Abstract
Objective
Previous research has shown that pulse pressure (PP) has a significant role in the start and development of type 2 diabetes mellitus. However, there is little proof that PP and pre-diabetes mellitus (Pre-DM) are related. Our study aimed to investigate the relationship between PP and incident pre-DM in a substantial cohort of Chinese participants.
Design
The ‘DATADRYAD’ database (www.Datadryad.org) was used to retrieve the data for this secondary retrospective cohort analysis.
Participants
Data from 182 672 Chinese individuals who participated in the medical examination programme were recorded in this retrospective cohort study between 2010 and 2016 across 32 sites and 11 cities in China.
Setting
PP assessed at baseline and incident pre-DM during follow-up were the target-independent and dependent variables. The association between PP and pre-DM was investigated using Cox proportional hazards regression.
Primary outcome measures
The outcome was incident pre-DM. Impaired fasting glucose levels (fasting blood glucose between 5.6 and 6.9 mmol/L) were used to define pre-DM.
Results
After controlling for confounding variables, PP was positively correlated with incident pre-DM among Chinese adults (HR 1.009, 95% CI 1.007 to 1.010). Additionally, at a PP inflection point of 29 mm Hg, a non-linear connection between the PP and incident pre-DM was discovered. Increased PP was an independent risk factor for developing pre-DM when PP was greater than 29 mm Hg. However, their association was not significant when PP was less than 29 mm Hg. According to subgroup analyses, females, never-smokers and non-obesity correlated more significantly with PP and pre-DM.
Conclusion
We discovered that higher PP independently correlated with pre-DM risk in this study of Chinese participants. The connection between PP and incident pre-DM was also non-linear. High PP levels were related to a higher risk of pre-DM when PP was above 29 mm Hg.
Article focus
Our study investigated the relationship between PP and incident pre-DM in a secondary retrospective cohort of Chinese participants.
Keywords: blood pressure, China, general diabetes
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Our research leveraged a substantial sample size, drawing participants from multiple centres, thus ensuring a robust representation of the Chinese population.
We elucidated a non-linear relationship, marking the pioneering effort to pinpoint the inflection point of Pulse pressure (PP’s) impact on pre-diabetes mellitus (pre-DM).
Our subgroup analysis allowed us to delve into other potential risk factors within the PP and incident pre-DM association.
Our study did not incorporate a 2-hour oral glucose tolerance test or glycosylated haemoglobin level measurements, which could potentially lead to an underestimation of the incidence of pre-DM.
Introduction
Pre-diabetes mellitus (pre-DM) is intermediate hyperglycaemia below the diagnostic cut-off for type 2 DM (T2DM). Patients with pre-DM have been reported to have a higher risk for cardiovascular disease (CVD) and nephropathy, indicating that impaired glucose begins to have a pathogenic effect at this early stage of diabetes.1 The prevalence of pre-DM is increasing globally with an ageing population, urbanisation and changing lifestyles. From 2008 to 2017, the prevalence of pre-DM in China has climbed from 15.5%2 to 35.2%,3 creating a significant public health burden. Approximately 70% of subjects with pre-DM will eventually get T2DM.4 Numerous studies looked for ways to pinpoint the causes of diabetes and pre-DM to prevent and cure the disease in its earliest stages.
Pulse pressure (PP) is referred to as the difference between systolic blood pressure (SBP) and diastolic blood pressure (DBP). Clinically, PP is the manifestation of atherosclerosis.5 Compared with their non-diabetic contemporaries, people with T2DM have greater atherosclerosis, which results in a broad PP.5 A greater risk of CVD exists in patients with T2DM.6 In addition, an increase in PP is another risk factor for CVD incidence and positively correlates with mortality.7–9 Additionally, compared with healthy individuals, patients with pre-DM have a greater burden from coronary atherosclerosis.10 Notably, the atherosclerosis burden began to develop even before T2DM’s clinical symptoms.10 PP was found to be a significant risk factor for T2DM in a recent retrospective cohort investigation of a sizeable sample.11 However, whether pre-DM in Chinese adults is associated with PP is unknown. This study sought to analyse the precise correlation between PP and the likelihood of developing pre-DM in Chinese participants.
Methods
Data source
Researchers can obtain original research data for free via the Dryad Digital Repository. The Dryad data repository’s data on 211 833 Chinese persons were downloaded.12 The current research employed openly available data from a medical examination programme as a secondary inquiry. Researchers may use the data for secondary analysis in accordance with the Dryad terms of service without interfering with the interests of the authors.
Study population
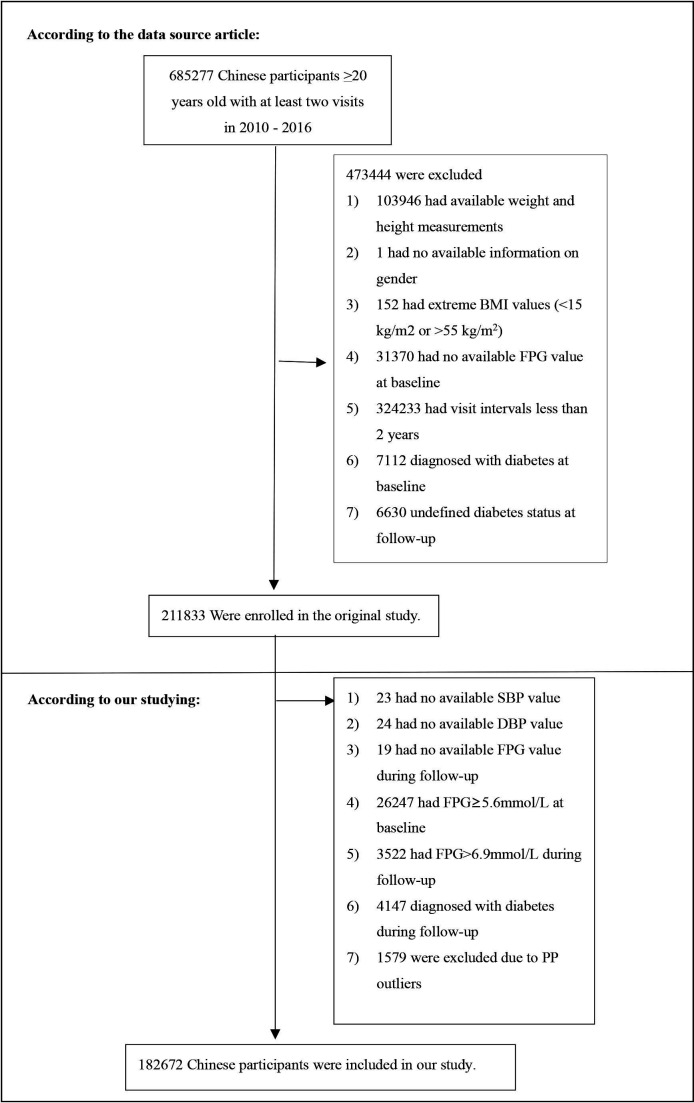
Individuals were excluded from the investigation under these conditions: (1) diabetes at baseline; (2) diabetes or not defined diabetes status at follow-up; (3) abnormal body mass index (BMI) values (BMI over 55 or less than 15 kg/m²); (4) lacking data on baseline fasting plasma glucose (FPG), FPG at follow-up, height, DBP, gender, weight and SBP; (5) FPG>6.9 mmol/L during follow-up and FPG≥5.6 mmol/L at baseline; (6) follow-up interval <2 years and (7) PP outliers (3 SD above or below the mean). Finally, 182 672 subjects eventually entered the study. The study’s design and participant flow are shown in figure 1.
