Abstract
Background
Sleep disturbance is a prevalent condition among people living with dementia (PLwD) or mild cognitive impairment (MCI). Its assessment and management within primary care is complex because of the comorbidities, older age, and cognitive impairment typical of this patient group.
Aim
To explore how primary care clinicians assess, understand, and manage sleep disturbance for PLwD or MCI; if and why such initiatives work; and how people and their carers experience sleep disturbance and its treatment.
Design and setting
A realist review of existing literature conducted in 2022.
Method
Six bibliographic databases were searched. Context–mechanism–outcome configurations (CMOCs) were developed and refined.
Results
In total, 60 records were included from 1869 retrieved hits and 19 CMOCs were developed. Low awareness of and confidence in the treatment of sleep disturbance among primary care clinicians and patients, combined with time and resource constraints, meant that identifying sleep disturbance was difficult and not prioritised. Medication was perceived by clinicians and patients as the primary management tool, resulting in inappropriate or long-term prescription. Rigid nursing routines in care homes were reportedly not conducive to good-quality sleep.
Conclusion
In primary care, sleep disturbance among PLwD or MCI is not adequately addressed. Over-reliance on medication, underutilisation of non-pharmacological strategies, and inflexible care home routines were reported as a result of low confidence in sleep management and resource constraints. This does not constitute effective and person-centred care. Future work should consider ways to tailor the assessment and management of sleep disturbance to the needs of individuals and their informal carers without overstretching services.
Keywords: caregivers, cognitive dysfunction, community health services, family practice, primary health care, sleep wake disorders
Introduction
Sleep disturbance can be defined as any condition that affects the quality, timing, or length of sleep so there is an impact on a person’s daily functioning.1 Sleep disturbance includes but is not limited to insomnia, narcolepsy, obstructive sleep apnoea, restless leg syndrome, periodic limb movements, and rapid-eye movement sleep behavioural disorder.2,3 Sleep disturbance, especially insomnia, is a common symptom of dementia and mild cognitive impairment (MCI), although as for people without dementia, factors such as pain, low mood, or a combination of causes may also disrupt sleep in this population.3 Some studies suggest that sleep disturbance has a key role in the progression of dementia4–6 or is associated with worsening symptoms.7
In a meta-analysis of 11 studies to explore the prevalence of sleep disturbance in people living with dementia (PLwD), 26% of the pooled population experienced sleep disturbance symptoms and 19% had clinically significant cases of sleep disturbance.8 Another review of 55 studies estimated a 38% prevalence of sleep disturbance among PLwD who were living in nursing homes based on symptoms, and 20% based on clinical measures.9 One literature review reported a 60% prevalence of any sleep disturbance among the MCI population, although studies exploring the prevalence of sleep disturbance among people with MCI are comparatively few.10–12
In primary care, the assessment and management of sleep disturbance among PLwD or MCI is complex because of a lack of clear diagnostic criteria and this population’s characteristics, which typically comprises older people living with multiple comorbidities, and polypharmacy.13 Although preliminary evidence suggests that non-pharmacological interventions are effective,14–16 these approaches are often not available, are not used or trusted by clinicians, and are not embedded within usual primary care practice.17
How this fits in
| Existing literature reports that the management of sleep disturbance among people living with dementia or mild cognitive impairment is a challenging problem. This realist review indicates why, how, and in which circumstances primary care is hindered when managing sleep disturbance in this population. Medication is often considered the primary management option because of a range of complex factors, despite limited efficacy and problematic side effects. Alternative management techniques, including evidence-based non-pharmacological strategies tailored to the individual and their carer/s, may improve sleep disturbance within this population. |
The treatment of sleep disturbance among PLwD or MCI is a challenging or ‘wicked’ problem.18 Wicked problems originate from social planning and are defined as problems difficult or impossible to solve because of incomplete, contradictory, or changing requirements that are hard to define, and where solutions to one problem likely generate another problem.19 The assessment and management of sleep disturbance in primary care may therefore benefit from being examined carefully by considering all potential solutions, contexts, and experiences. A realist approach was therefore used to explore how primary care clinicians assess, understand, and manage sleep disturbance for PLwD or MCI; if and why such initiatives work; and how people and their carers experience sleep disturbance and its treatment.
Method
This realist review is part of a larger National Institute for Health and Care Research funded research project.20 The review protocol is registered with PROSPERO (CRD42022304679) and published elsewhere.21
The realist approach recognises that an intervention’s impact is heavily dependent on its context and that a complex array of contextual factors therefore needs to be examined. Compared with other structured review approaches, the realist approach to review allows a wider array of search terms and broader inclusion criteria to be used, allowing for a more thorough identification and comparison of potential contextual factors.22
The bibliographic database search strategy was developed in Ovid MEDLINE by an information specialist (one of the authors) using a combination of free-text terms and controlled vocabulary (see Supplementary Box S1). No date limit was applied. The MEDLINE search was translated for use in an appropriate selection of bibliographic databases in total comprising APA, PsycINFO, HMIC, CINAHL, and ASSIA. The final searches were conducted on 4 July 2022. Forward citation searching and a reference list search of the included studies was also conducted.
The retrieved documents were imported to an EndNote X9 file and were examined for inclusion and exclusion (Box 1) through title, abstract, and then full-text screening (by three authors). Of the articles, 15% were randomly cross-checked by two authors for consistency at the title, abstract, and full-text screening stage. The recommended rate for cross-checking in realist reviews is 10%–20%.23 Discrepancies were discussed and screening strategies agreed on. The included articles were imported into NVivo (version 1.6.1).
Box 1.
Inclusion and exclusion criteria
| Criteria | Include | Exclude |
|---|---|---|
| Population | PLwD or MCI, primary/community-based clinicians, and carers (family, friends, unpaid, informal, or paid carers) | Trauma cases |
| Phenomenon of interest | Assessment and management of sleep | End-of-life care (life expectancy <3 months) |
| Context and setting | Primary care centres, community in the widest sense of the word (that is, live at home, sheltered accommodation, care homes including residential and nursing homes, or supported/assisted living setting) | Hospital-based/secondary/tertiary care or interventions, hospice care, non-Organisation for Economic Co-operation and Development nations |
| Evaluation | Experience of sleep disturbance, diagnosis, assessment, management, approaches, or interventions (what, how, why, by whom, for whom, what extent), experience with drug prescriptions, or medication usage | Quantitative findings from randomised controlled trials of effectiveness studies |
| Study design/type of document | Original research (published articles, conference abstracts, books, and unpublished and grey literature), reviews, viewpoints, policy documents, websites of professional bodies, and any relevant clinical or non-clinical guideline | — |
| Other | — | Non-English language |
MCI = mild cognitive impairment. PLwD = people living with dementia.
