582 | 0.817 |
| ADG, g/d | 345 | 342 | 339 | 336 | 23 | 0.606 | 0.993 |
| ADFI, g/d | 736 | 748 | 716 | 732 | 31 | 0.263 | 0.833 |
| G:F | 0.47 | 0.46 | 0.48 | 0.46 | 0.03 | 0.828 | 0.809 |
1One unit of β-mannanase activity is defined as the amount of enzyme required to releases 1 μmol of mannose reducing sugars equivalents per minute from locust bean gum (1.0%) in sodium phosphate buffer (200 mM), pH 6.0 at 50°C.
Table 4.
Jejunal digesta viscosity and AID in nursery pigs fed with β-mannanase in experiment 11.
| Item | β-mannanase, U2/kg | SEM | P value | ||||
|---|---|---|---|---|---|---|---|
| 0 | 200 | 400 | 600 | Linear | Quadratic | ||
| Jejunal digesta viscosity, cP3 | 5.23 | 4.42 | 3.83 | 4.38 | 0.42 | 0.076 | 0.085 |
| AID4, % | |||||||
| Dry matter | 66.0 | 55.7 | 66.3 | 65.1 | 3.7 | 0.571 | 0.134 |
| Energy | 64.3 | 53.4 | 64.5 | 64.7 | 3.9 | 0.395 | 0.093 |
| NDF5 | 35.0 | 36.2 | 35.0 | 33.7 | 8.4 | 0.800 | 0.770 |
| ADF6 | 20.3 | 23.6 | 20.8 | 19.0 | 8.9 | 0.785 | 0.630 |
1Each value in the table represents means of 12 pigs (1 pig per metabolic cage).
2One unit of β-mannanase activity was defined as the amount of enzyme required to releases 1 µmole of mannose reducing sugars equivalents per minute from 1.0% locust bean gum in 200 mM sodium phosphate buffer, pH 6.0 at 50 °C.
3Centipoise.
4AID: apparent ileal digestibility.
5NDF: neutral detergent fiber.
6ADF: acid detergent fiber.
Experiment 2
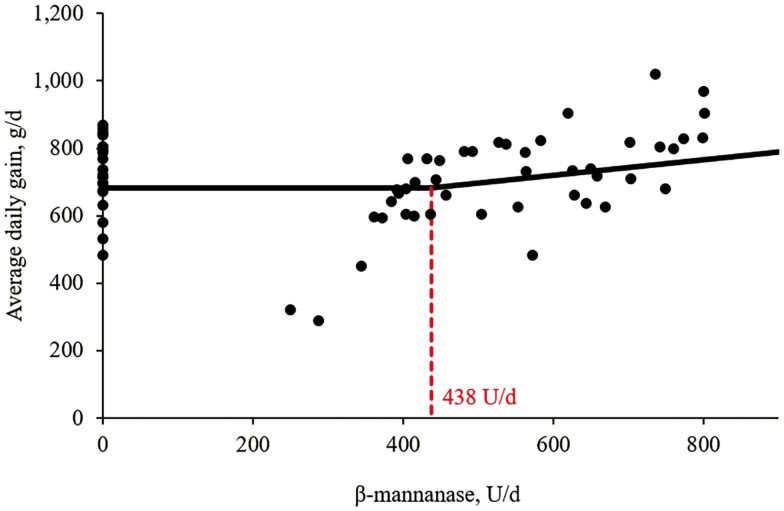
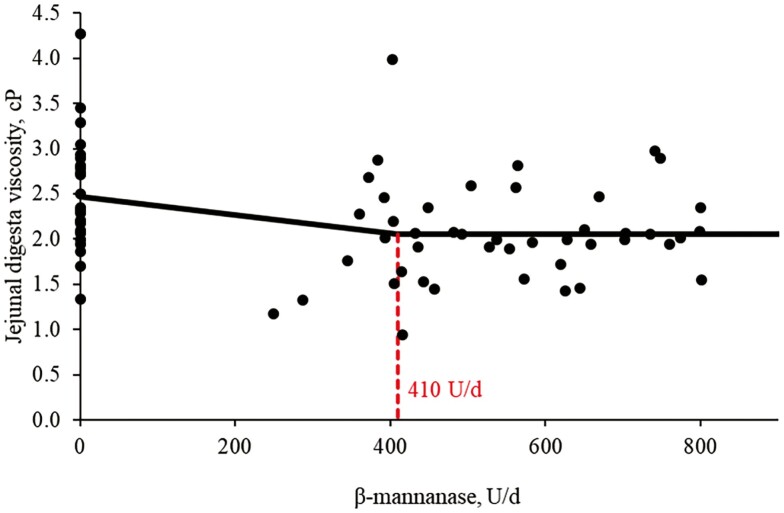
Increasing supplemental levels of β-mannanase from 0 to 600 U/kg in the feeds tended to have quadratic effects (P = 0.080) on ADG of nursery pigs (Table 5). However, there was no difference in BW on day 10, ADFI, and G:F in the pigs by increasing supplemental levels of β-mannanase from 0 to 600 U/kg in the feeds. According to broken-line analysis, ADG was increased (P < 0.05) when β-mannanase was supplemented over 386 U/kg of feeds (Figure 1). Increasing supplemental levels of β-mannanase from 0 to 600 U/kg in the feeds linearly reduced (P < 0.05) digesta viscosity (2.50 to 2.10 cP) in the jejunum of pigs. According to broken-line analysis, jejunal digesta viscosity of the pigs was reduced (P < 0.05) when β-mannanase was supplemented at 360 U/kg of feed (Figure 2).
Table 5.
Growth performance and jejunal digesta viscosity of nursery pigs fed with β-mannanase in experiment 2.
| Item | β-mannanase, U1/kg | SEM | P value | |||
|---|---|---|---|---|---|---|
| 0 | 400 | 600 | Linear | Quadratic | ||
| Body weight, kg | ||||||
| day 0 | 15.4 | 15.5 | 15.5 | 0.8 | 0.546 | 0.424 |
| day 10 | 22.8 | 22.5 | 23.0 | 1.0 | 0.574 | 0.192 |
| ADG, g/d | 737 | 699 | 750 | 28 | 0.632 | 0.080 |
| ADFI, g/d | 1,162 | 1,105 | 1,132 | 39 | 0.507 | 0.295 |
| G:F | 0.64 | 0.64 | 0.67 | 0.02 | 0.192 | 0.383 |
| Jejunal digesta viscosity, cP2 | 2.50 | 2.09 | 2.10 | 0.15 | 0.022 | 0.170 |
1One unit of β-mannanase activity is defined as the amount of enzyme required to releases 1 μmol of mannose reducing sugars equivalents per minute from locust bean gum (1.0%) in sodium phosphate buffer (200 mM), pH 6.0 at 50 °C.
2cP: centipoise (1 cP = 1/100 dyne s/cm2).
Figure 1.
