Abstract
Multiple randomized controlled trials have extensively examined the therapeutic effectiveness of sodium-glucose cotransporter 2 (SGLT2) inhibitors, ushering in a transformative approach to treating individuals with type 2 diabetes mellitus (DM). Notably, emerging reports have drawn attention to the potential positive impacts of SGLT2 inhibitors in nondiabetic patients. In an effort to delve into this phenomenon, a comprehensive systematic literature review spanning PubMed (NLM), Medline (Ovid), and Cochrane Library, covering publications from 2000 to 2024 was undertaken. This systematic review encompassed twenty-six randomized control trials (RCTs) involving 35,317 participants. The findings unveiled a multifaceted role for SGLT2 inhibitors, showcasing their ability to enhance metabolic control and yield cardioprotective effects through a reduction in cardiovascular death (CVD) and hospitalization related to heart failure (HF). Additionally, a renalprotective effect was observed, evidenced by a slowdown in chronic kidney disease (CKD) progression and a decrease in albuminuria. Importantly, these benefits were coupled with an acceptable safety profile. The literature also points to various biological plausibility and underlying mechanistic pathways, offering insights into the association between SGLT2 inhibitors and these positive outcomes in nondiabetic individuals. Current research trends indicate a continual exploration of additional role for SGLT2 inhibitors in. Nevertheless, further research is imperative to fully elucidate the mechanisms and long-term outcomes associated with the nondiabetic use of SGLT2 inhibitors.
Keywords: SGLT2, Chronic kidney disease, Cardiovascular disease, IgA nephropathy, Metabolism, Systematic review
Introduction
Sodium-glucose Cotransporter 2 (SGLT2) inhibitors are hypoglycemic agents with a unique mechanism of action toward lowering blood sugar independent of insulin [1]. SGLT2 inhibitors were first derived from phlorizin isolated from the root bark of apple trees in 1835 [2]. In addition to anti-inflammatory, antioxidant, and anticancer effects, phlorizin is also reported to have anti-glycemic effects [2], [3]. Although phlorizin has poor bioavailability, novel phlorizin-based analogs derived from C glucoside have increased bioavailability, selectivity, and stability [4], [5]. Commonly, SGLT2 inhibitors are approved for marketing for the treatment of hyperglycemia by the European Medicines Agency (EMA), Food and Drug Administration (FDA), Pharmaceutical and Medical Devices Agency, Japan (PMDA) and National Medical Products Administration, China (NMPA) [6]. To date, four SGLT2 inhibitors, canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin, are currently approved for use in adults in the US [7]. Canagliflozin was the first SGLT2 inhibitor approved in March 2013, followed by dapagliflozin in January 2014, empagliflozin in August 2014, and the latest being ertugliflozin in 2017 [7]. Among the four FDA-approved drugs, empagliflozin has the greatest selectivity for SGLT2 compared to SGLT1, while canagliflozin is the least selective [8].
Sodium-glucose cotransporters (SGLT) are transport proteins that transport glucose into cells via a sodium concentration gradient established by a Na+/K+ ATPase pump [9]. SGLT1 and SGLT2 are the two essential transport proteins in the SGLT family. SGLT2 is predominantly expressed in proximal convoluted tubules [6], [9]. It is a low affinity/high-capacity protein located in the apical membrane of renal proximal tubules (S1 & S2 segment) for most of the glucose reabsorption (80–90 %) in kidneys [6], [9]. SGLT1 is a high affinity/low-capacity protein expressed in the S3 segment of the renal proximal tubule and responsible for the reabsorption of 10 to 20 % of glucose not absorbed in the S1 and S2 segments of the tubule [10], [11], [12], [13]. In addition, SGLT1 is also expressed in the small intestine, heart, and brain [14], [15]. It is located in the brush border membrane of the small intestine, in cardiomyocytes of the heart and pyramidal and purkinje cells, blood–brain barrier, and endothelium of intracerebral capillaries [14], [15]. SGLT2 inhibitors are well absorbed from the gastrointestinal tract. They have high plasma protein binding (PPB) with extensive tissue distribution (dapagliflozin 91 % PPB, empagliflozin 86 % PPB, ertugliflozin 93 % PPB and canagliflozin 99 % PPB [16]. SGLT2 inhibitors undergo biotransformation by UDP-glucuronosyltransferases (UGTs)-mediated glucuronidation with minimal metabolism by cytochrome P450 and are excreted through urine [17], [18]. SGLT2 inhibitors are administered as daily doses. The currently available doses include canagliflozin (100 mg & 300 mg), dapagliflozin (5 & 10 mg), empagliflozin (10 & 25 mg) and ertugliflozin (5 & 15 mg) [19], [20], [21], [22].
SGLT2 inhibitors are among the latest FDA-approved antihyperglycemic agents [1]. These are weak glucose-lowering agents that are either used as monotherapy or in combination with metformin, sulfonylurea, pioglitazone, or insulin and reduce the mean hemoglobin A1c (HbA1c) level between 0.4 and 1.1 % as compared to placebo [23], [24], [25], [26], [27], [28], [29]. A meta-analysis of 10 randomized controlled clinical trials [71] showed that SGLT2 inhibitors were associated with a lower occurrence of cardiovascular death or hypertensive heart failure (HHF) by 33 % in high-risk diabetic patients [30]. Multiple large-scale randomized controlled clinical trials, including CREDENCE (Effects of Canagliflozin on Renal & Cardiovascular Outcome in Participants with Diabetic Nephropathy), SCORED (Sotagliflozin in Patients with Diabetic and Chronic Kidney Disease), CANVAS (Canagliflozin Cardiovascular Assessment Study), EMPA-REG OUTCOME(Empagliflozin Cardiovascular Outcome events trial in Type 2 Diabetes Mellitus patients), DECLARE-TIMI 58 (Dapagliflozin & Cardiovascular outcome in type 2 Diabetes), VERTIS-CV (Cardiovascular outcome with Empagliflozin in type 2 Diabetes), SOLOIST-WHF (Sotagliflozin in patients with diabetes and recent worsening heart failure) evaluated the cardiovascular benefits of SGLT2 inhibitor use in patients with diabetes mellitus and found significant reduction in cardiovascular death and heart failure in patients with SGLT2 inhibitors [31], [32], [33], [34], [35], [36], [37]. CKD trials, including CREDENCE and SCORED trials, are randomized controlled trials that evaluated the effects of SGLT2 inhibitors on primary kidney endpoints in diabetic patients [31]. CREDENCE trial showed a 30 % reduction in doubling of creatinine, end stage renal disease (ESRD), and death from renal disease in patients treated with canagliflozin [31]. Although historically used for the management of diabetes and diabetes-related complications, recently nondiabetic use of SGLT2 inhibitors has been emphasized. This study will review the available evidence for using SGLT2 inhibitors in nondiabetic patients.
Methods
A systematic review was conducted to assess the evidence for nondiabetic use of SGLT2 inhibitors.
Eligibility criteria
The eligibility criteria for inclusion included all randomized controlled trials (RCTs) looking at the nondiabetic use of SGLT2 inhibitors published in peer-reviewed journals between 2000 and 2024. Studies were excluded if they were (i) not randomized controlled trials, (ii) not related to nondiabetic use of SGLT2 inhibitors, (iii) animal studies, (iv) ongoing randomized controlled trials with no results, (v) genetic study or modeling study or bench work, (vi) laboratory-based studies & (vii) descriptive studies or systemic reviews or metanalysis.
Search strategy and selection process
Only relevant randomized controlled trials published in english between 2000 and 2024 with available full texts were included in the final review. We systematically searched articles included in PubMed (NLM), Medline (Ovid), and the Cochrane Library using a broad set of keywords and mesh terms to maximize sensitivity; the last search date was Jan 7th, 2024. Concepts that made up the search term included – SGLT2 inhibitor, nondiabetic use, canagliflozin, empagliflozin, dapagliflozin, ertugliflozin, CKD, and heart failure. Also, the bibliography of identified articles and Scopus (Elsevier) were searched for any additional studies not found through the initial search of the database. An auto-alert was also set up on Medline (Ovid) to notify users of related articles that matched the search term. Two authors independently reviewed all full-text articles and abstracts, and any discrepancies were resolved by consensus. Citation manager RefWorks (ProQuest, Ann Arbor, MI) was used to manage citations, including removing internal and external duplicates among the three databases. An MS Excel workbook was used to screen abstracts. Two authors independently appraised and extracted details from all eligible full-text articles and findings integrated into the descriptive summary table.
Data gathering and risk of bias assessment
Findings from selected studies included author, year of publication, country, study design, sample size and characteristics of participants, primary and secondary outcomes, duration of follow-up, intervention and comparison group, selection criteria, effect size, and conclusion. Methodologic quality was assessed using the Cochrane Collaboration risk of bias tool [38]. Quality control during the article searching process was accomplished by (i) a database search conducted by experienced authors, (ii) an independent search of all abstracts and titles by two authors, (iii) screeners were blinded to study author, (iv) independent review of all full-text articles by two authors, and (v) a high Cohen's kappa coefficient for agreement between authors screening the abstracts.
Outcomes
The primary and secondary cardiovascular and metabolic outcomes included left ventricular end diastolic volume (LVEDV), left ventricular end systolic volume (LVESV), worsening heart failure (HF), hospitalization for HF, change in cardiac function, cardiovascular death, variation in blood pressure,composite of cardiovascular and renal outcome, left ventricular mass, admission for HF, change in body weight, percent change in BMI, Hba1C, weight etc. Some of the renal outcome status variables included % change in 24 h proteinuria, change in eGFR, composite of renal function and cardiovascular function, death from renal disease, safety outcome etc.
Results
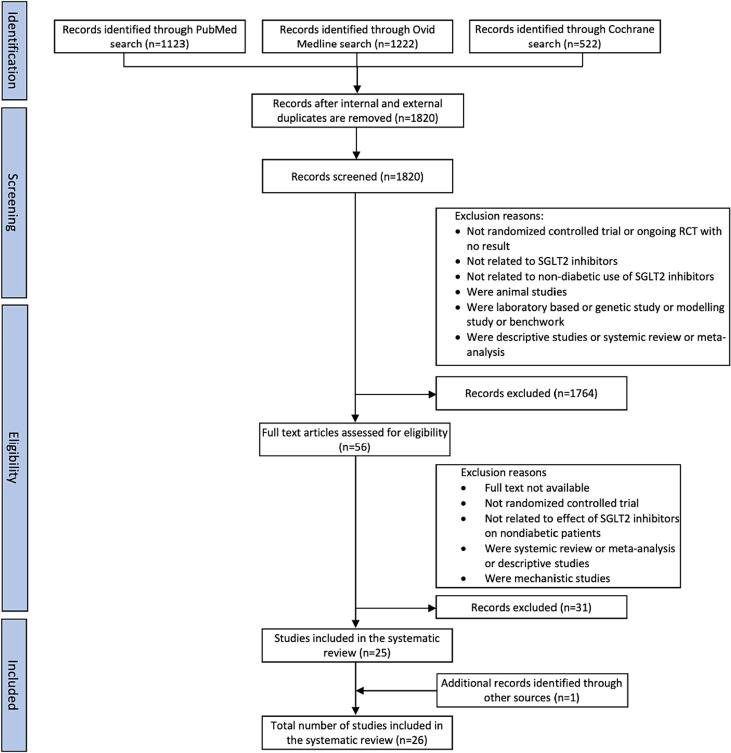
The primary objective of this study was to evaluate the nondiabetic use of SGLT2 inhibitors. The initial search retrieved 2867 articles. A total of 2811 articles were excluded based on the title and abstract review. The process for literature search and inclusion of studies in the review is identified in Fig. 1. Fifty-six full-text articles were reviewed, of which 25 articles fulfilled all the inclusion and exclusion criteria and were included in the study. One additional study was identified through a bibliography search. A total of 26 randomized controlled trials involving 35,317 participants published in english between 2000 and 2024 from the US and other countries were included in the study. The Cohen’s kappa of agreement between the two authors was 98 % (Cohen’s kappa for inter-rater reliability was κ = 0.96). The characteristics of the 26 RCTs were included in Table 1, Table 2, Table 3. We also assessed the methodological quality and risk of bias using the Cochrane collaborative methodological quality assessment result of all included RCTs in the study. The RCTs for non diabetic use of SGLT2 inhibitors only compared the efficacy of dapagliflozin, empagliflozin and canagliflozin. To the best of our knowledge, no study looked at the non diabetic efficacy of ertugliflozin.
