Abstract
Background
Dementia affects 5–8% of the population aged over 65 years (~50 million worldwide). Several factors are associated with increased risk, including diet. The Mediterranean diet (MedDiet) has shown potential protective effects against several chronic diseases.
Aims
This systematic review with meta-analysis aim was to assess the association between adherence to the MedDiet and the risk of dementia in the elderly.
Methods
PRISMA-2020 guidelines were followed. PubMed/MEDLINE and Scopus were searched on 17 July 2023. The Newcastle–Ottawa Scale tool was used to assess the risk of bias. The protocol was pre-registered in PROSPERO (registration number: CRD 42023444368). Heterogeneity was assessed using the I2 test. Publication bias was assessed by visual inspection of the funnel plot and by Egger’s regression asymmetry test. The final effect size was reported as OR or HR, depending on the study design of the included studies.
Results
Out of 682 records, 21 were included in the analysis. The pooled OR was 0.89 (95% CI = 0.84–0.94) based on 65,955 participants (I2 = 69.94). When only cohort studies were included, HR was 0.84 (95% CI = 0.76–0.94) based on 55,205 participants (I2 = 89.70). When only Alzheimer Disease was considered OR was 0.73 (95% CI = 0.62–0.85) based on 38,292 participants (I2 = 63.85).
Discussion
Despite the relatively low risk reduction associated with higher adherence to MedDiet among elderly, it should be considered that this population is the most affected.
Conclusions
Adherence to MedDiet could be an effective non-pharmacological measure to reduce the burden of dementia, even among elderly.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40520-024-02718-6.
Keywords: Mediterranean diet, Elderly, Dementia, Alzheimer disease
Introduction
Dementia represents a group of several brain degenerative diseases that impair memory, thinking and the ability to perform daily tasks. These diseases are characterized by the destruction of nerve cells and damage to the brain, which in turn leads to a progressive deterioration of cognitive function over time [1]. Dementia can affect people of any age, but it is predominant among the elderly, despite not being a natural part of the aging process. In 2014, approximately 5 million people over the age of 65 lived with dementia, and projections estimate an increase to near to 14 million by 2060 [2]. Moreover, dementia ranks as the 7th leading cause of death and is one of the major causes of disability and dependency in the elderly population, with women suffering the most, especially in terms of higher disability-adjusted life years (DALYs) and mortality [3].
Alzheimer's disease (AD) is the most common cause of dementia, accounting for at least two-thirds of all dementia cases in people over the age of 65 [4]. It is caused by a slowly progressing neurodegenerative accumulation of amyloid-beta peptides (Aβ) which cause neuritic plaques and neurofibrillary tangles [5]. Several factors have been associated with a higher or lower risk of dementia, including Alzheimer’s Disease. Older age, genetic factors, traumatic head injury [6], depression [7], cardiovascular and cerebrovascular disease [8], smoking [9], family history of dementia [10], increased homocysteine levels and Apolipoprotein E (APO-E) ε4 allele have been recognized as potential risk factors [11]. On the contrary, higher education [12], use of anti-inflammatory agents [13], cognitive engagement [14], regular aerobic exercise [14] and healthy diet have been reported to decrease the risk of Alzheimer's disease [4].
Among healthy dietary patterns, the Mediterranean diet has been associated with beneficial effects on several health-related outcomes, including cognitive function [15]. The Mediterranean diet is characterized by a high consumption of whole grains, fruits, vegetables, legumes, and olive oil, with a moderate intake of cheese and fish and a limited intake of meat (especially red and processed meat), sweets and alcohol. Several studies have highlighted the anti-inflammatory effect of the Mediterranean Diet which is considered as one of the main biological pathways through which beneficial effects are mediated [16]. However, results from the literature are not concordant, with some studies reporting that higher adherence to the Mediterranean Diet can improve physical performance and cognitive function [17], delay the onset or prevent dementia and reduce the risk of Alzheimer disease [18], while other studies have not reported any protective effects [19]. Moreover, most of the previous studies refer to adults in general, instead of specifically focusing on the elderly.
Therefore, the primary aim of this systematic review with meta-analysis was to retrieve, collect and collate all the existing evidence in the literature to obtain a comprehensive view on the association between adherence to a Mediterranean diet and risk of dementia among elderly people. Second, this review aims to evaluate critically existing literature, assessing the quality of the studies included and potential biases. Third, using a meta-analytical approach, this review aimed to provide a summary statistical estimation of the strengths of the association between adherence to a Mediterranean diet and dementia, also conducting sensitivity analysis, considering the type of dementia and study design. Lastly, this review also conducted subgroup analyses based on the geographical area and methods used to assess diet.
Methods
The current systematic review with meta-analysis was conducted according to the guidelines of the Cochrane Collaboration [20], and the results were reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 (PRISMA-2020) and the Meta-Analysis of Observational Studies in Epidemiology guidelines [21]. The research protocol was defined in advance and shared among the research team. Therefore, the protocol was registered in the international database of prospectively registered systematic reviews (PROSPERO; registration number: CRD 42023444368).
Literature search strategy
The literature search was conducted simultaneously on PubMed/MEDLINE and Scopus on 17 July 2023, based on the following research question: “Is higher adherence to the Mediterranean diet associated with a lower risk of dementia in the elderly?”. Therefore, the search strategy was developed considering three aspects: the elderly (as population), adherence to the Mediterranean diet (as exposure), and dementia (as outcome of interest). Selected keywords, both MeSH terms and Title/Abstract, were combined using the Boolean operators AND and OR. The search strategy was first developed in PubMed/MEDLINE and therefore adopted for Scopus. The search strategy used for each database is presented in the supplementary Table 1. The searches were performed blindly by two researchers (VG and DN) and an equal number of records were retrieved. Potential additional relevant articles were searched by screening the reference lists of the included articles and consulting experts in the field.
Inclusion/exclusion criteria
Inclusion/exclusion criteria were defined according to the following guidelines: Population (P), Exposure (E), Comparison (C), Outcome (O), Study design (S). In particular, only observational epidemiological studies in elderly people (over 60 years of age), assessing the association between adherence to the Mediterranean diet and dementia (any type), published in English in an international, peer-reviewed scientific journal, were considered eligible. In contrast, non-original or interventional studies assessing the association between adherence to any other type of diet and a health outcome other than dementia in people younger than 60 years, not published in English and not in a peer-reviewed journal were excluded. A detailed description of the inclusion/exclusion criteria, defined according to PECOS, is provided in the supplementary Table 2.
Study selection and data extraction
As previously done [22, 23], the selection of studies was carried out in two stages. First, titles and abstracts of records retrieved using the search strategy and those retrieved from additional sources were screened independently by two reviewers using the inclusion/exclusion criteria above. Secondly, the full-text was searched and downloaded only for potentially eligible articles. These were then assessed independently by two reviewers. Any disagreements about the eligibility and inclusion of articles were resolved by discussion between the reviewers. If disagreement persisted, a third senior researcher was involved to make the final decision. The extracted data were collected using a standardized, and pre-defined spreadsheet using Excel (Microsoft Excel® for Microsoft 365 MSO, USA, 2019). To improve the quality of data extraction, the spreadsheet was pre-tested on five randomly selected studies. The following information was extracted from each included study: first author, year of publication, the country in which the study was conducted, study period, study design, number of participants, age and sex, main population characteristics, number of participants lost (attrition rate), dietary assessment tool used, whether or not the tool was validated, Mediterranean diet score used to assess adherence, diagnostic tool used to diagnose dementia, type of dementia, maxim adjusted effect size measurements along with the corresponding 95% CIs, variables used for adjustment, whether funding was received for conducting the original study, and declared conflicts of interest. Data extraction was performed in duplicate and discrepancies were resolved by discussion. Missing data were obtained by contacting the corresponding author.
Data synthesis
Following the PRIMA 2020 guidelines, the selection process was documented using a “flow diagram” showing the number of references excluded at each step. Reasons for study exclusion after full-text assessment are reported in detail. In addition, the extracted data were tabulated and summarized in text. Moreover, the results of the statistical analysis are presented in both tables and figures (detailed below).
Quality assessment
The Newcastle–Ottawa Scale (NOS) was used to assess the methodological quality and risk of bias of the included studies. The scale is based on a 'star system' in which studies are graded on three main aspects: the selection of study groups, the comparability of the groups, and the ascertainment of either the exposure or the outcome of interest. The overall quality score was considered as a continuous variable; however, taking into account the previously adopted cut-off, the studies were considered to be of high quality if the NOS score was equal to or greater than 7 points.
Statistical analysis
Data were pooled using a meta-analysis focusing on the overall association between higher adherence to the Mediterranean diet and the risk of any type of dementia (including mild cognitive impairment). The summary effect size was calculated based on the odds ratio (OR), hazard ratio (HR), and risk ratio (RR) of the included studies. The final effect size was reported as OR or HR based on the study design of the included studies. In particular, an OR was reported in the subgroup analysis that included only cross-sectional studies. Conversely, in the subgroup that included only longitudinal studies, the effect size was calculated using HR. In the current meta-analysis, random and fixed effect models were used. Heterogeneity was assessed using the I2 test, which measures the proportion of total variance between studies that is beyond random error. Based on the results obtained, heterogeneity was classified as high when I2 values were equal to or greater than 75%, moderate when I2 values were between 50 and 75%, low when I2 values were between 25 and 50%, and finally, no heterogeneity when I2 values were equal to or less than 25%. Publication bias was assessed by both visual inspection of the funnel plot and by Egger’s regression asymmetry test, with statistical significance set at p < 0.10. If publication bias was detected, the trim and fill method was used to adjust for it by searching for missing studies to the right of the total. All data analyses were performed using Prometa3® software (Internovi, Cesena, Italy).
Sensitivity analyses
A sensitivity analysis was conducted based on type of dementia (including only unspecified dementia and mild cognitive impairment; only unspecified dementia; only Alzheimer disease; only mild cognitive impairment). Moreover, a sensitivity analysis was performed that included only studies of high methodological quality. Finally, studies based on the same population were excluded to avoid potential overlapping effects. In this case, only studies with the highest NOS score or, in case of a tie, the study with the larger sample size were selected.
Subgroup analyses
Subgroup analysis was performed by study design, country in which the study took place (including only studies conducted in Mediterranean countries), sex, and only including studies that used validated tools. Subgroup analyses were only performed when three or more studies were available.
Results
Literature search
A total of 682 records were identified by searching Pubmed/MEDLINE (n = 257) and Scopus (n = 425). No additional articles were included based on reference screening and expert consultation.