Changes in the ADG (g/d) with supplementation of β-mannanase using a broken-line analysis. The breakpoint was 438 U/d of β-mannanase supplementation when ADG was 683 g/d. The equation for ADG was Y = 683 − 0.41 × zl; if β-mannanase supplementation is lower than the breakpoint, then z = 0; if β-mannanase supplementation is higher than the breakpoint, then zl = breakpoint − β-mannanase supplementation. The P values for the plateau was <0.0001, for the slope was 0.0415, and for the breaking point was 0.0003. The breakpoint was converted from 438 U/d to 386 U/kg of feed by dividing it by the overall average feed intake (1.133 kg/d).
Figure 2.
Changes in the jejunal digesta viscosity (centipoise [cP]) with supplementation of β-mannanase using a broken-line analysis. One cP is equal to the viscosity of water (=0.01 dyne*s/cm2). The breakpoint was 410 U/d of β-mannanase supplementation when the digesta viscosity was 2.06 cP. The equation for jejunal digesta viscosity was Y = 2.058 − 0.001 × zl; if β-mannanase supplementation is higher than the breakpoint, then z = 0; if β-mannanase supplementation is lower than the breakpoint, then zl = breakpoint − β-mannanase supplementation. The P value for the plateau was <0.001, for the slope was 0.021, and for the breaking point was 0.014. The breakpoint was converted from 410 U/d to 360 U/kg of feed by dividing it with the overall average feed intake (1.13 kg/d).
Increasing supplemental levels of β-mannanase from 0 to 600 U/kg in the feeds linearly reduced (P < 0.05) TNF-α (5.81 to 3.81 ng/g of protein), IgG (1.44 to 1.07 mg/g), and MDA (1.46 to 1.26 μmol/g) in the duodenum of pigs (Table 6). Increasing supplemental levels of β-mannanase from 0 to 600 U/kg in the feeds also tended to have quadratic effects on IgG (P = 0.081) and PC (P = 0.093) in the duodenum of pigs. Increasing supplemental levels of β-mannanase from 0 to 600 U/kg in the feeds linearly reduced (P < 0.05) TNF-α (6.23 to 4.19 ng/g of protein), IgG (1.20 to 0.80 mg/g), MDA (1.06 to 0.69 μmol/g), and PC (7.11 to 4.22 μmol/g) in the jejunum of pigs. Increasing supplemental levels of β-mannanase from 0 to 600 U/kg in the feeds linearly reduced (P < 0.05) TNF-α (167.4 to 81.0 pg/mL) and IgG (1.54 to 1.03 pg/mL) in plasma of the pigs. Increasing supplemental levels of β-mannanase from 0 to 600 U/kg in the feeds had quadratic effects (P < 0.05) on MDA and tended to have quadratic effects (P = 0.071) on TNF-α in the plasma of pigs.
Table 6.
Immune response and oxidative stress status in the duodenum, jejunum, and plasma of nursery pigs fed with β-mannanase in experiment 2.
| Item1 | β-mannanase, U2/kg | SEM | P value | |||
|---|---|---|---|---|---|---|
| 0 | 400 | 600 | Linear | Quadratic | ||
| Duodenum | ||||||
| TNF-α, pg/mg protein | 5.81 | 5.19 | 3.81 | 0.58 | 0.033 | 0.342 |
| IgG, μg/mg protein | 1.44 | 1.45 | 1.07 | 0.20 | 0.038 | 0.081 |
| MDA, nmol/mg protein | 1.46 | 1.31 | 1.26 | 0.10 | 0.046 | 0.859 |
| PC, nmol/mg protein | 3.71 | 3.89 | 3.22 | 0.30 | 0.249 | 0.093 |
| Jejunum | ||||||
| TNF-α, pg/mg protein | 6.23 | 5.51 | 4.19 | 0.79 | 0.046 | 0.438 |
| IgG, μg/mg protein | 1.20 | 1.07 | 0.80 | 0.10 | 0.008 | 0.250 |
| MDA, nmol/mg protein | 1.06 | 0.96 | 0.69 | 0.12 | 0.002 | 0.123 |
| PC, nmol/mg protein | 7.11 | 4.61 | 4.22 | 0.41 | <0.001 | 0.268 |
| Plasma | ||||||
| TNF-α, pg/mL | 167.4 | 69.9 | 81.0 | 19.8 | <0.001 | 0.071 |
| IgG, mg/mL | 1.54 | 1.35 | 1.03 | 0.12 | 0.004 | 0.275 |
| MDA, nmol/mL | 19.8 | 11.9 | 17.6 | 2.2 | 0.187 | 0.011 |
| PC, nmol/mg protein | 3.76 | 3.33 | 3.68 | 0.44 | 0.737 | 0.375 |
1TNF-α: tumor necrosis factor-α; IgG: immunoglobulin G; MDA: malondialdehyde; PC: protein carbonyl.
2One unit of β-mannanase activity is defined as the amount of enzyme required to releases 1 μmol of mannose reducing sugars equivalents per minute from locust bean gum (1.0%) in sodium phosphate buffer (200 mM), pH 6.0 at 50°C.
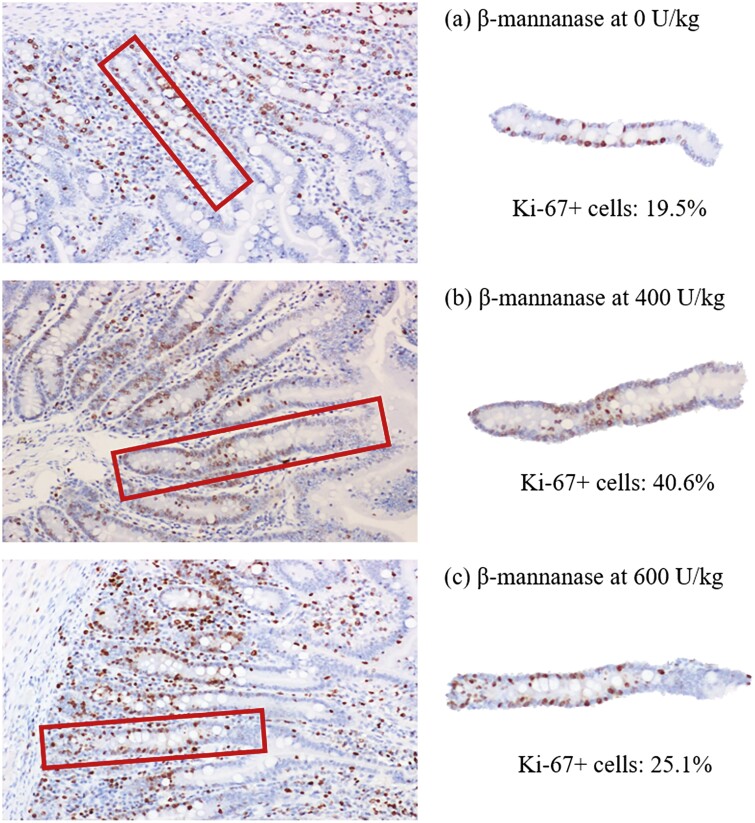
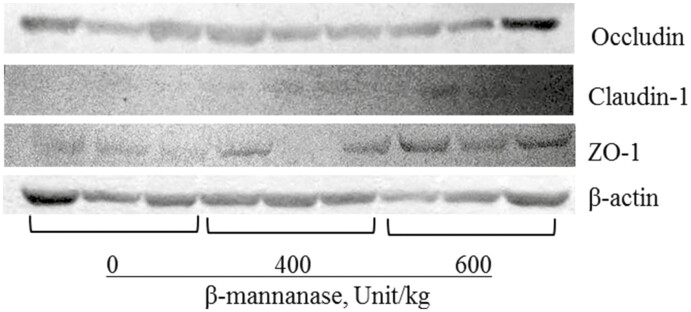
Increasing supplemental levels of β-mannanase from 0 to 600 U/kg in the feeds linearly increased (P < 0.05) VH (579 to 651 μm) and CD (281 to 301 μm) in the duodenum of nursery pigs (Table 7). Increasing supplemental levels of β-mannanase from 0 to 600 U/kg in the feeds linearly increased (P < 0.05) VH (426 to 516 μm), CD (175 to 246 μm), VH:CD (2.48 to 2.13) in the jejunum of pigs. Increasing supplemental levels of β-mannanase from 0 to 600 U/kg in the feeds linearly increased (P < 0.05) crypt cell proliferation (29.3 to 35.5%) and also had quadratic effects (P < 0.05) on crypt cell proliferation in the jejunum of pigs (Figure 3). Increasing supplemental levels of β-mannanase from 0 to 600 U/kg in the feeds did not affect expressions of occludin and claudin-1 in the jejunum of nursery pigs. However, increasing supplemental levels of β-mannanase from 0 to 600 U/kg in the feeds tended to linearly improve (P = 0.083) the expression of ZO-1 in the jejunum of pigs (Figure 4).