The content was initially coded by two authors into three broad areas:
assessment and diagnosis;
management; and
patient and carer experience and influence.
These categories were then refined into subcategories. Links between the data in each subcategory were discussed and arranged in an initial web of causation (see Supplementary Figure S1) from which the same two authors began drafting initial context–mechanism–outcome configurations (CMOCs).
CMOCs are the way that causal statements are expressed in realist reviews. Briefly, they set out how something that functions as context, is linked to an outcome, via a causal process called a mechanism. In other words, in a realist review, something that functions as context, ‘triggers’ a mechanism, which in turn causes an outcome.24
The developed CMOCs were refined with thorough cross-checking and reference to the primary data by three authors. Regular and substantial input was given throughout the process by one of the authors who is an expert in realist review methodology. The review followed RAMESES standards (see Supplementary Table S1).24
RESULTS
Study characteristics
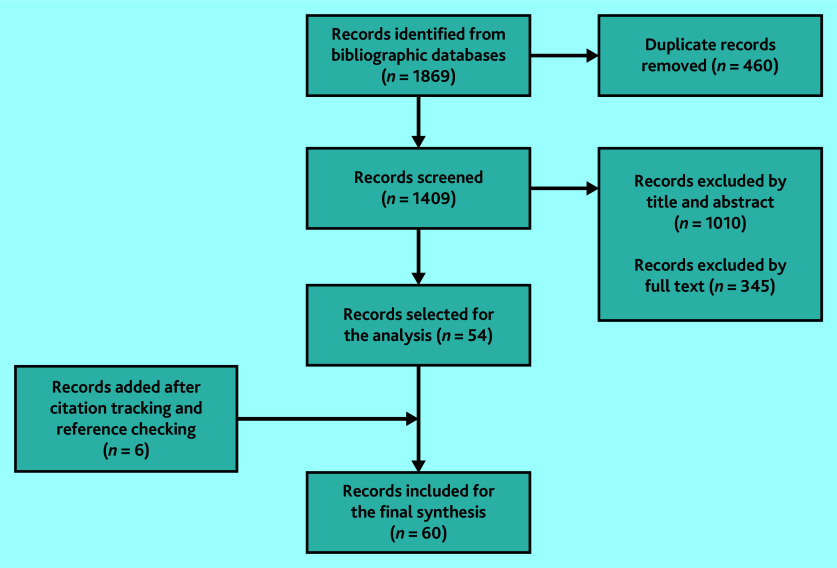
The screening process (Figure 1) ended with 60 included records (see Supplementary Table S2). Most studies focused on the challenges of providing effective care. Reported challenges included problems in care homes,25–39 limited awareness or knowledge among clinicians,39–46 inappropriate use of medication,16,36,44,47–58 assessment and diagnosis difficulties,32,39–41,43–46,59–62 and negative experiences reported by PLwD or MCI and their carers.31,34,41,57,63–78 Few studies explored tailored care as a management strategy.15,78
Figure 1.
PRISMA flow diagram of screened documents.
Publication dates ranged between 1995 and 2022, with 50% of the included studies published within the past decade. Among the documents included, 36 were primary research, 15 were review articles, four were commentaries, and three book chapters. Additionally, one review protocol and one PhD thesis were incorporated, the latter of which had not been published in a peer-reviewed journal at the time of this review. Approximately one-third of the primary studies employed qualitative methodologies. Additionally, qualitative data and information were incorporated from the discussion and conclusion sections of the included studies.
In terms of geographical origin, most of the included articles were from the US (25 articles), followed by the UK (12 articles). Australia and Belgium each accounted for five documents, while Germany and Norway contributed three documents each. Canada, Austria, Ireland, Japan, the Netherlands, and New Zealand were each represented by one document, and one document was developed through international collaboration (UK and Canada).
Context–mechanism–outcome configurations
In total, 19 CMOCs (see Supplementary Table S3) were developed, which are presented under four main themes:
barriers to detection of sleep disturbance;
long-term or inappropriate medication use;
care homes’ role in the management of sleep disturbance; and
positive role of informal carers.
Barriers to detection of sleep disturbance
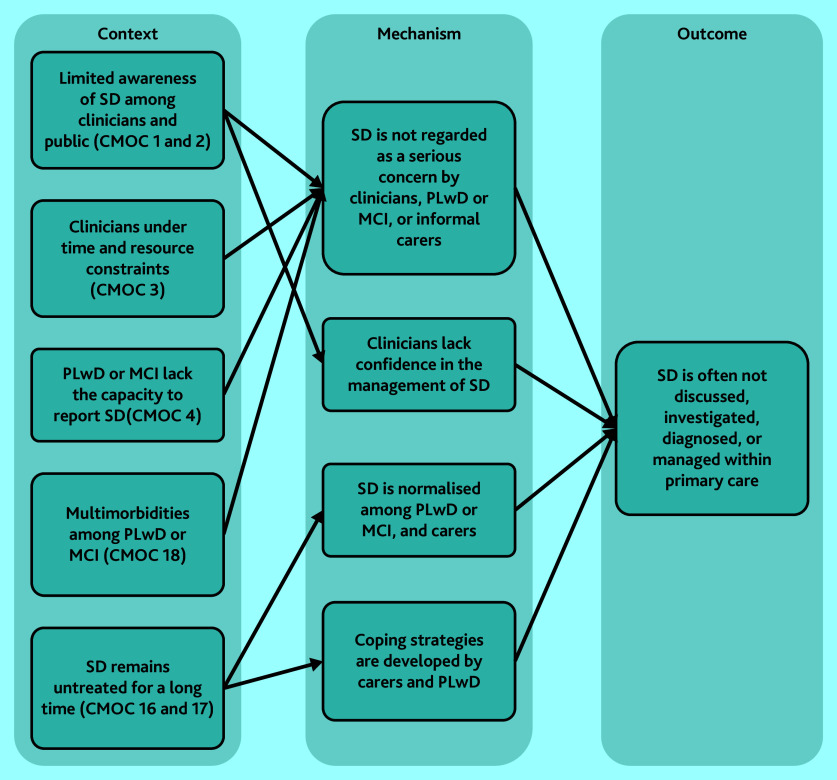
Multiple factors were found to limit discussion, investigation, detection, diagnoses, and management of sleep disturbance within primary care. Most CMOCs (1–4 and 16–18) explain the various reasons behind barriers or challenges and how they hinder appropriate intervention (Figure 2). These challenges result in sleep disturbance being unidentified or deprioritised as a health concern.32,39–41,43–46,59–62
Figure 2.
CMOCs that explain lack of sleep disturbance diagnosis among PLwD or MCI. CMOC = context–mechanism–outcome configuration; see Supplementary Table S3 for CMOC numbering. MCI = mild cognitive impairment. PLwD = people living with dementia. SD = sleep deprivation.