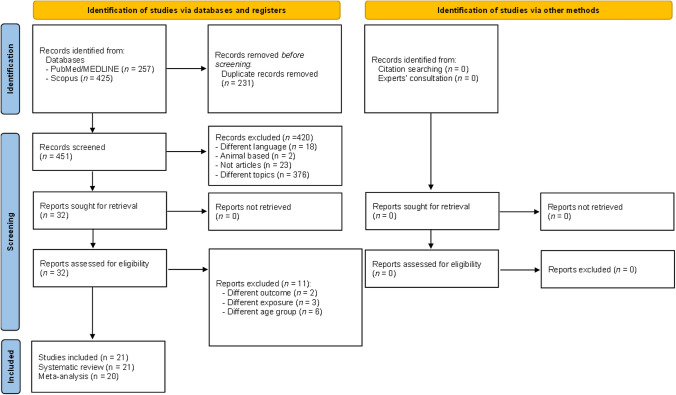
After preliminary exclusion of duplicates (n = 231), a total of 451 records were screened based on title and abstract. Based on the initial screening, 420 records were removed due to different language (n = 18), non-human studies (n = 2), non-original work (n = 23) and focus on different topics (n = 376), leaving 32 records eligible for inclusion. Based on full-text assessment, 11 records were excluded (reasons for exclusion are detailed in the supplementary Table 3) [24–34], resulting in 21 records included in the current systematic review [19, 35–54]; however, one record did not provide analytical data and, therefore, could not be included in the meta-analysis [37]. The selection process is shown in Fig. 1.
Fig. 1.
PRISMA flow diagram showing the selection process
Main characteristics of included studies
Almost all continents were represented with Europe with eight studies (Greece n = 3 [36, 39, 46], Sweden n = 2 [42, 49], Netherlands n = 1 [40], France n = 1 [19], Italy n = 1 [48]), followed by the United States of America with seven studies [43, 44, 47, 50–53], three studies were conducted in Australia [35, 41, 45], one study was conducted in Brazil [37], one study in Hong Kong [38], and one study in Morocco [54]. Regarding the study period, the first cohort was established in 1970 [49], while the most recent study was conducted in 2022 [35]. In terms of study design, half of the included studies were cohort studies (n = 13) [19, 39, 40, 42–47, 49, 50, 52, 53], followed by cross-sectional studies (n = 6) [35–38, 41, 54], one study performed both cross-sectional and longitudinal analysis [48], and lastly one study is a case–control study nested within a cohort study [51]. Sample sizes ranged from 96 [37] to 28,025 participants [42]. The attrition rate (based on non-compliance or loss to follow-up) ranged from 0 [37] to 76.7% [48]; however, three studies did not report this information [36, 41, 46]. Dietary assessment was mainly based on food frequency questionnaires (FFQ), with the number of items ranging from 14 [35] to 389 [40]. Only two studies used a combination of FFQ and 24-h recall [19] or a 7-day food diary [42], while one study used a 7-day food diary [49]. All questionnaires used were validated, but two studies did not report this information [37, 42]. Adherence to the Mediterranean diet was estimated using different types of scores. In particular, eight studies used the score proposed by Trichopoulou et al. [19, 38, 41, 43, 50–53]; seven studies used the score proposed by Panagiotakos et al. [36, 37, 39, 40, 46, 47, 54], one study used both scores [45], two studies used the modified Mediterranean Diet Score (mMDS) [42, 49], one study used the alternate Mediterranean Diet Score (aMDS) [44], one study used the 14-item Mediterranean Diet Adherence Screener (MEDAS) [35], and one study calculated adherence using defined self-defined score [48]. Details are given in Table 1.
Table 1.
Main characteristics of included studies, reported in alphabetical order
| Author, year | Country | Study period’ | Study design | Sample size | Attrition | Tool for dietary assessment | Validation | MedDiet score | Dementia type | Diagnostic assessment |
|---|---|---|---|---|---|---|---|---|---|---|
| Allcock, 2022 | Australia | 2022 | Cross-sectional | 294 | 9 (2.9%) | 14 items MEDAS | Yes | 14-item MEDAS | All type of dementia | AD8 |
| Anastasiou CA, 2017 | Greece | n.a. | Cross-sectional | 1864 | n.a. | 69 items FFQ | Yes | MedDietScore, proposed by Panagiotakos et al. | AD, vascular dementia, Lewy body and frontotemporal dementias | Semi-structured interview of the CDR |
| Calil Silvia RB, 2018 | Brazil | n.a. | Cross-sectional | 96 | 0 | 98 items FFQ | n.a. | MedDietScore, proposed by Panagiotakos et al. | MCI and AD | Clinical assessments performed by a geriatrician or neurologist |
| Chan R, 2013 | Hong Kong | 2001–2003 | Cross-sectional | 3670 | 330 (8.2%) | 280 items FFQ | Yes | MedDietScore, proposed by Trichopoulou et al. | Cognitive impairment | CSI-D |
| Charisis, 2021 | Greece | n.a.; 3.1 ± 0.9 years of FU | Cohort | 1046 | 2.4% | 69 items FFQ | Yes | MedDietScore, proposed by Panagiotakos et al. | AD, VaD, Lewy body and frontotemporal dementias | CDR |
| de Crom TO, 2022 | Netherlands |
I sub-cohort 1989–1993 baseline II sub-cohort 2009–2012 baseline. No data on FU |
Cohort |
I sub-cohort 5375 II sub-cohort 2861 |
I sub-cohort: 32.7% II sub-cohort: 5% |
I sub-cohort: 170 items FFQ II sub-cohort: 389 items FFQ |
Yes | MedDietScore, proposed by Panagiotakos et al. | All-type of dementia | Clinical assessment based on DSM-III-R and NINCDS-ADRDA for AD |
| Féart, 2009 | France | 2001–2007 | Cohort | 1410 | 1213 (14%) | FFQ and 24 h recall | Yes | MedDietScore, proposed by Trichopoulou et al. | Dementia and AD | Battery of neuropsychological tests + neurological evaluation |
| Gardener, 2012 | Australia | n.a. | Cross-sectional | 970 | n.a. | 74 items CCVFFQ | Yes | MedDietScore, proposed by Trichopoulou et al. | MCI and AD | Clinical assessment based on diagnostic criteria DSM-IV and ICD-10 |
| Glans I, 2023 | Sweden | 1991–2014; 23 years of FU | Cohort | 28,025 | 2421 (7.9%) | 7-day food diary, detailed FFQ, and 1-h interview | n.a. | mMDS | All-type dementia, AD, VaD | Registered dementia diagnoses from the Swedish National Patient Register (Inpatient + hospital) |
| Gu Y, 2010 | USA | 1992–2008; 3.8 year of FU | Cohort | 1219 | 1559 (56.1%) | 61 items version of Willett’s FFQ | Yes | MedDietScore, proposed by Trichopoulou et al. | AD | DSM-III-R and criteria of the NINCDS-ADRDA |
| Haring, 2016 | USA | 1996–1999; 9.11 years of FU | Cohort | 6425 | 1054 (14.1%) | WHI FFQ | Yes | aMDS | Probable dementia | Functional test + clinical evaluation + noncontrast CT brain scan and laboratory blood tests |
| Hosking DE, 2019 | Australia | 2001–2014; 12 years of FU | Cohort | 1220 | 1331 (52.2%) | CSIRO semi-quantitative FFQ | Yes | MedDietScore, proposed by Trichopoulou et al. and proposed by Panagiotakos et al. | AD and MCI (combined) | Neuropsychological testing and MMSE |
| Mamalaki E, 2022 | Greece | n.a. | Cohort | 1018 | n.a. | 69 items FFQ | Yes | MedDietScore, proposed by Panagiotakos et al. | All-type of dementia | Multiple neuropsychological validated tests and clinical assessment |
| Morris, 2015 | USA | 2004–2013; 9 years of FU | Cohort | 923 | 40.26% | Modified version of the Harvard semi-quantitative FFQ | Yes | MedDietScore, proposed by Panagiotakos et al. | AD | Neurological examination, medical history and cognitive performance tests |
| Nicoli, 2021 | Italy | 2003–2018; 12 years FU | Cross-sectional and cohort | Cross-sectional = 1390; cohort = 512 | 76.7% | FFQ | Yes | MDS* | All-type of dementia | Clinical assessment based on DSM-IV, and MMSE |
| Olsson, 2014 | Sweden | 1970–1994; 12 years FU | Cohort | 1038 | 55% | Optically readable form of a 7-day food record based on a validated pre-coded menu book | Yes | mMDS | AD and all-type dementia | Clinical assesment and MMSE |
| Roberts, 2010 | USA | 2004; 2.2 years OF fu | Cohort | 1233 | 736 (37.4%) | 128 items modified Block 1995 Revision of the HHH Questionnaire | Yes | MedDietScore, proposed by Trichopoulou et al. | MCI and dementia | According to the DSM-IV criteria |
| Scarmeas, 2006a | USA | 1992–1999 | Case–control study nested within a community-based cohort | 1984: 194 patients with AD vs 1790 nondemented subjects | 52% | 61 items Willett’s semiquantitative FFQ | Yes | MedDietScore, proposed by Trichopoulou et al. | AD | CDR and clinical assessment. Alzheimer disease was categorized based on NINCDS-ADRDA |
| Scarmeas, 2006b | USA | Baseline 1992–1999; 4 ± 3 years of FU | Cohort | 2258 | 1908 (45%) | 61 items Willett’s semiquantitative FFQ | Yes | MedDietScore, proposed by Trichopoulou et al. | AD | CDR and clinical assessment. Alzheimer disease was categorized based on NINCDS-ADRDA |
| Scarmeas, 2009 | USA | Baseline 1992–1999; 10 years of FU | Cohort | 1875 | 1818 (49%) | 61 items FFQ | Yes | MedDietScore, proposed by Trichopoulou et al. | MCI and AD | Clinical assessment |
| Talhaoui, A, 2023 | Morocco | March 2017–May 2018 | Cross-sectional | 151 | 86 (36.3%) | Questionnaire of weekly food consumption | Yes | MedDietScore, proposed by Panagiotakos et al. | Cognitive impairment | MMSE |
’For cohort studies, the duration of follow-up was also reported
AD Alzheimer's Disease, AD8 dementia screening intervention, aMDS alternate Mediterranean diet score, CCVFFQ Cancer Council of Victoria Food Frequency Questionnaire, CDR Clinical Dementia Rating Scale, CSI-D Community Screening Instrument for Dementia, CSIRO Commonwealth Scientific and Industrial Research Organization, CT computed tomography; HHH Health Habits and History, DSM-III-R Diagnostic and Statistical Manual of Mental Disorders 3rd edition, DSM-IV Diagnostic and Statistical Manual of Mental Disorders 4th edition, FFQ food frequency questionnaire, FU follow-up, ICD-10 International Classification diseases version 10, MCI mild cognitive impairment, MDS* Mediterranean diet score calculated as the sum of intakes of nine dietary components: high intake of cereals, fruits, vegetables, legumes, fish, monounsaturated/saturated fat ratio, low intake of milk and meat, and moderate alcohol intake, MEDAS Mediterranean Diet Adherence Screener, MedDietScore Mediterranean Dietary Score, mMDS modified Mediterranean diet score, MMSE Mini-mental state examination, NINCDS-ADRDA National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association, VaD vascular dementia, WHI Women’s Health Initiative
Main characteristics of the study population
Recruited participants were all over 60 years of age (due to our inclusion criteria) with the oldest population being 93 years of age [48]. In the majority of included studies, participants were community-dwelling; however, four studies only included Medicare beneficiaries [43, 51–53] and three studies included participants from healthcare institutions (neurology outpatients [37], retirement communities [47], and nursing homes [54]). All studies included both women and men, but one study included only postmenopausal women [44], and one study included only men [49]. More details are given in Table 2.