Fig. 1.
PRISMA Flow Diagram.
Table 1.
SGLT2 inhibitor trials looking at the efficacy of SGLT2 inhibitors on cardiovascular outcomes in patients without diabetes.
| Author, year, country | N, Median age, F (%), DM (%) | Type of Study | Selection criteria | Treatment group | Comparison group | Median follow-up | Primary outcome | Secondary outcome | Effect size | Study conclusion |
|---|---|---|---|---|---|---|---|---|---|---|
| Gallego et al., 2021, Canada [39] | 84, 62, 36 %, DM (0 %) | EMPA-TROPISM trial— Randomized double-blind placebo- controlled trial | Adult age > 18 with NYHA class II and III HF with LVEF < 50 % with stable s/s and medical treatment within 3 months | Empagliflozin (10 mg/day) | Placebo | 6 months | Left ventricular end diastolic volume (LVEDV)and left ventricular end systolic volume (LVESV) | Left ventricular mass, left ventricular ejection fraction, quality of life, peak oxygen consumption | Significant reduction in LVEDV (-25.1 ± 260 ml) vs (-1.5 ± 254 ml) (p < 0.001); LVESV (-26.6 ± 20.5) vs (-0.5 ± 21.9) (p < 0.001); Reduction of LV mass (p < 0.001); improvement of LV ejection fraction (p < 0.001); improvement of peak O2 consumption and quality of life | Role of SGLT2 inhibitor in treatment of heart failure with reduced ejection fraction independent of glycemic status |
| Anker et al., 2020, Germany [40] | 1874, 67.6, 24.8 %, DM (50 %) |
Randomized double-blind parallel group placebo-controlled event driven trial | Adult age 18 years with NYHA class II, III and IV HF with LVEF of 40 % | Empagliflozin (10 mg/day) | Placebo | 16 months | Cardiovascular death, hospitalization for HF, total hospitalization for HF, adverse renal outcome | Time for cardiovascular death; time for first renal composite outcome | The hazard ratio for time to first event of cardiovascular death 0.78 (0.64 – 0.97). Hazard ratio for first and recurrent hypertensive HF 0.76 (0.57 – 1.01) Time to cardiovascular death 0.92 (0.68 – 1.24) | Empagliflozin decreased the risk of primary outcome and total hospitalization for HF by 25–30 % and decreased the rate of decline of eGFR and risk of adverse effect by 50 % |
| McMurray et al., 2019, UK [41] | 4744, 66.2, 23.8 %, DM (55 %) | DAPA-HF— Randomized double-blinded, placebo-controlled clinical trialPhase 3 | Patients with NYHA class II, III and IVLVEF of 40 % | Dapagliflozin (10 mg/day) | Placebo | 24 months | Worsening heart failure (hospitalization or urgent visit resulting in intravenous treatment for HF Cardiovascular death | Composite of hospitalization for HF or cardiovascular death.Total number of hospitalizations for HF and cardiovascular deaths | HR for primary outcome in treatment group compared to placebo is 0.74 (0.65–0.85)Worsening HF in 10 % of Dapagliflozin vs 13.7 % of placebo with HR of 0.70 (0.59–0.83)HR of death from CVD is 0.82 (0.69–0.98)Similar finding similar in patient with and without diabetes | Among patients with HF with reduced EF, the risk of worsening HF or death from cardiovascular disease was lower among those who received Dapagliflozin compared to those who received placebo irrespective of diabetes. |
| Nassif et al., 2019, US [42] | 263, 62.2, 27.5 %, DM 61.8 %) | DEFINE-HF—Randomized double-blinded, placebo-controlled linical trial | HF with NYHA class II & III LVEF of 40 %eGFR 30 ml/min/1.73 m2 | Dapagliflozin (10 mg/day) | Placebo | 12 weeks | Mean NT-proBNPProportion of patients with 5 points increase in HF specific health status on the Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score or a 20 % decrease in NT-proBNP | Proportion of patients with meaningful change in KCCQ, mean BNP, change in SBP and HbA1c | Patients treated with Dapagliflozin had a clinically meaningful improvement of 5 points in KCCQ or at least a 20 % reduction in NT-proBNP compared to placebo (p = 0.003)Similar results seen in patients with or without diabetes | Addition of Dapagliflozin for 12 weeks did not affect mean NT-proBNP but significant increased proportion of patients experienced clinically meaningful improvement in HF disease specific health status and natriuretic peptide |
| Dayem et al., 2023, Egypt [43] | 100, 55.24, 16 %, DM (0 %) | DACAMI trial—Double blinded randomized controlled trial | Patients with anterior ST elevation MI (STEMI) patient who underwent PCI with LVEF < 50 % | Dapagliflozin (10 mg/day) | Placebo | 12 weeks | Change in cardiac function determined by measure of cardiac function change between baseline and 12 weeks post cardiac event and echo parameter (LVEF, LVEDV, LV mass) at baseline four weeks and 12 weeks post event | Not reported | Mean drop in NT ProBNP in treatment group compared to control group is 10.17 % (p = 0.03)Decrease left ventricular mass by 11.46 % (p = 0.02) | Dapagliflozin plays a role in prevention of left ventricular dysfunction and maintain cardiac function post ant STEMI |
| Diaz-Cruz et al., 2020, Mexico [44] | 30, 50, 53.3 %, DM (0 %) | Double blinded placebo-controlled linical trial | Patients with pre-diabetes and prehypertension and were sedentary with the no diabetes, hypertension, renal, cardiac, thyroid disease | Dapagliflozin (10 mg/day) | Placebo | 12 weeks | Variability in blood pressure | Not reported | Significant decrease in 24-hour SBP (p = 0.04) and nighttime SBP (p = 0.01) and deep circadian blood pressure pattern (p = 0.04) | 3 months use of Dapagliflozin decreased blood pressure by lowering 24 h SBP, nighttime SBP, mean arterial pressure, nocturnal hypertensionCervicovaginal infection and UTI did not affect adherence to treatment |
| Packer et al., 2020, US [45] | 3730, 67.2, 23.5 %, DM (49.8 %) | Double blinded parallel group placebo-controlled event driven trial | Patients with NYHA class II, III and IV HF with LVEF of 40 % | Empagliflozin (10 mg/day) | Placebo | 16 months | Composite of cardiovascular deaths or hospitalization for worsening HF | Total number of hospitalization due to HF | HR for CVD or hospitalization for HF is 0.75 (0.65–0.86) (p < 0.001)Decrease in total number of hospitalizations for HF in treatment group is 0.70 (0.58–0.85) (p < 0.001) | Empagliflozin decreased risk of cardiovascular or hospitalization for HF irrespective of diabetes |
| Zanchi et al., 2020, Switzerland [46] | 40, 34.1, 40 %, DM (0 %) | Double-blind, randomized placebo-controlled trial | Adult 18–50 years of age with HbA1c < 6.5 %, urine albumin-creatinine ratio < 3.3 mg/mmol. Normal urine dipstick, hematology, chemistry and normal ultrasound | Empagliflozin (10 mg/day) | Placebo | 1 month | Acute and chronic effect on renal tissue oxygenation | Effect on body weight, blood pressure, renal tubular function, hematocrit | No acute or sustained changes were found in renal cortical or medullary tissue oxygenation 24-hour SBP and DBP decreased significantly after 1 month of Empagliflozin therapy | Empagliflozin has effect on blood pressure reduction |
| Petrie et al., 2020, UK [47] | 4744, 66, 23 %, DM (55 %) | Double-blind, randomized placebo-controlled trial | Adult 18–85 years with NYHA class II and II HF with LVEF < 50 % | Dapagliflozin (10 mg/day) | Placebo | 24 months | Composite of first episode of worsening HF or cardiovascular death | Hospital admission for worsening HF or CVDTotal number of hospital admission for HF and cardiovascular death | Significant reduction of risk of primary composite outcome of first episode of worsening HF in nondiabetics (HR of 0.73; p = 0.002) | Dapagliflozin was effective in reducing cardiovascular mortality and morbidity in patients with HF and reduced EF |
| Ibanez et al., 2021, US [48] | 84, 62, 36 %, DM (0 %) | EMPA-TROPISM-Double-blind, randomized placebo-controlled trial | Adult aged 18–85 years with NYHA class II and IIIHF with LVEF < 50 % | Empagliflozin (10 mg/day) | Placebo | 6 months | Effect of SGLT2 in nondiabetic patient with HFrEFInterstitial myocardial fibrosisAortic stenosisEpicardial adipose tissue | Not reported | Significant reduction in epicardial adipose tissue (p < 0.05), decreased interstitial myocardial fibrosis (p < 0.01) and significant reduction in aortic stiffness (p < 0.01) Significant reduction in inflammatory biomarker | Empagliflozin significantly improved adiposity, interstitial myocardial fibrosis, aortic stiffness and inflammatory markers in nondiabetic patient with HFrEF |
| Anker et al., 2021, Germany [49] | 5988, 71.8, 44.6 %, DM (49 %) |
EMPEROR- Preserved- Randomized double blind parallel group placebo-controlled event driven trial |
Adult 18 years with HF with class II to IV with LVEF of > 40 % | Empagliflozin (10 mg/day) | Placebo | 26.2 months | Composite of cardiovascular death or hospitalization due to HF | Adjudicated hospitalization for HF Rate of decline in eGFR |
The hazard of primary outcome (hospitalization for HF) was lower in Empagliflozin group HR 0.79 (p < 0.001) and the effect appeared consistent in patients with or without diabetes | Empagliflozin reduced the risk of cardiovascular deaths or hospitalization for HF in patients with LVEF of 40 % regardless of presence or absence of diabetes |
HF – heart failure, LVEF – left ventricular ejection fraction, HR – hazards ratio, SBP – systolic blood pressure, DBP – diastolic blood pressure, BNP – brain natriuretic peptide, HFrEF – heart failure with reduced ejection fraction, PCI – percutaneous intervention, STEMI – ST elevation MI, eGFR – estimated GFR.
Table 2.
