Abstract
Context.
Treatment of chronic viral hepatitis C (HCV) infection with direct-acting antiviral agents (DAAs) results in cure, or sustained viral response (SVR), in more than 90% of patients. However, there are subsets of patients who have persistent liver inflammation and fibrosis and develop hepatocellular carcinoma (HCC) despite achieving SVR. A possible reason for these phenomena may be the presence of virus particles in liver tissue but not blood, otherwise defined as occult infection.
Objective.
To describe liver histologic findings following successful DAA therapy, test HCV RNA by (liver) tissue polymerase chain reaction in treated cases, and identify predictive markers for HCC development in treated cases.
Design.
A total of 96 identified patients were divided into 4 groups, each differentiated by the presence or absence of SVR and HCC. Groups were compared for several clinicopathologic variables, including degree of inflammation and fibrosis, and the ‘directionality’ of fibrosis in cirrhotic livers using the novel progressive-indeterminate-regressive scoring system.
Results.
Overall, we found a significant decrease in inflammation in SVR patients. None of the patients showed regression of their cirrhosis following treatment. No evidence of occult HCV infection was seen in 40 livers tested, including 21 with HCC. The number of patients who developed HCC was similar in the SVR and non-SVR groups, and increased inflammation and fibrosis were associated with HCC development.
Conclusions.
Following DAA-SVR there appears to be an overall decrease in inflammation, but the fibrosis tends to persist, at least in the short term (median follow-up of 20.2 months).
The advent of direct-acting antiviral agents (DAAs) represents a landmark achievement in the management of chronic hepatitis C virus (HCV) infection. More than 90% of patients treated with DAAs achieve sustained virologic response (SVR) and are effectively cured.1–3 Scant data exist on histologic changes seen after DAA therapy; studies have reported persistent inflammation and fibrosis in some patients, despite cure.4 In addition, the impact of eliminating the virus on tumorigenesis remains controversial: achieving SVR may not necessarily correlate with a reduction in the risk of development or recurrence of hepatocellular carcinoma (HCC), especially in patients with cirrhosis.5–7
During and after treatment, therapeutic response is determined by measuring serum HCV RNA levels using various nucleic acid amplification tests. At 12 weeks after the completion of treatment, undetectable serum HCV RNA is indicative of SVR, and relapse after this period rarely occurs with DAA therapy.8 DAA-associated SVR (hereafter referred to as DAA-SVR) correlates with a significantly decreased risk of liver-related morbidity and all-cause mortality.9,10 However, despite undetectable HCV RNA in serum, virus has been detected in peripheral blood mononuclear cells or hepatic tissue in a subset of patients.11 Referred to as occult HCV infection, this phenomenon was first described in 2004 by Castillo et al12 when they showed that 57 of 100 patients with persistently abnormal liver function test results and no identifiable cause, including negative results for serum HCV antibody and RNA levels, had detectable HCV RNA in hepatic tissue. Subsequently, the concept of occult HCV infection has been broadened to encompass HCV antibody–positive patients with undetectable serum HCV RNA in the context of spontaneous clearance or treatment, but detectable virus in the tissues. Whether residual inflammation, fibrosis, and risk of HCC in patients after DAA-SVR is related to occult HCV infection has not been explored.
Another issue pertaining to DAA-SVR is its impact on patients who already have cirrhosis. Reversal of fibrosis has been well documented in the setting of interferon (IFN) treatment of HCV-infected and hepatitis B virus–infected patients.13–15 Assessment of the change in severity of fibrosis in response to therapy remains challenging. Most studies have done this using conventional fibrosis staging systems that have been in use for several decades, and these work well when there is a definite change in stage despite issues of sampling. However, these systems fail to evaluate any changes within a given stage, especially cirrhosis, which traditionally has been labeled as the “end stage.” We now recognize that even cirrhosis is a spectrum that varies from mild to severe disease. For patients with cirrhosis treated with DAA, any changes in the severity of clinical disease cannot be assessed by commonly used pathologic staging schemes for chronic hepatitis.16 Several systems have been developed to subclassify cirrhosis into mild and severe disease, but even these systems fail to predict the course of fibrosis based on a single liver biopsy.17,18 The recently proposed progressive-indeterminate-regressive (or PIR) system takes into account several previously underrecognized histologic characteristics and provides insight into the quality or directionality—progressive or regressive—of fibrosis in patients with cirrhosis based on a single liver biopsy.14,19 This system has shown excellent interobserver variability and can be assessed easily on hematoxylin-eosin–stained and trichome-stained slides obtained from formalin-fixed, paraffin-embedded tissue. Importantly, it has already been shown to correlate with liver-related morbidity and mortality in the setting of treated chronic hepatitis B.14,19 The PIR classification system has yet to be validated and has not been studied in HCV-infected patients. It is possible that the nature of persistent inflammation and fibrosis after DAA-SVR may be the histologic predictor of prognosis.
The goals of this study were to (1) evaluate hepatic histopathologic changes in patients who achieved DAA-SVR; specifically, the inflammatory grade, fibrosis stage, and quality of advanced fibrosis using the novel PIR system; (2) evaluate the presence of HCV RNA in liver tissue by using a highly sensitive nucleic acid amplification test to confirm or rule out occult HCV infection; and (3) evaluate risk factors for development of HCC after DAA-SVR.
MATERIALS AND METHODS
Patient and Pathology Case Selection
After obtaining approval from the Yale University Institutional Review Board, all patients with chronic HCV infection who had liver biopsies or resections performed or reviewed at Yale-New Haven Hospital from December 2014 to November 2017 were screened for history of treatment with DAAs. All patients who had received or were receiving DAA therapy were included in the study. A total of 154 individuals with available biographic data, detailed treatment courses, and accessible tissue samples were divided into 4 groups consisting of those who (1) achieved SVR and later developed biopsy-proven HCC (SVR/+HCC), (2) had active infection with concomitant biopsy-proven HCC (+HCV/+HCC), (3) achieved SVR without evidence of HCC (SVR/−HCC), and (4) had active infection without evidence of HCC (+HCV/−HCC). The pathologic and serologic data taken from +HCV or untreated patients were taken prior to initiation of DAA therapy. A total of 46 patients with concomitant history of other chronic liver conditions, such as alcoholic liver disease, drug-induced liver injury, and hepatitis B virus infection, were excluded from analysis of histologic parameters but not excluded from testing of tissue for HCV RNA. There were 6 cases with other concomitant malignancies in the liver, such as cholangiocarcinoma or metastatic neuroendocrine tumor, that were also excluded. Four cases of +HCV/−HCC had HCC treated in the past and were also excluded from analysis. This left a total of 96 cases for the study, which included 31 cases with SVR and 65 cases with active HCV infection. The final split into various subgroups taking HCC into consideration was: 19 SVR/+HCC, 13 +HCV/+HCC, 12 SVR/−HCC, and 52 +HCV/−HCC. There were 76 biopsies and 21 resections (including wedge resections, lobectomies, or explants) reviewed. The proportion of resections, explants, and biopsies varied among the different groups. A total of 13 of the 19 SVR/+HCC cases (68%) were resections or explants. Only 1 of the 12 SVR/−HCC cases (8%) was an explant. A total of 5 of the 13 +HCV/+HCC cases (38%) were resections. A total of 2 of the 52 +HCV/−HCC cases (4%) were explants. Initial analysis combined all tissues; however, subgroup analysis was subsequently performed because biopsies only sample a very limited portion of total liver tissue and may inaccurately reflect changes in the overall parenchyma.