Figure 1.
Study population. BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; SBP, systolic blood pressure.
Data collection
Trained staff members gathered and compiled all of the data. Data from laboratory inspections were gathered in the original study under uniform conditions using standardised handling procedures. The skilled personnel, including height, blood pressure, body weight and age, gathered demographic information. Professional trainees without light clothing and shoes measure individuals for weight and height. Weight/height2 (kg/m2) was used to compute BMI. Trained staff members took blood pressure using a standard mercury sphygmomanometer. A Beckman 5800 autoanalyser was used to measure laboratory data, such as low-density lipoprotein cholesterol (LDL-C), FPG, total cholesterol (TC), aspartate aminotransferase (AST), blood urea nitrogen (BUN), triglyceride (TG), alanine aminotransferase (ALT), serum creatinine (Scr) and high-density lipoprotein cholesterol (HDL-C). PP was calculated as the difference between SBP and DBP.
Diagnosis of pre-DM
Impaired fasting glucose levels (FPG between 5.6 and 6.9 mmol/L) were used to define pre-DM.13
Patient and public involvement
Given this was a secondary retrospective cohort study, no patient was involved in the study.
Statistical analysis
R software V.3.4.3 and EmpowerStats (R) V.4.0 were used for all statistical analyses.
We initially assessed the baseline data distribution by categorising it into quartiles based on the PP (Q1≤36; 36<Q2≤43; 43<Q3 ≤50; 50<Q4). Continuous data were reported as medians with IQRs (25th–75th percentile) or means with SD, while categorical data were expressed as frequencies and percentages. The Kruskal-Wallis H test, χ2 test and one-way analysis of variance were employed to assess disparities between PP groups. The cumulative incidence and terms person-year were used to represent incidence rates.14 Comparisons of survival and cumulative event rates were done using the Kaplan-Meier method. Using the log-rank test, we also examined the Kaplan-Meier HRs of unfavourable events.15
There were 133 257 (72.32%), 4240 (2.30%), 1541 (0.84%), 4209 (2.28%), 107 684 (58.44%), 82 879 (44.98%), 18 563 (10.07%), 83 382 (45.25%), 9759 (5.30%) and 133 257 (72.32%) individuals with missing data for smoking status, TG, ALT, TC, AST, LDL-C, BUN, HDL-C, SCr and drinking status, respectively. The present study employed multiple imputations to handle the missing data of covariants. The imputation model included smoking status, BMI, TG, ALT, TC, AST, LDL-C, BUN, HDL-C, SCr, FPG, sex, family history of diabetes, drinking status and age. Processes for missing data analysis employ the assumption of missing at random.16
This analysis assessed each factor’s impact on incident pre-DM using univariate Cox proportional hazards regression models. The multivariate Cox regression analysis also examined the precise connection between the PP and incident pre-DM. In addition, we created three models (fully adjusted, minimally adjusted and non-adjusted) to evaluate the connection between PP and incident pre-DM. Suppose the HR is changed by at least 10% after the covariance is included in the model. At this point, the covariance should be adjusted.17
The current analysis conducted several sensitivity analyses to determine if the findings were trustworthy. We converted PP into a categorical variable based on the quartile. We computed the P for the trend to verify the outcomes of the PP as the continuous variable and test for non-linearity. Obesity and older adults were connected to a greater occurrence of pre-DM. Thus, we excluded individuals with BMI ≥25 kg/m2 or age ≥60 years for subsequent sensitivity analyses to examine the connection between PP and pre-DM risk. Additionally, we incorporated the continuous covariate as a curve to the equation using a generalised additive model (GAM) to confirm the validity of the results. We also calculated E-values to examine the possibility of unmeasured confounding between PP and the risk of pre-DM.
We used Cox proportional hazards regression with cubic spline functions and smooth curve fitting to explore the nonlinear relationship between PP and pre-DM. We first used a recursive technique to locate the inflection point if a non-linear relationship was discovered.18 The recursive algorithm commences with an arbitrary initialisation and subsequently undergoes a series of filtering and smoothing steps in order to identify the inflection point accurately. Following this, we construct a two-piece Cox proportional hazards regression model, separately analysing the data on either side of the inflection point. Ultimately, the study determined the most appropriate model for PP’s connection with pre-DM through log-likelihood ratio analysis.
Subgroup analysis, using the Cox proportional hazard model, was also conducted. First, these variables were selected based on a combination of clinical relevance, literature review and the availability of data within our cohort. Second, the interaction test between these variables and PP was performed before the subgroup analysis. The likelihood ratio test was used to compare models with and without the multiplicative interaction term. Second, stratification was performed based on medians or established clinical cut points,19 and variables such as age (<60, ≥60 years) and BMI (<25, ≥25 kg/m2) were converted into categorical factors. Third, a fully adjusted analysis was performed for each stratum, except for the stratification factor. Ultimately, the likelihood ratio test was used to determine whether interaction terms existed in models with and without interaction terms.20 21 The study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement for all outcomes.17 22 Statistical significance was determined by a p<0.05, using two-tailed tests.