SGLT2 inhibitor trials looking at the efficacy of SGLT2 inhibitors on metabolic outcome in patients without diabetes.
| Author, year, country | N, Median age, Female (%), DM (%) | Type of study | Selection criteria | Treatment group | Comparison group | Median follow-up | Primary outcome | Secondary outcome | Effect size | Study conclusion |
|---|---|---|---|---|---|---|---|---|---|---|
| Bays et al., 2013, US [56] | 376, 44.8, 88 %, DM (0 %) | Intent to treatRandomized double blind placebo- controlled trial | Adult aged 18–65 years with BMI between 30 and < 50 kg/m2 or with wither lower BMI in presence of hypertension | Canagliflozin (50 mg,100mgor300mgoncedaily) | Placebo | 12 weeks | % change in body weight from baseline to week 12 | Absolute change in body weight% change in body weightChange in BMI | Canagliflozin increase urinary glucose excretion in a dose dependent manner and results in statistically reduction in body weight compared to placebo.Least square mean percent changes from baseline of −2.2 %, −2.9 %, 2.7 % and 1.3 % at doses of 50, 100, 300 mg and placebo (p < 0.05) | In overweight and obese subjects without diabetes, Canagliflozin significantly reduce body weight compared to placebo |
| Hollander et al., 2017, ermany [57] | 334, 45.7, 81.7 %, DM (0 %) | Randomized control double-blind parallel group placebo-controlled multicenter parallel-group trial | Overweight and obese adults aged 18–65 years without type 2 diabetes who had BMI 30 and < 50 kg/m2 or had BMI 27 and < 50 with comorbidities like HTN and/or dyslipidemia without diabetes | Canagliflozin (300 mg/day) Or Phentermine OrCanagliflozin + Phentermine | Placebo | 26 weeks | % change in body weight from baseline to week 26 | Proportion of individual achieving weight loss 5 % and change from baseline in SBP | Statistically superior weight loss from baseline for Canagliflozin/Phentermine compared to placebo at 26 weeks (mean difference −6.9 % with p < 0.001Statistically significant achievement of weight loss 5 % and reduction of SBP for Canagliflozin/Phentermine | Canagliflozin/Phentermine produced meaningful reduction in bodyweight and well tolerated in overweight/obese individuals with type II diabetes |
| Lundkvist et al., 2016, Sweden [58] | 50, 52, 61 %, DM (0 %) | Double blinded randomized controlled trial | Obese man and woman aged 15–70 years without diabetes, hypertension, and BMI of 30–45 kg/m2 | Dapagliflozin (10 mg/day) + subcutaneous long acting exenatide 2 mg once daily | Placebo | 24 weeks | Change in body weight from baseline to 24 weeks | Percent change in body weight from baseline. Efficacy on SBP, waist hip ratio, glycemic measures | The difference of body weight change was −4.13 kg (p < 0.001) attributable to adipose tissue reduction 36 % vs 4.2 % of participants achieved 5 % body weight loss | Dual treatment with Dapagliflozin/exenatide dual therapy reduced body weight, frequency of prediabetes and SBP over 24 weeksWell tolerated with minimal side effects |
| Gonzalez-Ortiz et al., 2017, exico [59] | 26, 45, 84.6 %, DM (0 %) | Randomized double-blinded, placebo-controlled clinical trial | Overweight subjects with BMI of 25–29.9 kg/m2 without hypertension | Dapagliflozin (10 mg/day) | Placebo | 12 weeks | Change in body weight at 12 weeks | Renal composite outcomeDeath from any causeHospitalization for HF | Significant improvement of weight, BMI, fat mass, SBP, visceral adiposity.No significant effect on DBP, glucose changes | Dapagliflozin has significant effect on reduction in body weight, visceral adiposity, BMI and SBP in overweight subjects |
| Neeland et al., 2020, US [60] | 35, 53, 62.9 %, DM (0 %) | Randomized double blind placebo-controlled trial | Age 18 years, obese, BMI 30, nondiabetic, able to undergo neck to knee MRI scan for body fat assessment | Empagliflozin (10 mg/day) | Placebo | 3 months | Change in body weightDecrease in HbA1cGlycerol derived gluconeogenesis | Fasting blood glucose, serum insulin level, TG, plasma free glycerol, adiponectin, alanine aminotransferase, norepinephrine | 6.5 % increase (p = 0.005) increase in area under curve for glycerol-derived 13C enrichment in treatment group | Empagliflozin reduced endogenous glycerol-gluconeogenesis in obese adults without diabetes. SGLT2 inhibitors may prevent type 2 diabetes in obesity |
| Ramirez-Rodriguez et al., 2020, Mexico [50] | 24, 46.7, 70.83 %, DM (0 %) | Randomized double blinded placebo-controlled clinical trial | Subjects with prediabetes | Dapagliflozin (10 mg/day) | Placebo | 3 months | Change in body weight, BMI, waist circumference, fasting glucose | Change in uric acid level | Significant decrease in body weight (p = 0.01), BMI (p = 0.023), waist circumference (p = 0.003), fasting blood sugar (p < 0.001) and uric acid (p = 0.03) | Dapagliflozin in patients with prediabetes significantly decreased body weight, BMI, waist circumference, fasting blood sugar, uric acid and increase insulin sensitivity |
| Ryan et al., 020, US [51] | 50, 35, 76 %, DM (0 %) | Randomized double blinded placebo-controlled clinical trial | Adults of age 18–65 years with BMI > 27.5 kg/m2 sedentary, with no known metabolic disease | Dapagliflozin (5 mg/day) for first 14 days and then increased to Dapagliflozin (10 mg/day) for rest of the study period | Placebo | 12 weeks | Fat free massRise in HDL cholesterol | Dietary preference, hunger, appetite | Dapagliflozin combined with dietary counselling resulted in significant reduction in fat free mass (p = 0.04) and attenuated the rise in HDL cholesterol (p = 0.028) | Careful consideration of decrease in fat free mass is indicatedLonger duration of SGLT2 inhibitors evoked measurable effects on dietary preference, hunger and appetite |
| Elkind- Hirsch et al., 2021, US [52] | 92, 28, 100 %, DM (0 %) | Randomized single blind trial | Subjects with BMI of 30–45 kg/m2 and PCOS | Exenatide (EQW) (2mgweekly)Dapagliflozin (DAPA (10 mg/day)Exenatide plus DapagliflozinDapagliflozin plus metforminPhentermine topiramate (PHEN/TPM) | – | 24 weeks | OGTTFasting insulinWeightBlood pressureWaist circumferenceBody composition | Not reported | Significant difference among the five different treatment groups for mean blood glucose (p < 0.02), BMI (p < 0.005), waist circumference (p < 0.035), fasting blood glucose (p < 0.0001) and OGTT (p < 0.0001)Dual treatment with EQW/DAPA was superior to either DAPA alone, DAPA/metformin and PHEN/TPM | Dual treatment with EQW/DAPA was superior to either DAPA alone, DAPA/metformin and PHEN/TPM in terms of clinical and metabolic benefits |
| Faerch et al., 2020, Denmark [53] | 112, 57.2, 55.83 %, DM (0 %) |
PRE-D trial—Randomized controlled parallel multicenter open-label non blinded study | Overweight subjects with BMI 25 kg/m2 and prediabetes | Dapagliflozin (10 mg/day)Metformin (1700mgdaily)Interval-based exercise | Placebo | 26 weeks | Change in the mean amplitude of glycemic excursions (MAGE), measure of glycemic variability between baseline and 13 weeks | Change from baseline to mid-point of treatment, end of treatment and follow up for MAGE, fasting plasma glucose, fasting serum insulin, body weight, waist hip ratio, blood lipids, OGTT | Compared to control group, there was a small difference in MAGE in Dapagliflozin group (p = 0.04) and a small non-significant reduction in exercise group (p = 0.067) and unchanged in Metformin. | Treatment with Dapagliflozin and interval-based exercise lead to similar but small improvements in glycemic variability compared to control and metformin therapy |
| Kullmann et al., 2022, Germany [54] | 40, 62.5, 60 %, DM (0 %) | Double-blind, randomized placebo-controlled trial | Adult aged 30–75 years with BMI 25 and < 40 kg/m2 and impaired fasting glucose | Empagliflozin (25 mg/day) | Placebo | 8 weeks | Insulin responsiveness of brain | Fasting plasma glucose, total adipose tissue content, liver fat content | Empagliflozin increased hypothalamic insulin responsivity and decrease in fasting blood glucose and liver fat | Empagliflozin may reverse brain insulin resistance with potential benefits for adiposity |
| Veelen et al., 2022, Netherlands [55] | 14, 42.85, 66.3 %, DM (0 %) | Randomized double blind placebo-controlled crossover trial | Adult 40–75 years with BMI 27 to 38 with prediabetes and sedentary lifestyle | Dapagliflozin (10 mg/day) | Placebo | 2 weeks | Body weight, HbA1c, SBP, DBP, 24-hour urinary glucose excretion, 24-hour energy expenditure, energy metabolism during daytime and night-time | Hepatic and skeletal muscle glycogen, mitochondrial oxidative metabolism, hepatic lipid content | Compared to placebo, Dapagliflozin had higher 24-hour urinary glucose excretion (p < 0.0001), daytime respiratory exchange ratio (p = 0.046), nighttime respiratory exchange ratio (p = 0.019), higher maximal mitochondrial oxidative capacity (p = 0.007) and lowers SBP (p = 0.025), DBP (p = 0.023).No difference for skeletal muscle glycogen, fasting plasma glucose, free fatty acid, and food preference | Dapagliflozin treatment of prediabetic insulin resistant individuals for 14 days resulted in significant metabolic adaptations, skeletal muscle metabolism, improved fat oxidation and mitochondrial oxidative capacity. |
BMI – body mass index; SBP – systolic blood pressure; DBP – diastolic blood pressure.
Table 3.
SGLT2 inhibitor trials looking at the efficacy of SGLT2 inhibitors on renal outcome in patients without diabetes.
| Author, year, country | N, Median age, F (%), DM (%) | Type of study | Selection criteria | Treatment group | Comparison group | Median follow-up | Primary outcome | Secondary outcome | Effect size | Study conclusion |
|---|---|---|---|---|---|---|---|---|---|---|
| Cherney et al., 2020, Canada [61] | 53, 51, 32 %, DM (0 %) | DIAMOND trial—Randomized double blind placebo- controlled crossover trial done at six hospitals in Canada, Malaysia, and Netherlands | Adult 18–75 years with CKD without diagnosis of DM and 24-hour urine protein excretion (>500 mg to ≤ 3500) with eGFR of 25 ml/min/1.73 m2 and were on stable ACEI | Dapagliflozin (10 mg/day) | Placebo | 18 weeks | % change in baseline 24-hour proteinuria during Dapagliflozin treatment relative to placebo | Changes in measured GFR, bodyweight, blood pressure, concentration of neurohormonal biomarker | Mean proteinuria change between treatment and placebo 0.9 % (p = 0.93), mGFR change −6.6 ml/min/1.73 m2 (P= <0.0001). Reduction in body weight 1.5 kg (p = 0.046) | 6 weeks treatment did not affect proteinuria in CKD patient without DM. Acute and reversible decline in mGFR and reduction in body weight |
| Heerspink et al, 2020, Netherlands [62] | 4304, 61.9, 33 %, DM (67.6 %) | DAPA-CKD trial— Randomized double-blind placebo controlled multicenter clinical trial | Patient with estimated GFR of 25 to 75 ml/min/1.73 m2 and urinary albumin creatinine ratio of 200 to 5000 in both diabetic and nondiabetic | Dapagliflozin (10 mg/day) | Placebo | 2.4 years | Composite of sustained decline in eGFR of at least 50 %, ESRD or death from renal or cardiac disease | Composite cardiovascular outcome defined as hospitalization for HF or death from cardiovascular disease. Death from other cause | The hazard ratio for composite of sustained decline in eGFR of at least 50 %, ESRD or death from renal cause was 0.56 (P < 0.0001) | The effect of Dapagliflozin were similar in participants with or without diabetes. Most effective class of drug to prevent CKD progression since discovery of RAS inhibitor |
| Herrington et al, 2022, UK [63] | 6609, 63.9, 33.2 %, DM (46.2 %) | EMPA- KIDNEY trial—International randomized parallel group double-blind, placebo-controlled clinical trial | Patient with CKD with eGFR of at least 20 but < 45 ml/min/1.73 m2 or eGFR of at least 45 but less than 90 ml/min with urine albumin creatinine ratio of at least 200 | Empagliflozin (10 mg/day) | Placebo | 2 years | Composite of progression of kidney disease (ESRD with sustained decrease in eGFR to < 10 ml/min, a sustained decrease in eGFR of ≥ 40 % from baseline or death from renal causes) or death from cardiovascular disease | Hospitalization from heart failure; hospitalization from any cause | The hazard ratio for progression of kidney disease or death from CVD in Empaglitazone vs. placebo was 0.86 (p = 0.003). No difference in composite outcome for hospitalization due to heart failure or death from cardiovascular disease | Empagliflozin lowered risk of disease progression from kidney disease or death from CVD compared to placebo |
| Causland et al., 2022, US [64] | 6262, 72, 43 %, DM (45 %) | DELIVER trial (prespecified analysis)- International multicenter randomized, parallel-group, event driven controlled trial | Adult 40 years or older with symptomatic LVEF > 40 % with evidence of structural heart disease (LVH, LA enlargement) | Dapagliflozin (10 mg/day) | Placebo | 2.3 years | Renal specific outcome- sustained 50 % or greater decline in eGFR compared to baseline, development of ESRD or death due to kidney disease. Cardiovascular disease | Safety outcome | Following the initial expected acute decline in eGFR, Dapagliflozin slowed the long-term decline in eGFR compared to placebo and was more pronounced in patients with diabetics compared to nondiabetics | Baseline kidney function did not modify the benefit of Dapagliflozin in patients with heart failure. DAPA did not significantly reduce the frequency of renal composite outcome |
CKD – chronic kidney disease, ESRD – end stage renal disease, eGFR – estimated GFR, mGFR – measured GFR, LVH – left ventricular hypertrophy, LA – left atrium.
