Abstract
Background:
The Society for Surgical Oncology choosing wisely guidelines recommend against sentinel lymph node biopsy (SLNB) in favor of observation in this population. Recent analyses reveal this has not been widely adopted. The purpose of this cost-effectiveness analysis is to compare the costs and benefits associated observation or SLNB in women > 70 years old with hormone-receptor positive, clinically node-negative, operable breast cancer.
Methods:
A decision tree with Markov modeling was created to compare treatment strategies using long-term follow up data from clinical trials in this population. Costs were estimated from published literature and publicly-available databases. Breast cancer-specific health-state utilities were derived from the literature and expert opinion. One-way, two-way, and probabilistic sensitivity analyses were conducted. A structural sensitivity analysis was performed to assess the effect of functional status and anxiety from non-evaluation of the axilla on cost-effectiveness. Costs and benefits, measured in life years (LYs) and quality-adjusted life years (QALYs), were tabulated across 10, 15, and 20 years and compared using incremental cost-effectiveness ratios.
Results:
SLNB is not cost-effective from the payer or societal perspectives with an ICER of $138,374/LY and $131,900/LY, respectively. When considering QALYs, SLNB provided fewer QALYs (SLNB=10.33 QALYs, Observation=10.53 QALYs) at a higher cost (SLNB=$15,845, Observation=$4,020). Structural sensitivity analysis revealed SLNB was cost-effective in certain patients with significant anxiety related to axillary observation (ICER=$39,417/QALY).
Conclusions:
Routine SLNB in this population is not cost-effective. The cost-effectiveness of SLNB, however, is dependent on individual patient factors including functional status as well as patient preference.
Keywords: Cost-utility analysis, Breast neoplasms, Sentinel lymph node biopsy, Surgical oncology
INTRODUCTION
Breast cancer remains the most common malignancy and a leading cause of cancer-related death among women, despite significant diagnostic and therapeutic advances.1 Patient-level factors, specifically age, are associated with differences in disease pathophysiology and contribute to variability in treatment and outcomes.2,3 Although surgical management, which includes consideration of both the breast and axilla, is the mainstay of treatment for elderly patients with breast cancer, the utility of sentinel lymph node biopsy (SLNB) in clinically node negative disease has been called into question as it has been shown to improve neither overall nor breast cancer-specific survival.4–7 Thus, the American Board of Internal Medicine (ABIM) Foundation and Society for Surgical Oncology (SSO) Choosing Wisely Guidelines recommend against the routine use of SLNB in clinically node-negative women ≥70 years old with early-stage, hormone-receptor positive, HER2 negative, invasive breast cancer.
This recommendation notwithstanding, a recent analysis of national practice patterns revealed most elderly patients meeting guideline criteria undergo SLNB.8 Resistance to de-escalation of surgical axillary staging may stem from a fear of undertreatment and a belief among providers that nodal status will influence further treatment decisions including adjuvant chemotherapy and radiation. Furthermore, providers may hesitate to change their approach to management based solely on patient age.9 While age is a surrogate for increasing comorbidity and functional deficits, disease progression in elderly patients who are healthy may more closely mirror that of younger patients. Consequently, in women 70 or older with few health problems, SLNB may be associated with reduced risk of local recurrence or provide valuable information to inform adjuvant therapy decisions. Therefore, a personalized approach to operative planning should be adopted weighing the risk of complications against the utility of information gained from performing SLNB. On the other hand, sparing women unnecessary SLNB may reduce costs to the healthcare system without compromising treatment efficacy or quality of life.
