Abstract
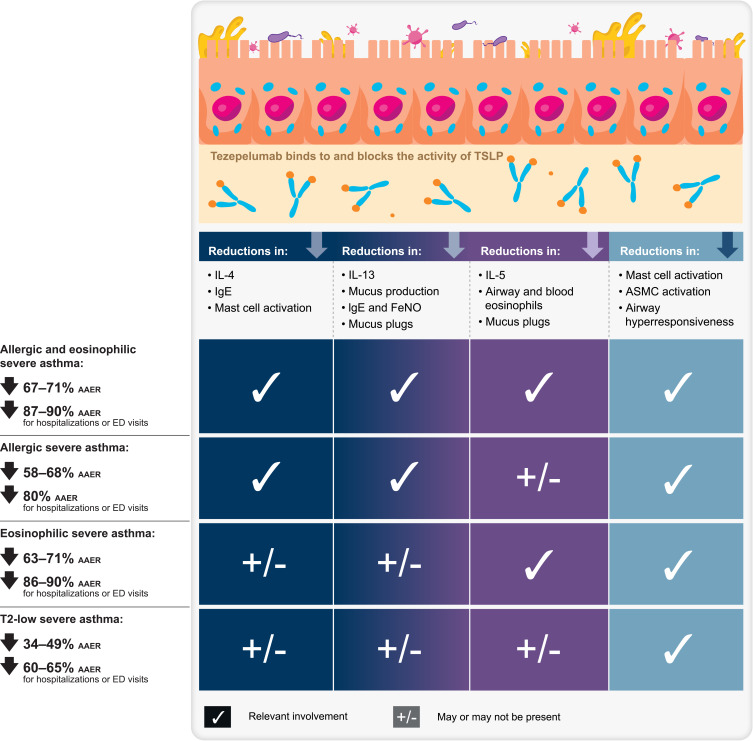
Asthma is a heterogeneous inflammatory disease of the airways, affecting many children, adolescents, and adults worldwide. Up to 10% of people with asthma have severe disease, associated with a higher risk of hospitalizations, greater healthcare costs, and poorer outcomes. Patients with severe asthma generally require high-dose inhaled corticosteroids and additional controller medications to achieve disease control; however, many patients remain uncontrolled despite this intensive treatment. The treatment of severe uncontrolled asthma has improved with greater understanding of asthma pathways and phenotypes as well as the advent of targeted biologic therapies. Tezepelumab, a monoclonal antibody, blocks thymic stromal lymphopoietin, an epithelial cytokine that has multifaceted effects on the initiation and persistence of asthma inflammation and pathophysiology. Unlike other biologic treatments, tezepelumab has demonstrated efficacy across severe asthma phenotypes, with the magnitude of effects varying by phenotype. Here we describe the anti-inflammatory effects and efficacy of tezepelumab across the most relevant phenotypes of severe asthma. Across clinical studies, tezepelumab reduced annualized asthma exacerbation rates versus placebo by 63–71% in eosinophilic severe asthma, by 58–68% in allergic severe asthma, by 67–71% in allergic and eosinophilic severe asthma, by 34–49% in type 2-low asthma, and by 31–41% in oral corticosteroid-dependent asthma. Furthermore, in all these asthma phenotypes, tezepelumab demonstrated higher efficacy in reducing exacerbations requiring hospitalizations or emergency department visits versus placebo. In patients with severe uncontrolled asthma, who commonly have multiple drivers of inflammation and disease, tezepelumab may modulate airway inflammation more extensively, as other available biologics block only specific downstream components of the inflammatory cascade.
Keywords: exacerbations, eosinophilic, allergic, type 2, oral corticosteroid-dependent, airway hyperresponsiveness
Plain Language Summary
Asthma is characterized by an immune response leading to airway inflammation. People with severe asthma may react to different triggers and develop different types of airway inflammation. In patients with asthma, a protein called thymic stromal lymphopoietin (TSLP) plays an important role in the immune response that leads to the signs and symptoms of asthma. TSLP is released by the airway lining in response to different asthma triggers, driving an immune chain reaction, leading to airway narrowing and tightening, increased airway inflammation, worsening asthma symptoms, and asthma attack. Tezepelumab is a monoclonal antibody (a type of protein) that prevents TSLP from attaching to its receptor, thereby blocking its activity, reducing airway inflammation and asthma symptoms. Tezepelumab is an add-on medicine for the treatment of people aged 12 years or older with severe asthma that is not controlled with their current medicines.
In this article, we discuss how tezepelumab may work in different types of asthma, for example allergic asthma, eosinophilic asthma, and T2-low asthma. We also describe how effective tezepelumab is in these different asthma types, through the reduction of asthma attacks and improvement in lung function, symptom control, and quality of life, leading to fewer emergency department visits and hospitalizations for asthma.
Introduction
Asthma, a complex and heterogeneous inflammatory disease of the airways, affects approximately 262 million individuals worldwide, equating to around 3416 cases per 100,000 population.1–3 Between a third and a half of children, adolescents, and adults with asthma have symptoms that regularly interfere with everyday life.3 Up to 10% of patients have severe asthma requiring high-dose inhaled corticosteroids and additional controller medications to achieve disease control (per Global Initiative for Asthma 2019 guidelines).2,4,5 Despite standard-of-care treatment, 20–50% of patients in the United States (US) with severe asthma have disease that remains uncontrolled, which is associated with more frequent symptoms, a greater burden of disease, a higher risk of hospitalizations, greater healthcare costs, and poorer outcomes.2,6
Biologic treatments (ie, monoclonal antibodies) that block individual components of the asthma inflammatory cascade have been developed over the past 20 years. Each reduces the rate of exacerbations in specific populations of patients with severe uncontrolled asthma.7 Most biologics for severe asthma target downstream components of the inflammatory cascade, including interleukin (IL)-5 receptor α (benralizumab, leading to eosinophil depletion), IL-5 (mepolizumab, reslizumab), immunoglobulin (Ig) E (omalizumab), and IL-4 receptor α (dupilumab, leading to blockade of IL-4 andIL-13 activity).8–12 These biologics were approved for moderate-to-severe or severe cases of allergic asthma, eosinophilic asthma, and/or oral corticosteroid (OCS)-dependent asthma based on their mechanisms of action and efficacy in randomized, placebo-controlled trials.8–13
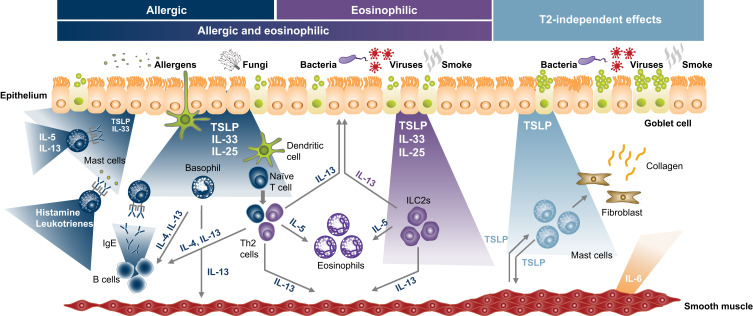
Largely as a result of these approvals, three central phenotypes have emerged with regard to treatment of severe uncontrolled asthma with targeted biologic therapies: eosinophilic asthma, allergic asthma, and type 2 (T2)-low asthma. Eosinophilic asthma is defined by elevated blood eosinophil count (BEC; ≥150 or ≥300 cells/µL); allergic asthma involves sensitivity to perennial aeroallergens (usually with a history of allergy-driven clinical symptoms); and T2-low asthma is characterized by the absence of prominent allergic and eosinophilic inflammation (Figure 1).1,14,15 However, considerable overlap between the allergic and eosinophilic phenotypes exists, and there are other inflammatory and pathophysiologic drivers of severe uncontrolled asthma beyond those strictly classified as allergic or eosinophilic.15 Any classification of individual patients into phenotypic categories represents a simplification of patients’ inflammatory states since each patient will have varying degrees of inflammation from the multiple pathways associated with asthma. Additionally, patients’ inflammatory characteristics may change over time, owing to changes in physiology as well as changes in medications, treatment adherence, exacerbations, and environmental exposure to allergens, viruses, irritants, and other airway insults.1,14,15 The heterogeneity of severe asthma challenges disease management, and many patients have an insufficient response to intensive treatments.2
Figure 1.
Epithelial cytokines and other inflammatory mediators and cell types involved in asthma pathogenesis.4,16–19
Note: Adapted from Thymic stromal lymphopoietin: its role and potential as a therapeutic target in asthma, by Gail M. Gauvreau, Roma Sehmi, Christopher S. Ambrose & Janet M. Griffiths © 2020 The Author(s) taken from Expert Opinion on Therapeutic Targets © 2020, 24:8 © 2020 Taylor & Francis Ltd, adapted by permission of the publisher, Taylor & Francis Ltd http://www.tandfonline.com.4
Abbreviations: Ig, immunoglobulin; IL, interleukin; ILC2, innate lymphoid cell type 2; T2, type 2; Th, T helper; TSLP, thymic stromal lymphopoietin.4
Targeting Thymic Stromal Lymphopoietin in Asthma
Thymic stromal lymphopoietin (TSLP) is an epithelial cytokine that has broad and multifaceted effects on the initiation and persistence of asthma airway inflammation. While it is primarily expressed by epithelial cells, it is also expressed by mast cells, dendritic cells, monocytes/macrophages, airway smooth muscle cells, fibroblasts, and other cell types (Figures 1 and 2).2,16,20–22 Conversely, TSLP acts on a wide range of effector cells, including dendritic cells, T and B cells, natural killer and regulatory T cells, innate lymphoid cells (ILCs) type 2 (ILC2s), eosinophils, basophils, mast cells, monocytes, macrophages, airway smooth muscle cells, and fibroblasts.2,20 As a result, TSLP can drive numerous aspects of asthma pathophysiology, with effects across the eosinophilic, allergic, and T2-low phenotypes.2
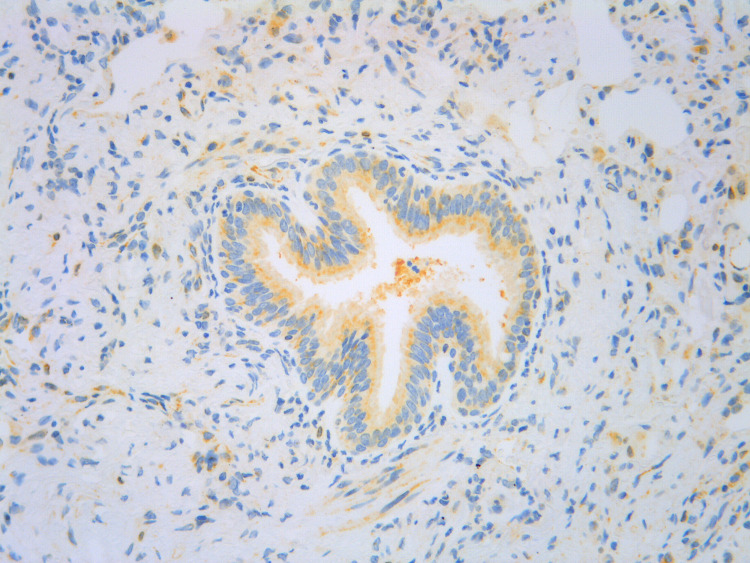
Figure 2.