Table 2.
Main characteristics of studied population
| Author, year | Population characteristics | Age | Sex | Effect size (95% CI) p-value | Adjustments |
|---|---|---|---|---|---|
| Allcock, 2022 | Community-dwelling older adults | 70.4 ± 6.2 | F = 205 (67.7%) | Beta = −0.134 (−0.198, −0.007) 0.035 | Age, gender, BMI, smoking status, average sleep duration/night, average physical activity duration/day, diabetes status and education status |
| Anastasiou CA, 2017 | Community-dwelling adults aged ≥ 65 years old from HELIAD | 73.0 ± 6.1 | M = 757; F = 1107 | OR = 0.92 (0.87–0.97) 0.004 | Age, sex, education, number of clinical co-morbidities, energy intake |
| Calil Silvia RB, 2018 | Adults over the age of 60 years recruited and assessed at the Paulista IPGG and in the Neurology Outpatient clinic of the Santa Marcelina Hospital | ≥ 60 | M = 27; F = 69 | n.a. | n.a. |
| Chan R, 2013 | Adults aged ≥ 65 years old of a cohort study examining the risk factors for osteoporosis, volunteers and able to walk or take public transport to the study site | ≥65 | M = 1926; F = 1744 | M: OR = 0.89 (0.56–1.41) 0.925 F: OR = 1.02 (0.75–1.41) 0.952 | Age, BMI, PA, energy intake, education level, Hong Kong ladder, community ladder, smoking status, alcohol use, activities of Daily Living, depression, self-reported history of DM, hypertension, CVD/ stroke |
| Charisis, 2021 | Community-dwelling adults aged ≥ 65 years old from HELIAD | 73.1 ± 5.0 | M = 40.3% | HR 0.10 (0.01–0.78) 0.029 | Age, sex, years of education, energy intake, BMI, number of clinical comorbidities, MCI at baseline, center of evaluation, PA, Apo E genotype |
| de Crom TO, 2022 | The first two subcohorts of the Rotterdam Study (RS), among inhabitants from the suburb Ommoord in Rotterdam | RS-I 67.7 ± 7.8 and RS-II 75.3 ± 5.9 | F = 59.0% | I sub-cohort ( 1989–993) N 1188/5375 HR 1.04 (0.97–1.10)II sub-cohort( 2009–2012) N 248/2861 HR = 0.75 (0.66–0.86) | Sex, age, educational level, smoking status, physical activity, and energy intake, BMI, diabetes, hypercholesterolemia, and hypertension |
| Féart, 2009 | Community dwellers from Bordeaux, France, included in The Three-City (3C) study | 75.9 | M = 527; F = 883 | Dementia HR 0.92 (0.51–1.66) p = 0.78; Alzheimer's disease HR 0.70 (0.34–1.43) p 0.33 | Sex, education, marital status, total energy intake, practice of physical exercise, taking ≥ 5 drugs/d, Center for Epidemiological Studies-Depression scale score, Apolipoprotein E genotype, BMI, hypertension, hypercholesterolemia, diabetes, tobacco, stroke |
| Gardener, 2012 | Adults over the age of 60 years | 71.72 ± 7.86 | M = 407; F = 563 | MCI OR 0.87 (0.75–1.00) p < 0.05AD OR 0.81 (0.71–0.92) p < 0.01 | Age, sex, country of birth, education, apolipoprotein E allele status, current smoking status, caloric intake, BMI, history of stroke, diabetes, hypertension, angina and heart attack |
| Glans I, 2023 | Nondemented individuals born 1923–1950 and living in Malmo (Malmo Diet and Cancer study) | ≥ 60 | F = 16,992; M = 11,033 |
All-cause dementia HR 0.95 (0.76–1.18) AD HR 0.87 (0.66–1.16) |
Sex, age, education, dietary assessment method, season and total calorie intake, smoking, physical activity, alcohol consumption and BMI |
| Gu Y, 2010 | Medicare-eligible northern Manhattan residents | 76.7 ± 6.4 | F = 812; M = 407 | HR 0.87 (0.78–0.97) | Age, gender, race, education, fasting insulin, adiponectin level, high-sensitivity C-reactive protein |
| Haring, 2016 | Postmenopausal women enrolled in the WHIMS study | 65–79 | F = 6425 | HR 1.13 (0.79, 1.63) | Age, race, education level, WHI Hormone Trial Randomization assignment (HTR arm), baseline 3MSE level, smoking status, physical activity, diabetes status, hypertension status, BMI, family income, depression, history of CVD and total energy intake |
| Hosking DE, 2019 | PATH through life study | 62.5 ± 1.5 | n.a. |
Using MedDietScore by Trichopoulou OR = 0.77 (0.43–1.39) Using MedDietScore by Panagiotakos et al. (1.30 (0.79–2.15) |
Energy intake, age, sex, and APOE3 allel 4, education, mental activity, physical activity, smoking status, depression, diabetes, BMI, hypertension, heart disease, and stroke |
| Mamalaki, 2022 | Community-dwelling adults aged ≥ 65 years old from HELIAD | 73.1 ± 5.0 | F = 59.7% | RR 0.968 (0.955–0.982) < 0.001 | Age, sex and years of education |
| Morris, 2015 | Volunteers living in retirement communities and senior public housing units in the Chicago area enrolled in the Rush MAP | 81 | M and F (no data) | HR 0.46 (0.29, 0.74) p < 0.001 | Age, sex, education, APOE-ε4, total energy intake, physical activity and participation in cognitively stimulating activities |
| Nicoli, 2021 | Adults aged ≥ 80 years old residents in the province of Varese | 93.2 | F = 73.0% | OR = 0.97 (0.71–1.31) p = 0.008; HR = 1.17 (0.82–1.66) p = 0.369 | Age, sex, education, total intake of kilocalories, smoke, alcohol, physical activity, hypertension, chronic obstructive pulmonary disease, diabetes, lifetime depression, previous stroke, previous transient ischemic attack |
| Olsson, 2014 | Men born in 1920–24 and living in Uppsala | 71 ± 0.6 | M |
AD: HR = 0.99 (0.44–2.26) p = 0.95; dementia HR = 0.84 (0.46–1.53) p = 0.69 |
Energy, educational level, apolipoprotein E (APOE) genotype and PA |
| Roberts, 2010 | Residents from Olmsted County, USA, aged 70–89 years | 70–89 | F = 592 | OR 0.78 (0.51–1.22) p = 0.28 | Age, years of education, total energy (continuous variables), sex, ApoE ε4 (ε4+ vs. ε4−), stroke, coronary heart disease, and depressive symptoms |
| Scarmeas, 2006a | Medicare beneficiaries residing in Manhattan | 76.3 ± 6.6 | M = 630; F = 1354 | OR 0.31 (0.16–0.58) p < 0.001 | Cohort, age at intake in the study, sex, ethnicity, education, APOE genotype, smoking, comorbidity index, and BMI |
| Scarmeas, 2006b | Medicare beneficiaries residing in Manhattan | 77.2 ± 6.6 | M = 720 (32%); F = 1538 (68%) | HR 0.60 (0.42–0.87) | Cohort, age, sex, ethnicity, education, apolipoprotein E genotype, caloric intake, smoking, comorbidity index, and body mass index |
| Scarmeas, 2009 | Medicare beneficiaries residing in Manhattan | 76.9 ± 6.5 | M = 603 (32%); F = 1272 (68%) |
AD: HR = 0.52 (0.30–0.91); p = 0.02; MCI: HR = 0.72 (0.52–1.00) p = 0.05 |
Cohort, age at intake in the study, gender, ethnicity, education, APOE, BMI and time between 1st dietary and 1st cognitive status assessment |
| Talhaoui A, 2023 | Moroccan elderly subjects from three nursing homes | ≥60 | F = 61 (40.45) | OR = 0.96 (0.85–1.08) p > 0.05 | Gender, profession, pension, education, nutritional status, Fat-Free-masse, calf circumference, GDS-15 score, and walking or cycling PA |
AD Alzheimer disease, F female, HELIAD Hellenic Longitudinal Investigation of Aging and Diet, HR Hazard ratio, IPGG Institute of Geriatrics and Gerontology Jose Ermírio de Moraes, M male, MAP Memory and Aging Project, MCI Mild Cognitive Impairment, OR Odds ratio, PATH Personality and Total Health, WHIMS Women’s Health Initiative Memory Study
Quality assessment
All included studies scored 7 or higher and were therefore considered to be of high quality. Only the study not included in meta-analysis was considered as moderate quality (main reasons were attributable to the statistical analysis). Approximately half of the included studies (n = 12) reported no conflicts of interest, five studies did not report this information [43, 44, 51–53], while four studies reported conflicts of interest [47–49, 54]. However, 15 studies received funding to conduct the research, four studies did not report this information [43, 49, 52, 53], and finally two studies did not receive funding [19, 54]. Detailed quality assessment, reported item by item, is described in Supplementary Table 4. Inter-rater reliability was assessed and the discrepancy between the two reviewers was approximately 5%. Disagreements were resolved by discussion, and final agreement was reached for all included studies.
Meta-analysis: MedDiet adherence and all type of dementia
As one study reported results separately for males and females [38], and one study reported data separately for the two included cohorts [40], they were considered to be independent studies. Finally, one study did not report quantitative data [37], and another reported data using the beta coefficient [35] (which is not statistically comparable with all other risk estimates collected), and for these reasons they were excluded from the meta-analysis. Therefore, a total of 21 data sets were included in the main analysis.
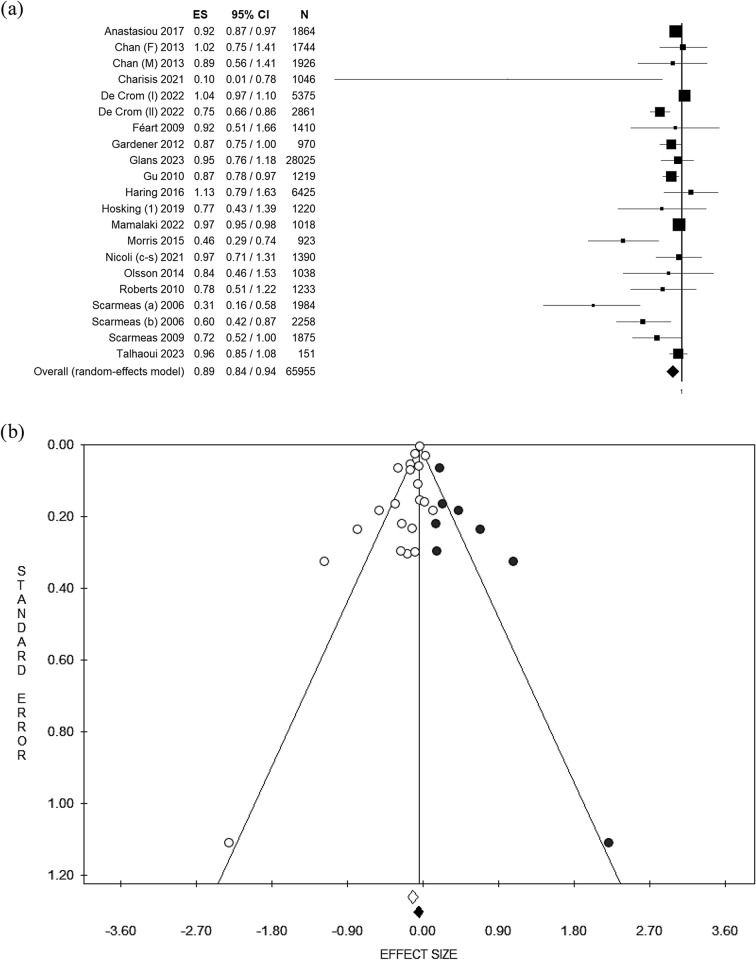
Considering all 21 data sets and using the random effect model, the pooled OR was 0.89 [(95% CI = 0.84–0.94); p-value < 0.001] based on 65,955 participants (Fig. 2a) with moderate statistical heterogeneity (df = 20, I2 = 69.94, p-value ≤ 0.001). Potential publication bias was identified by visual assessment of the funnel plot (Fig. 2b) and confirmed by Egger’s linear regression test (intercept −1.08, p-value = 0.013). After applying the trim and fill method, the estimated effect sizes were not significantly different from the main result. Given that one study estimated the adherence to the MedDiet using two different scores [45], and given the heterogeneity of the MedDiet scoring systems used in all included studies, and to improve the consistency and comparability between studies, we decided to perform an additional analysis alternatively pooling the two scores. However, the results did not change. The results for both the fixed and random effect models are shown in Table 3.
