ABSTRACT
The type VI secretion system (T6SS) is a multiprotein machine that uses a spring-like mechanism to inject effectors into target cells. The injection apparatus is composed of a baseplate on which is built a contractile tail tube/sheath complex. The inner tube, topped by the spike complex, is propelled outside of the cell by the contraction of the sheath. The injection system is anchored to the cell envelope and oriented towards the cell exterior by a trans-envelope complex. Effectors delivered by the T6SS are loaded within the inner tube or on the spike complex and can target prokaryotic and/or eukaryotic cells. Here we summarize the structure, assembly, and mechanism of action of the T6SS. We also review the function of effectors and their mode of recruitment and delivery.
INTRODUCTION
The type VI secretion system (T6SS) is a multiprotein machine that belongs to the versatile family of contractile injection systems (CISs) (1–4). CISs deliver effectors into target cells using a spring-like mechanism (4–6). Briefly, CISs assemble a needle-like structure, loaded with effectors, wrapped into a sheath built in an extended, metastable conformation (Fig. 1). Contraction of the sheath propels the needle toward the competitor cell. Genomes of Gram-negative bacteria usually encode one or several T6SSs, with an overrepresentation in Proteobacteria and Bacteroidetes (8–10; for a review on the role of T6SS in gut-associated Bacteroidales, see the chapter by Coyne and Comstock [7]). The broad arsenal of effectors delivered by T6SSs includes antibacterial proteins such as peptidoglycan hydrolases, eukaryotic effectors that act on cell cytoskeleton, and toxins that can target all cell types, such as DNases, phospholipases, and NAD+ hydrolases (11–14). Consequently, the T6SS plays a critical role in reshaping bacterial communities and, directly or indirectly, in pathogenesis (15–19). Destroying bacterial competitors also provides exogenous DNA that can be acquired in naturally competent bacteria and that serves as a reservoir for antibiotic resistance gene spread (20). This chapter lists the major effector families and summarizes the current knowledge on the assembly and mode of action of the T6SS.
FIGURE 1.
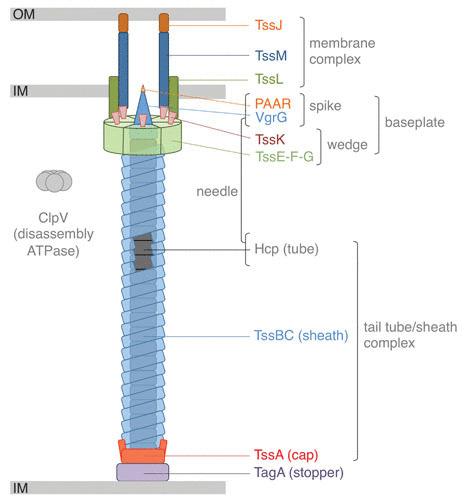
Schematic representation of the T6SS. The different subunits are labeled, as are the different subcomplexes. IM, inner membrane; OM, outer membrane.
TYPE VI SECRETION SYSTEM EFFECTORS
Several T6SSs have been shown to target eukaryotic cells (21–23). By promoting or preventing cytoskeleton rearrangements through the action of specific effectors that target actin or tubulin, the T6SSs of Vibrio cholerae, Aeromonas hydrophila, and Pseudomonas aeruginosa disable phagocytic cells or stimulate internalization into nonphagocytic cells (21, 22, 24–26). Other T6SSs have been demonstrated to manipulate host cells, although the molecular determinants are not yet entirely understood (27–30). However, T6SS gene clusters are widespread in Gram-negative bacterial genomes and not restricted to pathogens (10). Most of them encode proteins with potent antibacterial activities, such as enzymes that cleave essential macromolecules such as DNA, phospholipids, or the peptidoglycan mesh or essential metabolites such as NAD+/NADP+ (31–36). Additional T6SS antibacterial effectors include ADP-ribosyltransferases that specifically target the Z ring and hence inhibit cell division (37). Antibacterial effectors are active in the periplasm or cytoplasm of the target cell and are coproduced with immunity proteins that remain in the producing cell and act as antitoxins to prevent autointoxication during dueling between sister cells (11–13). More recently, T6SS effectors that collect manganese or zinc in the environment to provide metals to the cell have been described (38–40). By deploying antibacterial effectors or scavenging metals, T6SSs play an important role in bacterial communities, and hence T6SS gene clusters are usually highly represented in species present in multispecies microbiota such as the human gut (7, 16–18, 41). In general, the regulatory mechanisms and signals underlying expression of T6SS genes, production of T6SS subunits, or posttranslational activation of the secretion apparatus are tightly linked to environmental cues in the niche in which the T6SS is required to destroy competitors (42–45).
TYPE VI SECRETION MECHANISM OF ACTION
T6SSs use a contractile mechanism to inject effectors (Fig. 2). This mechanism is shared with all CISs: a sheath, assembled in an extended conformation, wraps a needle. Contraction of the sheath into a stable state propels the needle (1, 3–5). The needle is composed of an inner tube capped by the spike complex that pierces the membrane of the target cell (Fig. 1). The tail tube/sheath complex (TTC) is built on an assembly platform named the baseplate (BP) (Fig. 1). The TTC and BP are collectively called the tail, a structure that is conserved among all CISs. In addition to this theme common to all CISs, T6SSs have evolved (i) a membrane complex (MC), which docks the tail to the cell envelope and serves as a channel for the passage of the needle upon sheath contraction, and (ii) a specialized BP component to properly orient the needle toward the cell exterior, by recognizing and binding the MC (2–5, 46–48) (Fig. 1).
FIGURE 2.
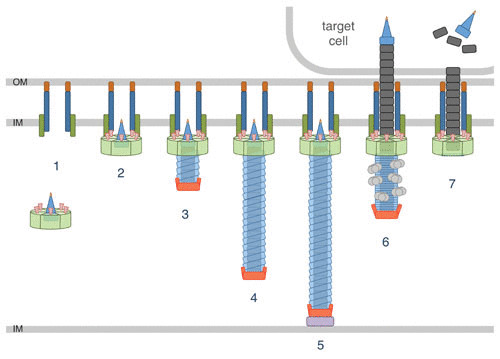
Assembly and mechanism of firing of the T6SS. T6SS biogenesis starts with the positioning and assembly of the membrane complex and the assembly of the BP (1). The recruitment and docking of the BP on the membrane complex (2) initiate the TssA-mediated polymerization of the tail tube/sheath tubular structure (3 to 5), which is stopped when hitting the opposite membrane by the TagA stopper (5). Sheath contraction propels the tube/spike needle into the target (6). The ClpV ATPase is recruited to the contracted sheath to recycle sheath subunits (6). Needle components, and effectors associated with them, are delivered inside the target (7).
T6SS biogenesis starts with the assembly of the MC in the cell envelope and that of the BP in the cytoplasm (49–51) (Fig. 2). Once the BP is docked to the MC, the inner tube and sheath are coordinately assembled (49–52) (Fig. 2).
ARCHITECTURE OF THE TYPE VI SECRETION SYSTEM
The Membrane Complex
The vast majority of T6SS gene clusters of proteobacterial species encode three membrane proteins: TssJ, TssL, and TssM (8–10, 53) (Fig. 1). TssJ is an outer membrane-associated lipoprotein that protrudes in the periplasm (54). TssL and TssM are anchored in the inner membrane (55–57). The structures of several TssJ homologues have been reported: they all share a classical transthyretin fold with an additional loop, of variable length and composition, located between β-strands 1 and 2 (58–60). TssL bears a single C-terminal membrane-spanning segment (56) and a cytoplasmic domain that comprises two bundles of α-helices (61–63). TssM possesses three transmembrane helices followed by a large periplasmic region (55, 57). The periplasmic region of TssM comprises three domains, including the C-terminal domain that engages in interaction with the TssJ extra loop (49, 58). TssL and TssM interact through their transmembrane segments (55, 64, 65). The cytoplasmic domains of TssL and TssM mediate contacts with the BP (50, 57, 64, 66, 67).
