Summary
Immune-checkpoint blockade has revolutionized cancer treatment, but some cancers such as acute myeloid leukemia (AML) do not respond or develop resistance. A potential mode of resistance is immune evasion of T-cell immunity involving aberrant MHC-I antigen presentation (AP). To map such mechanisms of resistance, we identified key MHC-I regulators using specific peptide-MHC-I-guided CRISPR/Cas9 screens in AML. The top-ranked negative regulators were surface protein Sushi Domain Containing 6 (SUSD6), Transmembrane Protein 127 (TMEM127), and the E3 ubiquitin ligase WWP2. SUSD6 is abundantly expressed in AML and multiple solid cancers, and its ablation enhanced MHC-I AP and reduced tumor growth in CD8+ T cell-dependent manner. Mechanistically, SUSD6 forms a tri-molecular complex with TMEM127 and MHC-I, which recruits WWP2 for MHC-I ubiquitination and lysosomal degradation. Together with the SUSD6-TMEM127-WWP2 gene signature negatively-correlates with cancer survival, our findings define a membrane-associated MHC-I inhibitory axis as potential therapeutic targets for both leukemia and solid cancers.
Keywords: Cancer, Immune evasion, Antigen presentation, MHC-I, T cell, SUSD6, TMEM127, WWP2, AML, Solid tumor, Ubiquitination, Lysosomal degradation, Immunotherapy
Introduction
Advances in immune checkpoint blockade (ICB) immunotherapy have reshaped the cancer treatment landscape.1 Anti-PD-1/PD-L1 (collectively shortened to PD) therapy has demonstrated durable clinical responses across numerous tumor types, by targeting general immune-evasion mechanisms in the tumor microenvironment (TME) to potently restore tumor-specific T cell immunity.2,3 The successes of anti-PD therapy are generally thought to arise from “inflamed” tumors with abundant tumor-specific CD8+ T cell responses, which are needed to effectively mount a cytotoxic anti-tumor response. However, many tumors are immunologically “cold” and lack significant T cell infiltration, resulting in a large subset of patients not responding to anti-PD therapy or developing acquired resistance.2 These results emphasize the need to identify novel therapeutic strategies beyond the PD pathway for immunologically cold tumors to enhance the efficacy of cancer immunotherapy.
The major histocompatibility complex class I (MHC-I or human leukocyte antigens (HLA) in human) antigen presentation (AP) pathway determines the specificity of CD8+ T cells and is essential for their activation and proliferation.4–7 Myeloid cells, as professional antigen-presenting cells (APCs), and some cancer cells are capable of processing self or tumor antigens to form stable peptide-MHC-I (pMHC-I) complexes on the cell surface, which engage CD8+ T cells through antigen-specific T cell receptors (TCR) and trigger CD8+ T cell responses. Genetic mutations and/or loss of expression in essential genes that positively regulate various stages of MHC-I AP have been implicated in tumor progression, as well as the development of resistance to ICB,8–10 such as MHC-I and Beta-2 microglobulin (B2M) (MHC-I complex),10–12 Transporter associated with antigen processing 1 and 2 (TAP1/2),13–15 Calreticulin (CALR), and Tapasin (TAPBP) (peptide transportation and loading),16–19 CITA (also known as NLRC5) and IRF2 (MHC-I transcription activator),20,21 as well as JAK1/JAK2 (MHC-I induction).8,22 These examples, however, may not fully represent the general mechanisms leading to low immunogenicity in most cancers. Further, reintroducing or overexpressing these factors specifically in the TME may be difficult as a therapeutic strategy.
In addition to the downregulation of essential positive regulators for the MHC-I pathway, tumors may actively escape the cytotoxic CD8+ T cell response through the upregulation of key negative regulators for MHC-I AP, a typical immune evasion mechanism seen in many viral infections but much less studied in cancer. Viruses can produce several viral factors that actively inhibit various steps of MHC-I AP pathways, including a subset that directly engages with membrane-associated MHC-I, including GP48,24 ORF7a (SARS-CoV-2),23 and BILF1 (EBV).25 In cancer, a few recent studies have suggested that autophagy or cholesterol-regulating soluble protein PCSK9 can mediate MHC-I degradation or disrupt MHC-I recycling, respectively.26,27 Transcriptional/epigenetic repressors TRAF3 and EZH2 as well as the thymidylate synthase (also required for DNA replication and repair) were identified as negative regulators of MHC-I expression.28–30 However, these factors may not maintain the specificity in MHC-I modulation as they had shown in many other important cellular functions, and their precise roles in regulating tumor-associated MHC-I AP await further validation. Together with emerging evidence suggesting low and variable percentages of tumor-reactive T cells in the TMEs,31 there is an increasing need to identify more cancer-specific regulators of the MHC-I AP pathway, especially those directly associated with membrane MHC-I, which could be used as potential therapeutic targets to increase the quality and quantity of tumor-specific CD8+ T cells.
To systematically identify general cancer-associated MHC-I inhibitors, we performed cell surface pMHC-I-guided CRISPR screens in both human and mouse acute myeloid leukemia (AML) cell lines. We started with AML given 1) its poor immunogenicity and low mutation burden;32 and 2) it represents the malignant counterpart of myeloid progenitors which can differentiate into professional APCs, with mechanisms that can be potentially extended to other cancer types. Through these screens, we constructed positive and negative regulatory networks of MHC-I modulation. Notably, we identified a membrane-associated inhibitory axis of MHC-I surface expression that involves Sushi Domain Containing 6 (SUSD6) and Transmembrane Protein 127 (TMEM127), which together recruit the E3 ubiquitin ligase WWP2 for MHC-I lysosomal degradation. TMEM127, a proposed 4-transmembrane protein33 involved in susceptibility to rare neuroendocrine tumors,34,35 has been suggested as a Nedd4-family E3 ligase adaptor that degrades MHC-II in the presence of the Salmonella effector protein, SteD;36 while SUSD6, a single-pass transmembrane protein, has no known immunological functions. We also characterized the function and mechanism of the SUSD6-TMEM127-WWP2 inhibitory axis in modulating anti-tumor immunity in both AML and solid cancers.
Results
Cell Surface pMHC-I-Guided CRISPR/Cas9 Screens Reveal Key MHC-I Regulatory Networks in AML
To systematically identify key cancer-associated regulators of the MHC-I AP pathway, we first engineered antigen-specific pMHC-I reporters in cell lines of AML, the APC precursor-derived cancer with a low antigen presentation machinery (APM) score37 compared to other cancer types (Figure S1A). Specifically, we engineered the Cas9-expressing THP-1, a well-characterized MLL-AF9+ TP53mut NRASG12D HLA-A*02:01+ human AML cell line, to express a truncated alpha-fetoprotein (AFP) tumor antigen fused with the blue fluorescence protein (BFP), which served as a quantitative reporter of AFP antigen expression (Figure 1A). To evaluate antigen-specific pMHC-I in this reporter system, we used a unique human TCR-mimicking antibody that binds the immunogenic AFP158–166 peptide presented by the HLA-A*02:01 molecule (HLA-A*02:01:AFP158–166, hereafter referred to as HLA-A2:AFP).38 We observed a positive correlation between the surface level of HLA-A2:AFP and AFP antigen expression as indicated by the BFP intensity (Figure S1B), validating the robustness of our system to quantitatively monitor MHC-I AP triggered by a specific antigen. Using the same strategy, we then generated another reporter using RN2 (MLL-AF9+ NRASG12D H-2Kb+ mouse AML cell line)39,40 and the model antigen, chicken ovalbumin (OVA) (Figure 1A). We also observed a positive correlation between OVA expression, as measured by the BFP intensity, and the surface level of the pMHC-I complex (H-2Kb:OVA257–264, hereafter referred to as H-2Kb:OVA) (Figure S1C).
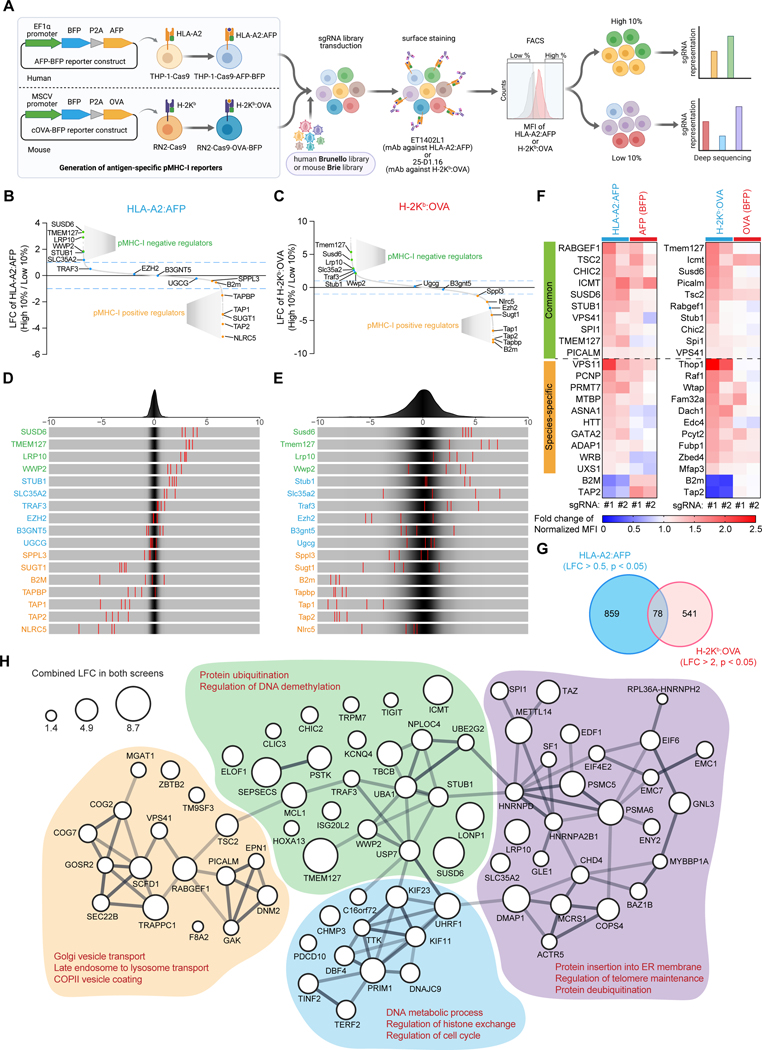
Figure 1. Systematic identification of antigen-specific pMHC-I inhibitors in AML. See also Figure S1.
(A) Schematic of the antigen-specific pMHC-I CRISPR screens.
(B-E) Waterfall plots (B and C) and frequency histograms (D and E) depicting the log2-fold changes (LFCs) of the selected regulators in the human (HLA-A2:AFP, B and D) and the mouse (H-2Kb:OVA, C and E) antigen-specific pMHC-I screens. Green: novel pMHC-I negative regulators, blue: published MHC-I negative regulators, orange: known pMHC-I positive regulators.
(F) Heatmap showing the surface levels of HLA-A2:AFP or H-2Kb:OVA and the expression levels of AFP or OVA antigen (denoted by the BFP level) in human or mouse reporter cells transduced with sgRNAs targeting the selected candidates. The mean values from three biological replicates were used to plot the heatmap.
(G) Venn diagram of the common negative AP regulators in the human HLA-A2:AFP screen and the mouse H-2Kb:OVA screen.
(H) STRING protein-protein interaction (PPI) network of the 78 common pMHC-I negative regulators as defined in (G). The minimum required interaction score was set to 0.4. k-means clustering was applied. Bubble size denoted the combined LFCs in both screens and line thickness denoted the STRING PPI score/confidence.
Using our engineered antigen-specific pMHC-I reporter cell lines, we then performed genome-wide CRISPR/Cas9 loss-of-function screens to uncover key pMHC-I regulators. These human and mouse reporter cell lines were transduced with either the Brunello sgRNA library or the Brie library,41 respectively. Cells were then sorted based on the differential surface expression of antigen-specific pMHC-I, and individual sgRNA read counts were then evaluated by deep sequencing (Figure 1A). Our screening strategy allowed us to identify previously unknown positive and negative regulators of pMHC-I expression in both human and mouse leukemia cells (Figures 1B–1E and Table S1). While the majority of these gene candidates were found to specifically modulate pMHC-I levels, some (e.g., ICMT, TSC2) affected pMHC-I expression by simultaneously regulating antigen levels, as indicated by the BFP expression (Figure 1F).
Integrating both human and mouse cell line screens, we identified 105 evolutionarily-conserved common pMHC-I positive regulators, whose ablation reduced surface levels of both HLA-A2:AFP and H-2Kb:OVA (Figure S1D and Table S2). Notably, key canonical regulators of MHC-I expression, antigen processing and presentation (e.g., B2M, TAP1, TAP2, TAPBP, NLRC5) were highly enriched in both screens (Figures 1B–1E). STRING protein-protein interaction network analysis further revealed novel molecules involved in transcription, RNA processing, mTOR signaling, and mitochondria function (Figure S1E). Interestingly, mTOR signaling has been reported to impact the MHC-I immunopeptidome,42 while mitochondrial dynamics and genomic integrity have been linked to neoantigen generation and MHC-I expression.43–45 Conversely, 78 genes were identified as common pMHC-I negative regulators (Figure 1G and Table S2). Network analysis showed that these genes were involved in protein ubiquitination, vesicle trafficking and transport, as well as DNA metabolic processes and cell cycle (Figure 1H). Together, our antigen-specific pMHC-I-based CRISPR screens systematically revealed evolutionally-conserved pMHC-I regulators.
Although the antigen-specific pMHC-I reporter system allows us to discover key regulators of the MHC-I AP pathway, it limits our findings to a specific subtype of the MHC-I family (HLA-A*02:01 in human and H-2Kb in mouse), as well as a given model antigen (AFP in human and OVA in mouse). Given that the majority of the HLA/MHC-I molecules on the cell surface are loaded with a vast array of peptides,46 we performed an additional CRISPR screen in the Cas9-expressing THP-1 cells to further dissect regulators also affecting pan-HLA surface expression to generalize our findings (Figure S2A). We used the W6/32 monoclonal antibody recognizing the major HLA family (HLA-ABC),47 enabling us to unbiasedly pinpoint general MHC-I regulators across different HLA subtypes and diverse antigens. Integrating our antigen-specific pMHC-I and pan-HLA screens, we identified 21 out of 105 genes as common regulators that positively modulate both antigen-specific pMHC-I and general MHC-I surface expression (Figures 2A, 2B and S2B). The majority of these common MHC-I positive regulators are key mediators of canonical antigen presentation (Figure S2C), including NLRC5, TAP1, TAP2, TAPBP, and B2M, as well as recently identified positive regulators of MHC-I expression (SUGT1)28 and glycosphingolipid-related pMHC-I/TCR recognition (SPPL3)47 (Figure 1B-E and 2A-B). We only found a few overlaps between these common MHC-I positive regulators from our screens (21 genes) and the recently reported MHC-I positive regulators in lymphoma (SUGT1, SRSF6, MOGS, and the canonical AP factors)28 (Figure S2B). Interestingly, 84 out of 105 positive pMHC-I regulators from our screens were only associated with antigen-specific pMHC-I, but not the pan-HLA (Figure S2B), these genes were strongly enriched in biological processes that control transcription, RNA processing, translation, and mitochondria function (Figure S2D).
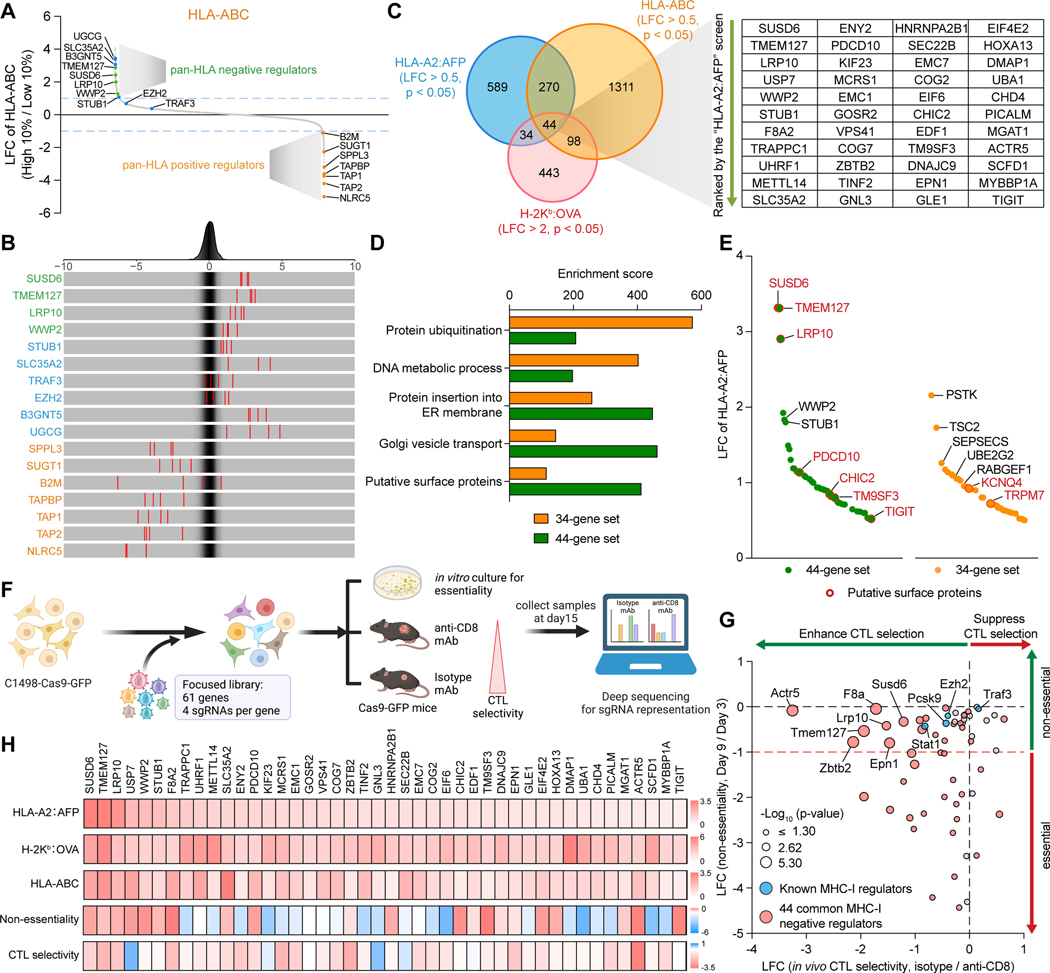
Figure 2. Integration of pan-HLA and in vivo CRISPR screen identifies functional MHC-I inhibitors. See also Figure S2.
(A and B) Waterfall plot (A) and frequency histograms (B) depicting the LFCs of the selected regulators in the human pan-HLA (HLA-ABC) screen. Green: novel pan-HLA negative regulators, blue: published MHC-I negative regulators, orange: known pan-HLA positive regulators.
(C) Venn diagram of the negative regulators in all three screens.
(D) Enrichment analyses comparing the 44-gene set and the 34-gene set in different categories of biological functions defined in Figure 1H and putative surface expression.
(E) Waterfall plot depicting the LFCs of the negative regulators from the 44-gene set and the 34-gene set in the HLA-A2:AFP screen. Putative surface proteins were highlighted in red.
(F) Schematic of the in vivo focused screens in the C1498 murine AML cell line.
(G) Scatter plots showing the non-essentiality of each candidate identified by in vitro cell growth (Y-axis) and the in vivo CTL selectivity of each targeted gene identified by comparison of tumors from Isotype versus anti-CD8 mAb-treated mice (X-axis).
(H) Heatmaps showing the LFCs of the 44 common negative AP regulators in the indicated screens.
Similarly, we validated several recently identified negative regulators for glycosphingolipid-related pMHC-I/TCR recognition (e.g., UGCG, SLC35A2, B3GNT5)47 as top candidates only in our pan-HLA screen but not in the antigen-specific pMHC-I screens (Figures 1B–1E, 2A and 2B). Interestingly, other recently characterized MHC-I inhibitors, EZH228,30 and TRAF3,29 were not top hits in either screen (Figures 2A and 2B). Although these results suggest some differential MHC-I regulatory mechanisms potentially related to diverse antigens, cell types, or variable HLA/MHC-I subtypes, our data collectively identified comprehensive MHC-I regulatory networks in AML.
We next subcategorized the 78 common pMHC-I negative regulators into two groups: 1) the 44-gene set modulating both pMHC-I and pan-HLA (common MHC-I negative regulators), and 2) the 34-gene set modulating only pMHC-I (pMHC-I-specific negative regulators) (Figure 2C). We found that the common MHC-I negative regulators from the 44-gene set were enriched in pathways related to protein trafficking organelles, including ER, Golgi, and endosome, while the pMHC-I specific negative regulators from the 34-gene set were involved in regulating protein ubiquitination, DNA metabolic processes, and cell cycle (Figures 2D, S2E and S2F). Notably, the 44-gene set had a larger portion of membrane proteins (SUSD6, TMEM127, LRP10, PDCD10, CHIC2, TM9SF3, and TIGIT) compared to the 34-gene set (Figures 2D and 2E), with SUSD6 and TMEM127 serving as the top 2 gene candidates in the HLA-A2:AFP screen (Figure 2E). Moreover, the molecules from the 44-gene set were, in general, more potent modulators of pMHC-I (Figures 2E and S2G). Considering MHC-I is a membrane-localized complex, these membrane-associated candidates may provide more direct and general mechanistic links to MHC-I downregulation. Although the pMHC-I-specific negative regulators are of significant interest and may be related to antigen processing and/or loading, we decided to focus on the 44 common MHC-I negative regulators for this study.