There was limited awareness of sleep disturbance among clinicians, including its potential role in people’s health, possible assessment techniques, and non-pharmacological interventions, which contributed to sleep disturbance being unaddressed for many PLwD or MCI.39–46 Awareness of sleep disturbance among PLwD or MCI and their informal carers also appeared to be limited.40,63,74,75 The symptoms were sometimes normalised and coped with by the patient–carer dyad,76,79,80 or went unrecognised by the PLwD because of cognitive or communication impairments.78 This overall lack of awareness of sleep disturbance meant that discussions about sleep during primary care appointments did not frequently occur.
Long-term or inappropriate medication use
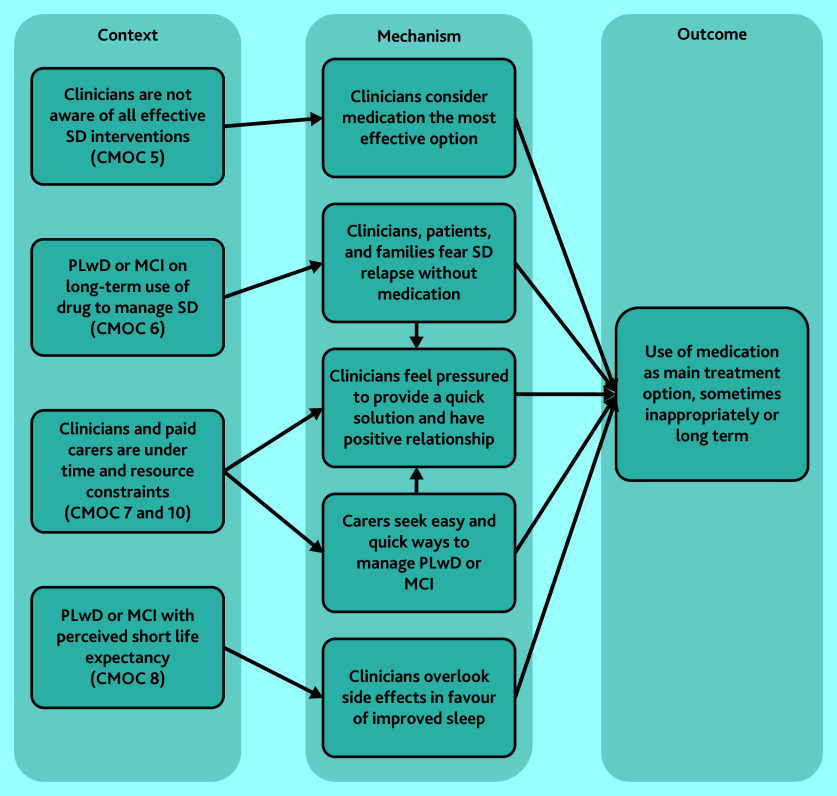
In the instances where sleep disturbance is addressed in primary care, CMOCs 5–8 and 10 reveal how and why inappropriate prescription of sleep medication could manifest for PLwD or MCI (Figure 3). Clinicians’ limited awareness or lack of confidence in non-pharmaceutical interventions15,16,54 can result in medication being the preferred choice. Concerns about withdrawal effects if prescribed medications are ceased, from both clinicians and carers, also contributed to inappropriate long-term medication use.47,50 Carers relied on medication to relieve the stress of sleep deprivation,25,81 and prescribers reportedly felt compelled to prescribe medication to maintain positive relationships with their patients.41,43 The side effects of some medications were sometimes less of a concern for prescribers if the PLwD or MCI had a short life expectancy.34,44,58
Figure 3.
CMOCs that explain the causes for inappropriate or long-term use of medication. CMOC = context–mechanism–outcome configuration; see Supplementary Table S3 for CMOC numbering. MCI = mild cognitive impairment. PLwD = people living with dementia. SD = sleep deprivation.
Care homes’ role in the management of sleep disturbance
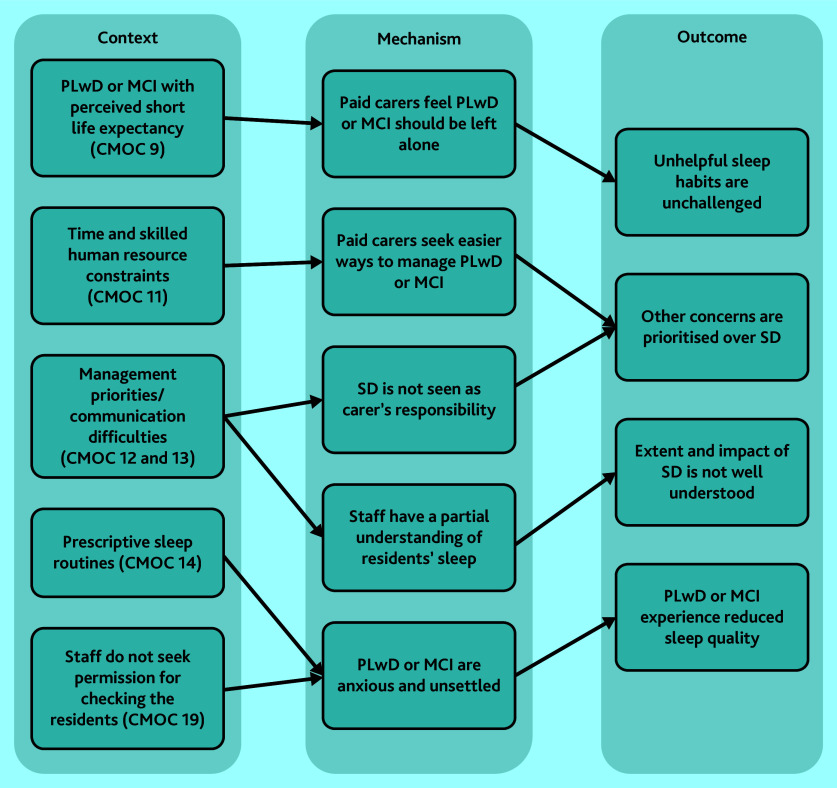
Figure 4 demonstrates the relationships between CMOCs that explore care homes’ role in the management of sleep disturbance (CMOC 9, 11–14, and 19). Some concerns from the themes above, such as limited awareness of sleep disturbance, also apply to staff working in these settings.28,29,31,32,37 Care home staff were reluctant to implement sleep hygiene practices for PLwD or MCI for a range of reasons.64 As a result of environmental, resource, and policy-driven challenges, care homes reportedly imposed rigid routines for residents regardless of their individual circumstances.33,38 In some cases, night-time checks occurred without seeking consent, which was disruptive and anxiety-inducing for residents.31,64
Figure 4.
