Abstract
Immune responses to vector-corrected cells have limited the application of gene therapy for treatment of chronic disorders such as inherited deficiency states. We have found that recombinant adeno-associated virus (AAV) efficiently transduces muscle fibers in vivo without activation of cellular and humoral immunity to neoantigenic transgene products such as β-galactosidase, which differs from the experience with recombinant adenovirus, where vibrant T-cell responses to the transgene product destroy the targeted muscle fibers. T cells activated following intramuscular administration of adenovirus expressing lacZ (AdlacZ) can destroy AAVlacZ-transduced muscle fibers, indicating a prior state of immunologic nonresponsiveness in the context of AAV gene therapy. Adoptive transfer of dendritic cells infected with AdlacZ leads to immune mediated elimination of AAVlacZ-transduced muscle fibers. AAVlacZ-transduced antigen-presenting cells fail to demonstrate β-galactosidase activity and are unable to elicit transgene immunity in adoptive transfer experiments. These studies indicate that vector-mediated transduction of dendritic cells is necessary for cellular immune responses to muscle gene therapy, a step which AAV avoids, providing a useful biological niche for its use in gene therapy.
Somatic gene transfer is a powerful way to elicit cellular and humoral immune responses to a foreign protein. While this has been exploited for the development of vaccines for cancer (5, 29, 32–35, 43) and infectious disease (25, 37, 40), it is a substantial problem in the treatment of chronic diseases, such as autosomal recessive disorders, where prolonged transgene expression may be desired (4, 44). This problem has been most extensively documented following in vivo gene transfer with recombinant adenoviruses. Adenoviruses expressing the lacZ gene elicit vibrant cellular and humoral immune responses to cytosolic β-galactosidase following delivery to liver, lung, muscle, and joint that often contribute to destruction of the genetically corrected target cells and lead to inflammation and loss of transgene (14, 41, 46, 48, 52). Similar problems were encountered following low-density lipoprotein receptor gene transfer in a murine model of familial hypercholesterolemia (27). Target cell destruction is mediated, in part, by antigen-specific class I-restricted cytotoxic T lymphocytes (CTL) to both transgene product and newly expressed viral protein (39, 46, 47, 51, 52, 54). Activation of CD4+ T cells, presumably of the TH1 subset, is required for the full realization of the destructive CTL effect (22, 28, 48, 50).
Initial studies with recombinant adeno-associated virus (AAV) delivered to skeletal muscle have yielded unexpected results in terms of the stability of gene transfer and ensuing immune responses. This human parvovirus can be rendered defective by completely eliminating all viral open reading frames, leaving the viral capsid proteins and the product of the transgene as the only sources of antigen (26). In most cases, gene transfer with AAV has been good; however, transgene expression is often poor (9, 10, 12, 31, 36, 42). Two exceptions are skeletal muscle (11, 23, 45) and the central nervous system (21), where postmitotic, differentiated cells such as muscle fibers and neurons are efficiently targeted with AAV, leading to high-level and stable transgene expression. It was particularly surprising that AAV failed to elicit immune responses to highly expressed neoantigenic transgene products when injected into muscle (11, 23, 45) whereas other vector systems expressing the identical transgene, such as adenovirus (52) and naked DNA, do. We have evaluated the mechanisms by which AAV evades immunologic responses following injection into muscle in the context of rate-limiting steps of immune activation by adenovirus.
MATERIALS AND METHODS
Animals.
C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, Maine). In this study, 4- to 5-week-old male mice were used.
Production of recombinant AAV.
Recombinant AAV expressing lacZ (AAVlacZ) was generated by plasmid transfection in 293 cells infected with E1-deleted adenovirus as described previously (10). The cytomegalovirus promoter drives expression of lacZ in this vector. A brief description of the method is provided. 293 cells were infected with AdALP (multiplicity of infection [MOI] of 10) for 2 h in Dulbecco modified Eagle medium (DMEM)–2% fetal bovine serum (FBS). At 2 h postinfection, the transfection cocktail (containing, per 15-cm-diameter plate, 0.125 ml of 2.5 M CaCl, 37.5 μg of trans plasmid [providing Rep/Cap], 12.5 μg of cis plasmid [pAV.CMVlacZ], and 1.25 ml of 2× HEPES) was added. The cells were incubated for 12 to 16 h at 37°C. Medium was replaced with fresh DMEM–2% FBS, and the infected/transfected cells were harvested 40 to 48 h postinfection.
Purification of recombinant AAV.
Frozen cell suspensions were subjected to three rounds of freeze-thaw cycles to release virus from the cells. On completion of the final thaw, bovine pancreatic DNase (1,000 U [0.5 mg] per 15-cm plate) and RNase (0.2 mg/ml, final concentration) were added, and the extract was incubated at 37°C for 30 min. Sodium deoxycholate (10% stock) was added to the sample to a final concentration of 0.5%, and the sample was incubated for 10 min at 37°C. CsCl (0.454 g/ml of sample) was added, and the sample was applied to a step gradient composed of equal volumes of CsCl in 10 mM Tris-Cl at 1.6 g/ml (bottom tier) and 1.45 g/ml (middle tier). Viral particles were banded at 25,000 rpm in a Beckman SW28 rotor for 8 h at 4°C. Fractions (1 ml) were collected from the bottom of the tube. Peak fractions that contained AAVlacZ (r = 1.41 g/ml) were combined and banded to equilibrium overnight in CsCl, using a Beckman Ty-70.1 rotor. Peak fractions (0.5 ml/fraction) of AAVlacZ were collected (r = 1.41 g/ml) and loaded onto a three-tier gradient consisting of 3 ml of 1.6-g/ml CsCl (bottom tier), 3 ml 1.45-g/ml CsCl (middle tier) and 3 ml of 1.33-g/ml CsCl (upper tier). Samples were spun in a Beckman SW41 rotor for 24 h at 35,000 rpm. Peak fractions (0.5 ml) were again collected (r = 1.41 g/ml) and loaded onto a three-tier gradient as described above. Peak fractions were dialyzed against 20 mM HEPES (pH 7.6)–150 mM NaCl.
Intramuscular injections.
Mice were anesthetized with ketamine-xylazine (70 and 10 mg/kg of body weight, respectively). Recombinant AAVlacZ (7 × 1011 particles/ml) or E1-deleted adenovirus (H5.010CMVlacZ, 1012 particles/ml) was injected into the tibialis anterior in a volume of 25 μl after a small incision was made to lay open the muscle. Incisions were closed with Vicryl suture. This E1-deleted adenovirus will subsequently be called AdlacZ. Muscle was harvested on days 10, 28, and 60 by placing the tissue on OCT embedding compound, freezing it in nitrogen-cooled isopentane for 7 s, and transferring it to liquid nitrogen. Frozen sections were analyzed for β-galactosidase activity by 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) histochemistry.
Morphological analyses.
For X-Gal staining, muscle sections were fixed in 0.5% glutaraldehyde for 10 min, washed three times with phosphate-buffered saline (PBS) containing 1 mM MgCl2, and incubated in 1 mg of X-Gal per ml–5 mM K3Fe(CN)6–5 mM K4Fe(CN)6–1 mM MgCl2 in PBS for 6 h. Tissue was counterstained with neutral red.
Cytotoxicity assay.
Splenocytes and cells from regional lymph node were isolated from C57BL/6 animals 10 days after intramuscular injection of AdlacZ and/or AAVlacZ and restimulated for 5 days at 5 × 106 cells/well in a 24-well plate with AdlacZ (MOI of 0.8). These cells were assayed on MC57 target cells at different effector/target cell ratios (starting at 25:1) in a 6-h 51Cr release assay. As target cells, MC57 cells were infected with AdlacZ at an MOI of 100 for 12 h, or a lacZ-expressing cell line was used (53). Uninfected MC57 cells were used as a negative control. Before incubation with the effectors, target cells were labeled with 100 μCi of 51Cr (Na251CrO4; NEN) for 1 h and then washed three times with DMEM without FBS. After the 6-h incubation of effector and target cells, 100 μl of supernatant was removed from each well and counted in a Packard Cobra II gamma counter. Percentage of 51Cr release was calculated as following: [(cpm of sample − cpm of spontaneous release)/(cpm of maximal release − cpm of spontaneous release)] × 100. All samples were measured as quadruplicates; for maximum release, 5% sodium dodecyl sulfate was added to the target cells and spontaneous release was determined from target cells incubated without effector cells.
Cytokine ELISA.
Lymphocytes (6 × 106 cells) were restimulated for 48 h with either UV-inactivated AdlacZ (5 × 109 particles/well), AAVlacZ (2 × 108 particles/well), purified β-galactosidase protein (10 μg/well), or medium in 24-well Costar plates. Cell-free supernatants (100 μl) were assayed for the secretion of interleukin-10 (IL-10) or gamma interferon (IFN-γ) by enzyme-linked immunosorbent assay (ELISA) as recommended by the manufacturer of the ELISA kit (Pharmingen).