To fully understand how payer priorities and patient-level factors may influence circumstances that favor de-escalation of axillary management in elderly patients with clinically node-negative, hormone-receptor positive breast cancers, we performed a cost-effectiveness analysis investigating the relative costs and benefits afforded by adherence to the SSO Choosing Wisely guidelines. We hypothesize that although routine surgical evaluation of the axilla with SLNB is generally not cost-effective in this population, this relationship may be altered if patients have a higher than average life-expectancy or increased anxiety associated with de-escalating surgical management.
METHODS
Model Design and Assumptions
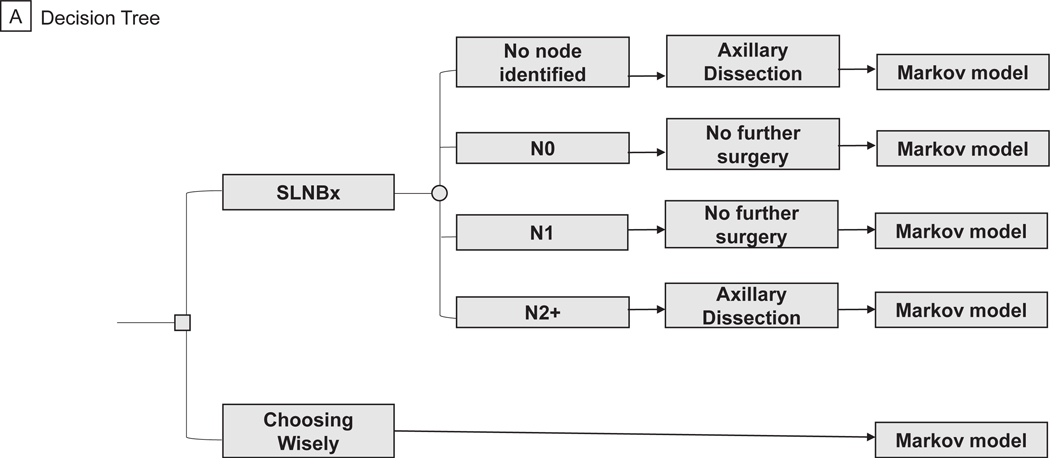
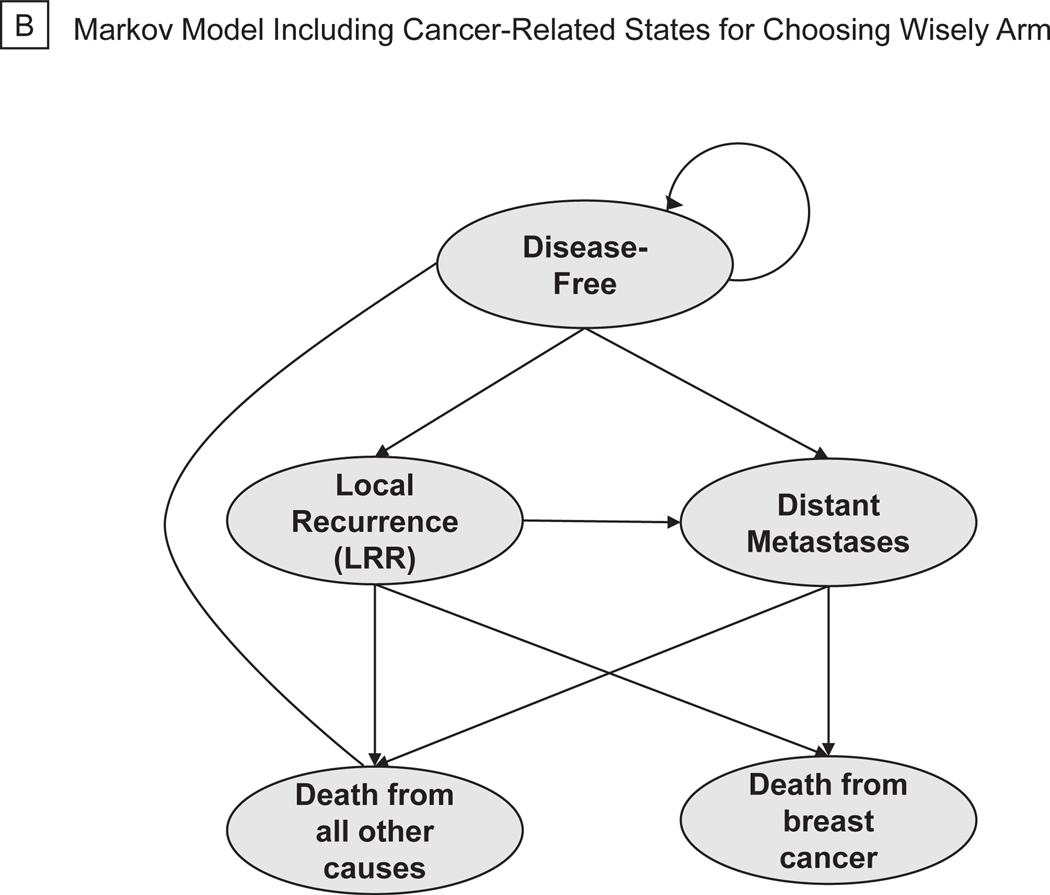
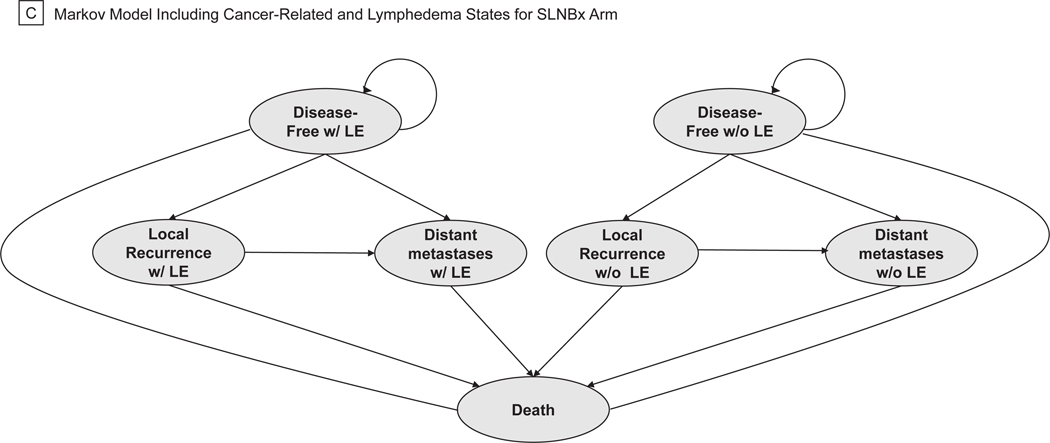
A decision-analytic tree was created to model costs and efficacy of two approaches to axillary management in breast cancer using TreeAge Pro Software (TreeAge Software, 2020, Williamstown, MA). The model was created using society guidelines, expert opinion, and randomized trial data to reflect the actual, real-world management of patients as they progress through standard breast-cancer treatment algorithms (Figure 1A). All patients entered the model at the time of surgery for their primary breast tumor and had no clinical evidence of axillary lymph node involvement on routine preoperative staging. At the decision node, patients could undergo SLNB or axillary observation. All patients relegated to axillary observation entered a Markov process at the time of surgery (Figure 1B). Further management of patients in the SLNB arm was dictated by the presence or absence of metastatic disease in sentinel nodes on pathologic analysis. Patients with no macro- or micrometastases detected in their sentinel nodes underwent no further axillary surgery. We assumed patients with macro- or micrometastatic disease in 1–2 sentinel nodes met American College of Surgeons Oncology Groups Z0011 trial inclusion criteria and underwent no further axillary surgery.10 Patients with metastases detected in ≥ 3 sentinel nodes or those with non-detection of sentinel nodes on SLNB underwent completion axillary dissection (ALND) of all level I/II nodes. All patients with positive sentinel nodes received adjuvant systemic chemotherapy for 6 months, and patients with ≥3 positive nodes received adjuvant axillary radiation (5000 Gray in 25 fractions).4 At this stage, patients entered a Markov model where, based on probabilities derived from the literature, they could transition between four health states: disease-free, locoregional recurrence (LRR), distant metastatic recurrence (DMR), and death. Locoregional recurrence included both ipsilateral breast and axillary recurrences. Patients undergoing SNLB or ALND could enter any disease state with or without lymphedema (Figure 1C).
Figure 1.
Simplified Decision-Analytic Tree and Markov Transition States. (a) Decision Tree. (b) Markov Model Including Cancer-Related States for Choosing Wisely Arm. (c) Markov Model Including Cancer-Related and Lymphedema States for SLNBx Arm.
Patient characteristics for the base case scenario were derived from clinical trials cited as evidence for the Choosing Wisely guidelines (eMethods 1, eTable 1). The Markov cycle length was set to one year. The longest follow-up of clinical trial data used in this model was 15 years and the base case scenario was therefore modeled over a 15-year time horizon. A half-cycle correction was applied to the model and all costs and benefits were discounted at a rate of 3% per year. Two separate analyses were performed from the healthcare payer and societal perspectives, taking into consideration direct and indirect cost associated with each treatment.11
The primary outcome was the incremental cost-effectiveness ratio (ICER) of SLNB, measured in United States dollars (USD) per unadjusted life-years (LYs), which was obtained by dividing the difference in costs between SLNB and axillary observation by the difference in benefits. The secondary outcome was the ICER measured in USD per quality-adjusted life years (QALYs) using health-state utilities. The willingness to pay (WTP) threshold for cost-effectiveness was set at $100,000/LY.