The electron microscopy structure of the fully assembled 1.7-MDa TssJLM MC from enteroaggregative Escherichia coli has been reported (49, 68, 69). The complex has a rocket-like structure: a large base, which contains the cytoplasmic and membrane domains of TssL and TssM, is followed by arches and pillars which correspond to the TssM periplasmic domains and TssJ (68). The TssJLM complex, which has 5-fold symmetry in vivo and after purification, comprises 15 copies of TssJ and 10 copies of TssL and of TssM (49, 58). The MC delimits an internal lumen with a diameter insufficient for the passage of the tail tube. In addition, this lumen is partly occluded by a periplasmic constriction gate, suggesting that large conformational changes occur upon BP docking or sheath contraction (49, 58).
The MC can be accessorized by additional subunits, such as peptidoglycan-binding proteins (53, 70, 71). MC anchorage to the cell wall likely stabilizes the MC to resist the forces generated during sheath contraction (70). Finally, recent studies have shown that proper assembly of the MC requires the activity of peptidoglycan-degrading enzymes (72, 73).
Interestingly, while the tail complex is evolutionarily related to contractile injection machines, the evolution history of the MC is less clear. TssL and TssM present significant homologies with two accessory subunits associated with type IVb secretion systems, DotU and IcmF, respectively (8, 9). No homologue of TssJ is associated with DotU/IcmF complexes, suggesting that TssJ is from a different ancestry. Indeed, while essential when present, TssJ is lacking in some T6SSs, such as those of Agrobacterium and Acinetobacter. Further studies are required to understand whether other proteins can compensate for the absence of TssJ in these species. The fact that the MC has a distinct history compared to the tail is also exemplified by the observation that no TssJLM complex is present in Bacteroidales T6SSs (74, 75). However, putative uncharacterized membrane proteins are encoded within these T6SS gene clusters, suggesting that a different transenvelope complex has been domesticated to anchor the tail (74, 75).
The Tail
The baseplate
The BP (Fig. 1) is a large complex, 2.7 MDa, comprising >60 polypeptides of at least six different proteins (50). The role of the BP is to initiate the polymerization of the TTC. While it has not been formally shown yet, the T6SS BP is believed to trigger sheath contraction, as demonstrated in other CISs. A specific role of the T6SS BP is to anchor the TTC to the MC. The BP is composed of six wedge subcomplexes organized around the central hub, i.e., the N-terminal domain of the VgrG spike (76, 77) (Fig. 1). VgrG hence belongs to two tail subcomplexes: it constitutes the tip of the needle and the hub for the BP. The wedge complex is composed of four proteins: TssE, -F, -G, and -K. These four proteins assemble a structure of 1:2:1:6 stoichiometry, the TssG peptide being the central core (77–79). Two TssF subunits wrap TssG to form a triangular shape called the trifurcation unit, whereas two extensions of TssG make contacts with two TssK trimers (77). TssE, -F, -G are, respectively, homologues of phage T4 gp25, gp6, and gp7 and phage Mu Mup46, Mup47, and Mup48 (50, 77, 79, 80), which also constitute the inner part of phage BPs (79–81). TssK has no homologue in Myoviridae but shares architectural homologies with receptor-binding proteins (RBP) of Siphoviridae phages (67). The structure of the N-terminal domain of TssK is superimposable with that of Siphoviridae RBP shoulder domains that are anchored into the BP (67). Indeed, the TssK N-terminal domain establishes extensive contacts with the TssF2G complex (67). The TssK C-terminal domain has evolved to bind to the MC and specifically to the TssL and TssM cytoplasmic domains (57, 64, 66, 67). Similar to the MC, the BP can be accessorized by additional subunits, such as TssA1 in P. aeruginosa (82), that may stabilize the complex or provide additional functions.
The tail tube/sheath complex
The TTC comprises the needle and the contractile sheath (Fig. 1). It forms an ∼1-μm-long tubular structure in the cytoplasm that is assembled in 30 to 50 s (52, 83).
The needle is composed of the inner tube topped by the spike complex. The inner tube is made of hexamers of the Hcp protein (84–86). These donut-shaped hexameric Hcp rings (87, 88) stack on each other in a head-to-tail orientation to form a hollow tube (86). Interestingly, despite very low sequence similarities between T6SS Hcps and tube proteins from other CISs, their structure is strictly conserved (5). Hcp tube polymerization starts at the BP, through direct recruitment of the first ring to the base of the VgrG hub/spike (89). The spike complex is composed of a trimer of the VgrG protein and, in most instances, of the PAAR repeat protein (85, 90). VgrG contains several conserved domains (24, 85). The N-terminal domain resembles the phage T4 gp27 protein and acts as a symmetry adaptor between the 6-fold symmetry of the inner tube and the 3-fold symmetry of the VgrG central and C-terminal domains, which share homologies with the phage T4 gp5 N-terminal and β-prism domains (89, 91, 92). The VgrG β-prism domain is a triangular β-helix that forms, together with the conical PAAR protein, the penetration device of the T6SS needle (90, 93). The VgrG trimer and the PAAR protein can be extended by additional domains that may act as effectors or as adaptors for effectors (24, 90).
The sheath polymerizes from the BP. It is proposed that, similarly to its gp25-like homologues in Myoviridae, the TssE BP subunit constitutes the sheath polymerization initiator (79, 91). In contrast to other CISs, the T6SS sheath is composed of two proteins, TssB and TssC (1, 52, 85, 94, 95), forming a stable dimer that is the repeat unit for sheath polymerization (96–98). Six TssBC dimers form a strand that wraps an Hcp hexameric ring. The TssBC dimer can be divided into three regions: domains 1 and 2 resemble CIS sheath proteins, and domain 3 is inserted into domain 2 (99, 100). Extensive contacts between TssBC dimers from the same strand and from the neighboring −1 and +1 strands stabilize the extended conformation of the sheath polymer (100, 101).
In the T6SS, assembly of the inner tube and that of the extended sheath are interdependent (86, 102). The TssA protein coordinates the polymerization of the TTC (103) (Fig. 2). TssA localizes at the distal extremity of the growing tail tube/sheath (103), at the location in which hexameric tube rings and TssBC strands are incorporated (104). TssA presents a 6-arm starfish-like structure with a central core (103). Protein-protein interaction studies have suggested that the central core of TssA may undergo large conformational changes to insert new Hcp hexamers, whereas the arms may facilitate sheath polymerization (103, 105). Tail tube/sheath polymerization proceeds in the cytoplasm and is stopped when the distal end hits the membrane on the opposite membrane of the bacterial cell (104, 106). A recent study has identified TagA, a protein that interacts with TssA to stop the assembly of the tail and to maintain the sheath under the extended conformation (106) (Fig. 2). However, the TssA cap protein and the TagA stopper are not conserved in T6SS gene clusters, suggesting that different mechanisms control tail tube/sheath assembly and termination in different T6SS+ species (105–107).
