Abstract
To investigate the involvement of various cellular and humoral aspects of immunity in the clearance of rabies virus from the central nervous system, (CNS), we studied the development of clinical signs and virus clearance from the CNS in knockout mice lacking either B and T cells, CD8+ cytotoxic T cells, B cells, alpha/beta interferon (IFN-α/β) receptors, IFN-γ receptors, or complement components C3 and C4. Following intranasal infection with the attenuated rabies virus CVS-F3, normal adult mice of different genetic backgrounds developed a transient disease characterized by loss of body weight and appetite depression which peaked at 13 days postinfection (p.i.). While these animals had completely recovered by day 21 p.i., mice lacking either B and T cells or B cells alone developed a progressive disease and succumbed to infection. Mice lacking either CD8+ T cells, IFN receptors, or complement components C3 and C4 showed no significant differences in the development of clinical signs by comparison with intact counterparts having the same genetic background. However, while infectious virus and viral RNA could be detected in normal control mice only until day 8 p.i., in all of the gene knockout mice studied except those lacking C3 and C4, virus infection persisted through day 21 p.i. Analysis of rabies virus-specific antibody production together with histological assessment of brain inflammation in infected animals revealed that clearance of CVS-F3 by 21 days p.i. correlated with both a strong inflammatory response in the CNS early in the infection (day 8 p.i.), and the rapid (day 10 p.i.) production of significant levels of virus-neutralizing antibody (VNA). These studies confirm that rabies VNA is an absolute requirement for clearance of an established rabies virus infection. However, for the latter to occur in a timely fashion, collaboration between VNA and inflammatory mechanisms is necessary.
Immune defense against viral infections of the central nervous system (CNS) is limited by the blood-brain barrier as well as by constraints on the expression in the CNS of essential elements of immunity. Nevertheless, viral infections of the CNS are often contained, likely by the cooperative action of diverse effectors of immunity including soluble factors, antibody, and cytotoxic T cells. Of the many soluble factors involved in the generation and control of immune responses, the type 1 interferons (IFNs) are important contributors to defense against virus infection due to their direct antiviral activity. It is also evident that both type 1 and type 2 IFNs collaborate in the cell-mediated antiviral response, as demonstrated by the fact that mice lacking IFN-α, -β, and -γ receptors are unable to mount a cytotoxic T-lymphocyte response to lymphocytic choriomeningitis virus (LCMV), which results in persistence of the virus (22). An important role for IFN-γ in antiviral defense in the CNS has been confirmed in a variety of other systems. For example, neutralization of IFN-γ impairs the clearance of measles virus from the CNS (8) and increases demyelination in the spinal cord induced by Theiler’s virus (16). A major influence of IFN-γ is on cellular immunity. The CD8+ T effector cells of this arm of the immune response have been shown to be effective in reducing virus titers in the brain after experimental infection with coronavirus (19), Theiler’s virus (13), and LCMV (7, 14).
While cellular immunity and IFNs may reduce virus load, virus-specific antibody, and particularly virus-neutralizing antibody (VNA), plays an essential role in the control of most, if not all, virus infections of the CNS. For example, treatment of LCMV-infected mice with a virus-neutralizing monoclonal antibody (MAb) could suppress virus replication and protect against infection (26). Furthermore, treatment of rabies virus-infected rats with a virus-neutralizing MAb protected the animals against a lethal infection and cleared the virus from the CNS (3). Antibodies also contribute to the recovery from infection with Theiler’s virus (16) and reduce the virus load in SCID mice persistently infected with Sindbis virus (12). It is clear that virus-specific antibodies are essential for the elimination of free virus. In addition, antibody may participate in the removal of virus-infected cells through antibody-dependent cell-mediated cytotoxicity or complement-dependent lysis (1). In the case of the CNS, where cytolytic mechanisms would likely have devastating effects on neural function, recent studies suggest that antibody may participate in the elimination of virus from infected cells in the absence of significant cell destruction. Several mechanisms to explain how this may occur have been proposed (2, 3, 12). Regardless of the mechanisms involved in antibody-mediated virus clearance, enabling antibody and possibly other necessary effector cells and molecules access to the CNS, as opposed to other sites, requires crossing the blood-brain barrier. We speculate that the interaction of several immune functions is therefore a prerequisite for virus clearance from the CNS.
It is well known that infection of humans with the highly neurotrophic rabies virus is lethal in the absence of postexposure prophylaxis which, to be efficacious, must consist of both the administration of rabies virus-specific VNA and active immunization against rabies virus. Based on the fact that rabies virus quickly enters the CNS in animal models (20), it is very likely that the virus also reaches the CNS in infected humans prior to treatment which is often given some days after exposure. We therefore consider that active immunization in rabies postexposure prophylaxis provides an element of immunity, distinct from VNA production, which is essential for the clearance of rabies virus from the CNS. To test this hypothesis, we have chosen a model system based on the infection of adult mice with the attenuated rabies virus variant CVS-F3, as infection of nonimmune mice with other laboratory or street rabies virus strains is rapidly lethal and preimmunization evidently prevents virus spread to the CNS. By comparison with the highly neuropathogenic wild-type CVS-24 rabies virus, which is virtually the same antigenically, the CVS-F3 variant has a single mutation at amino acid 333 which is reflected in reduced cell-cell spread in vitro and limited replication in the CNS of immunocompetent mice (5). To delineate the immune effector mechanisms involved in containing or clearing CVS-F3 infection from the CNS, we have compared the course of CVS-F3 infection and the development of immunity in gene knockout (k.o.) mice lacking either B and T cells, CD8+ T cells, B cells, IFN-α/β receptors (IFN-α/βR) IFN-γR, or complement components C3 and C4 as well as their normal counterparts. Intranasal instillation of virus was selected as the means of infection, as this provides ready access to the CNS while minimizing any contribution to immunity from local responses triggered by invasive inoculation procedures. In this regard, it should be noted that aerosol transmission of rabies virus to humans has been documented (24) and has not been excluded as a route of infection in recent cases of cryptic rabies in the United States.
MATERIALS AND METHODS
Mice.