Health State Transition Probabilities
The probabilities of pathologic nodal stage N0, N1, N2 or N3 disease on SLNB were determined from clinical trial results of patients undergoing full axillary dissection in this population (Table 1).5,7 Annual health state transition probabilities were determined from pragmatic clinical trial data and their external validation studies.5,7,10,12,13–19 Cumulative incidences for the outcomes of interest reported in these studies including recurrence, metastases, and death were converted to rates and subsequently to annual probabilities using rate to probability equations as previously described (eMethods 2, eTable 2).20 The probability of progression from LRR to DMR was derived from a meta-analysis of clinical trials evaluating prognosis after LRR.21 Breast-cancer specific mortality rates were obtained from a variety of published studies looking at prognosis after LRR and DMR.22–26 Age-dependent annual all-cause mortality rates were obtained from Life Tables for United States females.27 The incidence and severity of lymphedema following SLNB and ALND were obtained from large-scale studies that included both subjective and objective measures of arm swelling, and only cases of moderate to severe lymphedema were considered in this analysis.28–30
Table 1.
Model inputs for the base case and sensitivity analyses from the payer and societal perspectives
|
| ||||
|---|---|---|---|---|
| Input Variable | Base Case | Range | Distribution | Source |
|
| ||||
| Sex | Female | NA | NA | N/A |
| Age | 70 | 60–90 | Triangular | 5,6,7 |
| Time Horizon | 15 years | 10–25 years | NA | 5 |
| Discount Rate | 3% | NA | NA | 11 |
| Probabilities | ||||
| High burden disease | 0.071 | 0.010–0.100 | Beta | 5,7 |
| Low burden disease | 0.183 | 0.050–0.500 | Beta | 5,7 |
| No axillary disease | 0.746 | 0.600–0.900 | Beta | 5,7 |
| Probability of sentinel node non-detection | 0.010 | 0.000–0.100 | Beta | 49 |
| Lymphedema after SLNB | 0.057 | 0.010–0.200 | Beta | 28,29,30 |
| Lymphedema after ALND | 0.140 | 0.050–0.300 | Beta | 28,29,30 |
| LRR with no axillary evaluation | 0.008 | 0.006–0.010 | Beta | 5,6,7 |
| DMR with no axillary evaluation | 0.010 | 0.009–0.011 | Beta | 5,6,7 |
| LRR with no positive SLNs | 0.003 | 0.002–0.006 | Beta | 13,14 |
| DMR with no positive SLNs | 0.007 | 0.006–0.009 | Beta | 13,14 |
| LRR with 1–2 positive SLNs | 0.004 | 0.003–0.007 | Beta | 10,15,16 |
| DMR with 1–2 positive SLNs | 0.008 | 0.005–0.009 | Beta | 10,15,16 |
| LRR with > 2 positive SLNs | 0.011 | 0.009–0.013 | Beta | 18,19 |
| DMR with > 2 positive SLNs | 0.064 | 0.020–0.080 | Beta | 18,19 |
| DMR after LRR | 0.146 | 0.050–0.250 | Beta | 21 |
| Cancer specific mortality after DMR | 0.320 | 0.200–0.500 | Beta | 23,24 |
| Cancer specific mortality after LRR | 0.048 | 0.030–0.060 | Beta | 22,25,26 |
| All-cause mortality | Varies by age | N/A | Table | 27 |
| Health State Utilities | ||||
| Disease-free | 0.977 | 0.800–1.00 | Beta | 39,40 |
| LRR, year 1 | 0.836 | 0.700–0.950 | Beta | 39,40 |
| LRR, after year 1 | 0.878 | 0.750–0.975 | Beta | 39,40 |
| DMR, annual | 0.719 | 0.600–0.800 | Beta | 39,40 |
| Disutility Estimates | ||||
| Age-related quality of life | Variable | 0.820–0.900 | N/A | 42 |
| Adjuvant radiation or chemotherapy | 0.30 | 0.100–0.400 | Beta | 50 |
| Lymphedema | 0.20 | 0.150–0.300 | Beta | 41 |
| Costs | ||||
| Injection for SLNB | $88 | $25-$200 | Gamma | 31,32 |
| SLNB | $6,937 | $3,000-$10,000 | Gamma | 31,32 |
| ALND | $7,948 | $4,000-$11,000 | Gamma | 31,32 |
| LR recurrence, year 1 | $8,247 | $5,000-$12,000 | Gamma | 34 |
| LR recurrence, after year 1 | Variable | NA | NA | 34 |
| Distant metastases | $6,790 | $3,000-$10,000 | Gamma | 34 |
| Adjuvant chemotherapy | $12,394 | $5,164-$18,590 | Gamma | 31,33 |
| Adjuvant axillary radiation | $14,000 | $5,500–20,000 | Gamma | 31,33 |
| Lymphedema, year 1 | $2,268 | $500-$5,000 | Gamma | 33 |
| Lymphedema, after year 1 | $1,465 | $250-$2,500 | Gamma | 33 |
| Costs, Societal Perspective Only | ||||
| Travel for Lymphedema treatment, year 1 | $24 | $30–55 | Gamma | 35,36 |
| Travel for lymphedema, treatment, after year 1 | $22 | $18–42 | Gamma | 35,36 |
| Informal caregiver costs for LR recurrence, year 1 | $4,200 | $2,000-$6,000 | Gamma | 37 |
| Informal caregiver costs for LR recurrence, after year 1 | $2,900 | $1,000-$5,000 | Gamma | 37 |
| Informal caregiver costs for DMR | $4,200 | $2,000-$6,000 | Gamma | 37 |
SLNB = sentinel lymph node biopsy, ALND = axillary lymph node dissection, SLNs = sentinel lymph nodes, LRR = locoregional recurrence, DMR = distant metastatic recurrence
Costs
Costs associated with the surgical treatment of patients’ primary breast tumors were assumed equivalent across arms and therefore excluded from the model. The cost of the localizing injection for SLNB as well as the total estimated costs of SLNB and ALND were obtained from the published literature and the CMS Physician Fee Schedule.31,32 The initial and annual cost of lymphedema treatments were estimated from an analysis of lymphedema costs under active and passive surveillance strategies.33 The annual cost of LRR and DMR were obtained from the literature. The model accounted for the early concentration and unequal distribution of costs in the recurrence state across years from the time of diagnosis.34 Additional costs considered in the societal perspective analysis included travel costs for lymphedema treatment, out-of-pocket lymphedema costs, and the cost of informal caregiving for patients with active or recurrent disease.35–38 All costs were converted to December 2020 USD using the U.S Bureau of Labor Statistic’s Consumer Price Index.38
Health state utilities
Utilities for breast cancer-related health states were derived from patient interviews, which included a large cohort of postmenopausal women with breast cancer, using a chained standard gamble approach.39,40 The model accounts for changes in breast-cancer recurrence-related quality of life over time with the largest quality-of-life decrement occurring near the time of diagnosis and increasing after treatment. For the base case analysis, lymphedema was assigned a disutility value of 0.20 and varied over sensitivity analysis.41 Additionally, the model accounted for health-related quality of life decrements associated with aging.42
Sensitivity Analyses
One-way sensitivity analyses were performed on every input parameter to assess the strength of model conclusions to wide variations in individual inputs. The results of these analyses were compiled in a tornado diagram to evaluate the relative effect size of each variable on overall outcomes. A Monte-Carlo probabilistic sensitivity analysis was performed for 10,000 iterations of the model where input values were randomly sampled from all variable distributions simultaneously. Distributions were fit to model variables to capture roughly 20% variation from the base-case estimate. Gamma distributions were used for probabilities and beta distributions for costs and utilities (eMethods 3, eTable 3). The results of this sensitivity analysis were compiled to create a cost-effectiveness acceptability curve where the proportion of iterations favoring each strategy were tabulated at various WTP thresholds.