Contraction of the T6SS sheath, which occurs in less than 2–5 ms, is believed to start at the BP. The cryo-electron microscopy structure of the Vibrio cholerae T6SS sheath has been solved in the two states, extended and contracted, allowing a reconstitution of the molecular events leading to contraction (99, 100). Contraction consists of a reorganization of the TssBC strands and, notably, an outward rotation of the sheath subunits (100). By doing so, the sheath compacts on the BP and contacts with the inner tube are abolished, thus promoting its expulsion (5, 100, 101). The free energy released during contraction is estimated to >44,000 kcal·mol−1 for a 1-μm-long sheath (100).
After contraction, the sheath is disassembled by a dedicated AAA+ ATPase, ClpV (94, 102) (Fig. 2). ClpV binds to an N-terminal helix of TssC that belongs to sheath domain 3 (108, 109), which is accessible only in the contracted conformation (98, 100). Although this is not clearly established, it is proposed that contracted sheath subunits are recycled rather than conveyed to degradation (102).
LOADING AND TRANSPORT OF EFFECTORS
As summarized above, a broad repertoire of antibacterial and antihost activities has been described already for T6SS effectors. In addition, the mode of loading and transfer of these effectors into target cells is also variable. The common theme is that these effectors are associated with needle components, as the needle is the only portion of the T6SS to be propelled into the target cell (12, 13) (Fig. 3). Effectors can be additional domains fused to needle components such as Hcp, VgrG, or PAAR or independent proteins that directly or indirectly bind to Hcp, VgrG, or PAAR (12, 13). Recruitment of these independent cargo effectors to Hcp, VgrG, or PAAR can be mediated by adaptors, which are themselves domains of the needle components, or independent proteins (110) (Fig. 3).
FIGURE 3.
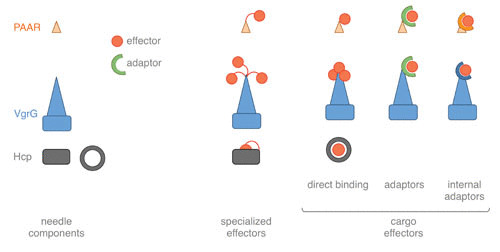
Schematic representation of the mechanisms of effector loading. Effectors are depicted as red circles. Specialized effectors are chimeric needle proteins with extensions encoding the effector. Cargo effectors are independent proteins that associate with needle components (Hcp, VgrG, and PAAR). Binding of cargo effectors to needle components could be direct or mediated by adaptor modules that are independent proteins (adaptors) or extensions of VgrG and PAAR (internal adaptors).
Specialized Hcp, VgrG, and PAAR
When the effector module is on the same polypeptide as the needle component, the T6SS subunit is called “specialized” or “evolved.” Although effectors fused to Hcp or PAAR have been described (36, 90, 111), the best-characterized examples are C-terminal extensions of specialized VgrGs such as V. cholerae VgrG1, which cross-links actin, and VgrG3, which has peptidoglycan glycoside hydrolase activity; A. hydrophila VgrG1, which ADP-ribosylates actin; P. aeruginosa VgrG2b, which interacts with the tubule cap complex; and Burkholderia pseudomallei VgrG5, which induces host cell membrane fusion (21–26, 112, 113).
Cargo Effectors
Cargo effectors are independent proteins that need to recognize their Hcp, VgrG, or PAAR carrier for transfer. This recognition could be direct, such as the case of effectors that bind Hcp, or may require an additional adaptor module that binds VgrG or PAAR (12, 13, 110) (Fig. 3). Usually the effector genes are genetically linked to genes encoding their vehicle, their adaptors (if any), and, in the case of antibacterial toxins, their immunity proteins. These genetic elements could be found within T6SS gene clusters or as Hcp-VgrG islands scattered on the genome (9, 10).
When associated with Hcp, the effector is embedded in the lumen of the hexameric ring and is thus likely found inside the channel of the inner tube during T6SS assembly (16, 114). Consequently, it is protected and stabilized (114, 115). However, the available space in the Hcp ring lumen limits the size of the effector to be transported, which is estimated to be <25 kDa (114).
Adaptors can be isolated proteins or domains fused to the cargo or the vehicle (110). Adaptors from distinct families, such as DUF1795 (EagT6 and EagR), DUF2169, DUF4123 (Tap-1 and Tec), transthyretin, and recombination hot spot (Rhs), have been described and studied (35, 90, 116–125). When several copies of VgrG or PAAR proteins are encoded within the genome, these adaptor modules specify the carriers on which the effector should be mounted (112, 118, 122, 123, 126). In addition to loading the effector on the vehicle, some of these adaptors have been shown to act as chaperones to stabilize the effector or to wrap hydrophobic transmembrane segments to prevent effector aggregation (112, 124, 125).
CONCLUDING REMARKS
Although the T6SS is one of the most recently identified secretion apparatuses, we now have a detailed view on how the system is assembled, how it is structurally arranged, and how effectors are loaded and transported. The broad repertoire of effectors has only recently started to emerge, and it is likely that many effectors with interesting activities will be identified and characterized in the next years. The discovery of the T6SS 13 years ago and its role as an antibacterial weapon have altered our view of bacterial communities. It is now broadly admitted that not only do bacteria cohabitate peacefully but also complex interactions are established to maintain stable ecosystems, such as the human gut microbiota. Further fundamental and translational works are required to better understand how T6SS activation or inhibition may impact microbial communities and may perturb complex ecosystems.
ACKNOWLEDGMENTS
We thank the laboratory members for helpful discussions and support.
Our work on the T6SS is supported by the Centre National de la Recherche Scientifique (CNRS), the Aix-Marseille Université (AMU), and grants from the Agence Nationale de la Recherche (ANR-14-CE14-0006 and ANR-17-CE11-0039 to E.C., ANR-18-CE15-0013 to L.J., and ANR-18-CE11-0023 to E.D.), the Fondation pour la Recherche Médicale (DEQ20180339165), and the Fondation Bettencourt-Schueller. Y.C. is supported by the FRM (FRM-ECO20160736014).
Contributor Information
Yassine Cherrak, Laboratoire d’Ingénierie des Systèmes Macromoléculaires (LISM), Institut de Microbiologie de la Méditerranée (IMM), Aix-Marseille Université, CNRS, UMR 7255, 13402 Marseille Cedex 20, France; Y.C. and N.F. contributed equally to this review..
Nicolas Flaugnatti, Laboratoire d’Ingénierie des Systèmes Macromoléculaires (LISM), Institut de Microbiologie de la Méditerranée (IMM), Aix-Marseille Université, CNRS, UMR 7255, 13402 Marseille Cedex 20, France; Y.C. and N.F. contributed equally to this review.; Present address: Laboratory of Molecular Microbiology, Global Health Institute, School of Life Sciences, Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland.
Eric Durand, Laboratoire d’Ingénierie des Systèmes Macromoléculaires (LISM), Institut de Microbiologie de la Méditerranée (IMM), Aix-Marseille Université, CNRS, UMR 7255, 13402 Marseille Cedex 20, France.
Laure Journet, Laboratoire d’Ingénierie des Systèmes Macromoléculaires (LISM), Institut de Microbiologie de la Méditerranée (IMM), Aix-Marseille Université, CNRS, UMR 7255, 13402 Marseille Cedex 20, France.
Eric Cascales, Laboratoire d’Ingénierie des Systèmes Macromoléculaires (LISM), Institut de Microbiologie de la Méditerranée (IMM), Aix-Marseille Université, CNRS, UMR 7255, 13402 Marseille Cedex 20, France.
Maria Sandkvist, Department of Microbiology and Immunology, University of Michigan, Ann Arbor, Michigan.