Histopathological staining for TSLP (yellow) in asthmatic bronchiolar epithelial and stromal cells.
Note: Image source: AstraZeneca, in-house data.
Abbreviation: TSLP, thymic stromal lymphopoietin.
Key Tezepelumab Clinical Studies
Tezepelumab is a first-in-class monoclonal antibody (IgG2λ) that blocks the activity of TSLP.23 Major primary clinical trials of tezepelumab in patients with asthma include PATHWAY (NCT02054130),24 NAVIGATOR (NCT03347279),25,26 SOURCE (NCT03406078),27,28 UPSTREAM (NCT02698501),29 CASCADE (NCT03688074),30,31 and PATH-HOME (NCT03968978),32 which in total enrolled over 2000 patients. In addition, the DESTINATION extension study (NCT03706079) followed patients from NAVIGATOR and SOURCE for up to 104 weeks of treatment.33 An overview of these studies is provided in Supplementary Table 1.
Tezepelumab has been demonstrated to block TSLP and thus down-regulate multiple aspects of the asthma inflammatory cascade, including IL-4, IL-5, and IL-13 activity with respective declines in serum IgE levels, blood and airway eosinophil counts, and mucus production and fractional exhaled nitric oxide (FeNO) levels.24,26,29,30,34,35 In addition, tezepelumab consistently reduces airway hyperresponsiveness (AHR; to mannitol and methacholine) compared with placebo, presumably via mast cell and airway smooth muscle effects.29,30
Overall, in randomized, placebo-controlled studies, tezepelumab reduced asthma exacerbations by 56–71% compared with placebo in patients with severe, uncontrolled asthma across the spectrum of inflammatory phenotypes, and improved lung function, asthma control, and health-related quality of life (HRQoL).2,23,24,26 Tezepelumab was also well tolerated, with a similar overall frequency of adverse events (AEs) between tezepelumab and placebo.24 In a pooled analysis of PATHWAY and NAVIGATOR, AEs occurring at a slightly higher frequency with tezepelumab compared with placebo were pharyngitis, arthralgia, and back pain (each 4% with tezepelumab versus 3% with placebo).23 As a result of these data, tezepelumab was approved by the US Food and Drug Administration (FDA) in 2021 as the first and, at the time of writing, the only biologic for use in all types of severe asthma.23
Aim of the Review
The objective of this review is to summarize the anti-inflammatory effects and efficacy of tezepelumab across the phenotypes most relevant to the treatment of severe uncontrolled asthma: eosinophilic, allergic, allergic and eosinophilic, T2-low, and OCS-dependent.
Eosinophilic Severe Asthma
Eosinophilic asthma, particularly that not driven by allergy, usually develops in adulthood and is often severe. It can be associated with comorbid chronic rhinosinusitis and nasal polyps (NPs).17 For eosinophilic inflammation in the absence of a classical T2-mediated allergic response, ILC2s play a central role.17,36 In eosinophilic inflammation, TSLP can activate ILC2s, which in turn produce IL-5 and IL-13, leading to eosinophilia and mucus hypersecretion. TSLP may also have direct effects on eosinophils.4
Anti-Inflammatory Effects of Tezepelumab in Eosinophilic Severe Asthma
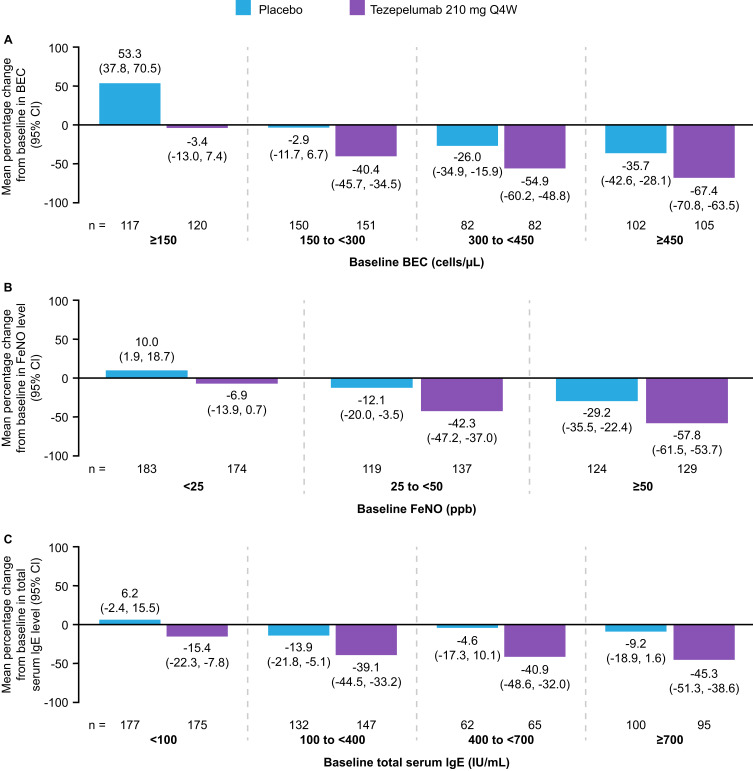
By blocking TSLP and reducing ILC2 activation, tezepelumab reduces inflammatory biomarkers such as serum IL-5 and IL-13 levels and blood and airway eosinophil levels (Figures 1 and 3).4,30,37 The Phase 2 PATHWAY and Phase 3 NAVIGATOR studies reported substantial and persistent decreases in blood eosinophil levels with tezepelumab versus placebo in patients with severe, uncontrolled asthma, consistent with a regulation and potential normalization of inflammation.24,26 Post hoc analysis from NAVIGATOR (Figure 4A–C) revealed that reductions in BECs with tezepelumab by Week 52 were greater in patients with higher baseline levels than those with lower baseline levels, with a 67.4% mean reduction in BECs in patients with baseline BEC ≥450 cells/µL versus a 40.4% mean reduction in patients with baseline BEC 150 to <300 cells/µL (Figure 4A).38 The same pattern was observed when analyzing levels of FeNO in patients treated with tezepelumab, with greater reductions from baseline in those with higher baseline levels than those with lower baseline levels (Figure 4B).38 FeNO is associated with allergic and/or eosinophilic inflammation and is a biomarker of IL-13 activity, as epithelial exposure to IL-13 induces nitric oxide synthase to increase production of nitric oxide.36,39 Post hoc exploratory analysis of the NAVIGATOR study also demonstrated reductions in patient-reported phlegm/mucus production and frequent productive cough with tezepelumab compared with placebo, overall and across subgroups based on baseline BEC and FeNO levels.34
Figure 3.
Summary of mechanisms and efficacy of tezepelumab in severe asthma, by asthma subtype.
Note: AAER reductions cited are based primarily on pooled PATHWAY/NAVIGATOR results but with inclusion of additional subgroup results, when relevant, from NAVIGATOR, as described in the text.
Abbreviations: AAER, annualized asthma exacerbation rate; ASMC, airway smooth muscle cell; ED, emergency department; FeNO, fractional exhaled nitric oxide; IgE, immunoglobulin E; IL, interleukin; T2, type 2; TSLP, thymic stromal lymphopoietin.
Figure 4.
Mean percentage changes from baseline to Week 52 in BEC (A), FeNO levels (B), and total serum IgE levels (C), in patients receiving tezepelumab 210 mg Q4W or placebo, grouped by baseline level of the respective biomarker. A post hoc analysis of the NAVIGATOR Phase 3 study. Adapted from Effect of tezepelumab on asthma inflammatory biomarker levels varies by baseline biomarker levels. J Corren J, J Spahn, C Ambrose, N Martin, G Colice, N Molfino, B Cook. Ann Allergy Asthma Immunol 129(5)(Suppl):S36. Copyright 2022, with permission from Elsevier.38
Abbreviations: BEC, blood eosinophil count; CI, confidence interval; FeNO, fractional exhaled nitric oxide; IgE, immunoglobulin E; Q4W, every 4 weeks.
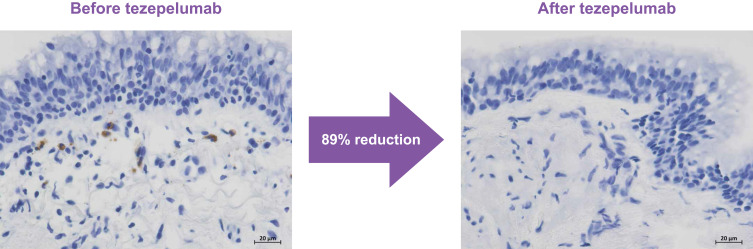
In the mechanistic CASCADE study, tezepelumab treatment of patients with uncontrolled moderate-to-severe asthma resulted in an 89% reduction in airway submucosal eosinophils from baseline to the end of treatment versus a 25% reduction with placebo (p<0.001; Figure 5).30 Across patients, the effect was similar regardless of baseline eosinophil count.30 Similarly, in the UPSTREAM study, airway tissue eosinophils, sputum eosinophils, and bronchoalveolar lavage (BAL) eosinophils decreased by 74%, 69%, and 75% with tezepelumab, respectively, while among placebo recipients there was a 28% increase, 26% increase, and 7% decrease in airway tissue, sputum, and BAL eosinophils, respectively (all p≤0.01).29
Figure 5.