CMOCs explaining the role of care homes in the management of sleep disturbance. CMOC = context–mechanism–outcome configuration; see Supplementary Table S3 for CMOC numbering. MCI = mild cognitive impairment. PLwD = people living with dementia. SD = sleep deprivation.
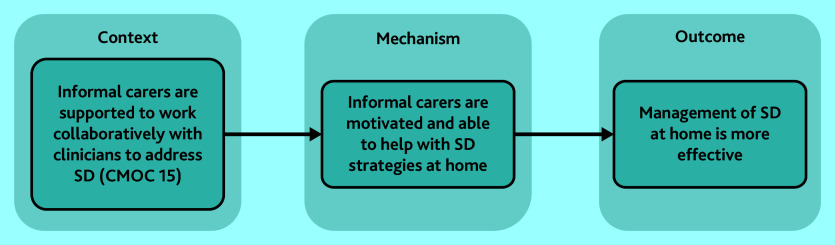
Positive role of informal carers
When informal carers were supported by health systems with information and active assistance (CMOC 15), they were able to follow recommended care plans for sleep management (Figure 5). Interventions for managing sleep disturbance among PLwD or MCI appeared to be dependent on carers, either informal or formal.27,34,42,53,57,61,63,66,68–70,73,76,80,81 Informal carers who were supported with information and guidance reported feeling more able and motivated to implement sleep management strategies at home. Carers also implemented coping strategies for handling sleep disturbance, which reportedly occurred independent of healthcare providers.71,74,76,78,79 However, the efficacy of these strategies was not explored in the literature.
Figure 5.
Role of informal carers in caring for a PLwD or MCI with sleep disturbance. CMOC = context–mechanism–outcome configuration; see Supplementary Table S3 for CMOC numbering. MCI = mild cognitive impairment. PLwD = people living with dementia. SD = sleep deprivation.
DISCUSSION
Summary
The findings in this review highlight numerous challenges and barriers that impair effective assessment, diagnosis, and clinical management of sleep disturbance for PLwD or MCI. The diagnosis of sleep disturbance among this population is hindered by limited awareness of the issue among primary care clinicians and care home staff, compounded by time and resource constraints. PLwD or MCI who are unable to accurately report sleep disturbance can further challenge its identification. Long-term and inappropriate prescribing practices result from clinicians’ and patients’ beliefs that medication is the most effective and easiest ‘quick-fix’ management option. This is exacerbated by clinicians’ lack of confidence in the effectiveness or availability of evidence-based alternative, non-pharmacological interventions such as light therapy, cognitive behavioural therapy, physical activities, social relations with people, or even robopets, or sleep hygiene.15,36,62,70,77,82,83 In care homes, a lack of sufficiently trained staff and other resources, communication challenges, and conflicting organisational priorities resulted in rigid routines that have a negative impact on residents’ sleep. For those living in their own homes, informal carers play a significant role in the implementation of sleep management interventions.
Strengths and limitations
This realist review was a detailed account of current literature on this topic. The review was led by one author who is a leading expert in realist review, and input was received throughout from those with expertise in the subject area. Arguably, the breadth of the inclusion criteria was also a limitation, as the large number of articles included from outside the UK made it difficult to ascertain whether the findings are wholly relevant to UK health provision. For example, there were some discrepancies between countries when reporting prescribing practices.47,48,52 However, many identified themes, such as low levels of awareness of sleep disturbance and the use of medication as the primary treatment method, were common across countries and healthcare systems.
Although including articles without a limit on date of publication allowed for a more comprehensive search, the authors of the current study cannot be sure that the findings detailed are entirely contemporary to current practice. Attitudes towards certain practices, such as the prescription of antipsychotic medications to treat sleep disturbance, have changed in recent years, with the UK Government pledging to reduce the prescription of antipsychotic medications for PLwD by two-thirds.84 Earlier UK-based research may not reflect this policy initiative and inappropriate use of this medication may no longer be contemporaneous with UK practice. However, there is evidence to suggest that although GPs are now aware of the negative consequences of sleep medication, there is a variable attitude to their use because of a perceived lack of alternatives and time constraints.85 Further, although many articles included detailed insight into sleep management practices in care homes, few studies explored current practices stemming from within GP surgeries and also few primary care professionals, excluding care home staff, were included as participants within the reviewed studies. More work is therefore required to accurately determine clinicians’ perspectives, and how current practice within primary care operates outside care homes, including general practice. A detailed exploration of current practice in the UK, including GP surgeries, is currently being conducted as the second phase of the TIMES project.
Comparison with existing literature
Research exploring the general management of sleep disturbance in primary care identifies similar concerns regarding the inappropriate prescription of sleep medication and underutilisation of more tailored non-pharmacological solutions.86–88 Although practitioners may perceive a pressure to prescribe, this practice may actually deter patients with sleep disturbance from seeking further help.89 The use of sleep medication for PLwD or MCI can cause unintended consequences such as the progression of dementia, falls, and loss of functional abilities.13,90 Management of comorbidities, because of issues such as polypharmacy or use of medications for >1 condition, may further hinder deprescribing for different conditions.91 Like the findings in the current study, other studies indicate that when GPs work under time pressures they are prone to suboptimal prescribing practices.91,92 Resource pressures in care homes, such as understaffing and poor communication, was also linked to long-term medication use to manage sleep disturbance.93,94
Implications for research and practice
The current review identified significant gaps within the literature. Further research is therefore needed to explore how sleep disturbance is assessed and managed for people attending GP surgeries and living in their own homes. Little information was available on how sleep disturbance is managed among people with MCI, and the literature did not readily distinguish between people with a dementia or MCI diagnosis. Future research could investigate sleep disturbance in the MCI population and focus on whether approaches do or should differ between PLwD and those with MCI. Finally, although much of the literature focused on the barriers to effective sleep management, few explored current and effective community-based sleep management practices. The authors of the current study would recommend that the perspectives of those implementing any sleep disturbance interventions, such as clinicians or, more likely, carers, are explored before any intervention is developed and more widely implemented. An understanding of carer perspectives is key to developing any effective sleep management tool within primary care.
The deprioritisation of sleep disturbance and lack of confidence in effective assessment and management strategies highlight the need to increase awareness of this area. This awareness should include instilling confidence in evidence-based non-pharmacological interventions, such as light therapy, cognitive behavioural therapy, physical activities, social engagement, and sleep hygiene.15,36,62,70,77,82 Integrating these components as part of a tailored approach to care may enable more effective interventions for sleep disturbance that are sensitive to the person’s unique circumstances and goals. It is essential, however, that any proposal for a new approach to care is implemented with due consideration for the time and resource constraints faced by healthcare and social service providers. The authors of the current study believe that patient care should include timely assessments of PLwD or MCI for possible sleep disturbances, a holistic approach to addressing sleep issues alongside various health conditions in this population, and the provision of continuous and context-specific care.