Integration of in vivo Screens Identifies Functional MHC-I Inhibitors
To further validate the immune regulatory function of the 44 common MHC-I negative regulators in the context of anti-cancer immunity, we performed focused in vivo CRISPR screens in a syngeneic C1498 murine AML model,48 using a focused library containing sgRNAs targeting these molecules as well as several published MHC-I regulators (e.g., Traf3, Ezh2, Pcsk9).27–30 sgRNA-transduced AML cells were transplanted into either CD8+ T cell-depleted mice or immune-competent mice, or cultured in vitro to assess their roles in affecting cell fitness (essentiality) (Figure 2F). The majority of the 44 candidates were negatively enriched under CD8+ cytotoxic T cell (CTL) selection at day 15 post-transplantation. This included the top-ranked candidates Susd6, Tmem127, Lrp10, Wwp2 and Stub1 (Figures 2G and 2H, and Table S3). These results suggest that targeting these functional MHC-I inhibitors could potentiate cancer cell elimination by CTLs. Notably, most of these genes were not essential to cancer cell-intrinsic fitness (Figures 2G and 2H). Collectively, our comprehensive in vitro genome-wide and in vivo focused screens systematically identified functional common negative regulators of MHC-I in AML that were associated with anti-cancer immunity.
SUSD6 Loss Enhances MHC-I Expression and Facilities T Cell-Mediated Immunosurveillance in AML and Solid Cancers.
Among the common MHC-I negative regulators, we were particularly interested in SUSD6, given that: 1) it was one of the top hits in all screen settings (Figure 2H), and 2) it represents a single-pass transmembrane protein with our validated expression on the cell surface (Figures S3A and S3B). Using validated sgRNAs for human and mouse SUSD6 (Figures S3C and S3D), we confirmed SUSD6 suppresses both antigen-specific pMHC-I and general HLA/MHC-I expression in both human and mouse AML cells lines (Figures 3A–3D). Moreover, restoring SUSD6 expression in SUSD6-deficient cells fully reversed its MHC-I-enhancing phenotype (Figure S3E). In addition, we confirmed the HLA inhibitory effects of SUSD6 in two additional human AML cell lines (MV4–11 and KASUMI-1) (Figures S3F and S3G), as well as in the context of interferon treatment (Figure S3H). Taken together, our findings suggest a functional role of the membrane protein SUSD6 in suppressing both antigen-specific pMHC-I and general MHC-I expression.
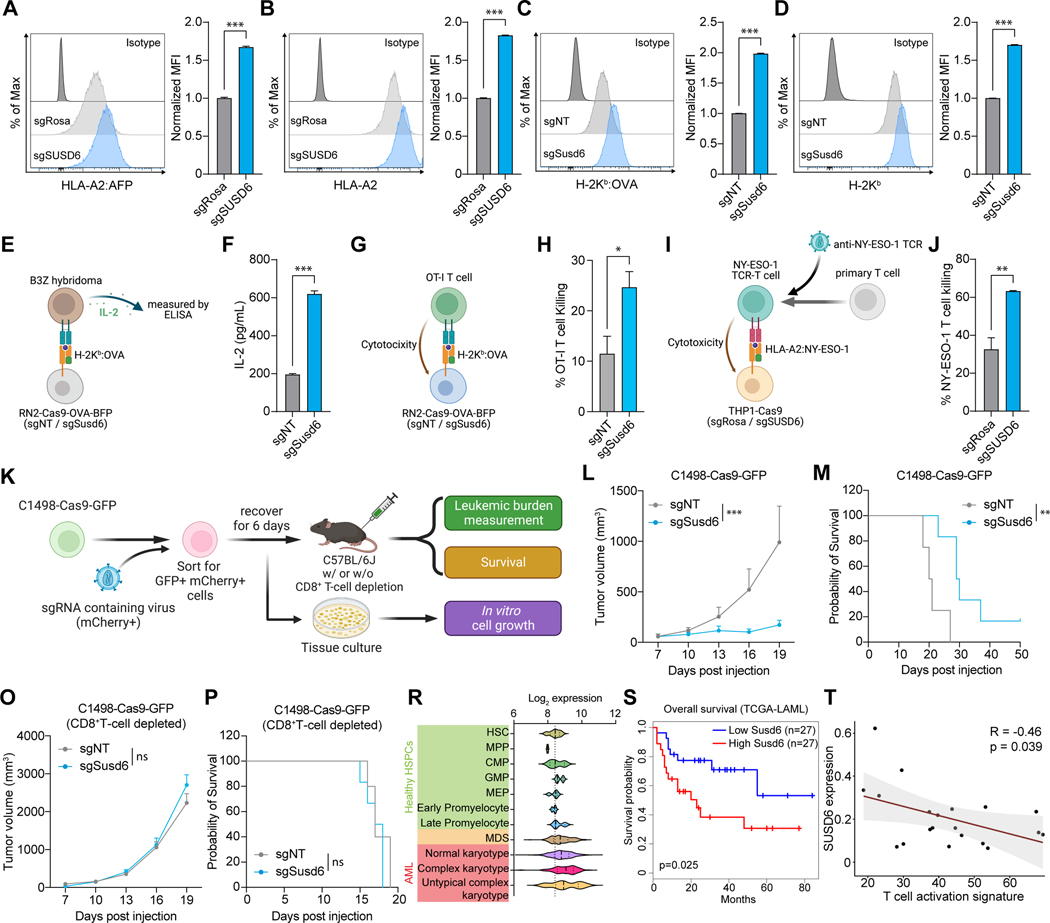
Figure 3. SUSD6 suppresses MHC-I expression and CD8+ T cell immunity in AML. See also Figure S3.
(A-D) Representative histograms (left) and bar plots (right) showing the surface levels of HLA-A2:AFP (A) or HLA-A2 (B) in human THP-1-Cas9-AFP-BFP cells, and the surface levels of H-2Kb:OVA (C) or H-2Kb (D) in mouse RN2-Cas9-OVA-BFP cells transduced with the indicated sgRNAs. (n=3)
(E and F) Schematic of the T cell activation assay (E) and bar plot showing the IL-2 secreted by the B3Z T cell hybridoma incubated with sgRNA-transduced RN2-Cas9-OVA-BFP cells (F). (n=3)
(G and H) Schematic of the mouse T cell killing assay (G) and bar plot showing the percentages of sgRNA-transduced RN2-Cas9-OVA-BFP cells killed by the OT-I T cells (H). (n=3)
(I and J) Schematic of the human T cell killing assay (I) and bar plot showing the percentages of sgRNA-transduced NY-ESO-1-expressing THP-1-Cas9 cells killed by the NY-ESO-1 TCR-T cells (J). (n=3)
(K) Schematic of the in vivo validations of SUSD6 functions in a mouse syngeneic AML model.
(L-P) Quantification of the tumor volumes (L and O) and Kaplan-Meier survival curves (M and P) of immunocompetent (L and M) or CD8+ T cell-depleted (O and P) mice transplanted with sgRNA-transduced C1498-Cas9-GFP cells as described in (K). (for L and M: n=4 for sgNT and n=6 for sgSusd6; for O and P: n=5 for sgNT and n=6 for sgSusd6)
(R) Violin plot of SUSD6 mRNA levels in normal HSPCs, MDS cells, and AML cells with different karyotypes from patient samples. HSPCs, hematopoietic stem and progenitor cells; HSC, hematopoietic stem cell; MPP, multipotent progenitor; CMP, common myeloid progenitor; GMP, granulocyte-monocyte progenitor; MEP, megakaryocyte-erythrocyte progenitor; MDS, myelodysplastic syndromes. Data were obtained from BloodSpot.51
(S) Survival of AML patients with high or low expression of SUSD6 in the TCGA-LAML cohort.
(T) Pearson correlation of SUSD6 expression levels in AML cells and T cell activation signature in CD8+ T cells from the bone marrow immuno-microenvironments from AML patients. Data were generated by single-cell RNA-seq.53
Data are presented as the mean ± SEM. ns, not significant; *, p< 0.05; **, p< 0.01; and ***, p< 0.001 by two-tailed unpaired Student’s t-test (A-D, F, H, and J), two-way ANOVA for the last time point (L and O), or Log-rank Mantel-Cox test (M and P). Rosa26-targeting sgRNA (sgRosa, for human) and non-targeting sgRNA (sgNT, for mouse) were used as controls.
Considering MHC-I AP is one of the major determinants that control CD8+ T cell activation and function,6,7 we then tested whether SUSD6 deficiency could promote antigen-specific CD8+ T cell responses. By co-culturing the H-2Kb:OVA-specific B3Z CD8+ T cell hybridoma49,50 with RN2-Cas9-OVA-BFP (RN2-OVA) mouse AML cells, we confirmed that SUSD6 deficiency in the AML cells potentiated T cell responses as measured by IL-2 secretion (Figures 3E and 3F). Concordantly, Susd6-deficient RN2-OVA cells were more susceptible to OT-I T cell-mediated killing in a co-culture assay (Figures 3G and 3H). Similarly, SUSD6-deficient human THP-1 AML cells, treated with the hypo-methylating agent azacytidine to induce NY-ESO-1 antigen expression, were more susceptible to killing by human peripheral T cells that were engineered to express a NY-ESO-1 specific TCR recognizing the NY-ESO-1 SLLMWITQC peptide-HLA-A*02:01 complex (Figures. 3I and 3J).
We next examined the in vivo function of SUSD6 using the C1498 murine AML model.48 Control or Susd6-deficient C1498-Cas9-GFP AML cells were sorted, expanded, cultured in vitro, or transplanted into immune-competent mice (Figure 3K). Susd6 knockout efficiency and its MHC-I enhancement effect were confirmed before transplantation (Figures S3I and S3J). The loss of Susd6 significantly delayed leukemia progression in vivo without impairing in vitro cell growth (Figures 3L and S3K), leading to prolonged animal survival (Figure 3M). Notably, the AML-suppressive activity from Susd6 ablation was dependent on CD8+ T cells (Figures 3O and 3P), suggesting SUSD6 plays a critical role in controlling T cell immunity in AML. To further establish the clinical significance of SUSD6 in AML, we analyzed the BloodSpot database51 and found significantly higher expression of SUSD6 in myelodysplastic syndromes (MDS) and AML compared to normal hematopoietic stem and progenitor cells (HSPCs) (Figure 3R). Moreover, SUSD6 expression was higher in AML patients with complex karyotypes, who have adverse prognoses (Figure 3R).52 Survival analysis also suggested that high expression of SUSD6 was associated with poor clinical outcomes in AML patients (Figure 3S). Evaluation of our in-house single-cell RNA-seq profiling of the bone marrow immune microenvironment in AML patients53 revealed that the levels of SUSD6 expression in AML malignant cells were negatively correlated with the T cell activation signature (CD3E, GZMB, CX3CR1, FGFBP2, PRF1) in CD8+ T cells (Figure 3T). Collectively, our findings suggest SUSD6 suppresses the MHC-I expression and immunogenicity of AML cells and thus promotes their immune evasion of T-cell responses.
The SUSD6 gene is primarily enriched in cells of the immune system, particularly the myeloid cell subsets and NK cells, with modest or low expression in most human organs, based on the BIOGPS database (Figure S4A). In addition to AML, SUSD6 was found to be significantly upregulated in a series of human solid cancers, such as pancreatic cancer (PAAD), Cholangiocarcinoma (CHOL), glioblastoma (GBM), and brain low-grade glioma (LGG) (Figure S4B). Therefore, we tested if our findings on SUSD6 could be extended beyond AML to solid tumors, as aberrant expression of the MHC-I pathway had been suggested in many cancer types.4,5,10,54,55 We first confirmed that SUSD6 ablation enhanced MHC-I expression in multiple solid tumor cell lines, including B16F10-OVA (melanoma), CMT167 (lung carcinoma), KPC (pancreatic cancer), as well as MC38 and CT26 (colon cancer) (Figure S4C). Two representative solid tumor lines (B16F10-OVA and CT26), with distinct genetic backgrounds, were selected to further validate the functions of SUSD6 in vivo (Figure S4D). We then used lentiviral-based shRNA-mediated gene silencing instead of CRISPR/Cas9 gene editing to manipulate the expression of Susd6, given the high immunogenicity of Cas9 in the selected solid tumor models.56 We confirmed successful knockdown (KD) of Susd6 by western blot and RT-qPCR and demonstrated significant MHC-I-boosting effects associated with reduced Susd6 expression (Figures S4E–S4J). Similar to our AML data, Susd6 KD delayed tumor progression and improved animal survival in both tumor models (Figures 4A–4D) without affecting in vitro cell growth (Figures S4K and S4L). In addition, CD8+ T cell depletion completely impaired the tumor-suppressive function of Susd6 KD in both models (Figures 4E–4H). To further validate that the tumor-suppressive effect of Susd6 KD was directly related to the upregulation of MHC-I expression, we decreased MHC-I surface expression in Susd6-KD B16F10-OVA cells back to the parental cell level using B2m-targeting shRNAs (Figure S4M). Notably, reducing MHC-I surface expression in Susd6-KD B16F10-OVA cells completely blunted the tumor-suppressive effect of Susd6 KD (Figures 4I and 4J), without affecting the in vitro cell growth and in vivo tumor progression in immune-deficient Rag2−/− Il2rg−/− mice (Figures S4N–S4P). Similar phenomena were observed in the MHC-I reduced Susd6-KD AML (C1498-Cas9-GFP) cells (Figures S4Q–S4T). Furthermore, MHC-I reduction completely abolished the enhanced OT-I T cell cytotoxicity upon Susd6 KD in both AML and solid tumor cell lines (Figures S4U–S4W). Together, these data suggest that the tumor-suppressive activity upon Susd6 knockdown is a consequence of the enhanced MHC-I surface expression and is dependent on CD8+ T cell immunity.
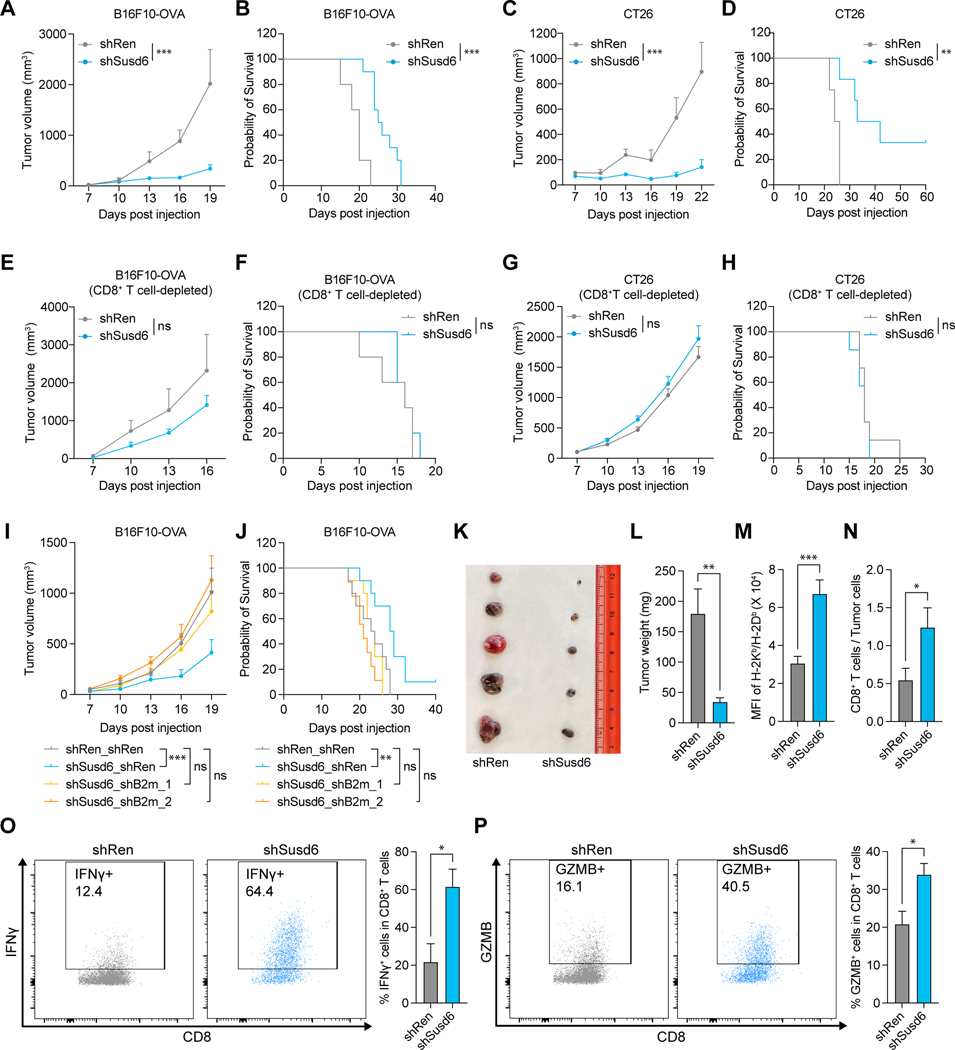
Figure 4. SUSD6 suppresses MHC-I expression and T cell evasion in solid tumor models. See also Figure S4.
(A-J) Quantification of the tumor volumes (A, C, E, G, I) and Kaplan-Meier survival curves (B, D, F, H, J) of immunocompetent (A-D, I and J) or CD8+ T cell-depleted (E-H) mice transplanted with B16F10-OVA (A, B, E, F, I, J) or CT26 (C, D, G, H) cells transduced with the indicated shRNAs as described in Figure S4D. (for A and B: n=5 for shRen, n=10 for shSusd6; for C and D: n=4 for shRen, n=6 for shSusd6; for E and F: n=5; for G and H: n=7; for I and J: n=10 for shRen_shRen, shSusd6_shRen and shSusd6_shB2m_1, n=9 for shSusd6_shB2m_2)
(K-P) shRNA-transduced B16F10-OVA tumors were transplanted and harvested for analyses on day 15.
(K) Representative image of the B16F10-OVA tumors.
(L) Weights of the B16F10-OVA tumors. (n=9)
(M) Surface expression of H-2Kb/H-2Db in B16F10-OVA tumors. (n=9)
(N) Quantification of tumor-infiltrating CD8+ T cells. (n=9)
(O and P) Representative dot plots (left) and quantifications (right) of the proportions of IFN-γ (O) and Granzyme B (P) expressing tumor-infiltrating CD8+ T cells. (n=8)
Data are presented as the mean ± SEM. ns, not significant; *, p< 0.05; **, p< 0.01; and ***, p< 0.001 by two-way ANOVA for the last time point (A, C, E, G and I), Log-rank Mantel-Cox test (B, D, F, H and J), or two-tailed unpaired Student’s t-test (L-P). Renilla-targeting shRNA (shRen) was used as a control.
In line with the reduced tumor burden (Figures 4K and 4L), ex vivo analysis of Susd6 KD tumors revealed enhanced MHC-I surface expression on tumor cells (Figure 4M), accompanied by increased percentages of CD8+ T cells in the TME (Figure 4N). Furthermore, the tumor-infiltrating CD8+ T cells in the Susd6-KD tumors had an increased effector function compared to those from the parental tumors, evidenced by their capacities to secrete granzyme B and interferon-γ (Figures 4O and 4P). Collectively, our data suggest SUSD6 inhibits MHC-I expression and T-cell immunity in both leukemia and solid tumors.
SUSD6 Facilitates MHC-I Lysosomal Degradation
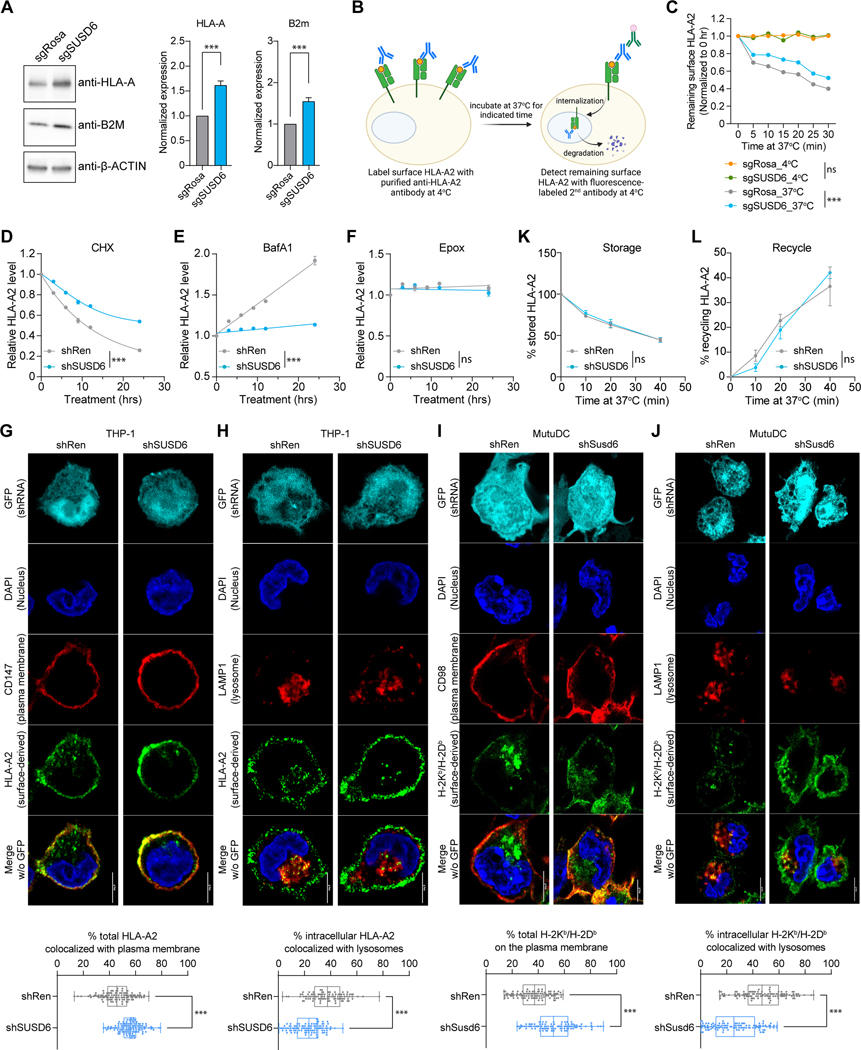
To investigate the mechanisms by which SUSD6 regulates MHC-I surface expression, we first demonstrated that SUSD6 did not affect the transcription of the major components involved in antigen presentation and processing (Figure S5A). Additionally, its ablation resulted in a substantial increase in the total amount of HLA protein (Figure 5A) without affecting the global translation (Figures S5B and S5C). Thus, we hypothesized that SUSD6 might affect MHC-I through post-translational mechanisms. Indeed, SUSD6 ablation reduced surface HLA-A2 internalization (Figures 5B and 5C). Further, SUSD6 KD slowed down the reduction of surface HLA-A2 in the presence of a translation inhibitor, cycloheximide (CHX) (Figures 5D and S5D), suggesting that a potential role of SUSD6 in surface HLA degradation. Moreover, a substantial increase in surface HLA-A2 was observed in control but not SUSD6-KD THP-1 cells under treatment with the lysosome inhibitor Bafilomycin A1 (BafA1) (Figures 5E and S5D) but not with the proteasomal inhibitor Epoxomicin (Epox, Figures 5F and S5D). showcasing the importance of lysosomal degradation in surface MHC-I regulation and the essential role of SUSD6 in this process.
