Abstract
Alcohol use disorders (AUDs) are characterized by repeated episodes of binge drinking. Based on reports that exposure to predator odor stress (PS) consistently increases ethanol intake, the present studies examined whether prior binge drinking differentially altered responsivity to PS and subsequent ethanol intake in male and female mice, when compared to mice without prior binge exposure. Initial studies in naïve male and female C57BL/6J mice confirmed that 30 min exposure to dirty rat bedding significantly increased plasma corticosterone (CORT) levels and anxiety-related behavior, justifying the use of dirty rat bedding as PS in the subsequent drinking studies. Next, separate groups of male and female C57BL/6J mice received 7 binge ethanol sessions (binge) or drank water (controls), followed by a 1 month period of abstinence. Then, 2-bottle choice ethanol intake (10% or 10E versus water, 23 h/day) was measured in lickometer chambers for 4 weeks. After baseline intake stabilized, exposure to intermittent PS (2X/week X 2 weeks) significantly enhanced ethanol intake after the 2nd PS in male, but not female, binge mice versus baseline and versus the increase in controls. However in a subgroup of females (with low baselines), PS produced a similar increase in 10E intake in control and binge mice versus baseline. Analysis of lick behavior determined that the enhanced 10E intake in binge male mice and in the female low baseline subgroup was associated with a significant increase in 10E bout frequency and 10E licks throughout the circadian dark phase. Thus, PS significantly increased 10E intake and had a synergistic interaction with prior binge drinking in males, whereas PS produced a similar significant increase in 10E intake in the low baseline subgroup of binge and control females. Plasma CORT levels were increased significantly in both binge and control animals after PS. CORT levels at 24 h withdrawal from daily 10E intake were highest in the groups with elevated 10E licks (i.e., binge males and control females). At 24 h withdrawal, protein levels of GABAA receptor α1 subunit, corticotropin releasing factor receptor 1, and glucocorticoid receptor in prefrontal cortex (PFC) and hippocampus (HC) were differentially altered in the male and female mice versus levels in separate groups of age-matched naïve mice, with more changes in HC than in PFC and in females than in males. Importantly, the sexually divergent changes in protein levels in PFC and HC add to evidence for sex differences in the neurochemical systems influenced by stress and binge drinking and argue for sex-specific pharmacological strategies to treat AUD.
Keywords: predator odor, anxiety, GABAA receptors, corticotrophin releasing factor receptor 1, prefrontal cortex, hippocampus
Introduction
Alcohol use disorders (AUDs) are characterized by repeated episodes of binge drinking, which is a pattern of drinking that brings blood alcohol (ethanol) concentration (BEC) ≥ 80 mg% (i.e., 80 mg/dL or 0.80 mg/mL). Notably, a history of binge drinking can be a strong predictor of subsequent ethanol dependence (Hingson et al., 2006). Excessive ethanol use is the 4th leading preventable cause of death in the US, but globally, it accounts for 5.9% of all deaths (~ 3.3 million in 2012) and is the leading risk factor for premature death and disability among people between the ages of 15 and 49 (NIAAA, 2017).
The experience of stress may represent a risk factor that shifts ethanol consumption from recreational to excessive (see Becker et al., 2011; Chester et al., 2006; Gilpin & Weiner, 2017; Lynch et al., 1999; Sillaber & Henniger, 2004 and references therein). Human studies document a link between ethanol consumption and stress, with higher levels of consumption in those individuals that experience higher levels of stress (reviewed in Keyes et al., 2012). In rodent animal models, exposure to stress during early development produces a consistent increase in ethanol consumption in adulthood (see Campbell et al., 2009 and references therein). Results are more variable regarding the effects of various stressors in adolescent and adult animals on ethanol self-administration when tested during adulthood (e.g., Becker et al., 2011; Brunell & Spear, 2005; Chester et al., 2004, 2006, 2008; Doremus et al., 2005; Lynch et al., 1999; Sillaber & Henniger, 2004; Tambour et al., 2008). However, recent work found that exposure to predator odor stress (PS) increases subsequent ethanol intake in male and female mice and male rats (Cozzoli et al., 2014; Edwards et al., 2013; Manjoch et al., 2016; Roltsch et al., 2014). Exposure to various forms of PS also increases thermal nociception, startle reactivity, and anxiety-related behaviors in ethanol naïve rats and mice (Belzung et al., 2001; Cohen & Zohar, 2004; Cohen et al., 2008; Gilpin & Weiner, 2017; Hebb et al., 2003; Roltsch et al., 2014; Whitaker & Gilpin, 2015), suggesting that a high arousal and anxiety state may contribute to the PS-induced increase in subsequent ethanol intake. One hallmark of PS is that behavioral and neuroendocrine responses to repeated PS exposure often lead to sensitization rather than habituation, similar to what occurs with post-traumatic stress disorder (PTSD; Staples, 2010). As a result, exposure to PS is considered a traumatic stress, and it is used as a model of PTSD (reviewed in Cohen & Zohar, 2004; Dielenberg & McGregor, 2001; Gilpin & Weiner, 2017; Matar et al, 2013).
PTSD is now classified as a trauma- or stressor-related disorder, and several studies document an association between PTSD and the development of an AUD (reviewed in Gilpin & Weiner, 2017). While PTSD symptoms may precede the onset of AUD (Gilpin & Weiner, 2017), the prevalence of AUD among those with PTSD is estimated at 28% for women and 52% for men (Norman et al., 2012). In general, comorbid PTSD/AUD is associated with greater psychological distress, diminished social functioning, poorer treatment response, more frequent hospitalizations, more physical health problems, and increased AUD-related problems than with either disorder alone (Norman et al., 2012). Thus, PTSD symptoms appear to promote excessive ethanol drinking, whereas ethanol abuse worsens PTSD symptoms. This comorbidity negatively influences recovery prognosis and effective pharmacotherapeutic strategies.
Stress activates the hypothalamic-pituitary-adrenal (HPA) axis, and abnormalities in the HPA axis are observed in both AUD and PTSD, suggesting that the overlap in HPA involvement may constitute a neurobiological mechanism underlying the comorbidity of these disorders (Gilpin & Weiner, 2017; Norman et al., 2012). For example, some individuals with PTSD have an increased number and sensitivity of glucocorticoid receptors (GRs), enhanced negative feedback of the HPA axis, and elevated corticotropin releasing factor (CRF) levels in cerebrospinal fluid (Pittman et al., 2012; Zoladz & Diamond, 2013). Using the PS model of PTSD, exposure to PS significantly increased plasma corticosterone (CORT) and adrenocorticotropic hormone (ACTH) levels in male and female rodents (Cozzoli et al., 2014; Whitaker & Gilpin, 2015), with a greater increase in female versus (vs) male mice (Cozzoli et al., 2014) and a greater increase in “Avoider” rats with a high anxiety phenotype (Whitaker & Gilpin, 2015). CRF peptide levels also were elevated significantly following PS in the central nucleus of the amygdala and the ventromedial prefrontal cortex (vmPFC) of these rats (Itoga et al., 2016; Schreiber et al., 2017). Thus, PS produces similar changes in HPA axis responsivity as seen in PTSD, supporting the suggestion that PS may be used to model PTSD in rodent studies.
There are sex differences in HPA axis responsivity to stress and in CRF receptor 1 (CRF-R1) signaling, which may influence susceptibility to PTSD and AUD. Epidemiological studies indicate that lifetime prevalence of PTSD is twice as high in females as in males (11% vs 5.4%; 9.7% vs 3.6%, respectively; Bangasser & Valentino, 2014; Kilpatrick et al., 2013; Valentino et al., 2013a). Using animal models, sex differences in the coupling of CRF-R1 with second messenger cascades render females more responsive to acute stress and less able to adapt to chronic stress as a result of compromised CRF-R1 internalization (Valentino et al., 2013a, 2013b). In female rats, the decreased ability of CRF-R1 to associate with β-arrestin 2 results in less internalization of CRF-R1 and biased signaling through stimulatory G-protein (Gs) and protein kinase A pathways following stress in locus coeruleus (LC) neurons. In male rats, stress promotes a greater association of CRF-R1 with β-arrestin 2, facilitating internalization of CRF-R1 in LC neurons and a bias toward signaling through β-arrestin 2 (i.e., Gs independent). These sex differences in CRF-R1 signaling, which are unrelated to adult hormone status of male or females, have a functional influence on LC neurons, with the magnitude of LC activation by stress being greater in female vs male rats (Valentino et al., 2013a, 2013b). Thus, mechanisms underlying the PS-associated increase in ethanol intake likely differ in males and females.
Despite the comorbidity of AUD and PTSD in humans, few studies have examined the possibility that binge ethanol exposure could accentuate the physiological and behavioral effects of stress. We contend that prior binge ethanol drinking is a risk factor that interacts with stress to augment subsequent ethanol drinking behavior. To test this assertion, initial studies in naïve male and female C57BL/6J mice confirmed that 30 min exposure to dirty rat bedding significantly increased plasma CORT levels and anxiety-related behavior, which provided a rationale for the use of dirty rat bedding as PS in the current studies. Then, the subsequent drinking studies examined whether prior binge drinking differentially altered responsivity to intermittent PS and subsequent ethanol intake in adult male and female C57BL/6J mice, when compared to mice without previous binge ethanol exposure. Accompanying changes in levels of select proteins in the PFC and hippocampus (HC) also were examined and compared with levels in a separate group of age-matched naïve mice to explore neurochemical responses following prior binge drinking and intermittent PS exposure. The proteins examined were related to HPA axis and stress (CRF-R1 and GR, see above) and a γ-aminobutyric acidA receptor (GABAAR) subunit that is the most prominent subtype in the adult brain and is important in ethanol dependence (GABAAR α1 subunit; reviewed in Kumar et al., 2009) and that exhibits sex differences following chronic ethanol administration (Devaud et al., 1998). We predicted that prior binge ethanol drinking would differentially alter subsequent ethanol intake and the pattern of changes in protein levels in male and female mice.
Materials and Methods
Animals and General Procedure
Adult male and female C57BL/6J mice were purchased from Jackson Laboratories West (Sacramento, CA) at 8 weeks of age, and each sex was tested in a separate study. Upon arrival mice were group housed, and acclimated to a regular 12 h light/dark cycle (lights off at 1900) in a temperature (22 ± 2°C) and humidity controlled environment. Rodent chow (Labdiet 5001 rodent diet; PMI International, Richmond IN) and water were available ad libitum, with the exception of the mild fluid restriction during the Scheduled High Alcohol Consumption (SHAC) procedure for binge drinking (see below). Animals were 10 weeks old at the start of the study and were individually housed throughout the experiments with nestlets. See Table 1 for Experimental Timeline. For the SHAC procedure, mice (15/treatment/sex) had 7 binge drinking sessions every 3rd day (binge group) or consumed water (control group). During the one month period of abstinence (after binge drinking and prior to lickometer drinking), mice were acclimated to a 12 h reverse light/dark cycle (lights off at 1100) and to consuming food and water in lickometer chambers (see below) during the final week of abstinence. Due to the number of lickometer chambers available (n=24), group size was 12/treatment/sex for the lickometer portion of the study. After measuring water intake for 1 week, ethanol intake (10% v/v ethanol in tap water, 10E vs water; 23 h/day) was measured in lickometer chambers for approximately 4 weeks, with exposure to intermittent PS during weeks 2 and 3 (Table 1). Mice were euthanized 24 h after the final lickometer drinking session with brains rapidly removed, quickly frozen over dry ice, and then stored at −80°C for Western Blot analysis. Brains from naïve animals of a similar age were collected and used for comparisons in the Western Blot analysis. All procedures complied with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were conducted under Institutional Animal Care and Use Committee approved protocols.
Table 1.
