Summary
Pakistan, among the top five most water-stressed nations globally, grapples with water scarcity owing to inadequate treatment infrastructure and groundwater overextraction. We demonstrate a successful nature-based closed-loop system to treat wastewater from urban vehicle-washing facilities, previously reliant on groundwater. An eco-friendly integrated system containing floating treatment wetlands (FTWs), subsurface flow constructed wetlands (SSF-CWs), and sand filtration (SF) was designed and installed at three vehicle-washing facilities for wastewater treatment and reuse in a loop. While the system is still operational after years, a consistent and significant reduction in water quality indicators is recorded, successfully meeting the national environmental quality standards of Pakistan. By reducing per unit water treatment costs to as low as $0.0163/m³ and achieving payback periods under a year, the embrace of these closed-loop strategies vividly underscores the imperative of transitioning to a circular economy in the domains of wastewater treatment and resource conservation.
Subject areas: Environmental science, Environmental Management, Aquatic Sciences
Graphical abstract
Highlights
-
•
Nature-based system effectively reclaims wastewater in urban car-wash facilities
-
•
System performance was validated by improved water quality indicators
-
•
FTIR confirmed hydrocarbons removal; microbial analysis exhibited degradation
-
•
A solution to water scarcity challenges in Pakistan and similar economies globally
Environmental science, Environmental Management, Aquatic Sciences
Introduction
The transition from a linear to a circular economy aligns with the United Nations Sustainable Development Goals (SDGs), which provide a global framework for sustainable development.1 By embracing circularity in wastewater treatment, we can promote SDG 6 (Clean Water and Sanitation) which ensures efficient use of water resources and minimizes pollution. In addition, circular economy practices contribute to SDG 12 (Responsible Consumption and Production) by reducing waste generation and promoting resource efficiency. The use of nature-based solutions is encouraged worldwide, and the United Nations Water Action Decade (2018–2028) and the Decade of Ecosystem Restoration (2020–2030) further emphasize these efforts.2
Using freshwater in automobile service stations and vehicle-wash facilities places an unnecessary burden on aquatic resources.3 A case-in-point is the wastewater generated at vehicle-washing stations that contains nearly 1% of contamination (mud, grease, oil, sand, and detergents) and can be easily recycled using a nature-based closed-loop system. Approximately 150–600 L of freshwater is consumed by vehicle-wash facilities to perform a complete professional wash on a single vehicle, and the same amount of water is discharged without treatment.4,5,6 Freshwater use restrictions by vehicle-wash facilities are in place in many countries including the Netherlands, Brazil, and several Scandinavian countries3; however, in Pakistan, authorities have been unsuccessful in ensuring the installation of water recycling plants at vehicle-wash service stations. Recently, the Water and Sanitation Agency (WASA) of Pakistan initiated a crackdown due to the lack of compliance, but a counterargument by vehicle-wash station owners was the unavailability of sustainable and affordable technology to meet the regulatory requirements and reduce environmental pressures.7 In Pakistan, the groundwater level is continuously decreasing in metropolitan cities, and approximately 3 billion liters of freshwater is wasted annually which could meet the drinking needs of approximately 666,000 people. Pakistan is among the top five countries worldwide in terms of wastewater production with only 1.2% of wastewater in the country undergoing treatment.8
Traditional methods, which are mainly based on coagulation, flocculation, sedimentation, and filtration, for the treatment and reuse of vehicle-wash wastewater, are not sustainable because of their high chemical and energy consumption, and production of waste.9 Therefore, nature-based low-cost technologies are encouraged for the wastewater remediation and reuse of wastewater.10 The use of natural wetlands for wastewater treatment is not encouraged as they are recognized as valued ecosystem components; hence, artificial wetlands that incorporate natural processes and functions are being researched as an alternative means to reclaim wastewater.11 To this end, subsurface flow constructed wetlands (SSF-CWs) and floating treatment wetlands (FTWs) are gaining tremendous popularity treating different types of wastewater.12 At the core of their functionality, SSF-CWs and FTWs exploit the synergistic interaction between aquatic plants, microbial communities, and the physical environment to facilitate a range of treatment processes. Specifically, microbial biofilms associated with plant roots play a crucial role in biotransformation and biodegradation of organic and inorganic pollutants.13 The rhizosphere, the region surrounding plant roots, creates a microenvironment conducive for microbial activity and enhances nutrient uptake.14 Aquatic plants in FTWs directly absorb pollutants and also release oxygen into the water, aiding aerobic microbial degradation of contaminants. As a result, the rhizosphere of SSF-CWs supports water purification through a process known as the Root Zone Method (German: Wurzelraumverfahren).15 Additionally, the sedimentation of suspended solids and the sorption of pollutants onto plant roots and soil particles also contribute to the purification process.16 These naturally occurring processes in SSF-CWs and FTWs not only ensure efficient wastewater treatment but also minimize the ecological footprint of such interventions.17,18 Sand filtration (SF) is used to remove microbial contamination from water and has been extensively applied.19 However, an integrated system involving the combined use of FTWs, SSF-CWs, and SF has not been previously applied at vehicle-wash facilities for the treatment and reuse of wastewater. By in large, combining these nature-based approaches could offer low-cost, sustainable, and environmentally friendly means wastewater reclamation.12
As per the regulations set by the WASA, vehicle-wash facilities are required to recycle a minimum of 70% of the used water; failure to meet this requirement could result in suspension of their operations and penalties.7 To this end, we developed and implemented a nature-based closed-loop system composed of FTWs, SSF-CWs, and SF, and evaluated the treatment efficiency from three vehicle-wash facilities (two car washes and one container wash). The performance of the systems was studied by analyzing water samples collected from the outlet for two years toward physicochemical and microbiological parameters. At the end of two years, we found that the system’s performance was excellent as the same water is being reused for multiple operations. This led us to conclude that this technology has tremendous potential to address water-related challenges in developing countries related to wastewater use and allocation, environmental integrity, water scarcity, groundwater depletion, and sustainable water facilities.
Results and discussion
Characteristics of vehicle-wash wastewater
A comprehensive analysis of untreated wastewater demonstrated that it exhibited elevated levels of chemical oxygen demand (COD), biochemical oxygen demand (BOD), hydrocarbons, detergents, and total suspended solids (TSS), exceeding the permissible limits established by the National Environmental Quality Standards of Pakistan (Table 1). Multiple studies have consistently shown that wastewater from vehicle-washing facilities typically displays heightened water quality parameters when benchmarked against the discharge standards set forth in Pakistan.20,21,22 Conversely, concentrations of heavy metals, essential cations (sodium, potassium, calcium, and magnesium), and anions (chlorides, sulfate, etc.) were found to be below the wastewater discharge standards. One plausible explanation for this observation could be the use of uncontaminated groundwater sources for the vehicle-washing processes. Upon comparing wastewater from three different vehicle-wash stations, it was determined that the wastewater from Interloop Logistics Momentum (ILM) exhibited the highest contamination levels. It might be due to the reason that this station washes containers which are may be more polluted than the cars, or it has lesser professional staff to clean the vehicles.
Table 1.
Characteristics of untreated wastewater from vehicle-wash bay of Toyota Lyallpur Motors (TLM), Toyota Chenab Motors (TCM), and Interloop Logistics Momentum (ILM)
| Parameter | Unit | Value |
NEQS | ||
|---|---|---|---|---|---|
| TLM | TCM | ILM | |||
| pH | NA | 8.64 (0.35) | 7.43 (0.28) | 8.12 (0.27) | 6–9 |
| Chemical oxygen demand | mg/L | 387 (83) | 335 (56) | 555 (72) | 150 |
| Biochemical oxygen demand | mg/L | 191 (39) | 178 (42) | 232 (43) | 80 |
| Total organic carbon | mg/L | 135 (22) | 116 (19) | 178 (26) | NG |
| Hydrocarbons | mg/L | 218 (87) | 195 (48) | 285 (58) | 10 |
| Detergents | mg/L | 160 (61) | 148 (56) | 192 (48) | 20 |
| Electrical conductivity | mS/cm | 3.62 (0.57) | 1.27 (0.35) | 2.16 (0.46) | NG |
| Turbidity | FAU | 140 (43) | 128 (36) | 154 (32) | NG |
| Total solids | mg/L | 3340 (781) | 830 (115) | 1435 (350) | NG |
| Total dissolved solids | mL/L | 2135 (743) | 750 (95) | 1285 (208) | 3500 |
| Total settleable solids | mL/L | 1.72 (0.37) | 2.18 (0.37) | 2.42 (0.31) | NG |
| TSS | mg/L | 260 (52) | 103 (25) | 310 (55) | 150 |
| Nitrogen | mg/L | 7.42 (1.80) | 4.58 (2.62) | 5.74 (1.57) | NG |
| Phosphorus | mg/L | 3.27 (0.4) | 1.42 (0.52) | 1.58 (0.33) | NG |
| Sulfate | mg/L | 177 (21) | 68 (16) | 108 (16) | 600 |
| Chlorides | mg/L | 481 (64) | 180 (32) | 342 (45) | 1000 |
| Calcium | mg/L | 101 (27) | 28 (8.4) | 76 (18) | NG |
| Magnesium | mg/L | 54 (22) | 30 (5.7) | 42 (10) | NG |
| Sodium | mg/L | 259 (23) | 270 (18) | 176 (16) | NG |
| Potassium | mg/L | 46 (9.1) | 68 (11.3) | 35 (7.2) | NG |
| Chromium | mg/L | 0.12 (0.33) | 0.14 (0.28) | 0.08 (0.01) | 1 |
| Iron | mg/L | 1.06 (0.27) | 1.17 (0.32) | 2.13 (0.31) | 8 |
| Arsenic | mg/L | 0.15 (0.03) | 0.08 (0.01) | 0.05 (0.01) | 1 |
| Cadmium | mg/L | 0.05 (0.01) | 0.06 (0.01) | 0.13 (0.03) | 0.1 |
| Lead | mg/L | 0.25 (0.08) | 0.17 (0.05) | 0.28 (0.05) | 0.5 |
| Manganese | mg/L | <0.01 | <0.01 | <0.01 | 1.5 |
| Total coliforms | CFU/mL | 4016 (899) | 3552 (735) | 1460 (950) | NG |
| Escherichia coli | CFU/mL | 268 (59) | 301 (61) | 232 (50) | NG |
Performance evaluation
The system’s performance was evaluated by examining water samples for parameters including pH, electrical conductivity, turbidity, COD, BOD, total organic carbon (TOC), total solids, total dissolved solids (TDS), TSS, nitrogen, phosphorus, E. coli, total coliform, sulfate, chloride, calcium, magnesium, detergents, hydrocarbons, sodium, potassium, oil, grease, and toxicity.
