Abstract
Alzheimer disease (AD) is the most common cause of dementia in older individuals. AD is characterized pathologically by amyloid-β (Aβ) plaques and tau neurofibrillary tangles in the brain, with associated loss of synapses and neurons, which eventually results in dementia. Many of the early attempts to develop treatments for AD focused on Aβ, but a lack of efficacy of these treatments in terms of slowing disease progression led to a change of strategy toward targeting of tau pathology. Given that tau shows a stronger correlation with symptom severity than does Aβ, targeting of tau is more likely to be efficacious once cognitive decline begins. Anti-tau therapies initially focused on post-translational modifications, inhibition of tau aggregation and stabilization of microtubules. However, trials of many potential drugs were discontinued because of toxicity and/or lack of efficacy. Currently, the majority of tau-targeting agents in clinical trials are immunotherapies. In this Review, we provide an update on the results from the initial immunotherapy trials and an overview of new therapeutic candidates that are in clinical development, as well as considering future directions for tau-targeting therapies.
Introduction
Since the publication of our previous review on tau-targeting therapies in 20181, the number of people in the USA with Alzheimer disease (AD) has increased from an estimated 5.4 million to 6.5 million, making it a major health issue2. Worldwide, around 57 million people are thought to have dementia, with AD probably contributing to 60–70% of these cases3. In addition to the direct impact on patients, AD and related illnesses cost hundreds of billions of dollars to care-givers and the health-care system2. As the population continues to age, the need for effective therapies will only increase.
Early efforts to find a disease-modifying therapies for AD focused on amyloid-β (Aβ), the main component of the extracellular plaques that accumulate in the brain in this condition. However, both immunotherapies and secretase modifiers have been largely ineffective or detrimental4,5. The main exceptions are lecanemab6,7 and donanemab8,9, both of which produced modest but significant slowing of cognitive decline in phase III trials. The limited success of Aβ-targeting therapies led to a change in focus towards the tau protein — the main component of the neurofibrillary tangles (NFTs) that comprise the other major pathological hallmark of AD. This decision was supported by the fact that tau pathology correlates better with the degree of dementia than does Aβ deposition10–17.
The presence of tau pathology in many conditions other than AD, including the primary tauopathies progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), Pick disease, frontotemporal dementia (FTD), and primary age-related tauopathy18,19, makes it an appealing target for therapeutic development. The progression of tau lesions is thought to involve both loss and gain of function for the protein, offering multiple points for intervention. In this Review, we briefly discuss these aspects of tau pathology, highlighting data published since our previous Review1. We summarize the latest results from ongoing and completed clinical trials and provide information on trials that have recently been initiated. In addition, we discuss strategies for improving tau-targeting therapies, in particular immunotherapies, and future directions for the field.
Targetable aspects of tau pathology
Numerous aspects of tau pathology could be targeted in AD (Fig. 1). Phosphorylated tau (p-tau) pre-tangles and neuropil threads can be seen in brain tissue decades before the symptoms of AD manifest20. The pathology commonly begins in the entorhinal cortex and hippocampus and spreads in a stereotypical pattern; however, several atypical variants of AD exist, accounting for up to 45% of all cases21,22. Monomeric, oligomeric and aggregated tau species are observed in all tauopathies, although AD and the various primary tauopathies differ with regard to tau isoform composition and multimer morphology18,19,23,24.
Figure 1 |. Tau-related therapeutic targets.
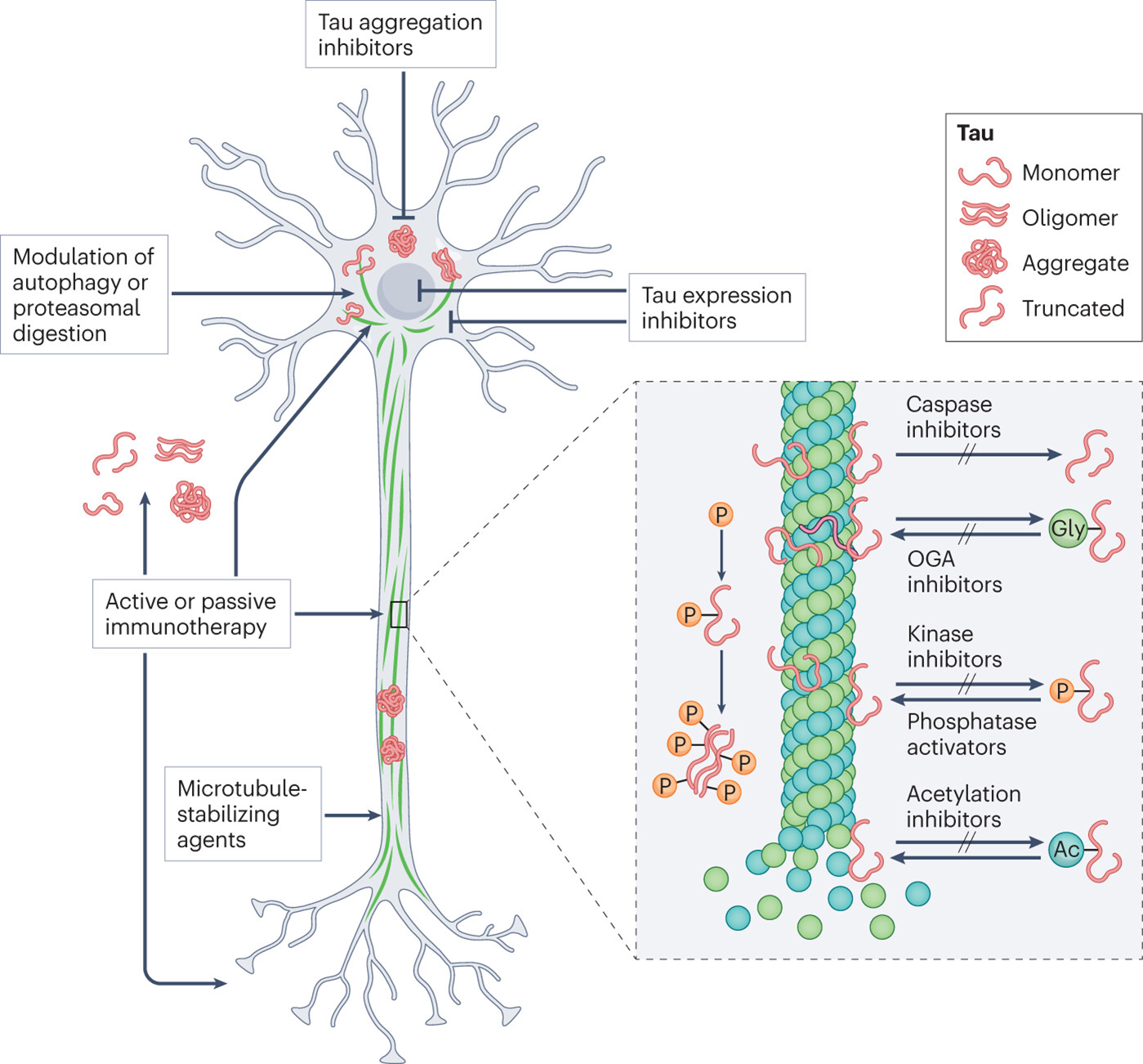
The figure shows the various tau-targeting approaches that are in preclinical or clinical development for the treatment of Alzheimer disease and primary tauopathies. Antisense oligonucleotides can be used to reduce tau expression. Inhibitors of tau aggregation include curcumin and the methylene blue derivative LMTX. Microtubule stabilizers such as TPI-287 and NAP can be used to compensate for loss of the normal microtubule-stabilizing function of tau. Clearance of pathological tau can be enhanced using modulators of autophagy or proteasomal degradation. Active and passive immunotherapies use antibodies to target pathological tau intracellularly or extracellularly and promote its degradation and clearance. Pathological tau is characterized by extensive post-translational modifications, including hyperphosphorylation, acetylation, truncation. Glycosylation can be protective or detrimental. The inset shows various inhibitors that target the enzymes involved in these modifications. Ac, acetyl group, Gly, glycosyl group; OGA, O-GlcNAcase; P, phosphate. Adapted from ref.1
Post-translational modifications
The pathological tau in AD is characterized in part by extensive post-translational modifications (Fig. 1). Here, we focus on the modifications that have been the subject of clinical development, namely, hyperphosphorylation, acetylation, truncation and glycosylation.
Hyperphosphorylation.
The tau hyperphosphorylation that is seen in AD results from increased activity of tau kinases25–30, combined with reduced activity of protein phosphatase 2A (PP2A)31,32. Tau kinases have been shown to be activated directly or indirectly by Aβ, and Aβ can induce tau phosphorylation and aggregation in vivo33–44. The outcome is increased occupancy at multiple phosphorylation sites, the prevelance of phosphorylation at specific sites and overall extent of phosphorylation changes with disease stage20,45–53. Patterns of phosphorylation also differ between tauopathies, and familial mutations in the tau-encoding gene MAPT can promote phosphorylation54–56. The consequences of increased phosphorylation include mislocalization of tau to the somatogenic compartment, decreased microtubule binding and promotion of tau misfolding57.
Acetylation.
Although not seen as consistently as hyperphosphorylation, enhanced tau acetylation in AD and other tauopathies can impair microtubule binding, decrease solubility, promote cleavage and impair degradation of the protein58–60. Salsalate and diflunisal reduce tau acetylation through inhibition of p300 acetyltransferase61,62, and were initially identified owing to their association with decreased incidence of AD in patients62–65.
Truncation.
The distribution of cleaved tau fragments is complex, with some species appearing in both AD and healthy individuals, some only in AD and other tauopathies others only in non-AD tauopathies66. Aβ can promote tau truncation through caspase activation but is not required for this process, as truncated tau is also found in non-AD tauopathies. Cleavage promotes tau assembly, reduces microtubule binding, promotes synaptic and organelle dysfunction, and acetylation of tubulin, and might promote tau secretion66,67. Two caspase inhibitors, minocycline and VX-765, have shown positive results in AD models68–71, and minocycline has entered clinical trials in patients with AD (see below).
Glycosylation.
O-GlcNAcylation, a specialized protective type of O-glycosylation, promotes microtubule binding, prevents phosphorylation and reduces aggregation of tau, and is found to be reduced in AD60,72. By contrast, N-glycosylation and non-enzymatic glycosylation (glycation) are increased in AD and other tauopathies. These modifications promote tau phosphorylation and misfolding while impairing microtubule binding and protein digestion60. To date, O-GlcNAcylation is the only glycosylation mechanism to be targeted in clinical trials.
Tau aggregation
Tau multimers include small soluble aggregates, paired helical filaments (PHFs), straight filaments and twisted ribbons. Post-translational modifications and mutations influence the structure of these aggregates, which can be faithfully transmitted during seeding60,73–79.
Oligomeric tau has emerged as the primary pathogenic species, resulting in acute toxicity80–83 as well as impairments in nuclear stability and gene transcription, mitochondrial health, neurotransmission, synaptic function and protein degradation80,81,83–86. Extracellular oligomers can initiate templated seeding of tau following uptake into naive cells80–84. Larger NFT aggregates might initially represent a compensatory protective mechanism, but in the longer term, NFT-bearing neurons exhibit changes in gene expression, as well as synapse loss, inhibition of axonal transport and energy deficits87–90. Multiple groups have developed small-molecule inhibitors with the goal of preventing or reversing tau aggregation and reducing the spread of pathology91–93.
Cytoskeletal dysfunction
Compared with control neurons, NFT-bearing neurons from patients with AD show reduced tubulin expression, microtubule length and overall tubule numbers, as well as increased acetylation of tubulin90,94,95. Tubulin from patients with AD is slower to assemble and has increased GTPase activity compared with that from healthy controls96. In AD, the dynamic removal and restoration of the external tyrosine residues of tubulin are impaired leading, to a build-up of detyrosinated tubulin97,98. Together, these data point towards disruption of the microtubule network, resulting from the loss of tau binding and other pathological processes, as a potential target for therapeutic intervention.
Protein degradation pathway impairment
Defects in macroautophagy (autophagy), endosomal microautophagy and chaperone-mediated autophagy have been observed in AD and other tauopathies99–101. Reduced expression of autophagy and endosomal microautophagy components, reduced chaperone-mediated autophagy activity, impaired lysosomal fusion, decreased lysosomal activity, increased concentrations of ubiquitinated protein and disruption of key signalling pathways are all observed in AD58,99–107.
Once established, tau pathology can also affect its own clearance. Tau can inhibit autophagy induction and autophagosome formation, impair autophagosome–lysosome fusion, and sequester pathway components108–113., and can also prevent endosomal uptake of proteins and increase endosomal leakage114–119. These effects underscore the importance of removing tau aggregates from neurons.
Targeting tau pathology
Since our previous Review on tau-targeting therapies1, several trials have concluded or been initiated. In this section, we discuss strategies that target various aspects of tau pathology [Fig. 1], and in the next section, we focus specifically on immunotherapies, in particular, antibody-based therapies, which have been the subject of most of the clinical trials to date Fig. 2. Our group has worked extensively in the tau immunotherapy field from its infancy so we are ideally placed to provide an expert opinion on this topic. Figure 2 provides a breakdown of the different treatment strategies, and how far each has advanced in clinical testing. Supplementary Tables 1 and 2 list the non-immunotherapy and immunotherapy trials, respectively, and Table 1 lists the potential advantages and disadvantages of each treatment type.
Figure 2 |. Current status of clinical trials of tau-targeting drugs.
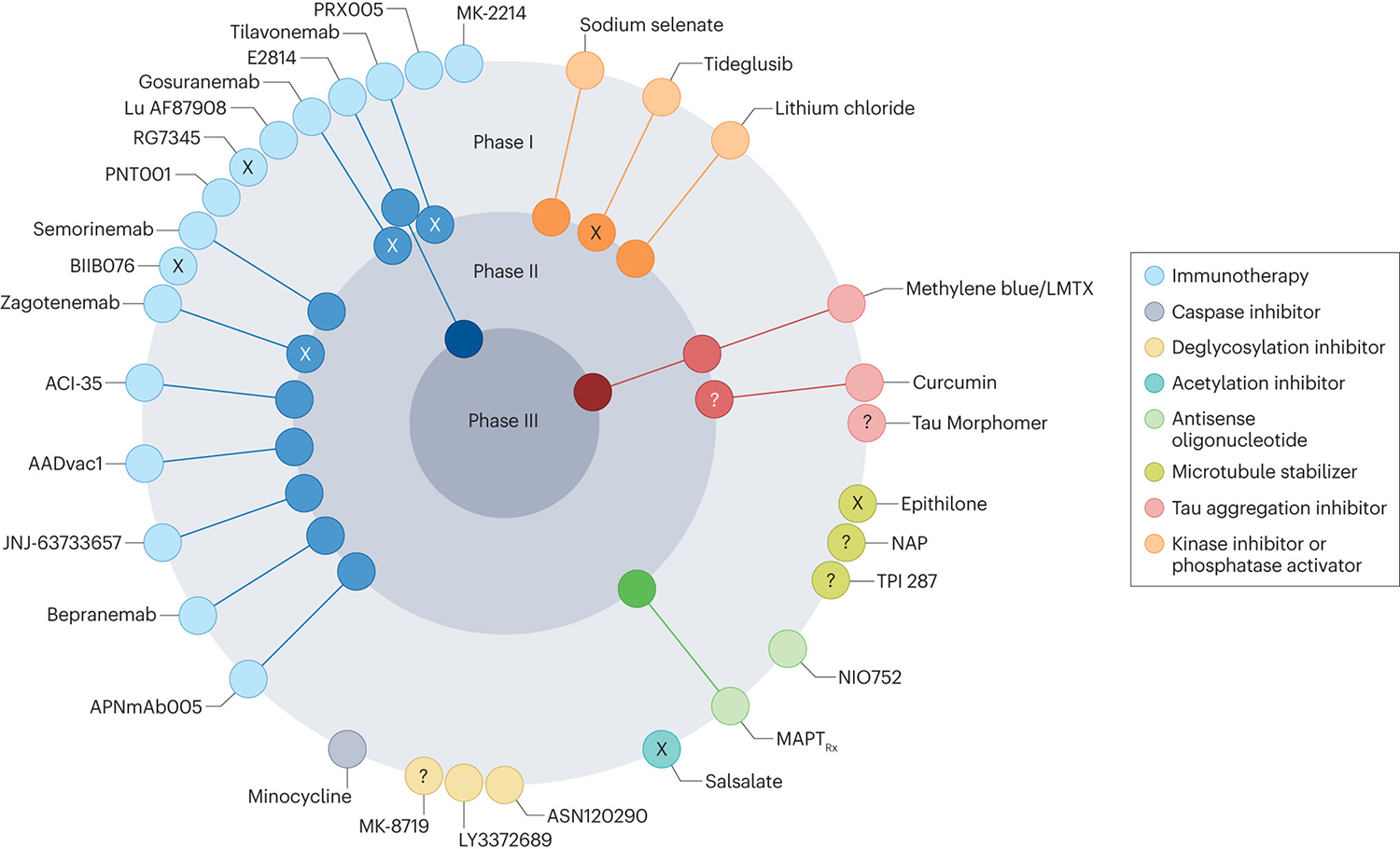
At the time of writing, the most active field is tau immunotherapy, with two active vaccines (AADvac1 and ACI-35) and nine antibodies (APNmAb005, E2814, JNJ-63733657, Lu AF87908, MK-2214, PNT001, PRX005, semorinemab and bepranemab) in ongoing clinical trials. Several of the other compounds in trials have complex or incompletely defined mechanisms of action; in this diagram, these compounds are categorized according to their presumed tau-related mode of action. X indicates trials that, to our knowledge, have been halted or terminated, as detailed in the main text, although their current status is sometimes difficult to determine, ? reflects uncertainty about the current status of trials. Adapted from ref.1.
Table 1|.
Advantages and disadvantages tau-targeting therapies
| Drug type | Advantages | Disadvantages |
|---|---|---|
| Antisense oligonucleotides | Lowering total tau levels might lead to dissociation of aggregates and reduce pathology Specifically targets tau Safe and well tolerated in human testing Reduces CSF tau levels below baseline in humans |
Also reduces levels of non-pathological tau, which might have unforeseen consequences Tau knockdown or knockout produces cognitive and motor deficits in some animal models |
| Phosphatase activators | Dephosphorylation increases tau–microtubule binding and makes tau less prone to aggregation PP2A is responsible for most tau dephosphorylation |
PP2A is also involved in metabolism, gene expression, signal transduction, apoptosis and cell cycle entry, so global inhibition might have off-target effects In mild-to-moderate AD, PP2A inhibition does not reduce AD biomarker levels in CSF |
| Kinase inhibitors | Reduced phosphorylation increases tau–microtubule binding and makes tau less prone to aggregation The kinase inhibitor lithium is already approved for humans and is widely used for other conditions |
The targeted kinases are involved in other signalling pathways; for example, GSK3β affects metabolism, autophagy, DNA repair and apoptosis, so global inhibition might have off-target effects Long-term lithium use has serious adverse effects Low doses of lithium produced no change in GSK3β activity |
| Acetylation inhibitors | Reduced acetylation increases tau–microtubule binding, regulates phosphorylation and promotes tau cleavage, making tau less prone to aggregation The acetylation inhibitor salsalate reduceds acetylated and total tau levels, prevents hippocampal atrophy and improves memory in tauopathy mice, and is safe and well tolerated in humans |
Inhibiting acetylation will affect various molecules with unknown consequences Salsalate failed to improve cognition, prevent reductions in volumetric imaging and reduce AD biomarker levels in CSF in humans Salsalate is not tau-specific |
| Deglycosylation inhibitors |
O-glcNAcylation of tau is protective and prevents phosphorylation and aggregation OGA inhibitors showed good brain penetration and safety in clinical trials |
OGA inhibitors does not just target tau; they are involved in metabolic and signalling functions The biological relevance of O-glcNAcylation to other proteins is unknown |
| Caspase inhibitors | Blocking tau truncation promotes its binding to microtubules, thereby inhibiting tau aggregation and toxicity and resulting in improved function of synapses and organelles | The targeted caspases do not just cleave tau; for example, caspase 1 is involved in cytokine activation and apoptosis and caspase 3 affects tissue regeneration and differentiation as well as apoptosis The caspase inhibitor minocycline produces adverse effects, particularly with long-term treatment |
| Tau aggregation inhibitors | Overall, reduced tau aggregation diminishes tau toxicity Small molecules might be easier and cheaper to synthesize than antibodies Medicinal chemistry can be used to modify tau binding and brain uptake |
Small-molecule tau aggregation inhibitors with efficacy in culture often show toxicity and/or lack of blood–brain barrier permeation in vivo These compounds typically bind to β-sheets that are found in various proteins, including normal ones To some extent, tau aggregation might be a defence mechanism to prevent toxicity of smaller aggregates such as oligomers |
| Microtubule stabilizers | Stabilizing microtubules improves axonal transport and supports the maintenance of neuronal processes and dendritic spines | Microtubule stabilizers have primarily been used for cancer treatment and have a narrow therapeutic window and substantial toxicity The microtubule stabilizer TPI-287 was poorly tolerated in human testing and was shown to worsen dementia symptoms; its brain penetrance could not be confirmed |
| Active immunotherapy | Specifically targets tau Active immunotherapy is more cost-effective and longer lasting than passive immunotherapy Inducing a polyclonal response in patients might further improve efficacy |
Possible adverse immune responses, which could be irreversible Antibodies generated might not target optimal epitopes |
| Passive immunotherapy | Specifically targets tau Antibodies can be designed to target specific pathological epitopes, and treatment can potentially be tailored to disease stage Antibodies or antibody fragments can be further modified to enhance clearance Adverse effects are likely to be reversible because the antibodies will be cleared if treatment is stopped |
Possible adverse immune response Choice of epitope is important, as not all epitopes are present at any given time Optimal efficacy requires both extracellular and intracellular clearance Humanization might change antibody properties |
AD, Alzheimer disease; CSF, cerebrospinal fluid; GSK3β, glycogen synthase kinase 3β; OGA, O-GlcNAcase;PP2A, protein phosphatase 2A.
Reducing tau expression
Tau antisense oligonucleotides (ASOs) target human MAPT mRNA to reduce the expression of tau120. In 2017, a phase Ib trial (NCT03186989) was initiated to study the safety, tolerability, pharmacokinetics and pharmacodynamics of the tau ASO MAPTRx (also known as BIIB080) in patients with mild AD. At a 2021 press conference, the drug was reported to be safe and to reduce total tau (t-tau) and p-tau levels in the cerebrospinal fluid (CSF) in a dose-dependent manner121. Additional data from phase I testing, presented in 2023, showed dose-dependent decreases in t-tau and p-tau in the CSF122. PET scans from participants who received a high dose of the drug showed a decrease in tau levels to below baseline values in all brain regions analysed122. Adverse events were predominately mild to moderate. In addition, reductions in tau phosphorylated at residue 181 (p-tau181), the inflammatory marker YKL40 and the ratio of t-tau to Aβ42 in the CSF were reported123. Despite the decrease in CSF measures, however, no significant improvements in cognitive, functional, psychiatric or neurological impairments were observed123. Phase II testing has been initiated in patients with mild cognitive impairment (MCI) due to AD or with mild AD (NCT05399888), with cognitive changes as the primary outcome, and will run through to December 2026.
NIO752 is another tau ASO that is currently in two trials to examine safety, tolerability, and pharmacokinetics in people with PSP (NCT04539041), MCI or early AD (NCT05469360). The results are anticipated in 2023 and 2024, respectively.
Targeting tau protein modifications
Phosphatase modifiers.
As we outlined in our previous Review, memantine has various mechanisms of action, including enhancement of PP2A activity1,124. No updates on clinical trials of memantine in AD or other tauopathies have been provided since our earlier article.