For PLwD or MCI with sleep disturbance living in care homes, a flexible and context-sensitive approach to managing sleep disturbance may help mitigate some of the negative experiences reported by care home residents at night. This might include adjusting sleep routines, limiting the number of night-time checks, and interventions relevant to residents’ preferences. Night-time care in care homes should be considered no less important than care during the day, and support and training for night staff to recognise, manage, and communicate sleep disturbance should be readily available.
PLwD or MCI, informal carers, and family members may also benefit from a greater awareness and understanding of the impact of poor sleep, good sleep hygiene practices, and advice on the potential side effects of sleep medication, including potentially severe adverse events.13 This could enable some elements of sleep disturbance to be effectively self-managed and alter the expectation or concern that doctors will prescribe as a first port of call. Notably, the current assessment and management of sleep disturbance for PLwD or MCI living at home is often dependent on the input of informal carers and families. Informal carers often experience high levels of burden associated with caring for a PLwD.79,95,96 Any intervention package involving informal carers should therefore be formulated collaboratively and with consideration as to how any proposed intervention can be feasibly implemented given their capabilities and available resources.
Acknowledgments
The authors would like to acknowledge the support of the following research networks: NIHR Applied Research Collaboration South West Peninsula and East of England, the NIHR Exeter Biomedical Research Centre, and the Exeter Clinical Trials Unit. The authors gratefully acknowledge the local principal investigators and researchers involved in participant recruitment, assessment, and intervention. The authors are grateful to the TIMES study participants for their participation in the study and to members of the patient and public involvement and engagement group for their support and contribution throughout the study.
Funding
This study is funded by the National Institute for Health and Care Research (NIHR) under its Programme Grants for Applied Research (grant reference number: NIHR202345). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Ethical approval
Not applicable.
Data
Not applicable.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article:
References
- 1.Sleep Foundation Sleep disorders. 2022. https://www.sleepfoundation.org/sleep-disorders (accessed 7 Mar 2024).
- 2.Alzheimer’s Society Sleep problems and treatments for people with dementia. 2021. https://www.alzheimers.org.uk/about-dementia/symptoms-and-diagnosis/sleep-problems-treatments-dementia (accessed 7 Mar 2024).
- 3.Mayo Clinic Sleep disorders. 2019. https://www.mayoclinic.org/diseases-conditions/sleep-disorders/symptoms-causes/syc-20354018 (accessed 7 Mar 2024).
- 4.Caputo M, Monastero R, Mariani E, et al. Neuropsychiatric symptoms in 921 elderly subjects with dementia: a comparison between vascular and neurodegenerative types. Acta Psychiatr Scand. 2008;117(6):455–464. doi: 10.1111/j.1600-0447.2008.01175.x. [DOI] [PubMed] [Google Scholar]
- 5.Huang S-S, Wang W-F, Liao Y-C. Severity and prevalence of behavioral and psychological symptoms among patients of different dementia stages in Taiwan. Arch Clin Psychiatry. 2017;44(4):89–93. [Google Scholar]
- 6.Shi L, Chen S-J, Ma M-Y, et al. Sleep disturbances increase the risk of dementia: a systematic review and meta-analysis. Sleep Med Rev. 2018;40:4–16. doi: 10.1016/j.smrv.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Bubu OM, Andrade AG, Umasabor-Bubu OQ, et al. Obstructive sleep apnea, cognition and Alzheimer’s disease: a systematic review integrating three decades of multidisciplinary research. Sleep Med Rev. 2020;50:101250. doi: 10.1016/j.smrv.2019.101250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koren T, Fisher E, Webster L, et al. Prevalence of sleep disturbances in people with dementia living in the community: a systematic review and meta-analysis. Ageing Res Rev. 2023;83:101782. doi: 10.1016/j.arr.2022.101782. [DOI] [PubMed] [Google Scholar]
- 9.Webster L, Costafreda Gonzalez S, Stringer A, et al. Measuring the prevalence of sleep disturbances in people with dementia living in care homes: a systematic review and meta-analysis. Sleep. 2020;43(4):zsz251. doi: 10.1093/sleep/zsz251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guarnieri B, Adorni F, Musicco M, et al. Prevalence of sleep disturbances in mild cognitive impairment and dementing disorders: a multicenter Italian clinical cross-sectional study on 431 patients. Dement Geriatr Cogn Disord. 2012;33(1):50–58. doi: 10.1159/000335363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKinnon A, Terpening Z, Hickie IB, et al. Prevalence and predictors of poor sleep quality in mild cognitive impairment. J Geriatr Psychiatry Neurol. 2014;27(3):204–211. doi: 10.1177/0891988714527516. [DOI] [PubMed] [Google Scholar]
- 12.Mubashir T, Abrahamyan L, Niazi A, et al. The prevalence of obstructive sleep apnea in mild cognitive impairment: a systematic review. BMC Neurol. 2019;19(1):195. doi: 10.1186/s12883-019-1422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson K, Loke YK, Fox C, et al. Adverse effects of Z-drugs for sleep disturbance in people living with dementia: a population-based cohort study. BMC Med. 2020;18(1):351. doi: 10.1186/s12916-020-01821-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson R, Dowell A, Jones L, Gander P. Non-pharmacological interventions a feasible option for addressing dementia-related sleep problems in the context of family care. Pilot Feasibility Stud. 2021;7(1):114. doi: 10.1186/s40814-021-00851-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livingston G, Barber JA, Kinnunen KM, et al. DREAMS-START (Dementia RElAted Manual for Sleep; STrAtegies for RelaTives) for people with dementia and sleep disturbances: a single-blind feasibility and acceptability randomized controlled trial. Int Psychogeriatr. 2019;31(2):251–265. doi: 10.1017/S1041610218000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Caoimh R, Mannion H, Sezgin D, et al. Non-pharmacological treatments for sleep disturbance in mild cognitive impairment and dementia: a systematic review and meta-analysis. Maturitas. 2019;127:82–94. doi: 10.1016/j.maturitas.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Kinnunen KM, Vikhanova A, Livingston G. The management of sleep disorders in dementia: an update. Curr Opin Psychiatry. 2017;30(6):491–497. doi: 10.1097/YCO.0000000000000370. [DOI] [PubMed] [Google Scholar]
- 18.Burns D, Hyde P, Killett A. Wicked problems or wicked people? Reconceptualising institutional abuse. Sociol Health Illn. 2013;35(4):514–528. doi: 10.1111/j.1467-9566.2012.01511.x. [DOI] [PubMed] [Google Scholar]
- 19.Rittel HW, Webber MM. Dilemmas in a general theory of planning. Policy Sci. 1973;4(2):155–169. [Google Scholar]
- 20.Fox C. Developing tailored primary care management of sleep problems in people with dementia and mild cognitive impairment. https://arc-eoe.nihr.ac.uk/research-implementation/research-themes/ageing-and-multi-morbidity/times-tailored-management-sleep (accessed 7 Mar 2024).