Fig. 2.
Forest plot (a) and Funnel plot (b) using the random effect model of the main analysis. The white dots represent the included studies. The white diamond represents the calculated Effect size. The black dots represent the estimated studies after the trim and fill method. The black diamond represents the estimated ES after the trim and fill method. ES effect size, 95% CI 95% confidence interval
Table 3.
Summary statistics of the main, sensitivity and subgroup analyses
| Summary statistics | Publication bias | ||||||
|---|---|---|---|---|---|---|---|
| Analysis | Studies included [Ref.] | No. of participants | df | ES (95% CI); p-value | I2; p-value | Intercept’; p-value | Estimateda ES; p-value |
| All type of dementia: including unspecified dementia, Alzheimer disease and mild cognitive impairment | |||||||
| All type of dementiab | [19, 35, 36, 38–54] | 65,955 | 20 | OR^ = 0.96 (0.95–0.97); <0.001 | 69.55; <0.001 | −1.19; 0.005 | OR^ = 0.97 (0.95–0.98); <0.001 |
| OR” = 0.89 (0.84–0.94); <0.001 | OR” = 0.95 (0.89–1.02); 0.145 | ||||||
| All type of dementiac | [19, 35, 36, 38–54] | 65,955 | 20 | OR^ = 0.96 (0.95–0.97); <0.001 | 69.94; <0.001 | −1.08; 0.013 | OR^ = 0.97 (0.95–0.98); <0.001 |
| OR” = 0.89 (0.84–0.95); <0.001 | OR” = 0.92 (0.86–0.99); 0.019 | ||||||
| All type of dementia cross-sectional | [36, 38, 41, 48, 51, 54] | 10,029 | 6 | OR^ = 0.92 (0.88–0.96); <0.001 | 52.25; 0.050 | −0.78; 0.394 | n.a. |
| OR” = 0.91 (0.82–1.00); 0.055 | |||||||
| All type of dementia cohort studies | [19, 39, 40, 42–50, 52, 53] | 55,205 | 14 | HR^ = 0.97 (0.96–0.99); 0.001 | 89.70; <0.001 | −1.76; 0.053 | HR^ = 0.99 (0.97–1.00); 0.039 |
| HR” = 0.84 (0.76–0.94); 0.002 | HR” = 0.85 (0.76–0.95); 0.003 | ||||||
| Dementia: including diagnosis of unspecified Dementia and/or mild cognitive impairment | |||||||
| Dementiab | [36, 38–42, 44–46, 48–50, 52, 54] | 59,571 | 16 | OR^ = 0.96 (0.95–0.98); <0.001 | 53.56, 0.005 | −0.72; 0.078 | OR^ = 0.97 (0.96–0.98); <0.001 |
| OR” = 0.93 (0.88–0.98); 0.005 | OR” = 0.96 (0.91–1.02); 0.196 | ||||||
| Dementiac | [19, 36, 38–42, 44–46, 48–50, 52, 54] | 59,571 | 16 | OR^ = 0.96 (0.95–0.98); <0.001 | 54.62; 0.004 | −0.58; 0.170 | OR^ = 0.97 (0.96–0.98); <0.001 |
| OR” = 0.93 (0.89–0.98); 0.009 | OR” = 0.94 (0.89–0.99); 0.024 | ||||||
| Dementia cross sectional | [36, 38, 41, 48, 54] | 8045 | 5 | OR^ = 0.92 (0.88–0.97); <0.001 | 0.00; 0.903 | 0.21; 0.631 | n.a. |
| OR” = 0.92 (0.88–0.97); <0.001 | |||||||
| Dementia cohort studies | [19, 39, 40, 42, 44–46, 48–50, 52] | 50,805 | 11 | HR^ = 0.98 (0.97–1.00); 0.025 | 63.07; 0.002 | −0.86; 0.113 | HR^ = 0.99 (0.98–1.00); 0.025 |
| HR” = 0.94 (0.87–1.01); 0.083 | HR” = 1.00 (0.92–1.08); 0.912 | ||||||
| Dementia: only including diagnosis of unspecified Dementia | |||||||
| Dementia | [19, 36, 38–41, 44, 46, 48, 49] | 55,092 | 12 | OR^ = 0.96 (0.95–0.98); <0.001 | 59.86; 0.003 | −0.62; 0.239 | OR^ = 0.97 (0.95–0.98); <0.001 |
| OR” = 0.94 (0.88–0.99); 0.021 | OR” = 0.94 (0.88–0.99); 0.021 | ||||||
| Dementia cross sectional | [36, 38, 41, 44, 48] | 14,319 | 5 | OR^ = 0.92 (0.88–0.97); <0.001 | 0.00; 0.797 | 0.41; 0.379 | n.a. |
| OR” = 0.92 (0.88–0.97); <0.001 | |||||||
| Dementia cohort studies | [19, 39, 40, 42, 46, 48, 49] | 41,285 | 7 | OR^ = 0.99 (0.97–1.00); =0.033 | 70.74; 0.001 | −0.91; 0.273 | OR^ = 0.99 (0.98–1.00); =0.033 |
| OR” = 0.95 (0.88–1.03); =0.196 | OR” = 0.99 (0.91–1.09); 0.900 | ||||||
| Only including diagnosis of Alzheimer’s disease | |||||||
| Overall Alzheimer's disease | [41–43, 47, 49, 51–53] | 38,292 | 7 | OR^ = 0.81 (0.75–0.87); <0.001 | 63.85; 0.007 | −1.88; 0.055 | OR^ = 0.83 (0.77–0.89); <0.001 |
| OR” = 0.73 (0.62–0.85); <0.001 | OR” = 0.76 (0.63–0.90); 0.002 | ||||||
| Alzheimer’s disease (cohort studies) | [42, 43, 47, 49, 52, 53] | 34,300 | 5 | HR^ = 0.79 (0.75–0.84); <0.001 | 91.78; <0.001 | −1.57; 0.629 | HR^ = 0.80 (0.76–0.85); <0.001 |
| HR” = 0.73 (0.58–0.93); 0.010 | HR” = 0.82 (0.66–1.02); 0.075 | ||||||
| Only including diagnosis of mild cognitive impairment | |||||||
| Mild cognitive impairement (all) | [41, 52, 54] | 2996 | 2 | OR^ = 0.91 (0.83–0.99); =0.028 | 35.49; 0.212 | −2.72; 0.259 | OR^ = 0.96 (0.89–1.03); =0.276 |
| OR” = 0.89 (0.79–1.01); 0.063 | OR” = 0.96 (0.85–01.09); 0.525 | ||||||
| Mediterranean area | [19, 36, 39, 46, 48, 54] | 6879 | 5 | OR^ = 0.96 (0.95–0.98); <0.001 | 31.91; 0.196 | −0.86: 0.171 | OR^ = 0.97 (0.96–0.98); <0.001 |
| OR” = 0.95 (0.91–0.99); 0.021 | OR” = 0.97 (0.92–1.01); 0.142 | ||||||
| Validated exposure | [19, 35, 36, 38–41, 43–54] | 37,930 | 19 | OR^ = 0.96 (0.95–0.97); <0.001 | 71.07; <0.001 | −1.24; 0.005 | OR^ = 0.97 (0.95–0.98); <0.001 |
| OR” = 0.89 (0.83–0.94); <0.001 | OR” = 0.95 (0.89–1.02); 0.126 | ||||||
aEstimated using the trim and fill analysis
bHosking et al. assessed adherence to the Mediterranean diet using two different scores, therefore, in this analysis effect size using Trichopoulou’s score was used
cHosking et al. assessed adherence to the Mediterranean diet using two different scores, therefore, in this analysis effect size using Panagiotakos’ score was used
’Calculated using Egger’s linear regression test, ^fixed effects model;” random effects model
CI confident interval, df degree of freedom, ES effect size, n.a. not applicable, OR odds ratio, HR hazard ratio
Sensitivity analyses
Sensitivity analyses were performed based on the type of dementia. In particular, the pooled effect size for the risk of dementia, including only unspecified dementia and mild cognitive impairment, was calculated based on 17 studies. Using the random effect model, the pooled OR was 0.93 [(95% CI = 0.88–0.98); p-value = 0.005] based on 59,571 participants with moderate statistical heterogeneity (df = 16, I2 = 53.56, p-value = 0.005). Similar results were found using the fixed effect model. Potential publication bias was identified by visual assessment of the funnel plot and confirmed by Egger’s linear regression test (intercept −0.72, p-value = 0.078). After applying the trim and fill method, the estimated effect size of the fixed effect was not significantly different from the main result, while the estimated effect size of the random effect was not statistically significant (Table 3). Again, an alternative analysis was performed using the two MedDiet scores calculated by Hosking et al. [45], but the results did not change.
A sensitivity analysis focusing only on unspecified dementia (excluding Alzheimer’s disease and mild cognitive impairment) was performed. In this analysis, 13 studies were included and using the random effects model, the pooled OR was 0.94 [(95% CI = 0.88–0.99); p-value = 0.005] based on 59,571 participants, with moderate statistical heterogeneity (df = 12, I2 = 59.86, p-value = 0.003). Similar results were found using the fixed effects model. No potential publication bias was detected by visual assessment of the funnel plot and confirmed by Egger’s linear regression test (intercept −0.62, p-value = 0.239) (Table 3).
A sensitivity analysis focusing on Alzheimer’ disease only was performed. In this analysis, eight studies were included and using the random effects model, the pooled OR was 0.73 [(95% CI = 0.62–0.85); p-value < 0.001] based on 38,292 participants, with moderate statistical heterogeneity (df = 7, I2 = 63.85, p-value = 0.007). Similar results were found using the fixed effects model. Potential publication bias was detected by visual assessment of the funnel plot and confirmed by Egger’s linear regression test (intercept −1.88, p-value = 0.055) (Table 3). After applying the trim and fill method, the estimated effect sizes for both fixed and random effects remain relatively consistent (Table 3).