Clinical Variables
Clinical variables included in the analysis were patient demographics and typical laboratory markers for hepatic function with corresponding prognostic indices (including the model for end-stage liver disease score and Child-Pugh class). Hepatitis C virus genotype and viral load, both routinely performed by polymerase chain reaction (PCR) for new patients, were collected. In addition, metabolic parameters, including standard measures for obesity, diabetes mellitus, hypertension, dyslipidemia, and substance use disorders, were collected. Charts were also reviewed for personal histories of other types of liver disease, other malignancies, human immunodeficiency virus infection, and other immunocompromising conditions, although these conditions were rarely encountered. Laboratory values incorporated in the study were derived from blood work that was collected nearest to the date of biopsy or resection. All patients had available demographic data, viral loads, and liver function test values. Remaining clinical variables were available for most patients except for HDL values (not available for 38 patients) and hemoglobin A1c values (not available for 51 patients). Sustained virologic response was defined as undetectable HCV RNA in serum, performed at least 12 weeks following DAA therapy.8
Histologic Evaluation
Pathologic variables included in the analysis were the inflammatory grade and fibrosis stage of liver disease, as per the Batts-Ludwig staging system.20 In resections for HCC, background tissue taken for analysis was farthest away from the tumor. In addition, the novel progressive, indeterminate, regressive (PIR) system, as described by Sun et al,14 was used to describe the quality of fibrosis (predominantly progressive, indeterminate, regressive) in each specimen showing cirrhosis.
PIR Classification System
As defined by Sun et al,14 the PIR system to qualify liver fibrosis in the setting of cirrhosis is divided into 3 reporting categories: P, I, and R. These categories are thought to more faithfully reflect the dynamic process of scar deposition and to better discriminate between irreparable fibrous replacement and a healing reaction. In predominantly progressive fibrosis (P), more than 50% of the fibrous septa are composed of wide bands of loosely aggregated collagen; these septa have moderate to marked infiltration by inflammatory cells and ductular reaction. Predominantly regressive fibrosis (R) is characterized by more than 50% of septa showing thin and compact fibrosis; these septa are relatively acellular and devoid of significant ductular reaction. Indeterminate fibrosis (I) is a mix of progressive and regressive fibrosis with no clear majority of one over the other. Figure 1 shows examples of each category. One representative hematoxylin-eosin–stained slide and 1 trichrome-stained slide were reviewed per case. When needed and available, a second slide for a given case was reviewed. All histologic variables included in this study were evaluated retrospectively at a multiheaded microscope by 2 pathologists with training in hepatopathology, and a consensus score was given for each case. The pathologists were blinded to the study group of each case.
Figure 1.
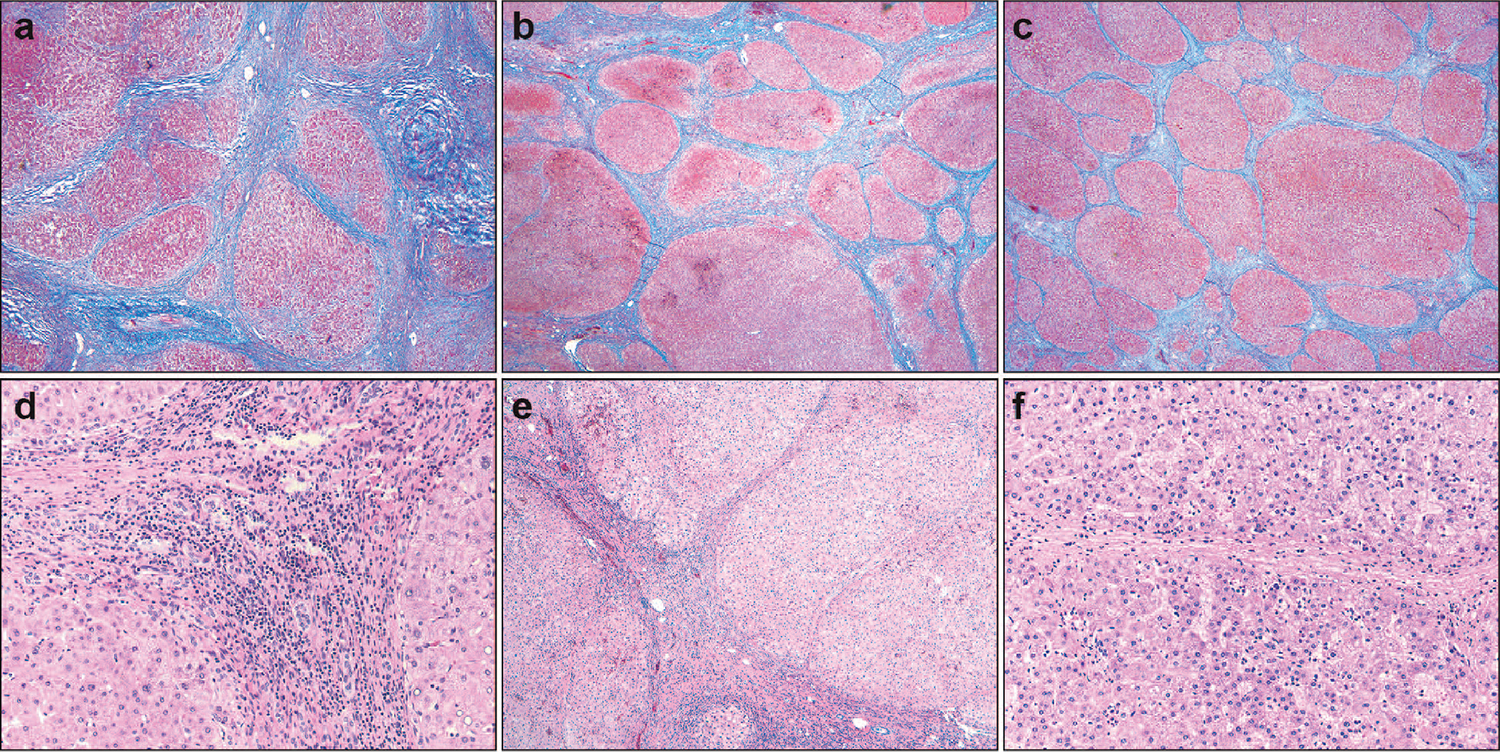
Representations of progressive-indeterminate-regressive (PIR) scores. a through c, Predominantly progressive (P), indeterminate (I), and regressive (R) fibrosis, respectively. d through f, Higher-power images showing increased thickness, inflammation, and cellularity in progressive septae compared with those undergoing regression (trichrome, original magnification ×40 [a through c]; hematoxylin eosin, original magnifications ×200 [d and f] and ×100 [e]).
HCV RNA Detection in Tissue Samples
Qualitative reverse transcriptase PCR using a primer set designed against a highly conserved region of the HCV genome was performed on a subset of 45 biopsy samples: 19 from SVR/−HCC patients, 21 from SVR/+HCC patients, and 5 control samples from patients who had active HCV infection without evidence of HCC.21 The limit of detection of HCV RNA of the assay is less than 10 IU/mL. Housekeeping gene RNP3 was amplified to ensure the quality, quantity, and integrity of nucleic acid extraction.22
Statistical Analysis
To test differences in proportions (categoric variables) and means (continuous variables) among the 4 different study groups, Pearson χ2 and Kruskal-Wallis tests were performed, respectively. Fisher exact and Student t-tests were employed when comparing categoric variables and continuous variables, respectively, between 2 groups. Data analysis was performed using the STATA statistical software program (2015; Stata Statistical Software release 14, StataCorp LP, College Station, Texas).