Effect on Cardiovascular diseases
Eleven RCTs comprising a combined cohort of 18,793 patients were included in the review (Table 1) [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49]. The sample size for the trials ranged between 30 and 5988. The participants' baseline characteristics included a median age of 34.1 to 71.8, predominantly male (only one study had 53.3 % female) [44]. Four trials had participants without diabetes [39], [43], [44], [46]. Most trials had at least 50 % or more participants with diabetes. All the trials were double-blinded randomized placebo-controlled trials. Across the eleven studies, the SGLT2 inhibitors used included dapagliflozin in 5 studies and empagliflozin in 6 studies. Dapagliflozin and empagliflozin were administered at a dosage of 10 mg given once daily and compared with a control group receiving placebo. The median length of follow-up ranged between 12 weeks to 26.2 months. Most trials had participants aged ≥ 18 years with NYHA Class II, III & IV HF with LV ejection fraction (LVEF) ranging between 40 and 50 %. The study by Nassif et al. also had estimated GFR (eGFR) of ≥ 30 ml/min/1.73 m2 body surface area as inclusion criteria [42]. Another study had urine albumin/creatinine ratio < 3.3 mg/mmol, normal urine dipstick, hematology, chemistry, and normal ultrasound [46]. Dayem et al. included participants with anterior ST elevation MI (STEMI) who underwent percutaneous coronary intervention (PCI) but with LVEF < 50 % [43]. A study by Diaz-Cruz et al. had study participants who were prediabetic, prehypertensive, non-smoker without hypertension, renal, cardiac, and thyroid diseases [44]. Intervention with empagliflozin was used in RCTs [39], [40], [45], [46], [48], [49], [50], [51], [52], [53], [54], [55].
All studies showed significant reduction in left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic volume (LVESV), left ventricular (LV) mass (P < 0.001); hazard ratio (HR) of 0.78 (CI:0.64–0.97) for time to first event of cardiovascular death (CVD); HR for hospitalization for HF 0.75 (CI:0.65-0.086) & significant decrease in 24 hrs. systolic BP (SBP) and diastolic BP (DBP) [40], [45], [46], [48]. All six studies concluded that in patients with heart failure with reduced ejection fraction (HFrEF), SGLT2 inhibitors reduce cardiovascular mortality and total hospital admission and help reduce blood pressure (BP) regardless of diabetic status. A study by Ibanez et al. showed a significant decrease in epicardial adipose tissue, interstitial myocardial fibrosis, and aortic stiffness in participants with empagliflozin compared to placebo [48]. Intervention with dapagliflozin was used in 5 RCTs [47], [41], [42], [43], [44]. The RCTs showed a hazard ratio for worsening HF 0.74 (CI:0.65–0.85); clinically meaningful improvement of 5 points in HF disease-specific health status; significant reduction in NT-proBNP (P = 0.003), and reduction in LV mass (P = 0.002) [41], [42]. Dayem et al. concluded that dapagliflozin significantly prevents LV dysfunction and maintains cardiac function post anterior STEMI [43]. Trials with dapagliflozin concluded that the risk of worsening HF or CVD was considerably lower among those who received dapagliflozin compared to those who received a placebo, irrespective of diabetic status [47], [41], [42], [43], [44].
Effect on Metabolic outcome
Eleven RCTs consisting of a cohort of 1153 participants were included in the review of the metabolic effect of SGLT2 inhibitors (Table 2) [56], [57], [58], [59], [60]. The sample size for the experimental trials ranged between 14 and 376. The baseline characteristics of the participants included a mean age range of 28 to 66.3 with female predominance. A study by Elkind et al. had all female participants [52]. All participants were nondiabetic; however, some trials had prediabetic participants which might result in bias in the study generalization [50], [53], [54]. Among the eleven RCTs, nine trials were randomized double-blinded placebo-controlled trials. One of the trials was randomized single-blinded (Elkind et al.), and another was an open-labeled non-blinded trial [52], [53]. Dapagliflozin, canagliflozin, and empagliflozin were the SGLT2 inhibitors used in 7, 2, and 2 studies, respectively. Canagliflozin was used at doses of 50 mg, 100 mg, and 300 mg; dapagliflozin 10 mg and empagliflozin 10 mg were given once daily and compared with a control group receiving placebo.
The Elkind et al. trial had study participants assigned to one of five investigation groups: once weekly exenatide (EQW) 2 mg, dapagliflozin 10 mg, co-administered EQW/Dapagliflozin (2 mg/10 mg daily, combined dapagliflozin/metformin (10 mg/2000 mg), and phentermine-topiramate extended-release (PHEN/TPM) (7.5 mg/46 mg) [52]. Some of the RCTs included participants aged ≥18 years with BMI ≥30 and <50 or lower BMI with comorbidities [51], [60], [56], [57], [58]. One study included subjects with a BMI between 30 and 45 Kg/m2 with polycystic ovarian syndrome (PCOS) [52]. Two trials included overweight subjects with BMI [53], [59]. Faerch et al. showed that dapagliflozin had a small significant improvement (p = 0.04) in glycemic variability compared to metformin [53]. The other two studies included adults aged 30 to 75 with a BMI of 25 to 40 [54], [55]. The major primary outcome was a percent change in body weight. Other outcomes included changes in HbA1c, BMI, waist circumference, fasting glucose, body composition, and insulin resistance. Median follow-up was between 2 weeks to 26 weeks. A trial with canagliflozin identified statistically significant weight loss from baseline (P < 0.001), and dose-dependent increased urinary glucose with a conclusion that canagliflozin produced meaningful weight loss in overweight or obese individuals without type 2 DM. Trials with dapagliflozin showed a significant reduction in body weight (P < 0.001), fat-free mass (P = 0.04), oral glucose tolerance test (OGTT) (P < 0.0001), BMI (P = 0.019), waist circumference (P = 0.003), fasting blood sugar (P < 0.001) and uric acid (P = 0.02). There was a significant increase in urinary glucose excretion (p < 0.0001), and an improvement in systolic BP and visceral adiposity. No significant effect in diastolic BP was seen. Elkind et al. also reported that dual treatment with EQW/Dapagliflozin was superior to either dapagliflozin alone or dapagliflozin combination with metformin in reducing OGTT or improving fasting insulin [52].
Studies with dapagliflozin concluded that this drug, either as monotherapy or combination therapy, caused a significant reduction of body weight, waist circumference, glucose tolerance test, fasting blood sugar, and uric acid. In addition, longer duration of SGLT2 inhibitors were found to evoke measurable effects on dietary preference, hunger, and appetite [51]. Trials with intervention with empagliflozin concluded that empagliflozin reduced endogenous glycerol gluconeogenesis in obese adults without DM. Neeland et al. commented on the possible role of SGLT2 inhibitors in preventing T2DM in obese individuals [60]. Also, empagliflozin may reverse brain insulin resistance with potential benefits for adiposity and whole-body metabolism [54].
Effect on Chronic Kidney Disease (CKD)
Four RCTs looked at the role of SGLT2 inhibitors on CKD in a cohort of 17,228 participants (Table 3) [61], [62], [63], [64], [65]. The baseline characteristics of the participants included a mean age ranging between 51 and 72 years and male predominance. Among the four studies, the DIAMOND trial was done on nondiabetic participants [61]. EMPA-KIDNEY, DAPA-CKD, and DELIVER trials included 46.2 %, 67.6 %, and 45 % diabetic population [62], [63], [65]. All four trials were randomized double-blinded placebo-controlled clinical trials. Three trials had dapagliflozin as an intervention, and the fourth trial studied empagliflozin. The median duration of follow-up ranged between 18 weeks to 2.4 years. The DIAMOND trial included nondiabetic participants aged 18 to 75 with CKD with urine protein excretion > 500 to 3500 mg/gm with eGFR of 25 ml/min/1.73 m2 body surface area. Dapagliflozin showed no significant effect on the primary outcome of percentage change in 24 hrs proteinuria, but there was a significant change in measured GFR (P < 0.0001). The EMPA-KIDNEY trial done on patients with CKD with eGFR of at least 20 to < 45 ml/min/1.73 m2 body surface area showed that empagliflozin reduced the progression of kidney disease or death with an HR of 0.86 (P = 0.003) [63]. The DAPA-CKD trial showed a significant reduction of a composite of sustained decline in eGFR of at least 50 %, ESRD, or death from renal cause with ann HR 0.56 (P < 0.0001) [62]. The DELIVER trial by Solomon et al. and post-analysis by Causland et al. included adults 40 years or older with left ventricular ejection fraction (LVEF) ≥ 40 % with evidence of structural heart disease [64], [65]. This trial showed a slowed long-term decline in eGFR in dapagliflozin vs. placebo with no reduction in frequency of kidney composite outcome. Among the four trials, three concluded that dapagliflozin or empagliflozin reduced the hazard of progression of kidney disease irrespective of diabetic status. Heerspink et al. also reported that SGLT2 inhibitors were the most effective drug to prevent CKD progression since the discovery of RAS inhibitors [62]. Causland et al. concluded that dapagliflozin did not significantly reduce the frequency of kidney composite outcomes [64].
Effect of SGLT2 inhibitors in IgA nephropathy
Most SGLT2 inhibitor trials did not primarily explore its effect on patients with glomerulonephritis. However, some of these trials included a large number of patients with glomerulonephritis as a cause of CKD. In the DAPA-CKD trial, around 270 patients had IgA nephropathy, and it was observed that dapagliflozin significantly reduced the risk of CKD progression as well as reduced the urine albumin-to-creatinine ratio [62]. A combined result from EMPA-KIDNEY & DAPA-CKD showed a 51 % reduction in the risk of CKD progression in IgA nephropathy [66].
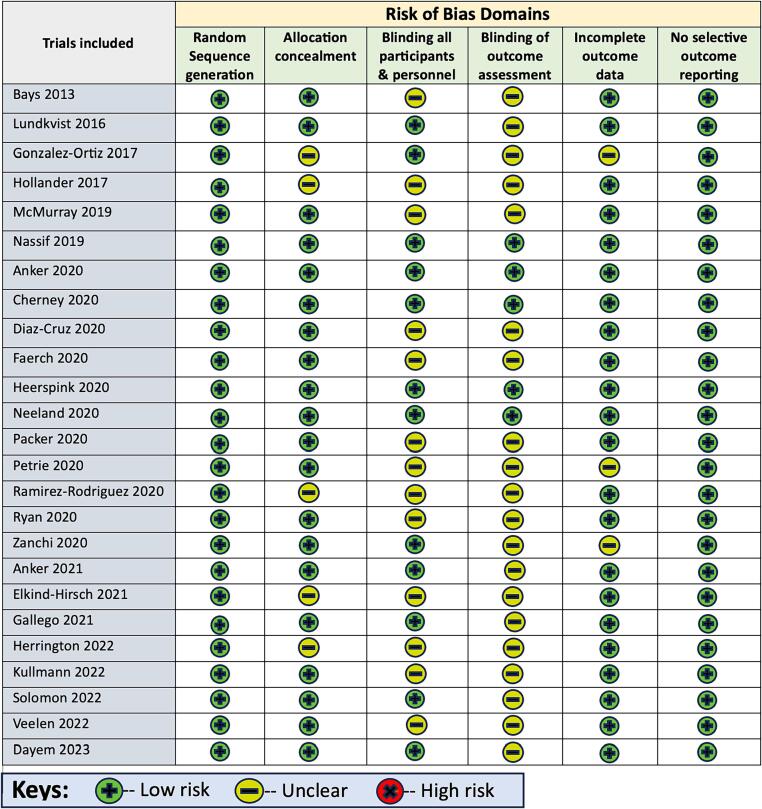
Risk of bias assessment of included trials
The Cochrane risk of bias assessment tool was used to determine the risk of bias for the trials included. The risk of bias for trials is differentiated as low risk of bias, uncertain risk of bias, or high risk of bias. The individual breakdown of risk of bias was summarized in Fig. 2. Most of the studies were assessed to have a low risk of selection bias, sampling bias, reporting bias, and other biases. Few of the studies had an uncertain risk of bias. Two studies had potential attrition bias with high dropout rates (31 % in the Hollander et al. study and 25 % in Bays et al. study) [56], [57].