A sensitivity analysis focusing on mild cognitive impairment only was performed. In this analysis, only three studies were included and using the random effect model, the pooled OR was 0.89 [(95% CI = 0.79–1.01); p-value = 0.063] based on 2996 participants, with low statistical heterogeneity (df = 2, I2 = 35.49, p-value = 0.212). A statistically significant result was found using the fixed effects model. Potential publication bias was identified by visual assessment of the funnel plot and confirmed by Egger’s linear regression test (intercept −2.72, p-value = 0.259). After applying the trim and fill method, the estimated effect size for both the fixed and random effects was no longer statistically significant (Table 3).
A sensitivity analysis based on methodological quality was not performed as all included studies were of high quality. Finally, studies based on the same population were excluded to avoid potential overlapping effects. In this analysis, only three studies were included and using the random effects model, the pooled OR was 0.89 [(95% CI = 0.82–0.97); p-value = 0.005] based on 58,813 participants, with moderate statistical heterogeneity (df = 15, I2 = 62.80, p-value < 0.001). Similar results were found using the fixed effects model. Potential publication bias was identified by visual assessment of the funnel plot and confirmed by Egger’s linear regression test (intercept −0.93, p-value = 0.126). After applying the trim and fill method, the estimated effect sizes remain similar for both fixed and random effects (Table 3).
Subgroup analyses
A subgroup analysis by study design was performed for the main analysis (all types of dementia) and for each type of dementia. In particular, considering cross-sectional studies focusing on all types of dementia, seven studies were included and using the random effect model, the pooled OR was 0.91 [(95% CI = 0.82–1.00); p-value = 0.055] based on 10,029 participants, with moderate statistical heterogeneity (df = 7, I2 = 52.25, p-value = 0.050). More statistically significant results were found using the fixed effects model. No potential publication bias was identified by visual assessment of the funnel plot and confirmed by Egger’s linear regression test (intercept −0.78, p-value = 0.394) (Table). Focusing on cohort studies, 15 studies were included and using the random effect model, the pooled HR was 0.84 [(95% CI = 0.76–0.94); p-value = 0.002] based on 55,205 participants, with high statistical heterogeneity (df = 14, I2 = 89.70, p-value < 0.001). After applying the trim and fill method, the estimated effect size for both fixed and random effects did not change (Table 3).
Subgroup analyses by study design for dementia, unspecified dementia, Alzheimer’s disease and MCI are reported in Table 3. Subgroup analysis by geographical area was performed by grouping countries in the Mediterranean area. In this case, six studies were included and the pooled OR was 0.95 [(95% CI = 0.91–0.99); p-value < 0.001] based on 6879 participants, with low statistical heterogeneity (df = 5, I2 = 31.91, p-value = 0.196). Potential publication bias was identified by visual assessment of the funnel plot and confirmed by Egger’s linear regression test (intercept −0.86, p-value = 0.171). After applying the trim and fill method, the estimated effect size for both fixed and random effects did not change (Table 3).
Subgroup analysis by sex was not possible because less than three studies reported data for sex separately. Finally, as all the studies used validated tools to diagnose dementia, only a sensitivity analysis based on studies that used validated tools to assess diet was performed. In this case, 20 studies were included and the pooled OR was 0.89 [(95% CI = 0.83–0.94); p-value < 0.001] based on 37,930 participants, with moderate statistical heterogeneity (df = 19, I2 = 71.07, p-value < 0.001). After applying the trim and fill method, the estimated effect size of the fixed effect was not significantly different, while the estimated effect size of the random effect was not statistically significant (Table 3).
Discussion
In this systematic review and meta-analysis, we assessed the association between the highest level of adherence to the MedDiet and the likelihood of developing dementia. The main results suggest that the highest adherence to the MedDiet is associated with an approximate 11% reduction in the likelihood of developing dementia in a population of 65,955 older adults. Despite the apparently small protective effect, it should be borne in mind that dementia is a frequently diagnosed disease, especially in the elderly. Furthermore, dementia has a high burden in terms of cost of care and quality of life. Furthermore, it is important to consider that the world's population is undergoing a progressive aging process, wherein the elderly constitute a significant proportion of the population. Therefore, even though the estimated effect size may be relatively modest, this applies to a rather large segment of the population, possibly on the rise. Additionally, it is crucial to bear in mind that the estimated effect in this meta-analysis is attributable solely to adherence to the MedDiet. This implies that by improving one's diet alone, the risk of developing dementia could be significantly reduced. Greater results could be achieved by implementing multiple healthy lifestyle choices [55]. Lastly, it should not be underestimated that this effect is linked to a primary prevention effect, the cost of which is negligible, especially when considering the high burden of dementia. Our results can be considered reliable insofar as we took into account several methodological aspects. First, we used both fixed effects and random effects models, the latter of which are recommended in the case of high to moderate statistical heterogeneity; however, the results did not change significantly. Secondly, given the heterogeneity of the MedDiet scores used in the original studies, we performed supplementary analyses using the MedDiet scores as an alternative, without this affecting the results. This type of analysis was performed primarily to test whether the use of different scales might affect the strength of the association. The same rationale was applied in the sensitivity analysis, for which we only considered studies that reported using a validated dietary assessment tool. In addition, as some studies were conducted based on the same population, only those with the largest sample size were included, thus eliminating the potential overlap effect. Even in this case, the results did not change significantly, confirming the robustness of our findings. To strengthen our results, we also estimated the risks separately by type of dementia, looking specifically at the risk of all types of dementia, all types of dementia without Alzheimer’s disease, dementia without Alzheimer’s disease and MCI, Alzheimer’s disease only, and MCI only. The association was found to be stronger only when only Alzheimer’s disease was considered, with the results suggesting that higher adherence to the MedDiet is associated with an approximately 27% lower risk of Alzheimer’s disease. On the contrary, the association was no longer significant when looking at MCI alone. However, only three studies reported separate data for MCI, so this result should be interpreted with caution. In fact, the sample size was relatively small, which may have affected the statistical power. Moreover, to assess the risk of prevalent and incident dementia separately, subgroup analyses were performed using only cross-sectional or cohort studies, respectively. When only cross-sectional studies were considered, the results remained relatively consistent for each of the type of dementia considered. Conversely, when only cohort studies were included, the association was borderline significant. This could be explained by the smaller sample size or by an inherent methodological weakness of cohort studies. In fact, longitudinal studies may be prone to selection bias, especially in the elderly population, who may be lost to follow-up for various reasons, including death. Moreover, specifically for dementia, it can be difficult or less accurate to assess exposure using questionnaires, thus affecting the certainty of results. From this perspective, case–control studies could also be a valid instrument for assessing the association between MedDiet and dementia, also considering that only one case–control study was retrieved and included in the current meta-analysis. Furthermore, dietary intake, by definition, is characterized by intrinsic methodological challenges. Dietary intake is measured and then quantified using self-reported data (questionnaires, diaries or 24-h recalls) which may be subject to recall bias, social desirability bias and even misreporting or misclassification. Moreover, dietary habits are culturally specific and assessing adherence to the MedDiet even in non-Mediterranean countries can be more complicated because certain Mediterranean foods are consumed less frequently or not at all, or, conversely, others may be consumed more frequently but not considered in the MedDiet scores, thus altering dietary intake. Moreover, due to the generally low adherence to the MedDiet, especially in non-Mediterranean countries, differences between groups can be more difficult to define, and the possible association between exposure and outcome(s) can be blurred. Therefore, we performed a subgroup analysis that only included studies conducted in the Mediterranean area. In this case, higher adherence to the MedDiet was associated with a 4–5% reduced risk of all types of dementia (considering fixed and random effects model, respectively). This risk reduction is lower than in the main analysis and when only Alzheimer’s disease was considered, probably because it included only six studies with a small sample size (n = 6879 subjects).
Lastly, it was not possible to analyse the subgroups by sex because fewer than three studies reported data for both sexes separately. In this respect, further research is needed to assess differences between the two sexes in elderly people. However, a previous meta-analysis assessing the association between MedDiet and cognitive health highlights attenuated results when only women were considered [56]; which potentially suggests that the cognitive effect of the MedDiet differs between sexes. Moreover, despite some differences in terms of inclusion/exclusion criteria, our results are similar to previously published meta-analyses, which mostly involve only cohort studies (usually with at least 1 year of follow-up) and including adults in general (not only the elderly, as in our case). In particular, the meta-analysis conducted by Cao et al. included only 4 studies and the estimated risk reduction was around 31% [RR = 0.69 (95% CI 0.57–0.84)] considering dementia or MCI [57]. The meta-analysis conducted by Singh et al. found a 33% risk reduction between the higher tertiles of MedDiet adherence and MCI or Alzheimer [HR = 0.67 (95% CI 0.55–0.81)], based on five studies [58]. Wu et al. also conducted a meta-analysis assessing the association between MedDiet and all types of dementia. This included nine cohort studies and the estimated risk reduction was 21% [RR = 0.79 (95% CI 0.70–0.90)], with no evidence of significant heterogeneity. However, the meta-analysis conducted by Coelho-Júnior et al. specifically focused on the elderly (adults over 60). However, despite finding a significant association between higher adherence to the MedDiet and multiple functional and cognitive functions (such as walking speed, knee muscle strength, global cognition and memory), they failed to find a significant association with all types of dementia (seven studies), Alzheimer’s (five studies) and MCI (three studies) [17].
Potential biological mechanisms
Cardiovascular risk factors such as hypertension, obesity (mainly abdominal obesity), dyslipidaemia, and type 2 diabetes are considered to have a significant impact on the risk of dementia [59]. These factors are indeed associated with chronic inflammation and metabolic dysfunctions such as insulin resistance and consequent hyperinsulinemia that could be detrimental to the brain [60–62]. Findings from several studies have shown that a high adherence to the MedDiet can lead to a reduction in several biomarkers of inflammation known to be implicated in the onset of AD, such as pro-inflammatory cytokines interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumour necrosis factor-alfa (TNF-α) [63–65]. The role of the MedDiet in reducing chronic inflammation seems to be mediated by the anti-oxidant and anti-inflammatory action of the numerous bioactive compounds such as vitamins, minerals, phytochemicals, and essential fatty acids, provided by foods typically consumed as part of this dietary pattern [66]. In addition, the lower energy density of the MedDiet, as compared to western dietary pattern, improves weight management and helps to reduce adipose tissue (especially abdominal fat) leading to a decrease in the production of pro-inflammatory cytokines, improved insulin-resistance and hyperinsulinemia, and improvements in other parameters such as hypertension and fasting glucose levels [67].
Moreover, there are other possible factors that could explain the positive effect of the MedDiet in preventing cognitive disorders. In particular, the MedDiet seems to help regulate the structure and function of the gut microbiota [68]. Dysbiotic gut microbiota are believed to play a role in the pathogenesis of cognitive decline, and in particular in AD, leading to synaptic dysfunction and neuroinflammation [69]. The MedDiet, in which is rich in fibre, plant protein and healthy fats (mainly from seafood, nuts and olive oil), along with a limited amount of saturated fat, animal protein and refined sugar, has been shown to have a positive impact on microbiota composition by increasing the bacteria that produce short-chain fatty acids, which are metabolites with anti-inflammatory effect [68]. Moreover, higher adherence to MedDiet has been associated with higher biodiversity of microbiota, which in turn seems to be associated with a regulation of cognitive functions [70]. In addition, a state of eubiosis has been shown to help reduce endothelial dysfunction, which is another known risk factor of cognitive [71].