β2-Microglobulin (β2m) k.o. mice (C57BL/6J-B2m), which fail to express major histocompatibility complex (MHC) class I antigens and as a result are deficient in CD4− CD8+ T cells (11), as well as control C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, Maine). Rag-2 k.o. mice, which are T- and B-cell deficient (129/SvEv Tac/BR-[KO] rag 2), and the corresponding congenic control 129/SvEv mice were purchased from Taconic (Germantown, N.Y.). Mice lacking IFN-α/βR (IFN-α/βR k.o.) or IFN-γR (IFN-γR k.o.) were kindly provided by Michel Aguet (Swiss Institute for Experimental Cancer Research, Lausanne, Switzerland). Both IFN-α/βR k.o. and IFN-γR k.o. mice have a 129/SvEv genetic background. Antibody-deficient (JHD k.o.) mice on a C57BL/6J background were provided by Randy Hardy (Fox Chase Cancer Center, Philadelphia, Pa.). C3/C4 k.o. mice, which lack the complement components C3 and C4 (genetic background C57BL/6J), were provided by Michael Carroll (Harvard Medical School, Boston, Mass.). All mice were maintained under pathogen-free conditions and used at 8 to 10 weeks of age.
Virus infection of mice.
The rabies virus escape mutant CVS-F3, which has an arginine-to-glutamine substitution at amino acid position 333 of the G protein and is nonpathogenic for immunocompetent mice when administered via the oral, intramuscular, or intracranial route (6), was propagated in BHK-21 cells.
Groups of 15 8- to 10-week-old normal control mice or gene k.o. mice were infected intranasally (i.n.) under anesthesia with 10 μl of phosphate-buffered saline (PBS) containing 105 focus-forming units (FFU) of CVS-F3. Following infection, the mice were examined for appearance of clinical signs of disease, and their weights were recorded on a daily basis.
Virus isolation and virus titration.
At different times after i.n. infection, mice were sacrificed, their brains were removed, and a 20% brain suspension in PBS was prepared. To determine the virus yield, monolayers of mouse neuroblastoma cells in 96-well plates were infected with 50 μl of brain suspension at serial 10-fold dilutions and incubated for 1 h at 37°C to allow for virus adsorption. The virus inoculum was then removed, and the cultures were replenished with 100 μl of culture medium, and incubated at 34°C. Forty-eight hours postinfection (p.i.), the cells were fixed in 80% acetone and subjected to a fluorescent staining technique with rabies N-protein-specific antibody (23). Foci were counted using a fluorescence microscope. All titrations were carried out in triplicate.
RNA extraction, RT-PCR, and Southern blot analysis.
Total RNA was isolated from CVS-F3-infected mouse brains according to the manufacturer’s manual for the RNAzol B method (Biotecx Laboratories, Inc., Houston, Tex.). Reverse transcription (RT) reactions were performed at 42°C for 1 h, using avian myeloblastosis virus reverse transcriptase (Promega, Madison, Wis.) as previously described (21). To examine for the presence of rabies virus genomic RNA, total mouse brain RNA (3 μg) and 1 μM rabies virus-specific primer C5-a (5-CTTCACTCAAGGGTCTTC-3) were used in the RT reaction. A portion of the RT product was subjected to PCR amplification using primers C5-a and C3-a (5-TTGTTGAAGTTCACCTCC-3). Amplification was carried out for 35 cycles (denaturation at 94°C for 1 min, annealing at 50°C for 1 min, and polymerization at 72°C for 1 min), with Taq DNA polymerase (Fisher Scientific, Pittsburgh, Pa.), as described previously (21). As an internal control of RNA preparations, a 348-bp segment of glyceraldehyde-3-phosphate dehydrogenase (G3PDH) mRNA was also amplified by using antisense primer 5-GGCCATGAGGTCCACCACCCTGTT-3 and sense primer 5-TGCCAAGGCTGTGGGCAAGGTCAT-3. The PCR products were electrophoresed on 1% agarose gel (Sigma, St. Louis, Mo.). G3PDH-specific PCR products were detected by ethidium bromide staining. Rabies virus-specific PCR products were blotted onto GeneScreen Plus membrane (DuPont, Boston, Mass.), hybridized with 32P-labeled internal oligonucleotide probe C5-c (5-TCCATCATGACCACCAAGTC-3), and exposed to autoradiography film. Oligonucleotide probes were labeled with [γ-32P]ATP (specific activity, 4,500 Ci/mmol; ICN Pharmaceuticals, Inc., Irvine, Calif.), using T4 polynucleotide kinase (Promega).
Determination of VNA and rabies-specific antibody isotypes.
Ten and 20 days p.i. blood was collected from five animals of each experimental group. The mouse sera were heat inactivated at 56°C for 30 min, and neutralizing activity was determined as described previously (25). The isotypes of rabies virus-specific antibodies made by the various mouse strains were assessed in direct enzyme-linked immunosorbent assay (ELISA) using β-propriolactone-inactivated ERA rabies virus as the trapping antigen as previously described (27). Isotype-specific antibodies were either alkaline phosphatase conjugated (immunoglobulin G [IgG; Cappel], IgG1 [Pharmingen], IgG2a [Cappel], IgG2b [Cappel], and IgG3 [Cappel]) or horseradish peroxidase-conjugated (IgM [Sigma]). p-Nitrophenyl phosphate substrate (Sigma) was used for color development of alkaline phosphatase-conjugated antibodies, and activity was read spectrophotometrically at 405 nm. 3,3′,5,5′-Tetramethylbenzidine dihydrochloride substrate (Sigma) was used for color development of the peroxidase-conjugated antibody, and activity was read spectrophotometrically at 450 nm.
Immunohistochemical and histological analysis.