Results
Characteristics of individuals
In the current research, 182 672 individuals deemed free of pre-DM at baseline were included. The average age was 40.832±11.864 years, and 53.082% of individuals were male. A total of 20 284 individuals eventually got pre-DM after an average of 3.143 years of follow-up. Table 1 displays comprehensive clinical measurements, biochemical tests and various parameters. We categorised participants into subgroups based on PP quartiles (Q1≤36; 36<Q2≤43; 43<Q3≤50; 50<Q4). Compared with the Q1 group, the other groups (Q2 group, Q3 group, Q4 group) had higher ALT, BMI, age, TG, Scr, TC, AST, BUN, LDL-C and lower HDL-C. Additionally, the Q4 group had a higher proportion of men, smokers and drinkers.
Table 1.
The baseline characteristics of participants
| PP | Q1 (≤36) | Q2 (36 to ≤43) | Q3 (43 to ≤50) | Q4 (>50) | P value |
| Participants | 39 914 | 47 771 | 43 811 | 51 176 | |
| Gender | <0.001 | ||||
| Male | 16 960 (42.491%) | 22 739 (47.600%) | 24 830 (56.675%) | 32 437 (63.383%) | |
| Female | 22 954 (57.509%) | 25 032 (52.400%) | 18 981 (43.325%) | 18 739 (36.617%) | |
| Age(years) | 39.837±9.945 | 39.905±10.269 | 40.186±11.236 | 43.028±14.560 | <0.001 |
| Drinking status | |||||
| Current drinker | 526 (1.318%) | 712 (1.490%) | 682 (1.557%) | 1004 (1.962%) | |
| Ex-drinker | 3923 (9.829%) | 5436 (11.379%) | 5777 (13.186%) | 7498 (14.651%) | |
| Never-drinker | 35 465 (88.854%) | 41 623 (87.130%) | 37 352 (85.257%) | 42 674 (83.387%) | |
| Smoking status | <0.001 | ||||
| Current smoker | 5468 (13.699%) | 7191 (15.053%) | 7324 (16.717%) | 9441 (18.448%) | |
| Ex-smoker | 1164 (2.916%) | 1536 (3.215%) | 1707 (3.896%) | 2159 (4.219%) | |
| Never-smoker | 33 282 (83.384%) | 39 044 (81.732%) | 34 780 (79.386%) | 39 576 (77.333%) | |
| Family history of diabetes | <0.001 | ||||
| No | 38 974 (97.645%) | 46 764 (97.892%) | 42 922 (97.971%) | 50 379 (98.443%) | |
| Yes | 940 (2.355%) | 1007 (2.108%) | 889 (2.029%) | 797 (1.557%) | |
| SBP (mm Hg) | 104.383±10.737 | 111.554±10.604 | 119.000±10.592 | 131.740±12.760 | <0.001 |
| DBP (mm Hg) | 73.782±10.685 | 72.461±10.398 | 73.164±10.347 | 74.508±10.733 | <0.001 |
| BMI (kg/m2) | 22.205±3.119 | 22.654±3.134 | 23.100±3.184 | 23.773±3.358 | <0.001 |
| AST(U/L) | 21.2 (17, 26.6) | 21.5 (17.2, 27) | 22 (17.7, 27.8) | 23 (18.1, 28.7) | <0.001 |
| ALT (U/L) | 16 (11.6, 24) | 16.6 (12, 25.3) | 18 (12, 27.4) | 19.3 (14, 29) | <0.001 |
| HDL-C (mmol/L) | 1.394±0.312 | 1.384±0.310 | 1.365±0.304 | 1.351±0.303 | <0.001 |
| TG (mmol/L) | 0.94 (0.67, 1.40) | 0.99 (0.70, 1.47) | 1.04 (0.72, 1.55) | 1.12 (0.79, 1.69) | <0.001 |
| LDL-C (mmol/L) | 2.651±0.656 | 2.663±0.660 | 2.685±0.671 | 2.734±0.690 | <0.001 |
| TC (mmol/L) | 4.617±0.858 | 4.632±0.863 | 4.659±0.880 | 4.725±0.916 | <0.001 |
| BUN (mmol/L) | 4.502±1.142 | 4.538±1.148 | 4.612±1.156 | 4.732±1.199 | <0.001 |
| SCr (umol/L) | 67.116±14.932 | 68.284±15.134 | 70.240±15.137 | 72.093±16.223 | <0.001 |
| FPG (mmol/L) | 4.691±0.513 | 4.730±0.497 | 4.772±0.480 | 4.849±0.450 | <0.001 |
| PP (mm Hg) | 30.601±3.971 | 39.093±1.979 | 45.836±1.986 | 57.232±6.395 | <0.001 |
Values are n (%) or mean±SD.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BUN, blood urea nitrogen; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PP, pulse pressure; SBP, systolic blood pressure; Scr, serum creatinine; TC, total cholesterol; TG, triglycerides.
The incidence rate of pre-DM
During the follow-up, 20 284 individuals developed incident pre-DM, as outlined in online supplemental table 1. All people had a prevalence rate of 11.10%. The four PP groups’ prevalence rates were 8.92%, 9.47% (9.21%–9.73%), 10.82% and 14.58%. In addition, the cumulative incidence rate of the overall population and four PP groups were 3532.68, 2779.24, 2999.95, 3470.06 and 4701.37 per 100 000 person-years, respectively. Individuals in the Q2, Q3 and Q4 groups exhibited significantly greater cumulative incidence and prevalence rates of pre-DM than those in the Q1 group.
bmjopen-2023-080018supp001.pdf (82.3KB, pdf)
bmjopen-2023-080018supp002.pdf (92.2KB, pdf)
The Kaplan-Meier curves for the propensity to survive without pre-DM are shown in online supplemental figure 1. There was a significant difference between the four PP groups regarding the likelihood of developing pre-DM (p<0.001). As PP levels increased, the chance of living without pre-DM steadily dropped. As a result, pre-DM risk was highest among those in the highest PP categories.