Enrichment of antigen-presenting cells (APCs) from spleen.
A single-cell suspension of spleen was washed three times and then incubated for 2 h at 37°C (cells from two spleens/75-cm2 flask). All nonadherent cells were removed and discarded, and fresh DMEM–5% FBS and granulocyte/macrophage colony-stimulating factor (GM-CSF) (5 ng/ml) were added to the adherent cell population. The next day, nonadherent cells were harvested, washed, and used for infection. For adenovirus infection, cells were infected with an MOI of 100, whereas cells were exposed to purified AAVlacZ at an MOI of 5 based on LacZ-forming units. After 2 h of infection, cells were extensively washed and 5 × 106 cells were adoptively transferred into the tail vein of each animal.
Enrichment of dendritic cells from mouse spleen.
Splenocytes isolated from C57BL/6 mice were treated with ammonium chloride-Tris buffer for 2 min at 37°C to deplete erythrocytes. Cells were incubated for 90 min at 37°C (four spleens/150-cm2 flask). After the incubation, nonadherent cells were removed and discarded, fresh DMEM–10% FBS and GM-CSF (5 ng/ml) were added, and the sample was incubated overnight at 37°C. Nonadherent cells were pooled, and 3 ml at 5 × 107 cells/ml was layered over a 3-ml metrizamide gradient (14.5% in PBS) or a 30% Percoll gradient and centrifuged at 500 × g for 10 min. The dendritic cell-enriched fraction was obtained from the interface. The purity of the fraction was determined by immunohistochemistry using antibody 33D1.
Purification of B cells from mouse spleen.
A single-cell suspension from spleen of C57BL/6 animals was prepared at a density of 107 cells/ml in DMEM–10% FBS, 4-ml volumes of cell suspensions were underlaid with 3 ml of Histopaque 1083 (Sigma), and gradients were spun at 700 × g for 15 min at room temperature. The lymphocytes banded at the interface were harvested, pooled, washed once, and resuspended to a cell density of 107 cells/ml in DMEM–10% FBS. Cells were treated with a 1/1,000 dilution of anti-mouse Thy1.2 monoclonal antibody ascites plus 20 mg of anti-CD11b per ml for 30 to 45 min on ice. Cells were pelleted, resuspended in medium, and treated with rabbit complement for 60 min at 37°C. Purified B cells were pelleted and resuspended in medium to the appropriate cell density.
Isolation of macrophages.
A single-cell suspension of splenic cells was allowed to adhere onto plastic tissue culture flasks for 90 min at 37°C. The adherent cell population was scraped off and allowed to readhere for another 60 min at 37°C. The readhered population was dislodged and resuspended in DMEM–10% FBS. Flow cytometric analysis using a monoclonal antibody to MacI revealed that this population contained >70% macrophages.
Adoptive transfer.
Subpopulations of APCs were purified as described above, infected ex vivo with AdlacZ at an MOI of 100 for 2 h, washed six to eight times with DMEM-FBS, and adoptively transferred (106 cells/animal) into animals which had been infected intramuscularly with AAVlacZ the same day. Control animals received the same number of uninfected purified APCs. Muscles were isolated 10 or 28 days after adoptive transfer and stained for β-galactosidase activity.
Cytospins.
Purified populations of APCs were infected ex vivo with AdlacZ at an MOI of 100 for 2 h and washed six to eight times with DMEM-FBS. After 48 h, infected cells were washed once with PBS, and 100 μl of cell suspension (106 to 107 cells/ml) was cytospun onto glass slides and then fixed and stained for β-galactosidase activity as described above.
FISH.
Fixed specimens were washed briefly in PBS and then rinsed three times in 2× SSC (1× SSC is 150 mM NaCl plus 30 mM Na citrate) for 10 min at room temperature. They were placed directly into 50% formamide–2× SSC for 10 min at room temperature and transferred to prehybridization solution (4× SSC, 0.4% bovine serum albumin, 0.05% Tween 20, 50% formamide, 10 μg of tRNA per ml [pH 7.0]) for 1 h at 37°C. Specimens were denatured at 95°C for 10 min on a heat block, plunged into ice-cold 70% ethanol, dehydrated, and air dried. Fluorescent in situ hybridization (FISH) was carried out with digoxygenin-labeled and biotin-labeled DNA probes (labeled by using a Random prime labeling kit from Boehringer Mannheim). Labeled probe was denatured at 70°C for 5 min. Hybridization was allowed to proceed overnight at 37°C in a humid environment with 20 μl of probe solution per 22- by 22-mm coverslip sealed with rubber cement. After hybridization, specimens were washed as follows: 50% formamide–2× SSC for 15 min at 40°C, 2× SSC at 40°C for 15 min, 4× SSC containing 0.05% Tween 20 (SSC-Tween buffer) for 10 min at room temperature, and 3% bovine serum albumin in 4× SSC with 0.5% Tween 20 to block nonspecific binding of detection reagents. Detection was performed with streptavidin-fluorescein isothiocyanate and rhodamine antibodies for 20 min at 37°C. After three washes in 4× SSC-Tween buffer, coverslips were mounted in Vectashield antifade mounting solution (Vector Laboratories, Burlingame, Calif.) containing 200 ng of DAPI (4′, 6-diamidine-2-phenylindole) as a counterstain.
Immunoperoxidase staining method.
Frozen sections were fixed with acetone for 10 min, air dried, and rehydrated in PBS. Sections were then blocked in 20% goat serum in PBS for 30 min at room temperature, incubated for 1 h in primary antibody at room temperature, washed three times in 2% goat serum, and then placed in a biotinylated secondary antibody complementary to the species the primary antibody was produced in for 45 min at room temperature. The sections were washed three times, incubated for 25 min in ABC (avidin-biotin-chromagen) solution (Vector Laboratories), and washed three times in PBS before being immersed in diaminobenzidine (Sigma) for 2 min, after which they were washed three times in water, counterstained with hematoxylin, dehydrated, and coverslipped.
RESULTS
AAV-transduced muscle fibers expressing a neoantigen are nonresponsive to immune activation.
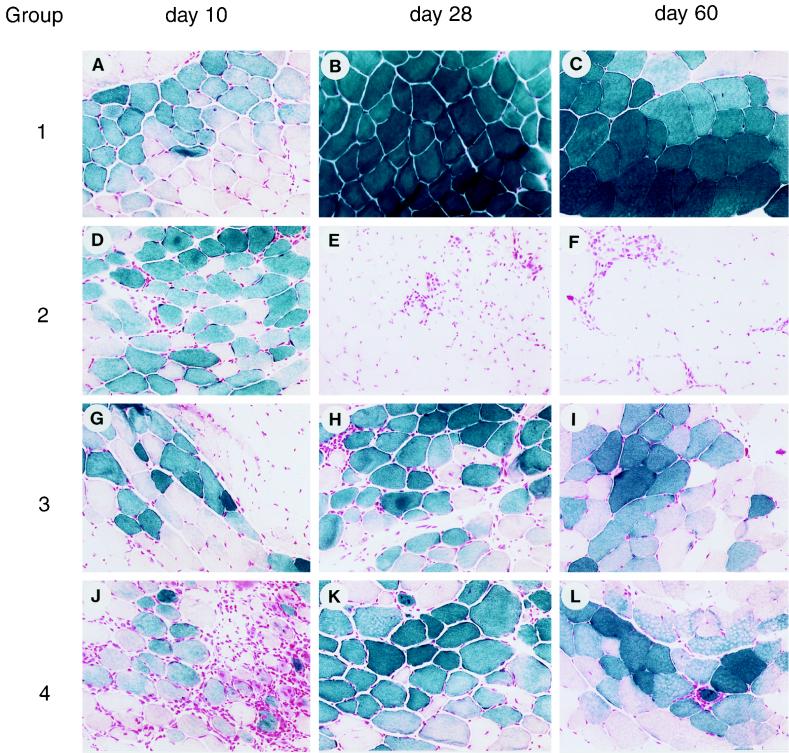
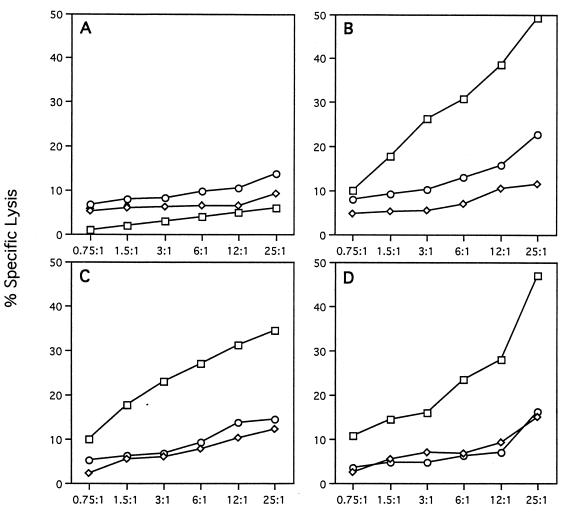
One approach used in this study for understanding the mechanism(s) by which animals respond to AAV- and adenovirus-encoded neoantigens in muscle was to inject AAVlacZ into the tibialis anterior of C57BL/6 mice and reconstitute components of the immunologic responses to AdlacZ into the AAVlacZ-treated animals (experimental groups are listed in Table 1). This will define critical steps that occur in response to adenovirus vectors that are not associated with AAV vectors. Injection of AAVlacZ alone (group 1) confers stable transgene expression (Fig. 1A to C), without activating CTL (Fig. 2A) or CD4+ T helper cells (Table 2) to β-galactosidase; CD4+ T cells secrete IL-10 in response to AAV virions (Table 2) and neutralizing antibodies to AAV are elicited (Table 3), suggesting activation of TH2 and B cells to viral capsid proteins. Several hypotheses were considered to explain the T-cell activation to adenovirus-encoded β-galactosidase in muscle that is not observed with AAV-derived β-galactosidase, including (i) cytokine-induced expression of major histocompatibility complex (MHC) class I and II in muscle fibers from adenovirus leading to direct activation of T cells by the transduced muscle fiber, (ii) adenovirus-induced lysis of muscle fibers and third-party presentation of leaked β-galactosidase, and (iii) preferential transduction or activation of APC with adenovirus but not AAV.