Sodium selenate has been shown to reduce tau phosphorylation in animal models125–127, but in a clinical trial in people with mild-to-moderate AD, only modest benefits were detected on diffusion MRI128. A phase Ib open-label study (ACTRN12617001218381) in 12 individuals with behavioural variant FTD was completed in 2021129. Small declines in MRI, cognitive and behavioural measures were observed, with no changes in t-tau, p-tau or the neurodegeneration biomarker neurofilament light chain (NfL) in the CSF. Two phase IIb trials are examining the safety, tolerability and efficacy of sodium selenate in FTD (ANZCTR12620000236998) and PSP (ACTRN12620001254987)130,131. Outcome measures will include tau levels in CSF, serum and plasma; tau PET; MRI for brain atrophy and midbrain mean diffusivity; and cognitive and functional measures. These studies are expected to be completed in 2025.
Kinase inhibitors.
Lithium chloride is widely used to treat bipolar disorder and has also been shown to inhibit glycogen synthase kinase 3β (GSK3β) — an enzyme that phosphorylates tau132,133. In a pilot study in patients with MCI (NCT02601859), no adverse reactions to lithium chloride treatment were reported, although GSK3β activity was not significantly changed, suggesting that the dose was too low to be effective 134. A phase II trial (NCT02862210) has been extended to 2023 to assess the effects of this drug on behavioural symptoms of FTD. Additional outcomes include changes in motor symptoms, adverse events and serum biomarkers. The results of this trial have yet to be reported.
Acetylation inhibitors.
Salsalate is a small-molecule non-steroidal anti-inflammatory drug that has been shown to inhibit tau acetylation 62. In a transgenic mouse model of tauopathy, this drug reduced levels of t-tau and acetylated tau, prevented hippocampal atrophy and reduced memory deficits 61. A phase I open-label study (NCT02422485) evaluated safety, tolerability and CSF biomarkers in patients with PSP who were treated with salsalate135. The drug was well tolerated but failed to elicit any significant improvements. A second phase I trial in patients with AD (NCT03277573) is assessing adverse effects, changes in CSF biomarkers, and imaging and cognitive measures. Although the estimated completion date was December 2021, the results have yet to be reported.
Deglycosylation inhibitors.
O-GlcNAcylation of tau produces protective effects in tauopathies by preventing tau phosphorylation and aggregation136,137. ASN120290 is an O-GlcNAcase (OGA) inhibitor that was determined to be safe and well-tolerated in a phase I trial conducted in healthy adults138,139. Drug concentrations were comparable in the CSF and plasma, indicating that the compound readily enters the brain. In 2018, ASN120290 was given an orphan drug designation for the treatment of PSP. ASN-51 is a second-generation, longer-lasting version of ASN120290 that was assessed in a phase I trial (NCT04759365) aimed at evaluating its safety, tolerability, pharmacokinetics and pharmacodynamics in healthy individuals. The trial was terminated in August 2022 citing various logistic reasons, and the findings from enrolled patients have yet to be reported.
Through the use of PET radioligands, the OGA inhibitor LY3372689 was shown to efficiently penetrate the brain after a single dose in rats, and this research is now being extended to healthy volunteers with LY3372689 showing brain penetration and occupancy140,141. Additional studies of both single (NCT03819270) and multiple (NCT04106206) ascending doses in healthy volunteers have shown that LY3372689 is safe142–144. An ongoing phase II trial, which includes cognitive assessments and imaging, is determining the efficacy, safety and tolerability of this drug in patients with early AD (NCT05063539) will be completed in 2024.
Another OGA inhibitor, MK-8719 was discussed our previous Review1, but no further updates are available on this drug at present.
Caspase inhibitors.
A multicentre phase II study of two different doses of the caspase inhibitor minocycline (ISRCTN16105064) was conducted in patients over 50 years of age with mild AD145. The treatment failed to slow cognitive decline, and the higher dose was associated with increased adverse effects; and treatment was discontinued.
Tau aggregation inhibitors
Curcumin reduces tauopathy in animal models and prevents tau aggregation in vitro146–148. A phase II clinical trial (NCT01383161) examined the effects of curcumin treatment in patients with MCI and healthy adults149. Improvements in long-term memory, visual memory and attention were noted in the individuals who received the drug. Furthermore, significant associations were observed between improved cognition and decreases in PET ligand binding to pathological tau and Aβ. A second phase II study (NCT01811381) is examining the effects of curcumin, alone or with yoga, in people with MCI or subjective cognitive impairment. The endpoints include blood-based biomarkers, changes on PET imaging and adverse events. The study was scheduled to be completed in 2020 but the results have yet to be released.
LMTX (also known as TRx0237) is a derivative of methylene blue that crosses the blood–brain barrier (BBB), and In animal models, reduced tau aggregation and improved cognition150,151. Several phase III trials of this drug have been initiated in people with mild-to-moderate AD, along with an open-label extension study for individuals who completed the earlier trials152–155. Although none of the trials produced positive results, a revised report was released that purported to show efficacy153,155,156. However, this report used statistical analysis based on a small subset of the total sample that lacked proper control groups154,155. The open-label extension study (NCT02245568) was terminated early.
A phase III trial of LMTX in patients with AD (NCT03446001) was completed in April 2023 and will be followed by a 1-year open-label extension study. The primary outcomes are adverse events and changes in cognition and daily functioning. Additional outcomes include brain atrophy and findings on 18F-fluorodeoxyglucose-PET scans. The results have yet to be reported.
Tau Morphomer (also known as ACI3024) is a small-molecule inhibitor that selectively targets tau aggregates. A phase I trial (ISRCTN18150742) to examine safety and tolerability in healthy adults was completed in 2020. The drug was found to enter the brain and its levels in the CSF increased in a dose-dependent manner157. No further data or future clinical trial plans have been reported.
Microtubule stabilizers
TPI-287 (also known as abeotaxane) is a microtubule stabilizer that has been shown to be safe and effective in cancer trials158. Two phase I trials, one in people with in AD (NCT01966666) and the other in people with CBD or PSP (NCT02133846), were combined to examine the safety, tolerability, pharmacokinetics and pharmacodynamics of the drug159. The AD group had a lower maximum tolerated dose than the CBD and PSP groups. In the AD group, the treatment was associated with reduced cognitive decline compared with placebo, however the authors attributed this to the greater than expected cognitive decline in the placebo group. Although the patients with CBD or PSP patients tolerated a higher dose, the treated individuals showed increased falls and worsening dementia symptoms. Brain penetrance of TPI-287 could not be confirmed as it was not detected in the CSF 1 week after the final infusion. No trials of this drug are currently in progress.
NAP (also known as davunetide) is a neuroprotective peptide that has been shown to reduce tau and Aβ burden and improve cognition in animals160. A phase II trial (NCT01056965), examining its safety, tolerability, cognition, imaging and CSF biomarkers in various tauopathies, including PSP, FTD, CBD and non-fluent aphasia, was reported to be completed in 2012, but the results have yet to be released.
Tau immunotherapies
The first successful reports of vaccine and antibody therapies targeting tau were made by our group161–164. Subsequently, numerous papers have reported effective targeting of multiple tau epitopes, including the amino-terminus, the mid-domain, the microtubule-binding region, misfolded tau, p-tau202, p-tau231, p-tau396/404, p-tau409, p-tau413, p-tau422 and oligomeric tau species57,165–172.
Immunotherapy can be active or passive and can function through extracellular or intracellular mechanisms (Fig. 3). Active immunotherapy delivers a tau immunogen as a vaccine, and the advantages of this approach are its low cost, promotion of a polyclonal antibody response and lasting efficacy. However, because tau is an endogenous protein, the potential exists for adverse and potentially irreversible autoimmune responses. Detrimental effects were seen in early mouse studies that used full-length recombinant tau with strong adjuvants that would never be approved for human use173,174. Multiple immunizations with the p-tau396/404 immunogen in alum adjuvant led to increased mortality in 3×Tg but not JNPL3 mice162,175. With fewer immunizations, 3×Tg mice showed a strong sustained antibody response with clearance of Aβ and tau and the animals remained healthy. No safety issues have been reported in other preclinical studies or ongoing clinical trials of active tau immunization.
Figure 3 |. Proposed modes of action of anti-tau antibodies.
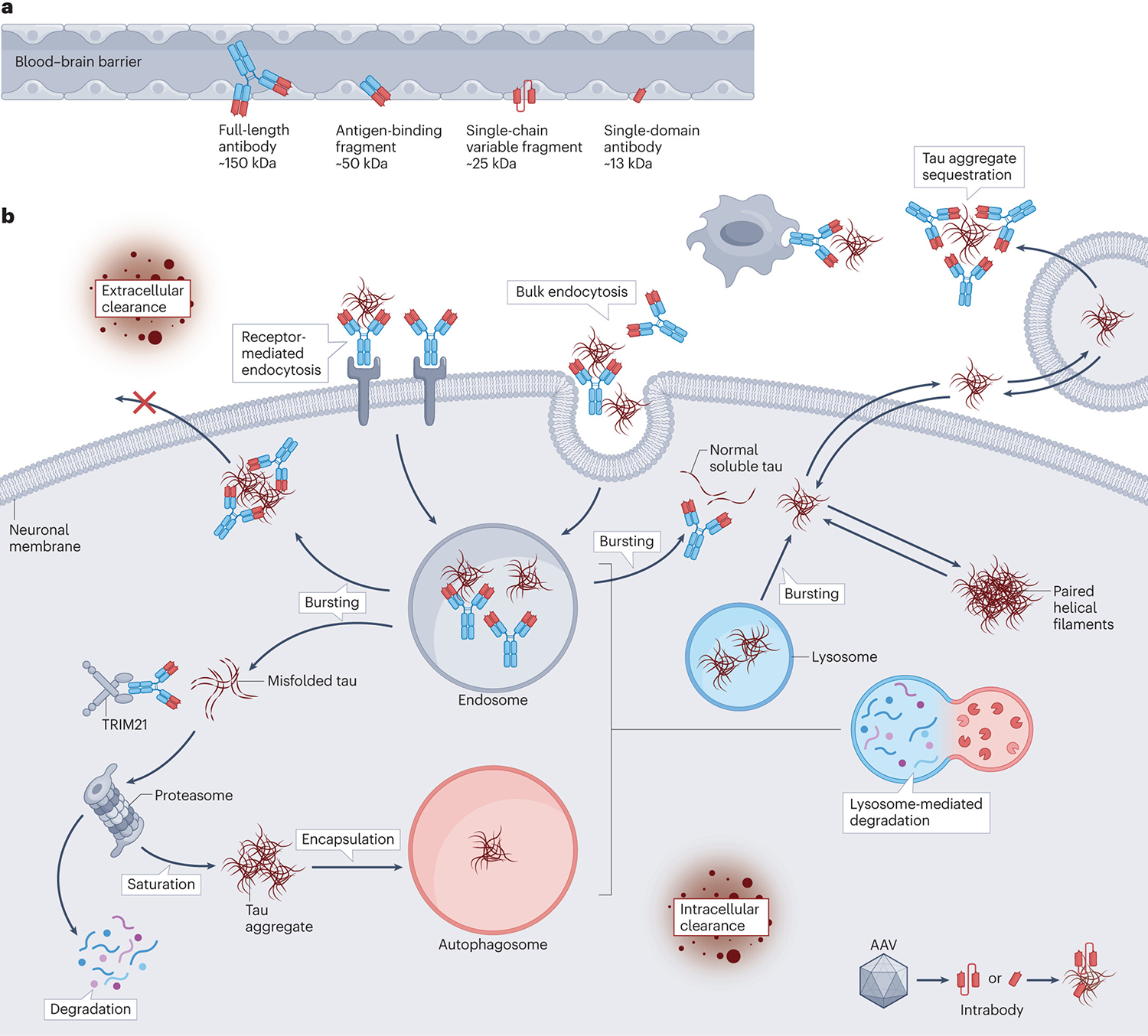
a | Tau antibodies can consist of whole IgGs (~150 kDa) antibody fragments such as antigen-binding fragments (50 kDa), single-chain variable fragments (~25 kDa), and single-domain antibodies (~13 kDa). b | Antibodies might target tau intracellularly or extracellularly, and should ideally act in both compartments to maximize efficacy. Extracellularly, antibodies could sequester tau aggregates, prevent tau aggregation, promote microglial phagocytosis of tau–antibody complexes and/or facilitate removal of tau to the periphery. These mechanisms would all reduce the spread of tau between neurons and subsequent pathological seeding. Antibodies, with or without tau, can also be internalized by neurons through either receptor-mediated or bulk endocytosis. Inside the neuron, these antibodies can bind to tau aggregates within the endosomal–lysosomal system. There, they can promote disassembly of tau aggregates, allowing greater access for lysosomal enzymes. Formation of tau–antibody complexes within the endosomal–lysosomal system might also prevent tau from disrupting endosomal membranes and escaping back into the cytosol, thereby aiding complete tau degradation. Some antibodies might also enter the cytosol, where they can sequester misfolded tau or promote proteasomal clearance through tripartite motif containing 21 (TRIM21) binding, thereby enhancing clearance and preventing tau secretion. Antibodies bound to larger tau aggregates could be cleared via the autophagosome. Antibody fragments, either administered or encoded by adeno-associated virus (AAV) vectors, also have therapeutic potential. Astrocytic tau pathology can presumably be targeted using the same mechanisms of action, although experimental confirmation is required.
The main advantage of passive immunotherapy is flexibility. Premade antibodies can target specific epitopes, which could allow treatment to be tailored to the disease stage. The antibodies can also be optimized, for example, by changing the IgG subclass or through modifications to improve uptake and/or alter tau binding. Passive immunotherapy is also relatively reversible as any adverse effects should subside following antibody clearance, with minimal to no T cell activation. Disadvantages include the high cost and the need for chronic administration, which increases the likelihood of an anti-idiotypic response and associated adverse effects. Monoclonal targeting might also be less effective than the active polyclonal response. Antibodies could prevent or clear tau pathology through several mechanisms, as reviewed extensively elsewhere167. In brief, antibodies can work extracellularly to sequester pathological tau or promote its clearance via the periphery or by microglia. Intracellularly, tau antibodies could promote disaggregation of tau polymers and their degradation through cellular clearance pathways.
In preclinical testing, pretreatment of pathological tau with antibodies or addition of an antibody and tau to cultures simultaneously dampened seeding of pathological tau176–186, possibly through the formation of tau–antibody complexes that prevented uptake of the tau seeds by neighbouring neurons. These complexes could be phagocytosed by microglia or cleared from the interstitial space via the BBB and/or the circumventricular organs. Antibody treatment can increase serum tau levels, which might indicate removal from the brain via Fc receptors or, more likely, the increased half-life of antibody bound tau187–189. Microglia also phagocytose antibody–tau complexes, typically in an Fc-dependent manner, with clearance being impaired by effectorless antibodies or fragments or by blocking the Fc receptors181,189–194.
Despite concerns about antibodies with effector functions promoting glial activation, either human IgG1 antibodies did not increase cytokine levels in cultured glia compared with tau alone, or the activation pattern was different from that induced by bacterial antigens191,192. However, glia might behave differently in culture than in situ, and differentiating the impact of antibody treatments from the effects of tau-induced cytokine production is difficult. Furthermore, an effectorless tau antibody, gosuranemab, seemed to promote glial activation in humans, although the small number of patients prevents firm conclusions from being drawn195. Data from patients with tauopathy demonstrate the complexity of the glial response. Glial activation has been observed in early stages and is thought to be both a result and a mediator of pathology196–198. Activated glia are found in association with plaques and NFT-bearing cells, with some studies showing a stronger correlation of glial activation with tau pathology compared to Aβ196,199–203. In some studies, a progressive increase in glial activation was seen over time199. However, some reports suggest that increased baseline rates of glial tracer binding early in disease reflect a protective response, and other studies found distinct waves of glial activation in MCI and AD201,204–207. Ultimately, these data highlight the need for further research, ideally in vivo, into the role of glia in tauopathies.
Multiple groups have shown that antibodies can enter neurons and colocalize with pathological tau162,182–184,208–216. This uptake could occur through several different mechanisms (Fig. 3). Neurons express Fcγ receptors and tripartite motif containing 21 (TRIM21), a high-affinity cytosolic Fc receptor162,208,212,217–226. Antibodies or antibody-bound tau can bind to surface receptors, which facilitate uptake from the extracellular space in a clathrin-dependent manner and enable delivery to the endosomal–lysosomal system. Antibodies or antibody–tau complexes can also enter the cell via non-specific bulk endocytosis. Colocalization between antibodies and tau or other protein targets in this compartment has been shown in cell and animal models182,183,208,209,211,213–215,227–230. Multiple groups have demonstrated neuronal uptake of therapeutic antibodies against other intracellular targets, and of autoantibodies and circulating IgGs228–242. Once internalized, antibodies might exert protective effects in the endosome or the cytosol. By binding to endosomal tau, antibodies could prevent tau-induced endosomal membrane disruption and promote disassociation of aggregates, thereby facilitating lysosomal enzyme access for digestion. Antibodies might also enter the cytosol following disruption of the endosomal membrane or through translocation. Endosomal–lysosomal membrane integrity is compromised in the presence of pathological tau, Aβ, exosomes or reactive oxygen species, and any increased permeability might also allow antibodies to escape114,117–119,243–248 Antigens and antigen–antibody complexes are transported from the endosome to the cytosol in dendritic cells249–251, and a similar mechanism could exist in neurons.
Once in the cytosol, antibody-bound tau can be ubiquitinated for proteasomal degradation through its association with TRIM21194,212. Specifically, an antibody against p-tau422 has been shown to reduce the levels of insoluble tau in vivo, but its efficacy is lost in the absence of TRIM21, further highlighting the importance of antibody-mediated intracellular clearance of tau194. Both endosomal–lysosomal and proteasomal clearance can also be aided by antibody-mediated blockage or reversal of tau polymerization, as smaller aggregates or monomers are more easily cleared227,252–254.
In the sections that follow, we discuss the clinical trials of tau immunotherapies that have been initiated or published since our previous Review1.
Active immunotherapy
AADvac1.
AADvac1 is an active vaccine designed to target N-terminally truncated tau fragments255,256. It consists of a synthetic peptide encompassing amino acids 294–305 of the tau protein coupled to keyhole limpet hemocyanin with an aluminum hydroxide adjuvant. Four clinical studies of AADvac1 have been completed, three in patients with mild-to-moderate AD and the fourth in patients with non-fluent, agrammatic variant progressive aphasia (naPPA), the pathology of which resembles AD and FTD257.
In a phase I trial (NCT01850238) found that AADvac1 was safe and well tolerated in patients with AD256. No deleterious immunological responses were elicited. All but one of 30 patients developed an IgG response with no encephalitis or vasogenic oedema. Five patients in the treatment group experienced serious adverse events, with two patients from this group withdrawing from the trial with complications thought to be unrelated to treatment. Cognitive scores remained stable in all patients. Overall, AADvac1 had excellent immunogenicity and a favourable safety profile.
A follow-up phase I study (NCT02031198, FUNDAMANT) revealed a similar safety profile258. Antibody titres declined after the six-dose vaccination regimen, but booster doses restored IgG levels. Higher IgG titres were significantly correlated with reduced hippocampal atrophy and cognitive decline. An association between cognitive benefit and IgG titre was observed in patients with positive AD biomarkers.
A phase II trial (NCT02579252, ADAMANT) was conducted in patients with mild AD259–261. AADvac1 was safe and well tolerated and induced a strong IgG response. The vaccine did not alter cognition or brain atrophy rates but was associated with a 58% attenuation of plasma NfL increase. In patients who provided CSF samples, levels of the p-tau217 epitope were significantly reduced, with trends for clearance of p-tau181 and t-tau, in the AADvac1 group. In a subgroup of patients who were predicted to have both Aβ and tau pathology, AADvac1 reduced clinical and functional decline and plasma NfL levels260. These results suggested that larger stratified studies are needed to evaluate the clinical efficacy of this vaccine.
A separate 24-month open-label phase I pilot trial (NCT03174886) was conducted in patients with naPPA. The primary objective was to assess the safety of AADvac1 with immunogenicity as a second objective. Exploratory outcomes included clinical, cognitive, and biomarker readouts. The results have not yet been disclosed.
ACI-35.
ACI-35 is a liposome-based vaccine that targets the p-tau396/404 epitope262. A phase Ib study (ISRCTN13033912) completed in 2017 found ACI-35 to be safe and well tolerated in patients with mild-to-moderate AD. However, the immune response was weak, even after booster shots261. A second-generation vaccine, ACI-35.030, was developed, which included a second adjuvant and an epitope to activate T helper cells. The redesigned vaccine has better immunogenicity, and the antibodies generated specifically bind to p-tau and recognize PHFs from the brains of individuals with AD261.
A phase Ib/IIa trial (NCT04445831) to test the safety and immunogenicity of ACI-35.030 in early AD is ongoing. A separate arm was added to evaluate JACI-35.054, which uses the same p-tau396/404 peptide linked to a carrier protein. The primary outcomes are adverse events and plasma antibody titres, with cognition and behaviour as secondary outcomes. The interim results from the ACI-35.030 cohorts showed that all groups developed a potent antibody response that was specific for p-tau and PHFs263–267. The ACI-35.030-induced immune response was sustained when boosted periodically for up to 72 weeks, with no clinically relevant safety concerns268. JACI-35.054 also generated encouraging interim safety, tolerability and immunogenicity results in the low-dose cohort266. However, ACI-35.030 has been selected for further development, given that its antibody response was stonger relative to JACI-35.054268.
Passive immunotherapy
APNmAb005.
APNmAb005 is an anti-tau IgG antibody (subclass not reported). According to a preclinical preprint, the mouse version of this antibody preferentially recognized synaptic oligomeric and insoluble tau in brain lysates from individuals with AD and pathological tau in brain tissue from people with 3R and 4R tauopathies269. The antibody prevented tau seeding in culture and partially rescued synaptic and neuronal loss and increased tau levels in brain lysate in a mouse model of tauopathy, indicating that the antibody prevented toxicity in vivo without promoting tau clearance.
In May 2022, a phase I study (NCT05344989) was initiated to evaluate the safety profile of a single dose of APNmAb005 in healthy participants. The trial is expected to be completed in July 2024.
Bepranemab.
Bepranemab (UCB0107) is an IgG4 antibody that binds to amino acids 235–250 of tau near the microtubule-binding region. The mouse version was found to block tau seeding in culture270 and in two mouse models of tauopathy when pre-incubated with tau 271.
Three phase I trials evaluated the safety, tolerability and pharmacokinetics of bepranemab. The first trial (NCT03464227) in healthy individuals showed no drug-related safety issues or anti-drug antibodies, and a dose-dependent increase in UCB0107 levels was observed in serum and CSF272,273. A second phase I trial (NCT03605082), also in healthy individuals, had safety and pharmacokinetics as primary endpoints. The results have not been released. The third phase I trial (NCT04185415), in patients with PSP, raised no safety issues274. An open-label extension study (NCT04658199) was registered to evaluate the long-term safety and tolerability of UCB0107 in patients with PSP and is scheduled to run until 2025.
A phase II trial of bepranemab in patients with MCI or mild AD (NCT04867616) is ongoing and expected to run until 2025. The primary outcome is the cognitive score, and the secondary outcomes are adverse events, other cognitive measures, tau PET and pharmacokinetics.
BIIB076.
BIIB076 is an IgG1 antibody that recognizes the mid-domain of tau. It was reported to block tau seeding in culture after immunodepletion and to inhibit tau propagation between neurons177.
