Abstract
Objective
To develop and externally validate a prediction model for severe cisplatin associated acute kidney injury (CP-AKI).
Design
Multicenter cohort study.
Setting
Six geographically diverse major academic cancer centers across the US.
Participants
Adults (≥18 years) receiving their first dose of intravenous cisplatin, 2006-22.
Main outcome measures
The primary outcome was CP-AKI, defined as a twofold or greater increase in serum creatinine or kidney replacement therapy within 14 days of a first dose of intravenous cisplatin. Independent predictors of CP-AKI were identified using a multivariable logistic regression model, which was developed in a derivation cohort and tested in an external validation cohort. For the primary model, continuous variables were examined using restricted cubic splines. A simple risk model was also generated by converting the odds ratios from the primary model into risk points. Finally, a multivariable Cox model was used to examine the association between severity of CP-AKI and 90 day survival.
Results
A total of 24 717 adults were included, with 11 766 in the derivation cohort (median age 59 (interquartile range (IQR) 50-67)) and 12 951 in the validation cohort (median age 60 (IQR 50-67)). The incidence of CP-AKI was 5.2% (608/11 766) in the derivation cohort and 3.3% (421/12 951) in the validation cohort. Each of the following factors were independently associated with CP-AKI in the derivation cohort: age, hypertension, diabetes mellitus, serum creatinine level, hemoglobin level, white blood cell count, platelet count, serum albumin level, serum magnesium level, and cisplatin dose. A simple risk score consisting of nine covariates was shown to predict a higher risk of CP-AKI in a monotonic fashion in both the derivation cohort and the validation cohort. Compared with patients in the lowest risk category, those in the highest risk category showed a 24.00-fold (95% confidence interval (CI) 13.49-fold to 42.78-fold) higher odds of CP-AKI in the derivation cohort and a 17.87-fold (10.56-fold to 29.60-fold) higher odds in the validation cohort. The primary model had a C statistic of 0.75 and showed better discrimination for CP-AKI than previously published models, the C statistics for which ranged from 0.60 to 0.68 (DeLong P<0.001 for each comparison). Greater severity of CP-AKI was monotonically associated with shorter 90 day survival (adjusted hazard ratio 4.63 (95% CI 3.56 to 6.02) for stage 3 CP-AKI versus no CP-AKI).
Conclusion
This study found that a simple risk score based on readily available variables from patients receiving intravenous cisplatin could predict the risk of severe CP-AKI, the occurrence of which is strongly associated with death.
Introduction
Cisplatin is a potent chemotherapeutic drug used to treat a wide range of cancers.1 Despite efforts to find less toxic yet equally effective alternatives, cisplatin remains a preferred treatment option for advanced bladder cancer2 and non-small cell lung cancer,3 and it is widely used in the treatment of mesothelioma,4 head and neck cancer,5 6 gynecologic cancers,7 8 and testicular cancers.9 10 Acute kidney injury is one of the most common and serious toxicities due to cisplatin use. Cisplatin associated acute kidney injury (CP-AKI) can increase susceptibility to extrarenal toxicities from cisplatin and other renally cleared chemotherapies, as well as jeopardize eligibility for further treatment with cisplatin, or participation in clinical trials of other cancer treatments.11 12
Given the frequency with which cisplatin is administered globally and the high burden of nephrotoxicity associated with its use, understanding which patients are at highest risk for CP-AKI is important. Accurate assessment of susceptibility to CP-AKI can help clinicians weigh the risks and benefits of cisplatin, adjust the dose as needed, identify those who should be monitored more frequently, and allow researchers to enrich prospective patient cohorts (eg, for clinical trials testing novel interventions for the prevention of CP-AKI). Previous studies that investigated risk factors for CP-AKI were limited by small sample size, lack of external validation, non-contemporary data, heterogeneous definitions of acute kidney injury, and inclusion of biomarkers that are not readily available in clinical practice.13 14 15 16 17 18 Moreover, most studies used liberal definitions of acute kidney injury based on small changes in serum creatinine levels and thus did not assess the more severe and clinically relevant manifestations of CP-AKI.
To deal with these limitations and fill a key knowledge gap, we derived and externally validated a prediction model for moderate-to-severe CP-AKI using data from six large contemporary cohorts.
Methods
Study design
We conducted a multicenter cohort study of adults (≥18 years) treated with intravenous cisplatin at six major academic cancer centers across the US: Memorial Sloan Kettering Cancer Center, Massachusetts General Hospital, Dana-Farber Cancer Institute, MD Anderson Cancer Center, University of Colorado, and Northwell Health.
Study population
The study sample included adults (≥18 years) receiving a first dose of intravenous cisplatin as an inpatient or outpatient between 2006 and 2022. We excluded patients with end stage kidney disease, those with a missing baseline serum creatinine value (defined as the value in the 30 days that was closest to, and preceding, the first dose of intravenous cisplatin), and those without at least one follow-up serum creatinine value in the first 14 days after a first dose of intravenous cisplatin.
Data collection
We collected data on age, sex, race, ethnicity, body mass index, comorbidities (hypertension, diabetes mellitus, chronic obstructive pulmonary disease, congestive heart failure, cirrhosis), status as a current or former smoker, baseline laboratory values (serum creatinine, white blood cell and platelet counts, hemoglobin, and serum magnesium, calcium, and albumin), date and dose of administered cisplatin, and receipt of other nephrotoxic chemotherapy (immune checkpoint inhibitors, pemetrexed, cetuximab, and ifosfamide) administered within 30 days before cisplatin. Baseline laboratory values were defined as the values in the 30 days closest to and preceding the first dose of intravenous cisplatin. To assess outcomes, we collected data on serum creatinine level, kidney replacement therapy, and survival after treatment with cisplatin.
Data on comorbidities were extracted using ICD-9 and ICD-10 (international classification of diseases, ninth revision and 10th revision, respectively) codes (see supplemental table S1). Baseline estimated glomerular filtration rate was calculated using the 2021 Chronic Kidney Disease Epidemiology Collaboration equation,19 which incorporates age, sex, and serum creatinine level.
Primary outcome
The primary outcome was CP-AKI, defined as a twofold or greater increase in serum creatinine level compared with baseline or kidney replacement therapy within 14 days after the first dose of cisplatin, consistent with stage 2 or 3 acute kidney injury defined by the Kidney Disease: Improving Global Outcomes (KDIGO) consensus guidelines.20 For the primary outcome we focused on moderate-to-severe acute kidney injury, as it is more clinically relevant than milder forms of acute kidney injury.
Secondary outcomes
Two of the secondary outcomes were based on alternative definitions for CP-AKI—one more liberal than the definition used for our primary outcome and one more strict. For the more liberal definition (the first secondary outcome), we defined CP-AKI according to modified KDIGO acute kidney injury criteria as an increase in serum creatinine concentration ≥26.5 µmol/L compared with baseline, a ≥1.5-fold increase in serum creatinine level compared with baseline, or kidney replacement therapy within 14 days of a first dose of intravenous cisplatin. For the stricter definition (the second secondary outcome), we defined CP-AKI according to modified KDIGO stage 3 criteria for acute kidney injury as a threefold or greater increase in serum creatinine level compared with baseline or kidney replacement therapy within 14 days after a first dose of intravenous cisplatin. For a third secondary outcome, we examined the composite outcome of major adverse kidney events within 90 days, defined as death within 90 days, kidney replacement therapy within 90 days, or persistent kidney dysfunction (increase in serum creatinine level ≥100% compared with baseline) at day 90 (defined as the closest value within 30 days before or after day 90) after a first dose of intravenous cisplatin.
