Abstract
Background & Aims
The liver is one of the organs most commonly affected by metastasis. The presence of liver metastases has been reported to be responsible for an immunosuppressive microenvironment and diminished immunotherapy efficacy. Herein, we aimed to investigate the role of IL-10 in liver metastasis and to determine how its modulation could affect the efficacy of immunotherapy in vivo.
Methods
To induce spontaneous or forced liver metastasis in mice, murine cancer cells (MC38) or colon tumor organoids were injected into the cecum or the spleen, respectively. Mice with complete and cell type-specific deletion of IL-10 and IL-10 receptor alpha were used to identify the source and the target of IL-10 during metastasis formation. Programmed death ligand 1 (PD-L1)-deficient mice were used to test the role of this checkpoint. Flow cytometry was applied to characterize the regulation of PD-L1 by IL-10.
Results
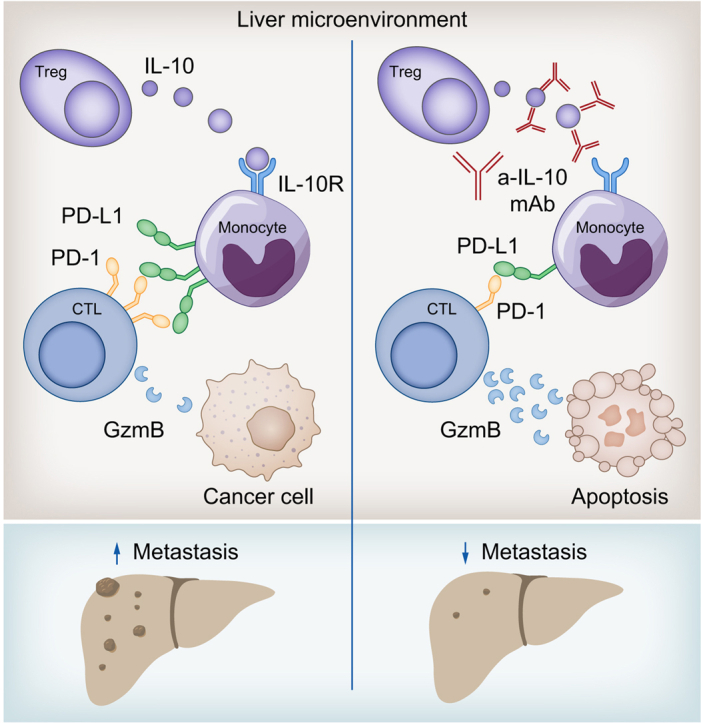
We found that Il10-deficient mice and mice treated with IL-10 receptor alpha antibodies were protected against liver metastasis formation. Furthermore, by using IL-10 reporter mice, we demonstrated that Foxp3+ regulatory T cells (Tregs) were the major cellular source of IL-10 in liver metastatic sites. Accordingly, deletion of IL-10 in Tregs, but not in myeloid cells, led to reduced liver metastasis. Mechanistically, IL-10 acted on Tregs in an autocrine manner, thereby further amplifying IL-10 production. Furthermore, IL-10 acted on myeloid cells, i.e. monocytes, and induced the upregulation of the immune checkpoint protein PD-L1. Finally, the PD-L1/PD-1 axis attenuated CD8-dependent cytotoxicity against metastatic lesions.
Conclusions
Treg-derived IL-10 upregulates PD-L1 expression in monocytes, which in turn reduces CD8+ T-cell infiltration and related antitumor immunity in the context of colorectal cancer-derived liver metastases. These findings provide the basis for future monitoring and targeting of IL-10 in colorectal cancer-derived liver metastases.
Impact and implications
Liver metastasis diminishes the effectiveness of immunotherapy and increases the mortality rate in patients with colorectal cancer. We investigated the role of IL-10 in liver metastasis formation and assessed its impact on the effectiveness of immunotherapy. Our data show that IL-10 is a pro-metastatic factor involved in liver metastasis formation and that it acts as a regulator of PD-L1. This provides the basis for future monitoring and targeting of IL-10 in colorectal cancer-derived liver metastasis.
Keywords: liver metastasis, IL-10, PD-L1, immunotherapy, immune cells
Graphical abstract
Highlights
-
•
IL-10 promotes colorectal cancer-derived liver metastasis.
-
•
Foxp3+Tregs are the main source of IL-10 during liver metastasis formation.
-
•
IL-10 acts on myeloid cells thereby dampening antitumor immunity.
-
•
The effect of IL-10 is at least in part mediated by PD-L1.
Introduction
Liver metastasis is one of the major causes of cancer-associated mortality.1 Among cancer entities, colorectal cancer (CRC) is one of the most common cancers worldwide with a high metastatic rate – mainly to the liver.2 Furthermore, other entities like lung cancer, breast cancer, and skin cancer, metastasize to the liver.3 Despite decades of cancer research, treating metastatic cancers still poses a challenge. Thus, further research focusing on metastasis formation and progression is essential. The presence of liver metastases can also influence the selection of appropriate therapeutic regimens, since it has been associated with reduced responsiveness to immune checkpoint inhibitors, for example, anti-programmed death 1 (PD-1) and anti-programmed death ligand 1 (PD-L1) antibodies.4 The roles and regulation of immune checkpoints in liver metastases have not yet been well described. Interestingly, PD-L1 expression was found to be higher in liver metastases compared to primary CRC, while positively correlating with the number of infiltrating T cells.5 Additionally, it was demonstrated that radiotherapy, specifically on the liver, could increase tumor-specific T-cell survival and restore immunotherapy efficacy.4 Thus, it is critical to decipher the regulation of immune checkpoint molecules in liver metastasis to increase the effectiveness of immunotherapy.
IL-10, the founding member of the IL-10 cytokine family, is known as a key cytokine for immune regulation.6 In the last decade, several studies analyzed the role of IL-10 in primary cancer.[7], [8], [9], [10] IL-10 administration in preclinical models8,9 and clinical trials11,12 showed a beneficial effect on primary tumor sites, thereby identifying IL-10 as a new therapeutic approach for patients with different cancer entities. In fact, IL-10 seems to play a protective role in tumor immunology by increasing the survival and cytotoxicity of CD8+ T cells.13 In line with this, IL-10 was reported to be similarly protective in lung metastases.10 In contrast, the role of IL-10 in liver metastasis has so far been poorly described, with the exception of a recent in vitro study. In this study, organotypic slice cultures from human CRC-derived liver metastases were used and antitumor effects of a neutralizing antibody against IL-10 both alone as treatment and in combination with exogenously administered carcinoembryonic antigen-specific chimeric antigen receptor T (CAR-T) cells were analyzed. Interestingly, the authors found that IL-10 blockade could enhance the effectiveness of CAR-T cell treatment.14 However, the cellular source of IL-10, its function and mechanism of action in vivo was not analyzed in this study. This was the aim of our work. Using several mouse models, we found that despite the protective effect on primary tumors, IL-10 instead promotes liver metastasis. IL-10 does not affect cancer cell extravasation, but rather influences later stages of the metastatic cascade. Specifically, Foxp3+Treg-derived IL-10 acts on Foxp3+Tregs themselves, thereby amplifying IL-10 production. Furthermore, IL-10 promotes PD-L1 upregulation on monocytes, which subsequently suppresses CD8+ T-cell-mediated immune surveillance. Accordingly, the deletion of PD-L1 resulted in a reduction of liver metastases. In conclusion, these data identify IL-10 as a pro-metastatic factor in liver metastasis formation and characterize this cytokine as a regulator of PD-L1.
