Abstract
Introduction
Thromboembolic events have occurred in clinical trials of roxadustat. This post hoc analysis explored potential factors related to thromboembolic events in dialysis-dependent patients treated with roxadustat in four phase 3 clinical trials in Japan.
Methods
Thromboembolic events with onset before and after week 12 were evaluated. Baseline risk factors for thromboembolic events were investigated by Cox regression analyses. Nested case-control analyses using conditional logistic models with matched pairs of case-control data explored relationships between thromboembolic events and laboratory parameters.
Results
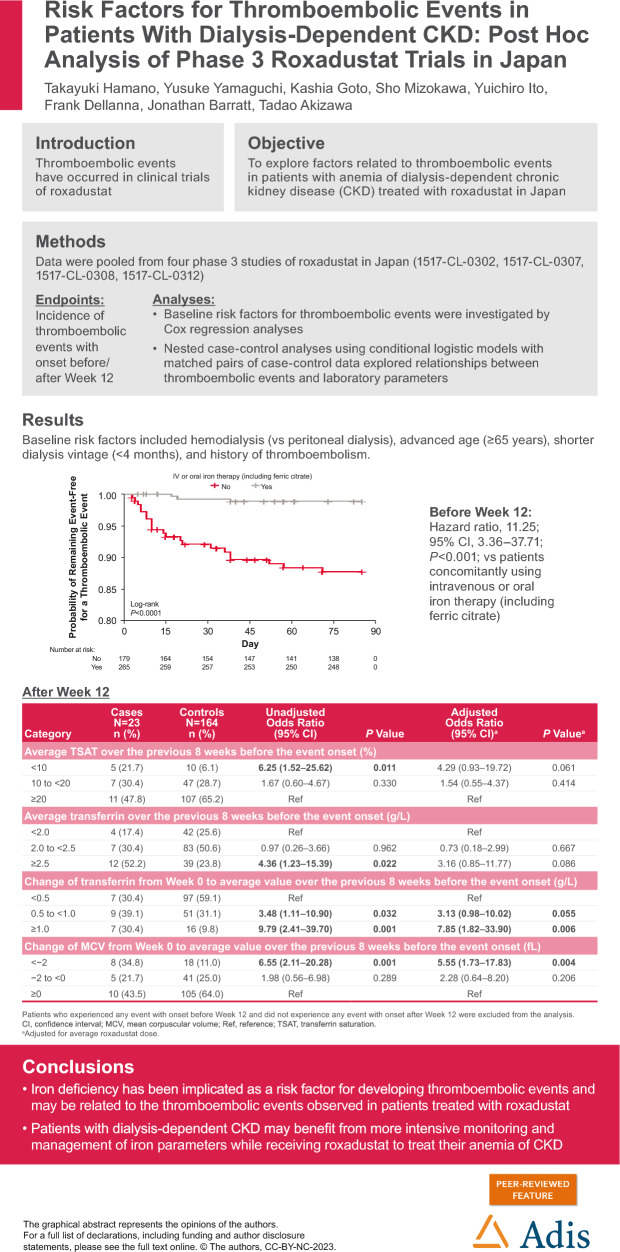
Of the 444 patients, 56 thromboembolic events were observed in 44 patients during ≤ 52 weeks of treatment. The proportion of venous and arterial thromboembolic events gradually increased after week 12. Baseline risk factors included hemodialysis (vs peritoneal dialysis), advanced age (≥ 65 years), shorter dialysis vintage (< 4 months), and history of thromboembolism. The absence of concomitant intravenous or oral iron therapy (including ferric citrate) was associated with thromboembolic events before week 12 (hazard ratio 11.25; 95% confidence interval [CI] 3.36–37.71; vs presence). Case-control analysis revealed that low average transferrin saturation (< 10%; unadjusted odds ratio [OR] 6.25; 95% CI 1.52–25.62; vs ≥ 20%), high average transferrin level (≥ 2.5 g/L; unadjusted OR 4.36; 95% CI 1.23–15.39; vs < 2.0 g/L), and high average roxadustat dose (≥ 150 mg; unadjusted OR 5.95; 95% CI 1.07–33.16; vs < 50 mg) over the previous 8 weeks before the event onset were associated with thromboembolic events after week 12. However, adjustment for iron status extinguished the significant relationship between roxadustat dose and events. Multivariate case-control analysis showed that increased transferrin from baseline (≥ 1.0 g/L; adjusted OR 7.85; 95% CI 1.82–33.90; vs < 0.5 g/dL) and decreased mean corpuscular volume (< − 2 fL; adjusted OR 5.55; 95% CI 1.73–17.83; vs ≥ 0 fL) were associated with increased risk of thromboembolic events.
Conclusion
In addition to established risk factors, iron deficiency may be related to thromboembolic events.
Graphical Abstract available for this article.
Trial Registration
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-023-02727-3.
Keywords: Anemia, Chronic kidney disease, Dialysis, Hypoxia-inducible factor prolyl hydroxylase inhibitor, Iron deficiency, Roxadustat, Thromboembolism
Plain Language Summary
Roxadustat is an oral medicine that treats anemia in patients with chronic kidney disease (CKD). Thromboembolic events, or blood vessels blocked by a blood clot, have occurred in clinical trials of roxadustat. This study explored potential factors that may be related to thromboembolic events in roxadustat-treated patients with anemia of CKD on dialysis before and after week 12. This study found that hemodialysis (vs peritoneal dialysis), advanced age (older than 65 years), short amount of time on dialysis (less than 4 months), previous history of thromboembolic events, and not receiving iron therapy were risk factors for thromboembolic events before week 12. Iron deficiency and high roxadustat dose were risk factors for thromboembolic events after week 12. When iron status was also considered, we did not find that roxadustat dose was related to thromboembolic events. A different model found that increased levels of transferrin, a protein that transports iron, from baseline and decreased mean corpuscular volume, or smaller red blood cells, increased the risk of thromboembolic events. Patients with anemia of CKD on dialysis may benefit from more intentional monitoring and management of iron while receiving roxadustat.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-023-02727-3.
Key Summary Points
| Why carry out this study? |
| Roxadustat, an oral medication, is a hypoxia-inducible factor prolyl hydroxylase inhibitor approved for the treatment of anemia of chronic kidney disease (CKD). |
| Thromboembolic events have occurred in clinical trials of roxadustat, resulting in a warning for potential development of thromboembolism in the prescribing information for roxadustat in Japan. |
| The current study explored the factors related to the occurrence of thromboembolic events in patients with anemia of dialysis-dependent CKD treated with roxadustat in Japan. |
| What was learned from the study? |
| Baseline risk factors for thromboembolic events before week 12 were found to be hemodialysis (vs peritoneal dialysis) and shorter dialysis vintage (< 4 months); risk factors for events after week 12 were advanced age (≥ 65 years) and history of thromboembolism. |
| The absence of intravenous or oral iron therapy early in treatment, before week 12, was associated with an increased risk of developing thromboembolic events; low transferrin saturation and increased transferrin levels were associated with an increased risk of developing thromboembolic events after week 12. |
| Decrease in mean corpuscular hemoglobin/mean corpuscular volume was associated with an increased risk of thromboembolic events in the later treatment period. |
Digital Features
This article is published with digital features, including a Graphical Abstract, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.24466225.
Introduction
Anemia, a common complication for patients with chronic kidney disease (CKD), is associated with decreased health-related quality of life and increased risk of cardiovascular events [1, 2]. Patients with advanced kidney disease have an increased risk of thromboembolism compared with those without kidney disease [3]. For adults with anemia of CKD on hemodialysis (HD), the Japanese Society for Dialysis Therapy (JSDT) recommends target hemoglobin levels be maintained in the range of 10–12 g/dL, and they recommend initiating treatment in this patient population when hemoglobin levels are < 10 g/dL at multiple examinations [4]. In adults on peritoneal dialysis (PD), JSDT recommends target hemoglobin levels be maintained in the range of 11–13 g/dL; treatment in this population should be initiated when hemoglobin levels are < 11 g/dL at multiple examinations [4].
Erythropoiesis-stimulating agents (ESAs) have been used to treat patients with anemia of CKD since the early 1990s; however, they are associated with safety concerns including hypertension, stroke, vascular access thrombosis, and thromboembolic complications [5]. Successful treatment for anemia of CKD requires increasing hemoglobin levels to, or maintaining hemoglobin levels within, the recommended target range and may require oral and/or intravenous (IV) iron supplementation [2]. Roxadustat, an oral medication, is a hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHI) that is approved in multiple countries and regions for the treatment of anemia of CKD [6].
Thromboembolic events have occurred in clinical trials of roxadustat, with varied incidence and severity depending on the patient population and thromboembolic site (e.g., artery or vein) [7, 8]. The Japanese prescribing information for roxadustat includes a warning for potential development of thromboembolism [9]. A meta-analysis of 30 studies comprising 13,146 patients found that patients treated with HIF-PHIs were more likely to experience thromboembolic events compared with patients treated with ESAs [10]. There is little information available about the potential relationship between thromboembolic events and clinical characteristics, including laboratory values, of patients with dialysis-dependent (DD) CKD who are administered roxadustat in Japan.
The objective of the current study was to explore factors related to the occurrence of arterial and venous thromboembolic events in patients with anemia of DD CKD treated with roxadustat in Japan.