A phase I trial of BIIB076 (NCT03056729) was conducted in healthy volunteers and individuals with mild or probable AD. In June 2019, the trial protocol was modified by eliminating the more advanced AD cohort and adopting adverse events as the sole primary outcome. Adverse events prompted the investigators to reduce the highest dose275,276. BIIB076 reduced mid-region-containing tau in CSF 1 week after infusion, suggesting target engagement275. However, the development of BIIB076 was terminated in July 2022 for business reasons277.
E2814.
E2814 is an IgG1 antibody that recognizes HVPGG motifs in the second and fourth repeats of the tau microtubule-binding domain and binds to extracellular tau178. This antibody (or its murine version) has been reported to prevent tau seeding and aggregation in vitro, attenuate deposition of tau aggregates in mice injected with tau fibrils, and reduce free tau containing the mid-domain in non-human primates178,278. Interestingly, in mice that had received intracerebral tau seed injections, a 3 week course of intraperitoneal injections of the mouse version of E2814 reduced insoluble tau levels on the contralateral but not the ipsilateral side of the seed injections, raising efficacy concerns178. A longer-term study using the same seeding method and peripheral antibody injections for 12 weeks showed significant reductions in insoluble tau on both the ipsilateral and contralateral sides at the highest dose, and target engagement in the CSF279. Neither study reported on soluble tau levels.
A phase I trial (NCT04231513) completed in 2020 tested the safety, tolerability and immunogenicity of E2814 in healthy individuals. No significant drug-related adverse events were reported, although two participants developed anti-E2814 antibodies. Serum and CSF pharmacokinetics were proportional to antibody dose, with a dose-related increase of antibody–tau association in the CSF, which persisted for at least 1 month278,280,281. In 2021, a multiple-ascending-dose phase was added to the study. The trial ended in March 2023, and the results have yet to be released.
In 2021, E2814 was chosen to be evaluated in the Dominantly Inherited Alzheimer’s Network Trials Unit (DIAN-TU) prevention trial, the participants of which carry pathogenic amyloid precursor protein or presenilin mutations282. A phase Ib/II trial (NCT04971733) aims to enroll thirteen DIAN patients with mild-to-moderate cognitive impairment. This trial will assess safety, tolerability, target engagement and pharmacokinetics, as well as anti-drug antibodies, and will run until April 2025. Preliminary results from this trial were presented at the Alzheimer’s Association International Conference (AAIC) in 2023283,284. The antibody was safe and well tolerated with favourable pharmacokinetics, and showed target engagement with tau in the CSF. After 3 months, the treated individuals showed a significant decrease in CSF tau 243–254, a tau fragment that strongly correlates with tau PET scan data283,285. Importantly, E2814 binds outside the 243–254 region, thereby ensuring that the CSF data are not confounded by the treatment itself283. In vivo, the antibody might bind to a larger tau fragment extracellularly and/or intracellularly.
Further phase II/III trials (NCT05269394 and NCT01760005) will test E2814 treatment alone or concurrently with anti-Aβ treatment (lecanemab) in DIAN patients with early-onset AD. These trials will evaluate safety, tolerability, biomarkers and cognitive and other functional efficacy of E2814 alone or with lecanemab. Both trials are expected to be completed in October 2027.
Gosuranemab.
Gosuranemab (BIIB092) is an IgG4 antibody that binds human tau at residues 15–22286. It was raised against extracellular N-terminal tau fragments (eTau) isolated from human neurons differentiated from pluripotent stem cells derived from patients with familial AD186,287. The antibody was shown to decrease free tau in brain interstitial fluid and CSF in tauopathy mice after intraperitoneal injections but its potential effect on clearing tau in brain tissue was not reported186.
In a phase I trial to evaluate the safety of gosuranemab in healthy volunteers (NCT02294851), no severe adverse events were reported288. The antibody significantly decreased unbound tau in CSF, with sustained reduction of eTau fragments for up to 12 weeks at higher doses. In a phase Ib trial in patients with PSP (NCT02460094), gosuranemab was safe and well tolerated, with mild-to-moderate adverse effects, and showed dose-dependent accumulation in serum and plasma289. All doses decreased free eTau by more than 90%, and this decrease was sustained for 85 days after treatment. An open-label extension (NCT02658916) was offered to phase Ib study participants to evaluate long-term safety and tolerability. However, this trial was terminated when a follow-up phase II trial (NCT03068468, PASSPORT) failed to meet its primary endpoint290. The phase II study was conducted to evaluate the efficacy of gosuranemab in 490 patients with PSP. In December 2019, it was announced that gosuranemab showed no efficacy, as assessed on the PSP Rating Scale, which measures movement difficulties. However, the antibody did reduce CSF free N-terminal tau fragments by 98%.
Gosuranemab also failed to show efficacy in a phase Ib ‘basket’ trial (NCT03658135) in four different primary tauopathies: Aβ PET-negative corticobasal syndrome, naPPA, frontotemporal lobar degeneration with MAPT mutation and traumatic encephalopathy syndrome287,291. No adverse events were reported and the treatment cleared most of the free eTau from the CSF but had no effect on exploratory measures of disease severity. Both this trial and the aforementioned phase II trial were terminated in December 2019291,292.
In a preliminary study of tissue from gosuranemab-treated individuals with primary tauopathies, treatment-related glial responses were reported, with no clearance of neuronal tau inclusions195. However, only a few individuals underwent autopsies and their treatment regimens and times to death following the last dose differed substantially.
Another phase II study (NCT03352557, TANGO) was conducted in patients with MCI or mild AD. This trial was designed to evaluate long-term safety and efficacy of three different doses of gosuranemab and generation of anti-drug antibodies. The treatment either failed to change or worsened cognitive scores275,293, and all three dose groups had poorer cognitive outcomes than the placebo group275. This trial and further development of gosuranemab were terminated293.
JNJ-63733657.
JNJ-63733657 is an IgG1 antibody with a high affinity for p-tau217. It has been reported to neutralize tau seeds and inhibit pathological spreading in mouse models of tauopathy, but these data have not been peer reviewed294.
A phase I trial of JNJ-63733657 (NCT03375697) was conducted in healthy individuals and patients with prodromal or mild AD294,295. No safety or tolerability issues were raised. The pharmacokinetics were similar between healthy participants and those with AD, with dose-dependent reductions of p-tau217 in the CSF. Two other phase I trials (NCT03689153 and NCT05407818) to assess the safety, tolerability and pharmacokinetics of JNJ-63733657 in healthy participants have been completed but the results have not yet been published.
A phase II study (NCT04619420) is also ongoing to evaluate efficacy, safety, and tolerability of JNJ-63733657 in patients with early-stage AD who have a positive tau PET scan is also ongoing. The primary outcome is change in cognition, and secondary outcomes include other functional measures, brain tau burden, CSF tau, safety and pharmacokinetics. This trial will run until 2025.
Lu AF87908.
Lu AF87908 is an IgG1 antibody raised against p-tau396/404. The mouse version, C10.2, reduced tau seeding in vitro and in mice when pathological tau was pre-incubated with or immunodepleted by the antibody179. In cultured microglia, C10.2 promoted tau uptake and lysosome-mediated degradation191. The humanized version showed highly specific and sensitive tau binding in post-mortem brain tissue from people with AD or primary tauopathies296. A phase I study (NCT04149860) to test the safety, tolerability and pharmacokinetics of Lu AF87908 in healthy individuals and patients with AD concluded in July 2023.
MK-2214.
The precise epitope for MK-2214 has not been reported, but this antibody might be derived from a mouse antibody that recognizes p-tau413 and was found to bind AD tau and showed efficacy in animal models297,298. Two phase I trials have been initiated to examine its safety, tolerability, pharmacokinetics and pharmacodynamics in healthy individuals (jRCT2031220627) or patients with MCI or mild-to-moderate AD (NCT05466422), and are expected to be completed in 2024298.
PNT001.
PNT001 recognizes cis p-tau231 which is reported to be a highly neurotoxic form of pathological tau 214,299. Cis p-tau231 has been detected in brain tissue from people with AD or traumatic brain injury (TBI) and was shown to have a role in tau aggregation and neurodegeneration214,300–302. Preclinical studies indicated that peripheral anti-cis p-tau231 treatment cleared pathological tau from the brain and ameliorated neuronal degeneration and some cognitive impairments in mouse models of tauopathy, vascular dementia and TBI214,300,303.
A phase II study (NCT04096287) evaluated the safety and tolerability of PNT001 in healthy individuals. The antibody was well tolerated, with dose-dependent serum and CSF exposure274. Another phase I trial (NCT04677829) to examine safety and tolerability in patients with acute TBI was also registered, but was terminated soon after the first participant enrolled and no results have been disclosed.
PRX005.
PRX005 is an IgG1 antibody targeting the microtubule-binding region in both the 3R and 4R tau isoforms304,305. According to a conference presentation, PRX005 recognizes both unphosphorylated and phosphorylated tau, and NFTs and dystrophic neurites in AD brain tissue304,305. It blocks binding of tau to heparan sulfate proteoglycan, thereby preventing tau transmission between cells, and was also shown to inhibit tau aggregation and p-tau accumulation in mouse models of tauopathy and amyloidosis.
A phase I study to evaluate the safety and tolerability of PRX005 is ongoing. The top-line results from a single-ascending-dose study in healthy individuals was announced in a press release and in poster at the AAIC in 2023305,306. The antibody was shown to be safe, with dose-dependent plasma and CSF exposure. A multiple-ascending-dose study in patients with AD has been initiated.
RG7345.
RG7345 targets p-tau422, and, preclinical studies, chronic administration reduced tau pathology in transgenic mice211. A phase I trial (NCT02281786) was initiated in healthy individuals to assess the safety, tolerability and pharmacokinetics of this antibody. Presumably it did not assess target engagement because the pSer422 epitope is found at very low levels or not at all in healthy individuals45,307,308. This trial was discontinued, probably because of unfavourable pharmacokinetics, and the results have not been published309.
Semorinemab.
Semorinemab (RO7105705) is an IgG4 antibody that targets the N-terminus of monomeric and oligomeric tau. The mouse version was shown to target extracellular tau and reduced one phospho-tau epitope on brain sections, but had no effect on tau in western in a mouse model of tauopathy193,310. Effects on insoluble tau and on behaviour were not reported. Interestingly, the version of semorinemab with effector function cleared tau at a lower dose than the effectorless version, and neither antibody subclass increased astrogliosis or microgliosis193,310. Nevertheless, the effectorless version was selected for clinical trials, presumably because unlike the version with effector function, its mouse version does not cause fragmentation of microtubule-associated protein 2 — a protein that is important for microtubule assembly and stabilization in neurons in culture193,310.
A phase I trial of semorinemab (NCT02820896) was conducted in healthy individuals and patients with mild-to-moderate AD. All dosing and administration paradigms were safe and well tolerated311. No severe adverse effects were reported, and dose-dependent plasma and CSF antibody exposure was observed.
A phase II trial (NCT03289143, TAURIEL) was conducted in patients with prodromal or mild AD. The antibody was safe; however, it missed both the primary and secondary efficacy endpoints312. Antibody administration also failed to slow NFT accumulation, although its pharmacokinetics were dose-proportional274.
Another phase II trial (NCT03828747, LAURIET) enrolled patients with moderate AD. This study was completed in August 2023. The primary outcome was change in cognitive scores, and secondary outcomes include additional cognitive tests and behaviour, adverse events, serum concentration and immunogenicity. The top-line results showed that semorinemab treatment slowed decline on one cognitive test, but no changes in other cognitive and functional outcomes were noted313, and tau burden based on PET signal was not altered although CSF tau was reduced313,314. A decision on phase III testing is pending.
Tilavonemab.
Tilavonemab (CN2–8E12) is an IgG4 antibody that recognizes an N-terminal tau epitope comprising residues 25–30 and has been reported to work extracellularly180,181,185,315. In culture, tilavonemab blocked tau seeding and prevented propagation of tau pathology when preincubated with the tau seeds181,185. In a mouse model of tauopathy, the drug substantially reduced levels of p-tau and insoluble tau and rescued contextual fear conditioning deficits180. A second study showed reduced levels of insoluble tau and decreased brain atrophy, as well as improved motor function, in mice treated with tilavonemab189.
In a phase I trial in patients with PSP (NCT02494024), tilavonemab was shown to be safe316. The drug had a serum half-life of 27–37 days and dose-dependent blood exposure. An open-label extension study (NCT03413319) was performed to determine the long-term safety and tolerability of the drug, as well as the eligibility of participants for a subsequent phase II trial.
A phase II trial (NCT02985879) to evaluate the safety and efficacy of tilavonemab was conducted in patients with PSP. A 4-year extension (NCT03391765) was initiated in participants who had completed the placebo-controlled treatment phase. Tilavonemab provided no benefit over placebo, although it had target engagement and a favourable tolerability profile317,318. The extension studies, as well as an expanded access programme in patients with CBD, were subsequently halted317,319.
Another phase II trial of tilavonemab (NCT02880956) was conducted in patients with early-stage AD. An extension study (NCT03712787) on long-term safety and tolerability was offered to participants who completed the initial testing. In the extension study, which ended in July 2021, the treatment did not halt cognitive decline or improve functional outcomes, nor did it slow brain atrophy or lower plasma NfL levels275,320. Given the lack of efficacy in all trials, development of tilavonemab was terminated315.
Zagotenemab.
Zagotenemab (LY3303560) is a humanized form of the MC1 antibody, which recognizes an early form of misfolded tau321,322. In preclinical testing in tau transgenic mice, chronic injections of zagotenemab reduced insoluble p-tau levels in the spinal cord and p-tau immunoreactivity in brainstem and spinal cord, and improved motor phenotypes321. The single-chain variable fragment (scFv) of the antibody also reduced tau pathology in mice when administered in an AAV construct as a gene therapy323.
Two phase I trials assessed the safety and pharmacokinetics of zagotenemab. The first (NCT02754830) evaluated safety and serum drug concentration in healthy individuals and patients with MCI or mild-to-moderate AD. A second trial (NCT03019536) assessed multiple ascending doses in the same AD cohort. Adverse effects and pharmacokinetics were evaluated. The results have not been published.
A phase II efficacy trial of zagotenemab (NCT03518073) was also conducted in patients with gradual and progressive memory decline. Primary outcomes included changes in cognition and secondary outcomes were additional functional measures and anti-drug antibodies. This trial missed its primary endpoint, and development of zagotenemab was discontinued324.
Factors influencing antibody efficacy
The results from human testing raise the issue of how to maximize antibody efficacy. Multiple factors affect antibody efficacy, including mechanism of action, IgG subclass, epitope and the patient population being treated.
Tilavonemab, gosuranemab, semorinemab, and zagotenemab which were found in preclinical testing to work only extracellularly and to solely or partially target the N-terminus of tau, have not provided functional benefits in clinical trials, suggesting that extracellular targeting of tau epitopes will not be sufficient. Although tau spreading is a valid clinical target, extracellular tau is only a small proportion of the tau in AD325,326. Most tau, including its pathological forms, is found within neurons. Therefore, removing extracellular tau is unlikely to reverse intracellular pathology, although the extracellular and intracellular pools might exist in equilibrium. In addition, in the CSF patients with AD or primary tauopathy, N-terminal tau is found at much lower levels than tau containing the mid-domain327–330. Furthermore, tilavonemab and gosuranemab were tested in patients with PSP, who, unlike patients with AD, do not have elevated CSF tau levels328,331–336. These factors could all have contributed to the lack of efficacy of the antibodies. Patients with primary tauopathy were shown to have decreased CSF levels of the microtubule-binding region of tau337, suggesting that extracellular antibodies against this region would not be beneficial in these individuals.
The optimal tau epitopes to target in AD remain open to debate. Mass spectroscopy has revealed relatively low levels of carboxy-terminal tau in the CSF in AD, indicating that extracellular antibodies targeting this region are likely to be ineffective280,328–330,337,338. Mid-domain tau (approximately aa 150–250) comprises the largest fraction of tau in the CSF, suggesting that this region would be a better target327,328; however, as stated above, even the largest fraction of extracellular tau is a minuscule proportion of the tau in the brain.
Intracellularly, the N-terminus might not be the optimal target for immunotherapies339. Preventing or reversing aggregation of tau can reduce seeding and make the protein easier to digest; therefore, selecting epitopes from the core of aggregates could be more beneficial, but these epitopes often have limited accessibility owing to their hydrophobic nature. Cryo-electron microscopy has clarified the core structure and sequence of filaments from different tauopathies55,340–342. Although the structures differ, they all consist of the microtubule-binding region and C-terminus of the molecule. Thus, antibodies targeting epitopes in these regions might be appealing candidates, assuming that they can work intracellularly. Like C-terminal tau, the microtubule-binding region of tau is primarily found intraneuronally. An additional challenge for immunotherapies that target phospho-epitopes is the shifting prevalence of these epitopes over time, with some being more prominent in early-stage disease and others increasing during disease progression.
In addition to differences in CSF tau, the pathology seen in primary tauopathies and AD differs in many respects, including the brain regions affected and the types of tau lesions18,19. Therefore, these patient populations should not be considered interchangeable during the development and testing of immunotherapies. Tau filaments can assume different morphologies with unique core structures, and phospho-epitope profiles might also vary between and within tauopathies 54,55,340–343. In addition to neuronal tau, primary tauopathies also feature glial tau deposits. These inclusions, such as oligodendrocyte coiled bodies and tufted astrocytes, are distinct from the pathological tau that is seen in neurons. The type of astrocytic pathology is also disease-dependent18,344, which has consequences for synaptic function, provision of trophic support, inflammation and maintenance of myelination344,345. Glial tau pathology might propagate independently of neurons346,347. Targeting of non-neuronal inclusions with immunotherapies is unlikely to be straightforward, as the optimal epitopes might be different from those in neurons, and antibodies optimized for neurons may not be internalized by glia. Clearing this pool of tau may require a more direct focus, such as using gene therapy to specifically express antibodies in glial cells323.
Current human trials utilize either IgG1 or IgG4 antibody subclasses. Unlike IgG1, IgG4 antibodies mostly lack effector function, which might increase safety but reduce their efficacy. Although Lee at al. argued that effector function was unnecessary, their antibody with effector functions promoted tau clearance at a lower dose than its effectorless counterpart193,310. Mukadam et al. showed that in slice cultures, an antibody mutated to lack Fc binding, which mediates effector function, was less effective than its unmodified counterpart194. In cultured microglia, a direct comparison between humanized IgG1 and IgG4 versions of the same antibody showed that the IgG1 variant was more efficacious at promoting tau phagocytosis192. When the same tau binding region was cloned into all four mouse IgG subclasses (IgG1, IgG2a, IgG2b and IgG3), IgG1 and IgG2a (the human IgG1 analogue) were the most effective at preventing tau toxicity and promoting tau clearance, and the effectorless variant IgG3 (human IgG4 analogue) was the least effective348. Some of those efficacy differences might relate to variable Fc-mediated neuronal uptake of the IgG subclasses. In addition to Fc binding, the IgG subclass influences antibody catabolism rates, self-association and stability349–352, and changing the IgG subclass can affect antigen binding even when the variable regions are unchanged182,348,353–368. These findings could have major implications for antibodies that underwent preclinical testing before being humanized, sometimes into a different subclass. IgG4 can also split and form heterodimers with other IgG4 antibodies, and it is unclear whether this possibility was considered for the antibodies reviewed above 369,370.
Thorough testing of humanized antibodies in mouse models is not feasible because species differences increase the likelihood of development of anti-idiotypic antibodies and related adverse effects. These problems could be minimized to some extent by using mouse models with humanized immune systems crossed with tauopathy models, although no such hybrid models have been described in the literature. Some humanized antibodies have been examined in non-human primates, but they have limited utility as these animals are not prone to develop tau pathology.
Antibody charge and affinity should also be considered during antibody design, although. affinity for the antigen and efficacy do not necessarily correspond. For example, a lower-affinity antibody against p-tau396/404 was more efficacious than a higher affinity antibody against the same epitope182,183,348,371. Though not directly comparable, the low-affinity MC1 antibody showed greater efficacy in vivo than a higher-affinity antibody against a different tau epitope372. However, such findings are not universal, as a higher-affinity antibody specific for tau truncated at Asp421 was more effective than a lower-affinity antibody against the same epitope (E.E.C., E.M.S. et al, unpublished work). Charge also influences every aspect of antibody function, including binding, uptake into cells or across the BBB, what cell type the antibody targets, and how quickly the antibody is degraded182,373–378. Of note, an antibody’s ability to prevent tau seeding does not necessarily relate to its ability to prevent tau toxicity182,325.
These findings highlight the need for further research into optimal antibody design and demonstrate the challenge of translating results from the laboratory to human patients. Many of these questions have been explored more thoroughly in other fields, notably in the development of cancer immunotherapies, and merit greater study. In the sections that follow, we discuss how antibodies and their fragments might be modified to enhance tau clearance379–402.
New immunotherapy approaches
Antibody fragments
Currently, only whole IgGs are being tested in clinical trials, but antibody fragments show potential as therapeutic and imaging agents. scFvs and single-domain antibodies (sdAbs) are much smaller (approximately 25 kDa and 13 kDa, respectively) than whole IgGs (150 kDa). The smaller size could enable enhanced BBB penetration and targeting of cryptic epitopes that are inaccessible to whole antibodies. In addition, sdAbs are stable and easier to produce in large quantities than whole antibodies. Preclinical testing of scFvs and sdAbs in cell and animal models has shown that they can prevent the formation of tau polymers, act as imaging agents and reduce tau pathology215,252,254,323,348,403–409. One potential complicating factor for using unmodified antibody fragments as long-term therapies is that they can have a half-life in the order of hours, compared with 1–3 weeks for whole antibodies. However, we have observed fluorescent signal from tau scFvs and sdAbs in the brains of tauopathy mice several days after injection215,407. Thus, with a sufficient quantity of target to bind, antibody fragments seem to be retained in tissue. Moreover, scFvs and sdAbs can be delivered as gene therapies, which have been shown to reduce polymerization and clear tau in vivo253,254,404,408,410. Antibody fragment gene therapy is also supported by results from other neurodegenerative disease models410–414.
Modified immunotherapy
Tau antibodies and antibody fragments can be incorporated into molecular complexes to promote targeted tau degradation through the proteasome or the endosomal–lysosomal system. Whole IgGs and antibody fragments have been used as the binding agents for extracellular, surface-bound or intracellular targets (Fig. 4). Although no modified, multivalent sdAbs have yet been used to target tau in clinical trials, some have been tested for the treatment of cancer, as well as infectious and autoimmune diseases415,416.
Figure 4 |. Modified immunotherapy strategies.