Materials and methods
Mice
C57BL/6J, Il10-/-, Il10flox/flox;Foxp3cre+, Il10flox/flox;Lysmcre+, Rag-/-(Yale), Il10eGFP;Foxp3RFP, Il10raflox/flox;Cdh5cre+, Il17acre+;Rosa26floxSTOPfloxYFP;Foxp3RFP, Il10raflox/flox;Lysmcre+, Il10raflox/flox;Cd11ccre+, Il10raflox/flox;Il17acre+, DEREG, Pdl1-/- were housed in the animal facility of the University Medical Center Hamburg-Eppendorf under specific pathogen-free conditions. Il10raflox/flox mice (kindly provided by Richard A. Flavell) and Il10flox/flox were validated and characterized before.15,16 Details of the sources of these mouse lines were listed in the supplementary CTAT table. Age- (8-14 weeks) and sex-matched littermates were used for experiments. All animal experiments were approved by the Institutional Review Board “Behörde für Justiz und Verbraucherschutz, Lebensmittelsicherheit und Veterinärwesen” (Hamburg, Germany).
Cancer cell lines and organoids
Lewis lung carcinoma (LLC) and colon adenocarcinoma (MC38) cancer cells were cultured in DMEM containing 10% FBS and penicillin-streptomycin. MC38 cells were lentivirally transduced to express the green fluorescence protein (MC38-GFP) and puromycin resistance. As an alternative neutral control, MC38 cells were lentivirally transduced to express a non-targeted short hairpin RNA (MC38-shC) and a puromycin resistance. Transduced MC38 were cultured with 10% FBS DMEM in the presence of penicillin-streptomycin and puromycin.17 Lentiviral vectors were produced following our established protocols.18 Cells were harvested at around 80% confluency for in vivo injections. Mouse tumor organoids 129 (MTO129) were kindly provided by Prof. Eduard Batlle. MTO culture was performed as previously described.19
Mouse models for liver metastasis induction
Spontaneous liver metastasis induction
Spontaneous liver metastasis was established by injecting cancer cells into the cecum (2 × 105 cells per mouse), as described previously.20 The humane endpoint of mice was in accordance with the approved animal protocol. Mice were anesthetized with isoflurane and an incision was made in the middle of the abdomen to expose the cecum. This was followed by an orthotopic injection of cancer cells dissolved in 50 μl PBS into the cecum using a 32-G needle until a solid bubble was formed on the caecum wall. Four weeks post injection, the caecum and liver were harvested for further analysis.
Forced liver metastasis induction
Forced liver metastasis was induced by injecting cancer cells intrasplenically. Under anesthesia, the spleen was exposed and subsequently, 3.5 × 105 MC38 cancer cells dissolved in 100 μl PBS were intrasplenically injected using a 27-G needle, followed by a partial splenectomy. For MTOs, 3.5-5 x 105 cells dissolved in 100 μl HBSS were injected into each mouse. After 3 weeks, the mice were euthanized and the livers were harvested for further analysis. For anti-PD-L1 antibody treatment, 250 μg per mouse was injected intraperitoneally every 3 days. Anti-PD-L1 antibody administration was started 1 day post liver metastasis induction. For Foxp3+ Treg depletion, DEREG mice received 600 ng diphtheria toxin intraperitoneally, starting 1 day before liver metastasis induction, followed by weekly injections of 200 ng diphtheria toxin.
Immune cell isolation
Murine livers were harvested after PBS perfusion and gallbladder removal. Human samples were obtained from patients with CRC and resectable liver metastases at the Surgical Department of the University Medical Center Hamburg-Eppendorf. Murine and human tissues were cut into small pieces and digested in HBSS (with Ca2+ and Mg2+) containing 10 U/ml DNase and 1 mg/ml collagenase, in a shaking incubator at 37 °C for 25 minutes. After digestion, the livers were smashed and washed using PBS (1% FBS) through a cell strainer to a single cell resolution, and the pellet was collected after centrifugation at 400 g for 8 minutes. Immune cells were then enriched from the pellet by Percoll gradient centrifugation (GE Healthcare, Chicago, IL). Human samples were obtained under the approval codes PV-3578 and PV-3548, which were approved by the local ethical committee “Ethik-Kommission der Ärztekammer Hamburg”. Written informed consent was received from all participants prior to inclusion in this study.
Flow cytometry and fluorescent activated cell sorting
Hepatic immune cells or liver sinusoid endothelial cells (LSECs) were isolated as mentioned above. A monoclonal antibody (clone 2.4G2) was used to block the Fc-γ receptors. Then, the cells were washed and stained with fluorochrome-conjugated antibodies (Table S2). The BD LSRFortessa (BD Biosciences, San Jose, CA) was used for flow cytometry and FACSAria IIIu (BD Biosciences, San Jose, CA) was used for cell sorting. Data analysis was performed using FlowJo v.6.1 (TreeStar, Ashland, OR).
qPCR
Total RNA from cells or tissue was extracted with the TRIzol reagent (Invitrogen) and reverse transcribed with the High Capacity cDNA Reverse Transcription Kit according to the manufacturer’s protocols (Life Technologies, Darmstadt, Germany). Real-time PCR was performed with the StepOne Plus (Life Technologies) using TaqMan probes, including Il10 (Mm00439614_m1), Il10ra (Mm00434151_m1), Il10rb (Mm00434157_m1), Hprt (Mm03024075_m1), and Granzyme B (Mm00442837_m1). The level of gene expression was normalized to that of Hprt.
Statistical analysis
All data were analyzed using the GraphPad Prism statistical software (GraphPad software, San Diego, CA, USA). Mouse data are presented as mean ± SEM. The mRNA analysis is shown using a base 10 logarithm. Comparison of means was performed using the Mann-Whitney U test for paired group comparisons or the one-way ANOVA (Bonferroni) for multiple group comparisons. P values below 0.05 were considered significant.