RESULTS
Clinical Variables
The 4 study groups were compared, and the summary of their demographics, characterization of liver disease, laboratory values, and metabolic parameters is presented in Table 1. Patients with HCC were significantly older than those without, both overall and when subgroup analysis was performed among DAA-SVR and untreated patients. No difference was seen in sex or ethnicity. Most patients in all groups were infected with genotype 1 HCV (overall mean, 87%), with no significant difference between groups. The alanine aminotransferase, aspartate aminotransferase, and platelet levels were significantly lower among DAA-SVR than untreated patients. Platelet count was lower in patients with HCC than those without, irrespective of treatment status (P = .01). There were no statistically significant differences among several metabolic parameters, including glycemic control, antihypertensive medication use, body mass index, and tobacco use.
Table 1.
Summarized Clinical Variables of All Patient Groups
| Untreated |
DAA-SVR |
P Value | |||
|---|---|---|---|---|---|
| −CC (n = 52) | +HCC (n = 13) | −CC (n = 12) | +HCC (n = 19) | ||
|
| |||||
| Patient demographics | |||||
| Male sex, No. (%) | 37 (71) | 10 (77) | 10 (83) | 17 (89) | .42 |
| Mean age, y | 54 | 67 | 54 | 62 | <.001a |
| Ethnicity, No. (% white) | 24 (46) | 7 (54) | 8 (67) | 12 (63) | .87 |
| HCV genotype 1, % | 81 | 94 | 83 | 88 | |
| Laboratory values | |||||
| Mean AST, U/L | 72 | 103 | 90 | 75 | <.001a |
| Mean ALT, U/L | 72 | 95 | 63 | 50 | <.001a |
| Mean bilirubin, mg/dL | 1.0 | 1.0 | 3.2 | 1.1 | .05 |
| Mean albumin, mg/dL | 3.9 | 3.7 | 3.6 | 3.6 | .30 |
| Mean creatinine, mg/dL | 1.5 | 0.9 | 2.4 | 1.1 | .07 |
| Median INR | 1.1 | 1.0 | 1.2 | 1.3 | .10 |
| Median platelets, 103/μL | 200 | 158 | 147 | 120 | .01a |
| Metabolic parameters | |||||
| Mean BMI, kg/m2 | 30 | 28 | 30 | 30 | .56 |
| Mean hemoglobin A1c, % | 6.6 | 6.2 | 5.3 | 6.5 | .10 |
| Hypertension, No. (%) | 25 (48) | 9 (69) | 6 (50) | 6 (32) | .47 |
| Tobacco use, No. (%) | 16 (31) | 7 (54) | 2 (17) | 2 (11) | .15 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DAA-SVR, direct acting antiviral–induced sustained viral response; −CC, no hepatocellular carcinoma; +HCC, with hepatocellular carcinoma; HCV, hepatitis C virus; INR, international normalized ratio.
Significant P value (<.05).
Pathologic Variables
Table 2 summarizes the distribution of inflammatory grade and fibrosis stage among patients from all 4 groups. The distribution of PIR score is shown for all patients with stage 4 fibrosis.
Table 2.
Summarized Pathologic Variables of All Patient Groups
| Untreated |
DAA-SVR |
P Valuea | P Valueb | |||||
|---|---|---|---|---|---|---|---|---|
| −CC (n = 52) | +HCC (n = 13) | Total (n = 65) | −CC (n = 12) | +HCC (n = 19) | Total (n = 31) | |||
|
| ||||||||
| Inflammatory grade, No. (%) | ||||||||
| 0 | 1 (2) | 0 | 1 (2) | 2 (17) | 2 (10) | 4 (13) | .04c | .45 |
| 1 | 27 (52) | 2 (15) | 29 (45) | 8 (67) | 10 (53) | 18 (58) | ||
| 2 | 19 (37) | 8 (62) | 27 (41) | 1 (8) | 6 (32) | 7 (23) | ||
| 3 | 5 (10) | 3 (23) | 8 (12) | 1 (8) | 1 (5) | 2 (6) | ||
| High inflammatory grade (2 or 3), No. (%) | 24 (46) | 11 (85) | 35 (53) | 2 (17) | 7 (37) | 9 (29) | .04c | .10 |
| Fibrosis stage, No. (%) | ||||||||
| 0 | 3 (6) | 0 | 3 (4) | 0 | 0 | 0 | ||
| 1 | 9 (17) | 0 | 9 (14) | 0 | 0 | 0 | ||
| 2 | 15 (29) | 1 (8) | 16 (25) | 5 (42) | 3 (16) | 8 (26) | ||
| 3 | 7 (13) | 2 (15) | 9 (14) | 0 | 2 (10) | 2 (6) | ||
| 4 | 18 (35) | 10 (77) | 28 (43) | 7 (58) | 14 (74) | 21 (68) | ||
| Advanced fibrosis (stage 3 or 4), No. (%) | 25 (48) | 12 (92) | 37 (57) | 7 (58) | 16 (84) | 23 (74) | .12 | <.001c |
| PIR score, No. (%)d | .54 | .04c | ||||||
| P | 7 (42) | 5 (56) | 12 (46) | 4 (57) | 4 (29) | 8 (38) | ||
| I | 5 (29) | 4 (44) | 9 (35) | 0 (0) | 6 (43) | 6 (29) | ||
| R | 5 (29) | 0 | 5 (19) | 3 (43) | 4 (29) | 7 (33) | ||
| P or I | 12 (71) | 9 (100) | 21 (81) | 4 (57) | 10 (71) | 14 (67) | .33 | .08 |
Abbreviations: DAA-SVR, direct acting antiviral–induced sustained viral response; −CC, no hepatocellular carcinoma; +HCC, with hepatocellular carcinoma; PIR, progressive, indeterminate, regressive.
P values in this column correspond to tests comparing DAA-SVR versus untreated patients.
P values in this column correspond to tests comparing +HCC versus no-HCC patients.
Significant P value (< .05).
Two cases of stage 4 cirrhosis were not analyzed for PIR because of (1) 1 being a consult slide that was sent back to the original institution, and (2) material considered inadequate for analysis.
Untreated Versus DAA-SVR Patients
Cases from untreated patients showed higher grades of inflammation (grades 2 to 3) compared with DAA-SVR cases (P = .04). However, 22 of 31 DAA-SVR cases (71%) showed at least grade 1 inflammation, with 9 (29%) showing grade 2 or 3. There was no significant difference in the incidence of advanced fibrosis between untreated and DAA-SVR patients (74% versus 57%, P = .12). Subgroup analysis of biopsy and resection specimens showed similar findings; however, statistical significance could not be reached because of the smaller numbers. High-grade inflammation was seen more commonly in untreated compared with DAA-SVR patients (53% versus 29%, P = .04). A similar trend was seen among resection specimens (57% versus 29%, P = .35).
+HCC Versus −HCC Patients
Advanced fibrosis was more commonly seen in patients with HCC versus those without (89% versus 52%, P < .001), irrespective of treatment status. There was no significant difference in the rate of high-grade inflammation (grade 2 or 3) among patients with or without HCC (62% versus 40%, P = .10). In subgroup analysis of biopsy specimens, the rate of advanced fibrosis was significantly higher in biopsies from HCC cases versus those without (89% versus 52%, P < .001).
PIR Scoring and Relationship to Clinical Features, Inflammation, and HCC
Among all patients with cirrhosis in the cohort, those who demonstrated predominantly progressive or indeterminate fibrosis (now referred to as P-I fibrosis) showed no significant difference compared with those with regressive-type fibrosis among all clinical characteristics collected.