Univariate analysis
Online supplemental table 2 presents the findings of the univariate analysis. DBP, TC, BMI, SBP, FPG, age, TG, SCr, LDL-C, PP and BUN were correlated with pre-DM risk. HDL-C exhibits an inverse relationship with pre-DM risk. Individuals who never drink or smoke also have a lower risk of developing pre-DM. Pre-DM risk was shown to be greater in men than in women.
The results of the connection between PP and pre-DM
The Cox proportional hazard regression models for the association between PP and pre-DM are shown in table 2. In the non-adjusted model, the HR (95% CI) for the relationship between pre-DM and PP was 1.025 (1.023 to 1.026). The HR (95% CI) in the minimally adjusted model was 1.013 (1.011 to 1.014) after adjusting for smoking status, age, BMI, family history of diabetes, gender and drinking status. The HR (95% CI) was 1.009 (1.007 to 1.010) in the fully adjusted model after controlling for smoking status, BMI, TG, ALT, TC, AST, LDL-C, BUN, HDL-C, SCr, sex, FPG, family history of diabetes, drinking status and age. The findings showed that for every 1 mm Hg rise in PP, the risk of pre-DM rose by 0.9%.
Table 2.
Relationship between PP and incident pre-diabetes in different models
| Variable | Non-adjusted model (HR, 95% CI, p value) | Minimally adjusted model (HR, 95% CI, p value) | Fully adjusted model (HR, 95% CI, p value) | GAM (HR, 95% CI, p value) |
| Total PP |
1.025 (1.023 to 1.026), <0.001 | 1.013 (1.011 to 1.014), <0.001 | 1.009 (1.007 to 1.010), <0.001 | 1.008 (1.007 to 1.010), <0.001 |
| PP (quartile) | ||||
| Q1 | ref | ref | ref | ref |
| Q2 | 1.125 (1.077 to 1.176), <0.001 | 1.070 (1.024 to 1.118), 0.003 | 1.043 (0.998 to 1.090), 0.061 | 1.046 (1.001 to 1.093), 0.045 |
| Q3 | 1.347 (1.290 to 1.407), <0.001 | 1.208 (1.156 to 1.262), <0.001 | 1.131 (1.083 to 1.181), <0.001 | 1.129 (1.080 to 1.179), <0.001 |
| Q4 | 1.860 (1.787 to 1.935), <0.001 | 1.408 (1.352 to 1.467), <0.001 | 1.246 (1.197 to 1.298), <0.001 | 1.238 (1.188 to 1.291), <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 | <0.001 |
| Gender | ||||
| Male | ref | ref | ||
| Female | 0.777 (0.751 to 0.803), <0.001 | 0.877 (0.843 to 0.913), <0.001 | ||
| Age(years) | 1.027 (1.026 to 1.028), <0.001 | 1.022 (1.021 to 1.023), <0.001 | ||
| Drinking status | ||||
| Current drinker | ref | ref | ||
| Ex-drinker | 0.965 (0.877 to 1.061), 0.457 | 1.013 (0.920 to 1.114), 0.799 | ||
| Never-drinker | 0.911 (0.832 to 0.997), 0.042 | 1.075 (0.981 to 1.178), 0.122 | ||
| Smoking status | ||||
| Current smoker | ref | ref | ||
| Ex-smoker | 1.020 (0.948 to 1.097), 0.596 | 1.021 (0.949 to 1.098), 0.578 | ||
| Never-smoker | 1.055 (1.016 to 1.095), 0.005 | 1.023 (0.985 to 1.062), 0.246 | ||
| Family history of diabetes | ||||
| No | ref | ref | ||
| Yes | 1.157 (1.058 to 1.265), 0.001 | 1.120 (1.024 to 1.225), 0.013 | ||
| BMI (kg/m2) | 1.091 (1.086 to 1.095), <0.001 | 1.060 (1.055 to 1.065), <0.001 | ||
| AST(U/L) | 0.999 (0.997 to 1.000), 0.150 | |||
| ALT (U/L) | 1.003 (1.002 to 1.004), <0.001 | |||
| HDL-C (mmol/L) | 1.429 (1.353 to 1.509), <0.001 | |||
| TG (mmol/L) | 1.145 (1.129 to 1.161), <0.001 | |||
| LDL-C (mmol/L) | 1.309 (1.248 to 1.373), <0.001 | |||
| TC (mmol/L) | 0.772 (0.741 to 0.803), <0.001 | |||
| BUN (mmol/L) | 0.976 (0.964 to 0.988), <0.001 | |||
| SCr (umol/L) | 1.003 (1.002 to 1.004), <0.001 | |||
| FPG (mmol/L) | 4.613 (4.451 to 4.780), <0.001 | |||
Crude model: we did not adjust for other covariants.
Minimally adjusted model: we adjusted for gender, age, family history of diabetes, drinking status, smoking status and BMI.
Fully adjusted model: we adjusted for gender, age, family history of diabetes, drinking status, smoking status, BMI, TC, TG, HDL-C, LDL-C, AST, ALT, SCr, BUN and FPG.
GAM: All covariates listed in table 1 were adjusted. However, continuous covariates were adjusted as non-linearity.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BUN, blood urea nitrogen; FPG, fasting plasma glucose; GAM, generalised additive mode; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PP, pulse pressure; Ref, reference; SCr, serum creatinine; TC, total cholesterol; TG, triglyceride.
Sensitivity analyses
We used several sensitivity analyses to evaluate how reliable our results were. PP was changed from a continuous to a categorical variable before being reintroduced into the model. Following the translation of PP into categorical variables, the trend p was not equal, suggesting a potential nonlinear connection between PP and the chance of developing pre-DM. Additionally, a GAM added the continuity covariate to the equation. Results for the GAM model showed a positive connection between PP and the probability of developing pre-DM (HR 1.008, 95% CI 1.007 to 1.010) (table 2).