Fig. 2.
The Cochrane Risk of Bias assessment tool.
Discussion
In this systemic review, SGLT2 inhibitors, including empagliflozin, dapagliflozin, and canagliflozin, demonstrated a significant reduction in LVEDV, LVESV, LV mass, decreased risk of CVD, hospitalization for heart failure (HF) and blood pressure reduction irrespective of diabetic status. Also, there is a significant reduction in epicardial adipose tissue, interstitial myocardial fibrosis, and aortic root stiffness. In addition, treatment with SGLT2 inhibitors was associated with a substantial reduction of weight, fat-free mass, endogenous glycerol gluconeogenesis, BMI, improvement of SBP and visceral adiposity, and reduction of brain insulin resistance in overweight or obese individuals without diabetes. A study by Elkind et al. also reported a significant decrease in OGTT and improved fasting insulin in nondiabetic women with PCOS. No difference was observed in serum NT-proBNP level, HbA1c, and LDL cholesterol.
The incidence and burden of HF is increasing over time. Studies have shown that at least one-third of the adults in the US can be defined as having at least stage A HF or at risk for HF or have at least one risk factor for HF [67], [68]. Currently, more than 6.7 million Americans over the age of 20 years have HF, with the prevalence predicted to rise to 8.5 million by 2030, with a lifetime risk of 24 % [68]. Further, patients with HF are at increased risk of recurrent hospitalization and mortality [69]. HF accounts for approximately 900,000 hospitalizations per year, costing $11 billion or more [70], [71]. Thus, an unmet need exists to identify effective pharmacological therapy focusing on improved symptoms, reduced hospitalization, and increased survival. As per current guidelines, SGLT2 inhibitors are the third line of management for refractory and persistent symptomatic HF in patients with diabetes mellitus [72].
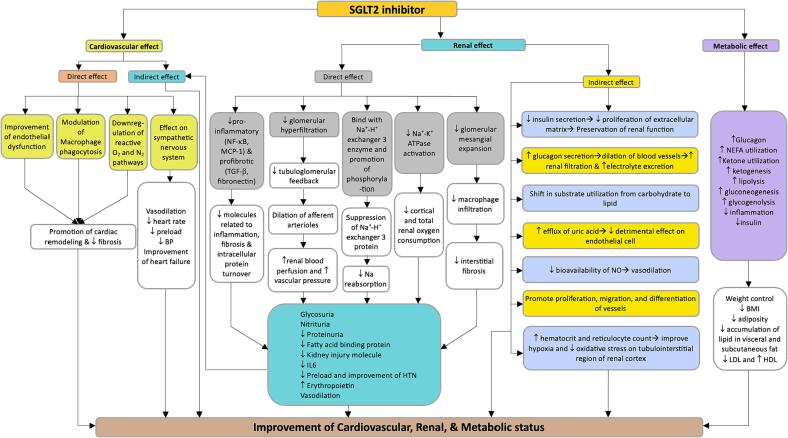
In this review, treatment with various SGLT2 inhibitors resulted in a significant reduction in the risk of cardiovascular mortality and HF-associated hospitalizations in patients without diabetes mellitus. The postulated mechanism for the role of SGLT2 inhibitors in HF includes direct cardiovascular effect, cardiovascular profile improvement, energy metabolism, and anti-inflammatory properties (Fig. 3) [72]. Direct cardiovascular effects of SGLT2 inhibitors include the effect on cardiac remodeling and fibrosis by modulating macrophage phagocytosis and downregulation of reactive oxygen and nitrogen-specific pathways [73]. Through action on the renal system (glycosuria, natriuresis, and tubuloglomerular feedback), SGLT2 inhibitors promote a reduction in body weight, regulation of blood pressure, and improvement of HbA1c resulting in healthy cardiovascular and metabolic profile [74], [75]. Glycosuria may also promote a shift towards ketone metabolism, the preferable metabolism in the heart of patients with HF [76]. In addition, SGLT2 inhibitors may decrease the inflammatory process by activating the SIRT1/AMPK pathway, reducing oxidative stress, and enhancing antioxidant activity [77]. Furthermore, SGLT2 inhibitors has shown to ameliorate endoplasmic reticulum stress [78], [79]. Although all trials reported a significant cardiovascular benefit for SGLT2 inhibitors, there is growing evidence of the role of SGLT1 inhibitors in the cardiovascular system [80], [81], [82], [83]. The potential mechanism of SGLT1 inhibitors in diabetes-related heart injury includes reduction of cardiomyocyte fibrosis and apoptosis through JNK, p38 MAPK pathway, and activation of Rac1 pathway. SGLT1 inihibitors may reduce cardiomyocyte hypertrophy and oxidative stress and decrease inflammatory response. SGLT1 inhibitors reduce vascular endothelial cell glucose sensitivity and downstream complications [84]. Potential mechanisms for SGLT1 inhibitors in reducing nondiabetic heart injury included reduction of oxidative stress, cardiomyocyte hypertrophy, and fibrosis through inhibition of the AMPK-ERK-EGFR-PKC-NOX2 pathway, downregulation of ANP and BNP and inhibition of excessive activation of TLR4/CaMKII pathways respectively [85], [86], [87]. Thus, available evidence and animal models highlight the potential cardiovascular benefit of SGLT1 inhibitors. However, inhibition of SGLT1 in the heart can be a double-edged sword, as nonspecific blockage of SGLT1 receptors in other organ systems may result in delirious side effects [88].
Fig. 3.
Postulated mechanisms for the nondiabetic action of SGLT2 inhibitors. SGLT2 inhibitors have both cardioprotective and renoprotective effect in addition to metabolic effect. The cardioprotective effect includes direct cardiovascular effect as well as indirectly through action on renal system and is manifested through improvement of cardiac parameters, decrease in heart failure as well as promotion of cardiac remodeling. Renal effect is through both direct and indirect pathways resulting in glycosuria, nitrituria, decrease in proteinuria, inflammation, vasodilation, decrease preload, hormonal effect as well as increased hematocrit and erythropoietin. Some of the metabolic effects included decreased BMI, decreased adiposity, change in appetite and weight among others. (Abbreviations: NF-κB – Nuclear factor kappa B; MCP-1 – Monocyte chemoattractant protein-1; TGF-β – Transforming growth factor β; NEFA – Non-esterified fatty acids; NO – Nitric oxide; Na – Sodium; O2 – Oxygen; N2 – Nitrogen; LDL – Low density lipoprotein; HDL – High density Lipoprotein;↑– increase in; ↓ – decrease in).
Multiple clinical trials reported the mortality benefit and reduced hospitalization rate with SGLT2 inhibitors in nondiabetic patients. However, a retrospective cohort study by Yan et al. showed no difference in length of hospital stay and all-cause readmission with heart failure, irrespective of diabetic status [89]. Thus, more clinical trials are required to establish the actual benefit of SGLT2 inhibitors in nondiabetic patients and delineate their role in different aspects of the cardiovascular system. Also, there is a need for further studies on the role of SGLT2 inhibitors on metabolic profile, vascular health, and other potential systemic benefits.
Studies have shown that dapagliflozin, empagliflozin, and canagliflozin have consistent and biologically plausible class effects on cardiorenal outcomes in diabetic patients [90]. Two indicators, including glomerular filtration fraction and degree of albuminuria were identified as the most common risk factor for cardiorenal events [90]. Our current review detected a significant effect of SGLT2 inhibitors in delaying the progression of renal disease and reducing albuminuria in nondiabetic patients, and hence, it may help to mitigate cardiorenal symptoms (Fig. 3).
Chronic kidney disease (CKD) is a progressive disease that affects around 10 % of the world's population and is the 18th leading cause of death [91]. In the United States, the prevalence of CKD in males is 12.3 %, and in females is 14.9 % [92]. Although CKD is more prevalent among the diabetic population (24.5 %), a small but significant percentage of CKD can be found in nondiabetic individuals (4.9 %) [91]. The unadjusted yearly healthcare costs of CKD is around $24.6 billion [93]. In a significant portion of CKD patients, there is a considerable propensity for delayed or underdiagnosis and the ultimate progression to ESRD [91]. Some of the common and robust risk factors for CKD progression include the level of GFR, proteinuria, and hypertension [94]. The consequence of CKD progression is devastating and condemning patients to various degrees of chronic lifelong disabilities. To date, angiotensin converting enzyme inhibitors (ACEi) are the standard of care for delaying the progression of CKD [95]. However, recently, SGLT2 inhibitors have been found to decrease the progression of CKD in the diabetic population, with little known about their role in the nondiabetic population. This review highlighted the role of SGLT2 inhibitors in nondiabetics.
The majority of the trials related to CKD included in this review concluded that dapagliflozin or empagliflozin reduced the hazard of progression of kidney disease irrespective of diabetic status. This review found SGLT2 inhibitors offer cardiovascular and renal protection in diabetic and nondiabetic patients with CKD, making them among the first-line therapy for CKD [96]. We also found that in nondiabetic patients, SGLT2 inhibitors not only improved cardiovascular outcomes in CKD patients but also improved renal status in patients with cardiovascular disease. An SGLT2 Inhibitor Meta-Analysis Cardio-Renal Trialists Consortium (SMART-C) concluded that SGLT2 inhibitors reduced the risk of CKD progression, ESRD, or death from HF by 37 %, irrespective of diabetic status [66]. They also reported 40 % protection from progression of CKD in diabetic kidney disease, 30 % in patients with ischemic/hypertensive kidney disease, 40 % in patients with glomerulonephritis, and 26 % in patients with CKD of unknown etiology [66]. Most SGLT2 inhibitor trials didn't primarily focus on glomerulonephritis, but EMPA-KIDNEY & DAPA-CKD trials showed reduction of CKD progression and improvement of urine albumin-to-creatinine ratio in patients with IgA nephropathy [62], [66]. The reno-protective effect of SGLT2 inhibitors can be both direct and indirect. The direct mechanisms include (i) reduction in hyperfiltration and glomerular injury through both glucose-related and unrelated pathways, (ii) reduction in energy consumption and hypoxia through reduced NA+/K+ ATPase, and (iii) inhibition of inflammatory, fibrotic, and pro-apoptotic response through reduction in inflammatory mediators [97], [98], [99], [100], [101], [102]. Some indirect effects include glycosuria, natriuresis, tubuloglomerular feedback, sympathoinhibition, protective effect on endothelial cells, vasodilation, increased hematocrit, reduction in uric acid level, etc. (Fig. 3) [97].
Various studies related to SGLT2 inhibitors have shown minimal adverse effects in the diabetic population and are expected to manifest similar or less effects in nondiabetic individuals. Some of the most common side effects include hypotension, UTI, Fournier's gangrene, AKI, euglycemic ketoacidosis, bone fractures, and bladder cancer [72], [103]. Though no significant side effects were observed in the clinical trials reviewed for this study, there is a need for active monitoring and close surveillance.
Some of the strengths of this study include the review of all available RCTs to date that looked at the nondiabetic role of SGLT2 inhibitors, the use of the PRISMA diagram, and the maintenance of high Cohen's Kappa of agreement. In addition, this review summarizes the current scientific knowledge, informs therapeutic guidelines related to SGLT2 inhibitors, and promotes the potential benefit of SGLT2 inhibitors as an essential therapeutic milestone in nondiabetic patients.