Peter J. Christie, Department of Microbiology and Molecular Genetics, McGovern Medical School, Houston, Texas
REFERENCES
- 1.Bönemann G, Pietrosiuk A, Mogk A. 2010. Tubules and donuts: a type VI secretion story. Mol Microbiol 76:815–821. 10.1111/j.1365-2958.2010.07171.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 2.Zoued A, Brunet YR, Durand E, Aschtgen MS, Logger L, Douzi B, Journet L, Cambillau C, Cascales E. 2014. Architecture and assembly of the type VI secretion system. Biochim Biophys Acta 1843:1664–1673. 10.1016/j.bbamcr.2014.03.018. [PubMed] [DOI] [PubMed] [Google Scholar]
- 3.Cascales E. 2017. Microbiology: and Amoebophilus invented the machine gun! Curr Biol 27:R1170–R1173. 10.1016/j.cub.2017.09.025. [PubMed] [DOI] [PubMed] [Google Scholar]
- 4.Taylor NMI, van Raaij MJ, Leiman PG. 2018. Contractile injection systems of bacteriophages and related systems. Mol Microbiol 108:6–15. 10.1111/mmi.13921. [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Brackmann M, Nazarov S, Wang J, Basler M. 2017. Using force to punch holes: mechanics of contractile nanomachines. Trends Cell Biol 27:623–632. 10.1016/j.tcb.2017.05.003. [PubMed] [DOI] [PubMed] [Google Scholar]
- 6.Cianfanelli FR, Monlezun L, Coulthurst SJ. 2016. Aim, load, fire: the type VI secretion system, a bacterial nanoweapon. Trends Microbiol 24:51–62. 10.1016/j.tim.2015.10.005. [PubMed] [DOI] [PubMed] [Google Scholar]
- 7.Coyne MJ, Comstock LE. 2019. Type VI secretion systems and the gut microbiota. Microbiol Spectr 7:PSIB-0009-2018. 10.1128/microbiolspec.PSIB-0009-2018. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bingle LE, Bailey CM, Pallen MJ. 2008. Type VI secretion: a beginner’s guide. Curr Opin Microbiol 11:3–8. 10.1016/j.mib.2008.01.006. [PubMed] [DOI] [PubMed] [Google Scholar]
- 9.Cascales E. 2008. The type VI secretion toolkit. EMBO Rep 9:735–741. 10.1038/embor.2008.131. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyer F, Fichant G, Berthod J, Vandenbrouck Y, Attree I. 2009. Dissecting the bacterial type VI secretion system by a genome wide in silico analysis: what can be learned from available microbial genomic resources? BMC Genomics 10:104. 10.1186/1471-2164-10-104. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell AB, Peterson SB, Mougous JD. 2014. Type VI secretion system effectors: poisons with a purpose. Nat Rev Microbiol 12:137–148. 10.1038/nrmicro3185. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durand E, Cambillau C, Cascales E, Journet L. 2014. VgrG, Tae, Tle, and beyond: the versatile arsenal of type VI secretion effectors. Trends Microbiol 22:498–507. 10.1016/j.tim.2014.06.004. [PubMed] [DOI] [PubMed] [Google Scholar]
- 13.Alcoforado Diniz J, Liu YC, Coulthurst SJ. 2015. Molecular weaponry: diverse effectors delivered by the type VI secretion system. Cell Microbiol 17:1742–1751. 10.1111/cmi.12532. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hachani A, Wood TE, Filloux A. 2016. Type VI secretion and anti-host effectors. Curr Opin Microbiol 29:81–93. 10.1016/j.mib.2015.11.006. [PubMed] [DOI] [PubMed] [Google Scholar]
- 15.Fu Y, Waldor MK, Mekalanos JJ. 2013. Tn-Seq analysis of Vibrio cholerae intestinal colonization reveals a role for T6SS-mediated antibacterial activity in the host. Cell Host Microbe 14:652–663. 10.1016/j.chom.2013.11.001. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sana TG, Flaugnatti N, Lugo KA, Lam LH, Jacobson A, Baylot V, Durand E, Journet L, Cascales E, Monack DM. 2016. Salmonella Typhimurium utilizes a T6SS-mediated antibacterial weapon to establish in the host gut. Proc Natl Acad Sci U S A 113:E5044–E5051. 10.1073/pnas.1608858113. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sana TG, Lugo KA, Monack DM. 2017. T6SS: the bacterial “fight club” in the host gut. PLoS Pathog 13:e1006325. 10.1371/journal.ppat.1006325. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chassaing B, Cascales E. 2018. Antibacterial weapons: targeted destruction in the microbiota. Trends Microbiol 26:329–338. 10.1016/j.tim.2018.01.006. [PubMed] [DOI] [PubMed] [Google Scholar]
- 19.García-Bayona L, Comstock LE. 2018. Bacterial antagonism in host-associated microbial communities. Science 361:eaat2456. 10.1126/science.aat2456. [PubMed] [DOI] [PubMed] [Google Scholar]
- 20.Veening JW, Blokesch M. 2017. Interbacterial predation as a strategy for DNA acquisition in naturally competent bacteria. Nat Rev Microbiol 15:621–629. 10.1038/nrmicro.2017.66. [PubMed] [DOI] [PubMed] [Google Scholar]
- 21.Pukatzki S, Ma AT, Sturtevant D, Krastins B, Sarracino D, Nelson WC, Heidelberg JF, Mekalanos JJ. 2006. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc Natl Acad Sci U S A 103:1528–1533. 10.1073/pnas.0510322103. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sana TG, Baumann C, Merdes A, Soscia C, Rattei T, Hachani A, Jones C, Bennett KL, Filloux A, Superti-Furga G, Voulhoux R, Bleves S. 2015. Internalization of Pseudomonas aeruginosa strain PAO1 into epithelial cells is promoted by interaction of a T6SS effector with the microtubule network. mBio 6:e00712-15. 10.1128/mBio.00712-15. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwarz S, Singh P, Robertson JD, LeRoux M, Skerrett SJ, Goodlett DR, West TE, Mougous JD. 2014. VgrG-5 is a Burkholderia type VI secretion system-exported protein required for multinucleated giant cell formation and virulence. Infect Immun 82:1445–1452. 10.1128/IAI.01368-13. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pukatzki S, Ma AT, Revel AT, Sturtevant D, Mekalanos JJ. 2007. Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proc Natl Acad Sci U S A 104:15508–15513. 10.1073/pnas.0706532104. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suarez G, Sierra JC, Erova TE, Sha J, Horneman AJ, Chopra AK. 2010. A type VI secretion system effector protein, VgrG1, from Aeromonas hydrophila that induces host cell toxicity by ADP ribosylation of actin. J Bacteriol 192:155–168. 10.1128/JB.01260-09. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durand E, Derrez E, Audoly G, Spinelli S, Ortiz-Lombardia M, Raoult D, Cascales E, Cambillau C. 2012. Crystal structure of the VgrG1 actin cross-linking domain of the Vibrio cholerae type VI secretion system. J Biol Chem 287:38190–38199. 10.1074/jbc.M112.390153. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aubert DF, Xu H, Yang J, Shi X, Gao W, Li L, Bisaro F, Chen S, Valvano MA, Shao F. 2016. A Burkholderia type VI effector deamidates Rho GTPases to activate the pyrin inflammasome and trigger inflammation. Cell Host Microbe 19:664–674. 10.1016/j.chom.2016.04.004. [PubMed] [DOI] [PubMed] [Google Scholar]