Methods
Component Studies
This post hoc exploratory analysis pooled data from four phase 3 clinical trials (1517-CL-0302, 1517-CL-0307, 1517-CL-0308, and 1517-CL-0312), which are all the phase 3 trials of roxadustat conducted in Japan in the DD CKD patient population (Table 1) [6, 7, 11]. The data pooling was performed to supplement the limited number of patients and events observed in each study and to increase the statistical power for identifying risk factors. Studies 1517-CL-0302 and 1517-CL-0308 were open-label, 24-week studies. 1517-CL-0307 was a randomized, 24-week, double-blind study. 1517-CL-0312 was a 52-week, open-label study. Prior to enrollment, patients in 1517-CL-0308 were naive to ESA treatment, whereas patients in 1517-CL-0307 and 1517-CL-0312 were treated with ESA prior to enrollment. Study 1517-CL-0302 included two patient populations: patients who had been treated with an ESA, as well as patients who had not been treated with an ESA, prior to enrollment. Patients in 1517-CL-0308 were randomized to receive a starting dose of either 50 mg or 70 mg roxadustat. In 1517-CL-0302, ESA-naive patients were randomized to receive a starting dose of either 50 mg or 70 mg roxadustat, and ESA-converted patients received a conversion dose of either 70 mg or 100 mg roxadustat. Patients in 1517-CL-0307 were randomized to receive a conversion dose of either 70 mg or 100 mg roxadustat, or an active comparator. Roxadustat doses and dose conversions for all studies are presented in Table S1 in the electronic supplementary material.
Table 1.
Component studies
| Design feature | 1517-CL-0302 | 1517-CL-0307 | 1517-CL-0308 | 1517-CL-0312 |
|---|---|---|---|---|
| Open-label | Yes | Double-blind | Yes | Yes |
| Comparator | None | DA | None | None |
| Patients treated with roxadustat, n | 56 | 150 | 75 | 163 |
| Hemodialysis, n | 0 | 150 | 75 | 163 |
| Peritoneal dialysis, n | 56 | 0 | 0 | 0 |
| Baseline hemoglobin, g/dL |
< 10.5 for ESA-naive patients 10.0 to ≤ 12.0 for ESA-converted patients |
10.0 to ≤ 12.0 | ≤ 10.0 | 10.0 to ≤ 12.0 |
| Previous ESA treatment | ESA-naive/ ESA-converted | ESA-converted | ESA-naive | ESA-converted |
| Iron-related inclusion criteria |
TSAT ≥ 5% or serum ferritin ≥ 30 ng/mL for ESA-naive patients TSAT ≥ 20% or serum ferritin ≥ 100 ng/mL for ESA-converted patients |
TSAT ≥ 20% or serum ferritin ≥ 100 ng/mL | TSAT ≥ 5% or serum ferritin ≥ 30 ng/mL | TSAT ≥ 20% or serum ferritin ≥ 100 ng/mL |
| Study duration | 24 weeks | 24 weeks | 24 weeks | 52 weeks |
DA darbepoetin alfa, ESA erythropoiesis-stimulating agent, TSAT transferrin saturation
All studies included in this post hoc analysis were conducted in accordance with the Declaration of Helsinki of 1964 and its later amendments, the International Conference on Harmonisation of Technical Requirements for Pharmaceuticals for Human Use guidelines, Good Clinical Practice, and applicable local laws and regulations. Individual study details are available at https://clinicaltrials.gov/ct2/show/NCT02780726, https://clinicaltrials.gov/ct2/show/NCT02952092, https://clinicaltrials.gov/ct2/show/NCT02780141, and https://clinicaltrials.gov/ct2/show/NCT02779764, and their associated publications [6, 7, 11].
As this was a pooled post hoc analysis, approval by an ethics committee for this analysis was not necessary; however, institutional review board approval was obtained prior to initiating each of the studies. All participants provided written informed consent.
Participants
All eligible patients were ≥ 20 years of age, had anemia of CKD, and were receiving either HD (1517-CL-0307, 1517-CL-0308, 1517-CL-0312) or PD (1517-CL-0302). Eligible patients from 1517-CL-0302 (ESA-converted patients), 1517-CL-0307, and 1517-CL-0312 had transferrin saturation (TSAT) ≥ 20% or serum ferritin levels ≥ 100 ng/mL. Eligible patients from 1517-CL-0302 (ESA-naive patients) and 1517-CL-0308 had TSAT ≥ 5% or serum ferritin ≥ 30 ng/mL. These studies included patients who had been on dialysis for ≤ 4 months (incident-dialysis [ID] patients) and > 4 months (stable dialysis [SD] patients).
Exclusion criteria included prior treatment with roxadustat, uncontrolled hypertension, concurrent congestive heart failure (New York Heart Association Class III or higher), history of hospitalization for treatment of stroke, myocardial infarction, or pulmonary embolism within 12 weeks before screening, and recent red blood cell transfusion and/or surgical procedure that promotes anemia. During dialysis sessions, patients were undergoing anticoagulation according to standard practice.
Iron Administration Protocols
IV iron therapy was permitted at the discretion of the investigator if patients had TSAT < 20% or serum ferritin < 100 ng/mL in 1517-CL-0302 (ESA-converted patients), 1517-CL-0307, and 1517-CL-0312, and was permitted at the discretion of the investigator if patients had TSAT < 5% or serum ferritin < 30 ng/mL in 1517-CL-0302 (ESA-naive patients) and 1517-CL-0308. There were no restrictions on the concomitant oral administration of iron products in any of the studies.
Definition of Thromboembolic Events
Thromboembolic events were defined according to preferred terms selected by Standardized MedDRA Queries: embolic and thrombotic events (narrow). Thromboembolic events were categorized as arterial thromboembolic events, venous thromboembolic events, and shunt-related thromboembolic events on the basis of internal physician determination. Thromboembolic events with onset before week 12 were defined as any event with onset before day 85 (exclusive), and thromboembolic events with onset after week 12 were defined as any event with onset day after day 85 (inclusive).
Analysis Method and Statistical Analysis
Two different approaches were used to evaluate risk factors that may potentially be associated with a thromboembolic event: a Cox regression analysis and a nested case-control analysis (see Fig. S1a in the electronic supplementary material). The thromboembolic events were analyzed as one event instead of being analyzed separately with arterial, venous, and shunt-related events. Analyses were performed separately for events with onset before and after week 12.
Cox Regression Analysis
The time to first onset of thromboembolic events before and after week 12, including time to censoring, was analyzed using the Kaplan-Meier method by subgroups, and the association between the thromboembolic event and each subgroup was evaluated by log-rank test. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using a Cox proportional hazards model for each subgroup factor. The Cox regression is a common technique to analyze time-to-event data and is known to have an advantage of not requiring distributional assumption for baseline hazard. The subgroup factors included patient demographic and clinical characteristics and ESA treatment history.
Nested Case-Control Analysis
Patients with a thromboembolic event were classified as cases, and patients without a thromboembolic event were classified as controls. For a patient with an event at a given time point (days from first study drug taken), potential controls who had not experienced any events at the onset time of the case were selected. A matching algorithm was used to select controls with similar characteristics with respect to important confounding variables from potential controls (see Fig. S1b in the electronic supplementary material). We selected the matching variables based on the results from the Cox regression analysis. Cases were matched to up to 10 controls with the same level of binary matching variables and the smallest Mahalanobis distance of continuous matching variables. The patients experiencing thromboembolic events and their matched controls were compared in terms of the potential risk factors. By case and matched control group, numbers and percentages of patients were calculated for binary and categorical factors. A conditional logistic regression model was used to calculate an odds ratio (OR) for cases compared with matched controls with 95% CIs and P values. The advantage of nested case-control analysis is the flexibility to control for confounding effects via matching techniques, and thus relatively high statistical power is expected even in this pooled analysis with only 44 patients experiencing the thromboembolic events, where naive model adjustment for multiple confounding factors may be challenging. More details on the methodology are available in the Methods section of the electronic supplementary material.
Results
Overview of Thromboembolic Events
The mean age of the 444 patients in this pooled analysis was 64.2 years. Patients had a history of diabetes (37.4%), thromboembolism (25.9%), and cardiovascular disease (14.0%). The mean (SD) baseline hemoglobin level was 10.5 (1.2) g/dL (Table 2).
Table 2.