Micrograph images of airway eosinophils (brown staining) from CASCADE, before and after treatment with tezepelumab.
Note: Image source: AstraZeneca, in-house data.
Airway inflammation driven by IL-5 and IL-13 activity can also drive the formation of airway mucus plugs, via IL-13 induction of goblet cell hyperplasia, mucus hypersecretion and IL-5-driven eosinophil recruitment and degranulation, resulting in cross-linked mucin and mucus plugs that can occlude the large and small airways. In the CASCADE study, TSLP inhibition by tezepelumab reduced occlusive mucus plugs versus placebo (change in mean [standard deviation] mucus plug score was –1.7 [2.6] with tezepelumab versus 0.0 [1.4] with placebo). Reductions in mucus plug scores with tezepelumab were correlated with improvements in lung function and reductions in eosinophilic inflammation.40,41
Efficacy of Tezepelumab in Eosinophilic Severe Asthma
In PATHWAY, annualized asthma exacerbation rates (AAERs) were lower with tezepelumab 210 mg every 4 weeks (Q4W) versus placebo by 65% in patients with baseline BEC ≥250 cells/µL.24 In NAVIGATOR, AAERs were lower with tezepelumab versus placebo by 61% and 70% for baseline BECs ≥150 cells/µL and ≥300 cells/µL, respectively.26 Post hoc pooled analyses of PATHWAY and NAVIGATOR subgroups further demonstrated that tezepelumab can substantially reduce asthma exacerbations versus placebo in patients with eosinophilic asthma, with 63% and 71% reductions in exacerbations with tezepelumab versus placebo in patients with BECs ≥150 and ≥300 cells/µL, respectively.42 Tezepelumab reduced AAERs that required hospitalizations or emergency department (ED) visits over 52 weeks by 86% and 90% versus placebo, in patients with BECs ≥150 and ≥300 cells/µL, respectively.43
Treatment with tezepelumab improved lung function, HRQoL, and asthma symptoms versus placebo in patients with baseline BECs 150 to <300, 300 to <450, and ≥450 cells/µL.43 Least squares (LS) mean differences (95% confidence interval [CI]) in prebronchodilator forced expiratory volume in one second (FEV1) with tezepelumab versus placebo was 0.10 (0.02–0.18), 0.21 (0.11–0.31), and 0.27 (0.18–0.36) for the three BEC subgroups, respectively. LS mean differences (95% CI) in the six-item Asthma Control Questionnaire (ACQ-6) score was –0.28 (−0.48 to −0.08), −0.56 (−0.82 to −0.29), and −0.46 (−0.69 to −0.23) for the three BEC subgroups, respectively. LS mean differences (95% CI) in Asthma Quality of Life Questionnaire for 12 Years and Older (AQLQ(S)+12) was 0.27 (0.05–0.49), 0.49 (0.20–0.78), and 0.49 (0.23–0.74) for the three BEC subgroups, respectively.43 In an exploratory analysis of HRQoL changes in NAVIGATOR, tezepelumab treatment was associated with improvements in St George’s Respiratory Questionnaire (SGRQ) score versus placebo in patients with baseline BECs <300 and ≥300 cells/µL; LS mean differences (95% CI) with tezepelumab versus placebo were −3.80 (−7.25 to −0.34) and −8.91 (–13.08 to −4.75), respectively.44
Efficacy of tezepelumab in patients with eosinophilic asthma was further demonstrated in patients with high baseline BEC in the NAVIGATOR study, with a 74–77% reduction of exacerbations in patients with BECs of ≥500, ≥750, and ≥1000 cells/µL.45 The LS mean (95% CI) improvements in prebronchodilator FEV1 compared with placebo were 0.26 (0.15–0.37) L, 0.21 (0.02–0.40) L, and 0.41 (0.13–0.69) L in patients with baseline BECs of ≥500, ≥750, and ≥1000 cells/μL, respectively.45 Tezepelumab also demonstrated high efficacy in patients with severe uncontrolled asthma and comorbid NPs, which is a phenotypic marker of eosinophilic disease.46–48 In NAVIGATOR, tezepelumab reduced exacerbations by 86% (95% CI: 70–93),46 and improved SNOT-22 scores with an LS mean change from baseline of −11.08 (−17.80 to −4.35) versus placebo in patients with severe uncontrolled asthma and a history of NPs, over 52 weeks of treatment.47
Allergic Severe Asthma
Allergic asthma frequently starts in childhood and is often associated with other atopic diseases such as atopic dermatitis. The pathophysiology of allergic severe asthma is driven by type 2 T helper (Th2) lymphocytes. Eosinophilia can be present, but, in patients with primary allergic disease, the eosinophilia is driven by the allergic response.17,36
TSLP acts as a key player in allergen-induced airway responses and persistent airway inflammation in patients with allergic asthma.49 In allergic inflammation, TSLP activates Th2 cells that can drive the production of IL-4, IL-5, and IL-13.4 Together, IL-4 and IL-13 production leads to T cell proliferation, B cell differentiation to plasma cells, and antibody isotype switching to IgE. As noted above, IL-5 and IL-13 production evokes eosinophilic inflammation, mucus hypersecretion, and airway mucus plugs (Figures 1 and 3).4,40
Anti-Inflammatory Effects of Tezepelumab in Allergic Severe Asthma
In a Phase 1 proof-of-concept study in patients with mild allergic asthma (NCT01405963), tezepelumab attenuated the early and late asthmatic responses to allergen challenge and reduced biomarkers of inflammation compared with placebo, implicating TSLP in allergen-induced airway responses and persistent airway inflammation in patients with allergic asthma.49 Compared with placebo, tezepelumab reduced sputum eosinophils over the course of the study and also inhibited the influx of eosinophils into the airway following the allergen challenge; similarly, tezepelumab reduced FeNO levels throughout the study and following allergen challenge.49 In these patients, tezepelumab treatment also significantly reduced AHR at end of treatment (Day 83 pre-allergen challenge; p<0.05), as measured by the response to inhaled methacholine.
The PATHWAY and NAVIGATOR studies reported progressive decreases in total serum IgE levels with tezepelumab versus placebo in patients with severe, uncontrolled asthma.24,26 As observed with blood eosinophil and FeNO reductions, post hoc analyses from NAVIGATOR revealed that reductions in total serum IgE levels with tezepelumab by Week 52 were greater in patients with higher baseline levels than those with lower baseline levels: 15.4% mean reduction with baseline IgE <100 IU/mL, 39.1% and 40.9% mean reduction with baseline IgE 100 to <400 IU/mL and 400 to <700 IU/mL, respectively, and 45.3% mean reduction with baseline IgE ≥700 IU/mL (Figure 4C).38 In the DESTINATION extension study (recruiting from NAVIGATOR and SOURCE), tezepelumab resulted in progressive reductions in serum IgE levels over a 2-year period, demonstrating long-term suppression of IgE production.33 Total serum IgE levels remained below baseline for 40 weeks after tezepelumab cessation, suggesting a potential post-treatment disease-modifying effect, although there was an upward trend in serum IgE levels at 28–40 weeks after tezepelumab cessation.50
A double-blind, parallel-design trial in patients with cat allergen-induced nasal allergy (CATNIP; NCT02237196) reported that inhibition of TSLP by tezepelumab augmented the efficacy of subcutaneous allergen immunotherapy (SCIT).51 Reduction in total nasal symptom scores (TNSS) following nasal cat allergen challenge during, and 1 year following, allergen immunotherapy suggested that tezepelumab may have promoted tolerance after a 1-year course of treatment.51 Patients receiving tezepelumab and SCIT combination therapy or SCIT monotherapy had comparable increases in serum cat-specific IgE at Week 12, followed by a decline at Week 26.51 This reduction then plateaued at Week 52 in the SCIT group, but the tezepelumab and SCIT group, and tezepelumab‑monotherapy group experienced continued reductions in cat-specific IgE levels through Week 104 (1 year after stopping treatment).51 At Week 52, nasal allergen challenge (NAC)-induced TNSS were reduced in patients receiving tezepelumab and SCIT combination therapy versus SCIT alone. At Week 104, TNSS was not significantly different in patients receiving tezepelumab and SCIT versus SCIT alone, but peak TNSS was significantly lower in the tezepelumab and SCIT group.51 Transcriptomic analysis of nasal epithelial samples demonstrated that treatment with the combination of tezepelumab and SCIT persistently downregulated a gene network related to T2 inflammation that was associated with improvement in NAC responses.51 This included the gene coding for tryptase alpha beta 1, a well-established and important mast cell mediator of the immediate allergic response. Furthermore, levels of nasal fluid tryptase were found to correlate with the gene expression, with levels decreasing significantly by Week 52 in the tezepelumab and SCIT group compared with the SCIT alone group.51
Eosinophilic inflammation and FeNO elevation are also potential features of allergic asthma due to IL-5 and IL-13 production by Th2 cells. As noted above, tezepelumab inhibition of TSLP activity reduces eosinophilia and FeNO levels in patients with severe asthma, including those with confirmed allergic disease.24,26,30,52,53
Efficacy of Tezepelumab in Allergic Severe Asthma
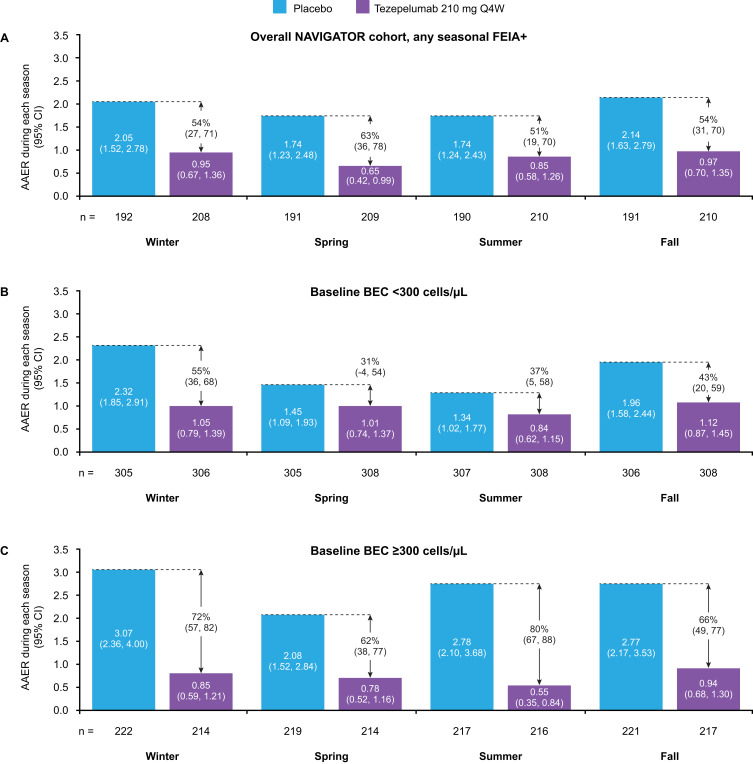
Post hoc analysis of the PATHWAY study found that tezepelumab 210 mg Q4W reduced AAER versus placebo by 66–78% in patients with any perennial allergy.53 In NAVIGATOR, tezepelumab reduced AAER over 52 weeks versus placebo by 58–68% in patients with severe allergic asthma, across the subgroups of those with perennial aeroallergen sensitivity, confirmed symptomatic allergy, and eligible for omalizumab.52 Furthermore, in a post hoc analysis of seasonal variations in asthma exacerbations, tezepelumab blunted seasonal variability in AAER across all seasons in patients with seasonal aeroallergen sensitization (51–63% reduction versus placebo), in those with baseline BEC <300 cells/µL (31–55% reduction versus placebo), and in those with baseline BEC ≥300 cells/µL (62–80% reduction versus placebo) (Figure 6).54,55
Figure 6.