- 21.Greene L, Aryankhesal A, Megson M, et al. Understanding primary care diagnosis and management of sleep disturbance for people with dementia or mild cognitive impairment: a realist review protocol. BMJ Open. 2022;12(11):e067424. doi: 10.1136/bmjopen-2022-067424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pawson R, Greenhalgh T, Harvey G, Walshe K. Realist review-a new method of systematic review designed for complex policy interventions. J Health Serv Res Policy. 2005;10(1_suppl):21–34. doi: 10.1258/1355819054308530. [DOI] [PubMed] [Google Scholar]
- 23.Hunter R, Gorely T, Beattie M, Harris K. Realist review. Int Rev Sport Exerc Psychol. 2022;15(1):242–265. [Google Scholar]
- 24.Wong G, Greenhalgh T, Westhorp G, et al. RAMESES publication standards: realist syntheses. BMC Med. 2013;11:21. doi: 10.1186/1741-7015-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webster L, Powell K, Costafreda SG, Livingston G. The impact of sleep disturbances on care home residents with dementia: the SIESTA qualitative study. Int Psychogeriatr. 2020;32(7):839–847. doi: 10.1017/S1041610220000642. [DOI] [PubMed] [Google Scholar]
- 26.Agnew T. Common sense solutions for sleepless nights. Nurs Older People. 2008;20(5):7–8. [PubMed] [Google Scholar]
- 27.Alessi CA, Schnelle JF. Approach to sleep disorders in the nursing home setting: review article. Sleep Med Rev. 2000;4(1):45–56. doi: 10.1053/smrv.1999.0066. [DOI] [PubMed] [Google Scholar]
- 28.Blytt KM, Bjorvatn B, Husebo B, Flo E. Clinically significant discrepancies between sleep problems assessed by standard clinical tools and actigraphy. BMC Geriatr. 2017;17(1):253. doi: 10.1186/s12877-017-0653-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blytt KM, Flo-Groeneboom E. New knowledge on the impact of sleep disturbances illustrates the urgent need to address sleep problems in nursing home residents. Int Psychogeriatr. 2020;32(7):795–797. doi: 10.1017/S1041610220001106. [DOI] [PubMed] [Google Scholar]
- 30.Janus SI, Kosters J, van den Bosch KA, et al. Sounds in nursing homes and their effect on health in dementia: a systematic review. Int Psychogeriatr. 2021;33(6):627–644. doi: 10.1017/S1041610220000952. [DOI] [PubMed] [Google Scholar]
- 31.Kerr D, Wilkinson H, Cunningham C. Supporting older people in care homes at night. 2008 https://www.pure.ed.ac.uk/ws/portalfiles/portal/10247770/Supporting_older_people_in_care_homes_at_night.pdf (accessed 7 Mar 2024). [Google Scholar]
- 32.Kontos P, Martin W. Embodiment and dementia: exploring critical narratives of selfhood, surveillance, and dementia care. Dementia (London) 2013;12(3):288–302. doi: 10.1177/1471301213479787. [DOI] [PubMed] [Google Scholar]
- 33.Nunez KM, Khan Z, Testad I, et al. Current practice and challenges in night-time care for people with dementia living in care homes: a qualitative study. Int J Geriatr Psychiatry. 2018;33(1):e140–e149. doi: 10.1002/gps.4737. [DOI] [PubMed] [Google Scholar]
- 34.Nygaard A, Halvorsrud L, Grov EK, Bergland A. ‘What matters to you?’ — a qualitative study on the views of nursing home residents with dementia regarding the health care they receive. J Clin Nurs. 2022;31(1–2):262–274. doi: 10.1111/jocn.15904. [DOI] [PubMed] [Google Scholar]
- 35.Snow AL, Loup J, Morgan RO, et al. Enhancing sleep quality for nursing home residents with dementia: a pragmatic randomized controlled trial of an evidence-based frontline huddling program. BMC Geriatr. 2021;21(1):281. doi: 10.1186/s12877-021-02189-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song Y, Dowling GA, Wallhagen MI, et al. Sleep in older adults with Alzheimer’s disease. J Neurosci Nurs. 2010;42(4):190–200. doi: 10.1097/jnn.0b013e3181e26b1d. [DOI] [PubMed] [Google Scholar]
- 37.Lamp Group Nurses impress at royal commission: evidence from nurses was well received at the inquiry into Australia’s aged care system. Lamp. 2019;76(5):8–9. [Google Scholar]
- 38.Webster L, Costafreda SG, Powell K, Livingston G. How do care home staff use non-pharmacological strategies to manage sleep disturbances in residents with dementia: the SIESTA qualitative study. PLoS One. 2022;17(8):e0272814. doi: 10.1371/journal.pone.0272814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yesavage JA, Friedman L, Ancoli-Israel S, et al. Development of diagnostic criteria for defining sleep disturbance in Alzheimer’s disease. J Geriatr Psychiatry Neurol. 2003;16(3):131–139. doi: 10.1177/0891988703255684. [DOI] [PubMed] [Google Scholar]
- 40.Brown CA, Wielandt P, Wilson D, et al. Healthcare providers’ knowledge of disordered sleep, sleep assessment tools, and nonpharmacological sleep interventions for persons living with dementia: a national survey. Sleep Disord. 2014;2014:286274. doi: 10.1155/2014/286274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flick U, Garms-Homolová V, Röhnsch G. “And mostly they have a need for sleeping pills”: physicians’ views on treatment of sleep disorders with drugs in nursing homes. J Aging Stud. 2012;26(4):484–494. doi: 10.1016/j.jaging.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. doi: 10.1136/bmj.h369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee DR, Thomas AJ. Sleep in dementia a caregiving – assessment and treatment implications: a review. Int Psychogeriatr. 2011;23(2):190–201. doi: 10.1017/S1041610210001894. [DOI] [PubMed] [Google Scholar]
- 44.Lichstein KL, Morin CM. Treatment of late-life insomnia. London: Sage; 2000. [Google Scholar]
- 45.Papp KK, Penrod CE, Strohl KP. Knowledge and attitudes of primary care physicians toward sleep and sleep disorders. Sleep Breath. 2002;6(3):103–109. doi: 10.1007/s11325-002-0103-3. [DOI] [PubMed] [Google Scholar]
- 46.Stoppe G, Sandholzer H, Staedt J, et al. Sleep disturbances in the demented elderly: treatment in ambulatory care. Sleep. 1995;18(10):844–848. doi: 10.1093/sleep/18.10.844. [DOI] [PubMed] [Google Scholar]