Baseline demographics and clinical characteristics
| Characteristic | Roxadustat (N = 444) |
|---|---|
| Age (years), mean (SD) | 64.2 (11.7) |
| Sex (male), n (%) | 291 (65.5) |
| Dialysis vintage (months), mean (SD) | 69.1 (80.2) |
| Dialysis vintage, n (%) | |
| ≤ 4 months | 83 (18.7) |
| > 4 months | 361 (81.3) |
| History of diabetes, n (%) | 166 (37.4) |
| History of thromboembolism, n (%) | 115 (25.9) |
| History of cardiovascular disease, n (%) | 62 (14.0) |
| Body mass index (kg/m2), mean (SD) | 22.8 (3.7) |
| Previous ESA treatment, n (%) | |
| Naive | 88 (19.8) |
| Conversion | 356 (80.2) |
| Concomitant antiplatelet agent use, n (%) | 202 (45.5) |
| Concomitant anticoagulant use, n (%) | 18 (4.1) |
| Concomitant IV or oral iron therapy use (including ferric citrate), n (%) | 265 (59.7) |
| Concomitant IV iron therapy use, n (%) | 110 (24.8) |
| Concomitant oral iron therapy use (including ferric citrate), n (%) | 173 (39.0) |
| Hemoglobin at week 0 (g/dL), mean (SD) | 10.5 (1.2) |
| Ferritin at week 0 (ng/mL), median (min, max) | 90.8 (6.9, 572.0) |
| TSAT at week 0 (%), mean (SD) | 29.9 (11.9) |
| Transferrin at week 0 (g/L), median (min, max) | 1.8 (0.9, 3.3) |
| Platelets at week 0 (104/µL), mean (SD) | 20.5 (6.3) |
| hsCRP at week 0 (mg/dL), median (min, max) | 0.06 (0.0, 6.3) |
ESA erythropoiesis-stimulating agent, hsCRP high-sensitivity C-reactive protein, max maximum, min minimum, SD standard deviation, TSAT transferrin saturation
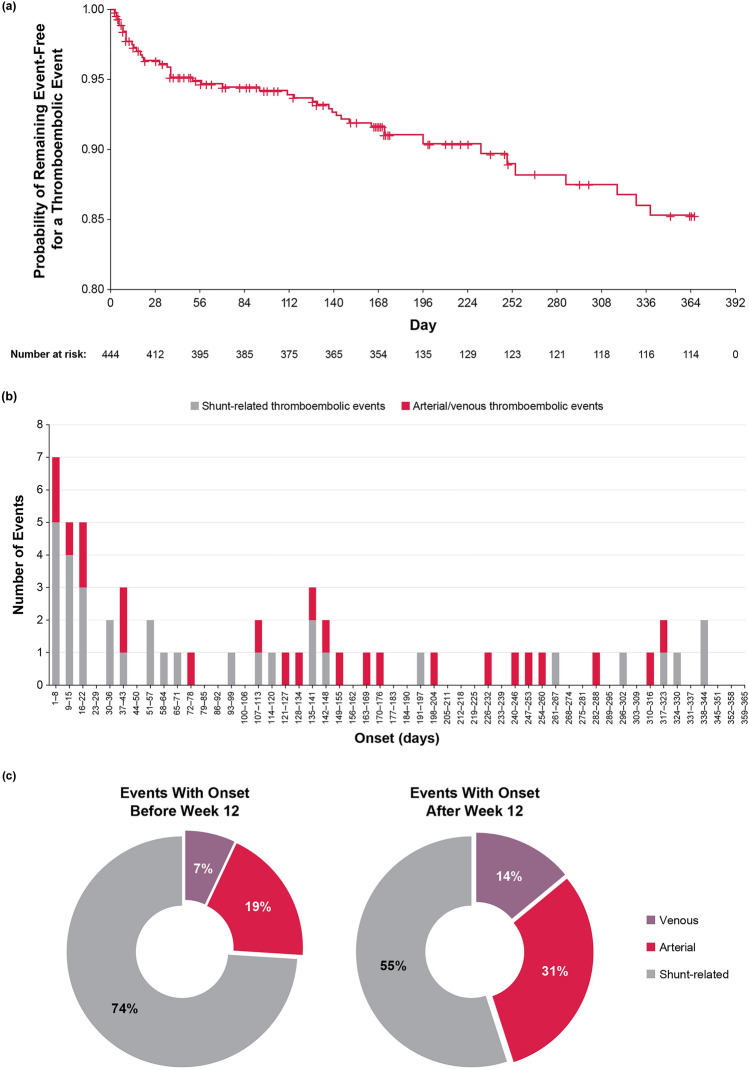
Of the 444 patients, 56 events were observed in 44 patients during the ≤ 52-week treatment period. There was a peak in event frequency in the early treatment period (from 0 to 8 weeks), and more shunt-related events were observed during this period. The event frequency flattened from 8 to 16 weeks and then gradually increased until the end of the treatment period (up to 52 weeks). The breakdown of events differed between the early and late stages of treatment (Fig. 1a). Most events were shunt-related. There were 27 events with onset before week 12 (shunt-related, 74%; arterial, 19%; venous, 7%) and 29 events with onset after week 12 (shunt-related, 55%; arterial, 31%; venous, 14%; Fig. 1b, c). The proportion of venous and arterial thromboembolic events gradually increased as time passed.
Fig. 1.
a Time to first onset of thromboembolic event and cumulative incidence. b Onset timing of thromboembolic events. c Number and percentage of events with onset before/after week 12
Clinical Variables Measured Over Entire Treatment Period
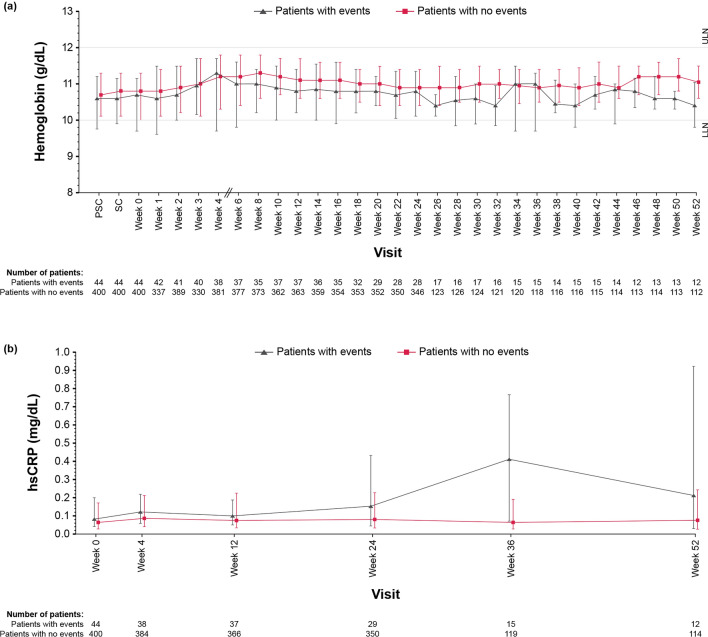
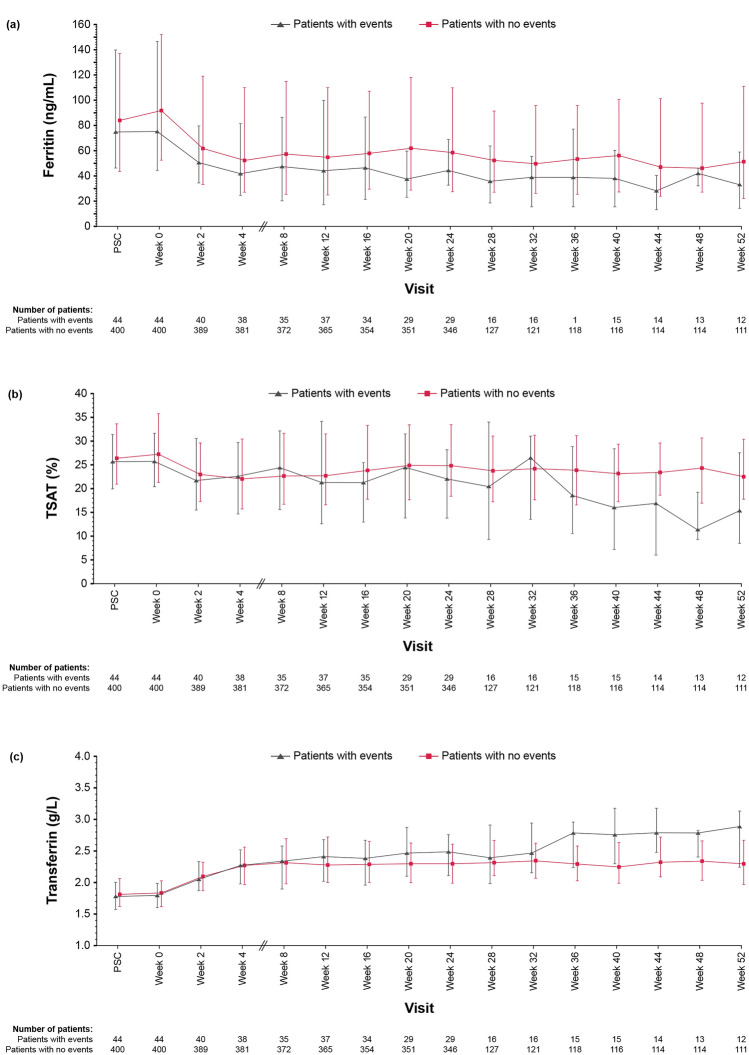
Hemoglobin levels, high-sensitivity C-reactive protein (hsCRP), ferritin, TSAT, and transferrin over the entire treatment period are displayed in Figs. 2 and 3. The median hemoglobin levels were slightly lower in patients with thromboembolic events compared with those without thromboembolic events at most evaluation time points during the treatment period. Patients with thromboembolic events had slightly higher median hsCRP, lower median ferritin, lower median TSAT, and higher median transferrin compared with those without thromboembolic events, especially at the late stage (weeks 36–52) of the treatment period.
Fig. 2.
Medians ± interquartile ranges plot of a hemoglobin levels and b hsCRP levels in patient subgroups with and without thromboembolic events. hsCRP, high-sensitivity C-reactive protein; LLN, lower limit of normal; PSC, prescreening; SC, screening; ULN, upper limit of normal
Fig. 3.
Medians ± interquartile ranges plot of a ferritin, b TSAT, and c transferrin in patient subgroups with and without thromboembolic events. PSC, prescreening; TSAT, transferrin saturation
Mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), MCH concentration (MCHC), and platelets over the entire treatment period are displayed in Figs. S2 and S3 in the electronic supplementary material. No notable differences were observed for platelets, and patients with thromboembolic events had slightly lower median MCV, lower median MCH, and lower median MCHC compared with those without thromboembolic events, especially at the late stage of the treatment period (see Fig. S2 in the electronic supplementary material).
Thromboembolic Events With Onset Before Week 12
Cox Regression Analysis
Patients who were not concomitantly using IV or oral iron therapy (including ferric citrate) had an increased risk of thromboembolic events (HR 11.25; 95% CI 3.36–37.71) compared with patients who were concomitantly using IV or oral iron therapy (including ferric citrate) (Table 3, Fig. 4).
Table 3.