At different times after infection, mice were anesthetized and perfused transcardially with PBS containing procain-HCl (5 g/liter) and heparin (20,000 IU/liter) followed by Bouin-Hollande fixation solution (28). Brains were removed and postfixed for 24 h in the same fixative. After dehydration in a graded series of 2-propanal, tissues were embedded in Paraplast Plus (Merck, Darmstadt, Germany) and cut into 7-μm-thick coronal sections. Histological analysis was performed on coronal sections through the hippocampus. For immunohistochemical analysis, the sections were incubated with a monospecific rabbit antibody which recognizes rabies virus ribonucleoprotein. Reactions were visualized with biotinylated sheep anti-rabbit IgG and streptavidin-peroxidase (Amersham), using the nickel-enhanced diaminobenzidine reaction described previously (28). For histology and examination, sections were stained with hematoxylin-eosin.
RESULTS
Development of clinical signs in normal and gene k.o. mice infected with CVS-F3.
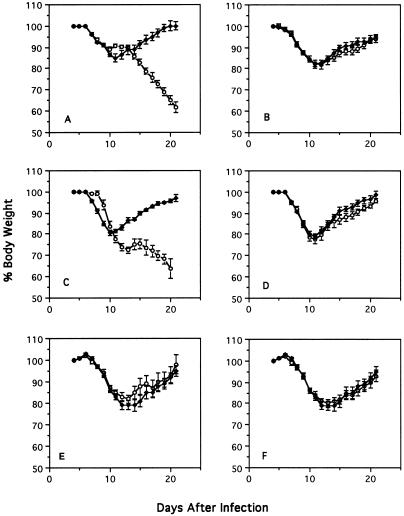
Intramuscular and intracranial infection of normal adult mice with CVS-F3 elicits the rapid production of high VNA titers in the absence of any overt signs of disease (data not shown). In contrast, i.n. inoculation of normal adult mice of different genetic backgrounds with CVS-F3 causes a transient disease characterized by appetite depression and body weight loss (Fig. 1). In the experiment shown in Fig. 1, normal adult 129/SvEv (Fig. 1A, E, and F) and C57BL/6J (Fig. 1B to D) mice exhibited weight loss which peaked 11 to 12 days following i.n. infection with CVS-F3 (105 FFU). While these animals recovered and almost completely regained their original body weight by day 21 p.i., mice lacking B and T lymphocytes (rag-2 k.o. [Fig. 1A]) and mice lacking B lymphocytes (JHD k.o. [Fig. 1C]) developed a progressive disease and succumbed to infection between 21 and 24 days p.i. On the other hand, mice lacking either CD8+ T lymphocytes (β2m k.o. [Fig. 1B]), the complement components C3 and C4 (C3/C4 k.o. [Fig. 1D]), IFN-α/βR (IFN-α/βR k.o. [Fig. 1E), or IFN-γR (IFN-γR k.o. [Fig. 1F]) showed no significant differences in the course of clinical disease by comparison with immunologically intact mice having the same genetic background.
FIG. 1.
Effect of i.n. infection with CVS-F3 on body weight of mice with different gene defects. Normal 129/SvEv (A, E, and F) and C57BL/6J (B to D) mice (⧫) and the gene k.o. mice (○), rag-2 k.o. (A), β2m k.o. (B), JHD k.o. (C) C3/C4 k.o. (D), IFN-α/βR k.o. (E), and IFN-γR k.o. (F), were infected i.n. with CVS-F3 as described in Materials and Methods. Mice were weighed on a daily basis. Individual body weights were transformed into percentages, taking the weight at day 5 p.i. as 100%. The results are expressed as mean plus standard error of the mean percent body weight of 15 mice per group.
Clearance of infectious virus from the brain.
To examine the fate of CVS-F3 virus in the brain after i.n. infection, brain suspensions were prepared 8, 13, and 21 days p.i. from two mice at each time point and tested for the presence of infectious virus. Table 1 shows that at day 8 p.i., infectious virus was present in the brains of all normal as well as all gene k.o. mice. However, by days 13 and 21 p.i., infectious virus remained detectable only in the brains of rag-2 k.o. and JHD k.o. mice. Nevertheless, while virus titers in rag-2 k.o. mice increased approximately 10-fold between days 13 and 21 days p.i., virus titers in the brains of the JHD k.o. mice remained constant over the entire time period examined. All mice lacking the capacity to make antibody died between days 21 and 24 p.i.
TABLE 1.
Virus titers in mouse brains following i.n. infection with CVS-F3
| Mouse strain | Virus titer (FFU/g of brain)a at indicated days p.i.
|
|||||
|---|---|---|---|---|---|---|
| 8
|
13
|
21
|
||||
| 1 | 2 | 1 | 2 | 1 | 2 | |
| Rag-2 k.o. | 5.0 × 105 | 1.0 × 105 | 2.0 × 105 | 2.5 × 105 | 1.0 × 106 | 5.0 × 106 |
| β2m k.o. | 7.5 × 103 | 1.8 × 104 | <10 | <10 | <10 | <10 |
| JHD k.o. | 2.0 × 104 | 5.0 × 104 | 1.5 × 104 | 5.0 × 104 | 5.0 × 104 | 5.0 × 103 |
| C3/C4 k.o. | 1.5 × 104 | 1.0 × 105 | <10 | <10 | <10 | <10 |
| IFN-α/βR k.o. | 2.5 × 105 | 2.5 × 105 | <10 | <10 | <10 | <10 |
| IFN-γR k.o. | 4.0 × 105 | 5.0 × 104 | <10 | <10 | <10 | <10 |
| C57BL/6J | 1.0 × 105 | 1.5 × 105 | <10 | <10 | <10 | <10 |
| 129/SvEv | 7.5 × 103 | 2.6 × 103 | <10 | <10 | <10 | <10 |
Determined in two mice (1 and 2) for each time point and mouse strain.
Clearance of viral RNA and viral antigen from the brain.