A structural sensitivity analysis was performed to test several critical model assumptions. First, we assumed there was a probability that a patient undergoing axillary observation would experience anxiety related to non-evaluation of the axilla and that this anxiety was associated with a quality of life disutility. This assumption was based on studies that demonstrate anxiety regarding perceived risk and residual disease may drive patients’ operative decision making.43,44 Additionally, although existing investigations substantiate foregoing SLNB, patients may not fully understand these data and, therefore, overestimate their risk of LRR or DMR.45 Next, we adjusted the model to represent patients with exceptional health and functional status for their chronologic age by reducing the probability of all-cause mortality for each round of the Markov model by 10%. This structural sensitivity analysis was carried out to model elderly patients with breast cancer who are most likely to request or be offered SLNB based on survey of breast-cancer experts.9 Two-way sensitivity analyses were performed on select interrelated model inputs by varying these input parameters simultaneously.
RESULTS
Total costs and benefits accrued over a 15-year time horizon for the base-case analysis from the payer perspective were $15,503 and 12.08 LYs for SLNB and $4,255 and 12.00 LYs for axillary observation, resulting in an ICER of $138,374/LY. The payer-perspective analysis was performed over variable time horizons. SLNB did not become cost-effective until the analysis was carried out over 20 years, at which point women undergoing SLNB gained an additional 0.17 LYs at an additional cost of $11,033 resulting in an ICER of $63,161 (Table 2).
Table 2.
Incremental costs, life and quality-adjusted life years, and cost-effectiveness ratios
| Benefits measured in life year (LYs) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Strategy | Time Horizon | Total Cost | Incremental Cost | Total LYs | Incremental LYs | ICER | |||
|
| |||||||||
| Payer Perspective | |||||||||
| SLNB | 10 years | $14,141 | $11,504 | 8.91 | 0.02 | $652,636 | |||
| Observation | $2,637 | 8.89 | |||||||
| SLNB | 15 years | $15,503 | $11,248 | 12.08 | 0.08 | $138,374 | |||
| Observation | $4,255 | 12.00 | |||||||
| SLNB | 20 years | $16,402 | $11,033 | 14.20 | 0.17 | $63,161 | |||
| Observation | $5,368 | 14.02 | |||||||
| Societal Perspective | |||||||||
| SLNB | 15 years | $17,285 | $10,552 | 12.08 | 0.08 | $131,900 | |||
| Observation | $6,733 | 12.00 | |||||||
|
| |||||||||
| Benefits measured in quality-adjusted life years (QALYs) | |||||||||
|
| |||||||||
| Strategy | Time Horizon | Total Cost | Incremental Cost | Total QALYs | Incremental QALYs | ICER | |||
|
| |||||||||
| Payer Perspective | |||||||||
| SLNB | 15 years | $15,845 | $11,825 | 10.33 | −0.20 | −$60,025 | |||
| Observation | $4,020 | 10.53 | |||||||
| SLNB | 20 years | $17,065 | $11,727 | 12.66 | −0.18 | −$65,657 | |||
| Observation | $5,339 | 12.84 | |||||||
| SLNB | 25 years | $17,888 | $11,642 | 14.27 | −0.13 | −91,701 | |||
| Observation | $6,246 | 14.39 | |||||||
|
| |||||||||
| Structural sensitivity analysis assuming 25% probability of anxiety and an associated disutility of 0.20 associated with axillary observation. | |||||||||
|
| |||||||||
| Payer Perspective | |||||||||
| SLNB | 15 years | $15,845 | $11,533 | 10.33 | 0.30 | $39,417 | |||
| Observation | $4,020 | 10.03 | |||||||
|
| |||||||||
| Structural sensitivity analysis assuming 25% probability of anxiety, an associated disutility of 0.20 associated with axillary observation, and a 10% reduction in the annual probability of all-cause mortality | |||||||||
|
| |||||||||
| Payer Perspective | |||||||||
| SLNB | 15 years | $15,546 | $11,206 | 9.70 | 0.29 | $38,658 | |||
| Observation | $4,340 | 9.41 | |||||||
SLNB = sentinel lymph node biopsy
The ICER decreased to $131,900/LY under the societal perspective over 15 years. Increased costs incurred by patients undergoing SLNB for lymphedema-related travel and treatment were offset by the slight increase in LRR rates and higher associated informal caregiver costs under this strategy (Table 2).
Considering quality of life, SLNB and axillary observation provided 10.33 and 10.53 QALYs over a 15-year time horizon, respectively. Axillary observation was the dominant strategy in this analysis as it provided greater QALYs at a lower cost. Axillary observation remained the dominant strategy when the model was extended over a 25-year time horizon (Table 2).
Sensitivity Analyses
Assuming a 25% probability of anxiety related to axillary observation and an associated quality-of-life decrement of 0.20 for every recurrence-free year, SLNB and axillary observation provided 10.33 and 10.03 QALYs, respectively, resulting in an ICER of $39,417/QALY. Applying a 10% reduction to yearly all-cause mortality made SLNB slightly more cost-effective at 15 years with an ICER of $38,658/QALY (Table 2).