Univariate Cox regression analysis for thromboembolic events with onset before week 12
| Category | Number | No. of events (%) |
Hazard ratio (95% CI)a |
P valueb |
|---|---|---|---|---|
| Age (years) | ||||
| < 65 | 206 | 7 (3.4) | Ref | 0.071 |
| ≥ 65 | 238 | 17 (7.1) | 2.21 (0.92–5.32) | |
| Previous ESA treatment | ||||
| Naive | 88 | 8 (9.1) | 2.11 (0.90–4.93) | 0.078 |
| Conversion | 356 | 16 (4.5) | Ref | |
| Previous ESA treatment, monthly dose (µg)c | ||||
| < 40 | 137 | 10 (7.3) | Ref | 0.276 |
| 40 to < 160 | 254 | 10 (3.9) | 0.52 (0.22–1.24) | |
| ≥ 160 | 53 | 4 (7.5) | 0.98 (0.31–3.13) | |
| Type of dialysis | ||||
| Hemodialysis | 388 | 24 (6.2) | Inf | 0.059 |
| Peritoneal dialysis | 56 | 0 (0.0) | Ref | |
| Dialysis vintage (months) | ||||
| ≤ 4 | 83 | 9 (10.8) | 2.74 (1.20–6.26) | 0.013 |
| > 4 | 361 | 15 (4.2) | Ref | |
| History of thromboembolism | ||||
| No | 329 | 16 (4.9) | Ref | 0.387 |
| Yes | 115 | 8 (7.0) | 1.45 (0.62–3.39) | |
| History of cardiovascular event | ||||
| No | 382 | 21 (5.5) | Ref | 0.822 |
| Yes | 62 | 3 (4.8) | 0.87 (0.26–2.92) | |
| Concomitant use of IV iron therapy | ||||
| No | 334 | 24 (7.2) | Inf | 0.003 |
| Yes | 110 | 0 (0.0) | Ref | |
| Concomitant use of oral iron therapy (including ferric citrate) | ||||
| No | 271 | 21 (7.7) | 4.61 (1.38–15.46) | 0.006 |
| Yes | 173 | 3 (1.7) | Ref | |
| Concomitant use of IV or oral iron therapy (including ferric citrate) | ||||
| No | 179 | 21 (11.7) | 11.25 (3.36–37.71) | < 0.001 |
| Yes | 265 | 3 (1.1) | Ref | |
CI confidence interval, ESA erythropoiesis-stimulating agent, Inf infinite, IV intravenous, Ref reference
aEstimated using Cox proportional hazards model
bP values based on log-rank test to test the null hypothesis of no difference in incidence across subgroup categories
cESA monthly dose was calculated by converting to darbepoetin alfa unit
Fig. 4.
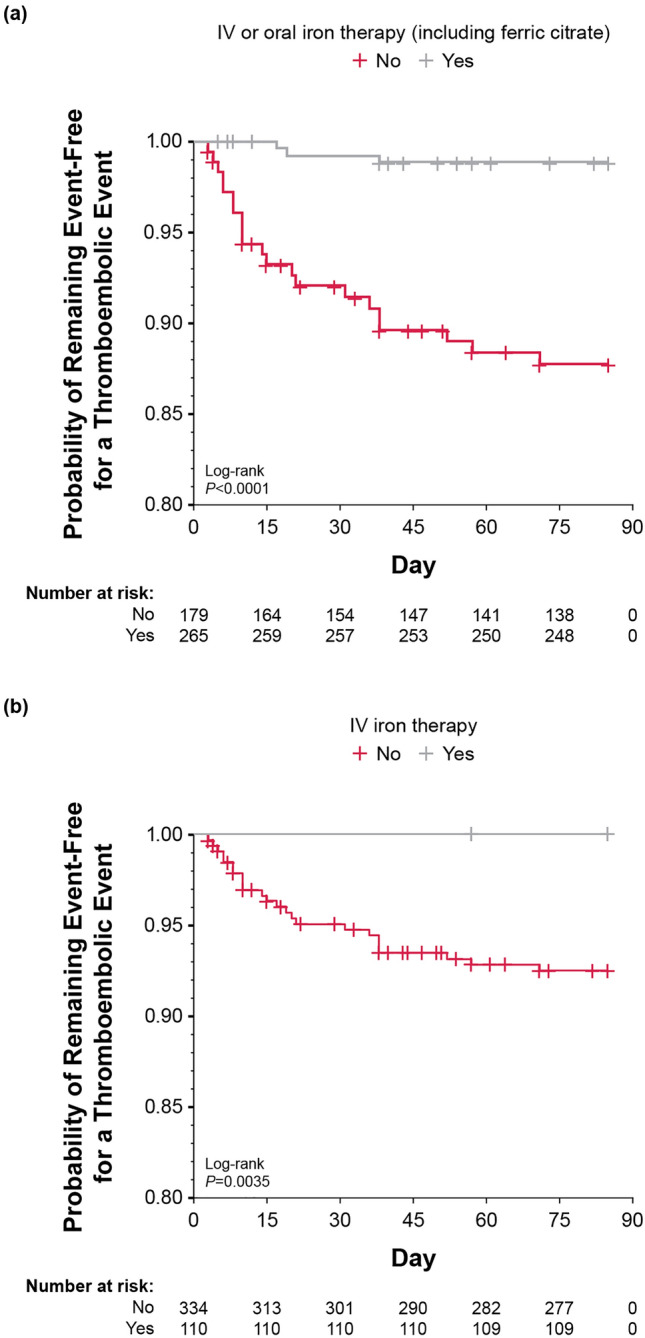
Kaplan-Meier plot of time to first onset of thromboembolic events with onset before week 12: a concomitant IV or oral iron therapy (including ferric citrate); b concomitant IV therapy. IV, intravenous
Patients with shorter dialysis vintage (≤ 4 months) also had an increased risk of thromboembolic events (HR 2.74; 95% CI 1.20–6.26; vs > 4 months; Table 3). In relation to the shorter dialysis vintage, ESA-naive patients may be associated with thromboembolic events (HR 2.11; 95% CI 0.90–4.93 vs ESA-converted; Table 3).
Thromboembolic events were observed in 24 out of 388 patients on HD, while no thromboembolic events occurred in 56 patients on PD. Advanced age (≥ 65 years) may also be associated with thromboembolic events (HR 2.21; 95% CI 0.92–5.32; vs < 65 years; Table 3).
Other factors that were not associated with thromboembolic events are shown in Table S2 in the electronic supplementary material.
Nested Case-Control Analysis
The case and control groups were well balanced in terms of matching variables and other baseline characteristics (see Table S3 in the electronic supplementary material). There was a trend towards higher rate of hemoglobin rise (≥ 0.5 g/dL/week) with increased risk of thromboembolic events (unadjusted OR 2.40; 95% CI 0.73–7.86; vs < 0.5 g/dL/week; Table 4).
Table 4.
Nested case-control analysis for thromboembolic events with onset before week 12
| Category | Cases N = 24 n (%) |
Controls N = 223 n (%) |
Unadjusted odds ratio (95% CI) |
P value |
|---|---|---|---|---|
| Hemoglobin rate of change (g/dL/week) | ||||
| < 0.5 | 19 (79.2) | 200 (89.7) | Ref | |
| ≥ 0.5 | 5 (20.8) | 23 (10.3) | 2.40 (0.73–7.86) | 0.149 |
| Hemoglobin at end of week 4 (g/dL) | ||||
| < 10 | 7 (29.2) | 53 (23.8) | Ref | |
| 10 to < 12 | 15 (62.5) | 141 (63.2) | 0.80 (0.23–2.78) | 0.731 |
| ≥ 12 | 2 (8.3) | 29 (13.0) | 0.50 (0.08–3.10) | 0.457 |
| Ferritin at end of week 4 (ng/mL) | ||||
| < 30 | 7 (29.2) | 78 (35.0) | 0.49 (0.17–1.38) | 0.178 |
| 30 to < 50 | 3 (12.5) | 55 (24.7) | 0.33 (0.09–1.21) | 0.096 |
| ≥ 50 | 7 (30.4) | 90 (54.9) | Ref | |
| TSAT at end of week 4 (%) | ||||
| < 10 | 3 (12.5) | 23 (10.3) | 1.02 (0.27–3.76) | 0.982 |
| 10 to < 20 | 7 (29.2) | 87 (39.0) | 0.55 (0.20–1.51) | 0.246 |
| ≥ 20 | 14 (58.3) | 113 (50.7) | Ref | |
| Transferrin at end of week 4 (g/L) | ||||
| < 2.2 | 9 (37.5) | 87 (39.0) | Ref | |
| 2.2 to < 2.6 | 10 (41.7) | 83 (37.2) | 1.09 (0.41–2.95) | 0.860 |
| ≥ 2.6 | 5 (20.8) | 53 (23.8) | 0.94 (0.30–2.91) | 0.912 |
| hsCRP at end of week 4 (%) | ||||
| < 0.1 | 9 (37.5) | 111 (49.8) | Ref | |
| 0.1 to < 0.3 | 11 (45.8) | 77 (34.5) | 1.71 (0.67–4.35) | 0.259 |
| ≥ 0.3 | 4 (16.7) | 35 (15.7) | 1.32 (0.38–4.50) | 0.661 |
| MCV at end of week 4 (fL) | ||||
| < 95 | 12 (50.0) | 129 (57.8) | Ref | |
| 95 to < 100 | 6 (25.0) | 61 (27.4) | 1.13 (0.40–3.17) | 0.815 |
| ≥ 100 | 6 (25.0) | 33 (14.8) | 2.06 (0.71–5.92) | 0.181 |
| MCH at end of week 4 (pg) | ||||
| < 30 | 9 (37.5) | 76 (34.1) | Ref | |
| 30 to < 32 | 7 (29.2) | 98 (43.9) | 0.64 (0.23–1.80) | 0.399 |
| ≥ 32 | 8 (33.3) | 49 (22.0) | 1.60 (0.54–4.69) | 0.396 |
| MCHC at end of week 4 (g/L) | ||||
| < 325 | 12 (50.0) | 110 (49.3) | 1.17 (0.41–3.37) | 0.770 |
| 325 to < 330 | 6 (25.0) | 47 (21.1) | 1.36 (0.42–4.43) | 0.605 |
| ≥ 330 | 6 (25.0) | 66 (29.6) | Ref | |
| Platelets at end of week 4 (104/µL) | ||||
| < 20 | 12 (50.0) | 119 (53.4) | Ref | |
| 20 to < 28 | 8 (33.3) | 75 (33.6) | 1.02 (0.40–2.60) | 0.969 |
| ≥ 28 | 4 (16.7) | 29 (13.0) | 1.25 (0.37–4.14) | 0.720 |
| Concomitant use of IV iron therapy | ||||
| No | 24 (100.0) | 215 (96.4) | Inf | NE |
| Yes | 0 (0.0) | 8 (3.6) | Ref | |
| Concomitant use of oral iron therapy (including ferric citrate) | ||||
| No | 21 (87.5) | 166 (74.4) | 2.46 (0.69–8.73) | 0.163 |
| Yes | 3 (12.5) | 57 (25.6) | Ref | |
| Concomitant use of IV or oral iron therapy (including ferric citrate) | ||||
| No | 21 (87.5) | 159 (71.3) | 2.85 (0.81–9.99) | 0.103 |
| Yes | 3 (12.5) | 64 (28.7) | Ref | |
CI confidence interval, hsCRP high-sensitivity C-reactive protein, Inf infinite, IV intravenous, MCH mean corpuscular hemoglobin, MCHC mean corpuscular hemoglobin concentration, MCV mean corpuscular volume, NE not evaluable, Ref reference, TSAT transferrin saturation
There were no thromboembolic events observed in patients who had concomitantly used IV or oral iron therapy (not including ferric citrate). When including ferric citrate administration as iron therapy, the trend for increased risk for thromboembolic events in patients without concomitant IV or oral iron therapy (including ferric citrate) persisted (unadjusted OR 2.85; 95% CI 0.81–9.99; vs patients with concomitant IV or oral iron therapy [including ferric citrate]; Table 4).