Experimental Timeline
| SHAC Procedure | Abstinence | Lickometer Drinking | Naïve Mice |
|---|---|---|---|
|
|
|
|
NOTE: Male and female mice were tested in separate studies. The only difference between the Binge and Control groups was their treatment during the SHAC procedure. These groups were treated similarly during abstinence and the lickometer drinking phases of the study. Separate groups of experimentally naïve male and female mice were used to measure CORT levels, to measure anxiety levels on the EPM, or were euthanized as a comparator group for the Western Blot analyses.
BEC = blood ethanol concentration; CORT = corticosterone; 5E = 5% v/v ethanol; 10E = 10% v/v ethanol; PS = predator odor stress; SHAC = Scheduled High Alcohol Consumption; EPM = elevated plus maze
SHAC procedure
The SHAC procedure was used to model binge drinking, as we have shown that scheduling periods of fluid access produces consistent and high ethanol intake in male and female mice (≥ 2 g/kg in 30 min) and BECs ≥ 100 mg/dL (or 1.0 mg/mL), which exceeds the NIAAA criteria for binge drinking (e.g., Cozzoli et al., 2016; Finn et al., 2005; Strong et al., 2010; Tanchuck et al., 2011). Mice in the “binge” groups received 7 binge ethanol sessions for 30 min/session (single bottle with a 5% v/v ethanol solution in tap water) every 3rd day, with water consumption the remainder of the time. Retro-orbital sinus blood (20 μL) was collected immediately following the 7th binge session and analyzed for ethanol content via headspace gas chromatography (Finn et al., 2007). Mice in the “control” groups received the same schedule of fluid access but drank only tap water.
Lickometer drinking procedure
During the final week of the 30 day ethanol abstinence period following the SHAC procedure, all animals were habituated to consuming tap water and food in lickometer chambers (see Ford et al., 2005; Ramaker et al., 2015). Briefly, each lickometer chamber was a 4-walled Plexiglas insert (7 × 4 × 7 in) that contained two holes where the sippers of the drinking tubes entered the cage. The insert was positioned on an elevated wire grid floor inside a mouse shoebox cage with bedding. All mice had access to a bottle containing 10E and one containing tap water for 23 h/day for approximately 4 weeks. The 23 h period of access to 10E and water began approximately 30 min prior to lights off. Intake was measured 7 days/week. The first week of 10E intake represented baseline. During the next 2 weeks, mice were exposed to PS (see below) during the 1 h period prior to the next measurement of 23 h 10E intake. Post-stress intake was measured during week 4 (Table 1).
Drinking patterns (10E vs water) were monitored with lickometer circuits (Med Associates, Inc., St. Albans, VT) that were attached to each fluid sipper and that recorded time-stamped licks for each mouse and each fluid presented. Based on our prior work indicating that 10E licks and g/kg ethanol intake are highly correlated when hourly intake was measured over 24 h (r=0.694, p<0.01, n=24; Figure 1 in Ramaker et al., 2011), only 10E licks were recorded and analyzed. After the measurement of 10E intake on day 24, the 10E tube was removed for a 24 h period of withdrawal (i.e., mice drank only water), followed by euthanasia on day 25.
Figure 1. Similar increase in anxiety-related behavior after 2nd exposure to predator odor stress (PS) in naïve male (A) and female (B) mice.
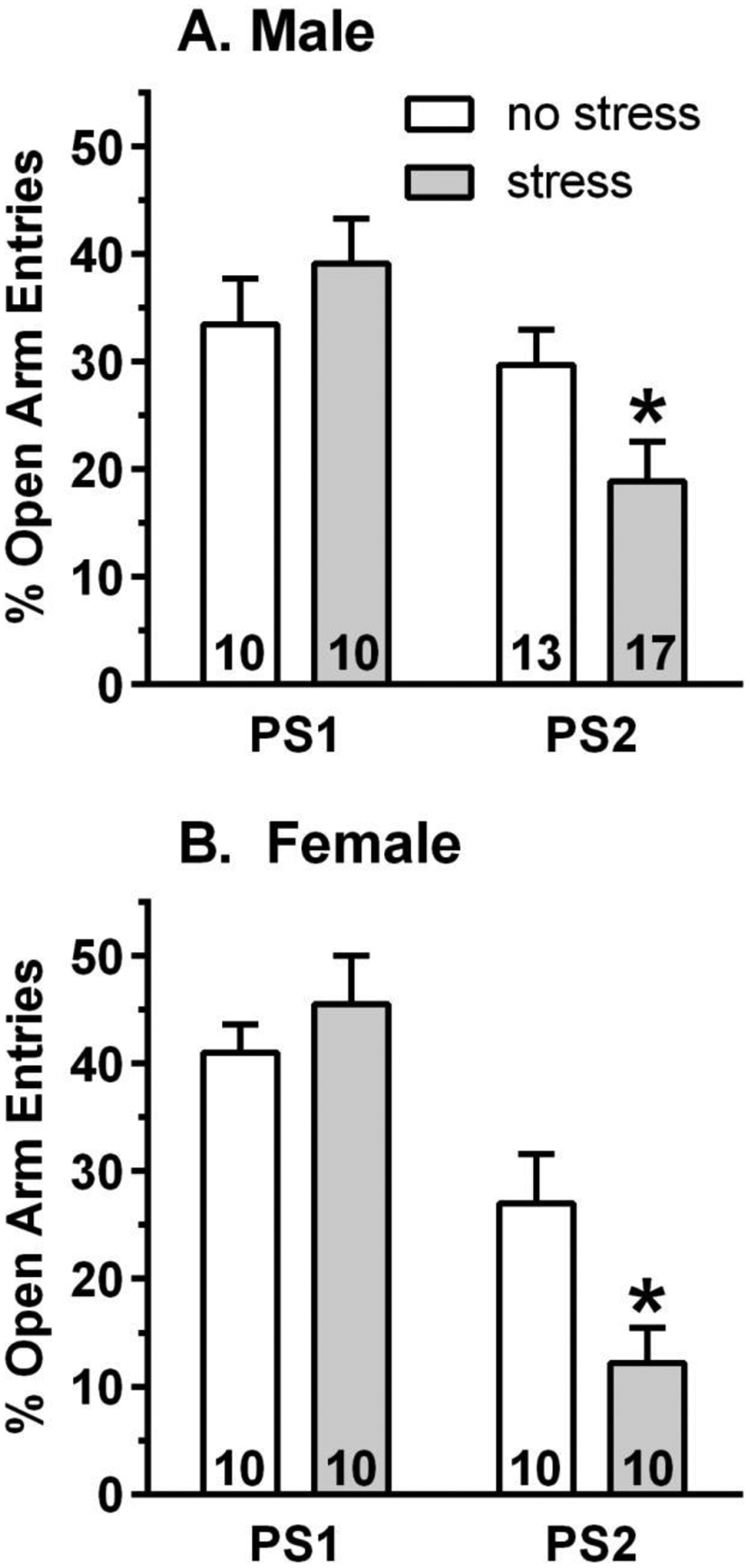
Separate groups of naïve male and female mice were tested on the elevated plus maze at 2 – 2.5 h after 1 or 2 exposures to PS (stress) or following no PS exposure (no stress). Compared to the no stress mice, 2 exposures to PS was required to observe a significant increase in anxiety-related behavior, measured by a significant decrease in percent (%) open arm entries. Following the 2nd PS, % open arm entries were decreased by 36% in males and by 55% in females. Values are the mean ± SEM for the number of animals contained in each bar. *p<0.05 vs respective no stress group.
PS exposure
Mice were exposed to dirty rat bedding for 30 min on Tuesday and Friday of the stress weeks (i.e., 2X/week x 2 weeks) at approximately 1 h prior to lights off. Briefly, mice were removed from their lickometer chambers, placed into a clean polycarbonate cage (28 × 18 × 13 in) with Ecofresh bedding, and transported to the procedure room. Then, mice were placed into another polycarbonate cage of the same size filled with soiled rat bedding that had been obtained from male and female rat cages after the rats had been housed in their cages for 1 week (Cozzoli et al., 2014). Immediately following the 1st and 4th PS, tail blood samples (~ 20 μL) were collected for subsequent plasma CORT measurements in 5 μL plasma with a commercially available 125I radioimmunoassay kit (ImmuChem Double Antibody Corticosterone for rodents; MP Biomedicals, Santa Ana, CA), as recently described (Cozzoli et al., 2014). In a separate group of naïve male and female mice, tail blood samples were collected after the 1st and 3rd PS for subsequent plasma CORT measurements (Table 1).
Elevated plus maze (EPM) test
Anxiety-related behavior was tested in separate groups of naïve adult male and female C57BL/6J mice following 1 or 2 exposures to PS for 30 min or following no PS exposure (Table 1). Mice were moved to a separate room for the EPM test and allowed to habituate to that room for at least 1 h. The EPM test was conducted at 2 – 2.5 h post-PS in a dimly lit room (21 lux). Mice were placed on the center of the EPM for a 5 min test. Open and closed arm entries and time were recorded.
Western blot analysis of relative brain protein levels
Immunoblotting was performed on PFC and HC tissue that was dissected from mouse brains obtained from the binge and control groups and from age matched naïve mice. Tissue was homogenized, and a total particulate fraction was collected by centrifugation. Protein concentrations for each tissue were determined using the Thermo Scientific (Rockport, IL) BCA procedure. Sample homogenates (20 μg protein/sample) were diluted with sample loading buffer (Bio Rad, Hercules, CA), denatured for 3 min and then separated using a 10-well 4–15% Tris-glycine gel in a Bio Rad Western Blot apparatus. Most gels had 3 naïve, 3 control and 3 binge samples from the same tissue and sex (i.e., male PFC) for within-blot comparisons. After separation, proteins were transferred to PVDF membranes (Bio Rad immuno-blot PVDF). Blots were incubated with selected antibodies: CRF-R1 (1:400) or GR (1:50) (Thermo Scientific) and the GABAAR α1 subunit (1:200) or the housekeeping protein, β-actin (1:500) (Santa Cruz Biotechnologies, Dallas TX), followed by incubation with the appropriate HRP-conjugated secondary antibody (1:8000). Blots were then incubated in chemiluminescent substrate (HyGlo, Denville Sci, Holliston MA), and signals were captured using a Bio Rad ChemiDoc MP imager. Relative density measurements were collected and quantified using the Image Lab software. Measurements were normalized to the β-actin signal for equivalent protein loading. Three – five data points were collected from each treatment group animal across independent immunoblots, converted to percent of naive values, and then the converted values were averaged for each animal for summary and statistical analysis.
Statistical analysis
Because the male and female mice were tested in separate studies, data for each sex were analyzed separately. For the SHAC portion of the study, t-tests determined the influence of treatment (binge vs control) on body weight and total fluid consumption. Ethanol intake and BEC also are provided for the binge groups. For the Lickometer portion of the study, the dependent variables were 10E licks/23 h, hourly 10E licks, bout frequency, bout size, and plasma CORT levels. Two way ANOVA determined the effect of prior binge (binge) or water (control) consumption (factor = treatment) on the dependent variables, with time (either day or h) as a repeated measures factor. Because we were predicting treatment differences in the lickometer portion of the study, planned comparisons were conducted with or without the presence of a significant interaction. A similar strategy was used for the female data that was divided into subgroup of high vs low baseline, since the distribution of 10E licks clustered into 2 groups, with > 900 10E lick differences separating the high vs low baseline subgroup distributions of 10E licks. Thus, the decision to separate mice into the high vs low baseline subgroups was based on a > 900 lick difference separating the mouse with the highest baseline in the “low” subgroup and the lowest baseline in the “high” subgroup. Separate studies in naïve mice determined the effect of PS vs no PS on EPM behavior (% open arm entries and % open arm time) and plasma CORT levels with one-way ANOVA. Plasma CORT levels after PS exposure in naïve mice vs mice in the lickometer studies (i.e., drinking ethanol) were compared with two-way ANOVA. Western blot data for each sex and brain region were analyzed for treatment effects (naïve, control, binge) using one-way ANOVA with Tukey’s post hoc tests.