TABLE 1.
Overview of experimental strategya
| Group | Left leg | Right leg | Group | Left leg | Adoptive transfer | |
|---|---|---|---|---|---|---|
| 1 | AAVlacZ | 5 | AAVlacZ | APC AdlacZ | ||
| 2 | AAVlacZ | AdlacZ | 6 | AAVlacZ | APC naive | |
| 3 | AAVlacZ + AdBglII | 7 | AAVlacZ | APC AdBglII | ||
| 4 | AAVlacZ + AdALP | 8 | AAVlacZ | APC AAVlacZ | ||
| 9 | AAVlacZ | APC AAVlacZ/ AdBglII | ||||
| 10 | AAVlacZ | APC AdlacZ/ AAV ApoE | ||||
Animals were injected with AAVlacZ into the left tibialis anterior alone (group 1) and either coinjected into the same muscle with an E1-deleted adenovirus not expressing any transgene (i.e., AdBglII [group 3]) or expressing the ALP transgene (group 4), or injected separately with an adenovirus expressing the lacZ transgene into the right leg (group 2). The second set of experiments (groups 5 to 9) were based on adoptive transfer into C57BL/6 mice of 5 × 106 splenic APCs infected with various combinations of vectors ex vivo; all animals were injected with AAVlacZ in the left tibialis anterior at the time of adoptive transfer. These include APCs infected with AdlacZ (group 5), AdBglII (group 7), AAVlacZ (group 8), AAVlacZ and AdBglII (group 9), and AdlacZ and AAV ApoE (group 10). Group 6 received nontransduced APCs. The left tibialis anterior was harvested 10, 28, or 60 days later, cryosectioned, and stained for β-galactosidase activity.
FIG. 1.
Impact of adenovirus on intramuscular AAVlacZ. C57BL/6 mice were injected in the left tibialis anterior with AAVlacZ in combination with adenovirus vectors. Representative macrographs of X-Gal histochemical stains of the left tibialis anterior harvested 10, 28, and 60 days after gene transfer are presented. Group 1, AAVlacZ alone; group 2, AAVlacZ with AdlacZ in the contralateral leg; group 3, AAVlacZ mixed with AdBglII; group 4, AAVlacZ mixed with AdALP. Magnification is ×45.
FIG. 2.
CTL responses to intramuscular gene transfer. Lymphocytes were harvested from inguinal lymph nodes and spleen, restimulated in vitro for 5 days with AdlacZ, and analyzed for specific lysis using mock-infected (diamonds), AdlacZ-infected (squares), and pLJ-lacZ-infected (retrovirus transduced and selected to express lacZ; circles) syngeneic target cells (MC57). Groups studied: (A) AAVlacZ (left leg); (B) AAVlacZ (left leg) and AdlacZ (right leg); (C) mixture of AAVlacZ and AdBglII (left leg); (D) mixture of AAVlacZ and AdALP (left leg).
TABLE 2.
Cytokine secretion upon intramuscular administration of AAVlacZ and various adenovirusesa
| Group | Left leg | Right leg | IFN-γ (pg/ml)
|
IL-10 (pg/ml)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mock | Ad5 | β-Gal | AAV | Mock | Ad5 | β-Gal | AAV | |||
| 1 | AAVlacZ | 0 | 0 | 0 | 0 | 0.5 | 0.5 | 3.1 | 86 | |
| 2 | AAVlacZ | AdlacZ | 0 | 279 | 504 | 0 | 0.8 | 310 | 770 | 9 |
| 3 | AAVlacZ + AdBglII | 0 | 170 | 0 | 1.7 | 0.7 | 480 | 34 | 15 | |
| 4 | AAVlacZ + AdALP | 9 | 699 | 0 | 5.1 | 0.8 | 860 | 25 | 101 | |
Lymphocytes from C57BL/6 animals harvested 10 days after infection were restimulated with purified β-galactosidase (β-Gal), AAVlacZ (AAV), UV-inactivated adenovirus type 5 (Ad5), or medium (Mock) for 48 h, and the supernatants were assayed for the secretion of IL-10 and IFN-γ by cytokine ELISA. The data are presented as the amount of cytokine secreted following stimulation with antigen. This experiment was repeated three times with identical results; representative data are presented.
TABLE 3.
Antibody responses to vector capsid proteins and β-galactosidasea
| Group | Left leg | Right leg | Adenovirus
|
AAV
|
β-Galactosidase, IgG-ELISA | ||
|---|---|---|---|---|---|---|---|
| IgG-ELISA (absorbance) | Neutralizing antibody (dilution) | IgG-ELISA (absorbance) | Neutralizing antibody (dilution) | ||||
| 1 | AAVlacZ | 0.10 | 0 | 0.66 | 1:1,280 | 0.05 | |
| 2 | AAVlacZ | AdlacZ | 0.49 | 1:320 | 0.42 | 1:1,280 | 0.55 |
| 3 | AAVlacZ + AdBglII | 0.32 | 1:160 | 0.39 | 1:1,280 | 0.26 | |
| 4 | AAVlacZ + AdALP | 0.38 | 1:160 | 0.35 | 1:1,280 | 0.2 | |
| Uninfected | ND | 0 | 0.09 | 0 | 0.05 | ||
Serum samples harvested 28 days after intramuscular infection of C57BL/6 mice with various combinations of AAV and adenovirus as indicated were tested for the presence of neutralizing antibodies (anti-AAV or antiadenovirus) or serum IgG (specific for AAV, adenovirus, or β-Gal). The neutralizing antibody titers are presented as reciprocal dilutions which inhibit infection of AdlacZ (HeLa cells) or AAVlacZ (E1- and E4-expressing cell line derived from 293 cells) by 50% based on number of lacZ-positive cells. Serum IgG (IgG-ELISA) measures the relative amounts (absorbance at 405 nm) of antibodies in the samples at a serum dilution of 1:100. ND, not determined.
Transduction of dendritic cells by adenovirus vectors is required for immunity against the transgene product.
Muscle fibers normally express little MHC class I and no MHC class II, raising questions as to their suitability as CTL targets or potential as APC in the activation of CTL. Injection of AdlacZ contralateral to the leg that received AAVlacZ (group 2) resulted in substantial inflammation and loss of transgene expression in the AAVlacZ-transduced leg (Fig. 1D to F) associated with infiltration of CD8+ and CD4+ T cells (data not shown), as well as full CTL (Fig. 2B), TH1/TH2 (Table 2), and B-cell (Table 3) responses to both adenovirus and β-galactosidase. This finding suggests that AAV-transduced fibers are suitable T-cell targets when CTL are appropriately activated to antigen, a situation that does not appear to occur following AAV injection into muscle. These experiments were repeated with an extended interval between the initial AAVlacZ transduction and subsequent contralateral administration of AdlacZ to determine if AAVlacZ induced tolerance to β-galactosidase. The same result was achieved (i.e., apparent destruction of AAVlacZ- and AdlacZ-expressing muscle fibers), suggesting that the animals are nonresponsive to β-galactosidase when it is provided by an AAV vector rather than being tolerant to it (data not shown).