Thromboembolic Events With Onset After Week 12
Cox Regression Analysis
Patients aged ≥ 65 years (HR 3.27; 95% CI 1.28–8.31; vs < 65 years) and patients with a history of thromboembolism (HR 3.05; 95% CI 1.34–6.93; vs patients without a history of thromboembolism) were associated with increased risk of thromboembolic events (Table 5). A history of a cardiovascular event may be associated with the occurrence of thromboembolic events (HR 2.30; 95% CI 0.90–5.83; vs patients without a history of a cardiovascular event; Table 5). Thromboembolic events were observed in 23 out of 367 patients on HD, while no thromboembolic events occurred in 56 patients on PD. Other factors that were not associated with thromboembolic events are shown in Table S4 in the electronic supplementary material.
Table 5.
Univariate Cox regression analysis for thromboembolic events with onset after week 12
| Category | Number | No. of events (%) | Hazard ratio (95% CI)a |
P valueb |
|---|---|---|---|---|
| Age (years) | ||||
| < 65 | 199 | 6 (3.0) | Ref | 0.009 |
| ≥ 65 | 224 | 17 (7.6) | 3.27 (1.28–8.31) | |
| Previous ESA treatment | ||||
| Naive | 80 | 2 (2.5) | Ref | 0.712 |
| Conversion | 343 | 21 (6.1) | 1.33 (0.29–5.99) | |
| Previous ESA treatment, monthly dose (µg)c | ||||
| < 40 | 127 | 5 (3.9) | Ref | 0.150 |
| 40 to < 160 | 246 | 12 (4.9) | 0.91 (0.32–2.61) | |
| ≥ 160 | 50 | 6 (12.0) | 2.28 (0.69–7.54) | |
| Type of dialysis | ||||
| Hemodialysis | 367 | 23 (6.3) | Inf | 0.156 |
| Peritoneal dialysis | 56 | 0 (0.0) | Ref | |
| Dialysis vintage (months) | ||||
| ≤ 4 | 74 | 2 (2.7) | 0.81 (0.18–3.61) | 0.778 |
| > 4 | 349 | 21 (6.0) | Ref | |
| History of thromboembolism | ||||
| No | 314 | 12 (3.8) | Ref | 0.005 |
| Yes | 109 | 11 (10.1) | 3.05 (1.34–6.93) | |
| History of cardiovascular event | ||||
| No | 364 | 17 (4.7) | Ref | 0.072 |
| Yes | 59 | 6 (10.2) | 2.30 (0.90–5.83) | |
| Concomitant use of IV iron therapy | ||||
| No | 312 | 12 (3.8) | 0.64 (0.28–1.47) | 0.286 |
| Yes | 111 | 11 (9.9) | Ref | |
| Concomitant use of oral iron therapy (including ferric citrate) | ||||
| No | 253 | 17 (6.7) | 2.01 (0.79–5.09) | 0.134 |
| Yes | 170 | 6 (3.5) | Ref | |
| Concomitant use of IV or oral iron therapy (including ferric citrate) | ||||
| No | 160 | 8 (5.0) | 1.40 (0.58–3.36) | 0.455 |
| Yes | 263 | 15 (5.7) | Ref | |
Patients who experienced any event with onset before week 12 and did not experience any event with onset after week 12 were excluded from the analysis
CI confidence interval, ESA erythropoiesis-stimulating agent, Inf infinite, IV intravenous, Ref reference
aEstimated using Cox proportional hazards model
bBased on log-rank test
cESA monthly dose was calculated by converting to darbepoetin alfa unit
Nested Case-Control Analysis
The case and control groups were well balanced in terms of matching variables and other baseline characteristics (Table S5 in the electronic supplementary material).
Higher rate of hemoglobin decline (< − 0.25 g/dL/week) may be associated with increased risk of thromboembolic events (unadjusted OR 3.31; 95% CI 0.813–13.4; vs ≥ − 0.25 g/dL/week). There was a trend towards low average hemoglobin levels (< 10.0 g/dL) over the previous 8 weeks before event onset with an increased risk of thromboembolic events (unadjusted OR 3.38; 95% CI 0.76–14.97; vs ≥ 11.0 g/dL; Table 6).
Table 6.
Nested case-control analysis for thromboembolic events with onset after week 12
| Category | Cases N = 23 n (%) |
Controls N = 164 n (%) |
Unadjusted odds ratio (95% CI) |
P value | Adjusted odds ratio (95% CI)a |
P valuea |
|---|---|---|---|---|---|---|
| Average hemoglobin over the previous 8 weeks before the event onset (g/dL) | ||||||
| < 10.0 | 4 (17.4) | 9 (5.5) | 3.38 (0.76–14.97) | 0.108 | 1.68 (0.28–9.98) | 0.566 |
| 10.0 to < 11.0 | 10 (43.5) | 60 (36.6) | 1.57 (0.59–4.19) | 0.369 | 1.14 (0.39–3.38) | 0.811 |
| ≥ 11.0 | 9 (39.1) | 95 (57.9) | Ref | Ref | ||
| Geometric mean ferritin over the previous 8 weeks before the event onset (ng/mL) | ||||||
| < 30 | 9 (39.1) | 41 (25.0) | 2.77 (0.93–8.25) | 0.066 | 2.32 (0.77–6.98) | 0.136 |
| 30 to < 50 | 7 (30.4) | 33 (20.1) | 2.25 (0.72–7.06) | 0.163 | 1.73 (0.51–5.86) | 0.380 |
| ≥ 50 | 7 (30.4) | 90 (54.9) | Ref | Ref | ||
| Average TSAT over the previous 8 weeks before the event onset (%) | ||||||
| < 10 | 5 (21.7) | 10 (6.1) | 6.25 (1.52–25.62) | 0.011 | 4.29 (0.93–19.72) | 0.061 |
| 10 to < 20 | 7 (30.4) | 47 (28.7) | 1.67 (0.60–4.67) | 0.330 | 1.54 (0.55–4.37) | 0.414 |
| ≥ 20 | 11 (47.8) | 107 (65.2) | Ref | Ref | ||
| Average transferrin over the previous 8 weeks before the event onset (g/L) | ||||||
| < 2.0 | 4 (17.4) | 42 (25.6) | Ref | Ref | ||
| 2.0 to < 2.5 | 7 (30.4) | 83 (50.6) | 0.97 (0.26–3.66) | 0.962 | 0.73 (0.18–2.99) | 0.667 |
| ≥ 2.5 | 12 (52.2) | 39 (23.8) | 4.36 (1.23–15.39) | 0.022 | 3.16 (0.85–11.77) | 0.086 |
| Geometric mean hsCRP over the previous 16 weeks before the event onsetb (mg/dL) | ||||||
| < 0.1 | 8 (34.8) | 88 (53.7) | Ref | Ref | ||
| 0.1 to < 0.3 | 7 (30.4) | 47 (28.7) | 1.58 (0.52–4.80) | 0.417 | 1.55 (0.49–4.87) | 0.453 |
| ≥ 0.3 | 8 (34.8) | 29 (17.7) | 2.85 (0.98–8.35) | 0.056 | 2.86 (0.97–8.40) | 0.056 |
| Average MCV over the previous 8 weeks before the event onset (fL) | ||||||
| < 90 | 5 (21.7) | 21 (12.8) | 1.90 (0.60–6.04) | 0.275 | 1.69 (0.51–5.60) | 0.389 |
| 90 to < 95 | 6 (26.1) | 56 (34.1) | 1.83 (0.30–2.30) | 0.717 | 1.14 (0.38–3.37) | 0.819 |
| ≥ 95 | 12 (52.2) | 87 (53.0) | Ref | Ref | ||
| Average MCH over the previous 8 weeks before the event onset (pg) | ||||||
| < 29 | 6 (26.1) | 19 (11.6) | 3.53 (1.07–11.58) | 0.038 | 3.00 (0.88–10.27) | 0.080 |
| 29 to < 31 | 7 (30.4) | 52 (31.7) | 1.44 (0.50–4.11) | 0.498 | 1.60 (0.55–4.66) | 0.391 |
| ≥ 31 | 10 (43.5) | 93 (56.7) | Ref | Ref | ||
| Average MCHC over the previous 8 weeks before the event onset (g/L) | ||||||
| < 320 | 10 (43.5) | 40 (24.4) | 2.63 (0.85–8.11) | 0.092 | 2.15 (0.68–6.75) | 0.191 |
| 320 to < 330 | 7 (30.4) | 64 (39.0) | 1.11 (0.36–3.42) | 0.858 | 1.08 (0.35–3.39) | 0.893 |
| ≥ 330 | 6 (26.1) | 60 (36.6) | Ref | Ref | ||
| Average platelets over the previous 8 weeks before the event onset (104/µL) | ||||||
| < 15.0 | 6 (26.1) | 38 (23.2) | Ref | Ref | ||
| 15.0 to < 25.0 | 12 (52.2) | 95 (57.9) | 0.86 (0.29–2.54) | 0.783 | 0.77 (0.26–2.34) | 0.648 |
| ≥ 25.0 | 5 (21.7) | 31 (18.9) | 0.94 (0.23–3.86) | 0.933 | 0.82 (0.20–3.36) | 0.779 |
| Average roxadustat dose over the previous 8 weeks before event onset (mg) | ||||||
| < 50 | 6 (26.1) | 56 (34.1) | Ref | Ref | ||
| 50 to < 150 | 14 (60.9) | 103 (62.8) | 1.59 (0.54–4.66) | 0.394 | 1.35 (0.45–4.07) | 0.598 |
| ≥ 150 | 3 (13.0) | 5 (3.0) | 5.95 (1.07–33.16) | 0.042 | 3.77 (0.57–25.11) | 0.170 |
| Concomitant use of IV or oral iron therapy (including ferric citrate) | ||||||
| No | 8 (34.8) | 70 (42.7) | 0.96 (0.38–2.43) | 0.934 | 1.00 (0.38–2.61) | 0.995 |
| Yes | 15 (65.2) | 94 (57.3) | Ref | Ref | ||
Patients who experienced any event with onset before week 12 and did not experience any event with onset after week 12 were excluded from the analysis
CI confidence interval, hsCRP high-sensitivity C-reactive protein, IV intravenous, MCH mean corpuscular hemoglobin, MCHC mean corpuscular hemoglobin concentration, MCV mean corpuscular volume, Ref reference, TSAT transferrin saturation