Statistical analysis
Categorical data are shown as numbers (percentages) and continuous variables as median (interquartile range (IQR)). The derivation cohort comprised patients treated at Memorial Sloan Kettering Cancer Center, and the external validation cohort comprised those treated at the remaining five hospitals. The supplemental methods present sample size calculations for optimism in apparent model fit and for model validation.
Development of the primary model—Using multivariable logistic regression, we identified independent predictors of CP-AKI in the derivation cohort. Candidate variables were selected for consideration for inclusion in the final model based on clinical knowledge, biologic plausibility, univariate associations, and parsimony. We examined continuous variables using restricted cubic splines, with knots placed at the 5th, 27.5th, 50th, 72.5th, and 95th centiles of each variable. Variables were selected for the final model using backward elimination using a significance threshold of P=0.1. Multiple imputation by chained equations was used to impute missing data, with 20 complete datasets created and results pooled using Rubin’s rules.21 Spline terms were identified on one imputed dataset and imposed on all imputed datasets.
Development of a simple risk model—Next, we sought to develop a parsimonious and clinically useful integer based score for CP-AKI based on the primary multivariable model. To do so, we evaluated each of the continuous variables from the primary model in categories based on clinically relevant cut-offs and their association with CP-AKI (eg, age ≤45, 46-60, 61-70 years; hemoglobin concentration <110, 110-119, ≥120 g/L; white blood cell count ≤12.0, >12.0 x109/L; albumin <33, 33-38, >38 g/L; magnesium <0.82, ≥0.82 mmol/L; and cisplatin dose ≤50, 51-75, 76-100, 101-125, 126-150, 151-200, >200 mg). We then assigned each covariate an integer or half integer score derived by dividing the odds ratio for that variable by the smallest odds ratio in the model.22 The score for the reference category for each variable was set at 0. We calculated the total score for each patient by summing the individual scores. Patients were then split into four risk groups according to the distribution of their total score: low, moderate, high, and very high. We used logistic regression to calculate the odds ratios for CP-AKI according to each risk score category, with the low risk category serving as the reference group. In an additional analysis, we divided the risk score into fourths and calculated the odds ratio for CP-AKI, with the lowest fourth serving as the reference group.
Calibration, discrimination, internal validation, and comparison with previous models—Model calibration was assessed with calibration plots, along with estimates of calibration slope and intercept. Discrimination was assessed by calculating the area under the receiver operating characteristic curve (C statistic) for the derivation and validation cohorts. The model was internally validated with 500 bootstrap samples. Model performance was evaluated by comparing the C statistic for the primary model with the C statistics from previous multivariable models for CP-AKI13 14 23 using the DeLong method and utilizing data from the entire cohort (the derivation and validation cohorts combined). Additionally, decision curves were used to compare the performance of both the primary and the simple models (Gupta et al) with three previously published models (Bhat et al, de Jongh et al, and Motwani et al).13 14 23 These three models were chosen as comparators because they were the largest models to date evaluating predictors of CP-AKI using multivariable modeling.
Additional analyses and secondary outcomes—we used similar methodology to assess the performance of our primary model across a series of additional analyses and to develop models for our three secondary outcomes. First, we assessed CP-AKI or death in the 14 days after a first dose of intravenous cisplatin as a composite outcome, because death is a competing risk for acute kidney injury.24 25 26 Second, we modified the time period for CP-AKI assessment to 10 days and 21 days after a first dose of intravenous cisplatin. Third, we repeated the primary analysis but limited it to patients treated with cisplatin in 2016 or later to determine whether the performance of our model differed based on earlier versus more contemporary data. Finally, we repeated the primary analysis using complete case analysis rather than multiple imputation for missing data. We also performed an additional analysis where we excluded patients who only had a follow-up serum creatinine value in the first four days after a first dose of intravenous cisplatin but no additional values.
CP-AKI severity and survival—Because acute kidney injury is a strong predictor of mortality in other contexts such as critical illness,27 28 29 30 31 we examined the association between severity of CP-AKI and survival. We categorized CP-AKI into four groups: no acute kidney injury, stage 1 acute kidney injury (an increase in serum creatinine concentration ≥26.5 µmol/L or a 1.5-1.9-fold increase in serum creatinine level), stage 2 acute kidney injury (2-2.9-fold increase in serum creatinine level), or stage 3 acute kidney injury (≥3-fold increase in serum creatinine, or kidney replacement therapy), each assessed within 14 days after a first dose of intravenous cisplatin. We then used Kaplan-Meier curves and multivariable Cox regression models to examine the association between CP-AKI stage with 90 day and one year survival. We also examined the longer term association between CP-AKI and the composite outcome of major adverse kidney events within 365 days, defined as death or kidney replacement therapy in the first 365 days, or persistent kidney dysfunction (twofold or greater increase in serum creatinine compared with baseline) at day 365 (defined as the closest value within 180 days before or after day 365) after a first dose of intravenous cisplatin. Models were adjusted for age, sex, body mass index, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, current or former smoker, baseline serum creatinine level, hemoglobin level, white blood cell count, platelet count, serum albumin level, serum magnesium level, cisplatin dose, and concomitant nephrotoxic chemotherapy (pemetrexed, cetuximab, ifosfamide, or immune checkpoint inhibitors within 30 days before a first dose of intravenous cisplatin).
Data analysis—Analyses were performed using R version 3.6.3 (R Foundation).
Patient and public involvement
Patients were involved in the study through participation in a focus group, as well as an anonymous survey (see supplemental methods), where their perceptions of the study’s key findings were assessed. Their feedback was also considered in the design of an algorithm based on the risk prediction model. Twenty seven patients who had previously received intravenous cisplatin were asked to fill out a survey (see supplemental methods), and 20 patients (74%) completed it. Most patients (85%; 17/20) thought that the findings of this study were important to the scientific community, and that they would want to know about this information when discussing the risks and benefits of cisplatin with their oncologist (see supplemental figure S1).
Results
Baseline characteristics and CP-AKI incidence—The initial study population included 34 122 patients across six sites, comprising 15 752 patients in the derivation cohort and 18 370 in the validation cohort. After applying the exclusion criteria, the final dataset consisted of 11 766 patients in the derivation cohort and 12 951 in the validation cohort (fig 1; also see supplemental figure S2). Baseline characteristics were largely similar between the two cohorts, though the proportion of patients with hypertension, diabetes mellitus, congestive heart failure, and cirrhosis was higher in the validation cohort, and the proportion with chronic obstructive pulmonary disease was higher in the derivation cohort (table 1). Patients in the derivation cohort also received a higher median dose of cisplatin than those in the validation cohort. Supplemental table S2 shows baseline characteristics by outcome status.
Fig 1.