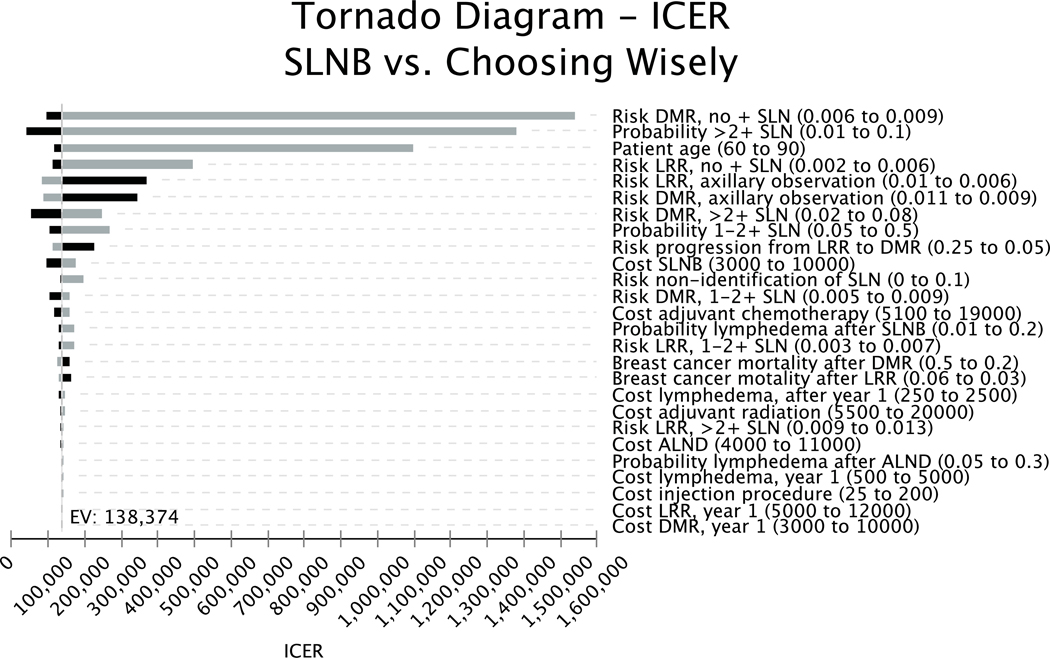
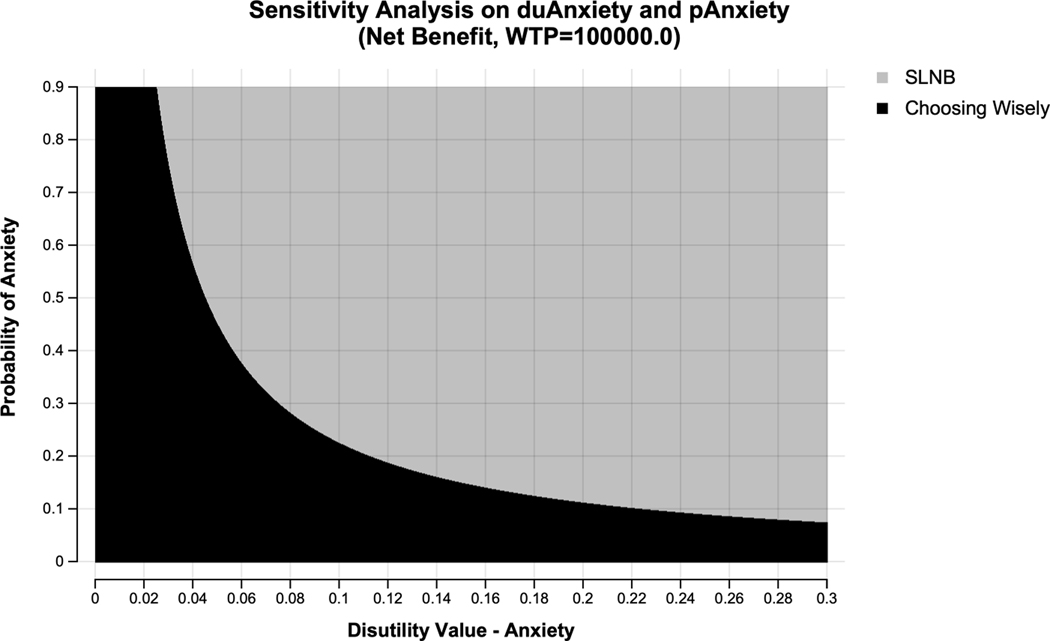
One-way sensitivity analysis revealed the model was most sensitive to the risk of local and distant recurrence in patients with no positive sentinel nodes on SLNB or those undergoing axillary observation, patient age, and the probability of positive lymph nodes discovered on SLNB (Figure 2). Two-way sensitivity analysis of the probability of anxiety with axillary observation and the disutility of anxiety revealed SLNB became the preferred strategy at a WTP threshold of $100,000/QALY if the disutility associated with patient anxiety secondary to abstaining from axillary surgery was only 0.06 and the probability of anxiety was high at 90%. SLNB was the preferred strategy when the quality-of-life decrement associated with anxiety exceeded the disutility of lymphedema (0.30) and the probability of significant anxiety was low at 15% (Figure 3).
Figure 2.
Tornado diagram showing the effect of changes in input values on the incremental cost-effectiveness ratio (ICER) measured in life-years. Black bars represent decreases and grey bars represent increases in each variable.
Figure 3.
Two-way sensitivity analysis showing the cost-effective strategy at each combination of variable values. The probability of a patient having anxiety from axillary observation is shown on the Y axis and the disutility associated with this anxiety is represented on the X axis. The black area represents combinations where axillary observation (Choosing Wisely) is the preferred strategy, and the grey area represents combinations of variables values where sentinel lymph node biopsy (SLNB) is the preferred strategy at a willingness-to-pay threshold of $100,000/life-year.
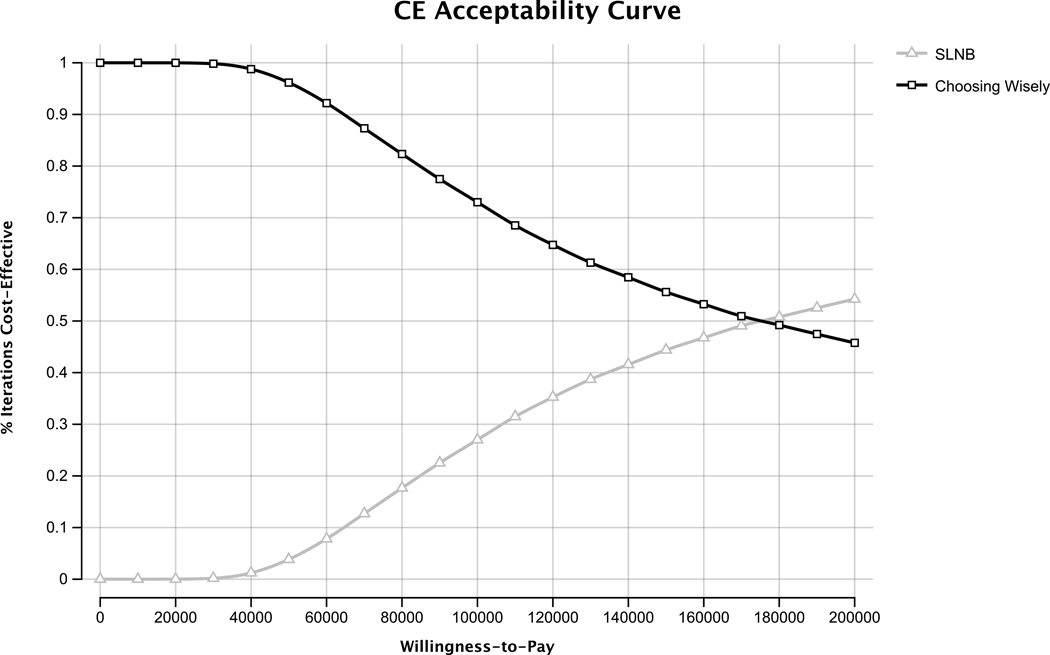
Monte-Carlo probabilistic sensitivity analysis from the payer perspective revealed axillary observation was the preferred strategy in approximately 82% of iterations at a WTP threshold of $100,000/LY. Taking into consideration the variability and uncertainty in model inputs, SLNB did not become the preferred strategy until a WTP threshold of $155,000/LY (Figure 4).