aFor average roxadustat dose, we adjusted for average TSAT. For concomitant use of IV or oral iron therapy (including ferric citrate), we adjusted for ferritin and TSAT just prior to the event onset. For other factors, we adjusted for average roxadustat dose
bTo account for less frequent hsCRP assessments in each trial, the average hsCRP over the previous 16 weeks before event onset was included in this analysis
Low average TSAT (< 10%) over the previous 8 weeks before event onset (unadjusted OR 6.25; 95% CI 1.52–25.62; vs ≥ 20%) and high average transferrin (≥ 2.5 g/L) over the previous 8 weeks before event onset (unadjusted OR 4.36; 95% CI 1.23–15.39; vs < 2.0 g/L) were associated with thromboembolic events (Table 6). There was a trend towards lower geometric mean ferritin levels (< 30 ng/mL) over the previous 8 weeks before event onset with an increased risk of thromboembolic events (unadjusted OR 2.77; 95% CI 0.93–8.25; vs ≥ 50.0 ng/mL; Table 6).
High geometric mean hsCRP (≥ 0.3 mg/dL) over the previous 16 weeks before event onset tended to be associated with an increased risk of thromboembolic events (unadjusted OR 2.85; 95% CI 0.98–8.35; vs < 0.1 mg/dL; Table 6). Low average MCH (< 29 pg) over the previous 8 weeks before event onset was associated with thromboembolic events (unadjusted OR 3.53; 95% CI 1.07–11.58; vs ≥ 31 pg; Table 6).
High roxadustat dose (≥ 150 mg) over the previous 8 weeks before event onset was associated with thromboembolic events (unadjusted OR 5.95; 95% CI 1.07–33.16; vs < 50 mg; Table 6). However, when adjusted for average TSAT over the previous 8 weeks before event onset, the association between high roxadustat dose (≥ 150 mg) and thromboembolic events was attenuated (adjusted OR 3.77; 95% CI 0.57–25.11; vs < 50 mg; Table 6). Conversely, even after adjustment for average roxadustat dose over the previous 8 weeks before event onset, high average transferrin levels (≥ 2.5 g/L; adjusted OR 3.16; 95% CI 0.85–11.77; vs < 2.0 g/L), low average TSAT levels (< 10%; adjusted OR 4.29; 95% CI 0.93–19.72; vs ≥ 20%), high geometric mean hsCRP levels (≥ 0.3 mg/dL; adjusted OR 2.86; 95% CI 0.97–8.40; vs < 0.1 mg/dL), and low average MCH (< 29 pg; adjusted OR 3.00; 95% CI 0.88–10.27; vs ≥ 31 pg) were numerically associated with thromboembolic events (Table 6).
Results of nested case-control analyses of change from week 0 in each laboratory parameter are shown in Table 7. Large increases in average transferrin levels (≥ 1.0 g/L) over the previous 8 weeks before event onset (adjusted OR 7.85; 95% CI 1.82–33.90; vs < 0.5 g/L) and large decreases in average MCV (< − 2 fL) over the previous 8 weeks before event onset (adjusted OR 5.55; 95% CI 1.73–17.83; vs ≥ 0 fL) were associated with thromboembolic events (Table 7).
Table 7.
Nested case-control analysis for thromboembolic events with onset after week 12: change from week 0 in laboratory parameters
| Category | Cases N = 23 n (%) |
Controls N = 164 n (%) |
Unadjusted odds ratio (95% CI) |
P value | Adjusted odds ratio (95% CI)a |
P valuea |
|---|---|---|---|---|---|---|
| Change of hemoglobin from week 0 to average value over the previous 8 weeks before the event onset (g/dL) | ||||||
| < − 0.5 | 6 (26.1) | 37 (22.6) | 0.97 (0.32–2.91) | 0.956 | 0.67 (0.20–2.26) | 0.518 |
| − 0.5 to < 0.0 | 5 (21.7) | 36 (22.0) | 0.94 (0.30–2.95) | 0.920 | 0.73 (0.22–2.43) | 0.614 |
| ≥ 0.0 | 12 (52.2) | 91 (55.5) | Ref | Ref | ||
| Change of ferritin from week 0 to geometric mean over the previous 8 weeks before the event onset (ng/mL) | ||||||
| < − 50 | 6 (26.1) | 56 (34.1) | 1.22 (0.31–4.73) | 0.777 | 1.36 (0.34–5.39) | 0.662 |
| − 50 to < 0 | 13 (56.5) | 67 (40.9) | 2.83 (0.81–9.92) | 0.103 | 2.68 (0.74–9.68) | 0.132 |
| ≥ 0 | 4 (17.4) | 41 (25.0) | Ref | Ref | ||
| Change of TSAT from week 0 to average value over the previous 8 weeks before the event onset (%) | ||||||
| < − 15 | 7 (30.4) | 31 (18.9) | 1.55 (0.49–4.88) | 0.451 | 0.95 (0.26–3.43) | 0.942 |
| − 15 to < 0 | 7 (30.4) | 78 (47.6) | 0.64 (0.21–1.95) | 0.431 | 0.48 (0.15–1.53) | 0.212 |
| ≥ 0 | 9 (39.1) | 55 (33.5) | Ref | Ref | ||
| Change of transferrin from week 0 to average value over the previous 8 weeks before the event onset (g/L) | ||||||
| < 0.5 | 7 (30.4) | 97 (59.1) | Ref | Ref | ||
| 0.5 to < 1.0 | 9 (39.1) | 51 (31.1) | 3.48 (1.11–10.90) | 0.032 | 3.13 (0.98–10.02) | 0.055 |
| ≥ 1.0 | 7 (30.4) | 16 (9.8) | 9.79 (2.41–39.70) | 0.001 | 7.85 (1.82–33.90) | 0.006 |
| Change of hsCRP from week 0 to geometric mean over the previous 16 weeks before the event onsetb (mg/dL) | ||||||
| < 0.0 | 7 (30.4) | 50 (30.5) | Ref | Ref | ||
| 0.0 to < 0.1 | 10 (43.5) | 80 (48.8) | 0.87 (0.31–2.45) | 0.790 | 1.06 (0.36–3.17) | 0.912 |
| ≥ 0.1 | 6 (26.1) | 34 (20.7) | 1.13 (0.34–3.73) | 0.837 | 1.34 (0.40–4.50) | 0.640 |
| Change of MCV from week 0 to average value over the previous 8 weeks before the event onset (fL) | ||||||
| < − 2 | 8 (34.8) | 18 (11.0) | 6.55 (2.11–20.28) | 0.001 | 5.55 (1.73–17.83) | 0.004 |
| − 2 to < 0 | 5 (21.7) | 41 (25.0) | 1.98 (0.56–6.98) | 0.289 | 2.28 (0.64–8.20) | 0.206 |
| ≥ 0 | 10 (43.5) | 105 (64.0) | Ref | Ref | ||
| Change of MCH from week 0 to average value over the previous 8 weeks before the event onset (pg) | ||||||
| < − 2 | 5 (21.7) | 16 (9.8) | 5.12 (1.40–18.71) | 0.014 | 3.83 (0.98–14.87) | 0.053 |
| − 2 to < 0 | 10 (43.5) | 48 (29.3) | 4.09 (1.26–13.30) | 0.019 | 3.80 (1.16–12.44) | 0.028 |
| ≥ 0 | 8 (34.8) | 100 (61.0) | Ref | Ref | ||
| Change of MCHC from week 0 to average value over the previous 8 weeks before the event onset (g/L) | ||||||
| < − 15 | 6 (26.1) | 14 (8.5) | 3.03 (0.93–9.87) | 0.065 | 2.00 (0.51–7.80) | 0.316 |
| − 15 to < 0 | 7 (30.4) | 81 (49.4) | 0.64 (0.23–1.79) | 0.397 | 0.61 (0.21–1.73) | 0.349 |
| ≥ 0 | 10 (43.5) | 69 (42.1) | Ref | Ref | ||
| Change of platelets from week 0 to average value over the previous 8 weeks before the event onset (104/µL) | ||||||
| < 0 | 8 (34.8) | 75 (45.7) | Ref | Ref | ||
| 0 to < 2 | 10 (43.5) | 40 (24.4) | 2.75 (0.89–8.49) | 0.078 | 2.63 (0.83–8.31) | 0.100 |
| ≥ 2 | 5 (21.7) | 49 (29.9) | 0.87 (0.25–2.98) | 0.825 | 0.80 (0.23–2.77) | 0.719 |
Patients who experienced any event with onset before week 12 and did not experience any event with onset after week 12 were excluded from the analysis
CI confidence interval, hsCRP high-sensitivity C-reactive protein, MCH mean corpuscular hemoglobin, MCHC mean corpuscular hemoglobin concentration, MCV mean corpuscular volume, Ref reference, TSAT transferrin saturation
aAdjusted for average roxadustat dose
bTo account for less frequent hsCRP assessments in each trial, the average hsCRP over the previous 16 weeks before event onset was included in this analysis
Discussion
In this exploratory post hoc analysis, the factor associated with the highest probability of a thromboembolic event occurrence was the absence of concomitant iron supplementation. HD, advanced age, dialysis vintage, history of thromboembolism, and history of cardiovascular disease were also identified as factors associated with thromboembolic events in this analysis [12–14]. High frequency of thromboembolic events occurred during the initial stage of study drug administration (from 0 to 8 weeks) and they were mostly shunt-related. Most of the arterial-related and venous-related events occurred during the later period (after week 12).