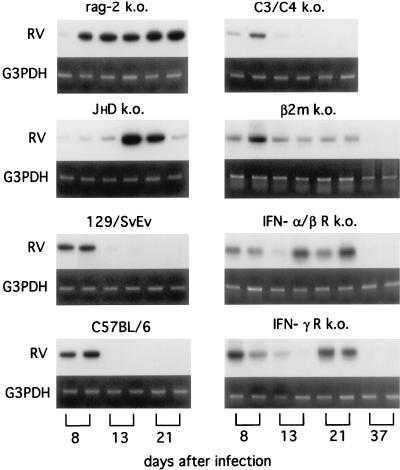
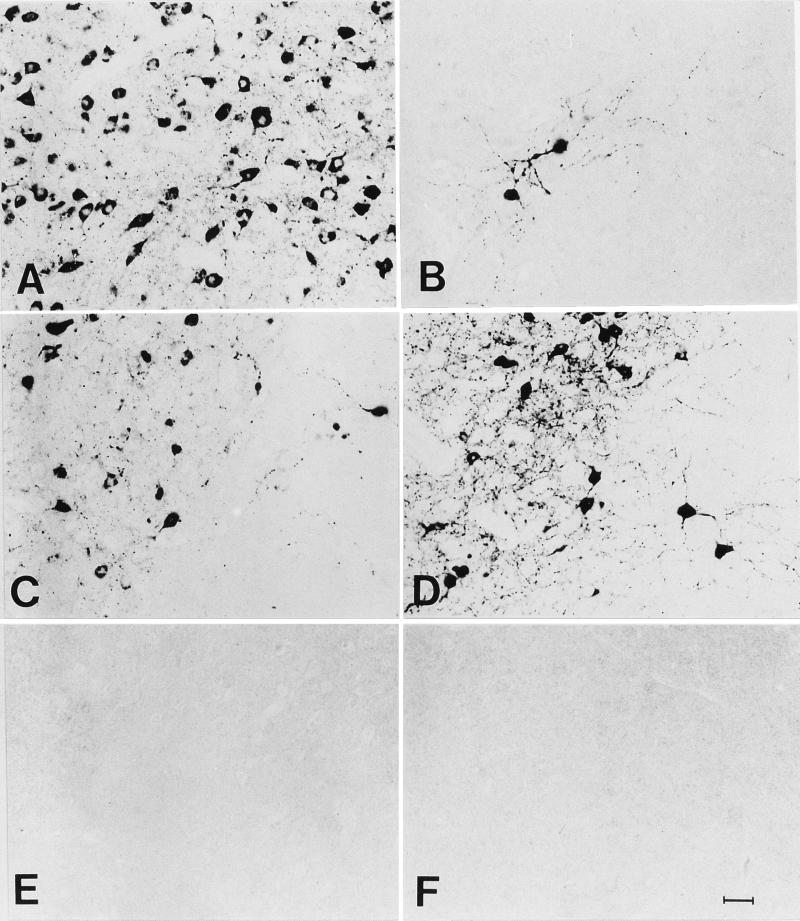
It is conceivable that the development of a neutralizing antibody response approximately 1 week after infection may interfere with the subsequent isolation of infectious virus from brain tissue, thereby leading to the false perception that virus had been completely eliminated. To control against this possibility, brain tissue was analyzed for the presence of viral RNA or viral antigens by using RT-PCR analysis and immunohistochemistry. As is evident from the results of RT-PCR analysis shown in Fig. 2, genomic rabies virus RNA, like infectious virus, was present in the brains of all normal and gene k.o. mice 8 days p.i. By day 13 p.i., viral RNA had been cleared from the brains of C57BL/6J, 129/SvEv, and C3/C4 k.o. mice but could still be detected until day 21 p.i. in the brains of rag-2 k.o., JHD k.o., β2m k.o., IFN-α/β R k.o., and IFN-γ R k.o. mice. However, viral RNA could no longer be detected in the brains of all surviving mice 37 days p.i. These differences in the rate of clearance of all evidence of rabies virus infection from the brain are supported by the results of immunohistochemical analysis for rabies virus N protein in the brain. N protein could be detected in brain sections from all normal and gene k.o. mice at 8 and 13 days p.i. (Fig. 3) but by day 21 p.i. was completely absent from the brains of normal C57BL/6J (Fig. 3P), 129/SvEv (Fig. 3S), and C3/C4 k.o. (data not shown) mice. Like viral RNA, rabies virus N protein could be readily detected at low magnification at day 21 in brain sections prepared from rag-2 k.o. (Fig. 3C) and at a lower level in sections from JHD k.o. (Fig. 3I), β2m k.o. (Fig. 3F), and IFN-γR k.o. (Fig. 3M) mice. Analysis of the sections shown in Fig. 3C, F, I, M, P, and S at high magnification (Fig. 4) confirms this pattern, clearly demonstrating the presence of rabies antigen-positive neurons at 21 days p.i. in rag-2 k.o. (Fig. 4A), β2m k.o. (Fig. 4B), JHD k.o. (Fig. 4C), and IFN-γR k.o. (Fig. 4D) mice but not in sections from C57BL/6J (Fig. 4E) or 129/SvEv (Fig. 4F) mice.
FIG. 2.
RT-PCR analysis of rabies virus genomic RNA in brain tissue from mice infected i.n. with CVS-F3. Brains from two mice of each of the indicated strains were collected at 8, 13, 21, and 37 days after i.n. infection with CVS-F3, and RNA was extracted and subjected to RT-PCR analysis for rabies virus genomic RNA (RV) as described in Materials and Methods. Amplification of G3PDH mRNA served as an internal control.
FIG. 3.
Immunohistochemical analysis for rabies virus N protein in coronal sections through the hippocampus of rag-2 k.o. (A to C), β2m k.o. (D to F), JHD k.o. (G to I), IFN-γR k.o. (K to M), C57BL/6J (N to P), and 129/SvEv (R and S) mice infected with CVS-F3. Also shown is a section from a noninfected 129/SvEv mouse (Q). Mice were sacrificed at 8 (A, D, G, K, and N), 13 (B, E, H, L, O, and R), and 21 (C, F, I, M, P, and S) days p.i., and brain sections were prepared and stained with antibodies specific for rabies virus N protein as described in Materials and Methods. Arrows indicate N-protein-positive cells. Areas shown at higher magnification in Fig. 4 are marked by asterisks. Bar = 500 μm.
FIG. 4.