- 47.Azermai M, Elseviers M, Petrovic M, et al. Assessment of antipsychotic prescribing in Belgian nursing homes. Int psychogeriatr. 2011;23(8):1240–1248. doi: 10.1017/S104161021100024X. [DOI] [PubMed] [Google Scholar]
- 48.Azermai M, Elseviers M, Petrovic M, et al. Geriatric drug utilisation of psychotropics in Belgian nursing homes. Hum Psychopharmacol. 2011;26(1):12–20. doi: 10.1002/hup.1160. [DOI] [PubMed] [Google Scholar]
- 49.Borson S, Scanlan JM, Doane K, Gray S. Antidepressant prescribing in nursing homes: is there a place for tricyclics? Int J Geriatr Psychiatry. 2002;17(12):1140–1145. doi: 10.1002/gps.766. [DOI] [PubMed] [Google Scholar]
- 50.Bourgeois J, Elseviers M, Azermai M, et al. Benzodiazepine use in Belgian nursing homes: a closer look into indications and dosages. Eur J Clin Pharmacol. 2012;68(5):833–844. doi: 10.1007/s00228-011-1188-z. [DOI] [PubMed] [Google Scholar]
- 51.Bourgeois J, Elseviers MM, Van Bortel L, et al. The use of antidepressants in Belgian nursing homes: focus on indications and dosages in the PHEBE study. Drugs Aging. 2012;29(9):759–769. doi: 10.1007/s40266-012-0003-6. [DOI] [PubMed] [Google Scholar]
- 52.Brimelow RE, Wollin JA, Byrne GJ, Dissanayaka NN. Prescribing of psychotropic drugs and indicators for use in residential aged care and residents with dementia. Int Psychogeriatr. 2019;31(6):837–847. doi: 10.1017/S1041610218001229. [DOI] [PubMed] [Google Scholar]
- 53.Class CA, Schneider L, Farlow MR. Optimal management of behavioural disorders associated with dementia. Drugs Aging. 1997;10(2):95–106. doi: 10.2165/00002512-199710020-00003. [DOI] [PubMed] [Google Scholar]
- 54.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mann E, Köpke S, Haastert B, et al. Psychotropic medication use among nursing home residents in Austria: a cross-sectional study. BMC Geriatr. 2009;9:18. doi: 10.1186/1471-2318-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGee N, Hart AM, Burman M. Reconsidering benzodiazepines and z-drug prescriptions: responsible prescribing and deprescribing. J Nurse Pract. 2021;17(1):76–83. [Google Scholar]
- 57.Polenick CA, Leggett AN, Maust DT, Kales HC. Medical care tasks among spousal dementia caregivers: links to care-related sleep disturbances. Am J Geriatr Psychiatry. 2018;26(5):589–597. doi: 10.1016/j.jagp.2018.01.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shuster JL. Palliative care for advanced dementia. Clin Geriatr Med. 2000;16(2):373–386. doi: 10.1016/s0749-0690(05)70062-8. [DOI] [PubMed] [Google Scholar]
- 59.Anderson KN, Catt M, Collerton J, et al. Assessment of sleep and circadian rhythm disorders in the very old: the Newcastle 85+ Cohort Study. Age Ageing. 2014;43(1):57–63. doi: 10.1093/ageing/aft153. [DOI] [PubMed] [Google Scholar]
- 60.Benca RM. Diagnosis and treatment of chronic insomnia: a review. Psychiatr Serv. 2005;56(3):332–343. doi: 10.1176/appi.ps.56.3.332. [DOI] [PubMed] [Google Scholar]
- 61.Jan DL, Delfine D, Eileen VDP, et al. The management of dementia by flemish GPs: it remains a difficult job. Acta Clin Belg. 2021;76(4):264–271. doi: 10.1080/17843286.2020.1716462. [DOI] [PubMed] [Google Scholar]
- 62.McCrae CS, Dzierzewski JM, Kay DB. Treatment of late-life insomnia. Sleep Med Clin. 2009;4(4):593–604. doi: 10.1016/j.jsmc.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corey KL, McCurry MK. When caregiving ends: the experiences of former family caregivers of people with dementia. Gerontologist. 2018;58(2):e87–e96. doi: 10.1093/geront/gnw205. [DOI] [PubMed] [Google Scholar]
- 64.Flick U, Garms-Homolova V, Rohnsch G. ‘When they sleep, they sleep’: daytime activities and sleep disorders in nursing homes. J Health Psychol. 2010;15(5):755–764. doi: 10.1177/1359105310368182. [DOI] [PubMed] [Google Scholar]
- 65.Koch S, Haesler E, Tiziani A, Wilson J. Effectiveness of sleep management strategies for residents of aged care facilities: findings of a systematic review. J Clin Nurs. 2006;15(10):1267–1275. doi: 10.1111/j.1365-2702.2006.01385.x. [DOI] [PubMed] [Google Scholar]
- 66.Kotronoulas G, Wengstrom Y, Kearney N. Sleep and sleep-wake disturbances in care recipient-caregiver dyads in the context of a chronic illness: a critical review of the literature. J Pain Symptom Manage. 2013;45(3):579–594. doi: 10.1016/j.jpainsymman.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y, Leggett AN, Kim K, et al. Daily sleep, well-being, and adult day services use among dementia care dyads. Aging Ment Health. 2022;26(12):2472–2480. doi: 10.1080/13607863.2021.1998354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Logsdon RG, McCurry SM, Teri L. Behavioral treatment of affective disorders and associated symptoms. In: Attix DK, Welsh-Bohmer KA, editors. Geriatric neuropsychology: assessment and intervention. New York, NY: Guilford Press; 2006. pp. 349–366. [Google Scholar]
- 69.Matsuda O, Hasebe N, Ikehara K, et al. Longitudinal study of the mental health of caregivers caring for elderly patients with dementia: effect of institutional placement on mental health. Psychiatry Clin Neurosci. 1997;51(5):289–293. doi: 10.1111/j.1440-1819.1997.tb03200.x. [DOI] [PubMed] [Google Scholar]
- 70.McCurry SM, Gibbons LE, Logsdon RG, et al. Training caregivers to change the sleep hygiene practices of patients with dementia: the NITE-AD Project. J Am Geriatr Soc. 2003;51(10):1455–1460. doi: 10.1046/j.1532-5415.2003.51466.x. [DOI] [PubMed] [Google Scholar]