One of the limitations of this study includes limited information regarding the drug and dose-dependent efficacy and determination of relative benefit across the SGLT2 inhibitors due to a lack of head-to-head clinical trials. Secondly, most of the studies had combined diabetic and nondiabetic populations with no reported baseline characteristics for individual groups. The diversity in baseline characteristics of the study population and heterogeneity in sample size and background clinical comorbidities may also account for differences in study outcomes and generalizability. Some of the limitations of CKD trials also include lack of inclusion of various underlying causes of CKD, limited/no trial on patients receiving our renal replacement therapy (RRT) or kidney transplant, and quantification of therapeutic disease-specific role of SGLT2 inhibitors. Additionally, some studies had high dropout rates, contributing to an increased risk of attrition bias. Thus, more studies are warranted to identify other nondiabetic uses of these drugs and to characterize the unbiased role of different SGLT2 inhibitors in homogeneous and standard populations. Further, long-term follow-up studies are needed to identify the side effect profile and develop therapeutic guidelines correctly.
Conclusion and future direction
In conclusion, SGLT2 inhibitors stand as indispensable pillars in medical practice, seamlessly integrated into various guideline-driven treatment plans for diabetic patients. This comprehensive review extends beyond their conventional diabetic applications, shedding light on the potential of SGLT2 inhibitors in addressing cardiac, renal, and metabolic conditions in nondiabetic individuals. The amalgamation of findings from numerous clinical trials highlights a noteworthy reduction in cardiovascular mortality, a decline in hospitalization rates, and enhanced metabolic parameters across various SGLT2 inhibitors. Moreover, while stirring controversy regarding their antihypertensive effects, shine in impeding CKD progression and positioning them as a formidable class of medication alongside RAS inhibitors. In the realm of therapeutic medicine for nondiabetic patients, SGLT2 inhibitors emerge as a beacon of promise, suggesting their potential inclusion in guideline-based protocols for comprehensive patient care.
Currently, multiple trials are ongoing to further classify the nondiabetic role of SGLT2 inhibitors not only in CKD and cardiovascular diseases but also in other disease processes, including acute kidney injury, transplant recipients, patients on renal replacement therapies, PCOS, SIADH, adrenal disease, nonalcoholic fatty liver disease, and valvular heart disease. Results from these trials and other trials will help to broaden the horizon of SGLT2 inhibitors and help inform various management guidelines. Newer trials may consider studying other SGLT inhibitors, particularly SGLT 1 inhibitors.
Furthermore, trials comparing additive effects of SGLT2 inhibitors with GLP-1 agonist, fenerenone, or endothelin receptor antagonist in non-diabetics may prove to be very helpful in improving renal outcomes.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Irtiza Hasan: Writing – review & editing, Writing – original draft, Investigation, Data curation. Tasnuva Rashid: Writing – review & editing, Writing – original draft, Methodology, Investigation, Data curation. Vishal Jaikaransingh: Writing – review & editing. Charles Heilig: Writing – review & editing. Emaad M. Abdel-Rahman: Writing – review & editing. Alaa S. Awad: Conceptualization, Writing – review & editing, Writing – original draft.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Hsia D.S., Grove O., Cefalu W.T. An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73–79. doi: 10.1097/MED.0000000000000311. PMCID: PMC6028052. PMID: 27898586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Habtemariam S. The Molecular Pharmacology of Phloretin: Anti-Inflammatory Mechanisms of Action. Biomedicines. 2023;11(1). PMCID: PMC9855955. PMID: 36672652. [DOI] [PMC free article] [PubMed]
- 3.Rossetti L., Smith D., Shulman G.I., Papachristou D., DeFronzo R.A. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987;79(5):1510–1515. doi: 10.1172/JCI112981. PMCID: PMC424427. PMID: 3571496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowie M.R., Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol. 2020;17(12):761–772. doi: 10.1038/s41569-020-0406-8. [DOI] [PubMed] [Google Scholar]
- 5.Hardman T.C., Dubrey S.W. Development and potential role of type-2 sodium-glucose transporter inhibitors for management of type 2 diabetes. Diabetes Ther. 2011;2(3):133–145. doi: 10.1007/s13300-011-0004-1. PMCID: PMC3173594. PMID: 22127823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalra J., Mangali S.B., Dasari D., Bhat A., Goyal S., Dhar I., et al. SGLT1 inhibition boon or bane for diabetes-associated cardiomyopathy. Fundam Clin Pharmacol. 2020;34(2):173–188. doi: 10.1111/fcp.12516. PMID: 31698522. [DOI] [PubMed] [Google Scholar]
- 7.Nespoux J., Vallon V. Renal effects of SGLT2 inhibitors: an update. Curr Opin Nephrol Hypertens. 2020;29(2):190–198. doi: 10.1097/MNH.0000000000000584. PMCID: PMC7224333. PMID: 31815757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shubrook J.H., Bokaie B.B., Adkins S.E. Empagliflozin in the treatment of type 2 diabetes: evidence to date. Drug Des Devel Ther. 2015;9:5793–5803. doi: 10.2147/DDDT.S69926. PMCID: PMC4634822. PMID: 26586935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao M., Li N., Zhou H. SGLT1: a potential drug Target for Cardiovascular disease. Drug Des Devel Ther. 2023;17:2011–2023. doi: 10.2147/DDDT.S418321. PMCID: PMC10332373. PMID: 37435096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song P., Onishi A., Koepsell H., Vallon V. Sodium glucose cotransporter SGLT1 as a therapeutic target in diabetes mellitus. Expert Opin Ther Targets. 2016;20(9):1109–1125. doi: 10.1517/14728222.2016.1168808. PMCID: PMC5045806. PMID: 26998950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrannini E. Sodium-glucose co-transporters and their inhibition: clinical physiology. Cell Metab. 2017;26(1):27–38. doi: 10.1016/j.cmet.2017.04.011. PMID: 28506519. [DOI] [PubMed] [Google Scholar]
- 12.Ghezzi C., Loo D.D.F., Wright E.M. Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia. 2018;61(10):2087–2097. doi: 10.1007/s00125-018-4656-5. PMCID: PMC6133168. PMID: 30132032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hummel C.S., Lu C., Loo D.D., Hirayama B.A., Voss A.A., Wright E.M. Glucose transport by human renal Na+/D-glucose cotransporters SGLT1 and SGLT2. Am J Physiol Cell Physiol. 2011;300(1):C14–C21. doi: 10.1152/ajpcell.00388.2010. PMCID: PMC3023189. PMID: 20980548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poppe R., Karbach U., Gambaryan S., Wiesinger H., Lutzenburg M., Kraemer M., et al. Expression of the Na+-D-glucose cotransporter SGLT1 in neurons. J Neurochem. 1997;69(1):84–94. doi: 10.1046/j.1471-4159.1997.69010084.x. PMID: 9202297. [DOI] [PubMed] [Google Scholar]
- 15.Zhou L., Cryan E.V., D'Andrea M.R., Belkowski S., Conway B.R., Demarest K.T. Human cardiomyocytes express high level of Na+/glucose cotransporter 1 (SGLT1) J Cell Biochem. 2003;90(2):339–346. doi: 10.1002/jcb.10631. PMID: 14505350. [DOI] [PubMed] [Google Scholar]
- 16.Padda IS, Mahtani AU, Parmar M. Sodium-Glucose Transport Protein 2 (SGLT2) Inhibitors. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC.; 2023. [PubMed]
- 17.Scheen A.J. Pharmacokinetics, Pharmacodynamics and clinical use of SGLT2 inhibitors in patients with type 2 diabetes mellitus and chronic kidney disease. Clin Pharmacokinet. 2015;54(7):691–708. doi: 10.1007/s40262-015-0264-4. PMID: 25805666. [DOI] [PubMed] [Google Scholar]
- 18.Wright E.M. SGLT2 inhibitors: physiology and Pharmacology. Kidney360. 2021;2(12):2027–2037. doi: 10.34067/KID.0002772021. PMCID: PMC8986039. PMID: 35419546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deeks E.D., Scheen A.J. Canagliflozin: a review in type 2 diabetes. Drugs. 2017;77(14):1577–1592. doi: 10.1007/s40265-017-0801-6. PMID: 28836175. [DOI] [PubMed] [Google Scholar]
- 20.Dhillon S. Dapagliflozin: a review in type 2 diabetes. Drugs. 2019;79(10):1135–1146. doi: 10.1007/s40265-019-01148-3. PMCID: PMC6879440. PMID: 31236801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frampton J.E. Empagliflozin: a review in type 2 diabetes. Drugs. 2018;78(10):1037–1048. doi: 10.1007/s40265-018-0937-z. PMID: 29946963. [DOI] [PubMed] [Google Scholar]
- 22.Marrs J.C., Anderson S.L. Ertugliflozin in the treatment of type 2 diabetes mellitus. drugs. Context. 2020:9. doi: 10.7573/dic.2020-7-4. PMCID: PMC7707814. PMID: 33293984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clar C, Gill JA, Court R, Waugh N. Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes. BMJ Open. 2012;2(5). PMCID: PMC3488745. PMID: 23087012. [DOI] [PMC free article] [PubMed]
- 24.Musso G., Gambino R., Cassader M., Pagano G. A novel approach to control hyperglycemia in type 2 diabetes: sodium glucose co-transport (SGLT) inhibitors: systematic review and meta-analysis of randomized trials. Ann Med. 2012;44(4):375–393. doi: 10.3109/07853890.2011.560181. PMID: 21495788. [DOI] [PubMed] [Google Scholar]
- 25.Stenlöf K., Cefalu W.T., Kim K.A., Alba M., Usiskin K., Tong C., et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15(4):372–382. doi: 10.1111/dom.12054. PMCID: PMC3593184. PMID: 23279307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenstock J., Jelaska A., Frappin G., Salsali A., Kim G., Woerle H.J., et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37(7):1815–1823. doi: 10.2337/dc13-3055. PMID: 24929430. [DOI] [PubMed] [Google Scholar]
- 27.Ridderstråle M., Andersen K.R., Zeller C., Kim G., Woerle H.J., Broedl U.C. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2(9):691–700. doi: 10.1016/S2213-8587(14)70120-2. PMID: 24948511. [DOI] [PubMed] [Google Scholar]
- 28.Pratley R.E., Eldor R., Raji A., Golm G., Huyck S.B., Qiu Y., et al. Ertugliflozin plus sitagliptin versus either individual agent over 52 weeks in patients with type 2 diabetes mellitus inadequately controlled with metformin: the VERTIS FACTORIAL randomized trial. Diabetes Obes Metab. 2018;20(5):1111–1120. doi: 10.1111/dom.13194. PMCID: PMC5947297. PMID: 29266675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollander P, Liu J, Hill J, Johnson J, Jiang ZW, Golm G, Huyck S, Terra SG, Mancuso JP, Engel SS, Lauring B. Ertugliflozin Compared with Glimepiride in Patients with Type 2 Diabetes Mellitus Inadequately Controlled on Metformin: The VERTIS SU Randomized Study. Diabetes Ther. 2018;9(1):193-207. PMCID: PMC5801240. PMID: 29282633. [DOI] [PMC free article] [PubMed]