Immunohistochemical analysis for rabies virus N protein in coronal sections through the hippocampus of rag-2 k.o. (A), β2m k.o. (B), JHD k.o. (C), IFN-γ R k.o. (D), C57BL/6J (E), and 129/SvEv (F) mice 21 days following infection with CVS-F3. Areas from sections marked by asterisks in Fig. 3 are shown at higher magnification (bar = 25 μm).
Production of virus-specific antibodies after infection with CVS-F3.
Clearance of rabies virus from the brain is well known to be dependent on the presence of VNA. It is therefore not surprising that rag-2 and JHD k.o. mice, which cannot make VNA (Table 2), do not clear CVS-F3 and eventually succumb to the infection. With the exception of rag-2 and JHD k.o. mice, all mouse strains tested developed high VNA titers by day 21 p.i. (Table 2). However, the development of the VNA response was apparently delayed in IFN-α/βR k.o. and IFN-γR k.o. mice, since at day 10 p.i., VNA titers in these animals were approximately 10 times lower than in fully immunocompetent controls. Surprisingly, despite failing to clear the virus infection by day 13, β2m k.o. mice developed titers of neutralizing antibodies which were by 10 days p.i. higher than those seen in the controls. Thus, in addition to the quantity of VNA produced another factor, conceivably the quality of the antibodies, evidently plays a major role in virus clearance. As antibodies with different isotypes differ functionally, it may be that the ability of VNA to clear rabies virus from the CNS is highly dependent on their isotype. Since the pattern of T-cell cytokines made in response to infection dictates the classes of antibodies produced, it is likely that several of the gene k.o. mouse strains studied in this investigation differ from intact animals in the array of rabies virus-specific antibodies produced in response to infection. This may culminate in differences in the ability to clear the virus from the brain. Significant differences were seen between the strains in the overall magnitude of their rabies virus-specific antibody response as well as in the profile of rabies virus-specific antibody isotypes produced during infection with CVS-F3 (Table 3). Genetic background evidently contributes to the antibody response to rabies CVS-F3 infection, as 129/SvEv mice made low levels of IgM rabies virus-specific antibodies but very high levels of antibodies of the IgG2a, IgG2b, and IgA isotypes, while C57/BL6 mice made high levels of IgM, low to moderate levels of IgG2b, and little IgG2a or IgA (Table 3). The patterns of rabies virus-specific antibody isotypes produced by IFN-α/βR and IFN-γR k.o. mice were similar in having elevated levels of IgM and IgG1 but lower levels of IgG2a, IgG2b, and IgA by comparison with the background 129/SvEv strain. While both C57BL/6J and congenic β2m k.o. mice produced predominantly IgM antibodies in response to infection with CVS-F3, the low to moderate levels of rabies virus-specific IgG2a, IgG2b, and IgA antibodies seen in C57BL/6J mice were virtually absent from the β2m k.o. mice.
TABLE 2.
Titers of VNA from mice infected i.n. with CVS-F3
| Mouse strain | VNA geometric mean titera (range)
|
|
|---|---|---|
| 10 days p.i. | 20 days p.i. | |
| Rag-2 k.o. | 0 | 0 |
| β2m k.o. | 1,265 (810–2430) | 3,028 (1,800–8,100) |
| JHD k.o. | 0 | 0 |
| IFN-α/βR k.o. | 44 (30–90) | 4,446 (1,800–16,200) |
| IFN-γR k.o. | 68 (30–270) | 6,005 (2,700–8,100) |
| C57BL/6J | 581 (135–2,430) | 1466 (600–1,800) |
| 129/SvEv | 455 (270–810) | 16236 (5,460–48,600) |
Geometric mean of the reciprocal titer (reciprocal of the highest serum dilution yielding a 50% reduction in test virus, as determined by the fluorescent-focus inhibition assay) of five mice.
TABLE 3.
Distribution of isotypes of rabies virus-specific antibody elicited by infection with CVS-F3
| Isotype | Geometric mean rabies virus-specific antibody titera in indicated mouse strain
|
||||
|---|---|---|---|---|---|
| 129/SvEv | IFN-α/βR k.o. | IFN-γR k.o. | C57BL/6J | β2m k.o. | |
| IgM | 5,649 (2,440–9,760) | 34,995 (17,920–71,680) | 37,670 (40,960–110,080) | 29,512 (25,320–32,805) | 21,135 (5,603–38,641) |
| IgG1 | 724 (0–40,960) | 40,551 (21,760–117,760) | 16,143 (3,520–125,440) | 29 (0–640) | 3 (0–130) |
| IgG2a | 444,631 (102,400–1,392,640) | 11,272 (9,122–24,762) | 45,920 (23,680–140,760) | 1,148 (586–1,813) | 92 (55–340) |
| IgG2b | 95,499 (27,520–547,840) | 40,738 (9,880–94,720) | 62,517 (29,440–209,920) | 7,244 (2,141–29,789) | 772 (480–2,382) |
| IgG3 | 0 (0–40) | 2 (0–110) | 0 | 1 (0–568) | 0 |
| IgA | 171,396 (38,560–7,536,640) | 17,219 (6,880–33,280) | 26,546 (3,040–79,360) | 876 (370–2,320) | 99 (0–5,920) |
Determined 20 days p.i. as the inverse of the last dilution of serum in ELISA which gave an optical density greater than twice the background optical density of wells with secondary isotype-specific antibody but without serum. Groups consisted of five mice each. Numbers in parentheses represent the range of antibody titers found within each group of animals. IFN-α/βR and IFN-γR k.o. mice are on a 129SvEv background, while β2m k.o. mice are on a C57/BL6 background.
Development of CNS inflammation in CVS-F3-infected mice.