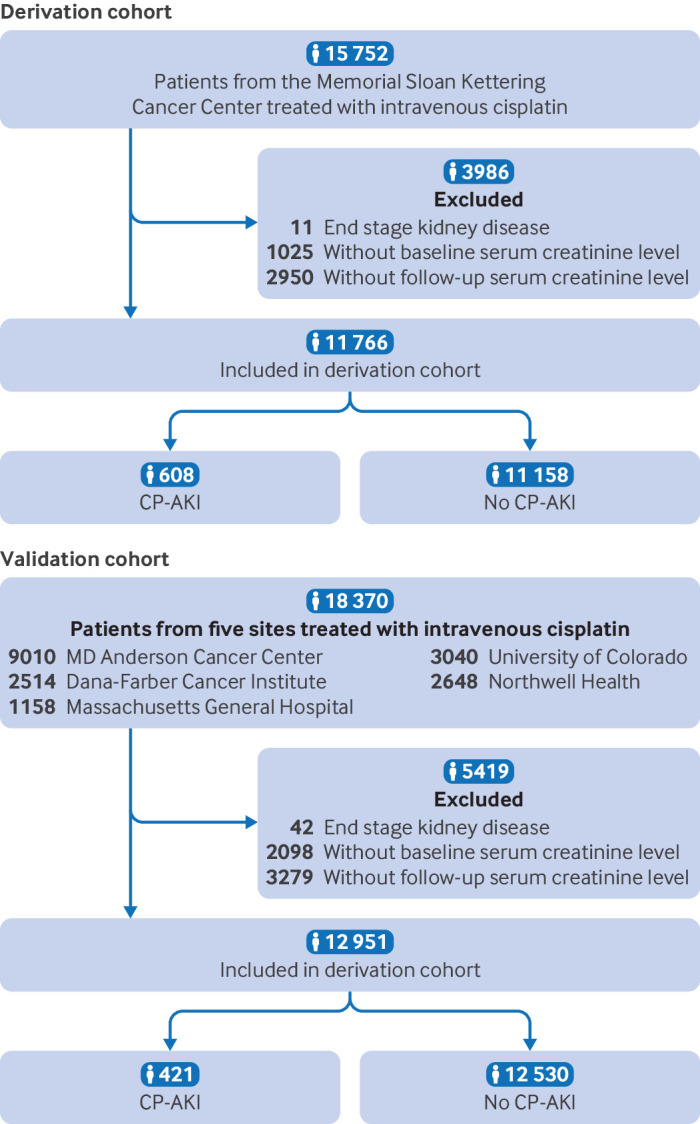
Flow of participants in derivation cohort and validation cohort. CP-AKI=cisplatin associated acute kidney injury
Table 1.
Baseline characteristics of participants used to derive a simple risk score for predicting severe cisplatin associated acute kidney injury. Values are number (percentage) unless stated otherwise
| Characteristics | All patients (n=24 717) | Derivation cohort (n=11 766) | Validation cohort (n=12 951) | |
|---|---|---|---|---|
| Personal information | ||||
| Median (IQR) age (years) | 60 (50-67) | 59 (50-67) | 60 (50-67) | |
| Male sex | 14 275 (57.8) | 6935 (58.9) | 7340 (56.7) | |
| Race: | ||||
| White | 19 378 (78.4) | 9495 (80.7) | 9,883 (76.3) | |
| Black | 1673 (6.8) | 793 (6.7) | 880 (6.8) | |
| Asian/Pacific Islander | 1449 (5.9) | 803 (6.8) | 646 (5.0) | |
| Other/Unknown | 2217 (9.0) | 675 (5.7) | 1542 (11.9) | |
| Ethnicity: | ||||
| Non-Hispanic | 19 597 (79.3) | 9532 (81.0) | 10 065 (77.7) | |
| Hispanic | 2045 (8.3) | 708 (6.0) | 1337 (10.3) | |
| Median (IQR) body mass index | 26.8 (23.5-30.6) | 26.7 (23.5-30.4) | 27.0 (23.6-30.9) | |
| Coexisting conditions | ||||
| Diabetes mellitus | 3259 (13.2) | 1426 (12.1) | 1833 (14.2) | |
| Hypertension | 7223 (29.2) | 2900 (24.6) | 4323 (33.4) | |
| Chronic obstructive pulmonary disease | 2368 (9.6) | 1516 (12.9) | 852 (6.6) | |
| Current or former smoker | 10 810 (43.7) | 6762 (57.5) | 4048 (31.3) | |
| Congestive heart failure | 462 (1.9) | 91 (0.8) | 371 (2.9) | |
| Cirrhosis | 247 (1.0) | 67 (0.6) | 180 (1.4) | |
| Baseline eGFR (mL/min/1.73m2)*: | ||||
| Median (IQR) eGFR | 91 (76-102) | 90 (75-101) | 92 (77-104) | |
| ≥90 | 12 856 (52.0) | 5844 (49.7) | 7012 (54.1) | |
| 60-89 | 9752 (39.5) | 4908 (41.7) | 4844 (37.4) | |
| 45-59 | 1763 (7.1) | 865 (7.4) | 898 (6.9) | |
| <45 | 346 (1.4) | 149 (1.3) | 197 (1.5) | |
| Laboratory values (median (IQR)) | ||||
| WBC count (x109/L) | 7.1 (5.6-9.2) | 7.1 (5.6-9.1) | 7.1 (5.6-9.2) | |
| Hemoglobin (g/L) | 128 (113-140) | 128 (114-140) | 127 (112-140) | |
| Platelet count (x109/L) | 250 (200-314) | 255 (204-322) | 245 (197-306) | |
| Creatinine (µmol/L) | 72.5 (61.9-88.4) | 79.6 (61.9-88.4) | 72.5 (60.1-86.6) | |
| Magnesium (mmol/L) | 0.8 (0.8-0.9) | 0.9 (0.8-0.9) | 0.8 (0.8-0.9) | |
| Calcium (mmol/L) | 2.3 (2.3-2.4) | 2.3 (2.3-2.4) | 2.4 (2.3-2.4) | |
| Albumin (g/L) | 41 (37-43) | 41 (37-44) | 41 (37-43) | |
| Chemotherapy | ||||
| Median (IQR) cisplatin dose (mg) | 75 (55-130) | 90 (60-160) | 70 (47-97) | |
| Nephrotoxic chemotherapy† | 2064 (8.4) | 1043 (8.9) | 1021 (7.9) | |
| Pemetrexed | 935 (3.8) | 673 (5.7) | 262 (2.0) | |
| Immune checkpoint inhibitors | 325 (1.3) | 139 (1.2) | 186 (1.4) | |
| Cetuximab | 46 (0.2) | 0 (0.0) | 46 (0.4) | |
| Ifosfamide | 798 (3.2) | 257 (2.2) | 541 (4.2) | |
Missing data: In the overall cohort (n=24 717), ethnicity was missing in 3075 (12.4%), body mass index in 3889 (15.7%), smoking in 1639 (6.6%), WBC count in 275 (1.1%), hemoglobin in 270 (1.1%), platelet count in 470 (1.9%), serum magnesium in 6577 (26.6%), serum calcium in 3356 (13.6%), serum albumin in 1938 (7.8%), cisplatin dose in 83 (0.3%), and immune checkpoint inhibitors in 5 (<0.1%).
eGFR=estimated glomerular infiltration rate; IQR=interquartile range; WBC=white blood cell.
eGFR was calculated using the 2021 Chronic Kidney Disease Epidemiology Collaboration equation.19
Receipt of concomitant nephrotoxic chemotherapy was assessed within 30 days before receipt of cisplatin, and included pemetrexed, immune checkpoint inhibitors (pembrolizumab, nivolumab, atezolizumab, durvalumab, avelumab, ipilimumab, and cemiplimab), cetuximab, and ifosfamide.