Among all patients with cirrhosis in the cohort, there was no significant difference in P, I, and R score distribution between untreated and DAA-SVR patients (P = .54). However, there was a significantly increased proportion of P-I fibrosis among HCC patients compared with patients without HCC (90% versus 64%, P = .04, 1-tailed analysis). Among DAA-SVR patients, the rate of P-I fibrosis in those with HCC was 71% versus 57% in those without (not statistically significant, P = .32). There was no statistically significant difference in the distribution of PIR scores between resections and biopsies (P = .41) or in the percentage of P-I cases (75% versus 74%, P > .99). Subgroup analysis (biopsy and resection) for PIR scoring was not performed because the total number of patients in each group was considered too low.
Summary of Paired Pre–DAA-SVR and Post–DAA-SVR Tissue Sampling
Fourteen patients with paired pretreatment and post–DAA-SVR biopsies were identified from our files. The findings of these biopsies are summarized in Table 3. All pretreatment biopsies were performed either at the time of initial diagnosis for HCV infection or at the time of an unexplained increase in liver function tests, and all post–DAA-SVR biopsies were performed for fibrosis staging or confirmation of cirrhosis. A decrease in inflammatory grade was identified in 10 of 14 posttreatment cases (71%; Figure 2). Fibrosis stage decreased in 2 patients, increased in 3, and stayed the same in 9 (Figure 3). Seven patients had cirrhosis on the initial biopsy. These biopsies were characterized by either predominantly P (n=5), I (n=2), or R (n=0) fibrosis. Post–DAA-SVR regression was seen in 2 of these patients, with final scores being P (n = 3), I (n = 2), or R (n = 2). The median duration between completion of treatment and biopsy in these patients was 6 months.
Table 3.
Results of Paired Pre–Direct-Acting Antiviral Agent (DAA) Therapy and Post–DAA Therapy Biopsies
| Sex | Age, y | Pre-Tx Grade | Pre-Tx Stage | Pre-Tx PIR | DAA-SVR Grade | DAA-SVR Stage | DAA-SVR PIR | Duration of Tx, wk | Time Interval Between Biopsies, y |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Male | 41 | 1 | 1 | 2 | 2 | 12 | 18 | ||
| Male | 60 | 2 | 2 | 1 | 2 | 12 | 2 | ||
| Female | 56 | 3 | 2 | 1 | 4 | 12 | 6 | ||
| Male | 62 | 2 | 4 | I | 1 | 4 | P | 12 | 2 |
| Female | 53 | 2 | 2 | 0 | 2 | 12 | 1 | ||
| Male | 66 | 3 | 3 | 0 | 2 | 12 | 5 | ||
| Male | 61 | 4 | 3 | 2 | 2 | N/A | 17 | ||
| Male | 64 | 3 | 4 | P | 3 | 4 | P | 24 | 2 |
| Male | 64 | 2 | 2 | 2 | 4 | I | 12 | 2 | |
| Male | 61 | 2 | 4 | P | 2 | 4 | I | 24 | 16 |
| Male | 64 | 2 | 4 | P | 1 | 4 | R | 24 | 1 |
| Male | 63 | 3 | 4 | P | 2 | 4 | P | 24 | 11 |
| Male | 50 | 3 | 4 | P | 1 | 4 | P | 26 | 10 |
| Male | 66 | 2 | 4 | P | 1 | 3–4 | R | 45 | 2 |
Abbreviations: N/A, not available; PIR, progressive, indeterminate, and regressive fibrosis; SVR, sustained viral response; Tx, therapy.
Figure 2.
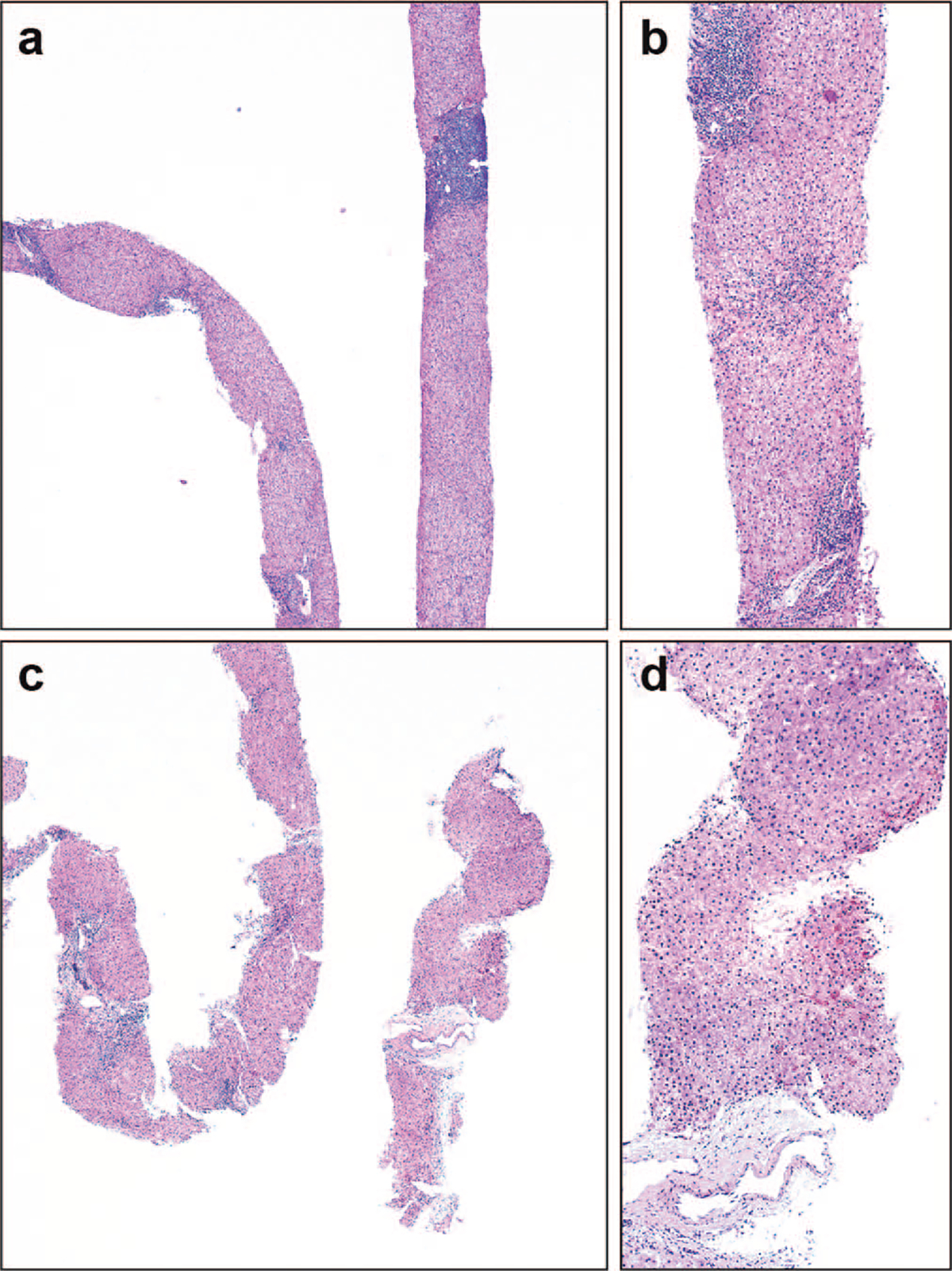
Pretreatment and posttreatment biopsies from a patient. a and b, One set of diagnostic biopsies showing mild to moderate inflammatory grade. c and d, Posttreatment biopsies demonstrate decreased inflammation (hematoxylin-eosin, original magnifications ×100 [a and c] and ×200 [b and d]).