While differences in the ability to clear CVS-F3 from the brain may be the consequence of variability in VNA production, it is also conceivable that differences in a local cellular response, reflected by the level of cell infiltration into the CNS, are involved. To investigate this possibility, we examined coronal sections through the hippocampus for the presence of cellular infiltrates which are usually highest in the hippocampal fissure. Examination of brain sections from C57BL/6J mice (Fig. 5N and O) as well as from 129/SvEv mice (Fig. 5R) revealed massive cell infiltration in the hippocampal fissure between days 10 and 13 p.i. At day 21 p.i., when viral RNA could no longer be detected (Fig. 2), inflammation was also nearly completely resolved (Fig. 5P and S), with histological sections resembling those of uninfected controls (Fig. 5Q). In β2m k.o. mice, a significant inflammatory reaction developed in this area (Fig. 5D to F), but its appearance was delayed with respect to that seen in control C57BL/6J mice. In contrast, examination of matching areas in brain sections from rag-2 k.o. (Fig. 5A to C), JHD k.o. (Fig. 5G to I), and IFN-γR k.o. (Fig. 5K to M) mice revealed no inflammatory processes at day 8 p.i. and either little or no inflammation at days 13 and 21.
FIG. 5.
Histological analysis of coronal sections through the hippocampus of rag-2 k.o. (A to C), β2m k.o. (D to F), JHD k.o. (G to I), IFN-γR k.o. (K to M), C57BL/6J (N to P), and 129/SvEv (R and S) mice infected with CVS-F3. Also shown is a section from a noninfected 129/SvEv mouse (Q). Mice were sacrificed at 8 (A, D, G, K, and N), 13 (B, E, H, L, O, and R), and 21 (C, F, I, M, P, and S) days p.i., and brain sections were prepared and stained with hematoxylin-eosin as described in Materials and Methods. Panels show matching areas of the hippocampal fissure of different mice, magnified approximately 40-fold. Bar = 25 μm.
DISCUSSION
Our results clearly demonstrate that i.n. infection of mice with the attenuated rabies virus CVS-F3 leads to replication of the virus in the CNS which is accompanied by signs of mild disease even in fully immunocompetent mice. While infectious virus, viral antigen, and viral RNA could be readily demonstrated in the CNS of all of the mouse strains tested 8 days after i.n. infection, no evidence of rabies infection remained detectable in immunocompetent C57BL/6 and 129/SvEv mice by 13 days p.i. As expected, a rabies virus-specific antibody response is an absolute requirement for recovery from disease caused by CVS-F3, as mice lacking B cells do not clear the virus and succumb to the infection. However, the production of VNA alone, regardless of isotype of the antibodies, was not sufficient for the rapid elimination of virus from the CNS. β2m k.o. mice produced higher levels of VNA than fully immunocompetent congenic C57/BL6 mice yet cleared the virus in a considerably delayed fashion, as shown by immunohistochemistry. Unlike C57/BL6 mice, β2m k.o. mice failed to produce significant titers of IgG2a, IgG2b, and IgA rabies virus-specific antibodies. On the other hand, in response to CVS-F3 infection IFN-α/βR k.o. and IFN-γR k.o. mice were slow to produce VNA but eventually made much higher levels of IgG1, IgG2a, IgG2b, as well as IgA isotype antibodies, than C57/BL6 mice. With respect to their 129/SvEv congenic control mice, IFN-α/βR k.o. and IFN-γR k.o. mice produced substantially lower levels of IgG2a and IgA but higher levels of IgM, and IgG1 in response to CVS-F3 infection. Only relatively minor differences were observed in the levels of rabies virus-specific IgG2b produced by these mice. These findings do not provide any clear basis to consider that elimination of rabies virus from the CNS is solely due to the production of rabies virus-specific antibody of any particular isotype. Another element of immune responsiveness to rabies virus must collaborate with antibody in virus clearance and be responsible for the observed differences between the strains studied. This is unlikely to involve either the classical or alternative complement cascades, as mice unable to express the central complement components, C3 and C4, recovered from disease and cleared the virus like normal controls. Together with the observation that depletion of C3 with cobra venom factor had no effect on the clearance of Sindbis virus from infected mouse brain (12), these findings are consistent with the hypothesis that antibody-mediated clearance of virus from the CNS is not dependent on the participation of the better-known complement cascades. This does not exclude the possibility of a contribution from complement component C1, which is strongly upregulated in microglia during virus-induced CNS inflammation (4).
The finding that the infectious virus load increases between days 13 and 21 p.i. in mice lacking both T cells and antibody (rag-2 k.o.), but remains constant from day 8 p.i. until the animals’ death in at least some mice lacking antibody alone (JHD k.o.), argues that there is a contribution to the antiviral response from T cells in addition to their important role in helping antibody production. In this regard, it is noteworthy that mice lacking β2m and, consequently functional CD8+ T cells, were also slow to clear CVS-F3 from their CNS, with evidence of infection remaining detectable 21 days p.i. despite the presence of high levels of VNA in these animals from at least 10 days p.i. Nevertheless, the fact that the virus is cleared from the CNS in the absence of functional CD8+ cells indicates that these cells are not necessary for the clearance of CVS-F3. However, the presence of CD8+ cells clearly accelerates the elimination of virus, possibly through the production of soluble factors, as has been previously suggested for LCMV (14). It is conceivable that due to the low levels of MHC class II antigens expressed in the brain, MHC class I-restricted CD8+ T cells are required for the early production of cytokines at the site of infection where relatively high levels of antigen are available to stimulate the immune response. This possibility is supported by the finding that the absence of functional CD8+ T cells during infection with rabies CVS-F3 is reflected in the failure to produce significant titers of rabies virus-specific antibodies of the IgG2a and IgG2b isotypes. Because of the known role of IFN-γ in isotype switching to IgG2a (22), low levels of this class of antibody likely are the result of inadequate production of IFN-γ. Further evidence that IFN-γ production may be limited in β2m k.o. mice comes from the observation that CNS inflammation in response to CVS-F3 infection is delayed in these animals. A strong CNS inflammatory response, which is well known to be dependent on IFN-γ, was first seen in β2m k.o. mice some 5 days after its appearance in congenic C57BL/6J mice. Based on these results and the absence of a clear correlation between rabies virus clearance and the presence of antibodies of any particular isotype, we conclude that CD8+ T cells likely contribute to the antibody-mediated clearance of rabies virus from the CNS by enhancing IFN-γ production and the CNS inflammatory response. Whether cytolytic destruction of infected neurons by CD8+ T cells plays any role remains to be discerned.