All data are presented as mean ± SEM, and analysis was conducted with SYSTAT (versions 11 & 13, SYSTAT Software, Inc., Richmond, CA). The level of significance was set at p≤0.05, and p≤0.09 was considered a statistical trend.
Results
Significant increase in plasma CORT levels in naïve male and female mice after PS exposure
The purpose of this initial study was to determine whether dirty rat bedding as PS exposure activated the HPA axis, measured by a significant increase in plasma CORT levels. Plasma CORT levels were measured in separate groups of experimentally naïve mice at baseline and following PS exposures. For PS exposure, mice were individually housed, and plasma CORT levels were measured after 1 or 3 exposures to dirty rat bedding (30 min; Table 1). The results are depicted in Table 2. While absolute values were higher in female vs male mice, PS exposure significantly increased plasma CORT levels in males [F(2,12)=39.39, p<0.001 and post-hoc tests] vs baseline. Similarly, PS exposure significantly increased plasma CORT levels in females [F(2,14)=19.51, p<0.001 and post-hoc tests] vs baseline. Plasma CORT levels did not differ between a single or repeated PS exposures in naïve mice (Table 2), indicating that dirty rat bedding as PS exposure produced a significant activation of the HPA axis that was similar across repeated PS exposures.
Table 2.
Predator Odor Stress (PS)-Induced Changes in Plasma Corticosterone (CORT) Levels in Naïve Male and Female Mice
| Sex | Baseline | PS1 | PS3 |
|---|---|---|---|
| Male | 8.56 ± 3.36 μg/dL (n=10) | 41.39 ± 2.83 μg/dL*** (n=5) | 46.18 ± 3.87 μg/dL*** (n=4) |
| Female | 12.54 ± 2.48 μg/dL (n=7) | 53.43 ± 4.16 μg/dL*** (n=5) | 53.43 ± 9.66 μg/dL*** (n=5) |
Experimentally naïve mice had tail blood samples taken for baseline CORT levels. Separate groups of experimentally naïve mice had tail blood samples taken after 1 or 3 PS exposures. While absolute values were higher in females vs males, exposure to PS significantly increased CORT levels in male and female mice vs respective baseline. Values are mean ± SEM for the number of mice in parentheses.
p<0.001 vs respective baseline
Similar change in anxiety-related behavior following PS in naïve male and female mice
Since dirty rat bedding exposure increased anxiety-related behavior in male CD-1 mice (Hebb et al., 2003), we measured EPM behavior in separate groups of naïve male and female mice following 1 or 2 PS exposures (30 min exposure to dirty rat bedding) or no PS exposure. Compared to unstressed mice, 2 PS exposures (2X/week, as with the lickometer drinking studies) were required to observe a significant increase in anxiety-related behavior, measured by a significant decrease in percent (%) open arm entries in male [F(1,28)=4.47, p<0.05] and female [F(1,18)=6.88, p<0.05] mice (Figure 1). Similar results were observed for % open arm time (data not shown). Following the 2nd PS exposure, % open arm entries and % open arm time were decreased by 36% and 65% in males and by 55% and 30% in females, respectively. There was no significant change in % open arm entries or % open arm time after the 1st PS exposure versus the no stress group. One or two exposures to PS did not alter closed arm entries or total entries (used as an index of activity) in males or females, when compared to the respective no stress groups (data not shown). Thus, 2 exposures to PS significantly increased anxiety-related behavior in ethanol naïve male and female mice without altering activity.
Binge drinking
Male and female mice underwent 7 binge ethanol sessions (binge, n=15/sex) or consumed water (control, n=15/sex). In male mice, mean ± SEM ethanol intake and BEC during the final binge session was 1.94 ± 0.12 g/kg and 1.37 ± 0.05 mg/mL, respectively, and BEC was significantly correlated with ethanol intake (r=0.5457, p<0.05, n=15). Overall ethanol intake across binge sessions was 2.17 ± 0.06 g/kg. In female mice, mean ± SEM ethanol intake and BEC during the final binge session was 1.97 ± 0.11 g/kg and 1.42 ± 0.08 mg/mL, respectively, and there was a strong trend for a correlation between BEC and ethanol intake (r=0.4964, p<0.06, n=15). The overall ethanol intake across the 7 binge sessions was 2.39 ± 0.10 g/kg. These results confirm that both male and female mice in the binge groups consumed high doses of ethanol in the 30 min sessions and achieved BECs that greatly exceeded the NIAAA criteria for binge drinking (i.e., 0.80 mg/mL vs 1.37 and 1.42 mg/mL in the present study).
Body weights in the control and binge groups did not differ at the start or finish of the SHAC procedure. In male mice, mean ± SEM body weights on day 1 were 24.04 ± 0.54 g (control) and 23.71 ± 0.52 g (binge) and on day 21 were 25.11 ± 0.58 g (control) and 24.55 ± 0.42 g (binge), representing a 3.5 – 4% increase in body weight across time in both groups. In female mice, mean ± SEM body weights on day 1 were 18.06 ± 0.29 g (control) and 18.37 ± 0.27 g (binge) and on day 21 were 19.18 ± 0.36 g (control) and 19.35 ± 0.22 g (binge), representing a 5 – 6% increase in body weight across time in both groups. Overall total fluid intake also did not differ in the control and binge groups for the male (water = 3.70 ± 0.09 mL; binge = 3.73 ± 0.12 mL) and female (water = 3.69 ± 0.13 mL; binge 3.52 ± 0.14 mL) mice. Thus, treatment (binge vs water control) did not significantly alter body weight or total fluid intake in either sex.
Sex differences in the influence of prior binge drinking on subsequent ethanol intake and patterns of drinking following intermittent PS
Based on the CORT and EPM results in naïve mice (Figure 1 and Table 2), the lickometer studies used dirty rat bedding as the PS. As depicted in Figure 2, prior binge drinking experience produced a greater enhancement in 10E intake after PS in male but not in female mice, when the analysis was conducted across all animals. The initial analysis was conducted on the daily 10E lick data (days 8 – 24) and averaged baseline (days 5–7, when baseline drinking had stabilized). In male mice, 10E licks were significantly influenced by treatment (binge vs control) [F(1,20)=5.97, p<0.05] and were significantly altered by time/PS [F(17,340)=9.46, p<0.001]. The significant interaction between treatment and time/PS [F(17,340)=2.05, p<0.01] confirmed the significant divergent change in 10E licks following PS in male binge vs control mice (Figure 2A). Post-hoc tests indicated that 10E licks were significantly higher in binge vs control male mice after PS2 – PS4. In contrast, 10E licks in female mice were changing across time/PS [F(17,374)=5.84, p<0.001], but there was no main effect of treatment nor interaction with binge exposure (Figure 2C).
Figure 2. Prior binge drinking alters responsivity to predator odor stress (PS) in male mice and in a subgroup of female mice with low baselines.
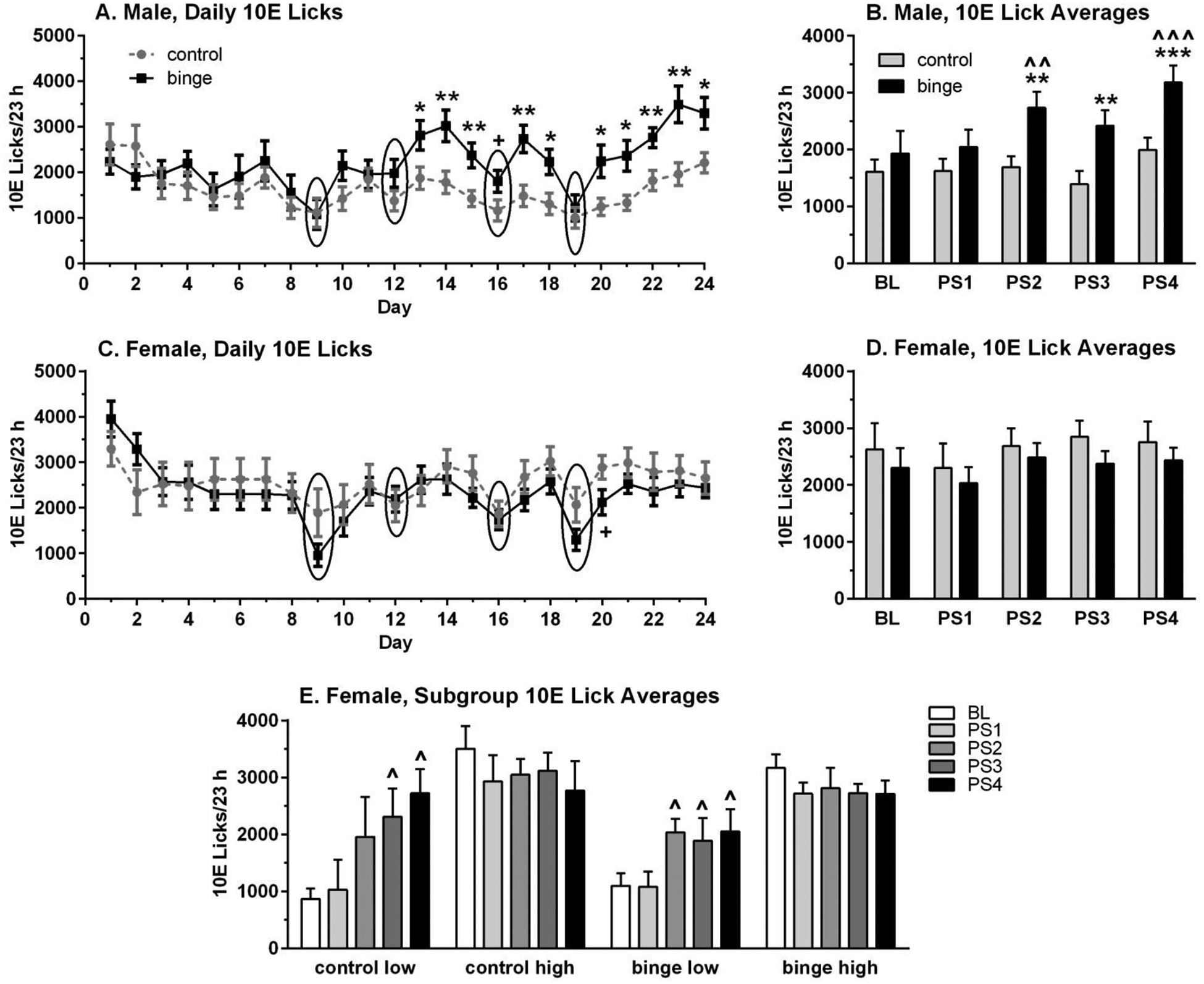
Depicted are mean ± SEM licks on the ethanol sipper (10% ethanol, 10E)/23 h for n=12/group/sex for panels A – D, and labels for each panel refer to prior binge ethanol (binge) or water (control) intake. Group size for female subgroups in panel E is: n=4 (control low), n=8 (control high), n=5 (binge low), n=7 (binge high). Overall, 10E intake began diverging after the 2nd PS in males with prior binge drinking experience, but only began diverging after the 2nd PS in binge and control female subgroups with low baseline 10E intake. Panels A & C: Daily 10E licks are shown; circles highlight days with exposure to PS prior to the assessment of 23 h 10E intake. Panels B, D & E: 10E licks were averaged into 5 blocks: baseline (BL, days 5 – 7), PS1 (after 1st PS, days 10 – 11), PS2 (after 2nd PS, days 13–15), PS3 (after 3rd PS, days 17 – 18), and PS4 (after 4th PS, days 22 – 24). +p<0.07, *p<0.05, **p<0.01, ***p<0.001 vs respective control; ^p<0.05, ^^p<0.01, ^^^p<0.001 vs respective baseline.