Implications for policy, practice and future research
Our data show an 11% reduction in the risk of all types of dementia and a 27% reduction in the risk of Alzheimer’s disease in people who follow the MedDiet. However, moderate heterogeneity was observed in the main analysis and in almost all sensitivity and subgroup analyses. Furthermore, when only cohort studies were considered individually, the statistical significance was found to be borderline or no longer significant. One interpretation of this finding could be that diet, and in particular stronger adherence to the Mediterranean diet, may have a greater protective effect against Alzheimer’s disease than against dementia in general. Although the underlying pathophysiological mechanisms of the protective role of the MedDiet against dementia are not clear, some studies found a significant correlation between increased adherence to the MedDiet and lower Alzheimer’s disease biomarker burden. Specifically, Hill et al. found that higher adherence to the MedDiet was associated with a lower deposition [0.11 (95% CI 0.04–0.17)] of beta-amyloid (Aβ), a key protein in the Alzheimer disease pathogenesis [72]. Furthermore, in another study, adherence to a MedDiet was found to be inversely correlated with brain positron emission tomography (PET) for both beta-amyloid plaques and tau tangles, meaning that higher adherence to the MedDiet is associated not only with lower deposition of beta-amyloid, but also with lower protein tau accumulation [73]. Consequently, higher adherence to the MedDiet could have a preventive impact on both the main pathogenetic pathways of Alzheimer's disease.
It is also important to bear in mind that our study population is made up of individuals over the age of 60. In such individuals, it is conceivable that the effects of recently adopted dietary habits may have less influence on health outcomes such as dementia (which require long-term exposure), as compared with dietary habits followed throughout their lifetime. However, the over-60s are the population most affected by dementia. It is, therefore, very important to consider diet as a potential exposure factor that can modify the risk of this disease, both in terms of prevention and healthcare policies, especially considering that there is still no direct treatment for dementia. Moreover, it should also consider that adherence to MedDiet is decreasing overtime, with the exception registered during COVID-19 pandemic [74]. These data prompt reflections in terms of public health. Specifically, if spending more time at home during the COVID-19 pandemic has led to increased adherence to the MedDiet [74], public health strategies should focus not only on greater nutrition education closely tied to the characteristics of the MedDiet itself [75, 76]; but also, on promoting policies that facilitate adherence to the MedDiet in all settings. In this regard, much is being done to facilitate the availability of healthy food options for consumption during meals outside the home [77]. However, more should be done to promote culinary skills that enable individuals to prepare healthy dishes [78, 79], as well as invest time in their preparation, possibly facilitating the sharing of these moments with friends and family (conviviality). These are essential elements of the MedDiet that, although supported by scientific knowledge, are not yet effectively integrated into health policies and campaigns for promoting and educating about nutrition and health. This applies to both the general population and specific target groups, as well as professionals in the food and health sector.
Like many other lifestyle recommendations, adopting a Mediterranean diet confers many additional health benefits, such as the positive effects on mental health, as well the associated reduced incidence of cardiovascular diseases and diabetes [80–86]. This means that the decision to implement health policies aimed at promoting this type of diet can be advantageous in the long term as it has positive repercussions on numerous health outcomes of public interest. Efforts should not only be directed towards changing the population's eating habits; the promotion of a healthy lifestyle must also be supported by much broader policies to make healthy choices easier. For example, adopting an effective food nutrition labelling system could help consumers make healthier food choices. Furthermore, the MedDiet has been shown to be a sustainable and cost-effective way of reducing the risk of many health conditions [87].
Lastly, starting from our results, future studies need to more deeply explore the underlying biological mechanisms through which the MedDiet may influence dementia, and therefore comparing the effectiveness of the MedDiet with other dietary patterns in reducing the risk of dementia. As well as, investigate the potential synergistic effects of combining adherence to the MedDiet with other healthy lifestyle factors, such as physical activity, mental stimulation, and social engagement still remain an important aspect to be further assessed in future. Lastly, scientific collaboration across countries might contribute in definition of a globally recognised method for assessing MedDiet adherence. This could reduce the high heterogeneity found regarding MedDiet scores and methods, improve comparisons among different regions, and foster scientific collaborations.
Limitations and strengths
Our results should be interpreted with caution because they do involve some limitations: firstly, this is a secondary analysis (review of original studies) and, therefore, it is automatically influenced by the limitations of each of the included studies, such as potential selection bias or bias in exposure or outcome assessment. These limitations include the fact that dietary intake was self-reported, with potentially risk of recall or social desirability bias, and that several types of MedDiet scores were used. There was also high heterogeneity, probably because of the different types of scores used, or because of the different type of potential confounders considered in each included study; a third limitation is that most of the included studies were cross-sectional, in which causality might not be assessed by definition.
Nonetheless, the current systematic review has certain strengths: first, we followed the PRISMA guidelines which allow us to use a comprehensive approach both for conduct and reporting; we also consulted three different databases in order to retrieve all eligible studies (more than the minimum required by guidelines). We conducted multiple sensitivity and subgroup analyses to assess the association between several types of dementia, as well as the study design, geographical area, or MedDiet score used. In contrast with previous meta-analysis, we did not exclude cross-sectional studies, which are a valuable study design especially when cohort studies are difficult to perform. As mentioned before, longitudinal studies among the elderly might be biased because of the potentially high number of lost to follow-up. Moreover, even if in cross-sectional studies exposure and outcome are measured at the same time-point, it is challenging to consider that a higher adherence to MedDiet has occurred due to dementia.
Conclusions
In conclusion, the present study assessed the association between adherence to the Mediterranean diet and all types of dementia (stratifying the results by type of diagnosis) in the elderly population aged over 60 years. There is a protective effect of the Mediterranean diet when all types of dementia are considered together and when only Alzheimer's disease is considered individually. Specifically, there is an 11% reduction in the risk of all types of dementia and a 27% reduction if only Alzheimer's disease is considered. Given the moderate heterogeneity observed and the limitations mentioned above, these results should be interpreted with caution. However, even if the risk reduction is minimal, especially when all types of dementia are considered, it is true that it affects a relatively large number of people, especially the elderly. Therefore, even a small percentage reduction would represent a significant number of people who could potentially prevent dementia just by increasing their adherence to the Mediterranean diet. Consequently, our results confirm the importance of promoting adherence to the Mediterranean diet in order to improve cognitive health in aging populations.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
Conceptualisation: V.G. and D.N.; methodology: V.G.; Software: V.G.; Studies quality assessment: A.S. (Andea Sommariva), L.M.D, G.G., M.M., F.N. and A.S. (Antonella Savoia); formal analysis, V.G.; investigation: A.C., R.F., M.M., A.S. (Antonella Savoia); data curation: V.G. and D.N.; writing—original draft preparation: D.N. and V.G.; writing—review and editing: F.E.P., S.C., G.G. and V.G.; Tables and Figures: V.G. and D.N.; supervision, V.G. All authors have read and agreed to the published version of the manuscript.
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement. Authors did not received any funding for this work.
Data availability
Not applicable.
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Blaszczyk JW. Pathogenesis of Dementia. Int J Mol Sci. 2022;24:543. doi: 10.3390/ijms24010543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans DA. Population estimate of people with clinical Alzheimer's disease and mild cognitive impairment in the United States (2020–2060) Alzheimer's Dementia. 2021;17:1966–1975. doi: 10.1002/alz.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doblhammer G, Fritze T, Reinke C, Fink A. Can dementia become the most prevalent disease at the time of death in Germany? Projections up to the year 2060 for the five most important diseases at the time of death. J Popul Ageing. 2022;15:523–540. doi: 10.1007/s12062-022-09365-7. [DOI] [Google Scholar]
- 4.Kumar A, Sidhu J, Goyal A, Tsao JW. Alzheimer Disease. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Jaskirat Sidhu declares no relevant financial relationships with ineligible companies. Disclosure: Amandeep Goyal declares no relevant financial relationships with ineligible companies. Disclosure: Jack Tsao declares no relevant financial relationships with ineligible companies. StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC.; 2023.