The possibility that T-cell-mediated, IFN-γ-dependent CNS inflammation contributes to the clearance of rabies virus from the CNS is supported by the fact that mice with a targeted defect in the expression of receptors for IFN-γ showed no marked CNS inflammation at either 8, 13, or 21 days p.i. and failed to clear the virus within 21 days p.i. Thus, the response to the proinflammatory cytokine IFN-γ is essential to generate a CNS inflammatory response to rabies virus. Nevertheless, the IFN-γR k.o. mice exhibit high levels of VNA by day 21 p.i., recover from the disease, and eventually clear the virus. In this case, it may be argued that the absence of the IFN-γ-dependent inflammatory response is manifested in a delay in the production of VNA as well as major changes in the ratios of the various antibody isotypes produced but culminates in only a slowed clearance of rabies virus from the CNS rather than a lethal outcome. IFN-α and -β appear to play an important role in the IFN-γ-dependent response pathway to rabies CVS-F3 virus infection. The perturbations in antibody production, both the delay in the appearance of VNA and in the display of an aberrant isotype pattern, are shared by IFN-α/βR and IFN-γR k.o. mice, as is the late clearance of CVS-F3 from the CNS. While we do not know the effect of targeted disruption of IFN-α/βR on CNS inflammation after infection with rabies CVS-F3, it is notable that cooperation between IFN-α/β and IFN-γ has been observed in the activation of mouse macrophages to produce NO, an important inflammatory mediator (10). We therefore speculate that the absence of a response to IFN-α/β results in a reduction in the aspect of the IFN-γ response to rabies virus infection that is responsible for rapid clearance of the virus.
We conclude from the results presented above that a cell-mediated inflammatory response may contribute to the rapid clearance of rabies virus from the CNS as has been inferred in Borna disease virus infection (17). A strong local CNS inflammatory response may promote contact between immune effectors and infected cells or enhance antibody production more directly through factor production. While our evidence is in general agreement with the concept that antibody is the primary immune effector of rabies virus clearance, it is evident that mice lacking B cells retain some ability to limit virus replication which appears to be T-cell dependent. Moreover, a contribution to CNS inflammation from antibody or B cells is supported by the observation that JHD k.o. mice fail to make a significant inflammatory response to CVS-F3 infection. Alternatively, there may be a T-cell developmental, maturation, or functional defect, resulting from the absence of B cells (9), in the JHD k.o. mice which is reflected in the inability to mount a CNS inflammatory response to rabies CVS-F3 infection. The observation that IFN-γ production by T cells is enhanced and the ratio of IgG2a to IgG1 antibodies increased when activated antigen-specific B cells are present during the induction of an immune response (15) supports the possibility that antigen-specific inflammatory responses in JHD k.o. mice are compromised due to a deficit in IFN-γ production by T cells.
Our studies confirm that rabies virus-specific antibodies are essential for clearance of an established rabies virus infection from the CNS, as likely occurs in human postexposure treatment situations. For the latter to occur in a timely fashion, an intact IFN-α/β- and IFN-γ-dependent inflammatory pathway appears to be necessary. The cooperation of different immune mechanisms in the clearance of rabies CVS-F3 from the CNS could explain why immunocompetent mice with different backgrounds rapidly clear the virus despite very large differences in the levels and qualities of rabies virus-specific antibodies that they make. Furthermore, CVS-F3-infected mice with different immunological deficits produced rabies-specific antibodies with widely disparate isotype profiles yet eventually cleared the virus. These findings indicate that clearance of rabies virus from the CNS is not dependent on the production of any particular antibody isotype, as previously reported for rabies and other viruses (3, 12, 26). The antibody isotype profiles made by the different animals are likely due to differences in the balance of Th1 plus CD8 versus Th2 factor production and therefore a reflection of whether there is a concomitant IFN-γ-dependent inflammatory response. These results support the notion that some mechanism associated with the inflammatory response facilitates rabies virus clearance from the CNS. In the early stage of infection, inflammatory processes in the CNS contribute to the response which rapidly clears rabies virus, indicating that any use of anti-inflammatory agents concomitant with rabies postexposure prophylaxis may lead to delay in the clearance of rabies virus and ultimately to the failure of rabies postexposure treatment.
ACKNOWLEDGMENTS
We thank Heather Carbaugh, Jean M. Champion, Gregory M. Dickson, and Rhonda B. Kean for excellent technical help in the animal experiments; H. Preibsch, A. Rospert, S. Roscher, P. Sack, E. Rodenberg, P. Latterman, and H. Schneider for performing the immunohistochemical analysis of mouse brain tissue; and Michel Aguet, Randy Hardy, and Michel Carroll for providing gene k.o. mice.
This work was supported by Public Health Service grant AI 09706, the Volkswagen-Siftung, and the German Research Foundation (SFB 297).