Table 7.
Morphology and crypt cell proliferation in the duodenum and jejunum of nursery pigs fed with β-mannanase in experiment 2.
| Item | β-mannanase, U1/kg | SEM | P value | |||
|---|---|---|---|---|---|---|
| 0 | 400 | 600 | Linear | Quadratic | ||
| Duodenum | ||||||
| Villus height, μm | 579 | 671 | 651 | 43 | 0.023 | 0.174 |
| Crypt depth, μm | 281 | 303 | 301 | 8.1 | 0.042 | 0.329 |
| VH:CD2 | 2.06 | 2.20 | 2.15 | 0.12 | 0.313 | 0.379 |
| Jejunum | ||||||
| Villus height, μm | 426 | 487 | 516 | 16 | <0.001 | 0.944 |
| Crypt depth, μm | 175 | 224 | 246 | 7.8 | <0.001 | 0.864 |
| VH:CD | 2.48 | 2.19 | 2.13 | 0.11 | 0.022 | 0.687 |
| Crypt cell proliferation, % | 29.3 | 40.7 | 35.5 | 1.99 | 0.014 | 0.011 |
| Tight junction proteins3 | ||||||
| Occludin | 0.80 | 0.72 | 0.88 | 0.09 | 0.577 | 0.294 |
| Claudin-1 | 0.57 | 0.52 | 0.54 | 0.18 | 0.791 | 0.772 |
| Zonula occludens -1 | 0.84 | 0.84 | 1.18 | 0.13 | 0.083 | 0.307 |
1One unit of β-mannanase activity is defined as the amount of enzyme required to releases 1 μmol of mannose reducing sugars equivalents per minute from locust bean gum (1.0%) in sodium phosphate buffer (200 mM), pH 6.0 at 50°C.
2VH:CD:villus height to crypt depth ratio.
3Relative band density represents each tight junction’s band ratio compared with β-action’s band density.
Figure 3.
Representing images of crypts and crypt cells expressing Ki-67 proteins in the jejunum of pigs fed diets with (a) 0 U/kg of β-mannanase, (b) 400 U/kg, and (c) 600 U/kg. Using immunohistochemistry, cells expressing Ki-67 protein are shown in dark round dots. Percent of proliferating cells in a crypt is measured.
Figure 4.
Tight junction proteins in the jejunum of nursery pigs fed with β-mannanase in experiment 2.
Discussion
Intestinal health is comprehensive, considering the aspects of nutrient digestion and absorption, the capacity for intestinal antioxidation, immune responses, and the balance of intestinal microbiota (Jang, 2022). These factors collectively also contribute to the integrity of the intestinal barrier, a crucial aspect of nutrient utilization and a defense system against invasions by potentially harmful bacteria, toxins, or viral pathogens. The barrier function drives through complex interactions among the intestinal immune system, commensal bacteria, and the epithelial layer, effectively allowing the absorption of nutrients and transport of substances and preventing the entry of undesirable substances into the circulatory system of the body (Vancamelbeke and Vermeire, 2017). However, nursery pig generally has immature intestine insufficient to break down complex carbohydrates commonly present in feedstuffs and also susceptible to intestinal barrier dysfunction with the immune system and microbiota imbalance (Jang and Kim, 2022). In particular, β-mannans, as one of the complex carbohydrates, can contribute to an increase in digesta viscosity, which can inhibit nutrient absorption and impair the growth of pigs (Kiarie et al., 2013). β-Mannanase, an NSPase enzyme, specifically targets β-mannans and hydrolysis the β-1,4-mannosidic bonds, thereby improving the digestibility of feed ingredients and reducing digesta viscosity (Ximenes et al., 2005; Genova et al., 2023). This study shows that supplementation of β-mannanase at 361 U/kg of nursery feed could minimize the digesta viscosity in the jejunum of nursery pigs. In addition, β-mannanase supplementation showed benefits on intestinal health by reducing TNF-a, IgG, MDA, and PC, enhancing crypt cell proliferation, and increasing the expression of tight junction proteins in the jejunum of nursery pigs.
In this study, no differences in the feed efficiency might be related to possible aspects: 1) the period of supplementation was insufficient to detect the changes in feed efficiency, or 2) the baseline diet was already enough to drive improvement by the effects of β-mannanase in the short term. Future studies with longer durations, challenging conditions, or varied dietary conditions may provide further understanding of the effects of β-mannanase on feed efficiency. In this study, supplementation of β-mannanase from 0 to 600 U/kg also shows interesting patterns in BW gain of nursery pigs. Initially, as the supplemental levels were at 0 to 400 U/kg, there was a decrease of 38 g/d in ADG. However, with supplementation levels at 400 to 600 U/kg, ADG increased to 51 g/d. In other words, in the initial range, there was a decrease of 0.1 g per 1 U enzyme, but in the 400 to 600 U/kg range, there was an increase of 0.26 g per 1 U enzyme, which was opposite and more than double the initial rate. Considering the distance among the supplementation levels, this could indicate that the BW gain response to β-mannanase supplementation would fit a plateau model rather than a quadratic regression model. According to broken-line analysis, β-mannanase supplementation at over 386 U/kg could enhance the BW gain of nursery pigs. This study also shows that supplementation of β-mannanase at 361 U/kg of nursery feed could minimize the digesta viscosity in the jejunum of nursery pigs with benefits on intestinal health by reducing TNF-a, IgG, MDA, and PC, enhancing crypt cell proliferation, and increasing the expression of tight junction proteins in the small intestine of nursery pigs. These findings suggest that β-mannanase supplementation can effectively alleviate the negative effects of β-mannans on the growth and intestinal health of nursery pigs.