- 5.Breijyeh Z, Karaman R. Comprehensive review on Alzheimer's disease: causes and treatment. Molecules. 2020;25:5789. doi: 10.3390/molecules25245789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanrahan JG, Burford C, Nagappan P, Adegboyega G, Rajkumar S, Kolias A, Helmy A, Hutchinson PJ. Is dementia more likely following traumatic brain injury? Syst Rev J Neurol. 2023;270:3022–3051. doi: 10.1007/s00415-023-11614-4. [DOI] [PubMed] [Google Scholar]
- 7.Leung DKY, Chan WC, Spector A, Wong GHY. Prevalence of depression, anxiety, and apathy symptoms across dementia stages: A systematic review and meta-analysis. Int J Geriatr Psychiatry. 2021;36:1330–1344. doi: 10.1002/gps.5556. [DOI] [PubMed] [Google Scholar]
- 8.McGuinness B, Todd S, Passmore P, Bullock R. Blood pressure lowering in patients without prior cerebrovascular disease for prevention of cognitive impairment and dementia. Cochrane Database Syst Rev. 2009;2009:CD004034. doi: 10.1002/14651858.CD004034.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters R, Poulter R, Warner J, Beckett N, Burch L, Bulpitt C. Smoking, dementia and cognitive decline in the elderly, a systematic review. BMC Geriatr. 2008;8:36. doi: 10.1186/1471-2318-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vrijsen J, Abu-Hanna A, de Rooij SE, Smidt N. Association between dementia parental family history and mid-life modifiable risk factors for dementia: a cross-sectional study using propensity score matching within the Lifelines cohort. BMJ Open. 2021;11:e049918. doi: 10.1136/bmjopen-2021-049918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharp ES, Gatz M. Relationship between education and dementia: an updated systematic review. Alzheimer Dis Assoc Disord. 2011;25:289–304. doi: 10.1097/WAD.0b013e318211c83c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang C, Wang Y, Wang D, Zhang J, Zhang F. NSAID exposure and risk of Alzheimer's disease: an updated meta-analysis from Cohort Studies. Front Aging Neurosci. 2018;10:83. doi: 10.3389/fnagi.2018.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bransby L, Buckley RF, Rosenich E, Franks KH, Yassi N, Maruff P, Pase MP, Lim YY. The relationship between cognitive engagement and better memory in midlife. Alzheimer Dement (Amst) 2022;14:e12278. doi: 10.1002/dad2.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr. 2010;92:1189–1196. doi: 10.3945/ajcn.2010.29673. [DOI] [PubMed] [Google Scholar]
- 16.Tuttolomondo A, Simonetta I, Daidone M, Mogavero A, Ortello A, Pinto A. Metabolic and vascular effect of the Mediterranean diet. Int J Mol Sci. 2019;20:4716. doi: 10.3390/ijms20194716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coelho-Júnior HJ, Trichopoulou A, Panza F. Cross-sectional and longitudinal associations between adherence to Mediterranean diet with physical performance and cognitive function in older adults: A systematic review and meta-analysis. Ageing Res Rev. 2021;70:101395. doi: 10.1016/j.arr.2021.101395. [DOI] [PubMed] [Google Scholar]
- 18.Wu L, Sun D. Adherence to Mediterranean diet and risk of developing cognitive disorders: An updated systematic review and meta-analysis of prospective cohort studies. Sci Rep. 2017;7:41317. doi: 10.1038/srep41317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Féart C, Samieri C, Rondeau V, Amieva H, Portet F, Dartigues JF, Scarmeas N, Barberger-Gateau P. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA. 2009;302:638–648. doi: 10.1001/jama.2009.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods Group, Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi:10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed]
- 21.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hrobjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gianfredi V, Bragazzi NL, Nucci D, Villarini M, Moretti M. Cardiovascular diseases and hard drinking waters: implications from a systematic review with meta-analysis of case-control studies. J Water Health. 2017;15:31–40. doi: 10.2166/wh.2016.131. [DOI] [PubMed] [Google Scholar]
- 23.Gianfredi V, Nucci D, Vannini S, Villarini M, Moretti M. In vitro Biological effects of sulforaphane (SFN), epigallocatechin-3-gallate (EGCG), and curcumin on breast cancer cells: a systematic review of the literature. Nutr Cancer. 2017;69:969–978. doi: 10.1080/01635581.2017.1359322. [DOI] [PubMed] [Google Scholar]
- 24.Andreu-Reinón ME, Chirlaque MD, Gavrila D, Amiano P, Mar J, Tainta M, Ardanaz E, Larumbe R, Colorado-Yohar SM, Navarro-Mateu F, Navarro C, Huerta JM. Mediterranean diet and risk of dementia and Alzheimer's disease in the EPIC-Spain Dementia Cohort Study. Nutrients. 2021;13:700. doi: 10.3390/nu13020700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen H, Dhana K, Huang Y, Huang L, Tao Y, Liu X, Melo van Lent D, Zheng Y, Ascherio A, Willett W, Yuan C. Association of the Mediterranean dietary approaches to stop hypertension intervention for neurodegenerative delay (MIND) diet with the risk of Dementia. JAMA Psychiat. 2023;80:630–638. doi: 10.1001/jamapsychiatry.2023.0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cornelis MC, Agarwal P, Holland TM, van Dam RM. MIND dietary pattern and its association with cognition and incident Dementia in the UK Biobank. Nutrients. 2022;15:32. doi: 10.3390/nu15010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dhana K, Evans DA, Rajan KB, Bennett DA, Morris MC. Healthy lifestyle and the risk of Alzheimer dementia: Findings from 2 longitudinal studies. Neurology. 2020;95:e374–e383. doi: 10.1212/wnl.0000000000009816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filippini T, Adani G, Malavolti M, Garuti C, Cilloni S, Vinceti G, Zamboni G, Tondelli M, Galli C, Costa M, Chiari A, Vinceti M. Dietary habits and risk of early-onset Dementia in an Italian case-control study. Nutrients. 2020;12:3682. doi: 10.3390/nu12123682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu EA, Wu A, Dearborn JL, Gottesman RF, Sharrett AR, Steffen LM, Coresh J, Rebholz CM. Adherence to dietary patterns and risk of incident dementia: findings from the atherosclerosis risk in communities study. J Alzheimer Dis. 2020;78:827–835. doi: 10.3233/jad-200392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ince N, Gelener P, Besler HT. Does Mediterranean diet correlate with cognitive performance among elderly? A cross-sectional study from Cyprus. Prog Nutr. 2020;22:75–83. doi: 10.23751/pn.v22i1.7801. [DOI] [Google Scholar]
- 31.Scarmeas N, Luchsinger JA, Mayeux R, Stern Y. Mediterranean diet and Alzheimer disease mortality. Neurology. 2007;69:1084–1093. doi: 10.1212/01.wnl.0000277320.50685.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeuchi H, Kawashima R. Diet and Dementia: a prospective study. Nutrients. 2021;13:4500. doi: 10.3390/nu13124500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas A, Lefèvre-Arbogast S, Féart C, Foubert-Samier A, Helmer C, Catheline G, Samieri C. Association of a MIND diet with brain structure and Dementia in a French population. J Prevent Alzheimer Dis. 2022;9:655–664. doi: 10.14283/jpad.2022.67. [DOI] [PubMed] [Google Scholar]
- 34.White SA, Ward N, Verghese J, Kramer AF, Grandjean da Costa K, Liu CK, Kowaleski C, Reid KF. NUTRITIONAL RISK STATUS, DIETARY INTAKE AND COGNITIVE PERFORMANCE IN OLDER ADULTS WITH MOTORIC COGNITIVE RISK SYNDROME. JAR Life. 2020;9:47–54. doi: 10.14283/jarlife.2020.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allcock L, Mantzioris E, Villani A. Adherence to a Mediterranean Diet is associated with physical and cognitive health: A cross-sectional analysis of community-dwelling older Australians. Front Public Health. 2022;10:1017078. doi: 10.3389/fpubh.2022.1017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anastasiou CA, Yannakoulia M, Kosmidis MH, Dardiotis E, Hadjigeorgiou GM, Sakka P, Arampatzi X, Bougea A, Labropoulos I, Scarmeas N. Mediterranean diet and cognitive health: Initial results from the Hellenic Longitudinal Investigation of Ageing and Diet. PLoS ONE. 2017;12:e0182048. doi: 10.1371/journal.pone.0182048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calil SRB, Brucki SMD, Nitrini R, Yassuda MS. Adherence to the Mediterranean and MIND diets is associated with better cognition in healthy seniors but not in MCI or AD. Clin Nutr ESPEN. 2018;28:201–207. doi: 10.1016/j.clnesp.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Chan R, Chan D, Woo J. A cross sectional study to examine the association between dietary patterns and cognitive impairment in older Chinese people in Hong Kong. J Nutr Health Aging. 2013;17:757–765. doi: 10.1007/s12603-013-0348-5. [DOI] [PubMed] [Google Scholar]
- 39.Charisis S, Ntanasi E, Yannakoulia M, Anastasiou CA, Kosmidis MH, Dardiotis E, Hadjigeorgiou G, Sakka P, Veskoukis AS, Kouretas D, Scarmeas N. Plasma GSH levels and Alzheimer's disease. A prospective approach: Results from the HELIAD study. Free Radic Biol Med. 2021;162:274–282. doi: 10.1016/j.freeradbiomed.2020.10.027. [DOI] [PubMed] [Google Scholar]
- 40.de Crom TOE, Mooldijk SS, Ikram MK, Ikram MA, Voortman T. MIND diet and the risk of dementia: a population-based study. Alzheimer Res Therapy. 2022;14:8. doi: 10.1186/s13195-022-00957-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gardener S, Gu Y, Rainey-Smith SR, Keogh JB, Clifton PM, Mathieson SL, Taddei K, Mondal A, Ward VK, Scarmeas N, Barnes M, Ellis KA, Head R, Masters CL, Ames D, Macaulay SL, Rowe CC, Szoeke C, Martins RN. Adherence to a Mediterranean diet and Alzheimer's disease risk in an Australian population. Transl Psychiatry. 2012;2:e164. doi: 10.1038/tp.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glans I, Sonestedt E, Nägga K, Gustavsson AM, González-Padilla E, Borne Y, Stomrud E, Melander O, Nilsson PM, Palmqvist S, Hansson O. Association between dietary habits in midlife with Dementia incidence over a 20-year period. Neurology. 2023;100:E28–E37. doi: 10.1212/WNL.0000000000201336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gu Y, Luchsinger JA, Stern Y, Scarmeas N. Mediterranean diet, inflammatory and metabolic biomarkers, and risk of Alzheimer's disease. J Alzheimer Dis. 2010;22:483–492. doi: 10.3233/jad-2010-100897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haring B, Wu C, Mossavar-Rahmani Y, Snetselaar L, Brunner R, Wallace RB, Neuhouser ML, Wassertheil-Smoller S. No association between dietary patterns and risk for cognitive decline in older women with 9-year follow-up: data from the women's health initiative memory study. J Acad Nutr Diet. 2016;116:921–930.e1. doi: 10.1016/j.jand.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hosking DE, Eramudugolla R, Cherbuin N, Anstey KJ. MIND not Mediterranean diet related to 12-year incidence of cognitive impairment in an Australian longitudinal cohort study. Alzheimer's Dementia. 2019;15:581–589. doi: 10.1016/j.jalz.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 46.Mamalaki E, Charisis S, Anastasiou CA, Ntanasi E, Georgiadi K, Balomenos V, Kosmidis MH, Dardiotis E, Hadjigeorgiou G, Sakka P, Scarmeas N, Yannakoulia M. The longitudinal association of lifestyle with cognitive health and dementia risk: findings from the HELIAD Study. Nutrients. 2022;14:2818. doi: 10.3390/nu14142818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer's disease. Alzheimer Dementia. 2015;11:1007–1014. doi: 10.1016/j.jalz.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicoli C, Galbussera AA, Bosetti C, Franchi C, Gallus S, Mandelli S, Marcon G, Quadri P, Riso P, Riva E, Lucca U, Tettamanti M. The role of diet on the risk of dementia in the oldest old: The Monzino 80-plus population-based study. Clin Nutr. 2021;40:4783–4791. doi: 10.1016/j.clnu.2021.06.016. [DOI] [PubMed] [Google Scholar]