Follow-up analyses were conducted on data that were averaged into 5 blocks: baseline (days 5 – 7), PS1 (days 10 – 11), PS2 (days 13 – 15), PS3 (days 17 – 18), and PS4 (days 22 – 24). As with the analysis of daily 10E licks in male mice, the averaged 10E licks were significantly influenced by treatment [F(1,22)=6.85, p<0.02] and time/PS [F(4,88)=7.23, p<0.001], with a significant interaction between treatment and time/PS [F(4,88)=2.45, p=0.05] (Figure 2B). Averaged 10E licks in the binge male mice were significantly increased across time/PS [F(4,44)=6.44, p<0.001], with post-hoc tests confirming that 10E licks were significantly increased after PS2 (↑ 42%) and PS4 (↑ 65%) vs baseline. In contrast, averaged 10E licks in the control male mice were not significantly altered across time/PS [F(4,44)=2.04, p=0.106], despite a 24% increase in 10E licks after PS4 vs baseline. Extrapolation of 10E licks to g/kg dose indicated that averaged baseline 10E intake on days 5 – 7 was ~8 g/kg in the controls and ~9 g/kg in the binge male mice, and that it increased to ~10 g/kg (control) and ~14 g/kg (binge) when averaged across days 22 – 24. Importantly, the increase in 10E intake over baseline after PS4 on days 22 – 24 was 24 – 25% in controls vs 65 – 55% in binge mice, when 10E licks and g/kg were used for the calculations, respectively.
Results in females differed from those in males, as the averaged 10E intake in female mice was not significantly altered by prior binge experience or by PS (no main effect of treatment or time/PS and no interaction; Figure 2D). Extrapolation of 10E licks to g/kg dose revealed that averaged 10E increased from ~12 g/kg to ~12.5 g/kg in controls and from ~10.5 g/kg to 11.0 g/kg in binge females (days 5 – 7 vs days 22 – 24). These slight increases in 10E intake over baseline after PS4 on days 22 – 24 were similar when calculations were made on 10E licks or estimated g/kg intake (5 – 4% for control, 6 – 5% for binge, respectively).
Inspection of the baseline 10E lick data revealed a continuous distribution in male mice, but there appeared to be 2 subgroups in females with > 900 lick differences separating averaged low vs high baseline subgroups (see data analysis section for subgroup criterion). Therefore, preliminary analysis examined the data in the female subgroups (Figure 2E). The initial 3-way ANOVA revealed a main effect of subgroup [F(1,20)=17.32, p<0.001] and time/PS [F(4,80)=3.95, p<0.01], with an interaction between subgroup and time/PS [F(4,80)=8.87, p<0.001] on averaged 10E licks. In the females with prior binge exposure, averaged 10E licks were significantly influenced by subgroup [F(1,10)=16.79, p<0.01], tended to be influenced by time/PS [F(4,40)=2.19, p<0.09], and the interaction between subgroup and time/PS was significant [F(4,40)=4.52, p<0.01]. This result was due to the significant increase in averaged 10E licks across time/PS in the binge females with low baselines [F(4,16)=8.68, p=0.001], with post-hoc tests confirming that 10E licks were significantly increased by 72 – 87% vs baseline after PS2, PS3, and PS4 (Figure 2E). In the control females, averaged 10E licks tended to be influenced by time/PS [F(4,40)=2.13, p=0.09], but there was a significant interaction between subgroup and time/PS [F(4,40)=4.78, p<0.01]. The interaction was due to the significant increase in averaged 10E licks across time/PS in the control females with low baselines [F(4,12)=3.86, p<0.05], with post-hoc tests confirming that averaged 10E licks were increased significantly after PS3 (↑ 166%) and PS4 (↑ 214%). Averaged 10E licks were increased by 126% after PS2, but this difference vs baseline was not significant.
Hourly 10E licks were analyzed for one baseline day (day 7 in males and day 8 in females, since hourly 10E licks on days 5 – 7 were lost in females due to a program malfunction) and for one day after the 4th PS (day 23). In general, the majority of drinking behavior occurred during the circadian dark phase in all groups, and the pattern of hourly consumption on the representative baseline day was not significantly different in the binge vs control male and female mice (Supplemental Figure 1A & 1C; only main effect of time: F(22,484)=9.85, p<0.001 for males; F(22,484)=11.10, p<0.001 for females). However, hourly 10E licks were significantly increased throughout the circadian dark phase after the 4th PS in binge vs control male mice (Supplemental Figure 1B). This conclusion is supported by the main effect of treatment [F(1,22)=10.48, p<0.01], time [F(22,484)=14.09, p<0.001] and significant interaction [F(22,484)=2.23, p=0.001]. Notably, the increase started at the first hour measurement, which began approximately 30 min prior to lights off. Inspection of the daily lick data also indicated that there was a transient increase in 10E licks during the circadian dark phase after PS1 (i.e., on day 10) in binge males, with more persistent increases in 10E licks during the circadian dark after each subsequent PS. After the 4th PS in female mice, hourly 10E licks remained similar in the binge and control groups (Supplemental Figure 1D; only main effect of time, F(22,484)=19.72, p<0.001). However, when female subgroups were examined, hourly 10E licks were significantly increased throughout the circadian dark phase after the 4th PS in both binge and control female mice with low baselines (data not shown). This conclusion is supported by the main effect of day [day 8 vs day 23: F(1,14)=10.61, p<0.01], time [F(22,308)=9.46, p<0.001] and significant interaction [F(22,308)=3.32, p<0.001]. Thus, the elevation in hourly 10E licks during the circadian dark phase after intermittent PS in binge male mice and in binge and control females with low baselines contributed to the significant enhancement in 23 h ethanol intake in these animals.
Analysis of the microarchitecture of drinking revealed that the enhanced drinking following PS in binge male mice was due to an increase in bout frequency and bout size (Supplemental Figure 2A & 2B). Note that a bout is experimentally defined as a minimum of 20 licks with no more than a 60 sec pause between licks (Ford et al., 2005, 2008; Ramaker et al., 2011, 2012, 2015). Analysis was conducted on the daily 10E lick data (days 8 – 24) and averaged baseline (days 1 – 7, since hourly data for days 5 – 7 were lost in the female mice). In male mice, there was a significant main effect of treatment on bout frequency [F(1,19)=4.73, p<0.05] and a trend for an increase in bout size [F(1,19)=3.28, p=0.086]. Bout frequency also changed significantly across time/PS [F(17,323)=12.12, p<0.001], and there was a trend for an interaction between treatment and time/PS [F(17,323)=1.52, p=0.085]. The number of bouts/23 h session began increasing after the 2nd PS in binge males, and bout size began increasing after the 1st PS (Supplemental Figure 2A & 2B). Average lick rate (licks/min) also increased, beginning with the 1st PS in binge males, but average bout duration was unchanged (data not shown). Thus, the increase in bout size in male mice with prior binge experience was due to their enhanced lick rate. In female mice, bout frequency and bout size changed significantly across time/PS [Fs(17,340)>2.03, ps≤0.09] (Supplemental Figure 2C & 2D). While there was no main effect of treatment on either bout parameter in females, there was a transient increase in bout frequency and size in the control vs binge females after the 4th exposure to PS. However, in the subgroups of control and binge females with low baselines, there was a significant increase in bout frequency across time/PS, when compared with the pattern in the subgroups with high baselines [binge: time/PS: F(20,160)=3.79, p<0.001, interaction: F(20,160)=2.01, p<0.01; control: time/PS: F(20,200)=2.12, p<0.01, interaction: F(20,200)=1.79, p<0.05]. Bout frequency was significantly lower in the low vs high baseline subgroups, but increased across time after intermittent PS so that bout frequencies did not differ after PS4 in the two subgroups of control and binge females (data not shown). Overall, the PS-induced augmentation in bout frequency and size in male mice with prior binge drinking experience and in bout frequency in female subgroups with low baseline (both control and binge) also contributed to the significant enhancement in 23 h ethanol intake in these animals.
A history of ethanol drinking blunts the PS-induced increase in plasma CORT levels and produces sex differences in the pattern of changes in plasma CORT levels during withdrawal
Plasma CORT levels were measured in all mice following the 1st and 4th exposure to PS as well as at 24 h withdrawal after the final drinking session (i.e., upon euthanasia). The results are depicted in Figure 3. For this study, we did not measure baseline CORT levels, but we will refer to an estimate of “baseline” CORT levels from Cozzoli et al. (2014) that was determined prior to any stress exposures (and following 15 days of 2 h 10E intake in mice on a similar light/dark cycle). These mean ± SEM baseline estimates were 13.085 ± 3.335 μg/dL for males (n=9) and 15.21 ± 2.98 μg/dL for females (n=9). As depicted in Figure 3, CORT levels following PS1 and PS4 were higher than these baseline values.
Figure 3. Divergent effects of prior binge vs control treatment on plasma corticosterone (CORT) levels during withdrawal but not following predator odor stress (PS) in male (A) and female (B) mice.
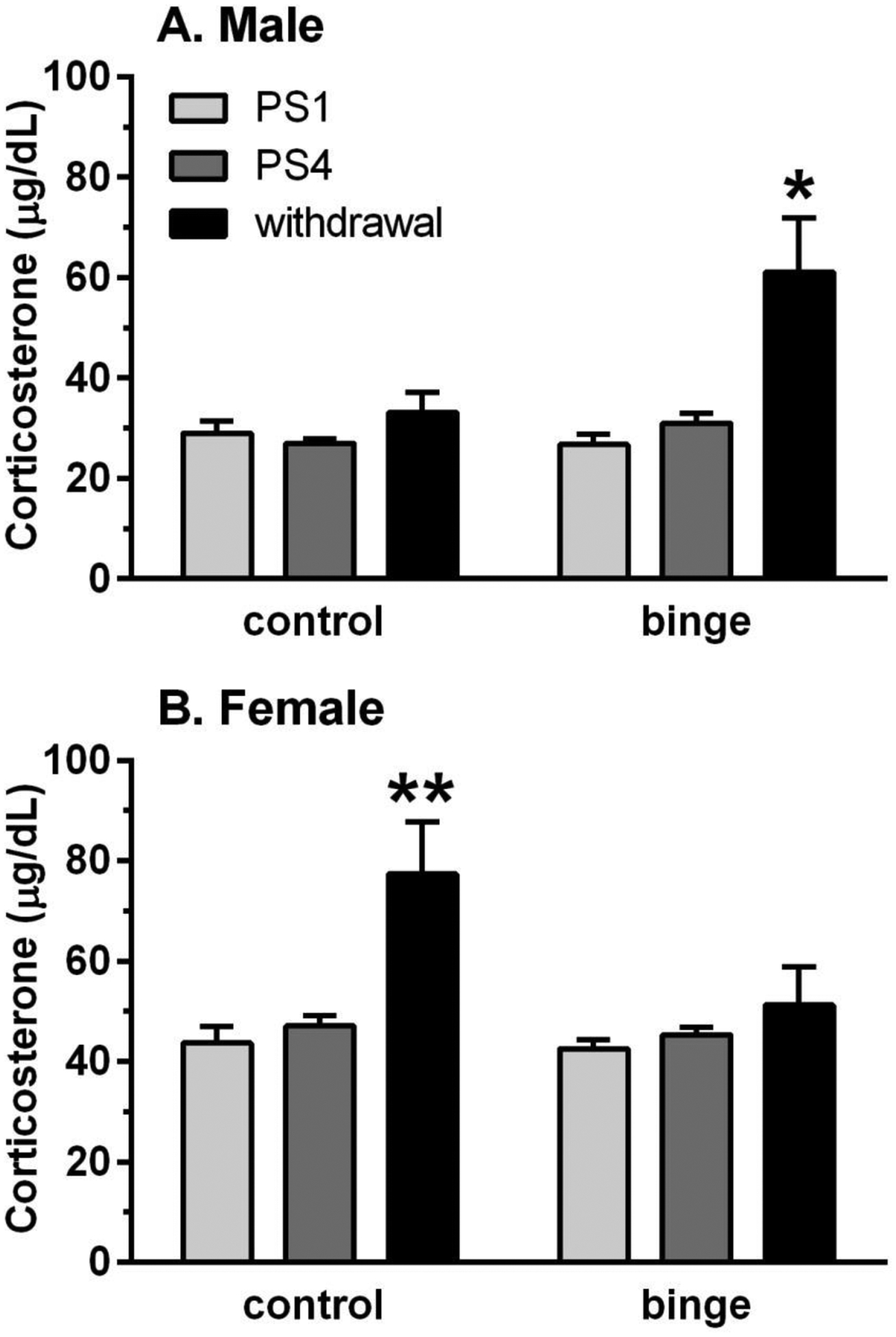
Plasma CORT levels were measured in all mice following the 1st and 4th PS exposure and at 24 h withdrawal after the final drinking session. For comparative purposes, mean “baseline” CORT levels (from Cozzoli et al., 2014) were 13.085 μg/dL for males and 15.21 μg/dL for females. CORT levels following PS1 and PS4 were higher than these baseline values, and the PS-induced increase in CORT levels was not influenced significantly by prior binge drinking or repeated PS. In contrast, the withdrawal-induced increase in CORT levels was higher in binge vs control males and in control vs binge females. Depicted are mean ± SEM for n=15/group/sex except control males (n=7) due to a technical difficulty with that assay. *p<0.05, **p<0.01 vs respective PS1 and PS4.