CP-AKI occurred in 608 patients (5.2%; 608/11 766) in the derivation cohort and 421 patients (3.3%; 421/12 951) in the validation cohort. Rates of CP-AKI were largely unchanged over time (see supplemental figure S3).
Primary model—Each of the following was independently associated with risk of CP-AKI in the primary model: age, hypertension, diabetes mellitus, serum creatinine level, hemoglobin level, white blood cell count, platelet count, serum albumin level, serum magnesium level, and cisplatin dose. Supplemental table S3 shows a full description of the primary model. Supplemental figure S4 shows partial effect plots for the restricted cubic spline analyses.
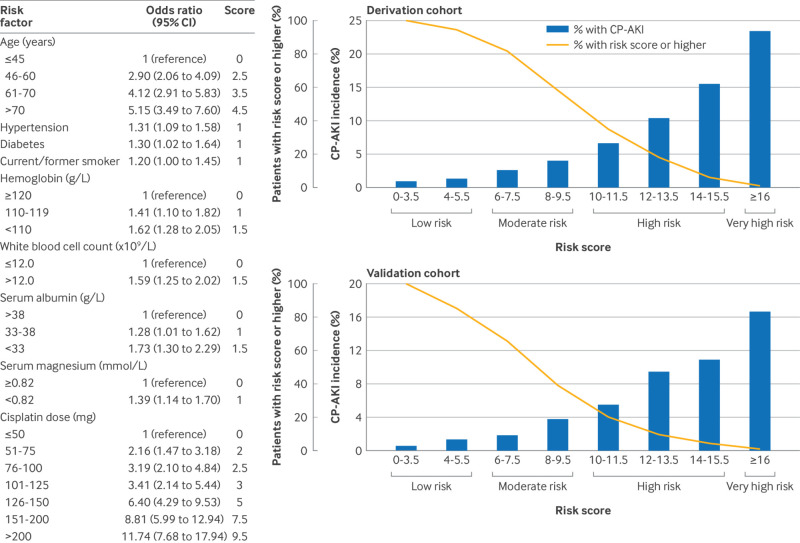
Simple risk model—Nine covariates were included in the simple risk model; fig 2 shows the odds ratios and associated risk points. With the addition of serum creatinine to the simple risk model, the area under the curve of the simplified model remained unchanged, and therefore serum creatinine was not included in the interest of parsimony. The total number of points a patient could be assigned ranged from 0 to 22.5, whereas the actual range was 0 to 20.5 (ie, no patient had a score of ≥21). In both the derivation cohort and the validation cohort, a higher score monotonically predicted a higher risk of CP-AKI, the incidence of which ranged from 0.9% to 23.5% in the derivation cohort and 0.7% to 16.7% in the validation cohort (fig 2). Supplemental figure S5 shows additional model characteristics.
Fig 2.
Risk factors for CP-AKI in the simple risk model, and incidence of CP-AKI according to risk score in the derivation and validation cohorts. The left panel shows the risk factors for CP-AKI in the simple risk model, along with their odds ratios and associated score points. The top right and bottom right panels show the incidence of CP-AKI in the derivation and validation cohorts, respectively, according to risk score. CI=confidence interval; CP-AKI=cisplatin associated acute kidney injury
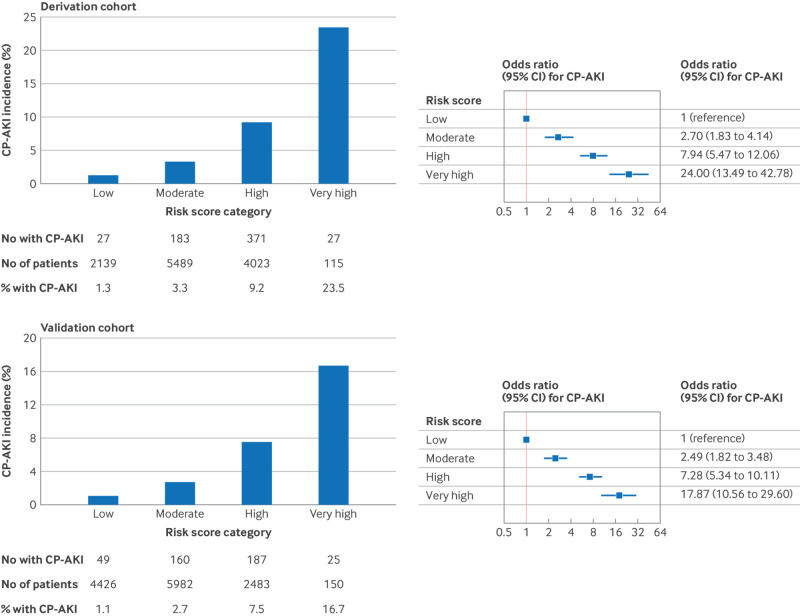
CP-AKI by risk score category—In the derivation cohort, rates of CP-AKI ranged from 1.3% in the low risk category to 23.5% in the very high risk category (odds ratio 24.00 (95% CI 13.49 to 42.78) for the very high versus low risk category; fig 3). In the validation cohort, rates of CP-AKI ranged from 1.1% in the low risk category to 16.7% in the very high risk category (17.87 (10.56 to 29.60) for the very high versus low risk category; fig 3). Supplemental figure S6 shows the incidence and odds ratios for CP-AKI according to risk score categories in fourths.
Fig 3.
Incidence of CP-AKI according to risk score category in the derivation cohort and validation cohort. CI=confidence interval; CP-AKI=cisplatin associated acute kidney injury
Diagnostic accuracy and comparison with previous models—We internally validated our model using bootstrapping, and the optimism corrected area under the curve was 0.74. To assess generalizability of the model, we performed external validation in an independent validation cohort composed of data from five academic medical centers. The C statistic for the primary model was 0.76 (95% CI 0.74 to 0.78) in the derivation cohort, 0.72 (0.70 to 0.75) in the validation cohort, and 0.75 (0.73 to 0.76) in the overall dataset. The primary model for the validation cohort was well calibrated (see supplemental figure S7). The primary model also had superior performance in predicting CP-AKI compared with the three existing models, with the Motwani et al,23 de Jongh et al,14 and Bhat et al13 models having C statistics of 0.68, 0.60, and 0.60, respectively, when tested in our dataset (DeLong P<0.001 for each comparison) (fig 4). Supplemental figure S8 shows the decision curves for the primary model, simple model, and three existing models, and the net benefit for both the primary and the simple model compared with the existing models.
Fig 4.