Figure 4.
Cost-effectiveness acceptability curves generated from probabilistic sensitivity analysis across a range of willingness-to-pay (WTP) thresholds. Axillary observation is the cost-effective strategy in 82% of iterations at a WTP thresholds of $100,000 per life-year (LY). Axillary observation and sentinel node biopsy are cost-effective 50% of the time at a WTP threshold of $155,000/LY.
DISCUSSION
This cost-utility analysis demonstrates SLNB is not cost-effective in patients ≥ 70 years old with hormone-receptor positive, clinically node-negative, operable breast cancer at the commonly invoked WTP threshold of $100,000/LY when considering all costs to the healthcare system and society. Furthermore, when considering the quality of life associated with various breast cancer-specific health states including lymphedema, axillary observation becomes the dominant strategy by providing more QALYs at a lower cost. Sensitivity analyses demonstrate SLNB may be cost-effective in certain patients with excellent functional status, greater than average life expectancy, and high anxiety associated with abstaining from SLNB.
Our base-case analysis is consistent with trials of ALND versus observation in elderly patients, which demonstrate a low incidence of lymph node metastases, modest reductions in locoregional recurrence, and no associated mortality benefit following axillary surgery.5–7 As demonstrated in the tornado diagram in Figure 2, the results of our model were highly sensitive to the true incidence of nodal metastases in this population. SLNB becomes cost-effective only when the probability of high or low nodal burden significantly exceeds that reported in clinical trials.
Despite aforementioned trials substantiating the safety of forgoing SLNB in this population, an analysis of 68,205 women ≥ 65 year old with clinically node-negative, stage I-II breast cancer revealed 91.2% underwent axillary surgery.8 A recently published survey of breast surgeons revealed consideration of axillary observation in patients ≥ 70 years of age with early stage disease was the most commonly disregarded Choosing Wisely guideline.9 Interestingly, providers indicated recommending against this guideline if they estimated a patient’s life-expectancy would be exceed five years from the time of surgery. Our analysis, however, suggests an incremental gain of 0.09 life years (1.08 months) with SLNB realized over a 15-year period. SLNB did not become cost-effective until the model was extended over a 20-year time horizon, affording an additional 0.17 life years (2.04 months). The cost effectiveness of SLNB in this scenario is predominantly driven more by the number of years over which the small incremental cost of the procedure is distributed rather than a clinically meaningful extension of life. Furthermore, we show SLNB delivers fewer benefits than observation when outcomes are measured in QALYs.
In the previous survey, providers endorsed pursuing SLNB out of respect for patient autonomy. We demonstrate that SLNB is cost-effective in select situations where patient preference for surgery and functional status are high. The psychological impact of axillary treatment is an important, although incompletely characterized, consideration when recommending for or against SLNB. There are no studies to date that attempt to quantify these potential impacts or any associated quality of life benefit or decrement. Comparative evidence from the watch-and-wait (WW) literature in non-breast malignancies, which may shed light on how different approaches to axillary staging influence emotional well-being, is conflicting. A survey of 1,152 patients with chronic lymphocytic leukemia revealed those placed on WW protocols were 15% more likely to feel anxious or depressed than those starting treatment. Patients who were provided clear, comprehensible information regarding WW protocols, however, were much less likely to experience negative psychological effects, suggesting education and support may ameliorate the anxiety associated with WW.46 A systematic review including 966 men with prostate cancer demonstrated superior health-related quality of life and equivalent anxiety and depression scores in men managed by active surveillance compared to radical resection.47 It is therefore unclear whether women managed with axillary observation will experience any significant anxiety or negative psychological effects related to de-escalation of axillary staging or whether these potential effects may be mitigated with improved education.
In addition to a demonstrated risk of operative morbidity associated with axillary surgery, there is a risk of financial toxicity that must be weighed against expected benefits. A survey of more than 600 women with breast cancer revealed 28% considered cost over cosmesis or breast conservation in selecting a treatment strategy.48 A large percentage of patients endorsed experiencing significant financial burden as a result of cancer treatment and 78% stated that costs were never discussed during the course of their workup or treatment.48 At a minimum, patients should receive information regarding evidence to support their treatment plan and estimated costs borne by the patients so that an informed decision can be made and patient autonomy truly respected.
Adherence to the Choosing Wisely guidelines without individualized consideration for SLNB for the subset of individuals at highest risk for occult lymph node metastases and recurrence may be problematic. While this study does include a sensitivity analysis that theoretically considers a high-risk cohort, existing data precludes incorporating a separate high-risk arm in the model. Long term follow up by Martelli et al. of 671 patients randomized to axillary clearance versus observation failed to demonstrate any difference in 15-year cumulative incidence of breast cancer-specific mortality and reported no significant axillary treatment effect on distant metastases and cancer-specific mortality.5 The authors concluded this result was based on the low incidence of occult nodal disease and receipt of adjuvant tamoxifen in this population. Similarly, the International Breast Cancer Study Group Trial 10–93, which randomized 473 patients ≥ 60 years old with clinically node-negative breast tumors to ALND or observation, reported similar overall and disease-free survival between groups.7 Although these data do not specifically address a separate high-risk cohort within their considered study demographic, the results reflect outcomes for all patients meeting inclusion criteria and show that foregoing axillary surgery does not result in significant undertreatment in this population or drive disparate outcomes.