In addition, the current research excluded participants with BMI<25 kg/m2 for sensitivity analysis. After controlling for confounding factors, we observed a positive association between PP and pre-DM risk (HR 1.011, 95% CI 1.009 to 1.013) (online supplemental table 3). Moreover, we considered participants with ages <60 years for sensitivity studies. After adjusting for smoking status, BMI, TG, ALT, TC, AST, LDL-C, BUN, HDL-C, SCr, sex, FPG, family history of diabetes, drinking status and age, the results showed that PP remained positively correlated with the likelihood of developing pre-DM (HR 1.008, 95% CI 1.007 to 1.010). (online supplemental table 3). According to the sensitivity analysis, our findings appeared to be solid.
Additionally, an E-value was computed to assess the vulnerability of the study results to potential unobserved confounding factors. The resulting E-value (1.21) demonstrated a higher level of statistical significance in comparison to the relative risk (1.05) associated with unmeasured confounders and PP. This suggests that the impact of unmeasured or unidentified confounders on the relationship between PP and the occurrence of pre-DM was negligible.
The analysis of the non-linear connection
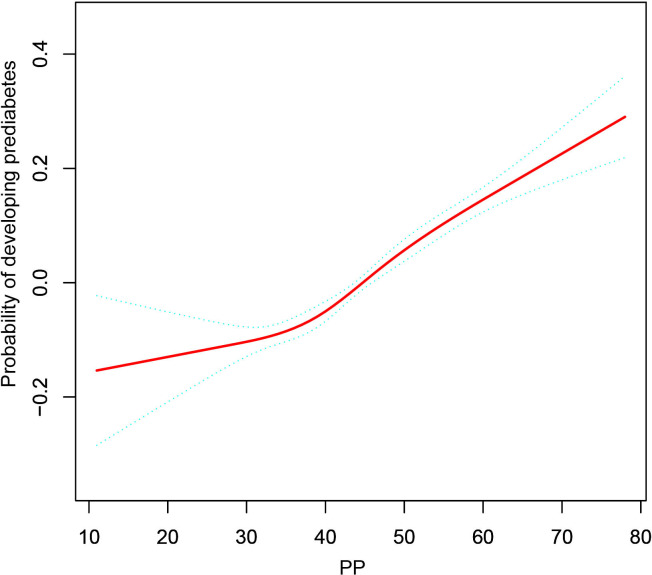
The non-linear connection between PP and incident pre-DM is illustrated in figure 2. After correcting for confounding factors, there was a non-linear link between PP and incident pre-DM (table 3). Based on a two-piecewise Cox proportional hazards regression model, the PP’s inflection point was 29 mm Hg (P for log-likelihood ratio test=0.008). When PP was more than 29 mm Hg, PP was strongly linked with incident pre-DM (HR 1.009, 95%CI 1.008 to 1.011, p<0.001). However, their correlation was not significant when PP was less than 29 mm Hg (HR 0.990, 95% CI 0.977 to 1.003, p=0.149).
Figure 2.
The non-linear relationship between PP and incident pre-diabetes. A non-linear relationship between PP and incident pre-diabetes was detected after adjusting for smoking status, BMI, TG, ALT, TC, AST, LDL-C, BUN, HDL-C, SCr, sex, FPG, family history of diabetes, drinking status and age. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BUN, blood urea nitrogen; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PP, pulse pressure; SCr, serum creatinine; TG, triglyceride.
Table 3.
The result of the two-piecewise Cox proportional hazards regression model
| Incident pre-diabetes | HR (95%CI) | P value |
| Fitting model by standard linear regression | 1.009 (1.007 to 1.010) | <0.001 |
| Fitting model by two-piecewise Cox proportional hazards regression | ||
| The inflection point of PP (mm Hg) | 29 | |
| ≤29 | 0.990 (0.977 to 1.003) | 0.149 |
| >29 | 1.009 (1.008 to 1.011) | <0.001 |
| P for the log-likelihood ratio test | 0.008 | |
We adjusted for gender, age, family history of diabetes, drinking status, smoking status, BMI, TC, TG, HDL-C, LDL-C, AST, ALT, SCr, BUN and FPG.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BUN, blood urea nitrogen; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PP, pulse pressure; SCr, serum creatinine; TC, total cholesterol; TG, triglyceride.
The results of the subgroup analysis
Interaction tests performed before subgroup analyses showed that age, BMI, gender, family history of diabetes, smoking status and drinking status interacted with PP (p<0.001) (online supplemental table 4). We selected age, BMI, gender, family history of diabetes, smoking status and drinking status as stratification variables and examined the changes in their impact sizes (online supplemental table 5). Age, drinking status and family history of diabetes had no impact on the correlation between PP and the risk of pre-DM. Females, never-smokers, ever-smokers and subjects with BMI<25 kg/m2 were more likely to be associated with pre-DM risk. Conversely, there was a weaker connection in males, current smokers and individuals with BMI≥25 kg/m2.
Discussion
The current study’s main goal was to investigate the connection between PP and incident pre-DM in Chinese participants. The findings demonstrated a correlation between increased PP and a higher risk of pre-DM. The correlation between PP and pre-DM was also investigated on the left and right sides of the inflection point. PP level and incident pre-DM have a non-linear relationship. It was found that never-smokers, ever-smokers, females and individuals with BMI<25 kg/m2 had a greater correlation between PP and incident pre-DM.