Removal of COD, BOD, and TOC
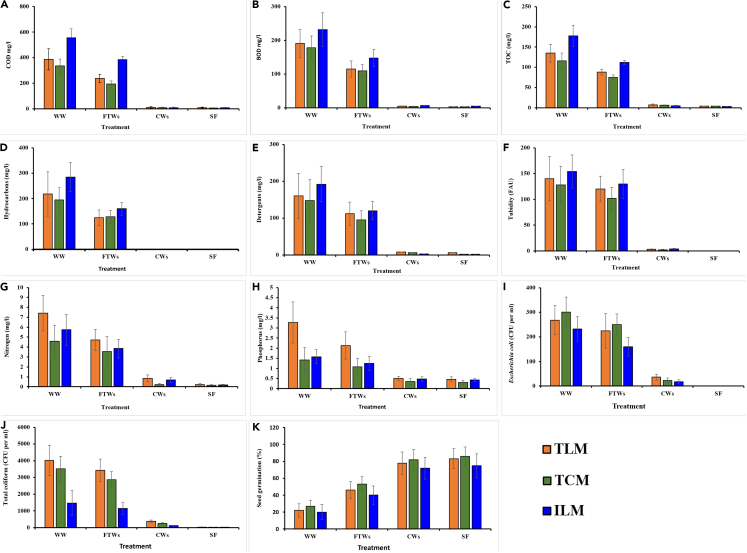
The COD of the wastewater of the three vehicle-wash facilities was 335–555 mg/L. There was 15%, 42%, and 31% reduction in COD of the wastewater of Toyota Lyallpur Motors (TLM), Toyota Chenab Motors (TCM), and ILM after passing it through FTWs, respectively. This reduction was further increased to 97%, 98%, and 98% of the wastewater of TLM, TCM, and ILM after passing through SSF-CWs, respectively. However, after passing through SF, there was no further significant reduction (Figure 1A). Similarly, the BOD of the wastewater was 178–132 mg/L, and there was 40%, 38%, and 36% reduction in the wastewater of TLM, TCM, and ILM, respectively, after passing the wastewater through FTWs, which further decreased to 97%, 98%, and 97%, respectively, after passing through SSF-CWs. There was no further reduction after passing through the SF (Figure 1B). Similarly, TOC was decreased to 97%, 96%, and 98% of wastewater of TLM, TCM, and ILM after passing through the FTWs, SSF-CWs, and SF (Figure 1C). It is a well-known fact that FTWs and SSF-CWs remove the COD, BOD, and TOC from the wastewater.23 Plant-associated microbial communities have been recognized for their integral role in organic pollutant degradation, resonating with our observations on the effectiveness of FTWs and SSF-CWs.14 Principally, organic pollutants are adsorbed/absorbed at the root surface, where the microbial community associated with the plants degrades them.24,25 Sedimentation, along with biodegradation, plays a synergistic role in pollutant removal, as emphasized by research findings, aligning with our observations on the importance of these processes in SSF-CWs.26,27 Between FTWs and SSF-CWs, there was more reduction in COD, BOD, and TOC by passing the wastewater through SSF-CWs than the FTWs. SSF-CWs typically demonstrate superior removal efficiency of contaminants like COD, BOD, and TOC when compared to other treatment systems, a phenomenon attributed to the prolonged retention time and complex microbial processes within these wetlands.13,24,28
Figure 1.
Assessment of physicochemcial and biological indicators in influent and effluent
Removal of chemical oxygen demand (A), biochemical oxygen demand (B) total organic carbon (C) hydrocarbons (D), detergents (E), turbidity (F), nitrogen (G), and phosphorus (H) and Escherichia coli (I) and total coliforms (J) during different treatment processes. The untreated wastewater (WW), the outlet of floating treatment wetlands (FTWs), constructed wetland (CWs), and sand filtration (SF). Germination (%) of wheat (Triticum sativum) seeds irrigated with wastewater treated by different treatments (K). The untreated wastewater (WW), the outlet of floating treatment wetlands (FTWs), constructed wetland (CWs), and sand filtration (SF). All these values are the mean of 24 different samples collected every month for two years. Error bars indicate the standard error.
Removal of hydrocarbons, detergents, and turbidity
The wastewater samples analyses revealed hydrocarbon concentrations ranging from 195 to 285 mg/L. The prevalence of hydrocarbons in vehicle-wash wastewater can be attributed to the residues of oils, fuels, and lubricants from vehicles, a common concern for many urban wastewater management systems.29 After FTWs treatment, hydrocarbon reductions were observed at 43%, 34%, and 44% for TLM, TCM, and ILM, respectively. Remarkably, this was augmented to a complete reduction (100%) for all three samples post-SSFCWs treatment (Figure 1D). Such significant reduction can be attributed to microbial activity inherent in these systems. In FTWs and SSF-CWs, microorganisms form a biofilm on roots, building material, and gravel, and use hydrocarbons as a sole source of carbon and energy.30,31 Plants not only offer essential nutrients but also create an environment conducive for microbial proliferation, which in turn facilitates pollutant degradation via mineralization or transformation into simpler compounds32
The concentration of detergents in the wastewater of the three vehicle-wash stations was 148–192 mg/L. There was 30%, 36%, and 37% reduction in its quantity in the wastewater of TLM, TCM, and ILM, respectively, after passing through FTWs, and this reduction was further increased to 95%, 96%, and 98% after SSF-CWs, respectively (Figure 1E). After passing through SF, this reduction was increased to 99% in all the wastewater. Earlier investigations reported that FTWs and SSF-CWs efficiently removed detergents such as sodium dodecyl sulfate from the wastewater.33,34
The turbidity of the wastewater was 128–154 FAU. High turbidity levels, often resulting from suspended particles in vehicle wash-off, can severely affect the receiving waterbodies.35 Post-FTWs treatment, turbidity reductions of 14%, 20%, and 15% were observed for TLM, TCM, and ILM, respectively. SSF-CWs treatment further amplified these reductions to 98%, 98%, and 97% for the respective samples, achieving a complete reduction (99.9%) post-SF treatment (Figure 1F). Prior research has confirmed the capability of FTWs in substantially reducing water turbidity.36 SSF-CWs, in particular, have been lauded for their effectiveness in turbidity removal, predominantly attributed to the entrapment of suspended particles within the extensive plant root systems.37
Removal of TDS and TSS
The concentration of TDS was 2135 mg/L, 750 mg/L, and 1285 mg/L in the wastewater of TLM, TCM, and ILM, respectively. Subsequent treatment through FTWs, SSF-CWs, and SF led to reductions in TDS to 1213, 485, and 765 mg/L, respectively (Table 2). Such reductions in TDS concentrations are crucial, as elevated levels of dissolved solids can significantly alter the osmotic balance in aquatic systems and adversely impact aquatic organisms.38 The removal of TDS may be due to the adsorption/absorption of cations and anions by the plants and microbes that colonize on the roots, gravel, and sand.39 Plant and microorganisms take up nutrients (nitrogen and phosphorus) and other elements for their growth and development.40 Sedimentation also plays an important role in the removal of TDS from the water. Similarly, the wastewater samples from TLM, TCM, and ILM witnessed a 100% removal in TSS after undergoing treatment through FTWs and SSF-CWs. The efficacy in TSS removal can largely be credited to the filtration capacities inherent to the extensive root systems in FTWs and SSF-CWs, as well as the sedimentation of suspended particles.41 The extensive root systems of the plants in FTWs and SSF-CWs work as filter and enhance the removal of the suspended material from the water.36
Table 2.