Figure 3.
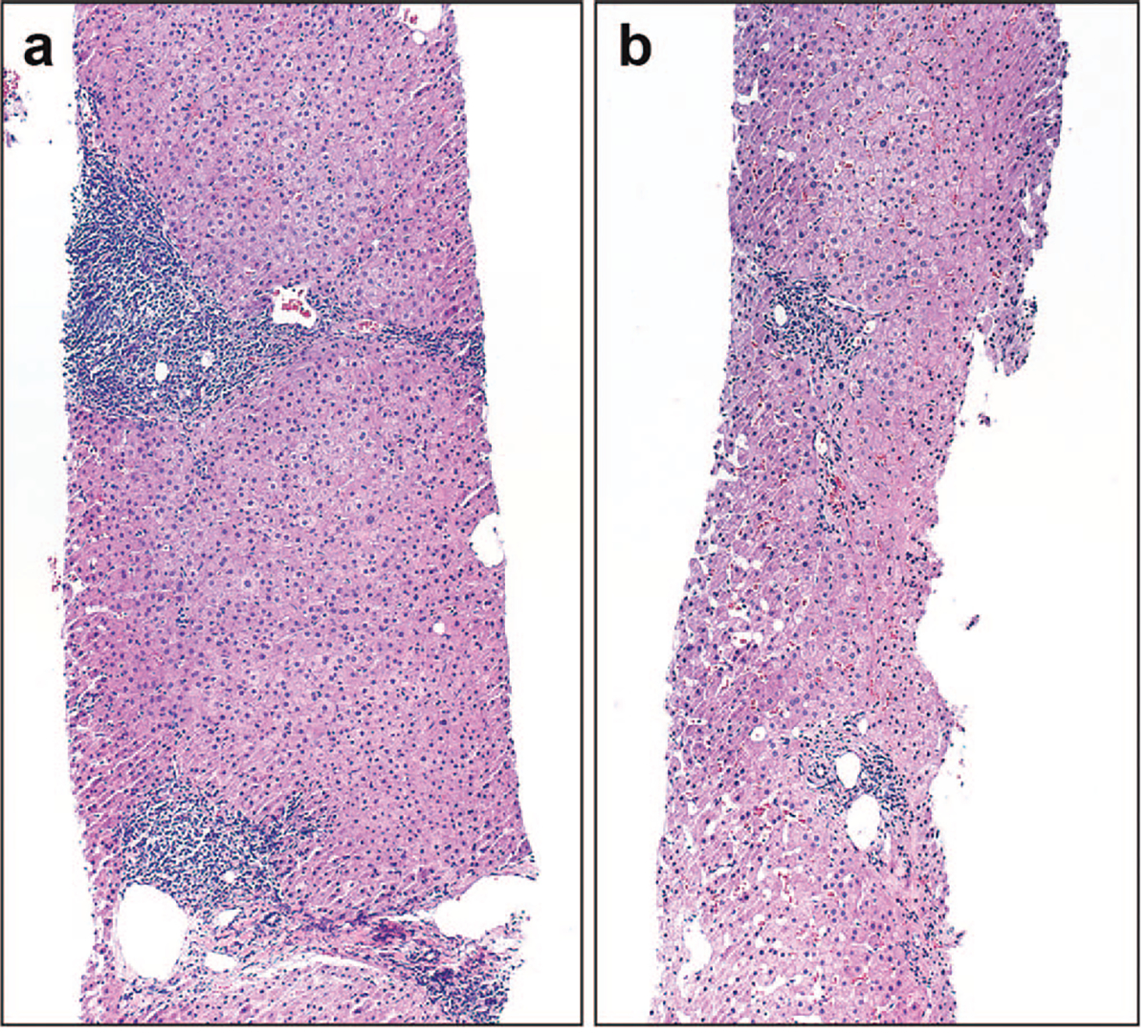
Pretreatment and posttreatment biopsies from a patient. a, This patient had mild to moderate inflammatory grade and periportal to bridging fibrosis on his pretreatment biopsy, both of which demonstrated remission in his (b) posttreatment biopsy (hematoxylin-eosin, original magnifications ×100 [a] and ×200 [b]).
Relationship of Inflammatory Grade and Time Since Treatment Cessation
Among DAA-SVR patients, the median time interval from the date of treatment cessation and date of tissue acquisition was 20.2 months (range, 5–126.3 months). When we analyzed the relationship between inflammatory grade and time interval from treatment cessation to tissue acquisition, a trend was seen with decreased inflammation over time (Figure 4), although this was not statistically significant (analysis of variance, P = .48). There was no significant difference in the prevalence of grade 2 or 3 inflammation between patients whose tissue was collected before and after the median of 20.2 months (21% versus 33%, P = .66).
Figure 4.
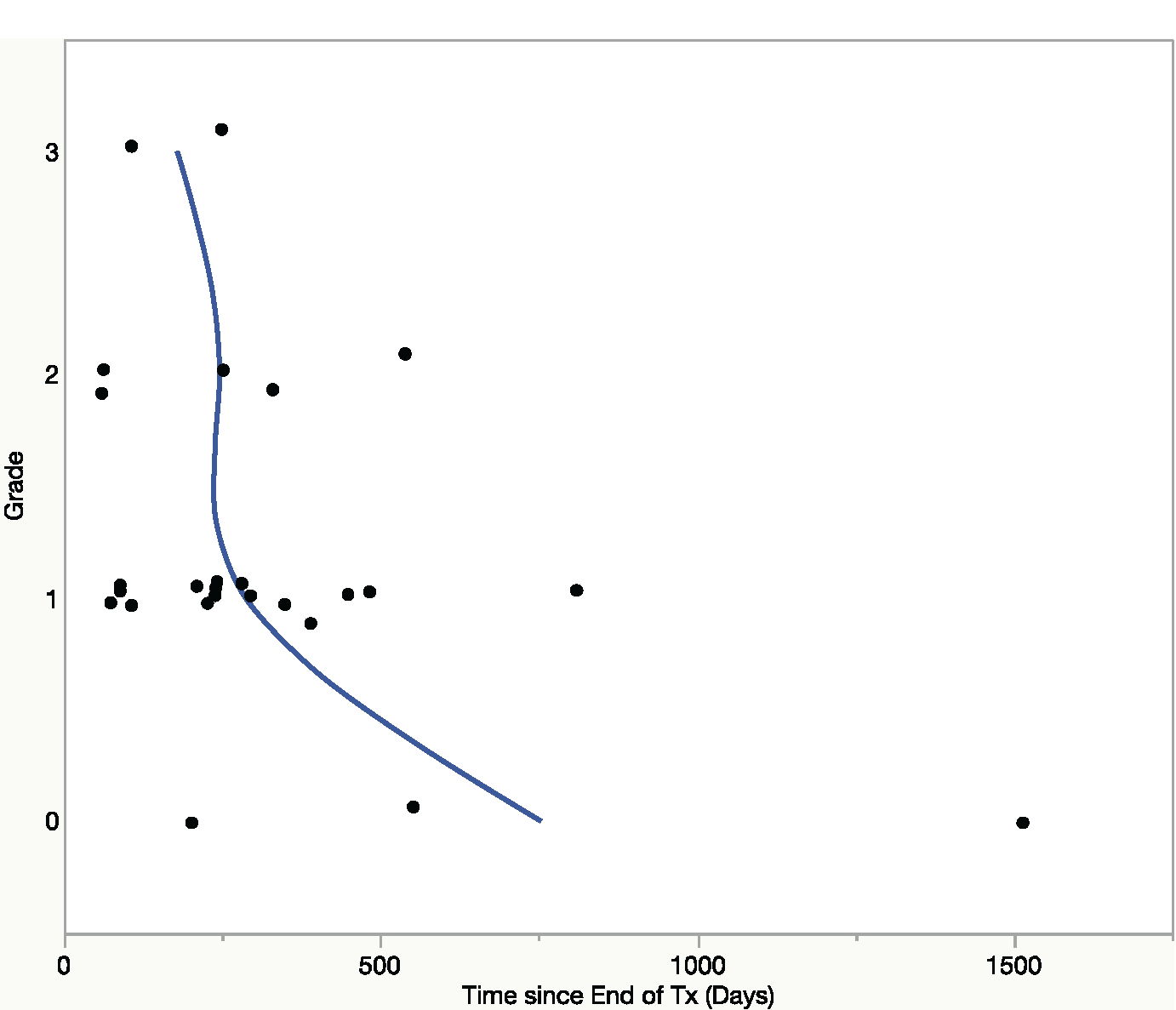
Inflammatory grade of tissue specimens (y-axis) and time, since treatment cessation, of tissue acquisition (x-axis). There was a trend toward decreased inflammation over time, although by analysis of variance statistic there was no significant difference in the mean time (P = .48) among the 4 categories of inflammation.