- 49.Olsson E, Karlström B, Kilander L, Byberg L, Cederholm T, Sjögren P. Dietary patterns and cognitive dysfunction in a 12-year follow-up study of 70 year old men. J Alzheimers Dis. 2014;43:109–119. doi: 10.3233/JAD-140867. [DOI] [PubMed] [Google Scholar]
- 50.Roberts RO, Geda YE, Cerhan JR, Knopman DS, Cha RH, Christianson TJH, Pankratz VS, Ivnik RJ, Boeve BF, O'Connor HM, Petersen RC. Vegetables, unsaturated fats, moderate alcohol intake, and mild cognitive impairment. Dement Geriatr Cogn Disord. 2010;29:413–423. doi: 10.1159/000305099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scarmeas N, Stern Y, Mayeux R, Luchsinger JA. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch Neurol. 2006;63:1709–1717. doi: 10.1001/archneur.63.12.noc60109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66:216–225. doi: 10.1001/archneurol.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer's disease. Ann Neurol. 2006;59:912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Talhaoui A, Aboussaleh Y, Bikri S, Zohra Rouim F, Ahami A. The relationship between adherence to a Mediterranean diet and cognitive impairment among the elderly in Morocco. Acta Neuropsychologica. 2023;21:125–138. doi: 10.5604/01.3001.0053.4197. [DOI] [Google Scholar]
- 55.Ferravante C, Gianfredi V, Bietta C. [Nutrition in Umbria: adherence to five-a-day] Recenti Prog Med. 2020;111:539–545. doi: 10.1701/3421.34069. [DOI] [PubMed] [Google Scholar]
- 56.Aridi YS, Walker JL, Wright ORL. The association between the Mediterranean dietary pattern and cognitive health: a systematic review. Nutrients. 2017;9:674. doi: 10.3390/nu9070674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cao L, Tan L, Wang H-F, Jiang T, Zhu X-C, Lu H, Tan M-S, Yu J-T. Dietary patterns and risk of Dementia: a systematic review and meta-analysis of cohort studies. Mol Neurobiol. 2016;53:6144–6154. doi: 10.1007/s12035-015-9516-4. [DOI] [PubMed] [Google Scholar]
- 58.Singh B, Parsaik AK, Mielke MM, Erwin PJ, Knopman DS, Petersen RC, Roberts RO. Association of Mediterranean diet with mild cognitive impairment and Alzheimer's disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;39:271–282. doi: 10.3233/jad-130830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim YJ, Kim SM, Jeong DH, Lee SK, Ahn ME, Ryu OH. Associations between metabolic syndrome and type of dementia: analysis based on the National Health Insurance Service database of Gangwon province in South Korea. Diabetol Metab Syndr. 2021;13:4. doi: 10.1186/s13098-020-00620-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schrijvers EM, Witteman JC, Sijbrands EJ, Hofman A, Koudstaal PJ, Breteler MM. Insulin metabolism and the risk of Alzheimer disease: the Rotterdam Study. Neurology. 2010;75:1982–1987. doi: 10.1212/WNL.0b013e3181ffe4f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kellar D, Craft S. Brain insulin resistance in Alzheimer's disease and related disorders: mechanisms and therapeutic approaches. Lancet Neurol. 2020;19:758–766. doi: 10.1016/S1474-4422(20)30231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Femminella GD, Livingston NR, Raza S, van der Doef T, Frangou E, Love S, Busza G, Calsolaro V, Carver S, Holmes C, Ritchie CW, Lawrence RM, McFarlane B, Tadros G, Ridha BH, Bannister C, Walker Z, Archer H, Coulthard E, Underwood B, Prasanna A, Koranteng P, Karim S, Junaid K, McGuinness B, Passmore AP, Nilforooshan R, Macharouthu A, Donaldson A, Thacker S, Russell G, Malik N, Mate V, Knight L, Kshemendran S, Tan T, Holscher C, Harrison J, Brooks DJ, Ballard C, Edison P. Does insulin resistance influence neurodegeneration in non-diabetic Alzheimer’s subjects? Alzheimers Res Therapy. 2021;13:47. doi: 10.1186/s13195-021-00784-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonaccio M, Costanzo S, Di Castelnuovo A, Gialluisi A, Ruggiero E, De Curtis A, Persichillo M, Cerletti C, Donati MB, de Gaetano G, Iacoviello L. Increased adherence to a Mediterranean diet is associated with reduced low-grade inflammation after a 12.7-year period results from the Moli-sani Study. J Acad Nutr Diet. 2023;123:783–795.e7. doi: 10.1016/j.jand.2022.12.005. [DOI] [PubMed] [Google Scholar]
- 64.Koelman L, Egea Rodrigues C, Aleksandrova K. Effects of dietary patterns on biomarkers of inflammation and immune responses: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2022;13:101–115. doi: 10.1093/advances/nmab086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hart MJ, Torres SJ, McNaughton SA, Milte CM. Dietary patterns and associations with biomarkers of inflammation in adults: a systematic review of observational studies. Nutr J. 2021;20:24. doi: 10.1186/s12937-021-00674-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lourida I, Soni M, Thompson-Coon J, Purandare N, Lang IA, Ukoumunne OC, Llewellyn DJ. Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology. 2013;24:479–489. doi: 10.1097/ede.0b013e3182944410. [DOI] [PubMed] [Google Scholar]
- 67.Dinu M, Pagliai G, Angelino D, Rosi A, Dall'Asta M, Bresciani L, Ferraris C, Guglielmetti M, Godos J, Del Bo C, Nucci D, Meroni E, Landini L, Martini D, Sofi F. Effects of popular diets on anthropometric and Cardiometabolic parameters: an umbrella review of meta-analyses of randomized controlled trials. Adv Nutr. 2020;11:815–833. doi: 10.1093/advances/nmaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ghosh TS, Rampelli S, Jeffery IB, Santoro A, Neto M, Capri M, Giampieri E, Jennings A, Candela M, Turroni S, Zoetendal EG, Hermes GDA, Elodie C, Meunier N, Brugere CM, Pujos-Guillot E, Berendsen AM, De Groot L, Feskins EJM, Kaluza J, Pietruszka B, Bielak MJ, Comte B, Maijo-Ferre M, Nicoletti C, De Vos WM, Fairweather-Tait S, Cassidy A, Brigidi P, Franceschi C, O'Toole PW. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut. 2020;69:1218–1228. doi: 10.1136/gutjnl-2019-319654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bairamian D, Sha S, Rolhion N, Sokol H, Dorothee G, Lemere CA, Krantic S. Microbiota in neuroinflammation and synaptic dysfunction: a focus on Alzheimer's disease. Mol Neurodegener. 2022;17:19. doi: 10.1186/s13024-022-00522-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maggi S, Ticinesi A, Limongi F, Noale M, Ecarnot F. The role of nutrition and the Mediterranean diet on the trajectories of cognitive decline. Exp Gerontol. 2023;173:112110. doi: 10.1016/j.exger.2023.112110. [DOI] [PubMed] [Google Scholar]
- 71.Maiuolo J, Carresi C, Gliozzi M, Mollace R, Scarano F, Scicchitano M, Macri R, Nucera S, Bosco F, Oppedisano F, Ruga S, Coppoletta AR, Guarnieri L, Cardamone A, Bava I, Musolino V, Paone S, Palma E, Mollace V. The contribution of gut microbiota and endothelial dysfunction in the development of arterial hypertension in animal models and in humans. Int J Mol Sci. 2022;23:3698. doi: 10.3390/ijms23073698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hill E, Goodwill AM, Gorelik A, Szoeke C. Diet and biomarkers of Alzheimer's disease: a systematic review and meta-analysis. Neurobiol Aging. 2019;76:45–52. doi: 10.1016/j.neurobiolaging.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 73.Merrill DA, Siddarth P, Raji CA, Emerson ND, Rueda F, Ercoli LM, Miller KJ, Lavretsky H, Harris LM, Burggren AC, Bookheimer SY, Barrio JR, Small GW. Modifiable risk factors and brain positron emission tomography measures of amyloid and tau in Nondemented adults with memory complaints. Am J Geriatr Psychiatry. 2016;24:729–737. doi: 10.1016/j.jagp.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Della Valle PG, Mosconi G, Nucci D, Vigezzi GP, Gentile L, Gianfredi V, Bonaccio M, Gianfagna F, Signorelli C, Iacoviello L, Odone A. Adherence to the Mediterranean Diet during the COVID-19 national lockdowns: a systematic review of observational studies. Acta Biomed. 2021;92:e2021440. doi: 10.23750/abm.v92iS6.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aureli V, Rossi L. Nutrition knowledge as a driver of adherence to the Mediterranean diet in Italy. Front Nutr. 2022;9:804865. doi: 10.3389/fnut.2022.804865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Turner A, LaMonica HM, Moroney C, O'Leary F, Naismith SL, Flood VM. Knowledge, attitudes, and behaviours concerning the Mediterranean diet among older adults in Australia. J Community Health. 2023;48:951–962. doi: 10.1007/s10900-023-01237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sotos-Prieto M, Del Rio D, Drescher G, Estruch R, Hanson C, Harlan T, Hu FB, Loi M, McClung JP, Mojica A, Puglielli D, Toong K, Yangarber F, Kales SN. Mediterranean diet - promotion and dissemination of healthy eating: proceedings of an exploratory seminar at the Radcliffe institute for advanced study. Int J Food Sci Nutr. 2022;73:158–171. doi: 10.1080/09637486.2021.1941804. [DOI] [PubMed] [Google Scholar]
- 78.Hughes G, Bennett KM, Hetherington MM. Old and alone: barriers to healthy eating in older men living on their own. Appetite. 2004;43:269–276. doi: 10.1016/j.appet.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 79.Tani Y, Fujiwara T, Kondo K. Cooking skills related to potential benefits for dietary behaviors and weight status among older Japanese men and women: a cross-sectional study from the JAGES. Int J Behav Nutr Phys Act. 2020;17:82. doi: 10.1186/s12966-020-00986-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Uusitupa M, Khan TA, Viguiliouk E, Kahleova H, Rivellese AA, Hermansen K, Pfeiffer A, Thanopoulou A, Salas-Salvadó J, Schwab U, Sievenpiper JL. Prevention of type 2 diabetes by lifestyle changes: a systematic review and meta-analysis. Nutrients. 2019;11:2611. doi: 10.3390/nu11112611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosato V, Temple NJ, La Vecchia C, Castellan G, Tavani A, Guercio V. Mediterranean diet and cardiovascular disease: a systematic review and meta-analysis of observational studies. Eur J Nutr. 2019;58:173–191. doi: 10.1007/s00394-017-1582-0. [DOI] [PubMed] [Google Scholar]
- 82.Psaltopoulou T, Sergentanis TN, Panagiotakos DB, Sergentanis IN, Kosti R, Scarmeas N. Mediterranean diet, stroke, cognitive impairment, and depression: a meta-analysis. Ann Neurol. 2013;74:580–591. doi: 10.1002/ana.23944. [DOI] [PubMed] [Google Scholar]
- 83.Dinu M, Lotti S, Napoletano A, Corrao A, Pagliai G, Tristan Asensi M, Gianfredi V, Nucci D, Colombini B, Sofi F. Association between psychological disorders, Mediterranean diet, and Chronotype in a group of Italian adults. Int J Environ Res Public Health. 2022;20:335. doi: 10.3390/ijerph20010335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gianfredi V, Nucci D, Tonzani A, Amodeo R, Benvenuti AL, Villarini M, Moretti M. Sleep disorder, Mediterranean Diet and learning performance among nursing students: inSOMNIA, a cross-sectional study. Ann Ig. 2018;30:470–481. doi: 10.7416/ai.2018.2247. [DOI] [PubMed] [Google Scholar]
- 85.Nucci D, Nardi M, Cinnirella A, Campagnoli E, Maffeo M, Perrone PM, Shishmintseva V, Grosso FM, Castrofino A, Castaldi S, Romano L, Gianfredi V. Adherence to Mediterranean diet and risk of pancreatic cancer: systematic review and meta-analysis. Int J Environ Res Public Health. 2023;20:2403. doi: 10.3390/ijerph20032403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gianfredi V, Koster A, Odone A, Amerio A, Signorelli C, Schaper NC, Bosma H, Kohler S, Dagnelie PC, Stehouwer CDA, Schram MT, Dongen M, Eussen S. Associations of dietary patterns with incident depression: the Maastricht study. Nutrients. 2021;13:1034. doi: 10.3390/nu13031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Germani A, Vitiello V, Giusti AM, Pinto A, Donini LM, del Balzo V. Environmental and economic sustainability of the Mediterranean Diet. Int J Food Sci Nutr. 2014;65:1008–1012. doi: 10.3109/09637486.2014.945152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.