- 28.Eshraghi A, Kim J, Walls AC, Ledvina HE, Miller CN, Ramsey KM, Whitney JC, Radey MC, Peterson SB, Ruhland BR, Tran BQ, Goo YA, Goodlett DR, Dove SL, Celli J, Veesler D, Mougous JD. 2016. Secreted effectors encoded within and outside of the Francisella pathogenicity island promote intramacrophage growth. Cell Host Microbe 20:573–583. 10.1016/j.chom.2016.10.008. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ledvina HE, Kelly KA, Eshraghi A, Plemel RL, Peterson SB, Lee B, Steele S, Adler M, Kawula TH, Merz AJ, Skerrett SJ, Celli J, Mougous JD. 2018. A phosphatidylinositol 3-kinase effector alters phagosomal maturation to promote intracellular growth of Francisella. Cell Host Microbe 24:285–295.e8. 10.1016/j.chom.2018.07.003. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lennings J, West TE, Schwarz S. 2019. The Burkholderia type VI secretion system 5: composition, regulation and role in virulence. Front Microbiol 9:3339. 10.3389/fmicb.2018.03339. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hood RD, Singh P, Hsu F, Güvener T, Carl MA, Trinidad RR, Silverman JM, Ohlson BB, Hicks KG, Plemel RL, Li M, Schwarz S, Wang WY, Merz AJ, Goodlett DR, Mougous JD. 2010. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe 7:25–37. 10.1016/j.chom.2009.12.007. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell AB, Hood RD, Bui NK, LeRoux M, Vollmer W, Mougous JD. 2011. Type VI secretion delivers bacteriolytic effectors to target cells. Nature 475:343–347. 10.1038/nature10244. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russell AB, LeRoux M, Hathazi K, Agnello DM, Ishikawa T, Wiggins PA, Wai SN, Mougous JD. 2013. Diverse type VI secretion phospholipases are functionally plastic antibacterial effectors. Nature 496:508–512. 10.1038/nature12074. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma LS, Hachani A, Lin JS, Filloux A, Lai EM. 2014. Agrobacterium tumefaciens deploys a superfamily of type VI secretion DNase effectors as weapons for interbacterial competition in planta. Cell Host Microbe 16:94–104. 10.1016/j.chom.2014.06.002. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitney JC, Quentin D, Sawai S, LeRoux M, Harding BN, Ledvina HE, Tran BQ, Robinson H, Goo YA, Goodlett DR, Raunser S, Mougous JD. 2015. An interbacterial NAD(P)(+) glycohydrolase toxin requires elongation factor Tu for delivery to target cells. Cell 163:607–619. 10.1016/j.cell.2015.09.027. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pissaridou P, Allsopp LP, Wettstadt S, Howard SA, Mavridou DAI, Filloux A. 2018. The Pseudomonas aeruginosa T6SS-VgrG1b spike is topped by a PAAR protein eliciting DNA damage to bacterial competitors. Proc Natl Acad Sci U S A 115:12519–12524. 10.1073/pnas.1814181115. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ting SY, Bosch DE, Mangiameli SM, Radey MC, Huang S, Park YJ, Kelly KA, Filip SK, Goo YA, Eng JK, Allaire M, Veesler D, Wiggins PA, Peterson SB, Mougous JD. 2018. Bifunctional immunity proteins protect bacteria against FtsZ-targeting ADP-ribosylating toxins. Cell 175:1380–1392.e14. 10.1016/j.cell.2018.09.037. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang T, Si M, Song Y, Zhu W, Gao F, Wang Y, Zhang L, Zhang W, Wei G, Luo ZQ, Shen X. 2015. Type VI secretion system transports Zn2+ to combat multiple stresses and host immunity. PLoS Pathog 11:e1005020. 10.1371/journal.ppat.1005020. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Si M, Zhao C, Burkinshaw B, Zhang B, Wei D, Wang Y, Dong TG, Shen X. 2017. Manganese scavenging and oxidative stress response mediated by type VI secretion system in Burkholderia thailandensis. Proc Natl Acad Sci U S A 114:E2233–E2242. 10.1073/pnas.1614902114. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Si M, Wang Y, Zhang B, Zhao C, Kang Y, Bai H, Wei D, Zhu L, Zhang L, Dong TG, Shen X. 2017. The type VI secretion system engages a redox-regulated dual-functional heme transporter for zinc acquisition. Cell Rep 20:949–959. 10.1016/j.celrep.2017.06.081. [PubMed] [DOI] [PubMed] [Google Scholar]
- 41.Verster AJ, Ross BD, Radey MC, Bao Y, Goodman AL, Mougous JD, Borenstein E. 2017. The landscape of type VI secretion across human gut microbiomes reveals its role in community composition. Cell Host Microbe 22:411–419.e4. 10.1016/j.chom.2017.08.010. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernard CS, Brunet YR, Gueguen E, Cascales E. 2010. Nooks and crannies in type VI secretion regulation. J Bacteriol 192:3850–3860. 10.1128/JB.00370-10. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silverman JM, Brunet YR, Cascales E, Mougous JD. 2012. Structure and regulation of the type VI secretion system. Annu Rev Microbiol 66:453–472. 10.1146/annurev-micro-121809-151619. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyata ST, Bachmann V, Pukatzki S. 2013. Type VI secretion system regulation as a consequence of evolutionary pressure. J Med Microbiol 62:663–676. 10.1099/jmm.0.053983-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 45.LeRoux M, Peterson SB, Mougous JD. 2015. Bacterial danger sensing. J Mol Biol 427:3744–3753. 10.1016/j.jmb.2015.09.018. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ho BT, Dong TG, Mekalanos JJ. 2014. A view to a kill: the bacterial type VI secretion system. Cell Host Microbe 15:9–21. 10.1016/j.chom.2013.11.008. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basler M. 2015. Type VI secretion system: secretion by a contractile nanomachine. Philos Trans R Soc Lond B Biol Sci 370:20150021. 10.1098/rstb.2015.0021. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coulthurst S. 2019. The type VI secretion system: a versatile bacterial weapon. Microbiology 165:503–515. 10.1099/mic.0.000789. [PubMed] [DOI] [PubMed] [Google Scholar]
- 49.Durand E, Nguyen VS, Zoued A, Logger L, Péhau-Arnaudet G, Aschtgen MS, Spinelli S, Desmyter A, Bardiaux B, Dujeancourt A, Roussel A, Cambillau C, Cascales E, Fronzes R. 2015. Biogenesis and structure of a type VI secretion membrane core complex. Nature 523:555–560. 10.1038/nature14667. [PubMed] [DOI] [PubMed] [Google Scholar]
- 50.Brunet YR, Zoued A, Boyer F, Douzi B, Cascales E. 2015. The type VI secretion TssEFGK-VgrG phage-like baseplate is recruited to the TssJLM membrane complex via multiple contacts and serves as assembly platform for tail tube/sheath polymerization. PLoS Genet 11:e1005545. 10.1371/journal.pgen.1005545. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gerc AJ, Diepold A, Trunk K, Porter M, Rickman C, Armitage JP, Stanley-Wall NR, Coulthurst SJ. 2015. Visualization of the Serratia type VI secretion system reveals unprovoked attacks and dynamic assembly. Cell Rep 12:2131–2142. 10.1016/j.celrep.2015.08.053. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Basler M, Pilhofer M, Henderson GP, Jensen GJ, Mekalanos JJ. 2012. Type VI secretion requires a dynamic contractile phage tail-like structure. Nature 483:182–186. 10.1038/nature10846. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aschtgen MS, Gavioli M, Dessen A, Lloubès R, Cascales E. 2010. The SciZ protein anchors the enteroaggregative Escherichia coli type VI secretion system to the cell wall. Mol Microbiol 75:886–899. 10.1111/j.1365-2958.2009.07028.