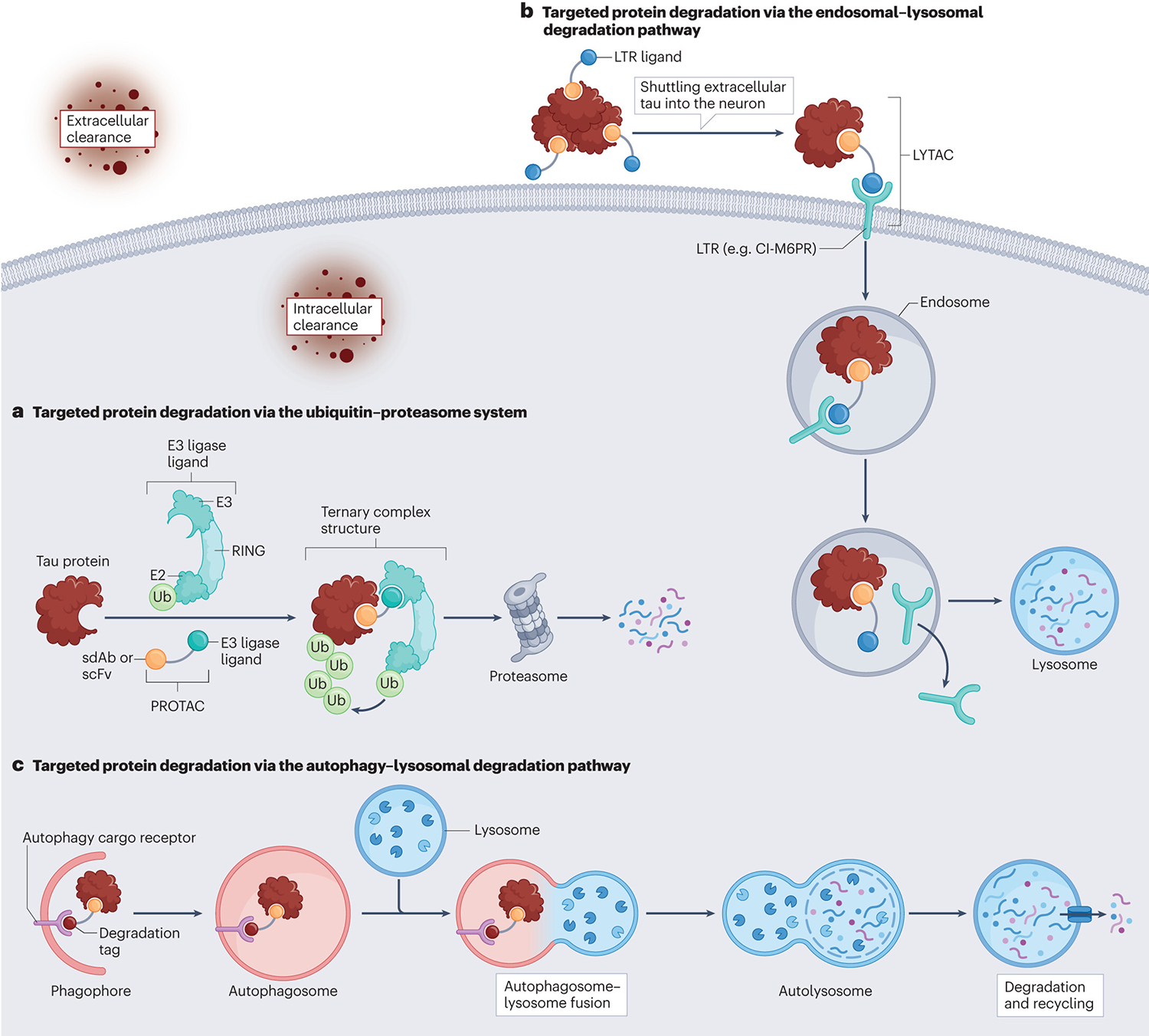
a | Targeted protein degradation via the ubiquitin–proteasome system. Proteolysis-targeting chimaeras (PROTACs) are hetero-bivalent complexes comprising a target binder (single-domain antibody (sdAb) or single-chain variable fragment (scFv)), a short linker and an E3 ligase-recruiting molecule. These complexes bring the target protein (in this case, tau) and E3 ligase into close proximity and trigger proteasome-based degradation. b | Targeted protein degradation via the endosomal–lysosomal degradation pathway. Lysosome-targeting chimaeras (LYTACs) are hetero-bivalent complexes are comprising a target binder (sdAb or scFv), a short linker and a ligand for the lysosome-targeting receptor (LTR). They shuttle extracellular tau into the neuron for degradation. c | Targeted protein degradation via the autophagy–lysosomal degradation pathway. Autophagy-targeting chimaeras are hetero-bivalent complexes comprising a target binder (sdAb, or scFv), a short linker and a degradation tag, which can enhance tau degradation through the autophagy–lysosomal degradation pathway. CI-M6PR, cation-independent mannose-6-phosphate receptor; RING, really interesting new gene; Ub, ubiquitin.
Targeted protein degradation via the ubiquitin–proteasome system.
scFvs and sdAbs can be modified to enhance target ubiquitination and proteasomal clearance. Gallardo et al. developed anti-tau scFv chimaeras by fusing scFvs to ubiquitin at either Lys48 or Lys63, which directs proteins to the proteasome or lysosome, respectively389. Both scFvs reduced intracellular tau levels in culture but only the proteasome-targeting scFv was effective in vivo.
Proteolysis-targeting chimaeras (PROTACs) are hetero-bivalent complexes comprised of a target binder (small molecule or antibody), a short linker and an E3 ligase-recruiting molecule. The complex brings the target protein and ubiquitination machinery into close proximity, leading to polyubiquitination and proteasome-mediated degradation of the protein. Small-molecule-based and peptide-based PROTACs have been shown to degrade tau in cultured cells384–388. In mouse models of AD and tauopathy, a tau-targeting PROTAC reduced t-tau and p-tau levels, preserved dendritic arborization and improved cognitive performance384,388. Several studies have successfully incorporated sdAbs as the target binding portion of the PROTAC to rapidly degrade a range of proteins381–383.
A third strategy fuses the PEST (Proline (P), Glutamic Acid (E) / or Aspartic Acid (D), Serine (S), and Threonine (T)) proteasome-targeting motif to the antibody fragment of interest. This modification enhanced the efficacy of an anti-huntingtin scFv in transgenic mice390. sdAbs fused to the same PEST motif prevented α-synuclein-induced toxicity in cultured cells and reduced α-synuclein pathology in vivo379,380.
Targeted protein degradation via the endosomal–lysosomal system.
Cell-surface lysosome-targeting receptors (LTRs) such as the cation-independent mannose-6-phosphate receptor (CI-M6PR) have been reported to facilitate transport of proteins to lysosomes417. CI-M6PR shuttles cargo to pre-lysosomal compartments, where the cargo dissociates and progresses to the lysosome while the receptor is recycled. CI-M6PR has been targeted to treat lysosomal storage disorders418 and is highly expressed in neurons419–421. Tau-targeting antibodies could be modified to bind to CI-M6PR to enhance tau clearance.
So-called sweeping antibodies have a modified pH-sensitive variable domain that releases the target protein into acidic compartments to be digested, and the unbound antibody is recycled back to the cell surface391. The constant domain of the antibody can be modified to enhance Fc binding, which protects it from being degraded and enhances cellular uptake of the antibody–protein complexes. This approach could enhance the ability of tau antibodies to clear tau from the extracellular space through microglial phagocytosis.
Lysosome-targeting chimaeras (LYTACs) consist of an antibody or small molecule fused to a synthetic mannose-6-phosphonate glycopeptide that acts as an LTR ligand. The LYTAC molecule can simultaneously bind to a membrane-bound or extracellular target protein and the LTR. Once the antibody–target complex has been endocytosed, the target protein is released and the LYTAC is recycled back to the cell surface392,393.
Antibody-based PROTACs (AbTACs) are antibody derivatives that promote lysosomal degradation. One arm of the engineered bispecific AbTAC antibody binds to an extracellular or membrane target and the other arm binds to a membrane-bound E3 ligase such as ring finger protein 43. As proof of concept, an AbTAC targeting programmed death ligand 1 successfully promoted lysosomal targeting and clearance of the protein397.
Targeted protein degradation via the autophagy–lysosomal pathway.
The autophagy targeting chimaera (AUTAC) complex is composed of a cGMP-based degradation tag, a linker and a small molecule or antibody to bind to the target394,395. The use of a cGMP derivative was based on findings that 8-nitro-cGMP promoted Lys63 polyubiquitination and, thus, clearance through the autophagy–lysosomal system396. This method was successfully used to promote the autophagic degradation of mitochondria in human fibroblasts395.
AUTOTACs, a second type of autophagy-targeting chimaera, are bidirectional complexes consisting of a module that interacts with the autophagy cargo receptor p62/SQSTM1 and a portion that binds the target399. This arrangement creates a link between the target and p62, leading to the oligomerization and activation of p62 and, in turn, target degradation by the autophagy–lysosome pathway. Using a modified 4-phenylbutyric acid molecular chaperone as a tau binder, AUTOTACs have successfully targeted misfolded tau in cells and tauopathy mice.
The tauopathy-homing and autophagy-activating nanoassembly (THN) has a magnetic mesoporous silica nanoparticle core embedded with PEGylated cerium oxide bound to the AT8 tau antibody401. AT8 binds to p-tau202/205 and cerium oxide promotes autophagy400. In cultured cells, THN particles colocalized with autophagosomes and promoted clearance of tau, and in a tauopathy rat model, THN particles were internalized by neurons and bound to pathological tau401. The treated animals showed amelioration of cognitive deficits.
Enhancing tau dephosphorylation.
Dephosphorylation-targeting chimaeras (DEPTACs) contain a tau-binding portion connected to a PP2A recruiter via a linker, with an added motif to increase cellular uptake402. This arrangement brings tau in close proximity to PP2A, thereby facilitating dephosphorylation. In tauopathy mice, treatment with a β-tubulin peptide-based DEPTAC lowered pathological tau levels while improving memory and microtubule assembly and increasing dendritic spine density. This approach would also avoid the dephosphorylation of unrelated proteins that could result if PP2A was targeted globally.
Conclusions
In our previous Review on tau-targeting therapies1, we stated that the outcomes of pending trials would provide a clearer picture of the landscape of these therapies. Some therapies that showed promise in preclinical testing have failed to translate into benefits for patients. Other drugs have advanced to or within trials. Of the non-immunotherapy approaches, sodium selenate, lithium chloride and some OGA inhibitors have ongoing clinical trials, but many others have either failed or not advanced. In addition, several candidates completed clinical trials but no results were released.
Among the recent clinical trials that were reported, ASOs produced promising results, safely reducing CSF tau levels below baseline in patients with mild AD123. Other measures, such as clinical presentation and brain volume, were not significantly different between the placebo and treatment groups; however, this study was relatively short (23 weeks) and had a small number of participants, with safety and pharmacokinetics being the principal endpoints. A larger multi-year phase II study is underway, with a focus on cognitive outcomes, the results are eagerly awaited.
The failure of some anti-tau antibodies might be attributed to several factors, including the choice of epitope, the study population and the mechanism of action, as well as limited information on how the properties of humanized antibodies relate to the mouse prototypes. Many studies have focused on N-terminal-tau-targeting antibodies that act extracellularly, despite the fact that over 99% of tau is intracellular and few N-terminal tau fragments are found in the extracellular space. In addition, these antibodies were trialed in patients with primary tauopathy even though extracellular tau levels are not increased in these individuals. Multiple groups, including ours, have highlighted the unsuitability of the N-terminus of tau as a therapeutic target325,339,422–424. The C-terminus of tau might also be an inappropriate target for extracellular antibodies.
The microtubule-binding region and C-terminus of tau are appealing targets for intracellular antibodies, as these regions make up the core of tau polymers. The efficacy of targeting specific phospho-epitopes might depend on the disease stage. Further development of peripheral and imaging biomarkers to identify the disease stage before treatment could allow antibody treatments to be tailored to the pathology of individual patients.
Immunotherapies and non-immunotherapy candidates face several common challenges. Both antibodies and small molecules must cross the BBB and enter neurons. Studies must also be long enough for the clinical benefits of the therapies to become apparent. Moreover, the choice of outcome measures must be carefully considered.
The failed immunotherapy candidates highlight the need for more research into how to select and optimize antibodies. We have long argued that to maximize efficacy, antibodies should be able to target both intracellular and extracellular pathological tau. Our group and others have demonstrated the importance of neuronal uptake of antibodies, and data on the role of TRIM21 further supports intracellular mechanisms182–184,194,209,211,212,214,227,348,425. Antigen binding, charge and cellular uptake can all be altered by changing constant domains — either swapping between murine IgGs or from murine to human — even if the variable region remains the same182,348. Following humanization, antibodies must be thoroughly re-evaluated, which is feasible in culture models but difficult in vivo owing to a lack of suitable human-like animal models.
Combination trials of therapies targeting both tau and Aβ should become more common in the near future. However, such approaches might not be applicable to all patients; for example, individuals who carry the apolipoprotein E ε4 have an increased risk of amyloid-related imaging abnormalities after treatment with lecanemab or donanemab. We also expect modified antibody approaches, as well as antibody-based gene therapies and/or gene-editing strategies, to enter trials. Advances in tau biomarkers should enable suitable patient populations to be identified at earlier stages of the disease, thereby increasing the likelihood of a positive outcome.
Progress in the field of tau-targeting therapies has not been as rapid as we had hoped. However, the intracellular location, size and complexity of tau makes it a more challenging target than Aβ, and it took nearly 40 years from the discovery of Aβ to an approved therapy (which, notably, is an antibody). We believe that it is time for tau-targeting therapies to receive a similar degree of support to their Aβ-targeting counterparts. Watching large companies with extensive resources periodically scale back their research into therapies for neurodegeneration has been worrying, but is understandable to some extent in view of the difficulty and high cost of these studies. As always, more basic research is needed, both to test potential therapies, and to explore the pathological mechanisms of AD and other tauopathies, which will help us to determine how best to optimize existing candidates and to identify new targets for intervention.
Supplementary Material
Key points.
Therapies targeting expression, post-translational modifications, aggregation and clearance of tau have advanced to human testing. These have been largely safe and well tolerated.
Clinical efficacy of tau targeting therapies has yet to be established and some trials have failed. However, multiple trials are ongoing and new candidates continue to enter trials.
Antisense oligonucleotides have recently shown promising results in human testing in reducing tau expression. Larger studies will determine whether this translates into clinical benefits.
Most of the ongoing trials are immunotherapies. These can target tau intra- and/or extracellularly, but targeting tau only extracellularly is less likely to be effective.
Choice of epitope, antibody subclass and its charge, patient population, and mechanism of action must all be carefully considered when selecting antibodies and vaccines for clinical trials. Ideally, antibodies should be thoroughly retested after humanization, as this process may alter their properties.
Acknowledgements
The authors were supported by NIH grants R01 AG032611, NS077239, RF1 NS120488, R21 AG069475 and T32 AG052909, and Alzheimer’s Association grants AARF-22-926735 and AARF-22-924783.
Footnotes
Competing interests
E. M. S. in an inventor on various patents related to the topic of this review that are assigned to New York University. Some of the patents on tau immunotherapies are licensed to H. Lundbeck. The other authors declare no competing interests.
Peer review information
Nature Reviews Neurology thanks S. Fowler, who co-reviewed with S. Bez; R. Kayed; and L. Vivash for their contribution to the peer review of this work.
Supplementary information
Supplementary information is available for this paper at https://doi.org/10.1038/s415XX-XXX-XXXX-X
References
- 1.Congdon EE & Sigurdsson EM Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 14, 399–415, doi: 10.1038/s41582-018-0013-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimer’s Association. Alzheimer’s disease Facts and Figures 2022. Alzheimer Dement 18, 700–789 (2022). [DOI] [PubMed] [Google Scholar]
- 3.Collaborators, G. B. D. D. F. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health 7, e105–e125, doi: 10.1016/S2468-2667(21)00249-8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panza F, Lozupone M, Logroscino G & Imbimbo BP A critical appraisal of amyloid-beta-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 15, 73–88, doi: 10.1038/s41582-018-0116-6 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Jeremic D, Jimenez-Diaz L & Navarro-Lopez JD Past, present and future of therapeutic strategies against amyloid-beta peptides in Alzheimer’s disease: a systematic review. Ageing Res Rev 72, 101496, doi: 10.1016/j.arr.2021.101496 (2021). [DOI] [PubMed] [Google Scholar]
- 6.van Dyck CH et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 388, 9–21, doi: 10.1056/NEJMoa2212948 (2023). [DOI] [PubMed] [Google Scholar]
- 7.Alzforum Report. U.S. FDA Gives Green Light to Leqembi, aka Lecanemab. https://www.alzforum.org/news/research-news/us-fda-gives-green-light-leqembi-aka-lecanemab (06 Jan 2023).
- 8.Mintun M, Ritchie CW, Solomon P, Sims JR, Salloway S, Hansson O, Apostolova LG, Zimmer JA, Evans CD, Lu M, Ardayfio PA, Sparks JD, Wessels AM, Shcherbinin S, Wang H, Nery ESM, Collins EC, Dennehy EB, Brooks DA, Skovronsky DM Donanemab in Early Symptomatic Alzheimer’s Disease: Efficacy and Safety in TRAILBLAZER-ALZ 2, a Phase 3 Randomized Clinical Trial. Alzheimer’s Association International Conference Amsterdam, Netherlands, and Online 2023 (2023). [Google Scholar]
- 9.Company., P. r. E. L. a. Lilly’s Donanemab Significantly Slowed Cognitive and Functional Decline in Phase 3 Study of Early Alzheimer’s Disease. https://investor.lilly.com/news-releases/news-release-details/lillys-donanemab-significantly-slowed-cognitive-and-functional (May 3 2023). [Google Scholar]
- 10.Tissot C et al. Association between regional tau pathology and neuropsychiatric symptoms in aging and dementia due to Alzheimer’s disease. Alzheimers Dement (N Y) 7, e12154, doi: 10.1002/trc2.12154 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ossenkoppele R et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain 139, 1551–1567, doi: 10.1093/brain/aww027 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ge X et al. Association of Tau Pathology With Clinical Symptoms in the Subfields of Hippocampal Formation. Front. Aging Neurosci. 13, 672077, doi: 10.3389/fnagi.2021.672077 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arriagada PV, Growdon JH, Hedley-Whyte ET & Hyman BT Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 42, 631–639 (1992). [DOI] [PubMed] [Google Scholar]
- 14.Arriagada PV, Marzloff K & Hyman BT Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology 42, 1681–1688 (1992). [DOI] [PubMed] [Google Scholar]
- 15.Nelson PT et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J. Neuropathol. Exp. Neurol. 71, 362–381, doi: 10.1097/NEN.0b013e31825018f7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dronse J et al. In vivo Patterns of Tau Pathology, Amyloid-beta Burden, and Neuronal Dysfunction in Clinical Variants of Alzheimer’s Disease. J. Alzheimers Dis. 55, 465–471, doi: 10.3233/JAD-160316 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Robinson JL et al. Primary Tau Pathology, Not Copathology, Correlates With Clinical Symptoms in PSP and CBD. J. Neuropathol. Exp. Neurol. 79, 296–304, doi: 10.1093/jnen/nlz141 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung DC, Roemer S, Petrucelli L & Dickson DW Cellular and pathological heterogeneity of primary tauopathies. Mol. Neurodegener. 16, 57, doi: 10.1186/s13024-021-00476-x (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Wu KM, Yang L, Dong Q & Yu JT Tauopathies: new perspectives and challenges. Mol. Neurodegener. 17, 28, doi: 10.1186/s13024-022-00533-z (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H & Del Tredici K Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 112, 389–404, doi: 10.1007/s00401-006-0127-z (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferreira D et al. The hippocampal sparing subtype of Alzheimer’s disease assessed in neuropathology and in vivo tau positron emission tomography: a systematic review. Acta neuropathologica communications 10, 166, doi: 10.1186/s40478-022-01471-z (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferreira D, Nordberg A & Westman E Biological subtypes of Alzheimer disease: A systematic review and meta-analysis. Neurology 94, 436–448, doi: 10.1212/WNL.0000000000009058 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crary JF et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 128, 755–766, doi: 10.1007/s00401-014-1349-0 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arima K Ultrastructural characteristics of tau filaments in tauopathies: immuno-electron microscopic demonstration of tau filaments in tauopathies. Neuropathology 26, 475–483 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Jin N et al. Truncation and activation of GSK-3beta by calpain I: a molecular mechanism links to tau hyperphosphorylation in Alzheimer’s disease. Sci. Rep. 5, 8187, doi: 10.1038/srep08187 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leroy K, Yilmaz Z & Brion JP Increased level of active GSK-3beta in Alzheimer’s disease and accumulation in argyrophilic grains and in neurones at different stages of neurofibrillary degeneration. Neuropathol. Appl. Neurobiol. 33, 43–55, doi: 10.1111/j.1365-2990.2006.00795.x (2007). [DOI] [PubMed] [Google Scholar]
- 27.Pei JJ et al. Accumulation of cyclin-dependent kinase 5 (cdk5) in neurons with early stages of Alzheimer’s disease neurofibrillary degeneration. Brain Res. 797, 267–277 (1998). [DOI] [PubMed] [Google Scholar]
- 28.Tseng HC, Zhou Y, Shen Y & Tsai LH A survey of Cdk5 activator p35 and p25 levels in Alzheimer’s disease brains. FEBS Lett. 523, 58–62 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Patrick GN et al. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 402, 615–622, doi: 10.1038/45159 (1999). [DOI] [PubMed] [Google Scholar]
- 30.Yarza R, Vela S, Solas M & Ramirez MJ c-Jun N-terminal Kinase (JNK) Signaling as a Therapeutic Target for Alzheimer’s Disease. Front. Pharmacol. 6, 321, doi: 10.3389/fphar.2015.00321 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sontag JM & Sontag E Protein phosphatase 2A dysfunction in Alzheimer’s disease. Front. Mol. Neurosci. 7, 16, doi: 10.3389/fnmol.2014.00016 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taleski G & Sontag E Protein phosphatase 2A and tau: an orchestrated ‘Pas de Deux’. FEBS Lett. 592, 1079–1095, doi: 10.1002/1873-3468.12907 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Frautschy SA, Baird A & Cole GM Effects of injected Alzheimer beta-amyloid cores in rat brain. Proc. Natl. Acad. Sci. U. S. A. 88, 8362–8366, doi: 10.1073/pnas.88.19.8362 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kowall NW, McKee AC, Yankner BA & Beal MF In vivo neurotoxicity of beta-amyloid [beta(1–40)] and the beta(25–35) fragment. Neurobiol. Aging 13, 537–542, doi: 10.1016/0197-4580(92)90053-z (1992). [DOI] [PubMed] [Google Scholar]
- 35.Hernandez P, Lee G, Sjoberg M & Maccioni RB Tau phosphorylation by cdk5 and Fyn in response to amyloid peptide Abeta (25–35): involvement of lipid rafts. J. Alzheimers Dis. 16, 149–156, doi: 10.3233/JAD-2009-0933 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Kirouac L, Rajic AJ, Cribbs DH & Padmanabhan J Activation of Ras-ERK Signaling and GSK-3 by Amyloid Precursor Protein and Amyloid Beta Facilitates Neurodegeneration in Alzheimer’s Disease. eNeuro 4, doi: 10.1523/ENEURO.0149-16.2017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma QL et al. Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: suppression by omega-3 fatty acids and curcumin. J. Neurosci. 29, 9078–9089, doi: 10.1523/JNEUROSCI.1071-09.2009 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nassif M et al. Beta-amyloid peptide toxicity in organotypic hippocampal slice culture involves Akt/PKB, GSK-3beta, and PTEN. Neurochem. Int. 50, 229–235, doi: 10.1016/j.neuint.2006.08.008 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Otth C et al. AbetaPP induces cdk5-dependent tau hyperphosphorylation in transgenic mice Tg2576. J. Alzheimers Dis. 4, 417–430, doi: 10.3233/jad-2002-4508 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Sigurdsson EM, Lee JM, Dong XW, Hejna MJ & Lorens SA Bilateral injections of amyloid-beta 25–35 into the amygdala of young Fischer rats: behavioral, neurochemical, and time dependent histopathological effects. Neurobiol. Aging 18, 591–608 (1997). [DOI] [PubMed] [Google Scholar]