PP, the arithmetic difference between SBP and DBP, is determined by arterial wall elasticity and is related to all-cause mortality, cardiovascular events, stroke, kidney injury, severe eye illness and arterial stiffness.23–28 In comparison to blood pressure, PP has a better predictive capacity for poor cardiovascular outcomes in people with diabetes, according to several previous pieces of evidence.6 29 In addition, some studies found that PP demonstrated strong predictive ability in the homeostatic model assessment of insulin resistance index, diabetes and metabolic syndrome.30–32 In a retrospective study involving 211 814 Chinese participants, after controlling for BMI, smoking and drinking status, age, TC, gender, family history of diabetes, FPG, TG and BUN and ALT, Higher PP levels independently connect with increased T2DM risk (HR :1.003, 95%CI 1.001 to 1.005).11 In a longitudinal study involving 12 272 Chinese, Zhang et al30 found that high PP in Chinese women may be related to the development of T2DM after adjusting for confounding covariates. In a national cross-sectional study involving 6187 Korean older adults, Kwon et al32 discovered a positive association between PP and metabolic syndrome after adjusting for alcohol consumption, smoking, age, regular exercise and mean arterial blood pressure. In another cross-sectional cohort study that included 38 708 rural Chinese participants, compared with the lowest group, the OR (95%CI) of PP in the highest quartile of risk for metabolic syndrome was found to be 1.81 (1.67 to 1.95) after controlling for confounders.33 In addition, in a prospective research enrolling 32 917 Chinese, the HR (95%CI) for diabetes in the Q3 and Q4 groups were 1.13 (1.04 to 1.22) and 1.14 (1.05 to 1.24), respectively, after adjusting for covariates compared with the Q1 group.34 However, in a multicentre, longitudinal cohort study that included 18 619 adults, high PP was not related to an increased risk of diabetes after adjusting for BMI, mean arterial pressure, gender, high-sensitivity C reactive protein, age, exercise, smoking, blood pressure lowering agents, drinking, hyperlipidaemia and family history of diabetes.35 A retrospective study of 178 individuals with hypertension found that PP was not associated with the risk of new-onset diabetes after adjusting for potential confounders.36 This retrospective cohort study involved 182 672 Chinese individuals and revealed a higher incidence of pre-DM at increased PP levels. After adjusting for smoking status, BMI, TG, ALT, TC, AST, LDL-C, BUN, HDL-C, SCr, sex, FPG, family history of diabetes, drinking status and age, the results indicated that each unit of the LAP raised the risk of pre-DM by 0.9%. Moreover, sensitivity analysis has demonstrated that this correlation remains observable in Chinese adults with age <60 years or BMI<25 kg/m2. The efforts mentioned above have demonstrated the consistency of the connection between PP and pre-DM risk. The findings offered a clinical PP-level intervention guideline to decrease pre-DM risk.
Few previous studies have investigated the probable curvilinear link between PP and pre-DM. The current study first examined the non-linear association between PP and pre-DM. After controlling for smoking status, BMI, TG, ALT, TC, AST, LDL-C, BUN, HDL-C, SCr, sex, FPG, family history of diabetes, drinking status and age, the findings revealed that the connection between PP and pre-DM was nonlinear. Based on a two-piecewise Cox proportional hazards regression model, we identified the inflection point of PP as 29 mm Hg. When PP levels exceeded 29 mm Hg, a 1-unit increase in PP correlated with a 0.9% increase in the HR for individuals with pre-DM (HR 1.009, 95% CI 1.008 to 1.011, p<0.001). However, no significant correlation was observed between PP levels below 29 mm Hg and the incident pre-DM (HR 0.990, 95% CI 0.977 to 1.003, p=0.149). Elevated PP serves as a valuable indicator for identifying high-risk participants likely to develop pre-DM during follow-up. Moreover, our analysis revealed that the relationship between PP and the emergence of pre-DM was more pronounced in never-smokers, ever-smokers, females and individuals with BMI<25 kg/m2. In contrast, this association appeared attenuated in males, current smokers and individuals with BMI≥25 kg/m2. Prior research has consistently identified obesity, smoking and male as contributors to insulin resistance,37 38 which is a precursor to pre-DM. We postulated that the attenuated association observed in these subgroups may be attributable to the overriding influence of these risk factors on the pathogenesis of pre-DM. The direct impact of PP on pre-DM risk may be somewhat eclipsed by the more substantial effects of obesity, active smoking and the male sex on insulin resistance and subsequent pre-DM development. This information can remind individuals to adopt healthier lifestyle habits sooner, ultimately improving their outcomes.
The mechanism behind the association between PP and pre-DM is yet unknown. Several explanations currently exist for PP leading to pre-DM. First, endothelial cell dysfunction may result in microvascular dysfunction,39 which in turn causes dysfunctional glucose metabolism, insulin resistance, poor tissue perfusion and arterial stiffness.40–42 Additionally, arterial stiffness may exacerbate microvascular lesions, creating a vicious cycle.42 43 Second, normal arteries can reduce PP, but arterial stiffness increases blood flow through low-resistance organs (such as the kidney and brain), which will cause organ dysfunction.44 As a low-resistance, high-blood-flow organ with a mean tissue perfusion of 250–300 mL/min/100 g, the pancreas may be negatively impacted by arterial stiffness in terms of its endocrine function.
The current research possesses several notable advantages. First, we delved deeper into the non-linear relationship between PP and pre-DM. Second, we minimised the impact of residual confounding factors through rigorous statistical adjustments. Third, we conducted sensitivity analyses to ensure the robustness of our findings. Lastly, we performed a group analysis to evaluate other potential risk covariates that could affect the link between PP and pre-DM.
The present study has certain limitations. First, this study is based on a Chinese population, and while it offers valuable insights, the findings might not be directly generalisable to other populations due to genetic, lifestyle and environmental differences. In the future, we will explore the relationship between PP and pre-DM risk in diverse populations. Second, as our study was a secondary analysis, we cannot guarantee data quality monitoring and variable control. In forthcoming research endeavours, we will endeavour to construct prospective cohort studies with enhanced data quality oversight and variable control, thereby mitigating bias. Third, excluding over 500 000 individuals from a final cohort of 182 672 subjects could have selection bias and implications for generalisability. In the future, we will design prospective studies in a more diverse population to validate our findings. Fourth, pre-DM was defined based on impaired fasting glucose levels in our study. This could potentially lead to underestimation of pre-DM incidence. This is a secondary retrospective study, and the raw data did not provide information regarding 2-hour oral glucose tolerance test or glycosylated haemoglobin level measurements. In the future, we will consider designing our study to document more variables, including 2-hour oral glucose tolerance test or glycosylated haemoglobin level measurements. Fifth, as with all observational studies, there may be uncontrolled or unmeasured confounding factors, such as diet, exercise, atherosclerosis, the use of antihypertensive medications and the presence of hypertension, despite controlling for known potential confounders such as BMI, TC, LDL-C, AST, ALT, Scr, BUN and FPG. However, we used the E-value to evaluate the impact of unmeasured confounders and determined it unlikely that they fully explained the results. Sixth, SBP and DBP were only measured at baseline in the original study, and we did not assess how SBP and DBP changed over time. In the future, we will consider designing our own study and documenting more information, such as diet, exercise, atherosclerosis, antihypertensive medications, the presence of hypertension and changes in blood pressure over time, and then using a GAM model to explore the impact of changes in PP on pre-DM risk.