The absence of IV or oral iron therapy early in treatment, before week 12, was associated with increased risk of developing thromboembolic events. Low TSAT and increased transferrin levels were associated with increased risk of developing thromboembolic events after week 12. Prior studies have identified an association between iron deficiency anemia and thromboembolic events [15, 16]. A previous report [17] concluded that an increase in transferrin induced by iron deficiency was associated with increased hypercoagulability and risk of thromboembolism. This potential mechanism could explain, in part, the increased risk for thromboembolism in patients treated with roxadustat who had increased transferrin levels. Another study in patients with anemia receiving chemotherapy suggested that treatment with IV iron was associated with a reduced risk of thromboembolic events by ESA [18]. Overall, the current analysis suggests that iron deficiency during treatment with roxadustat may be a risk factor related to thromboembolic events. Indeed, many previous reports have indicated that iron deficiency (measured by serum ferritin and/or TSAT levels) increases the risk for negative patient outcomes in patients with anemia of CKD [19–24]. In contrast, excess iron can cause organ damage, as well as exacerbation of an ongoing infection [25–27]. Clinicians need to weigh the benefits versus the risks of concomitant iron therapy with roxadustat treatment, and awareness of iron levels prior to initiating ESA or roxadustat may optimize patient outcomes with treatment for anemia. Further investigation is required to understand the potential association between iron deficiency and the risk of thromboembolic events in patients treated with roxadustat.
In this post hoc analysis, incident-dialysis (ID; i.e., initiated dialysis < 4 months ago) patients had a higher risk of developing thromboembolic events early in treatment with roxadustat. It is well established that patients have an increased risk of mortality during the first 4 months of dialysis [28]. Insufficient maturation of shunts may increase the risk of shunt-related thromboembolic events in ID patients [29]. Stable dialysis patients typically require lower doses of ESAs to maintain hemoglobin target levels compared with ID patients. It has been previously reported that ESA-naive patients had reduced levels of ferritin after the administration of roxadustat compared with ESA-converted patients treated with roxadustat [11]. This is theorized to be due to the increased amount of iron required to correct low hemoglobin levels in patients who are ESA-naive [30]. Lower levels of supplemental iron may be needed to maintain hemoglobin levels within the target range for ESA-converted patients, which could explain the absence of iron therapy being a risk factor for thromboembolic events only before week 12.
This post hoc analysis identified decreases in MCH/MCV as being correlated with the risk of thromboembolic events in the later treatment period. This is consistent with a previous report determining that low MCH levels were associated with an increased risk for venous thrombosis [31]. Interestingly, there were no cases of thromboembolic events in patients with anemia of CKD on PD. However, this study included only 56 patients on PD treated with roxadustat. HD was one of the factors associated with thromboembolic events both before and after week 12 in this analysis. Most of the thromboembolic events that occurred in this analysis were shunt-related. This may explain, in part, the increased risk of thromboembolic events observed in patients on HD, compared with patients on PD, who did not have an arteriovenous fistula. It cannot be ruled out that this result may be inflated as a result of there being no cases of thromboembolic events in patients on PD; however, this finding warrants further investigation.
Owing to the post hoc nature of this analysis, these results should be cautiously interpreted and viewed as hypothesis-generating. Multiplicity adjustment was not performed for any statistical testing given the hypothesis-generating purpose of these analyses. The numbers of patients and thromboembolic events within the studies were limited, and the studies were not well powered to identify all potential risk factors. For example, a higher rate of hemoglobin rise (≥ 0.5 g/dL/week) was numerically associated with increased risk of thromboembolic events before week 12, and in contrast, a higher rate of hemoglobin decline was numerically associated with an increased risk of thromboembolic events after week 12. These findings should be further investigated with larger patient populations in global studies. The current analyses did not include any comparison of safety data versus ESAs, as the risk and safety profile for ESAs has already been well established [32–34]. Because of the small number of events, these analyses were conducted with all thromboembolic events (as opposed to a separate analysis with arterial, venous, and shunt-related events). The majority of thromboembolic events in this post hoc analysis were shunt-related. Generally, shunt-related thromboembolic events are associated with better patient outcomes and can often be managed in the clinic compared with systemic arterial or venous thromboembolic events [35, 36]. Thrombosis in patients with anemia of CKD is complex and multifactorial, and the analysis may be limited because of confounding, missing data, and clinical correlation between variables such as hemoglobin, iron, and inflammatory markers.
Conclusion
This post hoc analysis found that iron deficiency, as well as other known risk factors, may be related to thromboembolic events in patients with DD CKD treated with roxadustat. Patients treated with roxadustat who concomitantly used IV or oral iron therapy (including ferric citrate) early in treatment were less likely to experience a thromboembolic event. Patients with DD CKD in higher-risk groups may benefit from more intentional monitoring and management of iron parameters while receiving roxadustat to treat their anemia of CKD.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to sincerely thank the patients and their family members for their support, as well as the investigators who participated in these trials. We would also like to acknowledge Keiko Tanaka (Medical Affairs, Astellas Pharma Inc.) and Masataka Morita (Medical Affairs, Astellas Pharma, Inc.), who performed study management, and Tomihisa Kawasaki (Project Management, Development, Astellas Pharma, Inc.) who contributed to study design.
Medical Writing/Editorial Assistance.
Medical writing/editorial support was provided by Lindsay Achzet, PhD, Drayton Hammond, PharmD, and Carol Cadmus, ELS, from Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, USA, and funded by the study sponsor, Astellas Pharma, Inc.
Author Contributions
Study design: Takayuki Hamano, Yusuke Yamaguchi, Tadao Akizawa. Study investigator: All authors (Takayuki Hamano, Yusuke Yamaguchi, Kashia Goto, Sho Mizokawa, Yuichiro Ito, Frank Dellanna, Jonathan Barratt, Tadao Akizawa). Collection and assembly of data: Takayuki Hamano, Yusuke Yamaguchi. Data analysis: Takayuki Hamano, Yusuke Yamaguchi. Data interpretation; Manuscript preparation, review and revisions; Final approval of manuscript: All authors.
Funding
This study was sponsored by Astellas Pharma, Inc. The Rapid Service Fee and the Open Access Fee were funded by Astellas Pharma, Inc.
Data Availability
Researchers may request access to anonymized participant level data, trial level data and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
Declarations
Conflict of Interest
Takayuki Hamano has received grants for physician-initiated research, consulting fees, and honoraria for lectures from Astellas Pharma, Inc. Yusuke Yamaguchi is an employee of Astellas Pharma, Inc. Kashia Goto has participated in company stock ownership plan through Astellas Pharma, Inc. Sho Mizokawa is an employee of Astellas Pharma, Inc. Yuichiro Ito is an employee of Astellas Pharma, Inc. Frank Dellanna has nothing to disclose. Jonathan Barratt has received fees for invited lectures from Astellas Pharma Inc. Tadao Akizawa has received personal consulting fees from Astellas Pharma, Inc., Kyowa Kirin, Kissei Pharmaceutical, Ono Pharmaceutical, Fuso Pharmaceutical, Torii Pharmaceutical, GlaxoSmithKline, JT Pharmaceutical, Nipro Corporation, Otsuka, Sanwa Kagaku, and Bayer and has also received personal payment or honoraria for lectures from Astellas Pharma, Inc., Kyowa Kirin, Kissei Pharmaceutical, Ono Pharmaceutical, Fuso Pharmaceutical, Torii Pharmaceutical, Chugai Pharmaceutical, Mitsubishi Tanabe, and Bayer.