- 71.McCurry SM, LaFazia DM, Pike KC, et al. Managing sleep disturbances in adult family homes: recruitment and implementation of a behavioral treatment program. Geriatr Nurs. 2009;30(1):36–44. doi: 10.1016/j.gerinurse.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCurry SM, LaFazia DM, Pike KC, et al. Development and evaluation of a sleep education program for older adults with dementia living in adult family homes. Am J Geriatr Psychiatry. 2012;20(6):494–504. doi: 10.1097/JGP.0b013e318248ae79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McCurry SM, Pike KC, Vitiello MV, et al. Factors associated with concordance and variability of sleep quality in persons with Alzheimer’s disease and their caregivers. Sleep. 2008;31(5):741–748. doi: 10.1093/sleep/31.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moore RC, Harmell AL, Chattillion E, et al. PEAR model and sleep outcomes in dementia caregivers: influence of activity restriction and pleasant events on sleep disturbances. Int Psychogeriatr. 2011;23(9):1462–1469. doi: 10.1017/S1041610211000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brown DT, Westbury JL, Schüz B. Sleep and agitation in nursing home residents with and without dementia. Int Psychogeriatr. 2015;27(12):1945–1955. doi: 10.1017/S1041610215001568. [DOI] [PubMed] [Google Scholar]
- 76.Rapaport P, Webster L, Horsley R, et al. An intervention to improve sleep for people living with dementia: Reflections on the development and co-production of DREAMS:START (Dementia RElAted Manual for Sleep: STrAtegies for RelaTives) Dementia (London) 2018;17(8):976–989. doi: 10.1177/1471301218789559. [DOI] [PubMed] [Google Scholar]
- 77.Richards KC, Beck C, O’Sullivan PS, Shue VM. Effect of individualized social activity on sleep in nursing home residents with dementia. J Am Geriatr Soc. 2005;53(9):1510–1517. doi: 10.1111/j.1532-5415.2005.53460.x. [DOI] [PubMed] [Google Scholar]
- 78.Svendsboe EJ. Carers to people with Lewy body dementia and Alzheimer’s disease: experiences and coping strategies. 2018 https://openarchive.ki.se/xmlui/bitstream/handle/10616/46481/Thesis_Ellen_Svendsboe.pdf (accessed 7 Mar 2024). [Google Scholar]
- 79.Gibson RH, Gander PH, Jones LM. Understanding the sleep problems of people with dementia and their family caregivers. Dementia (London) 2014;13(3):350–365. doi: 10.1177/1471301212473884. [DOI] [PubMed] [Google Scholar]
- 80.Kaskie B, Bobitt J, Herrera J, et al. Cannabis use among persons with dementia and their caregivers: Lighting up an emerging issue for clinical gerontologists. Clin Gerontol. 2021;44(1):42–52. doi: 10.1080/07317115.2020.1852465. [DOI] [PubMed] [Google Scholar]
- 81.Wade R, Pachana NA, Dissanayaka N. Factors related to sleep disturbances for informal carers of individuals with PD and dyadic relationship: a rural perspective. J Geriatr Psychiatry Neurol. 2021;34(5):389–396. doi: 10.1177/0891988720944250. [DOI] [PubMed] [Google Scholar]
- 82.Jin JW, Nowakowski S, Taylor A, et al. Cognitive behavioral therapy for mood and insomnia in persons with dementia: a systematic review. Alzheimer Dis Assoc Disord. 2021;35(4):366–373. doi: 10.1097/WAD.0000000000000454. [DOI] [PubMed] [Google Scholar]
- 83.Kohn JN, Lee EE. Improving sleep by fostering social connection for dementia patients in long-term care. Int Psychogeriatr. 2021;33(10):1005–1007. doi: 10.1017/S1041610221000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Medicines and Healthcare products Regulatory Agency Antipsychotics: initiative to reduce prescribing to older people with dementia. 2014. https://www.gov.uk/drug-safety-update/antipsychotics-initiative-to-reduce-prescribing-to-older-people-with-dementia (accessed 7 Mar 2024).
- 85.Sirdifield C, Anthierens S, Creupelandt H, et al. General practitioners’ experiences and perceptions of benzodiazepine prescribing: systematic review and meta-synthesis. BMC Fam Pract. 2013;14:191. doi: 10.1186/1471-2296-14-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Doherty AJ, Boland P, Reed J, et al. Barriers and facilitators to deprescribing in primary care: a systematic review. BJGP Open. 2020. DOI: [DOI] [PMC free article] [PubMed]
- 87.Wallis KA, Andrews A, Henderson M. Swimming against the tide: primary care physicians’ views on deprescribing in everyday practice. Ann Fam Med. 2017;15(4):341–346. doi: 10.1370/afm.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sirdifield C, Chipchase SY, Owen S, Siriwardena AN. A systematic review and meta-synthesis of patients’ experiences and perceptions of seeking and using benzodiazepines and Z-drugs: towards safer prescribing. Patient. 2017;10(1):1–15. doi: 10.1007/s40271-016-0182-z. [DOI] [PubMed] [Google Scholar]
- 89.Cheung JM, Bartlett DJ, Armour CL, Saini B. The insomnia patient perspective, a narrative review. Behav Sleep Med. 2013;11(5):369–389. doi: 10.1080/15402002.2012.694382. [DOI] [PubMed] [Google Scholar]
- 90.Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38(11):2340–2372. doi: 10.1016/j.clinthera.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 91.Cadogan CA, Ryan C, Francis JJ, et al. Improving appropriate polypharmacy for older people in primary care: selecting components of an evidence-based intervention to target prescribing and dispensing. Implement Sci. 2015;10:161. doi: 10.1186/s13012-015-0349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luijks HD, Loeffen MJ, Lagro-Janssen AL, et al. GPs’ considerations in multimorbidity management: a qualitative study. Br J Gen Pract. 2012. DOI: [DOI] [PMC free article] [PubMed]
- 93.Bell HT, Granas AG, Enmarker I, et al. Nurses’ and pharmacists’ learning experiences from participating in interprofessional medication reviews for elderly in primary health care–a qualitative study. BMC Fam Pract. 2017;18(1):30. doi: 10.1186/s12875-017-0598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Palagyi A, Keay L, Harper J, et al. Barricades and brickwalls–a qualitative study exploring perceptions of medication use and deprescribing in long-term care. BMC Geriatr. 2016;16:15. doi: 10.1186/s12877-016-0181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lawson S, Mullan J, Wong G, et al. Family carers’ experiences of managing older relative’s medications: insights from the MEMORABLE study. Patient Educ Couns. 2022;105(7):2573–2580. doi: 10.1016/j.pec.2021.12.017. [DOI] [PubMed] [Google Scholar]
- 96.Parkinson M, Carr SM, Rushmer R, Abley C. Investigating what works to support family carers of people with dementia: a rapid realist review. J Public Health (Oxf) 2017;39(4):e290–e301. doi: 10.1093/pubmed/fdw100. [DOI] [PMC free article] [PubMed] [Google Scholar]