When the analysis was conducted across the 3 time points, CORT levels tended to be higher in the binge vs control males [F(1,68)=3.74, p<0.06], they were significantly altered across time points [F(2,68)=7.13, p<0.01], and the significant interaction [F(2,68)=3.65, p<0.05] was due to the higher CORT levels in the binge males during withdrawal. This conclusion is supported by a subsequent significant ANOVA in the binge males [F(2,34)=6.01, p<0.01] and post-hoc tests where the CORT levels during withdrawal were significantly higher than those following PS1 and PS4 (Figure 3A). A different pattern of results was found in females, with CORT levels significantly higher in control vs binge mice [F(1,83)=4.49, p<0.05] and a main effect of time point [F(2,83)=8.44, p<0.001]. However, the significant interaction [F(2,83)=3.24, p<0.05] was due to the significantly higher CORT levels in the control females during withdrawal vs PS1 and PS4, which was confirmed with a subsequent significant ANOVA in the control females [F(2,42)=8.48, p=0.001] and post-hoc tests (Figure 3B). Similar results were found when female subgroups were examined (data not shown). Collectively, the results indicate that treatment did not significantly alter the PS-induced elevation in CORT levels following PS1 and PS4 in male and female mice, yet it produced divergent changes in CORT levels during withdrawal, with an enhanced CORT response in binge males and control females.
Based on evidence that abnormalities in the HPA axis are observed in both AUD and PTSD (see Introduction; Gilpin & Weiner, 2017; Norman et al., 2012), a separate analysis compared CORT levels after a single or repeated PS in naïve mice (Table 2) vs in mice with ethanol drinking experience (i.e., lickometer study; Figure 3). In male mice, treatment (naïve, binge, control) significantly altered CORT levels when the analysis was conducted across both PS exposures [F(2,47)=22.77, p<0.001]. Subsequent analyses indicated that CORT levels were significantly altered following a single or repeated PS [PS1: F(2,16)=9.71, p<0.01; PS3/4: F(2,31)=15.15, p<0.001], with post-hoc tests confirming that CORT levels were significantly higher in naïve vs binge mice and naïve vs control mice following PS1 (ps<0.01) or PS3/4 (ps<0.001). In female mice, treatment (naïve, binge, control) significantly altered CORT levels when the analysis was conducted across both PS exposures [F(2,63)=3.48, p<0.05]. However, subsequent analyses indicated that treatment tended to alter CORT levels following a single PS [F(2,31)=2.32, p=0.11], with trends for higher CORT levels in naïve vs binge mice and naïve vs control mice following PS1 (ps=0.10). Treatment did not significantly alter CORT levels following repeated PS. We were unable to conduct this comparison across female subgroups, given that the naïve mice had not consumed ethanol and could not be subdivided. Collectively, these analyses suggest that a history of ethanol drinking may dampen the CORT response to PS, particularly in male mice.
Sex and brain regional differences in the relative density of select protein levels after ethanol drinking and exposure to intermittent PS
Western blot analysis was employed to determine whether sex differences in ethanol drinking and in responsivity to intermittent PS were associated with alterations in protein levels in PFC and HC at 24 h withdrawal from the final drinking session, when compared to protein levels in naïve mice. As described in the Introduction, the proteins examined were CRF-R1, GR, and GABAAR α1 subunit.
Figure 4 summarizes the results of PFC protein levels in male and female mice. Treatment did not significantly alter protein levels of CRF-R1 or GABAAR α1 subunit in either male or female mice, despite a 16 – 17% decrease in protein levels of the GABAAR α1 subunit in control and binge female mice vs naïve. However, sexually divergent effects of treatment on the relative density of GR were found. There was no significant effect of treatment in male PFC, despite a 15% reduction in protein levels in control vs naïve mice. However, in female PFC, treatment significantly influenced GR levels [F(2,25)=9.46, p=0.001], with post-hoc test confirming the significant increase in GR levels in control (↑ 90%) and binge (↑ 70%) female mice vs naïve. Changes in protein levels were similar in the binge and control female subgroups.
Figure 4. Sex differences in the effects of ethanol drinking and intermittent predator odor stress on glucocorticoid receptor (GR) protein levels in prefrontal cortex (PFC).
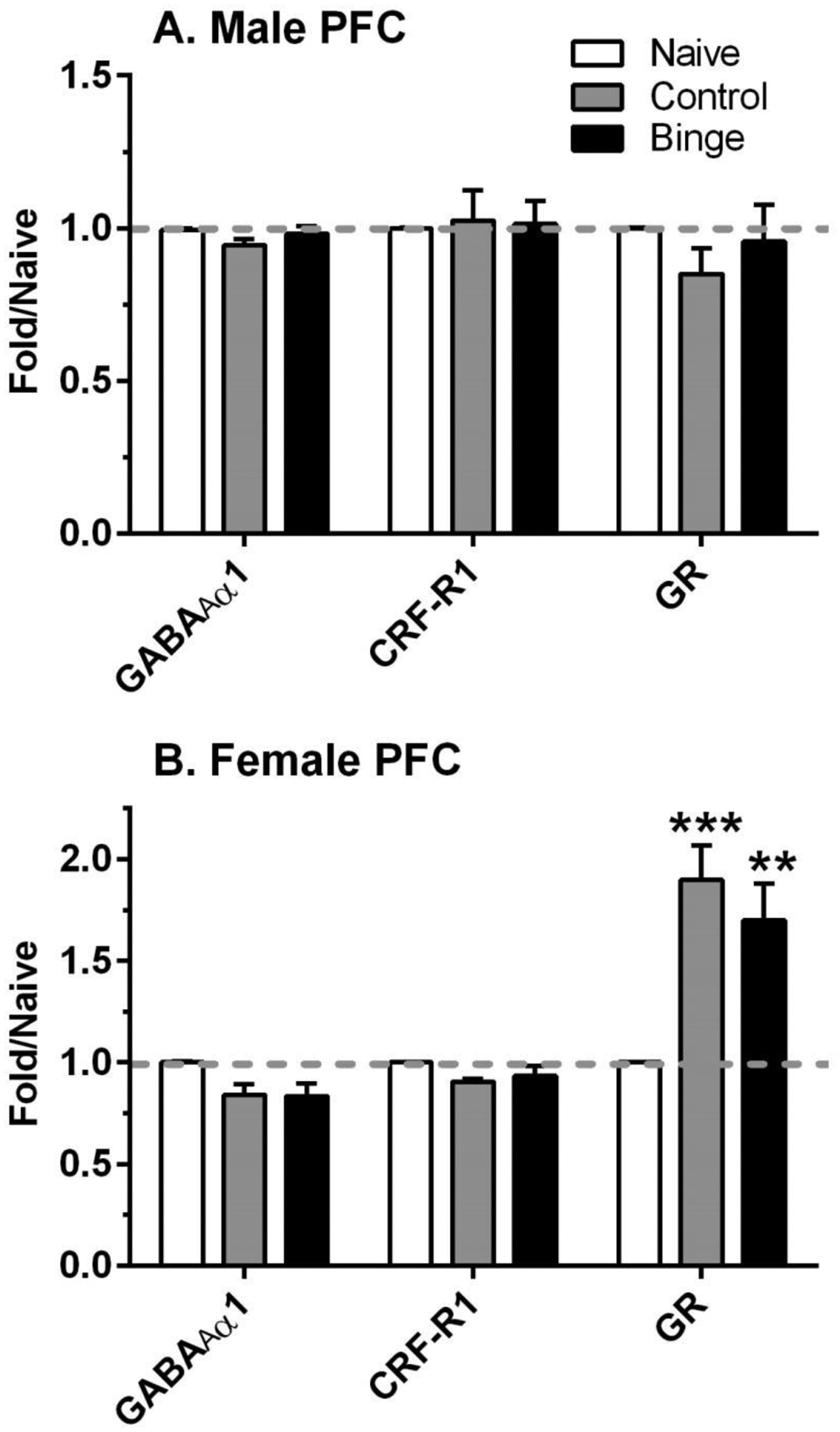
Western blot analysis was conducted on dissected PFC tissue at 24 h after the final lickometer session (binge = prior binge ethanol intake; control = prior water intake) and compared to values from similarly aged naïve mice (naïve). Divergent effects of treatment were observed on protein levels of GR in male and female PFC. Values are mean ± SEM for each group; in some cases, SEM is contained within the mean: male: n=6–10 (naïve), n=11 (control), n=12 (binge); female: n=6–9 (naïve), n=8 (control), n=9–11 (binge). All levels were initially normalized to β-actin. Fold regulation was then determined by normalizing individual values to the mean of the relative expression of the respective naïve group (dashed gray line). **p<0.01, ***p=0.001 vs respective naïve. GABAAR α1 = GABAA receptor α1 subunit; CRF-R1 = corticotropin releasing factor receptor 1
Results in the HC indicate that there were sexually divergent effects of treatment for all 3 proteins examined (Figure 5). Levels of the GABAAR α1 subunit were significantly altered by treatment in females [F(2,32)=4.07, p<0.05] and tended to be altered by treatment in males [F(2,32)=2.58, p=0.09]. Post-hoc tests confirmed the opposite effects of sex and treatment on GABAAR α1 levels: significant decrease of 16% in control females and trend for increase of 23% in binge males, vs respective naïve. And, while CRF-R1 protein levels were significantly increased by treatment in male [F(2,28)=4.02, p<0.05] and female [F(2,28)=9.48, p=0.001] HC, the pattern of significant post-hoc changes differed. In male HC, the 20% increase in CRF-R1 in the control group was significantly higher than in the binge group and tended to be higher (p=0.10) than the naïve group. In female HC, CRF-R1 protein levels were significantly increased by 90% and 101% in the control and binge mice vs naïve. Treatment also significantly altered GR levels only in female [F(2.27)=3.56, p<0.05] but not in male HC. And while there was an increase in GR levels of 55% and 71% in the control and binge female mice vs naïve, respectively, only the increase in binge females reached significance. Changes in protein levels were similar in the binge and control female subgroups. In summary, this neurochemical analysis suggests that there are sex and brain regional alterations in the proteins examined that may be involved in responses to ethanol drinking and/or PS.
Figure 5. Sexually divergent changes in protein levels related to stress and a GABAA receptor subunit in hippocampus (HC) after ethanol drinking and intermittent predator odor stress.