Inflammatory cytokines, such as IFN-γ, induce MHC expression in cultured human myoblasts, suggesting that the inflammation associated with intramuscular injection of adenovirus may convert the muscle fiber to a bona fide APC, leading to direct activation of CTL (15, 18, 30, 38). This was ruled out by mixing an empty E1-deleted adenovirus (i.e., transgene negative called AdBglII) with AAVlacZ prior to injection into muscle (group 3). The localized presence of adenovirus elicited inflammation (Fig. 1G and H) but did not lead to CTL (Fig. 2C) or CD4+ T-cell (Table 2) responses to β-galactosidase, and transgene expression was stable (Fig. 1G to I). Low-level antibody to β-galactosidase was detected (Table 3).
Adenovirus-induced damage to the transduced fiber may leak β-galactosidase to APCs, facilitating activation of both CD4+ and CD8+ T cells. Furthermore, adenovirus could function as an adjuvant mobilizing and activating the cells necessary for T-cell activation. In this experiment, AAVlacZ was mixed with an adenovirus expressing a different neoantigenic reporter gene, human alkaline phosphatase, which is a target of destructive immune responses and should enhance leakage and third-party presentation of β-galactosidase in cotransduced fibers (group 4). This was associated with substantial inflammation at the site of injection (Fig. 1J), infiltration of CD4+ and CD8+ T cells (data not shown), stable transgene expression (Fig. 1J to L), and T- and B-cell activation to adenovirus without significant immune responses to β-galactosidase (Fig. 2D; Tables 2 and 3), suggesting that third-party antigen presentation or adjuvant effect of the vector is not significant.
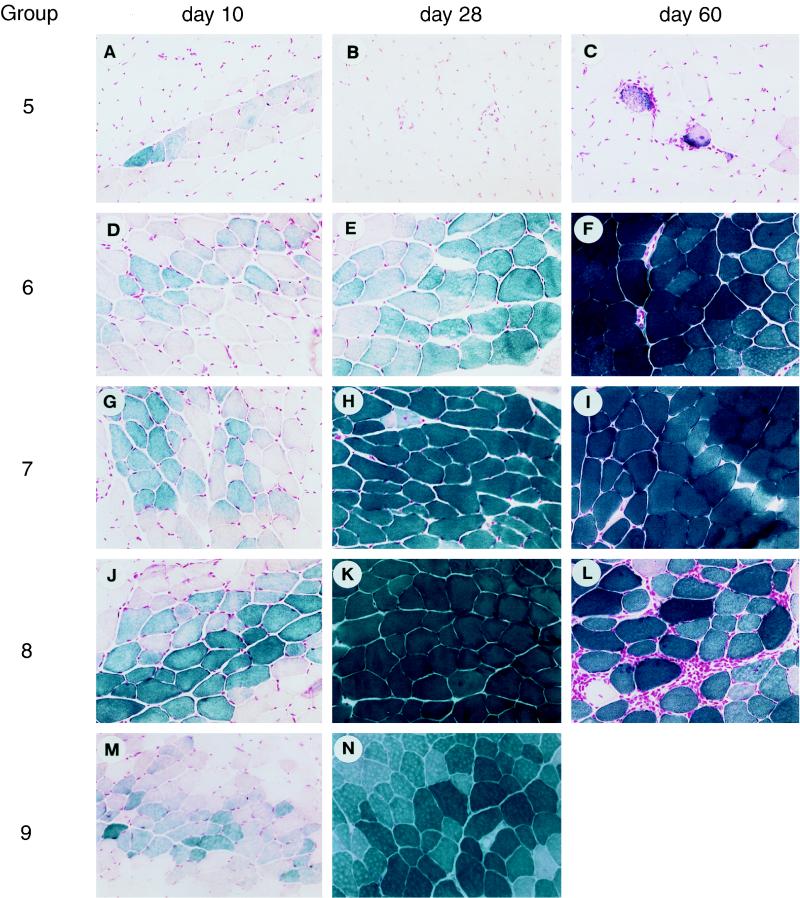
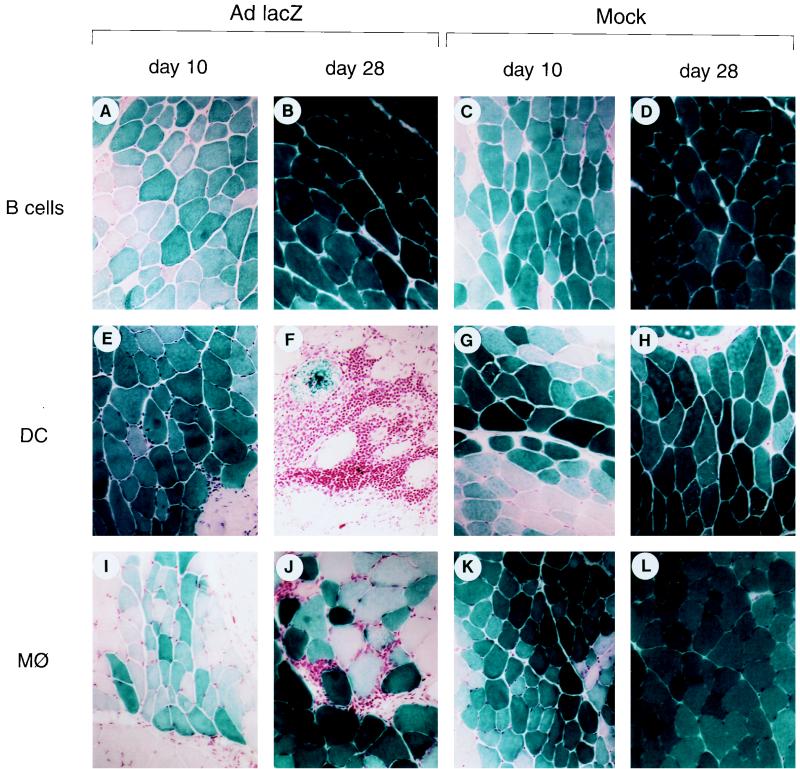
The remaining hypothesis is that vector transduction of APCs and presentation of endogenously produced β-galactosidase is necessary for CTL activation; this would predict that adenovirus transduces APCs. A mixture of APCs purified from spleen of naive C57BL/6 mice was mock infected (group 6) or infected ex vivo with AdlacZ (group 5) or AdBglII (group 7) prior to adoptive transfer into C57BL/6 mice that received an intramuscular injection of AAVlacZ. Animals receiving AdlacZ-infected APCs quickly exhibited an impressive mononuclear inflammatory response localized to the AAVlacZ-transduced muscle fibers (Fig. 3A to C) that was associated with activation of CD4+ T cells (i.e., TH1 and TH2) to β-galactosidase (Table 4) and precipitous loss of transgene (Fig. 3A to C). Transgene expression was stable without detectable inflammatory or β-galactosidase specific T-cell responses following adoptive transfer of mock-infected APCs (Fig. 3D to F; Table 4); adoptive transfer of APCs infected with AdBglII resulted in modest activation of IL-10 secretion from T helper cells to β-galactosidase without detectable inflammation or diminution in lacZ expression (Fig. 3G to I; Table 4). These data are consistent with an essential role for transduction of the APCs and presentation of endogenously produced antigen following intramuscular injection of adenovirus vectors.
FIG. 3.
Impact of APC adoptive transfer on intramuscular AAVlacZ. C57BL/6 mice were injected with AAVlacZ in the left tibialis anterior in combination with adoptive transfer of APCs exposed to a variety of vectors. Representative macrographs of X-Gal histochemical stains of the left tibialis anterior harvested 10, 28, and 60 days after gene transfer are presented. APCs were mock infected (group 6) or infected with the following vectors prior to adoptive transfer: group 5, AdlacZ; group 7, AdBglII; group 8, AAVlacZ; group 9, AAVlacZ and AdBglII. Magnification is ×45.
TABLE 4.
Cytokine secretion following adoptive transfer of virally infected APCs into animals transduced intramuscularly with AAVlacZa
| Group | Left leg | Adoptive transfer | IFN-γ (pg/ml)
|
IL-10 (pg/ml)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mock | Ad5 | β-Gal | AAV | Mock | Ad5 | β-Gal | AAV | |||
| 5 | AAVlacZ | APC AdlacZ | 113 | 423 | 418 | 265 | 54 | 850 | 1,020 | 230 |
| 6 | AAVlacZ | APC naive | 1.5 | 4.5 | 70 | 9.2 | 24 | 23 | 66 | 59 |
| 7 | AAVlacZ | APC AdBglII | 34 | 219 | 80 | 120 | 54 | 813 | 340 | 320 |
| 8 | AAVlacZ | APC AAVlacZ | 2 | 3.4 | 5.8 | 9.2 | 34 | 35 | 70 | 160 |
| 9 | AAVlacZ | APC AAVlacZ/AdBglII | 51 | 195 | 92 | 95 | 57 | 605 | 291 | 214 |
Lymphocytes from regional lymph nodes and spleen were harvested 10 days after intramuscular infection with AAVlacZ and adoptive transfer of 5 × 106 APCs infected ex vivo with AdlacZ (group 5), AdBglII (group 7), AAVlacZ (group 8), or AAVlacZ plus AdBglII (group 9) and mock infected (group 6). Cells were restimulated with purified β-galactosidase (β-Gal), AAVlacZ (AAVlacZ), UV-inactivated adenovirus type 5 (Ad5), or medium (Mock) for 48 h, and the supernatants were assayed for the secretion of IL-10 and IFN-γ by cytokine ELISA. A representative experiment is shown; this study was repeated twice with identical results.