AAER during each season in patients with any seasonal aeroallergen sensitization/allergy overall (A), with baseline BEC <300 cells/μL (B), and with baseline BEC ≥300 cells/μL (C), receiving tezepelumab 210 mg Q4W or placebo. A post hoc analysis of the NAVIGATOR Phase 3 study.
Notes: Calendar seasons were defined as winter (January to March), spring (April to June), summer (July to September), and fall (October to December).
Abbreviations: AAER, annualized asthma exacerbation rate; BEC, blood eosinophil count; FEIA+, fluorescence enzyme immunoassay positive; Q4W, every 4 weeks.
Pooled analysis of data from PATHWAY and NAVIGATOR similarly demonstrated that tezepelumab substantially reduced asthma exacerbations versus placebo in patients with seasonal or perennial allergic asthma. A 62% reduction in exacerbations with tezepelumab versus placebo was observed in patients with perennial aeroallergen sensitivity.43 Tezepelumab treatment reduced the rate of exacerbations that were associated with hospitalizations or ED visits versus placebo by 80% in patients with perennial aeroallergen sensitivity.43 Tezepelumab also reduced AAER versus placebo in patients with evidence of severe allergic asthma52 during all seasons,56 and irrespective of omalizumab eligibility,52 number of aeroallergen sensitizations,57 serum aeroallergen-specific IgE level,58 or the presence or absence of allergic comorbidities.59 Treatment with tezepelumab was associated with improvements in lung function, HRQoL, and asthma symptoms versus placebo in patients with perennial allergy. The LS mean difference (95% CI) with tezepelumab versus placebo was 0.09 (0.03–0.14), −0.25 (–0.39 to −0.10), and 0.28 (0.12–0.44) for prebronchodilator FEV1, ACQ-6 score, and AQLQ(S)+12, respectively.43 In an exploratory analysis of HRQoL changes in NAVIGATOR, tezepelumab treatment was associated with improvements in SGRQ score versus placebo in patients with any perennial allergy; LS mean difference with tezepelumab versus placebo was –5.42 (95% CI: −8.71 to −2.12).44
Patients with Both Allergic and Eosinophilic Severe Asthma
While allergic and eosinophilic asthma represent two distinct asthma phenotypes, many patients exhibit both.36,60 In an analysis of 935 adults with non-subtype-selected, uncontrolled, moderate-to-severe asthma, 587 (63%) had both allergic and eosinophilic (BEC ≥150 cells/µL) disease, highlighting the relevance of this population.61 In patients with allergic and eosinophilic inflammation, the primary TSLP-related mechanisms implicated are those outlined above. Allergen exposure evokes release of TSLP, which activates dendritic cells and Th2 cells and stimulates production of IL-4, IL-5, and IL-13, inducing T cell proliferation, B cell differentiation to plasma cells, and antibody isotype switching to IgE, as well as eosinophilic inflammation and increased mucus production.4 In addition, TSLP activation of ILC2 further drives airway eosinophilia and mucus production via IL-5 and IL-13 production (Figures 1 and 3).
Anti-Inflammatory Effects of Tezepelumab in Allergic and Eosinophilic Severe Asthma
Blockade of TSLP with tezepelumab can regulate inflammation mediated by IL-4, IL-5, and IL‑13, reducing IgE, eosinophils, and mucus production and FeNO, respectively.4 Tezepelumab is unique among biologics in its ability to meaningfully reduce the activity of all three of these T2 cytokines, which can make it uniquely suited to patients with allergic and eosinophilic disease. In addition, tezepelumab’s ability to reduce AHR and mucus plugs is also highly relevant to patients with allergic and eosinophilic asthma.
As noted above, tezepelumab attenuated the early and late asthmatic responses to allergen challenge and reduced biomarkers of allergic and eosinophilic inflammation in an allergen challenge study. In the PATHWAY, NAVIGATOR, DESTINATION, and CATNIP studies, tezepelumab recipients demonstrated progressive decreases in serum IgE levels,24,26,33,50 which remained below baseline for 40 to 52 weeks after tezepelumab cessation.33,50 In the PATHWAY, NAVIGATOR, and DESTINATION studies, tezepelumab recipients demonstrated substantial and persistent decreases in blood eosinophil and FeNO levels, consistent with normalization of inflammation.24,26,33 In the CASCADE and UPSTREAM studies, tezepelumab treatment resulted in a 74–89% reduction in airway submucosal eosinophils (Figure 5), with similar effects regardless of baseline eosinophil count;29,30 CASCADE also demonstrated a meaningful reduction in airway mucus plugs compared with placebo.40,41
Efficacy of Tezepelumab in Allergic and Eosinophilic Severe Asthma
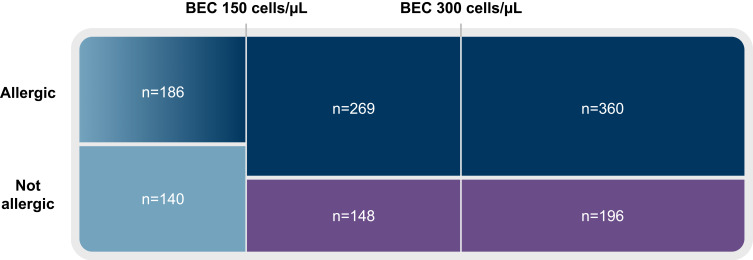
Post hoc analyses of pooled PATHWAY and NAVIGATOR subgroups demonstrated that tezepelumab can substantially reduce asthma exacerbations versus placebo in patients with both eosinophilic and allergic disease. In the pooled population, allergy to a perennial aeroallergen was confirmed in 629/1334 (47%) patients with baseline BEC ≥150 cells/µL and 360/1334 (27%) patients with baseline BEC ≥300 cells/µL (Figure 7).43 Tezepelumab reduced AAER versus placebo by 67% in patients with baseline BEC ≥150 cells/µL and perennial aeroallergen sensitization, and by 71% in those with baseline BEC ≥300 cells/µL and perennial aeroallergen sensitization.42,43 Exacerbations associated with hospitalizations or ED visits were reduced by 87–90% with tezepelumab versus placebo (AstraZeneca in-house data). Tezepelumab improved prebronchodilator FEV1 at Week 52 versus placebo with an LS mean difference (95% CI)) of 0.13 (0.06–0.19) in patients with baseline BEC ≥150 cells/µL and perennial aeroallergen sensitization, and 0.21 (0.12–0.29) in patients with baseline BEC ≥300 cells/µL and perennial aeroallergen sensitization.43 Furthermore, improvements in ACQ-6 score at Week 52 were observed with tezepelumab versus placebo in patients with baseline BEC ≥300 cells/µL and perennial aeroallergen sensitization: LS mean difference of −0.37 (95% CI: −0.58, −0.15). In an exploratory analysis of HRQoL changes in NAVIGATOR, tezepelumab treatment was associated with improvements in SGRQ score versus placebo in patients with baseline BEC ≥300 cells/µL and perennial aeroallergen sensitization; LS mean difference with tezepelumab versus placebo was −9.00 (95% CI: −13.98 to −4.02).44
Figure 7.
Proportional representation of patients by baseline BEC and allergy to perennial aeroallergens in the pooled PATHWAY and NAVIGATOR populations.
Note: Excludes 35 patients with no allergy testing results.
Abbreviation: BEC, blood eosinophil count.