HCV RNA Detection in Tissue Samples
Hepatitis C virus RNA was detected in all 5 control samples and none of the tissue samples collected from 40 patients who achieved SVR. Housekeeping gene RNP3 was detected in all 45 samples, ensuring the quality of the PCR reaction.
DISCUSSION
There are 3 major findings in this study of consecutive pathologic specimens from chronic HCV patients during a 3-year period. First, there appears to be a global decrease in histologic inflammatory grade and mean alanine aminotransferase and aspartate aminotransferase upon achieving DAA-SVR; however, nearly one-third (9 of 31; 29%) of DAA-SVR patients in this cohort continued to show grade 2 or 3 inflammation. Our findings suggest that the recession of inflammatory changes following SVR is time dependent, although the median follow-up of 20.2 months is short for such an analysis. Second, our data do not show any significant difference in the number of SVR/+HCC and SVR/−HCC patients in the relatively short follow-up period of less than 3 years. Furthermore, there is an inherent selection bias in that patients with longstanding cirrhosis were among the first patients to be offered DAA therapy. Finally, none of the liver tissues from DAA-SVR patients (n = 40) were positive for HCV by nucleic acid amplification test. Therefore, occult HCV infection in liver is unlikely to be a prevalent mechanism of persistent inflammation, worsening fibrosis, or HCC development in successfully treated patients.
Until now, there have been limited published data on histologic changes in post–DAA-SVR livers.4 In our large cohort of consecutive liver specimens from patients with chronic HCV, we show a significant decrease in inflammatory grade in post–DAA-SVR livers. However, 29% of DAA-SVR cases showed high grades (2 or 3+) of inflammation, and 38% of DAA-SVR cases with cirrhosis continued to show a predominantly progressive pattern of fibrosis, which by definition requires septal inflammation to be present. By comparison, as expected, a higher percentage of untreated patients (53%) had high-grade (2 or 3+) inflammation and predominantly progressive pattern of fibrosis (46%). Similarly, in 14 patients with paired pre– and post–DAA-SVR biopsies, 10 showed decreases in inflammatory grade. Although not statistically signficant, likely due to a small number of cases, there was certainly a trend toward decreasing necroinflammation over time following DAA treatment (Figure 4). Previous studies in the setting of IFN-based treatment and SVR have shown somewhat similar results. Tsubota et al23 showed a significant decrease in necroinflammation in a cohort of 93 post–IFN therapy-induced SVR patients, with histologic improvement occurring in a time-dependent fashion. For those cases with persistent high-grade inflammation after treatment, the possibility of an effect of immune reconstitution, such as that seen in HIV clearance, should be considered for future studies.
Novel histology-based classification systems have been developed that evaluate various features of fibrosis and architecture in cirrhosis and that predict the clinical severity of cirrhosis. These schemes, namely the Laennec subclassification of cirrhosis, “the collagen proportionate area,” and the Jain-Garcia systems are based on previously underappreciated histologic parameters in cirrhosis, such as septal thickness and cellularity, nodule size, and fibrosis area, among other properties.17,18,24 However, the utility of these systems for predicting the impact of highly effective antiviral therapy has not been tested. The PIR system was created to more thoroughly describe the dynamic process of fibrosis in the liver, thereby providing some “directionality” to the disease process in cases of advanced fibrosis. We chose to use the PIR classification in this study for 2 reasons: (1) it previously correlated to fibrosis regression with clinical improvement in the setting of another viral hepatitis (hepatitis B virus), and (2) it demonstrated low rates of pathologist interobserver variability.14 Our data reveal no significant decrease in the incidence of P-I fibrosis after DAA-SVR in a large cohort. However, when we examined livers after DAA-SVR from patients who had cirrhosis confirmed on pretreatment biopsies (Table 3), 3 of 7 cases showed some degree of regression. A total of 2 of the 7 showed a major change in fibrosis pattern: they were previously predominantly progressive and became predominantly regressive following treatment. In a recent study by Putra et al,4 the Laennec subclassification of cirrhosis system was applied to liver explants after DAA-SVR, with a median SVR period of 24 weeks. Among 25 cirrhotic DAA-SVR livers, only 20% showed histologic evidence of fibrosis regression. Based on our use of the PIR classification, we had a comparable proportion of regressive changes (7 of 21; 33%) in fibrosis among DAA-SVR cases with cirrhosis. This estimate is also similar to those previously published in the setting of SVR with IFN-based therapies.13,25 Bernuth et al26 provided additional support for the concept of DAA-induced fibrosis regression using serum and imaging markers of fibrosis.4 Our study was hindered by a selection bias in that most of the biopsies in the setting of DAA-SVR were performed to confirm the presence of advanced fibrosis based on clinical suspicion. In current clinical practice most patients who show clinical and laboratory improvement after DAA-SVR do not undergo biopsy. This explains the higher prevalence of advanced fibrosis in our DAA-SVR patients compared with the untreated patients. We believe these cases may be enriched for those patients who have irreversible/advanced tissue injury/fibrosis or longstanding disease. In fact, 2 of 3 patients who had progressive fibrosis on both pre- and post-SVR biopsies had a duration of HCV infection (prior to initiation of therapy) of 10 years or more. In contrast, the duration of HCV infection for both patients who showed a change from progressive to regressive fibrosis was 2 years or less. Overall, our data suggest that a subset of patients with HCV-related cirrhosis undergo some degree of fibrosis regression secondary to DAA therapy. That degree may be related to length and extent of disease prior to initiation of therapy.