- 41.Sigurdsson EM, Lorens SA, Hejna MJ, Dong XW & Lee JM Local and distant histopathological effects of unilateral amyloid-beta 25–35 injections into the amygdala of young F344 rats. Neurobiol. Aging 17, 893–901 (1996). [DOI] [PubMed] [Google Scholar]
- 42.Takashima A et al. Exposure of rat hippocampal neurons to amyloid beta peptide (25–35) induces the inactivation of phosphatidyl inositol-3 kinase and the activation of tau protein kinase I/glycogen synthase kinase-3 beta. Neurosci. Lett. 203, 33–36 (1996). [DOI] [PubMed] [Google Scholar]
- 43.Terwel D et al. Amyloid activates GSK-3beta to aggravate neuronal tauopathy in bigenic mice. Am. J. Pathol. 172, 786–798, doi: 10.2353/ajpath.2008.070904 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Town T et al. p35/Cdk5 pathway mediates soluble amyloid-beta peptide-induced tau phosphorylation in vitro. J. Neurosci. Res. 69, 362–372, doi: 10.1002/jnr.10299 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Augustinack JC, Schneider A, Mandelkow EM & Hyman BT Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol. 103, 26–35 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Luna-Munoz J, Chavez-Macias L, Garcia-Sierra F & Mena R Earliest stages of tau conformational changes are related to the appearance of a sequence of specific phospho-dependent tau epitopes in Alzheimer’s disease. J. Alzheimers Dis. 12, 365–375 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Wesseling H et al. Tau PTM Profiles Identify Patient Heterogeneity and Stages of Alzheimer’s Disease. Cell 183, 1699–1713 e1613, doi: 10.1016/j.cell.2020.10.029 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moloney CM et al. Phosphorylated tau sites that are elevated in Alzheimer’s disease fluid biomarkers are visualized in early neurofibrillary tangle maturity levels in the post mortem brain. Alzheimer’s & dementia : the journal of the Alzheimer’s Association, doi: 10.1002/alz.12749 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Braak H, Thal DR, Ghebremedhin E & Del Tredici K Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 70, 960–969, doi: 10.1097/NEN.0b013e318232a379 (2011). [DOI] [PubMed] [Google Scholar]
- 50.Neddens J et al. Phosphorylation of different tau sites during progression of Alzheimer’s disease. Acta neuropathologica communications 6, 52, doi: 10.1186/s40478-018-0557-6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kimura S & Shiota K Sequential changes of programmed cell death in developing fetal mouse limbs and its possible roles in limb morphogenesis. J. Morphol. 229, 337–346, doi: (1996). [DOI] [PubMed] [Google Scholar]
- 52.Mair W et al. FLEXITau: Quantifying Post-translational Modifications of Tau Protein in Vitro and in Human Disease. Anal. Chem. 88, 3704–3714, doi: 10.1021/acs.analchem.5b04509 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Regalado-Reyes M et al. Phospho-Tau Changes in the Human CA1 During Alzheimer’s Disease Progression. J. Alzheimers Dis. 69, 277–288, doi: 10.3233/JAD-181263 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Samimi N et al. Distinct phosphorylation profiles of tau in brains of patients with different tauopathies. Neurobiol. Aging 108, 72–79, doi: 10.1016/j.neurobiolaging.2021.08.011 (2021). [DOI] [PubMed] [Google Scholar]
- 55.Falcon B et al. Structures of filaments from Pick’s disease reveal a novel tau protein fold. Nature 561, 137–140, doi: 10.1038/s41586-018-0454-y (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noble W, Hanger DP, Miller CC & Lovestone S The importance of tau phosphorylation for neurodegenerative diseases. Front. Neurol. 4, 83, doi: 10.3389/fneur.2013.00083 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xia Y, Prokop S & Giasson BI “Don’t Phos Over Tau”: recent developments in clinical biomarkers and therapies targeting tau phosphorylation in Alzheimer’s disease and other tauopathies. Mol. Neurodegener. 16, 37, doi: 10.1186/s13024-021-00460-5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caballero B et al. Acetylated tau inhibits chaperone-mediated autophagy and promotes tau pathology propagation in mice. Nature communications 12, 2238, doi: 10.1038/s41467-021-22501-9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alquezar C et al. TSC1 loss increases risk for tauopathy by inducing tau acetylation and preventing tau clearance via chaperone-mediated autophagy. Sci Adv 7, eabg3897, doi: 10.1126/sciadv.abg3897 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alquezar C, Arya S & Kao AW Tau Post-translational Modifications: Dynamic Transformers of Tau Function, Degradation, and Aggregation. Front. Neurol. 11, 595532, doi: 10.3389/fneur.2020.595532 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Min SW et al. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat. Med. 21, 1154–1162, doi: 10.1038/nm.3951 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shin MK et al. Reducing acetylated tau is neuroprotective in brain injury. Cell 184, 2715–2732 e2723, doi: 10.1016/j.cell.2021.03.032 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vlad SC, Miller DR, Kowall NW & Felson DT Protective effects of NSAIDs on the development of Alzheimer disease. Neurology 70, 1672–1677, doi: 10.1212/01.wnl.0000311269.57716.63 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Craen AJ, Gussekloo J, Vrijsen B & Westendorp RG Meta-analysis of nonsteroidal antiinflammatory drug use and risk of dementia. Am. J. Epidemiol. 161, 114–120, doi: 10.1093/aje/kwi029 (2005). [DOI] [PubMed] [Google Scholar]
- 65.Camu F, Van de Velde A & Vanlersberghe C Nonsteroidal anti-inflammatory drugs and paracetamol in children. Acta Anaesthesiol. Belg. 52, 13–20 (2001). [PubMed] [Google Scholar]
- 66.Quinn JP, Corbett NJ, Kellett KAB & Hooper NM Tau Proteolysis in the Pathogenesis of Tauopathies: Neurotoxic Fragments and Novel Biomarkers. J. Alzheimers Dis. 63, 13–33, doi: 10.3233/JAD-170959 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Plouffe V et al. Hyperphosphorylation and cleavage at D421 enhance tau secretion. PLoS One 7, e36873, doi: 10.1371/journal.pone.0036873 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Noble W et al. Minocycline reduces the development of abnormal tau species in models of Alzheimer’s disease. FASEB J. 23, 739–750, doi: 10.1096/fj.08-113795 (2009). [DOI] [PubMed] [Google Scholar]
- 69.Tan MS, Liu Y, Hu H, Tan CC & Tan L Inhibition of caspase-1 ameliorates tauopathy and rescues cognitive impairment in SAMP8 mice. Metab. Brain Dis. 37, 1197–1205, doi: 10.1007/s11011-022-00914-9 (2022). [DOI] [PubMed] [Google Scholar]
- 70.Flores J, Noel A, Foveau B, Beauchet O & LeBlanc AC Pre-symptomatic Caspase-1 inhibitor delays cognitive decline in a mouse model of Alzheimer disease and aging. Nature communications 11, 4571, doi: 10.1038/s41467-020-18405-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Flores J et al. Caspase-1 inhibition alleviates cognitive impairment and neuropathology in an Alzheimer’s disease mouse model. Nature communications 9, 3916, doi: 10.1038/s41467-018-06449-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cantrelle FX et al. Phosphorylation and O-GlcNAcylation of the PHF-1 Epitope of Tau Protein Induce Local Conformational Changes of the C-Terminus and Modulate Tau Self-Assembly Into Fibrillar Aggregates. Front. Mol. Neurosci. 14, 661368, doi: 10.3389/fnmol.2021.661368 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arakhamia T et al. Posttranslational Modifications Mediate the Structural Diversity of Tauopathy Strains. Cell 180, 633–644 e612, doi: 10.1016/j.cell.2020.01.027 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oakley SS et al. Tau Filament Self-Assembly and Structure: Tau as a Therapeutic Target. Front. Neurol. 11, 590754, doi: 10.3389/fneur.2020.590754 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sanders DW et al. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron 82, 1271–1288, doi: 10.1016/j.neuron.2014.04.047 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ferrer I, Andres-Benito P, Carmona M & Del Rio JA Common and Specific Marks of Different Tau Strains Following Intra-Hippocampal Injection of AD, PiD, and GGT Inoculum in hTau Transgenic Mice. International journal of molecular sciences 23, doi: 10.3390/ijms232415940 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weitzman SA et al. Insoluble Tau From Human FTDP-17 Cases Exhibit Unique Transmission Properties In Vivo. J. Neuropathol. Exp. Neurol. 79, 941–949, doi: 10.1093/jnen/nlaa086 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaufman SK et al. Tau Prion Strains Dictate Patterns of Cell Pathology, Progression Rate, and Regional Vulnerability In Vivo. Neuron 92, 796–812, doi: 10.1016/j.neuron.2016.09.055 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clavaguera F et al. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc. Natl. Acad. Sci. U. S. A. 110, 9535–9540, doi: 10.1073/pnas.1301175110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Niewiadomska G, Niewiadomski W, Steczkowska M & Gasiorowska A Tau Oligomers Neurotoxicity. Life (Basel) 11, doi: 10.3390/life11010028 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gerson JE, Mudher A & Kayed R Potential mechanisms and implications for the formation of tau oligomeric strains. Crit. Rev. Biochem. Mol. Biol. 51, 482–496, doi: 10.1080/10409238.2016.1226251 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shafiei SS, Guerrero-Munoz MJ & Castillo-Carranza DL Tau Oligomers: Cytotoxicity, Propagation, and Mitochondrial Damage. Front. Aging Neurosci. 9, 83, doi: 10.3389/fnagi.2017.00083 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cardenas-Aguayo Mdel C, Gomez-Virgilio L, DeRosa S & Meraz-Rios MA The role of tau oligomers in the onset of Alzheimer’s disease neuropathology. ACS Chem. Neurosci. 5, 1178–1191, doi: 10.1021/cn500148z (2014). [DOI] [PubMed] [Google Scholar]
- 84.Guerrero-Munoz MJ, Gerson J & Castillo-Carranza DL Tau Oligomers: The Toxic Player at Synapses in Alzheimer’s Disease. Front. Cell. Neurosci. 9, 464, doi: 10.3389/fncel.2015.00464 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cheng Y & Bai F The Association of Tau With Mitochondrial Dysfunction in Alzheimer’s Disease. Front. Neurosci. 12, 163, doi: 10.3389/fnins.2018.00163 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Montalbano M et al. Tau oligomers mediate aggregation of RNA-binding proteins Musashi1 and Musashi2 inducing Lamin alteration. Aging cell 18, e13035, doi: 10.1111/acel.13035 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Otero-Garcia M et al. Molecular signatures underlying neurofibrillary tangle susceptibility in Alzheimer’s disease. Neuron 110, 2929–2948 e2928, doi: 10.1016/j.neuron.2022.06.021 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vazquez A Metabolic states following accumulation of intracellular aggregates: implications for neurodegenerative diseases. PLoS One 8, e63822, doi: 10.1371/journal.pone.0063822 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mandelkow EM, Stamer K, Vogel R, Thies E & Mandelkow E Clogging of axons by tau, inhibition of axonal traffic and starvation of synapses. Neurobiol. Aging 24, 1079–1085 (2003). [DOI] [PubMed] [Google Scholar]
- 90.Brion JP & Flament-Durand J Distribution and expression of the alpha-tubulin mRNA in the hippocampus and the temporal cortex in Alzheimer’s disease. Pathol. Res. Pract. 191, 490–498, doi: 10.1016/s0344-0338(11)80867-8 (1995). [DOI] [PubMed] [Google Scholar]
- 91.Cisek K, Cooper GL, Huseby CJ & Kuret J Structure and mechanism of action of tau aggregation inhibitors. Current Alzheimer research 11, 918–927, doi: 10.2174/1567205011666141107150331 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dominguez-Meijide A, Vasili E & Outeiro TF Pharmacological Modulators of Tau Aggregation and Spreading. Brain Sci 10, doi: 10.3390/brainsci10110858 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aillaud I & Funke SA Tau Aggregation Inhibiting Peptides as Potential Therapeutics for Alzheimer Disease. Cell. Mol. Neurobiol, doi: 10.1007/s10571-022-01230-7 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martinez-Hernandez J et al. Crosstalk between acetylation and the tyrosination/detyrosination cycle of alpha-tubulin in Alzheimer’s disease. Front Cell Dev Biol 10, 926914, doi: 10.3389/fcell.2022.926914 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang F et al. Posttranslational modifications of alpha-tubulin in alzheimer disease. Translational neurodegeneration 4, 9, doi: 10.1186/s40035-015-0030-4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rajaei S et al. Conformational change and GTPase activity of human tubulin: A comparative study on Alzheimer’s disease and healthy brain. J. Neurochem. 155, 207–224, doi: 10.1111/jnc.15009 (2020). [DOI] [PubMed] [Google Scholar]
- 97.Peris L et al. Tubulin tyrosination regulates synaptic function and is disrupted in Alzheimer’s disease. Brain 145, 2486–2506, doi: 10.1093/brain/awab436 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vu HT, Akatsu H, Hashizume Y, Setou M & Ikegami K Increase in alpha-tubulin modifications in the neuronal processes of hippocampal neurons in both kainic acid-induced epileptic seizure and Alzheimer’s disease. Sci. Rep. 7, 40205, doi: 10.1038/srep40205 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Caponio DV, Zhang K, Shi S, Wong L, Vyhnalek G, Fang M, F E. Compromised autophagy and mitophagy in brain ageing and Alzheimer’s diseases. Aging Brain 2, doi: 10.1016/j.nbas.2022.100056 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Filippone A, Esposito E, Mannino D, Lyssenko N & Pratico D The contribution of altered neuronal autophagy to neurodegeneration. Pharmacol. Ther. 238, 108178, doi: 10.1016/j.pharmthera.2022.108178 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nixon RA The aging lysosome: An essential catalyst for late-onset neurodegenerative diseases. Biochim Biophys Acta Proteins Proteom 1868, 140443, doi: 10.1016/j.bbapap.2020.140443 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bourdenx M et al. Chaperone-mediated autophagy prevents collapse of the neuronal metastable proteome. Cell 184, 2696–2714 e2625, doi: 10.1016/j.cell.2021.03.048 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee JH et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell 141, 1146–1158, doi: 10.1016/j.cell.2010.05.008 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Urbanelli L et al. Cathepsin D expression is decreased in Alzheimer’s disease fibroblasts. Neurobiol. Aging 29, 12–22, doi: 10.1016/j.neurobiolaging.2006.09.005 (2008). [DOI] [PubMed] [Google Scholar]
- 105.Mori H, Kondo J & Ihara Y Ubiquitin is a component of paired helical filaments in Alzheimer’s disease. Science 235, 1641–1644 (1987). [DOI] [PubMed] [Google Scholar]
- 106.Keller JN, Hanni KB & Markesbery WR Impaired proteasome function in Alzheimer’s disease. J. Neurochem. 75, 436–439 (2000). [DOI] [PubMed] [Google Scholar]
- 107.Perry G, Friedman R, Shaw G & Chau V Ubiquitin is detected in neurofibrillary tangles and senile plaque neurites of Alzheimer disease brains. Proc. Natl. Acad. Sci. U. S. A. 84, 3033–3036 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Perez M et al. Tau--an inhibitor of deacetylase HDAC6 function. J. Neurochem. 109, 1756–1766, doi: 10.1111/j.1471-4159.2009.06102.x (2009). [DOI] [PubMed] [Google Scholar]
- 109.Li MZ et al. Intracellular accumulation of tau inhibits autophagosome formation by activating TIA1-amino acid-mTORC1 signaling. Mil Med Res 9, 38, doi: 10.1186/s40779-022-00396-x (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Feng Q et al. MAPT/Tau accumulation represses autophagy flux by disrupting IST1-regulated ESCRT-III complex formation: a vicious cycle in Alzheimer neurodegeneration. Autophagy, 1–18, doi: 10.1080/15548627.2019.1633862 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Funk KE, Mrak RE & Kuret J Granulovacuolar degeneration (GVD) bodies of Alzheimer’s disease (AD) resemble late-stage autophagic organelles. Neuropathol. Appl. Neurobiol. 37, 295–306, doi: 10.1111/j.1365-2990.2010.01135.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Midani-Kurcak JS, Dinekov M, Puladi B, Arzberger T & Kohler C Effect of tau-pathology on charged multivesicular body protein 2b (CHMP2B). Brain Res. 1706, 224–236, doi: 10.1016/j.brainres.2018.11.008 (2019). [DOI] [PubMed] [Google Scholar]
- 113.Yamazaki Y et al. Immunopositivity for ESCRT-III subunit CHMP2B in granulovacuolar degeneration of neurons in the Alzheimer’s disease hippocampus. Neurosci. Lett. 477, 86–90, doi: 10.1016/j.neulet.2010.04.038 (2010). [DOI] [PubMed] [Google Scholar]
- 114.Jones EM et al. Interaction of tau protein with model lipid membranes induces tau structural compaction and membrane disruption. Biochemistry 51, 2539–2550, doi: 10.1021/bi201857v (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Calafate S, Flavin W, Verstreken P & Moechars D Loss of Bin1 Promotes the Propagation of Tau Pathology. Cell reports 17, 931–940, doi: 10.1016/j.celrep.2016.09.063 (2016). [DOI] [PubMed] [Google Scholar]
- 116.Caballero B et al. Interplay of pathogenic forms of human tau with different autophagic pathways. Aging cell 17, doi: 10.1111/acel.12692 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Polanco JC, Hand GR, Briner A, Li C & Gotz J Exosomes induce endolysosomal permeabilization as a gateway by which exosomal tau seeds escape into the cytosol. Acta Neuropathol. 141, 235–256, doi: 10.1007/s00401-020-02254-3 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Flavin WP et al. Endocytic vesicle rupture is a conserved mechanism of cellular invasion by amyloid proteins. Acta Neuropathol. 134, 629–653, doi: 10.1007/s00401-017-1722-x (2017). [DOI] [PubMed] [Google Scholar]
- 119.Chen JJ et al. Compromised function of the ESCRT pathway promotes endolysosomal escape of tau seeds and propagation of tau aggregation. J. Biol. Chem. 294, 18952–18966, doi: 10.1074/jbc.RA119.009432 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Scoles DR, Minikel EV & Pulst SM Antisense oligonucleotides: A primer. Neurology Genetics 5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alzforum Alzheimer’s Association International Conference Report. Antisense Therapy Stifles CSF Tau in Mild Alzheimer’s Disease. https://www.alzforum.org/news/conference-coverage/antisense-therapy-stifles-csf-tau-mild-alzheimers-disease-0 (2021).
- 122.Alzforum International Conference on Alzheimer’s and Parkinson’s Diseases 2023. First Hit on Aggregated Tau: Antisense Oligonucleotide Lowers Tangles. https://www.alzforum.org/news/conference-coverage/first-hit-aggregated-tau-antisense-oligonucleotide-lowers-tangles (07 Apr 2023).
- 123.Mummery CJ et al. Tau-targeting antisense oligonucleotide MAPT(Rx) in mild Alzheimer’s disease: a phase 1b, randomized, placebo-controlled trial. Nat. Med. 29, 1437–1447, doi: 10.1038/s41591-023-02326-3 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chohan MO, Khatoon S, Iqbal IG & Iqbal K Involvement of I2PP2A in the abnormal hyperphosphorylation of tau and its reversal by Memantine. FEBS Lett. 580, 3973–3979, doi: 10.1016/j.febslet.2006.06.021 (2006). [DOI] [PubMed] [Google Scholar]
- 125.Corcoran NM et al. Sodium selenate specifically activates PP2A phosphatase, dephosphorylates tau and reverses memory deficits in an Alzheimer’s disease model. J. Clin. Neurosci. 17, 1025–1033, doi: 10.1016/j.jocn.2010.04.020 (2010). [DOI] [PubMed] [Google Scholar]
- 126.Rueli RHLH e. a. Selenprotein S reduces endoplasmic reticulum stress-induced phosphorylation of tau: potential selenate mitigation of tau pathology. J Alz Dis 55, 749–762 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.van Eersel J et al. Sodium selenate mitigates tau pathology, neurodegeneration, and functional deficits in Alzheimer’s disease models. P Natl Acad Sci USA 107, 13888–13893, doi: 10.1073/pnas.1009038107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Malpas CB e. a. A Phase IIa randomized control trial of VEL (sodium selenate) in mild-moderate Alzheiemr’s disease. J. Alzheimers Dis. 54, 223–232 (2016). [DOI] [PubMed] [Google Scholar]
- 129.Vivash L et al. A phase 1b open-label study of sodium selenate as a disease-modifying treatment for possible behavioral variant frontotemporal dementia. Alzheimers Dement (N Y) 8, e12299, doi: 10.1002/trc2.12299 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vivash L et al. A study protocol for a phase II randomised, double-blind, placebo-controlled trial of sodium selenate as a disease-modifying treatment for behavioural variant frontotemporal dementia. BMJ Open 10, e040100, doi: 10.1136/bmjopen-2020-040100 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vivash L et al. Sodium selenate as a disease-modifying treatment for progressive supranuclear palsy: protocol for a phase 2, randomised, double-blind, placebo-controlled trial. BMJ Open 11, e055019, doi: 10.1136/bmjopen-2021-055019 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Muñoz-B. S, Tornero-Écija AR, Vincent O & Escalante R. VPS13A is closely associated with mitochondria and is required for efficient lysosomal degradation. Disease models & mechanisms 12, dmm036681 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fu Z-Q et al. LiCl attenuates thapsigargin-induced tau hyperphosphorylation by inhibiting GSK-3β in vivo and in vitro. Journal of Alzheimer’s Disease 21, 1107–1117 (2010). [DOI] [PubMed] [Google Scholar]
- 134.Duthie A et al. Recruitment, retainment, and biomarkers of response; a pilot trial of lithium in humans with mild cognitive impairment. Frontiers in molecular neuroscience 12, 163 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.VandeVrede L et al. Open-Label Phase 1 Futility Studies of Salsalate and Young Plasma in Progressive Supranuclear Palsy. Movement Disorders Clinical Practice 7, 440–447 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Robertson LA, Moya KL & Breen KC The potential role of tau protein O-glycosylation in Alzheimer’s disease. Journal of Alzheimer’s Disease 6, 489–495 (2004). [DOI] [PubMed] [Google Scholar]
- 137.Liu F, Iqbal K, Grundke-Iqbal I, Hart GW & Gong C-X O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer’s disease. Proceedings of the National Academy of Sciences 101, 10804–10809 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Therapeutics Alzforum. ASN120290. https://www.alzforum.org/therapeutics/asn90 (Accessed 2023).
- 139.Ryan JM, Quattropani A, Abd-Elaziz K, den Daas I, Schneider M, Ousson S, Neny M, Sand A, Hantson J, Permanne B, Beher D PHASE 1 STUDY IN HEALTHY VOLUNTEERS OF THE O-GLCNACASE INHIBITOR ASN120290 AS A NOVEL THERAPY FOR PROGRESSIVE SUPRANUCLEAR PALSY AND RELATED TAUOPATHIES. Alzheimer’s Association International Conference O1–12-05 Philadelphia, Pennsylvania (2018). [Google Scholar]
- 140.Shcherbinin S et al. Brain target occupancy of LY3372689, an inhibitor of the O-GlcNAcase (OGA) enzyme: Translation from rat to human: Neuroimaging/evaluating treatments. Alzheimer’s & Dementia 16, e040558 (2020). [Google Scholar]
- 141.Alzforum Therapeutics. LY3372689. https://www.alzforum.org/therapeutics/ly3372689 (Accessed 2023).