Effect on different physico-chemical parameters of vehicle-wash wastewater treated by the integrated system installed at Toyota Lyallpur Motors (TLM), Toyota Chenab Motors (TCM), and Interloop Logistics Momentum (ILM)
| Treatment/parameter | Untreated WW |
FTWs |
CWs |
SF |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TLM | TCM | ILM | TLM | TCM | ILM | TLM | TCM | ILM | TLM | TCM | ILM | |
| pH | 8.62 (0.42) | 7.43 (0.38) | 8.12 (0.47) | 7.03 (0.59) | 6.65 (0.42) | 7.25 (0.52) | 6.70 (0.45) | 6.32 (0.34) | 6.63 (0.40) | 6.54 (0.44) | 6.28 (0.35) | 6.55 (0.45) |
| Chlorides (mg/L) | 481 (27) | 180 (15) | 342 (18) | 406 (51) | 88 (22) | 178 (52) | 326 (58) | 64 (15) | 166 (55) | 304 (42) | 60 (12) | 158 (42) |
| Sulfates (mg/L) | 177 (22) | 68 (17) | 108 (20) | 143 (34) | 53 (14) | 94 (22) | 107 (26) | 46 (12) | 65 (38) | 98 (27) | 43 (10) | 62 (28) |
| Sodium (mg/L) | 259 (23) | 270 (18) | 76 (15) | 204 (36) | 160 (32) | 48 (15) | 120 (52) | 117 (26) | 36 (13) | 106 (45) | 105 (18) | 31 (10) |
| Potassium (mg/L) | 46 (9.15) | 68 (11.3) | 37 (7.2) | 32 (9.5) | 39 (14) | 25 (9.4) | 23 (11) | 26 (16) | 23 (8.4) | 18 (11) | 23 (13) | 20 (7.4) |
| Total dissolved solids (mg/L) | 2135 (462) | 750 (95) | 1285 (208) | 1643 (310) | 570 (45) | 850 (210) | 1299 (152) | 510 (30) | 780 (110) | 1213 (160) | 485 (27) | 765 (80) |
| Total suspended solids (mg/L) | 260 (64) | 103 (25) | 310 (55) | 78 (29) | 58 (17) | 105 (30) | 0 | 0 | 0 | 0 | 0 | 0 |
All these values are the mean of 24 different samples collected every month for two years. Values in parentheses represent standard deviation. The untreated wastewater (WW), the outlet of floating treatment wetlands (FTWs), constructed wetland (CWs), and sand filtration (SF).
Effect on pH
Initial analysis revealed that the wastewater from TLM, TCM, and ILM exhibited pH values of 8.64, 7.43, and 8.12, respectively (Table 2). After treatment via the integrated system encompassing FTWs, SSF-CWs, and SF, a significant drop in pH was observed, recording values of 6.54, 6.28, and 6.55 for TLM, TCM, and ILM, respectively. Worth noting is that pH values indicative of alkalinity, such as those recorded, are often associated with the use of alkaline detergents in car-washing facilities.42 The observed decrease in pH post-treatment can also be ascribed to the formation of root exudates and the microbial breakdown of organic compounds that culminate in acid production, corroborating findings by Ijaz et al..43 Such transformations in pH are essential in ensuring the treated water’s compatibility with its intended end use, minimizing ecological impacts.
Removal of nutrients and other elements
Preliminary analyses identified total nitrogen concentrations in the wastewater samples from TLM, TCM, and ILM as 7.42, 4.58, and 5.74 mg/L, respectively. Upon processing through the combined FTWs, SSF-CWs, and SF system, these concentrations significantly reduced, registering values of 0.23, 0.15, and 0.17 mg/L for TLM, TCM, and ILM, respectively (Figure 1G). Nitrogen removal from wastewater is pivotal, as its excess release into natural water systems can trigger eutrophication, severely affecting aquatic ecosystems.44 In a parallel observation, phosphorus concentrations in the wastewater from TLM, TCM, and ILM underwent marked reductions post-treatment, declining from initial values of 3.27, 1.42, and 1.58 mg/L to 0.46, 0.31, and 0.43 mg/L, respectively (Figure 1H). It is well documented that phosphorus also plays a significant role in eutrophication processes, emphasizing the importance of its effective removal.45 Comparative analysis between the two wetland systems elucidated that SSF-CWs demonstrated superior efficiency in nitrogen and phosphorus removal compared to FTWs. In a similar vein, other water quality parameters, including chlorides, sulfates, sodium, and potassium, witnessed substantial reductions when treated through the integrated FTWs, SSF-CWs, and SF system (Table 2).
Fourier transform infrared spectroscopy
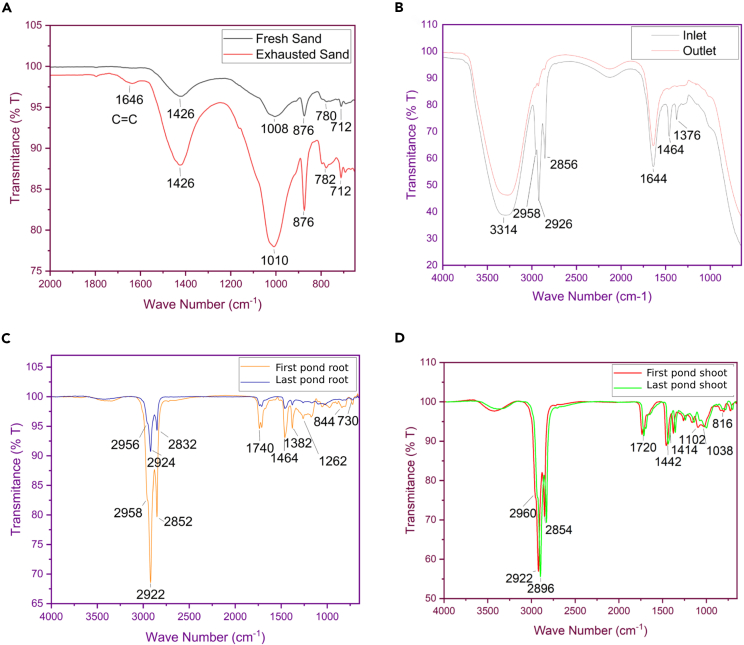
Fourier transform infrared spectroscopy (FTIR) analysis was performed on both fresh and exhausted sand samples (Figure 2A). The spectrum of fresh sand displayed a peak at 692 cm−1, indicative of the Si-O asymmetric bending vibration.46 Additional peaks at 874 cm−1 and 711 cm−1 were observed, corresponding to the Si-O stretching vibrations,47 and a peak at 1,004 cm−1 was attributed to the Si-O-Si asymmetric stretch vibration.48 A notable distinction in the exhausted sand’s spectrum was the presence of a peak at 1,625 cm−1, suggesting the accumulation of plant residues via a C=C bond,49 which appears to be the primary cause of filter exhaustion. For the carwash system inlet wastewater (Figure 2B), FTIR peaks at 2,954, 2,922, 2,854, and 1,376 cm−1 were identified, which are characteristic of the aldehyde functional group and C–H bond stretching vibrations.50 The peaks at 3,295 and 1,638 cm−1 were associated with the hydroxyl group’s (O–H) stretching vibrations.51 Hydrocarbons concentration was quantified using the standard curve method, with a calculated concentration of 301 mg/L. Conversely, the outlet water post-treatment showed no hydrocarbon peaks, indicating complete hydrocarbon removal with only the hydroxyl group peaks remaining. Further, FTIR analyses were conducted on the root endosphere from the first pond of the FTW (Figure 2C), where a hydrocarbon presence was detected in a 10 g root sample, with a concentration of 0.696 mg/g. This was evidenced by peaks at 724 and 839 cm−1, which are typically associated with meta-dispersed aromatic C–H bands. However, in the final pond (Figure 2D), also known as CWs, the hydrocarbon concentration in the root endosphere was significantly lower at 0.172 mg/g. Additional studies on the shoot endosphere in the first pond revealed a hydrocarbon concentration of 1.090 mg/g in the shoots, a level comparable to that of plants not exposed to hydrocarbons. The final pond’s shoot endosphere analysis showed a slightly reduced hydrocarbon concentration of 1.048 mg/g. These findings suggest varying levels of hydrocarbon presence and degradation throughout the treatment process, with the final water treatment stages effectively reducing hydrocarbon content.
Figure 2.
FTIR spectral analysis of hydrocarbon degradation
(A) Displays FTIR spectra with higher peaks indicative of exhausted sand.
(B) Presents a comparison between inlet and outlet water spectra, with the outlet spectrum showing hydrocarbon removal.
(C) Depicts hydrocarbon levels in root endospheres from the initial and final ponds.
(D) Illustrates hydrocarbon degradation in shoot endospheres, evidencing the efficacy of the wastewater treatment process.
Microbial analyses
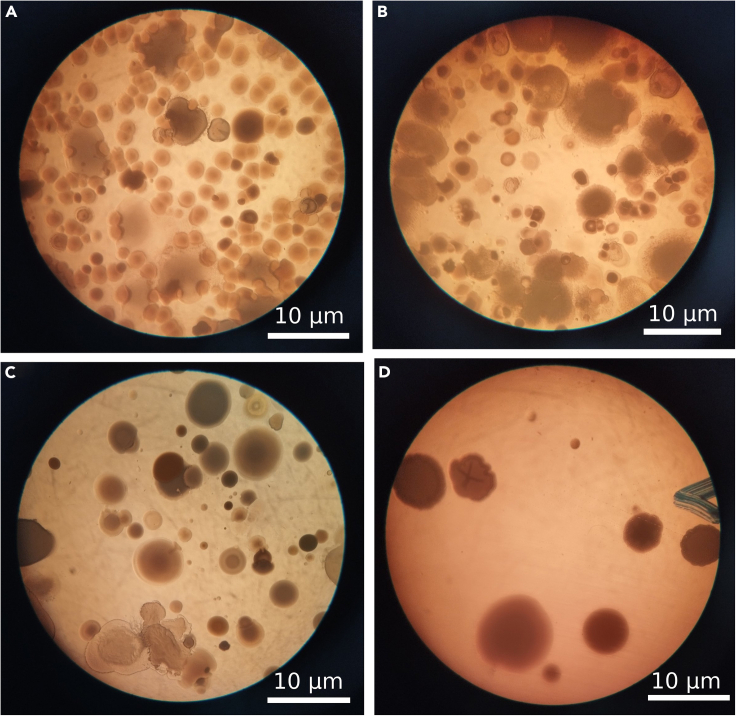
The stereoscopic examination of plant roots and shoots from the initial and terminal ponds, combined with quantitative PCR analysis, has provided a comprehensive view of microbial dynamics in the treatment of vehicle-wash station wastewater (Figure 3). Dense clusters of hydrocarbon-degrading bacterial colonies were evident in the roots from the first pond, aligning with the high pollution load characterized by the presence of oil and grease (Figure 3A). The last pond showed a marked reduction in these bacterial colonies, signifying effective hydrocarbon removal (Figure 3B). A similar pattern was observed in shoot samples, where the first pond’s variety of bacterial colonies diminished by the final pond, corroborating the reduced pollution levels (Figures 3C and 3D).