T2-Low Severe Asthma
Whilst the mechanisms involved in T2-high asthma are well characterized, those implicated in T2-low asthma are less defined. T2-low asthma generally involves either neutrophilic or paucigranulocytic inflammation,62 and tends to be more resistant to inhaled corticosteroids. Commonly, it is associated with obesity, smoking, pollutants, viral or bacterial infections, and advanced age.60 T2-low populations are most frequently defined by low BEC and/or low FeNO levels.63,64 Importantly, even T2-low inflammation manifests with some component of IL-5-, IL-13-, and IL-4-dependent inflammation.65 While specific inflammatory pathways can be described as non-T2, few, if any, patients are truly non-T2 as all should have some degree of T2 airway inflammation.66 This was well demonstrated by a bronchial biopsy study of patients with severe uncontrolled asthma, in which 82% of patients with low BECs had evidence of airway submucosal eosinophilia.66
Anti-Inflammatory Effects of Tezepelumab in T2-Low Severe Asthma
Although the mechanisms responsible for tezepelumab’s reduction of exacerbations in patients with T2-low severe asthma is not precisely known, reductions in AHR coupled with reductions in any low levels of T2 inflammation may contribute.64 TSLP can directly activate mast cells independently of IgE, inducing the release of pro-inflammatory cytokines and chemokines. TSLP can also mediate cross-talk between airway smooth muscle cells, mast cells, and other immune and structural cells.4,67,68 By blocking TSLP activity, tezepelumab may reduce airway smooth muscle and mast cell activation (Figures 1 and 3).4 Plausibly, tezepelumab may also intervene in the neutrophilic non-T2 inflammatory pathway via interaction with ILCs type 3 and IL-17, although clinical data to support these effects are limited.20
Multiple observations support the mechanisms proposed above. With regard to AHR, exploratory data from CASCADE revealed that the reduction in AHR to mannitol was significantly greater with tezepelumab versus placebo (nominal p=0.03).30 In addition, a numerically greater proportion of patients in the tezepelumab group had a negative mannitol test at the end of treatment versus placebo (43% versus 25%).30 This observation was independent of baseline BEC, suggesting that tezepelumab’s AHR reduction is a T2-independent effect;64 direct action on mast cell activation is believed to play a role since mannitol triggers mast cell-dependent bronchoconstriction. Similarly, in the UPSTREAM study, tezepelumab reduced the proportion of patients with AHR to mannitol versus placebo.29 In an early mechanistic study, tezepelumab also reduced AHR measured by challenge with methacholine, which acts directly on airway smooth muscle, consistent with TSLP’s ability to directly activate smooth muscle cells.49
In CASCADE, tezepelumab treatment of patients with uncontrolled moderate-to-severe asthma resulted in reduced airway submucosal eosinophils versus placebo even among patients with T2-low disease and BEC <150 cells/µL.30 Post hoc analysis of patients with baseline BEC <150 cells/µL in NAVIGATOR also revealed a net reduction in BEC with tezepelumab versus placebo by Week 52; there was a 53.3% mean increase in BEC in placebo recipients with baseline BEC <150 cells/µL compared with a 3.4% mean reduction in BEC among tezepelumab recipients.38
Efficacy of Tezepelumab in T2-Low Severe Asthma
In PATHWAY, tezepelumab 210 mg Q4W reduced AAER versus placebo by 79% in patients with baseline BEC <250 cells/µL, by 64% in patients with FeNO <24 ppb, and by 84% in patients with a prespecified definition of low Th2 status (IgE ≤100 IU/mL or eosinophil count <140 cells/µL).24 In NAVIGATOR, tezepelumab reduced AAER over 52 weeks versus placebo by 39% in patients with BEC <150 cells/µL and by 32% in patients with FeNO levels <25 ppb.26 Analyses of pooled PATHWAY and NAVIGATOR subgroups similarly demonstrated that tezepelumab can meaningfully reduce asthma exacerbations versus placebo in patients with severe, uncontrolled asthma with markers of T2-low disease.42 Tezepelumab reduced exacerbations versus placebo by 48% in those with baseline BEC <150 cells/µL and by 40% in patients with baseline FeNO <25 ppb.42 Tezepelumab was also effective in the double and triple T2-low subgroups, defined by low BEC, low FeNO levels, and the absence of perennial aeroallergen sensitization (reduction of 34–49% versus placebo).42 Tezepelumab reduced exacerbations associated with hospitalizations or ED visits versus placebo by 60% and 65% in patients with BEC <150 cells/µL and FeNO <25 ppb, respectively.42
The pooled PATHWAY and NAVIGATOR studies generally demonstrated improvements with tezepelumab versus placebo for other secondary endpoints among patients with T2-low asthma, although at a lower magnitude than seen among patients with allergic or eosinophilic asthma.43 In patients with BEC <150 cells/µL, change from baseline to Week 52 (tezepelumab versus placebo) in prebronchodilator FEV1 was 0.07 for both, in ACQ-6 was −1.12 versus −1.01, and in AQLQ(S)+12 was 1.06 versus 0.94.43 In patients with FeNO <25 ppb, a change from baseline to Week 52 (tezepelumab versus placebo) in prebronchodilator FEV1 was 0.13 versus 0.08, in ACQ-6 was −1.26 versus −1.14, and in AQLQ(S)+12 was 1.20 versus 1.11.43 In patients with T2-low asthma, tezepelumab’s meaningful reductions in asthma exacerbations but more limited improvements versus placebo in these secondary endpoints, point to differing pathophysiology underlying exacerbations, lung function, and patient-reported symptoms and quality of life. The greater precision inherent in measuring exacerbations compared with patient-reported outcomes may also contribute to the observed differences across endpoints.69
OCS-Dependent Severe Asthma
Some patients with severe asthma require long-term daily maintenance treatment with OCSs in order to control their asthma. However, long-term use of systemic corticosteroids can lead to multiple AEs and an increased risk of mortality.70 In patients with severe OCS-dependent asthma, one of the aims of treatment is to enable tapering and discontinuation of daily OCSs.28 The prevalence of severe OCS-dependent asthma varies greatly, depending on the definition used and the patient population. In the CHRONICLE study of contemporary US specialist-treated severe asthma, 12% of patients enrolled were receiving maintenance systemic corticosteroid therapy.71
Anti-Inflammatory Effects of Tezepelumab in OCS-Dependent Severe Asthma
The SOURCE study evaluated the OCS-sparing effect of tezepelumab in patients with OCS-dependent severe asthma.28 At Week 48, tezepelumab treatment led to reduced eosinophil counts, FeNO, and total serum IgE versus placebo. Reductions were similar to those observed in other severe asthma populations, with an LS mean difference (95% CI) of −117 (−164 to −70) cells/µL, −10.31 (−18.06 to −2.55) ppb, and −118.43 (−219.31 to −17.55) IU/mL for BECs, FeNO, and total serum IgE, respectively. As in other studies, reductions from baseline in BECs and FeNO with tezepelumab were observed as early as Week 4, while total serum IgE levels gradually decreased over 48 weeks.28
Efficacy of Tezepelumab in OCS-Dependent Severe Asthma
Tezepelumab has demonstrated efficacy in patients with OCS-dependent asthma enrolled in PATHWAY and NAVIGATOR and is approved for use in this population; however, it has not definitively demonstrated OCS sparing in this population given the results of the SOURCE study.28 SOURCE enrolled patients with asthma, who required daily OCSs and had been receiving medium-dose (250–500 µg daily) or high-dose (>500 µg daily) inhaled corticosteroids for ≥12 months.28 The reduction in daily OCS dose from baseline was statistically similar in patients receiving tezepelumab or placebo at Week 48 (odds ratio [OR] 1.28 [95% CI: 0.69–2.35]; p=0.43) in the overall population.28 The lack of difference appeared to be related to the high response rate in both groups: 54% of the tezepelumab group and 46% of the placebo group reduced their daily OCS dose by 90–100%. Despite this high placebo response rate, an improvement was observed with tezepelumab versus placebo in daily OCS reduction in patients with baseline BEC ≥150 cells/µL (OR 2.58 [95% CI: 1.16–5.75]). Tezepelumab reduced AAER by 31% versus placebo over 48 weeks,28 and the rate of exacerbations associated with ED visits or hospitalizations was reduced by 41% with tezepelumab versus placebo.28 Furthermore, early and sustained improvements in prebronchodilator FEV1 and ACQ-6 score were observed with tezepelumab. For FEV1, there was an LS mean change from baseline at Week 48 of 0.21 L in the tezepelumab group and −0.04 L in the placebo group (LS mean difference 0.26 L [95% CI: 0.13–0.39]).28 For ACQ-6, the LS mean change from baseline at Week 48 of −0.87 in the tezepelumab group and −0.51 in the placebo group (LS mean difference −0.37 [95% CI: −0.71 to −0.02]).28
A pooled analysis of the NAVIGATOR and PATHWAY studies also examined patients with severe uncontrolled asthma receiving daily, maintenance OCS treatment. In this subgroup, treatment with tezepelumab versus placebo resulted in a non-significant 41% reduction in the rate of exacerbations and a 79% reduction in exacerbations requiring hospitalizations or ED visits.42 Treatment with tezepelumab was associated with improvements in lung function, HRQoL, and asthma symptoms versus placebo in those with maintenance OCS use.43 LS mean difference (95% CI) with tezepelumab versus placebo was 0.21 (0.06–0.37), −0.66 (−1.05 to −0.26), and 0.50 (0.06–0.94) for prebronchodilator FEV1, ACQ-6 score, and AQLQ(S)+12, respectively.43 Similarly, in the DESTINATION extension study, tezepelumab induced a non-significant 39% reduction in AAER over 104 weeks versus placebo in patients receiving maintenance OCS.33 In DESTINATION, reductions of background medications, including daily OCSs, were allowed at the discretion of investigators as per usual clinical practice.33 Among patients from NAVIGATOR, a numerically higher proportion in the tezepelumab group than in the placebo group discontinued OCSs by Week 104 (37.9% versus 7.7%).72 Among patients from SOURCE, a numerically higher proportion in the tezepelumab group than in the placebo group discontinued OCSs by Week 104 (66.7% versus 46.9%).72