- 142.Kielbasa W et al. Brain target occupancy of LY3372689, an inhibitor of the O-GlcNAcase (OGA) enzyme, following administration of single and multiple doses to healthy volunteers. Alzheimer’s & Dementia 17, e057774 (2021). [Google Scholar]
- 143.Kielbasa W et al. A single ascending dose study in healthy volunteers to assess the safety and PK of LY3372689, an inhibitor of O-GlcNAcase (OGA) enzyme: Human/Human trials: Anti-tau. Alzheimer’s & Dementia 16, e040473 (2020). [Google Scholar]
- 144.Lowe SL et al. Single and multiple ascending dose studies in healthy volunteers to assess the safety and PK of LY3372689, an inhibitor of the O-GlcNAcase (OGA) enzyme. Alzheimer’s & Dementia 17, e057728 (2021). [Google Scholar]
- 145.Howard R et al. Minocycline at 2 Different Dosages vs Placebo for Patients With Mild Alzheimer Disease: A Randomized Clinical Trial. JAMA neurology 77, 164–174, doi: 10.1001/jamaneurol.2019.3762 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ma Q-L et al. Curcumin suppresses soluble tau dimers and corrects molecular chaperone, synaptic, and behavioral deficits in aged human tau transgenic mice. Journal of Biological Chemistry 288, 4056–4065 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rane JS, Bhaumik P & Panda D Curcumin inhibits tau aggregation and disintegrates preformed tau filaments in vitro. Journal of Alzheimer’s Disease 60, 999–1014 (2017). [DOI] [PubMed] [Google Scholar]
- 148.Goel A, Kunnumakkara AB & Aggarwal BB Curcumin as “Curecumin”: from kitchen to clinic. Biochemical pharmacology 75, 787–809 (2008). [DOI] [PubMed] [Google Scholar]
- 149.Small GW et al. Memory and Brain Amyloid and Tau Effects of a Bioavailable Form of Curcumin in Non-Demented Adults: A Double-Blind, Placebo-Controlled 18-Month Trial. Am. J. Geriatr. Psychiatry 26, 266–277, doi: 10.1016/j.jagp.2017.10.010 (2018). [DOI] [PubMed] [Google Scholar]
- 150.Hosokawa M et al. Methylene blue reduced abnormal tau accumulation in P301L tau transgenic mice. PLoS One 7, e52389, doi: 10.1371/journal.pone.0052389 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hochgräfe K et al. Preventive methylene blue treatment preserves cognition in mice expressing full-length pro-aggregant human Tau. Acta neuropathologica communications 3, 1–22 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gauthier S et al. Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer’s disease: a randomised, controlled, double-blind, parallel-arm, phase 3 trial. Lancet 388, 2873–2884, doi: 10.1016/S0140-6736(16)31275-2 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wilcock GK et al. Potential of Low Dose Leuco-Methylthioninium Bis(Hydromethanesulphonate) (LMTM) Monotherapy for Treatment of Mild Alzheimer’s Disease: Cohort Analysis as Modified Primary Outcome in a Phase III Clinical Trial. J. Alzheimers Dis. 61, 435–457, doi: 10.3233/JAD-170560 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Alzforum Clinical Trials on Alzheimer’s Disease. Tau Inhibitor Fails Again—Subgroup Analysis Irks Clinicians at CTAD. https://www.alzforum.org/news/conference-coverage/tau-inhibitor-fails-again-subgroup-analysis-irks-clinicians-ctad (16 Dec 2016).
- 155.Alzforum Alzheimer’s Association International Conference Report. In First Phase 3 Trial, the Tau Drug LMTM Did Not Work. Period. http://www.alzforum.org/news/conference-coverage/first-phase-3-trial-tau-drug-lmtm-did-not-work-period#show-more (29 July 2016). [Google Scholar]
- 156.Alzforum International Conference on Frontotemporal Dementias. First Round of FTD Therapeutics Fell Short, But Many More Are Up and Running. https://www.alzforum.org/news/conference-coverage/first-round-ftd-therapeutics-fell-short-many-more-are-and-running (27 Sep 2016). [Google Scholar]
- 157.Alzforum therapeutics. ACI-3024. https://www.alzforum.org/therapeutics/aci-3024 (Accessed 2023).
- 158.Fitzgerald DP et al. TPI-287, a New Taxane Family Member, Reduces the Brain Metastatic Colonization of Breast Cancer CellsTPI-287 Reduces Brain Metastatic Colonization. Molecular cancer therapeutics 11, 1959–1967 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tsai RM et al. Reactions to Multiple Ascending Doses of the Microtubule Stabilizer TPI-287 in Patients With Alzheimer Disease, Progressive Supranuclear Palsy, and Corticobasal Syndrome: A Randomized Clinical Trial. JAMA neurology 77, 215–224, doi: 10.1001/jamaneurol.2019.3812 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Magen I & Gozes I Microtubule-stabilizing peptides and small molecules protecting axonal transport and brain function: focus on davunetide (NAP). Neuropeptides 47, 489–495 (2013). [DOI] [PubMed] [Google Scholar]
- 161.Asuni AA, Quartermain D, Sigurdsson EM Tau-based immunotherapy for dementia. Alzheimer Dement 2, S40–41 (2006). [Google Scholar]
- 162.Asuni AA, Boutajangout A, Quartermain D & Sigurdsson EM Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J. Neurosci. 27, 9115–9129, doi: 10.1523/JNEUROSCI.2361-07.2007 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Boutajangout A, Ingadottir J, Davies P, Sigurdsson EM Passive tau immuntherapy diminishes functional decline and clears tau aggregates in a mouse model of tauopathy. Alzheimer Dement 6, S578 (2010). [Google Scholar]
- 164.Boutajangout A, Ingadottir J, Davies P & Sigurdsson EM Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. J. Neurochem. 118, 658–667, doi: 10.1111/j.1471-4159.2011.07337.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bittar A, Bhatt N & Kayed R Advances and considerations in AD tau-targeted immunotherapy. Neurobiol. Dis. 134, 104707, doi: 10.1016/j.nbd.2019.104707 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Colin M et al. From the prion-like propagation hypothesis to therapeutic strategies of anti-tau immunotherapy. Acta Neuropathol. 139, 3–25, doi: 10.1007/s00401-019-02087-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Congdon EE, Jiang Y & Sigurdsson EM Targeting tau only extracellularly is likely to be less efficacious than targeting it both intra- and extracellularly. Semin. Cell Dev. Biol. 126, 125–137, doi: 10.1016/j.semcdb.2021.12.002 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ji C & Sigurdsson EM Current Status of Clinical Trials on Tau Immunotherapies. Drugs 81, 1135–1152, doi: 10.1007/s40265-021-01546-6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sandusky-Beltran LA & Sigurdsson EM Tau immunotherapies: Lessons learned, current status and future considerations. Neuropharmacology 175, 108104, doi: 10.1016/j.neuropharm.2020.108104 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ng PY, Chang IS, Koh RY & Chye SM Recent advances in tau-directed immunotherapy against Alzheimer’s disease: an overview of pre-clinical and clinical development. Metab. Brain Dis. 35, 1049–1066, doi: 10.1007/s11011-020-00591-6 (2020). [DOI] [PubMed] [Google Scholar]
- 171.Karimi N, Bayram Catak F, Arslan E, Saghazadeh A & Rezaei N Tau immunotherapy in Alzheimer’s disease and progressive supranuclear palsy. Int. Immunopharmacol. 113, 109445, doi: 10.1016/j.intimp.2022.109445 (2022). [DOI] [PubMed] [Google Scholar]
- 172.Guo Y, Li S, Zeng L-H & Tan J Tau-targeting therapy in Alzheimer’s disease: critical advances and future opportunities. Ageing and Neurodegenerative Diseases 2, 11, doi: 10.20517/and.2022.16 (2022). [DOI] [Google Scholar]
- 173.Rosenmann H et al. Tauopathy-like abnormalities and neurologic deficits in mice immunized with neuronal tau protein. Arch. Neurol. 63, 1459–1467, doi: 10.1001/archneur.63.10.1459 (2006). [DOI] [PubMed] [Google Scholar]
- 174.Rozenstein-Tsalkovich L et al. Repeated immunization of mice with phosphorylated-tau peptides causes neuroinflammation. Exp. Neurol. 248, 451–456, doi: 10.1016/j.expneurol.2013.07.006 (2013). [DOI] [PubMed] [Google Scholar]
- 175.Rajamohamedsait H, Rasool S, Rajamohamedsait W, Lin Y & Sigurdsson EM Prophylactic Active Tau Immunization Leads to Sustained Reduction in Both Tau and Amyloid-beta Pathologies in 3xTg Mice. Sci. Rep. 7, 17034, doi: 10.1038/s41598-017-17313-1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nicholls SB et al. Characterization of TauC3 antibody and demonstration of its potential to block tau propagation. PLoS One 12, e0177914, doi: 10.1371/journal.pone.0177914 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nobuhara CK et al. Tau Antibody Targeting Pathological Species Blocks Neuronal Uptake and Interneuron Propagation of Tau in Vitro. Am. J. Pathol. 187, 1399–1412, doi: 10.1016/j.ajpath.2017.01.022 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Roberts M et al. Pre-clinical characterisation of E2814, a high-affinity antibody targeting the microtubule-binding repeat domain of tau for passive immunotherapy in Alzheimer’s disease. Acta neuropathologica communications 8, 13, doi: 10.1186/s40478-020-0884-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rosenqvist N et al. Highly specific and selective anti-pS396-tau antibody C10.2 targets seeding-competent tau. Alzheimers Dement (N Y) 4, 521–534, doi: 10.1016/j.trci.2018.09.005 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yanamandra K et al. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron 80, 402–414, doi: 10.1016/j.neuron.2013.07.046 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Funk KE, Mirbaha H, Jiang H, Holtzman DM & Diamond MI Distinct Therapeutic Mechanisms of Tau Antibodies: PROMOTING MICROGLIAL CLEARANCE VERSUS BLOCKING NEURONAL UPTAKE. J. Biol. Chem. 290, 21652–21662, doi: 10.1074/jbc.M115.657924 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Congdon EE, Chukwu JE, Shamir DB, Deng J, Ujla D, Sait HBR, Neubert TA, Kong XP, Sigurdsson EM Tau antibody chimerization alters its charge and binding, thereby reduces its cellular uptake and efficacy. eBioMedicine 42, 157–173 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Congdon EE et al. Affinity of Tau antibodies for solubilized pathological Tau species but not their immunogen or insoluble Tau aggregates predicts in vivo and ex vivo efficacy. Mol. Neurodegener. 11, 62–86, doi: 10.1186/s13024-016-0126-z (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Shamir DB et al. Dynamics of Internalization and Intracellular Interaction of Tau Antibodies and Human Pathological Tau Protein in a Human Neuron-Like Model. Front. Neurol. 11, 602292, doi: 10.3389/fneur.2020.602292 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kfoury N, Holmes BB, Jiang H, Holtzman DM & Diamond MI Trans-cellular propagation of Tau aggregation by fibrillar species. J. Biol. Chem. 287, 19440–19451, doi: 10.1074/jbc.M112.346072 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bright J et al. Human secreted tau increases amyloid-beta production. Neurobiol. Aging 36, 693–709, doi: 10.1016/j.neurobiolaging.2014.09.007 (2015). [DOI] [PubMed] [Google Scholar]
- 187.Castillo-Carranza DL et al. Passive immunization with Tau oligomer monoclonal antibody reverses tauopathy phenotypes without affecting hyperphosphorylated neurofibrillary tangles. J. Neurosci. 34, 4260–4272, doi: 10.1523/JNEUROSCI.3192-13.2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.d’Abramo C et al. Detecting tau in serum of transgenic animal models after tau immunotherapy treatment. Neurobiol. Aging 37, 58–65, doi: 10.1016/j.neurobiolaging.2015.09.017 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yanamandra K, Jiang H, Mahan TE, Maloney SE, Wozniak DF, Diamond M I, Holtzman DM Anti-tau antibody reduces insoluble tau and decreases brain atrophy. Ann Clin Transl Neurol 2, 278–288 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Luo W et al. Microglial internalization and degradation of pathological tau is enhanced by an anti-tau monoclonal antibody. Sci. Rep. 5, 11161, doi: 10.1038/srep11161 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Andersson CR et al. Antibody-mediated clearance of tau in primary mouse microglial cultures requires Fcgamma-receptor binding and functional lysosomes. Sci. Rep. 9, 4658, doi: 10.1038/s41598-019-41105-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zilkova M et al. Humanized tau antibodies promote tau uptake by human microglia without any increase of inflammation. Acta neuropathologica communications 8, 74, doi: 10.1186/s40478-020-00948-z (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lee SH et al. Antibody-Mediated Targeting of Tau In Vivo Does Not Require Effector Function and Microglial Engagement. Cell reports 16, 1690–1700, doi: 10.1016/j.celrep.2016.06.099 (2016). [DOI] [PubMed] [Google Scholar]
- 194.Mukadam AS et al. Cytosolic antibody receptor TRIM21 is required for effective tau immunotherapy in mouse models. Science 379, 1336–1341, doi: 10.1126/science.abn1366 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kim B et al. Tau immunotherapy is associated with glial responses in FTLD-tau. Acta Neuropathol. 142, 243–257, doi: 10.1007/s00401-021-02318-y (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Leyns CEG & Holtzman DM Glial contributions to neurodegeneration in tauopathies. Mol. Neurodegener. 12, 50, doi: 10.1186/s13024-017-0192-x (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Leng F & Edison P Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat. Rev. Neurol. 17, 157–172, doi: 10.1038/s41582-020-00435-y (2021). [DOI] [PubMed] [Google Scholar]
- 198.Uddin MS & Lim LW Glial cells in Alzheimer’s disease: From neuropathological changes to therapeutic implications. Ageing Res Rev 78, 101622, doi: 10.1016/j.arr.2022.101622 (2022). [DOI] [PubMed] [Google Scholar]
- 199.Serrano-Pozo A et al. Reactive glia not only associates with plaques but also parallels tangles in Alzheimer’s disease. Am. J. Pathol. 179, 1373–1384, doi: 10.1016/j.ajpath.2011.05.047 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dani M et al. Microglial activation correlates in vivo with both tau and amyloid in Alzheimer’s disease. Brain 141, 2740–2754, doi: 10.1093/brain/awy188 (2018). [DOI] [PubMed] [Google Scholar]
- 201.Ismail R et al. The relationships between neuroinflammation, beta-amyloid and tau deposition in Alzheimer’s disease: a longitudinal PET study. J. Neuroinflammation 17, 151, doi: 10.1186/s12974-020-01820-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Maeda J et al. In vivo positron emission tomographic imaging of glial responses to amyloid-beta and tau pathologies in mouse models of Alzheimer’s disease and related disorders. J. Neurosci. 31, 4720–4730, doi: 10.1523/JNEUROSCI.3076-10.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Appleton J, Funk Q, Bradbury K, Yu M, Faridar A, Beers D, Appel SH, Fujita M, Masdeu JC, Pascual B Neuroinflammation co-localizes highly with tau in amnestic mild cognitive impairment. Alzheimer Dement 18, doi:DOI: 10.1002/alz.068025 (2022). [DOI] [Google Scholar]
- 204.Hamelin L et al. Early and protective microglial activation in Alzheimer’s disease: a prospective study using 18F-DPA-714 PET imaging. Brain 139, 1252–1264, doi: 10.1093/brain/aww017 (2016). [DOI] [PubMed] [Google Scholar]
- 205.Hamelin L et al. Distinct dynamic profiles of microglial activation are associated with progression of Alzheimer’s disease. Brain 141, 1855–1870, doi: 10.1093/brain/awy079 (2018). [DOI] [PubMed] [Google Scholar]
- 206.Fan Z, Brooks DJ, Okello A & Edison P An early and late peak in microglial activation in Alzheimer’s disease trajectory. Brain 140, 792–803, doi: 10.1093/brain/aww349 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Femminella GD et al. Microglial activation in early Alzheimer trajectory is associated with higher gray matter volume. Neurology 92, e1331–e1343, doi: 10.1212/WNL.0000000000007133 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Congdon EE, Gu J, Sait HB & Sigurdsson EM Antibody uptake into neurons occurs primarily via clathrin-dependent Fcgamma receptor endocytosis and is a prerequisite for acute tau protein clearance. J. Biol. Chem. 288, 35452–35465, doi: 10.1074/jbc.M113.491001 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Gu J, Congdon EE & Sigurdsson EM Two novel Tau antibodies targeting the 396/404 region are primarily taken up by neurons and reduce Tau protein pathology. J. Biol. Chem. 288, 33081–33095, doi: 10.1074/jbc.M113.494922 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wu Q, Lin Y, Gu J & Sigurdsson EM Dynamic assessment of tau immunotherapies in the brains of live animals by two-photon imaging. EBioMedicine 35, 270–278, doi: 10.1016/j.ebiom.2018.08.041 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Collin L et al. Neuronal uptake of tau/pS422 antibody and reduced progression of tau pathology in a mouse model of Alzheimer’s disease. Brain 137, 2834–2846, doi: 10.1093/brain/awu213 (2014). [DOI] [PubMed] [Google Scholar]
- 212.McEwan WA et al. Cytosolic Fc receptor TRIM21 inhibits seeded tau aggregation. Proc. Natl. Acad. Sci. U. S. A. 114, 574–579, doi: 10.1073/pnas.1607215114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Krishnamurthy PK, Deng Y & Sigurdsson EM Mechanistic Studies of Antibody-Mediated Clearance of Tau Aggregates Using an ex vivo Brain Slice Model. Front Psychiatry 2, 59, doi: 10.3389/fpsyt.2011.00059 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kondo A et al. Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature 523, 431–436, doi: 10.1038/nature14658 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Krishnaswamy S et al. Antibody-derived in vivo imaging of tau pathology. J. Neurosci. 34, 16835–16850, doi: 10.1523/JNEUROSCI.2755-14.2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Shamir DB, Rosenqvist N, Rasool S, Pedersen JT & Sigurdsson EM Internalization of tau antibody and pathological tau protein detected with a flow cytometry multiplexing approach. Alzheimer’s and Dement 12, 1098–1107, doi: 10.1016/j.jalz.2016.01.013 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fuller JP, Stavenhagen JB & Teeling JL New roles for Fc receptors in neurodegeneration-the impact on Immunotherapy for Alzheimer’s Disease. Front. Neurosci. 8, 235, doi: 10.3389/fnins.2014.00235 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.van der Kleij H et al. Evidence for neuronal expression of functional Fc (epsilon and gamma) receptors. J. Allergy Clin. Immunol. 125, 757–760, doi: 10.1016/j.jaci.2009.10.054 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Nakamura K et al. CD3 and immunoglobulin G Fc receptor regulate cerebellar functions. Mol. Cell. Biol. 27, 5128–5134, doi: 10.1128/MCB.01072-06 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Stamou M, Grodzki AC, van Oostrum M, Wollscheid B & Lein PJ Fc gamma receptors are expressed in the developing rat brain and activate downstream signaling molecules upon cross-linking with immune complex. J. Neuroinflammation 15, 7, doi: 10.1186/s12974-017-1050-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Suemitsu S et al. Fcgamma receptors contribute to pyramidal cell death in the mouse hippocampus following local kainic acid injection. Neuroscience 166, 819–831, doi: 10.1016/j.neuroscience.2010.01.004 (2010). [DOI] [PubMed] [Google Scholar]
- 222.Andoh T & Kuraishi Y Direct action of immunoglobulin G on primary sensory neurons through Fc gamma receptor I. FASEB J. 18, 182–184, doi: 10.1096/fj.02-1169fje (2004). [DOI] [PubMed] [Google Scholar]
- 223.Andoh T & Kuraishi Y Expression of Fc epsilon receptor I on primary sensory neurons in mice. Neuroreport 15, 2029–2031 (2004). [DOI] [PubMed] [Google Scholar]
- 224.Qu L, Zhang P, LaMotte RH & Ma C Neuronal Fc-gamma receptor I mediated excitatory effects of IgG immune complex on rat dorsal root ganglion neurons. Brain. Behav. Immun. 25, 1399–1407, doi: 10.1016/j.bbi.2011.04.008 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Jiang H et al. Nociceptive neuronal Fc-gamma receptor I is involved in IgG immune complex induced pain in the rat. Brain. Behav. Immun. 62, 351–361, doi: 10.1016/j.bbi.2017.03.001 (2017). [DOI] [PubMed] [Google Scholar]
- 226.Wang L et al. Neuronal FcgammaRI mediates acute and chronic joint pain. J. Clin. Invest. 129, 3754–3769, doi: 10.1172/JCI128010 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chandupatla RR, Flatley A, Feederle R, Mandelkow EM & Kaniyappan S Novel antibody against low-n oligomers of tau protein promotes clearance of tau in cells via lysosomes. Alzheimers Dement (N Y) 6, e12097, doi: 10.1002/trc2.12097 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Masliah E et al. Effects of alpha-synuclein immunization in a mouse model of Parkinson’s disease. Neuron 46, 857–868, doi: 10.1016/j.neuron.2005.05.010 (2005). [DOI] [PubMed] [Google Scholar]
- 229.Masliah E et al. Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS One 6, e19338, doi: 10.1371/journal.pone.0019338 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 230.Pozzi S et al. Monoclonal full-length antibody against TAR DNA binding protein 43 reduces related proteinopathy in neurons. JCI Insight 5, doi: 10.1172/jci.insight.140420 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Karpiak SE & Mahadik SP Selective uptake by Purkinje neurons of antibodies to S-100 protein. Exp. Neurol. 98, 453–457, doi: 10.1016/0014-4886(87)90254-8 (1987). [DOI] [PubMed] [Google Scholar]
- 232.Fabian RH & Ritchie TC Intraneuronal IgG in the central nervous system. J. Neurol. Sci. 73, 257–267, doi: 10.1016/0022-510x(86)90150-4 (1986). [DOI] [PubMed] [Google Scholar]
- 233.Greenlee JE, Burns JB, Rose JW, Jaeckle KA & Clawson S Uptake of systemically administered human anticerebellar antibody by rat Purkinje cells following blood-brain barrier disruption. Acta Neuropathol. 89, 341–345, doi: 10.1007/BF00309627 (1995). [DOI] [PubMed] [Google Scholar]
- 234.Graus F et al. Effect of intraventricular injection of an anti-Purkinje cell antibody (anti-Yo) in a guinea pig model. J. Neurol. Sci. 106, 82–87, doi: 10.1016/0022-510x(91)90198-g (1991). [DOI] [PubMed] [Google Scholar]
- 235.Hill KE, Clawson SA, Rose JW, Carlson NG & Greenlee JE Cerebellar Purkinje cells incorporate immunoglobulins and immunotoxins in vitro: implications for human neurological disease and immunotherapeutics. J. Neuroinflammation 6, 31, doi: 10.1186/1742-2094-6-31 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Greenlee JE et al. Neuronal uptake of anti-Hu antibody, but not anti-Ri antibody, leads to cell death in brain slice cultures. J. Neuroinflammation 11, 160, doi: 10.1186/s12974-014-0160-0 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Greenlee JE et al. Purkinje cell death after uptake of anti-Yo antibodies in cerebellar slice cultures. J. Neuropathol. Exp. Neurol. 69, 997–1007, doi: 10.1097/NEN.0b013e3181f0c82b (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Rocchi A et al. Autoantibodies to synapsin I sequestrate synapsin I and alter synaptic function. Cell Death Dis. 10, 864, doi: 10.1038/s41419-019-2106-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Goldwaser EL et al. Evidence that Brain-Reactive Autoantibodies Contribute to Chronic Neuronal Internalization of Exogenous Amyloid-beta1–42 and Key Cell Surface Proteins During Alzheimer’s Disease Pathogenesis. J. Alzheimers Dis. 74, 345–361, doi: 10.3233/JAD-190962 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Gustafsson G et al. Cellular Uptake of alpha-Synuclein Oligomer-Selective Antibodies is Enhanced by the Extracellular Presence of alpha-Synuclein and Mediated via Fcgamma Receptors. Cell. Mol. Neurobiol. 37, 121–131, doi: 10.1007/s10571-016-0352-5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Nguyen L et al. Antibody Therapy Targeting RAN Proteins Rescues C9 ALS/FTD Phenotypes in C9orf72 Mouse Model. Neuron 105, 645–662 e611, doi: 10.1016/j.neuron.2019.11.007 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Benkler C et al. Aggregated SOD1 causes selective death of cultured human motor neurons. Sci. Rep. 8, 16393, doi: 10.1038/s41598-018-34759-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zaretsky DV, Zaretskaia MV & Molkov YI Membrane channel hypothesis of lysosomal permeabilization by beta-amyloid. Neurosci. Lett. 770, 136338, doi: 10.1016/j.neulet.2021.136338 (2022). [DOI] [PubMed] [Google Scholar]
- 244.Umeda T et al. Intraneuronal amyloid beta oligomers cause cell death via endoplasmic reticulum stress, endosomal/lysosomal leakage, and mitochondrial dysfunction in vivo. J. Neurosci. Res. 89, 1031–1042, doi: 10.1002/jnr.22640 (2011). [DOI] [PubMed] [Google Scholar]
- 245.Yang AJ, Chandswangbhuvana D, Margol L & Glabe CG Loss of endosomal/lysosomal membrane impermeability is an early event in amyloid Abeta1–42 pathogenesis. J. Neurosci. Res. 52, 691–698, doi: (1998). [DOI] [PubMed] [Google Scholar]
- 246.Lee JH et al. Faulty autolysosome acidification in Alzheimer’s disease mouse models induces autophagic build-up of Abeta in neurons, yielding senile plaques. Nat. Neurosci. 25, 688–701, doi: 10.1038/s41593-022-01084-8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ling D, Song HJ, Garza D, Neufeld TP & Salvaterra PM Abeta42-induced neurodegeneration via an age-dependent autophagic-lysosomal injury in Drosophila. PLoS One 4, e4201, doi: 10.1371/journal.pone.0004201 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Dingjan I et al. Lipid peroxidation causes endosomal antigen release for cross-presentation. Sci. Rep. 6, 22064, doi: 10.1038/srep22064 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zehner M et al. The translocon protein Sec61 mediates antigen transport from endosomes in the cytosol for cross-presentation to CD8(+) T cells. Immunity 42, 850–863, doi: 10.1016/j.immuni.2015.04.008 (2015). [DOI] [PubMed] [Google Scholar]
- 250.Embgenbroich M & Burgdorf S Current Concepts of Antigen Cross-Presentation. Front. Immunol. 9, 1643, doi: 10.3389/fimmu.2018.01643 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Gros M & Amigorena S Regulation of Antigen Export to the Cytosol During Cross-Presentation. Front. Immunol. 10, 41, doi: 10.3389/fimmu.2019.00041 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Abskharon R et al. Crystal structure of a conformational antibody that binds tau oligomers and inhibits pathological seeding by extracts from donors with Alzheimer’s disease. J. Biol. Chem. 295, 10662–10676, doi: 10.1074/jbc.RA120.013638 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Li S et al. A Single-Chain Variable Fragment Antibody Inhibits Aggregation of Phosphorylated Tau and Ameliorates Tau Toxicity in vitro and in vivo. J. Alzheimers Dis. 79, 1613–1629, doi: 10.3233/JAD-191266 (2021). [DOI] [PubMed] [Google Scholar]
- 254.Krishnaswamy S, Huang HW, Marchal IS, Ryoo HD & Sigurdsson EM Neuronally expressed anti-tau scFv prevents tauopathy-induced phenotypes in Drosophila models. Neurobiol. Dis. 137, 104770, doi: 10.1016/j.nbd.2020.104770 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kontsekova E, Zilka N, Kovacech B, Novak P & Novak M First-in-man tau vaccine targeting structural determinants essential for pathological tau-tau interaction reduces tau oligomerisation and neurofibrillary degeneration in an Alzheimer’s disease model. Alzheimers Res. Ther. 6, 44, doi: 10.1186/alzrt278 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Novak P et al. Safety and immunogenicity of the tau vaccine AADvac1 in patients with Alzheimer’s disease: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Neurol 16, 123–134, doi: 10.1016/s1474-4422(16)30331-3 (2017). [DOI] [PubMed] [Google Scholar]
- 257.Grossman M The non-fluent/agrammatic variant of primary progressive aphasia. Lancet Neurol. 11, 545–555, doi: 10.1016/S1474-4422(12)70099-6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Novak P et al. FUNDAMANT: an interventional 72-week phase 1 follow-up study of AADvac1, an active immunotherapy against tau protein pathology in Alzheimer’s disease. Alzheimers Res. Ther. 10, 108, doi: 10.1186/s13195-018-0436-1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Press release Axon Neuroscience. Axon announces positive results from Phase II ADAMANT trial for AADvac1 in Alzheimer’s Disease. https://www.axon-neuroscience.eu/docs/press_release_Axon_announces_positive_result_9-9-2019 (09 Sept 2019).