- 30.Bhattarai M., Salih M., Regmi M., Al-Akchar M., Deshpande R., Niaz Z., et al. Association of Sodium-Glucose Cotransporter 2 inhibitors with Cardiovascular outcomes in patients with type 2 diabetes and other risk factors for Cardiovascular disease: a meta-analysis. JAMA Netw Open. 2022;5(1) doi: 10.1001/jamanetworkopen.2021.42078. e2142078-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perkovic V., Jardine M.J., Neal B., Bompoint S., Heerspink H.J.L., Charytan D.M., et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744. PMID: 30990260. [DOI] [PubMed] [Google Scholar]
- 32.Bhatt D.L., Szarek M., Pitt B., Cannon C.P., Leiter L.A., McGuire D.K., et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021;384(2):129–139. doi: 10.1056/NEJMoa2030186. PMID: 33200891. [DOI] [PubMed] [Google Scholar]
- 33.Neal B., Perkovic V., Mahaffey K.W., de Zeeuw D., Fulcher G., Erondu N., et al. Canagliflozin and Cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. PMID: 28605608. [DOI] [PubMed] [Google Scholar]
- 34.Zinman B., Wanner C., Lachin J.M., Fitchett D., Bluhmki E., Hantel S., et al. Empagliflozin, Cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. PMID: 26378978. [DOI] [PubMed] [Google Scholar]
- 35.Wiviott S.D., Raz I., Bonaca M.P., Mosenzon O., Kato E.T., Cahn A., et al. Dapagliflozin and Cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389. PMID: 30415602. [DOI] [PubMed] [Google Scholar]
- 36.Cannon C.P., Pratley R., Dagogo-Jack S., Mancuso J., Huyck S., Masiukiewicz U., et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383(15):1425–1435. doi: 10.1056/NEJMoa2004967. PMID: 32966714. [DOI] [PubMed] [Google Scholar]
- 37.Bhatt D.L., Szarek M., Steg P.G., Cannon C.P., Leiter L.A., McGuire D.K., et al. Sotagliflozin in patients with diabetes and recent worsening Heart failure. N Engl J Med. 2021;384(2):117–128. doi: 10.1056/NEJMoa2030183. PMID: 33200892. [DOI] [PubMed] [Google Scholar]
- 38.Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santos-Gallego C.G., Vargas-Delgado A.P., Requena-Ibanez J.A., Garcia-Ropero A., Mancini D., Pinney S., et al. Randomized trial of empagliflozin in nondiabetic patients with Heart failure and reduced ejection Fraction. J Am Coll Cardiol. 2021;77(3):243–255. doi: 10.1016/j.jacc.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 40.Anker S.D., Butler J., Filippatos G., Khan M.S., Marx N., Lam C.S.P., et al. Effect of empagliflozin on Cardiovascular and renal outcomes in patients with Heart failure by baseline diabetes status: results from the EMPEROR-reduced trial. Circulation. 2021;143(4):337–349. doi: 10.1161/CIRCULATIONAHA.120.051824. PMCID: PMC7834911. PMID: 33175585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McMurray J.J.V., Solomon S.D., Inzucchi S.E., Køber L., Kosiborod M.N., Martinez F.A., et al. Dapagliflozin in patients with Heart failure and reduced ejection Fraction. N Engl J Med. 2019;381(21):1995–2008. doi: 10.1056/NEJMoa1911303. PMID: 31535829. [DOI] [PubMed] [Google Scholar]
- 42.Nassif M.E., Windsor S.L., Tang F., Khariton Y., Husain M., Inzucchi S.E., et al. Dapagliflozin effects on Biomarkers, symptoms, and functional status in patients with Heart failure with reduced ejection Fraction: the DEFINE-HF trial. Circulation. 2019;140(18):1463–1476. doi: 10.1161/CIRCULATIONAHA.119.042929. PMID: 31524498. [DOI] [PubMed] [Google Scholar]
- 43.Dayem K.A., Younis O., Zarif B., Attia S., AbdelSalam A. Impact of dapagliflozin on cardiac function following anterior myocardial infarction in non-diabetic patients - DACAMI (a randomized controlled clinical trial) Int J Cardiol. 2023;379:9–14. doi: 10.1016/j.ijcard.2023.03.002. PMID: 36889650. [DOI] [PubMed] [Google Scholar]
- 44.Díaz-Cruz C., González-Ortiz M., Rosales-Rivera L.Y., Patiño-Laguna A.J., Ramírez-Rodríguez Z.G., Díaz-Cruz K., et al. Effects of dapagliflozin on blood pressure variability in patients with prediabetes and prehypertension without pharmacological treatment: a randomized trial. Blood Press Monit. 2020;25(6):346–350. doi: 10.1097/MBP.0000000000000479. PMID: 32815921. [DOI] [PubMed] [Google Scholar]
- 45.Packer M., Anker S.D., Butler J., Filippatos G., Pocock S.J., Carson P., et al. Cardiovascular and renal outcomes with empagliflozin in Heart failure. N Engl J Med. 2020;383(15):1413–1424. doi: 10.1056/NEJMoa2022190. PMID: 32865377. [DOI] [PubMed] [Google Scholar]
- 46.Zanchi A., Burnier M., Muller M.E., Ghajarzadeh-Wurzner A., Maillard M., Loncle N., et al. Acute and chronic effects of SGLT2 inhibitor empagliflozin on renal oxygenation and blood pressure control in nondiabetic normotensive subjects: a randomized, placebo-controlled trial. J Am Heart Assoc. 2020;9(13) doi: 10.1161/JAHA.119.016173. PMCID: PMC7670540. PMID: 32567439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petrie M.C., Verma S., Docherty K.F., Inzucchi S.E., Anand I., Belohlávek J., et al. Effect of dapagliflozin on worsening Heart failure and Cardiovascular death in patients with Heart failure with and without diabetes. JAMA. 2020;323(14):1353–1368. doi: 10.1001/jama.2020.1906. PMCID: PMC7157181. PMID: 32219386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Requena-Ibáñez J.A., Santos-Gallego C.G., Rodriguez-Cordero A., Vargas-Delgado A.P., Mancini D., Sartori S., et al. Mechanistic insights of empagliflozin in nondiabetic patients with HFrEF: from the EMPA-TROPISM study. JACC Heart Fail. 2021;9(8):578–589. doi: 10.1016/j.jchf.2021.04.014. PMID: 34325888. [DOI] [PubMed] [Google Scholar]
- 49.Anker S.D., Butler J., Filippatos G., Ferreira J.P., Bocchi E., Böhm M., et al. Empagliflozin in Heart failure with a preserved ejection Fraction. N Engl J Med. 2021;385(16):1451–1461. doi: 10.1056/NEJMoa2107038. PMID: 34449189. [DOI] [PubMed] [Google Scholar]
- 50.Ramírez-Rodríguez A.M., González-Ortiz M., Martínez-Abundis E. Effect of dapagliflozin on insulin secretion and insulin sensitivity in patients with prediabetes. Exp Clin Endocrinol Diabetes. 2020;128(8):506–511. doi: 10.1055/a-0664-7583. PMID: 30149417. [DOI] [PubMed] [Google Scholar]
- 51.Ryan S.P.P., Newman A.A., Wilburn J.R., Rhoades L.D., Trikha S.R.J., Godwin E.C., et al. Sodium glucose co-transporter 2 inhibition does not favorably modify the physiological responses to Dietary Counselling in diabetes-free, Sedentary overweight and obese adult humans. Nutrients. 2020;12(2) doi: 10.3390/nu12020510. PMCID: PMC7071188. PMID: 32085394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elkind-Hirsch K.E., Chappell N., Seidemann E., Storment J., Bellanger D. Exenatide, dapagliflozin, or phentermine/topiramate differentially affect metabolic profiles in polycystic Ovary syndrome. J Clin Endocrinol Metab. 2021;106(10):3019–3033. doi: 10.1210/clinem/dgab408. PMID: 34097062. [DOI] [PubMed] [Google Scholar]
- 53.Færch K., Blond M.B., Bruhn L., Amadid H., Vistisen D., Clemmensen K.K.B., et al. The effects of dapagliflozin, metformin or exercise on glycaemic variability in overweight or obese individuals with prediabetes (the PRE-D trial): a multi-arm, randomised, controlled trial. Diabetologia. 2021;64(1):42–55. doi: 10.1007/s00125-020-05306-1. PMID: 33064182. [DOI] [PubMed] [Google Scholar]
- 54.Kullmann S., Hummel J., Wagner R., Dannecker C., Vosseler A., Fritsche L., et al. Empagliflozin improves insulin sensitivity of the hypothalamus in humans with prediabetes: a randomized, double-blind, placebo-controlled, phase 2 trial. Diabetes Care. 2022;45(2):398–406. doi: 10.2337/dc21-1136. PMCID: PMC8914418. PMID: 34716213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Veelen A., Andriessen C., Op den Kamp Y., Erazo-Tapia E., de Ligt M., Mevenkamp J., et al. Effects of the sodium-glucose cotransporter 2 inhibitor dapagliflozin on substrate metabolism in prediabetic insulin resistant individuals: a randomized, double-blind crossover trial. Metabolism. 2023;140 doi: 10.1016/j.metabol.2022.155396. PMID: 36592688. [DOI] [PubMed] [Google Scholar]
- 56.Bays H.E., Weinstein R., Law G., Canovatchel W. Canagliflozin: effects in overweight and obese subjects without diabetes mellitus. Obesity (Silver Spring) 2014;22(4):1042–1049. doi: 10.1002/oby.20663. PMCID: PMC4285787. PMID: 24227660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hollander P., Bays H.E., Rosenstock J., Frustaci M.E., Fung A., Vercruysse F., et al. Coadministration of canagliflozin and phentermine for weight Management in Overweight and Obese Individuals without Diabetes: a randomized clinical trial. Diabetes Care. 2017;40(5):632–639. doi: 10.2337/dc16-2427. [DOI] [PubMed] [Google Scholar]
- 58.Lundkvist P., Sjöström C.D., Amini S., Pereira M.J., Johnsson E., Eriksson J.W. Dapagliflozin once-daily and exenatide once-weekly dual therapy: a 24-week randomized, placebo-controlled, phase II study examining effects on body weight and prediabetes in obese adults without diabetes. Diabetes Obes Metab. 2017;19(1):49–60. doi: 10.1111/dom.12779. PMCID: PMC5215525. PMID: 27550386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.González-Ortiz M., Grover-Páez F., Díaz-Cruz C. de J.P.-L.A., López-Murillo L.D., Martínez-Abundis E. Dapagliflozin administration on visceral adiposity, blood pressure and aortic central pressure in overweight patients without type 2 diabetes. Minerva Med. 2017;108(4):384–386. doi: 10.23736/S0026-4806.17.05048-0. PMID: 28677364. [DOI] [PubMed] [Google Scholar]
- 60.Neeland I.J., de Albuquerque R.N., Hughes C., Ayers C.R., Malloy C.R., Jin E.S. Effects of empagliflozin treatment on glycerol-derived hepatic gluconeogenesis in adults with obesity: a randomized clinical trial. Obesity (Silver Spring) 2020;28(7):1254–1262. doi: 10.1002/oby.22854. PMCID: PMC7316140. PMID: 32568464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cherney D.Z.I., Dekkers C.C.J., Barbour S.J., Cattran D., Abdul Gafor A.H., Greasley P.J., et al. Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (DIAMOND): a randomised, double-blind, crossover trial. Lancet Diabetes Endocrinol. 2020;8(7):582–593. doi: 10.1016/S2213-8587(20)30162-5. PMID: 32559474. [DOI] [PubMed] [Google Scholar]
- 62.Heerspink H.J.L., Stefánsson B.V., Correa-Rotter R., Chertow G.M., Greene T., Hou F.F., et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816. PMID: 32970396. [DOI] [PubMed] [Google Scholar]
- 63.Herrington W.G., Staplin N., Wanner C., Green J.B., Hauske S.J., Emberson J.R., et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117–127. doi: 10.1056/NEJMoa2204233. PMCID: PMC7614055. PMID: 36331190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mc Causland F.R., Claggett B.L., Vaduganathan M., Desai A.S., Jhund P., de Boer R.A., et al. Dapagliflozin and kidney outcomes in patients with Heart failure with mildly reduced or preserved ejection Fraction: a prespecified analysis of the DELIVER randomized clinical trial. JAMA Cardiol. 2023;8(1):56–65. doi: 10.1001/jamacardio.2022.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Solomon S.D., McMurray J.J.V., Claggett B., de Boer R.A., DeMets D., Hernandez A.F., et al. Dapagliflozin in Heart failure with mildly reduced or preserved ejection Fraction. N Engl J Med. 2022;387(12):1089–1098. doi: 10.1056/NEJMoa2206286. PMID: 36027570. [DOI] [PubMed] [Google Scholar]