REFERENCES
- 1.Davis D R, Metzger H. Structural basis of antibody function. Annu Rev Immunol. 1983;1:87–117. doi: 10.1146/annurev.iy.01.040183.000511. [DOI] [PubMed] [Google Scholar]
- 2.Dietzschold B. Antibody-mediated clearance of viruses from the mammalian nervous system. Trends Microbiol. 1993;1:63–66. doi: 10.1016/0966-842X(93)90035-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dietzschold B, Kao M, Zheng Y M, Chen Z Y, Maul G, Fu Z F, Rupprecht C, Koprowski H. Delineation of putative mechanisms involved in the antibody-mediated clearance of rabies virus from the central nervous system. Proc Natl Acad Sci USA. 1992;89:7252–7256. doi: 10.1073/pnas.89.15.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dietzschold B, Schwaeble W, Schaeffer M K-H, Hooper D C, Zheng Y M, Petry F, Sheng H, Fink T, Loos M, Koprowski H, Weihe E. Expression of C1q, a subcomponent of the rat complement system, is dramatically enhanced in brains of rats with either Borna disease or experimental allergic encephalomyelitis. J Neurol Sci. 1995;130:11–16. doi: 10.1016/0022-510x(94)00269-t. [DOI] [PubMed] [Google Scholar]
- 5.Dietzschold B, Wiktor T J, Trojanowski J R, Macfarlan R I, Wunner W H, Torres-Anjel M J, Koprowski H. Differences in cell-to-cell spread of pathogenic and apathogenic rabies virus in vivo and in vitro. J Virol. 1985;56:12–18. doi: 10.1128/jvi.56.1.12-18.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietzschold B, Wunner W H, Wiktor T J, Lopes A D, Lafon M, Smith C, Koprowki H. Characterization of an antigenic determinant of the glycoprotein which correlates with pathogenicity of rabies virus. Proc Natl Acad Sci USA. 1983;80:70–74. doi: 10.1073/pnas.80.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doherty P C, Allan J E, Lynch F, Ceredig R. Dissection of an inflammatory process induced by CD8+ T cells. Immunol Today. 1990;11:55–59. doi: 10.1016/0167-5699(90)90019-6. [DOI] [PubMed] [Google Scholar]
- 8.Finke D, Brinckmann U G, Ter Meulen V, Liebert U G. Gamma interferon is a major mediator of anti-viral defense in experimental measles virus-induced encephalitis. J Virol. 1995;69:5469–5474. doi: 10.1128/jvi.69.9.5469-5474.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janeway C A, Jr, Murgita R A, Weinbaum F I, Asofsky R, Wigzell H. Evidence for an immunoglobulin-dependent antigen-specific helper T cell. Proc Natl Acad Sci USA. 1977;74:4582–4586. doi: 10.1073/pnas.74.10.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamijo R, Shapiro J, Le J, Huang S, Aguet M, Vilcek J. Generation of nitric oxide and induction of major histocompatibility complex class II antigen in macrophages from mice lacking the interferon γ receptor. Proc Natl Acad Sci USA. 1993;90:6626–6630. doi: 10.1073/pnas.90.14.6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koller B H, Marrack P, Kappler J W, Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science. 1992;248:1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 12.Levine B, Hardwick J M, Trapp B D, Crawford T O, Bollinger R C, Griffin D E. Antibody-mediated clearance of alpha-virus infection from neurons. Science. 1991;254:856–859. doi: 10.1126/science.1658936. [DOI] [PubMed] [Google Scholar]
- 13.Nash A A. Virological and pathological processes involved in Theiler’s virus infection of the central nervous system. Semin Neurosci. 1991;3:109–116. [Google Scholar]
- 14.Oldstone M B A, Blount P, Southern P J, Lampert P W. Cytoimmunotherapy for persistent virus infection reveals a unique clearance pattern from the central nervous system. Nature. 1986;321:239–243. doi: 10.1038/321239a0. [DOI] [PubMed] [Google Scholar]
- 15.Pasare C, Morafo V, Entringer M, Bansal P, George A, Bal V, Rath S, Durdik J M. Presence of activated antigen-binding B cells during immunization enhances relative levels of IFN-γ in T cell responses. J Immunol. 1998;160:778–787. [PubMed] [Google Scholar]
- 16.Patrick A K, Lindsley M D, Rodriguez M. Differential pathogenesis between mouse strains resistant and susceptible to Theiler’s virus-induced demyelination. Semin Virol. 1990;1:281–288. [Google Scholar]
- 17.Richt J A, Schmeel A, Freese K, Carbone K M, Narayan O, Rott R. Borna disease virus-specific T cells protect against or cause immunopathological Borna disease. J Exp Med. 1994;170:1467–1473. doi: 10.1084/jem.179.5.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez M, Pavelko K, Coffman R L. Gamma interferon is critical for resistance to Theiler’s virus-induced demyelination. J Virol. 1995;69:7286–7290. doi: 10.1128/jvi.69.11.7286-7290.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sedgwick J D, Doerries R. The immune system response to viral infection of the CNS. Semin Neurosci. 1991;3:93–100. [Google Scholar]
- 20.Shankar V, Dietzschold B, Koprowski H. Direct entry of rabies virus into the central nervous system without prior replication. J Virol. 1991;55:2736–2738. doi: 10.1128/jvi.65.5.2736-2738.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shankar V, Kao M, Hamir A N, Sheng H, Koprowski H, Dietzschold B. Kinetics of virus spread and changes in levels of several cytokine mRNAs in the brain after intranasal infection of rats with Borna disease virus. J Virol. 1992;66:992–998. doi: 10.1128/jvi.66.2.992-998.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snapper C M, Paul W E. Interferon-γ and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 23.Van den Broek M F, Mueller U, Huang S, Aguet M, Zinkernagel R M. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J Virol. 1995;69:4792–4796. doi: 10.1128/jvi.69.8.4792-4796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wandeler A I, Nadin-Davis S A, Tinline R R, Rupprecht C E. Rabies epidemiology: some ecological and evolutionary perspectives. In: Rupprecht C E, Dietzschold B, Koprowski H, editors. Lyssaviruses. Berlin, Germany: Springer-Verlag; 1994. pp. 297–324. [DOI] [PubMed] [Google Scholar]
- 25.Wiktor T J, Macfarlan R I, Foggin C M, Koprowski H. Antigenic analysis of rabies and Mokola virus from Zimbabwe using monoclonal antibodies. Dev Biol Stand. 1984;57:199–211. [PubMed] [Google Scholar]
- 26.Wright K E, Buchmeier M J. Antiviral antibodies attenuate T-cell-mediated immunopathology following acute lymphocytic choriomeningitis virus infection. J Virol. 1991;65:3001–3006. doi: 10.1128/jvi.65.6.3001-3006.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yusibov V, Modelska A, Steplewski K, Agadjanyan M, Weiner D, Hooper D C, Koprowski H. Antigens produced in plants by infection with chimeric plant viruses immunize against rabies virus and HIV-1. Proc Natl Acad Sci USA. 1997;94:5784–5788. doi: 10.1073/pnas.94.11.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zentel H J, Weihe E. The neuro-B cell link of peptidergic innervation in bursa fabricii. Brain Behav Immun. 1991;5:132–147. doi: 10.1016/0889-1591(91)90012-y. [DOI] [PubMed] [Google Scholar]