Figure 5. SUSD6 targets MHC-I for lysosomal degradation. See also Figure S5.
(A) Representative western blots (left) and normalized band intensities (right) of HLA-A and B2m in sgRNA-transduced THP-1 cells. (n=5).
(B) Schematic of the surface HLA-A2 internalization assay in sgRNA-transduced THP-1 cells.
(C) Quantifications of the surface-remaining HLA-A2. (n=3)
(D-F) Time course studies of the surface HLA-A2 expression on shRNA-transduced THP-1 cells treated with Cycloheximide (CHX, D), Bafilomycin A1 (BafA1, E), or Epoxomicin (Epox, F) (n=4).
(G-J) Representative confocal images (top) and quantifications (bottom) of the surface-derived MHC-I colocalized with the plasma membrane markers (G and I) or the lysosomal marker (H and J) in THP-1 cells (G and H) or MutuDC cells (I and J) transduced with indicated shRNAs. At least 100 cells were quantified in each group. All scale bars: 5 μm.
(K and L) Flow cytometric analyses of intracellular MHC-I storage (K) and recycle (L) in shRNA-transduced THP-1 cells as described in Figures S5H and S5I, respectively. (for K: n=6; for L: n=4)
Data are presented as the mean ± SEM (A-F, K, and L) or box and whiskers with all data points (G-J). ns, not significant; ***, p<0.001 by two-tailed unpaired Student’s t-test (A), two-way ANOVA for the last time point (C-F, K, and L), or Mann-Whitney test (G-J). sgRosa and shRen were used as controls.
We also performed MHC-I labeling experiments to monitor cell surface MHC-I trafficking from the plasma membrane into different intracellular organelles. In line with the increased surface MHC-I expression upon Susd6-KD (Figure S5E), we observed increased colocalization of MHC-I with the plasma membrane (Figures 5G and 5I) and decreased colocalization with the lysosomal marker LAMP-1 (Figures 5H and 5J). Further, we did not notice a significant difference in the colocalization of the internalized MHC-I with EEA1+ early endosomes (Figure S5F) or TfR1+ recycling endosomes (Figure S5G). To further study the potential role of SUSD6 in MHC-I storage and recycling, which have previously been implicated in regulating MHC-I surface expression (Figure S5D),57 we performed well-established MHC-I recycling and storage assays by flow cytometry.58,59 We demonstrated that SUSD6 was not involved in either the recycling or the storage of the internalized MHC-I (Figures 5K and 5L, S5H and S5I).
SUSD6 and TMEM127 complex Targets MHC-I for Lysosomal Degradation through Direct Interaction and Recruitment of E3 Ligases
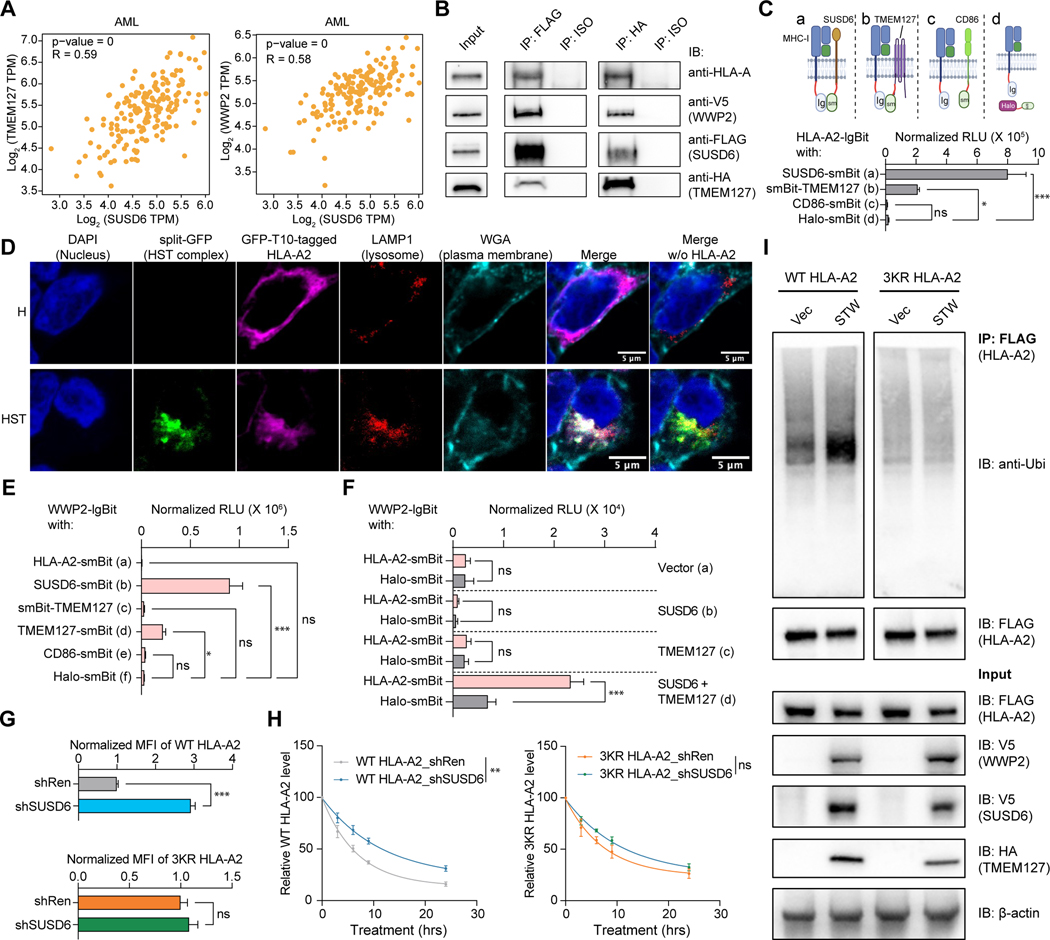
TMEM127 and WWP2, two other top hits identified by our screens (Figures 1 and 2), have previously been reported to be involved in protein ubiquitination and degradation of surface MHC-II in the presence of a bacterial effector protein.36 Interestingly, the expression of both genes is highly correlated with SUSD6 expression in AML and solid tumors (Figures 6A, S6A, and S6B), highlighting the possibility that TMEM127 and WWP2 may associate with SUSD6 for the degradation of surface MHC-I. Indeed, co-immunoprecipitation (Co-IP) experiments revealed that SUSD6, TMEM127, and WWP2 could simultaneously and specifically form a complex with HLA molecules (Figures 6B and S6C). To further validate this data, we employed a split-luciferase system,60 and demonstrated direct interactions among HLA-A2, SUSD6, and TMEM127 (Figures 6C and S6D). Additionally, we confirmed the simultaneous interaction between these three molecules via a tripartite split-GFP system,61,62 where the GFP signal could only be detected when these molecules were in close proximity (Figures 6D and S6E). Notably, the reconstituted GFP signals denoting the HLA/SUSD6/TMEM127 (HST) complexes colocalized with the lysosomal marker LAMP1 (Figure 6D). Moreover, the percentage of the split-GFP+ cells significantly increased upon BafA1 treatment only in cells expressing both SUSD6 and TMEM127 but not in those expressing SUSD6 and CD86 (control plasma membrane protein), further confirming that the HST complex was targeted for lysosomal degradation (Figures S6E and S6F).
Figure 6. The SUSD6/TMEM127/WWP2 complex mediates MHC-I ubiquitination and degradation. See also Figure S6.
(A) mRNA co-expression analysis of TMEM127 (left) and WWP2 (right) with SUSD6 in AML. Data were obtained from the TCGA database and analyzed by GEPIA2.69
(B) Immunoprecipitation using anti-FLAG antibody or anti-HA antibody in 293T cells transduced with SUSD6-FLAG, TMEM127-HA, and WWP2-V5. IP, immunoprecipitation; IB, immunoblotting; ISO, isotype.
(C) Schematics (top) of the split-luciferase experiments and the bar plot showing the normalized relative luminescence units (RLU) (bottom) in 293T cells co-transfected with the indicated tagged proteins. (n=6)
(D) Representative confocal images showing colocalization of the HLA-A2/SUSD6/TMEM127 complex with LAMP1+ lysosomes in 293T cells co-transduced with GFP-T10-tagged HLA-A2 alone (H) or together with GFP1–9-tagged SUSD6, and GFP-T11-tagged TMEM127 (HST). All scale bars: 5μm.
(E) Bar plot showing the RLU in 293T cells co-transfected with WWP2-lgBit and the indicated smBit-tagged proteins as described in Figure S6L. (n=6)
(F) Bar plot showing the RLU in 293T cells co-transfected with WWP2-lgBit and HLA-A2-smBit or Halo-smBit in the presence or absence of SUSD6 and/or TMEM127 overexpression as described in Figure S6M. (n=5)
(G and H) THP-1 cells were transduced with FLAG-tagged WT HLA-A2 or 3KR HLA-A2 mutant and with the indicated shRNAs, followed by the indicated analyses.
(G) Surface expression of FLAG-tagged HLA-A2. (n=3)
(H) Time course studies of surface HLA-A2 expression (by FLAG staining) upon CHX treatment. (n=3)
(I) FLAG-tagged WT HLA-A2- or 3KR HLA-A2-expressing THP-1 cells were transduced with the STW complex (SUSD6-V5, TMEM127-HA, and WWP2-V5) or control vectors (Vec), followed by immunoprecipitation using anti-FLAG beads.
Data are presented as the mean ± SEM. ns, not significant; *, p< 0.05; **, p< 0.01; and ***, p< 0.001 by one-way ANOVA (C and E), two-tailed unpaired Student’s t-test (F and G), or two-way ANOVA for the last time point (H). shRen was used as a control.
Considering that WWP2 is the key E3 ligase in the SUSD6/TMEM127/WWP2 (STW) complex to potentially execute MHC-I ubiquitination, we decided to further explore the role of WWP2 in downregulating surface MHC-I expression. We first confirmed the increase in both surface and total protein levels of MHC-I in the absence of WWP2 (Figures S6G and S6H). Similar to what was observed in the kinetics studies performed in SUSD6-KD THP-1 cells (Figures 5D–5F), WWP2 was also involved in the degradation of surface MHC-I through lysosomal but not proteasomal pathways (Figures S6I-S6K). Furthermore, the split-luciferase analysis revealed the direct interaction between WWP2 and SUSD6 (Figures 6E and S6L), and between WWP2 and TMEM127 (Figures 6E and S6L) as described before.36 Surprisingly, the presence of both SUSD6 and TMEM127 was essential for the direct interaction between WWP2 and HLA (Figures 6E, 6F, S6L and S6M), implying that SUSD6 and TMEM127 cooperate to recruit WWP2 to the vicinity of the HLA molecules. Accordingly, decreased HLA poly-ubiquitination was observed in the absence of either SUSD6 or WWP2 (Figures S6N and S6O). To further examine if the degradation of surface MHC-I is a consequence of MHC-I ubiquitination, we mutated all three lysine residues in the cytosolic tail of HLA-A2 to arginine residues (K335R/K340R/K364R, hereafter referred to as 3KR mutant) to prevent the lysine ubiquitination at its intracellular domain.63 As expected, the reduction of SUSD6 increased the surface expression of wild-type (WT) HLA-A2 but not the 3KR mutant (Figure 6G). Accordingly, SUSD6 KD decreased the degradation rate of WT HLA-A2 rather than the 3KR mutant in the presence of CHX treatment (Figure 6H). Furthermore, we demonstrated that the ubiquitination of WT HLA-A2 but not the 3KR mutant was increased when the STW complex was overexpressed (Figure 6I). Collectively, these data demonstrate that SUSD6 and TMEM127 form a tri-molecular complex with MHC-I and recruit WWP2 for MHC-I ubiquitination and subsequent lysosomal degradation.
Notably, WWP2 ablation did not augment surface HLA expression to the degree observed in the absence of SUSD6 or TMEM127 (Figure S6P), suggesting that additional E3 ligases may play a role in the degradation of HLA. Indeed, another E3 ubiquitin ligase STUB1, which was identified in our screen (Figures 1B–1E and 2C) and previously shown to affect the MHC-I expression through downregulating IFNGR1,64 was pulled down together with SUSD6 or TMEM127 (Figure S6Q). Taken together, our mechanistic studies demonstrate that SUSD6 and TMEM127 simultaneously interact with the MHC-I. E3 ligases, such as WWP2 and STUB1, may be recruited during the interaction to ubiquitinate plasma membrane HLA for lysosomal degradation.
Targeting TMEM127 in the SUSD6/TMEM127/WWP2 Complex Restores Tumor MHC-I Expression and Controls Tumor Growth
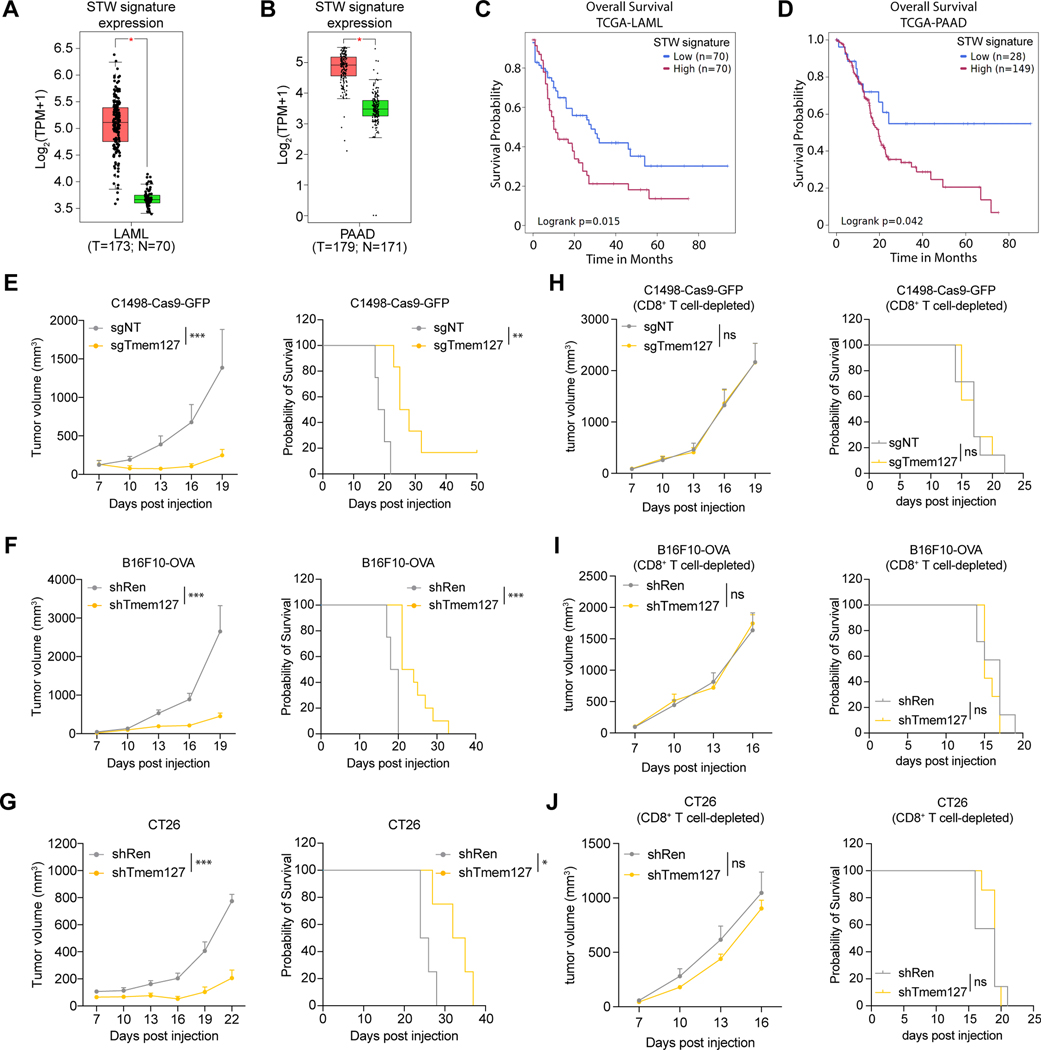
Given the highly correlated expression of the STW gene signature in various tumor types (Figures 6A, S6A, and S6B), we explored the clinical relevance of the STW gene signature using the TCGA database. We found that the STW gene signature was significantly upregulated in AML and PAAD (Figures 7A and 7B) and negatively correlated with patients’ overall survival (Figures 7C and 7D). Since SUSD6, TMEM127, and WWP2 work together to mediate the ubiquitination and lysosomal degradation of surface MHC-I, we tested whether tumor cell surface MHC-I levels could be restored by targeting the STW complex instead of SUSD6 alone. As a proof of concept, we have shown that knocking down SUSD6 in AML (C1498) or solid tumors (B16F10-OVA and CT26) enhanced tumor MHC-I surface expression, delayed tumor growth, and extended animal survival (Figures 3 and 4). Additionally, knocking down TMEM127 also resulted in similar phenotypes in these tumor models (Figures 7E-7G and S7A–S7F) without impairing the tumor cell growth in vitro (Figure S7G). The in vivo tumor-suppressing effects of TMEM127 ablation were also CD8+ T cell-dependent (Figures 7H-7J), recapitulating what we observed in the Susd6-deficient AML and solid tumor models (Figures 3 and 4).
Figure 7. Targeting the SUSD6/TMEM127/WWP2 complex enhances MHC-I expression and cancer immunity. See also Figure S7.
(A-B) Expression of the STW gene signature in AML (A) and PAAD (B). Red, cancer cells; Green, normal tissue. Data were obtained from the TCGA database and analyzed by GEPIA2.69
(C-D) Association of high or low STW gene signature with the overall survival of AML (C) or PAAD (D) patients in the TCGA database by SurvivalGenie.70
(E-J) Quantifications of the tumor volumes (left) and Kaplan-Meier survival curves (right) of immunocompetent (E-G) or CD8+ T cell-depleted (H-J) mice transplanted with C1498-Cas9-GFP (E and H), B16F10-OVA (F and I), or CT26 (G and J) cells transduced with the indicated sgRNAs or shRNAs. (for E: n=4 for sgNT and n=6 for sgTmem127; for F: n=4 for shRen and n=10 for shTmem127; for G: n=4 for shRen and n=5 for shTmem127; for H-J: n=7)
Data are presented as box and whiskers with all data points (A-B) or the mean ± SEM (E-J left). ns, not significant; *, p< 0.05; **, p< 0.01; and ***, p< 0.001 by two-way ANOVA for the last time point (E-J left), or Log-rank Mantel-Cox test (E-J right). shRen and sgNT were used as controls.
Taken together, our in vitro and in vivo studies collectively confirm our hypothesis that targeting the membrane-associated STW MHC-I inhibitory axis can restore tumor MHC-I expression and enhance immune surveillance.
Discussion
In this study, we performed the first genome-wide cell surface pMHC-I-guided CRISPR screens and identified key regulators that: 1) control both antigen-specific pMHC-I AP and general MHC-I surface expression; 2) are evolutionarily conserved in both human and mouse systems; and 3) are largely shared by AML and solid cancers. Given the fact that the endogenous MHC-I AP inhibition mechanisms in immune homeostasis and/or cancers remain unclear, our findings collectively identified and characterized SUSD6 and TMEM127 as a pan-cancer membrane-associated regulatory axis of MHC-I. To our knowledge, the host membrane molecule(s) that can directly engage and inhibit surface MHC-I expression has never been suggested. The SUSD6/TMEM127/MHC-I tri-molecular complex recruited specific E3 ligase(s), such as WWP2, for MHC-I ubiquitination and lysosomal degradation, advancing our understanding of MHC-I AP inhibition mechanisms in cancer.