Figure 3.
Stereoscopic microscopy of bacterial colonization and hydrocarbon removal
(A) Dense bacterial colonies in the roots from the final pond, indicative of high pollution load.
(B) Roots from the first pond with fewer bacterial colonies, suggesting preliminary hydrocarbon removal.
(C) Varied bacterial colony density in the shoots from the initial pond.
(D) A reduction in bacterial presence in the shoots from the final pond, aligning with decreased pollution levels.
Colony-forming units (CFUs) performed on M9 media containing diesel as sole carbon source, and quantitative PCR analyses conducted to assess the abundance and expression of the alkB gene, a marker for hydrocarbon degradation, further confirmed these observations. Specifically, in the first pond, the abundance of hydrocarbon-degrading bacteria and alkB gene was significantly high. In contrast, water samples obtained after treatment showed a considerable decline in the CFU counts and alkB gene abundance and expression, indicating that the bulk of hydrocarbons had been degraded. Notably, SF stages were devoid of detectable hydrocarbon-degrading bacteria or alkB gene expression, evidencing the sand filter’s capacity to attenuate residual bacterial populations (Table S1). This integrative approach underscores the efficacy of the treatment system in reducing hydrocarbon pollutants and highlights the potential of molecular techniques in monitoring environmental bioremediation processes.
We also tested the abundance of E. coli and total coliforms in the untreated wastewater. Their abundance in treated water was 2,300 and 4,000 CFU/100 mL, respectively. Their high abundance in Faisalabad’s car-wash wastewater, sourced from groundwater, was likely due to regional contamination, including sewage leakages and fecal-contaminated runoff, and from unsafely storing water in open containers, which can attract bird droppings. Post-treatment via the FTWs, SSF-CWs, and SF system led to a marked reduction in these concentrations for all stations (Figures 1I and 1J). There was a 100% reduction in their numbers after passing the wastewater of TLM, TCM, and ILM through the system. Between two wetland units, a better removal of pathogen indicators was observed by passing the wastewater through SSF-CWs when compared to the FTWs.
Phytotoxicity reduction
Ingredients such as surfactants, solvents, and fragrances commonly found in detergents can potentially disrupt aquatic life, alter natural water chemistries, and magnify the challenges of wastewater treatment.52 Therefore, phytotoxicity assay was performed to evaluate the detoxification potential of the treatment system. The seeds of wheat (T. sativum) were subjected to the untreated wastewater and the treated water of TLM, TCM, and ILM. There was lesser germination of seeds irrigated with untreated wastewater as compared to germination of seeds irrigated with the treated water (Figure 1K). In a previous study, inhibition in germination of seeds of various other crops with untreated water was also reported.53 The significantly higher germination success with treated water suggests not only the removal of pollutants, but also the substantial detoxification, enhancing its compatibility with vegetation in the receiving waterbody/ecosystem.
Cost-effectiveness
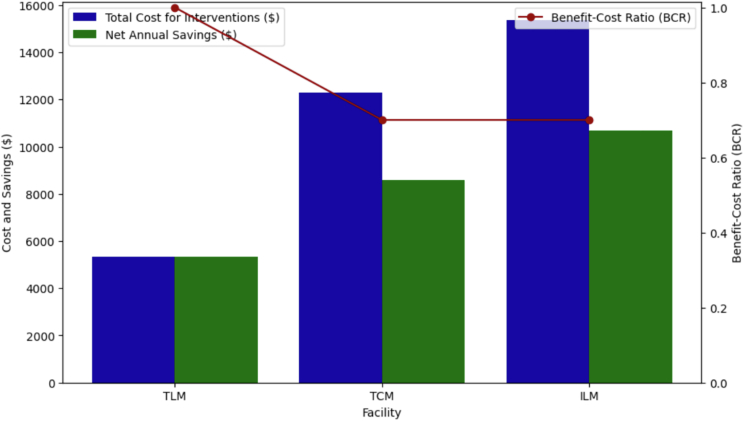
Water recycling initiatives across three vehicle-washing stations have demonstrated not only environmental responsibility but also notable cost-effectiveness. The adoption of these systems resulted in drastic reductions in water treatment costs: from $0.8/m³ to $0.03/m³ at TLM, to $0.036/m³ at TCM, and to $0.0396/m³ at ILM (Figure 4; Table 3, supplementary information). Such findings emphasized the economic viability of water recycling in regions confronted with water scarcity.54,55 Furthermore, overextraction of groundwater is globally recognized as a critical issue, leading to ecological challenges like land subsidence and deterioration of aquatic habitats.56 Each of these stations’ initiatives, by reducing the annual groundwater extraction by thousands of cubic meters, not only offers a solution to economic constraints but also combats the detrimental environmental impacts of overextraction. As these practices align with the urgent calls for sustainable water management,57 they set a benchmark for industries particularly in developing countries, emphasizing the confluence of economic prudence and ecological responsibility.
Figure 4.
Cost-effectiveness analysis for groundwater recycling initiatives at three facilities
The bar graph represents the total cost of interventions and the net annual savings for Toyota Lyallpur Motors (TLM), Toyota Chenab Motors (TCM), and Interloop Logistics Momentum (ILM), while the green line illustrates the benefit-cost ratio (BCR) for each facility.
Table 3.
Comparative analysis of metrics across Toyota Lyallpur Motors (TLM), Toyota Chenab Motors (TCM), and Interloop Logistics Momentum (ILM), highlighting economic and resource efficiency of groundwater recycling initiatives
| Attributes | TLM | TCM | ILM |
|---|---|---|---|
| Total cost for interventions ($) | 5,341 | 12,283 | 15,373 |
| Annual groundwater savings (m³) | 6,935 | 11,096 | 13,870 |
| Power for groundwater extraction (kWh/m³) | 0.8 | 0.8 | 0.8 |
| Electricity cost ($/kWh) | 0.4 | 0.4 | 0.4 |
| Revised annual maintenance cost ($) | 225 | 300 | 400 |
| Cost to extract and treat without recycling ($) | 5,548 | 8,876.80 | 11,096 |
| Total cost for first year ($) | 5,566 | 12,583 | 15,773 |
| Annual expenses for subsequent years ($) | 225 | 300 | 400 |
| Net annual savings ($) | 5,323 | 8,576.80 | 10,696 |
| Cost per m³ before recycling ($) | 0.8 | 0.8 | 0.8 |
| Cost per m³ after recycling ($) | 0.0325 | 0.027 | 0.0288 |
| Derived metrics | |||
| Payback period (years) | 1 | 1.43 | 1.44 |
| Return on investment (%) | 99.7 | 69.8 | 69.6 |
| Benefit-cost ratio (BCR) | 1 | 0.7 | 0.7 |
| Economic Value Index (EVI) (m³/$) | 1.24 | 0.88 | 0.88 |
All values are reported in the US dollars.
The analysis of water recycling interventions further illuminated profound economic and environmental dividends. The payback periods for TLM exhibit an impressive payback period of only 1 year, signifying a rapid recoupment of costs (Figure 3). The payback periods for ILM and TCM were slightly higher (1.44 and 1.43 years, respectively), but still suggest a relatively quick recovery of the initial investment in just over a year (Table 3). The return on investment for TLM stands out at an astounding 99.7%, nearly the ideal value, while TCM and ILM hover around the 70% mark (Table 3). The benefit-cost ratio (BCR) for TLM is also exemplary at 1.0, which means the benefits derived equal the costs incurred, rendering it the most economically sustainable of the three (Table 3; Figure 2). A BCR greater than 1 is typically indicative of a project’s economic viability, as it demonstrates that the benefits surpass the costs.58 Finally, the Economic Value Index (EVI) underscores the cost-effectiveness of water treatment at each station, with TLM leading at 1.24 m³/$. The EVI offers a novel perspective on resource efficiency in relation to cost, a metric that is increasingly gaining traction in environmental economics literature.59 In conclusion, while all three stations present commendable metrics, TLM stands out in terms of economic and resource efficiency. However, it is imperative to note that external factors, such as regional variations in groundwater extraction costs and maintenance, with higher inflation rates, may influence these metrics and should be revised with respect to the evolving situations. Cumulatively, our results champion the indispensable role of water recycling in navigating contemporary ecological and economic challenges.
Implications
The implications of the proposed closed-loop wastewater treatment system for vehicle-washing facilities are significant. This system offers several benefits and positive outcomes, including.