- 260.Novak P. KB, Katina S, Schmidt R, Scheltens P, Kontsekova E, Ropele S, Fialova L, Kramberger M, Paulenka-Ivanovova N, Smisek M, Hanes J, Stevens E, Kovac A, Sutovsky S, Parrak V, Koson P, Prcina M, Galba J, Cente M, Hromadka T, Filipcik P, Piestansky J, Samcova M, Prenn-Gologranc C, Sivak R, Froelich L, Fresser M, Rakusa M, Harrison J, Hort J, Otto M, Tosun D, Ondrus M, Winblad B, Novak M, Zilka N. ADAMANT: a placebo-controlled randomized phase 2 study of AADvac1, an active immunotherapy against pathological tau in Alzheimer’s disease. Nature Aging 1, 521–534 (2021). [DOI] [PubMed] [Google Scholar]
- 261.Alzforum AAT-AD/PD Focus Meeting 2020. Active Tau Vaccine: Hints of Slowing Neurodegeneration. https://www.alzforum.org/news/conference-coverage/active-tau-vaccine-hints-slowing-neurodegeneration (15 Apr 2020). [Google Scholar]
- 262.Hickman DT et al. Sequence-independent control of peptide conformation in liposomal vaccines for targeting protein misfolding diseases. J Biol Chem 286, 13966–13976, doi: 10.1074/jbc.M110.186338 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Press release AC Immune. AC Immune Announces Interim Phase 1b/2a Data Showing That Its ACI-35.030 Anti-PTau Alzheimer’s Vaccine Generates A Potent Immune Response. https://ir.acimmune.com/news-releases/news-release-details/ac-immune-announces-interim-phase-1b2a-data-showing-its-aci (12 Nov 2021). [Google Scholar]
- 264.Press release AC Immune. AC Immune ACI-35.030 Phase 1b/2a Trial Interim Data Confirm Consistent Safety And Potent Immunogenicity Of PTau Alzheimer’s Vaccine In High-Dose Cohort. https://ir.acimmune.com/news-releases/news-release-details/ac-immune-aci-35030-phase-1b2a-trial-interim-data-confirm (15 Feb 2022). [Google Scholar]
- 265.Press release AC Immune. AC Immune Advances Phospho-Tau Alzheimer’s Vaccine In Phase 1b/2a Study. https://ir.acimmune.com/news-releases/news-release-details/ac-immune-advances-phospho-tau-alzheimers-vaccine-phase-1b2a (16 Jul 2020). [Google Scholar]
- 266.Press release AC Immune. AC Immune Announces Expansion Of Phase 1b/2a Phospho-Tau Alzheimer’s Vaccine Trial And Provides A Program Update. https://ir.acimmune.com/news-releases/news-release-details/ac-immune-announces-expansion-phase-1b2a-phospho-tau-alzheimers (17 May 2021). [Google Scholar]
- 267.Press release AC Immune. AC Immune’s Alzheimer’s Vaccine Generates Potent Anti-PTau Antibody Response In A Phase 1b/2a Study. https://ir.acimmune.com/news-releases/news-release-details/ac-immunes-alzheimers-vaccine-generates-potent-anti-ptau (11 Feb 2021). [Google Scholar]
- 268.Press release AC Immune. AC Immune’s Alzheimer’s Disease Vaccine-Candidate ACI-35.030 Selected For Further Development. https://ir.acimmune.com/news-releases/news-release-details/ac-immunes-alzheimers-disease-vaccine-candidate-aci-35030 (30 Nov 2022). [Google Scholar]
- 269.Tai HC et al. The tau oligomer antibody APNmAb005 detects early-stage pathological tau enriched at synapses and rescues neuronal loss in long-term treatments. bioRxiv. (June 26, 2022). [Google Scholar]
- 270.Courade JP et al. Epitope determines efficacy of therapeutic anti-Tau antibodies in a functional assay with human Alzheimer Tau. Acta Neuropathol. 136, 729–745, doi: 10.1007/s00401-018-1911-2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Albert M et al. Prevention of tau seeding and propagation by immunotherapy with a central tau epitope antibody. Brain 142, 1736–1750, doi: 10.1093/brain/awz100 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Buchanan T, De Bruyn S, Fadini T, Watanabe S, Germani M, Boyden A, Rebollo Mesa I, Meno-Tetang G, Zanigni S, Colson A, Famodimu O, Ewan C A randomised, placebo-controlled, first-in-human study with a central Tau epitope antibody - UCB0107. International Congress of the Parkinson’s Disease and Movement Disorders Society Nice, France Late-Breaking Abstracts LBA3 (2019). [Google Scholar]
- 273.Press release UCB. UCB presents UCB0107 anti-Tau immunotherapy Phase I study results at World Movement Disorders Conference®. https://www.ucb.com/stories-media/Press-Releases/article/UCB-presents-UCB0107-anti-Tau-immunotherapy-Phase-I-study-results-at-World-Movement-Disorders-Conference (25 Sep 2019). [Google Scholar]
- 274.Alzforum International Conference on Alzheimer’s and Parkinson’s Diseases 2021. N-Terminal Tau Antibodies Fade, Mid-Domain Ones Push to the Fore. https://www.alzforum.org/news/conference-coverage/n-terminal-tau-antibodies-fade-mid-domain-ones-push-fore (27 Mar 2021). [Google Scholar]
- 275.Alzforum clinical Trials on Alzheimer’s Disease 2021. More Tau Antibodies Bid Adieu; Semorinemab Keeps Foot in Door. https://www.alzforum.org/news/conference-coverage/more-tau-antibodies-bid-adieu-semorinemab-keeps-foot-door (13 Nov 2021).
- 276.Alzforum Therapeutics. BIIB076. https://www.alzforum.org/therapeutics/biib076 (Accessed 2023).
- 277.Biogen (BIIB) Q2 2022. Earnings Call Transcript. [Google Scholar]
- 278.Press release Eisai. EISAI PRESENTS DATA SHOWING QUANTIFICATION OF TAU MICROTUBULE BINDING REGION IN CEREBROSPINAL FLUID AND THE IDENTIFICATION OF A TARGET ENGAGEMENT BIOMARKER FOR THE NEW ANTI-TAU ANTIBODY E2814 AT ALZHEIMER’S ASSOCIATION INTERNATIONAL CONFERENCE (AAIC)2019. https://www.eisai.com/news/2019/news201955.html (19 Jul 2019). [Google Scholar]
- 279.Talma S, Gartlon J, Takahashi E, Horie K, Aoyama M, Wildsmith K, Staddon J, de Silva R, Roberts M Efficacy of the murine version of E2814 in a validated AD brain seed-injection model in hTau mice. Alzheimer’s Association International Conference Amsterdam, Netherlands, and Online P4–673 (2023). [Google Scholar]
- 280.Horie K, Barthelemy NR, Sato C & Bateman RJ CSF tau microtubule binding region identifies tau tangle and clinical stages of Alzheimer’s disease. Brain 144, 515–527, doi: 10.1093/brain/awaa373 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Horie KW TE, Aoyama M, Nakatani, Roberts M, Staddon J, de Silva R, Koyama A. in Alzheimer’s Association International Conference, July 2019 P4–696 (2019). [Google Scholar]
- 282.Alzforum Report. Aiming at the Tangle’s Heart? DIAN-TU Trial to Torpedo Tau’s Core. https://www.alzforum.org/news/research-news/aiming-tangles-heart-dian-tu-trial-torpedo-taus-core (18 Mar 2021).
- 283.Zhou J, Rawal S, Yagi T, Wildsmith KR, Takahashi E, Horie K, Barthelemy NR, Aluri J, Lalovic B, Boyd P, Bateman RJ, Reyderman L E2814: an anti-tau therapy engages its CNS target and affects the downstream tangle-specific biomarker MTBR-tau243 in Dominantly Inherited Alzheimer’s Disease. Alzheimer’s Association International Conference Amsterdam, Netherlands, and Online 2023 (2023). [Google Scholar]
- 284.Rawal S, Yagi T, Boyd P, Aluri J, Wildsmith KR, Reyderman L Safety, Pharmacokinetics and Immunogenicity of Single and Multiple Ascending Doses of the Anti-Tau Therapeutic Antibody E2814: A Phase 1, First-In-Human (FIH) Study in Healthy Subjects. Alzheimer’s Association International Conference Amsterdam, Netherlands, and Online P1–909 (2023). [Google Scholar]
- 285.Horie K et al. CSF MTBR-tau243 is a specific biomarker of tau tangle pathology in Alzheimer’s disease. Nat. Med, doi: 10.1038/s41591-023-02443-z (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Sopko R et al. Characterization of tau binding by gosuranemab. Neurobiol. Dis. 146, 105120, doi: 10.1016/j.nbd.2020.105120 (2020). [DOI] [PubMed] [Google Scholar]
- 287.Alzforum Therapeutics. Gosuranemab. https://www.alzforum.org/therapeutics/gosuranemab (Accessed 2023).
- 288.Qureshi IA et al. A randomized, single ascending dose study of intravenous BIIB092 in healthy participants. Alzheimers Dement (N Y) 4, 746–755, doi: 10.1016/j.trci.2018.10.007 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Boxer AL et al. Safety of the tau-directed monoclonal antibody BIIB092 in progressive supranuclear palsy: a randomised, placebo-controlled, multiple ascending dose phase 1b trial. Lancet Neurol 18, 549–558, doi: 10.1016/s1474-4422(19)30139-5 (2019). [DOI] [PubMed] [Google Scholar]
- 290.Dam T et al. Safety and efficacy of anti-tau monoclonal antibody gosuranemab in progressive supranuclear palsy: a phase 2, randomized, placebo-controlled trial. Nat Med 27, 1451–1457, doi: 10.1038/s41591-021-01455-x (2021). [DOI] [PubMed] [Google Scholar]
- 291.Press release Biogen. Biogen Reports Top-Line Results from Phase 2 Study in Progressive Supranuclear Palsy. https://investors.biogen.com/news-releases/news-release-details/biogen-reports-top-line-results-phase-2-study-progressive (13 Dec 2019). [Google Scholar]
- 292.Alzforum Report. Gosuranemab, Biogen’s Anti-Tau Immunotherapy, Does Not Fly for PSP. https://www.alzforum.org/news/research-news/gosuranemab-biogens-anti-tau-immunotherapy-does-not-fly-psp (13 Dec 2019).
- 293.Press release Biogen. Biogen Announces Topline Results From Phase 2 Study of Gosuranemab, an Anti-Tau Antibody, for Alzheimer’s Disease. https://investors.biogen.com/news-releases/news-release-details/biogen-announces-topline-results-phase-2-study-gosuranemab-anti (16 Jun 2021). [Google Scholar]
- 294.Alzforum Therapeutics. JNJ-63733657. https://www.alzforum.org/therapeutics/jnj-63733657 (Accessed 2023).
- 295.Galpern WR, Mercken M, Van Kolen K, Timmers M, Haeverans K, Janssens L, Triana-Baltzer G, Kolb HC, Jacobs T, Nandy P, Malia T, Sun H, Van Nueten L P1–052: A SINGLE ASCENDING DOSE STUDY TO EVALUATE THE SAFETY, TOLERABILITY, PHARMACOKINETICS, AND PHARMACODYNAMICS OF THE ANTI-PHOSPHO-TAU ANTIBODY JNJ-63733657 IN HEALTHY SUBJECTS. Alzheimer Dement 15, P252–P253 (2019). [Google Scholar]
- 296.Helboe L et al. Highly Specific and Sensitive Target Binding by the Humanized pS396-Tau Antibody hC10.2 Across a Wide Spectrum of Alzheimer’s Disease and Primary Tauopathy Postmortem Brains. J. Alzheimers Dis. 88, 207–228, doi: 10.3233/JAD-220125 (2022). [DOI] [PubMed] [Google Scholar]
- 297.Umeda T, E. H, Kunori Y, Matsumoto Y, Taniguchi T, Mori H, Tomiyama T. Passive immunothrapy of tauopathy targeting pSer413-tau: a pilot study in mice. Ann Clin Transl Neurol 2, 241–255 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Alzforum Therapeutics. MK-2214. https://www.alzforum.org/therapeutics/mk-2214 (Accessed 2023).
- 299.Naserkhaki R et al. cis pT231-Tau Drives Neurodegeneration in Bipolar Disorder. ACS Chem. Neurosci. 10, 1214–1221, doi: 10.1021/acschemneuro.8b00629 (2019). [DOI] [PubMed] [Google Scholar]
- 300.Albayram O et al. Cis P-tau is induced in clinical and preclinical brain injury and contributes to post-injury sequelae. Nature communications 8, 1000, doi: 10.1038/s41467-017-01068-4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Mohsenian Sisakht A et al. Pathogenic cis p-tau levels in CSF reflects severity of traumatic brain injury. Neurol. Res. 44, 496–502, doi: 10.1080/01616412.2021.2022921 (2022). [DOI] [PubMed] [Google Scholar]
- 302.Nakamura K et al. Proline isomer-specific antibodies reveal the early pathogenic tau conformation in Alzheimer’s disease. Cell 149, 232–244, doi: 10.1016/j.cell.2012.02.016 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Qiu C et al. Cis P-tau underlies vascular contribution to cognitive impairment and dementia and can be effectively targeted by immunotherapy in mice. Sci. Transl. Med. 13, doi: 10.1126/scitranslmed.aaz7615 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Alzforum Therapeutics. PRX005. https://www.alzforum.org/therapeutics/prx005 (Accessed 2023).
- 305.Press release prothena. Prothena Reports Topline Phase 1 Single Ascending Dose Study Results of PRX005, a Novel Anti-MTBR-Tau Antibody for the Potential Treatment of Alzheimer’s Disease. https://ir.prothena.com/investors/press-releases/news-details/2023/Prothena-Reports-Topline-Phase-1-Single-Ascending-Dose-Study-Results-of-PRX005-a-Novel-Anti-MTBR-Tau-Antibody-for-the-Potential-Treatment-of-Alzheimers-Disease/default.aspx (1 Jan 2023). [Google Scholar]
- 306.Martenyi F, Campbell B, Kinney GG, Lee J, Johnson AD, Swanson C, Zago W, Garren H P1–727 - PRX005, a novel anti-MTBR tau monoclonal antibody: results from a first-in-human double-blind, placebo-controlled, single ascending dose phase 1 study. Alzheimer’s Association International Conference Amsterdam, Netherlands, and Online P1–03 (2023). [Google Scholar]
- 307.Hasegawa M et al. Characterization of mAb AP422, a novel phosphorylation-dependent monoclonal antibody against tau protein. FEBS Lett. 384, 25–30, doi: 10.1016/0014-5793(96)00271-2 (1996). [DOI] [PubMed] [Google Scholar]
- 308.Bussiere T et al. Phosphorylated serine422 on tau proteins is a pathological epitope found in several diseases with neurofibrillary degeneration. Acta Neuropathol. 97, 221–230, doi: 10.1007/s004010050978 (1999). [DOI] [PubMed] [Google Scholar]
- 309.Alzforum Therapeutics. RG7345. https://www.alzforum.org/therapeutics/rg7345 (Accessed 2023).
- 310.Ayalon G et al. Antibody semorinemab reduces tau pathology in a transgenic mouse model and engages tau in patients with Alzheimer’s disease. Sci. Transl. Med. 13, doi: 10.1126/scitranslmed.abb2639 (2021). [DOI] [PubMed] [Google Scholar]
- 311.Kerchner GA et al. A Phase I study to evaluate the safety and tolerability of RO7105705 in healthy volunteers and patients with mild-to-moderate AD. Alzheimers Dement. 13, doi: 10.1016/j.jalz.2017.07.243 (2017). [DOI] [Google Scholar]
- 312.Press release AC Immune. AC Immune Reports Top Line Results from TAURIEL Phase 2 Trial Evaluating Semorinemab in Early Alzheimer’s Disease. https://ir.acimmune.com/news-releases/news-release-details/ac-immune-reports-top-line-results-tauriel-phase-2-trial (September 23, 2020). [Google Scholar]
- 313.Alzforum Report. First Cognitive Signal that Tau Immunotherapy Works? https://www.alzforum.org/news/research-news/first-cognitive-signal-tau-immunotherapy-works (02 Sep 2021).
- 314.Monteiro C et al. Randomized Phase II Study of the Safety and Efficacy of Semorinemab in Participants With Mild-to-Moderate Alzheimer Disease: Lauriet. Neurology 101, e1391–e1401, doi: 10.1212/WNL.0000000000207663 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Alzforum therapeutics. Tilvonemab. https://www.alzforum.org/therapeutics/tilavonemab (Accessed 2023).
- 316.West T et al. Preclinical and Clinical Development of ABBV-8E12, a Humanized Anti-Tau Antibody, for Treatment of Alzheimer’s Disease and Other Tauopathies. J Prev Alzheimers Dis 4, 236–241, doi: 10.14283/jpad.2017.36 (2017). [DOI] [PubMed] [Google Scholar]
- 317.Hoglinger GU et al. Safety and efficacy of tilavonemab in progressive supranuclear palsy: a phase 2, randomised, placebo-controlled trial. Lancet Neurol. 20, 182–192, doi: 10.1016/S1474-4422(20)30489-0 (2021). [DOI] [PubMed] [Google Scholar]
- 318.Koga S, Dickson DW & Wszolek ZK Neuropathology of progressive supranuclear palsy after treatment with tilavonemab. Lancet Neurol. 20, 786–787, doi: 10.1016/S1474-4422(21)00283-0 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Alzforum report. AbbVie’s Tau Antibody Flops in Progressive Supranuclear Palsy. https://www.alzforum.org/news/research-news/abbvies-tau-antibody-flops-progressive-supranuclear-palsy (26 Jul 2019).