The PIR score is a novel system and is an advancement over prior systems for assessing fibrosis. However, several pitfalls became obvious during the study. In the initial study all assessments were performed on biopsies; hence, the tissue heterogeneity was less and the system showed excellent interobserver variability. There are 3 histologic criteria to evaluate on every case, which tend to demonstrate concordance in terms of the overall score. For example, thick fibrous septae are likely to demonstrate inflammation and bile ductular reaction. However, occasionally there are cases in which only 1 or 2 of the features are present, making the scoring difficult. Some cases demonstrate heterogeneity of the 3 features throughout the tissue. Although the predominant features were taken for the final scoring, it was challenging to identify these in some cases. As a result, we found PIR scoring to be more difficult on resections where a large area was available for evaluation and tissue heterogeneity became more evident. This also raises sampling issues on biopsies that represent a minute fragment of the total liver volume. To control for this, we performed subgroup analysis of biopsies and resections separately. Further studies, potentially using quantitative image analysis techniques, may provide the level of objectivity needed to establish the hard criteria required for clinical application of this system.27,28
Our results show that among continuous pathology specimens received for HCC during a 3-year period, there was no significant difference in the number of cases from DAA-SVR versus untreated patients in our cohort. This is intriguing because it is known that SVR achieved with non-DAA therapies is associated with decreased risk of HCC.29,30 The same would be expected of DAA-SVR. We speculate that most of the patients with cirrhosis who developed HCC within the study period had either (1) occult HCV infection, (2) early HCC that existed before the initiation of therapy, (3) irreversible inflammatory injury and tissue damage that continued despite SVR, or (4) selection bias at a tertiary care medical center where many HCC-SVR patients are evaluated for liver transplantation or HCC.
Occult viral infection has been implicated in cases of recurrent viral hepatitis after SVR under immunosuppression, or HCC developing in cryptogenic cirrhosis or without any underlying identifiable chronic liver disease.31,32 In 2008, Maylin et al33 showed that in patients who achieved SVR with IFN-based regimens, the rate of occult HCV infection was approximately 1.7% among a sample of 114 individuals who had liver biopsies at a mean duration of approximately 3 years after the completion of treatment. The impact of DAAs on the immune system compared with IFN-based regimens or HCV detection in reservoir sites like the liver or mononuclear phagocyte system is poorly understood. One leading theory for the development of early and aggressive HCC in the context of DAA-SVR is that the rapid systemic destruction of HCV virions may also attenuate the antitumor immune response.7 It has been suggested that this more likely occurs in patients who have preexisting microtumors that are not readily seen on imaging. Although occult HCV infection has been thought to be responsible for sustained or increased HCC risk, our evidence suggests that occult infection is unlikely to be a major factor. The results of HCV detection after SVR (after treatment or spontaneous) have yielded variable results.11 Some studies show persistent virus, whereas others do not. This variability has been attributed to patient selection, choice of sample selection (liver tissue, peripheral blood mononuclear cells, plasma, or serum), and methodology (real-time qualitative reverse transcriptase PCR, in situ hybridization, etc). To the best of our knowledge, 2 prior studies have evaluated the possibility of occult HCV infection in post-SVR patients in native liver tissue. One showed 29 of 30 cases were negative,34 whereas Wang et al35 showed that 15% in their cohort were positive.
Other factors have previously been shown to be important in predicting HCC development in the setting of IFN-mediated SVR, like the presence of diabetes mellitus, alcohol use, and the presence of cirrhosis at 24 weeks following SVR.36,37 In our study we excluded any other concomitant liver disease or alcohol abuse, and we feel the increased risk of HCC, at least in the short term (2 years), is related to underlying persistent inflammation and advanced fibrosis.
Persistence of marked inflammatory grade and “progressive” pattern of fibrosis in many cases of DAA-SVR would seem to support that. Our results showed that there was a trend of higher incidence of HCC in patients who had a higher grade of inflammation and either a progressive or an indeterminate pattern of fibrosis (Table 2). It is also possible that these HCCs were at very early stages of evolution when the DAA therapy was started, and they escaped detection. It is possible that if one only looks at HCV patients without cirrhosis or at longer term outcome in cirrhotic patients post DAA-SVR, the HCC risk may decline. In our study, we validate higher age and the presence of advanced fibrosis as patient characteristics associated with HCC, even in the setting of SVR.38,39 Taken together, our findings suggest that HCV tissue infection-independent mechanisms, including tissue inflammation and progressive-type fibrosis, are likely more significant biologic mediators of clinical prognosis and HCC development.
Our study has some limitations. We did not distinguish between incident HCC following DAA therapy (as defined as a new HCC following at least 15 months after DAA-SVR) and HCC presentation within 15 months after DAA-SVR, which is likely not treatment related. To mitigate this effect, we excluded all patients with a prior history of HCC. Because the DAAs have been in use for only a few years, the number of cases is small and follow-up is short. The median follow-up time in our study is only 20.2 months following DAA-SVR, which makes it difficult to make a more definitive statement about changes secondary to treatment and their relationship with time. Other limitations include the selection bias for patients with advanced fibrosis in the setting of DAA-SVR, as discussed above, and the difficulties in applying a relatively new histologic scoring scheme (PIR), as discussed above. Because of this selection bias this study can neither evaluate the overall incidence of HCC in the setting of DAA-SVR nor outline the natural history of all HCV patients treated with DAA.
In conclusion, we show that tissue response to DAA-SVR in the setting of chronic hepatitis C is generally favorable; however, persistent high-grade inflammation is seen in a subset of patients. Further studies are required to identify parameters that may predict histologic response to DAA treatment. Together, persistent inflammation and fibrosis appear to be more biologically significant than the presence of occult HCV infection in mediating continued tissue injury, clinical disease, and evolution of HCC in the setting of DAA-induced SVR.
Footnotes
The authors have no relevant financial interest in the products or companies described in this article.
Presented in part at the United States and Canadian Academy of Pathology (USCAP) National Meeting; March 17–23, 2018; Vancouver, Canada; and at the American Association for the Study of Liver Diseases (AASLD) National Meeting; October 20–24, 2017; Washington, DC.
Contributor Information
Romulo Celli, Department of Pathology, Yale School of Medicine, New Haven, Connecticut.
Saad Saffo, Section of Digestive Diseases, Department of Internal Medicine, Yale School of Medicine, New Haven, Connecticut.
Saleem Kamili, Division of Viral Hepatitis, Centers for Disease Control and Prevention, Atlanta, Georgia.
Nicholas Wiese, Division of Viral Hepatitis, Centers for Disease Control and Prevention, Atlanta, Georgia.
Tonya Hayden, Division of Viral Hepatitis, Centers for Disease Control and Prevention, Atlanta, Georgia.
Tamar Taddei, Section of Digestive Diseases, Department of Internal Medicine, Yale School of Medicine, New Haven, Connecticut.
Dhanpat Jain, Section of Gastrointestinal and Liver Pathology, Yale School of Medicine, New Haven, Connecticut.