-
(1)
Cost-effectiveness: Nature-based solutions often offer a low-cost alternative for wastewater treatment, making them particularly attractive for vehicle-washing facilities in terms of operational expenses. By leveraging natural processes, these systems can reduce reliance on energy-intensive processes and costly infrastructures.60
-
(2)
Sustainability and resource conservation: Closed-loop systems align with the principles of sustainability and resource conservation.61 By recycling and reusing water within the facility, these systems minimize the need for freshwater intake and reduce overall water consumption. This is particularly crucial in regions facing water scarcity or where water resources need to be managed efficiently.62
-
(3)
Valorization of biomass: Closed-loop systems can enhance the valorization of residual biomass. This biomass can be utilized to produce value-added products, such as biofuels, fertilizers, and other useful materials. This concept of circular economy has tremendous significance.63
-
(4)
Alignment with UN Sustainability Goals: This study not only showcases the economic feasibility of our approach but also its environmental sustainability, making a compelling case for its adoption in line with the United Nations’ directives for the current and upcoming decades. Specifically, our findings resonate with the objectives of the 2018–2028 International Decade for Action – “Water for Sustainable Development,” and anticipate contributions to the 2020–2030 UN Decade on Ecosystem Restoration. This positions our study not only as a report on a scalable system, but also as a timely response to global calls for sustainable development practices.
Overall, implementing a nature-based closed-loop wastewater treatment system in a vehicle-washing facility could contribute to environmental sustainability, resource efficiency, and cost-effectiveness while reducing the facility’s carbon footprint and promoting the circular economy.64 This study represents a significant advancement in combining different wetlands and SF to develop an integrated system for wastewater treatment and reuse system. The developed process exhibits key qualities aligned with the requirements of this era, including low capital cost, sustainability, environmental friendliness, absence of operational and maintenance costs, and a smaller spatial footprint than traditional wastewater treatment technologies.
Limitations of the study
This study acknowledges certain limitations in its approach to substrate regeneration within biofilters. Primarily, our methodology was confined to two modes of sand management in the treatment system: replacement and regeneration. The replacement procedure involved substituting the sand after 60 days of continuous use. In contrast, the regeneration process was initiated upon reaching a predefined pressure differential, indicated by the water level at the inlet. This process entailed the careful removal of the top 8–10 cm layer of sand above the non-woven geotextile membrane, followed by the washing of the membrane and the refilling of the sand filter with either fresh or recycled sand. The removed sand was rigorously washed using water from the treated water storage tank, and this water was then recycled through the car-wash wastewater treatment system. The washed sand was sun-dried over a period of approximately 6 to 7 days before being stored for future use. While this methodology ensures effective maintenance and operation of the sand filter within the vehicle-wash wastewater treatment system, its application was restricted to this specific context. The study did not explore alternative substrate regeneration techniques or assess the effectiveness of these procedures in different types of biofilters. Consequently, the findings have limited applicability beyond the specific conditions and systems tested. Further research is needed to evaluate the efficacy of these methods in diverse settings and with varying types of biofilters to broaden the scope of these findings.
Conclusions
In conclusion, the research showcases a groundbreaking, cost-effective, and sustainable solution for water scarcity in Pakistan through the implementation of an integrated system comprising FTWs, SSF-CWs, and SF in urban vehicle-washing facilities. This system not only effectively removes various contaminants such as BOD, COD, TOC, TSS, and pathogens, but also reclaims wastewater to a high-quality standard suitable for reuse. The study highlights the superior efficiency of constructed wetlands in contaminant removal, marking a significant advancement in combining different wetland technologies and SF for wastewater treatment. This innovative approach addresses critical environmental challenges, offering a scalable and environmentally friendly alternative to traditional wastewater treatment methods. Further research is recommended to investigate the application of green materials as substrates in SSF-CWs. This exploration aims to improve the regeneration lifespan of the system and minimize the need for frequent sand replacement, thereby enhancing efficiency and sustainability.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals | ||
| M9 minimal salts | Sigma-Aldrich | M6030 |
| Diesel (for media plates) | Local market | N/A |
| Sodium chloride | Sigma-Aldrich | S9888 |
| Biological samples | ||
| Local and indigenous wetland plants, i.e., Phragmites australis, Typha domingensis, Canna indica | natural wetlands/ponds/drains | Mansoor Hameed, Ph.D. Faculty of Sciences, Department of Botany, UAF |
| Triticum sativum seeds | Local market | N/A |
| Others | ||
| PVC pipes and pots | Local market | N/A |
| Coconut shavings | Local market | N/A |
| Loamy texture soil | Local market | N/A |
| Gravel (pebbles: 4 to 64 mm) | Local market | N/A |
| Metal wire (6-foot length) | Local market | N/A |
| Bamboos | Local market | N/A |
| Nut bolts and washers | Local market | N/A |
| Concrete anchors | Local market | N/A |
| Polyethylene-based or polystyrene-based roof insulation rolls | Diamond Foam Company | N/A |
| Non-woven geotextile membrane | Local market | N/A |
| Whatman® qualitative filter paper, Grade 1 | Sigma-Aldrich | WHA1001325 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Muhammad Afzal (manibge@yahoo.com).
Materials availability
This study did not generate new unique reagents.
Data and code availability
Data: No new data were generated.
Code: No codes were generated.
Any additional information is available from the lead contact upon request.
Method details
Designing and developing vehicle-wash wastewater treatment and reuse system
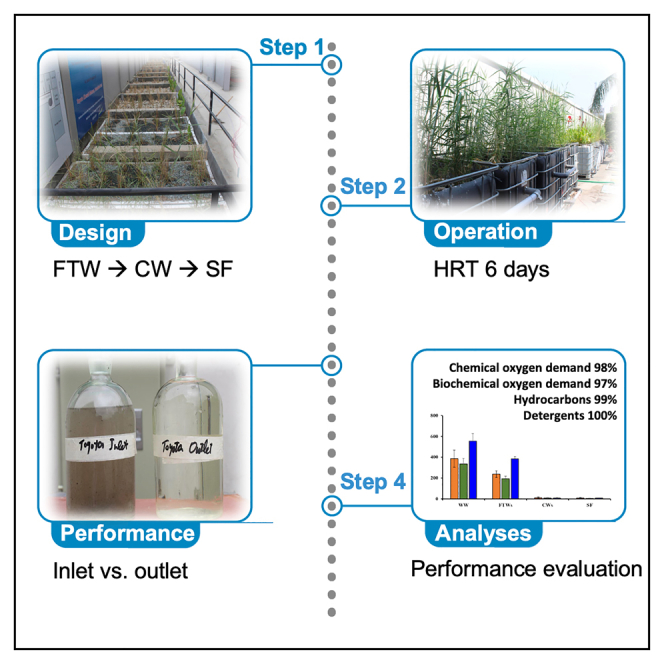
The system for the treatment and reuse of vehicle-wash wastewater was installed at (1): Toyota Lyallpur Motors (TLM), Faisalabad (geographic coordinates 31.507559872844087, 73.06845123208316), in March 2019, (2): Toyota Chenab Motors (TCM), Faisalabad (31.478719811567508, 73.22460090744954), in June 2019, and (3): Interloop Logistics Momentum (ILM), Khanewal (30.332008483865824, 71.78934270934977) in February 2020. The car wash facilities at TLM and TCM clean approximately 30 small vehicles (cars) and produce approximately 4,000- and 8,000-L wastewater per day, respectively, whereas the containers-wash facility at ILM wash about 10–15 containers and produce approximately 9,000 L wastewater per day. All wash stations operate six days a week, including a half day on Fridays. At both car-wash facilities, wastewater from the car wash bay was stored in a storage tank named an “untreated wastewater storage tank (UWST)”. A pump was used to supply the wastewater from the UWST into the FTWs, from which it was gravimetrically allowed to sequentially flow into the SSF-CWs and SF unit, and finally was stored in the tank named as “treated water storage tank (TWST)”. The total retention time of the wastewater in the system was approximately six days. The average flow rates observed were 0.1667 m³/hr (equivalent to 4 m³/day) at TLM, 0.333 m³/hr (8 m³/day) at TCM, and 0.375 m³/hr (9 m³/day) at ILM. The design of all tanks, ranging from those for treated water to storage tanks, incorporates elevated side walls to ensure a sufficient head for effluent to flow by gravity. The flow diagrams of the integrated wastewater treatment and recycling systems installed at TLM, TCM, and ILM are shown in Figures S1–S3, respectively. Each system comprised a series of ponds and wetlands engineered for effective flow and volume capacities. Specifically, for TLM Faisalabad, a system designed to handle 4000 L per day with a six-day retention time includes a singular untreated wastewater collection tank measuring 7.9 m by 1.73 m by 1.52 m. This system also incorporated five floating treatment wetlands (FTWs) and a constructed wetland (CW), each one cubic meter in size with volumes of 0.9 m³ and 0.3 m³, respectively. A sand filter of equal dimensions as the wetlands provided additional purification, and the treated water is stored in a tank with dimensions of 4.87 m by 2.4 m by 1.22 m. TCM Faisalabad comprised a system for treating 8000 L per day, also with a six-day retention time. It featured a wastewater collection tank with an effective volume of 12.5 m³, 2 FTW, and 10 CWs, each significantly larger than those at TLM, with volumes of 6.33 m³ and 2.113 m³, respectively. It also included a larger sand filter and two treated water storage tanks, each with a capacity of 10 m³. Lastly, ILM system treats 9000 L per day. It has a collection tank with a volume of 19 m³, three FTW with a volume of 5.10 m³ each, and 18 CW divided into two size groups with volumes of 1.39 m³ and 0.91 m³. A sand filter of the smaller dimension and a significantly larger treated water storage tank measuring 2.44 m by 2.13 m by 3.9 m with an effective volume of 18.7 m³ complete the system.
Untreated wastewater storage tank
An underground UWST was designed to collect and store the wastewater. This tank provided a retention time for the non-dissolved particles to settle. This tank was also equipped with a level sensor to switch water pump operation according to the logic provided (Table S2; Figure S4A). A water pump was used to maintain the level of the FTWs by feeding wastewater from the UWST. The pump was operated using three logics.