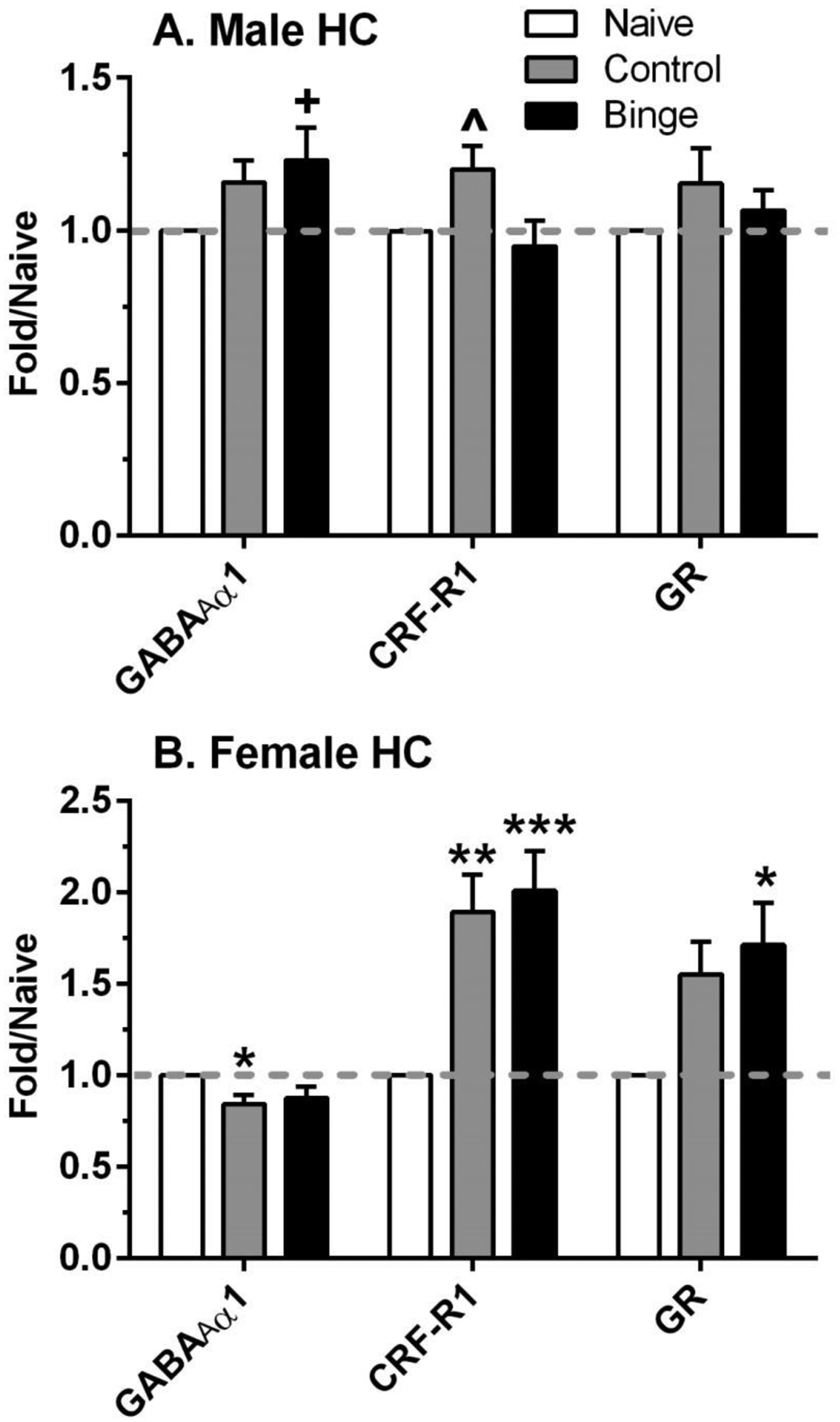
Western blot analysis was conducted on dissected HC tissue at 24 h after the final lickometer session (binge = prior binge ethanol intake; control = prior water intake) and compared to values from similarly aged naïve mice (naïve). Treatment significantly altered protein levels of the GABAA receptor α1 subunit (GABAAR α1), corticotropin releasing factor receptor 1 (CRF-R1), and glucocorticoid receptor (GR) in female HC, whereas fewer changes were observed in male HC. Values are mean ± SEM for each group; in some cases, SEM is contained within the mean: male: n=10–12 (naïve), n=11–12 (control), n=10–11 (binge); female: n=8–13 (naïve), n=11 (control), n=10–11 (binge). All levels were initially normalized to β-actin. Fold regulation was then determined by normalizing individual values to the mean of the relative expression of the respective naïve group (dashed gray line). +p=0.085, *p<0.05, **p<0.01, ***p=0.001 vs respective naïve; ^p<0.05 vs respective binge.
Discussion
The overall intent of the present studies was to assess effects of a PTSD-like stress and prior binge-like ethanol consumption on subsequent drinking patterns, stress hormone concentrations and alterations in several relevant brain protein levels. The results from the initial studies in naïve mice, documenting that 30 min exposure to dirty rat bedding activated the HPA axis and increased anxiety-like behavior, provided a strong rationale for the use of dirty rat bedding as PS in the subsequent drinking studies. Notably, the drinking study results provide strong evidence that the ability of PS to increase 10E intake was enhanced by prior binge drinking experience in adult male mice. In control male mice, 10E licks increased by 24% after PS4 in a 23 h access paradigm, consistent with our recent report of a 30 – 35% increase in 2 h 10E intake on the 2nd day following PS in 2 cohorts of adult male mice without prior binge experience (Cozzoli et al., 2014). Moreover, in the binge male mice, 10E licks were increased over baseline by 65% following the last PS exposure. This synergistic effect of prior binge drinking and intermittent PS on ethanol drinking in male mice was due to an increase in bout frequency and bout size as well as an elevation in hourly 10E licks across the majority of the circadian dark phase. Thus, repeated PS increased multiple parameters of ethanol intake patterns during the active phase in the binge-exposed male mice.
Epidemiological evidence indicates that women develop ethanol-related heart disease, liver damage, and peripheral neuropathy after fewer years of heavy drinking, and that women may be more vulnerable to AUD-induced brain damage (Wiren, 2013). Additionally, lifetime prevalence of PTSD is reported to be twice as high in females as in males, whereas prevalence of AUD with PTSD is higher in males than in females (See Introduction). Therefore, we wanted to evaluate responses in male and female mice in the present set of studies. In contrast to the results in males, prior binge drinking experience did not produce a synergistic increase in subsequent ethanol intake after intermittent PS in female mice, when the entire cohort of mice was analyzed. However, when subgroups of females that were based on low vs high baselines were examined, both control and binge females with low baselines exhibited a significant increase in 10E licks after intermittent PS that was due to an increase in bout frequency and an elevation in hourly 10E licks across the majority of the circadian dark phase. Notably, 10E licks were increased after PS4 by 87% in binge females and by 214% in control females, vs respective baseline, suggesting an enhanced response to intermittent PS in female mice without prior binge ethanol experience. These increases were greater than the 25 – 36% increase in 2 h 10E intake on the 2nd day following PS in 2 cohorts of adult female mice without prior binge experience (Cozzoli et al., 2014) and could reflect differences in period of ethanol access between the two studies. Regardless, changes in ethanol intake patterns contributing to the PS-enhancement of 10E intake in the subgroup of females with low baselines was similar to that in males with prior binge exposure.
Binge ethanol intake with the SHAC procedure was comparable in the male and female mice, yet 10E intake during the lickometer portion of the study was higher in females than in males, consistent with evidence that female rodents consume higher doses of ethanol than male rodents in a variety of ethanol drinking and self-administration procedures (see Cozzoli et al., 2014 and references therein). So, it is not clear if the overall lack of PS-enhancement in ethanol intake in females was due in part, to their higher baselines (when the entire data set was considered). However, our earlier work determined that prior binge drinking increased later ethanol intake in some female mice, primarily adolescent animals (Strong et al., 2010), and inspection of the data revealed two separable baseline subgroups in the binge and control female mice that were not evident in the male mice. It is unlikely that these subgroups were due to estrous-cycle differences in drinking, given that plasma estradiol levels, which were measured by gas chromatography-mass spectrometry (Jensen et al., 2017), did not differ between the low vs high baseline subgroups of the binge and control females. Mean ± SEM estradiol levels (pg/mL) at the end of the study were: 3.93 ± 0.27 (binge low), 3.25 ± 0.52 (binge high), 3.36 ± 1.12 (control low), and 2.75 ± 0.32 (control high). Additionally, limited available data suggest that stage of estrous cycle did not alter ethanol self-administration or binge ethanol intake in randomly cycling female rats (Roberts et al., 1998) or C57BL/6J female mice (Satta et al., 2018), respectively. Moreover, exposure to cat odor PS did not disrupt estrous cycling in female rats (Perrot-Sinal et al., 2004), nor did long term binge drinking alter the estrous cycle in female C57BL/6J mice (Satta et al., 2018). Consistent with this evidence, the range in variation in 10E intake across days did not differ in males and females (e.g., Figure 2A & 2C), suggesting that estrous cycle phase did not contribute significant variability in the present studies. Thus, the mechanism underlying the distinct baseline drinking subgroups in females is unclear, but large individual variability in ethanol intake (Wilcox et al., 2014) as well as in conditioned fear expression (Hager et al., 2014) in C57BL/6J mice has been reported. Additionally, the above heterogeneity in response is consistent with results in male rats following other PS, where an increase in ethanol consumption or self-administration was observed only in animals characterized as exhibiting an “extreme behavioral response” (EBR; high anxiety) following cat odor PS (Manjoch et al., 2016) or as “Avoiders” due to their avoidance of a bobcat urine paired context (Edwards et al., 2013; Roltsch et al., 2014).
Exposure to PS (cat odor, bobcat urine, dirty rat bedding) has been reported to increase anxiety-related behavior in naïve male mice and rats, measured 2 – 7 days following PS exposure (Belzung et al., 2001; Cohen & Zohar, 2004; Cohen et al., 2008; Hebb et al., 2003; Roltsch et al., 2014; Whitaker & Gilpin, 2015). As mentioned above, a high anxiety phenotype was observed in “Avoider” rats (Roltsch et al., 2014; Whitaker & Gilpin, 2015) or in “EBR” rats (Cohen et al., 2013). Comparisons across 6 – 9 inbred mouse strains classified male C57BL/6 mice as a highly reactive strain, based on anxiogenic responses following cat odor PS (Belzung et al., 2001; Cohen et al., 2008). Consistent with a report that exposure to dirty rat bedding as PS significantly increased anxiety-related behavior in male CD-1 mice (Hebb et al., 2003), the present findings indicate that 2 exposures to dirty rat bedding as PS significantly increased anxiety-related behavior in naïve male and female C57BL/6J mice, when measured on the EPM. However, it is not known whether this PS-induced increase in anxiety-related behavior in mice prior to ethanol exposure would correspond with subsequent enhanced ethanol intake after repeated PS exposure. Existing data indicate that a PS-induced high anxiety phenotype in naïve animals was associated with subsequent enhanced ethanol intake in “EBR” and “Avoider” male rats (Edwards et al., 2013; Manjoch et al., 2016; Roltsch et al., 2014). So, we do not know if the PS-enhancement of ethanol intake in male mice and a subgroup of female mice in the present study was associated with an increase in anxiety-related behavior after PS exposure or if it represented a compensatory mechanism to offset a PS-induced increase in anxiety. Future studies will determine whether PS-enhancement of ethanol intake is associated with a change in anxiety-related behavior. We also plan to develop a pre-screen to identify PS “sensitive” male and female mice, based on measurements of anxiety and odor avoidance, and to determine the correspondence with PS-enhancement of ethanol intake.