Based on our screening results, we identified positive and negative regulators for antigen-specific pMHC-I modulation. Many of these hits are associated with epigenetic modulation, transcriptional regulation, and RNA processing, which could also control various cellular functions beyond specific pMHC-I modulation. Therefore, we performed an additional screen on pan-HLA surface expression, which enabled us to unbiasedly pinpoint general MHC-I regulators across different HLA subtypes and diverse antigens. Our results also clearly suggest some differential regulatory mechanisms by comparing regulators affecting either or both pMHC-I/pan-HLA. In this study, we focused on the common MHC-I negative regulators with more membrane-associated proteins and a robust ability to modulate both antigen-specific pMHC-I and pan-HLA expression. The remaining regulators that primarily affected antigen-specific pMHC-I but not pan-HLA, which may be related to antigen processing and/or presentation, awaits further investigation. Of note, although the majority of the MHC-I molecules on the cell surface are thought to be preloaded with peptides, some unstable empty MHC-I molecules could still reach the surface in variable amounts in different cell types.65,66 Therefore, it would be important to further examine the role of these MHC-I regulators in the presence of exogenous peptide loading and to distinguish peptide-specific MHC-I regulators.
It is well-established that many viruses use membrane-associated viral factors to directly hijack MHC-I expression. For instance, the murine cytomegalovirus protein GP48/M06 and the SARS-CoV-2 protein ORF7A, which are single-pass transmembrane proteins, can directly interact with the α chain of MHC-I and destabilize its association with B2M.23,24 Additionally, the Epstein-Barr Virus G-protein-coupled receptor BILF1 contributes to viral immune evasion by specifically targeting MHC-I internalization and degradation.25 Interestingly, SUSD6 is a single-pass transmembrane protein, while TMEM127 is a 4-pass transmembrane protein. In this study, we validated their functions in modulating MHC-I surface expression through directly engaging with MHC-I to form a tri-molecular complex. Therefore, we believe SUSD6 and TMEM127 comprise a new class of host membrane molecule(s) that directly bind to and inhibit MHC-I surface expression. The exact interacting domains and interfaces involved in the interactions of SUSD6 and TMEM127 with MHC-I warrant further biochemical and structural analysis. Moreover, whether these molecules can regulate different human HLA subtypes, especially the minor HLA-E/F/G, deserves careful investigation. TMEM127 has been well characterized as an adaptor for WWP2,36 which was validated by our split-luciferase assays. However, we also identified a strong interaction between SUSD6 and WWP2, and both TMEM127 and SUSD6 are required for the interaction between MHC-I and WWP2. Other than WWP2, we found that STUB1 could engage with TMEM127 and SUSD6. Therefore, it is very likely that TMEM127 and SUSD6 served as a “regulasome” at the membrane level, to bridge certain E3 ligases to the proximity of MHC-I for its degradation. Further studies should investigate whether there are conserved motifs in the cytoplasmic tails of TMEM127 and SUSD6 that are involved in the interaction with particular ubiquitination-related molecules.
The roles of SUSD6 and TMEM127 in modulating MHC-I surface expression appear to be quite consistent across different cancer subtypes, as indicated by our data involving multiple AML and solid cancer cell lines. SUSD6 mRNA is enriched in immune subsets under homeostatic conditions and is upregulated in AML and several solid cancers, yet the regulation of its expression is unknown. We found a strong positive correlation among the expression of TMEM127, SUSD6 and WWP2 in multiple cancer types, suggesting a common induction mechanism of these molecules. The cross-priming of T cells by dendritic cells in the tumor-draining lymph node is generally considered to be the initiation step of the anti-tumor immune response.67 Thus, it will also be critical to study the role of the SUSD6-TMEM127-WWP2 regulatory axis in non-transformed cell types, especially professional antigen-presenting cells, Given that SUSD6 or TMEM127 deficiency increased MHC-I expression in the MutuDC myeloid cell line, these molecules may likely represent a universal mechanism of MHC-I inhibition beyond cancer cells.
Our findings also support STW-mediated MHC-I lysosomal degradation as a key mechanism regulating MHC-I expression. Further research will be required to determine how the SUSD6/TMEM127/MHC-I complex is transported to the lysosome and whether autophagy is involved in this process as previously suggested.26 Although SUSD6 does not regulate MHC-I recycling or storage under the current experimental settings, it is still possible that the STW axis might regulate MHC-I trafficking,57 especially in different cancer cells or immune cell subsets. Other than STW, it would be also important to explore other molecules that came out from our initial screen that may be involved in MHC-I recycling and/or storage. Notably, TMEM127 and SUSD6 did not affect the intrinsic growth of cancer cells we tested; however, TMEM127 loss of function mutations predispose to rare, predominantly benign neuroendocrine tumors, pheochromocytomas,34,35 while SUSD6 has been reported to suppress the activity of certain cancer cells.68 These observations also emphasize the need to study their potential roles in cancer biology beyond MHC-I modulation.
Our findings also suggest the SUSD6-TMEM127-WWP2 axis as a cancer-associated immune evasion mechanism and potential therapeutic target for “cold” tumors that involve insufficient CD8+ T cell immunity due to low MHC-I expression, particularly in cancers with high-expression of these molecule(s). Therapeutic targeting of these molecules, such as antibodies against SUSD6 or TMEM127 to disrupt the SUSD6/TMEM127/MHC-I tri-molecular complex, intra-tumoral delivery of shRNA encapsulating nanoparticles, and small molecule inhibitors to E3 ligases, could be feasible approaches to enhance MHC-I antigen presentation and CD8+ T cell immunity. In summary, we have identified SUSD6, TMEM127 and other common MHC-I inhibitors through comprehensive CRISPR screens. Our study advances our understanding of cancer-associated immune evasion of the MHC-I AP pathway and provides new candidates for immunotherapy of cancer and potentially for viral infection or autoimmunity.
Limitations of the study
Our genome-wide CRISPR screens were mainly based on human and mouse AML cell lines, followed by validation in solid cancer lines. Thus, these results may not fully capture specific mechanisms in some particular cancer types. In this study, we focused on the membrane-associated STW axis that negatively regulated surface MHC-I levels through MHC-I ubiquitination and lysosomal degradation. Other regulators, such as those involving MHC-I trafficking, as well as antigen processing and presentation, warrant further investigation. It is also possible that SUSD6, TMEM127, or WWP2 may have other important functions beyond MHC-I modulation. Further, the structural details of the STW complex interaction with MHC-I, and whether other molecules beyond the STW molecules can be involved in the complex remain unclear. Lastly, the detailed mechanisms of action and therapeutic strategies targeting these molecules for cancer immunotherapy require careful evaluation, especially in different cancer types with profound genetic or immune heterogeneity.
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact: Jun Wang (jun.wang@nyulangone.org).
Materials availability
All materials generated or analyzed during this study are included in this article and its supplementary information files. Please contact the lead contact for unique material requests. Any material that can be shared will be released via a Material Transfer Agreement for non-commercial usage.
Data and Code Availability
This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cell lines
HEK293T cells (ATCC, CRL-3216, RRID: CVCL_0063, Female), MC38 (Kerafast; ENH204-FP, RRID: CVCL_B288, Female), B16F10-OVA (gift from Hongbo Chi), KPC (gift from Alec Kimmelman), CMT167 (ECACC, 10032302, RRID: CVCL_2405, Female), EMT6 (ATCC, CRL-2755, RRID: CVCL_1923, Female), and CT26 (ATCC, CRL-2638, RRID: CVCL_7256) were cultured in complete Dulbecco’s Modified Eagle Medium (DMEM, Corning), supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM L-Glutamine, 1X Penicillin/Streptomycin, 1 mM Sodium Pyruvate, and 20 mM HEPES. THP-1 cells (ATCC, TIB-202, RRID: CVCL_0006, Male), RN2 cells (gift from Chris Vakoc), C1498 (ATCC, TIB-49, RRID: CVCL_3494, Female) were cultured in RPMI 1640 medium (Corning) supplemented with 10% FBS, 2 mM L-Glutamine, 20 mM HEPES, and 1X Penicillin/Streptomycin. MutuDC1940 (Applied Biological Materials, T0528) were cultured in Prigrow V (Applied Biological Materials, TM015), supplemented with 4 mM Glutamax (Gibco), 10% FBS, 1% of 7.5% Sodium Bicarbonate Solution, 50 μM β-mercaptoethanol (β-ME), 10 mM HEPES, 1X Penicillin/Streptomycin. All cells were maintained at 37°C and in a 5% CO2 atmosphere. All the cell lines were determined negative for mycoplasma using the LookOut Mycoplasma PCR Detection Kit (Sigma). Cells were used for experiments within 15 to 20 passages from thawing. The H-2Kb:OVA-specific B3Z (gift from Dr. Peter Cresswell) hybridoma cells were cultured at 37 °C and 5% CO2 in RPMI-1640, supplemented with 10% FBS, 25 mM HEPES, 1 mM sodium pyruvate, 1x Pen/Strep, and 50 μM β-ME.
Cell lines transduced with lentiviral MSCV-Cas9–2A-Blast were selected with blasticidin (InvivoGen) 48 hrs after transduction. All transfections were performed in HEK293T cells using Lipofectamine3000 (Invitrogen) reagent at 4:2:3 ratios of sgRNA construct: pVSVG: pPax2 in OPTI-MEM solution. Viral supernatant was collected 48 hrs and 72 hrs post-transfection. Spin-infections were performed at 32°C with 1,500 RCF for 30 min with polybrene reagent (1:2000 dilution) (Fisher Scientific). For SUSD6/ TMEM127/ WWP2/ STUB1 overexpression, a cassette containing human-codon-optimized FLAG/HA/V5-tagged SUSD6/ TMEM127/ WWP2/ STUB1 CDSs and the selective markers, such as EGFP-P2A-PURO, mCherry, BFP, or URFP were cloned into the lentiviral vector pCDH-EF1s. Lentivirus were generated using HEK293T, and spin-infections were performed to transduce AML cells.
The tripartite split-GFP system61,62 was adapted to detect the HLA-A2/SUSD6/TMEM127 complex. Briefly, HLA-A2 was fused to the tenth (T10) β-strand of GFP at its C-terminal (HLA-A2-T10), while TMEM127 and CD86 were fused to the eleventh (T11) β-strand of GFP at its C-terminal (TMEM127-T11 and CD86-T11). SUSD6 was fused to the first to ninth β-strand of GFP (GFP1–9) at its C-terminal (SUSD6-GFP1–9). 293T cells were transduced with either the HLA-A2-T10 alone (H) or the combination of HLA-A2-T10, SUSD6-GFP1–9, and TMEM127-T11 (HST) or CD86-T11 (HSC). Transduced cells were sorted and expanded before proceeding to flow cytometric analysis and immunofluorescence staining.
All expression vectors (including vector maps and sequences) are available upon request.
Animals
C57BL/6J (000664), BALB/cJ (000651), Cas9-GFP (024858), OT-I (003831) were produced using breeders bought from the Jackson Laboratory. The immunodeficient Rag2/Il2rg double knockout mice (4111-F) were produced using breeders bought from the Taconic. Mice were bred and maintained in individually ventilated cages and fed with autoclaved food and sterile water at NYU School of Medicine Animal Facility. All animal experiments were performed in accordance with protocols approved by the New York University Institutional Animal Care and Use Committee (IACUC ID: IA16–00008_TR1 and TR202300011), according to national and institutional guidelines.
Cloning and transduction of shRNAs and sgRNAs
shRNAs were cloned into the LT3GEPIR shRNA vector (Addgene #111177) or the modified SGEP shRNA vector (Addgene #111170), where GFP was replaced by mCherry, as described.75 Briefly, the 97-mer oligonucleotides were synthesized by Integrated DNA Technologies (IDT), followed by PCR amplified using the primers miRE-Xho-fw (TGAACTCGAGAAGGTATATTGCTGTTGACAGTGAGCG) and miRE-EcoOligo-rev (TCTCGAATTCTAGCCCCTTGAAGTCCGAGGCAGTAGGC), and the Q5® High-Fidelity DNA Polymerase (NEB). Final vectors were obtained by XhoI/EcoRI cloning of the 125 nt purified PCR products into recipient vectors. sgRNAs were designed and cloned into the LRG2.1-GFP sgRNA vector (Addgene #108098), or LRG2.1-TagBFP2 sgRNA vector (Addgene #124773), or LRCherry2.1 sgRNA vector (Addgene #108099) following the shRNA/sgRNA Cloning Protocol from the Broad Institute. All shRNA/sgRNA expression vectors (including vector maps and sequences) are available upon request. All shRNAs and sgRNAs used in this study are listed in Table S4.
Lentivirus for shRNA- and sgRNA-transduction were generated using HEK293T, and spin-infections were performed to transduce target cells. shRNA-transduced cells were either selected with puromycin (Sigma # P7255–25MG, 1 μg/ml) or FACS sorting before downstream applications. Doxycycline (Takara Bio # 631311, 1 μg/ml) and doxycycline-containing diets (inotiv # TD.05298, 1 g/kg) were used to induce the expression of the shRNAs in vitro and in vivo, respectively. sgRNA-transduced cells were FACS sorted before downstream applications.
Surface antigen-guided Genome-wide CRISPR Screens
THP-1-Cas9-AFP-BFP and RN2-Cas9-cOVA-BFP cells were transduced with the Brunello sgRNA library (Addgene #73179) or Brie sgRNA library (Addgene #73632) virus,41 respectively, at a low MOI (~0.3). On day 2 post-transduction, GFP+ percentage was assessed to determine infection efficiency and sgRNA coverage (~1,000X). Then, puromycin (Sigma # P7255–25MG, 1 μg/ml) was added for 5 days to select infected (GFP+) cells. After selection, viable infected cells were isolated by Histopaque 1077 (Sigma Aldrich #10771) and grown without antibiotics. On day 12 post-transduction of the genome-wide libraries, THP-1-Cas9-AFP-BFP and RN2-OVA cells were stained with human TruStain Fcblock (BioLegend #422302, dilution 1:200) and mouse TruStain Fcblock (BioLegend #101320, dilution 1:200), respectively, for 10 mins at room temperature and then subsequently stained the THP-1-Cas9-AFP-BFP cells for APC-ET1402L138 or APC-HLA-ABC (BioLegend #311410, dilution 1:200), and stained the RN2-Cas9-cOVA-BFP cells for APC-H-2Kb:OVA (BioLegend #141606, dilution 1:200), for 40 mins at 4°C followed by flow cytometry gating on high and low 10% of the population. 3–5 million cells were collected for each sorted population followed by genomic DNA (gDNA) extraction using the QIAGEN DNA kit (#51306) according to manufacturer’s protocol. For library construction, gDNA was amplified for 25 cycles using EX-Taq (TakaraBio # RR001A) and primer pairs that contain barcodes. PCR products were size-selected using AMPure XP beads (Beckman Coulter #A63880). Barcoded libraries were then sequenced using the Next-Seq instrument (single-end, 80 cycles). The log2-fold changes (LFCs) of each screen were calculated by the average log2-fold change (High10% / Low 10%) of all sgRNAs targeting a given gene. We plotted the LFCs of selected sgRNAs using non-targeting control as background. The plots were generated with R package ggplot2 (version 3.3.6).
Focused in vivo CRISPR screens
C1498-Cas9-GFP cells were transduced with a focused library containing sgRNAs targeting the 44 common AP repressors and several published controls (4 sgRNAs per gene). Transduced cells were sorted by FACS and then expanded for 6 days before being injected in either CD8+ T cell-depleted mice or immune-competent Cas9-GFP mice. A portion of the cells was cultured in vitro to assess the non-essentiality of each candidate. Transplanted cancer cells were then collected on day15. gDNA was extracted for library preparation and sgRNA representations were evaluated using the Next-Seq instrument (single-end, 80 cycles). The LFCs of immune selection were calculated by the average log2-fold change (Isotype / anti-CD8) of all sgRNAs targeting a given gene, while the non-essentialities were calculated by the average log2-fold change (Day 9 /Day 3) of all sgRNAs targeting a given gene.
Flow cytometry
THP-1-Cas9-AFP-BFP and RN2-OVA cells were transduced with the indicated sgRNAs, harvested on day 4 and day 8, followed by washing with FACS buffer (PBS + 2% FBS). Human TruStain FcX™ (BioLegend #422302, dilution 1:100) or mouse TruStain FcX™ (BioLegend #101320, dilution 1:100) was used for Fc receptor blocking at room temperature for 15 min. THP-1-Cas9-AFP-BFP cells were then stained using the following fluorescently labeled antibodies: APC-ET1402L1, 38 PE-HLA-ABC (BioLegend #311406, dilution 1:100), PerCP/Cy5.5-CD147 (BioLegend # 306220, dilution 1:100) and APC/Cy7-HLA-A2 (BioLegend #343310, dilution 1:100) for 40 mins at 4 °C, while RN2-Cas9-cOVA-BFP cells were stained with: APC-H-2Kb:OVA (BioLegend #141606, dilution 1:100), PE-H-2Kb (BioLegend #116508, dilution 1:100) and APC-CD98 (BioLegend #128212, dilution 1:100) for 40 mins at 4 °C. Data were acquired on a BD LSRFortessa™ Cell Analyzer or BD ® LSR II Flow Cytometer and analyzed by the FlowJo software.
Intracellular staining was performed using the eBioscience™ Intracellular Fixation & Permeabilization Buffer Set (Invitrogen # 88–8824-00) following the manufacture’s protocol.
Surface HLA internalization
THP-1-Cas9 cells were transduced with the indicated sgRNAs and cultured for 8 days. Cells were then stained for purified antibodies against human HLA-A2 (BioLegend #343302, dilution 1:100) for 40 mins at 4 °C. Cells were then incubated at 37 °C for the indicated time before putting on ice and staining for APC Goat anti-mouse IgG (BioLegend # 405308, dilution 1:100) for 30 mins on ice. DAPI (Sigma Aldrich #D9542–1MG, 1 μg/ml) was used to exclude dead cells. Data were acquired on a BD LSRFortessa™ Cell Analyzer and analyzed by the FlowJo software.
T cell activity assay by B3Z T cell hybridoma co-culture
RN2-Cas9-OVA cells were transduced with indicated sgRNA, sorted and expanded for 8 days, followed by co-culturing with B3Z T cell hybridoma as described previously.49,50 Briefly, 1 × 105 transduced RN2-Cas9-OVA cells were fixed in 1% paraformaldehyde at 37 °C for 10 min, followed by incubation with 200 mM glycine (dissolved in PBS, pH 7.5) at 37 °C for 5 min to stop the fixation. Cells were then washed three times with B3Z medium. A total of 5 × 104 B3Z T cell hybridoma cells were added per well and cultured for 10 h at 37 °C. The supernatant was harvested and secreted IL-2 was then measured by ELISA following the manufacturer’s protocol (BD Biosciences #555148).
OT-I T cell killing assay
RN2-OVA cells were transduced with the indicated sgRNAs and shRNAs, sorted and expanded for 8 days followed by co-culturing with OT-I C8+ T cells. Briefly, 50,000 sgRNA-transduced RN2-OVA cells were seeded into each well of a 96-well U-bottom plate together with 4,000 CD8+ T cells freshly isolated from the lymph nodes of OT-I mice or C57BL/6J mice in RPMI-1640, supplemented with 10% FBS, 25 mM HEPES, 1 mM sodium pyruvate, 1x Pen/Strep, 10ng/ml murine IL-2 (PeproTech #212–12) and 50 μm β-ME. Flow cytometric analyses were applied to assess viable cell numbers for the tumor cells using AccuCheck counting beads (Invitrogen #PCB100) at 44 hours post-co-culture. DAPI (Sigma Aldrich #D9542–1MG, 1 μg/ml) was used to exclude dead cells. “% OT-I T cell killing” was calculated as: 1 – (cell # in the OT-I group/cell # in the C57BL/6J group).
NY-ESO-1 TCR-T cell killing assay
Human CD8+ T cells were purchased from STEMCELL Technologies. Cells were thawed and cultured at 1,000,000 cells/ml in RPMI-1640, supplemented with 20% FBS, 25 mM HEPES, 1 mM sodium pyruvate, 1x Pen/Strep, 1x NEAA, 10ng/ml human IL-2 (PeproTech #200–02) and 50 μm β-ME. Dynabeads™ Human T-Activator CD3/CD28 (Gibco #11161D) were added to stimulate T cells. After 48 hours of activation, T cells were lentiviraly transduced with the NY-ESO-1 TCR to generate NY-ESO-1 TCR-T cells. T cells were then rested for 3 days without Dynabeads before setting up the co-culture system.
For setting up the co-culture assay, THP-1-Cas9-AFP-BFP cells were transduced with the indicated sgRNA, sorted and expanded for 8 days, then induced with Azacitidine (Selleckchem #S1782, 5 μM) for 3 days, followed by co-culturing with NY-ESO-1 TCR-T cells. Briefly, 50,000 sgRNA-transduced THP-1-Cas9-AFP-BFP cells were seeded into each well of a 96-well U-bottom plate together with 2,500 non-transduced human CD8+ T cells or human NY-ESO-1 TCR-T cells generated as described above in RPMI-1640, supplemented with 20% FBS, 25 mM HEPES, 1 mM sodium pyruvate, 1x Pen/Strep, 1x NEAA, 10ng/ml human IL-2 (PeproTech #200–02) and 50 μm β-ME. Flow cytometric analyses were applied to assess viable cell numbers for the tumor cells using AccuCheck counting beads (Invitrogen #PCB100) at 96 hours post-co-culture. DAPI (Sigma Aldrich #D9542–1MG, 1 μg/ml) was used to exclude dead cells. “% NY-ESO-1 T cell killing” was calculated as 1 - (cell # in the NY-ESO-1 T cell group/cell # in the non-transduced group).
OP-Puro assay
OP-Puro assay was performed as previously described.76 Briefly, THP-1-Cas9 cells were transduced with indicated sgRNA. On day 8 post-transduction, cells were harvested and the Click-iT Plus OPP Alexa Fluor 647 Protein Synthesis Assay Kit (Invitrogen #C10458) was used to assess the global translation following the manufacture’s protocol.