Conclusion
This cohort study of the Chinese population shows that PP was inversely and non-linearly associated with the incidence of pre-DM after adjusting for other confounding factors. High PP levels were related to pre-DM risk when PP was above 29 mm Hg. From a therapeutic standpoint, lowering the PP below the inflection point represents a cost-effective and straightforward approach for the early prevention and intervention of pre-DM.
Supplementary Material
Footnotes
CC and YH contributed equally.
Contributors: CC and YH contributed to the study concept and design, researched and interpreted the data and drafted the manuscript. HH, YH and JL analysed the data and reviewed the manuscript. CC and YH oversaw the project’s progress, contributed to the discussion and reviewed the manuscript. HH, YH and JL were the guarantors of this work. As such, they had full access to all data in the study and were responsible for the data integrity and analysis accuracy. All authors read and approved the final manuscript.
Funding: This study was supported by the Natural Science Funding of China (No.82272598, No.81901470) and the Natural Science Foundation of Guangdong Province, China (No. 2020A1515011203). This study was also supported by the Science, Technology and Innovation Commission of Shenzhen (JCYJ20210324135804012, JCYJ20220530150407015).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
The original study followed guidelines outlined by the Declaration of Helsinki and was approved by the Rich Healthcare Group Review Board. In addition, the Rich Healthcare Group Review Board has waived informed consent for the current retrospective study. All methods were performed in accordance with the relevant Declaration of Helsinki.
References
- 1.Ali MK, Bullard KM, Saydah S, et al. Cardiovascular and renal burdens of Prediabetes in the USA: analysis of data from serial cross-sectional surveys, 1988-2014. Lancet Diabetes Endocrinol 2018;6:392–403. 10.1016/S2213-8587(18)30027-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med 2010;362:1090–101. 10.1056/NEJMoa0908292 [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Teng D, Shi X, et al. Prevalence of diabetes recorded in Mainland China using 2018 diagnostic criteria from the American diabetes Association: national cross sectional study. BMJ 2020:m997. 10.1136/bmj.m997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabák AG, Herder C, Rathmann W, et al. Prediabetes: a high-risk state for diabetes development. Lancet 2012;379:2279–90. 10.1016/S0140-6736(12)60283-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Somaratne JB, Whalley GA, Poppe KK, et al. Screening for left ventricular hypertrophy in patients with type 2 diabetes mellitus in the community. Cardiovasc Diabetol 2011;10:29. 10.1186/1475-2840-10-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kodama S, Horikawa C, Fujihara K, et al. Meta-analysis of the quantitative relation between pulse pressure and mean arterial pressure and cardiovascular risk in patients with diabetes mellitus. Am J Cardiol 2014;113:1058–65. 10.1016/j.amjcard.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 7.Laugesen E, Knudsen ST, Hansen KW, et al. Invasive aortic pulse pressure is not superior to cuff pulse pressure in cardiovascular risk prediction. J Hypertens 2021;39:607–13. 10.1097/HJH.0000000000002694 [DOI] [PubMed] [Google Scholar]
- 8.Rojas RJ, Chávez-Sosa JV, Gutierrez-Ajalcriña R, et al. Association between Dehydroepiandrosterone levels and cardiovascular risk in public sector health workers in a Peruvian region. Cardiovasc Endocrinol Metab 2021;10:51–5. 10.1097/XCE.0000000000000219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee CJ, Yoon M, Ha J, et al. Kang SM: comparison of the association between arterial stiffness indices and heart failure in patients with high cardiovascular risk: A retrospective study. Front Cardiovasc Med 2021;8:782849. 10.3389/fcvm.2021.782849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Açar B, Ozeke O, Karakurt M, et al. Association of Prediabetes with higher coronary Atherosclerotic burden among patients with first diagnosed acute coronary syndrome. Angiology 2019;70:174–80. 10.1177/0003319718772420 [DOI] [PubMed] [Google Scholar]
- 11.Wu C, Ma D, Chen Y. Association of pulse pressure difference and diabetes mellitus in Chinese people: A cohort study. Int J Gen Med 2021;14:6601–8. 10.2147/IJGM.S327841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Zhang X-P, Yuan J, et al. Association of body mass index and age with incident diabetes in Chinese adults: a population-based cohort study. BMJ Open 2018;8:e021768. 10.1136/bmjopen-2018-021768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Classification and diagnosis of diabetes: standards of medical care in Diabetes-2018. Diabetes Care 2018;41:S13–27. [DOI] [PubMed] [Google Scholar]
- 14.Qin H, Chen Z, Zhang Y, et al. Triglyceride to high-density lipoprotein cholesterol ratio is associated with incident diabetes in men: A retrospective study of Chinese individuals. J Diabetes Investig 2020;11:192–8. 10.1111/jdi.13087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robson ME, Tung N, Conte P, et al. Olympiad final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician’s choice in patients with a Germline BRCA Mutation and Her2-negative metastatic breast cancer. Ann Oncol 2019;30:558–66. 10.1093/annonc/mdz012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377–99. 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 17.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg 2014;12:1500–24. 10.1016/j.ijsu.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 18.Zhu F, Chen C, Zhang Y, et al. Elevated blood mercury level has a non-linear association with infertility in U.S. women: data from the Nhanes 2013-2016. Reprod Toxicol 2020;91:53–8. 10.1016/j.reprotox.2019.11.005 [DOI] [PubMed] [Google Scholar]