In the present studies, CORT levels were measured as an index of hormonal responses to stress and HPA axis responsivity and revealed several important results. First, PS exposure significantly increased plasma CORT levels in male and female mice that were naïve and that had a history of ethanol drinking, with higher levels in females vs males (Table 2 & Figure 3). These results are consistent with our recent report (Cozzoli et al., 2014) and with evidence for sex differences in HPA axis responsivity to stress (reviewed in Bourke et al., 2012; Panagiotakopoulos & Neigh, 2014; Valentino et al., 2013a, 2013b and references therein). Second, there was no significant difference in CORT levels in naïve mice following 1 or 3 PS exposures or in ethanol drinking mice following 1 or 4 PS exposures, suggesting that repeated PS exposure did not produce a sensitized CORT response. Third, the CORT response to PS was significantly lower in male mice and tended to be lower in female mice with a history of ethanol drinking vs naïve mice (Figure 3, Table 2). This blunted response to PS in mice with ethanol drinking history is consistent with the report that ethanol dependence leads to a dampened neuroendocrine state (Richardson et al., 2008). An alternate interpretation is that the lower CORT response to PS in mice with enhanced ethanol intake was due to PS sensitivity, since “Avoider” rats (Whitaker & Gilpin, 2015) and “EBR” rats (Cohen et al., 2013) exhibit lower CORT levels after PS. Finally, CORT levels were higher during withdrawal than following PS1 or PS4, and the withdrawal-induced increase was greatest in the mice with the highest prior drinking (binge males and control females). Although we did not measure withdrawal in the present studies, recent work has shown that 24 h withdrawal following 2 weeks of binge drinking or 1 week of 24 h ethanol drinking produced anxiety and hyperalgesia in male C57BL/6J mice, respectively (Lee et al., 2016; Smith et al., 2016). Given the longer history of ethanol drinking in the present study, we presume that mice were experiencing some negative affective symptoms during withdrawal, but we do not know how that would correspond to the group differences in CORT levels during withdrawal. Collectively, the CORT results suggest that the HPA axis is dysregulated following a history of ethanol drinking and that there are sex differences in HPA axis responsivity.
Western blot analysis revealed alterations in relative levels of select proteins in PFC and HC at 24 h after the final day of 10E access and 5 days after the final PS exposure that diverged by sex as well as brain area (Figures 4 and 5). Protein levels of the GABAAR α1 subunit in PFC were unchanged in binge and control males and females vs respective naïve mice. In contrast, levels of this receptor subunit protein in HC were significantly decreased in control females and tended to be increased in binge males vs respective naïve mice. The present results in PFC are consistent with early work showing that 24 h withdrawal following 3 or 9 days of liquid ethanol diet consumption did not significantly alter GABAAR α1 subunit protein levels in male and female cerebral cortex vs controls (Devaud & Alele, 2004). However, longer periods of liquid diet consumption (14 & 40 days) significantly decreased cortical levels of the GABAAR α1 subunit in male but not female rats vs respective controls (Devaud et al., 1998; Matthews et al., 1998) and did not alter HC levels of this subunit protein in male rats (Matthews et al., 1998). In terms of potential stress effects on GABAAR α1 subunit expression, 4 – 6 weeks of post-weaning social isolation stress significantly decreased α1 subunit expression in PFC and HC of male Swiss Webster mice (Pinna et al., 2006) but did not alter HC GABAAR α1 subunit expression in male C57BL/6J mice (Sanna et al., 2011) vs respective group housed mice. Thus, it is not known if the significant decrease in GABAAR α1 subunit protein levels in female controls is due to their length of ethanol consumption or the exposure to intermittent PS or both. Overall, the findings suggest that α1 subunit GABAA receptors have a sex-selective role in the HC response to a repeated stress and ongoing ethanol consumption in our mouse model.
Sexually divergent and brain regional differences in the effects of ethanol drinking and intermittent PS on relative protein levels of CRF-R1 and GR also were observed. CRF-R1 levels were significantly increased in binge and control female HC vs respective naïve mice, while levels in HC in control males were increased significantly vs binge males and tended to be increased vs naïve males. Taken in conjunction with evidence for increased CRF-R1 signaling and decreased CRF-R1 internalization in females vs males (Valentino et al., 2013a, 2013b), the significant upregulation of CRF-R1 in binge and control female HC suggests that females may be more sensitive to repeated activation of the stress response via CRFergic mechanisms than males (Bangasser & Valentino, 2014). It is possible that these differences contribute to the higher stress-induced CORT levels in females vs males in the present study. Moreover, GR protein levels were significantly increased in control and binge female PFC vs naïve and were significantly increased in binge female HC with a similar non-significant increase in control female HC vs naïve. In contrast, treatment did not significantly alter GR levels in male PFC or HC. Because GR is a transcription factor, dysregulation of GR activity likely modulates a variety of systems that regulate stress and ethanol use and that may differ in males and females. Distinct regulatory mechanisms also exist for GR activation, where high glucocorticoids (e.g., CORT) reduce CRF expression in the paraventricular nucleus of the hypothalamus (PVN) and increase CRF expression in the amygdala via an effect at nuclear GR (Edwards et al., 2015; Makino et al., 2002), and thus contribute to negative and positive feedback of the HPA axis, respectively. Additionally, GR activation suppresses HC output, which should disinhibit the HPA axis and exert a positive feedback effect on the HPA axis, given that HC excitability relays GABAergic inhibitory tones to the PVN (see Makino et al., 2002 and references therein). However, data also suggest that feedback inhibition through GR in the PVN may override a positive feedback effect through GR in the HC (Makino et al., 2002 and references therein), and it is unclear whether activation of GR in PFC would contribute to feedback inhibition of the HPA axis. Collectively, the clear sex differences in CRFR1 and GR levels suggest that sexually divergent mechanisms may contribute to a dampened neuroendocrine state following a history of ethanol drinking and to recovery of the HPA axis from the long-term ethanol access as well as the repeated stress exposure. As a result, pharmacological strategies targeting the CRFR1 or GR systems may be differentially effective in males vs females.
Overall, PS significantly increased ethanol intake and had a synergistic interaction with prior binge drinking in males, whereas PS produced a similar increase in 10E intake in a subgroup of binge and control females. Additional studies are necessary to determine how baseline intake in females influences responsivity to PS exposure. Nonetheless, the PS-induced increase in anxiety-related behavior in naïve mice along with the ability of repeated PS to increase ethanol intake in mice with ethanol drinking experience is consistent with the idea that a high anxiety state may contribute to the ability of a traumatic stress to increase ethanol intake in patients with comorbid PTSD/AUD. Furthermore, our findings suggest that multiple neuronal responses may be activated by stress and ethanol drinking and that sex influences these neurochemical response pathways. Notably, the sexually divergent changes in protein levels in PFC and HC add to evidence for sex differences in the neurochemical systems affected by stress and a history of ethanol drinking and argue for sex-specific pharmacological strategies to treat AUD.
Supplementary Material
Supplemental Figure 1. Significant enhancement in hourly ethanol drinking during the circadian dark phase after intermittent predator odor stress (PS) contributes to the significant enhancement in 23 h ethanol intake depicted in Figure 2. Depicted are mean ± SEM hourly licks on the ethanol sipper (10% ethanol, 10E) for n=12/group/sex that had prior binge ethanol experience (binge) or drank only water (control). The 23 h period of 10E intake began at approximately 30 min prior to lights off. In general, 10E licks were higher during the circadian dark phase (1 – 12 h). Panels A & C: Hourly 10E licks for a representative baseline day are shown (day 7 in males and day 8 in females due to a computer malfunction where hourly data were lost in females for days 5 – 7), and they did not differ in the binge vs control groups. Panels B & D: Hourly 10E licks after 4 exposures to PS (e.g., day 23) were significantly increased in male mice with prior binge ethanol experience. Not shown: Hourly 10E licks also were significantly increased throughout the circadian dark phase after the 4th PS in binge and control females with low baselines (see Results for details). +p≤0.07, *p≤0.05, **p<0.01, ***p=0.001 vs respective control.
Supplemental Figure 2. Alteration in ethanol bout characteristics after intermittent predator odor stress (PS) exposure contributes to the significant enhancement in 23 h ethanol intake depicted in Figure 2. Values are the mean ± SEM for 12/group/sex that had prior binge drinking experience (binge) or drank only water (control). Circles highlight data on days with exposure to PS. Panels A & C depict bout frequency (number of ethanol bouts over 23 h). Panels B & D depict bout size (licks per bout). A bout is experimentally defined as a minimum of 20 licks with no more than a 60 sec pause between licks. Hourly data in female mice are missing for days 5 – 7 due to a computer malfunction. There was a significant increase in bout frequency and bout size after intermittent PS in male mice with prior binge ethanol experience. Not shown: Bout frequency also was significantly increased across time/PS in the binge and control females with low baselines (see Results for details). +p≤0.08, *p≤0.05, **p≤0.01 vs respective control.
ACKNOLWEDGEMENTS
This research was supported by grants (I01 BX001070; I01 BX002966) and resources from the Department of Veteran Affairs (DAF) and a Pacific University School of Pharmacy award (LLD). AC was awarded a VA Summer Fellowship. The contributions of Andres Garcia, Megan Perius and Mehrdad Tafti of the Devaud Lab are greatly appreciated.