-
(1)
Start at a low level of FTWs.
-
(2)
Stop at a high level of FTWs.
-
(3)
Stop at a low level of the FTWs.
Using the truth and Karnaugh map (Tables S2 and S3), a Boolean expression was performed, and a logic circuit (Figure S4A) was drawn for the 24/7 operation of the water pump. This feature allows the system to operate automatically.
Floating treatment wetlands (FTWs)
The FTW units were developed in series in hard-plastic tanks cemented on the ground (Figure S4B). A floating mat was developed using a locally available polyethylene material with eight holes at an equal distance from each other. These holes had a diameter of 10 cm on the upper side and 7.5 cm at the lower side of the mat. The edges of the mat were covered with aluminum foil to protect it from sunlight. The seedlings of the native plants, Phragmites australis (five seedlings per hole), Typha domingensis (one seedling per hole), and Canna indica (one seedling per hole), were fixed in the holes of the mats with coconut shaving. Seedlings of the plants were grown at the National Institute for Biotechnology and Genetic Engineering (NIBGE). Agricultural soil was placed over the mat up to 1 inch, and thin layers of sand, round pebbles, and gravel were applied over the soil. The vegetated mats were transferred into the tanks with vehicle-wash wastewater.
Subsurface flow constructed wetlands (SSF-CWs)
Subsurface flow CWs units were developed and connected in series (Figure S4C). In each CW unit, the effluent was collected from the bottom and fed to the next tank at the top. The CW unit was developed in such a way that there was a 15 cm layer of round pebbles at the bottom with an average diameter of 12.5 cm. The second layer of each unit was filled to a thickness of 10 cm with round flat pebbles having an average diameter of 9 cm. The third layer was filled up to 43 cm thickness with “road constructing gravel” having an average diameter of 11 cm. The 4th layer was filled up to 20 cm thickness with “building constructing gravel” having an average diameter of 1.6 cm. The fifth and last layer was filled up to 5 cm thickness with gravel having an average diameter of 0.48 cm. The seedlings of three different plants (P. australis, T. domingensis, and C. indica) were vegetated in the 4th layer at an equal distance of 15.24 cm. These seedlings were already grown in the vicinity of NIBGE and were removed from the soil, and their roots were washed with tap water, before planting in the gravel.
Sand filter (SF)
The SF was designed to have a single baffled shape (Figure S4D). At the bottom of the tank, the first layer (7.5 cm thickness) was developed using construction-grade gravel having an average size (diameter) of 1.6 cm. The second layer (15 cm thickness) consisted of round shape gravel having an average diameter of 0.8 cm. The third layer (4 cm thickness) was comprised of flat shape gravel having an average diameter of 0.67 cm. The fourth layer (6 cm thickness) consisted of round shape gravel having an average diameter of 0.48 cm. The fifth layer (sand layer) was divided into three parts, with a thickness of 7.5 cm, 10 cm, and 17.5 cm, respectively. Between 1st two layers (7.5 cm and 10 cm), there was a non-woven geotextile membrane, and it was to replace the upper 7.5 cm layer of sand after every two months or by observing the pressure difference between the inlet and outlet of SF. The main function of this SF is to remove pathogenic bacteria from the treated water. The integrated system for the treatment and reuse of vehicle-wash wastewater at TLM Faisalabad is shown in Figures S5A–S5N; whereas development of an integrated system of FTW, SSF-CWs, and SF at ILM Khanewal for the treatment and reuse of the containers-wash station is shown in Figures S5O and S5P.
Collection of water samples and their analysis
The water samples were collected during the first week of each month for two years from Toyota Lyallpur Motors (June 2019 to May 2021), and from Toyota Chenab Motors and Momentum Logistics (March 2020-2022). Water sampling was started after three months of plant growth. Water samples were collected in glass bottles from five different points, the outlet of the wastewater storage tank, the outlet of the FTWs, SSF-CWs, and SF by using the composite sample technique (8 h sampling) and transported to NIBGE.
Turbidity of the water samples was determined by using Spectro Quadrant Nova 60 (Merck Millipore). A benchtop digital pH meter (Accumet model 25, Denver Instrument Company, USA) was used to determine the pH of the water samples. A benchtop digital meter (CON 500 COLE- PARMER, Singapore) was used to measure the electrical conductivity (EC) of the water samples. The COD, TOC, total phosphorus, and sulfates were analyzed using the colorimetric method as described earlier (APHA, 2005). For COD and TOC measurements, the samples were heated at 150°C in the presence of K2Cr2O7 and H2SO4 (2 h for COD and 1.5 h for TOC). Biochemical oxygen demand was determined by a 5-Day BOD test. Chlorides, calcium, and magnesium contents in the water samples were determined using the titration method (APHA, 2005). Sodium and potassium levels in the filtered samples were determined by using a flame photometer (Model 410, Sherwood Scientific). Total nitrogen was measured using the Kjeldahl digestion method. The total suspended solids were determined gravimetrically using pre-dried Whatman’s filter paper. Total solids were determined gravimetrically using a pre-dried beaker. Total dissolved solids were determined in a similar way with filtered samples. For hydrocarbons assessment, Fourier Transform Infrared (FTIR) spectroscopy analysis was conducted utilizing a PerkinElmer Spectrum II instrument (PerkinElmer, USA) within a controlled dry atmospheric condition. The Horizontal Attenuated Total Reflectance (HATR) technique was employed, incorporating a Zinc Selenide (ZnSe) flat plate as the reflective medium. For plant tissues analyses, a representative sample comprising either 10 g of root or shoot material was subjected to a thorough cleansing process involving both water and methanol. This was followed by a manual grinding process using a pestle and mortar, persisting approximately 20 min, to achieve a fine paste consistency. Subsequent to the grinding, 10 mL of n-Hexane was added to each sample, which was then vigorously agitated using a vortex shaker for a duration of 2 min. Upon settling, a 24 μL aliquot of the supernatant was carefully transferred onto the FTIR ZnSe flat plate for spectroscopic analysis, culminating in the generation of a detailed spectral graph. The spectral data acquisition spanned a wavenumber range from 600 to 4000 cm−1, adopting a resolution of 4 cm−1. The concentration of detergents in the water was determined using the solvent extraction method as described earlier.65
Determination of plant growth
The plants were harvested ten cm above the surface of the mat of the FTWs and gravel of the SSF-CWs twice a year. Shoot lengths and weights (dried) were determined as reported earlier (Rehman et al., 2019). Briefly, shoot length was determined using a scale and dried mass was determined by placing the plant material in an oven at 60°C for 72 h, where the remaining mass after the evaporation loss was considered as the dried biomass.
Phytotoxicity assay
The toxicity of the water samples was also determined as previously described (Rehman et al., 2019). Briefly, wheat (Triticum sativum) seeds were sown in Petri dishes with sterilized cotton and 3 mL of distilled water as the control and 3 mL of the water sample. After seven days, the seeds were checked for germination.
Microbial analyses
Stereoscopic microscopy was conducted to compare the microbial abundance between the initial and final wetland sections. For this analysis, sterile blades disinfected with 70% ethanol were used to harvest plant roots and shoots from samples taken from the rhizosphere. These samples were then treated with 2% bleach, followed by a triple rinse with distilled water. Each sample was ground by hand in a sterilized mortar and pestle. Three drops of a 0.9% NaCl solution were added to the resulting paste and spread onto M9 agar media plates, with diesel as sole carbon source. The plates were incubated at 37°C overnight and subsequently examined with a stereoscope to assess microbial populations.
In this study, the enumeration of hydrocarbon-degrading bacteria within the effluent water of FTWs, CWs, and SF, as well as on the rhizoplane of plants was performed using the plate count method known as colony forming units (CFU) analysis. The bacteria were isolated from the rhizoplane by vigorous shaking of the roots in a saline solution with glass beads measuring 0.1 cm in diameter. Serial dilutions of these suspensions, as well as those from the water samples, were then cultured on minimal media plates supplemented with 1% diesel as a carbon source and incubated at 37°C for bacterial growth.25
The quantification and expression levels of the alkB gene, which codes for an alkane hydroxylase involved in hydrocarbon degradation, were assessed from both water samples and rhizoplane washings. This was achieved through the extraction of DNA and RNA using commercial extraction kits, followed by the synthesis of cDNA from the RNA with reverse transcriptase. Subsequently, real-time PCR (iCycler IQ5, BioRad) was employed using the synthesized DNA and cDNA as templates to determine the abundance and expression levels of the alkB gene, in accordance with methodologies detailed in previous literature.
We also calculated the numbers of the total coliforms and Escherichia coli in the treated water samples, which could serve as indicators of potential pathogens removal upon treatment (APHA, 20025). Here, the water samples were plated on Chromocult coliform agar to determine total coliform and E. coli. The plates were incubated for 24–48 h at 37°C, and the numbers of the colonies was counted.
Cost-effectiveness
In this study, the economic and environmental implications of water recycling systems across three stations: Lyallpur, Toyota Chenab Motors, and Logistics Momentum Khanewal, were critically assessed. Initially, the annual volume of treated water post-recycling was documented for each station representing the amount of groundwater that would have been otherwise extracted. For cost evaluations, the expense to treat groundwater without recycling was determined using the product of volume (Table 3), energy required (0.8 kWh/m3), and electricity cost ($0.4/kWh). Post-implementation costs encompassed the annual maintenance and intervention costs. Derived metrics included the payback period (ratio of initial investment to net annual savings), Return on Investment (ROI = (Net Annual Savings/Initial Investment) × 100), Benefit-Cost Ratio (BCR = net savings/total costs), and Economic Value Index (EVI = Annual Groundwater Volume/Total Annual Cost). Throughout the analysis, comparisons with existing scientific literature ensured a robust contextual understanding and validation of our results.