Tumor inoculation and dissection
For tumor inoculation, 1 – 1.5 × 106 cancer cells (C1498-Cas9-GFP, B16F10-OVA, CT26) were subcutaneously injected into the C57BL/6J, BALB/cJ or Rag2/Il2rg double knockout mice. Tumor sizes were measured every three days.
For tumor dissection, B16F10-OVA tumors were isolated on day 15 post-inoculation. Tumors were subjected to image and weight upon harvest. Liberase™ TM (Roche #5401119001, 7.7 μg/ml) and DNase I (Invitrogen #EN0521, 100 μg/ml) were then used to digest the tumor chunks. Single-cell suspensions were generated by smashing the digested tumor chunks through 70 μm strainers (BD Biosciences). Tumor cells were then subjected to flow cytometric analyses.
For measuring the tumor-infiltrating lymphocytes (TILs), mouse TruStain FcX™ (BioLegend #101320, dilution 1:100) was used for Fc receptor blocking at room temperature for 15 min. TILs were then stained using the following fluorescently labeled antibodies: BV605-CD3ε (BD Biosciences #563004, dilution 1:200), PE-B220 (BD Biosciences #553090, dilution 1:200), PerCP/Cy5.5-NK1.1 (BioLegend #108728, dilution 1:200), PE/Cy7-CD4 (BioLegend #100422, dilution 1:200), APC-CD8 (Thermo Fisher Scientific #17–0081-82, dilution 1:200), APC/Cy7-CD45.2 (BioLegend #109824, dilution 1:200) for 40 mins at 4 °C. DAPI (Sigma Aldrich #D9542–1MG, 1 μg/ml) was used to exclude dead cells. Data were acquired on a BD LSRFortessa™ Cell Analyzer or BD ® LSR II Flow Cytometer and analyzed by the FlowJo software.
For measuring MHC-I expression on tumor cells, mouse TruStain FcX™ (BioLegend #101320, dilution 1:100) was used for Fc receptor blocking at room temperature for 15 min. Tumor cells were then stained using the following fluorescently labeled antibodies: PE/Cy7-H-2Kb/Db (BioLegend #114616, dilution 1:200), APC-CD98-APC (BioLegend #128212, dilution 1:200) for 40 mins at 4 °C. DAPI (Sigma Aldrich # D9542–1MG, 1 μg/ml) was used to exclude dead cells. Data were acquired on a BD LSRFortessa™ Cell Analyzer or BD ® LSR II Flow Cytometer and analyzed by the FlowJo software.
Immunofluorescence imaging
For MHC-I labeling assays in MutuDC cells, shRNA-transduced cells were seeded onto poly-L-Lysine (Sigma) precoated coverslips 20 hrs before the MHC-I labeling assay. Before staining, cells were washed once with PBS + 2% BSA at room temperature. Cells were then stained with Fc blocker (BD Biosciences #553142, dilution 1:100) for 5 min at room temperature, followed by labeling with PE-conjugated H-2Kb/H-2Db (Miltenyi #130–115-586, dilution 1:100) for 15 min at room temperature. Next, cell-coated coverslips were incubated at 37°C for 30 min (for EEA1) or 90 min (for LAMP1, TfR1 and CD98). After incubation, cells were washed once with room temperature PBS, then fixed with 4% (vol/vol) paraformaldehyde for 10 min at room temperature, followed by washing three times with PBS. For plasma membrane staining, Coverslips were stained overnight at 4 °C with APC anti-mouse CD98 (BioLegend #128212, dilution 1:100) in PBS + 2% BSA. For intracellular staining, cells were permeabilized with 0.1% saponin in PBS + 2% BSA containing Fc blocker for 30 min at room temperature, then stained overnight at 4 °C with anti-mouse EEA1 antibody (Thermo Fisher Scientific # MA5–31575, dilution 1:100), or APC anti-mouse CD107a (LAMP1) (Miltenyi #130–111-505, dilution 1:50), or APC anti-mouse CD71 (Miltenyi #130–119-133, dilution 1:50). To reveal the EEA1 staining, cells were incubated with AF647-conjugated goat anti-mouse IgG (BioLegend #405322, dilution 1:500) for 1 hr. After washing, cells were counterstained with DAPI (Sigma Aldrich # D9542–1MG, 1 μg/ml) and then coverslips were mounted with 10 μl of Immu-Mount™ mountant (Thermo Fisher Scientific) onto slides and examined with a Leica Stellaris 8 confocal microscope using the lightening mode. Surface-derived MHC-I translocation was analyzed using a macro in ImageJ calculating the ratio between H-2Kb/H-2Db fluorescence onto indicated organelles and H-2Kb/H-2Db fluorescence in the cytoplasm (for LAMP1, EEA1, CD71) or of the whole cells (for CD98).
For HLA labeling assays in THP-1 cells, shRNA-transduced cells were washed and stained with Fc blocker (Biolegend #422302, dilution 1:100) for 5 min at room temperature, followed by labeling with PE-conjugated HLA-A2 (Miltenyi # 130–116-493, dilution 1:100) for 15 min at room temperature. Cells were then washed, seeded on poly-L-Lysine (Sigma) precoated coverslips and incubated at 37°C for 90 min. After incubation, cells were washed once with room temperature PBS, then fixed with 4% (vol/vol) paraformaldehyde for 10 min at room temperature, followed by washing three times with PBS. For plasma membrane staining, Coverslips were stained overnight at 4 °C with purified anti-human CD147 (BioLegend #306202, dilution 1:100) in PBS + 2% BSA. For intracellular staining, cells were permeabilized with 0.1% saponin in PBS + 2% BSA containing Fc blocker for 30 min at room temperature, then stained overnight at 4 °C with purified anti-human CD107a (LAMP1) (Thermo Fisher Scientific #14–1079-80, dilution 1:100). To reveal primary antibody staining, cells were incubated with AF647-conjugated goat anti-mouse IgG (BioLegend #405322, dilution 1:500) for 1 hr. After washing, cells were counterstained with DAPI (Sigma Aldrich # D9542–1MG, 1 μg/ml) and then coverslips were mounted with 10 μl of Immu-Mount™ mountant (Thermo Fisher Scientific) onto slides and examined with a Leica Stellaris 8 confocal microscope using the lightening mode. Surface-derived HLA-A2 translocation was analyzed using a macro in ImageJ calculating the ratio between HLA-A2 fluorescence onto indicated organelles and HLA-A2 fluorescence in the cytoplasm (for LAMP1) or of the whole cells (for CD147).
For the split-GFP experiments, sorted SUSD6-GFP1–9, HLA-A2-T10 and TMEM127-T11 transduced 293T and HLA-A2-T10 transduced cells were seeded onto poly-L-Lysine (Sigma) precoated coverslips and cultured overnight. Cells were stained with WGA-CF770 (Biotium # 29059–1, 5ug/ml) at 37 °C for 15min, washed with PBS, then fixed with 4% (vol/vol) paraformaldehyde for 10 min at room temperature. Cells were then permeabilized with 0.15% Triton X-100 and blocked with 2% BSA in PBST (PBS + 0.1% Tween 20). Coverslips were stained overnight at 4 °C with PE-anti-huCD107a (Miltenyi #130–111-621, dilution 1:100) and APC-anti-HA (BioLegend #901523, dilution 1:100) diluted in blocking buffer. After washing, cells were stained with DAPI (Sigma Aldrich # D9542–1MG, 1 μg/ml) for 5 min at room temperature and then coverslips were mounted with 10 μl of Immu-Mount™ mountant (Thermo Fisher Scientific) onto slides. Slides were analyzed on a Leica Stellaris 8 confocal microscope. ImageJ software was used to analyze the data.
RNA extraction and quantitative PCR
Total RNA was extracted from cells using a commercially available RNA extraction Kit (QIAGEN #74136). Reverse transcription was performed with a High-Capacity RT kit (Applied Biosystems #4368813). Quantitative PCR (qPCR) was performed using the CFX96 Touch Real-Time PCR Detection System (Bio-Rad) with a 20 μL reaction, composed of 100 ng of cDNA, 10 μL Power SYBR Green master mix (Applied Biosystems #4309155), and 250 nM of each forward and reverse primer. All SYBR Green primers used in this study are listed in Table S5.
Surface HLA-A2 kinetics assay
To determine the involvement of SUSD6 and WWP2 in the degradation of surface MHC-I molecule, THP-1-Cas9 cells transduced with shRen (control shRNA) versus shSUSD6 or sgRosa (control sgRNA) versus sgWWP2 were treated with 20 μg/ml Cycloheximide (CHX, Sigma #01810) to inhibit protein biosynthesis, 20μM Bafilomycin A1 (BafA1, MCE #HY-100558) to inhibit lysosomal degradation, or 1 μM Epoxomicin (Epox, APExBIo #A2606) to inhibit proteasomal degradation, at 37 °C for 0 h, 3 h, 6 h, 9 h, 12 h, and 24 h. Cells were then stained and checked by flow cytometry by using the following antibodies: human TruStain FcX™ (BioLegend #422302, dilution 1:100), APC/Cy7-HLA-A2 (BioLegend #343310, dilution 1:100) and PerCP/Cy5.5-CD147 (BioLegend #306220, dilution 1:100). DAPI (Sigma Aldrich # D9542–1MG, 1 μg/ml) was used to exclude dead cells. Data were acquired on a BD LSRFortessa™ Cell Analyzer or BD ® LSR II Flow Cytometer and analyzed by the FlowJo software.
MHC-I recycling and storage assay
To measure MHC-I recycling to the plasma membrane, we performed a recycling assay as described.58 Briefly, THP-1 cells with or without knocking down SUSD6 by shRNA were Fc blocked (BioLegend #422302, dilution 1:100) and stained by purified anti-human HLA-A2 Antibody (BioLegend #343302, dilution 1:500) on ice for 30 min. Cells were washed with ice-cold media followed by incubation at 37°C for 30 min to allow the internalization of antibody-bound MHC-I. Surface remaining antibodies were stripped with a low pH buffer (IMDM supplemented with 1x Pen/Strep and 50 mM sodium citrate, pH 3.5). Cells were washed twice in PBS and twice in complete media. Part of the cells were processed immediately after the washing step (t0), and the rest of the cells were incubated in complete media at 37°C for the indicated time (tx) to allow the recycling of MHC-I. To reveal the surface pool of MHC-I, a set of cells was fixed at the end of the incubation and incubated with an APC-conjugated goat anti-mouse IgG (BioLegend #405308, dilution 1:200). To reveal the total residual pool of antibody-labeled MHC-I, another set of cells were fixed and permeabilized with PBS supplemented with 0.1% saponin and 0.1% BSA, followed by incubation with APC-anti-mouse IgG at room temperature for 45 min. Cells were then washed twice with permeabilization buffer and twice with PBS supplemented with 2% BSA, followed by flow cytometry. The level of recycling was calculated as below:
To examine the MHC-I storage post-internalization, we performed an assay modified from a previously described.59 Basically, THP-1 cells were Fc blocked, stained using APC-conjugated anti-HLA-A2 antibody (BioLegend # 343307, dilution 1:200), and allowed for internalization, and the uninternalized antibody was removed by acid stripping as described above. Cells were then resuspended in complete media and incubated at 37°C. Cells were taken out at different time points and chilled on ice immediately to stop the biological processing of the internalized MHC-I (e.g. recycling and degradation). Recycled MHC-I-bound antibodies were removed by acid stripping (in this case, only the internalized and stored MHC-I-bound antibodies would be detected) and cells were washed followed by flow cytometry. The level of recycling was calculated as below:
Western blot
Cas9-expressing THP-1 and RN2 cells were transduced with indicated sgRNA. B16F10-OVA and CT26 cells were transduced with indicated shRNA for silencing the targeted genes. Cells were lysed at 7–10 days post-transduction and the lysates were harvested for western blot using the following antibodies: from Abcam (Waltham, MA): rabbit anti-HLA-A antibody (ab52922, dilution 1:2,000) and rabbit anti-beta 2 Microglobulin antibody (ab75853, dilution 1:2,000); from Invitrogen (Waltham, MA): rabbit anti-KIAA0247 antibody (PA5–56481, 1:200–1:500); from Bethyl Laboratories (Montgomery, TX): rabbit anti-TMEM127 antibody (A303–450A, dilution 1:1,000); from BioLegend (San Diego, CA): Direct-Blot™ HRP anti-β-actin antibody (664804, dilution 1:10,000); and from Enzo Life Sciences (Farmingdale, NY): rat anti-Grp94 antibody (ADI-SPA-850-F, dilution 1:2,000).
For Co-immunoprecipitation samples, the following antibodies were used: from BioLegend (San Diego, CA): Direct-Blot™ HRP anti-V5-tag Antibody (680603, dilution 1:2,000), Direct-Blot™ HRP anti-FLAG Tag Antibody (637311, 1:2,000), Direct-Blot™ HRP anti-HA.11 Epitope Tag Antibody (901519, dilution 1:2,500), Direct-Blot™ HRP anti-β-actin antibody (664804, dilution 1:10,000), and Direct-Blot™ HRP anti-Ubiquitin Antibody (646303, dilution 1:2,000); from Abcam (Waltham, MA): rabbit anti-HLA-A antibody (ab52922, dilution 1:2,000); from Enzo Life Sciences (Farmingdale, NY): rat anti-Grp94 antibody (ADI-SPA-850-F, dilution 1:2,000); and from Proteintech (Rosemont, IL): rabbit anti-PD-L1 antibody (17952–1-AP, dilution 1:500).
Co-immunoprecipitation (Co-IP)
293T cells were lentiviraly transduced to express SUSD6-FLAG, TMEM127-HA, and/or WWP2-V5. 4–7 days post-transduction, cells were treated with 20μM Bafilomycin A1 (MCE #HY-100558) for 6 hours to inhibit lysosomal degradation and subsequently cross-linked by DSP (Sigma #22586) to stabilize transient molecular interactions following the manufacture’s protocol. Cells were then lysed in TBS (20mM Tris-HCl, 150mM NaCl, pH 7.6) supplemented with 2mM CaCl2, 1% digitonin (Sigma #D141), and protease inhibitor cocktail (Roche #11836153001) for 1 hour at 4 °C. Cell lysate was pre-cleared with Protein G beads (Invitrogen #10003D) for 1 hour at 4 °C. Antibody was added to the pre-cleared lysate and incubated at 4 °C for overnight. Protein G beads were then added for immunoprecipitation and incubated at 4 °C for 3 h. Following that, beads were washed three times with lysis buffer and twice with TBS. After boiling the beads in Laemmli Sample Buffer (Bio-rad #1610747) containing 2-Mercaptoethanol (Sigma #M6250), the supernatant was used for the western blot.
For ubiquitin pull-down, THP-1 cells with or without knocking down SUSD6 or knocking out WWP2 were treated with 20μM Bafilomycin A1 (MCE #HY-100558) for 6 hrs and then lysed in TBS (50mM Tris, 150mM NaCl, pH=7.5) supplemented with 1mM EDTA, 1% NP-40, and protease inhibitors cocktail for 1 hr at 4 °C. Cell lysate was pre-cleared with Protein G beads (Invitrogen #10003D) for 1 hr at 4 °C. TUBE2 agarose (LifeSensors #UM402) or control agarose (LifeSensors #UM400) was added to the pre-cleared lysate and incubated at 4 °C for overnight, followed by washing twice with lysis buffer and three times with TBS supplemented with 0.1% Tween-20 and 1mM EDTA. Agarose was boiled in Laemmli Sample Buffer (Bio-rad #1610747) containing 2-Mercaptoethanol (Sigma #M6250), and the supernatant was used for the western blot.
To examine STW complex-mediated ubiquitination of MHC-I (WT or 3KR mutant), 293T cells were co-transfected with FLAG-tagged HLA-A2 (WT or 3KR mutant) with vector control or STW complex (SUSD6-V5, TMEM127-HA, and WWP2-V5). Cells were treated with 20μM Bafilomycin A1 (MCE #HY-100558) for 6 hrs and then lysed in denaturing buffer (50 mM Tris, pH=7.5, 1% SDS, 0.2 % NP-40, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 2 mM MgCl2, 10% glycerol, 0.5 mM DTT, 0.2 mM Sodium orthovanadate (NAV, Sigma #450243), 20 nM Okadaic acid (OA, Sigma #O8010), 10 mM N-Ethylmaleimide (NEM, Sigma #E3876), and Protease inhibitor cocktail (Roche #11836153001)) at 95 °C for 15 min. FLAG-tagged HLA-A2 was pulled down using Anti-FLAG® M2 Magnetic Beads (Sigma #M8823) with rotation at 4 °C for 3 hrs. Beads were washed three times with wash buffer 1 (denaturing buffer without SDS) and three times with wash buffer 2 (denaturing buffer without SDS and glycerol), followed by elution with 3 x FLAG peptide at 0.5 mg/ml. Beads were removed by magnet and the elutes were mixed with Laemmli Sample Buffer (Bio-rad #1610747) containing 2-Mercaptoethanol (Sigma #M6250) and used for western blot.
Split-luciferase assay
293T cells were transfected in a 384-well plate with indicated lgBit- or smBit-tagged HLA-A2, SUSD6, TMEM127, or WWP2 for 18 to 24 h. Media was then changed to Opti-Men (Gibco #31985062), and Nano-Glo® Live Cell Assay System (Promega #N2011) was added to each well following the manufacture’s protocol. Luminescence was detected and quantified using an EnSpire plate reader (PerkinElmer).
Gene Ontology Analyses
Gene Ontology (GO) analyses were performed for genes that were positively or negatively selected in the screen using R package clusterProfiler (3.10.0). Enrichment scores were then plotted with R package ggplot2 (version 3.3.6).
Enrichment Score
Fisher exact test was used to calculate the enrichment scores (odd ratios) of the 5 functional gene sets (defined in Figure 1H plus the putative surface proteins) among the 44-gene set or the 34-gene set using the whole genome as background.
Statistical analysis of non-scRNAseq data
Two-tailed student’s t-test, one-way ANOVA, two-way ANOVA, or Kaplan-Meier were applied for statistical analysis in GraphPad Prism. P values were calculated and reported as follows: ns, not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001. The error bars in each figure represented the standard error of the mean (SEM).
Supplementary Material
Supplementary Figure 1. Genome-wide CRISPR screens of antigen-specific pMHC-I inhibitors in human and mouse cell lines, Related to Figure 1
(A) Antigen presentation machinery (AMP) scores among various cancer types calculated as described.37 Data were derived from the TCGA database.(B and C) Flow-cytometric analyses of the human THP-1-Cas9-AFP-BFP reporter cell line (B) and the mouse RN2-Cas9-OVA-BFP reporter cell line (C) on the levels of antigen expression (denoted by BFP, x-axis) and cell surface antigen-specific pMHC-I (y-axis).(D) Venn diagram of the common positive AP regulators in the human HLA-A2:AFP screen and the mouse H-2Kb:OVA screen.(E) STRING protein-protein interaction (PPI) network of the 105 commonly selected hits defined in (D). The minimum required interaction score was set to 0.4. k-means clustering was applied. Bubble size denoted the combined LFCs in both screens and line thickness denoted the STRING PPI score/confidence.
Supplementary Figure 2. Analyses of in vitro CRISPR screens, Related to Figure 2
(A) Schematic of the pan-HLA screen in Cas9-expressing human THP-1 cells.(B) Venn diagram of the positive regulators in the human antigen-specific pMHC-I screen, the human pan-HLA screen, and the mouse antigen-specific pMHC-I screen. The 21 common positive regulators from the three screens were listed in order of their enrichment scores by human antigen-specific pMHC-I screen.(C and D) Gene Ontology analysis of the 21 common MHC-I positive regulators (C) and the 84 pMHC-I-specific positive regulators, as defined in (B).(E and F) Gene Ontology analysis of the 44 common MHC-I negative regulators (E) and the 34 pMHC-I-specific negative regulators, as defined in Figure 2C.(G) Violin plots comparing the LFCs of the HLA-A2:AFP screen for the candidates from the 44-gene set with those from the 34-gene set. *, p< 0.05 by two-tailed unpaired Student’s t-test.
Figure S7. Targeting TMEM127 for MHC-I modulation, Related to Figure 7
(A-E) Western blot (A, B, and D) and RT-qPCR (C and E) to validate Tmem127 knockout (A) or knockdown (B-E) efficiency in C1498-Cas9 (A), B16F10-OVA (B-C), and CT26 (D-E). For C and E, n=3.(F) Flow cytometry analysis of surface MHC-I in C1498-Cas9 cells (left), B16F10-OVA cells (middle), and CT26 cells (right) transduced with the indicated sgRNAs or shRNAs. (n=3)(G) In vitro growth of C1498-Cas9 cells (left), B16F10-OVA cells (middle) and CT26 cells (right) transduced with the indicated sgRNAs or shRNAs. (for C1498-Cas9-GFP: n=3 for sgNT and n=6 for sgTmem127; for B16F10-OVA: n=6; for CT26: n=3)Data are presented as the mean ± SEM. ns, not significant; and ***, p< 0.001 by two-tailed unpaired Student’s t-test (C, E, and F), or two-way ANOVA for the last time point (G). Renilla-targeting shRNA (shRen) and non-targeting sgRNA (sgNT) were used as controls.
Table S2. STRING analysis for the 78 negative and 105 positive AP regulators. Related to Figures 1 and S1.
Table S3. Raw and analyzed data for the in vivo CRISPR screen. Related to Figure 2.
Table S4. sgRNAs and shRNAs used in this study. Related to STAR Methods.