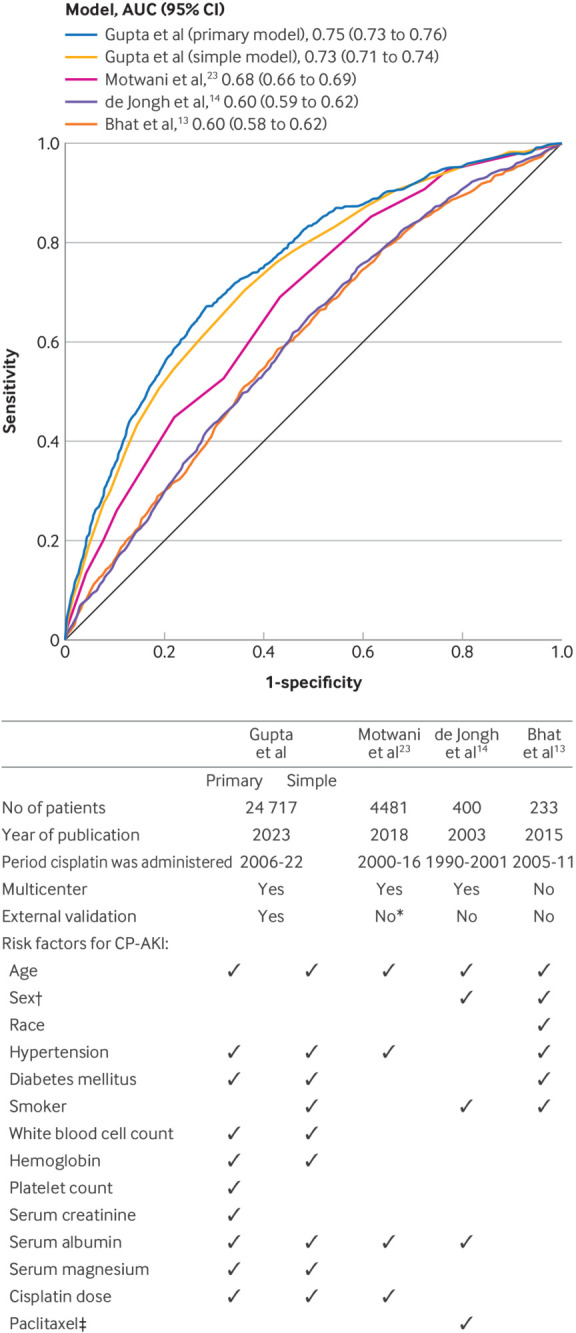
Performance of the primary and simple models compared with three previous models. Top panel shows a comparison of the discrimination of the primary and simple models (Gupta et al) with three existing models (Bhat et al,13 de Jongh et al,14 and Motwani et al.23 for CP-AKI. DeLong P<0.001 for comparison of the Gupta et al models to each of the other three models. Bottom panel shows a comparison of the study characteristics and risk factors for CP-AKI identified in the Gupta et al models versus three previous models. *In the study by Motwani et al,23 development and validation cohorts were used, but patients were derived from two hospitals within the same integrated healthcare system. †In the study by de Jongh et al,14 female sex was associated with a higher risk of CP-AKI, whereas in the study by Bhat et al,13 male sex was associated with a higher risk. ‡Data on paclitaxel were not available in the current dataset. AUC=area under the curve; CI=confidence interval; CP-AKI=cisplatin associated acute kidney injury
Additional analyses and secondary outcomes—Discrimination of the primary model was similar across all five additional analyses (see supplemental figure S9). Moreover, the C statistic remained unchanged (0.75 (95% CI 0.74 to 0.77)) upon exclusion of patients with only a follow-up serum creatinine value in the four days after a first dose of intravenous cisplatin (1581/36 483 (4.3%) excluded). When the model was refit for the first two secondary outcomes (see supplemental table S3), the C statistics were 0.71 and 0.74 for CP-AKI defined using a more liberal or a stricter definition, respectively, and it outperformed each of the three previously published models (see supplemental figure S10; DeLong P<0.001 for each comparison). For the secondary outcome of the composite of major adverse kidney events within 90 days, 9.3% of patients (1841/19 732) with data available at day 90 met the composite outcome, and the C statistic of the model was 0.79 (95% CI 0.78 to 0.81) (DeLong P<0.001; see supplemental figure S10).
CP-AKI severity and survival—Greater severity of CP-AKI was monotonically associated with decreased survival at 90 days (log-rank P<0.001; adjusted hazard ratio 4.63 (95% CI 3.56 to 6.02) for stage 3 acute kidney injury versus no acute kidney injury; fig 5) and one year (see supplemental figure S11). Higher stage of CP-AKI was also monotonically associated with an increased risk for the composite outcome of major adverse kidney events within 365 days (see supplemental figure S12).
Fig 5.
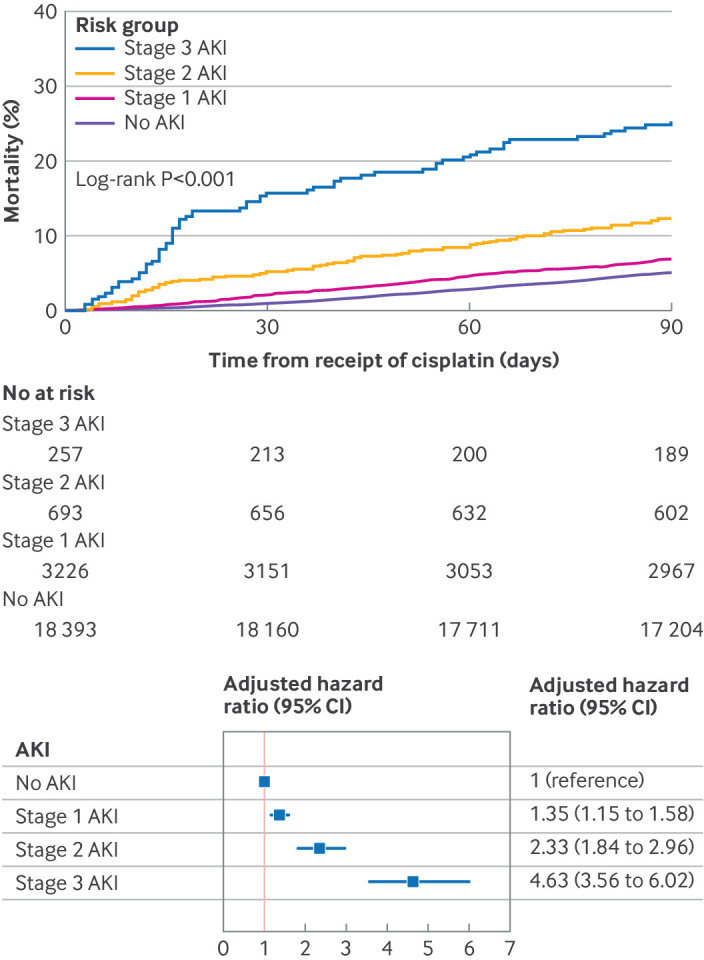
CP-AKI severity and mortality. Top panel shows cumulative incidence of death in the first 90 days after cisplatin was administered according to CP-AKI stage. CP-AKI severity was categorized into four groups according to stage of AKI: no AKI, stage 1 AKI (an increase in serum creatinine ≥26.5 µmol/L or a 1.5-1.9-fold increase in serum creatinine level), stage 2 AKI (2-2.9-fold increase in serum creatinine), or stage 3 AKI (≥3-fold increase in serum creatinine, or kidney replacement therapy), each assessed within 14 days after cisplatin was administered. Bottom panel shows a multivariable Cox model for 90 day survival according to CP-AKI stage. The model was adjusted for age, male sex, body mass index, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, current or former smoker status, serum creatinine level, hemoglobin level, white blood cell count, platelet count, serum albumin level, serum magnesium level, cisplatin dose, and concomitant nephrotoxic chemotherapy (binary variable that included pemetrexed, cetuximab, ifosfamide, or immune checkpoint inhibitors within 30 days before a first dose of intravenous cisplatin). AKI=acute kidney injury; CI=confidence interval; CP-AKI=cisplatin associated acute kidney injury
Discussion
In a multicenter cohort study of >24 000 adults treated with intravenous cisplatin between 2006 and 2022, we identified key risk factors for severe acute kidney injury based on readily available variables at the time cisplatin was administered. Using these data, we derived and characterized a simple clinical prediction score for CP-AKI comprising nine components that distinguishes patients at low risk versus high risk. Further, we externally validated our score using data from patients treated at five geographically diverse hospitals across the US, and we showed the superior discrimination of our model in predicting CP-AKI compared with three existing models.13 14 23 Lastly, we found a strong monotonic and independent association between CP-AKI and death, underscoring the importance of identifying those at highest risk for this condition.