References
- 1.Kowdley KV, Lawitz E, Poordad F, et al. Phase 2b trial of interferon-free therapy for hepatitis C virus genotype 1. N Engl J Med. 2014;370(3):222–232. [DOI] [PubMed] [Google Scholar]
- 2.Lawitz E, Poordad FF, Pang PS, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2014;383(9916):515–523. [DOI] [PubMed] [Google Scholar]
- 3.Muir AJ, Poordad F, Lalezari J, et al. Daclatasvir in combination with asunaprevir and beclabuvir for hepatitis C virus genotype 1 infection with compensated cirrhosis. JAMA. 2015;313(17):1736–1744. [DOI] [PubMed] [Google Scholar]
- 4.Putra J, Schiano TD, Fiel MI. Histological assessment of the liver explant in transplanted hepatitis C virus patients achieving sustained virological response with direct-acting antiviral agents. Histopathology. 2018;72(6):990–996. [DOI] [PubMed] [Google Scholar]
- 5.ANRS Collaborative Study Group on Hepatocellular Carcinoma (ANRS CO22 HEPATHER, CO12 CirVir and CO23 CUPILT cohorts). Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: data from three ANRS cohorts. J Hepatol. 2016;65(4):734–740. [DOI] [PubMed] [Google Scholar]
- 6.Reig M, Marino Z, Perello C, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65(4):719–726. [DOI] [PubMed] [Google Scholar]
- 7.Alberti A, Piovesan S. Increased incidence of liver cancer after successful DAA treatment of chronic hepatitis C: fact or fiction? Liver Int. 2017;37(6):802–808. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida EM, Sulkowski MS, Gane EJ, et al. Concordance of sustained virological response 4, 12, and 24 weeks post-treatment with sofosbuvir-containing regimens for hepatitis C virus. Hepatology. 2015;61(1):41–45. [DOI] [PubMed] [Google Scholar]
- 9.Backus LI, Belperio PS, Shahoumian TA, Mole LA. Impact of sustained virologic response with direct-acting antiviral treatment on mortality in patients with advanced liver disease. Hepatology. 2019;69(2):487–497. [DOI] [PubMed] [Google Scholar]
- 10.Backus LI, Belperio PS, Shahoumian TA, Mole LA. Direct-acting antiviral sustained virologic response: impact on mortality in patients without advanced liver disease. Hepatology. 2018;68(3):827–838. [DOI] [PubMed] [Google Scholar]
- 11.Vidimliski PD, Nikolov I, Geshkovska NM, Dimovski A, Rostaing L, Sikole A. Review: occult hepatitis C virus infection: still remains a controversy. J Med Virol. 2014;86(9):1491–1498. [DOI] [PubMed] [Google Scholar]
- 12.Castillo I, Pardo M, Bartolome J, et al. Occult hepatitis C virus infection in patients in whom the etiology of persistently abnormal results of liver-function tests is unknown. J Infect Dis. 2004;189(1):7–14. [DOI] [PubMed] [Google Scholar]
- 13.Shiratori Y, Imazeki F, Moriyama M, et al. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000;132(7):517–524. [DOI] [PubMed] [Google Scholar]
- 14.Sun Y, Zhou J, Wang L, et al. New classification of liver biopsy assessment for fibrosis in chronic hepatitis B patients before and after treatment. Hepatology. 2017;65(5):1438–1450. [DOI] [PubMed] [Google Scholar]
- 15.Chang TT, Liaw YF, Wu SS, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52(3):886–893. [DOI] [PubMed] [Google Scholar]
- 16.Serpaggi J, Carnot F, Nalpas B, et al. Direct and indirect evidence for the reversibility of cirrhosis. Hum Pathol. 2006;37(12):1519–1526. [DOI] [PubMed] [Google Scholar]
- 17.Kim SU, Oh HJ, Wanless IR, Lee S, Han KH, Park YN. The Laennec staging system for histological sub-classification of cirrhosis is useful for stratification of prognosis in patients with liver cirrhosis. J Hepatol. 2012;57(3):556–563. [DOI] [PubMed] [Google Scholar]
- 18.Tsochatzis E, Bruno S, Isgro G, et al. Collagen proportionate area is superior to other histological methods for sub-classifying cirrhosis and determining prognosis. J Hepatol. 2014;60(5):948–954. [DOI] [PubMed] [Google Scholar]
- 19.Theise ND, Jia J, Sun Y, Wee A, You H. Progression and regression of fibrosis in viral hepatitis in the treatment era: the Beijing classification. Mod Pathol. 2018;31(8):1191–1200. [DOI] [PubMed] [Google Scholar]
- 20.Batts KP, Ludwig J. Chronic hepatitis: an update on terminology and reporting. Am J Surg Pathol. 1995;19(12):1409–1417. [DOI] [PubMed] [Google Scholar]
- 21.Mixson-Hayden T, Lee D, Ganova-Raeva L, et al. Hepatitis B virus and hepatitis C virus infections in United States-bound refugees from Asia and Africa. Am J Trop Med Hyg. 2014;90(6):1014–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoddard RA, Gee JE, Wilkins PP, McCaustland K, Hoffmaster AR. Detection of pathogenic Leptospira spp. through TaqMan polymerase chain reaction targeting the LipL32 gene. Diagn Microbiol Infect Dis. 2009;64(3):247–255. [DOI] [PubMed] [Google Scholar]
- 23.Tsubota A, Kumada H, Chayama K, et al. Time course of histological changes in patients with a sustained biochemical and virological response to interferon-alpha therapy for chronic hepatitis C virus infection. J Hepatol. 1997;27(1):49–55. [DOI] [PubMed] [Google Scholar]
- 24.Nagula S, Jain D, Groszmann RJ, Garcia-Tsao G. Histological-hemodynamic correlation in cirrhosis-a histological classification of the severity of cirrhosis. J Hepatol. 2006;44(1):111–117. [DOI] [PubMed] [Google Scholar]
- 25.Poynard T, McHutchison J, Manns M, et al. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122(5):1303–1313. [DOI] [PubMed] [Google Scholar]
- 26.Bernuth S, Yagmur E, Schuppan D, et al. Early changes in dynamic biomarkers of liver fibrosis in hepatitis C virus-infected patients treated with sofosbuvir. Dig Liver Dis. 2016;48(3):291–297. [DOI] [PubMed] [Google Scholar]
- 27.Perveen S, Shahbaz M, Keshavjee K, Guergachi A. A systematic machine learning based approach for the diagnosis of non-alcoholic fatty liver disease risk and progression. Sci Rep. 2018;8(1):2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janowczyk A, Madabhushi A. Deep learning for digital pathology image analysis: a comprehensive tutorial with selected use cases. J Pathol Inform. 2016. Jul 26;7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2017; S0168-8278(17)32273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu CS, Huang CJ, Kao JH, et al. Interferon-based therapy decreases risks of hepatocellular carcinoma and complications of cirrhosis in chronic hepatitis C patients. PLoS One. 2013;8(7):e70458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carreno V, Bartolome J, Castillo I, Quiroga JA. Occult hepatitis B virus and hepatitis C virus infections. Rev Med Virol. 2008;18(3):139–157. [DOI] [PubMed] [Google Scholar]
- 32.Marrero JA, Lok AS. Occult hepatitis B virus infection in patients with hepatocellular carcinoma: innocent bystander, cofactor, or culprit? Gastroenterology. 2004;126(1):347–350. [DOI] [PubMed] [Google Scholar]
- 33.Maylin S, Martinot-Peignoux M, Ripault MP, et al. Sustained virological response is associated with clearance of hepatitis C virus RNA and a decrease in hepatitis C virus antibody. Liver Int. 2009;29(4):511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitcomb E, Choi WT, Jerome KR, et al. Biopsy specimens from allograft liver contain histologic features of hepatitis C virus infection after virus eradication. Clin Gastroenterol Hepatol. 2017;15(8):1279–1285. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Rao H, Chi X, et al. Detection of residual HCV-RNA in patients who have achieved sustained virological response is associated with persistent histological abnormality. EBioMedicine. 2019;46:227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toyoda H, Kumada T, Tada T, et al. Risk factors of hepatocellular carcinoma development in non-cirrhotic patients with sustained virologic response for chronic hepatitis C virus infection. J Gastroenterol Hepatol. 2015;30(7):1183–1189. [DOI] [PubMed] [Google Scholar]
- 37.El-Serag HB, Kanwal F, Richardson P, Kramer J. Risk of hepatocellular carcinoma after sustained virological response in Veterans with hepatitis C virus infection. Hepatology. 2016;64(1):130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153(4):996–1005.e1. [DOI] [PubMed] [Google Scholar]
- 39.Asahina Y, Tsuchiya K, Tamaki N, et al. Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology. 2010;52(2):518–527. [DOI] [PubMed] [Google Scholar]