Digesta viscosity is critical in nutrient absorption and overall intestinal health of nursery pigs (Moita and Kim, 2022). High viscosity can impair nutrient absorption, negatively impacting growth performance and intestinal health (Chen et al., 2020; Duarte and Kim, 2021; Moita et al., 2022). The results in this study showed that supplementation of β-mannanase clearly reduced the viscosity of jejunal digesta in nursery pigs. Viscosity of jejunal digesta measured showed different values between experiments 1 and 2 possibly due to differences in NDF (12.7% in experiment 1 vs. 8.6% in experiment 2) whereas these values were within viscosity recently observed in the jejunal digesta of nursery pigs (Passos et al., 2015b; Duarte et al., 2019; Moita et al., 2022). This could be supported by the β-mannanase hydrolysis into a smaller degree of polymerization, which can be involved in the viscosity of a substance (Kauzmann and Eyring, 1940). In general, larger molecules tend to increase the viscosity compared with smaller molecules because larger molecules can have more interaction with other external compounds (Kauzmann and Eyring, 1940). Interestingly, the broken-line analysis showed that the jejunal digesta viscosity could be reduced when β-mannanase was supplemented at 360 U/kg feed, indicating that the impact on viscosity caused by β-mannans disappeared when β-mannans were degraded by β-mannanase into smaller units of mannan oligosaccharides up to a certain degree. However, the information on the degree of polymerization that increases viscosity is limited and further research is needed to explore the effect of the degree or size of the hydrolysis products on viscosity (Baker et al., 2021).
This study primarily aimed to investigate the responses to the small intestine of nursery pigs because antinutritional soluble NSP including galactomannan and β-mannans causing digesta viscosity mainly affect the small intestine of nursery pigs (Choi et al. 2023; Deng et al. 2023; Duarte et al. 2023). Thus, this study investigated the impacts of β-mannanase supplementation on responses to the small intestine where the nutrient intervention and the challenges effectively work, although β-mannanase could have potential benefits through the entire gastrointestinal tract. Hydrolysis β-mannans can effectively enhance nutrient absorption by reducing digesta viscosity and increasing the accessibility of digestive enzymes to nutrients (Chen et al., 2017; Jang et al., 2023a). However, in this study, β-mannanase supplementation did not show significant changes in nutrient digestibility of the pigs. The result could be attributed to antinutritional compounds from soybean meal as it contains not only β-mannans but also antinutritional and allergenic proteins including trypsin inhibitors, lectins, glycinin, and β-conglycinin (Hong et al., 2004; Kim et al., 2010). These antinutritional compounds in feedstuffs could exacerbate intestinal inflammation and impair nutrient digestion, potentially leading to diarrhea in nursery pigs (Li et al., 1990; Roh et al., 2015). The β-mannans are typically present in the middle lamella and primary cell wall, where β-mannans contribute to the structure and rigidity (Buckeridge, 2010; Ahl et al., 2019). The β-mannans interact with other NSP and proteins to form a complex that provides the cell strength and flexibility (Carpita and Gibeaut, 1993; Cosgrove, 2005; Scheller and Ulvskov, 2010). Previous studies have shown that minor mixed-linked mannans could combine with allergenic protein components as the primary N-linked carbohydrate structure of β-conglycinin (Koshiyama, 1966; Kimura et al., 1997). Interestingly, the levels of IgG and PC in the duodenum were numerically increased by β-mannanase supplementation, indicating that β-mannanase supplementation could potentially exacerbate immune responses and oxidative stress in the duodenum of nursery pigs when the supplementation level is insufficient. Therefore, insufficient supplementation of β-mannanase may offset the positive effects of hydrolyzing β-mannans as a part of releasing antinutritional soluble NSP on nutrient digestion, possibly due to the release of antinutritional compounds in the feeds.
Intestinal barrier function was evaluated using multiple parameters known to have roles in intestinal barrier function. The protein expression of tight junction protein was measured as the primary indicator to determine the barrier function because these proteins play critical roles in maintaining the paracellular barrier, preventing the passage of unexpected substances, such as viruses, antinutritional compounds, allergenic proteins, pathogens, etc., into the body. In addition, inflammatory cytokines (TNF-α and IL-6) and oxidative damage products (MDA and PC) as well as jejunal morphology and crypt cell proliferation were measured for this study. These parameters have been used to evaluate barrier function. In addition, the mucosal tissue from the small intestine was collected to measure intestinal localized markers of intestinal immune response and serum to evaluate systemic immune response in the body. The localized markers, including TNF-α and IgG, refer to the immune status in the small intestine, indicating direct responses to the enzyme supplementation, whereas systemic markers represent the overall immune response in the body because localized and systemic immune responses are closely linked, with immune cells and signaling molecules or proteins, to efficiently response to immunostimulants from the external environment. This connection could provide an understanding of the beneficial effects of β-mannanase on intestinal health and then connected to the immune response in the whole body. If β-mannanase changes the systemic markers without affecting the localized markers, it can be assumed that β-mannanase supplementation in feeds indirectly causes changes in the systemic immune response by affecting immune cells from other tissues. In this study, β-mannanase supplementation clearly reduced the production of both localized and systemic markers. This could indicate that β-mannanase could reduce the intestinal immune response and thus positively affect the overall immune status in the body.
Increasing supplemental levels of β-mannanase linearly reduced TNF-α, IgG, MDA, and PC both in the duodenum and the jejunum of nursery pigs. The TNF-α, a pro-inflammatory cytokine, along with IgG, an immunoglobulin, are key mediators of inflammatory response, and MDA and PC are products of oxidative damage (Duarte and Kim, 2022; Deng et al., 2023b; Jang et al., 2023b). Previous studies have shown that the decreases in TNF-α and IgG (Holanda and Kim, 2022; Deng et al., 2023a) and MDA and PC (Chen et al. 2017; Frame et al. 2020; ) concentrations in the jejunal mucosa of pigs correlate with improved growth performance and also correlate with positive changes in other indicators of intestinal health including the expressions of tight junction proteins, villi structures, and intestinal oxidative stress. The findings, which align with the changes in these indicators, suggest that β-mannanase supplementation could enhance intestinal health through reductions in TNF-α, IgG, MDA, and PC concentrations in the small intestine of the pigs. Thus, this may be partly due to mannan oligosaccharides from the hydrolysis of β-mannans which can help reduce intestinal inflammation and oxidative damage by potentially multiple mechanisms. Firstly, by promoting the growth of beneficial bacteria, mannan oligosaccharides may suppress harmful bacteria that trigger the activation of intestinal immune cells, subsequently reducing inflammatory cytokines (Yang et al., 2021). Additionally, by enhancing intestinal barrier function, mannan oligosaccharides may prevent the infiltration of pathogenic bacteria and their toxins into the circulatory, leading to a potential decrease in reducing the activation of immune cells, especially B lymphocytes for immunoglobulin production (Kim et al., 2016; Yang et al., 2021; Lu et al., 2022). According to Lu et al. (2022), the recognition of mannan oligosaccharides as pathogen-associated molecular patterns by immune cells can effectively suppress immune responses by reducing the production of cytokines and immunoglobulins. Arsenault et al. (2017) also showed that supplementation of β-mannanase to hydrolyze β-mannans positively regulates the majority of this immune-related signaling, indicating that the feed-induced immune response is suppressed by the addition of β-mannanase. Therefore, supplemental β-mannanase in swine feed hydrolyzes β-mannans, mitigating their negative impact on nutrient utilization and immune response while enhancing metabolic efficiency, thereby supporting more sustainable and antibiotic-free animal protein production (Arsenault and Kogut. 2015; Kiarie et al., 2022).