Implementation of additional preoperative staging may identify patients at particularly highest-risk for recurrence. In our study model, preoperative staging protocols and associated costs were based on current NCCN staging recommendations, which do not include routine axillary imaging or genetic testing. In their recent study, McEvoy et al. analyzed the cost-effectiveness of axillary ultrasound as an alternative to SLNB for axillary staging in postmenopausal females. This analysis assumed subgroups of patients with early invasive breast cancer (cT1bN0-T2N0 and cN1) received Oncotype Dx guided systemic therapy even in the context of undiagnosed nodal disease. Even with consideration of a high-risk subgroup as defined by ultrasound and genomic testing, these data are consistent with our analysis that observation was associated with lower costs and higher quality of life compared to SLNB.32
This study has several important limitations. Data used to determine transition probabilities was extrapolated from several trials, resulting in some heterogeneity between results. To date, there are no clinical trials designed specifically to evaluate the Choosing Wisely guidelines. As mentioned previously, existing studies, and, accordingly, our model, are restricted with regard to data for patients at particularly high risk for recurrence based on genomic profiling or occult axillary disease. Any results that extrapolate beyond 15 years are theoretical given the lack of follow-up reported in clinical trials past this time frame. This analysis did not consider the incidence of axillary infection, nerve damage, or range of motion restriction that may further increase costs and reduce quality of life. The annual probability of LRR and DMR were considered constant overtime, and the model did not account for the change in prognosis following recurrence based on time to recurrence. The model did not consider length of hospitalization or hospital-related costs beyond those associated with the operation nor did it consider the impact of arm function, sensory loss, and time form surgery to return of normal activity on quality-of-life. Adjustment for any of these factors, however, would likely make SLNB increasingly cost-ineffective and would not significantly change the conclusions of this analysis. The model was based on clinical trial evidence. The results are therefore specific to the populations included in these trials and may not be valid for certain higher risk populations, such as predominantly clinically-detected rather than screening-detected cancers.
CONCLUSION
Although routine SLNB is not cost-effective compared to axillary observation in women ≥ 70 years old with hormone-receptor positive, clinically node-negative breast cancer at a willingness to pay threshold of $100,000/LY, this strategy may be cost-effective in select patients with high anxiety from axillary observation and good functional status. These data highlight the need for additional quality-of-life assessments among patients managed by various axillary treatment strategies as well as patient and provider education regarding potential outcomes and implications of various treatment strategies.
Supplementary Material
Precis:
Despite the Society for Surgical Oncology Choosing Wisely Guidelines’ recommendation against routine surgical axillary staging in women over 70 years old with early-stage, hormone receptor-positive breast cancer, this practice remains widespread. This rigorous cost-utility analysis with Markov modeling shows axillary observation is cost-effective compared to surgical axillary staging for most women in this population.
Acknowledgements
We would like to acknowledge the contribution of Kristina J. Nicholson, MD, MSc (UPMC) for her technical assistance during model creation.
Research support/funding:
Dr. Downs-Canner is supported by NIH Grant 5K12CA120780-12.
Footnotes
Conflicts of Interest: the authors have nothing to disclose.
REFERENCES
- 1.American Cancer Society. Cancer Facts & Figures 2021. Atlanta: American Cancer Society; 2021. [Google Scholar]
- 2.Dominici LS, King TA. How do age and molecular subtypes impact surgical decisions? Breast Cancer Manag. 2018. February; [Epub ahead of print]. Accessed 12/15/20. Available at: 10.2217/bmt-2017-0023. [DOI] [Google Scholar]
- 3.Jenkins EO, Deal AM, Anders CK, et al. Age-specific changes in intrinsic breast cancer subtypes: a focus on older women. Oncologist. 2014;19:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. Breast Cancer (Version 3.2021) [NCCN web site]. Available at: http://www.nccn.org/professionals/physician_gls/pdf/breast_blocks.pdf. Accessed April 5, 2021. [Google Scholar]
- 5.Martelli G, Miceli R, Daidone MG, et al. Axillary dissection versus no axillary dissection in elderly patients with breast cancer and no palpable axillary nodes: results after 15 years of follow-up. Ann Surg Oncol. 2011;18(1):125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 7 years or older with early breast cancer: long-term follow-up of CALBG 9343. J Clin Oncol. 2013;31(19):2382–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.International Breast Cancer Study Group; Rudenstam C, Zahrieh D, Forbes JF, et al. Randomized trial comparing axillary clearance versus no axillary clearance in older patients with breast cancer: first results of International Breast Cancer Study Group Trial 10–93. J Clin Oncol. 2006;24(3):337–344. [DOI] [PubMed] [Google Scholar]
- 8.Dominici LS, Sineshaw HM, Jemal A, et al. Patterns of axillary evaluation in older patients with breast cancer and associations with adjuvant therapy receipt. Breast Cancer Res Treat. 2018;167(2):555–566. [DOI] [PubMed] [Google Scholar]
- 9.Smith ME, Vitous CA, Hughes TM, et al. Barriers and facilitators to de-implementation of the Choosing Wisely Guidelines for low-value breast cancer surgery. Ann Surg Oncol. 2020;27(8):2653–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: The American College of Surgeons Oncology Groups Z0011 randomized trial. Ann Surg. 2010;252(3):426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders GD, Neumann PJ, Basu A, et al. Methodological practices, and reports of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103. [DOI] [PubMed] [Google Scholar]
- 12.Wang M, Ren J, Tang L, et al. Molecular features in young vs elderly breast cancer patients and the impacts on survival disparities by age at diagnosis. Cancer Med. 2018;7(7):3269–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Tang Y, Jing H, et al. Risk stratification for prediction of locoregional recurrence in patients with pathologic T1–2N0 breast cancer after mastectomy. BMC Cancer. 2020;20(1):1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortesi L, Proietto M, Cirilli C, et al. Prognosis and treatment of micrometastatic breast cancer sentinel lymph node: a population-based study. J Surg Oncol. 2012;106(4):399–405. [DOI] [PubMed] [Google Scholar]
- 15.Jung J, Han W, Lee ES, et al. Retrospectively validating the results of the ACOSOG Z0011 trial in a large Asian Z0011 eligible cohort. Breast Cancer Res and Treat. 2019;175:203–215. [DOI] [PubMed] [Google Scholar]