- 19.Zheng X, Cao C, He Y, et al. Association between Nonalcoholic fatty liver disease and incident diabetes mellitus among Japanese: a retrospective cohort study using propensity score matching. Lipids Health Dis 2021;20:59. 10.1186/s12944-021-01485-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mullee A, Romaguera D, Pearson-Stuttard J, et al. Association between soft drink consumption and mortality in 10 European countries. Jama Intern Med 2019;179:1479–90. 10.1001/jamainternmed.2019.2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keidel D, Anto JM, Basagaña X, et al. The role of socioeconomic status in the Association of lung function and air pollution-A pooled analysis of three adult ESCAPE cohorts. IJERPH 2019;16:1901. 10.3390/ijerph16111901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–9. 10.1016/j.ijsu.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto M, Fujihara K, Ishizawa M, et al. Pulse pressure is a stronger Predictor than systolic blood pressure for severe eye diseases in diabetes mellitus. J AM HEART ASSOC 2019;8:e010627. 10.1161/JAHA.118.010627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Qamari A, Adeleke I, Kretzer A, et al. Pulse pressure and perioperative stroke. Curr Opin Anaesthesiol 2019;32:57–63. 10.1097/ACO.0000000000000673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Huang J-Y, Lo K, et al. Association of pulse pressure with all-cause mortality in young adults. Postgrad Med J 2020;96:461–6. 10.1136/postgradmedj-2019-137070 [DOI] [PubMed] [Google Scholar]
- 26.St-Louis E, Sudarshan M, Al-Habboubi M, et al. The outcomes of the elderly in acute care general surgery. Eur J Trauma Emerg Surg 2016;42:107–13. 10.1007/s00068-015-0517-9 [DOI] [PubMed] [Google Scholar]
- 27.Brown CH, Neuman MD. Optimizing perioperative care for older adults. Anesthesiology Clinics 2015;33:xv–xvi. 10.1016/j.anclin.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 28.Gavish B, Bursztyn M. Ambulatory pulse pressure components: concept, determination and clinical relevance. J Hypertens 2019;37:765–74. 10.1097/HJH.0000000000001920 [DOI] [PubMed] [Google Scholar]
- 29.Payne RA, Wilkinson IB, Webb DJ. Arterial stiffness and hypertension: emerging concepts. Hypertension 2010;55:9–14. 10.1161/HYPERTENSIONAHA.107.090464 [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Wang B, Wang C, et al. High pulse pressure is related to risk of type 2 diabetes mellitus in Chinese middle-aged females. Int J Cardiol 2016;220:467–71. 10.1016/j.ijcard.2016.06.233 [DOI] [PubMed] [Google Scholar]
- 31.Yasuno S, Ueshima K, Oba K, et al. Is pulse pressure a Predictor of new-onset diabetes in high-risk hypertensive patients?: a Subanalysis of the Candesartan antihypertensive survival evaluation in Japan (CASE-J) trial. Diabetes Care 2010;33:1122–7. 10.2337/dc09-1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon Y-J, Chung T-H, Shim J-Y, et al. The Association of pulse pressure with metabolic syndrome in Korean elderly: A nationwide population-based study. Diabetes Res Clin Pract 2017;123:75–81. 10.1016/j.diabres.2016.11.027 [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Li Y, Wang F, et al. Independent and combined effects of resting heart rate and pulse pressure with metabolic syndrome in Chinese rural population: the Henan rural cohort study. Clinica Chimica ACTA 2018;484:246–52. 10.1016/j.cca.2018.06.010 [DOI] [PubMed] [Google Scholar]
- 34.Li WJ, Fang W, Cai ZF, et al. Association between pulse pressure and new-onset diabetes in hypertensive patients. Zhonghua Xin Xue Guan Bing Za Zhi 2021;49:673–9. 10.3760/cma.j.cn112148-20200729-00603 [DOI] [PubMed] [Google Scholar]
- 35.Wu Y, Han X, Gao J, et al. Individual and combined contributions of age-specific and sex-specific pulse pressure and brachial-ankle pulse wave velocity to the risk of new-onset diabetes mellitus. BMJ Open Diabetes Res Care 2021;9:e001942. 10.1136/bmjdrc-2020-001942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen J-Y, Chou C-H, Lee YL, et al. Association of central aortic pressures indexes with development of diabetes mellitus in essential hypertension. Am J Hypertens 2010;23:1069–73. 10.1038/ajh.2010.145 [DOI] [PubMed] [Google Scholar]
- 37.Juneja A, Dwivedi S, Srivastava DK, et al. Insulin resistance in young obese subjects and its relation to smoking (A pilot study). Ind J CLIN Biochem 2017;32:99–102. 10.1007/s12291-016-0579-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ciarambino T, Crispino P, Guarisco G, et al. Gender differences in insulin resistance: new knowledge and perspectives. Curr Issues Mol Biol 2023;45:7845–61. 10.3390/cimb45100496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muris DMJ, Houben AJHM, Schram MT, et al. Stehouwer CD: Microvascular dysfunction is associated with a higher incidence of type 2 diabetes mellitus: a systematic review and meta-analysis. Arterioscler Thromb Vasc Biol 2012;32:3082–94. 10.1161/ATVBAHA.112.300291 [DOI] [PubMed] [Google Scholar]
- 40.Levy BI, Schiffrin EL, Mourad J-J, et al. Struijker-Boudier HA: impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation 2008;118:968–76. 10.1161/CIRCULATIONAHA.107.763730 [DOI] [PubMed] [Google Scholar]
- 41.Vincent MA, Clerk LH, Lindner JR, et al. Microvascular recruitment is an early insulin effect that regulates Skeletal muscle glucose uptake in vivo. Diabetes 2004;53:1418–23. 10.2337/diabetes.53.6.1418 [DOI] [PubMed] [Google Scholar]
- 42.Malik AR, Kondragunta V, Kullo IJ. Forearm vascular reactivity and arterial stiffness in asymptomatic adults from the community. Hypertension 2008;51:1512–8. 10.1161/HYPERTENSIONAHA.107.106088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol 2007;50:1–13. 10.1016/j.jacc.2006.12.050 [DOI] [PubMed] [Google Scholar]
- 44.Ungvari Z, Csiszar A, Kaminski PM, et al. Chronic high pressure-induced arterial oxidative stress: involvement of protein kinase C-dependent NAD(P)H oxidase and local renin-angiotensin system. Am J Pathol 2004;165:219–26. 10.1016/S0002-9440(10)63290-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-080018supp001.pdf (82.3KB, pdf)
bmjopen-2023-080018supp002.pdf (92.2KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.