- 320.Florian H et al. Tilavonemab in early Alzheimer’s disease: results from a phase 2, randomized, double-blind study. Brain 146, 2275–2284, doi: 10.1093/brain/awad024 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Chai X et al. Passive immunization with anti-Tau antibodies in two transgenic models: reduction of Tau pathology and delay of disease progression. J. Biol. Chem. 286, 34457–34467, doi: 10.1074/jbc.M111.229633 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Jicha GA, Bowser R, Kazam IG & Davies P Alz-50 and MC-1, a new monoclonal antibody raised to paired helical filaments, recognize conformational epitopes on recombinant tau. J. Neurosci. Res. 48, 128–132, doi: (1997). [DOI] [PubMed] [Google Scholar]
- 323.Vitale F et al. Anti-tau conformational scFv MC1 antibody efficiently reduces pathological tau species in adult JNPL3 mice. Acta neuropathologica communications 6, 82, doi: 10.1186/s40478-018-0585-2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Lilly Q3 2021. Earnings Call. [Google Scholar]
- 325.Sigurdsson EM Alzheimer’s therapy development: A few points to consider. Prog. Mol. Biol. Transl. Sci. 168, 205–217, doi: 10.1016/bs.pmbts.2019.06.001 (2019). [DOI] [PubMed] [Google Scholar]
- 326.Han P et al. A Quantitative Analysis of Brain Soluble Tau and the Tau Secretion Factor. J. Neuropathol. Exp. Neurol. 76, 44–51, doi: 10.1093/jnen/nlw105 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Sato C et al. Tau Kinetics in Neurons and the Human Central Nervous System. Neuron 97, 1284–1298 e1287, doi: 10.1016/j.neuron.2018.02.015 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Barthelemy NR et al. Differential Mass Spectrometry Profiles of Tau Protein in the Cerebrospinal Fluid of Patients with Alzheimer’s Disease, Progressive Supranuclear Palsy, and Dementia with Lewy Bodies. J. Alzheimers Dis. 51, 1033–1043, doi: 10.3233/JAD-150962 (2016). [DOI] [PubMed] [Google Scholar]
- 329.Barthelemy NR et al. Tau Protein Quantification in Human Cerebrospinal Fluid by Targeted Mass Spectrometry at High Sequence Coverage Provides Insights into Its Primary Structure Heterogeneity. J. Proteome Res. 15, 667–676, doi: 10.1021/acs.jproteome.5b01001 (2016). [DOI] [PubMed] [Google Scholar]
- 330.Barthelemy NR, Mallipeddi N, Moiseyev P, Sato C & Bateman RJ Tau Phosphorylation Rates Measured by Mass Spectrometry Differ in the Intracellular Brain vs. Extracellular Cerebrospinal Fluid Compartments and Are Differentially Affected by Alzheimer’s Disease. Front. Aging Neurosci. 11, 121, doi: 10.3389/fnagi.2019.00121 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Wagshal D et al. Divergent CSF tau alterations in two common tauopathies: Alzheimer’s disease and progressive supranuclear palsy. J. Neurol. Neurosurg. Psychiatry 86, 244–250, doi: 10.1136/jnnp-2014-308004 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Hall S et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch. Neurol. 69, 1445–1452, doi: 10.1001/archneurol.2012.1654 (2012). [DOI] [PubMed] [Google Scholar]
- 333.Hu WT, Trojanowski JQ & Shaw LM Biomarkers in frontotemporal lobar degenerations--progress and challenges. Prog. Neurobiol. 95, 636–648, doi: 10.1016/j.pneurobio.2011.04.012 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Olsson B et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol. 15, 673–684, doi: 10.1016/S1474-4422(16)00070-3 (2016). [DOI] [PubMed] [Google Scholar]
- 335.Bian H et al. CSF biomarkers in frontotemporal lobar degeneration with known pathology. Neurology 70, 1827–1835, doi: 10.1212/01.wnl.0000311445.21321.fc (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Grossman M et al. Cerebrospinal fluid profile in frontotemporal dementia and Alzheimer’s disease. Ann. Neurol. 57, 721–729, doi: 10.1002/ana.20477 (2005). [DOI] [PubMed] [Google Scholar]
- 337.Horie K et al. CSF tau microtubule-binding region identifies pathological changes in primary tauopathies. Nat. Med. 28, 2547–2554, doi: 10.1038/s41591-022-02075-9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Kanmert D. et al. C-Terminally Truncated Forms of Tau, But Not Full-Length Tau or Its C-Terminal Fragments, Are Released from Neurons Independently of Cell Death. J. Neurosci. 35, 10851–10865, doi: 10.1523/JNEUROSCI.0387-15.2015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Jadhav S et al. A walk through tau therapeutic strategies. Acta neuropathologica communications 7, 22, doi: 10.1186/s40478-019-0664-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Zhang W et al. Novel tau filament fold in corticobasal degeneration. Nature 580, 283–287, doi: 10.1038/s41586-020-2043-0 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Falcon B et al. Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature 568, 420–423, doi: 10.1038/s41586-019-1026-5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Scheres SH, Zhang W, Falcon B & Goedert M Cryo-EM structures of tau filaments. Curr. Opin. Struct. Biol. 64, 17–25, doi: 10.1016/j.sbi.2020.05.011 (2020). [DOI] [PubMed] [Google Scholar]
- 343.Wu L, Gilyazova N, Ervin JF, Wang SJ & Xu B Site-Specific Phospho-Tau Aggregation-Based Biomarker Discovery for AD Diagnosis and Differentiation. ACS Chem. Neurosci. 13, 3281–3290, doi: 10.1021/acschemneuro.2c00342 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Reid MJ, Beltran-Lobo P, Johnson L, Perez-Nievas BG & Noble W Astrocytes in Tauopathies. Front. Neurol. 11, 572850, doi: 10.3389/fneur.2020.572850 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Kahlson MA & Colodner KJ Glial Tau Pathology in Tauopathies: Functional Consequences. J. Exp. Neurosci. 9, 43–50, doi: 10.4137/JEN.S25515 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Ferrer I et al. Involvement of Oligodendrocytes in Tau Seeding and Spreading in Tauopathies. Front. Aging Neurosci. 11, 112, doi: 10.3389/fnagi.2019.00112 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Narasimhan S et al. Human tau pathology transmits glial tau aggregates in the absence of neuronal tau. J. Exp. Med. 217, doi: 10.1084/jem.20190783 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Congdon EE et al. Single domain antibodies targeting pathological tau protein: Influence of four IgG subclasses on efficacy and toxicity. EBioMedicine 84, 104249, doi: 10.1016/j.ebiom.2022.104249 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Morell A, Terry WD & Waldmann TA Metabolic properties of IgG subclasses in man. J. Clin. Invest. 49, 673–680, doi: 10.1172/JCI106279 (1970). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Handlogten MW et al. Prevention of Fab-arm exchange and antibody reduction via stabilization of the IgG4 hinge region. MAbs 12, 1779974, doi: 10.1080/19420862.2020.1779974 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Heads JT et al. Electrostatic interactions modulate the differential aggregation propensities of IgG1 and IgG4P antibodies and inform charged residue substitutions for improved developability. Protein Eng. Des. Sel. 32, 277–288, doi: 10.1093/protein/gzz046 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Pepinsky RB et al. Improving the solubility of anti-LINGO-1 monoclonal antibody Li33 by isotype switching and targeted mutagenesis. Protein Sci. 19, 954–966, doi: 10.1002/pro.372 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Cooper LJ et al. Role of heavy chain constant domains in antibody-antigen interaction. Apparent specificity differences among streptococcal IgG antibodies expressing identical variable domains. J. Immunol. 150, 2231–2242 (1993). [PubMed] [Google Scholar]
- 354.Pritsch O et al. Can immunoglobulin C(H)1 constant region domain modulate antigen binding affinity of antibodies? J. Clin. Invest. 98, 2235–2243, doi: 10.1172/JCI119033 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Pritsch O et al. Can isotype switch modulate antigen-binding affinity and influence clonal selection? Eur. J. Immunol. 30, 3387–3395, doi: (2000). [DOI] [PubMed] [Google Scholar]
- 356.Hovenden M et al. IgG subclass and heavy chain domains contribute to binding and protection by mAbs to the poly gamma-D-glutamic acid capsular antigen of Bacillus anthracis. PLoS Pathog. 9, e1003306, doi: 10.1371/journal.ppat.1003306 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Motley MP, Diago-Navarro E, Banerjee K, Inzerillo S & Fries BC The Role of IgG Subclass in Antibody-Mediated Protection against Carbapenem-Resistant Klebsiella pneumoniae. mBio 11, doi: 10.1128/mBio.02059-20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Tudor D et al. Isotype modulates epitope specificity, affinity, and antiviral activities of anti-HIV-1 human broadly neutralizing 2F5 antibody. Proc. Natl. Acad. Sci. U. S. A. 109, 12680–12685, doi: 10.1073/pnas.1200024109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Xia Y, Janda A, Eryilmaz E, Casadevall A & Putterman C The constant region affects antigen binding of antibodies to DNA by altering secondary structure. Mol. Immunol. 56, 28–37, doi: 10.1016/j.molimm.2013.04.004 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Dam TK, Torres M, Brewer CF & Casadevall A Isothermal titration calorimetry reveals differential binding thermodynamics of variable region-identical antibodies differing in constant region for a univalent ligand. J. Biol. Chem. 283, 31366–31370, doi: 10.1074/jbc.M806473200 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Janda A & Casadevall A Circular Dichroism reveals evidence of coupling between immunoglobulin constant and variable region secondary structure. Mol. Immunol. 47, 1421–1425, doi: 10.1016/j.molimm.2010.02.018 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Kato K et al. Carbon-13 NMR study of switch variant anti-dansyl antibodies: antigen binding and domain-domain interactions. Biochemistry 30, 6604–6610, doi: 10.1021/bi00240a033 (1991). [DOI] [PubMed] [Google Scholar]
- 363.McLean GR, Torres M, Elguezabal N, Nakouzi A & Casadevall A Isotype can affect the fine specificity of an antibody for a polysaccharide antigen. J. Immunol. 169, 1379–1386, doi: 10.4049/jimmunol.169.3.1379 (2002). [DOI] [PubMed] [Google Scholar]
- 364.Torres M, Fernandez-Fuentes N, Fiser A & Casadevall A The immunoglobulin heavy chain constant region affects kinetic and thermodynamic parameters of antibody variable region interactions with antigen. J. Biol. Chem. 282, 13917–13927, doi: 10.1074/jbc.M700661200 (2007). [DOI] [PubMed] [Google Scholar]
- 365.Torres M, Fernandez-Fuentes N, Fiser A & Casadevall A Exchanging murine and human immunoglobulin constant chains affects the kinetics and thermodynamics of antigen binding and chimeric antibody autoreactivity. PLoS One 2, e1310, doi: 10.1371/journal.pone.0001310 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Torres M, May R, Scharff MD & Casadevall A Variable-region-identical antibodies differing in isotype demonstrate differences in fine specificity and idiotype. J. Immunol. 174, 2132–2142, doi: 10.4049/jimmunol.174.4.2132 (2005). [DOI] [PubMed] [Google Scholar]
- 367.Xia Y et al. The constant region contributes to the antigenic specificity and renal pathogenicity of murine anti-DNA antibodies. J. Autoimmun. 39, 398–411, doi: 10.1016/j.jaut.2012.06.005 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Yuan RR et al. Isotype switching increases efficacy of antibody protection against Cryptococcus neoformans infection in mice. Infect. Immun. 66, 1057–1062, doi: 10.1128/IAI.66.3.1057-1062.1998 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Labrijn AF et al. Therapeutic IgG4 antibodies engage in Fab-arm exchange with endogenous human IgG4 in vivo. Nat. Biotechnol. 27, 767–771, doi: 10.1038/nbt.1553 (2009). [DOI] [PubMed] [Google Scholar]
- 370.Young E et al. Estimation of polyclonal IgG4 hybrids in normal human serum. Immunology 142, 406–413, doi: 10.1111/imm.12265 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Boutajangout A, Quartermain D & Sigurdsson EM Immunotherapy targeting pathological tau prevents cognitive decline in a new tangle mouse model. J. Neurosci. 30, 16559–16566, doi: 10.1523/JNEUROSCI.4363-10.2010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.d’Abramo C, Acker CM, Jimenez HT & Davies P Tau passive immunotherapy in mutant P301L mice: antibody affinity versus specificity. PLoS One 8, e62402, doi: 10.1371/journal.pone.0062402 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Hintersteiner B et al. Charge heterogeneity: Basic antibody charge variants with increased binding to Fc receptors. MAbs 8, 1548–1560, doi: 10.1080/19420862.2016.1225642 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Schoch A et al. Charge-mediated influence of the antibody variable domain on FcRn-dependent pharmacokinetics. Proc. Natl. Acad. Sci. U. S. A. 112, 5997–6002, doi: 10.1073/pnas.1408766112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Khawli LA, Glasky MS, Alauddin MM & Epstein AL Improved tumor localization and radioimaging with chemically modified monoclonal antibodies. Cancer Biother. Radiopharm. 11, 203–215, doi: 10.1089/cbr.1996.11.203 (1996). [DOI] [PubMed] [Google Scholar]
- 376.Kobayashi H et al. The pharmacokinetic characteristics of glycolated humanized anti-Tac Fabs are determined by their isoelectric points. Cancer Res. 59, 422–430 (1999). [PubMed] [Google Scholar]
- 377.Datta-Mannan A et al. Balancing charge in the complementarity-determining regions of humanized mAbs without affecting pI reduces non-specific binding and improves the pharmacokinetics. MAbs 7, 483–493, doi: 10.1080/19420862.2015.1016696 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Li B et al. Framework selection can influence pharmacokinetics of a humanized therapeutic antibody through differences in molecule charge. MAbs 6, 1255–1264, doi: 10.4161/mabs.29809 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Chatterjee D et al. Proteasome-targeted nanobodies alleviate pathology and functional decline in an alpha-synuclein-based Parkinson’s disease model. NPJ Parkinsons Dis 4, 25, doi: 10.1038/s41531-018-0062-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Butler DC et al. Bifunctional Anti-Non-Amyloid Component alpha-Synuclein Nanobodies Are Protective In Situ. PLoS One 11, e0165964, doi: 10.1371/journal.pone.0165964 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Lim S et al. bioPROTACs as versatile modulators of intracellular therapeutic targets including proliferating cell nuclear antigen (PCNA). Proc. Natl. Acad. Sci. U. S. A. 117, 5791–5800, doi: 10.1073/pnas.1920251117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Ibrahim AFM et al. Antibody RING-Mediated Destruction of Endogenous Proteins. Mol. Cell 79, 155–166 e159, doi: 10.1016/j.molcel.2020.04.032 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Roth S et al. Targeting Endogenous K-RAS for Degradation through the Affinity-Directed Protein Missile System. Cell Chem Biol 27, 1151–1163 e1156, doi: 10.1016/j.chembiol.2020.06.012 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Wang W et al. A novel small-molecule PROTAC selectively promotes tau clearance to improve cognitive functions in Alzheimer-like models. Theranostics 11, 5279–5295, doi: 10.7150/thno.55680 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Silva MC et al. Targeted degradation of aberrant tau in frontotemporal dementia patient-derived neuronal cell models. Elife 8, doi: 10.7554/eLife.45457 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Chu TT et al. Specific Knockdown of Endogenous Tau Protein by Peptide-Directed Ubiquitin-Proteasome Degradation. Cell Chem Biol 23, 453–461, doi: 10.1016/j.chembiol.2016.02.016 (2016). [DOI] [PubMed] [Google Scholar]
- 387.Lu M et al. Discovery of a Keap1-dependent peptide PROTAC to knockdown Tau by ubiquitination-proteasome degradation pathway. Eur. J. Med. Chem. 146, 251–259, doi: 10.1016/j.ejmech.2018.01.063 (2018). [DOI] [PubMed] [Google Scholar]
- 388.Bhatia S, Singh M, Singh T & Singh V Scrutinizing the Therapeutic Potential of PROTACs in the Management of Alzheimer’s Disease. Neurochem. Res. 48, 13–25, doi: 10.1007/s11064-022-03722-w (2023). [DOI] [PubMed] [Google Scholar]
- 389.Gallardo G et al. Targeting tauopathy with engineered tau-degrading intrabodies. Mol. Neurodegener. 14, 38, doi: 10.1186/s13024-019-0340-6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Butler DC & Messer A Bifunctional anti-huntingtin proteasome-directed intrabodies mediate efficient degradation of mutant huntingtin exon 1 protein fragments. PLoS One 6, e29199, doi: 10.1371/journal.pone.0029199 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Igawa T, Haraya K & Hattori K Sweeping antibody as a novel therapeutic antibody modality capable of eliminating soluble antigens from circulation. Immunol. Rev. 270, 132–151, doi: 10.1111/imr.12392 (2016). [DOI] [PubMed] [Google Scholar]
- 392.Banik SM et al. Lysosome-targeting chimaeras for degradation of extracellular proteins. Nature 584, 291–297, doi: 10.1038/s41586-020-2545-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Ahn G et al. LYTACs that engage the asialoglycoprotein receptor for targeted protein degradation. Nat. Chem. Biol. 17, 937–946, doi: 10.1038/s41589-021-00770-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Takahashi D et al. AUTACs: Cargo-Specific Degraders Using Selective Autophagy. Mol. Cell 76, 797–810 e710, doi: 10.1016/j.molcel.2019.09.009 (2019). [DOI] [PubMed] [Google Scholar]
- 395.Takahashi D & Arimoto H Targeting selective autophagy by AUTAC degraders. Autophagy 16, 765–766, doi: 10.1080/15548627.2020.1718362 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Sawa T et al. Protein S-guanylation by the biological signal 8-nitroguanosine 3’,5’-cyclic monophosphate. Nat. Chem. Biol. 3, 727–735, doi: 10.1038/nchembio.2007.33 (2007). [DOI] [PubMed] [Google Scholar]
- 397.Cotton AD, Nguyen DP, Gramespacher JA, Seiple IB & Wells JA Development of Antibody-Based PROTACs for the Degradation of the Cell-Surface Immune Checkpoint Protein PD-L1. J. Am. Chem. Soc. 143, 593–598, doi: 10.1021/jacs.0c10008 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Cha-Molstad H et al. p62/SQSTM1/Sequestosome-1 is an N-recognin of the N-end rule pathway which modulates autophagosome biogenesis. Nature communications 8, 102, doi: 10.1038/s41467-017-00085-7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Ji CH et al. The AUTOTAC chemical biology platform for targeted protein degradation via the autophagy-lysosome system. Nature communications 13, 904, doi: 10.1038/s41467-022-28520-4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Song W et al. Ceria nanoparticles stabilized by organic surface coatings activate the lysosome-autophagy system and enhance autophagic clearance. ACS Nano 8, 10328–10342, doi: 10.1021/nn505073u (2014). [DOI] [PubMed] [Google Scholar]
- 401.Sun H et al. A Tauopathy-Homing and Autophagy-Activating Nanoassembly for Specific Clearance of Pathogenic Tau in Alzheimer’s Disease. ACS Nano 15, 5263–5275, doi: 10.1021/acsnano.0c10690 (2021). [DOI] [PubMed] [Google Scholar]
- 402.Zheng J et al. A novel dephosphorylation targeting chimera selectively promoting tau removal in tauopathies. Signal Transduct Target Ther 6, 269, doi: 10.1038/s41392-021-00669-2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Krishnaswamy S et al. In Vivo Imaging of Tauopathy in Mice. Methods Mol. Biol. 1779, 513–526, doi: 10.1007/978-1-4939-7816-8_32 (2018). [DOI] [PubMed] [Google Scholar]
- 404.Ising C et al. AAV-mediated expression of anti-tau scFvs decreases tau accumulation in a mouse model of tauopathy. J. Exp. Med. 214, 1227–1238, doi: 10.1084/jem.20162125 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Nisbet RM et al. Combined effects of scanning ultrasound and a tau-specific single chain antibody in a tau transgenic mouse model. Brain, doi: 10.1093/brain/awx052 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Spencer B et al. Selective targeting of 3 repeat Tau with brain penetrating single chain antibodies for the treatment of neurodegenerative disorders. Acta Neuropathol. 136, 69–87, doi: 10.1007/s00401-018-1869-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Jiang Y et al. Single-domain antibody-based noninvasive in vivo imaging of alpha-synuclein or tau pathology. Sci Adv 9, eadf3775, doi: 10.1126/sciadv.adf3775 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Danis C et al. Inhibition of Tau seeding by targeting Tau nucleation core within neurons with a single domain antibody fragment. Mol. Ther. 30, 1484–1499, doi: 10.1016/j.ymthe.2022.01.009 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Li T et al. Camelid single-domain antibodies: A versatile tool for in vivo imaging of extracellular and intracellular brain targets. J. Control. Release 243, 1–10, doi: 10.1016/j.jconrel.2016.09.019 (2016). [DOI] [PubMed] [Google Scholar]
- 410.Marino M & Holt MG AAV Vector-Mediated Antibody Delivery (A-MAD) in the Central Nervous System. Front. Neurol. 13, 870799, doi: 10.3389/fneur.2022.870799 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Chen YH et al. Administration of AAV-Alpha Synuclein NAC Antibody Improves Locomotor Behavior in Rats Overexpressing Alpha Synuclein. Genes (Basel) 12, doi: 10.3390/genes12060948 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Butler YR et al. alpha-Synuclein fibril-specific nanobody reduces prion-like alpha-synuclein spreading in mice. Nature communications 13, 4060, doi: 10.1038/s41467-022-31787-2 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Chen YH et al. Downregulation of alpha-Synuclein Protein Levels by an Intracellular Single-Chain Antibody. J. Parkinsons Dis. 10, 573–590, doi: 10.3233/JPD-191787 (2020). [DOI] [PubMed] [Google Scholar]
- 414.Zhou C, Emadi S, Sierks MR & Messer A A human single-chain Fv intrabody blocks aberrant cellular effects of overexpressed alpha-synuclein. Mol. Ther. 10, 1023–1031, doi: 10.1016/j.ymthe.2004.08.019 (2004). [DOI] [PubMed] [Google Scholar]
- 415.Wang J et al. Research Progress and Applications of Multivalent, Multispecific and Modified Nanobodies for Disease Treatment. Front. Immunol. 12, 838082, doi: 10.3389/fimmu.2021.838082 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Jovcevska I & Muyldermans S The Therapeutic Potential of Nanobodies. Biodrugs 34, 11–26, doi: 10.1007/s40259-019-00392-z (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Ghosh P, Dahms NM & Kornfeld S Mannose 6-phosphate receptors: new twists in the tale. Nat. Rev. Mol. Cell Biol. 4, 202–212, doi: 10.1038/nrm1050 (2003). [DOI] [PubMed] [Google Scholar]
- 418.Gary-Bobo M, Nirde P, Jeanjean A, Morere A & Garcia M Mannose 6-phosphate receptor targeting and its applications in human diseases. Curr. Med. Chem. 14, 2945–2953, doi: 10.2174/092986707782794005 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Kyttala A, Heinonen O, Peltonen L & Jalanko A Expression and endocytosis of lysosomal aspartylglucosaminidase in mouse primary neurons. J. Neurosci. 18, 7750–7756, doi: 10.1523/JNEUROSCI.18-19-07750.1998 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Hawkes C & Kar S Insulin-like growth factor-II/mannose-6-phosphate receptor: widespread distribution in neurons of the central nervous system including those expressing cholinergic phenotype. J. Comp. Neurol. 458, 113–127, doi: 10.1002/cne.10578 (2003). [DOI] [PubMed] [Google Scholar]
- 421.Couce ME, Weatherington AJ & McGinty JF Expression of insulin-like growth factor-II (IGF-II) and IGF-II/mannose-6-phosphate receptor in the rat hippocampus: an in situ hybridization and immunocytochemical study. Endocrinology 131, 1636–1642, doi: 10.1210/endo.131.4.1396308 (1992). [DOI] [PubMed] [Google Scholar]
- 422.Jabbari E & Duff KE Tau-targeting antibody therapies: too late, wrong epitope or wrong target? Nat. Med. 27, 1341–1342, doi: 10.1038/s41591-021-01465-9 (2021). [DOI] [PubMed] [Google Scholar]
- 423.Bespalov A, Courade JP, Khiroug L, Terstappen GC & Wang Y A call for better understanding of target engagement in Tau antibody development. Drug Discov Today 27, 103338, doi: 10.1016/j.drudis.2022.103338 (2022). [DOI] [PubMed] [Google Scholar]
- 424.Sigurdsson EM Tau Immunotherapies for Alzheimer’s Disease and Related Tauopathies: Progress and Potential Pitfalls. J. Alzheimers Dis. 64, S555–S565, doi: 10.3233/JAD-179937 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Wu Q et al. Increased neuronal activity in motor cortex reveals prominent calcium dyshomeostasis in tauopathy mice. Neurobiol. Dis. 147, 105165, doi: 10.1016/j.nbd.2020.105165 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