- 16.Yi M, Kuerer HM, Mittendorg EA, et al. Impact of the American College of Surgeons Oncology Group Z0011 criteria applied to a contemporary patient population. J Am Col Surg. 2013;216(1):105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrow M, Van Zee KJ, Patil S, et al. Axillary dissection and nodal irradiation can be avoided for most node-positive Z0011-eligible breast cancers: a prospective validation study of 793 patients. Ann Surg. 2017;266(3):457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong Y, Yang A, Xie X, et al. Impact of the extent of axillary surgery in patients with N2–3 disease in the de-escalation era: a propensity score-matched study. Clin Trans Onc. 2021;23:526–535. [DOI] [PubMed] [Google Scholar]
- 19.Kim YY, Park HK, Lee KH, et al. Prognostically distinctive subgroup in pathologic N3 breast cancer. J Breast Cancer. 2016;19(2):163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleurence RL, Hollenbeak CS. Rates and probabilities in economic modelling. Pharmacoeconomics. 2007;25(1):3–6. [DOI] [PubMed] [Google Scholar]
- 21.Wapnir IL, Anderson SJ, Mamounas EP, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five national surgical adjuvant breast and bowel project node-positive adjuvant breast cancer trials. J Clin Oncol. 2005;24(13):2028–2037. [DOI] [PubMed] [Google Scholar]
- 22.Abner AL, Recht A, Eberlein T, et al. Prognosis following salvage mastectomy for recurrence in the breast after conservative surgery and radiation therapy for early-stage breast cancer. J Clin Oncol. 1993;11(1):44–48. [DOI] [PubMed] [Google Scholar]
- 23.Gennari A, Conte P, Rosso R, et al. Survival of metastatic breast carcinoma patients over a 20-year period. Cancer. 2005;104(8):1742–1750. [DOI] [PubMed] [Google Scholar]
- 24.Foukakis T, Fornander T, Lekberg T, et al. Age-specific trends of survival in metastatic breast cancer: 26 years longitudinal data from a population-based cancer registry in Stockholm, Sweden. Breast Cancer Res and Treat. 2011;130:553–560. [DOI] [PubMed] [Google Scholar]
- 25.Lee LA, Silverstein MJ, Chung CT, et al. Breast cancer-specific mortality after invasive local recurrence in patients with ductal carcinoma-in-situ of the breast. Am J Surg. 2006;192(4):416–419 [DOI] [PubMed] [Google Scholar]
- 26.Harris EE, Hwang WT, Seyednejad F, et al. Prognosis after regional lymph node recurrence in patients with stage I-II breast carcinoma treated with breast conservation therapy. Cancer. 2003;98(10):2144–2151. [DOI] [PubMed] [Google Scholar]
- 27.Arias E, Xu JQ. Unites States life tables, 2017. National Vital Statistics Reports: vol 68 no 7. Hyattsville, MD: National Center for Health Statistics, 2019. [PubMed] [Google Scholar]
- 28.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC trial. J Nat Cancer Inst. 2006;98(9):599–609. [DOI] [PubMed] [Google Scholar]
- 29.Mclaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measures. J Clin Oncol. 2008;26(32):5213–5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashikaga T, Krag DN, Land SR, et al. , and the National Surgical Adjuvant Breast and Bowel Project (NSABP). Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J Surg Oncol. 2010;102(2):111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Physician Fee Schedule Lookup Tool [U.S. Centers for Medicare & Medicaid Services web site]. January 20, 2021. Available at: https://www.cms.gov/medicare/physician-fee-schedule/search. Accessed March 10, 2021. [Google Scholar]
- 32.McEvoy AM, Poplack S, Nickel K, et al. Cost-effectiveness analyses demonstrate that observation is superior to sentinel lymph node biopsy for postmenopausal women with HR+ breast cancer and negative axillary ultrasound. Breast Cancer Res Treat. 2020;183(2):251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stout NL, Pfalzer LA, Spinger B, et al. Breast cancer-related lymphedema: comparing direct costs of a prospective surveillance model and a traditional model of care. Phys Ther. 2012;92(1):152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stokes ME, Thompson D, Montoya El, et al. Ten-year survival and cost following breast cancer recurrence: estimates from SEER-medicare data. Val Health. 2008;11(2):213–220. [DOI] [PubMed] [Google Scholar]
- 35.Lam O, Boderick B, Toor S. How far Americans live from the closest hospital differs by community type [Pew Research Center web site]. January 4, 2019. Available at https://www.pewresearch.org/fact-tank/2018/12/12/how-far-americans-live-from-the-closest-hospital-differs-by-community-type. Accessed March 23, 2021. [Google Scholar]
- 36.IRS issues standard mileage rates for 2020. IR-2019–215 [IRS web site]. December 21, 2019. Available at https://www.irs.gov/newsroom/irs-issues-standard-mileage-rates-for-2020. Accessed March 23, 2021. [Google Scholar]
- 37.Hayman JA, Langa KM, Kabeto MU, et al. Estimating the cost of informal caregiving for elderly patients with cancer. J Clin Oncol. 2001;19(13):3219–25. [DOI] [PubMed] [Google Scholar]
- 38.US Bureau of Labor Statistics. CPI Inflation Calculator [Bureau of Labor Statistics web site] Available at http://www.bls.gov/data/inflation_calculator.htm. Accessed March 20, 2021. [Google Scholar]
- 39.Karnon J, Delea T, Barghout V. Cost utility analysis of early adjuvant Letrozole or Anastrozole versus Tamoxifen in postmenopausal women with early invasive breast cancer: the UK perspective. Eur J Health Econ. 2008;9(2):171–183. [DOI] [PubMed] [Google Scholar]
- 40.Sorensen SV, Brown R, Benedict A, et al. Patient-rated utilities in postmenopausal early breast cancer (EBC): a cross-country comparison. 2004. Value Health. 7(6):641. [Google Scholar]
- 41.Cheville AL, Almoza M, Courmier JN, et al. A prospective cohort study defining utilities using time trade-offs and the Euroqol-5D to assess the impact of cancer-related lymphedema. Cancer. 2010;116(15):3722–3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gold MR, Franks P, McCoy KI, et al. Toward consistency in cost-utility analyses: using national measures to create condition-specific values. Med Care. 1998;36(6):778–792. [DOI] [PubMed] [Google Scholar]
- 43.Rosenberg SM, Greaney ML, Patenaude AF, et al. Factors affecting surgical decisions in newly diagnosed young women with early-stage breast cancer. J Adolesc Young Adult Oncol. 2019; 8(4):463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parker PA, Peterson SK, Bedrosian I, et al. Prospective study of surgical decision-making processes for contralateral prophylactic mastectomy in women with breast cancer. Ann Surg. 2016;263(1):178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grimm LJ, Shelb RA, Knippa EE, et al. Patient perceptions of breast cancer risk in imaging-detected low-risk scenarios and thresholds for desired intervention: a multi-institutional survey. J Am Coll Radiol. 2018;15(6):911–919. [DOI] [PubMed] [Google Scholar]
- 46.Pemberton-Whitely Z, Martin C. The emotional impact of watch and wait for CLL. HemaSphere. 2019;3(S1):690–691. [Google Scholar]
- 47.Bellardita L, Valdagni R, van den Bergh R, et al. How does active surveillance for prostate cancer affect quality of life? A systematic review. Euro Uro. 2015;67(4):2015. [DOI] [PubMed] [Google Scholar]
- 48.Greenup RA, Rushing C, Fish L, et al. Financial cost and burden related to decisions of breast cancer surgery. J Oncology Practice. 2019;15(8):e666–e676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teal CB, Slocum JP, Akin EA. Evaluation of the benefit of using blue dye in addition to radioisotope for sentinel lymph node biopsy in patients with breast cancer. Breast J. 2005;11(6):391–393. [DOI] [PubMed] [Google Scholar]
- 50.Kim SH, Jo M, Ock M, Lee H, et al. Estimation of health state utilities in breast cancer. Patient Pref Adherence. 2017;14:531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.