This study also shows that β-mannanase supplementation increased VH:CD and crypt cell proliferation in the jejunum of nursery pigs, indicating an enhancement in intestinal absorptive capacity. Furthermore, this study also evaluated the expression of tight junction proteins, including both cytosolic (ZO-1) and membrane-associated types (occludin and claudin-1) to evaluate the intestinal barrier integrity by β-mannanase supplementation. The ZO-1, occludin, and claudin-1 in the tight junction complex are important in maintaining barrier selectivity and permeability, essential for nutrient absorption and pathogen defense. Interestingly, the protein expression of ZO-1 tended to be increased by increasing β-mannanase supplementation. The ZO-1 is a scaffolding protein that is one of the multidomain proteins usually localized at sites of intercellular junctions and can interact with other noncytosolic tight junction proteins to regulate barrier function (Meyer et al., 2022). Based on these changes by β-mannanase supplementation, it could be expected that mannan oligosaccharides may stimulate the proliferation of enterocytes, which can enhance the structure and barrier function of the intestinal epithelium. This stimulation could be also due to the modulation of intestinal microbiota by mannan oligosaccharides for the growth of beneficial bacteria, producing short-chain fatty acids that provide energy for enterocytes and promote their proliferation (Jana et al., 2021).
In addition, the direct interaction of mannan oligosaccharides with intestinal epithelium can stimulate enterocyte proliferation. According to Mavrogeni et al. (2022), nondigestible oligosaccharides, including mannan oligosaccharides, could directly interact with intestinal epithelial and immune cells via the mitogen-activated protein kinases (MAPKs)-associated pathways involved in cellular proliferation. Furthermore, the ratio of crypt cell proliferation in the jejunum could be also partly increased by β-mannanase supplementation with the levels of MDA and TNF-α in the plasma showing minimum following the quadratic responses. Although crypt cell proliferation is important for maintaining intestinal structure and barrier function, excessive proliferation may lead to a high turnover of enterocytes, possibly resulting in immature enterocytes with increasing energy or nutrient mobilization for growth, thereby undesirably impacting growth (Williams et al., 2015). The supplementation of high levels of β-mannanase may lead to excessive production of mannan oligosaccharides that activate functional receptors on the enterocytes regarding the immune response and cell proliferation, causing overstimulation of the immune system and oxidative stress in the body (Nochta et al., 2009; Zhou et al., 2019). The overstimulation of the immune response and oxidative stress could negatively affect the health and growth of pigs, which are diverting energy from growth to immune response, causing tissue inflammation and damage, and possibly reducing the effectiveness against actual pathogens. This imbalance may also cause additional oxidative stress, leading to cellular damage. This could indicate that there is a need for careful dosage optimization to achieve beneficial effects without adverse outcomes. Therefore, this study could indicate that supplementation of β-mannanase could positively affect the intestinal structure and barrier function by activating crypt cell proliferation through direct or indirect mannan oligosaccharides interaction with the intestinal epithelium of nursery pigs.
In conclusion, supplementation of β-mannanase at 360 U/kg in the corn-soybean meal-based feeds could minimize digesta viscosity and up to 600 U/kg positively affected growth performance and intestinal health of pigs by reducing intestinal inflammation and oxidative stress and enhancing the intestinal structure and barrier function.
Acknowledgments
This research was funded by North Carolina Agricultural Foundation (Raleigh, NC, USA), USDA-NIFA (Hatch #02893 and National Needs Fellowship #2019-38420-28970, Washington DC, USA), and CTCBIO Inc. (Seoul, Korea). We acknowledge technical supports from Dr. Hongyu Chen, Dr. Jiyao Guo, Dr. In Kyung Park, Dr. Wanpuech Parnsen, and the members of the Kim lab at North Carolina State University.
Glossary
Abbreviations
- ADF
acid detergent fiber
- ADFI
average daily feed intake
- ADG
average daily gain
- AID
apparent ileal digestibility
- BW
body weight
- cP
centipoise
- DDGS
distiller’s dried grains with solubles
- DM
dry matter
- ELISA
enzyme-linked immunoassay
- G:F
gain to feed ratio; immunoglobulin G
- IgG
immunoglobulin G
- MAPKs
mitogen-activated protein kinases
- MDA
malondialdehyde
- NDF
neutral detergent fiber
- NSP
non-starch polysaccharides
- PC
protein carbonyl
- SID
standard ileal digestibility
- TNF-α
tumor necrosis factor alpha
- U
unit
- VH:CD
, villus height to crypt depth
- ZO-1
zonula occludens-1
Contributor Information
Ki Beom Jang, Department of Animal Science, North Carolina State University, Raleigh, NC 27695, USA.
Young Ihn Kim, Department of Animal Science, North Carolina State University, Raleigh, NC 27695, USA.
Marcos Elias Duarte, Department of Animal Science, North Carolina State University, Raleigh, NC 27695, USA.
Sung Woo Kim, Department of Animal Science, North Carolina State University, Raleigh, NC 27695, USA.
Author Contributions
Conceptualization and design: S.W.K.; Methodology: S.W.K.; Formal analysis: S.W.K., K.B.J., M.E.D., and Y.I.K.; Investigation: S.W.K., K.B.J., M.E.D., and Y.I.K.; Data interpretation: S.W.K., K.B.J., M.E.D., and Y.I.K.; Writing-original draft preparation: S.W.K. and K.B.J.; Writing-review and editing: S.W.K., K.B.J., M.E.D., and Y.I.K.; Supervision: S.W.K.; Funding acquisition: S.W.K. All authors have agreed to the published version of the manuscript.