Safety of Tezepelumab in Severe Asthma
Aggregated safety data on tezepelumab use in patients with severe asthma are available from the pooled analysis of NAVIGATOR and PATHWAY,43 as well as the long-term extension study DESTINATION.33 In the pooled analysis of NAVIGATOR and PATHWAY, the safety of tezepelumab was consistent with that in the individual studies, with no clinically meaningful differences in the frequency of AEs with tezepelumab versus placebo (75% versus 77%).43 The most common AEs (incidence ≥3%) with a higher frequency among tezepelumab recipients are pharyngitis, arthralgia, and back pain.23 Among patients with arthralgia AEs, severity/intensity was mild or moderate in all cases and study drug was discontinued in only one tezepelumab recipient due to arthralgia. The slightly higher incidence with tezepelumab may have been associated with OCS use, as daily OCS use at baseline was reported in 24% of tezepelumab recipients compared with 0% of placebo recipients.43 Asthma, upper respiratory tract infection, and sinusitis were reported less frequently with tezepelumab compared with placebo. The overall number of patients experiencing any on-treatment serious AEs (SAEs) was low (9% and 13% with tezepelumab and placebo, respectively), with 0.8% versus 0.3% reporting cardiac SAEs, respectively.43 Injection site reactions were reported in 4% and 3% of patients in the tezepelumab and placebo groups, respectively.43 No tezepelumab-related anaphylactic reactions were reported.43 Overall, treatment discontinuation due to an AE was reported in 2% and 3% of patients in the tezepelumab and placebo groups, respectively (1% and 2%, respectively due to SAEs); no on-treatment deaths were reported.43
Tezepelumab’s long-term safety was confirmed over 104 weeks of treatment in the DESTINATION extension study (NAVIGATOR and SOURCE) and was consistent with previously reported data.33 The most common AEs reported in ≥10% of patients were nasopharyngitis, upper respiratory tract infection and headache.33 The on-study pooled incidence of deaths was 0.80 and 0.58 per 100 patient-years in the tezepelumab and placebo groups, respectively (difference 0.22 [95% CI: −0.61 to 0.94]).33 No patterns were identified in the causes of the deaths, and no deaths were considered causally related to tezepelumab by a masked independent adjudication committee.33 The exposure-adjusted incidence of any AEs and SAEs was lower with tezepelumab than with placebo. The incidence of respiratory, thoracic, and mediastinal SAEs was lower in tezepelumab recipients than in placebo recipients, while the incidence of cardiac SAEs was higher in tezepelumab recipients than in placebo recipients.33 No pattern was identified in either the cause or timing of cardiac SAEs in relation to study drug administration.33 Neither investigators nor a masked independent adjudication committee attributed causality to tezepelumab for any cardiac SAE.33 The incidence of cardiac disorder system organ class AEs, independently adjudicated major adverse cardiovascular events, and cardiovascular deaths was in each case similar in tezepelumab and placebo recipients. The incidence of SAEs in the infections and infestations system organ class was also similar with tezepelumab and placebo.33
Role of Tezepelumab in Severe Asthma Relative to Other Biologics
Tezepelumab was approved by the FDA in 2021 as an add-on maintenance treatment for adult and pediatric patients aged ≥12 years with severe asthma regardless of phenotype.23 Tezepelumab is the most recently approved biologic for severe asthma, and a common question relates to its role relative to other biologics in the treatment of severe asthma. The anti-inflammatory effects of tezepelumab encompass the mechanisms of all other biologics, in that blocking TSLP leads to reductions in IgE, IL-5, blood and airway eosinophils, and the IL-4 and IL-13 pathways. It is the only biologic to meaningfully reduce the inflammatory pathways associated with IL-4, IL-13, and IL-5, which are central to allergic and eosinophilic inflammation.4 Thus, tezepelumab has the most extensive anti-inflammatory effects of any biologic in severe asthma.4
Within the T2 inflammatory pathways, TSLP inhibition results in the regulation and potential normalization of inflammation rather than complete blockade of individual downstream inflammatory pathways. In addition, tezepelumab is the only biologic to consistently demonstrate an improvement in AHR in randomized, placebo-controlled trials, which may explain, at least in part, its unique efficacy in patients with T2-low asthma.4,29,30 As noted above, tezepelumab’s multi-faceted anti-inflammatory effects could have significant benefits in patients with multiple upregulated inflammatory pathways, specifically those with eosinophilic and allergic inflammation as well as those with upregulation of IL-5 and IL-13 activity, as evidenced by elevated blood eosinophils and elevated FeNO.24,26,30
Based on the PATHWAY and NAVIGATOR trials, the efficacy of tezepelumab across the spectrum of severe asthma is comparable to, or greater than, that of other biologics with regard to exacerbation reduction and improvement in lung function, asthma symptoms, and asthma-related quality of life.43 Although head-to-head trials are lacking, independent network meta-analysis comparisons have confirmed this observation in patients with eosinophilic severe asthma or those with elevated T2 biomarkers.73,74 Among patients with allergic severe asthma, tezepelumab reduced the AAER versus placebo by 60–68% in participants with severe allergic asthma who met US or European Union eligibility criteria for omalizumab treatment,52 which compares favorably to the 25% reduction observed with omalizumab in patients with severe asthma in the EXTRA study.75 In patients with T2-low disease, specifically baseline BEC <150 cells/µL or FeNO <25 ppb, only tezepelumab has demonstrated a statistically significant and clinically meaningful reduction in asthma exacerbations.43 Additionally, tezepelumab has demonstrated high efficacy in reducing exacerbations associated with ED visits or hospitalizations, to a greater degree than that observed with other biologics, in a systematic review.76 The primary shortcoming of the current evidence with tezepelumab in severe asthma is that an OCS-sparing benefit among patients with OCS-dependent disease has not been definitively demonstrated with tezepelumab in a placebo-controlled, randomized trial.28 However, exploratory analyses from SOURCE and DESTINATION are suggestive of an OCS-sparing effect and additional studies are underway to further examine OCS-sparing efficacy with tezepelumab in OCS-dependent patients (WAYFINDER [NCT05274815] and SUNRISE [NCT05398263]).
Conclusion
In summary, tezepelumab treatment has demonstrated efficacy across the most relevant phenotypes of severe asthma, including eosinophilic, allergic, allergic and eosinophilic, T2 low, and OCS-dependent. With unique multi-faceted anti-inflammatory effects, tezepelumab is a therapeutic agent that can regulate airway inflammation across multiple inflammatory pathways, more extensively than other available biologics for severe asthma.
Acknowledgments
The authors thank Sarah Amir and Emma East from Lucid Group, Marlow, Buckinghamshire, UK, for providing medical writing and editorial support, which was funded by AstraZeneca in accordance with Good Publication Practice 2022 (GPP22) guidelines.
Funding Statement
Funding for this article was provided by AstraZeneca.
Abbreviations
AAER, annualized asthma exacerbation rate; ACQ-6, asthma control questionnaire; AE, adverse event; AHR, airway hyperresponsiveness; AQLQ(S)+12, asthma quality of life questionnaire; ASMC, airway smooth muscle cell; BAL, bronchoalveolar lavage; BEC, blood eosinophil count; CI, confidence interval; ED, emergency department; FDA, US Food and Drug Administration; FEIA+, fluorescence enzyme immunoassay positive; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in one second; HRQoL, health-related quality of life; Ig, immunoglobulin; IL, interleukin; ILC, innate lymphoid cell; ILC2, innate lymphoid cell type 2; LS, least squares; NAC, nasal allergen challenge; NP, nasal polyp; OCS, oral corticosteroid; OR, odds ratio; Q4W, every 4 weeks; SAE, serious adverse event; SCIT, subcutaneous allergen immunotherapy; SGRQ, St George’s Respiratory Questionnaire; T2, type 2; Th2, type 2 T helper; TNSS, total nasal symptom score; TSLP, thymic stromal lymphopoietin; US, United States.
Author Contributions
All authors made a significant contribution to the work reported, through the conception, study design, execution, acquisition of data, and/or analysis and interpretation; took part in drafting, revising and/or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Disclosure
RP Jr received consulting/advisory board fees from AstraZeneca, Genentech, Praesidia Biotherapies Inc., RIFM, and TEVA; fees for speaking from AstraZeneca, Merck & Co, and Sanofi; and research grants from ACTIV-1, AgoMab, AstraZeneca, Janssen, Medimmune, RIFM, TEVA, and Vault Health. NL received consulting fees from Amgen, AstraZeneca, Avillion, Genentech, GSK, Novartis, Regeneron, Sanofi, and Teva; honoraria for non-speaker bureau presentations from GSK and Astra Zeneca; and travel support from Astra Zeneca, Sanofi and GSK; her institution received research support from Amgen, AstraZeneca, Avillion, Evidera, Gossamer Bio, Genentech, GSK, Janssen, Regeneron, Sanofi, Novartis and Teva. She is an honorary faculty member of Observational and Pragmatic Research Institute (OPRI) but does not receive compensation for this role. JC received grants and personal fees from AstraZeneca, Genentech and Vectura, and has received grants from Optinose, Regeneron, Novartis, Pulmatrix, Sanofi and Teva Pharmaceuticals. CA is an employee of AstraZeneca and holds stock and stock options. The authors report no other conflicts of interest in this work.