Ethical Approval
All studies included in this post hoc analysis were conducted in accordance with the ethical principles of the Declaration of Helsinki of 1964 and its later amendments, the International Conference on Harmonisation of Technical Requirements for Pharmaceuticals for Human Use guidelines, Good Clinical Practice, and applicable local laws and regulations. As this was a pooled post hoc analysis, approval by an ethics committee for this analysis was not necessary; however, institutional review board approval was obtained prior to initiating each of the studies. All participants provided written informed consent.
Footnotes
Prior Presentation: This manuscript is based on work that has been previously presented. Hamano T, et al. Arterial and venous thromboembolic events in patients with anemia of dialysis-dependent CKD treated with roxadustat: exploratory post hoc analysis. TH-PO978. Presented at the Annual Meeting for the American Society of Nephrology (Kidney Week), November 3–6, 2022, Orlando, Florida, USA.
References
- 1.Nakhoul G, Simon JF. Anemia of chronic kidney disease: treat it, but not too aggressively. Cleve Clin J Med. 2016;83(8):613–624. doi: 10.3949/ccjm.83a.15065. [DOI] [PubMed] [Google Scholar]
- 2.Locatelli F, Fishbane S, Block GA, Macdougall IC. Targeting hypoxia-inducible factors for the treatment of anemia in chronic kidney disease patients. Am J Nephrol. 2017;45(3):187–199. doi: 10.1159/000455166. [DOI] [PubMed] [Google Scholar]
- 3.Lutz J, Menke J, Sollinger D, Schinzel H, Thurmel K. Haemostasis in chronic kidney disease. Nephrol Dial Transplant. 2014;29(1):29–40. doi: 10.1093/ndt/gft209. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto H, Nishi S, Tomo T, et al. 2015 Japanese Society for Dialysis Therapy: Guidelines for Renal Anemia in Chronic Kidney Disease. Ren Replace Ther. 2017;3(1).
- 5.Del Vecchio L, Locatelli F. An overview on safety issues related to erythropoiesis-stimulating agents for the treatment of anaemia in patients with chronic kidney disease. Expert Opin Drug Saf. 2016;15(8):1021–1030. doi: 10.1080/14740338.2016.1182494. [DOI] [PubMed] [Google Scholar]
- 6.Akizawa T, Otsuka T, Reusch M, Ueno M. Intermittent oral dosing of roxadustat in peritoneal dialysis chronic kidney disease patients with anemia: a randomized, phase 3, multicenter, open-label study. Ther Apher Dial. 2020;24(2):115–125. doi: 10.1111/1744-9987.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akizawa T, Iwasaki M, Yamaguchi Y, Majikawa Y, Reusch M. Phase 3, randomized, double-blind, active-comparator (darbepoetin alfa) study of oral roxadustat in CKD patients with anemia on hemodialysis in Japan. J Am Soc Nephrol. 2020;31(7):1628–1639. doi: 10.1681/ASN.2019060623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barratt J, Sulowicz W, Schomig M, et al. Efficacy and cardiovascular safety of roxadustat in dialysis-dependent chronic kidney disease: pooled analysis of four phase 3 studies. Adv Ther. 2021;38(10):5345–5360. doi: 10.1007/s12325-021-01903-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pharmaceuticals and Medical Devices Agency (Japan). Report on the deliberation results–Evrenzo (roxadustat). September 2019.
- 10.Chen H, Cheng Q, Wang J, Zhao X, Zhu S. Long-term efficacy and safety of hypoxia-inducible factor prolyl hydroxylase inhibitors in anaemia of chronic kidney disease: a meta-analysis including 13,146 patients. J Clin Pharm Ther. 2021;46(4):999–1009. doi: 10.1111/jcpt.13385. [DOI] [PubMed] [Google Scholar]
- 11.Akizawa T, Ueno M, Shiga T, Reusch M. Oral roxadustat three times weekly in ESA-naive and ESA-converted patients with anemia of chronic kidney disease on hemodialysis: results from two phase 3 studies. Ther Apher Dial. 2020;24(6):628–641. doi: 10.1111/1744-9987.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molnar AO, Bota SE, McArthur E, et al. Risk and complications of venous thromboembolism in dialysis patients. Nephrol Dial Transplant. 2018;33(5):874–880. doi: 10.1093/ndt/gfx212. [DOI] [PubMed] [Google Scholar]
- 13.Anderson F. Risk factors for venous thromboembolism. Circulation. 2003;107:I9–I16. doi: 10.1161/01.CIR.0000078469.07362.E6. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh MY, Lee CK, Lo CM, Chen CH, Chuang SY, Wu CC. Temporal distribution and biological determinants of thrombotic events after interventions for dialysis vascular access. Sci Rep. 2019;9(1):10720. doi: 10.1038/s41598-019-47293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hung SH, Lin HC, Chung SD. Association between venous thromboembolism and iron-deficiency anemia: a population-based study. Blood Coagul Fibrinolysis. 2015;26(4):368–372. doi: 10.1097/MBC.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 16.Coutinho JM. Association between anemia and cerebral venous thrombosis. Stroke. 2015;46:5. doi: 10.1161/STROKEAHA.115.009843. [DOI] [PubMed] [Google Scholar]
- 17.Tang X, Fang M, Cheng R, et al. Iron-deficiency and estrogen are associated with ischemic stroke by up-regulating transferrin to induce hypercoagulability. Circ Res. 2020;127(5):651–663. doi: 10.1161/CIRCRESAHA.119.316453. [DOI] [PubMed] [Google Scholar]
- 18.Henry DH, Dahl NV, Auerbach MA. Thrombocytosis and venous thromboembolism in cancer patients with chemotherapy induced anemia may be related to ESA induced iron restricted erythropoiesis and reversed by administration of IV iron. Am J Hematol. 2012;87(3):308–310. doi: 10.1002/ajh.22262. [DOI] [PubMed] [Google Scholar]
- 19.Guedes M, Muenz DG, Zee J, et al. Serum biomarkers of iron stores are associated with increased risk of all-cause mortality and cardiovascular events in nondialysis CKD patients, with or without anemia. J Am Soc Nephrol. 2021;32(8):2020–2030. doi: 10.1681/ASN.2020101531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuragano T, Joki N, Hase H, et al. Low transferrin saturation (TSAT) and high ferritin levels are significant predictors for cerebrovascular and cardiovascular disease and death in maintenance hemodialysis patients. PLoS ONE. 2020;15(9):e0236277. doi: 10.1371/journal.pone.0236277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisenga MF, Nolte IM, van der Meer P, Bakker SJL, Gaillard C. Association of different iron deficiency cutoffs with adverse outcomes in chronic kidney disease. BMC Nephrol. 2018;19(1):225. doi: 10.1186/s12882-018-1021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Awan AA, Walther CP, Richardson PA, Shah M, Winkelmayer WC, Navaneethan SD. Prevalence, correlates and outcomes of absolute and functional iron deficiency anemia in nondialysis-dependent chronic kidney disease. Nephrol Dial Transplant. 2021;36(1):129–136. doi: 10.1093/ndt/gfz192. [DOI] [PubMed] [Google Scholar]
- 23.Chang YL, Hung SH, Ling W, Lin HC, Li HC, Chung SD. Association between ischemic stroke and iron-deficiency anemia: a population-based study. PLoS one. 2013;8(12):e82952. doi: 10.1371/journal.pone.0082952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuo KL, Hung SC, Tseng WC, et al. Association of anemia and iron parameters with mortality among patients undergoing prevalent hemodialysis in Taiwan: the AIM-HD study. J Am Heart Assoc. 2018;7(15):e009206. doi: 10.1161/JAHA.118.009206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu CC, Senussi NH, Fertrin KY, Kowdley KV. Iron overload disorders. Hepatol Commun. 2022;6(8):1842–1854. doi: 10.1002/hep4.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganz T. Iron administration, infection, and anemia management in CKD: untangling the effects of intravenous iron therapy on immunity and infection risk. Kidney Med. 2020;2(3):341–353. doi: 10.1016/j.xkme.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macdougall IC, White C, Anker SD, et al. Intravenous iron in patients undergoing maintenance hemodialysis. N Engl J Med. 2019;380(5):447–458. doi: 10.1056/NEJMoa1810742. [DOI] [PubMed] [Google Scholar]
- 28.Bradbury BD, Fissell RB, Albert JM, et al. Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Clin J Am Soc Nephrol. 2007;2(1):89–99. doi: 10.2215/CJN.01170905. [DOI] [PubMed] [Google Scholar]
- 29.MacRae JM, Dipchand C, Oliver M, et al. Arteriovenous access failure, stenosis, and thrombosis. Can J Kidney Health Dis. 2016;3:2054358116669126. doi: 10.1177/2054358116669126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, Shou X, Xu Y, et al. A network meta-analysis of the efficacy of hypoxia-inducible factor prolyl-hydroxylase inhibitors in dialysis chronic kidney disease. Aging. 2023;15(6):2237–2274. doi: 10.18632/aging.204611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rezende SM, Lijfering WM, Rosendaal FR, Cannegieter SC. Hematologic variables and venous thrombosis: red cell distribution width and blood monocyte count are associated with an increased risk. Haematologica. 2014;99(1):194–200. doi: 10.3324/haematol.2013.083840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Besarab A. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339(9):584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 33.Drueke TB. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355(20):2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 34.Szczech LA, Barnhart HX, Inrig JK, et al. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int. 2008;74(6):791–798. doi: 10.1038/ki.2008.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lucena J, Rico A, Vázquez R, et al. Pulmonary embolism and sudden–unexpected death: prospective study on 2477 forensic autopsies performed at the Institute of Legal Medicine in Seville. J Forensic Leg Med. 2009;16(4):196–201. doi: 10.1016/j.jflm.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 36.Quencer KB, Oklu R. Hemodialysis access thrombosis. Cardiovasc Diagn Ther. 2017;7(Suppl 3):S299–S308. doi: 10.21037/cdt.2017.09.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Researchers may request access to anonymized participant level data, trial level data and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.