Results
Impaired IL-10 signaling protects mice against CRC-derived liver metastasis
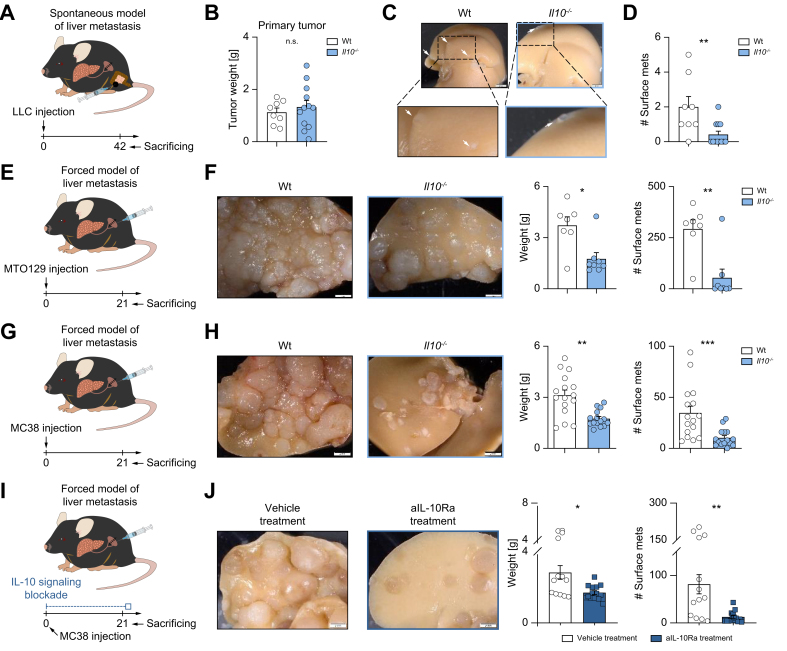
We first aimed to test the role of IL-10 in liver metastasis. To this end, LLC cancer cells were injected orthotopically into the cecum. Although the primary tumor weight was comparable in Il10-deficient and wild-type (WT) control mice (Fig. 1A,B), Il10-deficient mice developed less liver metastases compared to WT controls (Fig. 1C,D). We then confirmed this finding using murine colorectal liver metastasis (CRLM)-derived organoids, namely MTO129 (Fig. 1E,F), and MC38 cells (Fig. 1G,H). We observed reduced liver metastasis formation in Il10-deficient mice compared to WT littermates in both models. Next, to specifically investigate the role of IL-10 in CRLM development, we used an IL-10 receptor alpha (IL-10Ra) antibody, which was administered 1 day before metastasis induction, followed by injections every 3 days after induction. Indeed, IL-10Ra blockade during liver metastasis formation significantly decreased liver metastases (Fig. 1I,J). Finally, to test the impact of IL-10 on the primary tumor, we injected MC38 colon cancer cells intracecally and overall survival (humane endpoint) was assessed as a primary endpoint (Fig. S1A,B). In line with previous publications,10 Il10-deficient mice had a lower survival rate and increased primary tumor growth compared to WT controls (Fig. S1A-C), a finding indicating a protective function of IL-10 in primary tumor development.
Fig. 1.
Impaired IL-10 signaling protects mice against colorectal cancer-derived liver metastasis.
(A) Schematic overview of intracecal LLC cancer cell injection for spontaneous liver metastasis induction. (B) Primary tumor weight in the cecum. (C) Representative pictures and (D) number of liver metastases in WT and Il10-/- mice (n ≥8 mice per group). (E) Schematic overview of MTO129 intrasplenic injection for forced liver metastasis induction. (F) Representative pictures of liver metastasis, metastatic load, including liver weight and number of macroscopic liver metastases in WT and Il10-/- mice (n ≥ 7 mice per group). (G) Schematic overview of intrasplenic MC38 cancer cell injection. (H) Representative pictures of liver metastasis, metastatic load in WT and Il10-/- mice (n ≥12 mice per group). (I) Schematic overview of WT mice receiving aIL-10Ra or isotype treatment during intrasplenic MC38 cell injection. (J) Representative pictures and analysis of liver weight and number of liver metastases (n ≥12 mice per group). Scale bar: 2 mm. Data are presented as mean ± SEM. Non-significant (n.s.): p >0.05; ∗∗p <0.01; ∗∗∗p <0.001 calculated by Mann-Whitney U test. aIL-10Ra, IL-10 receptor alpha antibody; LLC, Lewis lung carcinoma; MC38, murine colon cancer cells; MTO, mouse tumor organoid; WT, wild-type. (This figure appears in color on the web.)
Taken together, these results demonstrated a pathogenic role of IL-10 signaling during liver metastasis formation.
The pathogenic role of IL-10 in liver metastasis formation is independent of colitis severity and the microbiome
Il10-deficient mice are known to be highly susceptible to spontaneous colitis manifestation.6 Consequently, we next studied the impact of colitis development on liver metastasis. Il10-/- mice exhibited reduced liver metastases compared to littermate controls (Fig. S2A,B). Interestingly, these mice did not develop colitis during metastasis formation (Fig. S2C). We therefore induced colitis in Il10-/- mice and littermate controls using a colitogenic microbiome (Fig. S2D-F).21 However, despite higher colitis severity observed in Il10-/- mice compared to littermate controls, the former were still protected from liver metastasis (Fig. S2G,H).
Taken together, Il10-deficient mice are protected from CRLM independently of colitis development.
Foxp3+Tregs are the major source of IL-10 in liver metastasis formation
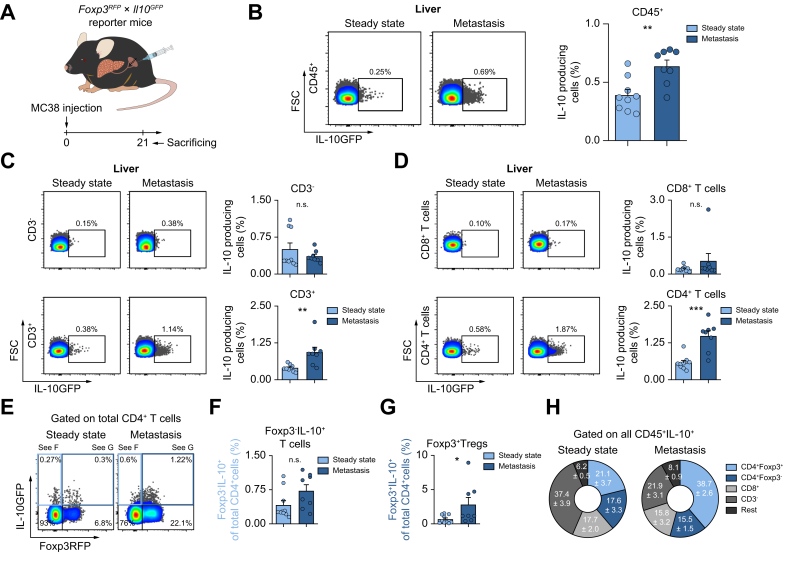
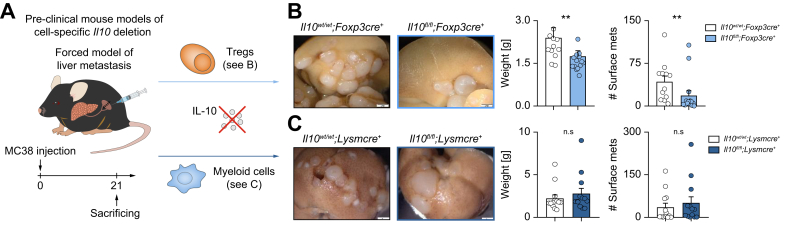
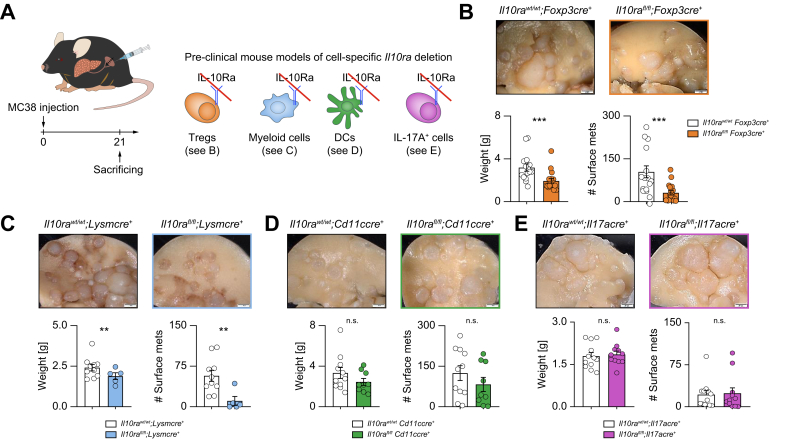
Next, we aimed to identify the cellular source of IL-10 during liver metastasis formation. IL-10 is known to be widely expressed in various tissues and cells.6 To define the cellular source of IL-10, a Il10GFP;Foxp3RFP reporter mouse was used.22 Increased IL-10 expression in hepatic immune cells, especially in CD4+ T cells, upon liver metastasis induction was observed (Fig. 2A-D). Of note, this upregulation was not seen in CD3-cells or CD8+ T cells. More specifically, an upregulation of IL-10 was found in Foxp3+Tregs, but not in Foxp3-IL-10+ cells (Fig. 2E-G). Immune cells, especially Foxp3+Tregs were also the major source of IL-10 in murine livers at steady state (Fig. S3A). Foxp3+Tregs further expanded along with liver metastasis formation (Fig. S3B,C), with a dynamically increased number that was IL-10+ (Fig. S3D,E). Indeed, Foxp3+Tregs contributed mostly to IL-10 production in established liver metastasis (Fig. 2H). Moreover, Foxp3+Treg depletion reduced liver metastatic load (Fig. S3F). Interestingly, the composition of IL-10-producing cells in primary CRC differed from that in liver metastases. Using the cecum cancer model, we found that the frequency of IL-10+ cells was mainly increased in CD3-cells, but not T cells (Fig. S4). Indeed, human samples from CRC and CRLM present distinct immune cell composition and therefore might account for different phenotypes regarding the different roles of IL-10 in primary CRC and CRLM (Fig. S5). Thus, our data indicated that the main IL-10-producing cells in primary CRC were non-T cells, as opposed to Foxp3+Tregs in liver metastases. To test the functional relevance of this finding, mice with a Foxp3+Treg-specific IL-10 deletion underwent liver metastasis induction via intrasplenic MC38 cell injection (Fig. 3A). Interestingly, mice bearing a Foxp3+Treg-specific IL-10 deletion were protected against liver metastasis compared to littermate controls (Fig. 3B). As a control, we also used mice with a myeloid cell-specific IL-10 deletion (LysmCre). Importantly, the liver metastatic burden did not differ between these groups (Fig. 3C).