References
- 1.Busse WW. Biological treatments for severe asthma: a major advance in asthma care. Allergol Int. 2019;68(2):158–166. doi: 10.1016/j.alit.2019.01.004 [DOI] [PubMed] [Google Scholar]
- 2.Menzies-Gow A, Wechsler ME, Brightling CE. Unmet need in severe, uncontrolled asthma: can anti-TSLP therapy with tezepelumab provide a valuable new treatment option? Respir Res. 2020;21(1):268. doi: 10.1186/s12931-020-01505-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The global asthma report 2022. Int J Tuberc Lung Dis. 2022;26(1):1–104. doi: 10.5588/ijtld.22.1010 [DOI] [PubMed] [Google Scholar]
- 4.Gauvreau GM, Sehmi R, Ambrose CS, Griffiths JM. Thymic stromal lymphopoietin: its role and potential as a therapeutic target in asthma. Expert Opin Ther Targets. 2020;24(8):777–792. doi: 10.1080/14728222.2020.1783242 [DOI] [PubMed] [Google Scholar]
- 5.Backman H, Jansson SA, Stridsman C, et al. Severe asthma-A population study perspective. Clin Exp Allergy. 2019;49(6):819–828. doi: 10.1111/cea.13378 [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Golam S, Myers J, Bly C, Smolen H, Xu X. Systematic literature review of the clinical, humanistic, and economic burden associated with asthma uncontrolled by GINA steps 4 or 5 treatment. Curr Med Res Opin. 2018;34(12):2075–2088. doi: 10.1080/03007995.2018.1505352 [DOI] [PubMed] [Google Scholar]
- 7.Reibman J, Tan L, Ambrose C, et al. Clinical and economic burden of severe asthma among US patients treated with biologic therapies. Ann Allergy Asthma Immunol. 2021;127(3):318–325.e2. doi: 10.1016/j.anai.2021.03.015 [DOI] [PubMed] [Google Scholar]
- 8.FasenraTM (benralizumab) Prescribing Information. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2023. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761070s000lbl.pdf. Accessed February 26, 2024 [Google Scholar]
- 9.Cinqair(R) (reslizumab) Prescribing Information. Teva Pharmaceutical Industries Ltd., Teva Respiratory, LLC: Frazer, PA; 2023. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761033lbl.pdf. Accessed February 26, 2024 [Google Scholar]
- 10.Xolair(R) (omalizumab) Prescribing Information. CA: Genentech Inc; 2023. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/103976s5238lbl.pdf. Accessed February 26, 2024 [Google Scholar]
- 11.Nucala (mepolizumab) Prescribing Information. PA: GlaxoSmithKline LLC; 2023. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761122s008,125526s019lbl.pdf.Accessed February 26, 2024 [Google Scholar]
- 12.Dupixent (dupilumab) Prescribing Information. NJ: Regeneron Pharmaceuticals Inc NY, Sanofi-aventis U.S. LLC; 2023. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761055s040lbl.pdf. Accessed February 26, 2024 [Google Scholar]
- 13.Wang E, Wechsler ME, Tran TN, et al. Characterization of severe asthma worldwide: data from the International Severe Asthma Registry. Chest. 2020;157(4):790–804. doi: 10.1016/j.chest.2019.10.053 [DOI] [PubMed] [Google Scholar]
- 14.Kupczyk M, Dahlen B, Sterk PJ, et al. Stability of phenotypes defined by physiological variables and biomarkers in adults with asthma. Allergy. 2014;69(9):1198–1204. doi: 10.1111/all.12445 [DOI] [PubMed] [Google Scholar]
- 15.Tran TN, Zeiger RS, Peters SP, et al. Overlap of atopic, eosinophilic, and TH2-high asthma phenotypes in a general population with current asthma. Ann Allergy Asthma Immunol. 2016;116(1):37–42. doi: 10.1016/j.anai.2015.10.027 [DOI] [PubMed] [Google Scholar]
- 16.Porsbjerg CM, Sverrild A, Lloyd CM, Menzies-Gow AN, Bel EH. Anti-alarmins in asthma: targeting the airway epithelium with next-generation biologics. Eur Respir J. 2020;56(5):2000260. doi: 10.1183/13993003.00260-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brusselle GG, Maes T, Bracke KR. Eosinophils in the spotlight: eosinophilic airway inflammation in nonallergic asthma. Nat Med. 2013;19(8):977–979. doi: 10.1038/nm.3300 [DOI] [PubMed] [Google Scholar]
- 18.Brusselle G, Bracke K. Targeting immune pathways for therapy in asthma and chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(5):S322–8. doi: 10.1513/AnnalsATS.201403-118AW [DOI] [PubMed] [Google Scholar]
- 19.Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16(1):45–56. doi: 10.1038/ni.3049 [DOI] [PubMed] [Google Scholar]
- 20.Plaza V, Cañete C, Domingo C, Martínez Rivera C, Muñoz X. Efficacy and potential positioning of tezepelumab in the treatment of severe asthma. Open Respir Arch. 2023;5(2):100231. doi: 10.1016/j.opresp.2022.100231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roan F, Obata-Ninomiya K, Ziegler SF. Epithelial cell-derived cytokines: more than just signaling the alarm. J Clin Invest. 2019;129(4):1441–1451. doi: 10.1172/JCI124606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varricchi G, Pecoraro A, Marone G, et al. Thymic stromal lymphopoietin isoforms, inflammatory disorders, and cancer. Front Immunol. 2018;9:1595. doi: 10.3389/fimmu.2018.01595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tezpire(TM) (tezepelumab-ekko) Prescribing Information. CA: Amgen Inc; 2023. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761224s000lbl.pdf. Accessed February 26, 2024 [Google Scholar]
- 24.Corren J, Parnes JR, Wang L, et al. Tezepelumab in adults with uncontrolled Asthma. N Engl J Med. 2017;377(10):936–946. doi: 10.1056/NEJMoa1704064 [DOI] [PubMed] [Google Scholar]
- 25.Menzies-Gow A, Colice G, Griffiths JM, et al. NAVIGATOR: a phase 3 multicentre, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the efficacy and safety of tezepelumab in adults and adolescents with severe, uncontrolled asthma. Respir Res. 2020;21(1):266. doi: 10.1186/s12931-020-01526-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menzies-Gow A, Corren J, Bourdin A, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med. 2021;384(19):1800–1809. doi: 10.1056/NEJMoa2034975 [DOI] [PubMed] [Google Scholar]
- 27.Wechsler ME, Colice G, Griffiths JM, et al. SOURCE: a phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel group trial to evaluate the efficacy and safety of tezepelumab in reducing oral corticosteroid use in adults with oral corticosteroid dependent asthma. Respir Res. 2020;21(1):264. doi: 10.1186/s12931-020-01503-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wechsler ME, Menzies-Gow A, Brightling CE, et al. Evaluation of the oral corticosteroid-sparing effect of tezepelumab in adults with oral corticosteroid-dependent asthma (SOURCE): a randomised, placebo-controlled, phase 3 study. Lancet Respir Med. 2022;10(7):650–660. doi: 10.1016/S2213-2600(21)00537-3 [DOI] [PubMed] [Google Scholar]
- 29.Sverrild A, Hansen S, Hvidtfeldt M, et al. The effect of tezepelumab on airway hyperresponsiveness to mannitol in asthma (UPSTREAM). Eur Respir J. 2021;59(1):2101296. doi: 10.1183/13993003.01296-2021 [DOI] [PubMed] [Google Scholar]
- 30.Diver S, Khalfaoui L, Emson C, et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2021;9(11):1299–1312. doi: 10.1016/S2213-2600(21)00226-5 [DOI] [PubMed] [Google Scholar]
- 31.Emson C, Diver S, Chachi L, et al. CASCADE: a phase 2, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the effect of tezepelumab on airway inflammation in patients with uncontrolled asthma. Respir Res. 2020;21(1):265. doi: 10.1186/s12931-020-01513-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alpizar S, Megally A, Chen C, Raj A, Downie J, Colice G. Functionality and performance of an accessorized pre-filled syringe and an autoinjector for at-home administration of tezepelumab in patients with severe, uncontrolled asthma. J Asthma Allergy. 2021;14:381–392. doi: 10.2147/JAA.S305114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menzies-Gow A, Wechsler ME, Brightling CE, et al. Long-term safety and efficacy of tezepelumab in people with severe, uncontrolled asthma (DESTINATION): a randomised, placebo-controlled extension study. Lancet Respir Med. 2023;11(5):425–483. doi: 10.1016/S2213-2600(22)00492-1 [DOI] [PubMed] [Google Scholar]
- 34.Castro M, Kraft M, Ambrose CS, et al. Tezepelumab reduces patient-reported cough and phlegm production in patients with severe, uncontrolled asthma: results from the phase 3 NAVIGATOR study. Presented at: American Thoracic Society (ATS). Washington DC, USA; 2023. [Google Scholar]
- 35.Sverrild A, Cerps S, Nieto-Fontarigo J, et al. Effects of tezepelumab on host epithelial tolerance to virus in patients with uncontrolled asthma. Presented at: European Respiratory Society (ERS). Virtual meeting; 2021. [Google Scholar]
- 36.Oppenheimer J, Hoyte FCL, Phipatanakul W, Silver J, Howarth P, Lugogo NL. Allergic and eosinophilic asthma in the era of biomarkers and biologics: similarities, differences and misconceptions. Ann Allergy Asthma Immunol. 2022;129(2):169–180. doi: 10.1016/j.anai.2022.02.021 [DOI] [PubMed] [Google Scholar]
- 37.Griffiths JM, Pham T-H, Wang E, et al. Tezepelumab reduces inflammatory biomarkers as early as week 2 and maintains reductions through week 52 in the phase 3 NAVIGATOR severe asthma trial. Presented at: American Academy of Allergy, Asthma and Immunology (AAAAI). Phoenix, AZ, USA; 2022. [Google Scholar]
- 38.Corren J, Spahn JD, Ambrose CS, et al. Effect of tezepelumab on asthma inflammatory biomarker levels varies by baseline biomarker levels. Presented at: American College of Allergy, Asthma and Immunology (ACAAI) Annual Scientific Meeting. Louisville, KY, USA; 2022. [Google Scholar]
- 39.Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–615. doi: 10.1164/rccm.9120-11ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nordenmark L, Emson C, Hellqvist Å, et al. Tezepelumab reduced mucus plugs in patients with moderate-to-severe asthma. Presented at: European Respiratory Society (ERS). Barcelona, Spain; 2022. [Google Scholar]