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 54.Aschtgen MS, Bernard CS, De Bentzmann S, Lloubès R, Cascales E. 2008. SciN is an outer membrane lipoprotein required for type VI secretion in enteroaggregative Escherichia coli. J Bacteriol 190:7523–7531. 10.1128/JB.00945-08. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma LS, Lin JS, Lai EM. 2009. An IcmF family protein, ImpLM, is an integral inner membrane protein interacting with ImpKL, and its walker a motif is required for type VI secretion system-mediated Hcp secretion in Agrobacterium tumefaciens. J Bacteriol 191:4316–4329. 10.1128/JB.00029-09. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aschtgen MS, Zoued A, Lloubès R, Journet L, Cascales E. 2012. The C-tail anchored TssL subunit, an essential protein of the enteroaggregative Escherichia coli Sci-1 type VI secretion system, is inserted by YidC. Microbiologyopen 1:71–82. 10.1002/mbo3.9. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Logger L, Aschtgen MS, Guérin M, Cascales E, Durand E. 2016. Molecular dissection of the interface between the type VI secretion TssM cytoplasmic domain and the TssG baseplate component. J Mol Biol 428:4424–4437. 10.1016/j.jmb.2016.08.032. [PubMed] [DOI] [PubMed] [Google Scholar]
- 58.Felisberto-Rodrigues C, Durand E, Aschtgen MS, Blangy S, Ortiz-Lombardia M, Douzi B, Cambillau C, Cascales E. 2011. Towards a structural comprehension of bacterial type VI secretion systems: characterization of the TssJ-TssM complex of an Escherichia coli pathovar. PLoS Pathog 7:e1002386. 10.1371/journal.ppat.1002386. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rao VA, Shepherd SM, English G, Coulthurst SJ, Hunter WN. 2011. The structure of Serratia marcescens Lip, a membrane-bound component of the type VI secretion system. Acta Crystallogr D Biol Crystallogr 67:1065–1072. 10.1107/S0907444911046300. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robb CS, Assmus M, Nano FE, Boraston AB. 2013. Structure of the T6SS lipoprotein TssJ1 from Pseudomonas aeruginosa. Acta Crystallogr Sect F Struct Biol Cryst Commun 69:607–610. 10.1107/S1744309113012220. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Durand E, Zoued A, Spinelli S, Watson PJ, Aschtgen MS, Journet L, Cambillau C, Cascales E. 2012. Structural characterization and oligomerization of the TssL protein, a component shared by bacterial type VI and type IVb secretion systems. J Biol Chem 287:14157–14168. 10.1074/jbc.M111.338731. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robb CS, Nano FE, Boraston AB. 2012. The structure of the conserved type six secretion protein TssL (DotU) from Francisella novicida. J Mol Biol 419:277–283. 10.1016/j.jmb.2012.04.003. [PubMed] [DOI] [PubMed] [Google Scholar]
- 63.Chang JH, Kim YG. 2015. Crystal structure of the bacterial type VI secretion system component TssL from Vibrio cholerae. J Microbiol 53:32–37. 10.1007/s12275-015-4539-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 64.Zoued A, Cassaro CJ, Durand E, Douzi B, España AP, Cambillau C, Journet L, Cascales E. 2016. Structure-function analysis of the TssL cytoplasmic domain reveals a new interaction between the type VI secretion baseplate and membrane complexes. J Mol Biol 428:4413–4423. 10.1016/j.jmb.2016.08.030. [PubMed] [DOI] [PubMed] [Google Scholar]
- 65.Zoued A, Duneau JP, Durand E, España AP, Journet L, Guerlesquin F, Cascales E. 2018. Tryptophan-mediated dimerization of the TssL transmembrane anchor is required for type VI secretion system activity. J Mol Biol 430:987–1003. 10.1016/j.jmb.2018.02.008. [PubMed] [DOI] [PubMed] [Google Scholar]
- 66.Zoued A, Durand E, Bebeacua C, Brunet YR, Douzi B, Cambillau C, Cascales E, Journet L. 2013. TssK is a trimeric cytoplasmic protein interacting with components of both phage-like and membrane anchoring complexes of the type VI secretion system. J Biol Chem 288:27031–27041. 10.1074/jbc.M113.499772. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nguyen VS, Logger L, Spinelli S, Legrand P, Huyen Pham TT, Nhung Trinh TT, Cherrak Y, Zoued A, Desmyter A, Durand E, Roussel A, Kellenberger C, Cascales E, Cambillau C. 2017. Type VI secretion TssK baseplate protein exhibits structural similarity with phage receptor-binding proteins and evolved to bind the membrane complex. Nat Microbiol 2:17103. 10.1038/nmicrobiol.2017.103. [PubMed] [DOI] [PubMed] [Google Scholar]
- 68.Rapisarda C, Cherrak Y, Kooger R, Schmidt V, Pellarin R, Logger L, Cascales E, Pilhofer M, Durand E, Fronzes R. 2019. In situ and high-resolution cryo-EM structure of a bacterial type VI secretion system membrane complex. EMBO J 38:e100886. 10.15252/embj.2018100886. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yin M, Yan Z, Li X. 2019. Architecture of type VI secretion system membrane core complex. Cell Res 29:251–253. 10.1038/s41422-018-0130-7. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aschtgen MS, Thomas MS, Cascales E. 2010. Anchoring the type VI secretion system to the peptidoglycan: TssL, TagL, TagP... what else? Virulence 1:535–540. 10.4161/viru.1.6.13732. [PubMed] [DOI] [PubMed] [Google Scholar]
- 71.Santin YG, Camy CE, Zoued A, Doan T, Aschtgen MS, Cascales E. 2019. Role and recruitment of the TagL peptidoglycan-binding protein during type VI secretion system biogenesis. J Bacteriol 201:e00173-19. 10.1128/JB.00173-19. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weber BS, Hennon SW, Wright MS, Scott NE, de Berardinis V, Foster LJ, Ayala JA, Adams MD, Feldman MF. 2016. Genetic dissection of the type VI secretion system in Acinetobacter and identification of a novel peptidoglycan hydrolase, TagX, required for its biogenesis. mBio 7:e01253-16. 10.1128/mBio.01253-16. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Santin YG, Cascales E. 2017. Domestication of a housekeeping transglycosylase for assembly of a type VI secretion system. EMBO Rep 18:138–149. 10.15252/embr.201643206. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Russell AB, Wexler AG, Harding BN, Whitney JC, Bohn AJ, Goo YA, Tran BQ, Barry NA, Zheng H, Peterson SB, Chou S, Gonen T, Goodlett DR, Goodman AL, Mougous JD. 2014. A type VI secretion-related pathway in Bacteroidetes mediates interbacterial antagonism. Cell Host Microbe 16:227–236. 10.1016/j.chom.2014.07.007. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coyne MJ, Roelofs KG, Comstock LE. 2016. Type VI secretion systems of human gut Bacteroidales segregate into three genetic architectures, two of which are contained on mobile genetic elements. BMC Genomics 17:58. 10.1186/s12864-016-2377-z. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nazarov S, Schneider JP, Brackmann M, Goldie KN, Stahlberg H, Basler M. 2018. Cryo-EM reconstruction of type VI secretion system baseplate and sheath distal end. EMBO J 37:e97103. 10.15252/embj.201797103. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cherrak Y, Rapisarda C, Pellarin R, Bouvier G, Bardiaux B, Allain F, Malosse C, Rey M, Chamot-Rooke J, Cascales E, Fronzes R, Durand E. 2018. Biogenesis and structure of a type VI secretion baseplate. Nat Microbiol 3:1404–1416. 10.1038/s41564-018-0260-1. [PubMed] [DOI] [PubMed] [Google Scholar]