Comparison with other studies
The C statistic of our model was 0.75, outperforming previous clinical prediction models for CP-AKI, which achieved values ranging from 0.60 to 0.68. These previously published prediction models had important limitations that we sought to address, including modest sample size, lack of external validation, non-contemporary data, and use of liberal definitions of acute kidney injury (based on small changes in serum creatinine levels) that may lack clinical relevance. The largest of these studies, by Motwani et al,23 assessed CP-AKI in 4481 patients treated with cisplatin at two centers within an integrated healthcare system. The study found that older age, hypertension, lower serum albumin level, and higher cisplatin dose were each associated with a higher risk of CP-AKI, similar to the findings of our model. In that study, however, only a limited number of predictors was assessed, true external validation was lacking, and CP-AKI was defined liberally as a 26.5 µmol/L increase in serum creatinine within 14 days of a first dose of intravenous cisplatin. In contrast, we focused on the more clinically relevant outcome of doubling of serum creatinine level or receipt of kidney replacement therapy.
Other studies that examined risk factors for CP-AKI were limited by similar issues and considerably smaller sample sizes. De Jongh et al14 and Bhat et al13 examined risk factors for CP-AKI in 400 and 233 patients, respectively. Risk factors identified included older age, female sex, black race, hypertension, diabetes mellitus, current smoking, hypoalbuminemia, and concomitant treatment with paclitaxel. Interestingly, neither study identified cisplatin dose as a risk factor for CP-AKI, perhaps because the patients all received similar doses. Zhu et al recently studied CP-AKI among 256 patients who received cisplatin at a single hospital in China.32 In addition to age, hypertension, and diabetes mellitus, the authors found that serum cystatin C and urinary kidney injury molecule-1 levels were each associated with CP-AKI. These biomarkers were measured after multiple cycles, however, raising concerns about reverse causality.
Unlike previous studies, in which serum albumin was the only routinely available laboratory value identified as a risk factor for CP-AKI, we identified several novel risk factors, including white blood cell count, hemoglobin level, platelet count, serum creatinine level, and serum magnesium level. Though some of these risk factors may simply reflect overall health, serum magnesium is particularly intriguing. Hypomagnesemia due to renal magnesium wasting is a well recognized manifestation of CP-AKI, but hypomagnesemia as a risk factor for CP-AKI has not been well documented. Hypomagnesemia is a risk factor for CP-AKI in animal studies, possibly as a result of downregulation of key transporters expressed in the proximal renal tubule (multidrug resistance proteins 4 and 6).33 34 Because these transporters are responsible for secreting cisplatin into the tubular lumen, their downregulation leads to intracellular accumulation of cisplatin and therefore acute kidney injury. Alternatively, patients may have had pre-existing tubular dysfunction from previous exposure to alternative nephrotoxic chemotherapy, thereby leading to hypomagnesemia and predisposing to acute kidney injury. Future studies should examine whether overall kidney health, assessed not only by serum creatinine but also by proteinuria and electrolyte abnormalities indicative of tubular dysfunction, aid in risk prediction of CP-AKI.
In addition to the risk factors identified for CP-AKI, we also found that greater severity of CP-AKI is associated with decreased 90 day and one year survival. Acute kidney injury has been associated with an increased risk of death in other contexts, such as critically ill patients, 27 28 29 30 31 but it has not been examined on a large scale in patients receiving cisplatin. The association between CP-AKI and death may be due to premature discontinuation of cisplatin therapy or ineligibility for other treatments that are renally cleared. Additionally, acute kidney injury can predispose to cardiovascular disease and infection, which may also explain the association between CP-AKI and death.35 These findings are important, as life expectancy among patients with cancer has increased over time,36 and these gains may be offset in those who develop CP-AKI. Accordingly, tools that allow clinicians to readily identify those at highest risk of CP-AKI may lead to better patient selection and implementation of prophylactic measures.
Strengths and limitations of this study
Our study has several notable strengths. First, the large sample size enabled us to confirm previously discovered risk factors for CP-AKI, to identify novel ones, and to develop a model that can predict risk of CP-AKI across a wide spectrum (fig 2). Second, our study focused on severe CP-AKI, with previous studies examining milder forms of kidney injury of less clinical relevance. Third, we were able to rigorously externally validate our model by including data from six geographically diverse centers from across the US, thereby increasing the generalizability and reproducibility of our findings. Fourth, our findings were consistent across five additional analyses and three secondary outcomes, further confirming the validity of our findings. Fifth, we included patients treated with cisplatin from 2006 to 2022, and an additional analysis limited to patients treated in 2016 or later found similar model discrimination compared with our primary model. Accordingly, our findings reflect contemporary practice patterns and are applicable to patients being treated today.
We also acknowledge several limitations. First, data on medications used at home were not available. Second, because cancer type could not be reliably differentiated based on ICD codes (since a patient could have multiple malignancies), the association we report between severity of CP-AKI and survival should be interpreted cautiously as these analyses were not adjusted for cancer type or stage. Nevertheless, cisplatin dose is likely a much more important predictor of CP-AKI than the underlying type of malignancy, and we are unaware of any studies that found cancer type to be an important risk factor for CP-AKI. Third, our study was limited to centers within the US. CP-AKI is, however, common both within and outside the US. Moreover, unlike novel anticancer agents that are often costly and therefore may not be as widely available, cisplatin is cheap, effective, and used in patients with cancer worldwide. Accordingly, our results are likely to be generalizable to patients outside the US. Fourth, discrimination of the primary model was modest, with a C statistic of 0.75. However, our model considerably outperformed all previous models, both for the primary outcome as well as for each of the secondary outcomes. It is likely that major improvements in risk prediction of CP-AKI will only become available when other factors (eg, genetics) are considered, or with the addition of novel biomarkers. However, novel AKI biomarkers such as urinary kidney injury molecule-1 and neutrophil gelatinase associated lipocalin may only be able to predict CP-AKI if measured after cisplatin has been administered, in which case their utility for a priori risk stratification will be limited. Moreover, many of these markers are not routinely available in current clinical practice.