Fig. 2.
Foxp3+Tregs are the major IL-10-producing cells during liver metastasis formation.
(A) Schematic overview of intrasplenic MC38 cancer cell injection for forced liver metastasis induction in Foxp3RFP;Il10GFP mice (n ≥8 mice per group). Frequency of IL-10+ cells in the fraction of (B) CD45+ cells, (C) CD3-and T cells, and in (D) CD8+ T cells and CD4+ T cells. (E) IL-10 expression in (F) Foxp3-IL-10+ cells and (G) Foxp3+Tregs. (H) General distribution of all IL-10-producing CD45+ cells in healthy liver and liver with metastasis. Data are presented as mean ± SEM. Non-significant (n.s.): p >0.05; ∗p ≤0.05; ∗∗p <0.01; ∗∗∗p <0.001, as calculated by Mann-Whitney U test. MC38, murine colon cancer cells; Tregs, regulatory T cells. (This figure appears in color on the web.)
Fig. 3.
Foxp3+Treg-derived IL-10 facilitates liver metastasis formation.
(A) MC38 cells were intrasplenically injected into mice with cell-specific Il10 deletion in Foxp3+Tregs and myeloid cells and their littermate controls (n ≥10 mice per group). Livers were harvested 21 days post injection. (B, C) Representative images, liver weight and number of liver metastases in mice with (B) Treg-specific or (C) myeloid cell-specific Il10 deletion and their littermates. Scale bar: 2 mm. Data are presented as mean ± SEM. Non-significant (n.s.): p >0.05; ∗∗p <0.01, as calculated by Mann-Whitney U test. MC38, murine colon cancer cells; Tregs, regulatory T cells. (This figure appears in color on the web.)
Collectively, our results suggested that Foxp3+Tregs are the major functionally relevant source of IL-10 in liver metastasis.
IL-10 signaling in Foxp3+Tregs and myeloid cells promotes CRLM development
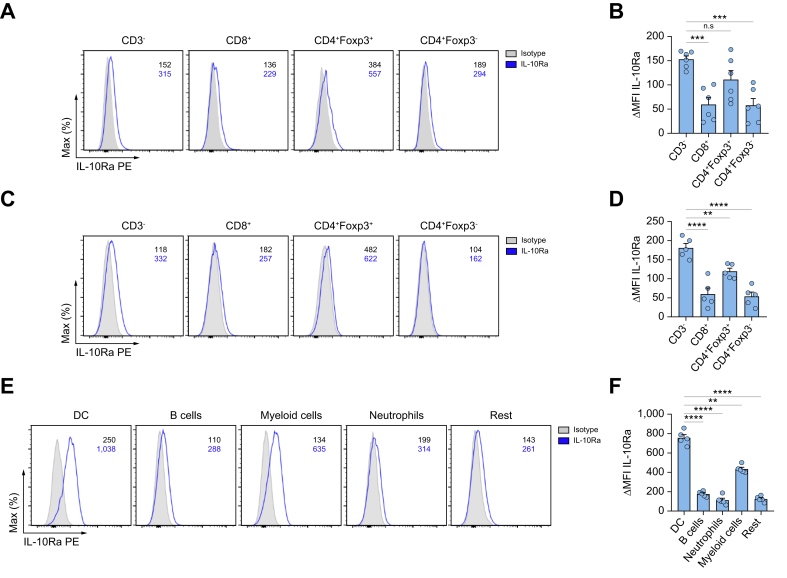
To further identify potential target cells of IL-10 during liver metastasis formation, IL-10Ra expression was analyzed in immune cells, cancer cells, hepatocytes and LSECs using qPCR and flow cytometry. Interestingly, innate cells and Foxp3+Tregs expressed the highest IL-10Ra levels among different immune cell subsets at steady state (Fig. 4A,B). Furthermore, they maintained high IL-10Ra expression in established liver metastasis (Fig. 4C,D). MC38 and LLC cancer cells showed no IL-10Ra expression (Fig. S6A,B). In liver cells, LSECs showed IL-10Ra expression, while the rest of the cells including hepatocytes showed only low or no IL-10Ra expression (Fig. S6C,D). We further examined STAT3 activation in these cells upon IL-10 stimulation in vitro. MC38 and LLC cancer cells did not respond to IL-10 (Fig. S6E), while LSECs (Fig. S6F,G) and immune cells (Fig. S6F) exhibited higher STAT3 phosphorylation after IL-10 stimulation in vitro. We have previously demonstrated that IL-22-mediated interactions between LSECs and immune cells promoted liver metastasis via enhancing cancer cell extravasation.20 On this basis, we tested the role of IL-10 signaling in cancer cell extravasation using an intrasplenic injection of MC38-GFP cells in Il10+/+ and Il10-/- mice (Fig. S6H). We found that extravasated cancer cells in the liver were comparable between these two groups 1 day post injection (Fig. S6I,J). Likewise, IL-10Ra deletion in LSECs did not significantly reduce liver metastases in vivo (Fig. S6K,L). We further analyzed different subsets of immune cells in response to IL-10 in vitro. We found that STAT3 signaling was activated in innate cells and Foxp3+Tregs, rather than CD8+ T cells and Foxp3-IL-10+ cells, upon IL-10 stimulation in vitro (Fig. S6M,N). In terms of non-T cells, myeloid cells, and especially dendritic cells (DCs), exhibited increased IL-10Ra expression compared to neutrophils, B cells, and other examined immune cell populations (Fig. 4E,F). Hence, myeloid cells and Foxp3+Tregs were identified as the main target cells of IL-10 during liver metastasis formation.