Table S5. RT-qPCR primers used in this study. Related to STAR Methods.
Supplementary Figure 3. SUSD6 potently inhibits MHC-I expression in AML, Related to Figure 3
(A) Schematic of the topological domains of human SUSD6.(B) Representative flow cytometry surface staining of KASUMI-1 cells transduced with Vector control, FLAG-SUSD6 (FLAG tag fused to the extracellular N-terminal of SUSD6), and SUSD6-FLAG (FLAG tag fused to the intracellular C-terminal of SUSD6) by an anti-FLAG antibody.(C and D) Western blots of SUSD6 protein levels from the whole cell lysates of THP-1-Cas9-AFP-BFP (C) and RN2-Cas9-OVA-BFP (D) cells transduced with the indicated sgRNAs.(E) Flow cytometric analyses of surface HLA-ABC (left) and HLA-A2 (right) levels rescued by overexpressing the SUSD6 coding sequence (CDS) in SUSD6-deficient Cas9-expressing THP-1 cells. (n=3)(F and G) Flow cytometric analyses of surface HLA-ABC and HLA-Bw6 levels in Cas9-expressing MV4–11 (F) and KASUMI-1 (G) cells transduced with the indicated sgRNAs. (n=3 for sgRosa, n=6 for sgSUSD6)(H) Flow cytometric analyses of surface HLA-A2:AFP (left) and HLA-ABC (right) levels in THP-1-Cas9-AFP-BFP cells transduced with the indicated sgRNAs with or without IFN-γ treatment (0.5 ng/ml). (n=6)(I) Western blots of Susd6 protein levels of the whole cell lysates from C1498-Cas9-GFP cells transduced with the indicated sgRNAs (Blue arrow, Susd6 band; orange arrow, non-specific band).(J) Flow cytometric analysis of surface H-2Kb/H-2Db levels in C1498-Cas9-GFP cells transduced with the indicated sgRNAs. (n=3)(K) In vitro cell growth of C1498-Cas9-GFP transduced with the indicated sgRNAs as described in Figure 3K. (n=8)Data are presented as the mean ± SEM. ns, not significant; ***, p< 0.001 by one-way ANOVA (E), two-tailed unpaired Student’s t-test (F, G, and J), two-way ANOVA (H), or two-way ANOVA for the last time point (K). Rosa26-targeting sgRNA (sgRosa, for human) and non-targeting sgRNA (sgNT, for mouse) were used as controls.
Supplementary Figure 4. SUSD6 as a cancer-associated MHC-I inhibitor, Related to Figure 4
(A) SUSD6 mRNA levels in normal human tissues (grey) and immune subsets (blue) from BioGPS database.71,72(B) SUSD6 mRNA levels in human cancers (red) and the corresponding normal tissues (green). CHOL, Cholangiocarcinoma; GBM, Glioblastoma multiforme; LAML, Acute Myeloid Leukemia; LGG, Brain Lower Grade Glioma; PAAD, Pancreatic adenocarcinoma. Data were obtained from the TCGA database and analyzed by GEPIA2.69(C) Flow cytometry analyses of surface H-2Kb/H-2Db and H-2Kd/H-2Dd in Cas9-expressing tumor cell lines transduced with the indicated sgRNAs. (n=3)(D) Schematic of the mouse models for in vivo studies to validate the function of Susd6 using syngeneic solid tumor cell lines.(E-H) Representative western blots (E and G) and RT-qPCR (F and H) analyses of Susd6 expression levels in B16F10-OVA cells (E-F) or CT26 cells (G-H) transduced with the indicated shRNAs. (for F, n=3; and for H, n=4)(I and J) Flow cytometric analyses of surface H-2Kb/H-2Db levels in B16F10-OVA cells (I) and surface H-2Kd/H-2Dd levels in CT26 cells (J) transduced with the indicated shRNAs. (n=3)(K and L) In vitro cell growth of B16F10-OVA (K) and CT26 (L) transduced with the indicated shRNAs. (n=4)(M) Flow cytometry analyses of surface H-2Kb/H-2Db in B16F10-OVA cells transduced with indicated shRNAs. (n=3)(N) In vitro cell growth of B16F10-OVA transduced with the indicated shRNAs. (n=3)(O and P) Quantification of the tumor volumes (O) and Kaplan-Meier survival curves (P) of mice transplanted with B16F10-OVA cells transduced with the indicated shRNAs. (n=5 for shRen_shRen and shSusd6_shRen, n=4 for shSusd6_shB2m_1 and shSusd6_shB2m_2)(Q) Flow cytometry analyses of surface H-2Kb/H-2Db in C1498-Cas9-GFP cells transduced with the indicated sgRNAs and shRNAs. (n=3)(R) In vitro cell growth of C1498-Cas9-GFP transduced with the indicated sgRNAs and shRNAs. (n=4)(S and T) Quantification of the tumor volumes (S) and Kaplan-Meier survival curves (T) of mice transplanted with C1498-Cas9-GFP cells transduced with the indicated sgRNAs and shRNAs. (n=4)(U) Bar plot showing the percentages of shRNA-transduced B16F10-OVA cells killed by the OT-I T cells. (n=3)(V) Bar plot showing the surface levels of H-2Kb/H-2Db in RN2-Cas9-OVA-BFP cells transduced with the indicated sgRNAs and shRNAs. (n=3)(W) Bar plot showing the percentages of sgRNA/shRNA-transduced RN2-Cas9-OVA-BFP cells killed by the OT-I T cells. (n=3)Data are presented as the mean ± SEM (A, C, F, H-O, Q-S, and U-W) or box and whiskers with all data points (B). ns, not significant; *, p< 0.05; **, p< 0.01; and ***, p< 0.001 by two-tailed unpaired Student’s t-test (C, F, H, I, and J), two-way ANOVA for the last time point (K, L, N, O, R, and S), one-way ANOVA (M, Q, and U-W), or Log-rank Mantel-Cox test (P and T). Renilla-targeting shRNA (shRen) and non-targeting sgRNA (sgNT) were used as controls.
Figure S5. SUSD6 regulates MHC-I through post-translational mechanisms, Related to Figure 5
(A) RT-qPCR analysis of genes involved in antigen processing and presentation upon SUSD6 knockout in THP-1 cells. (n=3)(B-C) Representative dot plots (B) and quantifications (C) showing the OP-Puro-labeled (as an indicator of active translation) THP-1 cells transduced with the indicated sgRNAs by flow cytometry. (n=3 for sgRosa, n=6 for sgSUSD6)(D) Schematic of the pathways involved in the regulation of surface MHC-I expression. Modified from Sebastian et al, 2018.57(E) Representative histogram (left) and bar plot (right) showing the surface levels of H-2Kb/H-2Db in mouse MutuDC cells transduced with the indicated shRNAs. (n=4)(F and G) Representative confocal images (top) showing the subcellular localization of the surface-derived MHC-I and quantifications (bottom) of the surface-derived MHC-I translocated into early endosomes (F) and recycling endosomes (G) relative to the internalized MHC-I in MutuDC cells transduced with indicated shRNAs. At least 100 cells were quantified in each group. All scale bars: 5 μm.(H and I) Schematics of the experimental design for flow-cytometry-based pMHC-I storage (H) and recycling (I) assays. The equations for calculating the storage efficiency and recycling efficiency were listed in the box of each schematic.Data are presented as the mean ± SEM (A, C, and E) or box and whiskers with all data points (F and G). ns, not significant; ***, p<0.001 by two-tailed unpaired Student’s t-test (A, C and E) or Mann-Whitney test (F and G). Rosa26-targeting sgRNA (sgRosa) and Renilla-targeting shRNA (shRen) were used as controls.
Figure S6. MHC-I downregulation through STW-mediated ubiquitination, Related to Figure 6
(A and B) mRNA co-expression analysis of TMEM127 (A) and WWP2 (B) with SUSD6 in COAD and LIHC tumors. COAD, Colon adenocarcinoma; LIHC; Liver hepatocellular carcinoma. Data were obtained from the TCGA database and analyzed by GEPIA2.69(C) Immunoprecipitation using anti-FLAG or anti-HA antibodies in 293T cells transduced with SUSD6-FLAG and TMEM127-HA. Grp94 (an ER lumen protein), PD-L1 (a membrane protein), and β-actin (a cytosolic protein) served as negative controls.(D) Schematics (left) of the split-luciferase experiments and bar plots showing the normalized relative luminescence units (RLU) (right) in 293T cells co-transfected with the indicated small NanoBit (smBit)- or large NanoBit (lgBit)- tagged proteins (n=6).(E and F) Detection of GFP signal in 293T cells transduced with the indicated genes and treated with or without BafA1 by flow cytometry. Representative flow cytometry plots were shown in (E) and the quantification was shown in (F). (n=3)(G) Flow cytometry analysis of surface HLA-A2 in THP-1-Cas9 cells transduced with the indicated sgRNAs. (n=7).(H) Representative images (top) and quantification (bottom) of the Western blot analysis of total HLA-A in THP-1-Cas9 cells transduced with the indicated sgRNAs (the expression values from sgWWP2 was normalized to sgRosa). (n=4)(I-K) Time course studies of the surface HLA-A2 expression on THP-1-Cas9 cells transduced with the indicated sgRNAs and treated with Cycloheximide (CHX, I), Bafilomycin A1 (BafA1, J), or Epoxomicin (Epox, K). (n=4)(L) Schematics of the split-luciferase experiments to demonstrate the interaction between WWP2 and SUSD6 or TMEM127 in transfected 293T cells.(M) Schematics of the split-luciferase experiments to demonstrate the interaction between WWP2 and HLA-A2 in transfected 293T cells with or without SUSD6 and/or TMEM127 overexpression.(N and O) Immunoprecipitation using Tandem Ubiquitin Binding Entities 2 (TUBE2) agarose or Control (Ctrl) agarose in THP-1 cells with or without SUSD6 knockdown (N) or WWP2 knockout (O). Red right brackets; poly-ubiquitinated HLA-A. (P) Flow cytometry analysis of the surface HLA-A2 expression in THP-1-Cas9 cells transduced with the indicated sgRNA. (n=3)(Q) Immunoprecipitation using anti-FLAG antibody in 293T cells transduced with SUSD6-FLAG and STUB1-V5 (blue words), or TMEM127-FLAG and STUB1-V5 (orange words). Data are presented as the mean ± SEM. ns, not significant; *, p< 0.05; and ***, p< 0.001 by one-way ANOVA (D and P), two-way ANOVA (F), two-tailed unpaired Student’s t-test (G and H), or two-way ANOVA for the last time point (I-K). In C, N, O, and Q: IP, immunoprecipitation; IB, immunoblotting; ISO, isotype. Rosa26-targeting sgRNA (sgRosa) and Renilla-targeting shRNA (shRen) were used as controls.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-HLA-A*02:01:AFP158–166, Clone ET1402L1 | A gift from Eureka Therapeutics38 | |
| Rabbit anti-KIAA0247 antibody | Invitrogen | Cat# PA5–56481; RRID: AB_2642999 |
| Rabbit anti-TMEM127 antibody | Bethyl Laboratories | Cat# A303–450A; RRID: AB_10952702 |
| Rabbit anti-HLA-A antibody, Clone EP1395Y | Abcam | Cat# ab52922; RRID: AB_881225 |
| Rabbit anti-beta 2 Microglobulin antibody, Clone EP2978Y | Abcam | Cat# ab75853; RRID: AB_1523204 |
| ANTI-FLAG antibody, Clone M2 | Sigma | Cat# F3165; RRID: AB_259529 |
| Direct-Blot™ HRP anti-β-actin Antibody, Clone W16197A |
BioLegend | Cat# 664804; RRID: AB_2728496 |
| Direct-Blot™ HRP anti-V5-tag Antibody, Clone 7/4 | BioLegend | Cat# 680603; RRID: AB_2572008 |
| Direct-Blot™ HRP anti-FLAG Tag Antibody, Clone L5 | BioLegend | Cat# 637311; RRID: AB_2566706 |
| Direct-Blot™ HRP anti-HA.11 Epitope Tag Antibody, Clone 16B12 | BioLegend | Cat# 901519; RRID: AB_2686981 |
| Direct-Blot™ HRP anti-Ubiquitin Antibody, Clone P4D1 | BioLegend | Cat# 646303; RRID: AB_2629597 |
| Rat anti-Grp94 antibody, Clone 9G10 | Enzo Life Sciences | Cat# ADI-SPA-850-F; RRID: AB_11179746 |
| Rabbit anti-PD-L1 antibody | Proteintech | Cat# 17952–1-AP; RRID: AB_10597552 |
| Rat anti-mouse CD8β, Clone Lyt 3.2 | BioXCell | Cat# BE0223; RRID: AB_2687706 |
| Rat IgG1 isotype control, anti-horseradish peroxidase | BioXCell | Cat# BE0088; RRID: AB_1107775 |
| Human TruStain FcX™ | BioLegend | Cat# 422302; RRID: AB_2818986 |
| TruStain FcX™ (anti-mouse CD16/32) | BioLegend | Cat# 101320; RRID: AB_1574975 |
| Purified Rat Anti-Mouse CD16/CD32 (Mouse BD Fc Block™) | BD Biosciences | Cat# 553142; RRID: AB_394657 |
| APC anti-human HLA-A,B,C, Clone W6/32 | BioLegend | Cat# 311410; RRID: AB_314879 |
| PE anti-human HLA-A,B,C, Clone W6/32 | BioLegend | Cat # 311406; RRID: AB_314875 |
| APC anti-mouse H-2Kb bound to SIINFEKL, Clone 25-D1.16 |
BioLegend | Cat # 141606; RRID: AB_11219595 |
| PerCP/Cyanine5.5 anti-human CD147, Clone HIM6 |
BioLegend | Cat # 306220; RRID: AB_2750176 |
| APC/Cyanine7 anti-human HLA-A2, Clone BB7.2 |
BioLegend | Cat# 343310; RRID: AB_2561568 |
| PE anti-mouse H-2Kb, Clone AF6–88.5 | BioLegend | Cat# 116508; RRID: AB_313735 |
| Brilliant Violet 605 hamster anti-mouse CD3ε, Clone 145–2C11 | BD Biosciences | Cat# 563004; RRID: AB_2737945 |
| PE anti-mouse CD45R/B220, Clone RA3–6B2 | BD Biosciences | Cat# 553090; RRID: AB_394620 |
| PerCP/Cyanine5.5 anti-mouse NK-1.1, Clone PK136 | BioLegend | Cat# 108728; RRID: AB_2132705 |
| PE/Cyanine7 anti-mouse CD4, Clone GK1.5 | BioLegend | Cat# 100422; RRID: AB_312707 |
| CD8a Monoclonal Antibody (53–6.7), APC, eBioscience, Clone 53–6.7 | Thermo Fisher Scientific | Cat# 17–0081-82; RRID: AB_469335 |
| APC/Cyanine7 anti-mouse CD45.2, Clone 104 | BioLegend | Cat# 109824; RRID: AB_830789 |
| PE/Cyanine7 anti-mouse H-2Kb/H-2Db, Clone 28–8-6 | BioLegend | Cat# 114616; RRID: AB_2750196 |
| APC anti-mouse CD98 (4F2), Clone RL388 | BioLegend | Cat# 128212; RRID: AB_2750545 |
| Purified anti-human CD147, Clone HIM6 | BioLegend | Cat# 306202; RRID: AB_314586 |
| Purified anti-human HLA-A2, Clone BB7.2 | BioLegend | Cat# 343302; RRID: AB_1659243 |
| APC Goat anti-mouse IgG (minimal x-reactivity) | BioLegend | Cat# 405308; RRID: AB_315011 |
| Alexa Fluor(R) 647 Goat anti-mouse IgG (minimal x-reactivity) | BioLegend | Cat# 405322; RRID: AB_2563045 |
| APC anti-HA.11 Epitope Tag, Clone 16B12 |
BioLegend | Cat# 901523; RRID: AB_2734657 |
| CD107a (LAMP-1) Antibody, anti-human, PE, REAfinity™ | Miltenyi Biotec | Cat# 130–111-621; RRID: AB_2654474 |
| APC anti-human HLA-A2 Antibody, Clone BB7.2 | BioLegend | Cat# 343307; RRID: AB_2561566 |
| EEA1 Monoclonal Antibody, Clone GT10811 | Thermo Fisher Scientific | Cat# MA5–31575; RRID: AB_2787202 |
| CD71 Antibody, anti-mouse, APC, REAfinity™, Clone REA627 | Miltenyi Biotec | Cat# 130–119-133; RRID: AB_2751633 |
| H-2Kb/H-2Db Antibody, anti-mouse, PE, REAfinity™, Clone REA932 | Miltenyi Biotec | Cat# 130–115-586; RRID: AB_2727102 |
| CD107a (LAMP-1) Antibody, anti-mouse, APC, REAfinity™, Clone REA777 | Miltenyi Biotec | Cat# 130–111-505; RRID: AB_2654465 |
| CD107a (LAMP-1) Monoclonal Antibody (eBioH4A3), eBioscience™, Clone H4A3 | Thermo Fisher Scientific | Cat# 14–1079-80; RRID: AB_467426 |
| HLA-A2 Antibody, anti-human, PE, REAfinity™, Clone REA517 | Miltenyi Biotec | Cat# 130–116-493; RRID: AB_2727568 |
| Bacterial and virus strains | ||
| MegaX DH10B Electrocomp Cells | Life Technologies | Cat# C640003 |
| One Shot™ Stbl3™ Chemically Competent E. coli | Invitrogen | Cat# C737303 |
| Biological samples | ||
| Chemicals, peptides, and recombinant proteins | ||
| Cycloheximide | Sigma | Cat# 01810 |
| Bafilomycin A1 | MCE | Cat# HY-100558 |
| Epoxomicin | APExBIo | Cat# A2606 |
| cOmplete™, Mini Protease Inhibitor Cocktail | Roche | Cat# 11836153001 |
| DSP (dithiobis(succinimidyl propionate)), Lomant’s Reagent | Thermo Scientific | Cat# 22586 |
| Digitonin | Sigma | Cat# D141 |
| 4x Laemmli Sample Buffer | Bio-rad | Cat# 1610747 |
| 2-Mercaptoethanol | Sigma | Cat# M6250 |
| Lipofectamine™ 3000 Transfection Reagent | Invitrogen | Cat# L3000008 |
| Dynabeads™ Protein G for Immunoprecipitation | Invitrogen | Cat# 10003D |
| DAPI | Sigma Aldrich | Cat# D9542–1MG |
| Puromycin | Sigma Aldrich | Cat# P7255–25MG |
| Polyethylenimine (PEI) | Polysciences, Inc | Cat# 23966 |
| Polybrene transfection reagent | Fisher Scientific | Cat# TR1003G |
| Dimethyl sulfoxide (DMSO) | Fisher | Cat# D128–500 |
| Poly-L-lysine solution | Sigma Aldrich | Cat# P8920 |
| SlowFade Glass Soft-set antifade mountant | Invitrogen | Cat# S36917 |
| Recombinant Human IL-2 | PeproTech | Cat# 200–02 |
| Recombinant Murine IL-2 | PeproTech | Cat# 212–12 |
| MEM Non-essential Amino Acid Solution (100×) | Sigma Aldrich | Cat# M7145–100ML |
| Sodium Pyruvate (100 mM) | Gibco | Cat# 11360070 |
| Dynabeads™ Human T-Activator CD3/CD28 for T Cell Expansion and Activation | Gibco | Cat# 11161D |
| Blasticidin | InvivoGen | Cat# ant-bl-05 |
| TUBE2 agarose | LifeSensors | Cat# UM402 |
| Control agarose | LifeSensors | Cat# UM400 |
| Anti-FLAG® M2 Magnetic Beads | Sigma Aldrich | Cat# M8823 |
| Q5® High-Fidelity DNA Polymerase | NEB | Cat# M0491S |
| CF770-conjugated Wheat Germ Agglutinin (WGA) | Biotium | Cat# 29059–1 |
| TaKaRa Ex Taq® DNA Polymerase | TakaraBio | Cat# RR001A |
| Histopaque®−1077 | Sigma | Cat# 10771 |
| AccuCheck Counting Beads | Invitrogen | Cat# PCB100 |
| Sodium orthovanadate | Sigma | Cat# 450243 |
| Okadaic acid ammonium salt from Prorocentrum concavum | Sigma | Cat# O8010 |
| N-Ethylmaleimide | Sigma | Cat# E3876 |
| Opti-MEM™ I Reduced Serum Medium | Gibco | Cat# 31985062 |
| AMPure XP beads | Beckman Coulter | Cat# A63880 |
| Azacitidine | Selleckchem | Cat# S1782 |
| Liberase™ TM Research Grade | Sigma Aldrich | Cat# 5401119001 |
| DNase I, RNase-free | Invitrogen | Cat# EN0521 |
| Doxycycline | Takara Bio | Cat# 631311 |
| doxycycline-containing diets (1 g/kg) | inotiv | Cat# TD.05298 |
| Critical commercial assays | ||
| RNeasy Plus Mini Kit | QIAGEN | Cat# 74136 |
| High-Capacity cDNA Reverse Transcription Kit | Applied Biosystems | Cat# 4368813 |
| SYBR Green PCR Master Mix | Applied Biosystems | Cat# 4309155 |
| Nano-Glo® Live Cell Assay System | Promega | Cat# N2011 |
| QIAamp DNA Mini Kit | Qiagen | Cat# 51304 |
| Rneasy Plus Mini Kit | Qiagen | Cat# 74136 |
| 2x Phusion Master Mix | Thermo Scientific | Cat# F-548 |
| Dneasy Blood & Tissue Kit of Qiagen | QIAGEN | Cat# 69504 |
| Ex-Taq DNA Polymerase | Takara Bio | Cat# RR001B |
| eBioscience™ Intracellular Fixation & Permeabilization Buffer Set | Invitrogen | Cat# 88–8824-00 |
| Mouse IL-2 ELISA Set | BD Biosciences | Cat# 555148 |
| LookOut Mycoplasma PCR Detection Kit | Sigma Aldrich | Cat# MP0040A-1KT |
| Click-iT™ Plus OPP Alexa Fluor™ 647 Protein Synthesis Assay Kit | Invitrogen | Cat# C10458 |
| Deposited data | ||
| Experimental models: cell lines | ||
| Human: THP-1 | ATCC | Cat# TIB-202; RRID: CVCL_0006 |
| Human: MV4–11 | ATCC | Cat# CRL-9591; RRID: CVCL_0064 |
| Human: KASUMI-1 | ATCC | Cat# CRL-2724; RRID: CVCL_0589 |
| Human: 293T | ATCC | Cat# CRL-3216 RRID: CVCL_0063 |
| Mouse: MLL-AF9 NrasG12 (RN2) Cas9 | A gift from Dr. Chris Vakoc | N/A |
| Mouse: CT26 | ATCC | Cat# CRL-2638 RRID: CVCL_7256 |
| Mouse: B16F10-OVA | A gift from Dr. Hongbo Chi | N/A |
| Mouse: C1498 | ATCC | Cat# TIB-49 RRID: CVCL_3494 |
| Mouse: MC38 | Kerafast | Cat# ENH204-FP RRID: CVCL_B288 |
| Mouse: KPC | A gift from Dr. Alec Kimmelman | N/A |
| Mouse: CMT167 | ECACC | Cat# 10032302 RRID: CVCL_2405 |
| Mouse: EMT6 | ATCC | Cat# CRL-2755 RRID: CVCL_1923 |
| Mouse: MutuDC1940 | Applied Biological Materials | Cat# T0528 |
| Mouse: B3Z T cell hybridoma | A gift from Dr. Peter Cresswell | RRID: CVCL_6277 |
| Experimental models: organisms/strains | ||
| Mouse: C57BL/6J | Jackson Laboratory | Cat# 000664 |
| Mouse: BALB/cJ | Jackson Laboratory | Cat# 000651 |
| Mouse: Cas9-GFP | Jackson Laboratory | Cat# 024858 |
| Mouse: OT-I | Jackson Laboratory | Cat# 003831 |
| Mouse: Rag2/Il2rg Double Knockout | Taconic | Cat# 4111-F |
| Oligonucleotides | ||
| sgRNA and shRNA sequences, see Table S4 | This paper | N/A |