Implications
Our study has important implications for patients with cancer who are treated with cisplatin. Cisplatin continues to be widely used, and acute kidney injury is one of its most common and important complications, as it can lead to discontinuation of therapy and ineligibility for other treatments. Using data readily available before cisplatin was administered, we identified several risk factors for severe CP-AKI. These patient and treatment specific risk factors for severe CP-AKI can be used to identify those patients who may possibly benefit from preventive measures, close monitoring, and consideration for alternative treatments. We shared our results during a patient centered focus group that included six patients who had received cisplatin, of whom two (33%) developed CP-AKI. We then designed an algorithm that outlines our recommendations for providers according to different risk categories for CP-AKI (see supplemental figure S13). Future studies should validate these recommendations.
Conclusion
We developed a simple, externally validated risk score for severe CP-AKI. This model should help providers weigh the risks and benefits of cisplatin and will allow for enrichment of prospective studies designed to prevent CP-AKI.
What is already known on this topic
Studies of cisplatin associated acute kidney injury (CP-AKI) have identified risk factors such as older age, hypertension, and higher cisplatin dose
These studies were limited by modest sample size, lack of external validation, non-contemporary data, and use of liberal definitions of acute kidney injury
What this study adds
In this study, a simple risk score for predicting severe CP-AKI was derived based on readily available date from >24 000 adults treated with a first dose of intravenous cisplatin across six US cancer centers during 2006-22
This risk score can be used to identify those who may benefit from closer monitoring, preventive measures, and consideration for alternative treatments
Web extra.
Extra material supplied by authors
Supplementary information: Additional methods, tables S1-S3, figures S1-S13, and references
Contributors: SG, IGG, and JSH contributed equally as first authors. AB, AA, and DEL contributed equally as senior authors. SG, IGG, JSH, AB, AA, and DEL designed the study. SG and DEL wrote the initial draft of the manuscript. KLC did the statistical analysis. ND, SLW, RHS, SAK, AMJ, SPR, JLO, OGL, DFC, and VDP cleaned and organized data and designed tables and graphs. RH made an online risk calculator. PKB, MES, MHA, KDJ, SSM, WX, KS, and KLR assisted with data collection and revised the manuscript for critical content. SG is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: None received.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work. Outside of the submitted work, SG reports research support from the National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) K23DK125672. She also reports research funding from BTG International, GE HealthCare, and AstraZeneca outside the submitted work. She is a member of GlaxoSmithKline’s (GSK) Global Anemia Council, a consultant for Secretome and Proletariat Therapeutics, and founder and president emeritus of the American Society of Onconephrology (unpaid). IG is supported by a Memorial Sloan Kettering Cancer Center support grant/core grant (P30CA008748). JSH serves on chronic kidney disease scientific advisory boards for Boehringer Ingelheim and the Kinetix Group. MES reports research support from the NIH, NIDDK R01DK140839. She also reports research funding from Gilead, Abbvie, Merck, Roche/Genetech, Novartis, Cabaletta, EMD-Serono, Otsuka, and Angion outside of the submitted work. She has served on a scientific advisory board for Travere, Novartis, Vera, Calliditas, and Mallinckrodt, is a data monitoring committee member for Alpine Immunosciences, and consults for Resonance. KDJ is a cofounder of the American Society of Onconephrology and reports consultancy agreements with Secretome, George Clinicals, PMV pharmaceuticals, Otsuka, and Calliditas, reports honorariums from the American Society of Nephrology, Lexicomp, and UpToDate.com; and reports serving as editor in chief of ASN Kidney News, and section editor for onconephrology for Nephrology Dialysis Transplantation. WX is supported by Department of Defense W81XWH2210951 and reports research funding from Oncohost outside the scope of this work, advisory board fees from Exelixis and Jazz Pharmaceuticals, and continuing medical education honorariums from MedNet, Harborside Press, MJH Healthcare Holdings, and Academy for Continued Healthcare Learning. KS reports receiving consulting fees from Scholar Rock and Equinox Group, personal fees for consulting or advisory board participation from Exelexis and MedScape, research funding to the institution from Merck, and travel honorarium from Merck. KR receives fees for organizing educational initiatives from Medscape and CME outfitters and previously served on the advisory board for SAGA diagnostics. KR also receives funds for institutional research from Bristol Myers Squibb. DEL is supported by NIH grants R01HL144566, R01DK125786, and R01DK126685 and reports research support from BioPorto, BTG International, and Metro International Biotech, and has served as a consultant for Sidereal Therapeutics, Casma Therapeutics, and MexBrain.
Data sharing No additional data available.
The lead author (SG) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: A risk calculator was created based on the prediction model and will be made available online at MDCalc.com. We will work with cancer advocacy groups (eg, American Cancer Society) to share our findings with patients. Dissemination to the public will also be achieved through media outreach, and lay summaries will be posted on publicly searchable websites.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
The study was approved with a waiver of informed consent by the institutional review board at each participating site, and by the Mass General Brigham institutional review board for the overall study, which waived the need for informed consent.