Fig. 4.
Dendritic cells, myeloid cells and Foxp3+Tregs can respond to IL-10.
(A–D) Representative FACS plot and ΔMFI quantification of IL-10Ra expression in immune cells isolated from (A, B) a healthy liver or (C, D) liver metastasis 21 days post intrasplenic cancer cell injection (n ≥5 mice per group). (E) Representative FACS plot and (F) ΔMFI quantification of IL-10Ra expression from different innate subsets (CD3-) in liver metastasis. Data are presented as mean ± SEM. Non-significant (n.s.): p >0.05; ∗∗∗p <0.001, as calculated by Mann-Whitney U test or one-way ANOVA (Bonferroni) with Bonferroni post hoc tests. IL-10Ra, IL-10 receptor alpha; MFI, mean fluorescence intensity; Tregs, regulatory T cells. (This figure appears in color on the web.)
To further understand the outcome of IL-10 signaling on immune cells in the context of liver metastasis, we used cell-specific IL-10Ra-deficient mouse models (Fig. 5A). We injected MC38 cancer cells intrasplenically to establish liver metastasis in these mouse models and then compared their liver metastatic burden. Interestingly, IL-10 ablation in both Foxp3+Tregs (Fig. 5B) and myeloid cells (Fig. 5C) protected mice against liver metastasis. However, this protection was absent in mice with IL-10Ra deletion in DCs (Fig. 5D), suggesting a dispensable role of IL-10R signaling in DCs during liver metastasis formation. Additionally, IL-10Ra deletion in Foxp3+Tregs did not alter the frequency (Fig. S6O) of Foxp3+Tregs but decreased their Il10 production upon IL-10 stimulation (Fig. S6P). Of note, IL-10 signaling ablation in IL-17-producing cells did not affect liver metastasis formation (Fig. 5E).
Fig. 5.
IL-10 signaling in Foxp3+Tregs and myeloid cells promotes colorectal cancer-derived liver metastasis.
(A) Schematic overview of intrasplenic MC38 cancer cell injection in mice with distinct cell-specific Il10ra deletion (n ≥5 mice per group). Representative images, liver weight and number of liver metastases in mice with (B) Treg-, (C) myeloid-, (D) DC-, or (E) Th17-specific Il10ra deletion. Scale bar: 2 mm. Data are presented as mean ± SEM. Non-significant (n.s.): p >0.05; ∗p ≤0.05; ∗∗p <0.01; ∗∗∗p <0.001, as calculated by Mann-Whitney U test. DC, dendritic cell; Il10ra, IL-10 receptor alpha; MC38, murine colon cancer cells; Th17, T helper 17 cells; Tregs, regulatory T cells. (This figure appears in color on the web.)
Taken together, IL-10 signaling in Foxp3+Tregs promotes their production of IL-10, which then acts on myeloid cells, thereby promoting CRLM.
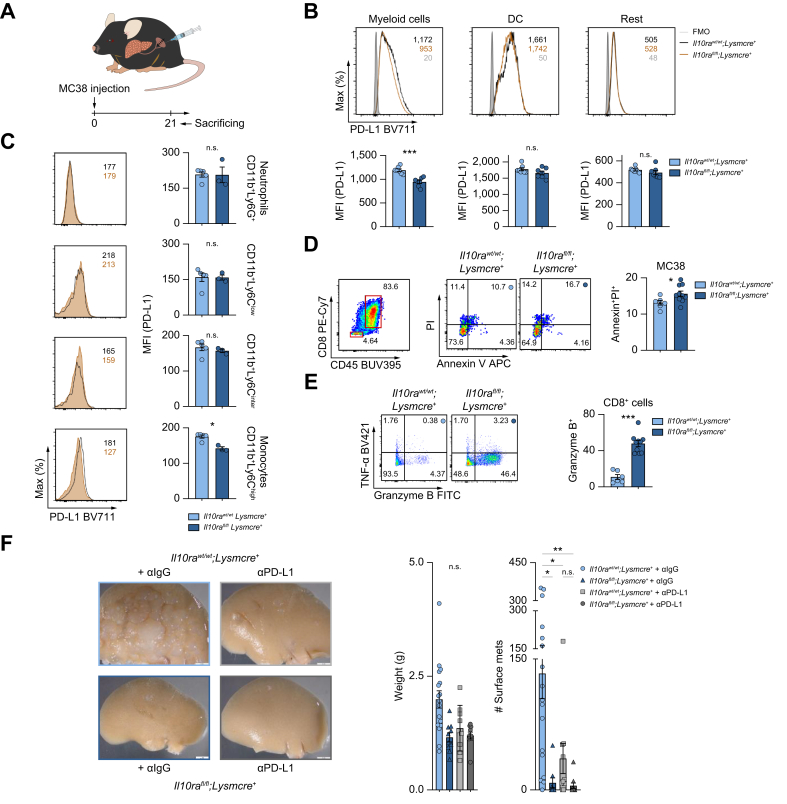
IL-10 induces PD-L1 upregulation in monocytes, thereby attenuating CD8+ T-cell mediated immune surveillance
Finally, we aimed to decipher the underlying mechanism. Bulk sequencing of myeloid cells indicated that one of the inhibitory checkpoint ligands, PD-L1, was reduced in Il10rafl/fl;Lysmcre+ mice compared to littermate controls (Fig. S7A). Thus, we hypothesized that IL-10 may regulate the PD-L1/PD-1 axis between myeloid cells and cytotoxic T cells, thereby affecting antitumor immunity. To test this hypothesis, PD-L1 expression was assessed in hepatic myeloid cells isolated from myeloid cell-specific IL-10Ra-deficient mice and littermate controls after liver metastasis induction (Fig. 6A). PD-L1 expression in myeloid cells was downregulated in the absence of IL-10Ra in myeloid cells (Fig. 6B). Additionally, this IL-10 signaling-dependent PD-L1 regulation was further validated in myeloid cells, specifically monocytes (Fig. 6C and Fig. S7B,C). Furthermore, PD-L1 deletion protected mice against liver metastasis formation (Fig. S7D). Next, we aimed to test whether this IL-10 signaling in myeloid cells indeed affects antitumor immunity of CD8+ T cells and cancer cell killing. We first induced liver metastasis using an intrasplenic injection of MC38 cells in myeloid cell-specific IL-10Ra-deficient mice and littermate controls. From these mice we then isolated CD8+ T cells from liver metastases, and cocultured them with MC38 cancer cells. Subsequently, antitumor factor granzyme B within CD8+ T cells was assessed, as well as cancer cell apoptosis via Annexin V and PI staining (Fig. 6D,E). Higher CD8+ T cell-produced granzyme B levels and increased cancer cell apoptosis were observed in Il10rafl/fl;Lysmcre+ mice compared to their littermate controls (Fig. 6D,E). To confirm if the effect on CD8+ T cells and cancer cells was dependent on PD-L1, we characterized CD8+ T cells in Pdl1+/+ and Pdl1-/- mice after injecting MC38 cells intrasplenically. Increased tumor-infiltrating CD8+ T cells with similar granzyme B mRNA production was detected in Pdl1-/- mice compared to littermate control (Pdl1+/+) mice (Fig. S7E,F). After coculture with MC38 cells, tumor-infiltrating CD8+ cells in Pdl1-/- mice expressed much higher amounts of granzyme B compared to Pdl1+/+ mice (Fig. S7G). To confirm that the pro-metastatic role of myeloid cell-dependent IL-10 signaling was mediated by PD-L1, PD-L1 blockade treatment was administrated to Il10rafl/fl;Lysmcre+ mice and their littermate controls upon liver metastasis induction. Deletion of IL-10Ra in myeloid cells reduced metastasis formation in the absence of a PD-L1 antibody. However, in the presence of a PD-L1 antibody, the deletion of IL-10Ra did not impact metastasis formation (Fig. 6F). These data indicate that the effect of IL-10 signaling in myeloid cells is, at least in part, dependent on PD-L1 expression.