Quantification and statistical analysis
Results presented are mean of three replicates, the standard error of three replicates is presented in parentheses.
Acknowledgments
We gratefully acknowledge the generous funding provided by Toyota Lyallpur Motors, Toyota Chenab Motors, Interloop Limited, and Bahauddin Siddiqui (Faisalabad, Pakistan) and Partners, and Higher Education Commission, Pakistan for supporting the execution of this study.
Author contributions
Conceptualization, M. Afzal and S.I.; experimentation, S.Y. and M.Y.; formal analysis, S.Y. and M.Y.; software, M. Arslan and M.T.; validation, M. Arslan, M.U., and J.A.M.; resources, M. Afzal and T.M.; original draft writing, M. Afzal, M. Arslan, and S.Y.; review and editing, J.A.M., S.I., E.I., M.A.M., and M. Arslan; funding acquisition, M. Afzal.
Declaration of interests
The authors declare no competing interests.
Published: March 6, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.109361.
Contributor Information
Muhammad Afzal, Email: manibge@yahoo.com.
Muhammad Arslan, Email: marslan@ualberta.ca.
Supplemental information
References
- 1.de Oliveira C.T., Oliveira G.G.A. What Circular economy indicators really measure? An overview of circular economy principles and sustainable development goals. Resour. Conserv. Recycl. 2023;190 doi: 10.1016/j.resconrec.2022.106850. [DOI] [Google Scholar]
- 2.Cooke S.J., Frempong-Manso A., Piczak M.L., Karathanou E., Clavijo C., Ajagbe S.O., Akeredolu E., Strauch A.M., Piccolo J. A freshwater perspective on the United Nations decade for ecosystem restoration. Conserv. Sci. Pract. 2022;4 doi: 10.1111/csp2.12787. [DOI] [Google Scholar]
- 3.Monney I., Donkor E.A., Buamah R. Clean vehicles, polluted waters: empirical estimates of water consumption and pollution loads of the carwash industry. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e03952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asha M., Chandan K., Harish H., NikhileswarReddy S., Sharath K., Liza G.M. Recycling of waste water collected from automobile service station. Procedia Environmental Sciences. 2016;35:289–297. [Google Scholar]
- 5.Lau W., Ismail A., Firdaus S. Car wash industry in Malaysia: Treatment of car wash effluent using ultrafiltration and nanofiltration membranes. Separ. Purif. Technol. 2013;104:26–31. [Google Scholar]
- 6.Lyu S., Chen W., Zhang W., Fan Y., Jiao W. Wastewater reclamation and reuse in China: Opportunities and challenges. J. Environ. Sci. 2016;39:86–96. doi: 10.1016/j.jes.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Shah W. 2022. WASA to Take Action against Service Stations for Wasting Water. [Google Scholar]
- 8.Iqbal Z., Abbas F., Ibrahim M., Mahmood A., Gul M., Qureshi T.I. Ecological risk assessment of soils under different wastewater irrigation farming system in Punjab, Pakistan. Int. J. Environ. Sci. Technol. 2022;19:1925–1936. doi: 10.1007/s13762-021-03237-x. [DOI] [Google Scholar]
- 9.Singh P., Berawala N., Patil Y. Automobile service station waste assessment and promising biological treatment alternatives: a review. Environ. Monit. Assess. 2022;194:753. doi: 10.1007/s10661-022-10387-z. [DOI] [PubMed] [Google Scholar]
- 10.Mittal Y., Srivastava P., Pandey S., Yadav A.K. Development of nature-based sustainable passive technologies for treating and disinfecting municipal wastewater: Experiences from constructed wetlands and slow sand filter. Sci. Total Environ. 2023;900 doi: 10.1016/j.scitotenv.2023.165320. [DOI] [PubMed] [Google Scholar]
- 11.Arslan M., Wilkinson S., Naeth M.A., Gamal El-Din M., Khokhar Z., Walker C., Lucke T. Performance of constructed floating wetlands in a cold climate waste stabilization pond. Sci. Total Environ. 2023;880 doi: 10.1016/j.scitotenv.2023.163115. [DOI] [PubMed] [Google Scholar]
- 12.Wu H., Wang R., Yan P., Wu S., Chen Z., Zhao Y., Cheng C., Hu Z., Zhuang L., Guo Z., et al. Constructed wetlands for pollution control. Nat. Rev. Earth Environ. 2023;4:218–234. doi: 10.1038/s43017-023-00395-z. [DOI] [Google Scholar]
- 13.Vymazal J. Constructed wetlands for wastewater treatment: five decades of experience. Environ. Sci. Technol. 2011;45:61–69. doi: 10.1021/es101403q. [DOI] [PubMed] [Google Scholar]
- 14.Brix H. Do macrophytes play a role in constructed treatment wetlands? Water Sci. Technol. 1997;35:11–17. [Google Scholar]
- 15.Arslan M. Dissertation, RWTH Aachen University; 2019. Antimicrobials in Constructed Wetlands Can Cause in Planta Dysbiosis. [DOI] [Google Scholar]
- 16.Stottmeister U., Wießner A., Kuschk P., Kappelmeyer U., Kästner M., Bederski O., Müller R.A., Moormann H. Effects of plants and microorganisms in constructed wetlands for wastewater treatment. Biotechnol. Adv. 2003;22:93–117. doi: 10.1016/j.biotechadv.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Kadlec R.H., Wallace S. CRC press; 2008. Treatment Wetlands. [Google Scholar]
- 18.Malyan S.K., Yadav S., Sonkar V., Goyal V.C., Singh O., Singh R. Mechanistic understanding of the pollutant removal and transformation processes in the constructed wetland system. Water Environ. Res. 2021;93:1882–1909. doi: 10.1002/wer.1599. [DOI] [PubMed] [Google Scholar]
- 19.Mortula M.M., Fattah K.P., Iqbal F., Khan Z. Effects of adsorption and filtration processes on greywater microbiological contamination and the potential human health risk reduction. Water Reuse. 2023;13:329–344. doi: 10.2166/wrd.2023.029. [DOI] [Google Scholar]
- 20.Ahmad J., Umar M., Akhtar K., Shah F. Effect of coagulation flocculation and sand & gravel filtration on the quality of car wash waste water. Journal of the Pakistan Institute of Chemical Engineers. 2018;45 [Google Scholar]
- 21.Asha M., Chandan K., Harish H., NikhileswarReddy S., Sharath K., Liza G.M. Recycling of waste water collected from automobile service station. Environ. Sci. 2016;35:289–297. [Google Scholar]
- 22.Baddor I.M., Farhoud N., Mohammed I., Abdel-Magid D., Alshami S., hassan Ahmad F., Asaad E. 2014. Study of Car Wash Wastewater Treatment by Adsorption; pp. 2–22. [Google Scholar]
- 23.Vymazal J., Zhao Y., Mander Ü. Recent research challenges in constructed wetlands for wastewater treatment: A review. Ecol. Eng. 2021;169 doi: 10.1016/j.ecoleng.2021.106318. [DOI] [Google Scholar]
- 24.Afzal M., Arslan M., Müller J.A., Shabir G., Islam E., Tahseen R., Anwar-ul-Haq M., Hashmat A.J., Iqbal S., Khan Q.M. Floating treatment wetlands as a suitable option for large-scale wastewater treatment. Nat. Sustain. 2019;2:863–871. [Google Scholar]
- 25.Afzal M., Rehman K., Shabir G., Tahseen R., Ijaz A., Hashmat A.J., Brix H. Large-scale remediation of oil-contaminated water using floating treatment wetlands. NPJ Clean Water. 2019;2:3. [Google Scholar]
- 26.Kumar V., Bera T., Roy S., Vuong P., Jana C., Sarkar D.J., Devi M.S., Jana A.K., Rout A.K., Kaur P., et al. Investigating bio-remediation capabilities of a constructed wetland through spatial successional study of the sediment microbiome. NPJ Clean Water. 2023;6:8. doi: 10.1038/s41545-023-00225-1. [DOI] [Google Scholar]
- 27.Zhang D.-Q., Jinadasa K.B.S.N., Gersberg R.M., Liu Y., Tan S.K., Ng W.J. Application of constructed wetlands for wastewater treatment in tropical and subtropical regions (2000–2013) J. Environ. Sci. 2015;30:30–46. doi: 10.1016/j.jes.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Arslan M., Siddique K., Muller J.A., Tahseen R., Iqbal S., Islam E., Abbasi S.A., Usman M., Gamal El-Din M., Afzal M. ACS ES&T Water; 2023. Full-Scale Floating Treatment Wetlands in Pakistan: From Performance Evaluation to Public Acceptance. [Google Scholar]
- 29.Qamar Z., Khan S., Khan A., Aamir M., Nawab J., Waqas M. Appraisement, source apportionment and health risk of polycyclic aromatic hydrocarbons (PAHs) in vehicle-wash wastewater, Pakistan. Sci. Total Environ. 2017;605–606:106–113. doi: 10.1016/j.scitotenv.2017.06.152. [DOI] [PubMed] [Google Scholar]
- 30.Ayres J., Awad J., Walker C., Page D., van Leeuwen J., Beecham S. In: Regional Perspectives of Nature-based Solutions for Water: Benefits and Challenges. Pachova N., Velasco P., Torrens A., Jegatheesan V., editors. Springer International Publishing; 2022. Constructed Floating Wetlands for the Treatment of Surface Waters and Industrial Wastewaters; pp. 35–66. [DOI] [Google Scholar]