Additional experiments were performed to determine the specific type of APC required for immune activation. Three types of APCs (macrophages, B cells, and dendritic cells) were isolated from spleens of naive mice. Immunophenotype analysis was performed on the enriched fractions of cells to assess their level of purity (Table 5). The B-cell fraction demonstrated the B-cell-specific marker B220+ on 89% of cells; the macrophage fraction had the macrophage-specific markers MacI and F4-80 in >70% of cells, with B and T cells constituting the remaining cells; and the dendritic cell fraction showed positive staining with dendritic cell marker 33D1 on >70% of cells, with contamination by 5% macrophages and 15% B cells.
TABLE 5.
Immunophenotype analysis of enriched fraction of cellsa
| Fraction | % of total
|
|||
|---|---|---|---|---|
| T cell, CD3+ | B cell, B220+ | Macrophage, F4-80/Mac1 | Dendritic cell, 33D1 | |
| APC | 12 | 55 | 25 | ND |
| B cell | 8 | 89 | 3 | 15 |
| Macrophage | 10 | 15 | >70 | <5 |
| Dendritic cell | 0 | 15 | 5 | 70–80 |
A mixed population of APCs or enriched populations of B cells, macrophages, and dendritic cells was isolated from naive spleen. The purity of the APC, B-cell, and macrophage fractions was determined in performing FACS analysis by staining the cells with anti-CD3+ for T cells, anti-B220+ for B cells, and anti-F4-80 or anti-MacI for macrophages. The purity of the dendritic cell population was determined in cytospinning the cells onto slides and subsequently performing immunohistochemical staining, utilizing the dendritic cell-specific antibody 33D1. ND, not determined.
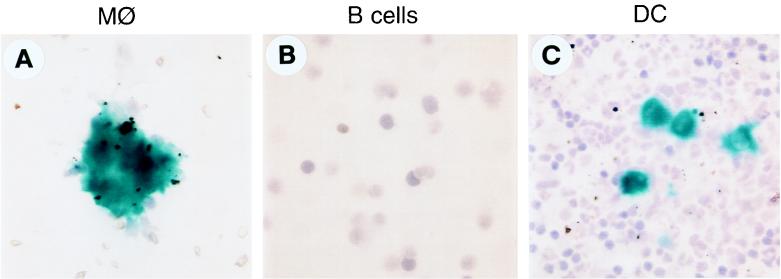
Cultured APC fractions were analyzed directly for AdlacZ transduction. The enriched fractions were evaluated by X-Gal histochemistry 2 days after exposure to AdlacZ (Fig. 4). No transduction was observed in the B-cell fraction (Fig. 4B), and rare lacZ-positive cells (<0.19%) were found in the macrophage fraction (Fig. 4A). In contrast, the dendritic cell fraction revealed lacZ expression in >10% of the cells (Fig. 4C). Adoptive transfer of the enriched APC fractions, infected with AdlacZ, was performed in C57BL/6 mice that were stably expressing lacZ in muscle following injection with AAVlacZ. The AdlacZ-transduced B-cell fraction failed to activate CD4+ T cells to either adenovirus or β-galactosidase (Table 6); AAVlacZ-transduced muscle fibers stably expressed β-galactosidase without evidence of inflammation (Fig. 5A and B). Adoptive transfer of AdlacZ-transduced macrophages did activate T cells to adenovirus and β-galactosidase (Table 6); mononuclear cell infiltrate was noted in the AAVlacZ-transduced muscle, although lacZ expression was stable (Fig. 5I and J). Inflammation or CD4+ T-cell activation did not occur when naive macrophages were adoptively transferred (Table 6; Fig. 5K and L). The results for AdlacZ-transduced dendritic cells were qualitatively different with activation of TH1 cells to β-galactosidase (Table 6), as well as destruction of fibers and loss of transgene expression (Fig. 5E and F). Naive dendritic cells produced none of these findings (Table 6; Fig. 5G and H). These data indicate that adenovirus-transduced dendritic cells are capable of eliciting a vibrant T-cell response capable of destroying lacZ-expressing muscle fibers. Activation of T cells to β-galactosidase observed with adenovirus-transduced macrophages was insufficient to destroy lacZ-expressing fibers by day 28. We cannot rule out the possibility that the partial T-cell activation noted with the macrophage fraction is due to the small amount of contaminating dendritic cells. Previous experiments with purified fractions of activated T cells indicated that the number of T cells adoptively transferred in the dendritic cell fraction are insufficient to target AAVlacZ-transduced muscle fibers (data not shown). It is unlikely that the effect observed with the dendritic cell fraction is due to contaminating B cells, T cells, and macrophages.
FIG. 4.
AdlacZ infectivity of various subpopulations of APCs. Purified or enriched populations of APCs were infected with AdlacZ (MOI of 100) for 48 h, cytospun on slides, and X-Gal stained. MØ, macrophages; DC, dendritic cells. Magnification is ×50.
TABLE 6.
Cytokine secretion following adoptive transfer of purified APCs into AAVlacZ-infected animalsa
| APCs | IFN-γ (pg/ml)
|
IL-10 (pg/ml)
|
||||
|---|---|---|---|---|---|---|
| Mock | Ad5 | β-Gal | Mock | Ad5 | β-Gal | |
| B cell | ||||||
| lacZ | 3.3 | 0.7 | 0.3 | 15 | 4.8 | 9.8 |
| Naive | 0.2 | 0.2 | 1 | 19 | 23 | 4 |
| Macrophages | ||||||
| lacZ | 76 | 291 | 247 | 2 | 34 | 16 |
| Naive | 3.7 | 4.8 | 8.3 | 0.9 | 3.6 | 3.4 |
| DC | ||||||
| lacZ | 14 | 274 | 268 | 0.7 | 9.5 | 1.7 |
| Naive | 6.7 | 12.5 | 13.3 | 2.6 | 8.4 | 2.6 |
Lymphocytes from regional lymph nodes and spleen were harvested 10 days after adoptive transfer of 106 purified B cells, macrophages, or dendritic cells (DC) either infected ex vivo with H5.010.CMVlacZ (lacZ) or uninfected (naive). Cells were restimulated with UV-inactivated adenovirus type 5 (Ad5), purified β-galactosidase protein (β-Gal), or medium (Mock) for 48 h, and the supernatants were assayed for the secretion of IL-10 or IFN-γ by cytokine ELISA.
FIG. 5.
Adoptive transfer of purified APC fractions. C57BL/6 mice were injected with AAVlacZ in the left tibialis anterior in combination with adoptive transfer of purified fractions of APCs infected with AdlacZ (columns 1 and 2) or mock infected (columns 3 and 4). In each case, the left tibialis anterior was removed for X-Gal histochemistry 10 and 28 days after adoptive transfer. The following enriched fractions were adoptively transferred: B cells; dendritic cells (DC); and macrophages (MØ). Magnification is ×45.
Inefficient transduction of APCs allows AAV to evade immunity.
The lack of detectable immune responses to AAV-encoded neoantigens is of interest in terms of basic mechanisms of T-cell activation as well as applications to gene therapy. The experience with recombinant adenovirus is that transduction of APCs is required for activation of T cells to β-galactosidase. We hypothesize that this step is defective in the context of AAV, which was confirmed in the following experiments. Naive APCs purified from spleen were exposed to high-titer AAVlacZ prior to adoptive transfer into C57BL/6 mice previously injected intramuscularly with AAVlacZ (group 8). Direct analysis of the APCs failed to reveal evidence of β-galactosidase expression (data not shown). Furthermore, recipient animals failed to activate T cells to β-galactosidase, and expression of the transgene was stable (Fig. 3J to L; Table 4); a delayed and self-limited CD4+ T-cell inflammatory response was noted at day 60 (Fig. 3L), resolving by day 120 (data not shown).