- 66.Podestà M.A., Sabiu G., Galassi A., Ciceri P., Cozzolino M. SGLT2 inhibitors in diabetic and non-diabetic chronic kidney disease. Biomedicines. 2023;11(2) doi: 10.3390/biomedicines11020279. PMCID: PMC9953060. PMID: 36830815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xanthakis V., Enserro D.M., Larson M.G., Wollert K.C., Januzzi J.L., Levy D., et al. Neurohormonal Correlates, and prognosis of Heart failure stages in the community. JACC. Heart Fail. 2016;4(10):808–815. doi: 10.1016/j.jchf.2016.05.001. PMCID: PMC8450920. PMID: 27395350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bozkurt B., Ahmad T., Alexander K.M., Baker W.L., Bosak K., Breathett K., et al. Heart failure epidemiology and outcomes statistics: a report of the Heart Failure Society of America. J Card Fail. 2023;29(10):1412–1451. doi: 10.1016/j.cardfail.2023.07.006. PMID: 37797885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bui A.L., Horwich T.B., Fonarow G.C. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8(1):30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anderson A., Mukashev N., Zhou D., Bigler W. The costs of Disparities in preventable Heart failure hospitalizations in the US south, 2015–17. Health Aff. 2023;42(5):693–701. doi: 10.1377/hlthaff.2022.01314. [DOI] [PubMed] [Google Scholar]
- 71.Jackson SL, Tong X, King RJ, Loustalot F, Hong Y, Ritchey MD. National Burden of Heart Failure Events in the United States, 2006 to 2014. Circulation: Heart Failure. 2018;11(12):e004873. [DOI] [PMC free article] [PubMed]
- 72.Jessica Audet J.P., Moten S.h., Rauf A. SGLT2 inhibitors use in non-diabetic patients: a Narrative review. J Clinical Nephrol Kidney Diseases. 2021;6(1):6. [Google Scholar]
- 73.Lee T.M., Chang N.C., Lin S.Z. Dapagliflozin, a selective SGLT2 inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic Biol Med. 2017;104:298–310. doi: 10.1016/j.freeradbiomed.2017.01.035. PMID: 28132924. [DOI] [PubMed] [Google Scholar]
- 74.List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32(4):650-7. PMCID: PMC2660449. PMID: 19114612. [DOI] [PMC free article] [PubMed]
- 75.Dekkers C.C.J., Sjöström C.D., Greasley P.J., Cain V., Boulton D.W., Heerspink H.J.L. Effects of the sodium-glucose co-transporter-2 inhibitor dapagliflozin on estimated plasma volume in patients with type 2 diabetes. Diabetes Obes Metab. 2019;21(12):2667–2673. doi: 10.1111/dom.13855. PMCID: PMC6899523. PMID: 31407856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Crawford P.A. Refueling the failing Heart: a case for sodium-glucose Cotransporter 2 inhibition in Cardiac energy homeostasis. JACC Basic Transl Sci. 2018;3(5):588–590. doi: 10.1016/j.jacbts.2018.08.002. PMCID: PMC6234513. PMID: 30456330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Packer M. SGLT2 inhibitors produce Cardiorenal benefits by promoting adaptive Cellular reprogramming to induce a state of fasting mimicry: a Paradigm shift in understanding their mechanism of action. Diabetes Care. 2020;43(3):508–511. doi: 10.2337/dci19-0074. PMID: 32079684. [DOI] [PubMed] [Google Scholar]
- 78.Ren F.F., Xie Z.Y., Jiang Y.N., Guan X., Chen Q.Y., Lai T.F., et al. Dapagliflozin attenuates pressure overload-induced myocardial remodeling in mice via activating SIRT1 and inhibiting endoplasmic reticulum stress. Acta Pharmacol Sin. 2022;43(7):1721–1732. doi: 10.1038/s41401-021-00805-2. PMCID: PMC9253115. PMID: 34853445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang C.C., Li Y., Qian X.Q., Zhao H., Wang D., Zuo G.X., et al. Empagliflozin alleviates myocardial I/R injury and cardiomyocyte apoptosis via inhibiting ER stress-induced autophagy and the PERK/ATF4/Beclin1 pathway. J Drug Target. 2022;30(8):858–872. doi: 10.1080/1061186X.2022.2064479. PMID: 35400245. [DOI] [PubMed] [Google Scholar]
- 80.Murtaza G., Virk H.U.H., Khalid M., Lavie C.J., Ventura H., Mukherjee D., et al. Diabetic cardiomyopathy - a comprehensive updated review. Prog Cardiovasc Dis. 2019;62(4):315–326. doi: 10.1016/j.pcad.2019.03.003. PMID: 30922976. [DOI] [PubMed] [Google Scholar]
- 81.Jia G., DeMarco V.G., Sowers J.R. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol. 2016;12(3):144–153. doi: 10.1038/nrendo.2015.216. PMCID: PMC4753054. PMID: 26678809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin N., Lin H., Yang Q., Lu W., Sun Z., Sun S., et al. SGLT1 inhibition attenuates apoptosis in diabetic Cardiomyopathy via the JNK and p38 pathway. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.598353. PMCID: PMC7883645. PMID: 33597877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dasari D., Bhat A., Mangali S., Ghatage T., Lahane G.P., Sriram D., et al. Canagliflozin and dapagliflozin attenuate glucolipotoxicity-induced oxidative stress and apoptosis in Cardiomyocytes via inhibition of sodium-glucose Cotransporter-1. ACS Pharmacol Transl Sci. 2022;5(4):216–225. doi: 10.1021/acsptsci.1c00207. PMCID: PMC9003386. PMID: 35434529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nishikawa T., Edelstein D., Du X.L., Yamagishi S., Matsumura T., Kaneda Y., et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404(6779):787–790. doi: 10.1038/35008121. PMID: 10783895. [DOI] [PubMed] [Google Scholar]
- 85.Sawa Y., Saito M., Ishida N., Ibi M., Matsushita N., Morino Y., et al. Pretreatment with KGA-2727, a selective SGLT1 inhibitor, is protective against myocardial infarction-induced ventricular remodeling and heart failure in mice. J Pharmacol Sci. 2020;142(1):16–25. doi: 10.1016/j.jphs.2019.11.001. PMID: 31776072. [DOI] [PubMed] [Google Scholar]
- 86.Li Z., Agrawal V., Ramratnam M., Sharma R.K., D'Auria S., Sincoular A., et al. Cardiac sodium-dependent glucose cotransporter 1 is a novel mediator of ischaemia/reperfusion injury. Cardiovasc Res. 2019;115(11):1646–1658. doi: 10.1093/cvr/cvz037. PMCID: PMC6704393. PMID: 30715251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bode D., Semmler L., Wakula P., Hegemann N., Primessnig U., Beindorff N., et al. Dual SGLT-1 and SGLT-2 inhibition improves left atrial dysfunction in HFpEF. Cardiovasc Diabetol. 2021;20(1):7. doi: 10.1186/s12933-020-01208-z. PMCID: PMC7792219. PMID: 33413413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsimihodimos V., Filippas-Ntekouan S., Elisaf M. SGLT1 inhibition: pros and cons. Eur J Pharmacol. 2018;838:153–156. doi: 10.1016/j.ejphar.2018.09.019. PMID: 30240793. [DOI] [PubMed] [Google Scholar]
- 89.Yan C.L., Erben A., Sancassani R. Evaluation of inpatient sodium-glucose co-Transporter-2 inhibitor use in patients hospitalized for acute Heart failure. Am J Cardiol. 2024;211:175–179. doi: 10.1016/j.amjcard.2023.11.005. [DOI] [PubMed] [Google Scholar]
- 90.Kluger A.Y., Tecson K.M., Lee A.Y., Lerma E.V., Rangaswami J., Lepor N.E., et al. Class effects of SGLT2 inhibitors on cardiorenal outcomes. Cardiovasc Diabetol. 2019;18(1):99. doi: 10.1186/s12933-019-0903-4. PMCID: PMC6683461. PMID: 31382965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kovesdy C.P. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. 2022;12(1):7–11. doi: 10.1016/j.kisu.2021.11.003. PMCID: PMC9073222. PMID: 35529086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mills K.T., Xu Y., Zhang W., Bundy J.D., Chen C.S., Kelly T.N., et al. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2015;88(5):950–957. doi: 10.1038/ki.2015.230. PMCID: PMC4653075. PMID: 26221752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ozieh M.N., Bishu K.G., Dismuke C.E., Egede L.E. Trends in healthcare expenditure in United States adults with chronic kidney disease: 2002–2011. BMC Health Serv Res. 2017;17(1):368. doi: 10.1186/s12913-017-2303-3. PMCID: PMC5441091. PMID: 28532412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Staples A., Wong C. Risk factors for progression of chronic kidney disease. Curr Opin Pediatr. 2010;22(2):161–169. doi: 10.1097/MOP.0b013e328336ebb0. PMCID: PMC2948868. PMID: 20090523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ward F., Holian J., Murray P.T. Drug therapies to delay the progression of chronic kidney disease. Clin Med (Lond) 2015;15(6):550–557. doi: 10.7861/clinmedicine.15-6-550. PMCID: PMC4953257. PMID: 26621944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fernandez-Fernandez B., Sarafidis P., Kanbay M., Navarro-González J.F., Soler M.J., Górriz J.L., et al. SGLT2 inhibitors for non-diabetic kidney disease: drugs to treat CKD that also improve glycaemia. Clin Kidney J. 2020;13(5):728–733. doi: 10.1093/ckj/sfaa198. PMCID: PMC7577767. PMID: 33123352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Skrabic R., Kumric M., Vrdoljak J., Rusic D., Skrabic I., Vilovic M., et al. SGLT2 inhibitors in chronic kidney disease: from mechanisms to clinical Practice. Biomedicines. 2022;10(10) doi: 10.3390/biomedicines10102458. PMCID: PMC9598622. PMID: 36289720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vallon V., Gerasimova M., Rose M.A., Masuda T., Satriano J., Mayoux E., et al. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol. 2014;306(2):F194–F204. doi: 10.1152/ajprenal.00520.2013. PMCID: PMC3920018. PMID: 24226524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Korner A., Eklof A.C., Celsi G., Aperia A. Increased renal metabolism in diabetes. Mechanism and functional implications Diabetes. 1994;43(5):629–633. doi: 10.2337/diab.43.5.629. PMID: 8168637. [DOI] [PubMed] [Google Scholar]
- 100.Tahara A., Kurosaki E., Yokono M., Yamajuku D., Kihara R., Hayashizaki Y., et al. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur J Pharmacol. 2013;715(1–3):246–255. doi: 10.1016/j.ejphar.2013.05.014. PMID: 23707905. [DOI] [PubMed] [Google Scholar]
- 101.Heerspink H.J.L., Perco P., Mulder S., Leierer J., Hansen M.K., Heinzel A., et al. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia. 2019;62(7):1154–1166. doi: 10.1007/s00125-019-4859-4. PMCID: PMC6560022. PMID: 31001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gallo L.A., Ward M.S., Fotheringham A.K., Zhuang A., Borg D.J., Flemming N.B., et al. Once daily administration of the SGLT2 inhibitor, empagliflozin, attenuates markers of renal fibrosis without improving albuminuria in diabetic db/db mice. Sci Rep. 2016;6 doi: 10.1038/srep26428. PMCID: PMC4881020. PMID: 27226136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bersoff-Matcha S.J., Chamberlain C., Cao C., Kortepeter C., Chong W.H. Fournier gangrene associated with sodium-glucose Cotransporter-2 inhibitors: a review of spontaneous Postmarketing cases. Ann Intern Med. 2019;170(11):764–769. doi: 10.7326/M19-0085. PMID: 31060053. [DOI] [PubMed] [Google Scholar]