Fig. 6.
IL-10-mediated PD-L1 upregulation in monocytes attenuates CD8+ T-cell infiltration and antitumor immunity.
(A) Schematic overview of intrasplenic MC38 cancer cell injection in mice with myeloid-specific Il10ra deletion and their littermate controls (n ≥3 mice per group). (B, C) Representative FACS plot and MFI quantification of PD-L1 expression in (B) myeloid cells, DCs and remaining innate cells, as well as in (C) indicated subpopulations within myeloid cells. 14 days post injection, (D) apoptosis of MC38 cells and (E) antitumor immunity (TNF-α and granzyme B) of CD8+ T cells were analyzed using flow cytometry. (F) Mice with myeloid-specific Il10ra deletion and their littermate controls, receiving anti-IgG or anti-PD-L1 (250 μg/mouse) were injected intrasplenically with MC38 cancer cells (n ≥9 mice per group). Number of liver metastases was quantified 21 days post liver metastasis induction. Scale bar: 2 mm. Data are presented as mean ± SEM. Non-significant (n.s.): p >0.05; ∗p ≤0.05; ∗∗p <0.01; ∗∗∗p <0.001, as calculated by one-way ANOVA (Bonferroni) with Bonferroni post hoc tests. . IL-10Ra, IL-10 receptor alpha; MC38, murine colon cancer cells; MFI, mean fluorescence intensity; (a)PD-L1, programmed cell death ligand 1 (antibody); TNF-α, tumor necrosis factor-alpha. (This figure appears in color on the web.)
Overall, we demonstrated that IL-10 induces PD-L1 expression in monocytes, thereby attenuating CD8+ T-cell infiltration and antitumor immunity, ultimately facilitating liver metastasis formation.
Discussion
The liver is one of the most susceptible sites to metastasis from various malignancies, for example, CRC, breast cancer and melanoma. Overall, CRC remains the most common primary tumor leading to liver metastasis.23 Most patients with CRC develop CRLM during the course of disease, even after curative resection of the primary tumor. Currently, standard treatment options against liver metastasis consist of surgical resection and chemotherapy. However, even if detected at an early stage, a considerable number of liver metastases cannot be surgically removed.24 Thus, controlling liver metastasis remains challenging and metastasis is still the major contributor to CRC-related death.25
Notably, immunotherapy has, in certain instances, led to remarkable responses in inoperable malignancies, such as melanoma and small cell lung cancer.26 Specifically, immune checkpoint inhibitors have been found to restore antitumor T cells and suppress tumor development. Anti-PD-1 and anti-PD-L1 antibodies are the most studied and attractive immune checkpoint inhibitors that have shown therapeutic potential against primary cancers and metastases.25,26 However, the presence of liver metastasis has been reported to reduce cytotoxic CD8+ T-cell numbers and restrain the effect of immunotherapy.4 Unfortunately, the underlying mechanism is unclear.
Interestingly, a recent in vitro study found that IL-10 blockade in organotypic slides from patients with metastatic CRC increased activated CD8+ frequency and enhanced the activation and cytotoxicity of CAR-T cells, leading to higher cancer cell apoptosis.27 These data suggest that IL-10 signaling may play an important role in CRLM. However, the in vivo relevancy of IL-10, the source and potential mechanism of action remain unclear. Furthermore, IL-10 has been implicated in the pathogenesis of multiple diseases, such as chronic infectious diseases and cancer.6,28,29 A recent study reported upregulated IL-10 levels in murine liver metastasis compared to healthy controls.30 Interestingly, lower serum IL-10 levels correlated with higher occurrence and poorer prognosis of CRC.31 Furthermore, a protective role of IL-10 in lung metastasis was reported.10 However, the role of IL-10 in liver metastasis in vivo was unclear. Therefore, in this study, we aimed to decipher the role of IL-10 in liver metastasis and to determine how its modulation could be used to enhance the efficacy of immunotherapy in vivo.
In line with previous findings,28 we observed that IL-10 was upregulated in metastatic sites compared to healthy liver in mouse. The role of IL-10 in various cancer types seems to be controversial.32 Herein, we used spontaneous and forced mouse models of liver metastasis. We found that IL-10 inhibited primary CRC, while it promoted CRLM. Interestingly, the cellular source of IL-10 was different between the primary CRC and CRLM: we identified Foxp3+Tregs as the main cellular source of IL-10 during liver metastasis formation, while innate cells were the main cellular source of IL-10 in primary CRC in our model. This different cellular source may explain the different roles of IL-10. This is in line with recent studies demonstrating different immune cell compositions in human CRC and CRLM.33,34 To provide more evidence, we also characterized the immune composition in human samples from CRC and CRLM. In CRC, B-cell frequency decreased in tumor tissue compared to peritumor tissue. In contrast, in CRLM, reduced innate cells and increased T cells were present in metastases. Within T cells, CD4+ and CD8+ cell frequency was not altered in CRC, while a higher CD4+ T-cell and lower CD8+ T-cell frequency was observed in CRLM compared to peri-metastatic liver. However, further studies will be important to address this point. Next, we performed flow cytometry to measure IL-10Ra expression among immune cells, cancer cells, hepatocytes and LSECs. We found that T cells do express lower amounts of IL-10Ra and thus have a lower STAT3 activation upon IL-10 treatment compared to innate cells. This is in line with our previous publications.35 As the IL-10Ra expression was low in T cells, we conducted functional in vitro and in vivo experiments in order to show that the expression is still biologically relevant. By using cell-specific IL-10Ra-deficient mouse models, we found that IL-10Ra depletion in myeloid cells and Foxp3+Tregs resulted in significantly less liver metastasis. Consistent with previous studies analyzing the role of IL-10Ra on Foxp3+Tregs during colitis,28 we showed that IL-10 signaling in Foxp3+Tregs was important to boost IL-10 production. Furthermore, impaired IL-10 signaling in Foxp3+Tregs did not affect the frequency of Foxp3+Tregs. Thus, we suggest that IL-10Ra depletion on Foxp3+Tregs influences IL-10 production, which subsequently impairs IL-10 signaling in myeloid cells and thereby affects liver metastatic burden.
Next, we aimed to identify the mechanism underlying the pathogenic myeloid cell-mediated role of IL-10 signaling in liver metastasis formation. It was reported that Tregs, together with CD11b+ monocytes, are associated with immune suppression in liver metastasis.36 In addition, a combination of Treg depletion and an anti-PD-1 therapy, instead of an anti-PD-1 monotherapy, was shown to improve antitumor immunity.36 However, the molecular mechanism underlying this immune suppression is not clear. Surprisingly, our data shows a downregulation of PD-L1 in myeloid cells upon IL-10Ra deletion during liver metastasis formation. Specifically, monocytes are the most affected myeloid cell population. Moreover, it has previously been reported that anti-PD-L1 blockade on monocytes can lead to a greater T-cell expansion in the context of asymptomatic multiple myeloma.37 Previous studies have demonstrated that PD-L1 depletion in DCs increases CD8+ T-cell responses and reduces tumor growth.38 However, in CRC-derived liver metastasis, IL-10-mediated PD-L1 regulation was not found in DCs but in monocytes. Additionally, IL-10Ra deficiency in DCs did not alter liver metastatic burden in our mouse models.