- 78.English G, Byron O, Cianfanelli FR, Prescott AR, Coulthurst SJ. 2014. Biochemical analysis of TssK, a core component of the bacterial type VI secretion system, reveals distinct oligomeric states of TssK and identifies a TssK-TssFG subcomplex. Biochem J 461:291–304. 10.1042/BJ20131426. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taylor NM, Prokhorov NS, Guerrero-Ferreira RC, Shneider MM, Browning C, Goldie KN, Stahlberg H, Leiman PG. 2016. Structure of the T4 baseplate and its function in triggering sheath contraction. Nature 533:346–352. 10.1038/nature17971. [PubMed] [DOI] [PubMed] [Google Scholar]
- 80.Büttner CR, Wu Y, Maxwell KL, Davidson AR. 2016. Baseplate assembly of phage Mu: defining the conserved core components of contractile-tailed phages and related bacterial systems. Proc Natl Acad Sci U S A 113:10174–10179. 10.1073/pnas.1607966113. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kostyuchenko VA, Leiman PG, Chipman PR, Kanamaru S, van Raaij MJ, Arisaka F, Mesyanzhinov VV, Rossmann MG. 2003. Three-dimensional structure of bacteriophage T4 baseplate. Nat Struct Biol 10:688–693. 10.1038/nsb970. [PubMed] [DOI] [PubMed] [Google Scholar]
- 82.Planamente S, Salih O, Manoli E, Albesa-Jové D, Freemont PS, Filloux A. 2016. TssA forms a gp6-like ring attached to the type VI secretion sheath. EMBO J 35:1613–1627. 10.15252/embj.201694024. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brunet YR, Espinosa L, Harchouni S, Mignot T, Cascales E. 2013. Imaging type VI secretion-mediated bacterial killing. Cell Rep 3:36–41. 10.1016/j.celrep.2012.11.027. [PubMed] [DOI] [PubMed] [Google Scholar]
- 84.Ballister ER, Lai AH, Zuckermann RN, Cheng Y, Mougous JD. 2008. In vitro self-assembly of tailorable nanotubes from a simple protein building block. Proc Natl Acad Sci U S A 105:3733–3738. 10.1073/pnas.0712247105. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leiman PG, Basler M, Ramagopal UA, Bonanno JB, Sauder JM, Pukatzki S, Burley SK, Almo SC, Mekalanos JJ. 2009. Type VI secretion apparatus and phage tail-associated protein complexes share a common evolutionary origin. Proc Natl Acad Sci U S A 106:4154–4159. 10.1073/pnas.0813360106. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brunet YR, Hénin J, Celia H, Cascales E. 2014. Type VI secretion and bacteriophage tail tubes share a common assembly pathway. EMBO Rep 15:315–321. 10.1002/embr.201337936. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mougous JD, Cuff ME, Raunser S, Shen A, Zhou M, Gifford CA, Goodman AL, Joachimiak G, Ordoñez CL, Lory S, Walz T, Joachimiak A, Mekalanos JJ. 2006. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science 312:1526–1530. 10.1126/science.1128393. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Douzi B, Spinelli S, Blangy S, Roussel A, Durand E, Brunet YR, Cascales E, Cambillau C. 2014. Crystal structure and self-interaction of the type VI secretion tail-tube protein from enteroaggregative Escherichia coli. PLoS One 9:e86918. 10.1371/journal.pone.0086918. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Renault MG, Zamarreno Beas J, Douzi B, Chabalier M, Zoued A, Brunet YR, Cambillau C, Journet L, Cascales E. 2018. The gp27-like hub of VgrG serves as adaptor to promote Hcp tube assembly. J Mol Biol 430(18 Part B):3143–3156. 10.1016/j.jmb.2018.07.018. [PubMed] [DOI] [PubMed] [Google Scholar]
- 90.Shneider MM, Buth SA, Ho BT, Basler M, Mekalanos JJ, Leiman PG. 2013. PAAR-repeat proteins sharpen and diversify the type VI secretion system spike. Nature 500:350–353. 10.1038/nature12453. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leiman PG, Shneider MM. 2012. Contractile tail machines of bacteriophages. Adv Exp Med Biol 726:93–114. 10.1007/978-1-4614-0980-9_5. [PubMed] [DOI] [PubMed] [Google Scholar]
- 92.Spínola-Amilibia M, Davó-Siguero I, Ruiz FM, Santillana E, Medrano FJ, Romero A. 2016. The structure of VgrG1 from Pseudomonas aeruginosa, the needle tip of the bacterial type VI secretion system. Acta Crystallogr D Struct Biol 72:22–33. 10.1107/S2059798315021142. [PubMed] [DOI] [PubMed] [Google Scholar]
- 93.Uchida K, Leiman PG, Arisaka F, Kanamaru S. 2014. Structure and properties of the C-terminal β-helical domain of VgrG protein from Escherichia coli O157. J Biochem 155:173–182. 10.1093/jb/mvt109. [PubMed] [DOI] [PubMed] [Google Scholar]
- 94.Bönemann G, Pietrosiuk A, Diemand A, Zentgraf H, Mogk A. 2009. Remodelling of VipA/VipB tubules by ClpV-mediated threading is crucial for type VI protein secretion. EMBO J 28:315–325. 10.1038/emboj.2008.269. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lossi NS, Dajani R, Freemont P, Filloux A. 2011. Structure-function analysis of HsiF, a gp25-like component of the type VI secretion system, in Pseudomonas aeruginosa. Microbiology 157:3292–3305. 10.1099/mic.0.051987-0. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bröms JE, Ishikawa T, Wai SN, Sjöstedt A. 2013. A functional VipA-VipB interaction is required for the type VI secretion system activity of Vibrio cholerae O1 strain A1552. BMC Microbiol 13:96. 10.1186/1471-2180-13-96. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang XY, Brunet YR, Logger L, Douzi B, Cambillau C, Journet L, Cascales E. 2013. Dissection of the TssB-TssC interface during type VI secretion sheath complex formation. PLoS One 8:e81074. 10.1371/journal.pone.0081074. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kube S, Kapitein N, Zimniak T, Herzog F, Mogk A, Wendler P. 2014. Structure of the VipA/B type VI secretion complex suggests a contraction-state-specific recycling mechanism. Cell Rep 8:20–30. 10.1016/j.celrep.2014.05.034. [PubMed] [DOI] [PubMed] [Google Scholar]
- 99.Kudryashev M, Wang RY, Brackmann M, Scherer S, Maier T, Baker D, DiMaio F, Stahlberg H, Egelman EH, Basler M. 2015. Structure of the type VI secretion system contractile sheath. Cell 160:952–962. 10.1016/j.cell.2015.01.037. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang J, Brackmann M, Castaño-Díez D, Kudryashev M, Goldie KN, Maier T, Stahlberg H, Basler M. 2017. Cryo-EM structure of the extended type VI secretion system sheath-tube complex. Nat Microbiol 2:1507–1512. 10.1038/s41564-017-0020-7. [PubMed] [DOI] [PubMed] [Google Scholar]
- 101.Brackmann M, Wang J, Basler M. 2018. Type VI secretion system sheath inter-subunit interactions modulate its contraction. EMBO Rep 19:225–233. 10.15252/embr.201744416. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kapitein N, Bönemann G, Pietrosiuk A, Seyffer F, Hausser I, Locker JK, Mogk A. 2013. ClpV recycles VipA/VipB tubules and prevents non-productive tubule formation to ensure efficient type VI protein secretion. Mol Microbiol 87:1013–1028. 10.1111/mmi.12147. [PubMed] [DOI] [PubMed] [Google Scholar]