Conflict of Interest Statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Agazzi, A., Perricone V., Omodei Zorini F., Sandrini S., Mariani E., Jiang X. -R., Ferrari A., Crestani M., Nguyen T. X., Bontempo V.,. et al. 2020. Dietary mannan oligosaccharides modulate gut inflammatory response and improve duodenal villi height in post-weaning piglets improving feed efficiency. Animals. 10:1283. doi: 10.3390/ani10081283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahl, L. I., Mravec J., Jørgensen B., Rudall P. J., Rønsted N., and Grace O. M... 2019. Dynamics of intracellular mannan and cell wall folding in the drought responses of succulent Aloe species. Plant Cell Environ. 42:2458–2471. doi: 10.1111/pce.13560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC. 2006. Official methods of analysis of AOAC International. doi: 10.3109/15563657608988149 [DOI] [Google Scholar]
- Arsenault, R. J., and Kogut M. H... 2015. Immunometabolism and the kinome peptide array: a new perspective and tool for the study of gut health. Front. Vet. Sci. 2:44. doi: 10.3389/fvets.2015.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenault, R. J., Lee J. T., Latham R., Carter B., and Kogut M. H... 2017. Changes in immune and metabolic gut response in broilers fed β-mannanase in β-mannan-containing diets. Poult. Sci. 96:4307–4316. doi: 10.3382/ps/pex246 [DOI] [PubMed] [Google Scholar]
- Baker, J. T., Duarte M. E., Holanda D. M., and Kim S. W... 2021. Friend or foe? Impacts of dietary xylans, xylooligosaccharides, and xylanases on intestinal health and growth performance of monogastric animals. Animals. 11:609. doi: 10.3390/ani11030609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian, B., Ingale S. L., Park J. H., Rathi P. C., Shanmugam S., and Kim I. H... 2018. Inclusion of dietary β-mannanase improves performance and ileal digestibility and reduces ileal digesta viscosity of broilers fed corn-soybean meal based diet. Poult. Sci. 97:3097–3101. doi: 10.3382/ps/pey157 [DOI] [PubMed] [Google Scholar]
- Buckeridge, M. S. 2010. Seed cell wall storage polysaccharides: models to understand cell wall biosynthesis and degradation. Plant Physiol. 154:1017–1023. doi: 10.1104/pp.110.158642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita, N. C., and Gibeaut D. M... 1993. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. 3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x [DOI] [PubMed] [Google Scholar]
- Chen, H., Zhang S., Park I., and Kim S. W... 2017. Impacts of energy feeds and supplemental protease on growth performance, nutrient digestibility, and gut health of pigs from 18 to 45 kg body weight. Anim. Nutr. 3:359–365. doi: 10.1016/j.aninu.2017.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H., Zhang S., and Kim S. W... 2020. Effects of supplemental xylanase on gut health and growth performance of nursery pigs fed diets with corn distillers’ dried grains with solubles. J. Anim. Sci. 98:skaa185. doi: 10.1093/jas/skaa185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, H., Chen Y., Longo F., and Kim S. W... 2023. Comparative effects of benzoic acid and sodium benzoate in diets for nursery pigs on growth performance and acidification of digesta and urine. J. Anim. Sci. 101:skad116. doi: 10.1093/jas/skad116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove, D. J. 2005. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 6:850–861. doi: 10.1038/nrm1746 [DOI] [PubMed] [Google Scholar]
- Deng, Z., Duarte M. E., Jang K. B., and Kim S. W... 2022. Soy protein concentrate replacing animal protein supplements and its impacts on intestinal immune status, intestinal oxidative stress status, nutrient digestibility, mucosa-associated microbiota, and growth performance of nursery pigs. J. Anim. Sci. 100:skac255. doi: 10.1093/jas/skac255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, Z., Jang K. B., Jalukar S., Du X., and Kim S. W... 2023a. Efficacy of feed additive containing bentonite and enzymatically hydrolyzed yeast on intestinal health and growth of newly weaned pigs under chronic dietary challenges of fumonisin and aflatoxin. Toxins (Basel). 15:433. doi: 10.3390/toxins15070433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, Z., Duarte M. E., and Kim S. W... 2023b. Efficacy of soy protein concentrate replacing animal protein supplements in mucosa-associated microbiota, intestinal health, and growth performance of nursery pigs. Anim. Nutr. 14:235–248. doi: 10.1016/j.aninu.2023.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte, M. E., and Kim S. W... 2021. Modulation of jejunal mucosa-associated microbiota in relation to nutrient digestibility and intestinal health of pigs by supplementation of β-glucanase to corn soybean meal-based diets with xylanase. J. Anim. Sci. 99:1–13. doi: 10.1093/jas/skab190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte, M. E., and Kim S. W... 2022. Phytobiotics from oregano extracts enhance the intestinal health and growth performance of pigs. Antioxidants. 11:2066. doi: 10.3390/antiox11102066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte, M. E., Zhou F. X., W. M.Dutra, Jr, and Kim S. W... 2019. Dietary supplementation of xylanase and protease on growth performance, digesta viscosity, nutrient digestibility, immune and oxidative stress status, and gut health of newly weaned pigs. Anim. Nutr. 5:351–358. doi: 10.1016/j.aninu.2019.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte, M. E., Stahl C. H., and Kim S. W... 2023. Intestinal oxidative damages by F18+ Escherichia coli and its amelioration with an antibacterial bacitracin fed to nursery pigs. Antioxidants. 12:1040. doi: 10.3390/antiox12051040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federation of Animal Science Societies. 2020. Chapter 9: Swine. In: Guide for the care and use of agricultural animals in research and teaching. Ag Guide. 4th ed. Champaign (IL): American Dairy Science Association. [Google Scholar]
- Frame, C. A., Johnson E., Kilburn L., Huff-Lonergan E., Kerr B. J., and Serao M. R... 2020. Impact of dietary oxidized protein on oxidative status and performance in growing pigs. J. Anim. Sci. 98:skaa097. doi: 10.1093/jas/skaa097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genova, J. L., Rupolo P. E., de Azevedo L. B., Henz D., Carvalho S. T., Kipper M., Gonçalves G. de A. C., Vilela H. L. O., Pasquetti T. J., de Oliveira N. T. E.,. et al. 2023. β-Mannanase supplementation in diets reduced in 85 kcal metabolizable energy/kg containing xylanase-phytase improves gain to feed ratio, nutrient usage, and backfat thickness in finisher pigs. Front. Vet. Sci. 10:692. doi: 10.3389/fvets.2023.1144692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halas, V., and Nochta I... 2012. Mannan oligosaccharides in nursery pig nutrition and their potential mode of action. Animals. 2:261–274. doi: 10.3390/ani2020261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holanda, D. M., and Kim S. W... 2022. Impacts of weaning weights and mycotoxin challenges on jejunal mucosa-associated microbiota, intestinal and systemic health, and growth performance of nursery pigs. J. Anim. Sci. Biotechnol. 13:43. doi: 10.1186/s40104-022-00691-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, K. J., Lee C. H., and Kim S. W... 2004. Aspergillus oryzae GB-107 fermentation improves nutritional quality of food soybeans and feed soybean meals. J. Med. Food. 7:430–435. doi: 10.1089/jmf.2004.7.430 [DOI] [PubMed] [Google Scholar]