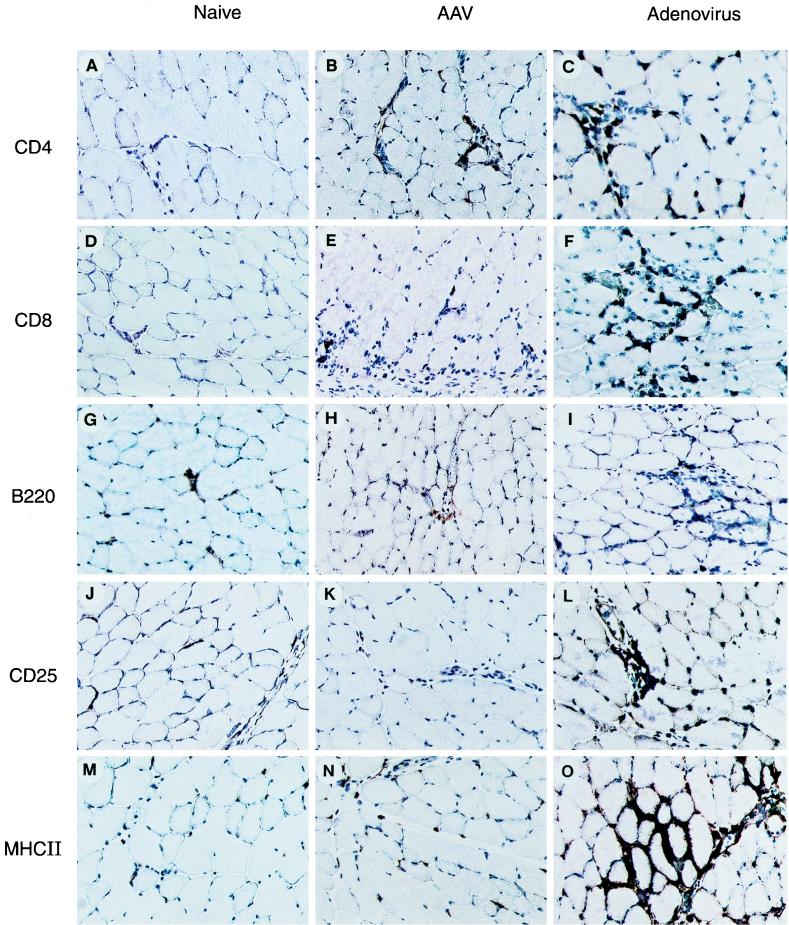
In an attempt to further evaluate mechanisms by which AAV evades immunity, skeletal muscle was characterized for indices of inflammation and immune activation, using techniques of immunocytochemistry. Representative micrographs for several markers are presented in Fig. 6; the relative abundance of each marker quantified by morphometry is shown in Table 7. Recombinant adenovirus elicits a substantial and mixed infiltrate (T cells [CD4 and CD8], B cells [B220], and dendritic cell and macrophages [CD11c]), with evidence for activated lymphocytes (CD25) and activated macrophages/dendritic cells (MHC class II, B7-2, and CD11c). AAV elicited substantially less inflammation, with modest, if any, lymphocyte or APC activation markers.
FIG. 6.
Characterization of cells infiltrating virus-infected muscle. Muscle tissues from C57BL/6 animals were harvested 10 days after injection of either AAVlacZ (middle column) or AdlacZ (right column) in the left tibialis anterior. Uninfected muscle tissue (first column) served as a baseline staining. Sections were stained with the primary antibodies indicated on the left. The signal of the primary antibodies was amplified by using an ABC-peroxidase kit. Magnification is ×45.
TABLE 7.
Immunohistochemical analysis of muscle for markers of inflammation and immune activationa
| Marker | Naive | AAV | Adenovirus |
|---|---|---|---|
| CD4 | 1 | 4 | 40 |
| CD8 | 1 | 2 | 55 |
| B220 | 1 | 6 | 12 |
| CD25 | 1 | 8 | 44 |
| MHC II | 1 | 12 | 52 |
| B7-2 | 1 | 1 | 9 |
| CD11c | 1 | 2.1 | 4.6 |
C57BL/6 mice were uninfected (mock) or injected with AAVlacZ or AdlacZ into the tibialis anterior. Ten days later, muscle was harvested and evaluated for the presence of the markers listed, using techniques of immunohistochemistry. Expression of the markers was quantified from multiple samples using morphometric techniques as described in Materials and Methods. Each sample is normalized to the level found in naive animals.
From these experiments, we propose several mechanisms by which AAV may avoid the productive presentation of antigen by APCs. These hypotheses include insufficient activation of the APC for presentation of antigen, suppression of the antigen presentation, or inability of AAV to transduce APC. Additional in vitro and in vivo experiments were performed to evaluate these positive mechanisms.
Adenovirus-injected muscle demonstrated higher levels of APC-derived antigens and markers of APC and T-cell activation than what was observed with AAV (Fig. 6; Table 7). It is possible that the recombinant adenovirus activates the transduced APC more effectively than AAV, explaining differences in immune activation to these vectors. To study this hypothesis, naive APCs were infected with both AAVlacZ and AdBglII (an E1-deleted adenovirus not expressing a transgene) prior to adoptive transfer (group 9). T cells were not activated to β-galactosidase (Table 4), nor was there an effect on transgene stability (Fig. 3M and N). Another explanation is that AAV efficiently transduces cells but also actively suppresses the presentation of antigens. How this could occur is unclear since the only open reading frame in the recombinant genome is the transgene (26); it is possible that viral capsid proteins or packaged viral proteins (i.e., Rep) either are toxic to APCs or suppress APC activation. To formally evaluate this mechanism, naive APCs were infected with both AdlacZ and a recombinant AAV expressing an irrelevant gene (i.e., Apo E) prior to adoptive transfer (group 10). The presence of high-titer recombinant AAV during the APC infection did not suppress the ability of AdlacZ to activate APCs so that following adoptive transfer, lacZ-expressing muscle fibers were destroyed in the same manner as occurred with AdlacZ-infected APCs (data not shown).
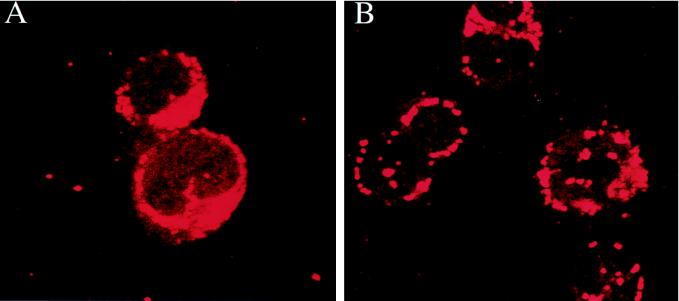
The most likely explanation for the difference between AAV and Ad in eliciting transgene immunity is selective transduction of the APCs. Transduction of fractionated populations of APCs with AdlacZ demonstrated lacZ expression in dendritic cells and some macrophages (Fig. 4), which effectively activated T cells to β-galactosidase following adoptive transfer (Fig. 5). LacZ expression was not detected in APCs following exposure to AAVlacZ (data not shown), nor were these cells capable of activating T cells in vivo (Fig. 3J to L). This finding is consistent with the notion that AAV efficiently enters a number of cell types, most of which are not permissive for transduction due to postentry blocks. The block to AAV transduction in APCs was studied in vitro by using FISH to localize the vector genome within the cell. Previous studies in cultured epithelial cell lines indicated that AAV vectors efficiently enter the cell but do not localize to the nucleus and do not replicate to form transcriptionally active replicated or integrated forms (9, 10). Coinfection with an E1- and E4-expressing adenovirus mobilized the AAV genome into intranuclear replication centers which are transcriptionally active. FISH analysis of dendritic cells infected with AAVlacZ demonstrated vector genome in a perinuclear distribution (Fig. 7A) that was not seen in adenovirus-infected cells hybridized with the AAV probe (data not shown). Exposure of dendritic cells with the wild-type adenovirus and AAVlacZ resulted in the formation of replication centers within the nucleus (Fig. 7B). Toxicity of the wild-type adenovirus to the dendritic cells precluded their adoptive transfer. These data indicate that AAV enters APCs in a nonproductive transduction.
FIG. 7.
Localization of AAV in virus-infected dendritic cells. Purified dendritic cells were either infected with AAVlacZ (A) or coinfected with AAVlacZ and wild-type adenovirus (B) for 48 h. The cells were cytospun on slides, and FISH analysis was performed with a biotinylated AAV-specific probe. Magnification is ×168.
DISCUSSION
This study underscores the critical role of antigen presentation, and the context in which it occurs, as defining the nature of the ensuing immune responses to gene therapy. Our data indicate that presentation of Escherichia coli β-galactosidase expressed in the APC is necessary for both CTL and CD4+ T-cell responses following intramuscular injection of vector. Fractionation experiments indicated that transduction of dendritic cells may be a limiting step in the activation of T cells to vector-encoded antigens.
Immune responses to tumor-associated antigens provide a substantially different biological context in which similar questions have been asked. Potential mechanisms of T-cell activation are simplified in that endogenous production of tumor-associated antigens in professional APCs is not possible. The most informative results have emerged from experiments in which bone marrow chimeras that had APCs expressing MHC molecules of a separate haplotype from those on the immunizing tumor cells were created (20). These studies supported the “cross priming” hypothesis in which tumor antigens are taken up by APCs, processed, and presented on the MHC class I molecule. For this to be operative, exogenous antigens must be shunted into the transporter-associated protein (TAP)-dependent pathways for MHC class I processing (19).