To understand the role of PD-L1 in CRLM, we first used PD-L1-deficient mice and showed that PD-L1 deficiency resulted in significantly lower liver metastatic burden, a higher frequency of tumor-infiltrating CD8+ T cells as well as a higher total amount of granzyme B expression. Consistent with this, tumor-infiltrating CD8+ T cells from myeloid cell-specific IL-10Ra-deficient mice aggravated cancer cell apoptosis, while also exhibiting a higher granzyme B expression. Importantly, using anti-PD-L1 in myeloid cell-specific Il10ra-deficient mice, we observed that the effect of IL-10 signaling in myeloid cells on liver metastasis formation was at least in part dependent on PD-L1. However, our data do not exclude an additional role of other factors, such as IFN-γ. Further studies are required to test the role of these factors. Taken together, we demonstrated that IL-10 signaling mediated the regulation of PD-L1 on monocytes, and thus, affected cancer cell apoptosis through modulation of CD8+ T cell-mediated antitumor immunity. This suggests a potential mechanism underlying resistance to immunotherapy in patients with liver metastasis and emphasizes the importance of controlling IL-10 levels in such patients.
There have been contrasting reports regarding PD-L1/PD-1 interactions between monocytes and T cells.[39], [40], [41] It was reported that monocyte-expressed PD-1 could affect T memory cell-mediated antitumor immunity.41 Interestingly, we showed that monocyte-expressed PD-L1 could attenuate CD8+ T-cell infiltration, and thus antitumor immunity, specifically via granzyme B expression. Of note, the combination of anti-PD-L1 and IL-10Ra depletion in myeloid cells produced the best clinical outcome upon metastasis induction in mice. This provides a novel treatment option for combining anti-PD-L1 and anti-IL-10Ra in patients with CRLM, especially those who do not respond to immunotherapy.
In conclusion, our data provide evidence that Foxp3+Treg-produced IL-10 upregulates PD-L1 expression in monocytes, which in turn reduces CD8+ T-cell infiltration and related antitumor immunity, thereby promoting CRLM formation. These findings highlight the potential therapeutic benefit of a novel combined anti-IL-10Ra and anti-PD-L1 approach against CRLM.
Abbreviations
CAR-T, chimeric antigen receptor T; CRC, colorectal cancer; CRLM, colorectal liver metastasis; DCs, dendritic cells; IL-10Ra, IL-10 receptor alpha; LLC, Lewis lung carcinoma; LSECs, liver sinusoidal endothelial cells; MC38, murine colon cancer cells; MTO, mouse tumor organoid; PD-1, programmed death 1; PD-L1, programmed death ligand 1; Tregs, regulatory T cells; WT, wild-type.
Financial support
This work was supported in part by the Deutsche Forschungsgemeinschaft (DFG) (SFB841 to S.H.), the European Research Council (CoG 865466 to S.H.), Else Kröner Memorial Stipendium (A.D.G. and J.K), Erich und Gertrud Roggenbuck-Stiftung (A.D.G.), Hamburger Krebsgesellschaft Stiftung (A.D.G.) and the Jung Foundation for Science and Research (A.D.G.), Werner Otto Stiftung (J.K.), China Scholarship Council (T.Z.), Mildred Scheel Cancer Career Center Hamburg HaTriCS4 (funded by Deutsche Krebshilfe; J.K.). P.M.L. receives speaker fees from MSD. N.G. receives research grants from F. Hoffmann-La Roche and DZIF. S.H. has an endowed Heisenberg-Professorship awarded by the Deutsche Forschungsgemeinschaft.
Conflict of interest
N.G. reports financial support from F. Hoffmann-La Roche. This is outside the submitted work. Other authors declare no conflict of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Authors’ contributions
A.M.S., T.Z., and A.D.G. conceived, designed, and carried out most experiments, analyzed data, and wrote the manuscript; T.B., D.E.Z., L.Z., J.L., J.K., M.N. carried out in vivo experiments and flow cytometry assays; A.F. performed organoids culture and preparation for injection. S.Z. performed qPCR. D.V.T., E.B. provided mouse organoids and provided technical consult. B.S. analyzed RNA sequencing data. K.R. and T.A. constructed expression vectors and performed transfection experiments; Y.X. and L.B. performed fluorescence-activated cell sorting; I.L. performed in vivo experiments; L.K., L.B., B.M., P.S., N.K., P.M.L, A.H., P.C.A., B.F., P.B., R.G., O.M., J.R.I, T.H. provided critical intellectual input; N.G. designed experiments, provided critical intellectual input, and edited the paper; A.D.G. and S.H. conceived the idea and supervised the study, designed experiments, and wrote the manuscript. All authors reviewed and concurred with the submitted manuscript.
Data availability statement
All data from this study are provided in the source data. Primary data from flow cytometry or qPCR are available upon reasonable request. The accession number for the bulk seq data is GSE247304.
Acknowledgements
The authors thank Cathleen Haueis, Sandra Wende, and Tom Blankenburg for technical assistance, the in vivo Optical Imaging Core Facility and the Cytometry und Cell Sorting Core Unit at the University Medical Center Hamburg-Eppendorf for their technical assistance. Pdl1-/- mice were kindly provided by Dr. Gisa Tiegs as part of an ongoing collaboration.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2023.12.015.
Contributor Information
Anastasios D. Giannou, Email: a.giannou@uke.de.
Samuel Huber, Email: s.huber@uke.de.