- 103.Zoued A, Durand E, Brunet YR, Spinelli S, Douzi B, Guzzo M, Flaugnatti N, Legrand P, Journet L, Fronzes R, Mignot T, Cambillau C, Cascales E. 2016. Priming and polymerization of a bacterial contractile tail structure. Nature 531:59–63. 10.1038/nature17182. [PubMed] [DOI] [PubMed] [Google Scholar]
- 104.Vettiger A, Winter J, Lin L, Basler M. 2017. The type VI secretion system sheath assembles at the end distal from the membrane anchor. Nat Commun 8:16088. 10.1038/ncomms16088. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zoued A, Durand E, Santin YG, Journet L, Roussel A, Cambillau C, Cascales E. 2017. TssA: the cap protein of the type VI secretion system tail. Bioessays 39:10. 10.1002/bies.201600262. [PubMed] [DOI] [PubMed] [Google Scholar]
- 106.Santin YG, Doan T, Lebrun R, Espinosa L, Journet L, Cascales E. 2018. In vivo TssA proximity labelling during type VI secretion biogenesis reveals TagA as a protein that stops and holds the sheath. Nat Microbiol 3:1304–1313. 10.1038/s41564-018-0234-3. [PubMed] [DOI] [PubMed] [Google Scholar]
- 107.Dix SR, Owen HJ, Sun R, Ahmad A, Shastri S, Spiewak HL, Mosby DJ, Harris MJ, Batters SL, Brooker TA, Tzokov SB, Sedelnikova SE, Baker PJ, Bullough PA, Rice DW, Thomas MS. 2018. Structural insights into the function of type VI secretion system TssA subunits. Nat Commun 9:4765. 10.1038/s41467-018-07247-1. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pietrosiuk A, Lenherr ED, Falk S, Bönemann G, Kopp J, Zentgraf H, Sinning I, Mogk A. 2011. Molecular basis for the unique role of the AAA+ chaperone ClpV in type VI protein secretion. J Biol Chem 286:30010–30021. 10.1074/jbc.M111.253377. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Douzi B, Brunet YR, Spinelli S, Lensi V, Legrand P, Blangy S, Kumar A, Journet L, Cascales E, Cambillau C. 2016. Structure and specificity of the type VI secretion system ClpV-TssC interaction in enteroaggregative Escherichia coli. Sci Rep 6:34405. 10.1038/srep34405. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Unterweger D, Kostiuk B, Pukatzki S. 2017. Adaptor proteins of type VI secretion system effectors. Trends Microbiol 25:8–10. 10.1016/j.tim.2016.10.003. [PubMed] [DOI] [PubMed] [Google Scholar]
- 111.Ma J, Pan Z, Huang J, Sun M, Lu C, Yao H. 2017. The Hcp proteins fused with diverse extended-toxin domains represent a novel pattern of antibacterial effectors in type VI secretion systems. Virulence 8:1189–1202. 10.1080/21505594.2017.1279374. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brooks TM, Unterweger D, Bachmann V, Kostiuk B, Pukatzki S. 2013. Lytic activity of the Vibrio cholerae type VI secretion toxin VgrG-3 is inhibited by the antitoxin TsaB. J Biol Chem 288:7618–7625. 10.1074/jbc.M112.436725. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Toesca IJ, French CT, Miller JF. 2014. The type VI secretion system spike protein VgrG5 mediates membrane fusion during intercellular spread by pseudomallei group Burkholderia species. Infect Immun 82:1436–1444. 10.1128/IAI.01367-13. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Silverman JM, Agnello DM, Zheng H, Andrews BT, Li M, Catalano CE, Gonen T, Mougous JD. 2013. Haemolysin coregulated protein is an exported receptor and chaperone of type VI secretion substrates. Mol Cell 51:584–593. 10.1016/j.molcel.2013.07.025. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Whitney JC, Beck CM, Goo YA, Russell AB, Harding BN, De Leon JA, Cunningham DA, Tran BQ, Low DA, Goodlett DR, Hayes CS, Mougous JD. 2014. Genetically distinct pathways guide effector export through the type VI secretion system. Mol Microbiol 92:529–542. 10.1111/mmi.12571. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Unterweger D, Miyata ST, Bachmann V, Brooks TM, Mullins T, Kostiuk B, Provenzano D, Pukatzki S. 2014. The Vibrio cholerae type VI secretion system employs diverse effector modules for intraspecific competition. Nat Commun 5:3549. 10.1038/ncomms4549. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liang X, Moore R, Wilton M, Wong MJ, Lam L, Dong TG. 2015. Identification of divergent type VI secretion effectors using a conserved chaperone domain. Proc Natl Acad Sci U S A 112:9106–9111. 10.1073/pnas.1505317112. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Unterweger D, Kostiuk B, Ötjengerdes R, Wilton A, Diaz-Satizabal L, Pukatzki S. 2015. Chimeric adaptor proteins translocate diverse type VI secretion system effectors in Vibrio cholerae. EMBO J 34:2198–2210. 10.15252/embj.201591163. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Alcoforado Diniz J, Coulthurst SJ. 2015. Intraspecies competition in Serratia marcescens is mediated by type VI-secreted Rhs effectors and a conserved effector-associated accessory protein. J Bacteriol 197:2350–2360. 10.1128/JB.00199-15. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Flaugnatti N, Le TT, Canaan S, Aschtgen MS, Nguyen VS, Blangy S, Kellenberger C, Roussel A, Cambillau C, Cascales E, Journet L. 2016. A phospholipase A1 antibacterial type VI secretion effector interacts directly with the C-terminal domain of the VgrG spike protein for delivery. Mol Microbiol 99:1099–1118. 10.1111/mmi.13292. [PubMed] [DOI] [PubMed] [Google Scholar]
- 121.Bondage DD, Lin JS, Ma LS, Kuo CH, Lai EM. 2016. VgrG C terminus confers the type VI effector transport specificity and is required for binding with PAAR and adaptor-effector complex. Proc Natl Acad Sci U S A 113:E3931–E3940. 10.1073/pnas.1600428113. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cianfanelli FR, Alcoforado Diniz J, Guo M, De Cesare V, Trost M, Coulthurst SJ. 2016. VgrG and PAAR proteins define distinct versions of a functional type VI secretion system. PLoS Pathog 12:e1005735. 10.1371/journal.ppat.1005735. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ma J, Sun M, Dong W, Pan Z, Lu C, Yao H. 2017. PAAR-Rhs proteins harbor various C-terminal toxins to diversify the antibacterial pathways of type VI secretion systems. Environ Microbiol 19:345–360. 10.1111/1462-2920.13621. [PubMed] [DOI] [PubMed] [Google Scholar]
- 124.Quentin D, Ahmad S, Shanthamoorthy P, Mougous JD, Whitney JC, Raunser S. 2018. Mechanism of loading and translocation of type VI secretion system effector Tse6. Nat Microbiol 3:1142–1152. 10.1038/s41564-018-0238-z. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Burkinshaw BJ, Liang X, Wong M, Le ANH, Lam L, Dong TG. 2018. A type VI secretion system effector delivery mechanism dependent on PAAR and a chaperone-co-chaperone complex. Nat Microbiol 3:632–640. 10.1038/s41564-018-0144-4. [PubMed] [DOI] [PubMed] [Google Scholar]
- 126.Hachani A, Allsopp LP, Oduko Y, Filloux A. 2014. The VgrG proteins are “à la carte” delivery systems for bacterial type VI effectors. J Biol Chem 289:17872–17884. 10.1074/jbc.M114.563429. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]