Direct intramuscular injection of plasmid based DNA is a well-characterized system for eliciting immune responses to a recombinant gene product (7). This approach is being considered for a number of vaccines for viral diseases. Injection of DNA into muscle results in low-level transduction of muscle fibers and activation of both cellular and humoral immune responses to the transgene product (7). Expression of the transgene is usually transient, although stable gene expression has been reported, in some cases, despite immune activation. Several mechanisms have been considered. The classic bone marrow chimera experiments, developed to study tumor-associated antigens, suggest that cross priming from the transfected muscle fiber to the APC contributes to T-cell activation (6, 13). A recent study noted that cotransfection of the immunizing plasmid with one expressing B7-2 was associated with enhanced T- and B-cell responses, indicating that the muscle fiber may function as an APC under the right conditions (24). Direct transfection of the APC has not been ruled out in any of these studies.
Of potential relevance to the present study is previous work on the nature of antigen presentation to viral infection in animal models. Intratracheal infection of mice with influenza virus was associated with infection of dendritic cells from mediastinal lymph nodes at efficiencies sufficient to activate naive T cells in vitro (16). Analysis of influenza virus-infected APCs from humans demonstrated that dendritic cells are 20-fold more effective than macrophages in activating T cells (2). Similar results have been obtained with vaccinia virus as an antitumor vaccine in murine models. In vivo activation of T cells to the tumor-associated antigen was dependent on the promoter driving its expression in the virus, which correlated with the ability of infected dendritic cells to activate T cells in vitro (3).
The critical role of APC transduction in gene therapy with DNA viral vectors has important implications in the development of safe and effective adenovirus vectors. This hypothesis would predict that the transcriptional unit responsible for expression of the transgene in an adenovirus could have a substantial effect on the nature of the ensuing immune response. Most experiments have used active constitutive promoters, which may express very efficiently in dendritic cells. A blunted immune response may be achieved with vectors that contain more specific promoters, which are not active in APCs. Vector-specific differences in transgene expression within APCs due to dose, route of administration, promoter, etc., could explain some of the variation in immune responses that have characterized in vivo applications (1, 8, 49). A similar outcome could be achieved with adenovirus vectors whose tropism is restricted through modifications in the capsid proteins.
The ability of AAV injected into muscle to evade immune responses is of immediate value to the use of this vector in gene therapy where stable expression is desired. Stable transgene expression has been achieved in murine models with a number of constructs, including those that express factor IX (17) and Apo E (unpublished data), despite the fact that antibodies were generated to these secreted human proteins. We conclude that intramuscular injection of AAV evades destructive immune responses to vector-encoded antigens although a humoral immune response may develop if the antigen is secreted. The low efficiency of APC transduction by AAV observed in this study is consistent with previous work that identified a postentry block of AAV-mediated gene transfer in most cell types except muscle fibers and neurons (9, 10). The exquisite cell specific restriction of AAV transduction appears to have created a biological niche for gene therapy in which muscle fibers are efficiently transduced without activating APCs.
ACKNOWLEDGMENTS
The first two authors contributed equally to this work.
The technical support of Qin Su and the Cell Morphology and Vector Cores of the Institute for Human Gene Therapy was appreciated. The following antibodies were kindly provided by Ellen Pure: B220+, 33D1, and CD11C.
Support was derived from the National Institutes of Health (P30 DK47757 and P01 AR/NS43648) and Genovo, Inc. Karin Jooss is supported by the Human Frontier Science Program Organization, Strasbourg, France.
REFERENCES
- 1.Armentano D, Zabner J, Sacks C, Sookedo C S, Smith M P, St. George J A, Wadsworth S C, Smith A E, Gregory R J. Effect of the E4 region on the persistence of transgene expression from adenovirus vectors. J Virol. 1997;71:2408–2416. doi: 10.1128/jvi.71.3.2408-2416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhardwaj N, Bender A, Gonzalez N, Bui L K, Garrett M C, Steinman R M. Influenza virus-infected dendritic cells stimulate strong proliferative and cytolytic responses from human CD8+ T cells. J Clin Invest. 1994;94:797–807. doi: 10.1172/JCI117399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bronte V, Carroll M W, Goletz T J, Wang M, Overwijk W W, Marincola F, Rosenberg S A, Moss B, Restifo N P. Antigen expression by dendritic cells correlates with the therapeutic effectiveness of a model recombinant poxvirus tumor vaccine. Proc Natl Acad Sci USA. 1997;94:3183–3188. doi: 10.1073/pnas.94.7.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crystal R. Transfer of genes to humans: early lessons and obstacles to success. Science. 1995;270:404–410. doi: 10.1126/science.270.5235.404. [DOI] [PubMed] [Google Scholar]
- 5.Descamps V, Duffour M T, Mathieu M C, Fernandez N, Cordier L, Abina M A, Kremer E, Perricaudet M, Haddada H. Strategies for cancer gene therapy using adenoviral vectors. J Mol Med. 1996;74:183–189. doi: 10.1007/BF00204748. [DOI] [PubMed] [Google Scholar]
- 6.Doe B, Selby M, Barnett S, Baenziger J, Walker C M. Induction of cytotoxic T lymphocytes by intramuscular immunization with plasmid DNA is facilitated by bone marrow-derived cells. Proc Natl Acad Sci USA. 1996;93:8578–8583. doi: 10.1073/pnas.93.16.8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ertl H C, Xiang Z. Novel vaccine approaches. J Immunol. 1996;156:3579–3582. [PubMed] [Google Scholar]
- 8.Fang B, Wang H, Gordon G, Bellinger D A, Read M S, Brinkhous K M, Woo S L C, Eisensmith R C. Lack of persistence of E1-recombinant adenoviral vectors containing a temperature-sensitive E2a mutation in immunocompetent mice and hemophilia B dogs. Gene Ther. 1996;3:217–222. [PubMed] [Google Scholar]
- 9.Ferrari F K, Samulski T, Shenk T, Samulski R J. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher K J, Gao G-P, Weitzman M D, DeMatteo R, Burda J F, Wilson J M. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher K J, Jooss K, Alston J, Yang Y, Haecker S E, High K, Pathak R, Raper S E, Wilson J M. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 12.Flotte T R, Afione S A, Conrad C, McGrath S A, Solow R, Oka H, Zeitlin P L, Guggino W B, Carter B J. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc Natl Acad Sci USA. 1993;90:10613–10617. doi: 10.1073/pnas.90.22.10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu T-M, Ulmer J B, Caulfield M J, Deck R R, Friedman A, Wang S, Liu X, Donnelly J J, Liu M A. Priming of cytotoxic T lymphocytes by DNA vaccines: requirement for professional antigen presenting cells and evidence for antigen transfer from myocytes. Mol Med. 1997;3:362–371. [PMC free article] [PubMed] [Google Scholar]
- 14.Gao G-P, Yang Y, Wilson J M. Biology of adenovirus vectors with E1 and E4 deletions for liver-directed gene therapy. J Virol. 1996;70:8934–8943. doi: 10.1128/jvi.70.12.8934-8943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goebels N, Michaelis D, Wekerly H, Hohlfeld R. Human myoblasts as antigen-presenting cells. J Immunol. 1992;149:661–667. [PubMed] [Google Scholar]
- 16.Hamilton-Easton A M, Eichelberger M. Virus-specific antigen presentation by different subsets of cells from lung and mediastinal lymph node tissues of influenza virus-infected mice. J Virol. 1995;69:6359–6366. doi: 10.1128/jvi.69.10.6359-6366.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herzog R W, Hagstrom J N, Kung S-H, Tai S J, Wilson J M, Fisher K J, High K A. Stable gene transfer and expression of human factor IX following intramuscular injection of recombinant AAV. Proc Natl Acad Sci USA. 1997;94:5804–5809. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hohlfeld R, Engel A G. Induction of HLA-DR expression on human myoblasts with interferon-γ. Am J Pathol. 1990;136:503–508. [PMC free article] [PubMed] [Google Scholar]