| Real time-PCR primer sequences, see Table S5 | This paper | N/A |
| Human pooled genome-wide sgRNA library (Brunello) sequences | Addgene | Addgene: 73179 |
| Mouse pooled genome-wide sgRNA library (Brie) sequences | Addgene | Addgene: 73632 |
| Recombinant DNA | ||
| pCDH-EF1s | N/A | Addgene: 72484 |
| psPAX2 | N/A | Addgene: 12260 |
| pVSVG | N/A | Addgene: 12259 |
| LRG2.1-GFP sgRNA vector | N/A | Addgene: 108098 |
| LRG2.1-TagBFP2 sgRNA vector | N/A | Addgene: 124773 |
| LRCherry2.1 sgRNA vector | N/A | Addgene: 108099 |
| LT3GEPIR shRNA vector | N/A | Addgene: 111177 |
| MSCV-Cas9–2A-Blast | Chen et al., 201940 | N/A |
| pLenti-SUSD6-GFP(1–9)-V5 | This paper | N/A |
| pLenti-HLA-A2-T10-HA | This paper | N/A |
| pLenti-TMEM127-T11-FLAG | This paper | N/A |
| pLenti-hSUSD6-FLAG-IRES-Puro-T2A-GFP | This paper | N/A |
| pLenti-mCherry-T2A-V5-hSUSD6 | This paper | N/A |
| pLenti-hTMEM127-FLAG-IRES-Puro-T2A-GFP | This paper | N/A |
| pLenti-mCherry-T2A-hTMEM127-HA | This paper | N/A |
| pLenti-tagBFP-T2A-hWWP2-V5 | This paper | N/A |
| pLenti-tagBFP-T2A-hSTUB1-V5 | This paper | N/A |
| pLenti-hSUSD6-lgBit | This paper | N/A |
| pLenti-hSUSD6-smBit | This paper | N/A |
| pLenti-hTMEM127-lgBit | This paper | N/A |
| pLenti-hTMEM127-smBit | This paper | N/A |
| pLenti-lgBit-hTMEM127 | This paper | N/A |
| pLenti-smBit-hTMEM127 | This paper | N/A |
| pLenti-HLA-A2-lgBit | This paper | N/A |
| pLenti-HLA-A2-smBit | This paper | N/A |
| pLenti-CD86-smBit | This paper | N/A |
| pLenti-HaloTag-smBit | This paper | N/A |
| pLenti-WWP2-lgBit | This paper | N/A |
| pLenti-smURFP-T2A-FLAG-HLA-A2 (WT) | This paper | N/A |
| pLenti-smURFP-T2A-FLAG-HLA-A2 (3KR) | This paper | N/A |
| SREP shRNA vector | This paper | N/A |
| Software and algorithms | ||
| ImageJ | Schneider et al., 201273 | https://imagej.nih.gov/ij/ |
| GraphPad Prism 9 version 9.3.1 | GraphPad Software | https://www.graphpad.com/ |
| R package ggplot2 version 3.3.6 | R package | https://cran.r-project.org/web/packages/ggplot2/index.html |
| R package clusterProfiler version 3.10.0 | R package | https://bioconductor.org/packages/release/bioc/html/clusterProfiler.html |
| FlowJo version 10.8 | FlowJo | https://www.flowjo.com/ |
| Cytoscape version 3.7.2 | Shannon et al., 200374 | https://cytoscape.org/ |
| Other | ||
| BD LSR FORTESSA | N/A | N/A |
| SY3200 cell sorter | N/A | N/A |
| BD LSR II Flow Cytometer | N/A | N/A |
| Illumina HiSeq 4000 | N/A | N/A |
Highlights:
Specific peptide-MHC-I-guided CRISPR screens reveal key MHC-I regulatory networks
SUSD6 inhibits MHC-I surface expression and T-cell activity in AML and solid tumors
The SUSD6/TMEM127/WWP2 axis induces MHC-I ubiquitination and lysosomal degradation
Targeting the membrane-associated STW complex promotes anti-cancer immunity
Acknowledgments
We thank all members of the Wang and Aifantis laboratories for helpful discussions and technical assistance. A. Heguy and the NYU Genome Technology Center (supported in part by NCI grant P30CA016087-30) for expertise with sequencing experiments, the NYU Flow Cytometry facility for expert cell sorting, and the Applied Bioinformatics Laboratory for computational assistance. This work is supported by Mark Foundation ASPIRE Award to J.W. I.A. is supported by NIH (NCI and NHLBI) (R01CA216421, R01 CA173636, 1R01CA228135, R01CA242020, 1R01HL159175), Vogelstein Foundation, and the Evans MDS Foundation. J.W. is supported by the NIH (R01CA269898, R37CA273333-01, R21AI163924-02), V foundation, Mark Foundation, and Melanoma Research Alliance. X.C. is supported by Leukemia Research Foundation New Investigator Research Grant and the American Society of Hematology Scholar Award. Q.L. is supported by Cancer Research Institute Irvington Postdoctoral Fellowship.
Footnotes
Declaration of Interests
J.W., I.A., X.C., and Q.L. are named inventors on a patent application related to this study. J.W. is on the Scientific Advisory Board of RootPath Inc., and is a consultant for Bristol Myers Squibb (Relatlimab Advisory Council). D.S. is currently an employee at Rubius Therapeutics. Other authors declare no conflict of interests.
Inclusion and Diversity
We support inclusive, diverse, and equitable conduct of research.
References
- 1.Sharpe AH, and Pauken KE (2018). The diverse functions of the PD1 inhibitory pathway. Nature Reviews Immunology 18, 153–167. 10.1038/nri.2017.108. [DOI] [PubMed] [Google Scholar]
- 2.Sanmamed MF, and Chen L. (2018). A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell 175, 313–326. 10.1016/j.cell.2018.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J, Yuan R, Song W, Sun J, Liu D, and Li Z. (2017). PD-1, PD-L1 (B7-H1) and Tumor-Site Immune Modulation Therapy: The Historical Perspective. Journal of Hematology & Oncology 10, 34. 10.1186/s13045-017-0403-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jhunjhunwala S, Hammer C, and Delamarre L. (2021). Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat Rev Cancer 21, 298–312. 10.1038/s41568-021-00339-z. [DOI] [PubMed] [Google Scholar]
- 5.Dhatchinamoorthy K, Colbert JD, and Rock KL (2021). Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front Immunol 12, 636568. 10.3389/fimmu.2021.636568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peaper DR, and Cresswell P. (2008). Regulation of MHC class I assembly and peptide binding. Annu Rev Cell Dev Biol 24, 343–368. 10.1146/annurev.cellbio.24.110707.175347. [DOI] [PubMed] [Google Scholar]
- 7.Dockree T, Holland CJ, Clement M, Ladell K, McLaren JE, van den Berg HA, Gostick E, K LM, Llewellyn-Lacey S, Bridgeman JS, et al. (2017). CD8(+) T-cell specificity is compromised at a defined MHCI/CD8 affinity threshold. Immunol Cell Biol 95, 68–76. 10.1038/icb.2016.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, Grasso CS, Hugo W, Sandoval S, Torrejon DY, et al. (2017). Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov 7, 188–201. 10.1158/2159-8290.CD-16-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nowicki TS, Hu-Lieskovan S, and Ribas A. (2018). Mechanisms of Resistance to PD-1 and PD-L1 Blockade. Cancer J 24, 47–53. 10.1097/PPO.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gettinger S, Choi J, Hastings K, Truini A, Datar I, Sowell R, Wurtz A, Dong W, Cai G, Melnick MA, et al. (2017). Impaired HLA Class I Antigen Processing and Presentation as a Mechanism of Acquired Resistance to Immune Checkpoint Inhibitors in Lung Cancer. Cancer Discov 7, 1420–1435. 10.1158/2159-8290.CD-17-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rooney MS, Shukla SA, Wu CJ, Getz G, and Hacohen N. (2015). Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 160, 48–61. 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shukla SA, Rooney MS, Rajasagi M, Tiao G, Dixon PM, Lawrence MS, Stevens J, Lane WJ, Dellagatta JL, Steelman S, et al. (2015). Comprehensive analysis of cancer-associated somatic mutations in class I HLA genes. Nat Biotechnol 33, 1152–1158. 10.1038/nbt.3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maeurer MJ, Gollin SM, Martin D, Swaney W, Bryant J, Castelli C, Robbins P, Parmiani G, Storkus WJ, and Lotze MT (1996). Tumor escape from immune recognition: lethal recurrent melanoma in a patient associated with downregulation of the peptide transporter protein TAP-1 and loss of expression of the immunodominant MART-1/Melan-A antigen. J Clin Invest 98, 1633–1641. 10.1172/JCI118958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ling A, Lofgren-Burstrom A, Larsson P, Li X, Wikberg ML, Oberg A, Stenling R, Edin S, and Palmqvist R. (2017). TAP1 down-regulation elicits immune escape and poor prognosis in colorectal cancer. Oncoimmunology 6, e1356143. 10.1080/2162402X.2017.1356143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cromme FV, Airey J, Heemels MT, Ploegh HL, Keating PJ, Stern PL, Meijer CJ, and Walboomers JM (1994). Loss of transporter protein, encoded by the TAP-1 gene, is highly correlated with loss of HLA expression in cervical carcinomas. J Exp Med 179, 335–340. 10.1084/jem.179.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, Avezov E, Li J, Kollmann K, Kent DG, et al. (2013). Somatic CALR Mutations in Myeloproliferative Neoplasms with Nonmutated JAK2. New England Journal of Medicine 369, 2391–2405. 10.1056/NEJMoa1312542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arshad N, and Cresswell P. (2018). Tumor-associated calreticulin variants functionally compromise the peptide loading complex and impair its recruitment of MHC-I. J Biol Chem 293, 9555–9569. 10.1074/jbc.RA118.002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shionoya Y, Kanaseki T, Miyamoto S, Tokita S, Hongo A, Kikuchi Y, Kochin V, Watanabe K, Horibe R, Saijo H, et al. (2017). Loss of tapasin in human lung and colon cancer cells and escape from tumor-associated antigen-specific CTL recognition. Oncoimmunology 6, e1274476. 10.1080/2162402X.2016.1274476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sokol L, Koelzer VH, Rau TT, Karamitopoulou E, Zlobec I, and Lugli A. (2015). Loss of tapasin correlates with diminished CD8(+) T-cell immunity and prognosis in colorectal cancer. J Transl Med 13, 279. 10.1186/s12967-015-0647-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshihama S, Roszik J, Downs I, Meissner TB, Vijayan S, Chapuy B, Sidiq T, Shipp MA, Lizee GA, and Kobayashi KS (2016). NLRC5/MHC class I transactivator is a target for immune evasion in cancer. Proc Natl Acad Sci U S A 113, 5999–6004. 10.1073/pnas.1602069113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kriegsman BA, Vangala P, Chen BJ, Meraner P, Brass AL, Garber M, and Rock KL (2019). Frequent Loss of IRF2 in Cancers Leads to Immune Evasion through Decreased MHC Class I Antigen Presentation and Increased PD-L1 Expression. J Immunol 203, 1999–2010. 10.4049/jimmunol.1900475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroder K, Hertzog PJ, Ravasi T, and Hume DA (2004). Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol 75, 163–189. 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 23.Arshad N, Laurent-Rolle M, Ahmed WS, Hsu JC-C, Mitchell SM, Pawlak J, Sengupta D, Biswas KH, and Cresswell P. (2022). SARS-CoV-2 accessory proteins ORF7a and ORF3a use distinct mechanisms to downregulate MHC-I surface expression. bioRxiv, 2022.2005.2017.492198. 10.1101/2022.05.17.492198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sgourakis NG, May NA, Boyd LF, Ying J, Bax A, and Margulies DH (2015). A Novel MHC-I Surface Targeted for Binding by the MCMV m06 Immunoevasin Revealed by Solution NMR. J Biol Chem 290, 28857–28868. 10.1074/jbc.M115.689661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zuo J, Currin A, Griffin BD, Shannon-Lowe C, Thomas WA, Ressing ME, Wiertz EJ, and Rowe M. (2009). The Epstein-Barr virus G-protein-coupled receptor contributes to immune evasion by targeting MHC class I molecules for degradation. PLoS Pathog 5, e1000255. 10.1371/journal.ppat.1000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto K, Venida A, Yano J, Biancur DE, Kakiuchi M, Gupta S, Sohn ASW, Mukhopadhyay S, Lin EY, Parker SJ, et al. (2020). Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature 581, 100–105. 10.1038/s41586-020-2229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Bao X, Hu M, Chang H, Jiao M, Cheng J, Xie L, Huang Q, Li F, and Li CY (2020). Inhibition of PCSK9 potentiates immune checkpoint therapy for cancer. Nature 588, 693–698. 10.1038/s41586-020-2911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dersh D, Phelan JD, Gumina ME, Wang B, Arbuckle JH, Holly J, Kishton RJ, Markowitz TE, Seedhom MO, Fridlyand N, et al. (2021). Genome-wide Screens Identify Lineage- and Tumor-Specific Genes Modulating MHC-I- and MHC-II-Restricted Immunosurveillance of Human Lymphomas. Immunity 54, 116–131 e110. 10.1016/j.immuni.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu SS, Zhang W, Wang X, Jiang P, Traugh N, Li Z, Meyer C, Stewig B, Xie Y, Bu X, et al. (2021). Therapeutically Increasing MHC-I Expression Potentiates Immune Checkpoint Blockade. Cancer Discov 11, 1524–1541. 10.1158/2159-8290.CD-20-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burr ML, Sparbier CE, Chan KL, Chan YC, Kersbergen A, Lam EYN, Azidis-Yates E, Vassiliadis D, Bell CC, Gilan O, et al. (2019). An Evolutionarily Conserved Function of Polycomb Silences the MHC Class I Antigen Presentation Pathway and Enables Immune Evasion in Cancer. Cancer Cell 36, 385–401 e388. 10.1016/j.ccell.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheper W, Kelderman S, Fanchi LF, Linnemann C, Bendle G, de Rooij MAJ, Hirt C, Mezzadra R, Slagter M, Dijkstra K, et al. (2019). Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers. Nat Med 25, 89–94. 10.1038/s41591-018-0266-5. [DOI] [PubMed] [Google Scholar]
- 32.Jimbu L, Mesaros O, Popescu C, Neaga A, Berceanu I, Dima D, Gaman M, and Zdrenghea M. (2021). Is There a Place for PD-1-PD-L Blockade in Acute Myeloid Leukemia? Pharmaceuticals (Basel) 14. 10.3390/ph14040288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flores SK, Deng Y, Cheng Z, Zhang X, Tao S, Saliba A, Chu I, Burnichon N, Gimenez-Roqueplo AP, Wang E, et al. (2020). Functional Characterization of TMEM127 Variants Reveals Novel Insights into Its Membrane Topology and Trafficking. J Clin Endocrinol Metab 105, e3142–3156. 10.1210/clinem/dgaa396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin Y, Yao L, King EE, Buddavarapu K, Lenci RE, Chocron ES, Lechleiter JD, Sass M, Aronin N, Schiavi F, et al. (2010). Germline mutations in TMEM127 confer susceptibility to pheochromocytoma. Nat Genet 42, 229–233. 10.1038/ng.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qin Y, Deng Y, Ricketts CJ, Srikantan S, Wang E, Maher ER, and Dahia PL (2014). The tumor susceptibility gene TMEM127 is mutated in renal cell carcinomas and modulates endolysosomal function. Hum Mol Genet 23, 2428–2439. 10.1093/hmg/ddt638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alix E, Godlee C, Cerny O, Blundell S, Tocci R, Matthews S, Liu M, Pruneda JN, Swatek KN, Komander D, et al. (2020). The Tumour Suppressor TMEM127 Is a Nedd4-Family E3 Ligase Adaptor Required by Salmonella SteD to Ubiquitinate and Degrade MHC Class II Molecules. Cell Host Microbe 28, 54–68 e57. 10.1016/j.chom.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang S, He Z, Wang X, Li H, and Liu XS (2019). Antigen presentation and tumor immunogenicity in cancer immunotherapy response prediction. Elife 8. 10.7554/eLife.49020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu H, Xu Y, Xiang J, Long L, Green S, Yang Z, Zimdahl B, Lu J, Cheng N, Horan LH, et al. (2017). Targeting Alpha-Fetoprotein (AFP)-MHC Complex with CAR T-Cell Therapy for Liver Cancer. Clin Cancer Res 23, 478–488. 10.1158/1078-0432.CCR-16-1203. [DOI] [PubMed] [Google Scholar]
- 39.Zuber J, McJunkin K, Fellmann C, Dow LE, Taylor MJ, Hannon GJ, and Lowe SW (2011). Toolkit for evaluating genes required for proliferation and survival using tetracycline-regulated RNAi. Nat Biotechnol 29, 79–83. 10.1038/nbt.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen X, Glytsou C, Zhou H, Narang S, Reyna DE, Lopez A, Sakellaropoulos T, Gong Y, Kloetgen A, Yap YS, et al. (2019). Targeting Mitochondrial Structure Sensitizes Acute Myeloid Leukemia to Venetoclax Treatment. Cancer Discov 9, 890–909. 10.1158/2159-8290.CD-19-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doench JG, Fusi N, Sullender M, Hegde M, Vaimberg EW, Donovan KF, Smith I, Tothova Z, Wilen C, Orchard R, et al. (2016). Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat Biotechnol 34, 184–191. 10.1038/nbt.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caron E, Vincent K, Fortier MH, Laverdure JP, Bramoulle A, Hardy MP, Voisin G, Roux PP, Lemieux S, Thibault P, and Perreault C. (2011). The MHC I immunopeptidome conveys to the cell surface an integrative view of cellular regulation. Mol Syst Biol 7, 533. 10.1038/msb.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prota G, Lechuga-Vieco AV, and De Libero G. (2022). Mitochondrial Proteins as Source of Cancer Neoantigens. Int J Mol Sci 23. 10.3390/ijms23052627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mardis ER (2019). Neoantigens and genome instability: impact on immunogenomic phenotypes and immunotherapy response. Genome Med 11, 71. 10.1186/s13073-019-0684-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lei X, Lin H, Wang J, Ou Z, Ruan Y, Sadagopan A, Chen W, Xie S, Chen B, Li Q, et al. (2022). Mitochondrial fission induces immunoescape in solid tumors through decreasing MHC-I surface expression. Nature Communications 13, 3882. 10.1038/s41467-022-31417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas C, and Tampe R. (2021). MHC I assembly and peptide editing - chaperones, clients, and molecular plasticity in immunity. Curr Opin Immunol 70, 48–56. 10.1016/j.coi.2021.02.004. [DOI] [PubMed] [Google Scholar]
- 47.Jongsma MLM, de Waard AA, Raaben M, Zhang T, Cabukusta B, Platzer R, Blomen VA, Xagara A, Verkerk T, Bliss S, et al. (2021). The SPPL3-defined glycosphingolipid repertoire orchestrates HLA class I-mediated immune responses. Immunity 54, 387. 10.1016/j.immuni.2021.01.016. [DOI] [PubMed] [Google Scholar]
- 48.Mopin A, Driss V, and Brinster C. (2016). A Detailed Protocol for Characterizing the Murine C1498 Cell Line and its Associated Leukemia Mouse Model. J Vis Exp. 10.3791/54270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu Q, Grotzke JE, and Cresswell P. (2018). A novel probe to assess cytosolic entry of exogenous proteins. Nat Commun 9, 3104. 10.1038/s41467-018-05556-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karttunen J, and Shastri N. (1991). Measurement of ligand-induced activation in single viable T cells using the lacZ reporter gene. Proc Natl Acad Sci U S A 88, 3972–3976. 10.1073/pnas.88.9.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bagger FO, Sasivarevic D, Sohi SH, Laursen LG, Pundhir S, Sonderby CK, Winther O, Rapin N, and Porse BT (2016). BloodSpot: a database of gene expression profiles and transcriptional programs for healthy and malignant haematopoiesis. Nucleic Acids Res 44, D917–924. 10.1093/nar/gkv1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stolzel F, Mohr B, Kramer M, Oelschlagel U, Bochtler T, Berdel WE, Kaufmann M, Baldus CD, Schafer-Eckart K, Stuhlmann R, et al. (2016). Karyotype complexity and prognosis in acute myeloid leukemia. Blood Cancer J 6, e386. 10.1038/bcj.2015.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lasry A, Nadorp B, Fornerod M, Nicolet D, Wu H, Walker CJ, Sun Z, Witkowski MT, Tikhonova AN, Guillamot-Ruano M, et al. (2023). An inflammatory state remodels the immune microenvironment and improves risk stratification in acute myeloid leukemia. Nat Cancer 4, 27–42. 10.1038/s43018-022-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lima A, and Rodriguez TA (2021). MHC-I presents: tumor surveillance in the epithelia by cell competition. Nat Immunol 22, 1358–1360. 10.1038/s41590-021-01053-6. [DOI] [PubMed] [Google Scholar]