Supplementary data
The following are the supplementary data to this article:
:
References
- 1.Esposito M., Ganesan S., Kang Y. Emerging strategies for treating metastasis. Nat Cancer. 2021;2:258–270. doi: 10.1038/s43018-021-00181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clinicians. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Obenauf A.C., Massagué J. Surviving at a distance: organ-specific metastasis. Trends Cancer. 2015;1:76–91. doi: 10.1016/j.trecan.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu J., Green M.D., Li S., et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med. 2021;27:152–164. doi: 10.1038/s41591-020-1131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei X.L., Luo X., Sheng H., et al. PD-L1 expression in liver metastasis: its clinical significance and discordance with primary tumor in colorectal cancer. J Transl Med. 2020;18:475. doi: 10.1186/s12967-020-02636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bedke T., Muscate F., Soukou S., et al. IL-10-producing T cells and their dual functions. Semin Immunol. 2019;44 doi: 10.1016/j.smim.2019.101335. [DOI] [PubMed] [Google Scholar]
- 7.Guo Y., Xie Y.Q., Gao M., et al. Metabolic reprogramming of terminally exhausted CD8(+) T cells by IL-10 enhances anti-tumor immunity. Nat Immunol. 2021;22:746–756. doi: 10.1038/s41590-021-00940-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mumm J.B., Emmerich J., Zhang X., et al. IL-10 elicits IFNγ-dependent tumor immune surveillance. Cancer Cell. 2011;20:781–796. doi: 10.1016/j.ccr.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Qiao J., Liu Z., Dong C., et al. Targeting tumors with IL-10 prevents dendritic cell-mediated CD8(+) T cell apoptosis. Cancer Cell. 2019;35:901–915.e904. doi: 10.1016/j.ccell.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Tanikawa T., Wilke C.M., Kryczek I., et al. Interleukin-10 ablation promotes tumor development, growth, and metastasis. Cancer Res. 2012;72:420–429. doi: 10.1158/0008-5472.CAN-10-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naing A., Wong D.J., Infante J.R., et al. Pegilodecakin combined with pembrolizumab or nivolumab for patients with advanced solid tumours (IVY): a multicentre, multicohort, open-label, phase 1b trial. Lancet Oncol. 2019;20:1544–1555. doi: 10.1016/S1470-2045(19)30514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spigel D., Jotte R., Nemunaitis J., et al. Randomized phase 2 studies of checkpoint inhibitors alone or in combination with pegilodecakin in patients with metastatic NSCLC (CYPRESS 1 and CYPRESS 2) J Thorac Oncol. 2021;16:327–333. doi: 10.1016/j.jtho.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Oft M. Immune regulation and cytotoxic T cell activation of IL-10 agonists - preclinical and clinical experience. Semin Immunol. 2019;44 doi: 10.1016/j.smim.2019.101325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan K.M., Jiang X., Guha P., et al. Blockade of interleukin 10 potentiates antitumour immune function in human colorectal cancer liver metastases. Gut. 2023 Feb;72(2):325–337. doi: 10.1136/gutjnl-2021-325808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubtsov Y.P., Rasmussen J.P., Chi E.Y., et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 16.Yogev N., Bedke T., Kobayashi Y., et al. CD4(+) T-cell-derived IL-10 promotes CNS inflammation in mice by sustaining effector T cell survival. Cell Rep. 2022;38 doi: 10.1016/j.celrep.2022.110565. [DOI] [PubMed] [Google Scholar]
- 17.Giannou A.D., Marazioti A., Spella M., et al. Mast cells mediate malignant pleural effusion formation. J Clin Invest. 2015;125:2317–2334. doi: 10.1172/JCI79840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber K., Bartsch U., Stocking C., et al. A multicolor panel of novel lentiviral "gene ontology" (LeGO) vectors for functional gene analysis. Mol Ther. 2008;16:698–706. doi: 10.1038/mt.2008.6. [DOI] [PubMed] [Google Scholar]
- 19.Tauriello D.V.F., Palomo-Ponce S., Stork D., et al. TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554:538–543. doi: 10.1038/nature25492. [DOI] [PubMed] [Google Scholar]
- 20.Giannou A.D., Kempski J., Shiri A.M., et al. Tissue resident iNKT17 cells facilitate cancer cell extravasation in liver metastasis via interleukin-22. Immunity. 2023;56:125–142 e112. doi: 10.1016/j.immuni.2022.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palm N.W., de Zoete M.R., Cullen T.W., et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158:1000–1010. doi: 10.1016/j.cell.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamanaka M., Kim S.T., Wan Y.Y., et al. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25:941–952. doi: 10.1016/j.immuni.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Tsilimigras D.I., Brodt P., Clavien P.A., et al. Liver metastases. Nat Rev Dis Primers. 2021;7:27. doi: 10.1038/s41572-021-00261-6. [DOI] [PubMed] [Google Scholar]
- 24.Hu D.D., Pan Y.X., Chen G. Colorectal cancer liver metastases: an update of treatment strategy and future perspectives. Surg Pract Sci. 2021:7. [Google Scholar]
- 25.Zhou H., Liu Z., Wang Y., et al. Colorectal liver metastasis: molecular mechanism and interventional therapy. Signal Transduct Target Ther. 2022;7:70. doi: 10.1038/s41392-022-00922-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edwards S.C., Hoevenaar W.H.M., Coffelt S.B. Emerging immunotherapies for metastasis. Br J Cancer. 2021;124:37–48. doi: 10.1038/s41416-020-01160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sullivan K.M., Jiang X., Guha P., et al. Blockade of interleukin 10 potentiates antitumour immune function in human colorectal cancer liver metastases. Gut. 2023;72:325–337. doi: 10.1136/gutjnl-2021-325808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rallis K.S., Corrigan A.E., Dadah H., et al. IL-10 in cancer: an essential thermostatic regulator between homeostatic immunity and inflammation - a comprehensive review. Future Oncol. 2022;18:3349–3365. doi: 10.2217/fon-2022-0063. [DOI] [PubMed] [Google Scholar]
- 29.Saraiva M., Vieira P., O'Garra A. Biology and therapeutic potential of interleukin-10. J Exp Med. 2020:217. doi: 10.1084/jem.20190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang X.M., Zhang N.R., Lin X.T., et al. Antitumor immunity of low-dose cyclophosphamide: changes in T cells and cytokines TGF-beta and IL-10 in mice with colon-cancer liver metastasis. Gastroenterol Rep (Oxf) 2020;8:56–65. doi: 10.1093/gastro/goz060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abtahi S., Davani F., Mojtahedi Z., et al. Dual association of serum interleukin-10 levels with colorectal cancer. J Cancer Res Ther. 2017;13:252–256. doi: 10.4103/0973-1482.199448. [DOI] [PubMed] [Google Scholar]
- 32.Mannino M.H., Zhu Z., Xiao H., et al. The paradoxical role of IL-10 in immunity and cancer. Cancer Lett. 2015;367:103–107. doi: 10.1016/j.canlet.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Wu Y., Yang S., Ma J., et al. Spatiotemporal immune landscape of colorectal cancer liver metastasis at single-cell level. Cancer Discov. 2022;12:134–153. doi: 10.1158/2159-8290.CD-21-0316. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y., Zhang Q., Xing B., et al. Immune phenotypic linkage between colorectal cancer and liver metastasis. Cancer Cell. 2022;40:424–437 e425. doi: 10.1016/j.ccell.2022.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Kamanaka M., Huber S., Zenewicz L.A., et al. Memory/effector (CD45RB(lo)) CD4 T cells are controlled directly by IL-10 and cause IL-22-dependent intestinal pathology. J Exp Med. 2011;208:1027–1040. doi: 10.1084/jem.20102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J.C., Mehdizadeh S., Smith J., et al. Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.aba0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bar N., Costa F., Das R., et al. Differential effects of PD-L1 versus PD-1 blockade on myeloid inflammation in human cancer. JCI Insight. 2020;5 doi: 10.1172/jci.insight.129353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh S.A., Wu D.C., Cheung J., et al. PD-L1 expression by dendritic cells is a key regulator of T-cell immunity in cancer. Nat Cancer. 2020;1:681–691. doi: 10.1038/s43018-020-0075-x. [DOI] [PubMed] [Google Scholar]
- 39.Diskin B., Adam S., Cassini M.F., et al. PD-L1 engagement on T cells promotes self-tolerance and suppression of neighboring macrophages and effector T cells in cancer. Nat Immunol. 2020;21:442–454. doi: 10.1038/s41590-020-0620-x. [DOI] [PubMed] [Google Scholar]
- 40.Strauss L., Mahmoud M.A.A., Weaver J.D., et al. Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.aay1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akbay E.A., Koyama S., Liu Y., et al. Interleukin-17A promotes lung tumor progression through neutrophil attraction to tumor sites and mediating resistance to PD-1 blockade. J Thorac Oncol. 2017;12:1268–1279. doi: 10.1016/j.jtho.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
:
Data Availability Statement
All data from this study are provided in the source data. Primary data from flow cytometry or qPCR are available upon reasonable request. The accession number for the bulk seq data is GSE247304.