REFERENCES
- Bangasser DA, Valentino RJ (2014) Sex differences in stress-related psychiatric disorders: Neurobiological perspectives. Front Neuroendocrinology 35:303–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, Doremus-Fitzwater TL (2011) Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology 218:131–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzung C, El Hage W, Moindrot N, Griebel G (2001) Behavioral and neurochemical changes following predatory stress in mice. Neuropharmacology 41:400–408. [DOI] [PubMed] [Google Scholar]
- Bourke CH, Harrell CS, Neigh GN (2012) Stress-induced sex differences: Adaptations mediated by the glucocorticoid receptor. Horm Behav 62:210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunell SC, Spear LP (2005) Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin Exp Res 29:1641–1653. [DOI] [PubMed] [Google Scholar]
- Campbell JC, Szumlinski KK, Kippin TE (2009) Contribution of early environmental stress to alcoholism vulnerability. Alcohol 43:547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Barrenha GD, DeMaria A, Finegan A (2006) Different effects of stress on alcohol drinking behavior in male and female mice selectively bred for high alcohol preference. Alcohol Alcohol 41:44–53. [DOI] [PubMed] [Google Scholar]
- Chester JA, Barrenha GD, Hughes ML, Keuneke KJ (2008) Age- and sex-dependent effects of footshock stress on subsequent alcohol drinking and acoustic startle behavior in mice selectively bred for high-alcohol preference. Alcohol Clin Exp Res 32:1782–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Zweifel M, Froehlich JC (2004) Effects of stress on alcohol consumption in rats selectively bred for high or low alcohol drinking. Alcohol Clin Exp Res 28:385–393. [DOI] [PubMed] [Google Scholar]
- Cohen H, Zohar J (2004) An animal model of posttraumatic stress disorder: The use of cut-off behavioral criteria. Ann NY Acad Sci 1032:167–178. [DOI] [PubMed] [Google Scholar]
- Cohen H, Geva AB, Matar MA, Zohar J, Kaplan Z (2008) Post-traumatic stress behavioural responses in inbred mouse strains: can genetic predisposition explain phenotypic vulnerability? Int J Neuropsychopharmacology 11:331–349. [DOI] [PubMed] [Google Scholar]
- Cohen H, Matar MA, Zohar J (2013) Animal models of post-traumatic stress disorder. Curr Protocols Neurosci 9.45.1–9.45.18 [DOI] [PubMed] [Google Scholar]
- Cozzoli DK, Kaufman MN, Nipper MA, Hashimoto JG, Wiren KM, Finn DA (2016) Functional regulation of PI3K-associated signaling in the accumbens by binge drinking in male but not female mice. Neuropharmacology 105:164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Tanchuck-Nipper MA, Kaufman MN, Horowitz CB, Finn DA (2014) Environmental stressors influence limited-access ethanol consumption by C57BL/6J mice in a sex-dependent manner. Alcohol 48:741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud LL, Alele P (2004) Differential effects of chronic ethanol administration and withdrawal on γ-aminobutyric acid type A and NMDA receptor subunit proteins in male and female rat brain. Alcohol Clin Exp Res 28: 957–965. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Fritschy J-M, Morrow AL (1998) Influence of gender on GABAA receptor alterations elicited by ethanol dependence in rats. Brain Res 796: 222–230. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, McGregor IS (2001) Defensive behavior in rats toward predatory odor: a review. Neurosci Biobehav Rev 25:597–609. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP (2005) Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res 29:1796–1808. [DOI] [PubMed] [Google Scholar]
- Edwards S, Baynes BB, Carmichael CY, Zamora-Martinez ER, Barrus M, Koob GF, Gilpin NW (2013) Traumatic stress reactivity promotes excessive alcohol drinking and alters the balance of prefrontal cortex-amygdala activity. Transl Psychiatry 3:e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Little HJ, Richardson HN, Vendruscolo LF (2015) Divergent regulation of distinct glucocorticoid systems in alcohol dependence. Alcohol 49:811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Belknap JK, Cronise K, Yoneyama N, Murillo A, Crabbe JC (2005) A procedure to produce high alcohol intake in mice. Psychopharmacology 178:471–480. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ (2007) Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12–41). Alcohol Clin Exp Res 31:939–949. [DOI] [PubMed] [Google Scholar]
- Ford MM, Beckley EH, Nickel JD, Eddy S, Finn DA (2008) Ethanol intake patterns in female mice: Influence of allopregnanolone and the inhibition of its synthesis. Drug Alcohol Depend 97:73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Phillips TJ, Finn DA (2005) Neurosteroid modulators of GABAA receptors differentially modulate ethanol intake patterns in male C57BL/6J mice. Alcohol Clin Exp Res 29:1630–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Weiner JL (2017) Neurobiology of comorbid post-traumatic stress disorder and alcohol-use disorder. Genes Brain Behav 16:15–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagar T, Jansen RF, Pieneman AW, Manivannan SN, Golani I, van der Sluis S, Smit AB, Verhage M, Stiedl O (2014) Display of individuality in avoidance behavior and risk assessment of inbred mice. Front Behav Neurosci 8:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb ALO, Zacharko RM, Dominguez H, Laforest S, Gauthier M, Levac C, Drolet G (2003) Changes in brain cholecystokinin and anxiety-like behavior following exposure of mice to predator odor. Neuroscience 116:539–551. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR (2006) Age at drinking onset and alcohol dependence: age at onset, duration and severity. Arch Pediatr Adolesc Med 160:739–746. [DOI] [PubMed] [Google Scholar]
- Itoga CA, Roltsch Hellard EA, Whitaker AM, Lu Y-L, Schreiber AL, Baynes BB, Baiamonte BA, Richardson HN, Gilpin NW (2016) Traumatic stress promotes hyperalgesia via corticotropin-releasing factor-1 receptor (CRFR1) signaling in central amygdala. Neuropsychopharmacology 41:2463–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JP, Nipper MA, Helms ML, Ford MM, Crabbe JC, Rossi DJ, Finn DA (2017) Ethanol withdrawal-induced dysregulation of neurosteroid levels in plasma, cortex, and hippocampus in genetic animal models of high and low withdrawal. Psychopharmacology 234:2793–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Hatzenbuehler ML, Grant BF, Hasin DS (2012) Stress and alcohol: epidemiologic evidence. Alcohol Research 34:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ (2013) National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress 26:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL (2009) The role of GABAA receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology 205:529–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coelho MA, McGregor HA, Solton NR, Cohen M, Szumlinski KK (2016) Adolescent mice are resilient to alcohol withdrawal-induced anxiety and changes in indices of glutamate function within the nucleus accumbens. Front Cell Neurosci 10:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Kushner MG, Rawleigh JM, Fiszdon J, Carroll ME (1999) The effects of restraint stress on voluntary ethanol consumption in rats. Exp Clin Psychopharmacology 7:318–323. [DOI] [PubMed] [Google Scholar]
- Makino S, Hashimoto K, Gold PW (2002) Multiple feedback mechanisms activating corticotropin-releasing hormone system in the brain during stress. Pharmacol Biochem Behav 73:147–158. [DOI] [PubMed] [Google Scholar]
- Manjoch H, Vainer E, Matar M, Ifergane F, Zohar J, Kaplan Z, Cohen H (2016) Predator-scent stress, ethanol consumption and the opioid system in an animal model of PTSD. Behav Brain Res 306:91–105 [DOI] [PubMed] [Google Scholar]
- Matar MA, Zohar J, Cohen H (2013) Translationally relevant modeling of PTSD in rodents. Cell Tissue Res 354:127–139. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Devaud LL, Fritschy JM, Sieghart W, Morrow AL (1998) Differential regulation of GABAA receptor gene expression by ethanol in the rat hippocampus versus cerebral cortex. J Neurochem 70:1160–1166. [DOI] [PubMed] [Google Scholar]
- NIAAA (2017) Alcohol Facts and Statistics. https://pubs.niaaa.gov/publications/AlcoholFacts&Stats/AlcoholFacts&Stats.pdf, downloaded 4/25/17.
- Norman SB, Myers US, Wilkins KC, Goldsmith AA, Hristova V, Huang Z, McCullough KC, Robinson SK (2012) Review of biological mechanisms and pharmacological treatments of comorbid PTSD and substance use disorder. Neuropharmacology 62:542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagiotakopoulos L, Neigh GN (2014) Development of the HPA axis: Where and when do sex differences manifest? Front Neuroendocrinology 35:285–302. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Gregus A, Boudreau D, Kalynchuck LE (2004) Sex and repeated restraint stress interact to affect cat odor-induced defensive behavior in adult rats. Brain Res 1027:161–172. [DOI] [PubMed] [Google Scholar]
- Pinna G, Agis-Balboa RC, Zhubi A, Matsumoto K, Grayson DR, Costa E, Guidotti A (2006) Imidazenil and diazepam increase locomotor activity in mice exposed to protracted social isolation. Proc Natl Acad Sci 103:4275–4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman RK, Rasmusson AM, Koenen K, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I (2012) Biological studies of post-traumatic stress disorder. Nature Rev Neurosci 13:769–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaker MJ, Ford MM, Fretwell AM, Finn DA (2011) Alteration of ethanol drinking in mice via modulation of the GABAA receptor with ganaxolone, finasteride and gaboxadol. Alcohol Clin Exp Res 35:1994–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaker MJ, Strong MN, Ford MM, Finn DA (2012) Effect of ganaxolone and THIP on operant and limited-access ethanol self-administration. Neuropharmacology 63:555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaker MJ, Strong-Kaufman MN, Ford MM, Phillips TJ, Finn DA (2015) Effect of nucleus accumbens shell infusions of ganaxolone or gaboxadol on ethanol consumption in mice. Psychopharmacology 232:1415–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL (2008) Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci 28:1641–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Smith AD, Weiss F, Rivier C, Koob GF (1998) Estrous cycle effects on operant responding for ethanol in female rats. Alcohol Clin Exp Res 22:1564–1569. [PubMed] [Google Scholar]
- Roltsch EA, Baynes BB, Mayeux JP, Whitaker AM, Baiamonte BA, Gilpin NW (2014) Predator odor stress alters corticotropin-releasing factor-1 receptor (CRF1R)-dependent behaviors in rats. Neuropharmacology 79:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Talani G, Obili N, Mascia PM, Mostallino MC, Secci PP, Pisu MG, Biggio F, Utzeri C, Olla P, Biggio G, Follesa P (2011) Voluntary ethanol consumption induced by social isolation reverses the increase of α4/δ GABAA receptor gene expression and function in the hippocampus of C57BL/6J mice. Front Neurosci 5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satta R, Hilderbrand ER, Lasek AW (2018) Ovarian hormones contribute to high levels of binge-like drinking by female mice. Alcohol Clin Exp Res 42:286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber AL, Lu Y-L, Baynes BB, Richardson HN, Gilpin NW (2017) Corticotropin-releasing factor in ventromedial prefrontal cortex mediates avoidance of a traumatic stress-paired context. Neuropharmacology 113:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillaber I, Henniger MSH (2004) Stress and alcohol drinking. Ann Med 36:596–605. [DOI] [PubMed] [Google Scholar]
- Smith ML, Hostetler CM, Heinricher MM, Ryabinin AE (2016) Social transfer of pain in mice. Sci Adv 2:e1600855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples LG (2010) Predator odor avoidance as a rodent model of anxiety: Learning-mediated consequences beyond the initial exposure. Neurobiol Learning Memory 94:435–445. [DOI] [PubMed] [Google Scholar]
- Strong MN, Yoneyama N, Fretwell AM, Snelling C, Tanchuck MA, Finn DA (2010) “Binge” drinking experience in adolescent mice shows sex differences and elevated ethanol intake in adulthood. Horm Behav 58:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambour S, Brown LL, Crabbe JC (2008) Gender and age at drinking onset affect voluntary alcohol consumption but neither the alcohol deprivation effect nor the response to stress in mice. Alcohol Clin Exp Res 32:2100–2106. [DOI] [PubMed] [Google Scholar]
- Tanchuck MA, Yoneyama N, Ford MM, Fretwell AM, Finn DA (2011) Assessment of GABA-B, metabotropic glutamate and opioid receptor involvement in an animal model of binge drinking. Alcohol 45:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Bangasser D, Van Bockstaele EJ (2013a) Sex-biased stress signaling: The corticotropin-releasing factor receptor as a model. Mol Pharmacol 83:737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E, Bangasser D (2013b) Sex-specific cell signaling: the corticotropin-releasing factor receptor model. Trends Pharmacol Sci 34:437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker AM, Gilpin NW (2015) Blunted hypothalamo-pituitary-adrenal axis response to predator odor predicts high stress reactivity. Physiol Behav 147:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox MV, Cuzon Carlson VC, Sherazee N, Sprow GM, Bock R, Thiele TE, Lovinger DM, Alvarez VA (2014) Repeated binge-like ethanol drinking alters ethanol drinking patterns and depresses striatal GABAergic transmission. Neuropsychopharmacology 39:579–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiren KM (2013) Males and females are just different: Sexually dimorphic responses to chronic ethanol exposure in hippocampal slice cultures. Neurosci Lett 550:1–5. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Diamond DM (2013) Current status on behavioral and biological markers of PTSD: A search for clarity in a conflicting literature. Neurosci Biobehav Rev 37:860–895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Significant enhancement in hourly ethanol drinking during the circadian dark phase after intermittent predator odor stress (PS) contributes to the significant enhancement in 23 h ethanol intake depicted in Figure 2. Depicted are mean ± SEM hourly licks on the ethanol sipper (10% ethanol, 10E) for n=12/group/sex that had prior binge ethanol experience (binge) or drank only water (control). The 23 h period of 10E intake began at approximately 30 min prior to lights off. In general, 10E licks were higher during the circadian dark phase (1 – 12 h). Panels A & C: Hourly 10E licks for a representative baseline day are shown (day 7 in males and day 8 in females due to a computer malfunction where hourly data were lost in females for days 5 – 7), and they did not differ in the binge vs control groups. Panels B & D: Hourly 10E licks after 4 exposures to PS (e.g., day 23) were significantly increased in male mice with prior binge ethanol experience. Not shown: Hourly 10E licks also were significantly increased throughout the circadian dark phase after the 4th PS in binge and control females with low baselines (see Results for details). +p≤0.07, *p≤0.05, **p<0.01, ***p=0.001 vs respective control.
Supplemental Figure 2. Alteration in ethanol bout characteristics after intermittent predator odor stress (PS) exposure contributes to the significant enhancement in 23 h ethanol intake depicted in Figure 2. Values are the mean ± SEM for 12/group/sex that had prior binge drinking experience (binge) or drank only water (control). Circles highlight data on days with exposure to PS. Panels A & C depict bout frequency (number of ethanol bouts over 23 h). Panels B & D depict bout size (licks per bout). A bout is experimentally defined as a minimum of 20 licks with no more than a 60 sec pause between licks. Hourly data in female mice are missing for days 5 – 7 due to a computer malfunction. There was a significant increase in bout frequency and bout size after intermittent PS in male mice with prior binge ethanol experience. Not shown: Bout frequency also was significantly increased across time/PS in the binge and control females with low baselines (see Results for details). +p≤0.08, *p≤0.05, **p≤0.01 vs respective control.