- 41.Nordenmark LH, Hellqvist Å, Emson C, et al. Tezepelumab and mucus plugs in patients with moderate-to-severe asthma. NEJM Evidence. 2023;2(10). doi: 10.1056/EVIDoa2300135 [DOI] [PubMed] [Google Scholar]
- 42.Corren J, Cook B, Ambrose CS, et al. Efficacy of tezepelumab in patients with severe, uncontrolled asthma: a pooled analysis of the phase 2b PATHWAY and phase 3 NAVIGATOR studies. Presented at: American Academy of Allergy, Asthma and Immunology (AAAAI) Annual Meeting. Phoenix, AZ, USA; 2022. [Google Scholar]
- 43.Corren J, Menzies-Gow A, Chupp G, et al. Efficacy of tezepelumab in severe, uncontrolled asthma: pooled analysis of PATHWAY and NAVIGATOR studies. Am J Respir Crit Care Med. 2023;208(1):13–24. doi: 10.1164/rccm.202210-2005OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kraft M, Corren J, Ambrose C, et al. Clinically meaningful improvements in St George’s Respiratory Questionnaire score with tezepelumab versus placebo in patients with severe, uncontrolled asthma: results from the phase 3 NAVIGATOR study. Presented at: American Thoracic Society (ATS). San Francisco, CA, USA; 2022. [Google Scholar]
- 45.Jain JP, Ambrose CS, Cook B, Ponnarambil S, Martin N, Webb L. Efficacy of tezepelumab in patients with severe, uncontrolled asthma and high baseline blood eosinophil counts. Presented at: American College of Allergy, Asthma and Immunology (ACAAI) Annual Scientific Meeting. Louisville, KY, USA; 2022. [Google Scholar]
- 46.Menzies-Gow ACJ, Corren J, Israel E, et al. Tezepelumab efficacy in patients with severe, uncontrolled asthma and comorbid nasal polyps in NAVIGATOR. Presented at: European Respiratory Society (ERS) International Congress. Virtual meeting; 2021. [Google Scholar]
- 47.Jacobs JS, Hoyte FCL, Spahn JD, et al. Tezepelumab efficacy by SNOT-22 score in patients with severe, uncontrolled asthma and comorbid nasal polyps in NAVIGATOR. Presented at: American Academy of Allergy, Asthma and Immunology (AAAAI). San Antonio, TX, USA; 2023. [Google Scholar]
- 48.Spahn JD, Jacobs JS, Hoyte FCL, et al. Tezepelumab efficacy by SNOT-22 domain scores in patients with severe, uncontrolled asthma and comorbid nasal polyps in the phase 3 NAVIGATOR study. Poster presentation presented at: American Thoracic Society (ATS). Washington DC, USA; 2023. [Google Scholar]
- 49.Gauvreau GM, O’Byrne PM, Boulet LP, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014;370(22):2102–2110. doi: 10.1056/NEJMoa1402895 [DOI] [PubMed] [Google Scholar]
- 50.Caminati M, Llanos JP, Spahn J, et al. Changes in serum total IgE after cessation of tezepelumab after 2 years of treatment (DESTINATION). Presented at: European Respiratory Society (ERS) International Congress. Milan, Italy; 2023. [Google Scholar]
- 51.Corren J, Larson D, Altman MC, et al. Effects of combination treatment with tezepelumab and allergen immunotherapy on nasal responses to allergen: a randomized controlled trial. J Allergy Clin Immunol. 2023;151(1):192–201. doi: 10.1016/j.jaci.2022.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corren J, Ambrose CS, Griffiths JM, et al. Efficacy of tezepelumab in patients with evidence of severe allergic asthma: results from the phase 3 NAVIGATOR study. Clin Exp Allergy. 2023;53(4):417–428. doi: 10.1111/cea.14256 [DOI] [PubMed] [Google Scholar]
- 53.Corren J, Ambrose CS, Sałapa K, et al. Efficacy of tezepelumab in patients with severe, uncontrolled asthma and perennial allergy. J Allergy Clin Immunol Pract. 2021;9(12):4334–4342.e6. doi: 10.1016/j.jaip.2021.07.045 [DOI] [PubMed] [Google Scholar]
- 54.Corren J, Menzies-Gow A, Ambrose C, et al. Effect of tezepelumab on seasonal exacerbations in patients with severe, uncontrolled asthma grouped by blood eosinophil count. Presented at: European Respiratory Society (ERS). Barcelona, Spain; 2022. [Google Scholar]
- 55.Corren J, Lindsley AW, Spahn J, et al. Tezepelumab reduces exacerbations across all seasons in patients with severe, uncontrolled asthma with seasonal allergy: results from the phase 3 NAVIGATOR study. Presented at: American Academy of Allergy, Asthma and Immunology (AAAAI). San Antonio, TX, USA; 2023. [DOI] [PubMed] [Google Scholar]
- 56.Corren J, Karpefors M, Hellqvist Å, Parnes JR, Colice G. Tezepelumab reduces exacerbations across all seasons in patients with severe, uncontrolled asthma: a post hoc analysis of the PATHWAY phase 2b study. J Asthma Allergy. 2021;14:1–11. doi: 10.2147/JAA.S286036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corren J, Welte T, Cook B, et al. Tezepelumab efficacy by number of sensitivities to perennial aeroallergens in NAVIGATOR. Presented at: American College of Allergy, Asthma and Immunology (ACAAI) Annual Scientific Meeting. New Orleans, LA, USA; 2021. [Google Scholar]
- 58.Lindsley AW, Colice G, Spahn J, Martin N, Ambrose CS, Hoyte FCL. Efficacy of tezepelumab in patients with severe, uncontrolled asthma by specific perennial allergen immunoglobulin E thresholds. Poster presentation presented at: American Academy of Allergy, Asthma and Immunology (AAAAI). San Antonio, TX, USA; 2023. [Google Scholar]
- 59.Menzies-Gow A, Corren J, Cook B, et al. Efficacy of tezepelumab in patients with severe, uncontrolled asthma, according to baseline blood eosinophil count and allergic status. Presented at: European Academy of Allergy and Clinical Immunology (EAACI) Hybrid Congress; 2021. [Google Scholar]
- 60.Denton E, Price DB, Tran TN, et al. Cluster analysis of inflammatory biomarker expression in the International Severe Asthma Registry. J Allergy Clin Immunol Pract. 2021;9(7):2680–2688 e7. doi: 10.1016/j.jaip.2021.02.059 [DOI] [PubMed] [Google Scholar]
- 61.Chen M, Shepard K, Yang M, et al. Overlap of allergic, eosinophilic and type 2 inflammatory subtypes in moderate-to-severe asthma. Clin Exp Allergy. 2021;51(4):546–555. doi: 10.1111/cea.13790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kyriakopoulos C, Gogali A, Bartziokas K, Kostikas K. Identification and treatment of T2-low asthma in the era of biologics. ERJ Open Res. 2021;7(2):00309–2020. doi: 10.1183/23120541.00309-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peters MC, Mekonnen ZK, Yuan S, Bhakta NR, Woodruff PG, Fahy JV. Measures of gene expression in sputum cells can identify TH2-high and TH2-low subtypes of asthma. J Allergy Clin Immunol. 2014;133(2):388–394. doi: 10.1016/j.jaci.2013.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Corren J, Brightling CE, Boulet LP, et al. Not just an anti-eosinophil drug: tezepelumab treatment for type 2 asthma and beyond. Eur Respir J. 2023;61(3):2202202. doi: 10.1183/13993003.02202-2022 [DOI] [PubMed] [Google Scholar]
- 65.Peters MC, Ringel L, Dyjack N, et al. A transcriptomic method to determine airway immune dysfunction in T2-high and T2-low asthma. Am J Respir Crit Care Med. 2019;199(4):465–477. doi: 10.1164/rccm.201807-1291OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cosío BG, Shafiek H, Iglesias A, et al. Validation of a pathological score for the assessment of bronchial biopsies in severe uncontrolled asthma: beyond blood eosinophils. Arch Bronconeumol. 2023;59(8):502–509. doi: 10.1016/j.arbres.2023.05.014 [DOI] [PubMed] [Google Scholar]
- 67.Comeau MR, Ziegler SF. The influence of TSLP on the allergic response. Mucosal Immunol. 2010;3(2):138–147. doi: 10.1038/mi.2009.134 [DOI] [PubMed] [Google Scholar]
- 68.Han NR, Oh HA, Nam SY, et al. TSLP induces mast cell development and aggravates allergic reactions through the activation of MDM2 and STAT6. J Invest Dermatol. 2014;134(10):2521–2530. doi: 10.1038/jid.2014.198 [DOI] [PubMed] [Google Scholar]
- 69.Ambrose CS, Israel E, Bowen K, et al. Reply to Lipworth and Chan. Am J Respir Crit Care Med. 2023;208(2):212–213. doi: 10.1164/rccm.202305-0843LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee H, Ryu J, Nam E, et al. Increased mortality in patients with corticosteroid-dependent asthma: a nationwide population-based study. Eur Respir J. 2019;54(5):1900804. doi: 10.1183/13993003.00804-2019 [DOI] [PubMed] [Google Scholar]
- 71.Ambrose CS, Chipps BE, Moore WC, et al. The CHRONICLE study of US adults with subspecialist-treated severe asthma: objectives, design, and initial results. Pragmat Obs Res. 2020;11:77–90. doi: 10.2147/POR.S251120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wechsler ME, Menzies-Gow A, Brightling CE, et al. Oral corticosteroid-sparing effect of tezepelumab in adults with severe asthma: results from the DESTINATION study. Presented at: American Thoracic Society (ATS). Washington, DC, USA; 2023. [Google Scholar]
- 73.Ando K, Fukuda Y, Tanaka A, Sagara H. Comparative efficacy and safety of tezepelumab and other biologics in patients with inadequately controlled asthma according to thresholds of type 2 inflammatory biomarkers: a systematic review and network meta-analysis. Cells. 2022;11(5):819. doi: 10.3390/cells11050819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nopsopon T, Lassiter G, Chen ML, et al. Comparative efficacy of tezepelumab to mepolizumab, benralizumab, and dupilumab in eosinophilic asthma: a Bayesian network meta-analysis. J Allergy Clin Immunol. 2023;151(3):747–755. doi: 10.1016/j.jaci.2022.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hanania NA, Alpan O, Hamilos DL, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011;154(9):573–582. doi: 10.7326/0003-4819-154-9-201105030-00002 [DOI] [PubMed] [Google Scholar]
- 76.Korn S, Cook B, Simpson LJ, Llanos JP, Ambrose CS. Efficacy of biologics in severe, uncontrolled asthma stratified by blood eosinophil count: a systematic review. Adv Ther. 2023;40(7):2944–2964. doi: 10.1007/s12325-023-02514-0 [DOI] [PMC free article] [PubMed] [Google Scholar]