- 55.Dersh D, Holly J, and Yewdell JW (2021). A few good peptides: MHC class I-based cancer immunosurveillance and immunoevasion. Nat Rev Immunol 21, 116–128. 10.1038/s41577-020-0390-6. [DOI] [PubMed] [Google Scholar]
- 56.Dubrot J, Lane-Reticker SK, Kessler EA, Ayer A, Mishra G, Wolfe CH, Zimmer MD, Du PP, Mahapatra A, Ockerman KM, et al. (2021). In vivo screens using a selective CRISPR antigen removal lentiviral vector system reveal immune dependencies in renal cell carcinoma. Immunity 54, 571–585 e576. 10.1016/j.immuni.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 57.Montealegre S, and van Endert PM (2018). Endocytic Recycling of MHC Class I Molecules in Non-professional Antigen Presenting and Dendritic Cells. Front Immunol 9, 3098. 10.3389/fimmu.2018.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weigert R, Yeung AC, Li J, and Donaldson JG (2004). Rab22a regulates the recycling of membrane proteins internalized independently of clathrin. Mol Biol Cell 15, 3758–3770. 10.1091/mbc.e04-04-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cebrian I, Croce C, Guerrero NA, Blanchard N, and Mayorga LS (2016). Rab22a controls MHC-I intracellular trafficking and antigen cross-presentation by dendritic cells. EMBO Rep 17, 1753–1765. 10.15252/embr.201642358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dixon AS, Schwinn MK, Hall MP, Zimmerman K, Otto P, Lubben TH, Butler BL, Binkowski BF, Machleidt T, Kirkland TA, et al. (2016). NanoLuc Complementation Reporter Optimized for Accurate Measurement of Protein Interactions in Cells. ACS Chem Biol 11, 400–408. 10.1021/acschembio.5b00753. [DOI] [PubMed] [Google Scholar]
- 61.Foglieni C, Papin S, Salvade A, Afroz T, Pinton S, Pedrioli G, Ulrich G, Polymenidou M, and Paganetti P. (2017). Split GFP technologies to structurally characterize and quantify functional biomolecular interactions of FTD-related proteins. Sci Rep 7, 14013. 10.1038/s41598-017-14459-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cabantous S, Nguyen HB, Pedelacq JD, Koraichi F, Chaudhary A, Ganguly K, Lockard MA, Favre G, Terwilliger TC, and Waldo GS (2013). A new protein-protein interaction sensor based on tripartite split-GFP association. Sci Rep 3, 2854. 10.1038/srep02854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burr ML, van den Boomen DJ, Bye H, Antrobus R, Wiertz EJ, and Lehner PJ (2013). MHC class I molecules are preferentially ubiquitinated on endoplasmic reticulum luminal residues during HRD1 ubiquitin E3 ligase-mediated dislocation. Proc Natl Acad Sci U S A 110, 14290–14295. 10.1073/pnas.1303380110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ng S, Lim S, Sim ACN, Mangadu R, Lau A, Zhang C, Martinez SB, Chandramohan A, Lim UM, Ho SSW, et al. (2022). STUB1 is an intracellular checkpoint for interferon gamma sensing. Sci Rep 12, 14087. 10.1038/s41598-022-18404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu YY, Myers NB, Hilbert CM, Harris MR, Balendiran GK, and Hansen TH (1999). Definition and transfer of a serological epitope specific for peptide-empty forms of MHC class I. Int Immunol 11, 1897–1906. 10.1093/intimm/11.12.1897. [DOI] [PubMed] [Google Scholar]
- 66.Ljunggren HG, Stam NJ, Ohlen C, Neefjes JJ, Hoglund P, Heemels MT, Bastin J, Schumacher TN, Townsend A, Karre K, and et al. (1990). Empty MHC class I molecules come out in the cold. Nature 346, 476–480. 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- 67.Sanchez-Paulete AR, Teijeira A, Cueto FJ, Garasa S, Perez-Gracia JL, Sanchez-Arraez A, Sancho D, and Melero I. (2017). Antigen cross-presentation and T-cell cross-priming in cancer immunology and immunotherapy. Ann Oncol 28, xii44–xii55. 10.1093/annonc/mdx237. [DOI] [PubMed] [Google Scholar]
- 68.Huang CJ, Yang SH, Huang SM, Lin CM, Chien CC, Chen YC, Lee CL, Wu HH, and Chang CC (2011). A predicted protein, KIAA0247, is a cell cycle modulator in colorectal cancer cells under 5-FU treatment. J Transl Med 9, 82. 10.1186/1479-5876-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang Z, Kang B, Li C, Chen T, and Zhang Z. (2019). GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res 47, W556–W560. 10.1093/nar/gkz430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dwivedi B, Mumme H, Satpathy S, Bhasin SS, and Bhasin M. (2022). Survival Genie, a web platform for survival analysis across pediatric and adult cancers. Sci Rep 12, 3069. 10.1038/s41598-022-06841-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu C, Macleod I, and Su AI (2013). BioGPS and MyGene.info: organizing online, gene-centric information. Nucleic Acids Res 41, D561–565. 10.1093/nar/gks1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, et al. (2004). A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci U S A 101, 6062–6067. 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schneider CA, Rasband WS, and Eliceiri KW (2012). NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9, 671–675. 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, and Ideker T. (2003). Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13, 2498–2504. 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fellmann C, Hoffmann T, Sridhar V, Hopfgartner B, Muhar M, Roth M, Lai DY, Barbosa IA, Kwon JS, Guan Y, et al. (2013). An optimized microRNA backbone for effective single-copy RNAi. Cell Rep 5, 1704–1713. 10.1016/j.celrep.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 76.Liu J, Xu Y, Stoleru D, and Salic A. (2012). Imaging protein synthesis in cells and tissues with an alkyne analog of puromycin. Proc Natl Acad Sci U S A 109, 413–418. 10.1073/pnas.1111561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Genome-wide CRISPR screens of antigen-specific pMHC-I inhibitors in human and mouse cell lines, Related to Figure 1
(A) Antigen presentation machinery (AMP) scores among various cancer types calculated as described.37 Data were derived from the TCGA database.(B and C) Flow-cytometric analyses of the human THP-1-Cas9-AFP-BFP reporter cell line (B) and the mouse RN2-Cas9-OVA-BFP reporter cell line (C) on the levels of antigen expression (denoted by BFP, x-axis) and cell surface antigen-specific pMHC-I (y-axis).(D) Venn diagram of the common positive AP regulators in the human HLA-A2:AFP screen and the mouse H-2Kb:OVA screen.(E) STRING protein-protein interaction (PPI) network of the 105 commonly selected hits defined in (D). The minimum required interaction score was set to 0.4. k-means clustering was applied. Bubble size denoted the combined LFCs in both screens and line thickness denoted the STRING PPI score/confidence.
Supplementary Figure 2. Analyses of in vitro CRISPR screens, Related to Figure 2
(A) Schematic of the pan-HLA screen in Cas9-expressing human THP-1 cells.(B) Venn diagram of the positive regulators in the human antigen-specific pMHC-I screen, the human pan-HLA screen, and the mouse antigen-specific pMHC-I screen. The 21 common positive regulators from the three screens were listed in order of their enrichment scores by human antigen-specific pMHC-I screen.(C and D) Gene Ontology analysis of the 21 common MHC-I positive regulators (C) and the 84 pMHC-I-specific positive regulators, as defined in (B).(E and F) Gene Ontology analysis of the 44 common MHC-I negative regulators (E) and the 34 pMHC-I-specific negative regulators, as defined in Figure 2C.(G) Violin plots comparing the LFCs of the HLA-A2:AFP screen for the candidates from the 44-gene set with those from the 34-gene set. *, p< 0.05 by two-tailed unpaired Student’s t-test.
Figure S7. Targeting TMEM127 for MHC-I modulation, Related to Figure 7
(A-E) Western blot (A, B, and D) and RT-qPCR (C and E) to validate Tmem127 knockout (A) or knockdown (B-E) efficiency in C1498-Cas9 (A), B16F10-OVA (B-C), and CT26 (D-E). For C and E, n=3.(F) Flow cytometry analysis of surface MHC-I in C1498-Cas9 cells (left), B16F10-OVA cells (middle), and CT26 cells (right) transduced with the indicated sgRNAs or shRNAs. (n=3)(G) In vitro growth of C1498-Cas9 cells (left), B16F10-OVA cells (middle) and CT26 cells (right) transduced with the indicated sgRNAs or shRNAs. (for C1498-Cas9-GFP: n=3 for sgNT and n=6 for sgTmem127; for B16F10-OVA: n=6; for CT26: n=3)Data are presented as the mean ± SEM. ns, not significant; and ***, p< 0.001 by two-tailed unpaired Student’s t-test (C, E, and F), or two-way ANOVA for the last time point (G). Renilla-targeting shRNA (shRen) and non-targeting sgRNA (sgNT) were used as controls.
Table S2. STRING analysis for the 78 negative and 105 positive AP regulators. Related to Figures 1 and S1.
Table S3. Raw and analyzed data for the in vivo CRISPR screen. Related to Figure 2.
Table S4. sgRNAs and shRNAs used in this study. Related to STAR Methods.
Table S5. RT-qPCR primers used in this study. Related to STAR Methods.
Supplementary Figure 3. SUSD6 potently inhibits MHC-I expression in AML, Related to Figure 3
(A) Schematic of the topological domains of human SUSD6.(B) Representative flow cytometry surface staining of KASUMI-1 cells transduced with Vector control, FLAG-SUSD6 (FLAG tag fused to the extracellular N-terminal of SUSD6), and SUSD6-FLAG (FLAG tag fused to the intracellular C-terminal of SUSD6) by an anti-FLAG antibody.(C and D) Western blots of SUSD6 protein levels from the whole cell lysates of THP-1-Cas9-AFP-BFP (C) and RN2-Cas9-OVA-BFP (D) cells transduced with the indicated sgRNAs.(E) Flow cytometric analyses of surface HLA-ABC (left) and HLA-A2 (right) levels rescued by overexpressing the SUSD6 coding sequence (CDS) in SUSD6-deficient Cas9-expressing THP-1 cells. (n=3)(F and G) Flow cytometric analyses of surface HLA-ABC and HLA-Bw6 levels in Cas9-expressing MV4–11 (F) and KASUMI-1 (G) cells transduced with the indicated sgRNAs. (n=3 for sgRosa, n=6 for sgSUSD6)(H) Flow cytometric analyses of surface HLA-A2:AFP (left) and HLA-ABC (right) levels in THP-1-Cas9-AFP-BFP cells transduced with the indicated sgRNAs with or without IFN-γ treatment (0.5 ng/ml). (n=6)(I) Western blots of Susd6 protein levels of the whole cell lysates from C1498-Cas9-GFP cells transduced with the indicated sgRNAs (Blue arrow, Susd6 band; orange arrow, non-specific band).(J) Flow cytometric analysis of surface H-2Kb/H-2Db levels in C1498-Cas9-GFP cells transduced with the indicated sgRNAs. (n=3)(K) In vitro cell growth of C1498-Cas9-GFP transduced with the indicated sgRNAs as described in Figure 3K. (n=8)Data are presented as the mean ± SEM. ns, not significant; ***, p< 0.001 by one-way ANOVA (E), two-tailed unpaired Student’s t-test (F, G, and J), two-way ANOVA (H), or two-way ANOVA for the last time point (K). Rosa26-targeting sgRNA (sgRosa, for human) and non-targeting sgRNA (sgNT, for mouse) were used as controls.
Supplementary Figure 4. SUSD6 as a cancer-associated MHC-I inhibitor, Related to Figure 4
(A) SUSD6 mRNA levels in normal human tissues (grey) and immune subsets (blue) from BioGPS database.71,72(B) SUSD6 mRNA levels in human cancers (red) and the corresponding normal tissues (green). CHOL, Cholangiocarcinoma; GBM, Glioblastoma multiforme; LAML, Acute Myeloid Leukemia; LGG, Brain Lower Grade Glioma; PAAD, Pancreatic adenocarcinoma. Data were obtained from the TCGA database and analyzed by GEPIA2.69(C) Flow cytometry analyses of surface H-2Kb/H-2Db and H-2Kd/H-2Dd in Cas9-expressing tumor cell lines transduced with the indicated sgRNAs. (n=3)(D) Schematic of the mouse models for in vivo studies to validate the function of Susd6 using syngeneic solid tumor cell lines.(E-H) Representative western blots (E and G) and RT-qPCR (F and H) analyses of Susd6 expression levels in B16F10-OVA cells (E-F) or CT26 cells (G-H) transduced with the indicated shRNAs. (for F, n=3; and for H, n=4)(I and J) Flow cytometric analyses of surface H-2Kb/H-2Db levels in B16F10-OVA cells (I) and surface H-2Kd/H-2Dd levels in CT26 cells (J) transduced with the indicated shRNAs. (n=3)(K and L) In vitro cell growth of B16F10-OVA (K) and CT26 (L) transduced with the indicated shRNAs. (n=4)(M) Flow cytometry analyses of surface H-2Kb/H-2Db in B16F10-OVA cells transduced with indicated shRNAs. (n=3)(N) In vitro cell growth of B16F10-OVA transduced with the indicated shRNAs. (n=3)(O and P) Quantification of the tumor volumes (O) and Kaplan-Meier survival curves (P) of mice transplanted with B16F10-OVA cells transduced with the indicated shRNAs. (n=5 for shRen_shRen and shSusd6_shRen, n=4 for shSusd6_shB2m_1 and shSusd6_shB2m_2)(Q) Flow cytometry analyses of surface H-2Kb/H-2Db in C1498-Cas9-GFP cells transduced with the indicated sgRNAs and shRNAs. (n=3)(R) In vitro cell growth of C1498-Cas9-GFP transduced with the indicated sgRNAs and shRNAs. (n=4)(S and T) Quantification of the tumor volumes (S) and Kaplan-Meier survival curves (T) of mice transplanted with C1498-Cas9-GFP cells transduced with the indicated sgRNAs and shRNAs. (n=4)(U) Bar plot showing the percentages of shRNA-transduced B16F10-OVA cells killed by the OT-I T cells. (n=3)(V) Bar plot showing the surface levels of H-2Kb/H-2Db in RN2-Cas9-OVA-BFP cells transduced with the indicated sgRNAs and shRNAs. (n=3)(W) Bar plot showing the percentages of sgRNA/shRNA-transduced RN2-Cas9-OVA-BFP cells killed by the OT-I T cells. (n=3)Data are presented as the mean ± SEM (A, C, F, H-O, Q-S, and U-W) or box and whiskers with all data points (B). ns, not significant; *, p< 0.05; **, p< 0.01; and ***, p< 0.001 by two-tailed unpaired Student’s t-test (C, F, H, I, and J), two-way ANOVA for the last time point (K, L, N, O, R, and S), one-way ANOVA (M, Q, and U-W), or Log-rank Mantel-Cox test (P and T). Renilla-targeting shRNA (shRen) and non-targeting sgRNA (sgNT) were used as controls.
Figure S5. SUSD6 regulates MHC-I through post-translational mechanisms, Related to Figure 5
(A) RT-qPCR analysis of genes involved in antigen processing and presentation upon SUSD6 knockout in THP-1 cells. (n=3)(B-C) Representative dot plots (B) and quantifications (C) showing the OP-Puro-labeled (as an indicator of active translation) THP-1 cells transduced with the indicated sgRNAs by flow cytometry. (n=3 for sgRosa, n=6 for sgSUSD6)(D) Schematic of the pathways involved in the regulation of surface MHC-I expression. Modified from Sebastian et al, 2018.57(E) Representative histogram (left) and bar plot (right) showing the surface levels of H-2Kb/H-2Db in mouse MutuDC cells transduced with the indicated shRNAs. (n=4)(F and G) Representative confocal images (top) showing the subcellular localization of the surface-derived MHC-I and quantifications (bottom) of the surface-derived MHC-I translocated into early endosomes (F) and recycling endosomes (G) relative to the internalized MHC-I in MutuDC cells transduced with indicated shRNAs. At least 100 cells were quantified in each group. All scale bars: 5 μm.(H and I) Schematics of the experimental design for flow-cytometry-based pMHC-I storage (H) and recycling (I) assays. The equations for calculating the storage efficiency and recycling efficiency were listed in the box of each schematic.Data are presented as the mean ± SEM (A, C, and E) or box and whiskers with all data points (F and G). ns, not significant; ***, p<0.001 by two-tailed unpaired Student’s t-test (A, C and E) or Mann-Whitney test (F and G). Rosa26-targeting sgRNA (sgRosa) and Renilla-targeting shRNA (shRen) were used as controls.
Figure S6. MHC-I downregulation through STW-mediated ubiquitination, Related to Figure 6
(A and B) mRNA co-expression analysis of TMEM127 (A) and WWP2 (B) with SUSD6 in COAD and LIHC tumors. COAD, Colon adenocarcinoma; LIHC; Liver hepatocellular carcinoma. Data were obtained from the TCGA database and analyzed by GEPIA2.69(C) Immunoprecipitation using anti-FLAG or anti-HA antibodies in 293T cells transduced with SUSD6-FLAG and TMEM127-HA. Grp94 (an ER lumen protein), PD-L1 (a membrane protein), and β-actin (a cytosolic protein) served as negative controls.(D) Schematics (left) of the split-luciferase experiments and bar plots showing the normalized relative luminescence units (RLU) (right) in 293T cells co-transfected with the indicated small NanoBit (smBit)- or large NanoBit (lgBit)- tagged proteins (n=6).(E and F) Detection of GFP signal in 293T cells transduced with the indicated genes and treated with or without BafA1 by flow cytometry. Representative flow cytometry plots were shown in (E) and the quantification was shown in (F). (n=3)(G) Flow cytometry analysis of surface HLA-A2 in THP-1-Cas9 cells transduced with the indicated sgRNAs. (n=7).(H) Representative images (top) and quantification (bottom) of the Western blot analysis of total HLA-A in THP-1-Cas9 cells transduced with the indicated sgRNAs (the expression values from sgWWP2 was normalized to sgRosa). (n=4)(I-K) Time course studies of the surface HLA-A2 expression on THP-1-Cas9 cells transduced with the indicated sgRNAs and treated with Cycloheximide (CHX, I), Bafilomycin A1 (BafA1, J), or Epoxomicin (Epox, K). (n=4)(L) Schematics of the split-luciferase experiments to demonstrate the interaction between WWP2 and SUSD6 or TMEM127 in transfected 293T cells.(M) Schematics of the split-luciferase experiments to demonstrate the interaction between WWP2 and HLA-A2 in transfected 293T cells with or without SUSD6 and/or TMEM127 overexpression.(N and O) Immunoprecipitation using Tandem Ubiquitin Binding Entities 2 (TUBE2) agarose or Control (Ctrl) agarose in THP-1 cells with or without SUSD6 knockdown (N) or WWP2 knockout (O). Red right brackets; poly-ubiquitinated HLA-A. (P) Flow cytometry analysis of the surface HLA-A2 expression in THP-1-Cas9 cells transduced with the indicated sgRNA. (n=3)(Q) Immunoprecipitation using anti-FLAG antibody in 293T cells transduced with SUSD6-FLAG and STUB1-V5 (blue words), or TMEM127-FLAG and STUB1-V5 (orange words). Data are presented as the mean ± SEM. ns, not significant; *, p< 0.05; and ***, p< 0.001 by one-way ANOVA (D and P), two-way ANOVA (F), two-tailed unpaired Student’s t-test (G and H), or two-way ANOVA for the last time point (I-K). In C, N, O, and Q: IP, immunoprecipitation; IB, immunoblotting; ISO, isotype. Rosa26-targeting sgRNA (sgRosa) and Renilla-targeting shRNA (shRen) were used as controls.
Data Availability Statement
This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.