- Hyun, Y., Ellis M., McKeith F. K., and Wilson E. R... 1997. Feed intake pattern of group-housed growing-finishing pigs monitored using a computerized feed intake recording system. J. Anim. Sci. 75:1443–1451. doi: 10.2527/1997.7561443x [DOI] [PubMed] [Google Scholar]
- Jana, U. K., Kango N., and Pletschke B... 2021. Hemicellulose-derived oligosaccharides: emerging prebiotics in disease alleviation. Front. Nutr. 8:670817. doi: 10.3389/fnut.2021.670817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, K. B. 2022. Postnatal growth and intestinal health of pigs through nutritional intervention [Doctoral dissertation]. North Carolina State University. NC State Library. https://www.lib.ncsu.edu/resolver/1840.20/39521 [Google Scholar]
- Jang, K. B., and Kim S. W... 2022. Role of milk carbohydrates in intestinal health of nursery pigs: a review. J. Anim. Sci. Biotechnol. 13:6. doi: 10.1186/s40104-021-00650-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, K. B., Kim J. H., Purvis J. M., Chen J., Ren P., Vazquez-Anon M., and Kim S. W.. 2020b. Effects of mineral methionine hydroxy analog chelate in sow diets on epigenetic modification and growth of progeny. J. Anim. Sci. 98:1–12. doi: 10.1093/JAS/SKAA271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, K. B., Purvis J. M., and Kim S. W... 2021. Dose–response and functional role of whey permeate as a source of lactose and milk oligosaccharides on intestinal health and growth of nursery pigs. J. Anim. Sci. 99:skab008. doi: 10.1093/jas/skab008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, K. B., Zhao Y., Kim Y. I., Pasquetti T., and Kim S. W... 2023a. Effects of bacterial β-mannanase on apparent total tract digestibility of nutrients in various feedstuffs fed to growing pigs. Anim. Biosci. 36:1700–1708. doi: 10.5713/ab.23.0158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, K. B., Moita V. H. C., Martinez N., Sokale A., and Kim S. W... 2023b. Efficacy of zinc glycinate reducing zinc oxide on intestinal health and growth of nursery pigs challenged with F18 + Escherichia coli. J. Anim. Sci. 101:1–17. doi: 10.1093/jas/skad035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon, S. M., Hosseindoust A., Choi Y. H., Kim M. J., Kim K. Y., Lee J. H., Kil D. Y., Kim B. G., and Chae B. J... 2019. Comparative standardized ileal amino acid digestibility and metabolizable energy contents of main feed ingredients for growing pigs when adding dietary β-mannanase. Anim. Nutr. 5:359–365. doi: 10.1016/j.aninu.2019.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha, R., and Mishra P... 2021. Dietary fiber in poultry nutrition and their effects on nutrient utilization, performance, gut health, and on the environment: a review. J. Anim. Sci. Biotechnol. 12:51. doi: 10.1186/s40104-021-00576-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauzmann, W., and Eyring H... 1940. The viscous flow of large molecules. J. Am. Chem. Soc. 62:3113–3125. doi: 10.1021/ja01868a059 [DOI] [Google Scholar]
- Kiarie, E., Romero L. F., and Nyachoti C. M... 2013. The role of added feed enzymes in promoting gut health in swine and poultry. Nutr. Res. Rev. 26:71–88. doi: 10.1017/S0954422413000048 [DOI] [PubMed] [Google Scholar]
- Kiarie, E. G., Steelman S., Martinez M., and Livingston K... 2021. Significance of single β-mannanase supplementation on performance and energy utilization in broiler chickens, laying hens, turkeys, sows, and nursery-finish pigs: a meta-analysis and systematic review. Transl. Anim. Sci. 5:txab160. doi: 10.1093/tas/txab160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiarie, G. K., Steelman S., and Martinez M... 2022. Does supplementing β-mannanase modulate the feed-induced immune response and gastrointestinal ecology in poultry and pigs? An appraisal. Front. Anim. Sci. 3:875095. doi: 10.3389/fanim.2022.875095 [DOI] [Google Scholar]
- Kim, S. W., and Duarte M. E... 2021. Understanding intestinal health in nursery pigs and the relevant nutritional strategies. Anim. Biosci. 34:338–344. doi: 10.5713/ab.21.0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. W., van Heugten E., Ji F., Lee C. H., and Mateo R. D... 2010. Fermented soybean meal as a vegetable protein source for nursery pigs: I. Effects on growth performance of nursery pigs. J. Anim. Sci. 88:214–224. doi: 10.2527/jas.2009-1993 [DOI] [PubMed] [Google Scholar]
- Kim, S. W., Brooks K. L., and Weaver A. C... 2016. Effects of supplemental β-mannanase to provide substrates for binding to aflatoxin in feed on growth and health of pigs. Proceedings of the 17th Asian-Australasian Association of Animal Production. p. 399.
- Kim, J. S., Ingale S. L., Hosseindoust A. R., Lee S. H., Lee J. H., and Chae B. J... 2017. Effects of mannan level and β-mannanase supplementation on growth performance, apparent total tract digestibility and blood metabolites of growing pigs. Animal. 11:202–208. doi: 10.1017/S1751731116001385 [DOI] [PubMed] [Google Scholar]
- Kim, S. W., Holanda D. M., Gao X., Park I., and Yiannikouris A... 2019. Efficacy of a yeast cell wall extract to mitigate the effect of naturally co-occurring mycotoxins contaminating feed ingredients fed to young pigs: impact on gut health, microbiome, and growth. Toxins. 11:633. doi: 10.3390/toxins11110633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, Y., Ohno A., and Takagi S... 1997. Structural analysis of N-glycans of storage glycoproteins in soybean (Glycine max L) seed. Biosci. Biotechnol. Biochem. 61:1866–1871. doi: 10.1271/bbb.61.1866 [DOI] [PubMed] [Google Scholar]
- Koshiyama, I. 1966. Carbohydrate component in 7S protein of soybean casein fraction. Agric. Biol. Chem. 30:646–650. doi: 10.1080/00021369.1966.10858660 [DOI] [Google Scholar]
- Lallès, J. P., Bosi P., Smidt H., and Stokes C. R... 2007. Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 66:260–268. doi: 10.1017/S0029665107005484 [DOI] [PubMed] [Google Scholar]
- Li, D. F., Nelssen J. L., Reddy P. G., Blecha F., Hancock J. D., Allee G. L., Goodband R. D., and Klemm R. D... 1990. Transient hypersensitivity to soybean meal in the early-weaned pig. J. Anim. Sci. 68:1790–1799. doi: 10.2527/1990.6861790x [DOI] [PubMed] [Google Scholar]