- 31.Qiu Y., Ji Y., Tian Y., Li H., Li J., Li Z., Liao M., Liu G., Feng Y. Engineering demonstration of the remediation of urban water using a novel MES enhanced ecological floating bed: From construction to long-term performance. Chem. Eng. J. 2023;454 doi: 10.1016/j.cej.2022.140024. [DOI] [Google Scholar]
- 32.Kumar A., Mittal R.K., Goel R. CRC Press; 2023. Waste Recovery and Management: An Approach toward Sustainable Development Goals. [Google Scholar]
- 33.Bawiec A. Efficiency of nitrogen and phosphorus compounds removal in hydroponic wastewater treatment plant. Environ. Technol. 2019;40:2062–2072. doi: 10.1080/09593330.2018.1436595. [DOI] [PubMed] [Google Scholar]
- 34.Yasin M., Tauseef M., Zafar Z., Rahman M., Islam E., Iqbal S., Afzal M. Plant-Microbe Synergism in Floating Treatment Wetlands for the Enhanced Removal of Sodium Dodecyl Sulphate from Water. Sustainability. 2021;13:2883. [Google Scholar]
- 35.Davies-Colley R.J., Smith D.G. Turbidity suspeni) ed sediment, and water clarity: a review 1. J. American Water Resour. Assoc. 2001;37:1085–1101. [Google Scholar]
- 36.Borne K.E., Théron F., Andrès Y. Turbidity reduction induced by Floating Treatment Wetlands (FTW): A flume experiment to assess the impact of flow velocity. Ecol. Eng. 2021;168 doi: 10.1016/j.ecoleng.2021.106275. [DOI] [Google Scholar]
- 37.Kasenene A.J., Machunda R.L., Njau K.N. Performance of inclined plates settler integrated with constructed wetland for high turbidity water treatment. Water Pract. Technol. 2021;16:516–529. doi: 10.2166/wpt.2021.009. [DOI] [Google Scholar]
- 38.Maupin M.A., Barber N.L. US Department of the Interior, US Geological Survey; 2005. Estimated Withdrawals from Principal Aquifers in the United States, 2000. [Google Scholar]
- 39.Higgins J., Mattes A., Stiebel W., Wootton B. CRC Press; 2017. Eco-Engineered Bioreactors: Advanced Natural Wastewater Treatment. [Google Scholar]
- 40.Vymazal J. Removal of nutrients in various types of constructed wetlands. Sci. Total Environ. 2007;380:48–65. doi: 10.1016/j.scitotenv.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 41.Shukla R., Gupta D., Mishra V.K. Investigation of treatment potential of horizontal subsurface flow constructed wetland for the treatment of secondary treated sewage. Int. J. Environ. Sci. Technol. 2023;21:2965–2974. doi: 10.1007/s13762-023-05108-z. [DOI] [Google Scholar]
- 42.Kurniawan T.A., Chan G.Y.S., Lo W.-h., Babel S. Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci. Total Environ. 2006;366:409–426. doi: 10.1016/j.scitotenv.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Ijaz A., Shabir G., Khan Q.M., Afzal M. Enhanced remediation of sewage effluent by endophyte-assisted floating treatment wetlands. Ecol. Eng. 2015;84:58–66. [Google Scholar]
- 44.Smith V.H. Eutrophication of freshwater and coastal marine ecosystems a global problem. Environ. Sci. Pollut. Res. Int. 2003;10:126–139. doi: 10.1065/espr2002.12.142. [DOI] [PubMed] [Google Scholar]
- 45.Carpenter S.R. Eutrophication of aquatic ecosystems: bistability and soil phosphorus. Proc. Natl. Acad. Sci. USA. 2005;102:10002–10005. doi: 10.1073/pnas.0503959102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan W., Liu D., Tan D., Yuan P., Chen M. FTIR spectroscopy study of the structure changes of palygorskite under heating. Spectrochim. Acta Mol. Biomol. Spectrosc. 2012;97:1052–1057. doi: 10.1016/j.saa.2012.07.085. [DOI] [PubMed] [Google Scholar]
- 47.Li Y., Cai J., Song G., Ji J. DRIFT spectroscopic study of diagenetic organic–clay interactions in argillaceous source rocks. Spectrochim. Acta Mol. Biomol. Spectrosc. 2015;148:138–145. doi: 10.1016/j.saa.2015.03.131. [DOI] [PubMed] [Google Scholar]
- 48.Stavola M. Infrared spectrum of interstitial oxygen in silicon. Appl. Phys. Lett. 1984;44:514–516. [Google Scholar]
- 49.Calderón F., Haddix M., Conant R., Magrini-Bair K., Paul E. Diffuse-reflectance Fourier-transform mid-infrared spectroscopy as a method of characterizing changes in soil organic matter. Soil Sci. Soc. Am. J. 2013;77:1591–1600. [Google Scholar]
- 50.Boyekong G.O., Mengounou G.M., Nkouetcha E.T., Imano A.M. Analysis of the dielectric and physicochemical performances of thermally aged natural monoester insulating liquids. IEEE Trans. Dielectr. Electr. Insul. 2023;30:2498–2506. https://ieeexplore.ieee.org/document/10292901 [Google Scholar]
- 51.Soonmin H. Analysis of thin films by infrared spectroscopy. Ind. J. Nat. Sci. 2020;10:27593–27599. [Google Scholar]
- 52.Czarnota M., Thomas P.A. 2010. Using Surfactants, Wetting Agents, and Adjuvants in the Greenhouse. [Google Scholar]
- 53.Li X.-N., Song H.-L., Li W., Lu X.-W., Nishimura O. An integrated ecological floating-bed employing plant, freshwater clam and biofilm carrier for purification of eutrophic water. Ecol. Eng. 2010;36:382–390. [Google Scholar]
- 54.Grant S.B., Saphores J.-D., Feldman D.L., Hamilton A.J., Fletcher T.D., Cook P.L.M., Stewardson M., Sanders B.F., Levin L.A., Ambrose R.F., et al. Taking the “waste” out of “wastewater” for human water security and ecosystem sustainability. Sci. Technol. Humanit. 2012;337:681–686. doi: 10.1126/science.1216852. [DOI] [PubMed] [Google Scholar]
- 55.Qadir M., Wichelns D., Raschid-Sally L., McCornick P.G., Drechsel P., Bahri A., Minhas P. The challenges of wastewater irrigation in developing countries. Agric. Water Manag. 2010;97:561–568. [Google Scholar]
- 56.Famiglietti J.S. The global groundwater crisis. Nat. Clim. Change. 2014;4:945–948. [Google Scholar]
- 57.Poff N.L., Brown C.M., Grantham T.E., Matthews J.H., Palmer M.A., Spence C.M., Wilby R.L., Haasnoot M., Mendoza G.F., Dominique K.C., Baeza A. Sustainable water management under future uncertainty with eco-engineering decision scaling. Nat. Clim. Change. 2016;6:25–34. [Google Scholar]
- 58.Johnson K.A., Wing O.E.J., Bates P.D., Fargione J., Kroeger T., Larson W.D., Sampson C.C., Smith A.M. A benefit–cost analysis of floodplain land acquisition for US flood damage reduction. Nat. Sustain. 2019;3:56–62. [Google Scholar]
- 59.Onyeaka H., Tamasiga P., Nwauzoma U.M., Miri T., Juliet U.C., Nwaiwu O., Akinsemolu A.A. Using artificial intelligence to tackle food waste and enhance the circular economy: Maximising resource efficiency and Minimising environmental impact: A review. Sustainability. 2023;15 [Google Scholar]
- 60.Qiu Y., Schertzer D., Tchiguirinskaia I. Assessing cost-effectiveness of nature-based solutions scenarios: Integrating hydrological impacts and life cycle costs. J. Clean. Prod. 2021;329 doi: 10.1016/j.jclepro.2021.129740. [DOI] [Google Scholar]
- 61.Hapuwatte B.M., Jawahir I.S. Closed-loop sustainable product design for circular economy. J. Ind. Ecol. 2021;25:1430–1446. doi: 10.1111/jiec.13154. [DOI] [Google Scholar]
- 62.Hunjra A.I., Hassan M.K., Zaied Y.B., Managi S. Nexus between green finance, environmental degradation, and sustainable development: Evidence from developing countries. Resour. Pol. 2023;81 doi: 10.1016/j.resourpol.2023.103371. [DOI] [Google Scholar]
- 63.Kuppan P., Sudharsanam A., Venkateswarlu K., Megharaj M. Solar technology‒closed loop synergy facilitates low-carbon circular bioeconomy in microalgal wastewater treatment. NPJ Clean Water. 2023;6:43. doi: 10.1038/s41545-023-00256-8. [DOI] [Google Scholar]
- 64.Lehmann S. In: Urban Energy Transition. Second Edition. Droege P., editor. Elsevier; 2018. 2.10 - Conceptualizing the Urban Nexus Framework for a Circular Economy: Linking Energy, Water, Food, and Waste (EWFW) in Southeast-Asian cities; pp. 371–398. [DOI] [Google Scholar]
- 65.Motomizu S., Fujiwara S., Fujiwara A., Toei K. Solvent extraction-spectrophotometric determination of anionic surfactants with ethyl violet. Anal. Chem. 1982;54:392–397. doi: 10.1021/ac00240a011. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data: No new data were generated.
Code: No codes were generated.
Any additional information is available from the lead contact upon request.