- 19.Huang A Y C, Bruce A T, Pardoll D M, Levitsky H Z. In vivo cross-priming of MHC class I-restricted antigens requires the TAP transporter. Immunity. 1996;4:349–355. doi: 10.1016/s1074-7613(00)80248-4. [DOI] [PubMed] [Google Scholar]
- 20.Huang A Y C, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 21.Kaplitt M G, Leone P, Samulski R J, Xiao X, Pfaff D W, O’Malley K L, During M J. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994;8:148–153. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- 22.Kay M A, Holterman A-X, Meuse L, Gown A, Ochs H D, Linsley P S, Wilson C B. Long-term hepatic adenovirus-mediated gene expression in mice following CTLA4Ig administration. Nat Genet. 1995;11:191–197. doi: 10.1038/ng1095-191. [DOI] [PubMed] [Google Scholar]
- 23.Kessler P D, Podsakoff G, Chen X, McQuiston S A, Colosi P C, Matelis L A, Kurtzman G J, Byrne B J. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc Natl Acad Sci USA. 1996;93:14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim J J, Bagarazzi M L, Trivedi N, Hu Y, Kazahaya K, Wilson D M, Ciccarelli R, Chattergoon M A, Dang K, Mahalingam S, Chalian A A, Agadjanyan M G, Boyer J D, Wang B, Weiner D B. Engineering of in vivo immune responses to DNA immunization via co-delivery of costimulatory molecule genes. Nat Biotechnol. 1997;15:641–646. doi: 10.1038/nbt0797-641. [DOI] [PubMed] [Google Scholar]
- 25.Kohn D B, Sarver N. Gene therapy for HIV-1 infection. Adv Exp Med Biol. 1996;394:421–428. doi: 10.1007/978-1-4757-9209-6_39. [DOI] [PubMed] [Google Scholar]
- 26.Kotin R M. Prospects for the use of adeno-associated virus as a vector for human gene therapy. Hum Gene Ther. 1994;5:793–801. doi: 10.1089/hum.1994.5.7-793. [DOI] [PubMed] [Google Scholar]
- 27.Kozarsky K F, Jooss K, Donahee M, Strauss J F, Wilson J M. Effective treatment of familial hypercholesterolaemia in the mouse model using adenovirus-mediated transfer of the VLDL receptor gene. Nat Genet. 1996;13:54–61. doi: 10.1038/ng0596-54. [DOI] [PubMed] [Google Scholar]
- 28.Lei D, Lehmann M, Shellito J E, Nelson S, Siegling A, Volk H-D, Kolls J K. Nondepleting anti-CD4 antibody treatment prolongs lung-directed E1-deleted adenovirus-mediated gene expression in rats. Hum Gene Ther. 1996;7:2273–2279. doi: 10.1089/hum.1996.7.18-2273. [DOI] [PubMed] [Google Scholar]
- 29.Lew D, Parker S E, Latimer T, Abai A M, Kuwahara-Rundell A, Doh S G, Yang Z-Y, Laface D, Gromkowski S H, Nabel G J, Manthorpe M, Norman J. Cancer gene therapy using plasmid DNA: pharmacokinetic study of DNA following injection in mice. Hum Gene Ther. 1995;6:553–564. doi: 10.1089/hum.1995.6.5-553. [DOI] [PubMed] [Google Scholar]
- 30.Mantegazza R, Hughes S M, Mitchell D, Travis M, Blau H M, Steinman L. Modulation of MHC class II antigen expression in human myoblasts after treatment with IFN-γ. Neurology. 1991;41:1128–1132. doi: 10.1212/wnl.41.7.1128. [DOI] [PubMed] [Google Scholar]
- 31.Miller J L, Donahue R E, Sellers S E, Samulski R J, Young N S, Nienhuis A W. Recombinant adeno-associated virus (rAAV)-mediated expression of a human γ-globin gene in human progenitor-derived erythroid cells. Proc Natl Acad Sci USA. 1994;91:10183–10187. doi: 10.1073/pnas.91.21.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mullen C A, Blaese R M. Gene therapy of cancer. Cancer Chemother Biol Response Modifiers. 1996;16:285–294. [PubMed] [Google Scholar]
- 33.Nabel G J, Yang Z Y, Nabel E G, Bishop K, Marquet M, Felgner M, Gordon D, Chang A E. Direct gene transfer for treatment of human cancer. Ann NY Acad Sci. 1995;772:227–231. doi: 10.1111/j.1749-6632.1995.tb44748.x. [DOI] [PubMed] [Google Scholar]
- 34.Parker S E, Vahlsing H L, Serfilippi L M, Franklin C L, Doh S G, Gromkowski S H, Lew D, Manthorpe M, Norman J. Cancer gene therapy using plasmid DNA: safety evaluation in rodents and non-human primates. Hum Gene Ther. 1995;6:575–590. doi: 10.1089/hum.1995.6.5-575. [DOI] [PubMed] [Google Scholar]
- 35.Plautz G E, Yang Z-Y, Wu B-Y, Gao X, Huang L, Nabel G J. Immunotherapy of malignancy by in vivo gene transfer into tumors. Proc Natl Acad Sci USA. 1993;90:4645–4649. doi: 10.1073/pnas.90.10.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Podsakoff G, Wong K K, Chatterjee S. Efficient gene transfer into nondividing cells by adeno-associated virus-based vectors. J Virol. 1994;68:5656–5666. doi: 10.1128/jvi.68.9.5656-5666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poeschla E M, Wong-Staal F. Gene therapy and HIV disease. In: Volberg P, Jacobson M A, editors. AIDS clinical review, 1995/1996. New York, N.Y: Marcel Dekker, Inc.; 1996. pp. 1–45. [PubMed] [Google Scholar]
- 38.Roy R, Danserau G, Tremblay J B, Belles-Isles M, Huard J, Labrecque C, Bouchard J P. Expression of major histocompatibility complex antigens on human myoblasts. Transplant Proc. 1991;23:799–801. [PubMed] [Google Scholar]
- 39.Tripathy S K, Black H B, Goldwasser E, Leiden J M. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 40.Ulmer J B, Donnelley J J, Parker S E, Rhodes G H, Felgner P L, Dwarki V J, Gromkwoski S J, Deck R R, DeWitt C M, Friedman A, Hawe L A, Leander K R, Martinez D, Perry H C, Shiver J W, Montgomery D L, Liu M A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 41.VanGinkel F W, Liu C, Simecka J W, Dong J-Y, Greenway T, Frizzell R A, Kiyono H, McGhee J R, Pascual D W. Intratracheal gene delivery with adenoviral vector induces elevated systemic IgG and mucosal IgA antibodies to adenovirus and β-galactosidase. Hum Gene Ther. 1995;6:895–903. doi: 10.1089/hum.1995.6.7-895. [DOI] [PubMed] [Google Scholar]
- 42.Walsh C E, Neinhuis A W, Samulski R J, Brown M G, Miller J L, Young N S, Liu J M. Phenotypic correction of fanconi anemia in human hematopoietic cells with a recombinant adeno-associated virus vector. J Clin Invest. 1994;94:1440–1448. doi: 10.1172/JCI117481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whartenby K A, Abbound C N, Marrogi A J, Ramesh R, Freeman S M. The biology of cancer gene therapy. Lab Invest. 1995;72:131–145. [PubMed] [Google Scholar]
- 44.Wilson J M. Gene therapy for cystic fibrosis: challenges and future directions. J Clin Invest. 1995;96:2574–2554. doi: 10.1172/JCI118318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao X, Li J, Samulski R J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Y, Ertl H C J, Wilson J M. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 47.Yang Y, Xiang Z, Ertl H C J, Wilson J M. Upregulation of class I major histocompatibility complex antigens by interferon γ is necessary for T-cell-mediated elimination of recombinant adenoviruses-infected hepatocytes in vivo. Proc Natl Acad Sci USA. 1995;92:7257–7261. doi: 10.1073/pnas.92.16.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Y, Li Q, Ertl H C J, Wilson J M. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Y, Nunes F A, Berencsi K, Gonczol E, Engelhardt J F, Wilson J M. Inactivation of E2a in recombinant adenovirus improves the prospect for gene therapy in cystic fibrosis. Nat Genet. 1995;7:362–369. doi: 10.1038/ng0794-362. [DOI] [PubMed] [Google Scholar]
- 50.Yang Y, Trinchieri G, Wilson J M. Recombinant IL-12 prevents formation of blocking IgA antibodies to recombinant adenovirus and allows repeated gene therapy to mouse lung. Nat Med. 1995;1:890–893. doi: 10.1038/nm0995-890. [DOI] [PubMed] [Google Scholar]
- 51.Yang Y, Su Q, Wilson J M. Role of viral antigens in destructive cellular immune responses to adenovirus vector-transduced cells in mouse lungs. J Virol. 1996;70:7209–7212. doi: 10.1128/jvi.70.10.7209-7212.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang Y, Haecker S E, Su Q, Wilson J M. Immunology of gene therapy with adenoviral vectors in mouse skeletal muscle. Hum Mol Gen. 1996;5:1703–1712. doi: 10.1093/hmg/5.11.1703. [DOI] [PubMed] [Google Scholar]
- 53.Yang Y, Jooss K, Su Q, Ertl H C J, Wilson J M. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 1996;3:137–144. [PubMed] [Google Scholar]
- 54.Zsengeller Z K, Wert S E, Hull W M, Hu X, Yei S, Trapnell B C, Whitsett J A. Persistence of replication-deficient adenovirus-mediated gene transfer in lungs of immune-deficient (nu/nu) mice. Hum Gene Ther. 1995;6:457–467. doi: 10.1089/hum.1995.6.4-457. [DOI] [PubMed] [Google Scholar]