References
- 1. Brown A, Kumar S, Tchounwou PB. Cisplatin-Based Chemotherapy of Human Cancers. J Cancer Sci Ther 2019;11:97. [PMC free article] [PubMed] [Google Scholar]
- 2. Witjes JA, Bruins HM, Cathomas R, et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur Urol 2021;79:82-104. 10.1016/j.eururo.2020.03.055 [DOI] [PubMed] [Google Scholar]
- 3. Griesinger F, Korol EE, Kayaniyil S, Varol N, Ebner T, Goring SM. Efficacy and safety of first-line carboplatin-versus cisplatin-based chemotherapy for non-small cell lung cancer: A meta-analysis. Lung Cancer 2019;135:196-204. 10.1016/j.lungcan.2019.07.010 [DOI] [PubMed] [Google Scholar]
- 4. Richards WG, Zellos L, Bueno R, et al. Phase I to II study of pleurectomy/decortication and intraoperative intracavitary hyperthermic cisplatin lavage for mesothelioma. J Clin Oncol 2006;24:1561-7. 10.1200/JCO.2005.04.6813 [DOI] [PubMed] [Google Scholar]
- 5. Posner MR, Hershock DM, Blajman CR, et al. TAX 324 Study Group . Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 2007;357:1705-15. 10.1056/NEJMoa070956 [DOI] [PubMed] [Google Scholar]
- 6. Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008;359:1116-27. 10.1056/NEJMoa0802656 [DOI] [PubMed] [Google Scholar]
- 7. Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med 1999;340:1144-53. 10.1056/NEJM199904153401502 [DOI] [PubMed] [Google Scholar]
- 8. Moore DH, Blessing JA, McQuellon RP, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol 2004;22:3113-9. 10.1200/JCO.2004.04.170 [DOI] [PubMed] [Google Scholar]
- 9. de Wit R, Roberts JT, Wilkinson PM, et al. Equivalence of three or four cycles of bleomycin, etoposide, and cisplatin chemotherapy and of a 3- or 5-day schedule in good-prognosis germ cell cancer: a randomized study of the European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group and the Medical Research Council. J Clin Oncol 2001;19:1629-40. 10.1200/JCO.2001.19.6.1629 [DOI] [PubMed] [Google Scholar]
- 10. Grimison PS, Stockler MR, Chatfield M, et al. Australian and New Zealand Urogenital and Prostate Cancer Trials Group . Accelerated BEP for metastatic germ cell tumours: a multicenter phase II trial by the Australian and New Zealand Urogenital and Prostate Cancer Trials Group (ANZUP). Ann Oncol 2014;25:143-8. 10.1093/annonc/mdt369 [DOI] [PubMed] [Google Scholar]
- 11. Tsao CK, Moshier E, Seng SM, et al. Impact of the CKD-EPI equation for estimating renal function on eligibility for cisplatin-based chemotherapy in patients with urothelial cancer. Clin Genitourin Cancer 2012;10:15-20. 10.1016/j.clgc.2011.10.004 [DOI] [PubMed] [Google Scholar]
- 12. Dash A, Galsky MD, Vickers AJ, et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer 2006;107:506-13. 10.1002/cncr.22031 [DOI] [PubMed] [Google Scholar]
- 13. Bhat ZY, Cadnapaphornchai P, Ginsburg K, et al. Understanding the risk factors and long- term consequences of cisplatin-associated acute kidney injury: An observational cohort study. PLoS One 2015;10:e0142225. 10.1371/journal.pone.0142225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Jongh FE, van Veen RN, Veltman SJ, et al. Weekly high-dose cisplatin is a feasible treatment option: analysis on prognostic factors for toxicity in 400 patients. Br J Cancer 2003;88:1199-206. 10.1038/sj.bjc.6600884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Faig J, Haughton M, Taylor RC, et al. Retrospective Analysis of Cisplatin Nephrotoxicity in Patients With Head and Neck Cancer Receiving Outpatient Treatment With Concurrent High-dose Cisplatin and Radiotherapy. Am J Clin Oncol 2018;41:432-40. 10.1097/COC.0000000000000301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van der Vorst MJDL, Neefjes ECW, Toffoli EC, et al. Incidence and risk factors for acute kidney injury in head and neck cancer patients treated with concurrent chemoradiation with high-dose cisplatin. BMC Cancer 2019;19:1066. 10.1186/s12885-019-6233-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kidera Y, Kawakami H, Sakiyama T, et al. Risk factors for cisplatin-induced nephrotoxicity and potential of magnesium supplementation for renal protection. PLoS One 2014;9:e101902. 10.1371/journal.pone.0101902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhu X, Li J, Tang Y, et al. Acute kidney injury after multiple cycles of cisplatin chemotherapy: A nomogram for risk assessment. Kidney Blood Press Res 2023;48:485-94. 10.1159/000531289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Inker LA, Eneanya ND, Coresh J, et al. Chronic Kidney Disease Epidemiology Collaboration . New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N Engl J Med 2021;385:1737-49. 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kellum JA, Lameire N, Aspelin P, et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2:1-138. [Google Scholar]
- 21. Rubin D. Multiple imputation for nonresponse in surveys. In: Wiley Series in Probability and Statistics. Wiley, 1987. [Google Scholar]
- 22. Driver JA, Gaziano JM, Gelber RP, Lee IM, Buring JE, Kurth T. Development of a risk score for colorectal cancer in men. Am J Med 2007;120:257-63. 10.1016/j.amjmed.2006.05.055 [DOI] [PubMed] [Google Scholar]
- 23. Motwani SS, McMahon GM, Humphreys BD, Partridge AH, Waikar SS, Curhan GC. Development and validation of a risk prediction model for acute kidney injury after the first course of cisplatin. J Clin Oncol 2018;36:682-8. 10.1200/JCO.2017.75.7161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weisbord SD, Gallagher M, Jneid H, et al. PRESERVE Trial Group . Outcomes after Angiography with Sodium Bicarbonate and Acetylcysteine. N Engl J Med 2018;378:603-14. 10.1056/NEJMoa1710933 [DOI] [PubMed] [Google Scholar]
- 25. Semler MW, Self WH, Wanderer JP, et al. SMART Investigators and the Pragmatic Critical Care Research Group . Balanced Crystalloids versus Saline in Critically Ill Adults. N Engl J Med 2018;378:829-39. 10.1056/NEJMoa1711584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leaf DE, Waikar SS. End Points for Clinical Trials in Acute Kidney Injury. Am J Kidney Dis 2017;69:108-16. 10.1053/j.ajkd.2016.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bagshaw SM, Mortis G, Doig CJ, Godinez-Luna T, Fick GH, Laupland KB. One-year mortality in critically ill patients by severity of kidney dysfunction: a population-based assessment. Am J Kidney Dis 2006;48:402-9. 10.1053/j.ajkd.2006.06.002 [DOI] [PubMed] [Google Scholar]
- 28. Bagshaw SM, Laupland KB, Doig CJ, et al. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: a population-based study. Crit Care 2005;9:R700-9. 10.1186/cc3879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clermont G, Acker CG, Angus DC, Sirio CA, Pinsky MR, Johnson JP. Renal failure in the ICU: comparison of the impact of acute renal failure and end-stage renal disease on ICU outcomes. Kidney Int 2002;62:986-96. 10.1046/j.1523-1755.2002.00509.x [DOI] [PubMed] [Google Scholar]
- 30. Ostermann M, Chang R, Riyadh ICU Program Users Group . Correlation between the AKI classification and outcome. Crit Care 2008;12:R144. 10.1186/cc7123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morgera S, Schneider M, Neumayer HH. Long-term outcomes after acute kidney injury. Crit Care Med 2008;36(Suppl):S193-7. 10.1097/CCM.0b013e318168cae2 [DOI] [PubMed] [Google Scholar]
- 32. Guan W-J, Ni Z-Y, Hu Y, et al. China Medical Treatment Expert Group for Covid-19 . Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020;382:1708-20. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Solanki MH, Chatterjee PK, Gupta M, et al. Magnesium protects against cisplatin-induced acute kidney injury by regulating platinum accumulation. Am J Physiol Renal Physiol 2014;307:F369-84. 10.1152/ajprenal.00127.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Solanki MH, Chatterjee PK, Xue X, et al. Magnesium protects against cisplatin-induced acute kidney injury without compromising cisplatin-mediated killing of an ovarian tumor xenograft in mice. Am J Physiol Renal Physiol 2015;309:F35-47. 10.1152/ajprenal.00096.2015 [DOI] [PubMed] [Google Scholar]
- 35. Silver SA, Harel Z, McArthur E, et al. Causes of death after a hospitalization with AKI. J Am Soc Nephrol 2018;29:1001-10. 10.1681/ASN.2017080882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Capocaccia R, Gatta G, Dal Maso L. Life expectancy of colon, breast, and testicular cancer patients: an analysis of US-SEER population-based data. Ann Oncol 2015;26:1263-8. 10.1093/annonc/mdv131 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: Additional methods, tables S1-S3, figures S1-S13, and references