- Lu, Z. Y., Feng L., Jiang W. D., Wu P., Liu Y., Jiang J., Kuang S. Y., Tang L., Li S. W., Zhong C. B.,. et al. 2022. Dietary mannan oligosaccharides strengthens intestinal immune barrier function via multipath cooperation during aeromonas hydrophila infection in grass carp (Ctenopharyngodon idella). Front. Immunol. 13:1010221. doi: 10.3389/fimmu.2022.1010221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv, J. N., Chen Y. Q., Guo X. J., Piao X. S., Cao Y. H., and Dong B... 2013. Effects of supplementation of β-mannanase in corn-soybean meal diets on performance and nutrient digestibility in growing pigs. Asian Australas. J. Anim. Sci. 26:579–587. doi: 10.5713/ajas.2012.12612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrogeni, M. E., Asadpoor M., Henricks P. A. J., Keshavarzian A., Folkerts G., and Braber S... 2022. Direct action of non-digestible oligosaccharides against a leaky gut. Nutrients. 14:4699. doi: 10.3390/nu14214699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, T. N., Schwesinger C., and Denker B. M... 2022. Zonula occludens-1 is a scaffolding protein for signaling molecules. Galpha(12) directly binds to the Src homology 3 domain and regulates paracellular permeability in epithelial cells. J. Biol. Chem. 277:24855–24858. doi: 10.1074/jbc.C200240200. [DOI] [PubMed] [Google Scholar]
- Moita, V. H. C., and Kim S. W... 2022. Nutritional and functional roles of phytase and xylanase enhancing the intestinal health of nursery pigs and broiler chickens. Animals. 12:3322. doi: 10.3390/ani12233322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moita, V. H. C., and Kim S. W... 2023. Efficacy of a bacterial 6-phytase supplemented beyond traditional dose levels on jejunal mucosa-associated microbiota, ileal nutrient digestibility, bone and intestinal health, and growth performance of nursery pigs. J. Anim. Sci. 101:skad134. doi: 10.1093/jas/skad134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moita, V. H. C., Duarte M. E., and Kim S. W... 2022. Functional roles of xylanase enhancing intestinal health and growth performance of nursery pigs by reducing the digesta viscosity and modulating the mucosa-associated microbiota in the jejunum. J. Anim. Sci. 100:1–15. doi: 10.1093/jas/skac116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nochta, I., Tuboly T., Halas V., and Babinszky L... 2009. Effect of different levels of mannan-oligosaccharide supplementation on some immunological variables in weaned piglets. J. Anim. Physiol. Anim. Nutr. 93:496–504. doi: 10.1111/j.1439-0396.2008.00835.x [DOI] [PubMed] [Google Scholar]
- NRC. 2012. Nutrient requirements of swine: 11th revised edition. Washington (DC): The National Academies Press. [Google Scholar]
- Nyachoti, C. M., Zijlstra R. T., de Lange C. F. M., and Patience J. F... 2004. Voluntary feed intake in growing-finishing pigs: a review of the main determining factors and potential approaches for accurate predictions. Can. J. Anim. Sci. 84:549–566. doi: 10.4141/a04-001 [DOI] [Google Scholar]
- Passos, A. A., Andrade C., Phillips C. E., Coffey M. T., and Kim S. W... 2015a. Nutrient value of spray field forages fed to pigs and the use of fibrolytic enzymes to enhance nutrient digestibility. J. Anim. Sci. 93:1721–1728. doi: 10.2527/jas.2014-8435 [DOI] [PubMed] [Google Scholar]
- Passos, A. A., Park I., Ferket P., von Heimendahl E., and Kim S. W... 2015b. Effect of dietary supplementation of xylanase on apparent ileal digestibility of nutrients, viscosity of digesta, and intestinal morphology of growing pigs fed corn and soybean meal based diet. Anim. Nutr. 1:19–23. doi: 10.1016/j.aninu.2015.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh, S. -G., Carroll J. A., and Kim S. W... 2015. Effects of fermented soybean meal on innate immunity-related gene expressions in nursery pigs acutely challenged with lipopolysaccharides. Anim. Sci. J. 86:508–516. doi: 10.1111/asj.12319 [DOI] [PubMed] [Google Scholar]
- Scheller, H. V., and Ulvskov P... 2010. Hemicelluloses. Annu. Rev. Plant Biol. 61:263–289. doi: 10.1146/annurev-arplant-042809-112315 [DOI] [PubMed] [Google Scholar]
- Tiwari, U. P., Chen H., Kim S. W., and Jha R... 2018. Supplemental effect of xylanase and mannanase on nutrient digestibility and gut health of nursery pigs studied using both in vivo and in vitro model. Anim. Feed Sci. Technol. 245:77–90. doi: 10.1016/j.anifeedsci.2018.07.002 [DOI] [Google Scholar]
- Vancamelbeke, M., and Vermeire S... 2017. The intestinal barrier: a fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 11:821–834. doi: 10.1080/17474124.2017.1343143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Soest, P. J., Robertson J. B., and Lewis B. A... 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2 [DOI] [PubMed] [Google Scholar]
- Williams, C. H., David D. J., and Iismaa O... 1962. The determination of chromic oxide in faeces samples by atomic absorption spectrophotometry. J. Agric. Sci. 59:381–385. doi: 10.1017/s002185960001546x [DOI] [Google Scholar]
- Williams, J. M., Duckworth C. A., Burkitt M. D., Watson A. J. M., Campbell B. J., and Pritchard D. M... 2015. Epithelial cell shedding and barrier function: a matter of life and death at the small intestinal villus tip. Vet. Pathol. 52:445–455. doi: 10.1177/0300985814559404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ximenes, E. A., Chen H., Kataeva I. A., Cotta M. A., Felix C. R., Ljungdahl L. G., and Li X. L... 2005. A mannanase, ManA, of the polycentric anaerobic fungus Orpinomyces sp. strain PC-2 has carbohydrate binding and docking modules. Can. J. Microbiol. 51:559–568. doi: 10.1139/w05-033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, C., Zhang T., Tian Q., Cheng Y., Gebeyew K., Liu G., Tan Z., and He Z... 2021. Supplementing mannan oligosaccharide reduces the passive transfer of immunoglobulin g and improves antioxidative capacity, immunity, and intestinal microbiota in neonatal goats. Front. Microbiol. 12:795081. doi: 10.3389/fmicb.2021.795081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon, S. Y., Yang Y. X., Shinde P. L., Choi J. Y., Kim J. S., Kim Y. W., Yun K., Jo J. K., Lee J. H., Ohh S. J.,. et al. 2010. Effects of mannanase and distillers dried grain with solubles on growth performance, nutrient digestibility, and carcass characteristics of grower-finisher pigs. J. Anim. Sci. 88:181–191. doi: 10.2527/jas.2008-1741 [DOI] [PubMed] [Google Scholar]
- Zheng, L., Duarte M. E., Sevarolli Loftus A., and Kim S. W... 2021. Intestinal health of pigs upon weaning: challenges and nutritional intervention. Front. Vet. Sci. 8:91. doi: 10.3389/fvets.2021.628258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, M., Tao Y., Lai C., Huang C., Zhou Y., and Yong Q... 2019. Effects of mannanoligosaccharide supplementation on the growth performance, immunity, and oxidative status of partridge shank chickens. Animals. 9:817. doi: 10.3390/ani9100817 [DOI] [PMC free article] [PubMed] [Google Scholar]